User login
Despite enthusiasm for the use of molecular testing and molecularly targeted agents in patients with advanced non–small cell lung cancer (NSCLC), most patients are not candidates for upfront treatment with molecular agents. Chemotherapy therefore remains the backbone of treatment for this patient population.
This article presents the best available evidence for selection of chemotherapy for patients with advanced NSCLC and examines the controversy surrounding maintenance therapy.
EVOLUTION OF CHEMOTHERAPY IN NSCLC
The first evidence that chemotherapy produced a significant survival benefit in patients with advanced NSCLC came in 1995 when a meta-analysis showed that platinum-based chemotherapy conferred a 2-month improvement in median survival over best supportive care.1
This finding led to a decade of randomized phase 3 clinical trials that compared different platinum-based regimens. The quintessential trial in this regard was Eastern Cooperative Oncology Group (ECOG) 1594, in which three platinum doublets were compared with cisplatin and paclitaxel on the end point of overall survival (OS) in patients with advanced NSCLC. No significant differences were found among the regimens tested.2
Bevacizumab adds to platinum doublet in nonsquamous NSCLC
With the introduction of bevacizumab, an antibody against vascular endothelial growth factor, knowledge of NSCLC histology became important. In the ECOG 4599 trial, published in 2006, bevacizumab added to platinum doublet chemotherapy in patients with advanced nonsquamous NSCLC significantly improved the response rate, progression-free survival (PFS), and median OS compared with platinum doublet chemotherapy alone.3 This trial was limited to patients with nonsquamous NSCLC because its predecessor trial had revealed an excess of life-threatening pulmonary hemorrhage in association with bevacizumab in patients with squamous cell carcinoma.
Pemetrexed superior to docetaxel in nonsquamous histology
In 2004, Hanna et al4 demonstrated pemetrexed to be noninferior to docetaxel on efficacy outcomes as second-line therapy in advanced NSCLC. Pemetrexed had a significantly better toxicity profile, however, which led to its approval for this indication. Post hoc analyses of this trial suggested a differential effect of pemetrexed based on histology. In the pemetrexed arm, patients with nonsquamous histology appeared to have superior survival compared with patients who had squamous histology, whereas in the docetaxel arm, histology did not affect outcome.5 Further, the nonsquamous histologic subgroup had superior OS with pemetrexed compared with docetaxel.6
Pemetrexed and gemcitabine perform differently based on histology
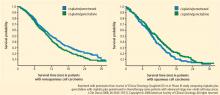
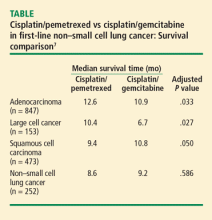
A phase 3 trial with a noninferiority design compared cisplatin/pemetrexed with cisplatin/gemcitabine as first-line therapy on OS. The noninferiority criteria were met, with no difference in median OS between the two groups (median OS: 10.3 months in both arms).7
Based on this evidence, NSCLC is no longer an adequate pathologic diagnosis. Pathologists must differentiate squamous from nonsquamous histology to take full advantage of the safety of angiogenesis inhibitors and the efficacy of pemetrexed.
OPTIMAL FIRST-LINE REGIMEN FOR NONSQUAMOUS NSCLC
Both cisplatin/pemetrexed and carboplatin/paclitaxel plus bevacizumab have level 1 evidence to support their use as first-line treatment of NSCLC with nonsquamous histology. Carboplatin/pemetrexed/bevacizumab is also being used in the community despite the absence of randomized trial evidence to support its use for this indication.
A single-arm phase 2 trial of carboplatin/pemetrexed/bevacizumab followed by maintenance pemetrexed/bevacizumab in 49 patients produced a response rate of 55%, PFS of 7.8 months, and OS of 14 months.10 Although the results are impressive, they should be considered hypothesis-generating rather than treatment-changing in light of the small number of patients enrolled and the single-arm design.
An open-label randomized phase 3 trial, Point-Break, is comparing two regimens in patients who have advanced nonsquamous NSCLC: (1) carboplatin/pemetrexed/bevacizumab followed by maintenance pemetrexed/bevacizumab and (2) carboplatin/paclitaxel/bevacizumab followed by bevacizumab.11 Although potentially practice-changing, PointBreak will not answer whether bevacizumab adds benefit to cisplatin and pemetrexed, nor will it determine which first-line regimen is superior because of the different maintenance regimens.
SQUAMOUS NSCLC: PLATINUM DOUBLET OPTIMAL
No agents are currently approved specifically for the treatment of squamous cell carcinoma, which appears to have a high level of expression of insulin-like growth factor receptor (IGF-1R). A 64% response rate observed with an IGF-1R antagonist added to paclitaxel/carboplatin in patients with NSCLC of squamous cell histology in a phase 2 trial led to the design of a phase 3 trial in which patients were randomized to carboplatin/paclitaxel with or without the IGF-1R antagonist figitumumab. The trial ended prematurely in 2009 because of an imbalance of deaths in the experimental arm.12 As expected, hyperglycemia was more common in the experimental arm. Unexpectedly, the incidences of grade 5 infections and cardiovascular events were also significantly higher in the experimental arm.
