User login
Ablation cuts AF recurrence 2.5-fold vs. amiodarone in heart failure
SAN DIEGO – Catheter ablation proved superior to amiodarone for treatment of persistent atrial fibrillation in patients with systolic heart failure in the randomized AATAC-AF trial.
The rate of the primary study endpoint – freedom from recurrent AF through 26 months of prospective follow-up– was 70% in the catheter ablation group, twice the 34% rate with amiodarone, Dr. Luigi Di Biase reported at the annual meeting of the American College of Cardiology. After covariate adjustment, the investigators found that recurrence was 2.5 times more likely in the patients treated with amiodarone.
But he added a major caveat: pulmonary vein antrum isolation (PVI) alone was no better than the antiarrhythmic drug. The high overall treatment success rate seen with catheter ablation in the trial was achieved by operators who performed PVI plus some additional form of ablation of their own choosing, such as elimination of non–pulmonary vein triggers, ablation of complex fractionated electrograms, and/or additional linear ablation lesions, according to Dr. Di Biase, head of electrophysiology at the Albert Einstein College of Medicine, New York.
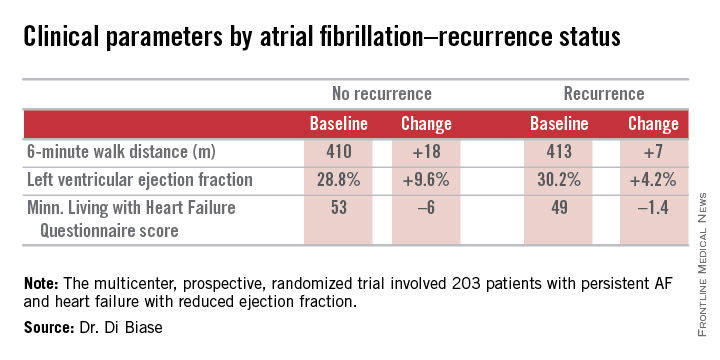
AATAC-AF (Ablation versus Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD/CRTD) was a multicenter, prospective, randomized trial involving 203 patients with persistent AF and heart failure with reduced ejection fraction. Patients randomized to ablation had to receive PVI at a minimum; operators could perform additional ablation according to their preference. Twenty percent of patients randomized to ablation received PVI alone; 80% underwent additional posterior wall and non–pulmonary vein trigger ablation. The 26-month rate of freedom from recurrence of AF was 36% in patients who received PVI alone and 79% in those who underwent more extensive ablations. A particular strength of the AATAC study was that all participants had an implantable cardioverter-defibrillator and/or cardiac resynchronization therapy device, permitting detection of AF with a much higher degree of accuracy than possible in most AF ablation trials.
Any recurrent AF episodes during the first 3 months of follow-up were excluded from the analysis, regardless of whether patients were in the ablation or amiodarone arms, in accord with the 3-month blanking period that’s standard among electrophysiologists. Patients averaged 1.4 ablation sessions during the first 3 months of the trial.
Regardless of treatment, patients in whom AF did not recur showed significantly greater improvement in left ventricular ejection fraction, exercise capacity, and heart failure–related quality of life.
In addition, all-cause mortality during follow-up was significantly lower in the ablation group: 8%, compared with 18% in patients assigned to amiodarone. Moreover, the rate of hospitalization for arrhythmia or worsening heart failure was 31% in the ablation group versus 57% in patients on amiodarone. The economic implications of this sharp reduction in hospitalizations will be the subject of further study, according to Dr. Di Biase.
Also noteworthy was the finding that seven patients had to discontinue amiodarone due to serious side effects: four because of thyroid toxicity, two for pulmonary toxicity, and one owing to hepatic dysfunction, he continued.
Discussant Dr. Richard I. Fogel, current president of the Heart Rhythm Society, commented that “the 70% arrhythmia-free follow-up was a little surprising to me.”
“That seems a little bit high, particularly in a group with persistent atrial fibrillation,” observed Dr. Fogel, who is chief executive officer at St. Vincent Medical Group, Indianapolis.
Dr. Di Biase attributed the high success rate to two factors: One, only highly experienced operators participated in AATAC, and two, most of them weren’t content to stick to PVI alone.
“If you try to do a more extensive procedure addressing non–pulmonary vein triggers in other areas in the left atrium, the success rate is increased by far,” the electrophysiologist said.
As for a possible mechanism for the mortality benefit seen with ablation, “several studies have shown that in a population with heart failure with reduced ejection fraction, atrial fibrillation is an independent predictor of mortality,” Dr. Di Biase said. “So I believe that staying in sinus rhythm may have affected the long-term mortality. If you have a treatment that reduces the amount of time in atrial fibrillation, you may reduce mortality.”
While catheter ablation is an increasingly popular treatment strategy in patients with drug-refractory paroxysmal AF, it has been understudied in the setting of AF and comorbid heart failure. These two conditions are commonly coexistent, and they feed on each other in a destructive way: AF worsens heart failure, and heart failure tends to make AF worse.
AATAC was funded by the participating investigators and institutions without external financial support. Dr. Di Biase reported serving as a consultant to Biosense Webster and St. Jude Medical and serving as a paid speaker for Atricure, Biotronik, Medtronic, Boston Scientific, and Epi EP.
SAN DIEGO – Catheter ablation proved superior to amiodarone for treatment of persistent atrial fibrillation in patients with systolic heart failure in the randomized AATAC-AF trial.
The rate of the primary study endpoint – freedom from recurrent AF through 26 months of prospective follow-up– was 70% in the catheter ablation group, twice the 34% rate with amiodarone, Dr. Luigi Di Biase reported at the annual meeting of the American College of Cardiology. After covariate adjustment, the investigators found that recurrence was 2.5 times more likely in the patients treated with amiodarone.

But he added a major caveat: pulmonary vein antrum isolation (PVI) alone was no better than the antiarrhythmic drug. The high overall treatment success rate seen with catheter ablation in the trial was achieved by operators who performed PVI plus some additional form of ablation of their own choosing, such as elimination of non–pulmonary vein triggers, ablation of complex fractionated electrograms, and/or additional linear ablation lesions, according to Dr. Di Biase, head of electrophysiology at the Albert Einstein College of Medicine, New York.
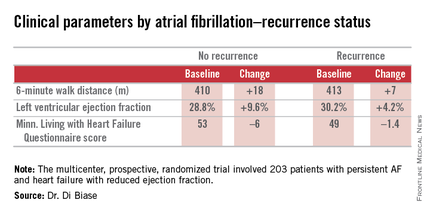
AATAC-AF (Ablation versus Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD/CRTD) was a multicenter, prospective, randomized trial involving 203 patients with persistent AF and heart failure with reduced ejection fraction. Patients randomized to ablation had to receive PVI at a minimum; operators could perform additional ablation according to their preference. Twenty percent of patients randomized to ablation received PVI alone; 80% underwent additional posterior wall and non–pulmonary vein trigger ablation. The 26-month rate of freedom from recurrence of AF was 36% in patients who received PVI alone and 79% in those who underwent more extensive ablations. A particular strength of the AATAC study was that all participants had an implantable cardioverter-defibrillator and/or cardiac resynchronization therapy device, permitting detection of AF with a much higher degree of accuracy than possible in most AF ablation trials.
Any recurrent AF episodes during the first 3 months of follow-up were excluded from the analysis, regardless of whether patients were in the ablation or amiodarone arms, in accord with the 3-month blanking period that’s standard among electrophysiologists. Patients averaged 1.4 ablation sessions during the first 3 months of the trial.
Regardless of treatment, patients in whom AF did not recur showed significantly greater improvement in left ventricular ejection fraction, exercise capacity, and heart failure–related quality of life.
In addition, all-cause mortality during follow-up was significantly lower in the ablation group: 8%, compared with 18% in patients assigned to amiodarone. Moreover, the rate of hospitalization for arrhythmia or worsening heart failure was 31% in the ablation group versus 57% in patients on amiodarone. The economic implications of this sharp reduction in hospitalizations will be the subject of further study, according to Dr. Di Biase.
Also noteworthy was the finding that seven patients had to discontinue amiodarone due to serious side effects: four because of thyroid toxicity, two for pulmonary toxicity, and one owing to hepatic dysfunction, he continued.
Discussant Dr. Richard I. Fogel, current president of the Heart Rhythm Society, commented that “the 70% arrhythmia-free follow-up was a little surprising to me.”
“That seems a little bit high, particularly in a group with persistent atrial fibrillation,” observed Dr. Fogel, who is chief executive officer at St. Vincent Medical Group, Indianapolis.
Dr. Di Biase attributed the high success rate to two factors: One, only highly experienced operators participated in AATAC, and two, most of them weren’t content to stick to PVI alone.
“If you try to do a more extensive procedure addressing non–pulmonary vein triggers in other areas in the left atrium, the success rate is increased by far,” the electrophysiologist said.
As for a possible mechanism for the mortality benefit seen with ablation, “several studies have shown that in a population with heart failure with reduced ejection fraction, atrial fibrillation is an independent predictor of mortality,” Dr. Di Biase said. “So I believe that staying in sinus rhythm may have affected the long-term mortality. If you have a treatment that reduces the amount of time in atrial fibrillation, you may reduce mortality.”
While catheter ablation is an increasingly popular treatment strategy in patients with drug-refractory paroxysmal AF, it has been understudied in the setting of AF and comorbid heart failure. These two conditions are commonly coexistent, and they feed on each other in a destructive way: AF worsens heart failure, and heart failure tends to make AF worse.
AATAC was funded by the participating investigators and institutions without external financial support. Dr. Di Biase reported serving as a consultant to Biosense Webster and St. Jude Medical and serving as a paid speaker for Atricure, Biotronik, Medtronic, Boston Scientific, and Epi EP.
SAN DIEGO – Catheter ablation proved superior to amiodarone for treatment of persistent atrial fibrillation in patients with systolic heart failure in the randomized AATAC-AF trial.
The rate of the primary study endpoint – freedom from recurrent AF through 26 months of prospective follow-up– was 70% in the catheter ablation group, twice the 34% rate with amiodarone, Dr. Luigi Di Biase reported at the annual meeting of the American College of Cardiology. After covariate adjustment, the investigators found that recurrence was 2.5 times more likely in the patients treated with amiodarone.

But he added a major caveat: pulmonary vein antrum isolation (PVI) alone was no better than the antiarrhythmic drug. The high overall treatment success rate seen with catheter ablation in the trial was achieved by operators who performed PVI plus some additional form of ablation of their own choosing, such as elimination of non–pulmonary vein triggers, ablation of complex fractionated electrograms, and/or additional linear ablation lesions, according to Dr. Di Biase, head of electrophysiology at the Albert Einstein College of Medicine, New York.
AATAC-AF (Ablation versus Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD/CRTD) was a multicenter, prospective, randomized trial involving 203 patients with persistent AF and heart failure with reduced ejection fraction. Patients randomized to ablation had to receive PVI at a minimum; operators could perform additional ablation according to their preference. Twenty percent of patients randomized to ablation received PVI alone; 80% underwent additional posterior wall and non–pulmonary vein trigger ablation. The 26-month rate of freedom from recurrence of AF was 36% in patients who received PVI alone and 79% in those who underwent more extensive ablations. A particular strength of the AATAC study was that all participants had an implantable cardioverter-defibrillator and/or cardiac resynchronization therapy device, permitting detection of AF with a much higher degree of accuracy than possible in most AF ablation trials.
Any recurrent AF episodes during the first 3 months of follow-up were excluded from the analysis, regardless of whether patients were in the ablation or amiodarone arms, in accord with the 3-month blanking period that’s standard among electrophysiologists. Patients averaged 1.4 ablation sessions during the first 3 months of the trial.
Regardless of treatment, patients in whom AF did not recur showed significantly greater improvement in left ventricular ejection fraction, exercise capacity, and heart failure–related quality of life.
In addition, all-cause mortality during follow-up was significantly lower in the ablation group: 8%, compared with 18% in patients assigned to amiodarone. Moreover, the rate of hospitalization for arrhythmia or worsening heart failure was 31% in the ablation group versus 57% in patients on amiodarone. The economic implications of this sharp reduction in hospitalizations will be the subject of further study, according to Dr. Di Biase.
Also noteworthy was the finding that seven patients had to discontinue amiodarone due to serious side effects: four because of thyroid toxicity, two for pulmonary toxicity, and one owing to hepatic dysfunction, he continued.
Discussant Dr. Richard I. Fogel, current president of the Heart Rhythm Society, commented that “the 70% arrhythmia-free follow-up was a little surprising to me.”
“That seems a little bit high, particularly in a group with persistent atrial fibrillation,” observed Dr. Fogel, who is chief executive officer at St. Vincent Medical Group, Indianapolis.
Dr. Di Biase attributed the high success rate to two factors: One, only highly experienced operators participated in AATAC, and two, most of them weren’t content to stick to PVI alone.
“If you try to do a more extensive procedure addressing non–pulmonary vein triggers in other areas in the left atrium, the success rate is increased by far,” the electrophysiologist said.
