User login
Although not every patient with locally advanced non–small cell lung cancer (NSCLC) is a surgical candidate, surgery is worth considering for a subpopulation of patients who can benefit. For patients with stage III disease, choosing the optimal treatment is difficult and best done by a team skilled in managing this type of cancer.
Accurate clinical staging is extremely important to optimize treatment and outcome. The most common staging system used is the tumor, node, metastasis (TNM) system, which was revised in 2009. Surgery as a potential option for patients with lung cancer is becoming more accepted for patients with N2 disease but is still controversial for N3 disease. For the most part, surgical candidates are those with N2 or T4N1 disease. This article therefore focuses on staging and the utility of surgery in patients with N2 disease.
NONINVASIVE STAGING
Computed tomography (CT) of the chest and upper abdomen with intravenous contrast, including the liver and adrenal glands, is standard procedure for noninvasive staging of NSCLC. Contrast CT or magnetic resonance imaging of the brain is necessary to rule out brain metastasis in the patient with NSCLC for whom surgery is being considered.
At Cleveland Clinic, we have found that PET stage and pathologic stage correlate less than 70% of the time, which reflects the high incidence of histoplasmosis and other endemic inflammatory diseases of the mediastinum.
MEDIASTINAL SAMPLING
The staging evaluation includes an assessment of the mediastinal lymph nodes. Nodal sampling of the mediastinum is advised for every patient with potentially resectable NSCLC, even in the absence of an enlarged mediastinal lymph node on CT, because mediastinal lymph node involvement is a negative prognostic indicator; absence of tumor involvement of the mediastinal lymph nodes confers a more favorable prognosis.
Cervical mediastinoscopy was first introduced for lung cancer staging in 1959 and is considered the gold standard in mediastinal staging. The sensitivity and specificity approach 100% in experienced hands. A single-center experience of 2,137 cervical mediastinoscopies revealed a complication rate of 0.6% and death from mediastinoscopy in 0.05%.2 Node stations obtainable with cervical mediastinoscopy are 2R, 4R, 3, 2L, and 4L, and sometimes 10R. These are central mediastinal nodal stations.
De Leyn et al3 demonstrated that even small tumors can have lymph node involvement. Cervical mediastinoscopy was positive in their series in 9.5% of stage T1 tumors, 17.7% of T2, 31.2% of T3, and 33.3% of T4.
ENDOBRONCHIAL ULTRASOUND (EBUS) AND EBUS STAGING
Endobronchial ultrasound involves the use of a bronchoscope with an ultrasound probe mounted on it to evaluate nodal stations for suspicious lesions that require biopsy. Needle aspiration biopsy is performed by advancing a needle housed in a sheath of the endoscope, using ultrasound to identify target nodal tissue and obtain sample tissue for evaluation.
BRAIN IMAGING
Although brain metastases are uncommon, occurring in only 1% to 5% of asymptomatic patients with NSCLC, their identification is paramount when the treatment for stage III NSCLC is potentially high-morbidity surgery.
PHYSIOLOGIC EVALUATION
When the treatment plan potentially includes surgery, a multidisciplinary evaluation is essential and should involve specialists in medical oncology, radiation oncology, pulmonary medicine, thoracic surgery, and pathology.
Because surgery for stage III NSCLC is aggressive, prior physiologic evaluation is necessary to assess operative risk. Pulmonary function evaluation should include spirometry, measurement of arterial blood gas values, diffusion capacity (transfer factor of the lung for carbon monoxide), 6-minute walk test, and cardiopulmonary exercise testing.
Stress testing, whether by nuclear imaging or dobutamine echocardiogram, is also indicated, especially if considering pneumonectomy. A quantitative ventilation-perfusion scan is indicated for a more definitive evaluation of pulmonary function.
