User login
Popular theory of mast cell development is wrong, team says
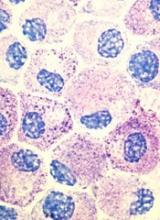
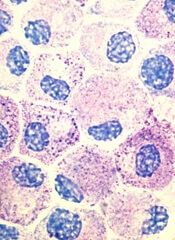
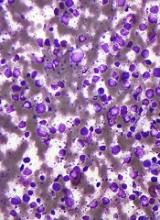
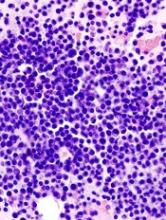
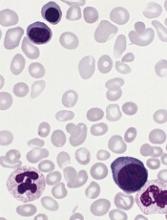
Stem cell factor (SCF) and KIT signaling are not necessary for early mast cell development, according to research published in Blood.
It has been assumed that the differentiation of hematopoietic progenitors to mast cells requires SCF and KIT signaling.
However, researchers found that mast cell progenitors can survive, mature, and proliferate in the absence of SCF and KIT signaling.
The researchers began this work by analyzing mast cell progenitor populations in samples from healthy subjects, patients with chronic myeloid leukemia (CML) or gastrointestinal stromal tumors (GIST) who were treated with imatinib, and patients with systemic mastocytosis carrying the D816V KIT mutation.
Imatinib inhibits KIT signaling, and the D816V KIT mutation causes KIT signaling to be constitutively active.
The researchers found the imatinib-treated CML and GIST patients and the patients with systemic mastocytosis all had mast cell progenitor populations similar to those observed in healthy subjects.
The team therefore concluded that dysfunctional KIT signaling does not affect the frequency of circulating mast cell progenitors in vivo.
On the other hand, the researchers also found that circulating mast cells were sensitive to imatinib in patients with CML. The patients had higher numbers of peripheral blood mast cells at diagnosis than they did after treatment with imatinib.
“When the patients were treated with the drug imatinib, which blocks the effect of stem cell factor, the number of mature mast cells dropped, while the number of progenitor cells did not change,” said study author Gunnar Nilsson, PhD, of Karolinska Institutet in Stockholm, Sweden.
Subsequent experiments showed that mast cell progenitors can survive in vitro without KIT signaling and without SCF. In addition, mast cell progenitors were able to mature and proliferate in vitro without SCF.
In fact, the researchers said they found that interleukin 3 was sufficient to promote the survival of mast cell progenitors in vitro.
“The study increases our understanding of how mast cells are formed and could be important in the development of new therapies, for example, for mastocytosis . . . ,” said study author Joakim Dahlin, PhD, of the University of Cambridge in the UK.
“One hypothesis that we will now test is whether interleukin 3 can be a new target in the treatment of mast cell-driven diseases.” ![]()
Stem cell factor (SCF) and KIT signaling are not necessary for early mast cell development, according to research published in Blood.
It has been assumed that the differentiation of hematopoietic progenitors to mast cells requires SCF and KIT signaling.
However, researchers found that mast cell progenitors can survive, mature, and proliferate in the absence of SCF and KIT signaling.
The researchers began this work by analyzing mast cell progenitor populations in samples from healthy subjects, patients with chronic myeloid leukemia (CML) or gastrointestinal stromal tumors (GIST) who were treated with imatinib, and patients with systemic mastocytosis carrying the D816V KIT mutation.
Imatinib inhibits KIT signaling, and the D816V KIT mutation causes KIT signaling to be constitutively active.
The researchers found the imatinib-treated CML and GIST patients and the patients with systemic mastocytosis all had mast cell progenitor populations similar to those observed in healthy subjects.
The team therefore concluded that dysfunctional KIT signaling does not affect the frequency of circulating mast cell progenitors in vivo.
On the other hand, the researchers also found that circulating mast cells were sensitive to imatinib in patients with CML. The patients had higher numbers of peripheral blood mast cells at diagnosis than they did after treatment with imatinib.
“When the patients were treated with the drug imatinib, which blocks the effect of stem cell factor, the number of mature mast cells dropped, while the number of progenitor cells did not change,” said study author Gunnar Nilsson, PhD, of Karolinska Institutet in Stockholm, Sweden.
Subsequent experiments showed that mast cell progenitors can survive in vitro without KIT signaling and without SCF. In addition, mast cell progenitors were able to mature and proliferate in vitro without SCF.
In fact, the researchers said they found that interleukin 3 was sufficient to promote the survival of mast cell progenitors in vitro.
“The study increases our understanding of how mast cells are formed and could be important in the development of new therapies, for example, for mastocytosis . . . ,” said study author Joakim Dahlin, PhD, of the University of Cambridge in the UK.
“One hypothesis that we will now test is whether interleukin 3 can be a new target in the treatment of mast cell-driven diseases.” ![]()
Stem cell factor (SCF) and KIT signaling are not necessary for early mast cell development, according to research published in Blood.
It has been assumed that the differentiation of hematopoietic progenitors to mast cells requires SCF and KIT signaling.
However, researchers found that mast cell progenitors can survive, mature, and proliferate in the absence of SCF and KIT signaling.
The researchers began this work by analyzing mast cell progenitor populations in samples from healthy subjects, patients with chronic myeloid leukemia (CML) or gastrointestinal stromal tumors (GIST) who were treated with imatinib, and patients with systemic mastocytosis carrying the D816V KIT mutation.
Imatinib inhibits KIT signaling, and the D816V KIT mutation causes KIT signaling to be constitutively active.
The researchers found the imatinib-treated CML and GIST patients and the patients with systemic mastocytosis all had mast cell progenitor populations similar to those observed in healthy subjects.
The team therefore concluded that dysfunctional KIT signaling does not affect the frequency of circulating mast cell progenitors in vivo.
On the other hand, the researchers also found that circulating mast cells were sensitive to imatinib in patients with CML. The patients had higher numbers of peripheral blood mast cells at diagnosis than they did after treatment with imatinib.
“When the patients were treated with the drug imatinib, which blocks the effect of stem cell factor, the number of mature mast cells dropped, while the number of progenitor cells did not change,” said study author Gunnar Nilsson, PhD, of Karolinska Institutet in Stockholm, Sweden.
Subsequent experiments showed that mast cell progenitors can survive in vitro without KIT signaling and without SCF. In addition, mast cell progenitors were able to mature and proliferate in vitro without SCF.
In fact, the researchers said they found that interleukin 3 was sufficient to promote the survival of mast cell progenitors in vitro.
“The study increases our understanding of how mast cells are formed and could be important in the development of new therapies, for example, for mastocytosis . . . ,” said study author Joakim Dahlin, PhD, of the University of Cambridge in the UK.
“One hypothesis that we will now test is whether interleukin 3 can be a new target in the treatment of mast cell-driven diseases.” ![]()
Cancer patients perceive their abilities differently than caregivers do
New research suggests older cancer patients and their caregivers often differ in their assessment of the patients’ abilities.
In this study, patients generally rated their physical and mental function higher than caregivers did.
The study also showed the differences in assessment of patients’ physical abilities were associated with greater caregiver burden.
This research was published in The Oncologist.
“Caregivers are such an important part of our healthcare system, particularly for older adults with cancer,” said study author Arti Hurria, MD, of City of Hope National Medical Center in Duarte, California.
“We wanted to further understand the factors that are associated with caregiver burden.”
One factor Dr Hurria and her colleagues thought might be important is differences in assessments of patient health and physical abilities between patients and their caregivers.
“In daily practice, we sometimes see a disconnect between what the patient perceives their general health and abilities to be in comparison to what the caregiver thinks,” Dr Hurria said. “We wanted to see whether this disconnect impacted caregiver burden.”
To do this, Dr Hurria and her colleagues questioned 100 older cancer patients and their caregivers.
Subjects were asked about the patients’ general health and physical function, meaning their ability to perform everyday activities. The researchers then compared the answers given by the patients and their respective caregivers.
The researchers also assessed the level of caregiver burden (defined as a subjective feeling of stress caused by being overwhelmed by the demands of caring) by administering a standard questionnaire on topics such as sleep disturbance, physical effort, and patient behavior.
The 100 cancer patients, ages 65 to 91, were suffering from a variety of cancers. The most common were lymphoma (n=26), breast cancer (n=19), and gastrointestinal cancers (n=15). Twelve patients had leukemia, and 10 had myeloma.
The ages of the caregivers ranged from 28 to 85, and the majority were female (73%). They were mainly either the spouse of the patient (68%) or an adult child (18%).
Results
There was no significant difference in patient and caregiver accounts of the patients’ comorbidities (P=0.68), falls in the last 6 months (P=0.71), or percent weight change in the last 6 months (P=0.21).
However, caregivers consistently rated patients as having poorer physical function and mental health and requiring more social support than the patients themselves did.
There was a significant difference (P<0.05) in caregiver and patient accounts when it came to the following measures:
- Need for help with instrumental activities of daily living
- Karnofsky Performance Status
- Medical Outcomes Study-Physical Function
- Medical Outcomes Study-Social Support Survey
- Mental Health Inventory.
Only the disparity in the assessment of physical function was significantly associated with greater caregiver burden (P<0.001). What is still unclear is the cause of this disparity.
“I think there are 2 possible explanations,” said study author Tina Hsu, MD, of the University of Ottawa in Ontario, Canada.
“One is that older adults with cancer either don’t appreciate how much help they require or, more likely, they are able preserve their sense of independence and dignity through a perception that they feel they can do more than they really can.”
“Alternatively, it is possible that caregivers who are more stressed out perceive their loved one to require more help than they actually do need. Most likely, the truth of how much help the patient actually needs lies somewhere between what patients and caregivers report.”
Based on their findings, Drs Hsu and Hurria and their colleagues advise that clinicians consider assessing caregiver burden in those caregivers who report the patient as being more dependent than the patient does themselves.
“Caregivers play an essential role in supporting older adults with cancer,” Dr Hsu said. “We plan to further explore factors associated with caregiver burden in this population, particularly in those who are frailer and thus require even more hands-on support. We also hope to explore what resources caregivers of older adults with cancer feel they need to better help them with their role.” ![]()
New research suggests older cancer patients and their caregivers often differ in their assessment of the patients’ abilities.
In this study, patients generally rated their physical and mental function higher than caregivers did.
The study also showed the differences in assessment of patients’ physical abilities were associated with greater caregiver burden.
This research was published in The Oncologist.
“Caregivers are such an important part of our healthcare system, particularly for older adults with cancer,” said study author Arti Hurria, MD, of City of Hope National Medical Center in Duarte, California.
“We wanted to further understand the factors that are associated with caregiver burden.”
One factor Dr Hurria and her colleagues thought might be important is differences in assessments of patient health and physical abilities between patients and their caregivers.
“In daily practice, we sometimes see a disconnect between what the patient perceives their general health and abilities to be in comparison to what the caregiver thinks,” Dr Hurria said. “We wanted to see whether this disconnect impacted caregiver burden.”
To do this, Dr Hurria and her colleagues questioned 100 older cancer patients and their caregivers.
Subjects were asked about the patients’ general health and physical function, meaning their ability to perform everyday activities. The researchers then compared the answers given by the patients and their respective caregivers.
The researchers also assessed the level of caregiver burden (defined as a subjective feeling of stress caused by being overwhelmed by the demands of caring) by administering a standard questionnaire on topics such as sleep disturbance, physical effort, and patient behavior.
