User login
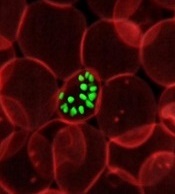
Donor screening assays more sensitive than diagnostic assays for Zika
New research suggests assays used to screen donated blood for the Zika virus are more sensitive than assays used to help physicians diagnose Zika infection.
The study showed that donor screening assays could detect Zika virus RNA with greater sensitivity than diagnostic real-time polymerase chain reaction (RT-PCR) assays.
However, the evidence also indicated that increasing the volume of blood analyzed can increase the sensitivity of diagnostic assays.
This research was published in Transfusion in a special issue focusing on Zika and other transfusion-transmitted viruses.
The researchers compared 17 nucleic acid amplification technology assays used at 11 different labs. (Some of the 17 assays were actually the same assays used at different labs.)
One of the donor screening assays was the Procleix Zika virus assay, which was co-developed by Hologic, Inc. and Grifols Diagnostic Solutions, Inc. and used at Hologic.
The other donor screening assay was the cobas Zika test, which was developed by Roche Molecular Systems, Inc. and used in the company’s lab.
The RT-PCR diagnostic assays included the US Centers for Disease Control and Prevention’s (CDC) Singleplex (1087, 4481) and Trioplex assays—both low input (LI) and high input (HI)—which were used at the CDC labs in Puerto Rico (PR) and Fort Collins (FC).
Modified versions of the CDC’s assays were also used at the Blood Systems Research Institute (BSRI) in San Francisco, the University of California (UC) Davis, the Institut Louis Malarde (ILM) in French Polynesia, and 2 labs in Brazil—Fundação Pró-Sangue and Laboratório Richet.
The US Food and Drug Administration (FDA) used its own RT-PCR test, and the Etablissement Francais du Sang (EFS) in France used the Altona RealStar ZIKV RT-PCR assay.
Results
The various assays were used on plasma samples positive for 2 different strains of Zika virus—1 from Brazil and 1 from French Polynesia—as well as Zika-negative control samples.
The researchers found the donor screening assays provided comparable sensitivity and were more sensitive than each of the diagnostic RT-PCR assays.
So the team compared results with the 2 donor screening assays combined to results with the RT-PCR assays, which they grouped into 9 categories based on similar intended applications, methodologies, and results.
The 95% limit of detection (LOD95) and 50% limit of detection (LOD50) for the assays were as follows.
| Donor screening assays | CDC PR Trioplex-LI | CDC PR Trioplex-HI | CDC FC 1087-LI | CDC FC 1087-HI | BSRI/UC Davis | FDA | EFS | ILM | Brazil labs | |
| Brazil LOD95 | 13.7 | 540 | 22.8 | 220 | 43.9 | 2189 | 6343 | 312 | 107 | 165 |
| Brazil LOD50 | 2.5 | 411 | 19.6 | 43.9 | 32.3 | 326 | 523 | 46.3 | 19.6 | 124 |
| French Polynesia LOD95 | 17.9 | 1529 | 28.8 | 205 | 20.3 | 1102 | 4918 | 466 | 135 | 1351 |
| French Polynesia LOD50 | 2.5 | 123 | 24.8 | 152 | 15.1 | 81.7 | 321 | 49.6 | 24.8 | 248 |
The researchers noted that the donor screening assays were about 10-fold to 100-fold more sensitive than the standard input RT-PCR assays.
However, the CDC’s assays were performed with low and high inputs of plasma. And increasing the sample input volume increased the limit of detection by 10-fold to 30-fold.
“The results of this study, that evaluated 17 Zika virus assays in 11 laboratories and documented excellent sensitivity of the 2 donor screening assays manufactured by Roche and Grifols, were critical to support the decision by the US Food and Drug Administration and blood industry to implement investigational screening of donors in Puerto Rico in April 2016 and the entire US by the end of 2016,” said study author Michael Busch, MD, PhD, of BSRI.
“Given the sensitivity of these assays, the FDA approved clinical trials using individual donation screening and rescinded earlier policies precluding transfusion of blood collected in Puerto Rico and deferral from donation by donors who had traveled to Zika risk countries throughout the US. This screening has detected over 350 infected blood donations in Puerto Rico and dozens of infected donations in the continental US.” ![]()
New research suggests assays used to screen donated blood for the Zika virus are more sensitive than assays used to help physicians diagnose Zika infection.
The study showed that donor screening assays could detect Zika virus RNA with greater sensitivity than diagnostic real-time polymerase chain reaction (RT-PCR) assays.
However, the evidence also indicated that increasing the volume of blood analyzed can increase the sensitivity of diagnostic assays.
This research was published in Transfusion in a special issue focusing on Zika and other transfusion-transmitted viruses.
The researchers compared 17 nucleic acid amplification technology assays used at 11 different labs. (Some of the 17 assays were actually the same assays used at different labs.)
One of the donor screening assays was the Procleix Zika virus assay, which was co-developed by Hologic, Inc. and Grifols Diagnostic Solutions, Inc. and used at Hologic.
The other donor screening assay was the cobas Zika test, which was developed by Roche Molecular Systems, Inc. and used in the company’s lab.
The RT-PCR diagnostic assays included the US Centers for Disease Control and Prevention’s (CDC) Singleplex (1087, 4481) and Trioplex assays—both low input (LI) and high input (HI)—which were used at the CDC labs in Puerto Rico (PR) and Fort Collins (FC).
Modified versions of the CDC’s assays were also used at the Blood Systems Research Institute (BSRI) in San Francisco, the University of California (UC) Davis, the Institut Louis Malarde (ILM) in French Polynesia, and 2 labs in Brazil—Fundação Pró-Sangue and Laboratório Richet.
The US Food and Drug Administration (FDA) used its own RT-PCR test, and the Etablissement Francais du Sang (EFS) in France used the Altona RealStar ZIKV RT-PCR assay.
Results
The various assays were used on plasma samples positive for 2 different strains of Zika virus—1 from Brazil and 1 from French Polynesia—as well as Zika-negative control samples.
The researchers found the donor screening assays provided comparable sensitivity and were more sensitive than each of the diagnostic RT-PCR assays.
So the team compared results with the 2 donor screening assays combined to results with the RT-PCR assays, which they grouped into 9 categories based on similar intended applications, methodologies, and results.
The 95% limit of detection (LOD95) and 50% limit of detection (LOD50) for the assays were as follows.
| Donor screening assays | CDC PR Trioplex-LI | CDC PR Trioplex-HI | CDC FC 1087-LI | CDC FC 1087-HI | BSRI/UC Davis | FDA | EFS | ILM | Brazil labs | |
| Brazil LOD95 | 13.7 | 540 | 22.8 | 220 | 43.9 | 2189 | 6343 | 312 | 107 | 165 |
| Brazil LOD50 | 2.5 | 411 | 19.6 | 43.9 | 32.3 | 326 | 523 | 46.3 | 19.6 | 124 |
| French Polynesia LOD95 | 17.9 | 1529 | 28.8 | 205 | 20.3 | 1102 | 4918 | 466 | 135 | 1351 |
| French Polynesia LOD50 | 2.5 | 123 | 24.8 | 152 | 15.1 | 81.7 | 321 | 49.6 | 24.8 | 248 |
The researchers noted that the donor screening assays were about 10-fold to 100-fold more sensitive than the standard input RT-PCR assays.
