User login
Intermittent High-Dose Inhaled Corticosteroid Works for Wheezing Toddlers
SAN FRANCISCO – Intermittent courses of high-dose inhaled budesonide were as effective and as safe as daily low-dose inhaled budesonide in wheezing toddlers but exposed them to a lower cumulative dose of the corticosteroid in a year-long study of 278 children.
The two groups did not differ significantly in the frequency of exacerbations that required systemic corticosteroids, the frequency or severity of respiratory tract illness, the number of urgent or emergent visits for care, or other efficacy and safety measures, Dr. Leonard B. Bacharier and his associates reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Children in the daily low-dose budesonide group, however, were exposed to more than three times the cumulative dose of budesonide, compared with the intermittent high-dose therapy group – 150 mg vs. 46 mg.
The multicenter, randomized, double-blind, placebo-controlled trial, called the Maintenance Versus Intermittent Inhaled Steroids in Wheezing Toddlers (MIST) study, is the last of eight clinical trials performed by the National Heart, Lung, and Blood Institute’s Childhood Asthma Research and Education Network.
The NHLBI’s 2007 "Expert Panel Report 3: Guidelines for Diagnosis and Management of Asthma" recommended using daily low-dose inhaled corticosteroids to treat children who have a positive modified Asthma Predictive Index.
The MIST study is the first to compare the currently recommended daily low-dose regimen with the intermittent high-dose regimen, said Dr. Bacharier of Washington University, St. Louis.
On the basis of the MIST results, Dr. Bacharier and his associates recommended instead that clinicians consider using intermittent high-dose inhaled corticosteroids in the subset of children identified in the MIST study, who were not the most severe asthma cases. They suggested starting a 7-day course of high-dose budesonide early during respiratory tract illnesses in wheezing children who have a positive modified Asthma Predictive Index, have had at least one exacerbation in the past year, use albuterol less than 3 days per week, and have had no more than one night awakening in the prior 2 weeks.
The study enrolled children 12-53 months of age, nearly half of whom were in the lower age range of 12-32 months. All children had a history of at least four wheezing episodes in the prior year (or at least three if they’d had 3 months or more of asthma controller therapy) and a positive modified Asthma Predictive Index. Each child also had at least one severe exacerbation that required systemic corticosteroids or an unscheduled urgent or emergent visit or hospitalization in the previous year.
The study excluded children who had more than two hospitalizations for wheezing or more than six courses of oral corticosteroids.
During a 2-week run-in period, all children used a nebulized placebo nightly and albuterol as needed. During this period, children who had persistent asthma symptoms or who did not follow the protocol for more than 25% of days also were excluded.
The children were then randomized for 52 weeks of therapy. The daily low-dose budesonide group used 0.5 mg of nebulized budesonide once daily at night except during respiratory tract illness, when they switched to "respiratory tract illness kits" that gave them nebulized placebo in the morning and 0.5 mg of budesonide at night for 7 days. The intermittent high-dose budesonide group used nebulized placebo once daily at night except during respiratory tract illness, when their kits gave them 1 mg of budesonide in the morning and at night for 7 days.
Previous studies have shown that daily inhaled corticosteroids have "a small but statistically significant class effect on reducing linear growth in preschool-aged children, which only partially reverses after discontinuation of study treatments," which was a main reason for studying the intermittent-therapy alternative, Dr. Bacharier said.
In the MIST study, children in the daily-therapy group grew an average of 7.76 cm, compared with 8.01 cm in the intermittent-therapy group, but the 0.25-cm greater growth with intermittent therapy was not statistically significant.
There were no significant differences between groups in baseline characteristics, adherence to therapy during the study, declines in levels of exhaled nitric oxide, time to first exacerbation, or time to treatment failure.
The NHLBI funded the study. AstraZeneca provided the budesonide and placebo for the study. Dr. Bacharier has been a consultant for AstraZeneca (which markets budesonide), Merck, and Novartis, and has received honoraria from GlaxoSmithKline.
SAN FRANCISCO – Intermittent courses of high-dose inhaled budesonide were as effective and as safe as daily low-dose inhaled budesonide in wheezing toddlers but exposed them to a lower cumulative dose of the corticosteroid in a year-long study of 278 children.
The two groups did not differ significantly in the frequency of exacerbations that required systemic corticosteroids, the frequency or severity of respiratory tract illness, the number of urgent or emergent visits for care, or other efficacy and safety measures, Dr. Leonard B. Bacharier and his associates reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Children in the daily low-dose budesonide group, however, were exposed to more than three times the cumulative dose of budesonide, compared with the intermittent high-dose therapy group – 150 mg vs. 46 mg.
The multicenter, randomized, double-blind, placebo-controlled trial, called the Maintenance Versus Intermittent Inhaled Steroids in Wheezing Toddlers (MIST) study, is the last of eight clinical trials performed by the National Heart, Lung, and Blood Institute’s Childhood Asthma Research and Education Network.
The NHLBI’s 2007 "Expert Panel Report 3: Guidelines for Diagnosis and Management of Asthma" recommended using daily low-dose inhaled corticosteroids to treat children who have a positive modified Asthma Predictive Index.
The MIST study is the first to compare the currently recommended daily low-dose regimen with the intermittent high-dose regimen, said Dr. Bacharier of Washington University, St. Louis.
On the basis of the MIST results, Dr. Bacharier and his associates recommended instead that clinicians consider using intermittent high-dose inhaled corticosteroids in the subset of children identified in the MIST study, who were not the most severe asthma cases. They suggested starting a 7-day course of high-dose budesonide early during respiratory tract illnesses in wheezing children who have a positive modified Asthma Predictive Index, have had at least one exacerbation in the past year, use albuterol less than 3 days per week, and have had no more than one night awakening in the prior 2 weeks.
The study enrolled children 12-53 months of age, nearly half of whom were in the lower age range of 12-32 months. All children had a history of at least four wheezing episodes in the prior year (or at least three if they’d had 3 months or more of asthma controller therapy) and a positive modified Asthma Predictive Index. Each child also had at least one severe exacerbation that required systemic corticosteroids or an unscheduled urgent or emergent visit or hospitalization in the previous year.
The study excluded children who had more than two hospitalizations for wheezing or more than six courses of oral corticosteroids.
During a 2-week run-in period, all children used a nebulized placebo nightly and albuterol as needed. During this period, children who had persistent asthma symptoms or who did not follow the protocol for more than 25% of days also were excluded.
The children were then randomized for 52 weeks of therapy. The daily low-dose budesonide group used 0.5 mg of nebulized budesonide once daily at night except during respiratory tract illness, when they switched to "respiratory tract illness kits" that gave them nebulized placebo in the morning and 0.5 mg of budesonide at night for 7 days. The intermittent high-dose budesonide group used nebulized placebo once daily at night except during respiratory tract illness, when their kits gave them 1 mg of budesonide in the morning and at night for 7 days.
Previous studies have shown that daily inhaled corticosteroids have "a small but statistically significant class effect on reducing linear growth in preschool-aged children, which only partially reverses after discontinuation of study treatments," which was a main reason for studying the intermittent-therapy alternative, Dr. Bacharier said.
In the MIST study, children in the daily-therapy group grew an average of 7.76 cm, compared with 8.01 cm in the intermittent-therapy group, but the 0.25-cm greater growth with intermittent therapy was not statistically significant.
There were no significant differences between groups in baseline characteristics, adherence to therapy during the study, declines in levels of exhaled nitric oxide, time to first exacerbation, or time to treatment failure.
The NHLBI funded the study. AstraZeneca provided the budesonide and placebo for the study. Dr. Bacharier has been a consultant for AstraZeneca (which markets budesonide), Merck, and Novartis, and has received honoraria from GlaxoSmithKline.
SAN FRANCISCO – Intermittent courses of high-dose inhaled budesonide were as effective and as safe as daily low-dose inhaled budesonide in wheezing toddlers but exposed them to a lower cumulative dose of the corticosteroid in a year-long study of 278 children.
The two groups did not differ significantly in the frequency of exacerbations that required systemic corticosteroids, the frequency or severity of respiratory tract illness, the number of urgent or emergent visits for care, or other efficacy and safety measures, Dr. Leonard B. Bacharier and his associates reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Children in the daily low-dose budesonide group, however, were exposed to more than three times the cumulative dose of budesonide, compared with the intermittent high-dose therapy group – 150 mg vs. 46 mg.
The multicenter, randomized, double-blind, placebo-controlled trial, called the Maintenance Versus Intermittent Inhaled Steroids in Wheezing Toddlers (MIST) study, is the last of eight clinical trials performed by the National Heart, Lung, and Blood Institute’s Childhood Asthma Research and Education Network.
The NHLBI’s 2007 "Expert Panel Report 3: Guidelines for Diagnosis and Management of Asthma" recommended using daily low-dose inhaled corticosteroids to treat children who have a positive modified Asthma Predictive Index.
The MIST study is the first to compare the currently recommended daily low-dose regimen with the intermittent high-dose regimen, said Dr. Bacharier of Washington University, St. Louis.
On the basis of the MIST results, Dr. Bacharier and his associates recommended instead that clinicians consider using intermittent high-dose inhaled corticosteroids in the subset of children identified in the MIST study, who were not the most severe asthma cases. They suggested starting a 7-day course of high-dose budesonide early during respiratory tract illnesses in wheezing children who have a positive modified Asthma Predictive Index, have had at least one exacerbation in the past year, use albuterol less than 3 days per week, and have had no more than one night awakening in the prior 2 weeks.
The study enrolled children 12-53 months of age, nearly half of whom were in the lower age range of 12-32 months. All children had a history of at least four wheezing episodes in the prior year (or at least three if they’d had 3 months or more of asthma controller therapy) and a positive modified Asthma Predictive Index. Each child also had at least one severe exacerbation that required systemic corticosteroids or an unscheduled urgent or emergent visit or hospitalization in the previous year.
The study excluded children who had more than two hospitalizations for wheezing or more than six courses of oral corticosteroids.
During a 2-week run-in period, all children used a nebulized placebo nightly and albuterol as needed. During this period, children who had persistent asthma symptoms or who did not follow the protocol for more than 25% of days also were excluded.
The children were then randomized for 52 weeks of therapy. The daily low-dose budesonide group used 0.5 mg of nebulized budesonide once daily at night except during respiratory tract illness, when they switched to "respiratory tract illness kits" that gave them nebulized placebo in the morning and 0.5 mg of budesonide at night for 7 days. The intermittent high-dose budesonide group used nebulized placebo once daily at night except during respiratory tract illness, when their kits gave them 1 mg of budesonide in the morning and at night for 7 days.
Previous studies have shown that daily inhaled corticosteroids have "a small but statistically significant class effect on reducing linear growth in preschool-aged children, which only partially reverses after discontinuation of study treatments," which was a main reason for studying the intermittent-therapy alternative, Dr. Bacharier said.
In the MIST study, children in the daily-therapy group grew an average of 7.76 cm, compared with 8.01 cm in the intermittent-therapy group, but the 0.25-cm greater growth with intermittent therapy was not statistically significant.
There were no significant differences between groups in baseline characteristics, adherence to therapy during the study, declines in levels of exhaled nitric oxide, time to first exacerbation, or time to treatment failure.
The NHLBI funded the study. AstraZeneca provided the budesonide and placebo for the study. Dr. Bacharier has been a consultant for AstraZeneca (which markets budesonide), Merck, and Novartis, and has received honoraria from GlaxoSmithKline.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF ALLERGY, ASTHMA, AND IMMUNOLOGY
Intermittent High-Dose Inhaled Corticosteroid Works for Wheezing Toddlers
SAN FRANCISCO – Intermittent courses of high-dose inhaled budesonide were as effective and as safe as daily low-dose inhaled budesonide in wheezing toddlers but exposed them to a lower cumulative dose of the corticosteroid in a year-long study of 278 children.
The two groups did not differ significantly in the frequency of exacerbations that required systemic corticosteroids, the frequency or severity of respiratory tract illness, the number of urgent or emergent visits for care, or other efficacy and safety measures, Dr. Leonard B. Bacharier and his associates reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Children in the daily low-dose budesonide group, however, were exposed to more than three times the cumulative dose of budesonide, compared with the intermittent high-dose therapy group – 150 mg vs. 46 mg.
The multicenter, randomized, double-blind, placebo-controlled trial, called the Maintenance Versus Intermittent Inhaled Steroids in Wheezing Toddlers (MIST) study, is the last of eight clinical trials performed by the National Heart, Lung, and Blood Institute’s Childhood Asthma Research and Education Network.
The NHLBI’s 2007 "Expert Panel Report 3: Guidelines for Diagnosis and Management of Asthma" recommended using daily low-dose inhaled corticosteroids to treat children who have a positive modified Asthma Predictive Index.
The MIST study is the first to compare the currently recommended daily low-dose regimen with the intermittent high-dose regimen, said Dr. Bacharier of Washington University, St. Louis.
On the basis of the MIST results, Dr. Bacharier and his associates recommended instead that clinicians consider using intermittent high-dose inhaled corticosteroids in the subset of children identified in the MIST study, who were not the most severe asthma cases. They suggested starting a 7-day course of high-dose budesonide early during respiratory tract illnesses in wheezing children who have a positive modified Asthma Predictive Index, have had at least one exacerbation in the past year, use albuterol less than 3 days per week, and have had no more than one night awakening in the prior 2 weeks.
The study enrolled children 12-53 months of age, nearly half of whom were in the lower age range of 12-32 months. All children had a history of at least four wheezing episodes in the prior year (or at least three if they’d had 3 months or more of asthma controller therapy) and a positive modified Asthma Predictive Index. Each child also had at least one severe exacerbation that required systemic corticosteroids or an unscheduled urgent or emergent visit or hospitalization in the previous year.
The study excluded children who had more than two hospitalizations for wheezing or more than six courses of oral corticosteroids.
During a 2-week run-in period, all children used a nebulized placebo nightly and albuterol as needed. During this period, children who had persistent asthma symptoms or who did not follow the protocol for more than 25% of days also were excluded.
The children were then randomized for 52 weeks of therapy. The daily low-dose budesonide group used 0.5 mg of nebulized budesonide once daily at night except during respiratory tract illness, when they switched to "respiratory tract illness kits" that gave them nebulized placebo in the morning and 0.5 mg of budesonide at night for 7 days. The intermittent high-dose budesonide group used nebulized placebo once daily at night except during respiratory tract illness, when their kits gave them 1 mg of budesonide in the morning and at night for 7 days.
Previous studies have shown that daily inhaled corticosteroids have "a small but statistically significant class effect on reducing linear growth in preschool-aged children, which only partially reverses after discontinuation of study treatments," which was a main reason for studying the intermittent-therapy alternative, Dr. Bacharier said.
In the MIST study, children in the daily-therapy group grew an average of 7.76 cm, compared with 8.01 cm in the intermittent-therapy group, but the 0.25-cm greater growth with intermittent therapy was not statistically significant.
There were no significant differences between groups in baseline characteristics, adherence to therapy during the study, declines in levels of exhaled nitric oxide, time to first exacerbation, or time to treatment failure.
