User login
VIDEO: Start varenicline during hospitalization for heart attack
ORLANDO – Initiating varenicline while smokers are hospitalized for acute coronary syndrome (ACS) is an effective strategy for boosting 6-month smoking abstinence rates in this extremely high–cardiovascular risk population, Dr. Mark J. Eisenberg said in an interview at the American Heart Association scientific sessions.
At the meeting, Dr. Eisenberg presented the results of the EVITA trial, a multicenter, randomized, double-blind study of 303 U.S. and Canadian patients, all longtime smokers, who began a 12-week course of varenicline (Chantix) at 1 mg twice daily or placebo while hospitalized for ACS. Participants had been smokers for an average of 36 years and smoked 22 cigarettes per day at the time of their ACS.
The 24-week rate of biochemically confirmed abstinence in the placebo group was 32.5%. That’s typical. Numerous studies have shown that less than one-third of smokers remain abstinent once discharged from the hospital following an ACS.
In contrast, the 24-week abstinence rate in the varenicline group was 47.3%. The number needed to treat with varenicline to achieve one additional quitter through 6 months of follow-up was 6.8, according to Dr. Eisenberg, professor of medicine at McGill University and director of the cardiovascular health services research program at Jewish General Hospital, both in Montreal.
Observers hailed this as a practice-changing study, and Dr. Eisenberg concurred. Noting that cardiologists are already comfortable in starting ACS patients on statins, beta-blockers, and aspirin while in hospital, he predicted physicians will seize this unique opportunity to help patients quit smoking as well.
“This is a teachable moment,” Dr. Eisenberg observed. “The public health benefit for smoking cessation in this population is huge. You can cut their risk of death and significant morbidity in half if you can get them to stop smoking.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Initiating varenicline while smokers are hospitalized for acute coronary syndrome (ACS) is an effective strategy for boosting 6-month smoking abstinence rates in this extremely high–cardiovascular risk population, Dr. Mark J. Eisenberg said in an interview at the American Heart Association scientific sessions.
At the meeting, Dr. Eisenberg presented the results of the EVITA trial, a multicenter, randomized, double-blind study of 303 U.S. and Canadian patients, all longtime smokers, who began a 12-week course of varenicline (Chantix) at 1 mg twice daily or placebo while hospitalized for ACS. Participants had been smokers for an average of 36 years and smoked 22 cigarettes per day at the time of their ACS.
The 24-week rate of biochemically confirmed abstinence in the placebo group was 32.5%. That’s typical. Numerous studies have shown that less than one-third of smokers remain abstinent once discharged from the hospital following an ACS.
In contrast, the 24-week abstinence rate in the varenicline group was 47.3%. The number needed to treat with varenicline to achieve one additional quitter through 6 months of follow-up was 6.8, according to Dr. Eisenberg, professor of medicine at McGill University and director of the cardiovascular health services research program at Jewish General Hospital, both in Montreal.
Observers hailed this as a practice-changing study, and Dr. Eisenberg concurred. Noting that cardiologists are already comfortable in starting ACS patients on statins, beta-blockers, and aspirin while in hospital, he predicted physicians will seize this unique opportunity to help patients quit smoking as well.
“This is a teachable moment,” Dr. Eisenberg observed. “The public health benefit for smoking cessation in this population is huge. You can cut their risk of death and significant morbidity in half if you can get them to stop smoking.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Initiating varenicline while smokers are hospitalized for acute coronary syndrome (ACS) is an effective strategy for boosting 6-month smoking abstinence rates in this extremely high–cardiovascular risk population, Dr. Mark J. Eisenberg said in an interview at the American Heart Association scientific sessions.
At the meeting, Dr. Eisenberg presented the results of the EVITA trial, a multicenter, randomized, double-blind study of 303 U.S. and Canadian patients, all longtime smokers, who began a 12-week course of varenicline (Chantix) at 1 mg twice daily or placebo while hospitalized for ACS. Participants had been smokers for an average of 36 years and smoked 22 cigarettes per day at the time of their ACS.
The 24-week rate of biochemically confirmed abstinence in the placebo group was 32.5%. That’s typical. Numerous studies have shown that less than one-third of smokers remain abstinent once discharged from the hospital following an ACS.
In contrast, the 24-week abstinence rate in the varenicline group was 47.3%. The number needed to treat with varenicline to achieve one additional quitter through 6 months of follow-up was 6.8, according to Dr. Eisenberg, professor of medicine at McGill University and director of the cardiovascular health services research program at Jewish General Hospital, both in Montreal.
Observers hailed this as a practice-changing study, and Dr. Eisenberg concurred. Noting that cardiologists are already comfortable in starting ACS patients on statins, beta-blockers, and aspirin while in hospital, he predicted physicians will seize this unique opportunity to help patients quit smoking as well.
“This is a teachable moment,” Dr. Eisenberg observed. “The public health benefit for smoking cessation in this population is huge. You can cut their risk of death and significant morbidity in half if you can get them to stop smoking.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AHA SCIENTIFIC SESSIONS
Most Women Remain Unconcerned About Heart Risk
ORLANDO – Women’s awareness of the health risk posed by heart disease has stalled, with fully 45% of participants in a nationally representative survey being unaware that heart disease is the number-one killer of U.S. women.
The survey of 1,011 women was commissioned by the Women’s Heart Alliance. The results have provided the group with fresh ideas about how to increase awareness and motivate women to ask their physicians about their heart health, Dr. Holly S. Andersen said in an interview at the American Heart Association scientific sessions.
A key survey finding was that only 27% of women were able to name a woman in their life with heart disease. Even fewer – a mere 11% – could name a woman who has died from it. But the women who had that personal connection to heart disease were 50% more likely to describe themselves as “somewhat or very concerned” about their own risk, and they were also more likely to have asked their physicians about it.
One important strategy going forward will be to focus public education efforts on making heart disease more real and personal for women in an effort to encourage them to learn their personal risk status and take action as warranted, according to Dr. Andersen, scientific adviser to the Women’s Heart Alliance and a cardiologist at New York-Presbyterian Hospital.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Women’s awareness of the health risk posed by heart disease has stalled, with fully 45% of participants in a nationally representative survey being unaware that heart disease is the number-one killer of U.S. women.
The survey of 1,011 women was commissioned by the Women’s Heart Alliance. The results have provided the group with fresh ideas about how to increase awareness and motivate women to ask their physicians about their heart health, Dr. Holly S. Andersen said in an interview at the American Heart Association scientific sessions.
A key survey finding was that only 27% of women were able to name a woman in their life with heart disease. Even fewer – a mere 11% – could name a woman who has died from it. But the women who had that personal connection to heart disease were 50% more likely to describe themselves as “somewhat or very concerned” about their own risk, and they were also more likely to have asked their physicians about it.
One important strategy going forward will be to focus public education efforts on making heart disease more real and personal for women in an effort to encourage them to learn their personal risk status and take action as warranted, according to Dr. Andersen, scientific adviser to the Women’s Heart Alliance and a cardiologist at New York-Presbyterian Hospital.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Women’s awareness of the health risk posed by heart disease has stalled, with fully 45% of participants in a nationally representative survey being unaware that heart disease is the number-one killer of U.S. women.
The survey of 1,011 women was commissioned by the Women’s Heart Alliance. The results have provided the group with fresh ideas about how to increase awareness and motivate women to ask their physicians about their heart health, Dr. Holly S. Andersen said in an interview at the American Heart Association scientific sessions.
A key survey finding was that only 27% of women were able to name a woman in their life with heart disease. Even fewer – a mere 11% – could name a woman who has died from it. But the women who had that personal connection to heart disease were 50% more likely to describe themselves as “somewhat or very concerned” about their own risk, and they were also more likely to have asked their physicians about it.
One important strategy going forward will be to focus public education efforts on making heart disease more real and personal for women in an effort to encourage them to learn their personal risk status and take action as warranted, according to Dr. Andersen, scientific adviser to the Women’s Heart Alliance and a cardiologist at New York-Presbyterian Hospital.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AHA SCIENTIFIC SESSIONS
VIDEO: Most women remain unconcerned about heart risk
ORLANDO – Women’s awareness of the health risk posed by heart disease has stalled, with fully 45% of participants in a nationally representative survey being unaware that heart disease is the number-one killer of U.S. women.
The survey of 1,011 women was commissioned by the Women’s Heart Alliance. The results have provided the group with fresh ideas about how to increase awareness and motivate women to ask their physicians about their heart health, Dr. Holly S. Andersen said in an interview at the American Heart Association scientific sessions.
A key survey finding was that only 27% of women were able to name a woman in their life with heart disease. Even fewer – a mere 11% – could name a woman who has died from it. But the women who had that personal connection to heart disease were 50% more likely to describe themselves as “somewhat or very concerned” about their own risk, and they were also more likely to have asked their physicians about it.
One important strategy going forward will be to focus public education efforts on making heart disease more real and personal for women in an effort to encourage them to learn their personal risk status and take action as warranted, according to Dr. Andersen, scientific adviser to the Women’s Heart Alliance and a cardiologist at New York-Presbyterian Hospital.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Women’s awareness of the health risk posed by heart disease has stalled, with fully 45% of participants in a nationally representative survey being unaware that heart disease is the number-one killer of U.S. women.
The survey of 1,011 women was commissioned by the Women’s Heart Alliance. The results have provided the group with fresh ideas about how to increase awareness and motivate women to ask their physicians about their heart health, Dr. Holly S. Andersen said in an interview at the American Heart Association scientific sessions.
A key survey finding was that only 27% of women were able to name a woman in their life with heart disease. Even fewer – a mere 11% – could name a woman who has died from it. But the women who had that personal connection to heart disease were 50% more likely to describe themselves as “somewhat or very concerned” about their own risk, and they were also more likely to have asked their physicians about it.
One important strategy going forward will be to focus public education efforts on making heart disease more real and personal for women in an effort to encourage them to learn their personal risk status and take action as warranted, according to Dr. Andersen, scientific adviser to the Women’s Heart Alliance and a cardiologist at New York-Presbyterian Hospital.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Women’s awareness of the health risk posed by heart disease has stalled, with fully 45% of participants in a nationally representative survey being unaware that heart disease is the number-one killer of U.S. women.
The survey of 1,011 women was commissioned by the Women’s Heart Alliance. The results have provided the group with fresh ideas about how to increase awareness and motivate women to ask their physicians about their heart health, Dr. Holly S. Andersen said in an interview at the American Heart Association scientific sessions.
A key survey finding was that only 27% of women were able to name a woman in their life with heart disease. Even fewer – a mere 11% – could name a woman who has died from it. But the women who had that personal connection to heart disease were 50% more likely to describe themselves as “somewhat or very concerned” about their own risk, and they were also more likely to have asked their physicians about it.
One important strategy going forward will be to focus public education efforts on making heart disease more real and personal for women in an effort to encourage them to learn their personal risk status and take action as warranted, according to Dr. Andersen, scientific adviser to the Women’s Heart Alliance and a cardiologist at New York-Presbyterian Hospital.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AHA SCIENTIFIC SESSIONS
Answers elusive in quest for better chlamydia treatment
SAN DIEGO – The hottest topic today in the treatment of sexually transmitted diseases caused by Chlamydia trachomatis is the unresolved question of whether azithromycin is still as effective as doxycycline, the other current guideline-recommended, first-line therapy, Dr. Kimberly Workowski said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“This is important, because doxycycline is administered twice a day for 7 days, and azithromycin is given as a single pill suitable for directly observed therapy,” noted Dr. Workowski, professor of medicine at Emory University in Atlanta and lead author of the 2015 Centers for Disease Control and Prevention STD treatment guidelines.
Several recent retrospective case series have suggested azithromycin is less effective, with the biggest efficacy gap being seen in rectal C. trachomatis infections. These nonrandomized studies were further supported by an Australian meta-analysis of six randomized, controlled trials comparing the two antibiotics for the treatment of genital chlamydia. The investigators found roughly 3% greater efficacy for doxycycline, compared with azithromycin, for urogenital chlamydia, and a 7% advantage for doxycycline in treating symptomatic urethral infection in men.