However, the CDC’s assays were performed with low and high inputs of plasma. And increasing the sample input volume increased the limit of detection by 10-fold to 30-fold.
“The results of this study, that evaluated 17 Zika virus assays in 11 laboratories and documented excellent sensitivity of the 2 donor screening assays manufactured by Roche and Grifols, were critical to support the decision by the US Food and Drug Administration and blood industry to implement investigational screening of donors in Puerto Rico in April 2016 and the entire US by the end of 2016,” said study author Michael Busch, MD, PhD, of BSRI.
“Given the sensitivity of these assays, the FDA approved clinical trials using individual donation screening and rescinded earlier policies precluding transfusion of blood collected in Puerto Rico and deferral from donation by donors who had traveled to Zika risk countries throughout the US. This screening has detected over 350 infected blood donations in Puerto Rico and dozens of infected donations in the continental US.” ![]()
New research suggests assays used to screen donated blood for the Zika virus are more sensitive than assays used to help physicians diagnose Zika infection.
The study showed that donor screening assays could detect Zika virus RNA with greater sensitivity than diagnostic real-time polymerase chain reaction (RT-PCR) assays.
However, the evidence also indicated that increasing the volume of blood analyzed can increase the sensitivity of diagnostic assays.
This research was published in Transfusion in a special issue focusing on Zika and other transfusion-transmitted viruses.
The researchers compared 17 nucleic acid amplification technology assays used at 11 different labs. (Some of the 17 assays were actually the same assays used at different labs.)
One of the donor screening assays was the Procleix Zika virus assay, which was co-developed by Hologic, Inc. and Grifols Diagnostic Solutions, Inc. and used at Hologic.
The other donor screening assay was the cobas Zika test, which was developed by Roche Molecular Systems, Inc. and used in the company’s lab.
The RT-PCR diagnostic assays included the US Centers for Disease Control and Prevention’s (CDC) Singleplex (1087, 4481) and Trioplex assays—both low input (LI) and high input (HI)—which were used at the CDC labs in Puerto Rico (PR) and Fort Collins (FC).
Modified versions of the CDC’s assays were also used at the Blood Systems Research Institute (BSRI) in San Francisco, the University of California (UC) Davis, the Institut Louis Malarde (ILM) in French Polynesia, and 2 labs in Brazil—Fundação Pró-Sangue and Laboratório Richet.
The US Food and Drug Administration (FDA) used its own RT-PCR test, and the Etablissement Francais du Sang (EFS) in France used the Altona RealStar ZIKV RT-PCR assay.
Results
The various assays were used on plasma samples positive for 2 different strains of Zika virus—1 from Brazil and 1 from French Polynesia—as well as Zika-negative control samples.
The researchers found the donor screening assays provided comparable sensitivity and were more sensitive than each of the diagnostic RT-PCR assays.
So the team compared results with the 2 donor screening assays combined to results with the RT-PCR assays, which they grouped into 9 categories based on similar intended applications, methodologies, and results.
The 95% limit of detection (LOD95) and 50% limit of detection (LOD50) for the assays were as follows.
| Donor screening assays | CDC PR Trioplex-LI | CDC PR Trioplex-HI | CDC FC 1087-LI | CDC FC 1087-HI | BSRI/UC Davis | FDA | EFS | ILM | Brazil labs | |
| Brazil LOD95 | 13.7 | 540 | 22.8 | 220 | 43.9 | 2189 | 6343 | 312 | 107 | 165 |
| Brazil LOD50 | 2.5 | 411 | 19.6 | 43.9 | 32.3 | 326 | 523 | 46.3 | 19.6 | 124 |
| French Polynesia LOD95 | 17.9 | 1529 | 28.8 | 205 | 20.3 | 1102 | 4918 | 466 | 135 | 1351 |
| French Polynesia LOD50 | 2.5 | 123 | 24.8 | 152 | 15.1 | 81.7 | 321 | 49.6 | 24.8 | 248 |
The researchers noted that the donor screening assays were about 10-fold to 100-fold more sensitive than the standard input RT-PCR assays.
However, the CDC’s assays were performed with low and high inputs of plasma. And increasing the sample input volume increased the limit of detection by 10-fold to 30-fold.
“The results of this study, that evaluated 17 Zika virus assays in 11 laboratories and documented excellent sensitivity of the 2 donor screening assays manufactured by Roche and Grifols, were critical to support the decision by the US Food and Drug Administration and blood industry to implement investigational screening of donors in Puerto Rico in April 2016 and the entire US by the end of 2016,” said study author Michael Busch, MD, PhD, of BSRI.
“Given the sensitivity of these assays, the FDA approved clinical trials using individual donation screening and rescinded earlier policies precluding transfusion of blood collected in Puerto Rico and deferral from donation by donors who had traveled to Zika risk countries throughout the US. This screening has detected over 350 infected blood donations in Puerto Rico and dozens of infected donations in the continental US.” ![]()
Drug induces remission in patient with severe TA-TMA
MARSEILLE—An investigational drug has successfully treated a severe case of transplant-associated thrombotic microangiopathy (TA-TMA), according to a presentation at the 43rd Annual Meeting of the European Society for Blood and Marrow Transplantation.
The drug is OMS721, a monoclonal antibody targeting mannan-binding lectin-associated serine protease-2 (MASP-2), the effector enzyme of the lectin pathway of the complement system.
The patient received OMS721, which is being developed by Omeros Corporation, under a compassionate-use protocol.
Marco Zecca, MD, of the Fondazione IRCCS Policlinico San Matteo in Italy, and his colleagues provided details on this patient in a poster presented at the meeting (Physician Poster Abstracts-Day 1, abstract A437).
The female patient had undergone a hematopoietic stem cell transplant to treat Diamond‐Blackfan anemia. At age 14, she received a transplant from an HLA-compatible, unrelated donor.
Seven months later, she was diagnosed with TA-TMA. The patient was initially treated with eculizumab but had to stop taking the drug after she developed acute pulmonary edema.
She was then treated with plasma exchange but experienced a TA-TMA relapse at 11 months. The patient was again treated with eculizumab and again had to discontinue the drug after developing acute pulmonary edema.
The patient’s condition continued to worsen, and she soon required hemodialysis 3 times a week as well as daily platelet transfusions.
Dr Zecca requested OMS721 as compassionate-use treatment for the patient, and Omeros complied.
Two months after starting OMS721, the patient was able to discontinue hemodialysis and decrease her platelet transfusion requirements. She did not experience any adverse events related to OMS721.
Recently, the patient’s dose was tapered, but she developed a viral infection that reactivated her TA-TMA.
A return to the original dose of OMS721 was successful. Now, the patient no longer requires dialysis or transfusions.
“This patient had severe TMA that I believe would have caused her death,” Dr Zecca said. “Her positive response to OMS721 treatment, both initially and following her virus-induced relapse during tapering, was impressive.”
“The results of OMS721 treatment in this challenge-rechallenge scenario underscore the important effects of the drug. Since the poster was produced, her TMA has remained in remission, and we have been able to discontinue her platelet transfusions. Her rapid response has been heartening, and we all are grateful for this remarkable outcome.” ![]()
MARSEILLE—An investigational drug has successfully treated a severe case of transplant-associated thrombotic microangiopathy (TA-TMA), according to a presentation at the 43rd Annual Meeting of the European Society for Blood and Marrow Transplantation.