RESULTS OBTAINED WITH MULTIMODALITY THERAPY
Southwest Oncology Group 8805
The Southwest Oncology Group (SWOG) study 8805 used a trimodality approach in patients with bulky stage III NSCLC: induction chemoradiation with concurrent cisplatin, etoposide, and radiotherapy (45 Gy) followed by surgical resection.4 The 3-year survival rate with this treatment strategy was 26%. Patients in this trial who were downstaged following induction therapy so that they had node-negative disease at the time of surgery had a superior prognosis, with a 3-year survival rate of 41%. Therefore, a subset of patients with stage III NSCLC stands to benefit from surgery, but identifying this group prior to surgery may not be possible.
Trial of accelerated multimodality therapy
An accelerated multimodality induction regimen given over 12 days was tested in 105 patients with stage IIIa (n = 78) and stage IIIb (n = 27) NSCLC, 97% of whom had mediastinal involvement.5 Seven patients had T4 disease. The induction regimen consisted of a 12-day course of concurrent cisplatin, paclitaxel, and radiotherapy. A 4-day continuous infusion of cisplatin (20 mg/m2/day) and a 24-hour continuous infusion of paclitaxel (175 mg/m2) were administered on day 1. Concurrent accelerated fractionated radiotherapy consisted of twice-daily fractions of 1.5 Gy.
All patients completed induction therapy. Of the 105 patients, 98 were candidates for surgical treatment and 83 underwent curative resections (lobectomy, n = 42; pneumonectomy, n = 36; and bilobectomy, n = 5).
Surgical mortality was 7% and morbidity was 31% (supraventricular arrhythmia, 18%; recurrent laryngeal nerve palsy, 6%; pneumonia or adult respiratory distress syndrome, 3%; bronchopleural fistula, 3%; wound infection, 2%; reoperation for bleeding, 1%).
Profiles of patients with favorable and unfavorable prognoses were developed. A younger patient with adenocarcinoma whose disease was downstaged with induction therapy had a favorable prognosis, whereas an older patient with squamous carcinoma that did not respond to treatment and continued in pathologic stage IIIb had an unfavorable prognosis.
SURVIVAL DATA FAVOR SURGERY
An accurate head-to-head comparison of chemoradiation with or without surgery in patients with resectable NSCLC is difficult because patients selected for surgery must meet performance status criteria, whereas an evaluation of performance status is not mandated for patients treated with definitive chemoradiation alone. The quality of postoperative care and the management of postoperative complications also differ from institution to institution.
A controlled trial in which patients with stage IIIa NSCLC were randomized to chemoradiation with or without surgical resection was performed by Albain et al.6 The induction regimen consisted of 2 cycles of cisplatin and etoposide plus radiotherapy (45 Gy). At 5 years, overall survival was 27% in patients who underwent resection and 20% in those who continued radiotherapy without resection, a difference that did not achieve statistical significance. Progression-free survival was superior in the group assigned to surgery compared with those not undergoing resection (median: 12.8 months vs 10.5 months).
A Surveillance Epidemiology and End Results registry of more than 48,000 patients with stage III NSCLC revealed significantly better overall survival in those who received neoadjuvant radiotherapy plus surgery compared with radiation therapy alone, postoperative radiation therapy, and surgery alone.7
CLEVELAND CLINIC EXPERIENCE WITH ACCELERATED PROTOCOL
At Cleveland Clinic, the current protocol for stage IIIa and IIIb NSCLC is an accelerated multimodality regimen consisting of paclitaxel, 50 mg/m2 twice weekly for 3 weeks; carboplatin (target area under the concentration vs time curve dosing) twice weekly for 3 weeks; and daily erlotinib (phase 1 dose escalation protocol) with concurrent radiotherapy, 1.5 Gy twice daily, as induction therapy, followed by a preoperative evaluation and surgery if local control is achieved with induction treatment.
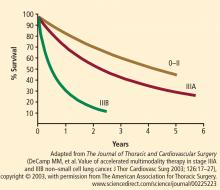
This protocol has been used in 30 patients with stage IIIa disease (median age: 61 years) with no operative mortality (62% lobectomy, 38% pneumonectomy) and a median length of stay of 6.2 days. Forty percent of patients had their disease downstaged following induction therapy. Three-year survival is approximately 60% and, at 5 years, survival is still 55%.
