User login
Clinical Endocrinology News is an independent news source that provides endocrinologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the endocrinologist's practice. Specialty topics include Diabetes, Lipid & Metabolic Disorders Menopause, Obesity, Osteoporosis, Pediatric Endocrinology, Pituitary, Thyroid & Adrenal Disorders, and Reproductive Endocrinology. Featured content includes Commentaries, Implementin Health Reform, Law & Medicine, and In the Loop, the blog of Clinical Endocrinology News. Clinical Endocrinology News is owned by Frontline Medical Communications.
addict
addicted
addicting
addiction
adult sites
alcohol
antibody
ass
attorney
audit
auditor
babies
babpa
baby
ban
banned
banning
best
bisexual
bitch
bleach
blog
blow job
bondage
boobs
booty
buy
cannabis
certificate
certification
certified
cheap
cheapest
class action
cocaine
cock
counterfeit drug
crack
crap
crime
criminal
cunt
curable
cure
dangerous
dangers
dead
deadly
death
defend
defended
depedent
dependence
dependent
detergent
dick
die
dildo
drug abuse
drug recall
dying
fag
fake
fatal
fatalities
fatality
free
fuck
gangs
gingivitis
guns
hardcore
herbal
herbs
heroin
herpes
home remedies
homo
horny
hypersensitivity
hypoglycemia treatment
illegal drug use
illegal use of prescription
incest
infant
infants
job
ketoacidosis
kill
killer
killing
kinky
law suit
lawsuit
lawyer
lesbian
marijuana
medicine for hypoglycemia
murder
naked
natural
newborn
nigger
noise
nude
nudity
orgy
over the counter
overdosage
overdose
overdosed
overdosing
penis
pimp
pistol
porn
porno
pornographic
pornography
prison
profanity
purchase
purchasing
pussy
queer
rape
rapist
recall
recreational drug
rob
robberies
sale
sales
sex
sexual
shit
shoot
slut
slutty
stole
stolen
store
sue
suicidal
suicide
supplements
supply company
theft
thief
thieves
tit
toddler
toddlers
toxic
toxin
tragedy
treating dka
treating hypoglycemia
treatment for hypoglycemia
vagina
violence
whore
withdrawal
without prescription
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-imn')]
div[contains(@class, 'pane-pub-home-imn')]
div[contains(@class, 'pane-pub-topic-imn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Bone density loss in lean male runners parallels similar issue in women
Similar to a phenomenon already well documented in women, inadequate nutrition appears to be linked to hormonal abnormalities and potentially preventable tibial cortical bone density loss in athletic men, according to results of a small, prospective study.
Based on these findings, “we suspect that a subset of male runners might not be fueling their bodies with enough nutrition and calories for their physical activity,” reported Melanie S. Haines, MD, at the annual meeting of the Endocrine Society.
This is not the first study to suggest male athletes are at risk of a condition equivalent to what has been commonly referred to as the female athlete triad, but it enlarges the objective data that the phenomenon is real, and it makes insufficient availability of energy the likely cause.
In women, the triad is described as a lack of adequate stored energy, irregular menses, and bone density loss. In men, menstrual cycles are not relevant, of course, but this study like others suggests a link between the failure to maintain adequate stores of energy, disturbances in hormone function, and decreased bone density in both men and women, Dr. Haines explained.
RED-S vs. male or female athlete triad
“There is now a move away from the term female athlete triad or male athlete triad,” Dr. Haines reported. Rather the factors of failing to maintain adequate energy for metabolic demands, hormonal disturbances, and bone density loss appear to be relevant to both sexes, according to Dr. Haines, an endocrinologist at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. She said several groups, including the International Olympic Committee (IOC), have transitioned to the term RED-S to apply to both sexes.
“RED-S is an acronym for relative energy deficiency in sport, and it appears to be gaining traction,” Dr. Haines said in an interview.
According to her study and others, excessive lean body mass from failure to supply sufficient energy for physiological needs “negatively affects hormones and bone,” Dr. Haines explained. In men and women, endocrine disturbances are triggered when insufficient calories lead to inadequate macro- and micronutrients.
In this study, 31 men aged 16-30 years were evaluated. Fifteen were in the athlete group, defined by running at least 30 miles per week for at least the previous 6 months. There were 16 control subjects; all exercised less than 2 hours per week and did not participate in team sports, but they were not permitted in the study if their body mass index exceeded 27.5 kg/m2.
Athletes vs. otherwise healthy controls
Conditions that affect bone health were exclusion criteria in both groups, and neither group was permitted to take medications affecting bone health other than dietary calcium or vitamin D supplements for 2 months prior to the study.
Tibial cortical porosity was significantly greater – signaling deterioration in microarchitecture – in athletes, compared with control subjects (P = .003), according to quantitative computed tomography measurements. There was also significantly lower tibial cortical bone mineral density (P = .008) among athletes relative to controls.
Conversely, tibial trabecular measures of bone density and architecture were better among athletes than controls, but this was expected and did not contradict the hypothesis of the study.
“Trabecular bone refers to the inner part of the bone, which increases with weight-bearing exercise, but cortical bone is the outer shell, and the source of stress fractures,” Dr. Haines explained.
The median age of both the athletes and the controls was 24 years. Baseline measurements were similar. Body mass index, fat mass, estradiol, and leptin were all numerically lower in the athletes than controls, but none were significant, although there was a trend for the difference in leptin (P = .085).
Hormones correlated with tibial failure load
When these characteristics were evaluated in the context of mean tibial failure load, a metric related to strength, there was a strongly significant positive association with lean body mass (R = 0.85; P < 0.001) and estradiol level (R = 0.66; P = .007). The relationship with leptin also reached significance (R = 0.59; P = .046).
Unexpectedly, there was no relationship between testosterone and tibial failure load. The reason is unclear, but Dr. Haines’s interpretation is that the relationship between specific hormonal disturbances and bone density loss “might not be as simple” as once hypothesized.
The next step is a longitudinal evaluation of the same group of athletes to follow changes in the relationship between these variables over time, according to Dr. Haines.
Eventually, with evidence that there is a causal relationship between nutrition, hormonal changes, and bone loss, the research in this area will focus on better detection of risk and prophylactic strategies.
“Intervention trials to show that we can prevent stress factors will be difficult to perform,” Dr. Haines acknowledged, but she said that preventing adverse changes in bone at relatively young ages could have implications for long-term bone health, including protection from osteoporosis later in life.
The research presented by Dr. Haines is consistent with an area of research that is several decades old, at least in females, according to Siobhan M. Statuta, MD, a sports medicine primary care specialist at the University of Virginia, Charlottesville. The evidence that the same phenomenon occurs in men is more recent, but she said that it is now well accepted the there is a parallel hormonal issue in men and women.
“It is not a question of not eating enough. Often, athletes continue to consume the same diet, but their activity increases,” Dr. Statuta explained. “The problem is that they are not supplying enough of the calories they need to sustain the energy they are expending. You might say they are not fueling their engines appropriately.”
In 2014, the International Olympic Committee published a consensus statement on RED-S. They described this as a condition in which a state of energy deficiency leads to numerous complications in athletes, not just osteoporosis. Rather, a host of physiological systems, ranging from gastrointestinal complaints to cardiovascular events, were described.
RED-S addresses health beyond bones
“The RED-S theory is better described as a spoke-and-wheel concept rather than a triad. While inadequate energy availability is important to both, RED-S places this at the center of the wheel with spokes leading to all the possible complications rather than as a first event in a limited triad,” Dr. Statuta said in an interview.
However, she noted that the term RED-S is not yet appropriate to replace that of the male and female athlete triad.
“More research is required to hash out the relationship of a body in a state of energy deficiency and how it affects the entire body, which is the principle of RED-S,” Dr. Statuta said. “There likely are scientific effects, and we are currently investigating these relationships more.”
“These are really quite similar entities but have different foci,” she added. Based on data collected over several decades, “the triad narrows in on two body systems affected by low energy – the reproductive system and bones. RED-S incorporates these same systems yet adds on many more organ systems.
The original group of researchers have remained loyal to the concept of the triad that involves inadequate availability of energy followed by hormonal irregularities and osteoporosis. This group, the Female and Male Athlete Triad Coalition, has issued publications on this topic several times. Consensus statements were updated last year.
“The premise is that the triad leading to bone loss is shared by both men and women, even if the clinical manifestations differ,” said Dr. Statuta. The most notable difference is that men do not experience menstrual irregularities, but Dr. Statuta suggested that the clinical consequences are not necessarily any less.
“Males do not have menstrual cycles as an outward marker of an endocrine disturbance, so it is harder to recognize clinically, but I think there is agreement that not having enough energy available is the trigger of endocrine changes and then bone loss is relevant to both sexes,” she said. She said this is supported by a growing body of evidence, including the data presented by Dr. Haines at the Endocrine Society meeting.
Dr. Haines and Dr. Statuta report no potential conflicts of interest.
Similar to a phenomenon already well documented in women, inadequate nutrition appears to be linked to hormonal abnormalities and potentially preventable tibial cortical bone density loss in athletic men, according to results of a small, prospective study.
Based on these findings, “we suspect that a subset of male runners might not be fueling their bodies with enough nutrition and calories for their physical activity,” reported Melanie S. Haines, MD, at the annual meeting of the Endocrine Society.
This is not the first study to suggest male athletes are at risk of a condition equivalent to what has been commonly referred to as the female athlete triad, but it enlarges the objective data that the phenomenon is real, and it makes insufficient availability of energy the likely cause.
In women, the triad is described as a lack of adequate stored energy, irregular menses, and bone density loss. In men, menstrual cycles are not relevant, of course, but this study like others suggests a link between the failure to maintain adequate stores of energy, disturbances in hormone function, and decreased bone density in both men and women, Dr. Haines explained.
RED-S vs. male or female athlete triad
“There is now a move away from the term female athlete triad or male athlete triad,” Dr. Haines reported. Rather the factors of failing to maintain adequate energy for metabolic demands, hormonal disturbances, and bone density loss appear to be relevant to both sexes, according to Dr. Haines, an endocrinologist at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. She said several groups, including the International Olympic Committee (IOC), have transitioned to the term RED-S to apply to both sexes.
“RED-S is an acronym for relative energy deficiency in sport, and it appears to be gaining traction,” Dr. Haines said in an interview.
According to her study and others, excessive lean body mass from failure to supply sufficient energy for physiological needs “negatively affects hormones and bone,” Dr. Haines explained. In men and women, endocrine disturbances are triggered when insufficient calories lead to inadequate macro- and micronutrients.
In this study, 31 men aged 16-30 years were evaluated. Fifteen were in the athlete group, defined by running at least 30 miles per week for at least the previous 6 months. There were 16 control subjects; all exercised less than 2 hours per week and did not participate in team sports, but they were not permitted in the study if their body mass index exceeded 27.5 kg/m2.
Athletes vs. otherwise healthy controls
Conditions that affect bone health were exclusion criteria in both groups, and neither group was permitted to take medications affecting bone health other than dietary calcium or vitamin D supplements for 2 months prior to the study.
Tibial cortical porosity was significantly greater – signaling deterioration in microarchitecture – in athletes, compared with control subjects (P = .003), according to quantitative computed tomography measurements. There was also significantly lower tibial cortical bone mineral density (P = .008) among athletes relative to controls.
Conversely, tibial trabecular measures of bone density and architecture were better among athletes than controls, but this was expected and did not contradict the hypothesis of the study.
“Trabecular bone refers to the inner part of the bone, which increases with weight-bearing exercise, but cortical bone is the outer shell, and the source of stress fractures,” Dr. Haines explained.
The median age of both the athletes and the controls was 24 years. Baseline measurements were similar. Body mass index, fat mass, estradiol, and leptin were all numerically lower in the athletes than controls, but none were significant, although there was a trend for the difference in leptin (P = .085).
Hormones correlated with tibial failure load
When these characteristics were evaluated in the context of mean tibial failure load, a metric related to strength, there was a strongly significant positive association with lean body mass (R = 0.85; P < 0.001) and estradiol level (R = 0.66; P = .007). The relationship with leptin also reached significance (R = 0.59; P = .046).
Unexpectedly, there was no relationship between testosterone and tibial failure load. The reason is unclear, but Dr. Haines’s interpretation is that the relationship between specific hormonal disturbances and bone density loss “might not be as simple” as once hypothesized.
The next step is a longitudinal evaluation of the same group of athletes to follow changes in the relationship between these variables over time, according to Dr. Haines.
Eventually, with evidence that there is a causal relationship between nutrition, hormonal changes, and bone loss, the research in this area will focus on better detection of risk and prophylactic strategies.
“Intervention trials to show that we can prevent stress factors will be difficult to perform,” Dr. Haines acknowledged, but she said that preventing adverse changes in bone at relatively young ages could have implications for long-term bone health, including protection from osteoporosis later in life.
The research presented by Dr. Haines is consistent with an area of research that is several decades old, at least in females, according to Siobhan M. Statuta, MD, a sports medicine primary care specialist at the University of Virginia, Charlottesville. The evidence that the same phenomenon occurs in men is more recent, but she said that it is now well accepted the there is a parallel hormonal issue in men and women.
“It is not a question of not eating enough. Often, athletes continue to consume the same diet, but their activity increases,” Dr. Statuta explained. “The problem is that they are not supplying enough of the calories they need to sustain the energy they are expending. You might say they are not fueling their engines appropriately.”
In 2014, the International Olympic Committee published a consensus statement on RED-S. They described this as a condition in which a state of energy deficiency leads to numerous complications in athletes, not just osteoporosis. Rather, a host of physiological systems, ranging from gastrointestinal complaints to cardiovascular events, were described.
RED-S addresses health beyond bones
“The RED-S theory is better described as a spoke-and-wheel concept rather than a triad. While inadequate energy availability is important to both, RED-S places this at the center of the wheel with spokes leading to all the possible complications rather than as a first event in a limited triad,” Dr. Statuta said in an interview.
However, she noted that the term RED-S is not yet appropriate to replace that of the male and female athlete triad.
“More research is required to hash out the relationship of a body in a state of energy deficiency and how it affects the entire body, which is the principle of RED-S,” Dr. Statuta said. “There likely are scientific effects, and we are currently investigating these relationships more.”
“These are really quite similar entities but have different foci,” she added. Based on data collected over several decades, “the triad narrows in on two body systems affected by low energy – the reproductive system and bones. RED-S incorporates these same systems yet adds on many more organ systems.
The original group of researchers have remained loyal to the concept of the triad that involves inadequate availability of energy followed by hormonal irregularities and osteoporosis. This group, the Female and Male Athlete Triad Coalition, has issued publications on this topic several times. Consensus statements were updated last year.
“The premise is that the triad leading to bone loss is shared by both men and women, even if the clinical manifestations differ,” said Dr. Statuta. The most notable difference is that men do not experience menstrual irregularities, but Dr. Statuta suggested that the clinical consequences are not necessarily any less.
“Males do not have menstrual cycles as an outward marker of an endocrine disturbance, so it is harder to recognize clinically, but I think there is agreement that not having enough energy available is the trigger of endocrine changes and then bone loss is relevant to both sexes,” she said. She said this is supported by a growing body of evidence, including the data presented by Dr. Haines at the Endocrine Society meeting.
Dr. Haines and Dr. Statuta report no potential conflicts of interest.
Similar to a phenomenon already well documented in women, inadequate nutrition appears to be linked to hormonal abnormalities and potentially preventable tibial cortical bone density loss in athletic men, according to results of a small, prospective study.
Based on these findings, “we suspect that a subset of male runners might not be fueling their bodies with enough nutrition and calories for their physical activity,” reported Melanie S. Haines, MD, at the annual meeting of the Endocrine Society.
This is not the first study to suggest male athletes are at risk of a condition equivalent to what has been commonly referred to as the female athlete triad, but it enlarges the objective data that the phenomenon is real, and it makes insufficient availability of energy the likely cause.
In women, the triad is described as a lack of adequate stored energy, irregular menses, and bone density loss. In men, menstrual cycles are not relevant, of course, but this study like others suggests a link between the failure to maintain adequate stores of energy, disturbances in hormone function, and decreased bone density in both men and women, Dr. Haines explained.
RED-S vs. male or female athlete triad
“There is now a move away from the term female athlete triad or male athlete triad,” Dr. Haines reported. Rather the factors of failing to maintain adequate energy for metabolic demands, hormonal disturbances, and bone density loss appear to be relevant to both sexes, according to Dr. Haines, an endocrinologist at Massachusetts General Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. She said several groups, including the International Olympic Committee (IOC), have transitioned to the term RED-S to apply to both sexes.
“RED-S is an acronym for relative energy deficiency in sport, and it appears to be gaining traction,” Dr. Haines said in an interview.
According to her study and others, excessive lean body mass from failure to supply sufficient energy for physiological needs “negatively affects hormones and bone,” Dr. Haines explained. In men and women, endocrine disturbances are triggered when insufficient calories lead to inadequate macro- and micronutrients.
In this study, 31 men aged 16-30 years were evaluated. Fifteen were in the athlete group, defined by running at least 30 miles per week for at least the previous 6 months. There were 16 control subjects; all exercised less than 2 hours per week and did not participate in team sports, but they were not permitted in the study if their body mass index exceeded 27.5 kg/m2.