However, the investigators were quick to add the caveat that “the quality of the evidence varies considerably” (Clin Infect Dis. 2014 Jul 15;59(2):193-205).
There’s a pressing need for better data. Dr. Workowski and her colleagues on the STD guidelines panel are eagerly awaiting the results of a well-structured randomized trial led by Dr. William M. Geisler, professor of medicine at the University of Alabama, Birmingham. The investigators randomized more than 300 chlamydia-infected male and female inmates in youth correctional facilities to guideline-recommended azithromycin at 1 g orally in a single dose or oral doxycycline at 100 mg twice daily for 7 days. The results, which are anticipated soon, should influence clinical practice, Dr. Workowski said.
“There is something going on here that we’re trying to understand. It may have something to do with organism load,” according to Dr. Workowski.
Here’s what else is new in chlamydia:
•Pregnancy: For treatment of chlamydia in pregnancy, amoxicillin at 500 mg orally t.i.d. for 7 days has been demoted from a first-line recommended therapy to alternative-regimen status. Now, the sole recommended first-line treatment in pregnancy is oral azithromycin at 1 g orally in a single dose.
“We did this based on in vitro studies showing Chlamydia trachomatis is not well-killed by amoxicillin. Instead, the drug induces persistent viable noninfectious forms which can sometimes reactivate,” Dr. Workowski explained.
•Delayed-release doxycycline: This FDA-approved drug, known as Doryx, administered as a 200-mg tablet once daily for 7 days, “might be an alternative” to the standard generic doxycycline regimen of 100 mg twice daily for 7 days, according to the current Centers for Disease Control and Prevention guidelines. In a randomized, double-blind trial, the new agent was as effective as twice-daily generic doxycycline in men and women with urogenital C. trachomatis infection, and it had fewer gastrointestinal side effects. Doryx is costlier than the twice-daily alternatives.
• Lymphogranuloma venereum: The current guidelines repeat a point made in previous editions, but one Dr. Workowski believes remains underappreciated and thus worthy of emphasis: Rectal exposure to C. trachomatis serovars L1, L2, and L3 in men who have sex with men or in women who have rectal sex can cause lymphogranuloma venereum, which takes the form of proctocolitis mimicking inflammatory bowel disease.
At the time of the initial visit, before results of diagnostic tests for chlamydia are available, patients suspected of having lymphogranuloma venereum should be started presumptively on the recommended regimen for this STD, which is oral doxycycline at 100 mg b.i.d. for 21 days.
“If you also see painful ulcers or, on anoscopy, mucosal ulcers, you should also treat empirically for herpes simplex until your culture results come back,” she added.
Dr. Workowski reported having no financial conflicts of interest.
SAN DIEGO – The hottest topic today in the treatment of sexually transmitted diseases caused by Chlamydia trachomatis is the unresolved question of whether azithromycin is still as effective as doxycycline, the other current guideline-recommended, first-line therapy, Dr. Kimberly Workowski said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“This is important, because doxycycline is administered twice a day for 7 days, and azithromycin is given as a single pill suitable for directly observed therapy,” noted Dr. Workowski, professor of medicine at Emory University in Atlanta and lead author of the 2015 Centers for Disease Control and Prevention STD treatment guidelines.
Several recent retrospective case series have suggested azithromycin is less effective, with the biggest efficacy gap being seen in rectal C. trachomatis infections. These nonrandomized studies were further supported by an Australian meta-analysis of six randomized, controlled trials comparing the two antibiotics for the treatment of genital chlamydia. The investigators found roughly 3% greater efficacy for doxycycline, compared with azithromycin, for urogenital chlamydia, and a 7% advantage for doxycycline in treating symptomatic urethral infection in men.
However, the investigators were quick to add the caveat that “the quality of the evidence varies considerably” (Clin Infect Dis. 2014 Jul 15;59(2):193-205).
There’s a pressing need for better data. Dr. Workowski and her colleagues on the STD guidelines panel are eagerly awaiting the results of a well-structured randomized trial led by Dr. William M. Geisler, professor of medicine at the University of Alabama, Birmingham. The investigators randomized more than 300 chlamydia-infected male and female inmates in youth correctional facilities to guideline-recommended azithromycin at 1 g orally in a single dose or oral doxycycline at 100 mg twice daily for 7 days. The results, which are anticipated soon, should influence clinical practice, Dr. Workowski said.
“There is something going on here that we’re trying to understand. It may have something to do with organism load,” according to Dr. Workowski.
Here’s what else is new in chlamydia:
•Pregnancy: For treatment of chlamydia in pregnancy, amoxicillin at 500 mg orally t.i.d. for 7 days has been demoted from a first-line recommended therapy to alternative-regimen status. Now, the sole recommended first-line treatment in pregnancy is oral azithromycin at 1 g orally in a single dose.
“We did this based on in vitro studies showing Chlamydia trachomatis is not well-killed by amoxicillin. Instead, the drug induces persistent viable noninfectious forms which can sometimes reactivate,” Dr. Workowski explained.
•Delayed-release doxycycline: This FDA-approved drug, known as Doryx, administered as a 200-mg tablet once daily for 7 days, “might be an alternative” to the standard generic doxycycline regimen of 100 mg twice daily for 7 days, according to the current Centers for Disease Control and Prevention guidelines. In a randomized, double-blind trial, the new agent was as effective as twice-daily generic doxycycline in men and women with urogenital C. trachomatis infection, and it had fewer gastrointestinal side effects. Doryx is costlier than the twice-daily alternatives.
• Lymphogranuloma venereum: The current guidelines repeat a point made in previous editions, but one Dr. Workowski believes remains underappreciated and thus worthy of emphasis: Rectal exposure to C. trachomatis serovars L1, L2, and L3 in men who have sex with men or in women who have rectal sex can cause lymphogranuloma venereum, which takes the form of proctocolitis mimicking inflammatory bowel disease.
At the time of the initial visit, before results of diagnostic tests for chlamydia are available, patients suspected of having lymphogranuloma venereum should be started presumptively on the recommended regimen for this STD, which is oral doxycycline at 100 mg b.i.d. for 21 days.
“If you also see painful ulcers or, on anoscopy, mucosal ulcers, you should also treat empirically for herpes simplex until your culture results come back,” she added.
Dr. Workowski reported having no financial conflicts of interest.
SAN DIEGO – The hottest topic today in the treatment of sexually transmitted diseases caused by Chlamydia trachomatis is the unresolved question of whether azithromycin is still as effective as doxycycline, the other current guideline-recommended, first-line therapy, Dr. Kimberly Workowski said at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
“This is important, because doxycycline is administered twice a day for 7 days, and azithromycin is given as a single pill suitable for directly observed therapy,” noted Dr. Workowski, professor of medicine at Emory University in Atlanta and lead author of the 2015 Centers for Disease Control and Prevention STD treatment guidelines.
Several recent retrospective case series have suggested azithromycin is less effective, with the biggest efficacy gap being seen in rectal C. trachomatis infections. These nonrandomized studies were further supported by an Australian meta-analysis of six randomized, controlled trials comparing the two antibiotics for the treatment of genital chlamydia. The investigators found roughly 3% greater efficacy for doxycycline, compared with azithromycin, for urogenital chlamydia, and a 7% advantage for doxycycline in treating symptomatic urethral infection in men.
However, the investigators were quick to add the caveat that “the quality of the evidence varies considerably” (Clin Infect Dis. 2014 Jul 15;59(2):193-205).
There’s a pressing need for better data. Dr. Workowski and her colleagues on the STD guidelines panel are eagerly awaiting the results of a well-structured randomized trial led by Dr. William M. Geisler, professor of medicine at the University of Alabama, Birmingham. The investigators randomized more than 300 chlamydia-infected male and female inmates in youth correctional facilities to guideline-recommended azithromycin at 1 g orally in a single dose or oral doxycycline at 100 mg twice daily for 7 days. The results, which are anticipated soon, should influence clinical practice, Dr. Workowski said.
“There is something going on here that we’re trying to understand. It may have something to do with organism load,” according to Dr. Workowski.
Here’s what else is new in chlamydia:
•Pregnancy: For treatment of chlamydia in pregnancy, amoxicillin at 500 mg orally t.i.d. for 7 days has been demoted from a first-line recommended therapy to alternative-regimen status. Now, the sole recommended first-line treatment in pregnancy is oral azithromycin at 1 g orally in a single dose.
“We did this based on in vitro studies showing Chlamydia trachomatis is not well-killed by amoxicillin. Instead, the drug induces persistent viable noninfectious forms which can sometimes reactivate,” Dr. Workowski explained.
•Delayed-release doxycycline: This FDA-approved drug, known as Doryx, administered as a 200-mg tablet once daily for 7 days, “might be an alternative” to the standard generic doxycycline regimen of 100 mg twice daily for 7 days, according to the current Centers for Disease Control and Prevention guidelines. In a randomized, double-blind trial, the new agent was as effective as twice-daily generic doxycycline in men and women with urogenital C. trachomatis infection, and it had fewer gastrointestinal side effects. Doryx is costlier than the twice-daily alternatives.
• Lymphogranuloma venereum: The current guidelines repeat a point made in previous editions, but one Dr. Workowski believes remains underappreciated and thus worthy of emphasis: Rectal exposure to C. trachomatis serovars L1, L2, and L3 in men who have sex with men or in women who have rectal sex can cause lymphogranuloma venereum, which takes the form of proctocolitis mimicking inflammatory bowel disease.
At the time of the initial visit, before results of diagnostic tests for chlamydia are available, patients suspected of having lymphogranuloma venereum should be started presumptively on the recommended regimen for this STD, which is oral doxycycline at 100 mg b.i.d. for 21 days.
“If you also see painful ulcers or, on anoscopy, mucosal ulcers, you should also treat empirically for herpes simplex until your culture results come back,” she added.
Dr. Workowski reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM ICAAC 2015
Dengue disease is here and U.S. physicians need to get to know it
SAN DIEGO – With dengue disease now knocking on the door of the United States, it’s a good time for American physicians to get up to speed regarding the most rapidly spreading mosquito-borne viral disease in the world.
That’s a particularly sound idea if they – or their patients – plan to visit anywhere in the Caribbean, Central America, Brazil, East Asia, or large swathes of Africa, where the disease is a major and rapidly growing public health problem. The World Health Organization estimates 3.6 billion people worldwide are at risk for dengue disease, with up to 100 million symptomatic infections occurring annually, 250,000-500,000 cases of severe dengue, and 21,000 deaths due to the disease. There have been recent outbreaks in South Florida, Texas, and Hawaii, Dr. Federico Narvaez noted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The most important thing for clinicians to know about dengue disease is how to identify the subset of up to 10% of symptomatic dengue patients who – absent appropriate intervention – will progress to severe disease marked by pronounced plasma leakage leading to shock, respiratory failure, severe hemorrhage, and/or organ failure, he stressed. This is a disease that can cause death within the space of 24-48 hours in a person who was healthy just a few days before. And there are a handful of warning signs that predictably occur on day 3 or 4 of the illness, when the initial high fever comes down, before things take a dramatic turn for the worse.
“Timely diagnosis improves prognosis. If properly managed, the case fatality rate of severe dengue is less than 1%,” said Dr. Narvaez of the National Pediatric Reference Hospital and the Nicaragua Ministry of Health in Managua.
The traditional WHO classification for dengue into self-limited dengue fever, dengue hemorrhagic fever, and dengue shock syndrome was replaced in 2009 by a system that Dr. Narvaez and other experts consider a big step forward in guiding clinical management. Under the revised WHO classification, dengue disease is divided into dengue without warning signs, dengue with warning signs, and severe dengue.
