User login
L-ascorbic acid, more commonly know as vitamin C, is an essential micronutrient used in numerous metabolic pathways. It functions physiologically as a water-soluble antioxidant by virtue of its high reducing power, playing a key role in the function of leukocytes, protein metabolism, and production of neurotransmitters.1-3 Vitamin C also contributes to musculoskeletal health through biosynthesis of carnitine and collagen4 and enhancement of intestinal absorption of dietary iron5 from plants and vegetables. Unlike most animals, humans are unable to synthesize this essential vitamin and therefore require intake from natural dietary sources or supplements.6 The ability of vitamin C to prevent or treat disease has been an area of research interest since the vitamin was identified and isolated by Szent-Györgyi in the 1930s.7-16 Research in orthopedic surgery has focused on the effects of vitamin C on fracture healing, its potential use in preventing complex regional pain syndrome (CRPS), and its role in the pathophysiology of osteoarthritis. In this article, we review the basics of vitamin C metabolism and summarize the evidence surrounding the role of vitamin C supplementation in orthopedics.
Sources and Metabolism
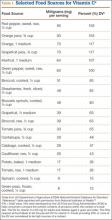
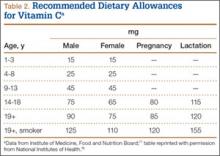
Vitamin C is found naturally in many fruits and vegetables (Table 1) and is a common fortification in cereals, juices, and multivitamins. Daily recommended intake (Table 2) depends on age and smoking status. Absorption occurs in the distal small intestine, with blood plasma vitamin C concentrations reflecting dietary intake. Pharmacokinetic studies have shown that vitamin C concentrations are tightly regulated through absorption, tissue accumulation, and renal resorption, with plasma concentrations rarely exceeding 100 μmol/L without additional supplementation.17 Although the usual dietary doses of 100 mg/d (adult) are almost completely absorbed, producing a plasma concentration of 60 μmol/L, higher intake results in an increasingly smaller fraction absorbed.1,18 Intake of more than 1000 mg/d results in less than 50% absorption19 (unmetabolized vitamin C is excreted in stool and urine1). Even at higher doses, vitamin C has low toxicity3; the most common complaints are diarrhea, nausea, and abdominal cramps caused by the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract.1
Vitamin C Deficiency
The relationship between vitamin C deficiency and the development of scurvy has been documented for centuries. Symptoms are described in the ancient Egyptian, Greek, and Roman literature.20 Ascorbic acid is essential for normal collagen function, as it is a required cofactor for enzymatic transfer of hydroxyl groups to select proline and lysine residues during procollagen formation. Hydroxylysine contributes to the intermolecular cross-links in collagen, and hydroxyproline stabilizes the triple-helix structure of collagen.21 Insufficient vitamin C during this process results in collagen that is non-cross-linked, nonhelical, structurally unstable, and weak.21 Clinical manifestations of scurvy stem from an underlying impairment of collagen production causing a systemic decrease in connective tissue integrity, capillary fragility, poor wound healing, fatigue, myalgias, arthritis, and even death.22 Vitamin C deficiency has also been implicated as a cause of diffuse bleeding in surgical patients with normal coagulation parameters secondary to capillary fragility.23 In the United States, the 2003–2004 National Health and Nutrition Examination Survey (NHANES) measured serum vitamin C concentrations in 7277 noninstitutionalized patients 6 years old or older.24 Age-adjusted incidence of subnormal serum vitamin C levels (<28 μmol/L) was 19.6%, and incidence of frank vitamin C deficiency (<11.4 μmol/L) was 7.1%. Reported rates of vitamin C deficiency in hospitalized patients are much higher, with 47% to 60% having subnormal values (<28 μmol/L) and 17% to 19% being vitamin C–deficient (<11.4 μmol/L).22,25 Identified risk factors for hypovitaminosis C include advanced age, obesity, low socioeconomic status, unemployment, male sex, and concomitant alcohol and tobacco consumption.22,24,25
Fracture Healing and Prevention
The effects of vitamin C deficiency on bone healing have been studied with animal models as early as the 1940s.26,27 Early experiments using guinea pigs demonstrated failure of bone graft incorporation, delayed collagen maturation, and decreased collagen and callus formation in scorbutic animals compared with controls that received vitamin C supplementation.26,27 Based on his work with guinea pigs, Bourne26 reported in 1942 that vitamin C deficiency significantly inhibited the reparative process in damaged bone and that patients with fractures should receive vitamin C supplementation. Building on this early research, Yilmaz and colleagues28 found faster histologic healing for tibia fractures in a rat model for animals that received a single injection of vitamin C 0.5 mg/kg compared with a nonscorbutic control group, and Sarisözen and colleagues29 showed significantly accelerated histologic bone formation and mineralization at the fracture site for rats that received vitamin C supplementation. Moreover, Kipp and colleagues30 found that scorbutic guinea pigs had lower bone mineral density (BMD), decreased bone mineral content, and impaired collagen synthesis of articular cartilage and tendons compared with nondeficient controls.
Besides promoting bone formation, vitamin C improves the mechanical strength of callus formation. Alcantara-Martos and colleagues31 used an osteogenic disorder Shionogi (ODS) rat model to examine the effects of vitamin C intake on femoral fracture healing. This particular animal model is unable to produce its own vitamin C. The groups with lower serum vitamin C levels demonstrated lower mechanical resistance of the fracture callus to torsional loads 5 weeks after fracture. Moreover, the group that received vitamin C supplementation showed higher histologic grade of callus formation and demonstrated faster healing rates. The authors suggested that subclinical vitamin C deficiency can delay fracture healing and that vitamin C supplementation in nondeficient patients would improve bone healing.
Other research has demonstrated a link between vitamin C and mesenchymal cell differentiation. Mohan and colleagues32 used an sfx mouse model to show that vitamin C deficiency results in decreased bone formation secondary to impaired osteoblast differentiation, diminished bone density, and development of spontaneous fractures. The authors indicated that not only is vitamin C essential for maintenance of differentiated functions of osteoblasts, but deficiency during early active growth may affect peak BMD levels in humans. Additional studies have demonstrated the role of vitamin C in endochondral bone formation through both induction of osteoblast differentiation and modulation of gene expression in hypertrophic chondrocytes.33-36 Chronic vitamin C deficiency has been found to depress osteoblast function and differentiation of chondrocytes.37 More recently, Kim and colleagues38 examined the effect of vitamin C insufficiency in Gulo-deficient mice, which are unable to synthesize ascorbic acid. Ascorbic acid insufficiency over 4 weeks led to decreased plasma levels of osteocalcin and bone formation in vivo as well as significantly diminished metaphyseal trabecular bone. Despite all the evidence demonstrating the importance of vitamin C in bone formation and maintenance, many of the underlying processes in this relationship have yet to be determined.
Bone Mineral Density
Several observational studies have found a positive association between vitamin C intake and BMD in postmenopausal women. In a retrospective, cross-sectional study by Hall and Greendale,39 a positive association was found between vitamin C intake and BMD of the femoral neck in 775 participants in the Postmenopausal Estrogen/Progestin Interventions trial. After calcium intake, physical activity level, smoking, estrogen use, age, and body mass index were adjusted for, each 100-mg increase in dietary vitamin C was associated with a 0.017 g/cm2 increase in BMD. Wang and colleagues40 found a positive association between dietary vitamin C intake and femoral neck BMD in a retrospective analysis of 125 postmenopausal Mexican American women. Other observational studies have reported that decreased intake of vitamin C is associated with osteoporosis41 and increased rates of BMD loss42 and that supplementation with vitamin C may suppress bone resorption in postmenopausal women.43
The results of these studies contrast with the findings of Leveille and colleagues,44 who examined the relationship between dietary vitamin C and hip BMD in 1892 postmenopausal women. Although the authors found that women (age, 55-64 years) using vitamin C supplements for more than 10 years had an average BMD 6.7% higher than that of nonusers, they did not find any association between dietary vitamin C intake and BMD. Moreover, NHANES III also found inconsistent associations between vitamin C and BMD among 13,080 adults surveyed in the United States.45 Although for premenopausal women dietary ascorbic acid was associated with increased BMD, for postmenopausal women with a history of smoking and estrogen replacement, it was actually associated with lower BMD values. For other subgroups in the study, the relationship was also inconsistent or nonlinear.