As for a possible mechanism for the mortality benefit seen with ablation, “several studies have shown that in a population with heart failure with reduced ejection fraction, atrial fibrillation is an independent predictor of mortality,” Dr. Di Biase said. “So I believe that staying in sinus rhythm may have affected the long-term mortality. If you have a treatment that reduces the amount of time in atrial fibrillation, you may reduce mortality.”
While catheter ablation is an increasingly popular treatment strategy in patients with drug-refractory paroxysmal AF, it has been understudied in the setting of AF and comorbid heart failure. These two conditions are commonly coexistent, and they feed on each other in a destructive way: AF worsens heart failure, and heart failure tends to make AF worse.
AATAC was funded by the participating investigators and institutions without external financial support. Dr. Di Biase reported serving as a consultant to Biosense Webster and St. Jude Medical and serving as a paid speaker for Atricure, Biotronik, Medtronic, Boston Scientific, and Epi EP.
AT ACC 15
Key clinical point: Catheter ablation is hands down more effective than amiodarone for the treatment of persistent atrial fibrillation in patients with systolic heart failure.
Major finding: The rate of freedom from recurrent atrial fibrillation during 26 months of follow-up was 70% in patients randomized to catheter ablation, compared with 34% in those assigned to amiodarone.
Data source: The AATAC-AF study was a multicenter, randomized, prospective clinical trial inc 203 patients.
Disclosures: The trial was funded by the participating investigators and institutions without commercial support. Dr. Di Biase reported serving as a consultant to Biosense Webster and St. Jude Medical and serving as a paid speaker for Atricure, Biotronik, Medtronic, Boston Scientific, and Epi EP.
VIDEO: Novel Three-shot Immunotherapy Regimen
HOUSTON– Dr. Amber M. Patterson of Nationwide Children’s Hospital and Research Institute in Columbus, Ohio, provides the lowdown on intralymphatic immunotherapy, in which a three-injection protocol of ultrasound-guided lymph node injections spaced 4 weeks apart is being investigated as a treatment for grass pollen-induced rhinoconjunctivitis in adolescents. It’s an attractive alternative to the conventional 3-year regimen of weekly, then monthly subcutaneous immunotherapy injections.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON– Dr. Amber M. Patterson of Nationwide Children’s Hospital and Research Institute in Columbus, Ohio, provides the lowdown on intralymphatic immunotherapy, in which a three-injection protocol of ultrasound-guided lymph node injections spaced 4 weeks apart is being investigated as a treatment for grass pollen-induced rhinoconjunctivitis in adolescents. It’s an attractive alternative to the conventional 3-year regimen of weekly, then monthly subcutaneous immunotherapy injections.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON– Dr. Amber M. Patterson of Nationwide Children’s Hospital and Research Institute in Columbus, Ohio, provides the lowdown on intralymphatic immunotherapy, in which a three-injection protocol of ultrasound-guided lymph node injections spaced 4 weeks apart is being investigated as a treatment for grass pollen-induced rhinoconjunctivitis in adolescents. It’s an attractive alternative to the conventional 3-year regimen of weekly, then monthly subcutaneous immunotherapy injections.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Baroreflex activation therapy improves heart failure symptoms
SAN DIEGO– Baroreflex activation therapy showed promise as an important new device therapy for patients with New York Heart Association Class III heart failure, according to researchers.
In a preliminary randomized trial, the investigational therapy demonstrated safety comparable to that of established device therapies for heart failure. And it significantly improved functional status, exercise capacity, and quality of life while reducing levels of the biomarker N-terminal pro-brain natriuretic peptide (NT-proBNP) and days-in-hospital for worsening heart failure, Dr. William T. Abraham said at the annual meeting of the American College of Cardiology.
“The trial showed important results,” he said. “If these observations are confirmed in larger studies, baroreflex activation therapy may offer a new addition for the treatment of advanced heart failure patients with a reduced left ventricular ejection fraction.”
Novel therapies for patients with NYHA class III heart failure with a reduced ejection fraction (HFrEF) are sorely needed, he added, as 25%-35% of patients with HFrEF remain in NYHA class III despite current drug and device therapies. These patients are moderately symptomatic, meaning they are sick enough that their quality of life is sharply diminished, but not sufficiently ill to qualify for advanced heart therapies, such as cardiac transplantation or a left ventricular assist device.
Dr. Abraham presented the findings of a multinational, prospective, randomized, 6-month, controlled trial involving 140 NYHA class III HFrEF patients in the United States, Canada, Germany, and France. They were randomized to optimal guideline-directed medical therapy alone or in conjunction with baroreflex activation therapy (BAT), a form of neuromodulatory therapy involving electrical stimulation of the carotid baroreflex baroreceptor delivered by an implanted device similar to a pacemaker.
Progressive heart failure is characterized by increased sympathetic and reduced parasympathetic nerve activity. BAT addresses both abnormalities.
“This form of neuromodulation differs from other forms of neuromodulation in that it does not target a peripheral efferent nerve, but rather it targets the carotid baroreceptor. It targets afferent signals to the brain, which then produce an integrated autonomic nervous system response resulting in inhibition of sympathetic activity and enhancement of parasympathetic activity. So this is a physiologic form of autonomic rebalancing that is mediated via the CNS,” explained Dr. Abraham, professor of medicine and director of the division of cardiovascular medicine at Ohio State University, Columbus.
The primary safety endpoint was freedom from system- and procedure-related major adverse neurologic and cardiovascular events at 6 months. The rate was 97.2%. There were no deaths, and complications – all of which occurred within the first 7 days – were few and short lived, with rates similar to those seen with implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy (CRT). The BAT device, known as the CVRx Barostim neo, did not interact with the ICDs and CRT devices present in a large number of participants. No hypotension occurred in this normotensive HFrEF population.
To place the efficacy outcomes in perspective, Dr. Abraham continued, it’s worth noting that the average 19.5-point between-group difference favoring BAT in scores on the Minnesota Living with Heart Failure Quality of Life Questionnaire (MLHFQ) at 6 months compared to a baseline of 45 points dwarfs the benefits obtainable with standard therapies.
“Please remember that our best drug therapies for HFrEF – ACE inhibitors and beta blockers– improve the MLHFQ score by 4 or 5 points, and cardiac resynchronization therapy improves that score by an average of 9 or 10 points,” he said. From a baseline of 300 meters, the 6-minute hall walk distance improved by 58 meters more in the BAT group than in controls. By comparison, CRT improves the distance walked in 6 minutes by about 30 meters.
“The magnitude of benefit of BAT exceeds that seen with standard therapies, it was seen on top of those standard therapies, and it certainly falls into a range that would be considered clinically meaningful,” the cardiologist asserted.
Left ventricular ejection fraction improved in the BAT group by an average of 2.4% from a baseline of 24% while decreasing by 0.1% in controls. The between-group difference in NT-proBNP at 6 months was 342 pg/mL in favor of the BAT group, starting from a baseline level of roughly 1,300 pg/mL.
The BAT group averaged 6.95 hospital days per year for worsening heart failure during the 6 months prior to enrollment and 0.67 days per year in the 6 months following device activation, for an adjusted 82% relative risk reduction. In contrast, hospital days for heart failure remained steady in controls. The possibility of a new treatment that reduces heart failure hospitalizations is of particular interest in light of the enormous financial burden such hospitalizations currently place upon the Medicare system.
Simultaneously with Dr. Abraham’s presentation at ACC 15, the study manuscript was published online (JACC: Heart Failure 2015 [doi: 10.1016/j.jchf.2015.02.006]).
Dr. Abraham is a consultant to CVRx (which funded the study), as well as Novartis, St. Jude Medical, CardioMEMS, and Abbott Vascular.
SAN DIEGO– Baroreflex activation therapy showed promise as an important new device therapy for patients with New York Heart Association Class III heart failure, according to researchers.
In a preliminary randomized trial, the investigational therapy demonstrated safety comparable to that of established device therapies for heart failure. And it significantly improved functional status, exercise capacity, and quality of life while reducing levels of the biomarker N-terminal pro-brain natriuretic peptide (NT-proBNP) and days-in-hospital for worsening heart failure, Dr. William T. Abraham said at the annual meeting of the American College of Cardiology.
“The trial showed important results,” he said. “If these observations are confirmed in larger studies, baroreflex activation therapy may offer a new addition for the treatment of advanced heart failure patients with a reduced left ventricular ejection fraction.”
Novel therapies for patients with NYHA class III heart failure with a reduced ejection fraction (HFrEF) are sorely needed, he added, as 25%-35% of patients with HFrEF remain in NYHA class III despite current drug and device therapies. These patients are moderately symptomatic, meaning they are sick enough that their quality of life is sharply diminished, but not sufficiently ill to qualify for advanced heart therapies, such as cardiac transplantation or a left ventricular assist device.
Dr. Abraham presented the findings of a multinational, prospective, randomized, 6-month, controlled trial involving 140 NYHA class III HFrEF patients in the United States, Canada, Germany, and France. They were randomized to optimal guideline-directed medical therapy alone or in conjunction with baroreflex activation therapy (BAT), a form of neuromodulatory therapy involving electrical stimulation of the carotid baroreflex baroreceptor delivered by an implanted device similar to a pacemaker.
Progressive heart failure is characterized by increased sympathetic and reduced parasympathetic nerve activity. BAT addresses both abnormalities.
“This form of neuromodulation differs from other forms of neuromodulation in that it does not target a peripheral efferent nerve, but rather it targets the carotid baroreceptor. It targets afferent signals to the brain, which then produce an integrated autonomic nervous system response resulting in inhibition of sympathetic activity and enhancement of parasympathetic activity. So this is a physiologic form of autonomic rebalancing that is mediated via the CNS,” explained Dr. Abraham, professor of medicine and director of the division of cardiovascular medicine at Ohio State University, Columbus.
The primary safety endpoint was freedom from system- and procedure-related major adverse neurologic and cardiovascular events at 6 months. The rate was 97.2%. There were no deaths, and complications – all of which occurred within the first 7 days – were few and short lived, with rates similar to those seen with implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy (CRT). The BAT device, known as the CVRx Barostim neo, did not interact with the ICDs and CRT devices present in a large number of participants. No hypotension occurred in this normotensive HFrEF population.
To place the efficacy outcomes in perspective, Dr. Abraham continued, it’s worth noting that the average 19.5-point between-group difference favoring BAT in scores on the Minnesota Living with Heart Failure Quality of Life Questionnaire (MLHFQ) at 6 months compared to a baseline of 45 points dwarfs the benefits obtainable with standard therapies.
“Please remember that our best drug therapies for HFrEF – ACE inhibitors and beta blockers– improve the MLHFQ score by 4 or 5 points, and cardiac resynchronization therapy improves that score by an average of 9 or 10 points,” he said. From a baseline of 300 meters, the 6-minute hall walk distance improved by 58 meters more in the BAT group than in controls. By comparison, CRT improves the distance walked in 6 minutes by about 30 meters.
“The magnitude of benefit of BAT exceeds that seen with standard therapies, it was seen on top of those standard therapies, and it certainly falls into a range that would be considered clinically meaningful,” the cardiologist asserted.
Left ventricular ejection fraction improved in the BAT group by an average of 2.4% from a baseline of 24% while decreasing by 0.1% in controls. The between-group difference in NT-proBNP at 6 months was 342 pg/mL in favor of the BAT group, starting from a baseline level of roughly 1,300 pg/mL.
The BAT group averaged 6.95 hospital days per year for worsening heart failure during the 6 months prior to enrollment and 0.67 days per year in the 6 months following device activation, for an adjusted 82% relative risk reduction. In contrast, hospital days for heart failure remained steady in controls. The possibility of a new treatment that reduces heart failure hospitalizations is of particular interest in light of the enormous financial burden such hospitalizations currently place upon the Medicare system.
Simultaneously with Dr. Abraham’s presentation at ACC 15, the study manuscript was published online (JACC: Heart Failure 2015 [doi: 10.1016/j.jchf.2015.02.006]).
Dr. Abraham is a consultant to CVRx (which funded the study), as well as Novartis, St. Jude Medical, CardioMEMS, and Abbott Vascular.
SAN DIEGO– Baroreflex activation therapy showed promise as an important new device therapy for patients with New York Heart Association Class III heart failure, according to researchers.
In a preliminary randomized trial, the investigational therapy demonstrated safety comparable to that of established device therapies for heart failure. And it significantly improved functional status, exercise capacity, and quality of life while reducing levels of the biomarker N-terminal pro-brain natriuretic peptide (NT-proBNP) and days-in-hospital for worsening heart failure, Dr. William T. Abraham said at the annual meeting of the American College of Cardiology.
“The trial showed important results,” he said. “If these observations are confirmed in larger studies, baroreflex activation therapy may offer a new addition for the treatment of advanced heart failure patients with a reduced left ventricular ejection fraction.”
Novel therapies for patients with NYHA class III heart failure with a reduced ejection fraction (HFrEF) are sorely needed, he added, as 25%-35% of patients with HFrEF remain in NYHA class III despite current drug and device therapies. These patients are moderately symptomatic, meaning they are sick enough that their quality of life is sharply diminished, but not sufficiently ill to qualify for advanced heart therapies, such as cardiac transplantation or a left ventricular assist device.