The 100 cancer patients, ages 65 to 91, were suffering from a variety of cancers. The most common were lymphoma (n=26), breast cancer (n=19), and gastrointestinal cancers (n=15). Twelve patients had leukemia, and 10 had myeloma.
The ages of the caregivers ranged from 28 to 85, and the majority were female (73%). They were mainly either the spouse of the patient (68%) or an adult child (18%).
Results
There was no significant difference in patient and caregiver accounts of the patients’ comorbidities (P=0.68), falls in the last 6 months (P=0.71), or percent weight change in the last 6 months (P=0.21).
However, caregivers consistently rated patients as having poorer physical function and mental health and requiring more social support than the patients themselves did.
There was a significant difference (P<0.05) in caregiver and patient accounts when it came to the following measures:
- Need for help with instrumental activities of daily living
- Karnofsky Performance Status
- Medical Outcomes Study-Physical Function
- Medical Outcomes Study-Social Support Survey
- Mental Health Inventory.
Only the disparity in the assessment of physical function was significantly associated with greater caregiver burden (P<0.001). What is still unclear is the cause of this disparity.
“I think there are 2 possible explanations,” said study author Tina Hsu, MD, of the University of Ottawa in Ontario, Canada.
“One is that older adults with cancer either don’t appreciate how much help they require or, more likely, they are able preserve their sense of independence and dignity through a perception that they feel they can do more than they really can.”
“Alternatively, it is possible that caregivers who are more stressed out perceive their loved one to require more help than they actually do need. Most likely, the truth of how much help the patient actually needs lies somewhere between what patients and caregivers report.”
Based on their findings, Drs Hsu and Hurria and their colleagues advise that clinicians consider assessing caregiver burden in those caregivers who report the patient as being more dependent than the patient does themselves.
“Caregivers play an essential role in supporting older adults with cancer,” Dr Hsu said. “We plan to further explore factors associated with caregiver burden in this population, particularly in those who are frailer and thus require even more hands-on support. We also hope to explore what resources caregivers of older adults with cancer feel they need to better help them with their role.” ![]()
New research suggests older cancer patients and their caregivers often differ in their assessment of the patients’ abilities.
In this study, patients generally rated their physical and mental function higher than caregivers did.
The study also showed the differences in assessment of patients’ physical abilities were associated with greater caregiver burden.
This research was published in The Oncologist.
“Caregivers are such an important part of our healthcare system, particularly for older adults with cancer,” said study author Arti Hurria, MD, of City of Hope National Medical Center in Duarte, California.
“We wanted to further understand the factors that are associated with caregiver burden.”
One factor Dr Hurria and her colleagues thought might be important is differences in assessments of patient health and physical abilities between patients and their caregivers.
“In daily practice, we sometimes see a disconnect between what the patient perceives their general health and abilities to be in comparison to what the caregiver thinks,” Dr Hurria said. “We wanted to see whether this disconnect impacted caregiver burden.”
To do this, Dr Hurria and her colleagues questioned 100 older cancer patients and their caregivers.
Subjects were asked about the patients’ general health and physical function, meaning their ability to perform everyday activities. The researchers then compared the answers given by the patients and their respective caregivers.
The researchers also assessed the level of caregiver burden (defined as a subjective feeling of stress caused by being overwhelmed by the demands of caring) by administering a standard questionnaire on topics such as sleep disturbance, physical effort, and patient behavior.
The 100 cancer patients, ages 65 to 91, were suffering from a variety of cancers. The most common were lymphoma (n=26), breast cancer (n=19), and gastrointestinal cancers (n=15). Twelve patients had leukemia, and 10 had myeloma.
The ages of the caregivers ranged from 28 to 85, and the majority were female (73%). They were mainly either the spouse of the patient (68%) or an adult child (18%).
Results
There was no significant difference in patient and caregiver accounts of the patients’ comorbidities (P=0.68), falls in the last 6 months (P=0.71), or percent weight change in the last 6 months (P=0.21).
However, caregivers consistently rated patients as having poorer physical function and mental health and requiring more social support than the patients themselves did.
There was a significant difference (P<0.05) in caregiver and patient accounts when it came to the following measures:
- Need for help with instrumental activities of daily living
- Karnofsky Performance Status
- Medical Outcomes Study-Physical Function
- Medical Outcomes Study-Social Support Survey
- Mental Health Inventory.
Only the disparity in the assessment of physical function was significantly associated with greater caregiver burden (P<0.001). What is still unclear is the cause of this disparity.
“I think there are 2 possible explanations,” said study author Tina Hsu, MD, of the University of Ottawa in Ontario, Canada.
“One is that older adults with cancer either don’t appreciate how much help they require or, more likely, they are able preserve their sense of independence and dignity through a perception that they feel they can do more than they really can.”
“Alternatively, it is possible that caregivers who are more stressed out perceive their loved one to require more help than they actually do need. Most likely, the truth of how much help the patient actually needs lies somewhere between what patients and caregivers report.”
Based on their findings, Drs Hsu and Hurria and their colleagues advise that clinicians consider assessing caregiver burden in those caregivers who report the patient as being more dependent than the patient does themselves.
“Caregivers play an essential role in supporting older adults with cancer,” Dr Hsu said. “We plan to further explore factors associated with caregiver burden in this population, particularly in those who are frailer and thus require even more hands-on support. We also hope to explore what resources caregivers of older adults with cancer feel they need to better help them with their role.” ![]()
VSTs can treat 5 different viral infections after HSCT
New research suggests virus-specific T cells (VSTs) can protect patients from severe viral infections that sometimes occur after hematopoietic stem cell transplant (HSCT).
The VSTs proved effective against 5 different viruses—Epstein-Barr virus (EBV), adenovirus (AdV), cytomegalovirus (CMV), BK virus (BKV), and human herpesvirus 6 (HHV-6).
Ifigeneia Tzannou, MD, of Baylor College of Medicine in Houston, Texas, and her colleagues reported these findings in the Journal of Clinical Oncology.
“In this study, we continued our previous work . . . in which we showed that patients who had developed an Epstein-Barr virus infection after a transplant . . . could be helped by receiving immune cells specialized in eliminating that particular virus,” Dr Tzannou said. “Then, we and others successfully targeted other viruses—namely, adenoviruses and cytomegalovirus.”
“The novel contribution of this study is that we have targeted additional viruses, the BK virus and the HHV-6 virus, which had not been targeted this way before,” added study author Bilal Omer, MD, of Baylor College of Medicine.
“This is important because the BK virus does not have an effective treatment, and the complications are significant, including severe pain and bleeding. These patients are in the hospital for weeks, months sometimes, and, now, we have a treatment option.”
The researchers tested their VSTs in a phase 2 trial of 38 HSCT recipients with at least 1 of the aforementioned viruses.
“[To prepare the VSTs,] we take blood from healthy donors who have already been exposed to these viruses and who we have confirmed have immune cells that can fight the infections,” Dr Tzannou said.
“We isolate the cells and let them multiply in culture. The final product is a mixture of cells that, together, can target all 5 viruses. We prepared 59 sets of virus-specific cells from different donors following this procedure.”
“Our strategy is to prepare a number of sets of virus-specific cells ahead of time and store them in a freezer, ready to use when a patient needs them,” Dr Omer noted. “To match patient and donor, we use elaborate matching algorithms.”
Patients
The trial included 38 patients who had undergone HSCT to treat acute myeloid leukemia/myelodysplastic syndromes (n=20), acute lymphoblastic leukemia (n=9), lymphoma/myeloma (n=3), or nonmalignant disorders (n=6).
These 38 patients had a total of 45 infections—CMV (n=17), EBV (n=2), AdV (n=7), BKV (n=16), and HHV-6 (n=3).
Response
The researchers monitored virus levels and other clinical responses in the 37 evaluable patients.
Six weeks after the first VST infusion, the overall response rate was 91.9%.
Seventeen patients received VSTs for persistent CMV. Sixteen of these patients (94.1%) responded, 6 with complete responses (CRs) and 10 with partial responses (PRs).
Two patients received VSTs for EBV, and both achieved a virologic CR.
Seven patients received VSTs for persistent AdV. The response rate was 71.4%. Four patients achieved a CR, 1 had a PR, and 2 patients did not respond.
Three patients received VSTs to treat HHV-6 reactivations. The response rate was 67%. Two patients had a PR, and 1 was not evaluable.
Sixteen patients received VSTs for BKV-associated hemorrhagic cystitis (n= 14) or BKV-associated nephritis (n=2).
All 16 patients responded. One had a clinical and virologic CR. Six had a clinical CR but a virologic PR. Seven had a virologic and clinical PR. And 2 patients had only a virologic PR.
A total of 15 patients received a second VST infusion—1 due to lack of response, 7 who had a PR, and 7 due to recurrence. Ten of these patients responded to the second infusion—1 with a CR and 9 with a PR.
Four patients received a third infusion of VSTs. Two achieved a CR, 1 had a PR, and 1 did not respond.
Toxicity
One patient developed an isolated fever within 24 hours of VST infusion, but the researchers did not observe any other immediate toxicities.
One of the patients with BKV-associated hemorrhagic cystitis experienced transient hydronephrosis and a decrease in renal function associated with a concomitant bacterial urinary tract infection.
Nineteen patients had prior grade 2 to 4 graft-versus-host disease (GVHD)—15 with grade 2 and 4 with grade 3. All GVHD was quiescent at the time of VST infusion.
One patient developed recurrent grade 3 gastrointestinal GVHD after VST infusion and rapid corticosteroid taper. Five patients developed recurrent (n=3) or de novo (n=2) grade 1 to 2 skin GVHD, which resolved with topical treatment (n=4) and reinitiation of corticosteroid treatment (n=1).
Two patients had a flare of upper-gastrointestinal GVHD, which resolved after a brief corticosteroid course.
“We didn’t have any significant toxicities,” Dr Tzannou said. “Taken together, the results of this trial suggest that it is reasonable to consider this treatment as an early option for these patients. We hope that the results of a future multicenter, phase 3 clinical trial will help raise awareness in both physicians and patients that this treatment, which is safe and effective, is available.” ![]()
New research suggests virus-specific T cells (VSTs) can protect patients from severe viral infections that sometimes occur after hematopoietic stem cell transplant (HSCT).
The VSTs proved effective against 5 different viruses—Epstein-Barr virus (EBV), adenovirus (AdV), cytomegalovirus (CMV), BK virus (BKV), and human herpesvirus 6 (HHV-6).
Ifigeneia Tzannou, MD, of Baylor College of Medicine in Houston, Texas, and her colleagues reported these findings in the Journal of Clinical Oncology.
“In this study, we continued our previous work . . . in which we showed that patients who had developed an Epstein-Barr virus infection after a transplant . . . could be helped by receiving immune cells specialized in eliminating that particular virus,” Dr Tzannou said. “Then, we and others successfully targeted other viruses—namely, adenoviruses and cytomegalovirus.”
“The novel contribution of this study is that we have targeted additional viruses, the BK virus and the HHV-6 virus, which had not been targeted this way before,” added study author Bilal Omer, MD, of Baylor College of Medicine.
“This is important because the BK virus does not have an effective treatment, and the complications are significant, including severe pain and bleeding. These patients are in the hospital for weeks, months sometimes, and, now, we have a treatment option.”
