User login
A 24‐year‐old man presented to the emergency room with a 3‐month history of bright red blood per rectum and increasing fatigue. Review of systems was significant for intermittent hematuria, swelling, and pain in his lower extremities. He denied abdominal pain, nausea, or vomiting, and was otherwise asymptomatic. He was not taking any medicines. He said that he has had this bleeding problem on and off since he was a child. The chronic intermittent rectal bleeding usually resolved spontaneously. Previous treatments have consisted of blood transfusions, small bowel resections, and a partial colectomy.
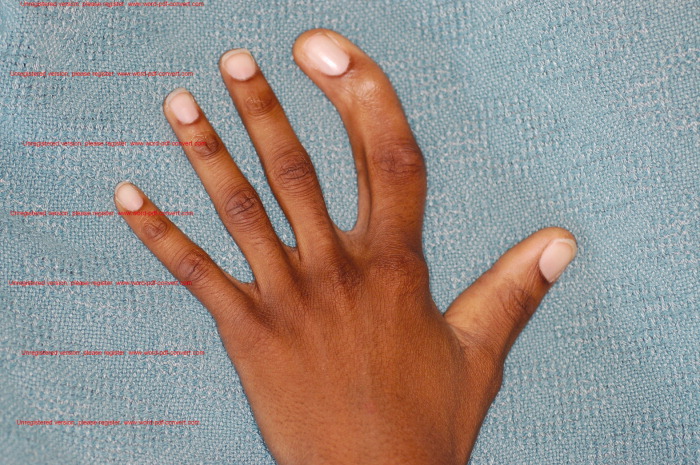
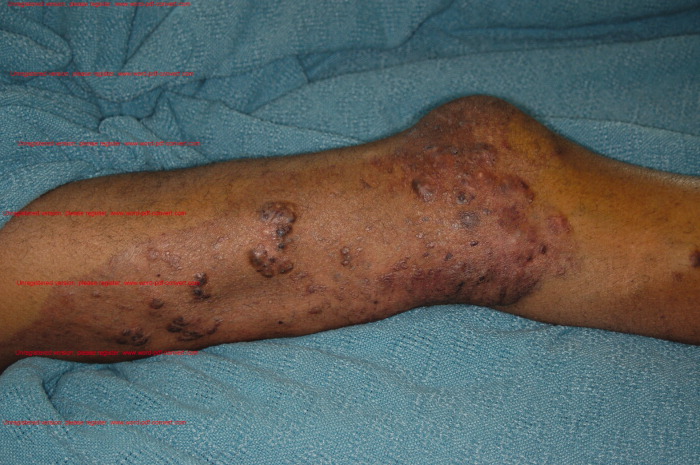
Physical exam demonstrates a thin and well‐nourished African American male in no distress. Temperature 36.7C, blood pressure while sitting was 111/65 mmHg, with a pulse of 117 beats per minute; on standing his blood pressure was 103/54 mmHg, with a pulse of 137 beats per minute, and respirations were 18 breaths per minute. Abdominal examination revealed splenomegaly. Rectal exam revealed the presence of bright red blood. Other significant findings include unilateral limb skeletal asymmetry with the right upper and lower extremity being longer than the left side. There was significant hypertrophy of several digits of the hands and feet bilaterally (Figure 1). Notable was the presence of raised, hyperpigmented irregular linear plaques, extending from his right medial forearm to his chest and also from his abdomen to right medial thigh. Additional skin examination was remarkable for well‐demarcated, raised vascular areas on the lateral thighs and knees bilaterally (Figure 2), as well as the dorsum of both the feet. Laboratory workup was notable for hemoglobin of 2.7gm/dl, a hematocrit of 9%, and mean corpuscular volume (MCV) of 58 fl. Normal coagulation parameters, and profound iron deficiency (iron level 16 mcg/dl and ferritin <20 ng/ml).

Other routine laboratory results including coagulation parameters were unremarkable.
Discussion
Based on the classic examination findings and history of gastrointestinal bleeding, this patient has Klippel‐Trenaunay‐Weber syndrome (KTWS), which is characterized by cutaneous malformations of the capillary and venous systems, bony and soft tissue hypertrophy, and arteriovenous malformations (AVMs).1 Many patients with KTWS suffer recurrent bleeding from gastrointestinal AVMs.
