User login
Despite the recent advances in systemic therapy, metastatic spread to the brain continues to be the most common neurologic complication of many cancers. The clinical incidence of brain metastases varies with primary cancer diagnosis, with estimates ranging from 1.2%-19.8%.1,2 Metastatic spread to the brain is even more prevalent at autopsy, with evidence of intracranial tumor being found in 26% of patients in some series.3 It is possible that the clinical incidence of metastatic disease to the brain will continue to increase as newer therapeutic agents improve survival and imaging techniques continue to improve.
The management of brain metastases has changed rapidly as technological improvements have made treatment increasingly safe and efficacious. Traditionally, treatment consisted of radiotherapy to the whole brain, with or without surgical resection.4,5 More recently, stereotactic radiosurgery (SRS) has been adopted on the basis of evidence that it is safe and efficacious alone or in combination with radiotherapy to the whole brain.6 Further evidence is emerging that neurocognitive outcomes are improved when whole-brain radiotherapy (WBRT) is omitted, which possibly contributes to improved patient quality of life.7 Taking into account this and other data, the American Society for Radiation Oncology’s Choosing Wisely campaign now recommends not routinely adding WBRT to radiosurgery in patients with limited brain metastases.8
Despite this recommendation, many patients continue to benefit from WBRT, and it remains a common treatment in radiation oncology clinics across the US for several reasons. Many patients present with multiple brain metastases and are ineligible for radiosurgery. Even for technically eligible patients, WBRT has been shown to improve local control and decrease the rate of distant brain failure over radiosurgery alone.6 With higher rates of subsequent failures, patients receiving radiosurgery alone must adhere to more rigorous follow-up and imaging schedules, which can be difficult for many rural patients who have to travel long distances to centers. Furthermore, there is some suggestion that this decreased failure rate may result in improved survival in highly selected patients with excellent disease and performance status.9 Controversies exist, however, and strong institutional biases persist, contributing to significant differences in practice. We surveyed academic radiation oncologists and in an effort to identify and describe practice patterns in the delivery of WBRT at academic centers.
Methods
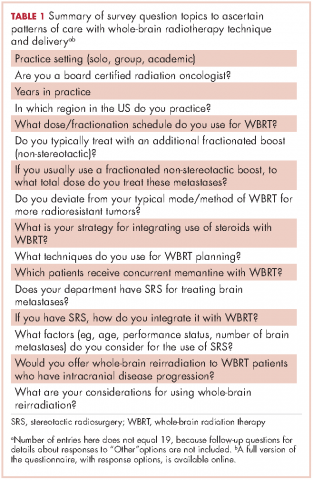
We conducted a thorough review of available literature on radiation for brain metastases and based on our findings, devised a survey 19 questions to ascertain practice patterns and treatment delivery among US academic physicians (Table 1). After obtaining institutional review board approval to do the study, we sent the survey to program coordinators at radiation oncology programs that are accredited by the Accreditation Council for Graduate Medical Education. We instructed coordinators to e-mail the survey to their practicing resident and attending physicians. The surveys were created using SurveyMonkey software. We obtained informed consent from the providers. A total of 3 follow-up e-mails were sent to each recipient of the survey to solicit responses, similar to the Dillman Total Design Survey Method.10
SPSS version 22.0 was used to analyze the data in an exploratory fashion. Statistical methods were used to assess the association of demographic data with SRS and WBRT delivery and treatment technique items when the analyses involved percentages that included the Pearson chi-square statistic and the chi-square test for linear trend. When the analysis focused on ranking data, the Kruskal-Wallis test, Mann-Whitney U test, the Jonckheere-Terpstra and the Kendall tau-b rank correlation were used as appropriate. If there were small sample sizes within some groups, then exact significant levels were assessed. Statistical significance was set by convention at P < .05.
Results
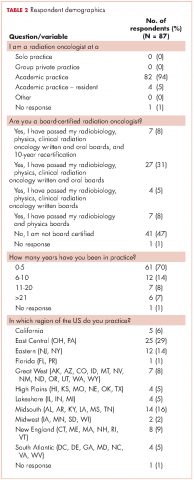
We received 95 responses of which 87 were considered complete for analysis. Forty-seven percent of the 87 respondents were not board-certified, and the remainder had passed their radiobiology and physics boards exams. A majority of respondents (70%, 61 of 87) were physicians who had been in practice for ≤5 years. Fifty-four percent of respondents were located in the Northeast US, 22% in the South, 14% in the West, and 10% in the Midwest and Hawaii (Table 2).