The exact mechanism by which ascorbic acid contributes to BMD is not fully delineated. However, it likely is related to the known role of vitamin C in collagen formation, bone matrix development, osteoblast differentiation, and its antioxidant effects limiting bone resorption.44,46
Hip Fractures
Besides demonstrating positive effects of vitamin C on bone healing and BMD, epidemiologic studies have found evidence of a protective effect of vitamin C on hip fracture risk. In a study of the Swedish Mammography cohort, 66,651 women (age, 40-76 years) were prospectively followed.47 The authors found that the odds ratio (OR) for hip fractures among smokers with a low intake of vitamin E (median intake, ≤6.2 mg/d) was 3.0 (95% CI, 1.6-5.4) and for vitamin C (median intake, ≤67 mg/d) was 3.0 (95% CI, 1.6-5.6). Moreover, in smokers with a low intake of both vitamins E and C, OR increased to 4.9 (95% CI, 2.2-11.0). In addition, the Utah Study of Nutrition and Bone Health matched 1215 cases of hip fractures in patients who had ever smoked (age, >50 years) with 1349 controls and found that vitamin C intake above 159 mg/d had a significant protective effect on the incidence of hip fracture; however, a graded relationship was not observed.48 Despite the inconsistencies in the NHANES III study regarding the relationship between vitamin C and BMD, Simon and Hudes45 found that serum vitamin C was associated with lower risk for self-reported fracture in postmenopausal women who had ever smoked and had a history of estrogen therapy (OR, 0.51; 95% CI, 0.36-0.70). Finally, Sahni and colleagues49 followed 958 Framingham cohort men and women (mean age, 75 years) over 17 years and found that those in the highest tertile of total vitamin C intake (median, 313 mg/d) had significantly fewer hip fractures and nonvertebral fractures compared with those in the lowest tertile of intake (median, 94 mg/d). Dietary vitamin C intake was not associated with fracture risk in this study.
Complex Regional Pain Syndrome
Type 1 CRPS is a debilitating condition characterized by severe pain, swelling, and vasomotor instability. It is commonly precipitated by an injury or surgery to an extremity and is a dreaded sequelae in orthopedics,50 with incidence rates of 10% to 22% in wrist fractures51-53 and 10% after foot and ankle surgery.54 Although the pathophysiology of CRPS remains unknown, dysregulation and increased permeability of the vasculature caused by free radicals are thought to play an important role.55 In dermal burns, high doses of vitamin C therapy slowed progression of vascular permeability and therefore reduced extravascular leakage of fluids and protein.56,57 The ability of vitamin C to prevent CRPS has been studied in only a handful of trials.
In a double-blind trial, Zollinger and colleagues51 randomized 127 conservatively treated distal radius fractures to receive either vitamin C 500 mg or placebo daily for 50 days starting on day of injury. Incidence of CRPS (using the diagnostic criteria proposed by Veldman and colleagues58) at 1-year follow-up was 22% in the placebo group and 7% in the vitamin C group (95% CI for difference, 2%-26%). Complaints while wearing the cast and fracture type increased the risk for developing CRPS. This initial study was followed up by a prospective, randomized, double-blind multicenter trial by the same authors,52 who had 416 patients with 427 wrist fractures receive either placebo or vitamin C 200 mg/d, 500 mg/d, or 1500 mg/d for 50 days. This follow-up study included both operative (11%) and nonoperative (89%) distal radius fractures. Incidence of CRPS was 10.1% in the placebo group and 2.4% in the vitamin C group (P < .002). Although there was an appreciable drop in the relative risk (RR) of developing CRPS between the vitamin C 200-mg/d and 500-mg/d groups (0.41-0.17), there was no additional benefit in the 1500-mg/d group. Pooling the data for these 2 randomized trials showed that the overall RR for developing CRPS was lower with vitamin C supplementation (RR, 0.28; 95% CI, 0.14-0.56; P = .0003).59
Results of the 2 trials by Zollinger and colleagues51,52 have been met with several concerns.60-62 As a corollary to the unclear etiology of CRPS, several different sets of diagnostic criteria exist, and the criteria are somewhat subjective and imprecise. Although both trials used the Veldman criteria,58 the incidence of CRPS in the placebo group dropped unexpectedly between trials, from 22% to 10.1%, and the results may have been different had other criteria been used. Moreover, the idea that toxic oxygen radicals have a role in CRPS and that vitamin C can scavenge these radicals is based on limited data.61 In the absence of a clear pathophysiologic explanation, some surgeons have been reluctant to treat patients with vitamin C supplementation.
Cazeneuve and colleagues53 also studied the effect of vitamin C supplementation on CRPS in patients with distal radius fractures treated with reduction and intrafocal pinning. Group 1 consisted of 100 patients (treated from 1995 to 1998) who did not receive vitamin C supplementation, and group 2 consisted of 95 patients (treated from 1998 to 2002) who received vitamin C 1000 mg/d for 45 days starting on day of fracture. Patients were followed for up to 90 days after surgery. Incidence of CRPS type 1 was 10% in the untreated group and 2.1% in the group that received vitamin C supplementation.
Vitamin C prophylaxis for CRPS has also been studied in foot and ankle surgery. Besse and colleagues54 prospectively compared 2 chronologically successive groups that received (235 feet) or did not receive (185 feet) vitamin C 1000-mg/d supplementation for 45 days. Incidence of CRPS type 1 as diagnosed with International Association for the Study of Pain (IASP) criteria dropped from 9.6% to 1.7% with vitamin C supplementation. In a case series, Zollinger and colleagues63 examined CRPS type 1 rates after performing cementless total trapeziometacarpal semiconstrained joint prosthesis implantations for trapeziometacarpal arthritis. Forty implantations were performed in 34 patients. All patients received vitamin C 500 mg/d for CRPS prevention starting 2 days before surgery for 50 days. There were no cases of CRPS in the postoperative period, according to Veldman or IASP criteria. Although the results of the studies by Cazeneuve and colleagues53 and Besse and colleagues54 agree with those of the distal radius fracture trials by Zollinger and colleagues,51,52 the quasi-experimental design and the lack of blinding and randomization temper the conclusions that can be drawn because of the risk for significant bias.
In a recent systematic review examining the effectiveness of vitamin C supplementation in preventing CRPS in trauma and surgery in the extremities, Shibuya and colleagues64 concluded that taking at least 500 mg of vitamin C daily for 45 to 50 days after injury or surgery may help decrease the incidence of CRPS after a traumatic event.
Osteoarthritis
Damage caused by free radicals has long been thought to play an important role in osteoarthritis (OA).65-67 A cross-sectional study in knee OA found that amounts of joint fluid antioxidants were lower in patients with severe arthritis than in those with intact cartilage, further implicating free radicals in the pathophysiology of OA.68 Use of vitamin C for prophylaxis against development or progression of OA is therefore a hot research topic. Thus far, animal studies have had mixed results—several showing a chondroprotective effect of vitamin C69,70 and others finding either no effect or even a positive association with the development of arthritis.71
The literature on human subjects, chiefly observational studies, is just as controversial. Wang and colleagues40 found vitamin C intake associated with both a 50% risk reduction of bone marrow lesions on magnetic resonance imaging over a 10-year interval (OR, 0.5; 95% CI, 0.29-0.87) and inversely associated with the tibial plateau bone area. Similarly, the Clearwater Osteoarthritis Study, which followed 1023 patients (age, >40 years), showed that participants who took vitamin C supplements were 11% less likely to develop radiographic evidence of OA (RR, 0.89; 95% CI, 0.85-0.93).72 Nonetheless, other studies have failed to show such associations73 or have demonstrated the opposite effect. Chaganti and colleagues74 analyzed levels of vitamins C and E in the Multicenter Osteoarthritis Study (MOST) cohort of 3026 men and women (age, 50-79 years) and found higher vitamin levels were not protective against incidence of radiographic whole-knee OA and may even have been associated with increased risk.
Conclusion
Vitamin C is an essential micronutrient and a powerful water-soluble antioxidant in numerous biochemical pathways that influence bone health. It has been implicated in the biology of fracture healing, and vitamin C supplementation has been proposed as prophylaxis against hip fractures based on observational data. Results of 2 high-quality double-blind randomized trials support use of vitamin C as prophylaxis against CRPS in wrist fractures treated conservatively and operatively; the evidence for foot and ankle surgery is weaker. Use of vitamin C in OA prevention has tremendous potential, though animal and human study results are controversial. Heterogeneous results and lack of prospective trials preclude any recommendation at this time.
1. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5(2):66-74.
2. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377-6381.
3. Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637-640.
4. Padh H. Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991;49(3):65-70.
5. Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993;51(11):313-326.
6. Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171-2184.
7. Szent-Györgyi A. On the function of hexuronic acid in the respiration of the cabbage leaf. J Biol Chem. 1931;90(1):385-393.
8. Svirbely JL, Szent-Györgyi A. The chemical nature of vitamin C. Biochem J. 1933;27(1):279-285.
9. Pauling L. Vitamin C and the Common Cold. San Francisco, CA: Freeman; 1970.
10. Spittle CR. Atherosclerosis and vitamin C. Lancet. 1971;2(7737):1280-1281.
11. Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354(9181):810-816.
12. Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53(1 suppl):270S-282S.
13. Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687-690.
14. Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980.
15. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145-1154.
16. Roberts JM, Myatt L, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282-1291.
17. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
18. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415-1423.
19. Glatthaar BE, Hornig DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357-377.
20. Carpenter KJ. The History of Scurvy and Vitamin C. New York, NY: Cambridge University Press; 1986.
21. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879-2882.
22. Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14(7):419-425.
23. Blee TH, Cogbill TH, Lambert PJ. Hemorrhage associated with vitamin C deficiency in surgical patients. Surgery. 2002;131(4):408-412.
24. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252-1263.
25. Gan R, Eintracht S, Hoffer LJ. Vitamin C deficiency in a university teaching hospital. J Am Coll Nutr. 2008;27(3):428-433.
