User login
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
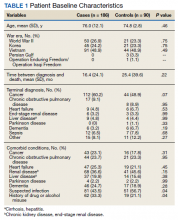
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
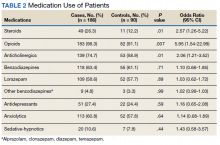
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
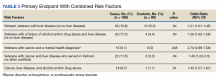
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
Delirium is a condition commonly exhibited by hospitalized patients and by those who are approaching the end of life.1 Patients who experience a disturbance in attention that develops over a relatively short period and represents an acute change may have delirium.2 Furthermore, there is often an additional cognitive disturbance, such as disorientation, memory deficit, language deficits, visuospatial deficit, or perception. Terminal delirium is defined as delirium that occurs in the dying process and implies that reversal is less likely.3 When death is anticipated, diagnostic workups are not recommended, and treatment of the physiologic abnormalities that contribute to delirium is generally ineffective.4
Background
Delirium is often underdiagnosed and undetected by the clinician. Some studies have shown that delirium is not detected in 22 to 50% of cases.5 Factors that contribute to the underdetection of delirium include preexisting dementia, older age, presence of visual or hearing impairment, and hypoactive presentation of delirium. Other possible reasons for nondetection of delirium are its fluctuating nature and lack of formal cognitive assessment as part of a routine screening across care settings.5 Another study found that 41% of health care providers (HCPs) felt that screening for delirium was burdensome.6
To date, there are no veteran-focused studies that investigate prevalence or risk factors for terminal delirium in US Department of Veterans Affairs (VA) long-term care hospice units. Most long-term care hospice units in the VA are in community living centers (CLCs) that follow regulatory guidelines for using antipsychotic medications. The Centers for Medicare and Medicaid Services state that if antipsychotics are prescribed, documentation must clearly show the indication for the antipsychotic medication, the multiple attempts to implement planned care, nonpharmacologic approaches, and ongoing evaluation of the effectiveness of these interventions.7 The symptoms of terminal delirium cause significant distress to patients, family and caregivers, and nursing staff. Literature suggests that delirium poses significant relational challenges for patients, families, and HCPs in end-of-life situations.8,9 We hypothesize that the early identification of risk factors for the development of terminal delirium in this population may lead to increased use of nonpharmacologic measures to prevent terminal delirium, increase nursing vigilance for development of symptoms, and reduce symptom burden should terminal delirium develop.
Prevalence of delirium in the long-term care setting has ranged between 1.4 and 70.3%.10 The rate was found to be much higher in institutionalized populations compared with that of patients classified as at-home. In a study of the prevalence, severity, and natural history of neuropsychiatric syndromes in terminally ill veterans enrolled in community hospice, delirium was found to be present in only 4.1% on the initial visit and 42.5% during last visit. Also, more than half had at least 1 episode of delirium during the 90-day study period.11 In a study of the prevalence of delirium in terminal cancer patients admitted to hospice, 80% experienced delirium in their final days.12
Risk factors for the development of delirium that have been identified in actively dying patients include bowel or bladder obstruction, fluid and electrolyte imbalances, suboptimal pain management, medication adverse effects and toxicity (eg, benzodiazepines, opioids, anticholinergics, and steroids), the addition of ≥ 3 medications, infection, hepatic and renal failure, poor glycemic control, hypoxia, and hematologic disturbances.4,5,13 A high percentage of patients with a previous diagnosis of dementia were found to exhibit terminal delirium.14
There are 2 major subtypes of delirium: hyperactive and hypoactive.4 Patients with hypoactive delirium exhibit lethargy, reduced motor activity, lack of interest, and/or incoherent speech. There is currently little evidence to guide the treatment of hypoactive delirium. By contrast, hyperactive delirium is associated with hallucinations, agitation, heightened arousal, and inappropriate behavior. Many studies suggest both nonpharmacologic and pharmacologic treatment modalities for the treatment of hyperactive delirium.4,13 Nonpharmacologic interventions may minimize the risk and severity of symptoms associated with delirium. Current guidelines recommend these interventions before pharmacologic treatment.4 Nonpharmacologic interventions include but are not limited to the following: engaging the patient in mentally stimulating activities; surrounding the patient with familiar materials (eg, photos); ensuring that all individuals identify themselves when they encounter a patient; minimizing the intensity of stimulation, providing family or volunteer presence, soft lighting and warm blankets; and ensuring the patient uses hearing aids and glasses if needed.4,14