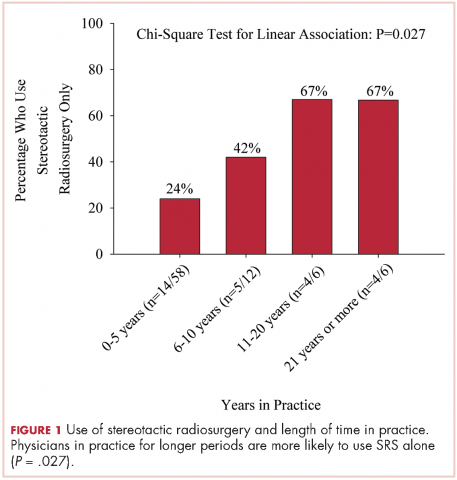
We used the chi-square test for linear trends to assess for a relationship between years of practice and whether respondents deviated from their typical method of WBRT therapy when treating more radioresistant tumors (melanoma, renal cell carcinoma). Respondents were classified by years in practice: 0-5, 6-10, 11-20, and >21 years. The results showed a linear association, with those in practice for longer periods more likely to use SRS alone, P = .027 (Figure 1).
Discussion
The incidence of brain metastases is increasing because of improvements in diagnostic imaging techniques and advancements in systemic therapy control of extracranial disease but not of intracranial disease or metastasis, because therapies do not cross the blood-brain barrier.11,12 Brain metastases are the most common type of brain tumor. Given that most chemotherapeutic agents cannot cross the blood-brain barrier, radiotherapy is considered a means of treatment and of controlling brain metastases. Early data from the 1950s13 and 1960s14 have suggested clinical improvement with brain radiation, making radiotherapy the cornerstone for treatment of brain metastases.
The Radiation Therapy Oncology Group (RTOG) has evaluated several fractionation schedules, with 5 schemas evaluated by the RTOG 6901 and 7361 studies: 30 Gy in 10 fractions, 30 Gy in 15 fractions, 40 Gy in 15 fractions, 40 Gy in 20 fractions, and 20 Gy in 5 fractions. The combined results from these two trials showed that outcomes were similar for patients treated with a shorter regimen than for those treated with a more protracted schedule. In our study, respondents reported that they most frequently treated brain metastases to a total dose of 30 Gy in 10 fractions. Given the results of the aforementioned RTOG trials and practice patterns among academic physicians, we recommend all practitioners consider a shorter hypofractioned course when treating brain metastases with WBRT. This will also reduce delays for patients who are likely to benefit greatly from earlier enrollment into hospice care, because protracted radiation schedules typically are not covered while a patient is in hospice.
Pharmacologic management for patients with brain metastases is important for symptomatic improvement. Glucocorticoids are important for palliation of symptoms from edema and increased intracranial pressure.15 However, steroids have a multitude of side effects and their use in asymptomatic patients is unnecessary. Improvements in imaging and detection11 have allowed us to find smaller and asymptomatic brain tumors. In our survey, it was promising to see a change in former practice patterns, with only 8% of academic practitioners regularly prescribing steroids to all of their patients receiving whole-brain radiation.
Diminished cognitive function and short-term memory loss are troublesome side effects of WBRT. As cancer patients live longer, such cognitive dysfunction will become more than just a nuisance. The RTOG has investigated the use of prophylactic memantine for patients receiving whole-brain radiation to determine if it would aid in the preservation of cognition. It found that patients who received memantine did better and had delayed time to cognitive decline and a reduced rate of memory decline, executive function, and processing speed.16 In our study, about a third of practitioners prescribed memantine and it was reserved for patients who had an otherwise favorable prognosis.
The RTOG has also investigated adjusting treatment technique for patients who receive WBRT. RTOG 0933 was a phase 2 trial that evaluated hippocampal avoidance during deliverance of WBRT with intensity-modulated radiation therapy (IMRT). Results showed that avoiding the hippocampus during WBRT was associated with improved memory preservation and patient quality of life.17 In a survey of practicing radiation oncologists in the US, most reported that they did not use memantine or IMRT for hippocampal sparing when delivering whole-brain radiation.18 Given the positive results of RTOG 0933 and 0614, the NRG Oncology research organization is conducting a phase 3 randomized trial that compares memantine use for patients receiving whole-brain radiation with or without hippocampal sparing to determine if patients will have reduced cognitive decline. All patients receiving WBRT should be considered for enrolment on this trial if they are eligible.
