User login
Surgical resection for patients with stage I non–small cell lung cancer (NSCLC) is typically associated with survival rates of 60% to 70% after 5 years, and as high as 80% in some series.1 Although lobectomy or pneumonectomy improves outcomes compared with sublobar resection for many patients, a substantial number are ineligible for standard surgical resection because of cardiovascular disease or other conditions that are associated with unacceptably high perioperative risk. Observation alone is not a good strategy for patients who are ineligible for surgery. Studies comparing treatment outcomes associated with resection, radiation, and observation have demonstrated much shorter survival times and higher mortality for patients treated with observation only.2
Stereotactic body radiotherapy (SBRT) is the new standard of care for patients with medically inoperable stage I NSCLC. SBRT differs from standard radiation therapy in terms of dose, fractionation, field size, and targeting. Compared with standard radiation, SBRT offers a shorter and more convenient treatment regimen with improved local control and survival while lowering treatment cost.3,4 Although cancer-specific outcomes of patients in SBRT series are similar to those in surgical groups, they are not truly comparable because of dissimilarities between the two populations. The inoperable group has higher rates of comorbidity and death compared with the medically operable group; as many as one-third die from comorbid conditions rather than cancer, leading to short follow-up in many SBRT series. Surgical resection remains the standard of care for operable stage I NSCLC.
STEREOTACTIC RADIATION FOR PATIENTS WITH INOPERABLE LUNG CANCER
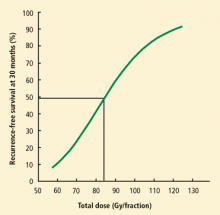
Modern standard external beam radiation doses without chemotherapy for stage I lung cancer are approximately 60 to 74 Gy. The dose fractionation schedule used with SBRT delivers much higher equivalent doses (83 Gy to 150 Gy), although the true biologically equivalent dose (BED) is not yet perfectly understood.8 Most clinical studies that have examined the effectiveness of SBRT have demonstrated local control rates in excess of 90% to 95% when an adequate dose (BED ≥ 100 Gy) is utilized, since the dose-response curve appears to plateau at this level.9 These response rates are higher than the 50% to 60% rate observed with conventional radiation.3,4 Efforts to confirm these comparative results in randomized trials have been largely abandoned because of the perceived advantage with SBRT.
PERIPHERAL VERSUS CENTRAL TUMORS
Stereotactic body radiotherapy has been referred to as “radiosurgery,” in part because the extremely high doses used to treat tumor are ablative to the immediate surrounding tissue. The consequences of ablation depend on whether the treatment involves parallel or serial tissue. Parallel tissue, such as lung, kidney, or liver, remains functional after the ablation or removal of small subunits if adequate volume of functional organ remains. With serial tissue such as the spinal cord or bowel, damage to one section results in loss of function at distal sites. Although the lung is parallel tissue, it includes serial structures such as the trachea and proximal bronchial tree. Tumors located within 2 cm of the proximal bronchial tree are classified as central, whereas tumors outside this zone are peripheral.
Peripheral tumors
Peripheral lung tumors are surrounded by only parallel tissue, and no maximum point-dose limit has been identified for their treatment. A recent cooperative group study (Radiation Therapy Oncology Group [RTOG] 0236) enrolled 55 patients, 80% with tumor stage IA (T1 N0) and 20% with stage IB (T2 N0).10 Patients with bronchoalveolar histology were excluded from the study. Patients received three radiation treatments of 20 Gy each (BED of 180 Gy) to their known tumor with a small margin, and were followed with serial computed tomography (CT). After a median follow-up of 34 months, only one of the 55 evaluable patients had a local tumor failure, for a local control rate of 97.6%. Three patients had recurrences in the initially involved lobe for a 3-year local control rate of 90.6%; two patients had nodal failures for a 3-year local regional control rate of 87.2%; and 11 patients had disseminated recurrences, for a 3-year distant failure rate of 22.1%.
Survival after 3 years was approximately 50%, which is much better than the survival rate typically attained with standard radiation therapy. Further, only 10 of the 26 deaths were attributed to lung cancer while 16 patients died of comorbid conditions such as stroke or myocardial infarction, illustrating the difficulty in tracking overall survival as a measure of efficacy in this medically fragile population.
Adverse events in this study were relatively rare. Seven patients had grade 3 or higher pulmonary complications, including hypoxia, pneumonitis, and pulmonary function test changes. Of note, the study scored changes in pulmonary function as toxicity; however, in this population, where nearly all patients have underlying lung disease, chronic obstructive pulmonary disease (COPD) exacerbations are also common.
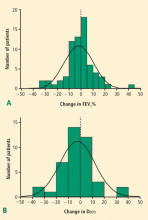
Our own analysis of pulmonary function changes in patients treated with SBRT at Cleveland Clinic demonstrated that while there was no significant change in average baseline, pulmonary function in almost 10% of patients met criteria for a grade 3 pulmonary toxicity. A similar number of patients had a proportional improvement in pulmonary function, however. Given a nearly comparable distribution of pulmonary function changes in both directions with no significant deviation from baseline in aggregate, most of these fluctuations may be related to changes in the patient’s underlying comorbidities rather than effects of treatment.
RTOG 0236 demonstrated an excellent level of local control (97.6%) using 3 fractions of 20 Gy each (BED 180 Gy total). As noted, the dose response may plateau at 100 Gy BED,9 which raises the question of whether the radiation dose levels used in this study were higher than necessary. A recently completed randomized phase 2 clinical trial conducted by the RTOG compared 34 Gy in a single fraction versus 48 Gy in 4 fractions, and a similar study by Roswell Park Cancer Institute, Buffalo, New York, and Cleveland Clinic is comparing 60 Gy in 3 fractions versus 30 Gy in a single fraction. These studies, once mature, should help define the optimal radiation dose and treatment schedule for patients with inoperable peripheral tumors.
Central tumors
Centrally located tumors are in proximity to both parallel tissues (normal lung) and serial tissues (trachea, bronchial tree, or esophagus), as well as imperfectly categorized tissues (heart and great vessels). An important question is whether it is possible to reach a radiation dose level of 100 Gy BED or higher in these tumors without causing excessive toxicity to normal tissues. Although there is a potential risk of cardiotoxicity with chest radiotherapy, clinical studies of SBRT for lung cancer have not demonstrated any evidence of toxicity to the heart or the great vessels with focal radiation. Some studies have suggested that radiotherapy of central lung tumors may be associated with other adverse events.