CONCLUSION
Multimodality therapy for NSCLC is effective and achieves favorable survival. Pathologic downstaging is an important predictor for survival but patients with residual N2 disease still have meaningful survival with resection.
A team approach to evaluation and treatment among medical oncology, radiation oncology, pulmonary medicine, and thoracic surgery is critical to successful outcome.
- Pieterman RM, van Putten JWG, Meuzelaar JL, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 2000; 343:254–261.
- Hammoud Z, Anderson RC, Meyers BF, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg 1999; 118:894–899.
- De Leyn P, Vansteenkiste J, Cuypers P, et al. Role of cervical mediastinoscopy in staging of non-small cell lung cancer without enlarged mediastinal lymph nodes on CT scan. Eur J Cardiothor Surg 1997; 12:706–712.
- Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995; 13:1880–1892.
- De Camp MM, Rice TW, Adelstein DJ, et al. Value of accelerated multimodality therapy in stage IIIA and IIIB non–small cell lung cancer. J Thor Cardiovasc Surg 2003; 126:17–27.
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009; 374:379–386.
- Koshy M, Goloubeva O, Suntharalingam M. Impact of neoadjuvant radiation on survival in stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 79:1388–1394.
Although not every patient with locally advanced non–small cell lung cancer (NSCLC) is a surgical candidate, surgery is worth considering for a subpopulation of patients who can benefit. For patients with stage III disease, choosing the optimal treatment is difficult and best done by a team skilled in managing this type of cancer.
Accurate clinical staging is extremely important to optimize treatment and outcome. The most common staging system used is the tumor, node, metastasis (TNM) system, which was revised in 2009. Surgery as a potential option for patients with lung cancer is becoming more accepted for patients with N2 disease but is still controversial for N3 disease. For the most part, surgical candidates are those with N2 or T4N1 disease. This article therefore focuses on staging and the utility of surgery in patients with N2 disease.
NONINVASIVE STAGING
Computed tomography (CT) of the chest and upper abdomen with intravenous contrast, including the liver and adrenal glands, is standard procedure for noninvasive staging of NSCLC. Contrast CT or magnetic resonance imaging of the brain is necessary to rule out brain metastasis in the patient with NSCLC for whom surgery is being considered.
At Cleveland Clinic, we have found that PET stage and pathologic stage correlate less than 70% of the time, which reflects the high incidence of histoplasmosis and other endemic inflammatory diseases of the mediastinum.
MEDIASTINAL SAMPLING
The staging evaluation includes an assessment of the mediastinal lymph nodes. Nodal sampling of the mediastinum is advised for every patient with potentially resectable NSCLC, even in the absence of an enlarged mediastinal lymph node on CT, because mediastinal lymph node involvement is a negative prognostic indicator; absence of tumor involvement of the mediastinal lymph nodes confers a more favorable prognosis.
Cervical mediastinoscopy was first introduced for lung cancer staging in 1959 and is considered the gold standard in mediastinal staging. The sensitivity and specificity approach 100% in experienced hands. A single-center experience of 2,137 cervical mediastinoscopies revealed a complication rate of 0.6% and death from mediastinoscopy in 0.05%.2 Node stations obtainable with cervical mediastinoscopy are 2R, 4R, 3, 2L, and 4L, and sometimes 10R. These are central mediastinal nodal stations.
De Leyn et al3 demonstrated that even small tumors can have lymph node involvement. Cervical mediastinoscopy was positive in their series in 9.5% of stage T1 tumors, 17.7% of T2, 31.2% of T3, and 33.3% of T4.