In a study of 544 laboratory-confirmed cases of pediatric dengue in Managua, Dr. Narvaez and coinvestigators compared the former and revised WHO classifications and demonstrated that the 2009 revised system boosted the positive predictive value for need for inpatient care from 43% to 67% (PLoS Negl Trop Dis. 2011 Nov;5[11]:e1397. doi: 10.1371/journal.pntd.0001397. Epub 2011 Nov 8).
Some key points about dengue disease: It has two distinct mosquito vectors, Aedis aegypti and A. albonictus. There are four cocirculating serotypes; infection with one doesn’t protect against infection with the others. Three-quarters of infections are asymptomatic. Symptomatic infections follow a three-stage course: the febrile, critical, and recovery phases. And the primary pathophysiology of dengue disease is plasma leakage.
The febrile phase is marked by abrupt onset of high fever plus various combinations of severe headache, facial flushing, a transient macular or maculopapular rash, retro-orbital pain, and/or the intense arthralgias/myalgias which have led to dengue being known as ‘breakbone fever.’
The critical phase begins around the time of defervescence. This is when clinically significant plasma leakage can occur, with resultant compensated or decompensated shock and other severe complications. The critical phase is the time for vigilance regarding the appearance of the 2009 WHO warning signs of increased risk for shock: abdominal pain, an abrupt rise in hematocrit concurrent with a rapid drop in platelets, mucosal bleeding, development of ascites or other clinically apparent fluid accumulation, liver enlargement of more than 2 cm, persistent vomiting, and restlessness/lethargy.
In a soon-to-be-published study of 812 Nicaraguan dengue patients, 220 of whom developed shock, Dr. Narvaez and coworkers found that the presence of any of the warning signs except persistent vomiting was associated with significantly increased likelihood of subsequent shock, with the magnitude of increased risk ranging from 1.31 to 2.3. Moreover, other studies have demonstrated that by acting upon these warning signs by means of cautious administration of intravenous fluids and other supportive measures, the risk of developing shock is reduced.
The WHO warning signs are particularly valuable in the often resource-poor countries where dengue is most common. In such settings most front-line primary care physicians lack ready access to ultrasound imaging of the gallbladder looking for evidence of wall thickening. A thickened gallbladder wall is an expression of subclinical plasma leakage, which has been shown in multiple studies to be even better at identifying patients at risk for severe dengue than the WHO warning signs.
For example, a prospective hospital-based study in which Dutch and Indonesian investigators utilized serial daily bedside ultrasonography with a hand-held imaging device found that gallbladder wall edema at enrollment had a 35% positive predictive value and a 90% negative predictive value for subsequent severe dengue (PLoS Negl Trop Dis. 2013 Jun 13;7[6]:e2277. doi: 10.1371/journal.pntd.0002277).
The critical phase typically lasts from day 3 or 4 through day 6 of the illness. This is followed by the recovery phase, marked by reabsorption of extravasated fluid over the course of 48-72 hours, increased diuresis, and stabilization of hemodynamic status. The appearance of a highly pruritic and erythematous rash with small islands of normal skin is another common finding that indicates the patient’s condition will continue to improve. A temporary bradycardia is also quite common during the recovery phase, according to Dr. Narvaez.
He reported having no financial conflicts of interest regarding his presentation.
SAN DIEGO – With dengue disease now knocking on the door of the United States, it’s a good time for American physicians to get up to speed regarding the most rapidly spreading mosquito-borne viral disease in the world.
That’s a particularly sound idea if they – or their patients – plan to visit anywhere in the Caribbean, Central America, Brazil, East Asia, or large swathes of Africa, where the disease is a major and rapidly growing public health problem. The World Health Organization estimates 3.6 billion people worldwide are at risk for dengue disease, with up to 100 million symptomatic infections occurring annually, 250,000-500,000 cases of severe dengue, and 21,000 deaths due to the disease. There have been recent outbreaks in South Florida, Texas, and Hawaii, Dr. Federico Narvaez noted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The most important thing for clinicians to know about dengue disease is how to identify the subset of up to 10% of symptomatic dengue patients who – absent appropriate intervention – will progress to severe disease marked by pronounced plasma leakage leading to shock, respiratory failure, severe hemorrhage, and/or organ failure, he stressed. This is a disease that can cause death within the space of 24-48 hours in a person who was healthy just a few days before. And there are a handful of warning signs that predictably occur on day 3 or 4 of the illness, when the initial high fever comes down, before things take a dramatic turn for the worse.
“Timely diagnosis improves prognosis. If properly managed, the case fatality rate of severe dengue is less than 1%,” said Dr. Narvaez of the National Pediatric Reference Hospital and the Nicaragua Ministry of Health in Managua.
The traditional WHO classification for dengue into self-limited dengue fever, dengue hemorrhagic fever, and dengue shock syndrome was replaced in 2009 by a system that Dr. Narvaez and other experts consider a big step forward in guiding clinical management. Under the revised WHO classification, dengue disease is divided into dengue without warning signs, dengue with warning signs, and severe dengue.
In a study of 544 laboratory-confirmed cases of pediatric dengue in Managua, Dr. Narvaez and coinvestigators compared the former and revised WHO classifications and demonstrated that the 2009 revised system boosted the positive predictive value for need for inpatient care from 43% to 67% (PLoS Negl Trop Dis. 2011 Nov;5[11]:e1397. doi: 10.1371/journal.pntd.0001397. Epub 2011 Nov 8).
Some key points about dengue disease: It has two distinct mosquito vectors, Aedis aegypti and A. albonictus. There are four cocirculating serotypes; infection with one doesn’t protect against infection with the others. Three-quarters of infections are asymptomatic. Symptomatic infections follow a three-stage course: the febrile, critical, and recovery phases. And the primary pathophysiology of dengue disease is plasma leakage.
The febrile phase is marked by abrupt onset of high fever plus various combinations of severe headache, facial flushing, a transient macular or maculopapular rash, retro-orbital pain, and/or the intense arthralgias/myalgias which have led to dengue being known as ‘breakbone fever.’
The critical phase begins around the time of defervescence. This is when clinically significant plasma leakage can occur, with resultant compensated or decompensated shock and other severe complications. The critical phase is the time for vigilance regarding the appearance of the 2009 WHO warning signs of increased risk for shock: abdominal pain, an abrupt rise in hematocrit concurrent with a rapid drop in platelets, mucosal bleeding, development of ascites or other clinically apparent fluid accumulation, liver enlargement of more than 2 cm, persistent vomiting, and restlessness/lethargy.
In a soon-to-be-published study of 812 Nicaraguan dengue patients, 220 of whom developed shock, Dr. Narvaez and coworkers found that the presence of any of the warning signs except persistent vomiting was associated with significantly increased likelihood of subsequent shock, with the magnitude of increased risk ranging from 1.31 to 2.3. Moreover, other studies have demonstrated that by acting upon these warning signs by means of cautious administration of intravenous fluids and other supportive measures, the risk of developing shock is reduced.
The WHO warning signs are particularly valuable in the often resource-poor countries where dengue is most common. In such settings most front-line primary care physicians lack ready access to ultrasound imaging of the gallbladder looking for evidence of wall thickening. A thickened gallbladder wall is an expression of subclinical plasma leakage, which has been shown in multiple studies to be even better at identifying patients at risk for severe dengue than the WHO warning signs.
For example, a prospective hospital-based study in which Dutch and Indonesian investigators utilized serial daily bedside ultrasonography with a hand-held imaging device found that gallbladder wall edema at enrollment had a 35% positive predictive value and a 90% negative predictive value for subsequent severe dengue (PLoS Negl Trop Dis. 2013 Jun 13;7[6]:e2277. doi: 10.1371/journal.pntd.0002277).
The critical phase typically lasts from day 3 or 4 through day 6 of the illness. This is followed by the recovery phase, marked by reabsorption of extravasated fluid over the course of 48-72 hours, increased diuresis, and stabilization of hemodynamic status. The appearance of a highly pruritic and erythematous rash with small islands of normal skin is another common finding that indicates the patient’s condition will continue to improve. A temporary bradycardia is also quite common during the recovery phase, according to Dr. Narvaez.
He reported having no financial conflicts of interest regarding his presentation.
SAN DIEGO – With dengue disease now knocking on the door of the United States, it’s a good time for American physicians to get up to speed regarding the most rapidly spreading mosquito-borne viral disease in the world.
That’s a particularly sound idea if they – or their patients – plan to visit anywhere in the Caribbean, Central America, Brazil, East Asia, or large swathes of Africa, where the disease is a major and rapidly growing public health problem. The World Health Organization estimates 3.6 billion people worldwide are at risk for dengue disease, with up to 100 million symptomatic infections occurring annually, 250,000-500,000 cases of severe dengue, and 21,000 deaths due to the disease. There have been recent outbreaks in South Florida, Texas, and Hawaii, Dr. Federico Narvaez noted at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
The most important thing for clinicians to know about dengue disease is how to identify the subset of up to 10% of symptomatic dengue patients who – absent appropriate intervention – will progress to severe disease marked by pronounced plasma leakage leading to shock, respiratory failure, severe hemorrhage, and/or organ failure, he stressed. This is a disease that can cause death within the space of 24-48 hours in a person who was healthy just a few days before. And there are a handful of warning signs that predictably occur on day 3 or 4 of the illness, when the initial high fever comes down, before things take a dramatic turn for the worse.
“Timely diagnosis improves prognosis. If properly managed, the case fatality rate of severe dengue is less than 1%,” said Dr. Narvaez of the National Pediatric Reference Hospital and the Nicaragua Ministry of Health in Managua.
The traditional WHO classification for dengue into self-limited dengue fever, dengue hemorrhagic fever, and dengue shock syndrome was replaced in 2009 by a system that Dr. Narvaez and other experts consider a big step forward in guiding clinical management. Under the revised WHO classification, dengue disease is divided into dengue without warning signs, dengue with warning signs, and severe dengue.
In a study of 544 laboratory-confirmed cases of pediatric dengue in Managua, Dr. Narvaez and coinvestigators compared the former and revised WHO classifications and demonstrated that the 2009 revised system boosted the positive predictive value for need for inpatient care from 43% to 67% (PLoS Negl Trop Dis. 2011 Nov;5[11]:e1397. doi: 10.1371/journal.pntd.0001397. Epub 2011 Nov 8).
Some key points about dengue disease: It has two distinct mosquito vectors, Aedis aegypti and A. albonictus. There are four cocirculating serotypes; infection with one doesn’t protect against infection with the others. Three-quarters of infections are asymptomatic. Symptomatic infections follow a three-stage course: the febrile, critical, and recovery phases. And the primary pathophysiology of dengue disease is plasma leakage.
The febrile phase is marked by abrupt onset of high fever plus various combinations of severe headache, facial flushing, a transient macular or maculopapular rash, retro-orbital pain, and/or the intense arthralgias/myalgias which have led to dengue being known as ‘breakbone fever.’
The critical phase begins around the time of defervescence. This is when clinically significant plasma leakage can occur, with resultant compensated or decompensated shock and other severe complications. The critical phase is the time for vigilance regarding the appearance of the 2009 WHO warning signs of increased risk for shock: abdominal pain, an abrupt rise in hematocrit concurrent with a rapid drop in platelets, mucosal bleeding, development of ascites or other clinically apparent fluid accumulation, liver enlargement of more than 2 cm, persistent vomiting, and restlessness/lethargy.
In a soon-to-be-published study of 812 Nicaraguan dengue patients, 220 of whom developed shock, Dr. Narvaez and coworkers found that the presence of any of the warning signs except persistent vomiting was associated with significantly increased likelihood of subsequent shock, with the magnitude of increased risk ranging from 1.31 to 2.3. Moreover, other studies have demonstrated that by acting upon these warning signs by means of cautious administration of intravenous fluids and other supportive measures, the risk of developing shock is reduced.
The WHO warning signs are particularly valuable in the often resource-poor countries where dengue is most common. In such settings most front-line primary care physicians lack ready access to ultrasound imaging of the gallbladder looking for evidence of wall thickening. A thickened gallbladder wall is an expression of subclinical plasma leakage, which has been shown in multiple studies to be even better at identifying patients at risk for severe dengue than the WHO warning signs.