Dr. Abraham presented the findings of a multinational, prospective, randomized, 6-month, controlled trial involving 140 NYHA class III HFrEF patients in the United States, Canada, Germany, and France. They were randomized to optimal guideline-directed medical therapy alone or in conjunction with baroreflex activation therapy (BAT), a form of neuromodulatory therapy involving electrical stimulation of the carotid baroreflex baroreceptor delivered by an implanted device similar to a pacemaker.
Progressive heart failure is characterized by increased sympathetic and reduced parasympathetic nerve activity. BAT addresses both abnormalities.
“This form of neuromodulation differs from other forms of neuromodulation in that it does not target a peripheral efferent nerve, but rather it targets the carotid baroreceptor. It targets afferent signals to the brain, which then produce an integrated autonomic nervous system response resulting in inhibition of sympathetic activity and enhancement of parasympathetic activity. So this is a physiologic form of autonomic rebalancing that is mediated via the CNS,” explained Dr. Abraham, professor of medicine and director of the division of cardiovascular medicine at Ohio State University, Columbus.
The primary safety endpoint was freedom from system- and procedure-related major adverse neurologic and cardiovascular events at 6 months. The rate was 97.2%. There were no deaths, and complications – all of which occurred within the first 7 days – were few and short lived, with rates similar to those seen with implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy (CRT). The BAT device, known as the CVRx Barostim neo, did not interact with the ICDs and CRT devices present in a large number of participants. No hypotension occurred in this normotensive HFrEF population.
To place the efficacy outcomes in perspective, Dr. Abraham continued, it’s worth noting that the average 19.5-point between-group difference favoring BAT in scores on the Minnesota Living with Heart Failure Quality of Life Questionnaire (MLHFQ) at 6 months compared to a baseline of 45 points dwarfs the benefits obtainable with standard therapies.
“Please remember that our best drug therapies for HFrEF – ACE inhibitors and beta blockers– improve the MLHFQ score by 4 or 5 points, and cardiac resynchronization therapy improves that score by an average of 9 or 10 points,” he said. From a baseline of 300 meters, the 6-minute hall walk distance improved by 58 meters more in the BAT group than in controls. By comparison, CRT improves the distance walked in 6 minutes by about 30 meters.
“The magnitude of benefit of BAT exceeds that seen with standard therapies, it was seen on top of those standard therapies, and it certainly falls into a range that would be considered clinically meaningful,” the cardiologist asserted.
Left ventricular ejection fraction improved in the BAT group by an average of 2.4% from a baseline of 24% while decreasing by 0.1% in controls. The between-group difference in NT-proBNP at 6 months was 342 pg/mL in favor of the BAT group, starting from a baseline level of roughly 1,300 pg/mL.
The BAT group averaged 6.95 hospital days per year for worsening heart failure during the 6 months prior to enrollment and 0.67 days per year in the 6 months following device activation, for an adjusted 82% relative risk reduction. In contrast, hospital days for heart failure remained steady in controls. The possibility of a new treatment that reduces heart failure hospitalizations is of particular interest in light of the enormous financial burden such hospitalizations currently place upon the Medicare system.
Simultaneously with Dr. Abraham’s presentation at ACC 15, the study manuscript was published online (JACC: Heart Failure 2015 [doi: 10.1016/j.jchf.2015.02.006]).
Dr. Abraham is a consultant to CVRx (which funded the study), as well as Novartis, St. Jude Medical, CardioMEMS, and Abbott Vascular.
AT ACC 15
Key clinical point: Baroreflex activation therapy is safe and improves multiple aspects of NYHA class III heart failure with reduced ejection fraction.
Major finding: Fifty-five percent of patients assigned to baroreflex activation therapy plus optimal guideline-directed medical therapy showed at least a one-class-rank improvement at 6 months of follow-up, compared with 24% of controls on optimal medical therapy alone.
Data source: A multinational, prospective, randomized, 6-month, controlled clinical trial of 140 patients with NYHA class III HFrEF.
Disclosures: Dr. Abraham is a consultant to CVRx (which funded the study), as well as Novartis, St. Jude Medical, CardioMEMS, and Abbott Vascular.
Evolocumab halved cardiovascular events in OSLER study
SAN DIEGO– The investigational PCSK9 inhibitor evolucumab dramatically reduced the 1-year cardiovascular event rate, by 53%, compared with standard statin-based lipid-lowering in the randomized OSLER study.
This reduction in adverse cardiovascular events was accompanied by a robust 61% reduction in LDL compared with standard therapy. The LDL level in evolocumab-treated patients dropped from 120 mg/dL to an average of 48 mg/dL, Dr. Marc S. Sabatine reported at the annual meeting of the American College of Cardiology.
The safety profile of patients on evolocumab plus standard, statin-based lipid lowering was essentially the same as in controls on standard therapy alone. Moreover, no gradient was seen for any adverse events on the basis of the extent of LDL lowering. For example, the types and incidence of adverse events and laboratory abnormalities in the 773 evolocumab-treated OSLER participants with an achieved LDL less than 25 mg/dL were closely similar to those seen with an LDL above 40 mg/dL, according to Dr. Sabatine, chairman of the Thrombolysis In Myocardial Infarction Study Group at Brigham and Women’s Hospital in Boston.
“These data, in conjuction with epidemiological and genetic data, offer further support for the potential for PCSK9 inhibition as a safe and effective means to reduce major adverse cardiovascular outcomes through particularly robust LDL cholesterol lowering,” he added.
OSLER (Open-Label Study of Long-Term Evaluation against LDL Cholesterol) included 4,465 patients who had completed one of a dozen earlier phase II or III studies of evolucumab, a monoclonal antibody that inhibits proprotein convertase subtilisin kexin type 9 (PCSK9), and were then randomized 2:1 to 1 year of open-label evolucumab administered subcutaneously at either 140 mg every 2 weeks or 420 mg once monthly plus standard lipid-lowering, or to standard lipid-lowering alone, generally with moderate- or high-dose statins.
The PCSK9 inhibitor reduced LDL cholesterol by 73 mg/dL compared to the control arm. In addition, the evolocumab group experienced a 26% decrease in Lp(a), a 47% drop in ApoB, a 13% reduction in triglycerides, and a 7% rise in HDL relative to standard care.
The 1-year composite cardiovascular event rate was just 0.95% in the evolucumab group, a 53% reduction relative to the 2.18% rate in the control arm. The composite outcome was comprised of death, MI, stroke or TIA, hospitalization for heart failure, coronary revascularization, or hospitalization for unstable angina. The reduction in events seen with evolocumab was consistent across all the components of the composite outcome.
Rates of most adverse events were closely similar in the two study arms. The exception was self-reported neurocognitive events such as forgetfulness or confusion, where the 0.9% incidence in the evolucumab group, while quite low in an absolute sense, was nonetheless threefold higher than in controls.
While the OSLER findings are highly encouraging in terms of both efficacy and safety, the study was too small and too brief at 1 year to be considered definitive, especially in terms of cardiovascular event rates, of which there only 60 in total. All eyes are now on the ongoing 27,500-patient, randomized, placebo-controlled FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) study. Participants in FOURIER must have a history of MI, stroke, or peripheral artery disease plus at least one additional high-risk feature. Results are expected in 2017.
Similar massive pivotal phase III studies of two other highly promising PCSK9 inhibitors – alirocumab and bococizumab – are ongoing. The PCSK9 inhibitors were the talk of ACC 2015 in light of the mounting impressive safety and efficacy data. The Food and Drug Administration is now considering whether to approve PCSK9 inhibition for patients with familial hypercholesterolemia whose LDL is not adequately controlled by statins, with a decision expected later this year.
Discussant Dr. Christopher P. Cannon called OSLER “a terrific study.”
The OSLER findings, coupled with a previously reported preliminary analysis suggesting reduced cardiovascular events in clinical trials of alirocumab, provide strong support for the notion that the lower the achieved LDL – whether accomplished by statins or other agents – the greater the cardiovascular benefit.
“This is so exciting. Lowering cholesterol is definitely a passion of mine,” said Dr. Cannon, professor of medicine at Harvard Medical School, Boston.
Discussant Dr. Judith S. Hochman was equally enthusiastic about the OSLER outcomes.
“This is really impressive and very encouraging for this class of cholesterol-lowering agents. And I want to congratulate you for enrolling 50% women. But we need more study about the potential for neurocognitive events,” commented Dr. Hochman, professor of cardiology and senior associate dean for clinical sciences at New York University.
Dr. Sabatine agreed. While neurocognitive events in OSLER were self-reported by patients, FOURIER includes a substudy featuring formal neurocognitive testing using validated instruments. And while that should provide definitive answers, he said he’s not too concerned about the neurocognitive issue.
“Biologically, LDL cholesterol is not used as a source of cholesterol for cells, and that’s especially true for cells in the CNS because of the blood-brain barrier. And very low LDL levels don’t appear to have any effect on the CNS in animal models,” he said.
A more comprehensive picture of evolucumab’s safety was provided elsewhere at ACC 15 by Dr. Peter P. Toth. His safety analysis incorporated more than 6,000 evolucumab-treated patients and 3,569 controls who participated in a dozen phase II and III trials as well as OSLER.
The short version: This pooled analysis demonstrated no increase in any clinical adverse events or laboratory abnormalities with evolocumab, compared with controls. That includes muscle-related adverse events, liver or renal toxicity, impairment in glucose metabolism, infections, injection-site reactions, and neurocognitive events, said Dr. Toth of the University of Illinois, Peoria.
Simultaneous with Dr. Sabatine’s presentation at ACC 15, the OSLER results were published online (N. Engl. J. Med. 2015 [doi: 10.1056/NEJMoa1500858]).
Both OSLER and Dr. Toth’s broader safety analysis were funded by Amgen. Dr. Sabatine and Dr. Toth reported having received research grant support from and serving as consultants to Amgen and numerous other pharmaceutical companies. Dr. Hochman is a consultant to GlaxoSmithKline. Dr. Cannon has received research grant support from and served as a consultant or advisor to many pharmaceutical companies.
SAN DIEGO– The investigational PCSK9 inhibitor evolucumab dramatically reduced the 1-year cardiovascular event rate, by 53%, compared with standard statin-based lipid-lowering in the randomized OSLER study.
This reduction in adverse cardiovascular events was accompanied by a robust 61% reduction in LDL compared with standard therapy. The LDL level in evolocumab-treated patients dropped from 120 mg/dL to an average of 48 mg/dL, Dr. Marc S. Sabatine reported at the annual meeting of the American College of Cardiology.
The safety profile of patients on evolocumab plus standard, statin-based lipid lowering was essentially the same as in controls on standard therapy alone. Moreover, no gradient was seen for any adverse events on the basis of the extent of LDL lowering. For example, the types and incidence of adverse events and laboratory abnormalities in the 773 evolocumab-treated OSLER participants with an achieved LDL less than 25 mg/dL were closely similar to those seen with an LDL above 40 mg/dL, according to Dr. Sabatine, chairman of the Thrombolysis In Myocardial Infarction Study Group at Brigham and Women’s Hospital in Boston.
“These data, in conjuction with epidemiological and genetic data, offer further support for the potential for PCSK9 inhibition as a safe and effective means to reduce major adverse cardiovascular outcomes through particularly robust LDL cholesterol lowering,” he added.
OSLER (Open-Label Study of Long-Term Evaluation against LDL Cholesterol) included 4,465 patients who had completed one of a dozen earlier phase II or III studies of evolucumab, a monoclonal antibody that inhibits proprotein convertase subtilisin kexin type 9 (PCSK9), and were then randomized 2:1 to 1 year of open-label evolucumab administered subcutaneously at either 140 mg every 2 weeks or 420 mg once monthly plus standard lipid-lowering, or to standard lipid-lowering alone, generally with moderate- or high-dose statins.
The PCSK9 inhibitor reduced LDL cholesterol by 73 mg/dL compared to the control arm. In addition, the evolocumab group experienced a 26% decrease in Lp(a), a 47% drop in ApoB, a 13% reduction in triglycerides, and a 7% rise in HDL relative to standard care.
The 1-year composite cardiovascular event rate was just 0.95% in the evolucumab group, a 53% reduction relative to the 2.18% rate in the control arm. The composite outcome was comprised of death, MI, stroke or TIA, hospitalization for heart failure, coronary revascularization, or hospitalization for unstable angina. The reduction in events seen with evolocumab was consistent across all the components of the composite outcome.
Rates of most adverse events were closely similar in the two study arms. The exception was self-reported neurocognitive events such as forgetfulness or confusion, where the 0.9% incidence in the evolucumab group, while quite low in an absolute sense, was nonetheless threefold higher than in controls.
While the OSLER findings are highly encouraging in terms of both efficacy and safety, the study was too small and too brief at 1 year to be considered definitive, especially in terms of cardiovascular event rates, of which there only 60 in total. All eyes are now on the ongoing 27,500-patient, randomized, placebo-controlled FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) study. Participants in FOURIER must have a history of MI, stroke, or peripheral artery disease plus at least one additional high-risk feature. Results are expected in 2017.