Awareness of central versus peripheral tumor locations was first raised in an early phase 2 study in which patients were treated with 60 to 66 Gy in 3 fractions over a period of 1 to 2 weeks. Grade 3 or higher toxicity during 2 years of follow-up was noted for 46% of patients with central tumors and 17% of patients with peripheral tumors.11 Six deaths that occurred during the study were considered to be possibly treatment-related, including four cases of bacterial pneumonia, one patient with pericardial effusion, and one patient with hemoptysis that was later ascribed to carinal recurrence.
Other studies using lower fraction sizes, however, have demonstrated excellent efficacy and safety in treating central tumors with SBRT. In early Japanese studies12,13 that used smaller fractions without tissue constraints, no differences in toxicity were noted with treatment of central versus peripheral tumors. A European study similarly demonstrated more than 90% local control at 3 years for a regimen of 60 Gy in 8 fractions (7.5 Gy/fraction).14 Currently the RTOG is conducting a dose escalation study examining doses from 50 Gy to 60 Gy (10 Gy to 12 Gy per fraction in 5 fractions). The study has reached its highest level (60 Gy in 5 fractions) with no evidence of excessive toxicity reported.
SAFETY AND TOLERABILITY
Radiation pneumonitis (an inflammatory complication of radiation frequently characterized by cough, fever, and shortness of breath) is rare—less than 5% in most series. An outlier is a single series that utilized 48 Gy in 4 fractions, a common and well-tolerated dose; the investigators reported a 30% rate of grade 2 through 5 (symptomatic) pneumonitis.18 Pneumonitis was significantly associated with the conformality index, a measure of how tightly the radiation beam is focused on the target tumor, emphasizing the importance of treatment technique on outcomes.
Other notes of caution for patients receiving SBRT include chest wall toxicity and neuropathy. Chest wall toxicity may include a variety of adverse events such as rib fractures, chest wall pain, and skin changes. These events have been described at chest wall radiation doses greater than 30 Gy.19 One study reported brachial plexopathy in 7 of 37 patients who received doses above 100 Gy BED delivered to the brachial plexus.20 Another recent study found that the probability of chest wall toxicity increased as the volume of chest wall receiving a 60 Gy dose increased above 15 to 20 cc.21 Esophagitis and skin reactions are rare except in cases where the patient is being treated for a tumor in extremely close proximity to the esophagus or skin.22
Computed tomography after SBRT often reveals substantial focal fibrosis in the region of high-dose lung radiation.23,24 Despite the often striking radiographic appearance, symptoms are rare and fibrosis may sometimes be mistaken for tumor recurrence. CT images should be read by those experienced in following post-SBRT changes. Findings suspicious for recurrence are typically evaluated by positron emission tomography (PET) followed by biopsy only if PET demonstrates sufficient hypermetabolism.
OPERABLE PATIENTS
Surgical resection is the standard of care for operable patients with lung cancer. Some studies are beginning to examine whether SBRT may also be useful in potentially operable patients. A Japanese study examined outcomes for 87 operable patients who underwent SBRT for stage I NSCLC and who were followed over a 55-month period.25 The local control rate was 92% for T1 tumors, a success rate approaching that of lobectomy. The success rate decreased to 73% for T2 tumors. Five-year overall survival rates were 72% for stage IA and 62% for stage IB, paralleling the surgical experience. Similar early results have been reported from the Netherlands.26 An RTOG study of medically operable patients recently completed enrollment after accruing 33 patients, with final results pending maturation of the data.
A major barrier to the introduction of SBRT to the operable population is the limited nature of the available data; SBRT technology has been implemented only recently and follow-up has been modest, owing to the nature of the medically inoperable population. In addition, it is difficult to determine during the first few months after SBRT which patients will be well controlled. Waiting for response to become apparent is an appropriate strategy for an inoperable patient with no alternatives, but operable patients need a trigger to indicate initiation of salvage therapies.27 In addition, lymph node dissection during surgery often provides information that is essential to tumor staging, and this information might be unavailable for patients treated with SBRT. It is also difficult to weigh the efficacy and tolerability of SBRT against surgical management because the two patient populations are not comparable.
High-risk operable patients
Comparisons of surgery and SBRT for stage I NSCLC are in their infancy and subject to extreme selection bias. Some attempts to create matched populations have demonstrated similar outcomes in matched patients.28,29 Markov modeling suggests improved efficacy for surgery overall, but the model turns in favor of SBRT in patients whose predicted surgical mortality exceeds 4%.30
High-risk operable patients are currently eligible for the American College of Surgeons Oncology group (ACOSOG)/RTOG 0870/Cancer and Leukemia Group B (CALGB) 140503 study; a randomized phase 3 clinical trial that is comparing lobectomy versus sublobar resection for small (< 2 cm) peripheral NSCLC. This study should help to clarify how this higher-risk patient group should be managed.
CLEVELAND CLINIC EXPERIENCE
At Cleveland Clinic, more than 700 patients with stage I NSCLC have been treated with SBRT since 2003. Peripheral tumors are typically treated with a radiation dose of 60 Gy in 3 fractions spaced over 8 to 14 days, or alternatively 30 Gy to 34 Gy in a single fraction. Occasional large tumors near the chest wall or spinal cord are treated with doses up to 50 Gy in 5 fractions over 5 consecutive days. For central tumors, radiation dose regimens include 50 Gy (5 fractions over 5 consecutive days) or 60 Gy (8 fractions over 10 days), depending upon tumor size and proximity to critical structures.
SUMMARY AND CONCLUSIONS
Many patients with NSCLC are ineligible for surgery because of COPD, cardiovascular disease, or other conditions associated with unacceptably high perioperative risk. SBRT is the standard of care for patients with medically inoperable stage I NSCLC. Modern standard radiation doses are typically between 50 to 60 Gy in 3 to 5 fractions. Local control rates in excess of 90% to 95% have been reported with these doses. SBRT is generally well tolerated by patients with both peripheral and centrally located tumors. On average, lung function is not substantially altered by SBRT, although individual patients may exhibit increased or decreased FEV1 and Dlco values after treatment. Pneumonitis has been relatively rare in most studies, with typical rates of 0% to 5%. SBRT has been shown to produce reasonable rates of local control in potentially operable patients, although data are extremely limited in this population and there are important questions about salvage therapy and postprocedural evaluation in these patients. Several ongoing clinical trials are continuing to define the efficacy and safety of different radiation dosing procedures for patients with inoperable NSCLC.