The researchers tested their VSTs in a phase 2 trial of 38 HSCT recipients with at least 1 of the aforementioned viruses.
“[To prepare the VSTs,] we take blood from healthy donors who have already been exposed to these viruses and who we have confirmed have immune cells that can fight the infections,” Dr Tzannou said.
“We isolate the cells and let them multiply in culture. The final product is a mixture of cells that, together, can target all 5 viruses. We prepared 59 sets of virus-specific cells from different donors following this procedure.”
“Our strategy is to prepare a number of sets of virus-specific cells ahead of time and store them in a freezer, ready to use when a patient needs them,” Dr Omer noted. “To match patient and donor, we use elaborate matching algorithms.”
Patients
The trial included 38 patients who had undergone HSCT to treat acute myeloid leukemia/myelodysplastic syndromes (n=20), acute lymphoblastic leukemia (n=9), lymphoma/myeloma (n=3), or nonmalignant disorders (n=6).
These 38 patients had a total of 45 infections—CMV (n=17), EBV (n=2), AdV (n=7), BKV (n=16), and HHV-6 (n=3).
Response
The researchers monitored virus levels and other clinical responses in the 37 evaluable patients.
Six weeks after the first VST infusion, the overall response rate was 91.9%.
Seventeen patients received VSTs for persistent CMV. Sixteen of these patients (94.1%) responded, 6 with complete responses (CRs) and 10 with partial responses (PRs).
Two patients received VSTs for EBV, and both achieved a virologic CR.
Seven patients received VSTs for persistent AdV. The response rate was 71.4%. Four patients achieved a CR, 1 had a PR, and 2 patients did not respond.
Three patients received VSTs to treat HHV-6 reactivations. The response rate was 67%. Two patients had a PR, and 1 was not evaluable.
Sixteen patients received VSTs for BKV-associated hemorrhagic cystitis (n= 14) or BKV-associated nephritis (n=2).
All 16 patients responded. One had a clinical and virologic CR. Six had a clinical CR but a virologic PR. Seven had a virologic and clinical PR. And 2 patients had only a virologic PR.
A total of 15 patients received a second VST infusion—1 due to lack of response, 7 who had a PR, and 7 due to recurrence. Ten of these patients responded to the second infusion—1 with a CR and 9 with a PR.
Four patients received a third infusion of VSTs. Two achieved a CR, 1 had a PR, and 1 did not respond.
Toxicity
One patient developed an isolated fever within 24 hours of VST infusion, but the researchers did not observe any other immediate toxicities.
One of the patients with BKV-associated hemorrhagic cystitis experienced transient hydronephrosis and a decrease in renal function associated with a concomitant bacterial urinary tract infection.
Nineteen patients had prior grade 2 to 4 graft-versus-host disease (GVHD)—15 with grade 2 and 4 with grade 3. All GVHD was quiescent at the time of VST infusion.
One patient developed recurrent grade 3 gastrointestinal GVHD after VST infusion and rapid corticosteroid taper. Five patients developed recurrent (n=3) or de novo (n=2) grade 1 to 2 skin GVHD, which resolved with topical treatment (n=4) and reinitiation of corticosteroid treatment (n=1).
Two patients had a flare of upper-gastrointestinal GVHD, which resolved after a brief corticosteroid course.
“We didn’t have any significant toxicities,” Dr Tzannou said. “Taken together, the results of this trial suggest that it is reasonable to consider this treatment as an early option for these patients. We hope that the results of a future multicenter, phase 3 clinical trial will help raise awareness in both physicians and patients that this treatment, which is safe and effective, is available.” ![]()
New research suggests virus-specific T cells (VSTs) can protect patients from severe viral infections that sometimes occur after hematopoietic stem cell transplant (HSCT).
The VSTs proved effective against 5 different viruses—Epstein-Barr virus (EBV), adenovirus (AdV), cytomegalovirus (CMV), BK virus (BKV), and human herpesvirus 6 (HHV-6).
Ifigeneia Tzannou, MD, of Baylor College of Medicine in Houston, Texas, and her colleagues reported these findings in the Journal of Clinical Oncology.
“In this study, we continued our previous work . . . in which we showed that patients who had developed an Epstein-Barr virus infection after a transplant . . . could be helped by receiving immune cells specialized in eliminating that particular virus,” Dr Tzannou said. “Then, we and others successfully targeted other viruses—namely, adenoviruses and cytomegalovirus.”
“The novel contribution of this study is that we have targeted additional viruses, the BK virus and the HHV-6 virus, which had not been targeted this way before,” added study author Bilal Omer, MD, of Baylor College of Medicine.
“This is important because the BK virus does not have an effective treatment, and the complications are significant, including severe pain and bleeding. These patients are in the hospital for weeks, months sometimes, and, now, we have a treatment option.”
The researchers tested their VSTs in a phase 2 trial of 38 HSCT recipients with at least 1 of the aforementioned viruses.
“[To prepare the VSTs,] we take blood from healthy donors who have already been exposed to these viruses and who we have confirmed have immune cells that can fight the infections,” Dr Tzannou said.
“We isolate the cells and let them multiply in culture. The final product is a mixture of cells that, together, can target all 5 viruses. We prepared 59 sets of virus-specific cells from different donors following this procedure.”
“Our strategy is to prepare a number of sets of virus-specific cells ahead of time and store them in a freezer, ready to use when a patient needs them,” Dr Omer noted. “To match patient and donor, we use elaborate matching algorithms.”
Patients
The trial included 38 patients who had undergone HSCT to treat acute myeloid leukemia/myelodysplastic syndromes (n=20), acute lymphoblastic leukemia (n=9), lymphoma/myeloma (n=3), or nonmalignant disorders (n=6).
These 38 patients had a total of 45 infections—CMV (n=17), EBV (n=2), AdV (n=7), BKV (n=16), and HHV-6 (n=3).
Response
The researchers monitored virus levels and other clinical responses in the 37 evaluable patients.
Six weeks after the first VST infusion, the overall response rate was 91.9%.
Seventeen patients received VSTs for persistent CMV. Sixteen of these patients (94.1%) responded, 6 with complete responses (CRs) and 10 with partial responses (PRs).
Two patients received VSTs for EBV, and both achieved a virologic CR.
Seven patients received VSTs for persistent AdV. The response rate was 71.4%. Four patients achieved a CR, 1 had a PR, and 2 patients did not respond.
Three patients received VSTs to treat HHV-6 reactivations. The response rate was 67%. Two patients had a PR, and 1 was not evaluable.
Sixteen patients received VSTs for BKV-associated hemorrhagic cystitis (n= 14) or BKV-associated nephritis (n=2).
All 16 patients responded. One had a clinical and virologic CR. Six had a clinical CR but a virologic PR. Seven had a virologic and clinical PR. And 2 patients had only a virologic PR.
A total of 15 patients received a second VST infusion—1 due to lack of response, 7 who had a PR, and 7 due to recurrence. Ten of these patients responded to the second infusion—1 with a CR and 9 with a PR.
Four patients received a third infusion of VSTs. Two achieved a CR, 1 had a PR, and 1 did not respond.
Toxicity
One patient developed an isolated fever within 24 hours of VST infusion, but the researchers did not observe any other immediate toxicities.
One of the patients with BKV-associated hemorrhagic cystitis experienced transient hydronephrosis and a decrease in renal function associated with a concomitant bacterial urinary tract infection.
Nineteen patients had prior grade 2 to 4 graft-versus-host disease (GVHD)—15 with grade 2 and 4 with grade 3. All GVHD was quiescent at the time of VST infusion.
One patient developed recurrent grade 3 gastrointestinal GVHD after VST infusion and rapid corticosteroid taper. Five patients developed recurrent (n=3) or de novo (n=2) grade 1 to 2 skin GVHD, which resolved with topical treatment (n=4) and reinitiation of corticosteroid treatment (n=1).
Two patients had a flare of upper-gastrointestinal GVHD, which resolved after a brief corticosteroid course.
“We didn’t have any significant toxicities,” Dr Tzannou said. “Taken together, the results of this trial suggest that it is reasonable to consider this treatment as an early option for these patients. We hope that the results of a future multicenter, phase 3 clinical trial will help raise awareness in both physicians and patients that this treatment, which is safe and effective, is available.” ![]()
Researchers find higher opioid use among cancer survivors
A study of residents in Ontario, Canada, showed that opioid prescription use was more common in cancer survivors than in individuals without a history of cancer.
This was true even among survivors who were 10 or more years past their cancer diagnosis.
Rinku Sutradhar, PhD, of the University of Toronto in Ontario, Canada, and her colleagues reported these findings in Cancer.
The researchers said little is known about prescribing opioids to relieve pain in individuals who have survived cancer.
To investigate, the team looked at opioid prescribing among residents of Ontario, Canada, with and without a history of cancer.
The study included 8601 adults who were at least 5 years past a cancer diagnosis. These subjects were were matched with 8601 individuals without a prior cancer diagnosis. The subjects were matched based on sex and calendar year of birth.
The researchers looked for opioid prescriptions filled at a pharmacy during the observation period. Follow-up was stopped at any indication of cancer recurrence, second malignancy, or new cancer diagnosis.
The rate of opioid prescribing was 1.22 times higher among cancer survivors than corresponding matched controls.
Over a 36-month period, the average number of opioid prescriptions filled by cancer survivors was 7.7, compared with 6.3 for controls.
This increased rate of opioid prescribing was also seen among survivors who were 10 or more years past their cancer diagnosis.
Individuals with lower income and those who were younger, from rural neighborhoods, and with more comorbidities had significantly higher prescribing rates. Sex was not associated with prescribing rates.
“Our research findings raise concerns about the diagnosis and management of chronic pain problems among survivors stemming from their cancer diagnosis or treatment,” Dr Sutradhar said. “Physicians providing primary care to cancer survivors should consider close examination of reasons for continued opioid use to differentiate chronic pain from dependency.” ![]()
A study of residents in Ontario, Canada, showed that opioid prescription use was more common in cancer survivors than in individuals without a history of cancer.
This was true even among survivors who were 10 or more years past their cancer diagnosis.
Rinku Sutradhar, PhD, of the University of Toronto in Ontario, Canada, and her colleagues reported these findings in Cancer.
The researchers said little is known about prescribing opioids to relieve pain in individuals who have survived cancer.
To investigate, the team looked at opioid prescribing among residents of Ontario, Canada, with and without a history of cancer.
The study included 8601 adults who were at least 5 years past a cancer diagnosis. These subjects were were matched with 8601 individuals without a prior cancer diagnosis. The subjects were matched based on sex and calendar year of birth.
The researchers looked for opioid prescriptions filled at a pharmacy during the observation period. Follow-up was stopped at any indication of cancer recurrence, second malignancy, or new cancer diagnosis.
The rate of opioid prescribing was 1.22 times higher among cancer survivors than corresponding matched controls.
Over a 36-month period, the average number of opioid prescriptions filled by cancer survivors was 7.7, compared with 6.3 for controls.
This increased rate of opioid prescribing was also seen among survivors who were 10 or more years past their cancer diagnosis.
Individuals with lower income and those who were younger, from rural neighborhoods, and with more comorbidities had significantly higher prescribing rates. Sex was not associated with prescribing rates.