For example, a prospective hospital-based study in which Dutch and Indonesian investigators utilized serial daily bedside ultrasonography with a hand-held imaging device found that gallbladder wall edema at enrollment had a 35% positive predictive value and a 90% negative predictive value for subsequent severe dengue (PLoS Negl Trop Dis. 2013 Jun 13;7[6]:e2277. doi: 10.1371/journal.pntd.0002277).
The critical phase typically lasts from day 3 or 4 through day 6 of the illness. This is followed by the recovery phase, marked by reabsorption of extravasated fluid over the course of 48-72 hours, increased diuresis, and stabilization of hemodynamic status. The appearance of a highly pruritic and erythematous rash with small islands of normal skin is another common finding that indicates the patient’s condition will continue to improve. A temporary bradycardia is also quite common during the recovery phase, according to Dr. Narvaez.
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM ICAAC 2015
Promising nonvaccine approaches to controlling dengue
SAN DIEGO – In the aftermath of the latest somewhat disappointing report on the effort to develop a dengue vaccine, novel nonvaccine approaches aimed at curbing this rapidly growing public health problem are drawing renewed attention, Eva Harris, Ph.D., declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
These promising nonvaccine tools fall into several categories: vector control using genetically modified mosquitoes, development of new and better insecticides, and communitywide nonpesticide-based source reduction programs, explained Dr. Harris, professor of infectious diseases and vaccinology and director of the Center for Global Public Health at the University of California, Berkeley.
Many dengue experts, Dr. Harris among them, were disappointed in the recently reported long-term results of several large clinical trials of the candidate dengue vaccine furthest along in the developmental pipeline. The Sanofi Pasteur vaccine, known as CYD-TDV, is due for review and potential registration by the World Health Organization next year. The latest results show favorable safety but suboptimal efficacy. On the plus side, the data showed that children aged 9-16 years continued to benefit 3-4 years after vaccination. There was, however, a disturbing finding: children younger than 9 years of age at vaccination had an increased risk of hospitalization for dengue when they were naturally infected in the third year following vaccination (N Engl J Med. 2015;373[13]:1195-206).
In an accompanying editorial titled “A Candidate Dengue Vaccine Walks a Tightrope,” Dr. Cameron P. Simmons called the latter finding “a particularly unwelcome outcome” that, if not due to chance, raises the possibility that immunization of young children elicits only transient antibody-mediated immunity. As their antibody titers wane over time, these vaccinated children may, through sensitization, be predisposed to clinical dengue infections that are sufficiently serious to warrant hospitalization. It’s possible, but unproved, that booster doses of the vaccine might circumvent this problem, he added.
“The bumpy road to a vaccine-based solution for dengue continues,” observed Dr. Simmons of the University of Melbourne (N Engl J Med. 2015;373[13]:1263-4).
Dr. Harris noted that two other vaccines, one sponsored by the National Institutes of Health and the other by Takeda, are due to start large, long-term phase-III clinical trials in the next few months. And Merck and GlaxoSmithKline have next-generation vaccines in phase-I studies. But possible consideration of any of these vaccines for regulatory approval is a long ways off, and her focus at ICAAC 2015 was on nonvaccine solutions.
She and her coinvestigators recently published positive results of their landmark randomized controlled trial of a pesticide-free, community-based mobilization program for dengue prevention known as the Camino Verde, or Green Way (BMJ. 2015;351:h3267).
The impetus for this program was recognition of the shortcomings of current dengue control efforts, which rely heavily upon massive use of the organophosphate pesticide temephos (Abate) in household water containers where the mosquito vectors breed. The pesticide program hasn’t prevented ongoing rapid growth of the dengue pandemic. Moreover, the associated human toxicity and negative environmental effects are a mounting concern.
Camino Verde is a nonchemical alternative approach in which facilitators run intervention design groups in neighborhoods to inform community leaders and other residents about the scope of their local dengue mosquito problem based upon entomologic survey results and then help develop consensus regarding community-specific programs for chemical-free prevention of mosquito reproduction. Popular options included introduction of fish into water storage containers, cleanup campaigns targeting abandoned tires and other standing-water sources, and scrubbing and covering water tanks. Allowing each participating site to select its own interventions encouraged strong community support, Dr. Harris explained.
The prospective Camino Verde study involved nearly 19,000 households with more than 85,000 residents in Nicaragua and Mexico. Clusters of households were randomized to continuation of the temephos-based, government-run dengue control program with or without adding on the Camino Verde intervention.
Among the key findings: There was a 30% lower risk of serologic infection with dengue virus among children from intervention sites, as well as a 25% reduction in dengue illness among people of all ages. The numbers needed to treat were 30 for a reduced risk of infection in children and 71 for a lower risk of illness. Investigators also documented a 44% reduction in houses containing Aedes aegypti larvae or pupae in Camino Verde–participating sites, compared with control communities.
This study provides the first-ever solid serologic evidence that a pesticide-free community mobilization effort has a positive impact on dengue infection. The logical next step is for governments in dengue-endemic countries to adopt such an approach, she said.
At least three different strategies of genetic modification of dengue-vector mosquitoes are being pursued in an effort to reduce dengue transmission. All show promise as partial solutions, in Dr. Harris’ view.
Release of sterile transgenic A. aegypti males over the course of a year in a Brazilian suburb resulted in a 95% reduction in the size of the local A. aegypti population, compared with an adjacent control area (PLoS Negl Trop Dis. 2015;9[7]:e0003864). Investigators at Colorado State University in Fort Collins have developed a transgenic strain of A. aegypti males carrying a dominant lethal gene resulting in next-generation flightless females that can’t mate or avoid predators (Proc Natl Acad Sci U S A. 2011;108[12]:4772-5).
In another approach, an international collaborative group has performed field-release trials of A. aegypti mosquitoes deliberately infected with a strain of the intracellular bacterium Wolbachia, which renders the insects resistant to dengue virus infection. The group’s mathematical modeling suggested widespread adoption of this approach would reduce dengue virus transmission by 66%-75%. The investigators predicted this would be sufficient to eliminate dengue in low- or moderate-transmission areas but probably wouldn’t accomplish complete control in the highest-risk areas (Sci Transl Med. 2015;7[279]:279ra37).
Dr. Harris reported that the Camino Verde trial was funded by the UBS Optimus Foundation. She reported having no financial conflicts of interest.
SAN DIEGO – In the aftermath of the latest somewhat disappointing report on the effort to develop a dengue vaccine, novel nonvaccine approaches aimed at curbing this rapidly growing public health problem are drawing renewed attention, Eva Harris, Ph.D., declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
These promising nonvaccine tools fall into several categories: vector control using genetically modified mosquitoes, development of new and better insecticides, and communitywide nonpesticide-based source reduction programs, explained Dr. Harris, professor of infectious diseases and vaccinology and director of the Center for Global Public Health at the University of California, Berkeley.
Many dengue experts, Dr. Harris among them, were disappointed in the recently reported long-term results of several large clinical trials of the candidate dengue vaccine furthest along in the developmental pipeline. The Sanofi Pasteur vaccine, known as CYD-TDV, is due for review and potential registration by the World Health Organization next year. The latest results show favorable safety but suboptimal efficacy. On the plus side, the data showed that children aged 9-16 years continued to benefit 3-4 years after vaccination. There was, however, a disturbing finding: children younger than 9 years of age at vaccination had an increased risk of hospitalization for dengue when they were naturally infected in the third year following vaccination (N Engl J Med. 2015;373[13]:1195-206).
In an accompanying editorial titled “A Candidate Dengue Vaccine Walks a Tightrope,” Dr. Cameron P. Simmons called the latter finding “a particularly unwelcome outcome” that, if not due to chance, raises the possibility that immunization of young children elicits only transient antibody-mediated immunity. As their antibody titers wane over time, these vaccinated children may, through sensitization, be predisposed to clinical dengue infections that are sufficiently serious to warrant hospitalization. It’s possible, but unproved, that booster doses of the vaccine might circumvent this problem, he added.
“The bumpy road to a vaccine-based solution for dengue continues,” observed Dr. Simmons of the University of Melbourne (N Engl J Med. 2015;373[13]:1263-4).
Dr. Harris noted that two other vaccines, one sponsored by the National Institutes of Health and the other by Takeda, are due to start large, long-term phase-III clinical trials in the next few months. And Merck and GlaxoSmithKline have next-generation vaccines in phase-I studies. But possible consideration of any of these vaccines for regulatory approval is a long ways off, and her focus at ICAAC 2015 was on nonvaccine solutions.
She and her coinvestigators recently published positive results of their landmark randomized controlled trial of a pesticide-free, community-based mobilization program for dengue prevention known as the Camino Verde, or Green Way (BMJ. 2015;351:h3267).
The impetus for this program was recognition of the shortcomings of current dengue control efforts, which rely heavily upon massive use of the organophosphate pesticide temephos (Abate) in household water containers where the mosquito vectors breed. The pesticide program hasn’t prevented ongoing rapid growth of the dengue pandemic. Moreover, the associated human toxicity and negative environmental effects are a mounting concern.
Camino Verde is a nonchemical alternative approach in which facilitators run intervention design groups in neighborhoods to inform community leaders and other residents about the scope of their local dengue mosquito problem based upon entomologic survey results and then help develop consensus regarding community-specific programs for chemical-free prevention of mosquito reproduction. Popular options included introduction of fish into water storage containers, cleanup campaigns targeting abandoned tires and other standing-water sources, and scrubbing and covering water tanks. Allowing each participating site to select its own interventions encouraged strong community support, Dr. Harris explained.
The prospective Camino Verde study involved nearly 19,000 households with more than 85,000 residents in Nicaragua and Mexico. Clusters of households were randomized to continuation of the temephos-based, government-run dengue control program with or without adding on the Camino Verde intervention.
Among the key findings: There was a 30% lower risk of serologic infection with dengue virus among children from intervention sites, as well as a 25% reduction in dengue illness among people of all ages. The numbers needed to treat were 30 for a reduced risk of infection in children and 71 for a lower risk of illness. Investigators also documented a 44% reduction in houses containing Aedes aegypti larvae or pupae in Camino Verde–participating sites, compared with control communities.
This study provides the first-ever solid serologic evidence that a pesticide-free community mobilization effort has a positive impact on dengue infection. The logical next step is for governments in dengue-endemic countries to adopt such an approach, she said.
At least three different strategies of genetic modification of dengue-vector mosquitoes are being pursued in an effort to reduce dengue transmission. All show promise as partial solutions, in Dr. Harris’ view.
Release of sterile transgenic A. aegypti males over the course of a year in a Brazilian suburb resulted in a 95% reduction in the size of the local A. aegypti population, compared with an adjacent control area (PLoS Negl Trop Dis. 2015;9[7]:e0003864). Investigators at Colorado State University in Fort Collins have developed a transgenic strain of A. aegypti males carrying a dominant lethal gene resulting in next-generation flightless females that can’t mate or avoid predators (Proc Natl Acad Sci U S A. 2011;108[12]:4772-5).
In another approach, an international collaborative group has performed field-release trials of A. aegypti mosquitoes deliberately infected with a strain of the intracellular bacterium Wolbachia, which renders the insects resistant to dengue virus infection. The group’s mathematical modeling suggested widespread adoption of this approach would reduce dengue virus transmission by 66%-75%. The investigators predicted this would be sufficient to eliminate dengue in low- or moderate-transmission areas but probably wouldn’t accomplish complete control in the highest-risk areas (Sci Transl Med. 2015;7[279]:279ra37).
Dr. Harris reported that the Camino Verde trial was funded by the UBS Optimus Foundation. She reported having no financial conflicts of interest.
