User login
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
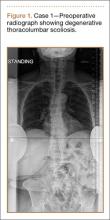
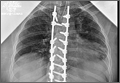
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
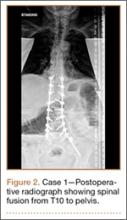
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
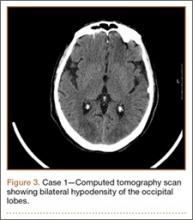
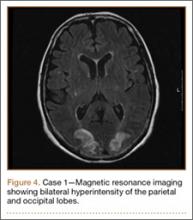
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
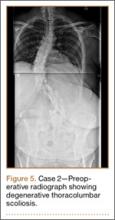
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
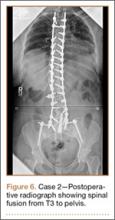
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
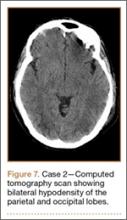
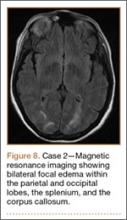
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
First described in 1996, posterior reversible encephalopathy syndrome (PRES) exhibits a wide clinical spectrum and is definitively diagnosed through computed tomography (CT) and/or magnetic resonance imaging (MRI) studies of the brain.1 Clinical presentation may include a spectrum of symptoms, including nausea, emesis, seizures, visual loss, paralysis, and headaches.2,3 The most common imaging finding of PRES is bilateral foci of vasogenic edema located in the parieto-occipital white matter.2-6 Other areas of the brain are frequently affected as well, with the frontal and temporal lobes and the basal or cortical ganglia showing signs of distinctly noncytotoxic edema in 12.5% to 54.2% of all cases.3 With the symptom of visual loss being present in 20% to 62.5% of patients with PRES, the syndrome constitutes a rare potential cause for postoperative visual loss (POVL) after spinal surgery, which has a generally good prognosis because most patients will completely regain their eyesight.2,3
We present a unique account of 2 patients who underwent extensive spinal surgery and received a timely diagnosis and treatment of PRES at a single institution. We aim to elucidate the difference in clinical and radiographic presentation of PRES in relation to other known causes of POVL after spinal surgery. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
Clinical Presentation. A 78-year-old woman presented to the outpatient clinic with disability due to severe lower back pain. Her surgical history was significant for breast lumpectomy and cataract excision. Her medical history was significant for hypertension, obesity (body mass index, 31.5), hypercholesterolemia, emphysema, and anemia. She had undergone spinal surgery, specifically laminectomies from L2 to S1. The radiographic examination showed degenerative thoracolumbar scoliosis with severe spondylosis, disc space collapse, and ankylosis of L4-L5 (Figure 1).
Operative Procedure. The patient underwent transpsoas lumbar interbody fusion (XLIF, NuVasive) from L1 to L4 and posterior spinal fusion from T10 to pelvis (Expedium, Depuy Synthes) (Figure 2). Operative time was 553 minutes; estimated blood loss was 2000 mL due to intraoperative coagulopathy (platelets, 40,000/µL) near the end of the posterior portion of the procedure. Intraoperative hypotension was treated by volume resuscitation and transient use of vasopressor agents. She was transfused with 1700 mL of blood, 150 mL of saline solution, and 420 mL of Lactated Ringer’s solution. No intraoperative complications occurred. The patient was extubated uneventfully on postoperative day 1 and was at baseline neurologically with no visual disturbances.
Development and Diagnosis of PRES. The patient made significant progress with physical therapy and developed episodes of hypertension at night on postoperative days 4 to 6. Her mean peak systolic blood pressure was 180 mm Hg. This improved after oral beta-blocker therapy. On postoperative day 6, the patient was ambulating with physical therapy and the aid of a walker. She was found to be neurologically intact, was resting comfortable in a chair reading a book, and was cleared for transfer to a rehabilitation facility the next day. During the morning on postoperative day 7, she developed confusion and visual loss. The patient reported blurry vision followed by complete bilateral painless loss of vision aside from mild light perception. She was unable to identify any objects. She had extinction to double simultaneous stimuli and evidence of agraphesthesia in the left hand. Her neurologic examination was otherwise at baseline. Upon emergent imaging, head CT showed bilateral symmetric areas of hypodensity involving the cortical and subcortical white matter of both occipital lobes (Figure 3). MRI showed extensive bilateral cortical and subcortical signal hyperintensity involving the parietal and occipital lobes (Figure 4). No evidence of petechial or lobar hemorrhage was found.
Treatment and Clinical Course. The patient was transferred to the neurology intensive care unit for neurologic monitoring. She was treated aggressively for recurrent hypertensive episodes. Twenty-four hours after initial blood pressure optimization therapy, she partially recovered her eyesight. She exhibited complete recovery after 48 hours. The patient was discharged to a rehabilitation facility in stable condition on postoperative day 11.
Case 2
Clinical Presentation. A 51-year-old woman presented to the outpatient clinic with progressive low back pain and decompensation due to degenerative adult scoliosis. Her surgical history was significant for an uneventful Caesarean section. Her medical history was significant for borderline hypertension and obesity (body mass index, 34.4). The radiographic examination showed an S-shaped thoracolumbar curve from T4 to L4 (Figure 5).
