User login
Biomarkers can help psychiatrists monitor abstinence and detect relapse in patients with alcohol use disorders. A blood test that measures carbohydrate-deficient transferrin percentage (%CDT) is FDA-approved for detecting heavy drinking (Table 1)1 and has shown effectiveness in several medical and surgical uses.
Table 1
%CDT test: Fast facts
| Brand names: |
| Axis-Shield %CDT, Bio-Rad %CDT TIA, Tina-quant (a) %CDT |
| FDA-approved indication: |
| Testing for excessive alcohol use |
| Manufacturers: |
| Axis-Shield PLC, Bio-Rad Laboratories, Roche Diagnostics |
| Recommended use: |
| Detecting heavy alcohol consumption, monitoring abstinence, and identifying elapse in patients with alcohol use disorders |
| Laboratories that process %CDT results: |
|
HOW %CDT SIGNALS ALCOHOL ABUSE
In 1976, Swedish researchers detected transferrin fractions in the CSF of alcoholic patients but not in nonalcoholics.2 One of these fractions was also present in alcoholics’ serum. This discovery led to the CDT biomarker.
%CDT testing is based on the finding that consuming an average >60 grams of alcohol (about 5 standard drinks) daily for ≥ 2 weeks causes a higher percentage of transferrin—a glycoprotein that transports iron in the blood—to lack its usual carbohydrate content. Transferrin appears in 6 isoforms, and studies have shown that heavy drinking increases three of these—asialo, mono sialo, and disialo transferrin—a state collectively called “carbohydrate-deficient.”
A %CDT reading ≥ 2.6 indicates that a patient may have had on average at least 5 alcoholic drinks daily for ≥ 2 weeks. Because CDT has a short mean half-life (7 to 14 days), readings >2.6 may suggest much heavier drinking at some time before the blood sample was taken.
Microcolumn chromatography separation assays that measure CDT as a percentage of total circulating transferrin have replaced initial CDT assays that used isoelectric focusing separation and immunoassay systems. This advance corrected for individual transferrin level variations.
CLINICAL USE
The %CDT test has shown effectiveness in several medical and surgical uses, including
- screening patients with diseases possibly triggered by alcohol use, such as treatment-resistant hypertension, gastroesophageal reflux disease, or depression
- detecting alcohol-use disorders in hospitalized patients
- screening presurgical and trauma patients to predict alcohol withdrawal syndrome and/or postsurgical complications.3,4
Although no alcohol biomarker alone reliably confirms alcohol abuse/dependence, %CDT can corroborate initial clinical impressions, especially when the patient’s self-report is suspect or information from significant others is not available.
Among biomarkers, %CDT most accurately predicts alcohol withdrawal syndrome in men (mean corpuscular volume [MCV] measurements are more accurate in women).4 During psychiatric consults, the test may help confirm suspected alcohol use in patients admitted to inpatient medical, surgical, or trauma units.
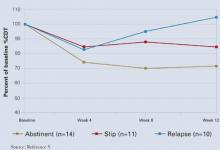
%CDT may also help monitor abstinence when treating an alcohol use disorder. In a major prospective treatment outcome study, %CDT was more sensitive than gamma-glutamyltransferase (GGT) in detecting relapse in male but not female alcoholics.5 Patients who abstained from drinking for 12 weeks showed a 30% decrease in %CDT. Subjects who relapsed during treatment later showed a 60% increase in %CDT, indicating sustained heavy drinking.
%CDT decreased over the first 4 weeks of treatment and continued to decline over time for abstinent patients (Figure). After week 4, %CDT increased again for those who consumed ≥ 5 drinks per day or had a full-blown relapse.5
Together, routine %CDT and GGT testing during treatment and follow-up can help clinicians monitor a patient’s progress and provide accurate reports to courts, child welfare agencies, and programs for impaired professionals. A 30% reduction from baseline in either biomarker indicates abstinence or significantly reduced alcohol consumption, whereas a 30% increase suggests relapse.6 Consider all clinical information in the final analysis, however.