The NHLBI funded the study. AstraZeneca provided the budesonide and placebo for the study. Dr. Bacharier has been a consultant for AstraZeneca (which markets budesonide), Merck, and Novartis, and has received honoraria from GlaxoSmithKline.
SAN FRANCISCO – Intermittent courses of high-dose inhaled budesonide were as effective and as safe as daily low-dose inhaled budesonide in wheezing toddlers but exposed them to a lower cumulative dose of the corticosteroid in a year-long study of 278 children.
The two groups did not differ significantly in the frequency of exacerbations that required systemic corticosteroids, the frequency or severity of respiratory tract illness, the number of urgent or emergent visits for care, or other efficacy and safety measures, Dr. Leonard B. Bacharier and his associates reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Children in the daily low-dose budesonide group, however, were exposed to more than three times the cumulative dose of budesonide, compared with the intermittent high-dose therapy group – 150 mg vs. 46 mg.
The multicenter, randomized, double-blind, placebo-controlled trial, called the Maintenance Versus Intermittent Inhaled Steroids in Wheezing Toddlers (MIST) study, is the last of eight clinical trials performed by the National Heart, Lung, and Blood Institute’s Childhood Asthma Research and Education Network.
The NHLBI’s 2007 "Expert Panel Report 3: Guidelines for Diagnosis and Management of Asthma" recommended using daily low-dose inhaled corticosteroids to treat children who have a positive modified Asthma Predictive Index.
The MIST study is the first to compare the currently recommended daily low-dose regimen with the intermittent high-dose regimen, said Dr. Bacharier of Washington University, St. Louis.
On the basis of the MIST results, Dr. Bacharier and his associates recommended instead that clinicians consider using intermittent high-dose inhaled corticosteroids in the subset of children identified in the MIST study, who were not the most severe asthma cases. They suggested starting a 7-day course of high-dose budesonide early during respiratory tract illnesses in wheezing children who have a positive modified Asthma Predictive Index, have had at least one exacerbation in the past year, use albuterol less than 3 days per week, and have had no more than one night awakening in the prior 2 weeks.
The study enrolled children 12-53 months of age, nearly half of whom were in the lower age range of 12-32 months. All children had a history of at least four wheezing episodes in the prior year (or at least three if they’d had 3 months or more of asthma controller therapy) and a positive modified Asthma Predictive Index. Each child also had at least one severe exacerbation that required systemic corticosteroids or an unscheduled urgent or emergent visit or hospitalization in the previous year.
The study excluded children who had more than two hospitalizations for wheezing or more than six courses of oral corticosteroids.
During a 2-week run-in period, all children used a nebulized placebo nightly and albuterol as needed. During this period, children who had persistent asthma symptoms or who did not follow the protocol for more than 25% of days also were excluded.
The children were then randomized for 52 weeks of therapy. The daily low-dose budesonide group used 0.5 mg of nebulized budesonide once daily at night except during respiratory tract illness, when they switched to "respiratory tract illness kits" that gave them nebulized placebo in the morning and 0.5 mg of budesonide at night for 7 days. The intermittent high-dose budesonide group used nebulized placebo once daily at night except during respiratory tract illness, when their kits gave them 1 mg of budesonide in the morning and at night for 7 days.
Previous studies have shown that daily inhaled corticosteroids have "a small but statistically significant class effect on reducing linear growth in preschool-aged children, which only partially reverses after discontinuation of study treatments," which was a main reason for studying the intermittent-therapy alternative, Dr. Bacharier said.
In the MIST study, children in the daily-therapy group grew an average of 7.76 cm, compared with 8.01 cm in the intermittent-therapy group, but the 0.25-cm greater growth with intermittent therapy was not statistically significant.
There were no significant differences between groups in baseline characteristics, adherence to therapy during the study, declines in levels of exhaled nitric oxide, time to first exacerbation, or time to treatment failure.
The NHLBI funded the study. AstraZeneca provided the budesonide and placebo for the study. Dr. Bacharier has been a consultant for AstraZeneca (which markets budesonide), Merck, and Novartis, and has received honoraria from GlaxoSmithKline.
SAN FRANCISCO – Intermittent courses of high-dose inhaled budesonide were as effective and as safe as daily low-dose inhaled budesonide in wheezing toddlers but exposed them to a lower cumulative dose of the corticosteroid in a year-long study of 278 children.
The two groups did not differ significantly in the frequency of exacerbations that required systemic corticosteroids, the frequency or severity of respiratory tract illness, the number of urgent or emergent visits for care, or other efficacy and safety measures, Dr. Leonard B. Bacharier and his associates reported at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Children in the daily low-dose budesonide group, however, were exposed to more than three times the cumulative dose of budesonide, compared with the intermittent high-dose therapy group – 150 mg vs. 46 mg.
The multicenter, randomized, double-blind, placebo-controlled trial, called the Maintenance Versus Intermittent Inhaled Steroids in Wheezing Toddlers (MIST) study, is the last of eight clinical trials performed by the National Heart, Lung, and Blood Institute’s Childhood Asthma Research and Education Network.
The NHLBI’s 2007 "Expert Panel Report 3: Guidelines for Diagnosis and Management of Asthma" recommended using daily low-dose inhaled corticosteroids to treat children who have a positive modified Asthma Predictive Index.
The MIST study is the first to compare the currently recommended daily low-dose regimen with the intermittent high-dose regimen, said Dr. Bacharier of Washington University, St. Louis.
On the basis of the MIST results, Dr. Bacharier and his associates recommended instead that clinicians consider using intermittent high-dose inhaled corticosteroids in the subset of children identified in the MIST study, who were not the most severe asthma cases. They suggested starting a 7-day course of high-dose budesonide early during respiratory tract illnesses in wheezing children who have a positive modified Asthma Predictive Index, have had at least one exacerbation in the past year, use albuterol less than 3 days per week, and have had no more than one night awakening in the prior 2 weeks.
The study enrolled children 12-53 months of age, nearly half of whom were in the lower age range of 12-32 months. All children had a history of at least four wheezing episodes in the prior year (or at least three if they’d had 3 months or more of asthma controller therapy) and a positive modified Asthma Predictive Index. Each child also had at least one severe exacerbation that required systemic corticosteroids or an unscheduled urgent or emergent visit or hospitalization in the previous year.
The study excluded children who had more than two hospitalizations for wheezing or more than six courses of oral corticosteroids.
During a 2-week run-in period, all children used a nebulized placebo nightly and albuterol as needed. During this period, children who had persistent asthma symptoms or who did not follow the protocol for more than 25% of days also were excluded.
The children were then randomized for 52 weeks of therapy. The daily low-dose budesonide group used 0.5 mg of nebulized budesonide once daily at night except during respiratory tract illness, when they switched to "respiratory tract illness kits" that gave them nebulized placebo in the morning and 0.5 mg of budesonide at night for 7 days. The intermittent high-dose budesonide group used nebulized placebo once daily at night except during respiratory tract illness, when their kits gave them 1 mg of budesonide in the morning and at night for 7 days.
Previous studies have shown that daily inhaled corticosteroids have "a small but statistically significant class effect on reducing linear growth in preschool-aged children, which only partially reverses after discontinuation of study treatments," which was a main reason for studying the intermittent-therapy alternative, Dr. Bacharier said.
In the MIST study, children in the daily-therapy group grew an average of 7.76 cm, compared with 8.01 cm in the intermittent-therapy group, but the 0.25-cm greater growth with intermittent therapy was not statistically significant.
There were no significant differences between groups in baseline characteristics, adherence to therapy during the study, declines in levels of exhaled nitric oxide, time to first exacerbation, or time to treatment failure.
The NHLBI funded the study. AstraZeneca provided the budesonide and placebo for the study. Dr. Bacharier has been a consultant for AstraZeneca (which markets budesonide), Merck, and Novartis, and has received honoraria from GlaxoSmithKline.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF ALLERGY, ASTHMA, AND IMMUNOLOGY
Major Finding: Wheezing toddlers on intermittent high-dose budesonide or daily low-dose budesonide had similar results regarding frequency of exacerbations that required systemic corticosteroids, frequency or severity of respiratory tract illness, and number of urgent or emergent visits for care.
Data Source: Year-long multicenter, randomized, double-blind, placebo-controlled study of 278 children.
Disclosures: The National Heart, Lung, and Blood Institute funded the study. AstraZeneca provided the budesonide and placebo for the study. Dr. Bacharier has been a consultant for AstraZeneca (which markets budesonide), Merck, and Novartis, and has received honoraria from GlaxoSmithKline.
Cerebral Microbleeds Common in Elderly
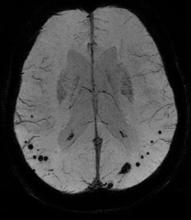
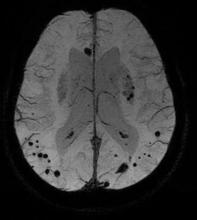
LOS ANGELES – The prevalence of cerebral microbleeds increased from 24% to 28%, and microbleeds rarely disappeared over a mean of 3 years, in a study of 831 older adults in the general Dutch population.
A subset of nondemented adults aged 60 years or older in the Rotterdam Study underwent brain MRI scans and other examinations in 2005-2006 and again in 2008-2010. Independent raters looked for microbleeds in side-by-side comparisons of the baseline and follow-up scans without knowing which was which and without access to other imaging or test results. The mean time between scans was 3.4 years.
People with microbleeds at baseline were five times more likely to develop new microbleeds. Among 203 people with microbleeds on the first scan, 25% had new microbleeds on the second scan compared with 5% of 628 people without microbleeds at baseline, Dr. Mariëlle M.F. Poels said at the International Stroke Conference.
The risk was even higher in people who had multiple microbleeds at baseline, who were seven times more likely to develop new microbleeds compared with people with no microbleeds on the initial scan, said Dr. Poels of Erasmus University, Rotterdam, the Netherlands.
The incidence of microbleeds increased with age, from 8% in people aged 60-69 years to 19% in people older than 80 years (Stroke 2011;42:656-61).
Previous longitudinal studies of cerebral microbleeds were smaller and focused on patients seen at memory clinics or patients with cerebral amyloid angiopathy instead of the general population.
The study used a three-dimensional T2*-weighted gradient-recalled echo sequence to detect microbleeds, defined as focal areas of very low signal intensity. The investigators used other MRI sequences to rate infarcts and used a validated tissue classification technique to assess white matter lesion volume. They collected DNA samples for apolipoprotein E genotyping.
Only six people (3%) had fewer microbleeds at follow-up compared with baseline. Four of these six had one microbleed on the initial scan and none at follow-up. The fifth person had two microbleeds at baseline and one at follow-up. The number of microbleeds in the sixth person decreased from 11 at baseline to 6 at follow-up, Dr. Poels said at the conference, sponsored by the American Heart Association.
In addition, another six people had some microbleeds on the initial scan that had disappeared at follow-up, but they also had new microbleeds, so their total number of microbleeds did not decrease over time. All but one of these six had more than five microbleeds at baseline and a higher number at follow-up.
In the entire cohort, 258 new microbleeds developed between the first and second scans, and only 18 microbleeds seemed to disappear. "Microbleeds rarely disappear," she said.
The location of microbleeds at baseline strongly predicted the location of new microbleeds. Of the new microbleeds, 60% were strictly lobar and 40% were either deep or infratentorial microbleeds.
Certain vascular risk factors for microbleeds were associated with the location of new microbleeds, suggesting that deep or infratentorial microbleeds are independent indicators of hypertensive vasculopathy, the investigators suggested.
High systolic blood pressure, high pulse pressure, and hypertension were associated with new deep or infratentorial microbleeds but not with new lobar microbleeds. Increasing serum total cholesterol was associated with a decreasing incidence of deep or infratentorial microbleeds on the follow-up scan.
The presence of lacunar infarcts at baseline was associated with a fourfold higher risk for new deep or infratentorial microbleeds at follow-up than in people without infarcts. A fourfold higher risk for new strictly lobar microbleeds was seen in people with the apolipoprotein E4 genotype. People with larger white matter lesion volume at baseline had double the likelihood of new microbleeds in any of the locations, compared with people with smaller white matter lesion volume at baseline.
"Microbleed assessment on T2*-weighted MRI may serve as a possible marker of both cerebral amyloid angiopathy and hypertensive vasculopathy progression," Dr. Poels said.
Controlling vascular risk factors in people who already have microbleeds may slow the progression of pathology and prevent symptomatic intracerebral hemorrhage, she suggested.
The 831 participants who completed both MRI scans were younger and healthier than the 231 people who dropped out of the study after the first scan, either because they refused a second scan or were ineligible to continue. This may have resulted in underestimation of the true incidence of microbleeds in the general population and underestimation of associations between risk factors and incident cerebral microbleeds, the investigators noted.
Dr. Poels and her associates said they had no relevant financial disclosures.
LOS ANGELES – The prevalence of cerebral microbleeds increased from 24% to 28%, and microbleeds rarely disappeared over a mean of 3 years, in a study of 831 older adults in the general Dutch population.
A subset of nondemented adults aged 60 years or older in the Rotterdam Study underwent brain MRI scans and other examinations in 2005-2006 and again in 2008-2010. Independent raters looked for microbleeds in side-by-side comparisons of the baseline and follow-up scans without knowing which was which and without access to other imaging or test results. The mean time between scans was 3.4 years.
People with microbleeds at baseline were five times more likely to develop new microbleeds. Among 203 people with microbleeds on the first scan, 25% had new microbleeds on the second scan compared with 5% of 628 people without microbleeds at baseline, Dr. Mariëlle M.F. Poels said at the International Stroke Conference.
The risk was even higher in people who had multiple microbleeds at baseline, who were seven times more likely to develop new microbleeds compared with people with no microbleeds on the initial scan, said Dr. Poels of Erasmus University, Rotterdam, the Netherlands.
The incidence of microbleeds increased with age, from 8% in people aged 60-69 years to 19% in people older than 80 years (Stroke 2011;42:656-61).
Previous longitudinal studies of cerebral microbleeds were smaller and focused on patients seen at memory clinics or patients with cerebral amyloid angiopathy instead of the general population.
The study used a three-dimensional T2*-weighted gradient-recalled echo sequence to detect microbleeds, defined as focal areas of very low signal intensity. The investigators used other MRI sequences to rate infarcts and used a validated tissue classification technique to assess white matter lesion volume. They collected DNA samples for apolipoprotein E genotyping.
Only six people (3%) had fewer microbleeds at follow-up compared with baseline. Four of these six had one microbleed on the initial scan and none at follow-up. The fifth person had two microbleeds at baseline and one at follow-up. The number of microbleeds in the sixth person decreased from 11 at baseline to 6 at follow-up, Dr. Poels said at the conference, sponsored by the American Heart Association.
In addition, another six people had some microbleeds on the initial scan that had disappeared at follow-up, but they also had new microbleeds, so their total number of microbleeds did not decrease over time. All but one of these six had more than five microbleeds at baseline and a higher number at follow-up.
In the entire cohort, 258 new microbleeds developed between the first and second scans, and only 18 microbleeds seemed to disappear. "Microbleeds rarely disappear," she said.
The location of microbleeds at baseline strongly predicted the location of new microbleeds. Of the new microbleeds, 60% were strictly lobar and 40% were either deep or infratentorial microbleeds.
