User login
ITL: Physician Reviews of HM-Relevant Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prediction tool for neurological outcomes after in-hospital cardiac arrest
- Radiation exposure in integrated healthcare systems, 1996-2010
- Postoperative troponin predicts 30-day mortality
- Clinical prediction model of mortality in acute heart failure
- Indwelling pleural catheter vs. talc pleurodesis via chest tube
- Early surgery for high-risk, native-valve endocarditis patients
- Risk factors after ED visit for syncope
- Acute hyperglycemia in CAP patients
- Hospital delirium associated with cognitive decline, institutionalization, and death
- Seven-day ciprofloxacin effective against acute pyelonephritis
- Advance directives in community patients with heart failure
- Chlorhexidine bathing effective against CVC-associated bloodstream infections
- Simulation training improves lumbar puncture skills
- PCP referrals to hospitals and publicly reported data
- Medication reconciliation best practices
Prediction Tool Validated for Prognosticating Favorable Neurological Outcome after In-Hospital Cardiac Arrest
Clinical question: Does the Cardiac Arrest Survival Post Resuscitation In-Hospital (CASPRI) score accurately predict favorable neurological outcomes?
Background: Previous cardiac arrest prediction models have been focused on survival to discharge without consideration of neurological status and have not been translated into valid bedside prognostication tools. Neurologic prognosis can assist patients, families, and physicians in decisions about continued goals of care post-arrest.
Study design: Retrospective cohort study.
Setting: Acute-care hospitals.
Synopsis: Using the Get with the Guidelines Resuscitation Registry, 551 hospitals identified 42,957 patients who were successfully resuscitated from an in-hospital cardiac arrest from January 2000 to October 2009. Researchers developed a simple prediction tool for favorable neurological outcomes (defined as “no” or “moderate” neurological disability) at discharge. The 11 predictors used to calculate the CASPRI score are age; time to defibrillation; pre-arrest neurological status; hospital location; duration of resuscitation; and pre-arrest comorbidities: mechanical ventilation, renal insufficiency, hepatic insufficiency, sepsis, malignancy,
and hypotension.
Rates of favorable neurological outcome were similar between derivation cohort (24.6%) and validation cohort (24.5%). The model had excellent discrimination with a C score of 0.80. Probability of favorable neurological survival ranged from 70.7% in the top decile of patients (CASPRI <10) and 2.8% in bottom decile (CASPRI ≥ 28).
This tool is not generalizable to patients with out-of-hospital arrest or undergoing therapeutic hypothermia.
Bottom line: CASPRI is a simple bedside tool validated to estimate probability of favorable neurological outcome after in-hospital cardiac arrest.
Citation: Chan PS, Spertus JA, Krumholz HA, et al. A validated prediction tool for initial survivors in in-hospital cardiac arrest. Arch Intern Med. 2012;172(12):947-953.
Increased Use of Radiologic Imaging and Associated Radiation Exposure in Integrated Healthcare Systems, 1996-2010
Clinical question: How much has imaging utilization and associated radiation exposure increased over 15 years in integrated healthcare systems independent of financial incentives in a fee-for-service system?
Background: Use of diagnostic imaging has increased significantly within fee-for-service healthcare models. The associated radiation exposure has increased the risk of radiation-induced malignancies. Little is known about the pattern of imaging use in integrated healthcare systems without the financial incentives seen in other models of care.
Study design: Retrospective cohort study.
Setting: Six integrated healthcare systems in the U.S.
Synopsis: The number of diagnostic imaging studies performed and estimated radiation exposure were determined from analysis of electronic medical records from member patients enrolled in health systems in the HMO Research Network from 1996 to 2010. Annual increases in use of advanced diagnostics were noted in CT (7.8% annual growth), MRI (10%), ultrasound (3.9%), and PET (57%) studies.
Increased CT use over the 15-year study period resulted in increased radiation exposure, doubling mean per capita effective dose (1.2 mSv to 2.3 mSv), as well as those receiving high exposure (1.2% to 2.5%) and very high exposure (0.6% to 1.4%).
The increased imaging use and radiation exposure among HMO enrollees was similar to that of fee-for-service Medicare patients in previous studies.
Bottom line: There is a significant increase in use of diagnostic imaging studies and associated radiation exposure among integrated healthcare system enrollees from 1996 to 2010, similar to patients in fee-for-service health plans.
Citation: Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409.
Postoperative Troponin Predicts 30-Day Mortality
Clinical question: Does postoperative peak troponin level predict 30-day mortality in patients undergoing noncardiac surgery?
Background: The use of postoperative peak troponin levels in predicting 30-day mortality for patients undergoing noncardiac surgery has not been studied extensively. Identifying patients at high risk for death following noncardiac surgery could facilitate appropriate postoperative care and improve survival.
Study design: Prospective cohort study.
Setting: International university and nonuniversity hospitals.
Synopsis: The Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study is a large, international, multicenter, prospective cohort study designed to evaluate the major complications of noncardiac surgery. More than 15,100 patients ages 45 and older requiring at least an overnight hospitalization were enrolled following noncardiac surgery.
Peak troponin measurements during the first three postoperative days of 0.01 ng/ml or less, 0.02 ng/ml, 0.03 ng/ml to 0.29 ng/ml, and 0.3 ng/ml or greater had 30-day mortality rates of 1.0%, 4.0%, 9.3%, and 16.9%, respectively.
This study demonstrates the sensitivity of troponin measurement for predicting postoperative 30-day mortality in patients undergoing noncardiac surgery. The study does not address interventions based on an increased postoperative troponin level. Future studies might investigate postoperative modifiable risk factors.
Bottom line: Postoperative peak troponin level predicts 30-day mortality in patients undergoing noncardiac surgery.
Citation: Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
Clinical Prediction Model of Mortality in Acute Heart Failure
Clinical question: Can a clinical prediction model accurately risk-stratify patients presenting to the ED with acute heart failure?
Background: Accurately prognosticating mortality is essential when determining whether to hospitalize or discharge patients presenting to the ED with acute heart failure. Evidence-based clinical prediction models enable physicians to risk-stratify patients and optimize care.
Study design: Retrospective cohort study.
Setting: Multicenter study of 86 hospitals in Ontario, Canada.
Synopsis: Data collected from 12,591 patients who presented to EDs with acute heart failure in Ontario were analyzed. A clinical prediction model of seven-day mortality of discharged and hospitalized patients was derived and validated. The Emergency Heart Failure Mortality Risk Grade (EHMRG) found an increased mortality based on higher triage heart rate, lower triage systolic blood pressure, initial oxygen saturation, and elevated troponin levels. This model uses readily available data collected in ED visits. The high-risk EHMRG score predicted about 8% seven-day mortality versus 0.3% in the low-risk score.
This model was not applied to chronic heart failure, did not utilize left ventricular function, and does not differentiate between systolic and diastolic heart failure.
Bottom line: The Emergency Heart Failure Mortality Risk Grade predicts seven-day mortality in acute heart failure in the emergent setting.
Citation: Lee DS, Stitt A, Austin PC, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156(11):767-775.
Indwelling Pleural Catheter Is as Effective as Talc Pleurodesis Via Chest Tube in Relieving Dyspnea in Patients with Malignant Pleural Effusion
Clinical question: Is indwelling pleural catheter (IPC) as effective as chest tube and talc pleurodesis (talc) in improving dyspnea from malignant pleural effusion in patients who had no previous pleurodesis?
Background: Despite guidelines recommending chest tube insertion with pleurodesis as a first-line treatment for symptom palliation from malignant pleural effusion, there has been no randomized trial comparing indwelling pleural catheter with chest tube and talc pleurodesis.
Study design: Open-label, randomized controlled trial.
Setting: Seven hospitals in the United Kingdom.
Synopsis: One hundred six patients with malignant pleural effusion were randomized to undergo either IPC or talc treatment, and their daily mean dyspnea was measured. There was a clinically significant improvement of dyspnea in both IPC and talc groups over the first 42 days of the trial, without any significant difference in dyspnea between the two groups. After six months, researchers found a clinically significant decrease in dyspnea in the IPC group compared with the talc group. Chest pain and global quality of life were improved and were similar in both groups throughout the trial period. Length of hospital stay was significantly shorter in the IPC group compared with the talc group, but more patients in the IPC group experienced adverse events.
Bottom line: Indwelling pleural catheter is as effective as talc pleurodesis in reliving dyspnea from malignant pleural effusion; however, IPC is associated with increased adverse events despite shorter length of hospital stay.
Citation: Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383-2389.
Early Surgery Better than Conventional Treatment in High-Risk Native-Valve Endocarditis
Clinical question: Is early cardiac surgery better than conventional treatment for patients with left-sided, native-valve, infective endocarditis?
Background: Although guidelines strongly recommend early surgery for patients with infective endocarditis and congestive heart failure, the timing of surgery for patients with large vegetations and high risk of embolism without heart failure symptoms remains controversial.
Study design: Prospective, randomized trial.
Setting: Two medical centers in South Korea.
Synopsis: Seventy-six patients with left-sided, native-valve, infective endocarditis with a high risk of embolism (defined as vegetation with a diameter greater than 10 mm or severe mitral or aortic valve disease) were randomized to undergo early surgery (within 48 hours of enrollment) or conventional treatment (antibiotic therapy and surgery only if complications required urgent surgery). The primary outcome of composite in-hospital death or
clinical embolic events within six weeks of the trial occurred in only one patient in the early surgery group, compared with nine patients in the conventional group (hazard ratio 0.10, 95% CI, 0.01-0.82, P=0.03).
There was no difference in all-cause mortality at six months between the two groups, but the rate of composite endpoint of death from any cause, embolic events, or recurrence of infective endocarditis at six months was significantly lower in the early surgery group compared with the conventional group.
Bottom line: Early cardiac surgery for patients with left-sided, native-valve infective endocarditis with a high risk of embolism significantly improved the composite outcome of all-cause mortality, embolic events, or recurrence of endocarditis compared with the conventional therapy.
Citation: Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366(26):2466-2473.
Risk Factors for Short-Term Mortality after Emergency Department Visit for Syncope
Clinical question: What are the risk factors for short-term mortality after an ED evaluation for syncope or near-syncope?
Background: Syncope accounts for 1% to 2% of all ED visits and an equal number of hospital admissions. The risk of death after an ED visit for syncope is poorly understood, resulting in frequent hospital admissions.
Study design: Retrospective cohort study.
Setting: EDs in Southern California.
Synopsis: Authors evaluated 23,951 ED visits resulting in syncope as sole primary diagnosis. Age was identified as the most significant risk factor for short-term mortality. Cumulative survival data revealed that more than 1% of patients 60 or older died by 30 days. There were 215 deaths (2.84%) in patients hospitalized from the ED and 66 deaths (0.45%) among patients not hospitalized.
Pre-existing comorbidities significantly associated with increased mortality included heart failure (HR=14.3 in ages 18-53; HR=3.09 in ages 60-79; HR=2.34 in ages 80-plus), diabetes (HR=1.49), seizure (HR=1.65), dementia (HR=1.41), and a recent prior visit for syncope (HR=1.86). The risk of death by 30 days was less than 0.2% in patients under 60 without heart failure and more than 2.5% in patients of all ages with heart failure.
Bottom line: After an ED visit for syncope, patients with a history of heart failure and patients 60 and older have a significantly increased risk of short-term mortality.
Citation: Derose SF, Gabayan GZ, Chiu VY, Sun BC. Patterns and preexisting risk factors of 30-day mortality after a primary discharge diagnosis of syncope or near syncope. Acad Emerg Med. 2012;19(5):488-496.
Acute Hyperglycemia Associated with Increased Mortality in Community-Acquired Pneumonia
Clinical question: In patients admitted to the hospital for community-acquired pneumonia, is serum glucose level on admission associated with mortality?
Background: Some retrospective studies have shown an association between alterations in serum glucose levels or pre-existing diabetes and higher mortality due to infections, while other studies have shown no clear association.
Study design: Multicenter, prospective cohort study.
Setting: Hospitals and private practices in Germany, Switzerland, and Austria.
Synopsis: Prospective data from 6,891 patients were included in the analysis. Patients without diabetes and normal serum glucose levels had the lowest mortality after 90 days. Patients without diabetes but with mild acute hyperglycemia (108 mg/dL to 198 mg/dL) had a significantly increased risk of death at 90 days (HR 1.56), and patients without diabetes but with more severe acute hyperglycemia (over 252 mg/dL) had an even higher risk of death at 90 days (HR 2.37).
The 90-day mortality rate was significantly higher in patients with pre-existing diabetes (HR 2.47), although this was not affected by serum glucose levels on admission.
Bottom line: Acute hyperglycemia, as well as pre-existing diabetes, was associated with an increased risk of 90-day mortality in patients with community acquired pneumonia.
Citation: Lepper PM, Ott S, Nüesch E, et al. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ. 2012;344:e3397.
Hospital Delirium Associated with Cognitive Decline, Institutionalization, and Death
Clinical question: What is the risk of subsequent cognitive decline, institutionalization, or death due to delirium in patients with dementia?
Background: Patients suffering delirium during hospitalization can suffer additional cognitive decline. Whether this is due to additional damage from the delirium state or reflects pre-existing cognitive vulnerability remains uncertain.
Study design: Prospective analysis of a cohort of Alzheimer’s patients.
Setting: Massachusetts community-based disease registry.
Synopsis: The analysis compared nonhospitalized individuals to patients hospitalized with, and without, delirium. In 771 individuals with dementia, at least one adverse outcome (including cognitive decline, institutionalization, or death) occurred in 32% of those not hospitalized, 55% of those hospitalized without delirium, and 79% of those hospitalized with delirium. Even after adjusting for confounders, hospitalization increased the risk for each of the adverse outcomes; the highest risk was in those with delirium.
Among hospitalized patients, the authors estimated 1 in 5 cases of cognitive decline, 1 in 7 institutionalizations, and 1 in 16 deaths were attributable to delirium. Some of the attributed risk could be the result of residual confounding from unmeasured variables, limiting conclusions of causality. Despite these limitations, this study supports the hypothesis that delirium prevention measures could improve important patient outcomes.
Bottom line: Hospitalization is associated with high rates of adverse outcomes in elderly patients with dementia, the worst of which occurs in those who experience delirium.
Citation: Fong TG, Jones RN, Marcantonio ER, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Int Med. 2012;156:848-856.
In Acute Pyelonephritis, a Seven-Day Course of Ciprofloxacin is Effective in Obtaining Clinical Cure
Clinical question: What is the efficacy of ciprofloxacin for seven days compared with 14 days in women with community-acquired acute pyelonephritis?
Background: Community-acquired acute pyelonephritis is a common and sometimes serious infection in women. In an era of increasing antibiotic resistance worldwide, it is prudent to reduce antibiotic utilization. There are limited controlled trials to assess the optimum duration of antibiotic treatment for this common infection.
Study design: Prospective, randomized, double-blind, noninferiority trial.
Setting: Twenty-one infectious-disease centers in Sweden.
Synopsis: Researchers randomly assigned 284 women 18 or older with a presumptive diagnosis of acute pyelonephritis to ciprofloxacin treatment for seven or 14 days. The primary endpoint was clinical and bacteriological cure 10 to 14 days after the completion of the treatment regimen. Short-term clinical cure occurred in 97% of the patients treated for seven days and 96% treated for 14 days. Long-term follow-up showed cumulative efficacy of 93% in each group. Both regimens were well tolerated.
Patients in this study had a low occurrence of complicated (9%) and recurrent (13%) infections. Whether short courses of antibiotics are effective in more complicated infections cannot be ascertained from this study. Also, the high cure rate obtained with a seven-day course of ciprofloxacin should not be extrapolated to other classes of antibiotics. Fluoroquinolones, such as ciprofloxacin, are recommended as first-line agents for empiric oral treatment of acute pyelonephritis if the resistance rate of the uropathogens remains lower than 10%; however, there is growing evidence that E. coli strains are becoming increasingly resistant to ciprofloxacin, limiting its usefulness.
Bottom line: Acute pyelonephritis in women can be treated successfully and safely with a seven-day course of ciprofloxacin, in areas with low ciprofloxacin resistance.
Citation: Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomized, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380:484-490.
Advance Directives in Community Patients with Heart Failure
Clinical question: How prevalent are advance directives in heart-failure patients, and does a completed advance directive decrease end-of-life resource use (hospitalizations, ICU admissions, mechanical ventilation)?
Background: Heart failure is a common chronic and fatal disease. End-of-life care in heart-failure patients is associated with extremely high healthcare utilization. Heart failure guidelines recommend completing advance directives in all patients.
Study design: Population-based longitudinal cohort study.
Setting: Rochester Epidemiology Project in Olmstead County, Minn.
Synopsis: Investigators enrolled 608 patients presenting with heart failure between October 2007 and October 2011. At the time of enrollment, only 41% of the patients had existing advance directives. Independent predictors of advance directive completion included older age, history of malignancy, and renal dysfunction.
After a mean follow-up of 1.8 years, 164 patients (27%) had died. Among those patients, 106 had an advance directive (64.6%) at time of death—75 had an advance directive at the time of enrollment and another 31 completed an advance directive after enrollment.
Twenty-five patients (23.6%) specified DNR/DNI and another 39 (36.8%) denoted limitations on aggressiveness of care if death was imminent. Among the patients who died, 88 (53.7%) were hospitalized in the last month of their life and 50 (30.5%) died in the hospital. There was no difference in hospitalizations between those with an advance directive specifying limits and those who did not specify limits (OR 1.26, 95% CI 0.64-2.48). However, those with an advance directive specifying limits were less frequently mechanically ventilated (OR 0.26, 95% CI 0.06-0.88), and there was a trend toward them being less frequently admitted into the ICU (OR 0.45, 95% CI 0.16-1.29).
Bottom line: Less than half of community patients with heart failure had an advance directive, and many of these failed to address end-of-life decisions. Patients with an advance directive that specified limits in care were less likely to receive mechanical ventilation.
Citation: Dunlay SM, Swetz KM, Mueller PS, Roger VL. Advance directives in community patients with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5:283-289.
Chlorhexidine Bathing Associated with Significant, Sustainable Reductions in Central-Venous-Catheter-Associated Bloodstream Infection
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511.
Simulation Training Improves Lumbar Puncture Skills
Clinical question: What effect does simulation have on lumbar puncture (LP) skills of PGY1 internal-medicine (IM) residents compared with PGY2-4 neurology residents who have not received simulation training?
Background: LPs are common procedures. The American College of General Medical Education does not define competency; neither do the internal-medicine (IM) or neurology board certifications. Simulation can improve skills in many areas but has not been well studied in LPs.
Study design: Pre-test-post-test.
Setting: Northwestern University’s Feinberg School of Medicine in Chicago.
Synopsis: The intervention group included 58 PGY1 IM residents, while the control group was 49 PGY2-to-PGY4 neurology residents. The pre-test consisted of a 21-point checklist. IM residents watched a three-hour video, performed LPs on simulators, and received feedback. The post-test was a clinical skills examination using the checklist. If this exam was failed, the participant practiced and was retested. Neurology residents completed the pre-test and demonstrated an LP using the simulator.
Pre-test passing was achieved by only 2% of IM residents and 6% of neurology residents. Post-test passing was achieved by 95% of the IM residents on the first trial and 100% of IM residents after an hour of additional training. IM mean scores increased to 95.7% from 46.3%, while the mean score of neurology residents was 65.4%.
This study is limited by its single-center nature, as education is variable from center to center. The study evaluated the proficiency on simulators only, and it did not evaluate the proficiency of the participants on patients.
Bottom line: Simulation training improves lumbar puncture skills.
Citation: Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137.
Primary-Care Physicians Do Not Use Publicly Reported Data When Referring Patients to Hospitals
Clinical question: When referring patients with pneumonia to the hospital, what factors do primary-care physicians (PCPs) consider?
Background: Publicly reported data are widely available. Pneumonia has publicly reported quality measures and is a common reason for hospitalization. Fewer PCPs are attending in the hospital due to the hospitalist movement; therefore, PCPs refer patients to a hospital when the need arises.
Study design: Online survey.
Setting: PCPs within 10 miles of Springfield, Mass.
Synopsis: A total of 92 PCPs responded to the survey, which included presentation of a case regarding a patient with pneumonia. PCPs were asked the importance of multiple factors leading to their decision to refer to a hospital. Familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%) were considered to be very important to the PCPs that responded to the survey. Publicly reported data were very important to only 18% of respondents, and zero reported using publicly reported data when referring patients.
Importance of specific quality measures also was queried; antibiotics given within six hours of arrival (66%), appropriate choice of antibiotics (63%), and blood cultures prior to antibiotic administration (51%) were very important to respondents. Prestige, such as magnet status and U.S. News and World Report “Best Hospital” status, were deemed important by about 40% of PCPs.
Bottom line: Despite the availability of publicly reported data, PCPs do not use this information to refer patients to the hospital.
Citation: Morsi E, Lindenauer PK, Rothberg MB. Primary care physicians’ use of publicly reported quality data in hospital referral decisions. J Hosp Med. 2012;7(5):370-375.
What Works for Medication Reconciliation?
Clinical question: What are the most effective practices for medication reconciliation in the hospital setting?
Background: Medication discrepancies are common, occurring in as many as 70% of patients at hospital admission or discharge. Up to a third of these discrepancies have potential to cause patient harm, including prolonged hospital stays, ED visits, hospital recidivism, and use of other healthcare resources. Medication reconciliation (“med rec”) is a strategy for reducing these errors, though previous literature has not systematically reviewed best practices for hospital-based med rec.
Study design: Systematic review of literature.
Setting: Controlled studies from the U.S., Canada, Australia, New Zealand, Northern Ireland, United Kingdom, Belgium, Denmark, the Netherlands, and Sweden.
Synopsis: Investigators identified 26 controlled studies using a systematic search of English-language articles on med rec during inpatient hospitalizations published between Jan. 1, 1966, and Oct. 31, 2010. Fifteen studies reported on pharmacist-related interventions; six reported on technology-specific interventions; and five reported on other types of interventions, including staff education and use of standardized med-rec tools.
Analysis of these studies revealed that all of these interventions successfully decreased medication discrepancies and potential adverse drug events, but there was inconsistent benefit with regard to adverse drug events and healthcare utilization compared with usual care. The literature was most supportive of pharmacist-related interventions, including but not limited to comprehensive medication history at admission, med rec at discharge, patient counseling, discharge communication with outpatient providers, and post-discharge communication with the patient and post-hospital providers.
Bottom line: Successful med rec requires multiple interventions at various transitions of care and involves a variety of medical professionals. Patient-targeted interventions, including pharmacists, have the potential to decrease errors and adverse events.
Citation: Mueller S, Sponsler K, Kripalani S, Schnipper J. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prediction tool for neurological outcomes after in-hospital cardiac arrest
- Radiation exposure in integrated healthcare systems, 1996-2010
- Postoperative troponin predicts 30-day mortality
- Clinical prediction model of mortality in acute heart failure
- Indwelling pleural catheter vs. talc pleurodesis via chest tube
- Early surgery for high-risk, native-valve endocarditis patients
- Risk factors after ED visit for syncope
- Acute hyperglycemia in CAP patients
- Hospital delirium associated with cognitive decline, institutionalization, and death
- Seven-day ciprofloxacin effective against acute pyelonephritis
- Advance directives in community patients with heart failure
- Chlorhexidine bathing effective against CVC-associated bloodstream infections
- Simulation training improves lumbar puncture skills
- PCP referrals to hospitals and publicly reported data
- Medication reconciliation best practices
Prediction Tool Validated for Prognosticating Favorable Neurological Outcome after In-Hospital Cardiac Arrest
Clinical question: Does the Cardiac Arrest Survival Post Resuscitation In-Hospital (CASPRI) score accurately predict favorable neurological outcomes?
Background: Previous cardiac arrest prediction models have been focused on survival to discharge without consideration of neurological status and have not been translated into valid bedside prognostication tools. Neurologic prognosis can assist patients, families, and physicians in decisions about continued goals of care post-arrest.
Study design: Retrospective cohort study.
Setting: Acute-care hospitals.
Synopsis: Using the Get with the Guidelines Resuscitation Registry, 551 hospitals identified 42,957 patients who were successfully resuscitated from an in-hospital cardiac arrest from January 2000 to October 2009. Researchers developed a simple prediction tool for favorable neurological outcomes (defined as “no” or “moderate” neurological disability) at discharge. The 11 predictors used to calculate the CASPRI score are age; time to defibrillation; pre-arrest neurological status; hospital location; duration of resuscitation; and pre-arrest comorbidities: mechanical ventilation, renal insufficiency, hepatic insufficiency, sepsis, malignancy,
and hypotension.
Rates of favorable neurological outcome were similar between derivation cohort (24.6%) and validation cohort (24.5%). The model had excellent discrimination with a C score of 0.80. Probability of favorable neurological survival ranged from 70.7% in the top decile of patients (CASPRI <10) and 2.8% in bottom decile (CASPRI ≥ 28).
This tool is not generalizable to patients with out-of-hospital arrest or undergoing therapeutic hypothermia.
Bottom line: CASPRI is a simple bedside tool validated to estimate probability of favorable neurological outcome after in-hospital cardiac arrest.
Citation: Chan PS, Spertus JA, Krumholz HA, et al. A validated prediction tool for initial survivors in in-hospital cardiac arrest. Arch Intern Med. 2012;172(12):947-953.
Increased Use of Radiologic Imaging and Associated Radiation Exposure in Integrated Healthcare Systems, 1996-2010
Clinical question: How much has imaging utilization and associated radiation exposure increased over 15 years in integrated healthcare systems independent of financial incentives in a fee-for-service system?
Background: Use of diagnostic imaging has increased significantly within fee-for-service healthcare models. The associated radiation exposure has increased the risk of radiation-induced malignancies. Little is known about the pattern of imaging use in integrated healthcare systems without the financial incentives seen in other models of care.
Study design: Retrospective cohort study.
Setting: Six integrated healthcare systems in the U.S.
Synopsis: The number of diagnostic imaging studies performed and estimated radiation exposure were determined from analysis of electronic medical records from member patients enrolled in health systems in the HMO Research Network from 1996 to 2010. Annual increases in use of advanced diagnostics were noted in CT (7.8% annual growth), MRI (10%), ultrasound (3.9%), and PET (57%) studies.
Increased CT use over the 15-year study period resulted in increased radiation exposure, doubling mean per capita effective dose (1.2 mSv to 2.3 mSv), as well as those receiving high exposure (1.2% to 2.5%) and very high exposure (0.6% to 1.4%).
The increased imaging use and radiation exposure among HMO enrollees was similar to that of fee-for-service Medicare patients in previous studies.
Bottom line: There is a significant increase in use of diagnostic imaging studies and associated radiation exposure among integrated healthcare system enrollees from 1996 to 2010, similar to patients in fee-for-service health plans.
Citation: Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409.
Postoperative Troponin Predicts 30-Day Mortality
Clinical question: Does postoperative peak troponin level predict 30-day mortality in patients undergoing noncardiac surgery?
Background: The use of postoperative peak troponin levels in predicting 30-day mortality for patients undergoing noncardiac surgery has not been studied extensively. Identifying patients at high risk for death following noncardiac surgery could facilitate appropriate postoperative care and improve survival.
Study design: Prospective cohort study.
Setting: International university and nonuniversity hospitals.
Synopsis: The Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study is a large, international, multicenter, prospective cohort study designed to evaluate the major complications of noncardiac surgery. More than 15,100 patients ages 45 and older requiring at least an overnight hospitalization were enrolled following noncardiac surgery.
Peak troponin measurements during the first three postoperative days of 0.01 ng/ml or less, 0.02 ng/ml, 0.03 ng/ml to 0.29 ng/ml, and 0.3 ng/ml or greater had 30-day mortality rates of 1.0%, 4.0%, 9.3%, and 16.9%, respectively.
This study demonstrates the sensitivity of troponin measurement for predicting postoperative 30-day mortality in patients undergoing noncardiac surgery. The study does not address interventions based on an increased postoperative troponin level. Future studies might investigate postoperative modifiable risk factors.
Bottom line: Postoperative peak troponin level predicts 30-day mortality in patients undergoing noncardiac surgery.
Citation: Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
Clinical Prediction Model of Mortality in Acute Heart Failure
Clinical question: Can a clinical prediction model accurately risk-stratify patients presenting to the ED with acute heart failure?
Background: Accurately prognosticating mortality is essential when determining whether to hospitalize or discharge patients presenting to the ED with acute heart failure. Evidence-based clinical prediction models enable physicians to risk-stratify patients and optimize care.
Study design: Retrospective cohort study.
Setting: Multicenter study of 86 hospitals in Ontario, Canada.
Synopsis: Data collected from 12,591 patients who presented to EDs with acute heart failure in Ontario were analyzed. A clinical prediction model of seven-day mortality of discharged and hospitalized patients was derived and validated. The Emergency Heart Failure Mortality Risk Grade (EHMRG) found an increased mortality based on higher triage heart rate, lower triage systolic blood pressure, initial oxygen saturation, and elevated troponin levels. This model uses readily available data collected in ED visits. The high-risk EHMRG score predicted about 8% seven-day mortality versus 0.3% in the low-risk score.
This model was not applied to chronic heart failure, did not utilize left ventricular function, and does not differentiate between systolic and diastolic heart failure.
Bottom line: The Emergency Heart Failure Mortality Risk Grade predicts seven-day mortality in acute heart failure in the emergent setting.
Citation: Lee DS, Stitt A, Austin PC, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156(11):767-775.
Indwelling Pleural Catheter Is as Effective as Talc Pleurodesis Via Chest Tube in Relieving Dyspnea in Patients with Malignant Pleural Effusion
Clinical question: Is indwelling pleural catheter (IPC) as effective as chest tube and talc pleurodesis (talc) in improving dyspnea from malignant pleural effusion in patients who had no previous pleurodesis?
Background: Despite guidelines recommending chest tube insertion with pleurodesis as a first-line treatment for symptom palliation from malignant pleural effusion, there has been no randomized trial comparing indwelling pleural catheter with chest tube and talc pleurodesis.
Study design: Open-label, randomized controlled trial.
Setting: Seven hospitals in the United Kingdom.
Synopsis: One hundred six patients with malignant pleural effusion were randomized to undergo either IPC or talc treatment, and their daily mean dyspnea was measured. There was a clinically significant improvement of dyspnea in both IPC and talc groups over the first 42 days of the trial, without any significant difference in dyspnea between the two groups. After six months, researchers found a clinically significant decrease in dyspnea in the IPC group compared with the talc group. Chest pain and global quality of life were improved and were similar in both groups throughout the trial period. Length of hospital stay was significantly shorter in the IPC group compared with the talc group, but more patients in the IPC group experienced adverse events.
Bottom line: Indwelling pleural catheter is as effective as talc pleurodesis in reliving dyspnea from malignant pleural effusion; however, IPC is associated with increased adverse events despite shorter length of hospital stay.
Citation: Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383-2389.
Early Surgery Better than Conventional Treatment in High-Risk Native-Valve Endocarditis
Clinical question: Is early cardiac surgery better than conventional treatment for patients with left-sided, native-valve, infective endocarditis?
Background: Although guidelines strongly recommend early surgery for patients with infective endocarditis and congestive heart failure, the timing of surgery for patients with large vegetations and high risk of embolism without heart failure symptoms remains controversial.
Study design: Prospective, randomized trial.
Setting: Two medical centers in South Korea.
Synopsis: Seventy-six patients with left-sided, native-valve, infective endocarditis with a high risk of embolism (defined as vegetation with a diameter greater than 10 mm or severe mitral or aortic valve disease) were randomized to undergo early surgery (within 48 hours of enrollment) or conventional treatment (antibiotic therapy and surgery only if complications required urgent surgery). The primary outcome of composite in-hospital death or
clinical embolic events within six weeks of the trial occurred in only one patient in the early surgery group, compared with nine patients in the conventional group (hazard ratio 0.10, 95% CI, 0.01-0.82, P=0.03).
There was no difference in all-cause mortality at six months between the two groups, but the rate of composite endpoint of death from any cause, embolic events, or recurrence of infective endocarditis at six months was significantly lower in the early surgery group compared with the conventional group.
Bottom line: Early cardiac surgery for patients with left-sided, native-valve infective endocarditis with a high risk of embolism significantly improved the composite outcome of all-cause mortality, embolic events, or recurrence of endocarditis compared with the conventional therapy.
Citation: Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366(26):2466-2473.
Risk Factors for Short-Term Mortality after Emergency Department Visit for Syncope
Clinical question: What are the risk factors for short-term mortality after an ED evaluation for syncope or near-syncope?
Background: Syncope accounts for 1% to 2% of all ED visits and an equal number of hospital admissions. The risk of death after an ED visit for syncope is poorly understood, resulting in frequent hospital admissions.
Study design: Retrospective cohort study.
Setting: EDs in Southern California.
Synopsis: Authors evaluated 23,951 ED visits resulting in syncope as sole primary diagnosis. Age was identified as the most significant risk factor for short-term mortality. Cumulative survival data revealed that more than 1% of patients 60 or older died by 30 days. There were 215 deaths (2.84%) in patients hospitalized from the ED and 66 deaths (0.45%) among patients not hospitalized.
Pre-existing comorbidities significantly associated with increased mortality included heart failure (HR=14.3 in ages 18-53; HR=3.09 in ages 60-79; HR=2.34 in ages 80-plus), diabetes (HR=1.49), seizure (HR=1.65), dementia (HR=1.41), and a recent prior visit for syncope (HR=1.86). The risk of death by 30 days was less than 0.2% in patients under 60 without heart failure and more than 2.5% in patients of all ages with heart failure.
Bottom line: After an ED visit for syncope, patients with a history of heart failure and patients 60 and older have a significantly increased risk of short-term mortality.
Citation: Derose SF, Gabayan GZ, Chiu VY, Sun BC. Patterns and preexisting risk factors of 30-day mortality after a primary discharge diagnosis of syncope or near syncope. Acad Emerg Med. 2012;19(5):488-496.
Acute Hyperglycemia Associated with Increased Mortality in Community-Acquired Pneumonia
Clinical question: In patients admitted to the hospital for community-acquired pneumonia, is serum glucose level on admission associated with mortality?
Background: Some retrospective studies have shown an association between alterations in serum glucose levels or pre-existing diabetes and higher mortality due to infections, while other studies have shown no clear association.
Study design: Multicenter, prospective cohort study.
Setting: Hospitals and private practices in Germany, Switzerland, and Austria.
Synopsis: Prospective data from 6,891 patients were included in the analysis. Patients without diabetes and normal serum glucose levels had the lowest mortality after 90 days. Patients without diabetes but with mild acute hyperglycemia (108 mg/dL to 198 mg/dL) had a significantly increased risk of death at 90 days (HR 1.56), and patients without diabetes but with more severe acute hyperglycemia (over 252 mg/dL) had an even higher risk of death at 90 days (HR 2.37).
The 90-day mortality rate was significantly higher in patients with pre-existing diabetes (HR 2.47), although this was not affected by serum glucose levels on admission.
Bottom line: Acute hyperglycemia, as well as pre-existing diabetes, was associated with an increased risk of 90-day mortality in patients with community acquired pneumonia.
Citation: Lepper PM, Ott S, Nüesch E, et al. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ. 2012;344:e3397.
Hospital Delirium Associated with Cognitive Decline, Institutionalization, and Death
Clinical question: What is the risk of subsequent cognitive decline, institutionalization, or death due to delirium in patients with dementia?
Background: Patients suffering delirium during hospitalization can suffer additional cognitive decline. Whether this is due to additional damage from the delirium state or reflects pre-existing cognitive vulnerability remains uncertain.
Study design: Prospective analysis of a cohort of Alzheimer’s patients.
Setting: Massachusetts community-based disease registry.
Synopsis: The analysis compared nonhospitalized individuals to patients hospitalized with, and without, delirium. In 771 individuals with dementia, at least one adverse outcome (including cognitive decline, institutionalization, or death) occurred in 32% of those not hospitalized, 55% of those hospitalized without delirium, and 79% of those hospitalized with delirium. Even after adjusting for confounders, hospitalization increased the risk for each of the adverse outcomes; the highest risk was in those with delirium.
Among hospitalized patients, the authors estimated 1 in 5 cases of cognitive decline, 1 in 7 institutionalizations, and 1 in 16 deaths were attributable to delirium. Some of the attributed risk could be the result of residual confounding from unmeasured variables, limiting conclusions of causality. Despite these limitations, this study supports the hypothesis that delirium prevention measures could improve important patient outcomes.
Bottom line: Hospitalization is associated with high rates of adverse outcomes in elderly patients with dementia, the worst of which occurs in those who experience delirium.
Citation: Fong TG, Jones RN, Marcantonio ER, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Int Med. 2012;156:848-856.
In Acute Pyelonephritis, a Seven-Day Course of Ciprofloxacin is Effective in Obtaining Clinical Cure
Clinical question: What is the efficacy of ciprofloxacin for seven days compared with 14 days in women with community-acquired acute pyelonephritis?
Background: Community-acquired acute pyelonephritis is a common and sometimes serious infection in women. In an era of increasing antibiotic resistance worldwide, it is prudent to reduce antibiotic utilization. There are limited controlled trials to assess the optimum duration of antibiotic treatment for this common infection.
Study design: Prospective, randomized, double-blind, noninferiority trial.
Setting: Twenty-one infectious-disease centers in Sweden.
Synopsis: Researchers randomly assigned 284 women 18 or older with a presumptive diagnosis of acute pyelonephritis to ciprofloxacin treatment for seven or 14 days. The primary endpoint was clinical and bacteriological cure 10 to 14 days after the completion of the treatment regimen. Short-term clinical cure occurred in 97% of the patients treated for seven days and 96% treated for 14 days. Long-term follow-up showed cumulative efficacy of 93% in each group. Both regimens were well tolerated.
Patients in this study had a low occurrence of complicated (9%) and recurrent (13%) infections. Whether short courses of antibiotics are effective in more complicated infections cannot be ascertained from this study. Also, the high cure rate obtained with a seven-day course of ciprofloxacin should not be extrapolated to other classes of antibiotics. Fluoroquinolones, such as ciprofloxacin, are recommended as first-line agents for empiric oral treatment of acute pyelonephritis if the resistance rate of the uropathogens remains lower than 10%; however, there is growing evidence that E. coli strains are becoming increasingly resistant to ciprofloxacin, limiting its usefulness.
Bottom line: Acute pyelonephritis in women can be treated successfully and safely with a seven-day course of ciprofloxacin, in areas with low ciprofloxacin resistance.
Citation: Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomized, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380:484-490.
Advance Directives in Community Patients with Heart Failure
Clinical question: How prevalent are advance directives in heart-failure patients, and does a completed advance directive decrease end-of-life resource use (hospitalizations, ICU admissions, mechanical ventilation)?
Background: Heart failure is a common chronic and fatal disease. End-of-life care in heart-failure patients is associated with extremely high healthcare utilization. Heart failure guidelines recommend completing advance directives in all patients.
Study design: Population-based longitudinal cohort study.
Setting: Rochester Epidemiology Project in Olmstead County, Minn.
Synopsis: Investigators enrolled 608 patients presenting with heart failure between October 2007 and October 2011. At the time of enrollment, only 41% of the patients had existing advance directives. Independent predictors of advance directive completion included older age, history of malignancy, and renal dysfunction.
After a mean follow-up of 1.8 years, 164 patients (27%) had died. Among those patients, 106 had an advance directive (64.6%) at time of death—75 had an advance directive at the time of enrollment and another 31 completed an advance directive after enrollment.
Twenty-five patients (23.6%) specified DNR/DNI and another 39 (36.8%) denoted limitations on aggressiveness of care if death was imminent. Among the patients who died, 88 (53.7%) were hospitalized in the last month of their life and 50 (30.5%) died in the hospital. There was no difference in hospitalizations between those with an advance directive specifying limits and those who did not specify limits (OR 1.26, 95% CI 0.64-2.48). However, those with an advance directive specifying limits were less frequently mechanically ventilated (OR 0.26, 95% CI 0.06-0.88), and there was a trend toward them being less frequently admitted into the ICU (OR 0.45, 95% CI 0.16-1.29).
Bottom line: Less than half of community patients with heart failure had an advance directive, and many of these failed to address end-of-life decisions. Patients with an advance directive that specified limits in care were less likely to receive mechanical ventilation.
Citation: Dunlay SM, Swetz KM, Mueller PS, Roger VL. Advance directives in community patients with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5:283-289.
Chlorhexidine Bathing Associated with Significant, Sustainable Reductions in Central-Venous-Catheter-Associated Bloodstream Infection
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511.
Simulation Training Improves Lumbar Puncture Skills
Clinical question: What effect does simulation have on lumbar puncture (LP) skills of PGY1 internal-medicine (IM) residents compared with PGY2-4 neurology residents who have not received simulation training?
Background: LPs are common procedures. The American College of General Medical Education does not define competency; neither do the internal-medicine (IM) or neurology board certifications. Simulation can improve skills in many areas but has not been well studied in LPs.
Study design: Pre-test-post-test.
Setting: Northwestern University’s Feinberg School of Medicine in Chicago.
Synopsis: The intervention group included 58 PGY1 IM residents, while the control group was 49 PGY2-to-PGY4 neurology residents. The pre-test consisted of a 21-point checklist. IM residents watched a three-hour video, performed LPs on simulators, and received feedback. The post-test was a clinical skills examination using the checklist. If this exam was failed, the participant practiced and was retested. Neurology residents completed the pre-test and demonstrated an LP using the simulator.
Pre-test passing was achieved by only 2% of IM residents and 6% of neurology residents. Post-test passing was achieved by 95% of the IM residents on the first trial and 100% of IM residents after an hour of additional training. IM mean scores increased to 95.7% from 46.3%, while the mean score of neurology residents was 65.4%.
This study is limited by its single-center nature, as education is variable from center to center. The study evaluated the proficiency on simulators only, and it did not evaluate the proficiency of the participants on patients.
Bottom line: Simulation training improves lumbar puncture skills.
Citation: Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137.
Primary-Care Physicians Do Not Use Publicly Reported Data When Referring Patients to Hospitals
Clinical question: When referring patients with pneumonia to the hospital, what factors do primary-care physicians (PCPs) consider?
Background: Publicly reported data are widely available. Pneumonia has publicly reported quality measures and is a common reason for hospitalization. Fewer PCPs are attending in the hospital due to the hospitalist movement; therefore, PCPs refer patients to a hospital when the need arises.
Study design: Online survey.
Setting: PCPs within 10 miles of Springfield, Mass.
Synopsis: A total of 92 PCPs responded to the survey, which included presentation of a case regarding a patient with pneumonia. PCPs were asked the importance of multiple factors leading to their decision to refer to a hospital. Familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%) were considered to be very important to the PCPs that responded to the survey. Publicly reported data were very important to only 18% of respondents, and zero reported using publicly reported data when referring patients.
Importance of specific quality measures also was queried; antibiotics given within six hours of arrival (66%), appropriate choice of antibiotics (63%), and blood cultures prior to antibiotic administration (51%) were very important to respondents. Prestige, such as magnet status and U.S. News and World Report “Best Hospital” status, were deemed important by about 40% of PCPs.
Bottom line: Despite the availability of publicly reported data, PCPs do not use this information to refer patients to the hospital.
Citation: Morsi E, Lindenauer PK, Rothberg MB. Primary care physicians’ use of publicly reported quality data in hospital referral decisions. J Hosp Med. 2012;7(5):370-375.
What Works for Medication Reconciliation?
Clinical question: What are the most effective practices for medication reconciliation in the hospital setting?
Background: Medication discrepancies are common, occurring in as many as 70% of patients at hospital admission or discharge. Up to a third of these discrepancies have potential to cause patient harm, including prolonged hospital stays, ED visits, hospital recidivism, and use of other healthcare resources. Medication reconciliation (“med rec”) is a strategy for reducing these errors, though previous literature has not systematically reviewed best practices for hospital-based med rec.
Study design: Systematic review of literature.
Setting: Controlled studies from the U.S., Canada, Australia, New Zealand, Northern Ireland, United Kingdom, Belgium, Denmark, the Netherlands, and Sweden.
Synopsis: Investigators identified 26 controlled studies using a systematic search of English-language articles on med rec during inpatient hospitalizations published between Jan. 1, 1966, and Oct. 31, 2010. Fifteen studies reported on pharmacist-related interventions; six reported on technology-specific interventions; and five reported on other types of interventions, including staff education and use of standardized med-rec tools.
Analysis of these studies revealed that all of these interventions successfully decreased medication discrepancies and potential adverse drug events, but there was inconsistent benefit with regard to adverse drug events and healthcare utilization compared with usual care. The literature was most supportive of pharmacist-related interventions, including but not limited to comprehensive medication history at admission, med rec at discharge, patient counseling, discharge communication with outpatient providers, and post-discharge communication with the patient and post-hospital providers.
Bottom line: Successful med rec requires multiple interventions at various transitions of care and involves a variety of medical professionals. Patient-targeted interventions, including pharmacists, have the potential to decrease errors and adverse events.
Citation: Mueller S, Sponsler K, Kripalani S, Schnipper J. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prediction tool for neurological outcomes after in-hospital cardiac arrest
- Radiation exposure in integrated healthcare systems, 1996-2010
- Postoperative troponin predicts 30-day mortality
- Clinical prediction model of mortality in acute heart failure
- Indwelling pleural catheter vs. talc pleurodesis via chest tube
- Early surgery for high-risk, native-valve endocarditis patients
- Risk factors after ED visit for syncope
- Acute hyperglycemia in CAP patients
- Hospital delirium associated with cognitive decline, institutionalization, and death
- Seven-day ciprofloxacin effective against acute pyelonephritis
- Advance directives in community patients with heart failure
- Chlorhexidine bathing effective against CVC-associated bloodstream infections
- Simulation training improves lumbar puncture skills
- PCP referrals to hospitals and publicly reported data
- Medication reconciliation best practices
Prediction Tool Validated for Prognosticating Favorable Neurological Outcome after In-Hospital Cardiac Arrest
Clinical question: Does the Cardiac Arrest Survival Post Resuscitation In-Hospital (CASPRI) score accurately predict favorable neurological outcomes?
Background: Previous cardiac arrest prediction models have been focused on survival to discharge without consideration of neurological status and have not been translated into valid bedside prognostication tools. Neurologic prognosis can assist patients, families, and physicians in decisions about continued goals of care post-arrest.
Study design: Retrospective cohort study.
Setting: Acute-care hospitals.
Synopsis: Using the Get with the Guidelines Resuscitation Registry, 551 hospitals identified 42,957 patients who were successfully resuscitated from an in-hospital cardiac arrest from January 2000 to October 2009. Researchers developed a simple prediction tool for favorable neurological outcomes (defined as “no” or “moderate” neurological disability) at discharge. The 11 predictors used to calculate the CASPRI score are age; time to defibrillation; pre-arrest neurological status; hospital location; duration of resuscitation; and pre-arrest comorbidities: mechanical ventilation, renal insufficiency, hepatic insufficiency, sepsis, malignancy,
and hypotension.
Rates of favorable neurological outcome were similar between derivation cohort (24.6%) and validation cohort (24.5%). The model had excellent discrimination with a C score of 0.80. Probability of favorable neurological survival ranged from 70.7% in the top decile of patients (CASPRI <10) and 2.8% in bottom decile (CASPRI ≥ 28).
This tool is not generalizable to patients with out-of-hospital arrest or undergoing therapeutic hypothermia.
Bottom line: CASPRI is a simple bedside tool validated to estimate probability of favorable neurological outcome after in-hospital cardiac arrest.
Citation: Chan PS, Spertus JA, Krumholz HA, et al. A validated prediction tool for initial survivors in in-hospital cardiac arrest. Arch Intern Med. 2012;172(12):947-953.
Increased Use of Radiologic Imaging and Associated Radiation Exposure in Integrated Healthcare Systems, 1996-2010
Clinical question: How much has imaging utilization and associated radiation exposure increased over 15 years in integrated healthcare systems independent of financial incentives in a fee-for-service system?
Background: Use of diagnostic imaging has increased significantly within fee-for-service healthcare models. The associated radiation exposure has increased the risk of radiation-induced malignancies. Little is known about the pattern of imaging use in integrated healthcare systems without the financial incentives seen in other models of care.
Study design: Retrospective cohort study.
Setting: Six integrated healthcare systems in the U.S.
Synopsis: The number of diagnostic imaging studies performed and estimated radiation exposure were determined from analysis of electronic medical records from member patients enrolled in health systems in the HMO Research Network from 1996 to 2010. Annual increases in use of advanced diagnostics were noted in CT (7.8% annual growth), MRI (10%), ultrasound (3.9%), and PET (57%) studies.
Increased CT use over the 15-year study period resulted in increased radiation exposure, doubling mean per capita effective dose (1.2 mSv to 2.3 mSv), as well as those receiving high exposure (1.2% to 2.5%) and very high exposure (0.6% to 1.4%).
The increased imaging use and radiation exposure among HMO enrollees was similar to that of fee-for-service Medicare patients in previous studies.
Bottom line: There is a significant increase in use of diagnostic imaging studies and associated radiation exposure among integrated healthcare system enrollees from 1996 to 2010, similar to patients in fee-for-service health plans.
Citation: Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409.
Postoperative Troponin Predicts 30-Day Mortality
Clinical question: Does postoperative peak troponin level predict 30-day mortality in patients undergoing noncardiac surgery?
Background: The use of postoperative peak troponin levels in predicting 30-day mortality for patients undergoing noncardiac surgery has not been studied extensively. Identifying patients at high risk for death following noncardiac surgery could facilitate appropriate postoperative care and improve survival.
Study design: Prospective cohort study.
Setting: International university and nonuniversity hospitals.
Synopsis: The Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) Study is a large, international, multicenter, prospective cohort study designed to evaluate the major complications of noncardiac surgery. More than 15,100 patients ages 45 and older requiring at least an overnight hospitalization were enrolled following noncardiac surgery.
Peak troponin measurements during the first three postoperative days of 0.01 ng/ml or less, 0.02 ng/ml, 0.03 ng/ml to 0.29 ng/ml, and 0.3 ng/ml or greater had 30-day mortality rates of 1.0%, 4.0%, 9.3%, and 16.9%, respectively.
This study demonstrates the sensitivity of troponin measurement for predicting postoperative 30-day mortality in patients undergoing noncardiac surgery. The study does not address interventions based on an increased postoperative troponin level. Future studies might investigate postoperative modifiable risk factors.
Bottom line: Postoperative peak troponin level predicts 30-day mortality in patients undergoing noncardiac surgery.
Citation: Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
Clinical Prediction Model of Mortality in Acute Heart Failure
Clinical question: Can a clinical prediction model accurately risk-stratify patients presenting to the ED with acute heart failure?
Background: Accurately prognosticating mortality is essential when determining whether to hospitalize or discharge patients presenting to the ED with acute heart failure. Evidence-based clinical prediction models enable physicians to risk-stratify patients and optimize care.
Study design: Retrospective cohort study.