Similar massive pivotal phase III studies of two other highly promising PCSK9 inhibitors – alirocumab and bococizumab – are ongoing. The PCSK9 inhibitors were the talk of ACC 2015 in light of the mounting impressive safety and efficacy data. The Food and Drug Administration is now considering whether to approve PCSK9 inhibition for patients with familial hypercholesterolemia whose LDL is not adequately controlled by statins, with a decision expected later this year.
Discussant Dr. Christopher P. Cannon called OSLER “a terrific study.”
The OSLER findings, coupled with a previously reported preliminary analysis suggesting reduced cardiovascular events in clinical trials of alirocumab, provide strong support for the notion that the lower the achieved LDL – whether accomplished by statins or other agents – the greater the cardiovascular benefit.
“This is so exciting. Lowering cholesterol is definitely a passion of mine,” said Dr. Cannon, professor of medicine at Harvard Medical School, Boston.
Discussant Dr. Judith S. Hochman was equally enthusiastic about the OSLER outcomes.
“This is really impressive and very encouraging for this class of cholesterol-lowering agents. And I want to congratulate you for enrolling 50% women. But we need more study about the potential for neurocognitive events,” commented Dr. Hochman, professor of cardiology and senior associate dean for clinical sciences at New York University.
Dr. Sabatine agreed. While neurocognitive events in OSLER were self-reported by patients, FOURIER includes a substudy featuring formal neurocognitive testing using validated instruments. And while that should provide definitive answers, he said he’s not too concerned about the neurocognitive issue.
“Biologically, LDL cholesterol is not used as a source of cholesterol for cells, and that’s especially true for cells in the CNS because of the blood-brain barrier. And very low LDL levels don’t appear to have any effect on the CNS in animal models,” he said.
A more comprehensive picture of evolucumab’s safety was provided elsewhere at ACC 15 by Dr. Peter P. Toth. His safety analysis incorporated more than 6,000 evolucumab-treated patients and 3,569 controls who participated in a dozen phase II and III trials as well as OSLER.
The short version: This pooled analysis demonstrated no increase in any clinical adverse events or laboratory abnormalities with evolocumab, compared with controls. That includes muscle-related adverse events, liver or renal toxicity, impairment in glucose metabolism, infections, injection-site reactions, and neurocognitive events, said Dr. Toth of the University of Illinois, Peoria.
Simultaneous with Dr. Sabatine’s presentation at ACC 15, the OSLER results were published online (N. Engl. J. Med. 2015 [doi: 10.1056/NEJMoa1500858]).
Both OSLER and Dr. Toth’s broader safety analysis were funded by Amgen. Dr. Sabatine and Dr. Toth reported having received research grant support from and serving as consultants to Amgen and numerous other pharmaceutical companies. Dr. Hochman is a consultant to GlaxoSmithKline. Dr. Cannon has received research grant support from and served as a consultant or advisor to many pharmaceutical companies.
SAN DIEGO– The investigational PCSK9 inhibitor evolucumab dramatically reduced the 1-year cardiovascular event rate, by 53%, compared with standard statin-based lipid-lowering in the randomized OSLER study.
This reduction in adverse cardiovascular events was accompanied by a robust 61% reduction in LDL compared with standard therapy. The LDL level in evolocumab-treated patients dropped from 120 mg/dL to an average of 48 mg/dL, Dr. Marc S. Sabatine reported at the annual meeting of the American College of Cardiology.
The safety profile of patients on evolocumab plus standard, statin-based lipid lowering was essentially the same as in controls on standard therapy alone. Moreover, no gradient was seen for any adverse events on the basis of the extent of LDL lowering. For example, the types and incidence of adverse events and laboratory abnormalities in the 773 evolocumab-treated OSLER participants with an achieved LDL less than 25 mg/dL were closely similar to those seen with an LDL above 40 mg/dL, according to Dr. Sabatine, chairman of the Thrombolysis In Myocardial Infarction Study Group at Brigham and Women’s Hospital in Boston.
“These data, in conjuction with epidemiological and genetic data, offer further support for the potential for PCSK9 inhibition as a safe and effective means to reduce major adverse cardiovascular outcomes through particularly robust LDL cholesterol lowering,” he added.
OSLER (Open-Label Study of Long-Term Evaluation against LDL Cholesterol) included 4,465 patients who had completed one of a dozen earlier phase II or III studies of evolucumab, a monoclonal antibody that inhibits proprotein convertase subtilisin kexin type 9 (PCSK9), and were then randomized 2:1 to 1 year of open-label evolucumab administered subcutaneously at either 140 mg every 2 weeks or 420 mg once monthly plus standard lipid-lowering, or to standard lipid-lowering alone, generally with moderate- or high-dose statins.
The PCSK9 inhibitor reduced LDL cholesterol by 73 mg/dL compared to the control arm. In addition, the evolocumab group experienced a 26% decrease in Lp(a), a 47% drop in ApoB, a 13% reduction in triglycerides, and a 7% rise in HDL relative to standard care.
The 1-year composite cardiovascular event rate was just 0.95% in the evolucumab group, a 53% reduction relative to the 2.18% rate in the control arm. The composite outcome was comprised of death, MI, stroke or TIA, hospitalization for heart failure, coronary revascularization, or hospitalization for unstable angina. The reduction in events seen with evolocumab was consistent across all the components of the composite outcome.
Rates of most adverse events were closely similar in the two study arms. The exception was self-reported neurocognitive events such as forgetfulness or confusion, where the 0.9% incidence in the evolucumab group, while quite low in an absolute sense, was nonetheless threefold higher than in controls.
While the OSLER findings are highly encouraging in terms of both efficacy and safety, the study was too small and too brief at 1 year to be considered definitive, especially in terms of cardiovascular event rates, of which there only 60 in total. All eyes are now on the ongoing 27,500-patient, randomized, placebo-controlled FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) study. Participants in FOURIER must have a history of MI, stroke, or peripheral artery disease plus at least one additional high-risk feature. Results are expected in 2017.
Similar massive pivotal phase III studies of two other highly promising PCSK9 inhibitors – alirocumab and bococizumab – are ongoing. The PCSK9 inhibitors were the talk of ACC 2015 in light of the mounting impressive safety and efficacy data. The Food and Drug Administration is now considering whether to approve PCSK9 inhibition for patients with familial hypercholesterolemia whose LDL is not adequately controlled by statins, with a decision expected later this year.
Discussant Dr. Christopher P. Cannon called OSLER “a terrific study.”
The OSLER findings, coupled with a previously reported preliminary analysis suggesting reduced cardiovascular events in clinical trials of alirocumab, provide strong support for the notion that the lower the achieved LDL – whether accomplished by statins or other agents – the greater the cardiovascular benefit.
“This is so exciting. Lowering cholesterol is definitely a passion of mine,” said Dr. Cannon, professor of medicine at Harvard Medical School, Boston.
Discussant Dr. Judith S. Hochman was equally enthusiastic about the OSLER outcomes.
“This is really impressive and very encouraging for this class of cholesterol-lowering agents. And I want to congratulate you for enrolling 50% women. But we need more study about the potential for neurocognitive events,” commented Dr. Hochman, professor of cardiology and senior associate dean for clinical sciences at New York University.
Dr. Sabatine agreed. While neurocognitive events in OSLER were self-reported by patients, FOURIER includes a substudy featuring formal neurocognitive testing using validated instruments. And while that should provide definitive answers, he said he’s not too concerned about the neurocognitive issue.
“Biologically, LDL cholesterol is not used as a source of cholesterol for cells, and that’s especially true for cells in the CNS because of the blood-brain barrier. And very low LDL levels don’t appear to have any effect on the CNS in animal models,” he said.
A more comprehensive picture of evolucumab’s safety was provided elsewhere at ACC 15 by Dr. Peter P. Toth. His safety analysis incorporated more than 6,000 evolucumab-treated patients and 3,569 controls who participated in a dozen phase II and III trials as well as OSLER.
The short version: This pooled analysis demonstrated no increase in any clinical adverse events or laboratory abnormalities with evolocumab, compared with controls. That includes muscle-related adverse events, liver or renal toxicity, impairment in glucose metabolism, infections, injection-site reactions, and neurocognitive events, said Dr. Toth of the University of Illinois, Peoria.
Simultaneous with Dr. Sabatine’s presentation at ACC 15, the OSLER results were published online (N. Engl. J. Med. 2015 [doi: 10.1056/NEJMoa1500858]).
Both OSLER and Dr. Toth’s broader safety analysis were funded by Amgen. Dr. Sabatine and Dr. Toth reported having received research grant support from and serving as consultants to Amgen and numerous other pharmaceutical companies. Dr. Hochman is a consultant to GlaxoSmithKline. Dr. Cannon has received research grant support from and served as a consultant or advisor to many pharmaceutical companies.
AT ACC 15
Key clinical point: Evolocumab shows impressive LDL lowering and slashes cardiovascular events.
Major finding: The PCSK9 inhibitor lowered LDL by an average of 73 mg/dL more than standard lipid-lowering therapy alone while reducing the 1-year rate of composite cardiovascular events by 53%.
Data source: The OSLER study included 4,465 patients who were randomized to 1 year of open-label evolocumab plus standard statin-based lipid-lowering or to standard lipid-lowering alone.
Disclosures: OSLER was funded by Amgen. The presenter reported receiving research grant support from and serving as a consultant to Amgen and numerous other pharmaceutical companies.
MI survivors face higher cancer risk
SAN DIEGO– The risk of developing cancer is significantly higher in survivors of an acute MI compared to the general population, according to a large Danish national registry study.
“Greater focus on long-term cancer risk is warranted in MI survivors. This could potentially have implications on future patient care for MI patients, outpatient follow-up strategies, and distribution of health care resources,” Morten Winther Malmborg said at the annual meeting of the American College of Cardiology.
He presented a nationwide cohort study including 3,005,734 Danish adults with no baseline history of MI or cancer who were followed for up to 17 years in the comprehensive Danish National Patient Registry. During the study period, 125,926 of these individuals had a nonfatal MI.
The subsequent incidence of cancer in the MI survivors was 167 cases per 10,000 person-years compared with 95 per 10,000 person-years in the control group, reported Mr. Malmborg, a fourth-year medical student at the University of Copenhagen.
Cancer diagnoses of all types were highest by far in the first 6 months post-MI, which he attributed to surveillance bias, since that was a period of increased medical contact. However, after he and his coinvestigators excluded the cancers diagnosed during that initial 6-month period, the post-MI group still had a highly significant 11% increased relative risk for cancer overall during the period from 6 months through 17 years post-MI.
The younger a patient was when the MI occurred, the greater the subsequent cancer risk. Individuals who had a nonfatal MI at age 30-54 had a 44% greater risk of cancer overall at 6 months–17 years post-MI compared, with the control group. Those who had an MI at age 55-69 had a 19% increased cancer risk compared to controls, while those whose MI occurred at age 70-99 had a modest but still statistically significant 5% increase in cancer risk.
Particularly striking, according to Mr. Malmborg, was the MI survivors’ 44% increased relative risk for lung cancer and 31% increase in bladder cancer during the period from 6 months–17 years post-MI compared with the general population. In contrast, rates of breast, prostate, and colon cancer weren’t significantly different between MI survivors and the general population with no history of MI.
This observational study didn’t address the mechanisms involved in MI survivors’ increased cancer risk. Although the Danish registry didn’t include information of smoking status, Mr. Malmborg speculated that smoking may figure prominently, since it is a major shared risk factor for cardiovascular disease as well as lung and bladder cancer in particular. Other shared risk factors for cardiovascular disease and cancer include obesity, sedentary lifestyle, and excessive alcohol use.
This is the first large-scale study to look at cancer risk post-MI. It’s an increasingly relevant issue because the advances in cardiac care that have brought improved long-term survival following acute MI means more patients with a history of MI are likely to die from noncardiac causes, Mr. Malmborg observed.
He and his coinvestigators are now performing a number-needed-to-screen analysis to help them determine whether structured, formal creening for cancer following an MI should be done routinely.
The study was supported by Danish national medical research funds. The presenter reported having no financial conflicts.
SAN DIEGO– The risk of developing cancer is significantly higher in survivors of an acute MI compared to the general population, according to a large Danish national registry study.
“Greater focus on long-term cancer risk is warranted in MI survivors. This could potentially have implications on future patient care for MI patients, outpatient follow-up strategies, and distribution of health care resources,” Morten Winther Malmborg said at the annual meeting of the American College of Cardiology.
He presented a nationwide cohort study including 3,005,734 Danish adults with no baseline history of MI or cancer who were followed for up to 17 years in the comprehensive Danish National Patient Registry. During the study period, 125,926 of these individuals had a nonfatal MI.
The subsequent incidence of cancer in the MI survivors was 167 cases per 10,000 person-years compared with 95 per 10,000 person-years in the control group, reported Mr. Malmborg, a fourth-year medical student at the University of Copenhagen.