Certain vascular risk factors for microbleeds were associated with the location of new microbleeds, suggesting that deep or infratentorial microbleeds are independent indicators of hypertensive vasculopathy, the investigators suggested.
High systolic blood pressure, high pulse pressure, and hypertension were associated with new deep or infratentorial microbleeds but not with new lobar microbleeds. Increasing serum total cholesterol was associated with a decreasing incidence of deep or infratentorial microbleeds on the follow-up scan.
The presence of lacunar infarcts at baseline was associated with a fourfold higher risk for new deep or infratentorial microbleeds at follow-up than in people without infarcts. A fourfold higher risk for new strictly lobar microbleeds was seen in people with the apolipoprotein E4 genotype. People with larger white matter lesion volume at baseline had double the likelihood of new microbleeds in any of the locations, compared with people with smaller white matter lesion volume at baseline.
"Microbleed assessment on T2*-weighted MRI may serve as a possible marker of both cerebral amyloid angiopathy and hypertensive vasculopathy progression," Dr. Poels said.
Controlling vascular risk factors in people who already have microbleeds may slow the progression of pathology and prevent symptomatic intracerebral hemorrhage, she suggested.
The 831 participants who completed both MRI scans were younger and healthier than the 231 people who dropped out of the study after the first scan, either because they refused a second scan or were ineligible to continue. This may have resulted in underestimation of the true incidence of microbleeds in the general population and underestimation of associations between risk factors and incident cerebral microbleeds, the investigators noted.
Dr. Poels and her associates said they had no relevant financial disclosures.
LOS ANGELES – The prevalence of cerebral microbleeds increased from 24% to 28%, and microbleeds rarely disappeared over a mean of 3 years, in a study of 831 older adults in the general Dutch population.
A subset of nondemented adults aged 60 years or older in the Rotterdam Study underwent brain MRI scans and other examinations in 2005-2006 and again in 2008-2010. Independent raters looked for microbleeds in side-by-side comparisons of the baseline and follow-up scans without knowing which was which and without access to other imaging or test results. The mean time between scans was 3.4 years.
People with microbleeds at baseline were five times more likely to develop new microbleeds. Among 203 people with microbleeds on the first scan, 25% had new microbleeds on the second scan compared with 5% of 628 people without microbleeds at baseline, Dr. Mariëlle M.F. Poels said at the International Stroke Conference.
The risk was even higher in people who had multiple microbleeds at baseline, who were seven times more likely to develop new microbleeds compared with people with no microbleeds on the initial scan, said Dr. Poels of Erasmus University, Rotterdam, the Netherlands.
The incidence of microbleeds increased with age, from 8% in people aged 60-69 years to 19% in people older than 80 years (Stroke 2011;42:656-61).
Previous longitudinal studies of cerebral microbleeds were smaller and focused on patients seen at memory clinics or patients with cerebral amyloid angiopathy instead of the general population.
The study used a three-dimensional T2*-weighted gradient-recalled echo sequence to detect microbleeds, defined as focal areas of very low signal intensity. The investigators used other MRI sequences to rate infarcts and used a validated tissue classification technique to assess white matter lesion volume. They collected DNA samples for apolipoprotein E genotyping.
Only six people (3%) had fewer microbleeds at follow-up compared with baseline. Four of these six had one microbleed on the initial scan and none at follow-up. The fifth person had two microbleeds at baseline and one at follow-up. The number of microbleeds in the sixth person decreased from 11 at baseline to 6 at follow-up, Dr. Poels said at the conference, sponsored by the American Heart Association.
In addition, another six people had some microbleeds on the initial scan that had disappeared at follow-up, but they also had new microbleeds, so their total number of microbleeds did not decrease over time. All but one of these six had more than five microbleeds at baseline and a higher number at follow-up.
In the entire cohort, 258 new microbleeds developed between the first and second scans, and only 18 microbleeds seemed to disappear. "Microbleeds rarely disappear," she said.
The location of microbleeds at baseline strongly predicted the location of new microbleeds. Of the new microbleeds, 60% were strictly lobar and 40% were either deep or infratentorial microbleeds.
Certain vascular risk factors for microbleeds were associated with the location of new microbleeds, suggesting that deep or infratentorial microbleeds are independent indicators of hypertensive vasculopathy, the investigators suggested.
High systolic blood pressure, high pulse pressure, and hypertension were associated with new deep or infratentorial microbleeds but not with new lobar microbleeds. Increasing serum total cholesterol was associated with a decreasing incidence of deep or infratentorial microbleeds on the follow-up scan.
The presence of lacunar infarcts at baseline was associated with a fourfold higher risk for new deep or infratentorial microbleeds at follow-up than in people without infarcts. A fourfold higher risk for new strictly lobar microbleeds was seen in people with the apolipoprotein E4 genotype. People with larger white matter lesion volume at baseline had double the likelihood of new microbleeds in any of the locations, compared with people with smaller white matter lesion volume at baseline.
"Microbleed assessment on T2*-weighted MRI may serve as a possible marker of both cerebral amyloid angiopathy and hypertensive vasculopathy progression," Dr. Poels said.
Controlling vascular risk factors in people who already have microbleeds may slow the progression of pathology and prevent symptomatic intracerebral hemorrhage, she suggested.
The 831 participants who completed both MRI scans were younger and healthier than the 231 people who dropped out of the study after the first scan, either because they refused a second scan or were ineligible to continue. This may have resulted in underestimation of the true incidence of microbleeds in the general population and underestimation of associations between risk factors and incident cerebral microbleeds, the investigators noted.
Dr. Poels and her associates said they had no relevant financial disclosures.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: Cerebral microbleeds were present in 24% of elderly people in the general population, increased in number with age, and rarely disappeared over time.
Data Source: Repeat brain MRI scans a mean of 3 years apart in 831 elderly non-demented adults within the longitudinal Rotterdam Study.
Disclosures: Dr. Poels and her associates said they had no relevant financial disclosures.
Cerebral Microbleeds Common in Elderly
LOS ANGELES – The prevalence of cerebral microbleeds increased from 24% to 28%, and microbleeds rarely disappeared over a mean of 3 years, in a study of 831 older adults in the general Dutch population.
A subset of nondemented adults aged 60 years or older in the Rotterdam Study underwent brain MRI scans and other examinations in 2005-2006 and again in 2008-2010. Independent raters looked for microbleeds in side-by-side comparisons of the baseline and follow-up scans without knowing which was which and without access to other imaging or test results. The mean time between scans was 3.4 years.
People with microbleeds at baseline were five times more likely to develop new microbleeds. Among 203 people with microbleeds on the first scan, 25% had new microbleeds on the second scan compared with 5% of 628 people without microbleeds at baseline, Dr. Mariëlle M.F. Poels said at the International Stroke Conference.
The risk was even higher in people who had multiple microbleeds at baseline, who were seven times more likely to develop new microbleeds compared with people with no microbleeds on the initial scan, said Dr. Poels of Erasmus University, Rotterdam, the Netherlands.
The incidence of microbleeds increased with age, from 8% in people aged 60-69 years to 19% in people older than 80 years (Stroke 2011;42:656-61).
Previous longitudinal studies of cerebral microbleeds were smaller and focused on patients seen at memory clinics or patients with cerebral amyloid angiopathy instead of the general population.
The study used a three-dimensional T2*-weighted gradient-recalled echo sequence to detect microbleeds, defined as focal areas of very low signal intensity. The investigators used other MRI sequences to rate infarcts and used a validated tissue classification technique to assess white matter lesion volume. They collected DNA samples for apolipoprotein E genotyping.
Only six people (3%) had fewer microbleeds at follow-up compared with baseline. Four of these six had one microbleed on the initial scan and none at follow-up. The fifth person had two microbleeds at baseline and one at follow-up. The number of microbleeds in the sixth person decreased from 11 at baseline to 6 at follow-up, Dr. Poels said at the conference, sponsored by the American Heart Association.
In addition, another six people had some microbleeds on the initial scan that had disappeared at follow-up, but they also had new microbleeds, so their total number of microbleeds did not decrease over time. All but one of these six had more than five microbleeds at baseline and a higher number at follow-up.
In the entire cohort, 258 new microbleeds developed between the first and second scans, and only 18 microbleeds seemed to disappear. "Microbleeds rarely disappear," she said.
The location of microbleeds at baseline strongly predicted the location of new microbleeds. Of the new microbleeds, 60% were strictly lobar and 40% were either deep or infratentorial microbleeds.
Certain vascular risk factors for microbleeds were associated with the location of new microbleeds, suggesting that deep or infratentorial microbleeds are independent indicators of hypertensive vasculopathy, the investigators suggested.
High systolic blood pressure, high pulse pressure, and hypertension were associated with new deep or infratentorial microbleeds but not with new lobar microbleeds. Increasing serum total cholesterol was associated with a decreasing incidence of deep or infratentorial microbleeds on the follow-up scan.
The presence of lacunar infarcts at baseline was associated with a fourfold higher risk for new deep or infratentorial microbleeds at follow-up than in people without infarcts. A fourfold higher risk for new strictly lobar microbleeds was seen in people with the apolipoprotein E4 genotype. People with larger white matter lesion volume at baseline had double the likelihood of new microbleeds in any of the locations, compared with people with smaller white matter lesion volume at baseline.
"Microbleed assessment on T2*-weighted MRI may serve as a possible marker of both cerebral amyloid angiopathy and hypertensive vasculopathy progression," Dr. Poels said.
Controlling vascular risk factors in people who already have microbleeds may slow the progression of pathology and prevent symptomatic intracerebral hemorrhage, she suggested.
The 831 participants who completed both MRI scans were younger and healthier than the 231 people who dropped out of the study after the first scan, either because they refused a second scan or were ineligible to continue. This may have resulted in underestimation of the true incidence of microbleeds in the general population and underestimation of associations between risk factors and incident cerebral microbleeds, the investigators noted.
Dr. Poels and her associates said they had no relevant financial disclosures.
LOS ANGELES – The prevalence of cerebral microbleeds increased from 24% to 28%, and microbleeds rarely disappeared over a mean of 3 years, in a study of 831 older adults in the general Dutch population.
A subset of nondemented adults aged 60 years or older in the Rotterdam Study underwent brain MRI scans and other examinations in 2005-2006 and again in 2008-2010. Independent raters looked for microbleeds in side-by-side comparisons of the baseline and follow-up scans without knowing which was which and without access to other imaging or test results. The mean time between scans was 3.4 years.
People with microbleeds at baseline were five times more likely to develop new microbleeds. Among 203 people with microbleeds on the first scan, 25% had new microbleeds on the second scan compared with 5% of 628 people without microbleeds at baseline, Dr. Mariëlle M.F. Poels said at the International Stroke Conference.
The risk was even higher in people who had multiple microbleeds at baseline, who were seven times more likely to develop new microbleeds compared with people with no microbleeds on the initial scan, said Dr. Poels of Erasmus University, Rotterdam, the Netherlands.
The incidence of microbleeds increased with age, from 8% in people aged 60-69 years to 19% in people older than 80 years (Stroke 2011;42:656-61).
Previous longitudinal studies of cerebral microbleeds were smaller and focused on patients seen at memory clinics or patients with cerebral amyloid angiopathy instead of the general population.
The study used a three-dimensional T2*-weighted gradient-recalled echo sequence to detect microbleeds, defined as focal areas of very low signal intensity. The investigators used other MRI sequences to rate infarcts and used a validated tissue classification technique to assess white matter lesion volume. They collected DNA samples for apolipoprotein E genotyping.
Only six people (3%) had fewer microbleeds at follow-up compared with baseline. Four of these six had one microbleed on the initial scan and none at follow-up. The fifth person had two microbleeds at baseline and one at follow-up. The number of microbleeds in the sixth person decreased from 11 at baseline to 6 at follow-up, Dr. Poels said at the conference, sponsored by the American Heart Association.
In addition, another six people had some microbleeds on the initial scan that had disappeared at follow-up, but they also had new microbleeds, so their total number of microbleeds did not decrease over time. All but one of these six had more than five microbleeds at baseline and a higher number at follow-up.
In the entire cohort, 258 new microbleeds developed between the first and second scans, and only 18 microbleeds seemed to disappear. "Microbleeds rarely disappear," she said.
The location of microbleeds at baseline strongly predicted the location of new microbleeds. Of the new microbleeds, 60% were strictly lobar and 40% were either deep or infratentorial microbleeds.
Certain vascular risk factors for microbleeds were associated with the location of new microbleeds, suggesting that deep or infratentorial microbleeds are independent indicators of hypertensive vasculopathy, the investigators suggested.
High systolic blood pressure, high pulse pressure, and hypertension were associated with new deep or infratentorial microbleeds but not with new lobar microbleeds. Increasing serum total cholesterol was associated with a decreasing incidence of deep or infratentorial microbleeds on the follow-up scan.
The presence of lacunar infarcts at baseline was associated with a fourfold higher risk for new deep or infratentorial microbleeds at follow-up than in people without infarcts. A fourfold higher risk for new strictly lobar microbleeds was seen in people with the apolipoprotein E4 genotype. People with larger white matter lesion volume at baseline had double the likelihood of new microbleeds in any of the locations, compared with people with smaller white matter lesion volume at baseline.
"Microbleed assessment on T2*-weighted MRI may serve as a possible marker of both cerebral amyloid angiopathy and hypertensive vasculopathy progression," Dr. Poels said.
Controlling vascular risk factors in people who already have microbleeds may slow the progression of pathology and prevent symptomatic intracerebral hemorrhage, she suggested.
The 831 participants who completed both MRI scans were younger and healthier than the 231 people who dropped out of the study after the first scan, either because they refused a second scan or were ineligible to continue. This may have resulted in underestimation of the true incidence of microbleeds in the general population and underestimation of associations between risk factors and incident cerebral microbleeds, the investigators noted.
Dr. Poels and her associates said they had no relevant financial disclosures.
LOS ANGELES – The prevalence of cerebral microbleeds increased from 24% to 28%, and microbleeds rarely disappeared over a mean of 3 years, in a study of 831 older adults in the general Dutch population.
A subset of nondemented adults aged 60 years or older in the Rotterdam Study underwent brain MRI scans and other examinations in 2005-2006 and again in 2008-2010. Independent raters looked for microbleeds in side-by-side comparisons of the baseline and follow-up scans without knowing which was which and without access to other imaging or test results. The mean time between scans was 3.4 years.
People with microbleeds at baseline were five times more likely to develop new microbleeds. Among 203 people with microbleeds on the first scan, 25% had new microbleeds on the second scan compared with 5% of 628 people without microbleeds at baseline, Dr. Mariëlle M.F. Poels said at the International Stroke Conference.
The risk was even higher in people who had multiple microbleeds at baseline, who were seven times more likely to develop new microbleeds compared with people with no microbleeds on the initial scan, said Dr. Poels of Erasmus University, Rotterdam, the Netherlands.
The incidence of microbleeds increased with age, from 8% in people aged 60-69 years to 19% in people older than 80 years (Stroke 2011;42:656-61).
Previous longitudinal studies of cerebral microbleeds were smaller and focused on patients seen at memory clinics or patients with cerebral amyloid angiopathy instead of the general population.