SAN DIEGO – In the aftermath of the latest somewhat disappointing report on the effort to develop a dengue vaccine, novel nonvaccine approaches aimed at curbing this rapidly growing public health problem are drawing renewed attention, Eva Harris, Ph.D., declared at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
These promising nonvaccine tools fall into several categories: vector control using genetically modified mosquitoes, development of new and better insecticides, and communitywide nonpesticide-based source reduction programs, explained Dr. Harris, professor of infectious diseases and vaccinology and director of the Center for Global Public Health at the University of California, Berkeley.
Many dengue experts, Dr. Harris among them, were disappointed in the recently reported long-term results of several large clinical trials of the candidate dengue vaccine furthest along in the developmental pipeline. The Sanofi Pasteur vaccine, known as CYD-TDV, is due for review and potential registration by the World Health Organization next year. The latest results show favorable safety but suboptimal efficacy. On the plus side, the data showed that children aged 9-16 years continued to benefit 3-4 years after vaccination. There was, however, a disturbing finding: children younger than 9 years of age at vaccination had an increased risk of hospitalization for dengue when they were naturally infected in the third year following vaccination (N Engl J Med. 2015;373[13]:1195-206).
In an accompanying editorial titled “A Candidate Dengue Vaccine Walks a Tightrope,” Dr. Cameron P. Simmons called the latter finding “a particularly unwelcome outcome” that, if not due to chance, raises the possibility that immunization of young children elicits only transient antibody-mediated immunity. As their antibody titers wane over time, these vaccinated children may, through sensitization, be predisposed to clinical dengue infections that are sufficiently serious to warrant hospitalization. It’s possible, but unproved, that booster doses of the vaccine might circumvent this problem, he added.
“The bumpy road to a vaccine-based solution for dengue continues,” observed Dr. Simmons of the University of Melbourne (N Engl J Med. 2015;373[13]:1263-4).
Dr. Harris noted that two other vaccines, one sponsored by the National Institutes of Health and the other by Takeda, are due to start large, long-term phase-III clinical trials in the next few months. And Merck and GlaxoSmithKline have next-generation vaccines in phase-I studies. But possible consideration of any of these vaccines for regulatory approval is a long ways off, and her focus at ICAAC 2015 was on nonvaccine solutions.
She and her coinvestigators recently published positive results of their landmark randomized controlled trial of a pesticide-free, community-based mobilization program for dengue prevention known as the Camino Verde, or Green Way (BMJ. 2015;351:h3267).
The impetus for this program was recognition of the shortcomings of current dengue control efforts, which rely heavily upon massive use of the organophosphate pesticide temephos (Abate) in household water containers where the mosquito vectors breed. The pesticide program hasn’t prevented ongoing rapid growth of the dengue pandemic. Moreover, the associated human toxicity and negative environmental effects are a mounting concern.
Camino Verde is a nonchemical alternative approach in which facilitators run intervention design groups in neighborhoods to inform community leaders and other residents about the scope of their local dengue mosquito problem based upon entomologic survey results and then help develop consensus regarding community-specific programs for chemical-free prevention of mosquito reproduction. Popular options included introduction of fish into water storage containers, cleanup campaigns targeting abandoned tires and other standing-water sources, and scrubbing and covering water tanks. Allowing each participating site to select its own interventions encouraged strong community support, Dr. Harris explained.
The prospective Camino Verde study involved nearly 19,000 households with more than 85,000 residents in Nicaragua and Mexico. Clusters of households were randomized to continuation of the temephos-based, government-run dengue control program with or without adding on the Camino Verde intervention.
Among the key findings: There was a 30% lower risk of serologic infection with dengue virus among children from intervention sites, as well as a 25% reduction in dengue illness among people of all ages. The numbers needed to treat were 30 for a reduced risk of infection in children and 71 for a lower risk of illness. Investigators also documented a 44% reduction in houses containing Aedes aegypti larvae or pupae in Camino Verde–participating sites, compared with control communities.
This study provides the first-ever solid serologic evidence that a pesticide-free community mobilization effort has a positive impact on dengue infection. The logical next step is for governments in dengue-endemic countries to adopt such an approach, she said.
At least three different strategies of genetic modification of dengue-vector mosquitoes are being pursued in an effort to reduce dengue transmission. All show promise as partial solutions, in Dr. Harris’ view.
Release of sterile transgenic A. aegypti males over the course of a year in a Brazilian suburb resulted in a 95% reduction in the size of the local A. aegypti population, compared with an adjacent control area (PLoS Negl Trop Dis. 2015;9[7]:e0003864). Investigators at Colorado State University in Fort Collins have developed a transgenic strain of A. aegypti males carrying a dominant lethal gene resulting in next-generation flightless females that can’t mate or avoid predators (Proc Natl Acad Sci U S A. 2011;108[12]:4772-5).
In another approach, an international collaborative group has performed field-release trials of A. aegypti mosquitoes deliberately infected with a strain of the intracellular bacterium Wolbachia, which renders the insects resistant to dengue virus infection. The group’s mathematical modeling suggested widespread adoption of this approach would reduce dengue virus transmission by 66%-75%. The investigators predicted this would be sufficient to eliminate dengue in low- or moderate-transmission areas but probably wouldn’t accomplish complete control in the highest-risk areas (Sci Transl Med. 2015;7[279]:279ra37).
Dr. Harris reported that the Camino Verde trial was funded by the UBS Optimus Foundation. She reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM ICAAC 2015
EADV: Venous leg ulcers herald increased cancer risk
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
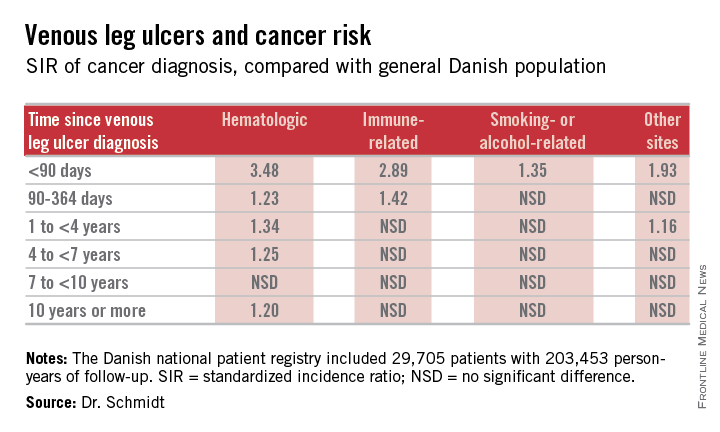
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.
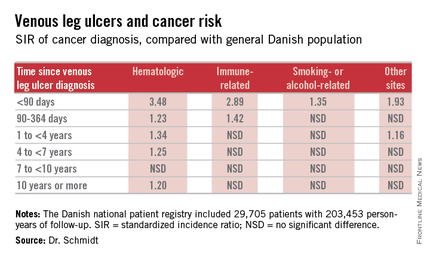
It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.

It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
COPENHAGEN – Patients with venous leg ulcers have an increased risk of occult cancer – especially hematologic and immune-related malignancies, according to a Danish nationwide cohort study.
The risk of newly detected cancer was greatest during the first 89 days after diagnosis of a venous leg ulcer. Indeed, during that initial period the risk of being diagnosed with a hematologic cancer was 3.48-fold greater than expected based upon Danish Cancer Registry data, Dr. Sigrun Alba Johannesdottir Schmidt reported at the annual congress of the European Academy of Dermatology and Venereology.

It’s reasonable to assume that the vast majority of cancers identified within a year following diagnosis of a venous leg ulcer were probably present at the time when the ulcer was first diagnosed, meaning venous leg ulcers can serve as a red flag for occult cancer.
However, it’s also worth noting that the increased risk of hematologic malignancies persisted, albeit to a lesser degree, for up to 10 years. This suggests that venous ulceration could also have a carcinogenic effect. It’s biologically plausible that a venous leg ulcer could promote development of cancer through a variety of mechanisms, including inflammation, alteration of plasma viscosity and the adhesive properties of blood cells, and disruptions of venous pressure that encourage direct neoplastic invasion, according to Dr. Schmidt of Aarhus (Denmark) University.
She presented a Danish national patient registry study, which included all 29,705 patients with a first-time inpatient, outpatient, or emergency department diagnosis of a venous leg ulcer during 1982-2010. Fifty-five percent of them were age 70 years or older at the time of ulcer diagnosis. And 42% had moderate to very severe comorbid conditions based upon their Charlson Comorbidity Index score. During a median of 5.1 years of follow-up, or a total of 203,453 person-years, their overall risk of a first-time cancer diagnosis was significantly increased by 11%,compared with the general Danish population.
The malignancy risk was strongly time-dependent . However, the absolute risk of cancer was relatively low: less than 1% within the first 90 days after diagnosis of a venous leg ulcer. The number of patients who would need to be examined for a possible malignancy at the time of diagnosis of a venous leg ulcer in order to diagnosis one excess cancer was 146.
Dr. Schmidt indicated she would defer to experts in cost-benefit analysis as to whether an extensive work-up for occult malignancy is worthwhile in patients with a newly diagnosed venous leg ulcer, given the low absolute risk of cancer.
She reported having no financial conflicts of interest regarding her study, which was conducted with Danish institutional research funds.
AT THE EADV CONGRESS
Key clinical point: Venous leg ulceration appears to be a red flag for occult malignancy.
Major finding: During the first 89 days following diagnosis of a venous leg ulcer, affected patients are at a 3.48-fold increased risk of being diagnosed with a hematologic malignancy and a 2.89-fold greater risk of immune-related cancer compared with the general population.
Data source: This Danish nationwide cohort study compared standardized incidence ratios for various types of cancer during more than 200,000 person-years of follow-up in 29,705 patients with a venous leg ulcer vs. the general population.
Disclosures: The presenter reported having no financial conflicts of interest regarding her study, which was supported by Danish institutional funds.
EADV: Intralesional therapy for scleroderma dystrophic calcifications
COPENHAGEN – Intralesional sodium thiosulfate injections are an effective treatment for the painful and disabling dystrophic calcifications associated with systemic sclerosis, lupus, and other autoimmune diseases, as well as with nephrogenic systemic fibrosis, Dr. Jane Baumgartner-Nielsen said at the annual congress of the European Academy of Dermatology and Venereology.
“We suggest that intralesional injections of sodium thiosulfate may be considered in severe or ulcerated lesions before surgery or amputation,” said Dr. Baumgartner-Nielsen of Aarhus (Denmark) University.
Treatment of these often ulcerated cutaneous lesions has traditionally been challenging. While surgery is common, it’s problematic because wound healing is often prolonged in patients with autoimmune disease, she observed.
She presented a case series of six patients who underwent interlesional injections of sodium thiosulfate for painful and disabling dystrophic calcifications. The lesions were located on extensor surfaces or the fingertips. They were extremely painful: patients rated their pain as 9 on a 10-point scale. All six patients were women. Five had anticentromere antibody–positive systemic sclerosis; other investigators have reported that dystrophic calcifications occur in roughly 70% of such patients. The sixth patient had nephrogenic systemic fibrosis.
The six patients underwent a total of 21 injections of eight lesions. The injections were placed at the base of the calcifications. The concentration of sodium thiosulfate employed was 150 mg/mL. Dystrophic calcifications less than 5 mm in diameter on the fingertips received a single injection. Larger lesions complicated by ulceration got four injections at 4-week intervals.
The lesions decreased in size by an average of 67% at 4 weeks and 90% at 12 weeks. Complete remission was achieved by week 12 in half of patients; the other half had 80% reduction of their lesions. All patients reported dramatically less pain and improved physical function, compared with baseline. There were no serious side effects.
Audience member Dr. Alice B. Gottlieb inquired as to how painful the injections are.
“About 9 or 10 on a 10-point scale, but the pain disappears very quickly. In 30 seconds the patient is smiling again,” Dr. Baumgartner-Nielsen replied.
Dr. Gottlieb said she was interested in the intralesional therapy for her pediatric lupus patients with dystrophic calcifications. “But if there’s that much injection site pain, you might have to put the kid out,” noted Dr. Gottlieb, professor and dermatologist-in-chief at Tufts Medical Center, Boston.