26. Bourne G. The effect of graded doses of vitamin C upon the regeneration of bone in guinea-pigs on a scorbutic diet. J Physiol. 1942;101(3):327-336.
27. Bourne GH. The relative importance of periosteum and endosteum in bone healing and the relationship of vitamin C to their activities. Proc R Soc Med. 1944;37(6):275-279.
28. Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121(7):426-428.
29. Sarisözen B, Durak K, Dinçer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30(3):309-313.
30. Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18(3):281-288.
31. Alcantara-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly osteogenic disorder Shionogi rats. J Bone Joint Surg Br. 2007;89(3):402-407.
32. Mohan S, Kapoor A, Singgih A, et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J Bone Miner Res. 2005;20(9):1597-1610.
33. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143(2):213-221.
34. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7(2):235-246.
35. Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264(29):17281-17286.
36. Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid–dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11(8):1103-1113.
37. Sakamoto Y, Takano Y. Morphological influence of ascorbic acid deficiency on endochondral ossification in osteogenic disorder Shionogi rat. Anat Rec. 2002;268(2):93-104.
38. Kim W, Bae S, Kim H, et al. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anat Cell Biol. 2013;46(4):254-261.
39. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63(3):183-189.
40. Wang Y, Hodge AM, Wluka AE, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66.
41. Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523-1527.
42. Kaptoge S, Welch A, McTaggart A, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis Int. 2003;14(5):418-428.
43. Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health. 2006;15(3):295-300.
44. Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington state, USA. J Epidemiol Community Health. 1997;51(5):479-485.
45. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154(5):427-433.
46. Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):581-588.
47. Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14(1):129-135.
48. Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol. 2006;163(1):9-17.
49. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24(6):1086-1094.
50. Rho RH, Brewer RP, Lamer TJ, Wilson PR. Complex regional pain syndrome. Mayo Clin Proc. 2002;77(2):174-180.
51. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 1999;354(9195):2025-2028.
52. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose–response study. J Bone Joint Surg Am. 2007;89(7):1424-1431.
53. Cazeneuve JF, Leborgne JM, Kermad K, Hassan Y. Vitamin C and prevention of reflex sympathetic dystrophy following surgical management of distal radius fractures [in French]. Acta Orthop Belg. 2002;68(5):481-484.
54. Besse JL, Gadeyne S, Galand-Desme S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009;15(4):179-182.
55. Goris RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun. 1987;3(1-5):13-18.
56. Matsuda T, Tanaka H, Shimazaki S, et al. High-dose vitamin C therapy for extensive deep dermal burns. Burns. 1992;18(2):127-131.
57. Matsuda T, Tanaka H, Hanumadass M, et al. Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil. 1992;13(5):560-566.
58. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012-1016.
59. Zollinger PE. The administration of vitamin C in prevention of CRPS-I after distal radial fractures and hand surgery—a review of two RCTs and one observational prospective study. Open Conference Proc J. 2011;2:1-4.
60. Rogers BA, Ricketts DM. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2008;90(2):447-448.
61. Amadio PC. Vitamin C reduced the incidence of reflex sympathetic dystrophy after wrist fracture. J Bone Joint Surg Am. 2000;82(6):873.
62. Frolke JP. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2007;89(11):2550-2551.
63. Zollinger PE, Unal H, Ellis ML, Tuinebreijer WE. Clinical results of 40 consecutive basal thumb prostheses and no CRPS type I after vitamin C prophylaxis. Open Orthop J. 2010;4:62-66.
64. Shibuya N, Humphers JM, Agarwal MR, Jupiter DC. Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery—systematic review and meta-analysis. J Foot Ankle Surg. 2013;52(1):62-66.
65. Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32(7):562-567.
66. McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648-656.
67. Kaiki G, Tsuji H, Yonezawa T, et al. Osteoarthrosis induced by intra-articular hydrogen peroxide injection and running load. J Orthop Res. 1990;8(5):731-740.
68. Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515-521.
69. Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol. 1990;71(2):279-293.
70. Kurz B, Jost B, Schunke M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthritis Cartilage. 2002;10(2):119-126.
71. Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50(6):1822-1831.
72. Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14(4):709-715.
73. Hill J, Bird HA. Failure of selenium-ace to improve osteoarthritis. Br J Rheumatol. 1990;29(3):211-213.
74. Chaganti RK, Tolstykh I, Javaid MK, et al; Multicenter Osteoarthritis Study Group (MOST). High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190-196.
75. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Release 26. http://www.ars.usda.gov/Services/docs.htm?docid=24936. Published August 2013. Revised November 2013. Accessed May 14, 2015.
76. National Institutes of Health, Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. National Institutes of Health website. http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/. Reviewed June 5, 2013. Accessed May 14, 2015.
77. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000.
L-ascorbic acid, more commonly know as vitamin C, is an essential micronutrient used in numerous metabolic pathways. It functions physiologically as a water-soluble antioxidant by virtue of its high reducing power, playing a key role in the function of leukocytes, protein metabolism, and production of neurotransmitters.1-3 Vitamin C also contributes to musculoskeletal health through biosynthesis of carnitine and collagen4 and enhancement of intestinal absorption of dietary iron5 from plants and vegetables. Unlike most animals, humans are unable to synthesize this essential vitamin and therefore require intake from natural dietary sources or supplements.6 The ability of vitamin C to prevent or treat disease has been an area of research interest since the vitamin was identified and isolated by Szent-Györgyi in the 1930s.7-16 Research in orthopedic surgery has focused on the effects of vitamin C on fracture healing, its potential use in preventing complex regional pain syndrome (CRPS), and its role in the pathophysiology of osteoarthritis. In this article, we review the basics of vitamin C metabolism and summarize the evidence surrounding the role of vitamin C supplementation in orthopedics.
Sources and Metabolism
Vitamin C is found naturally in many fruits and vegetables (Table 1) and is a common fortification in cereals, juices, and multivitamins. Daily recommended intake (Table 2) depends on age and smoking status. Absorption occurs in the distal small intestine, with blood plasma vitamin C concentrations reflecting dietary intake. Pharmacokinetic studies have shown that vitamin C concentrations are tightly regulated through absorption, tissue accumulation, and renal resorption, with plasma concentrations rarely exceeding 100 μmol/L without additional supplementation.17 Although the usual dietary doses of 100 mg/d (adult) are almost completely absorbed, producing a plasma concentration of 60 μmol/L, higher intake results in an increasingly smaller fraction absorbed.1,18 Intake of more than 1000 mg/d results in less than 50% absorption19 (unmetabolized vitamin C is excreted in stool and urine1). Even at higher doses, vitamin C has low toxicity3; the most common complaints are diarrhea, nausea, and abdominal cramps caused by the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract.1
Vitamin C Deficiency
The relationship between vitamin C deficiency and the development of scurvy has been documented for centuries. Symptoms are described in the ancient Egyptian, Greek, and Roman literature.20 Ascorbic acid is essential for normal collagen function, as it is a required cofactor for enzymatic transfer of hydroxyl groups to select proline and lysine residues during procollagen formation. Hydroxylysine contributes to the intermolecular cross-links in collagen, and hydroxyproline stabilizes the triple-helix structure of collagen.21 Insufficient vitamin C during this process results in collagen that is non-cross-linked, nonhelical, structurally unstable, and weak.21 Clinical manifestations of scurvy stem from an underlying impairment of collagen production causing a systemic decrease in connective tissue integrity, capillary fragility, poor wound healing, fatigue, myalgias, arthritis, and even death.22 Vitamin C deficiency has also been implicated as a cause of diffuse bleeding in surgical patients with normal coagulation parameters secondary to capillary fragility.23 In the United States, the 2003–2004 National Health and Nutrition Examination Survey (NHANES) measured serum vitamin C concentrations in 7277 noninstitutionalized patients 6 years old or older.24 Age-adjusted incidence of subnormal serum vitamin C levels (<28 μmol/L) was 19.6%, and incidence of frank vitamin C deficiency (<11.4 μmol/L) was 7.1%. Reported rates of vitamin C deficiency in hospitalized patients are much higher, with 47% to 60% having subnormal values (<28 μmol/L) and 17% to 19% being vitamin C–deficient (<11.4 μmol/L).22,25 Identified risk factors for hypovitaminosis C include advanced age, obesity, low socioeconomic status, unemployment, male sex, and concomitant alcohol and tobacco consumption.22,24,25
Fracture Healing and Prevention
The effects of vitamin C deficiency on bone healing have been studied with animal models as early as the 1940s.26,27 Early experiments using guinea pigs demonstrated failure of bone graft incorporation, delayed collagen maturation, and decreased collagen and callus formation in scorbutic animals compared with controls that received vitamin C supplementation.26,27 Based on his work with guinea pigs, Bourne26 reported in 1942 that vitamin C deficiency significantly inhibited the reparative process in damaged bone and that patients with fractures should receive vitamin C supplementation. Building on this early research, Yilmaz and colleagues28 found faster histologic healing for tibia fractures in a rat model for animals that received a single injection of vitamin C 0.5 mg/kg compared with a nonscorbutic control group, and Sarisözen and colleagues29 showed significantly accelerated histologic bone formation and mineralization at the fracture site for rats that received vitamin C supplementation. Moreover, Kipp and colleagues30 found that scorbutic guinea pigs had lower bone mineral density (BMD), decreased bone mineral content, and impaired collagen synthesis of articular cartilage and tendons compared with nondeficient controls.
Besides promoting bone formation, vitamin C improves the mechanical strength of callus formation. Alcantara-Martos and colleagues31 used an osteogenic disorder Shionogi (ODS) rat model to examine the effects of vitamin C intake on femoral fracture healing. This particular animal model is unable to produce its own vitamin C. The groups with lower serum vitamin C levels demonstrated lower mechanical resistance of the fracture callus to torsional loads 5 weeks after fracture. Moreover, the group that received vitamin C supplementation showed higher histologic grade of callus formation and demonstrated faster healing rates. The authors suggested that subclinical vitamin C deficiency can delay fracture healing and that vitamin C supplementation in nondeficient patients would improve bone healing.
Other research has demonstrated a link between vitamin C and mesenchymal cell differentiation. Mohan and colleagues32 used an sfx mouse model to show that vitamin C deficiency results in decreased bone formation secondary to impaired osteoblast differentiation, diminished bone density, and development of spontaneous fractures. The authors indicated that not only is vitamin C essential for maintenance of differentiated functions of osteoblasts, but deficiency during early active growth may affect peak BMD levels in humans. Additional studies have demonstrated the role of vitamin C in endochondral bone formation through both induction of osteoblast differentiation and modulation of gene expression in hypertrophic chondrocytes.33-36 Chronic vitamin C deficiency has been found to depress osteoblast function and differentiation of chondrocytes.37 More recently, Kim and colleagues38 examined the effect of vitamin C insufficiency in Gulo-deficient mice, which are unable to synthesize ascorbic acid. Ascorbic acid insufficiency over 4 weeks led to decreased plasma levels of osteocalcin and bone formation in vivo as well as significantly diminished metaphyseal trabecular bone. Despite all the evidence demonstrating the importance of vitamin C in bone formation and maintenance, many of the underlying processes in this relationship have yet to be determined.
Bone Mineral Density
Several observational studies have found a positive association between vitamin C intake and BMD in postmenopausal women. In a retrospective, cross-sectional study by Hall and Greendale,39 a positive association was found between vitamin C intake and BMD of the femoral neck in 775 participants in the Postmenopausal Estrogen/Progestin Interventions trial. After calcium intake, physical activity level, smoking, estrogen use, age, and body mass index were adjusted for, each 100-mg increase in dietary vitamin C was associated with a 0.017 g/cm2 increase in BMD. Wang and colleagues40 found a positive association between dietary vitamin C intake and femoral neck BMD in a retrospective analysis of 125 postmenopausal Mexican American women. Other observational studies have reported that decreased intake of vitamin C is associated with osteoporosis41 and increased rates of BMD loss42 and that supplementation with vitamin C may suppress bone resorption in postmenopausal women.43
The results of these studies contrast with the findings of Leveille and colleagues,44 who examined the relationship between dietary vitamin C and hip BMD in 1892 postmenopausal women. Although the authors found that women (age, 55-64 years) using vitamin C supplements for more than 10 years had an average BMD 6.7% higher than that of nonusers, they did not find any association between dietary vitamin C intake and BMD. Moreover, NHANES III also found inconsistent associations between vitamin C and BMD among 13,080 adults surveyed in the United States.45 Although for premenopausal women dietary ascorbic acid was associated with increased BMD, for postmenopausal women with a history of smoking and estrogen replacement, it was actually associated with lower BMD values. For other subgroups in the study, the relationship was also inconsistent or nonlinear.
The exact mechanism by which ascorbic acid contributes to BMD is not fully delineated. However, it likely is related to the known role of vitamin C in collagen formation, bone matrix development, osteoblast differentiation, and its antioxidant effects limiting bone resorption.44,46
Hip Fractures
Besides demonstrating positive effects of vitamin C on bone healing and BMD, epidemiologic studies have found evidence of a protective effect of vitamin C on hip fracture risk. In a study of the Swedish Mammography cohort, 66,651 women (age, 40-76 years) were prospectively followed.47 The authors found that the odds ratio (OR) for hip fractures among smokers with a low intake of vitamin E (median intake, ≤6.2 mg/d) was 3.0 (95% CI, 1.6-5.4) and for vitamin C (median intake, ≤67 mg/d) was 3.0 (95% CI, 1.6-5.6). Moreover, in smokers with a low intake of both vitamins E and C, OR increased to 4.9 (95% CI, 2.2-11.0). In addition, the Utah Study of Nutrition and Bone Health matched 1215 cases of hip fractures in patients who had ever smoked (age, >50 years) with 1349 controls and found that vitamin C intake above 159 mg/d had a significant protective effect on the incidence of hip fracture; however, a graded relationship was not observed.48 Despite the inconsistencies in the NHANES III study regarding the relationship between vitamin C and BMD, Simon and Hudes45 found that serum vitamin C was associated with lower risk for self-reported fracture in postmenopausal women who had ever smoked and had a history of estrogen therapy (OR, 0.51; 95% CI, 0.36-0.70). Finally, Sahni and colleagues49 followed 958 Framingham cohort men and women (mean age, 75 years) over 17 years and found that those in the highest tertile of total vitamin C intake (median, 313 mg/d) had significantly fewer hip fractures and nonvertebral fractures compared with those in the lowest tertile of intake (median, 94 mg/d). Dietary vitamin C intake was not associated with fracture risk in this study.
Complex Regional Pain Syndrome
Type 1 CRPS is a debilitating condition characterized by severe pain, swelling, and vasomotor instability. It is commonly precipitated by an injury or surgery to an extremity and is a dreaded sequelae in orthopedics,50 with incidence rates of 10% to 22% in wrist fractures51-53 and 10% after foot and ankle surgery.54 Although the pathophysiology of CRPS remains unknown, dysregulation and increased permeability of the vasculature caused by free radicals are thought to play an important role.55 In dermal burns, high doses of vitamin C therapy slowed progression of vascular permeability and therefore reduced extravascular leakage of fluids and protein.56,57 The ability of vitamin C to prevent CRPS has been studied in only a handful of trials.
In a double-blind trial, Zollinger and colleagues51 randomized 127 conservatively treated distal radius fractures to receive either vitamin C 500 mg or placebo daily for 50 days starting on day of injury. Incidence of CRPS (using the diagnostic criteria proposed by Veldman and colleagues58) at 1-year follow-up was 22% in the placebo group and 7% in the vitamin C group (95% CI for difference, 2%-26%). Complaints while wearing the cast and fracture type increased the risk for developing CRPS. This initial study was followed up by a prospective, randomized, double-blind multicenter trial by the same authors,52 who had 416 patients with 427 wrist fractures receive either placebo or vitamin C 200 mg/d, 500 mg/d, or 1500 mg/d for 50 days. This follow-up study included both operative (11%) and nonoperative (89%) distal radius fractures. Incidence of CRPS was 10.1% in the placebo group and 2.4% in the vitamin C group (P < .002). Although there was an appreciable drop in the relative risk (RR) of developing CRPS between the vitamin C 200-mg/d and 500-mg/d groups (0.41-0.17), there was no additional benefit in the 1500-mg/d group. Pooling the data for these 2 randomized trials showed that the overall RR for developing CRPS was lower with vitamin C supplementation (RR, 0.28; 95% CI, 0.14-0.56; P = .0003).59
Results of the 2 trials by Zollinger and colleagues51,52 have been met with several concerns.60-62 As a corollary to the unclear etiology of CRPS, several different sets of diagnostic criteria exist, and the criteria are somewhat subjective and imprecise. Although both trials used the Veldman criteria,58 the incidence of CRPS in the placebo group dropped unexpectedly between trials, from 22% to 10.1%, and the results may have been different had other criteria been used. Moreover, the idea that toxic oxygen radicals have a role in CRPS and that vitamin C can scavenge these radicals is based on limited data.61 In the absence of a clear pathophysiologic explanation, some surgeons have been reluctant to treat patients with vitamin C supplementation.
Cazeneuve and colleagues53 also studied the effect of vitamin C supplementation on CRPS in patients with distal radius fractures treated with reduction and intrafocal pinning. Group 1 consisted of 100 patients (treated from 1995 to 1998) who did not receive vitamin C supplementation, and group 2 consisted of 95 patients (treated from 1998 to 2002) who received vitamin C 1000 mg/d for 45 days starting on day of fracture. Patients were followed for up to 90 days after surgery. Incidence of CRPS type 1 was 10% in the untreated group and 2.1% in the group that received vitamin C supplementation.