The study used a three-dimensional T2*-weighted gradient-recalled echo sequence to detect microbleeds, defined as focal areas of very low signal intensity. The investigators used other MRI sequences to rate infarcts and used a validated tissue classification technique to assess white matter lesion volume. They collected DNA samples for apolipoprotein E genotyping.
Only six people (3%) had fewer microbleeds at follow-up compared with baseline. Four of these six had one microbleed on the initial scan and none at follow-up. The fifth person had two microbleeds at baseline and one at follow-up. The number of microbleeds in the sixth person decreased from 11 at baseline to 6 at follow-up, Dr. Poels said at the conference, sponsored by the American Heart Association.
In addition, another six people had some microbleeds on the initial scan that had disappeared at follow-up, but they also had new microbleeds, so their total number of microbleeds did not decrease over time. All but one of these six had more than five microbleeds at baseline and a higher number at follow-up.
In the entire cohort, 258 new microbleeds developed between the first and second scans, and only 18 microbleeds seemed to disappear. "Microbleeds rarely disappear," she said.
The location of microbleeds at baseline strongly predicted the location of new microbleeds. Of the new microbleeds, 60% were strictly lobar and 40% were either deep or infratentorial microbleeds.
Certain vascular risk factors for microbleeds were associated with the location of new microbleeds, suggesting that deep or infratentorial microbleeds are independent indicators of hypertensive vasculopathy, the investigators suggested.
High systolic blood pressure, high pulse pressure, and hypertension were associated with new deep or infratentorial microbleeds but not with new lobar microbleeds. Increasing serum total cholesterol was associated with a decreasing incidence of deep or infratentorial microbleeds on the follow-up scan.
The presence of lacunar infarcts at baseline was associated with a fourfold higher risk for new deep or infratentorial microbleeds at follow-up than in people without infarcts. A fourfold higher risk for new strictly lobar microbleeds was seen in people with the apolipoprotein E4 genotype. People with larger white matter lesion volume at baseline had double the likelihood of new microbleeds in any of the locations, compared with people with smaller white matter lesion volume at baseline.
"Microbleed assessment on T2*-weighted MRI may serve as a possible marker of both cerebral amyloid angiopathy and hypertensive vasculopathy progression," Dr. Poels said.
Controlling vascular risk factors in people who already have microbleeds may slow the progression of pathology and prevent symptomatic intracerebral hemorrhage, she suggested.
The 831 participants who completed both MRI scans were younger and healthier than the 231 people who dropped out of the study after the first scan, either because they refused a second scan or were ineligible to continue. This may have resulted in underestimation of the true incidence of microbleeds in the general population and underestimation of associations between risk factors and incident cerebral microbleeds, the investigators noted.
Dr. Poels and her associates said they had no relevant financial disclosures.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: Cerebral microbleeds were present in 24% of elderly people in the general population, increased in number with age, and rarely disappeared over time.
Data Source: Repeat brain MRI scans a mean of 3 years apart in 831 elderly non-demented adults within the longitudinal Rotterdam Study.
Disclosures: Dr. Poels and her associates said they had no relevant financial disclosures.
Cerebral Microbleeds Common in Elderly
LOS ANGELES – The prevalence of cerebral microbleeds increased from 24% to 28%, and microbleeds rarely disappeared over a mean of 3 years, in a study of 831 older adults in the general Dutch population.
A subset of nondemented adults aged 60 years or older in the Rotterdam Study underwent brain MRI scans and other examinations in 2005-2006 and again in 2008-2010. Independent raters looked for microbleeds in side-by-side comparisons of the baseline and follow-up scans without knowing which was which and without access to other imaging or test results. The mean time between scans was 3.4 years.
People with microbleeds at baseline were five times more likely to develop new microbleeds. Among 203 people with microbleeds on the first scan, 25% had new microbleeds on the second scan compared with 5% of 628 people without microbleeds at baseline, Dr. Mariëlle M.F. Poels said at the International Stroke Conference.
The risk was even higher in people who had multiple microbleeds at baseline, who were seven times more likely to develop new microbleeds compared with people with no microbleeds on the initial scan, said Dr. Poels of Erasmus University, Rotterdam, the Netherlands.
The incidence of microbleeds increased with age, from 8% in people aged 60-69 years to 19% in people older than 80 years (Stroke 2011;42:656-61).
Previous longitudinal studies of cerebral microbleeds were smaller and focused on patients seen at memory clinics or patients with cerebral amyloid angiopathy instead of the general population.
The study used a three-dimensional T2*-weighted gradient-recalled echo sequence to detect microbleeds, defined as focal areas of very low signal intensity. The investigators used other MRI sequences to rate infarcts and used a validated tissue classification technique to assess white matter lesion volume. They collected DNA samples for apolipoprotein E genotyping.
Only six people (3%) had fewer microbleeds at follow-up compared with baseline. Four of these six had one microbleed on the initial scan and none at follow-up. The fifth person had two microbleeds at baseline and one at follow-up. The number of microbleeds in the sixth person decreased from 11 at baseline to 6 at follow-up, Dr. Poels said at the conference, sponsored by the American Heart Association.
In addition, another six people had some microbleeds on the initial scan that had disappeared at follow-up, but they also had new microbleeds, so their total number of microbleeds did not decrease over time. All but one of these six had more than five microbleeds at baseline and a higher number at follow-up.
In the entire cohort, 258 new microbleeds developed between the first and second scans, and only 18 microbleeds seemed to disappear. "Microbleeds rarely disappear," she said.
The location of microbleeds at baseline strongly predicted the location of new microbleeds. Of the new microbleeds, 60% were strictly lobar and 40% were either deep or infratentorial microbleeds.
Certain vascular risk factors for microbleeds were associated with the location of new microbleeds, suggesting that deep or infratentorial microbleeds are independent indicators of hypertensive vasculopathy, the investigators suggested.
High systolic blood pressure, high pulse pressure, and hypertension were associated with new deep or infratentorial microbleeds but not with new lobar microbleeds. Increasing serum total cholesterol was associated with a decreasing incidence of deep or infratentorial microbleeds on the follow-up scan.
The presence of lacunar infarcts at baseline was associated with a fourfold higher risk for new deep or infratentorial microbleeds at follow-up than in people without infarcts. A fourfold higher risk for new strictly lobar microbleeds was seen in people with the apolipoprotein E4 genotype. People with larger white matter lesion volume at baseline had double the likelihood of new microbleeds in any of the locations, compared with people with smaller white matter lesion volume at baseline.
"Microbleed assessment on T2*-weighted MRI may serve as a possible marker of both cerebral amyloid angiopathy and hypertensive vasculopathy progression," Dr. Poels said.
Controlling vascular risk factors in people who already have microbleeds may slow the progression of pathology and prevent symptomatic intracerebral hemorrhage, she suggested.
The 831 participants who completed both MRI scans were younger and healthier than the 231 people who dropped out of the study after the first scan, either because they refused a second scan or were ineligible to continue. This may have resulted in underestimation of the true incidence of microbleeds in the general population and underestimation of associations between risk factors and incident cerebral microbleeds, the investigators noted.
Dr. Poels and her associates said they had no relevant financial disclosures.
LOS ANGELES – The prevalence of cerebral microbleeds increased from 24% to 28%, and microbleeds rarely disappeared over a mean of 3 years, in a study of 831 older adults in the general Dutch population.
A subset of nondemented adults aged 60 years or older in the Rotterdam Study underwent brain MRI scans and other examinations in 2005-2006 and again in 2008-2010. Independent raters looked for microbleeds in side-by-side comparisons of the baseline and follow-up scans without knowing which was which and without access to other imaging or test results. The mean time between scans was 3.4 years.
People with microbleeds at baseline were five times more likely to develop new microbleeds. Among 203 people with microbleeds on the first scan, 25% had new microbleeds on the second scan compared with 5% of 628 people without microbleeds at baseline, Dr. Mariëlle M.F. Poels said at the International Stroke Conference.
The risk was even higher in people who had multiple microbleeds at baseline, who were seven times more likely to develop new microbleeds compared with people with no microbleeds on the initial scan, said Dr. Poels of Erasmus University, Rotterdam, the Netherlands.
The incidence of microbleeds increased with age, from 8% in people aged 60-69 years to 19% in people older than 80 years (Stroke 2011;42:656-61).
Previous longitudinal studies of cerebral microbleeds were smaller and focused on patients seen at memory clinics or patients with cerebral amyloid angiopathy instead of the general population.
The study used a three-dimensional T2*-weighted gradient-recalled echo sequence to detect microbleeds, defined as focal areas of very low signal intensity. The investigators used other MRI sequences to rate infarcts and used a validated tissue classification technique to assess white matter lesion volume. They collected DNA samples for apolipoprotein E genotyping.
Only six people (3%) had fewer microbleeds at follow-up compared with baseline. Four of these six had one microbleed on the initial scan and none at follow-up. The fifth person had two microbleeds at baseline and one at follow-up. The number of microbleeds in the sixth person decreased from 11 at baseline to 6 at follow-up, Dr. Poels said at the conference, sponsored by the American Heart Association.
In addition, another six people had some microbleeds on the initial scan that had disappeared at follow-up, but they also had new microbleeds, so their total number of microbleeds did not decrease over time. All but one of these six had more than five microbleeds at baseline and a higher number at follow-up.
In the entire cohort, 258 new microbleeds developed between the first and second scans, and only 18 microbleeds seemed to disappear. "Microbleeds rarely disappear," she said.
The location of microbleeds at baseline strongly predicted the location of new microbleeds. Of the new microbleeds, 60% were strictly lobar and 40% were either deep or infratentorial microbleeds.
Certain vascular risk factors for microbleeds were associated with the location of new microbleeds, suggesting that deep or infratentorial microbleeds are independent indicators of hypertensive vasculopathy, the investigators suggested.
High systolic blood pressure, high pulse pressure, and hypertension were associated with new deep or infratentorial microbleeds but not with new lobar microbleeds. Increasing serum total cholesterol was associated with a decreasing incidence of deep or infratentorial microbleeds on the follow-up scan.
The presence of lacunar infarcts at baseline was associated with a fourfold higher risk for new deep or infratentorial microbleeds at follow-up than in people without infarcts. A fourfold higher risk for new strictly lobar microbleeds was seen in people with the apolipoprotein E4 genotype. People with larger white matter lesion volume at baseline had double the likelihood of new microbleeds in any of the locations, compared with people with smaller white matter lesion volume at baseline.
"Microbleed assessment on T2*-weighted MRI may serve as a possible marker of both cerebral amyloid angiopathy and hypertensive vasculopathy progression," Dr. Poels said.
Controlling vascular risk factors in people who already have microbleeds may slow the progression of pathology and prevent symptomatic intracerebral hemorrhage, she suggested.
The 831 participants who completed both MRI scans were younger and healthier than the 231 people who dropped out of the study after the first scan, either because they refused a second scan or were ineligible to continue. This may have resulted in underestimation of the true incidence of microbleeds in the general population and underestimation of associations between risk factors and incident cerebral microbleeds, the investigators noted.
Dr. Poels and her associates said they had no relevant financial disclosures.
LOS ANGELES – The prevalence of cerebral microbleeds increased from 24% to 28%, and microbleeds rarely disappeared over a mean of 3 years, in a study of 831 older adults in the general Dutch population.
A subset of nondemented adults aged 60 years or older in the Rotterdam Study underwent brain MRI scans and other examinations in 2005-2006 and again in 2008-2010. Independent raters looked for microbleeds in side-by-side comparisons of the baseline and follow-up scans without knowing which was which and without access to other imaging or test results. The mean time between scans was 3.4 years.
People with microbleeds at baseline were five times more likely to develop new microbleeds. Among 203 people with microbleeds on the first scan, 25% had new microbleeds on the second scan compared with 5% of 628 people without microbleeds at baseline, Dr. Mariëlle M.F. Poels said at the International Stroke Conference.
The risk was even higher in people who had multiple microbleeds at baseline, who were seven times more likely to develop new microbleeds compared with people with no microbleeds on the initial scan, said Dr. Poels of Erasmus University, Rotterdam, the Netherlands.
The incidence of microbleeds increased with age, from 8% in people aged 60-69 years to 19% in people older than 80 years (Stroke 2011;42:656-61).
Previous longitudinal studies of cerebral microbleeds were smaller and focused on patients seen at memory clinics or patients with cerebral amyloid angiopathy instead of the general population.
The study used a three-dimensional T2*-weighted gradient-recalled echo sequence to detect microbleeds, defined as focal areas of very low signal intensity. The investigators used other MRI sequences to rate infarcts and used a validated tissue classification technique to assess white matter lesion volume. They collected DNA samples for apolipoprotein E genotyping.
Only six people (3%) had fewer microbleeds at follow-up compared with baseline. Four of these six had one microbleed on the initial scan and none at follow-up. The fifth person had two microbleeds at baseline and one at follow-up. The number of microbleeds in the sixth person decreased from 11 at baseline to 6 at follow-up, Dr. Poels said at the conference, sponsored by the American Heart Association.
In addition, another six people had some microbleeds on the initial scan that had disappeared at follow-up, but they also had new microbleeds, so their total number of microbleeds did not decrease over time. All but one of these six had more than five microbleeds at baseline and a higher number at follow-up.
In the entire cohort, 258 new microbleeds developed between the first and second scans, and only 18 microbleeds seemed to disappear. "Microbleeds rarely disappear," she said.
The location of microbleeds at baseline strongly predicted the location of new microbleeds. Of the new microbleeds, 60% were strictly lobar and 40% were either deep or infratentorial microbleeds.
Certain vascular risk factors for microbleeds were associated with the location of new microbleeds, suggesting that deep or infratentorial microbleeds are independent indicators of hypertensive vasculopathy, the investigators suggested.
High systolic blood pressure, high pulse pressure, and hypertension were associated with new deep or infratentorial microbleeds but not with new lobar microbleeds. Increasing serum total cholesterol was associated with a decreasing incidence of deep or infratentorial microbleeds on the follow-up scan.
The presence of lacunar infarcts at baseline was associated with a fourfold higher risk for new deep or infratentorial microbleeds at follow-up than in people without infarcts. A fourfold higher risk for new strictly lobar microbleeds was seen in people with the apolipoprotein E4 genotype. People with larger white matter lesion volume at baseline had double the likelihood of new microbleeds in any of the locations, compared with people with smaller white matter lesion volume at baseline.
"Microbleed assessment on T2*-weighted MRI may serve as a possible marker of both cerebral amyloid angiopathy and hypertensive vasculopathy progression," Dr. Poels said.
Controlling vascular risk factors in people who already have microbleeds may slow the progression of pathology and prevent symptomatic intracerebral hemorrhage, she suggested.
The 831 participants who completed both MRI scans were younger and healthier than the 231 people who dropped out of the study after the first scan, either because they refused a second scan or were ineligible to continue. This may have resulted in underestimation of the true incidence of microbleeds in the general population and underestimation of associations between risk factors and incident cerebral microbleeds, the investigators noted.
Dr. Poels and her associates said they had no relevant financial disclosures.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: Cerebral microbleeds were present in 24% of elderly people in the general population, increased in number with age, and rarely disappeared over time.