A randomized phase 3 trial of carboplatin/paclitaxel compared with carboplatin and nanoparticle albumin-bound (nab) paclitaxel was conducted in 1,052 patients with stage IIIb/IV NSCLC with the primary end point being overall response rate (ORR).13 The rationale for substituting nab-paclitaxel for paclitaxel was that paclitaxel is dissolved in polyoxyethylated castor oil. This decreases the efficacy of paclitaxel and contributes to its toxicities, including hypersensitivity reactions and neuropathy. In metastatic breast cancer, nab-paclitaxel was shown to be more efficacious than solvent-based paclitaxel.14
The nab-paclitaxel trial met its primary end point of superior response rate: 33% in the nab-paclitaxel arm versus 25% in the standard paclitaxel arm. However, on final analysis there was no difference in PFS or OS between the two arms, making the difference in ORR of little clinical significance. No hypersensitivity reactions occurred in the nab-paclitaxel arm, while three occurred in the paclitaxel arm. Grade 3 sensory neuropathy occurred significantly less often in the group assigned to nab-paclitaxel compared with paclitaxel (3 vs 10, respectively; P < .001). Although there appears to be no efficacy advantage of nab-paclitaxel over standard paclitaxel for advanced NSCLC patients, use of nab-paclitaxel might be considered in patients with stage IV NSCLC who have poorly controlled diabetes or who already suffer significant neuropathy.
Although not a prespecified end point, the response rate in patients with squamous cell histology nearly doubled among those treated with nab-paclitaxel compared with standard paclitaxel. However, this did not translate into significant differences in PFS or OS in this subgroup, and the use of nab-paclitaxel in this patient population specifically is not advised.
In light of the data, the standard treatment for squamous NSCLC remains a platinum doublet other than pemetrexed. A phase 3 clinical trial, ECLIPSE, is currently enrolling patients at the Cleveland Clinic. The trial will randomize chemotherapy-naïve patients with stage IIIb/IV NSCLC and a Karnofsky performance status of 0 or 1 to receive carboplatin and gemcitabine with or without the polyadenosine diphosphate–ribose polymerase inhibitor iniparib.
MAINTENANCE THERAPY
The utility of maintenance therapy—the uninterrupted continuation of therapy for patients who do not progress after completing first-line chemotherapy—in patients with advanced NSCLC is controversial. Two kinds of maintenance therapy have emerged.
Switch maintenance, also known as early second-line therapy, is so termed because patients are immediately switched to a second-line agent different from the first-line doublet therapy.
Continuation maintenance is the continuation of one or more drugs from the induction regimen, the best example being continuation of single-agent evacizumab in the ECOG 4599 regimen.
Five major trials of successful maintenance therapy for nonprogressors after first-line chemotherapy have been presented over the past 4 years, and these have led to new indications for the maintenance drugs.8,15–18 PFS in favor of active maintenance has been documented in trials of early versus delayed maintenance docetaxel,15 pemetrexed versus placebo,8 erlotinib versus placebo,16 bevacizumab/erlotinib versus bevacizumab/placebo (ATLAS trial),17 and gemcitabine or erlotinib versus placebo (IFCT-GFPC 0502).18 The magnitude of improved PFS associated with each treatment has been similar. As a result, improved of PFS with maintenance therapy is now widely accepted.
To improve survival: maintenance therapy or better second-line therapy?
The utility of maintenance therapy can be confounded by the lack of a predefined second-line treatment. Some argue that the benefit to maintenance may also be realized by appropriate use of the same agent as salvage therapy in the case of disease progression.
Overall survival improved with maintenance therapy in only two trials; one compared pemetrexed with placebo and the other compared erlotinib with placebo.8,16 The validity of these findings, however, remains in question. In the trial of pemetrexed, only 19% of the patients in the control arm ever received pemetrexed, and in the erlotinib trial, only 21% of the patients in the control arm ever received erlotinib after progression. Whether maintenance therapy was responsible for an improvement in OS or whether ineffective second-line therapy dampened survival in patients in the control arms is unknown.
In the IFCT-GFPC 0502 phase 3 study, patients received four cycles of cisplatin/gemcitabine.19 If patients did not progress, they were randomized to observation, continuation maintenance with gemcitabine, or switch maintenance to erlotinib. Predefined second-line therapy in all arms was pemetrexed. The primary end point chosen was PFS, even though maintenance therapy had already been established to extend PFS. The median PFS in the gemcitabine maintenance arm was 3.8 months, compared with 1.9 months in the observation arm. The hazard ratio (HR) of 0.55 (P < .0001) was similar to that observed with pemetrexed in the maintenance trial noted above.19 Subgroup analysis showed improvement in PFS with the gemcitabine maintenance group compared with observation in all subgroups, including those based on histology. The HR in the erlotinib maintenance arm was 0.82 (P = .002), similar to that observed with erlotinib in the Sequential Tarceva in Unresectable NSCLC (SATURN) trial.16
In the IFCT-GFPC 0502 study, 60.4% of patients in the gemcitabine arm, 63.2 % of those in the erlotinib arm, and 76.1% in the observation arm received postmaintenance treatment with pemetrexed.19 Nearly one-half (49.6%) of patients in the observation arm received third-line treatment with erlotinib.
In the overall study population, a trend toward improved OS was observed with maintenance gemcitabine or erlotinib compared with observation, but did not achieve statistical significance, possibly because the trial lacked adequate power to detect a difference on this end point.
A subgroup analysis of patients who received second-line pemetrexed showed a significant improvement in OS in the maintenance arms compared with the observation arm. About 25% of the patients never advanced to second-line pemetrexed. Because patients who never received second-line pemetrexed may represent the sickest patients, the relevance of this finding to the overall population of patients with stage IV NSCLC is unknown.
Ongoing maintenance trials
Two ongoing clinical trials of maintenance are exploring bevacizumab with or without pemetrexed as maintenance following first-line cisplatin/pemetrexed/bevacizumab (AVAPERL) and bevacizumab alone, pemetrexed alone, or a combination of the two as maintenance following first-line therapy with carboplatin/paclitaxel/bevacizumab (Eastern Cooperative Oncology Group). Preliminary results from the AVAPERL study were reported recently and support the use of pemetrexed and bevacizumab as maintenance therapy compared with bevacizumab alone.20
CONCLUSIONS ABOUT MAINTENANCE THERAPY
Pemetrexed and erlotinib significantly prolong OS survival compared with placebo when used as maintenance therapy in advanced NSCLC patients who do not progress after four cycles of first-line chemotherapy. Whether this improvement in OS can be attributed to maintenance therapy or more effective second-line therapy is open to debate.
