User login
Progressive multifocal leukoencephalopathy (PML) is a rare, typically fatal, opportunistic infection caused by the JC virus (JCV). Formerly an example of neurologic arcana, PML became an important clinical concern when it developed in patients with human immunodeficiency virus (HIV) infection. More recently, PML has attracted the attention of rheumatologists following reports of its being associated with the use of targeted therapies such as natalizumab and rituximab.1
A recent survey of rheumatologists’ knowledge of and attitudes towards PML revealed that concerns over PML affect decisions on the use of biologic agents. Further, rheumatologists have important real and perceived learning gaps regarding PML; for example, 41% of those surveyed could not identify the diagnostic test of choice for PML.2
PML IN RHEUMATIC DISEASES
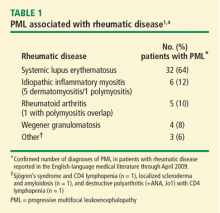
Examination of the immunosuppressive therapies prescribed to these patients within 6 months of the onset of neurologic symptoms attributed to PML revealed that low-dose (≤ 15 mg/d) prednisone, with or without an antimalarial agent, was the only immunosuppressive therapy in 31% of patients with SLE and in 11% of patients with rheumatic diseases other than SLE. Three patients had no documented immunosuppressive therapy in the 6 months prior to the onset of PML. Two patients with SLE were prescribed rituximab; no cases were reported in association with biologic therapies other than rituximab.4
In order to circumvent reporting bias, a nationwide hospital discharge database representing nearly 300 million patient discharges was used to determine the relative frequency of PML in patients with rheumatic diseases.5 Because of the reliance on diagnostic coding, rheumatic diseases were likely underreported in this sample; information on therapies was unavailable. After excluding patients who had HIV or cancer or were organ transplant recipients, four cases of PML were identified per 100,000 SLE discharges. This rate was 10-fold higher than the rate associated with rheumatoid arthritis and 20-fold higher than that of the background population.
These data show that PML is a rare occurrence in patients with rheumatic diseases, and SLE appears to be associated with a predisposition to PML. This predisposition in patients with SLE does not appear to be proportional to the degree of iatrogenic immunosuppression, emphasizing the role of host factors.
DISEASE-MODIFYING DRUGS AND PML RISK
In addition to certain disease states, disease-modifying biologic drugs have recently been associated with rare instances of PML.
Rituximab
The first case of rituximab-associated PML in the setting of rheumatoid arthritis was recorded in September 2008.6 The patient had longstanding rheumatoid arthritis and Sjögren syndrome. She received four courses of rituximab and was diagnosed with PML 18 months after the last dose; she died 1 month later. Her therapy for rheumatoid arthritis included a tumor necrosis factor (TNF) antagonist prior to rituximab initiation and treatment with methotrexate and steroids before, during, and after rituximab therapy. Oropharyngeal cancer developed in this patient 9 months prior to the onset of PML and was treated with chemotherapy and radiation therapy.
Another case of PML in a patient with rheumatic disease who had been treated with rituximab was notable because it was the first in which the patient had not previously been treated with an anti-TNF agent.7
Ascertaining cause of PML in patients treated with rituximab is difficult because the potential pathogenic mechanism remains unknown. Humoral immunity is not protective against PML, leading to speculation that the loss of other B-cell functions, such as those of antigen-presenting cells or cytokine production, may lead to a defect in cell-mediated immunity. Another theory posits that reconstitution of naïve B cells with latent JCV infection following B-cell depletion from rituximab therapy may somehow facilitate the development of PML.
Efalizumab
Efalizumab is a monoclonal antibody that targets CD11a, the alpha subunit of lymphocyte function–associated antigen 1. Efalizumab blocks binding to intercellular adhesion molecule 1, and thereby blocks T-cell adhesion and migration. CD11a is also expressed on a variety of other leukocytes and lymphocytes such as B cells, monocytes, and natural killer cells.
Efalizumab was approved in 2003 by the FDA for the treatment of moderate to severe plaque psoriasis. It is estimated that 46,000 patients have been treated with efalizumab worldwide since its approval. In 2008, a black box warning was added to the efalizumab prescribing information following the occurrence of serious infections, including pulmonary tuberculosis, necrotizing fasciitis, and invasive fungal infections.8 Subsequently, four cases of PML, three of which were fatal, were reported in psoriasis patients treated with efalizumab. Of note, these were the first cases of PML reported in patients with psoriasis. Of more concern, the affected patients were among a group of approximately 1,100 patients who had been treated with efalizumab for more than 3 years. In February 2009, a public health advisory was issued by the FDA,9 and efalizumab was voluntarily withdrawn by its manufacturer 2 months later.
Belatacept
Belatacept is a recombinant soluble fusion protein of the extracellular domain of human cytotoxic T-lymphocyte antigen-4 with a fragment of a modified Fc domain of immunoglobulin G1. Recently approved by the FDA for prophylaxis of renal transplant rejection, it is a second-generation, higher-avidity version of abatacept. Abatacept is licensed for the treatment of rheumatoid arthritis and is under investigation for the treatment of vasculitis and SLE. Belatacept differs from abatacept by only two amino acids.
Two cases of PML have been reported in association with belatacept, one in a patient following renal transplantation and the other in a patient following liver transplantation. Both patients had been treated with other standard immunosuppressive therapies for prophylaxis of organ transplant rejection, including mycophenolate mofetil.
Mycophenolate mofetil
Mycophenolate mofetil is the prodrug of mycophenolic acid. Both have been the subjects of FDA alerts regarding PML, based on a January 2008 report of 10 definite and 7 possible cases of PML occurring with mycophenolate mofetil. The patients affected included four with SLE, none of whom underwent a renal transplant.10
In a retrospective cohort study of 32,757 renal transplant patients, Neff et al11 found 14 cases of PML per 100,000 person-years among patients treated with mycophenolate mofetil following kidney transplant compared with none in patients who did not receive mycophenolate mofetil. It is difficult to ascertain risk with mycophenolate mofetil because it is standard therapy among renal transplant patients, leaving few patients in these groups unexposed.