- Smolle-Juettner FM, Maier A, Lindenmann J, Matzi V, Neuböck N. Resection in stage I/II non-small cell lung cancer. In:Heide J, Schmittel A, Kaiser D, Hinkelbein W, eds. Controversies in the Treatment of Lung Cancer. Basel, Switzerland: Karger; 2010:71–77.
- McGarry RC, Song G, des Rosiers P, Timmerman R. Observationonly management of early stage, medically inoperable lung cancer: poor outcome. Chest 2002; 121:1155–1158.
- Lanni TB, Grills IS, Kestin LL, Robertson JM. Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol 2011; 34:494–498.
- Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis [published online ahead of print September 3, 2009]. Radiother Oncol 2010; 95:32–40. doi: 10.1016/jradonc.2009.08.003
- Noordijk EM, vd Poest Clement E, Hermans J, Wever AMJ, Leer JWH. Radiotherapy as an alternative to surgery in elderly patients with resectable lung cancer. Radiother Oncol 1988; 13:83–89.
- Sibley GS. Radiotherapy for patients with medically inoperable stage I nonsmall cell lung carcinoma: smaller volumes and higher doses—a review. Cancer 1998; 82:433–438.
- Mehta M, Manon R. Are more aggressive therapies able to improve treatment of locally advanced non-small cell lung cancer: combined modality treatment? Semin Oncol 2005; 32 (2 suppl 3):S25–S34.
- Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 2008; 70:847–852.
- Wulf J, Baier K, Mueller G, Flentje MP. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol 2005; 77:83–87.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303:1070–1076.
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006; 24:4833–4839.
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007; 2 (7 suppl 3):S94–S100.
- Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame [published online ahead of print September 19, 2005]. Int J Radiat Oncol Biol Phys 2005; 63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011; 6:2036–2043.
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003; 124:1946–1955.
- Henderson M, McGarry R, Yiannoutsos C, et al. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 72:404–409.
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009; 4:838–844.
- Yamashita H, Nakagawa K, Nakamura N, et al. Exceptionally high incidence of symptomatic grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol 2007; 2:21.
- Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving > 30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76:796–801.
- Forquer JA, Fakiris AJ, Timmerman RD, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: dose-limiting toxicity in apical tumor sites. Radiother Oncol 2009; 93:408–413.
- Stephans KL, Djemil T, Tendulkar RD, Robinson CG, Reddy CA, Videtic GM. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT) [published online ahead of print February 6, 2011]. Int J Radiat Oncol Biol Phys 2012; 82:974–980. doi: 10.1016/j.ijrobp.2010.12.002
- Hoppe BS, Laser B, Kowalski AV, et al. Acute skin toxicity following stereotactic body radiation therapy for stage I non-small-cell lung cancer: who’s at risk? Int J Radiat Oncol Biol Phys 2008; 72:1283–1286.
- Bradley J. Radiographic response and clinical toxicity following SBRT for stage I lung cancer. J Thorac Oncol 2007; 2 (7 suppl 3):S118–S124.
- Linda A, Trovo M, Bradley JD. Radiation injury of the lung after stereotactic body radiation therapy (SBRT) for lung cancer: a timeline and pattern of CT changes [published online ahead of print December 1, 2009]. Eur J Radiol 2011; 79:147–154. doi: 10.1016/j.ejrad.2009.10.029
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery [published online ahead of print July 16, 2011]? Int J Radiat Oncol Biol Phys 2011; 81:1352–1358. doi: 10.1016/j.ijrobp.2009.07.1751
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small-cell lung cancer [published online ahead of print November 19, 2011.] Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2011.06.2003.
- Neri S, Takahashi Y, Terashi T, et al. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol 2010; 5:2003–2007.
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer [published online ahead of print April 18, 2010]. J Thorac Cardiovasc Surg 2010; 140:377–386. doi: 10.1016/j.jtcvs.2009.12.054
- Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of stage I non-small-cell lung acancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review [published online ahead of print June 2, 2011]. Int J Radiat Oncol Biol Phys 2012; 82:1149–1156. doi: 10.1016/j.ijrobp.2011.03.005
- Louie AV, Rodrigues G, Hannouf M, et al. Stereotactic body radiotherapy versus surgery for medically operable stage I non-small-cell lung cancer: a Markov model-based decision analysis [published online ahead of print October 6, 2010]. Int J Radiat Oncol Biol Phys 2011; 81:964–973. doi: 10.1016/j.ijrobp.2010.06.040
- Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 1999; 24:31–37.
Surgical resection for patients with stage I non–small cell lung cancer (NSCLC) is typically associated with survival rates of 60% to 70% after 5 years, and as high as 80% in some series.1 Although lobectomy or pneumonectomy improves outcomes compared with sublobar resection for many patients, a substantial number are ineligible for standard surgical resection because of cardiovascular disease or other conditions that are associated with unacceptably high perioperative risk. Observation alone is not a good strategy for patients who are ineligible for surgery. Studies comparing treatment outcomes associated with resection, radiation, and observation have demonstrated much shorter survival times and higher mortality for patients treated with observation only.2
Stereotactic body radiotherapy (SBRT) is the new standard of care for patients with medically inoperable stage I NSCLC. SBRT differs from standard radiation therapy in terms of dose, fractionation, field size, and targeting. Compared with standard radiation, SBRT offers a shorter and more convenient treatment regimen with improved local control and survival while lowering treatment cost.3,4 Although cancer-specific outcomes of patients in SBRT series are similar to those in surgical groups, they are not truly comparable because of dissimilarities between the two populations. The inoperable group has higher rates of comorbidity and death compared with the medically operable group; as many as one-third die from comorbid conditions rather than cancer, leading to short follow-up in many SBRT series. Surgical resection remains the standard of care for operable stage I NSCLC.
STEREOTACTIC RADIATION FOR PATIENTS WITH INOPERABLE LUNG CANCER
Modern standard external beam radiation doses without chemotherapy for stage I lung cancer are approximately 60 to 74 Gy. The dose fractionation schedule used with SBRT delivers much higher equivalent doses (83 Gy to 150 Gy), although the true biologically equivalent dose (BED) is not yet perfectly understood.8 Most clinical studies that have examined the effectiveness of SBRT have demonstrated local control rates in excess of 90% to 95% when an adequate dose (BED ≥ 100 Gy) is utilized, since the dose-response curve appears to plateau at this level.9 These response rates are higher than the 50% to 60% rate observed with conventional radiation.3,4 Efforts to confirm these comparative results in randomized trials have been largely abandoned because of the perceived advantage with SBRT.