Setting: Multicenter study of 86 hospitals in Ontario, Canada.
Synopsis: Data collected from 12,591 patients who presented to EDs with acute heart failure in Ontario were analyzed. A clinical prediction model of seven-day mortality of discharged and hospitalized patients was derived and validated. The Emergency Heart Failure Mortality Risk Grade (EHMRG) found an increased mortality based on higher triage heart rate, lower triage systolic blood pressure, initial oxygen saturation, and elevated troponin levels. This model uses readily available data collected in ED visits. The high-risk EHMRG score predicted about 8% seven-day mortality versus 0.3% in the low-risk score.
This model was not applied to chronic heart failure, did not utilize left ventricular function, and does not differentiate between systolic and diastolic heart failure.
Bottom line: The Emergency Heart Failure Mortality Risk Grade predicts seven-day mortality in acute heart failure in the emergent setting.
Citation: Lee DS, Stitt A, Austin PC, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156(11):767-775.
Indwelling Pleural Catheter Is as Effective as Talc Pleurodesis Via Chest Tube in Relieving Dyspnea in Patients with Malignant Pleural Effusion
Clinical question: Is indwelling pleural catheter (IPC) as effective as chest tube and talc pleurodesis (talc) in improving dyspnea from malignant pleural effusion in patients who had no previous pleurodesis?
Background: Despite guidelines recommending chest tube insertion with pleurodesis as a first-line treatment for symptom palliation from malignant pleural effusion, there has been no randomized trial comparing indwelling pleural catheter with chest tube and talc pleurodesis.
Study design: Open-label, randomized controlled trial.
Setting: Seven hospitals in the United Kingdom.
Synopsis: One hundred six patients with malignant pleural effusion were randomized to undergo either IPC or talc treatment, and their daily mean dyspnea was measured. There was a clinically significant improvement of dyspnea in both IPC and talc groups over the first 42 days of the trial, without any significant difference in dyspnea between the two groups. After six months, researchers found a clinically significant decrease in dyspnea in the IPC group compared with the talc group. Chest pain and global quality of life were improved and were similar in both groups throughout the trial period. Length of hospital stay was significantly shorter in the IPC group compared with the talc group, but more patients in the IPC group experienced adverse events.
Bottom line: Indwelling pleural catheter is as effective as talc pleurodesis in reliving dyspnea from malignant pleural effusion; however, IPC is associated with increased adverse events despite shorter length of hospital stay.
Citation: Davies HE, Mishra EK, Kahan BC, et al. Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA. 2012;307(22):2383-2389.
Early Surgery Better than Conventional Treatment in High-Risk Native-Valve Endocarditis
Clinical question: Is early cardiac surgery better than conventional treatment for patients with left-sided, native-valve, infective endocarditis?
Background: Although guidelines strongly recommend early surgery for patients with infective endocarditis and congestive heart failure, the timing of surgery for patients with large vegetations and high risk of embolism without heart failure symptoms remains controversial.
Study design: Prospective, randomized trial.
Setting: Two medical centers in South Korea.
Synopsis: Seventy-six patients with left-sided, native-valve, infective endocarditis with a high risk of embolism (defined as vegetation with a diameter greater than 10 mm or severe mitral or aortic valve disease) were randomized to undergo early surgery (within 48 hours of enrollment) or conventional treatment (antibiotic therapy and surgery only if complications required urgent surgery). The primary outcome of composite in-hospital death or
clinical embolic events within six weeks of the trial occurred in only one patient in the early surgery group, compared with nine patients in the conventional group (hazard ratio 0.10, 95% CI, 0.01-0.82, P=0.03).
There was no difference in all-cause mortality at six months between the two groups, but the rate of composite endpoint of death from any cause, embolic events, or recurrence of infective endocarditis at six months was significantly lower in the early surgery group compared with the conventional group.
Bottom line: Early cardiac surgery for patients with left-sided, native-valve infective endocarditis with a high risk of embolism significantly improved the composite outcome of all-cause mortality, embolic events, or recurrence of endocarditis compared with the conventional therapy.
Citation: Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med. 2012;366(26):2466-2473.
Risk Factors for Short-Term Mortality after Emergency Department Visit for Syncope
Clinical question: What are the risk factors for short-term mortality after an ED evaluation for syncope or near-syncope?
Background: Syncope accounts for 1% to 2% of all ED visits and an equal number of hospital admissions. The risk of death after an ED visit for syncope is poorly understood, resulting in frequent hospital admissions.
Study design: Retrospective cohort study.
Setting: EDs in Southern California.
Synopsis: Authors evaluated 23,951 ED visits resulting in syncope as sole primary diagnosis. Age was identified as the most significant risk factor for short-term mortality. Cumulative survival data revealed that more than 1% of patients 60 or older died by 30 days. There were 215 deaths (2.84%) in patients hospitalized from the ED and 66 deaths (0.45%) among patients not hospitalized.
Pre-existing comorbidities significantly associated with increased mortality included heart failure (HR=14.3 in ages 18-53; HR=3.09 in ages 60-79; HR=2.34 in ages 80-plus), diabetes (HR=1.49), seizure (HR=1.65), dementia (HR=1.41), and a recent prior visit for syncope (HR=1.86). The risk of death by 30 days was less than 0.2% in patients under 60 without heart failure and more than 2.5% in patients of all ages with heart failure.
Bottom line: After an ED visit for syncope, patients with a history of heart failure and patients 60 and older have a significantly increased risk of short-term mortality.
Citation: Derose SF, Gabayan GZ, Chiu VY, Sun BC. Patterns and preexisting risk factors of 30-day mortality after a primary discharge diagnosis of syncope or near syncope. Acad Emerg Med. 2012;19(5):488-496.
Acute Hyperglycemia Associated with Increased Mortality in Community-Acquired Pneumonia
Clinical question: In patients admitted to the hospital for community-acquired pneumonia, is serum glucose level on admission associated with mortality?
Background: Some retrospective studies have shown an association between alterations in serum glucose levels or pre-existing diabetes and higher mortality due to infections, while other studies have shown no clear association.
Study design: Multicenter, prospective cohort study.
Setting: Hospitals and private practices in Germany, Switzerland, and Austria.
Synopsis: Prospective data from 6,891 patients were included in the analysis. Patients without diabetes and normal serum glucose levels had the lowest mortality after 90 days. Patients without diabetes but with mild acute hyperglycemia (108 mg/dL to 198 mg/dL) had a significantly increased risk of death at 90 days (HR 1.56), and patients without diabetes but with more severe acute hyperglycemia (over 252 mg/dL) had an even higher risk of death at 90 days (HR 2.37).
The 90-day mortality rate was significantly higher in patients with pre-existing diabetes (HR 2.47), although this was not affected by serum glucose levels on admission.
Bottom line: Acute hyperglycemia, as well as pre-existing diabetes, was associated with an increased risk of 90-day mortality in patients with community acquired pneumonia.
Citation: Lepper PM, Ott S, Nüesch E, et al. Serum glucose levels for predicting death in patients admitted to hospital for community acquired pneumonia: prospective cohort study. BMJ. 2012;344:e3397.
Hospital Delirium Associated with Cognitive Decline, Institutionalization, and Death
Clinical question: What is the risk of subsequent cognitive decline, institutionalization, or death due to delirium in patients with dementia?
Background: Patients suffering delirium during hospitalization can suffer additional cognitive decline. Whether this is due to additional damage from the delirium state or reflects pre-existing cognitive vulnerability remains uncertain.
Study design: Prospective analysis of a cohort of Alzheimer’s patients.
Setting: Massachusetts community-based disease registry.
Synopsis: The analysis compared nonhospitalized individuals to patients hospitalized with, and without, delirium. In 771 individuals with dementia, at least one adverse outcome (including cognitive decline, institutionalization, or death) occurred in 32% of those not hospitalized, 55% of those hospitalized without delirium, and 79% of those hospitalized with delirium. Even after adjusting for confounders, hospitalization increased the risk for each of the adverse outcomes; the highest risk was in those with delirium.
Among hospitalized patients, the authors estimated 1 in 5 cases of cognitive decline, 1 in 7 institutionalizations, and 1 in 16 deaths were attributable to delirium. Some of the attributed risk could be the result of residual confounding from unmeasured variables, limiting conclusions of causality. Despite these limitations, this study supports the hypothesis that delirium prevention measures could improve important patient outcomes.
Bottom line: Hospitalization is associated with high rates of adverse outcomes in elderly patients with dementia, the worst of which occurs in those who experience delirium.
Citation: Fong TG, Jones RN, Marcantonio ER, et al. Adverse outcomes after hospitalization and delirium in persons with Alzheimer disease. Ann Int Med. 2012;156:848-856.
In Acute Pyelonephritis, a Seven-Day Course of Ciprofloxacin is Effective in Obtaining Clinical Cure
Clinical question: What is the efficacy of ciprofloxacin for seven days compared with 14 days in women with community-acquired acute pyelonephritis?
Background: Community-acquired acute pyelonephritis is a common and sometimes serious infection in women. In an era of increasing antibiotic resistance worldwide, it is prudent to reduce antibiotic utilization. There are limited controlled trials to assess the optimum duration of antibiotic treatment for this common infection.
Study design: Prospective, randomized, double-blind, noninferiority trial.
Setting: Twenty-one infectious-disease centers in Sweden.
Synopsis: Researchers randomly assigned 284 women 18 or older with a presumptive diagnosis of acute pyelonephritis to ciprofloxacin treatment for seven or 14 days. The primary endpoint was clinical and bacteriological cure 10 to 14 days after the completion of the treatment regimen. Short-term clinical cure occurred in 97% of the patients treated for seven days and 96% treated for 14 days. Long-term follow-up showed cumulative efficacy of 93% in each group. Both regimens were well tolerated.
Patients in this study had a low occurrence of complicated (9%) and recurrent (13%) infections. Whether short courses of antibiotics are effective in more complicated infections cannot be ascertained from this study. Also, the high cure rate obtained with a seven-day course of ciprofloxacin should not be extrapolated to other classes of antibiotics. Fluoroquinolones, such as ciprofloxacin, are recommended as first-line agents for empiric oral treatment of acute pyelonephritis if the resistance rate of the uropathogens remains lower than 10%; however, there is growing evidence that E. coli strains are becoming increasingly resistant to ciprofloxacin, limiting its usefulness.
Bottom line: Acute pyelonephritis in women can be treated successfully and safely with a seven-day course of ciprofloxacin, in areas with low ciprofloxacin resistance.
Citation: Sandberg T, Skoog G, Hermansson AB, et al. Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomized, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet. 2012;380:484-490.
Advance Directives in Community Patients with Heart Failure
Clinical question: How prevalent are advance directives in heart-failure patients, and does a completed advance directive decrease end-of-life resource use (hospitalizations, ICU admissions, mechanical ventilation)?
Background: Heart failure is a common chronic and fatal disease. End-of-life care in heart-failure patients is associated with extremely high healthcare utilization. Heart failure guidelines recommend completing advance directives in all patients.
Study design: Population-based longitudinal cohort study.
Setting: Rochester Epidemiology Project in Olmstead County, Minn.
Synopsis: Investigators enrolled 608 patients presenting with heart failure between October 2007 and October 2011. At the time of enrollment, only 41% of the patients had existing advance directives. Independent predictors of advance directive completion included older age, history of malignancy, and renal dysfunction.
After a mean follow-up of 1.8 years, 164 patients (27%) had died. Among those patients, 106 had an advance directive (64.6%) at time of death—75 had an advance directive at the time of enrollment and another 31 completed an advance directive after enrollment.
Twenty-five patients (23.6%) specified DNR/DNI and another 39 (36.8%) denoted limitations on aggressiveness of care if death was imminent. Among the patients who died, 88 (53.7%) were hospitalized in the last month of their life and 50 (30.5%) died in the hospital. There was no difference in hospitalizations between those with an advance directive specifying limits and those who did not specify limits (OR 1.26, 95% CI 0.64-2.48). However, those with an advance directive specifying limits were less frequently mechanically ventilated (OR 0.26, 95% CI 0.06-0.88), and there was a trend toward them being less frequently admitted into the ICU (OR 0.45, 95% CI 0.16-1.29).
Bottom line: Less than half of community patients with heart failure had an advance directive, and many of these failed to address end-of-life decisions. Patients with an advance directive that specified limits in care were less likely to receive mechanical ventilation.
Citation: Dunlay SM, Swetz KM, Mueller PS, Roger VL. Advance directives in community patients with heart failure. Circ Cardiovasc Qual Outcomes. 2012;5:283-289.
Chlorhexidine Bathing Associated with Significant, Sustainable Reductions in Central-Venous-Catheter-Associated Bloodstream Infection
Clinical question: What is the impact, and sustainability, of chlorhexidine bathing on central-venous-catheter-associated bloodstream infections?
Background: Chlorhexidine bathing has been associated with reductions in healthcare-associated bloodstream infections, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. No prospective studies have evaluated the impact and sustainability of chlorhexidine bathing.
Study design: Prospective, three-phase study.
Setting: Medical-surgical ICUs and respiratory-care units at five New York hospitals.
Synopsis: In the pre-intervention phase (six to nine months, 1,808 admissions), patients were bathed with soap and water or nonmedicated bathing cloths. In the intervention phase (eight months, 1,832 admissions), patients were bathed with 2% chlorhexidine cloths. In the post-intervention phase (12 months, 2,834 admissions), chlorhexidine bathing was continued without oversight by researchers.
During the intervention phase, there were significantly fewer central-venous-catheter-associated bloodstream infections (2.6/1,000 catheter days vs. 6.4/1,000 pre-intervention). The reductions in bloodstream infections were sustained during the post-intervention period (2.9/1,000 catheter days). Compliance with chlorhexidine bathing was 82% and 88% during the intervention and post-intervention phases, and was well tolerated by the patients.
Limitations of this study include lack of patient-specific data and severity of illness data, as well as lack of randomization and blinding. Although not evaluated in this study, the savings associated with decreased bloodstream infections likely outweigh the cost of chlorhexidine bathing.
Bottom line: Chlorhexidine bathing is a well-tolerated, sustainable intervention that significantly reduces central-venous-catheter-associated bloodstream infections.
Citation: Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511.
Simulation Training Improves Lumbar Puncture Skills
Clinical question: What effect does simulation have on lumbar puncture (LP) skills of PGY1 internal-medicine (IM) residents compared with PGY2-4 neurology residents who have not received simulation training?
Background: LPs are common procedures. The American College of General Medical Education does not define competency; neither do the internal-medicine (IM) or neurology board certifications. Simulation can improve skills in many areas but has not been well studied in LPs.
Study design: Pre-test-post-test.
Setting: Northwestern University’s Feinberg School of Medicine in Chicago.
Synopsis: The intervention group included 58 PGY1 IM residents, while the control group was 49 PGY2-to-PGY4 neurology residents. The pre-test consisted of a 21-point checklist. IM residents watched a three-hour video, performed LPs on simulators, and received feedback. The post-test was a clinical skills examination using the checklist. If this exam was failed, the participant practiced and was retested. Neurology residents completed the pre-test and demonstrated an LP using the simulator.
Pre-test passing was achieved by only 2% of IM residents and 6% of neurology residents. Post-test passing was achieved by 95% of the IM residents on the first trial and 100% of IM residents after an hour of additional training. IM mean scores increased to 95.7% from 46.3%, while the mean score of neurology residents was 65.4%.
This study is limited by its single-center nature, as education is variable from center to center. The study evaluated the proficiency on simulators only, and it did not evaluate the proficiency of the participants on patients.
Bottom line: Simulation training improves lumbar puncture skills.
Citation: Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137.
Primary-Care Physicians Do Not Use Publicly Reported Data When Referring Patients to Hospitals
Clinical question: When referring patients with pneumonia to the hospital, what factors do primary-care physicians (PCPs) consider?
Background: Publicly reported data are widely available. Pneumonia has publicly reported quality measures and is a common reason for hospitalization. Fewer PCPs are attending in the hospital due to the hospitalist movement; therefore, PCPs refer patients to a hospital when the need arises.
Study design: Online survey.
Setting: PCPs within 10 miles of Springfield, Mass.
Synopsis: A total of 92 PCPs responded to the survey, which included presentation of a case regarding a patient with pneumonia. PCPs were asked the importance of multiple factors leading to their decision to refer to a hospital. Familiarity with the hospital (70%), patient preference (62%), and admitting arrangements with a hospitalist group (62%) were considered to be very important to the PCPs that responded to the survey. Publicly reported data were very important to only 18% of respondents, and zero reported using publicly reported data when referring patients.
Importance of specific quality measures also was queried; antibiotics given within six hours of arrival (66%), appropriate choice of antibiotics (63%), and blood cultures prior to antibiotic administration (51%) were very important to respondents. Prestige, such as magnet status and U.S. News and World Report “Best Hospital” status, were deemed important by about 40% of PCPs.
Bottom line: Despite the availability of publicly reported data, PCPs do not use this information to refer patients to the hospital.
Citation: Morsi E, Lindenauer PK, Rothberg MB. Primary care physicians’ use of publicly reported quality data in hospital referral decisions. J Hosp Med. 2012;7(5):370-375.
What Works for Medication Reconciliation?
Clinical question: What are the most effective practices for medication reconciliation in the hospital setting?
Background: Medication discrepancies are common, occurring in as many as 70% of patients at hospital admission or discharge. Up to a third of these discrepancies have potential to cause patient harm, including prolonged hospital stays, ED visits, hospital recidivism, and use of other healthcare resources. Medication reconciliation (“med rec”) is a strategy for reducing these errors, though previous literature has not systematically reviewed best practices for hospital-based med rec.
Study design: Systematic review of literature.
Setting: Controlled studies from the U.S., Canada, Australia, New Zealand, Northern Ireland, United Kingdom, Belgium, Denmark, the Netherlands, and Sweden.
Synopsis: Investigators identified 26 controlled studies using a systematic search of English-language articles on med rec during inpatient hospitalizations published between Jan. 1, 1966, and Oct. 31, 2010. Fifteen studies reported on pharmacist-related interventions; six reported on technology-specific interventions; and five reported on other types of interventions, including staff education and use of standardized med-rec tools.
Analysis of these studies revealed that all of these interventions successfully decreased medication discrepancies and potential adverse drug events, but there was inconsistent benefit with regard to adverse drug events and healthcare utilization compared with usual care. The literature was most supportive of pharmacist-related interventions, including but not limited to comprehensive medication history at admission, med rec at discharge, patient counseling, discharge communication with outpatient providers, and post-discharge communication with the patient and post-hospital providers.
Bottom line: Successful med rec requires multiple interventions at various transitions of care and involves a variety of medical professionals. Patient-targeted interventions, including pharmacists, have the potential to decrease errors and adverse events.
Citation: Mueller S, Sponsler K, Kripalani S, Schnipper J. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069.
Improving Stroke Alert Response Time
In‐hospital strokes account for a significant proportion of the almost 800,000 cerebrovascular accidents that occur each year in the United States.1 Although inpatient strokes are thought to be under‐recognized and under‐reported, between 4% and 17% of all stroke patients in the hospital experienced stroke onset during hospitalization.2, 3 Estimates place the number of in‐hospital strokes at 35,000‐75,000 each year in the United States.4
As a result of the exquisite sensitivity of brain tissue to ischemic events, stroke is a medical emergency and time‐to‐treatment is of the essence. With each minute of ischemia, 1.9 million neurons are destroyed.5 Evidence suggests benefit of treatment with intravenous thrombolysis up to 4.5 hours after symptom onset, with lower disability associated with more rapid initiation of therapy.6, 7 To facilitate timely thrombolytic therapy, the American Stroke Association (ASA) recommends that imaging of the brain be initiated within 25 minutes of presentation for patients with suspected stroke.8
Studies demonstrate greater delays in the evaluation of hospitalized patients suffering from stroke compared to stroke patients presenting to the Emergency Department (ED).9, 10 Performance of timely evaluation of in‐hospital stroke rarely meets ASA goals. Analysis of a Michigan stroke registry found that only 3.1% of patients with in‐hospital strokes received computed tomography (CT) scan within 25 minutes of symptom recognition, and a Colorado stroke registry found time‐to‐evaluation to be more than twice the recommended benchmark.11, 12 Data from a multicenter stroke registry in Spain showed that half of all thrombolysis‐eligible, in‐hospital stroke patients could not be treated due to delays in evaluation.13
Our prior work demonstrated that the use of an in‐hospital stroke response team significantly reduced time to evaluation for true ischemic strokes.10 Even with this rapid response mechanism, the evaluation time for in‐hospital stroke was still more than twice that observed in the ED despite using the same team to respond to both settings. Hospital rapid response systems, specifically for patients with suspected stroke, have been described in the literature and outline in‐hospital response systems capable of meeting evaluation time goals.1415 How to optimize a stroke response system has not been previously described. The aim of this quality improvement (QI) initiative was to reduce time‐to‐evaluation for strokes occurring in patients already hospitalized using systems analysis and modification. We describe key elements and tools for implementing institutional QI for in‐hospital stroke.
METHODS
The QI initiative was implemented at the University of Colorado Hospital (UCH), a tertiary care academic medical center. The Colorado Multiple Institutional Review Board determined this project to be in the exempt category. UCH uses a protocol in which all stroke alerts undergo non‐contrast CT of the brain. If no intracranial bleeding is found, and the patient is a thrombolytic candidate, advanced CT imaging including CT perfusion and CT angiogram will also be performed during the alert. Magnetic resonance imaging (MRI) with diffusion weighted imaging is done non‐emergently for subsequent stroke evaluation, but is not part of the stroke alert protocol. The primary endpoint of time from alert to initiation of CT was chosen because it represents an unambiguous interval which is present for all stroke alerts. Pre‐intervention data was gathered for 6 months, from September 2008 to February 2009. During this period, the process through which in‐hospital strokes were identified, referred for evaluation, and treated was mapped to identify inefficient or unreliable steps, and the process was redesigned to enhance efficiency. The intervention was rolled out over a 3‐month period from March 2009 to May 2009. During the intervention roll‐out period, the refined stroke alert process and a checklist containing the optimal in‐hospital stroke alert response system was implemented. An education campaign was initiated, for acute stroke team members and nursing staff, on signs of stroke and each individual's role in response to symptoms of in‐hospital stroke based on the new process. During the roll‐out period, each unit in the hospital was provided in‐hospital stroke alert posters and a packet containing specific stroke education on the in‐hospital stroke alert process. Unit educators were empowered to determine how to best deliver the education to their staff, and many chose to invite the stroke program coordinator to give an hour‐long presentation on stroke prior to shift or during lunch. Each unit educator kept record of the stroke instruction provided and submitted staff signatures to the stroke program. Nursing staff was also provided with in‐hospital stroke protocol badge cards that outlined optimal approach to stroke identification and treatment using the revised protocol. Interventions were being implemented in a progressive fashion throughout the roll‐out period. Starting during the roll‐out and continuing into the post‐intervention period, feedback on all in‐hospital stroke alerts was provided to the stroke team and front‐line providers. The impact of the intervention was followed for 6 months post‐intervention from June 2009 to November 2009. The QI tools used in this project are well described by the Institute of Healthcare Improvement, and each step in the QI process is outlined in detail below:16
Step 1: Process Map With Identification of Unreliable and Reliably Slow Steps
A detailed process map was created to outline steps in the existing stroke alert process (see Supporting Figures, Process Maps, in the online version of this article). One investigator (R.Z.) interviewed key members of the multidisciplinary stroke team, including representatives from the departments of neurology, nursing, hospital medicine, neurosurgery, radiology, and transportation. Interviews with key stakeholders and frequent participants in stroke alerts revealed evidence of episodic unreliable steps. Stakeholders were noted to have slightly different conceptions of how the process flow was intended to occur, and where responsibility lay for certain tasks. The interviews aided in identification of pitfalls, bottlenecks, misconceptions, and areas that needed clarification or change in the alert process.
Examples of unreliable and bottleneck steps include: In the pre‐intervention process, the transportation department was responsible for moving patients to radiology; this step was identified as reliably slow. Investigation revealed that the transportation department did not have a mechanism for rapid response to emergency transport requests. Analysis also revealed that 2 key steps necessary for treating in‐hospital stroke were occasionally neglected: ensuring adequate intravenous (IV) access, and ordering of the correct panel of laboratory tests. Finally, a process communication deficit was identified, with CT technicians periodically unaware of the pending arrival of an in‐hospital stroke patient, thus preventing the scan from being cleared for the emergent stroke imaging.
Direct observation of real‐time stroke alerts in both the inpatient and ED settings was also employed to outline the process and identify areas of inefficiency. Direct observation of stroke alerts in progress verified the unified picture of process flow developed from stakeholder interviews (see Supporting Figures, Process Maps, in the online version of this article). Particular note was made of differences between the stroke alert process in the ED and the inpatient setting.
Step 2: System Redesign With Input From All Stakeholders
Proposed interventions were presented to hospital governing councils, including the interdisciplinary Stroke Council and Nurse Managers Council. After verification of the shortcomings of the existing alert process and obtaining buy‐in from key participants and governing departments, a new process was designed (see Supporting Figures, Process Maps, in the online version of this article). Specific changes include the following examples: First, electrocardiogram was moved to occur after CT scan. Second, investigation revealed that the transportation department within the hospital was designed for non‐emergent transportation and not amenable to change. The mechanism of patient transportation was changed such that, rather than using the transportation department, patients were now transported by the neurology resident responding to the stroke alert, accompanied by the patient's ward nurse. This both removed a bottleneck step and assured critical staff presence during the transportation of a potentially unstable patient. Third, to ensure effective communication, CT technicians were provided with stroke alert pagers that receive text messages regarding incoming in‐hospital stroke alert patients. Fourth, a time limit was set for IV attempts prior to transportation. The new protocol, along with explicit expectations for the role of the patient's nurse in in‐hospital stroke alerts, was described in a hospital‐wide nursing stroke education initiative.
Step 3: In‐Hospital Stroke Alert Checklist
A new standardized protocol for optimal in‐hospital stroke care was detailed on a laminated pocket card. The checklist described exactly what steps were to be performed, by whom, how to make them occur, and in what order. The checklist was designed to reduce the incidence of omitted steps, such as ordering of correct laboratory evaluations. The laminated cards highlighted the benchmark time to evaluation of 25 minutes. Process checklist cards were distributed to all members of the acute stroke alert response team, and short versions designed specifically for nursing staff were distributed as badge cards and posted on clinical care units (Supporting Information Appendix I).
Step 4: Real‐Time Feedback
During the intervention roll‐out and post‐intervention periods, feedback was provided from the stroke program to the front‐line providers following each in‐hospital stroke alert. The clinicians involved were notified of the final diagnosis and patient outcome, and were provided with feedback about how the patient's evaluation times compared with benchmark goals. Feedback may serve to motivate, based on clinician professionalism, but performance in the alert was not tied to rewards or penalties for the providers involved. The feedback process was designed to be bi‐directional, with requests for input from staff on barriers to rapid evaluation experienced and suggestions for future process improvement (Supporting Information Appendix II).
Statistical Analysis
The primary outcome was the change in time from stroke alert to CT scan (alert‐to‐CT), comparing pre‐intervention and post‐intervention periods. This time interval was chosen because its calculation involved unambiguous time points, which are available for all patients for whom an in‐hospital alert is called. It is a measure of process efficiency, with minimal expected variation based on differences in patient characteristics (ie, hemorrhagic vs ischemic stroke). Non‐overlapping Kaplan‐Meier curves confirmed the proportional hazards assumption for 2 Cox proportional hazards models: unadjusted and adjusted by group characteristics with P‐value <0.10. Relative hazards and estimates for the percent of patients with alert‐to‐CT scan 25 minutes, according to intervention groups, were obtained from these models. For analyses, admit unit was re‐categorized as intensive care unit (ICU), Med/Surg, or Other. Analyses were conducted using SAS Version 9.2 (SAS Institute, Inc, Cary, NC).
RESULTS
During the study intervals, there were 82 inpatient stroke alerts. Of these alerts, 75 were included in the analysis. Seven were excluded for the following reasons: alert canceled by the stroke team (3), time of alert was not recorded (1), patient identifiers not recorded (1), or stroke alert was preceded by CT imaging (2).
During the 6 months prior to intervention, the median inpatient stroke alert‐to‐CT time (n = 31) was 69.0 minutes (Table 1). Nineteen percent of these alerts met the goal of 25 minutes from alert‐to‐CT time. During the 6‐month post‐intervention period, the median inpatient alert‐to‐CT time (n = 44) was 29.5 minutes. Thirty‐two percent of these alerts met the 25‐minute alert‐to‐CT time benchmark. In the unadjusted model, patients during the post‐intervention period were significantly more likely to have alert‐to‐CT scan time 25 minutes compared to patients prior to the intervention (post‐intervention compared to pre‐intervention, Relative Hazard (RH): 3.03; 95% confidence interval [CI]: 1.76‐5.20; log‐rank P < 0.0001). This remained significant after adjustment for hyperlipidemia, active cancer, final diagnosis of ischemic brain injury, and final diagnosis of stroke mimic (RH: 4.96; 95% CI: 2.65‐9.32; P < 0.0001); data not shown. Admit unit was not included in the adjusted model since there was no indication of differences in the 3‐level variable according to intervention group (P = 0.27). In addition to reduction in median response times, the variability of response times was markedly reduced, and no patient in the 6‐month post‐intervention period had delay to CT sufficient to preclude use of IV thrombolysis (Figure 1).
| Pre‐Intervention (n = 31) | Post‐Intervention (n = 44) | P Value | |
|---|---|---|---|
| |||
| Stroke alert to CT time, median [95% CI] | 69 min [34, 103] | 29.5 min [26, 40] | P < 0.0001 |
| Age, median [IQR] | 61.0 [54.0, 70.0] | 60.5 [48.5, 70.5] | 0.94 |
| Female (%) | 19 (61.3) | 23 (52.3) | 0.44 |
| Race (%) | |||
| Asian | 1 (3.2) | 1 (2.3) | 0.31 |
| Black | 4 (12.9) | 6 (13.6) | |
| Caucasian | 21 (67.7) | 27 (61.4) | |
| Hispanic | 3 (9.7) | 10 (22.7) | |
| Unknown | 2 (6.5) | 0 (0) | |
| Admit unit (%) | |||
| Intensive care | 12 (38.7) | 10 (22.7) | 0.07 |
| Medicine/surgery | 15 (48.4) | 24 (54.6) | |
| Neurology | 0 (0) | 5 (11.4) | |
| Post‐acute care | 3 (9.7) | 0 (0) | |
| Rehabilitation | 1 (3.2) | 2 (4.6) | |
| Women's and maternal care | 0 (0) | 2 (4.6) | |
| Cardiology | 0 (0) | 1 (2.3) | |
| Case mix index, median [IQR] | n = 29 2.6 [1.1, 5.0] | n = 42 2.2 [1.6, 4.5] | 0.82 |
| Prior cerebrovascular accident (%) | 5 (16.1) | 8 (18.2) | 0.82 |
| Hypertension (%) | 17 (54.8) | 24 (54.6) | 0.98 |
| Diabetes mellitus (%) | 7 (22.6) | 11 (25.0) | 0.81 |
| Hyperlipidemia (%) | 15 (48.4) | 9 (20.5) | 0.01 |
| Tobacco abuse, current (%) | 4 (12.9) | 1 (2.3) | 0.15 |
| Alcohol abuse (%) | 2 (6.5) | 0 (0) | 0.17 |
| Active cancer (%) | 8 (25.8) | 5 (11.4) | 0.10 |
| Peripheral vascular disease (%) | 2 (6.5) | 3 (6.8) | 1.0 |
| Coronary artery disease (%) | 6 (19.4) | 7 (15.9) | 0.70 |
| Congestive heart failure (%) | n = 30 5 (16.7) | 4 (9.1) | 0.47 |
| Valvulopathy (%) | 0 (0) | 1 (2.3) | 1.0 |
| Atrial fibrillation (%) | 3 (9.7) | 10 (22.7) | 0.14 |
| Anticoagulation (%) | 7 (22.6) | 7 (15.9) | 0.47 |
| Final diagnosis ischemic brain injury (%) | 15 (48.4) | 11 (25.0) | 0.04 |
| Final diagnosis hemorrhagic brain injury (%) | 3 (9.7) | 4 (9.1) | 1.0 |
| Final diagnosis stroke mimic (symptoms not due to ischemic or hemorrhagic brain injury) (%) | 13 (41.9) | 29 (65.9) | 0.04 |
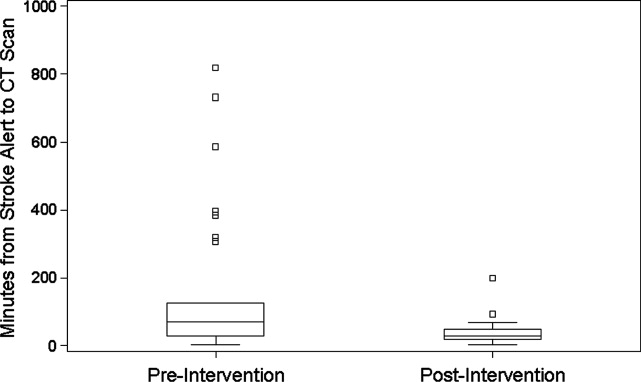
CONCLUSIONS
In‐hospital strokes represent an emergency for which response time is critical. Neurologic injury progresses with every minute of ischemia, and current recommendations offer a limited time window for intravenous thrombolysis. For stroke with symptom onset in the monitored setting of the hospital, there is a compelling imperative to reduce all delays from system inefficiencies. The findings of the current QI initiative suggest that dramatic improvements are possible through systematic evaluation and redesign of hospital response processes, a checklist for in‐hospital stroke carried by front‐line responders, and ongoing real‐time feedback.
Limitations of this study include a prepost design. The necessity of implementing system change hospital‐wide precluded use of a concurrent control group. The time goals for evaluation are derived from American Stroke Association targets for patients arriving in the Emergency Department. There are differences in process between the hospital ward and the Emergency Department, but the fundamental concept of minimizing time to evaluation once patient symptoms are recognized by hospital staff remains valid.
The possibility of system improvements not due to this QI initiative cannot be excluded. In 2006, this hospital expanded the responsibility of the stroke response team to include acute neurologic deficits outside of the ED without other changes to the in‐hospital stroke alert process. This reduced time to evaluation for in‐hospital ischemic strokes compared to usual care, but even with the same acute stroke response team responding to stroke alerts in both settings, in‐hospital stroke response times remained significantly longer than response times for stroke in the ED.10 The presence of an in‐hospital stroke alert response team alone was not capable of reducing evaluation times to goal. Minimal improvement in median in‐hospital stroke alert evaluation time was seen in the intervening year, following the completion of our previously published analysis, suggesting explicit system QI was necessary.
The Hawthorne effect, in which individuals who know they are being observed modify behavior while such monitoring is in effect, is a major limitation of interpreting QI initiatives. By committing to continuous and ongoing feedback to front‐line providers, this phenomenon can be harnessed to sustain improvement.17 In effect, the study of efficient response to the in‐hospital stroke never ceases. UCH has continued to employ the post‐intervention stroke alert protocol and engage in ongoing feedback after each stroke alert. In the 12 months following the conclusion of this study, the median response time to in‐hospital strokes continues to be 30 minutes, and 7 additional in‐hospital stroke patients have been treated with thrombolysis.
This inpatient stroke alert initiative decreased median inpatient alert‐to‐CT time by 57%, and demonstrates that quality of in‐hospital stroke care can be improved. Decrease in stroke alert‐to‐CT time facilitates earlier thrombolytic therapy. Analysis of treatment and patient outcomes was outside of the scope of the current study, but earlier treatment has potential to significantly improve clinical outcomes.
The Society of Hospital Medicine defines one of the goals of QI to be the change in processes with reduction in variation, thus improving the care for all patients rather than focusing exclusively on outlier events.18 This initiative markedly reduced evaluation variability, allowing a greater percentage of patients to be eligible for treatment within the critical time window. Prior to the intervention, almost a quarter of patients had delays in evaluation sufficient to preclude IV thrombolysis, whereas in the 6 months after the intervention was initiated, not a single patient had evaluation delayed to the point that IV thrombolysis would not have been an option (Figure 1). The goal of in‐hospital stroke QI must be to improve the speed of the process for all patients, and assure that no patient is denied the potential for therapy as a result of inefficiencies in hospital systems.
Acknowledgements
The authors thank Traci Yamashita, PRA, for her work in the statistical analysis for this publication, and Dr Jeffrey Glasheen for development of the University of Colorado Hospital's Hospitalist Training Track Quality Improvement Program of which this work is a product.
- ,,, et al.Heart disease and stroke statistics—2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,.Characteristics of in‐hospital onset ischemic stroke.Eur Neurol.2006;55:155–159.
- ,.Inpatient and community ischemic strokes in a community hospital.Neuroepidemiology.2007;28:86–92.
- .In‐hospital stroke.Lancet Neurol.2003;2:741–746.
- .Time is brain‐quantified.Stroke.2006;37:263–266.
- ,,, et al.Ultra‐early thrombolysis in acute ischemic stroke is associated with better outcomes and lower mortality.Stroke.2010;41:712–716.
- ,,,.Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association.Stroke.2009;40:2945–2948.
- ,,, et al.Guidelines for early management of adults with ischemic stroke.Stroke.2007;38;1655–1711.
- ,,, et al.In‐hospital stroke treated with intravenous tissue plasminogen activator.Stroke.2008;39:2614–2616.
- ,,,,.Stroke alert program improves recognition and evaluation time of in‐hospital ischemic stroke.J Stroke Cerebrovasc Dis.2009;19:494–496.
- ,,,,,.In‐hospital stroke in a statewide stroke registry.Cerebrovasc Dis.2008;25:12–20.
- ,,,,.Quality of care for in‐hospital stroke: analysis of a statewide registry.Stroke.2011;42:207–210.
- ,,, et al.In‐hospital stroke: a multi‐center prospective registry.Eur J Neurol.2011;18:170–176.
- ,,.Code Gray—an organized approach to inpatient stroke.Crit Care Nurs Q.2003;26:296–302.
- ,,.ID, stat‐rapid response to in‐hospital stroke patients.Nurs Manage.2009;40:34–38.
- Institute of Healthcare Improvement. Quality Improvement Tools. Available at: http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/Tools/. Accessed December 1,2010.
- ,,,,,.Variability in the Hawthorne effect with regard to hand hygiene performance in high‐ and low‐performing inpatient care units.Infect Control Hosp Epidemiol.2009;30:222–225.
- Society of Hospital Medicine Quality Improvement Resources. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/32. Accessed December 1,2010.
In‐hospital strokes account for a significant proportion of the almost 800,000 cerebrovascular accidents that occur each year in the United States.1 Although inpatient strokes are thought to be under‐recognized and under‐reported, between 4% and 17% of all stroke patients in the hospital experienced stroke onset during hospitalization.2, 3 Estimates place the number of in‐hospital strokes at 35,000‐75,000 each year in the United States.4
As a result of the exquisite sensitivity of brain tissue to ischemic events, stroke is a medical emergency and time‐to‐treatment is of the essence. With each minute of ischemia, 1.9 million neurons are destroyed.5 Evidence suggests benefit of treatment with intravenous thrombolysis up to 4.5 hours after symptom onset, with lower disability associated with more rapid initiation of therapy.6, 7 To facilitate timely thrombolytic therapy, the American Stroke Association (ASA) recommends that imaging of the brain be initiated within 25 minutes of presentation for patients with suspected stroke.8
Studies demonstrate greater delays in the evaluation of hospitalized patients suffering from stroke compared to stroke patients presenting to the Emergency Department (ED).9, 10 Performance of timely evaluation of in‐hospital stroke rarely meets ASA goals. Analysis of a Michigan stroke registry found that only 3.1% of patients with in‐hospital strokes received computed tomography (CT) scan within 25 minutes of symptom recognition, and a Colorado stroke registry found time‐to‐evaluation to be more than twice the recommended benchmark.11, 12 Data from a multicenter stroke registry in Spain showed that half of all thrombolysis‐eligible, in‐hospital stroke patients could not be treated due to delays in evaluation.13
Our prior work demonstrated that the use of an in‐hospital stroke response team significantly reduced time to evaluation for true ischemic strokes.10 Even with this rapid response mechanism, the evaluation time for in‐hospital stroke was still more than twice that observed in the ED despite using the same team to respond to both settings. Hospital rapid response systems, specifically for patients with suspected stroke, have been described in the literature and outline in‐hospital response systems capable of meeting evaluation time goals.1415 How to optimize a stroke response system has not been previously described. The aim of this quality improvement (QI) initiative was to reduce time‐to‐evaluation for strokes occurring in patients already hospitalized using systems analysis and modification. We describe key elements and tools for implementing institutional QI for in‐hospital stroke.
METHODS
The QI initiative was implemented at the University of Colorado Hospital (UCH), a tertiary care academic medical center. The Colorado Multiple Institutional Review Board determined this project to be in the exempt category. UCH uses a protocol in which all stroke alerts undergo non‐contrast CT of the brain. If no intracranial bleeding is found, and the patient is a thrombolytic candidate, advanced CT imaging including CT perfusion and CT angiogram will also be performed during the alert. Magnetic resonance imaging (MRI) with diffusion weighted imaging is done non‐emergently for subsequent stroke evaluation, but is not part of the stroke alert protocol. The primary endpoint of time from alert to initiation of CT was chosen because it represents an unambiguous interval which is present for all stroke alerts. Pre‐intervention data was gathered for 6 months, from September 2008 to February 2009. During this period, the process through which in‐hospital strokes were identified, referred for evaluation, and treated was mapped to identify inefficient or unreliable steps, and the process was redesigned to enhance efficiency. The intervention was rolled out over a 3‐month period from March 2009 to May 2009. During the intervention roll‐out period, the refined stroke alert process and a checklist containing the optimal in‐hospital stroke alert response system was implemented. An education campaign was initiated, for acute stroke team members and nursing staff, on signs of stroke and each individual's role in response to symptoms of in‐hospital stroke based on the new process. During the roll‐out period, each unit in the hospital was provided in‐hospital stroke alert posters and a packet containing specific stroke education on the in‐hospital stroke alert process. Unit educators were empowered to determine how to best deliver the education to their staff, and many chose to invite the stroke program coordinator to give an hour‐long presentation on stroke prior to shift or during lunch. Each unit educator kept record of the stroke instruction provided and submitted staff signatures to the stroke program. Nursing staff was also provided with in‐hospital stroke protocol badge cards that outlined optimal approach to stroke identification and treatment using the revised protocol. Interventions were being implemented in a progressive fashion throughout the roll‐out period. Starting during the roll‐out and continuing into the post‐intervention period, feedback on all in‐hospital stroke alerts was provided to the stroke team and front‐line providers. The impact of the intervention was followed for 6 months post‐intervention from June 2009 to November 2009. The QI tools used in this project are well described by the Institute of Healthcare Improvement, and each step in the QI process is outlined in detail below:16
Step 1: Process Map With Identification of Unreliable and Reliably Slow Steps
A detailed process map was created to outline steps in the existing stroke alert process (see Supporting Figures, Process Maps, in the online version of this article). One investigator (R.Z.) interviewed key members of the multidisciplinary stroke team, including representatives from the departments of neurology, nursing, hospital medicine, neurosurgery, radiology, and transportation. Interviews with key stakeholders and frequent participants in stroke alerts revealed evidence of episodic unreliable steps. Stakeholders were noted to have slightly different conceptions of how the process flow was intended to occur, and where responsibility lay for certain tasks. The interviews aided in identification of pitfalls, bottlenecks, misconceptions, and areas that needed clarification or change in the alert process.
Examples of unreliable and bottleneck steps include: In the pre‐intervention process, the transportation department was responsible for moving patients to radiology; this step was identified as reliably slow. Investigation revealed that the transportation department did not have a mechanism for rapid response to emergency transport requests. Analysis also revealed that 2 key steps necessary for treating in‐hospital stroke were occasionally neglected: ensuring adequate intravenous (IV) access, and ordering of the correct panel of laboratory tests. Finally, a process communication deficit was identified, with CT technicians periodically unaware of the pending arrival of an in‐hospital stroke patient, thus preventing the scan from being cleared for the emergent stroke imaging.
Direct observation of real‐time stroke alerts in both the inpatient and ED settings was also employed to outline the process and identify areas of inefficiency. Direct observation of stroke alerts in progress verified the unified picture of process flow developed from stakeholder interviews (see Supporting Figures, Process Maps, in the online version of this article). Particular note was made of differences between the stroke alert process in the ED and the inpatient setting.
Step 2: System Redesign With Input From All Stakeholders
Proposed interventions were presented to hospital governing councils, including the interdisciplinary Stroke Council and Nurse Managers Council. After verification of the shortcomings of the existing alert process and obtaining buy‐in from key participants and governing departments, a new process was designed (see Supporting Figures, Process Maps, in the online version of this article). Specific changes include the following examples: First, electrocardiogram was moved to occur after CT scan. Second, investigation revealed that the transportation department within the hospital was designed for non‐emergent transportation and not amenable to change. The mechanism of patient transportation was changed such that, rather than using the transportation department, patients were now transported by the neurology resident responding to the stroke alert, accompanied by the patient's ward nurse. This both removed a bottleneck step and assured critical staff presence during the transportation of a potentially unstable patient. Third, to ensure effective communication, CT technicians were provided with stroke alert pagers that receive text messages regarding incoming in‐hospital stroke alert patients. Fourth, a time limit was set for IV attempts prior to transportation. The new protocol, along with explicit expectations for the role of the patient's nurse in in‐hospital stroke alerts, was described in a hospital‐wide nursing stroke education initiative.
Step 3: In‐Hospital Stroke Alert Checklist
A new standardized protocol for optimal in‐hospital stroke care was detailed on a laminated pocket card. The checklist described exactly what steps were to be performed, by whom, how to make them occur, and in what order. The checklist was designed to reduce the incidence of omitted steps, such as ordering of correct laboratory evaluations. The laminated cards highlighted the benchmark time to evaluation of 25 minutes. Process checklist cards were distributed to all members of the acute stroke alert response team, and short versions designed specifically for nursing staff were distributed as badge cards and posted on clinical care units (Supporting Information Appendix I).
Step 4: Real‐Time Feedback
During the intervention roll‐out and post‐intervention periods, feedback was provided from the stroke program to the front‐line providers following each in‐hospital stroke alert. The clinicians involved were notified of the final diagnosis and patient outcome, and were provided with feedback about how the patient's evaluation times compared with benchmark goals. Feedback may serve to motivate, based on clinician professionalism, but performance in the alert was not tied to rewards or penalties for the providers involved. The feedback process was designed to be bi‐directional, with requests for input from staff on barriers to rapid evaluation experienced and suggestions for future process improvement (Supporting Information Appendix II).
Statistical Analysis
The primary outcome was the change in time from stroke alert to CT scan (alert‐to‐CT), comparing pre‐intervention and post‐intervention periods. This time interval was chosen because its calculation involved unambiguous time points, which are available for all patients for whom an in‐hospital alert is called. It is a measure of process efficiency, with minimal expected variation based on differences in patient characteristics (ie, hemorrhagic vs ischemic stroke). Non‐overlapping Kaplan‐Meier curves confirmed the proportional hazards assumption for 2 Cox proportional hazards models: unadjusted and adjusted by group characteristics with P‐value <0.10. Relative hazards and estimates for the percent of patients with alert‐to‐CT scan 25 minutes, according to intervention groups, were obtained from these models. For analyses, admit unit was re‐categorized as intensive care unit (ICU), Med/Surg, or Other. Analyses were conducted using SAS Version 9.2 (SAS Institute, Inc, Cary, NC).
RESULTS
During the study intervals, there were 82 inpatient stroke alerts. Of these alerts, 75 were included in the analysis. Seven were excluded for the following reasons: alert canceled by the stroke team (3), time of alert was not recorded (1), patient identifiers not recorded (1), or stroke alert was preceded by CT imaging (2).
During the 6 months prior to intervention, the median inpatient stroke alert‐to‐CT time (n = 31) was 69.0 minutes (Table 1). Nineteen percent of these alerts met the goal of 25 minutes from alert‐to‐CT time. During the 6‐month post‐intervention period, the median inpatient alert‐to‐CT time (n = 44) was 29.5 minutes. Thirty‐two percent of these alerts met the 25‐minute alert‐to‐CT time benchmark. In the unadjusted model, patients during the post‐intervention period were significantly more likely to have alert‐to‐CT scan time 25 minutes compared to patients prior to the intervention (post‐intervention compared to pre‐intervention, Relative Hazard (RH): 3.03; 95% confidence interval [CI]: 1.76‐5.20; log‐rank P < 0.0001). This remained significant after adjustment for hyperlipidemia, active cancer, final diagnosis of ischemic brain injury, and final diagnosis of stroke mimic (RH: 4.96; 95% CI: 2.65‐9.32; P < 0.0001); data not shown. Admit unit was not included in the adjusted model since there was no indication of differences in the 3‐level variable according to intervention group (P = 0.27). In addition to reduction in median response times, the variability of response times was markedly reduced, and no patient in the 6‐month post‐intervention period had delay to CT sufficient to preclude use of IV thrombolysis (Figure 1).
| Pre‐Intervention (n = 31) | Post‐Intervention (n = 44) | P Value | |
|---|---|---|---|
| |||
| Stroke alert to CT time, median [95% CI] | 69 min [34, 103] | 29.5 min [26, 40] | P < 0.0001 |
| Age, median [IQR] | 61.0 [54.0, 70.0] | 60.5 [48.5, 70.5] | 0.94 |
| Female (%) | 19 (61.3) | 23 (52.3) | 0.44 |
| Race (%) | |||
| Asian | 1 (3.2) | 1 (2.3) | 0.31 |
| Black | 4 (12.9) | 6 (13.6) | |
| Caucasian | 21 (67.7) | 27 (61.4) | |
| Hispanic | 3 (9.7) | 10 (22.7) | |
| Unknown | 2 (6.5) | 0 (0) | |
| Admit unit (%) | |||
| Intensive care | 12 (38.7) | 10 (22.7) | 0.07 |
| Medicine/surgery | 15 (48.4) | 24 (54.6) | |
| Neurology | 0 (0) | 5 (11.4) | |
| Post‐acute care | 3 (9.7) | 0 (0) | |
| Rehabilitation | 1 (3.2) | 2 (4.6) | |
| Women's and maternal care | 0 (0) | 2 (4.6) | |
| Cardiology | 0 (0) | 1 (2.3) | |
| Case mix index, median [IQR] | n = 29 2.6 [1.1, 5.0] | n = 42 2.2 [1.6, 4.5] | 0.82 |
| Prior cerebrovascular accident (%) | 5 (16.1) | 8 (18.2) | 0.82 |
| Hypertension (%) | 17 (54.8) | 24 (54.6) | 0.98 |
| Diabetes mellitus (%) | 7 (22.6) | 11 (25.0) | 0.81 |
| Hyperlipidemia (%) | 15 (48.4) | 9 (20.5) | 0.01 |
| Tobacco abuse, current (%) | 4 (12.9) | 1 (2.3) | 0.15 |
| Alcohol abuse (%) | 2 (6.5) | 0 (0) | 0.17 |
| Active cancer (%) | 8 (25.8) | 5 (11.4) | 0.10 |
| Peripheral vascular disease (%) | 2 (6.5) | 3 (6.8) | 1.0 |
| Coronary artery disease (%) | 6 (19.4) | 7 (15.9) | 0.70 |
| Congestive heart failure (%) | n = 30 5 (16.7) | 4 (9.1) | 0.47 |
| Valvulopathy (%) | 0 (0) | 1 (2.3) | 1.0 |
| Atrial fibrillation (%) | 3 (9.7) | 10 (22.7) | 0.14 |
| Anticoagulation (%) | 7 (22.6) | 7 (15.9) | 0.47 |
| Final diagnosis ischemic brain injury (%) | 15 (48.4) | 11 (25.0) | 0.04 |
| Final diagnosis hemorrhagic brain injury (%) | 3 (9.7) | 4 (9.1) | 1.0 |
| Final diagnosis stroke mimic (symptoms not due to ischemic or hemorrhagic brain injury) (%) | 13 (41.9) | 29 (65.9) | 0.04 |

CONCLUSIONS
In‐hospital strokes represent an emergency for which response time is critical. Neurologic injury progresses with every minute of ischemia, and current recommendations offer a limited time window for intravenous thrombolysis. For stroke with symptom onset in the monitored setting of the hospital, there is a compelling imperative to reduce all delays from system inefficiencies. The findings of the current QI initiative suggest that dramatic improvements are possible through systematic evaluation and redesign of hospital response processes, a checklist for in‐hospital stroke carried by front‐line responders, and ongoing real‐time feedback.