Data Source: Repeat brain MRI scans a mean of 3 years apart in 831 elderly non-demented adults within the longitudinal Rotterdam Study.
Disclosures: Dr. Poels and her associates said they had no relevant financial disclosures.
Study Finds Icatibant Relieves Acute Angioedema
SAN FRANCISCO – The experimental drug icatibant relieved moderate or severe acute attacks of hereditary angioedema faster than placebo in the third randomized, double-blind, controlled trial of the experimental drug in 98 patients.
Among 88 patients with cutaneous and/or abdominal acute attacks, the 43 patients randomized to treatment with a single subcutaneous injection of 30 mg of icatibant reported that symptom relief started a median of 2 hours after treatment, compared with 20 hours for the 45 patients randomized to saline placebo injection. Symptom relief was defined as at least a 50% decrease in the combined Visual Analog Scale (VAS) symptom score, compared with baseline.
"I think this represents a very exciting, important advance in the treatment of an underserved population," Dr. William R. Lumry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
A significant difference between groups was seen as early as 1 hour after treatment, but the 50% or greater reduction in VAS symptom scores was not achieved until 2 hours after treatment, Dr. Lumry and his associates reported.
The third For Angioedema Subcutaneous Treatment (FAST-3) trial followed two earlier phase III trials of the drug for acute attacks of hereditary angioedema, FAST-1 and FAST-2 (N. Engl. J. Med. 2010;363:532-41).
A "not approvable" letter from the Food and Drug Administration prompted Shire, the company developing icatibant, to provide more data to the FDA. The company’s response and results from FAST-3 are scheduled to be reviewed by the FDA in August 2011.
The FAST-3 study enrolled adults in 11 countries with moderate to very severe swelling from type I or type II hereditary angioedema that had been going on for no more than 12 hours and included at least one VAS score of 30 mm or greater. Patients were observed for 8 hours after injection, and their skin swelling, skin pain, abdominal pain, difficulty swallowing, and voice changes were assessed every 30 minutes for 4 hours after injection and at 5, 6, 8, and 12 hours. Rescue therapy was permitted but withheld if possible for 8-9 hours after injection.
Only the cutaneous and abdominal attacks were considered in the primary results, said Dr. Lumry, medical director of the Allergy and Asthma Research Associates Research Center, Dallas.
The 10 patients with laryngeal swelling attacks provided additional data. Five patients with severe laryngeal attacks immediately received open-label icatibant and reported onset of relief of symptoms a median of 2.3 hours later. Among five patients with mild to moderate laryngeal attacks, three patients who were randomized to icatibant treatment reported onset of symptom relief a median of 2.5 hours after treatment.
The two patients with mild to moderate attacks who were randomized to placebo both ended up getting icatibant, one whose attack was severe enough to warrant treatment, and the other a patient who received icatibant as rescue therapy 3.4 hours after randomization. Combined, the two patients with mild to moderate laryngeal attacks who were randomized to placebo reported onset of symptom relief a median of 3.2 hours after treatment.
Secondary outcomes in the nonlaryngeal cohort were significantly improved in the icatibant group, compared with the placebo group, Dr. Lumry said. The median time to initial symptom relief (assessed by the patients and the investigators) was less than 1 hour in the icatibant group and about 3.5 hours in the placebo group. The median time to onset of relief of the primary symptom (skin pain, skin swelling, or abdominal pain) was 1.5 hours in the icatibant group and 18.5 hours in the placebo group. The median time to almost complete symptom relief was less than 1 hour with icatibant and 36 hours with placebo.
Safety measures were recorded daily for 5 days and at a final 14-day safety visit.
Rescue medications were needed before the onset of symptom relief by 17 patients randomized to placebo – 16 in the nonlaryngeal cohort and 1 in the laryngeal cohort – compared with none in the icatibant groups. In the nonlaryngeal cohort, 3 patients in the icatibant group and 18 in the placebo group used rescue medications at some time during the attack or within 5 days of the injection.
All patients who got icatibant developed injection site reactions that resolved within 4 hours. Of the patients who initially received placebo, 10 developed at least one severe adverse event, compared with 2 patients who were randomized to icatibant and none who received open-label icatibant, a significant difference between the drug and placebo groups. One patient in the placebo group died of a myocardial infarction around 10 days later.
Because many of the adverse events that were most commonly reported are associated with hereditary angioedema, "we could not separate whether this was due to drug effect or due to the attack," Dr. Lumry said.
The mean age of patients in the study’s different arms ranged from 36 to 50 years. Most of the patients were women, and most were white.
The study was funded by Shire, which is developing icatibant. Dr. Lumry has been a consultant or speaker for, or received research grants from, Shire, CSL Behring, Dyax, Pharming, and ViroPharma.
SAN FRANCISCO – The experimental drug icatibant relieved moderate or severe acute attacks of hereditary angioedema faster than placebo in the third randomized, double-blind, controlled trial of the experimental drug in 98 patients.
Among 88 patients with cutaneous and/or abdominal acute attacks, the 43 patients randomized to treatment with a single subcutaneous injection of 30 mg of icatibant reported that symptom relief started a median of 2 hours after treatment, compared with 20 hours for the 45 patients randomized to saline placebo injection. Symptom relief was defined as at least a 50% decrease in the combined Visual Analog Scale (VAS) symptom score, compared with baseline.
"I think this represents a very exciting, important advance in the treatment of an underserved population," Dr. William R. Lumry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
A significant difference between groups was seen as early as 1 hour after treatment, but the 50% or greater reduction in VAS symptom scores was not achieved until 2 hours after treatment, Dr. Lumry and his associates reported.
The third For Angioedema Subcutaneous Treatment (FAST-3) trial followed two earlier phase III trials of the drug for acute attacks of hereditary angioedema, FAST-1 and FAST-2 (N. Engl. J. Med. 2010;363:532-41).
A "not approvable" letter from the Food and Drug Administration prompted Shire, the company developing icatibant, to provide more data to the FDA. The company’s response and results from FAST-3 are scheduled to be reviewed by the FDA in August 2011.
The FAST-3 study enrolled adults in 11 countries with moderate to very severe swelling from type I or type II hereditary angioedema that had been going on for no more than 12 hours and included at least one VAS score of 30 mm or greater. Patients were observed for 8 hours after injection, and their skin swelling, skin pain, abdominal pain, difficulty swallowing, and voice changes were assessed every 30 minutes for 4 hours after injection and at 5, 6, 8, and 12 hours. Rescue therapy was permitted but withheld if possible for 8-9 hours after injection.
Only the cutaneous and abdominal attacks were considered in the primary results, said Dr. Lumry, medical director of the Allergy and Asthma Research Associates Research Center, Dallas.
The 10 patients with laryngeal swelling attacks provided additional data. Five patients with severe laryngeal attacks immediately received open-label icatibant and reported onset of relief of symptoms a median of 2.3 hours later. Among five patients with mild to moderate laryngeal attacks, three patients who were randomized to icatibant treatment reported onset of symptom relief a median of 2.5 hours after treatment.
The two patients with mild to moderate attacks who were randomized to placebo both ended up getting icatibant, one whose attack was severe enough to warrant treatment, and the other a patient who received icatibant as rescue therapy 3.4 hours after randomization. Combined, the two patients with mild to moderate laryngeal attacks who were randomized to placebo reported onset of symptom relief a median of 3.2 hours after treatment.
Secondary outcomes in the nonlaryngeal cohort were significantly improved in the icatibant group, compared with the placebo group, Dr. Lumry said. The median time to initial symptom relief (assessed by the patients and the investigators) was less than 1 hour in the icatibant group and about 3.5 hours in the placebo group. The median time to onset of relief of the primary symptom (skin pain, skin swelling, or abdominal pain) was 1.5 hours in the icatibant group and 18.5 hours in the placebo group. The median time to almost complete symptom relief was less than 1 hour with icatibant and 36 hours with placebo.
Safety measures were recorded daily for 5 days and at a final 14-day safety visit.
Rescue medications were needed before the onset of symptom relief by 17 patients randomized to placebo – 16 in the nonlaryngeal cohort and 1 in the laryngeal cohort – compared with none in the icatibant groups. In the nonlaryngeal cohort, 3 patients in the icatibant group and 18 in the placebo group used rescue medications at some time during the attack or within 5 days of the injection.
All patients who got icatibant developed injection site reactions that resolved within 4 hours. Of the patients who initially received placebo, 10 developed at least one severe adverse event, compared with 2 patients who were randomized to icatibant and none who received open-label icatibant, a significant difference between the drug and placebo groups. One patient in the placebo group died of a myocardial infarction around 10 days later.
Because many of the adverse events that were most commonly reported are associated with hereditary angioedema, "we could not separate whether this was due to drug effect or due to the attack," Dr. Lumry said.
The mean age of patients in the study’s different arms ranged from 36 to 50 years. Most of the patients were women, and most were white.
The study was funded by Shire, which is developing icatibant. Dr. Lumry has been a consultant or speaker for, or received research grants from, Shire, CSL Behring, Dyax, Pharming, and ViroPharma.
SAN FRANCISCO – The experimental drug icatibant relieved moderate or severe acute attacks of hereditary angioedema faster than placebo in the third randomized, double-blind, controlled trial of the experimental drug in 98 patients.
Among 88 patients with cutaneous and/or abdominal acute attacks, the 43 patients randomized to treatment with a single subcutaneous injection of 30 mg of icatibant reported that symptom relief started a median of 2 hours after treatment, compared with 20 hours for the 45 patients randomized to saline placebo injection. Symptom relief was defined as at least a 50% decrease in the combined Visual Analog Scale (VAS) symptom score, compared with baseline.
"I think this represents a very exciting, important advance in the treatment of an underserved population," Dr. William R. Lumry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
A significant difference between groups was seen as early as 1 hour after treatment, but the 50% or greater reduction in VAS symptom scores was not achieved until 2 hours after treatment, Dr. Lumry and his associates reported.
The third For Angioedema Subcutaneous Treatment (FAST-3) trial followed two earlier phase III trials of the drug for acute attacks of hereditary angioedema, FAST-1 and FAST-2 (N. Engl. J. Med. 2010;363:532-41).
A "not approvable" letter from the Food and Drug Administration prompted Shire, the company developing icatibant, to provide more data to the FDA. The company’s response and results from FAST-3 are scheduled to be reviewed by the FDA in August 2011.
The FAST-3 study enrolled adults in 11 countries with moderate to very severe swelling from type I or type II hereditary angioedema that had been going on for no more than 12 hours and included at least one VAS score of 30 mm or greater. Patients were observed for 8 hours after injection, and their skin swelling, skin pain, abdominal pain, difficulty swallowing, and voice changes were assessed every 30 minutes for 4 hours after injection and at 5, 6, 8, and 12 hours. Rescue therapy was permitted but withheld if possible for 8-9 hours after injection.
Only the cutaneous and abdominal attacks were considered in the primary results, said Dr. Lumry, medical director of the Allergy and Asthma Research Associates Research Center, Dallas.
The 10 patients with laryngeal swelling attacks provided additional data. Five patients with severe laryngeal attacks immediately received open-label icatibant and reported onset of relief of symptoms a median of 2.3 hours later. Among five patients with mild to moderate laryngeal attacks, three patients who were randomized to icatibant treatment reported onset of symptom relief a median of 2.5 hours after treatment.
The two patients with mild to moderate attacks who were randomized to placebo both ended up getting icatibant, one whose attack was severe enough to warrant treatment, and the other a patient who received icatibant as rescue therapy 3.4 hours after randomization. Combined, the two patients with mild to moderate laryngeal attacks who were randomized to placebo reported onset of symptom relief a median of 3.2 hours after treatment.
Secondary outcomes in the nonlaryngeal cohort were significantly improved in the icatibant group, compared with the placebo group, Dr. Lumry said. The median time to initial symptom relief (assessed by the patients and the investigators) was less than 1 hour in the icatibant group and about 3.5 hours in the placebo group. The median time to onset of relief of the primary symptom (skin pain, skin swelling, or abdominal pain) was 1.5 hours in the icatibant group and 18.5 hours in the placebo group. The median time to almost complete symptom relief was less than 1 hour with icatibant and 36 hours with placebo.
Safety measures were recorded daily for 5 days and at a final 14-day safety visit.
Rescue medications were needed before the onset of symptom relief by 17 patients randomized to placebo – 16 in the nonlaryngeal cohort and 1 in the laryngeal cohort – compared with none in the icatibant groups. In the nonlaryngeal cohort, 3 patients in the icatibant group and 18 in the placebo group used rescue medications at some time during the attack or within 5 days of the injection.
All patients who got icatibant developed injection site reactions that resolved within 4 hours. Of the patients who initially received placebo, 10 developed at least one severe adverse event, compared with 2 patients who were randomized to icatibant and none who received open-label icatibant, a significant difference between the drug and placebo groups. One patient in the placebo group died of a myocardial infarction around 10 days later.
Because many of the adverse events that were most commonly reported are associated with hereditary angioedema, "we could not separate whether this was due to drug effect or due to the attack," Dr. Lumry said.
The mean age of patients in the study’s different arms ranged from 36 to 50 years. Most of the patients were women, and most were white.
The study was funded by Shire, which is developing icatibant. Dr. Lumry has been a consultant or speaker for, or received research grants from, Shire, CSL Behring, Dyax, Pharming, and ViroPharma.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF ALLERGY, ASTHMA, AND IMMUNOLOGY
Major Finding: Patients with acute attacks of hereditary angioedema reported onset of relief of symptoms a median of 2 hours after injection of the investigational drug icatibant or 20 hours after placebo injection.
Data Source: FAST-3, a phase III randomized, double-blind, placebo-controlled study of icatibant or placebo injections in 98 patients with acute attacks from type I or type II hereditary angioedema.
Disclosures: The study was funded by Shire, which is developing icatibant. Dr. Lumry has been a consultant or speaker for, or received research grants from, Shire, CSL Behring, Dyax, Pharming, and ViroPharma.
Study Finds Icatibant Relieves Acute Angioedema
SAN FRANCISCO – The experimental drug icatibant relieved moderate or severe acute attacks of hereditary angioedema faster than placebo in the third randomized, double-blind, controlled trial of the experimental drug in 98 patients.
Among 88 patients with cutaneous and/or abdominal acute attacks, the 43 patients randomized to treatment with a single subcutaneous injection of 30 mg of icatibant reported that symptom relief started a median of 2 hours after treatment, compared with 20 hours for the 45 patients randomized to saline placebo injection. Symptom relief was defined as at least a 50% decrease in the combined Visual Analog Scale (VAS) symptom score, compared with baseline.
"I think this represents a very exciting, important advance in the treatment of an underserved population," Dr. William R. Lumry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
A significant difference between groups was seen as early as 1 hour after treatment, but the 50% or greater reduction in VAS symptom scores was not achieved until 2 hours after treatment, Dr. Lumry and his associates reported.
The third For Angioedema Subcutaneous Treatment (FAST-3) trial followed two earlier phase III trials of the drug for acute attacks of hereditary angioedema, FAST-1 and FAST-2 (N. Engl. J. Med. 2010;363:532-41).