PERIPHERAL VERSUS CENTRAL TUMORS
Stereotactic body radiotherapy has been referred to as “radiosurgery,” in part because the extremely high doses used to treat tumor are ablative to the immediate surrounding tissue. The consequences of ablation depend on whether the treatment involves parallel or serial tissue. Parallel tissue, such as lung, kidney, or liver, remains functional after the ablation or removal of small subunits if adequate volume of functional organ remains. With serial tissue such as the spinal cord or bowel, damage to one section results in loss of function at distal sites. Although the lung is parallel tissue, it includes serial structures such as the trachea and proximal bronchial tree. Tumors located within 2 cm of the proximal bronchial tree are classified as central, whereas tumors outside this zone are peripheral.
Peripheral tumors
Peripheral lung tumors are surrounded by only parallel tissue, and no maximum point-dose limit has been identified for their treatment. A recent cooperative group study (Radiation Therapy Oncology Group [RTOG] 0236) enrolled 55 patients, 80% with tumor stage IA (T1 N0) and 20% with stage IB (T2 N0).10 Patients with bronchoalveolar histology were excluded from the study. Patients received three radiation treatments of 20 Gy each (BED of 180 Gy) to their known tumor with a small margin, and were followed with serial computed tomography (CT). After a median follow-up of 34 months, only one of the 55 evaluable patients had a local tumor failure, for a local control rate of 97.6%. Three patients had recurrences in the initially involved lobe for a 3-year local control rate of 90.6%; two patients had nodal failures for a 3-year local regional control rate of 87.2%; and 11 patients had disseminated recurrences, for a 3-year distant failure rate of 22.1%.
Survival after 3 years was approximately 50%, which is much better than the survival rate typically attained with standard radiation therapy. Further, only 10 of the 26 deaths were attributed to lung cancer while 16 patients died of comorbid conditions such as stroke or myocardial infarction, illustrating the difficulty in tracking overall survival as a measure of efficacy in this medically fragile population.
Adverse events in this study were relatively rare. Seven patients had grade 3 or higher pulmonary complications, including hypoxia, pneumonitis, and pulmonary function test changes. Of note, the study scored changes in pulmonary function as toxicity; however, in this population, where nearly all patients have underlying lung disease, chronic obstructive pulmonary disease (COPD) exacerbations are also common.
Our own analysis of pulmonary function changes in patients treated with SBRT at Cleveland Clinic demonstrated that while there was no significant change in average baseline, pulmonary function in almost 10% of patients met criteria for a grade 3 pulmonary toxicity. A similar number of patients had a proportional improvement in pulmonary function, however. Given a nearly comparable distribution of pulmonary function changes in both directions with no significant deviation from baseline in aggregate, most of these fluctuations may be related to changes in the patient’s underlying comorbidities rather than effects of treatment.
RTOG 0236 demonstrated an excellent level of local control (97.6%) using 3 fractions of 20 Gy each (BED 180 Gy total). As noted, the dose response may plateau at 100 Gy BED,9 which raises the question of whether the radiation dose levels used in this study were higher than necessary. A recently completed randomized phase 2 clinical trial conducted by the RTOG compared 34 Gy in a single fraction versus 48 Gy in 4 fractions, and a similar study by Roswell Park Cancer Institute, Buffalo, New York, and Cleveland Clinic is comparing 60 Gy in 3 fractions versus 30 Gy in a single fraction. These studies, once mature, should help define the optimal radiation dose and treatment schedule for patients with inoperable peripheral tumors.
Central tumors
Centrally located tumors are in proximity to both parallel tissues (normal lung) and serial tissues (trachea, bronchial tree, or esophagus), as well as imperfectly categorized tissues (heart and great vessels). An important question is whether it is possible to reach a radiation dose level of 100 Gy BED or higher in these tumors without causing excessive toxicity to normal tissues. Although there is a potential risk of cardiotoxicity with chest radiotherapy, clinical studies of SBRT for lung cancer have not demonstrated any evidence of toxicity to the heart or the great vessels with focal radiation. Some studies have suggested that radiotherapy of central lung tumors may be associated with other adverse events.
Awareness of central versus peripheral tumor locations was first raised in an early phase 2 study in which patients were treated with 60 to 66 Gy in 3 fractions over a period of 1 to 2 weeks. Grade 3 or higher toxicity during 2 years of follow-up was noted for 46% of patients with central tumors and 17% of patients with peripheral tumors.11 Six deaths that occurred during the study were considered to be possibly treatment-related, including four cases of bacterial pneumonia, one patient with pericardial effusion, and one patient with hemoptysis that was later ascribed to carinal recurrence.
Other studies using lower fraction sizes, however, have demonstrated excellent efficacy and safety in treating central tumors with SBRT. In early Japanese studies12,13 that used smaller fractions without tissue constraints, no differences in toxicity were noted with treatment of central versus peripheral tumors. A European study similarly demonstrated more than 90% local control at 3 years for a regimen of 60 Gy in 8 fractions (7.5 Gy/fraction).14 Currently the RTOG is conducting a dose escalation study examining doses from 50 Gy to 60 Gy (10 Gy to 12 Gy per fraction in 5 fractions). The study has reached its highest level (60 Gy in 5 fractions) with no evidence of excessive toxicity reported.
SAFETY AND TOLERABILITY
Radiation pneumonitis (an inflammatory complication of radiation frequently characterized by cough, fever, and shortness of breath) is rare—less than 5% in most series. An outlier is a single series that utilized 48 Gy in 4 fractions, a common and well-tolerated dose; the investigators reported a 30% rate of grade 2 through 5 (symptomatic) pneumonitis.18 Pneumonitis was significantly associated with the conformality index, a measure of how tightly the radiation beam is focused on the target tumor, emphasizing the importance of treatment technique on outcomes.
Other notes of caution for patients receiving SBRT include chest wall toxicity and neuropathy. Chest wall toxicity may include a variety of adverse events such as rib fractures, chest wall pain, and skin changes. These events have been described at chest wall radiation doses greater than 30 Gy.19 One study reported brachial plexopathy in 7 of 37 patients who received doses above 100 Gy BED delivered to the brachial plexus.20 Another recent study found that the probability of chest wall toxicity increased as the volume of chest wall receiving a 60 Gy dose increased above 15 to 20 cc.21 Esophagitis and skin reactions are rare except in cases where the patient is being treated for a tumor in extremely close proximity to the esophagus or skin.22
Computed tomography after SBRT often reveals substantial focal fibrosis in the region of high-dose lung radiation.23,24 Despite the often striking radiographic appearance, symptoms are rare and fibrosis may sometimes be mistaken for tumor recurrence. CT images should be read by those experienced in following post-SBRT changes. Findings suspicious for recurrence are typically evaluated by positron emission tomography (PET) followed by biopsy only if PET demonstrates sufficient hypermetabolism.