%CDT results also provide an objective basis for discussing relapses with patients. Those who are responding to treatment often welcome %CDT testing as proof they are abstaining from alcohol.
Figure %CDT changes with alcohol use or abstinence
CLINICAL PRACTICALITY
The %CDT test is an immunoassay with ion-exchange column separation followed by turbidimetric measurement. Because the procedure is complex, laboratories generally require 24 to 72 hours to test the sample.
Because the %CDT test is relatively new, pricing, reimbursement rates, and availability are not well established. Pricing will likely vary widely from state to state and among laboratories and insurers. Medicare reimburses approximately $25 for %CDT testing, compared with $10 for GGT testing.
Only select reference laboratories offer %CDT testing, but the test should become more widely available over time (Table 1).
SENSITIVITY AND SPECIFICITY
%CDT test sensitivity is 70% to 90% in male inpatient alcoholics (usually drinking within 4 to 7 days of blood draw), with specificities >90%. Sensitivity is 12% to 40% in general populations—among whom underreporting of drinking may lead to more false positives—and 40% to 60% in outpatient alcoholics.7,8 Specificity, however, remains high—80% to 90%—when sensitivity is reduced.
Overall, %CDT is as sensitive as GGT and more sensitive than other biomarkers, including MCV, aspartate transaminase, and alanine transaminase.9 %CDT is more specific than GGT (92% vs. 75%) and other biomarkers. %CDT appears to be more sensitive in men, whereas GGT is more sensitive in women (Table 2).10
Ample evidence, however, suggests that %CDT and GGT readings together may provide a comprehensive picture of recent alcohol abuse.10 Whereas frequent drinking alters %CDT, GGT signals drinking intensity and is more effective at detecting episodic binge drinking (Table 2). This probably explains why problem drinkers age <20—who typically binge-drink—show little or no change in %CDT.
Table 2
%CDT vs GGT testing for recent alcohol abuse
| %CDT | GGT | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Cutoff value |
|
|
| %CDT: Carbohydrate-deficient transferrin percentage | ||
| GGT: Gamma-glutamyltransferase | ||
FACTORS THAT ALTER RESULTS
Unlike GGT and MCV, few medical conditions distort %CDT,11 meaning the test is accurate in patients with most medical conditions. Only end-stage liver disease, biliary cirrhosis, and rare genetic transferrin variants alter %CDT.
Women tend to have higher and more-variable CDT values than do men, possibly because of variability in normal transferrin levels, anemia secondary to iron deficiency, pregnancy, use of oral contraceptives, or menopause. %CDT cutoff scores are not gender-specific, however, because percentage rather than absolute CDT is typically measured.
%CDT seems to decrease slightly as body mass index increases, suggesting a small but significant inverse relationship between weight and %CDT. Smoking seems to raise %CDT values slightly. The specifics of and reasons for these relationships are unclear.
Related resources
- Axis-Shield. www.axis-shield.com. Click on “Lab diagnostics,” then “Alcohol-related.”
Disclosure
Dr. Anton is a consultant to Axis-Shield.
Dr. Miller and Ms. Dominick report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Anton RF, Dominick C, Bigelow M, Westby C. CDTect Research Group. Comparison of Bio-Rad %CDT TIA and CDTect as laboratory markers of heavy alcohol use and their relationships with gamma-glutamyltransferase. Clin Chem 2001;47:1769-75.
2. Stibler H, Kjellin KG. Isoelectric focusing and electrophoresis of the CSF proteins on tremor of different origins. J Neurol 1976;30:269-85.
3. Miller PM. Recent developments in alcohol biomarkers: Implications for managing alcohol-sensitive diseases. Resid Staff Physician 2004;50:11-17.
4. Spies CD, Kissner M, Neumann T, et al. Elevated carbohydrate-deficient transferrin predicts prolonged intensive care unit stay in traumatized men. Alcohol Alcohol 1998;33:661-9.
