User login
According to the literature, the rate of deep venous thrombosis after total hip arthroplasty (THA) can be high (45%-63%) without prophylactic anticoagulation.1-6 A meta-analysis of 13 studies found a rate of 51%.7 As lower extremity deep venous thrombi are the initial source of symptomatic pulmonary emboli in about 90% of cases,8 THA patients are usually given medication postoperatively focused on prevention of these thromboembolic events.9 Chemoprophylaxis may involve warfarin, enoxaparin, or their combination in an anticoagulation bridge. Enoxaparin is one of many low-molecular-weight heparins (LMWHs). All LMWHs exert their anticoagulant effect by binding to antithrombin III.10 The binding of LMWH to antithrombin III catalyzes the inhibition of factor Xa by antithrombin III, disrupting clot formation.11
In its hydroquinone form, vitamin K is essential as a cofactor for carboxylation of the glutamic acid residues of the amino-terminals of the coagulation proteins II, VII, IX, and X, leading to their activation. Anticoagulation by warfarin is achieved by the inhibition of the reductase enzymes that produce vitamin K hydroquinone in the liver from vitamin K epoxide.12 This inhibition prevents activation of the clotting proteins.12,13 Prophylaxis with enoxaparin or warfarin can reduce the rate of venous thromboembolic disease to 3.6% and 3.7%, respectively.2 However, these medications inhibit the clotting cascade, and their use risks prolonging the healing process.9 The delay increases the risk for wound infection,14 which can lead to a longer hospital stay and therefore higher costs.
We conducted a study to compare patients who received warfarin only with patients who received warfarin bridged with enoxaparin as antithrombotic chemoprophylaxis after THA. Outcomes of interest were number of days until a dry wound was observed and length of hospital stay. We hypothesized that, compared with warfarin-only therapy, bridged therapy would increase the risk for prolonged wound healing and result in longer hospital stays.
Materials and Methods
At our 746-bed academic medical center, 121 THAs were performed between January 1, 2008 and December 31, 2009. This study was approved by the center’s Office for Human Subjects Protections institutional review board (IRB). The research involved collecting or studying existing data, documents, and records recorded anonymously by the investigator in such a manner that subjects could not be identified, directly or through identifiers linked to the subjects, and therefore patient consent was not needed. Therefore, the IRB waived the need for consent. Relevant data included in this study were extracted from patient medical records, given within 35 days of surgery. For each patient, discharge notes provided data on the hospital course, and nurses’ notes provided data on wound status after THA.
Propensity Score Matching
For accurate analysis, it was important to consider confounding factors in both patient groups. Some covariates that may influence accurate analysis are age,15 diabetes,16 sex,15,17 hypertension,18 and body mass index.15,19Propensity score, defined as the conditional probability of receiving treatment, given the observed background covariates, was initially defined by Rosenbaum20 and Rubin.21 The motivation behind propensity scores can be understood by considering an idealized situation in which the 2 groups are similar on all background characteristics. In nonexperimental studies, researchers aim to find for each treated individual a comparison individual who looks exactly the same as the treated individual with respect to observed pretreatment covariates. Thus, assuming no hidden bias, any difference in outcomes within these pairs can be attributed to the variable of interest and not to any other differences between the treated and comparison individuals. Our study is a typical nonexperimental retrospective study in which the 2 groups being compared are patients receiving warfarin only or warfarin bridged with enoxaparin. To minimize the influence of background covariates, we used matching procedures and present our results both with and without the use of matching techniques.