Given the FDA alert with respect to mycophenolate mofetil and PML,10 the frequent use of mycophenolate mofetil in the setting of SLE, and the concerns about possible predisposition to PML among patients with SLE, it will be important to clarify the level of risk in patients with SLE who are treated with mycophenolate mofetil.
AGGREGATE EXPERIENCE: REVIEW OF FEDERAL DATABASE
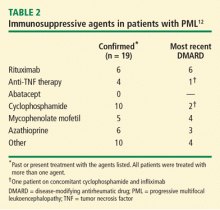
Ten cases of PML were confirmed with cyclophosphamide treatment, and cyclophosphamide was the most recent DMARD prescribed in two of these cases. Five cases were confirmed with mycophenolate mofetil (in four of which it was the most recently prescribed DMARD) and six with azathioprine (in three of which it was the most recently prescribed DMARD).
Risk of PML with DMARD therapy
Rituximab. The confirmation of six cases of PML among rituximab-treated rheumatoid arthritis patients is a source of concern. Nevertheless, PML is a rare adverse event. It occurs in fewer than 1 in 10,000 rituximab-treated patients who have rheumatoid arthritis, among a total of approximately 130,000 such patients. A better understanding of the potential mechanism responsible for the increased risk of developing PML may help in risk prediction and to guide patient selection for this agent.
Anti-TNF therapy. A paucity of confirmed cases in patients treated with anti-TNF therapy argues against a significant risk of PML associated with this therapy, especially considering the estimated 2 to 3 million rheumatoid arthritis patients who are receiving treatment with anti-TNF agents. A note of caution is sounded by a recent case report of PML in a rheumatoid arthritis patient. The patient had been treated with infliximab, with the only background therapy being methotrexate.13 Ongoing vigilance is therefore necessary.
Mycophenolate mofetil. All five confirmed cases of PML in mycophenolate mofetil-treated patients had earlier received treatment with cyclophosphamide. These data indicate no clear signal of excess risk with mycophenolate mofetil above that seen with other nonbiologic immunosuppressive agents, such as cyclophosphamide or azathioprine.
CONCLUSION AND RECOMMENDATIONS
PML has been reported in association with a variety of disease states, although a predisposition in patients with SLE has become apparent. Synthetic and biologic immunosuppressive therapies have also been implicated, but PML may also occur in the setting of minimal iatrogenic immunosuppression.
Until greater clarity can be achieved, all patients with systemic rheumatic diseases should be considered at risk for PML, regardless of the nature or intensity of their immunosuppressive therapy. In this context, differentiating PML from neurologic syndromes related to the underlying rheumatic disease (eg, neuropsychiatric SLE, cerebral vasculitis) is critical, particularly given the markedly different approaches to management.
PML should be considered in patients with unexplained subacute progressive focal and diffuse neurologic deficits, especially if their clinical or radiologic status worsens in the face of increased intensity of immunosuppressive therapy. Spinal cord or optic nerve involvement argues against PML. A normal magnetic resonance image (MRI) has a high negative predictive value, and frank infarction is not a feature of PML. In classic PML, contrast enhancement is typically absent and routine cerebrospinal fluid (CSF) analysis is typically normal. However, contrast enhancement and edema on MRI, lymphocytic CSF pleocytosis, and elevated CSF protein may be seen in the more recently described “inflammatory PML,” in which case the distinction from cerebral vasculitis or neuropsychiatric SLE may be more difficult. Angiography appears normal in patients with PML.
The diagnostic test of choice is a polymerase chain reaction (PCR) assay for JCV in CSF. If the PCR is repeatedly negative, then a brain biopsy should be considered, especially in the setting of progressive neurologic decline in patients receiving immunosuppressive therapy.
DISCUSSION
Dr. Simpson: To what extent are these lesions in the brain being attributed to the underlying vasculitis, particularly in SLE, as opposed to pursuing a PML diagnosis, and how might this result in dramatic underreporting of the complication?
Dr. Molloy: We found that PML is almost certainly underdiagnosed, particularly in SLE patients. If a patient succumbs to assumed neuropsychiatric SLE, how often is an autopsy undertaken? One telling paper from Sweden documented four cases of PML in SLE patients.14 In one of these, the diagnosis was made retrospectively from autopsy tissue that had been banked 20 years previously. It undoubtedly is underdiagnosed.
Dr. Calabrese: Even in the most recent rituximab-associated cases of PML, several patients were empirically given additional immunosuppressive therapy because it was presumed that they had a comorbid neuropsychiatric rheumatic complication. The presence of neuropsychiatric complications ascribed to an autoinflammatory disease generally warrants escalation of immunosuppressive therapy. It has always been standard practice for us to rule out opportunistic infection, but JCV infection has not been on the radar screen until very recently.
Dr. Molloy: I’d like to emphasize that, in our literature review, 50% of the rheumatic disease patients diagnosed with PML had been treated with more intensive immunosuppressive therapy. It was only after they continued to deteriorate that JCV infection was suspected and PML ultimately diagnosed.
Dr. Berger: Is it fair to say that the incidence of PML in SLE is about 10 times that in rheumatoid arthritis?
Dr. Molloy: In the hospital discharge database, it was 10-fold higher in SLE than in rheumatoid arthritis, but we can’t draw a conclusion from the AERS database because we don’t have a denominator. The database consists of voluntary submission of cases.
Dr. Calabrese: The information that we can expect to glean from the database is profoundly limited, for all the reasons that you enumerated. Despite the flaws, we’re obligated to continuously examine it because sometimes a case or two may provide some special insight.