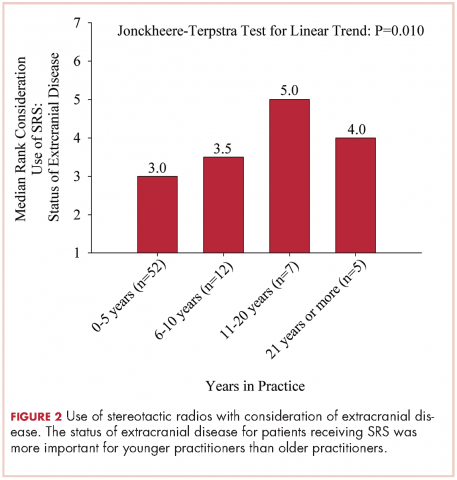
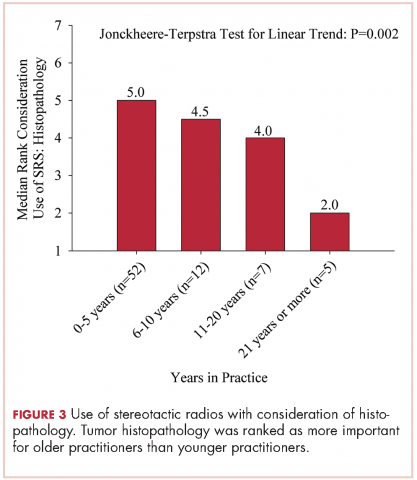
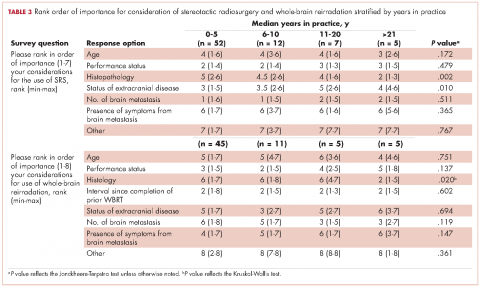
The delivery of brain radiation has continued to change, especially with the introduction of SRS. Recent publication of a meta-analysis of three phase 3 trials evaluating SRS with or without WBRT for 1-4 brain metastases showed that patients aged 50 years or younger experienced a survival benefit with SRS, and the omission of whole-brain radiation did not affect distant brain relapse rates. 19 The authors recommended that for this population, SRS alone is the preferred treatment. In our study, physicians who had been in practice for a longer time were more likely to treat using SRS alone. The results showed a linear association, with those in practice for a longer time being more likely to use SRS alone compared with those practicing for a shorter time (P = .027). Accordingly, 67% of respondents (8 of 12) who had been in practice for 11 or more years used SRS alone, whereas 24% (14 of 58) who had practiced for 0-5 years and 42% (5 of 12) who had practice from 6-10 years used SRS alone (Figure 1). When treating with SRS, younger practitioners placed more importance on the status of extracranial disease, whereas older practitioners placed more importance on tumor histopathology.
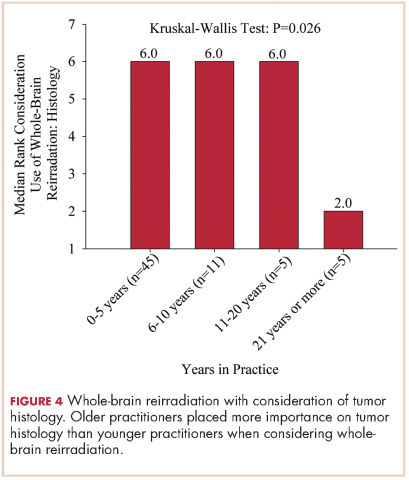
The use of repeat whole-brain reirradiation is more controversial among practitioners.20-22 Son and colleagues evaluated patients who needed whole-brain reirradiation after intracranial disease progression.22 The authors noted that patients with stable extracranial disease benefited from reirradiation. In our study, we found that when considering whole-brain reirradiation, older practitioners placed more importance on tumor histology than other factors.
As far as we know, this is the first study evaluating the practices and patterns of care with regard to the delivery of brain radiation in academic centers in the US. We found that time in practice was the most significant predictor of treatment technique and delivery. We also found that older practitioners place more importance on tumor histopathology compared with younger practitioners. A limitation of this study is that we had contact information only for program coordinators at ACGME-accredited programs. As such, we were not able to assess practice patterns among community practitioners. In addition, it seemed that residents and junior faculty were more likely to respond to this survey, likely because of the dissemination pattern. Given the evolution and diversity of treatment regimens for brain metastases, we believe that patients with brain metastases should be managed individually using a multidisciplinary approach.
1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872.
2. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705.
3. Takakura K. Metastatic tumors of the central nervous system. Tokyo: Igaku-Shoin; 1982.
4. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
5. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med. 1990;322(8):494-500.
6. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710-717.
7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
8. Choosing Wisely [ASTRO]. Don’t routinely add adjuvant whole-brain radiation therapy to stereotactic radiosurgery for limited brain metastases. http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-adjunct-whole-brain-radiation-therapy/. Updated June 21, 2016. Accessed November 10, 2016.
9. Aoyama H, Tago M, Shirato H, Japanese Radiation Oncology Study Group I. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464.
10. Hoddinott SN, Bass MJ. The Dillman total design survey method. Can Fam Physician. 1986;32:2366-2368.
11. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54.
12. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5-14.
13. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682-689.
14. Nieder C, Niewald M, Schnabel K. Treatment of brain metastases from hypernephroma. Urol Int. 1996;57(1):17-20.
15. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
16. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437.
17. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816.
18. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: The US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole-brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74-77.
19. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2015;91(4):710-717.
20. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988;15(2):433-437.
21. Sadikov E, Bezjak A, Yi QL, et al. Value of whole-brain re-irradiation for brain metastases — single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-538.
22. Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA. outcomes after whole-brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):e167-e172.
Despite the recent advances in systemic therapy, metastatic spread to the brain continues to be the most common neurologic complication of many cancers. The clinical incidence of brain metastases varies with primary cancer diagnosis, with estimates ranging from 1.2%-19.8%.1,2 Metastatic spread to the brain is even more prevalent at autopsy, with evidence of intracranial tumor being found in 26% of patients in some series.3 It is possible that the clinical incidence of metastatic disease to the brain will continue to increase as newer therapeutic agents improve survival and imaging techniques continue to improve.
The management of brain metastases has changed rapidly as technological improvements have made treatment increasingly safe and efficacious. Traditionally, treatment consisted of radiotherapy to the whole brain, with or without surgical resection.4,5 More recently, stereotactic radiosurgery (SRS) has been adopted on the basis of evidence that it is safe and efficacious alone or in combination with radiotherapy to the whole brain.6 Further evidence is emerging that neurocognitive outcomes are improved when whole-brain radiotherapy (WBRT) is omitted, which possibly contributes to improved patient quality of life.7 Taking into account this and other data, the American Society for Radiation Oncology’s Choosing Wisely campaign now recommends not routinely adding WBRT to radiosurgery in patients with limited brain metastases.8
Despite this recommendation, many patients continue to benefit from WBRT, and it remains a common treatment in radiation oncology clinics across the US for several reasons. Many patients present with multiple brain metastases and are ineligible for radiosurgery. Even for technically eligible patients, WBRT has been shown to improve local control and decrease the rate of distant brain failure over radiosurgery alone.6 With higher rates of subsequent failures, patients receiving radiosurgery alone must adhere to more rigorous follow-up and imaging schedules, which can be difficult for many rural patients who have to travel long distances to centers. Furthermore, there is some suggestion that this decreased failure rate may result in improved survival in highly selected patients with excellent disease and performance status.9 Controversies exist, however, and strong institutional biases persist, contributing to significant differences in practice. We surveyed academic radiation oncologists and in an effort to identify and describe practice patterns in the delivery of WBRT at academic centers.
Methods
We conducted a thorough review of available literature on radiation for brain metastases and based on our findings, devised a survey 19 questions to ascertain practice patterns and treatment delivery among US academic physicians (Table 1). After obtaining institutional review board approval to do the study, we sent the survey to program coordinators at radiation oncology programs that are accredited by the Accreditation Council for Graduate Medical Education. We instructed coordinators to e-mail the survey to their practicing resident and attending physicians. The surveys were created using SurveyMonkey software. We obtained informed consent from the providers. A total of 3 follow-up e-mails were sent to each recipient of the survey to solicit responses, similar to the Dillman Total Design Survey Method.10
SPSS version 22.0 was used to analyze the data in an exploratory fashion. Statistical methods were used to assess the association of demographic data with SRS and WBRT delivery and treatment technique items when the analyses involved percentages that included the Pearson chi-square statistic and the chi-square test for linear trend. When the analysis focused on ranking data, the Kruskal-Wallis test, Mann-Whitney U test, the Jonckheere-Terpstra and the Kendall tau-b rank correlation were used as appropriate. If there were small sample sizes within some groups, then exact significant levels were assessed. Statistical significance was set by convention at P < .05.
Results
We received 95 responses of which 87 were considered complete for analysis. Forty-seven percent of the 87 respondents were not board-certified, and the remainder had passed their radiobiology and physics boards exams. A majority of respondents (70%, 61 of 87) were physicians who had been in practice for ≤5 years. Fifty-four percent of respondents were located in the Northeast US, 22% in the South, 14% in the West, and 10% in the Midwest and Hawaii (Table 2).