Limitations of this study include a prepost design. The necessity of implementing system change hospital‐wide precluded use of a concurrent control group. The time goals for evaluation are derived from American Stroke Association targets for patients arriving in the Emergency Department. There are differences in process between the hospital ward and the Emergency Department, but the fundamental concept of minimizing time to evaluation once patient symptoms are recognized by hospital staff remains valid.
The possibility of system improvements not due to this QI initiative cannot be excluded. In 2006, this hospital expanded the responsibility of the stroke response team to include acute neurologic deficits outside of the ED without other changes to the in‐hospital stroke alert process. This reduced time to evaluation for in‐hospital ischemic strokes compared to usual care, but even with the same acute stroke response team responding to stroke alerts in both settings, in‐hospital stroke response times remained significantly longer than response times for stroke in the ED.10 The presence of an in‐hospital stroke alert response team alone was not capable of reducing evaluation times to goal. Minimal improvement in median in‐hospital stroke alert evaluation time was seen in the intervening year, following the completion of our previously published analysis, suggesting explicit system QI was necessary.
The Hawthorne effect, in which individuals who know they are being observed modify behavior while such monitoring is in effect, is a major limitation of interpreting QI initiatives. By committing to continuous and ongoing feedback to front‐line providers, this phenomenon can be harnessed to sustain improvement.17 In effect, the study of efficient response to the in‐hospital stroke never ceases. UCH has continued to employ the post‐intervention stroke alert protocol and engage in ongoing feedback after each stroke alert. In the 12 months following the conclusion of this study, the median response time to in‐hospital strokes continues to be 30 minutes, and 7 additional in‐hospital stroke patients have been treated with thrombolysis.
This inpatient stroke alert initiative decreased median inpatient alert‐to‐CT time by 57%, and demonstrates that quality of in‐hospital stroke care can be improved. Decrease in stroke alert‐to‐CT time facilitates earlier thrombolytic therapy. Analysis of treatment and patient outcomes was outside of the scope of the current study, but earlier treatment has potential to significantly improve clinical outcomes.
The Society of Hospital Medicine defines one of the goals of QI to be the change in processes with reduction in variation, thus improving the care for all patients rather than focusing exclusively on outlier events.18 This initiative markedly reduced evaluation variability, allowing a greater percentage of patients to be eligible for treatment within the critical time window. Prior to the intervention, almost a quarter of patients had delays in evaluation sufficient to preclude IV thrombolysis, whereas in the 6 months after the intervention was initiated, not a single patient had evaluation delayed to the point that IV thrombolysis would not have been an option (Figure 1). The goal of in‐hospital stroke QI must be to improve the speed of the process for all patients, and assure that no patient is denied the potential for therapy as a result of inefficiencies in hospital systems.
Acknowledgements
The authors thank Traci Yamashita, PRA, for her work in the statistical analysis for this publication, and Dr Jeffrey Glasheen for development of the University of Colorado Hospital's Hospitalist Training Track Quality Improvement Program of which this work is a product.
In‐hospital strokes account for a significant proportion of the almost 800,000 cerebrovascular accidents that occur each year in the United States.1 Although inpatient strokes are thought to be under‐recognized and under‐reported, between 4% and 17% of all stroke patients in the hospital experienced stroke onset during hospitalization.2, 3 Estimates place the number of in‐hospital strokes at 35,000‐75,000 each year in the United States.4
As a result of the exquisite sensitivity of brain tissue to ischemic events, stroke is a medical emergency and time‐to‐treatment is of the essence. With each minute of ischemia, 1.9 million neurons are destroyed.5 Evidence suggests benefit of treatment with intravenous thrombolysis up to 4.5 hours after symptom onset, with lower disability associated with more rapid initiation of therapy.6, 7 To facilitate timely thrombolytic therapy, the American Stroke Association (ASA) recommends that imaging of the brain be initiated within 25 minutes of presentation for patients with suspected stroke.8
Studies demonstrate greater delays in the evaluation of hospitalized patients suffering from stroke compared to stroke patients presenting to the Emergency Department (ED).9, 10 Performance of timely evaluation of in‐hospital stroke rarely meets ASA goals. Analysis of a Michigan stroke registry found that only 3.1% of patients with in‐hospital strokes received computed tomography (CT) scan within 25 minutes of symptom recognition, and a Colorado stroke registry found time‐to‐evaluation to be more than twice the recommended benchmark.11, 12 Data from a multicenter stroke registry in Spain showed that half of all thrombolysis‐eligible, in‐hospital stroke patients could not be treated due to delays in evaluation.13
Our prior work demonstrated that the use of an in‐hospital stroke response team significantly reduced time to evaluation for true ischemic strokes.10 Even with this rapid response mechanism, the evaluation time for in‐hospital stroke was still more than twice that observed in the ED despite using the same team to respond to both settings. Hospital rapid response systems, specifically for patients with suspected stroke, have been described in the literature and outline in‐hospital response systems capable of meeting evaluation time goals.1415 How to optimize a stroke response system has not been previously described. The aim of this quality improvement (QI) initiative was to reduce time‐to‐evaluation for strokes occurring in patients already hospitalized using systems analysis and modification. We describe key elements and tools for implementing institutional QI for in‐hospital stroke.
METHODS
The QI initiative was implemented at the University of Colorado Hospital (UCH), a tertiary care academic medical center. The Colorado Multiple Institutional Review Board determined this project to be in the exempt category. UCH uses a protocol in which all stroke alerts undergo non‐contrast CT of the brain. If no intracranial bleeding is found, and the patient is a thrombolytic candidate, advanced CT imaging including CT perfusion and CT angiogram will also be performed during the alert. Magnetic resonance imaging (MRI) with diffusion weighted imaging is done non‐emergently for subsequent stroke evaluation, but is not part of the stroke alert protocol. The primary endpoint of time from alert to initiation of CT was chosen because it represents an unambiguous interval which is present for all stroke alerts. Pre‐intervention data was gathered for 6 months, from September 2008 to February 2009. During this period, the process through which in‐hospital strokes were identified, referred for evaluation, and treated was mapped to identify inefficient or unreliable steps, and the process was redesigned to enhance efficiency. The intervention was rolled out over a 3‐month period from March 2009 to May 2009. During the intervention roll‐out period, the refined stroke alert process and a checklist containing the optimal in‐hospital stroke alert response system was implemented. An education campaign was initiated, for acute stroke team members and nursing staff, on signs of stroke and each individual's role in response to symptoms of in‐hospital stroke based on the new process. During the roll‐out period, each unit in the hospital was provided in‐hospital stroke alert posters and a packet containing specific stroke education on the in‐hospital stroke alert process. Unit educators were empowered to determine how to best deliver the education to their staff, and many chose to invite the stroke program coordinator to give an hour‐long presentation on stroke prior to shift or during lunch. Each unit educator kept record of the stroke instruction provided and submitted staff signatures to the stroke program. Nursing staff was also provided with in‐hospital stroke protocol badge cards that outlined optimal approach to stroke identification and treatment using the revised protocol. Interventions were being implemented in a progressive fashion throughout the roll‐out period. Starting during the roll‐out and continuing into the post‐intervention period, feedback on all in‐hospital stroke alerts was provided to the stroke team and front‐line providers. The impact of the intervention was followed for 6 months post‐intervention from June 2009 to November 2009. The QI tools used in this project are well described by the Institute of Healthcare Improvement, and each step in the QI process is outlined in detail below:16
Step 1: Process Map With Identification of Unreliable and Reliably Slow Steps
A detailed process map was created to outline steps in the existing stroke alert process (see Supporting Figures, Process Maps, in the online version of this article). One investigator (R.Z.) interviewed key members of the multidisciplinary stroke team, including representatives from the departments of neurology, nursing, hospital medicine, neurosurgery, radiology, and transportation. Interviews with key stakeholders and frequent participants in stroke alerts revealed evidence of episodic unreliable steps. Stakeholders were noted to have slightly different conceptions of how the process flow was intended to occur, and where responsibility lay for certain tasks. The interviews aided in identification of pitfalls, bottlenecks, misconceptions, and areas that needed clarification or change in the alert process.
Examples of unreliable and bottleneck steps include: In the pre‐intervention process, the transportation department was responsible for moving patients to radiology; this step was identified as reliably slow. Investigation revealed that the transportation department did not have a mechanism for rapid response to emergency transport requests. Analysis also revealed that 2 key steps necessary for treating in‐hospital stroke were occasionally neglected: ensuring adequate intravenous (IV) access, and ordering of the correct panel of laboratory tests. Finally, a process communication deficit was identified, with CT technicians periodically unaware of the pending arrival of an in‐hospital stroke patient, thus preventing the scan from being cleared for the emergent stroke imaging.
Direct observation of real‐time stroke alerts in both the inpatient and ED settings was also employed to outline the process and identify areas of inefficiency. Direct observation of stroke alerts in progress verified the unified picture of process flow developed from stakeholder interviews (see Supporting Figures, Process Maps, in the online version of this article). Particular note was made of differences between the stroke alert process in the ED and the inpatient setting.
Step 2: System Redesign With Input From All Stakeholders
Proposed interventions were presented to hospital governing councils, including the interdisciplinary Stroke Council and Nurse Managers Council. After verification of the shortcomings of the existing alert process and obtaining buy‐in from key participants and governing departments, a new process was designed (see Supporting Figures, Process Maps, in the online version of this article). Specific changes include the following examples: First, electrocardiogram was moved to occur after CT scan. Second, investigation revealed that the transportation department within the hospital was designed for non‐emergent transportation and not amenable to change. The mechanism of patient transportation was changed such that, rather than using the transportation department, patients were now transported by the neurology resident responding to the stroke alert, accompanied by the patient's ward nurse. This both removed a bottleneck step and assured critical staff presence during the transportation of a potentially unstable patient. Third, to ensure effective communication, CT technicians were provided with stroke alert pagers that receive text messages regarding incoming in‐hospital stroke alert patients. Fourth, a time limit was set for IV attempts prior to transportation. The new protocol, along with explicit expectations for the role of the patient's nurse in in‐hospital stroke alerts, was described in a hospital‐wide nursing stroke education initiative.
Step 3: In‐Hospital Stroke Alert Checklist
A new standardized protocol for optimal in‐hospital stroke care was detailed on a laminated pocket card. The checklist described exactly what steps were to be performed, by whom, how to make them occur, and in what order. The checklist was designed to reduce the incidence of omitted steps, such as ordering of correct laboratory evaluations. The laminated cards highlighted the benchmark time to evaluation of 25 minutes. Process checklist cards were distributed to all members of the acute stroke alert response team, and short versions designed specifically for nursing staff were distributed as badge cards and posted on clinical care units (Supporting Information Appendix I).
Step 4: Real‐Time Feedback
During the intervention roll‐out and post‐intervention periods, feedback was provided from the stroke program to the front‐line providers following each in‐hospital stroke alert. The clinicians involved were notified of the final diagnosis and patient outcome, and were provided with feedback about how the patient's evaluation times compared with benchmark goals. Feedback may serve to motivate, based on clinician professionalism, but performance in the alert was not tied to rewards or penalties for the providers involved. The feedback process was designed to be bi‐directional, with requests for input from staff on barriers to rapid evaluation experienced and suggestions for future process improvement (Supporting Information Appendix II).
Statistical Analysis
The primary outcome was the change in time from stroke alert to CT scan (alert‐to‐CT), comparing pre‐intervention and post‐intervention periods. This time interval was chosen because its calculation involved unambiguous time points, which are available for all patients for whom an in‐hospital alert is called. It is a measure of process efficiency, with minimal expected variation based on differences in patient characteristics (ie, hemorrhagic vs ischemic stroke). Non‐overlapping Kaplan‐Meier curves confirmed the proportional hazards assumption for 2 Cox proportional hazards models: unadjusted and adjusted by group characteristics with P‐value <0.10. Relative hazards and estimates for the percent of patients with alert‐to‐CT scan 25 minutes, according to intervention groups, were obtained from these models. For analyses, admit unit was re‐categorized as intensive care unit (ICU), Med/Surg, or Other. Analyses were conducted using SAS Version 9.2 (SAS Institute, Inc, Cary, NC).
RESULTS
During the study intervals, there were 82 inpatient stroke alerts. Of these alerts, 75 were included in the analysis. Seven were excluded for the following reasons: alert canceled by the stroke team (3), time of alert was not recorded (1), patient identifiers not recorded (1), or stroke alert was preceded by CT imaging (2).
During the 6 months prior to intervention, the median inpatient stroke alert‐to‐CT time (n = 31) was 69.0 minutes (Table 1). Nineteen percent of these alerts met the goal of 25 minutes from alert‐to‐CT time. During the 6‐month post‐intervention period, the median inpatient alert‐to‐CT time (n = 44) was 29.5 minutes. Thirty‐two percent of these alerts met the 25‐minute alert‐to‐CT time benchmark. In the unadjusted model, patients during the post‐intervention period were significantly more likely to have alert‐to‐CT scan time 25 minutes compared to patients prior to the intervention (post‐intervention compared to pre‐intervention, Relative Hazard (RH): 3.03; 95% confidence interval [CI]: 1.76‐5.20; log‐rank P < 0.0001). This remained significant after adjustment for hyperlipidemia, active cancer, final diagnosis of ischemic brain injury, and final diagnosis of stroke mimic (RH: 4.96; 95% CI: 2.65‐9.32; P < 0.0001); data not shown. Admit unit was not included in the adjusted model since there was no indication of differences in the 3‐level variable according to intervention group (P = 0.27). In addition to reduction in median response times, the variability of response times was markedly reduced, and no patient in the 6‐month post‐intervention period had delay to CT sufficient to preclude use of IV thrombolysis (Figure 1).
| Pre‐Intervention (n = 31) | Post‐Intervention (n = 44) | P Value | |
|---|---|---|---|
| |||
| Stroke alert to CT time, median [95% CI] | 69 min [34, 103] | 29.5 min [26, 40] | P < 0.0001 |
| Age, median [IQR] | 61.0 [54.0, 70.0] | 60.5 [48.5, 70.5] | 0.94 |
| Female (%) | 19 (61.3) | 23 (52.3) | 0.44 |
| Race (%) | |||
| Asian | 1 (3.2) | 1 (2.3) | 0.31 |
| Black | 4 (12.9) | 6 (13.6) | |
| Caucasian | 21 (67.7) | 27 (61.4) | |
| Hispanic | 3 (9.7) | 10 (22.7) | |
| Unknown | 2 (6.5) | 0 (0) | |
| Admit unit (%) | |||
| Intensive care | 12 (38.7) | 10 (22.7) | 0.07 |
| Medicine/surgery | 15 (48.4) | 24 (54.6) | |
| Neurology | 0 (0) | 5 (11.4) | |
| Post‐acute care | 3 (9.7) | 0 (0) | |
| Rehabilitation | 1 (3.2) | 2 (4.6) | |
| Women's and maternal care | 0 (0) | 2 (4.6) | |
| Cardiology | 0 (0) | 1 (2.3) | |
| Case mix index, median [IQR] | n = 29 2.6 [1.1, 5.0] | n = 42 2.2 [1.6, 4.5] | 0.82 |
| Prior cerebrovascular accident (%) | 5 (16.1) | 8 (18.2) | 0.82 |
| Hypertension (%) | 17 (54.8) | 24 (54.6) | 0.98 |
| Diabetes mellitus (%) | 7 (22.6) | 11 (25.0) | 0.81 |
| Hyperlipidemia (%) | 15 (48.4) | 9 (20.5) | 0.01 |
| Tobacco abuse, current (%) | 4 (12.9) | 1 (2.3) | 0.15 |
| Alcohol abuse (%) | 2 (6.5) | 0 (0) | 0.17 |
| Active cancer (%) | 8 (25.8) | 5 (11.4) | 0.10 |
| Peripheral vascular disease (%) | 2 (6.5) | 3 (6.8) | 1.0 |
| Coronary artery disease (%) | 6 (19.4) | 7 (15.9) | 0.70 |
| Congestive heart failure (%) | n = 30 5 (16.7) | 4 (9.1) | 0.47 |
| Valvulopathy (%) | 0 (0) | 1 (2.3) | 1.0 |
| Atrial fibrillation (%) | 3 (9.7) | 10 (22.7) | 0.14 |
| Anticoagulation (%) | 7 (22.6) | 7 (15.9) | 0.47 |
| Final diagnosis ischemic brain injury (%) | 15 (48.4) | 11 (25.0) | 0.04 |
| Final diagnosis hemorrhagic brain injury (%) | 3 (9.7) | 4 (9.1) | 1.0 |
| Final diagnosis stroke mimic (symptoms not due to ischemic or hemorrhagic brain injury) (%) | 13 (41.9) | 29 (65.9) | 0.04 |

CONCLUSIONS
In‐hospital strokes represent an emergency for which response time is critical. Neurologic injury progresses with every minute of ischemia, and current recommendations offer a limited time window for intravenous thrombolysis. For stroke with symptom onset in the monitored setting of the hospital, there is a compelling imperative to reduce all delays from system inefficiencies. The findings of the current QI initiative suggest that dramatic improvements are possible through systematic evaluation and redesign of hospital response processes, a checklist for in‐hospital stroke carried by front‐line responders, and ongoing real‐time feedback.
Limitations of this study include a prepost design. The necessity of implementing system change hospital‐wide precluded use of a concurrent control group. The time goals for evaluation are derived from American Stroke Association targets for patients arriving in the Emergency Department. There are differences in process between the hospital ward and the Emergency Department, but the fundamental concept of minimizing time to evaluation once patient symptoms are recognized by hospital staff remains valid.
The possibility of system improvements not due to this QI initiative cannot be excluded. In 2006, this hospital expanded the responsibility of the stroke response team to include acute neurologic deficits outside of the ED without other changes to the in‐hospital stroke alert process. This reduced time to evaluation for in‐hospital ischemic strokes compared to usual care, but even with the same acute stroke response team responding to stroke alerts in both settings, in‐hospital stroke response times remained significantly longer than response times for stroke in the ED.10 The presence of an in‐hospital stroke alert response team alone was not capable of reducing evaluation times to goal. Minimal improvement in median in‐hospital stroke alert evaluation time was seen in the intervening year, following the completion of our previously published analysis, suggesting explicit system QI was necessary.
The Hawthorne effect, in which individuals who know they are being observed modify behavior while such monitoring is in effect, is a major limitation of interpreting QI initiatives. By committing to continuous and ongoing feedback to front‐line providers, this phenomenon can be harnessed to sustain improvement.17 In effect, the study of efficient response to the in‐hospital stroke never ceases. UCH has continued to employ the post‐intervention stroke alert protocol and engage in ongoing feedback after each stroke alert. In the 12 months following the conclusion of this study, the median response time to in‐hospital strokes continues to be 30 minutes, and 7 additional in‐hospital stroke patients have been treated with thrombolysis.
This inpatient stroke alert initiative decreased median inpatient alert‐to‐CT time by 57%, and demonstrates that quality of in‐hospital stroke care can be improved. Decrease in stroke alert‐to‐CT time facilitates earlier thrombolytic therapy. Analysis of treatment and patient outcomes was outside of the scope of the current study, but earlier treatment has potential to significantly improve clinical outcomes.
The Society of Hospital Medicine defines one of the goals of QI to be the change in processes with reduction in variation, thus improving the care for all patients rather than focusing exclusively on outlier events.18 This initiative markedly reduced evaluation variability, allowing a greater percentage of patients to be eligible for treatment within the critical time window. Prior to the intervention, almost a quarter of patients had delays in evaluation sufficient to preclude IV thrombolysis, whereas in the 6 months after the intervention was initiated, not a single patient had evaluation delayed to the point that IV thrombolysis would not have been an option (Figure 1). The goal of in‐hospital stroke QI must be to improve the speed of the process for all patients, and assure that no patient is denied the potential for therapy as a result of inefficiencies in hospital systems.
Acknowledgements
The authors thank Traci Yamashita, PRA, for her work in the statistical analysis for this publication, and Dr Jeffrey Glasheen for development of the University of Colorado Hospital's Hospitalist Training Track Quality Improvement Program of which this work is a product.
- ,,, et al.Heart disease and stroke statistics—2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,.Characteristics of in‐hospital onset ischemic stroke.Eur Neurol.2006;55:155–159.
- ,.Inpatient and community ischemic strokes in a community hospital.Neuroepidemiology.2007;28:86–92.
- .In‐hospital stroke.Lancet Neurol.2003;2:741–746.
- .Time is brain‐quantified.Stroke.2006;37:263–266.
- ,,, et al.Ultra‐early thrombolysis in acute ischemic stroke is associated with better outcomes and lower mortality.Stroke.2010;41:712–716.
- ,,,.Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association.Stroke.2009;40:2945–2948.
- ,,, et al.Guidelines for early management of adults with ischemic stroke.Stroke.2007;38;1655–1711.
- ,,, et al.In‐hospital stroke treated with intravenous tissue plasminogen activator.Stroke.2008;39:2614–2616.
- ,,,,.Stroke alert program improves recognition and evaluation time of in‐hospital ischemic stroke.J Stroke Cerebrovasc Dis.2009;19:494–496.
- ,,,,,.In‐hospital stroke in a statewide stroke registry.Cerebrovasc Dis.2008;25:12–20.
- ,,,,.Quality of care for in‐hospital stroke: analysis of a statewide registry.Stroke.2011;42:207–210.
- ,,, et al.In‐hospital stroke: a multi‐center prospective registry.Eur J Neurol.2011;18:170–176.
- ,,.Code Gray—an organized approach to inpatient stroke.Crit Care Nurs Q.2003;26:296–302.
- ,,.ID, stat‐rapid response to in‐hospital stroke patients.Nurs Manage.2009;40:34–38.
- Institute of Healthcare Improvement. Quality Improvement Tools. Available at: http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/Tools/. Accessed December 1,2010.
- ,,,,,.Variability in the Hawthorne effect with regard to hand hygiene performance in high‐ and low‐performing inpatient care units.Infect Control Hosp Epidemiol.2009;30:222–225.
- Society of Hospital Medicine Quality Improvement Resources. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/32. Accessed December 1,2010.
- ,,, et al.Heart disease and stroke statistics—2010 update: a report from the American Heart Association.Circulation.2010;121:e46–e215.
- ,,.Characteristics of in‐hospital onset ischemic stroke.Eur Neurol.2006;55:155–159.
- ,.Inpatient and community ischemic strokes in a community hospital.Neuroepidemiology.2007;28:86–92.
- .In‐hospital stroke.Lancet Neurol.2003;2:741–746.
- .Time is brain‐quantified.Stroke.2006;37:263–266.
- ,,, et al.Ultra‐early thrombolysis in acute ischemic stroke is associated with better outcomes and lower mortality.Stroke.2010;41:712–716.
- ,,,.Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association.Stroke.2009;40:2945–2948.
- ,,, et al.Guidelines for early management of adults with ischemic stroke.Stroke.2007;38;1655–1711.
- ,,, et al.In‐hospital stroke treated with intravenous tissue plasminogen activator.Stroke.2008;39:2614–2616.
- ,,,,.Stroke alert program improves recognition and evaluation time of in‐hospital ischemic stroke.J Stroke Cerebrovasc Dis.2009;19:494–496.
- ,,,,,.In‐hospital stroke in a statewide stroke registry.Cerebrovasc Dis.2008;25:12–20.
- ,,,,.Quality of care for in‐hospital stroke: analysis of a statewide registry.Stroke.2011;42:207–210.
- ,,, et al.In‐hospital stroke: a multi‐center prospective registry.Eur J Neurol.2011;18:170–176.
- ,,.Code Gray—an organized approach to inpatient stroke.Crit Care Nurs Q.2003;26:296–302.
- ,,.ID, stat‐rapid response to in‐hospital stroke patients.Nurs Manage.2009;40:34–38.
- Institute of Healthcare Improvement. Quality Improvement Tools. Available at: http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/Tools/. Accessed December 1,2010.
- ,,,,,.Variability in the Hawthorne effect with regard to hand hygiene performance in high‐ and low‐performing inpatient care units.Infect Control Hosp Epidemiol.2009;30:222–225.
- Society of Hospital Medicine Quality Improvement Resources. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/32. Accessed December 1,2010.
Patient Knowledge of Hospital Medication
Inpatient medication errors represent an important patient safety issue. The magnitude of the problem is staggering, with 1 review finding almost 1 in every 5 medication doses in error, with 7% having potential for adverse drug events.1 While mistakes made at the ordering stage are frequently intercepted by pharmacist or nursing review, administration errors are particularly difficult to prevent.2 The patient, as the last link in the medication administration chain, represents the final individual capable of preventing an incorrect medication administration. It is perhaps surprising then that patients generally lack a formal role in detecting and preventing adverse medication administration events.3
There have been some ambitious attempts to improve patient education regarding hospital medications and involve selected patients in the medication administration process. Such initiatives may result in increased patient participation and satisfaction.47 There is also potential that increased patient knowledge of their hospital medications could promote the goal of medication safety, as the actively involved patient may be able to catch medication errors in the hospital.
Knowledge of prescribed medications is a prerequisite to patient involvement in prevention of inpatient medication errors and yet there is little research on patient knowledge of their hospital medications. Furthermore, as the experience of hospitalization may be disorienting and disempowering for patients, it remains to be seen if patient attitudes toward participation in inpatient medication safety are favorable. To that end, we conducted a pilot study in which we assessed current patient awareness of their in‐hospital medications and surveyed attitudes toward increased patient knowledge of hospital medications.
PATIENTS AND METHODS
We conducted a cross‐sectional study of 50 cognitively intact adult internal medicine inpatients at the University of Colorado Hospital, a tertiary‐care academic teaching hospital. This study was part of a larger project designed to examine potential for patient involvement in the medication reconciliation process. A professional research assistant approached eligible patients within 24 hours of admission. To be eligible, patients had to self‐identify as knowing their outpatient medications, speak English, and have been admitted from the community. Nursing home residents and patients with a past medical history of dementia were excluded. Enrollment was tracked during the first half of the study to estimate effect of inclusion/exclusion criteria. Thirty‐eight percent of hospital admissions to medicine services were excluded based on the specified criteria. Thirty‐four percent of eligible patients were approached and 50% of approached patients agreed to participate in the study. Patient knowledge of their outpatient medication regimen was compared to admitting physician medication reconciliation to assess accuracy of patient self‐report of outpatient medication knowledge.
After consenting to participate, study patients completed a structured list of their outpatient medications and a survey of attitudes about being shown their in‐hospital medications, hospital medication errors, and patient involvement in hospital safety. They then completed a list of the medications they believed to be prescribed to them in the hospital.
The primary outcomes were the proportions of as needed (PRN), scheduled, and total hospital medications omitted by the patient, compared to the inpatient medication administration record (MAR) (patient errors of omission). Secondary outcomes included the number of in‐hospital medications listed by the patient that did not appear on the inpatient MAR (patient errors of commission), as well as patient attitudes measured on a 5‐point Likert scale (1 indicated strongly disagree and 5 indicated strongly agree.) Descriptive data included age, race, gender, and number of inpatient medications prescribed. Separate analysis of variance (ANOVA) models provided mean estimates of the primary outcomes and tested differences according to each of the patient characteristics: age in years (<65 or 65), self‐reported knowledge of hospital medications, and self‐reported desire to be involved in medication safety. Similar ANOVA models adjusted for number of medications were also examined to determine whether the relationship between the primary outcomes according to patient characteristics were altered by the number of medications. The protocol was approved by the Colorado Multiple Institutional Review Board.
RESULTS
Participants averaged 54 years of age (standard deviation [SD] = 17, range = 21‐89). Forty‐six percent (23/50) were male, and 74% (37/50) were non‐Hispanic white. Using a structured, patient‐completed, outpatient medication list, patients in the study were on an average of 5.3 outpatient prescription medications (range = 0‐17), 2.2 over‐the‐counter medications (range = 0‐8), and 0.2 herbal medications (range = 0‐7). The admitting physician's medication reconciliation list demonstrated similar number of outpatient prescription medications (average = 5.7) to the patient‐generated list. Fifty‐four percent of patient‐completed home medication lists included all of the prescription medications on the physician's medication reconciliation at admission. According to the inpatient MAR, study patients were prescribed an average of 11.3 scheduled and PRN hospital medications (range = 2‐26) at time of study enrollment.
Patient Knowledge of Their Hospital Medication List
Ninety‐six percent (48/50) of study patients omitted 1 or more of their hospital medications. On average, patients omitted 6.8 medications (range = 0‐22) (Table 1). Among scheduled medications, patients most commonly omitted antibiotics (17%), cardiovascular medications (16%), and antithrombotics (15%) (Figure 1). Among PRN medications, patients most commonly omitted analgesics (33%) and gastrointestinal medications (29%) (Figure 2).
| Total Medications | Scheduled Medications | PRN Medications | |
|---|---|---|---|
| |||
| Percent of patients with at least 1 hospital medication they could not name (95% CI) | 96% (90‐100%) | 94% (87‐100%) | 80% (69‐92%) |
| Average number of hospital medications omitted by patient (range) | 6.8 (0‐22) | 5.2 (0‐15) | 1.6 (0‐7) |
| Percentage of hospital medications omitted by patient (95% CI) | 60% (52‐67%) | 60% (52‐67%) | 68% (57‐78%) |
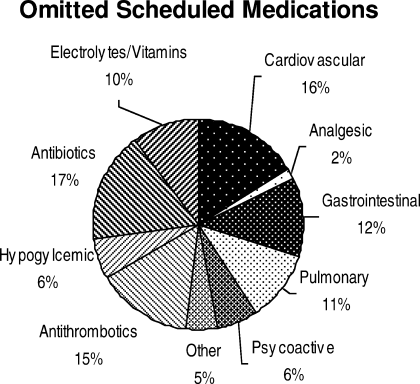
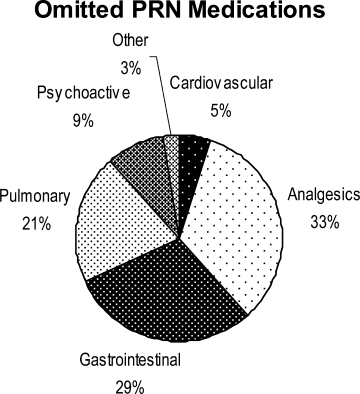
Patients less than 65 years omitted 60% of their PRN medications whereas patients greater than 65 years omitted 88% (P = 0.01). This difference remained even after adjustment for number of medications. There were no significant differences, based on age, in ability to name scheduled or total medications. Forty‐four percent of patients (22/50) believed they were receiving a medication in the hospital that was not actually prescribed.
Patient Attitudes Toward Increased Knowledge of Hospital Medications
Only 28% (14/50) of patients reported having seen their hospital medication list, although 78% (39/50) favored being given such a list, and 81% (39/48) reported that this would improve their satisfaction with care. Ninety percent (45/50) wanted to review their hospital medication list for accuracy and 94% (47/50) felt patient participation in reviewing hospital medications had potential to reduce errors. No associations were found between self‐reported knowledge of hospital medications or self‐reported desire to be involved in medication safety and the proportion of PRN, scheduled, or total medications omitted.
DISCUSSION
Overall, patients in the study were able to name fewer than one‐half of their hospital medications. Our study suggests that adult medicine inpatients believe learning about their hospital medications would increase their satisfaction and has potential to promote medication safety. At the same time, patients did not know many of their hospital medications and this would limit their ability to fully participate in the medication safety process. Study patients frequently committed both errors of omission (ie, they did not know which medications were prescribed), and errors of commission (ie, they believed they were prescribed medications that were not prescribed). Younger patients were aware of more of their PRN medications than older patients, potentially reflecting greater patient care involvement in younger generations. However, study patients, regardless of age, were able to name fewer than one‐half of their PRN hospital medications. The most common scheduled hospital medications that patients were unable to name come from medication classes which can be associated with significant adverse events, including antibiotics, cardiovascular medications, and antithrombotics.
We posit that without systematically educating patients about their hospital medications, significant deficits in patient knowledge are inevitable. Some might argue that patients should not be asked to know their hospital medications or identify medication errors while sick and vulnerable. Certainly with multiple medication changes, formulary substitutions, and frequent modifications based on changes in clinical status, inpatient medication education could be time consuming and potentially introduce patient confusion or anxiety. Incorrect patient feedback could have potential to introduce new errors. An educational program might use graded participation based on patient interest and ability. Models for this exist in the literature, even extending to patient medication self‐administration.57 In our sample of inpatients, the majority desired a more active role in learning about their hospital medications and believed that their involvement might prevent hospital medication errors from occurring.
Medication literacy, education, and active patient involvement in medication monitoring as a means to improve patient outcomes has received significant attention in the outpatient setting, with lessons applicable to the hospital.8, 9 More broadly, the Joint Commission has established a Hospital National Patient Safety Goal to encourage patients' active involvement in their own care as a patient safety strategy.10 Examples set forth by the Joint Commission include involving patients in infection control measures, marking of procedural sites, and reporting of safety concerns relating to treatment.
While this study identifies patient knowledge deficit as a barrier to utilizing patients as part of the hospital medication safety process, it does not test whether reducing this knowledge deficit would actually reduce medication error. Our study population was limited to cognitively intact adult medicine patients at a single institution, limiting the generalizability of our conclusions. Our enrollment process may have resulted in a study population with less serious illness, greater knowledge of their hospital medications, and greater interest in participating in medication safety potentially overestimating patient knowledge of hospital medications. Finally, our small sample size limits the power to find differences in study comparisons.
Our findings are striking in that we found significant deficits in patient understanding of their hospital medications even among patients who believed they knew, or desired to know, what is being prescribed to them in the hospital. Without a system to incorporate the patient into hospital medication management, these patients will be disenfranchised from participating in inpatient medication safety. These results are a call to reexamine how we educate and involve patients regarding hospital medications. Mechanisms to allow patients to provide feedback to the medical team on their hospital medications might identify errors or improve patient satisfaction with their care. However, the systems and cultural changes needed to provide education on inpatient medications are considerable. Future research is needed to determine if increasing patient knowledge regarding their hospital medications would reduce medication errors in the inpatient setting and how this could be effectively implemented.
Acknowledgements
The authors thank Sue Felton, MA, Professional Research Assistant, for enrolling patients in this trial, and Traci Yamashita, MS, Professional Research Assistant, for statistical analysis.
- ,,,,.Medication errors observed in 36 health care facilities.Arch Intern Med.2002;162:1897–1903.
- ,,, et al.Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group.JAMA.1995;274:29–34.
- ,.Patient Safety: what about the patient?Qual Saf Health Care.2002;11:76–80.
- ,,, et al.Pharmacist involvement in a multidisciplinary inpatient medication education program.Am J Health Syst Pharm.2003;60:1012–1018.
- ,,,.Self‐administration of medication by patients and family members during hospitalization.Patient Educ Couns.1996;27:103–112.
- ,,,.Hospital inpatient self‐administration of medicine programmes: a critical literature review.Pharm World Sci.2006;28:140–151.
- ,,,.Self‐administration of medication in hospital: patients' perspectives.J Adv Nurs.2004;46:194–203.
- ,.Outpatient drug safety: new steps in an old direction.Pharmacoepidemiol Drug Saf.2007;16:160–165.
- ,,.Impact of health literacy on health outcomes in ambulatory care patients: a systematic review.Phamacosociology.2008;42:1272–1281.
- Joint Commission.2009. Standards Improvement Initiative. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed June 2009.
Inpatient medication errors represent an important patient safety issue. The magnitude of the problem is staggering, with 1 review finding almost 1 in every 5 medication doses in error, with 7% having potential for adverse drug events.1 While mistakes made at the ordering stage are frequently intercepted by pharmacist or nursing review, administration errors are particularly difficult to prevent.2 The patient, as the last link in the medication administration chain, represents the final individual capable of preventing an incorrect medication administration. It is perhaps surprising then that patients generally lack a formal role in detecting and preventing adverse medication administration events.3
There have been some ambitious attempts to improve patient education regarding hospital medications and involve selected patients in the medication administration process. Such initiatives may result in increased patient participation and satisfaction.47 There is also potential that increased patient knowledge of their hospital medications could promote the goal of medication safety, as the actively involved patient may be able to catch medication errors in the hospital.
Knowledge of prescribed medications is a prerequisite to patient involvement in prevention of inpatient medication errors and yet there is little research on patient knowledge of their hospital medications. Furthermore, as the experience of hospitalization may be disorienting and disempowering for patients, it remains to be seen if patient attitudes toward participation in inpatient medication safety are favorable. To that end, we conducted a pilot study in which we assessed current patient awareness of their in‐hospital medications and surveyed attitudes toward increased patient knowledge of hospital medications.
PATIENTS AND METHODS
We conducted a cross‐sectional study of 50 cognitively intact adult internal medicine inpatients at the University of Colorado Hospital, a tertiary‐care academic teaching hospital. This study was part of a larger project designed to examine potential for patient involvement in the medication reconciliation process. A professional research assistant approached eligible patients within 24 hours of admission. To be eligible, patients had to self‐identify as knowing their outpatient medications, speak English, and have been admitted from the community. Nursing home residents and patients with a past medical history of dementia were excluded. Enrollment was tracked during the first half of the study to estimate effect of inclusion/exclusion criteria. Thirty‐eight percent of hospital admissions to medicine services were excluded based on the specified criteria. Thirty‐four percent of eligible patients were approached and 50% of approached patients agreed to participate in the study. Patient knowledge of their outpatient medication regimen was compared to admitting physician medication reconciliation to assess accuracy of patient self‐report of outpatient medication knowledge.
After consenting to participate, study patients completed a structured list of their outpatient medications and a survey of attitudes about being shown their in‐hospital medications, hospital medication errors, and patient involvement in hospital safety. They then completed a list of the medications they believed to be prescribed to them in the hospital.
The primary outcomes were the proportions of as needed (PRN), scheduled, and total hospital medications omitted by the patient, compared to the inpatient medication administration record (MAR) (patient errors of omission). Secondary outcomes included the number of in‐hospital medications listed by the patient that did not appear on the inpatient MAR (patient errors of commission), as well as patient attitudes measured on a 5‐point Likert scale (1 indicated strongly disagree and 5 indicated strongly agree.) Descriptive data included age, race, gender, and number of inpatient medications prescribed. Separate analysis of variance (ANOVA) models provided mean estimates of the primary outcomes and tested differences according to each of the patient characteristics: age in years (<65 or 65), self‐reported knowledge of hospital medications, and self‐reported desire to be involved in medication safety. Similar ANOVA models adjusted for number of medications were also examined to determine whether the relationship between the primary outcomes according to patient characteristics were altered by the number of medications. The protocol was approved by the Colorado Multiple Institutional Review Board.
RESULTS
Participants averaged 54 years of age (standard deviation [SD] = 17, range = 21‐89). Forty‐six percent (23/50) were male, and 74% (37/50) were non‐Hispanic white. Using a structured, patient‐completed, outpatient medication list, patients in the study were on an average of 5.3 outpatient prescription medications (range = 0‐17), 2.2 over‐the‐counter medications (range = 0‐8), and 0.2 herbal medications (range = 0‐7). The admitting physician's medication reconciliation list demonstrated similar number of outpatient prescription medications (average = 5.7) to the patient‐generated list. Fifty‐four percent of patient‐completed home medication lists included all of the prescription medications on the physician's medication reconciliation at admission. According to the inpatient MAR, study patients were prescribed an average of 11.3 scheduled and PRN hospital medications (range = 2‐26) at time of study enrollment.
Patient Knowledge of Their Hospital Medication List
Ninety‐six percent (48/50) of study patients omitted 1 or more of their hospital medications. On average, patients omitted 6.8 medications (range = 0‐22) (Table 1). Among scheduled medications, patients most commonly omitted antibiotics (17%), cardiovascular medications (16%), and antithrombotics (15%) (Figure 1). Among PRN medications, patients most commonly omitted analgesics (33%) and gastrointestinal medications (29%) (Figure 2).
| Total Medications | Scheduled Medications | PRN Medications | |
|---|---|---|---|
| |||
| Percent of patients with at least 1 hospital medication they could not name (95% CI) | 96% (90‐100%) | 94% (87‐100%) | 80% (69‐92%) |
| Average number of hospital medications omitted by patient (range) | 6.8 (0‐22) | 5.2 (0‐15) | 1.6 (0‐7) |
| Percentage of hospital medications omitted by patient (95% CI) | 60% (52‐67%) | 60% (52‐67%) | 68% (57‐78%) |


Patients less than 65 years omitted 60% of their PRN medications whereas patients greater than 65 years omitted 88% (P = 0.01). This difference remained even after adjustment for number of medications. There were no significant differences, based on age, in ability to name scheduled or total medications. Forty‐four percent of patients (22/50) believed they were receiving a medication in the hospital that was not actually prescribed.
Patient Attitudes Toward Increased Knowledge of Hospital Medications
Only 28% (14/50) of patients reported having seen their hospital medication list, although 78% (39/50) favored being given such a list, and 81% (39/48) reported that this would improve their satisfaction with care. Ninety percent (45/50) wanted to review their hospital medication list for accuracy and 94% (47/50) felt patient participation in reviewing hospital medications had potential to reduce errors. No associations were found between self‐reported knowledge of hospital medications or self‐reported desire to be involved in medication safety and the proportion of PRN, scheduled, or total medications omitted.
DISCUSSION
Overall, patients in the study were able to name fewer than one‐half of their hospital medications. Our study suggests that adult medicine inpatients believe learning about their hospital medications would increase their satisfaction and has potential to promote medication safety. At the same time, patients did not know many of their hospital medications and this would limit their ability to fully participate in the medication safety process. Study patients frequently committed both errors of omission (ie, they did not know which medications were prescribed), and errors of commission (ie, they believed they were prescribed medications that were not prescribed). Younger patients were aware of more of their PRN medications than older patients, potentially reflecting greater patient care involvement in younger generations. However, study patients, regardless of age, were able to name fewer than one‐half of their PRN hospital medications. The most common scheduled hospital medications that patients were unable to name come from medication classes which can be associated with significant adverse events, including antibiotics, cardiovascular medications, and antithrombotics.
We posit that without systematically educating patients about their hospital medications, significant deficits in patient knowledge are inevitable. Some might argue that patients should not be asked to know their hospital medications or identify medication errors while sick and vulnerable. Certainly with multiple medication changes, formulary substitutions, and frequent modifications based on changes in clinical status, inpatient medication education could be time consuming and potentially introduce patient confusion or anxiety. Incorrect patient feedback could have potential to introduce new errors. An educational program might use graded participation based on patient interest and ability. Models for this exist in the literature, even extending to patient medication self‐administration.57 In our sample of inpatients, the majority desired a more active role in learning about their hospital medications and believed that their involvement might prevent hospital medication errors from occurring.
Medication literacy, education, and active patient involvement in medication monitoring as a means to improve patient outcomes has received significant attention in the outpatient setting, with lessons applicable to the hospital.8, 9 More broadly, the Joint Commission has established a Hospital National Patient Safety Goal to encourage patients' active involvement in their own care as a patient safety strategy.10 Examples set forth by the Joint Commission include involving patients in infection control measures, marking of procedural sites, and reporting of safety concerns relating to treatment.
While this study identifies patient knowledge deficit as a barrier to utilizing patients as part of the hospital medication safety process, it does not test whether reducing this knowledge deficit would actually reduce medication error. Our study population was limited to cognitively intact adult medicine patients at a single institution, limiting the generalizability of our conclusions. Our enrollment process may have resulted in a study population with less serious illness, greater knowledge of their hospital medications, and greater interest in participating in medication safety potentially overestimating patient knowledge of hospital medications. Finally, our small sample size limits the power to find differences in study comparisons.
Our findings are striking in that we found significant deficits in patient understanding of their hospital medications even among patients who believed they knew, or desired to know, what is being prescribed to them in the hospital. Without a system to incorporate the patient into hospital medication management, these patients will be disenfranchised from participating in inpatient medication safety. These results are a call to reexamine how we educate and involve patients regarding hospital medications. Mechanisms to allow patients to provide feedback to the medical team on their hospital medications might identify errors or improve patient satisfaction with their care. However, the systems and cultural changes needed to provide education on inpatient medications are considerable. Future research is needed to determine if increasing patient knowledge regarding their hospital medications would reduce medication errors in the inpatient setting and how this could be effectively implemented.
Acknowledgements
The authors thank Sue Felton, MA, Professional Research Assistant, for enrolling patients in this trial, and Traci Yamashita, MS, Professional Research Assistant, for statistical analysis.
Inpatient medication errors represent an important patient safety issue. The magnitude of the problem is staggering, with 1 review finding almost 1 in every 5 medication doses in error, with 7% having potential for adverse drug events.1 While mistakes made at the ordering stage are frequently intercepted by pharmacist or nursing review, administration errors are particularly difficult to prevent.2 The patient, as the last link in the medication administration chain, represents the final individual capable of preventing an incorrect medication administration. It is perhaps surprising then that patients generally lack a formal role in detecting and preventing adverse medication administration events.3
There have been some ambitious attempts to improve patient education regarding hospital medications and involve selected patients in the medication administration process. Such initiatives may result in increased patient participation and satisfaction.47 There is also potential that increased patient knowledge of their hospital medications could promote the goal of medication safety, as the actively involved patient may be able to catch medication errors in the hospital.
Knowledge of prescribed medications is a prerequisite to patient involvement in prevention of inpatient medication errors and yet there is little research on patient knowledge of their hospital medications. Furthermore, as the experience of hospitalization may be disorienting and disempowering for patients, it remains to be seen if patient attitudes toward participation in inpatient medication safety are favorable. To that end, we conducted a pilot study in which we assessed current patient awareness of their in‐hospital medications and surveyed attitudes toward increased patient knowledge of hospital medications.
PATIENTS AND METHODS
We conducted a cross‐sectional study of 50 cognitively intact adult internal medicine inpatients at the University of Colorado Hospital, a tertiary‐care academic teaching hospital. This study was part of a larger project designed to examine potential for patient involvement in the medication reconciliation process. A professional research assistant approached eligible patients within 24 hours of admission. To be eligible, patients had to self‐identify as knowing their outpatient medications, speak English, and have been admitted from the community. Nursing home residents and patients with a past medical history of dementia were excluded. Enrollment was tracked during the first half of the study to estimate effect of inclusion/exclusion criteria. Thirty‐eight percent of hospital admissions to medicine services were excluded based on the specified criteria. Thirty‐four percent of eligible patients were approached and 50% of approached patients agreed to participate in the study. Patient knowledge of their outpatient medication regimen was compared to admitting physician medication reconciliation to assess accuracy of patient self‐report of outpatient medication knowledge.
After consenting to participate, study patients completed a structured list of their outpatient medications and a survey of attitudes about being shown their in‐hospital medications, hospital medication errors, and patient involvement in hospital safety. They then completed a list of the medications they believed to be prescribed to them in the hospital.
The primary outcomes were the proportions of as needed (PRN), scheduled, and total hospital medications omitted by the patient, compared to the inpatient medication administration record (MAR) (patient errors of omission). Secondary outcomes included the number of in‐hospital medications listed by the patient that did not appear on the inpatient MAR (patient errors of commission), as well as patient attitudes measured on a 5‐point Likert scale (1 indicated strongly disagree and 5 indicated strongly agree.) Descriptive data included age, race, gender, and number of inpatient medications prescribed. Separate analysis of variance (ANOVA) models provided mean estimates of the primary outcomes and tested differences according to each of the patient characteristics: age in years (<65 or 65), self‐reported knowledge of hospital medications, and self‐reported desire to be involved in medication safety. Similar ANOVA models adjusted for number of medications were also examined to determine whether the relationship between the primary outcomes according to patient characteristics were altered by the number of medications. The protocol was approved by the Colorado Multiple Institutional Review Board.
RESULTS
Participants averaged 54 years of age (standard deviation [SD] = 17, range = 21‐89). Forty‐six percent (23/50) were male, and 74% (37/50) were non‐Hispanic white. Using a structured, patient‐completed, outpatient medication list, patients in the study were on an average of 5.3 outpatient prescription medications (range = 0‐17), 2.2 over‐the‐counter medications (range = 0‐8), and 0.2 herbal medications (range = 0‐7). The admitting physician's medication reconciliation list demonstrated similar number of outpatient prescription medications (average = 5.7) to the patient‐generated list. Fifty‐four percent of patient‐completed home medication lists included all of the prescription medications on the physician's medication reconciliation at admission. According to the inpatient MAR, study patients were prescribed an average of 11.3 scheduled and PRN hospital medications (range = 2‐26) at time of study enrollment.
Patient Knowledge of Their Hospital Medication List
Ninety‐six percent (48/50) of study patients omitted 1 or more of their hospital medications. On average, patients omitted 6.8 medications (range = 0‐22) (Table 1). Among scheduled medications, patients most commonly omitted antibiotics (17%), cardiovascular medications (16%), and antithrombotics (15%) (Figure 1). Among PRN medications, patients most commonly omitted analgesics (33%) and gastrointestinal medications (29%) (Figure 2).
| Total Medications | Scheduled Medications | PRN Medications | |
|---|---|---|---|
| |||
| Percent of patients with at least 1 hospital medication they could not name (95% CI) | 96% (90‐100%) | 94% (87‐100%) | 80% (69‐92%) |
| Average number of hospital medications omitted by patient (range) | 6.8 (0‐22) | 5.2 (0‐15) | 1.6 (0‐7) |
| Percentage of hospital medications omitted by patient (95% CI) | 60% (52‐67%) | 60% (52‐67%) | 68% (57‐78%) |


Patients less than 65 years omitted 60% of their PRN medications whereas patients greater than 65 years omitted 88% (P = 0.01). This difference remained even after adjustment for number of medications. There were no significant differences, based on age, in ability to name scheduled or total medications. Forty‐four percent of patients (22/50) believed they were receiving a medication in the hospital that was not actually prescribed.