Dr. Simpson: As neurologists, we often lag behind rheumatologists in the use of new treatments, including intravenous immune globulin (IVIG) and now rituximab. Rituximab is becoming the go-to drug for a number of neurologic diseases. I’m using it quite a bit and have observed some dramatic responses in patients with chronic inflammatory demyelinating polyneuropathy, for example, in whom IVIG or plasmapheresis was failing. Anecdotally, some of the turnarounds in polymyositis and even myasthenia gravis are remarkable as well. I’m not sure to what extent neurologists—particularly peripheral neurologists—who use rituximab are recognizing PML.
Dr. Fox: The index of suspicion is probably vastly different among multiple sclerosis (MS) specialists and general neurologists. Neurologists who treat MS will be acutely aware of PML because of its association with natalizumab.
Dr. Berger: Yes, but you’re talking about possibly two orders of magnitude difference between natalizumab and rituximab. In fact, PML is rarely reported in the setting of neurologic disease. It’s mostly reported in the setting of rheumatologic disease.
Dr. Rudick: I don’t necessarily agree with you. Ascertaining the true incidence of PML with agents other than natalizumab is difficult. One is unlikely to miss a case of PML in an MS patient treated with natalizumab, but most cases stemming from the use of these other disease-modifying drugs are probably being missed.
Dr. Calabrese: I get two messages out of this body of work. Number one is that while PML is rare, it is seen across the spectrum of immunosuppressive agents, including biologic and nonbiologic drugs. Number two is that PML is seriously underreported and underrecognized, which is probably leading to suboptimal patient care. Rituximab was recently approved for treatment of Wegener granulomatosis, and this disease is heavily pretreated with cyclophosphamide. You would expect that PML is on the radar among clinicians caring for patients whose diseases warrant the use of increasingly complex, potent, and novel immunosuppressives.
Dr. Berger: There is one other biologic agent you left out—alemtuzumab. It wipes out all of the B cells and T cells; the B cells repopulate but the T cells remain suppressed for a long period. If ever there was a drug whose action mirrors what happens in HIV, alemtuzumab is that drug. Yet, PML is rarely seen with alemtuzumab. Alemtuzumab-associated PML has not been reported in the MS population, and it has only been seen in two transplantations that I’m aware of. I’m not saying that it doesn’t occur, but we’re not seeing it with the same frequency that one would predict given its profile.
- Calabrese LH, Molloy ES, Huang D, Ransohoff RM. Progressive multifocal leukoencephalopathy in rheumatic diseases: evolving clinical and pathologic patterns of disease. Arthritis Rheum 2007; 6:2116–2128.
- Calabrese LH, Molloy ES, Taege AJ. Rheumatologists’ knowledge, attitudes and concerns regarding progressive multifocal encephalopathy (PML) [abstract]. Arthritis Rheum 2009; 60 (suppl 10):130.
- Rituxan (rituximab). U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150747.htm. Updated June 19, 2009. Accessed September 15, 2011.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun Rev 2008; 8:144–146.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy: a national estimate of frequency in systemic lupus erythematosus and other rheumatic diseases. Arthritis Rheum 2009; 60:3761–3765.
- Fleischmann RM. Progressive multifocal leukoencephalopathy following rituximab treatment in a patient with rheumatoid arthritis. Arthritis Rheum 2009; 60:3225–3228.
- Rituxan (rituximab) – PML. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm187791.htm. Updated October 23, 2009. Accessed September 15, 2011.
- Raptiva (efalizumab) October 2008. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm092089.htm. Updated June 19, 2009. Accessed September 15, 2011.
- Raptiva (efalizumab) Feb 2009. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm149675.htm. Updated June 16, 2009. Accessed September 15, 2011.
- Communication about an ongoing safety review of CellCept (mycophenolate mofetil) and Myfortic (mycophenolic acid). U.S. Food and Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm072438.htm. Updated October 30, 2009. Accessed September 15, 2011.
- Neff RT, Hurst FP, Falta EM, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation 2008; 86:1474–1478.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy and biologic therapy in rheumatic diseases: an analysis of the FDA AERS database [ACR abstract 700]. Arthritis Rheum 2010; 62 (suppl):S292.
- Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum 2010; 62:3191–3195.
- Nived O, Bengtsson AA, Jonsen A, Sturfelt G. Progressive multifocal leukoencephalopathy—the importance of early diagnosis illustrated in four cases. Lupus 2008; 17:1036–1041.
Progressive multifocal leukoencephalopathy (PML) is a rare, typically fatal, opportunistic infection caused by the JC virus (JCV). Formerly an example of neurologic arcana, PML became an important clinical concern when it developed in patients with human immunodeficiency virus (HIV) infection. More recently, PML has attracted the attention of rheumatologists following reports of its being associated with the use of targeted therapies such as natalizumab and rituximab.1
A recent survey of rheumatologists’ knowledge of and attitudes towards PML revealed that concerns over PML affect decisions on the use of biologic agents. Further, rheumatologists have important real and perceived learning gaps regarding PML; for example, 41% of those surveyed could not identify the diagnostic test of choice for PML.2
PML IN RHEUMATIC DISEASES
Examination of the immunosuppressive therapies prescribed to these patients within 6 months of the onset of neurologic symptoms attributed to PML revealed that low-dose (≤ 15 mg/d) prednisone, with or without an antimalarial agent, was the only immunosuppressive therapy in 31% of patients with SLE and in 11% of patients with rheumatic diseases other than SLE. Three patients had no documented immunosuppressive therapy in the 6 months prior to the onset of PML. Two patients with SLE were prescribed rituximab; no cases were reported in association with biologic therapies other than rituximab.4
In order to circumvent reporting bias, a nationwide hospital discharge database representing nearly 300 million patient discharges was used to determine the relative frequency of PML in patients with rheumatic diseases.5 Because of the reliance on diagnostic coding, rheumatic diseases were likely underreported in this sample; information on therapies was unavailable. After excluding patients who had HIV or cancer or were organ transplant recipients, four cases of PML were identified per 100,000 SLE discharges. This rate was 10-fold higher than the rate associated with rheumatoid arthritis and 20-fold higher than that of the background population.