A "not approvable" letter from the Food and Drug Administration prompted Shire, the company developing icatibant, to provide more data to the FDA. The company’s response and results from FAST-3 are scheduled to be reviewed by the FDA in August 2011.
The FAST-3 study enrolled adults in 11 countries with moderate to very severe swelling from type I or type II hereditary angioedema that had been going on for no more than 12 hours and included at least one VAS score of 30 mm or greater. Patients were observed for 8 hours after injection, and their skin swelling, skin pain, abdominal pain, difficulty swallowing, and voice changes were assessed every 30 minutes for 4 hours after injection and at 5, 6, 8, and 12 hours. Rescue therapy was permitted but withheld if possible for 8-9 hours after injection.
Only the cutaneous and abdominal attacks were considered in the primary results, said Dr. Lumry, medical director of the Allergy and Asthma Research Associates Research Center, Dallas.
The 10 patients with laryngeal swelling attacks provided additional data. Five patients with severe laryngeal attacks immediately received open-label icatibant and reported onset of relief of symptoms a median of 2.3 hours later. Among five patients with mild to moderate laryngeal attacks, three patients who were randomized to icatibant treatment reported onset of symptom relief a median of 2.5 hours after treatment.
The two patients with mild to moderate attacks who were randomized to placebo both ended up getting icatibant, one whose attack was severe enough to warrant treatment, and the other a patient who received icatibant as rescue therapy 3.4 hours after randomization. Combined, the two patients with mild to moderate laryngeal attacks who were randomized to placebo reported onset of symptom relief a median of 3.2 hours after treatment.
Secondary outcomes in the nonlaryngeal cohort were significantly improved in the icatibant group, compared with the placebo group, Dr. Lumry said. The median time to initial symptom relief (assessed by the patients and the investigators) was less than 1 hour in the icatibant group and about 3.5 hours in the placebo group. The median time to onset of relief of the primary symptom (skin pain, skin swelling, or abdominal pain) was 1.5 hours in the icatibant group and 18.5 hours in the placebo group. The median time to almost complete symptom relief was less than 1 hour with icatibant and 36 hours with placebo.
Safety measures were recorded daily for 5 days and at a final 14-day safety visit.
Rescue medications were needed before the onset of symptom relief by 17 patients randomized to placebo – 16 in the nonlaryngeal cohort and 1 in the laryngeal cohort – compared with none in the icatibant groups. In the nonlaryngeal cohort, 3 patients in the icatibant group and 18 in the placebo group used rescue medications at some time during the attack or within 5 days of the injection.
All patients who got icatibant developed injection site reactions that resolved within 4 hours. Of the patients who initially received placebo, 10 developed at least one severe adverse event, compared with 2 patients who were randomized to icatibant and none who received open-label icatibant, a significant difference between the drug and placebo groups. One patient in the placebo group died of a myocardial infarction around 10 days later.
Because many of the adverse events that were most commonly reported are associated with hereditary angioedema, "we could not separate whether this was due to drug effect or due to the attack," Dr. Lumry said.
The mean age of patients in the study’s different arms ranged from 36 to 50 years. Most of the patients were women, and most were white.
The study was funded by Shire, which is developing icatibant. Dr. Lumry has been a consultant or speaker for, or received research grants from, Shire, CSL Behring, Dyax, Pharming, and ViroPharma.
SAN FRANCISCO – The experimental drug icatibant relieved moderate or severe acute attacks of hereditary angioedema faster than placebo in the third randomized, double-blind, controlled trial of the experimental drug in 98 patients.
Among 88 patients with cutaneous and/or abdominal acute attacks, the 43 patients randomized to treatment with a single subcutaneous injection of 30 mg of icatibant reported that symptom relief started a median of 2 hours after treatment, compared with 20 hours for the 45 patients randomized to saline placebo injection. Symptom relief was defined as at least a 50% decrease in the combined Visual Analog Scale (VAS) symptom score, compared with baseline.
"I think this represents a very exciting, important advance in the treatment of an underserved population," Dr. William R. Lumry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
A significant difference between groups was seen as early as 1 hour after treatment, but the 50% or greater reduction in VAS symptom scores was not achieved until 2 hours after treatment, Dr. Lumry and his associates reported.
The third For Angioedema Subcutaneous Treatment (FAST-3) trial followed two earlier phase III trials of the drug for acute attacks of hereditary angioedema, FAST-1 and FAST-2 (N. Engl. J. Med. 2010;363:532-41).
A "not approvable" letter from the Food and Drug Administration prompted Shire, the company developing icatibant, to provide more data to the FDA. The company’s response and results from FAST-3 are scheduled to be reviewed by the FDA in August 2011.
The FAST-3 study enrolled adults in 11 countries with moderate to very severe swelling from type I or type II hereditary angioedema that had been going on for no more than 12 hours and included at least one VAS score of 30 mm or greater. Patients were observed for 8 hours after injection, and their skin swelling, skin pain, abdominal pain, difficulty swallowing, and voice changes were assessed every 30 minutes for 4 hours after injection and at 5, 6, 8, and 12 hours. Rescue therapy was permitted but withheld if possible for 8-9 hours after injection.
Only the cutaneous and abdominal attacks were considered in the primary results, said Dr. Lumry, medical director of the Allergy and Asthma Research Associates Research Center, Dallas.
The 10 patients with laryngeal swelling attacks provided additional data. Five patients with severe laryngeal attacks immediately received open-label icatibant and reported onset of relief of symptoms a median of 2.3 hours later. Among five patients with mild to moderate laryngeal attacks, three patients who were randomized to icatibant treatment reported onset of symptom relief a median of 2.5 hours after treatment.
The two patients with mild to moderate attacks who were randomized to placebo both ended up getting icatibant, one whose attack was severe enough to warrant treatment, and the other a patient who received icatibant as rescue therapy 3.4 hours after randomization. Combined, the two patients with mild to moderate laryngeal attacks who were randomized to placebo reported onset of symptom relief a median of 3.2 hours after treatment.
Secondary outcomes in the nonlaryngeal cohort were significantly improved in the icatibant group, compared with the placebo group, Dr. Lumry said. The median time to initial symptom relief (assessed by the patients and the investigators) was less than 1 hour in the icatibant group and about 3.5 hours in the placebo group. The median time to onset of relief of the primary symptom (skin pain, skin swelling, or abdominal pain) was 1.5 hours in the icatibant group and 18.5 hours in the placebo group. The median time to almost complete symptom relief was less than 1 hour with icatibant and 36 hours with placebo.
Safety measures were recorded daily for 5 days and at a final 14-day safety visit.
Rescue medications were needed before the onset of symptom relief by 17 patients randomized to placebo – 16 in the nonlaryngeal cohort and 1 in the laryngeal cohort – compared with none in the icatibant groups. In the nonlaryngeal cohort, 3 patients in the icatibant group and 18 in the placebo group used rescue medications at some time during the attack or within 5 days of the injection.
All patients who got icatibant developed injection site reactions that resolved within 4 hours. Of the patients who initially received placebo, 10 developed at least one severe adverse event, compared with 2 patients who were randomized to icatibant and none who received open-label icatibant, a significant difference between the drug and placebo groups. One patient in the placebo group died of a myocardial infarction around 10 days later.
Because many of the adverse events that were most commonly reported are associated with hereditary angioedema, "we could not separate whether this was due to drug effect or due to the attack," Dr. Lumry said.
The mean age of patients in the study’s different arms ranged from 36 to 50 years. Most of the patients were women, and most were white.
The study was funded by Shire, which is developing icatibant. Dr. Lumry has been a consultant or speaker for, or received research grants from, Shire, CSL Behring, Dyax, Pharming, and ViroPharma.
SAN FRANCISCO – The experimental drug icatibant relieved moderate or severe acute attacks of hereditary angioedema faster than placebo in the third randomized, double-blind, controlled trial of the experimental drug in 98 patients.
Among 88 patients with cutaneous and/or abdominal acute attacks, the 43 patients randomized to treatment with a single subcutaneous injection of 30 mg of icatibant reported that symptom relief started a median of 2 hours after treatment, compared with 20 hours for the 45 patients randomized to saline placebo injection. Symptom relief was defined as at least a 50% decrease in the combined Visual Analog Scale (VAS) symptom score, compared with baseline.
"I think this represents a very exciting, important advance in the treatment of an underserved population," Dr. William R. Lumry said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
A significant difference between groups was seen as early as 1 hour after treatment, but the 50% or greater reduction in VAS symptom scores was not achieved until 2 hours after treatment, Dr. Lumry and his associates reported.
The third For Angioedema Subcutaneous Treatment (FAST-3) trial followed two earlier phase III trials of the drug for acute attacks of hereditary angioedema, FAST-1 and FAST-2 (N. Engl. J. Med. 2010;363:532-41).
A "not approvable" letter from the Food and Drug Administration prompted Shire, the company developing icatibant, to provide more data to the FDA. The company’s response and results from FAST-3 are scheduled to be reviewed by the FDA in August 2011.
The FAST-3 study enrolled adults in 11 countries with moderate to very severe swelling from type I or type II hereditary angioedema that had been going on for no more than 12 hours and included at least one VAS score of 30 mm or greater. Patients were observed for 8 hours after injection, and their skin swelling, skin pain, abdominal pain, difficulty swallowing, and voice changes were assessed every 30 minutes for 4 hours after injection and at 5, 6, 8, and 12 hours. Rescue therapy was permitted but withheld if possible for 8-9 hours after injection.
Only the cutaneous and abdominal attacks were considered in the primary results, said Dr. Lumry, medical director of the Allergy and Asthma Research Associates Research Center, Dallas.
The 10 patients with laryngeal swelling attacks provided additional data. Five patients with severe laryngeal attacks immediately received open-label icatibant and reported onset of relief of symptoms a median of 2.3 hours later. Among five patients with mild to moderate laryngeal attacks, three patients who were randomized to icatibant treatment reported onset of symptom relief a median of 2.5 hours after treatment.
The two patients with mild to moderate attacks who were randomized to placebo both ended up getting icatibant, one whose attack was severe enough to warrant treatment, and the other a patient who received icatibant as rescue therapy 3.4 hours after randomization. Combined, the two patients with mild to moderate laryngeal attacks who were randomized to placebo reported onset of symptom relief a median of 3.2 hours after treatment.
Secondary outcomes in the nonlaryngeal cohort were significantly improved in the icatibant group, compared with the placebo group, Dr. Lumry said. The median time to initial symptom relief (assessed by the patients and the investigators) was less than 1 hour in the icatibant group and about 3.5 hours in the placebo group. The median time to onset of relief of the primary symptom (skin pain, skin swelling, or abdominal pain) was 1.5 hours in the icatibant group and 18.5 hours in the placebo group. The median time to almost complete symptom relief was less than 1 hour with icatibant and 36 hours with placebo.
Safety measures were recorded daily for 5 days and at a final 14-day safety visit.
Rescue medications were needed before the onset of symptom relief by 17 patients randomized to placebo – 16 in the nonlaryngeal cohort and 1 in the laryngeal cohort – compared with none in the icatibant groups. In the nonlaryngeal cohort, 3 patients in the icatibant group and 18 in the placebo group used rescue medications at some time during the attack or within 5 days of the injection.
All patients who got icatibant developed injection site reactions that resolved within 4 hours. Of the patients who initially received placebo, 10 developed at least one severe adverse event, compared with 2 patients who were randomized to icatibant and none who received open-label icatibant, a significant difference between the drug and placebo groups. One patient in the placebo group died of a myocardial infarction around 10 days later.
Because many of the adverse events that were most commonly reported are associated with hereditary angioedema, "we could not separate whether this was due to drug effect or due to the attack," Dr. Lumry said.
The mean age of patients in the study’s different arms ranged from 36 to 50 years. Most of the patients were women, and most were white.
The study was funded by Shire, which is developing icatibant. Dr. Lumry has been a consultant or speaker for, or received research grants from, Shire, CSL Behring, Dyax, Pharming, and ViroPharma.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF ALLERGY, ASTHMA, AND IMMUNOLOGY
Major Finding: Patients with acute attacks of hereditary angioedema reported onset of relief of symptoms a median of 2 hours after injection of the investigational drug icatibant or 20 hours after placebo injection.
Data Source: FAST-3, a phase III randomized, double-blind, placebo-controlled study of icatibant or placebo injections in 98 patients with acute attacks from type I or type II hereditary angioedema.
Disclosures: The study was funded by Shire, which is developing icatibant. Dr. Lumry has been a consultant or speaker for, or received research grants from, Shire, CSL Behring, Dyax, Pharming, and ViroPharma.
Imaging Informs Prognosis After Intracerebral Hemorrhage
LOS ANGELES – MRI results showing brain lesions on diffusion-weighted imaging after primary intracerebral hemorrhage predicted a more than sixfold increase in risk of poor function 1 year later in a prospective, longitudinal study of 62 patients.
A combination of diffusion-weighted imaging (DWI) MRI results, glucose levels at baseline, and scores on the National Institutes of Health Stroke Scale (NIHSS) provided 86% accuracy in predicting good or poor outcome 1 year later, Dr. Richard E. Burgess said at the International Stroke Conference sponsored by the American Heart Association.
Recent studies have shown that remote ischemic lesions in patients with intracerebral hemorrhage are more common than previously thought. In the study by Dr. Burgess and his associates, 29% of patients had lesions on DWI when scanned a median of 2 days after primary intracerebral hemorrhage.
That rate is "in accord with other studies," said Dr. Burgess of Georgetown University, Washington, D.C. One recent study reported lesions on DWI in up to 30% of patients after intracerebral hemorrhage. A separate study found an association between these lesions and intracerebral hemorrhage related to cerebral amyloid angiopathy.
Patients in the current study had a median of two lesions each; 68% were deep lesions and 32% were lobar. The lesions were ipsilateral in 50% of patients, contralateral in 17%, and bilateral in 33%.
The location of the intracerebral hemorrhage was deep in 68% of patients, and the mean hemorrhage volume was 27 cc.
In the study, called the Differences in the Imaging of Primary Hemorrhage Based on Ethnicity or Race (DECIPHER) study, all patients underwent a history, a neurologic examination, multiple MRI sequences, and a battery of predictive outcome scales at baseline. The MRI exam was repeated at 30 days, 1 year, and 3 years, and the outcome scales assessments were repeated at these time points and at 2 and 4 years.
The investigators assessed outcomes at 1 year using a modified Rankin Scale and designated patients as having either a good outcome (scoring 0-3 on the scale) or a poor outcome (scores of 4-6).
The study participants had a mean age of 62 years and a median NIHSS score of 5 at baseline; 52% were women, 66% were black, and 84% had hypertension.
Three factors at baseline significantly predicted poor outcome at 1 year in a multivariate analysis, Dr. Burgess said. The presence of DWI lesions increased the risk for a poor outcome 6.6-fold, compared with no lesions on DWI. A higher NIHSS score increased the risk for a poor outcome by 15%. A higher glucose level increased the risk by 2%. Combined with older age, which was not a significant factor by itself, the combined factors correctly predicted 1-year outcome in 86% of patients.
Overall, among 43 patients who were predicted to have a good outcome at 1 year, 40 did and 3 had a poor outcome, for 93% accuracy in predictions. Among 19 patients who were predicted to have a poor outcome, 13 did and 6 had a good outcome, for a 68% accuracy rate, he said.