Athletes vs. otherwise healthy controls
Conditions that affect bone health were exclusion criteria in both groups, and neither group was permitted to take medications affecting bone health other than dietary calcium or vitamin D supplements for 2 months prior to the study.
Tibial cortical porosity was significantly greater – signaling deterioration in microarchitecture – in athletes, compared with control subjects (P = .003), according to quantitative computed tomography measurements. There was also significantly lower tibial cortical bone mineral density (P = .008) among athletes relative to controls.
Conversely, tibial trabecular measures of bone density and architecture were better among athletes than controls, but this was expected and did not contradict the hypothesis of the study.
“Trabecular bone refers to the inner part of the bone, which increases with weight-bearing exercise, but cortical bone is the outer shell, and the source of stress fractures,” Dr. Haines explained.
The median age of both the athletes and the controls was 24 years. Baseline measurements were similar. Body mass index, fat mass, estradiol, and leptin were all numerically lower in the athletes than controls, but none were significant, although there was a trend for the difference in leptin (P = .085).
Hormones correlated with tibial failure load
When these characteristics were evaluated in the context of mean tibial failure load, a metric related to strength, there was a strongly significant positive association with lean body mass (R = 0.85; P < 0.001) and estradiol level (R = 0.66; P = .007). The relationship with leptin also reached significance (R = 0.59; P = .046).
Unexpectedly, there was no relationship between testosterone and tibial failure load. The reason is unclear, but Dr. Haines’s interpretation is that the relationship between specific hormonal disturbances and bone density loss “might not be as simple” as once hypothesized.
The next step is a longitudinal evaluation of the same group of athletes to follow changes in the relationship between these variables over time, according to Dr. Haines.
Eventually, with evidence that there is a causal relationship between nutrition, hormonal changes, and bone loss, the research in this area will focus on better detection of risk and prophylactic strategies.
“Intervention trials to show that we can prevent stress factors will be difficult to perform,” Dr. Haines acknowledged, but she said that preventing adverse changes in bone at relatively young ages could have implications for long-term bone health, including protection from osteoporosis later in life.
The research presented by Dr. Haines is consistent with an area of research that is several decades old, at least in females, according to Siobhan M. Statuta, MD, a sports medicine primary care specialist at the University of Virginia, Charlottesville. The evidence that the same phenomenon occurs in men is more recent, but she said that it is now well accepted the there is a parallel hormonal issue in men and women.
“It is not a question of not eating enough. Often, athletes continue to consume the same diet, but their activity increases,” Dr. Statuta explained. “The problem is that they are not supplying enough of the calories they need to sustain the energy they are expending. You might say they are not fueling their engines appropriately.”
In 2014, the International Olympic Committee published a consensus statement on RED-S. They described this as a condition in which a state of energy deficiency leads to numerous complications in athletes, not just osteoporosis. Rather, a host of physiological systems, ranging from gastrointestinal complaints to cardiovascular events, were described.
RED-S addresses health beyond bones
“The RED-S theory is better described as a spoke-and-wheel concept rather than a triad. While inadequate energy availability is important to both, RED-S places this at the center of the wheel with spokes leading to all the possible complications rather than as a first event in a limited triad,” Dr. Statuta said in an interview.
However, she noted that the term RED-S is not yet appropriate to replace that of the male and female athlete triad.
“More research is required to hash out the relationship of a body in a state of energy deficiency and how it affects the entire body, which is the principle of RED-S,” Dr. Statuta said. “There likely are scientific effects, and we are currently investigating these relationships more.”
“These are really quite similar entities but have different foci,” she added. Based on data collected over several decades, “the triad narrows in on two body systems affected by low energy – the reproductive system and bones. RED-S incorporates these same systems yet adds on many more organ systems.
The original group of researchers have remained loyal to the concept of the triad that involves inadequate availability of energy followed by hormonal irregularities and osteoporosis. This group, the Female and Male Athlete Triad Coalition, has issued publications on this topic several times. Consensus statements were updated last year.
“The premise is that the triad leading to bone loss is shared by both men and women, even if the clinical manifestations differ,” said Dr. Statuta. The most notable difference is that men do not experience menstrual irregularities, but Dr. Statuta suggested that the clinical consequences are not necessarily any less.
“Males do not have menstrual cycles as an outward marker of an endocrine disturbance, so it is harder to recognize clinically, but I think there is agreement that not having enough energy available is the trigger of endocrine changes and then bone loss is relevant to both sexes,” she said. She said this is supported by a growing body of evidence, including the data presented by Dr. Haines at the Endocrine Society meeting.
Dr. Haines and Dr. Statuta report no potential conflicts of interest.
FROM ENDO 2022
Biden moves to limit nicotine levels in cigarettes
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
The Department of Health and Human Services posted a notice that details plans for a new rule to create a maximum allowed amount of nicotine in certain tobacco products. The Food and Drug Administration would take the action, the notice said, “to reduce addictiveness to certain tobacco products, thus giving addicted users a greater ability to quit.” The product standard would also help keep nonsmokers interested in trying tobacco, mainly youth, from starting to smoke and become regulars.
“Lowering nicotine levels to minimally addictive or non-addictive levels would decrease the likelihood that future generations of young people become addicted to cigarettes and help more currently addicted smokers to quit,” FDA Commissioner Robert Califf, MD, said in a statement.
The FDA, in charge of regulating cigarettes, issues a proposed rule when changes are discussed. That would be followed by a period for public comments before a final rule could be issued.
The proposed rule was first reported by The Washington Post.
The FDA in 2018 published a study in the New England Journal of Medicine that estimated that a potential limit on nicotine in cigarettes could, by the year 2100, prevent more than 33 million people from becoming regular smokers, and prevent the deaths of more than 8 million people from tobacco-related illnesses.
The action to reduce nicotine levels would fit in with President Joe Biden’s goal of reducing cancer death rates by half over 25 years. Each year, according to the American Cancer Society, about 480,000 deaths (about 1 in 5) are related to smoking. Currently, about 34 million American adults still smoke cigarettes.
Matthew Myers, president of the Campaign for Tobacco-Free Kids, called the proposed rule a “truly game-changing proposal.”
“There is no other single action our country can take that would prevent more young people from becoming addicted to tobacco or have a greater impact on reducing deaths from cancer, cardiovascular disease and respiratory disease,” Mr. Myers said in a statement.
However, he said, “these gains will only be realized if the administration and the FDA demonstrate a full-throated commitment to finalizing and implementing this proposal.”
The FDA proposed the nicotine reduction strategy in talks with the White House and the Department of Health and Human Services early in 2021, according to the Post.
Earlier this year, the FDA issued a proposed rule to ban menthol flavoring in cigarettes. The agency is accepting public comments though July 5.
The action of reducing nicotine levels would likely take years to complete, Mitch Zeller, JD, recently retired director of the FDA Center for Tobacco Products, told the Post.
In 2018, the FDA issued a proposed ruling to set a standard for maximum nicotine levels in cigarettes.
Advocates say the action of slashing nicotine, the active – and addictive – ingredient in cigarettes, would save millions of lives for generations to come. Opponents liken it to the prohibition of alcohol in the 1920s and predict the action will fail.
Others say that if limits are put on nicotine levels, adults should have greater access to noncombustible alternatives.
A version of this article first appeared on WebMD.com.
COVID-19 Pandemic stress affected ovulation, not menstruation
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ATLANTA – Disturbances in ovulation that didn’t produce any actual changes in the menstrual cycle of women were extremely common during the first year of the COVID-19 pandemic and were linked to emotional stress, according to the findings of an “experiment of nature” that allowed for comparison with women a decade earlier.
Findings from two studies of reproductive-age women, one conducted in 2006-2008 and the other in 2020-2021, were presented by Jerilynn C. Prior, MD, at the annual meeting of the Endocrine Society.
The comparison of the two time periods yielded several novel findings. “I was taught in medical school that when women don’t eat enough they lose their period. But what we now understand is there’s a graded response to various stressors, acting through the hypothalamus in a common pathway. There is a gradation of disturbances, some of which are subclinical or not obvious,” said Dr. Prior, professor of endocrinology and metabolism at the University of British Columbia, Vancouver.
Moreover, women’s menstrual cycle lengths didn’t differ across the two time periods, despite a dramatic 63% decrement in normal ovulatory function related to increased depression, anxiety, and outside stresses that the women reported in diaries.
“Assuming that regular cycles need normal ovulation is something we should just get out of our minds. It changes our concept about what’s normal if we only know about the cycle length,” she observed.
It will be critical going forward to see whether the ovulatory disturbances have resolved as the pandemic has shifted “because there’s strong evidence that ovulatory disturbances, even with normal cycle length, are related to bone loss and some evidence it’s related to early heart attacks, breast and endometrial cancers,” Dr. Prior said during a press conference.
Asked to comment, session moderator Genevieve Neal-Perry, MD, PhD, told this news organization: “I think what we can take away is that stress itself is a modifier of the way the brain and the gonads communicate with each other, and that then has an impact on ovulatory function.”
Dr. Neal-Perry noted that the association of stress and ovulatory disruption has been reported in various ways previously, but “clearly it doesn’t affect everyone. What we don’t know is who is most susceptible. There have been some studies showing a genetic predisposition and a genetic anomaly that actually makes them more susceptible to the impact of stress on the reproductive system.”
But the lack of data on weight change in the study cohorts is a limitation. “To me one of the more important questions was what was going on with weight. Just looking at a static number doesn’t tell you whether there were changes. We know that weight gain or weight loss can stress the reproductive axis,” noted Dr. Neal-Parry of the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill.
‘Experiment of nature’ revealed invisible effect of pandemic stress
The women in both cohorts of the Menstruation Ovulation Study (MOS) were healthy volunteers aged 19-35 years recruited from the metropolitan Vancouver region. All were menstruating monthly and none were taking hormonal birth control. Recruitment for the second cohort had begun just prior to the March 2020 COVID-19 pandemic lockdown.
Interviewer-administered questionnaires (CaMos) covering demographics, socioeconomic status, and reproductive history, and daily diaries kept by the women (menstrual cycle diary) were identical for both cohorts.
Assessments of ovulation differed for the two studies but were cross-validated. For the earlier time period, ovulation was assessed by a threefold increase in follicular-to-luteal urinary progesterone (PdG). For the pandemic-era study, the validated quantitative basal temperature (QBT) method was used.
There were 301 women in the earlier cohort and 125 during the pandemic. Both were an average age of about 29 years and had a body mass index of about 24.3 kg/m2 (within the normal range). The pandemic cohort was more racially/ethnically diverse than the earlier one and more in-line with recent census data.
More of the women were nulliparous during the pandemic than earlier (92.7% vs. 80.4%; P = .002).
The distribution of menstrual cycle lengths didn’t differ, with both cohorts averaging about 30 days (P = .893). However, while 90% of the women in the earlier cohort ovulated normally, only 37% did during the pandemic, a highly significant difference (P < .0001).
Thus, during the pandemic, 63% of women had “silent ovulatory disturbances,” either with short luteal phases after ovulation or no ovulation, compared with just 10% in the earlier cohort, “which is remarkable, unbelievable actually,” Dr. Prior remarked.
The difference wasn’t explained by any of the demographic information collected either, including socioeconomic status, lifestyle, or reproductive history variables.
And it wasn’t because of COVID-19 vaccination, as the vaccine wasn’t available when most of the women were recruited, and of the 79 who were recruited during vaccine availability, only two received a COVID-19 vaccine during the study (and both had normal ovulation).
Employment changes, caring responsibilities, and worry likely causes
The information from the diaries was more revealing. Several diary components were far more common during the pandemic, including negative mood (feeling depressed or anxious, sleep problems, and outside stresses), self-worth, interest in sex, energy level, and appetite. All were significantly different between the two cohorts (P < .001) and between those with and without ovulatory disturbances.
“So menstrual cycle lengths and long cycles didn’t differ, but there was a much higher prevalence of silent or subclinical ovulatory disturbances, and these were related to the increased stresses that women recorded in their diaries. This means that the estrogen levels were pretty close to normal but the progesterone levels were remarkably decreased,” Dr. Prior said.
Interestingly, reported menstrual cramps were also significantly more common during the pandemic and associated with ovulatory disruption.
“That is a new observation because previously we’ve always thought that you needed to ovulate in order to even have cramps,” she commented.
Asked whether COVID-19 itself might have played a role, Dr. Prior said no woman in the study tested positive for the virus or had long COVID.
“As far as I’m aware, it was the changes in employment … and caring for elders and worry about illness in somebody you loved that was related,” she said.
Asked what she thinks the result would be if the study were conducted now, she said: “I don’t know. We’re still in a stressful time with inflation and not complete recovery, so probably the issue is still very present.”
Dr. Prior and Dr. Neal-Perry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ENDO 2022
Osteoporosis risk rises with air pollution levels
COPENHAGEN – Chronic exposure to high levels of particulate matter (PM) air pollution 2.5 mcm (PM2.5) or larger, and 10 mcm (PM10) or larger, in size is associated with a significantly higher likelihood of having osteoporosis, according to research presented at the annual European Congress of Rheumatology.
The results of the 7-year longitudinal study carried out across Italy from 2013 to 2019 dovetail with other recent published accounts from the same team of Italian researchers, led by Giovanni Adami, MD, of the rheumatology unit at the University of Verona (Italy). In addition to the current report presented at EULAR 2022, Dr. Adami and associates have reported an increased risk of flares of both rheumatoid arthritis and psoriasis following periods of elevated pollution, as well as an overall elevated risk for autoimmune diseases with higher concentrations of PM2.5 and PM10.
The pathogenesis of osteoporosis is thought to involve both genetic and environmental input, such as smoking, which is itself environmental air pollution, Dr. Adami said. The biological rationale for why air pollution might contribute to risk for osteoporosis comes from studies showing that exposure to indoor air pollution from biomass combustion raises serum levels of RANKL (receptor activator of nuclear factor-kappa ligand 1) but lowers serum osteoprotegerin – suggesting an increased risk of bone resorption – and that toxic metals such as lead, cadmium, mercury, and aluminum accumulate in the skeleton and negatively affect bone health.
In their study, Dr. Adami and colleagues found that, overall, the average exposure during the period 2013-2019 across Italy was 16.0 mcg/m3 for PM2.5 and 25.0 mcg/m3 for PM10.
“I can tell you that [25.0 mcg/m3 for PM10] is a very high exposure. It’s not very good for your health,” Dr. Adami said.
Data on more than 59,000 Italian women
Dr. Adami and colleagues used clinical characteristics and densitometric data from Italy’s osteoporosis fracture risk and osteoporosis screening reimbursement tool known as DeFRAcalc79, which has amassed variables from more than 59,000 women across the country. They used long-term average PM concentrations across Italy during 2013-2019 that were obtained from the Italian Institute for Environmental Protection and Research’s 617 air quality stations in 110 Italian provinces. The researchers linked individuals to a PM exposure value determined from the average concentration of urban, rural, and near-traffic stations in each person’s province of residence.
For 59,950 women across Italy who were at high risk for fracture, the researchers found 64.5% with bone mineral density that was defined as osteoporotic. At PM10 concentrations of 30 mcg/m3 or greater, there was a significantly higher likelihood of osteoporosis at both the femoral neck (odds ratio, 1.15) and lumbar spine (OR, 1.17).
The likelihood of osteoporosis was slightly greater with PM2.5 at concentrations of 25 mcg/m3 or more at the femoral neck (OR, 1.22) and lumbar spine (OR, 1.18). These comparisons were adjusted for age, body mass index (BMI), presence of prevalent fragility fractures, family history of osteoporosis, menopause, glucocorticoid use, comorbidities, and for residency in northern, central, or southern Italy.
Both thresholds of PM10 > 30 mcg/m3 and PM2.5 > 25 mcg/m3 “are considered safe … by the World Health Organization,” Dr. Adami pointed out.
“If you live in a place where the chronic exposure is less than 30 mcg/m3, you probably have slightly lower risk of osteoporosis as compared to those who live in a highly industrialized, polluted zone,” he explained.
“The cortical bone – femoral neck – seemed to be more susceptible, compared to trabecular bone, which is the lumbar spine. We have no idea why this is true, but we might speculate that somehow chronic inflammation like the [kind] seen in rheumatoid arthritis might be responsible for cortical bone impairment and not trabecular bone impairment,” Dr. Adami said.
One audience member, Kenneth Poole, BM, PhD, senior lecturer and honorary consultant in Metabolic Bone Disease and Rheumatology at the University of Cambridge (England), asked whether it was possible to account for the possibility of confounding caused by areas with dense housing in places where the particulate matter would be highest, and where residents may be less active and use stairs less often.
Dr. Adami noted that confounding is indeed a possibility, but he said Italy is unique in that its most polluted area – the Po River valley – is also its most wealthy area and in general has less crowded living situations with a healthier population, which could have attenuated, rather than reinforced, the results.
Does air pollution have an immunologic effect?
In interviews with this news organization, session comoderators Filipe Araújo, MD, and Irene Bultink, MD, PhD, said that the growth in evidence for the impact of air pollution on risk for, and severity of, various diseases suggests air pollution might have an immunologic effect.
“I think it’s very important to point this out. I also think it’s very hard to rule out confounding, because when you’re living in a city with crowded housing you may not walk or ride your bike but instead go by car or metro, and [the lifestyle is different],” said Dr. Bultink of Amsterdam University Medical Centers.