Although involvement is usually unilateral, this patient had bilateral limb hypertrophy and hemangiomas. His nevus flammeus was unilateral and incidentally was present over the lower abdomen and posterior thigh and buttock, with significant underlying varices in the pelvis and rectum. His hematuria was secondary to AVMs in the bladder and resolved by itself. The size and extent of his pelvic and rectal varices presented a therapeutic challenge. With blood transfusions and a conservative approach, his bleeding diminished spontaneously. A rectal artery was thought to be contributing to the problem, so a prophylactic embolization was performed by interventional radiology. Follow‐up at 2 months revealed no further bleeding.
Hospitalists treat common causes of gastrointestinal (GI) bleeding such as ulcers, polyps, malignancies, varices, inflammatory bowel disease, AVMs, and, rarely, mucosal Kaposi sarcoma. However, they may occasionally encounter an adult with skin manifestations of a congenital cause of GI bleeding. The 4 most common congenital disorders with primary cutaneous manifestations that also involve the GI tract are reviewed below (also see Table 1).
| Vascular Malformation Syndromes | Characteristics |
|---|---|
| Klippel‐Trenaunay‐Weber | Soft tissue; bony, vascular lesions; and varices |
| Mafucci | Enchondromas, subcutaneous visceral lesions |
| Blue rubber bleb nevus | Bluish black sessile venous malformations |
| Osler‐Maffuci‐Weber‐Rendu | Mucocutaneous telangiectasias |
Blue rubber bleb nevus syndrome, also known as Bean syndrome, is the rarest of these disorders, characterized by cutaneous and intestinal cavernous hemangiomas that may occasionally be painful and tender.2 Hemangiomas may measure from a few millimeters to approximately 5 cm and are raised, blue‐purple, and rubbery in consistency, with a wrinkled surface. They are usually located on the trunk, extremities, face, and any part of the GI tract, with the small intestine and distal colon being the most common sites involved. Given that the lesions may involve the full thickness of the bowel wall, surgery is often required, as less invasive measures such as endoscopic laser coagulation may be inadequate. Orthopedic problems such as scoliosis arise from pressure exerted by large vascular malformations.
Maffucci syndrome is characterized by skeletal and vascular malformations manifested as enchondromas in the metaphyseal and diaphyseal portion of long bones. The vascular lesions, which may involve mucous membranes or viscera, are compressible blue‐purple hemangiomas that follow the rate of the growth of the child. Limb deformities, pathological fractures, and malignant transformation into chondrosarcomas are common complications.3
Osler‐Weber‐Rendu syndrome is also known as hereditary hemorrhagic telangiectasia. In this disorder, mucocutaneous telangiectatic lesions usually develop by puberty and may involve the conjunctiva, respiratory tract, brain, liver, GI tract, and genitourinary (GU) tract. Most patients exhibit only epistaxis, yet massive hemorrhage may occur in the lung, GI tract, and GU tract. These hemorrhages can usually be managed by cautery or electrocoagulation but pulmonary and GI lesions may need excision.4
KTWS consists of the triad of cutaneous vascular malformations of the capillary, venous and lymphatic systems, bony and soft tissue hypertrophy, and venous varicosities in association with AVMs. The name Weber is added when patients have AVMs that are clinically significant; otherwise, it is simply known as Klippel‐Trenaunay syndrome. The most common cutaneous vascular lesion is a capillary hemangioma known as a nevus flammeus. The distribution of the nevus flammeus usually indicates underlying vascular malformations that may extend as deep as the bone, causing limb or digit hypertrophy, as seen in this patient.5 Delineation of the extent of vascular abnormalities is accomplished by noninvasive methods such as color ultrasonography, magnetic resonance imaging, and computer‐aided angiography. Symptomatic GI or GU involvement is rare (1%), but can cause significant hemorrhage.6 Surgical correction is often difficult and the lesions tend to recur.
In the largest published series of Klippel‐Trenaunay patients, followed over 30 years at the Mayo Clinic, most patients were treated conservatively, with surgery limited to epiphysiodesis to prevent excessive leg length in the affected limbs and selected superficial vein stripping in patients with large venous varicosities with preserved deep venous systems.7, 8 For the treatment of AVMs, nonsurgical measures such as foam embolization and radiotherapy are increasingly being used due to their safety and precise application.9, 10
- ,,, et al.Klippel‐Trenaunay syndrome.Am J Med Genet.1998;79(4):319–326.
- .Blue rubber bleb nevus syndrome.Curr Treat Options Gastroenterol.2001;4(5):433–440.
- ,.Maffucci's syndrome, functional and neoplastic significance. Case report and review of the literature.J Bone Joint Surg Am.1973;55:1465–1479.
- ,,.Hereditary hemorrhagic telangiectasia (Osler‐Weber‐Rendu syndrome): a view from the 21st century.Postgrad Med J.2003;79:18–24.
- ,.Klippel Trenaunay syndrome: the importance of “geographic stains” in identifying lymphatic disease and risk of complications.J Am Acad Dermatol.2004;51(3):391–398.
- ,,,.Klippel‐Trenaunay syndrome with involvement of cecum and rectum: a rare cause of lower gastrointestinal bleeding.Eur J Med Res.2004;9(11):515–517.
- ,,.Klippel‐Trenaunay syndrome: spectrum and management.Mayo Clinic Proc.1998;73:28–36.
- ,,,,,.Surgical treatment of venous malformations in Klippel‐Trenaunay syndrome.J Vasc Surg.2000;32:840–847.
- .Radiotherapy in the management of Klippel‐Trenaunay‐Weber syndrome: report of two cases.Ann Vasc Surg.2005;19(4):566–571.
- ,,.Venous angiomata: treatment with sclerosant foam.Ann Vasc Surg.2005;19:457–464.
A 24‐year‐old man presented to the emergency room with a 3‐month history of bright red blood per rectum and increasing fatigue. Review of systems was significant for intermittent hematuria, swelling, and pain in his lower extremities. He denied abdominal pain, nausea, or vomiting, and was otherwise asymptomatic. He was not taking any medicines. He said that he has had this bleeding problem on and off since he was a child. The chronic intermittent rectal bleeding usually resolved spontaneously. Previous treatments have consisted of blood transfusions, small bowel resections, and a partial colectomy.
Physical exam demonstrates a thin and well‐nourished African American male in no distress. Temperature 36.7C, blood pressure while sitting was 111/65 mmHg, with a pulse of 117 beats per minute; on standing his blood pressure was 103/54 mmHg, with a pulse of 137 beats per minute, and respirations were 18 breaths per minute. Abdominal examination revealed splenomegaly. Rectal exam revealed the presence of bright red blood. Other significant findings include unilateral limb skeletal asymmetry with the right upper and lower extremity being longer than the left side. There was significant hypertrophy of several digits of the hands and feet bilaterally (Figure 1). Notable was the presence of raised, hyperpigmented irregular linear plaques, extending from his right medial forearm to his chest and also from his abdomen to right medial thigh. Additional skin examination was remarkable for well‐demarcated, raised vascular areas on the lateral thighs and knees bilaterally (Figure 2), as well as the dorsum of both the feet. Laboratory workup was notable for hemoglobin of 2.7gm/dl, a hematocrit of 9%, and mean corpuscular volume (MCV) of 58 fl. Normal coagulation parameters, and profound iron deficiency (iron level 16 mcg/dl and ferritin <20 ng/ml).


Other routine laboratory results including coagulation parameters were unremarkable.
Discussion
Based on the classic examination findings and history of gastrointestinal bleeding, this patient has Klippel‐Trenaunay‐Weber syndrome (KTWS), which is characterized by cutaneous malformations of the capillary and venous systems, bony and soft tissue hypertrophy, and arteriovenous malformations (AVMs).1 Many patients with KTWS suffer recurrent bleeding from gastrointestinal AVMs.
Although involvement is usually unilateral, this patient had bilateral limb hypertrophy and hemangiomas. His nevus flammeus was unilateral and incidentally was present over the lower abdomen and posterior thigh and buttock, with significant underlying varices in the pelvis and rectum. His hematuria was secondary to AVMs in the bladder and resolved by itself. The size and extent of his pelvic and rectal varices presented a therapeutic challenge. With blood transfusions and a conservative approach, his bleeding diminished spontaneously. A rectal artery was thought to be contributing to the problem, so a prophylactic embolization was performed by interventional radiology. Follow‐up at 2 months revealed no further bleeding.
Hospitalists treat common causes of gastrointestinal (GI) bleeding such as ulcers, polyps, malignancies, varices, inflammatory bowel disease, AVMs, and, rarely, mucosal Kaposi sarcoma. However, they may occasionally encounter an adult with skin manifestations of a congenital cause of GI bleeding. The 4 most common congenital disorders with primary cutaneous manifestations that also involve the GI tract are reviewed below (also see Table 1).
| Vascular Malformation Syndromes | Characteristics |
|---|---|
| Klippel‐Trenaunay‐Weber | Soft tissue; bony, vascular lesions; and varices |
| Mafucci | Enchondromas, subcutaneous visceral lesions |
| Blue rubber bleb nevus | Bluish black sessile venous malformations |
| Osler‐Maffuci‐Weber‐Rendu | Mucocutaneous telangiectasias |
Blue rubber bleb nevus syndrome, also known as Bean syndrome, is the rarest of these disorders, characterized by cutaneous and intestinal cavernous hemangiomas that may occasionally be painful and tender.2 Hemangiomas may measure from a few millimeters to approximately 5 cm and are raised, blue‐purple, and rubbery in consistency, with a wrinkled surface. They are usually located on the trunk, extremities, face, and any part of the GI tract, with the small intestine and distal colon being the most common sites involved. Given that the lesions may involve the full thickness of the bowel wall, surgery is often required, as less invasive measures such as endoscopic laser coagulation may be inadequate. Orthopedic problems such as scoliosis arise from pressure exerted by large vascular malformations.
Maffucci syndrome is characterized by skeletal and vascular malformations manifested as enchondromas in the metaphyseal and diaphyseal portion of long bones. The vascular lesions, which may involve mucous membranes or viscera, are compressible blue‐purple hemangiomas that follow the rate of the growth of the child. Limb deformities, pathological fractures, and malignant transformation into chondrosarcomas are common complications.3
Osler‐Weber‐Rendu syndrome is also known as hereditary hemorrhagic telangiectasia. In this disorder, mucocutaneous telangiectatic lesions usually develop by puberty and may involve the conjunctiva, respiratory tract, brain, liver, GI tract, and genitourinary (GU) tract. Most patients exhibit only epistaxis, yet massive hemorrhage may occur in the lung, GI tract, and GU tract. These hemorrhages can usually be managed by cautery or electrocoagulation but pulmonary and GI lesions may need excision.4
KTWS consists of the triad of cutaneous vascular malformations of the capillary, venous and lymphatic systems, bony and soft tissue hypertrophy, and venous varicosities in association with AVMs. The name Weber is added when patients have AVMs that are clinically significant; otherwise, it is simply known as Klippel‐Trenaunay syndrome. The most common cutaneous vascular lesion is a capillary hemangioma known as a nevus flammeus. The distribution of the nevus flammeus usually indicates underlying vascular malformations that may extend as deep as the bone, causing limb or digit hypertrophy, as seen in this patient.5 Delineation of the extent of vascular abnormalities is accomplished by noninvasive methods such as color ultrasonography, magnetic resonance imaging, and computer‐aided angiography. Symptomatic GI or GU involvement is rare (1%), but can cause significant hemorrhage.6 Surgical correction is often difficult and the lesions tend to recur.
In the largest published series of Klippel‐Trenaunay patients, followed over 30 years at the Mayo Clinic, most patients were treated conservatively, with surgery limited to epiphysiodesis to prevent excessive leg length in the affected limbs and selected superficial vein stripping in patients with large venous varicosities with preserved deep venous systems.7, 8 For the treatment of AVMs, nonsurgical measures such as foam embolization and radiotherapy are increasingly being used due to their safety and precise application.9, 10
A 24‐year‐old man presented to the emergency room with a 3‐month history of bright red blood per rectum and increasing fatigue. Review of systems was significant for intermittent hematuria, swelling, and pain in his lower extremities. He denied abdominal pain, nausea, or vomiting, and was otherwise asymptomatic. He was not taking any medicines. He said that he has had this bleeding problem on and off since he was a child. The chronic intermittent rectal bleeding usually resolved spontaneously. Previous treatments have consisted of blood transfusions, small bowel resections, and a partial colectomy.
Physical exam demonstrates a thin and well‐nourished African American male in no distress. Temperature 36.7C, blood pressure while sitting was 111/65 mmHg, with a pulse of 117 beats per minute; on standing his blood pressure was 103/54 mmHg, with a pulse of 137 beats per minute, and respirations were 18 breaths per minute. Abdominal examination revealed splenomegaly. Rectal exam revealed the presence of bright red blood. Other significant findings include unilateral limb skeletal asymmetry with the right upper and lower extremity being longer than the left side. There was significant hypertrophy of several digits of the hands and feet bilaterally (Figure 1). Notable was the presence of raised, hyperpigmented irregular linear plaques, extending from his right medial forearm to his chest and also from his abdomen to right medial thigh. Additional skin examination was remarkable for well‐demarcated, raised vascular areas on the lateral thighs and knees bilaterally (Figure 2), as well as the dorsum of both the feet. Laboratory workup was notable for hemoglobin of 2.7gm/dl, a hematocrit of 9%, and mean corpuscular volume (MCV) of 58 fl. Normal coagulation parameters, and profound iron deficiency (iron level 16 mcg/dl and ferritin <20 ng/ml).


Other routine laboratory results including coagulation parameters were unremarkable.
Discussion
Based on the classic examination findings and history of gastrointestinal bleeding, this patient has Klippel‐Trenaunay‐Weber syndrome (KTWS), which is characterized by cutaneous malformations of the capillary and venous systems, bony and soft tissue hypertrophy, and arteriovenous malformations (AVMs).1 Many patients with KTWS suffer recurrent bleeding from gastrointestinal AVMs.
Although involvement is usually unilateral, this patient had bilateral limb hypertrophy and hemangiomas. His nevus flammeus was unilateral and incidentally was present over the lower abdomen and posterior thigh and buttock, with significant underlying varices in the pelvis and rectum. His hematuria was secondary to AVMs in the bladder and resolved by itself. The size and extent of his pelvic and rectal varices presented a therapeutic challenge. With blood transfusions and a conservative approach, his bleeding diminished spontaneously. A rectal artery was thought to be contributing to the problem, so a prophylactic embolization was performed by interventional radiology. Follow‐up at 2 months revealed no further bleeding.
Hospitalists treat common causes of gastrointestinal (GI) bleeding such as ulcers, polyps, malignancies, varices, inflammatory bowel disease, AVMs, and, rarely, mucosal Kaposi sarcoma. However, they may occasionally encounter an adult with skin manifestations of a congenital cause of GI bleeding. The 4 most common congenital disorders with primary cutaneous manifestations that also involve the GI tract are reviewed below (also see Table 1).
| Vascular Malformation Syndromes | Characteristics |
|---|---|
| Klippel‐Trenaunay‐Weber | Soft tissue; bony, vascular lesions; and varices |
| Mafucci | Enchondromas, subcutaneous visceral lesions |
| Blue rubber bleb nevus | Bluish black sessile venous malformations |
| Osler‐Maffuci‐Weber‐Rendu | Mucocutaneous telangiectasias |
Blue rubber bleb nevus syndrome, also known as Bean syndrome, is the rarest of these disorders, characterized by cutaneous and intestinal cavernous hemangiomas that may occasionally be painful and tender.2 Hemangiomas may measure from a few millimeters to approximately 5 cm and are raised, blue‐purple, and rubbery in consistency, with a wrinkled surface. They are usually located on the trunk, extremities, face, and any part of the GI tract, with the small intestine and distal colon being the most common sites involved. Given that the lesions may involve the full thickness of the bowel wall, surgery is often required, as less invasive measures such as endoscopic laser coagulation may be inadequate. Orthopedic problems such as scoliosis arise from pressure exerted by large vascular malformations.
Maffucci syndrome is characterized by skeletal and vascular malformations manifested as enchondromas in the metaphyseal and diaphyseal portion of long bones. The vascular lesions, which may involve mucous membranes or viscera, are compressible blue‐purple hemangiomas that follow the rate of the growth of the child. Limb deformities, pathological fractures, and malignant transformation into chondrosarcomas are common complications.3
Osler‐Weber‐Rendu syndrome is also known as hereditary hemorrhagic telangiectasia. In this disorder, mucocutaneous telangiectatic lesions usually develop by puberty and may involve the conjunctiva, respiratory tract, brain, liver, GI tract, and genitourinary (GU) tract. Most patients exhibit only epistaxis, yet massive hemorrhage may occur in the lung, GI tract, and GU tract. These hemorrhages can usually be managed by cautery or electrocoagulation but pulmonary and GI lesions may need excision.4
KTWS consists of the triad of cutaneous vascular malformations of the capillary, venous and lymphatic systems, bony and soft tissue hypertrophy, and venous varicosities in association with AVMs. The name Weber is added when patients have AVMs that are clinically significant; otherwise, it is simply known as Klippel‐Trenaunay syndrome. The most common cutaneous vascular lesion is a capillary hemangioma known as a nevus flammeus. The distribution of the nevus flammeus usually indicates underlying vascular malformations that may extend as deep as the bone, causing limb or digit hypertrophy, as seen in this patient.5 Delineation of the extent of vascular abnormalities is accomplished by noninvasive methods such as color ultrasonography, magnetic resonance imaging, and computer‐aided angiography. Symptomatic GI or GU involvement is rare (1%), but can cause significant hemorrhage.6 Surgical correction is often difficult and the lesions tend to recur.
In the largest published series of Klippel‐Trenaunay patients, followed over 30 years at the Mayo Clinic, most patients were treated conservatively, with surgery limited to epiphysiodesis to prevent excessive leg length in the affected limbs and selected superficial vein stripping in patients with large venous varicosities with preserved deep venous systems.7, 8 For the treatment of AVMs, nonsurgical measures such as foam embolization and radiotherapy are increasingly being used due to their safety and precise application.9, 10
- ,,, et al.Klippel‐Trenaunay syndrome.Am J Med Genet.1998;79(4):319–326.
- .Blue rubber bleb nevus syndrome.Curr Treat Options Gastroenterol.2001;4(5):433–440.
- ,.Maffucci's syndrome, functional and neoplastic significance. Case report and review of the literature.J Bone Joint Surg Am.1973;55:1465–1479.
- ,,.Hereditary hemorrhagic telangiectasia (Osler‐Weber‐Rendu syndrome): a view from the 21st century.Postgrad Med J.2003;79:18–24.
- ,.Klippel Trenaunay syndrome: the importance of “geographic stains” in identifying lymphatic disease and risk of complications.J Am Acad Dermatol.2004;51(3):391–398.
- ,,,.Klippel‐Trenaunay syndrome with involvement of cecum and rectum: a rare cause of lower gastrointestinal bleeding.Eur J Med Res.2004;9(11):515–517.
- ,,.Klippel‐Trenaunay syndrome: spectrum and management.Mayo Clinic Proc.1998;73:28–36.
- ,,,,,.Surgical treatment of venous malformations in Klippel‐Trenaunay syndrome.J Vasc Surg.2000;32:840–847.
- .Radiotherapy in the management of Klippel‐Trenaunay‐Weber syndrome: report of two cases.Ann Vasc Surg.2005;19(4):566–571.
- ,,.Venous angiomata: treatment with sclerosant foam.Ann Vasc Surg.2005;19:457–464.
- ,,, et al.Klippel‐Trenaunay syndrome.Am J Med Genet.1998;79(4):319–326.
- .Blue rubber bleb nevus syndrome.Curr Treat Options Gastroenterol.2001;4(5):433–440.
- ,.Maffucci's syndrome, functional and neoplastic significance. Case report and review of the literature.J Bone Joint Surg Am.1973;55:1465–1479.
- ,,.Hereditary hemorrhagic telangiectasia (Osler‐Weber‐Rendu syndrome): a view from the 21st century.Postgrad Med J.2003;79:18–24.
- ,.Klippel Trenaunay syndrome: the importance of “geographic stains” in identifying lymphatic disease and risk of complications.J Am Acad Dermatol.2004;51(3):391–398.
- ,,,.Klippel‐Trenaunay syndrome with involvement of cecum and rectum: a rare cause of lower gastrointestinal bleeding.Eur J Med Res.2004;9(11):515–517.
- ,,.Klippel‐Trenaunay syndrome: spectrum and management.Mayo Clinic Proc.1998;73:28–36.
- ,,,,,.Surgical treatment of venous malformations in Klippel‐Trenaunay syndrome.J Vasc Surg.2000;32:840–847.
- .Radiotherapy in the management of Klippel‐Trenaunay‐Weber syndrome: report of two cases.Ann Vasc Surg.2005;19(4):566–571.
- ,,.Venous angiomata: treatment with sclerosant foam.Ann Vasc Surg.2005;19:457–464.