The drug is OMS721, a monoclonal antibody targeting mannan-binding lectin-associated serine protease-2 (MASP-2), the effector enzyme of the lectin pathway of the complement system.
The patient received OMS721, which is being developed by Omeros Corporation, under a compassionate-use protocol.
Marco Zecca, MD, of the Fondazione IRCCS Policlinico San Matteo in Italy, and his colleagues provided details on this patient in a poster presented at the meeting (Physician Poster Abstracts-Day 1, abstract A437).
The female patient had undergone a hematopoietic stem cell transplant to treat Diamond‐Blackfan anemia. At age 14, she received a transplant from an HLA-compatible, unrelated donor.
Seven months later, she was diagnosed with TA-TMA. The patient was initially treated with eculizumab but had to stop taking the drug after she developed acute pulmonary edema.
She was then treated with plasma exchange but experienced a TA-TMA relapse at 11 months. The patient was again treated with eculizumab and again had to discontinue the drug after developing acute pulmonary edema.
The patient’s condition continued to worsen, and she soon required hemodialysis 3 times a week as well as daily platelet transfusions.
Dr Zecca requested OMS721 as compassionate-use treatment for the patient, and Omeros complied.
Two months after starting OMS721, the patient was able to discontinue hemodialysis and decrease her platelet transfusion requirements. She did not experience any adverse events related to OMS721.
Recently, the patient’s dose was tapered, but she developed a viral infection that reactivated her TA-TMA.
A return to the original dose of OMS721 was successful. Now, the patient no longer requires dialysis or transfusions.
“This patient had severe TMA that I believe would have caused her death,” Dr Zecca said. “Her positive response to OMS721 treatment, both initially and following her virus-induced relapse during tapering, was impressive.”
“The results of OMS721 treatment in this challenge-rechallenge scenario underscore the important effects of the drug. Since the poster was produced, her TMA has remained in remission, and we have been able to discontinue her platelet transfusions. Her rapid response has been heartening, and we all are grateful for this remarkable outcome.” ![]()
MARSEILLE—An investigational drug has successfully treated a severe case of transplant-associated thrombotic microangiopathy (TA-TMA), according to a presentation at the 43rd Annual Meeting of the European Society for Blood and Marrow Transplantation.
The drug is OMS721, a monoclonal antibody targeting mannan-binding lectin-associated serine protease-2 (MASP-2), the effector enzyme of the lectin pathway of the complement system.
The patient received OMS721, which is being developed by Omeros Corporation, under a compassionate-use protocol.
Marco Zecca, MD, of the Fondazione IRCCS Policlinico San Matteo in Italy, and his colleagues provided details on this patient in a poster presented at the meeting (Physician Poster Abstracts-Day 1, abstract A437).
The female patient had undergone a hematopoietic stem cell transplant to treat Diamond‐Blackfan anemia. At age 14, she received a transplant from an HLA-compatible, unrelated donor.
Seven months later, she was diagnosed with TA-TMA. The patient was initially treated with eculizumab but had to stop taking the drug after she developed acute pulmonary edema.
She was then treated with plasma exchange but experienced a TA-TMA relapse at 11 months. The patient was again treated with eculizumab and again had to discontinue the drug after developing acute pulmonary edema.
The patient’s condition continued to worsen, and she soon required hemodialysis 3 times a week as well as daily platelet transfusions.
Dr Zecca requested OMS721 as compassionate-use treatment for the patient, and Omeros complied.
Two months after starting OMS721, the patient was able to discontinue hemodialysis and decrease her platelet transfusion requirements. She did not experience any adverse events related to OMS721.
Recently, the patient’s dose was tapered, but she developed a viral infection that reactivated her TA-TMA.
A return to the original dose of OMS721 was successful. Now, the patient no longer requires dialysis or transfusions.
“This patient had severe TMA that I believe would have caused her death,” Dr Zecca said. “Her positive response to OMS721 treatment, both initially and following her virus-induced relapse during tapering, was impressive.”
“The results of OMS721 treatment in this challenge-rechallenge scenario underscore the important effects of the drug. Since the poster was produced, her TMA has remained in remission, and we have been able to discontinue her platelet transfusions. Her rapid response has been heartening, and we all are grateful for this remarkable outcome.” ![]()
NCCN launches radiation therapy resource
The National Comprehensive Cancer Network® (NCCN®) recently launched the NCCN Radiation Therapy Compendium™, which provides a single access point for NCCN recommendations pertaining to radiation therapy.
The compendium provides guidance on all radiation therapy modalities recommended within NCCN guidelines, including intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative radiotherapy, image-guided radiotherapy, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
“As a single source for all radiation therapy recommendations within the NCCN guidelines, the compendium benefits patients with cancer by assisting providers and payers in making evidence-based treatment and coverage decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
The NCCN Radiation Therapy Compendium™ includes recommendations for the following 24 cancer types:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
NCCN said additional cancer types will be published on a rolling basis over the coming months. ![]()
The National Comprehensive Cancer Network® (NCCN®) recently launched the NCCN Radiation Therapy Compendium™, which provides a single access point for NCCN recommendations pertaining to radiation therapy.
The compendium provides guidance on all radiation therapy modalities recommended within NCCN guidelines, including intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative radiotherapy, image-guided radiotherapy, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
“As a single source for all radiation therapy recommendations within the NCCN guidelines, the compendium benefits patients with cancer by assisting providers and payers in making evidence-based treatment and coverage decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
The NCCN Radiation Therapy Compendium™ includes recommendations for the following 24 cancer types:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
NCCN said additional cancer types will be published on a rolling basis over the coming months. ![]()
The National Comprehensive Cancer Network® (NCCN®) recently launched the NCCN Radiation Therapy Compendium™, which provides a single access point for NCCN recommendations pertaining to radiation therapy.
The compendium provides guidance on all radiation therapy modalities recommended within NCCN guidelines, including intensity modulated radiation therapy, intra-operative radiation therapy, stereotactic radiosurgery/stereotactic body radiotherapy/stereotactic ablative radiotherapy, image-guided radiotherapy, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
“As a single source for all radiation therapy recommendations within the NCCN guidelines, the compendium benefits patients with cancer by assisting providers and payers in making evidence-based treatment and coverage decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
The NCCN Radiation Therapy Compendium™ includes recommendations for the following 24 cancer types:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
NCCN said additional cancer types will be published on a rolling basis over the coming months. ![]()
Drug receives orphan designation for AML
The US Food and Drug Administration (FDA) has granted annamycin orphan designation for the treatment of acute myeloid leukemia (AML).
Annamycin is a liposomal anthracycline under development by Moleculin Biotech, Inc.
The company said it is working with the FDA on an investigative new drug application for a phase 1/2 trial of annamycin in patients with relapsed or refractory AML.
Annamycin has already been tested in 6 clinical trials, 3 of which focused on leukemia.
Results from one of these trials, in adults with relapsed/refractory acute lymphoblastic leukemia, were published in Clinical Lymphoma, Myeloma & Leukemia in 2013.
Annamycin has been under development by several other pharmaceutical companies. Moleculin Biotech, Inc. acquired rights and development assets relating to the drug in 2015.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted annamycin orphan designation for the treatment of acute myeloid leukemia (AML).
Annamycin is a liposomal anthracycline under development by Moleculin Biotech, Inc.