“It stresses that these diseases [that are associated with air pollution] although they are different in their pathophysiology, it points toward the systemic nature of rheumatic diseases, including osteoporosis,” said Dr. Araújo of Hospital Cuf Cascais (Portugal) and Hospital Ortopédico de Sant’Ana, Parede, Portugal.
The study was independently supported.Dr. Adami disclosed being a shareholder of Galapagos and Theramex.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Chronic exposure to high levels of particulate matter (PM) air pollution 2.5 mcm (PM2.5) or larger, and 10 mcm (PM10) or larger, in size is associated with a significantly higher likelihood of having osteoporosis, according to research presented at the annual European Congress of Rheumatology.
The results of the 7-year longitudinal study carried out across Italy from 2013 to 2019 dovetail with other recent published accounts from the same team of Italian researchers, led by Giovanni Adami, MD, of the rheumatology unit at the University of Verona (Italy). In addition to the current report presented at EULAR 2022, Dr. Adami and associates have reported an increased risk of flares of both rheumatoid arthritis and psoriasis following periods of elevated pollution, as well as an overall elevated risk for autoimmune diseases with higher concentrations of PM2.5 and PM10.
The pathogenesis of osteoporosis is thought to involve both genetic and environmental input, such as smoking, which is itself environmental air pollution, Dr. Adami said. The biological rationale for why air pollution might contribute to risk for osteoporosis comes from studies showing that exposure to indoor air pollution from biomass combustion raises serum levels of RANKL (receptor activator of nuclear factor-kappa ligand 1) but lowers serum osteoprotegerin – suggesting an increased risk of bone resorption – and that toxic metals such as lead, cadmium, mercury, and aluminum accumulate in the skeleton and negatively affect bone health.
In their study, Dr. Adami and colleagues found that, overall, the average exposure during the period 2013-2019 across Italy was 16.0 mcg/m3 for PM2.5 and 25.0 mcg/m3 for PM10.
“I can tell you that [25.0 mcg/m3 for PM10] is a very high exposure. It’s not very good for your health,” Dr. Adami said.
Data on more than 59,000 Italian women
Dr. Adami and colleagues used clinical characteristics and densitometric data from Italy’s osteoporosis fracture risk and osteoporosis screening reimbursement tool known as DeFRAcalc79, which has amassed variables from more than 59,000 women across the country. They used long-term average PM concentrations across Italy during 2013-2019 that were obtained from the Italian Institute for Environmental Protection and Research’s 617 air quality stations in 110 Italian provinces. The researchers linked individuals to a PM exposure value determined from the average concentration of urban, rural, and near-traffic stations in each person’s province of residence.
For 59,950 women across Italy who were at high risk for fracture, the researchers found 64.5% with bone mineral density that was defined as osteoporotic. At PM10 concentrations of 30 mcg/m3 or greater, there was a significantly higher likelihood of osteoporosis at both the femoral neck (odds ratio, 1.15) and lumbar spine (OR, 1.17).
The likelihood of osteoporosis was slightly greater with PM2.5 at concentrations of 25 mcg/m3 or more at the femoral neck (OR, 1.22) and lumbar spine (OR, 1.18). These comparisons were adjusted for age, body mass index (BMI), presence of prevalent fragility fractures, family history of osteoporosis, menopause, glucocorticoid use, comorbidities, and for residency in northern, central, or southern Italy.
Both thresholds of PM10 > 30 mcg/m3 and PM2.5 > 25 mcg/m3 “are considered safe … by the World Health Organization,” Dr. Adami pointed out.
“If you live in a place where the chronic exposure is less than 30 mcg/m3, you probably have slightly lower risk of osteoporosis as compared to those who live in a highly industrialized, polluted zone,” he explained.
“The cortical bone – femoral neck – seemed to be more susceptible, compared to trabecular bone, which is the lumbar spine. We have no idea why this is true, but we might speculate that somehow chronic inflammation like the [kind] seen in rheumatoid arthritis might be responsible for cortical bone impairment and not trabecular bone impairment,” Dr. Adami said.
One audience member, Kenneth Poole, BM, PhD, senior lecturer and honorary consultant in Metabolic Bone Disease and Rheumatology at the University of Cambridge (England), asked whether it was possible to account for the possibility of confounding caused by areas with dense housing in places where the particulate matter would be highest, and where residents may be less active and use stairs less often.
Dr. Adami noted that confounding is indeed a possibility, but he said Italy is unique in that its most polluted area – the Po River valley – is also its most wealthy area and in general has less crowded living situations with a healthier population, which could have attenuated, rather than reinforced, the results.
Does air pollution have an immunologic effect?
In interviews with this news organization, session comoderators Filipe Araújo, MD, and Irene Bultink, MD, PhD, said that the growth in evidence for the impact of air pollution on risk for, and severity of, various diseases suggests air pollution might have an immunologic effect.
“I think it’s very important to point this out. I also think it’s very hard to rule out confounding, because when you’re living in a city with crowded housing you may not walk or ride your bike but instead go by car or metro, and [the lifestyle is different],” said Dr. Bultink of Amsterdam University Medical Centers.
“It stresses that these diseases [that are associated with air pollution] although they are different in their pathophysiology, it points toward the systemic nature of rheumatic diseases, including osteoporosis,” said Dr. Araújo of Hospital Cuf Cascais (Portugal) and Hospital Ortopédico de Sant’Ana, Parede, Portugal.
The study was independently supported.Dr. Adami disclosed being a shareholder of Galapagos and Theramex.
A version of this article first appeared on Medscape.com.
COPENHAGEN – Chronic exposure to high levels of particulate matter (PM) air pollution 2.5 mcm (PM2.5) or larger, and 10 mcm (PM10) or larger, in size is associated with a significantly higher likelihood of having osteoporosis, according to research presented at the annual European Congress of Rheumatology.
The results of the 7-year longitudinal study carried out across Italy from 2013 to 2019 dovetail with other recent published accounts from the same team of Italian researchers, led by Giovanni Adami, MD, of the rheumatology unit at the University of Verona (Italy). In addition to the current report presented at EULAR 2022, Dr. Adami and associates have reported an increased risk of flares of both rheumatoid arthritis and psoriasis following periods of elevated pollution, as well as an overall elevated risk for autoimmune diseases with higher concentrations of PM2.5 and PM10.
The pathogenesis of osteoporosis is thought to involve both genetic and environmental input, such as smoking, which is itself environmental air pollution, Dr. Adami said. The biological rationale for why air pollution might contribute to risk for osteoporosis comes from studies showing that exposure to indoor air pollution from biomass combustion raises serum levels of RANKL (receptor activator of nuclear factor-kappa ligand 1) but lowers serum osteoprotegerin – suggesting an increased risk of bone resorption – and that toxic metals such as lead, cadmium, mercury, and aluminum accumulate in the skeleton and negatively affect bone health.
In their study, Dr. Adami and colleagues found that, overall, the average exposure during the period 2013-2019 across Italy was 16.0 mcg/m3 for PM2.5 and 25.0 mcg/m3 for PM10.
“I can tell you that [25.0 mcg/m3 for PM10] is a very high exposure. It’s not very good for your health,” Dr. Adami said.
Data on more than 59,000 Italian women
Dr. Adami and colleagues used clinical characteristics and densitometric data from Italy’s osteoporosis fracture risk and osteoporosis screening reimbursement tool known as DeFRAcalc79, which has amassed variables from more than 59,000 women across the country. They used long-term average PM concentrations across Italy during 2013-2019 that were obtained from the Italian Institute for Environmental Protection and Research’s 617 air quality stations in 110 Italian provinces. The researchers linked individuals to a PM exposure value determined from the average concentration of urban, rural, and near-traffic stations in each person’s province of residence.
For 59,950 women across Italy who were at high risk for fracture, the researchers found 64.5% with bone mineral density that was defined as osteoporotic. At PM10 concentrations of 30 mcg/m3 or greater, there was a significantly higher likelihood of osteoporosis at both the femoral neck (odds ratio, 1.15) and lumbar spine (OR, 1.17).
The likelihood of osteoporosis was slightly greater with PM2.5 at concentrations of 25 mcg/m3 or more at the femoral neck (OR, 1.22) and lumbar spine (OR, 1.18). These comparisons were adjusted for age, body mass index (BMI), presence of prevalent fragility fractures, family history of osteoporosis, menopause, glucocorticoid use, comorbidities, and for residency in northern, central, or southern Italy.
Both thresholds of PM10 > 30 mcg/m3 and PM2.5 > 25 mcg/m3 “are considered safe … by the World Health Organization,” Dr. Adami pointed out.
“If you live in a place where the chronic exposure is less than 30 mcg/m3, you probably have slightly lower risk of osteoporosis as compared to those who live in a highly industrialized, polluted zone,” he explained.
“The cortical bone – femoral neck – seemed to be more susceptible, compared to trabecular bone, which is the lumbar spine. We have no idea why this is true, but we might speculate that somehow chronic inflammation like the [kind] seen in rheumatoid arthritis might be responsible for cortical bone impairment and not trabecular bone impairment,” Dr. Adami said.
One audience member, Kenneth Poole, BM, PhD, senior lecturer and honorary consultant in Metabolic Bone Disease and Rheumatology at the University of Cambridge (England), asked whether it was possible to account for the possibility of confounding caused by areas with dense housing in places where the particulate matter would be highest, and where residents may be less active and use stairs less often.
Dr. Adami noted that confounding is indeed a possibility, but he said Italy is unique in that its most polluted area – the Po River valley – is also its most wealthy area and in general has less crowded living situations with a healthier population, which could have attenuated, rather than reinforced, the results.
Does air pollution have an immunologic effect?
In interviews with this news organization, session comoderators Filipe Araújo, MD, and Irene Bultink, MD, PhD, said that the growth in evidence for the impact of air pollution on risk for, and severity of, various diseases suggests air pollution might have an immunologic effect.
“I think it’s very important to point this out. I also think it’s very hard to rule out confounding, because when you’re living in a city with crowded housing you may not walk or ride your bike but instead go by car or metro, and [the lifestyle is different],” said Dr. Bultink of Amsterdam University Medical Centers.
“It stresses that these diseases [that are associated with air pollution] although they are different in their pathophysiology, it points toward the systemic nature of rheumatic diseases, including osteoporosis,” said Dr. Araújo of Hospital Cuf Cascais (Portugal) and Hospital Ortopédico de Sant’Ana, Parede, Portugal.
The study was independently supported.Dr. Adami disclosed being a shareholder of Galapagos and Theramex.
A version of this article first appeared on Medscape.com.
AT THE EULAR 2022 CONGRESS
Could a type 2 diabetes drug tackle kidney stones?
than patients who received placebo during a median 1.5 years of treatment.
These findings are from an analysis of pooled data from phase 1-4 clinical trials of empagliflozin for blood glucose control in 15,081 patients with type 2 diabetes.
Priyadarshini Balasubramanian, MD, presented the study as a poster at the annual meeting of the Endocrine Society; the study also was published online in the Journal of Clinical Endocrinology & Metabolism.
The researchers acknowledge this was a retrospective, post-hoc analysis and that urolithiasis – a stone in the urinary tract, which includes nephrolithiasis, a kidney stone – was an adverse event, not a primary or secondary outcome.
Also, the stone composition, which might help explain how the drug may affect stone formation, is unknown.
Therefore, “dedicated randomized prospective clinical trials are needed to confirm these initial observations in patients both with and without type 2 diabetes,” said Dr. Balasubramanian, a clinical fellow in the section of endocrinology & metabolism, department of internal medicine at Yale University, New Haven, Conn.
However, “if this association is proven, empagliflozin may be used to decrease the risk of kidney stones at least in those with type 2 diabetes, but maybe also in those without diabetes,” Dr. Balasubramanian said in an interview.
Further trials are also needed to determine if this is a class effect, which is likely, she speculated, and to unravel the potential mechanism.
This is important because of the prevalence of kidney stones, which affect up to 15% of the general population and 15%-20% of patients with diabetes, she explained.
‘Provocative’ earlier findings
The current study was prompted by a recent observational study by Kasper B. Kristensen, MD, PhD, and colleagues.
Because SGLT2 inhibitors increase urinary glucose excretion through reduced renal reabsorption of glucose leading to osmotic diuresis and increased urinary flow, they hypothesized that these therapies “may reduce the risk of upper urinary tract stones (nephrolithiasis) by reducing the concentration of lithogenic substances in urine.”
Using data from Danish Health registries, they matched 12,325 individuals newly prescribed an SGLT2 inhibitor 1:1 with patients newly prescribed a glucagonlike peptide-1 (GLP1) agonist, another new class of drugs for treating type 2 diabetes.
They found a hazard ratio of 0.51 (95% confidence interval, 0.37-0.71) for incident nephrolithiasis and a hazard ratio of 0.68 (95% CI, 0.48-0.97) for recurrent nephrolithiasis for patients taking SGLT2 inhibitors versus GLP-1 agonists.
These findings are “striking,” according to Dr. Balasubramanian and colleagues. However, “these data, while provocative, were entirely retrospective and therefore possibly prone to bias,” they add.
Pooled data from 20 trials
The current study analyzed data from 20 randomized controlled trials of glycemic control in type 2 diabetes, in which 10,177 patients had received empagliflozin 10 mg or 25 mg and 4,904 patients had received placebo.
Most patients (46.5%) had participated in the EMPA-REG OUTCOMES trial, which also had the longest follow-up (2.6 years).
The researchers identified patients with a new stone from the urinary tract (including the kidney, ureter, and urethra). Patients had received either the study drug for a median of 543 days or placebo for a median of 549 days.
During treatment, 104 of 10,177 patients in the pooled empagliflozin groups and 79 of 4,904 patients in the pooled placebo groups developed a stone in the urinary tract.
This was equivalent to 0.63 new urinary-tract stones per 100 patient-years in the pooled empagliflozin groups versus 1.01 new urinary-tract stones per 100 patient-years in the pooled placebo groups.
The incidence rate ratio was 0.64 (95% CI, 0.48-0.86), in favor of empagliflozin.
When the analysis was restricted to new kidney stones, the results were similar: 75 of 10,177 patients in the pooled empagliflozin groups and 57 of 4,904 patients in the pooled placebo groups developed a kidney stone.
This was equivalent to 0.45 new kidney stones per 100 patient-years in the pooled empagliflozin groups versus 0.72 new kidney stones per 100 patient-years in the pooled placebo groups.
The IRR was 0.65 (95% CI, 0.46-0.92), in favor of empagliflozin.
Upcoming small RCT in adults without diabetes
Invited to comment on the new study, Dr. Kristensen said: “The reduced risk of SGLT2 inhibitors towards nephrolithiasis is now reported in at least two studies with different methodology, different populations, and different exposure and outcome definitions.”
“I agree that randomized clinical trials designed specifically to confirm these findings appear warranted,” added Dr. Kristensen, from the Institute of Public Health, Clinical Pharmacology, Pharmacy, and Environmental Medicine, University of Southern Denmark in Odense.
There is a need for studies in patients with and without diabetes, he added, especially ones that focus on prevention of nephrolithiasis in patients with kidney stone disease.
A new trial should shed further light on this.
Results are expected by the end of 2022 for SWEETSTONE (Impact of the SGLT2 Inhibitor Empagliflozin on Urinary Supersaturations in Kidney Stone Formers), a randomized, double-blind crossover exploratory study in 46 patients without diabetes.
This should provide preliminary data to “establish the relevance for larger trials assessing the prophylactic potential of empagliflozin in kidney stone disease,” according to an article on the trial protocol recently published in BMJ.
The trials included in the pooled dataset were funded by Boehringer Ingelheim or the Boehringer Ingelheim and Eli Lilly Diabetes Alliance. Dr. Balasubramanian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than patients who received placebo during a median 1.5 years of treatment.
These findings are from an analysis of pooled data from phase 1-4 clinical trials of empagliflozin for blood glucose control in 15,081 patients with type 2 diabetes.
Priyadarshini Balasubramanian, MD, presented the study as a poster at the annual meeting of the Endocrine Society; the study also was published online in the Journal of Clinical Endocrinology & Metabolism.
The researchers acknowledge this was a retrospective, post-hoc analysis and that urolithiasis – a stone in the urinary tract, which includes nephrolithiasis, a kidney stone – was an adverse event, not a primary or secondary outcome.
Also, the stone composition, which might help explain how the drug may affect stone formation, is unknown.
Therefore, “dedicated randomized prospective clinical trials are needed to confirm these initial observations in patients both with and without type 2 diabetes,” said Dr. Balasubramanian, a clinical fellow in the section of endocrinology & metabolism, department of internal medicine at Yale University, New Haven, Conn.
However, “if this association is proven, empagliflozin may be used to decrease the risk of kidney stones at least in those with type 2 diabetes, but maybe also in those without diabetes,” Dr. Balasubramanian said in an interview.
Further trials are also needed to determine if this is a class effect, which is likely, she speculated, and to unravel the potential mechanism.
This is important because of the prevalence of kidney stones, which affect up to 15% of the general population and 15%-20% of patients with diabetes, she explained.
‘Provocative’ earlier findings
The current study was prompted by a recent observational study by Kasper B. Kristensen, MD, PhD, and colleagues.
Because SGLT2 inhibitors increase urinary glucose excretion through reduced renal reabsorption of glucose leading to osmotic diuresis and increased urinary flow, they hypothesized that these therapies “may reduce the risk of upper urinary tract stones (nephrolithiasis) by reducing the concentration of lithogenic substances in urine.”