Patient Attitudes Toward Increased Knowledge of Hospital Medications
Only 28% (14/50) of patients reported having seen their hospital medication list, although 78% (39/50) favored being given such a list, and 81% (39/48) reported that this would improve their satisfaction with care. Ninety percent (45/50) wanted to review their hospital medication list for accuracy and 94% (47/50) felt patient participation in reviewing hospital medications had potential to reduce errors. No associations were found between self‐reported knowledge of hospital medications or self‐reported desire to be involved in medication safety and the proportion of PRN, scheduled, or total medications omitted.
DISCUSSION
Overall, patients in the study were able to name fewer than one‐half of their hospital medications. Our study suggests that adult medicine inpatients believe learning about their hospital medications would increase their satisfaction and has potential to promote medication safety. At the same time, patients did not know many of their hospital medications and this would limit their ability to fully participate in the medication safety process. Study patients frequently committed both errors of omission (ie, they did not know which medications were prescribed), and errors of commission (ie, they believed they were prescribed medications that were not prescribed). Younger patients were aware of more of their PRN medications than older patients, potentially reflecting greater patient care involvement in younger generations. However, study patients, regardless of age, were able to name fewer than one‐half of their PRN hospital medications. The most common scheduled hospital medications that patients were unable to name come from medication classes which can be associated with significant adverse events, including antibiotics, cardiovascular medications, and antithrombotics.
We posit that without systematically educating patients about their hospital medications, significant deficits in patient knowledge are inevitable. Some might argue that patients should not be asked to know their hospital medications or identify medication errors while sick and vulnerable. Certainly with multiple medication changes, formulary substitutions, and frequent modifications based on changes in clinical status, inpatient medication education could be time consuming and potentially introduce patient confusion or anxiety. Incorrect patient feedback could have potential to introduce new errors. An educational program might use graded participation based on patient interest and ability. Models for this exist in the literature, even extending to patient medication self‐administration.57 In our sample of inpatients, the majority desired a more active role in learning about their hospital medications and believed that their involvement might prevent hospital medication errors from occurring.
Medication literacy, education, and active patient involvement in medication monitoring as a means to improve patient outcomes has received significant attention in the outpatient setting, with lessons applicable to the hospital.8, 9 More broadly, the Joint Commission has established a Hospital National Patient Safety Goal to encourage patients' active involvement in their own care as a patient safety strategy.10 Examples set forth by the Joint Commission include involving patients in infection control measures, marking of procedural sites, and reporting of safety concerns relating to treatment.
While this study identifies patient knowledge deficit as a barrier to utilizing patients as part of the hospital medication safety process, it does not test whether reducing this knowledge deficit would actually reduce medication error. Our study population was limited to cognitively intact adult medicine patients at a single institution, limiting the generalizability of our conclusions. Our enrollment process may have resulted in a study population with less serious illness, greater knowledge of their hospital medications, and greater interest in participating in medication safety potentially overestimating patient knowledge of hospital medications. Finally, our small sample size limits the power to find differences in study comparisons.
Our findings are striking in that we found significant deficits in patient understanding of their hospital medications even among patients who believed they knew, or desired to know, what is being prescribed to them in the hospital. Without a system to incorporate the patient into hospital medication management, these patients will be disenfranchised from participating in inpatient medication safety. These results are a call to reexamine how we educate and involve patients regarding hospital medications. Mechanisms to allow patients to provide feedback to the medical team on their hospital medications might identify errors or improve patient satisfaction with their care. However, the systems and cultural changes needed to provide education on inpatient medications are considerable. Future research is needed to determine if increasing patient knowledge regarding their hospital medications would reduce medication errors in the inpatient setting and how this could be effectively implemented.
Acknowledgements
The authors thank Sue Felton, MA, Professional Research Assistant, for enrolling patients in this trial, and Traci Yamashita, MS, Professional Research Assistant, for statistical analysis.
- ,,,,.Medication errors observed in 36 health care facilities.Arch Intern Med.2002;162:1897–1903.
- ,,, et al.Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group.JAMA.1995;274:29–34.
- ,.Patient Safety: what about the patient?Qual Saf Health Care.2002;11:76–80.
- ,,, et al.Pharmacist involvement in a multidisciplinary inpatient medication education program.Am J Health Syst Pharm.2003;60:1012–1018.
- ,,,.Self‐administration of medication by patients and family members during hospitalization.Patient Educ Couns.1996;27:103–112.
- ,,,.Hospital inpatient self‐administration of medicine programmes: a critical literature review.Pharm World Sci.2006;28:140–151.
- ,,,.Self‐administration of medication in hospital: patients' perspectives.J Adv Nurs.2004;46:194–203.
- ,.Outpatient drug safety: new steps in an old direction.Pharmacoepidemiol Drug Saf.2007;16:160–165.
- ,,.Impact of health literacy on health outcomes in ambulatory care patients: a systematic review.Phamacosociology.2008;42:1272–1281.
- Joint Commission.2009. Standards Improvement Initiative. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed June 2009.
- ,,,,.Medication errors observed in 36 health care facilities.Arch Intern Med.2002;162:1897–1903.
- ,,, et al.Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group.JAMA.1995;274:29–34.
- ,.Patient Safety: what about the patient?Qual Saf Health Care.2002;11:76–80.
- ,,, et al.Pharmacist involvement in a multidisciplinary inpatient medication education program.Am J Health Syst Pharm.2003;60:1012–1018.
- ,,,.Self‐administration of medication by patients and family members during hospitalization.Patient Educ Couns.1996;27:103–112.
- ,,,.Hospital inpatient self‐administration of medicine programmes: a critical literature review.Pharm World Sci.2006;28:140–151.
- ,,,.Self‐administration of medication in hospital: patients' perspectives.J Adv Nurs.2004;46:194–203.
- ,.Outpatient drug safety: new steps in an old direction.Pharmacoepidemiol Drug Saf.2007;16:160–165.
- ,,.Impact of health literacy on health outcomes in ambulatory care patients: a systematic review.Phamacosociology.2008;42:1272–1281.
- Joint Commission.2009. Standards Improvement Initiative. Available at: http://www.jointcommission.org/NR/rdonlyres/31666E86‐E7F4–423E‐9BE8‐F05BD1CB0AA8/0/HAP_NPSG.pdf. Accessed June 2009.
In the Literature
In This Edition
Literature at a Glance
A guide to this month’s studies
- PE and COPD exacerbations.
- Care bundles and readmission rates.
- Family history and VTE risk.
- Vasopressor choice and mortality in sepsis.
- Vitamin K use in overanticoagulation.
- Appropriate treatment of asymptomatic bacteriuria.
- Guideline adherence in thrombocytopenia.
Pulmonary Embolism Frequently Complicates COPD Exacerbations
Clinical question: What percentage of patients with acute chronic obstructive pulmonary disease (COPD) exacerbations has pulmonary emboli?
Background: As many as 30% of COPD exacerbations have no apparent precipitating event. Even in patients with evidence of a precipitating event, such as an upper-respiratory illness or increased environmental irritants, pulmonary emboli (PE) may coexist and warrant evaluation.
Study design: Literature review.
Setting: Multiple studies in Europe and the U.S.
Synopsis: This literature review included five studies to estimate the rate of PE in patients with a COPD exacerbation. Overall incidence of PE in COPD exacerbations was 19.9%, but of those patients requiring hospitalization, the incidence was as high as 25.5%. Incidence estimates varied based on interpretation of data that were missing or inconsistent between studies. Patients most commonly present with dyspnea, chest pain, hemoptysis, cough, and palpitations. Six percent of PE patients presented with syncope; no patients with an exacerbation without a PE presented with syncope.
Risk of mortality from PE is almost twice as high in patients with a COPD exacerbation compared with PE in other settings. A significant number of patients have PE without history or evidence of DVT, so in situ thrombosis is a significant factor. The interpretation of these results is limited by the heterogeneity of the study designs, and by the relatively low number of cases. Larger trials are necessary.
Bottom line: Pulmonary emboli are present in as many as 25% of all COPD exacerbations. Delay in diagnosis of PE in COPD patients affects morbidity and mortality. PE should be a consideration in many COPD exacerbations.
Citation: Rizkallah J, Man SF, Din DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009;135(3):786-793.
Targeted-Care Bundle Can Reduce ED Visits and Readmission Rates in High-Risk Elderly Patients
Clinical question: Can a care coordination bundle reduce length of stay (LOS), ED visits, or readmissions within 30 days of a hospital admission?
Background: Hospital-based care coordination interventions have shown mixed results in affecting LOS, post-discharge ED visits, and readmission rates. Although there has been some success with particular interventions, no consistent benefit has been demonstrated. Most notably, a recent meta-analysis of several different interventions showed no improvement in mortality, LOS, or readmission rates.
Study design: A randomized, controlled trial of select high-risk elderly patients.
Setting: A large teaching hospital at Baylor University Medical Center.
Synopsis: A “targeted-care bundle” was implemented with high-risk elderly patients to try to reduce LOS, readmissions, and ED visits. High-risk patients were identified by age, diagnosis-related group (DRG), number of medications at admission, comorbid conditions, and need for assistance in activities of daily living. Subjects were randomized to usual care or to receive a targeted-care bundle. The targeted-care bundle included multiple interventions. A study care coordinator provided daily patient education, including condition-specific teaching, discharge teaching and planning, and a follow-up phone call at five to seven days after discharge. A clinical pharmacist intervened for medication reconciliation at admission and discharge, medication teaching, and a follow-up phone call at five to seven days after discharge. Structured documents, including a personal health record and supplemental discharge form, were implemented.
The study had low enrollment, largely due to the requirement to obtain informed consent from all participants. Therefore, the study was underpowered to detect such target endpoints as LOS. A significant decrease in 30-day readmission rates/ED visits was noticed, but there was no persistent effect at 60 days.
The intervention was designed to use existing hospital staff in order to be practical for broad utilization. Future studies need to focus on increased enrollment to demonstrate beneficial effect.
Bottom line: Targeted health interventions focusing on education and coordination of care might effect some significant outcomes, most notably readmissions or ED visits within 30 days, but the nature of the clinical problem makes rigorous testing of interventions a challenge.
Citation: Kohler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218.
Family History Is a Risk Factor for Venous Thrombosis
Clinical question: Is family history of additional value in predicting an individual’s risk of venous thrombosis once a genetic risk factor is identified?
Background: A positive family history of venous thrombosis might suggest the presence of genetic risk factors in a given family. However, it is not known whether family history is of additional significance—once a risk factor is identified—in predicting an individual’s risk for venous thrombosis.
Study design: Population-based, case-control study.
Setting: Participants in the Multiple Environmental and Genetic Assessment (MEGA) of risk factors for venous thrombosis study.
Synopsis: Recruitment, data collection, and blood samples were obtained from individuals in the MEGA study. Participants completed a questionnaire about risk factors for venous thrombosis and family history. A positive family history more than doubled the risk of venous thrombosis, and when more than one family member was affected, the risk increased fourfold. The risk for venous thrombosis increased 64 times for individuals who had a family history, genetic risk factor, and environmental risk factor when compared with those with a negative family history and no known risk factors.
The underreporting or overestimation of the prevalence of a positive family history might limit this study.
Bottom line: Family history is a risk indictor for a first venous thrombosis, despite the presence of other risk factors.
Citation: Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med. 2009;169(6):610-615.
Vasopressor Choice Predicts Mortality in Septic Shock
Clinical question: Does vasopressor choice affect mortality in patients with community-acquired septic shock?
Background: Community-acquired septic shock is a common illness and, despite aggressive care, a leading cause of death. Randomized clinical control trials evaluating the efficacy and safety of different adrenergic supportive agents are lacking. Thus, both norepinephrine and dopamine are recommended as first-line agents in the treatment of septic shock by the Surviving Sepsis Campaign guidelines.
Study design: Multicenter, cohort observational study.
Setting: Seventeen intensive-care units in Portugal.
Synopsis: In adjusted analysis controlling for Simplified Acute Physiology Score (SAPS) II, use of norepinephrine in community-acquired septic shock was associated with higher hospital mortality and lower 28-day survival when compared with dopamine. Specifically, patients treated with norepinephrine had a statistically significant higher hospital mortality rate than those treated with dopamine (52% and 38.5%, respectively, P=0.002) and a lower 28-day survival (log rank=22.6; P<0.001). While this data is valuable, the nonrandomized, observational study design limits firm conclusions regarding vasopressor choice. Further results from three large trials comparing vasopressor use in septic shock should continue to shed light on this debate.
Bottom line: Norepinephrine administration is associated with higher hospital mortality and lower 28-day survival when compared with dopamine in patients with community-acquired septic shock.
Citation: Póvoa PR, Carneiro AH, Ribeiro OS, Pereira AC, Portuguese Community-Acquired Sepsis Study Group. Influence of vasopressor agent in septic shock mortality. Results from the Portuguese Community-Acquired Sepsis Study (SACiUCI study). Crit Care Med. 2009;37(2):410-416.
Oral Vitamin K Versus Placebo to Correct Excess Anticoagulation in Warfarin Patients
Clinical question: In nonbleeding patients with warfarin-associated coagulopathy, does oral vitamin K reduce bleeding events when compared to placebo?
Background: Warfarin is a common drug for primary and secondary prevention of thromboembolism, but it requires continued monitoring of the international normalized ratio (INR) value. INR values >4.0 are associated with an increase in bleeding complications, with specific concern for intracranial bleeding when INR values exceed 4.5. Small, randomized trials have shown that single, low-dose administration of oral vitamin K effectively reduces the INR in nonbleeding, overanticoagulated patients.
However, these studies have not shown if vitamin K reduces risk for bleeding without increasing the risk for thromboembolism.
Study design: Randomized, placebo-controlled trial.
Setting: Fourteen anticoagulation clinics in Canada, Italy, and the U.S.
Synopsis: Nonbleeding patients with supratherapeutic INR values between 4.5 and 10.0 were randomly assigned to receive 1.25 mg of oral vitamin K or placebo, then evaluated for all forms of bleeding for 90 days. Bleeding events were defined as “major bleeding,” “minor bleeding,” and “trivial bleeding.”
Though patients who received oral vitamin K had a significantly more rapid INR decrease, there were no differences between the two groups with regard to all bleeding events, thromboembolism, or death. The study was underpowered to detect differences in major bleeding.
Bottom line: Low-dose oral vitamin K leads to more rapid correction of the INR in overanticoagulated patients on warfarin therapy, but has little effect on clinical outcomes at 90 days.
Citation: Crowther MA, Ageno W, Garcia D, et al. Oral vitamin K versus placebo to correct excessive anticoagulation in patients receiving warfarin: a randomized trial. Ann Intern Med. 2009;150(5):293-300.
Inappropriate Treatment of Catheter-Associated Asymptomatic Bacteriuria
Clinical question: Are hospitalized patients with urinary catheters inappropriately treated with antibiotics for asymptomatic bacteriuria?
Background: Persons with catheters acquire bacteriuria at the rate of 3% to 10% per day, but in the majority of cases, no symptoms or secondary complications occur. Evidenced-based guidelines state that asymptomatic bacteriuria is not a clinically significant infection, and numerous studies have shown that treatment is unlikely to confer clinical benefit.
Study design: Retrospective cohort study.
Setting: A single-site Veterans Affairs hospital.
Synopsis: Using urine culture results over a three-month period from a single VA medical center, 280 cases were analyzed: 164 catheter-associated asymptomatic bacteriuria and 116 catheter-associated urinary tract infections (UTIs). A UTI was defined as having one or more of these symptoms: fever, urgency, frequency, dysuria, suprapubic tenderness, altered mental status, or hypotension in a patient without another recognized infection and a positive urine culture. Of the asymptomatic bacteriuria cases, 68% were managed appropriately with no antibiotic treatment; 32% were inappropriately treated with antibiotics.
In multivariate analysis, older patient age, predominance of gram-negative bacteria, and higher urine white blood cell count were significantly associated with inappropriate treatment.
This study highlights the fact that antibiotics continue to be used inappropriately in patients with catheters. Current guidelines do not distinguish well between asymptomatic bacteriuria and UTI, so there might be a knowledge gap. This study was based on urine culture data, not urinalysis of all patients with a catheter, so the symptomatic patients were likely over-represented.
An associated editorial observes that the study extrapolates data from studies that involved patients with uncomplicated UTIs and, therefore, might reach erroneous conclusions. Further, viewing catheter-associated symptomatic UTIs and catheter-associated asymptomatic bactiuria as dichotomous and warranting inherently different management fails to encompass a number of clinical factors, including co-infection, and further fails to acknowledge that removal of the catheter is the first step in treatment. However, the finding that antibiotics continue to be used inappropriately is useful.
Bottom line: A clinical determination of whether a patient with a catheter really has a symptomatic UTI/urosepsis or only has asymptomatic bacteriuria should precede starting antibiotics in hospitalized patients.
Citations: Cope M, Cevallos ME, Cadle RM, Darouiche RO, Musher DM, Trautner BW. Inappropriate treatment of catheter-associated asymptomatic bateriuria in a tertiary care hospital. Clin Infect Dis. 2009;48(9):1182-1188.
Kunin CM. Catheter-associated urinary tract infections: a syllogism compounded by a questionable dichotomy. Clin Infect Dis. 2009;48:1189-1190.
Current Practices in the Evaluation and Management of Thrombocytopenia in Heparin Patients
Clinical question: Are the current American College of Chest Physicians (ACCP) guidelines for the recognition, treatment, and prevention of heparin-induced thrombocytopenia (HIT) being followed?
Background: Heparin-based anticoagulation is frequently given to hospitalized patients, and approximately 1% to 5% of these patients develop HIT. In 2004, the ACCP published a consensus statement on the evaluation, management, and prevention of HIT.
Study design: Prospective, observational study.
Setting: Forty-eight U.S. hospitals in the Complications After Thrombocytopenia Caused by Heparin (CATCH) registry.
Synopsis: The CATCH trial enrolled patients receiving any form of heparin for >96 hours (n=2,420), cardiac-care-unit patients treated with heparin (n=1,090), and patients who had an HIT antibody assay performed (n=449), for a total of 3,536 total patients. The study included patients on unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH). Thrombocytopenia was defined at a platelet count <150,000, or a decrease of 50% when compared with admission.
In the prolonged heparin group, 36.4% of patients developed thrombocytopenia; however, HIT was suspected in only 19.8% of these high-risk patients. While physicians were more likely to consider HIT in the cardiac-care patients (37.6%), the diagnosis was considered>24 hours after the thrombocytopenia developed. Physicians often waited until after a thromboembolic complication occurred before evaluating for HIT. More often than not, preventive measures were missed (e.g., failing to check for HIT antibodies, continuing heparin after HIT was suspected).
Bottom line: Thrombocytopenia is a common occurrence in patients receiving heparin and, despite the risk of devastating complications from HIT, treatment infrequently conforms to the established guidelines.
Citation: Crespo EM, Oliveira GBF, Honeycutt EF, et al. Evaluation and management of thrombocytopenia and suspected heparin-induced thrombocytopenia in hospitalized patients: The Complications After Thrombocytopenia Caused by Heparin (CATCH) registry. Am Heart J. 2009;157(4):651-657. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- PE and COPD exacerbations.
- Care bundles and readmission rates.
- Family history and VTE risk.
- Vasopressor choice and mortality in sepsis.
- Vitamin K use in overanticoagulation.
- Appropriate treatment of asymptomatic bacteriuria.
- Guideline adherence in thrombocytopenia.
Pulmonary Embolism Frequently Complicates COPD Exacerbations
Clinical question: What percentage of patients with acute chronic obstructive pulmonary disease (COPD) exacerbations has pulmonary emboli?
Background: As many as 30% of COPD exacerbations have no apparent precipitating event. Even in patients with evidence of a precipitating event, such as an upper-respiratory illness or increased environmental irritants, pulmonary emboli (PE) may coexist and warrant evaluation.
Study design: Literature review.
Setting: Multiple studies in Europe and the U.S.
Synopsis: This literature review included five studies to estimate the rate of PE in patients with a COPD exacerbation. Overall incidence of PE in COPD exacerbations was 19.9%, but of those patients requiring hospitalization, the incidence was as high as 25.5%. Incidence estimates varied based on interpretation of data that were missing or inconsistent between studies. Patients most commonly present with dyspnea, chest pain, hemoptysis, cough, and palpitations. Six percent of PE patients presented with syncope; no patients with an exacerbation without a PE presented with syncope.
Risk of mortality from PE is almost twice as high in patients with a COPD exacerbation compared with PE in other settings. A significant number of patients have PE without history or evidence of DVT, so in situ thrombosis is a significant factor. The interpretation of these results is limited by the heterogeneity of the study designs, and by the relatively low number of cases. Larger trials are necessary.
Bottom line: Pulmonary emboli are present in as many as 25% of all COPD exacerbations. Delay in diagnosis of PE in COPD patients affects morbidity and mortality. PE should be a consideration in many COPD exacerbations.
Citation: Rizkallah J, Man SF, Din DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009;135(3):786-793.
Targeted-Care Bundle Can Reduce ED Visits and Readmission Rates in High-Risk Elderly Patients
Clinical question: Can a care coordination bundle reduce length of stay (LOS), ED visits, or readmissions within 30 days of a hospital admission?
Background: Hospital-based care coordination interventions have shown mixed results in affecting LOS, post-discharge ED visits, and readmission rates. Although there has been some success with particular interventions, no consistent benefit has been demonstrated. Most notably, a recent meta-analysis of several different interventions showed no improvement in mortality, LOS, or readmission rates.
Study design: A randomized, controlled trial of select high-risk elderly patients.
Setting: A large teaching hospital at Baylor University Medical Center.
Synopsis: A “targeted-care bundle” was implemented with high-risk elderly patients to try to reduce LOS, readmissions, and ED visits. High-risk patients were identified by age, diagnosis-related group (DRG), number of medications at admission, comorbid conditions, and need for assistance in activities of daily living. Subjects were randomized to usual care or to receive a targeted-care bundle. The targeted-care bundle included multiple interventions. A study care coordinator provided daily patient education, including condition-specific teaching, discharge teaching and planning, and a follow-up phone call at five to seven days after discharge. A clinical pharmacist intervened for medication reconciliation at admission and discharge, medication teaching, and a follow-up phone call at five to seven days after discharge. Structured documents, including a personal health record and supplemental discharge form, were implemented.
The study had low enrollment, largely due to the requirement to obtain informed consent from all participants. Therefore, the study was underpowered to detect such target endpoints as LOS. A significant decrease in 30-day readmission rates/ED visits was noticed, but there was no persistent effect at 60 days.
The intervention was designed to use existing hospital staff in order to be practical for broad utilization. Future studies need to focus on increased enrollment to demonstrate beneficial effect.
Bottom line: Targeted health interventions focusing on education and coordination of care might effect some significant outcomes, most notably readmissions or ED visits within 30 days, but the nature of the clinical problem makes rigorous testing of interventions a challenge.
Citation: Kohler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218.
Family History Is a Risk Factor for Venous Thrombosis
Clinical question: Is family history of additional value in predicting an individual’s risk of venous thrombosis once a genetic risk factor is identified?
Background: A positive family history of venous thrombosis might suggest the presence of genetic risk factors in a given family. However, it is not known whether family history is of additional significance—once a risk factor is identified—in predicting an individual’s risk for venous thrombosis.
Study design: Population-based, case-control study.
Setting: Participants in the Multiple Environmental and Genetic Assessment (MEGA) of risk factors for venous thrombosis study.
Synopsis: Recruitment, data collection, and blood samples were obtained from individuals in the MEGA study. Participants completed a questionnaire about risk factors for venous thrombosis and family history. A positive family history more than doubled the risk of venous thrombosis, and when more than one family member was affected, the risk increased fourfold. The risk for venous thrombosis increased 64 times for individuals who had a family history, genetic risk factor, and environmental risk factor when compared with those with a negative family history and no known risk factors.
The underreporting or overestimation of the prevalence of a positive family history might limit this study.
Bottom line: Family history is a risk indictor for a first venous thrombosis, despite the presence of other risk factors.
Citation: Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med. 2009;169(6):610-615.
Vasopressor Choice Predicts Mortality in Septic Shock
Clinical question: Does vasopressor choice affect mortality in patients with community-acquired septic shock?
Background: Community-acquired septic shock is a common illness and, despite aggressive care, a leading cause of death. Randomized clinical control trials evaluating the efficacy and safety of different adrenergic supportive agents are lacking. Thus, both norepinephrine and dopamine are recommended as first-line agents in the treatment of septic shock by the Surviving Sepsis Campaign guidelines.
Study design: Multicenter, cohort observational study.
Setting: Seventeen intensive-care units in Portugal.
Synopsis: In adjusted analysis controlling for Simplified Acute Physiology Score (SAPS) II, use of norepinephrine in community-acquired septic shock was associated with higher hospital mortality and lower 28-day survival when compared with dopamine. Specifically, patients treated with norepinephrine had a statistically significant higher hospital mortality rate than those treated with dopamine (52% and 38.5%, respectively, P=0.002) and a lower 28-day survival (log rank=22.6; P<0.001). While this data is valuable, the nonrandomized, observational study design limits firm conclusions regarding vasopressor choice. Further results from three large trials comparing vasopressor use in septic shock should continue to shed light on this debate.
Bottom line: Norepinephrine administration is associated with higher hospital mortality and lower 28-day survival when compared with dopamine in patients with community-acquired septic shock.
Citation: Póvoa PR, Carneiro AH, Ribeiro OS, Pereira AC, Portuguese Community-Acquired Sepsis Study Group. Influence of vasopressor agent in septic shock mortality. Results from the Portuguese Community-Acquired Sepsis Study (SACiUCI study). Crit Care Med. 2009;37(2):410-416.
Oral Vitamin K Versus Placebo to Correct Excess Anticoagulation in Warfarin Patients
Clinical question: In nonbleeding patients with warfarin-associated coagulopathy, does oral vitamin K reduce bleeding events when compared to placebo?
Background: Warfarin is a common drug for primary and secondary prevention of thromboembolism, but it requires continued monitoring of the international normalized ratio (INR) value. INR values >4.0 are associated with an increase in bleeding complications, with specific concern for intracranial bleeding when INR values exceed 4.5. Small, randomized trials have shown that single, low-dose administration of oral vitamin K effectively reduces the INR in nonbleeding, overanticoagulated patients.
However, these studies have not shown if vitamin K reduces risk for bleeding without increasing the risk for thromboembolism.
Study design: Randomized, placebo-controlled trial.
Setting: Fourteen anticoagulation clinics in Canada, Italy, and the U.S.
Synopsis: Nonbleeding patients with supratherapeutic INR values between 4.5 and 10.0 were randomly assigned to receive 1.25 mg of oral vitamin K or placebo, then evaluated for all forms of bleeding for 90 days. Bleeding events were defined as “major bleeding,” “minor bleeding,” and “trivial bleeding.”
Though patients who received oral vitamin K had a significantly more rapid INR decrease, there were no differences between the two groups with regard to all bleeding events, thromboembolism, or death. The study was underpowered to detect differences in major bleeding.
Bottom line: Low-dose oral vitamin K leads to more rapid correction of the INR in overanticoagulated patients on warfarin therapy, but has little effect on clinical outcomes at 90 days.
Citation: Crowther MA, Ageno W, Garcia D, et al. Oral vitamin K versus placebo to correct excessive anticoagulation in patients receiving warfarin: a randomized trial. Ann Intern Med. 2009;150(5):293-300.
Inappropriate Treatment of Catheter-Associated Asymptomatic Bacteriuria
Clinical question: Are hospitalized patients with urinary catheters inappropriately treated with antibiotics for asymptomatic bacteriuria?
Background: Persons with catheters acquire bacteriuria at the rate of 3% to 10% per day, but in the majority of cases, no symptoms or secondary complications occur. Evidenced-based guidelines state that asymptomatic bacteriuria is not a clinically significant infection, and numerous studies have shown that treatment is unlikely to confer clinical benefit.
Study design: Retrospective cohort study.
Setting: A single-site Veterans Affairs hospital.
Synopsis: Using urine culture results over a three-month period from a single VA medical center, 280 cases were analyzed: 164 catheter-associated asymptomatic bacteriuria and 116 catheter-associated urinary tract infections (UTIs). A UTI was defined as having one or more of these symptoms: fever, urgency, frequency, dysuria, suprapubic tenderness, altered mental status, or hypotension in a patient without another recognized infection and a positive urine culture. Of the asymptomatic bacteriuria cases, 68% were managed appropriately with no antibiotic treatment; 32% were inappropriately treated with antibiotics.
In multivariate analysis, older patient age, predominance of gram-negative bacteria, and higher urine white blood cell count were significantly associated with inappropriate treatment.
This study highlights the fact that antibiotics continue to be used inappropriately in patients with catheters. Current guidelines do not distinguish well between asymptomatic bacteriuria and UTI, so there might be a knowledge gap. This study was based on urine culture data, not urinalysis of all patients with a catheter, so the symptomatic patients were likely over-represented.
An associated editorial observes that the study extrapolates data from studies that involved patients with uncomplicated UTIs and, therefore, might reach erroneous conclusions. Further, viewing catheter-associated symptomatic UTIs and catheter-associated asymptomatic bactiuria as dichotomous and warranting inherently different management fails to encompass a number of clinical factors, including co-infection, and further fails to acknowledge that removal of the catheter is the first step in treatment. However, the finding that antibiotics continue to be used inappropriately is useful.
Bottom line: A clinical determination of whether a patient with a catheter really has a symptomatic UTI/urosepsis or only has asymptomatic bacteriuria should precede starting antibiotics in hospitalized patients.
Citations: Cope M, Cevallos ME, Cadle RM, Darouiche RO, Musher DM, Trautner BW. Inappropriate treatment of catheter-associated asymptomatic bateriuria in a tertiary care hospital. Clin Infect Dis. 2009;48(9):1182-1188.
Kunin CM. Catheter-associated urinary tract infections: a syllogism compounded by a questionable dichotomy. Clin Infect Dis. 2009;48:1189-1190.
Current Practices in the Evaluation and Management of Thrombocytopenia in Heparin Patients
Clinical question: Are the current American College of Chest Physicians (ACCP) guidelines for the recognition, treatment, and prevention of heparin-induced thrombocytopenia (HIT) being followed?
Background: Heparin-based anticoagulation is frequently given to hospitalized patients, and approximately 1% to 5% of these patients develop HIT. In 2004, the ACCP published a consensus statement on the evaluation, management, and prevention of HIT.
Study design: Prospective, observational study.
Setting: Forty-eight U.S. hospitals in the Complications After Thrombocytopenia Caused by Heparin (CATCH) registry.
Synopsis: The CATCH trial enrolled patients receiving any form of heparin for >96 hours (n=2,420), cardiac-care-unit patients treated with heparin (n=1,090), and patients who had an HIT antibody assay performed (n=449), for a total of 3,536 total patients. The study included patients on unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH). Thrombocytopenia was defined at a platelet count <150,000, or a decrease of 50% when compared with admission.
In the prolonged heparin group, 36.4% of patients developed thrombocytopenia; however, HIT was suspected in only 19.8% of these high-risk patients. While physicians were more likely to consider HIT in the cardiac-care patients (37.6%), the diagnosis was considered>24 hours after the thrombocytopenia developed. Physicians often waited until after a thromboembolic complication occurred before evaluating for HIT. More often than not, preventive measures were missed (e.g., failing to check for HIT antibodies, continuing heparin after HIT was suspected).
Bottom line: Thrombocytopenia is a common occurrence in patients receiving heparin and, despite the risk of devastating complications from HIT, treatment infrequently conforms to the established guidelines.
Citation: Crespo EM, Oliveira GBF, Honeycutt EF, et al. Evaluation and management of thrombocytopenia and suspected heparin-induced thrombocytopenia in hospitalized patients: The Complications After Thrombocytopenia Caused by Heparin (CATCH) registry. Am Heart J. 2009;157(4):651-657. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- PE and COPD exacerbations.
- Care bundles and readmission rates.
- Family history and VTE risk.
- Vasopressor choice and mortality in sepsis.
- Vitamin K use in overanticoagulation.
- Appropriate treatment of asymptomatic bacteriuria.
- Guideline adherence in thrombocytopenia.
Pulmonary Embolism Frequently Complicates COPD Exacerbations
Clinical question: What percentage of patients with acute chronic obstructive pulmonary disease (COPD) exacerbations has pulmonary emboli?
Background: As many as 30% of COPD exacerbations have no apparent precipitating event. Even in patients with evidence of a precipitating event, such as an upper-respiratory illness or increased environmental irritants, pulmonary emboli (PE) may coexist and warrant evaluation.
Study design: Literature review.
Setting: Multiple studies in Europe and the U.S.
Synopsis: This literature review included five studies to estimate the rate of PE in patients with a COPD exacerbation. Overall incidence of PE in COPD exacerbations was 19.9%, but of those patients requiring hospitalization, the incidence was as high as 25.5%. Incidence estimates varied based on interpretation of data that were missing or inconsistent between studies. Patients most commonly present with dyspnea, chest pain, hemoptysis, cough, and palpitations. Six percent of PE patients presented with syncope; no patients with an exacerbation without a PE presented with syncope.
Risk of mortality from PE is almost twice as high in patients with a COPD exacerbation compared with PE in other settings. A significant number of patients have PE without history or evidence of DVT, so in situ thrombosis is a significant factor. The interpretation of these results is limited by the heterogeneity of the study designs, and by the relatively low number of cases. Larger trials are necessary.
Bottom line: Pulmonary emboli are present in as many as 25% of all COPD exacerbations. Delay in diagnosis of PE in COPD patients affects morbidity and mortality. PE should be a consideration in many COPD exacerbations.
Citation: Rizkallah J, Man SF, Din DD. Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009;135(3):786-793.
Targeted-Care Bundle Can Reduce ED Visits and Readmission Rates in High-Risk Elderly Patients
Clinical question: Can a care coordination bundle reduce length of stay (LOS), ED visits, or readmissions within 30 days of a hospital admission?
Background: Hospital-based care coordination interventions have shown mixed results in affecting LOS, post-discharge ED visits, and readmission rates. Although there has been some success with particular interventions, no consistent benefit has been demonstrated. Most notably, a recent meta-analysis of several different interventions showed no improvement in mortality, LOS, or readmission rates.
Study design: A randomized, controlled trial of select high-risk elderly patients.
Setting: A large teaching hospital at Baylor University Medical Center.
Synopsis: A “targeted-care bundle” was implemented with high-risk elderly patients to try to reduce LOS, readmissions, and ED visits. High-risk patients were identified by age, diagnosis-related group (DRG), number of medications at admission, comorbid conditions, and need for assistance in activities of daily living. Subjects were randomized to usual care or to receive a targeted-care bundle. The targeted-care bundle included multiple interventions. A study care coordinator provided daily patient education, including condition-specific teaching, discharge teaching and planning, and a follow-up phone call at five to seven days after discharge. A clinical pharmacist intervened for medication reconciliation at admission and discharge, medication teaching, and a follow-up phone call at five to seven days after discharge. Structured documents, including a personal health record and supplemental discharge form, were implemented.
The study had low enrollment, largely due to the requirement to obtain informed consent from all participants. Therefore, the study was underpowered to detect such target endpoints as LOS. A significant decrease in 30-day readmission rates/ED visits was noticed, but there was no persistent effect at 60 days.
The intervention was designed to use existing hospital staff in order to be practical for broad utilization. Future studies need to focus on increased enrollment to demonstrate beneficial effect.
Bottom line: Targeted health interventions focusing on education and coordination of care might effect some significant outcomes, most notably readmissions or ED visits within 30 days, but the nature of the clinical problem makes rigorous testing of interventions a challenge.
Citation: Kohler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211-218.
Family History Is a Risk Factor for Venous Thrombosis
Clinical question: Is family history of additional value in predicting an individual’s risk of venous thrombosis once a genetic risk factor is identified?
Background: A positive family history of venous thrombosis might suggest the presence of genetic risk factors in a given family. However, it is not known whether family history is of additional significance—once a risk factor is identified—in predicting an individual’s risk for venous thrombosis.
Study design: Population-based, case-control study.
Setting: Participants in the Multiple Environmental and Genetic Assessment (MEGA) of risk factors for venous thrombosis study.
Synopsis: Recruitment, data collection, and blood samples were obtained from individuals in the MEGA study. Participants completed a questionnaire about risk factors for venous thrombosis and family history. A positive family history more than doubled the risk of venous thrombosis, and when more than one family member was affected, the risk increased fourfold. The risk for venous thrombosis increased 64 times for individuals who had a family history, genetic risk factor, and environmental risk factor when compared with those with a negative family history and no known risk factors.
The underreporting or overestimation of the prevalence of a positive family history might limit this study.
Bottom line: Family history is a risk indictor for a first venous thrombosis, despite the presence of other risk factors.
Citation: Bezemer ID, van der Meer FJ, Eikenboom JC, Rosendaal FR, Doggen CJ. The value of family history as a risk indicator for venous thrombosis. Arch Intern Med. 2009;169(6):610-615.
Vasopressor Choice Predicts Mortality in Septic Shock
Clinical question: Does vasopressor choice affect mortality in patients with community-acquired septic shock?
Background: Community-acquired septic shock is a common illness and, despite aggressive care, a leading cause of death. Randomized clinical control trials evaluating the efficacy and safety of different adrenergic supportive agents are lacking. Thus, both norepinephrine and dopamine are recommended as first-line agents in the treatment of septic shock by the Surviving Sepsis Campaign guidelines.
Study design: Multicenter, cohort observational study.
Setting: Seventeen intensive-care units in Portugal.
Synopsis: In adjusted analysis controlling for Simplified Acute Physiology Score (SAPS) II, use of norepinephrine in community-acquired septic shock was associated with higher hospital mortality and lower 28-day survival when compared with dopamine. Specifically, patients treated with norepinephrine had a statistically significant higher hospital mortality rate than those treated with dopamine (52% and 38.5%, respectively, P=0.002) and a lower 28-day survival (log rank=22.6; P<0.001). While this data is valuable, the nonrandomized, observational study design limits firm conclusions regarding vasopressor choice. Further results from three large trials comparing vasopressor use in septic shock should continue to shed light on this debate.
Bottom line: Norepinephrine administration is associated with higher hospital mortality and lower 28-day survival when compared with dopamine in patients with community-acquired septic shock.
Citation: Póvoa PR, Carneiro AH, Ribeiro OS, Pereira AC, Portuguese Community-Acquired Sepsis Study Group. Influence of vasopressor agent in septic shock mortality. Results from the Portuguese Community-Acquired Sepsis Study (SACiUCI study). Crit Care Med. 2009;37(2):410-416.
Oral Vitamin K Versus Placebo to Correct Excess Anticoagulation in Warfarin Patients
Clinical question: In nonbleeding patients with warfarin-associated coagulopathy, does oral vitamin K reduce bleeding events when compared to placebo?
Background: Warfarin is a common drug for primary and secondary prevention of thromboembolism, but it requires continued monitoring of the international normalized ratio (INR) value. INR values >4.0 are associated with an increase in bleeding complications, with specific concern for intracranial bleeding when INR values exceed 4.5. Small, randomized trials have shown that single, low-dose administration of oral vitamin K effectively reduces the INR in nonbleeding, overanticoagulated patients.
However, these studies have not shown if vitamin K reduces risk for bleeding without increasing the risk for thromboembolism.
Study design: Randomized, placebo-controlled trial.
Setting: Fourteen anticoagulation clinics in Canada, Italy, and the U.S.
Synopsis: Nonbleeding patients with supratherapeutic INR values between 4.5 and 10.0 were randomly assigned to receive 1.25 mg of oral vitamin K or placebo, then evaluated for all forms of bleeding for 90 days. Bleeding events were defined as “major bleeding,” “minor bleeding,” and “trivial bleeding.”
Though patients who received oral vitamin K had a significantly more rapid INR decrease, there were no differences between the two groups with regard to all bleeding events, thromboembolism, or death. The study was underpowered to detect differences in major bleeding.
Bottom line: Low-dose oral vitamin K leads to more rapid correction of the INR in overanticoagulated patients on warfarin therapy, but has little effect on clinical outcomes at 90 days.
Citation: Crowther MA, Ageno W, Garcia D, et al. Oral vitamin K versus placebo to correct excessive anticoagulation in patients receiving warfarin: a randomized trial. Ann Intern Med. 2009;150(5):293-300.
Inappropriate Treatment of Catheter-Associated Asymptomatic Bacteriuria
Clinical question: Are hospitalized patients with urinary catheters inappropriately treated with antibiotics for asymptomatic bacteriuria?
Background: Persons with catheters acquire bacteriuria at the rate of 3% to 10% per day, but in the majority of cases, no symptoms or secondary complications occur. Evidenced-based guidelines state that asymptomatic bacteriuria is not a clinically significant infection, and numerous studies have shown that treatment is unlikely to confer clinical benefit.
Study design: Retrospective cohort study.
Setting: A single-site Veterans Affairs hospital.
Synopsis: Using urine culture results over a three-month period from a single VA medical center, 280 cases were analyzed: 164 catheter-associated asymptomatic bacteriuria and 116 catheter-associated urinary tract infections (UTIs). A UTI was defined as having one or more of these symptoms: fever, urgency, frequency, dysuria, suprapubic tenderness, altered mental status, or hypotension in a patient without another recognized infection and a positive urine culture. Of the asymptomatic bacteriuria cases, 68% were managed appropriately with no antibiotic treatment; 32% were inappropriately treated with antibiotics.
In multivariate analysis, older patient age, predominance of gram-negative bacteria, and higher urine white blood cell count were significantly associated with inappropriate treatment.
This study highlights the fact that antibiotics continue to be used inappropriately in patients with catheters. Current guidelines do not distinguish well between asymptomatic bacteriuria and UTI, so there might be a knowledge gap. This study was based on urine culture data, not urinalysis of all patients with a catheter, so the symptomatic patients were likely over-represented.
An associated editorial observes that the study extrapolates data from studies that involved patients with uncomplicated UTIs and, therefore, might reach erroneous conclusions. Further, viewing catheter-associated symptomatic UTIs and catheter-associated asymptomatic bactiuria as dichotomous and warranting inherently different management fails to encompass a number of clinical factors, including co-infection, and further fails to acknowledge that removal of the catheter is the first step in treatment. However, the finding that antibiotics continue to be used inappropriately is useful.
Bottom line: A clinical determination of whether a patient with a catheter really has a symptomatic UTI/urosepsis or only has asymptomatic bacteriuria should precede starting antibiotics in hospitalized patients.
Citations: Cope M, Cevallos ME, Cadle RM, Darouiche RO, Musher DM, Trautner BW. Inappropriate treatment of catheter-associated asymptomatic bateriuria in a tertiary care hospital. Clin Infect Dis. 2009;48(9):1182-1188.
Kunin CM. Catheter-associated urinary tract infections: a syllogism compounded by a questionable dichotomy. Clin Infect Dis. 2009;48:1189-1190.
Current Practices in the Evaluation and Management of Thrombocytopenia in Heparin Patients
Clinical question: Are the current American College of Chest Physicians (ACCP) guidelines for the recognition, treatment, and prevention of heparin-induced thrombocytopenia (HIT) being followed?
Background: Heparin-based anticoagulation is frequently given to hospitalized patients, and approximately 1% to 5% of these patients develop HIT. In 2004, the ACCP published a consensus statement on the evaluation, management, and prevention of HIT.
Study design: Prospective, observational study.
Setting: Forty-eight U.S. hospitals in the Complications After Thrombocytopenia Caused by Heparin (CATCH) registry.
Synopsis: The CATCH trial enrolled patients receiving any form of heparin for >96 hours (n=2,420), cardiac-care-unit patients treated with heparin (n=1,090), and patients who had an HIT antibody assay performed (n=449), for a total of 3,536 total patients. The study included patients on unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH). Thrombocytopenia was defined at a platelet count <150,000, or a decrease of 50% when compared with admission.
In the prolonged heparin group, 36.4% of patients developed thrombocytopenia; however, HIT was suspected in only 19.8% of these high-risk patients. While physicians were more likely to consider HIT in the cardiac-care patients (37.6%), the diagnosis was considered>24 hours after the thrombocytopenia developed. Physicians often waited until after a thromboembolic complication occurred before evaluating for HIT. More often than not, preventive measures were missed (e.g., failing to check for HIT antibodies, continuing heparin after HIT was suspected).
Bottom line: Thrombocytopenia is a common occurrence in patients receiving heparin and, despite the risk of devastating complications from HIT, treatment infrequently conforms to the established guidelines.
Citation: Crespo EM, Oliveira GBF, Honeycutt EF, et al. Evaluation and management of thrombocytopenia and suspected heparin-induced thrombocytopenia in hospitalized patients: The Complications After Thrombocytopenia Caused by Heparin (CATCH) registry. Am Heart J. 2009;157(4):651-657. TH
Risk Stratification Tools for TIA
Transient ischemic attacks (TIAs) are common and represent a clarion call to action to prevent disabling stroke. Incidence estimates for TIA range from 37 to 107 per 100,000 persons each year.1 Extrapolating from these data, there are likely greater than 100,000 to 300,000 TIAs in the US annually. Within 3 months, approximately 10% of these patients will suffer a stroke, with approximately one‐half of these events occurring within the first 48 hours after the sentinel TIA.26 Nearly two‐thirds of secondary strokes result in disability and 21% are fatal.3 Hospitalists are frequently called to provide care for patients with TIA and, as such, in order to establish an appropriate care plan, they require tools to better predict the likelihood and timing of a disabling stroke.7 In this review we examine the rationale for early aggressive TIA evaluation and treatment in the hospital, overview risk stratification models to identify the patients at highest risk for early recurrent ischemia, and explore application of these tools to admission policy and individualized patient care planning.
Definition
TIA is defined as a brief episode of neurological dysfunction caused by focal brain or retinal ischemia with clinical symptoms typically lasting less than 1 hour and without evidence of brain infarction.8, 9 Prior arbitrary time limits are being abandoned as advanced imaging techniques demonstrate that clinical examination lacks the sensitivity to detect small cerebral infarctions leading to misclassification of as many as 30% to 40% of strokes as TIAs.811 For cases in which imaging is not available, the diagnosis of clinically probable TIA is suggested. Patients with imaging consistent with stroke appear to be at 4‐fold to 10‐fold higher risk for subsequent ischemic events, thus the presence of subclinical infarcts may have clinical importance.2, 12 The majority of TIAs resolve within 1 hour of onset and neurologic deficit continuance beyond this time frame is more consistent with a stroke.13 Continuing symptoms after 1 hour mandates aggressive therapy in lieu of withholding intervention in the hopes of a spontaneous recovery.
Rationale for Hospitalization
Urgent evaluation and treatment within 24 to 48 hours of a TIA is recommended by the National Stroke Association (Table 1).14 These guidelines also recommend hospital admission for high‐risk patients. There are a number of compelling arguments for the hospitalization of a patient at high risk for subsequent stroke.
| Test | Rationale | Therapy |
|---|---|---|
| ||
| Electrocardiogram and rhythm strip | To detect atrial fibrillation. | Long‐term warfarin indicated for suspected cardioembolic etiology. |
| Echocardiogram | To detect intracardiac thrombus or vegetations. Bubble study to detect patent foramen ovale in young patients. | Warfarin indicated for suspected cardioembolic etiology. Patent foramen ovale closure is an option for selected patients. |
| Carotid ultrasound | To detect large vessel atherosclerotic disease. | Antiplatelet therapy* indicated for atherosclerotic etiologies. Early carotid endarterectomy following TIA considered for 50% symptomatic ipsilateral stenosis. |
| Fasting lipid profile, complete blood count, serum electrolytes and creatinine | Secondary prevention of stroke by treating hyperlipidemia. Signs and symptoms associated with severe laboratory abnormalities may mimic TIA. | LDL >100 mg/dL (optional goal >70 mg/dL) is indication for cholesterol lowering therapy. |
| Neuroimaging‐MRI with diffusion images MRA or CT CTA | To detect clinically inapparent lesions of stroke. Useful in ruling out some mimics of TIA. | Patients with abnormal MRI diffusion images represent a population at increased risk for recurrent stroke. |
First, hospitalization offers potential for reduced time to thrombolysis for those patients who have a second ischemic event in the early period following TIA. Outpatients with new ischemic stroke may see hours pass between symptom onset and presentation to the emergency department (ED). This delay frequently places them outside of the thrombolytic window.1517 Hospitalization, assuming a well‐designed inpatient stroke care system, has great potential to reduce this delay. Approximately 50% of the stroke risk following a TIA is evident within 48 hours and rapid thrombolysis, available in an inpatient setting, is associated with improved outcome after stroke.3, 18 A cost‐utility analysis found that a 24‐hour admission for TIA patients to allow tissue plasminogen activator (t‐PA) for recurrent ischemia has a cost‐effectiveness ratio of $55,044 per quality‐adjusted life year with increasing cost effectiveness for the highest risk patients, such as those with a 24‐hour stroke risk of >5%.19
Second, hospital admission often facilitates the reliable and efficient evaluation for etiology and early initiation of secondary prevention. Neuroimaging, carotid ultrasound, echocardiography, and telemetry can be expedited with rapid initiation of proven secondary preventive therapies such as statin treatment, blood pressure control, and antithrombotic therapy. When indicated, carotid revascularization is recommended as soon as possible following TIA, with retrospective reviews suggesting improved outcomes when performed within 2 weeks of the event.1420 In one analysis, a negative association between hospitalization for TIA and subsequent stroke was discovered by review of Canadian population‐based administrative databases.5 While the mechanism for the negative association could not be established, the literature provides some support for hospitalization being associated with decreased risk for second strokes (hazard ratio [HR], 0.73; 95% confidence interval [CI], 0.570.95).5
Theoretically, much of this evaluation and treatment could occur in the outpatient setting but delays commonly seen in outpatient evaluation and the high potential for early second strokes for some patients may make this a risky care plan. Despite the high likelihood for serious outcomes following TIA and clear guidelines for early evaluation and management, current care often lacks a sense of urgency. A 2004 Canadian study revealed that three‐quarters of patients with a TIA were discharged directly from the ED with a resultant delay in diagnostic investigation.4 Over one‐third of patients were discharged without a prescription for antithrombotic therapy. American primary care practice patterns reveal even more significant delays in therapy, with only 2% of patients admitted to a hospital on the day of presentation for TIA, despite 80% of patients presenting for evaluation on the day of symptom onset.21 In this study less than one‐half of patients with atrial fibrillation were started on immediate anticoagulation.21 Further, as many as one‐third of patients did not have any evaluation in the month after the index event.21 Hospitalization for high‐risk patients has the potential to avoid these delays in outpatient evaluation and initiation of therapy.