We used the chi-square test for linear trends to assess for a relationship between years of practice and whether respondents deviated from their typical method of WBRT therapy when treating more radioresistant tumors (melanoma, renal cell carcinoma). Respondents were classified by years in practice: 0-5, 6-10, 11-20, and >21 years. The results showed a linear association, with those in practice for longer periods more likely to use SRS alone, P = .027 (Figure 1).
Discussion
The incidence of brain metastases is increasing because of improvements in diagnostic imaging techniques and advancements in systemic therapy control of extracranial disease but not of intracranial disease or metastasis, because therapies do not cross the blood-brain barrier.11,12 Brain metastases are the most common type of brain tumor. Given that most chemotherapeutic agents cannot cross the blood-brain barrier, radiotherapy is considered a means of treatment and of controlling brain metastases. Early data from the 1950s13 and 1960s14 have suggested clinical improvement with brain radiation, making radiotherapy the cornerstone for treatment of brain metastases.
The Radiation Therapy Oncology Group (RTOG) has evaluated several fractionation schedules, with 5 schemas evaluated by the RTOG 6901 and 7361 studies: 30 Gy in 10 fractions, 30 Gy in 15 fractions, 40 Gy in 15 fractions, 40 Gy in 20 fractions, and 20 Gy in 5 fractions. The combined results from these two trials showed that outcomes were similar for patients treated with a shorter regimen than for those treated with a more protracted schedule. In our study, respondents reported that they most frequently treated brain metastases to a total dose of 30 Gy in 10 fractions. Given the results of the aforementioned RTOG trials and practice patterns among academic physicians, we recommend all practitioners consider a shorter hypofractioned course when treating brain metastases with WBRT. This will also reduce delays for patients who are likely to benefit greatly from earlier enrollment into hospice care, because protracted radiation schedules typically are not covered while a patient is in hospice.
Pharmacologic management for patients with brain metastases is important for symptomatic improvement. Glucocorticoids are important for palliation of symptoms from edema and increased intracranial pressure.15 However, steroids have a multitude of side effects and their use in asymptomatic patients is unnecessary. Improvements in imaging and detection11 have allowed us to find smaller and asymptomatic brain tumors. In our survey, it was promising to see a change in former practice patterns, with only 8% of academic practitioners regularly prescribing steroids to all of their patients receiving whole-brain radiation.
Diminished cognitive function and short-term memory loss are troublesome side effects of WBRT. As cancer patients live longer, such cognitive dysfunction will become more than just a nuisance. The RTOG has investigated the use of prophylactic memantine for patients receiving whole-brain radiation to determine if it would aid in the preservation of cognition. It found that patients who received memantine did better and had delayed time to cognitive decline and a reduced rate of memory decline, executive function, and processing speed.16 In our study, about a third of practitioners prescribed memantine and it was reserved for patients who had an otherwise favorable prognosis.
The RTOG has also investigated adjusting treatment technique for patients who receive WBRT. RTOG 0933 was a phase 2 trial that evaluated hippocampal avoidance during deliverance of WBRT with intensity-modulated radiation therapy (IMRT). Results showed that avoiding the hippocampus during WBRT was associated with improved memory preservation and patient quality of life.17 In a survey of practicing radiation oncologists in the US, most reported that they did not use memantine or IMRT for hippocampal sparing when delivering whole-brain radiation.18 Given the positive results of RTOG 0933 and 0614, the NRG Oncology research organization is conducting a phase 3 randomized trial that compares memantine use for patients receiving whole-brain radiation with or without hippocampal sparing to determine if patients will have reduced cognitive decline. All patients receiving WBRT should be considered for enrolment on this trial if they are eligible.
The delivery of brain radiation has continued to change, especially with the introduction of SRS. Recent publication of a meta-analysis of three phase 3 trials evaluating SRS with or without WBRT for 1-4 brain metastases showed that patients aged 50 years or younger experienced a survival benefit with SRS, and the omission of whole-brain radiation did not affect distant brain relapse rates. 19 The authors recommended that for this population, SRS alone is the preferred treatment. In our study, physicians who had been in practice for a longer time were more likely to treat using SRS alone. The results showed a linear association, with those in practice for a longer time being more likely to use SRS alone compared with those practicing for a shorter time (P = .027). Accordingly, 67% of respondents (8 of 12) who had been in practice for 11 or more years used SRS alone, whereas 24% (14 of 58) who had practiced for 0-5 years and 42% (5 of 12) who had practice from 6-10 years used SRS alone (Figure 1). When treating with SRS, younger practitioners placed more importance on the status of extracranial disease, whereas older practitioners placed more importance on tumor histopathology.