The company said it is working with the FDA on an investigative new drug application for a phase 1/2 trial of annamycin in patients with relapsed or refractory AML.
Annamycin has already been tested in 6 clinical trials, 3 of which focused on leukemia.
Results from one of these trials, in adults with relapsed/refractory acute lymphoblastic leukemia, were published in Clinical Lymphoma, Myeloma & Leukemia in 2013.
Annamycin has been under development by several other pharmaceutical companies. Moleculin Biotech, Inc. acquired rights and development assets relating to the drug in 2015.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted annamycin orphan designation for the treatment of acute myeloid leukemia (AML).
Annamycin is a liposomal anthracycline under development by Moleculin Biotech, Inc.
The company said it is working with the FDA on an investigative new drug application for a phase 1/2 trial of annamycin in patients with relapsed or refractory AML.
Annamycin has already been tested in 6 clinical trials, 3 of which focused on leukemia.
Results from one of these trials, in adults with relapsed/refractory acute lymphoblastic leukemia, were published in Clinical Lymphoma, Myeloma & Leukemia in 2013.
Annamycin has been under development by several other pharmaceutical companies. Moleculin Biotech, Inc. acquired rights and development assets relating to the drug in 2015.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent rare diseases/disorders affecting fewer than 200,000 people in the US.
Orphan designation provides companies with certain incentives to develop products for rare diseases. This includes a 50% tax break on research and development, a fee waiver, access to federal grants, and 7 years of market exclusivity if the product is approved. ![]()
Team devises new way to manufacture RBCs
Researchers say they have generated the first human immortalized adult erythroid line that provides a sustainable supply of erythroid cells.
The team says this cell line, known as Bristol Erythroid Line Adult (BEL-A), is the first erythroid line to fully recapitulate normal erythropoiesis.
It produced mature reticulocytes that proved functionally and molecularly similar to adult reticulocytes cultured in vitro.
In addition, in vivo survival rates of BEL-A reticulocytes were similar to the survival rates of red blood cells (RBCs) from adult donors.
The researchers described their generation and testing of the BEL-A line in Nature Communications.
“Previous approaches to producing red blood cells have relied on various sources of stem cells, which can only presently produce very limited quantities,” said study author Jan Frayne, PhD, of the University of Bristol in the UK.
“By taking an alternative approach, we have generated the first human immortalized adult erythroid line (Bristol Erythroid Line Adult or BEL-A), and in doing so, have demonstrated a feasible way to sustainably manufacture red cells for clinical use from in vitro culture.”
Dr Frayne and her colleagues began with CD34+ cells from adult bone marrow. The researchers transduced these cells with an HPV16-E6/E7 construct and maintained them in the primary medium of the team’s erythroid culture system for 4 days.
On day 5, the researchers transferred the cells to an expansion medium containing doxycycline. After 190 days of continuous proliferation, the cells were frozen for storage.
Some cells were frozen throughout this time period as well, and all of the samples re-established efficiently in culture after thawing.
The researchers said these immortalized BEL-A cells were pro- to early basophilic erythroblasts, and there was no change in morphology over time.
After 100 days in continuous culture, the researchers transferred BEL-A cells to the primary erythroid culture medium containing doxycycline. The cells remained there for 6 days and were then moved to a medium without doxycycline to induce differentiation to mature erythroblasts and reticulocytes.
The researchers said the BEL-A cells exhibited an “unchanged ability to differentiate,” and were consistently able to expand.
The team also noted that BEL-A reticulocytes were similar to normal adult reticulocytes. The cells had similar diameters, comparable deformability indexes, and they bound and released oxygen in the same way.
The researchers tested the in vivo survival of BEL-A reticulocytes as well. They transfused BEL-A reticulocytes and donor RBCs into macrophage-depleted NSG mice. There was no difference in survival between the 2 cell types.
Dr Frayne and her colleagues said their results suggest immortalized erythroid lines can be used for the manufacture of RBCs for clinical use and for the study of erythropoiesis. ![]()
Researchers say they have generated the first human immortalized adult erythroid line that provides a sustainable supply of erythroid cells.
The team says this cell line, known as Bristol Erythroid Line Adult (BEL-A), is the first erythroid line to fully recapitulate normal erythropoiesis.
It produced mature reticulocytes that proved functionally and molecularly similar to adult reticulocytes cultured in vitro.
In addition, in vivo survival rates of BEL-A reticulocytes were similar to the survival rates of red blood cells (RBCs) from adult donors.
The researchers described their generation and testing of the BEL-A line in Nature Communications.
“Previous approaches to producing red blood cells have relied on various sources of stem cells, which can only presently produce very limited quantities,” said study author Jan Frayne, PhD, of the University of Bristol in the UK.
“By taking an alternative approach, we have generated the first human immortalized adult erythroid line (Bristol Erythroid Line Adult or BEL-A), and in doing so, have demonstrated a feasible way to sustainably manufacture red cells for clinical use from in vitro culture.”
Dr Frayne and her colleagues began with CD34+ cells from adult bone marrow. The researchers transduced these cells with an HPV16-E6/E7 construct and maintained them in the primary medium of the team’s erythroid culture system for 4 days.
On day 5, the researchers transferred the cells to an expansion medium containing doxycycline. After 190 days of continuous proliferation, the cells were frozen for storage.
Some cells were frozen throughout this time period as well, and all of the samples re-established efficiently in culture after thawing.
The researchers said these immortalized BEL-A cells were pro- to early basophilic erythroblasts, and there was no change in morphology over time.
After 100 days in continuous culture, the researchers transferred BEL-A cells to the primary erythroid culture medium containing doxycycline. The cells remained there for 6 days and were then moved to a medium without doxycycline to induce differentiation to mature erythroblasts and reticulocytes.
The researchers said the BEL-A cells exhibited an “unchanged ability to differentiate,” and were consistently able to expand.
The team also noted that BEL-A reticulocytes were similar to normal adult reticulocytes. The cells had similar diameters, comparable deformability indexes, and they bound and released oxygen in the same way.
The researchers tested the in vivo survival of BEL-A reticulocytes as well. They transfused BEL-A reticulocytes and donor RBCs into macrophage-depleted NSG mice. There was no difference in survival between the 2 cell types.
Dr Frayne and her colleagues said their results suggest immortalized erythroid lines can be used for the manufacture of RBCs for clinical use and for the study of erythropoiesis. ![]()
Researchers say they have generated the first human immortalized adult erythroid line that provides a sustainable supply of erythroid cells.
The team says this cell line, known as Bristol Erythroid Line Adult (BEL-A), is the first erythroid line to fully recapitulate normal erythropoiesis.
It produced mature reticulocytes that proved functionally and molecularly similar to adult reticulocytes cultured in vitro.
In addition, in vivo survival rates of BEL-A reticulocytes were similar to the survival rates of red blood cells (RBCs) from adult donors.
The researchers described their generation and testing of the BEL-A line in Nature Communications.
“Previous approaches to producing red blood cells have relied on various sources of stem cells, which can only presently produce very limited quantities,” said study author Jan Frayne, PhD, of the University of Bristol in the UK.
“By taking an alternative approach, we have generated the first human immortalized adult erythroid line (Bristol Erythroid Line Adult or BEL-A), and in doing so, have demonstrated a feasible way to sustainably manufacture red cells for clinical use from in vitro culture.”