Still, not all patients will require admission to a hospital setting. American EDs admit approximately one‐half of all TIAs, with regional variability not explained exclusively by clinical characteristics.22 Focusing on identifying the cohort of patients who would most benefit from hospitalization is paramount. In general, hospitalization should be reserved for patients with higher risk of an early secondary stroke. Specifically, admission is generally recommended for patients with crescendo symptoms, TIA on antithrombotic therapy, or symptoms lasting >1 hour.14 Additionally, patients with symptomatic carotid stenosis of 50% and presumed cardioembolic or hypercoagulable etiology merit hospital admission.14 In many cases these etiologies may not be known at time of presentation. Evaluation, such as carotid ultrasound, may not be readily available in the ED to inform the admission decision. Several new scoring systems that utilize routine clinical features available within an hour of presentation have been developed to more objectively assess the risk of secondary stroke following a TIA. The use of these prognostic scoring systems is recommended by the National Stroke Association to aid in triaging this cohort of patients.14
Prognostic Scoring Systems
California Score
The 2000 California Score (Tables 2 and 3) is a 5‐point tool retrospectively developed from a database of 1,707 TIA patients seen in the ED of a California hospital system to predict the likelihood of stroke within 90 days of an initial presentation with transient neurologic deficits.3 Multivariate logistic regression models were used to test the clinical factors associated with stroke risk, resulting in a tool using clinical features of age, diabetes, symptom duration, and the type of deficit to provide quantitative estimates of intermediate term risk. Within 90 days, none of the patients with a score of zero had a stroke whereas the highest score had a 34% risk of stroke (Table 3). While it is possible that some patients with lower scores had a nonischemic etiology for their transient neurological symptoms, clinical practice contains similar ambiguity, and such patients would be correctly predicted to have a low risk for subsequent strokes. Additionally, the derivation and validation of this tool did not include a neurologist confirmation of TIA diagnosis; however, this likely mirrors the practice in most EDs. The California Score has subsequently been validated and expanded upon during the development of the ABCD2 score.23 The California Score's utility is limited in the acute decision‐making process, especially regarding the decision to admit, as it focuses on 90‐day outcomes. For that, shorter‐term risk assessments are more useful.
| Clinical Feature | California Score (points) | ABCD (points) | ABCD2 (points) | |
|---|---|---|---|---|
| ||||
| Age | 60 years | 1 | 1 | 1 |
| Blood pressure | Systolic blood pressure 140 or diastolic blood pressure 90 mmHg | N/A | 1 | 1 |
| Clinical deficits* | Unilateral weakness (focal motor weakness of 1 or more of face, arm, hand, or leg) | 1 | 2 | 2 |
| Speech impairment (dysarthria, dysphasia, or both) | 1 | 1 | 1 | |
| Duration | 60 minutes | 1 | 2 | 2 |
| 10‐59 minutes | 1 | 1 | 1 | |
| Diabetes | Present | 1 | N/A | 1 |
| Maximum score | 5 | 6 | 7 | |
| California Score | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| ||||||
| 90‐day stroke risk (%) | 0 | 3 | 7 | 11 | 15 | 34 |
ABCD Score
The 2005 ABCD (Tables 2 and 4) is a 6‐point tool designed to predict 7‐day risk of stroke following TIA from the Oxfordshire Community Stroke Project, a UK prospective population‐based cohort of 209 patients with diagnosis of TIA made by a neurologist.24 It evaluated factors previously found to be independent predictors of stroke after TIA, and determined that risk factors of age, blood pressure, type of clinical deficit, and symptom duration predicted 7‐day risk of stroke following TIA. Unlike the California score, the ABCD authors found presenting blood pressure, but not diabetes, to be independent predictors of future events. The authors validated the score with a second population of TIA patients in the Oxford Vascular Study and in a hospital‐based TIA clinic cohort.24 In the validation cohorts the score was highly predictive of stroke at 7 days (P < 0.001). Patients with the lowest scores of 0 to 3 had no strokes in the week following the index TIA, whereas patients with the highest score of 6 carried a 35.5% risk of early second stroke. The primary limitation of the ABCD score lies in the small sample size, with only 18 recurrent strokes in the week after TIA in the derivation cohort.
| ABCD Score | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| |||||||
| 7‐Day stroke risk (%) | 0 | 0 | 0 | 0 | 2.2 | 16.3 | 35.5 |
The ABCD score has subsequently been tested in other settings with mixed results. Two studies found limited utility.25, 26 Other trials found high scores to be overly inclusive but highly predictive and thus the majority of evidence appears to support the validity of the ABCD score in predicting risk of early recurrent ischemic events.2729 The ABCD score has been used to triage patients with high scores to inpatient management although the benefit of this strategy on outcomes has not been proven.30
ABCD2 Score
The 2007 ABCD2 (Tables 2 and 5) is a 7‐point tool that uses the original ABCD criteria along with an additional point for diabetes.23 The ABCD2 derived a unified prognostic score for optimal prediction of the 2‐day stroke risk from multivariate analysis of the original data sets used to create the California and ABCD scores. This score was then validated by the authors in 4 independent cohorts from the United States and the United Kingdom.23 In sum, 4809 patients with TIA were included in the ABCD2 analysis. Similar to prior studies, stroke occurred in 9.2% of patients by 90 days of which 20% were fatal. The authors created low (03 points), intermediate (45 points), and high (67 points) risk categories. In the validation cohorts the prediction rule for the ABCD2 functioned better than the California or ABCD scores with c statistics of 0.62 to 0.83 (ideal prediction produces a c statistic of 1 and prediction no better than chance would have a c statistic of 0.5). They found a 2‐day risk of stroke of 1% for low, 4.1% for intermediate, and 8.1% for the high risk group. Data from the study suggest 34% of TIA patients will be in low‐risk, 45% in intermediate‐risk, and 21% in high‐risk categories. While the ABCD2 score overcomes some of the problems with the 2 prior systems it shares many of the limitations as it was derived from the combined data sets. All scoring systems lack the ability to provide guidance on the management of TIAs associated with rare conditions, such as vasculitis, that are underrepresented in the derivation data sets. The ABCD2 also does not incorporate imaging data and this will likely require further exploration.
| ABCD2 Score | 0‐3 | 4‐5 | 6‐7 |
|---|---|---|---|
| |||
| Risk stratification | Low | Intermediate | High |
| 2‐Day stroke risk (%) | 1 | 4.1 | 8.1 |
| 7‐Day stroke risk (%) | 1.2 | 5.9 | 11.7 |
| 90‐Day stroke risk (%) | 3.1 | 9.8 | 17.8 |
The ABCD2 score can be used to predict risk for a variety of time intervals, has now been validated in independent Greek and British populations, and appears to be the best performing tool at predicting early risk of stroke regardless of underlying etiology.23, 31, 32 The authors suggest that admission for patients in the high‐risk group is prudent whereas outpatient evaluation is reasonable for patients in the low‐risk group.23 Admission for patients in the intermediate‐risk group will depend on individualized decision making, local practice standards, and available community resources.
New Models of Care: An Opportunity for Hospitalists
The key to improving TIA outcomes appears to be more contingent on the speed of evaluation and initiation of appropriate therapy than on the location of the care. The EXPRESS trial studied the effect of an immediate access neurovascular clinic providing urgent evaluation and immediate treatment of nonhospitalized TIA patients versus usual care. Statistically significant reductions were seen in time to evaluation, first treatment prescription, and in 90‐day risk of recurrent stroke (10.3% versus 2.1%, P < 0.0001) after the clinic was changed to the rapid evaluation and treatment model.33
The SOS‐TIA study used a 24‐hour access hospital‐based TIA clinic to evaluate the effects of rapid assessment and interventions on hospital length of stay and clinical outcomes.34 The 90‐day stroke rate was 1.24% (95% CI, 0.712.12), which represents a 79% reduction compared to the predicted stroke rate from the ABCD2 scores. With expedited evaluation and treatment, 74% of patients were able to be sent home on the same day.
The results of these 2 new studies provide compelling evidence that rapid evaluation and treatment in the first 48 hours after TIA has the potential to alter outcomes. Unfortunately not all communities have access to same day TIA clinics. Still, these findings should embolden hospitalists to advocate for urgent evaluation, such as neurology and cardiac imaging and carotid evaluation, with immediate initiation of secondary preventive therapy and early surgical intervention when appropriate. In most cases these changes will require process transformations that present prime opportunities for hospitalists to reengineer systems of care.
Incorporating Prognostic Scores into Clinical Practice
Applying the evidence to practice requires calculation of the early risk but also awareness of the community resources available. High‐risk patients with an ABCD2 score of 6 or 7 have a very high 8.1% risk of stroke within the next 48 hours. Given the catastrophic outcomes frequently seen after second strokes, these patients warrant inpatient admission to facilitate the immediate initiation of appropriate secondary prevention and potentially shorten time to thrombolysis if an early stroke occurs. Intermediate‐risk patients with ABCD2 scores of 4 and 5 have a 4.1% 2‐day risk of stroke and may be considered for admission, hospital observation, or expedited clinic evaluation contingent on local availability. As many as one‐third of TIA patients will be categorized as low risk with a score of 0 to 3. These patients have a 2‐day risk of stroke of only 1% and are likely safe for prompt outpatient evaluation and management. The new, validated, ABCD2 score is not a substitute for individualized judgment, but is helpful in developing admission guidelines in cooperation between neurologists, emergency room physicians, and hospitalists, and in using evidence‐based medicine to provide optimal care for the patient presenting with a TIA.
Stroke and TIA arise from identical etiologies, respond to the same secondary preventive measures, and should be considered part of the spectrum of an ischemic cerebral syndrome. Recognizing TIA as a medical emergency with high rates of secondary stroke and subsequent disability allows institution of therapies with appropriate urgency. Hospitalization offers the ability to rapidly coordinate the testing and secondary prevention measures but also, for high‐risk patients, offers the opportunity to reduce the time to thrombolysis for early recurrent strokes. New, validated scoring systems such as the ABCD2 score help the hospitalist to decide which patients are appropriate for admission and which can be managed in progressive and traditional outpatient settings.
- ,,.Epidemiological impact in the United States of a tissue‐based definition of transient ischemic attack.Stroke.2003;34:919–924.
- ,,, et al.Head computed tomography findings predict short‐term stroke risk after transient ischemic attack.Stroke.2003;34:2894–2899.
- ,,, et al.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,, et al.Management and outcomes of transient ischemic attacks in Ontario.CMAJ.2004;170:1099–1104.
- ,,, et al.The high risk of stroke immediately after transient ischemic attack: a population based study.Neurology.2004;62:2015–2020.
- ,,, et al.Early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis.Arch Intern Med.2007;167:2417–2422.
- ,,, et al.The spectrum of community‐based hospitalist practice: a call to tailor internal medicine residency training.Arch Intern Med.2007;167:727–729.
- .Transient ischemic attack with abnormal diffusion‐weighted imaging results: what's in a name?Arch Neurol.2007;64:1080–1082.
- ,.A reappraisal of the definition and pathophysiology of the transient ischemic attack.Med Sci Monit.2007;13:RA50–53.
- ,,, et al.Diffusion‐weighted imaging‐negative patients with transient ischemic attack are at risk of recurrent transient events.Stroke.2007;38:2367–2369.
- ,,, et al.Transient ischemic attack—proposal for a new definition.N Engl J Med.2002;347:1713–1716.
- ,,, et al.Management and outcome of patients with transient ischemic attack admitted to a stroke unit.Cerebrovasc Dis.2007;24:80–85.
- .How transient are transient ischemic attacks.Neurology.1988;38:674–677.
- ,,, et al.National Stroke Association guidelines for the management of transient ischemic attacks.Ann Neurol.2006;60:301–313.
- ,,, et al.Out‐of‐hospital delays in patients with acute stroke.Ann Emerg Med.2004;44:476–483.
- ,,, et al.Factors associated with delayed admission to hospital and in‐hospital delays in acute stroke and TIA: a prospective multicenter study.Stroke.1999;30:40–48.
- ,,, et al.Prehospital and emergency department delays after acute stroke: the Genentech Stroke Presentation Survey.Stroke.2000;31:2585–2590.
- Tissue plasminogen activator for acute ischemic stroke.The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.N Engl J Med.1995;333:1581–1587.
- ,.Is hospitalization after TIA cost effective on the basis of treatment with tPA?Neurology.2005;65:1799–1801.
- ,,, et al.Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery.Lancet.2004;363:915–924.
- ,,, et al.New transient ischemic attack and stroke: outpatient management by primary care physicians.Arch Intern Med.2000;160:2941–2946.
- ,,, et al.Hospital and demographic influences on the disposition of transient ischemic attack.Acad Emerg Med.2008;15:171–176.
- ,,, et al.Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack.Lancet.2007;369:283–292.
- ,,, et al.A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack.Lancet.2005;366:29–36.
- ,,, et al.Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack?Stroke.2006;37:1710–1714.
- ,,, et al.Absence of usefulness of ABCD score in the early risk of recurrent stroke in transient ischemic attack patients.Stroke.2007;38:855–856.
- ,,, et al.Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital‐based case series study.Stroke.2006;37:2892–2897.
- ,.Rapid identification of high‐risk transient ischemic attacks: prospective validation of the ABCD score.Stroke.2008;39:297–302.
- ,,.Can the ABCD score be dichotomized to identify high‐risk patients with transient ischaemic attack in the emergency department?Emerg Med J.2007;24:92–95.
- ,,, et al.Can risk stratification of transient ischaemic attacks improve patient care in the emergency department?Emerg Med J.2007;24:637–640.
- ,,, et al.Prognosis in patients with transient ischaemic attack (TIA) and minor stroke attending TIA services in the north west of England: The NORTHSTAR Study.J Neurol Neurosurg Psychiatry.2007:1–6.
- ,,.Potential applicability of the ABCD2 in triaging TIA patients.Lancet.2007;369:1082.
- ,,, et al.Effect of urgent treatment of transient ischemic attack and minor stroke on early recurrent stroke (EXPRESS Study): a prospective population‐based sequential comparison.Lancet.2007;370;1432–1442.
- ,,, et al.A transient ischemic attack clinic with round‐the‐clock access (SOS‐TIA): feasibility and effects.Lancet Neurol.2007;6:953–960.
Transient ischemic attacks (TIAs) are common and represent a clarion call to action to prevent disabling stroke. Incidence estimates for TIA range from 37 to 107 per 100,000 persons each year.1 Extrapolating from these data, there are likely greater than 100,000 to 300,000 TIAs in the US annually. Within 3 months, approximately 10% of these patients will suffer a stroke, with approximately one‐half of these events occurring within the first 48 hours after the sentinel TIA.26 Nearly two‐thirds of secondary strokes result in disability and 21% are fatal.3 Hospitalists are frequently called to provide care for patients with TIA and, as such, in order to establish an appropriate care plan, they require tools to better predict the likelihood and timing of a disabling stroke.7 In this review we examine the rationale for early aggressive TIA evaluation and treatment in the hospital, overview risk stratification models to identify the patients at highest risk for early recurrent ischemia, and explore application of these tools to admission policy and individualized patient care planning.
Definition
TIA is defined as a brief episode of neurological dysfunction caused by focal brain or retinal ischemia with clinical symptoms typically lasting less than 1 hour and without evidence of brain infarction.8, 9 Prior arbitrary time limits are being abandoned as advanced imaging techniques demonstrate that clinical examination lacks the sensitivity to detect small cerebral infarctions leading to misclassification of as many as 30% to 40% of strokes as TIAs.811 For cases in which imaging is not available, the diagnosis of clinically probable TIA is suggested. Patients with imaging consistent with stroke appear to be at 4‐fold to 10‐fold higher risk for subsequent ischemic events, thus the presence of subclinical infarcts may have clinical importance.2, 12 The majority of TIAs resolve within 1 hour of onset and neurologic deficit continuance beyond this time frame is more consistent with a stroke.13 Continuing symptoms after 1 hour mandates aggressive therapy in lieu of withholding intervention in the hopes of a spontaneous recovery.
Rationale for Hospitalization
Urgent evaluation and treatment within 24 to 48 hours of a TIA is recommended by the National Stroke Association (Table 1).14 These guidelines also recommend hospital admission for high‐risk patients. There are a number of compelling arguments for the hospitalization of a patient at high risk for subsequent stroke.
| Test | Rationale | Therapy |
|---|---|---|
| ||
| Electrocardiogram and rhythm strip | To detect atrial fibrillation. | Long‐term warfarin indicated for suspected cardioembolic etiology. |
| Echocardiogram | To detect intracardiac thrombus or vegetations. Bubble study to detect patent foramen ovale in young patients. | Warfarin indicated for suspected cardioembolic etiology. Patent foramen ovale closure is an option for selected patients. |
| Carotid ultrasound | To detect large vessel atherosclerotic disease. | Antiplatelet therapy* indicated for atherosclerotic etiologies. Early carotid endarterectomy following TIA considered for 50% symptomatic ipsilateral stenosis. |
| Fasting lipid profile, complete blood count, serum electrolytes and creatinine | Secondary prevention of stroke by treating hyperlipidemia. Signs and symptoms associated with severe laboratory abnormalities may mimic TIA. | LDL >100 mg/dL (optional goal >70 mg/dL) is indication for cholesterol lowering therapy. |
| Neuroimaging‐MRI with diffusion images MRA or CT CTA | To detect clinically inapparent lesions of stroke. Useful in ruling out some mimics of TIA. | Patients with abnormal MRI diffusion images represent a population at increased risk for recurrent stroke. |
First, hospitalization offers potential for reduced time to thrombolysis for those patients who have a second ischemic event in the early period following TIA. Outpatients with new ischemic stroke may see hours pass between symptom onset and presentation to the emergency department (ED). This delay frequently places them outside of the thrombolytic window.1517 Hospitalization, assuming a well‐designed inpatient stroke care system, has great potential to reduce this delay. Approximately 50% of the stroke risk following a TIA is evident within 48 hours and rapid thrombolysis, available in an inpatient setting, is associated with improved outcome after stroke.3, 18 A cost‐utility analysis found that a 24‐hour admission for TIA patients to allow tissue plasminogen activator (t‐PA) for recurrent ischemia has a cost‐effectiveness ratio of $55,044 per quality‐adjusted life year with increasing cost effectiveness for the highest risk patients, such as those with a 24‐hour stroke risk of >5%.19
Second, hospital admission often facilitates the reliable and efficient evaluation for etiology and early initiation of secondary prevention. Neuroimaging, carotid ultrasound, echocardiography, and telemetry can be expedited with rapid initiation of proven secondary preventive therapies such as statin treatment, blood pressure control, and antithrombotic therapy. When indicated, carotid revascularization is recommended as soon as possible following TIA, with retrospective reviews suggesting improved outcomes when performed within 2 weeks of the event.1420 In one analysis, a negative association between hospitalization for TIA and subsequent stroke was discovered by review of Canadian population‐based administrative databases.5 While the mechanism for the negative association could not be established, the literature provides some support for hospitalization being associated with decreased risk for second strokes (hazard ratio [HR], 0.73; 95% confidence interval [CI], 0.570.95).5
Theoretically, much of this evaluation and treatment could occur in the outpatient setting but delays commonly seen in outpatient evaluation and the high potential for early second strokes for some patients may make this a risky care plan. Despite the high likelihood for serious outcomes following TIA and clear guidelines for early evaluation and management, current care often lacks a sense of urgency. A 2004 Canadian study revealed that three‐quarters of patients with a TIA were discharged directly from the ED with a resultant delay in diagnostic investigation.4 Over one‐third of patients were discharged without a prescription for antithrombotic therapy. American primary care practice patterns reveal even more significant delays in therapy, with only 2% of patients admitted to a hospital on the day of presentation for TIA, despite 80% of patients presenting for evaluation on the day of symptom onset.21 In this study less than one‐half of patients with atrial fibrillation were started on immediate anticoagulation.21 Further, as many as one‐third of patients did not have any evaluation in the month after the index event.21 Hospitalization for high‐risk patients has the potential to avoid these delays in outpatient evaluation and initiation of therapy.
Still, not all patients will require admission to a hospital setting. American EDs admit approximately one‐half of all TIAs, with regional variability not explained exclusively by clinical characteristics.22 Focusing on identifying the cohort of patients who would most benefit from hospitalization is paramount. In general, hospitalization should be reserved for patients with higher risk of an early secondary stroke. Specifically, admission is generally recommended for patients with crescendo symptoms, TIA on antithrombotic therapy, or symptoms lasting >1 hour.14 Additionally, patients with symptomatic carotid stenosis of 50% and presumed cardioembolic or hypercoagulable etiology merit hospital admission.14 In many cases these etiologies may not be known at time of presentation. Evaluation, such as carotid ultrasound, may not be readily available in the ED to inform the admission decision. Several new scoring systems that utilize routine clinical features available within an hour of presentation have been developed to more objectively assess the risk of secondary stroke following a TIA. The use of these prognostic scoring systems is recommended by the National Stroke Association to aid in triaging this cohort of patients.14
Prognostic Scoring Systems
California Score
The 2000 California Score (Tables 2 and 3) is a 5‐point tool retrospectively developed from a database of 1,707 TIA patients seen in the ED of a California hospital system to predict the likelihood of stroke within 90 days of an initial presentation with transient neurologic deficits.3 Multivariate logistic regression models were used to test the clinical factors associated with stroke risk, resulting in a tool using clinical features of age, diabetes, symptom duration, and the type of deficit to provide quantitative estimates of intermediate term risk. Within 90 days, none of the patients with a score of zero had a stroke whereas the highest score had a 34% risk of stroke (Table 3). While it is possible that some patients with lower scores had a nonischemic etiology for their transient neurological symptoms, clinical practice contains similar ambiguity, and such patients would be correctly predicted to have a low risk for subsequent strokes. Additionally, the derivation and validation of this tool did not include a neurologist confirmation of TIA diagnosis; however, this likely mirrors the practice in most EDs. The California Score has subsequently been validated and expanded upon during the development of the ABCD2 score.23 The California Score's utility is limited in the acute decision‐making process, especially regarding the decision to admit, as it focuses on 90‐day outcomes. For that, shorter‐term risk assessments are more useful.
| Clinical Feature | California Score (points) | ABCD (points) | ABCD2 (points) | |
|---|---|---|---|---|
| ||||
| Age | 60 years | 1 | 1 | 1 |
| Blood pressure | Systolic blood pressure 140 or diastolic blood pressure 90 mmHg | N/A | 1 | 1 |
| Clinical deficits* | Unilateral weakness (focal motor weakness of 1 or more of face, arm, hand, or leg) | 1 | 2 | 2 |
| Speech impairment (dysarthria, dysphasia, or both) | 1 | 1 | 1 | |
| Duration | 60 minutes | 1 | 2 | 2 |
| 10‐59 minutes | 1 | 1 | 1 | |
| Diabetes | Present | 1 | N/A | 1 |
| Maximum score | 5 | 6 | 7 | |
| California Score | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| ||||||
| 90‐day stroke risk (%) | 0 | 3 | 7 | 11 | 15 | 34 |
ABCD Score
The 2005 ABCD (Tables 2 and 4) is a 6‐point tool designed to predict 7‐day risk of stroke following TIA from the Oxfordshire Community Stroke Project, a UK prospective population‐based cohort of 209 patients with diagnosis of TIA made by a neurologist.24 It evaluated factors previously found to be independent predictors of stroke after TIA, and determined that risk factors of age, blood pressure, type of clinical deficit, and symptom duration predicted 7‐day risk of stroke following TIA. Unlike the California score, the ABCD authors found presenting blood pressure, but not diabetes, to be independent predictors of future events. The authors validated the score with a second population of TIA patients in the Oxford Vascular Study and in a hospital‐based TIA clinic cohort.24 In the validation cohorts the score was highly predictive of stroke at 7 days (P < 0.001). Patients with the lowest scores of 0 to 3 had no strokes in the week following the index TIA, whereas patients with the highest score of 6 carried a 35.5% risk of early second stroke. The primary limitation of the ABCD score lies in the small sample size, with only 18 recurrent strokes in the week after TIA in the derivation cohort.
| ABCD Score | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| |||||||
| 7‐Day stroke risk (%) | 0 | 0 | 0 | 0 | 2.2 | 16.3 | 35.5 |
The ABCD score has subsequently been tested in other settings with mixed results. Two studies found limited utility.25, 26 Other trials found high scores to be overly inclusive but highly predictive and thus the majority of evidence appears to support the validity of the ABCD score in predicting risk of early recurrent ischemic events.2729 The ABCD score has been used to triage patients with high scores to inpatient management although the benefit of this strategy on outcomes has not been proven.30
ABCD2 Score
The 2007 ABCD2 (Tables 2 and 5) is a 7‐point tool that uses the original ABCD criteria along with an additional point for diabetes.23 The ABCD2 derived a unified prognostic score for optimal prediction of the 2‐day stroke risk from multivariate analysis of the original data sets used to create the California and ABCD scores. This score was then validated by the authors in 4 independent cohorts from the United States and the United Kingdom.23 In sum, 4809 patients with TIA were included in the ABCD2 analysis. Similar to prior studies, stroke occurred in 9.2% of patients by 90 days of which 20% were fatal. The authors created low (03 points), intermediate (45 points), and high (67 points) risk categories. In the validation cohorts the prediction rule for the ABCD2 functioned better than the California or ABCD scores with c statistics of 0.62 to 0.83 (ideal prediction produces a c statistic of 1 and prediction no better than chance would have a c statistic of 0.5). They found a 2‐day risk of stroke of 1% for low, 4.1% for intermediate, and 8.1% for the high risk group. Data from the study suggest 34% of TIA patients will be in low‐risk, 45% in intermediate‐risk, and 21% in high‐risk categories. While the ABCD2 score overcomes some of the problems with the 2 prior systems it shares many of the limitations as it was derived from the combined data sets. All scoring systems lack the ability to provide guidance on the management of TIAs associated with rare conditions, such as vasculitis, that are underrepresented in the derivation data sets. The ABCD2 also does not incorporate imaging data and this will likely require further exploration.
| ABCD2 Score | 0‐3 | 4‐5 | 6‐7 |
|---|---|---|---|
| |||
| Risk stratification | Low | Intermediate | High |
| 2‐Day stroke risk (%) | 1 | 4.1 | 8.1 |
| 7‐Day stroke risk (%) | 1.2 | 5.9 | 11.7 |
| 90‐Day stroke risk (%) | 3.1 | 9.8 | 17.8 |
The ABCD2 score can be used to predict risk for a variety of time intervals, has now been validated in independent Greek and British populations, and appears to be the best performing tool at predicting early risk of stroke regardless of underlying etiology.23, 31, 32 The authors suggest that admission for patients in the high‐risk group is prudent whereas outpatient evaluation is reasonable for patients in the low‐risk group.23 Admission for patients in the intermediate‐risk group will depend on individualized decision making, local practice standards, and available community resources.
New Models of Care: An Opportunity for Hospitalists
The key to improving TIA outcomes appears to be more contingent on the speed of evaluation and initiation of appropriate therapy than on the location of the care. The EXPRESS trial studied the effect of an immediate access neurovascular clinic providing urgent evaluation and immediate treatment of nonhospitalized TIA patients versus usual care. Statistically significant reductions were seen in time to evaluation, first treatment prescription, and in 90‐day risk of recurrent stroke (10.3% versus 2.1%, P < 0.0001) after the clinic was changed to the rapid evaluation and treatment model.33
The SOS‐TIA study used a 24‐hour access hospital‐based TIA clinic to evaluate the effects of rapid assessment and interventions on hospital length of stay and clinical outcomes.34 The 90‐day stroke rate was 1.24% (95% CI, 0.712.12), which represents a 79% reduction compared to the predicted stroke rate from the ABCD2 scores. With expedited evaluation and treatment, 74% of patients were able to be sent home on the same day.
The results of these 2 new studies provide compelling evidence that rapid evaluation and treatment in the first 48 hours after TIA has the potential to alter outcomes. Unfortunately not all communities have access to same day TIA clinics. Still, these findings should embolden hospitalists to advocate for urgent evaluation, such as neurology and cardiac imaging and carotid evaluation, with immediate initiation of secondary preventive therapy and early surgical intervention when appropriate. In most cases these changes will require process transformations that present prime opportunities for hospitalists to reengineer systems of care.
Incorporating Prognostic Scores into Clinical Practice
Applying the evidence to practice requires calculation of the early risk but also awareness of the community resources available. High‐risk patients with an ABCD2 score of 6 or 7 have a very high 8.1% risk of stroke within the next 48 hours. Given the catastrophic outcomes frequently seen after second strokes, these patients warrant inpatient admission to facilitate the immediate initiation of appropriate secondary prevention and potentially shorten time to thrombolysis if an early stroke occurs. Intermediate‐risk patients with ABCD2 scores of 4 and 5 have a 4.1% 2‐day risk of stroke and may be considered for admission, hospital observation, or expedited clinic evaluation contingent on local availability. As many as one‐third of TIA patients will be categorized as low risk with a score of 0 to 3. These patients have a 2‐day risk of stroke of only 1% and are likely safe for prompt outpatient evaluation and management. The new, validated, ABCD2 score is not a substitute for individualized judgment, but is helpful in developing admission guidelines in cooperation between neurologists, emergency room physicians, and hospitalists, and in using evidence‐based medicine to provide optimal care for the patient presenting with a TIA.
Stroke and TIA arise from identical etiologies, respond to the same secondary preventive measures, and should be considered part of the spectrum of an ischemic cerebral syndrome. Recognizing TIA as a medical emergency with high rates of secondary stroke and subsequent disability allows institution of therapies with appropriate urgency. Hospitalization offers the ability to rapidly coordinate the testing and secondary prevention measures but also, for high‐risk patients, offers the opportunity to reduce the time to thrombolysis for early recurrent strokes. New, validated scoring systems such as the ABCD2 score help the hospitalist to decide which patients are appropriate for admission and which can be managed in progressive and traditional outpatient settings.
Transient ischemic attacks (TIAs) are common and represent a clarion call to action to prevent disabling stroke. Incidence estimates for TIA range from 37 to 107 per 100,000 persons each year.1 Extrapolating from these data, there are likely greater than 100,000 to 300,000 TIAs in the US annually. Within 3 months, approximately 10% of these patients will suffer a stroke, with approximately one‐half of these events occurring within the first 48 hours after the sentinel TIA.26 Nearly two‐thirds of secondary strokes result in disability and 21% are fatal.3 Hospitalists are frequently called to provide care for patients with TIA and, as such, in order to establish an appropriate care plan, they require tools to better predict the likelihood and timing of a disabling stroke.7 In this review we examine the rationale for early aggressive TIA evaluation and treatment in the hospital, overview risk stratification models to identify the patients at highest risk for early recurrent ischemia, and explore application of these tools to admission policy and individualized patient care planning.
Definition
TIA is defined as a brief episode of neurological dysfunction caused by focal brain or retinal ischemia with clinical symptoms typically lasting less than 1 hour and without evidence of brain infarction.8, 9 Prior arbitrary time limits are being abandoned as advanced imaging techniques demonstrate that clinical examination lacks the sensitivity to detect small cerebral infarctions leading to misclassification of as many as 30% to 40% of strokes as TIAs.811 For cases in which imaging is not available, the diagnosis of clinically probable TIA is suggested. Patients with imaging consistent with stroke appear to be at 4‐fold to 10‐fold higher risk for subsequent ischemic events, thus the presence of subclinical infarcts may have clinical importance.2, 12 The majority of TIAs resolve within 1 hour of onset and neurologic deficit continuance beyond this time frame is more consistent with a stroke.13 Continuing symptoms after 1 hour mandates aggressive therapy in lieu of withholding intervention in the hopes of a spontaneous recovery.
Rationale for Hospitalization
Urgent evaluation and treatment within 24 to 48 hours of a TIA is recommended by the National Stroke Association (Table 1).14 These guidelines also recommend hospital admission for high‐risk patients. There are a number of compelling arguments for the hospitalization of a patient at high risk for subsequent stroke.
| Test | Rationale | Therapy |
|---|---|---|
| ||
| Electrocardiogram and rhythm strip | To detect atrial fibrillation. | Long‐term warfarin indicated for suspected cardioembolic etiology. |
| Echocardiogram | To detect intracardiac thrombus or vegetations. Bubble study to detect patent foramen ovale in young patients. | Warfarin indicated for suspected cardioembolic etiology. Patent foramen ovale closure is an option for selected patients. |
| Carotid ultrasound | To detect large vessel atherosclerotic disease. | Antiplatelet therapy* indicated for atherosclerotic etiologies. Early carotid endarterectomy following TIA considered for 50% symptomatic ipsilateral stenosis. |
| Fasting lipid profile, complete blood count, serum electrolytes and creatinine | Secondary prevention of stroke by treating hyperlipidemia. Signs and symptoms associated with severe laboratory abnormalities may mimic TIA. | LDL >100 mg/dL (optional goal >70 mg/dL) is indication for cholesterol lowering therapy. |
| Neuroimaging‐MRI with diffusion images MRA or CT CTA | To detect clinically inapparent lesions of stroke. Useful in ruling out some mimics of TIA. | Patients with abnormal MRI diffusion images represent a population at increased risk for recurrent stroke. |
First, hospitalization offers potential for reduced time to thrombolysis for those patients who have a second ischemic event in the early period following TIA. Outpatients with new ischemic stroke may see hours pass between symptom onset and presentation to the emergency department (ED). This delay frequently places them outside of the thrombolytic window.1517 Hospitalization, assuming a well‐designed inpatient stroke care system, has great potential to reduce this delay. Approximately 50% of the stroke risk following a TIA is evident within 48 hours and rapid thrombolysis, available in an inpatient setting, is associated with improved outcome after stroke.3, 18 A cost‐utility analysis found that a 24‐hour admission for TIA patients to allow tissue plasminogen activator (t‐PA) for recurrent ischemia has a cost‐effectiveness ratio of $55,044 per quality‐adjusted life year with increasing cost effectiveness for the highest risk patients, such as those with a 24‐hour stroke risk of >5%.19
Second, hospital admission often facilitates the reliable and efficient evaluation for etiology and early initiation of secondary prevention. Neuroimaging, carotid ultrasound, echocardiography, and telemetry can be expedited with rapid initiation of proven secondary preventive therapies such as statin treatment, blood pressure control, and antithrombotic therapy. When indicated, carotid revascularization is recommended as soon as possible following TIA, with retrospective reviews suggesting improved outcomes when performed within 2 weeks of the event.1420 In one analysis, a negative association between hospitalization for TIA and subsequent stroke was discovered by review of Canadian population‐based administrative databases.5 While the mechanism for the negative association could not be established, the literature provides some support for hospitalization being associated with decreased risk for second strokes (hazard ratio [HR], 0.73; 95% confidence interval [CI], 0.570.95).5
Theoretically, much of this evaluation and treatment could occur in the outpatient setting but delays commonly seen in outpatient evaluation and the high potential for early second strokes for some patients may make this a risky care plan. Despite the high likelihood for serious outcomes following TIA and clear guidelines for early evaluation and management, current care often lacks a sense of urgency. A 2004 Canadian study revealed that three‐quarters of patients with a TIA were discharged directly from the ED with a resultant delay in diagnostic investigation.4 Over one‐third of patients were discharged without a prescription for antithrombotic therapy. American primary care practice patterns reveal even more significant delays in therapy, with only 2% of patients admitted to a hospital on the day of presentation for TIA, despite 80% of patients presenting for evaluation on the day of symptom onset.21 In this study less than one‐half of patients with atrial fibrillation were started on immediate anticoagulation.21 Further, as many as one‐third of patients did not have any evaluation in the month after the index event.21 Hospitalization for high‐risk patients has the potential to avoid these delays in outpatient evaluation and initiation of therapy.
Still, not all patients will require admission to a hospital setting. American EDs admit approximately one‐half of all TIAs, with regional variability not explained exclusively by clinical characteristics.22 Focusing on identifying the cohort of patients who would most benefit from hospitalization is paramount. In general, hospitalization should be reserved for patients with higher risk of an early secondary stroke. Specifically, admission is generally recommended for patients with crescendo symptoms, TIA on antithrombotic therapy, or symptoms lasting >1 hour.14 Additionally, patients with symptomatic carotid stenosis of 50% and presumed cardioembolic or hypercoagulable etiology merit hospital admission.14 In many cases these etiologies may not be known at time of presentation. Evaluation, such as carotid ultrasound, may not be readily available in the ED to inform the admission decision. Several new scoring systems that utilize routine clinical features available within an hour of presentation have been developed to more objectively assess the risk of secondary stroke following a TIA. The use of these prognostic scoring systems is recommended by the National Stroke Association to aid in triaging this cohort of patients.14
Prognostic Scoring Systems
California Score
The 2000 California Score (Tables 2 and 3) is a 5‐point tool retrospectively developed from a database of 1,707 TIA patients seen in the ED of a California hospital system to predict the likelihood of stroke within 90 days of an initial presentation with transient neurologic deficits.3 Multivariate logistic regression models were used to test the clinical factors associated with stroke risk, resulting in a tool using clinical features of age, diabetes, symptom duration, and the type of deficit to provide quantitative estimates of intermediate term risk. Within 90 days, none of the patients with a score of zero had a stroke whereas the highest score had a 34% risk of stroke (Table 3). While it is possible that some patients with lower scores had a nonischemic etiology for their transient neurological symptoms, clinical practice contains similar ambiguity, and such patients would be correctly predicted to have a low risk for subsequent strokes. Additionally, the derivation and validation of this tool did not include a neurologist confirmation of TIA diagnosis; however, this likely mirrors the practice in most EDs. The California Score has subsequently been validated and expanded upon during the development of the ABCD2 score.23 The California Score's utility is limited in the acute decision‐making process, especially regarding the decision to admit, as it focuses on 90‐day outcomes. For that, shorter‐term risk assessments are more useful.
| Clinical Feature | California Score (points) | ABCD (points) | ABCD2 (points) | |
|---|---|---|---|---|
| ||||
| Age | 60 years | 1 | 1 | 1 |
| Blood pressure | Systolic blood pressure 140 or diastolic blood pressure 90 mmHg | N/A | 1 | 1 |
| Clinical deficits* | Unilateral weakness (focal motor weakness of 1 or more of face, arm, hand, or leg) | 1 | 2 | 2 |
| Speech impairment (dysarthria, dysphasia, or both) | 1 | 1 | 1 | |
| Duration | 60 minutes | 1 | 2 | 2 |
| 10‐59 minutes | 1 | 1 | 1 | |
| Diabetes | Present | 1 | N/A | 1 |
| Maximum score | 5 | 6 | 7 | |
| California Score | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| ||||||
| 90‐day stroke risk (%) | 0 | 3 | 7 | 11 | 15 | 34 |
ABCD Score
The 2005 ABCD (Tables 2 and 4) is a 6‐point tool designed to predict 7‐day risk of stroke following TIA from the Oxfordshire Community Stroke Project, a UK prospective population‐based cohort of 209 patients with diagnosis of TIA made by a neurologist.24 It evaluated factors previously found to be independent predictors of stroke after TIA, and determined that risk factors of age, blood pressure, type of clinical deficit, and symptom duration predicted 7‐day risk of stroke following TIA. Unlike the California score, the ABCD authors found presenting blood pressure, but not diabetes, to be independent predictors of future events. The authors validated the score with a second population of TIA patients in the Oxford Vascular Study and in a hospital‐based TIA clinic cohort.24 In the validation cohorts the score was highly predictive of stroke at 7 days (P < 0.001). Patients with the lowest scores of 0 to 3 had no strokes in the week following the index TIA, whereas patients with the highest score of 6 carried a 35.5% risk of early second stroke. The primary limitation of the ABCD score lies in the small sample size, with only 18 recurrent strokes in the week after TIA in the derivation cohort.
| ABCD Score | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| |||||||
| 7‐Day stroke risk (%) | 0 | 0 | 0 | 0 | 2.2 | 16.3 | 35.5 |
The ABCD score has subsequently been tested in other settings with mixed results. Two studies found limited utility.25, 26 Other trials found high scores to be overly inclusive but highly predictive and thus the majority of evidence appears to support the validity of the ABCD score in predicting risk of early recurrent ischemic events.2729 The ABCD score has been used to triage patients with high scores to inpatient management although the benefit of this strategy on outcomes has not been proven.30
ABCD2 Score
The 2007 ABCD2 (Tables 2 and 5) is a 7‐point tool that uses the original ABCD criteria along with an additional point for diabetes.23 The ABCD2 derived a unified prognostic score for optimal prediction of the 2‐day stroke risk from multivariate analysis of the original data sets used to create the California and ABCD scores. This score was then validated by the authors in 4 independent cohorts from the United States and the United Kingdom.23 In sum, 4809 patients with TIA were included in the ABCD2 analysis. Similar to prior studies, stroke occurred in 9.2% of patients by 90 days of which 20% were fatal. The authors created low (03 points), intermediate (45 points), and high (67 points) risk categories. In the validation cohorts the prediction rule for the ABCD2 functioned better than the California or ABCD scores with c statistics of 0.62 to 0.83 (ideal prediction produces a c statistic of 1 and prediction no better than chance would have a c statistic of 0.5). They found a 2‐day risk of stroke of 1% for low, 4.1% for intermediate, and 8.1% for the high risk group. Data from the study suggest 34% of TIA patients will be in low‐risk, 45% in intermediate‐risk, and 21% in high‐risk categories. While the ABCD2 score overcomes some of the problems with the 2 prior systems it shares many of the limitations as it was derived from the combined data sets. All scoring systems lack the ability to provide guidance on the management of TIAs associated with rare conditions, such as vasculitis, that are underrepresented in the derivation data sets. The ABCD2 also does not incorporate imaging data and this will likely require further exploration.
| ABCD2 Score | 0‐3 | 4‐5 | 6‐7 |
|---|---|---|---|
| |||
| Risk stratification | Low | Intermediate | High |
| 2‐Day stroke risk (%) | 1 | 4.1 | 8.1 |
| 7‐Day stroke risk (%) | 1.2 | 5.9 | 11.7 |
| 90‐Day stroke risk (%) | 3.1 | 9.8 | 17.8 |
The ABCD2 score can be used to predict risk for a variety of time intervals, has now been validated in independent Greek and British populations, and appears to be the best performing tool at predicting early risk of stroke regardless of underlying etiology.23, 31, 32 The authors suggest that admission for patients in the high‐risk group is prudent whereas outpatient evaluation is reasonable for patients in the low‐risk group.23 Admission for patients in the intermediate‐risk group will depend on individualized decision making, local practice standards, and available community resources.
New Models of Care: An Opportunity for Hospitalists
The key to improving TIA outcomes appears to be more contingent on the speed of evaluation and initiation of appropriate therapy than on the location of the care. The EXPRESS trial studied the effect of an immediate access neurovascular clinic providing urgent evaluation and immediate treatment of nonhospitalized TIA patients versus usual care. Statistically significant reductions were seen in time to evaluation, first treatment prescription, and in 90‐day risk of recurrent stroke (10.3% versus 2.1%, P < 0.0001) after the clinic was changed to the rapid evaluation and treatment model.33
The SOS‐TIA study used a 24‐hour access hospital‐based TIA clinic to evaluate the effects of rapid assessment and interventions on hospital length of stay and clinical outcomes.34 The 90‐day stroke rate was 1.24% (95% CI, 0.712.12), which represents a 79% reduction compared to the predicted stroke rate from the ABCD2 scores. With expedited evaluation and treatment, 74% of patients were able to be sent home on the same day.
The results of these 2 new studies provide compelling evidence that rapid evaluation and treatment in the first 48 hours after TIA has the potential to alter outcomes. Unfortunately not all communities have access to same day TIA clinics. Still, these findings should embolden hospitalists to advocate for urgent evaluation, such as neurology and cardiac imaging and carotid evaluation, with immediate initiation of secondary preventive therapy and early surgical intervention when appropriate. In most cases these changes will require process transformations that present prime opportunities for hospitalists to reengineer systems of care.
Incorporating Prognostic Scores into Clinical Practice
Applying the evidence to practice requires calculation of the early risk but also awareness of the community resources available. High‐risk patients with an ABCD2 score of 6 or 7 have a very high 8.1% risk of stroke within the next 48 hours. Given the catastrophic outcomes frequently seen after second strokes, these patients warrant inpatient admission to facilitate the immediate initiation of appropriate secondary prevention and potentially shorten time to thrombolysis if an early stroke occurs. Intermediate‐risk patients with ABCD2 scores of 4 and 5 have a 4.1% 2‐day risk of stroke and may be considered for admission, hospital observation, or expedited clinic evaluation contingent on local availability. As many as one‐third of TIA patients will be categorized as low risk with a score of 0 to 3. These patients have a 2‐day risk of stroke of only 1% and are likely safe for prompt outpatient evaluation and management. The new, validated, ABCD2 score is not a substitute for individualized judgment, but is helpful in developing admission guidelines in cooperation between neurologists, emergency room physicians, and hospitalists, and in using evidence‐based medicine to provide optimal care for the patient presenting with a TIA.
Stroke and TIA arise from identical etiologies, respond to the same secondary preventive measures, and should be considered part of the spectrum of an ischemic cerebral syndrome. Recognizing TIA as a medical emergency with high rates of secondary stroke and subsequent disability allows institution of therapies with appropriate urgency. Hospitalization offers the ability to rapidly coordinate the testing and secondary prevention measures but also, for high‐risk patients, offers the opportunity to reduce the time to thrombolysis for early recurrent strokes. New, validated scoring systems such as the ABCD2 score help the hospitalist to decide which patients are appropriate for admission and which can be managed in progressive and traditional outpatient settings.
- ,,.Epidemiological impact in the United States of a tissue‐based definition of transient ischemic attack.Stroke.2003;34:919–924.
- ,,, et al.Head computed tomography findings predict short‐term stroke risk after transient ischemic attack.Stroke.2003;34:2894–2899.
- ,,, et al.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,, et al.Management and outcomes of transient ischemic attacks in Ontario.CMAJ.2004;170:1099–1104.
- ,,, et al.The high risk of stroke immediately after transient ischemic attack: a population based study.Neurology.2004;62:2015–2020.
- ,,, et al.Early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis.Arch Intern Med.2007;167:2417–2422.
- ,,, et al.The spectrum of community‐based hospitalist practice: a call to tailor internal medicine residency training.Arch Intern Med.2007;167:727–729.
- .Transient ischemic attack with abnormal diffusion‐weighted imaging results: what's in a name?Arch Neurol.2007;64:1080–1082.
- ,.A reappraisal of the definition and pathophysiology of the transient ischemic attack.Med Sci Monit.2007;13:RA50–53.
- ,,, et al.Diffusion‐weighted imaging‐negative patients with transient ischemic attack are at risk of recurrent transient events.Stroke.2007;38:2367–2369.
- ,,, et al.Transient ischemic attack—proposal for a new definition.N Engl J Med.2002;347:1713–1716.
- ,,, et al.Management and outcome of patients with transient ischemic attack admitted to a stroke unit.Cerebrovasc Dis.2007;24:80–85.
- .How transient are transient ischemic attacks.Neurology.1988;38:674–677.
- ,,, et al.National Stroke Association guidelines for the management of transient ischemic attacks.Ann Neurol.2006;60:301–313.
- ,,, et al.Out‐of‐hospital delays in patients with acute stroke.Ann Emerg Med.2004;44:476–483.
- ,,, et al.Factors associated with delayed admission to hospital and in‐hospital delays in acute stroke and TIA: a prospective multicenter study.Stroke.1999;30:40–48.
- ,,, et al.Prehospital and emergency department delays after acute stroke: the Genentech Stroke Presentation Survey.Stroke.2000;31:2585–2590.
- Tissue plasminogen activator for acute ischemic stroke.The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.N Engl J Med.1995;333:1581–1587.
- ,.Is hospitalization after TIA cost effective on the basis of treatment with tPA?Neurology.2005;65:1799–1801.
- ,,, et al.Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery.Lancet.2004;363:915–924.
- ,,, et al.New transient ischemic attack and stroke: outpatient management by primary care physicians.Arch Intern Med.2000;160:2941–2946.
- ,,, et al.Hospital and demographic influences on the disposition of transient ischemic attack.Acad Emerg Med.2008;15:171–176.
- ,,, et al.Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack.Lancet.2007;369:283–292.
- ,,, et al.A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack.Lancet.2005;366:29–36.
- ,,, et al.Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack?Stroke.2006;37:1710–1714.
- ,,, et al.Absence of usefulness of ABCD score in the early risk of recurrent stroke in transient ischemic attack patients.Stroke.2007;38:855–856.
- ,,, et al.Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital‐based case series study.Stroke.2006;37:2892–2897.
- ,.Rapid identification of high‐risk transient ischemic attacks: prospective validation of the ABCD score.Stroke.2008;39:297–302.
- ,,.Can the ABCD score be dichotomized to identify high‐risk patients with transient ischaemic attack in the emergency department?Emerg Med J.2007;24:92–95.
- ,,, et al.Can risk stratification of transient ischaemic attacks improve patient care in the emergency department?Emerg Med J.2007;24:637–640.
- ,,, et al.Prognosis in patients with transient ischaemic attack (TIA) and minor stroke attending TIA services in the north west of England: The NORTHSTAR Study.J Neurol Neurosurg Psychiatry.2007:1–6.
- ,,.Potential applicability of the ABCD2 in triaging TIA patients.Lancet.2007;369:1082.
- ,,, et al.Effect of urgent treatment of transient ischemic attack and minor stroke on early recurrent stroke (EXPRESS Study): a prospective population‐based sequential comparison.Lancet.2007;370;1432–1442.
- ,,, et al.A transient ischemic attack clinic with round‐the‐clock access (SOS‐TIA): feasibility and effects.Lancet Neurol.2007;6:953–960.
- ,,.Epidemiological impact in the United States of a tissue‐based definition of transient ischemic attack.Stroke.2003;34:919–924.
- ,,, et al.Head computed tomography findings predict short‐term stroke risk after transient ischemic attack.Stroke.2003;34:2894–2899.
- ,,, et al.Short‐term prognosis after emergency department diagnosis of TIA.JAMA.2000;284:2901–2906.
- ,,, et al.Management and outcomes of transient ischemic attacks in Ontario.CMAJ.2004;170:1099–1104.
- ,,, et al.The high risk of stroke immediately after transient ischemic attack: a population based study.Neurology.2004;62:2015–2020.
- ,,, et al.Early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis.Arch Intern Med.2007;167:2417–2422.
- ,,, et al.The spectrum of community‐based hospitalist practice: a call to tailor internal medicine residency training.Arch Intern Med.2007;167:727–729.
- .Transient ischemic attack with abnormal diffusion‐weighted imaging results: what's in a name?Arch Neurol.2007;64:1080–1082.
- ,.A reappraisal of the definition and pathophysiology of the transient ischemic attack.Med Sci Monit.2007;13:RA50–53.