Maintenance chemotherapy should be discussed with all patients whose tumors do not progress after four cycles of first-line chemotherapy. The use of maintenance therapy may be most reasonable in very symptomatic patients who receive palliative benefit from chemotherapy, or as a means of encouraging noncompliant patients to return for care.
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995; 311:899–909.
- Schiller JH, Harrington D, Belani C, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346:92–98.
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355:2542–2450.
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22:1589–1597.
- Peterson P, Park K, Fossella F, Gatzmeier U, John W, Scagliotti G. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC) [12th World Congress on Lung Cancer abstract P2-328]. J Thorac Oncol 2007; 2 (suppl 4): S851.
- Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist 2009; 14:253–263.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non–small-cell lung cancer. J Clin Oncol 2008; 26:3543–3551.
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009; 374:1432–1440.
- Belani CP, Brodowicz T, Ciuleanu T, et al. Maintenance pemetrexed (Pem) plus best supportive care (BSC) versus placebo (Plac) plus BSC: a randomized phase III study in advanced nonsmall cell lung cancer (NSCLC) [ASCO abstract CRA8000]. J Clin Oncol 2009; 27 (suppl).
- Patel JD, Hensing TA, Rademaker A, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer. J Clin Oncol 2009; 27:3284–3289.
- Patel JD, Bonomi P, Socinski MA, et al. Treatment rationale and study design for the PointBreak study: a randomized, open-label phase III study of pemetrexed/carboplatin/bevacizumab followed by maintenance pemetrexed/bevacizumab versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non–small-cell lung cancer. Clin Lung Cancer 2009; 10:252–256.
- Jassem J, Langer CJ, Karp DD, et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with nonsmall cell lung cancer (NSCLC). J Clin Oncol 2010; 28 (suppl): abstr 7500.
- Socinski MA, Bondarenko IN, Karaseva NA, et al. Survival results of a randomized, phase III trial of nab-paclitaxel and carboplatin compared with cremophor-based paclitaxel and carboplatin as first-line therapy in advanced non-small cell lung cancer [ASCO abstract 7551]. J Clin Oncol 2011; 29 (suppl).
- Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005; 23:7794–7803.
- Fidias PM, Dakhil SR, Lyss AP, et al Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non–small-cell lung cancer. J Clin Oncol 2008; 27:591–598.
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010; 11:521–529.
- Miller VA, O’Connor P, Soh C, Kabbinavar F; ATLAS investigators. A randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC) [ASCO abstract LBA8002]. J Clin Oncol 2009; 27 (suppl).
- Perol M, Zalcman G, Monnet I, et al. Final results from the IFCTGFPC 0502 phase III study: Maintenance therapy in advanced NSCLC with either gemcitabine (G) or erlotinib (E) versus observation (O) after cisplatin-gemcitabine induction chemotherapy (CT), with a predefined second-line treatment [ESMO abstract 370PD]. Ann Oncol 2010; 21 (suppl 8): viii124.
- Perol M, Chouaid C, Milleron J, et al. Maintenance with either gemcitabine or erlotinib versus observation with predefined second-line treatment after cisplatin-gemcitabine induction chemotherapy in advanced NSCLC: IFCT-GFPC 0502 phase III study [ASCO abstract 7507]. J Clin Oncol 2010; 28 (suppl).
- Barlesi F, de Castro J, Dvornichencko V, et al. AVAPERL (MO22089): Final efficacy outcomes for patients with advanced non-squamous non-small cell lung cancer randomized to continuation maintenance with bevacizumab (bev) or bev + pemetrexed (pem) after first-line bev-cisplatin-pem treatment. Paper presented at: The European Multidiscipinary Cancer Congress; September 24, 2011; Stockholm, Sweden.
Despite enthusiasm for the use of molecular testing and molecularly targeted agents in patients with advanced non–small cell lung cancer (NSCLC), most patients are not candidates for upfront treatment with molecular agents. Chemotherapy therefore remains the backbone of treatment for this patient population.
This article presents the best available evidence for selection of chemotherapy for patients with advanced NSCLC and examines the controversy surrounding maintenance therapy.
EVOLUTION OF CHEMOTHERAPY IN NSCLC
The first evidence that chemotherapy produced a significant survival benefit in patients with advanced NSCLC came in 1995 when a meta-analysis showed that platinum-based chemotherapy conferred a 2-month improvement in median survival over best supportive care.1
This finding led to a decade of randomized phase 3 clinical trials that compared different platinum-based regimens. The quintessential trial in this regard was Eastern Cooperative Oncology Group (ECOG) 1594, in which three platinum doublets were compared with cisplatin and paclitaxel on the end point of overall survival (OS) in patients with advanced NSCLC. No significant differences were found among the regimens tested.2
Bevacizumab adds to platinum doublet in nonsquamous NSCLC
With the introduction of bevacizumab, an antibody against vascular endothelial growth factor, knowledge of NSCLC histology became important. In the ECOG 4599 trial, published in 2006, bevacizumab added to platinum doublet chemotherapy in patients with advanced nonsquamous NSCLC significantly improved the response rate, progression-free survival (PFS), and median OS compared with platinum doublet chemotherapy alone.3 This trial was limited to patients with nonsquamous NSCLC because its predecessor trial had revealed an excess of life-threatening pulmonary hemorrhage in association with bevacizumab in patients with squamous cell carcinoma.
Pemetrexed superior to docetaxel in nonsquamous histology
In 2004, Hanna et al4 demonstrated pemetrexed to be noninferior to docetaxel on efficacy outcomes as second-line therapy in advanced NSCLC. Pemetrexed had a significantly better toxicity profile, however, which led to its approval for this indication. Post hoc analyses of this trial suggested a differential effect of pemetrexed based on histology. In the pemetrexed arm, patients with nonsquamous histology appeared to have superior survival compared with patients who had squamous histology, whereas in the docetaxel arm, histology did not affect outcome.5 Further, the nonsquamous histologic subgroup had superior OS with pemetrexed compared with docetaxel.6
Pemetrexed and gemcitabine perform differently based on histology
A phase 3 trial with a noninferiority design compared cisplatin/pemetrexed with cisplatin/gemcitabine as first-line therapy on OS. The noninferiority criteria were met, with no difference in median OS between the two groups (median OS: 10.3 months in both arms).7
Based on this evidence, NSCLC is no longer an adequate pathologic diagnosis. Pathologists must differentiate squamous from nonsquamous histology to take full advantage of the safety of angiogenesis inhibitors and the efficacy of pemetrexed.
OPTIMAL FIRST-LINE REGIMEN FOR NONSQUAMOUS NSCLC
Both cisplatin/pemetrexed and carboplatin/paclitaxel plus bevacizumab have level 1 evidence to support their use as first-line treatment of NSCLC with nonsquamous histology. Carboplatin/pemetrexed/bevacizumab is also being used in the community despite the absence of randomized trial evidence to support its use for this indication.
A single-arm phase 2 trial of carboplatin/pemetrexed/bevacizumab followed by maintenance pemetrexed/bevacizumab in 49 patients produced a response rate of 55%, PFS of 7.8 months, and OS of 14 months.10 Although the results are impressive, they should be considered hypothesis-generating rather than treatment-changing in light of the small number of patients enrolled and the single-arm design.
An open-label randomized phase 3 trial, Point-Break, is comparing two regimens in patients who have advanced nonsquamous NSCLC: (1) carboplatin/pemetrexed/bevacizumab followed by maintenance pemetrexed/bevacizumab and (2) carboplatin/paclitaxel/bevacizumab followed by bevacizumab.11 Although potentially practice-changing, PointBreak will not answer whether bevacizumab adds benefit to cisplatin and pemetrexed, nor will it determine which first-line regimen is superior because of the different maintenance regimens.
SQUAMOUS NSCLC: PLATINUM DOUBLET OPTIMAL
No agents are currently approved specifically for the treatment of squamous cell carcinoma, which appears to have a high level of expression of insulin-like growth factor receptor (IGF-1R). A 64% response rate observed with an IGF-1R antagonist added to paclitaxel/carboplatin in patients with NSCLC of squamous cell histology in a phase 2 trial led to the design of a phase 3 trial in which patients were randomized to carboplatin/paclitaxel with or without the IGF-1R antagonist figitumumab. The trial ended prematurely in 2009 because of an imbalance of deaths in the experimental arm.12 As expected, hyperglycemia was more common in the experimental arm. Unexpectedly, the incidences of grade 5 infections and cardiovascular events were also significantly higher in the experimental arm.
A randomized phase 3 trial of carboplatin/paclitaxel compared with carboplatin and nanoparticle albumin-bound (nab) paclitaxel was conducted in 1,052 patients with stage IIIb/IV NSCLC with the primary end point being overall response rate (ORR).13 The rationale for substituting nab-paclitaxel for paclitaxel was that paclitaxel is dissolved in polyoxyethylated castor oil. This decreases the efficacy of paclitaxel and contributes to its toxicities, including hypersensitivity reactions and neuropathy. In metastatic breast cancer, nab-paclitaxel was shown to be more efficacious than solvent-based paclitaxel.14
The nab-paclitaxel trial met its primary end point of superior response rate: 33% in the nab-paclitaxel arm versus 25% in the standard paclitaxel arm. However, on final analysis there was no difference in PFS or OS between the two arms, making the difference in ORR of little clinical significance. No hypersensitivity reactions occurred in the nab-paclitaxel arm, while three occurred in the paclitaxel arm. Grade 3 sensory neuropathy occurred significantly less often in the group assigned to nab-paclitaxel compared with paclitaxel (3 vs 10, respectively; P < .001). Although there appears to be no efficacy advantage of nab-paclitaxel over standard paclitaxel for advanced NSCLC patients, use of nab-paclitaxel might be considered in patients with stage IV NSCLC who have poorly controlled diabetes or who already suffer significant neuropathy.
Although not a prespecified end point, the response rate in patients with squamous cell histology nearly doubled among those treated with nab-paclitaxel compared with standard paclitaxel. However, this did not translate into significant differences in PFS or OS in this subgroup, and the use of nab-paclitaxel in this patient population specifically is not advised.
In light of the data, the standard treatment for squamous NSCLC remains a platinum doublet other than pemetrexed. A phase 3 clinical trial, ECLIPSE, is currently enrolling patients at the Cleveland Clinic. The trial will randomize chemotherapy-naïve patients with stage IIIb/IV NSCLC and a Karnofsky performance status of 0 or 1 to receive carboplatin and gemcitabine with or without the polyadenosine diphosphate–ribose polymerase inhibitor iniparib.
MAINTENANCE THERAPY
The utility of maintenance therapy—the uninterrupted continuation of therapy for patients who do not progress after completing first-line chemotherapy—in patients with advanced NSCLC is controversial. Two kinds of maintenance therapy have emerged.
Switch maintenance, also known as early second-line therapy, is so termed because patients are immediately switched to a second-line agent different from the first-line doublet therapy.
Continuation maintenance is the continuation of one or more drugs from the induction regimen, the best example being continuation of single-agent evacizumab in the ECOG 4599 regimen.