Although there are no US Food and Drug Administration-approved medications to treat hyperactive delirium, first-generation antipsychotics (eg, haloperidol, chlorpromazine) are considered the first-line treatment for patients exhibiting psychosis and psychomotor agitation.3,4,14-16 In terminally ill patients, there is limited evidence from clinical trials to support the efficacy of drug therapy.14 One study showed lack of efficacy with hydration and opioid rotation.17 In terminally ill patients experiencing hyperactive delirium, there is a significant increased risk of muscle tension, myoclonic seizures, and distress to the patient, family, and caregiver.1 Benzodiazepines can be considered first-line treatment for dying patients with terminal delirium in which the goals of treatment are to relieve muscle tension, ensure amnesia, reduce the risk of seizures, and decrease psychosis and agitation.18,19 Furthermore, in patients with history of alcohol misuse who are experiencing terminal delirium, benzodiazepines also may be the preferred pharmacologic treatment.20 Caution must be exercised with the use of benzodiazepines because they can also cause oversedation, increased confusion, and/or a paradoxical worsening of delirium.3,4,14
Methods
This was a retrospective case-control study of patients who died in the Edward Hines Jr. Veterans Affairs Hospital CLC in Hines, Illinois, under the treating specialty nursing home hospice from October 1, 2013 to September 30, 2015. Due to the retrospective nature of this trial, the use of antipsychotics within the last 2 weeks of life was a surrogate marker for development of terminal delirium. Cases were defined as patients who were treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Controls were defined as patients who were not treated with antipsychotics for terminal delirium within the last 2 weeks of their lives. Living hospice patients and patients who were discharged from the CLC before death were excluded.
The goals of this study were to (1) determine risk factors in the VA CLC hospice veteran population for the development of terminal delirium; (2) evaluate documentation by the nursing staff of nonpharmacologic interventions and indications for antipsychotic use in the treatment of terminal delirium; and (3) examine the current usage patterns of antipsychotics for the treatment of terminal delirium.
Veterans’ medical records were reviewed from 2 weeks before death until the recorded death date. Factors that were assessed included age, war era of service, date of death, terminal diagnosis, time interval from cancer diagnosis to death, comorbid conditions, prescribed antipsychotic medications, and other medications potentially contributing to delirium. Nursing documentation was reviewed for indications for administration of antipsychotic medications and nonpharmacologic interventions used to mitigate the symptoms of terminal delirium.
Statistical analysis was conducted in SAS Version 9.3. Cases were compared with controls using univariate and multivariate statistics as appropriate. Comparisons for continuous variables (eg, age) were conducted with Student t tests. Categorical variables (eg, PTSD diagnosis) were compared using χ2 analysis or Fisher exact test as appropriate. Variables with a P value < .1 in the univariate analysis were included in logistic regression models. Independent variables were removed from the models, using a backward selection process. Interaction terms were tested based on significance and clinical relevance. A P value < .05 was considered statistically significant.
Results
From October 1, 2013 to September 30, 2015, 307 patients were analyzed for inclusion in this study. Within this population, 186 received antipsychotic medications for the treatment of terminal delirium (cases), while 90 did not receive antipsychotics (controls). Of the 31 excluded patients, 13 were discharged to receive home hospice care, 11 were discharged to community nursing homes, 5 died in acute care units of Edward Hines, Jr. VA Hospital, and 2 died outside of the study period.
The mean age of all included patients was 75.5 years, and the most common terminal diagnosis was cancer, which occurred in 156 patients (56.5%) (Table 1). The baseline characteristics were similar between the cases and controls, including war era of veteran, terminal diagnosis, and comorbid conditions. The mean time between cancer diagnosis and death was not notably longer in the control group compared with that of the case group (25 vs 16 mo, respectively). There was no statistically significant difference in terminal diagnoses between cases and controls. Veterans in the control group spent more days (mean [SD]) in the hospice unit compared with veterans who experienced terminal delirium (48.5 [168.4] vs 28.2 [46.9]; P = .01). Patients with suspected infections were more likely found in the control group (P = .04; odds ratio [OR] = 1.70; 95% CI, 1.02-2.82).