These data show that PML is a rare occurrence in patients with rheumatic diseases, and SLE appears to be associated with a predisposition to PML. This predisposition in patients with SLE does not appear to be proportional to the degree of iatrogenic immunosuppression, emphasizing the role of host factors.
DISEASE-MODIFYING DRUGS AND PML RISK
In addition to certain disease states, disease-modifying biologic drugs have recently been associated with rare instances of PML.
Rituximab
The first case of rituximab-associated PML in the setting of rheumatoid arthritis was recorded in September 2008.6 The patient had longstanding rheumatoid arthritis and Sjögren syndrome. She received four courses of rituximab and was diagnosed with PML 18 months after the last dose; she died 1 month later. Her therapy for rheumatoid arthritis included a tumor necrosis factor (TNF) antagonist prior to rituximab initiation and treatment with methotrexate and steroids before, during, and after rituximab therapy. Oropharyngeal cancer developed in this patient 9 months prior to the onset of PML and was treated with chemotherapy and radiation therapy.
Another case of PML in a patient with rheumatic disease who had been treated with rituximab was notable because it was the first in which the patient had not previously been treated with an anti-TNF agent.7
Ascertaining cause of PML in patients treated with rituximab is difficult because the potential pathogenic mechanism remains unknown. Humoral immunity is not protective against PML, leading to speculation that the loss of other B-cell functions, such as those of antigen-presenting cells or cytokine production, may lead to a defect in cell-mediated immunity. Another theory posits that reconstitution of naïve B cells with latent JCV infection following B-cell depletion from rituximab therapy may somehow facilitate the development of PML.
Efalizumab
Efalizumab is a monoclonal antibody that targets CD11a, the alpha subunit of lymphocyte function–associated antigen 1. Efalizumab blocks binding to intercellular adhesion molecule 1, and thereby blocks T-cell adhesion and migration. CD11a is also expressed on a variety of other leukocytes and lymphocytes such as B cells, monocytes, and natural killer cells.
Efalizumab was approved in 2003 by the FDA for the treatment of moderate to severe plaque psoriasis. It is estimated that 46,000 patients have been treated with efalizumab worldwide since its approval. In 2008, a black box warning was added to the efalizumab prescribing information following the occurrence of serious infections, including pulmonary tuberculosis, necrotizing fasciitis, and invasive fungal infections.8 Subsequently, four cases of PML, three of which were fatal, were reported in psoriasis patients treated with efalizumab. Of note, these were the first cases of PML reported in patients with psoriasis. Of more concern, the affected patients were among a group of approximately 1,100 patients who had been treated with efalizumab for more than 3 years. In February 2009, a public health advisory was issued by the FDA,9 and efalizumab was voluntarily withdrawn by its manufacturer 2 months later.
Belatacept
Belatacept is a recombinant soluble fusion protein of the extracellular domain of human cytotoxic T-lymphocyte antigen-4 with a fragment of a modified Fc domain of immunoglobulin G1. Recently approved by the FDA for prophylaxis of renal transplant rejection, it is a second-generation, higher-avidity version of abatacept. Abatacept is licensed for the treatment of rheumatoid arthritis and is under investigation for the treatment of vasculitis and SLE. Belatacept differs from abatacept by only two amino acids.
Two cases of PML have been reported in association with belatacept, one in a patient following renal transplantation and the other in a patient following liver transplantation. Both patients had been treated with other standard immunosuppressive therapies for prophylaxis of organ transplant rejection, including mycophenolate mofetil.
Mycophenolate mofetil
Mycophenolate mofetil is the prodrug of mycophenolic acid. Both have been the subjects of FDA alerts regarding PML, based on a January 2008 report of 10 definite and 7 possible cases of PML occurring with mycophenolate mofetil. The patients affected included four with SLE, none of whom underwent a renal transplant.10
In a retrospective cohort study of 32,757 renal transplant patients, Neff et al11 found 14 cases of PML per 100,000 person-years among patients treated with mycophenolate mofetil following kidney transplant compared with none in patients who did not receive mycophenolate mofetil. It is difficult to ascertain risk with mycophenolate mofetil because it is standard therapy among renal transplant patients, leaving few patients in these groups unexposed.
Given the FDA alert with respect to mycophenolate mofetil and PML,10 the frequent use of mycophenolate mofetil in the setting of SLE, and the concerns about possible predisposition to PML among patients with SLE, it will be important to clarify the level of risk in patients with SLE who are treated with mycophenolate mofetil.
AGGREGATE EXPERIENCE: REVIEW OF FEDERAL DATABASE
Ten cases of PML were confirmed with cyclophosphamide treatment, and cyclophosphamide was the most recent DMARD prescribed in two of these cases. Five cases were confirmed with mycophenolate mofetil (in four of which it was the most recently prescribed DMARD) and six with azathioprine (in three of which it was the most recently prescribed DMARD).
Risk of PML with DMARD therapy
Rituximab. The confirmation of six cases of PML among rituximab-treated rheumatoid arthritis patients is a source of concern. Nevertheless, PML is a rare adverse event. It occurs in fewer than 1 in 10,000 rituximab-treated patients who have rheumatoid arthritis, among a total of approximately 130,000 such patients. A better understanding of the potential mechanism responsible for the increased risk of developing PML may help in risk prediction and to guide patient selection for this agent.
Anti-TNF therapy. A paucity of confirmed cases in patients treated with anti-TNF therapy argues against a significant risk of PML associated with this therapy, especially considering the estimated 2 to 3 million rheumatoid arthritis patients who are receiving treatment with anti-TNF agents. A note of caution is sounded by a recent case report of PML in a rheumatoid arthritis patient. The patient had been treated with infliximab, with the only background therapy being methotrexate.13 Ongoing vigilance is therefore necessary.
Mycophenolate mofetil. All five confirmed cases of PML in mycophenolate mofetil-treated patients had earlier received treatment with cyclophosphamide. These data indicate no clear signal of excess risk with mycophenolate mofetil above that seen with other nonbiologic immunosuppressive agents, such as cyclophosphamide or azathioprine.