- ,,, et al.Diffusion‐weighted imaging‐negative patients with transient ischemic attack are at risk of recurrent transient events.Stroke.2007;38:2367–2369.
- ,,, et al.Transient ischemic attack—proposal for a new definition.N Engl J Med.2002;347:1713–1716.
- ,,, et al.Management and outcome of patients with transient ischemic attack admitted to a stroke unit.Cerebrovasc Dis.2007;24:80–85.
- .How transient are transient ischemic attacks.Neurology.1988;38:674–677.
- ,,, et al.National Stroke Association guidelines for the management of transient ischemic attacks.Ann Neurol.2006;60:301–313.
- ,,, et al.Out‐of‐hospital delays in patients with acute stroke.Ann Emerg Med.2004;44:476–483.
- ,,, et al.Factors associated with delayed admission to hospital and in‐hospital delays in acute stroke and TIA: a prospective multicenter study.Stroke.1999;30:40–48.
- ,,, et al.Prehospital and emergency department delays after acute stroke: the Genentech Stroke Presentation Survey.Stroke.2000;31:2585–2590.
- Tissue plasminogen activator for acute ischemic stroke.The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.N Engl J Med.1995;333:1581–1587.
- ,.Is hospitalization after TIA cost effective on the basis of treatment with tPA?Neurology.2005;65:1799–1801.
- ,,, et al.Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery.Lancet.2004;363:915–924.
- ,,, et al.New transient ischemic attack and stroke: outpatient management by primary care physicians.Arch Intern Med.2000;160:2941–2946.
- ,,, et al.Hospital and demographic influences on the disposition of transient ischemic attack.Acad Emerg Med.2008;15:171–176.
- ,,, et al.Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack.Lancet.2007;369:283–292.
- ,,, et al.A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack.Lancet.2005;366:29–36.
- ,,, et al.Is the ABCD score useful for risk stratification of patients with acute transient ischemic attack?Stroke.2006;37:1710–1714.
- ,,, et al.Absence of usefulness of ABCD score in the early risk of recurrent stroke in transient ischemic attack patients.Stroke.2007;38:855–856.
- ,,, et al.Validation of the ABCD score in identifying individuals at high early risk of stroke after a transient ischemic attack: a hospital‐based case series study.Stroke.2006;37:2892–2897.
- ,.Rapid identification of high‐risk transient ischemic attacks: prospective validation of the ABCD score.Stroke.2008;39:297–302.
- ,,.Can the ABCD score be dichotomized to identify high‐risk patients with transient ischaemic attack in the emergency department?Emerg Med J.2007;24:92–95.
- ,,, et al.Can risk stratification of transient ischaemic attacks improve patient care in the emergency department?Emerg Med J.2007;24:637–640.
- ,,, et al.Prognosis in patients with transient ischaemic attack (TIA) and minor stroke attending TIA services in the north west of England: The NORTHSTAR Study.J Neurol Neurosurg Psychiatry.2007:1–6.
- ,,.Potential applicability of the ABCD2 in triaging TIA patients.Lancet.2007;369:1082.
- ,,, et al.Effect of urgent treatment of transient ischemic attack and minor stroke on early recurrent stroke (EXPRESS Study): a prospective population‐based sequential comparison.Lancet.2007;370;1432–1442.
- ,,, et al.A transient ischemic attack clinic with round‐the‐clock access (SOS‐TIA): feasibility and effects.Lancet Neurol.2007;6:953–960.
Case Report: Failure at the Transition of Care
The patient is an 86‐year‐old woman with a history of mild dementia, major depression with psychotic features, congestive heart failure, hypertension, hyperlipidemia, osteoporosis, and hypothyroidism. She presented to her primary care physician (PCP) complaining of 4 days of bilateral lower extremity edema and dyspnea on exertion. She was admitted to the hospitalist service for exacerbation of congestive heart failure.
MEDICATIONS
Donepezil, olanzapine, mirtazapine, sertraline, spironolactone, triamterene/hydrochlorothizide, simvastatin, alendronate, levothyroxine, multivitamin.
SOCIAL HISTORY
She lived alone in an independent‐living retirement apartment that provided meals but not medical care, and she was able to function independently in her activities of daily living. Her pharmacy delivered her medications via courier service, whereas visiting home nurses filled her medication box and checked on her status weekly.
HOSPITAL COURSE
Admission vitals were: heart rate, 83; blood pressure, 158/84; respiratory rate, 20; temperature, 36.4, and saturation, 95% on room air. Echocardiogram revealed intact ejection fraction, left ventricular hypertrophy, and impaired relaxation. A TSH of 6.6 demonstrated undertreated hypothyroidism. Telemetry monitoring was significant for frequent short bursts of narrow‐complex tachycardia without clear atrial activity. The etiology of her heart failure exacerbation was presumed to be paroxysmal atrial fibrillation in the setting of diastolic dysfunction. Given her mild hyperkalemia (5.1), her diuretics were changed to monotherapy with furosemide. Low‐dose beta blockade and antithrombotic therapy were started as well as increased supplementation of levothyroxine. After several days of diuresis, her potassium had normalized, she tolerated initiation of a new ACE inhibitor, and her dyspnea had resolved. On the last hospital day, her dentures were accidentally discarded with her breakfast tray, causing her great distress.
On discharge she was on 4 new medications, 2 old medications had been stopped, and 1 prior medication's dose had been increased. During medication reconciliation, the patient reported that she had not been taking olanzapine for weeks, and thus this was omitted from her home health medication orders, with instructions to discuss with her PCP on first follow‐up within the week. The patient was provided with congestive heart failure instructions and a complete medication list. Unfortunately, the day of discharge was the first day of a holiday weekend.
Case management was unavailable on the weekend; her out‐of‐state family member was unable to be reached by phone, and her usual pharmacy courier service was closed. As she did not have a friend or family member to pick up her prescriptions from an alternate pharmacy, her prescriptions were provided as handwritten scripts, called in to her pharmacy's voice mail, and written on the home health orders. The patient was discharged to her home with communication to her PCP via telephone, e‐mail, and electronic discharge summary.
POSTDISCHARGE
Medications were not delivered to the patient until the third postdischarge day. Three days after discharge, the daughter from out of state left a message for the PCP expressing concern that the patient was failingnot eating or taking any of her medications. An expedited home nursing visit was arranged. Five days after discharge, the pharmacist called the PCP stating he had not received a prescription for the beta‐blocker. Her PCP saw the patient in clinic 6 days after discharge and reconciled the medication list, restarting the olanzapine that the patient had stopped a few weeks before and the mirtazapine, which had not been restarted despite its presence on the discharge orders and patient instructions. She continued to have poor appetite and mood and was taking her medications only with great effort from her visiting nurse and staff at the retirement community. A major cause of this decline was the significant worsening of her depression brought on by hospitalization, lapses in her psychiatric medications, and emotional distress induced by the loss of her dentures during her hospital stay. She was readmitted 10 days after her discharge because she was unable to care for herself.
DISCUSSION
This case demonstrates numerous pitfalls in the transition process. Despite communication between the hospitalists and the PCP and a common electronic medical record, this patient failed the transition from the acute care hospital to the ambulatory setting. On the holiday weekend, ancillary support services were unavailable, including case management to contact her home care agency and her pharmacy to fill and deliver her new prescriptions. Despite efforts by the discharging physician, the out‐of‐town family could not be contacted. Thus, an elderly woman with cognitive impairment was left to process a new diagnosis and 7 medication changes with an unreliable mechanism to obtain her new medications.
With the rise of hospital medicine, it has increasingly been recognized that transitions represent a point of vulnerability in the care of geriatric patients. A change in physical location of care and handoffs between caregivers create the potential for error and loss of information. Prior research has demonstrated frequent quantitative and qualitative deficiencies in the information conveyed between inpatient and outpatient physicians, with direct communication occurring less than 20% of the time.1 In this case, communication occurred between the hospitalists and the outpatient physician, demonstrating that communication is just one element of successful transitions.
Components of effective care transitions have been described in the literature, including: preparation of the patient and caregiver for the transition, medication reconciliation, instructions to patient and caregiver about symptoms and signs of worsening, and an explicit follow‐up plan for tests and appointments.2 Optimally, there is interactive discussion between the hospitalist and the receiving clinician with a summary of events including an updated medication, allergy, and problem list, current advance directives, and a common plan of care.2 This case illustrates that these elements are necessary but may not be sufficient.
Some interventions have been found to be effective. A nurse‐led multidisciplinary approach to the discharge of elderly patients with congestive heart failure led to decreased readmission rates after 90 days and was found to be a cost‐saving measure.3 Similar results have been seen in geriatric patients with a variety of diagnoses in trials using advanced‐practice nurses to bridge the vulnerable period of discharge or by interventions to improve the ability of family caregivers to handle the challenges of the transition.46 Individualized attention to the unique needs of each patient and members of their social support structure, and investment in resources to do so, has the ability to decrease readmissions.
Medication errors, medication omissions, or the inability to fill medications on discharge represent a patient safety challenge. There has been increasing emphasis on medication reconciliation at admission and discharge, but in some cases the gold standard medication list is hard to determine. Electronic medical records would seem to be a natural solution to this problem, but as this case illustrates, the electronic record may not reflect the reality of patient adherence. As in this case, clarification may require another visit with the primary provider, leaving a period of time with an uncertain medication list and therefore a vulnerable patient. Access to medications after discharge was also a problem in this case, but this is not rare. A 2001 study found that 2 days after discharge from a general medicine hospital service, 1 in 5 patients had been unable to obtain all discharge medications.7 A pharmacist‐led medication reconciliation intervention in nursing home patients led to decreases in length of stay and discrepancy‐related adverse drug events. Furthermore, a follow‐up call allowed for clarification of medication questions in 25% of cases.7, 8
Patient characteristics such as depression and cognitive dysfunction have been found to affect readmission rates and are important to assess in addressing risk for poor outcomes after discharge. A 2000 study comparing readmission rates after discharge from a geriatric rehabilitation hospital found that patients with depression had an odds ratio of 3.5 for readmission compared with those without depression.9 Inadequate health literacy is also associated with decreased ability to self‐manage chronic disease and is associated with increased risk of mortality in community‐dwelling elderly such as the patient in this case.10 Asking patients or their proxies to explain their own understanding of the discharge plan can unmask comprehension issues that otherwise may go undetected.
Discharge on a weekend presents a period of critical vulnerability. Early recognition that a transition is susceptible to failure allows the events necessary for success to occur during the week when services are available. An example in the present case might include having had the pharmacy fill the prescriptions prior to the day of discharge. This does introduce a new opportunity for error in cases in which the plan of care changes but would have solved the inability to have prescriptions filled once the holiday weekend had begun.
In cases in which the usual mechanisms break down, increased effort on the part of the hospitalist can usually create a unique solution to the problem. Examples of creative solutions that did not occur in this case might include contacting the manager of the patient's retirement apartment to determine if this individual might be willing to fill prescriptions at an alternate pharmacy. A better alternative would be to change the system such that the solution is readily accessible and time efficient. Weekend availability of case management would be one such step. A means for the hospital's inpatient pharmacy to provide 2‐3 days of bridging medications would prevent weekend prescription access from affecting timely discharges of multiple patients over the course of a year. The hospitalist is in a unique position to take a leadership role in effecting system change to address these issues.
Ultimately, it is the duty of the hospitalist to take responsibility for the safety and well‐being of the patient, and if no solution can be found, it may be necessary to hold discharge or find an alternate disposition until logistical hurdles have been overcome. Indeed, for patients admitted for myocardial infarction, discharges were less likely to occur on weekends, presumably because of lack of ancillary services.11 With foresight, creative problem solving, and systems improvement, this should rarely be necessary.
Transitions continue to be a difficult time for the most vulnerable patients. Intense efforts have improved outcomes in selected populations but have not been broadly applied. Identification of patients at the highest risk, such as those with depression, poor social support, and cognitive limitations, would allow anticipation of difficult transitions and potential utilization of proven interventions, such as advanced‐practice nurses or follow‐up pharmacy contact. Processes such as these might have prevented some of the problems in this patient's discharge. Appreciation of the weekend discharge as a time of particular challenges allows barriers to be identified and solutions created during the week, when resources are still available. Attention to all elements of effective transitions should become part of the growing culture of patient safety.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial.Ann Intern Med.1994;120:999–1006.
- ,,, et al.Preparing patients and caregivers to participate in care delivered across settings: the care transitions intervention.J Am Geriatr Soc.2004;52:1817–1825.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111:26S–30S.
- ,,, et al.Medication reconciliation for reducing drug discrepancy adverse events.Am J Geriatr Pharmacother.2006;4:236–243.
- ,,, et al.Depression and activities of daily living predict rehospitalization within 6 months of discharge from geriatric rehabilitation.Rehabil Psychol.2004;49:219–223.
- ,,, et al.Health literacy and mortality among elderly persons.Arch Intern Med.2007;167:1503–1509.
- ,,, et al.Restricted weekend service inappropriately delays discharge after acute myocardial infarction.Heart.2002;87:216–219.
The patient is an 86‐year‐old woman with a history of mild dementia, major depression with psychotic features, congestive heart failure, hypertension, hyperlipidemia, osteoporosis, and hypothyroidism. She presented to her primary care physician (PCP) complaining of 4 days of bilateral lower extremity edema and dyspnea on exertion. She was admitted to the hospitalist service for exacerbation of congestive heart failure.
MEDICATIONS
Donepezil, olanzapine, mirtazapine, sertraline, spironolactone, triamterene/hydrochlorothizide, simvastatin, alendronate, levothyroxine, multivitamin.
SOCIAL HISTORY
She lived alone in an independent‐living retirement apartment that provided meals but not medical care, and she was able to function independently in her activities of daily living. Her pharmacy delivered her medications via courier service, whereas visiting home nurses filled her medication box and checked on her status weekly.
HOSPITAL COURSE
Admission vitals were: heart rate, 83; blood pressure, 158/84; respiratory rate, 20; temperature, 36.4, and saturation, 95% on room air. Echocardiogram revealed intact ejection fraction, left ventricular hypertrophy, and impaired relaxation. A TSH of 6.6 demonstrated undertreated hypothyroidism. Telemetry monitoring was significant for frequent short bursts of narrow‐complex tachycardia without clear atrial activity. The etiology of her heart failure exacerbation was presumed to be paroxysmal atrial fibrillation in the setting of diastolic dysfunction. Given her mild hyperkalemia (5.1), her diuretics were changed to monotherapy with furosemide. Low‐dose beta blockade and antithrombotic therapy were started as well as increased supplementation of levothyroxine. After several days of diuresis, her potassium had normalized, she tolerated initiation of a new ACE inhibitor, and her dyspnea had resolved. On the last hospital day, her dentures were accidentally discarded with her breakfast tray, causing her great distress.
On discharge she was on 4 new medications, 2 old medications had been stopped, and 1 prior medication's dose had been increased. During medication reconciliation, the patient reported that she had not been taking olanzapine for weeks, and thus this was omitted from her home health medication orders, with instructions to discuss with her PCP on first follow‐up within the week. The patient was provided with congestive heart failure instructions and a complete medication list. Unfortunately, the day of discharge was the first day of a holiday weekend.
Case management was unavailable on the weekend; her out‐of‐state family member was unable to be reached by phone, and her usual pharmacy courier service was closed. As she did not have a friend or family member to pick up her prescriptions from an alternate pharmacy, her prescriptions were provided as handwritten scripts, called in to her pharmacy's voice mail, and written on the home health orders. The patient was discharged to her home with communication to her PCP via telephone, e‐mail, and electronic discharge summary.
POSTDISCHARGE
Medications were not delivered to the patient until the third postdischarge day. Three days after discharge, the daughter from out of state left a message for the PCP expressing concern that the patient was failingnot eating or taking any of her medications. An expedited home nursing visit was arranged. Five days after discharge, the pharmacist called the PCP stating he had not received a prescription for the beta‐blocker. Her PCP saw the patient in clinic 6 days after discharge and reconciled the medication list, restarting the olanzapine that the patient had stopped a few weeks before and the mirtazapine, which had not been restarted despite its presence on the discharge orders and patient instructions. She continued to have poor appetite and mood and was taking her medications only with great effort from her visiting nurse and staff at the retirement community. A major cause of this decline was the significant worsening of her depression brought on by hospitalization, lapses in her psychiatric medications, and emotional distress induced by the loss of her dentures during her hospital stay. She was readmitted 10 days after her discharge because she was unable to care for herself.
DISCUSSION
This case demonstrates numerous pitfalls in the transition process. Despite communication between the hospitalists and the PCP and a common electronic medical record, this patient failed the transition from the acute care hospital to the ambulatory setting. On the holiday weekend, ancillary support services were unavailable, including case management to contact her home care agency and her pharmacy to fill and deliver her new prescriptions. Despite efforts by the discharging physician, the out‐of‐town family could not be contacted. Thus, an elderly woman with cognitive impairment was left to process a new diagnosis and 7 medication changes with an unreliable mechanism to obtain her new medications.
With the rise of hospital medicine, it has increasingly been recognized that transitions represent a point of vulnerability in the care of geriatric patients. A change in physical location of care and handoffs between caregivers create the potential for error and loss of information. Prior research has demonstrated frequent quantitative and qualitative deficiencies in the information conveyed between inpatient and outpatient physicians, with direct communication occurring less than 20% of the time.1 In this case, communication occurred between the hospitalists and the outpatient physician, demonstrating that communication is just one element of successful transitions.
Components of effective care transitions have been described in the literature, including: preparation of the patient and caregiver for the transition, medication reconciliation, instructions to patient and caregiver about symptoms and signs of worsening, and an explicit follow‐up plan for tests and appointments.2 Optimally, there is interactive discussion between the hospitalist and the receiving clinician with a summary of events including an updated medication, allergy, and problem list, current advance directives, and a common plan of care.2 This case illustrates that these elements are necessary but may not be sufficient.
Some interventions have been found to be effective. A nurse‐led multidisciplinary approach to the discharge of elderly patients with congestive heart failure led to decreased readmission rates after 90 days and was found to be a cost‐saving measure.3 Similar results have been seen in geriatric patients with a variety of diagnoses in trials using advanced‐practice nurses to bridge the vulnerable period of discharge or by interventions to improve the ability of family caregivers to handle the challenges of the transition.46 Individualized attention to the unique needs of each patient and members of their social support structure, and investment in resources to do so, has the ability to decrease readmissions.
Medication errors, medication omissions, or the inability to fill medications on discharge represent a patient safety challenge. There has been increasing emphasis on medication reconciliation at admission and discharge, but in some cases the gold standard medication list is hard to determine. Electronic medical records would seem to be a natural solution to this problem, but as this case illustrates, the electronic record may not reflect the reality of patient adherence. As in this case, clarification may require another visit with the primary provider, leaving a period of time with an uncertain medication list and therefore a vulnerable patient. Access to medications after discharge was also a problem in this case, but this is not rare. A 2001 study found that 2 days after discharge from a general medicine hospital service, 1 in 5 patients had been unable to obtain all discharge medications.7 A pharmacist‐led medication reconciliation intervention in nursing home patients led to decreases in length of stay and discrepancy‐related adverse drug events. Furthermore, a follow‐up call allowed for clarification of medication questions in 25% of cases.7, 8
Patient characteristics such as depression and cognitive dysfunction have been found to affect readmission rates and are important to assess in addressing risk for poor outcomes after discharge. A 2000 study comparing readmission rates after discharge from a geriatric rehabilitation hospital found that patients with depression had an odds ratio of 3.5 for readmission compared with those without depression.9 Inadequate health literacy is also associated with decreased ability to self‐manage chronic disease and is associated with increased risk of mortality in community‐dwelling elderly such as the patient in this case.10 Asking patients or their proxies to explain their own understanding of the discharge plan can unmask comprehension issues that otherwise may go undetected.
Discharge on a weekend presents a period of critical vulnerability. Early recognition that a transition is susceptible to failure allows the events necessary for success to occur during the week when services are available. An example in the present case might include having had the pharmacy fill the prescriptions prior to the day of discharge. This does introduce a new opportunity for error in cases in which the plan of care changes but would have solved the inability to have prescriptions filled once the holiday weekend had begun.
In cases in which the usual mechanisms break down, increased effort on the part of the hospitalist can usually create a unique solution to the problem. Examples of creative solutions that did not occur in this case might include contacting the manager of the patient's retirement apartment to determine if this individual might be willing to fill prescriptions at an alternate pharmacy. A better alternative would be to change the system such that the solution is readily accessible and time efficient. Weekend availability of case management would be one such step. A means for the hospital's inpatient pharmacy to provide 2‐3 days of bridging medications would prevent weekend prescription access from affecting timely discharges of multiple patients over the course of a year. The hospitalist is in a unique position to take a leadership role in effecting system change to address these issues.
Ultimately, it is the duty of the hospitalist to take responsibility for the safety and well‐being of the patient, and if no solution can be found, it may be necessary to hold discharge or find an alternate disposition until logistical hurdles have been overcome. Indeed, for patients admitted for myocardial infarction, discharges were less likely to occur on weekends, presumably because of lack of ancillary services.11 With foresight, creative problem solving, and systems improvement, this should rarely be necessary.
Transitions continue to be a difficult time for the most vulnerable patients. Intense efforts have improved outcomes in selected populations but have not been broadly applied. Identification of patients at the highest risk, such as those with depression, poor social support, and cognitive limitations, would allow anticipation of difficult transitions and potential utilization of proven interventions, such as advanced‐practice nurses or follow‐up pharmacy contact. Processes such as these might have prevented some of the problems in this patient's discharge. Appreciation of the weekend discharge as a time of particular challenges allows barriers to be identified and solutions created during the week, when resources are still available. Attention to all elements of effective transitions should become part of the growing culture of patient safety.
The patient is an 86‐year‐old woman with a history of mild dementia, major depression with psychotic features, congestive heart failure, hypertension, hyperlipidemia, osteoporosis, and hypothyroidism. She presented to her primary care physician (PCP) complaining of 4 days of bilateral lower extremity edema and dyspnea on exertion. She was admitted to the hospitalist service for exacerbation of congestive heart failure.
MEDICATIONS
Donepezil, olanzapine, mirtazapine, sertraline, spironolactone, triamterene/hydrochlorothizide, simvastatin, alendronate, levothyroxine, multivitamin.
SOCIAL HISTORY
She lived alone in an independent‐living retirement apartment that provided meals but not medical care, and she was able to function independently in her activities of daily living. Her pharmacy delivered her medications via courier service, whereas visiting home nurses filled her medication box and checked on her status weekly.
HOSPITAL COURSE
Admission vitals were: heart rate, 83; blood pressure, 158/84; respiratory rate, 20; temperature, 36.4, and saturation, 95% on room air. Echocardiogram revealed intact ejection fraction, left ventricular hypertrophy, and impaired relaxation. A TSH of 6.6 demonstrated undertreated hypothyroidism. Telemetry monitoring was significant for frequent short bursts of narrow‐complex tachycardia without clear atrial activity. The etiology of her heart failure exacerbation was presumed to be paroxysmal atrial fibrillation in the setting of diastolic dysfunction. Given her mild hyperkalemia (5.1), her diuretics were changed to monotherapy with furosemide. Low‐dose beta blockade and antithrombotic therapy were started as well as increased supplementation of levothyroxine. After several days of diuresis, her potassium had normalized, she tolerated initiation of a new ACE inhibitor, and her dyspnea had resolved. On the last hospital day, her dentures were accidentally discarded with her breakfast tray, causing her great distress.
On discharge she was on 4 new medications, 2 old medications had been stopped, and 1 prior medication's dose had been increased. During medication reconciliation, the patient reported that she had not been taking olanzapine for weeks, and thus this was omitted from her home health medication orders, with instructions to discuss with her PCP on first follow‐up within the week. The patient was provided with congestive heart failure instructions and a complete medication list. Unfortunately, the day of discharge was the first day of a holiday weekend.
Case management was unavailable on the weekend; her out‐of‐state family member was unable to be reached by phone, and her usual pharmacy courier service was closed. As she did not have a friend or family member to pick up her prescriptions from an alternate pharmacy, her prescriptions were provided as handwritten scripts, called in to her pharmacy's voice mail, and written on the home health orders. The patient was discharged to her home with communication to her PCP via telephone, e‐mail, and electronic discharge summary.
POSTDISCHARGE
Medications were not delivered to the patient until the third postdischarge day. Three days after discharge, the daughter from out of state left a message for the PCP expressing concern that the patient was failingnot eating or taking any of her medications. An expedited home nursing visit was arranged. Five days after discharge, the pharmacist called the PCP stating he had not received a prescription for the beta‐blocker. Her PCP saw the patient in clinic 6 days after discharge and reconciled the medication list, restarting the olanzapine that the patient had stopped a few weeks before and the mirtazapine, which had not been restarted despite its presence on the discharge orders and patient instructions. She continued to have poor appetite and mood and was taking her medications only with great effort from her visiting nurse and staff at the retirement community. A major cause of this decline was the significant worsening of her depression brought on by hospitalization, lapses in her psychiatric medications, and emotional distress induced by the loss of her dentures during her hospital stay. She was readmitted 10 days after her discharge because she was unable to care for herself.
DISCUSSION
This case demonstrates numerous pitfalls in the transition process. Despite communication between the hospitalists and the PCP and a common electronic medical record, this patient failed the transition from the acute care hospital to the ambulatory setting. On the holiday weekend, ancillary support services were unavailable, including case management to contact her home care agency and her pharmacy to fill and deliver her new prescriptions. Despite efforts by the discharging physician, the out‐of‐town family could not be contacted. Thus, an elderly woman with cognitive impairment was left to process a new diagnosis and 7 medication changes with an unreliable mechanism to obtain her new medications.
With the rise of hospital medicine, it has increasingly been recognized that transitions represent a point of vulnerability in the care of geriatric patients. A change in physical location of care and handoffs between caregivers create the potential for error and loss of information. Prior research has demonstrated frequent quantitative and qualitative deficiencies in the information conveyed between inpatient and outpatient physicians, with direct communication occurring less than 20% of the time.1 In this case, communication occurred between the hospitalists and the outpatient physician, demonstrating that communication is just one element of successful transitions.
Components of effective care transitions have been described in the literature, including: preparation of the patient and caregiver for the transition, medication reconciliation, instructions to patient and caregiver about symptoms and signs of worsening, and an explicit follow‐up plan for tests and appointments.2 Optimally, there is interactive discussion between the hospitalist and the receiving clinician with a summary of events including an updated medication, allergy, and problem list, current advance directives, and a common plan of care.2 This case illustrates that these elements are necessary but may not be sufficient.
Some interventions have been found to be effective. A nurse‐led multidisciplinary approach to the discharge of elderly patients with congestive heart failure led to decreased readmission rates after 90 days and was found to be a cost‐saving measure.3 Similar results have been seen in geriatric patients with a variety of diagnoses in trials using advanced‐practice nurses to bridge the vulnerable period of discharge or by interventions to improve the ability of family caregivers to handle the challenges of the transition.46 Individualized attention to the unique needs of each patient and members of their social support structure, and investment in resources to do so, has the ability to decrease readmissions.
Medication errors, medication omissions, or the inability to fill medications on discharge represent a patient safety challenge. There has been increasing emphasis on medication reconciliation at admission and discharge, but in some cases the gold standard medication list is hard to determine. Electronic medical records would seem to be a natural solution to this problem, but as this case illustrates, the electronic record may not reflect the reality of patient adherence. As in this case, clarification may require another visit with the primary provider, leaving a period of time with an uncertain medication list and therefore a vulnerable patient. Access to medications after discharge was also a problem in this case, but this is not rare. A 2001 study found that 2 days after discharge from a general medicine hospital service, 1 in 5 patients had been unable to obtain all discharge medications.7 A pharmacist‐led medication reconciliation intervention in nursing home patients led to decreases in length of stay and discrepancy‐related adverse drug events. Furthermore, a follow‐up call allowed for clarification of medication questions in 25% of cases.7, 8
Patient characteristics such as depression and cognitive dysfunction have been found to affect readmission rates and are important to assess in addressing risk for poor outcomes after discharge. A 2000 study comparing readmission rates after discharge from a geriatric rehabilitation hospital found that patients with depression had an odds ratio of 3.5 for readmission compared with those without depression.9 Inadequate health literacy is also associated with decreased ability to self‐manage chronic disease and is associated with increased risk of mortality in community‐dwelling elderly such as the patient in this case.10 Asking patients or their proxies to explain their own understanding of the discharge plan can unmask comprehension issues that otherwise may go undetected.
Discharge on a weekend presents a period of critical vulnerability. Early recognition that a transition is susceptible to failure allows the events necessary for success to occur during the week when services are available. An example in the present case might include having had the pharmacy fill the prescriptions prior to the day of discharge. This does introduce a new opportunity for error in cases in which the plan of care changes but would have solved the inability to have prescriptions filled once the holiday weekend had begun.
In cases in which the usual mechanisms break down, increased effort on the part of the hospitalist can usually create a unique solution to the problem. Examples of creative solutions that did not occur in this case might include contacting the manager of the patient's retirement apartment to determine if this individual might be willing to fill prescriptions at an alternate pharmacy. A better alternative would be to change the system such that the solution is readily accessible and time efficient. Weekend availability of case management would be one such step. A means for the hospital's inpatient pharmacy to provide 2‐3 days of bridging medications would prevent weekend prescription access from affecting timely discharges of multiple patients over the course of a year. The hospitalist is in a unique position to take a leadership role in effecting system change to address these issues.
Ultimately, it is the duty of the hospitalist to take responsibility for the safety and well‐being of the patient, and if no solution can be found, it may be necessary to hold discharge or find an alternate disposition until logistical hurdles have been overcome. Indeed, for patients admitted for myocardial infarction, discharges were less likely to occur on weekends, presumably because of lack of ancillary services.11 With foresight, creative problem solving, and systems improvement, this should rarely be necessary.
Transitions continue to be a difficult time for the most vulnerable patients. Intense efforts have improved outcomes in selected populations but have not been broadly applied. Identification of patients at the highest risk, such as those with depression, poor social support, and cognitive limitations, would allow anticipation of difficult transitions and potential utilization of proven interventions, such as advanced‐practice nurses or follow‐up pharmacy contact. Processes such as these might have prevented some of the problems in this patient's discharge. Appreciation of the weekend discharge as a time of particular challenges allows barriers to be identified and solutions created during the week, when resources are still available. Attention to all elements of effective transitions should become part of the growing culture of patient safety.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial.Ann Intern Med.1994;120:999–1006.
- ,,, et al.Preparing patients and caregivers to participate in care delivered across settings: the care transitions intervention.J Am Geriatr Soc.2004;52:1817–1825.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111:26S–30S.
- ,,, et al.Medication reconciliation for reducing drug discrepancy adverse events.Am J Geriatr Pharmacother.2006;4:236–243.
- ,,, et al.Depression and activities of daily living predict rehospitalization within 6 months of discharge from geriatric rehabilitation.Rehabil Psychol.2004;49:219–223.
- ,,, et al.Health literacy and mortality among elderly persons.Arch Intern Med.2007;167:1503–1509.
- ,,, et al.Restricted weekend service inappropriately delays discharge after acute myocardial infarction.Heart.2002;87:216–219.
- ,,, et al.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- .Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs.J Am Geriatr Soc.2003;51:549–555.
- ,,, et al.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333:1190–1195.
- ,,, et al.Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial.JAMA.1999;281:613–620.
- ,,, et al.Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial.Ann Intern Med.1994;120:999–1006.
- ,,, et al.Preparing patients and caregivers to participate in care delivered across settings: the care transitions intervention.J Am Geriatr Soc.2004;52:1817–1825.
- ,,, et al.The impact of follow‐up telephone calls to patients after hospitalization.Am J Med.2001;111:26S–30S.
- ,,, et al.Medication reconciliation for reducing drug discrepancy adverse events.Am J Geriatr Pharmacother.2006;4:236–243.
- ,,, et al.Depression and activities of daily living predict rehospitalization within 6 months of discharge from geriatric rehabilitation.Rehabil Psychol.2004;49:219–223.
- ,,, et al.Health literacy and mortality among elderly persons.Arch Intern Med.2007;167:1503–1509.
- ,,, et al.Restricted weekend service inappropriately delays discharge after acute myocardial infarction.Heart.2002;87:216–219.
Feedback Failures
On the evening of July 30, 1945, a U.S. battleship traveling from Guam to the Philippines was spotted in the Philippine Sea by a Japanese submarine crew. Six torpedoes sped across the black water with devastating effect.
Three hundred sailors died immediately as the stricken ship sank. Another 900 men were left floating in an oil slick in the shark-infested Pacific Ocean for four days.1
The events that led to the sinking represent a classic tale of systems failures. They ring familiar to any physician who has closely examined modern medical error.
The communication breakdown that prevented the ship’s captain from being aware of submarine activity on the same route four days before is no different than communication breakdowns in complex hospital systems. The bureaucratic decision by a remote administrator to withhold the safety measure of an escort to prevent such an attack on the grounds that it “lacked necessity” likely resonates with physicians who have struggled with getting authorization for care.
Why did those sailors remain in the water four days before rescue? Despite being only a two-hour flight from the nearest base, they were not recovered—or even missed—for what must have seemed an endless amount of time.
In the end, slightly more than 300 men were alive when they were spotted by a plane that happened to fly past. The rest succumbed to dehydration, exposure, and sharks. The failure of the Navy to rescue the sailors offers lessons to the clinician trying to improve transitions of care more than a half a century later.
The Feedback Loop
Hospital discharge is a complex process initiated by physician orders on charts, prescription pads, and patient instructions. Most often, the things we assume will be done out of our direct view are carried out satisfactorily. However, any hospitalist can easily recount stories of tests or follow-up that didn’t happen as ordered. Patients who fall through the cracks at discharge—like the stranded sailors of the USS Indianapolis—are, in part, the story of a simple omitted step: the feedback loop.
A feedback loop occurs when the results or consequences of an action are returned as an input loop to the initiating step in order to modify subsequent actions. This fundamental engineering concept can keep complex systems on course. The feedback loop allows the lack of completion of a portion of a process to be recognized—and corrective measures taken—before additional harm occurs.
In Guam, the island base from which the USS Indianapolis departed, the marker indicating the ship on the plotting board was removed when the ship left. Later, the Philippine port of Leyte failed to note that the ship didn’t arrive. Policy at the time was that all ships that left port were presumed to have arrived at their destination unless a call indicating trouble was received. The junior officer who noticed that the ship hadn’t arrived assumed there had been an order to divert to an alternate port. The Navy had no feedback mechanism to communicate between the two ports and raise an alarm when a ship did not arrive. In fact, a Navy directive discouraged communicating the arrival of combat ships as a matter of military secrecy. As a result, no rescue mission was launched—and the sharks began to arrive.
A closer examination of usual hospitalist discharge practice reveals too much similarity for comfort. Similar to how the USS Indianapolis was removed from the plotting board on Guam, discharged patients are removed from the hospitalist’s census list. Follow-up becomes the responsibility of the patient and primary care physician.
Communication with the primary care provider is recognized as a best practice for discharge, but research suggests direct communication occurs less than 20% of the time.2 These dismal statistic suggests the “port of arrival” is unaware our patients are expected in a significant percentage of discharges. Outpatient physicians, like the junior port director in Leyte, may assume that patients who do not call or arrive for appointments have been readmitted, seen by another physician, or otherwise diverted.
Even a superficial review reveals significant deficits in the feedback provided by our current discharge practice. When patients don’t arrive at follow-up appointments, most hospitalists lack any ability to recognize this failure of their transition plan. The assumption in most hospitalist groups is that patients who leave the hospital will achieve follow-up as directed. This “presumption of success” is ill-founded and may expose the patient to potential harm—and the physician to liability.
The case of Shirk v. Kelsey offers parallels to discharge situations hospitalists commonly encounter.3 In this malpractice case, a procedure was unsuccessful in terminating a patient’s pregnancy. The performing physician left follow-up of the pathology results to the patient’s usual outpatient obstetrical provider. The patient’s lack of follow-up was not recognized by the discharging obstetrician. This provider—not the practitioner with whom follow-up was intended—was found liable.
The American Medical Association’s code of ethics states: “Once having undertaken a case, the physician should not neglect the patient.” Hospitalists form physician-patient relationships with hospitalized patients that usually terminate on discharge. Our duty to not abandon or neglect a patient diminishes significantly after discharge, when we are no longer responsible for ongoing hospital care or exchanging information with patients on a daily basis.
But our duty does not disappear. Certainly, the responsibility falls to the hospitalist to be aware of and ensure a follow-up plan for important results such as pathology reports that return after discharge.
Allegations of improper post-discharge communication or failure to pass along critical results that become available once the patient has left the hospital, are common in medical malpractice claims.
Most are settled out of court, and many do not find the physician liable for malpractice. However, legal consequences are far less relevant than the safety and quality of care compromised when patients are failed by a system that lacks feedback loops to ensure safe transitions.
The ultimate goal of medicine is to improve the health and quality of life of our patients. Whether or not a lawsuit results, we need to recognize the commonality of patients who have their care compromised, delayed, or mismanaged because of our inability to recognize a foundering transition plan. Instead of looking at this as a failure of individual physicians to communicate, the problem needs to be addressed by creating effective, reliable systems.
Solutions
Providers discharging patients with follow-up needs should have a mechanism to identify those at highest risk for problems with transition. For these patients, follow-up with a post-discharge telephone call may be an effective feedback step.
One study looking at post-discharge phone contact found that 20% of patients had not filled new discharge prescriptions. Another post-discharge study revealed that a quarter of patients had medication questions that required clarification.4,5 Other research indicates more than one in 10 patients had new or worsening symptoms in the first five days after leaving the hospital.6 Despite these symptoms, 39% of these patients did not have a follow-up appointment established.
An integrated informatics system that prompts hospitalists and primary care physicians when patients do not arrive at expected follow-up or when test results return after discharge would be optimal. But a simple phone call to identify problems can be effective. Some hospitalist groups have incorporated routine post-discharge telephone contact into their practice—but most have not. Research identifying which patients would benefit is needed to allow targeted use of resources.7
While it’s understood that not all patient discharges will go smoothly, just as not all battleships will arrive at port without incident, there is frequently an opportunity to recover when things begin to go awry. A change in the common attitude that hospitalist responsibility ends when the patient leaves the hospital is necessary.
An element of the solution lies in the creation of feedback loops to identify patients who are not obtaining follow-up as expected. This step requires a commitment of resources—something our fragmented medical system, with location-based reimbursement, does not provide incentives for.
Creation of a feedback loop may be as complex as integrated medical informatics systems, or as simple as a follow-up phone call, but it is incumbent on each hospitalist to examine the environment in which they practice and ensure this vital element of a safe and reliable system is being addressed. TH
Drs. Cumbler and Egan are assistant professors in the Section of Hospital Medicine at the University of Colorado at Denver.
References
- Stanton D. In harms way: the sinking of the USS Indianapolis and the extraordinary story of its survivors. New York, NY: Henry Holt and Company LLC; 2001.
- Kripalani S, LeFevre F, Phillips F, Williams M, Basaviah P, Baker D. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841.
- Alpers A. Key legal principles for hospitalists. Am J Med. 1999;111(9):5-9.
- Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Am J Med. 2001;111:26S-30S.
- Boockvar K, LaCorte H, Giambanco V, Fridman B, Siu A. Medication reconciliation for reducing drug
- discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4:236-243.
- Epstein K, Juarez E, Loya K, Gorman MJ, Singer A. Frequency of new or worsening symptoms in the posthospitalization period. JHM. 2007;2:58-68.
- Mistiaen P, Poot E. Telephone follow-up, initiated by a hospital-based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database of Systemic Reviews 2006; 4. Article No.:CD004510. DOI:10.1002/14651858.CD004510.pub3.
On the evening of July 30, 1945, a U.S. battleship traveling from Guam to the Philippines was spotted in the Philippine Sea by a Japanese submarine crew. Six torpedoes sped across the black water with devastating effect.
Three hundred sailors died immediately as the stricken ship sank. Another 900 men were left floating in an oil slick in the shark-infested Pacific Ocean for four days.1
The events that led to the sinking represent a classic tale of systems failures. They ring familiar to any physician who has closely examined modern medical error.
The communication breakdown that prevented the ship’s captain from being aware of submarine activity on the same route four days before is no different than communication breakdowns in complex hospital systems. The bureaucratic decision by a remote administrator to withhold the safety measure of an escort to prevent such an attack on the grounds that it “lacked necessity” likely resonates with physicians who have struggled with getting authorization for care.
Why did those sailors remain in the water four days before rescue? Despite being only a two-hour flight from the nearest base, they were not recovered—or even missed—for what must have seemed an endless amount of time.
In the end, slightly more than 300 men were alive when they were spotted by a plane that happened to fly past. The rest succumbed to dehydration, exposure, and sharks. The failure of the Navy to rescue the sailors offers lessons to the clinician trying to improve transitions of care more than a half a century later.
The Feedback Loop
Hospital discharge is a complex process initiated by physician orders on charts, prescription pads, and patient instructions. Most often, the things we assume will be done out of our direct view are carried out satisfactorily. However, any hospitalist can easily recount stories of tests or follow-up that didn’t happen as ordered. Patients who fall through the cracks at discharge—like the stranded sailors of the USS Indianapolis—are, in part, the story of a simple omitted step: the feedback loop.
A feedback loop occurs when the results or consequences of an action are returned as an input loop to the initiating step in order to modify subsequent actions. This fundamental engineering concept can keep complex systems on course. The feedback loop allows the lack of completion of a portion of a process to be recognized—and corrective measures taken—before additional harm occurs.
In Guam, the island base from which the USS Indianapolis departed, the marker indicating the ship on the plotting board was removed when the ship left. Later, the Philippine port of Leyte failed to note that the ship didn’t arrive. Policy at the time was that all ships that left port were presumed to have arrived at their destination unless a call indicating trouble was received. The junior officer who noticed that the ship hadn’t arrived assumed there had been an order to divert to an alternate port. The Navy had no feedback mechanism to communicate between the two ports and raise an alarm when a ship did not arrive. In fact, a Navy directive discouraged communicating the arrival of combat ships as a matter of military secrecy. As a result, no rescue mission was launched—and the sharks began to arrive.
A closer examination of usual hospitalist discharge practice reveals too much similarity for comfort. Similar to how the USS Indianapolis was removed from the plotting board on Guam, discharged patients are removed from the hospitalist’s census list. Follow-up becomes the responsibility of the patient and primary care physician.
Communication with the primary care provider is recognized as a best practice for discharge, but research suggests direct communication occurs less than 20% of the time.2 These dismal statistic suggests the “port of arrival” is unaware our patients are expected in a significant percentage of discharges. Outpatient physicians, like the junior port director in Leyte, may assume that patients who do not call or arrive for appointments have been readmitted, seen by another physician, or otherwise diverted.
Even a superficial review reveals significant deficits in the feedback provided by our current discharge practice. When patients don’t arrive at follow-up appointments, most hospitalists lack any ability to recognize this failure of their transition plan. The assumption in most hospitalist groups is that patients who leave the hospital will achieve follow-up as directed. This “presumption of success” is ill-founded and may expose the patient to potential harm—and the physician to liability.
The case of Shirk v. Kelsey offers parallels to discharge situations hospitalists commonly encounter.3 In this malpractice case, a procedure was unsuccessful in terminating a patient’s pregnancy. The performing physician left follow-up of the pathology results to the patient’s usual outpatient obstetrical provider. The patient’s lack of follow-up was not recognized by the discharging obstetrician. This provider—not the practitioner with whom follow-up was intended—was found liable.
The American Medical Association’s code of ethics states: “Once having undertaken a case, the physician should not neglect the patient.” Hospitalists form physician-patient relationships with hospitalized patients that usually terminate on discharge. Our duty to not abandon or neglect a patient diminishes significantly after discharge, when we are no longer responsible for ongoing hospital care or exchanging information with patients on a daily basis.
But our duty does not disappear. Certainly, the responsibility falls to the hospitalist to be aware of and ensure a follow-up plan for important results such as pathology reports that return after discharge.
Allegations of improper post-discharge communication or failure to pass along critical results that become available once the patient has left the hospital, are common in medical malpractice claims.
Most are settled out of court, and many do not find the physician liable for malpractice. However, legal consequences are far less relevant than the safety and quality of care compromised when patients are failed by a system that lacks feedback loops to ensure safe transitions.
The ultimate goal of medicine is to improve the health and quality of life of our patients. Whether or not a lawsuit results, we need to recognize the commonality of patients who have their care compromised, delayed, or mismanaged because of our inability to recognize a foundering transition plan. Instead of looking at this as a failure of individual physicians to communicate, the problem needs to be addressed by creating effective, reliable systems.
Solutions
Providers discharging patients with follow-up needs should have a mechanism to identify those at highest risk for problems with transition. For these patients, follow-up with a post-discharge telephone call may be an effective feedback step.
One study looking at post-discharge phone contact found that 20% of patients had not filled new discharge prescriptions. Another post-discharge study revealed that a quarter of patients had medication questions that required clarification.4,5 Other research indicates more than one in 10 patients had new or worsening symptoms in the first five days after leaving the hospital.6 Despite these symptoms, 39% of these patients did not have a follow-up appointment established.
An integrated informatics system that prompts hospitalists and primary care physicians when patients do not arrive at expected follow-up or when test results return after discharge would be optimal. But a simple phone call to identify problems can be effective. Some hospitalist groups have incorporated routine post-discharge telephone contact into their practice—but most have not. Research identifying which patients would benefit is needed to allow targeted use of resources.7
While it’s understood that not all patient discharges will go smoothly, just as not all battleships will arrive at port without incident, there is frequently an opportunity to recover when things begin to go awry. A change in the common attitude that hospitalist responsibility ends when the patient leaves the hospital is necessary.
An element of the solution lies in the creation of feedback loops to identify patients who are not obtaining follow-up as expected. This step requires a commitment of resources—something our fragmented medical system, with location-based reimbursement, does not provide incentives for.
Creation of a feedback loop may be as complex as integrated medical informatics systems, or as simple as a follow-up phone call, but it is incumbent on each hospitalist to examine the environment in which they practice and ensure this vital element of a safe and reliable system is being addressed. TH
Drs. Cumbler and Egan are assistant professors in the Section of Hospital Medicine at the University of Colorado at Denver.
References
- Stanton D. In harms way: the sinking of the USS Indianapolis and the extraordinary story of its survivors. New York, NY: Henry Holt and Company LLC; 2001.
- Kripalani S, LeFevre F, Phillips F, Williams M, Basaviah P, Baker D. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841.
- Alpers A. Key legal principles for hospitalists. Am J Med. 1999;111(9):5-9.
- Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Am J Med. 2001;111:26S-30S.
- Boockvar K, LaCorte H, Giambanco V, Fridman B, Siu A. Medication reconciliation for reducing drug
- discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4:236-243.
- Epstein K, Juarez E, Loya K, Gorman MJ, Singer A. Frequency of new or worsening symptoms in the posthospitalization period. JHM. 2007;2:58-68.
- Mistiaen P, Poot E. Telephone follow-up, initiated by a hospital-based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database of Systemic Reviews 2006; 4. Article No.:CD004510. DOI:10.1002/14651858.CD004510.pub3.
On the evening of July 30, 1945, a U.S. battleship traveling from Guam to the Philippines was spotted in the Philippine Sea by a Japanese submarine crew. Six torpedoes sped across the black water with devastating effect.
Three hundred sailors died immediately as the stricken ship sank. Another 900 men were left floating in an oil slick in the shark-infested Pacific Ocean for four days.1
The events that led to the sinking represent a classic tale of systems failures. They ring familiar to any physician who has closely examined modern medical error.
The communication breakdown that prevented the ship’s captain from being aware of submarine activity on the same route four days before is no different than communication breakdowns in complex hospital systems. The bureaucratic decision by a remote administrator to withhold the safety measure of an escort to prevent such an attack on the grounds that it “lacked necessity” likely resonates with physicians who have struggled with getting authorization for care.
Why did those sailors remain in the water four days before rescue? Despite being only a two-hour flight from the nearest base, they were not recovered—or even missed—for what must have seemed an endless amount of time.
In the end, slightly more than 300 men were alive when they were spotted by a plane that happened to fly past. The rest succumbed to dehydration, exposure, and sharks. The failure of the Navy to rescue the sailors offers lessons to the clinician trying to improve transitions of care more than a half a century later.
The Feedback Loop
Hospital discharge is a complex process initiated by physician orders on charts, prescription pads, and patient instructions. Most often, the things we assume will be done out of our direct view are carried out satisfactorily. However, any hospitalist can easily recount stories of tests or follow-up that didn’t happen as ordered. Patients who fall through the cracks at discharge—like the stranded sailors of the USS Indianapolis—are, in part, the story of a simple omitted step: the feedback loop.
A feedback loop occurs when the results or consequences of an action are returned as an input loop to the initiating step in order to modify subsequent actions. This fundamental engineering concept can keep complex systems on course. The feedback loop allows the lack of completion of a portion of a process to be recognized—and corrective measures taken—before additional harm occurs.
In Guam, the island base from which the USS Indianapolis departed, the marker indicating the ship on the plotting board was removed when the ship left. Later, the Philippine port of Leyte failed to note that the ship didn’t arrive. Policy at the time was that all ships that left port were presumed to have arrived at their destination unless a call indicating trouble was received. The junior officer who noticed that the ship hadn’t arrived assumed there had been an order to divert to an alternate port. The Navy had no feedback mechanism to communicate between the two ports and raise an alarm when a ship did not arrive. In fact, a Navy directive discouraged communicating the arrival of combat ships as a matter of military secrecy. As a result, no rescue mission was launched—and the sharks began to arrive.
A closer examination of usual hospitalist discharge practice reveals too much similarity for comfort. Similar to how the USS Indianapolis was removed from the plotting board on Guam, discharged patients are removed from the hospitalist’s census list. Follow-up becomes the responsibility of the patient and primary care physician.
Communication with the primary care provider is recognized as a best practice for discharge, but research suggests direct communication occurs less than 20% of the time.2 These dismal statistic suggests the “port of arrival” is unaware our patients are expected in a significant percentage of discharges. Outpatient physicians, like the junior port director in Leyte, may assume that patients who do not call or arrive for appointments have been readmitted, seen by another physician, or otherwise diverted.
Even a superficial review reveals significant deficits in the feedback provided by our current discharge practice. When patients don’t arrive at follow-up appointments, most hospitalists lack any ability to recognize this failure of their transition plan. The assumption in most hospitalist groups is that patients who leave the hospital will achieve follow-up as directed. This “presumption of success” is ill-founded and may expose the patient to potential harm—and the physician to liability.
The case of Shirk v. Kelsey offers parallels to discharge situations hospitalists commonly encounter.3 In this malpractice case, a procedure was unsuccessful in terminating a patient’s pregnancy. The performing physician left follow-up of the pathology results to the patient’s usual outpatient obstetrical provider. The patient’s lack of follow-up was not recognized by the discharging obstetrician. This provider—not the practitioner with whom follow-up was intended—was found liable.