5. Anton RF, Moak DH, Latham P. Carbohydrate-deficient transferrin as an indicator of drinking status during a treatment outcome study. Alcohol Clin Exp Res 1996;20:841-6.
6. Anton RF, Lieber C, Tabakoff B. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol Clin Exp Res 2002;26:1215-22.
7. Allen JP, Litten RZ, Anton RF, Cross GM. Carbohydrate-deficient transferrin as a measure of immoderate drinking: remaining issues. Alcohol Clin Exp Res 1994;18:799-812.
8. Gronbaek M, Henriksen JH, Becker U. Carbohydrate-deficient transferrin—a valid marker of alcoholism in population studies? Results from the Copenhagen City Heart Study. Alcohol Clin Exp Res 1995;19:457-61.
9. Bell H, Tallaksen CM, Try K, Haug E. Carbohydrate-deficient transferrin and other markers of high alcohol consumption: A study of 502 patients admitted consecutively to a medical department. Alcohol Clin Exp Res 1994;18:1103-8.
10. Conigrave KM, Degenhardt LJ, Whitfield JB, et al. CDT, GGT, and AST as markers of alcohol use: The WHO/ISBRA Collaborative Project. Alcohol Clin Exp Res 2002;26:332-9.
11. Meerkerk GJ, Njoo KH, Bongers IM, et al. The specificity of the CDT assay in general practice: the influence of common chronic diseases and medication on the serum CDT concentration. Alcohol Clin Exp Res 1998;22:908-13.
Biomarkers can help psychiatrists monitor abstinence and detect relapse in patients with alcohol use disorders. A blood test that measures carbohydrate-deficient transferrin percentage (%CDT) is FDA-approved for detecting heavy drinking (Table 1)1 and has shown effectiveness in several medical and surgical uses.
Table 1
%CDT test: Fast facts
| Brand names: |
| Axis-Shield %CDT, Bio-Rad %CDT TIA, Tina-quant (a) %CDT |
| FDA-approved indication: |
| Testing for excessive alcohol use |
| Manufacturers: |
| Axis-Shield PLC, Bio-Rad Laboratories, Roche Diagnostics |
| Recommended use: |
| Detecting heavy alcohol consumption, monitoring abstinence, and identifying elapse in patients with alcohol use disorders |
| Laboratories that process %CDT results: |
|
HOW %CDT SIGNALS ALCOHOL ABUSE
In 1976, Swedish researchers detected transferrin fractions in the CSF of alcoholic patients but not in nonalcoholics.2 One of these fractions was also present in alcoholics’ serum. This discovery led to the CDT biomarker.
%CDT testing is based on the finding that consuming an average >60 grams of alcohol (about 5 standard drinks) daily for ≥ 2 weeks causes a higher percentage of transferrin—a glycoprotein that transports iron in the blood—to lack its usual carbohydrate content. Transferrin appears in 6 isoforms, and studies have shown that heavy drinking increases three of these—asialo, mono sialo, and disialo transferrin—a state collectively called “carbohydrate-deficient.”
A %CDT reading ≥ 2.6 indicates that a patient may have had on average at least 5 alcoholic drinks daily for ≥ 2 weeks. Because CDT has a short mean half-life (7 to 14 days), readings >2.6 may suggest much heavier drinking at some time before the blood sample was taken.
Microcolumn chromatography separation assays that measure CDT as a percentage of total circulating transferrin have replaced initial CDT assays that used isoelectric focusing separation and immunoassay systems. This advance corrected for individual transferrin level variations.
CLINICAL USE
The %CDT test has shown effectiveness in several medical and surgical uses, including
- screening patients with diseases possibly triggered by alcohol use, such as treatment-resistant hypertension, gastroesophageal reflux disease, or depression
- detecting alcohol-use disorders in hospitalized patients
- screening presurgical and trauma patients to predict alcohol withdrawal syndrome and/or postsurgical complications.3,4
Although no alcohol biomarker alone reliably confirms alcohol abuse/dependence, %CDT can corroborate initial clinical impressions, especially when the patient’s self-report is suspect or information from significant others is not available.