Data and Results
There are different matching algorithms aimed at matching groups. In our study, the optimal matching procedure alone could not produce adequately matched data, so we used both optimal matching20 and genetic matching.22,23 Genetic matching procedure with replacement22 can produce well-matched data—it matched each patient in the warfarin-only group with a patient in the bridged-therapy group and allowed different patients to be matched with 1 similar patient in the control group. However, as the same patients in the bridged-therapy group might be matched multiple times, it would complicate the after-matching analysis. We therefore used a 2-step matching procedure to obtain well-matched data, and a simplified analysis procedure after matching. In the first step, we implemented genetic matching with replacement, as introduced by Abadie and Imbens,22 to match each warfarin-only patient with 1 bridged-therapy patient. In the second step, we applied optimal matching to the 2 groups. This 2-step matching turned out to produce better matched pairs, as denoted by Rubin.21 Both matching steps were implemented using the MatchIt function in R.24
The balance of matching is checked using criteria suggested by Rubin21: (1) standardized difference of means of propensity score, (2) ratio of variances in propensity score in treated and control groups, and (3) for each covariate, ratio of variance in residuals orthogonal to propensity score in treated and control groups.
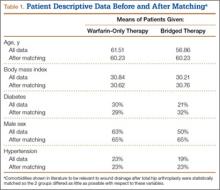
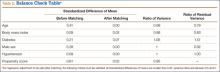
Table 1 lists the means of the background covariates for each group before and after matching. Table 2 lists the balance check results suggested by Rubin.21 After matching, all standardized differences of means are smaller than 0.25, and the variance ratios are between 0.5 and 2, which are the standards suggested21 for regression adjustment to be valid after matching.
After genetic matching, 31 bridged-therapy patients and 57 warfarin-only patients remained. After optimal matching, there were 31 patients in each group. Poisson regressions of datasets before and after matching adjustment were fitted.
Results
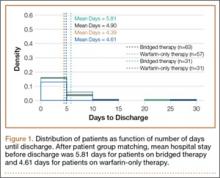
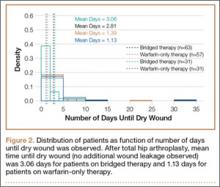
Wounds of bridged-therapy patients took longer to heal than wounds of warfarin-only patients both before (odds ratio, 2.16; P < .05) and after matching data (odds ratio, 2.39; P < .05) with respect to confounding factors. In addition, bridged-therapy patients had longer hospital stays both before (odds ratio 1.20; P < .05) and after matching data (odds ratio, 1.27; P < .05) with respect to confounding factors. Figures 1 and 2 are histograms displaying the 2 groups and their outcomes.
Discussion
For patients undergoing THA procedures, several important considerations should be taken into account. Colwell and colleagues2 showed that, compared with warfarin, enoxaparin offered a 0.1% higher rate of protection against venous thromboembolic disease after THA. However, patients given enoxaparin may face increased risks.25 Hallevi and colleagues26 demonstrated that, compared with warfarin, enoxaparin bridging increased the risk for serious bleeding in patients with cardioembolic stroke. In our review of the literature, we learned that the benefits of bridge therapy in thromboembolic disease have yet to be investigated in THA.
At our academic hospital, the extra costs associated with bridge therapy can be as much as about $200027 per day per patient. These costs can go much higher, depending on type of patient and types of resources used. Over the 2-year period covered by our study, the costs of using enoxaparin amounted to about $151,200 ($2000 × 1.2 days per patient). If bridging offers no significant protection against thromboembolic disease, then it would be more cost-effective to use a single anticoagulant, particularly enoxaparin, for high-risk patients.
There are significant risk factors associated with prolonged healing of surgical wounds. Protocols outlining these factors may help reduce costs. In addition, when deciding on the use of aggressive anticoagulation therapy, surgeons must consider the risks for prolonged leakage and infection in addition to the risk for thromboembolic disease. Protocols may aid in this process as well. Our study results showed that, compared with warfarin-only therapy, bridged therapy (enoxaparin and warfarin) was associated with longer hospital stays. Further research should examine whether there are advantages that justify the higher risks of delayed wound healing and subsequent infection. Improving our understanding of risk factors associated with anticoagulation therapy will make orthopedic surgery safer for patients.
1. Bergqvist D, Benoni G, Björgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335(10):696-700.
2. Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81(7):932-940.