In univariate analyses, the mean NIHSS score at baseline was significantly different between patients with good outcomes (score of 5) and bad outcomes (score of 14). Baseline glucose scores also differed significantly, measuring 113 mg/dL in the good-outcome group and 149 mg/dL in the poor-outcome group. Age also differed significantly in a univariate analysis, with a mean age of 60 years in patients with good outcomes and 67 years in those with poor outcomes.
"History, neurologic exam, laboratory data, and imaging findings – all have a role to play in predicting outcomes in patients," Dr. Burgess said. A larger study is needed to confirm the usefulness of acute MRI in this role and to assess other factors, including intracerebral hemorrhage volume and Glasgow Coma Scale score, that this small study could not properly analyze.
The MRI protocol in the study included DWI with apparent diffusion coefficient and fluid-attenuated inversion recovery sequences.
Dr. Burgess said he had no relevant financial disclosures.
LOS ANGELES – MRI results showing brain lesions on diffusion-weighted imaging after primary intracerebral hemorrhage predicted a more than sixfold increase in risk of poor function 1 year later in a prospective, longitudinal study of 62 patients.
A combination of diffusion-weighted imaging (DWI) MRI results, glucose levels at baseline, and scores on the National Institutes of Health Stroke Scale (NIHSS) provided 86% accuracy in predicting good or poor outcome 1 year later, Dr. Richard E. Burgess said at the International Stroke Conference sponsored by the American Heart Association.
Recent studies have shown that remote ischemic lesions in patients with intracerebral hemorrhage are more common than previously thought. In the study by Dr. Burgess and his associates, 29% of patients had lesions on DWI when scanned a median of 2 days after primary intracerebral hemorrhage.
That rate is "in accord with other studies," said Dr. Burgess of Georgetown University, Washington, D.C. One recent study reported lesions on DWI in up to 30% of patients after intracerebral hemorrhage. A separate study found an association between these lesions and intracerebral hemorrhage related to cerebral amyloid angiopathy.
Patients in the current study had a median of two lesions each; 68% were deep lesions and 32% were lobar. The lesions were ipsilateral in 50% of patients, contralateral in 17%, and bilateral in 33%.
The location of the intracerebral hemorrhage was deep in 68% of patients, and the mean hemorrhage volume was 27 cc.
In the study, called the Differences in the Imaging of Primary Hemorrhage Based on Ethnicity or Race (DECIPHER) study, all patients underwent a history, a neurologic examination, multiple MRI sequences, and a battery of predictive outcome scales at baseline. The MRI exam was repeated at 30 days, 1 year, and 3 years, and the outcome scales assessments were repeated at these time points and at 2 and 4 years.
The investigators assessed outcomes at 1 year using a modified Rankin Scale and designated patients as having either a good outcome (scoring 0-3 on the scale) or a poor outcome (scores of 4-6).
The study participants had a mean age of 62 years and a median NIHSS score of 5 at baseline; 52% were women, 66% were black, and 84% had hypertension.
Three factors at baseline significantly predicted poor outcome at 1 year in a multivariate analysis, Dr. Burgess said. The presence of DWI lesions increased the risk for a poor outcome 6.6-fold, compared with no lesions on DWI. A higher NIHSS score increased the risk for a poor outcome by 15%. A higher glucose level increased the risk by 2%. Combined with older age, which was not a significant factor by itself, the combined factors correctly predicted 1-year outcome in 86% of patients.
Overall, among 43 patients who were predicted to have a good outcome at 1 year, 40 did and 3 had a poor outcome, for 93% accuracy in predictions. Among 19 patients who were predicted to have a poor outcome, 13 did and 6 had a good outcome, for a 68% accuracy rate, he said.
In univariate analyses, the mean NIHSS score at baseline was significantly different between patients with good outcomes (score of 5) and bad outcomes (score of 14). Baseline glucose scores also differed significantly, measuring 113 mg/dL in the good-outcome group and 149 mg/dL in the poor-outcome group. Age also differed significantly in a univariate analysis, with a mean age of 60 years in patients with good outcomes and 67 years in those with poor outcomes.
"History, neurologic exam, laboratory data, and imaging findings – all have a role to play in predicting outcomes in patients," Dr. Burgess said. A larger study is needed to confirm the usefulness of acute MRI in this role and to assess other factors, including intracerebral hemorrhage volume and Glasgow Coma Scale score, that this small study could not properly analyze.
The MRI protocol in the study included DWI with apparent diffusion coefficient and fluid-attenuated inversion recovery sequences.
Dr. Burgess said he had no relevant financial disclosures.
LOS ANGELES – MRI results showing brain lesions on diffusion-weighted imaging after primary intracerebral hemorrhage predicted a more than sixfold increase in risk of poor function 1 year later in a prospective, longitudinal study of 62 patients.
A combination of diffusion-weighted imaging (DWI) MRI results, glucose levels at baseline, and scores on the National Institutes of Health Stroke Scale (NIHSS) provided 86% accuracy in predicting good or poor outcome 1 year later, Dr. Richard E. Burgess said at the International Stroke Conference sponsored by the American Heart Association.
Recent studies have shown that remote ischemic lesions in patients with intracerebral hemorrhage are more common than previously thought. In the study by Dr. Burgess and his associates, 29% of patients had lesions on DWI when scanned a median of 2 days after primary intracerebral hemorrhage.
That rate is "in accord with other studies," said Dr. Burgess of Georgetown University, Washington, D.C. One recent study reported lesions on DWI in up to 30% of patients after intracerebral hemorrhage. A separate study found an association between these lesions and intracerebral hemorrhage related to cerebral amyloid angiopathy.
Patients in the current study had a median of two lesions each; 68% were deep lesions and 32% were lobar. The lesions were ipsilateral in 50% of patients, contralateral in 17%, and bilateral in 33%.
The location of the intracerebral hemorrhage was deep in 68% of patients, and the mean hemorrhage volume was 27 cc.
In the study, called the Differences in the Imaging of Primary Hemorrhage Based on Ethnicity or Race (DECIPHER) study, all patients underwent a history, a neurologic examination, multiple MRI sequences, and a battery of predictive outcome scales at baseline. The MRI exam was repeated at 30 days, 1 year, and 3 years, and the outcome scales assessments were repeated at these time points and at 2 and 4 years.
The investigators assessed outcomes at 1 year using a modified Rankin Scale and designated patients as having either a good outcome (scoring 0-3 on the scale) or a poor outcome (scores of 4-6).
The study participants had a mean age of 62 years and a median NIHSS score of 5 at baseline; 52% were women, 66% were black, and 84% had hypertension.
Three factors at baseline significantly predicted poor outcome at 1 year in a multivariate analysis, Dr. Burgess said. The presence of DWI lesions increased the risk for a poor outcome 6.6-fold, compared with no lesions on DWI. A higher NIHSS score increased the risk for a poor outcome by 15%. A higher glucose level increased the risk by 2%. Combined with older age, which was not a significant factor by itself, the combined factors correctly predicted 1-year outcome in 86% of patients.
Overall, among 43 patients who were predicted to have a good outcome at 1 year, 40 did and 3 had a poor outcome, for 93% accuracy in predictions. Among 19 patients who were predicted to have a poor outcome, 13 did and 6 had a good outcome, for a 68% accuracy rate, he said.
In univariate analyses, the mean NIHSS score at baseline was significantly different between patients with good outcomes (score of 5) and bad outcomes (score of 14). Baseline glucose scores also differed significantly, measuring 113 mg/dL in the good-outcome group and 149 mg/dL in the poor-outcome group. Age also differed significantly in a univariate analysis, with a mean age of 60 years in patients with good outcomes and 67 years in those with poor outcomes.
"History, neurologic exam, laboratory data, and imaging findings – all have a role to play in predicting outcomes in patients," Dr. Burgess said. A larger study is needed to confirm the usefulness of acute MRI in this role and to assess other factors, including intracerebral hemorrhage volume and Glasgow Coma Scale score, that this small study could not properly analyze.
The MRI protocol in the study included DWI with apparent diffusion coefficient and fluid-attenuated inversion recovery sequences.
Dr. Burgess said he had no relevant financial disclosures.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: The presence of lesions on DWI MRI after intracerebral hemorrhage was a significant predictor of poor outcome 1 year later.
Data Source: Prospective, longitudinal study of 62 adults with primary intracerebral hemorrhage.
Disclosures: Dr. Burgess said he had no relevant financial disclosures.
Clinical Trial Validates First Pediatric Stroke Severity Scale
Major Finding: A pediatric version of the NIH Stroke Scale demonstrated excellent interrater reliability in a prospective validation study.
Data Source: Pediatric neurologists at 15 North American medical centers tested the PedNIHSS by using it to examine 113 children with acute arterial ischemic stroke, with interrater reliability tested in 25 patients.
Disclosures: Dr. Ichord and one of her associates in the study are on the clinical event committee for the Berlin Heart Trial for pediatric ventricular assist devices.
LOS ANGELES – For the first time, a pediatric stroke severity scale has been validated in a prospective clinical trial.
The study in 15 North American medical centers showed excellent interrater reliability when neurologists used a pediatric version of the National Institutes of Health Stroke Scale for adults to examine children aged 2-18 years with acute arterial ischemic stroke.
The neurologists used the Pediatric NIH Stroke Scale (PedNIHSS) on 113 patients who were examined daily from admission to discharge, or day 7 of hospitalization.
Interrater reliability was tested in a subset of 25 patients who underwent simultaneous examinations by two pediatric neurologists. Characteristics of the subgroup were similar to those of the entire cohort, Dr. Rebecca N. Ichord reported at the conference.
The simultaneous raters' scores were identical in 60% of ratings and were within a 1-point difference in 84% of ratings (Stroke 2011;42:613-7).
Research into potential ways of preventing or treating childhood stroke has been stymied in the past by the lack of a validated and reliable pediatric stroke scale. The PedNIHSS provides a way to index the severity of a child's stroke, to make comparisons across treatment groups, and to get a baseline for predicting outcome, said Dr. Ichord, director of the pediatric stroke program at the Children's Hospital of Philadelphia.
Clinicians, too, have wanted such a scale. “I have been asked over and over again [for a pediatric stroke scale] by clinicians who want to have a method of describing the severity of a child's stroke,” Dr. Ichord said at the meeting, which was sponsored by the American Heart Association. “It helps them with clinical decision making. It helps them to prepare parents and counsel parents.”
Characteristics of the patients and the strokes in the study were similar to those reported in previous pediatric stroke cohort studies, which suggests the current findings are generalizable and the PedNIHSS should be applicable in other settings. Because all of the raters in the current study were pediatric neurologists or trainees, a separate study is needed to assess the PedNIHSS in the hands of other specialists, she said.
The pediatric version also found good interrater reliability for facial weakness, dysarthria, and ataxia, which was not seen with the adult stroke scale. The reasons for this difference are unclear.
The use of the pediatric scale is limited to 2-18 years because younger children have limited language abilities, which are needed for use of the PedNIHSS. Neonates and children younger than 2 years of age with acute ischemic stroke may require a scale with less emphasis on focal sensorimotor deficits, the investigators suggested.
A video interview with Dr. Ichord about the scale can be viewed by using the QR code, or by visiting www.clinicalneurologynews.com
The PedNIHSS helps clinicians with clinical decision making and helps them to prepare and counsel parents.
Source DR. ICHORD
Major Finding: A pediatric version of the NIH Stroke Scale demonstrated excellent interrater reliability in a prospective validation study.
Data Source: Pediatric neurologists at 15 North American medical centers tested the PedNIHSS by using it to examine 113 children with acute arterial ischemic stroke, with interrater reliability tested in 25 patients.
Disclosures: Dr. Ichord and one of her associates in the study are on the clinical event committee for the Berlin Heart Trial for pediatric ventricular assist devices.
LOS ANGELES – For the first time, a pediatric stroke severity scale has been validated in a prospective clinical trial.
The study in 15 North American medical centers showed excellent interrater reliability when neurologists used a pediatric version of the National Institutes of Health Stroke Scale for adults to examine children aged 2-18 years with acute arterial ischemic stroke.
The neurologists used the Pediatric NIH Stroke Scale (PedNIHSS) on 113 patients who were examined daily from admission to discharge, or day 7 of hospitalization.
Interrater reliability was tested in a subset of 25 patients who underwent simultaneous examinations by two pediatric neurologists. Characteristics of the subgroup were similar to those of the entire cohort, Dr. Rebecca N. Ichord reported at the conference.
The simultaneous raters' scores were identical in 60% of ratings and were within a 1-point difference in 84% of ratings (Stroke 2011;42:613-7).
Research into potential ways of preventing or treating childhood stroke has been stymied in the past by the lack of a validated and reliable pediatric stroke scale. The PedNIHSS provides a way to index the severity of a child's stroke, to make comparisons across treatment groups, and to get a baseline for predicting outcome, said Dr. Ichord, director of the pediatric stroke program at the Children's Hospital of Philadelphia.
Clinicians, too, have wanted such a scale. “I have been asked over and over again [for a pediatric stroke scale] by clinicians who want to have a method of describing the severity of a child's stroke,” Dr. Ichord said at the meeting, which was sponsored by the American Heart Association. “It helps them with clinical decision making. It helps them to prepare parents and counsel parents.”
Characteristics of the patients and the strokes in the study were similar to those reported in previous pediatric stroke cohort studies, which suggests the current findings are generalizable and the PedNIHSS should be applicable in other settings. Because all of the raters in the current study were pediatric neurologists or trainees, a separate study is needed to assess the PedNIHSS in the hands of other specialists, she said.
The pediatric version also found good interrater reliability for facial weakness, dysarthria, and ataxia, which was not seen with the adult stroke scale. The reasons for this difference are unclear.
The use of the pediatric scale is limited to 2-18 years because younger children have limited language abilities, which are needed for use of the PedNIHSS. Neonates and children younger than 2 years of age with acute ischemic stroke may require a scale with less emphasis on focal sensorimotor deficits, the investigators suggested.
A video interview with Dr. Ichord about the scale can be viewed by using the QR code, or by visiting www.clinicalneurologynews.com
The PedNIHSS helps clinicians with clinical decision making and helps them to prepare and counsel parents.
Source DR. ICHORD
Major Finding: A pediatric version of the NIH Stroke Scale demonstrated excellent interrater reliability in a prospective validation study.
Data Source: Pediatric neurologists at 15 North American medical centers tested the PedNIHSS by using it to examine 113 children with acute arterial ischemic stroke, with interrater reliability tested in 25 patients.
Disclosures: Dr. Ichord and one of her associates in the study are on the clinical event committee for the Berlin Heart Trial for pediatric ventricular assist devices.
LOS ANGELES – For the first time, a pediatric stroke severity scale has been validated in a prospective clinical trial.
The study in 15 North American medical centers showed excellent interrater reliability when neurologists used a pediatric version of the National Institutes of Health Stroke Scale for adults to examine children aged 2-18 years with acute arterial ischemic stroke.
The neurologists used the Pediatric NIH Stroke Scale (PedNIHSS) on 113 patients who were examined daily from admission to discharge, or day 7 of hospitalization.