The use of repeat whole-brain reirradiation is more controversial among practitioners.20-22 Son and colleagues evaluated patients who needed whole-brain reirradiation after intracranial disease progression.22 The authors noted that patients with stable extracranial disease benefited from reirradiation. In our study, we found that when considering whole-brain reirradiation, older practitioners placed more importance on tumor histology than other factors.
As far as we know, this is the first study evaluating the practices and patterns of care with regard to the delivery of brain radiation in academic centers in the US. We found that time in practice was the most significant predictor of treatment technique and delivery. We also found that older practitioners place more importance on tumor histopathology compared with younger practitioners. A limitation of this study is that we had contact information only for program coordinators at ACGME-accredited programs. As such, we were not able to assess practice patterns among community practitioners. In addition, it seemed that residents and junior faculty were more likely to respond to this survey, likely because of the dissemination pattern. Given the evolution and diversity of treatment regimens for brain metastases, we believe that patients with brain metastases should be managed individually using a multidisciplinary approach.
Despite the recent advances in systemic therapy, metastatic spread to the brain continues to be the most common neurologic complication of many cancers. The clinical incidence of brain metastases varies with primary cancer diagnosis, with estimates ranging from 1.2%-19.8%.1,2 Metastatic spread to the brain is even more prevalent at autopsy, with evidence of intracranial tumor being found in 26% of patients in some series.3 It is possible that the clinical incidence of metastatic disease to the brain will continue to increase as newer therapeutic agents improve survival and imaging techniques continue to improve.
The management of brain metastases has changed rapidly as technological improvements have made treatment increasingly safe and efficacious. Traditionally, treatment consisted of radiotherapy to the whole brain, with or without surgical resection.4,5 More recently, stereotactic radiosurgery (SRS) has been adopted on the basis of evidence that it is safe and efficacious alone or in combination with radiotherapy to the whole brain.6 Further evidence is emerging that neurocognitive outcomes are improved when whole-brain radiotherapy (WBRT) is omitted, which possibly contributes to improved patient quality of life.7 Taking into account this and other data, the American Society for Radiation Oncology’s Choosing Wisely campaign now recommends not routinely adding WBRT to radiosurgery in patients with limited brain metastases.8
Despite this recommendation, many patients continue to benefit from WBRT, and it remains a common treatment in radiation oncology clinics across the US for several reasons. Many patients present with multiple brain metastases and are ineligible for radiosurgery. Even for technically eligible patients, WBRT has been shown to improve local control and decrease the rate of distant brain failure over radiosurgery alone.6 With higher rates of subsequent failures, patients receiving radiosurgery alone must adhere to more rigorous follow-up and imaging schedules, which can be difficult for many rural patients who have to travel long distances to centers. Furthermore, there is some suggestion that this decreased failure rate may result in improved survival in highly selected patients with excellent disease and performance status.9 Controversies exist, however, and strong institutional biases persist, contributing to significant differences in practice. We surveyed academic radiation oncologists and in an effort to identify and describe practice patterns in the delivery of WBRT at academic centers.
Methods
We conducted a thorough review of available literature on radiation for brain metastases and based on our findings, devised a survey 19 questions to ascertain practice patterns and treatment delivery among US academic physicians (Table 1). After obtaining institutional review board approval to do the study, we sent the survey to program coordinators at radiation oncology programs that are accredited by the Accreditation Council for Graduate Medical Education. We instructed coordinators to e-mail the survey to their practicing resident and attending physicians. The surveys were created using SurveyMonkey software. We obtained informed consent from the providers. A total of 3 follow-up e-mails were sent to each recipient of the survey to solicit responses, similar to the Dillman Total Design Survey Method.10
SPSS version 22.0 was used to analyze the data in an exploratory fashion. Statistical methods were used to assess the association of demographic data with SRS and WBRT delivery and treatment technique items when the analyses involved percentages that included the Pearson chi-square statistic and the chi-square test for linear trend. When the analysis focused on ranking data, the Kruskal-Wallis test, Mann-Whitney U test, the Jonckheere-Terpstra and the Kendall tau-b rank correlation were used as appropriate. If there were small sample sizes within some groups, then exact significant levels were assessed. Statistical significance was set by convention at P < .05.