The American Medical Association’s code of ethics states: “Once having undertaken a case, the physician should not neglect the patient.” Hospitalists form physician-patient relationships with hospitalized patients that usually terminate on discharge. Our duty to not abandon or neglect a patient diminishes significantly after discharge, when we are no longer responsible for ongoing hospital care or exchanging information with patients on a daily basis.
But our duty does not disappear. Certainly, the responsibility falls to the hospitalist to be aware of and ensure a follow-up plan for important results such as pathology reports that return after discharge.
Allegations of improper post-discharge communication or failure to pass along critical results that become available once the patient has left the hospital, are common in medical malpractice claims.
Most are settled out of court, and many do not find the physician liable for malpractice. However, legal consequences are far less relevant than the safety and quality of care compromised when patients are failed by a system that lacks feedback loops to ensure safe transitions.
The ultimate goal of medicine is to improve the health and quality of life of our patients. Whether or not a lawsuit results, we need to recognize the commonality of patients who have their care compromised, delayed, or mismanaged because of our inability to recognize a foundering transition plan. Instead of looking at this as a failure of individual physicians to communicate, the problem needs to be addressed by creating effective, reliable systems.
Solutions
Providers discharging patients with follow-up needs should have a mechanism to identify those at highest risk for problems with transition. For these patients, follow-up with a post-discharge telephone call may be an effective feedback step.
One study looking at post-discharge phone contact found that 20% of patients had not filled new discharge prescriptions. Another post-discharge study revealed that a quarter of patients had medication questions that required clarification.4,5 Other research indicates more than one in 10 patients had new or worsening symptoms in the first five days after leaving the hospital.6 Despite these symptoms, 39% of these patients did not have a follow-up appointment established.
An integrated informatics system that prompts hospitalists and primary care physicians when patients do not arrive at expected follow-up or when test results return after discharge would be optimal. But a simple phone call to identify problems can be effective. Some hospitalist groups have incorporated routine post-discharge telephone contact into their practice—but most have not. Research identifying which patients would benefit is needed to allow targeted use of resources.7
While it’s understood that not all patient discharges will go smoothly, just as not all battleships will arrive at port without incident, there is frequently an opportunity to recover when things begin to go awry. A change in the common attitude that hospitalist responsibility ends when the patient leaves the hospital is necessary.
An element of the solution lies in the creation of feedback loops to identify patients who are not obtaining follow-up as expected. This step requires a commitment of resources—something our fragmented medical system, with location-based reimbursement, does not provide incentives for.
Creation of a feedback loop may be as complex as integrated medical informatics systems, or as simple as a follow-up phone call, but it is incumbent on each hospitalist to examine the environment in which they practice and ensure this vital element of a safe and reliable system is being addressed. TH
Drs. Cumbler and Egan are assistant professors in the Section of Hospital Medicine at the University of Colorado at Denver.
References
- Stanton D. In harms way: the sinking of the USS Indianapolis and the extraordinary story of its survivors. New York, NY: Henry Holt and Company LLC; 2001.
- Kripalani S, LeFevre F, Phillips F, Williams M, Basaviah P, Baker D. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841.
- Alpers A. Key legal principles for hospitalists. Am J Med. 1999;111(9):5-9.
- Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Am J Med. 2001;111:26S-30S.
- Boockvar K, LaCorte H, Giambanco V, Fridman B, Siu A. Medication reconciliation for reducing drug
- discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4:236-243.
- Epstein K, Juarez E, Loya K, Gorman MJ, Singer A. Frequency of new or worsening symptoms in the posthospitalization period. JHM. 2007;2:58-68.
- Mistiaen P, Poot E. Telephone follow-up, initiated by a hospital-based health professional, for postdischarge problems in patients discharged from hospital to home. Cochrane Database of Systemic Reviews 2006; 4. Article No.:CD004510. DOI:10.1002/14651858.CD004510.pub3.
The Psychology of Error
What do your patient care errors have in common with financial mistakes that may compromise your retirement? Both have their underpinnings in the psychological strategies and tendencies we call heuristics.
The word derives from the Greek term “heuriskein” for discovery, but in the medical context we frequently think of these as these as mental shortcuts. Heuristics allow us to operate quickly despite the bewildering degree of complexity and uncertainty we encounter as we operate in the world but also lay the groundwork for disaster when they lead us astray. Let’s examine two mistakes and look at what they have in common: one that led to a drubbing in the stock market and the other that cost a patient his life.
A Market Misadventure
During the height of the market boom a young internist purchased shares of an exciting new biotech company poised at the forefront of tailored medical therapy based on genetic sequencing.
The stock nearly doubled, but as he rode the wild ride of the market’s fluctuations it became evident that the overall trend had changed. Almost daily monitoring of the press releases from the dynamic CEO helped reinforce his decision to hold the stock even after the dizzying drop that changed a strong gain to a significant loss. Finally after waiting months for the stock ticker to nudge back up to his entry point, he was glumly forced to face the loss.
The field of behavioral finance suggests humans are subject to cognitive predispositions leading to predictable errors. The first heuristic failure demonstrated by the unfortunate internist in our example is that of anchoring (see sidebar, p. 35).
The initial impression of the value of the company or particular price at which he purchased the stock has significance to him but is completely irrelevant to the value of the company once events and profit prospects changed. Thus, when new information about the company came to light, the focus should have been exclusively on the future valuation without regard to the past. That didn’t happen in this case. Our hapless investor had become anchored to the original price and refused to sell as it plummeted in the vain hopes that it would rise again despite the absence of evidence that this was likely.
Anchoring bias affects all of us and is as true in medicine as it is in the markets. The first diagnosis, which seems likely as we hear a case described, can be surprisingly hard to shake even when the facts on the ground have changed.
A second human tendency we see leading to both financial and medical calamity is the desire to be right. A strong self-image (and many physicians have a strong one, indeed) is bolstered by seeking information that confirms prior beliefs.
Unfortunately this confirmation bias can also cause us to overvalue the positive press about a company we are invested in and discount or not read at all things that might change our minds. Back in the clinical environment, examples abound where a physician becomes fixed on a diagnosis and orders tests designed to confirm the initial impression but fails to explore alternatives. The more invested in a diagnosis we become, the more selective we tend to be in seeking and interpreting data to reinforce our convictions.
Higher Stakes
Years later and hundreds of miles away a nocturnist gets a call from the emergency department (ED) on the seventh new admission of the night.
“I’ve got another rule-out myocardial infarction (MI) for you” said the ED physician, who briefly provided the assessment that the patient was low risk, with negative enzymes, chest X-ray, and electrocardiogram.
The nocturnist noted the atypical severity of the pain, systolic blood pressure more than 200, and positive cocaine history. But this did not alter the plan as the patient was passed from the ED physician to the nocturnist and then to the hospitalist who assumed care the next morning. Unfortunately, it took the patient experiencing a severe increase in tearing pain radiating to his back during the exercise stress test to prompt the discovery of his ascending aortic dissection. The patient died on the operating room table, leaving all three physicians wondering how they could have missed the diagnosis when in retrospect it seemed so obvious.
Present the same clinical scenario at grand rounds and the third-year medical students could tell you dissection should have been considered. How did three smart experienced people all make the same fatal mistake?
This case demonstrates a number of heuristic failures. Availability bias is a form of pattern recognition and arises from our habit of perceiving the things we see often as more likely than those which we have not seen or thought about recently. Hoof beats in Kentucky, as they say, are usually not a herd of zebra. ED physicians see what at times seems like hordes of patients with low-risk chest pain, the vast majority of which lack a life-threatening etiology. Thus, we can become complacent in assuming that the next admission for chest pain reflects the same cause as the seven before.
Pattern recognition serves a vital role. Most expert physicians rely on this more than classic deductive reasoning and, much less, Bayesian analysis. Casino operators exploit this tendency to see false patterns to their profit by installing displays that show the last 10 to 20 results over the roulette table. However, just as each turn of the roulette wheel is not influenced by prior spins, each patient is unique. One must beware of the misleading power of the availability bias.
Once the initial misdiagnosis had been made, the anchoring bias and confirmation bias continued the cascade of events—turning a mistake from a temporary error to a disaster. The phrase “chest pain rule out MI” not only encourages the physician to minimize the potential severity of the symptom via the framing effect but also telegraphs the anchoring phenomenon by fixing on a single disease concern for a symptom whose etiologies are legion.
However, even accepting that the initial diagnosis by the ED doctor was influenced by the availability bias, why was this not corrected by the nocturnist or by the hospitalist on the next day? The answer lies in diagnosis momentum.
Each physician does not evaluate the patient in isolation but rather has a tendency to include the assessment of the prior clinician as part of their own decision-making process. The more people who have seen the patient and agreed with the diagnosis, the higher the mental hurdle becomes to disagree and take the work-up in a different direction.
What You Can Do
Does the mere existence of these many heuristics condemn the physician to a career of repeating these potentially fatal errors? The obvious answer is no, but the solution requires a concerted effort on the part of the physician to avoid these mistakes.
Step one is to recognize that many heuristics are essentially abbreviations of full conscious reasoning. Now take a physician who is tired, stressed, or inundated with multiple tasks. In an effort to organize the seemingly chaotic world of medicine the mind seeks a crutch. These mental shortcuts allow us to quickly process massive amounts of information and come up with a reasonable plan that will be right most of the time.
When rushed, stressed, and distracted, we are most prone to use these shortcuts. These times of pressure are exactly when it is most important to pause and consider whether we’re acting on gut feeling or on full consideration of all the evidence. Awareness of the predictable circumstances that create the set-up for heuristic failures allows for a moment of reflection to prevent falling into one of these psychological traps. This process of deliberately considering our own decision-making is referred to as meta-cognition.
An additional familiar tool available to the physician is differential diagnosis. This is essentially a form of cognitive forcing strategy designed to guard against availability and anchoring biases. By deliberately creating a list of alternative possibilities, we become less prone to anchor on a single diagnosis.
By briefly reviewing the rare possibilities we have not seen recently and bringing them to the forefront of memory, we diminish the power of the availability bias. Spending a second or two considering the differential—even in seemingly routine cases—will defuse the hold of these particular heuristics.
Hospitalists by the nature of our practice tend to have multiple transitions in patient care. At times this offers a fresh perspective to correct mistakes, but it also offers potential to compound them via diagnosis momentum.
We habitually convey diagnosis and treatment plans to our partners at handoffs. Including a level of uncertainty as part of checkout would create a cue for the accepting physician to decrease the risk of this heuristic failure. One might imagine the patient in the case above would have had a greater probability of survival if the nocturnist had conveyed a diagnosis of “chest pain of uncertain etiology” to his partner rather than “chest pain rule-out MI.”
As illustrated by the cases above, heuristics are not mistakes in and of themselves. They are the assumptions and pattern-recognition techniques that serve us well the majority of the time in and out of medicine. Recognizing when you take one of these mental shortcuts, being aware of the circumstances that predispose to error creation, and evaluating your decision-making process allows the astute physician to guard against the times when they fail. Greater self-awareness of the process of your own cognition can make for a better clinician—and perhaps even make you a better investor. TH
Drs. Cumbler and Trosterman are assistant professors in the Section of Hospital Medicine at the University of Colorado.
What do your patient care errors have in common with financial mistakes that may compromise your retirement? Both have their underpinnings in the psychological strategies and tendencies we call heuristics.
The word derives from the Greek term “heuriskein” for discovery, but in the medical context we frequently think of these as these as mental shortcuts. Heuristics allow us to operate quickly despite the bewildering degree of complexity and uncertainty we encounter as we operate in the world but also lay the groundwork for disaster when they lead us astray. Let’s examine two mistakes and look at what they have in common: one that led to a drubbing in the stock market and the other that cost a patient his life.
A Market Misadventure
During the height of the market boom a young internist purchased shares of an exciting new biotech company poised at the forefront of tailored medical therapy based on genetic sequencing.
The stock nearly doubled, but as he rode the wild ride of the market’s fluctuations it became evident that the overall trend had changed. Almost daily monitoring of the press releases from the dynamic CEO helped reinforce his decision to hold the stock even after the dizzying drop that changed a strong gain to a significant loss. Finally after waiting months for the stock ticker to nudge back up to his entry point, he was glumly forced to face the loss.
The field of behavioral finance suggests humans are subject to cognitive predispositions leading to predictable errors. The first heuristic failure demonstrated by the unfortunate internist in our example is that of anchoring (see sidebar, p. 35).
The initial impression of the value of the company or particular price at which he purchased the stock has significance to him but is completely irrelevant to the value of the company once events and profit prospects changed. Thus, when new information about the company came to light, the focus should have been exclusively on the future valuation without regard to the past. That didn’t happen in this case. Our hapless investor had become anchored to the original price and refused to sell as it plummeted in the vain hopes that it would rise again despite the absence of evidence that this was likely.
Anchoring bias affects all of us and is as true in medicine as it is in the markets. The first diagnosis, which seems likely as we hear a case described, can be surprisingly hard to shake even when the facts on the ground have changed.
A second human tendency we see leading to both financial and medical calamity is the desire to be right. A strong self-image (and many physicians have a strong one, indeed) is bolstered by seeking information that confirms prior beliefs.
Unfortunately this confirmation bias can also cause us to overvalue the positive press about a company we are invested in and discount or not read at all things that might change our minds. Back in the clinical environment, examples abound where a physician becomes fixed on a diagnosis and orders tests designed to confirm the initial impression but fails to explore alternatives. The more invested in a diagnosis we become, the more selective we tend to be in seeking and interpreting data to reinforce our convictions.
Higher Stakes
Years later and hundreds of miles away a nocturnist gets a call from the emergency department (ED) on the seventh new admission of the night.
“I’ve got another rule-out myocardial infarction (MI) for you” said the ED physician, who briefly provided the assessment that the patient was low risk, with negative enzymes, chest X-ray, and electrocardiogram.
The nocturnist noted the atypical severity of the pain, systolic blood pressure more than 200, and positive cocaine history. But this did not alter the plan as the patient was passed from the ED physician to the nocturnist and then to the hospitalist who assumed care the next morning. Unfortunately, it took the patient experiencing a severe increase in tearing pain radiating to his back during the exercise stress test to prompt the discovery of his ascending aortic dissection. The patient died on the operating room table, leaving all three physicians wondering how they could have missed the diagnosis when in retrospect it seemed so obvious.
Present the same clinical scenario at grand rounds and the third-year medical students could tell you dissection should have been considered. How did three smart experienced people all make the same fatal mistake?
This case demonstrates a number of heuristic failures. Availability bias is a form of pattern recognition and arises from our habit of perceiving the things we see often as more likely than those which we have not seen or thought about recently. Hoof beats in Kentucky, as they say, are usually not a herd of zebra. ED physicians see what at times seems like hordes of patients with low-risk chest pain, the vast majority of which lack a life-threatening etiology. Thus, we can become complacent in assuming that the next admission for chest pain reflects the same cause as the seven before.
Pattern recognition serves a vital role. Most expert physicians rely on this more than classic deductive reasoning and, much less, Bayesian analysis. Casino operators exploit this tendency to see false patterns to their profit by installing displays that show the last 10 to 20 results over the roulette table. However, just as each turn of the roulette wheel is not influenced by prior spins, each patient is unique. One must beware of the misleading power of the availability bias.
Once the initial misdiagnosis had been made, the anchoring bias and confirmation bias continued the cascade of events—turning a mistake from a temporary error to a disaster. The phrase “chest pain rule out MI” not only encourages the physician to minimize the potential severity of the symptom via the framing effect but also telegraphs the anchoring phenomenon by fixing on a single disease concern for a symptom whose etiologies are legion.
However, even accepting that the initial diagnosis by the ED doctor was influenced by the availability bias, why was this not corrected by the nocturnist or by the hospitalist on the next day? The answer lies in diagnosis momentum.
Each physician does not evaluate the patient in isolation but rather has a tendency to include the assessment of the prior clinician as part of their own decision-making process. The more people who have seen the patient and agreed with the diagnosis, the higher the mental hurdle becomes to disagree and take the work-up in a different direction.
What You Can Do
Does the mere existence of these many heuristics condemn the physician to a career of repeating these potentially fatal errors? The obvious answer is no, but the solution requires a concerted effort on the part of the physician to avoid these mistakes.
Step one is to recognize that many heuristics are essentially abbreviations of full conscious reasoning. Now take a physician who is tired, stressed, or inundated with multiple tasks. In an effort to organize the seemingly chaotic world of medicine the mind seeks a crutch. These mental shortcuts allow us to quickly process massive amounts of information and come up with a reasonable plan that will be right most of the time.
When rushed, stressed, and distracted, we are most prone to use these shortcuts. These times of pressure are exactly when it is most important to pause and consider whether we’re acting on gut feeling or on full consideration of all the evidence. Awareness of the predictable circumstances that create the set-up for heuristic failures allows for a moment of reflection to prevent falling into one of these psychological traps. This process of deliberately considering our own decision-making is referred to as meta-cognition.
An additional familiar tool available to the physician is differential diagnosis. This is essentially a form of cognitive forcing strategy designed to guard against availability and anchoring biases. By deliberately creating a list of alternative possibilities, we become less prone to anchor on a single diagnosis.
By briefly reviewing the rare possibilities we have not seen recently and bringing them to the forefront of memory, we diminish the power of the availability bias. Spending a second or two considering the differential—even in seemingly routine cases—will defuse the hold of these particular heuristics.
Hospitalists by the nature of our practice tend to have multiple transitions in patient care. At times this offers a fresh perspective to correct mistakes, but it also offers potential to compound them via diagnosis momentum.
We habitually convey diagnosis and treatment plans to our partners at handoffs. Including a level of uncertainty as part of checkout would create a cue for the accepting physician to decrease the risk of this heuristic failure. One might imagine the patient in the case above would have had a greater probability of survival if the nocturnist had conveyed a diagnosis of “chest pain of uncertain etiology” to his partner rather than “chest pain rule-out MI.”
As illustrated by the cases above, heuristics are not mistakes in and of themselves. They are the assumptions and pattern-recognition techniques that serve us well the majority of the time in and out of medicine. Recognizing when you take one of these mental shortcuts, being aware of the circumstances that predispose to error creation, and evaluating your decision-making process allows the astute physician to guard against the times when they fail. Greater self-awareness of the process of your own cognition can make for a better clinician—and perhaps even make you a better investor. TH
Drs. Cumbler and Trosterman are assistant professors in the Section of Hospital Medicine at the University of Colorado.
What do your patient care errors have in common with financial mistakes that may compromise your retirement? Both have their underpinnings in the psychological strategies and tendencies we call heuristics.
The word derives from the Greek term “heuriskein” for discovery, but in the medical context we frequently think of these as these as mental shortcuts. Heuristics allow us to operate quickly despite the bewildering degree of complexity and uncertainty we encounter as we operate in the world but also lay the groundwork for disaster when they lead us astray. Let’s examine two mistakes and look at what they have in common: one that led to a drubbing in the stock market and the other that cost a patient his life.
A Market Misadventure
During the height of the market boom a young internist purchased shares of an exciting new biotech company poised at the forefront of tailored medical therapy based on genetic sequencing.
The stock nearly doubled, but as he rode the wild ride of the market’s fluctuations it became evident that the overall trend had changed. Almost daily monitoring of the press releases from the dynamic CEO helped reinforce his decision to hold the stock even after the dizzying drop that changed a strong gain to a significant loss. Finally after waiting months for the stock ticker to nudge back up to his entry point, he was glumly forced to face the loss.
The field of behavioral finance suggests humans are subject to cognitive predispositions leading to predictable errors. The first heuristic failure demonstrated by the unfortunate internist in our example is that of anchoring (see sidebar, p. 35).
The initial impression of the value of the company or particular price at which he purchased the stock has significance to him but is completely irrelevant to the value of the company once events and profit prospects changed. Thus, when new information about the company came to light, the focus should have been exclusively on the future valuation without regard to the past. That didn’t happen in this case. Our hapless investor had become anchored to the original price and refused to sell as it plummeted in the vain hopes that it would rise again despite the absence of evidence that this was likely.
Anchoring bias affects all of us and is as true in medicine as it is in the markets. The first diagnosis, which seems likely as we hear a case described, can be surprisingly hard to shake even when the facts on the ground have changed.
A second human tendency we see leading to both financial and medical calamity is the desire to be right. A strong self-image (and many physicians have a strong one, indeed) is bolstered by seeking information that confirms prior beliefs.
Unfortunately this confirmation bias can also cause us to overvalue the positive press about a company we are invested in and discount or not read at all things that might change our minds. Back in the clinical environment, examples abound where a physician becomes fixed on a diagnosis and orders tests designed to confirm the initial impression but fails to explore alternatives. The more invested in a diagnosis we become, the more selective we tend to be in seeking and interpreting data to reinforce our convictions.
Higher Stakes
Years later and hundreds of miles away a nocturnist gets a call from the emergency department (ED) on the seventh new admission of the night.
“I’ve got another rule-out myocardial infarction (MI) for you” said the ED physician, who briefly provided the assessment that the patient was low risk, with negative enzymes, chest X-ray, and electrocardiogram.
The nocturnist noted the atypical severity of the pain, systolic blood pressure more than 200, and positive cocaine history. But this did not alter the plan as the patient was passed from the ED physician to the nocturnist and then to the hospitalist who assumed care the next morning. Unfortunately, it took the patient experiencing a severe increase in tearing pain radiating to his back during the exercise stress test to prompt the discovery of his ascending aortic dissection. The patient died on the operating room table, leaving all three physicians wondering how they could have missed the diagnosis when in retrospect it seemed so obvious.
Present the same clinical scenario at grand rounds and the third-year medical students could tell you dissection should have been considered. How did three smart experienced people all make the same fatal mistake?
This case demonstrates a number of heuristic failures. Availability bias is a form of pattern recognition and arises from our habit of perceiving the things we see often as more likely than those which we have not seen or thought about recently. Hoof beats in Kentucky, as they say, are usually not a herd of zebra. ED physicians see what at times seems like hordes of patients with low-risk chest pain, the vast majority of which lack a life-threatening etiology. Thus, we can become complacent in assuming that the next admission for chest pain reflects the same cause as the seven before.
Pattern recognition serves a vital role. Most expert physicians rely on this more than classic deductive reasoning and, much less, Bayesian analysis. Casino operators exploit this tendency to see false patterns to their profit by installing displays that show the last 10 to 20 results over the roulette table. However, just as each turn of the roulette wheel is not influenced by prior spins, each patient is unique. One must beware of the misleading power of the availability bias.
Once the initial misdiagnosis had been made, the anchoring bias and confirmation bias continued the cascade of events—turning a mistake from a temporary error to a disaster. The phrase “chest pain rule out MI” not only encourages the physician to minimize the potential severity of the symptom via the framing effect but also telegraphs the anchoring phenomenon by fixing on a single disease concern for a symptom whose etiologies are legion.
However, even accepting that the initial diagnosis by the ED doctor was influenced by the availability bias, why was this not corrected by the nocturnist or by the hospitalist on the next day? The answer lies in diagnosis momentum.
Each physician does not evaluate the patient in isolation but rather has a tendency to include the assessment of the prior clinician as part of their own decision-making process. The more people who have seen the patient and agreed with the diagnosis, the higher the mental hurdle becomes to disagree and take the work-up in a different direction.
What You Can Do
Does the mere existence of these many heuristics condemn the physician to a career of repeating these potentially fatal errors? The obvious answer is no, but the solution requires a concerted effort on the part of the physician to avoid these mistakes.
Step one is to recognize that many heuristics are essentially abbreviations of full conscious reasoning. Now take a physician who is tired, stressed, or inundated with multiple tasks. In an effort to organize the seemingly chaotic world of medicine the mind seeks a crutch. These mental shortcuts allow us to quickly process massive amounts of information and come up with a reasonable plan that will be right most of the time.
When rushed, stressed, and distracted, we are most prone to use these shortcuts. These times of pressure are exactly when it is most important to pause and consider whether we’re acting on gut feeling or on full consideration of all the evidence. Awareness of the predictable circumstances that create the set-up for heuristic failures allows for a moment of reflection to prevent falling into one of these psychological traps. This process of deliberately considering our own decision-making is referred to as meta-cognition.
An additional familiar tool available to the physician is differential diagnosis. This is essentially a form of cognitive forcing strategy designed to guard against availability and anchoring biases. By deliberately creating a list of alternative possibilities, we become less prone to anchor on a single diagnosis.
By briefly reviewing the rare possibilities we have not seen recently and bringing them to the forefront of memory, we diminish the power of the availability bias. Spending a second or two considering the differential—even in seemingly routine cases—will defuse the hold of these particular heuristics.
Hospitalists by the nature of our practice tend to have multiple transitions in patient care. At times this offers a fresh perspective to correct mistakes, but it also offers potential to compound them via diagnosis momentum.
We habitually convey diagnosis and treatment plans to our partners at handoffs. Including a level of uncertainty as part of checkout would create a cue for the accepting physician to decrease the risk of this heuristic failure. One might imagine the patient in the case above would have had a greater probability of survival if the nocturnist had conveyed a diagnosis of “chest pain of uncertain etiology” to his partner rather than “chest pain rule-out MI.”
As illustrated by the cases above, heuristics are not mistakes in and of themselves. They are the assumptions and pattern-recognition techniques that serve us well the majority of the time in and out of medicine. Recognizing when you take one of these mental shortcuts, being aware of the circumstances that predispose to error creation, and evaluating your decision-making process allows the astute physician to guard against the times when they fail. Greater self-awareness of the process of your own cognition can make for a better clinician—and perhaps even make you a better investor. TH
Drs. Cumbler and Trosterman are assistant professors in the Section of Hospital Medicine at the University of Colorado.
In the Literature
In This Edition
- Cost sharing for prescription medications increases consumption of more costly healthcare services
- Community-acquired pneumonia core measures can lead to unintended consequences
- Prophylactic revascularization has no clear benefit for high-risk patients undergoing vascular surgery
- Aspirin resistance correlates with adverse clinical events
- Low-molecular-weight heparin appears to have greater efficacy as a prophylactic agent against deep-vein thrombosis and pulmonary embolism
- Antipsychotic medications appear to be associated with increased risk of death in demented patients
- Anticoagulation plus antiplatelet therapy fails to show benefit for peripheral arterial disease
- Transient atrial fibrillation following myocardial infarction increases the risk of recurrence and stroke
Do Incentives to Encourage Use of Certain Medications Affect Care?
Background: Insurers are increasingly using financial mechanisms to affect pharmaceutical usage. These practices may affect medication use and health outcomes in ways that are poorly defined and difficult to detect.
Study design: Literature review
Synopsis: There are numerous structures for drug-cost sharing, and this study evaluated co-payments, tiers/co-insurance, benefit caps, formulary limitations, and reference pricing strategies for their effect on prescription drug usage and healthcare outcomes.
Included articles varied widely in study design, making generalizable results difficult to isolate, and insurers may have instituted more than one cost-sharing mechanism simultaneously. Overall, for every 10% increase in cost sharing (via copayments or co-insurance) there was an associated 2%-6% decrease in prescription drug spending. Increasing consumer costs for medications clearly decreases usage.
Some studies demonstrated that the decrease in medication utilization was more pronounced for “nonessential” medications over “essential” medications. However, in specific chronic illnesses this is clearly associated with greater usage of inpatient and emergency medical services.
Cost sharing was also more likely to have adverse health consequences in vulnerable populations, particularly the elderly and poor. One in four Medicaid patients couldn’t fill at least one prescription in the past year, as opposed to one in 10 privately insured patients who couldn’t purchase one or more medications.
Further impact on healthcare consumption and outcomes may be masked because it is difficult to determine individual disease severity, and the effect on the more severely ill would be expected to be greater. These authors attempted to sort out a complex interaction between cost, consumption, and health, and they found important trends.
The goal of cost sharing is to align consumption more clearly with appropriate and economic products, thereby using cost sharing as a public health tool. The consequence of creating the incentives for ill patients to forego necessary treatments is a counterbalancing concern that is supported in some, but not all, of the literature.
Bottom line: Cost sharing for prescription medications decreases medication spending and utilization but disproportionately affects the disadvantaged and increases consumption of more costly healthcare services in patients with some chronic illnesses.
Citation: Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298(1):61-69.
Does Antibiotic Requirement for Suspected CAP Increase Misdiagnosis?
Background: Early administration of antibiotics in community-acquired pneumonia (CAP) improves patient outcomes. The Infectious Disease Society of America instituted guidelines that recommend initiation of antibiotics to all patients with suspected CAP within four hours of triage, and some payors are using this as a quality measure affecting reimbursement. However, this incentive may cause premature diagnosis of CAP and overuse of antibiotics.
Study design: Retrospective chart review
Setting: A large, high-volume teaching hospital with more than 500 beds and more than 112,000 annual emergency department (ED) visits
Synopsis: Charts of all patients with an admitting diagnosis of CAP were reviewed over two six-month periods. The initial review was prior to initiation of a four-hour antibiotics rule; the second was after a financial incentive to initiate antibiotics within four hours of triage was initiated.
After initiation of the four-hour rule, of the patients with an admitting diagnosis of CAP, significantly more patients received antibiotics within four hours of triage (66% versus 54%). However, the number of patients with abnormal chest X-ray findings associated with the diagnosis of CAP decreased from 28.5% to 20.6%, and the proportion of patients with a discharge diagnosis of CAP decreased from 75.9% to 58.9%.
The authors also used two diagnostic paradigms to make an independent diagnosis of CAP based on chart data. With the less rigorous independent analysis 44.7% of patients actually had CAP prior to the four-hour rule, and this fell to 36% after the four-hour rule. Using a more rigorous definition, only 32.7% of patients actually had CAP prior to initiation of the four-hour rule, and this fell to 27%.
There was no difference in length of stay or ICU transfers between the two analysis periods. The authors concluded that a four-hour rule increases premature diagnosis of CAP, presumably because providers felt compelled to initiate antibiotics before they had complete clinical data.
This tendency was associated with misuse and overuse of antibiotics, and increased laboratory testing, such as blood cultures, which had to be obtained before antibiotics were initiated. The authors emphasized the importance of reimbursement-associated quality measures creating incentives to treat the right patients for the correct diagnosis, and the potential harmful consequences of applying a quality-driven protocol to the wrong patient.
They suggest a six-hour rule would decrease the misdiagnosis of CAP. They also feel eliminating a mandatory time frame and requiring only that the first dose of antibiotics be administered in the ED will further ameliorate these effects.
Bottom line: Mandatory administration of antibiotics to patients with suspected CAP within four hours of triage increases the percentage of patients who receive antibiotics within four hours, but also increases the rate of misdiagnosis of CAP, inappropriate administration of antibiotics, and increased use of some laboratory services.
Citation: Kanwar M, Brar N, Khatib R, et al. Misdiagnosis of community-acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4-hour antibiotic administration rule. Chest. 2007 Jun;131(6):1865-1869.
Does prophylactic cardiac revascularization benefit patients undergoing vascular surgery?
Background: American College of Cardiology/American Heart Association Guidelines recommend referral for patients with multiple cardiac risk factors for non-invasive cardiac stress testing prior to surgery and prophylactic revascularization in high-risk patients. The authors performed a pilot analysis to determine how many patients would be needed to prospectively validate this recommendation in those with more significant ischemic cardiac disease.
Study design: Randomized controlled pilot study of 1,880 consecutive patients undergoing elective vascular surgery
Setting: Brazil, Belgium, the Netherlands, Italy, Serbia, and Montenegro
Synopsis: This was a pilot study to determine the necessary power to prove or disprove the benefit of the recommendation for cardiac revascularization in high-risk patients before major vascular surgery.
Prior research had shown that prophylactic revascularization is not of demonstrable benefit in this cohort. However, the majority of the patients in this previous trial had two-vessel disease and preserved left ventricular function. This study examined a sicker cohort of patients with more significant coronary artery disease and depressed left ventricular function.
This pilot screened all patients undergoing high-risk vascular surgery. All patients with three or more risk factors underwent non-invasive evaluation for cardiac ischemia. Patients with extensive ischemia were randomized to invasive evaluation and revascularization as appropriate or non-invasive management. Both arms received optimal medical management.
Prophylactic revascularization did not improve 30-day outcome after vascular surgery, demonstrated no difference in perioperative cardiac events, and found no difference in all-cause mortality or nonfatal myocardial infarction. Similarly, there was no evidence of long-term (at one year) difference between groups. The sample size needed to definitively establish that coronary revascularization is superior to medical therapy would be 300 patients per arm. That would require screening 9,000 patients.
Bottom line: Prophylactic revascularization has no clear benefit for high-risk patients undergoing vascular surgery, but a much larger sample size would be required to definitively prove or disprove benefit.
Citation: Poldermans D, Schouten O, Vidakovic R, et al. Clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V pilot study. J. Am Coll Cardiol. 2007;49(17):1763-1769.
How Does Aspirin Resistance Affect Patients with Coronary Artery Disease?
Background: Although aspirin is used to decrease the risk of ischemic events, up to 45% of patients do not derive adequate anti-platelet activity. Few prospective studies have used laboratory-measured aspirin resistance to assess clinical outcomes.
Study design: Blinded cohort
Setting: Patients affiliated with Queen Mary Hospital, the University of Hong Kong.
Synopsis: Aspirin-induced platelet inhibition was measured quantitatively on 468 patients with stable coronary artery disease who take 80-325 mg of aspirin per day. The study found 128 patients were aspirin resistant. Aspirin resistance was more prevalent with increased age, female gender, renal insufficiency, anemia, and with use of low-dose aspirin. At follow up, aspirin-resistant patients were more likely to develop a primary outcome event: cardiovascular deaths, myocardial infarction, stroke, transient ischemic attack, and unstable angina. Aspirin resistance was an independent risk factor for developing the aforementioned outcomes, as are diabetes, prior myocardial infarction, and low hemoglobin.
Bottom line: Aspirin resistance, as defined by an aggregation-based assay, is associated with adverse outcomes in patients with stable coronary artery disease.
Citation: Chen W, Cheng X, Lee PY, et al. Aspirin resistance and adverse clinical events in patients with coronary artery disease. Am J Med. 2007 Jul;120(7):631-635.
Which Agents Best Prevent Venous Thromboembolism?
Background: Pulmonary emboli have been linked to 10% of in-hospital deaths. There continues to be a strong emphasis on prevention. Unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and selective factor Xa inhibitors are used for prophylaxis.
Study design: A meta-analysis of randomized controlled trials
Synopsis: The meta-analysis included 36 studies of hospitalized medical patients that compared UFH with control, LMWH with control, LMWH with UFH, and a selective factor Xa inhibitor with a placebo.
When each was compared with a control, UFH and LMWH were associated with a decreased risk of deep venous thrombosis (DVT) (risk ratio=0.33; 0.56) and pulmonary embolism (PE) (risk ratio=0.64; 0.37). Compared with control, LMWH three times daily was more effective than twice-daily dosing (risk ratio=0.27, 0.52). Through direct comparison of UFH and LMWH, LMWH was shown to have decreased DVT risk (risk ratio=0.68) and fewer injection site hematomas (risk ratio=0.47).
Neither UFH nor LMWH reduced mortality. LMWH and UFH were associated with significantly more bleeding events than control, but this increased risk was significant only for minor bleeding.
Bottom line: LMWH appears to have greater efficacy than UFH as a prophylactic agent against DVT/PE. If UFH is used, three times daily dosing is preferred.
Citation: Wein L, Wein S, Haas SJ, et al. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients. Arch Intern Med. 2007;167(14):1476-1486.
What Is the Association Between Antipsychotic Drugs and Mortality?
Background: Atypical antipsychotics prescribed off-label for problematic behaviors in dementia have been associated with risks including diabetes, stroke, and increased mortality. This resulted in the FDA placing a “black box” warning on atypical antipsychotics used for dementia. Subsequent studies have suggested that conventional antipsychotics are perhaps even more problematic.
Study Design: Retrospective cohort study
Synopsis: This trial found a small but significant increase in the risk of death in patients taking an antipsychotic medication.
The adjusted hazard ratio for death with the use of atypical antipsychotics in community dwelling patients with dementia was 1.3 (confidence interval 1.02-1.70). Similar to prior research, the authors found that conventional antipsychotics carried a higher risk than atypical agents.
Patients in long-term care settings also suffered increased risk compared with community dwelling patients. Interestingly, the increased risk of death was apparent after as little as a month of treatment.
As with all retrospective observational cohort trials, there remains the risk that an unanticipated confounding factor could skew the data and create a false association. However, the findings of this research support prior concerns that antipsychotics carry risk of increased mortality. This research bolsters the argument that these agents should not be used lightly or without full discussion of risks and benefits with the patient and/or proxy.
Bottom line: Antipsychotic agents used in patients with dementia may create increased risk of death. Potential benefit needs to be carefully weighted against this serious harm.
Citation: Gill S, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007 June 5;146(11):775-786.
Does Combination Therapy Help Prevent Serious Vascular Ischemic Events?
Background: Peripheral arterial disease (PAD) manifests as claudication and limb ischemia affecting 8.5 million Americans. Atherosclerotic disease in the periphery also reflects increased risk for ischemic events in the coronary and cranial circulations. Both antiplatelet agents and anticoagulation will decrease the probability of thrombus formation, although this must be weighed against bleeding risk.
Study design: Randomized, open-label, multicenter trial
Setting: Eighty centers in Europe, Asia, Australia, and North America
Synopsis: This trial randomized more than 2,000 patients with PAD to treatment with antiplatelet therapy (aspirin, ticlopidine, or clopidogrel) with or without additional anticoagulation.
During the next 3.5 years serious vascular events occurred at approximately the same rate in both combination and monotherapy groups (15.9% versus 17.4%, p=0.37). There was no significant difference between the occurrence of the composite ischemic endpoints or any of the individual endpoints. There was, however, a significantly higher rate of both moderate and life-threatening bleeding in the combination therapy group.
The 4% risk of life-threatening hemorrhage in the combination group exceeded the 1.2% rate of the monotherapy group creating a relative risk for bleeding of 3.4.
This trial demonstrates that for patients with PAD on antiplatelet therapy, the increased rate of bleeding without significant added benefit makes addition of warfarin inadvisable.1 Evidence of utility of combination therapy from studies in other arterial systems provides mixed results.2-4 Based on the results of this study, combination therapy cannot be advocated if the primary symptoms are from PAD.
Bottom line: This study provides further evidence that more is not always better when it comes to preventing thrombosis and ischemia in the peripheral arterial system. Antiplatelet agents are preferable for PAD to combination antiplatelet plus anticoagulation.
Citations:
- The Warfarin Antiplatelet Vascular Evaluation Trial Investigators. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007 Jul 19;357(3):217-227.
- Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002 Sep 26;347(13):969-974.
- Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001 Nov 15;345(20):1444-1451.
- The ESPRIT Study Group. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurol. 2007 Feb;6:115-124.
Does Transient Atrial Fibrillation Increase Stroke Risk After ST-Elevation Myocardial Infarction?
Background: Prior research has demonstrated that 2.1% of patients will suffer a stroke in the year following a heart attack. Persistent and paroxysmal atrial fibrillation (AF) are well recognized as risk factors for stroke, but the significance of transient ischemia-induced AF is less clear.
Study design: Retrospective cohort study
Setting: Queen Mary Hospital, Hong Kong
Synopsis: The study involved patients admitted for acute inferior ST-segment-elevation myocardial infarction (MI) with preserved left ventricular ejection fraction.
Transient AF that had converted back to normal sinus rhythm by discharge was observed in 14% of patients after the MI. Over the next three years the transient AF patients were 15 times more likely than those who remained in sinus rhythm during the index hospitalization to have recurrent AF (34% versus 2%). Despite antiplatelet therapy in both groups, ischemic stroke developed in 22% of patients who had transient AF following their MI, compared with only 4% in patients who did not (HR 5.1, confidence interval 2.4-11.2). Cerebrovascular accidents generally occurred simultaneously with recurrence of paroxysmal AF.1-2
The finding that patients with transient-ischemia-induced AF represents a group with markedly higher risk of ischemic stroke is compelling. It suggests that these patients may be candidates for combined antiplatelet and anticoagulant therapy. Trials of combined therapy following MI demonstrate that this strategy reduces the rate of recurrent cardiac ischemia, stroke, or death but does carry significantly increased risk of bleeding.3-4
Bottom line: The presence of transient AF following MI represents a significant risk factor for the development of subsequent paroxysmal AF. These patients have a five-fold increased risk of ischemic stroke over the next three years and should be considered for combined antiplatelet and anticoagulant therapy.
Citations:
- Chung-Wah S, Man-Hong J, Hee-Hwa H, et al. Transient atrial fibrillation complicating acute inferior myocardial infarction: implications for future risk of ischemic stroke. Chest. 2007 Mar 30;132(1):44-49.
- Witt BJ, Ballman KV, Brown RD Jr., Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J. Med. 2006;119(4):354 e1-9.
- Van Es RF, Jonker J, Verheugt F, et al. Aspirin and Coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet. 2002 Jul 13;360(9327):109-113.
- Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002 Sep 26;347(13):969-974. TH
In This Edition
- Cost sharing for prescription medications increases consumption of more costly healthcare services
- Community-acquired pneumonia core measures can lead to unintended consequences
- Prophylactic revascularization has no clear benefit for high-risk patients undergoing vascular surgery
- Aspirin resistance correlates with adverse clinical events
- Low-molecular-weight heparin appears to have greater efficacy as a prophylactic agent against deep-vein thrombosis and pulmonary embolism
- Antipsychotic medications appear to be associated with increased risk of death in demented patients
- Anticoagulation plus antiplatelet therapy fails to show benefit for peripheral arterial disease
- Transient atrial fibrillation following myocardial infarction increases the risk of recurrence and stroke
Do Incentives to Encourage Use of Certain Medications Affect Care?
Background: Insurers are increasingly using financial mechanisms to affect pharmaceutical usage. These practices may affect medication use and health outcomes in ways that are poorly defined and difficult to detect.
Study design: Literature review
Synopsis: There are numerous structures for drug-cost sharing, and this study evaluated co-payments, tiers/co-insurance, benefit caps, formulary limitations, and reference pricing strategies for their effect on prescription drug usage and healthcare outcomes.
Included articles varied widely in study design, making generalizable results difficult to isolate, and insurers may have instituted more than one cost-sharing mechanism simultaneously. Overall, for every 10% increase in cost sharing (via copayments or co-insurance) there was an associated 2%-6% decrease in prescription drug spending. Increasing consumer costs for medications clearly decreases usage.
Some studies demonstrated that the decrease in medication utilization was more pronounced for “nonessential” medications over “essential” medications. However, in specific chronic illnesses this is clearly associated with greater usage of inpatient and emergency medical services.
Cost sharing was also more likely to have adverse health consequences in vulnerable populations, particularly the elderly and poor. One in four Medicaid patients couldn’t fill at least one prescription in the past year, as opposed to one in 10 privately insured patients who couldn’t purchase one or more medications.
Further impact on healthcare consumption and outcomes may be masked because it is difficult to determine individual disease severity, and the effect on the more severely ill would be expected to be greater. These authors attempted to sort out a complex interaction between cost, consumption, and health, and they found important trends.
The goal of cost sharing is to align consumption more clearly with appropriate and economic products, thereby using cost sharing as a public health tool. The consequence of creating the incentives for ill patients to forego necessary treatments is a counterbalancing concern that is supported in some, but not all, of the literature.
Bottom line: Cost sharing for prescription medications decreases medication spending and utilization but disproportionately affects the disadvantaged and increases consumption of more costly healthcare services in patients with some chronic illnesses.
Citation: Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298(1):61-69.
Does Antibiotic Requirement for Suspected CAP Increase Misdiagnosis?
Background: Early administration of antibiotics in community-acquired pneumonia (CAP) improves patient outcomes. The Infectious Disease Society of America instituted guidelines that recommend initiation of antibiotics to all patients with suspected CAP within four hours of triage, and some payors are using this as a quality measure affecting reimbursement. However, this incentive may cause premature diagnosis of CAP and overuse of antibiotics.
Study design: Retrospective chart review
Setting: A large, high-volume teaching hospital with more than 500 beds and more than 112,000 annual emergency department (ED) visits
Synopsis: Charts of all patients with an admitting diagnosis of CAP were reviewed over two six-month periods. The initial review was prior to initiation of a four-hour antibiotics rule; the second was after a financial incentive to initiate antibiotics within four hours of triage was initiated.
After initiation of the four-hour rule, of the patients with an admitting diagnosis of CAP, significantly more patients received antibiotics within four hours of triage (66% versus 54%). However, the number of patients with abnormal chest X-ray findings associated with the diagnosis of CAP decreased from 28.5% to 20.6%, and the proportion of patients with a discharge diagnosis of CAP decreased from 75.9% to 58.9%.
The authors also used two diagnostic paradigms to make an independent diagnosis of CAP based on chart data. With the less rigorous independent analysis 44.7% of patients actually had CAP prior to the four-hour rule, and this fell to 36% after the four-hour rule. Using a more rigorous definition, only 32.7% of patients actually had CAP prior to initiation of the four-hour rule, and this fell to 27%.
There was no difference in length of stay or ICU transfers between the two analysis periods. The authors concluded that a four-hour rule increases premature diagnosis of CAP, presumably because providers felt compelled to initiate antibiotics before they had complete clinical data.
This tendency was associated with misuse and overuse of antibiotics, and increased laboratory testing, such as blood cultures, which had to be obtained before antibiotics were initiated. The authors emphasized the importance of reimbursement-associated quality measures creating incentives to treat the right patients for the correct diagnosis, and the potential harmful consequences of applying a quality-driven protocol to the wrong patient.
They suggest a six-hour rule would decrease the misdiagnosis of CAP. They also feel eliminating a mandatory time frame and requiring only that the first dose of antibiotics be administered in the ED will further ameliorate these effects.
Bottom line: Mandatory administration of antibiotics to patients with suspected CAP within four hours of triage increases the percentage of patients who receive antibiotics within four hours, but also increases the rate of misdiagnosis of CAP, inappropriate administration of antibiotics, and increased use of some laboratory services.
Citation: Kanwar M, Brar N, Khatib R, et al. Misdiagnosis of community-acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4-hour antibiotic administration rule. Chest. 2007 Jun;131(6):1865-1869.
Does prophylactic cardiac revascularization benefit patients undergoing vascular surgery?
Background: American College of Cardiology/American Heart Association Guidelines recommend referral for patients with multiple cardiac risk factors for non-invasive cardiac stress testing prior to surgery and prophylactic revascularization in high-risk patients. The authors performed a pilot analysis to determine how many patients would be needed to prospectively validate this recommendation in those with more significant ischemic cardiac disease.
Study design: Randomized controlled pilot study of 1,880 consecutive patients undergoing elective vascular surgery
Setting: Brazil, Belgium, the Netherlands, Italy, Serbia, and Montenegro
Synopsis: This was a pilot study to determine the necessary power to prove or disprove the benefit of the recommendation for cardiac revascularization in high-risk patients before major vascular surgery.
Prior research had shown that prophylactic revascularization is not of demonstrable benefit in this cohort. However, the majority of the patients in this previous trial had two-vessel disease and preserved left ventricular function. This study examined a sicker cohort of patients with more significant coronary artery disease and depressed left ventricular function.
This pilot screened all patients undergoing high-risk vascular surgery. All patients with three or more risk factors underwent non-invasive evaluation for cardiac ischemia. Patients with extensive ischemia were randomized to invasive evaluation and revascularization as appropriate or non-invasive management. Both arms received optimal medical management.
Prophylactic revascularization did not improve 30-day outcome after vascular surgery, demonstrated no difference in perioperative cardiac events, and found no difference in all-cause mortality or nonfatal myocardial infarction. Similarly, there was no evidence of long-term (at one year) difference between groups. The sample size needed to definitively establish that coronary revascularization is superior to medical therapy would be 300 patients per arm. That would require screening 9,000 patients.
Bottom line: Prophylactic revascularization has no clear benefit for high-risk patients undergoing vascular surgery, but a much larger sample size would be required to definitively prove or disprove benefit.
Citation: Poldermans D, Schouten O, Vidakovic R, et al. Clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V pilot study. J. Am Coll Cardiol. 2007;49(17):1763-1769.
How Does Aspirin Resistance Affect Patients with Coronary Artery Disease?
Background: Although aspirin is used to decrease the risk of ischemic events, up to 45% of patients do not derive adequate anti-platelet activity. Few prospective studies have used laboratory-measured aspirin resistance to assess clinical outcomes.
Study design: Blinded cohort
Setting: Patients affiliated with Queen Mary Hospital, the University of Hong Kong.
Synopsis: Aspirin-induced platelet inhibition was measured quantitatively on 468 patients with stable coronary artery disease who take 80-325 mg of aspirin per day. The study found 128 patients were aspirin resistant. Aspirin resistance was more prevalent with increased age, female gender, renal insufficiency, anemia, and with use of low-dose aspirin. At follow up, aspirin-resistant patients were more likely to develop a primary outcome event: cardiovascular deaths, myocardial infarction, stroke, transient ischemic attack, and unstable angina. Aspirin resistance was an independent risk factor for developing the aforementioned outcomes, as are diabetes, prior myocardial infarction, and low hemoglobin.
Bottom line: Aspirin resistance, as defined by an aggregation-based assay, is associated with adverse outcomes in patients with stable coronary artery disease.
Citation: Chen W, Cheng X, Lee PY, et al. Aspirin resistance and adverse clinical events in patients with coronary artery disease. Am J Med. 2007 Jul;120(7):631-635.
Which Agents Best Prevent Venous Thromboembolism?
Background: Pulmonary emboli have been linked to 10% of in-hospital deaths. There continues to be a strong emphasis on prevention. Unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and selective factor Xa inhibitors are used for prophylaxis.
Study design: A meta-analysis of randomized controlled trials
Synopsis: The meta-analysis included 36 studies of hospitalized medical patients that compared UFH with control, LMWH with control, LMWH with UFH, and a selective factor Xa inhibitor with a placebo.
When each was compared with a control, UFH and LMWH were associated with a decreased risk of deep venous thrombosis (DVT) (risk ratio=0.33; 0.56) and pulmonary embolism (PE) (risk ratio=0.64; 0.37). Compared with control, LMWH three times daily was more effective than twice-daily dosing (risk ratio=0.27, 0.52). Through direct comparison of UFH and LMWH, LMWH was shown to have decreased DVT risk (risk ratio=0.68) and fewer injection site hematomas (risk ratio=0.47).
Neither UFH nor LMWH reduced mortality. LMWH and UFH were associated with significantly more bleeding events than control, but this increased risk was significant only for minor bleeding.
Bottom line: LMWH appears to have greater efficacy than UFH as a prophylactic agent against DVT/PE. If UFH is used, three times daily dosing is preferred.
Citation: Wein L, Wein S, Haas SJ, et al. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients. Arch Intern Med. 2007;167(14):1476-1486.
What Is the Association Between Antipsychotic Drugs and Mortality?