Operative Procedure. After discussions about the risks and benefits of the procedure, the patient underwent posterior spinal fusion from T3 to pelvis (Mesa, K2M) and interbody fusion from L4 to S1 via a presacral approach using the AxiaLIF system (TranS1) (Figure 6). The operation spanned 507 minutes. The patient lost approximately 2200 mL of blood. She was transfused with 1690 mL of blood, 1250 mL of Lactated Ringer’s solution, and 1 unit (50 mL) of albumin. No intraoperative complications occurred.
Development and Diagnosis of PRES. The patient was ambulatory with physical therapy and a walker on postoperative day 1. Her albumin levels were noted to be decreased postoperatively (28 mg/mL; normal, >35 mg/mL). She developed intermittent hypertensive episodes and experienced transient peripheral vision loss. After her ophthalmologic symptoms cleared, she was discharged and transferred to a rehabilitation facility on postoperative day 9. Eleven days later, the patient was emergently readmitted for a deep spine wound infection after an onset of wound swelling and fever. She underwent irrigation and débridement of the spine wound with an estimated blood loss of 400 mL. The patient continued to have fevers and was placed on ciprofloxacin and vancomycin, which was changed to levofloxacin on postoperative day 5. Elevated creatinine was noted, and the patient was diagnosed with acute renal failure. On postoperative day 7, oxacillin therapy was commenced. After her cultures grew methicillin-resistant Staphylococcus aureus, a peripherally inserted central catheter line was placed on postoperative day 9. As a result of nausea and constipation, the patient received feeding tubes on postoperative day 11. Additionally, she was diagnosed with a pleural effusion on postoperative day 14. Although her creatinine levels were decreasing, she continued to experience intermittent hypertensive episodes with a mean peak systolic blood pressure of 148 mm Hg. On postoperative day 15, she had a seizure and again developed visual loss. The patient was lethargic and followed only simple commands. She moved all extremities and withdrew symmetrically to noxious stimuli. Upon emergent imaging, head CT showed posterior subcortical white matter hypodensity within the occipital and parietal lobes bilaterally (Figure 7). MRI showed focal regions of symmetric hemispheric edema involving the parietal and occipital lobes in a predominantly subcortical white-matter distribution. Additionally, extensive involvement of the splenium and of the corpus callosum, left greater than right, was observed (Figure 8).
Treatment and Clinical Course. The patient was transferred to the intensive care unit for neuromonitoring. Her hypokalemia and hypertension were treated aggressively to normalize her potassium levels and blood pressure. Her oxacillin therapy was changed to daptomycin. On postoperative day 17, the patient was transferred to another institution for further medical management after achieving full recovery of her eyesight after electrolyte and blood pressure corrections.
Discussion
Posterior reversible encephalopathy syndrome is a rare but frequently devastating complication of spinal surgery, with an estimated incidence of 0.094% to 0.2%.7,8 Pediatric patients, as well as patients undergoing deformity correction surgery and posterior lumbar fusion, which necessitate prone positioning, have a significantly increased risk of POVL after spinal surgery.9 There are several causes of POVL after spinal surgery, each with a unique pathophysiology, clinical presentation, and prognosis.
The most common cause of POVL, accounting for 89% of all cases, is ischemic neuropathy.10 Ischemic neuropathy refers to a hypoperfusion or infarction of the anterior or posterior portion of the optic nerve and presents as painless bilateral vision loss or complete blindness on waking from the surgical procedure.11 Risk factors associated with anterior ischemic neuropathy are primarily diabetes mellitus, prone positioning, nocturnal hypotension, and blood loss.11 Posterior ischemic neuropathy has been most strongly correlated with anemia and hypotension.12 The exact etiology of this complication has not been established, although the prognosis is generally unfavorable, with most vision loss being permanent.10-12
Another potential cause of POVL after spinal surgery is retinal artery occlusion. It is most commonly observed in patients who were improperly positioned, resulting in compression of an orbit on the surface of the headrest or the operating table.13 Retinal artery occlusion characteristically presents as an irreversible unilateral complete loss of vision with a red spot on the macula and an afferent pupillary defect.14
Cortical blindness, another possible common cause of POVL, results from the hypoperfusion of the occipital cortex and has a slightly better prognosis. Cortical blindness generally results from an embolic event that can be visualized through neuroimaging and may be unilateral or bilateral, ranging from mild peripheral vision loss to complete blindness.15