Cancer diagnoses of all types were highest by far in the first 6 months post-MI, which he attributed to surveillance bias, since that was a period of increased medical contact. However, after he and his coinvestigators excluded the cancers diagnosed during that initial 6-month period, the post-MI group still had a highly significant 11% increased relative risk for cancer overall during the period from 6 months through 17 years post-MI.
The younger a patient was when the MI occurred, the greater the subsequent cancer risk. Individuals who had a nonfatal MI at age 30-54 had a 44% greater risk of cancer overall at 6 months–17 years post-MI compared, with the control group. Those who had an MI at age 55-69 had a 19% increased cancer risk compared to controls, while those whose MI occurred at age 70-99 had a modest but still statistically significant 5% increase in cancer risk.
Particularly striking, according to Mr. Malmborg, was the MI survivors’ 44% increased relative risk for lung cancer and 31% increase in bladder cancer during the period from 6 months–17 years post-MI compared with the general population. In contrast, rates of breast, prostate, and colon cancer weren’t significantly different between MI survivors and the general population with no history of MI.
This observational study didn’t address the mechanisms involved in MI survivors’ increased cancer risk. Although the Danish registry didn’t include information of smoking status, Mr. Malmborg speculated that smoking may figure prominently, since it is a major shared risk factor for cardiovascular disease as well as lung and bladder cancer in particular. Other shared risk factors for cardiovascular disease and cancer include obesity, sedentary lifestyle, and excessive alcohol use.
This is the first large-scale study to look at cancer risk post-MI. It’s an increasingly relevant issue because the advances in cardiac care that have brought improved long-term survival following acute MI means more patients with a history of MI are likely to die from noncardiac causes, Mr. Malmborg observed.
He and his coinvestigators are now performing a number-needed-to-screen analysis to help them determine whether structured, formal creening for cancer following an MI should be done routinely.
The study was supported by Danish national medical research funds. The presenter reported having no financial conflicts.
SAN DIEGO– The risk of developing cancer is significantly higher in survivors of an acute MI compared to the general population, according to a large Danish national registry study.
“Greater focus on long-term cancer risk is warranted in MI survivors. This could potentially have implications on future patient care for MI patients, outpatient follow-up strategies, and distribution of health care resources,” Morten Winther Malmborg said at the annual meeting of the American College of Cardiology.
He presented a nationwide cohort study including 3,005,734 Danish adults with no baseline history of MI or cancer who were followed for up to 17 years in the comprehensive Danish National Patient Registry. During the study period, 125,926 of these individuals had a nonfatal MI.
The subsequent incidence of cancer in the MI survivors was 167 cases per 10,000 person-years compared with 95 per 10,000 person-years in the control group, reported Mr. Malmborg, a fourth-year medical student at the University of Copenhagen.
Cancer diagnoses of all types were highest by far in the first 6 months post-MI, which he attributed to surveillance bias, since that was a period of increased medical contact. However, after he and his coinvestigators excluded the cancers diagnosed during that initial 6-month period, the post-MI group still had a highly significant 11% increased relative risk for cancer overall during the period from 6 months through 17 years post-MI.
The younger a patient was when the MI occurred, the greater the subsequent cancer risk. Individuals who had a nonfatal MI at age 30-54 had a 44% greater risk of cancer overall at 6 months–17 years post-MI compared, with the control group. Those who had an MI at age 55-69 had a 19% increased cancer risk compared to controls, while those whose MI occurred at age 70-99 had a modest but still statistically significant 5% increase in cancer risk.
Particularly striking, according to Mr. Malmborg, was the MI survivors’ 44% increased relative risk for lung cancer and 31% increase in bladder cancer during the period from 6 months–17 years post-MI compared with the general population. In contrast, rates of breast, prostate, and colon cancer weren’t significantly different between MI survivors and the general population with no history of MI.
This observational study didn’t address the mechanisms involved in MI survivors’ increased cancer risk. Although the Danish registry didn’t include information of smoking status, Mr. Malmborg speculated that smoking may figure prominently, since it is a major shared risk factor for cardiovascular disease as well as lung and bladder cancer in particular. Other shared risk factors for cardiovascular disease and cancer include obesity, sedentary lifestyle, and excessive alcohol use.
This is the first large-scale study to look at cancer risk post-MI. It’s an increasingly relevant issue because the advances in cardiac care that have brought improved long-term survival following acute MI means more patients with a history of MI are likely to die from noncardiac causes, Mr. Malmborg observed.
He and his coinvestigators are now performing a number-needed-to-screen analysis to help them determine whether structured, formal creening for cancer following an MI should be done routinely.
The study was supported by Danish national medical research funds. The presenter reported having no financial conflicts.
AT ACC 15
Key clinical point: Closer monitoring for development of cancer in MI survivors may be warranted.
Major finding: The incidence of cancer overall was 167 cases per 10,000 person-years in acute MI survivors, compared with 95 per 10,000 person-years in the general population without a history of MI or prior cancer.
Data source: An observational study of more than 3 million adults enrolled in the Danish National Patient Registry, roughly 126,000 of whom were diagnosed with a first nonfatal MI during the study period.
Disclosures: The study was supported by Danish national medical research funds. The presenter reported having no financial conflicts.
Post-TAVR brain health: An emerging concern
SNOWMASS, COLO. – New-onset brain lesions arising after transcatheter aortic valve replacement are the largely unacknowledged elephant in the room with regard to the boomingly popular procedure.
Multiple studies utilizing diffusion-weighted MRI have shown roughly a 70% incidence of new brain lesions following transcatheter aortic valve replacement (TAVR). And studies employing full neurocognitive test batteries have consistently shown a relationship between small brain infarcts much like these, cognitive decline, and dementia, Dr. David R. Holmes Jr. said at the Annual Cardiovascular Conference at Snowmass.
“This is an incredibly alarming piece of information. People should be aware of this. There is interest in doing TAVR in younger and younger patients. But there is indeed an issue with unintended consequences. If we take younger and less and less symptomatic patients, their chance of dementia in 20 years is probably going to be increased. We’re going to have to follow these patients for a long period of time to look at that specific endpoint,” noted Dr. Holmes of the Mayo Clinic in Rochester, Minn.
Speaking of unintended consequences, there is also the issue of TAVR-related stroke. Among the more than 27,000 patient records submitted to the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry through December 2014, the periprocedural stroke rate was 2.4%. One-year outcomes included 26.2% mortality and a 3.6% stroke rate.
Given that two-thirds of TAVR cases submitted to the TVT registry in 2014 involved patients age 80 or older, with New York Heart Association class III/IV symptoms present in 82%, and 50% of patients rated as being at extreme risk with a predicted 1-year mortality of 50% without intervention, a 3.6% stroke rate can be considered tolerable. But not so in the sort of younger asymptomatic patients with significant aortic stenosis increasingly under discussion as potential candidates for the procedure.
“Stroke rates are the real deal in patients undergoing TAVR. Maybe you’re going to take an asymptomatic person and give them a stroke rather than wait or give a surgical valve replacement,” the cardiologist said.
He predicted that within 10 years, the use of cerebral protection devices will be considered mandatory, not just during TAVR, but during percutaneous coronary intervention, CABG surgery, and probably during atrial fibrillation ablation as well. All of these procedures have been linked to new-onset brain lesions on diffusion-weighted MRI.
Promising new neuroprotection devices include Keystone Heart’s TriGuard, a filter-deflector that covers all three cerebral arteries, has no impact on cerebral blood flow, doesn’t require an additional access site, is supported by excellent safety data, and is approved in Europe but investigational in the United States, Dr. Holmes observed. Efficacy data are coming soon, when the results of the DETECT III (A Prospective Randomized Evaluation of The TriGuard HDH Embolic Deflection Device During Transcatheter Aortic Valve Replacement) will be presented at the ACC scientific sessions on March 15. In that study, 70 patients underwent neurocognitive testing before and 30 days after their TAVR procedure.
“We’re going to be using something – a filter or filter-deflector – in every single patient to prevent the abnormal brain hits that are seen with all of these procedures. The need for brain protection is not going away,” he forecast.
Dr. Holmes, who played a pivotal role in creating the TVT registry during his term as ACC president, pointed out an intriguing registry finding: Through 2014, only 36% of the procedures have been done percutaneously.
“When you go to meetings everybody says, ‘We do them all percutaneously with a dual Perclose.’ But when you look at the data, out of those 27,000 patients only about one-third were done percutaneously. Is that going to be different in the future? Probably. But it’s important to remember that we’re still not doing all that many percutaneous procedures,” the cardiologist said.
He reported serving as a consultant to Boston Scientific.
SNOWMASS, COLO. – New-onset brain lesions arising after transcatheter aortic valve replacement are the largely unacknowledged elephant in the room with regard to the boomingly popular procedure.
Multiple studies utilizing diffusion-weighted MRI have shown roughly a 70% incidence of new brain lesions following transcatheter aortic valve replacement (TAVR). And studies employing full neurocognitive test batteries have consistently shown a relationship between small brain infarcts much like these, cognitive decline, and dementia, Dr. David R. Holmes Jr. said at the Annual Cardiovascular Conference at Snowmass.
“This is an incredibly alarming piece of information. People should be aware of this. There is interest in doing TAVR in younger and younger patients. But there is indeed an issue with unintended consequences. If we take younger and less and less symptomatic patients, their chance of dementia in 20 years is probably going to be increased. We’re going to have to follow these patients for a long period of time to look at that specific endpoint,” noted Dr. Holmes of the Mayo Clinic in Rochester, Minn.
Speaking of unintended consequences, there is also the issue of TAVR-related stroke. Among the more than 27,000 patient records submitted to the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry through December 2014, the periprocedural stroke rate was 2.4%. One-year outcomes included 26.2% mortality and a 3.6% stroke rate.
Given that two-thirds of TAVR cases submitted to the TVT registry in 2014 involved patients age 80 or older, with New York Heart Association class III/IV symptoms present in 82%, and 50% of patients rated as being at extreme risk with a predicted 1-year mortality of 50% without intervention, a 3.6% stroke rate can be considered tolerable. But not so in the sort of younger asymptomatic patients with significant aortic stenosis increasingly under discussion as potential candidates for the procedure.
“Stroke rates are the real deal in patients undergoing TAVR. Maybe you’re going to take an asymptomatic person and give them a stroke rather than wait or give a surgical valve replacement,” the cardiologist said.
He predicted that within 10 years, the use of cerebral protection devices will be considered mandatory, not just during TAVR, but during percutaneous coronary intervention, CABG surgery, and probably during atrial fibrillation ablation as well. All of these procedures have been linked to new-onset brain lesions on diffusion-weighted MRI.
Promising new neuroprotection devices include Keystone Heart’s TriGuard, a filter-deflector that covers all three cerebral arteries, has no impact on cerebral blood flow, doesn’t require an additional access site, is supported by excellent safety data, and is approved in Europe but investigational in the United States, Dr. Holmes observed. Efficacy data are coming soon, when the results of the DETECT III (A Prospective Randomized Evaluation of The TriGuard HDH Embolic Deflection Device During Transcatheter Aortic Valve Replacement) will be presented at the ACC scientific sessions on March 15. In that study, 70 patients underwent neurocognitive testing before and 30 days after their TAVR procedure.
“We’re going to be using something – a filter or filter-deflector – in every single patient to prevent the abnormal brain hits that are seen with all of these procedures. The need for brain protection is not going away,” he forecast.
Dr. Holmes, who played a pivotal role in creating the TVT registry during his term as ACC president, pointed out an intriguing registry finding: Through 2014, only 36% of the procedures have been done percutaneously.
“When you go to meetings everybody says, ‘We do them all percutaneously with a dual Perclose.’ But when you look at the data, out of those 27,000 patients only about one-third were done percutaneously. Is that going to be different in the future? Probably. But it’s important to remember that we’re still not doing all that many percutaneous procedures,” the cardiologist said.
He reported serving as a consultant to Boston Scientific.
SNOWMASS, COLO. – New-onset brain lesions arising after transcatheter aortic valve replacement are the largely unacknowledged elephant in the room with regard to the boomingly popular procedure.
Multiple studies utilizing diffusion-weighted MRI have shown roughly a 70% incidence of new brain lesions following transcatheter aortic valve replacement (TAVR). And studies employing full neurocognitive test batteries have consistently shown a relationship between small brain infarcts much like these, cognitive decline, and dementia, Dr. David R. Holmes Jr. said at the Annual Cardiovascular Conference at Snowmass.
“This is an incredibly alarming piece of information. People should be aware of this. There is interest in doing TAVR in younger and younger patients. But there is indeed an issue with unintended consequences. If we take younger and less and less symptomatic patients, their chance of dementia in 20 years is probably going to be increased. We’re going to have to follow these patients for a long period of time to look at that specific endpoint,” noted Dr. Holmes of the Mayo Clinic in Rochester, Minn.
Speaking of unintended consequences, there is also the issue of TAVR-related stroke. Among the more than 27,000 patient records submitted to the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry through December 2014, the periprocedural stroke rate was 2.4%. One-year outcomes included 26.2% mortality and a 3.6% stroke rate.