Dr Frayne and her colleagues began with CD34+ cells from adult bone marrow. The researchers transduced these cells with an HPV16-E6/E7 construct and maintained them in the primary medium of the team’s erythroid culture system for 4 days.
On day 5, the researchers transferred the cells to an expansion medium containing doxycycline. After 190 days of continuous proliferation, the cells were frozen for storage.
Some cells were frozen throughout this time period as well, and all of the samples re-established efficiently in culture after thawing.
The researchers said these immortalized BEL-A cells were pro- to early basophilic erythroblasts, and there was no change in morphology over time.
After 100 days in continuous culture, the researchers transferred BEL-A cells to the primary erythroid culture medium containing doxycycline. The cells remained there for 6 days and were then moved to a medium without doxycycline to induce differentiation to mature erythroblasts and reticulocytes.
The researchers said the BEL-A cells exhibited an “unchanged ability to differentiate,” and were consistently able to expand.
The team also noted that BEL-A reticulocytes were similar to normal adult reticulocytes. The cells had similar diameters, comparable deformability indexes, and they bound and released oxygen in the same way.
The researchers tested the in vivo survival of BEL-A reticulocytes as well. They transfused BEL-A reticulocytes and donor RBCs into macrophage-depleted NSG mice. There was no difference in survival between the 2 cell types.
Dr Frayne and her colleagues said their results suggest immortalized erythroid lines can be used for the manufacture of RBCs for clinical use and for the study of erythropoiesis. ![]()
Protein may prevent transformation from MDS to AML
The protein p300 may prevent the transformation from myelodysplastic syndromes (MDS) to acute myeloid leukemia (AML), according to research published in Leukemia.
Researchers found that loss of p300 “markedly” increased leukemogenesis in a mouse model of MDS.
“The loss of p300 allows these defective [MDS] cells to grow and become leukemic,” said study author Stephen Nimer, MD, of Sylvester Comprehensive Cancer Center in Miami, Florida.
“This work offers us a window into AML, which we are now going to try to exploit.”
Previous research suggested that p300 and CBP (both histone lysine acetyltransferases) may be tumor suppressors. The current study indicates that, in the context of MDS, that is only true for p300.
The researchers evaluated the effects of deleting both p300 and CBP in Nup98-HoxD13 (NHD13) transgenic mice, a model of human MDS.
The team found that p300 deletion, but not CBP deletion, accelerated leukemogenesis in the mice.
“When we eliminated p300, 100% of the mice developed leukemia,” Dr Nimer said. “It indicated that, under this specific circumstance, p300 is a tumor suppressor, offering great insight into how MDS converts to leukemia. It was quite surprising that CBP plays no role at all.”
The researchers also found that deleting p300 restored the ability of NHD13-expressing hematopoietic stem and progenitor cells (HSPCs) to self-renew, and p300 deletion decreased apoptosis.
“While investigating how p300 functions in MDS cells, we found that MDS cells do not grow well in the lab,” Dr Nimer said. “However, when you eliminate p300, suddenly, the cells continue to grow.”
On the other hand, deletion of p300 did not have a significant effect on wild-type hematopoiesis.
Finally, the researchers found that p300 deletion enhanced cytokine signaling in NHD13-expressing HSPCs. They observed enhanced activation of the MAPK and JAK/STAT pathways in HSPCs isolated from NHD13 transgenic mice.
The team said more research is needed to understand exactly how p300 controls MDS cells, but these findings could ultimately help MDS patients avoid AML.
“Other than chemotherapy, right now, there’s no way to prevent MDS from developing into myeloid leukemia,” Dr Nimer said. “However, drugs are being developed that can promote p300 function and possibly prevent MDS patients from developing leukemia.” ![]()
The protein p300 may prevent the transformation from myelodysplastic syndromes (MDS) to acute myeloid leukemia (AML), according to research published in Leukemia.
Researchers found that loss of p300 “markedly” increased leukemogenesis in a mouse model of MDS.
“The loss of p300 allows these defective [MDS] cells to grow and become leukemic,” said study author Stephen Nimer, MD, of Sylvester Comprehensive Cancer Center in Miami, Florida.
“This work offers us a window into AML, which we are now going to try to exploit.”
Previous research suggested that p300 and CBP (both histone lysine acetyltransferases) may be tumor suppressors. The current study indicates that, in the context of MDS, that is only true for p300.
The researchers evaluated the effects of deleting both p300 and CBP in Nup98-HoxD13 (NHD13) transgenic mice, a model of human MDS.
The team found that p300 deletion, but not CBP deletion, accelerated leukemogenesis in the mice.
“When we eliminated p300, 100% of the mice developed leukemia,” Dr Nimer said. “It indicated that, under this specific circumstance, p300 is a tumor suppressor, offering great insight into how MDS converts to leukemia. It was quite surprising that CBP plays no role at all.”
The researchers also found that deleting p300 restored the ability of NHD13-expressing hematopoietic stem and progenitor cells (HSPCs) to self-renew, and p300 deletion decreased apoptosis.
“While investigating how p300 functions in MDS cells, we found that MDS cells do not grow well in the lab,” Dr Nimer said. “However, when you eliminate p300, suddenly, the cells continue to grow.”
On the other hand, deletion of p300 did not have a significant effect on wild-type hematopoiesis.
Finally, the researchers found that p300 deletion enhanced cytokine signaling in NHD13-expressing HSPCs. They observed enhanced activation of the MAPK and JAK/STAT pathways in HSPCs isolated from NHD13 transgenic mice.
The team said more research is needed to understand exactly how p300 controls MDS cells, but these findings could ultimately help MDS patients avoid AML.
“Other than chemotherapy, right now, there’s no way to prevent MDS from developing into myeloid leukemia,” Dr Nimer said. “However, drugs are being developed that can promote p300 function and possibly prevent MDS patients from developing leukemia.” ![]()
The protein p300 may prevent the transformation from myelodysplastic syndromes (MDS) to acute myeloid leukemia (AML), according to research published in Leukemia.
Researchers found that loss of p300 “markedly” increased leukemogenesis in a mouse model of MDS.
“The loss of p300 allows these defective [MDS] cells to grow and become leukemic,” said study author Stephen Nimer, MD, of Sylvester Comprehensive Cancer Center in Miami, Florida.
“This work offers us a window into AML, which we are now going to try to exploit.”
Previous research suggested that p300 and CBP (both histone lysine acetyltransferases) may be tumor suppressors. The current study indicates that, in the context of MDS, that is only true for p300.
The researchers evaluated the effects of deleting both p300 and CBP in Nup98-HoxD13 (NHD13) transgenic mice, a model of human MDS.
The team found that p300 deletion, but not CBP deletion, accelerated leukemogenesis in the mice.
“When we eliminated p300, 100% of the mice developed leukemia,” Dr Nimer said. “It indicated that, under this specific circumstance, p300 is a tumor suppressor, offering great insight into how MDS converts to leukemia. It was quite surprising that CBP plays no role at all.”
The researchers also found that deleting p300 restored the ability of NHD13-expressing hematopoietic stem and progenitor cells (HSPCs) to self-renew, and p300 deletion decreased apoptosis.
“While investigating how p300 functions in MDS cells, we found that MDS cells do not grow well in the lab,” Dr Nimer said. “However, when you eliminate p300, suddenly, the cells continue to grow.”
On the other hand, deletion of p300 did not have a significant effect on wild-type hematopoiesis.