Vitamin C prophylaxis for CRPS has also been studied in foot and ankle surgery. Besse and colleagues54 prospectively compared 2 chronologically successive groups that received (235 feet) or did not receive (185 feet) vitamin C 1000-mg/d supplementation for 45 days. Incidence of CRPS type 1 as diagnosed with International Association for the Study of Pain (IASP) criteria dropped from 9.6% to 1.7% with vitamin C supplementation. In a case series, Zollinger and colleagues63 examined CRPS type 1 rates after performing cementless total trapeziometacarpal semiconstrained joint prosthesis implantations for trapeziometacarpal arthritis. Forty implantations were performed in 34 patients. All patients received vitamin C 500 mg/d for CRPS prevention starting 2 days before surgery for 50 days. There were no cases of CRPS in the postoperative period, according to Veldman or IASP criteria. Although the results of the studies by Cazeneuve and colleagues53 and Besse and colleagues54 agree with those of the distal radius fracture trials by Zollinger and colleagues,51,52 the quasi-experimental design and the lack of blinding and randomization temper the conclusions that can be drawn because of the risk for significant bias.
In a recent systematic review examining the effectiveness of vitamin C supplementation in preventing CRPS in trauma and surgery in the extremities, Shibuya and colleagues64 concluded that taking at least 500 mg of vitamin C daily for 45 to 50 days after injury or surgery may help decrease the incidence of CRPS after a traumatic event.
Osteoarthritis
Damage caused by free radicals has long been thought to play an important role in osteoarthritis (OA).65-67 A cross-sectional study in knee OA found that amounts of joint fluid antioxidants were lower in patients with severe arthritis than in those with intact cartilage, further implicating free radicals in the pathophysiology of OA.68 Use of vitamin C for prophylaxis against development or progression of OA is therefore a hot research topic. Thus far, animal studies have had mixed results—several showing a chondroprotective effect of vitamin C69,70 and others finding either no effect or even a positive association with the development of arthritis.71
The literature on human subjects, chiefly observational studies, is just as controversial. Wang and colleagues40 found vitamin C intake associated with both a 50% risk reduction of bone marrow lesions on magnetic resonance imaging over a 10-year interval (OR, 0.5; 95% CI, 0.29-0.87) and inversely associated with the tibial plateau bone area. Similarly, the Clearwater Osteoarthritis Study, which followed 1023 patients (age, >40 years), showed that participants who took vitamin C supplements were 11% less likely to develop radiographic evidence of OA (RR, 0.89; 95% CI, 0.85-0.93).72 Nonetheless, other studies have failed to show such associations73 or have demonstrated the opposite effect. Chaganti and colleagues74 analyzed levels of vitamins C and E in the Multicenter Osteoarthritis Study (MOST) cohort of 3026 men and women (age, 50-79 years) and found higher vitamin levels were not protective against incidence of radiographic whole-knee OA and may even have been associated with increased risk.
Conclusion
Vitamin C is an essential micronutrient and a powerful water-soluble antioxidant in numerous biochemical pathways that influence bone health. It has been implicated in the biology of fracture healing, and vitamin C supplementation has been proposed as prophylaxis against hip fractures based on observational data. Results of 2 high-quality double-blind randomized trials support use of vitamin C as prophylaxis against CRPS in wrist fractures treated conservatively and operatively; the evidence for foot and ankle surgery is weaker. Use of vitamin C in OA prevention has tremendous potential, though animal and human study results are controversial. Heterogeneous results and lack of prospective trials preclude any recommendation at this time.
L-ascorbic acid, more commonly know as vitamin C, is an essential micronutrient used in numerous metabolic pathways. It functions physiologically as a water-soluble antioxidant by virtue of its high reducing power, playing a key role in the function of leukocytes, protein metabolism, and production of neurotransmitters.1-3 Vitamin C also contributes to musculoskeletal health through biosynthesis of carnitine and collagen4 and enhancement of intestinal absorption of dietary iron5 from plants and vegetables. Unlike most animals, humans are unable to synthesize this essential vitamin and therefore require intake from natural dietary sources or supplements.6 The ability of vitamin C to prevent or treat disease has been an area of research interest since the vitamin was identified and isolated by Szent-Györgyi in the 1930s.7-16 Research in orthopedic surgery has focused on the effects of vitamin C on fracture healing, its potential use in preventing complex regional pain syndrome (CRPS), and its role in the pathophysiology of osteoarthritis. In this article, we review the basics of vitamin C metabolism and summarize the evidence surrounding the role of vitamin C supplementation in orthopedics.
Sources and Metabolism
Vitamin C is found naturally in many fruits and vegetables (Table 1) and is a common fortification in cereals, juices, and multivitamins. Daily recommended intake (Table 2) depends on age and smoking status. Absorption occurs in the distal small intestine, with blood plasma vitamin C concentrations reflecting dietary intake. Pharmacokinetic studies have shown that vitamin C concentrations are tightly regulated through absorption, tissue accumulation, and renal resorption, with plasma concentrations rarely exceeding 100 μmol/L without additional supplementation.17 Although the usual dietary doses of 100 mg/d (adult) are almost completely absorbed, producing a plasma concentration of 60 μmol/L, higher intake results in an increasingly smaller fraction absorbed.1,18 Intake of more than 1000 mg/d results in less than 50% absorption19 (unmetabolized vitamin C is excreted in stool and urine1). Even at higher doses, vitamin C has low toxicity3; the most common complaints are diarrhea, nausea, and abdominal cramps caused by the osmotic effect of unabsorbed vitamin C in the gastrointestinal tract.1
Vitamin C Deficiency
The relationship between vitamin C deficiency and the development of scurvy has been documented for centuries. Symptoms are described in the ancient Egyptian, Greek, and Roman literature.20 Ascorbic acid is essential for normal collagen function, as it is a required cofactor for enzymatic transfer of hydroxyl groups to select proline and lysine residues during procollagen formation. Hydroxylysine contributes to the intermolecular cross-links in collagen, and hydroxyproline stabilizes the triple-helix structure of collagen.21 Insufficient vitamin C during this process results in collagen that is non-cross-linked, nonhelical, structurally unstable, and weak.21 Clinical manifestations of scurvy stem from an underlying impairment of collagen production causing a systemic decrease in connective tissue integrity, capillary fragility, poor wound healing, fatigue, myalgias, arthritis, and even death.22 Vitamin C deficiency has also been implicated as a cause of diffuse bleeding in surgical patients with normal coagulation parameters secondary to capillary fragility.23 In the United States, the 2003–2004 National Health and Nutrition Examination Survey (NHANES) measured serum vitamin C concentrations in 7277 noninstitutionalized patients 6 years old or older.24 Age-adjusted incidence of subnormal serum vitamin C levels (<28 μmol/L) was 19.6%, and incidence of frank vitamin C deficiency (<11.4 μmol/L) was 7.1%. Reported rates of vitamin C deficiency in hospitalized patients are much higher, with 47% to 60% having subnormal values (<28 μmol/L) and 17% to 19% being vitamin C–deficient (<11.4 μmol/L).22,25 Identified risk factors for hypovitaminosis C include advanced age, obesity, low socioeconomic status, unemployment, male sex, and concomitant alcohol and tobacco consumption.22,24,25
Fracture Healing and Prevention
The effects of vitamin C deficiency on bone healing have been studied with animal models as early as the 1940s.26,27 Early experiments using guinea pigs demonstrated failure of bone graft incorporation, delayed collagen maturation, and decreased collagen and callus formation in scorbutic animals compared with controls that received vitamin C supplementation.26,27 Based on his work with guinea pigs, Bourne26 reported in 1942 that vitamin C deficiency significantly inhibited the reparative process in damaged bone and that patients with fractures should receive vitamin C supplementation. Building on this early research, Yilmaz and colleagues28 found faster histologic healing for tibia fractures in a rat model for animals that received a single injection of vitamin C 0.5 mg/kg compared with a nonscorbutic control group, and Sarisözen and colleagues29 showed significantly accelerated histologic bone formation and mineralization at the fracture site for rats that received vitamin C supplementation. Moreover, Kipp and colleagues30 found that scorbutic guinea pigs had lower bone mineral density (BMD), decreased bone mineral content, and impaired collagen synthesis of articular cartilage and tendons compared with nondeficient controls.
Besides promoting bone formation, vitamin C improves the mechanical strength of callus formation. Alcantara-Martos and colleagues31 used an osteogenic disorder Shionogi (ODS) rat model to examine the effects of vitamin C intake on femoral fracture healing. This particular animal model is unable to produce its own vitamin C. The groups with lower serum vitamin C levels demonstrated lower mechanical resistance of the fracture callus to torsional loads 5 weeks after fracture. Moreover, the group that received vitamin C supplementation showed higher histologic grade of callus formation and demonstrated faster healing rates. The authors suggested that subclinical vitamin C deficiency can delay fracture healing and that vitamin C supplementation in nondeficient patients would improve bone healing.
Other research has demonstrated a link between vitamin C and mesenchymal cell differentiation. Mohan and colleagues32 used an sfx mouse model to show that vitamin C deficiency results in decreased bone formation secondary to impaired osteoblast differentiation, diminished bone density, and development of spontaneous fractures. The authors indicated that not only is vitamin C essential for maintenance of differentiated functions of osteoblasts, but deficiency during early active growth may affect peak BMD levels in humans. Additional studies have demonstrated the role of vitamin C in endochondral bone formation through both induction of osteoblast differentiation and modulation of gene expression in hypertrophic chondrocytes.33-36 Chronic vitamin C deficiency has been found to depress osteoblast function and differentiation of chondrocytes.37 More recently, Kim and colleagues38 examined the effect of vitamin C insufficiency in Gulo-deficient mice, which are unable to synthesize ascorbic acid. Ascorbic acid insufficiency over 4 weeks led to decreased plasma levels of osteocalcin and bone formation in vivo as well as significantly diminished metaphyseal trabecular bone. Despite all the evidence demonstrating the importance of vitamin C in bone formation and maintenance, many of the underlying processes in this relationship have yet to be determined.
Bone Mineral Density
Several observational studies have found a positive association between vitamin C intake and BMD in postmenopausal women. In a retrospective, cross-sectional study by Hall and Greendale,39 a positive association was found between vitamin C intake and BMD of the femoral neck in 775 participants in the Postmenopausal Estrogen/Progestin Interventions trial. After calcium intake, physical activity level, smoking, estrogen use, age, and body mass index were adjusted for, each 100-mg increase in dietary vitamin C was associated with a 0.017 g/cm2 increase in BMD. Wang and colleagues40 found a positive association between dietary vitamin C intake and femoral neck BMD in a retrospective analysis of 125 postmenopausal Mexican American women. Other observational studies have reported that decreased intake of vitamin C is associated with osteoporosis41 and increased rates of BMD loss42 and that supplementation with vitamin C may suppress bone resorption in postmenopausal women.43
The results of these studies contrast with the findings of Leveille and colleagues,44 who examined the relationship between dietary vitamin C and hip BMD in 1892 postmenopausal women. Although the authors found that women (age, 55-64 years) using vitamin C supplements for more than 10 years had an average BMD 6.7% higher than that of nonusers, they did not find any association between dietary vitamin C intake and BMD. Moreover, NHANES III also found inconsistent associations between vitamin C and BMD among 13,080 adults surveyed in the United States.45 Although for premenopausal women dietary ascorbic acid was associated with increased BMD, for postmenopausal women with a history of smoking and estrogen replacement, it was actually associated with lower BMD values. For other subgroups in the study, the relationship was also inconsistent or nonlinear.
The exact mechanism by which ascorbic acid contributes to BMD is not fully delineated. However, it likely is related to the known role of vitamin C in collagen formation, bone matrix development, osteoblast differentiation, and its antioxidant effects limiting bone resorption.44,46
Hip Fractures
Besides demonstrating positive effects of vitamin C on bone healing and BMD, epidemiologic studies have found evidence of a protective effect of vitamin C on hip fracture risk. In a study of the Swedish Mammography cohort, 66,651 women (age, 40-76 years) were prospectively followed.47 The authors found that the odds ratio (OR) for hip fractures among smokers with a low intake of vitamin E (median intake, ≤6.2 mg/d) was 3.0 (95% CI, 1.6-5.4) and for vitamin C (median intake, ≤67 mg/d) was 3.0 (95% CI, 1.6-5.6). Moreover, in smokers with a low intake of both vitamins E and C, OR increased to 4.9 (95% CI, 2.2-11.0). In addition, the Utah Study of Nutrition and Bone Health matched 1215 cases of hip fractures in patients who had ever smoked (age, >50 years) with 1349 controls and found that vitamin C intake above 159 mg/d had a significant protective effect on the incidence of hip fracture; however, a graded relationship was not observed.48 Despite the inconsistencies in the NHANES III study regarding the relationship between vitamin C and BMD, Simon and Hudes45 found that serum vitamin C was associated with lower risk for self-reported fracture in postmenopausal women who had ever smoked and had a history of estrogen therapy (OR, 0.51; 95% CI, 0.36-0.70). Finally, Sahni and colleagues49 followed 958 Framingham cohort men and women (mean age, 75 years) over 17 years and found that those in the highest tertile of total vitamin C intake (median, 313 mg/d) had significantly fewer hip fractures and nonvertebral fractures compared with those in the lowest tertile of intake (median, 94 mg/d). Dietary vitamin C intake was not associated with fracture risk in this study.
Complex Regional Pain Syndrome
Type 1 CRPS is a debilitating condition characterized by severe pain, swelling, and vasomotor instability. It is commonly precipitated by an injury or surgery to an extremity and is a dreaded sequelae in orthopedics,50 with incidence rates of 10% to 22% in wrist fractures51-53 and 10% after foot and ankle surgery.54 Although the pathophysiology of CRPS remains unknown, dysregulation and increased permeability of the vasculature caused by free radicals are thought to play an important role.55 In dermal burns, high doses of vitamin C therapy slowed progression of vascular permeability and therefore reduced extravascular leakage of fluids and protein.56,57 The ability of vitamin C to prevent CRPS has been studied in only a handful of trials.
In a double-blind trial, Zollinger and colleagues51 randomized 127 conservatively treated distal radius fractures to receive either vitamin C 500 mg or placebo daily for 50 days starting on day of injury. Incidence of CRPS (using the diagnostic criteria proposed by Veldman and colleagues58) at 1-year follow-up was 22% in the placebo group and 7% in the vitamin C group (95% CI for difference, 2%-26%). Complaints while wearing the cast and fracture type increased the risk for developing CRPS. This initial study was followed up by a prospective, randomized, double-blind multicenter trial by the same authors,52 who had 416 patients with 427 wrist fractures receive either placebo or vitamin C 200 mg/d, 500 mg/d, or 1500 mg/d for 50 days. This follow-up study included both operative (11%) and nonoperative (89%) distal radius fractures. Incidence of CRPS was 10.1% in the placebo group and 2.4% in the vitamin C group (P < .002). Although there was an appreciable drop in the relative risk (RR) of developing CRPS between the vitamin C 200-mg/d and 500-mg/d groups (0.41-0.17), there was no additional benefit in the 1500-mg/d group. Pooling the data for these 2 randomized trials showed that the overall RR for developing CRPS was lower with vitamin C supplementation (RR, 0.28; 95% CI, 0.14-0.56; P = .0003).59
Results of the 2 trials by Zollinger and colleagues51,52 have been met with several concerns.60-62 As a corollary to the unclear etiology of CRPS, several different sets of diagnostic criteria exist, and the criteria are somewhat subjective and imprecise. Although both trials used the Veldman criteria,58 the incidence of CRPS in the placebo group dropped unexpectedly between trials, from 22% to 10.1%, and the results may have been different had other criteria been used. Moreover, the idea that toxic oxygen radicals have a role in CRPS and that vitamin C can scavenge these radicals is based on limited data.61 In the absence of a clear pathophysiologic explanation, some surgeons have been reluctant to treat patients with vitamin C supplementation.
Cazeneuve and colleagues53 also studied the effect of vitamin C supplementation on CRPS in patients with distal radius fractures treated with reduction and intrafocal pinning. Group 1 consisted of 100 patients (treated from 1995 to 1998) who did not receive vitamin C supplementation, and group 2 consisted of 95 patients (treated from 1998 to 2002) who received vitamin C 1000 mg/d for 45 days starting on day of fracture. Patients were followed for up to 90 days after surgery. Incidence of CRPS type 1 was 10% in the untreated group and 2.1% in the group that received vitamin C supplementation.
Vitamin C prophylaxis for CRPS has also been studied in foot and ankle surgery. Besse and colleagues54 prospectively compared 2 chronologically successive groups that received (235 feet) or did not receive (185 feet) vitamin C 1000-mg/d supplementation for 45 days. Incidence of CRPS type 1 as diagnosed with International Association for the Study of Pain (IASP) criteria dropped from 9.6% to 1.7% with vitamin C supplementation. In a case series, Zollinger and colleagues63 examined CRPS type 1 rates after performing cementless total trapeziometacarpal semiconstrained joint prosthesis implantations for trapeziometacarpal arthritis. Forty implantations were performed in 34 patients. All patients received vitamin C 500 mg/d for CRPS prevention starting 2 days before surgery for 50 days. There were no cases of CRPS in the postoperative period, according to Veldman or IASP criteria. Although the results of the studies by Cazeneuve and colleagues53 and Besse and colleagues54 agree with those of the distal radius fracture trials by Zollinger and colleagues,51,52 the quasi-experimental design and the lack of blinding and randomization temper the conclusions that can be drawn because of the risk for significant bias.
In a recent systematic review examining the effectiveness of vitamin C supplementation in preventing CRPS in trauma and surgery in the extremities, Shibuya and colleagues64 concluded that taking at least 500 mg of vitamin C daily for 45 to 50 days after injury or surgery may help decrease the incidence of CRPS after a traumatic event.
Osteoarthritis
Damage caused by free radicals has long been thought to play an important role in osteoarthritis (OA).65-67 A cross-sectional study in knee OA found that amounts of joint fluid antioxidants were lower in patients with severe arthritis than in those with intact cartilage, further implicating free radicals in the pathophysiology of OA.68 Use of vitamin C for prophylaxis against development or progression of OA is therefore a hot research topic. Thus far, animal studies have had mixed results—several showing a chondroprotective effect of vitamin C69,70 and others finding either no effect or even a positive association with the development of arthritis.71
The literature on human subjects, chiefly observational studies, is just as controversial. Wang and colleagues40 found vitamin C intake associated with both a 50% risk reduction of bone marrow lesions on magnetic resonance imaging over a 10-year interval (OR, 0.5; 95% CI, 0.29-0.87) and inversely associated with the tibial plateau bone area. Similarly, the Clearwater Osteoarthritis Study, which followed 1023 patients (age, >40 years), showed that participants who took vitamin C supplements were 11% less likely to develop radiographic evidence of OA (RR, 0.89; 95% CI, 0.85-0.93).72 Nonetheless, other studies have failed to show such associations73 or have demonstrated the opposite effect. Chaganti and colleagues74 analyzed levels of vitamins C and E in the Multicenter Osteoarthritis Study (MOST) cohort of 3026 men and women (age, 50-79 years) and found higher vitamin levels were not protective against incidence of radiographic whole-knee OA and may even have been associated with increased risk.