Given that two-thirds of TAVR cases submitted to the TVT registry in 2014 involved patients age 80 or older, with New York Heart Association class III/IV symptoms present in 82%, and 50% of patients rated as being at extreme risk with a predicted 1-year mortality of 50% without intervention, a 3.6% stroke rate can be considered tolerable. But not so in the sort of younger asymptomatic patients with significant aortic stenosis increasingly under discussion as potential candidates for the procedure.
“Stroke rates are the real deal in patients undergoing TAVR. Maybe you’re going to take an asymptomatic person and give them a stroke rather than wait or give a surgical valve replacement,” the cardiologist said.
He predicted that within 10 years, the use of cerebral protection devices will be considered mandatory, not just during TAVR, but during percutaneous coronary intervention, CABG surgery, and probably during atrial fibrillation ablation as well. All of these procedures have been linked to new-onset brain lesions on diffusion-weighted MRI.
Promising new neuroprotection devices include Keystone Heart’s TriGuard, a filter-deflector that covers all three cerebral arteries, has no impact on cerebral blood flow, doesn’t require an additional access site, is supported by excellent safety data, and is approved in Europe but investigational in the United States, Dr. Holmes observed. Efficacy data are coming soon, when the results of the DETECT III (A Prospective Randomized Evaluation of The TriGuard HDH Embolic Deflection Device During Transcatheter Aortic Valve Replacement) will be presented at the ACC scientific sessions on March 15. In that study, 70 patients underwent neurocognitive testing before and 30 days after their TAVR procedure.
“We’re going to be using something – a filter or filter-deflector – in every single patient to prevent the abnormal brain hits that are seen with all of these procedures. The need for brain protection is not going away,” he forecast.
Dr. Holmes, who played a pivotal role in creating the TVT registry during his term as ACC president, pointed out an intriguing registry finding: Through 2014, only 36% of the procedures have been done percutaneously.
“When you go to meetings everybody says, ‘We do them all percutaneously with a dual Perclose.’ But when you look at the data, out of those 27,000 patients only about one-third were done percutaneously. Is that going to be different in the future? Probably. But it’s important to remember that we’re still not doing all that many percutaneous procedures,” the cardiologist said.
He reported serving as a consultant to Boston Scientific.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Diet’s link to RA risk centers on alcohol, fish, sugar consumption
SNOWMASS, COLO. – Moderate alcohol intake and fish consumption are the two dietary factors supported by the strongest epidemiologic evidence for a protective effect against development of rheumatoid arthritis, while sugar-sweetened sodas are associated with increased risk of the disease.
“Sugar-sweetened soft drinks are now the primary source of sugar in the American diet. This consumption has increased astronomically over the past 30 years,” Dr. Karen H. Costenbader observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Obesity, one consequence of America’s love affair with sugar, is a moderately potent independent risk factor for RA, according to several studies, including one carried out by Dr. Costenbader and her colleagues at Brigham and Women’s Hospital and Harvard University, Boston.
They followed nearly 220,000 women enrolled in the Nurses Health Study (NHS) and NHS II prospective cohort studies. During 4.7 million person-years of follow-up, women who were diagnosed with RA before age 55 were 45% more likely to be overweight and 65% more likely to be obese than were women without RA at that age. The association was stronger for seropositive than seronegative RA. Moreover, in an analysis that considered cumulative years of obesity as something akin to pack-years of smoking, the investigators found strong evidence of a dose effect, such that 10 cumulative years of being obese was associated with a 37% increased risk of RA by age 55 (Ann. Rheum. Dis. 2014;73:1914-22).
These findings were consistent with the results of a retrospective, population-based study of Olmsted County, Minn., residents conducted by investigators at the Mayo Clinic. They found that a history of obesity was associated with a 24% increased likelihood of developing RA. The investigators calculated that obesity could account for 52% of the sharp increase in the incidence of RA seen in the county, as elsewhere, between 1985 and 2007 (Arthritis Care Res. 2013;65:71-7).
The mechanism by which obesity may boost the development of RA is unknown. Speculation has focused on inflammation as the likely mediator. Visceral fat secretes proinflammatory cytokines, with resultant elevations in C-reactive protein, TNF-alpha, and other biomarkers of systemic inflammation, Dr. Costenbader said.
The importance of eating fish as a protector against developing RA is also supported by multiple epidemiologic studies. Investigators at the Karolinska Institute in Stockholm demonstrated in a study of 1,889 Swedish RA patients and 2,145 controls that eating oily fish on as few as 1-3 occasions per month was associated with a 20% reduction in the odds of developing RA (Epidemiology 2009;20:896-901).
The Swedish findings were confirmed in a recent, not-yet-published analysis of women enrolled in the Nurses’ Health Study and NHS II. Dr. Costenbader and coworkers showed that eating fish at least once per month was associated with a 28% reduction in RA risk, compared with never-eaters of fish.
The evidence for a preventive effect of eating fish and a contributory role of obesity in the development of RA is sufficiently strong that these two behavioral factors have been incorporated in the Personalized Risk Estimator for Rheumatoid Arthritis (PRE-RA) Family Study. This is an ongoing National Institutes of Health–funded, prospective, randomized, controlled trial being conducted by Dr. Costenbader and others at Brigham and Women’s Hospital.
The PRE-RA Family Study is designed to compare the willingness to change RA-associated behaviors among first-degree relatives of RA patients after exposure to an online personalized risk-estimation tool and education program. The four behavioral factors included in the risk estimator – and targeted for change in the online risk education program – are cigarette smoking, excess body weight, low fish intake, and poor oral health.
Dr. Costenbader said “the sweet spot” for alcohol consumption in terms of reducing RA risk was 5.0-9.9 g/day – equivalent to one-half to one drink – in an analysis of incident RA cases she and her coinvestigators performed in the NHS and NHS II cohorts (Arthritis Rheumatol. 2014;66:1998-2005).
In the sugar-sweetened soda study, she and her coworkers found that nurses who drank more than one serving per day had a 63% greater risk of developing seropositive RA compared with those who consumed less than one per month. Restricting the analysis to women with onset of RA after age 55, the association grew markedly stronger, with one or more soft drinks per day linked to a 2.64-fold increased risk (Am. J. Clin. Nutr. 2014;100:959-67).
Lots of other dietary factors, including coffee, tea, red meat, protein, vitamins and antioxidant-rich foods, and the Mediterranean diet pattern, have been looked at by various investigators. None have panned out.
Dr. Costenbader’s research is supported by the NIH and the Arthritis Foundation. She reported having no financial conflicts.
SNOWMASS, COLO. – Moderate alcohol intake and fish consumption are the two dietary factors supported by the strongest epidemiologic evidence for a protective effect against development of rheumatoid arthritis, while sugar-sweetened sodas are associated with increased risk of the disease.
“Sugar-sweetened soft drinks are now the primary source of sugar in the American diet. This consumption has increased astronomically over the past 30 years,” Dr. Karen H. Costenbader observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Obesity, one consequence of America’s love affair with sugar, is a moderately potent independent risk factor for RA, according to several studies, including one carried out by Dr. Costenbader and her colleagues at Brigham and Women’s Hospital and Harvard University, Boston.
They followed nearly 220,000 women enrolled in the Nurses Health Study (NHS) and NHS II prospective cohort studies. During 4.7 million person-years of follow-up, women who were diagnosed with RA before age 55 were 45% more likely to be overweight and 65% more likely to be obese than were women without RA at that age. The association was stronger for seropositive than seronegative RA. Moreover, in an analysis that considered cumulative years of obesity as something akin to pack-years of smoking, the investigators found strong evidence of a dose effect, such that 10 cumulative years of being obese was associated with a 37% increased risk of RA by age 55 (Ann. Rheum. Dis. 2014;73:1914-22).
These findings were consistent with the results of a retrospective, population-based study of Olmsted County, Minn., residents conducted by investigators at the Mayo Clinic. They found that a history of obesity was associated with a 24% increased likelihood of developing RA. The investigators calculated that obesity could account for 52% of the sharp increase in the incidence of RA seen in the county, as elsewhere, between 1985 and 2007 (Arthritis Care Res. 2013;65:71-7).
The mechanism by which obesity may boost the development of RA is unknown. Speculation has focused on inflammation as the likely mediator. Visceral fat secretes proinflammatory cytokines, with resultant elevations in C-reactive protein, TNF-alpha, and other biomarkers of systemic inflammation, Dr. Costenbader said.
The importance of eating fish as a protector against developing RA is also supported by multiple epidemiologic studies. Investigators at the Karolinska Institute in Stockholm demonstrated in a study of 1,889 Swedish RA patients and 2,145 controls that eating oily fish on as few as 1-3 occasions per month was associated with a 20% reduction in the odds of developing RA (Epidemiology 2009;20:896-901).
The Swedish findings were confirmed in a recent, not-yet-published analysis of women enrolled in the Nurses’ Health Study and NHS II. Dr. Costenbader and coworkers showed that eating fish at least once per month was associated with a 28% reduction in RA risk, compared with never-eaters of fish.
The evidence for a preventive effect of eating fish and a contributory role of obesity in the development of RA is sufficiently strong that these two behavioral factors have been incorporated in the Personalized Risk Estimator for Rheumatoid Arthritis (PRE-RA) Family Study. This is an ongoing National Institutes of Health–funded, prospective, randomized, controlled trial being conducted by Dr. Costenbader and others at Brigham and Women’s Hospital.
The PRE-RA Family Study is designed to compare the willingness to change RA-associated behaviors among first-degree relatives of RA patients after exposure to an online personalized risk-estimation tool and education program. The four behavioral factors included in the risk estimator – and targeted for change in the online risk education program – are cigarette smoking, excess body weight, low fish intake, and poor oral health.
Dr. Costenbader said “the sweet spot” for alcohol consumption in terms of reducing RA risk was 5.0-9.9 g/day – equivalent to one-half to one drink – in an analysis of incident RA cases she and her coinvestigators performed in the NHS and NHS II cohorts (Arthritis Rheumatol. 2014;66:1998-2005).
In the sugar-sweetened soda study, she and her coworkers found that nurses who drank more than one serving per day had a 63% greater risk of developing seropositive RA compared with those who consumed less than one per month. Restricting the analysis to women with onset of RA after age 55, the association grew markedly stronger, with one or more soft drinks per day linked to a 2.64-fold increased risk (Am. J. Clin. Nutr. 2014;100:959-67).
Lots of other dietary factors, including coffee, tea, red meat, protein, vitamins and antioxidant-rich foods, and the Mediterranean diet pattern, have been looked at by various investigators. None have panned out.
Dr. Costenbader’s research is supported by the NIH and the Arthritis Foundation. She reported having no financial conflicts.
SNOWMASS, COLO. – Moderate alcohol intake and fish consumption are the two dietary factors supported by the strongest epidemiologic evidence for a protective effect against development of rheumatoid arthritis, while sugar-sweetened sodas are associated with increased risk of the disease.
“Sugar-sweetened soft drinks are now the primary source of sugar in the American diet. This consumption has increased astronomically over the past 30 years,” Dr. Karen H. Costenbader observed at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Obesity, one consequence of America’s love affair with sugar, is a moderately potent independent risk factor for RA, according to several studies, including one carried out by Dr. Costenbader and her colleagues at Brigham and Women’s Hospital and Harvard University, Boston.
They followed nearly 220,000 women enrolled in the Nurses Health Study (NHS) and NHS II prospective cohort studies. During 4.7 million person-years of follow-up, women who were diagnosed with RA before age 55 were 45% more likely to be overweight and 65% more likely to be obese than were women without RA at that age. The association was stronger for seropositive than seronegative RA. Moreover, in an analysis that considered cumulative years of obesity as something akin to pack-years of smoking, the investigators found strong evidence of a dose effect, such that 10 cumulative years of being obese was associated with a 37% increased risk of RA by age 55 (Ann. Rheum. Dis. 2014;73:1914-22).
These findings were consistent with the results of a retrospective, population-based study of Olmsted County, Minn., residents conducted by investigators at the Mayo Clinic. They found that a history of obesity was associated with a 24% increased likelihood of developing RA. The investigators calculated that obesity could account for 52% of the sharp increase in the incidence of RA seen in the county, as elsewhere, between 1985 and 2007 (Arthritis Care Res. 2013;65:71-7).
The mechanism by which obesity may boost the development of RA is unknown. Speculation has focused on inflammation as the likely mediator. Visceral fat secretes proinflammatory cytokines, with resultant elevations in C-reactive protein, TNF-alpha, and other biomarkers of systemic inflammation, Dr. Costenbader said.
The importance of eating fish as a protector against developing RA is also supported by multiple epidemiologic studies. Investigators at the Karolinska Institute in Stockholm demonstrated in a study of 1,889 Swedish RA patients and 2,145 controls that eating oily fish on as few as 1-3 occasions per month was associated with a 20% reduction in the odds of developing RA (Epidemiology 2009;20:896-901).