Dr. Baumgartner-Nielsen reported having no financial conflicts regarding her study.
COPENHAGEN – Intralesional sodium thiosulfate injections are an effective treatment for the painful and disabling dystrophic calcifications associated with systemic sclerosis, lupus, and other autoimmune diseases, as well as with nephrogenic systemic fibrosis, Dr. Jane Baumgartner-Nielsen said at the annual congress of the European Academy of Dermatology and Venereology.
“We suggest that intralesional injections of sodium thiosulfate may be considered in severe or ulcerated lesions before surgery or amputation,” said Dr. Baumgartner-Nielsen of Aarhus (Denmark) University.
Treatment of these often ulcerated cutaneous lesions has traditionally been challenging. While surgery is common, it’s problematic because wound healing is often prolonged in patients with autoimmune disease, she observed.
She presented a case series of six patients who underwent interlesional injections of sodium thiosulfate for painful and disabling dystrophic calcifications. The lesions were located on extensor surfaces or the fingertips. They were extremely painful: patients rated their pain as 9 on a 10-point scale. All six patients were women. Five had anticentromere antibody–positive systemic sclerosis; other investigators have reported that dystrophic calcifications occur in roughly 70% of such patients. The sixth patient had nephrogenic systemic fibrosis.
The six patients underwent a total of 21 injections of eight lesions. The injections were placed at the base of the calcifications. The concentration of sodium thiosulfate employed was 150 mg/mL. Dystrophic calcifications less than 5 mm in diameter on the fingertips received a single injection. Larger lesions complicated by ulceration got four injections at 4-week intervals.
The lesions decreased in size by an average of 67% at 4 weeks and 90% at 12 weeks. Complete remission was achieved by week 12 in half of patients; the other half had 80% reduction of their lesions. All patients reported dramatically less pain and improved physical function, compared with baseline. There were no serious side effects.
Audience member Dr. Alice B. Gottlieb inquired as to how painful the injections are.
“About 9 or 10 on a 10-point scale, but the pain disappears very quickly. In 30 seconds the patient is smiling again,” Dr. Baumgartner-Nielsen replied.
Dr. Gottlieb said she was interested in the intralesional therapy for her pediatric lupus patients with dystrophic calcifications. “But if there’s that much injection site pain, you might have to put the kid out,” noted Dr. Gottlieb, professor and dermatologist-in-chief at Tufts Medical Center, Boston.
Dr. Baumgartner-Nielsen reported having no financial conflicts regarding her study.
COPENHAGEN – Intralesional sodium thiosulfate injections are an effective treatment for the painful and disabling dystrophic calcifications associated with systemic sclerosis, lupus, and other autoimmune diseases, as well as with nephrogenic systemic fibrosis, Dr. Jane Baumgartner-Nielsen said at the annual congress of the European Academy of Dermatology and Venereology.
“We suggest that intralesional injections of sodium thiosulfate may be considered in severe or ulcerated lesions before surgery or amputation,” said Dr. Baumgartner-Nielsen of Aarhus (Denmark) University.
Treatment of these often ulcerated cutaneous lesions has traditionally been challenging. While surgery is common, it’s problematic because wound healing is often prolonged in patients with autoimmune disease, she observed.
She presented a case series of six patients who underwent interlesional injections of sodium thiosulfate for painful and disabling dystrophic calcifications. The lesions were located on extensor surfaces or the fingertips. They were extremely painful: patients rated their pain as 9 on a 10-point scale. All six patients were women. Five had anticentromere antibody–positive systemic sclerosis; other investigators have reported that dystrophic calcifications occur in roughly 70% of such patients. The sixth patient had nephrogenic systemic fibrosis.
The six patients underwent a total of 21 injections of eight lesions. The injections were placed at the base of the calcifications. The concentration of sodium thiosulfate employed was 150 mg/mL. Dystrophic calcifications less than 5 mm in diameter on the fingertips received a single injection. Larger lesions complicated by ulceration got four injections at 4-week intervals.
The lesions decreased in size by an average of 67% at 4 weeks and 90% at 12 weeks. Complete remission was achieved by week 12 in half of patients; the other half had 80% reduction of their lesions. All patients reported dramatically less pain and improved physical function, compared with baseline. There were no serious side effects.
Audience member Dr. Alice B. Gottlieb inquired as to how painful the injections are.
“About 9 or 10 on a 10-point scale, but the pain disappears very quickly. In 30 seconds the patient is smiling again,” Dr. Baumgartner-Nielsen replied.
Dr. Gottlieb said she was interested in the intralesional therapy for her pediatric lupus patients with dystrophic calcifications. “But if there’s that much injection site pain, you might have to put the kid out,” noted Dr. Gottlieb, professor and dermatologist-in-chief at Tufts Medical Center, Boston.
Dr. Baumgartner-Nielsen reported having no financial conflicts regarding her study.
AT THE EADV CONGRESS
Key clinical point: Intralesional sodium thiosulfate is an effective alternative to surgery for disabling dystrophic calcifications in patients with systemic sclerosis or other autoimmune diseases.
Major finding: Fifty percent of patients with severely painful dystrophic calcifications experienced complete remission 12 weeks after their first intralesional injection of sodium thiosulfate; the remaining lesions were 80% smaller, compared with baseline.
Data source: This was a case series involving six patients with eight treated dystrophic calcifications secondary to systemic sclerosis of nephrogenic systemic fibrosis.
Disclosures: The study presenter reported having no financial conflicts of interest regarding her case series.
EADV: No cancer signal with ixekizumab
COPENHAGEN – Ixekizumab showed no hint of an increased malignancy risk of any sort in 4,208 patients with moderate to severe psoriasis who had 6,480 patient-years of exposure to the investigational interleukin-17A inhibitor in seven clinical trials.
“Patients were studied for an average of 18 months on ixekizumab. You may say that’s not enough time to assess the true risk for malignancy, and I would say you’re probably right. But 18 months isn’t shabby. From such an exposure, we can get some sense of the malignancy risk with this drug,” Dr. Bruce E. Strober asserted at the annual congress of the European Academy of Dermatology and Venereology.
That’s a key issue whenever a dermatologist prescribes a potent immune-altering anti-inflammatory medication for an indefinite period of time, he added.
In the randomized trials in which more than 2,300 patients who received 80 mg of ixekizumab every 2 or 4 weeks were compared with 739 patients assigned to etanercept (Enbrel) and 791 on placebo, malignancy rates were similarly low, at 0.1-0.3 cases per 100 patient-years across all four treatment arms, reported Dr. Strober, chair of the department of dermatology at the University of Connecticut, Farmington.
Moreover, in the long-term maintenance studies featuring 60 months of follow-up, the risks of both nonmelanoma skin cancer and other types of cancer were similarly low in patients randomized to ixekizumab at 80 mg every 2 weeks and those dosed every 4 weeks. That’s reassuring, because if a drug causes cancer one would expect to see that the more of the drug given, the higher the malignancy rate.
“You really don’t see any kind of a pattern or any difference between the various compared groups, including patients on etanercept, a drug that all of us feel very comfortable using in patients with psoriasis,” the dermatologist observed.
It’s thought that psoriasis patients have a higher background risk of malignancy than the general population due to the nature of the chronic disease, which involves immune dysregulation and chronic inflammation. In the studies to date, however, the malignancy risk in ixekizumab-treated patients is similar to those on placebo or etanercept.
“In my opinion, it’s comforting that the malignancy rates of the currently approved biologic agents for psoriasis are low and – one could argue – not above that which you would expect for the population of patients we’re treating. So I’ve always felt that monotherapy – and that’s an important point, not combination therapy with other immunosuppressive drugs – offers very little risk of de novo malignancy with the currently approved biologics,” Dr. Strober said.
Lumping together the results of the seven studies in his analysis, the incidence of squamous cell carcinoma was 0.1 cases per 100 patient-years of ixekizumab exposure, the risk of basal cell carcinoma was 0.3 cases per 100 patient-years, and the risk of malignancies other than nonmelanoma skin cancer was 0.5 per 100 patient-years of exposure to ixekizumab.
“That’s basically in line with every other biologic that I’ve examined with regard to malignancy rates: about 1 in 200 patients treated for 1 year gets a nonmelanoma skin cancer of some nature if you look at all data sources, including long-term registries and clinical trials. So it appears that this drug falls right in line with the other biologics in terms of rates of the more serious cancers, at least over the time period studied,” Dr. Strober commented.
He offered a caveat, however: “What will happen in patients given combination therapy with other immunosuppressive drugs? Or special populations, such as patients at higher risk for malignancy, be it skin cancer or other types of cancer? Or – and here’s the question we often have the most difficulty answering – what about people with a prior history of malignancy? What would be their risk of malignancy in being placed on a drug of this nature? Only time and really good registry data will allow us to answer these questions.”
Dr. Strober is a consultant to Eli Lilly, which sponsored the seven ixekizumab studies, as well as to numerous other pharmaceutical companies.
COPENHAGEN – Ixekizumab showed no hint of an increased malignancy risk of any sort in 4,208 patients with moderate to severe psoriasis who had 6,480 patient-years of exposure to the investigational interleukin-17A inhibitor in seven clinical trials.
“Patients were studied for an average of 18 months on ixekizumab. You may say that’s not enough time to assess the true risk for malignancy, and I would say you’re probably right. But 18 months isn’t shabby. From such an exposure, we can get some sense of the malignancy risk with this drug,” Dr. Bruce E. Strober asserted at the annual congress of the European Academy of Dermatology and Venereology.
That’s a key issue whenever a dermatologist prescribes a potent immune-altering anti-inflammatory medication for an indefinite period of time, he added.
In the randomized trials in which more than 2,300 patients who received 80 mg of ixekizumab every 2 or 4 weeks were compared with 739 patients assigned to etanercept (Enbrel) and 791 on placebo, malignancy rates were similarly low, at 0.1-0.3 cases per 100 patient-years across all four treatment arms, reported Dr. Strober, chair of the department of dermatology at the University of Connecticut, Farmington.
Moreover, in the long-term maintenance studies featuring 60 months of follow-up, the risks of both nonmelanoma skin cancer and other types of cancer were similarly low in patients randomized to ixekizumab at 80 mg every 2 weeks and those dosed every 4 weeks. That’s reassuring, because if a drug causes cancer one would expect to see that the more of the drug given, the higher the malignancy rate.
“You really don’t see any kind of a pattern or any difference between the various compared groups, including patients on etanercept, a drug that all of us feel very comfortable using in patients with psoriasis,” the dermatologist observed.
It’s thought that psoriasis patients have a higher background risk of malignancy than the general population due to the nature of the chronic disease, which involves immune dysregulation and chronic inflammation. In the studies to date, however, the malignancy risk in ixekizumab-treated patients is similar to those on placebo or etanercept.
“In my opinion, it’s comforting that the malignancy rates of the currently approved biologic agents for psoriasis are low and – one could argue – not above that which you would expect for the population of patients we’re treating. So I’ve always felt that monotherapy – and that’s an important point, not combination therapy with other immunosuppressive drugs – offers very little risk of de novo malignancy with the currently approved biologics,” Dr. Strober said.
Lumping together the results of the seven studies in his analysis, the incidence of squamous cell carcinoma was 0.1 cases per 100 patient-years of ixekizumab exposure, the risk of basal cell carcinoma was 0.3 cases per 100 patient-years, and the risk of malignancies other than nonmelanoma skin cancer was 0.5 per 100 patient-years of exposure to ixekizumab.
“That’s basically in line with every other biologic that I’ve examined with regard to malignancy rates: about 1 in 200 patients treated for 1 year gets a nonmelanoma skin cancer of some nature if you look at all data sources, including long-term registries and clinical trials. So it appears that this drug falls right in line with the other biologics in terms of rates of the more serious cancers, at least over the time period studied,” Dr. Strober commented.