The most common antipsychotic administered in the last 14 days of life was haloperidol. In the case group, 175 (94%) received haloperidol at least once in the last 2 weeks of life. Four (4.4%) veterans in the control group received haloperidol for the indication of nausea/vomiting; not terminal delirium. Atypical antipsychotics were infrequently used and included risperidone, olanzapine, quetiapine, and aripiprazole.
A total of 186 veterans received at least 1 dose of an antipsychotic for terminal delirium: 97 (52.2% ) veterans requiring antipsychotics for the treatment of terminal delirium required both scheduled and as-needed doses; 75 (40.3%) received only as-needed doses, and 14 (7.5%) required only scheduled doses. When the number of as-needed and scheduled doses were combined, each veteran received a mean 14.9 doses. However, for those veterans with antipsychotics ordered only as needed, a mean 5.8 doses were received per patient. Administration of antipsychotic doses was split evenly among the 3 nursing shifts (day-evening-night) with about 30% of doses administered on each shift.
Nurses were expected to document nonpharmacologic interventions that preceded the administration of each antipsychotic dose. Of the 1,028 doses administered to the 186 veterans who received at least 1 dose of an antipsychotic for terminal delirium, most of the doses (99.4%) had inadequate documentation based on current long-term care guidelines for prudent antipsychotic use.9
Several risk factors for terminal delirium were identified in this veteran population. Veterans with a history of drug or alcohol abuse were found to be at a significantly higher risk for terminal delirium (P = .04; OR, 1.87; 95% CI, 1.03-3.37). As noted in previous studies, steroid use (P = .01; OR, 2.57; 95% CI, 1.26-5.22); opioids (P = .007; OR, 5.94; 95% CI, 1.54-22.99), and anticholinergic medications (P = .01; OR, 2.06; 95% CI, 1.21-3.52) also increased the risk of delirium (Table 2).
When risk factors were combined, interaction terms were identified (Table 3). Those patients found to be at a higher risk of terminal delirium included Vietnam-era veterans with liver disease (P = .04; OR, 1.21; 95% CI, 1.01-1.45) and veterans with a history of drug or alcohol abuse plus comorbid liver disease (P = .03; OR, 1.26; 95% CI, 1.02-1.56). In a stratified analysis in veterans with a terminal diagnosis of cancer, those with a mental health condition (eg, PTSD, bipolar disorder, or schizophrenia) (P = .048; OR, 2.73; 95% CI, 0.98-7.58) also had higher risk of delirium, though not statistically significant. Within the cancer cohort, veterans with liver disease and a history of drug/alcohol abuse had increased risk of delirium (P = .01; OR, 1.43; 95% CI, 1.07-1.91).
Discussion
Terminal delirium is experienced by many individuals in their last days to weeks of life. Symptoms can present as hyperactive (eg, agitation, hallucinations, heightened arousal) or hypoactive (lethargy, reduced motor activity, incoherent speech). Hyperactive terminal delirium is particularly problematic because it causes increased distress to the patient, family, and caregivers. Delirium can lead to safety concerns, such as fall risk, due to patients’ decreased insight into functional decline.
Many studies suggest both nonpharmacologic and pharmacologic treatments for nonterminal delirium that may also apply to terminal delirium. Nonpharmacologic methods, such as providing a quiet and familiar environment, relieving urinary retention or constipation, and attending to sensory deficits may help prevent or minimize delirium. Pharmacologic interventions, such as antipsychotics or benzodiazepines, may benefit when other modalities have failed to assuage distressing symptoms of delirium. Because hypoactive delirium is usually accompanied by somnolence and reduced motor activity, medication is most often administered to individuals with hyperactive delirium.
The VA provides long-term care hospice beds in their CLCs for veterans who are nearing end of life and have inadequate caregiver support for comprehensive end-of-life care in the home (Case Presentation). Because of their military service and other factors common in their life histories, they may have a unique set of characteristics that are predictive of developing terminal delirium. Awareness of the propensity for terminal delirium will allow for early identification of symptoms, timely initiation of nonpharmacologic interventions, and potentially a decreased need for use of antipsychotic medications.