The Swedish findings were confirmed in a recent, not-yet-published analysis of women enrolled in the Nurses’ Health Study and NHS II. Dr. Costenbader and coworkers showed that eating fish at least once per month was associated with a 28% reduction in RA risk, compared with never-eaters of fish.
The evidence for a preventive effect of eating fish and a contributory role of obesity in the development of RA is sufficiently strong that these two behavioral factors have been incorporated in the Personalized Risk Estimator for Rheumatoid Arthritis (PRE-RA) Family Study. This is an ongoing National Institutes of Health–funded, prospective, randomized, controlled trial being conducted by Dr. Costenbader and others at Brigham and Women’s Hospital.
The PRE-RA Family Study is designed to compare the willingness to change RA-associated behaviors among first-degree relatives of RA patients after exposure to an online personalized risk-estimation tool and education program. The four behavioral factors included in the risk estimator – and targeted for change in the online risk education program – are cigarette smoking, excess body weight, low fish intake, and poor oral health.
Dr. Costenbader said “the sweet spot” for alcohol consumption in terms of reducing RA risk was 5.0-9.9 g/day – equivalent to one-half to one drink – in an analysis of incident RA cases she and her coinvestigators performed in the NHS and NHS II cohorts (Arthritis Rheumatol. 2014;66:1998-2005).
In the sugar-sweetened soda study, she and her coworkers found that nurses who drank more than one serving per day had a 63% greater risk of developing seropositive RA compared with those who consumed less than one per month. Restricting the analysis to women with onset of RA after age 55, the association grew markedly stronger, with one or more soft drinks per day linked to a 2.64-fold increased risk (Am. J. Clin. Nutr. 2014;100:959-67).
Lots of other dietary factors, including coffee, tea, red meat, protein, vitamins and antioxidant-rich foods, and the Mediterranean diet pattern, have been looked at by various investigators. None have panned out.
Dr. Costenbader’s research is supported by the NIH and the Arthritis Foundation. She reported having no financial conflicts.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Latest Valvular Disease Guidelines Bring Big Changes
SNOWMASS, COLO. – The 2014 American Heart Association/American College of Cardiology guidelines for the management of valvular heart disease break new ground in numerous ways, Dr. Rick A. Nishimura said at the Annual Cardiovascular Conference at Snowmass.
“We needed to do things differently. These guidelines were created in a different format from prior valvular heart disease guidelines. We wanted these guidelines to promote access to concise, relevant bytes of information at the point of care,” explained Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn., and cochair of the guidelines writing committee.
These guidelines – the first major revision in 8 years – introduce a new taxonomy and the first staging system for valvular heart disease. The guidelines also lower the threshold for intervention in asymptomatic patients, recommending surgical or catheter-based treatment at an earlier point in the disease process than ever before. And the guidelines introduce the concept of heart valve centers of excellence, offering a strong recommendation that patients be referred to those centers for procedures to be performed in the asymptomatic phase of disease (J. Am. Coll. Cardiol. 2014;63:2438-88).
These valvular heart disease guidelines place greater emphasis than before on the quality of the scientific evidence underlying recommendations. Since valvular heart disease is a field with a paucity of randomized trials, that meant cutting back.
“Our goal was, if there’s little evidence, don’t write a recommendation. So the number of recommendations went down, but at least the ones that were made were based on evidence,” the cardiologist noted.
Indeed, in the 2006 guidelines, more than 70% of the recommendations were Level of Evidence C and based solely upon expert opinion; in the new guidelines, that’s true for less than 50%. And the proportion of recommendations that are Level of Evidence B increased from 30% to 45%.
The 2014 update was prompted by huge changes in the field of valvular heart disease since 2006. For example, better data became available on the natural history of valvular heart disease. The old concept was not to operate on the asymptomatic patient with severe aortic stenosis and normal left ventricular function, but more recent natural history studies have shown that, left untreated, 72% of such patients will die or develop symptoms within 5 years.
So there has been a push to intervene earlier. Fortunately, that became doable, as recent years also brought improved noninvasive imaging, new catheter-based interventions, and refined surgical methods, enabling operators to safely lower the threshold for intervention in asymptomatic patients while at the same time extending procedural therapies to older, sicker populations.
Dr. Nishimura predicted that cardiologists and surgeons will find the new staging system clinically useful. The four stages, A-D, define the categories “at risk,” “progressive,” “asymptomatic severe,” and “symptomatic severe,” respectively. These categories are particularly helpful in determining how often to schedule patient follow-up and when to time intervention.
The guidelines recommend observation for patients who are Stage A or B and intervention when reasonable in patients who are Stage C2 or D. What bumps a patient with hemodynamically severe yet asymptomatic mitral regurgitation from Stage C1 to C2 is an left ventricular ejection fraction below 60% or a left ventricular end systolic dimension of 40 mm or more. In the setting of asymptomatic aortic stenosis, it’s a peak aortic valve velocity of 4.0 m/sec on Doppler echocardiography plus an LVEF of less than 50%.
The latest guidelines introduced the concept of heart valve centers of excellence in response to evidence of large variability across the country in terms of experience with valve operations. For example, the majority of centers perform fewer than 40 mitral valve repairs per year, and surgeons who perform mitral operations do a median of just five per year. The guideline committee, which included general and interventional cardiologists, surgeons, anesthesiologists, and imaging experts, was persuaded that those numbers are not sufficient to achieve optimal results in complex valve operations for asymptomatic patients.
The criteria for qualifying as a heart valve center of excellence, as defined in the guidelines, include having a multidisciplinary heart valve team, high patient volume, high-level surgical experience and expertise in complex valve procedures, and active participation in multicenter data registries and continuous quality improvement processes.
“The most important thing is you have to be very transparent with your data,” according to the cardiologist.
Ultimately, the most far-reaching change introduced in the current valvular heart disease guidelines is the switch from textbook format to what Dr. Nishimura calls structured data knowledge management.
“The AHA/ACC clinical practice guidelines are generally recognized as the flagship of U.S. cardiovascular medicine, but they’re like a library of old books. Clinically valuable knowledge is buried within documents that can be 200 pages long. What we need at the point of care is the gist: concise, relevant bytes of information that answer a specific clinical question, synthesized by experts,” Dr. Nishimura said.
The new approach is designed to counter the information overload that plagues contemporary medical practice. Each recommendation in the current valvular heart disease guidelines addresses a specific clinical question via a brief summary statement followed by a short explanatory paragraph, with accompanying references for those who seek additional details. This new format is designed to lead AHA/ACC clinical practice guidelines into the electronic information management future.
“In the future, you’ll go to your iPad or iPhone or whatever, type in search terms such as ‘anticoagulation for mechanical valves during pregnancy,’ and it will take you straight to the relevant knowledge byte. You can then click on ‘more’ and find out more and get to the supporting evidence tables. The knowledge chunks will be stored in a centralized knowledge management system. The nice thing about this is that it will be a living document that can easily be updated, instead of having to wait 8 years for a new version,” Dr. Nishimura explained.
He reported having no financial conflicts of interest.
SNOWMASS, COLO. – The 2014 American Heart Association/American College of Cardiology guidelines for the management of valvular heart disease break new ground in numerous ways, Dr. Rick A. Nishimura said at the Annual Cardiovascular Conference at Snowmass.
“We needed to do things differently. These guidelines were created in a different format from prior valvular heart disease guidelines. We wanted these guidelines to promote access to concise, relevant bytes of information at the point of care,” explained Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn., and cochair of the guidelines writing committee.
These guidelines – the first major revision in 8 years – introduce a new taxonomy and the first staging system for valvular heart disease. The guidelines also lower the threshold for intervention in asymptomatic patients, recommending surgical or catheter-based treatment at an earlier point in the disease process than ever before. And the guidelines introduce the concept of heart valve centers of excellence, offering a strong recommendation that patients be referred to those centers for procedures to be performed in the asymptomatic phase of disease (J. Am. Coll. Cardiol. 2014;63:2438-88).
These valvular heart disease guidelines place greater emphasis than before on the quality of the scientific evidence underlying recommendations. Since valvular heart disease is a field with a paucity of randomized trials, that meant cutting back.
“Our goal was, if there’s little evidence, don’t write a recommendation. So the number of recommendations went down, but at least the ones that were made were based on evidence,” the cardiologist noted.
Indeed, in the 2006 guidelines, more than 70% of the recommendations were Level of Evidence C and based solely upon expert opinion; in the new guidelines, that’s true for less than 50%. And the proportion of recommendations that are Level of Evidence B increased from 30% to 45%.
The 2014 update was prompted by huge changes in the field of valvular heart disease since 2006. For example, better data became available on the natural history of valvular heart disease. The old concept was not to operate on the asymptomatic patient with severe aortic stenosis and normal left ventricular function, but more recent natural history studies have shown that, left untreated, 72% of such patients will die or develop symptoms within 5 years.
So there has been a push to intervene earlier. Fortunately, that became doable, as recent years also brought improved noninvasive imaging, new catheter-based interventions, and refined surgical methods, enabling operators to safely lower the threshold for intervention in asymptomatic patients while at the same time extending procedural therapies to older, sicker populations.
Dr. Nishimura predicted that cardiologists and surgeons will find the new staging system clinically useful. The four stages, A-D, define the categories “at risk,” “progressive,” “asymptomatic severe,” and “symptomatic severe,” respectively. These categories are particularly helpful in determining how often to schedule patient follow-up and when to time intervention.
The guidelines recommend observation for patients who are Stage A or B and intervention when reasonable in patients who are Stage C2 or D. What bumps a patient with hemodynamically severe yet asymptomatic mitral regurgitation from Stage C1 to C2 is an left ventricular ejection fraction below 60% or a left ventricular end systolic dimension of 40 mm or more. In the setting of asymptomatic aortic stenosis, it’s a peak aortic valve velocity of 4.0 m/sec on Doppler echocardiography plus an LVEF of less than 50%.
The latest guidelines introduced the concept of heart valve centers of excellence in response to evidence of large variability across the country in terms of experience with valve operations. For example, the majority of centers perform fewer than 40 mitral valve repairs per year, and surgeons who perform mitral operations do a median of just five per year. The guideline committee, which included general and interventional cardiologists, surgeons, anesthesiologists, and imaging experts, was persuaded that those numbers are not sufficient to achieve optimal results in complex valve operations for asymptomatic patients.
The criteria for qualifying as a heart valve center of excellence, as defined in the guidelines, include having a multidisciplinary heart valve team, high patient volume, high-level surgical experience and expertise in complex valve procedures, and active participation in multicenter data registries and continuous quality improvement processes.
“The most important thing is you have to be very transparent with your data,” according to the cardiologist.
Ultimately, the most far-reaching change introduced in the current valvular heart disease guidelines is the switch from textbook format to what Dr. Nishimura calls structured data knowledge management.
“The AHA/ACC clinical practice guidelines are generally recognized as the flagship of U.S. cardiovascular medicine, but they’re like a library of old books. Clinically valuable knowledge is buried within documents that can be 200 pages long. What we need at the point of care is the gist: concise, relevant bytes of information that answer a specific clinical question, synthesized by experts,” Dr. Nishimura said.
The new approach is designed to counter the information overload that plagues contemporary medical practice. Each recommendation in the current valvular heart disease guidelines addresses a specific clinical question via a brief summary statement followed by a short explanatory paragraph, with accompanying references for those who seek additional details. This new format is designed to lead AHA/ACC clinical practice guidelines into the electronic information management future.
“In the future, you’ll go to your iPad or iPhone or whatever, type in search terms such as ‘anticoagulation for mechanical valves during pregnancy,’ and it will take you straight to the relevant knowledge byte. You can then click on ‘more’ and find out more and get to the supporting evidence tables. The knowledge chunks will be stored in a centralized knowledge management system. The nice thing about this is that it will be a living document that can easily be updated, instead of having to wait 8 years for a new version,” Dr. Nishimura explained.
He reported having no financial conflicts of interest.
SNOWMASS, COLO. – The 2014 American Heart Association/American College of Cardiology guidelines for the management of valvular heart disease break new ground in numerous ways, Dr. Rick A. Nishimura said at the Annual Cardiovascular Conference at Snowmass.
“We needed to do things differently. These guidelines were created in a different format from prior valvular heart disease guidelines. We wanted these guidelines to promote access to concise, relevant bytes of information at the point of care,” explained Dr. Nishimura, professor of medicine at the Mayo Clinic in Rochester, Minn., and cochair of the guidelines writing committee.
These guidelines – the first major revision in 8 years – introduce a new taxonomy and the first staging system for valvular heart disease. The guidelines also lower the threshold for intervention in asymptomatic patients, recommending surgical or catheter-based treatment at an earlier point in the disease process than ever before. And the guidelines introduce the concept of heart valve centers of excellence, offering a strong recommendation that patients be referred to those centers for procedures to be performed in the asymptomatic phase of disease (J. Am. Coll. Cardiol. 2014;63:2438-88).
These valvular heart disease guidelines place greater emphasis than before on the quality of the scientific evidence underlying recommendations. Since valvular heart disease is a field with a paucity of randomized trials, that meant cutting back.