Interrater reliability was tested in a subset of 25 patients who underwent simultaneous examinations by two pediatric neurologists. Characteristics of the subgroup were similar to those of the entire cohort, Dr. Rebecca N. Ichord reported at the conference.
The simultaneous raters' scores were identical in 60% of ratings and were within a 1-point difference in 84% of ratings (Stroke 2011;42:613-7).
Research into potential ways of preventing or treating childhood stroke has been stymied in the past by the lack of a validated and reliable pediatric stroke scale. The PedNIHSS provides a way to index the severity of a child's stroke, to make comparisons across treatment groups, and to get a baseline for predicting outcome, said Dr. Ichord, director of the pediatric stroke program at the Children's Hospital of Philadelphia.
Clinicians, too, have wanted such a scale. “I have been asked over and over again [for a pediatric stroke scale] by clinicians who want to have a method of describing the severity of a child's stroke,” Dr. Ichord said at the meeting, which was sponsored by the American Heart Association. “It helps them with clinical decision making. It helps them to prepare parents and counsel parents.”
Characteristics of the patients and the strokes in the study were similar to those reported in previous pediatric stroke cohort studies, which suggests the current findings are generalizable and the PedNIHSS should be applicable in other settings. Because all of the raters in the current study were pediatric neurologists or trainees, a separate study is needed to assess the PedNIHSS in the hands of other specialists, she said.
The pediatric version also found good interrater reliability for facial weakness, dysarthria, and ataxia, which was not seen with the adult stroke scale. The reasons for this difference are unclear.
The use of the pediatric scale is limited to 2-18 years because younger children have limited language abilities, which are needed for use of the PedNIHSS. Neonates and children younger than 2 years of age with acute ischemic stroke may require a scale with less emphasis on focal sensorimotor deficits, the investigators suggested.
A video interview with Dr. Ichord about the scale can be viewed by using the QR code, or by visiting www.clinicalneurologynews.com
The PedNIHSS helps clinicians with clinical decision making and helps them to prepare and counsel parents.
Source DR. ICHORD
Silent Strokes Common in Acute Anemic Events
Major Finding: Diffusion-weighted MRI in children hospitalized with acute anemic events showed evidence of acute silent infarction in 4 of 22 patients with sickle cell disease (18%) and in 2 of 30 patients without sickle cell disease (7%).
Data Source: Prospective, controlled study of 52 children.
Disclosures: Dr. Dowling said he had no relevant conflicts of interest. He has received research support from First American Real Estate Services Inc. The Children's Medical Center, Dallas, funded the study.
LOS ANGELES – Acute anemic events were associated with significantly increased risk for “silent” strokes not only in children with sickle cell disease but also in children with acute anemia due to other causes, a controlled study of 52 patients found.
Prospective screening of all children who were admitted to one hospital during a 30-month period found that 398 were having acute anemic events – accounting for nearly 1% of all admissions and 12% of children admitted with sickle cell disease.
Diffusion-weighted MRI in 52 of these children showed evidence of acute silent infarction in 4 (18%) of 22 patients with sickle cell disease and in 2 (7%) of 30 patients without sickle cell disease, Dr. Michael M. Dowling and his associates reported at the conference.
“I didn't initially plan a controlled study,” but funders insisted, and the comparison yielded unexpected information, said Dr. Dowling, a pediatric neurologist at the University of Texas Southwestern Medical Center, Dallas.
“I didn't think these children [without sickle cell disease] would be having silent infarctions.”
In addition, three patients with sickle cell disease showed evidence of what looked like remote silent infarctions on MRI (14%). Dr. Dowling said he was “fascinated” to also find evidence of remote silent infarctions in seven of the control patients (23%).
“These are not children with sickle cell disease. I'm worried that a lot of these children with acute anemic events were prone to recurrent anemic events from dysfunctional uterine bleeding, cancer, or repeat GI bleeds. I'm worried that at previous admissions they may have had an acute silent infarction in the setting of an acute anemic event that was missed,” Dr. Dowling said at the conference, which was sponsored by the American Heart Association.
Overall, 31% of all MRIs were abnormal, including acute and remote silent infarctions.
“I think we can't simply look at a severely anemic child in an emergency room and say he's hemodynamically stable, because I think these kids are losing brain cells. We need to respond to them more quickly,” he said.
He hopes that a future study will monitor these kinds of children at home via a pulse oximeter so that when their hemoglobin level falls, they can be brought in sooner for transfusion. “I think we can prevent injury,” he said.
Previous studies suggest that approximately 10% of children with sickle cell disease will have a clinically evident stroke unless they get prophylactic therapy, and up to 35% will have silent infarctions.
“It's a bad term because they're not silent,” Dr. Dowling said. “They cause significant cognitive impairment,” educational delays, and increased risk for new or progressive silent infarctions and overt stroke.
His working definition of silent infarction is an MRI lesion consistent with infarction without a focal deficit lasting more than 24 hours. The study defined an acute anemic event as either a hemoglobin level no higher than 5.5 g/dL or, for children with sickle cell anemia whose “normal” hemoglobin level may be 6 g/dL, a decline of at least 30% from baseline hemoglobin level.
Follow-up imaging in four patients with acute silent infarctions showed signals consistent with permanent brain injury in three of them, but not in the fourth. “Some of these might be reversible,” Dr. Dowling said.
He estimated that the incidence of acute silent infarction during acute anemic events is 633 per 100 patient-years in children with sickle cell disease, and 243 per 100 patient-years in children without sickle cell disease, based on the study's findings. His estimates are much larger than are those from the SIT (Silent Infarct Transfusion) study, which suggested an incidence of 43 per 100 patient-years in children with sickle cell disease as a whole, he said.
“We have identified unsuspected silent infarctions occurring during acute anemic events in 18% of children with sickle cell disease and 7% of controls. I think these events may be telling us” that children with acute anemia may need closer observation, he said.
The etiologies of acute anemia were a bit surprising in both the sickle cell and control groups. There were more oncology patients than anticipated among the 294 children who were admitted without sickle cell disease. Aplastic or hemolytic anemias were the leading etiology. Others included gastrointestinal bleed or liver problems, leukemias, lymphomas, and other cancers. Among patients with sickle cell disease and acute anemia, fewer than expected had acute chest syndrome, aplastic crisis, or splenic sequestration, although these still accounted for the majority. Some simply had infection, gastrointestinal bleed, or other problems.
Acute anemic events accounted for nearly 1% of all admissions and 12% of children admitted with sickle cell.
Source DR. DOWLING
Major Finding: Diffusion-weighted MRI in children hospitalized with acute anemic events showed evidence of acute silent infarction in 4 of 22 patients with sickle cell disease (18%) and in 2 of 30 patients without sickle cell disease (7%).
Data Source: Prospective, controlled study of 52 children.
Disclosures: Dr. Dowling said he had no relevant conflicts of interest. He has received research support from First American Real Estate Services Inc. The Children's Medical Center, Dallas, funded the study.
LOS ANGELES – Acute anemic events were associated with significantly increased risk for “silent” strokes not only in children with sickle cell disease but also in children with acute anemia due to other causes, a controlled study of 52 patients found.
Prospective screening of all children who were admitted to one hospital during a 30-month period found that 398 were having acute anemic events – accounting for nearly 1% of all admissions and 12% of children admitted with sickle cell disease.
Diffusion-weighted MRI in 52 of these children showed evidence of acute silent infarction in 4 (18%) of 22 patients with sickle cell disease and in 2 (7%) of 30 patients without sickle cell disease, Dr. Michael M. Dowling and his associates reported at the conference.
“I didn't initially plan a controlled study,” but funders insisted, and the comparison yielded unexpected information, said Dr. Dowling, a pediatric neurologist at the University of Texas Southwestern Medical Center, Dallas.
“I didn't think these children [without sickle cell disease] would be having silent infarctions.”
In addition, three patients with sickle cell disease showed evidence of what looked like remote silent infarctions on MRI (14%). Dr. Dowling said he was “fascinated” to also find evidence of remote silent infarctions in seven of the control patients (23%).
“These are not children with sickle cell disease. I'm worried that a lot of these children with acute anemic events were prone to recurrent anemic events from dysfunctional uterine bleeding, cancer, or repeat GI bleeds. I'm worried that at previous admissions they may have had an acute silent infarction in the setting of an acute anemic event that was missed,” Dr. Dowling said at the conference, which was sponsored by the American Heart Association.
Overall, 31% of all MRIs were abnormal, including acute and remote silent infarctions.
“I think we can't simply look at a severely anemic child in an emergency room and say he's hemodynamically stable, because I think these kids are losing brain cells. We need to respond to them more quickly,” he said.
He hopes that a future study will monitor these kinds of children at home via a pulse oximeter so that when their hemoglobin level falls, they can be brought in sooner for transfusion. “I think we can prevent injury,” he said.
Previous studies suggest that approximately 10% of children with sickle cell disease will have a clinically evident stroke unless they get prophylactic therapy, and up to 35% will have silent infarctions.
“It's a bad term because they're not silent,” Dr. Dowling said. “They cause significant cognitive impairment,” educational delays, and increased risk for new or progressive silent infarctions and overt stroke.
His working definition of silent infarction is an MRI lesion consistent with infarction without a focal deficit lasting more than 24 hours. The study defined an acute anemic event as either a hemoglobin level no higher than 5.5 g/dL or, for children with sickle cell anemia whose “normal” hemoglobin level may be 6 g/dL, a decline of at least 30% from baseline hemoglobin level.
Follow-up imaging in four patients with acute silent infarctions showed signals consistent with permanent brain injury in three of them, but not in the fourth. “Some of these might be reversible,” Dr. Dowling said.
He estimated that the incidence of acute silent infarction during acute anemic events is 633 per 100 patient-years in children with sickle cell disease, and 243 per 100 patient-years in children without sickle cell disease, based on the study's findings. His estimates are much larger than are those from the SIT (Silent Infarct Transfusion) study, which suggested an incidence of 43 per 100 patient-years in children with sickle cell disease as a whole, he said.
“We have identified unsuspected silent infarctions occurring during acute anemic events in 18% of children with sickle cell disease and 7% of controls. I think these events may be telling us” that children with acute anemia may need closer observation, he said.
The etiologies of acute anemia were a bit surprising in both the sickle cell and control groups. There were more oncology patients than anticipated among the 294 children who were admitted without sickle cell disease. Aplastic or hemolytic anemias were the leading etiology. Others included gastrointestinal bleed or liver problems, leukemias, lymphomas, and other cancers. Among patients with sickle cell disease and acute anemia, fewer than expected had acute chest syndrome, aplastic crisis, or splenic sequestration, although these still accounted for the majority. Some simply had infection, gastrointestinal bleed, or other problems.
Acute anemic events accounted for nearly 1% of all admissions and 12% of children admitted with sickle cell.
Source DR. DOWLING
Major Finding: Diffusion-weighted MRI in children hospitalized with acute anemic events showed evidence of acute silent infarction in 4 of 22 patients with sickle cell disease (18%) and in 2 of 30 patients without sickle cell disease (7%).
Data Source: Prospective, controlled study of 52 children.
Disclosures: Dr. Dowling said he had no relevant conflicts of interest. He has received research support from First American Real Estate Services Inc. The Children's Medical Center, Dallas, funded the study.
LOS ANGELES – Acute anemic events were associated with significantly increased risk for “silent” strokes not only in children with sickle cell disease but also in children with acute anemia due to other causes, a controlled study of 52 patients found.
Prospective screening of all children who were admitted to one hospital during a 30-month period found that 398 were having acute anemic events – accounting for nearly 1% of all admissions and 12% of children admitted with sickle cell disease.
Diffusion-weighted MRI in 52 of these children showed evidence of acute silent infarction in 4 (18%) of 22 patients with sickle cell disease and in 2 (7%) of 30 patients without sickle cell disease, Dr. Michael M. Dowling and his associates reported at the conference.
“I didn't initially plan a controlled study,” but funders insisted, and the comparison yielded unexpected information, said Dr. Dowling, a pediatric neurologist at the University of Texas Southwestern Medical Center, Dallas.
“I didn't think these children [without sickle cell disease] would be having silent infarctions.”
In addition, three patients with sickle cell disease showed evidence of what looked like remote silent infarctions on MRI (14%). Dr. Dowling said he was “fascinated” to also find evidence of remote silent infarctions in seven of the control patients (23%).
“These are not children with sickle cell disease. I'm worried that a lot of these children with acute anemic events were prone to recurrent anemic events from dysfunctional uterine bleeding, cancer, or repeat GI bleeds. I'm worried that at previous admissions they may have had an acute silent infarction in the setting of an acute anemic event that was missed,” Dr. Dowling said at the conference, which was sponsored by the American Heart Association.
Overall, 31% of all MRIs were abnormal, including acute and remote silent infarctions.
“I think we can't simply look at a severely anemic child in an emergency room and say he's hemodynamically stable, because I think these kids are losing brain cells. We need to respond to them more quickly,” he said.
He hopes that a future study will monitor these kinds of children at home via a pulse oximeter so that when their hemoglobin level falls, they can be brought in sooner for transfusion. “I think we can prevent injury,” he said.
Previous studies suggest that approximately 10% of children with sickle cell disease will have a clinically evident stroke unless they get prophylactic therapy, and up to 35% will have silent infarctions.
“It's a bad term because they're not silent,” Dr. Dowling said. “They cause significant cognitive impairment,” educational delays, and increased risk for new or progressive silent infarctions and overt stroke.
His working definition of silent infarction is an MRI lesion consistent with infarction without a focal deficit lasting more than 24 hours. The study defined an acute anemic event as either a hemoglobin level no higher than 5.5 g/dL or, for children with sickle cell anemia whose “normal” hemoglobin level may be 6 g/dL, a decline of at least 30% from baseline hemoglobin level.
Follow-up imaging in four patients with acute silent infarctions showed signals consistent with permanent brain injury in three of them, but not in the fourth. “Some of these might be reversible,” Dr. Dowling said.
He estimated that the incidence of acute silent infarction during acute anemic events is 633 per 100 patient-years in children with sickle cell disease, and 243 per 100 patient-years in children without sickle cell disease, based on the study's findings. His estimates are much larger than are those from the SIT (Silent Infarct Transfusion) study, which suggested an incidence of 43 per 100 patient-years in children with sickle cell disease as a whole, he said.
“We have identified unsuspected silent infarctions occurring during acute anemic events in 18% of children with sickle cell disease and 7% of controls. I think these events may be telling us” that children with acute anemia may need closer observation, he said.
The etiologies of acute anemia were a bit surprising in both the sickle cell and control groups. There were more oncology patients than anticipated among the 294 children who were admitted without sickle cell disease. Aplastic or hemolytic anemias were the leading etiology. Others included gastrointestinal bleed or liver problems, leukemias, lymphomas, and other cancers. Among patients with sickle cell disease and acute anemia, fewer than expected had acute chest syndrome, aplastic crisis, or splenic sequestration, although these still accounted for the majority. Some simply had infection, gastrointestinal bleed, or other problems.
Acute anemic events accounted for nearly 1% of all admissions and 12% of children admitted with sickle cell.
Source DR. DOWLING