OPERABLE PATIENTS
Surgical resection is the standard of care for operable patients with lung cancer. Some studies are beginning to examine whether SBRT may also be useful in potentially operable patients. A Japanese study examined outcomes for 87 operable patients who underwent SBRT for stage I NSCLC and who were followed over a 55-month period.25 The local control rate was 92% for T1 tumors, a success rate approaching that of lobectomy. The success rate decreased to 73% for T2 tumors. Five-year overall survival rates were 72% for stage IA and 62% for stage IB, paralleling the surgical experience. Similar early results have been reported from the Netherlands.26 An RTOG study of medically operable patients recently completed enrollment after accruing 33 patients, with final results pending maturation of the data.
A major barrier to the introduction of SBRT to the operable population is the limited nature of the available data; SBRT technology has been implemented only recently and follow-up has been modest, owing to the nature of the medically inoperable population. In addition, it is difficult to determine during the first few months after SBRT which patients will be well controlled. Waiting for response to become apparent is an appropriate strategy for an inoperable patient with no alternatives, but operable patients need a trigger to indicate initiation of salvage therapies.27 In addition, lymph node dissection during surgery often provides information that is essential to tumor staging, and this information might be unavailable for patients treated with SBRT. It is also difficult to weigh the efficacy and tolerability of SBRT against surgical management because the two patient populations are not comparable.
High-risk operable patients
Comparisons of surgery and SBRT for stage I NSCLC are in their infancy and subject to extreme selection bias. Some attempts to create matched populations have demonstrated similar outcomes in matched patients.28,29 Markov modeling suggests improved efficacy for surgery overall, but the model turns in favor of SBRT in patients whose predicted surgical mortality exceeds 4%.30
High-risk operable patients are currently eligible for the American College of Surgeons Oncology group (ACOSOG)/RTOG 0870/Cancer and Leukemia Group B (CALGB) 140503 study; a randomized phase 3 clinical trial that is comparing lobectomy versus sublobar resection for small (< 2 cm) peripheral NSCLC. This study should help to clarify how this higher-risk patient group should be managed.
CLEVELAND CLINIC EXPERIENCE
At Cleveland Clinic, more than 700 patients with stage I NSCLC have been treated with SBRT since 2003. Peripheral tumors are typically treated with a radiation dose of 60 Gy in 3 fractions spaced over 8 to 14 days, or alternatively 30 Gy to 34 Gy in a single fraction. Occasional large tumors near the chest wall or spinal cord are treated with doses up to 50 Gy in 5 fractions over 5 consecutive days. For central tumors, radiation dose regimens include 50 Gy (5 fractions over 5 consecutive days) or 60 Gy (8 fractions over 10 days), depending upon tumor size and proximity to critical structures.
SUMMARY AND CONCLUSIONS
Many patients with NSCLC are ineligible for surgery because of COPD, cardiovascular disease, or other conditions associated with unacceptably high perioperative risk. SBRT is the standard of care for patients with medically inoperable stage I NSCLC. Modern standard radiation doses are typically between 50 to 60 Gy in 3 to 5 fractions. Local control rates in excess of 90% to 95% have been reported with these doses. SBRT is generally well tolerated by patients with both peripheral and centrally located tumors. On average, lung function is not substantially altered by SBRT, although individual patients may exhibit increased or decreased FEV1 and Dlco values after treatment. Pneumonitis has been relatively rare in most studies, with typical rates of 0% to 5%. SBRT has been shown to produce reasonable rates of local control in potentially operable patients, although data are extremely limited in this population and there are important questions about salvage therapy and postprocedural evaluation in these patients. Several ongoing clinical trials are continuing to define the efficacy and safety of different radiation dosing procedures for patients with inoperable NSCLC.
Surgical resection for patients with stage I non–small cell lung cancer (NSCLC) is typically associated with survival rates of 60% to 70% after 5 years, and as high as 80% in some series.1 Although lobectomy or pneumonectomy improves outcomes compared with sublobar resection for many patients, a substantial number are ineligible for standard surgical resection because of cardiovascular disease or other conditions that are associated with unacceptably high perioperative risk. Observation alone is not a good strategy for patients who are ineligible for surgery. Studies comparing treatment outcomes associated with resection, radiation, and observation have demonstrated much shorter survival times and higher mortality for patients treated with observation only.2
Stereotactic body radiotherapy (SBRT) is the new standard of care for patients with medically inoperable stage I NSCLC. SBRT differs from standard radiation therapy in terms of dose, fractionation, field size, and targeting. Compared with standard radiation, SBRT offers a shorter and more convenient treatment regimen with improved local control and survival while lowering treatment cost.3,4 Although cancer-specific outcomes of patients in SBRT series are similar to those in surgical groups, they are not truly comparable because of dissimilarities between the two populations. The inoperable group has higher rates of comorbidity and death compared with the medically operable group; as many as one-third die from comorbid conditions rather than cancer, leading to short follow-up in many SBRT series. Surgical resection remains the standard of care for operable stage I NSCLC.
STEREOTACTIC RADIATION FOR PATIENTS WITH INOPERABLE LUNG CANCER
Modern standard external beam radiation doses without chemotherapy for stage I lung cancer are approximately 60 to 74 Gy. The dose fractionation schedule used with SBRT delivers much higher equivalent doses (83 Gy to 150 Gy), although the true biologically equivalent dose (BED) is not yet perfectly understood.8 Most clinical studies that have examined the effectiveness of SBRT have demonstrated local control rates in excess of 90% to 95% when an adequate dose (BED ≥ 100 Gy) is utilized, since the dose-response curve appears to plateau at this level.9 These response rates are higher than the 50% to 60% rate observed with conventional radiation.3,4 Efforts to confirm these comparative results in randomized trials have been largely abandoned because of the perceived advantage with SBRT.
PERIPHERAL VERSUS CENTRAL TUMORS
Stereotactic body radiotherapy has been referred to as “radiosurgery,” in part because the extremely high doses used to treat tumor are ablative to the immediate surrounding tissue. The consequences of ablation depend on whether the treatment involves parallel or serial tissue. Parallel tissue, such as lung, kidney, or liver, remains functional after the ablation or removal of small subunits if adequate volume of functional organ remains. With serial tissue such as the spinal cord or bowel, damage to one section results in loss of function at distal sites. Although the lung is parallel tissue, it includes serial structures such as the trachea and proximal bronchial tree. Tumors located within 2 cm of the proximal bronchial tree are classified as central, whereas tumors outside this zone are peripheral.