In this study, as noted in previous studies, certain medications (eg, steroids, opioids, and anticholinergics) increased the risk of developing terminal delirium in this veteran population. Steroids and opioids are commonly used in management of neoplasm-related pain and are prescribed throughout the course of terminal illness. The utility of these medications often outweighs potential adverse effects but should be considered when assessing the risk for development of delirium. Anticholinergics (eg, glycopyrrolate or scopolamine) are often prescribed in the last days of life for terminal secretions despite lack of evidence of patient benefit. Nonetheless, anticholinergics are used to reduce family and caregiver distress resulting from bothersome sounds from terminal secretions, referred to as the death rattle.21
It was found that veterans in the control group lived longer on the hospice unit. It is unclear whether the severity of illness was related to the development of terminal delirium or whether the development of terminal delirium contributed to a hastened death. Veterans with a suspected infection were identified by the use of antibiotics on admission to the hospice unit or when antibiotics were prescribed during the last 2 weeks of life. Thus, treatment of the underlying infection may have contributed to the finding of less delirium in the control group.
More than half the veterans in this study received at least 1 dose of an antipsychotic in the last 2 weeks of life for the treatment of terminal delirium. The most commonly administered medication was haloperidol, given either orally or subcutaneously. Atypical antipsychotics were used less often and were sometimes transitioned to subcutaneous haloperidol as the ability to swallow declined if symptoms persisted.
In this veteran population, having a history of drug or alcohol abuse (even if not recent) increased the risk of terminal delirium. Comorbid cancer and history of mental health disease (eg, PTSD, schizophrenia, bipolar disorder) and Vietnam-era veterans with liver disease (primary cancer, metastases, or cirrhosis) also were more likely to develop terminal delirium.
Just as hospice care is being provided in community settings, nurses are at the forefront of symptom management for veterans residing in VA CLCs under hospice care. Nonpharmacologic interventions are provided by the around-the-clock bedside team to provide comfort for veterans, families, and caregivers throughout the dying process. Nurses’ assessment skills and documentation inform the plan of care for the entire interdisciplinary hospice team. Because the treatment of terminal delirium often involves the administration of antipsychotic medications, scrutiny is applied to documentation surrounding these medications.7 This study suggested that there is a need for a more rigorous and consistent method of documenting the assessment of, and interventions for, terminal delirium.
Limitations
Limitations to the current study include hyperactive delirium that was misinterpreted and treated as pain; the probable underreporting of hypoactive delirium and associated symptoms; the use of antipsychotics as a surrogate marker for the development of terminal delirium; and lack of nursing documentation of assessment and interventions of terminal delirium. In addition, the total milligrams of antipsychotics administered per patient were not collected. Finally, there was the potential that other risk factors were not identified due to low numbers of veterans with certain diagnoses (eg, dementia).
Conclusions
Based on the findings in this study, several steps have been implemented to enhance the care of veterans under hospice care in this CLC: (1) Nurses providing direct patient care have been educated on the assessment by use of the mRASS and treatment of terminal delirium;22 (2) A hospice delirium note template has been created that details symptoms of terminal delirium, nonpharmacologic interventions, the use of antipsychotic medications if indicated, and the outcome of interventions; (3) Providers (eg, physician, advanced practice nurses) review each veteran’s medical history for the risk factors noted above; (4) Any risk factor(s) identified by this study will lead to a nursing order for delirium precautions, which requires completion of the delirium note template by nurses each shift.
The goal for this enhanced process is to identify veterans at risk for terminal delirium, observe changes that may indicate the onset of delirium, and intervene promptly to decrease symptom burden and improve quality of life and safety. Potentially, there will be less requirement for the use of antipsychotic medications to control the more severe symptoms of terminal delirium. A future study will evaluate the outcome of this enhanced process for the assessment and treatment of terminal delirium in this veteran population.
Acknowledgment
We thank Martin J. Gorbien, MD, associate chief of staff of Geriatrics and Extended Care, for his continued support throughout this project.
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138
1. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Intern Med. 2001;135(1):32-40.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC; 2013.
3. Grassi L, Caraceni A, Mitchell A, et al. Management of delirium in palliative care: a review. Curr Psychiatry Rep. 2015;17(13):1-9. doi:10.1007/s11920-015-0550-8
4. Bush S, Leonard M, Agar M, et al. End-of-life delirium: issues regarding the recognition, optimal management, and role of sedation in the dying phase. J Pain Symptom Manage. 2014;48 (2):215-230. doi:10.1016/j.jpainsymman. 2014.05.009
5. Moyer D. Terminal delirium in geriatric patients with cancer at end of life. Am J Hosp Palliat Med. 2010;28(1):44-51. doi:10.1177/1049909110376755
6. Lai X, Huang Z, Chen C, et al. Delirium screening in patients in a palliative care ward: a best practice implementation project. JBI Database System Rev Implement Rep. 2019;17(3):429-441. doi:10.11124/JBISRIR-2017-003646
7. Centers for Medicare and Medicaid Services. Medicare and Medicaid Programs; reform of requirements for long-term care facilities. Final rule. Fed Regist. 2016;81 (192):68688-68872. Accessed April 17, 2021. https://pubmed.ncbi.nlm.nih.gov/27731960
8. Wright D, Brajtman S, Macdonald M. A relational ethical approach to end-of-life delirium. J Pain Symptom Manage. 2014;48(2):191-198. doi:10.1016/j.jpainsymman.2013.08.015
9. Brajtman S, Higuchi K, McPherson C. Caring for patients with terminal delirium: palliative care unit and home care nurses’ experience. Int J Palliat Nurs. 2006;12(4):150-156. doi:10.12968/ijpn.2006.12.4.21010
10. Lange E, Verhaak P, Meer K. Prevalence, presentation, and prognosis of delirium in older people in the population, at home and in long-term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127-134. doi:10.1002/gps.3814
11. Goy E, Ganzini L. Prevalence and natural history of neuropsychiatric syndromes in veteran hospice patients. J Pain Symptom Manage. 2011;41(12):394-401. doi:10.1016/j.jpainsymman.2010.04.015
12. Bush S, Bruera E. The assessment and management of delirium in cancer patients. Oncologist. 2009;4(10):1039-1049. doi:10.1634/theoncologist.2009-0122
13. Clary P, Lawson P. Pharmacologic pearls for end-of-life care. Am Fam Physician. 2009;79(12):1059-1065.
14. Blinderman CD, Billings J. Comfort for patients dying in the hospital. N Engl J Med. 2015;373(26):2549-2561. doi:10.1056/NEJMra1411746
15. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris F.D. Clarifying delirium management: practical evidence-based, expert recommendation for clinical practice. J Palliat Med. 2013;16(4):423-435. doi:10.1089/jpm.2012.0319
16. Bobb B. Dyspnea and delirium at the end of life. Clin J Oncol Nurs. 2016;20(3):244-246. doi:10.1188/16.CJON.244-246
17. Morita T, Tei Y, Inoue S. Agitated terminal delirium and association with partial opioid substitution and hydration. J Palliat Med. 2003;6(4):557-563. doi:10.1089/109662103768253669
18. Attard A, Ranjith G, Taylor D. Delirium and its treatment. CNS Drugs. 2008;22(8):631-644-649. doi:10.2165/00023210-200822080-00002
19. Hui D. Benzodiazepines for agitation in patients with delirium: selecting the right patient, right time, and right indication. Curr Opin Support Palliat Care. 2018;12(4):489-494. doi:10.1097/SPC.0000000000000395
20. Irwin P, Murray S, Bilinski A, Chern B, Stafford B. Alcohol withdrawal as an underrated cause of agitated delirium and terminal restlessness in patients with advanced malignancy. J Pain Symptom Manage. 2005;29(1):104-108. doi:10.1016/j.jpainsymman.2004.04.010
21. Lokker ME, van Zuylen L, van der Rijt CCD, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;48:2-12. doi:10.1016/j.jpainsymman.2013.03.011
22. Sessler C, Gosnell M, Grap M, et al. The Richmond Agitation–Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002:166(10):1338-1344. doi:10.1164/rccm.2107138