“Our research findings raise concerns about the diagnosis and management of chronic pain problems among survivors stemming from their cancer diagnosis or treatment,” Dr Sutradhar said. “Physicians providing primary care to cancer survivors should consider close examination of reasons for continued opioid use to differentiate chronic pain from dependency.” ![]()
A study of residents in Ontario, Canada, showed that opioid prescription use was more common in cancer survivors than in individuals without a history of cancer.
This was true even among survivors who were 10 or more years past their cancer diagnosis.
Rinku Sutradhar, PhD, of the University of Toronto in Ontario, Canada, and her colleagues reported these findings in Cancer.
The researchers said little is known about prescribing opioids to relieve pain in individuals who have survived cancer.
To investigate, the team looked at opioid prescribing among residents of Ontario, Canada, with and without a history of cancer.
The study included 8601 adults who were at least 5 years past a cancer diagnosis. These subjects were were matched with 8601 individuals without a prior cancer diagnosis. The subjects were matched based on sex and calendar year of birth.
The researchers looked for opioid prescriptions filled at a pharmacy during the observation period. Follow-up was stopped at any indication of cancer recurrence, second malignancy, or new cancer diagnosis.
The rate of opioid prescribing was 1.22 times higher among cancer survivors than corresponding matched controls.
Over a 36-month period, the average number of opioid prescriptions filled by cancer survivors was 7.7, compared with 6.3 for controls.
This increased rate of opioid prescribing was also seen among survivors who were 10 or more years past their cancer diagnosis.
Individuals with lower income and those who were younger, from rural neighborhoods, and with more comorbidities had significantly higher prescribing rates. Sex was not associated with prescribing rates.
“Our research findings raise concerns about the diagnosis and management of chronic pain problems among survivors stemming from their cancer diagnosis or treatment,” Dr Sutradhar said. “Physicians providing primary care to cancer survivors should consider close examination of reasons for continued opioid use to differentiate chronic pain from dependency.” ![]()
Analysis reveals poor outcomes in refractory DLBCL
Results from the SCHOLAR-1 study revealed poor outcomes of salvage therapy in patients with refractory diffuse large B-cell lymphoma (DLBCL).
This retrospective study included data on patients enrolled in 2 randomized trials and 2 academic databases.
The patients had primary refractory disease, were refractory to second-line or later therapy, or had relapsed within 12 months of autologous stem cell transplant (ASCT).
Twenty-six percent of patients responded to salvage therapy, with 7% achieving a complete response (CR).
The median overall survival (OS) was 6.3 months, and 20% of patients were still alive at 2 years’ follow-up.
Christian Gisselbrecht, MD, of Saint Louis Hospital in Paris, France, and his colleagues reported these findings in Blood. SCHOLAR-1 was funded through an unrestricted grant from Kite Pharma.
“SCHOLAR-1 demonstrates the uniformly poor treatment outcomes for patients with aggressive non-Hodgkin lymphoma and emphasizes the need for breakthrough therapies for these refractory patients,” Dr Gisselbrecht said.
Patient characteristics
The study included pooled, patient-level data from 2 phase 3 trials and 2 databases:
- The Canadian Cancer Trials Group study LY.12 (n=219)
- The Lymphoma Academic Research Organization’s CORAL study (n=170)
- A cohort from MD Anderson Cancer Center (n=165)
- A cohort from the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (n=82).
There were a total of 636 patients who met criteria for refractory DLBCL, which included primary mediastinal B-cell lymphoma and transformed follicular lymphoma.
Twenty-eight percent of patients were primary refractory, 50% were refractory to second-line or later therapy, and 22% had relapsed within 12 months of transplant.
The patients’ median age was 55 (range, 19-81), and 64% were male. Seventy-three percent had an ECOG performance status of 0-1, 14% had a status of 2-4, and 13% were missing this data. Seventy-two percent of patients had stage III-IV disease, 27% had stage I-II disease, and less than 1% were missing this data.
Treatments
The MD Anderson cohort included patients who were relapsed/refractory to initial rituximab-containing chemotherapy, had failed salvage platinum-containing chemotherapy, and received a second salvage therapy at MD Anderson.
The University of Iowa/Mayo Clinic cohort included unselected, newly diagnosed patients with lymphoma who entered prospective documentation of primary and subsequent treatments and outcomes.
In the LY.12 study, patients were enrolled upon relapse after anthracycline-containing therapy and randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
The CORAL study enrolled patients in their first relapse or whose lymphoma was refractory to first-line therapy. They were randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
In the LY.12 and CORAL studies, eligible patients with CD20+ lymphoma were randomized to rituximab maintenance or observation post-ASCT.
Response
In all, 523 patients were evaluated for response. The overall response rate (ORR) was 26%, with a 7% CR rate and an 18% partial response rate.
Among patients with primary refractory disease, the ORR was 20%, and the CR rate was 3%.
Among patients who were refractory to second-line or later therapy, the ORR was 26%, and the CR rate was 10%.
Among patients who relapsed after transplant, the ORR was 34%, and the CR rate was 15%.
Survival
A total of 603 patients were evaluated for survival.
The median OS from the start of salvage therapy was 6.3 months (range, 5.9-7.0). The 1-year OS rate was 28%, and the 2-year OS was 20%.
Among primary refractory patients, the median OS was 7.1 months (range, 6.0-8.1), 1-year OS was 29%, and 2-year OS was 24%.
Among patient who were refractory to second-line or later therapy, the median OS was 6.1 months (range, 5.2-7.0), 1-year OS was 26%, and 2-year OS was 17%.
Among patients who relapsed after transplant, the median OS was 6.2 months (range, 5.2-7.6), 1-year OS was 32%, and 2-year OS was 19%.
“Although 60% to 70% of non-Hodgkin lymphoma patients survive 5 years after rituximab-based chemotherapy and autologous stem cell transplant, nearly half of them either do not respond or relapse shortly after transplant,” Dr Gisselbrecht noted.
“SCHOLAR-1 provides a rigorous measure of outcomes for these patients who do not benefit from currently available therapies, and this landmark study will serve as an important historical control for evaluating new therapeutic candidates in the field of non-Hodgkin lymphoma.” ![]()
Results from the SCHOLAR-1 study revealed poor outcomes of salvage therapy in patients with refractory diffuse large B-cell lymphoma (DLBCL).
This retrospective study included data on patients enrolled in 2 randomized trials and 2 academic databases.
The patients had primary refractory disease, were refractory to second-line or later therapy, or had relapsed within 12 months of autologous stem cell transplant (ASCT).
Twenty-six percent of patients responded to salvage therapy, with 7% achieving a complete response (CR).
The median overall survival (OS) was 6.3 months, and 20% of patients were still alive at 2 years’ follow-up.
Christian Gisselbrecht, MD, of Saint Louis Hospital in Paris, France, and his colleagues reported these findings in Blood. SCHOLAR-1 was funded through an unrestricted grant from Kite Pharma.
“SCHOLAR-1 demonstrates the uniformly poor treatment outcomes for patients with aggressive non-Hodgkin lymphoma and emphasizes the need for breakthrough therapies for these refractory patients,” Dr Gisselbrecht said.
Patient characteristics
The study included pooled, patient-level data from 2 phase 3 trials and 2 databases:
- The Canadian Cancer Trials Group study LY.12 (n=219)
- The Lymphoma Academic Research Organization’s CORAL study (n=170)
- A cohort from MD Anderson Cancer Center (n=165)
- A cohort from the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (n=82).
There were a total of 636 patients who met criteria for refractory DLBCL, which included primary mediastinal B-cell lymphoma and transformed follicular lymphoma.
Twenty-eight percent of patients were primary refractory, 50% were refractory to second-line or later therapy, and 22% had relapsed within 12 months of transplant.
The patients’ median age was 55 (range, 19-81), and 64% were male. Seventy-three percent had an ECOG performance status of 0-1, 14% had a status of 2-4, and 13% were missing this data. Seventy-two percent of patients had stage III-IV disease, 27% had stage I-II disease, and less than 1% were missing this data.
Treatments
The MD Anderson cohort included patients who were relapsed/refractory to initial rituximab-containing chemotherapy, had failed salvage platinum-containing chemotherapy, and received a second salvage therapy at MD Anderson.
The University of Iowa/Mayo Clinic cohort included unselected, newly diagnosed patients with lymphoma who entered prospective documentation of primary and subsequent treatments and outcomes.
In the LY.12 study, patients were enrolled upon relapse after anthracycline-containing therapy and randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
The CORAL study enrolled patients in their first relapse or whose lymphoma was refractory to first-line therapy. They were randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
In the LY.12 and CORAL studies, eligible patients with CD20+ lymphoma were randomized to rituximab maintenance or observation post-ASCT.
Response
In all, 523 patients were evaluated for response. The overall response rate (ORR) was 26%, with a 7% CR rate and an 18% partial response rate.
Among patients with primary refractory disease, the ORR was 20%, and the CR rate was 3%.
Among patients who were refractory to second-line or later therapy, the ORR was 26%, and the CR rate was 10%.
Among patients who relapsed after transplant, the ORR was 34%, and the CR rate was 15%.
Survival
A total of 603 patients were evaluated for survival.
The median OS from the start of salvage therapy was 6.3 months (range, 5.9-7.0). The 1-year OS rate was 28%, and the 2-year OS was 20%.
Among primary refractory patients, the median OS was 7.1 months (range, 6.0-8.1), 1-year OS was 29%, and 2-year OS was 24%.
Among patient who were refractory to second-line or later therapy, the median OS was 6.1 months (range, 5.2-7.0), 1-year OS was 26%, and 2-year OS was 17%.
Among patients who relapsed after transplant, the median OS was 6.2 months (range, 5.2-7.6), 1-year OS was 32%, and 2-year OS was 19%.
“Although 60% to 70% of non-Hodgkin lymphoma patients survive 5 years after rituximab-based chemotherapy and autologous stem cell transplant, nearly half of them either do not respond or relapse shortly after transplant,” Dr Gisselbrecht noted.
“SCHOLAR-1 provides a rigorous measure of outcomes for these patients who do not benefit from currently available therapies, and this landmark study will serve as an important historical control for evaluating new therapeutic candidates in the field of non-Hodgkin lymphoma.” ![]()
Results from the SCHOLAR-1 study revealed poor outcomes of salvage therapy in patients with refractory diffuse large B-cell lymphoma (DLBCL).
This retrospective study included data on patients enrolled in 2 randomized trials and 2 academic databases.
The patients had primary refractory disease, were refractory to second-line or later therapy, or had relapsed within 12 months of autologous stem cell transplant (ASCT).
Twenty-six percent of patients responded to salvage therapy, with 7% achieving a complete response (CR).
The median overall survival (OS) was 6.3 months, and 20% of patients were still alive at 2 years’ follow-up.
Christian Gisselbrecht, MD, of Saint Louis Hospital in Paris, France, and his colleagues reported these findings in Blood. SCHOLAR-1 was funded through an unrestricted grant from Kite Pharma.
“SCHOLAR-1 demonstrates the uniformly poor treatment outcomes for patients with aggressive non-Hodgkin lymphoma and emphasizes the need for breakthrough therapies for these refractory patients,” Dr Gisselbrecht said.
Patient characteristics
The study included pooled, patient-level data from 2 phase 3 trials and 2 databases:
- The Canadian Cancer Trials Group study LY.12 (n=219)
- The Lymphoma Academic Research Organization’s CORAL study (n=170)
- A cohort from MD Anderson Cancer Center (n=165)
- A cohort from the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (n=82).
There were a total of 636 patients who met criteria for refractory DLBCL, which included primary mediastinal B-cell lymphoma and transformed follicular lymphoma.
Twenty-eight percent of patients were primary refractory, 50% were refractory to second-line or later therapy, and 22% had relapsed within 12 months of transplant.
The patients’ median age was 55 (range, 19-81), and 64% were male. Seventy-three percent had an ECOG performance status of 0-1, 14% had a status of 2-4, and 13% were missing this data. Seventy-two percent of patients had stage III-IV disease, 27% had stage I-II disease, and less than 1% were missing this data.
Treatments
The MD Anderson cohort included patients who were relapsed/refractory to initial rituximab-containing chemotherapy, had failed salvage platinum-containing chemotherapy, and received a second salvage therapy at MD Anderson.
The University of Iowa/Mayo Clinic cohort included unselected, newly diagnosed patients with lymphoma who entered prospective documentation of primary and subsequent treatments and outcomes.
In the LY.12 study, patients were enrolled upon relapse after anthracycline-containing therapy and randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
The CORAL study enrolled patients in their first relapse or whose lymphoma was refractory to first-line therapy. They were randomized to 1 of 2 salvage regimens, with a goal of consolidative ASCT.
In the LY.12 and CORAL studies, eligible patients with CD20+ lymphoma were randomized to rituximab maintenance or observation post-ASCT.
Response
In all, 523 patients were evaluated for response. The overall response rate (ORR) was 26%, with a 7% CR rate and an 18% partial response rate.
Among patients with primary refractory disease, the ORR was 20%, and the CR rate was 3%.
Among patients who were refractory to second-line or later therapy, the ORR was 26%, and the CR rate was 10%.
Among patients who relapsed after transplant, the ORR was 34%, and the CR rate was 15%.
Survival
A total of 603 patients were evaluated for survival.
The median OS from the start of salvage therapy was 6.3 months (range, 5.9-7.0). The 1-year OS rate was 28%, and the 2-year OS was 20%.
Among primary refractory patients, the median OS was 7.1 months (range, 6.0-8.1), 1-year OS was 29%, and 2-year OS was 24%.
Among patient who were refractory to second-line or later therapy, the median OS was 6.1 months (range, 5.2-7.0), 1-year OS was 26%, and 2-year OS was 17%.
Among patients who relapsed after transplant, the median OS was 6.2 months (range, 5.2-7.6), 1-year OS was 32%, and 2-year OS was 19%.
“Although 60% to 70% of non-Hodgkin lymphoma patients survive 5 years after rituximab-based chemotherapy and autologous stem cell transplant, nearly half of them either do not respond or relapse shortly after transplant,” Dr Gisselbrecht noted.
“SCHOLAR-1 provides a rigorous measure of outcomes for these patients who do not benefit from currently available therapies, and this landmark study will serve as an important historical control for evaluating new therapeutic candidates in the field of non-Hodgkin lymphoma.” ![]()
Tests can produce confusing results after HSCT in MM
Tests used to assess treatment response in multiple myeloma (MM) may often produce confusing results after patients have undergone hematopoietic stem cell transplant (HSCT), a new study suggests.
The tests—serum protein electrophoresis/serum immunofixation electrophoresis (SPEP/SIFE) and serum free light chain assay (SFLCA)—can sometimes produce an oligoclonal pattern that can be mistaken for an M spike and suggest disease recurrence.
The study showed that this confusing result was significantly more likely to occur after patients underwent HSCT than after they received chemotherapy.
Gurmukh Singh, MD, PhD, of Augusta University in Augusta, Georgia, reported these findings in the Journal of Clinical Medicine Research.
For this study, Dr Singh looked at lab and clinical data on 251 MM patients treated from January 2010 to December 2016. One hundred and fifty-nine of those patients received autologous HSCTs.
Dr Singh compared results of SPEP/SIFE and/or SFLCA in patients who underwent HSCT and patients who did not. Each patient had at least 3 tests, and, for HSCT recipients, at least 2 of the tests occurred after transplant.
The incidence of oligoclonal patterns was significantly higher in HSCT recipients than in patients who had chemotherapy alone—57.9% and 8.8%, respectively (P=0.000003).
Only 5 of the 159 HSCT recipients had an oligoclonal pattern before treatment, but 92 of them had one afterward.
More than half of the oligoclonal patterns developed within the first year of HSCT. The earliest pattern was detected at 2 months—as soon as the first post-transplant tests were done—and a few occurred as late as 5 years later.
“We want to emphasize that oligoclonal bands should mostly be recognized as a response to treatment and not be mistaken as a recurrence of the original tumor,” Dr Singh said.
He explained that the key clarifier appears to be the location of the M spike when the diagnosis is made compared to the location of new spikes that may show up after HSCT.
“If the original peak was at location A, now the peak is location B, that allows us to determine that it is not the same abnormal, malignant antibody,” he said. “If it’s in a different location, it’s not the same protein. [T]his is just a normal response of recovery of the bone marrow that could be mistaken for recurrence of the disease.” ![]()
Tests used to assess treatment response in multiple myeloma (MM) may often produce confusing results after patients have undergone hematopoietic stem cell transplant (HSCT), a new study suggests.
The tests—serum protein electrophoresis/serum immunofixation electrophoresis (SPEP/SIFE) and serum free light chain assay (SFLCA)—can sometimes produce an oligoclonal pattern that can be mistaken for an M spike and suggest disease recurrence.
The study showed that this confusing result was significantly more likely to occur after patients underwent HSCT than after they received chemotherapy.
Gurmukh Singh, MD, PhD, of Augusta University in Augusta, Georgia, reported these findings in the Journal of Clinical Medicine Research.
For this study, Dr Singh looked at lab and clinical data on 251 MM patients treated from January 2010 to December 2016. One hundred and fifty-nine of those patients received autologous HSCTs.
Dr Singh compared results of SPEP/SIFE and/or SFLCA in patients who underwent HSCT and patients who did not. Each patient had at least 3 tests, and, for HSCT recipients, at least 2 of the tests occurred after transplant.
The incidence of oligoclonal patterns was significantly higher in HSCT recipients than in patients who had chemotherapy alone—57.9% and 8.8%, respectively (P=0.000003).
Only 5 of the 159 HSCT recipients had an oligoclonal pattern before treatment, but 92 of them had one afterward.
More than half of the oligoclonal patterns developed within the first year of HSCT. The earliest pattern was detected at 2 months—as soon as the first post-transplant tests were done—and a few occurred as late as 5 years later.
“We want to emphasize that oligoclonal bands should mostly be recognized as a response to treatment and not be mistaken as a recurrence of the original tumor,” Dr Singh said.
He explained that the key clarifier appears to be the location of the M spike when the diagnosis is made compared to the location of new spikes that may show up after HSCT.
“If the original peak was at location A, now the peak is location B, that allows us to determine that it is not the same abnormal, malignant antibody,” he said. “If it’s in a different location, it’s not the same protein. [T]his is just a normal response of recovery of the bone marrow that could be mistaken for recurrence of the disease.” ![]()
Tests used to assess treatment response in multiple myeloma (MM) may often produce confusing results after patients have undergone hematopoietic stem cell transplant (HSCT), a new study suggests.
The tests—serum protein electrophoresis/serum immunofixation electrophoresis (SPEP/SIFE) and serum free light chain assay (SFLCA)—can sometimes produce an oligoclonal pattern that can be mistaken for an M spike and suggest disease recurrence.
The study showed that this confusing result was significantly more likely to occur after patients underwent HSCT than after they received chemotherapy.
Gurmukh Singh, MD, PhD, of Augusta University in Augusta, Georgia, reported these findings in the Journal of Clinical Medicine Research.
For this study, Dr Singh looked at lab and clinical data on 251 MM patients treated from January 2010 to December 2016. One hundred and fifty-nine of those patients received autologous HSCTs.
Dr Singh compared results of SPEP/SIFE and/or SFLCA in patients who underwent HSCT and patients who did not. Each patient had at least 3 tests, and, for HSCT recipients, at least 2 of the tests occurred after transplant.
The incidence of oligoclonal patterns was significantly higher in HSCT recipients than in patients who had chemotherapy alone—57.9% and 8.8%, respectively (P=0.000003).
Only 5 of the 159 HSCT recipients had an oligoclonal pattern before treatment, but 92 of them had one afterward.
More than half of the oligoclonal patterns developed within the first year of HSCT. The earliest pattern was detected at 2 months—as soon as the first post-transplant tests were done—and a few occurred as late as 5 years later.
“We want to emphasize that oligoclonal bands should mostly be recognized as a response to treatment and not be mistaken as a recurrence of the original tumor,” Dr Singh said.
He explained that the key clarifier appears to be the location of the M spike when the diagnosis is made compared to the location of new spikes that may show up after HSCT.
“If the original peak was at location A, now the peak is location B, that allows us to determine that it is not the same abnormal, malignant antibody,” he said. “If it’s in a different location, it’s not the same protein. [T]his is just a normal response of recovery of the bone marrow that could be mistaken for recurrence of the disease.” ![]()
Insured cancer patients report ‘overwhelming’ financial distress
A study of 300 US cancer patients showed that paying for care can cause “overwhelming” financial distress, even when patients have health insurance.
Sixteen percent of the patients studied reported “high or overwhelming” financial distress, spending a median of 31% of their monthly household income on healthcare, not including insurance premiums.
They had a median monthly out-of-pocket cost of $728 (range, $6 to $47,250).
Fumiko Chino, MD, of Duke University Medical Center in Durham, North Carolina, and her colleagues reported these findings in a letter to JAMA Oncology.
The researchers interviewed 300 insured cancer patients for this study. They had a median age of 59.6, and 68.3% were married.
Fifty-six percent of patients had private insurance, 35.7% had Medicare, and 7.3% had Medicaid.
Annual household incomes were as follows:
- 45.7%, $60,000 or greater
- 15.7%, $40,000 to $59,999
- 17.7%, $20,000 to 39,999
- 13.7%, lower than $20,000
- 7.3%, unknown.
The median monthly out-of-pocket cost for care was $592 (range, $3-$47,250), not including insurance premiums. The median relative cost of care was 11% of a patient’s monthly household income.
“Those who spend more than 10% of their income on healthcare costs are considered underinsured,” Dr Chino said. “Learning about the cost-sharing burden on some insured patients is important right now, given the uncertainty in health insurance.”
Most of the patients studied (83.7%, n=251) reported no, low, or average financial distress. Their median relative cost of care was 10% of their monthly household income, and their median monthly out-of-pocket cost was $565 (range, $3 to $26,756). Six percent of these patients had Medicaid, 39% had Medicare, and 53.8% had private insurance.
For the 16.3% of patients (n=49) who reported high or overwhelming financial distress, 67.3% had private insurance, 18.4% had Medicare, and 14.3% had Medicaid. As stated above, their median relative cost of care was 31% of their monthly household income, and their median monthly out-of-pocket cost was $728 (range, $6 to $47,250).
“This study adds to the growing evidence that we need to intervene,” said study author Yousuf Zafar, MD, of Duke Cancer Institute.
“We know there are a lot of barriers that prevent patients from talking about cost with their providers. We need to create tools for patients at risk of financial toxicity and connect them with resources in a timely fashion so they can afford their care.” ![]()
A study of 300 US cancer patients showed that paying for care can cause “overwhelming” financial distress, even when patients have health insurance.
Sixteen percent of the patients studied reported “high or overwhelming” financial distress, spending a median of 31% of their monthly household income on healthcare, not including insurance premiums.
They had a median monthly out-of-pocket cost of $728 (range, $6 to $47,250).
Fumiko Chino, MD, of Duke University Medical Center in Durham, North Carolina, and her colleagues reported these findings in a letter to JAMA Oncology.
The researchers interviewed 300 insured cancer patients for this study. They had a median age of 59.6, and 68.3% were married.
Fifty-six percent of patients had private insurance, 35.7% had Medicare, and 7.3% had Medicaid.
Annual household incomes were as follows:
- 45.7%, $60,000 or greater
- 15.7%, $40,000 to $59,999
- 17.7%, $20,000 to 39,999
- 13.7%, lower than $20,000
- 7.3%, unknown.
The median monthly out-of-pocket cost for care was $592 (range, $3-$47,250), not including insurance premiums. The median relative cost of care was 11% of a patient’s monthly household income.
“Those who spend more than 10% of their income on healthcare costs are considered underinsured,” Dr Chino said. “Learning about the cost-sharing burden on some insured patients is important right now, given the uncertainty in health insurance.”
Most of the patients studied (83.7%, n=251) reported no, low, or average financial distress. Their median relative cost of care was 10% of their monthly household income, and their median monthly out-of-pocket cost was $565 (range, $3 to $26,756). Six percent of these patients had Medicaid, 39% had Medicare, and 53.8% had private insurance.
For the 16.3% of patients (n=49) who reported high or overwhelming financial distress, 67.3% had private insurance, 18.4% had Medicare, and 14.3% had Medicaid. As stated above, their median relative cost of care was 31% of their monthly household income, and their median monthly out-of-pocket cost was $728 (range, $6 to $47,250).
“This study adds to the growing evidence that we need to intervene,” said study author Yousuf Zafar, MD, of Duke Cancer Institute.
“We know there are a lot of barriers that prevent patients from talking about cost with their providers. We need to create tools for patients at risk of financial toxicity and connect them with resources in a timely fashion so they can afford their care.” ![]()
A study of 300 US cancer patients showed that paying for care can cause “overwhelming” financial distress, even when patients have health insurance.
Sixteen percent of the patients studied reported “high or overwhelming” financial distress, spending a median of 31% of their monthly household income on healthcare, not including insurance premiums.
They had a median monthly out-of-pocket cost of $728 (range, $6 to $47,250).
Fumiko Chino, MD, of Duke University Medical Center in Durham, North Carolina, and her colleagues reported these findings in a letter to JAMA Oncology.
The researchers interviewed 300 insured cancer patients for this study. They had a median age of 59.6, and 68.3% were married.
Fifty-six percent of patients had private insurance, 35.7% had Medicare, and 7.3% had Medicaid.
Annual household incomes were as follows:
- 45.7%, $60,000 or greater
- 15.7%, $40,000 to $59,999
- 17.7%, $20,000 to 39,999
- 13.7%, lower than $20,000
- 7.3%, unknown.
The median monthly out-of-pocket cost for care was $592 (range, $3-$47,250), not including insurance premiums. The median relative cost of care was 11% of a patient’s monthly household income.
“Those who spend more than 10% of their income on healthcare costs are considered underinsured,” Dr Chino said. “Learning about the cost-sharing burden on some insured patients is important right now, given the uncertainty in health insurance.”
Most of the patients studied (83.7%, n=251) reported no, low, or average financial distress. Their median relative cost of care was 10% of their monthly household income, and their median monthly out-of-pocket cost was $565 (range, $3 to $26,756). Six percent of these patients had Medicaid, 39% had Medicare, and 53.8% had private insurance.
For the 16.3% of patients (n=49) who reported high or overwhelming financial distress, 67.3% had private insurance, 18.4% had Medicare, and 14.3% had Medicaid. As stated above, their median relative cost of care was 31% of their monthly household income, and their median monthly out-of-pocket cost was $728 (range, $6 to $47,250).
“This study adds to the growing evidence that we need to intervene,” said study author Yousuf Zafar, MD, of Duke Cancer Institute.
“We know there are a lot of barriers that prevent patients from talking about cost with their providers. We need to create tools for patients at risk of financial toxicity and connect them with resources in a timely fashion so they can afford their care.”
Methotrexate could treat myeloproliferative neoplasms
Methotrexate (MTX) may be a feasible treatment option for myeloproliferative neoplasms (MPNs), according to preclinical research published in Haematologica.
Researchers tested low-dose MTX in mouse models that mimic human MPNs—essential thrombocythemia (ET) and polycythemia vera (PV).
The team found that MTX inhibited the JAK/STAT pathway, normalized some blood counts, and reduced splenomegaly in these mice.
“We have now shown pretty conclusively that we can use this approach to treat mouse models of human MPNs, results which provide a much more tangible prospect of success in humans,” said study author Martin Zeidler, PhD, of the University of Sheffield in Sheffield, UK.
Dr Zeidler and his colleagues tested MTX in transgenic mice whose endogenous JAK2 locus had been replaced by the human JAK2 V617F allele. These mice develop ET-like disease when heterozygous and PV-like disease when homozygous.
The researchers first treated wild-type mice and hJAK2 V617F age-matched littermates with either MTX or vehicle control for 28 days. The results indicated that low-dose MTX inhibits the JAK/STAT pathway.
The team then compared MTX and ruxolitinib in hJAK2 V617F-expressing mice.
MTX reduced hemoglobin levels, red blood cell counts, and hematocrit levels in heterozygous and homozygous hJAK2 V617F mice.
MTX also normalized white cell counts in heterozygous and homozygous mice, but platelet numbers and mean corpuscular volume were not significantly affected by treatment.
The researchers said MTX was as effective as ruxolitinib in these mice, but MTX treatment doesn’t result in general myelosuppression.
When the team analyzed bone marrow in the MTX-treated hJAK2 V617F mice, they found no reduction in cellularity or differentiation in any of the 3 hematopoietic lineages.
The researchers noted that homozygous mice have erythrocytic and megakaryocytic hyperplasia and polylobated nuclei in a proportion of megakaryocytes, and these phenotypes are subjectively reduced in MTX-treated mice. This suggests a disease-specific effect of MTX, rather than non-specific myelosuppression.
Both MTX and ruxolitinib reduced splenomegaly in hJAK2 V617F mice.
However, the researchers said they observed “modest increases” in reticulocyte numbers and spleen size in wild-type mice treated with MTX. These mice also had “slight increases” in pSTAT5 and PIM1 mRNA levels, but these same changes did not occur in hJAK2 V617F mice.
The researchers therefore concluded that a “detailed molecular analysis” of the interactions between MTX and wild-type JAK2 is needed.
The team is also hoping to start testing MTX in a clinical trial early next year, as these preclinical results suggest the drug could be effective in patients with ET or PV. And this might make MTX a low-cost alternative to therapies currently used to treat MPNs.
“Repurposing MTX has the potential to provide a new, molecularly targeted treatment for MPN patients within a budget accessible to healthcare systems throughout the world—a development that may ultimately provide substantial clinical and health economic benefits,” Dr Zeidler said.
Methotrexate (MTX) may be a feasible treatment option for myeloproliferative neoplasms (MPNs), according to preclinical research published in Haematologica.
Researchers tested low-dose MTX in mouse models that mimic human MPNs—essential thrombocythemia (ET) and polycythemia vera (PV).
The team found that MTX inhibited the JAK/STAT pathway, normalized some blood counts, and reduced splenomegaly in these mice.
“We have now shown pretty conclusively that we can use this approach to treat mouse models of human MPNs, results which provide a much more tangible prospect of success in humans,” said study author Martin Zeidler, PhD, of the University of Sheffield in Sheffield, UK.
Dr Zeidler and his colleagues tested MTX in transgenic mice whose endogenous JAK2 locus had been replaced by the human JAK2 V617F allele. These mice develop ET-like disease when heterozygous and PV-like disease when homozygous.
The researchers first treated wild-type mice and hJAK2 V617F age-matched littermates with either MTX or vehicle control for 28 days. The results indicated that low-dose MTX inhibits the JAK/STAT pathway.
The team then compared MTX and ruxolitinib in hJAK2 V617F-expressing mice.
MTX reduced hemoglobin levels, red blood cell counts, and hematocrit levels in heterozygous and homozygous hJAK2 V617F mice.
MTX also normalized white cell counts in heterozygous and homozygous mice, but platelet numbers and mean corpuscular volume were not significantly affected by treatment.
The researchers said MTX was as effective as ruxolitinib in these mice, but MTX treatment doesn’t result in general myelosuppression.
When the team analyzed bone marrow in the MTX-treated hJAK2 V617F mice, they found no reduction in cellularity or differentiation in any of the 3 hematopoietic lineages.
The researchers noted that homozygous mice have erythrocytic and megakaryocytic hyperplasia and polylobated nuclei in a proportion of megakaryocytes, and these phenotypes are subjectively reduced in MTX-treated mice. This suggests a disease-specific effect of MTX, rather than non-specific myelosuppression.
Both MTX and ruxolitinib reduced splenomegaly in hJAK2 V617F mice.
However, the researchers said they observed “modest increases” in reticulocyte numbers and spleen size in wild-type mice treated with MTX. These mice also had “slight increases” in pSTAT5 and PIM1 mRNA levels, but these same changes did not occur in hJAK2 V617F mice.
The researchers therefore concluded that a “detailed molecular analysis” of the interactions between MTX and wild-type JAK2 is needed.
The team is also hoping to start testing MTX in a clinical trial early next year, as these preclinical results suggest the drug could be effective in patients with ET or PV. And this might make MTX a low-cost alternative to therapies currently used to treat MPNs.
“Repurposing MTX has the potential to provide a new, molecularly targeted treatment for MPN patients within a budget accessible to healthcare systems throughout the world—a development that may ultimately provide substantial clinical and health economic benefits,” Dr Zeidler said.
Methotrexate (MTX) may be a feasible treatment option for myeloproliferative neoplasms (MPNs), according to preclinical research published in Haematologica.
Researchers tested low-dose MTX in mouse models that mimic human MPNs—essential thrombocythemia (ET) and polycythemia vera (PV).
The team found that MTX inhibited the JAK/STAT pathway, normalized some blood counts, and reduced splenomegaly in these mice.
“We have now shown pretty conclusively that we can use this approach to treat mouse models of human MPNs, results which provide a much more tangible prospect of success in humans,” said study author Martin Zeidler, PhD, of the University of Sheffield in Sheffield, UK.
Dr Zeidler and his colleagues tested MTX in transgenic mice whose endogenous JAK2 locus had been replaced by the human JAK2 V617F allele. These mice develop ET-like disease when heterozygous and PV-like disease when homozygous.
The researchers first treated wild-type mice and hJAK2 V617F age-matched littermates with either MTX or vehicle control for 28 days. The results indicated that low-dose MTX inhibits the JAK/STAT pathway.
The team then compared MTX and ruxolitinib in hJAK2 V617F-expressing mice.
MTX reduced hemoglobin levels, red blood cell counts, and hematocrit levels in heterozygous and homozygous hJAK2 V617F mice.
MTX also normalized white cell counts in heterozygous and homozygous mice, but platelet numbers and mean corpuscular volume were not significantly affected by treatment.
The researchers said MTX was as effective as ruxolitinib in these mice, but MTX treatment doesn’t result in general myelosuppression.
When the team analyzed bone marrow in the MTX-treated hJAK2 V617F mice, they found no reduction in cellularity or differentiation in any of the 3 hematopoietic lineages.
The researchers noted that homozygous mice have erythrocytic and megakaryocytic hyperplasia and polylobated nuclei in a proportion of megakaryocytes, and these phenotypes are subjectively reduced in MTX-treated mice. This suggests a disease-specific effect of MTX, rather than non-specific myelosuppression.
Both MTX and ruxolitinib reduced splenomegaly in hJAK2 V617F mice.
However, the researchers said they observed “modest increases” in reticulocyte numbers and spleen size in wild-type mice treated with MTX. These mice also had “slight increases” in pSTAT5 and PIM1 mRNA levels, but these same changes did not occur in hJAK2 V617F mice.
The researchers therefore concluded that a “detailed molecular analysis” of the interactions between MTX and wild-type JAK2 is needed.
The team is also hoping to start testing MTX in a clinical trial early next year, as these preclinical results suggest the drug could be effective in patients with ET or PV. And this might make MTX a low-cost alternative to therapies currently used to treat MPNs.
“Repurposing MTX has the potential to provide a new, molecularly targeted treatment for MPN patients within a budget accessible to healthcare systems throughout the world—a development that may ultimately provide substantial clinical and health economic benefits,” Dr Zeidler said.
Strategy could reduce myelosuppression in AML
Researchers believe they may have found a way to prevent chemotherapy-induced myelosuppression in acute myeloid leukemia (AML).
The team found that priming mice with the FLT3 inhibitor quizartinib protected multipotent progenitor cells (MPPs) from subsequent treatment with fluorouracil (5-FU) or gemcitabine.
And treatment with quizartinib followed by 5-FU proved more effective against AML than standard induction with cytarabine and doxorubicin.
Samuel Taylor, of the University of Western Australia in Crawley, Australia, and his colleagues reported these results in Science Translational Medicine.
The researchers first found that quizartinib induced “rapid and transient” quiescence of MPPs in C57BL/6 mice.
Quizartinib also provided MPPs with “marked protection” from 5-FU. In these experiments, a 10 mg/kg dose of quizartinib was given to mice at the same time as a 150 mg/kg dose of 5-FU. This treatment provided MPPs with 4- to 5-fold greater protection than vehicle control.
Subsequent experiments revealed the optimal dose and schedule for quizartinib. A priming dose of 30 mg/kg given 6 hours before 5-FU provided “slightly greater” protection to hematopoietic stem and progenitor cells than a 10 mg/kg dose, with significantly greater protection observed for short-term hematopoietic stem cells.
The researchers then showed that priming with quizartinib allowed for “rapid recovery of bone marrow cellularity” after treatment with 5-FU. Bone marrow cells were fully restored by day 8 after treatment in quizartinib-primed mice but not in vehicle-primed mice.
Quizartinib priming also protected mice from multiple rounds of treatment with 5-FU (15 cycles in some mice) and from myelosuppression induced by gemcitabine.
Finally, the researchers tested quizartinib followed by 5-FU in mouse models of AML. They found the treatment was more effective than treatment with cytarabine and doxorubicin in both FLT3-ITD(F692L)/NPM1c AML and NPM1c/NrasG12D AML.
FLT3-ITD(F692L)/NPM1c AML
The researchers transplanted 15 non-irradiated B6.CD45.1 mice with 3 × 105 spleen cells each from a FLT3-ITD(F691L)/NPM1c mouse that succumbed to AML at 6 weeks of age. Sixteen days after transplant, the mice were given one of the following:
- No treatment
- 10-day cycles of quizartinib (30 mg/kg) followed 6 hours later by 5-FU (150 mg/kg)
- Cytarabine plus doxorubicin (5+3).
All 5 of the untreated mice died within 30 days of transplantation, exhibiting high white blood cell (WBC) counts and splenomegaly.
The 5+3 mice received 2 cycles of treatment (days 16 to 21 and 36 to 41). All 5 had died by day 56 after transplantation, with high WBC counts and splenomegaly.
One the other hand, 4 of the 5 mice in the quizartinib/5-FU arm were still healthy at 176 days after transplantation and 80 days after stopping treatment. There were no detectable CD45.2+ AML cells when the mice were last bled on day 160, and they had normal WBC counts. There were no AML cells detectable in the animals’ bone marrow after they were killed at day 176.
The quizartinib/5-FU mouse that died before day 176 is believed to have developed resistance to 5-FU. This animal died 121 days after transplantation.
NPM1c/ NrasG12D AML
For another AML model, the researchers crossed NPM1c-mutant mice with NrasG12D-mutant mice. The team transplanted spleen cells from NPM1c/NrasG12D leukemic mice into 15 non-irradiated B6.CD45.1 recipient mice.
Fifteen days after transplantation, the NPM1c/ NrasG12D mice received one of the following:
- No treatment
- Quizartinib and 5-FU as above
- Cytarabine plus doxorubicin (5+3).
All 5 untreated mice died by day 32 after transplantation, and all 5 mice that received 5+3 died by day 35. Both groups of mice had high WBC counts and splenomegaly.
Mice in the quizartinib/5-FU arm initially received 4 cycles of treatment, starting on days 15, 25, 35, and 45 after transplantation. On day 53, they had minimal or undetectable numbers of CD45.2+ AML cells, and WBC counts were normal or slightly below normal.
At day 81—a month after stopping treatment—4 of the mice had detectable CD45.2+ AML cells in their blood. So they restarted treatment the next day. After 4 additional cycles, AML cells were undetectable in all 5 mice. At day 146—a month after stopping the second round of treatment—AML cells again became detectable in the blood.
The mice did not receive any additional treatment. One died at day 196, and 1 was killed at day 197 due to weight loss related to feeding difficulties (but this mouse did not show signs of AML).
The other 3 mice were “active and healthy” until they were killed at day 214. However, they had “high proportions” of CD45.2+ myeloid cells in their blood since day 183. And 2 of the mice had increased WBC counts from day 197.
Researchers believe they may have found a way to prevent chemotherapy-induced myelosuppression in acute myeloid leukemia (AML).
The team found that priming mice with the FLT3 inhibitor quizartinib protected multipotent progenitor cells (MPPs) from subsequent treatment with fluorouracil (5-FU) or gemcitabine.
And treatment with quizartinib followed by 5-FU proved more effective against AML than standard induction with cytarabine and doxorubicin.
Samuel Taylor, of the University of Western Australia in Crawley, Australia, and his colleagues reported these results in Science Translational Medicine.
The researchers first found that quizartinib induced “rapid and transient” quiescence of MPPs in C57BL/6 mice.
Quizartinib also provided MPPs with “marked protection” from 5-FU. In these experiments, a 10 mg/kg dose of quizartinib was given to mice at the same time as a 150 mg/kg dose of 5-FU. This treatment provided MPPs with 4- to 5-fold greater protection than vehicle control.
Subsequent experiments revealed the optimal dose and schedule for quizartinib. A priming dose of 30 mg/kg given 6 hours before 5-FU provided “slightly greater” protection to hematopoietic stem and progenitor cells than a 10 mg/kg dose, with significantly greater protection observed for short-term hematopoietic stem cells.
The researchers then showed that priming with quizartinib allowed for “rapid recovery of bone marrow cellularity” after treatment with 5-FU. Bone marrow cells were fully restored by day 8 after treatment in quizartinib-primed mice but not in vehicle-primed mice.
Quizartinib priming also protected mice from multiple rounds of treatment with 5-FU (15 cycles in some mice) and from myelosuppression induced by gemcitabine.
Finally, the researchers tested quizartinib followed by 5-FU in mouse models of AML. They found the treatment was more effective than treatment with cytarabine and doxorubicin in both FLT3-ITD(F692L)/NPM1c AML and NPM1c/NrasG12D AML.
FLT3-ITD(F692L)/NPM1c AML
The researchers transplanted 15 non-irradiated B6.CD45.1 mice with 3 × 105 spleen cells each from a FLT3-ITD(F691L)/NPM1c mouse that succumbed to AML at 6 weeks of age. Sixteen days after transplant, the mice were given one of the following:
- No treatment
- 10-day cycles of quizartinib (30 mg/kg) followed 6 hours later by 5-FU (150 mg/kg)
- Cytarabine plus doxorubicin (5+3).
All 5 of the untreated mice died within 30 days of transplantation, exhibiting high white blood cell (WBC) counts and splenomegaly.
The 5+3 mice received 2 cycles of treatment (days 16 to 21 and 36 to 41). All 5 had died by day 56 after transplantation, with high WBC counts and splenomegaly.
One the other hand, 4 of the 5 mice in the quizartinib/5-FU arm were still healthy at 176 days after transplantation and 80 days after stopping treatment. There were no detectable CD45.2+ AML cells when the mice were last bled on day 160, and they had normal WBC counts. There were no AML cells detectable in the animals’ bone marrow after they were killed at day 176.
The quizartinib/5-FU mouse that died before day 176 is believed to have developed resistance to 5-FU. This animal died 121 days after transplantation.
NPM1c/ NrasG12D AML
For another AML model, the researchers crossed NPM1c-mutant mice with NrasG12D-mutant mice. The team transplanted spleen cells from NPM1c/NrasG12D leukemic mice into 15 non-irradiated B6.CD45.1 recipient mice.
Fifteen days after transplantation, the NPM1c/ NrasG12D mice received one of the following:
- No treatment
- Quizartinib and 5-FU as above
- Cytarabine plus doxorubicin (5+3).
All 5 untreated mice died by day 32 after transplantation, and all 5 mice that received 5+3 died by day 35. Both groups of mice had high WBC counts and splenomegaly.
Mice in the quizartinib/5-FU arm initially received 4 cycles of treatment, starting on days 15, 25, 35, and 45 after transplantation. On day 53, they had minimal or undetectable numbers of CD45.2+ AML cells, and WBC counts were normal or slightly below normal.
At day 81—a month after stopping treatment—4 of the mice had detectable CD45.2+ AML cells in their blood. So they restarted treatment the next day. After 4 additional cycles, AML cells were undetectable in all 5 mice. At day 146—a month after stopping the second round of treatment—AML cells again became detectable in the blood.
The mice did not receive any additional treatment. One died at day 196, and 1 was killed at day 197 due to weight loss related to feeding difficulties (but this mouse did not show signs of AML).
The other 3 mice were “active and healthy” until they were killed at day 214. However, they had “high proportions” of CD45.2+ myeloid cells in their blood since day 183. And 2 of the mice had increased WBC counts from day 197.
Researchers believe they may have found a way to prevent chemotherapy-induced myelosuppression in acute myeloid leukemia (AML).
The team found that priming mice with the FLT3 inhibitor quizartinib protected multipotent progenitor cells (MPPs) from subsequent treatment with fluorouracil (5-FU) or gemcitabine.
And treatment with quizartinib followed by 5-FU proved more effective against AML than standard induction with cytarabine and doxorubicin.
Samuel Taylor, of the University of Western Australia in Crawley, Australia, and his colleagues reported these results in Science Translational Medicine.
The researchers first found that quizartinib induced “rapid and transient” quiescence of MPPs in C57BL/6 mice.
Quizartinib also provided MPPs with “marked protection” from 5-FU. In these experiments, a 10 mg/kg dose of quizartinib was given to mice at the same time as a 150 mg/kg dose of 5-FU. This treatment provided MPPs with 4- to 5-fold greater protection than vehicle control.
Subsequent experiments revealed the optimal dose and schedule for quizartinib. A priming dose of 30 mg/kg given 6 hours before 5-FU provided “slightly greater” protection to hematopoietic stem and progenitor cells than a 10 mg/kg dose, with significantly greater protection observed for short-term hematopoietic stem cells.
The researchers then showed that priming with quizartinib allowed for “rapid recovery of bone marrow cellularity” after treatment with 5-FU. Bone marrow cells were fully restored by day 8 after treatment in quizartinib-primed mice but not in vehicle-primed mice.
Quizartinib priming also protected mice from multiple rounds of treatment with 5-FU (15 cycles in some mice) and from myelosuppression induced by gemcitabine.
Finally, the researchers tested quizartinib followed by 5-FU in mouse models of AML. They found the treatment was more effective than treatment with cytarabine and doxorubicin in both FLT3-ITD(F692L)/NPM1c AML and NPM1c/NrasG12D AML.
FLT3-ITD(F692L)/NPM1c AML
The researchers transplanted 15 non-irradiated B6.CD45.1 mice with 3 × 105 spleen cells each from a FLT3-ITD(F691L)/NPM1c mouse that succumbed to AML at 6 weeks of age. Sixteen days after transplant, the mice were given one of the following:
- No treatment
- 10-day cycles of quizartinib (30 mg/kg) followed 6 hours later by 5-FU (150 mg/kg)
- Cytarabine plus doxorubicin (5+3).
All 5 of the untreated mice died within 30 days of transplantation, exhibiting high white blood cell (WBC) counts and splenomegaly.
The 5+3 mice received 2 cycles of treatment (days 16 to 21 and 36 to 41). All 5 had died by day 56 after transplantation, with high WBC counts and splenomegaly.
One the other hand, 4 of the 5 mice in the quizartinib/5-FU arm were still healthy at 176 days after transplantation and 80 days after stopping treatment. There were no detectable CD45.2+ AML cells when the mice were last bled on day 160, and they had normal WBC counts. There were no AML cells detectable in the animals’ bone marrow after they were killed at day 176.
The quizartinib/5-FU mouse that died before day 176 is believed to have developed resistance to 5-FU. This animal died 121 days after transplantation.
NPM1c/ NrasG12D AML
For another AML model, the researchers crossed NPM1c-mutant mice with NrasG12D-mutant mice. The team transplanted spleen cells from NPM1c/NrasG12D leukemic mice into 15 non-irradiated B6.CD45.1 recipient mice.
Fifteen days after transplantation, the NPM1c/ NrasG12D mice received one of the following:
- No treatment
- Quizartinib and 5-FU as above
- Cytarabine plus doxorubicin (5+3).
All 5 untreated mice died by day 32 after transplantation, and all 5 mice that received 5+3 died by day 35. Both groups of mice had high WBC counts and splenomegaly.
Mice in the quizartinib/5-FU arm initially received 4 cycles of treatment, starting on days 15, 25, 35, and 45 after transplantation. On day 53, they had minimal or undetectable numbers of CD45.2+ AML cells, and WBC counts were normal or slightly below normal.
At day 81—a month after stopping treatment—4 of the mice had detectable CD45.2+ AML cells in their blood. So they restarted treatment the next day. After 4 additional cycles, AML cells were undetectable in all 5 mice. At day 146—a month after stopping the second round of treatment—AML cells again became detectable in the blood.
The mice did not receive any additional treatment. One died at day 196, and 1 was killed at day 197 due to weight loss related to feeding difficulties (but this mouse did not show signs of AML).
The other 3 mice were “active and healthy” until they were killed at day 214. However, they had “high proportions” of CD45.2+ myeloid cells in their blood since day 183. And 2 of the mice had increased WBC counts from day 197.
Study suggests malaria is undertreated
A large study suggests the use of rapid diagnostic tests (RDTs) for malaria can reduce overuse of artemisinin combination therapies (ACTs), but malaria may also go untreated.
Researchers analyzed data from more than 500,000 patient visits across malaria-endemic regions of Africa and Afghanistan.
They found that, in most settings, the introduction of RDTs improved antimalarial targeting.
However, a substantial number of patients who tested positive for malaria appeared to go untreated, and negative test results prompted a shift to antibiotic prescriptions.
Katia Bruxvoort, PhD, of London School of Hygiene & Tropical Medicine in the UK, and her colleagues reported these results in the American Journal of Tropical Medicine and Hygiene.
The researchers analyzed drug prescriptions written from 2007 to 2013 in 562,368 patient encounters documented in 10 related studies. Eight studies were conducted in sub-Saharan Africa (Cameroon, Ghana, Nigeria, Tanzania, and Uganda), and 2 were conducted in Afghanistan.
Overall, RDTs appeared to limit—though not eliminate—routine prescription of ACTs to patients presenting with fever but not malaria.
In most cases, fewer than 30% of patients who tested negative for malaria still received ACTs. However, in Cameroon and Ghana, 39% to 49% of patients who tested negative for malaria received ACTs.
“[I]n many places, a reduction in the use of ACTs was accompanied by an increase in the use of antibiotics, which may drive up the risk of antibiotic-resistant infections,” Dr Bruxvoort noted.
Overall, 75% of patients studied left the clinic with either an antibiotic or an ACT.
In most areas studied, antibiotics were given to 40% to 80% of patients who had tested negative for malaria.
The researchers believe the shift to antibiotic use after ruling out malaria may indicate that many patients and providers are not comfortable treating a fever using only supportive care (taking a fever-reducing drug and drinking plenty of fluids).
“A key challenge is that we don’t currently have a reliable way to determine which fevers are evidence of a bacterial infection that requires a specific antibiotic treatment and which fevers will resolve with supportive care only,” Dr Bruxvoort said.
In addition, Dr Bruxvoort and her colleagues were surprised to find that, in 5 of the 8 African studies, more than 20% of patients who tested positive for malaria were not prescribed ACTs.
“Drug supply issues did not seem to be a problem in most of the areas where these patients sought treatment,” Dr Bruxvoort said. “There might be other reasons either patients or providers are not using ACTs in these contexts, but the issue of undertreating malaria, even when there is clear evidence of the disease, is troubling and deserves further study.”
A large study suggests the use of rapid diagnostic tests (RDTs) for malaria can reduce overuse of artemisinin combination therapies (ACTs), but malaria may also go untreated.
Researchers analyzed data from more than 500,000 patient visits across malaria-endemic regions of Africa and Afghanistan.
They found that, in most settings, the introduction of RDTs improved antimalarial targeting.
However, a substantial number of patients who tested positive for malaria appeared to go untreated, and negative test results prompted a shift to antibiotic prescriptions.
Katia Bruxvoort, PhD, of London School of Hygiene & Tropical Medicine in the UK, and her colleagues reported these results in the American Journal of Tropical Medicine and Hygiene.
The researchers analyzed drug prescriptions written from 2007 to 2013 in 562,368 patient encounters documented in 10 related studies. Eight studies were conducted in sub-Saharan Africa (Cameroon, Ghana, Nigeria, Tanzania, and Uganda), and 2 were conducted in Afghanistan.
Overall, RDTs appeared to limit—though not eliminate—routine prescription of ACTs to patients presenting with fever but not malaria.
In most cases, fewer than 30% of patients who tested negative for malaria still received ACTs. However, in Cameroon and Ghana, 39% to 49% of patients who tested negative for malaria received ACTs.
“[I]n many places, a reduction in the use of ACTs was accompanied by an increase in the use of antibiotics, which may drive up the risk of antibiotic-resistant infections,” Dr Bruxvoort noted.
Overall, 75% of patients studied left the clinic with either an antibiotic or an ACT.
In most areas studied, antibiotics were given to 40% to 80% of patients who had tested negative for malaria.
The researchers believe the shift to antibiotic use after ruling out malaria may indicate that many patients and providers are not comfortable treating a fever using only supportive care (taking a fever-reducing drug and drinking plenty of fluids).
“A key challenge is that we don’t currently have a reliable way to determine which fevers are evidence of a bacterial infection that requires a specific antibiotic treatment and which fevers will resolve with supportive care only,” Dr Bruxvoort said.
In addition, Dr Bruxvoort and her colleagues were surprised to find that, in 5 of the 8 African studies, more than 20% of patients who tested positive for malaria were not prescribed ACTs.
“Drug supply issues did not seem to be a problem in most of the areas where these patients sought treatment,” Dr Bruxvoort said. “There might be other reasons either patients or providers are not using ACTs in these contexts, but the issue of undertreating malaria, even when there is clear evidence of the disease, is troubling and deserves further study.”
A large study suggests the use of rapid diagnostic tests (RDTs) for malaria can reduce overuse of artemisinin combination therapies (ACTs), but malaria may also go untreated.
Researchers analyzed data from more than 500,000 patient visits across malaria-endemic regions of Africa and Afghanistan.
They found that, in most settings, the introduction of RDTs improved antimalarial targeting.
However, a substantial number of patients who tested positive for malaria appeared to go untreated, and negative test results prompted a shift to antibiotic prescriptions.
Katia Bruxvoort, PhD, of London School of Hygiene & Tropical Medicine in the UK, and her colleagues reported these results in the American Journal of Tropical Medicine and Hygiene.
The researchers analyzed drug prescriptions written from 2007 to 2013 in 562,368 patient encounters documented in 10 related studies. Eight studies were conducted in sub-Saharan Africa (Cameroon, Ghana, Nigeria, Tanzania, and Uganda), and 2 were conducted in Afghanistan.
Overall, RDTs appeared to limit—though not eliminate—routine prescription of ACTs to patients presenting with fever but not malaria.
In most cases, fewer than 30% of patients who tested negative for malaria still received ACTs. However, in Cameroon and Ghana, 39% to 49% of patients who tested negative for malaria received ACTs.
“[I]n many places, a reduction in the use of ACTs was accompanied by an increase in the use of antibiotics, which may drive up the risk of antibiotic-resistant infections,” Dr Bruxvoort noted.
Overall, 75% of patients studied left the clinic with either an antibiotic or an ACT.
In most areas studied, antibiotics were given to 40% to 80% of patients who had tested negative for malaria.
The researchers believe the shift to antibiotic use after ruling out malaria may indicate that many patients and providers are not comfortable treating a fever using only supportive care (taking a fever-reducing drug and drinking plenty of fluids).
“A key challenge is that we don’t currently have a reliable way to determine which fevers are evidence of a bacterial infection that requires a specific antibiotic treatment and which fevers will resolve with supportive care only,” Dr Bruxvoort said.
In addition, Dr Bruxvoort and her colleagues were surprised to find that, in 5 of the 8 African studies, more than 20% of patients who tested positive for malaria were not prescribed ACTs.
“Drug supply issues did not seem to be a problem in most of the areas where these patients sought treatment,” Dr Bruxvoort said. “There might be other reasons either patients or providers are not using ACTs in these contexts, but the issue of undertreating malaria, even when there is clear evidence of the disease, is troubling and deserves further study.”