Five major trials of successful maintenance therapy for nonprogressors after first-line chemotherapy have been presented over the past 4 years, and these have led to new indications for the maintenance drugs.8,15–18 PFS in favor of active maintenance has been documented in trials of early versus delayed maintenance docetaxel,15 pemetrexed versus placebo,8 erlotinib versus placebo,16 bevacizumab/erlotinib versus bevacizumab/placebo (ATLAS trial),17 and gemcitabine or erlotinib versus placebo (IFCT-GFPC 0502).18 The magnitude of improved PFS associated with each treatment has been similar. As a result, improved of PFS with maintenance therapy is now widely accepted.
To improve survival: maintenance therapy or better second-line therapy?
The utility of maintenance therapy can be confounded by the lack of a predefined second-line treatment. Some argue that the benefit to maintenance may also be realized by appropriate use of the same agent as salvage therapy in the case of disease progression.
Overall survival improved with maintenance therapy in only two trials; one compared pemetrexed with placebo and the other compared erlotinib with placebo.8,16 The validity of these findings, however, remains in question. In the trial of pemetrexed, only 19% of the patients in the control arm ever received pemetrexed, and in the erlotinib trial, only 21% of the patients in the control arm ever received erlotinib after progression. Whether maintenance therapy was responsible for an improvement in OS or whether ineffective second-line therapy dampened survival in patients in the control arms is unknown.
In the IFCT-GFPC 0502 phase 3 study, patients received four cycles of cisplatin/gemcitabine.19 If patients did not progress, they were randomized to observation, continuation maintenance with gemcitabine, or switch maintenance to erlotinib. Predefined second-line therapy in all arms was pemetrexed. The primary end point chosen was PFS, even though maintenance therapy had already been established to extend PFS. The median PFS in the gemcitabine maintenance arm was 3.8 months, compared with 1.9 months in the observation arm. The hazard ratio (HR) of 0.55 (P < .0001) was similar to that observed with pemetrexed in the maintenance trial noted above.19 Subgroup analysis showed improvement in PFS with the gemcitabine maintenance group compared with observation in all subgroups, including those based on histology. The HR in the erlotinib maintenance arm was 0.82 (P = .002), similar to that observed with erlotinib in the Sequential Tarceva in Unresectable NSCLC (SATURN) trial.16
In the IFCT-GFPC 0502 study, 60.4% of patients in the gemcitabine arm, 63.2 % of those in the erlotinib arm, and 76.1% in the observation arm received postmaintenance treatment with pemetrexed.19 Nearly one-half (49.6%) of patients in the observation arm received third-line treatment with erlotinib.
In the overall study population, a trend toward improved OS was observed with maintenance gemcitabine or erlotinib compared with observation, but did not achieve statistical significance, possibly because the trial lacked adequate power to detect a difference on this end point.
A subgroup analysis of patients who received second-line pemetrexed showed a significant improvement in OS in the maintenance arms compared with the observation arm. About 25% of the patients never advanced to second-line pemetrexed. Because patients who never received second-line pemetrexed may represent the sickest patients, the relevance of this finding to the overall population of patients with stage IV NSCLC is unknown.
Ongoing maintenance trials
Two ongoing clinical trials of maintenance are exploring bevacizumab with or without pemetrexed as maintenance following first-line cisplatin/pemetrexed/bevacizumab (AVAPERL) and bevacizumab alone, pemetrexed alone, or a combination of the two as maintenance following first-line therapy with carboplatin/paclitaxel/bevacizumab (Eastern Cooperative Oncology Group). Preliminary results from the AVAPERL study were reported recently and support the use of pemetrexed and bevacizumab as maintenance therapy compared with bevacizumab alone.20
CONCLUSIONS ABOUT MAINTENANCE THERAPY
Pemetrexed and erlotinib significantly prolong OS survival compared with placebo when used as maintenance therapy in advanced NSCLC patients who do not progress after four cycles of first-line chemotherapy. Whether this improvement in OS can be attributed to maintenance therapy or more effective second-line therapy is open to debate.
Maintenance chemotherapy should be discussed with all patients whose tumors do not progress after four cycles of first-line chemotherapy. The use of maintenance therapy may be most reasonable in very symptomatic patients who receive palliative benefit from chemotherapy, or as a means of encouraging noncompliant patients to return for care.
Despite enthusiasm for the use of molecular testing and molecularly targeted agents in patients with advanced non–small cell lung cancer (NSCLC), most patients are not candidates for upfront treatment with molecular agents. Chemotherapy therefore remains the backbone of treatment for this patient population.
This article presents the best available evidence for selection of chemotherapy for patients with advanced NSCLC and examines the controversy surrounding maintenance therapy.
EVOLUTION OF CHEMOTHERAPY IN NSCLC
The first evidence that chemotherapy produced a significant survival benefit in patients with advanced NSCLC came in 1995 when a meta-analysis showed that platinum-based chemotherapy conferred a 2-month improvement in median survival over best supportive care.1
This finding led to a decade of randomized phase 3 clinical trials that compared different platinum-based regimens. The quintessential trial in this regard was Eastern Cooperative Oncology Group (ECOG) 1594, in which three platinum doublets were compared with cisplatin and paclitaxel on the end point of overall survival (OS) in patients with advanced NSCLC. No significant differences were found among the regimens tested.2
Bevacizumab adds to platinum doublet in nonsquamous NSCLC
With the introduction of bevacizumab, an antibody against vascular endothelial growth factor, knowledge of NSCLC histology became important. In the ECOG 4599 trial, published in 2006, bevacizumab added to platinum doublet chemotherapy in patients with advanced nonsquamous NSCLC significantly improved the response rate, progression-free survival (PFS), and median OS compared with platinum doublet chemotherapy alone.3 This trial was limited to patients with nonsquamous NSCLC because its predecessor trial had revealed an excess of life-threatening pulmonary hemorrhage in association with bevacizumab in patients with squamous cell carcinoma.
Pemetrexed superior to docetaxel in nonsquamous histology
In 2004, Hanna et al4 demonstrated pemetrexed to be noninferior to docetaxel on efficacy outcomes as second-line therapy in advanced NSCLC. Pemetrexed had a significantly better toxicity profile, however, which led to its approval for this indication. Post hoc analyses of this trial suggested a differential effect of pemetrexed based on histology. In the pemetrexed arm, patients with nonsquamous histology appeared to have superior survival compared with patients who had squamous histology, whereas in the docetaxel arm, histology did not affect outcome.5 Further, the nonsquamous histologic subgroup had superior OS with pemetrexed compared with docetaxel.6
Pemetrexed and gemcitabine perform differently based on histology
A phase 3 trial with a noninferiority design compared cisplatin/pemetrexed with cisplatin/gemcitabine as first-line therapy on OS. The noninferiority criteria were met, with no difference in median OS between the two groups (median OS: 10.3 months in both arms).7
Based on this evidence, NSCLC is no longer an adequate pathologic diagnosis. Pathologists must differentiate squamous from nonsquamous histology to take full advantage of the safety of angiogenesis inhibitors and the efficacy of pemetrexed.
OPTIMAL FIRST-LINE REGIMEN FOR NONSQUAMOUS NSCLC
Both cisplatin/pemetrexed and carboplatin/paclitaxel plus bevacizumab have level 1 evidence to support their use as first-line treatment of NSCLC with nonsquamous histology. Carboplatin/pemetrexed/bevacizumab is also being used in the community despite the absence of randomized trial evidence to support its use for this indication.
A single-arm phase 2 trial of carboplatin/pemetrexed/bevacizumab followed by maintenance pemetrexed/bevacizumab in 49 patients produced a response rate of 55%, PFS of 7.8 months, and OS of 14 months.10 Although the results are impressive, they should be considered hypothesis-generating rather than treatment-changing in light of the small number of patients enrolled and the single-arm design.
An open-label randomized phase 3 trial, Point-Break, is comparing two regimens in patients who have advanced nonsquamous NSCLC: (1) carboplatin/pemetrexed/bevacizumab followed by maintenance pemetrexed/bevacizumab and (2) carboplatin/paclitaxel/bevacizumab followed by bevacizumab.11 Although potentially practice-changing, PointBreak will not answer whether bevacizumab adds benefit to cisplatin and pemetrexed, nor will it determine which first-line regimen is superior because of the different maintenance regimens.
SQUAMOUS NSCLC: PLATINUM DOUBLET OPTIMAL
No agents are currently approved specifically for the treatment of squamous cell carcinoma, which appears to have a high level of expression of insulin-like growth factor receptor (IGF-1R). A 64% response rate observed with an IGF-1R antagonist added to paclitaxel/carboplatin in patients with NSCLC of squamous cell histology in a phase 2 trial led to the design of a phase 3 trial in which patients were randomized to carboplatin/paclitaxel with or without the IGF-1R antagonist figitumumab. The trial ended prematurely in 2009 because of an imbalance of deaths in the experimental arm.12 As expected, hyperglycemia was more common in the experimental arm. Unexpectedly, the incidences of grade 5 infections and cardiovascular events were also significantly higher in the experimental arm.
A randomized phase 3 trial of carboplatin/paclitaxel compared with carboplatin and nanoparticle albumin-bound (nab) paclitaxel was conducted in 1,052 patients with stage IIIb/IV NSCLC with the primary end point being overall response rate (ORR).13 The rationale for substituting nab-paclitaxel for paclitaxel was that paclitaxel is dissolved in polyoxyethylated castor oil. This decreases the efficacy of paclitaxel and contributes to its toxicities, including hypersensitivity reactions and neuropathy. In metastatic breast cancer, nab-paclitaxel was shown to be more efficacious than solvent-based paclitaxel.14
The nab-paclitaxel trial met its primary end point of superior response rate: 33% in the nab-paclitaxel arm versus 25% in the standard paclitaxel arm. However, on final analysis there was no difference in PFS or OS between the two arms, making the difference in ORR of little clinical significance. No hypersensitivity reactions occurred in the nab-paclitaxel arm, while three occurred in the paclitaxel arm. Grade 3 sensory neuropathy occurred significantly less often in the group assigned to nab-paclitaxel compared with paclitaxel (3 vs 10, respectively; P < .001). Although there appears to be no efficacy advantage of nab-paclitaxel over standard paclitaxel for advanced NSCLC patients, use of nab-paclitaxel might be considered in patients with stage IV NSCLC who have poorly controlled diabetes or who already suffer significant neuropathy.
Although not a prespecified end point, the response rate in patients with squamous cell histology nearly doubled among those treated with nab-paclitaxel compared with standard paclitaxel. However, this did not translate into significant differences in PFS or OS in this subgroup, and the use of nab-paclitaxel in this patient population specifically is not advised.
In light of the data, the standard treatment for squamous NSCLC remains a platinum doublet other than pemetrexed. A phase 3 clinical trial, ECLIPSE, is currently enrolling patients at the Cleveland Clinic. The trial will randomize chemotherapy-naïve patients with stage IIIb/IV NSCLC and a Karnofsky performance status of 0 or 1 to receive carboplatin and gemcitabine with or without the polyadenosine diphosphate–ribose polymerase inhibitor iniparib.
MAINTENANCE THERAPY
The utility of maintenance therapy—the uninterrupted continuation of therapy for patients who do not progress after completing first-line chemotherapy—in patients with advanced NSCLC is controversial. Two kinds of maintenance therapy have emerged.
Switch maintenance, also known as early second-line therapy, is so termed because patients are immediately switched to a second-line agent different from the first-line doublet therapy.
Continuation maintenance is the continuation of one or more drugs from the induction regimen, the best example being continuation of single-agent evacizumab in the ECOG 4599 regimen.
Five major trials of successful maintenance therapy for nonprogressors after first-line chemotherapy have been presented over the past 4 years, and these have led to new indications for the maintenance drugs.8,15–18 PFS in favor of active maintenance has been documented in trials of early versus delayed maintenance docetaxel,15 pemetrexed versus placebo,8 erlotinib versus placebo,16 bevacizumab/erlotinib versus bevacizumab/placebo (ATLAS trial),17 and gemcitabine or erlotinib versus placebo (IFCT-GFPC 0502).18 The magnitude of improved PFS associated with each treatment has been similar. As a result, improved of PFS with maintenance therapy is now widely accepted.
To improve survival: maintenance therapy or better second-line therapy?
The utility of maintenance therapy can be confounded by the lack of a predefined second-line treatment. Some argue that the benefit to maintenance may also be realized by appropriate use of the same agent as salvage therapy in the case of disease progression.
Overall survival improved with maintenance therapy in only two trials; one compared pemetrexed with placebo and the other compared erlotinib with placebo.8,16 The validity of these findings, however, remains in question. In the trial of pemetrexed, only 19% of the patients in the control arm ever received pemetrexed, and in the erlotinib trial, only 21% of the patients in the control arm ever received erlotinib after progression. Whether maintenance therapy was responsible for an improvement in OS or whether ineffective second-line therapy dampened survival in patients in the control arms is unknown.
In the IFCT-GFPC 0502 phase 3 study, patients received four cycles of cisplatin/gemcitabine.19 If patients did not progress, they were randomized to observation, continuation maintenance with gemcitabine, or switch maintenance to erlotinib. Predefined second-line therapy in all arms was pemetrexed. The primary end point chosen was PFS, even though maintenance therapy had already been established to extend PFS. The median PFS in the gemcitabine maintenance arm was 3.8 months, compared with 1.9 months in the observation arm. The hazard ratio (HR) of 0.55 (P < .0001) was similar to that observed with pemetrexed in the maintenance trial noted above.19 Subgroup analysis showed improvement in PFS with the gemcitabine maintenance group compared with observation in all subgroups, including those based on histology. The HR in the erlotinib maintenance arm was 0.82 (P = .002), similar to that observed with erlotinib in the Sequential Tarceva in Unresectable NSCLC (SATURN) trial.16
In the IFCT-GFPC 0502 study, 60.4% of patients in the gemcitabine arm, 63.2 % of those in the erlotinib arm, and 76.1% in the observation arm received postmaintenance treatment with pemetrexed.19 Nearly one-half (49.6%) of patients in the observation arm received third-line treatment with erlotinib.
In the overall study population, a trend toward improved OS was observed with maintenance gemcitabine or erlotinib compared with observation, but did not achieve statistical significance, possibly because the trial lacked adequate power to detect a difference on this end point.
A subgroup analysis of patients who received second-line pemetrexed showed a significant improvement in OS in the maintenance arms compared with the observation arm. About 25% of the patients never advanced to second-line pemetrexed. Because patients who never received second-line pemetrexed may represent the sickest patients, the relevance of this finding to the overall population of patients with stage IV NSCLC is unknown.
Ongoing maintenance trials
Two ongoing clinical trials of maintenance are exploring bevacizumab with or without pemetrexed as maintenance following first-line cisplatin/pemetrexed/bevacizumab (AVAPERL) and bevacizumab alone, pemetrexed alone, or a combination of the two as maintenance following first-line therapy with carboplatin/paclitaxel/bevacizumab (Eastern Cooperative Oncology Group). Preliminary results from the AVAPERL study were reported recently and support the use of pemetrexed and bevacizumab as maintenance therapy compared with bevacizumab alone.20
CONCLUSIONS ABOUT MAINTENANCE THERAPY
Pemetrexed and erlotinib significantly prolong OS survival compared with placebo when used as maintenance therapy in advanced NSCLC patients who do not progress after four cycles of first-line chemotherapy. Whether this improvement in OS can be attributed to maintenance therapy or more effective second-line therapy is open to debate.
Maintenance chemotherapy should be discussed with all patients whose tumors do not progress after four cycles of first-line chemotherapy. The use of maintenance therapy may be most reasonable in very symptomatic patients who receive palliative benefit from chemotherapy, or as a means of encouraging noncompliant patients to return for care.
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995; 311:899–909.
- Schiller JH, Harrington D, Belani C, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346:92–98.
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355:2542–2450.
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22:1589–1597.
- Peterson P, Park K, Fossella F, Gatzmeier U, John W, Scagliotti G. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC) [12th World Congress on Lung Cancer abstract P2-328]. J Thorac Oncol 2007; 2 (suppl 4): S851.
- Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist 2009; 14:253–263.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non–small-cell lung cancer. J Clin Oncol 2008; 26:3543–3551.
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009; 374:1432–1440.
- Belani CP, Brodowicz T, Ciuleanu T, et al. Maintenance pemetrexed (Pem) plus best supportive care (BSC) versus placebo (Plac) plus BSC: a randomized phase III study in advanced nonsmall cell lung cancer (NSCLC) [ASCO abstract CRA8000]. J Clin Oncol 2009; 27 (suppl).
- Patel JD, Hensing TA, Rademaker A, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer. J Clin Oncol 2009; 27:3284–3289.
- Patel JD, Bonomi P, Socinski MA, et al. Treatment rationale and study design for the PointBreak study: a randomized, open-label phase III study of pemetrexed/carboplatin/bevacizumab followed by maintenance pemetrexed/bevacizumab versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non–small-cell lung cancer. Clin Lung Cancer 2009; 10:252–256.