Using data from Danish Health registries, they matched 12,325 individuals newly prescribed an SGLT2 inhibitor 1:1 with patients newly prescribed a glucagonlike peptide-1 (GLP1) agonist, another new class of drugs for treating type 2 diabetes.
They found a hazard ratio of 0.51 (95% confidence interval, 0.37-0.71) for incident nephrolithiasis and a hazard ratio of 0.68 (95% CI, 0.48-0.97) for recurrent nephrolithiasis for patients taking SGLT2 inhibitors versus GLP-1 agonists.
These findings are “striking,” according to Dr. Balasubramanian and colleagues. However, “these data, while provocative, were entirely retrospective and therefore possibly prone to bias,” they add.
Pooled data from 20 trials
The current study analyzed data from 20 randomized controlled trials of glycemic control in type 2 diabetes, in which 10,177 patients had received empagliflozin 10 mg or 25 mg and 4,904 patients had received placebo.
Most patients (46.5%) had participated in the EMPA-REG OUTCOMES trial, which also had the longest follow-up (2.6 years).
The researchers identified patients with a new stone from the urinary tract (including the kidney, ureter, and urethra). Patients had received either the study drug for a median of 543 days or placebo for a median of 549 days.
During treatment, 104 of 10,177 patients in the pooled empagliflozin groups and 79 of 4,904 patients in the pooled placebo groups developed a stone in the urinary tract.
This was equivalent to 0.63 new urinary-tract stones per 100 patient-years in the pooled empagliflozin groups versus 1.01 new urinary-tract stones per 100 patient-years in the pooled placebo groups.
The incidence rate ratio was 0.64 (95% CI, 0.48-0.86), in favor of empagliflozin.
When the analysis was restricted to new kidney stones, the results were similar: 75 of 10,177 patients in the pooled empagliflozin groups and 57 of 4,904 patients in the pooled placebo groups developed a kidney stone.
This was equivalent to 0.45 new kidney stones per 100 patient-years in the pooled empagliflozin groups versus 0.72 new kidney stones per 100 patient-years in the pooled placebo groups.
The IRR was 0.65 (95% CI, 0.46-0.92), in favor of empagliflozin.
Upcoming small RCT in adults without diabetes
Invited to comment on the new study, Dr. Kristensen said: “The reduced risk of SGLT2 inhibitors towards nephrolithiasis is now reported in at least two studies with different methodology, different populations, and different exposure and outcome definitions.”
“I agree that randomized clinical trials designed specifically to confirm these findings appear warranted,” added Dr. Kristensen, from the Institute of Public Health, Clinical Pharmacology, Pharmacy, and Environmental Medicine, University of Southern Denmark in Odense.
There is a need for studies in patients with and without diabetes, he added, especially ones that focus on prevention of nephrolithiasis in patients with kidney stone disease.
A new trial should shed further light on this.
Results are expected by the end of 2022 for SWEETSTONE (Impact of the SGLT2 Inhibitor Empagliflozin on Urinary Supersaturations in Kidney Stone Formers), a randomized, double-blind crossover exploratory study in 46 patients without diabetes.
This should provide preliminary data to “establish the relevance for larger trials assessing the prophylactic potential of empagliflozin in kidney stone disease,” according to an article on the trial protocol recently published in BMJ.
The trials included in the pooled dataset were funded by Boehringer Ingelheim or the Boehringer Ingelheim and Eli Lilly Diabetes Alliance. Dr. Balasubramanian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
than patients who received placebo during a median 1.5 years of treatment.
These findings are from an analysis of pooled data from phase 1-4 clinical trials of empagliflozin for blood glucose control in 15,081 patients with type 2 diabetes.
Priyadarshini Balasubramanian, MD, presented the study as a poster at the annual meeting of the Endocrine Society; the study also was published online in the Journal of Clinical Endocrinology & Metabolism.
The researchers acknowledge this was a retrospective, post-hoc analysis and that urolithiasis – a stone in the urinary tract, which includes nephrolithiasis, a kidney stone – was an adverse event, not a primary or secondary outcome.
Also, the stone composition, which might help explain how the drug may affect stone formation, is unknown.
Therefore, “dedicated randomized prospective clinical trials are needed to confirm these initial observations in patients both with and without type 2 diabetes,” said Dr. Balasubramanian, a clinical fellow in the section of endocrinology & metabolism, department of internal medicine at Yale University, New Haven, Conn.
However, “if this association is proven, empagliflozin may be used to decrease the risk of kidney stones at least in those with type 2 diabetes, but maybe also in those without diabetes,” Dr. Balasubramanian said in an interview.
Further trials are also needed to determine if this is a class effect, which is likely, she speculated, and to unravel the potential mechanism.
This is important because of the prevalence of kidney stones, which affect up to 15% of the general population and 15%-20% of patients with diabetes, she explained.
‘Provocative’ earlier findings
The current study was prompted by a recent observational study by Kasper B. Kristensen, MD, PhD, and colleagues.
Because SGLT2 inhibitors increase urinary glucose excretion through reduced renal reabsorption of glucose leading to osmotic diuresis and increased urinary flow, they hypothesized that these therapies “may reduce the risk of upper urinary tract stones (nephrolithiasis) by reducing the concentration of lithogenic substances in urine.”
Using data from Danish Health registries, they matched 12,325 individuals newly prescribed an SGLT2 inhibitor 1:1 with patients newly prescribed a glucagonlike peptide-1 (GLP1) agonist, another new class of drugs for treating type 2 diabetes.
They found a hazard ratio of 0.51 (95% confidence interval, 0.37-0.71) for incident nephrolithiasis and a hazard ratio of 0.68 (95% CI, 0.48-0.97) for recurrent nephrolithiasis for patients taking SGLT2 inhibitors versus GLP-1 agonists.
These findings are “striking,” according to Dr. Balasubramanian and colleagues. However, “these data, while provocative, were entirely retrospective and therefore possibly prone to bias,” they add.
Pooled data from 20 trials
The current study analyzed data from 20 randomized controlled trials of glycemic control in type 2 diabetes, in which 10,177 patients had received empagliflozin 10 mg or 25 mg and 4,904 patients had received placebo.
Most patients (46.5%) had participated in the EMPA-REG OUTCOMES trial, which also had the longest follow-up (2.6 years).
The researchers identified patients with a new stone from the urinary tract (including the kidney, ureter, and urethra). Patients had received either the study drug for a median of 543 days or placebo for a median of 549 days.
During treatment, 104 of 10,177 patients in the pooled empagliflozin groups and 79 of 4,904 patients in the pooled placebo groups developed a stone in the urinary tract.
This was equivalent to 0.63 new urinary-tract stones per 100 patient-years in the pooled empagliflozin groups versus 1.01 new urinary-tract stones per 100 patient-years in the pooled placebo groups.
The incidence rate ratio was 0.64 (95% CI, 0.48-0.86), in favor of empagliflozin.
When the analysis was restricted to new kidney stones, the results were similar: 75 of 10,177 patients in the pooled empagliflozin groups and 57 of 4,904 patients in the pooled placebo groups developed a kidney stone.
This was equivalent to 0.45 new kidney stones per 100 patient-years in the pooled empagliflozin groups versus 0.72 new kidney stones per 100 patient-years in the pooled placebo groups.
The IRR was 0.65 (95% CI, 0.46-0.92), in favor of empagliflozin.
Upcoming small RCT in adults without diabetes
Invited to comment on the new study, Dr. Kristensen said: “The reduced risk of SGLT2 inhibitors towards nephrolithiasis is now reported in at least two studies with different methodology, different populations, and different exposure and outcome definitions.”
“I agree that randomized clinical trials designed specifically to confirm these findings appear warranted,” added Dr. Kristensen, from the Institute of Public Health, Clinical Pharmacology, Pharmacy, and Environmental Medicine, University of Southern Denmark in Odense.
There is a need for studies in patients with and without diabetes, he added, especially ones that focus on prevention of nephrolithiasis in patients with kidney stone disease.
A new trial should shed further light on this.
Results are expected by the end of 2022 for SWEETSTONE (Impact of the SGLT2 Inhibitor Empagliflozin on Urinary Supersaturations in Kidney Stone Formers), a randomized, double-blind crossover exploratory study in 46 patients without diabetes.
This should provide preliminary data to “establish the relevance for larger trials assessing the prophylactic potential of empagliflozin in kidney stone disease,” according to an article on the trial protocol recently published in BMJ.
The trials included in the pooled dataset were funded by Boehringer Ingelheim or the Boehringer Ingelheim and Eli Lilly Diabetes Alliance. Dr. Balasubramanian has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ENDO 2022
What’s the best time of day to exercise? It depends on your goals
For most of us, the “best” time of day to work out is simple: When we can.
Maybe that’s before or after work. Or when the gym offers free daycare. Or when our favorite instructor teaches our favorite class.
That’s why we call it a “routine.” And if the results are the same, it’s hard to imagine changing it up.
But what if the results aren’t the same?
They may not be, according to a new study from a research team at Skidmore College in Saratoga Springs, N.Y.
Women who worked out in the morning lost more fat, while those who trained in the evening gained more upper-body strength and power. As for men, the performance improvements were similar no matter when they exercised. But those who did so in the evening had a significant drop in blood pressure, among other benefits.
The study is part of a growing body of research showing different results for different times of day among different populations. As it turns out, when you exercise can ultimately have a big effect, not just on strength and fat loss, but also heart health, mood, and quality of sleep.
An accidental discovery
The original goal of the Skidmore study was to test a unique fitness program with a group of healthy, fit, and extremely active adults in early middle age.
The program includes four workouts a week, each with a different focus: strength, steady-pace endurance, high-intensity intervals, and flexibility (traditional stretching combined with yoga and Pilates exercises).
But because the group was so large – 27 women and 20 men completed the 3-month program – they had to split them into morning and evening workout groups.
It wasn’t until researchers looked at the results that they saw the differences between morning and evening exercise, says lead author Paul Arciero, PhD.
Dr. Arciero stressed that participants in every group got leaner and stronger. But the women who worked out in the morning got much bigger reductions in body fat and body-fat percentage than the evening group. Meanwhile, women in the evening group got much bigger gains in upper-body strength, power, and muscular endurance than their morning counterparts.
Among the men, the evening group had significantly larger improvements in blood pressure, cholesterol levels, and the percentage of fat they burned for energy, along with a bigger drop in feelings of fatigue.
Strategic timing for powerful results
Some of these findings are consistent with previous research. For example, a study published in 2021 showed that the ability to exert high effort and express strength and power peaks in the late afternoon, about the same time that your core body temperature is at its highest point.
On the other hand, you’ll probably perform better in the morning when the activity requires a lot of skill and coordination or depends on strategic decision-making.
The findings apply to both men and women.
Performance aside, exercise timing might offer strong health benefits for men with type 2 diabetes, or at high risk for it.
A study showed that men who exercised between 3 p.m. and 6 p.m. saw dramatic improvements in blood sugar management and insulin sensitivity, compared to a group that worked out between 8 a.m. and 10 a.m.
They also lost more fat during the 12-week program, even though they were doing the exact same workouts.
Train consistently, sleep well
When you exercise can affect your sleep quality in many ways, said neuroscientist Jennifer Heisz, PhD, of McMaster University, Hamilton, Ont.
First, she said, “exercise helps you fall asleep faster and sleep deeper at night.” (The only exception is if you exercise so intensely or so close to bedtime that your heart rate is still elevated.)
Second, “exercising at a consistent time every day helps regulate the body’s circadian rhythms.” It doesn’t matter if the exercise is in the morning, evening, or anywhere in between. As long as it’s predictable, it will help you fall asleep and wake up at the same times.
Outdoor exercise is even better, she said. The sun is the most powerful regulator of the circadian clock and works in tandem with physical activity.
Third, exercising at specific times can help you overcome jet lag or adjust to an earlier or later shift at work.
“Exercising at 7 a.m. or between 1 and 4 p.m. helps your circadian clock to ‘fall back’ in time, making it easier to wake up earlier,” Dr. Heisz said. If you need to train your body to wake up later in the morning, try working out between 7 p.m. and 10 p.m.
All exercise is good, but the right timing can make it even better
“The best time to exercise is when you can fit it in,” Dr. Arciero said. “You’ve got to choose the time that fits your lifestyle best.”
But context matters, he noted.
“For someone needing to achieve an improvement in their risk for cardiometabolic disease,” his study shows an advantage to working out later in the day, especially for men. If you’re more focused on building upper-body strength and power, you’ll probably get better results from training in the afternoon or evening.
And for fat loss, the Skidmore study shows better results for women who did morning workouts.
And if you’re still not sure? Try sleeping on it – preferably after your workout.
A version of this article first appeared on WebMD.com.
For most of us, the “best” time of day to work out is simple: When we can.
Maybe that’s before or after work. Or when the gym offers free daycare. Or when our favorite instructor teaches our favorite class.
That’s why we call it a “routine.” And if the results are the same, it’s hard to imagine changing it up.
But what if the results aren’t the same?
They may not be, according to a new study from a research team at Skidmore College in Saratoga Springs, N.Y.
Women who worked out in the morning lost more fat, while those who trained in the evening gained more upper-body strength and power. As for men, the performance improvements were similar no matter when they exercised. But those who did so in the evening had a significant drop in blood pressure, among other benefits.
The study is part of a growing body of research showing different results for different times of day among different populations. As it turns out, when you exercise can ultimately have a big effect, not just on strength and fat loss, but also heart health, mood, and quality of sleep.
An accidental discovery
The original goal of the Skidmore study was to test a unique fitness program with a group of healthy, fit, and extremely active adults in early middle age.
The program includes four workouts a week, each with a different focus: strength, steady-pace endurance, high-intensity intervals, and flexibility (traditional stretching combined with yoga and Pilates exercises).
But because the group was so large – 27 women and 20 men completed the 3-month program – they had to split them into morning and evening workout groups.
It wasn’t until researchers looked at the results that they saw the differences between morning and evening exercise, says lead author Paul Arciero, PhD.
Dr. Arciero stressed that participants in every group got leaner and stronger. But the women who worked out in the morning got much bigger reductions in body fat and body-fat percentage than the evening group. Meanwhile, women in the evening group got much bigger gains in upper-body strength, power, and muscular endurance than their morning counterparts.
Among the men, the evening group had significantly larger improvements in blood pressure, cholesterol levels, and the percentage of fat they burned for energy, along with a bigger drop in feelings of fatigue.
Strategic timing for powerful results
Some of these findings are consistent with previous research. For example, a study published in 2021 showed that the ability to exert high effort and express strength and power peaks in the late afternoon, about the same time that your core body temperature is at its highest point.
On the other hand, you’ll probably perform better in the morning when the activity requires a lot of skill and coordination or depends on strategic decision-making.
The findings apply to both men and women.
Performance aside, exercise timing might offer strong health benefits for men with type 2 diabetes, or at high risk for it.
A study showed that men who exercised between 3 p.m. and 6 p.m. saw dramatic improvements in blood sugar management and insulin sensitivity, compared to a group that worked out between 8 a.m. and 10 a.m.
They also lost more fat during the 12-week program, even though they were doing the exact same workouts.
Train consistently, sleep well
When you exercise can affect your sleep quality in many ways, said neuroscientist Jennifer Heisz, PhD, of McMaster University, Hamilton, Ont.
First, she said, “exercise helps you fall asleep faster and sleep deeper at night.” (The only exception is if you exercise so intensely or so close to bedtime that your heart rate is still elevated.)
Second, “exercising at a consistent time every day helps regulate the body’s circadian rhythms.” It doesn’t matter if the exercise is in the morning, evening, or anywhere in between. As long as it’s predictable, it will help you fall asleep and wake up at the same times.
Outdoor exercise is even better, she said. The sun is the most powerful regulator of the circadian clock and works in tandem with physical activity.
Third, exercising at specific times can help you overcome jet lag or adjust to an earlier or later shift at work.
“Exercising at 7 a.m. or between 1 and 4 p.m. helps your circadian clock to ‘fall back’ in time, making it easier to wake up earlier,” Dr. Heisz said. If you need to train your body to wake up later in the morning, try working out between 7 p.m. and 10 p.m.
All exercise is good, but the right timing can make it even better
“The best time to exercise is when you can fit it in,” Dr. Arciero said. “You’ve got to choose the time that fits your lifestyle best.”
But context matters, he noted.
“For someone needing to achieve an improvement in their risk for cardiometabolic disease,” his study shows an advantage to working out later in the day, especially for men. If you’re more focused on building upper-body strength and power, you’ll probably get better results from training in the afternoon or evening.
And for fat loss, the Skidmore study shows better results for women who did morning workouts.
And if you’re still not sure? Try sleeping on it – preferably after your workout.
A version of this article first appeared on WebMD.com.
For most of us, the “best” time of day to work out is simple: When we can.
Maybe that’s before or after work. Or when the gym offers free daycare. Or when our favorite instructor teaches our favorite class.
That’s why we call it a “routine.” And if the results are the same, it’s hard to imagine changing it up.
But what if the results aren’t the same?
They may not be, according to a new study from a research team at Skidmore College in Saratoga Springs, N.Y.
Women who worked out in the morning lost more fat, while those who trained in the evening gained more upper-body strength and power. As for men, the performance improvements were similar no matter when they exercised. But those who did so in the evening had a significant drop in blood pressure, among other benefits.