“Our goal was, if there’s little evidence, don’t write a recommendation. So the number of recommendations went down, but at least the ones that were made were based on evidence,” the cardiologist noted.
Indeed, in the 2006 guidelines, more than 70% of the recommendations were Level of Evidence C and based solely upon expert opinion; in the new guidelines, that’s true for less than 50%. And the proportion of recommendations that are Level of Evidence B increased from 30% to 45%.
The 2014 update was prompted by huge changes in the field of valvular heart disease since 2006. For example, better data became available on the natural history of valvular heart disease. The old concept was not to operate on the asymptomatic patient with severe aortic stenosis and normal left ventricular function, but more recent natural history studies have shown that, left untreated, 72% of such patients will die or develop symptoms within 5 years.
So there has been a push to intervene earlier. Fortunately, that became doable, as recent years also brought improved noninvasive imaging, new catheter-based interventions, and refined surgical methods, enabling operators to safely lower the threshold for intervention in asymptomatic patients while at the same time extending procedural therapies to older, sicker populations.
Dr. Nishimura predicted that cardiologists and surgeons will find the new staging system clinically useful. The four stages, A-D, define the categories “at risk,” “progressive,” “asymptomatic severe,” and “symptomatic severe,” respectively. These categories are particularly helpful in determining how often to schedule patient follow-up and when to time intervention.
The guidelines recommend observation for patients who are Stage A or B and intervention when reasonable in patients who are Stage C2 or D. What bumps a patient with hemodynamically severe yet asymptomatic mitral regurgitation from Stage C1 to C2 is an left ventricular ejection fraction below 60% or a left ventricular end systolic dimension of 40 mm or more. In the setting of asymptomatic aortic stenosis, it’s a peak aortic valve velocity of 4.0 m/sec on Doppler echocardiography plus an LVEF of less than 50%.
The latest guidelines introduced the concept of heart valve centers of excellence in response to evidence of large variability across the country in terms of experience with valve operations. For example, the majority of centers perform fewer than 40 mitral valve repairs per year, and surgeons who perform mitral operations do a median of just five per year. The guideline committee, which included general and interventional cardiologists, surgeons, anesthesiologists, and imaging experts, was persuaded that those numbers are not sufficient to achieve optimal results in complex valve operations for asymptomatic patients.
The criteria for qualifying as a heart valve center of excellence, as defined in the guidelines, include having a multidisciplinary heart valve team, high patient volume, high-level surgical experience and expertise in complex valve procedures, and active participation in multicenter data registries and continuous quality improvement processes.
“The most important thing is you have to be very transparent with your data,” according to the cardiologist.
Ultimately, the most far-reaching change introduced in the current valvular heart disease guidelines is the switch from textbook format to what Dr. Nishimura calls structured data knowledge management.
“The AHA/ACC clinical practice guidelines are generally recognized as the flagship of U.S. cardiovascular medicine, but they’re like a library of old books. Clinically valuable knowledge is buried within documents that can be 200 pages long. What we need at the point of care is the gist: concise, relevant bytes of information that answer a specific clinical question, synthesized by experts,” Dr. Nishimura said.
The new approach is designed to counter the information overload that plagues contemporary medical practice. Each recommendation in the current valvular heart disease guidelines addresses a specific clinical question via a brief summary statement followed by a short explanatory paragraph, with accompanying references for those who seek additional details. This new format is designed to lead AHA/ACC clinical practice guidelines into the electronic information management future.
“In the future, you’ll go to your iPad or iPhone or whatever, type in search terms such as ‘anticoagulation for mechanical valves during pregnancy,’ and it will take you straight to the relevant knowledge byte. You can then click on ‘more’ and find out more and get to the supporting evidence tables. The knowledge chunks will be stored in a centralized knowledge management system. The nice thing about this is that it will be a living document that can easily be updated, instead of having to wait 8 years for a new version,” Dr. Nishimura explained.
He reported having no financial conflicts of interest.
VIDEO: Penicillin Skin Testing Improves Inpatient Antibiotic Stewardship
HOUSTON– Dr. Megan S. Motosue of the Mayo Clinic in Rochester, Minn., discusses the value of penicillin skin testing as a potent tool for improving inpatient antibiotic stewardship. By routinely performing penicillin skin testing using standard methods in a consecutive series of inpatients with a self-reported history of penicillin allergy, roughly 80% were found to not in fact be allergic. And this information changed their physicians’ behavior: prior to testing, vancomycin was the most commonly used antibiotic in the inpatients, but its use was reduced by more than half based upon the skin test results, with a commensurate increase in prescriptions for semi-synthetic penicillins.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON– Dr. Megan S. Motosue of the Mayo Clinic in Rochester, Minn., discusses the value of penicillin skin testing as a potent tool for improving inpatient antibiotic stewardship. By routinely performing penicillin skin testing using standard methods in a consecutive series of inpatients with a self-reported history of penicillin allergy, roughly 80% were found to not in fact be allergic. And this information changed their physicians’ behavior: prior to testing, vancomycin was the most commonly used antibiotic in the inpatients, but its use was reduced by more than half based upon the skin test results, with a commensurate increase in prescriptions for semi-synthetic penicillins.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
HOUSTON– Dr. Megan S. Motosue of the Mayo Clinic in Rochester, Minn., discusses the value of penicillin skin testing as a potent tool for improving inpatient antibiotic stewardship. By routinely performing penicillin skin testing using standard methods in a consecutive series of inpatients with a self-reported history of penicillin allergy, roughly 80% were found to not in fact be allergic. And this information changed their physicians’ behavior: prior to testing, vancomycin was the most commonly used antibiotic in the inpatients, but its use was reduced by more than half based upon the skin test results, with a commensurate increase in prescriptions for semi-synthetic penicillins.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Nailing VT as the cause of wide complex tachycardia
SNOWMASS, COLO. – An ECG can offer a handful of easily interpreted clues that raise to 99% the certainty of diagnosing ventricular tachycardia as the cause of a patient’s wide complex tachycardia, according to Dr. Samuel J. Asirvatham.
The first thing to realize about wide complex tachycardias (WCTs), as defined by a heart rate in excess of 100 bpm and a QRS duration greater than 120 milliseconds, is that the cause is ventricular tachycardia (VT) rather than supraventricular tachycardia in 80% of cases, he said at the Annual Cardiovascular Conference at Snowmass.
“When you diagnose wide complex tachycardia as not VT, you’re much more likely to make a mistake than if you just closed your eyes and said VT,” said Dr. Asirvatham, professor of medicine and pediatrics at the Mayo Clinic, Rochester, Minn.
The history also provides a valuable clue: “If there’s anything wrong with the heart – a prior MI, structural disease, a scar on the heart from previous surgery, sarcoid – there’s a 95% chance that the WCT is due to VT,” he said.
Key ECG features will boost the diagnostic certainty of VT from 95% to 99%. Here are Dr. Asirvatham’s favorites:
• Atrioventricular dissociation. When the ventricular rate is faster than the atrial rate, the result is atrioventricular dissocation. In the setting of WCT, it’s highly specific for VT, and it’s present on the ECGs of up to 50% of affected patients.
“This is a very useful clue. I like looking for this. If you see it, you can be convinced the patient has VT,” he said.
• The wide QRS. The wider the wide QRS, the more likely it’s VT.If the QRS duration is greater than 150 milliseconds, there’s nearly a 98% likelihood of VT.
• Northwest axis. If both lead I and the inferior leads show negative deflection – that is, the electrical wave is moving away from the positive electrodes located over the left shoulder and closest to the feet – that means the wave is moving toward the northwest on the frontal plane axis. The odds are extremely high that this is VT.
• Chest lead concordance. If all of the chest leads V1-6 are positive or they’re all negative, that’s “powerful information” indicating VT, according to Dr. Asirvatham.
• Time from onset of R wave to S wave nadir. Even if chest concordance isn’t present, VT can be diagnosed with near-absolute certainty simply by measuring the time from onset of the R wave to the lowest point on the S wave. If it’s longer than 100 milliseconds, that finding strongly favors VT.
“Remember these ECG features and 99% of the time you will correctly diagnose VT,” the cardiologist said.
Dr. Asirvatham reported serving as a consultant to a dozen medical device companies.
SNOWMASS, COLO. – An ECG can offer a handful of easily interpreted clues that raise to 99% the certainty of diagnosing ventricular tachycardia as the cause of a patient’s wide complex tachycardia, according to Dr. Samuel J. Asirvatham.
The first thing to realize about wide complex tachycardias (WCTs), as defined by a heart rate in excess of 100 bpm and a QRS duration greater than 120 milliseconds, is that the cause is ventricular tachycardia (VT) rather than supraventricular tachycardia in 80% of cases, he said at the Annual Cardiovascular Conference at Snowmass.
“When you diagnose wide complex tachycardia as not VT, you’re much more likely to make a mistake than if you just closed your eyes and said VT,” said Dr. Asirvatham, professor of medicine and pediatrics at the Mayo Clinic, Rochester, Minn.
The history also provides a valuable clue: “If there’s anything wrong with the heart – a prior MI, structural disease, a scar on the heart from previous surgery, sarcoid – there’s a 95% chance that the WCT is due to VT,” he said.
Key ECG features will boost the diagnostic certainty of VT from 95% to 99%. Here are Dr. Asirvatham’s favorites:
• Atrioventricular dissociation. When the ventricular rate is faster than the atrial rate, the result is atrioventricular dissocation. In the setting of WCT, it’s highly specific for VT, and it’s present on the ECGs of up to 50% of affected patients.
“This is a very useful clue. I like looking for this. If you see it, you can be convinced the patient has VT,” he said.
• The wide QRS. The wider the wide QRS, the more likely it’s VT.If the QRS duration is greater than 150 milliseconds, there’s nearly a 98% likelihood of VT.
• Northwest axis. If both lead I and the inferior leads show negative deflection – that is, the electrical wave is moving away from the positive electrodes located over the left shoulder and closest to the feet – that means the wave is moving toward the northwest on the frontal plane axis. The odds are extremely high that this is VT.
• Chest lead concordance. If all of the chest leads V1-6 are positive or they’re all negative, that’s “powerful information” indicating VT, according to Dr. Asirvatham.
• Time from onset of R wave to S wave nadir. Even if chest concordance isn’t present, VT can be diagnosed with near-absolute certainty simply by measuring the time from onset of the R wave to the lowest point on the S wave. If it’s longer than 100 milliseconds, that finding strongly favors VT.
“Remember these ECG features and 99% of the time you will correctly diagnose VT,” the cardiologist said.
Dr. Asirvatham reported serving as a consultant to a dozen medical device companies.
SNOWMASS, COLO. – An ECG can offer a handful of easily interpreted clues that raise to 99% the certainty of diagnosing ventricular tachycardia as the cause of a patient’s wide complex tachycardia, according to Dr. Samuel J. Asirvatham.
The first thing to realize about wide complex tachycardias (WCTs), as defined by a heart rate in excess of 100 bpm and a QRS duration greater than 120 milliseconds, is that the cause is ventricular tachycardia (VT) rather than supraventricular tachycardia in 80% of cases, he said at the Annual Cardiovascular Conference at Snowmass.
“When you diagnose wide complex tachycardia as not VT, you’re much more likely to make a mistake than if you just closed your eyes and said VT,” said Dr. Asirvatham, professor of medicine and pediatrics at the Mayo Clinic, Rochester, Minn.
The history also provides a valuable clue: “If there’s anything wrong with the heart – a prior MI, structural disease, a scar on the heart from previous surgery, sarcoid – there’s a 95% chance that the WCT is due to VT,” he said.
Key ECG features will boost the diagnostic certainty of VT from 95% to 99%. Here are Dr. Asirvatham’s favorites:
• Atrioventricular dissociation. When the ventricular rate is faster than the atrial rate, the result is atrioventricular dissocation. In the setting of WCT, it’s highly specific for VT, and it’s present on the ECGs of up to 50% of affected patients.
“This is a very useful clue. I like looking for this. If you see it, you can be convinced the patient has VT,” he said.
• The wide QRS. The wider the wide QRS, the more likely it’s VT.If the QRS duration is greater than 150 milliseconds, there’s nearly a 98% likelihood of VT.
• Northwest axis. If both lead I and the inferior leads show negative deflection – that is, the electrical wave is moving away from the positive electrodes located over the left shoulder and closest to the feet – that means the wave is moving toward the northwest on the frontal plane axis. The odds are extremely high that this is VT.
• Chest lead concordance. If all of the chest leads V1-6 are positive or they’re all negative, that’s “powerful information” indicating VT, according to Dr. Asirvatham.
• Time from onset of R wave to S wave nadir. Even if chest concordance isn’t present, VT can be diagnosed with near-absolute certainty simply by measuring the time from onset of the R wave to the lowest point on the S wave. If it’s longer than 100 milliseconds, that finding strongly favors VT.
“Remember these ECG features and 99% of the time you will correctly diagnose VT,” the cardiologist said.
Dr. Asirvatham reported serving as a consultant to a dozen medical device companies.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS