User login
Therapeutic hypothermia has been a central focus of research at the Safar Center for Resuscitation Research since the center was founded—as the International Resuscitation Research Center—at the University of Pittsburgh School of Medicine in 1979. In this article, which is based on my 2008 Bakken Lecture, I will discuss historical, contemporary, and futuristic applications of therapeutic hypothermia. Given that the key mission of the Safar Center is “to save hearts and brains too good to die,” the basis of my discussion will consist of how therapeutic hypothermia impacts both heart and brain—and the lessons that can be learned in each case.
THERAPEUTIC HYPOTHERMIA: A HISTORICAL PERSPECTIVE
Baron Dominique Jean Larrey, surgeon-in-chief of the Napoleonic armies and the father of modern military medicine, observed in 1814 that the wounded “privileged” soldiers lying closer to the campfire died sooner than those in more remote, colder areas.4 Similarly, Dr. Charles Phelps, surgeon to the New York City Police Department, in 1897 recommended the use of the “ice cap” for traumatic brain injury.5
In the 1980s, however, therapeutic hypothermia began to fall out of favor. This resulted, in part, from overzealous application in some patients, who were treated for durations longer than a week and at temperatures in the moderate (28°C to 32°C) rather than mild (33°C to 35°C) range. This led to an increase in complications.6 Laboratory studies in a rat model of global cerebral ischemia by Busto et al 7 in 1987 and in a canine model of cardiac arrest by Leonov et al8 in 1990 demonstrated that benefit could be produced using mild cooling after the insult. This and parallel work in neonatology led to the ultimate breakthrough that translated into improved outcomes with the use of mild therapeutic hypothermia in adults with cardiac arrest9,10 and in newborns with hypoxic-ischemic encephalopathy.11
Clinicians and scientists familiar with hypothermia might suggest that its potential therapeutic benefit has been known for decades, given the use of hypothermic circulatory arrest for neuroprotection and cardioprotection in open heart surgery. However, one of the most interesting aspects of neuroprotection provided by mild therapeutic hypothermia is that it is not clearly linked to attenuation of energy failure.7 Unlike the setting of deep hypothermic circulatory arrest—where the induction of hypothermia occurs before the insult, and levels of hypothermia are such that energy failure is prevented—mild cooling, applied after cardiac arrest, appears to confer benefit by other mechanisms. Effects on cell signaling, oxidative and nitrative stress, apoptosis, excitotoxicity, and other mechanisms appear to mediate this benefit.12,13
THERAPEUTIC HYPOTHERMIA: CONTEMPORARY APPLICATION
Use in cardiac arrest
Compared with normothermia, mild therapeutic hypothermia, induced immediately after restoration of spontaneous circulation in comatose survivors of ventricular fibrillation cardiac arrest, leads to 1 additional patient with intact neurological outcome for every 6 patients treated.9 This is a remarkable effect given the extremely poor overall outcomes observed after out-of-hospital cardiac arrest. Studies in animal models, however, suggest that the therapeutic potential of mild hypothermia can be maximized with application either during or as early as possible after the insult.14 However, clinicians in the field of cardiology appropriately have cause for concern about the possibility that even mild cooling could reduce that potential for successful defibrillation or lead to re-arrest. In 2005, an important paper by Boddicker et al15 explored the impact of mild hypothermia on defibrillation success in experimental ventricular fibrillation in pigs and found, remarkably, that the success rate actually improved with mild or moderate hypothermia! That report opened the door for a number of studies that are now focused on rapid cooling during cardiopulmonary resuscitation (CPR) and on the rapid induction of mild hypothermia using intravenous cooling.16,17
Support for the use of intra-arrest cooling came initially from work in animal models of cardiac arrest—first from the work of Abella et al18 in a mouse model of potassium-induced cardiac arrest, and later from a canine model in work by Nozari et al.19 In the latter study, delaying the onset of mild hypothermia during advanced cardiac life support markedly worsened both multisystem organ failure and survival. Cardiovascular function in that model appeared to be substantively improved by early intra-arrest cooling.
The potential for the use of intravenous cooling in patients with a bolus of crystalloid to induce mild hypothermia was pioneered in a seminal paper by Bernard et al.16 In that report, an approximately 2°C reduction in core temperature could be achieved with infusion of about 30 mL/kg of fluid over 30 minutes. Mean arterial blood pressure increased mildly, and the intervention was well tolerated when applied early after restoration of spontaneous circulation. Kim and colleagues20 built upon that initial work and demonstrated the feasibility of the use of intravenous iced normal saline to induce mild hypothermia by paramedics in the prehospital setting. This approach, and its impact on neurological outcome and survival, is currently being evaluated in a randomized controlled trial. Combining intra-arrest cooling with the use of intravenous fluids is the obvious next step. This could facilitate rapid induction, which could then be maintained with commercially available surface cooling devices.21
Cardiac arrest vs traumatic brain injury
One of the interesting aspects of the beneficial effects of mild therapeutic hypothermia in the setting of cardiac arrest relates to the following question: Why is hypothermia effective in improving neurological outcome after cardiac arrest while it has been more difficult to demonstrate benefit in other acute neurological insults, such as traumatic brain injury?22
Application of hypothermia in cardiac arrest may represent something of a “perfect storm.” First, a recent study by Berger et al23 provides some insight into the time course of neuronal death after cardiac arrest versus traumatic brain injury. In that study of children who suffered either cardiac arrest or severe traumatic brain injury requiring management in the intensive care unit, peak levels of the serum biomarker of neuronal death, neuron-specific enolase (NSE), occurred days after cardiac arrest, whereas they occurred generally within a few hours of traumatic brain injury. This suggests a broader therapeutic window for the application of mild hypothermia in cardiac arrest as opposed to traumatic brain injury. In addition, the only neuroprotective therapy used in cardiac arrest is mild hypothermia. In contrast, in traumatic brain injury, myriad therapies are part of standard of care, including intracranial pressure monitoring and cerebrospinal fluid drainage, mannitol, hypertonic saline, barbiturates, and surgical interventions such as decompressive craniectomy.24 These intracranial pressure–directed therapies in traumatic brain injury may confer a variety of neuroprotective actions, thus raising the bar for hypothermia to show benefit. A similar case could be made regarding the surgical treatment of subarachnoid hemorrhage, where hypothermia has been ineffective.25
Efforts to optimize hypothermia
Given the benefit of mild therapeutic hypothermia in cardiac arrest, we and other investigative teams are actively pursuing ways to further optimize its effects beyond the use of a more rapid induction, as discussed above.
One of the most overlooked areas of study relates to hypothermia’s profound effects on drug metabolism; despite the need for many drugs in critically ill patients after cardiac arrest, knowledge of how hypothermia alters drug metabolism and how best to adjust drug doses is limited. Therapeutic hypothermia has recently been shown, during cooling, to directly inhibit binding of drugs to the active site of the key drug-metabolizing enzyme, cytochrome P450.26 In contrast, in the setting of experimental cardiac arrest and resuscitation, mild hypothermia also protects against induction of cytokines such as interleukin-6, which downregulates cytochrome P450. Thus, mild hypothermia reduces drug metabolism during cooling but leads to a better recovery of drug metabolism after rewarming. This dichotomous effect will need to be studied at the bedside. Hypothermia can also reduce drug effects.26 Thus, until we know how to optimally dose various therapies in patients treated with hypothermia, it is probably best to carefully monitor levels (when possible) and also drug effects. The best example of this at the bedside is the use of monitoring neuromuscular blockade in patients treated with vecuronium or pancuronium during mild hypothermia.
Another interesting area of study involves defining the best anesthetics or sedatives to use with cooling. For example, a recent report by Statler et al27 showed that hypothermia was much less effective as a neuroprotectant after experimental traumatic brain injury in rats anesthetized with fentanyl than with isoflurane. In that study, fentanyl was unable to blunt the stress response to cooling. Given the variety of sedatives and analgesics used at the beside in both neurointensive care units and coronary care units, understanding which agents work best with hypothermia could further enhance hypothermia’s therapeutic benefit.
There is also a search for agents that may promote induction of hypothermia or create a poikilothermic state, thereby facilitating tolerance of the hypothermic state without a stress response. One agent that has shown some promise in the setting of experimental cardiac arrest is the endogenous peptide neurotensin, which has direct effects on temperature regulation at the hypothalamic level. In an experimental model of asphyxial cardiac arrest in rats, Katz et al28 reported that the neurotensin analog NT69L facilitated induction of hypothermia and improved outcome. Another agent that has been touted to induce a state of “hibernation on demand” is hydrogen sulfide gas. A recent experiment by Blackstone et al29 demonstrated induction of deep hypothermia and a hibernation-like state in mice allowed to breathe hydrogen sulfide gas at 80 parts per million. This state was completely reversible upon discontinuation of the agent. Unfortunately, studies in large animal models have not been able to demonstrate induction of hypothermia with this approach.30 Nevertheless, these drugs represent prototypes for future exploration; if the right agent is found, it could lead, in theory, to markedly enhanced efficacy of cooling.
FUTURISTIC APPLICATIONS OF THERAPEUTIC HYPOTHERMIA
Emergency preservation and resuscitation
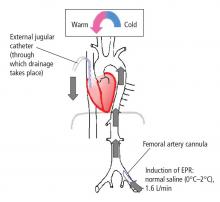
Exsanguination cardiac arrest is one of the most refractory types of cardiac arrest, with mortality rates generally greater than 95%.31 Obviously, therapies such as CPR are ineffective in the absence of an adequate circulating blood volume.
In initial reports,32–34 we targeted relatively brief insults ranging from 15 to 60 minutes. We determined that for insults at or beyond 60 minutes, profound levels of hypothermia (tympanic temperature of ~10°C) were most effective.34 In subsequent studies, we demonstrated that pharmacologic adjuncts to hypothermia were relatively ineffective. Indeed, only one agent, the brain-penetrating antioxidant Tempol, enhanced the efficacy of profound hypothermia.35 We also demonstrated that the prolonged use (36 to 48 hours) of mild hypothermia after the acute application of EPR further enhanced neurological outcomes as compared with more rapid rewarming.36 Similarly, unlike drugs, the addition of energy substrates (namely, dissolved oxygen and 2.5% dextrose) to the flush facilitated the ability to achieve remarkably long EPR durations in experimental exsanguination cardiac arrest—as long as 3 hours of preservation at approximately 10°C.37 These findings could also have important implications for optimizing conventional use of deep hypothermia circulatory arrest in cardiac or neurological surgery. We also have recently developed a rat model of EPR using a miniaturized cardiopulmonary bypass system. It is used to screen novel therapeutic adjuncts to EPR and to study mechanisms of neuroprotection in this special paradigm.38,39
Two other investigative teams, one at Harvard University and another at the Vienna General Hospital, have also been exploring the use of EPR-related technologies—and observing similar success. Alam et al40 have used a low-flow EPR approach in pigs to facilitate damage-control surgery after otherwise lethal traumatic insults. Janata et al41 have successfully used EPR in the setting of refractory normovolemic cardiac arrest, simulating the typical cardiac arrest victim who cannot be resuscitated in either the field or the emergency department.
Finally, the EPR concept recently received funding to proceed to a clinical trial in civilian trauma. The study, to be led by Dr. Samuel Tisherman, one of the pioneers of this approach at the Safar Center, will include several trauma centers in the United States and target otherwise lethally injured trauma victims with exsanguination cardiac arrest.
- Rosomoff HL, Safar P. Management of the comatose patient. In:Safar P, ed. Respiratory Therapy. Philadelphia, PA: FA Davis Co; 1965:244–258.
- Storm C, Schefold JC, Nibbe L, et al. Therapeutic hypothermia after cardiac arrest—the implementation of the ILCOR guidelines in clinical routine is possible! Crit Care 2006; 10:425.
- Safar P. Community-wide cardiopulmonary resuscitation. J Iowa Med Soc 1964:629–635.
- Larrey DJ. Memoirs of Military Surgery and Campaigns of the French Armies. Baltimore, MD: Joseph Cushing/University Press of Sergeant Hall;1814.
- Phelps C. Traumatic injuries of the brain and its membranes. In:Phelps C, ed. Traumatic Injuries of the Brain and Its Membranes. New York, NY: D Appleton & Co; 1897:223–224.
- Bohn DJ, Biggar WD, Smith CR, Conn AW, Barker GA. Influence of hypothermia, barbiturate therapy, and intracranial pressure monitoring on morbidity and mortality after near-drowning. Crit Care Med 1986; 14:529–534.
- Busto R, Dietrich WD, Globus MY, Valdés I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987; 7:729–738.
- Leonov Y, Sterz F, Safar P, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab 1990; 10:57–70.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005; 353:1574–1584.
- Kochanek PM, Jenkins LW, Clark RSB. Traumatic brain injury: laboratory studies. In:Tisherman SA, Sterz F, eds. Therapeutic Hypothermia. New York, NY: Springer; 2005:63–86.
- Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab 2007; 27:1879–1894.
- Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med 1993; 21:1348–1358.
- Boddicker KA, Zhang Y, Zimmerman MB, Davies LR, Kerber RE. Hypothermia improves defibrillation success and resuscitation outcomes from ventricular fibrillation. Circulation 2005; 111:3195–3201.
- Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation 2003; 56:9–13.
- Polderman KH, Rijnsburger ER, Peerdeman SM, Girbes AR. Induction of hypothermia in patients with various types of neurologic injury with use of large volumes of ice-cold intravenous fluid. Crit Care Med 2005; 33:2744–2751.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Nozari A, Safar P, Wu X, et al. Suspended animation can allow survival without brain damage after traumatic exsanguination cardiac arrest of 60 minutes in dogs. J Trauma 2004; 57:1266–1275.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Haugk M, Sterz F, Grassberger M, et al. Feasibility and efficacy of a new non-invasive surface cooling device in post-resuscitation intensive care medicine. Resuscitation 2007; 75:76–81.
- Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med 2008; 358:2447–2456.
- Berger RP, Adelson PD, Richichi R, Kochanek PM. Serum biomarkers after traumatic and hypoxemic brain injuries: insight into the biochemical response of the pediatric brain to inflicted brain injury. Dev Neurosci 2006; 28:327–335.
- , et al., Brain Trauma Foundation. Guidelines for the management of severe traumatic brain injury:. Introduction. J Neurotrauma 2007; 24( suppl 1):S1–S2.
- Todd MM, Hindman BJ, Clarke WR, Torner JCIntraoperative Hypothermia for Aneurysm Surgery Trial (IHAST) Investigators. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med 2005; 352:135–145.
- Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med 2007; 35:2196–2204.
- Statler KD, Alexander HL, Vagni VA, et al. Moderate hypothermia may be detrimental after traumatic brain injury in fentanylanesthetized rats. Crit Care Med 2003; 31:1134–1139.
- Katz LM, Wang Y, McMahon B, Richelson E. Neurotensin analog NT69L induces rapid and prolonged hypothermia after hypoxic ischemia. Acad Emerg Med 2001; 8:1115–1121.
- Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science 2005; 308:518.
- Li J, Zhang G, Cai S, Redington AN. Effect of inhaled hydrogen sulfide on metabolic responses in anesthetized, paralyzed, and mechanically ventilated piglets. Pediatr Crit Care Med 2008; 9:110–112.
- Rhee PM, Acosta J, Bridgeman A, Wang D, Jordan M, Rich N. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg 2000; 190:288–298.
- Behringer W, Prueckner S, Kentner R, et al. Rapid hypothermic aortic flush can achieve survival without brain damage after 30 minutes cardiac arrest in dogs. Anesthesiology 2000; 93:1491–1499.
- Behringer W, Kentner R, Wu X, et al. Fructose-1,6-bisphosphate and MK-801 by aortic arch flush for cerebral preservation during exsanguination cardiac arrest of 20 min in dogs: an exploratory study. Resuscitation 2001; 50:205–216.
- Behringer W, Safar P, Wu X, et al. Survival without brain damage after clinical death of 60–120 mins in dogs using suspended animation by profound hypothermia. Crit Care Med 2003; 31:1523–1531.
- Behringer W, Safar P, Kentner R, et al. Antioxidant Tempol enhances hypothermic cerebral preservation during prolonged cardiac arrest in dogs. J Cereb Blood Flow Metab 2002; 22:105–117.
- Wu X, Drabek T, Kochanek PM, et al. Induction of profound hypothermia for emergency preservation and resuscitation allows intact survival after cardiac arrest resulting from prolonged lethal hemorrhage and trauma in dogs. Circulation 2006; 113:1974–1982.
- Wu X, Drabek T, Tisherman SA, et al. Emergency preservation and resuscitation with profound hypothermia, oxygen, and glucose allows reliable neurological recovery after 3 h of cardiac arrest from rapid exsanguination in dogs. J Cereb Blood Flow Metab 2008; 28:302–311.
- Drabek T, Stezoski J, Garman RH, et al. Emergency preservation and delayed resuscitation allows normal recovery after exsanguination cardiac arrest in rats: a feasibility trial. Crit Care Med 2007; 35:532–537.
- Drabek T, Stezoski J, Garman RH, et al. Exsanguination cardiac arrest in rats treated by 60 min, but not 75 min, emergency preservation and delayed resuscitation is associated with intact outcome. Resuscitation 2007; 75:114–123.
- Alam HB, Chen Z, Honma K, et al. The rate of induction of hypothermic arrest determines the outcome in a swine model of lethal hemorrhage. J Trauma 2004; 57:961–969.
- Janata A, Bayegan K, Weihs W, et al. Emergency preservation and resuscitation improve survival after 15 minutes of normovolemic cardiac arrest in pigs. Crit Care Med 2007; 35:2785–2791.
Therapeutic hypothermia has been a central focus of research at the Safar Center for Resuscitation Research since the center was founded—as the International Resuscitation Research Center—at the University of Pittsburgh School of Medicine in 1979. In this article, which is based on my 2008 Bakken Lecture, I will discuss historical, contemporary, and futuristic applications of therapeutic hypothermia. Given that the key mission of the Safar Center is “to save hearts and brains too good to die,” the basis of my discussion will consist of how therapeutic hypothermia impacts both heart and brain—and the lessons that can be learned in each case.
THERAPEUTIC HYPOTHERMIA: A HISTORICAL PERSPECTIVE
Baron Dominique Jean Larrey, surgeon-in-chief of the Napoleonic armies and the father of modern military medicine, observed in 1814 that the wounded “privileged” soldiers lying closer to the campfire died sooner than those in more remote, colder areas.4 Similarly, Dr. Charles Phelps, surgeon to the New York City Police Department, in 1897 recommended the use of the “ice cap” for traumatic brain injury.5
In the 1980s, however, therapeutic hypothermia began to fall out of favor. This resulted, in part, from overzealous application in some patients, who were treated for durations longer than a week and at temperatures in the moderate (28°C to 32°C) rather than mild (33°C to 35°C) range. This led to an increase in complications.6 Laboratory studies in a rat model of global cerebral ischemia by Busto et al 7 in 1987 and in a canine model of cardiac arrest by Leonov et al8 in 1990 demonstrated that benefit could be produced using mild cooling after the insult. This and parallel work in neonatology led to the ultimate breakthrough that translated into improved outcomes with the use of mild therapeutic hypothermia in adults with cardiac arrest9,10 and in newborns with hypoxic-ischemic encephalopathy.11
Clinicians and scientists familiar with hypothermia might suggest that its potential therapeutic benefit has been known for decades, given the use of hypothermic circulatory arrest for neuroprotection and cardioprotection in open heart surgery. However, one of the most interesting aspects of neuroprotection provided by mild therapeutic hypothermia is that it is not clearly linked to attenuation of energy failure.7 Unlike the setting of deep hypothermic circulatory arrest—where the induction of hypothermia occurs before the insult, and levels of hypothermia are such that energy failure is prevented—mild cooling, applied after cardiac arrest, appears to confer benefit by other mechanisms. Effects on cell signaling, oxidative and nitrative stress, apoptosis, excitotoxicity, and other mechanisms appear to mediate this benefit.12,13
THERAPEUTIC HYPOTHERMIA: CONTEMPORARY APPLICATION
Use in cardiac arrest
Compared with normothermia, mild therapeutic hypothermia, induced immediately after restoration of spontaneous circulation in comatose survivors of ventricular fibrillation cardiac arrest, leads to 1 additional patient with intact neurological outcome for every 6 patients treated.9 This is a remarkable effect given the extremely poor overall outcomes observed after out-of-hospital cardiac arrest. Studies in animal models, however, suggest that the therapeutic potential of mild hypothermia can be maximized with application either during or as early as possible after the insult.14 However, clinicians in the field of cardiology appropriately have cause for concern about the possibility that even mild cooling could reduce that potential for successful defibrillation or lead to re-arrest. In 2005, an important paper by Boddicker et al15 explored the impact of mild hypothermia on defibrillation success in experimental ventricular fibrillation in pigs and found, remarkably, that the success rate actually improved with mild or moderate hypothermia! That report opened the door for a number of studies that are now focused on rapid cooling during cardiopulmonary resuscitation (CPR) and on the rapid induction of mild hypothermia using intravenous cooling.16,17
Support for the use of intra-arrest cooling came initially from work in animal models of cardiac arrest—first from the work of Abella et al18 in a mouse model of potassium-induced cardiac arrest, and later from a canine model in work by Nozari et al.19 In the latter study, delaying the onset of mild hypothermia during advanced cardiac life support markedly worsened both multisystem organ failure and survival. Cardiovascular function in that model appeared to be substantively improved by early intra-arrest cooling.
The potential for the use of intravenous cooling in patients with a bolus of crystalloid to induce mild hypothermia was pioneered in a seminal paper by Bernard et al.16 In that report, an approximately 2°C reduction in core temperature could be achieved with infusion of about 30 mL/kg of fluid over 30 minutes. Mean arterial blood pressure increased mildly, and the intervention was well tolerated when applied early after restoration of spontaneous circulation. Kim and colleagues20 built upon that initial work and demonstrated the feasibility of the use of intravenous iced normal saline to induce mild hypothermia by paramedics in the prehospital setting. This approach, and its impact on neurological outcome and survival, is currently being evaluated in a randomized controlled trial. Combining intra-arrest cooling with the use of intravenous fluids is the obvious next step. This could facilitate rapid induction, which could then be maintained with commercially available surface cooling devices.21
Cardiac arrest vs traumatic brain injury
One of the interesting aspects of the beneficial effects of mild therapeutic hypothermia in the setting of cardiac arrest relates to the following question: Why is hypothermia effective in improving neurological outcome after cardiac arrest while it has been more difficult to demonstrate benefit in other acute neurological insults, such as traumatic brain injury?22
Application of hypothermia in cardiac arrest may represent something of a “perfect storm.” First, a recent study by Berger et al23 provides some insight into the time course of neuronal death after cardiac arrest versus traumatic brain injury. In that study of children who suffered either cardiac arrest or severe traumatic brain injury requiring management in the intensive care unit, peak levels of the serum biomarker of neuronal death, neuron-specific enolase (NSE), occurred days after cardiac arrest, whereas they occurred generally within a few hours of traumatic brain injury. This suggests a broader therapeutic window for the application of mild hypothermia in cardiac arrest as opposed to traumatic brain injury. In addition, the only neuroprotective therapy used in cardiac arrest is mild hypothermia. In contrast, in traumatic brain injury, myriad therapies are part of standard of care, including intracranial pressure monitoring and cerebrospinal fluid drainage, mannitol, hypertonic saline, barbiturates, and surgical interventions such as decompressive craniectomy.24 These intracranial pressure–directed therapies in traumatic brain injury may confer a variety of neuroprotective actions, thus raising the bar for hypothermia to show benefit. A similar case could be made regarding the surgical treatment of subarachnoid hemorrhage, where hypothermia has been ineffective.25
Efforts to optimize hypothermia
Given the benefit of mild therapeutic hypothermia in cardiac arrest, we and other investigative teams are actively pursuing ways to further optimize its effects beyond the use of a more rapid induction, as discussed above.
One of the most overlooked areas of study relates to hypothermia’s profound effects on drug metabolism; despite the need for many drugs in critically ill patients after cardiac arrest, knowledge of how hypothermia alters drug metabolism and how best to adjust drug doses is limited. Therapeutic hypothermia has recently been shown, during cooling, to directly inhibit binding of drugs to the active site of the key drug-metabolizing enzyme, cytochrome P450.26 In contrast, in the setting of experimental cardiac arrest and resuscitation, mild hypothermia also protects against induction of cytokines such as interleukin-6, which downregulates cytochrome P450. Thus, mild hypothermia reduces drug metabolism during cooling but leads to a better recovery of drug metabolism after rewarming. This dichotomous effect will need to be studied at the bedside. Hypothermia can also reduce drug effects.26 Thus, until we know how to optimally dose various therapies in patients treated with hypothermia, it is probably best to carefully monitor levels (when possible) and also drug effects. The best example of this at the bedside is the use of monitoring neuromuscular blockade in patients treated with vecuronium or pancuronium during mild hypothermia.
Another interesting area of study involves defining the best anesthetics or sedatives to use with cooling. For example, a recent report by Statler et al27 showed that hypothermia was much less effective as a neuroprotectant after experimental traumatic brain injury in rats anesthetized with fentanyl than with isoflurane. In that study, fentanyl was unable to blunt the stress response to cooling. Given the variety of sedatives and analgesics used at the beside in both neurointensive care units and coronary care units, understanding which agents work best with hypothermia could further enhance hypothermia’s therapeutic benefit.
There is also a search for agents that may promote induction of hypothermia or create a poikilothermic state, thereby facilitating tolerance of the hypothermic state without a stress response. One agent that has shown some promise in the setting of experimental cardiac arrest is the endogenous peptide neurotensin, which has direct effects on temperature regulation at the hypothalamic level. In an experimental model of asphyxial cardiac arrest in rats, Katz et al28 reported that the neurotensin analog NT69L facilitated induction of hypothermia and improved outcome. Another agent that has been touted to induce a state of “hibernation on demand” is hydrogen sulfide gas. A recent experiment by Blackstone et al29 demonstrated induction of deep hypothermia and a hibernation-like state in mice allowed to breathe hydrogen sulfide gas at 80 parts per million. This state was completely reversible upon discontinuation of the agent. Unfortunately, studies in large animal models have not been able to demonstrate induction of hypothermia with this approach.30 Nevertheless, these drugs represent prototypes for future exploration; if the right agent is found, it could lead, in theory, to markedly enhanced efficacy of cooling.
FUTURISTIC APPLICATIONS OF THERAPEUTIC HYPOTHERMIA
Emergency preservation and resuscitation
Exsanguination cardiac arrest is one of the most refractory types of cardiac arrest, with mortality rates generally greater than 95%.31 Obviously, therapies such as CPR are ineffective in the absence of an adequate circulating blood volume.
In initial reports,32–34 we targeted relatively brief insults ranging from 15 to 60 minutes. We determined that for insults at or beyond 60 minutes, profound levels of hypothermia (tympanic temperature of ~10°C) were most effective.34 In subsequent studies, we demonstrated that pharmacologic adjuncts to hypothermia were relatively ineffective. Indeed, only one agent, the brain-penetrating antioxidant Tempol, enhanced the efficacy of profound hypothermia.35 We also demonstrated that the prolonged use (36 to 48 hours) of mild hypothermia after the acute application of EPR further enhanced neurological outcomes as compared with more rapid rewarming.36 Similarly, unlike drugs, the addition of energy substrates (namely, dissolved oxygen and 2.5% dextrose) to the flush facilitated the ability to achieve remarkably long EPR durations in experimental exsanguination cardiac arrest—as long as 3 hours of preservation at approximately 10°C.37 These findings could also have important implications for optimizing conventional use of deep hypothermia circulatory arrest in cardiac or neurological surgery. We also have recently developed a rat model of EPR using a miniaturized cardiopulmonary bypass system. It is used to screen novel therapeutic adjuncts to EPR and to study mechanisms of neuroprotection in this special paradigm.38,39
Two other investigative teams, one at Harvard University and another at the Vienna General Hospital, have also been exploring the use of EPR-related technologies—and observing similar success. Alam et al40 have used a low-flow EPR approach in pigs to facilitate damage-control surgery after otherwise lethal traumatic insults. Janata et al41 have successfully used EPR in the setting of refractory normovolemic cardiac arrest, simulating the typical cardiac arrest victim who cannot be resuscitated in either the field or the emergency department.
Finally, the EPR concept recently received funding to proceed to a clinical trial in civilian trauma. The study, to be led by Dr. Samuel Tisherman, one of the pioneers of this approach at the Safar Center, will include several trauma centers in the United States and target otherwise lethally injured trauma victims with exsanguination cardiac arrest.
Therapeutic hypothermia has been a central focus of research at the Safar Center for Resuscitation Research since the center was founded—as the International Resuscitation Research Center—at the University of Pittsburgh School of Medicine in 1979. In this article, which is based on my 2008 Bakken Lecture, I will discuss historical, contemporary, and futuristic applications of therapeutic hypothermia. Given that the key mission of the Safar Center is “to save hearts and brains too good to die,” the basis of my discussion will consist of how therapeutic hypothermia impacts both heart and brain—and the lessons that can be learned in each case.
THERAPEUTIC HYPOTHERMIA: A HISTORICAL PERSPECTIVE
Baron Dominique Jean Larrey, surgeon-in-chief of the Napoleonic armies and the father of modern military medicine, observed in 1814 that the wounded “privileged” soldiers lying closer to the campfire died sooner than those in more remote, colder areas.4 Similarly, Dr. Charles Phelps, surgeon to the New York City Police Department, in 1897 recommended the use of the “ice cap” for traumatic brain injury.5
In the 1980s, however, therapeutic hypothermia began to fall out of favor. This resulted, in part, from overzealous application in some patients, who were treated for durations longer than a week and at temperatures in the moderate (28°C to 32°C) rather than mild (33°C to 35°C) range. This led to an increase in complications.6 Laboratory studies in a rat model of global cerebral ischemia by Busto et al 7 in 1987 and in a canine model of cardiac arrest by Leonov et al8 in 1990 demonstrated that benefit could be produced using mild cooling after the insult. This and parallel work in neonatology led to the ultimate breakthrough that translated into improved outcomes with the use of mild therapeutic hypothermia in adults with cardiac arrest9,10 and in newborns with hypoxic-ischemic encephalopathy.11
Clinicians and scientists familiar with hypothermia might suggest that its potential therapeutic benefit has been known for decades, given the use of hypothermic circulatory arrest for neuroprotection and cardioprotection in open heart surgery. However, one of the most interesting aspects of neuroprotection provided by mild therapeutic hypothermia is that it is not clearly linked to attenuation of energy failure.7 Unlike the setting of deep hypothermic circulatory arrest—where the induction of hypothermia occurs before the insult, and levels of hypothermia are such that energy failure is prevented—mild cooling, applied after cardiac arrest, appears to confer benefit by other mechanisms. Effects on cell signaling, oxidative and nitrative stress, apoptosis, excitotoxicity, and other mechanisms appear to mediate this benefit.12,13
THERAPEUTIC HYPOTHERMIA: CONTEMPORARY APPLICATION
Use in cardiac arrest
Compared with normothermia, mild therapeutic hypothermia, induced immediately after restoration of spontaneous circulation in comatose survivors of ventricular fibrillation cardiac arrest, leads to 1 additional patient with intact neurological outcome for every 6 patients treated.9 This is a remarkable effect given the extremely poor overall outcomes observed after out-of-hospital cardiac arrest. Studies in animal models, however, suggest that the therapeutic potential of mild hypothermia can be maximized with application either during or as early as possible after the insult.14 However, clinicians in the field of cardiology appropriately have cause for concern about the possibility that even mild cooling could reduce that potential for successful defibrillation or lead to re-arrest. In 2005, an important paper by Boddicker et al15 explored the impact of mild hypothermia on defibrillation success in experimental ventricular fibrillation in pigs and found, remarkably, that the success rate actually improved with mild or moderate hypothermia! That report opened the door for a number of studies that are now focused on rapid cooling during cardiopulmonary resuscitation (CPR) and on the rapid induction of mild hypothermia using intravenous cooling.16,17
Support for the use of intra-arrest cooling came initially from work in animal models of cardiac arrest—first from the work of Abella et al18 in a mouse model of potassium-induced cardiac arrest, and later from a canine model in work by Nozari et al.19 In the latter study, delaying the onset of mild hypothermia during advanced cardiac life support markedly worsened both multisystem organ failure and survival. Cardiovascular function in that model appeared to be substantively improved by early intra-arrest cooling.
The potential for the use of intravenous cooling in patients with a bolus of crystalloid to induce mild hypothermia was pioneered in a seminal paper by Bernard et al.16 In that report, an approximately 2°C reduction in core temperature could be achieved with infusion of about 30 mL/kg of fluid over 30 minutes. Mean arterial blood pressure increased mildly, and the intervention was well tolerated when applied early after restoration of spontaneous circulation. Kim and colleagues20 built upon that initial work and demonstrated the feasibility of the use of intravenous iced normal saline to induce mild hypothermia by paramedics in the prehospital setting. This approach, and its impact on neurological outcome and survival, is currently being evaluated in a randomized controlled trial. Combining intra-arrest cooling with the use of intravenous fluids is the obvious next step. This could facilitate rapid induction, which could then be maintained with commercially available surface cooling devices.21
Cardiac arrest vs traumatic brain injury
One of the interesting aspects of the beneficial effects of mild therapeutic hypothermia in the setting of cardiac arrest relates to the following question: Why is hypothermia effective in improving neurological outcome after cardiac arrest while it has been more difficult to demonstrate benefit in other acute neurological insults, such as traumatic brain injury?22
Application of hypothermia in cardiac arrest may represent something of a “perfect storm.” First, a recent study by Berger et al23 provides some insight into the time course of neuronal death after cardiac arrest versus traumatic brain injury. In that study of children who suffered either cardiac arrest or severe traumatic brain injury requiring management in the intensive care unit, peak levels of the serum biomarker of neuronal death, neuron-specific enolase (NSE), occurred days after cardiac arrest, whereas they occurred generally within a few hours of traumatic brain injury. This suggests a broader therapeutic window for the application of mild hypothermia in cardiac arrest as opposed to traumatic brain injury. In addition, the only neuroprotective therapy used in cardiac arrest is mild hypothermia. In contrast, in traumatic brain injury, myriad therapies are part of standard of care, including intracranial pressure monitoring and cerebrospinal fluid drainage, mannitol, hypertonic saline, barbiturates, and surgical interventions such as decompressive craniectomy.24 These intracranial pressure–directed therapies in traumatic brain injury may confer a variety of neuroprotective actions, thus raising the bar for hypothermia to show benefit. A similar case could be made regarding the surgical treatment of subarachnoid hemorrhage, where hypothermia has been ineffective.25
Efforts to optimize hypothermia
Given the benefit of mild therapeutic hypothermia in cardiac arrest, we and other investigative teams are actively pursuing ways to further optimize its effects beyond the use of a more rapid induction, as discussed above.
One of the most overlooked areas of study relates to hypothermia’s profound effects on drug metabolism; despite the need for many drugs in critically ill patients after cardiac arrest, knowledge of how hypothermia alters drug metabolism and how best to adjust drug doses is limited. Therapeutic hypothermia has recently been shown, during cooling, to directly inhibit binding of drugs to the active site of the key drug-metabolizing enzyme, cytochrome P450.26 In contrast, in the setting of experimental cardiac arrest and resuscitation, mild hypothermia also protects against induction of cytokines such as interleukin-6, which downregulates cytochrome P450. Thus, mild hypothermia reduces drug metabolism during cooling but leads to a better recovery of drug metabolism after rewarming. This dichotomous effect will need to be studied at the bedside. Hypothermia can also reduce drug effects.26 Thus, until we know how to optimally dose various therapies in patients treated with hypothermia, it is probably best to carefully monitor levels (when possible) and also drug effects. The best example of this at the bedside is the use of monitoring neuromuscular blockade in patients treated with vecuronium or pancuronium during mild hypothermia.
Another interesting area of study involves defining the best anesthetics or sedatives to use with cooling. For example, a recent report by Statler et al27 showed that hypothermia was much less effective as a neuroprotectant after experimental traumatic brain injury in rats anesthetized with fentanyl than with isoflurane. In that study, fentanyl was unable to blunt the stress response to cooling. Given the variety of sedatives and analgesics used at the beside in both neurointensive care units and coronary care units, understanding which agents work best with hypothermia could further enhance hypothermia’s therapeutic benefit.
There is also a search for agents that may promote induction of hypothermia or create a poikilothermic state, thereby facilitating tolerance of the hypothermic state without a stress response. One agent that has shown some promise in the setting of experimental cardiac arrest is the endogenous peptide neurotensin, which has direct effects on temperature regulation at the hypothalamic level. In an experimental model of asphyxial cardiac arrest in rats, Katz et al28 reported that the neurotensin analog NT69L facilitated induction of hypothermia and improved outcome. Another agent that has been touted to induce a state of “hibernation on demand” is hydrogen sulfide gas. A recent experiment by Blackstone et al29 demonstrated induction of deep hypothermia and a hibernation-like state in mice allowed to breathe hydrogen sulfide gas at 80 parts per million. This state was completely reversible upon discontinuation of the agent. Unfortunately, studies in large animal models have not been able to demonstrate induction of hypothermia with this approach.30 Nevertheless, these drugs represent prototypes for future exploration; if the right agent is found, it could lead, in theory, to markedly enhanced efficacy of cooling.
FUTURISTIC APPLICATIONS OF THERAPEUTIC HYPOTHERMIA
Emergency preservation and resuscitation
Exsanguination cardiac arrest is one of the most refractory types of cardiac arrest, with mortality rates generally greater than 95%.31 Obviously, therapies such as CPR are ineffective in the absence of an adequate circulating blood volume.
In initial reports,32–34 we targeted relatively brief insults ranging from 15 to 60 minutes. We determined that for insults at or beyond 60 minutes, profound levels of hypothermia (tympanic temperature of ~10°C) were most effective.34 In subsequent studies, we demonstrated that pharmacologic adjuncts to hypothermia were relatively ineffective. Indeed, only one agent, the brain-penetrating antioxidant Tempol, enhanced the efficacy of profound hypothermia.35 We also demonstrated that the prolonged use (36 to 48 hours) of mild hypothermia after the acute application of EPR further enhanced neurological outcomes as compared with more rapid rewarming.36 Similarly, unlike drugs, the addition of energy substrates (namely, dissolved oxygen and 2.5% dextrose) to the flush facilitated the ability to achieve remarkably long EPR durations in experimental exsanguination cardiac arrest—as long as 3 hours of preservation at approximately 10°C.37 These findings could also have important implications for optimizing conventional use of deep hypothermia circulatory arrest in cardiac or neurological surgery. We also have recently developed a rat model of EPR using a miniaturized cardiopulmonary bypass system. It is used to screen novel therapeutic adjuncts to EPR and to study mechanisms of neuroprotection in this special paradigm.38,39
Two other investigative teams, one at Harvard University and another at the Vienna General Hospital, have also been exploring the use of EPR-related technologies—and observing similar success. Alam et al40 have used a low-flow EPR approach in pigs to facilitate damage-control surgery after otherwise lethal traumatic insults. Janata et al41 have successfully used EPR in the setting of refractory normovolemic cardiac arrest, simulating the typical cardiac arrest victim who cannot be resuscitated in either the field or the emergency department.
Finally, the EPR concept recently received funding to proceed to a clinical trial in civilian trauma. The study, to be led by Dr. Samuel Tisherman, one of the pioneers of this approach at the Safar Center, will include several trauma centers in the United States and target otherwise lethally injured trauma victims with exsanguination cardiac arrest.
- Rosomoff HL, Safar P. Management of the comatose patient. In:Safar P, ed. Respiratory Therapy. Philadelphia, PA: FA Davis Co; 1965:244–258.
- Storm C, Schefold JC, Nibbe L, et al. Therapeutic hypothermia after cardiac arrest—the implementation of the ILCOR guidelines in clinical routine is possible! Crit Care 2006; 10:425.
- Safar P. Community-wide cardiopulmonary resuscitation. J Iowa Med Soc 1964:629–635.
- Larrey DJ. Memoirs of Military Surgery and Campaigns of the French Armies. Baltimore, MD: Joseph Cushing/University Press of Sergeant Hall;1814.
- Phelps C. Traumatic injuries of the brain and its membranes. In:Phelps C, ed. Traumatic Injuries of the Brain and Its Membranes. New York, NY: D Appleton & Co; 1897:223–224.
- Bohn DJ, Biggar WD, Smith CR, Conn AW, Barker GA. Influence of hypothermia, barbiturate therapy, and intracranial pressure monitoring on morbidity and mortality after near-drowning. Crit Care Med 1986; 14:529–534.
- Busto R, Dietrich WD, Globus MY, Valdés I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987; 7:729–738.
- Leonov Y, Sterz F, Safar P, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab 1990; 10:57–70.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005; 353:1574–1584.
- Kochanek PM, Jenkins LW, Clark RSB. Traumatic brain injury: laboratory studies. In:Tisherman SA, Sterz F, eds. Therapeutic Hypothermia. New York, NY: Springer; 2005:63–86.
- Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab 2007; 27:1879–1894.
- Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med 1993; 21:1348–1358.
- Boddicker KA, Zhang Y, Zimmerman MB, Davies LR, Kerber RE. Hypothermia improves defibrillation success and resuscitation outcomes from ventricular fibrillation. Circulation 2005; 111:3195–3201.
- Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation 2003; 56:9–13.
- Polderman KH, Rijnsburger ER, Peerdeman SM, Girbes AR. Induction of hypothermia in patients with various types of neurologic injury with use of large volumes of ice-cold intravenous fluid. Crit Care Med 2005; 33:2744–2751.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Nozari A, Safar P, Wu X, et al. Suspended animation can allow survival without brain damage after traumatic exsanguination cardiac arrest of 60 minutes in dogs. J Trauma 2004; 57:1266–1275.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Haugk M, Sterz F, Grassberger M, et al. Feasibility and efficacy of a new non-invasive surface cooling device in post-resuscitation intensive care medicine. Resuscitation 2007; 75:76–81.
- Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med 2008; 358:2447–2456.
- Berger RP, Adelson PD, Richichi R, Kochanek PM. Serum biomarkers after traumatic and hypoxemic brain injuries: insight into the biochemical response of the pediatric brain to inflicted brain injury. Dev Neurosci 2006; 28:327–335.
- , et al., Brain Trauma Foundation. Guidelines for the management of severe traumatic brain injury:. Introduction. J Neurotrauma 2007; 24( suppl 1):S1–S2.
- Todd MM, Hindman BJ, Clarke WR, Torner JCIntraoperative Hypothermia for Aneurysm Surgery Trial (IHAST) Investigators. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med 2005; 352:135–145.
- Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med 2007; 35:2196–2204.
- Statler KD, Alexander HL, Vagni VA, et al. Moderate hypothermia may be detrimental after traumatic brain injury in fentanylanesthetized rats. Crit Care Med 2003; 31:1134–1139.
- Katz LM, Wang Y, McMahon B, Richelson E. Neurotensin analog NT69L induces rapid and prolonged hypothermia after hypoxic ischemia. Acad Emerg Med 2001; 8:1115–1121.
- Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science 2005; 308:518.
- Li J, Zhang G, Cai S, Redington AN. Effect of inhaled hydrogen sulfide on metabolic responses in anesthetized, paralyzed, and mechanically ventilated piglets. Pediatr Crit Care Med 2008; 9:110–112.
- Rhee PM, Acosta J, Bridgeman A, Wang D, Jordan M, Rich N. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg 2000; 190:288–298.
- Behringer W, Prueckner S, Kentner R, et al. Rapid hypothermic aortic flush can achieve survival without brain damage after 30 minutes cardiac arrest in dogs. Anesthesiology 2000; 93:1491–1499.
- Behringer W, Kentner R, Wu X, et al. Fructose-1,6-bisphosphate and MK-801 by aortic arch flush for cerebral preservation during exsanguination cardiac arrest of 20 min in dogs: an exploratory study. Resuscitation 2001; 50:205–216.
- Behringer W, Safar P, Wu X, et al. Survival without brain damage after clinical death of 60–120 mins in dogs using suspended animation by profound hypothermia. Crit Care Med 2003; 31:1523–1531.
- Behringer W, Safar P, Kentner R, et al. Antioxidant Tempol enhances hypothermic cerebral preservation during prolonged cardiac arrest in dogs. J Cereb Blood Flow Metab 2002; 22:105–117.
- Wu X, Drabek T, Kochanek PM, et al. Induction of profound hypothermia for emergency preservation and resuscitation allows intact survival after cardiac arrest resulting from prolonged lethal hemorrhage and trauma in dogs. Circulation 2006; 113:1974–1982.
- Wu X, Drabek T, Tisherman SA, et al. Emergency preservation and resuscitation with profound hypothermia, oxygen, and glucose allows reliable neurological recovery after 3 h of cardiac arrest from rapid exsanguination in dogs. J Cereb Blood Flow Metab 2008; 28:302–311.
- Drabek T, Stezoski J, Garman RH, et al. Emergency preservation and delayed resuscitation allows normal recovery after exsanguination cardiac arrest in rats: a feasibility trial. Crit Care Med 2007; 35:532–537.
- Drabek T, Stezoski J, Garman RH, et al. Exsanguination cardiac arrest in rats treated by 60 min, but not 75 min, emergency preservation and delayed resuscitation is associated with intact outcome. Resuscitation 2007; 75:114–123.
- Alam HB, Chen Z, Honma K, et al. The rate of induction of hypothermic arrest determines the outcome in a swine model of lethal hemorrhage. J Trauma 2004; 57:961–969.
- Janata A, Bayegan K, Weihs W, et al. Emergency preservation and resuscitation improve survival after 15 minutes of normovolemic cardiac arrest in pigs. Crit Care Med 2007; 35:2785–2791.
- Rosomoff HL, Safar P. Management of the comatose patient. In:Safar P, ed. Respiratory Therapy. Philadelphia, PA: FA Davis Co; 1965:244–258.
- Storm C, Schefold JC, Nibbe L, et al. Therapeutic hypothermia after cardiac arrest—the implementation of the ILCOR guidelines in clinical routine is possible! Crit Care 2006; 10:425.
- Safar P. Community-wide cardiopulmonary resuscitation. J Iowa Med Soc 1964:629–635.
- Larrey DJ. Memoirs of Military Surgery and Campaigns of the French Armies. Baltimore, MD: Joseph Cushing/University Press of Sergeant Hall;1814.
- Phelps C. Traumatic injuries of the brain and its membranes. In:Phelps C, ed. Traumatic Injuries of the Brain and Its Membranes. New York, NY: D Appleton & Co; 1897:223–224.
- Bohn DJ, Biggar WD, Smith CR, Conn AW, Barker GA. Influence of hypothermia, barbiturate therapy, and intracranial pressure monitoring on morbidity and mortality after near-drowning. Crit Care Med 1986; 14:529–534.
- Busto R, Dietrich WD, Globus MY, Valdés I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987; 7:729–738.
- Leonov Y, Sterz F, Safar P, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab 1990; 10:57–70.
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002; 346:549–556.
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002; 346:557–563.
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005; 353:1574–1584.
- Kochanek PM, Jenkins LW, Clark RSB. Traumatic brain injury: laboratory studies. In:Tisherman SA, Sterz F, eds. Therapeutic Hypothermia. New York, NY: Springer; 2005:63–86.
- Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab 2007; 27:1879–1894.
- Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med 1993; 21:1348–1358.
- Boddicker KA, Zhang Y, Zimmerman MB, Davies LR, Kerber RE. Hypothermia improves defibrillation success and resuscitation outcomes from ventricular fibrillation. Circulation 2005; 111:3195–3201.
- Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation 2003; 56:9–13.
- Polderman KH, Rijnsburger ER, Peerdeman SM, Girbes AR. Induction of hypothermia in patients with various types of neurologic injury with use of large volumes of ice-cold intravenous fluid. Crit Care Med 2005; 33:2744–2751.
- Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 2004; 109:2786–2791.
- Nozari A, Safar P, Wu X, et al. Suspended animation can allow survival without brain damage after traumatic exsanguination cardiac arrest of 60 minutes in dogs. J Trauma 2004; 57:1266–1275.
- Kim F, Olsufka M, Longstreth WT, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation 2007; 115:3064–3070.
- Haugk M, Sterz F, Grassberger M, et al. Feasibility and efficacy of a new non-invasive surface cooling device in post-resuscitation intensive care medicine. Resuscitation 2007; 75:76–81.
- Hutchison JS, Ward RE, Lacroix J, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med 2008; 358:2447–2456.
- Berger RP, Adelson PD, Richichi R, Kochanek PM. Serum biomarkers after traumatic and hypoxemic brain injuries: insight into the biochemical response of the pediatric brain to inflicted brain injury. Dev Neurosci 2006; 28:327–335.
- , et al., Brain Trauma Foundation. Guidelines for the management of severe traumatic brain injury:. Introduction. J Neurotrauma 2007; 24( suppl 1):S1–S2.
- Todd MM, Hindman BJ, Clarke WR, Torner JCIntraoperative Hypothermia for Aneurysm Surgery Trial (IHAST) Investigators. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med 2005; 352:135–145.
- Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med 2007; 35:2196–2204.
- Statler KD, Alexander HL, Vagni VA, et al. Moderate hypothermia may be detrimental after traumatic brain injury in fentanylanesthetized rats. Crit Care Med 2003; 31:1134–1139.
- Katz LM, Wang Y, McMahon B, Richelson E. Neurotensin analog NT69L induces rapid and prolonged hypothermia after hypoxic ischemia. Acad Emerg Med 2001; 8:1115–1121.
- Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science 2005; 308:518.
- Li J, Zhang G, Cai S, Redington AN. Effect of inhaled hydrogen sulfide on metabolic responses in anesthetized, paralyzed, and mechanically ventilated piglets. Pediatr Crit Care Med 2008; 9:110–112.
- Rhee PM, Acosta J, Bridgeman A, Wang D, Jordan M, Rich N. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg 2000; 190:288–298.
- Behringer W, Prueckner S, Kentner R, et al. Rapid hypothermic aortic flush can achieve survival without brain damage after 30 minutes cardiac arrest in dogs. Anesthesiology 2000; 93:1491–1499.
- Behringer W, Kentner R, Wu X, et al. Fructose-1,6-bisphosphate and MK-801 by aortic arch flush for cerebral preservation during exsanguination cardiac arrest of 20 min in dogs: an exploratory study. Resuscitation 2001; 50:205–216.
- Behringer W, Safar P, Wu X, et al. Survival without brain damage after clinical death of 60–120 mins in dogs using suspended animation by profound hypothermia. Crit Care Med 2003; 31:1523–1531.
- Behringer W, Safar P, Kentner R, et al. Antioxidant Tempol enhances hypothermic cerebral preservation during prolonged cardiac arrest in dogs. J Cereb Blood Flow Metab 2002; 22:105–117.
- Wu X, Drabek T, Kochanek PM, et al. Induction of profound hypothermia for emergency preservation and resuscitation allows intact survival after cardiac arrest resulting from prolonged lethal hemorrhage and trauma in dogs. Circulation 2006; 113:1974–1982.
- Wu X, Drabek T, Tisherman SA, et al. Emergency preservation and resuscitation with profound hypothermia, oxygen, and glucose allows reliable neurological recovery after 3 h of cardiac arrest from rapid exsanguination in dogs. J Cereb Blood Flow Metab 2008; 28:302–311.
- Drabek T, Stezoski J, Garman RH, et al. Emergency preservation and delayed resuscitation allows normal recovery after exsanguination cardiac arrest in rats: a feasibility trial. Crit Care Med 2007; 35:532–537.
- Drabek T, Stezoski J, Garman RH, et al. Exsanguination cardiac arrest in rats treated by 60 min, but not 75 min, emergency preservation and delayed resuscitation is associated with intact outcome. Resuscitation 2007; 75:114–123.
- Alam HB, Chen Z, Honma K, et al. The rate of induction of hypothermic arrest determines the outcome in a swine model of lethal hemorrhage. J Trauma 2004; 57:961–969.
- Janata A, Bayegan K, Weihs W, et al. Emergency preservation and resuscitation improve survival after 15 minutes of normovolemic cardiac arrest in pigs. Crit Care Med 2007; 35:2785–2791.