Peripheral tumors
Peripheral lung tumors are surrounded by only parallel tissue, and no maximum point-dose limit has been identified for their treatment. A recent cooperative group study (Radiation Therapy Oncology Group [RTOG] 0236) enrolled 55 patients, 80% with tumor stage IA (T1 N0) and 20% with stage IB (T2 N0).10 Patients with bronchoalveolar histology were excluded from the study. Patients received three radiation treatments of 20 Gy each (BED of 180 Gy) to their known tumor with a small margin, and were followed with serial computed tomography (CT). After a median follow-up of 34 months, only one of the 55 evaluable patients had a local tumor failure, for a local control rate of 97.6%. Three patients had recurrences in the initially involved lobe for a 3-year local control rate of 90.6%; two patients had nodal failures for a 3-year local regional control rate of 87.2%; and 11 patients had disseminated recurrences, for a 3-year distant failure rate of 22.1%.
Survival after 3 years was approximately 50%, which is much better than the survival rate typically attained with standard radiation therapy. Further, only 10 of the 26 deaths were attributed to lung cancer while 16 patients died of comorbid conditions such as stroke or myocardial infarction, illustrating the difficulty in tracking overall survival as a measure of efficacy in this medically fragile population.
Adverse events in this study were relatively rare. Seven patients had grade 3 or higher pulmonary complications, including hypoxia, pneumonitis, and pulmonary function test changes. Of note, the study scored changes in pulmonary function as toxicity; however, in this population, where nearly all patients have underlying lung disease, chronic obstructive pulmonary disease (COPD) exacerbations are also common.
Our own analysis of pulmonary function changes in patients treated with SBRT at Cleveland Clinic demonstrated that while there was no significant change in average baseline, pulmonary function in almost 10% of patients met criteria for a grade 3 pulmonary toxicity. A similar number of patients had a proportional improvement in pulmonary function, however. Given a nearly comparable distribution of pulmonary function changes in both directions with no significant deviation from baseline in aggregate, most of these fluctuations may be related to changes in the patient’s underlying comorbidities rather than effects of treatment.
RTOG 0236 demonstrated an excellent level of local control (97.6%) using 3 fractions of 20 Gy each (BED 180 Gy total). As noted, the dose response may plateau at 100 Gy BED,9 which raises the question of whether the radiation dose levels used in this study were higher than necessary. A recently completed randomized phase 2 clinical trial conducted by the RTOG compared 34 Gy in a single fraction versus 48 Gy in 4 fractions, and a similar study by Roswell Park Cancer Institute, Buffalo, New York, and Cleveland Clinic is comparing 60 Gy in 3 fractions versus 30 Gy in a single fraction. These studies, once mature, should help define the optimal radiation dose and treatment schedule for patients with inoperable peripheral tumors.
Central tumors
Centrally located tumors are in proximity to both parallel tissues (normal lung) and serial tissues (trachea, bronchial tree, or esophagus), as well as imperfectly categorized tissues (heart and great vessels). An important question is whether it is possible to reach a radiation dose level of 100 Gy BED or higher in these tumors without causing excessive toxicity to normal tissues. Although there is a potential risk of cardiotoxicity with chest radiotherapy, clinical studies of SBRT for lung cancer have not demonstrated any evidence of toxicity to the heart or the great vessels with focal radiation. Some studies have suggested that radiotherapy of central lung tumors may be associated with other adverse events.
Awareness of central versus peripheral tumor locations was first raised in an early phase 2 study in which patients were treated with 60 to 66 Gy in 3 fractions over a period of 1 to 2 weeks. Grade 3 or higher toxicity during 2 years of follow-up was noted for 46% of patients with central tumors and 17% of patients with peripheral tumors.11 Six deaths that occurred during the study were considered to be possibly treatment-related, including four cases of bacterial pneumonia, one patient with pericardial effusion, and one patient with hemoptysis that was later ascribed to carinal recurrence.
Other studies using lower fraction sizes, however, have demonstrated excellent efficacy and safety in treating central tumors with SBRT. In early Japanese studies12,13 that used smaller fractions without tissue constraints, no differences in toxicity were noted with treatment of central versus peripheral tumors. A European study similarly demonstrated more than 90% local control at 3 years for a regimen of 60 Gy in 8 fractions (7.5 Gy/fraction).14 Currently the RTOG is conducting a dose escalation study examining doses from 50 Gy to 60 Gy (10 Gy to 12 Gy per fraction in 5 fractions). The study has reached its highest level (60 Gy in 5 fractions) with no evidence of excessive toxicity reported.
SAFETY AND TOLERABILITY
Radiation pneumonitis (an inflammatory complication of radiation frequently characterized by cough, fever, and shortness of breath) is rare—less than 5% in most series. An outlier is a single series that utilized 48 Gy in 4 fractions, a common and well-tolerated dose; the investigators reported a 30% rate of grade 2 through 5 (symptomatic) pneumonitis.18 Pneumonitis was significantly associated with the conformality index, a measure of how tightly the radiation beam is focused on the target tumor, emphasizing the importance of treatment technique on outcomes.
Other notes of caution for patients receiving SBRT include chest wall toxicity and neuropathy. Chest wall toxicity may include a variety of adverse events such as rib fractures, chest wall pain, and skin changes. These events have been described at chest wall radiation doses greater than 30 Gy.19 One study reported brachial plexopathy in 7 of 37 patients who received doses above 100 Gy BED delivered to the brachial plexus.20 Another recent study found that the probability of chest wall toxicity increased as the volume of chest wall receiving a 60 Gy dose increased above 15 to 20 cc.21 Esophagitis and skin reactions are rare except in cases where the patient is being treated for a tumor in extremely close proximity to the esophagus or skin.22
Computed tomography after SBRT often reveals substantial focal fibrosis in the region of high-dose lung radiation.23,24 Despite the often striking radiographic appearance, symptoms are rare and fibrosis may sometimes be mistaken for tumor recurrence. CT images should be read by those experienced in following post-SBRT changes. Findings suspicious for recurrence are typically evaluated by positron emission tomography (PET) followed by biopsy only if PET demonstrates sufficient hypermetabolism.