He offered a caveat, however: “What will happen in patients given combination therapy with other immunosuppressive drugs? Or special populations, such as patients at higher risk for malignancy, be it skin cancer or other types of cancer? Or – and here’s the question we often have the most difficulty answering – what about people with a prior history of malignancy? What would be their risk of malignancy in being placed on a drug of this nature? Only time and really good registry data will allow us to answer these questions.”
Dr. Strober is a consultant to Eli Lilly, which sponsored the seven ixekizumab studies, as well as to numerous other pharmaceutical companies.
COPENHAGEN – Ixekizumab showed no hint of an increased malignancy risk of any sort in 4,208 patients with moderate to severe psoriasis who had 6,480 patient-years of exposure to the investigational interleukin-17A inhibitor in seven clinical trials.
“Patients were studied for an average of 18 months on ixekizumab. You may say that’s not enough time to assess the true risk for malignancy, and I would say you’re probably right. But 18 months isn’t shabby. From such an exposure, we can get some sense of the malignancy risk with this drug,” Dr. Bruce E. Strober asserted at the annual congress of the European Academy of Dermatology and Venereology.
That’s a key issue whenever a dermatologist prescribes a potent immune-altering anti-inflammatory medication for an indefinite period of time, he added.
In the randomized trials in which more than 2,300 patients who received 80 mg of ixekizumab every 2 or 4 weeks were compared with 739 patients assigned to etanercept (Enbrel) and 791 on placebo, malignancy rates were similarly low, at 0.1-0.3 cases per 100 patient-years across all four treatment arms, reported Dr. Strober, chair of the department of dermatology at the University of Connecticut, Farmington.
Moreover, in the long-term maintenance studies featuring 60 months of follow-up, the risks of both nonmelanoma skin cancer and other types of cancer were similarly low in patients randomized to ixekizumab at 80 mg every 2 weeks and those dosed every 4 weeks. That’s reassuring, because if a drug causes cancer one would expect to see that the more of the drug given, the higher the malignancy rate.
“You really don’t see any kind of a pattern or any difference between the various compared groups, including patients on etanercept, a drug that all of us feel very comfortable using in patients with psoriasis,” the dermatologist observed.
It’s thought that psoriasis patients have a higher background risk of malignancy than the general population due to the nature of the chronic disease, which involves immune dysregulation and chronic inflammation. In the studies to date, however, the malignancy risk in ixekizumab-treated patients is similar to those on placebo or etanercept.
“In my opinion, it’s comforting that the malignancy rates of the currently approved biologic agents for psoriasis are low and – one could argue – not above that which you would expect for the population of patients we’re treating. So I’ve always felt that monotherapy – and that’s an important point, not combination therapy with other immunosuppressive drugs – offers very little risk of de novo malignancy with the currently approved biologics,” Dr. Strober said.
Lumping together the results of the seven studies in his analysis, the incidence of squamous cell carcinoma was 0.1 cases per 100 patient-years of ixekizumab exposure, the risk of basal cell carcinoma was 0.3 cases per 100 patient-years, and the risk of malignancies other than nonmelanoma skin cancer was 0.5 per 100 patient-years of exposure to ixekizumab.
“That’s basically in line with every other biologic that I’ve examined with regard to malignancy rates: about 1 in 200 patients treated for 1 year gets a nonmelanoma skin cancer of some nature if you look at all data sources, including long-term registries and clinical trials. So it appears that this drug falls right in line with the other biologics in terms of rates of the more serious cancers, at least over the time period studied,” Dr. Strober commented.
He offered a caveat, however: “What will happen in patients given combination therapy with other immunosuppressive drugs? Or special populations, such as patients at higher risk for malignancy, be it skin cancer or other types of cancer? Or – and here’s the question we often have the most difficulty answering – what about people with a prior history of malignancy? What would be their risk of malignancy in being placed on a drug of this nature? Only time and really good registry data will allow us to answer these questions.”
Dr. Strober is a consultant to Eli Lilly, which sponsored the seven ixekizumab studies, as well as to numerous other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: In studies conducted in psoriasis patients to date, ixekizumab doesn’t raise any malignancy concerns.
Major finding: The risk of malignancies of any type was 0.9 cases per 100 patient-years of exposure to the investigational interleukin-17A inhibitor ixekizumab, which is in line with rates associated with the currently approved biologic agents.
Data source: This was an analysis of treatment-emergent malignancies among participants in seven clinical psoriasis trials representing a total of 6,480 patient-years of exposure to ixekizumab.
Disclosures: The presenter of this analysis is a consultant to Eli Lilly, which sponsored the seven ixekizumab studies, as well as to numerous other pharmaceutical companies.
EADV: Fractional CO2 laser called ‘unmatched’ for hypertrophic burn scars
COPENHAGEN – The fractional CO2 laser at 10,600 nm has emerged as the premier tool for improving the texture and elasticity of hypertrophic burn scars, Dr. Gerd G. Gauglitz asserted at the annual congress of the European Academy of Dermatology and Venereology.
“When it comes to improving the smoothness and the little elevations that are so bothersome to the burn patient, and especially when it comes to the stiffness of the scar, I think the fractional CO2 laser is the approach that has no competition. There might be other devices to come in the future, but I think the results we now see with the fractional CO2 laser are amazing,” said Dr. Gauglitz of Ludwig-Maximilian University, Munich.
It’s an opinion shared by other experts, he noted. For example, last year a panel of eight prominent American academic and military dermatologists and plastic surgeons with extensive experience in laser therapy of traumatic scars – including Dr. R. Rox Anderson of the Wellman Center for Photomedicine in Boston – published a consensus report that concluded “laser scar therapy, particularly fractional ablative laser resurfacing, represents a promising and vastly underused tool in the multidisciplinary treatment of traumatic scars” (JAMA Dermatol. 2014 Feb;150[2]:187-93).
Moreover, an international expert panel that issued updated clinical recommendations on scar management in 2014 declared that broader application of laser therapy constitutes “one of the most significant advances in scar management over the last 10 years.” The panel, which included Dr. Gauglitz, noted that “positive data for fractional lasers support their use for burn scar treatment” (Dermatol Surg. 2014 Aug;40[8]:817-24).
That being said, the current level of evidence supporting the use of fractional CO2 laser therapy for hypertrophic burn scars is poor, Dr. Gauglitz pointed out. No clear data-driven recommendations exist as to the optimal number of laser sessions, between-treatment intervals, or device settings. This was the impetus for his ongoing prospective, controlled study, evaluating a specific treatment protocol in 20 patients with severe, mature hypertrophic burn scars of an average 12.7 years duration.
For purposes of the study, as well as in his own clinical practice, Dr. Gauglitz utilizes the UltraPulse fractional CO2 laser to improve hypertrophic burn scars. He believes it offers compelling advantages over the alternative SuperPulse and continuous wave fractional CO2 laser technologies. The UltraPulse features the narrowest zone of thermal damage, meaning greater precision, less pain and down time; and a favorable safety profile. Most importantly, it has greater penetration capacity, causing microscopic thermal wounds to a depth of 4 mm below the skin surface, which is important for purposes of collagen remodeling, he explained.
When using CO2 lasers for burn scars, “clinically, we see completed reepithelialization after the procedure and then an ongoing collagen-remodeling phase lasting for up to 9 months. We see improvements in dermal architecture and creation of a collagen subtype profile that’s closer to nonwounded healthy skin,” according to the dermatologist.
Because other studies have relied heavily upon nonstandardized before and after photos and the Vancouver Scar Scale, with relatively short-term follow-up, Dr. Gauglitz and coinvestigators sought to advance the field by incorporating a variety of more objective measures, including the PRIMOS (Phaseshift Rapid in Vivo Measurement of the Skin) three-dimensional device widely utilized for wrinkle measurement, as well as the Cutometer, which evaluates skin elasticity. The investigators are also formally measuring the quality of life impact of their laser regimen.
The study consists of two parts. At the EADV congress, Dr. Gauglitz presented the results of the first part, which involved a single treatment of a 12-by-8 cm2 area, with a comparable untreated control area, and a 6-month follow-up. In the ongoing second part of the study, the same patients are undergoing three treatment sessions on a larger scarred area, with 3-month intervals between sessions. Again, a comparable untreated area of the body serves as the control.
Each treatment session involves three passes with the UltraPulse laser. The first is done at the most intense setting, known by the proprietary name of SCAAR FX (Synergistic Coagulation and Ablation for Advanced Resurfacing). This is aimed at inducing the collagen-remodeling process. The setting is 70-140 mJ/cm2, 250 Hz, with a density of 1%. The second pass utilizes the small-spot ACTIVE FX setting, designed to provide superficial ablative therapy to remove fine elevated scars. This involves 40-90 mJ/cm2 at high density and 300 Hz. The final pass is set at large-spot Active FX and employs 125 mJ/cm2 at low density and 125 Hz; the purpose is to smooth the scar and make the skin surface more homogeneous.
The most interesting aspect about the results, Dr. Gauglitz observed, is that 1 month after treatment, across-the-board improvements were documented, but then continued to grow in magnitude through the final 6-month follow-up – despite no further treatment. This was presumably the consequence of the collagen-remodeling process.
For example, scores on the Vancouver Scar Scale – still the most widely used scoring tool in the burn field – improved from roughly 7 at baseline to 2 at 6 months, compared with no change in the control area. Similarly, scores on the POSAS (Patient and Observer Scar Assessment Scale) showed highly significant and steadily growing improvement over 6 months of follow-up in most domains, including scar pigmentation, thickness, surface area, and flexibility.
Skin elasticity as measured by the Cutometer improved steadily by 28% at the 6-month mark. Likewise, evaluation with the PRIMOS device showed a steadily smoother scar surface over time.
Final results on the Dermatology Life Quality Index (DLQI) are still being tabulated, but there clearly is a strong improvement on this score – much of it attributed to the improved function resulting from greater flexibility.
“The results of fractional CO2 laser therapy are actually difficult to visualize, but patients are incredibly happy. For us, it might not be a big difference, but if you smoothen the scar surface and the patient is suddenly able to apply makeup without making the scar too visible, for those patients it’s a big thing,” Dr. Gauglitz said.
It’s clear from the ongoing multitreatment stage of the study that a single laser session is not optimal. “I think it’s going to be three, at least, for maximum benefit,” the dermatologist commented.
Asked if he thinks the fractional CO2 laser is the most effective tool for all aspects of scar treatment, Dr. Gauglitz was quick to say no.
“It depends, really, on the indication you want to treat. When it comes to erythema, a pulsed dye laser is much more effective than a fractional CO2 laser. For hyperpigmentation, a Q-switched ruby laser or the new picosecond laser might be a more interesting approach,” he replied.
Dr. Gauglitz reported serving on advisory boards and speakers’ bureaus for laser manufacturers Lumenis and Candela as well as for Merz Pharmaceuticals and Sinclair Pharma.
COPENHAGEN – The fractional CO2 laser at 10,600 nm has emerged as the premier tool for improving the texture and elasticity of hypertrophic burn scars, Dr. Gerd G. Gauglitz asserted at the annual congress of the European Academy of Dermatology and Venereology.
“When it comes to improving the smoothness and the little elevations that are so bothersome to the burn patient, and especially when it comes to the stiffness of the scar, I think the fractional CO2 laser is the approach that has no competition. There might be other devices to come in the future, but I think the results we now see with the fractional CO2 laser are amazing,” said Dr. Gauglitz of Ludwig-Maximilian University, Munich.
It’s an opinion shared by other experts, he noted. For example, last year a panel of eight prominent American academic and military dermatologists and plastic surgeons with extensive experience in laser therapy of traumatic scars – including Dr. R. Rox Anderson of the Wellman Center for Photomedicine in Boston – published a consensus report that concluded “laser scar therapy, particularly fractional ablative laser resurfacing, represents a promising and vastly underused tool in the multidisciplinary treatment of traumatic scars” (JAMA Dermatol. 2014 Feb;150[2]:187-93).
Moreover, an international expert panel that issued updated clinical recommendations on scar management in 2014 declared that broader application of laser therapy constitutes “one of the most significant advances in scar management over the last 10 years.” The panel, which included Dr. Gauglitz, noted that “positive data for fractional lasers support their use for burn scar treatment” (Dermatol Surg. 2014 Aug;40[8]:817-24).
That being said, the current level of evidence supporting the use of fractional CO2 laser therapy for hypertrophic burn scars is poor, Dr. Gauglitz pointed out. No clear data-driven recommendations exist as to the optimal number of laser sessions, between-treatment intervals, or device settings. This was the impetus for his ongoing prospective, controlled study, evaluating a specific treatment protocol in 20 patients with severe, mature hypertrophic burn scars of an average 12.7 years duration.
For purposes of the study, as well as in his own clinical practice, Dr. Gauglitz utilizes the UltraPulse fractional CO2 laser to improve hypertrophic burn scars. He believes it offers compelling advantages over the alternative SuperPulse and continuous wave fractional CO2 laser technologies. The UltraPulse features the narrowest zone of thermal damage, meaning greater precision, less pain and down time; and a favorable safety profile. Most importantly, it has greater penetration capacity, causing microscopic thermal wounds to a depth of 4 mm below the skin surface, which is important for purposes of collagen remodeling, he explained.
When using CO2 lasers for burn scars, “clinically, we see completed reepithelialization after the procedure and then an ongoing collagen-remodeling phase lasting for up to 9 months. We see improvements in dermal architecture and creation of a collagen subtype profile that’s closer to nonwounded healthy skin,” according to the dermatologist.
Because other studies have relied heavily upon nonstandardized before and after photos and the Vancouver Scar Scale, with relatively short-term follow-up, Dr. Gauglitz and coinvestigators sought to advance the field by incorporating a variety of more objective measures, including the PRIMOS (Phaseshift Rapid in Vivo Measurement of the Skin) three-dimensional device widely utilized for wrinkle measurement, as well as the Cutometer, which evaluates skin elasticity. The investigators are also formally measuring the quality of life impact of their laser regimen.
The study consists of two parts. At the EADV congress, Dr. Gauglitz presented the results of the first part, which involved a single treatment of a 12-by-8 cm2 area, with a comparable untreated control area, and a 6-month follow-up. In the ongoing second part of the study, the same patients are undergoing three treatment sessions on a larger scarred area, with 3-month intervals between sessions. Again, a comparable untreated area of the body serves as the control.
Each treatment session involves three passes with the UltraPulse laser. The first is done at the most intense setting, known by the proprietary name of SCAAR FX (Synergistic Coagulation and Ablation for Advanced Resurfacing). This is aimed at inducing the collagen-remodeling process. The setting is 70-140 mJ/cm2, 250 Hz, with a density of 1%. The second pass utilizes the small-spot ACTIVE FX setting, designed to provide superficial ablative therapy to remove fine elevated scars. This involves 40-90 mJ/cm2 at high density and 300 Hz. The final pass is set at large-spot Active FX and employs 125 mJ/cm2 at low density and 125 Hz; the purpose is to smooth the scar and make the skin surface more homogeneous.
The most interesting aspect about the results, Dr. Gauglitz observed, is that 1 month after treatment, across-the-board improvements were documented, but then continued to grow in magnitude through the final 6-month follow-up – despite no further treatment. This was presumably the consequence of the collagen-remodeling process.
For example, scores on the Vancouver Scar Scale – still the most widely used scoring tool in the burn field – improved from roughly 7 at baseline to 2 at 6 months, compared with no change in the control area. Similarly, scores on the POSAS (Patient and Observer Scar Assessment Scale) showed highly significant and steadily growing improvement over 6 months of follow-up in most domains, including scar pigmentation, thickness, surface area, and flexibility.
Skin elasticity as measured by the Cutometer improved steadily by 28% at the 6-month mark. Likewise, evaluation with the PRIMOS device showed a steadily smoother scar surface over time.
Final results on the Dermatology Life Quality Index (DLQI) are still being tabulated, but there clearly is a strong improvement on this score – much of it attributed to the improved function resulting from greater flexibility.
“The results of fractional CO2 laser therapy are actually difficult to visualize, but patients are incredibly happy. For us, it might not be a big difference, but if you smoothen the scar surface and the patient is suddenly able to apply makeup without making the scar too visible, for those patients it’s a big thing,” Dr. Gauglitz said.
It’s clear from the ongoing multitreatment stage of the study that a single laser session is not optimal. “I think it’s going to be three, at least, for maximum benefit,” the dermatologist commented.
Asked if he thinks the fractional CO2 laser is the most effective tool for all aspects of scar treatment, Dr. Gauglitz was quick to say no.
“It depends, really, on the indication you want to treat. When it comes to erythema, a pulsed dye laser is much more effective than a fractional CO2 laser. For hyperpigmentation, a Q-switched ruby laser or the new picosecond laser might be a more interesting approach,” he replied.
Dr. Gauglitz reported serving on advisory boards and speakers’ bureaus for laser manufacturers Lumenis and Candela as well as for Merz Pharmaceuticals and Sinclair Pharma.
COPENHAGEN – The fractional CO2 laser at 10,600 nm has emerged as the premier tool for improving the texture and elasticity of hypertrophic burn scars, Dr. Gerd G. Gauglitz asserted at the annual congress of the European Academy of Dermatology and Venereology.
“When it comes to improving the smoothness and the little elevations that are so bothersome to the burn patient, and especially when it comes to the stiffness of the scar, I think the fractional CO2 laser is the approach that has no competition. There might be other devices to come in the future, but I think the results we now see with the fractional CO2 laser are amazing,” said Dr. Gauglitz of Ludwig-Maximilian University, Munich.
It’s an opinion shared by other experts, he noted. For example, last year a panel of eight prominent American academic and military dermatologists and plastic surgeons with extensive experience in laser therapy of traumatic scars – including Dr. R. Rox Anderson of the Wellman Center for Photomedicine in Boston – published a consensus report that concluded “laser scar therapy, particularly fractional ablative laser resurfacing, represents a promising and vastly underused tool in the multidisciplinary treatment of traumatic scars” (JAMA Dermatol. 2014 Feb;150[2]:187-93).
Moreover, an international expert panel that issued updated clinical recommendations on scar management in 2014 declared that broader application of laser therapy constitutes “one of the most significant advances in scar management over the last 10 years.” The panel, which included Dr. Gauglitz, noted that “positive data for fractional lasers support their use for burn scar treatment” (Dermatol Surg. 2014 Aug;40[8]:817-24).
That being said, the current level of evidence supporting the use of fractional CO2 laser therapy for hypertrophic burn scars is poor, Dr. Gauglitz pointed out. No clear data-driven recommendations exist as to the optimal number of laser sessions, between-treatment intervals, or device settings. This was the impetus for his ongoing prospective, controlled study, evaluating a specific treatment protocol in 20 patients with severe, mature hypertrophic burn scars of an average 12.7 years duration.
For purposes of the study, as well as in his own clinical practice, Dr. Gauglitz utilizes the UltraPulse fractional CO2 laser to improve hypertrophic burn scars. He believes it offers compelling advantages over the alternative SuperPulse and continuous wave fractional CO2 laser technologies. The UltraPulse features the narrowest zone of thermal damage, meaning greater precision, less pain and down time; and a favorable safety profile. Most importantly, it has greater penetration capacity, causing microscopic thermal wounds to a depth of 4 mm below the skin surface, which is important for purposes of collagen remodeling, he explained.
When using CO2 lasers for burn scars, “clinically, we see completed reepithelialization after the procedure and then an ongoing collagen-remodeling phase lasting for up to 9 months. We see improvements in dermal architecture and creation of a collagen subtype profile that’s closer to nonwounded healthy skin,” according to the dermatologist.
Because other studies have relied heavily upon nonstandardized before and after photos and the Vancouver Scar Scale, with relatively short-term follow-up, Dr. Gauglitz and coinvestigators sought to advance the field by incorporating a variety of more objective measures, including the PRIMOS (Phaseshift Rapid in Vivo Measurement of the Skin) three-dimensional device widely utilized for wrinkle measurement, as well as the Cutometer, which evaluates skin elasticity. The investigators are also formally measuring the quality of life impact of their laser regimen.
The study consists of two parts. At the EADV congress, Dr. Gauglitz presented the results of the first part, which involved a single treatment of a 12-by-8 cm2 area, with a comparable untreated control area, and a 6-month follow-up. In the ongoing second part of the study, the same patients are undergoing three treatment sessions on a larger scarred area, with 3-month intervals between sessions. Again, a comparable untreated area of the body serves as the control.
Each treatment session involves three passes with the UltraPulse laser. The first is done at the most intense setting, known by the proprietary name of SCAAR FX (Synergistic Coagulation and Ablation for Advanced Resurfacing). This is aimed at inducing the collagen-remodeling process. The setting is 70-140 mJ/cm2, 250 Hz, with a density of 1%. The second pass utilizes the small-spot ACTIVE FX setting, designed to provide superficial ablative therapy to remove fine elevated scars. This involves 40-90 mJ/cm2 at high density and 300 Hz. The final pass is set at large-spot Active FX and employs 125 mJ/cm2 at low density and 125 Hz; the purpose is to smooth the scar and make the skin surface more homogeneous.
The most interesting aspect about the results, Dr. Gauglitz observed, is that 1 month after treatment, across-the-board improvements were documented, but then continued to grow in magnitude through the final 6-month follow-up – despite no further treatment. This was presumably the consequence of the collagen-remodeling process.
For example, scores on the Vancouver Scar Scale – still the most widely used scoring tool in the burn field – improved from roughly 7 at baseline to 2 at 6 months, compared with no change in the control area. Similarly, scores on the POSAS (Patient and Observer Scar Assessment Scale) showed highly significant and steadily growing improvement over 6 months of follow-up in most domains, including scar pigmentation, thickness, surface area, and flexibility.
Skin elasticity as measured by the Cutometer improved steadily by 28% at the 6-month mark. Likewise, evaluation with the PRIMOS device showed a steadily smoother scar surface over time.
Final results on the Dermatology Life Quality Index (DLQI) are still being tabulated, but there clearly is a strong improvement on this score – much of it attributed to the improved function resulting from greater flexibility.
“The results of fractional CO2 laser therapy are actually difficult to visualize, but patients are incredibly happy. For us, it might not be a big difference, but if you smoothen the scar surface and the patient is suddenly able to apply makeup without making the scar too visible, for those patients it’s a big thing,” Dr. Gauglitz said.
It’s clear from the ongoing multitreatment stage of the study that a single laser session is not optimal. “I think it’s going to be three, at least, for maximum benefit,” the dermatologist commented.
Asked if he thinks the fractional CO2 laser is the most effective tool for all aspects of scar treatment, Dr. Gauglitz was quick to say no.
“It depends, really, on the indication you want to treat. When it comes to erythema, a pulsed dye laser is much more effective than a fractional CO2 laser. For hyperpigmentation, a Q-switched ruby laser or the new picosecond laser might be a more interesting approach,” he replied.
Dr. Gauglitz reported serving on advisory boards and speakers’ bureaus for laser manufacturers Lumenis and Candela as well as for Merz Pharmaceuticals and Sinclair Pharma.
AT THE EADV CONGRESS
Key clinical point: Fractional CO2 laser therapy for hypertrophic burn scars provides unequaled improvements in skin softness and elasticity, as well as surface smoothness and relief.
Major finding: Scores on the Vancouver Scar Scale progressively dropped from roughly 7 to 2, with a 28% increase in scar elasticity as measured by the Cutometer, in the 6 months following one fractional CO2 laser treatment session in patients with severe hypertrophic burn scars.
Data source: This ongoing prospective study involves 20 patients with mature hypertrophic burn scars, treated with a fractional CO2 laser once or every 3 months, with untreated skin areas serving as the control.
Disclosures: Dr. Gauglitz reported serving on advisory boards and speakers’ bureaus for laser manufacturers Lumenis and Candela, as well as Merz Pharmaceuticals and Sinclair Pharma.