The study is part of a growing body of research showing different results for different times of day among different populations. As it turns out, when you exercise can ultimately have a big effect, not just on strength and fat loss, but also heart health, mood, and quality of sleep.
An accidental discovery
The original goal of the Skidmore study was to test a unique fitness program with a group of healthy, fit, and extremely active adults in early middle age.
The program includes four workouts a week, each with a different focus: strength, steady-pace endurance, high-intensity intervals, and flexibility (traditional stretching combined with yoga and Pilates exercises).
But because the group was so large – 27 women and 20 men completed the 3-month program – they had to split them into morning and evening workout groups.
It wasn’t until researchers looked at the results that they saw the differences between morning and evening exercise, says lead author Paul Arciero, PhD.
Dr. Arciero stressed that participants in every group got leaner and stronger. But the women who worked out in the morning got much bigger reductions in body fat and body-fat percentage than the evening group. Meanwhile, women in the evening group got much bigger gains in upper-body strength, power, and muscular endurance than their morning counterparts.
Among the men, the evening group had significantly larger improvements in blood pressure, cholesterol levels, and the percentage of fat they burned for energy, along with a bigger drop in feelings of fatigue.
Strategic timing for powerful results
Some of these findings are consistent with previous research. For example, a study published in 2021 showed that the ability to exert high effort and express strength and power peaks in the late afternoon, about the same time that your core body temperature is at its highest point.
On the other hand, you’ll probably perform better in the morning when the activity requires a lot of skill and coordination or depends on strategic decision-making.
The findings apply to both men and women.
Performance aside, exercise timing might offer strong health benefits for men with type 2 diabetes, or at high risk for it.
A study showed that men who exercised between 3 p.m. and 6 p.m. saw dramatic improvements in blood sugar management and insulin sensitivity, compared to a group that worked out between 8 a.m. and 10 a.m.
They also lost more fat during the 12-week program, even though they were doing the exact same workouts.
Train consistently, sleep well
When you exercise can affect your sleep quality in many ways, said neuroscientist Jennifer Heisz, PhD, of McMaster University, Hamilton, Ont.
First, she said, “exercise helps you fall asleep faster and sleep deeper at night.” (The only exception is if you exercise so intensely or so close to bedtime that your heart rate is still elevated.)
Second, “exercising at a consistent time every day helps regulate the body’s circadian rhythms.” It doesn’t matter if the exercise is in the morning, evening, or anywhere in between. As long as it’s predictable, it will help you fall asleep and wake up at the same times.
Outdoor exercise is even better, she said. The sun is the most powerful regulator of the circadian clock and works in tandem with physical activity.
Third, exercising at specific times can help you overcome jet lag or adjust to an earlier or later shift at work.
“Exercising at 7 a.m. or between 1 and 4 p.m. helps your circadian clock to ‘fall back’ in time, making it easier to wake up earlier,” Dr. Heisz said. If you need to train your body to wake up later in the morning, try working out between 7 p.m. and 10 p.m.
All exercise is good, but the right timing can make it even better
“The best time to exercise is when you can fit it in,” Dr. Arciero said. “You’ve got to choose the time that fits your lifestyle best.”
But context matters, he noted.
“For someone needing to achieve an improvement in their risk for cardiometabolic disease,” his study shows an advantage to working out later in the day, especially for men. If you’re more focused on building upper-body strength and power, you’ll probably get better results from training in the afternoon or evening.
And for fat loss, the Skidmore study shows better results for women who did morning workouts.
And if you’re still not sure? Try sleeping on it – preferably after your workout.
A version of this article first appeared on WebMD.com.
FROM FRONTIERS IN PHYSIOLOGY
Remnant cholesterol improves CV risk prediction
, a new study suggests.
The study, which followed almost 42,000 Danish individuals without a history of ischemic cardiovascular disease, diabetes, or statin use for more than 10 years, found that elevated remnant cholesterol appropriately reclassified up to 40% of those who later experienced myocardial infarction and ischemic heart disease.
“The clinical implications of our study include that doctors and patients should be aware of remnant cholesterol levels to prevent future risk of MI and ischemic heart disease,” the authors conclude.
They suggest that the development of a cardiovascular risk algorithm, including remnant cholesterol together with LDL cholesterol, would help to better identify high-risk individuals who could be candidates for statins in a primary prevention setting.
They note that physicians are encouraged to evaluate non-HDL cholesterol and/or apolipoprotein B rather than LDL cholesterol and certainly not yet remnant cholesterol, possibly because of the limited availability of remnant cholesterol values in some parts of the world.
However, they point out that remnant cholesterol can be calculated with a standard lipid profile without additional cost, which is currently already the standard procedure in the greater Copenhagen area.
“This means that the use of remnant cholesterol is easy to introduce into daily clinical practice,” they say.
The study was published online in the Journal of the American College of Cardiology.
The authors, Takahito Doi, MD, Anne Langsted, MD, and Børge Nordestgaard, from Copenhagen University Hospital, Denmark, explain that remnant cholesterol is total cholesterol minus LDL-cholesterol minus HDL-cholesterol and includes the cholesterol content of the triglyceride-rich very-low-density lipoproteins, intermediate-density lipoproteins, and chylomicron remnants in the nonfasting state.
“When these particles enter the arterial wall, they are taken up by macrophages to produce foam cells, and therefore elevated remnant cholesterol likely enhance accumulation of cholesterol in the arterial wall, leading to progression of atherosclerosis and in consequence ischemic heart disease,” they note.
They point out that most guidelines for assessment of the 10-year risk of ischemic heart and atherosclerotic cardiovascular disease include levels of total and HDL cholesterol, but remnant cholesterol levels are not included.
They conducted the current study to investigate whether elevated remnant cholesterol would lead to appropriate reclassification of individuals who later experienced MI or ischemic heart disease.
The researchers analyzed data from the Copenhagen General Population Study, which recruited individuals from the White Danish general population from 2003-2015 and followed them until 2018. Information on lifestyle, health, and medication, including statin therapy, was obtained through a questionnaire, and participants underwent physical examinations and had nonfasting blood samples drawn for biochemical measurements.
For the current study, they included 41,928 individuals aged 40-100 years enrolled before 2009 without a history of ischemic cardiovascular disease, diabetes, and statin use at baseline. The median follow-up time was 12 years. Information on diagnoses of MI and ischemic heart disease was collected from the national Danish Causes of Death Registry and all hospital admissions and diagnoses entered in the national Danish Patient Registry.
During the first 10 years of follow-up there were 1,063 MIs and 1,460 ischemic heart disease events (death of ischemic heart disease, nonfatal MI, and coronary revascularization).
Results showed that in models based on conventional risk factors estimating risk of heart disease of above or below 5% in 10 years, adding remnant cholesterol at levels above the 95th percentile, appropriately reclassified 23% of individuals who had an MI and 21% of individuals who had an ischemic heart disease event.
Using remnant cholesterol levels above the 75th percentile appropriately reclassified 10% of those who had an MI and 8% of those who had an ischemic heart disease event. No events were reclassified incorrectly.
Using measurements of remnant cholesterol also improved reclassification of individuals with heart disease risk above or below 7.5% or 10% in 10 years.
When reclassifications were combined from below to above 5%, 7.5%, and 10% risk of events, 42% of individuals with MI and 41% with ischemic heart disease events were reclassified appropriately.
In an editorial accompanying publication of the study in JACC, Peter Wilson, MD, Emory University School of Medicine, Atlanta, and Alan Remaley, MD, National Heart, Lung, and Blood Institute, say these findings rekindle interest in atherogenic nonfasting lipid measurements and emphasize an important role for elevated nonfasting remnant cholesterol as a value-added predictor of ischemic events.
The editorialists note that both fasting and nonfasting lipid values provide useful information for atherosclerotic cardiovascular disease (ASCVD) risk estimation, and elevated nonfasting remnant cholesterol appears to help identify persons at greater risk for an initial cardiovascular ischemic event.
They add that very elevated levels (above the 75th percentile) of nonfasting remnant cholesterol deserve further evaluation as a potentially valuable “modifier of ASCVD risk,” and replication of the results could move these findings forward to potentially improve prognostication and care for patients at risk for ischemic heart disease events.
An indirect measure of triglycerides
Dr. Wilson explained that remnant cholesterol is an indirect measure of triglycerides beyond LDL levels, and it is thus including a new lipid measurement in risk prediction.
“We are completely focused on LDL cholesterol,” he said. “This opens it up a bit by adding in another measure that takes into account triglycerides as well as LDL.”
He also pointed out that use of a nonfasting sample is another advantage of measuring remnant cholesterol.
“An accurate measure of LDL needs a fasting sample, which is a nuisance, whereas remnant cholesterol can be measured in a nonfasting blood sample, so it is more convenient,” Dr. Wilson said.
While this study shows this measure is helpful for risk prediction in the primary prevention population, Dr. Wilson believes remnant cholesterol could be most useful in helping to guide further medication choice in patients who are already taking statins.
“Statins mainly target LDL, but if we can also measure nonfasting triglycerides this will be helpful. It may help us select some patients who may need a different type of drug to use in addition to statins that lowers triglycerides,” he said.
This work was supported by the Global Excellence Programme, the Research Fund for the Capital Region of Denmark, the Japanese College of Cardiology Overseas Research Fellowship, and the Scandinavia Japan Sasakawa Foundation. Mr. Nordestgaard has reported consultancies or talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Amarin, Kowa, Denka, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. Dr. Doi has reported talks sponsored by MSD.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The study, which followed almost 42,000 Danish individuals without a history of ischemic cardiovascular disease, diabetes, or statin use for more than 10 years, found that elevated remnant cholesterol appropriately reclassified up to 40% of those who later experienced myocardial infarction and ischemic heart disease.
“The clinical implications of our study include that doctors and patients should be aware of remnant cholesterol levels to prevent future risk of MI and ischemic heart disease,” the authors conclude.
They suggest that the development of a cardiovascular risk algorithm, including remnant cholesterol together with LDL cholesterol, would help to better identify high-risk individuals who could be candidates for statins in a primary prevention setting.
They note that physicians are encouraged to evaluate non-HDL cholesterol and/or apolipoprotein B rather than LDL cholesterol and certainly not yet remnant cholesterol, possibly because of the limited availability of remnant cholesterol values in some parts of the world.
However, they point out that remnant cholesterol can be calculated with a standard lipid profile without additional cost, which is currently already the standard procedure in the greater Copenhagen area.
“This means that the use of remnant cholesterol is easy to introduce into daily clinical practice,” they say.
The study was published online in the Journal of the American College of Cardiology.
The authors, Takahito Doi, MD, Anne Langsted, MD, and Børge Nordestgaard, from Copenhagen University Hospital, Denmark, explain that remnant cholesterol is total cholesterol minus LDL-cholesterol minus HDL-cholesterol and includes the cholesterol content of the triglyceride-rich very-low-density lipoproteins, intermediate-density lipoproteins, and chylomicron remnants in the nonfasting state.
“When these particles enter the arterial wall, they are taken up by macrophages to produce foam cells, and therefore elevated remnant cholesterol likely enhance accumulation of cholesterol in the arterial wall, leading to progression of atherosclerosis and in consequence ischemic heart disease,” they note.
They point out that most guidelines for assessment of the 10-year risk of ischemic heart and atherosclerotic cardiovascular disease include levels of total and HDL cholesterol, but remnant cholesterol levels are not included.
They conducted the current study to investigate whether elevated remnant cholesterol would lead to appropriate reclassification of individuals who later experienced MI or ischemic heart disease.
The researchers analyzed data from the Copenhagen General Population Study, which recruited individuals from the White Danish general population from 2003-2015 and followed them until 2018. Information on lifestyle, health, and medication, including statin therapy, was obtained through a questionnaire, and participants underwent physical examinations and had nonfasting blood samples drawn for biochemical measurements.
For the current study, they included 41,928 individuals aged 40-100 years enrolled before 2009 without a history of ischemic cardiovascular disease, diabetes, and statin use at baseline. The median follow-up time was 12 years. Information on diagnoses of MI and ischemic heart disease was collected from the national Danish Causes of Death Registry and all hospital admissions and diagnoses entered in the national Danish Patient Registry.
During the first 10 years of follow-up there were 1,063 MIs and 1,460 ischemic heart disease events (death of ischemic heart disease, nonfatal MI, and coronary revascularization).
Results showed that in models based on conventional risk factors estimating risk of heart disease of above or below 5% in 10 years, adding remnant cholesterol at levels above the 95th percentile, appropriately reclassified 23% of individuals who had an MI and 21% of individuals who had an ischemic heart disease event.
Using remnant cholesterol levels above the 75th percentile appropriately reclassified 10% of those who had an MI and 8% of those who had an ischemic heart disease event. No events were reclassified incorrectly.
Using measurements of remnant cholesterol also improved reclassification of individuals with heart disease risk above or below 7.5% or 10% in 10 years.
When reclassifications were combined from below to above 5%, 7.5%, and 10% risk of events, 42% of individuals with MI and 41% with ischemic heart disease events were reclassified appropriately.
In an editorial accompanying publication of the study in JACC, Peter Wilson, MD, Emory University School of Medicine, Atlanta, and Alan Remaley, MD, National Heart, Lung, and Blood Institute, say these findings rekindle interest in atherogenic nonfasting lipid measurements and emphasize an important role for elevated nonfasting remnant cholesterol as a value-added predictor of ischemic events.
The editorialists note that both fasting and nonfasting lipid values provide useful information for atherosclerotic cardiovascular disease (ASCVD) risk estimation, and elevated nonfasting remnant cholesterol appears to help identify persons at greater risk for an initial cardiovascular ischemic event.
They add that very elevated levels (above the 75th percentile) of nonfasting remnant cholesterol deserve further evaluation as a potentially valuable “modifier of ASCVD risk,” and replication of the results could move these findings forward to potentially improve prognostication and care for patients at risk for ischemic heart disease events.
An indirect measure of triglycerides
Dr. Wilson explained that remnant cholesterol is an indirect measure of triglycerides beyond LDL levels, and it is thus including a new lipid measurement in risk prediction.
“We are completely focused on LDL cholesterol,” he said. “This opens it up a bit by adding in another measure that takes into account triglycerides as well as LDL.”
He also pointed out that use of a nonfasting sample is another advantage of measuring remnant cholesterol.
“An accurate measure of LDL needs a fasting sample, which is a nuisance, whereas remnant cholesterol can be measured in a nonfasting blood sample, so it is more convenient,” Dr. Wilson said.
While this study shows this measure is helpful for risk prediction in the primary prevention population, Dr. Wilson believes remnant cholesterol could be most useful in helping to guide further medication choice in patients who are already taking statins.
“Statins mainly target LDL, but if we can also measure nonfasting triglycerides this will be helpful. It may help us select some patients who may need a different type of drug to use in addition to statins that lowers triglycerides,” he said.
This work was supported by the Global Excellence Programme, the Research Fund for the Capital Region of Denmark, the Japanese College of Cardiology Overseas Research Fellowship, and the Scandinavia Japan Sasakawa Foundation. Mr. Nordestgaard has reported consultancies or talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Amarin, Kowa, Denka, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. Dr. Doi has reported talks sponsored by MSD.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The study, which followed almost 42,000 Danish individuals without a history of ischemic cardiovascular disease, diabetes, or statin use for more than 10 years, found that elevated remnant cholesterol appropriately reclassified up to 40% of those who later experienced myocardial infarction and ischemic heart disease.
“The clinical implications of our study include that doctors and patients should be aware of remnant cholesterol levels to prevent future risk of MI and ischemic heart disease,” the authors conclude.
They suggest that the development of a cardiovascular risk algorithm, including remnant cholesterol together with LDL cholesterol, would help to better identify high-risk individuals who could be candidates for statins in a primary prevention setting.
They note that physicians are encouraged to evaluate non-HDL cholesterol and/or apolipoprotein B rather than LDL cholesterol and certainly not yet remnant cholesterol, possibly because of the limited availability of remnant cholesterol values in some parts of the world.
However, they point out that remnant cholesterol can be calculated with a standard lipid profile without additional cost, which is currently already the standard procedure in the greater Copenhagen area.
“This means that the use of remnant cholesterol is easy to introduce into daily clinical practice,” they say.
The study was published online in the Journal of the American College of Cardiology.
The authors, Takahito Doi, MD, Anne Langsted, MD, and Børge Nordestgaard, from Copenhagen University Hospital, Denmark, explain that remnant cholesterol is total cholesterol minus LDL-cholesterol minus HDL-cholesterol and includes the cholesterol content of the triglyceride-rich very-low-density lipoproteins, intermediate-density lipoproteins, and chylomicron remnants in the nonfasting state.
“When these particles enter the arterial wall, they are taken up by macrophages to produce foam cells, and therefore elevated remnant cholesterol likely enhance accumulation of cholesterol in the arterial wall, leading to progression of atherosclerosis and in consequence ischemic heart disease,” they note.
They point out that most guidelines for assessment of the 10-year risk of ischemic heart and atherosclerotic cardiovascular disease include levels of total and HDL cholesterol, but remnant cholesterol levels are not included.
They conducted the current study to investigate whether elevated remnant cholesterol would lead to appropriate reclassification of individuals who later experienced MI or ischemic heart disease.
