User login
An 83-year-old Iraqi woman was transferred to our inpatient service from the intensive care unit (ICU). She had been admitted to the ICU for respiratory distress and hypotension, where she was treated with stress dose steroids, oseltamivir, vancomycin, piperacillin/tazobactam, and azithromycin. At our inpatient service, she complained of a new pruritic rash on her thighs, abdominal pain, and persistent diarrhea. Her medical history was notable for chronic interstitial lung disease, gastroesophageal reflux disease, and anemia.
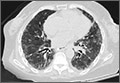
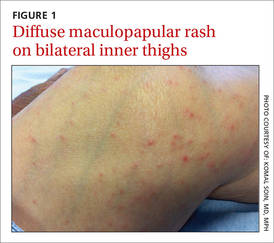
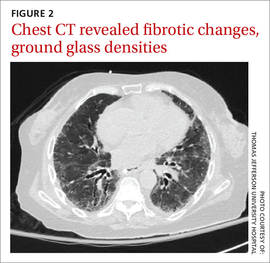
We noted a diffuse maculopapular rash on both of the patient’s inner thighs (FIGURE 1). Laboratory findings revealed leukocytosis and eosinophilia (total white blood cell count of 15,000, with 41% eosinophils). The patient’s eosinophil count—which had improved while she was on steroids in the ICU—had started to rise as steroids were tapered. Blood and cultures from a bronchoscopy were negative. Results from a bronchoalveolar lavage (BAL) were significant for a cell differential of 60% macrophages, 25% neutrophils, 5% lymphocytes, and 10% eosinophils. A stool sample for Clostridium difficile was negative. A computed tomography (CT) scan of the chest revealed bronchiectasis, fibrotic changes, and diffuse ground glass densities (FIGURE 2).
Our patient was a refugee who had arrived in the United States 5 years earlier. Per Centers for Disease Control and Prevention (CDC) guidelines, she had undergone routine stool ova and parasite (O&P) testing upon her arrival in the United States; the results were negative.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Strongyloides stercoralis hyperinfection syndrome
We suspected a parasitic infection because our patient was a refugee with pulmonary, gastrointestinal (GI), and skin complaints, as well as intermittent eosinophilia. Her negative O&P test upon arrival to the United States did not, however, eliminate the possibility of Strongyloides stercoralis, which often goes undetected in routine O&P samples. A serum test for Strongyloides immunoglobulin G (IgG) was positive at 6.39 IV (positive, >2.11 IV). Subsequently, multiple stool samples were tested specifically for the parasite and came back positive, confirming the diagnosis.
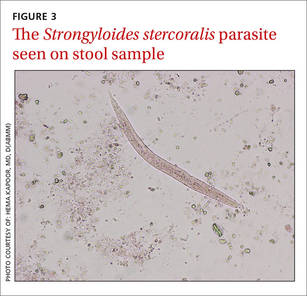
Strongyloidiasis is caused by the roundworm S. stercoralis (FIGURE 3), which infects approximately 30 to 100 million people worldwide.1 It is most common in warm, humid climates in subtropical and tropical regions. With increasing trends in migration and travel, strongyloidiasis is now often diagnosed in nonendemic areas.2
The disease is most prevalent in socioeconomically disadvantaged communities and in agricultural settings. Infection in humans occurs when bare skin comes into contact with contaminated soil. The human T-cell lymphotropic virus-1 (HTLV-1) also predisposes individuals to developing strongyloidiasis.1
Infected patients can be asymptomatic or have intermittent symptoms. Patients are likely to complain of a pruritic rash, cough, shortness of breath, abdominal pain, nausea, and/or diarrhea.3,4 The rash, called larva currens, results when the larvae invade the perianal region. The rash typically spreads to the buttocks, groin, and inner thighs.3
Immunosuppressed patients are at a heightened risk. Two serious forms of strongyloidiasis—hyperinfection syndrome (HS) and disseminated strongyloidiasis (DS)—can develop in immunosuppressed individuals. This can occur in patients receiving high-dose corticosteroids.3-5 Immunosuppression can lead to accelerated autoinfection and a large burden of migrating larvae in the body.