CONCLUSION AND RECOMMENDATIONS
PML has been reported in association with a variety of disease states, although a predisposition in patients with SLE has become apparent. Synthetic and biologic immunosuppressive therapies have also been implicated, but PML may also occur in the setting of minimal iatrogenic immunosuppression.
Until greater clarity can be achieved, all patients with systemic rheumatic diseases should be considered at risk for PML, regardless of the nature or intensity of their immunosuppressive therapy. In this context, differentiating PML from neurologic syndromes related to the underlying rheumatic disease (eg, neuropsychiatric SLE, cerebral vasculitis) is critical, particularly given the markedly different approaches to management.
PML should be considered in patients with unexplained subacute progressive focal and diffuse neurologic deficits, especially if their clinical or radiologic status worsens in the face of increased intensity of immunosuppressive therapy. Spinal cord or optic nerve involvement argues against PML. A normal magnetic resonance image (MRI) has a high negative predictive value, and frank infarction is not a feature of PML. In classic PML, contrast enhancement is typically absent and routine cerebrospinal fluid (CSF) analysis is typically normal. However, contrast enhancement and edema on MRI, lymphocytic CSF pleocytosis, and elevated CSF protein may be seen in the more recently described “inflammatory PML,” in which case the distinction from cerebral vasculitis or neuropsychiatric SLE may be more difficult. Angiography appears normal in patients with PML.
The diagnostic test of choice is a polymerase chain reaction (PCR) assay for JCV in CSF. If the PCR is repeatedly negative, then a brain biopsy should be considered, especially in the setting of progressive neurologic decline in patients receiving immunosuppressive therapy.
DISCUSSION
Dr. Simpson: To what extent are these lesions in the brain being attributed to the underlying vasculitis, particularly in SLE, as opposed to pursuing a PML diagnosis, and how might this result in dramatic underreporting of the complication?
Dr. Molloy: We found that PML is almost certainly underdiagnosed, particularly in SLE patients. If a patient succumbs to assumed neuropsychiatric SLE, how often is an autopsy undertaken? One telling paper from Sweden documented four cases of PML in SLE patients.14 In one of these, the diagnosis was made retrospectively from autopsy tissue that had been banked 20 years previously. It undoubtedly is underdiagnosed.
Dr. Calabrese: Even in the most recent rituximab-associated cases of PML, several patients were empirically given additional immunosuppressive therapy because it was presumed that they had a comorbid neuropsychiatric rheumatic complication. The presence of neuropsychiatric complications ascribed to an autoinflammatory disease generally warrants escalation of immunosuppressive therapy. It has always been standard practice for us to rule out opportunistic infection, but JCV infection has not been on the radar screen until very recently.
Dr. Molloy: I’d like to emphasize that, in our literature review, 50% of the rheumatic disease patients diagnosed with PML had been treated with more intensive immunosuppressive therapy. It was only after they continued to deteriorate that JCV infection was suspected and PML ultimately diagnosed.
Dr. Berger: Is it fair to say that the incidence of PML in SLE is about 10 times that in rheumatoid arthritis?
Dr. Molloy: In the hospital discharge database, it was 10-fold higher in SLE than in rheumatoid arthritis, but we can’t draw a conclusion from the AERS database because we don’t have a denominator. The database consists of voluntary submission of cases.
Dr. Calabrese: The information that we can expect to glean from the database is profoundly limited, for all the reasons that you enumerated. Despite the flaws, we’re obligated to continuously examine it because sometimes a case or two may provide some special insight.
Dr. Simpson: As neurologists, we often lag behind rheumatologists in the use of new treatments, including intravenous immune globulin (IVIG) and now rituximab. Rituximab is becoming the go-to drug for a number of neurologic diseases. I’m using it quite a bit and have observed some dramatic responses in patients with chronic inflammatory demyelinating polyneuropathy, for example, in whom IVIG or plasmapheresis was failing. Anecdotally, some of the turnarounds in polymyositis and even myasthenia gravis are remarkable as well. I’m not sure to what extent neurologists—particularly peripheral neurologists—who use rituximab are recognizing PML.
Dr. Fox: The index of suspicion is probably vastly different among multiple sclerosis (MS) specialists and general neurologists. Neurologists who treat MS will be acutely aware of PML because of its association with natalizumab.
Dr. Berger: Yes, but you’re talking about possibly two orders of magnitude difference between natalizumab and rituximab. In fact, PML is rarely reported in the setting of neurologic disease. It’s mostly reported in the setting of rheumatologic disease.
Dr. Rudick: I don’t necessarily agree with you. Ascertaining the true incidence of PML with agents other than natalizumab is difficult. One is unlikely to miss a case of PML in an MS patient treated with natalizumab, but most cases stemming from the use of these other disease-modifying drugs are probably being missed.
Dr. Calabrese: I get two messages out of this body of work. Number one is that while PML is rare, it is seen across the spectrum of immunosuppressive agents, including biologic and nonbiologic drugs. Number two is that PML is seriously underreported and underrecognized, which is probably leading to suboptimal patient care. Rituximab was recently approved for treatment of Wegener granulomatosis, and this disease is heavily pretreated with cyclophosphamide. You would expect that PML is on the radar among clinicians caring for patients whose diseases warrant the use of increasingly complex, potent, and novel immunosuppressives.
Dr. Berger: There is one other biologic agent you left out—alemtuzumab. It wipes out all of the B cells and T cells; the B cells repopulate but the T cells remain suppressed for a long period. If ever there was a drug whose action mirrors what happens in HIV, alemtuzumab is that drug. Yet, PML is rarely seen with alemtuzumab. Alemtuzumab-associated PML has not been reported in the MS population, and it has only been seen in two transplantations that I’m aware of. I’m not saying that it doesn’t occur, but we’re not seeing it with the same frequency that one would predict given its profile.