Finally, the researchers found that p300 deletion enhanced cytokine signaling in NHD13-expressing HSPCs. They observed enhanced activation of the MAPK and JAK/STAT pathways in HSPCs isolated from NHD13 transgenic mice.
The team said more research is needed to understand exactly how p300 controls MDS cells, but these findings could ultimately help MDS patients avoid AML.
“Other than chemotherapy, right now, there’s no way to prevent MDS from developing into myeloid leukemia,” Dr Nimer said. “However, drugs are being developed that can promote p300 function and possibly prevent MDS patients from developing leukemia.” ![]()
Model could advance fight against malaria
A new mathematical model could aid the development of drugs that target malaria parasites’ metabolic processes, according to researchers.
The team said they developed the first model of a malaria parasite that accurately integrates its genetics and metabolism.
They described this model in PLOS Computational Biology.
“The model integrates all available knowledge on the genetics and metabolism of the parasites and allows the formulation of testable hypotheses behind the parasites’ essential functions,” said study author Vassily Hatzimanikatis, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland.
“Ultimately, it can accelerate the discovery toward novel antimalarial drug targets.”
To create the model, Dr Hatzimanikatis and his colleagues studied Plasmodium falciparum parasites, focusing on the way they produce and use energy for their metabolic reactions.
This revealed which metabolic functions were essential at each stage of infection and which were energetically coupled through key metabolites.
The team was therefore able to model the bioenergetics of the metabolism of P falciparum, predicting which genes are indispensable for every biological function in the parasite.
By integrating metabolomics and genetics data, the model revealed the complex interactions between gene products, reactions, and metabolites in the parasite, and it revealed potential mechanisms to target with drugs.
“The design of efficient antimalarial drugs that target the parasite’s and not the patient’s metabolism requires an in-depth understanding of the mechanisms that make a particular enzyme essential,” said Anush Chiappino-Pepe, a PhD student at École Polytechnique Fédérale de Lausanne.
“So mathematical modeling of the parasite’s metabolism becomes a very powerful tool.”
The researchers said they will continue to calibrate and improve the predictive capabilities of their model with additional genetics and metabolomics data.
They hope to reveal the mechanisms behind host-pathogen interactions and gain insight into the physiology of P falciparum while it is dormant. ![]()
A new mathematical model could aid the development of drugs that target malaria parasites’ metabolic processes, according to researchers.
The team said they developed the first model of a malaria parasite that accurately integrates its genetics and metabolism.
They described this model in PLOS Computational Biology.
“The model integrates all available knowledge on the genetics and metabolism of the parasites and allows the formulation of testable hypotheses behind the parasites’ essential functions,” said study author Vassily Hatzimanikatis, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland.
“Ultimately, it can accelerate the discovery toward novel antimalarial drug targets.”
To create the model, Dr Hatzimanikatis and his colleagues studied Plasmodium falciparum parasites, focusing on the way they produce and use energy for their metabolic reactions.
This revealed which metabolic functions were essential at each stage of infection and which were energetically coupled through key metabolites.
The team was therefore able to model the bioenergetics of the metabolism of P falciparum, predicting which genes are indispensable for every biological function in the parasite.
By integrating metabolomics and genetics data, the model revealed the complex interactions between gene products, reactions, and metabolites in the parasite, and it revealed potential mechanisms to target with drugs.
“The design of efficient antimalarial drugs that target the parasite’s and not the patient’s metabolism requires an in-depth understanding of the mechanisms that make a particular enzyme essential,” said Anush Chiappino-Pepe, a PhD student at École Polytechnique Fédérale de Lausanne.
“So mathematical modeling of the parasite’s metabolism becomes a very powerful tool.”
The researchers said they will continue to calibrate and improve the predictive capabilities of their model with additional genetics and metabolomics data.
They hope to reveal the mechanisms behind host-pathogen interactions and gain insight into the physiology of P falciparum while it is dormant. ![]()
A new mathematical model could aid the development of drugs that target malaria parasites’ metabolic processes, according to researchers.
The team said they developed the first model of a malaria parasite that accurately integrates its genetics and metabolism.
They described this model in PLOS Computational Biology.
“The model integrates all available knowledge on the genetics and metabolism of the parasites and allows the formulation of testable hypotheses behind the parasites’ essential functions,” said study author Vassily Hatzimanikatis, PhD, of École Polytechnique Fédérale de Lausanne in Switzerland.
“Ultimately, it can accelerate the discovery toward novel antimalarial drug targets.”
To create the model, Dr Hatzimanikatis and his colleagues studied Plasmodium falciparum parasites, focusing on the way they produce and use energy for their metabolic reactions.
This revealed which metabolic functions were essential at each stage of infection and which were energetically coupled through key metabolites.
The team was therefore able to model the bioenergetics of the metabolism of P falciparum, predicting which genes are indispensable for every biological function in the parasite.
By integrating metabolomics and genetics data, the model revealed the complex interactions between gene products, reactions, and metabolites in the parasite, and it revealed potential mechanisms to target with drugs.
“The design of efficient antimalarial drugs that target the parasite’s and not the patient’s metabolism requires an in-depth understanding of the mechanisms that make a particular enzyme essential,” said Anush Chiappino-Pepe, a PhD student at École Polytechnique Fédérale de Lausanne.
“So mathematical modeling of the parasite’s metabolism becomes a very powerful tool.”
The researchers said they will continue to calibrate and improve the predictive capabilities of their model with additional genetics and metabolomics data.
They hope to reveal the mechanisms behind host-pathogen interactions and gain insight into the physiology of P falciparum while it is dormant.
Lower risk of heart attack with VKAs than with DOACs, aspirin
A large, retrospective study suggests patients with atrial fibrillation may have a lower risk of acute myocardial infarction (AMI) if they receive vitamin K antagonists (VKAs) rather than other anticoagulants.
Investigators found that patients taking direct oral anticoagulants (DOACs, rivaroxaban or dabigatran) had more than twice the AMI risk of patients taking VKAs.
And the AMI risk for patients taking low-dose aspirin was nearly double that of those taking VKAs.
Leo M. Stolk, PharmD, PhD, of Maastricht University Medical Centre in the Netherlands, and his colleagues reported these results in the British Journal of Clinical Pharmacology.
The investigators analyzed data on 30,146 adults with atrial fibrillation who were new users of DOACs (n=1266), VKAs (n=13,098), low-dose aspirin (n=15,400), or mixed anticoagulants (n=382). Most DOAC users were taking rivaroxaban (71.6%), but some were taking dabigatran (28.4%).
The mean follow-up was 0.95 years for DOAC users, 2.72 years for VKA users, 2.86 years for low-dose aspirin users, and 2.99 years for mixed medication users.
The investigators estimated the hazard ratio (HR) of AMI for users of DOACs, aspirin, or mixed medications versus VKAs. The team adjusted their analysis for age, sex, lifestyle, risk factors, comorbidities, and use of other medications.
Compared to VKA users, the risk of AMI was significantly higher for DOAC users (HR=2.11; 95% CI 1.08 – 4.12, P<0.05) and aspirin users (HR=1.91; 95% CI 1.45-2.51, P<0.05). The risk was not significantly higher for mixed medication users (HR=1.69; 95% CI 0.69, 4.16).