ENDOBRONCHIAL ULTRASOUND (EBUS) AND EBUS STAGING
Endobronchial ultrasound involves the use of a bronchoscope with an ultrasound probe mounted on it to evaluate nodal stations for suspicious lesions that require biopsy. Needle aspiration biopsy is performed by advancing a needle housed in a sheath of the endoscope, using ultrasound to identify target nodal tissue and obtain sample tissue for evaluation.
BRAIN IMAGING
Although brain metastases are uncommon, occurring in only 1% to 5% of asymptomatic patients with NSCLC, their identification is paramount when the treatment for stage III NSCLC is potentially high-morbidity surgery.
PHYSIOLOGIC EVALUATION
When the treatment plan potentially includes surgery, a multidisciplinary evaluation is essential and should involve specialists in medical oncology, radiation oncology, pulmonary medicine, thoracic surgery, and pathology.
Because surgery for stage III NSCLC is aggressive, prior physiologic evaluation is necessary to assess operative risk. Pulmonary function evaluation should include spirometry, measurement of arterial blood gas values, diffusion capacity (transfer factor of the lung for carbon monoxide), 6-minute walk test, and cardiopulmonary exercise testing.
Stress testing, whether by nuclear imaging or dobutamine echocardiogram, is also indicated, especially if considering pneumonectomy. A quantitative ventilation-perfusion scan is indicated for a more definitive evaluation of pulmonary function.
RESULTS OBTAINED WITH MULTIMODALITY THERAPY
Southwest Oncology Group 8805
The Southwest Oncology Group (SWOG) study 8805 used a trimodality approach in patients with bulky stage III NSCLC: induction chemoradiation with concurrent cisplatin, etoposide, and radiotherapy (45 Gy) followed by surgical resection.4 The 3-year survival rate with this treatment strategy was 26%. Patients in this trial who were downstaged following induction therapy so that they had node-negative disease at the time of surgery had a superior prognosis, with a 3-year survival rate of 41%. Therefore, a subset of patients with stage III NSCLC stands to benefit from surgery, but identifying this group prior to surgery may not be possible.
Trial of accelerated multimodality therapy
An accelerated multimodality induction regimen given over 12 days was tested in 105 patients with stage IIIa (n = 78) and stage IIIb (n = 27) NSCLC, 97% of whom had mediastinal involvement.5 Seven patients had T4 disease. The induction regimen consisted of a 12-day course of concurrent cisplatin, paclitaxel, and radiotherapy. A 4-day continuous infusion of cisplatin (20 mg/m2/day) and a 24-hour continuous infusion of paclitaxel (175 mg/m2) were administered on day 1. Concurrent accelerated fractionated radiotherapy consisted of twice-daily fractions of 1.5 Gy.
All patients completed induction therapy. Of the 105 patients, 98 were candidates for surgical treatment and 83 underwent curative resections (lobectomy, n = 42; pneumonectomy, n = 36; and bilobectomy, n = 5).
Surgical mortality was 7% and morbidity was 31% (supraventricular arrhythmia, 18%; recurrent laryngeal nerve palsy, 6%; pneumonia or adult respiratory distress syndrome, 3%; bronchopleural fistula, 3%; wound infection, 2%; reoperation for bleeding, 1%).
Profiles of patients with favorable and unfavorable prognoses were developed. A younger patient with adenocarcinoma whose disease was downstaged with induction therapy had a favorable prognosis, whereas an older patient with squamous carcinoma that did not respond to treatment and continued in pathologic stage IIIb had an unfavorable prognosis.
SURVIVAL DATA FAVOR SURGERY
An accurate head-to-head comparison of chemoradiation with or without surgery in patients with resectable NSCLC is difficult because patients selected for surgery must meet performance status criteria, whereas an evaluation of performance status is not mandated for patients treated with definitive chemoradiation alone. The quality of postoperative care and the management of postoperative complications also differ from institution to institution.