Progressive multifocal leukoencephalopathy (PML) is a rare, typically fatal, opportunistic infection caused by the JC virus (JCV). Formerly an example of neurologic arcana, PML became an important clinical concern when it developed in patients with human immunodeficiency virus (HIV) infection. More recently, PML has attracted the attention of rheumatologists following reports of its being associated with the use of targeted therapies such as natalizumab and rituximab.1
A recent survey of rheumatologists’ knowledge of and attitudes towards PML revealed that concerns over PML affect decisions on the use of biologic agents. Further, rheumatologists have important real and perceived learning gaps regarding PML; for example, 41% of those surveyed could not identify the diagnostic test of choice for PML.2
PML IN RHEUMATIC DISEASES
Examination of the immunosuppressive therapies prescribed to these patients within 6 months of the onset of neurologic symptoms attributed to PML revealed that low-dose (≤ 15 mg/d) prednisone, with or without an antimalarial agent, was the only immunosuppressive therapy in 31% of patients with SLE and in 11% of patients with rheumatic diseases other than SLE. Three patients had no documented immunosuppressive therapy in the 6 months prior to the onset of PML. Two patients with SLE were prescribed rituximab; no cases were reported in association with biologic therapies other than rituximab.4
In order to circumvent reporting bias, a nationwide hospital discharge database representing nearly 300 million patient discharges was used to determine the relative frequency of PML in patients with rheumatic diseases.5 Because of the reliance on diagnostic coding, rheumatic diseases were likely underreported in this sample; information on therapies was unavailable. After excluding patients who had HIV or cancer or were organ transplant recipients, four cases of PML were identified per 100,000 SLE discharges. This rate was 10-fold higher than the rate associated with rheumatoid arthritis and 20-fold higher than that of the background population.
These data show that PML is a rare occurrence in patients with rheumatic diseases, and SLE appears to be associated with a predisposition to PML. This predisposition in patients with SLE does not appear to be proportional to the degree of iatrogenic immunosuppression, emphasizing the role of host factors.
DISEASE-MODIFYING DRUGS AND PML RISK
In addition to certain disease states, disease-modifying biologic drugs have recently been associated with rare instances of PML.
Rituximab
The first case of rituximab-associated PML in the setting of rheumatoid arthritis was recorded in September 2008.6 The patient had longstanding rheumatoid arthritis and Sjögren syndrome. She received four courses of rituximab and was diagnosed with PML 18 months after the last dose; she died 1 month later. Her therapy for rheumatoid arthritis included a tumor necrosis factor (TNF) antagonist prior to rituximab initiation and treatment with methotrexate and steroids before, during, and after rituximab therapy. Oropharyngeal cancer developed in this patient 9 months prior to the onset of PML and was treated with chemotherapy and radiation therapy.
Another case of PML in a patient with rheumatic disease who had been treated with rituximab was notable because it was the first in which the patient had not previously been treated with an anti-TNF agent.7
Ascertaining cause of PML in patients treated with rituximab is difficult because the potential pathogenic mechanism remains unknown. Humoral immunity is not protective against PML, leading to speculation that the loss of other B-cell functions, such as those of antigen-presenting cells or cytokine production, may lead to a defect in cell-mediated immunity. Another theory posits that reconstitution of naïve B cells with latent JCV infection following B-cell depletion from rituximab therapy may somehow facilitate the development of PML.
Efalizumab
Efalizumab is a monoclonal antibody that targets CD11a, the alpha subunit of lymphocyte function–associated antigen 1. Efalizumab blocks binding to intercellular adhesion molecule 1, and thereby blocks T-cell adhesion and migration. CD11a is also expressed on a variety of other leukocytes and lymphocytes such as B cells, monocytes, and natural killer cells.
Efalizumab was approved in 2003 by the FDA for the treatment of moderate to severe plaque psoriasis. It is estimated that 46,000 patients have been treated with efalizumab worldwide since its approval. In 2008, a black box warning was added to the efalizumab prescribing information following the occurrence of serious infections, including pulmonary tuberculosis, necrotizing fasciitis, and invasive fungal infections.8 Subsequently, four cases of PML, three of which were fatal, were reported in psoriasis patients treated with efalizumab. Of note, these were the first cases of PML reported in patients with psoriasis. Of more concern, the affected patients were among a group of approximately 1,100 patients who had been treated with efalizumab for more than 3 years. In February 2009, a public health advisory was issued by the FDA,9 and efalizumab was voluntarily withdrawn by its manufacturer 2 months later.
Belatacept
Belatacept is a recombinant soluble fusion protein of the extracellular domain of human cytotoxic T-lymphocyte antigen-4 with a fragment of a modified Fc domain of immunoglobulin G1. Recently approved by the FDA for prophylaxis of renal transplant rejection, it is a second-generation, higher-avidity version of abatacept. Abatacept is licensed for the treatment of rheumatoid arthritis and is under investigation for the treatment of vasculitis and SLE. Belatacept differs from abatacept by only two amino acids.
Two cases of PML have been reported in association with belatacept, one in a patient following renal transplantation and the other in a patient following liver transplantation. Both patients had been treated with other standard immunosuppressive therapies for prophylaxis of organ transplant rejection, including mycophenolate mofetil.
Mycophenolate mofetil
Mycophenolate mofetil is the prodrug of mycophenolic acid. Both have been the subjects of FDA alerts regarding PML, based on a January 2008 report of 10 definite and 7 possible cases of PML occurring with mycophenolate mofetil. The patients affected included four with SLE, none of whom underwent a renal transplant.10
In a retrospective cohort study of 32,757 renal transplant patients, Neff et al11 found 14 cases of PML per 100,000 person-years among patients treated with mycophenolate mofetil following kidney transplant compared with none in patients who did not receive mycophenolate mofetil. It is difficult to ascertain risk with mycophenolate mofetil because it is standard therapy among renal transplant patients, leaving few patients in these groups unexposed.