HS was suspected in our patient based on her worsening lung disease, recent onset of diarrhea, and rash in the setting of increased eosinophils.
HS is usually limited to the pulmonary, GI, and skin systems, whereas DS can invade numerous other organs. Complications of HS and DS include disseminated bacterial and fungal infections; the mortality rate if either condition is left untreated is close to 90%.3-5
Strongyloidiasis can mimic other infections
The differential diagnosis for S. stercoralis infection includes the following:
Clostridium difficile infection must be excluded in a hospitalized patient with persistent diarrhea. A stool toxin and antigen test is used to make the diagnosis. Patients with C. difficile infection are more likely to have eosinopenia than eosinophilia.6
Schistosomiasis is a helminth infection that can also persist for decades. The signs and symptoms of a chronic infection can be similar to strongyloidiasis. However, patients with schistosomiasis will typically have large organ damage, bloody diarrhea, and/or urinary symptoms. Diagnosis is made from testing multiple stool samples, urine, and serology.6
Adrenal insufficiency occurs with complete or partial loss of endogenous glucocorticoids. There can be resultant eosinophilia, although it is usually not as marked as the eosinophilia observed in our patient. Adrenal insufficiency is diagnosed with an early morning serum cortisol test and a cosyntropin stimulation test.6
A high degree of suspicion in refugees is needed to make the diagnosis
When a patient is from an endemic area, such as Southeast Asia, Latin America, or sub-Saharan Africa7, one’s clinical suspicion should increase. Also, because signs and symptoms of strongyloidiasis are often nonspecific, a high suspicion for the disease is necessary to prompt testing. Eosinophilia may be present, but can be mild, and is not specific for the disease.
Available stool testing is not highly sensitive, and repeated specialized stool examinations are required, with sensitivity reaching close to 100% only after 7 serial samples are examined.3,8 Duodenal aspirate is more sensitive and larvae can also be seen through wet mount of bronchoalveolar lavage fluid. Serologic testing for Strongyloides IgG is available and has high sensitivity. However, specificity can be low because there can be cross-reactivity with other parasites, and the presence of the antibody does not differentiate between past and current infection.3,5,8
Imaging of the lungs is often variable and nonspecific. Findings on a chest x-ray or CT scan of the chest include diffuse alveolar opacities, interstitial infiltrates, pleural effusions, abscess or cavitation, or fibrotic changes.7 However, these findings can also be the result of a bacterial superinfection and not the parasite itself.3,6
Treatment begins with ivermectin
First-line treatment for strongyloidiasis is oral ivermectin, 200 mcg/kg/d.5 Optimal treatment duration is unknown because it is difficult to determine when S. stercoralis has been eradicated due to the low sensitivity of stool samples.4 For a patient with HS or DS, the CDC recommends treatment until stool and/or sputum samples are negative for 2 weeks.5
The CDC recommends that prior to arrival in the United States, all refugees should receive pre-departure treatment for parasites depending on their country of origin. For individuals arriving from the Middle East, the CDC recommends presumptive treatment with ivermectin for Strongyloides and albendazole for infections caused by soil-transmitted helminths.9 However, ivermectin was not routinely administered in the Middle East until January 2014.9,10 As a result of limited pre-departure treatment, US clinicians need to be cognizant of strongyloidiasis and have a high degree of suspicion in patients with nonspecific symptoms, especially when starting treatment with high-dose corticosteroids for other conditions.
We started our patient on a weight-based dose of ivermectin. Piperacillin/tazobactam 3.375 g (IV) every 6 hours was empirically started to cover enteric bacteria in the setting of HS, but was discontinued after blood cultures were negative. An HTLV-1/2 antibody test was negative. A repeat stool O&P test looking specifically for S. stercoralis came back positive on Day 6 of treatment. To determine the course of treatment, repeat O&Ps were done every 72 hours and ivermectin was continued until stool O&Ps were negative for 2 weeks. The total treatment course lasted 22 days.