Among biomarkers, %CDT most accurately predicts alcohol withdrawal syndrome in men (mean corpuscular volume [MCV] measurements are more accurate in women).4 During psychiatric consults, the test may help confirm suspected alcohol use in patients admitted to inpatient medical, surgical, or trauma units.
%CDT may also help monitor abstinence when treating an alcohol use disorder. In a major prospective treatment outcome study, %CDT was more sensitive than gamma-glutamyltransferase (GGT) in detecting relapse in male but not female alcoholics.5 Patients who abstained from drinking for 12 weeks showed a 30% decrease in %CDT. Subjects who relapsed during treatment later showed a 60% increase in %CDT, indicating sustained heavy drinking.
%CDT decreased over the first 4 weeks of treatment and continued to decline over time for abstinent patients (Figure). After week 4, %CDT increased again for those who consumed ≥ 5 drinks per day or had a full-blown relapse.5
Together, routine %CDT and GGT testing during treatment and follow-up can help clinicians monitor a patient’s progress and provide accurate reports to courts, child welfare agencies, and programs for impaired professionals. A 30% reduction from baseline in either biomarker indicates abstinence or significantly reduced alcohol consumption, whereas a 30% increase suggests relapse.6 Consider all clinical information in the final analysis, however.
%CDT results also provide an objective basis for discussing relapses with patients. Those who are responding to treatment often welcome %CDT testing as proof they are abstaining from alcohol.
Figure %CDT changes with alcohol use or abstinence
CLINICAL PRACTICALITY
The %CDT test is an immunoassay with ion-exchange column separation followed by turbidimetric measurement. Because the procedure is complex, laboratories generally require 24 to 72 hours to test the sample.
Because the %CDT test is relatively new, pricing, reimbursement rates, and availability are not well established. Pricing will likely vary widely from state to state and among laboratories and insurers. Medicare reimburses approximately $25 for %CDT testing, compared with $10 for GGT testing.
Only select reference laboratories offer %CDT testing, but the test should become more widely available over time (Table 1).
SENSITIVITY AND SPECIFICITY
%CDT test sensitivity is 70% to 90% in male inpatient alcoholics (usually drinking within 4 to 7 days of blood draw), with specificities >90%. Sensitivity is 12% to 40% in general populations—among whom underreporting of drinking may lead to more false positives—and 40% to 60% in outpatient alcoholics.7,8 Specificity, however, remains high—80% to 90%—when sensitivity is reduced.
Overall, %CDT is as sensitive as GGT and more sensitive than other biomarkers, including MCV, aspartate transaminase, and alanine transaminase.9 %CDT is more specific than GGT (92% vs. 75%) and other biomarkers. %CDT appears to be more sensitive in men, whereas GGT is more sensitive in women (Table 2).10
Ample evidence, however, suggests that %CDT and GGT readings together may provide a comprehensive picture of recent alcohol abuse.10 Whereas frequent drinking alters %CDT, GGT signals drinking intensity and is more effective at detecting episodic binge drinking (Table 2). This probably explains why problem drinkers age <20—who typically binge-drink—show little or no change in %CDT.
Table 2
%CDT vs GGT testing for recent alcohol abuse
| %CDT | GGT | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Cutoff value |
|
|
| %CDT: Carbohydrate-deficient transferrin percentage | ||
| GGT: Gamma-glutamyltransferase | ||
FACTORS THAT ALTER RESULTS
Unlike GGT and MCV, few medical conditions distort %CDT,11 meaning the test is accurate in patients with most medical conditions. Only end-stage liver disease, biliary cirrhosis, and rare genetic transferrin variants alter %CDT.
Women tend to have higher and more-variable CDT values than do men, possibly because of variability in normal transferrin levels, anemia secondary to iron deficiency, pregnancy, use of oral contraceptives, or menopause. %CDT cutoff scores are not gender-specific, however, because percentage rather than absolute CDT is typically measured.
%CDT seems to decrease slightly as body mass index increases, suggesting a small but significant inverse relationship between weight and %CDT. Smoking seems to raise %CDT values slightly. The specifics of and reasons for these relationships are unclear.
Related resources
- Axis-Shield. www.axis-shield.com. Click on “Lab diagnostics,” then “Alcohol-related.”
Disclosure
Dr. Anton is a consultant to Axis-Shield.
Dr. Miller and Ms. Dominick report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Biomarkers can help psychiatrists monitor abstinence and detect relapse in patients with alcohol use disorders. A blood test that measures carbohydrate-deficient transferrin percentage (%CDT) is FDA-approved for detecting heavy drinking (Table 1)1 and has shown effectiveness in several medical and surgical uses.
Table 1
%CDT test: Fast facts
| Brand names: |
| Axis-Shield %CDT, Bio-Rad %CDT TIA, Tina-quant (a) %CDT |
| FDA-approved indication: |
| Testing for excessive alcohol use |
| Manufacturers: |
| Axis-Shield PLC, Bio-Rad Laboratories, Roche Diagnostics |
| Recommended use: |
| Detecting heavy alcohol consumption, monitoring abstinence, and identifying elapse in patients with alcohol use disorders |
| Laboratories that process %CDT results: |
|
HOW %CDT SIGNALS ALCOHOL ABUSE
In 1976, Swedish researchers detected transferrin fractions in the CSF of alcoholic patients but not in nonalcoholics.2 One of these fractions was also present in alcoholics’ serum. This discovery led to the CDT biomarker.
%CDT testing is based on the finding that consuming an average >60 grams of alcohol (about 5 standard drinks) daily for ≥ 2 weeks causes a higher percentage of transferrin—a glycoprotein that transports iron in the blood—to lack its usual carbohydrate content. Transferrin appears in 6 isoforms, and studies have shown that heavy drinking increases three of these—asialo, mono sialo, and disialo transferrin—a state collectively called “carbohydrate-deficient.”
A %CDT reading ≥ 2.6 indicates that a patient may have had on average at least 5 alcoholic drinks daily for ≥ 2 weeks. Because CDT has a short mean half-life (7 to 14 days), readings >2.6 may suggest much heavier drinking at some time before the blood sample was taken.
Microcolumn chromatography separation assays that measure CDT as a percentage of total circulating transferrin have replaced initial CDT assays that used isoelectric focusing separation and immunoassay systems. This advance corrected for individual transferrin level variations.
CLINICAL USE
The %CDT test has shown effectiveness in several medical and surgical uses, including
- screening patients with diseases possibly triggered by alcohol use, such as treatment-resistant hypertension, gastroesophageal reflux disease, or depression
- detecting alcohol-use disorders in hospitalized patients
- screening presurgical and trauma patients to predict alcohol withdrawal syndrome and/or postsurgical complications.3,4
Although no alcohol biomarker alone reliably confirms alcohol abuse/dependence, %CDT can corroborate initial clinical impressions, especially when the patient’s self-report is suspect or information from significant others is not available.
Among biomarkers, %CDT most accurately predicts alcohol withdrawal syndrome in men (mean corpuscular volume [MCV] measurements are more accurate in women).4 During psychiatric consults, the test may help confirm suspected alcohol use in patients admitted to inpatient medical, surgical, or trauma units.
%CDT may also help monitor abstinence when treating an alcohol use disorder. In a major prospective treatment outcome study, %CDT was more sensitive than gamma-glutamyltransferase (GGT) in detecting relapse in male but not female alcoholics.5 Patients who abstained from drinking for 12 weeks showed a 30% decrease in %CDT. Subjects who relapsed during treatment later showed a 60% increase in %CDT, indicating sustained heavy drinking.
%CDT decreased over the first 4 weeks of treatment and continued to decline over time for abstinent patients (Figure). After week 4, %CDT increased again for those who consumed ≥ 5 drinks per day or had a full-blown relapse.5
Together, routine %CDT and GGT testing during treatment and follow-up can help clinicians monitor a patient’s progress and provide accurate reports to courts, child welfare agencies, and programs for impaired professionals. A 30% reduction from baseline in either biomarker indicates abstinence or significantly reduced alcohol consumption, whereas a 30% increase suggests relapse.6 Consider all clinical information in the final analysis, however.
%CDT results also provide an objective basis for discussing relapses with patients. Those who are responding to treatment often welcome %CDT testing as proof they are abstaining from alcohol.
Figure %CDT changes with alcohol use or abstinence
CLINICAL PRACTICALITY
The %CDT test is an immunoassay with ion-exchange column separation followed by turbidimetric measurement. Because the procedure is complex, laboratories generally require 24 to 72 hours to test the sample.
Because the %CDT test is relatively new, pricing, reimbursement rates, and availability are not well established. Pricing will likely vary widely from state to state and among laboratories and insurers. Medicare reimburses approximately $25 for %CDT testing, compared with $10 for GGT testing.
Only select reference laboratories offer %CDT testing, but the test should become more widely available over time (Table 1).
SENSITIVITY AND SPECIFICITY
%CDT test sensitivity is 70% to 90% in male inpatient alcoholics (usually drinking within 4 to 7 days of blood draw), with specificities >90%. Sensitivity is 12% to 40% in general populations—among whom underreporting of drinking may lead to more false positives—and 40% to 60% in outpatient alcoholics.7,8 Specificity, however, remains high—80% to 90%—when sensitivity is reduced.
Overall, %CDT is as sensitive as GGT and more sensitive than other biomarkers, including MCV, aspartate transaminase, and alanine transaminase.9 %CDT is more specific than GGT (92% vs. 75%) and other biomarkers. %CDT appears to be more sensitive in men, whereas GGT is more sensitive in women (Table 2).10
Ample evidence, however, suggests that %CDT and GGT readings together may provide a comprehensive picture of recent alcohol abuse.10 Whereas frequent drinking alters %CDT, GGT signals drinking intensity and is more effective at detecting episodic binge drinking (Table 2). This probably explains why problem drinkers age <20—who typically binge-drink—show little or no change in %CDT.
Table 2
%CDT vs GGT testing for recent alcohol abuse
| %CDT | GGT | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Cutoff value |
|
|
| %CDT: Carbohydrate-deficient transferrin percentage | ||
| GGT: Gamma-glutamyltransferase | ||
FACTORS THAT ALTER RESULTS
Unlike GGT and MCV, few medical conditions distort %CDT,11 meaning the test is accurate in patients with most medical conditions. Only end-stage liver disease, biliary cirrhosis, and rare genetic transferrin variants alter %CDT.
Women tend to have higher and more-variable CDT values than do men, possibly because of variability in normal transferrin levels, anemia secondary to iron deficiency, pregnancy, use of oral contraceptives, or menopause. %CDT cutoff scores are not gender-specific, however, because percentage rather than absolute CDT is typically measured.
%CDT seems to decrease slightly as body mass index increases, suggesting a small but significant inverse relationship between weight and %CDT. Smoking seems to raise %CDT values slightly. The specifics of and reasons for these relationships are unclear.
Related resources
- Axis-Shield. www.axis-shield.com. Click on “Lab diagnostics,” then “Alcohol-related.”
Disclosure
Dr. Anton is a consultant to Axis-Shield.
Dr. Miller and Ms. Dominick report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Anton RF, Dominick C, Bigelow M, Westby C. CDTect Research Group. Comparison of Bio-Rad %CDT TIA and CDTect as laboratory markers of heavy alcohol use and their relationships with gamma-glutamyltransferase. Clin Chem 2001;47:1769-75.
2. Stibler H, Kjellin KG. Isoelectric focusing and electrophoresis of the CSF proteins on tremor of different origins. J Neurol 1976;30:269-85.
3. Miller PM. Recent developments in alcohol biomarkers: Implications for managing alcohol-sensitive diseases. Resid Staff Physician 2004;50:11-17.
4. Spies CD, Kissner M, Neumann T, et al. Elevated carbohydrate-deficient transferrin predicts prolonged intensive care unit stay in traumatized men. Alcohol Alcohol 1998;33:661-9.
5. Anton RF, Moak DH, Latham P. Carbohydrate-deficient transferrin as an indicator of drinking status during a treatment outcome study. Alcohol Clin Exp Res 1996;20:841-6.
6. Anton RF, Lieber C, Tabakoff B. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol Clin Exp Res 2002;26:1215-22.
7. Allen JP, Litten RZ, Anton RF, Cross GM. Carbohydrate-deficient transferrin as a measure of immoderate drinking: remaining issues. Alcohol Clin Exp Res 1994;18:799-812.
8. Gronbaek M, Henriksen JH, Becker U. Carbohydrate-deficient transferrin—a valid marker of alcoholism in population studies? Results from the Copenhagen City Heart Study. Alcohol Clin Exp Res 1995;19:457-61.
9. Bell H, Tallaksen CM, Try K, Haug E. Carbohydrate-deficient transferrin and other markers of high alcohol consumption: A study of 502 patients admitted consecutively to a medical department. Alcohol Clin Exp Res 1994;18:1103-8.
10. Conigrave KM, Degenhardt LJ, Whitfield JB, et al. CDT, GGT, and AST as markers of alcohol use: The WHO/ISBRA Collaborative Project. Alcohol Clin Exp Res 2002;26:332-9.
11. Meerkerk GJ, Njoo KH, Bongers IM, et al. The specificity of the CDT assay in general practice: the influence of common chronic diseases and medication on the serum CDT concentration. Alcohol Clin Exp Res 1998;22:908-13.
1. Anton RF, Dominick C, Bigelow M, Westby C. CDTect Research Group. Comparison of Bio-Rad %CDT TIA and CDTect as laboratory markers of heavy alcohol use and their relationships with gamma-glutamyltransferase. Clin Chem 2001;47:1769-75.
2. Stibler H, Kjellin KG. Isoelectric focusing and electrophoresis of the CSF proteins on tremor of different origins. J Neurol 1976;30:269-85.
3. Miller PM. Recent developments in alcohol biomarkers: Implications for managing alcohol-sensitive diseases. Resid Staff Physician 2004;50:11-17.
4. Spies CD, Kissner M, Neumann T, et al. Elevated carbohydrate-deficient transferrin predicts prolonged intensive care unit stay in traumatized men. Alcohol Alcohol 1998;33:661-9.
5. Anton RF, Moak DH, Latham P. Carbohydrate-deficient transferrin as an indicator of drinking status during a treatment outcome study. Alcohol Clin Exp Res 1996;20:841-6.
6. Anton RF, Lieber C, Tabakoff B. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol Clin Exp Res 2002;26:1215-22.
7. Allen JP, Litten RZ, Anton RF, Cross GM. Carbohydrate-deficient transferrin as a measure of immoderate drinking: remaining issues. Alcohol Clin Exp Res 1994;18:799-812.
8. Gronbaek M, Henriksen JH, Becker U. Carbohydrate-deficient transferrin—a valid marker of alcoholism in population studies? Results from the Copenhagen City Heart Study. Alcohol Clin Exp Res 1995;19:457-61.
9. Bell H, Tallaksen CM, Try K, Haug E. Carbohydrate-deficient transferrin and other markers of high alcohol consumption: A study of 502 patients admitted consecutively to a medical department. Alcohol Clin Exp Res 1994;18:1103-8.
10. Conigrave KM, Degenhardt LJ, Whitfield JB, et al. CDT, GGT, and AST as markers of alcohol use: The WHO/ISBRA Collaborative Project. Alcohol Clin Exp Res 2002;26:332-9.
11. Meerkerk GJ, Njoo KH, Bongers IM, et al. The specificity of the CDT assay in general practice: the influence of common chronic diseases and medication on the serum CDT concentration. Alcohol Clin Exp Res 1998;22:908-13.