Background: Atypical antipsychotics prescribed off-label for problematic behaviors in dementia have been associated with risks including diabetes, stroke, and increased mortality. This resulted in the FDA placing a “black box” warning on atypical antipsychotics used for dementia. Subsequent studies have suggested that conventional antipsychotics are perhaps even more problematic.
Study Design: Retrospective cohort study
Synopsis: This trial found a small but significant increase in the risk of death in patients taking an antipsychotic medication.
The adjusted hazard ratio for death with the use of atypical antipsychotics in community dwelling patients with dementia was 1.3 (confidence interval 1.02-1.70). Similar to prior research, the authors found that conventional antipsychotics carried a higher risk than atypical agents.
Patients in long-term care settings also suffered increased risk compared with community dwelling patients. Interestingly, the increased risk of death was apparent after as little as a month of treatment.
As with all retrospective observational cohort trials, there remains the risk that an unanticipated confounding factor could skew the data and create a false association. However, the findings of this research support prior concerns that antipsychotics carry risk of increased mortality. This research bolsters the argument that these agents should not be used lightly or without full discussion of risks and benefits with the patient and/or proxy.
Bottom line: Antipsychotic agents used in patients with dementia may create increased risk of death. Potential benefit needs to be carefully weighted against this serious harm.
Citation: Gill S, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007 June 5;146(11):775-786.
Does Combination Therapy Help Prevent Serious Vascular Ischemic Events?
Background: Peripheral arterial disease (PAD) manifests as claudication and limb ischemia affecting 8.5 million Americans. Atherosclerotic disease in the periphery also reflects increased risk for ischemic events in the coronary and cranial circulations. Both antiplatelet agents and anticoagulation will decrease the probability of thrombus formation, although this must be weighed against bleeding risk.
Study design: Randomized, open-label, multicenter trial
Setting: Eighty centers in Europe, Asia, Australia, and North America
Synopsis: This trial randomized more than 2,000 patients with PAD to treatment with antiplatelet therapy (aspirin, ticlopidine, or clopidogrel) with or without additional anticoagulation.
During the next 3.5 years serious vascular events occurred at approximately the same rate in both combination and monotherapy groups (15.9% versus 17.4%, p=0.37). There was no significant difference between the occurrence of the composite ischemic endpoints or any of the individual endpoints. There was, however, a significantly higher rate of both moderate and life-threatening bleeding in the combination therapy group.
The 4% risk of life-threatening hemorrhage in the combination group exceeded the 1.2% rate of the monotherapy group creating a relative risk for bleeding of 3.4.
This trial demonstrates that for patients with PAD on antiplatelet therapy, the increased rate of bleeding without significant added benefit makes addition of warfarin inadvisable.1 Evidence of utility of combination therapy from studies in other arterial systems provides mixed results.2-4 Based on the results of this study, combination therapy cannot be advocated if the primary symptoms are from PAD.
Bottom line: This study provides further evidence that more is not always better when it comes to preventing thrombosis and ischemia in the peripheral arterial system. Antiplatelet agents are preferable for PAD to combination antiplatelet plus anticoagulation.
Citations:
- The Warfarin Antiplatelet Vascular Evaluation Trial Investigators. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007 Jul 19;357(3):217-227.
- Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002 Sep 26;347(13):969-974.
- Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001 Nov 15;345(20):1444-1451.
- The ESPRIT Study Group. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurol. 2007 Feb;6:115-124.
Does Transient Atrial Fibrillation Increase Stroke Risk After ST-Elevation Myocardial Infarction?
Background: Prior research has demonstrated that 2.1% of patients will suffer a stroke in the year following a heart attack. Persistent and paroxysmal atrial fibrillation (AF) are well recognized as risk factors for stroke, but the significance of transient ischemia-induced AF is less clear.
Study design: Retrospective cohort study
Setting: Queen Mary Hospital, Hong Kong
Synopsis: The study involved patients admitted for acute inferior ST-segment-elevation myocardial infarction (MI) with preserved left ventricular ejection fraction.
Transient AF that had converted back to normal sinus rhythm by discharge was observed in 14% of patients after the MI. Over the next three years the transient AF patients were 15 times more likely than those who remained in sinus rhythm during the index hospitalization to have recurrent AF (34% versus 2%). Despite antiplatelet therapy in both groups, ischemic stroke developed in 22% of patients who had transient AF following their MI, compared with only 4% in patients who did not (HR 5.1, confidence interval 2.4-11.2). Cerebrovascular accidents generally occurred simultaneously with recurrence of paroxysmal AF.1-2
The finding that patients with transient-ischemia-induced AF represents a group with markedly higher risk of ischemic stroke is compelling. It suggests that these patients may be candidates for combined antiplatelet and anticoagulant therapy. Trials of combined therapy following MI demonstrate that this strategy reduces the rate of recurrent cardiac ischemia, stroke, or death but does carry significantly increased risk of bleeding.3-4
Bottom line: The presence of transient AF following MI represents a significant risk factor for the development of subsequent paroxysmal AF. These patients have a five-fold increased risk of ischemic stroke over the next three years and should be considered for combined antiplatelet and anticoagulant therapy.
Citations:
- Chung-Wah S, Man-Hong J, Hee-Hwa H, et al. Transient atrial fibrillation complicating acute inferior myocardial infarction: implications for future risk of ischemic stroke. Chest. 2007 Mar 30;132(1):44-49.
- Witt BJ, Ballman KV, Brown RD Jr., Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J. Med. 2006;119(4):354 e1-9.
- Van Es RF, Jonker J, Verheugt F, et al. Aspirin and Coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet. 2002 Jul 13;360(9327):109-113.
- Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002 Sep 26;347(13):969-974. TH
In This Edition
- Cost sharing for prescription medications increases consumption of more costly healthcare services
- Community-acquired pneumonia core measures can lead to unintended consequences
- Prophylactic revascularization has no clear benefit for high-risk patients undergoing vascular surgery
- Aspirin resistance correlates with adverse clinical events
- Low-molecular-weight heparin appears to have greater efficacy as a prophylactic agent against deep-vein thrombosis and pulmonary embolism
- Antipsychotic medications appear to be associated with increased risk of death in demented patients
- Anticoagulation plus antiplatelet therapy fails to show benefit for peripheral arterial disease
- Transient atrial fibrillation following myocardial infarction increases the risk of recurrence and stroke
Do Incentives to Encourage Use of Certain Medications Affect Care?
Background: Insurers are increasingly using financial mechanisms to affect pharmaceutical usage. These practices may affect medication use and health outcomes in ways that are poorly defined and difficult to detect.
Study design: Literature review
Synopsis: There are numerous structures for drug-cost sharing, and this study evaluated co-payments, tiers/co-insurance, benefit caps, formulary limitations, and reference pricing strategies for their effect on prescription drug usage and healthcare outcomes.
Included articles varied widely in study design, making generalizable results difficult to isolate, and insurers may have instituted more than one cost-sharing mechanism simultaneously. Overall, for every 10% increase in cost sharing (via copayments or co-insurance) there was an associated 2%-6% decrease in prescription drug spending. Increasing consumer costs for medications clearly decreases usage.
Some studies demonstrated that the decrease in medication utilization was more pronounced for “nonessential” medications over “essential” medications. However, in specific chronic illnesses this is clearly associated with greater usage of inpatient and emergency medical services.
Cost sharing was also more likely to have adverse health consequences in vulnerable populations, particularly the elderly and poor. One in four Medicaid patients couldn’t fill at least one prescription in the past year, as opposed to one in 10 privately insured patients who couldn’t purchase one or more medications.
Further impact on healthcare consumption and outcomes may be masked because it is difficult to determine individual disease severity, and the effect on the more severely ill would be expected to be greater. These authors attempted to sort out a complex interaction between cost, consumption, and health, and they found important trends.
The goal of cost sharing is to align consumption more clearly with appropriate and economic products, thereby using cost sharing as a public health tool. The consequence of creating the incentives for ill patients to forego necessary treatments is a counterbalancing concern that is supported in some, but not all, of the literature.
Bottom line: Cost sharing for prescription medications decreases medication spending and utilization but disproportionately affects the disadvantaged and increases consumption of more costly healthcare services in patients with some chronic illnesses.
Citation: Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298(1):61-69.
Does Antibiotic Requirement for Suspected CAP Increase Misdiagnosis?
Background: Early administration of antibiotics in community-acquired pneumonia (CAP) improves patient outcomes. The Infectious Disease Society of America instituted guidelines that recommend initiation of antibiotics to all patients with suspected CAP within four hours of triage, and some payors are using this as a quality measure affecting reimbursement. However, this incentive may cause premature diagnosis of CAP and overuse of antibiotics.
Study design: Retrospective chart review
Setting: A large, high-volume teaching hospital with more than 500 beds and more than 112,000 annual emergency department (ED) visits
Synopsis: Charts of all patients with an admitting diagnosis of CAP were reviewed over two six-month periods. The initial review was prior to initiation of a four-hour antibiotics rule; the second was after a financial incentive to initiate antibiotics within four hours of triage was initiated.
After initiation of the four-hour rule, of the patients with an admitting diagnosis of CAP, significantly more patients received antibiotics within four hours of triage (66% versus 54%). However, the number of patients with abnormal chest X-ray findings associated with the diagnosis of CAP decreased from 28.5% to 20.6%, and the proportion of patients with a discharge diagnosis of CAP decreased from 75.9% to 58.9%.
The authors also used two diagnostic paradigms to make an independent diagnosis of CAP based on chart data. With the less rigorous independent analysis 44.7% of patients actually had CAP prior to the four-hour rule, and this fell to 36% after the four-hour rule. Using a more rigorous definition, only 32.7% of patients actually had CAP prior to initiation of the four-hour rule, and this fell to 27%.
There was no difference in length of stay or ICU transfers between the two analysis periods. The authors concluded that a four-hour rule increases premature diagnosis of CAP, presumably because providers felt compelled to initiate antibiotics before they had complete clinical data.
This tendency was associated with misuse and overuse of antibiotics, and increased laboratory testing, such as blood cultures, which had to be obtained before antibiotics were initiated. The authors emphasized the importance of reimbursement-associated quality measures creating incentives to treat the right patients for the correct diagnosis, and the potential harmful consequences of applying a quality-driven protocol to the wrong patient.
They suggest a six-hour rule would decrease the misdiagnosis of CAP. They also feel eliminating a mandatory time frame and requiring only that the first dose of antibiotics be administered in the ED will further ameliorate these effects.
Bottom line: Mandatory administration of antibiotics to patients with suspected CAP within four hours of triage increases the percentage of patients who receive antibiotics within four hours, but also increases the rate of misdiagnosis of CAP, inappropriate administration of antibiotics, and increased use of some laboratory services.
Citation: Kanwar M, Brar N, Khatib R, et al. Misdiagnosis of community-acquired pneumonia and inappropriate utilization of antibiotics: side effects of the 4-hour antibiotic administration rule. Chest. 2007 Jun;131(6):1865-1869.
Does prophylactic cardiac revascularization benefit patients undergoing vascular surgery?
Background: American College of Cardiology/American Heart Association Guidelines recommend referral for patients with multiple cardiac risk factors for non-invasive cardiac stress testing prior to surgery and prophylactic revascularization in high-risk patients. The authors performed a pilot analysis to determine how many patients would be needed to prospectively validate this recommendation in those with more significant ischemic cardiac disease.
Study design: Randomized controlled pilot study of 1,880 consecutive patients undergoing elective vascular surgery
Setting: Brazil, Belgium, the Netherlands, Italy, Serbia, and Montenegro
Synopsis: This was a pilot study to determine the necessary power to prove or disprove the benefit of the recommendation for cardiac revascularization in high-risk patients before major vascular surgery.
Prior research had shown that prophylactic revascularization is not of demonstrable benefit in this cohort. However, the majority of the patients in this previous trial had two-vessel disease and preserved left ventricular function. This study examined a sicker cohort of patients with more significant coronary artery disease and depressed left ventricular function.
This pilot screened all patients undergoing high-risk vascular surgery. All patients with three or more risk factors underwent non-invasive evaluation for cardiac ischemia. Patients with extensive ischemia were randomized to invasive evaluation and revascularization as appropriate or non-invasive management. Both arms received optimal medical management.
Prophylactic revascularization did not improve 30-day outcome after vascular surgery, demonstrated no difference in perioperative cardiac events, and found no difference in all-cause mortality or nonfatal myocardial infarction. Similarly, there was no evidence of long-term (at one year) difference between groups. The sample size needed to definitively establish that coronary revascularization is superior to medical therapy would be 300 patients per arm. That would require screening 9,000 patients.
Bottom line: Prophylactic revascularization has no clear benefit for high-risk patients undergoing vascular surgery, but a much larger sample size would be required to definitively prove or disprove benefit.
Citation: Poldermans D, Schouten O, Vidakovic R, et al. Clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: the DECREASE-V pilot study. J. Am Coll Cardiol. 2007;49(17):1763-1769.
How Does Aspirin Resistance Affect Patients with Coronary Artery Disease?
Background: Although aspirin is used to decrease the risk of ischemic events, up to 45% of patients do not derive adequate anti-platelet activity. Few prospective studies have used laboratory-measured aspirin resistance to assess clinical outcomes.
Study design: Blinded cohort
Setting: Patients affiliated with Queen Mary Hospital, the University of Hong Kong.
Synopsis: Aspirin-induced platelet inhibition was measured quantitatively on 468 patients with stable coronary artery disease who take 80-325 mg of aspirin per day. The study found 128 patients were aspirin resistant. Aspirin resistance was more prevalent with increased age, female gender, renal insufficiency, anemia, and with use of low-dose aspirin. At follow up, aspirin-resistant patients were more likely to develop a primary outcome event: cardiovascular deaths, myocardial infarction, stroke, transient ischemic attack, and unstable angina. Aspirin resistance was an independent risk factor for developing the aforementioned outcomes, as are diabetes, prior myocardial infarction, and low hemoglobin.
Bottom line: Aspirin resistance, as defined by an aggregation-based assay, is associated with adverse outcomes in patients with stable coronary artery disease.
Citation: Chen W, Cheng X, Lee PY, et al. Aspirin resistance and adverse clinical events in patients with coronary artery disease. Am J Med. 2007 Jul;120(7):631-635.
Which Agents Best Prevent Venous Thromboembolism?
Background: Pulmonary emboli have been linked to 10% of in-hospital deaths. There continues to be a strong emphasis on prevention. Unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and selective factor Xa inhibitors are used for prophylaxis.
Study design: A meta-analysis of randomized controlled trials
Synopsis: The meta-analysis included 36 studies of hospitalized medical patients that compared UFH with control, LMWH with control, LMWH with UFH, and a selective factor Xa inhibitor with a placebo.
When each was compared with a control, UFH and LMWH were associated with a decreased risk of deep venous thrombosis (DVT) (risk ratio=0.33; 0.56) and pulmonary embolism (PE) (risk ratio=0.64; 0.37). Compared with control, LMWH three times daily was more effective than twice-daily dosing (risk ratio=0.27, 0.52). Through direct comparison of UFH and LMWH, LMWH was shown to have decreased DVT risk (risk ratio=0.68) and fewer injection site hematomas (risk ratio=0.47).
Neither UFH nor LMWH reduced mortality. LMWH and UFH were associated with significantly more bleeding events than control, but this increased risk was significant only for minor bleeding.
Bottom line: LMWH appears to have greater efficacy than UFH as a prophylactic agent against DVT/PE. If UFH is used, three times daily dosing is preferred.
Citation: Wein L, Wein S, Haas SJ, et al. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients. Arch Intern Med. 2007;167(14):1476-1486.
What Is the Association Between Antipsychotic Drugs and Mortality?
Background: Atypical antipsychotics prescribed off-label for problematic behaviors in dementia have been associated with risks including diabetes, stroke, and increased mortality. This resulted in the FDA placing a “black box” warning on atypical antipsychotics used for dementia. Subsequent studies have suggested that conventional antipsychotics are perhaps even more problematic.
Study Design: Retrospective cohort study
Synopsis: This trial found a small but significant increase in the risk of death in patients taking an antipsychotic medication.
The adjusted hazard ratio for death with the use of atypical antipsychotics in community dwelling patients with dementia was 1.3 (confidence interval 1.02-1.70). Similar to prior research, the authors found that conventional antipsychotics carried a higher risk than atypical agents.
Patients in long-term care settings also suffered increased risk compared with community dwelling patients. Interestingly, the increased risk of death was apparent after as little as a month of treatment.
As with all retrospective observational cohort trials, there remains the risk that an unanticipated confounding factor could skew the data and create a false association. However, the findings of this research support prior concerns that antipsychotics carry risk of increased mortality. This research bolsters the argument that these agents should not be used lightly or without full discussion of risks and benefits with the patient and/or proxy.
Bottom line: Antipsychotic agents used in patients with dementia may create increased risk of death. Potential benefit needs to be carefully weighted against this serious harm.
Citation: Gill S, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007 June 5;146(11):775-786.
Does Combination Therapy Help Prevent Serious Vascular Ischemic Events?
Background: Peripheral arterial disease (PAD) manifests as claudication and limb ischemia affecting 8.5 million Americans. Atherosclerotic disease in the periphery also reflects increased risk for ischemic events in the coronary and cranial circulations. Both antiplatelet agents and anticoagulation will decrease the probability of thrombus formation, although this must be weighed against bleeding risk.
Study design: Randomized, open-label, multicenter trial
Setting: Eighty centers in Europe, Asia, Australia, and North America
Synopsis: This trial randomized more than 2,000 patients with PAD to treatment with antiplatelet therapy (aspirin, ticlopidine, or clopidogrel) with or without additional anticoagulation.
During the next 3.5 years serious vascular events occurred at approximately the same rate in both combination and monotherapy groups (15.9% versus 17.4%, p=0.37). There was no significant difference between the occurrence of the composite ischemic endpoints or any of the individual endpoints. There was, however, a significantly higher rate of both moderate and life-threatening bleeding in the combination therapy group.
The 4% risk of life-threatening hemorrhage in the combination group exceeded the 1.2% rate of the monotherapy group creating a relative risk for bleeding of 3.4.
This trial demonstrates that for patients with PAD on antiplatelet therapy, the increased rate of bleeding without significant added benefit makes addition of warfarin inadvisable.1 Evidence of utility of combination therapy from studies in other arterial systems provides mixed results.2-4 Based on the results of this study, combination therapy cannot be advocated if the primary symptoms are from PAD.
Bottom line: This study provides further evidence that more is not always better when it comes to preventing thrombosis and ischemia in the peripheral arterial system. Antiplatelet agents are preferable for PAD to combination antiplatelet plus anticoagulation.
Citations:
- The Warfarin Antiplatelet Vascular Evaluation Trial Investigators. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007 Jul 19;357(3):217-227.
- Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002 Sep 26;347(13):969-974.
- Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001 Nov 15;345(20):1444-1451.
- The ESPRIT Study Group. Medium intensity oral anticoagulants versus aspirin after cerebral ischaemia of arterial origin (ESPRIT): a randomised controlled trial. Lancet Neurol. 2007 Feb;6:115-124.
Does Transient Atrial Fibrillation Increase Stroke Risk After ST-Elevation Myocardial Infarction?
Background: Prior research has demonstrated that 2.1% of patients will suffer a stroke in the year following a heart attack. Persistent and paroxysmal atrial fibrillation (AF) are well recognized as risk factors for stroke, but the significance of transient ischemia-induced AF is less clear.
Study design: Retrospective cohort study
Setting: Queen Mary Hospital, Hong Kong
Synopsis: The study involved patients admitted for acute inferior ST-segment-elevation myocardial infarction (MI) with preserved left ventricular ejection fraction.
Transient AF that had converted back to normal sinus rhythm by discharge was observed in 14% of patients after the MI. Over the next three years the transient AF patients were 15 times more likely than those who remained in sinus rhythm during the index hospitalization to have recurrent AF (34% versus 2%). Despite antiplatelet therapy in both groups, ischemic stroke developed in 22% of patients who had transient AF following their MI, compared with only 4% in patients who did not (HR 5.1, confidence interval 2.4-11.2). Cerebrovascular accidents generally occurred simultaneously with recurrence of paroxysmal AF.1-2
The finding that patients with transient-ischemia-induced AF represents a group with markedly higher risk of ischemic stroke is compelling. It suggests that these patients may be candidates for combined antiplatelet and anticoagulant therapy. Trials of combined therapy following MI demonstrate that this strategy reduces the rate of recurrent cardiac ischemia, stroke, or death but does carry significantly increased risk of bleeding.3-4
Bottom line: The presence of transient AF following MI represents a significant risk factor for the development of subsequent paroxysmal AF. These patients have a five-fold increased risk of ischemic stroke over the next three years and should be considered for combined antiplatelet and anticoagulant therapy.
Citations:
- Chung-Wah S, Man-Hong J, Hee-Hwa H, et al. Transient atrial fibrillation complicating acute inferior myocardial infarction: implications for future risk of ischemic stroke. Chest. 2007 Mar 30;132(1):44-49.
- Witt BJ, Ballman KV, Brown RD Jr., Meverden RA, Jacobsen SJ, Roger VL. The incidence of stroke after myocardial infarction: a meta-analysis. Am J. Med. 2006;119(4):354 e1-9.
- Van Es RF, Jonker J, Verheugt F, et al. Aspirin and Coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet. 2002 Jul 13;360(9327):109-113.
- Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002 Sep 26;347(13):969-974. TH
What is the best medical therapy for the secondary prevention of stroke?
The Case
A 62-year-old obese woman with prior history of type 2 diabetes, hypertension, and a pack-a-day smoking habit presented to the emergency department (ED) for acute onset of right-side weakness and sensory loss noted on awakening from sleep.
She reports taking a baby aspirin daily to “prevent heart attacks” prior to her stroke. Her electrocardiogram demonstrates a left bundle branch block and frequent premature atrial contractions. She recovers with mild hemiparesis and is ready for discharge. What is the best medical therapy for secondary prevention of stroke?
Overview
Cerebrovascular accident (CVA) represents an important diagnosis for the hospitalist, with 700,000 people suffering a stroke in the U.S. each year.1 This translates to a stroke every 45 seconds. About 200,000 of these strokes are recurrent events.
Cardioembolism is the largest cause of ischemic strokes, representing 29% of all infarcts.2 Stasis from impaired contractile function, atrial fibrillation, or mechanical valves are significant risk factors. More rarely, a paradoxical embolus arising in the venous system may pass through a patent foramen ovale.
Large-artery atherosclerosis and lacunar infarcts each account for 16% of strokes. Risk factors for these forms of strokes are the same as those for atherosclerosis and include hypertension and diabetes. Rarer causes such as vasculitis, dissection, hypercoagulability, or hematological disorders account for 3% of strokes. Work-up for these should be driven by historical and atypical features such as young age, family history, or unusual distribution of ischemic zones. Despite appropriate work-up, the mechanism remains uncertain in 36% of strokes.
Regardless of the manifestation and residua of the index event, the hospitalist must initiate appropriate therapy to prevent a disabling CVA. While antithrombotic drugs are the mainstay of secondary prevention, it is a mistake to miss other opportunities for risk modification. Optimal management requires a tailored evaluation for etiology, identification of modifiable risk factors, and initiation of antiplatelet or anticoagulant therapy.
Cardioembolic Stroke
Treatment of stroke depends on the etiology of the original infarct. Evidence is strong that the optimal therapy for cardioembolic stroke is anticoagulation with warfarin.
The European Atrial Fibrillation Trial found that warfarin reduces the risk for second strokes in patients with atrial fibrillation by two-thirds and is superior to antiplatelet agents for preventing cardioembolic strokes.3 Warfarin increases the risk of extracranial bleeding, but not severely enough to negate the benefit of reducing stroke death and disability. The target international normalized ratio (INR) for non-valvular atrial fibrillation is generally two to three, although this may be higher for certain prosthetic valves.
Noncardioembolic Stroke
For large-vessel atherosclerotic and lacunar cerebral ischemia, the oldest—and still effective—treatment for recurrent stroke is aspirin. The use of low-dose aspirin after transient ischemic attack (TIA) or stroke reduces second strokes or death by approximately 15%-18%.4-5 Larger doses do not appear to be more effective, although the rate of gastrointestinal complaints is greater with increased dosage. The use of either 325 mg or 1,200 mg of aspirin produced the same 15% reduction in second ischemic events. Similar efficacy has been seen in comparisons between 30 mg and 283 mg dosing.6
While a subset of patients may experience aspirin resistance, reliable assays in clinical practice are not commonly available to guide management. Current recommendations suggest that use of between 50 mg and 325 mg of aspirin is appropriate for secondary prevention.7
Clopidogrel is another antiplatelet agent that can be given daily at 75 mg as alternate therapy for secondary prevention of non-cardioembolic stroke. The Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events trial comparing clopidogrel with aspirin in patients at risk of ischemic events demonstrated significant reduction in the annual rate of combined endpoint of stroke, myocardial infarction, and vascular death—from 5.83% with aspirin to 5.32% with clopidogrel.8 This study’s applicability to secondary prevention of stroke is limited by the fact that only 19% of the patients in this trial were included because of prior stroke, and the results were not significant for reduction of stroke as a lone endpoint. Clopidogrel is recommended as an acceptable agent for CVA secondary prevention and is preferred for patients with stroke and an aspirin allergy or with recent coronary stent.
The combination of a low-dose aspirin and extended-release dipyridamole has proved superior to aspirin monotherapy in multiple trials. Over two years, the European Stroke Prevention 2 trial found an 18% reduction with aspirin alone compared with 37% reduction with the combination therapy, and the European/Australasian Stroke Prevention in Reversible Ischaemia trial confirmed that the combination reduced the absolute rate of second ischemic events by 1% annually.9-10 Headache is a common side effect of dipyridamole and may limit use. Dypridamole/aspirin is recommended as another acceptable option for secondary prevention of non-cardioembolic stroke.
Evidence suggests that aspirin/dipyridamole and clopidogrel—although significantly more expensive—are more effective than aspirin monotherapy for preventing second cerebral ischemic events. Direct comparison between aspirin/dipyridamole and clopidogrel is ongoing in the Prevention Regimen for Effectively Avoiding Second Stroke trial, with results anticipated in 2008.
Things That Don’t Work
The Warfarin-Aspirin Recurrent Stroke Study trial demonstrated that warfarin was not better than aspirin for prevention of non-cardioembolic stroke, and the Warfarin–Aspirin Symptomatic Intracranial Disease trial found the same result for patients with intracranial stenosis.11-12 There is little evidence that warfarin should have a role in the treatment of most non-cardioembolic strokes. The MATCH trial failed to show benefit to adding aspirin to clopidogrel over clopidogrel monotherapy for secondary preventions of non-cardioembolic cerebral ischemia.13 Despite efficacy following coronary stenting, the combination of clopidogrel and aspirin can not be recommended for stroke prevention.
What To Do
Aggressive risk factor modification is key in the prevention of second ischemic events. One of the most promising therapies is the use of statins following a CVA. Maintaining low-density lipoprotein (LDL) at less than 100 mg/dL (or less than 70 mg/dL in the highest-risk patients) is recommended despite a relatively weak association between stroke and hyperlipidemia.
This stands in contrast to the strong relationship between elevated LDL and coronary disease. However, the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial utilized high-dose atorvastatin after acute CVA and was able to create an absolute risk reduction for second stroke of 2.2% over the next five years.14 It is possible that the findings of this trial may reflect actions of statin therapy on the endothelium independent of the lipid lowering effect.
Blood pressure commonly has a transient elevation following cerebral ischemia. This is managed permissively to preserve perfusion to the ischemic penumbra. Once the hyperacute period is over, reduction of blood pressure to less than 140/90 mm/Hg (130/80 mm/Hg for diabetics) is recommended.
Interventions to treat chronic hypertension have been demonstrated to reduce the rate of strokes by approximately 30% to 40% over four to five years.15-16 An optimal agent has not been determined, but therapy with angiotensin converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB), possibly in combination with a diuretic, have been effective. Close follow-up for titration to goal in the outpatient setting should be arranged. Diabetics should have optimization of glycemic control, and lifestyle counseling should occur regarding recognized risk factors for stroke such as smoking, inactivity, and alcohol abuse.
While antithrombotic therapy is the mainstay of what we think of in secondary prevention of stroke, treatment of these other modifiable risk factors have been shown to affect mortality and second strokes of a similar magnitude and should not be neglected.
How to Treat This Case
The patient described should undergo an MRI with diffusion (to define the area of ischemia) and targeted evaluation for etiology with cardiac monitoring, echocardiogram, and carotid ultrasound.
Assuming atrial fibrillation or intracardiac thrombus is ruled out, this likely represents atherosclerotic disease. MRI will help distinguish between large-vessel atherosclerotic etiology and lacunar infarct. If carotid stenosis of greater than 70% is found in the setting of large vessel atherosclerotic stroke, then she should be referred for carotid endarterectomy. At 50% to 69% stenosis, carotid endarterectomy would still be a consideration. Antithrombotic agent of choice for non-cardioembolic CVA is an anti-platelet agent. With a stroke occurring on a reasonable dose of aspirin, I would not recommend increasing the dose as there is little evidence that 325 mg is more effective than 81mg. The most appropriate step would be to change to an alternate anti-platelet agent such as combination dipyridamole/aspirin or clopidogrel.
In the absence of a direct comparison trial, either choice is acceptable. The evidence supporting dipyridamole/aspirin is stronger for secondary stroke prevention. Atorvastatin 80 mg daily is an evidence-based therapy after acute stroke and can be started immediately. Her hypertension should be managed permissively for the first few days after the acute event, but then an ACE-I or ARB—possibly in combination with a diuretic—would be appropriate. This patient’s goal blood pressure as a diabetic would be at least less than 130/80 mm/Hg.
Finally we would be remiss if we did not stress the importance of smoking cessation, exercise, and weight loss. TH
Dr. Cumbler is an assistant professor in the Section of Hospital Medicine at the University of Colorado, where he is a member of the Acute Stroke Service and serves on the Stroke Council.
References
- Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics-2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):e69-e171.
- Petty GW, Brown RD, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30:2513-2516.
- European Atrial Fibrillation Trial Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255-1262.
- Swedish Aspirin Low-Dose Trial Collaborative Group. Swedish aspirin low-dose aspirin trial (SALT) of 775 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet. 1991;338(8779):1345-1349.
- Farrell B, Godwin J, Richards S, et al. The United Kingdom Transient Ischaemic Attack (UK-TIA) aspirin trial: final results (abstract). J Neurol Neurosurg. Psychiatry 1991;54:1044-1054.
- Dutch TIA Trial Study Group. A comparison of two doses of aspirin (30 mg versus 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N Engl J Med. 1991 Oct 31;325(18):1261-1266.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Stroke. 2006 Feb;37(2):577-617.
- CAPRIE Steering Committee. A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events. Lancet 1996 Jan;348:1329-1339.
- Diener H, Cunha L, Forbes C, et al. European stroke prevention study 2: dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
- ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
- Mohr JP, Thompson JLP, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001 Nov 15; 345(20):1444-1451.
- Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005 Mar 31;352(13):1305-1316.
- Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo controlled trial. Lancet. 2004 Jul 24-30;36499431):331-337.
- Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559.
- The PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358(9287):1033-1041.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000 Jan 20;342:145-153.
The Case
A 62-year-old obese woman with prior history of type 2 diabetes, hypertension, and a pack-a-day smoking habit presented to the emergency department (ED) for acute onset of right-side weakness and sensory loss noted on awakening from sleep.
She reports taking a baby aspirin daily to “prevent heart attacks” prior to her stroke. Her electrocardiogram demonstrates a left bundle branch block and frequent premature atrial contractions. She recovers with mild hemiparesis and is ready for discharge. What is the best medical therapy for secondary prevention of stroke?
Overview
Cerebrovascular accident (CVA) represents an important diagnosis for the hospitalist, with 700,000 people suffering a stroke in the U.S. each year.1 This translates to a stroke every 45 seconds. About 200,000 of these strokes are recurrent events.
Cardioembolism is the largest cause of ischemic strokes, representing 29% of all infarcts.2 Stasis from impaired contractile function, atrial fibrillation, or mechanical valves are significant risk factors. More rarely, a paradoxical embolus arising in the venous system may pass through a patent foramen ovale.
Large-artery atherosclerosis and lacunar infarcts each account for 16% of strokes. Risk factors for these forms of strokes are the same as those for atherosclerosis and include hypertension and diabetes. Rarer causes such as vasculitis, dissection, hypercoagulability, or hematological disorders account for 3% of strokes. Work-up for these should be driven by historical and atypical features such as young age, family history, or unusual distribution of ischemic zones. Despite appropriate work-up, the mechanism remains uncertain in 36% of strokes.
Regardless of the manifestation and residua of the index event, the hospitalist must initiate appropriate therapy to prevent a disabling CVA. While antithrombotic drugs are the mainstay of secondary prevention, it is a mistake to miss other opportunities for risk modification. Optimal management requires a tailored evaluation for etiology, identification of modifiable risk factors, and initiation of antiplatelet or anticoagulant therapy.
Cardioembolic Stroke
Treatment of stroke depends on the etiology of the original infarct. Evidence is strong that the optimal therapy for cardioembolic stroke is anticoagulation with warfarin.
The European Atrial Fibrillation Trial found that warfarin reduces the risk for second strokes in patients with atrial fibrillation by two-thirds and is superior to antiplatelet agents for preventing cardioembolic strokes.3 Warfarin increases the risk of extracranial bleeding, but not severely enough to negate the benefit of reducing stroke death and disability. The target international normalized ratio (INR) for non-valvular atrial fibrillation is generally two to three, although this may be higher for certain prosthetic valves.
Noncardioembolic Stroke
For large-vessel atherosclerotic and lacunar cerebral ischemia, the oldest—and still effective—treatment for recurrent stroke is aspirin. The use of low-dose aspirin after transient ischemic attack (TIA) or stroke reduces second strokes or death by approximately 15%-18%.4-5 Larger doses do not appear to be more effective, although the rate of gastrointestinal complaints is greater with increased dosage. The use of either 325 mg or 1,200 mg of aspirin produced the same 15% reduction in second ischemic events. Similar efficacy has been seen in comparisons between 30 mg and 283 mg dosing.6
While a subset of patients may experience aspirin resistance, reliable assays in clinical practice are not commonly available to guide management. Current recommendations suggest that use of between 50 mg and 325 mg of aspirin is appropriate for secondary prevention.7
Clopidogrel is another antiplatelet agent that can be given daily at 75 mg as alternate therapy for secondary prevention of non-cardioembolic stroke. The Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events trial comparing clopidogrel with aspirin in patients at risk of ischemic events demonstrated significant reduction in the annual rate of combined endpoint of stroke, myocardial infarction, and vascular death—from 5.83% with aspirin to 5.32% with clopidogrel.8 This study’s applicability to secondary prevention of stroke is limited by the fact that only 19% of the patients in this trial were included because of prior stroke, and the results were not significant for reduction of stroke as a lone endpoint. Clopidogrel is recommended as an acceptable agent for CVA secondary prevention and is preferred for patients with stroke and an aspirin allergy or with recent coronary stent.
The combination of a low-dose aspirin and extended-release dipyridamole has proved superior to aspirin monotherapy in multiple trials. Over two years, the European Stroke Prevention 2 trial found an 18% reduction with aspirin alone compared with 37% reduction with the combination therapy, and the European/Australasian Stroke Prevention in Reversible Ischaemia trial confirmed that the combination reduced the absolute rate of second ischemic events by 1% annually.9-10 Headache is a common side effect of dipyridamole and may limit use. Dypridamole/aspirin is recommended as another acceptable option for secondary prevention of non-cardioembolic stroke.
Evidence suggests that aspirin/dipyridamole and clopidogrel—although significantly more expensive—are more effective than aspirin monotherapy for preventing second cerebral ischemic events. Direct comparison between aspirin/dipyridamole and clopidogrel is ongoing in the Prevention Regimen for Effectively Avoiding Second Stroke trial, with results anticipated in 2008.
Things That Don’t Work
The Warfarin-Aspirin Recurrent Stroke Study trial demonstrated that warfarin was not better than aspirin for prevention of non-cardioembolic stroke, and the Warfarin–Aspirin Symptomatic Intracranial Disease trial found the same result for patients with intracranial stenosis.11-12 There is little evidence that warfarin should have a role in the treatment of most non-cardioembolic strokes. The MATCH trial failed to show benefit to adding aspirin to clopidogrel over clopidogrel monotherapy for secondary preventions of non-cardioembolic cerebral ischemia.13 Despite efficacy following coronary stenting, the combination of clopidogrel and aspirin can not be recommended for stroke prevention.
What To Do
Aggressive risk factor modification is key in the prevention of second ischemic events. One of the most promising therapies is the use of statins following a CVA. Maintaining low-density lipoprotein (LDL) at less than 100 mg/dL (or less than 70 mg/dL in the highest-risk patients) is recommended despite a relatively weak association between stroke and hyperlipidemia.
This stands in contrast to the strong relationship between elevated LDL and coronary disease. However, the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial utilized high-dose atorvastatin after acute CVA and was able to create an absolute risk reduction for second stroke of 2.2% over the next five years.14 It is possible that the findings of this trial may reflect actions of statin therapy on the endothelium independent of the lipid lowering effect.
Blood pressure commonly has a transient elevation following cerebral ischemia. This is managed permissively to preserve perfusion to the ischemic penumbra. Once the hyperacute period is over, reduction of blood pressure to less than 140/90 mm/Hg (130/80 mm/Hg for diabetics) is recommended.
Interventions to treat chronic hypertension have been demonstrated to reduce the rate of strokes by approximately 30% to 40% over four to five years.15-16 An optimal agent has not been determined, but therapy with angiotensin converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB), possibly in combination with a diuretic, have been effective. Close follow-up for titration to goal in the outpatient setting should be arranged. Diabetics should have optimization of glycemic control, and lifestyle counseling should occur regarding recognized risk factors for stroke such as smoking, inactivity, and alcohol abuse.
While antithrombotic therapy is the mainstay of what we think of in secondary prevention of stroke, treatment of these other modifiable risk factors have been shown to affect mortality and second strokes of a similar magnitude and should not be neglected.
How to Treat This Case
The patient described should undergo an MRI with diffusion (to define the area of ischemia) and targeted evaluation for etiology with cardiac monitoring, echocardiogram, and carotid ultrasound.
Assuming atrial fibrillation or intracardiac thrombus is ruled out, this likely represents atherosclerotic disease. MRI will help distinguish between large-vessel atherosclerotic etiology and lacunar infarct. If carotid stenosis of greater than 70% is found in the setting of large vessel atherosclerotic stroke, then she should be referred for carotid endarterectomy. At 50% to 69% stenosis, carotid endarterectomy would still be a consideration. Antithrombotic agent of choice for non-cardioembolic CVA is an anti-platelet agent. With a stroke occurring on a reasonable dose of aspirin, I would not recommend increasing the dose as there is little evidence that 325 mg is more effective than 81mg. The most appropriate step would be to change to an alternate anti-platelet agent such as combination dipyridamole/aspirin or clopidogrel.
In the absence of a direct comparison trial, either choice is acceptable. The evidence supporting dipyridamole/aspirin is stronger for secondary stroke prevention. Atorvastatin 80 mg daily is an evidence-based therapy after acute stroke and can be started immediately. Her hypertension should be managed permissively for the first few days after the acute event, but then an ACE-I or ARB—possibly in combination with a diuretic—would be appropriate. This patient’s goal blood pressure as a diabetic would be at least less than 130/80 mm/Hg.
Finally we would be remiss if we did not stress the importance of smoking cessation, exercise, and weight loss. TH
Dr. Cumbler is an assistant professor in the Section of Hospital Medicine at the University of Colorado, where he is a member of the Acute Stroke Service and serves on the Stroke Council.
References
- Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics-2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):e69-e171.
- Petty GW, Brown RD, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30:2513-2516.
- European Atrial Fibrillation Trial Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255-1262.
- Swedish Aspirin Low-Dose Trial Collaborative Group. Swedish aspirin low-dose aspirin trial (SALT) of 775 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet. 1991;338(8779):1345-1349.
- Farrell B, Godwin J, Richards S, et al. The United Kingdom Transient Ischaemic Attack (UK-TIA) aspirin trial: final results (abstract). J Neurol Neurosurg. Psychiatry 1991;54:1044-1054.
- Dutch TIA Trial Study Group. A comparison of two doses of aspirin (30 mg versus 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N Engl J Med. 1991 Oct 31;325(18):1261-1266.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Stroke. 2006 Feb;37(2):577-617.
- CAPRIE Steering Committee. A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events. Lancet 1996 Jan;348:1329-1339.
- Diener H, Cunha L, Forbes C, et al. European stroke prevention study 2: dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
- ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
- Mohr JP, Thompson JLP, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001 Nov 15; 345(20):1444-1451.
- Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005 Mar 31;352(13):1305-1316.
- Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo controlled trial. Lancet. 2004 Jul 24-30;36499431):331-337.
- Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559.
- The PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358(9287):1033-1041.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000 Jan 20;342:145-153.
The Case
A 62-year-old obese woman with prior history of type 2 diabetes, hypertension, and a pack-a-day smoking habit presented to the emergency department (ED) for acute onset of right-side weakness and sensory loss noted on awakening from sleep.
She reports taking a baby aspirin daily to “prevent heart attacks” prior to her stroke. Her electrocardiogram demonstrates a left bundle branch block and frequent premature atrial contractions. She recovers with mild hemiparesis and is ready for discharge. What is the best medical therapy for secondary prevention of stroke?
Overview
Cerebrovascular accident (CVA) represents an important diagnosis for the hospitalist, with 700,000 people suffering a stroke in the U.S. each year.1 This translates to a stroke every 45 seconds. About 200,000 of these strokes are recurrent events.
Cardioembolism is the largest cause of ischemic strokes, representing 29% of all infarcts.2 Stasis from impaired contractile function, atrial fibrillation, or mechanical valves are significant risk factors. More rarely, a paradoxical embolus arising in the venous system may pass through a patent foramen ovale.
Large-artery atherosclerosis and lacunar infarcts each account for 16% of strokes. Risk factors for these forms of strokes are the same as those for atherosclerosis and include hypertension and diabetes. Rarer causes such as vasculitis, dissection, hypercoagulability, or hematological disorders account for 3% of strokes. Work-up for these should be driven by historical and atypical features such as young age, family history, or unusual distribution of ischemic zones. Despite appropriate work-up, the mechanism remains uncertain in 36% of strokes.
Regardless of the manifestation and residua of the index event, the hospitalist must initiate appropriate therapy to prevent a disabling CVA. While antithrombotic drugs are the mainstay of secondary prevention, it is a mistake to miss other opportunities for risk modification. Optimal management requires a tailored evaluation for etiology, identification of modifiable risk factors, and initiation of antiplatelet or anticoagulant therapy.
Cardioembolic Stroke
Treatment of stroke depends on the etiology of the original infarct. Evidence is strong that the optimal therapy for cardioembolic stroke is anticoagulation with warfarin.
The European Atrial Fibrillation Trial found that warfarin reduces the risk for second strokes in patients with atrial fibrillation by two-thirds and is superior to antiplatelet agents for preventing cardioembolic strokes.3 Warfarin increases the risk of extracranial bleeding, but not severely enough to negate the benefit of reducing stroke death and disability. The target international normalized ratio (INR) for non-valvular atrial fibrillation is generally two to three, although this may be higher for certain prosthetic valves.
Noncardioembolic Stroke
For large-vessel atherosclerotic and lacunar cerebral ischemia, the oldest—and still effective—treatment for recurrent stroke is aspirin. The use of low-dose aspirin after transient ischemic attack (TIA) or stroke reduces second strokes or death by approximately 15%-18%.4-5 Larger doses do not appear to be more effective, although the rate of gastrointestinal complaints is greater with increased dosage. The use of either 325 mg or 1,200 mg of aspirin produced the same 15% reduction in second ischemic events. Similar efficacy has been seen in comparisons between 30 mg and 283 mg dosing.6
While a subset of patients may experience aspirin resistance, reliable assays in clinical practice are not commonly available to guide management. Current recommendations suggest that use of between 50 mg and 325 mg of aspirin is appropriate for secondary prevention.7
Clopidogrel is another antiplatelet agent that can be given daily at 75 mg as alternate therapy for secondary prevention of non-cardioembolic stroke. The Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events trial comparing clopidogrel with aspirin in patients at risk of ischemic events demonstrated significant reduction in the annual rate of combined endpoint of stroke, myocardial infarction, and vascular death—from 5.83% with aspirin to 5.32% with clopidogrel.8 This study’s applicability to secondary prevention of stroke is limited by the fact that only 19% of the patients in this trial were included because of prior stroke, and the results were not significant for reduction of stroke as a lone endpoint. Clopidogrel is recommended as an acceptable agent for CVA secondary prevention and is preferred for patients with stroke and an aspirin allergy or with recent coronary stent.
The combination of a low-dose aspirin and extended-release dipyridamole has proved superior to aspirin monotherapy in multiple trials. Over two years, the European Stroke Prevention 2 trial found an 18% reduction with aspirin alone compared with 37% reduction with the combination therapy, and the European/Australasian Stroke Prevention in Reversible Ischaemia trial confirmed that the combination reduced the absolute rate of second ischemic events by 1% annually.9-10 Headache is a common side effect of dipyridamole and may limit use. Dypridamole/aspirin is recommended as another acceptable option for secondary prevention of non-cardioembolic stroke.
Evidence suggests that aspirin/dipyridamole and clopidogrel—although significantly more expensive—are more effective than aspirin monotherapy for preventing second cerebral ischemic events. Direct comparison between aspirin/dipyridamole and clopidogrel is ongoing in the Prevention Regimen for Effectively Avoiding Second Stroke trial, with results anticipated in 2008.
Things That Don’t Work
The Warfarin-Aspirin Recurrent Stroke Study trial demonstrated that warfarin was not better than aspirin for prevention of non-cardioembolic stroke, and the Warfarin–Aspirin Symptomatic Intracranial Disease trial found the same result for patients with intracranial stenosis.11-12 There is little evidence that warfarin should have a role in the treatment of most non-cardioembolic strokes. The MATCH trial failed to show benefit to adding aspirin to clopidogrel over clopidogrel monotherapy for secondary preventions of non-cardioembolic cerebral ischemia.13 Despite efficacy following coronary stenting, the combination of clopidogrel and aspirin can not be recommended for stroke prevention.
What To Do
Aggressive risk factor modification is key in the prevention of second ischemic events. One of the most promising therapies is the use of statins following a CVA. Maintaining low-density lipoprotein (LDL) at less than 100 mg/dL (or less than 70 mg/dL in the highest-risk patients) is recommended despite a relatively weak association between stroke and hyperlipidemia.
This stands in contrast to the strong relationship between elevated LDL and coronary disease. However, the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial utilized high-dose atorvastatin after acute CVA and was able to create an absolute risk reduction for second stroke of 2.2% over the next five years.14 It is possible that the findings of this trial may reflect actions of statin therapy on the endothelium independent of the lipid lowering effect.
Blood pressure commonly has a transient elevation following cerebral ischemia. This is managed permissively to preserve perfusion to the ischemic penumbra. Once the hyperacute period is over, reduction of blood pressure to less than 140/90 mm/Hg (130/80 mm/Hg for diabetics) is recommended.
Interventions to treat chronic hypertension have been demonstrated to reduce the rate of strokes by approximately 30% to 40% over four to five years.15-16 An optimal agent has not been determined, but therapy with angiotensin converting enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB), possibly in combination with a diuretic, have been effective. Close follow-up for titration to goal in the outpatient setting should be arranged. Diabetics should have optimization of glycemic control, and lifestyle counseling should occur regarding recognized risk factors for stroke such as smoking, inactivity, and alcohol abuse.
While antithrombotic therapy is the mainstay of what we think of in secondary prevention of stroke, treatment of these other modifiable risk factors have been shown to affect mortality and second strokes of a similar magnitude and should not be neglected.
How to Treat This Case
The patient described should undergo an MRI with diffusion (to define the area of ischemia) and targeted evaluation for etiology with cardiac monitoring, echocardiogram, and carotid ultrasound.
Assuming atrial fibrillation or intracardiac thrombus is ruled out, this likely represents atherosclerotic disease. MRI will help distinguish between large-vessel atherosclerotic etiology and lacunar infarct. If carotid stenosis of greater than 70% is found in the setting of large vessel atherosclerotic stroke, then she should be referred for carotid endarterectomy. At 50% to 69% stenosis, carotid endarterectomy would still be a consideration. Antithrombotic agent of choice for non-cardioembolic CVA is an anti-platelet agent. With a stroke occurring on a reasonable dose of aspirin, I would not recommend increasing the dose as there is little evidence that 325 mg is more effective than 81mg. The most appropriate step would be to change to an alternate anti-platelet agent such as combination dipyridamole/aspirin or clopidogrel.
In the absence of a direct comparison trial, either choice is acceptable. The evidence supporting dipyridamole/aspirin is stronger for secondary stroke prevention. Atorvastatin 80 mg daily is an evidence-based therapy after acute stroke and can be started immediately. Her hypertension should be managed permissively for the first few days after the acute event, but then an ACE-I or ARB—possibly in combination with a diuretic—would be appropriate. This patient’s goal blood pressure as a diabetic would be at least less than 130/80 mm/Hg.
Finally we would be remiss if we did not stress the importance of smoking cessation, exercise, and weight loss. TH
Dr. Cumbler is an assistant professor in the Section of Hospital Medicine at the University of Colorado, where he is a member of the Acute Stroke Service and serves on the Stroke Council.
References
- Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics-2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):e69-e171.
- Petty GW, Brown RD, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30:2513-2516.
- European Atrial Fibrillation Trial Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255-1262.
- Swedish Aspirin Low-Dose Trial Collaborative Group. Swedish aspirin low-dose aspirin trial (SALT) of 775 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet. 1991;338(8779):1345-1349.
- Farrell B, Godwin J, Richards S, et al. The United Kingdom Transient Ischaemic Attack (UK-TIA) aspirin trial: final results (abstract). J Neurol Neurosurg. Psychiatry 1991;54:1044-1054.
- Dutch TIA Trial Study Group. A comparison of two doses of aspirin (30 mg versus 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N Engl J Med. 1991 Oct 31;325(18):1261-1266.
- Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack. Stroke. 2006 Feb;37(2):577-617.
- CAPRIE Steering Committee. A randomized, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events. Lancet 1996 Jan;348:1329-1339.
- Diener H, Cunha L, Forbes C, et al. European stroke prevention study 2: dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1-13.
- ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665-1673.
- Mohr JP, Thompson JLP, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001 Nov 15; 345(20):1444-1451.
- Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005 Mar 31;352(13):1305-1316.
- Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo controlled trial. Lancet. 2004 Jul 24-30;36499431):331-337.
- Stroke Prevention by Aggressive Reduction in Cholesterol Levels Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549-559.
- The PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358(9287):1033-1041.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000 Jan 20;342:145-153.