A controlled trial in which patients with stage IIIa NSCLC were randomized to chemoradiation with or without surgical resection was performed by Albain et al.6 The induction regimen consisted of 2 cycles of cisplatin and etoposide plus radiotherapy (45 Gy). At 5 years, overall survival was 27% in patients who underwent resection and 20% in those who continued radiotherapy without resection, a difference that did not achieve statistical significance. Progression-free survival was superior in the group assigned to surgery compared with those not undergoing resection (median: 12.8 months vs 10.5 months).
A Surveillance Epidemiology and End Results registry of more than 48,000 patients with stage III NSCLC revealed significantly better overall survival in those who received neoadjuvant radiotherapy plus surgery compared with radiation therapy alone, postoperative radiation therapy, and surgery alone.7
CLEVELAND CLINIC EXPERIENCE WITH ACCELERATED PROTOCOL
At Cleveland Clinic, the current protocol for stage IIIa and IIIb NSCLC is an accelerated multimodality regimen consisting of paclitaxel, 50 mg/m2 twice weekly for 3 weeks; carboplatin (target area under the concentration vs time curve dosing) twice weekly for 3 weeks; and daily erlotinib (phase 1 dose escalation protocol) with concurrent radiotherapy, 1.5 Gy twice daily, as induction therapy, followed by a preoperative evaluation and surgery if local control is achieved with induction treatment.
This protocol has been used in 30 patients with stage IIIa disease (median age: 61 years) with no operative mortality (62% lobectomy, 38% pneumonectomy) and a median length of stay of 6.2 days. Forty percent of patients had their disease downstaged following induction therapy. Three-year survival is approximately 60% and, at 5 years, survival is still 55%.
CONCLUSION
Multimodality therapy for NSCLC is effective and achieves favorable survival. Pathologic downstaging is an important predictor for survival but patients with residual N2 disease still have meaningful survival with resection.
A team approach to evaluation and treatment among medical oncology, radiation oncology, pulmonary medicine, and thoracic surgery is critical to successful outcome.
Although not every patient with locally advanced non–small cell lung cancer (NSCLC) is a surgical candidate, surgery is worth considering for a subpopulation of patients who can benefit. For patients with stage III disease, choosing the optimal treatment is difficult and best done by a team skilled in managing this type of cancer.
Accurate clinical staging is extremely important to optimize treatment and outcome. The most common staging system used is the tumor, node, metastasis (TNM) system, which was revised in 2009. Surgery as a potential option for patients with lung cancer is becoming more accepted for patients with N2 disease but is still controversial for N3 disease. For the most part, surgical candidates are those with N2 or T4N1 disease. This article therefore focuses on staging and the utility of surgery in patients with N2 disease.
NONINVASIVE STAGING
Computed tomography (CT) of the chest and upper abdomen with intravenous contrast, including the liver and adrenal glands, is standard procedure for noninvasive staging of NSCLC. Contrast CT or magnetic resonance imaging of the brain is necessary to rule out brain metastasis in the patient with NSCLC for whom surgery is being considered.
At Cleveland Clinic, we have found that PET stage and pathologic stage correlate less than 70% of the time, which reflects the high incidence of histoplasmosis and other endemic inflammatory diseases of the mediastinum.
MEDIASTINAL SAMPLING
The staging evaluation includes an assessment of the mediastinal lymph nodes. Nodal sampling of the mediastinum is advised for every patient with potentially resectable NSCLC, even in the absence of an enlarged mediastinal lymph node on CT, because mediastinal lymph node involvement is a negative prognostic indicator; absence of tumor involvement of the mediastinal lymph nodes confers a more favorable prognosis.
Cervical mediastinoscopy was first introduced for lung cancer staging in 1959 and is considered the gold standard in mediastinal staging. The sensitivity and specificity approach 100% in experienced hands. A single-center experience of 2,137 cervical mediastinoscopies revealed a complication rate of 0.6% and death from mediastinoscopy in 0.05%.2 Node stations obtainable with cervical mediastinoscopy are 2R, 4R, 3, 2L, and 4L, and sometimes 10R. These are central mediastinal nodal stations.
