User login
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
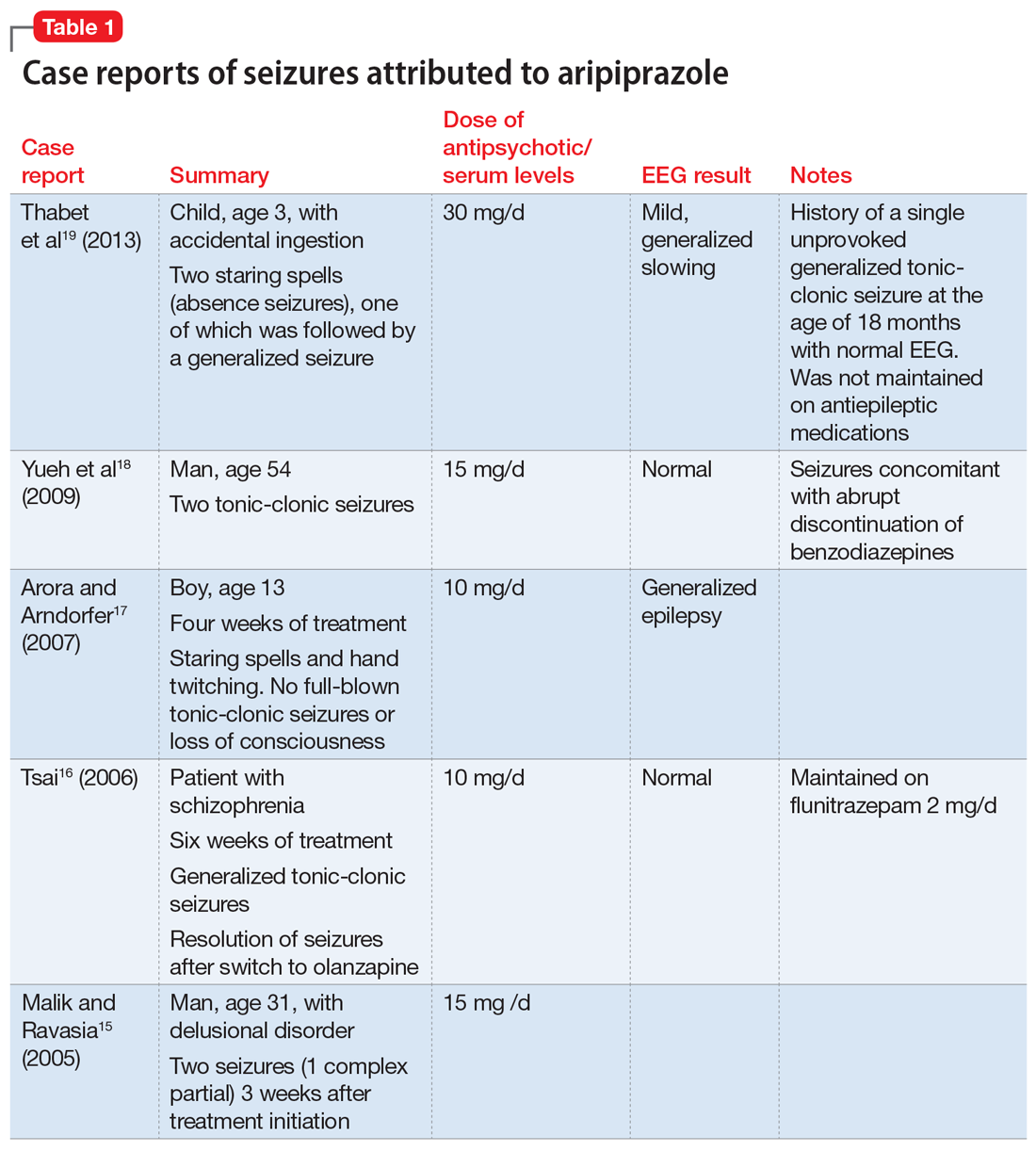
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
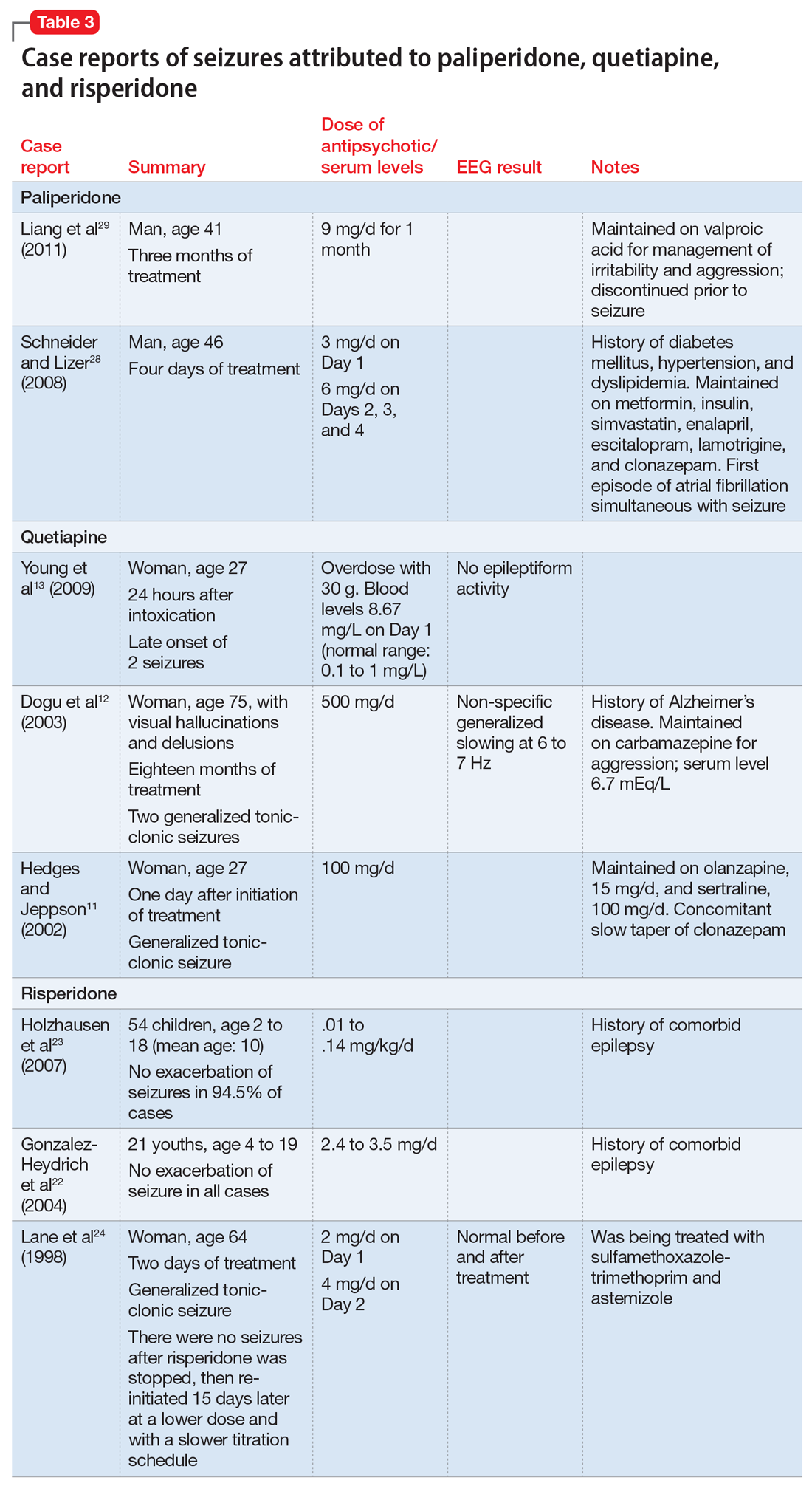
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
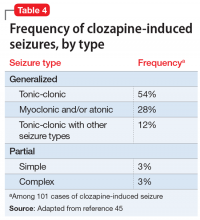
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
Antipsychotics, especially second-generation antipsychotics (SGAs), have been proven effective for treating psychosis as well as mood disorders.1,2 Because antipsychotics can lower the epileptogenic threshold, seizures are a serious potential adverse effect. Antipsychotics can cause isolated EEG abnormalities in 7% of patients with no history of epilepsy, and clinical seizures in .5% to 1.2% of such patients.3 Additionally, the neuropathophysiology underlying epilepsy can predispose patients to psychiatric disorders4; the estimated prevalence of psychosis in patients with epilepsy is approximately 7%.5 This review will shed light on the risk of clinical seizures related to antipsychotics.
Comparing seizure risk among antipsychotics
In a review of the World Health Organization’s adverse drug reactions database, Kumlien and Lundberg6 calculated the ratio of the number of reports of seizures to the total number of reports for each drug. They found that approximately 9% of all adverse drug reaction reports involving clozapine were due to seizures. Equivalent ratios were 5.90% for quetiapine, 4.91% for olanzapine, 3.68% for risperidone, 3.27% for haloperidol, and 2.59% for aripiprazole. Using the database of the Pharmacovigilance Unit of the Basque Country, Lertxundi et al7 reported a 3.2-fold increased risk of seizure with SGAs in comparison with first-generation antipsychotics (FGAs) (95% confidence interval [CI], 2.21 to 4.63), which went down to 2.08 (CI, 1.39 to 3.12) once clozapine was excluded. However, as the authors of both studies noted, the quality and relevance of this data are limited because it relies on spontaneous reporting.
Overall, the evidence regarding the seizure risk associated with antipsychotics is scarce. To the best of our knowledge, only 2 large observational studies have compared the seizure risks associated with different antipsychotics.
Using data from the UK-based Clinical Practice Research Datalink between 1998 and 2013, Bloechlinger et al8 examined the incidence rates of seizures among patients newly diagnosed with schizophrenia, affective disorders, or dementia who were prescribed antipsychotics. They excluded patients with a history of seizures or antiepileptic use. In the cohort of 60,121 patients, the incidence rates of seizures per 10,000 person-years were 11.7 (CI, 10.0 to 13.4) for those who did not use antipsychotics, 12.4 (CI, 10.9 to 13.8) for past users, 115.4 (CI, 50.1 to 180.7) for current users of haloperidol, 48.8 (CI, 30.7 to 66.9) for current users of quetiapine, 25.9 (CI, 11.8 to 40.0) for current users of risperidone, and 19.0 (CI, 8.7 to 29.3) for current users of olanzapine. No data were available about clozapine use.
In subsequent analyses, the authors found that among patients with affective disorders, only current use of medium- to high-potency FGAs (haloperidol, prochlorperazine, and trifluoperazine) was associated with a significantly increased risk of seizures (adjusted odds ratio: 2.51, CI, 1.51 to 4.18) compared with non-users.8 Among patients with dementia, current use of olanzapine or quetiapine and current use of any FGAs were associated with significantly increased odds of seizures. This study suggests that the underlying mental illness might modulate the seizure risk associated with antipsychotics.8
Wu et al9 conducted a study based on the National Health Insurance Research Database in Taiwan. They examined the 1-year incidence of new-onset seizures among patients diagnosed with schizophrenia or mood disorders who were new to antipsychotic treatment, and calculated the risk of seizure associated with each antipsychotic in reference to risperidone. They found that those receiving clozapine, thioridazine, and haloperidol were 2 to 3 times more likely to develop seizures than those treated with risperidone; risks associated with the rest of the FGAs were similar to that of risperidone.
The results of these 2 large cohort studies are somewhat concurrent in indicating that, other than clozapine, SGAs incur similar risks of seizures; furthermore, they specify that, contrary to earlier studies,10 haloperidol is associated with significantly higher odds of seizures. While both of these cohort studies controlled for several sociodemographic and clinical confounders, they have several limitations. First, diagnoses of seizures were based on information available in databases, which might be subject to inaccuracies. Second, neither study evaluated the effect of drug dosage and duration of exposure on new-onset seizures.
Continue to: Most evidence is from case reports
Most evidence is from case reports
Other than these 2 large studies, most of the evidence addressing the relationship between the use of antipsychotics and incidence of seizures is low quality and relies on case reports or expert opinions. Older studies found that, among FGAs, seizure risk is highest with chlorpromazine and promazine, and lowest with thioridazine and haloperidol.10 As for SGAs, case reports have described seizuresassociated with the use of quetiapine, aripiprazole, risperidone, paliperidone, and olanzapine.
Quetiapine. Three case reports published between 2002 and 2010 describe generalized
Aripiprazole. Five case reports described staring spells and tonic-clonic seizures in patients receiving 10 to 15 mg of aripiprazole.15-19 In the New Drug Application (NDA) for aripiprazole, the incidence of seizures was estimated to be .11% (1 of 926 patients) in placebo-controlled trials and .46% (3 of 859 patients) in haloperidol-controlled trials.20
Risperidone’s product labeling suggests the drug should be used with caution in patients with a history of seizures or conditions that could result in a lower seizure threshold. In Phase III placebo-controlled trials, seizures occurred in .3% of patients treated with risperidone, although in some cases, the seizures were induced by electrolyte disturbances such as hyponatremia.21 Gonzalez-Heydrich et al22 and Holzhausen et al23 found no increase in seizure activity among patients with epilepsy who were receiving risperidone. Lane et al24 published a case report of a geriatric woman who presented with a generalized tonic-clonic seizure related to rapid titration of risperidone; however, with slower titration and lower doses, she stopped having seizures without adding any antiepileptic drugs. Komossa et al25 found that risperidone is less epileptogenic than clozapine, with a relative risk of .22.
Paliperidone is the active metabolite of risperidone and does not have pharmacokinetic interactions with drugs metabolized by the cytochrome P450 (CYP) enzymes. Its labeling indicates that the drug should be used with caution in patients with a history of seizures.26 In Phase III placebo-controlled trials of paliperidone, the rate of seizures was .22%.27 Two case reports suggest close monitoring of seizure risk in patients receiving paliperidone.28,29 Liang et al29 reported that co-administration of valproic acid could mask an underlying decrease of the seizure threshold caused by antipsychotics such as paliperidone.
Continue to: Olanzapine
Olanzapine is a thienobenzodiazepine derivative and is chemically related to clozapine.30 The olanzapine NDA31 shows that 23 of 3,139 patients developed seizures, mainly tonic-clonic, with evidence suggesting that the seizures may have been due to confounding factors such as a history of seizures or metabolic abnormalities. There were no statistically significant differences in the rate of seizures associated with olanzapine compared with placebo or haloperidol (P = .252 and .168, respectively).
A literature review for olanzapine yielded 1 case report of repetitive focal seizures and lingual dystonia,32 5 case reports of generalized tonic-clonic seizures and myoclonus,33-37 and 2 case reports of status epilepticus.38,39 Olanzapine’s clearance is 25% to 30% lower in women, and most of these case reports occurred women.40
Details of the above case reports are summarized in Table 1 (aripiprazole15-19), Table 2 (olanzapine32-39), and Table 3 (paliperidone,28,29 quetiapine,11-13 and risperidone22-24).
Ziprasidone. According to the NDA safety database, the seizure rate attributed to ziprasidone was 1.8 per 100 subject-years or 0.54% of participants (12 of 2,588).41 No additional studies have been published regarding its seizure risk.
Clozapine has a black-box warning
To the best of our knowledge, clozapine is the only antipsychotic that carries an FDA “black-box” warning regarding its risk of inducing seizures.42 Devinsky and Pacia43 reported a cumulative risk of 10% after 3.8 years of treatment. The literature has described clozapine-induced generalized tonic-clonic, myoclonic, simple and complex partial, and absence seizures.44 Table 445 lists the estimated frequency of each seizure type based on 101 cases of clozapine-induced seizures. Myoclonic seizures and drop attacks could be precursors/warning signs of grand mal tonic-clonic seizures.46,47 Seizures have been observed at all stages of treatment, but were more common during initiation of clozapine, which emphasizes the importance of a progressive and slow titration.43,48 The incidence of seizures was estimated to be 6% in a sample of 216 patients with schizophrenia with no history of epilepsy who were prescribed clozapine.49
Continue to: Regarding a possible association between...
Regarding a possible association between clozapine dose or clozapine plasma levels and seizure risk, there is a positive linear relationship between the dose of clozapine and its serum concentration over a dosing range of 25 to 800 mg/d.50 However, the plasma concentration is also significantly affected by factors such as smoking, gender, age, drug interactions, and CYP genotypes. Therefore, the same clozapine dose will yield a lower serum concentration in an older male who smokes compared with a younger, non-smoking female.51 Perry et al52 suggested a dosing nomogram to calculate the influence of gender and smoking. Seizure risk, especially for tonic-clonic seizures, has been reported to increase with clozapine doses >600 mg/d,53 and with plasma concentrations exceeding 1,000 to 1,300 mg/L.54 However, in a 2011 regression analysis, Varma et al55 found no statistically significant relationship between seizure risk and clozapine oral dose; there was not enough data to test a correlation between clozapine plasma levels and the incidence of seizures.
How antipsychotics might lower the seizure threshold
Researchers have suggested several possible mechanisms to explain how antipsychotics might lower the seizure threshold. Antagonism of dopamine D4, histamine H1, and acetylcholine-muscarinic receptors seems to induce EEG alterations and increase the risk of seizures.56 Additionally, modulation of the N-methyl-
Watch for pharmacokinetic interactions
The CYP enzymes involved in drug metabolism include CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Most commonly used antiepileptics and antipsychotics are metabolized by CYP enzymes, and may also act as inhibitors or inducers of these enzymes.61 Drug interactions may impair seizure control, which is why monotherapy is preferable to combination treatment in patients with epilepsy.62 Carbamazepine and phenytoin are inducers of both CYP1A2 (which metabolizes olanzapine and clozapine), and CYP3A4 (which metabolizes haloperidol, risperidone, quetiapine, ziprasidone and clozapine). Paliperidone is not metabolized by CYP enzymes.62 Discontinuing an enzyme-inducing agent may result in increased antipsychotic plasma concentrations, which might lead to an increased risk of seizures.
Valproic acid, which is often used to prevent or treat clozapine-induced seizures, has an unclear effect on clozapine plasma concentrations.63 Although valproic acid is known to inhibit clozapine metabolism, 2 reports have suggested that the plasma concentrations of clozapine and its metabolites may decrease after adding valproic acid.64,65 Other studies have found that valproic acid increases plasma concentrations of clozapine while it decreases plasma concentrations of norclozapine; norclozapine is the main clozapine metabolite responsible for inducing seizures.66,67
Steps for minimizing seizure risk
Determining the seizure risk for a patient taking an antipsychotic is challenging because doing so depends not only on the seizurogenic potential of each drug but also on individualized predisposing factors.11,57,68 Choosing the “best” antipsychotic therefore largely depends on each patient’s profile. The predisposing factors consist mainly of the individually inherited seizure threshold (personal history of febrile convulsions or a family history of seizures) and other comorbid seizurogenic conditions, such as a history of head trauma, brain injury, intellectual disability, cerebral arteriosclerosis, neurodegenerative diseases, encephalopathy, chronic renal insufficiency, and hyponatremia. Furthermore, seizure risk depends on the antipsychotic dose administered and the rate of titration.11
Continue to: There is not enough evidence...
There is not enough evidence to recommend performing an EEG in all patients taking antipsychotics. Such testing is recommended only for patients who have predisposing factors for seizures. If an EEG shows any abnormality in a patient taking clozapine, consider decreasing the clozapine dose69,70 or adding an antiepileptic drug such as valproic acid or lamotrigine.44,70
Although clozapine carries a black-box warning of increased risk of causing seizures, there is no consensus regarding the efficacy of co-prescribing an antiepileptic. Some studies have suggested prescribing valproic acid prophylactically,71 after the occurrence of 1 seizure,59 or after 2 seizures.54,72 Others have recommended prescribing prophylactic valproic acid for patients taking ≥600 mg/d of clozapine or whose clozapine plasma levels are >500 mg/L.73 Varma et al55 recommended starting an antiepileptic medication if there are clear epileptiform discharges on EEG, if the patient develops stuttering or speech difficulties, or if seizures occur. Liukkonen et al72 advised initiating an antiepileptic at the start of clozapine treatment in patients who are taking other epileptogenic medications, patients with pre-existing seizure disorder, and patients with neurologic abnormalities. On the other hand, Caetano51 argued against primary prevention of seizures for patients receiving >600 mg/d of clozapine, suggesting that “the risk of seizures would be better managed by close clinical monitoring and measures of clozapine serum concentration rather than adding an anticonvulsant drug.”
Current recommendations for primary and secondary prevention of clozapine-induced seizures are detailed in Table 5.42,44,45,51,55,57,69,74,75
Studies addressing the seizurogenic potential of SGAs other than clozapine have a low level of evidence and include patients who had comorbid conditions and were taking other medications that could cause seizures. Additionally, clinical trials of SGAs rarely include patients with seizure disorders; this might underestimate the risk of seizures.4
The effect of the mental illness itself on the seizure threshold needs to be considered.43 Bloechlinger et al8 found that dementia might be inherently associated with a higher risk of antipsychotic-related seizures. Moreover, numerous qualitative EEG studies have found abnormalities in 20% to 60% of patients with schizophrenia.56 Other quantitative studies have reported mild and nonspecific EEG abnormalities, such as increased delta and/or theta activity, in many non-medicated patients with schizophrenia.10,76 Additionally, brain tissue analysis of deceased patients who had schizophrenia has shown a significant increase in dopamine concentrations in the left amygdala compared with controls, and this might be responsible for enhanced electrical activity in this region.10 Some studies have described EEG slowing in the frontal brain regions of patients with schizophrenia,77 and was selectively normalized in these areas with antipsychotics.78
As always, start low, go slow
Mounting evidence suggests that antipsychotic medications decrease the seizure threshold. Practitioners should thus be cautious in prescribing antipsychotics and should target reaching the minimal effective dose with slow titration, especially in patients with predisposing factors for epilepsy.
Continue to: Although evidence suggests...
Although evidence suggests antipsychotics can induce different types of epileptic seizures, the quality of this evidence is low. Randomized controlled trials are needed to determine which antipsychotics increase seizure risk and whether there is a dose-effect relationship.
Bottom Line
Among second-generation antipsychotics, clozapine appears to increase the risk of clinical seizure the most. Correlations with dosage and/or plasma levels have not been proven. Psychiatrists should be vigilant for pharmacokinetic interactions between antipsychotics and antiepileptics, notably via CYP1A2 and CYP3A4.
Related Resources
- Druschky K, Bleich S, Grohmann R, et al. Seizure rates under treatment with antipsychotic drugs: Data from the AMSP project. World J Biol Psychiatry. 2018;15:1-10.
- Epilepsy Foundation. For professionals: Antipsychotics. https://www.epilepsy.com/learn/professionals/diagnosistreatment/psychotropic-drugs-developmental-disabilities/comorbid-5.
Drug Brand Names
Aripiprazole • Abilify
Benztropine • Cogentin
Bethanechol • Duvoid
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cimetidine • Tagamet
Ciprofloxacin • Cipro
Citalopram • Celexa
Clonazepam • Klonopin
Clozapine • Clozaril
Donepezil • Aricept
Enalapril • Vasotec
Erythromycin • Erythrocin
Escitalopram • Lexapro
Flunitrazepam • Rohypnol
Fluvoxamine • Luvox
Gabapentin • Neurontin
Haloperidol • Haldol
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Metformin • Fortamet, Glucophage
Mirtazapine • Remeron
Nitrofurantoin • Furadantin
Olanzapine • Zyprexa
Paliperidone • Invega
Phenobarbital • Luminal
Phenytoin • Dilantin
Prochlorperazine • Compazine
Procyclidine • Kemadrin
Propranolol • Inderal
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Simvastatin • Zocor
Sulfamethoxazole/trimethoprim • Bactrim, Sulfatrim
Topiramate • Topamax
Trifluoperazine • Stelazine
Valproic acid • Depakene, Depakote
Ziprasidone • Geodon
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.
1. Bruijnzeel D, Suryadevara U, Tandon R. Antipsychotic treatment of schizophrenia: an update. Asian J Psychiatr. 2014;11:3-7.
2. Hrdlicka M, Dudova I. Atypical antipsychotics in the treatment of early-onset schizophrenia. Neuropsychiatr Dis Treat. 2015;11:907-913.
3. Koch-Stoecker S. Antipsychotic drugs and epilepsy: indications and treatment guidelines. Epilepsia. 2002;43(suppl 2):19-24.
4. Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345-354.
5. Torta R, Keller R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia. 1999;40(suppl 10):S2-S20.
6. Kumlien E, Lundberg PO. Seizure risk associated with neuroactive drugs: data from the WHO adverse drug reactions database. Seizure. 2010;19(2):69-73.
7. Lertxundi U, Hernandez R, Medrano J, et al. Antipsychotics and seizures: higher risk with atypicals? Seizure. 2013;22(2):141-143.
8. Bloechliger M, Rüegg S, Jick SS, et al. Antipsychotic drug use and the risk of seizures: follow-up study with a nested case-control analysis. CNS Drugs. 2015;29(7):591-603.
9. Wu CS, Wang SC, Yeh IJ, et al. Comparative risk of seizure with use of first- and second-generation antipsychotics in patients with schizophrenia and mood disorders. J Clin Psychiatry. 2016;77(5):e573-e579.
10. Cold JA, Wells BG, Froemming JH. Seizure activity associated with antipsychotic therapy. [Erratum in DICP. 1990;24(10):1012.] DICP. 1990;24(6):601-606.
11. Hedges DW, Jeppson KG. New-onset seizure associated with quetiapine and olanzapine. Ann Pharmacother. 2002;36(3):437-439.
12. Dogu O, Sevim S, Kaleagasi HS. Seizures associated with quetiapine treatment. Ann Pharmacother. 2003;37(9):1224-1227.
13. Young AC, Kleinschmidt KC, Wax PM. Late-onset seizures associated with quetiapine poisoning. J Med Toxicol. 2009;5(1):24-26.
14. US Food and Drug Administration. Recommendation of approvable action for quetiapine fumarate extended release (Seroquel® XR) for the treatment of schizophrenia. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022047Orig1s000MedR.pdf. April 24, 2007. Accessed January 28, 2019.
15. Malik AR, Ravasia S. Aripiprazole-induced seizure. Can J Psychiatry. 2005;50(3):186.
16. Tsai JF. Aripiprazole-associated seizure. J Clin Psychiatry. 2006;67(6):995-996.
17. Arora M, Arndorfer L. EEG abnormalities in a patient taking aripiprazole. Psychiatry (Edgmont). 2007;4(7):18-19.
18. Yueh CL, Yu SL, Chen HM, et al. Aripiprazole-induced seizure: a second case report. BMJ case reports. 2009;2009:bcr03.2009.1693. doi: 10.1136/bcr.03.2009.1693.
19. Thabet FI, Sweis RT, Joseph SA. Aripiprazole-induced seizure in a 3-year-old child: a case report and literature review. Clin Neuropharmacol. 2013;36(1):29-30.
20. US Food and Drug Administration. Abilify (Aripiprazole) tablets. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-436_Abilify_medr_P2.pdf. Published March 07, 2003. Accessed January 28, 2019.
21. US Food and Drug Administration. Drugs@FDA: FDA approved drug products. Risperdal tablets, Risperdal oral solution & Risperdal M-tab orally disintegrating tablets. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021444_S004_RISPERDAL_TABLETS.pdf. Published September 10, 2003. Accessed January 28, 2019.
22. Gonzalez-Heydrich J, Pandina GJ, Fleisher CA, et al. No seizure exacerbation from risperidone in youth with comorbid epilepsy and psychiatric disorders: a case series. J Child Adolesc Psychopharmacol. 2004;14(2):295-310.
23. Holzhausen SPF, Guerreiro MM, Baccin CE, et al. Use of risperidone in children with epilepsy. Epilepsy Behav. 2007;10(3):412-416.
24. Lane HY, Chang WH, Chou JC. Seizure during risperidone treatment in an elderly woman treated with concomitant medications. J Clinl Psychiatry. 1998;59(2):81-82.
25. Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):19:CD006626.
26. Paliperidone [package insert]. Mountainville, CA: Janssen Pharmaceuticals, Inc.; 2007.
27. Brugge, MD; US Food and Drug Administration. Paliperidone OROS oral formulation. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021999s000_MedR_Part4.pdf. Accessed January 28, 2019.
28. Schneider RA, Lizer MH. Apparent seizure and atrial fibrillation associated with paliperidone. Am J Health System Pharm. 2008;65(22):2122-2125.
29. Liang CS, Yang FW, Chiang KT. Paliperidone-associated seizure after discontinuation of sodium valproate: a case report. J Clin Psychopharmacol. 2011;31(2):246-247.
30. Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53(2):281-298.
31. US Food and Drug Administration. Drugs@FDA: FDA approved drug products: Zyprexa (olanzapine). ORIG-1. http://www.accessdata.fda.gov/drugsatfda_docs/nda/96/020592_Original_Approval_Pkg%20.pdf. Published September 30, 1996. Accessed January 28, 2019.
32. Anzellotti F, Capasso M, Frazzini V, et al. Olanzapine-related repetitive focal seizures with lingual dystonia. Epileptic Disord. 2016;18(1):83-86.
33. Lee JW, Crismon ML, Dorson PG. Seizure associated with olanzapine. Ann Pharmac. 1999;33(5):554-556.
34. Woolley J, Smith S. Lowered seizure threshold on olanzapine. Br J Psychiatry. 2001;178(1):85-86.
35. Behere RV, Anjith D, Rao NP, et al. Olanzapine-induced clinical seizure: a case report. Clin Neuropharmacol. 2009;32(5):297-298.
36. Camacho A, García-Navarro M, Martínez B, et al. Olanzapine-induced myoclonic status. Clin Neuropharmacol. 2005;28(3):145-147.
37. Rosen JB, Milstein MJ, Haut SR. Olanzapine-associated myoclonus. Epilepsy Res. 2012;98(2-3):247-250.
38. Wyderski RJ, Starrett WG, Abou-Saif A. Fatal status epilepticus associated with olanzapine therapy. Ann Pharmacother. 1999;33(7-8):787-789.
39. Spyridi S, Sokolaki S, Nimatoudis J, et al. Status epilepticus in a patient treated with olanzapine and mirtazapine. Int J Clin Pharmacol Ther. 2009;47(2):120-123.
40. Schatzberg AF, Nemeroff CB. Essentials of clinical psychopharmacology. 2nd ed. Arlington, Virginia: American Psychiatric Publishing; 2006.
41. US Food and Drug Administration. Drug approval package: Geodon (Ziprasidone HCI) Capsules. Medical Review Part 2. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/20-825_Geodan_medr_P2.pdf. Published February 5, 2001. Accessed January 29, 2019.
42. Clozaril [package insert]. East Hanover, NJ: Novartis; 2008.
43. Devinsky O, Pacia SV. Seizures during clozapine therapy. J Clin Psychiatry. 1994;55(suppl B):153-156.
44. Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101-111.
45. Wong J, Delva N. Clozapine-induced seizures: recognition and treatment. Can J Psychiatry. 2007;52(7):457-463.
46. Berman I, Zalma A, DuRand CJ, et al. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry. 1992;53(9):329-330.
47. Gouzoulis E, Ozdaglar A, Kasper J. Myoclonic seizures followed by grand mal seizures during clozapine treatment. Am J Psychiatry. 1993;150(7):1128.
48. Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry. 1996;39(5):367-370.
49. Grover S, Hazari N, Chakrabarti S, et al. Association of clozapine with seizures: a brief report involving 222 patients prescribed clozapine. East Asian Arch Psychiatry. 2015;25(2):73-78.
50. Byerly MJ, DeVane CL. Pharmacokinetics of clozapine and risperidone: a review of recent literature. J Clin Psychopharmacol. 1996;16(2):177-187.
51. Caetano D. Use of anticonvulsants as prophylaxis for seizures in patients on clozapine. Australas Psychiatry. 2014;22(1):78-83.
52. Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry.1998;44(8):733-738.
53. Dumortier G, Mahé V, Pons D, et al. Clonic seizure associated with high clozapine plasma level. J Neuropsychiatry Clin Neurosci. 2001;13(2):302-303.
54. Funderburg LG, Vertrees JE, True JE, et al. Seizure following addition of erythromycin to clozapine treatment. Am J Psychiatry. 1994;151(12):1840-1841.
55. Varma S, Bishara D, Besag FMC, et al. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol. 2011;1(2):47-66.
56. Amann BL, Pogarell O, Mergl R, et al. EEG abnormalities associated with antipsychotics: a comparison of quetiapine, olanzapine, haloperidol and healthy subjects. Hum Psychopharmacol. 2003;18(8):641-646.
57. Pisani F, Oteri G, Costa C, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25(2):91-110.
58. Maurice T, Phan VL, Urani A, et al. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn J Pharmacol. 1999;81(2):125-155.
59. Haller E, Binder RL. Clozapine and seizures. Am J Psychiatry. 1990;147(8):1069-1071.
60. Torta R, Monaco F. Atypical antipsychotics and serotoninergic antidepressants in patients with epilepsy: pharmacodynamic considerations. Epilepsia. 2002;43(suppl 2):8-13.
61. Spina E. Drug interactions. In: Shorvon S, Perucca E, Engel J Jr, eds. The treatment of epilepsy. 3rd ed. Oxford, UK: Blackwell Publishing; 2009:361-377.
62. Spina E, Perucca E. Clinical significance of pharmacokinetic interactions between antiepileptic and psychotropic drugs. Epilepsia. 2002;43(suppl 2):37-44.
63. de Leon J, Santoro V, D’Arrigo C, et al. Interactions between antiepileptics and second-generation antipsychotics. Expert Opin Drug Metab Toxicol. 2012;8(3):311-334.
64. Finley P, Warner D. Potential impact of valproic acid therapy on clozapine disposition. Biol Psychiatry. 1994;36(7):487-488.
65. Longo LP, Salzman C. Valproic acid effects on serum concentrations of clozapine and norclozapine. Am J Psychiatry. 1995;152(4):650.
66. Centorrino F, Baldessarini RJ, Kando J, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry. 1994;151(1):123-125.
67. Facciolà G, Avenoso A, Scordo MG, et al. Small effects of valproic acid on the plasma concentrations of clozapine and its major metabolites in patients with schizophrenic or affective disorders. Ther Drug Monit. 1999;21(3):341-345.
68. Hyde TM, Weinberger DR. Seizures and schizophrenia. Schizophr Bull. 1997;23(4):611-622.
69. Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract. 2010;16(2):125-128.
70. Kikuchi YS, Sato W, Ataka K, et al. Clozapine-induced seizures, electroencephalography abnormalities, and clinical responses in Japanese patients with schizophrenia. Neuropsychiatr Dis Treat. 2014;10:1973-1978.
71. Taner E, Coşar B, Işik E. Clozapine-induced myoclonic seizures and valproic acid. Int J Psychiatry Clin Pract. 1998;2(1):53-55.
72. Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long-term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res. 1992;44(2):107-112.
73. Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369-371.
74. Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol. 2005;19(1):93-96.
75. Gandelman-Marton R, Theitler J, Klein C, et al. Phenytoin intoxication in a clozapine-related prolonged seizure. J Emerg Med. 2008;35(4):407-409.
76. Primavera A, Giberti L, Scotto P, et al. Nonconvulsive status epilepticus as a cause of confusion in later life: a report of 5 cases. Neuropsychobiology. 1994;30(2-3):148-152.
77. Boutros NN, Arfken C, Galderisi S, et al. The status of spectral EEG abnormality as a diagnostic test for schizophrenia. Schizophrenia Res. 2008;99(1-3):225-237.
78. Takahashi T, Cho RY, Mizuno T, et al. Antipsychotics reverse abnormal EEG complexity in drug-naïve schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51(1):173-182.