Posterior reversible encephalopathy syndrome, the cause of POVL diagnosed in the 2 patients in this case report, is a neurologic syndrome that differs significantly in its clinical presentation and pathophysiology from the more well-known etiologies. The precise pathophysiologic mechanism of the syndrome is yet to be elucidated. One theory revolves around the failure of cerebral vascular autoregulation. It postulates that intracerebellar hypertension leads to the extravasation of proteins and fluid, resulting in the characteristic vasogenic edema.16,17 The other equally discussed theory postulates that cerebellar vasospasm and subsequent hypoperfusion leading to cellular hypoxemia and ischemia may be responsible.18-20 Posterior reversible encephalopathy syndrome has been reported with increasing frequency, particularly in connection with hypertension, acute renal failure associated with malignancy, cytotoxicity, and corticosteroids, as well as preeclampsia, eclampsia, and autoimmune disorders.1-3,21-23 Traditionally, patients display a combination of different symptoms, including vision changes ranging from slightly decreased perception to complete blindness. Unlike retinal artery occlusion and ischemic optic neuropathy, the onset of vision loss often does not happen immediately after surgery and may occur several hours to days after surgery. Visual disturbance may progressively worsen if the medical cause for the syndrome is not determined and corrected.2,3 In contrast to other known etiologies of POVL, PRES has a relatively favorable prognosis if managed appropriately. Reported case series determined a resolution of the characteristic parieto-occipital vasogenic edema in 83% to 88% of all patients in follow-up neuroimaging after aggressive control of seizures and arterial hypertension.2-3
Both patients undergoing spinal deformity surgery in this report suffered from intermittent hypertensive episodes in the postoperative period. One patient also developed acute renal failure during her hospital stay, and demonstrated low albumin levels postoperatively, which has also been associated with PRES.24 Through the immediate diagnosis and primary control of hypertension, both patients achieved complete neurologic recovery after a mean of 1.5 days (range, 1-2 days); this compares to a recovery period of an average 6.2 days (range, 1-14 days) reported by Ni and colleagues.3 The catastrophic effects of a misdiagnosis and incorrect or untimely treatment were well described in this case report. Several patients who were incorrectly diagnosed with demyelinating disorders or lupus encephalopathy received high doses of immunosuppressants and corticosteroids, known risk factors for the development of PRES.3 The patients subsequently rapidly deteriorated; no patients had a full recovery of their preoperative eyesight, and 1 patient developed complete permanent blindness.3 Optimized multidisciplinary collaboration allowing for a rapid neuro-ophthalmic examination and appropriate neuroimaging will permit an accurate and rapid diagnosis, leading to timely intervention and restoration of vision.
Conclusion
Temporary POVL is a potentially devastating complication of spinal surgery and general anesthesia. The more frequent causes such as ischemic optic neuropathy, retinal artery occlusion, and cortical blindness have very limited effective options for treatment and an overall poor prognosis. The inclusion of PRES in the differential diagnosis of POVL may allow early detection, management, and restoration of vision.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
2. Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
3. Ni J, Zhou LX, Hao HL, et al. The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: a retrospective series of 24 patients. J Neuroimaging. 2011;21(3):219-224.
4. Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566-1575.
5. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036-1042.
6. Yoon SD, Cho BM, Oh SM, et al. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013;15(3):206-213.
7. Patil CG, Lad EM, Lad SP, Ho C, Boakye M. Visual loss after spine surgery: a population-based study. Spine (Phila Pa 1976). 2008;33(13):1491-1496.
8. Stevens WR, Glazer PA, Kelley SD, Lietman TM, Bradford DS. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976). 1997;22(12):1319-1324.
9. Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spinal, orthopedic, cardiac, and general surgery. Anesth Analg. 2009;109(5):1534-1545.
10. Lee LA, Roth S, Posner KL, et al. The American Society of Anesthesiologists Postoperative Visual Loss Registry: analysis of 93 spine surgery cases with postoperative visual loss. Anesthesiology. 2006;105(4):652-659; quiz 867-868.
11. Hayreh SS. Ischemic optic neuropathies - where are we now? Graefes Arch Clin Exp Ophthalmol. 2013;251(8):1873-1884.
12. Buono LM, Foroozan R. Perioperative posterior ischemic optic neuropathy: review of the literature. Surv Ophthalmol. 2005;50(1):15-26.
13. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85.
14. Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87(1):75-78.
15. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531-546.
16. Primavera A, Audenino D, Mavilio N, Cocito L. Reversible posterior leucoencephalopathy syndrome in systemic lupus and vasculitis. Ann Rheum Dis. 2001;60(5):534-537.
17. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):447-455.
18. Ito T, Sakai T, Inagawa S, Utsu M, Bun T. MR angiography of cerebral vasospasm in preeclampsia. AJNR Am J Neuroradiol. 1995;16(6):1344-1346.
19. Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
20. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043-1049.
21. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205-210.
22. Ekawa Y, Shiota M, Tobiume T, et al. Reversible posterior leukoencephalopathy syndrome accompanying eclampsia: correct diagnosis using preoperative MRI. Tohoku J Exp Med. 2012;226(1):55-58.
23. Kur JK, Esdaile JM. Posterior reversible encephalopathy syndrome--an underrecognized manifestation of systemic lupus erythematosus. J Rheumatol. 2006;33(11):2178-2183.
24. Pirker A, Kramer L, Voller B, et al. Type of edema in posterior reversible encephalopathy syndrome depends on serum albumin levels: an MR imaging study in 28 patients. AJNR Am J Neuroradiol. 2011;32(3):527-531.