During the course of treatment, our patient gained weight and her rash, diarrhea, and abdominal pain improved. She was discharged home and followed up with an infectious disease specialist as an outpatient. Three months later, repeat Strongyloides IgG testing was negative.
CORRESPONDENCE
Komal Soin, MD, MPH, Kaiser Permanente Waipio Medical Office, 94-1480 Moaniani Street, Waipahu, HI 96797; komal.soin@gmail.com
1. Centers for Disease Control and Prevention. Parasites - Strongyloides. Epidemiology & risk factors. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/epi.html. Accessed September 4, 2015.
2. Buonfrate D, Angheben A, Gobbi F, et al. Imported strongyloidiasis: epidemiology, presentations, and treatment. Curr Infect Dis Rep. 2012;14:256-262.
3. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040-1047.
4. Buonfrate D, Requena-Mendez A, Angheben A, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013;13:78.
5. Centers for Disease Control and Prevention. Parasites - Strongyloides. Resources for health professionals. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed September 4, 2015.
6. UpToDate. Klion AD, Weller PF. Approach to the patient with unexplained eosinophilia. UpToDate Web site. Available at: http://www.uptodate.com/contents/approach-to-the-patientwith-unexplained-eosinophilia. Accessed August 27, 2015.
7. Mokhlesi B, Shulzhenko O, Garimella PS, et al. Pulmonary strongyloidiasis: the varied clinical presentations. Clin Pulm Med. 2004;11:6-13.
8. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7:e2002.
9. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html#me-asia-na-la-caribbean. Accessed April 3, 2014.
10. Centers for Disease Control and Prevention. Treatment options for presumptive parasitic infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/interventions/interventions.html. Accessed April 3, 2014.
An 83-year-old Iraqi woman was transferred to our inpatient service from the intensive care unit (ICU). She had been admitted to the ICU for respiratory distress and hypotension, where she was treated with stress dose steroids, oseltamivir, vancomycin, piperacillin/tazobactam, and azithromycin. At our inpatient service, she complained of a new pruritic rash on her thighs, abdominal pain, and persistent diarrhea. Her medical history was notable for chronic interstitial lung disease, gastroesophageal reflux disease, and anemia.
We noted a diffuse maculopapular rash on both of the patient’s inner thighs (FIGURE 1). Laboratory findings revealed leukocytosis and eosinophilia (total white blood cell count of 15,000, with 41% eosinophils). The patient’s eosinophil count—which had improved while she was on steroids in the ICU—had started to rise as steroids were tapered. Blood and cultures from a bronchoscopy were negative. Results from a bronchoalveolar lavage (BAL) were significant for a cell differential of 60% macrophages, 25% neutrophils, 5% lymphocytes, and 10% eosinophils. A stool sample for Clostridium difficile was negative. A computed tomography (CT) scan of the chest revealed bronchiectasis, fibrotic changes, and diffuse ground glass densities (FIGURE 2).
Our patient was a refugee who had arrived in the United States 5 years earlier. Per Centers for Disease Control and Prevention (CDC) guidelines, she had undergone routine stool ova and parasite (O&P) testing upon her arrival in the United States; the results were negative.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Strongyloides stercoralis hyperinfection syndrome
We suspected a parasitic infection because our patient was a refugee with pulmonary, gastrointestinal (GI), and skin complaints, as well as intermittent eosinophilia. Her negative O&P test upon arrival to the United States did not, however, eliminate the possibility of Strongyloides stercoralis, which often goes undetected in routine O&P samples. A serum test for Strongyloides immunoglobulin G (IgG) was positive at 6.39 IV (positive, >2.11 IV). Subsequently, multiple stool samples were tested specifically for the parasite and came back positive, confirming the diagnosis.