OPERABLE PATIENTS
Surgical resection is the standard of care for operable patients with lung cancer. Some studies are beginning to examine whether SBRT may also be useful in potentially operable patients. A Japanese study examined outcomes for 87 operable patients who underwent SBRT for stage I NSCLC and who were followed over a 55-month period.25 The local control rate was 92% for T1 tumors, a success rate approaching that of lobectomy. The success rate decreased to 73% for T2 tumors. Five-year overall survival rates were 72% for stage IA and 62% for stage IB, paralleling the surgical experience. Similar early results have been reported from the Netherlands.26 An RTOG study of medically operable patients recently completed enrollment after accruing 33 patients, with final results pending maturation of the data.
A major barrier to the introduction of SBRT to the operable population is the limited nature of the available data; SBRT technology has been implemented only recently and follow-up has been modest, owing to the nature of the medically inoperable population. In addition, it is difficult to determine during the first few months after SBRT which patients will be well controlled. Waiting for response to become apparent is an appropriate strategy for an inoperable patient with no alternatives, but operable patients need a trigger to indicate initiation of salvage therapies.27 In addition, lymph node dissection during surgery often provides information that is essential to tumor staging, and this information might be unavailable for patients treated with SBRT. It is also difficult to weigh the efficacy and tolerability of SBRT against surgical management because the two patient populations are not comparable.
High-risk operable patients
Comparisons of surgery and SBRT for stage I NSCLC are in their infancy and subject to extreme selection bias. Some attempts to create matched populations have demonstrated similar outcomes in matched patients.28,29 Markov modeling suggests improved efficacy for surgery overall, but the model turns in favor of SBRT in patients whose predicted surgical mortality exceeds 4%.30
High-risk operable patients are currently eligible for the American College of Surgeons Oncology group (ACOSOG)/RTOG 0870/Cancer and Leukemia Group B (CALGB) 140503 study; a randomized phase 3 clinical trial that is comparing lobectomy versus sublobar resection for small (< 2 cm) peripheral NSCLC. This study should help to clarify how this higher-risk patient group should be managed.
CLEVELAND CLINIC EXPERIENCE
At Cleveland Clinic, more than 700 patients with stage I NSCLC have been treated with SBRT since 2003. Peripheral tumors are typically treated with a radiation dose of 60 Gy in 3 fractions spaced over 8 to 14 days, or alternatively 30 Gy to 34 Gy in a single fraction. Occasional large tumors near the chest wall or spinal cord are treated with doses up to 50 Gy in 5 fractions over 5 consecutive days. For central tumors, radiation dose regimens include 50 Gy (5 fractions over 5 consecutive days) or 60 Gy (8 fractions over 10 days), depending upon tumor size and proximity to critical structures.
SUMMARY AND CONCLUSIONS
Many patients with NSCLC are ineligible for surgery because of COPD, cardiovascular disease, or other conditions associated with unacceptably high perioperative risk. SBRT is the standard of care for patients with medically inoperable stage I NSCLC. Modern standard radiation doses are typically between 50 to 60 Gy in 3 to 5 fractions. Local control rates in excess of 90% to 95% have been reported with these doses. SBRT is generally well tolerated by patients with both peripheral and centrally located tumors. On average, lung function is not substantially altered by SBRT, although individual patients may exhibit increased or decreased FEV1 and Dlco values after treatment. Pneumonitis has been relatively rare in most studies, with typical rates of 0% to 5%. SBRT has been shown to produce reasonable rates of local control in potentially operable patients, although data are extremely limited in this population and there are important questions about salvage therapy and postprocedural evaluation in these patients. Several ongoing clinical trials are continuing to define the efficacy and safety of different radiation dosing procedures for patients with inoperable NSCLC.
- Smolle-Juettner FM, Maier A, Lindenmann J, Matzi V, Neuböck N. Resection in stage I/II non-small cell lung cancer. In:Heide J, Schmittel A, Kaiser D, Hinkelbein W, eds. Controversies in the Treatment of Lung Cancer. Basel, Switzerland: Karger; 2010:71–77.
- McGarry RC, Song G, des Rosiers P, Timmerman R. Observationonly management of early stage, medically inoperable lung cancer: poor outcome. Chest 2002; 121:1155–1158.
- Lanni TB, Grills IS, Kestin LL, Robertson JM. Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol 2011; 34:494–498.
- Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis [published online ahead of print September 3, 2009]. Radiother Oncol 2010; 95:32–40. doi: 10.1016/jradonc.2009.08.003
- Noordijk EM, vd Poest Clement E, Hermans J, Wever AMJ, Leer JWH. Radiotherapy as an alternative to surgery in elderly patients with resectable lung cancer. Radiother Oncol 1988; 13:83–89.
- Sibley GS. Radiotherapy for patients with medically inoperable stage I nonsmall cell lung carcinoma: smaller volumes and higher doses—a review. Cancer 1998; 82:433–438.
- Mehta M, Manon R. Are more aggressive therapies able to improve treatment of locally advanced non-small cell lung cancer: combined modality treatment? Semin Oncol 2005; 32 (2 suppl 3):S25–S34.
- Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 2008; 70:847–852.
- Wulf J, Baier K, Mueller G, Flentje MP. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol 2005; 77:83–87.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303:1070–1076.
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006; 24:4833–4839.
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007; 2 (7 suppl 3):S94–S100.
- Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame [published online ahead of print September 19, 2005]. Int J Radiat Oncol Biol Phys 2005; 63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011; 6:2036–2043.
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003; 124:1946–1955.
- Henderson M, McGarry R, Yiannoutsos C, et al. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 72:404–409.
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009; 4:838–844.
- Yamashita H, Nakagawa K, Nakamura N, et al. Exceptionally high incidence of symptomatic grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol 2007; 2:21.
- Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving > 30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76:796–801.
- Forquer JA, Fakiris AJ, Timmerman RD, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: dose-limiting toxicity in apical tumor sites. Radiother Oncol 2009; 93:408–413.
- Stephans KL, Djemil T, Tendulkar RD, Robinson CG, Reddy CA, Videtic GM. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT) [published online ahead of print February 6, 2011]. Int J Radiat Oncol Biol Phys 2012; 82:974–980. doi: 10.1016/j.ijrobp.2010.12.002
- Hoppe BS, Laser B, Kowalski AV, et al. Acute skin toxicity following stereotactic body radiation therapy for stage I non-small-cell lung cancer: who’s at risk? Int J Radiat Oncol Biol Phys 2008; 72:1283–1286.