De Leyn et al3 demonstrated that even small tumors can have lymph node involvement. Cervical mediastinoscopy was positive in their series in 9.5% of stage T1 tumors, 17.7% of T2, 31.2% of T3, and 33.3% of T4.
ENDOBRONCHIAL ULTRASOUND (EBUS) AND EBUS STAGING
Endobronchial ultrasound involves the use of a bronchoscope with an ultrasound probe mounted on it to evaluate nodal stations for suspicious lesions that require biopsy. Needle aspiration biopsy is performed by advancing a needle housed in a sheath of the endoscope, using ultrasound to identify target nodal tissue and obtain sample tissue for evaluation.
BRAIN IMAGING
Although brain metastases are uncommon, occurring in only 1% to 5% of asymptomatic patients with NSCLC, their identification is paramount when the treatment for stage III NSCLC is potentially high-morbidity surgery.
PHYSIOLOGIC EVALUATION
When the treatment plan potentially includes surgery, a multidisciplinary evaluation is essential and should involve specialists in medical oncology, radiation oncology, pulmonary medicine, thoracic surgery, and pathology.
Because surgery for stage III NSCLC is aggressive, prior physiologic evaluation is necessary to assess operative risk. Pulmonary function evaluation should include spirometry, measurement of arterial blood gas values, diffusion capacity (transfer factor of the lung for carbon monoxide), 6-minute walk test, and cardiopulmonary exercise testing.
Stress testing, whether by nuclear imaging or dobutamine echocardiogram, is also indicated, especially if considering pneumonectomy. A quantitative ventilation-perfusion scan is indicated for a more definitive evaluation of pulmonary function.
RESULTS OBTAINED WITH MULTIMODALITY THERAPY
Southwest Oncology Group 8805
The Southwest Oncology Group (SWOG) study 8805 used a trimodality approach in patients with bulky stage III NSCLC: induction chemoradiation with concurrent cisplatin, etoposide, and radiotherapy (45 Gy) followed by surgical resection.4 The 3-year survival rate with this treatment strategy was 26%. Patients in this trial who were downstaged following induction therapy so that they had node-negative disease at the time of surgery had a superior prognosis, with a 3-year survival rate of 41%. Therefore, a subset of patients with stage III NSCLC stands to benefit from surgery, but identifying this group prior to surgery may not be possible.
Trial of accelerated multimodality therapy
An accelerated multimodality induction regimen given over 12 days was tested in 105 patients with stage IIIa (n = 78) and stage IIIb (n = 27) NSCLC, 97% of whom had mediastinal involvement.5 Seven patients had T4 disease. The induction regimen consisted of a 12-day course of concurrent cisplatin, paclitaxel, and radiotherapy. A 4-day continuous infusion of cisplatin (20 mg/m2/day) and a 24-hour continuous infusion of paclitaxel (175 mg/m2) were administered on day 1. Concurrent accelerated fractionated radiotherapy consisted of twice-daily fractions of 1.5 Gy.
All patients completed induction therapy. Of the 105 patients, 98 were candidates for surgical treatment and 83 underwent curative resections (lobectomy, n = 42; pneumonectomy, n = 36; and bilobectomy, n = 5).
Surgical mortality was 7% and morbidity was 31% (supraventricular arrhythmia, 18%; recurrent laryngeal nerve palsy, 6%; pneumonia or adult respiratory distress syndrome, 3%; bronchopleural fistula, 3%; wound infection, 2%; reoperation for bleeding, 1%).
Profiles of patients with favorable and unfavorable prognoses were developed. A younger patient with adenocarcinoma whose disease was downstaged with induction therapy had a favorable prognosis, whereas an older patient with squamous carcinoma that did not respond to treatment and continued in pathologic stage IIIb had an unfavorable prognosis.
SURVIVAL DATA FAVOR SURGERY
An accurate head-to-head comparison of chemoradiation with or without surgery in patients with resectable NSCLC is difficult because patients selected for surgery must meet performance status criteria, whereas an evaluation of performance status is not mandated for patients treated with definitive chemoradiation alone. The quality of postoperative care and the management of postoperative complications also differ from institution to institution.