3. Haake DA, Berkman SA. Venous thromboembolic disease after hip surgery. Risk factors, prophylaxis, and diagnosis. Clin Orthop Relat Res. 1989;(242):212-231.
4. Johnson R, Carmichael JH, Almond HG, Loynes RP. Deep venous thrombosis following Charnley arthroplasty. Clin Orthop Relat Res. 1978;(132):24-30.
5. Stamatakis JD, Kakkar VV, Sagar S, Lawrence D, Nairn D, Bentley PG. Femoral vein thrombosis and total hip replacement. Br Med J. 1977;2(6081):223-225.
6. Turpie AG, Levine MN, Hirsh J, et al. A randomized controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med. 1986;315(15):925-929.
7. Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S-334S.
8. Westrich GH, Sánchez PM. Prevention and treatment of thromboembolic disease: an overview. Instr Course Lect. 2002;51:471-480.
9. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
10. Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga JM. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol. 1998;82(5B):3L-10L.
11. Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20(7):771-775.
12. Kamali F, Wood P, Ward A. Vitamin K deficiency amplifies anticoagulation response to ximelagatran: possible implications for direct thrombin inhibitors and their clinical safety. Ann Hematol. 2009;88(2):141-149.
13. Choonara IA, Malia RG, Haynes BP, et al. The relationship between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol. 1988;25(1):1-7.
14. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515.
15. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
16. Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22(5):651-656.
17. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
18. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
19. Lübbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57(2):327-334.
20. Rosenbaum PR. A characterization of optimal designs for observational studies. J R Stat Soc Ser B. 1991;53(3):597-610.
21. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(1):169-188.
22. Abadie A, Imbens GW. Simple and Bias-Corrected Matching Estimators for Average Treatment Effects. Berkeley, CA: Department of Economics, University of California; 2002.
23. Diamond A, Sekhon J. Genetic matching for estimating causal effects: a new method of achieving balance in observational studies. Paper presented at: Annual Meeting of the Midwest Political Science Association; April 2005; Chicago, IL.
24. Imai K, King G, Lau O. logit: logistic regression for dichotomous dependent variables. In: Imai K, King G, Lau O. Zelig: Everyone’s Statistical Software. 2011; 238-244. http://gking.harvard.edu/zelig. Accessed May 26, 2015.
25. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
26. Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge? Arch Neurol. 2008;65(9):1169-1173.
27. Henry J. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day [2010]. http://kff.org/other/state-indicator/expenses-per-inpatient-day/#table. Accessed May 26, 2015.
According to the literature, the rate of deep venous thrombosis after total hip arthroplasty (THA) can be high (45%-63%) without prophylactic anticoagulation.1-6 A meta-analysis of 13 studies found a rate of 51%.7 As lower extremity deep venous thrombi are the initial source of symptomatic pulmonary emboli in about 90% of cases,8 THA patients are usually given medication postoperatively focused on prevention of these thromboembolic events.9 Chemoprophylaxis may involve warfarin, enoxaparin, or their combination in an anticoagulation bridge. Enoxaparin is one of many low-molecular-weight heparins (LMWHs). All LMWHs exert their anticoagulant effect by binding to antithrombin III.10 The binding of LMWH to antithrombin III catalyzes the inhibition of factor Xa by antithrombin III, disrupting clot formation.11
In its hydroquinone form, vitamin K is essential as a cofactor for carboxylation of the glutamic acid residues of the amino-terminals of the coagulation proteins II, VII, IX, and X, leading to their activation. Anticoagulation by warfarin is achieved by the inhibition of the reductase enzymes that produce vitamin K hydroquinone in the liver from vitamin K epoxide.12 This inhibition prevents activation of the clotting proteins.12,13 Prophylaxis with enoxaparin or warfarin can reduce the rate of venous thromboembolic disease to 3.6% and 3.7%, respectively.2 However, these medications inhibit the clotting cascade, and their use risks prolonging the healing process.9 The delay increases the risk for wound infection,14 which can lead to a longer hospital stay and therefore higher costs.
We conducted a study to compare patients who received warfarin only with patients who received warfarin bridged with enoxaparin as antithrombotic chemoprophylaxis after THA. Outcomes of interest were number of days until a dry wound was observed and length of hospital stay. We hypothesized that, compared with warfarin-only therapy, bridged therapy would increase the risk for prolonged wound healing and result in longer hospital stays.
Materials and Methods
At our 746-bed academic medical center, 121 THAs were performed between January 1, 2008 and December 31, 2009. This study was approved by the center’s Office for Human Subjects Protections institutional review board (IRB). The research involved collecting or studying existing data, documents, and records recorded anonymously by the investigator in such a manner that subjects could not be identified, directly or through identifiers linked to the subjects, and therefore patient consent was not needed. Therefore, the IRB waived the need for consent. Relevant data included in this study were extracted from patient medical records, given within 35 days of surgery. For each patient, discharge notes provided data on the hospital course, and nurses’ notes provided data on wound status after THA.
Propensity Score Matching
For accurate analysis, it was important to consider confounding factors in both patient groups. Some covariates that may influence accurate analysis are age,15 diabetes,16 sex,15,17 hypertension,18 and body mass index.15,19Propensity score, defined as the conditional probability of receiving treatment, given the observed background covariates, was initially defined by Rosenbaum20 and Rubin.21 The motivation behind propensity scores can be understood by considering an idealized situation in which the 2 groups are similar on all background characteristics. In nonexperimental studies, researchers aim to find for each treated individual a comparison individual who looks exactly the same as the treated individual with respect to observed pretreatment covariates. Thus, assuming no hidden bias, any difference in outcomes within these pairs can be attributed to the variable of interest and not to any other differences between the treated and comparison individuals. Our study is a typical nonexperimental retrospective study in which the 2 groups being compared are patients receiving warfarin only or warfarin bridged with enoxaparin. To minimize the influence of background covariates, we used matching procedures and present our results both with and without the use of matching techniques.
Data and Results
There are different matching algorithms aimed at matching groups. In our study, the optimal matching procedure alone could not produce adequately matched data, so we used both optimal matching20 and genetic matching.22,23 Genetic matching procedure with replacement22 can produce well-matched data—it matched each patient in the warfarin-only group with a patient in the bridged-therapy group and allowed different patients to be matched with 1 similar patient in the control group. However, as the same patients in the bridged-therapy group might be matched multiple times, it would complicate the after-matching analysis. We therefore used a 2-step matching procedure to obtain well-matched data, and a simplified analysis procedure after matching. In the first step, we implemented genetic matching with replacement, as introduced by Abadie and Imbens,22 to match each warfarin-only patient with 1 bridged-therapy patient. In the second step, we applied optimal matching to the 2 groups. This 2-step matching turned out to produce better matched pairs, as denoted by Rubin.21 Both matching steps were implemented using the MatchIt function in R.24
The balance of matching is checked using criteria suggested by Rubin21: (1) standardized difference of means of propensity score, (2) ratio of variances in propensity score in treated and control groups, and (3) for each covariate, ratio of variance in residuals orthogonal to propensity score in treated and control groups.
Table 1 lists the means of the background covariates for each group before and after matching. Table 2 lists the balance check results suggested by Rubin.21 After matching, all standardized differences of means are smaller than 0.25, and the variance ratios are between 0.5 and 2, which are the standards suggested21 for regression adjustment to be valid after matching.
After genetic matching, 31 bridged-therapy patients and 57 warfarin-only patients remained. After optimal matching, there were 31 patients in each group. Poisson regressions of datasets before and after matching adjustment were fitted.
Results
Wounds of bridged-therapy patients took longer to heal than wounds of warfarin-only patients both before (odds ratio, 2.16; P < .05) and after matching data (odds ratio, 2.39; P < .05) with respect to confounding factors. In addition, bridged-therapy patients had longer hospital stays both before (odds ratio 1.20; P < .05) and after matching data (odds ratio, 1.27; P < .05) with respect to confounding factors. Figures 1 and 2 are histograms displaying the 2 groups and their outcomes.
Discussion
For patients undergoing THA procedures, several important considerations should be taken into account. Colwell and colleagues2 showed that, compared with warfarin, enoxaparin offered a 0.1% higher rate of protection against venous thromboembolic disease after THA. However, patients given enoxaparin may face increased risks.25 Hallevi and colleagues26 demonstrated that, compared with warfarin, enoxaparin bridging increased the risk for serious bleeding in patients with cardioembolic stroke. In our review of the literature, we learned that the benefits of bridge therapy in thromboembolic disease have yet to be investigated in THA.
At our academic hospital, the extra costs associated with bridge therapy can be as much as about $200027 per day per patient. These costs can go much higher, depending on type of patient and types of resources used. Over the 2-year period covered by our study, the costs of using enoxaparin amounted to about $151,200 ($2000 × 1.2 days per patient). If bridging offers no significant protection against thromboembolic disease, then it would be more cost-effective to use a single anticoagulant, particularly enoxaparin, for high-risk patients.
There are significant risk factors associated with prolonged healing of surgical wounds. Protocols outlining these factors may help reduce costs. In addition, when deciding on the use of aggressive anticoagulation therapy, surgeons must consider the risks for prolonged leakage and infection in addition to the risk for thromboembolic disease. Protocols may aid in this process as well. Our study results showed that, compared with warfarin-only therapy, bridged therapy (enoxaparin and warfarin) was associated with longer hospital stays. Further research should examine whether there are advantages that justify the higher risks of delayed wound healing and subsequent infection. Improving our understanding of risk factors associated with anticoagulation therapy will make orthopedic surgery safer for patients.
According to the literature, the rate of deep venous thrombosis after total hip arthroplasty (THA) can be high (45%-63%) without prophylactic anticoagulation.1-6 A meta-analysis of 13 studies found a rate of 51%.7 As lower extremity deep venous thrombi are the initial source of symptomatic pulmonary emboli in about 90% of cases,8 THA patients are usually given medication postoperatively focused on prevention of these thromboembolic events.9 Chemoprophylaxis may involve warfarin, enoxaparin, or their combination in an anticoagulation bridge. Enoxaparin is one of many low-molecular-weight heparins (LMWHs). All LMWHs exert their anticoagulant effect by binding to antithrombin III.10 The binding of LMWH to antithrombin III catalyzes the inhibition of factor Xa by antithrombin III, disrupting clot formation.11
In its hydroquinone form, vitamin K is essential as a cofactor for carboxylation of the glutamic acid residues of the amino-terminals of the coagulation proteins II, VII, IX, and X, leading to their activation. Anticoagulation by warfarin is achieved by the inhibition of the reductase enzymes that produce vitamin K hydroquinone in the liver from vitamin K epoxide.12 This inhibition prevents activation of the clotting proteins.12,13 Prophylaxis with enoxaparin or warfarin can reduce the rate of venous thromboembolic disease to 3.6% and 3.7%, respectively.2 However, these medications inhibit the clotting cascade, and their use risks prolonging the healing process.9 The delay increases the risk for wound infection,14 which can lead to a longer hospital stay and therefore higher costs.
We conducted a study to compare patients who received warfarin only with patients who received warfarin bridged with enoxaparin as antithrombotic chemoprophylaxis after THA. Outcomes of interest were number of days until a dry wound was observed and length of hospital stay. We hypothesized that, compared with warfarin-only therapy, bridged therapy would increase the risk for prolonged wound healing and result in longer hospital stays.
Materials and Methods
At our 746-bed academic medical center, 121 THAs were performed between January 1, 2008 and December 31, 2009. This study was approved by the center’s Office for Human Subjects Protections institutional review board (IRB). The research involved collecting or studying existing data, documents, and records recorded anonymously by the investigator in such a manner that subjects could not be identified, directly or through identifiers linked to the subjects, and therefore patient consent was not needed. Therefore, the IRB waived the need for consent. Relevant data included in this study were extracted from patient medical records, given within 35 days of surgery. For each patient, discharge notes provided data on the hospital course, and nurses’ notes provided data on wound status after THA.
Propensity Score Matching
For accurate analysis, it was important to consider confounding factors in both patient groups. Some covariates that may influence accurate analysis are age,15 diabetes,16 sex,15,17 hypertension,18 and body mass index.15,19Propensity score, defined as the conditional probability of receiving treatment, given the observed background covariates, was initially defined by Rosenbaum20 and Rubin.21 The motivation behind propensity scores can be understood by considering an idealized situation in which the 2 groups are similar on all background characteristics. In nonexperimental studies, researchers aim to find for each treated individual a comparison individual who looks exactly the same as the treated individual with respect to observed pretreatment covariates. Thus, assuming no hidden bias, any difference in outcomes within these pairs can be attributed to the variable of interest and not to any other differences between the treated and comparison individuals. Our study is a typical nonexperimental retrospective study in which the 2 groups being compared are patients receiving warfarin only or warfarin bridged with enoxaparin. To minimize the influence of background covariates, we used matching procedures and present our results both with and without the use of matching techniques.
Data and Results
There are different matching algorithms aimed at matching groups. In our study, the optimal matching procedure alone could not produce adequately matched data, so we used both optimal matching20 and genetic matching.22,23 Genetic matching procedure with replacement22 can produce well-matched data—it matched each patient in the warfarin-only group with a patient in the bridged-therapy group and allowed different patients to be matched with 1 similar patient in the control group. However, as the same patients in the bridged-therapy group might be matched multiple times, it would complicate the after-matching analysis. We therefore used a 2-step matching procedure to obtain well-matched data, and a simplified analysis procedure after matching. In the first step, we implemented genetic matching with replacement, as introduced by Abadie and Imbens,22 to match each warfarin-only patient with 1 bridged-therapy patient. In the second step, we applied optimal matching to the 2 groups. This 2-step matching turned out to produce better matched pairs, as denoted by Rubin.21 Both matching steps were implemented using the MatchIt function in R.24
The balance of matching is checked using criteria suggested by Rubin21: (1) standardized difference of means of propensity score, (2) ratio of variances in propensity score in treated and control groups, and (3) for each covariate, ratio of variance in residuals orthogonal to propensity score in treated and control groups.
Table 1 lists the means of the background covariates for each group before and after matching. Table 2 lists the balance check results suggested by Rubin.21 After matching, all standardized differences of means are smaller than 0.25, and the variance ratios are between 0.5 and 2, which are the standards suggested21 for regression adjustment to be valid after matching.
After genetic matching, 31 bridged-therapy patients and 57 warfarin-only patients remained. After optimal matching, there were 31 patients in each group. Poisson regressions of datasets before and after matching adjustment were fitted.
Results
Wounds of bridged-therapy patients took longer to heal than wounds of warfarin-only patients both before (odds ratio, 2.16; P < .05) and after matching data (odds ratio, 2.39; P < .05) with respect to confounding factors. In addition, bridged-therapy patients had longer hospital stays both before (odds ratio 1.20; P < .05) and after matching data (odds ratio, 1.27; P < .05) with respect to confounding factors. Figures 1 and 2 are histograms displaying the 2 groups and their outcomes.
Discussion
For patients undergoing THA procedures, several important considerations should be taken into account. Colwell and colleagues2 showed that, compared with warfarin, enoxaparin offered a 0.1% higher rate of protection against venous thromboembolic disease after THA. However, patients given enoxaparin may face increased risks.25 Hallevi and colleagues26 demonstrated that, compared with warfarin, enoxaparin bridging increased the risk for serious bleeding in patients with cardioembolic stroke. In our review of the literature, we learned that the benefits of bridge therapy in thromboembolic disease have yet to be investigated in THA.
At our academic hospital, the extra costs associated with bridge therapy can be as much as about $200027 per day per patient. These costs can go much higher, depending on type of patient and types of resources used. Over the 2-year period covered by our study, the costs of using enoxaparin amounted to about $151,200 ($2000 × 1.2 days per patient). If bridging offers no significant protection against thromboembolic disease, then it would be more cost-effective to use a single anticoagulant, particularly enoxaparin, for high-risk patients.
There are significant risk factors associated with prolonged healing of surgical wounds. Protocols outlining these factors may help reduce costs. In addition, when deciding on the use of aggressive anticoagulation therapy, surgeons must consider the risks for prolonged leakage and infection in addition to the risk for thromboembolic disease. Protocols may aid in this process as well. Our study results showed that, compared with warfarin-only therapy, bridged therapy (enoxaparin and warfarin) was associated with longer hospital stays. Further research should examine whether there are advantages that justify the higher risks of delayed wound healing and subsequent infection. Improving our understanding of risk factors associated with anticoagulation therapy will make orthopedic surgery safer for patients.
1. Bergqvist D, Benoni G, Björgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335(10):696-700.
2. Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81(7):932-940.
3. Haake DA, Berkman SA. Venous thromboembolic disease after hip surgery. Risk factors, prophylaxis, and diagnosis. Clin Orthop Relat Res. 1989;(242):212-231.
4. Johnson R, Carmichael JH, Almond HG, Loynes RP. Deep venous thrombosis following Charnley arthroplasty. Clin Orthop Relat Res. 1978;(132):24-30.
5. Stamatakis JD, Kakkar VV, Sagar S, Lawrence D, Nairn D, Bentley PG. Femoral vein thrombosis and total hip replacement. Br Med J. 1977;2(6081):223-225.
6. Turpie AG, Levine MN, Hirsh J, et al. A randomized controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med. 1986;315(15):925-929.
7. Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S-334S.
8. Westrich GH, Sánchez PM. Prevention and treatment of thromboembolic disease: an overview. Instr Course Lect. 2002;51:471-480.
9. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
10. Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga JM. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol. 1998;82(5B):3L-10L.
11. Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20(7):771-775.
12. Kamali F, Wood P, Ward A. Vitamin K deficiency amplifies anticoagulation response to ximelagatran: possible implications for direct thrombin inhibitors and their clinical safety. Ann Hematol. 2009;88(2):141-149.
13. Choonara IA, Malia RG, Haynes BP, et al. The relationship between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol. 1988;25(1):1-7.
14. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515.
15. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
16. Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22(5):651-656.
17. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
18. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
19. Lübbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57(2):327-334.
20. Rosenbaum PR. A characterization of optimal designs for observational studies. J R Stat Soc Ser B. 1991;53(3):597-610.
21. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(1):169-188.
22. Abadie A, Imbens GW. Simple and Bias-Corrected Matching Estimators for Average Treatment Effects. Berkeley, CA: Department of Economics, University of California; 2002.
23. Diamond A, Sekhon J. Genetic matching for estimating causal effects: a new method of achieving balance in observational studies. Paper presented at: Annual Meeting of the Midwest Political Science Association; April 2005; Chicago, IL.
24. Imai K, King G, Lau O. logit: logistic regression for dichotomous dependent variables. In: Imai K, King G, Lau O. Zelig: Everyone’s Statistical Software. 2011; 238-244. http://gking.harvard.edu/zelig. Accessed May 26, 2015.
25. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
26. Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge? Arch Neurol. 2008;65(9):1169-1173.
27. Henry J. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day [2010]. http://kff.org/other/state-indicator/expenses-per-inpatient-day/#table. Accessed May 26, 2015.
1. Bergqvist D, Benoni G, Björgell O, et al. Low-molecular-weight heparin (enoxaparin) as prophylaxis against venous thromboembolism after total hip replacement. N Engl J Med. 1996;335(10):696-700.
2. Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am. 1999;81(7):932-940.
3. Haake DA, Berkman SA. Venous thromboembolic disease after hip surgery. Risk factors, prophylaxis, and diagnosis. Clin Orthop Relat Res. 1989;(242):212-231.
4. Johnson R, Carmichael JH, Almond HG, Loynes RP. Deep venous thrombosis following Charnley arthroplasty. Clin Orthop Relat Res. 1978;(132):24-30.
5. Stamatakis JD, Kakkar VV, Sagar S, Lawrence D, Nairn D, Bentley PG. Femoral vein thrombosis and total hip replacement. Br Med J. 1977;2(6081):223-225.
6. Turpie AG, Levine MN, Hirsh J, et al. A randomized controlled trial of a low-molecular-weight heparin (enoxaparin) to prevent deep-vein thrombosis in patients undergoing elective hip surgery. N Engl J Med. 1986;315(15):925-929.
7. Clagett GP, Anderson FA Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108(4 suppl):312S-334S.
8. Westrich GH, Sánchez PM. Prevention and treatment of thromboembolic disease: an overview. Instr Course Lect. 2002;51:471-480.
9. Colwell CW Jr, Froimson MI, Mont MA, et al. Thrombosis prevention after total hip arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92(3):527-535.
10. Fareed J, Jeske W, Hoppensteadt D, Clarizio R, Walenga JM. Low-molecular-weight heparins: pharmacologic profile and product differentiation. Am J Cardiol. 1998;82(5B):3L-10L.
11. Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20(7):771-775.
12. Kamali F, Wood P, Ward A. Vitamin K deficiency amplifies anticoagulation response to ximelagatran: possible implications for direct thrombin inhibitors and their clinical safety. Ann Hematol. 2009;88(2):141-149.
13. Choonara IA, Malia RG, Haynes BP, et al. The relationship between inhibition of vitamin K1 2,3-epoxide reductase and reduction of clotting factor activity with warfarin. Br J Clin Pharmacol. 1988;25(1):1-7.
14. Saleh K, Olson M, Resig S, et al. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20(3):506-515.
15. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005;87(6):844-850.
16. Lai K, Bohm ER, Burnell C, Hedden DR. Presence of medical comorbidities in patients with infected primary hip or knee arthroplasties. J Arthroplasty. 2007;22(5):651-656.
17. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991.
18. Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224.
19. Lübbeke A, Stern R, Garavaglia G, Zurcher L, Hoffmeyer P. Differences in outcomes of obese women and men undergoing primary total hip arthroplasty. Arthritis Rheum. 2007;57(2):327-334.
20. Rosenbaum PR. A characterization of optimal designs for observational studies. J R Stat Soc Ser B. 1991;53(3):597-610.
21. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(1):169-188.
22. Abadie A, Imbens GW. Simple and Bias-Corrected Matching Estimators for Average Treatment Effects. Berkeley, CA: Department of Economics, University of California; 2002.
23. Diamond A, Sekhon J. Genetic matching for estimating causal effects: a new method of achieving balance in observational studies. Paper presented at: Annual Meeting of the Midwest Political Science Association; April 2005; Chicago, IL.
24. Imai K, King G, Lau O. logit: logistic regression for dichotomous dependent variables. In: Imai K, King G, Lau O. Zelig: Everyone’s Statistical Software. 2011; 238-244. http://gking.harvard.edu/zelig. Accessed May 26, 2015.
25. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33-38.
26. Hallevi H, Albright KC, Martin-Schild S, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge? Arch Neurol. 2008;65(9):1169-1173.
27. Henry J. Kaiser Family Foundation. Hospital adjusted expenses per inpatient day [2010]. http://kff.org/other/state-indicator/expenses-per-inpatient-day/#table. Accessed May 26, 2015.