The researchers analyzed data from the Copenhagen General Population Study, which recruited individuals from the White Danish general population from 2003-2015 and followed them until 2018. Information on lifestyle, health, and medication, including statin therapy, was obtained through a questionnaire, and participants underwent physical examinations and had nonfasting blood samples drawn for biochemical measurements.
For the current study, they included 41,928 individuals aged 40-100 years enrolled before 2009 without a history of ischemic cardiovascular disease, diabetes, and statin use at baseline. The median follow-up time was 12 years. Information on diagnoses of MI and ischemic heart disease was collected from the national Danish Causes of Death Registry and all hospital admissions and diagnoses entered in the national Danish Patient Registry.
During the first 10 years of follow-up there were 1,063 MIs and 1,460 ischemic heart disease events (death of ischemic heart disease, nonfatal MI, and coronary revascularization).
Results showed that in models based on conventional risk factors estimating risk of heart disease of above or below 5% in 10 years, adding remnant cholesterol at levels above the 95th percentile, appropriately reclassified 23% of individuals who had an MI and 21% of individuals who had an ischemic heart disease event.
Using remnant cholesterol levels above the 75th percentile appropriately reclassified 10% of those who had an MI and 8% of those who had an ischemic heart disease event. No events were reclassified incorrectly.
Using measurements of remnant cholesterol also improved reclassification of individuals with heart disease risk above or below 7.5% or 10% in 10 years.
When reclassifications were combined from below to above 5%, 7.5%, and 10% risk of events, 42% of individuals with MI and 41% with ischemic heart disease events were reclassified appropriately.
In an editorial accompanying publication of the study in JACC, Peter Wilson, MD, Emory University School of Medicine, Atlanta, and Alan Remaley, MD, National Heart, Lung, and Blood Institute, say these findings rekindle interest in atherogenic nonfasting lipid measurements and emphasize an important role for elevated nonfasting remnant cholesterol as a value-added predictor of ischemic events.
The editorialists note that both fasting and nonfasting lipid values provide useful information for atherosclerotic cardiovascular disease (ASCVD) risk estimation, and elevated nonfasting remnant cholesterol appears to help identify persons at greater risk for an initial cardiovascular ischemic event.
They add that very elevated levels (above the 75th percentile) of nonfasting remnant cholesterol deserve further evaluation as a potentially valuable “modifier of ASCVD risk,” and replication of the results could move these findings forward to potentially improve prognostication and care for patients at risk for ischemic heart disease events.
An indirect measure of triglycerides
Dr. Wilson explained that remnant cholesterol is an indirect measure of triglycerides beyond LDL levels, and it is thus including a new lipid measurement in risk prediction.
“We are completely focused on LDL cholesterol,” he said. “This opens it up a bit by adding in another measure that takes into account triglycerides as well as LDL.”
He also pointed out that use of a nonfasting sample is another advantage of measuring remnant cholesterol.
“An accurate measure of LDL needs a fasting sample, which is a nuisance, whereas remnant cholesterol can be measured in a nonfasting blood sample, so it is more convenient,” Dr. Wilson said.
While this study shows this measure is helpful for risk prediction in the primary prevention population, Dr. Wilson believes remnant cholesterol could be most useful in helping to guide further medication choice in patients who are already taking statins.
“Statins mainly target LDL, but if we can also measure nonfasting triglycerides this will be helpful. It may help us select some patients who may need a different type of drug to use in addition to statins that lowers triglycerides,” he said.
This work was supported by the Global Excellence Programme, the Research Fund for the Capital Region of Denmark, the Japanese College of Cardiology Overseas Research Fellowship, and the Scandinavia Japan Sasakawa Foundation. Mr. Nordestgaard has reported consultancies or talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Amarin, Kowa, Denka, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. Dr. Doi has reported talks sponsored by MSD.
A version of this article first appeared on Medscape.com.
FDA approves setmelanotide for obesity in Bardet-Biedl syndrome
The Food and Drug Administration has approved a supplemental indication for setmelanotide (Imcivree, Rhythm Pharmaceuticals) injection for chronic weight management in adults and pediatric patients age 6 and older with obesity due to Bardet-Biedl Syndrome (BBS).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for BBS, a rare genetic disorder that impairs a hunger signal along the melanocortin-4 receptor (MC4R) pathway.
BBS affects an estimated 1,500-2,500 people in the United States.
Individuals with BBS typically have obesity that starts at age 1 along with insatiable hunger (hyperphagia). Available weight management options are generally unsuccessful.
Other symptoms may include retinal degeneration, reduced kidney function, or extra digits of the hands or feet.
Setmelanotide received priority review, orphan drug designation, and breakthrough designation for this new indication.
As previously reported, in November 2020, the FDA approved setmelanotide for weight management in adults and children as young as 6 years with obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing – who also have impaired hunger signaling from the brain.
These individuals have a normal weight at birth but develop persistent, severe obesity within months due to hyperphagia.
The FDA approval of Imcivree for BBS “represents a significant milestone for Rhythm [Pharmaceuticals], validating our strategy of developing Imcivree for people with hyperphagia and severe obesity caused by rare MC4R-pathway diseases and allowing us to provide our precision therapy to an established community of patients living with BBS and their families who are eagerly awaiting a new treatment option,” said David Meeker, MD, chair, president and CEO of Rhythm, in a press release.
Safety, effectiveness in 66-week trial in 44 patients
The safety and effectiveness of setmelanotidewas evaluated in a 66-week phase 3 clinical trial that enrolled 44 patients age 6 and older who had a diagnosis of BBS and obesity – defined as a body mass index greater than or equal to 30 kg/m2 or greater than or equal to 97th percentile for pediatric patients.
After an initial 14-week, randomized, double-blind, placebo-controlled treatment period, patients entered a 52-week, open-label period.
The trial met its primary endpoint and all key secondary endpoints, with statistically significant reductions in weight and hunger at 52 weeks on therapy.
- After 52 weeks of treatment, patients taking setmelanotide lost, on average, 7.9% of their initial BMI.
- 61% of patients lost 5% or more of their initial BMI, and 39% lost 10% or more of their initial BMI.
- In the 14-week, placebo-controlled treatment, on average, BMI dropped by 4.6% in the 22 patients treated with the study drug and dropped 0.1% in the 22 patients treated with placebo.
- At 52 weeks, the 14 patients aged 12 and older who were able to self-report their hunger had a significant –2.1 mean change in hunger score.
Setmelanotide is associated with the following warnings and precautions:
- Spontaneous penile erections in males and sexual adverse reactions in females. Instruct males with erection lasting longer than 4 hours to seek emergency medical attention.
- Depression and suicidal ideation. Monitor patients for new onset or worsening depression or suicidal thoughts or behaviors. Consider discontinuing the drug if patients have suicidal thoughts or behaviors or clinically significant or persistent depression symptoms.
- Skin pigmentation and darkening of preexisting nevi (moles). Examine skin before and during treatment.
- Setmelanotide is not approved for use in neonates or infants. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low-birth-weight infants treated with benzyl alcohol-preserved drugs.
The most common adverse reactions (with an incidence greater than or equal to 20%) included skin hyperpigmentation, injection site reactions, nausea, headache, diarrhea, abdominal pain, vomiting, depression, and spontaneous penile erection.
The FDA did not approve the company’s supplemental new drug application for setmelanotide in Alström syndrome.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a supplemental indication for setmelanotide (Imcivree, Rhythm Pharmaceuticals) injection for chronic weight management in adults and pediatric patients age 6 and older with obesity due to Bardet-Biedl Syndrome (BBS).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for BBS, a rare genetic disorder that impairs a hunger signal along the melanocortin-4 receptor (MC4R) pathway.
BBS affects an estimated 1,500-2,500 people in the United States.
Individuals with BBS typically have obesity that starts at age 1 along with insatiable hunger (hyperphagia). Available weight management options are generally unsuccessful.
Other symptoms may include retinal degeneration, reduced kidney function, or extra digits of the hands or feet.
Setmelanotide received priority review, orphan drug designation, and breakthrough designation for this new indication.
As previously reported, in November 2020, the FDA approved setmelanotide for weight management in adults and children as young as 6 years with obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing – who also have impaired hunger signaling from the brain.
These individuals have a normal weight at birth but develop persistent, severe obesity within months due to hyperphagia.
The FDA approval of Imcivree for BBS “represents a significant milestone for Rhythm [Pharmaceuticals], validating our strategy of developing Imcivree for people with hyperphagia and severe obesity caused by rare MC4R-pathway diseases and allowing us to provide our precision therapy to an established community of patients living with BBS and their families who are eagerly awaiting a new treatment option,” said David Meeker, MD, chair, president and CEO of Rhythm, in a press release.
Safety, effectiveness in 66-week trial in 44 patients
The safety and effectiveness of setmelanotidewas evaluated in a 66-week phase 3 clinical trial that enrolled 44 patients age 6 and older who had a diagnosis of BBS and obesity – defined as a body mass index greater than or equal to 30 kg/m2 or greater than or equal to 97th percentile for pediatric patients.
After an initial 14-week, randomized, double-blind, placebo-controlled treatment period, patients entered a 52-week, open-label period.
The trial met its primary endpoint and all key secondary endpoints, with statistically significant reductions in weight and hunger at 52 weeks on therapy.
- After 52 weeks of treatment, patients taking setmelanotide lost, on average, 7.9% of their initial BMI.
- 61% of patients lost 5% or more of their initial BMI, and 39% lost 10% or more of their initial BMI.
- In the 14-week, placebo-controlled treatment, on average, BMI dropped by 4.6% in the 22 patients treated with the study drug and dropped 0.1% in the 22 patients treated with placebo.
- At 52 weeks, the 14 patients aged 12 and older who were able to self-report their hunger had a significant –2.1 mean change in hunger score.
Setmelanotide is associated with the following warnings and precautions:
- Spontaneous penile erections in males and sexual adverse reactions in females. Instruct males with erection lasting longer than 4 hours to seek emergency medical attention.
- Depression and suicidal ideation. Monitor patients for new onset or worsening depression or suicidal thoughts or behaviors. Consider discontinuing the drug if patients have suicidal thoughts or behaviors or clinically significant or persistent depression symptoms.
- Skin pigmentation and darkening of preexisting nevi (moles). Examine skin before and during treatment.
- Setmelanotide is not approved for use in neonates or infants. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low-birth-weight infants treated with benzyl alcohol-preserved drugs.
The most common adverse reactions (with an incidence greater than or equal to 20%) included skin hyperpigmentation, injection site reactions, nausea, headache, diarrhea, abdominal pain, vomiting, depression, and spontaneous penile erection.
The FDA did not approve the company’s supplemental new drug application for setmelanotide in Alström syndrome.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a supplemental indication for setmelanotide (Imcivree, Rhythm Pharmaceuticals) injection for chronic weight management in adults and pediatric patients age 6 and older with obesity due to Bardet-Biedl Syndrome (BBS).
Setmelanotide, a melanocortin-4 receptor (MC4R) agonist, is the first FDA-approved therapy for BBS, a rare genetic disorder that impairs a hunger signal along the melanocortin-4 receptor (MC4R) pathway.
BBS affects an estimated 1,500-2,500 people in the United States.
Individuals with BBS typically have obesity that starts at age 1 along with insatiable hunger (hyperphagia). Available weight management options are generally unsuccessful.
Other symptoms may include retinal degeneration, reduced kidney function, or extra digits of the hands or feet.
Setmelanotide received priority review, orphan drug designation, and breakthrough designation for this new indication.
As previously reported, in November 2020, the FDA approved setmelanotide for weight management in adults and children as young as 6 years with obesity due to proopiomelanocortin (POMC), proprotein convertase subtilisin/kexin type 1 (PCSK1), or leptin receptor (LEPR) deficiency confirmed by genetic testing – who also have impaired hunger signaling from the brain.
These individuals have a normal weight at birth but develop persistent, severe obesity within months due to hyperphagia.
The FDA approval of Imcivree for BBS “represents a significant milestone for Rhythm [Pharmaceuticals], validating our strategy of developing Imcivree for people with hyperphagia and severe obesity caused by rare MC4R-pathway diseases and allowing us to provide our precision therapy to an established community of patients living with BBS and their families who are eagerly awaiting a new treatment option,” said David Meeker, MD, chair, president and CEO of Rhythm, in a press release.
Safety, effectiveness in 66-week trial in 44 patients
The safety and effectiveness of setmelanotidewas evaluated in a 66-week phase 3 clinical trial that enrolled 44 patients age 6 and older who had a diagnosis of BBS and obesity – defined as a body mass index greater than or equal to 30 kg/m2 or greater than or equal to 97th percentile for pediatric patients.
After an initial 14-week, randomized, double-blind, placebo-controlled treatment period, patients entered a 52-week, open-label period.
The trial met its primary endpoint and all key secondary endpoints, with statistically significant reductions in weight and hunger at 52 weeks on therapy.
- After 52 weeks of treatment, patients taking setmelanotide lost, on average, 7.9% of their initial BMI.
- 61% of patients lost 5% or more of their initial BMI, and 39% lost 10% or more of their initial BMI.
- In the 14-week, placebo-controlled treatment, on average, BMI dropped by 4.6% in the 22 patients treated with the study drug and dropped 0.1% in the 22 patients treated with placebo.
- At 52 weeks, the 14 patients aged 12 and older who were able to self-report their hunger had a significant –2.1 mean change in hunger score.
Setmelanotide is associated with the following warnings and precautions:
- Spontaneous penile erections in males and sexual adverse reactions in females. Instruct males with erection lasting longer than 4 hours to seek emergency medical attention.
- Depression and suicidal ideation. Monitor patients for new onset or worsening depression or suicidal thoughts or behaviors. Consider discontinuing the drug if patients have suicidal thoughts or behaviors or clinically significant or persistent depression symptoms.
- Skin pigmentation and darkening of preexisting nevi (moles). Examine skin before and during treatment.
- Setmelanotide is not approved for use in neonates or infants. Serious and fatal adverse reactions including “gasping syndrome” can occur in neonates and low-birth-weight infants treated with benzyl alcohol-preserved drugs.
The most common adverse reactions (with an incidence greater than or equal to 20%) included skin hyperpigmentation, injection site reactions, nausea, headache, diarrhea, abdominal pain, vomiting, depression, and spontaneous penile erection.
The FDA did not approve the company’s supplemental new drug application for setmelanotide in Alström syndrome.
A version of this article first appeared on Medscape.com.
Heart failure: Medicare cost sharing may put quadruple therapy out of reach
Out-of-pocket (OOP) costs for Medicare enrollees receiving quadruple drug therapy for heart failure with reduced ejection fraction were “substantially higher than regimens limited to generically available medications,” according to a new analysis of prescription drug plans.
“Despite the clinical benefit of quadruple therapy” consisting of beta-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors, “coverage was restricted primarily through cost sharing, and estimated annual OOP costs for beneficiaries were [over $2,000] per year under most plans,” wrote Kamil F. Faridi, MD, and associates. The findings were published in the Journal of the American College of Cardiology.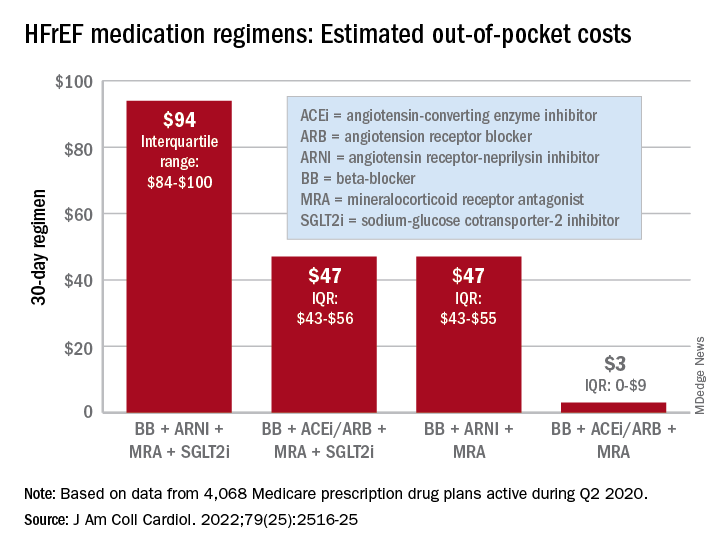
For just 1 month of quadruple drug therapy for heart failure with reduced ejection fraction (HFrEF), the estimated median OOP cost was $94 for individuals covered by a Medicare prescription drug plan during the second quarter of 2020, with the majority coming from the ARNI (median, $47) and the SGLT2 inhibitor (median, $45). Alternative HFrEF regimens were significantly less costly, ranging from $3 to $47 OOP, the investigators reported.
Almost all of the 4,068 plans participating in Medicare at that time covered quadruple therapy for HFrEF, but more than 99% restricted coverage by instituting cost sharing for medications at tier level 3 and above on the drug formularies. Such restrictions for ARNIs and SGLT2 inhibitors “might not be readily apparent to prescribing physicians,” wrote Dr. Faridi of Yale University, New Haven, Conn., and associates.
Other methods of regulating coverage were less common. Prior authorization of ARNIs was invoked by about a quarter of the plans, but none required authorization for any of the other drugs involved, and few plans used step therapy-requirements involving lower-cost alternatives, they noted.