A controlled trial in which patients with stage IIIa NSCLC were randomized to chemoradiation with or without surgical resection was performed by Albain et al.6 The induction regimen consisted of 2 cycles of cisplatin and etoposide plus radiotherapy (45 Gy). At 5 years, overall survival was 27% in patients who underwent resection and 20% in those who continued radiotherapy without resection, a difference that did not achieve statistical significance. Progression-free survival was superior in the group assigned to surgery compared with those not undergoing resection (median: 12.8 months vs 10.5 months).
A Surveillance Epidemiology and End Results registry of more than 48,000 patients with stage III NSCLC revealed significantly better overall survival in those who received neoadjuvant radiotherapy plus surgery compared with radiation therapy alone, postoperative radiation therapy, and surgery alone.7
CLEVELAND CLINIC EXPERIENCE WITH ACCELERATED PROTOCOL
At Cleveland Clinic, the current protocol for stage IIIa and IIIb NSCLC is an accelerated multimodality regimen consisting of paclitaxel, 50 mg/m2 twice weekly for 3 weeks; carboplatin (target area under the concentration vs time curve dosing) twice weekly for 3 weeks; and daily erlotinib (phase 1 dose escalation protocol) with concurrent radiotherapy, 1.5 Gy twice daily, as induction therapy, followed by a preoperative evaluation and surgery if local control is achieved with induction treatment.
This protocol has been used in 30 patients with stage IIIa disease (median age: 61 years) with no operative mortality (62% lobectomy, 38% pneumonectomy) and a median length of stay of 6.2 days. Forty percent of patients had their disease downstaged following induction therapy. Three-year survival is approximately 60% and, at 5 years, survival is still 55%.
CONCLUSION
Multimodality therapy for NSCLC is effective and achieves favorable survival. Pathologic downstaging is an important predictor for survival but patients with residual N2 disease still have meaningful survival with resection.
A team approach to evaluation and treatment among medical oncology, radiation oncology, pulmonary medicine, and thoracic surgery is critical to successful outcome.
- Pieterman RM, van Putten JWG, Meuzelaar JL, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 2000; 343:254–261.
- Hammoud Z, Anderson RC, Meyers BF, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg 1999; 118:894–899.
- De Leyn P, Vansteenkiste J, Cuypers P, et al. Role of cervical mediastinoscopy in staging of non-small cell lung cancer without enlarged mediastinal lymph nodes on CT scan. Eur J Cardiothor Surg 1997; 12:706–712.
- Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995; 13:1880–1892.
- De Camp MM, Rice TW, Adelstein DJ, et al. Value of accelerated multimodality therapy in stage IIIA and IIIB non–small cell lung cancer. J Thor Cardiovasc Surg 2003; 126:17–27.
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009; 374:379–386.
- Koshy M, Goloubeva O, Suntharalingam M. Impact of neoadjuvant radiation on survival in stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 79:1388–1394.
- Pieterman RM, van Putten JWG, Meuzelaar JL, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 2000; 343:254–261.
- Hammoud Z, Anderson RC, Meyers BF, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg 1999; 118:894–899.
- De Leyn P, Vansteenkiste J, Cuypers P, et al. Role of cervical mediastinoscopy in staging of non-small cell lung cancer without enlarged mediastinal lymph nodes on CT scan. Eur J Cardiothor Surg 1997; 12:706–712.
- Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 1995; 13:1880–1892.
- De Camp MM, Rice TW, Adelstein DJ, et al. Value of accelerated multimodality therapy in stage IIIA and IIIB non–small cell lung cancer. J Thor Cardiovasc Surg 2003; 126:17–27.
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009; 374:379–386.
- Koshy M, Goloubeva O, Suntharalingam M. Impact of neoadjuvant radiation on survival in stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 79:1388–1394.