Given the FDA alert with respect to mycophenolate mofetil and PML,10 the frequent use of mycophenolate mofetil in the setting of SLE, and the concerns about possible predisposition to PML among patients with SLE, it will be important to clarify the level of risk in patients with SLE who are treated with mycophenolate mofetil.
AGGREGATE EXPERIENCE: REVIEW OF FEDERAL DATABASE
Ten cases of PML were confirmed with cyclophosphamide treatment, and cyclophosphamide was the most recent DMARD prescribed in two of these cases. Five cases were confirmed with mycophenolate mofetil (in four of which it was the most recently prescribed DMARD) and six with azathioprine (in three of which it was the most recently prescribed DMARD).
Risk of PML with DMARD therapy
Rituximab. The confirmation of six cases of PML among rituximab-treated rheumatoid arthritis patients is a source of concern. Nevertheless, PML is a rare adverse event. It occurs in fewer than 1 in 10,000 rituximab-treated patients who have rheumatoid arthritis, among a total of approximately 130,000 such patients. A better understanding of the potential mechanism responsible for the increased risk of developing PML may help in risk prediction and to guide patient selection for this agent.
Anti-TNF therapy. A paucity of confirmed cases in patients treated with anti-TNF therapy argues against a significant risk of PML associated with this therapy, especially considering the estimated 2 to 3 million rheumatoid arthritis patients who are receiving treatment with anti-TNF agents. A note of caution is sounded by a recent case report of PML in a rheumatoid arthritis patient. The patient had been treated with infliximab, with the only background therapy being methotrexate.13 Ongoing vigilance is therefore necessary.
Mycophenolate mofetil. All five confirmed cases of PML in mycophenolate mofetil-treated patients had earlier received treatment with cyclophosphamide. These data indicate no clear signal of excess risk with mycophenolate mofetil above that seen with other nonbiologic immunosuppressive agents, such as cyclophosphamide or azathioprine.
CONCLUSION AND RECOMMENDATIONS
PML has been reported in association with a variety of disease states, although a predisposition in patients with SLE has become apparent. Synthetic and biologic immunosuppressive therapies have also been implicated, but PML may also occur in the setting of minimal iatrogenic immunosuppression.
Until greater clarity can be achieved, all patients with systemic rheumatic diseases should be considered at risk for PML, regardless of the nature or intensity of their immunosuppressive therapy. In this context, differentiating PML from neurologic syndromes related to the underlying rheumatic disease (eg, neuropsychiatric SLE, cerebral vasculitis) is critical, particularly given the markedly different approaches to management.
PML should be considered in patients with unexplained subacute progressive focal and diffuse neurologic deficits, especially if their clinical or radiologic status worsens in the face of increased intensity of immunosuppressive therapy. Spinal cord or optic nerve involvement argues against PML. A normal magnetic resonance image (MRI) has a high negative predictive value, and frank infarction is not a feature of PML. In classic PML, contrast enhancement is typically absent and routine cerebrospinal fluid (CSF) analysis is typically normal. However, contrast enhancement and edema on MRI, lymphocytic CSF pleocytosis, and elevated CSF protein may be seen in the more recently described “inflammatory PML,” in which case the distinction from cerebral vasculitis or neuropsychiatric SLE may be more difficult. Angiography appears normal in patients with PML.
The diagnostic test of choice is a polymerase chain reaction (PCR) assay for JCV in CSF. If the PCR is repeatedly negative, then a brain biopsy should be considered, especially in the setting of progressive neurologic decline in patients receiving immunosuppressive therapy.
DISCUSSION
Dr. Simpson: To what extent are these lesions in the brain being attributed to the underlying vasculitis, particularly in SLE, as opposed to pursuing a PML diagnosis, and how might this result in dramatic underreporting of the complication?
Dr. Molloy: We found that PML is almost certainly underdiagnosed, particularly in SLE patients. If a patient succumbs to assumed neuropsychiatric SLE, how often is an autopsy undertaken? One telling paper from Sweden documented four cases of PML in SLE patients.14 In one of these, the diagnosis was made retrospectively from autopsy tissue that had been banked 20 years previously. It undoubtedly is underdiagnosed.
Dr. Calabrese: Even in the most recent rituximab-associated cases of PML, several patients were empirically given additional immunosuppressive therapy because it was presumed that they had a comorbid neuropsychiatric rheumatic complication. The presence of neuropsychiatric complications ascribed to an autoinflammatory disease generally warrants escalation of immunosuppressive therapy. It has always been standard practice for us to rule out opportunistic infection, but JCV infection has not been on the radar screen until very recently.
Dr. Molloy: I’d like to emphasize that, in our literature review, 50% of the rheumatic disease patients diagnosed with PML had been treated with more intensive immunosuppressive therapy. It was only after they continued to deteriorate that JCV infection was suspected and PML ultimately diagnosed.
Dr. Berger: Is it fair to say that the incidence of PML in SLE is about 10 times that in rheumatoid arthritis?
Dr. Molloy: In the hospital discharge database, it was 10-fold higher in SLE than in rheumatoid arthritis, but we can’t draw a conclusion from the AERS database because we don’t have a denominator. The database consists of voluntary submission of cases.
Dr. Calabrese: The information that we can expect to glean from the database is profoundly limited, for all the reasons that you enumerated. Despite the flaws, we’re obligated to continuously examine it because sometimes a case or two may provide some special insight.
Dr. Simpson: As neurologists, we often lag behind rheumatologists in the use of new treatments, including intravenous immune globulin (IVIG) and now rituximab. Rituximab is becoming the go-to drug for a number of neurologic diseases. I’m using it quite a bit and have observed some dramatic responses in patients with chronic inflammatory demyelinating polyneuropathy, for example, in whom IVIG or plasmapheresis was failing. Anecdotally, some of the turnarounds in polymyositis and even myasthenia gravis are remarkable as well. I’m not sure to what extent neurologists—particularly peripheral neurologists—who use rituximab are recognizing PML.