When patients were stratified by gender, there was a significantly increased risk of AMI for aspirin users who were male (HR=1.60; 95% CI 1.10, 2.33, P<0.05) or female (HR=2.33; 95% CI 1.55, 3.50, P<0.05), when compared to VKA users. Neither DOACs nor mixed medications were associated with a significantly increased risk of AMI in this analysis.
The investigators also stratified patients according to their CHA2DS2-VASc score at the index date.
Among patients with a high score (≥4), there was a significantly increased risk of AMI for aspirin users compared to VKA users (HR=2.21; 95% CI 1.37,3.55, P<0.05).
Among patients with a medium score (>1 and <4), the risk of AMI was significantly higher for DOAC users (HR=2.67; 95% CI 1.11, 6.40, P<0.05) and aspirin users (HR=1.82; 95% CI 1.23, 2.68, P<0.05) compared to VKA users.
The investigators said this study suggests VKAs probably have greater beneficial effects on AMI than DOACs, and ongoing research is needed as the use of DOACs increases.
A large, retrospective study suggests patients with atrial fibrillation may have a lower risk of acute myocardial infarction (AMI) if they receive vitamin K antagonists (VKAs) rather than other anticoagulants.
Investigators found that patients taking direct oral anticoagulants (DOACs, rivaroxaban or dabigatran) had more than twice the AMI risk of patients taking VKAs.
And the AMI risk for patients taking low-dose aspirin was nearly double that of those taking VKAs.
Leo M. Stolk, PharmD, PhD, of Maastricht University Medical Centre in the Netherlands, and his colleagues reported these results in the British Journal of Clinical Pharmacology.
The investigators analyzed data on 30,146 adults with atrial fibrillation who were new users of DOACs (n=1266), VKAs (n=13,098), low-dose aspirin (n=15,400), or mixed anticoagulants (n=382). Most DOAC users were taking rivaroxaban (71.6%), but some were taking dabigatran (28.4%).
The mean follow-up was 0.95 years for DOAC users, 2.72 years for VKA users, 2.86 years for low-dose aspirin users, and 2.99 years for mixed medication users.
The investigators estimated the hazard ratio (HR) of AMI for users of DOACs, aspirin, or mixed medications versus VKAs. The team adjusted their analysis for age, sex, lifestyle, risk factors, comorbidities, and use of other medications.
Compared to VKA users, the risk of AMI was significantly higher for DOAC users (HR=2.11; 95% CI 1.08 – 4.12, P<0.05) and aspirin users (HR=1.91; 95% CI 1.45-2.51, P<0.05). The risk was not significantly higher for mixed medication users (HR=1.69; 95% CI 0.69, 4.16).
When patients were stratified by gender, there was a significantly increased risk of AMI for aspirin users who were male (HR=1.60; 95% CI 1.10, 2.33, P<0.05) or female (HR=2.33; 95% CI 1.55, 3.50, P<0.05), when compared to VKA users. Neither DOACs nor mixed medications were associated with a significantly increased risk of AMI in this analysis.
The investigators also stratified patients according to their CHA2DS2-VASc score at the index date.
Among patients with a high score (≥4), there was a significantly increased risk of AMI for aspirin users compared to VKA users (HR=2.21; 95% CI 1.37,3.55, P<0.05).
Among patients with a medium score (>1 and <4), the risk of AMI was significantly higher for DOAC users (HR=2.67; 95% CI 1.11, 6.40, P<0.05) and aspirin users (HR=1.82; 95% CI 1.23, 2.68, P<0.05) compared to VKA users.
The investigators said this study suggests VKAs probably have greater beneficial effects on AMI than DOACs, and ongoing research is needed as the use of DOACs increases.
A large, retrospective study suggests patients with atrial fibrillation may have a lower risk of acute myocardial infarction (AMI) if they receive vitamin K antagonists (VKAs) rather than other anticoagulants.
Investigators found that patients taking direct oral anticoagulants (DOACs, rivaroxaban or dabigatran) had more than twice the AMI risk of patients taking VKAs.
And the AMI risk for patients taking low-dose aspirin was nearly double that of those taking VKAs.
Leo M. Stolk, PharmD, PhD, of Maastricht University Medical Centre in the Netherlands, and his colleagues reported these results in the British Journal of Clinical Pharmacology.
The investigators analyzed data on 30,146 adults with atrial fibrillation who were new users of DOACs (n=1266), VKAs (n=13,098), low-dose aspirin (n=15,400), or mixed anticoagulants (n=382). Most DOAC users were taking rivaroxaban (71.6%), but some were taking dabigatran (28.4%).
The mean follow-up was 0.95 years for DOAC users, 2.72 years for VKA users, 2.86 years for low-dose aspirin users, and 2.99 years for mixed medication users.
The investigators estimated the hazard ratio (HR) of AMI for users of DOACs, aspirin, or mixed medications versus VKAs. The team adjusted their analysis for age, sex, lifestyle, risk factors, comorbidities, and use of other medications.
Compared to VKA users, the risk of AMI was significantly higher for DOAC users (HR=2.11; 95% CI 1.08 – 4.12, P<0.05) and aspirin users (HR=1.91; 95% CI 1.45-2.51, P<0.05). The risk was not significantly higher for mixed medication users (HR=1.69; 95% CI 0.69, 4.16).
When patients were stratified by gender, there was a significantly increased risk of AMI for aspirin users who were male (HR=1.60; 95% CI 1.10, 2.33, P<0.05) or female (HR=2.33; 95% CI 1.55, 3.50, P<0.05), when compared to VKA users. Neither DOACs nor mixed medications were associated with a significantly increased risk of AMI in this analysis.
The investigators also stratified patients according to their CHA2DS2-VASc score at the index date.
Among patients with a high score (≥4), there was a significantly increased risk of AMI for aspirin users compared to VKA users (HR=2.21; 95% CI 1.37,3.55, P<0.05).
Among patients with a medium score (>1 and <4), the risk of AMI was significantly higher for DOAC users (HR=2.67; 95% CI 1.11, 6.40, P<0.05) and aspirin users (HR=1.82; 95% CI 1.23, 2.68, P<0.05) compared to VKA users.
The investigators said this study suggests VKAs probably have greater beneficial effects on AMI than DOACs, and ongoing research is needed as the use of DOACs increases.
Company again withdraws application for pacritinib
CTI BioPharma has withdrawn its application for marketing authorization of pacritinib (Enpaxiq) in the European Union, according to the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP).
The company was seeking approval for pacritinib, a JAK2/FLT3 inhibitor, to treat splenomegaly or symptoms of myelofibrosis (MF) in adults with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
When the application was withdrawn, the CHMP was of the provisional opinion that pacritinib could not have been approved for this indication.
Last year, CTI BioPharma withdrew its application for approval of pacritinib in the US.
Issues preventing approval
The CHMP said it had a number of concerns related to the PERSIST-1 trial, which was used to support the application for approval in the European Union. In this trial, researchers compared pacritinib to best available therapy, excluding JAK inhibitors, in patients with MF.
The CHMP said the reduction in spleen size, which was the main efficacy outcome in the study, appeared to be lower with pacritinib than with another medicine of its class, with no improvement in symptom scores.
In addition, the incidence of thrombocytopenia was higher in patients treated with pacritinib.
And more deaths occurred in patients taking pacritinib than in those receiving best available therapy, including deaths due to bleeding and adverse effects on the heart.
The CHMP also said it needs more information about the starting materials used in the manufacture of pacritinib and how the drug acts on target proteins.