- Bradley J. Radiographic response and clinical toxicity following SBRT for stage I lung cancer. J Thorac Oncol 2007; 2 (7 suppl 3):S118–S124.
- Linda A, Trovo M, Bradley JD. Radiation injury of the lung after stereotactic body radiation therapy (SBRT) for lung cancer: a timeline and pattern of CT changes [published online ahead of print December 1, 2009]. Eur J Radiol 2011; 79:147–154. doi: 10.1016/j.ejrad.2009.10.029
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery [published online ahead of print July 16, 2011]? Int J Radiat Oncol Biol Phys 2011; 81:1352–1358. doi: 10.1016/j.ijrobp.2009.07.1751
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small-cell lung cancer [published online ahead of print November 19, 2011.] Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2011.06.2003.
- Neri S, Takahashi Y, Terashi T, et al. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol 2010; 5:2003–2007.
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer [published online ahead of print April 18, 2010]. J Thorac Cardiovasc Surg 2010; 140:377–386. doi: 10.1016/j.jtcvs.2009.12.054
- Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of stage I non-small-cell lung acancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review [published online ahead of print June 2, 2011]. Int J Radiat Oncol Biol Phys 2012; 82:1149–1156. doi: 10.1016/j.ijrobp.2011.03.005
- Louie AV, Rodrigues G, Hannouf M, et al. Stereotactic body radiotherapy versus surgery for medically operable stage I non-small-cell lung cancer: a Markov model-based decision analysis [published online ahead of print October 6, 2010]. Int J Radiat Oncol Biol Phys 2011; 81:964–973. doi: 10.1016/j.ijrobp.2010.06.040
- Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 1999; 24:31–37.
- Smolle-Juettner FM, Maier A, Lindenmann J, Matzi V, Neuböck N. Resection in stage I/II non-small cell lung cancer. In:Heide J, Schmittel A, Kaiser D, Hinkelbein W, eds. Controversies in the Treatment of Lung Cancer. Basel, Switzerland: Karger; 2010:71–77.
- McGarry RC, Song G, des Rosiers P, Timmerman R. Observationonly management of early stage, medically inoperable lung cancer: poor outcome. Chest 2002; 121:1155–1158.
- Lanni TB, Grills IS, Kestin LL, Robertson JM. Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol 2011; 34:494–498.
- Grutters JP, Kessels AG, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis [published online ahead of print September 3, 2009]. Radiother Oncol 2010; 95:32–40. doi: 10.1016/jradonc.2009.08.003
- Noordijk EM, vd Poest Clement E, Hermans J, Wever AMJ, Leer JWH. Radiotherapy as an alternative to surgery in elderly patients with resectable lung cancer. Radiother Oncol 1988; 13:83–89.
- Sibley GS. Radiotherapy for patients with medically inoperable stage I nonsmall cell lung carcinoma: smaller volumes and higher doses—a review. Cancer 1998; 82:433–438.
- Mehta M, Manon R. Are more aggressive therapies able to improve treatment of locally advanced non-small cell lung cancer: combined modality treatment? Semin Oncol 2005; 32 (2 suppl 3):S25–S34.
- Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 2008; 70:847–852.
- Wulf J, Baier K, Mueller G, Flentje MP. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol 2005; 77:83–87.
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303:1070–1076.
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006; 24:4833–4839.
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007; 2 (7 suppl 3):S94–S100.
- Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame [published online ahead of print September 19, 2005]. Int J Radiat Oncol Biol Phys 2005; 63:1427–1431. doi: 10.1016/j.ijrobp.2005.05.034
- Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011; 6:2036–2043.
- Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003; 124:1946–1955.
- Henderson M, McGarry R, Yiannoutsos C, et al. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 72:404–409.
- Stephans KL, Djemil T, Reddy CA, et al. Comprehensive analysis of pulmonary function test (PFT) changes after stereotactic body radiotherapy (SBRT) for stage I lung cancer in medically inoperable patients. J Thorac Oncol 2009; 4:838–844.
- Yamashita H, Nakagawa K, Nakamura N, et al. Exceptionally high incidence of symptomatic grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol 2007; 2:21.
- Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving > 30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76:796–801.
- Forquer JA, Fakiris AJ, Timmerman RD, et al. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: dose-limiting toxicity in apical tumor sites. Radiother Oncol 2009; 93:408–413.
- Stephans KL, Djemil T, Tendulkar RD, Robinson CG, Reddy CA, Videtic GM. Prediction of chest wall toxicity from lung stereotactic body radiotherapy (SBRT) [published online ahead of print February 6, 2011]. Int J Radiat Oncol Biol Phys 2012; 82:974–980. doi: 10.1016/j.ijrobp.2010.12.002
- Hoppe BS, Laser B, Kowalski AV, et al. Acute skin toxicity following stereotactic body radiation therapy for stage I non-small-cell lung cancer: who’s at risk? Int J Radiat Oncol Biol Phys 2008; 72:1283–1286.
- Bradley J. Radiographic response and clinical toxicity following SBRT for stage I lung cancer. J Thorac Oncol 2007; 2 (7 suppl 3):S118–S124.
- Linda A, Trovo M, Bradley JD. Radiation injury of the lung after stereotactic body radiation therapy (SBRT) for lung cancer: a timeline and pattern of CT changes [published online ahead of print December 1, 2009]. Eur J Radiol 2011; 79:147–154. doi: 10.1016/j.ejrad.2009.10.029
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery [published online ahead of print July 16, 2011]? Int J Radiat Oncol Biol Phys 2011; 81:1352–1358. doi: 10.1016/j.ijrobp.2009.07.1751
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small-cell lung cancer [published online ahead of print November 19, 2011.] Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2011.06.2003.
- Neri S, Takahashi Y, Terashi T, et al. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol 2010; 5:2003–2007.
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer [published online ahead of print April 18, 2010]. J Thorac Cardiovasc Surg 2010; 140:377–386. doi: 10.1016/j.jtcvs.2009.12.054
- Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of stage I non-small-cell lung acancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review [published online ahead of print June 2, 2011]. Int J Radiat Oncol Biol Phys 2012; 82:1149–1156. doi: 10.1016/j.ijrobp.2011.03.005
- Louie AV, Rodrigues G, Hannouf M, et al. Stereotactic body radiotherapy versus surgery for medically operable stage I non-small-cell lung cancer: a Markov model-based decision analysis [published online ahead of print October 6, 2010]. Int J Radiat Oncol Biol Phys 2011; 81:964–973. doi: 10.1016/j.ijrobp.2010.06.040
- Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer 1999; 24:31–37.