Conclusion
Vitamin C is an essential micronutrient and a powerful water-soluble antioxidant in numerous biochemical pathways that influence bone health. It has been implicated in the biology of fracture healing, and vitamin C supplementation has been proposed as prophylaxis against hip fractures based on observational data. Results of 2 high-quality double-blind randomized trials support use of vitamin C as prophylaxis against CRPS in wrist fractures treated conservatively and operatively; the evidence for foot and ankle surgery is weaker. Use of vitamin C in OA prevention has tremendous potential, though animal and human study results are controversial. Heterogeneous results and lack of prospective trials preclude any recommendation at this time.
1. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5(2):66-74.
2. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377-6381.
3. Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637-640.
4. Padh H. Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991;49(3):65-70.
5. Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993;51(11):313-326.
6. Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171-2184.
7. Szent-Györgyi A. On the function of hexuronic acid in the respiration of the cabbage leaf. J Biol Chem. 1931;90(1):385-393.
8. Svirbely JL, Szent-Györgyi A. The chemical nature of vitamin C. Biochem J. 1933;27(1):279-285.
9. Pauling L. Vitamin C and the Common Cold. San Francisco, CA: Freeman; 1970.
10. Spittle CR. Atherosclerosis and vitamin C. Lancet. 1971;2(7737):1280-1281.
11. Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354(9181):810-816.
12. Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53(1 suppl):270S-282S.
13. Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687-690.
14. Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980.
15. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145-1154.
16. Roberts JM, Myatt L, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282-1291.
17. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
18. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415-1423.
19. Glatthaar BE, Hornig DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357-377.
20. Carpenter KJ. The History of Scurvy and Vitamin C. New York, NY: Cambridge University Press; 1986.
21. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879-2882.
22. Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14(7):419-425.
23. Blee TH, Cogbill TH, Lambert PJ. Hemorrhage associated with vitamin C deficiency in surgical patients. Surgery. 2002;131(4):408-412.
24. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252-1263.
25. Gan R, Eintracht S, Hoffer LJ. Vitamin C deficiency in a university teaching hospital. J Am Coll Nutr. 2008;27(3):428-433.
26. Bourne G. The effect of graded doses of vitamin C upon the regeneration of bone in guinea-pigs on a scorbutic diet. J Physiol. 1942;101(3):327-336.
27. Bourne GH. The relative importance of periosteum and endosteum in bone healing and the relationship of vitamin C to their activities. Proc R Soc Med. 1944;37(6):275-279.
28. Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121(7):426-428.
29. Sarisözen B, Durak K, Dinçer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30(3):309-313.
30. Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18(3):281-288.
31. Alcantara-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly osteogenic disorder Shionogi rats. J Bone Joint Surg Br. 2007;89(3):402-407.
32. Mohan S, Kapoor A, Singgih A, et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J Bone Miner Res. 2005;20(9):1597-1610.
33. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143(2):213-221.
34. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7(2):235-246.
35. Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264(29):17281-17286.
36. Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid–dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11(8):1103-1113.
37. Sakamoto Y, Takano Y. Morphological influence of ascorbic acid deficiency on endochondral ossification in osteogenic disorder Shionogi rat. Anat Rec. 2002;268(2):93-104.
38. Kim W, Bae S, Kim H, et al. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anat Cell Biol. 2013;46(4):254-261.
39. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63(3):183-189.
40. Wang Y, Hodge AM, Wluka AE, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66.
41. Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523-1527.
42. Kaptoge S, Welch A, McTaggart A, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis Int. 2003;14(5):418-428.
43. Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health. 2006;15(3):295-300.
44. Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington state, USA. J Epidemiol Community Health. 1997;51(5):479-485.
45. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154(5):427-433.
46. Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):581-588.
47. Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14(1):129-135.
48. Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol. 2006;163(1):9-17.
49. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24(6):1086-1094.
50. Rho RH, Brewer RP, Lamer TJ, Wilson PR. Complex regional pain syndrome. Mayo Clin Proc. 2002;77(2):174-180.
51. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 1999;354(9195):2025-2028.
52. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose–response study. J Bone Joint Surg Am. 2007;89(7):1424-1431.
53. Cazeneuve JF, Leborgne JM, Kermad K, Hassan Y. Vitamin C and prevention of reflex sympathetic dystrophy following surgical management of distal radius fractures [in French]. Acta Orthop Belg. 2002;68(5):481-484.
54. Besse JL, Gadeyne S, Galand-Desme S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009;15(4):179-182.
55. Goris RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun. 1987;3(1-5):13-18.
56. Matsuda T, Tanaka H, Shimazaki S, et al. High-dose vitamin C therapy for extensive deep dermal burns. Burns. 1992;18(2):127-131.
57. Matsuda T, Tanaka H, Hanumadass M, et al. Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil. 1992;13(5):560-566.
58. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012-1016.
59. Zollinger PE. The administration of vitamin C in prevention of CRPS-I after distal radial fractures and hand surgery—a review of two RCTs and one observational prospective study. Open Conference Proc J. 2011;2:1-4.
60. Rogers BA, Ricketts DM. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2008;90(2):447-448.
61. Amadio PC. Vitamin C reduced the incidence of reflex sympathetic dystrophy after wrist fracture. J Bone Joint Surg Am. 2000;82(6):873.
62. Frolke JP. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2007;89(11):2550-2551.
63. Zollinger PE, Unal H, Ellis ML, Tuinebreijer WE. Clinical results of 40 consecutive basal thumb prostheses and no CRPS type I after vitamin C prophylaxis. Open Orthop J. 2010;4:62-66.
64. Shibuya N, Humphers JM, Agarwal MR, Jupiter DC. Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery—systematic review and meta-analysis. J Foot Ankle Surg. 2013;52(1):62-66.
65. Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32(7):562-567.
66. McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648-656.
67. Kaiki G, Tsuji H, Yonezawa T, et al. Osteoarthrosis induced by intra-articular hydrogen peroxide injection and running load. J Orthop Res. 1990;8(5):731-740.
68. Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515-521.
69. Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol. 1990;71(2):279-293.
70. Kurz B, Jost B, Schunke M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthritis Cartilage. 2002;10(2):119-126.
71. Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50(6):1822-1831.
72. Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14(4):709-715.
73. Hill J, Bird HA. Failure of selenium-ace to improve osteoarthritis. Br J Rheumatol. 1990;29(3):211-213.
74. Chaganti RK, Tolstykh I, Javaid MK, et al; Multicenter Osteoarthritis Study Group (MOST). High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190-196.
75. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Release 26. http://www.ars.usda.gov/Services/docs.htm?docid=24936. Published August 2013. Revised November 2013. Accessed May 14, 2015.
76. National Institutes of Health, Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. National Institutes of Health website. http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/. Reviewed June 5, 2013. Accessed May 14, 2015.
77. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000.
1. Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002;5(2):66-74.
2. Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86(16):6377-6381.
3. Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637-640.
4. Padh H. Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991;49(3):65-70.
5. Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993;51(11):313-326.
6. Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137(10):2171-2184.
7. Szent-Györgyi A. On the function of hexuronic acid in the respiration of the cabbage leaf. J Biol Chem. 1931;90(1):385-393.
8. Svirbely JL, Szent-Györgyi A. The chemical nature of vitamin C. Biochem J. 1933;27(1):279-285.
9. Pauling L. Vitamin C and the Common Cold. San Francisco, CA: Freeman; 1970.
10. Spittle CR. Atherosclerosis and vitamin C. Lancet. 1971;2(7737):1280-1281.
11. Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354(9181):810-816.
12. Block G. Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr. 1991;53(1 suppl):270S-282S.
13. Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301(13):687-690.
14. Hemila H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980.
15. Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH; Vitamins in Pre-eclampsia (VIP) Trial Consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367(9517):1145-1154.
16. Roberts JM, Myatt L, Spong CY, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362(14):1282-1291.
17. Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78-88.
18. Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281(15):1415-1423.
19. Glatthaar BE, Hornig DH, Moser U. The role of ascorbic acid in carcinogenesis. Adv Exp Med Biol. 1986;206:357-377.
20. Carpenter KJ. The History of Scurvy and Vitamin C. New York, NY: Cambridge University Press; 1986.
21. Murad S, Grove D, Lindberg KA, Reynolds G, Sivarajah A, Pinnell SR. Regulation of collagen synthesis by ascorbic acid. Proc Natl Acad Sci U S A. 1981;78(5):2879-2882.