- Jassem J, Langer CJ, Karp DD, et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with nonsmall cell lung cancer (NSCLC). J Clin Oncol 2010; 28 (suppl): abstr 7500.
- Socinski MA, Bondarenko IN, Karaseva NA, et al. Survival results of a randomized, phase III trial of nab-paclitaxel and carboplatin compared with cremophor-based paclitaxel and carboplatin as first-line therapy in advanced non-small cell lung cancer [ASCO abstract 7551]. J Clin Oncol 2011; 29 (suppl).
- Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005; 23:7794–7803.
- Fidias PM, Dakhil SR, Lyss AP, et al Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non–small-cell lung cancer. J Clin Oncol 2008; 27:591–598.
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010; 11:521–529.
- Miller VA, O’Connor P, Soh C, Kabbinavar F; ATLAS investigators. A randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC) [ASCO abstract LBA8002]. J Clin Oncol 2009; 27 (suppl).
- Perol M, Zalcman G, Monnet I, et al. Final results from the IFCTGFPC 0502 phase III study: Maintenance therapy in advanced NSCLC with either gemcitabine (G) or erlotinib (E) versus observation (O) after cisplatin-gemcitabine induction chemotherapy (CT), with a predefined second-line treatment [ESMO abstract 370PD]. Ann Oncol 2010; 21 (suppl 8): viii124.
- Perol M, Chouaid C, Milleron J, et al. Maintenance with either gemcitabine or erlotinib versus observation with predefined second-line treatment after cisplatin-gemcitabine induction chemotherapy in advanced NSCLC: IFCT-GFPC 0502 phase III study [ASCO abstract 7507]. J Clin Oncol 2010; 28 (suppl).
- Barlesi F, de Castro J, Dvornichencko V, et al. AVAPERL (MO22089): Final efficacy outcomes for patients with advanced non-squamous non-small cell lung cancer randomized to continuation maintenance with bevacizumab (bev) or bev + pemetrexed (pem) after first-line bev-cisplatin-pem treatment. Paper presented at: The European Multidiscipinary Cancer Congress; September 24, 2011; Stockholm, Sweden.
- Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ 1995; 311:899–909.
- Schiller JH, Harrington D, Belani C, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346:92–98.
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355:2542–2450.
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004; 22:1589–1597.
- Peterson P, Park K, Fossella F, Gatzmeier U, John W, Scagliotti G. Is pemetrexed more effective in adenocarcinoma and large cell lung cancer than in squamous cell carcinoma? A retrospective analysis of a phase III trial of pemetrexed vs docetaxel in previously treated patients with advanced non-small cell lung cancer (NSCLC) [12th World Congress on Lung Cancer abstract P2-328]. J Thorac Oncol 2007; 2 (suppl 4): S851.
- Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist 2009; 14:253–263.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non–small-cell lung cancer. J Clin Oncol 2008; 26:3543–3551.
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009; 374:1432–1440.
- Belani CP, Brodowicz T, Ciuleanu T, et al. Maintenance pemetrexed (Pem) plus best supportive care (BSC) versus placebo (Plac) plus BSC: a randomized phase III study in advanced nonsmall cell lung cancer (NSCLC) [ASCO abstract CRA8000]. J Clin Oncol 2009; 27 (suppl).
- Patel JD, Hensing TA, Rademaker A, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer. J Clin Oncol 2009; 27:3284–3289.
- Patel JD, Bonomi P, Socinski MA, et al. Treatment rationale and study design for the PointBreak study: a randomized, open-label phase III study of pemetrexed/carboplatin/bevacizumab followed by maintenance pemetrexed/bevacizumab versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non–small-cell lung cancer. Clin Lung Cancer 2009; 10:252–256.
- Jassem J, Langer CJ, Karp DD, et al. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with nonsmall cell lung cancer (NSCLC). J Clin Oncol 2010; 28 (suppl): abstr 7500.
- Socinski MA, Bondarenko IN, Karaseva NA, et al. Survival results of a randomized, phase III trial of nab-paclitaxel and carboplatin compared with cremophor-based paclitaxel and carboplatin as first-line therapy in advanced non-small cell lung cancer [ASCO abstract 7551]. J Clin Oncol 2011; 29 (suppl).
- Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol 2005; 23:7794–7803.
- Fidias PM, Dakhil SR, Lyss AP, et al Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non–small-cell lung cancer. J Clin Oncol 2008; 27:591–598.
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010; 11:521–529.
- Miller VA, O’Connor P, Soh C, Kabbinavar F; ATLAS investigators. A randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC) [ASCO abstract LBA8002]. J Clin Oncol 2009; 27 (suppl).
- Perol M, Zalcman G, Monnet I, et al. Final results from the IFCTGFPC 0502 phase III study: Maintenance therapy in advanced NSCLC with either gemcitabine (G) or erlotinib (E) versus observation (O) after cisplatin-gemcitabine induction chemotherapy (CT), with a predefined second-line treatment [ESMO abstract 370PD]. Ann Oncol 2010; 21 (suppl 8): viii124.
- Perol M, Chouaid C, Milleron J, et al. Maintenance with either gemcitabine or erlotinib versus observation with predefined second-line treatment after cisplatin-gemcitabine induction chemotherapy in advanced NSCLC: IFCT-GFPC 0502 phase III study [ASCO abstract 7507]. J Clin Oncol 2010; 28 (suppl).
- Barlesi F, de Castro J, Dvornichencko V, et al. AVAPERL (MO22089): Final efficacy outcomes for patients with advanced non-squamous non-small cell lung cancer randomized to continuation maintenance with bevacizumab (bev) or bev + pemetrexed (pem) after first-line bev-cisplatin-pem treatment. Paper presented at: The European Multidiscipinary Cancer Congress; September 24, 2011; Stockholm, Sweden.