Strongyloidiasis is caused by the roundworm S. stercoralis (FIGURE 3), which infects approximately 30 to 100 million people worldwide.1 It is most common in warm, humid climates in subtropical and tropical regions. With increasing trends in migration and travel, strongyloidiasis is now often diagnosed in nonendemic areas.2
The disease is most prevalent in socioeconomically disadvantaged communities and in agricultural settings. Infection in humans occurs when bare skin comes into contact with contaminated soil. The human T-cell lymphotropic virus-1 (HTLV-1) also predisposes individuals to developing strongyloidiasis.1
Infected patients can be asymptomatic or have intermittent symptoms. Patients are likely to complain of a pruritic rash, cough, shortness of breath, abdominal pain, nausea, and/or diarrhea.3,4 The rash, called larva currens, results when the larvae invade the perianal region. The rash typically spreads to the buttocks, groin, and inner thighs.3
Immunosuppressed patients are at a heightened risk. Two serious forms of strongyloidiasis—hyperinfection syndrome (HS) and disseminated strongyloidiasis (DS)—can develop in immunosuppressed individuals. This can occur in patients receiving high-dose corticosteroids.3-5 Immunosuppression can lead to accelerated autoinfection and a large burden of migrating larvae in the body.
HS was suspected in our patient based on her worsening lung disease, recent onset of diarrhea, and rash in the setting of increased eosinophils.
HS is usually limited to the pulmonary, GI, and skin systems, whereas DS can invade numerous other organs. Complications of HS and DS include disseminated bacterial and fungal infections; the mortality rate if either condition is left untreated is close to 90%.3-5
Strongyloidiasis can mimic other infections
The differential diagnosis for S. stercoralis infection includes the following:
Clostridium difficile infection must be excluded in a hospitalized patient with persistent diarrhea. A stool toxin and antigen test is used to make the diagnosis. Patients with C. difficile infection are more likely to have eosinopenia than eosinophilia.6
Schistosomiasis is a helminth infection that can also persist for decades. The signs and symptoms of a chronic infection can be similar to strongyloidiasis. However, patients with schistosomiasis will typically have large organ damage, bloody diarrhea, and/or urinary symptoms. Diagnosis is made from testing multiple stool samples, urine, and serology.6
Adrenal insufficiency occurs with complete or partial loss of endogenous glucocorticoids. There can be resultant eosinophilia, although it is usually not as marked as the eosinophilia observed in our patient. Adrenal insufficiency is diagnosed with an early morning serum cortisol test and a cosyntropin stimulation test.6
A high degree of suspicion in refugees is needed to make the diagnosis
When a patient is from an endemic area, such as Southeast Asia, Latin America, or sub-Saharan Africa7, one’s clinical suspicion should increase. Also, because signs and symptoms of strongyloidiasis are often nonspecific, a high suspicion for the disease is necessary to prompt testing. Eosinophilia may be present, but can be mild, and is not specific for the disease.
Available stool testing is not highly sensitive, and repeated specialized stool examinations are required, with sensitivity reaching close to 100% only after 7 serial samples are examined.3,8 Duodenal aspirate is more sensitive and larvae can also be seen through wet mount of bronchoalveolar lavage fluid. Serologic testing for Strongyloides IgG is available and has high sensitivity. However, specificity can be low because there can be cross-reactivity with other parasites, and the presence of the antibody does not differentiate between past and current infection.3,5,8
Imaging of the lungs is often variable and nonspecific. Findings on a chest x-ray or CT scan of the chest include diffuse alveolar opacities, interstitial infiltrates, pleural effusions, abscess or cavitation, or fibrotic changes.7 However, these findings can also be the result of a bacterial superinfection and not the parasite itself.3,6
Treatment begins with ivermectin
First-line treatment for strongyloidiasis is oral ivermectin, 200 mcg/kg/d.5 Optimal treatment duration is unknown because it is difficult to determine when S. stercoralis has been eradicated due to the low sensitivity of stool samples.4 For a patient with HS or DS, the CDC recommends treatment until stool and/or sputum samples are negative for 2 weeks.5
The CDC recommends that prior to arrival in the United States, all refugees should receive pre-departure treatment for parasites depending on their country of origin. For individuals arriving from the Middle East, the CDC recommends presumptive treatment with ivermectin for Strongyloides and albendazole for infections caused by soil-transmitted helminths.9 However, ivermectin was not routinely administered in the Middle East until January 2014.9,10 As a result of limited pre-departure treatment, US clinicians need to be cognizant of strongyloidiasis and have a high degree of suspicion in patients with nonspecific symptoms, especially when starting treatment with high-dose corticosteroids for other conditions.
We started our patient on a weight-based dose of ivermectin. Piperacillin/tazobactam 3.375 g (IV) every 6 hours was empirically started to cover enteric bacteria in the setting of HS, but was discontinued after blood cultures were negative. An HTLV-1/2 antibody test was negative. A repeat stool O&P test looking specifically for S. stercoralis came back positive on Day 6 of treatment. To determine the course of treatment, repeat O&Ps were done every 72 hours and ivermectin was continued until stool O&Ps were negative for 2 weeks. The total treatment course lasted 22 days.
During the course of treatment, our patient gained weight and her rash, diarrhea, and abdominal pain improved. She was discharged home and followed up with an infectious disease specialist as an outpatient. Three months later, repeat Strongyloides IgG testing was negative.
CORRESPONDENCE
Komal Soin, MD, MPH, Kaiser Permanente Waipio Medical Office, 94-1480 Moaniani Street, Waipahu, HI 96797; komal.soin@gmail.com
An 83-year-old Iraqi woman was transferred to our inpatient service from the intensive care unit (ICU). She had been admitted to the ICU for respiratory distress and hypotension, where she was treated with stress dose steroids, oseltamivir, vancomycin, piperacillin/tazobactam, and azithromycin. At our inpatient service, she complained of a new pruritic rash on her thighs, abdominal pain, and persistent diarrhea. Her medical history was notable for chronic interstitial lung disease, gastroesophageal reflux disease, and anemia.
We noted a diffuse maculopapular rash on both of the patient’s inner thighs (FIGURE 1). Laboratory findings revealed leukocytosis and eosinophilia (total white blood cell count of 15,000, with 41% eosinophils). The patient’s eosinophil count—which had improved while she was on steroids in the ICU—had started to rise as steroids were tapered. Blood and cultures from a bronchoscopy were negative. Results from a bronchoalveolar lavage (BAL) were significant for a cell differential of 60% macrophages, 25% neutrophils, 5% lymphocytes, and 10% eosinophils. A stool sample for Clostridium difficile was negative. A computed tomography (CT) scan of the chest revealed bronchiectasis, fibrotic changes, and diffuse ground glass densities (FIGURE 2).
Our patient was a refugee who had arrived in the United States 5 years earlier. Per Centers for Disease Control and Prevention (CDC) guidelines, she had undergone routine stool ova and parasite (O&P) testing upon her arrival in the United States; the results were negative.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Strongyloides stercoralis hyperinfection syndrome
We suspected a parasitic infection because our patient was a refugee with pulmonary, gastrointestinal (GI), and skin complaints, as well as intermittent eosinophilia. Her negative O&P test upon arrival to the United States did not, however, eliminate the possibility of Strongyloides stercoralis, which often goes undetected in routine O&P samples. A serum test for Strongyloides immunoglobulin G (IgG) was positive at 6.39 IV (positive, >2.11 IV). Subsequently, multiple stool samples were tested specifically for the parasite and came back positive, confirming the diagnosis.

Strongyloidiasis is caused by the roundworm S. stercoralis (FIGURE 3), which infects approximately 30 to 100 million people worldwide.1 It is most common in warm, humid climates in subtropical and tropical regions. With increasing trends in migration and travel, strongyloidiasis is now often diagnosed in nonendemic areas.2
The disease is most prevalent in socioeconomically disadvantaged communities and in agricultural settings. Infection in humans occurs when bare skin comes into contact with contaminated soil. The human T-cell lymphotropic virus-1 (HTLV-1) also predisposes individuals to developing strongyloidiasis.1
Infected patients can be asymptomatic or have intermittent symptoms. Patients are likely to complain of a pruritic rash, cough, shortness of breath, abdominal pain, nausea, and/or diarrhea.3,4 The rash, called larva currens, results when the larvae invade the perianal region. The rash typically spreads to the buttocks, groin, and inner thighs.3
Immunosuppressed patients are at a heightened risk. Two serious forms of strongyloidiasis—hyperinfection syndrome (HS) and disseminated strongyloidiasis (DS)—can develop in immunosuppressed individuals. This can occur in patients receiving high-dose corticosteroids.3-5 Immunosuppression can lead to accelerated autoinfection and a large burden of migrating larvae in the body.
HS was suspected in our patient based on her worsening lung disease, recent onset of diarrhea, and rash in the setting of increased eosinophils.
HS is usually limited to the pulmonary, GI, and skin systems, whereas DS can invade numerous other organs. Complications of HS and DS include disseminated bacterial and fungal infections; the mortality rate if either condition is left untreated is close to 90%.3-5
Strongyloidiasis can mimic other infections
The differential diagnosis for S. stercoralis infection includes the following:
Clostridium difficile infection must be excluded in a hospitalized patient with persistent diarrhea. A stool toxin and antigen test is used to make the diagnosis. Patients with C. difficile infection are more likely to have eosinopenia than eosinophilia.6
Schistosomiasis is a helminth infection that can also persist for decades. The signs and symptoms of a chronic infection can be similar to strongyloidiasis. However, patients with schistosomiasis will typically have large organ damage, bloody diarrhea, and/or urinary symptoms. Diagnosis is made from testing multiple stool samples, urine, and serology.6
Adrenal insufficiency occurs with complete or partial loss of endogenous glucocorticoids. There can be resultant eosinophilia, although it is usually not as marked as the eosinophilia observed in our patient. Adrenal insufficiency is diagnosed with an early morning serum cortisol test and a cosyntropin stimulation test.6
A high degree of suspicion in refugees is needed to make the diagnosis
When a patient is from an endemic area, such as Southeast Asia, Latin America, or sub-Saharan Africa7, one’s clinical suspicion should increase. Also, because signs and symptoms of strongyloidiasis are often nonspecific, a high suspicion for the disease is necessary to prompt testing. Eosinophilia may be present, but can be mild, and is not specific for the disease.
Available stool testing is not highly sensitive, and repeated specialized stool examinations are required, with sensitivity reaching close to 100% only after 7 serial samples are examined.3,8 Duodenal aspirate is more sensitive and larvae can also be seen through wet mount of bronchoalveolar lavage fluid. Serologic testing for Strongyloides IgG is available and has high sensitivity. However, specificity can be low because there can be cross-reactivity with other parasites, and the presence of the antibody does not differentiate between past and current infection.3,5,8
Imaging of the lungs is often variable and nonspecific. Findings on a chest x-ray or CT scan of the chest include diffuse alveolar opacities, interstitial infiltrates, pleural effusions, abscess or cavitation, or fibrotic changes.7 However, these findings can also be the result of a bacterial superinfection and not the parasite itself.3,6
Treatment begins with ivermectin
First-line treatment for strongyloidiasis is oral ivermectin, 200 mcg/kg/d.5 Optimal treatment duration is unknown because it is difficult to determine when S. stercoralis has been eradicated due to the low sensitivity of stool samples.4 For a patient with HS or DS, the CDC recommends treatment until stool and/or sputum samples are negative for 2 weeks.5
The CDC recommends that prior to arrival in the United States, all refugees should receive pre-departure treatment for parasites depending on their country of origin. For individuals arriving from the Middle East, the CDC recommends presumptive treatment with ivermectin for Strongyloides and albendazole for infections caused by soil-transmitted helminths.9 However, ivermectin was not routinely administered in the Middle East until January 2014.9,10 As a result of limited pre-departure treatment, US clinicians need to be cognizant of strongyloidiasis and have a high degree of suspicion in patients with nonspecific symptoms, especially when starting treatment with high-dose corticosteroids for other conditions.
We started our patient on a weight-based dose of ivermectin. Piperacillin/tazobactam 3.375 g (IV) every 6 hours was empirically started to cover enteric bacteria in the setting of HS, but was discontinued after blood cultures were negative. An HTLV-1/2 antibody test was negative. A repeat stool O&P test looking specifically for S. stercoralis came back positive on Day 6 of treatment. To determine the course of treatment, repeat O&Ps were done every 72 hours and ivermectin was continued until stool O&Ps were negative for 2 weeks. The total treatment course lasted 22 days.
During the course of treatment, our patient gained weight and her rash, diarrhea, and abdominal pain improved. She was discharged home and followed up with an infectious disease specialist as an outpatient. Three months later, repeat Strongyloides IgG testing was negative.
CORRESPONDENCE
Komal Soin, MD, MPH, Kaiser Permanente Waipio Medical Office, 94-1480 Moaniani Street, Waipahu, HI 96797; komal.soin@gmail.com
1. Centers for Disease Control and Prevention. Parasites - Strongyloides. Epidemiology & risk factors. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/epi.html. Accessed September 4, 2015.
2. Buonfrate D, Angheben A, Gobbi F, et al. Imported strongyloidiasis: epidemiology, presentations, and treatment. Curr Infect Dis Rep. 2012;14:256-262.
3. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040-1047.
4. Buonfrate D, Requena-Mendez A, Angheben A, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013;13:78.
5. Centers for Disease Control and Prevention. Parasites - Strongyloides. Resources for health professionals. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed September 4, 2015.
6. UpToDate. Klion AD, Weller PF. Approach to the patient with unexplained eosinophilia. UpToDate Web site. Available at: http://www.uptodate.com/contents/approach-to-the-patientwith-unexplained-eosinophilia. Accessed August 27, 2015.
7. Mokhlesi B, Shulzhenko O, Garimella PS, et al. Pulmonary strongyloidiasis: the varied clinical presentations. Clin Pulm Med. 2004;11:6-13.
8. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7:e2002.
9. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html#me-asia-na-la-caribbean. Accessed April 3, 2014.
10. Centers for Disease Control and Prevention. Treatment options for presumptive parasitic infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/interventions/interventions.html. Accessed April 3, 2014.
1. Centers for Disease Control and Prevention. Parasites - Strongyloides. Epidemiology & risk factors. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/epi.html. Accessed September 4, 2015.
2. Buonfrate D, Angheben A, Gobbi F, et al. Imported strongyloidiasis: epidemiology, presentations, and treatment. Curr Infect Dis Rep. 2012;14:256-262.
3. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040-1047.
4. Buonfrate D, Requena-Mendez A, Angheben A, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013;13:78.
5. Centers for Disease Control and Prevention. Parasites - Strongyloides. Resources for health professionals. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed September 4, 2015.
6. UpToDate. Klion AD, Weller PF. Approach to the patient with unexplained eosinophilia. UpToDate Web site. Available at: http://www.uptodate.com/contents/approach-to-the-patientwith-unexplained-eosinophilia. Accessed August 27, 2015.
7. Mokhlesi B, Shulzhenko O, Garimella PS, et al. Pulmonary strongyloidiasis: the varied clinical presentations. Clin Pulm Med. 2004;11:6-13.
8. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7:e2002.
9. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html#me-asia-na-la-caribbean. Accessed April 3, 2014.
10. Centers for Disease Control and Prevention. Treatment options for presumptive parasitic infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/interventions/interventions.html. Accessed April 3, 2014.