Given these concerns, the CHMP was of the opinion that pacritinib’s benefits had not been shown to outweigh its risks.
CTI BioPharma said it could address the CHMP’s concerns by providing data from a second study of pacritinib, PERSIST-2.
However, there was not enough time in the current application procedure to provide the data, so the company decided to withdraw the application.
CTI BioPharma said it intends to integrate data from PERSIST-2 into its current dossier before approaching the European Medicines Agency to discuss a new application.
The company also said the withdrawal of its application will not affect patients currently enrolled in clinical trials of pacritinib or compassionate use programs for the drug.
Previous withdrawal, clinical hold
CTI BioPharma withdrew its application for approval of pacritinib in the US after the US Food and Drug Administration (FDA) placed a full clinical hold on trials of the drug.
The FDA placed the hold on pacritinib trials in February 2016 after results from PERSIST-1 and PERSIST-2 showed excess mortality in patients who received pacritinib.
The FDA lifted the hold in January 2017 after CTI BioPharma agreed to conduct dose-exploration studies for pacritinib, submit final study reports and data sets for PERSIST-1 and PERSIST-2, and make modifications to protocols and study-related documents.
CTI BioPharma has withdrawn its application for marketing authorization of pacritinib (Enpaxiq) in the European Union, according to the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP).
The company was seeking approval for pacritinib, a JAK2/FLT3 inhibitor, to treat splenomegaly or symptoms of myelofibrosis (MF) in adults with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
When the application was withdrawn, the CHMP was of the provisional opinion that pacritinib could not have been approved for this indication.
Last year, CTI BioPharma withdrew its application for approval of pacritinib in the US.
Issues preventing approval
The CHMP said it had a number of concerns related to the PERSIST-1 trial, which was used to support the application for approval in the European Union. In this trial, researchers compared pacritinib to best available therapy, excluding JAK inhibitors, in patients with MF.
The CHMP said the reduction in spleen size, which was the main efficacy outcome in the study, appeared to be lower with pacritinib than with another medicine of its class, with no improvement in symptom scores.
In addition, the incidence of thrombocytopenia was higher in patients treated with pacritinib.
And more deaths occurred in patients taking pacritinib than in those receiving best available therapy, including deaths due to bleeding and adverse effects on the heart.
The CHMP also said it needs more information about the starting materials used in the manufacture of pacritinib and how the drug acts on target proteins.
Given these concerns, the CHMP was of the opinion that pacritinib’s benefits had not been shown to outweigh its risks.
CTI BioPharma said it could address the CHMP’s concerns by providing data from a second study of pacritinib, PERSIST-2.
However, there was not enough time in the current application procedure to provide the data, so the company decided to withdraw the application.
CTI BioPharma said it intends to integrate data from PERSIST-2 into its current dossier before approaching the European Medicines Agency to discuss a new application.
The company also said the withdrawal of its application will not affect patients currently enrolled in clinical trials of pacritinib or compassionate use programs for the drug.
Previous withdrawal, clinical hold
CTI BioPharma withdrew its application for approval of pacritinib in the US after the US Food and Drug Administration (FDA) placed a full clinical hold on trials of the drug.
The FDA placed the hold on pacritinib trials in February 2016 after results from PERSIST-1 and PERSIST-2 showed excess mortality in patients who received pacritinib.
The FDA lifted the hold in January 2017 after CTI BioPharma agreed to conduct dose-exploration studies for pacritinib, submit final study reports and data sets for PERSIST-1 and PERSIST-2, and make modifications to protocols and study-related documents.
CTI BioPharma has withdrawn its application for marketing authorization of pacritinib (Enpaxiq) in the European Union, according to the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP).
The company was seeking approval for pacritinib, a JAK2/FLT3 inhibitor, to treat splenomegaly or symptoms of myelofibrosis (MF) in adults with primary MF, post-polycythemia vera MF, or post-essential thrombocythemia MF.
When the application was withdrawn, the CHMP was of the provisional opinion that pacritinib could not have been approved for this indication.
Last year, CTI BioPharma withdrew its application for approval of pacritinib in the US.
Issues preventing approval
The CHMP said it had a number of concerns related to the PERSIST-1 trial, which was used to support the application for approval in the European Union. In this trial, researchers compared pacritinib to best available therapy, excluding JAK inhibitors, in patients with MF.
The CHMP said the reduction in spleen size, which was the main efficacy outcome in the study, appeared to be lower with pacritinib than with another medicine of its class, with no improvement in symptom scores.
In addition, the incidence of thrombocytopenia was higher in patients treated with pacritinib.
And more deaths occurred in patients taking pacritinib than in those receiving best available therapy, including deaths due to bleeding and adverse effects on the heart.
The CHMP also said it needs more information about the starting materials used in the manufacture of pacritinib and how the drug acts on target proteins.
Given these concerns, the CHMP was of the opinion that pacritinib’s benefits had not been shown to outweigh its risks.
CTI BioPharma said it could address the CHMP’s concerns by providing data from a second study of pacritinib, PERSIST-2.
However, there was not enough time in the current application procedure to provide the data, so the company decided to withdraw the application.
CTI BioPharma said it intends to integrate data from PERSIST-2 into its current dossier before approaching the European Medicines Agency to discuss a new application.
The company also said the withdrawal of its application will not affect patients currently enrolled in clinical trials of pacritinib or compassionate use programs for the drug.
Previous withdrawal, clinical hold
CTI BioPharma withdrew its application for approval of pacritinib in the US after the US Food and Drug Administration (FDA) placed a full clinical hold on trials of the drug.
The FDA placed the hold on pacritinib trials in February 2016 after results from PERSIST-1 and PERSIST-2 showed excess mortality in patients who received pacritinib.
The FDA lifted the hold in January 2017 after CTI BioPharma agreed to conduct dose-exploration studies for pacritinib, submit final study reports and data sets for PERSIST-1 and PERSIST-2, and make modifications to protocols and study-related documents.
CHMP recommends product for hemophilia B
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for nonacog beta pegol (N9-GP, Refixia®).
N9-GP is an extended half-life factor IX molecule intended for replacement therapy in patients with hemophilia B.
The CHMP has recommended N9-GP for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
The CHMP’s opinion has been forwarded to the European Commission, which will decide whether or not to grant marketing authorization for N9-GP. The product is being developed by Novo Nordisk.
Trial results
The CHMP’s recommendation for N9-GP is based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in a pair of phase 3 trials known as paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for nonacog beta pegol (N9-GP, Refixia®).
N9-GP is an extended half-life factor IX molecule intended for replacement therapy in patients with hemophilia B.
The CHMP has recommended N9-GP for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
The CHMP’s opinion has been forwarded to the European Commission, which will decide whether or not to grant marketing authorization for N9-GP. The product is being developed by Novo Nordisk.
Trial results
The CHMP’s recommendation for N9-GP is based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in a pair of phase 3 trials known as paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined.
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended marketing authorization for nonacog beta pegol (N9-GP, Refixia®).
N9-GP is an extended half-life factor IX molecule intended for replacement therapy in patients with hemophilia B.
The CHMP has recommended N9-GP for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
The CHMP’s opinion has been forwarded to the European Commission, which will decide whether or not to grant marketing authorization for N9-GP. The product is being developed by Novo Nordisk.
Trial results
The CHMP’s recommendation for N9-GP is based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in a pair of phase 3 trials known as paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined.