User login
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2
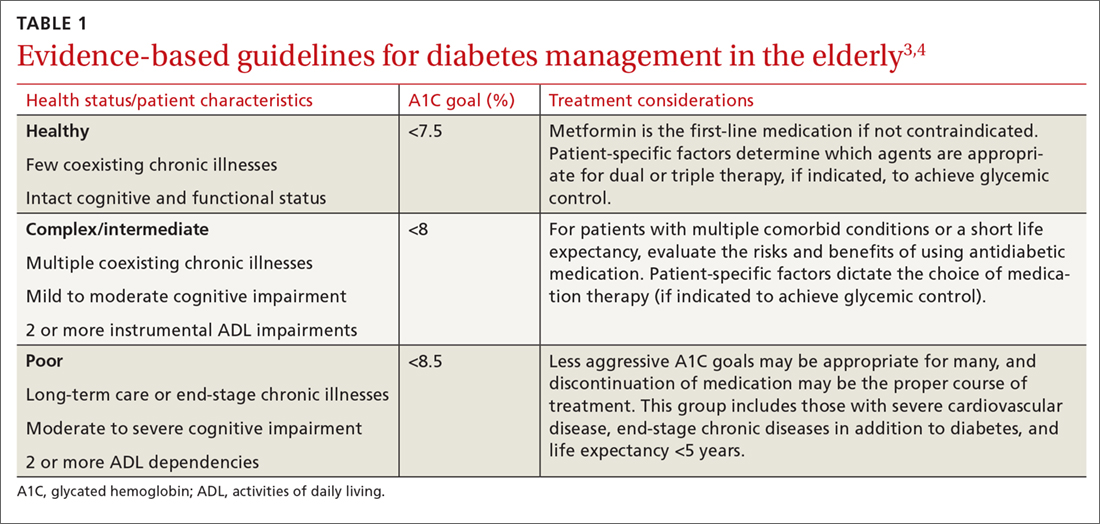
Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; bkeber@northwell.edu.
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2

Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; bkeber@northwell.edu.
As members of the baby boomer generation (adults ≥65 years) age, the number of people at risk for diabetes increases. Already nearly one-quarter of people over age 65 have type 2 diabetes (T2DM).1 With a proliferation of new medications to treat diabetes, deciding which ones to use in older patients is becoming complex.
In this article we review the important issues to consider when prescribing and monitoring diabetes medications in older adults. To provide optimal patient-centered care, it’s necessary to assess comorbid conditions as well as the costs, risks, and benefits of each medication. Determining appropriate goals of therapy and selecting agents that minimize the risk of hypoglycemia will help ensure safe and effective management of older patients with diabetes.
What makes elderly patients unique
The pathophysiology of T2DM in the elderly is unique in that it involves not just insulin resistance but also age-related loss of beta-cell function, leading to reduced insulin secretion and altered effectiveness of pharmacotherapy.2 The addition of second and third medications may be needed for those with longstanding T2DM, although these agents often reduce the A1C level to a lesser extent than when used as monotherapy in patients whose beta-cell function is still intact. In addition to physiologic changes, older adults with diabetes have varied general health statuses and care support systems. The goal for glycemic management should be personalized based on an individual’s comorbidities and physical and cognitive functional status (TABLE 13,4).2

Higher A1C goals can be acceptable for elderly patients with comorbid conditions such as cognitive dysfunction, dementia, or cardiovascular or renal disease. Evaluate cognition when determining appropriate pharmacotherapy. Assess a patient’s awareness of hypoglycemia and ability to adhere to the regimen prescribed. Visual impairment, decreased dexterity, baseline weight, nutritional and functional status, as well as social support, finances, and formulary restrictions should all be considered when determining the most appropriate regimen for a patient. Also take into account patient and family goals of care.2 TABLE 22-4 summarizes key risks and benefits of the medications we discuss next.

Metformin
Metformin is recommended as first-line therapy for those with T2DM for a number of reasons, including its potential to reduce cardiovascular events and mortality.3,5 It also significantly reduces A1C levels by 1% to 1.5%,6 while imparting a low risk of hypoglycemia. Metformin is cost effective and well tolerated, making it an excellent choice for use in older patients.
The most common adverse effects are abdominal discomfort, diarrhea, and weight loss. The use of extended-release preparations, as well as slow titration of dosing, can improve gastrointestinal (GI) tolerance. Weight loss may be an attractive side effect in patients who are overweight or obese, but weight loss and diarrhea are concerning effects in frail older adults who may have poor nutritional reserves.6
Monitor renal function frequently in older patients receiving metformin.3 Renal failure is a risk factor for adverse events such as lactic acidosis, and metformin is therefore contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.4 With this in mind, metformin should not be started in patients with an eGFR below 45 mL/min/1.73 m2. And for patients already taking metformin, reduce the total daily dose if the eGFR falls to between 30 and 45 mL/min/1.73 m2.4
Metformin can cause a reduction in vitamin B12 levels after long-term use in up to 30% of patients, likely due to decreased absorption from the ileum.7 Monitor vitamin B12 serum concentrations periodically with long-term therapy, particularly in patients with peripheral neuropathy or anemia, as these conditions may be exacerbated by vitamin B12 deficiency.3,4
Continue to: Sulfonylureas
Sulfonylureas
Sulfonylureas increase the secretion of insulin from pancreatic beta cells, significantly lower blood glucose, and reduce A1C levels by 1% to 2%.6 Because hypoglycemia is a serious risk with sulfonylureas, they should be used conservatively in the elderly.2 Avoid using sulfonylurea formulations with long half-lives or active metabolites, which can cause severe and prolonged hypoglycemia.8,9
Glyburide is broken down into active metabolites that accumulate in patients who have renal insufficiency; it should be avoided in older adults due to the risk of life-threatening hypoglycemic events.10 Glipizide has no active metabolites and has the lowest risk of hypoglycemia in the setting of decreased renal function, making it the preferred sulfonylurea for use in the elderly.3,10
Thiazolidinediones
Thiazolidinediones (TZDs) reduce insulin resistance and decrease hepatic glucose production without increasing the risk of hypoglycemia. These agents effectively lower A1C levels by 1% to 1.5%.11 Despite their efficacy, TZDs have limited benefit because of adverse effects. Serious complications include fluid retention that can exacerbate or lead to worsening heart failure, weight gain, macular edema, and hepatic failure.
Specifically, with pioglitazone, there is also a slightly increased risk of bladder cancer.2 In one study involving more than 30,000 patients taking pioglitazone, an increase in bladder cancer was noted among those using the medication for more than 2 years.12 Still, the hazard ratio was only 1.2, with 90 cases diagnosed over the course of the study. A prudent strategy would be to avoid its use in those with high risk of developing bladder cancer. TZDs are contraindicated in patients with New York Heart Association class III or IV heart failure.8
Increased fracture risk has been identified in both men and women and is a concerning adverse effect in the elderly.8 Fracture risk with TZDs has been approximately twice that of placebo, noted in a study of older women where the fracture rate was 5.1% vs 2.5%, respectively.11 TZDs can be of value in lowering A1C levels without the risk of hypoglycemia. But, due to their adverse effect profile, use TZDs cautiously in older adults at risk for heart failure, falls, or fractures.3
Continue to: DPP-4 inhibitors
DPP-4 inhibitors
Dipeptidyl peptidase-4 (DPP-4) inhibitors work by suppressing the enzyme that degrades 2 incretin hormones, glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP). The resulting enhancement of incretin activity increases glucose-dependent insulin secretion, decreases glucagon secretion, and promotes satiety.6 These agents have modest efficacy with the potential to lower A1C by 0.5% to 0.9%.8,13 Studies show that DPP-4 inhibitors are well tolerated with a minimal risk of hypoglycemia in the elderly.13 These agents are ideal for combination therapy or for monotherapy in older patients who are not good candidates for metformin or a sulfonylurea.
The safety profile, neutral effect on weight, and once-daily dosing make these agents advantageous for use in frail and debilitated elderly patients, as well as in patients with cognitive dysfunction, decreased dexterity, inconsistent meal patterns, or adherence issues. Dose adjustment is required in renal impairment, with the exception of linagliptin. High cost or formulary restrictions may impact use of these agents.
The DPP-4 inhibitors were well tolerated in short-term studies, but long-term safety has yet to be established.6 Reported post-marketing adverse effects include acute renal failure, allergic reactions, and acute pancreatitis.6,14 These agents should be avoided in any patient with a history of pancreatitis.14 In addition, trials investigating the cardiovascular safety and efficacy of DPP-4 inhibitors point to an increased risk of heart failure with the use of saxagliptin and alogliptin, regardless of age.15,16 The potential for adverse effects warrants increased patient monitoring when using these agents in older patients.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are injectable agents that potentiate the actions of the naturally occurring incretin GLP-1, which increases glucose-dependent insulin secretion, inhibits glucagon release, reduces hepatic glucose production, and delays gastric emptying. These agents have a pronounced effect on satiety and promote weight loss. The most common adverse effects are nausea, vomiting, and diarrhea, which occur most commonly during treatment initiation and titration. Studies in elderly patients confirm A1C reductions of 1% to 1.5% and a low risk of hypoglycemia when used alone.17,18
GLP-1 RAs can be used as monotherapy in older patients at risk for hypoglycemia or in those with hypoglycemic unawareness. They can also be used in combination therapy with other agents, including insulin, though concomitant use with insulin or insulin secretagogues increases the risk of hypoglycemia.3 Weight loss and GI adverse effects may limit the use of these agents in frail or undernourished elderly patients.6
Continue to: Since these agents are injected...
Since these agents are injected, they require intact visual, motor, and cognitive skills and thus may not be appropriate in older patients with cognitive or visual impairment or decreased dexterity. In addition, the high cost of these agents may limit their use.
Select a GLP-1 RA based on the frequency of administration, type of glucose control required (fasting or post-prandial), and the patient’s ability to use the administration device. Dose adjustment is required in renal impairment, except with dulaglutide and liraglutide. Use with caution in patients with a history of pancreatitis, and stop GLP-1 RAs if pancreatitis is suspected during treatment.4 Avoid GLP-1 RAs in patients with a personal or family history of thyroid-related cancers, as these agents have been associated with medullary thyroid tumors in animals.4
A new indication. Recent evidence suggests the GLP-1 RAs may offer additional cardiovascular benefit in patients with diabetes.18,19 In August 2017, liraglutide gained an additional FDA indication to reduce the risk of major adverse cardiovascular events in adults with T2DM and established cardiovascular disease.
This new indication was based on the Novo Nordisk- and National Institutes of Health-sponsored LEADER trial, in which liraglutide reduced the risk of cardiovascular death, nonfatal heart attack, or nonfatal stroke by 13% vs placebo (P=.01) with an absolute risk reduction (ARR) of 1.9%.19 Liraglutide demonstrated a 22% reduction in cardiovascular death and a 15% reduction in all-cause death (ARR 1.3%, 1.4% respectively).19 The new cardiovascular indication may impact the choice of add-on therapy to metformin in patients with preexisting cardiovascular conditions.
Continue to: Sodium glucose cotransporter-2 inhibitors
Sodium glucose cotransporter-2 inhibitors
SGLT-2 inhibitors prevent the reabsorption of renal-filtered glucose, resulting in decreased blood glucose levels and increased urinary excretion of glucose without stimulating insulin secretion, and therefore without increasing the risk of hypoglycemia. Additional effects include decreased blood pressure and weight loss.20 Dose adjustment is required in renal impairment.
SGLT-2 inhibitors can be used as monotherapy or in combination with other agents, including insulin, and the relatively low risk of hypoglycemia and moderate A1C lowering potential of 0.5% to 1% provide an oral option for select older patients.20 Common adverse events include hypotension, hyperkalemia, increased low-density lipoprotein (LDL) levels, acute kidney injury, genital mycotic infections, and hypoglycemia when used in combination with insulin or insulin secretagogues.20
Additional warnings have been issued by the FDA for the risk of urinary tract infection with sepsis, as well as diabetic ketoacidosis associated with SGLT-2 inhibitor use.21 The FDA has reported bone fracture risk and decreased bone mineral density with canagliflozin.21 Avoid using SGLT-2 inhibitors in patients with osteopenia or osteoporosis, as the risks outweigh the benefits. Drug-specific warnings may further impact individual use of an agent, with canagliflozin most recently having been associated with increased risk of leg and foot amputations.21
Given the adverse effect profile of SGLT-2 inhibitors, assess their risks and benefits in older patients on a case-by-case basis. Before initiating therapy, evaluate each patient’s volume status. A higher incidence of adverse effects related to intravascular volume depletion has been reported in those 65 or older, with a more prominent increase seen in patients 75 or older.22 However, the risk of hypoglycemia does not seem to increase with age.22
Although many adverse effects have been reported with SGLT-2 inhibitors, empagliflozin was associated with significantly lower rates of all-cause and cardiovascular death and lower risk of hospitalization for heart failure in the only SGLT-2 inhibitor cardiovascular outcomes trial reported to date.23 If this cardiovascular benefit is replicated in additional trials of the other SGLT-2 inhibitors, use of this drug class may increase.
Continue to: Insulin
Insulin
Many patients will ultimately require insulin due to the progressive loss of beta-cell function that occurs in advanced diabetes. Starting insulin therapy early on in the disease may actually restore beta-cell function and reduce glucotoxicity.24 In elderly patients with uncontrolled diabetes, early treatment with basal insulin results in better glycemic control and less hypoglycemia than continuing to titrate oral agents.25
Despite these benefits, however, insulin use often is not optimized in the elderly due to concerns about hypoglycemia and difficulty of administration. Safe use of insulin requires careful selection of an appropriate insulin regimen, since insulin use has been identified as an independent predictor of severe hypoglycemia in the elderly.8,26 Before initiating insulin therapy, evaluate whether an older patient is cognitively and physically able to safely use insulin.
Multiple daily injections may be challenging for some older adults. Limit such insulin regimens to use in high-functioning patients. Although all types of insulin can cause hypoglycemia, regimens that mimic insulin’s normal physiologic pattern introduce less hypoglycemic risk. Using basal insulin that mimics the body’s sustained insulin level throughout the day is associated with a lower frequency of hypoglycemia in older people with diabetes than conventional insulin regimens. Long-acting insulins such as glargine, detemir, and degludec offer a lower risk of hypoglycemia, particularly nocturnal hypoglycemia which may contribute to falls.2,27
Neutral protamine Hagedorn insulin and regular insulin are not recommended for use in the elderly, as they do not mimic the body’s natural basal-bolus insulin production and thus put patients at higher risk of hypoglycemia.4 If insulin intensification is needed after optimizing basal insulin, consider adding mealtime insulin with a bolus of rapid-acting insulin (insulin aspart, insulin lispro, or insulin glulisine). It is important to note that the kidneys are responsible for 30% to 80% of insulin clearance from the body.28 Because insulin action is prolonged in renal insufficiency, prevent hypoglycemia by decreasing basal and bolus doses when the eGFR is below 50 mL/min/1.73m2.28
Dosing errors. Whenever possible, use insulin preparations that minimize dosing errors. Insulin pen formulations, if financially feasible, allow more accurate dosing and are more acceptable to older patients compared with syringes and vials.29 Pen formulations are particularly preferable for older patients with impaired vision or dexterity.29 In addition, when patients must mix insulins, errors are more likely to occur. The use of premixed insulin vials has been shown to increase dosing accuracy when used by the elderly.30
Continue to: Combining antidiabetes agents
Combining antidiabetes agents
However, for older patients already taking metformin who are not at their A1C goal, consider adding a second agent, if not contraindicated. Potential agents include a GLP-1 RA, SGLT-2 inhibitor, DDP-4 inhibitor, or short-acting sulfonylurea (glipizide). Alternatively, basal insulin may be added. However, avoid combining a sulfonylurea with insulin, which greatly increases the risk of hypoglycemia.32 Consider adding a GLP-1 RA or basal insulin if the patient is not at his/her target A1C on oral therapy with multiple agents.3
CORRESPONDENCE
Barbara Keber, MD, Glen Cove Hospital, 101 St. Andrews Lane, Glen Cove, NY; bkeber@northwell.edu.
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
1. CDC. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2017. Atlanta, GA, U.S. Department of Health and Human Services, 2017.
2. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care. 2017;40:444-452.
3. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(Suppl 1):S1–S138.
4. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238.
5. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589.
6. Kezerle L, Shalev L, Barski L. Treating the elderly diabetic patient: special considerations. Diabetes Metab Syndr Obes. 2014;7:391-400.
7. Singh J, Tushar B. Metformin use and vitamin B12 deficiency in patients with type-2 diabetes mellitus. MVP J Med Sci. 2016:3:67-70.
8. Fravel MA, McDanel DL, Ross MB, et al. Special considerations for treatment of type 2 diabetes mellitus in the elderly. Am J Health Syst Pharm. 2011;68:500-509.
9. Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63:e8–e18.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Schernthaner G, Curie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155-S161.
12. Lewis JD, Ferrara A, Peng T, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916-922.
13. Avogaro A, Dardano A, de Kreutzenberg SV, et al. Dipeptidyl peptidase-4 inhibitors can minimize the hypoglycaemic burden and enhance safety in elderly people with diabetes. Diabetes Obes Metab. 2015;17:107-115.
14. DeVries JH, RosenstocK J. DPP-4 inhibitor-related pancreatitis: rare but real! Diabetes Care. 2017;40:161-163.
15. Leiter LA, Teoh H, Braunwald E, et al. Efficacy and safety of saxagliptin in older participants in the SAVOR-TIMI 53 trial. Diabetes Care. 2015;38:1145-1153.
16. , , , et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620-626.
17. Raccah D, Miossec P, Esposito V, et al. Efficacy and safety of lixisenatide in elderly (≥65 years old) and very elderly (≥75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204-211.
18. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
19. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee, LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
20. Lusk KA, Barnes NE. Role of sodium-glucose cotransporter 2 (SGLT2) inhibitors. US Pharm. 2016;41:26-29.
21. U.S. Food and Drug Administration. Sodium-glucose cotransporter-2 (SGLT2) inhibitors. Available at: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm446852.htm. Accessed May 18, 2018.
22. Miller EM. Overview of the efficacy and safety of SGLT-2 inhibitors in type 2 diabetes mellitus. J Fam Pract. 2017;66(2 Suppl):S5-S12.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
24. Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776-785.
25. Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53-59.
26. Fu H, Xie W, Curtis B, et al. Identifying factors associated with hypoglycemia-related hospitalizations among elderly patients with T2DM in the US: a novel approach using influential variable analysis. Curr Med Res Opin. 2014;30:1787-1793.
27. Sorli C, Warren M, Oyer D, et al. Elderly patients with diabetes experience a lower rate of nocturnal hypoglycaemia with insulin degludec than with insulin glargine: a meta-analysis of phase IIIa trials. Drugs Aging. 2013;30:1009-1018.
28. Sampanis CH. Management of hyperglycemia in patients with diabetes mellitus and chronic renal failure. Hippokratia. 2008;12:22-27.
29. Corsi A, Torre E, Coronel GA, et al. Pre-filled insulin pen in newly insulin-treated diabetic patients over 60 years old. Diab Nutr Metab. 1997;10:78-81.
30. Coscelli C, Calabrese G, Fedele D, et al. Use of premixed insulin among the elderly. Reduction of errors in patient preparation of mixtures. Diabetes Care. 1992;15:1628-1630.
31. American Geriatrics Society. Ten things clinicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/. Accessed May 18, 2018.
32. Mogensen UM, Andersson C, Fosbøl EL, et al. Sulfonylurea in combination with insulin is associated with increased mortality compared with a combination of insulin and metformin in a retrospective Danish nationwide study. Diabetologia. 2015;58:50-58.
From The Journal of Family Practice | 2018;67(7):408-410,412-415.
PRACTICE RECOMMENDATIONS
› Allow higher A1C goals for elderly patients who have such comorbid conditions as cognitive dysfunction, dementia, or cardiovascular or renal disease. B
› Look to metformin first in most instances if there are no contraindications. Monitor renal function frequently and vitamin B12 levels periodically. B
› Consider glucagon-like peptide-1 receptor agonists for patients who also have established cardiovascular disease, or consider starting basal insulin instead of using multiple oral agents. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