Results
We received 95 responses of which 87 were considered complete for analysis. Forty-seven percent of the 87 respondents were not board-certified, and the remainder had passed their radiobiology and physics boards exams. A majority of respondents (70%, 61 of 87) were physicians who had been in practice for ≤5 years. Fifty-four percent of respondents were located in the Northeast US, 22% in the South, 14% in the West, and 10% in the Midwest and Hawaii (Table 2).
We used the chi-square test for linear trends to assess for a relationship between years of practice and whether respondents deviated from their typical method of WBRT therapy when treating more radioresistant tumors (melanoma, renal cell carcinoma). Respondents were classified by years in practice: 0-5, 6-10, 11-20, and >21 years. The results showed a linear association, with those in practice for longer periods more likely to use SRS alone, P = .027 (Figure 1).
Discussion
The incidence of brain metastases is increasing because of improvements in diagnostic imaging techniques and advancements in systemic therapy control of extracranial disease but not of intracranial disease or metastasis, because therapies do not cross the blood-brain barrier.11,12 Brain metastases are the most common type of brain tumor. Given that most chemotherapeutic agents cannot cross the blood-brain barrier, radiotherapy is considered a means of treatment and of controlling brain metastases. Early data from the 1950s13 and 1960s14 have suggested clinical improvement with brain radiation, making radiotherapy the cornerstone for treatment of brain metastases.
The Radiation Therapy Oncology Group (RTOG) has evaluated several fractionation schedules, with 5 schemas evaluated by the RTOG 6901 and 7361 studies: 30 Gy in 10 fractions, 30 Gy in 15 fractions, 40 Gy in 15 fractions, 40 Gy in 20 fractions, and 20 Gy in 5 fractions. The combined results from these two trials showed that outcomes were similar for patients treated with a shorter regimen than for those treated with a more protracted schedule. In our study, respondents reported that they most frequently treated brain metastases to a total dose of 30 Gy in 10 fractions. Given the results of the aforementioned RTOG trials and practice patterns among academic physicians, we recommend all practitioners consider a shorter hypofractioned course when treating brain metastases with WBRT. This will also reduce delays for patients who are likely to benefit greatly from earlier enrollment into hospice care, because protracted radiation schedules typically are not covered while a patient is in hospice.
Pharmacologic management for patients with brain metastases is important for symptomatic improvement. Glucocorticoids are important for palliation of symptoms from edema and increased intracranial pressure.15 However, steroids have a multitude of side effects and their use in asymptomatic patients is unnecessary. Improvements in imaging and detection11 have allowed us to find smaller and asymptomatic brain tumors. In our survey, it was promising to see a change in former practice patterns, with only 8% of academic practitioners regularly prescribing steroids to all of their patients receiving whole-brain radiation.
Diminished cognitive function and short-term memory loss are troublesome side effects of WBRT. As cancer patients live longer, such cognitive dysfunction will become more than just a nuisance. The RTOG has investigated the use of prophylactic memantine for patients receiving whole-brain radiation to determine if it would aid in the preservation of cognition. It found that patients who received memantine did better and had delayed time to cognitive decline and a reduced rate of memory decline, executive function, and processing speed.16 In our study, about a third of practitioners prescribed memantine and it was reserved for patients who had an otherwise favorable prognosis.
The RTOG has also investigated adjusting treatment technique for patients who receive WBRT. RTOG 0933 was a phase 2 trial that evaluated hippocampal avoidance during deliverance of WBRT with intensity-modulated radiation therapy (IMRT). Results showed that avoiding the hippocampus during WBRT was associated with improved memory preservation and patient quality of life.17 In a survey of practicing radiation oncologists in the US, most reported that they did not use memantine or IMRT for hippocampal sparing when delivering whole-brain radiation.18 Given the positive results of RTOG 0933 and 0614, the NRG Oncology research organization is conducting a phase 3 randomized trial that compares memantine use for patients receiving whole-brain radiation with or without hippocampal sparing to determine if patients will have reduced cognitive decline. All patients receiving WBRT should be considered for enrolment on this trial if they are eligible.
The delivery of brain radiation has continued to change, especially with the introduction of SRS. Recent publication of a meta-analysis of three phase 3 trials evaluating SRS with or without WBRT for 1-4 brain metastases showed that patients aged 50 years or younger experienced a survival benefit with SRS, and the omission of whole-brain radiation did not affect distant brain relapse rates. 19 The authors recommended that for this population, SRS alone is the preferred treatment. In our study, physicians who had been in practice for a longer time were more likely to treat using SRS alone. The results showed a linear association, with those in practice for a longer time being more likely to use SRS alone compared with those practicing for a shorter time (P = .027). Accordingly, 67% of respondents (8 of 12) who had been in practice for 11 or more years used SRS alone, whereas 24% (14 of 58) who had practiced for 0-5 years and 42% (5 of 12) who had practice from 6-10 years used SRS alone (Figure 1). When treating with SRS, younger practitioners placed more importance on the status of extracranial disease, whereas older practitioners placed more importance on tumor histopathology.
The use of repeat whole-brain reirradiation is more controversial among practitioners.20-22 Son and colleagues evaluated patients who needed whole-brain reirradiation after intracranial disease progression.22 The authors noted that patients with stable extracranial disease benefited from reirradiation. In our study, we found that when considering whole-brain reirradiation, older practitioners placed more importance on tumor histology than other factors.
As far as we know, this is the first study evaluating the practices and patterns of care with regard to the delivery of brain radiation in academic centers in the US. We found that time in practice was the most significant predictor of treatment technique and delivery. We also found that older practitioners place more importance on tumor histopathology compared with younger practitioners. A limitation of this study is that we had contact information only for program coordinators at ACGME-accredited programs. As such, we were not able to assess practice patterns among community practitioners. In addition, it seemed that residents and junior faculty were more likely to respond to this survey, likely because of the dissemination pattern. Given the evolution and diversity of treatment regimens for brain metastases, we believe that patients with brain metastases should be managed individually using a multidisciplinary approach.
1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872.
2. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705.
3. Takakura K. Metastatic tumors of the central nervous system. Tokyo: Igaku-Shoin; 1982.
4. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
5. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med. 1990;322(8):494-500.
6. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710-717.
7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
8. Choosing Wisely [ASTRO]. Don’t routinely add adjuvant whole-brain radiation therapy to stereotactic radiosurgery for limited brain metastases. http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-adjunct-whole-brain-radiation-therapy/. Updated June 21, 2016. Accessed November 10, 2016.
9. Aoyama H, Tago M, Shirato H, Japanese Radiation Oncology Study Group I. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464.
10. Hoddinott SN, Bass MJ. The Dillman total design survey method. Can Fam Physician. 1986;32:2366-2368.
11. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54.
12. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5-14.
13. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682-689.
14. Nieder C, Niewald M, Schnabel K. Treatment of brain metastases from hypernephroma. Urol Int. 1996;57(1):17-20.
15. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
16. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437.
17. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816.
18. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: The US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole-brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74-77.
19. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2015;91(4):710-717.
20. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988;15(2):433-437.
21. Sadikov E, Bezjak A, Yi QL, et al. Value of whole-brain re-irradiation for brain metastases — single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-538.
22. Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA. outcomes after whole-brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):e167-e172.
1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872.
2. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705.
3. Takakura K. Metastatic tumors of the central nervous system. Tokyo: Igaku-Shoin; 1982.
4. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280(17):1485-1489.
5. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New Engl J Med. 1990;322(8):494-500.
6. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91(4):710-717.
7. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
8. Choosing Wisely [ASTRO]. Don’t routinely add adjuvant whole-brain radiation therapy to stereotactic radiosurgery for limited brain metastases. http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-adjunct-whole-brain-radiation-therapy/. Updated June 21, 2016. Accessed November 10, 2016.
9. Aoyama H, Tago M, Shirato H, Japanese Radiation Oncology Study Group I. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol. 2015;1(4):457-464.
10. Hoddinott SN, Bass MJ. The Dillman total design survey method. Can Fam Physician. 1986;32:2366-2368.
11. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54.
12. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5-14.
13. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7(4):682-689.
14. Nieder C, Niewald M, Schnabel K. Treatment of brain metastases from hypernephroma. Urol Int. 1996;57(1):17-20.
15. Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):103-114.
16. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429-1437.
17. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810-3816.
18. Slade AN, Stanic S. The impact of RTOG 0614 and RTOG 0933 trials in routine clinical practice: The US Survey of Utilization of Memantine and IMRT planning for hippocampus sparing in patients receiving whole-brain radiotherapy for brain metastases. Contemp Clin Trials. 2016;47:74-77.
19. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. International journal of radiation oncology, biology, physics. 2015;91(4):710-717.
20. Hazuka MB, Kinzie JJ. Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys. 1988;15(2):433-437.
21. Sadikov E, Bezjak A, Yi QL, et al. Value of whole-brain re-irradiation for brain metastases — single centre experience. Clin Oncol (R Coll Radiol). 2007;19(7):532-538.
22. Son CH, Jimenez R, Niemierko A, Loeffler JS, Oh KS, Shih HA. outcomes after whole-brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(2):e167-e172.