User login
Visual hallucinations: Differentiating psychiatric and neurologic causes
A visual hallucination is a visual percept experienced when awake that is not elicited by an external stimulus. Historically, hallucinations have been synonymous with psychiatric disease, most notably schizophrenia; however, over recent decades, hallucinations have been categorized based on their underlying etiology as psychodynamic (primary psychiatric), psychophysiologic (primary neurologic/structural), and psychobiochemical (neurotransmitter dysfunction).1 Presently, visual hallucinations are known to be caused by a wide variety of primary psychiatric, neurologic, ophthalmologic, and chemically-mediated conditions. Despite these causes, clinically differentiating the characteristics and qualities of visual hallucinations is often a lesser-known skillset among clinicians. The utility of this skillset is important for the clinician’s ability to differentiate the expected and unexpected characteristics of visual hallucinations in patients with both known and unknown neuropsychiatric conditions.
Though many primary psychiatric and neurologic conditions have been associated with and/or known to cause visual hallucinations, this review focuses on the following grouped causes:
- Primary psychiatric causes: psychiatric disorders with psychotic features and delirium; and
- Primary neurologic causes: neurodegenerative disease/dementias, seizure disorders, migraine disorders, vision loss, peduncular hallucinosis, and hypnagogic/hypnopompic phenomena.
Because the accepted definition of visual hallucinations excludes visual percepts elicited by external stimuli, drug-induced hallucinations would not qualify for either of these categories. Additionally, most studies reporting on the effects of drug-induced hallucinations did not control for underlying comorbid psychiatric conditions, dementia, or delirium, and thus the results cannot be attributed to the drug alone, nor is it possible to identify reliable trends in the properties of the hallucinations.2 The goals of this review are to characterize visual hallucinations experienced as a result of primary psychiatric and primary neurologic conditions and describe key grouping and differentiating features to help guide the diagnosis.
Visual hallucinations in the general population
A review of 6 studies (N = 42,519) reported that the prevalence of visual hallucinations in the general population is 7.3%.3 The prevalence decreases to 6% when visual hallucinations arising from physical illness or drug/chemical consumption are excluded. The prevalence of visual hallucinations in the general population has been associated with comorbid anxiety, stress, bereavement, and psychotic pathology.4,5 Regarding the age of occurrence of visual hallucinations in the general population, there appears to be a bimodal distribution.3 One peak appears in later adolescence and early adulthood, which corresponds with higher rates of psychosis, and another peak occurs late in life, which corresponds to a higher prevalence of neurodegenerative conditions and visual impairment.
Primary psychiatric causes
Most studies of visual hallucinations in primary psychiatric conditions have specifically evaluated patients with schizophrenia and mood disorders with psychotic features.6,7 In a review of 29 studies (N = 5,873) that specifically examined visual hallucinations in individuals diagnosed with schizophrenia, Waters et al3 found a wide range of reported prevalence (4% to 65%) and a weighted mean prevalence of 27%. In contrast, the prevalence of auditory hallucinations in these participants ranged from 25% to 86%, with a weighted mean of 59%.3
Hallucinations are a known but less common symptom of mood disorders that present with psychotic features.8 Waters et al3 also examined the prevalence of visual and auditory hallucinations in mood disorders (including mania, bipolar disorder, and depression) reported in 12 studies (N = 2,892).3 They found the prevalence of visual hallucinations in patients with mood disorders ranged from 6% to 27%, with a weighted mean of 15%, compared to the weighted mean of 28% who experienced auditory hallucinations. Visual hallucinations in primary psychiatric conditions are associated with more severe disease, longer hospitalizations, and poorer prognoses.9-11
Visual hallucinations of psychosis
In patients with psychotic symptoms, the characteristics of the visually hallucinated entity as well as the cognitive and emotional perception of the hallucinations are notably different than in patients with other, nonpsychiatric causes of visual hallucations.3
Continue to: Content and perceived physical properties
Content and perceived physical properties. Hallucinated entities are most often perceived as solid, 3-dimensional, well-detailed, life-sized people, animals, and objects (often fire) or events existing in the real world.3 The entity is almost always perceived as real, with accurate form and color, fine edges, and shadow; is often out of reach of the perceiver; and can be stationary or moving within the physical properties of the external environment.3
Timing and triggers. The temporal properties vary widely. Hallucinations can last from seconds to minutes and occur at any time of day, though by definition, they must occur while the individual is awake.3 Visual hallucinations in psychosis are more common during times of acute stress, strong emotions, and tiredness.3
Patient reaction and belief. Because of realistic qualities of the visual hallucination and the perception that it is real, patients commonly attempt to participate in some activity in relation to the hallucination, such as moving away from or attempting to interact with it.3 Additionally, patients usually perceive the hallucinated entity as uncontrollable, and are surprised when the entity appears or disappears. Though the content of the hallucination is usually impersonal, the meaning the patient attributes to the presence of the hallucinated entity is usually perceived as very personal and often requiring action. The hallucination may represent a harbinger, sign, or omen, and is often interpreted religiously or spiritually and accompanied by comorbid delusions.3
Visual hallucinations of delirium
Delirium is a syndrome of altered mentation—most notably consciousness, attention, and orientation—that occurs as a result of ≥1 metabolic, infectious, drug-induced, or other medical conditions and often manifests as an acute secondary psychotic illness.12 Multiple patient and environmental characteristics have been identified as risk factors for developing delirium, including multiple and/or severe medical illnesses, preexisting dementia, depression, advanced age, polypharmacy, having an indwelling urinary catheter, impaired sight or hearing, and low albumin levels.13-15 The development of delirium is significantly and positively associated with regular alcohol use, benzodiazepine withdrawal, and angiotensin receptor blocker and dopamine receptor agonist usage.15 Approximately 40% of patients with delirium have symptoms of psychosis, and in contrast to the hallucinations experienced by patients with schizophrenia, visual hallucinations are the most common type of hallucinations seen in delirium (27%).13 In a 2021 review that included 602 patients with delirium, Tachibana et al15 found that approximately 26% experienced hallucinations, 92% of which were visual hallucinations.
Content, perceived physical properties, and reaction. Because of the limited attention and cognitive function of patients with delirium, less is known about the content of their visual hallucinations. However, much like those with primary psychotic symptoms, patients with delirium often report seeing complex, normal-sized, concrete entities, most commonly people. Tachibana et al15 found that the hallucinated person is more often a stranger than a familiar person, but (rarely) may be an ethereal being such as a devil or ghost. The next most common visually hallucinated entities were creatures, most frequently insects and animals. Other common hallucinations were visions of events or objects, such as fires, falling ceilings, or water. Similar to those with primary psychotic illness such as schizophrenia, patients with delirium often experience emotional distress, anxiety, fear, and confusion in response to the hallucinated person, object, and/or event.15
Continue to: Primary neurologic causes
Primary neurologic causes
Visual hallucinations in neurodegenerative diseases
Patients with neurodegenerative diseases such as Parkinson disease (PD), dementia with Lewy bodies (DLB), or Creutzfeldt-Jakob disease (CJD) commonly experience hallucinations as a feature of their condition. However, the true cause of these hallucinations often cannot be directly attributed to any specific pathophysiology because these patients often have multiple coexisting risk factors, such as advanced age, major depressive disorder, use of neuroactive medications, and co-occurring somatic illness. Though the prevalence of visual hallucinations varies widely between studies, with 15% to 40% reported in patients with PD, the prevalence roughly doubles in patients with PD-associated dementia (30% to 60%), and is reported by 60% to 90% of those with DLB.16-18 Hallucinations are generally thought to be less common in Alzheimer disease; such patients most commonly experience visual hallucinations, although the reported prevalence ranges widely (4% to 59%).19,20 Notably, similarly to hallucinations experienced in patients with delirium, and in contrast to those with psychosis, visual hallucinations are more common than auditory hallucinations in neurodegenerative diseases.20 Hallucinations are not common in individuals with CJD but are a key defining feature of the He
Content, perceived physical properties, and reaction. Similar to the visual hallucinations experienced by patients with psychosis or delirium, those experienced in patients with PD, DLB, or CJD are often complex, most commonly of people, followed by animals and objects. The presence of “passage hallucinations”—in which a person or animal is seen in a patient’s peripheral vision, but passes out of their visual field before the entity can be directly visualized—is common.20 Those with PD also commonly have visual hallucinations in which the form of an object appears distorted (dysmorphopsia) or the color of an object appears distorted (metachromatopsia), though these would better be classified as illusions because a real object is being perceived with distortion.22
Hallucinations are more common in the evening and at night. “Presence hallucinations” are a common type of hallucination that cannot be directly related to a specific sensory modality such as vision, though they are commonly described by patients with PD as a seen or perceived image (usually a person) that is not directly in the individual’s visual field.17 These presence hallucinations are often described as being behind the patient or in a visualized scene of what was about to happen. Before developing the dementia and myoclonus also seen in sporadic CJD, patients with the Heidenhain variant of CJD describe illusions such as metachromatopsia, dysmorphia, and micropsia that eventually develop into frank visual hallucinations, which have been poorly reported in medical literature.22,23 There are no generalizable trends in the temporal nature of visual hallucinations in patients with neurodegenerative diseases. In most cases of visual hallucinations in patients with PD and dementia, insight relating to the perception varies widely based on the patient’s cognitive status. Subsequently, patients’ reactions to the hallucinations also vary widely.
Visual hallucinations in epileptic seizures
Occipital lobe epilepsies represent 1% to 4.6% of all epilepsies; however, these represent 20% to 30% of benign childhood partial epilepsies.24,25 These are commonly associated with various types of visual hallucinations depending upon the location of the seizure onset within the occipital lobe. These are referred to as visual auras.26 Visual auras are classified into simple visual hallucinations, complex visual hallucinations, visual illusions, and ictal amaurosis (hemifield blindness or complete blindness).
Content, perceived physical properties, and reaction. Simple visual hallucinations are often described as brief, stereotypical flashing lights of various shapes and colors. These images may flicker, change shape, or take on a geometric or irregular pattern. Appearances can be repetitive and stereotyped, are often reported as moving horizontally from the periphery to the center of the visual field, and can spread to the entire visual field. Most often, these hallucinations occur for 5 to 30 seconds, and have no discernible provoking factors. Complex visual hallucinations consist of formed images of animals, people, or elaborate scenes. These are believed to reflect activation of a larger area of cortex in the temporo-parieto-occipital region, which is the visual association cortex. Very rarely, occipital lobe seizures can manifest with ictal amaurosis.24
Continue to: Simple visual auras...
Simple visual auras have a very high localizing value to the occipital lobe. The primary visual cortex (Brodmann area 17) is situated in the banks of calcarine fissure and activation of this region produces these simple hallucinations. If the hallucinations are consistently lateralized, the seizures are very likely to be coming from the contralateral occipital lobe.
Visual hallucinations in brain tumors
In general, a tumor anywhere along the optic path can produce visual hallucinations; however, the exact causal mechanism of the hallucinations is unknown. Moreover, tumors in different locations—namely the occipital lobes, temporal lobes, and frontal lobes—appear to produce visual hallucinations with substantially different characteristics.27-29 Further complicating the search for the mechanism of these hallucinations is the fact that tumors are epileptogenic. In addition, 36% to 48% of patients with brain tumors have mood symptoms (depression/mania), and 22% to 24% have psychotic symptoms (delusions/hallucinations); these symptoms are considerably location-dependent.30-32
Content and associated signs/symptoms. There are some grouped symptoms and/or hallucination characteristics associated with cerebral tumors in different lobes of the brain, though these symptoms are not specific. The visual hallucinations associated with brain tumors are typically confined to the field of vision that corresponds to the location of the tumor. Additionally, many such patients have a baseline visual field defect to some extent due to the tumor location.
In patients with occipital lobe tumors, visual hallucinations closely resemble those experienced in occipital lobe seizures, specifically bright flashes of light in colorful simple and complex shapes. Interestingly, those with occipital lobe tumors report xanthopsia, a form of chromatopsia in which objects in their field of view appear abnormally colored a yellowish shade.26,27
In patients with temporal lobe tumors, more complex visual hallucinations of people, objects, and events occurring around them are often accompanied by auditory hallucinations, olfactory hallucinations, and/or anosmia.28In those with frontal lobe tumors, similar complex visual hallucinations of people, objects, and events are seen, and olfactory hallucinations and/or anosmia are often experienced. However, these patients often have a lower likelihood of experiencing auditory hallucinations, and a higher likelihood of developing personality changes and depression than other psychotic symptoms. The visual hallucinations experienced in those with frontal lobe tumors are more likely to have violent content.29
Continue to: Visual hallucinations in migraine with aura
Visual hallucinations in migraine with aura
The estimated prevalence of migraine in the general population is 15% to 29%; 31% of those with migraine experience auras.33-35 Approximately 99% of those with migraine auras experience some type of associated visual phenomena.33,36 The pathophysiology of migraine is believed to be related to spreading cortical depression, in which a slowly propagating wave of neuroelectric depolarization travels over the cortex, followed by a depression of normal brain activity. Visual aura is thought to occur due to the resulting changes in cortical activity in the visual cortex; however, the exact electrophysiology of visual migraine aura is not entirely known.37,38 Though most patients with visual migraine aura experience simple visual hallucinations, complex hallucinations have been reported in the (very rare) cases of migraine coma and familial hemiplegic migraine.39
Content and associated signs/symptoms. The most common hallucinated entities reported by patients with migraine with aura are zigzag, flashing/sparkling, black and white curved figure(s) in the center of the visual field, commonly called a scintillating phosphene or scintillating scotoma.36 The perceived entity is often singular and gradually moves from the center to the periphery of the visual field. These visual hallucinations appear in front of all other objects in the visual field and do not interact with the environment or observer, or resemble or morph into any real-world objects, though they may change in contour, size, and color. The scintillating nature of the hallucination often resolves within minutes, usually leaving a scotoma, or area of vision loss, in the area, with resolution back to baseline vision within 1 hour. The straight, zigzag, and usually black-and-white nature of the scintillating phosphenes of migraine are in notable contrast to the colorful, often circular visual hallucinations experienced in patients with occipital lobe seizures.25
Visual hallucinations in peduncular hallucinosis
Peduncular hallucinosis is a syndrome of predominantly dreamlike visual hallucinations that occurs in the setting of lesions in the midbrain and/or thalamus.40 A recent review of the lesion etiology found that approximately 63% are caused by focal infarction and approximately 15% are caused by mass lesions; subarachnoid hemorrhage, intracerebral hemorrhage, and demyelination cause approximately 5% of cases each.40 Additionally, a review of the affected brainstem anatomy showed almost all lesions were found in the paramedian reticular formations of the midbrain and pons, with the vast majority of lesions affecting or adjacent to the oculomotor and raphe nuclei of the midbrain.39 Due to the commonly involved visual pathway, some researchers have suggested these hallucinations may be the result of a release phenomenon.39
Content and associated signs/symptoms. The visual hallucinations of peduncular hallucinosis usually start 1 to 5 days after the causal lesion forms, last several minutes to hours, and most stop after 1 to 3 weeks; however, cases of hallucinations lasting for years have been reported. These hallucinations have a diurnal pattern of usually appearing while the patient is resting in the evening and/or preparing for sleep. The characteristics of visual hallucinations vary widely from simple distortions in how real objects appear to colorful and vivid hallucinated events and people who can interact with the observer. The content of the visual hallucinations often changes in nature during the hallucination, or from one hallucination to the next. The hallucinated entities can be worldly or extraterrestrial. Once these patients fall asleep, they often have equally vivid and unusual dreams, with content similar to their visual hallucinations. Due to the anatomical involvement of the nigrostriatal pathway and oculomotor nuclei, co-occurring parkinsonism, ataxia, and oculomotor nerve palsy are common and can be a key clinical feature in establishing the diagnosis. Though patients with peduncular hallucinations commonly fear their hallucinations, they often eventually gain insight, which eases their anxiety.39
Other causes
Visual hallucinations in visual impairment
Visual hallucinations are a diagnostic requirement for Charles Bonnet syndrome, in which individuals with vision loss experience visual hallucinations in the corresponding field of vision loss.41 A lesion at any point in the visual pathway that produces visual loss can lead to Charles Bonnet syndrome; however, age-related macular degeneration is the most common cause.42 The hallucinations of Charles Bonnet syndrome are believed to be a release phenomenon, given the defective visual pathway and resultant dysfunction in visual processing. The prevalence of Charles Bonnet syndrome ranges widely by study. Larger studies report a prevalence of 11% to 27% in patients with age-related macular degeneration, depending on the severity of vision loss.43,44 Because there are many causes of Charles Bonnet syndrome, and because a recent study found that only 15% of patients with this syndrome told their eye care clinician and that 21% had not reported their hallucinatory symptoms to anyone, the true prevalence is unknown.42 Though the onset of visual hallucinations correlates with the onset of vision loss, there appears to be no association between the nature or complexity of the hallucinations and the severity or progression of the patient’s vision loss.45 Some studies have reported either the onset of or a higher frequency of visual hallucinations at a time of visual recovery (for example, treatment or exudative age-related macular degeneration), which suggests that hallucinations may be triggered by fluctuations in visual acuity.46,47 Additional risk factors for experiencing visual hallucinations in the setting of visual pathway deficit include a history of stroke, social isolation, poor cognitive function, poor lighting, and age ≥65.
Continue to: Content and associated signs/symptoms
Content and associated signs/symptoms. The visual hallucinations of patients with Charles Bonnet syndrome appear almost exclusively in the defective visual field. Images tend to be complex, colored, with moving parts, and appear in front of the patient. The hallucinations are usually of familiar or normal-appearing people or mundane objects, and as such, the patient often does not realize the hallucinated entity is not real. In patients without comorbid psychiatric disease, visual hallucinations are not accompanied by any other types of hallucinations. The most commonly hallucinated entities are people, followed by simple visual hallucinations of geometric patterns, and then by faces (natural or cartoon-like) and inanimate objects. Hallucinations most commonly occur daily or weekly, and upon waking. These hallucinations most often last several minutes, though they can last just a few seconds or for hours. Hallucinations are usually emotionally neutral, but most patients report feeling confused by their appearance and having a fear of underlying psychiatric disease. They often gain insight to the unreal nature of the hallucinations after counseling.48
Visual hallucinations at the sleep/wake interface
Hypnagogic and hypnopompic hallucinations are fleeting perceptual experiences that occur while an individual is falling asleep or waking, respectively.49 Because by definition visual hallucinations occur while the individual is fully awake, categorizing hallucination-like experiences such as hypnagogia and hypnopompia is difficult, especially since these are similar to other states in which alterations in perception are expected (namely a dream state). They are commonly associated with sleep disorders such as narcolepsy, cataplexy, and sleep paralysis.50,51 In a study of 13,057 individuals in the general population, Ohayon et al4 found the overall prevalence of hypnagogic or hypnopompic hallucinations was 24.8% (5.3% visual) and 6.6% (1.5% visual), respectively. Approximately one-third of participants reported having experienced ≥1 hallucinatory experience in their lifetime, regardless of being asleep or awake.4 There was a higher prevalence of hypnagogic/hypnopompic experiences among those who also reported daytime hallucinations or other psychotic features.
Content and associated signs/symptoms. Unfortunately, because of the frequent co-occurrence of sleep disorders and psychiatric conditions, as well as the general paucity of research, it is difficult to characterize the visual phenomenology of hypnagogic/hypnopompic hallucinations. Some evidence suggests the nature of the perception of the objects hallucinated is substantially impacted by the presence of preexisting psychotic symptoms. Insight into the reality of these hallucinations also depends upon the presence of comorbid psychiatric disease. Hypnagogic/hypnopompic hallucinations are often described as complex, colorful, vivid, and dream-like, as if the patient was in a “half sleep” state.52 They are usually described as highly detailed events involving people and/or animals, though they may be grotesque in nature. Perceived entities are often described as undergoing a transformation or being mobile in their environment. Rarely do these perceptions invoke emotion or change the patient’s beliefs. Hypnagogia/hypnopompia also often have an auditory or haptic component to them. Visual phenomena can either appear to take place within an alternative background environment or appear superimposed on the patient’s actual physical environment.
How to determine the cause
In many of the studies cited in this review, the participants had a considerable amount of psychiatric comorbidity, which makes it difficult to discriminate between pure neurologic and pure psychiatric causes of hallucinations. Though the visual content of the hallucinations (people, objects, shapes, lights) can help clinicians broadly differentiate causes, many other characteristics of both the hallucinations and the patient can help determine the cause (Table3,4,12-39,41-52). The most useful characteristics for discerning the etiology of an individual’s visual hallucinations are the patient’s age, the visual field in which the hallucination occurs, and the complexity/simplicity of the hallucination.
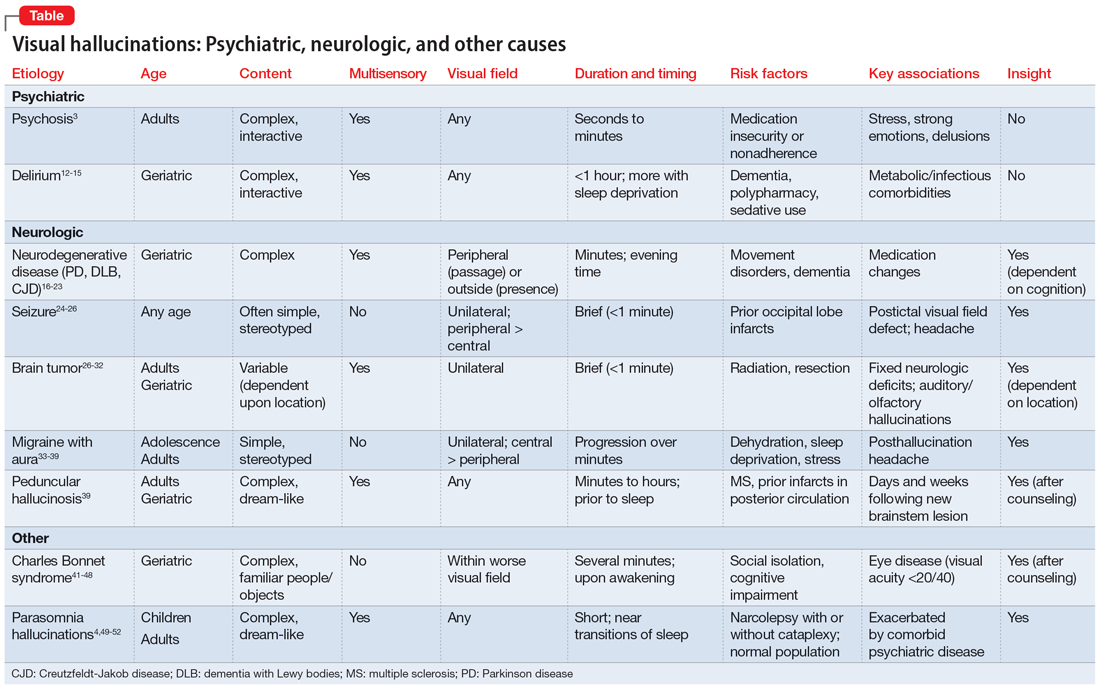
Patient age. Hallucinations associated with primary psychosis decrease with age. The average age of onset of migraine with aura is 21. Occipital lobe seizures occur in early childhood to age 40, but most commonly occur in the second decade.32,36 No trend in age can be reliably determined in individuals who experience hypnagogia/hypnopompia. In contrast, other potential causes of visual hallucinations, such as delirium, neurodegenerative disease, eye disease, and peduncular hallucinosis, are more commonly associated with advanced age.
Continue to: The visual field(s)
The visual field(s) in which the hallucination occurs can help differentiate possible causes in patients with seizure, brain tumor, migraine, or visual impairment. In patients with psychosis, delirium, peduncular hallucinosis, or hypnagogia/hypnopompia, hallucinations can occur in any visual field. Those with neurodegenerative disease, particularly PD, commonly describe seeing so-called passage hallucinations and presence hallucinations, which occur outside of the patient’s direct vision. Visual hallucinations associated with seizure are often unilateral (homonymous left or right hemifield), and contralateral to the affected neurologic structures in the visual neural pathway; they start in the left or right peripheral vision and gradually move to the central visual field. In hallucinations experienced by patients with brain tumors, the hallucinated entities typically appear on the visual field contralateral to the underlying tumor. Visual hallucinations seen in migraine often include a figure that moves from central vision to more lateral in the visual field. The visual hallucinations seen in eye disease (namely Charles Bonnet syndrome) are almost exclusively perceived in the visual fields affected by decreased visual acuity, though non-side-locked visual hallucinations are common in patients with age-related macular degeneration.
Content and complexity. The visual hallucinations perceived in those with psychosis, delirium, neurodegenerative disease, and sleep disorders are generally complex. These hallucinations tend to be of people, animals, scenes, or faces and include color and associated sound, with moving parts and interactivity with either the patient or the environment. These are in contrast to the simple visual hallucinations of visual cortex seizures, brain tumors, and migraine aura, which are often reported as brightly colored or black/white lights, flashes, and shapes, with or without associated auditory, olfactory, or somatic sensation. Furthermore, hallucinations due to seizure and brain tumor (also likely due to seizure) are often of brightly colored shapes and lights with curved edges, while patients with migraine more commonly report singular sparkling black/white objects with straight lines.
Bottom Line
Though there are no features known to be specific to only 1 cause of visual hallucinations, some characteristics of both the patient and the hallucinations can help direct the diagnostic differential. The most useful characteristics are the patient’s age, the visual field in which the hallucination occurs, and the complexity/ simplicity of the hallucination.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2020; 91(5):512-519. doi:10.1136/jnnp-2019-322702
1. Asaad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1987;143(9):1088-1097.
2. Taam MA, Boissieu P, Taam RA, et al. Drug-induced hallucination: a case/non-case study in the French Pharmacovigilance Database. Article in French. Eur J Psychiatry. 2015;29(1):21-31.
3. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and disease. Schizophr Bull. 2014;40(Suppl 4):S233-S245.
4. Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97(2-3):153-164.
5. Rees WD. The hallucinations of widowhood. Br Med J. 1971;4(5778):37-41.
6. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):97-104.
7. Gauntlett-Gilbert J, Kuipers E. Phenomenology of visual hallucinations in psychiatric conditions. J Nerv Ment Dis. 2003;191(3):203-205.
8. Goodwin FK, Jamison KR. Manic Depressive Illness. Oxford University Press, Inc.; 1999.
9. Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990;82(1):26-29.
10. McCabe MS, Fowler RC, Cadoret RJ, et al. Symptom differences in schizophrenia with good and bad prognosis. Am J Psychiatry. 1972;128(10):1239-1243.
11. Baethge C, Baldessarini RJ, Freudenthal K, et al. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136-145.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
13. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-333.
14. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
15. Tachibana M, Inada T, Ichida M, et al. Factors affecting hallucinations in patients with delirium. Sci Rep. 2021;11(1):13005. doi:10.1038/s41598-021-92578-1
16. Fenelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733-745.
17. Papapetropoulos S, Argyriou AA, Ellul J. Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol. 2005;252(10):1223-1228.
18. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79(6):652-655.
19. Linszen MMJ, Lemstra AW, Dauwan M, et al. Understanding hallucinations in probable Alzheimer’s disease: very low prevalence rates in a tertiary memory clinic. Alzheimers Dement (Amst). 2018;10:358-362.
20. Burghaus L, Eggers C, Timmermann L, et al. Hallucinations in neurodegenerative diseases. CNS Neurosci Ther. 2012;18(2):149-159.
21. Brar HK, Vaddigiri V, Scicutella A. Of illusions, hallucinations, and Creutzfeldt-Jakob disease (Heidenhain’s variant). J Neuropsychiatry Clin Neurosci. 2005;17(1):124-126.
22. Sasaki C, Yokoi K, Takahashi H, et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics. 2022;22(1):28-48.
23. Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999;56(1):55-61.
24. Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(Pt 4):753-769.
25. Caraballo R, Cersosimo R, Medina C, et al. Panayiotopoulos-type benign childhood occipital epilepsy: a prospective study. Neurology. 2000;5(8):1096-1100.
26. Chowdhury FA, Silva R, Whatley B, et al. Localisation in focal epilepsy: a practical guide. Practical Neurol. 2021;21(6):481-491.
27. Horrax G, Putnam TJ. Distortions of the visual fields in cases of brain tumour: the field defects and hallucinations produced by tumours of the occipital lobe. Brain. 1932;55(4):499-523.
28. Cushing H. Distortions of the visual fields in cases of brain tumor (6th paper): the field defects produced by temporal lobe lesions. Brain. 1922;44(4):341-396.
29. Fornazzari L, Farcnik K, Smith I, et al. Violent visual hallucinations and aggression in frontal lobe dysfunction: clinical manifestations of deep orbitofrontal foci. J Neuropsychiatry Clin Neurosci. 1992;4(1):42-44.
30. Madhusoodanan S, Opler MGA, Moise D, et al. Brain tumor location and psychiatric symptoms: is there an association? A meta-analysis of published cases studies. Expert Rev Neurother. 2010;10(10):1529-1536.
31. Madhusoodanan S, Sinha A, Moise D. Brain tumors and psychiatric manifestations: a review and analysis. Poster presented at: The American Association for Geriatric Psychiatry Annual Meeting; March 10-13; 2006; San Juan, Puerto Rico.
32. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors/gliomas. Rivistica Medica. 2007;13(4):209-215.
33. Kirchmann M. Migraine with aura: new understanding from clinical epidemiological studies. Curr Opin Neurol. 2006;19:286-293.
34. Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346(4):257-270.
35. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38(6):613-616.
36. Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996;119(Pt 2):355-361.
37. Cozzolino O, Marchese M, Trovato F, et al. Understanding spreading depression from headache to sudden unexpected death. Front Neurol. 2018;9:19.
38. Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687-4692.
39. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819-1840.
40. Galetta KM, Prasad S. Historical trends in the diagnosis of peduncular hallucinosis. J Neuroophthalmol. 2018;38(4):438-441.
41. Schadlu AP, Schadlu R, Shepherd JB III. Charles Bonnet syndrome: a review. Curr Opin Ophthalmol. 2009;20(3):219-222.
42. Vukicevic M, Fitzmaurice K. Butterflies and black lace patterns: the prevalence and characteristics of Charles Bonnet hallucinations in an Australian population. Clin Exp Ophthalmol. 2008;36(7):659-665.
43. Teunisse RJ, Cruysberg JR, Verbeek A, et al. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijmegen. Br J Psychiatry. 1995;166(2):254-257.
44. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucination in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
45. Khan JC, Shahid H, Thurlby DA, et al. Charles Bonnet syndrome in age-related macular degeneration: the nature and frequency of images in subjects with end-stage disease. Ophthalmic Epidemiol. 2008;15(3):202-208.
46. Cohen SY, Bulik A, Tadayoni R, et al. Visual hallucinations and Charles Bonnet syndrome after photodynamic therapy for age related macular degeneration. Br J Ophthalmol. 2003;87(8):977-979.
47. Meyer CH, Mennel S, Horle S, et al. Visual hallucinations after intravitreal injection of bevacizumab in vascular age-related macular degeneration. Am J Ophthalmol. 2007;143(1):169-170.
48. Jan T, Del Castillo J. Visual hallucinations: Charles Bonnet syndrome. West J Emerg Med. 2012;13(6):544-547. doi:10.5811/westjem.2012.7.12891
49. Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70:231-243.
50. Mitler MM, Hajdukovic R, Erman M, et al. Narcolepsy. J Clin Neurophysiol. 1990;7(1):93-118.
51. Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373-399.
52. Schultz SK, Miller DD, Oliver SE, et al. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23(1):15-23.
A visual hallucination is a visual percept experienced when awake that is not elicited by an external stimulus. Historically, hallucinations have been synonymous with psychiatric disease, most notably schizophrenia; however, over recent decades, hallucinations have been categorized based on their underlying etiology as psychodynamic (primary psychiatric), psychophysiologic (primary neurologic/structural), and psychobiochemical (neurotransmitter dysfunction).1 Presently, visual hallucinations are known to be caused by a wide variety of primary psychiatric, neurologic, ophthalmologic, and chemically-mediated conditions. Despite these causes, clinically differentiating the characteristics and qualities of visual hallucinations is often a lesser-known skillset among clinicians. The utility of this skillset is important for the clinician’s ability to differentiate the expected and unexpected characteristics of visual hallucinations in patients with both known and unknown neuropsychiatric conditions.
Though many primary psychiatric and neurologic conditions have been associated with and/or known to cause visual hallucinations, this review focuses on the following grouped causes:
- Primary psychiatric causes: psychiatric disorders with psychotic features and delirium; and
- Primary neurologic causes: neurodegenerative disease/dementias, seizure disorders, migraine disorders, vision loss, peduncular hallucinosis, and hypnagogic/hypnopompic phenomena.
Because the accepted definition of visual hallucinations excludes visual percepts elicited by external stimuli, drug-induced hallucinations would not qualify for either of these categories. Additionally, most studies reporting on the effects of drug-induced hallucinations did not control for underlying comorbid psychiatric conditions, dementia, or delirium, and thus the results cannot be attributed to the drug alone, nor is it possible to identify reliable trends in the properties of the hallucinations.2 The goals of this review are to characterize visual hallucinations experienced as a result of primary psychiatric and primary neurologic conditions and describe key grouping and differentiating features to help guide the diagnosis.
Visual hallucinations in the general population
A review of 6 studies (N = 42,519) reported that the prevalence of visual hallucinations in the general population is 7.3%.3 The prevalence decreases to 6% when visual hallucinations arising from physical illness or drug/chemical consumption are excluded. The prevalence of visual hallucinations in the general population has been associated with comorbid anxiety, stress, bereavement, and psychotic pathology.4,5 Regarding the age of occurrence of visual hallucinations in the general population, there appears to be a bimodal distribution.3 One peak appears in later adolescence and early adulthood, which corresponds with higher rates of psychosis, and another peak occurs late in life, which corresponds to a higher prevalence of neurodegenerative conditions and visual impairment.
Primary psychiatric causes
Most studies of visual hallucinations in primary psychiatric conditions have specifically evaluated patients with schizophrenia and mood disorders with psychotic features.6,7 In a review of 29 studies (N = 5,873) that specifically examined visual hallucinations in individuals diagnosed with schizophrenia, Waters et al3 found a wide range of reported prevalence (4% to 65%) and a weighted mean prevalence of 27%. In contrast, the prevalence of auditory hallucinations in these participants ranged from 25% to 86%, with a weighted mean of 59%.3
Hallucinations are a known but less common symptom of mood disorders that present with psychotic features.8 Waters et al3 also examined the prevalence of visual and auditory hallucinations in mood disorders (including mania, bipolar disorder, and depression) reported in 12 studies (N = 2,892).3 They found the prevalence of visual hallucinations in patients with mood disorders ranged from 6% to 27%, with a weighted mean of 15%, compared to the weighted mean of 28% who experienced auditory hallucinations. Visual hallucinations in primary psychiatric conditions are associated with more severe disease, longer hospitalizations, and poorer prognoses.9-11
Visual hallucinations of psychosis
In patients with psychotic symptoms, the characteristics of the visually hallucinated entity as well as the cognitive and emotional perception of the hallucinations are notably different than in patients with other, nonpsychiatric causes of visual hallucations.3
Continue to: Content and perceived physical properties
Content and perceived physical properties. Hallucinated entities are most often perceived as solid, 3-dimensional, well-detailed, life-sized people, animals, and objects (often fire) or events existing in the real world.3 The entity is almost always perceived as real, with accurate form and color, fine edges, and shadow; is often out of reach of the perceiver; and can be stationary or moving within the physical properties of the external environment.3
Timing and triggers. The temporal properties vary widely. Hallucinations can last from seconds to minutes and occur at any time of day, though by definition, they must occur while the individual is awake.3 Visual hallucinations in psychosis are more common during times of acute stress, strong emotions, and tiredness.3
Patient reaction and belief. Because of realistic qualities of the visual hallucination and the perception that it is real, patients commonly attempt to participate in some activity in relation to the hallucination, such as moving away from or attempting to interact with it.3 Additionally, patients usually perceive the hallucinated entity as uncontrollable, and are surprised when the entity appears or disappears. Though the content of the hallucination is usually impersonal, the meaning the patient attributes to the presence of the hallucinated entity is usually perceived as very personal and often requiring action. The hallucination may represent a harbinger, sign, or omen, and is often interpreted religiously or spiritually and accompanied by comorbid delusions.3
Visual hallucinations of delirium
Delirium is a syndrome of altered mentation—most notably consciousness, attention, and orientation—that occurs as a result of ≥1 metabolic, infectious, drug-induced, or other medical conditions and often manifests as an acute secondary psychotic illness.12 Multiple patient and environmental characteristics have been identified as risk factors for developing delirium, including multiple and/or severe medical illnesses, preexisting dementia, depression, advanced age, polypharmacy, having an indwelling urinary catheter, impaired sight or hearing, and low albumin levels.13-15 The development of delirium is significantly and positively associated with regular alcohol use, benzodiazepine withdrawal, and angiotensin receptor blocker and dopamine receptor agonist usage.15 Approximately 40% of patients with delirium have symptoms of psychosis, and in contrast to the hallucinations experienced by patients with schizophrenia, visual hallucinations are the most common type of hallucinations seen in delirium (27%).13 In a 2021 review that included 602 patients with delirium, Tachibana et al15 found that approximately 26% experienced hallucinations, 92% of which were visual hallucinations.
Content, perceived physical properties, and reaction. Because of the limited attention and cognitive function of patients with delirium, less is known about the content of their visual hallucinations. However, much like those with primary psychotic symptoms, patients with delirium often report seeing complex, normal-sized, concrete entities, most commonly people. Tachibana et al15 found that the hallucinated person is more often a stranger than a familiar person, but (rarely) may be an ethereal being such as a devil or ghost. The next most common visually hallucinated entities were creatures, most frequently insects and animals. Other common hallucinations were visions of events or objects, such as fires, falling ceilings, or water. Similar to those with primary psychotic illness such as schizophrenia, patients with delirium often experience emotional distress, anxiety, fear, and confusion in response to the hallucinated person, object, and/or event.15
Continue to: Primary neurologic causes
Primary neurologic causes
Visual hallucinations in neurodegenerative diseases
Patients with neurodegenerative diseases such as Parkinson disease (PD), dementia with Lewy bodies (DLB), or Creutzfeldt-Jakob disease (CJD) commonly experience hallucinations as a feature of their condition. However, the true cause of these hallucinations often cannot be directly attributed to any specific pathophysiology because these patients often have multiple coexisting risk factors, such as advanced age, major depressive disorder, use of neuroactive medications, and co-occurring somatic illness. Though the prevalence of visual hallucinations varies widely between studies, with 15% to 40% reported in patients with PD, the prevalence roughly doubles in patients with PD-associated dementia (30% to 60%), and is reported by 60% to 90% of those with DLB.16-18 Hallucinations are generally thought to be less common in Alzheimer disease; such patients most commonly experience visual hallucinations, although the reported prevalence ranges widely (4% to 59%).19,20 Notably, similarly to hallucinations experienced in patients with delirium, and in contrast to those with psychosis, visual hallucinations are more common than auditory hallucinations in neurodegenerative diseases.20 Hallucinations are not common in individuals with CJD but are a key defining feature of the He
Content, perceived physical properties, and reaction. Similar to the visual hallucinations experienced by patients with psychosis or delirium, those experienced in patients with PD, DLB, or CJD are often complex, most commonly of people, followed by animals and objects. The presence of “passage hallucinations”—in which a person or animal is seen in a patient’s peripheral vision, but passes out of their visual field before the entity can be directly visualized—is common.20 Those with PD also commonly have visual hallucinations in which the form of an object appears distorted (dysmorphopsia) or the color of an object appears distorted (metachromatopsia), though these would better be classified as illusions because a real object is being perceived with distortion.22
Hallucinations are more common in the evening and at night. “Presence hallucinations” are a common type of hallucination that cannot be directly related to a specific sensory modality such as vision, though they are commonly described by patients with PD as a seen or perceived image (usually a person) that is not directly in the individual’s visual field.17 These presence hallucinations are often described as being behind the patient or in a visualized scene of what was about to happen. Before developing the dementia and myoclonus also seen in sporadic CJD, patients with the Heidenhain variant of CJD describe illusions such as metachromatopsia, dysmorphia, and micropsia that eventually develop into frank visual hallucinations, which have been poorly reported in medical literature.22,23 There are no generalizable trends in the temporal nature of visual hallucinations in patients with neurodegenerative diseases. In most cases of visual hallucinations in patients with PD and dementia, insight relating to the perception varies widely based on the patient’s cognitive status. Subsequently, patients’ reactions to the hallucinations also vary widely.
Visual hallucinations in epileptic seizures
Occipital lobe epilepsies represent 1% to 4.6% of all epilepsies; however, these represent 20% to 30% of benign childhood partial epilepsies.24,25 These are commonly associated with various types of visual hallucinations depending upon the location of the seizure onset within the occipital lobe. These are referred to as visual auras.26 Visual auras are classified into simple visual hallucinations, complex visual hallucinations, visual illusions, and ictal amaurosis (hemifield blindness or complete blindness).
Content, perceived physical properties, and reaction. Simple visual hallucinations are often described as brief, stereotypical flashing lights of various shapes and colors. These images may flicker, change shape, or take on a geometric or irregular pattern. Appearances can be repetitive and stereotyped, are often reported as moving horizontally from the periphery to the center of the visual field, and can spread to the entire visual field. Most often, these hallucinations occur for 5 to 30 seconds, and have no discernible provoking factors. Complex visual hallucinations consist of formed images of animals, people, or elaborate scenes. These are believed to reflect activation of a larger area of cortex in the temporo-parieto-occipital region, which is the visual association cortex. Very rarely, occipital lobe seizures can manifest with ictal amaurosis.24
Continue to: Simple visual auras...
Simple visual auras have a very high localizing value to the occipital lobe. The primary visual cortex (Brodmann area 17) is situated in the banks of calcarine fissure and activation of this region produces these simple hallucinations. If the hallucinations are consistently lateralized, the seizures are very likely to be coming from the contralateral occipital lobe.
Visual hallucinations in brain tumors
In general, a tumor anywhere along the optic path can produce visual hallucinations; however, the exact causal mechanism of the hallucinations is unknown. Moreover, tumors in different locations—namely the occipital lobes, temporal lobes, and frontal lobes—appear to produce visual hallucinations with substantially different characteristics.27-29 Further complicating the search for the mechanism of these hallucinations is the fact that tumors are epileptogenic. In addition, 36% to 48% of patients with brain tumors have mood symptoms (depression/mania), and 22% to 24% have psychotic symptoms (delusions/hallucinations); these symptoms are considerably location-dependent.30-32
Content and associated signs/symptoms. There are some grouped symptoms and/or hallucination characteristics associated with cerebral tumors in different lobes of the brain, though these symptoms are not specific. The visual hallucinations associated with brain tumors are typically confined to the field of vision that corresponds to the location of the tumor. Additionally, many such patients have a baseline visual field defect to some extent due to the tumor location.
In patients with occipital lobe tumors, visual hallucinations closely resemble those experienced in occipital lobe seizures, specifically bright flashes of light in colorful simple and complex shapes. Interestingly, those with occipital lobe tumors report xanthopsia, a form of chromatopsia in which objects in their field of view appear abnormally colored a yellowish shade.26,27
In patients with temporal lobe tumors, more complex visual hallucinations of people, objects, and events occurring around them are often accompanied by auditory hallucinations, olfactory hallucinations, and/or anosmia.28In those with frontal lobe tumors, similar complex visual hallucinations of people, objects, and events are seen, and olfactory hallucinations and/or anosmia are often experienced. However, these patients often have a lower likelihood of experiencing auditory hallucinations, and a higher likelihood of developing personality changes and depression than other psychotic symptoms. The visual hallucinations experienced in those with frontal lobe tumors are more likely to have violent content.29
Continue to: Visual hallucinations in migraine with aura
Visual hallucinations in migraine with aura
The estimated prevalence of migraine in the general population is 15% to 29%; 31% of those with migraine experience auras.33-35 Approximately 99% of those with migraine auras experience some type of associated visual phenomena.33,36 The pathophysiology of migraine is believed to be related to spreading cortical depression, in which a slowly propagating wave of neuroelectric depolarization travels over the cortex, followed by a depression of normal brain activity. Visual aura is thought to occur due to the resulting changes in cortical activity in the visual cortex; however, the exact electrophysiology of visual migraine aura is not entirely known.37,38 Though most patients with visual migraine aura experience simple visual hallucinations, complex hallucinations have been reported in the (very rare) cases of migraine coma and familial hemiplegic migraine.39
Content and associated signs/symptoms. The most common hallucinated entities reported by patients with migraine with aura are zigzag, flashing/sparkling, black and white curved figure(s) in the center of the visual field, commonly called a scintillating phosphene or scintillating scotoma.36 The perceived entity is often singular and gradually moves from the center to the periphery of the visual field. These visual hallucinations appear in front of all other objects in the visual field and do not interact with the environment or observer, or resemble or morph into any real-world objects, though they may change in contour, size, and color. The scintillating nature of the hallucination often resolves within minutes, usually leaving a scotoma, or area of vision loss, in the area, with resolution back to baseline vision within 1 hour. The straight, zigzag, and usually black-and-white nature of the scintillating phosphenes of migraine are in notable contrast to the colorful, often circular visual hallucinations experienced in patients with occipital lobe seizures.25
Visual hallucinations in peduncular hallucinosis
Peduncular hallucinosis is a syndrome of predominantly dreamlike visual hallucinations that occurs in the setting of lesions in the midbrain and/or thalamus.40 A recent review of the lesion etiology found that approximately 63% are caused by focal infarction and approximately 15% are caused by mass lesions; subarachnoid hemorrhage, intracerebral hemorrhage, and demyelination cause approximately 5% of cases each.40 Additionally, a review of the affected brainstem anatomy showed almost all lesions were found in the paramedian reticular formations of the midbrain and pons, with the vast majority of lesions affecting or adjacent to the oculomotor and raphe nuclei of the midbrain.39 Due to the commonly involved visual pathway, some researchers have suggested these hallucinations may be the result of a release phenomenon.39
Content and associated signs/symptoms. The visual hallucinations of peduncular hallucinosis usually start 1 to 5 days after the causal lesion forms, last several minutes to hours, and most stop after 1 to 3 weeks; however, cases of hallucinations lasting for years have been reported. These hallucinations have a diurnal pattern of usually appearing while the patient is resting in the evening and/or preparing for sleep. The characteristics of visual hallucinations vary widely from simple distortions in how real objects appear to colorful and vivid hallucinated events and people who can interact with the observer. The content of the visual hallucinations often changes in nature during the hallucination, or from one hallucination to the next. The hallucinated entities can be worldly or extraterrestrial. Once these patients fall asleep, they often have equally vivid and unusual dreams, with content similar to their visual hallucinations. Due to the anatomical involvement of the nigrostriatal pathway and oculomotor nuclei, co-occurring parkinsonism, ataxia, and oculomotor nerve palsy are common and can be a key clinical feature in establishing the diagnosis. Though patients with peduncular hallucinations commonly fear their hallucinations, they often eventually gain insight, which eases their anxiety.39
Other causes
Visual hallucinations in visual impairment
Visual hallucinations are a diagnostic requirement for Charles Bonnet syndrome, in which individuals with vision loss experience visual hallucinations in the corresponding field of vision loss.41 A lesion at any point in the visual pathway that produces visual loss can lead to Charles Bonnet syndrome; however, age-related macular degeneration is the most common cause.42 The hallucinations of Charles Bonnet syndrome are believed to be a release phenomenon, given the defective visual pathway and resultant dysfunction in visual processing. The prevalence of Charles Bonnet syndrome ranges widely by study. Larger studies report a prevalence of 11% to 27% in patients with age-related macular degeneration, depending on the severity of vision loss.43,44 Because there are many causes of Charles Bonnet syndrome, and because a recent study found that only 15% of patients with this syndrome told their eye care clinician and that 21% had not reported their hallucinatory symptoms to anyone, the true prevalence is unknown.42 Though the onset of visual hallucinations correlates with the onset of vision loss, there appears to be no association between the nature or complexity of the hallucinations and the severity or progression of the patient’s vision loss.45 Some studies have reported either the onset of or a higher frequency of visual hallucinations at a time of visual recovery (for example, treatment or exudative age-related macular degeneration), which suggests that hallucinations may be triggered by fluctuations in visual acuity.46,47 Additional risk factors for experiencing visual hallucinations in the setting of visual pathway deficit include a history of stroke, social isolation, poor cognitive function, poor lighting, and age ≥65.
Continue to: Content and associated signs/symptoms
Content and associated signs/symptoms. The visual hallucinations of patients with Charles Bonnet syndrome appear almost exclusively in the defective visual field. Images tend to be complex, colored, with moving parts, and appear in front of the patient. The hallucinations are usually of familiar or normal-appearing people or mundane objects, and as such, the patient often does not realize the hallucinated entity is not real. In patients without comorbid psychiatric disease, visual hallucinations are not accompanied by any other types of hallucinations. The most commonly hallucinated entities are people, followed by simple visual hallucinations of geometric patterns, and then by faces (natural or cartoon-like) and inanimate objects. Hallucinations most commonly occur daily or weekly, and upon waking. These hallucinations most often last several minutes, though they can last just a few seconds or for hours. Hallucinations are usually emotionally neutral, but most patients report feeling confused by their appearance and having a fear of underlying psychiatric disease. They often gain insight to the unreal nature of the hallucinations after counseling.48
Visual hallucinations at the sleep/wake interface
Hypnagogic and hypnopompic hallucinations are fleeting perceptual experiences that occur while an individual is falling asleep or waking, respectively.49 Because by definition visual hallucinations occur while the individual is fully awake, categorizing hallucination-like experiences such as hypnagogia and hypnopompia is difficult, especially since these are similar to other states in which alterations in perception are expected (namely a dream state). They are commonly associated with sleep disorders such as narcolepsy, cataplexy, and sleep paralysis.50,51 In a study of 13,057 individuals in the general population, Ohayon et al4 found the overall prevalence of hypnagogic or hypnopompic hallucinations was 24.8% (5.3% visual) and 6.6% (1.5% visual), respectively. Approximately one-third of participants reported having experienced ≥1 hallucinatory experience in their lifetime, regardless of being asleep or awake.4 There was a higher prevalence of hypnagogic/hypnopompic experiences among those who also reported daytime hallucinations or other psychotic features.
Content and associated signs/symptoms. Unfortunately, because of the frequent co-occurrence of sleep disorders and psychiatric conditions, as well as the general paucity of research, it is difficult to characterize the visual phenomenology of hypnagogic/hypnopompic hallucinations. Some evidence suggests the nature of the perception of the objects hallucinated is substantially impacted by the presence of preexisting psychotic symptoms. Insight into the reality of these hallucinations also depends upon the presence of comorbid psychiatric disease. Hypnagogic/hypnopompic hallucinations are often described as complex, colorful, vivid, and dream-like, as if the patient was in a “half sleep” state.52 They are usually described as highly detailed events involving people and/or animals, though they may be grotesque in nature. Perceived entities are often described as undergoing a transformation or being mobile in their environment. Rarely do these perceptions invoke emotion or change the patient’s beliefs. Hypnagogia/hypnopompia also often have an auditory or haptic component to them. Visual phenomena can either appear to take place within an alternative background environment or appear superimposed on the patient’s actual physical environment.
How to determine the cause
In many of the studies cited in this review, the participants had a considerable amount of psychiatric comorbidity, which makes it difficult to discriminate between pure neurologic and pure psychiatric causes of hallucinations. Though the visual content of the hallucinations (people, objects, shapes, lights) can help clinicians broadly differentiate causes, many other characteristics of both the hallucinations and the patient can help determine the cause (Table3,4,12-39,41-52). The most useful characteristics for discerning the etiology of an individual’s visual hallucinations are the patient’s age, the visual field in which the hallucination occurs, and the complexity/simplicity of the hallucination.

Patient age. Hallucinations associated with primary psychosis decrease with age. The average age of onset of migraine with aura is 21. Occipital lobe seizures occur in early childhood to age 40, but most commonly occur in the second decade.32,36 No trend in age can be reliably determined in individuals who experience hypnagogia/hypnopompia. In contrast, other potential causes of visual hallucinations, such as delirium, neurodegenerative disease, eye disease, and peduncular hallucinosis, are more commonly associated with advanced age.
Continue to: The visual field(s)
The visual field(s) in which the hallucination occurs can help differentiate possible causes in patients with seizure, brain tumor, migraine, or visual impairment. In patients with psychosis, delirium, peduncular hallucinosis, or hypnagogia/hypnopompia, hallucinations can occur in any visual field. Those with neurodegenerative disease, particularly PD, commonly describe seeing so-called passage hallucinations and presence hallucinations, which occur outside of the patient’s direct vision. Visual hallucinations associated with seizure are often unilateral (homonymous left or right hemifield), and contralateral to the affected neurologic structures in the visual neural pathway; they start in the left or right peripheral vision and gradually move to the central visual field. In hallucinations experienced by patients with brain tumors, the hallucinated entities typically appear on the visual field contralateral to the underlying tumor. Visual hallucinations seen in migraine often include a figure that moves from central vision to more lateral in the visual field. The visual hallucinations seen in eye disease (namely Charles Bonnet syndrome) are almost exclusively perceived in the visual fields affected by decreased visual acuity, though non-side-locked visual hallucinations are common in patients with age-related macular degeneration.
Content and complexity. The visual hallucinations perceived in those with psychosis, delirium, neurodegenerative disease, and sleep disorders are generally complex. These hallucinations tend to be of people, animals, scenes, or faces and include color and associated sound, with moving parts and interactivity with either the patient or the environment. These are in contrast to the simple visual hallucinations of visual cortex seizures, brain tumors, and migraine aura, which are often reported as brightly colored or black/white lights, flashes, and shapes, with or without associated auditory, olfactory, or somatic sensation. Furthermore, hallucinations due to seizure and brain tumor (also likely due to seizure) are often of brightly colored shapes and lights with curved edges, while patients with migraine more commonly report singular sparkling black/white objects with straight lines.
Bottom Line
Though there are no features known to be specific to only 1 cause of visual hallucinations, some characteristics of both the patient and the hallucinations can help direct the diagnostic differential. The most useful characteristics are the patient’s age, the visual field in which the hallucination occurs, and the complexity/ simplicity of the hallucination.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2020; 91(5):512-519. doi:10.1136/jnnp-2019-322702
A visual hallucination is a visual percept experienced when awake that is not elicited by an external stimulus. Historically, hallucinations have been synonymous with psychiatric disease, most notably schizophrenia; however, over recent decades, hallucinations have been categorized based on their underlying etiology as psychodynamic (primary psychiatric), psychophysiologic (primary neurologic/structural), and psychobiochemical (neurotransmitter dysfunction).1 Presently, visual hallucinations are known to be caused by a wide variety of primary psychiatric, neurologic, ophthalmologic, and chemically-mediated conditions. Despite these causes, clinically differentiating the characteristics and qualities of visual hallucinations is often a lesser-known skillset among clinicians. The utility of this skillset is important for the clinician’s ability to differentiate the expected and unexpected characteristics of visual hallucinations in patients with both known and unknown neuropsychiatric conditions.
Though many primary psychiatric and neurologic conditions have been associated with and/or known to cause visual hallucinations, this review focuses on the following grouped causes:
- Primary psychiatric causes: psychiatric disorders with psychotic features and delirium; and
- Primary neurologic causes: neurodegenerative disease/dementias, seizure disorders, migraine disorders, vision loss, peduncular hallucinosis, and hypnagogic/hypnopompic phenomena.
Because the accepted definition of visual hallucinations excludes visual percepts elicited by external stimuli, drug-induced hallucinations would not qualify for either of these categories. Additionally, most studies reporting on the effects of drug-induced hallucinations did not control for underlying comorbid psychiatric conditions, dementia, or delirium, and thus the results cannot be attributed to the drug alone, nor is it possible to identify reliable trends in the properties of the hallucinations.2 The goals of this review are to characterize visual hallucinations experienced as a result of primary psychiatric and primary neurologic conditions and describe key grouping and differentiating features to help guide the diagnosis.
Visual hallucinations in the general population
A review of 6 studies (N = 42,519) reported that the prevalence of visual hallucinations in the general population is 7.3%.3 The prevalence decreases to 6% when visual hallucinations arising from physical illness or drug/chemical consumption are excluded. The prevalence of visual hallucinations in the general population has been associated with comorbid anxiety, stress, bereavement, and psychotic pathology.4,5 Regarding the age of occurrence of visual hallucinations in the general population, there appears to be a bimodal distribution.3 One peak appears in later adolescence and early adulthood, which corresponds with higher rates of psychosis, and another peak occurs late in life, which corresponds to a higher prevalence of neurodegenerative conditions and visual impairment.
Primary psychiatric causes
Most studies of visual hallucinations in primary psychiatric conditions have specifically evaluated patients with schizophrenia and mood disorders with psychotic features.6,7 In a review of 29 studies (N = 5,873) that specifically examined visual hallucinations in individuals diagnosed with schizophrenia, Waters et al3 found a wide range of reported prevalence (4% to 65%) and a weighted mean prevalence of 27%. In contrast, the prevalence of auditory hallucinations in these participants ranged from 25% to 86%, with a weighted mean of 59%.3
Hallucinations are a known but less common symptom of mood disorders that present with psychotic features.8 Waters et al3 also examined the prevalence of visual and auditory hallucinations in mood disorders (including mania, bipolar disorder, and depression) reported in 12 studies (N = 2,892).3 They found the prevalence of visual hallucinations in patients with mood disorders ranged from 6% to 27%, with a weighted mean of 15%, compared to the weighted mean of 28% who experienced auditory hallucinations. Visual hallucinations in primary psychiatric conditions are associated with more severe disease, longer hospitalizations, and poorer prognoses.9-11
Visual hallucinations of psychosis
In patients with psychotic symptoms, the characteristics of the visually hallucinated entity as well as the cognitive and emotional perception of the hallucinations are notably different than in patients with other, nonpsychiatric causes of visual hallucations.3
Continue to: Content and perceived physical properties
Content and perceived physical properties. Hallucinated entities are most often perceived as solid, 3-dimensional, well-detailed, life-sized people, animals, and objects (often fire) or events existing in the real world.3 The entity is almost always perceived as real, with accurate form and color, fine edges, and shadow; is often out of reach of the perceiver; and can be stationary or moving within the physical properties of the external environment.3
Timing and triggers. The temporal properties vary widely. Hallucinations can last from seconds to minutes and occur at any time of day, though by definition, they must occur while the individual is awake.3 Visual hallucinations in psychosis are more common during times of acute stress, strong emotions, and tiredness.3
Patient reaction and belief. Because of realistic qualities of the visual hallucination and the perception that it is real, patients commonly attempt to participate in some activity in relation to the hallucination, such as moving away from or attempting to interact with it.3 Additionally, patients usually perceive the hallucinated entity as uncontrollable, and are surprised when the entity appears or disappears. Though the content of the hallucination is usually impersonal, the meaning the patient attributes to the presence of the hallucinated entity is usually perceived as very personal and often requiring action. The hallucination may represent a harbinger, sign, or omen, and is often interpreted religiously or spiritually and accompanied by comorbid delusions.3
Visual hallucinations of delirium
Delirium is a syndrome of altered mentation—most notably consciousness, attention, and orientation—that occurs as a result of ≥1 metabolic, infectious, drug-induced, or other medical conditions and often manifests as an acute secondary psychotic illness.12 Multiple patient and environmental characteristics have been identified as risk factors for developing delirium, including multiple and/or severe medical illnesses, preexisting dementia, depression, advanced age, polypharmacy, having an indwelling urinary catheter, impaired sight or hearing, and low albumin levels.13-15 The development of delirium is significantly and positively associated with regular alcohol use, benzodiazepine withdrawal, and angiotensin receptor blocker and dopamine receptor agonist usage.15 Approximately 40% of patients with delirium have symptoms of psychosis, and in contrast to the hallucinations experienced by patients with schizophrenia, visual hallucinations are the most common type of hallucinations seen in delirium (27%).13 In a 2021 review that included 602 patients with delirium, Tachibana et al15 found that approximately 26% experienced hallucinations, 92% of which were visual hallucinations.
Content, perceived physical properties, and reaction. Because of the limited attention and cognitive function of patients with delirium, less is known about the content of their visual hallucinations. However, much like those with primary psychotic symptoms, patients with delirium often report seeing complex, normal-sized, concrete entities, most commonly people. Tachibana et al15 found that the hallucinated person is more often a stranger than a familiar person, but (rarely) may be an ethereal being such as a devil or ghost. The next most common visually hallucinated entities were creatures, most frequently insects and animals. Other common hallucinations were visions of events or objects, such as fires, falling ceilings, or water. Similar to those with primary psychotic illness such as schizophrenia, patients with delirium often experience emotional distress, anxiety, fear, and confusion in response to the hallucinated person, object, and/or event.15
Continue to: Primary neurologic causes
Primary neurologic causes
Visual hallucinations in neurodegenerative diseases
Patients with neurodegenerative diseases such as Parkinson disease (PD), dementia with Lewy bodies (DLB), or Creutzfeldt-Jakob disease (CJD) commonly experience hallucinations as a feature of their condition. However, the true cause of these hallucinations often cannot be directly attributed to any specific pathophysiology because these patients often have multiple coexisting risk factors, such as advanced age, major depressive disorder, use of neuroactive medications, and co-occurring somatic illness. Though the prevalence of visual hallucinations varies widely between studies, with 15% to 40% reported in patients with PD, the prevalence roughly doubles in patients with PD-associated dementia (30% to 60%), and is reported by 60% to 90% of those with DLB.16-18 Hallucinations are generally thought to be less common in Alzheimer disease; such patients most commonly experience visual hallucinations, although the reported prevalence ranges widely (4% to 59%).19,20 Notably, similarly to hallucinations experienced in patients with delirium, and in contrast to those with psychosis, visual hallucinations are more common than auditory hallucinations in neurodegenerative diseases.20 Hallucinations are not common in individuals with CJD but are a key defining feature of the He
Content, perceived physical properties, and reaction. Similar to the visual hallucinations experienced by patients with psychosis or delirium, those experienced in patients with PD, DLB, or CJD are often complex, most commonly of people, followed by animals and objects. The presence of “passage hallucinations”—in which a person or animal is seen in a patient’s peripheral vision, but passes out of their visual field before the entity can be directly visualized—is common.20 Those with PD also commonly have visual hallucinations in which the form of an object appears distorted (dysmorphopsia) or the color of an object appears distorted (metachromatopsia), though these would better be classified as illusions because a real object is being perceived with distortion.22
Hallucinations are more common in the evening and at night. “Presence hallucinations” are a common type of hallucination that cannot be directly related to a specific sensory modality such as vision, though they are commonly described by patients with PD as a seen or perceived image (usually a person) that is not directly in the individual’s visual field.17 These presence hallucinations are often described as being behind the patient or in a visualized scene of what was about to happen. Before developing the dementia and myoclonus also seen in sporadic CJD, patients with the Heidenhain variant of CJD describe illusions such as metachromatopsia, dysmorphia, and micropsia that eventually develop into frank visual hallucinations, which have been poorly reported in medical literature.22,23 There are no generalizable trends in the temporal nature of visual hallucinations in patients with neurodegenerative diseases. In most cases of visual hallucinations in patients with PD and dementia, insight relating to the perception varies widely based on the patient’s cognitive status. Subsequently, patients’ reactions to the hallucinations also vary widely.
Visual hallucinations in epileptic seizures
Occipital lobe epilepsies represent 1% to 4.6% of all epilepsies; however, these represent 20% to 30% of benign childhood partial epilepsies.24,25 These are commonly associated with various types of visual hallucinations depending upon the location of the seizure onset within the occipital lobe. These are referred to as visual auras.26 Visual auras are classified into simple visual hallucinations, complex visual hallucinations, visual illusions, and ictal amaurosis (hemifield blindness or complete blindness).
Content, perceived physical properties, and reaction. Simple visual hallucinations are often described as brief, stereotypical flashing lights of various shapes and colors. These images may flicker, change shape, or take on a geometric or irregular pattern. Appearances can be repetitive and stereotyped, are often reported as moving horizontally from the periphery to the center of the visual field, and can spread to the entire visual field. Most often, these hallucinations occur for 5 to 30 seconds, and have no discernible provoking factors. Complex visual hallucinations consist of formed images of animals, people, or elaborate scenes. These are believed to reflect activation of a larger area of cortex in the temporo-parieto-occipital region, which is the visual association cortex. Very rarely, occipital lobe seizures can manifest with ictal amaurosis.24
Continue to: Simple visual auras...
Simple visual auras have a very high localizing value to the occipital lobe. The primary visual cortex (Brodmann area 17) is situated in the banks of calcarine fissure and activation of this region produces these simple hallucinations. If the hallucinations are consistently lateralized, the seizures are very likely to be coming from the contralateral occipital lobe.
Visual hallucinations in brain tumors
In general, a tumor anywhere along the optic path can produce visual hallucinations; however, the exact causal mechanism of the hallucinations is unknown. Moreover, tumors in different locations—namely the occipital lobes, temporal lobes, and frontal lobes—appear to produce visual hallucinations with substantially different characteristics.27-29 Further complicating the search for the mechanism of these hallucinations is the fact that tumors are epileptogenic. In addition, 36% to 48% of patients with brain tumors have mood symptoms (depression/mania), and 22% to 24% have psychotic symptoms (delusions/hallucinations); these symptoms are considerably location-dependent.30-32
Content and associated signs/symptoms. There are some grouped symptoms and/or hallucination characteristics associated with cerebral tumors in different lobes of the brain, though these symptoms are not specific. The visual hallucinations associated with brain tumors are typically confined to the field of vision that corresponds to the location of the tumor. Additionally, many such patients have a baseline visual field defect to some extent due to the tumor location.
In patients with occipital lobe tumors, visual hallucinations closely resemble those experienced in occipital lobe seizures, specifically bright flashes of light in colorful simple and complex shapes. Interestingly, those with occipital lobe tumors report xanthopsia, a form of chromatopsia in which objects in their field of view appear abnormally colored a yellowish shade.26,27
In patients with temporal lobe tumors, more complex visual hallucinations of people, objects, and events occurring around them are often accompanied by auditory hallucinations, olfactory hallucinations, and/or anosmia.28In those with frontal lobe tumors, similar complex visual hallucinations of people, objects, and events are seen, and olfactory hallucinations and/or anosmia are often experienced. However, these patients often have a lower likelihood of experiencing auditory hallucinations, and a higher likelihood of developing personality changes and depression than other psychotic symptoms. The visual hallucinations experienced in those with frontal lobe tumors are more likely to have violent content.29
Continue to: Visual hallucinations in migraine with aura
Visual hallucinations in migraine with aura
The estimated prevalence of migraine in the general population is 15% to 29%; 31% of those with migraine experience auras.33-35 Approximately 99% of those with migraine auras experience some type of associated visual phenomena.33,36 The pathophysiology of migraine is believed to be related to spreading cortical depression, in which a slowly propagating wave of neuroelectric depolarization travels over the cortex, followed by a depression of normal brain activity. Visual aura is thought to occur due to the resulting changes in cortical activity in the visual cortex; however, the exact electrophysiology of visual migraine aura is not entirely known.37,38 Though most patients with visual migraine aura experience simple visual hallucinations, complex hallucinations have been reported in the (very rare) cases of migraine coma and familial hemiplegic migraine.39
Content and associated signs/symptoms. The most common hallucinated entities reported by patients with migraine with aura are zigzag, flashing/sparkling, black and white curved figure(s) in the center of the visual field, commonly called a scintillating phosphene or scintillating scotoma.36 The perceived entity is often singular and gradually moves from the center to the periphery of the visual field. These visual hallucinations appear in front of all other objects in the visual field and do not interact with the environment or observer, or resemble or morph into any real-world objects, though they may change in contour, size, and color. The scintillating nature of the hallucination often resolves within minutes, usually leaving a scotoma, or area of vision loss, in the area, with resolution back to baseline vision within 1 hour. The straight, zigzag, and usually black-and-white nature of the scintillating phosphenes of migraine are in notable contrast to the colorful, often circular visual hallucinations experienced in patients with occipital lobe seizures.25
Visual hallucinations in peduncular hallucinosis
Peduncular hallucinosis is a syndrome of predominantly dreamlike visual hallucinations that occurs in the setting of lesions in the midbrain and/or thalamus.40 A recent review of the lesion etiology found that approximately 63% are caused by focal infarction and approximately 15% are caused by mass lesions; subarachnoid hemorrhage, intracerebral hemorrhage, and demyelination cause approximately 5% of cases each.40 Additionally, a review of the affected brainstem anatomy showed almost all lesions were found in the paramedian reticular formations of the midbrain and pons, with the vast majority of lesions affecting or adjacent to the oculomotor and raphe nuclei of the midbrain.39 Due to the commonly involved visual pathway, some researchers have suggested these hallucinations may be the result of a release phenomenon.39
Content and associated signs/symptoms. The visual hallucinations of peduncular hallucinosis usually start 1 to 5 days after the causal lesion forms, last several minutes to hours, and most stop after 1 to 3 weeks; however, cases of hallucinations lasting for years have been reported. These hallucinations have a diurnal pattern of usually appearing while the patient is resting in the evening and/or preparing for sleep. The characteristics of visual hallucinations vary widely from simple distortions in how real objects appear to colorful and vivid hallucinated events and people who can interact with the observer. The content of the visual hallucinations often changes in nature during the hallucination, or from one hallucination to the next. The hallucinated entities can be worldly or extraterrestrial. Once these patients fall asleep, they often have equally vivid and unusual dreams, with content similar to their visual hallucinations. Due to the anatomical involvement of the nigrostriatal pathway and oculomotor nuclei, co-occurring parkinsonism, ataxia, and oculomotor nerve palsy are common and can be a key clinical feature in establishing the diagnosis. Though patients with peduncular hallucinations commonly fear their hallucinations, they often eventually gain insight, which eases their anxiety.39
Other causes
Visual hallucinations in visual impairment
Visual hallucinations are a diagnostic requirement for Charles Bonnet syndrome, in which individuals with vision loss experience visual hallucinations in the corresponding field of vision loss.41 A lesion at any point in the visual pathway that produces visual loss can lead to Charles Bonnet syndrome; however, age-related macular degeneration is the most common cause.42 The hallucinations of Charles Bonnet syndrome are believed to be a release phenomenon, given the defective visual pathway and resultant dysfunction in visual processing. The prevalence of Charles Bonnet syndrome ranges widely by study. Larger studies report a prevalence of 11% to 27% in patients with age-related macular degeneration, depending on the severity of vision loss.43,44 Because there are many causes of Charles Bonnet syndrome, and because a recent study found that only 15% of patients with this syndrome told their eye care clinician and that 21% had not reported their hallucinatory symptoms to anyone, the true prevalence is unknown.42 Though the onset of visual hallucinations correlates with the onset of vision loss, there appears to be no association between the nature or complexity of the hallucinations and the severity or progression of the patient’s vision loss.45 Some studies have reported either the onset of or a higher frequency of visual hallucinations at a time of visual recovery (for example, treatment or exudative age-related macular degeneration), which suggests that hallucinations may be triggered by fluctuations in visual acuity.46,47 Additional risk factors for experiencing visual hallucinations in the setting of visual pathway deficit include a history of stroke, social isolation, poor cognitive function, poor lighting, and age ≥65.
Continue to: Content and associated signs/symptoms
Content and associated signs/symptoms. The visual hallucinations of patients with Charles Bonnet syndrome appear almost exclusively in the defective visual field. Images tend to be complex, colored, with moving parts, and appear in front of the patient. The hallucinations are usually of familiar or normal-appearing people or mundane objects, and as such, the patient often does not realize the hallucinated entity is not real. In patients without comorbid psychiatric disease, visual hallucinations are not accompanied by any other types of hallucinations. The most commonly hallucinated entities are people, followed by simple visual hallucinations of geometric patterns, and then by faces (natural or cartoon-like) and inanimate objects. Hallucinations most commonly occur daily or weekly, and upon waking. These hallucinations most often last several minutes, though they can last just a few seconds or for hours. Hallucinations are usually emotionally neutral, but most patients report feeling confused by their appearance and having a fear of underlying psychiatric disease. They often gain insight to the unreal nature of the hallucinations after counseling.48
Visual hallucinations at the sleep/wake interface
Hypnagogic and hypnopompic hallucinations are fleeting perceptual experiences that occur while an individual is falling asleep or waking, respectively.49 Because by definition visual hallucinations occur while the individual is fully awake, categorizing hallucination-like experiences such as hypnagogia and hypnopompia is difficult, especially since these are similar to other states in which alterations in perception are expected (namely a dream state). They are commonly associated with sleep disorders such as narcolepsy, cataplexy, and sleep paralysis.50,51 In a study of 13,057 individuals in the general population, Ohayon et al4 found the overall prevalence of hypnagogic or hypnopompic hallucinations was 24.8% (5.3% visual) and 6.6% (1.5% visual), respectively. Approximately one-third of participants reported having experienced ≥1 hallucinatory experience in their lifetime, regardless of being asleep or awake.4 There was a higher prevalence of hypnagogic/hypnopompic experiences among those who also reported daytime hallucinations or other psychotic features.
Content and associated signs/symptoms. Unfortunately, because of the frequent co-occurrence of sleep disorders and psychiatric conditions, as well as the general paucity of research, it is difficult to characterize the visual phenomenology of hypnagogic/hypnopompic hallucinations. Some evidence suggests the nature of the perception of the objects hallucinated is substantially impacted by the presence of preexisting psychotic symptoms. Insight into the reality of these hallucinations also depends upon the presence of comorbid psychiatric disease. Hypnagogic/hypnopompic hallucinations are often described as complex, colorful, vivid, and dream-like, as if the patient was in a “half sleep” state.52 They are usually described as highly detailed events involving people and/or animals, though they may be grotesque in nature. Perceived entities are often described as undergoing a transformation or being mobile in their environment. Rarely do these perceptions invoke emotion or change the patient’s beliefs. Hypnagogia/hypnopompia also often have an auditory or haptic component to them. Visual phenomena can either appear to take place within an alternative background environment or appear superimposed on the patient’s actual physical environment.
How to determine the cause
In many of the studies cited in this review, the participants had a considerable amount of psychiatric comorbidity, which makes it difficult to discriminate between pure neurologic and pure psychiatric causes of hallucinations. Though the visual content of the hallucinations (people, objects, shapes, lights) can help clinicians broadly differentiate causes, many other characteristics of both the hallucinations and the patient can help determine the cause (Table3,4,12-39,41-52). The most useful characteristics for discerning the etiology of an individual’s visual hallucinations are the patient’s age, the visual field in which the hallucination occurs, and the complexity/simplicity of the hallucination.

Patient age. Hallucinations associated with primary psychosis decrease with age. The average age of onset of migraine with aura is 21. Occipital lobe seizures occur in early childhood to age 40, but most commonly occur in the second decade.32,36 No trend in age can be reliably determined in individuals who experience hypnagogia/hypnopompia. In contrast, other potential causes of visual hallucinations, such as delirium, neurodegenerative disease, eye disease, and peduncular hallucinosis, are more commonly associated with advanced age.
Continue to: The visual field(s)
The visual field(s) in which the hallucination occurs can help differentiate possible causes in patients with seizure, brain tumor, migraine, or visual impairment. In patients with psychosis, delirium, peduncular hallucinosis, or hypnagogia/hypnopompia, hallucinations can occur in any visual field. Those with neurodegenerative disease, particularly PD, commonly describe seeing so-called passage hallucinations and presence hallucinations, which occur outside of the patient’s direct vision. Visual hallucinations associated with seizure are often unilateral (homonymous left or right hemifield), and contralateral to the affected neurologic structures in the visual neural pathway; they start in the left or right peripheral vision and gradually move to the central visual field. In hallucinations experienced by patients with brain tumors, the hallucinated entities typically appear on the visual field contralateral to the underlying tumor. Visual hallucinations seen in migraine often include a figure that moves from central vision to more lateral in the visual field. The visual hallucinations seen in eye disease (namely Charles Bonnet syndrome) are almost exclusively perceived in the visual fields affected by decreased visual acuity, though non-side-locked visual hallucinations are common in patients with age-related macular degeneration.
Content and complexity. The visual hallucinations perceived in those with psychosis, delirium, neurodegenerative disease, and sleep disorders are generally complex. These hallucinations tend to be of people, animals, scenes, or faces and include color and associated sound, with moving parts and interactivity with either the patient or the environment. These are in contrast to the simple visual hallucinations of visual cortex seizures, brain tumors, and migraine aura, which are often reported as brightly colored or black/white lights, flashes, and shapes, with or without associated auditory, olfactory, or somatic sensation. Furthermore, hallucinations due to seizure and brain tumor (also likely due to seizure) are often of brightly colored shapes and lights with curved edges, while patients with migraine more commonly report singular sparkling black/white objects with straight lines.
Bottom Line
Though there are no features known to be specific to only 1 cause of visual hallucinations, some characteristics of both the patient and the hallucinations can help direct the diagnostic differential. The most useful characteristics are the patient’s age, the visual field in which the hallucination occurs, and the complexity/ simplicity of the hallucination.
Related Resources
- Wang J, Patel D, Francois D. Elaborate hallucinations, but is it a psychotic disorder? Current Psychiatry. 2021;20(2):46-50. doi:10.12788/cp.0091
- O’Brien J, Taylor JP, Ballard C, et al. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2020; 91(5):512-519. doi:10.1136/jnnp-2019-322702
1. Asaad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1987;143(9):1088-1097.
2. Taam MA, Boissieu P, Taam RA, et al. Drug-induced hallucination: a case/non-case study in the French Pharmacovigilance Database. Article in French. Eur J Psychiatry. 2015;29(1):21-31.
3. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and disease. Schizophr Bull. 2014;40(Suppl 4):S233-S245.
4. Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97(2-3):153-164.
5. Rees WD. The hallucinations of widowhood. Br Med J. 1971;4(5778):37-41.
6. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):97-104.
7. Gauntlett-Gilbert J, Kuipers E. Phenomenology of visual hallucinations in psychiatric conditions. J Nerv Ment Dis. 2003;191(3):203-205.
8. Goodwin FK, Jamison KR. Manic Depressive Illness. Oxford University Press, Inc.; 1999.
9. Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990;82(1):26-29.
10. McCabe MS, Fowler RC, Cadoret RJ, et al. Symptom differences in schizophrenia with good and bad prognosis. Am J Psychiatry. 1972;128(10):1239-1243.
11. Baethge C, Baldessarini RJ, Freudenthal K, et al. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136-145.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
13. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-333.
14. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
15. Tachibana M, Inada T, Ichida M, et al. Factors affecting hallucinations in patients with delirium. Sci Rep. 2021;11(1):13005. doi:10.1038/s41598-021-92578-1
16. Fenelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733-745.
17. Papapetropoulos S, Argyriou AA, Ellul J. Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol. 2005;252(10):1223-1228.
18. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79(6):652-655.
19. Linszen MMJ, Lemstra AW, Dauwan M, et al. Understanding hallucinations in probable Alzheimer’s disease: very low prevalence rates in a tertiary memory clinic. Alzheimers Dement (Amst). 2018;10:358-362.
20. Burghaus L, Eggers C, Timmermann L, et al. Hallucinations in neurodegenerative diseases. CNS Neurosci Ther. 2012;18(2):149-159.
21. Brar HK, Vaddigiri V, Scicutella A. Of illusions, hallucinations, and Creutzfeldt-Jakob disease (Heidenhain’s variant). J Neuropsychiatry Clin Neurosci. 2005;17(1):124-126.
22. Sasaki C, Yokoi K, Takahashi H, et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics. 2022;22(1):28-48.
23. Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999;56(1):55-61.
24. Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(Pt 4):753-769.
25. Caraballo R, Cersosimo R, Medina C, et al. Panayiotopoulos-type benign childhood occipital epilepsy: a prospective study. Neurology. 2000;5(8):1096-1100.
26. Chowdhury FA, Silva R, Whatley B, et al. Localisation in focal epilepsy: a practical guide. Practical Neurol. 2021;21(6):481-491.
27. Horrax G, Putnam TJ. Distortions of the visual fields in cases of brain tumour: the field defects and hallucinations produced by tumours of the occipital lobe. Brain. 1932;55(4):499-523.
28. Cushing H. Distortions of the visual fields in cases of brain tumor (6th paper): the field defects produced by temporal lobe lesions. Brain. 1922;44(4):341-396.
29. Fornazzari L, Farcnik K, Smith I, et al. Violent visual hallucinations and aggression in frontal lobe dysfunction: clinical manifestations of deep orbitofrontal foci. J Neuropsychiatry Clin Neurosci. 1992;4(1):42-44.
30. Madhusoodanan S, Opler MGA, Moise D, et al. Brain tumor location and psychiatric symptoms: is there an association? A meta-analysis of published cases studies. Expert Rev Neurother. 2010;10(10):1529-1536.
31. Madhusoodanan S, Sinha A, Moise D. Brain tumors and psychiatric manifestations: a review and analysis. Poster presented at: The American Association for Geriatric Psychiatry Annual Meeting; March 10-13; 2006; San Juan, Puerto Rico.
32. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors/gliomas. Rivistica Medica. 2007;13(4):209-215.
33. Kirchmann M. Migraine with aura: new understanding from clinical epidemiological studies. Curr Opin Neurol. 2006;19:286-293.
34. Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346(4):257-270.
35. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38(6):613-616.
36. Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996;119(Pt 2):355-361.
37. Cozzolino O, Marchese M, Trovato F, et al. Understanding spreading depression from headache to sudden unexpected death. Front Neurol. 2018;9:19.
38. Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687-4692.
39. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819-1840.
40. Galetta KM, Prasad S. Historical trends in the diagnosis of peduncular hallucinosis. J Neuroophthalmol. 2018;38(4):438-441.
41. Schadlu AP, Schadlu R, Shepherd JB III. Charles Bonnet syndrome: a review. Curr Opin Ophthalmol. 2009;20(3):219-222.
42. Vukicevic M, Fitzmaurice K. Butterflies and black lace patterns: the prevalence and characteristics of Charles Bonnet hallucinations in an Australian population. Clin Exp Ophthalmol. 2008;36(7):659-665.
43. Teunisse RJ, Cruysberg JR, Verbeek A, et al. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijmegen. Br J Psychiatry. 1995;166(2):254-257.
44. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucination in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
45. Khan JC, Shahid H, Thurlby DA, et al. Charles Bonnet syndrome in age-related macular degeneration: the nature and frequency of images in subjects with end-stage disease. Ophthalmic Epidemiol. 2008;15(3):202-208.
46. Cohen SY, Bulik A, Tadayoni R, et al. Visual hallucinations and Charles Bonnet syndrome after photodynamic therapy for age related macular degeneration. Br J Ophthalmol. 2003;87(8):977-979.
47. Meyer CH, Mennel S, Horle S, et al. Visual hallucinations after intravitreal injection of bevacizumab in vascular age-related macular degeneration. Am J Ophthalmol. 2007;143(1):169-170.
48. Jan T, Del Castillo J. Visual hallucinations: Charles Bonnet syndrome. West J Emerg Med. 2012;13(6):544-547. doi:10.5811/westjem.2012.7.12891
49. Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70:231-243.
50. Mitler MM, Hajdukovic R, Erman M, et al. Narcolepsy. J Clin Neurophysiol. 1990;7(1):93-118.
51. Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373-399.
52. Schultz SK, Miller DD, Oliver SE, et al. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23(1):15-23.
1. Asaad G, Shapiro B. Hallucinations: theoretical and clinical overview. Am J Psychiatry. 1987;143(9):1088-1097.
2. Taam MA, Boissieu P, Taam RA, et al. Drug-induced hallucination: a case/non-case study in the French Pharmacovigilance Database. Article in French. Eur J Psychiatry. 2015;29(1):21-31.
3. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and disease. Schizophr Bull. 2014;40(Suppl 4):S233-S245.
4. Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97(2-3):153-164.
5. Rees WD. The hallucinations of widowhood. Br Med J. 1971;4(5778):37-41.
6. Delespaul P, deVries M, van Os J. Determinants of occurrence and recovery from hallucinations in daily life. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):97-104.
7. Gauntlett-Gilbert J, Kuipers E. Phenomenology of visual hallucinations in psychiatric conditions. J Nerv Ment Dis. 2003;191(3):203-205.
8. Goodwin FK, Jamison KR. Manic Depressive Illness. Oxford University Press, Inc.; 1999.
9. Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990;82(1):26-29.
10. McCabe MS, Fowler RC, Cadoret RJ, et al. Symptom differences in schizophrenia with good and bad prognosis. Am J Psychiatry. 1972;128(10):1239-1243.
11. Baethge C, Baldessarini RJ, Freudenthal K, et al. Hallucinations in bipolar disorder: characteristics and comparison to unipolar depression and schizophrenia. Bipolar Disord. 2005;7(2):136-145.
12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing; 2013.
13. Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing. 2014;43(3):326-333.
14. Webster R, Holroyd S. Prevalence of psychotic symptoms in delirium. Psychosomatics. 2000;41(6):519-522.
15. Tachibana M, Inada T, Ichida M, et al. Factors affecting hallucinations in patients with delirium. Sci Rep. 2021;11(1):13005. doi:10.1038/s41598-021-92578-1
16. Fenelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733-745.
17. Papapetropoulos S, Argyriou AA, Ellul J. Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol. 2005;252(10):1223-1228.
18. Williams DR, Warren JD, Lees AJ. Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2008;79(6):652-655.
19. Linszen MMJ, Lemstra AW, Dauwan M, et al. Understanding hallucinations in probable Alzheimer’s disease: very low prevalence rates in a tertiary memory clinic. Alzheimers Dement (Amst). 2018;10:358-362.
20. Burghaus L, Eggers C, Timmermann L, et al. Hallucinations in neurodegenerative diseases. CNS Neurosci Ther. 2012;18(2):149-159.
21. Brar HK, Vaddigiri V, Scicutella A. Of illusions, hallucinations, and Creutzfeldt-Jakob disease (Heidenhain’s variant). J Neuropsychiatry Clin Neurosci. 2005;17(1):124-126.
22. Sasaki C, Yokoi K, Takahashi H, et al. Visual illusions in Parkinson’s disease: an interview survey of symptomatology. Psychogeriatrics. 2022;22(1):28-48.
23. Kropp S, Schulz-Schaeffer WJ, Finkenstaedt M, et al. The Heidenhain variant of Creutzfeldt-Jakob disease. Arch Neurol. 1999;56(1):55-61.
24. Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(Pt 4):753-769.
25. Caraballo R, Cersosimo R, Medina C, et al. Panayiotopoulos-type benign childhood occipital epilepsy: a prospective study. Neurology. 2000;5(8):1096-1100.
26. Chowdhury FA, Silva R, Whatley B, et al. Localisation in focal epilepsy: a practical guide. Practical Neurol. 2021;21(6):481-491.
27. Horrax G, Putnam TJ. Distortions of the visual fields in cases of brain tumour: the field defects and hallucinations produced by tumours of the occipital lobe. Brain. 1932;55(4):499-523.
28. Cushing H. Distortions of the visual fields in cases of brain tumor (6th paper): the field defects produced by temporal lobe lesions. Brain. 1922;44(4):341-396.
29. Fornazzari L, Farcnik K, Smith I, et al. Violent visual hallucinations and aggression in frontal lobe dysfunction: clinical manifestations of deep orbitofrontal foci. J Neuropsychiatry Clin Neurosci. 1992;4(1):42-44.
30. Madhusoodanan S, Opler MGA, Moise D, et al. Brain tumor location and psychiatric symptoms: is there an association? A meta-analysis of published cases studies. Expert Rev Neurother. 2010;10(10):1529-1536.
31. Madhusoodanan S, Sinha A, Moise D. Brain tumors and psychiatric manifestations: a review and analysis. Poster presented at: The American Association for Geriatric Psychiatry Annual Meeting; March 10-13; 2006; San Juan, Puerto Rico.
32. Madhusoodanan S, Danan D, Moise D. Psychiatric manifestations of brain tumors/gliomas. Rivistica Medica. 2007;13(4):209-215.
33. Kirchmann M. Migraine with aura: new understanding from clinical epidemiological studies. Curr Opin Neurol. 2006;19:286-293.
34. Goadsby PJ, Lipton RB, Ferrari MD. Migraine: current understanding and treatment. N Engl J Med. 2002;346(4):257-270.
35. Waters WE, O’Connor PJ. Prevalence of migraine. J Neurol Neurosurg Psychiatry. 1975;38(6):613-616.
36. Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996;119(Pt 2):355-361.
37. Cozzolino O, Marchese M, Trovato F, et al. Understanding spreading depression from headache to sudden unexpected death. Front Neurol. 2018;9:19.
38. Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687-4692.
39. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819-1840.
40. Galetta KM, Prasad S. Historical trends in the diagnosis of peduncular hallucinosis. J Neuroophthalmol. 2018;38(4):438-441.
41. Schadlu AP, Schadlu R, Shepherd JB III. Charles Bonnet syndrome: a review. Curr Opin Ophthalmol. 2009;20(3):219-222.
42. Vukicevic M, Fitzmaurice K. Butterflies and black lace patterns: the prevalence and characteristics of Charles Bonnet hallucinations in an Australian population. Clin Exp Ophthalmol. 2008;36(7):659-665.
43. Teunisse RJ, Cruysberg JR, Verbeek A, et al. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijmegen. Br J Psychiatry. 1995;166(2):254-257.
44. Holroyd S, Rabins PV, Finkelstein D, et al. Visual hallucination in patients with macular degeneration. Am J Psychiatry. 1992;149(12):1701-1706.
45. Khan JC, Shahid H, Thurlby DA, et al. Charles Bonnet syndrome in age-related macular degeneration: the nature and frequency of images in subjects with end-stage disease. Ophthalmic Epidemiol. 2008;15(3):202-208.
46. Cohen SY, Bulik A, Tadayoni R, et al. Visual hallucinations and Charles Bonnet syndrome after photodynamic therapy for age related macular degeneration. Br J Ophthalmol. 2003;87(8):977-979.
47. Meyer CH, Mennel S, Horle S, et al. Visual hallucinations after intravitreal injection of bevacizumab in vascular age-related macular degeneration. Am J Ophthalmol. 2007;143(1):169-170.
48. Jan T, Del Castillo J. Visual hallucinations: Charles Bonnet syndrome. West J Emerg Med. 2012;13(6):544-547. doi:10.5811/westjem.2012.7.12891
49. Foulkes D, Vogel G. Mental activity at sleep onset. J Abnorm Psychol. 1965;70:231-243.
50. Mitler MM, Hajdukovic R, Erman M, et al. Narcolepsy. J Clin Neurophysiol. 1990;7(1):93-118.
51. Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8(4):373-399.
52. Schultz SK, Miller DD, Oliver SE, et al. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23(1):15-23.
Iron deficiency in psychiatric patients
Nutritional deficiencies are one of the many causes of or contributors to symptoms in patients with psychiatric disorders. In this article, we discuss the prevalence of iron deficiency and its link to poor mental health, and how proper treatment may improve psychiatric symptoms. We also offer suggestions for how and when to test for and treat iron deficiency in psychiatric patients.
A common condition
Iron deficiency is the most common mineral deficiency in the world. According to the World Health Organization (WHO), approximately 25% of the global population is anemic and nearly one-half of those cases are the result of iron deficiency.1 While the WHO has published guidelines defining iron deficiency as it relates to ferritin levels (<15 ug/L in adults and <12 ug/L in children), this estimate might be low.2,3 Mei et al2 found that hemoglobin and soluble transferrin receptors can be used to determine iron-deficient erythropoiesis, which indicates a physiological definition of iron deficiency. According to a study of children and nonpregnant women by Mei et al,2 children with ferritin levels <20 ug/L and women with ferritin levels <25 ug/L should be considered iron-deficient. If replicated, this study suggests the prevalence of iron deficiency is higher than currently estimated.2 Overall, an estimated 1.2 billion people worldwide have iron-deficiency anemia.4 Additionally, patients can be iron deficient without being anemic, a condition thought to be at least twice as common.4
Essential for brain function
Research shows the importance of iron to proper brain function.5 Iron deficiency in pregnant women is associated with significant neuropsychological impairments in neonates. Rodent studies have demonstrated the importance of iron and the effects of iron deficiency on the hippocampus, corpus striatum, and production of monoamines.5 Specifically, iron is a necessary cofactor in the enzymes tryptophan hydroxylase and tyrosine hydroxylase, which produce serotonin, dopamine, and norepinephrine. In rodent studies, monoamine deficits secondary to iron deficiency persist into adulthood even with iron supplementation, which highlights the importance of preventing iron deficiency during pregnancy and early life.5 While most research has focused on the impact of iron deficiency in infancy and early childhood, iron deficiency has an ongoing impact into adulthood, even if treated.6
Iron deficiency and psychiatric symptoms
Current research suggests an association between iron deficiency or low ferritin levels and psychiatric disorders, specifically depression, anxiety, and schizophrenia. In a web survey of 11,876 adults, Hidese et al7 found an association between a self-reported history of iron deficiency anemia and a self-reported history of depression. Another study of 528 municipal employees found an association between low serum ferritin concentrations and a high prevalence of depressive symptoms among men; no statistically significant association was detected in women.8 In an analysis of the Taiwan National Health Insurance Database from 2000 to 2012, Lee et al9 found a statistically significant increased risk of anxiety disorders, depression, sleep disorders, and psychotic disorders in patients with iron deficiency anemia after controlling for multiple confounders. Xu et al10 used quantitative susceptibility mapping to assess the iron status in certain regions of the brain of 30 patients with first-episode psychosis. They found lower levels of iron in the bilateral substantia nigra, left red nucleus, and left thalamus compared to healthy controls.10 Kim et al11 found an association between iron deficiency and more severe negative symptoms in 121 patients with first-episode psychosis, which supports the hypothesis that iron deficiency may alter dopamine transmission in the brain.
Iron deficiency has been associated with psychopathology across the lifespan. In a population-based study in Taiwan, Chen et al12 found an association between iron deficiency anemia and psychiatric disorders in children and adolescents, including mood disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, and developmental disorders. At the other end of the age spectrum, in a survey of 1,875 older adults in England, Stewart et al13 found an association between low ferritin levels (<45 ng/mL) and depressive symptoms after adjusting for demographic factors and overall health status.
In addition to specific psychiatric disorders and symptoms, iron deficiency is often associated with nonspecific symptoms such as fatigue.14 Fatigue is a symptom of numerous psychiatric disorders and is included in the DSM diagnostic criteria for major depressive disorder and generalized anxiety disorder.15
Iron supplementation might improve psychiatric symptoms
Some evidence suggests that using iron supplementation to treat iron deficiency can improve psychiatric symptoms. In a 2013 systematic literature review of 10 studies, Greig et al16 found a link between low iron status and poor cognition, poor mental health scores, and fatigue among women of childbearing age. In this review, 7 studies demonstrated improvement in cognition and 3 demonstrated improvement in mental health with iron supplementation.16 In a 2021 prospective study, 19 children and adolescents age 6 to 15 who had serum ferritin levels <30 ng/mL were treated with oral iron supplementation for 12 weeks.17 Participants showed significant improvements in sleep quality, depressive symptoms, and general mood as assessed via the Pittsburgh Sleep Quality Index, Center for Epidemiologic Studies Depression Scale, and Profile of Mood States (POMS) questionnaires, respectively.17 A randomized controlled trial of 219 female soldiers who were given iron supplementation or placebo for 8 weeks during basic combat training found that compared to placebo, iron supplementation led to improvements in mood as measured by the POMS questionnaire.18 Lastly, in a 2016 observational study of 412 adult psychiatric patients, Kassir19 found most patients (81%) had iron deficiency, defined as a transferrin saturation coefficient <30% or serum ferritin <100 ng/mL. Although these cutoffs are not considered standard and thus may have overrepresented the percentage of patients considered iron-deficient, more than one-half of patients considered iron-deficient in this study experienced a reduction or elimination of psychiatric symptoms following treatment with iron supplementation and/or psychotropic medications.19
Continue to: Individuals with iron deficiency...
Individuals with iron deficiency without anemia also may see improvement in psychiatric symptoms with iron treatment. In a 2018 systematic review, Houston et al20 evaluated iron supplementation in 1,170 adults who were iron-deficient but not anemic. They found that in these patients, fatigue significantly improved but physical capacity did not.20 Additionally, 2 other studies found iron treatment improved fatigue in nonanemic women.21,22 In a 2016 systematic review, Pratt et al23 concluded, “There is emerging evidence that … nonanemic iron deficiency … is a disease in its own right, deserving of further research in the development of strategies for detection and treatment.” Al-Naseem et al24 suggested severity distinguishes iron deficiency with and without anemia.
Your role in assessing and treating iron deficiency
Testing for and treating iron deficiency generally is not a part of routine psychiatric practice. This might be due to apathy given the pervasiveness of iron deficiency, a belief that iron deficiency should be managed by primary care physicians, or a lack of familiarity with how to treat it and the benefits of such treatment for psychiatric patients. However, assessing for and treating iron deficiency in psychiatric patients is important, especially for individuals who are highly susceptible to inadequate iron levels. People at risk for iron deficiency include pregnant women, infants, young children, women with heavy menstrual bleeding, frequent blood donors, patients with cancer, individuals who have gastrointestinal (GI) surgeries or disorders, and those with heart failure.25
Assessment. Iron status can be assessed through an iron studies panel. Because a patient can have iron deficiency without anemia, a complete blood count (CBC) alone does not suffice.26 The iron panel includes serum iron, serum ferritin, serum transferrin or total iron-binding capacity (TIBC), and calculated transferrin saturation (TSAT), which is the ratio of serum iron to TIBC.
Iron deficiency is diagnosed if ferritin is <30 ng/mL, regardless of the hemoglobin concentration or underlying condition, and confirmed by a low TSAT.26 In most guidelines, the cutoff value for TSAT for iron deficiency is <20%. Because the TSAT can be influenced by iron supplements or iron-rich foods, wait several hours to obtain blood after a patient takes an oral iron supplement or eats iron-rich foods. If desired, clinicians can use either ferritin or TSAT alone to diagnose iron deficiency. However, because ferritin can be falsely normal in inflammatory conditions such as obesity and infection, a TSAT may be needed to confirm iron deficiency if there is a high clinical suspicion despite a normal ferritin level.26
Treatment. If iron deficiency is confirmed, instruct your patient to follow up with their primary care physician or the appropriate specialist to evaluate for any underlying etiologies.
Continue to: Iron deficiency should be treated...
Iron deficiency should be treated with supplementation because diet alone is insufficient for replenishing iron stores. Iron replacement can be oral or IV. Oral replacement is effective, safe, inexpensive, easy to obtain, and easy to administer.27 Oral replacement is recommended for adults whose anemia is not severe or who do not have a comorbid condition such as pregnancy, inflammatory bowel conditions, gastric surgery, or chronic kidney disease. When anemia is severe or a patient has one of these comorbid conditions, IV is the preferred method of replacement.27 In these cases, defer treatment to the patient’s primary care physician or specialist.
There are no clear recommendations on the amount of iron per dose to prescribe.27 The maximum amount of oral iron that can be absorbed is approximately 25 mg/d of elemental iron. A 325 mg ferrous sulfate tablet contains 65 mg of elemental iron, of which approximately 25 mg is absorbed and utilized.27
Emerging evidence suggests that excessive iron dosing may reduce iron absorption and increase adverse effects. In a study of 54 nonanemic young women with iron deficiency who were given iron supplementation, Moretti et al28 found that a large oral dose of iron taken in the morning increased hepcidin, which decreased the absorption of iron taken later for up to 48 hours. They found that 40 to 80 mg of elemental iron given on alternate days may maximize the fractional iron absorbed, increase dosage efficacy, reduce GI exposure to unabsorbed iron, and improve patients’ ability to tolerate iron supplementation.28
Adverse effects from iron supplements occur in up to 70% of patients.27 These can include metallic taste, nausea, vomiting, flatulence, diarrhea, epigastric pain, constipation, and dark stools.27 Using a liquid form may help reduce adverse effects because it can be more easily titrated.27 Tell patients to avoid enteric-coated or sustained-release iron capsules because these are poorly absorbed. Be cautious when prescribing iron supplementation to older adults because these patients tend to have more adverse effects, especially constipation, as well as reduced absorption, and may ultimately need IV treatment. Iron should not be taken with food, calcium supplements, antacids, coffee, tea, or milk.27
The amount of iron present, cost, and adverse effects vary by supplement. The Table27,29-33 provides more information on available forms of iron. Many forms of iron supplementation are available over-the-counter, and most are equally effective.27 Advise patients to use iron products that have been tested by an independent company, such as ConsumerLab.com. Such companies evaluate products to see if they contain the amount of iron listed on the product’s label; for contamination with lead, cadmium, or arsenic; and for the product’s ability to break apart for absorption.34
Six to 8 weeks of treatment with oral iron supplementation may be necessary before anemia is fully resolved, and it may take up to 6 months for iron stores to be repleted.27 If a patient cannot tolerate an iron supplement, reducing the dose or taking it with meals may help prevent adverse effects, but also will reduce absorption. Auerbach27 recommends assessing tolerability and rechecking the patient’s CBC 2 weeks after starting oral iron replacement, while also checking hemoglobin and the reticulocyte count to see if the patient is responding to treatment. An analysis of 5 studies found that a hemoglobin measurement on Day 14 that shows an increase ≥1.0 g/dL from baseline predicts longer-term and sustained treatment response to continued oral therapy.35 There is no clear consensus for target ferritin levels, but we suggest aiming for a ferritin level >100 ug/L based on recommendations for the treatment of restless legs syndrome.36 We recommend ongoing monitoring every 4 to 6 weeks.
Bottom Line
Iron deficiency is common and can cause or contribute to psychiatric symptoms and disorders. Consider screening patients for iron deficiency and treating it with oral supplementation in individuals without any comorbidities, or referring them to their primary care physician or specialist.
Related Resources
- Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2021;3(1):263-275.
1. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454.
2. Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8(8):e572-e582.
3. Snozek CLH, Spears GM, Porco AB, et al. Updated ferritin reference intervals for the Roche Elecsys® immunoassay. Clin Biochem. 2021;87:100-103. doi:10.1016/j.clinbiochem.2020.11.006
4. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. doi:10.1182/blood-2018-05-815944
5. Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158-165.
6. Shah HE, Bhawnani N, Ethirajulu A, et al. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus. 2021;13(9):e18138.
7. Hidese S, Saito K, Asano S, et al. Association between iron-deficiency anemia and depression: a web-based Japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513-521.
8. Yi S, Nanri A, Poudel-Tandukar K, et al. Association between serum ferritin concentrations and depressive symptoms in Japanese municipal employees. Psychiatry Res. 2011;189(3):368-372.
9. Lee HS, Chao HH, Huang WT, et al. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. 2020;20(1):216.
10. Xu M, Guo Y, Cheng J, et al. Brain iron assessment in patients with first-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31:102736.
11. Kim SW, Stewart R, Park WY, et al. Latent iron deficiency as a marker of negative symptoms in patients with first-episode schizophrenia spectrum disorder. Nutrients. 2018;10(11):1707.
12. Chen MH, Su TP, Chen YS, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161.
13. Stewart R, Hirani V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom Med. 2012;74(2):208-213.
14. Hanif N. Anwer F. Chronic iron deficiency. Updated September 10, 2022. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560876/
15.
16. Greig AJ, Patterson AJ, Collins CE, et al. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
17. Mikami K, Akama F, Kimoto K, et al. Iron supplementation for hypoferritinemia-related psychological symptoms in children and adolescents. J Nippon Med Sch. 2022;89(2):203-211.
18. McClung JP, Karl JP, Cable SJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124-131.
19. Kassir A. Iron deficiency: a diagnostic and therapeutic perspective in psychiatry. Article in French. Encephale. 2017;43(1):85-89.
20. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8(4):e019240. doi:10.1136/bmjopen-2017-019240
21. Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222-3227. doi:10.1182/blood-2011-04-346304
22. Vaucher P, Druais PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247-1254. doi:10.1503/cmaj.110950
23. Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618-628. doi:10.1111/ejh.12645
24. Al-Naseem A, Sallam A, Choudhury S, et al. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21(2):107-113. doi:10.7861/clinmed.2020-0582
25. National Institute of Health Office of Dietary Supplements. Iron. Fact sheet for health professionals. Updated April 5, 2022. Accessed January 31, 2023. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
26. Auerbach M. Causes and diagnosis of iron deficiency and iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/causes-and-diagnosis-of-iron-deficiency-and-iron-deficiency-anemia-in-adults
27. Auerbach M. Treatment of iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/treatment-of-iron-deficiency-anemia-in-adults
28. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981-1989.
29. Cooperman T. Iron supplements review (iron pills, liquids and chews). ConsumerLab.com. Published January 31, 2022. Updated December 19, 2022. Accessed January 31, 2023. https://www.consumerlab.com/reviews/iron-supplements-review/iron/
30. Okam MM, Koch TA, Tran MH. Iron deficiency anemia treatment response to oral iron therapy: a pooled analysis of five randomized controlled trials. Haematologica. 2016;101(1):e6-e7.
31. Silber MH. Management of restless legs syndrome and periodic limb movement disorder in adults. UpToDate. Accessed July 10, 2022. https://www.uptodate.com/contents/management-of-restless-legs-syndrome-and-periodic-limb-movement-disorder-in-adults
32. Harvard T.H. Chan School of Public Health. The nutrition source: iron. Accessed January 31, 2023. https://www.hsph.harvard.edu/nutritionsource/iron/
33. Little DR. Ambulatory management of common forms of anemia. Am Fam Physician. 1999;59(6):1598-1604.
34. Blood modifiers. In: Drug Facts and Comparisons. Facts and Comparisons. 1998:238-257.
35. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291-303.
36. Francés AM, Martínez-Bujanda JL. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Curr Med Res Opinion. 2020;36(4):613-623. doi:10.1080/03007995.2020.1716702
Nutritional deficiencies are one of the many causes of or contributors to symptoms in patients with psychiatric disorders. In this article, we discuss the prevalence of iron deficiency and its link to poor mental health, and how proper treatment may improve psychiatric symptoms. We also offer suggestions for how and when to test for and treat iron deficiency in psychiatric patients.
A common condition
Iron deficiency is the most common mineral deficiency in the world. According to the World Health Organization (WHO), approximately 25% of the global population is anemic and nearly one-half of those cases are the result of iron deficiency.1 While the WHO has published guidelines defining iron deficiency as it relates to ferritin levels (<15 ug/L in adults and <12 ug/L in children), this estimate might be low.2,3 Mei et al2 found that hemoglobin and soluble transferrin receptors can be used to determine iron-deficient erythropoiesis, which indicates a physiological definition of iron deficiency. According to a study of children and nonpregnant women by Mei et al,2 children with ferritin levels <20 ug/L and women with ferritin levels <25 ug/L should be considered iron-deficient. If replicated, this study suggests the prevalence of iron deficiency is higher than currently estimated.2 Overall, an estimated 1.2 billion people worldwide have iron-deficiency anemia.4 Additionally, patients can be iron deficient without being anemic, a condition thought to be at least twice as common.4
Essential for brain function
Research shows the importance of iron to proper brain function.5 Iron deficiency in pregnant women is associated with significant neuropsychological impairments in neonates. Rodent studies have demonstrated the importance of iron and the effects of iron deficiency on the hippocampus, corpus striatum, and production of monoamines.5 Specifically, iron is a necessary cofactor in the enzymes tryptophan hydroxylase and tyrosine hydroxylase, which produce serotonin, dopamine, and norepinephrine. In rodent studies, monoamine deficits secondary to iron deficiency persist into adulthood even with iron supplementation, which highlights the importance of preventing iron deficiency during pregnancy and early life.5 While most research has focused on the impact of iron deficiency in infancy and early childhood, iron deficiency has an ongoing impact into adulthood, even if treated.6
Iron deficiency and psychiatric symptoms
Current research suggests an association between iron deficiency or low ferritin levels and psychiatric disorders, specifically depression, anxiety, and schizophrenia. In a web survey of 11,876 adults, Hidese et al7 found an association between a self-reported history of iron deficiency anemia and a self-reported history of depression. Another study of 528 municipal employees found an association between low serum ferritin concentrations and a high prevalence of depressive symptoms among men; no statistically significant association was detected in women.8 In an analysis of the Taiwan National Health Insurance Database from 2000 to 2012, Lee et al9 found a statistically significant increased risk of anxiety disorders, depression, sleep disorders, and psychotic disorders in patients with iron deficiency anemia after controlling for multiple confounders. Xu et al10 used quantitative susceptibility mapping to assess the iron status in certain regions of the brain of 30 patients with first-episode psychosis. They found lower levels of iron in the bilateral substantia nigra, left red nucleus, and left thalamus compared to healthy controls.10 Kim et al11 found an association between iron deficiency and more severe negative symptoms in 121 patients with first-episode psychosis, which supports the hypothesis that iron deficiency may alter dopamine transmission in the brain.
Iron deficiency has been associated with psychopathology across the lifespan. In a population-based study in Taiwan, Chen et al12 found an association between iron deficiency anemia and psychiatric disorders in children and adolescents, including mood disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, and developmental disorders. At the other end of the age spectrum, in a survey of 1,875 older adults in England, Stewart et al13 found an association between low ferritin levels (<45 ng/mL) and depressive symptoms after adjusting for demographic factors and overall health status.
In addition to specific psychiatric disorders and symptoms, iron deficiency is often associated with nonspecific symptoms such as fatigue.14 Fatigue is a symptom of numerous psychiatric disorders and is included in the DSM diagnostic criteria for major depressive disorder and generalized anxiety disorder.15
Iron supplementation might improve psychiatric symptoms
Some evidence suggests that using iron supplementation to treat iron deficiency can improve psychiatric symptoms. In a 2013 systematic literature review of 10 studies, Greig et al16 found a link between low iron status and poor cognition, poor mental health scores, and fatigue among women of childbearing age. In this review, 7 studies demonstrated improvement in cognition and 3 demonstrated improvement in mental health with iron supplementation.16 In a 2021 prospective study, 19 children and adolescents age 6 to 15 who had serum ferritin levels <30 ng/mL were treated with oral iron supplementation for 12 weeks.17 Participants showed significant improvements in sleep quality, depressive symptoms, and general mood as assessed via the Pittsburgh Sleep Quality Index, Center for Epidemiologic Studies Depression Scale, and Profile of Mood States (POMS) questionnaires, respectively.17 A randomized controlled trial of 219 female soldiers who were given iron supplementation or placebo for 8 weeks during basic combat training found that compared to placebo, iron supplementation led to improvements in mood as measured by the POMS questionnaire.18 Lastly, in a 2016 observational study of 412 adult psychiatric patients, Kassir19 found most patients (81%) had iron deficiency, defined as a transferrin saturation coefficient <30% or serum ferritin <100 ng/mL. Although these cutoffs are not considered standard and thus may have overrepresented the percentage of patients considered iron-deficient, more than one-half of patients considered iron-deficient in this study experienced a reduction or elimination of psychiatric symptoms following treatment with iron supplementation and/or psychotropic medications.19
Continue to: Individuals with iron deficiency...
Individuals with iron deficiency without anemia also may see improvement in psychiatric symptoms with iron treatment. In a 2018 systematic review, Houston et al20 evaluated iron supplementation in 1,170 adults who were iron-deficient but not anemic. They found that in these patients, fatigue significantly improved but physical capacity did not.20 Additionally, 2 other studies found iron treatment improved fatigue in nonanemic women.21,22 In a 2016 systematic review, Pratt et al23 concluded, “There is emerging evidence that … nonanemic iron deficiency … is a disease in its own right, deserving of further research in the development of strategies for detection and treatment.” Al-Naseem et al24 suggested severity distinguishes iron deficiency with and without anemia.
Your role in assessing and treating iron deficiency
Testing for and treating iron deficiency generally is not a part of routine psychiatric practice. This might be due to apathy given the pervasiveness of iron deficiency, a belief that iron deficiency should be managed by primary care physicians, or a lack of familiarity with how to treat it and the benefits of such treatment for psychiatric patients. However, assessing for and treating iron deficiency in psychiatric patients is important, especially for individuals who are highly susceptible to inadequate iron levels. People at risk for iron deficiency include pregnant women, infants, young children, women with heavy menstrual bleeding, frequent blood donors, patients with cancer, individuals who have gastrointestinal (GI) surgeries or disorders, and those with heart failure.25
Assessment. Iron status can be assessed through an iron studies panel. Because a patient can have iron deficiency without anemia, a complete blood count (CBC) alone does not suffice.26 The iron panel includes serum iron, serum ferritin, serum transferrin or total iron-binding capacity (TIBC), and calculated transferrin saturation (TSAT), which is the ratio of serum iron to TIBC.
Iron deficiency is diagnosed if ferritin is <30 ng/mL, regardless of the hemoglobin concentration or underlying condition, and confirmed by a low TSAT.26 In most guidelines, the cutoff value for TSAT for iron deficiency is <20%. Because the TSAT can be influenced by iron supplements or iron-rich foods, wait several hours to obtain blood after a patient takes an oral iron supplement or eats iron-rich foods. If desired, clinicians can use either ferritin or TSAT alone to diagnose iron deficiency. However, because ferritin can be falsely normal in inflammatory conditions such as obesity and infection, a TSAT may be needed to confirm iron deficiency if there is a high clinical suspicion despite a normal ferritin level.26
Treatment. If iron deficiency is confirmed, instruct your patient to follow up with their primary care physician or the appropriate specialist to evaluate for any underlying etiologies.
Continue to: Iron deficiency should be treated...
Iron deficiency should be treated with supplementation because diet alone is insufficient for replenishing iron stores. Iron replacement can be oral or IV. Oral replacement is effective, safe, inexpensive, easy to obtain, and easy to administer.27 Oral replacement is recommended for adults whose anemia is not severe or who do not have a comorbid condition such as pregnancy, inflammatory bowel conditions, gastric surgery, or chronic kidney disease. When anemia is severe or a patient has one of these comorbid conditions, IV is the preferred method of replacement.27 In these cases, defer treatment to the patient’s primary care physician or specialist.
There are no clear recommendations on the amount of iron per dose to prescribe.27 The maximum amount of oral iron that can be absorbed is approximately 25 mg/d of elemental iron. A 325 mg ferrous sulfate tablet contains 65 mg of elemental iron, of which approximately 25 mg is absorbed and utilized.27
Emerging evidence suggests that excessive iron dosing may reduce iron absorption and increase adverse effects. In a study of 54 nonanemic young women with iron deficiency who were given iron supplementation, Moretti et al28 found that a large oral dose of iron taken in the morning increased hepcidin, which decreased the absorption of iron taken later for up to 48 hours. They found that 40 to 80 mg of elemental iron given on alternate days may maximize the fractional iron absorbed, increase dosage efficacy, reduce GI exposure to unabsorbed iron, and improve patients’ ability to tolerate iron supplementation.28
Adverse effects from iron supplements occur in up to 70% of patients.27 These can include metallic taste, nausea, vomiting, flatulence, diarrhea, epigastric pain, constipation, and dark stools.27 Using a liquid form may help reduce adverse effects because it can be more easily titrated.27 Tell patients to avoid enteric-coated or sustained-release iron capsules because these are poorly absorbed. Be cautious when prescribing iron supplementation to older adults because these patients tend to have more adverse effects, especially constipation, as well as reduced absorption, and may ultimately need IV treatment. Iron should not be taken with food, calcium supplements, antacids, coffee, tea, or milk.27
The amount of iron present, cost, and adverse effects vary by supplement. The Table27,29-33 provides more information on available forms of iron. Many forms of iron supplementation are available over-the-counter, and most are equally effective.27 Advise patients to use iron products that have been tested by an independent company, such as ConsumerLab.com. Such companies evaluate products to see if they contain the amount of iron listed on the product’s label; for contamination with lead, cadmium, or arsenic; and for the product’s ability to break apart for absorption.34

Six to 8 weeks of treatment with oral iron supplementation may be necessary before anemia is fully resolved, and it may take up to 6 months for iron stores to be repleted.27 If a patient cannot tolerate an iron supplement, reducing the dose or taking it with meals may help prevent adverse effects, but also will reduce absorption. Auerbach27 recommends assessing tolerability and rechecking the patient’s CBC 2 weeks after starting oral iron replacement, while also checking hemoglobin and the reticulocyte count to see if the patient is responding to treatment. An analysis of 5 studies found that a hemoglobin measurement on Day 14 that shows an increase ≥1.0 g/dL from baseline predicts longer-term and sustained treatment response to continued oral therapy.35 There is no clear consensus for target ferritin levels, but we suggest aiming for a ferritin level >100 ug/L based on recommendations for the treatment of restless legs syndrome.36 We recommend ongoing monitoring every 4 to 6 weeks.
Bottom Line
Iron deficiency is common and can cause or contribute to psychiatric symptoms and disorders. Consider screening patients for iron deficiency and treating it with oral supplementation in individuals without any comorbidities, or referring them to their primary care physician or specialist.
Related Resources
- Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2021;3(1):263-275.
Nutritional deficiencies are one of the many causes of or contributors to symptoms in patients with psychiatric disorders. In this article, we discuss the prevalence of iron deficiency and its link to poor mental health, and how proper treatment may improve psychiatric symptoms. We also offer suggestions for how and when to test for and treat iron deficiency in psychiatric patients.
A common condition
Iron deficiency is the most common mineral deficiency in the world. According to the World Health Organization (WHO), approximately 25% of the global population is anemic and nearly one-half of those cases are the result of iron deficiency.1 While the WHO has published guidelines defining iron deficiency as it relates to ferritin levels (<15 ug/L in adults and <12 ug/L in children), this estimate might be low.2,3 Mei et al2 found that hemoglobin and soluble transferrin receptors can be used to determine iron-deficient erythropoiesis, which indicates a physiological definition of iron deficiency. According to a study of children and nonpregnant women by Mei et al,2 children with ferritin levels <20 ug/L and women with ferritin levels <25 ug/L should be considered iron-deficient. If replicated, this study suggests the prevalence of iron deficiency is higher than currently estimated.2 Overall, an estimated 1.2 billion people worldwide have iron-deficiency anemia.4 Additionally, patients can be iron deficient without being anemic, a condition thought to be at least twice as common.4
Essential for brain function
Research shows the importance of iron to proper brain function.5 Iron deficiency in pregnant women is associated with significant neuropsychological impairments in neonates. Rodent studies have demonstrated the importance of iron and the effects of iron deficiency on the hippocampus, corpus striatum, and production of monoamines.5 Specifically, iron is a necessary cofactor in the enzymes tryptophan hydroxylase and tyrosine hydroxylase, which produce serotonin, dopamine, and norepinephrine. In rodent studies, monoamine deficits secondary to iron deficiency persist into adulthood even with iron supplementation, which highlights the importance of preventing iron deficiency during pregnancy and early life.5 While most research has focused on the impact of iron deficiency in infancy and early childhood, iron deficiency has an ongoing impact into adulthood, even if treated.6
Iron deficiency and psychiatric symptoms
Current research suggests an association between iron deficiency or low ferritin levels and psychiatric disorders, specifically depression, anxiety, and schizophrenia. In a web survey of 11,876 adults, Hidese et al7 found an association between a self-reported history of iron deficiency anemia and a self-reported history of depression. Another study of 528 municipal employees found an association between low serum ferritin concentrations and a high prevalence of depressive symptoms among men; no statistically significant association was detected in women.8 In an analysis of the Taiwan National Health Insurance Database from 2000 to 2012, Lee et al9 found a statistically significant increased risk of anxiety disorders, depression, sleep disorders, and psychotic disorders in patients with iron deficiency anemia after controlling for multiple confounders. Xu et al10 used quantitative susceptibility mapping to assess the iron status in certain regions of the brain of 30 patients with first-episode psychosis. They found lower levels of iron in the bilateral substantia nigra, left red nucleus, and left thalamus compared to healthy controls.10 Kim et al11 found an association between iron deficiency and more severe negative symptoms in 121 patients with first-episode psychosis, which supports the hypothesis that iron deficiency may alter dopamine transmission in the brain.
Iron deficiency has been associated with psychopathology across the lifespan. In a population-based study in Taiwan, Chen et al12 found an association between iron deficiency anemia and psychiatric disorders in children and adolescents, including mood disorders, autism spectrum disorder, attention-deficit/hyperactivity disorder, and developmental disorders. At the other end of the age spectrum, in a survey of 1,875 older adults in England, Stewart et al13 found an association between low ferritin levels (<45 ng/mL) and depressive symptoms after adjusting for demographic factors and overall health status.
In addition to specific psychiatric disorders and symptoms, iron deficiency is often associated with nonspecific symptoms such as fatigue.14 Fatigue is a symptom of numerous psychiatric disorders and is included in the DSM diagnostic criteria for major depressive disorder and generalized anxiety disorder.15
Iron supplementation might improve psychiatric symptoms
Some evidence suggests that using iron supplementation to treat iron deficiency can improve psychiatric symptoms. In a 2013 systematic literature review of 10 studies, Greig et al16 found a link between low iron status and poor cognition, poor mental health scores, and fatigue among women of childbearing age. In this review, 7 studies demonstrated improvement in cognition and 3 demonstrated improvement in mental health with iron supplementation.16 In a 2021 prospective study, 19 children and adolescents age 6 to 15 who had serum ferritin levels <30 ng/mL were treated with oral iron supplementation for 12 weeks.17 Participants showed significant improvements in sleep quality, depressive symptoms, and general mood as assessed via the Pittsburgh Sleep Quality Index, Center for Epidemiologic Studies Depression Scale, and Profile of Mood States (POMS) questionnaires, respectively.17 A randomized controlled trial of 219 female soldiers who were given iron supplementation or placebo for 8 weeks during basic combat training found that compared to placebo, iron supplementation led to improvements in mood as measured by the POMS questionnaire.18 Lastly, in a 2016 observational study of 412 adult psychiatric patients, Kassir19 found most patients (81%) had iron deficiency, defined as a transferrin saturation coefficient <30% or serum ferritin <100 ng/mL. Although these cutoffs are not considered standard and thus may have overrepresented the percentage of patients considered iron-deficient, more than one-half of patients considered iron-deficient in this study experienced a reduction or elimination of psychiatric symptoms following treatment with iron supplementation and/or psychotropic medications.19
Continue to: Individuals with iron deficiency...
Individuals with iron deficiency without anemia also may see improvement in psychiatric symptoms with iron treatment. In a 2018 systematic review, Houston et al20 evaluated iron supplementation in 1,170 adults who were iron-deficient but not anemic. They found that in these patients, fatigue significantly improved but physical capacity did not.20 Additionally, 2 other studies found iron treatment improved fatigue in nonanemic women.21,22 In a 2016 systematic review, Pratt et al23 concluded, “There is emerging evidence that … nonanemic iron deficiency … is a disease in its own right, deserving of further research in the development of strategies for detection and treatment.” Al-Naseem et al24 suggested severity distinguishes iron deficiency with and without anemia.
Your role in assessing and treating iron deficiency
Testing for and treating iron deficiency generally is not a part of routine psychiatric practice. This might be due to apathy given the pervasiveness of iron deficiency, a belief that iron deficiency should be managed by primary care physicians, or a lack of familiarity with how to treat it and the benefits of such treatment for psychiatric patients. However, assessing for and treating iron deficiency in psychiatric patients is important, especially for individuals who are highly susceptible to inadequate iron levels. People at risk for iron deficiency include pregnant women, infants, young children, women with heavy menstrual bleeding, frequent blood donors, patients with cancer, individuals who have gastrointestinal (GI) surgeries or disorders, and those with heart failure.25
Assessment. Iron status can be assessed through an iron studies panel. Because a patient can have iron deficiency without anemia, a complete blood count (CBC) alone does not suffice.26 The iron panel includes serum iron, serum ferritin, serum transferrin or total iron-binding capacity (TIBC), and calculated transferrin saturation (TSAT), which is the ratio of serum iron to TIBC.
Iron deficiency is diagnosed if ferritin is <30 ng/mL, regardless of the hemoglobin concentration or underlying condition, and confirmed by a low TSAT.26 In most guidelines, the cutoff value for TSAT for iron deficiency is <20%. Because the TSAT can be influenced by iron supplements or iron-rich foods, wait several hours to obtain blood after a patient takes an oral iron supplement or eats iron-rich foods. If desired, clinicians can use either ferritin or TSAT alone to diagnose iron deficiency. However, because ferritin can be falsely normal in inflammatory conditions such as obesity and infection, a TSAT may be needed to confirm iron deficiency if there is a high clinical suspicion despite a normal ferritin level.26
Treatment. If iron deficiency is confirmed, instruct your patient to follow up with their primary care physician or the appropriate specialist to evaluate for any underlying etiologies.
Continue to: Iron deficiency should be treated...
Iron deficiency should be treated with supplementation because diet alone is insufficient for replenishing iron stores. Iron replacement can be oral or IV. Oral replacement is effective, safe, inexpensive, easy to obtain, and easy to administer.27 Oral replacement is recommended for adults whose anemia is not severe or who do not have a comorbid condition such as pregnancy, inflammatory bowel conditions, gastric surgery, or chronic kidney disease. When anemia is severe or a patient has one of these comorbid conditions, IV is the preferred method of replacement.27 In these cases, defer treatment to the patient’s primary care physician or specialist.
There are no clear recommendations on the amount of iron per dose to prescribe.27 The maximum amount of oral iron that can be absorbed is approximately 25 mg/d of elemental iron. A 325 mg ferrous sulfate tablet contains 65 mg of elemental iron, of which approximately 25 mg is absorbed and utilized.27
Emerging evidence suggests that excessive iron dosing may reduce iron absorption and increase adverse effects. In a study of 54 nonanemic young women with iron deficiency who were given iron supplementation, Moretti et al28 found that a large oral dose of iron taken in the morning increased hepcidin, which decreased the absorption of iron taken later for up to 48 hours. They found that 40 to 80 mg of elemental iron given on alternate days may maximize the fractional iron absorbed, increase dosage efficacy, reduce GI exposure to unabsorbed iron, and improve patients’ ability to tolerate iron supplementation.28
Adverse effects from iron supplements occur in up to 70% of patients.27 These can include metallic taste, nausea, vomiting, flatulence, diarrhea, epigastric pain, constipation, and dark stools.27 Using a liquid form may help reduce adverse effects because it can be more easily titrated.27 Tell patients to avoid enteric-coated or sustained-release iron capsules because these are poorly absorbed. Be cautious when prescribing iron supplementation to older adults because these patients tend to have more adverse effects, especially constipation, as well as reduced absorption, and may ultimately need IV treatment. Iron should not be taken with food, calcium supplements, antacids, coffee, tea, or milk.27
The amount of iron present, cost, and adverse effects vary by supplement. The Table27,29-33 provides more information on available forms of iron. Many forms of iron supplementation are available over-the-counter, and most are equally effective.27 Advise patients to use iron products that have been tested by an independent company, such as ConsumerLab.com. Such companies evaluate products to see if they contain the amount of iron listed on the product’s label; for contamination with lead, cadmium, or arsenic; and for the product’s ability to break apart for absorption.34

Six to 8 weeks of treatment with oral iron supplementation may be necessary before anemia is fully resolved, and it may take up to 6 months for iron stores to be repleted.27 If a patient cannot tolerate an iron supplement, reducing the dose or taking it with meals may help prevent adverse effects, but also will reduce absorption. Auerbach27 recommends assessing tolerability and rechecking the patient’s CBC 2 weeks after starting oral iron replacement, while also checking hemoglobin and the reticulocyte count to see if the patient is responding to treatment. An analysis of 5 studies found that a hemoglobin measurement on Day 14 that shows an increase ≥1.0 g/dL from baseline predicts longer-term and sustained treatment response to continued oral therapy.35 There is no clear consensus for target ferritin levels, but we suggest aiming for a ferritin level >100 ug/L based on recommendations for the treatment of restless legs syndrome.36 We recommend ongoing monitoring every 4 to 6 weeks.
Bottom Line
Iron deficiency is common and can cause or contribute to psychiatric symptoms and disorders. Consider screening patients for iron deficiency and treating it with oral supplementation in individuals without any comorbidities, or referring them to their primary care physician or specialist.
Related Resources
- Berthou C, Iliou JP, Barba D. Iron, neuro-bioavailability and depression. EJHaem. 2021;3(1):263-275.
1. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454.
2. Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8(8):e572-e582.
3. Snozek CLH, Spears GM, Porco AB, et al. Updated ferritin reference intervals for the Roche Elecsys® immunoassay. Clin Biochem. 2021;87:100-103. doi:10.1016/j.clinbiochem.2020.11.006
4. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. doi:10.1182/blood-2018-05-815944
5. Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158-165.
6. Shah HE, Bhawnani N, Ethirajulu A, et al. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus. 2021;13(9):e18138.
7. Hidese S, Saito K, Asano S, et al. Association between iron-deficiency anemia and depression: a web-based Japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513-521.
8. Yi S, Nanri A, Poudel-Tandukar K, et al. Association between serum ferritin concentrations and depressive symptoms in Japanese municipal employees. Psychiatry Res. 2011;189(3):368-372.
9. Lee HS, Chao HH, Huang WT, et al. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. 2020;20(1):216.
10. Xu M, Guo Y, Cheng J, et al. Brain iron assessment in patients with first-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31:102736.
11. Kim SW, Stewart R, Park WY, et al. Latent iron deficiency as a marker of negative symptoms in patients with first-episode schizophrenia spectrum disorder. Nutrients. 2018;10(11):1707.
12. Chen MH, Su TP, Chen YS, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161.
13. Stewart R, Hirani V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom Med. 2012;74(2):208-213.
14. Hanif N. Anwer F. Chronic iron deficiency. Updated September 10, 2022. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560876/
15.
16. Greig AJ, Patterson AJ, Collins CE, et al. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
17. Mikami K, Akama F, Kimoto K, et al. Iron supplementation for hypoferritinemia-related psychological symptoms in children and adolescents. J Nippon Med Sch. 2022;89(2):203-211.
18. McClung JP, Karl JP, Cable SJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124-131.
19. Kassir A. Iron deficiency: a diagnostic and therapeutic perspective in psychiatry. Article in French. Encephale. 2017;43(1):85-89.
20. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8(4):e019240. doi:10.1136/bmjopen-2017-019240
21. Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222-3227. doi:10.1182/blood-2011-04-346304
22. Vaucher P, Druais PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247-1254. doi:10.1503/cmaj.110950
23. Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618-628. doi:10.1111/ejh.12645
24. Al-Naseem A, Sallam A, Choudhury S, et al. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21(2):107-113. doi:10.7861/clinmed.2020-0582
25. National Institute of Health Office of Dietary Supplements. Iron. Fact sheet for health professionals. Updated April 5, 2022. Accessed January 31, 2023. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
26. Auerbach M. Causes and diagnosis of iron deficiency and iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/causes-and-diagnosis-of-iron-deficiency-and-iron-deficiency-anemia-in-adults
27. Auerbach M. Treatment of iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/treatment-of-iron-deficiency-anemia-in-adults
28. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981-1989.
29. Cooperman T. Iron supplements review (iron pills, liquids and chews). ConsumerLab.com. Published January 31, 2022. Updated December 19, 2022. Accessed January 31, 2023. https://www.consumerlab.com/reviews/iron-supplements-review/iron/
30. Okam MM, Koch TA, Tran MH. Iron deficiency anemia treatment response to oral iron therapy: a pooled analysis of five randomized controlled trials. Haematologica. 2016;101(1):e6-e7.
31. Silber MH. Management of restless legs syndrome and periodic limb movement disorder in adults. UpToDate. Accessed July 10, 2022. https://www.uptodate.com/contents/management-of-restless-legs-syndrome-and-periodic-limb-movement-disorder-in-adults
32. Harvard T.H. Chan School of Public Health. The nutrition source: iron. Accessed January 31, 2023. https://www.hsph.harvard.edu/nutritionsource/iron/
33. Little DR. Ambulatory management of common forms of anemia. Am Fam Physician. 1999;59(6):1598-1604.
34. Blood modifiers. In: Drug Facts and Comparisons. Facts and Comparisons. 1998:238-257.
35. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291-303.
36. Francés AM, Martínez-Bujanda JL. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Curr Med Res Opinion. 2020;36(4):613-623. doi:10.1080/03007995.2020.1716702
1. McLean E, Cogswell M, Egli I, et al. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993-2005. Public Health Nutr. 2009;12(4):444-454.
2. Mei Z, Addo OY, Jefferds ME, et al. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. 2021;8(8):e572-e582.
3. Snozek CLH, Spears GM, Porco AB, et al. Updated ferritin reference intervals for the Roche Elecsys® immunoassay. Clin Biochem. 2021;87:100-103. doi:10.1016/j.clinbiochem.2020.11.006
4. Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. doi:10.1182/blood-2018-05-815944
5. Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158-165.
6. Shah HE, Bhawnani N, Ethirajulu A, et al. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus. 2021;13(9):e18138.
7. Hidese S, Saito K, Asano S, et al. Association between iron-deficiency anemia and depression: a web-based Japanese investigation. Psychiatry Clin Neurosci. 2018;72(7):513-521.
8. Yi S, Nanri A, Poudel-Tandukar K, et al. Association between serum ferritin concentrations and depressive symptoms in Japanese municipal employees. Psychiatry Res. 2011;189(3):368-372.
9. Lee HS, Chao HH, Huang WT, et al. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: a nationwide database analysis. BMC Psychiatry. 2020;20(1):216.
10. Xu M, Guo Y, Cheng J, et al. Brain iron assessment in patients with first-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021;31:102736.
11. Kim SW, Stewart R, Park WY, et al. Latent iron deficiency as a marker of negative symptoms in patients with first-episode schizophrenia spectrum disorder. Nutrients. 2018;10(11):1707.
12. Chen MH, Su TP, Chen YS, et al. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: a nationwide population-based study. BMC Psychiatry. 2013;13:161.
13. Stewart R, Hirani V. Relationship between depressive symptoms, anemia, and iron status in older residents from a national survey population. Psychosom Med. 2012;74(2):208-213.
14. Hanif N. Anwer F. Chronic iron deficiency. Updated September 10, 2022. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK560876/
15.
16. Greig AJ, Patterson AJ, Collins CE, et al. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: a systematic review. J Nutr Sci. 2013;2:e14.
17. Mikami K, Akama F, Kimoto K, et al. Iron supplementation for hypoferritinemia-related psychological symptoms in children and adolescents. J Nippon Med Sch. 2022;89(2):203-211.
18. McClung JP, Karl JP, Cable SJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr. 2009;90(1):124-131.
19. Kassir A. Iron deficiency: a diagnostic and therapeutic perspective in psychiatry. Article in French. Encephale. 2017;43(1):85-89.
20. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: a systematic review of randomised controlled trials. BMJ Open. 2018;8(4):e019240. doi:10.1136/bmjopen-2017-019240
21. Krayenbuehl PA, Battegay E, Breymann C, et al. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood. 2011;118(12):3222-3227. doi:10.1182/blood-2011-04-346304
22. Vaucher P, Druais PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184(11):1247-1254. doi:10.1503/cmaj.110950
23. Pratt JJ, Khan KS. Non-anaemic iron deficiency - a disease looking for recognition of diagnosis: a systematic review. Eur J Haematol. 2016;96(6):618-628. doi:10.1111/ejh.12645
24. Al-Naseem A, Sallam A, Choudhury S, et al. Iron deficiency without anaemia: a diagnosis that matters. Clin Med (Lond). 2021;21(2):107-113. doi:10.7861/clinmed.2020-0582
25. National Institute of Health Office of Dietary Supplements. Iron. Fact sheet for health professionals. Updated April 5, 2022. Accessed January 31, 2023. https://ods.od.nih.gov/factsheets/Iron-HealthProfessional/
26. Auerbach M. Causes and diagnosis of iron deficiency and iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/causes-and-diagnosis-of-iron-deficiency-and-iron-deficiency-anemia-in-adults
27. Auerbach M. Treatment of iron deficiency anemia in adults. UpToDate. Accessed July 8, 2022. https://www.uptodate.com/contents/treatment-of-iron-deficiency-anemia-in-adults
28. Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981-1989.
29. Cooperman T. Iron supplements review (iron pills, liquids and chews). ConsumerLab.com. Published January 31, 2022. Updated December 19, 2022. Accessed January 31, 2023. https://www.consumerlab.com/reviews/iron-supplements-review/iron/
30. Okam MM, Koch TA, Tran MH. Iron deficiency anemia treatment response to oral iron therapy: a pooled analysis of five randomized controlled trials. Haematologica. 2016;101(1):e6-e7.
31. Silber MH. Management of restless legs syndrome and periodic limb movement disorder in adults. UpToDate. Accessed July 10, 2022. https://www.uptodate.com/contents/management-of-restless-legs-syndrome-and-periodic-limb-movement-disorder-in-adults
32. Harvard T.H. Chan School of Public Health. The nutrition source: iron. Accessed January 31, 2023. https://www.hsph.harvard.edu/nutritionsource/iron/
33. Little DR. Ambulatory management of common forms of anemia. Am Fam Physician. 1999;59(6):1598-1604.
34. Blood modifiers. In: Drug Facts and Comparisons. Facts and Comparisons. 1998:238-257.
35. Cancelo-Hidalgo MJ, Castelo-Branco C, Palacios S, et al. Tolerability of different oral iron supplements: a systematic review. Curr Med Res Opin. 2013;29(4):291-303.
36. Francés AM, Martínez-Bujanda JL. Efficacy and tolerability of oral iron protein succinylate: a systematic review of three decades of research. Curr Med Res Opinion. 2020;36(4):613-623. doi:10.1080/03007995.2020.1716702
Managing patients with comorbid opioid and alcohol use disorders
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.
CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.

CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
When left untreated, opioid use disorder (OUD) is a debilitating and potentially lethal illness. Despite the availability of safe and effective medications for OUD, the prevalence of opioid use and overdose deaths has been increasing every year.1 An additional challenge in OUD treatment is the high prevalence of comorbid alcohol use disorder (AUD).2-6 A Clinical Trials Network survey from the National Institute on Drug Abuse found 38% of persons seeking treatment for OUD also had AUD.7 Other analyses have found alcohol was involved in approximately one-fifth of opioid-related deaths.8 Research also reveals that comorbid OUD and AUD contributes to poor treatment outcomes, more medical comorbidities, and a high risk of death (including overdose death).4,9 There is no standard of care for this particular patient population.3 This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
To illustrate the various decision points, we will follow 2 hypothetical patients through various stages of treatment (Figure), from their presentation in the emergency department (ED) or outpatient clinic, through their hospital admission (if needed), and into their outpatient follow-up treatment.

CASE REPORTS
Ms. A and Ms. B present to the ED for evaluation of nausea, vomiting, sweating, anxiety, and tremor. Both patients describe their most recent use of both alcohol and opioids approximately 12 hours ago, and each has been attempting to stop using both substances at home.
Decision-making in the emergency setting
In the ED, a few important decisions need to be made regarding treatment:
- Are the presenting symptoms primarily due to alcohol withdrawal syndrome (AWS), opioid withdrawal syndrome (OWS), or both?
- Does the patient require inpatient medical withdrawal management (detoxification) based on the history and severity of the withdrawal symptoms?
- What are the patient’s treatment goals for their AUD and OUD?
- Is maintenance medication for OUD indicated? If so, which medication is most appropriate?
In the ED, the presentation of individuals affected by both OUD and AUD can be challenging because OWS shares overlapping features with AWS, including nausea, vomiting, diarrhea, sweating, anxiety, and tremor. However, although acute OWS is typically very uncomfortable, it is rarely lethal. On the other hand, severe AWS may result in delirium, seizures, and death,10 which makes it essential to recognize and treat appropriately.
Both Ms. A and Ms. B should be medically evaluated and treated by an emergency medicine physician in conjunction with psychiatric (or addiction medicine) consultation. The ED assessment of a patient presenting with both AUD and OUD should include vital signs monitoring; physical examination; blood work including comprehensive metabolic panel, serum magnesium, and phosphorus; complete blood count; pregnancy test for women of reproductive age; urine drug screen (UDS); urinalysis; and serum ethanol level. Of note, sympathetic hyperactivity is found in both alcohol and opioid withdrawal, and patients with alcohol withdrawal may also have hypokalemia, a condition associated with an increased risk of arrhythmia. Furthermore, a prolonged QTc would affect clinical decision-making about medications for OUD (ie, methadone) and withdrawal management (ie, ondansetron, trazodone, and hydroxyzine). Therefore, an electrocardiogram should be conducted, where appropriate.
Initial treatment of AWS includes vitamin supplementation (thiamine, folic acid, and multivitamins) and benzodiazepine administration (symptom-triggered and/or scheduled taper). It may also include IV fluid resuscitation, analgesics for pain, ondansetron for nausea and vomiting, and other electrolyte repletion as indicated by the laboratory results.11 Additional measures for patients in opioid withdrawal should include alpha-2 agonists such as clonidine or lofexidine for adrenergic symptoms, antiemetics, antidiarrheals, muscle relaxants, anxiolytics such as hydroxyzine, and sleep medications such as trazodone.12
Continue to: The next decision...
The next decision is whether the patient needs to be admitted for inpatient treatment. This decision is based primarily on the risk assessment and severity of AWS, including a compelling history of complicated AWS such as seizures or delirium tremens as well as consideration of the complexity and severity of any comorbid medical or psychiatric conditions. Other indications for medical withdrawal management include a history of unsuccessful ambulatory withdrawal management and pregnancy. For severe AWS, a scheduled benzodiazepine taper in addition to the symptom-triggered protocol should be considered.13-15 A psychiatric evaluation may be obtained in the ED, as long as the patient is sober enough to meaningfully participate in the psychiatric interview. Wherever possible, psychiatric interviews should be supplemented by collateral information.
CASE REPORTS CONTINUED
Ms. A admits to a 5-year history of alcohol and opioid use that meets the criteria for severe AUD and severe OUD. She has previously required inpatient treatment for seizures related to AWS. Laboratory results are notable for a serum ethanol level of 380 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Disposition of patients in alcohol and opioid withdrawal
Given Ms. A’s history of seizures while withdrawing from alcohol, she is appropriate for hospital admission for medically managed withdrawal observation. As previously mentioned, there is clinical overlap between AWS and OWS, and differentiating between the 2 syndromes is essential and may be lifesaving. Whereas anxiety, agitation, diaphoresis, tachycardia, hypertension, and insomnia can be seen in both opioid and alcohol withdrawal, OWS-specific symptoms include mydriasis, lacrimation, rhinorrhea, bone or joint aches, yawning, and piloerection. AWS may present with visual or tactile hallucinations, delirium, and grand mal seizures.15
The details of inpatient management are beyond the scope of this article; however, both patients should be started on thiamine, folic acid, and a multivitamin. For patients in alcohol withdrawal with a history of poor diet who appear malnourished or have a history of malabsorption (such as gastric bypass surgery), thiamine 100 mg/d IV should be given for 3 to 5 days to prevent Wernicke encephalopathy.16 Where there is any concern the patient may be exhibiting signs of Wernicke-Korsakoff Syndrome (impaired cognition, evident malnourishment, ataxia, or eye movement abnormalities), high-dose thiamine IV should be given presumptively as follows: 500 mg IV 3 times a day for 3 days, 250 mg/d IV for 5 days, and then oral supplementation 100 mg/d for at least 30 days.17
In summary, on presentation to the ED, both patients should be medically stabilized and started on benzodiazepines for alcohol withdrawal. The risk assessment and the severity of the AWS often determines the level of care.
CASE REPORTS CONTINUED
On hospital Day 2, Ms. A tells the consulting psychiatrist she would like to start medications to treat her substance use disorders. She has a long history of failed attempts to achieve abstinence from opioids, so she and the psychiatrist agree to initiate a trial of buprenorphine/naloxone for her OUD, 4 mg/1 mg to 8 mg/2 mg for Day 1. Although buprenorphine/naloxone seems to help her alcohol cravings somewhat, she requests additional help. She experiences migraine headaches, which is in part why she began using opioid medications. Via joint decision making with her psychiatrist, she agrees to a trial of topiramate, with a slow titration schedule starting at 25 mg/d.
Continue to: Management decisions
Management decisions: Buprenorphine for OUD
The next issue is to determine the appropriate treatment for the patient’s OUD. Although treating OWS is important in improving the patient’s health, decreasing their discomfort, and facilitating their participation in a psychosocial treatment program,18 current evidence suggests that opioid withdrawal management alone without medication for OUD rarely leads to long-term recovery.19,20 Some research suggests that the risk of accidental opioid overdose immediately following acute withdrawal management may actually be increased due to decreased tolerance in these patients.12,21,22
Three medications have the most evidence for OUD treatment: buprenorphine, methadone, and naltrexone.15 The decision to use buprenorphine, methadone, or naltrexone depends on a variety of factors, including the severity of the OUD, patient history of prior treatment successes and failures, comorbid medical and psychiatric conditions, and patient preference.4 Treatment with buprenorphine or methadone is preferred over naltrexone for patients who do not want to or cannot tolerate the physical and emotional discomfort of the opioid withdrawal process, who experience moderate to severe OUD, who have a history of failed abstinence-based treatment, or who have more severe physiological tolerance/dependence.12 Buprenorphine is a mu opioid receptor partial agonist that has been shown to reduce opioid cravings,23 provide moderate pain relief,24 and ameliorate OWS.12 It does not typically result in significant respiratory depression, which is the biggest safety concern for opioid use.12 Buprenorphine may also treat comorbid AUD at higher doses; however, the data are inconclusive.25,26 Buprenorphine should be prescribed with caution to patients with comorbid, uncontrolled AUD, due to the risk of respiratory depression when combined with alcohol. Patients who continue to drink alcohol but are able to abstain from opioids may consider starting an AUD-specific medication. Pharmacologic options are discussed in more detail in the next section.
For patients who have higher physiological dependence or more severe OUD, methadone may be a reasonable alternative to buprenorphine. Methadone, a mu-opioid receptor agonist, ameliorates OWS, reduces opioid cravings, and reduces the euphoric effects of opioid ingestion if the patient relapses. However, methadone can only be dispensed for the treatment of OUD by a federally-certified treatment program governed by restrictive and federally mandated guidelines. Compared to buprenorphine, methadone is more dangerous in overdose, has more drug interactions, and is more commonly diverted for recreational use.27 Furthermore, methadone should be prescribed with caution to patients with comorbid, uncontrolled AUD, because both alcohol and methadone can result in respiratory depression.
By contrast, the first-line treatment for individuals experiencing moderateto severe AUD is typically naltrexone.28 Naltrexone is contraindicated in Ms. A because she has a severe OUD and is unlikely to tolerate the opioid withdrawal process. Research suggests that the use of naltrexone for OUD should be limited to patients who have a mild disorder or who show low physiological dependence.29 Alternatively, acamprosate, disulfiram, topiramate, or gabapentin should be considered for Ms. A.4,28,30 Because each of these medications have specific strengths and weaknesses, medication selection should be based on individual patient factors such as comorbid psychiatric and medical conditions and/or patient preference.28
Management decisions: AUD augmentation strategies
Naltrexone is contraindicated for patients who are receiving opioids, including opioid agonist therapy for OUD. Therefore, clinicians need to consider other options for these individuals. There are several medications with good evidence, including acamprosate, disulfiram, topiramate, and gabapentin. Acamprosate and disulfiram are FDA-approved for AUD; the latter 2 have been used off-label.
Continue to: Acamprosate is a glutamate receptor modulator...
Acamprosate is a glutamate receptor modulator that reduces alcohol cravings and is recommended for patients who have achieved and wish to maintain abstinence. It can be used in patients with liver disease, because it is not hepatically metabolized.30 Topiramate is also used to reduce alcohol cravings. It antagonizes glutamate at alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) and kainite receptors, facilitates gamma-aminobutyric acid (GABA) function, and reduces the extracellular release of dopamine in the mesocorticolimbic regions of the brain.30 Topiramate is a reasonable option for patients with a seizure disorder, a history of migraine headaches,30 or who are overweight or obese and wish to lose weight.31 In a nonrandomized study, topiramate reduced alcohol intake and cravings more than naltrexone.32
Disulfiram is another second-line therapy for AUD. It is best used under close supervision because it does not reduce alcohol cravings but makes ingesting alcohol extremely aversive by preventing the breakdown of the alcohol metabolite acetaldehyde, and in doing so causes a cluster of unpleasant symptoms, including sweating, palpitations, flushing, nausea/vomiting, and increased sympathetic tone.28 Disulfiram only works if it is taken daily, and it requires a high degree of motivation and/or daily supervision at home or in the clinic.33 It is not recommended to be used as a first-line treatment based on its potential toxicity, adverse effects, and mixed findings on its efficacy. In addition, it should not be given to medically vulnerable/fragile individuals.
Lastly, gabapentin, a voltage-gated calcium channel modulator, may also be used as a second-line agent for AUD. Patients who have started alcohol withdrawal management with gabapentin may wish to continue treatment to assist with craving suppression.30 It is also a good choice for patients who have comorbid diabetic neuropathy or other neuropathic pain conditions, anxiety, or insomnia.30,34 Of note, there have been reports of gabapentin misuse.
CASE REPORTS CONTINUED
Ms. B presents to the ED with a 5-year history of moderate AUD and a 2-year history of mild OUD. She denies a history of severe or complicated AWS. Her laboratory results are significant for a serum ethanol level of 250 mg/dL, UDS positive for opioids, and a negative pregnancy test.
Management decisions: Naltrexone for OUD
In contrast to Ms. A, Ms. B is likely able to complete the opioid withdrawal management process. It is reasonable to treat her uncomplicated, moderate alcohol withdrawal as an outpatient with gabapentin or a benzodiazepine taper. Had her AUD been as severe as Ms. A’s, or if she were unsuccessful with ambulatory withdrawal treatment attempts, Ms. B would also be a candidate for inpatient medical treatment for alcohol withdrawal regardless of the severity of her OUD. Ongoing pharmacotherapy for her AUD after withdrawal management is the same as previously outlined. After Ms. B completes the taper (typically 1 week after the ED visit), she should follow up for initiation of pharmacotherapy for AUD. Ms. B is an ideal candidate for naltrexone, which targets both AUD and OUD.
Continue to: Naltrexone is a semi-synthetic...
Naltrexone is a semi-synthetic competitive antagonist at mu-opioid receptors and a partial agonist at kappa receptors; it has little to no activity at delta receptors. Naltrexone has been shown to reduce alcohol cravings and diminish the euphoric effects of alcohol by reducing endogenous opioid release and receptor activation.35 Thus, even when patients do use alcohol while taking naltrexone, the amount of alcohol they use is typically substantially reduced.36 In fact, at a standard dose of 50 mg/d, 95% of mu-opioid receptors are occupied and are shown to yield approximately 40% alcohol abstinence rates at 1 year.36
Once Ms. B has completed withdrawal management from both alcohol and opioids, she should have a trial period of oral naltrexone to prove tolerability, and then transition to the long-acting injectable (LAI) formulation. Patients able to complete withdrawal management from opioids and transition to LAI naltrexone have been shown to have equivalent rates of successful abstinence from opioids compared to buprenorphine.37 Though Ms. B could opt to try buprenorphine to treat her mild OUD, naltrexone would be the preferred option because it has 3 advantages:
- it blocks the mu-opioid receptor, which prevents euphoria if an illicit substance is used
- it does not cause physiologic dependence or withdrawal syndrome if/when stopped
- if it is not effective, it is easy to switch to buprenorphine.
Lastly, all patients with OUD should be prescribed a rescue naloxone kit, in accordance with harm-reduction guidelines. Naloxone, a potent opioid receptor antagonist, is used to prevent or reverse respiratory depression in opioid overdose. Naloxone rescue kits include intranasal naloxone, which makes it easy for nonclinician bystanders to administer while waiting for emergency transport.38 Most states allow naloxone kits to be prescribed to individuals who have a concern for overdose among friends, family, or others in the community. The wide distribution and easy availability of naloxone rescue kits have been essential in decreasing overdose deaths among patients who misuse opioids.39
Take-home points
Patients with both OUD and AUD are relatively common and often pose significant management challenges when they present to the clinic or the ED in withdrawal. Because severe AWS can be life-threatening, hospitalization should be considered. OWS is often accompanied by intense cravings that can lead to relapse and the risk of accidental opioid overdose/death. As soon as patients are able to engage in a discussion about their treatment options, clinicians need to clarify the patient’s goals and priorities. In medications for OUD, the decision of whether to use buprenorphine, naltrexone, or methadone is guided by the severity of the OUD, the patient’s past treatment experience (illicit as well as prescribed), and patient preference. If the OUD is mild or if the patient prefers to avoid opioid agonist medications and can tolerate the opioid withdrawal process, both the AUD and OUD can be treated with naltrexone, preferably with the LAI formulation. Other AUD medications and outpatient psychotherapy may be used to augment treatment outcomes. For patients with a moderate to severe OUD, buprenorphine (preferably with immediate initiation) or methadone therapy should be offered. Patients with comorbid OUD and AUD who are treated with opioid agonists should be offered medication for AUD other than naltrexone, as outlined above. All patients with substance use disorders would benefit from psychosocial interventions, including group and individual therapy as well as community sober support groups.
Bottom Line
Patients with comorbid opioid use disorder (OUD) and alcohol use disorder (AUD) often pose significant management challenges when they present in withdrawal. This article reviews the evidence and summarizes practical considerations regarding the clinical management of patients with comorbid OUD and AUD.
Related Resources
- Chaney L, Mathia C, Cole T. Transitioning patients with opioid use disorder from methadone to buprenorphine. Current Psychiatry. 2022;21(12):23-24,28. doi:10.12788/ cp.0305
- Eatmon CV, Trent K. Pharmacotherapy for alcohol use disorder in patients with hepatic impairment. Current Psychiatry. 2021;20(12):25-28. doi:10.12788/cp.0068
Drug Brand Names
Acamprosate • Campral
Buprenorphine/naloxone • Suboxone, Zubsolv
Clonidine • Catapres
Disulfiram • Antabuse
Gabapentin • Neurontin
Hydroxyzine • Vistaril
Lofexidine • Lucemyra
Methadone • Methadose, Dolophine
Naloxone • Narcan
Naltrexone • ReVia, Vivitrol
Ondansetron • Zofran
Topiramate • Topamax
Trazodone • Desyrel, Oleptro
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
1. Mattson CL, Tanz LJ, Quinn K, et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths - United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70(6):202-207.
2. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
3. Nolan S, Klimas J, Wood E. Alcohol use in opioid agonist treatment. Addict Sci Clin Pract. 2016;11(1):17.
4. Hood LE, Leyrer-Hackson JM, Olive MF. Pharmacotherapeutic management of co-morbid alcohol and opioid use. Expert Opin Pharmacother. 2020;21(7):823-839.
5. Pikovsky M, Peacock A, Larney S, et al. Alcohol use disorder and associated physical health complications and treatment amongst individuals with and without opioid dependence: a case-control study. Drug Alcohol Depend. 2018;188:304-310.
6. Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019;197:78-82.
7. Hartzler B, Donovan DM, Huang Z. Comparison of opiate-primary treatment seekers with and without alcohol use disorder. J Subst Abuse Treat. 2010;39(2):114-123.
8. Jones CM, Paulozzi LJ, Mack KA; Centers for Disease Control and Prevention (CDC). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse-related emergency department visits and drug-related deaths - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(40):881-885.
9. Stapleton RD, Comiskey CM. Alcohol usage and associated treatment outcomes for opiate users entering treatment in Ireland. Drug Alcohol Depend. 2010;107(1):56-61.
10. Turner RC, Lichstein PR, Peden JG Jr, et al. Alcohol withdrawal syndromes: a review of pathophysiology, clinical presentation, and treatment. J Gen Intern Med. 1989;4(5):432-444.
11. Boba A. Management of acute alcohol intoxication. Am J Emerg Med. 1999;17(4):431.
12. The ASAM national practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14(2S Suppl1):1-91.
13. Shaw JM, Kolesar GS, Sellers EM, et al. Development of optimal treatment tactics for alcohol withdrawal. I. Assessment and effectiveness of supportive care. J Clin Psychopharmacol. 1981;1(6):382-389.
14. Naranjo CA, Sellers EM. Clinical assessment and pharmacotherapy of the alcohol withdrawal syndrome. Recent Dev Alcohol. 1986;4:265-281.
15. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367.
16. The ASAM clinical practice guideline on alcohol withdrawal management. J Addict Med. 2020;14(3S Suppl 1):1-72.
17. Isenberg-Grzeda E, Kutner HE, Nicolson SE. Wernicke-Korsakoff-syndrome: under-recognized and under-treated. Psychosomatics. 2012;53(6):507-516.
18. Schuckit MA. Treatment of opioid-use disorders. N Engl J Med. 2016;375(4):357-368.
19. Tang Y-L, Hao W. Improving drug addiction treatment in China. Addiction. 2007;102(7):1057-1063.
20. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622.
21. Wines JD Jr, Saitz R, Horton NJ, et al. Overdose after detoxification: a prospective study. Drug Alcohol Depend. 2007;89(2-3):161-169.
22. Maughan BC, Becker EA. Drug-related mortality after discharge from treatment: a record-linkage study of substance abuse clients in Texas, 2006-2012. Drug Alcohol Depend. 2019;204:107473.
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2002;(2):CD002025.
24. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12(5):379-384.
25. Nava F, Manzato E, Leonardi C, et al. Opioid maintenance therapy suppresses alcohol intake in heroin addicts with alcohol dependence: preliminary results of an open randomized study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1867-1872.
26. Srivastava A, Kahan M, Ross S. The effect of methadone maintenance treatment on alcohol consumption: a systematic review. J Subst Abuse Treat. 2008;34(2):215-223.
27. Davids E, Gastpar M. Buprenorphine in the treatment of opioid dependence. Eur Neuropsychopharmacol. 2004;14(3):209-216.
28. American Psychiatric Association. Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association; 2018.
29. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567.
30. Fairbanks J, Umbreit A, Kolla BP, et al. Evidence-based pharmacotherapies for alcohol use disorder: clinical pearls. Mayo Clin Proc. 2020;95(9):1964-1977.
31. Verrotti A, Scaparrotta A, Agostinelli S, et al. Topiramate-induced weight loss: a review. Epilepsy Res. 2011;95(3):189-199.
32. Flórez G, García-Portilla P, Alvarez S, et al. Using topiramate or naltrexone for the treatment of alcohol-dependent patients. Alcohol Clin Exp Res. 2008;32(7):1251-1259.
33. Jørgensen CH, Pedersen B, Tønnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35(10):1749-1758.
34. Mason BJ, Quello S, Shadan F. Gabapentin for the treatment of alcohol use disorder. Expert Opin Investig Drugs. 2018;27(1):113-124.
35. Sudakin D. Naltrexone: not just for opioids anymore. J Med Toxicol. 2016;12(1):71-75.
36. Rubio G, Jiménez-Arrieri MA, Ponce G, et al. Naltrexone versus acamprosate: one year follow-up of alcohol dependence treatment. Alcohol Alcohol. 2001;36(5):419-425.
37. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318.
38. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med. 2014;8(3):153-163.
39. Dunne RB. Prescribing naloxone for opioid overdose intervention. Pain Manag. 2018;8(3):197-208.
Evaluation after a suicide attempt: What to ask
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
In 2021, suicide was the 11th leading cause of death in the United States.1 Suicide resulted in 49,000 US deaths during 2021; it was the second most common cause of death in individuals age 10 to 34, and the fifth leading cause among children.1,2 Women are 3 to 4 times more likely than men to attempt suicide, but men are 4 times more likely to die by suicide.2
The evaluation of patients with suicidal ideation who have not made an attempt generally involves assessing 4 factors: the specific plan, access to lethal means, any recent social stressors, and the presence of a psychiatric disorder.3 The clinician should also assess which potential deterrents, such as religious beliefs or dependent children, might be present.
Mental health clinicians are often called upon to evaluate a patient after a suicide attempt to assess intent for continued self-harm and to determine appropriate disposition. Such an evaluation must consider multiple factors, including the method used, premeditation, consequences of the attempt, the presence of severe depression and/or psychosis, and the role of substance use. Assessment after a suicide attempt differs from the examination of individuals who harbor suicidal thoughts but have not made an attempt; the latter group may be more likely to respond to interventions such as intensive outpatient care, mobilization of family support, and religious proscriptions against suicide. However, for patients who make an attempt to end their life, whatever potential safeguards or deterrents to suicide that were in place obviously did not prevent the self-harm act. The consequences of the attempt, such as disabling injuries or medical complications, and possible involuntary commitment, need to be considered. Assessment of the patient’s feelings about having survived the attempt is important because the psychological impact of the attempt on family members may serve to intensify the patient’s depression and make a subsequent attempt more likely.
Many individuals who think of suicide have communicated self-harm thoughts or intentions, but such comments are often minimized or ignored. There is a common but erroneous belief that if patients are encouraged to discuss thoughts of self-harm, they will be more likely to act upon them. Because the opposite is true,4 clinicians should ask vulnerable patients about suicidal ideation or intent. Importantly, noncompliance with life-saving medical care, risk-taking behaviors, and substance use may also signal a desire for self-harm. Passive thoughts of death, typified by comments such as “I don’t care whether I wake up or not,” should also be elicited. Many patients who think of suicide speak of being in a “bad place” where reason and logic give way to an intense desire to end their misery.

The evaluation of a patient who has attempted suicide is an important component of providing psychiatric care. This article reflects our 45 years of evaluating such patients. As such, it reflects our clinical experience and is not evidence-based. We offer a checklist of 14 questions that we have found helpful when determining if it would be best for a patient to receive inpatient psychiatric hospitalization or a discharge referral for outpatient care (Table). Questions 1 through 6 are specific for patients who have made a suicide attempt, while questions 7 through 14 are helpful for assessing global risk factors for suicide.
1. Was the attempt premeditated?
Determining premeditation vs impulsivity is an essential element of the assessment following a suicide attempt. Many such acts may occur without forethought in response to an unexpected stressor, such as an altercation between partners or family conflicts. Impulsive attempts can occur when an individual is involved in a distressing event and/or while intoxicated. Conversely, premeditation involves forethought and planning, which may increase the risk of suicide in the near future.
Examples of premeditated behavior include:
- Contemplating the attempt days or weeks beforehand
- Researching the effects of a medication or combination of medications in terms of potential lethality
- Engaging in behavior that would decrease the likelihood of their body being discovered after the attempt
- Obtaining weapons and/or stockpiling pills
- Canvassing potential sites such as bridges or tall buildings
- Engaging in a suicide attempt “practice run”
- Leaving a suicide note or message on social media
- Making funeral arrangements, such as choosing burial clothing
- Writing a will and arranging for the custody of dependent children
- Purchasing life insurance that does not deny payment of benefits in cases of death by suicide.
Continue to: Patients with a premeditated...
Patients with a premeditated suicide attempt generally do not expect to survive and are often surprised or upset that the act was not fatal. The presence of indicators that the attempt was premeditated should direct the disposition more toward hospitalization than discharge. In assessing the impact of premeditation, it is important to gauge not just the examples listed above, but also the patient’s perception of these issues (such as potential loss of child custody). Consider how much the patient is emotionally affected by such thinking.
2. What were the consequences of the attempt?
Assessing the reason for the attempt (if any) and determining whether the inciting circumstance has changed due to the suicide attempt are an important part of the evaluation. A suicide attempt may result in reconciliation with and/or renewed support from family members or partners, who might not have been aware of the patient’s emotional distress. Such unexpected support often results in the patient exhibiting improved mood and affect, and possibly temporary resolution of suicidal thoughts. This “flight into health” may be short-lived, but it also may be enough to engage the patient in a therapeutic alliance. That may permit a discharge with safe disposition to the outpatient clinic while in the custody of a family member, partner, or close friend.
Alternatively, some people experience a troubling worsening of precipitants following a suicide attempt. Preexisting medical conditions and financial, occupational, and/or social woes may be exacerbated. Child custody determinations may be affected, assuming the patient understands the possibility of this adverse consequence. Violent methods may result in disfigurement and body image issues. Individuals from small, close-knit communities may experience stigmatization and unwanted notoriety because of their suicide attempt. Such negative consequences may render some patients more likely to make another attempt to die by suicide. It is crucial to consider how a suicide attempt may have changed the original stress that led to the attempt.
3. Which method was used?
Most fatal suicides in the US are by firearms, and many individuals who survive such attempts do so because of unfamiliarity with the weapon, gun malfunction, faulty aim, or alcohol use.5-7 Some survivors report intending to shoot themselves in the heart, but instead suffered shoulder injuries. Unfortunately, for a patient who survives self-inflicted gunshot wounds, the sequelae of chronic pain, multiple surgical procedures, disability, and disfigurement may serve as constant negative reminders of the event. Some individuals with suicidal intent eschew the idea of using firearms because they hope to avoid having a family member be the first to discover them. Witnessing the aftermath of a fatal suicide by gunshot can induce symptoms of posttraumatic stress disorder in family members and/or partners.8
For a patient with self-inflicted gunshot wounds, always determine whether the weapon has been secured or if the patient still has access to it. Asking about weapon availability is essential during the evaluation of any patient with depression, major life crises, or other factors that may yield a desire to die; this is especially true for individuals with substance use disorders (SUDs). Whenever readily available to such individuals, weapons need to be safely removed.
Continue to: Other self-harm methods...
Other self-harm methods with a high degree of lethality include jumping from bridges or buildings, poisonings, self-immolation, cutting, and hangings. Individuals who choose these approaches generally do not intend to survive. Many of these methods also entail premeditation, as in the case of individuals who canvass bridges and note time when traffic is light so they are less likely to be interrupted. Between 1937 and 2012, there were >1,600 deaths by suicide from San Francisco’s Golden Gate Bridge.9 Patients who choose highly lethal methods are often irritated during the postattempt evaluation because their plans were not fatal. Usually, patients who choose such potentially lethal methods are hospitalized initially on medical and surgical floors, and receive most of their psychiatric care from consultation psychiatrists. Following discharge, these patients may be at high risk for subsequent suicide attempts.
In the US, the most common method of attempting suicide is by overdose.4 Lethality is determined by the agent or combination of substances ingested, the amount taken, the person’s health status, and the length of time before they are discovered. Many patients mistakenly assume that readily available agents such as acetaminophen and aspirin are less likely to be fatal than prescription medications. Evaluators may want to assess for suicidality in individuals with erratic, risk-taking behaviors, who are at especially high risk for death. Learning about the method the patient used can help the clinician determine the imminent risk of another suicide attempt. The more potentially fatal the patient’s method, the more serious their suicide intent, and the higher the risk they will make another suicide attempt, possibly using an even more lethal method.
4. What was the intent?
“What did you want to happen when you made this attempt?” Many patients will respond that they wanted to die, sleep, not wake up, or did not care what happened. Others say it was a gesture to evoke a certain response from another person. If this is the case, it is important to know whether the desired outcome was achieved. These so-called gestures often involve making sure the intended person is aware of the attempt, often by writing a letter, sending a text, or posting on social media. Such behaviors may be exhibited by patients with personality disorders. While such attempts often are impulsive, if the attempt fails to generate the anticipated effect, the patient may try to gain more attention by escalating their suicide actions.
Conversely, if a spouse or partner reconciles with the patient solely because of a suicide attempt, this may set a pattern for future self-harm events in which the patient hopes to achieve the same outcome. Nevertheless, it is better to err for safety because some of these patients will make another attempt, just to prove that they should have been taken more seriously. An exploration of such intent can help the evaluation because even supposed “gestures” can have dangerous consequences. Acts that do not result in the desired outcome should precipitate hospitalization rather than discharge.
5. What facilitated the patient’s rescue?
“Why is this patient still alive?” Determine if the patient did anything to save themself, such as calling an ambulance, inducing emesis, telling someone what they did, or coming to the hospital on their own. If yes, asking them what changed their mind may provide information about what exists in their lives to potentially prevent future attempts, or about wishes to stay alive. These issues can be used to guide outpatient therapy.
Continue to: How does the patient feel about having survived?
6. How does the patient feel about having survived?
When a patient is asked how they feel about having survived a suicide attempt, some will label their act “stupid” and profess embarrassment. Others exhibit future-oriented thought, which is a very good prognostic sign. More ominous is subsequent dysphoria or lamenting that “I could not even do this right.” Patients often express anger toward anyone who rescued them, especially those whose attempts were carefully planned or were discovered by accident. Some patients might also express ambivalence about having survived.
The patient’s response to this question may be shaped by their desire to avoid hospitalization, so beyond their verbal answers, be attentive to clinical cues that may suggest the patient is not being fully transparent. Anger or ambivalence about having survived, a lack of future-oriented thought, and a restricted affect despite verbalizing joy about still being alive are features that suggest psychiatric hospitalization may be warranted.
7. Has the patient made previous suicide attempts?
Compared to individuals with no previous suicide attempts, patients with a history of suicide attempts are 30 to 40 times more likely to die by suicide.2 Many patients who present after a suicide attempt have tried to kill themselves multiple times. Exploring the number of past attempts, how recent the attempts were, and what dispositions were made can be of benefit. Reviewing the potential lethality of past attempts (eg, was hospitalization required, was the patient placed in an intensive care unit, and/or was intubation needed) is recommended. If outpatient care was suggested or medication prescribed, was the patient adherent? Consider asking about passive suicidal behavior, such as not seeking care for medical issues, discontinuing life-saving medication, or engaging in reckless behavior. While such behaviors may not have been classified as a suicide attempt, it might indicate a feeling of indifference toward staying alive. A patient with a past attempt, especially if recent, merits consideration for inpatient care. Once again, referring previously nonadherent patients to outpatient treatment is less likely to be effective.
8. Does the patient have a support network?
Before discharging a patient who has made a suicide attempt, consider the quality of their support network. Gauging the response of the family and friends to the patient’s attempt can be beneficial. Indifference or resentment on the part of loved ones is a bad sign. Some patients have access to support networks they either did not know were available or chose not to utilize. In other instances, after realizing how depressed the patient has been, the family might provide a new safety net. Strong religious affiliations can also be valuable because devout spirituality can be a deterrent to suicide behaviors.10 For an individual whose attempt was motivated by loneliness or feeling unloved or underappreciated, a newly realized support network can be an additional protective deterrent.
9. Does the patient have a family history of suicide?
There may be a familial component to suicide. Knowing about any suicide history in the family contributes to future therapeutic planning. The clinician may want to explore the patient’s family suicide history in detail because such information can have substantial impact on the patient’s motivation for attempting suicide. The evaluator may want to determine if the anniversary of a family suicide is coming. Triggers for a suicide attempt could include the anniversary of a death, birthdays, family-oriented holidays, and similar events. It is productive to understand how the patient feels about family members who have died by suicide. Some will empathize with the deceased, commenting that they did the “right thing.” Others, upon realizing how their own attempt affected others, will be remorseful and determined not to inflict more pain on their family. Such patients may need to be reminded of the misery associated with their family being left without them. These understandings are helpful at setting a safe disposition. However, a history of death by suicide in the family should always be thoroughly evaluated, regardless of the patient’s attitude about that death.
Continue to: Was the attempt the result of depression?
10. Was the attempt the result of depression?
For a patient experiencing depressive symptoms, the prognosis is less positive; they are more likely to harbor serious intent, premeditation, hopelessness, and social isolation, and less likely to express future-oriented thought. They often exhibit a temporary “flight into health.” Such progress is often transitory and may not represent recovery. Because mood disorders may still be present despite a temporary improvement, inpatient and pharmacologic treatment may be needed. If a patient’s suicide attempt occurred as a result of severe depression, it is possible they will make another suicide attempt unless their depression is addressed in a safe and secure setting, such as inpatient hospitalization, or through close family observation while the patient is receiving intensive outpatient treatment.
11. Does the patient have a psychotic disorder?
Many patients with a psychotic illness die following their first attempt without ever having contact with a mental health professional.11 Features of psychosis might include malevolent auditory hallucinations that suggest self-destruction.11 Such “voices” can be intense and self-deprecating; many patients with this type of hallucination report having made a suicide attempt “just to make the voices stop.”
Symptoms of paranoia can make it less likely for individuals with psychosis to confide in family members, friends, or medical personnel. Religious elements are often of a delusional nature and can be dangerous. Psychosis is more difficult to hide than depression and the presence of psychoses concurrent with major depressive disorder (MDD) increases the probability of suicidality.11 Psychosis secondary to substance use may diminish inhibitions and heighten impulsivity, thereby exacerbating the likelihood of self-harm. Usually, the presence of psychotic features precipitating or following a suicide attempt leads to psychiatric hospitalization.
12. Is the patient in a high-risk demographic group?
When evaluating a patient who has attempted suicide, it helps to consider not just what they did, but who they are. Specifically, does the individual belong to a demographic group that traditionally has a high rate of suicide? For example, patients who are Native American or Alaska Natives warrant extra caution.2 Older White males, especially those who are divorced, widowed, retired, and/or have chronic health problems, are also at greater risk. Compared to the general population, individuals age >80 have a massively elevated chance for self-induced death.12 Some of the reasons include:
- medical comorbidities make surviving an attempt less likely
- access to large amounts of medications
- more irreversible issues, such as chronic pain, disability, or widowhood
- living alone, which may delay discovery.
Patients who are members of any of these demographic groups may deserve serious consideration for inpatient psychiatric admission, regardless of other factors.
Continue to: Were drugs or alcohol involved?
13. Were drugs or alcohol involved?
This factor is unique in that it is both a chronic risk factor (SUDs) and a warning sign for imminent suicide, as in the case of an individual who gets intoxicated to disinhibit their fear of death so they can attempt suicide. Alcohol use disorders are associated with depression and suicide. Overdoses by fentanyl and other opiates have become more frequent.13 In many cases, fatalities are unintentional because users overestimate their tolerance or ingest contaminated substances.14 Disinhibition by alcohol and/or other drugs is a risk factor for attempting suicide and can intensify the depth of MDD. Some patients will ingest substances before an attempt just to give them the courage to act; many think of suicide only when intoxicated. Toxicology screens are indicated as part of the evaluation after a suicide attempt.
Depressive and suicidal thoughts often occur in people “coming down” from cocaine or other stimulants. These circumstances require determining whether to refer the patient for treatment for an SUD or psychiatric hospitalization.
In summary, getting intoxicated solely to diminish anxiety about suicide is a dangerous feature, whereas attempting suicide due to intoxication is less concerning. The latter patient may not consider suicide unless they become intoxicated again. When available, dual diagnosis treatment facilities can be an appropriate referral for such patients. Emergency department holding beds can allow these individuals to detoxify prior to the evaluation.
14. Does the patient have future-oriented thoughts?
When evaluating a patient who has attempted suicide, the presence of future planning and anticipation can be reassuring, but these features should be carefully assessed.14-16
After-the-fact comments may be more reliable when a patient offers them spontaneously, as opposed to in response to direct questioning.
- the specificity of the future plans
- corroboration from the family and others about the patient’s previous investment in the upcoming event
- whether the patient mentions such plans spontaneously or only in response to direct questioning
- the patient’s emotional expression or affect when discussing their future
- whether such plans are reasonable, grandiose, and/or unrealistic.
Bottom Line
When assessing a patient after a suicide attempt, both the patient’s presentation and history and the clinician’s instincts are important. Careful consideration of the method, stated intent, premeditation vs impulsivity, feelings about having survived, presence of psychiatric illness, high-risk demographic, postattempt demeanor and affect, quality of support, presence of self-rescue behaviors, future-oriented thoughts, and other factors can help in making the appropriate disposition.
Related Resources
- Kim H, Kim Y, Shin MH, et al. Early psychiatric referral after attempted suicide helps prevent suicide reattempts: a longitudinal national cohort study in South Korea. Front Psychiatry. 2022;13:607892. doi:10.3389/fpsyt.2022.607892
- Michaud L, Berva S, Ostertag L, et al. When to discharge and when to voluntary or compulsory hospitalize? Factors associated with treatment decision after self-harm. Psychiatry Res. 2022;317:114810. doi:10.1016/j.psychres.2022.114810
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
1. Ten Leading Causes of Death, United States 2020. Centers for Disease Control and Prevention WISQARS. Accessed March 4, 2022. https://wisqars.cdc.gov/data/lcd/home
2. Norris D, Clark MS. Evaluation and treatment of suicidal patients. Am Fam Physician. 2012;15;85(6):602-605.
3. Gliatto MF, Rai AK. Evaluation and treatment patients with suicidal ideation. Am Fam Phys. 1999;59(6):1500-1506.
4. Dazzi T, Gribble R, Wessely S, et al. Does asking about suicide and related behaviors induce suicidal ideation? What is the evidence? Psychol Med. 2014;44(16):3361-3363.
5. Lewiecki EM, Miller SA. Suicide, guns and public policy. Am J Public Health. 2013;103(1):27-31.
6. Frierson RL. Women who shoot themselves. Hosp Community Psychiatry. 1989;40(8):841-843.
7. Frierson RL, Lippmann SB. Psychiatric consultation for patients with self-inflicted gunshot wounds. Psychosomatics. 1990;31(1):67-74.
8. Mitchell AM, Terhorst L. PTSD symptoms in survivors bereaved by the suicide of a significant other. J Am Psychiatr Nurses Assoc. 2017;23(1):61-65.
9. Bateson J. The Golden Gate Bridge’s fatal flaw. Los Angeles Times. May 25, 2012. Accessed March 2, 2022. https://www.latimes.com/opinion/la-xpm-2012-may-25-la-oe-adv-bateson-golden-gate-20120525-story.html
10. Dervic K, Oquendoma MA, Grunebaum MF, et al. Religious affiliation and suicide attempt. Am J Psychiatry. 2004;161(12):2303-2308.
11. Nordentoft H, Madsen T, Fedyszyn IF. Suicidal behavior and mortality in first episode psychosis. J Nerv Ment Dis. 2015;203(5):387-392.
12. Frierson R, Lippmann S. Suicide attempts by the old and the very old. Arch Intern Med. 1991;151(1):141-144.
13. Braden JB, Edlund MJ, Sullivan MD. Suicide deaths with opiate poisonings in the United States: 1999-2014. Am J Public Health. 2017;107(3):421-426.
14. Morin KA, Acharya S, Eibl JK, et al: Evidence of increased fentanyl use during the COVID-19 pandemic among opioid agonist treated patients in Ontario, Canada. Int J Drug Policy. 2021;90:103088.
15. Shobassy A, Abu-Mohammad AS. Assessing imminent suicide risk: what about future planning? Current Psychiatry. 2022;21(2):12-17.
16. MacLeod AK, Pankhania B, Lee M, et al. Parasuicide, depression and the anticipation of positive and negative future experiences. Psychol Med. 1997;27(4):973-977.
17. Macleod AK, Tata P, Tyrer P, et al. Hopelessness and positive and negative future thinking in parasuicide. Br J Clin Psychol. 2010;44(Pt 4):495-504.
Gut microbiota and symptoms of psychosis: Is there a link?
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gutmicrobiome and the brain interact are collectively known as the gut-microbiota-brainaxis.7
How do we acquire our gut microbiome, and how does it come to influence ourbrain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.