“The use of cost sharing restricts access through high OOP costs for patients. Furthermore, these policies likely disadvantage relatively poorer patients (although the poorest Medicare patients will tend to be dual-enrolled in Medicaid and protected from cost sharing),” Jason H. Wasfy, MD, and Anna C. O’Kelly, MD, said in an accompanying editorial comment .
Since acceptable cost-effectiveness has been demonstrated for dapagliflozin, an SGLT1 inhibitor, and for the ARNIs, and because these medications have no generic equivalents, health plans should “use the discretion they have under Medicare Part D to reduce cost sharing for patients with HFrEF,” Dr. Wasfy and Dr. O’Kelly wrote, adding that the current study “demonstrates that without consensus on cost effectiveness from the societal perspective, costs can be imposed directly on patients in ways that slow uptake of cost-effective drugs.”
Data for all Medicare Advantage plans (n = 3,167) and standalone Part D plans (n = 901) came from the Medicare Prescription Drug Plan Formulary and Pricing Information Files. Annual OOP costs were estimated “using each phase of a 2020 Medicare part D standard benefit,” including deductible, standard coverage, coverage gap, and catastrophic coverage, the investigators explained.
Dr. Faridi and associates did not report any direct funding sources for their study. Dr Faridi received a grant from the National Institutes of Health outside the scope of the present work, and other investigators disclosed ties to the Food and Drug Administration, the Centers for Medicare and Medicaid Services, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Amgen, Cytokinetics, and the Institute for Clinical and Economic Review.
Dr. Wasfy is supported by the American Heart Association and has received consulting fees from Pfizer and honoraria from the Institute for Clinical and Economic Review. Dr. O’Kelly has no relevant disclosures.
Out-of-pocket (OOP) costs for Medicare enrollees receiving quadruple drug therapy for heart failure with reduced ejection fraction were “substantially higher than regimens limited to generically available medications,” according to a new analysis of prescription drug plans.
“Despite the clinical benefit of quadruple therapy” consisting of beta-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors, “coverage was restricted primarily through cost sharing, and estimated annual OOP costs for beneficiaries were [over $2,000] per year under most plans,” wrote Kamil F. Faridi, MD, and associates. The findings were published in the Journal of the American College of Cardiology.
For just 1 month of quadruple drug therapy for heart failure with reduced ejection fraction (HFrEF), the estimated median OOP cost was $94 for individuals covered by a Medicare prescription drug plan during the second quarter of 2020, with the majority coming from the ARNI (median, $47) and the SGLT2 inhibitor (median, $45). Alternative HFrEF regimens were significantly less costly, ranging from $3 to $47 OOP, the investigators reported.
Almost all of the 4,068 plans participating in Medicare at that time covered quadruple therapy for HFrEF, but more than 99% restricted coverage by instituting cost sharing for medications at tier level 3 and above on the drug formularies. Such restrictions for ARNIs and SGLT2 inhibitors “might not be readily apparent to prescribing physicians,” wrote Dr. Faridi of Yale University, New Haven, Conn., and associates.
Other methods of regulating coverage were less common. Prior authorization of ARNIs was invoked by about a quarter of the plans, but none required authorization for any of the other drugs involved, and few plans used step therapy-requirements involving lower-cost alternatives, they noted.
“The use of cost sharing restricts access through high OOP costs for patients. Furthermore, these policies likely disadvantage relatively poorer patients (although the poorest Medicare patients will tend to be dual-enrolled in Medicaid and protected from cost sharing),” Jason H. Wasfy, MD, and Anna C. O’Kelly, MD, said in an accompanying editorial comment .
Since acceptable cost-effectiveness has been demonstrated for dapagliflozin, an SGLT1 inhibitor, and for the ARNIs, and because these medications have no generic equivalents, health plans should “use the discretion they have under Medicare Part D to reduce cost sharing for patients with HFrEF,” Dr. Wasfy and Dr. O’Kelly wrote, adding that the current study “demonstrates that without consensus on cost effectiveness from the societal perspective, costs can be imposed directly on patients in ways that slow uptake of cost-effective drugs.”
Data for all Medicare Advantage plans (n = 3,167) and standalone Part D plans (n = 901) came from the Medicare Prescription Drug Plan Formulary and Pricing Information Files. Annual OOP costs were estimated “using each phase of a 2020 Medicare part D standard benefit,” including deductible, standard coverage, coverage gap, and catastrophic coverage, the investigators explained.
Dr. Faridi and associates did not report any direct funding sources for their study. Dr Faridi received a grant from the National Institutes of Health outside the scope of the present work, and other investigators disclosed ties to the Food and Drug Administration, the Centers for Medicare and Medicaid Services, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Amgen, Cytokinetics, and the Institute for Clinical and Economic Review.
Dr. Wasfy is supported by the American Heart Association and has received consulting fees from Pfizer and honoraria from the Institute for Clinical and Economic Review. Dr. O’Kelly has no relevant disclosures.
Out-of-pocket (OOP) costs for Medicare enrollees receiving quadruple drug therapy for heart failure with reduced ejection fraction were “substantially higher than regimens limited to generically available medications,” according to a new analysis of prescription drug plans.
“Despite the clinical benefit of quadruple therapy” consisting of beta-blockers, angiotensin receptor-neprilysin inhibitors (ARNIs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose cotransporter-2 (SGLT2) inhibitors, “coverage was restricted primarily through cost sharing, and estimated annual OOP costs for beneficiaries were [over $2,000] per year under most plans,” wrote Kamil F. Faridi, MD, and associates. The findings were published in the Journal of the American College of Cardiology.
For just 1 month of quadruple drug therapy for heart failure with reduced ejection fraction (HFrEF), the estimated median OOP cost was $94 for individuals covered by a Medicare prescription drug plan during the second quarter of 2020, with the majority coming from the ARNI (median, $47) and the SGLT2 inhibitor (median, $45). Alternative HFrEF regimens were significantly less costly, ranging from $3 to $47 OOP, the investigators reported.
Almost all of the 4,068 plans participating in Medicare at that time covered quadruple therapy for HFrEF, but more than 99% restricted coverage by instituting cost sharing for medications at tier level 3 and above on the drug formularies. Such restrictions for ARNIs and SGLT2 inhibitors “might not be readily apparent to prescribing physicians,” wrote Dr. Faridi of Yale University, New Haven, Conn., and associates.
Other methods of regulating coverage were less common. Prior authorization of ARNIs was invoked by about a quarter of the plans, but none required authorization for any of the other drugs involved, and few plans used step therapy-requirements involving lower-cost alternatives, they noted.
“The use of cost sharing restricts access through high OOP costs for patients. Furthermore, these policies likely disadvantage relatively poorer patients (although the poorest Medicare patients will tend to be dual-enrolled in Medicaid and protected from cost sharing),” Jason H. Wasfy, MD, and Anna C. O’Kelly, MD, said in an accompanying editorial comment .
Since acceptable cost-effectiveness has been demonstrated for dapagliflozin, an SGLT1 inhibitor, and for the ARNIs, and because these medications have no generic equivalents, health plans should “use the discretion they have under Medicare Part D to reduce cost sharing for patients with HFrEF,” Dr. Wasfy and Dr. O’Kelly wrote, adding that the current study “demonstrates that without consensus on cost effectiveness from the societal perspective, costs can be imposed directly on patients in ways that slow uptake of cost-effective drugs.”
Data for all Medicare Advantage plans (n = 3,167) and standalone Part D plans (n = 901) came from the Medicare Prescription Drug Plan Formulary and Pricing Information Files. Annual OOP costs were estimated “using each phase of a 2020 Medicare part D standard benefit,” including deductible, standard coverage, coverage gap, and catastrophic coverage, the investigators explained.
Dr. Faridi and associates did not report any direct funding sources for their study. Dr Faridi received a grant from the National Institutes of Health outside the scope of the present work, and other investigators disclosed ties to the Food and Drug Administration, the Centers for Medicare and Medicaid Services, Johnson & Johnson, AstraZeneca, Boehringer Ingelheim, Amgen, Cytokinetics, and the Institute for Clinical and Economic Review.
Dr. Wasfy is supported by the American Heart Association and has received consulting fees from Pfizer and honoraria from the Institute for Clinical and Economic Review. Dr. O’Kelly has no relevant disclosures.
FROM THE JOURNAL Of the AMERICAN COLLEGE OF CARDIOLOGY
Can the ketogenic diet treat polycystic ovary syndrome?
MADRID – During the International Scientific Symposium “New Frontiers in Scientific Research” that recently took place in Barcelona, specialists analyzed the role of the very-low-calorie ketogenic diet. This analysis was in relation to three comorbidities that have a higher incidence among overweight and obese patients: polycystic ovary syndrome, nonalcoholic fatty liver disease, and type 2 diabetes. The experts’ aim? To analyze and update the latest evidence on the benefits of this dietary choice.
Polycystic ovary syndrome
Alessandra Gambineri, MD, PhD, associate professor at the department of medicine and surgery (DIMEC) at the University of Bologna, Italy, addressed the link between obesity and polycystic ovary syndrome, which she described as a chronic disease that affects about 10% of women of childbearing age and that presents diverse phenotypes with different characteristics.
“The pathophysiology of this syndrome is characterized by the interaction of three factors: androgen excess, adipose tissue dysfunction, and insulin resistance. These factors interact with each other and are expressed differently in each phenotype,” said Dr. Gambineri.
She indicated that adipose tissue dysfunction is central to this pathology. This centrality results from its association with secretions, such as free fatty acids, proinflammatory cytokines, certain adipokines that promote insulin resistance, glucocorticosteroids, androgens, and oxidative stress.
“Similarly, the oxidative stress that characterizes this syndrome is increasingly present in obese individuals,” said Dr. Gambineri. “This oxidative stress also produces ovary hypotoxicity that aggravates ovulatory function. In this context, the very-low-calorie ketogenic diet can be useful in several ways: weight reduction; promoting the loss of mainly visceral/abdominal fat; decreasing lipotoxicity; and improving inflammation, hyperinsulinemia, and insulin resistance.”
This was the path followed to carry out a study that aimed to analyze the effects of the very-low-calorie ketogenic diet on manifestations of polycystic ovary syndrome in the obesity phenotype. Dr. Gambineri presented its results.
“The objective was to compare the effects of a very-low-calorie ketogenic diet and the standard low-calorie (hypocaloric) diet as a control group,” she said. “The effects studied include body weight, insulin resistance, menstrual cycle, ovulation, ovarian morphology, and hyperandrogenism in a population of 30 obese women with polycystic ovary syndrome and insulin resistance.”
Study participants had a diagnosis of polycystic ovary syndrome as defined by the National Institutes of Health criteria and were aged 18-45 years. These women were randomly assigned to two groups of equal size: experimental (very-low-calorie ketogenic diet) and control (hypocaloric diet). “The women assigned to the experimental group followed the ketogenic stage for eight weeks and then moved to the second, low-calorie diet phase for an additional eight weeks, while those in the control group (hypocaloric diet) followed the low-calorie diet for all 16 weeks.”
The primary outcomes were changes in weight and body composition, specifically fat mass and lean mass, measured by bioimpedance. “The changes observed in the following aspects were considered secondary outcomes: abdominal fat distribution, metabolic parameters, ovulation, ovarian morphology, hirsutism, hyperandrogenism, psychological well-being, and psychological distress,” said Dr. Gambineri. “Any reduction in the ovarian stroma, the area where androgens are synthesized, was also analyzed.”
The study authors found that although BMI decreased in both groups, this reduction was greater in the group that followed the very-low-calorie ketogenic diet. Significant weight loss was observed in both groups, 12.4 kg versus 4.7 kg. Significant differences were also observed in waist circumference (−8.1% in the experimental group vs. −2.2% in the control group), fat mass (−15.1% vs. −8.5%), and free testosterone (−30.3% vs. +10.6%). Only the experimental group saw a reduction in insulin.
“A key point regarding hyperandrogenism, especially regarding what’s referred to as free testosterone, there was only a significant reduction in the very-low-calorie ketogenic diet group,” said Dr. Gambineri. “This reduction was especially evident in the first part of the study, coinciding with the ketogenic period. The reason for this effect lies in the significant increase in the concentration of sex hormone-binding globulins, SHB6. Said globulins bind to the testosterone present in female blood, producing a reduction in free testosterone, a very important effect considering that this syndrome is an androgenic disorder. Furthermore, current treatments for polycystic ovary syndrome do not reduce free testosterone as much as this dietary approach does.”
For the specialist, among all these positive effects in these patients, perhaps most important is the notable improvement that occurs in ovulation. “At the beginning of the study, only 38.5% of the participants in the experimental group and 14.3% of those in the control group had ovulatory cycles. After the intervention, 84.6% managed to ovulate, compared to 35.7% who achieved this goal in the other group.”
Dr. Gambineri suggested that this method is “valid for reducing fat mass and rapidly improving hyperandrogenism and ovulatory dysfunction in women with obesity and polycystic ovary syndrome.”
Reversing type 2 diabetes?
Daniela Sofrà, MD, an endocrinologist specializing in diabetology at La Source Clinic, Lausanne, Switzerland, reviewed the current evidence on the role of the very-low-calorie ketogenic diet in the management of type 2 diabetes.
“It’s time to rethink diabetes treatment and focus efforts on managing obesity as an associated factor,” she said. “One of the hypotheses being examined in this regard is the twin cycle, which postulates that type 2 diabetes is the result of excess fat in the liver. This in turn is associated with insulin resistance with pancreas dysfunction.”
Dr. Sofrà added that there is a study documenting for the first time the reversibility of the morphology of the diabetic pancreas after caloric restriction with the very-low-calorie ketogenic diet. “The reason for this effect is the use of visceral and intrahepatic fat, which can lead to the remission of the clinical manifestation of type 2 diabetes, understanding as such the definition made by the American Diabetes Association: glycosylated hemoglobin < 6.5% without pharmacological therapy.”
Specifically, the results of this research showed that after following the very-low-calorie ketogenic diet and achieving a 15% weight loss (mean weight loss of the participants), liver glucose levels returned to normal levels within 7 days. Beta cell function returned to near normal within 8 weeks.
“Subsequent studies have shown the durability of remission of type 2 diabetes, thanks to the reactivation of the insulin-secreting function of beta cells that had become dedifferentiated in the face of chronic nutrient excess. Specifically, 6 out of 10 patients maintained glycosylated hemoglobin < 6% after 6 months without the need for pharmacological therapy,” Dr. Sofrà added.
Likewise, she highlighted that the probability of achieving remission is mainly determined by the duration of the disease. “The years with diabetes are one of the main predictors of the response that the patient will have with this dietary intervention. Studies have shown that remission is possible in patients with diabetes for less than 6 years, although there are other projects that indicate that it can be achieved with up to 10 years’ duration.”
Based on these data, Dr. Sofrà emphasized the pleiotropic effects of the very-low-calorie ketogenic diet on glycemic control, favoring the possible remission of diabetes or the reduction of drugs, as well as the reduction of the HOMA-IR index (insulin resistance) and waist circumference in people with type 2 diabetes.
Nonalcoholic fatty liver disease
The third comorbidity of obesity that may benefit from the very-low-calorie ketogenic diet is hepatic steatosis, or nonalcoholic fatty liver disease, said Hardy Walle, MD, an internal medicine specialist and director/founder of the Bodymed center, Kirkel, Germany, and one of the authors of this research.
“Recent research shows that ectopic fat and nonalcoholic fatty liver disease could be considered a cause, or at least one of the causes, of most of the diseases that affect the population as a consequence of overweight and obesity,” said Dr. Walle. “Some authors have stated that without fatty liver, there is no type 2 diabetes.”
Dr. Walle pointed out that between 30% and 40% of the adult population has nonalcoholic fatty liver disease, a percentage that increases considerably in people with obesity, reaching 70% prevalence and increasing, in the case of type 2 diabetes, to almost 90%. “Even normal weight does not rule out fatty liver; in fact, about 15% of people with nonalcoholic fatty liver disease are not overweight.”
In a setting where there are no approved drugs for the treatment of fatty liver (the current standard approach focuses on lifestyle interventions), a short-term hypocaloric diet (or liver fasting) is considered an effective method for management of this pathology. This principle was demonstrated by a study by the Saarland University, Saarbrücken, Germany, that Dr. Walle used to illustrate this statement.
“The participants (60 patients with hepatic steatosis) followed a hypocaloric diet (less than 1,000 kcal/day) for 14 days with a formula rich in protein and fiber specially developed for the treatment of nonalcoholic fatty liver disease. A fibroscan was then performed with controlled attenuation parameter measurement to quantify fatty liver disease. The results showed not only a significant improvement in nonalcoholic fatty liver disease parameters but also a marked improvement in all relevant metabolic parameters (serum lipids, liver enzymes),” explained Dr. Walle.
“This evidence leads us to affirm that the concept of hepatic fasting (by means of a hypocaloric diet) marks a point of reference for a future treatment approach for nonalcoholic fatty liver disease,” he concluded.
The study that Dr. Gambineri presented was carried out with the collaboration of the Pronokal Group (Nestlé Health Science). Dr. Gambineri, Dr. Sofrà, and Dr. Walle disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MADRID – During the International Scientific Symposium “New Frontiers in Scientific Research” that recently took place in Barcelona, specialists analyzed the role of the very-low-calorie ketogenic diet. This analysis was in relation to three comorbidities that have a higher incidence among overweight and obese patients: polycystic ovary syndrome, nonalcoholic fatty liver disease, and type 2 diabetes. The experts’ aim? To analyze and update the latest evidence on the benefits of this dietary choice.
Polycystic ovary syndrome
Alessandra Gambineri, MD, PhD, associate professor at the department of medicine and surgery (DIMEC) at the University of Bologna, Italy, addressed the link between obesity and polycystic ovary syndrome, which she described as a chronic disease that affects about 10% of women of childbearing age and that presents diverse phenotypes with different characteristics.
“The pathophysiology of this syndrome is characterized by the interaction of three factors: androgen excess, adipose tissue dysfunction, and insulin resistance. These factors interact with each other and are expressed differently in each phenotype,” said Dr. Gambineri.
She indicated that adipose tissue dysfunction is central to this pathology. This centrality results from its association with secretions, such as free fatty acids, proinflammatory cytokines, certain adipokines that promote insulin resistance, glucocorticosteroids, androgens, and oxidative stress.
“Similarly, the oxidative stress that characterizes this syndrome is increasingly present in obese individuals,” said Dr. Gambineri. “This oxidative stress also produces ovary hypotoxicity that aggravates ovulatory function. In this context, the very-low-calorie ketogenic diet can be useful in several ways: weight reduction; promoting the loss of mainly visceral/abdominal fat; decreasing lipotoxicity; and improving inflammation, hyperinsulinemia, and insulin resistance.”
This was the path followed to carry out a study that aimed to analyze the effects of the very-low-calorie ketogenic diet on manifestations of polycystic ovary syndrome in the obesity phenotype. Dr. Gambineri presented its results.
“The objective was to compare the effects of a very-low-calorie ketogenic diet and the standard low-calorie (hypocaloric) diet as a control group,” she said. “The effects studied include body weight, insulin resistance, menstrual cycle, ovulation, ovarian morphology, and hyperandrogenism in a population of 30 obese women with polycystic ovary syndrome and insulin resistance.”
Study participants had a diagnosis of polycystic ovary syndrome as defined by the National Institutes of Health criteria and were aged 18-45 years. These women were randomly assigned to two groups of equal size: experimental (very-low-calorie ketogenic diet) and control (hypocaloric diet). “The women assigned to the experimental group followed the ketogenic stage for eight weeks and then moved to the second, low-calorie diet phase for an additional eight weeks, while those in the control group (hypocaloric diet) followed the low-calorie diet for all 16 weeks.”
The primary outcomes were changes in weight and body composition, specifically fat mass and lean mass, measured by bioimpedance. “The changes observed in the following aspects were considered secondary outcomes: abdominal fat distribution, metabolic parameters, ovulation, ovarian morphology, hirsutism, hyperandrogenism, psychological well-being, and psychological distress,” said Dr. Gambineri. “Any reduction in the ovarian stroma, the area where androgens are synthesized, was also analyzed.”
The study authors found that although BMI decreased in both groups, this reduction was greater in the group that followed the very-low-calorie ketogenic diet. Significant weight loss was observed in both groups, 12.4 kg versus 4.7 kg. Significant differences were also observed in waist circumference (−8.1% in the experimental group vs. −2.2% in the control group), fat mass (−15.1% vs. −8.5%), and free testosterone (−30.3% vs. +10.6%). Only the experimental group saw a reduction in insulin.
“A key point regarding hyperandrogenism, especially regarding what’s referred to as free testosterone, there was only a significant reduction in the very-low-calorie ketogenic diet group,” said Dr. Gambineri. “This reduction was especially evident in the first part of the study, coinciding with the ketogenic period. The reason for this effect lies in the significant increase in the concentration of sex hormone-binding globulins, SHB6. Said globulins bind to the testosterone present in female blood, producing a reduction in free testosterone, a very important effect considering that this syndrome is an androgenic disorder. Furthermore, current treatments for polycystic ovary syndrome do not reduce free testosterone as much as this dietary approach does.”
For the specialist, among all these positive effects in these patients, perhaps most important is the notable improvement that occurs in ovulation. “At the beginning of the study, only 38.5% of the participants in the experimental group and 14.3% of those in the control group had ovulatory cycles. After the intervention, 84.6% managed to ovulate, compared to 35.7% who achieved this goal in the other group.”
Dr. Gambineri suggested that this method is “valid for reducing fat mass and rapidly improving hyperandrogenism and ovulatory dysfunction in women with obesity and polycystic ovary syndrome.”
Reversing type 2 diabetes?
Daniela Sofrà, MD, an endocrinologist specializing in diabetology at La Source Clinic, Lausanne, Switzerland, reviewed the current evidence on the role of the very-low-calorie ketogenic diet in the management of type 2 diabetes.
“It’s time to rethink diabetes treatment and focus efforts on managing obesity as an associated factor,” she said. “One of the hypotheses being examined in this regard is the twin cycle, which postulates that type 2 diabetes is the result of excess fat in the liver. This in turn is associated with insulin resistance with pancreas dysfunction.”
Dr. Sofrà added that there is a study documenting for the first time the reversibility of the morphology of the diabetic pancreas after caloric restriction with the very-low-calorie ketogenic diet. “The reason for this effect is the use of visceral and intrahepatic fat, which can lead to the remission of the clinical manifestation of type 2 diabetes, understanding as such the definition made by the American Diabetes Association: glycosylated hemoglobin < 6.5% without pharmacological therapy.”
Specifically, the results of this research showed that after following the very-low-calorie ketogenic diet and achieving a 15% weight loss (mean weight loss of the participants), liver glucose levels returned to normal levels within 7 days. Beta cell function returned to near normal within 8 weeks.
“Subsequent studies have shown the durability of remission of type 2 diabetes, thanks to the reactivation of the insulin-secreting function of beta cells that had become dedifferentiated in the face of chronic nutrient excess. Specifically, 6 out of 10 patients maintained glycosylated hemoglobin < 6% after 6 months without the need for pharmacological therapy,” Dr. Sofrà added.
Likewise, she highlighted that the probability of achieving remission is mainly determined by the duration of the disease. “The years with diabetes are one of the main predictors of the response that the patient will have with this dietary intervention. Studies have shown that remission is possible in patients with diabetes for less than 6 years, although there are other projects that indicate that it can be achieved with up to 10 years’ duration.”
Based on these data, Dr. Sofrà emphasized the pleiotropic effects of the very-low-calorie ketogenic diet on glycemic control, favoring the possible remission of diabetes or the reduction of drugs, as well as the reduction of the HOMA-IR index (insulin resistance) and waist circumference in people with type 2 diabetes.
Nonalcoholic fatty liver disease
The third comorbidity of obesity that may benefit from the very-low-calorie ketogenic diet is hepatic steatosis, or nonalcoholic fatty liver disease, said Hardy Walle, MD, an internal medicine specialist and director/founder of the Bodymed center, Kirkel, Germany, and one of the authors of this research.
“Recent research shows that ectopic fat and nonalcoholic fatty liver disease could be considered a cause, or at least one of the causes, of most of the diseases that affect the population as a consequence of overweight and obesity,” said Dr. Walle. “Some authors have stated that without fatty liver, there is no type 2 diabetes.”
Dr. Walle pointed out that between 30% and 40% of the adult population has nonalcoholic fatty liver disease, a percentage that increases considerably in people with obesity, reaching 70% prevalence and increasing, in the case of type 2 diabetes, to almost 90%. “Even normal weight does not rule out fatty liver; in fact, about 15% of people with nonalcoholic fatty liver disease are not overweight.”
In a setting where there are no approved drugs for the treatment of fatty liver (the current standard approach focuses on lifestyle interventions), a short-term hypocaloric diet (or liver fasting) is considered an effective method for management of this pathology. This principle was demonstrated by a study by the Saarland University, Saarbrücken, Germany, that Dr. Walle used to illustrate this statement.
“The participants (60 patients with hepatic steatosis) followed a hypocaloric diet (less than 1,000 kcal/day) for 14 days with a formula rich in protein and fiber specially developed for the treatment of nonalcoholic fatty liver disease. A fibroscan was then performed with controlled attenuation parameter measurement to quantify fatty liver disease. The results showed not only a significant improvement in nonalcoholic fatty liver disease parameters but also a marked improvement in all relevant metabolic parameters (serum lipids, liver enzymes),” explained Dr. Walle.
“This evidence leads us to affirm that the concept of hepatic fasting (by means of a hypocaloric diet) marks a point of reference for a future treatment approach for nonalcoholic fatty liver disease,” he concluded.
The study that Dr. Gambineri presented was carried out with the collaboration of the Pronokal Group (Nestlé Health Science). Dr. Gambineri, Dr. Sofrà, and Dr. Walle disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MADRID – During the International Scientific Symposium “New Frontiers in Scientific Research” that recently took place in Barcelona, specialists analyzed the role of the very-low-calorie ketogenic diet. This analysis was in relation to three comorbidities that have a higher incidence among overweight and obese patients: polycystic ovary syndrome, nonalcoholic fatty liver disease, and type 2 diabetes. The experts’ aim? To analyze and update the latest evidence on the benefits of this dietary choice.
Polycystic ovary syndrome
Alessandra Gambineri, MD, PhD, associate professor at the department of medicine and surgery (DIMEC) at the University of Bologna, Italy, addressed the link between obesity and polycystic ovary syndrome, which she described as a chronic disease that affects about 10% of women of childbearing age and that presents diverse phenotypes with different characteristics.
“The pathophysiology of this syndrome is characterized by the interaction of three factors: androgen excess, adipose tissue dysfunction, and insulin resistance. These factors interact with each other and are expressed differently in each phenotype,” said Dr. Gambineri.
She indicated that adipose tissue dysfunction is central to this pathology. This centrality results from its association with secretions, such as free fatty acids, proinflammatory cytokines, certain adipokines that promote insulin resistance, glucocorticosteroids, androgens, and oxidative stress.
“Similarly, the oxidative stress that characterizes this syndrome is increasingly present in obese individuals,” said Dr. Gambineri. “This oxidative stress also produces ovary hypotoxicity that aggravates ovulatory function. In this context, the very-low-calorie ketogenic diet can be useful in several ways: weight reduction; promoting the loss of mainly visceral/abdominal fat; decreasing lipotoxicity; and improving inflammation, hyperinsulinemia, and insulin resistance.”
This was the path followed to carry out a study that aimed to analyze the effects of the very-low-calorie ketogenic diet on manifestations of polycystic ovary syndrome in the obesity phenotype. Dr. Gambineri presented its results.
“The objective was to compare the effects of a very-low-calorie ketogenic diet and the standard low-calorie (hypocaloric) diet as a control group,” she said. “The effects studied include body weight, insulin resistance, menstrual cycle, ovulation, ovarian morphology, and hyperandrogenism in a population of 30 obese women with polycystic ovary syndrome and insulin resistance.”
Study participants had a diagnosis of polycystic ovary syndrome as defined by the National Institutes of Health criteria and were aged 18-45 years. These women were randomly assigned to two groups of equal size: experimental (very-low-calorie ketogenic diet) and control (hypocaloric diet). “The women assigned to the experimental group followed the ketogenic stage for eight weeks and then moved to the second, low-calorie diet phase for an additional eight weeks, while those in the control group (hypocaloric diet) followed the low-calorie diet for all 16 weeks.”
The primary outcomes were changes in weight and body composition, specifically fat mass and lean mass, measured by bioimpedance. “The changes observed in the following aspects were considered secondary outcomes: abdominal fat distribution, metabolic parameters, ovulation, ovarian morphology, hirsutism, hyperandrogenism, psychological well-being, and psychological distress,” said Dr. Gambineri. “Any reduction in the ovarian stroma, the area where androgens are synthesized, was also analyzed.”
The study authors found that although BMI decreased in both groups, this reduction was greater in the group that followed the very-low-calorie ketogenic diet. Significant weight loss was observed in both groups, 12.4 kg versus 4.7 kg. Significant differences were also observed in waist circumference (−8.1% in the experimental group vs. −2.2% in the control group), fat mass (−15.1% vs. −8.5%), and free testosterone (−30.3% vs. +10.6%). Only the experimental group saw a reduction in insulin.
“A key point regarding hyperandrogenism, especially regarding what’s referred to as free testosterone, there was only a significant reduction in the very-low-calorie ketogenic diet group,” said Dr. Gambineri. “This reduction was especially evident in the first part of the study, coinciding with the ketogenic period. The reason for this effect lies in the significant increase in the concentration of sex hormone-binding globulins, SHB6. Said globulins bind to the testosterone present in female blood, producing a reduction in free testosterone, a very important effect considering that this syndrome is an androgenic disorder. Furthermore, current treatments for polycystic ovary syndrome do not reduce free testosterone as much as this dietary approach does.”
For the specialist, among all these positive effects in these patients, perhaps most important is the notable improvement that occurs in ovulation. “At the beginning of the study, only 38.5% of the participants in the experimental group and 14.3% of those in the control group had ovulatory cycles. After the intervention, 84.6% managed to ovulate, compared to 35.7% who achieved this goal in the other group.”
Dr. Gambineri suggested that this method is “valid for reducing fat mass and rapidly improving hyperandrogenism and ovulatory dysfunction in women with obesity and polycystic ovary syndrome.”
Reversing type 2 diabetes?
Daniela Sofrà, MD, an endocrinologist specializing in diabetology at La Source Clinic, Lausanne, Switzerland, reviewed the current evidence on the role of the very-low-calorie ketogenic diet in the management of type 2 diabetes.
“It’s time to rethink diabetes treatment and focus efforts on managing obesity as an associated factor,” she said. “One of the hypotheses being examined in this regard is the twin cycle, which postulates that type 2 diabetes is the result of excess fat in the liver. This in turn is associated with insulin resistance with pancreas dysfunction.”
Dr. Sofrà added that there is a study documenting for the first time the reversibility of the morphology of the diabetic pancreas after caloric restriction with the very-low-calorie ketogenic diet. “The reason for this effect is the use of visceral and intrahepatic fat, which can lead to the remission of the clinical manifestation of type 2 diabetes, understanding as such the definition made by the American Diabetes Association: glycosylated hemoglobin < 6.5% without pharmacological therapy.”
Specifically, the results of this research showed that after following the very-low-calorie ketogenic diet and achieving a 15% weight loss (mean weight loss of the participants), liver glucose levels returned to normal levels within 7 days. Beta cell function returned to near normal within 8 weeks.
“Subsequent studies have shown the durability of remission of type 2 diabetes, thanks to the reactivation of the insulin-secreting function of beta cells that had become dedifferentiated in the face of chronic nutrient excess. Specifically, 6 out of 10 patients maintained glycosylated hemoglobin < 6% after 6 months without the need for pharmacological therapy,” Dr. Sofrà added.
Likewise, she highlighted that the probability of achieving remission is mainly determined by the duration of the disease. “The years with diabetes are one of the main predictors of the response that the patient will have with this dietary intervention. Studies have shown that remission is possible in patients with diabetes for less than 6 years, although there are other projects that indicate that it can be achieved with up to 10 years’ duration.”
Based on these data, Dr. Sofrà emphasized the pleiotropic effects of the very-low-calorie ketogenic diet on glycemic control, favoring the possible remission of diabetes or the reduction of drugs, as well as the reduction of the HOMA-IR index (insulin resistance) and waist circumference in people with type 2 diabetes.
Nonalcoholic fatty liver disease
The third comorbidity of obesity that may benefit from the very-low-calorie ketogenic diet is hepatic steatosis, or nonalcoholic fatty liver disease, said Hardy Walle, MD, an internal medicine specialist and director/founder of the Bodymed center, Kirkel, Germany, and one of the authors of this research.
“Recent research shows that ectopic fat and nonalcoholic fatty liver disease could be considered a cause, or at least one of the causes, of most of the diseases that affect the population as a consequence of overweight and obesity,” said Dr. Walle. “Some authors have stated that without fatty liver, there is no type 2 diabetes.”
Dr. Walle pointed out that between 30% and 40% of the adult population has nonalcoholic fatty liver disease, a percentage that increases considerably in people with obesity, reaching 70% prevalence and increasing, in the case of type 2 diabetes, to almost 90%. “Even normal weight does not rule out fatty liver; in fact, about 15% of people with nonalcoholic fatty liver disease are not overweight.”
In a setting where there are no approved drugs for the treatment of fatty liver (the current standard approach focuses on lifestyle interventions), a short-term hypocaloric diet (or liver fasting) is considered an effective method for management of this pathology. This principle was demonstrated by a study by the Saarland University, Saarbrücken, Germany, that Dr. Walle used to illustrate this statement.
“The participants (60 patients with hepatic steatosis) followed a hypocaloric diet (less than 1,000 kcal/day) for 14 days with a formula rich in protein and fiber specially developed for the treatment of nonalcoholic fatty liver disease. A fibroscan was then performed with controlled attenuation parameter measurement to quantify fatty liver disease. The results showed not only a significant improvement in nonalcoholic fatty liver disease parameters but also a marked improvement in all relevant metabolic parameters (serum lipids, liver enzymes),” explained Dr. Walle.
“This evidence leads us to affirm that the concept of hepatic fasting (by means of a hypocaloric diet) marks a point of reference for a future treatment approach for nonalcoholic fatty liver disease,” he concluded.
The study that Dr. Gambineri presented was carried out with the collaboration of the Pronokal Group (Nestlé Health Science). Dr. Gambineri, Dr. Sofrà, and Dr. Walle disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.