Dr. Fox: The index of suspicion is probably vastly different among multiple sclerosis (MS) specialists and general neurologists. Neurologists who treat MS will be acutely aware of PML because of its association with natalizumab.
Dr. Berger: Yes, but you’re talking about possibly two orders of magnitude difference between natalizumab and rituximab. In fact, PML is rarely reported in the setting of neurologic disease. It’s mostly reported in the setting of rheumatologic disease.
Dr. Rudick: I don’t necessarily agree with you. Ascertaining the true incidence of PML with agents other than natalizumab is difficult. One is unlikely to miss a case of PML in an MS patient treated with natalizumab, but most cases stemming from the use of these other disease-modifying drugs are probably being missed.
Dr. Calabrese: I get two messages out of this body of work. Number one is that while PML is rare, it is seen across the spectrum of immunosuppressive agents, including biologic and nonbiologic drugs. Number two is that PML is seriously underreported and underrecognized, which is probably leading to suboptimal patient care. Rituximab was recently approved for treatment of Wegener granulomatosis, and this disease is heavily pretreated with cyclophosphamide. You would expect that PML is on the radar among clinicians caring for patients whose diseases warrant the use of increasingly complex, potent, and novel immunosuppressives.
Dr. Berger: There is one other biologic agent you left out—alemtuzumab. It wipes out all of the B cells and T cells; the B cells repopulate but the T cells remain suppressed for a long period. If ever there was a drug whose action mirrors what happens in HIV, alemtuzumab is that drug. Yet, PML is rarely seen with alemtuzumab. Alemtuzumab-associated PML has not been reported in the MS population, and it has only been seen in two transplantations that I’m aware of. I’m not saying that it doesn’t occur, but we’re not seeing it with the same frequency that one would predict given its profile.
- Calabrese LH, Molloy ES, Huang D, Ransohoff RM. Progressive multifocal leukoencephalopathy in rheumatic diseases: evolving clinical and pathologic patterns of disease. Arthritis Rheum 2007; 6:2116–2128.
- Calabrese LH, Molloy ES, Taege AJ. Rheumatologists’ knowledge, attitudes and concerns regarding progressive multifocal encephalopathy (PML) [abstract]. Arthritis Rheum 2009; 60 (suppl 10):130.
- Rituxan (rituximab). U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150747.htm. Updated June 19, 2009. Accessed September 15, 2011.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun Rev 2008; 8:144–146.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy: a national estimate of frequency in systemic lupus erythematosus and other rheumatic diseases. Arthritis Rheum 2009; 60:3761–3765.
- Fleischmann RM. Progressive multifocal leukoencephalopathy following rituximab treatment in a patient with rheumatoid arthritis. Arthritis Rheum 2009; 60:3225–3228.
- Rituxan (rituximab) – PML. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm187791.htm. Updated October 23, 2009. Accessed September 15, 2011.
- Raptiva (efalizumab) October 2008. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm092089.htm. Updated June 19, 2009. Accessed September 15, 2011.
- Raptiva (efalizumab) Feb 2009. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm149675.htm. Updated June 16, 2009. Accessed September 15, 2011.
- Communication about an ongoing safety review of CellCept (mycophenolate mofetil) and Myfortic (mycophenolic acid). U.S. Food and Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm072438.htm. Updated October 30, 2009. Accessed September 15, 2011.
- Neff RT, Hurst FP, Falta EM, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation 2008; 86:1474–1478.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy and biologic therapy in rheumatic diseases: an analysis of the FDA AERS database [ACR abstract 700]. Arthritis Rheum 2010; 62 (suppl):S292.
- Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum 2010; 62:3191–3195.
- Nived O, Bengtsson AA, Jonsen A, Sturfelt G. Progressive multifocal leukoencephalopathy—the importance of early diagnosis illustrated in four cases. Lupus 2008; 17:1036–1041.
- Calabrese LH, Molloy ES, Huang D, Ransohoff RM. Progressive multifocal leukoencephalopathy in rheumatic diseases: evolving clinical and pathologic patterns of disease. Arthritis Rheum 2007; 6:2116–2128.
- Calabrese LH, Molloy ES, Taege AJ. Rheumatologists’ knowledge, attitudes and concerns regarding progressive multifocal encephalopathy (PML) [abstract]. Arthritis Rheum 2009; 60 (suppl 10):130.
- Rituxan (rituximab). U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm150747.htm. Updated June 19, 2009. Accessed September 15, 2011.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun Rev 2008; 8:144–146.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy: a national estimate of frequency in systemic lupus erythematosus and other rheumatic diseases. Arthritis Rheum 2009; 60:3761–3765.
- Fleischmann RM. Progressive multifocal leukoencephalopathy following rituximab treatment in a patient with rheumatoid arthritis. Arthritis Rheum 2009; 60:3225–3228.
- Rituxan (rituximab) – PML. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm187791.htm. Updated October 23, 2009. Accessed September 15, 2011.
- Raptiva (efalizumab) October 2008. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm092089.htm. Updated June 19, 2009. Accessed September 15, 2011.
- Raptiva (efalizumab) Feb 2009. U.S. Food and Drug Administration Web site. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm149675.htm. Updated June 16, 2009. Accessed September 15, 2011.
- Communication about an ongoing safety review of CellCept (mycophenolate mofetil) and Myfortic (mycophenolic acid). U.S. Food and Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm072438.htm. Updated October 30, 2009. Accessed September 15, 2011.
- Neff RT, Hurst FP, Falta EM, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation 2008; 86:1474–1478.
- Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy and biologic therapy in rheumatic diseases: an analysis of the FDA AERS database [ACR abstract 700]. Arthritis Rheum 2010; 62 (suppl):S292.
- Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis Rheum 2010; 62:3191–3195.
- Nived O, Bengtsson AA, Jonsen A, Sturfelt G. Progressive multifocal leukoencephalopathy—the importance of early diagnosis illustrated in four cases. Lupus 2008; 17:1036–1041.