22. Fain O, Pariés J, Jacquart B, et al. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14(7):419-425.
23. Blee TH, Cogbill TH, Lambert PJ. Hemorrhage associated with vitamin C deficiency in surgical patients. Surgery. 2002;131(4):408-412.
24. Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009;90(5):1252-1263.
25. Gan R, Eintracht S, Hoffer LJ. Vitamin C deficiency in a university teaching hospital. J Am Coll Nutr. 2008;27(3):428-433.
26. Bourne G. The effect of graded doses of vitamin C upon the regeneration of bone in guinea-pigs on a scorbutic diet. J Physiol. 1942;101(3):327-336.
27. Bourne GH. The relative importance of periosteum and endosteum in bone healing and the relationship of vitamin C to their activities. Proc R Soc Med. 1944;37(6):275-279.
28. Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B. The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg. 2001;121(7):426-428.
29. Sarisözen B, Durak K, Dinçer G, Bilgen OF. The effects of vitamins E and C on fracture healing in rats. J Int Med Res. 2002;30(3):309-313.
30. Kipp DE, McElvain M, Kimmel DB, Akhter MP, Robinson RG, Lukert BP. Scurvy results in decreased collagen synthesis and bone density in the guinea pig animal model. Bone. 1996;18(3):281-288.
31. Alcantara-Martos T, Delgado-Martinez AD, Vega MV, Carrascal MT, Munuera-Martinez L. Effect of vitamin C on fracture healing in elderly osteogenic disorder Shionogi rats. J Bone Joint Surg Br. 2007;89(3):402-407.
32. Mohan S, Kapoor A, Singgih A, et al. Spontaneous fractures in the mouse mutant sfx are caused by deletion of the gulonolactone oxidase gene, causing vitamin C deficiency. J Bone Miner Res. 2005;20(9):1597-1610.
33. Aronow MA, Gerstenfeld LC, Owen TA, Tassinari MS, Stein GS, Lian JB. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990;143(2):213-221.
34. Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7(2):235-246.
35. Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264(29):17281-17286.
36. Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid–dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11(8):1103-1113.
37. Sakamoto Y, Takano Y. Morphological influence of ascorbic acid deficiency on endochondral ossification in osteogenic disorder Shionogi rat. Anat Rec. 2002;268(2):93-104.
38. Kim W, Bae S, Kim H, et al. Ascorbic acid insufficiency induces the severe defect on bone formation via the down-regulation of osteocalcin production. Anat Cell Biol. 2013;46(4):254-261.
39. Hall SL, Greendale GA. The relation of dietary vitamin C intake to bone mineral density: results from the PEPI study. Calcif Tissue Int. 1998;63(3):183-189.
40. Wang Y, Hodge AM, Wluka AE, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects: a cross-sectional study. Arthritis Res Ther. 2007;9(4):R66.
41. Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523-1527.
42. Kaptoge S, Welch A, McTaggart A, et al. Effects of dietary nutrients and food groups on bone loss from the proximal femur in men and women in the 7th and 8th decades of age. Osteoporosis Int. 2003;14(5):418-428.
43. Pasco JA, Henry MJ, Wilkinson LK, Nicholson GC, Schneider HG, Kotowicz MA. Antioxidant vitamin supplements and markers of bone turnover in a community sample of nonsmoking women. J Womens Health. 2006;15(3):295-300.
44. Leveille SG, LaCroix AZ, Koepsell TD, Beresford SA, Van Belle G, Buchner DM. Dietary vitamin C and bone mineral density in postmenopausal women in Washington state, USA. J Epidemiol Community Health. 1997;51(5):479-485.
45. Simon JA, Hudes ES. Relation of ascorbic acid to bone mineral density and self-reported fractures among US adults. Am J Epidemiol. 2001;154(5):427-433.
46. Wolf RL, Cauley JA, Pettinger M, et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: results from the Women’s Health Initiative. Am J Clin Nutr. 2005;82(3):581-588.
47. Melhus H, Michaelsson K, Holmberg L, Wolk A, Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J Bone Miner Res. 1999;14(1):129-135.
48. Zhang J, Munger RG, West NA, Cutler DR, Wengreen HJ, Corcoran CD. Antioxidant intake and risk of osteoporotic hip fracture in Utah: an effect modified by smoking status. Am J Epidemiol. 2006;163(1):9-17.
49. Sahni S, Hannan MT, Blumberg J, Cupples LA, Kiel DP, Tucker KL. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: a 17-year follow-up from the Framingham Osteoporosis Study. J Bone Miner Res. 2009;24(6):1086-1094.
50. Rho RH, Brewer RP, Lamer TJ, Wilson PR. Complex regional pain syndrome. Mayo Clin Proc. 2002;77(2):174-180.
51. Zollinger PE, Tuinebreijer WE, Kreis RW, Breederveld RS. Effect of vitamin C on frequency of reflex sympathetic dystrophy in wrist fractures: a randomised trial. Lancet. 1999;354(9195):2025-2028.
52. Zollinger PE, Tuinebreijer WE, Breederveld RS, Kreis RW. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? A randomized, controlled, multicenter dose–response study. J Bone Joint Surg Am. 2007;89(7):1424-1431.
53. Cazeneuve JF, Leborgne JM, Kermad K, Hassan Y. Vitamin C and prevention of reflex sympathetic dystrophy following surgical management of distal radius fractures [in French]. Acta Orthop Belg. 2002;68(5):481-484.
54. Besse JL, Gadeyne S, Galand-Desme S, Lerat JL, Moyen B. Effect of vitamin C on prevention of complex regional pain syndrome type I in foot and ankle surgery. Foot Ankle Surg. 2009;15(4):179-182.
55. Goris RJ, Dongen LM, Winters HA. Are toxic oxygen radicals involved in the pathogenesis of reflex sympathetic dystrophy? Free Radic Res Commun. 1987;3(1-5):13-18.
56. Matsuda T, Tanaka H, Shimazaki S, et al. High-dose vitamin C therapy for extensive deep dermal burns. Burns. 1992;18(2):127-131.
57. Matsuda T, Tanaka H, Hanumadass M, et al. Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil. 1992;13(5):560-566.
58. Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012-1016.
59. Zollinger PE. The administration of vitamin C in prevention of CRPS-I after distal radial fractures and hand surgery—a review of two RCTs and one observational prospective study. Open Conference Proc J. 2011;2:1-4.
60. Rogers BA, Ricketts DM. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2008;90(2):447-448.
61. Amadio PC. Vitamin C reduced the incidence of reflex sympathetic dystrophy after wrist fracture. J Bone Joint Surg Am. 2000;82(6):873.
62. Frolke JP. Can vitamin C prevent complex regional pain syndrome in patients with wrist fractures? J Bone Joint Surg Am. 2007;89(11):2550-2551.
63. Zollinger PE, Unal H, Ellis ML, Tuinebreijer WE. Clinical results of 40 consecutive basal thumb prostheses and no CRPS type I after vitamin C prophylaxis. Open Orthop J. 2010;4:62-66.
64. Shibuya N, Humphers JM, Agarwal MR, Jupiter DC. Efficacy and safety of high-dose vitamin C on complex regional pain syndrome in extremity trauma and surgery—systematic review and meta-analysis. J Foot Ankle Surg. 2013;52(1):62-66.
65. Henrotin Y, Deby-Dupont G, Deby C, De Bruyn M, Lamy M, Franchimont P. Production of active oxygen species by isolated human chondrocytes. Br J Rheumatol. 1993;32(7):562-567.
66. McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39(4):648-656.
67. Kaiki G, Tsuji H, Yonezawa T, et al. Osteoarthrosis induced by intra-articular hydrogen peroxide injection and running load. J Orthop Res. 1990;8(5):731-740.
68. Regan EA, Bowler RP, Crapo JD. Joint fluid antioxidants are decreased in osteoarthritic joints compared to joints with macroscopically intact cartilage and subacute injury. Osteoarthritis Cartilage. 2008;16(4):515-521.
69. Meacock SC, Bodmer JL, Billingham ME. Experimental osteoarthritis in guinea-pigs. J Exp Pathol. 1990;71(2):279-293.
70. Kurz B, Jost B, Schunke M. Dietary vitamins and selenium diminish the development of mechanically induced osteoarthritis and increase the expression of antioxidative enzymes in the knee joint of STR/1N mice. Osteoarthritis Cartilage. 2002;10(2):119-126.
71. Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritis in a guinea pig model. Arthritis Rheum. 2004;50(6):1822-1831.
72. Peregoy J, Wilder FV. The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr. 2011;14(4):709-715.
73. Hill J, Bird HA. Failure of selenium-ace to improve osteoarthritis. Br J Rheumatol. 1990;29(3):211-213.
74. Chaganti RK, Tolstykh I, Javaid MK, et al; Multicenter Osteoarthritis Study Group (MOST). High plasma levels of vitamin C and E are associated with incident radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(2):190-196.
75. US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Release 26. http://www.ars.usda.gov/Services/docs.htm?docid=24936. Published August 2013. Revised November 2013. Accessed May 14, 2015.
76. National Institutes of Health, Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. National Institutes of Health website. http://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/. Reviewed June 5, 2013. Accessed May 14, 2015.
77. Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press; 2000.