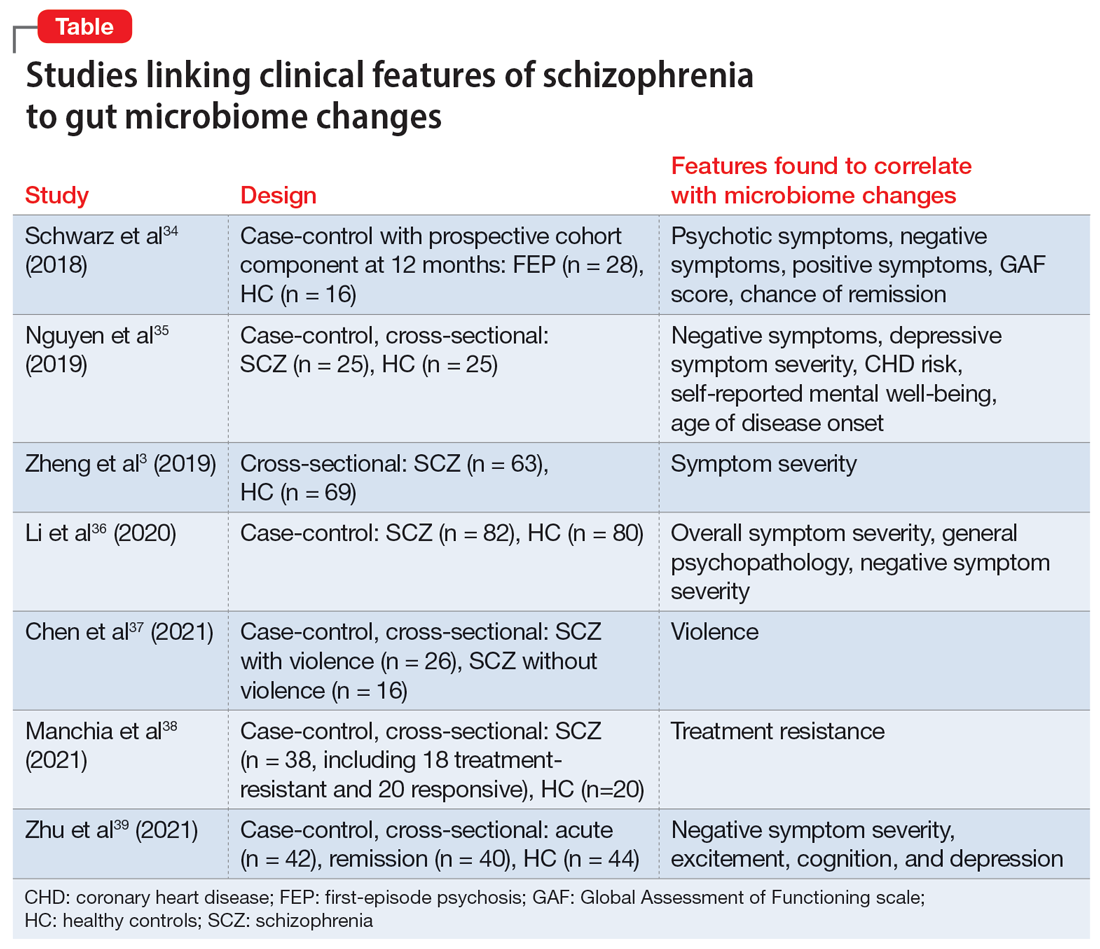
Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gutmicrobiome and the brain interact are collectively known as the gut-microbiota-brainaxis.7
How do we acquire our gut microbiome, and how does it come to influence ourbrain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.

Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
The human microbiota refers to the collection of bacteria, archaea, eukarya, and viruses that reside within the human body. The term gut microbiome indicates the composition of these microbes and genetic codes in the intestine.1 Harkening back to the ancient Greek physician Galen, who treated gastrointestinal (GI) symptoms to relieve mental disturbances such as psychosis, the gut has been a therapeutic target in schizophrenia long before antipsychotics and the DSM.2 In recent years, research into the gut microbiome has drastically increased, with genetic sequencing affording a more precise look into the specific bacteria that call the human intestines their home. This has led to the recognition that the gut microbiome may be severely disrupted in schizophrenia, a condition known as dysbiosis. Preliminary research suggests that gut bacteria are more helpful than many human genes in distinguishing individuals with schizophrenia from their healthy counterparts.3,4 In this article, we discuss the potential role of the gut microbiome in schizophrenia, including new research correlating clinical symptoms of psychosis with dysbiosis. We also provide recommendations for promoting a healthy gut microbiome.
The enteric brain across life
The composition of our bodies is far more microbiota than human. Strikingly, microbiota cells in the gut outnumber human cells, and the distal gut alone hosts bacteria with 100 times the genetic content of the entire genome.5 The intricate meshwork of nerves in the gut is often called the enteric brain because the gut consists of 100 million neurons and synthesizes many neuroactive chemicals implicated in mood disorders and psychosis, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and acetylcholine.6 The variety of neuroimmunologic, hormonal, and metabolic paths by which the gutmicrobiome and the brain interact are collectively known as the gut-microbiota-brainaxis.7
How do we acquire our gut microbiome, and how does it come to influence ourbrain and behavior? On the first day of life, as babies pass through the birth canal, they are bathed in their mother’s vaginal microbiota. In the following weeks, the microbiome expands and colonizes the gut as bacteria are introduced from environmental sources such as skin-to-skin contact and breastmilk.8 The microbiome continues to evolve throughout early life. As children expand their diets and navigate new aspects of the physical world, additional bacteria join the unseen ecosystem growing inside.9 The development of the microbiome coincides with the development of the brain. From preclinical studies, we know the gut microbiome mediates important aspects of neurodevelopment such as the formation of the blood-brain barrier (BBB), synaptic pruning, glial activation, and myelination.10 Interestingly, many of the risk factors for schizophrenia are associated with gut dysbiosis, including obstetric complications, infections treated with antibiotics, and urbanization.11-15
Throughout human life, the gut and brain remain in close communication. The gut microbiota continue to produce monoamines, along with other metabolites that are able to cross the BBB.6 The HPA axis, stimulation of the immune system, and the vagus nerve all provide highways of communication between the gut and the brain.7 The relationship between the enteric brain and cephalic brain continues through life, even up to a person’s final hour. One autopsy study that is often cited (but soberingly, cannot be found online) allegedly revealed that 92% of schizophrenia patients had developed colitis by the time of death.16,17
First-episode psychosis and antipsychotic treatment
For patients with schizophrenia, first-episode psychosis (FEP) represents a cocktail of mounting genetic and environmental factors. Typically, by the time a patient receives psychiatric care, they present with characteristic psychotic symptoms—hallucinations, delusions, bizarre behavior, and unusual thought process—along with a unique gut microbiome profile.
This disrupted microbiome coincides with a marked state of inflammation in the intestines. Inflammation triggers increased endothelial barrier permeability, similar to the way immune signals increase capillary permeability to allow immune cells into the periphery of the blood. Specific gut bacteria play specific roles in maintaining the gut barrier.18,19 Disruptions in the bacteria that maintain the gut barrier, combined with inflammation, contribute to a leaky gut. A leaky gut barrier allows bacterial and immune products to more easily enter the bloodstream and then the brain, which is a potential source of neuroinflammation in schizophrenia.20 This increase in gut permeability (leaky gut syndrome) is likely one of several reasons low-grade inflammation is common in schizophrenia—numerous studies show higher serum levels of proinflammatory cytokines along with antibacterial immunoglobulins in patients with FEP.21,22
Fortunately, antipsychotics, especially the second-generation agents, help restore a healthy gut microbiome and have substantial anti-inflammatory properties.23,24 These medications interact heavily with the gut microbiome: they have been found to have antibiotic properties, even in doses lower than would normally reach the gut microbiome.25 In humans, a randomized controlled trial of probiotic supplementation for schizophrenia patients taking antipsychotics showed a reduction in GI symptoms but no significant improvement in psychotic symptoms.26
Dysbiosis in schizophrenia: cause or effect?
There is no consensus on what constitutes a healthy gut microbiome because the gut microbiome is highly variable, even among healthy individuals, and can change quickly. Those who adopt new diets, for example, see drastic shifts in the gut microbiome within a few days.27 Despite this variation, the main separation between a healthy and dysbiotic gut comes from the diversity of bacteria present in the gut—a healthy gut microbiome is associated with increased diversity. Numerous disease states have been associated with decreased bacterial diversity, including Clostridium difficile infection, Parkinson disease, depression, Crohn disease, and schizophrenia spectrum disorders.28,29
Although there are ethical limitations to studying causality in humans directly, animal models have provided a great deal of insight into the gut microbiome’s role in the development of schizophrenia. A recent study used fecal transplant to provide the gut microbiome from patients with schizophrenia to a group of germ-free mice and compared these animals to a group of mice that received a fecal transplant from individuals with a healthy gut microbiome. The mice receiving the schizophrenia microbiome showed an increased startle response and hyperactivity.3 This was consistent with mouse models of schizophrenia, although with obvious limitations.30 In addition, the brains of these animals showed changes in glutamate, glutamine, and GABA in the hippocampus; these chemicals play a role in the neurophysiology of schizophrenia.3,31 This study has not yet been replicated, and considerable variation remains within the schizophrenia biosignature.
Continue to: Clinical symptoms of psychosis and the gut microbiome
Clinical symptoms of psychosis and the gut microbiome
Previous literature has grouped patients with schizophrenia spectrum disorders as 1 unified study group. But as is the case with many psychiatric conditions, there is a great deal of heterogeneity in neurobiology, genetics, and microbiome composition among individuals with schizophrenia.32
Researchers have begun to investigate ways in which the gut microbiome varies regarding the clinical symptoms of psychosis.33 The Table3,34-39 provides an overview of 7 human studies of gut microbiome changes relating to clinical features of schizophrenia. In these studies, researchers have found correlations between the gut microbiome and a tendency toward violence,37 cognitive deficits,34-36,39 depressive symptoms,35,39 and numerous other clinical features of psychosis. Most of these correlations have not yet been replicated by further studies. But among studies with similar clinical questions, 3 reported changes in gut microbiome correlated with overall symptom severity, and 4 studies correlated changes with negative symptom severity. In 2 studies,3,34Lachnospiraceae was correlated with worsened symptom severity. However, this may have been the result of poor control for antipsychotic use, as 1 study in bipolar patients found that Lachnospiraceae was increased in those taking antipsychotics compared to those who were not treated with antipsychotics.40 The specific shifts in bacteria seen for overall symptom and negative symptom severity were not consistent across studies. This is not surprising because the gut microbiome varies with diet and geographic region,41 and patients in these studies were from a variety of regions. Multiple studies demonstrated gut microbiome alterations for patients with more severe negative symptoms. This is particularly interesting because negative symptoms are often difficult to treat and do not respond to antipsychotics.42 This research suggests the gut microbiome may be helpful in developing future treatments for patients with negative symptoms that do not respond to existing treatments.
Research of probiotic supplementation for ameliorating symptoms of schizophrenia has yielded mixed results.43 It is possible that studies of probiotic supplementation have failed to consider the variations in the gut microbiome among individuals with schizophrenia. A better understanding of the variations in gut microbiome may allow for the development of more personalized interventions.

Recommendations for a healthy gut microbiome
In addition to antipsychotics, many other evidence-based interventions can be used to help restore a healthy gut microbiome in patients with schizophrenia. To improve the gut microbiome, we suggest discussing the following changes with patients:
- Quitting smoking. Smoking is common among patients with schizophrenia but decreases gut microbiome diversity.44
- Avoiding excessive alcohol use. Excessive alcohol use contributes to dysbiosis and increased intestinal permeability.45 Moderate alcohol consumption does not appear to have the same harmful effects on the microbiome.46
- Avoiding the use of recreational drugs, including marijuana, which impact the gut microbiome.47
- Consuming a diet rich in fiber.48 Presently, there is not enough evidence to recommend probiotic supplementation to reduce symptoms of schizophrenia.41 Similar to probiotics, fermented foods contain Lactobacillus, a bacterial species that produces lactic acid.49Lactobacillus is enriched in the gut microbiome in some neurodegenerative diseases, and lactic acid can be neurotoxic at high levels.50-52 Therefore, clinicians should not explicitly recommend fermented foods under the assumption of improved brain health. A diet rich in soluble fiber has been consistently shown to promote anti-inflammatory bacteria and is much more likely to be beneficial.53,54 Soluble fiber is found in foods such as fruits, vegetables, beans, and oats.
- Exercising can increase microbiome diversity and provide anti-inflammatory effects in the gut.55,56 A recent review found that steady-state aerobic and high-intensity exercise interventions have positive effects on mood, cognition, and other negative symptoms in patients with schizophrenia.55
- Minimizing stress. Psychological stress and physiological stress from untreated medical conditions are toxic to healthy gut bacteria and weaken the gut barrier.57
- Mitigating exposure to pollution. Environmental pollution, including exposures to air pollution, heavy metals, and pesticides, disrupts the gut microbiome.58
The American Heart Association publishes lifestyle recommendations for individuals with heart disease and the National Institutes of Health publishes lifestyle recommendations for patients with chronic kidney disease. This leads us to question why the American Psychiatric Association has not published lifestyle recommendations for those with severe mental illness. The effects of lifestyle on both the gut microbiome and symptom mitigation is critical. With increasingly shortened appointments, standardized guidelines would benefit psychiatrists and patients alike.
Bottom Line
The gut microbiome is connected to the clinical symptoms of psychosis via a variety of hormonal, neuroimmune, and metabolic mechanisms active across the lifespan. Despite advances in research, there is still much to be understood regarding this relationship. Clinicians should discuss with patients ways to promote a healthy gut microbiome, including consuming a diet rich in fiber, avoiding use of recreational drugs, and exercising regularly.
Related Resources
- Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci. 2022;12(4):89.
- Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
1. Bäckhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. doi:10.1126/science.1104816
2. Jackson SW. Galen—on mental disorders. J Hist Behav Sci. 1969;5(4):365-384. doi:10.1002/1520-6696(196910)5:4<365::AID-JHBS2300050408>3.0.CO;2-9
3. Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi:10.1126/sciadv.aau8317
4. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427. doi:10.1038/nature13595
5. Gill SR, Pop M, DeBoy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312(5778):1355-1359. doi:10.1126/science.1124234
6. Alam R, Abdolmaleky HM, Zhou JR. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet. 2017;174(6):651-660. doi:10.1002/ajmg.b.32567
7. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877-2013. doi:10.1152/physrev.00018.2018
8. Mueller NT, Bakacs E, Combellick J, et al. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109-117. doi:10.1016/j.molmed.2014.12.002
9. Fouhy F, Watkins C, Hill CJ, et al. Perinatal factors affect the gut microbiota up to four years after birth. Nat Commun. 2019;10(1):1517. doi:10.1038/s41467-019-09252-4
10. Sharon G, Sampson TR, Geschwind DH, et al. The central nervous system and the gut microbiome. Cell. 2016;167(4):915-932. doi:10.1016/j.cell.2016.10.027
11. Hill CJ, Lynch DB, Murphy K, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. 2017;5:4. doi:10.1186/s40168-016-0213-y
12. Gareau MG, Wine E, Rodrigues DM, et al. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307-317. doi:10.1136/gut.2009.202515
13. Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8(343):343ra82. doi:10.1126/scitranslmed.aad7121
14. Mancabelli L, Milani C, Lugli GA, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol. 2017;19(4):1379-1390. doi:10.1111/1462-2920.13692
15. Stilo SA, Murray RM. Non-genetic factors in schizophrenia. Curr Psychiatry Rep. 2019;21(10):100. doi:10.1007/s11920-019-1091-3
16. Buscaino VM. Patologia extraneurale della schizofrenia: fegato, tubo digerente, sistema reticolo-endoteliale. Acta Neurologica. 1953;VIII:1-60.
17. Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312-1313. doi:10.1016/S0140- 6736(04)17181-X
18. Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115-1118. doi:10.1126/science.1058709
19. Ewaschuk JB, Diaz H, Meddings L, et al. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol-Gastrointest Liver Physiol. 2008;295(5):G1025-G1034. doi:10.1152/ajpgi.90227.2008
20. Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914
21. Fillman SG, Cloonan N, Catts VS, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry. 2013;18(2):206-214. doi:10.1038/mp.2012.110
22. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671. doi:10.1016/j.biopsych.2011.04.013
23. Al-Amin M, Uddin MMN, Reza HM. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi:10.9758/cpn.2013.11.3.144
24. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299-306. doi:10.1016/j.schres.2018.05.017
25. Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623-628. doi:10.1038/nature25979
26. Dickerson FB, Stallings C, Origoni A, et al. Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: a randomized, placebo-controlled trial. Prim Care Companion CNS Disord. 2014;15(1):PCC.13m01579. doi:10.4088/PCC.13m01579
27. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559-563. doi:10.1038/nature12820
28. Bien J, Palagani V, Bozko P. The intestinal microbiota dysbiosis and Clostridium difficile infection: is there a relationship with inflammatory bowel disease? Ther Adv Gastroenterol. 2013;6(1):53-68. doi:10.1177/1756283X12454590
29. Cryan JF, O’Riordan KJ, Sandhu K, et al. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19(2):179-194. doi:10.1016/S1474-4422(19)30356-4
30. Jones CA, Watson DJG, Fone KCF. Animal models of schizophrenia. Br J Pharmacol. 2011;164(4):1162-1194. doi:10.1111/j.1476-5381.2011.01386.x
31. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40(1):190-206. doi:10.1038/npp.2014.95
32. Nasrallah, HA. The daunting challenge of schizophrenia: hundreds of biotypes and dozens of theories. Curr. Psychiatry 2018;17(12):4-6,50.
33. Nocera A, Nasrallah HA. The association of the gut microbiota with clinical features in schizophrenia. Behav Sci (Basel). 2022;12(4):89. doi:10.3390/bs12040089
34. Schwarz E, Maukonen J, Hyytiäinen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophr Res. 2018;192:398-403. doi:10.1016/j.schres.2017.04.017
35. Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res. 2019;204:23-29. doi:10.1016/j.schres.2018.09.014
36. Li S, Zhuo M, Huang X, et al. Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ. 2020;8:e9574. doi:10.7717/peerj.9574
37. Chen X, Xu J, Wang H, et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int J Legal Med. 2021;135(1):131-141. doi:10.1007/s00414-020-02439-1
38. Manchia M, Fontana A, Panebianco C, et al. Involvement of gut microbiota in schizophrenia and treatment resistance to antipsychotics. Biomedicines. 2021;9(8):875. doi:10.3390/biomedicines9080875
39. Zhu C, Zheng M, Ali U, et al. Association between abundance of haemophilus in the gut microbiota and negative symptoms of schizophrenia. Front Psychiatry. 2021;12:685910. doi:10.3389/fpsyt.2021.685910
40. Flowers SA, Evans SJ, Ward KM, et al. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy. 2017;37(3):261-267. doi:10.1002/phar.1890
41. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. doi:10.1038/nature11053
42. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. doi:10.1093/schbul/sb1057
43. Liu JCW, Gorbovskaya I, Hahn MK, et al. The gut microbiome in schizophrenia and the potential benefits of prebiotic and probiotic treatment. Nutrients. 2021;13(4):1152. doi:10.3390/nu13041152
44. Biedermann L, Zeitz J, Mwinyi J, et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PloS One. 2013;8(3):e59260. doi:10.1371/journal.pone.0059260
45. Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci. 2014;111(42):e4485-e4493. doi:10.1073/pnas.1415174111
46. Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, et al. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and ß-cell function. Alcohol. 2020;85:77-94. doi:10.1016/j.alcohol.2019.05.006
47. Panee J, Gerschenson M, Chang L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol. 2018;13(1):113-122. doi:10.1007/s11481-017-9767-0
48. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105-108. doi:10.1126/science.1208344
49. Rezac S, Kok CR, Heermann M, et al. Fermented foods as a dietary source of live organisms. Front Microbiol. 2018;9:1785. doi:10.3389/fmicb.2018.01785
50. Chen X, Zhang Y, Wang H, et al. The regulatory effects of lactic acid on neuropsychiatric disorders. Discover Ment Health. 2022;2(1). doi:10.1007/s44192-022-00011-4
51. Karbownik MS, Mokros Ł, Dobielska M, et al. Association between consumption of fermented food and food-derived prebiotics with cognitive performance, depressive, and anxiety symptoms in psychiatrically healthy medical students under psychological stress: a prospective cohort study. Front Nutr. 2022;9:850249. doi:10.3389/fnut.2022.850249
52. Romano S, Savva GM, Bedarf JR, et al. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis. 2021;7(1):27. doi:10.1038/s41531-021-00156-z
53. Bourassa MW, Alim I, Bultman SJ, et al. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci Lett. 2016;625:56-63. doi:10.1016/j.neulet.2016.02.009
54. Matt SM, Allen JM, Lawson MA, et al. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol. 2018;9:1832. doi:10.3389/fimmu.2018.01832
55. Mittal VA, Vargas T, Osborne KJ, et al. Exercise treatments for psychosis: a review. Curr Treat Options Psychiatry. 2017;4(2):152-166. doi:10.1007/s40501-017-0112-2
56. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. doi:10.1186/s40168-016-0189-7
57. Karl JP, Margolis LM, Madslien EH, et al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol-Gastrointest Liver Physiol. 2017;312(6):G559-G571. doi:10.1152/ajpgi.00066.2017
58. Claus SP, Guillou H, Ellero-Simatos S. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes. 2016;2:16003. doi:10.1038/npjbiofilms.2016.3
Treating PTSD: A review of 8 studies
Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder. The lifetime prevalence among American adults is 6.8%.1 Management of PTSD includes treating distressing symptoms, reducing avoidant behaviors, treating comorbid conditions (eg, depression, substance use disorders, or mood dysregulation), and improving adaptive functioning, which includes restoring a psychological sense of safety and trust. PTSD can be treated using evidence-based psychotherapies, pharmacotherapy, or a combination of both modalities. For adults, evidence-based treatment guidelines recommend the use of cognitive-behavioral therapy, cognitive processing therapy, cognitive therapy, and prolonged exposure therapy.2 These guidelines also recommend (with some reservations) the use of brief eclectic psychotherapy, eye movement desensitization and reprocessing, and narrative exposure therapy.2 Although the evidence base for the use of medications is not as strong as that for the psychotherapies listed above, the guidelines recommend the use of fluoxetine, paroxetine, sertraline, and venlafaxine.2
Currently available treatments for PTSD have significant limitations. For example, trauma-focused psychotherapies can have significant rates of nonresponse, partial response, or treatment dropout.3,4 Additionally, such therapies are not widely accessible. As for pharmacotherapy, very few available options are supported by evidence, and the efficacy of these options is limited, as shown by the reports that only 60% of patients with PTSD show a response to selective serotonin reuptake inhibitors (SSRIs), and only 20% to 30% achieve complete remission.5 Additionally, it may take months for patients to achieve an acceptable level of improvement with medications. As a result, a substantial proportion of patients who seek treatment continue to remain symptomatic, with impaired levels of functioning. This lack of progress in PTSD treatment has been labeled as a national crisis, calling for an urgent need to find effective pharmacologic treatments for PTSD.6
In this article, we review 8 randomized controlled trials (RCTs) of treatments for PTSD published within the last 5 years (Table7-14).

1. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202
Feder et al had previously found a significant and quick decrease in PTSD symptoms after a single dose of IV ketamine had. This is the first RCT to examine the effectiveness and safety of repeated IV ketamine infusions for the treatment of persistent PTSD.7
Study design
- This randomized, double-blind, parallel-arm controlled trial treated 30 individuals with chronic PTSD with 6 infusions of either ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg) over 2 consecutive weeks.
- Participants were individuals age 18 to 70 with a primary diagnosis of chronic PTSD according to the DSM-5 criteria and determined by The Structure Clinical Interview for DSM-5, with a score ≥30 on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5).
- Any severe or unstable medical condition, active suicidal or homicidal ideation, lifetime history of psychotic or bipolar disorder, current anorexia nervosa or bulimia, alcohol or substance use disorder within 3 months of screening, history of recreational ketamine or phencyclidine use on more than 1 occasion or any use in the previous 2 years, and ongoing treatment with a long-acting benzodiazepine or opioid medication were all considered exclusion criteria. Individuals who took short-acting benzodiazepines had their morning doses held on infusion days. Marijuana or cannabis derivatives were allowed.
- The primary outcome measure was a change in PTSD symptom severity as measured with CAPS-5. This was administered before the first infusion and weekly thereafter. The Impact of Event Scale-Revised, the Montgomery–Åsberg Depression Rating Scale, and adverse effect measurements were used as secondary outcome measures.
- Treatment response was defined as ≥30% symptom improvement 2 weeks after the first infusion as assessed with CAPS-5.
- Individuals who responded to treatment were followed naturalistically weekly for up to 4 weeks and then monthly until loss of responder status, or up to 6 months if there was no loss of response.
Outcomes
- At the second week, the mean CAPS-5 total score in the ketamine group was 11.88 points (SE = 3.96) lower than in the midazolam group (d = 1.13; 95% CI, 0.36 to 1.91).
- In the ketamine group, 67% of patients responded to therapy, compared to 20% in the midazolam group.
- Following the 2-week course of infusions, the median period until loss of response among ketamine responders was 27.5 days.
- Ketamine infusions showed good tolerability and safety. There were no clinically significant adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Repeated ketamine infusions are effective in reducing symptom severity in individuals with chronic PTSD.
- Limitations to this study include the exclusion of individuals with comorbid bipolar disorder, current alcohol or substance use disorder, or suicidal ideations, the small sample size, and a higher rate of transient dissociative symptoms in the ketamine group.
- Future studies could evaluate the efficacy of repeated ketamine infusions in individuals with treatment-resistant PTSD. Also, further studies are required to assess the efficacy of novel interventions to prevent relapse and evaluate the efficacy, safety, and tolerability of periodic IV ketamine use as maintenance.
- Additional research might determine whether pairing psychotherapy with ketamine administration can lessen the risk of recurrence for PTSD patients after stopping ketamine infusions.
2. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126
Clinical practice recommendations for PTSD have identified trauma-focused psychotherapies and SSRIs as very effective treatments. The few studies that have compared trauma-focused psychotherapy to SSRIs or to a combination of treatments are not generalizable, have significant limitations, or are primarily concerned with refractory disorders or augmentation techniques. This study evaluated the efficacy of prolonged exposure therapy (PE) plus placebo, PE plus sertraline, and sertraline plus enhanced medication management in the treatment of PTSD.8
Study design
- This randomized, 4-site, 24-week clinical trial divided participants into 3 subgroups: PE plus placebo, PE plus sertraline, and sertraline plus enhanced medication management.
- Participants were veterans or service members of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment as indicated by a CAPS score ≥50 for at least 3 months. The DSM-IV-TR version of CAPS was used because the DSM-5 version was not available at the time of the study.
- Individuals who had a current, imminent risk of suicide; active psychosis; alcohol or substance dependence in the past 8 weeks; inability to attend weekly appointments for the treatment period; prior intolerance to or failure of an adequate trial of PE or sertraline; medical illness likely to result in hospitalization or contraindication to study treatment; serious cognitive impairment; mild traumatic brain injury; or concurrent use of antidepressants, antipsychotics, benzodiazepines, prazosin, or sleep agents were excluded.
- Participants completed up to thirteen 90-minute sessions of PE.
- The sertraline dosage was titrated during a 10-week period and continued until Week 24. Dosages were adjusted between 50 and 200 mg/d, with the last dose increase at Week 10.
- The primary outcome measure was symptom severity of PTSD in the past month as determined by CAPS score at Week 24.
- The secondary outcome was self-reported symptoms of PTSD (PTSD checklist [PCL] Specific Stressor Version), clinically meaningful change (reduction of ≥20 points or score ≤35 on CAPS), response (reduction of ≥50% in CAPS score), and remission (CAPS score ≤35).
Outcomes
- At Week 24, 149 participants completed the study; 207 were included in the intent-to-treat analysis.
- PTSD symptoms significantly decreased over 24 weeks, according to a modified intent-to-treat analysis utilizing a mixed model of repeated measurements; nevertheless, slopes were similar across therapy groups.
Continue to: Conclusions/limitations
Conclusions/limitations
- Although the severity of PTSD symptoms decreased in all 3 subgroups, there was no difference in PTSD symptom severity or change in symptoms at Week 24 among all 3 subgroups.
- The main limitation of this study was the inclusion of only combat veterans.
- Further research should focus on enhancing treatment retention and should include administering sustained exposure therapy at brief intervals.
3. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924
First-line therapy for PTSD includes cognitive-behavioral therapies such as PE. However, because many people still have major adverse effects after receiving medication, improving treatment efficacy is a concern. Glucocorticoids promote extinction learning, and alterations in glucocorticoid signaling pathways have been associated with PTSD. Lehrner et al previously showed that adding hydrocortisone (HCORT) to PE therapy increased patients’ glucocorticoid sensitivity at baseline, improved treatment retention, and resulted in greater treatment improvements. This study evaluated HCORT in conjunction with PE for combat veterans with PTSD following deployment to Iraq and Afghanistan.9
Study design
- This randomized, double-blind, placebo-controlled trial administered HCORT 30 mg oral or placebo to 96 combat veterans 30 minutes before PE sessions.
- Participants were veterans previously deployed to Afghanistan or Iraq with deployment-related PTSD >6 months with a minimum CAPS score of 60. They were unmedicated or on a stable psychotropic regimen for ≥4 weeks.
- Exclusion criteria included a lifetime history of a primary psychotic disorder (bipolar I disorder or obsessive-compulsive disorder), medical or mental health condition other than PTSD that required immediate clinical attention, moderate to severe traumatic brain injury (TBI), substance abuse or dependence within the past 3 months, medical illness that contraindicated ingestion of hydrocortisone, acute suicide risk, and pregnancy or intent to become pregnant.
- The primary outcome measures included PTSD severity as assessed with CAPS.
- Secondary outcome measures included self-reported PTSD symptoms as assessed with the Posttraumatic Diagnostic Scale (PDS) and depression as assessed with the Beck Depression Inventory-II (BDI). These scales were administered pretreatment, posttreatment, and at 3-months follow-up.
Outcomes
- Out of 96 veterans enrolled, 60 were randomized and 52 completed the treatment.
- Five participants were considered recovered early and completed <12 sessions.
- Of those who completed treatment, 50 completed the 1-week posttreatment evaluations and 49 completed the 3-month follow-up evaluation.
- There was no difference in the proportion of dropouts (13.33%) across the conditions.
- HCORT failed to significantly improve either secondary outcomes or PTSD symptoms, according to an intent-to-treat analysis.
- However, exploratory analyses revealed that veterans with recent post-concussive symptoms and moderate TBI exposure saw a larger decrease in hyperarousal symptoms after PE therapy with HCORT augmentation.
- The reduction in avoidance symptoms with HCORT augmentation was also larger in veterans with higher baseline glucocorticoid sensitivity.
Continue to: Conclusions/limitations
Conclusions/limitations
- HCORT does not improve PTSD symptoms as assessed with the CAPS and PDS, or depression as assessed with the BDI.
- The main limitation of this study is generalizability.
- Further studies are needed to determine whether PE with HCORT could benefit veterans with indicators of enhanced glucocorticoid sensitivity, mild TBI, or postconcussive syndrome.
4. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952
PE, one of the most well-researched therapies for PTSD, is based on fear extinction. Exploring pharmacotherapies that improve fear extinction learning and their potential as supplements to PE is gaining increased attention. Such pharmacotherapies aim to improve the clinical impact of PE on the extent and persistence of symptom reduction. This study evaluated the effects of HCORT and D-cycloserine (DCS), a partial agonist of the N-methyl-D-aspartate (NMDA) receptor, on the learning and consolidation of fear extinction in patients with PTSD.10
Study design
- This double-blind, placebo-controlled, 3-group experimental design evaluated 90 individuals with PTSD who underwent fear conditioning with stimuli that was paired (CS+) or unpaired (CS−) with shock.
- Participants were veterans and civilians age 18 to 65 recruited from VA outpatient and community clinics and internet advertisements who met the criteria for PTSD or subsyndromal PTSD (according to DSM-IV criteria) for at least 3 months.
- Exclusion criteria included schizophrenia, bipolar disorder, substance abuse or dependence, alcohol dependence, previous moderate or severe head injury, seizure or neurological disorder, current infectious illness, systemic illness affecting CNS function, or other conditions known to affect psychophysiological responses. Excluded medications were antipsychotics, mood stabilizers, alpha- and beta-adrenergics, benzodiazepines, anticonvulsants, antihypertensives, sympathomimetics, anticholinergics, and steroids.
- Extinction learning took place 72 hours after extinction, and extinction retention was evaluated 1 week later. Placebo, HCORT 25 mg, or DCS 50 mg was given 1 hour before extinction learning.
- Clinical measures included PTSD diagnosis and symptom levels as determined by interview using CAPS and skin conduction response.
Outcomes
- The mean shock level, mean pre-stimulus skin conductance level (SCL) during habituation, and mean SC orienting response during the habituation phase did not differ between groups and were not associated with differential fear conditioning. Therefore, variations in shock level preference, resting SCL, or SC orienting response magnitude are unlikely to account for differences between groups during extinction learning and retention.
- During extinction learning, the DCS and HCORT groups showed a reduced differential CS+/CS− skin conductance response (SCR) compared to placebo.
- One week later, during the retention testing, there was a nonsignificant trend toward a smaller differential CS+/CS− SCR in the DCS group compared to placebo. HCORT and DCS administered as a single dosage facilitated fear extinction learning in individuals with PTSD symptoms.
Continue to: Conclusions/limitations
Conclusions/limitations
- In traumatized people with PTSD symptoms, a single dosage of HCORT or DCS enhanced the learning of fear extinction compared to placebo. A nonsignificant trend toward better extinction retention in the DCS group but not the HCORT group was also visible.
- These results imply that glucocorticoids and NMDA agonists have the potential to promote extinction learning in PTSD.
- Limitations include a lack of measures of glucocorticoid receptor sensitivity or FKBP5.
- Further studies could evaluate these findings with the addition of blood biomarker measures such as glucocorticoid receptor sensitivity or FKBP5.
5. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
Poor PTSD treatment results are associated with numerous comorbid conditions, such as dissociation, depression, alcohol and substance use disorders, childhood trauma, and suicidal ideation, which frequently leads to treatment resistance. Therefore, it is crucial to find a treatment that works for individuals with PTSD who also have comorbid conditions. In animal models, 3,4-methylenedioxymethamphetamine (MDMA), an empathogen/entactogen with stimulant properties, has been shown to enhance fear memory extinction and modulate fear memory reconsolidation. This study evaluated the efficacy and safety of MDMA-assisted therapy for treating patients with severe PTSD, including those with common comorbidities.11
Study design
- This randomized, double-blind, placebo-controlled, multi-site, phase 3 clinical trial evaluated individuals randomized to receive manualized therapy with MDMA or with placebo, combined with 3 preparatory and 9 integrative therapy sessions.
- Participants were 90 individuals (46 randomized to MDMA and 44 to placebo) with PTSD with a symptom duration ≥6 months and CAPS-5 total severity score ≥35 at baseline.
- Exclusion criteria included primary psychotic disorder, bipolar I disorder, eating disorders with active purging, major depressive disorder with psychotic features, dissociative identity disorder, personality disorders, current alcohol and substance use disorders, lactation or pregnancy, and any condition that could make receiving a sympathomimetic medication dangerous due to hypertension or tachycardia, including uncontrolled hypertension, history of arrhythmia, or marked baseline prolongation of QT and/or QTc interval.
- Three 8-hour experimental sessions of either therapy with MDMA assistance or therapy with a placebo control were given during the treatment period, and they were spaced approximately 4 weeks apart.
- In each session, participants received placebo or a single divided dose of MDMA 80 to 180 mg.
- At baseline and 2 months after the last experimental sessions, PTSD symptoms were measured with CAPS-5, and functional impairment was measured with Sheehan Disability Scale (SDS).
- The primary outcome measure was CAPS-5 total severity score at 18 weeks compared to baseline for MDMA-assisted therapy vs placebo-assisted therapy.
- The secondary outcome measure was clinician-rated functional impairment using the mean difference in SDS total scores from baseline to 18 weeks for MDMA-assisted therapy vs placebo-assisted therapy.
Outcomes
- MDMA was found to induce significant and robust attenuation in CAPS-5 score compared to placebo.
- The mean change in CAPS-5 score in completers was –24.4 in the MDMA group and –13.9 in the placebo group.
- MDMA significantly decreased the SDS total score.
- MDMA did not induce suicidality, misuse, or QT prolongation.
Continue to: Conclusions/limitations
Conclusions/limitations
- MDMA-assisted therapy is significantly more effective than manualized therapy with placebo in treating patients with severe PTSD, and it is also safe and well-tolerated, even in individuals with comorbidities.
- No major safety issues were associated with MDMA-assisted treatment.
- MDMA-assisted therapy should be promptly assessed for clinical usage because it has the potential to significantly transform the way PTSD is treated.
- Limitations of this study include a smaller sample size (due to the COVID-19 pandemic); lack of ethnic and racial diversity; short duration; safety data were collected by site therapist, which limited the blinding; and the blinding of participants was difficult due to the subjective effects of MDMA, which could have resulted in expectation effects.
6. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990
Sertraline and paroxetine are the only FDA-approved medications for treating PTSD. Some evidence suggests cannabis may provide a therapeutic benefit for PTSD.15 This study examined the effects of 3 different preparations of cannabis for treating PTSD symptoms.12
Study design
- This double-blind, randomized, placebo-controlled, crossover trial used 3 active treatment groups of cannabis: high delta-9-tetrahydrocannabinol (THC)/low cannabidiol (CBD), high CBD/low THC, and high THC/high CBD (THC+CBD). A low THC/low CBD preparation was used as a placebo. “High” content contained 9% to 15% concentration by weight of the respective cannabinoid, and “low” content contained <2% concentration by weight.
- Inclusion criteria included being a US military veteran, meeting DSM-5 PTSD criteria for ≥6 months, having moderate symptom severity (CAPS-5 score ≥25), abstaining from cannabis 2 weeks prior to study and agreeing not to use any non-study cannabis during the trial, and being stable on medications/therapy prior to the study.
- Exclusion criteria included women who were pregnant/nursing/child-bearing age and not taking an effective means of birth control; current/past serious mental illness, including psychotic and personality disorders; having a first-degree relative with a psychotic or bipolar disorder; having a high suicide risk based on Columbia-Suicide Severity Rating Scale; meeting DSM-5 criteria for moderate-severe cannabis use disorder; screening positive for illicit substances; or having significant medical disease.
- Participants in Stage 1 (n = 80) were randomized to 1 of the 3 active treatments or placebo for 3 weeks. After a 2-week washout, participants in Stage 2 (n = 74) were randomized to receive for 3 weeks 1 of the 3 active treatments they had not previously received.
- During each stage, participants had ad libitum use for a maximum of 1.8 g/d.
- The primary outcome was change in PTSD symptom severity by the end of Stage 1 as assessed with CAPS-5.
- Secondary outcomes included the PTSD Checklist for DSM-5 (PCL-5), the general depression subscale and anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms (IDAS), the Inventory of Psychosocial Functioning, and the Insomnia Severity Index.
Outcomes
- Six participants did not continue to Stage 2. Three participants did not finish Stage 2 due to adverse effects, and 7 did not complete outcome measurements. The overall attrition rate was 16.3%.
- There was no significant difference in total grams of smoked cannabis or placebo between the 4 treatment groups in Stage 1 at the end of 3 weeks. In Stage 2, there was a significant difference, with the THC+CBD group using more cannabis compared to the other 2 groups.
- Each of the 4 groups had significant reductions in total CAPS-5 scores at the end of Stage 1, and there was no significant difference in CAPS-5 severity scores between the 4 groups.
- In Stage 1, PCL-5 scores were not significantly different between treatment groups from baseline to the end of stage. There was a significant difference in Stage 2 between the high CBD and THC+CBD groups, with the combined group reporting greater improvement of symptoms.
- In Stage 2, the THC+CBD group reported greater reductions in pre/post IDAS social anxiety scores and IDAS general depression scores, and the high THC group reported greater reductions in pre/post IDAS social anxiety scores.
- In Stage 1, 37 of 60 participants in the active groups reported at least 1 adverse event, and 45 of the 74 Stage 2 participants reported at least 1 adverse event. The most common adverse events were cough, throat irritation, and anxiety. Participants in the Stage 1 high THC group had a significant increase in reported withdrawal symptoms after 1 week of stopping use.
Continue to: Conclusions/limitations
Conclusions/limitations
- This first randomized, placebo-control trial of cannabis in US veterans did not show a significant difference among treatment groups, including placebo, on the primary outcome of CAPS-5 score. All 4 groups had significant reductions in symptom severity on CAPS-5 and showed good tolerability.
- Prior beliefs about the effects of cannabis may have played a role in the reduction of PTSD symptoms in the placebo group.
- Many participants (n =34) were positive for THC during the screening process, so previous cannabis use/chronicity of cannabis use may have contributed.
- One limitation was that participants assigned to the Stage 1 high THC group had Cannabis Use Disorders Identification Test scores (which assesses cannabis use disorder risk) about 2 times greater than participants in other conditions.
- Another limitation was that total cannabis use was lower than expected, as participants in Stage 1 used 8.2 g to 14.6 g over 3 weeks, though they had access to up to 37.8 g.
- There was no placebo in Stage 2.
- Future studies should look at longer treatment periods with more participants.
7. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444
Bright light therapy is an inexpensive treatment approach that may affect serotonergic pathways.16 This study examined bright light therapy for reducing PTSD symptoms and examined if improvement of PTSD is related to a shift in circadian rhythm.13
Study design
- Veterans with combat-related PTSD had to have been stable on treatment for at least 8 weeks or to have not received any other PTSD treatments prior to the study.
- Participants were randomized to active treatment of 30 minutes daily 10,000 lux ultraviolet-filtered white light while sitting within 18 inches (n = 34) or a control condition of 30 minutes daily inactivated negative ion generator (n = 35) for 4 weeks.
- Inclusion criteria included a CAPS score ≥30.
- Exclusion criteria included high suicidality, high probability of alcohol/substance abuse in the past 3 months, bipolar disorder/mania/schizophrenia/psychosis, ophthalmologic deformities, shift work in past 2 months or travel across time zones in past 2 weeks, head trauma, high outdoor light exposure, history of winter depression, history of seizures, or myocardial infarction/stroke/cancer within 3 years.
- Primary outcomes were improvement on CAPS and Clinical Global Impressions-Improvement scale (CGI-IM) score at Week 4.
- Wrist actigraphy recordings measured sleep.
- Other measurements included the Hamilton Depression Rating Scale (HAM-D), Hamilton atypical symptoms (HAM-AS), PCL-Military (PCL-M), Pittsburg Sleep Quality Index (PSQI), BDI, Spielberger State-Trait Anxiety Inventory (STAI Form Y-2), Beck Suicide Scale, and Systematic Assessment for Treatment Emergent Effects questionnaire.
Outcomes
- There was a significant decrease in CAPS score in participants who received bright light therapy compared to controls. Treatment response (defined as ≥33% reduction in score) was significantly greater in the bright light (44%) vs control (8.6%) group. No participants achieved remission.
- There was a significant improvement in CGI-IM scores in the bright light group, but no significant difference in participants who were judged to improve “much” or “very much.”
- PCL-M scores did not change significantly between groups, although a significantly greater proportion of participants had treatment response in the bright light group (33%) vs control (6%).
- There were no significant changes in HAM-D, HAM-AS, STAI, BDI, actigraphic estimates of sleep, or PSQI scores.
- Bright light therapy resulted in phase advancement while control treatment had phase delay.
- There were no significant differences in adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Bright light therapy may be a treatment option or adjunct for combat-related PTSD as seen by improvement on CAPS and CGI scores, as well as a greater treatment response seen on CAPS and PCL-5 scores in the bright light group.
- There was no significant difference for other measures, including depression, anxiety, and sleep.
- Limitations include excluding patients with a wide variety of medical or psychiatric comorbidities, as well as limited long-term follow up data.
- Other limitations include not knowing the precise amount of time participants stayed in front of the light device and loss of some actigraphic data (data from only 49 of 69 participants).
8. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41 doi:10.1186/s12888-022-03699-4
Cognitive processing therapy (CPT), a type of trauma-focused psychotherapy, is an effective treatment for PTSD in the military population.17,18 However, patients may not be able to or want to participate in such therapy due to barriers such as difficulty arranging transportation, being homebound due to injury, concerns about COVID-19, stigma, familial obligations, and job constraints. This study looked at if CPT delivered face-to-face at the patient’s home or via telehealth in home would be effective and increase accessibility.14
Study design
- Participants (n = 120) were active-duty military and veterans who met DSM-5 criteria for PTSD. They were randomized to receive CPT in the office, in their home, or via telehealth. Participants could choose not to partake in 1 modality but were then randomized to 1 of the other 2.
- Exclusion criteria included suicide/homicide risk needing intervention, items/situations pertaining to danger (ie, aggressive pet or unsafe neighborhood), significant alcohol/substance use, active psychosis, and impaired cognitive functioning.
- The primary outcome measurement was change in PCL-5 and CAPS-5 score over 6 months. The BDI-II was used to assess depressive symptoms.
- Secondary outcomes included the Reliable Change Index (defined as “an improvement of 10 or more points that was sustained at all subsequent assessments”) on the PCL-5 and remission on the CAPS-5.
- CPT was delivered in 60-minute sessions twice a week for 6 weeks. Participants who did not have electronic resources were loaned a telehealth apparatus.
Outcomes
- Overall, 57% of participants opted out of 1 modality, which resulted in fewer participants being placed into the in-home arm (n = 32). Most participants chose not to do in-home treatments (54%), followed by in-office (29%), and telehealth (17%).
- There was a significant posttreatment improvement in PCL-5 scores in all treatment arms, with improvement greater with in-home (d = 2.1) and telehealth (d = 2.0) vs in-office (d=1.3). The in-home and telehealth scores were significantly improved compared to in-office, and the difference between in-home and telehealth PCL-5 scores was minimal.
- At 6 months posttreatment, the differences between the 3 treatment groups on PCL-5 score were negligible.
- CAPS-5 scores were significantly improved in all treatment arms, with improvement largest with in-home treatment; however, the differences between the groups were not significant.
- BDI-II scores improved in all modalities but were larger in the in-home (d = 1.2) and telehealth (d = 1.1) arms than the in-office arm (d = 0.52).
- Therapist time commitment was greater for the in-home and in-office arms (2 hours/session) than the telehealth arm (1 hour/session). This difference was due to commuting time for the patient or therapist.
- The dropout rate was not statistically significant between the groups.
- Adverse events did not significantly differ per group. The most commonly reported ones included nightmares, sleep difficulty, depression, anxiety, and irritability.
Conclusions/limitations
- Patients undergoing CPT had significant improvement in PTSD symptoms, with posttreatment PCL-5 improvement approximately twice as large in those who received the in-home and telehealth modalities vs in-office treatment.
- The group differences were not seen on CAPS-5 scores at posttreatment, or PCL-5 or CAPS-5 scores at 6 months posttreatment.
- In-home CPT was declined the most, which suggests that in-home distractions or the stigma of a mental health clinician being in their home played a role in patients’ decision-making. However, in-home CPT produced the greatest amount of improvement in PTSD symptoms. The authors concluded that in-home therapy should be reserved for those who are homebound or have travel limitations.
- This study shows evidence that telehealth may be a good modality for CPT, as seen by improvement in PTSD symptoms and good acceptability and retention.
- Limitations include more patients opting out of in-home CPT, and reimbursement for travel may not be available in the real-world setting.
1. Kessler RC, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019;74(5):596-607. doi: 10.1037/amp0000473
3. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
4. Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656-657.
5. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):169-180.
6. Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59.
7. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202. doi:10.1176/appi.ajp.2020.20050596
8. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126. doi:10.1001/jamapsychiatry.2018.3412
9. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924. doi:10.1016/j.brat.2021.103924
10. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952. doi:10.1038/s41386-021-01222-z
11. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
12. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. doi:10.1371/journal.pone.0246990
13. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444. doi:10.1093/milmed/usab014
14. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41. doi:10.1186/s12888-022-03699-4
15. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14:78-83. doi:10.1016/j.copsyc.2016.12.001
16. Neumeister A, Praschak-Rieder N, Besselmann B, et al. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54(2):133-138. doi:10.1001/archpsyc.1997.01830140043008
17. Kaysen D, Schumm J, Pedersen ER, et al. Cognitive processing therapy for veterans with comorbid PTSD and alcohol use disorders. Addict Behav. 2014;39(2):420-427. doi:10.1016/j.addbeh.2013.08.016
18. Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83(6):1058-1068. doi:10.1037/ccp0000016
Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder. The lifetime prevalence among American adults is 6.8%.1 Management of PTSD includes treating distressing symptoms, reducing avoidant behaviors, treating comorbid conditions (eg, depression, substance use disorders, or mood dysregulation), and improving adaptive functioning, which includes restoring a psychological sense of safety and trust. PTSD can be treated using evidence-based psychotherapies, pharmacotherapy, or a combination of both modalities. For adults, evidence-based treatment guidelines recommend the use of cognitive-behavioral therapy, cognitive processing therapy, cognitive therapy, and prolonged exposure therapy.2 These guidelines also recommend (with some reservations) the use of brief eclectic psychotherapy, eye movement desensitization and reprocessing, and narrative exposure therapy.2 Although the evidence base for the use of medications is not as strong as that for the psychotherapies listed above, the guidelines recommend the use of fluoxetine, paroxetine, sertraline, and venlafaxine.2
Currently available treatments for PTSD have significant limitations. For example, trauma-focused psychotherapies can have significant rates of nonresponse, partial response, or treatment dropout.3,4 Additionally, such therapies are not widely accessible. As for pharmacotherapy, very few available options are supported by evidence, and the efficacy of these options is limited, as shown by the reports that only 60% of patients with PTSD show a response to selective serotonin reuptake inhibitors (SSRIs), and only 20% to 30% achieve complete remission.5 Additionally, it may take months for patients to achieve an acceptable level of improvement with medications. As a result, a substantial proportion of patients who seek treatment continue to remain symptomatic, with impaired levels of functioning. This lack of progress in PTSD treatment has been labeled as a national crisis, calling for an urgent need to find effective pharmacologic treatments for PTSD.6
In this article, we review 8 randomized controlled trials (RCTs) of treatments for PTSD published within the last 5 years (Table7-14).

1. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202
Feder et al had previously found a significant and quick decrease in PTSD symptoms after a single dose of IV ketamine had. This is the first RCT to examine the effectiveness and safety of repeated IV ketamine infusions for the treatment of persistent PTSD.7
Study design
- This randomized, double-blind, parallel-arm controlled trial treated 30 individuals with chronic PTSD with 6 infusions of either ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg) over 2 consecutive weeks.
- Participants were individuals age 18 to 70 with a primary diagnosis of chronic PTSD according to the DSM-5 criteria and determined by The Structure Clinical Interview for DSM-5, with a score ≥30 on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5).
- Any severe or unstable medical condition, active suicidal or homicidal ideation, lifetime history of psychotic or bipolar disorder, current anorexia nervosa or bulimia, alcohol or substance use disorder within 3 months of screening, history of recreational ketamine or phencyclidine use on more than 1 occasion or any use in the previous 2 years, and ongoing treatment with a long-acting benzodiazepine or opioid medication were all considered exclusion criteria. Individuals who took short-acting benzodiazepines had their morning doses held on infusion days. Marijuana or cannabis derivatives were allowed.
- The primary outcome measure was a change in PTSD symptom severity as measured with CAPS-5. This was administered before the first infusion and weekly thereafter. The Impact of Event Scale-Revised, the Montgomery–Åsberg Depression Rating Scale, and adverse effect measurements were used as secondary outcome measures.
- Treatment response was defined as ≥30% symptom improvement 2 weeks after the first infusion as assessed with CAPS-5.
- Individuals who responded to treatment were followed naturalistically weekly for up to 4 weeks and then monthly until loss of responder status, or up to 6 months if there was no loss of response.
Outcomes
- At the second week, the mean CAPS-5 total score in the ketamine group was 11.88 points (SE = 3.96) lower than in the midazolam group (d = 1.13; 95% CI, 0.36 to 1.91).
- In the ketamine group, 67% of patients responded to therapy, compared to 20% in the midazolam group.
- Following the 2-week course of infusions, the median period until loss of response among ketamine responders was 27.5 days.
- Ketamine infusions showed good tolerability and safety. There were no clinically significant adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Repeated ketamine infusions are effective in reducing symptom severity in individuals with chronic PTSD.
- Limitations to this study include the exclusion of individuals with comorbid bipolar disorder, current alcohol or substance use disorder, or suicidal ideations, the small sample size, and a higher rate of transient dissociative symptoms in the ketamine group.
- Future studies could evaluate the efficacy of repeated ketamine infusions in individuals with treatment-resistant PTSD. Also, further studies are required to assess the efficacy of novel interventions to prevent relapse and evaluate the efficacy, safety, and tolerability of periodic IV ketamine use as maintenance.
- Additional research might determine whether pairing psychotherapy with ketamine administration can lessen the risk of recurrence for PTSD patients after stopping ketamine infusions.
2. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126
Clinical practice recommendations for PTSD have identified trauma-focused psychotherapies and SSRIs as very effective treatments. The few studies that have compared trauma-focused psychotherapy to SSRIs or to a combination of treatments are not generalizable, have significant limitations, or are primarily concerned with refractory disorders or augmentation techniques. This study evaluated the efficacy of prolonged exposure therapy (PE) plus placebo, PE plus sertraline, and sertraline plus enhanced medication management in the treatment of PTSD.8
Study design
- This randomized, 4-site, 24-week clinical trial divided participants into 3 subgroups: PE plus placebo, PE plus sertraline, and sertraline plus enhanced medication management.
- Participants were veterans or service members of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment as indicated by a CAPS score ≥50 for at least 3 months. The DSM-IV-TR version of CAPS was used because the DSM-5 version was not available at the time of the study.
- Individuals who had a current, imminent risk of suicide; active psychosis; alcohol or substance dependence in the past 8 weeks; inability to attend weekly appointments for the treatment period; prior intolerance to or failure of an adequate trial of PE or sertraline; medical illness likely to result in hospitalization or contraindication to study treatment; serious cognitive impairment; mild traumatic brain injury; or concurrent use of antidepressants, antipsychotics, benzodiazepines, prazosin, or sleep agents were excluded.
- Participants completed up to thirteen 90-minute sessions of PE.
- The sertraline dosage was titrated during a 10-week period and continued until Week 24. Dosages were adjusted between 50 and 200 mg/d, with the last dose increase at Week 10.
- The primary outcome measure was symptom severity of PTSD in the past month as determined by CAPS score at Week 24.
- The secondary outcome was self-reported symptoms of PTSD (PTSD checklist [PCL] Specific Stressor Version), clinically meaningful change (reduction of ≥20 points or score ≤35 on CAPS), response (reduction of ≥50% in CAPS score), and remission (CAPS score ≤35).
Outcomes
- At Week 24, 149 participants completed the study; 207 were included in the intent-to-treat analysis.
- PTSD symptoms significantly decreased over 24 weeks, according to a modified intent-to-treat analysis utilizing a mixed model of repeated measurements; nevertheless, slopes were similar across therapy groups.
Continue to: Conclusions/limitations
Conclusions/limitations
- Although the severity of PTSD symptoms decreased in all 3 subgroups, there was no difference in PTSD symptom severity or change in symptoms at Week 24 among all 3 subgroups.
- The main limitation of this study was the inclusion of only combat veterans.
- Further research should focus on enhancing treatment retention and should include administering sustained exposure therapy at brief intervals.
3. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924
First-line therapy for PTSD includes cognitive-behavioral therapies such as PE. However, because many people still have major adverse effects after receiving medication, improving treatment efficacy is a concern. Glucocorticoids promote extinction learning, and alterations in glucocorticoid signaling pathways have been associated with PTSD. Lehrner et al previously showed that adding hydrocortisone (HCORT) to PE therapy increased patients’ glucocorticoid sensitivity at baseline, improved treatment retention, and resulted in greater treatment improvements. This study evaluated HCORT in conjunction with PE for combat veterans with PTSD following deployment to Iraq and Afghanistan.9
Study design
- This randomized, double-blind, placebo-controlled trial administered HCORT 30 mg oral or placebo to 96 combat veterans 30 minutes before PE sessions.
- Participants were veterans previously deployed to Afghanistan or Iraq with deployment-related PTSD >6 months with a minimum CAPS score of 60. They were unmedicated or on a stable psychotropic regimen for ≥4 weeks.
- Exclusion criteria included a lifetime history of a primary psychotic disorder (bipolar I disorder or obsessive-compulsive disorder), medical or mental health condition other than PTSD that required immediate clinical attention, moderate to severe traumatic brain injury (TBI), substance abuse or dependence within the past 3 months, medical illness that contraindicated ingestion of hydrocortisone, acute suicide risk, and pregnancy or intent to become pregnant.
- The primary outcome measures included PTSD severity as assessed with CAPS.
- Secondary outcome measures included self-reported PTSD symptoms as assessed with the Posttraumatic Diagnostic Scale (PDS) and depression as assessed with the Beck Depression Inventory-II (BDI). These scales were administered pretreatment, posttreatment, and at 3-months follow-up.
Outcomes
- Out of 96 veterans enrolled, 60 were randomized and 52 completed the treatment.
- Five participants were considered recovered early and completed <12 sessions.
- Of those who completed treatment, 50 completed the 1-week posttreatment evaluations and 49 completed the 3-month follow-up evaluation.
- There was no difference in the proportion of dropouts (13.33%) across the conditions.
- HCORT failed to significantly improve either secondary outcomes or PTSD symptoms, according to an intent-to-treat analysis.
- However, exploratory analyses revealed that veterans with recent post-concussive symptoms and moderate TBI exposure saw a larger decrease in hyperarousal symptoms after PE therapy with HCORT augmentation.
- The reduction in avoidance symptoms with HCORT augmentation was also larger in veterans with higher baseline glucocorticoid sensitivity.
Continue to: Conclusions/limitations
Conclusions/limitations
- HCORT does not improve PTSD symptoms as assessed with the CAPS and PDS, or depression as assessed with the BDI.
- The main limitation of this study is generalizability.
- Further studies are needed to determine whether PE with HCORT could benefit veterans with indicators of enhanced glucocorticoid sensitivity, mild TBI, or postconcussive syndrome.
4. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952
PE, one of the most well-researched therapies for PTSD, is based on fear extinction. Exploring pharmacotherapies that improve fear extinction learning and their potential as supplements to PE is gaining increased attention. Such pharmacotherapies aim to improve the clinical impact of PE on the extent and persistence of symptom reduction. This study evaluated the effects of HCORT and D-cycloserine (DCS), a partial agonist of the N-methyl-D-aspartate (NMDA) receptor, on the learning and consolidation of fear extinction in patients with PTSD.10
Study design
- This double-blind, placebo-controlled, 3-group experimental design evaluated 90 individuals with PTSD who underwent fear conditioning with stimuli that was paired (CS+) or unpaired (CS−) with shock.
- Participants were veterans and civilians age 18 to 65 recruited from VA outpatient and community clinics and internet advertisements who met the criteria for PTSD or subsyndromal PTSD (according to DSM-IV criteria) for at least 3 months.
- Exclusion criteria included schizophrenia, bipolar disorder, substance abuse or dependence, alcohol dependence, previous moderate or severe head injury, seizure or neurological disorder, current infectious illness, systemic illness affecting CNS function, or other conditions known to affect psychophysiological responses. Excluded medications were antipsychotics, mood stabilizers, alpha- and beta-adrenergics, benzodiazepines, anticonvulsants, antihypertensives, sympathomimetics, anticholinergics, and steroids.
- Extinction learning took place 72 hours after extinction, and extinction retention was evaluated 1 week later. Placebo, HCORT 25 mg, or DCS 50 mg was given 1 hour before extinction learning.
- Clinical measures included PTSD diagnosis and symptom levels as determined by interview using CAPS and skin conduction response.
Outcomes
- The mean shock level, mean pre-stimulus skin conductance level (SCL) during habituation, and mean SC orienting response during the habituation phase did not differ between groups and were not associated with differential fear conditioning. Therefore, variations in shock level preference, resting SCL, or SC orienting response magnitude are unlikely to account for differences between groups during extinction learning and retention.
- During extinction learning, the DCS and HCORT groups showed a reduced differential CS+/CS− skin conductance response (SCR) compared to placebo.
- One week later, during the retention testing, there was a nonsignificant trend toward a smaller differential CS+/CS− SCR in the DCS group compared to placebo. HCORT and DCS administered as a single dosage facilitated fear extinction learning in individuals with PTSD symptoms.
Continue to: Conclusions/limitations
Conclusions/limitations
- In traumatized people with PTSD symptoms, a single dosage of HCORT or DCS enhanced the learning of fear extinction compared to placebo. A nonsignificant trend toward better extinction retention in the DCS group but not the HCORT group was also visible.
- These results imply that glucocorticoids and NMDA agonists have the potential to promote extinction learning in PTSD.
- Limitations include a lack of measures of glucocorticoid receptor sensitivity or FKBP5.
- Further studies could evaluate these findings with the addition of blood biomarker measures such as glucocorticoid receptor sensitivity or FKBP5.
5. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
Poor PTSD treatment results are associated with numerous comorbid conditions, such as dissociation, depression, alcohol and substance use disorders, childhood trauma, and suicidal ideation, which frequently leads to treatment resistance. Therefore, it is crucial to find a treatment that works for individuals with PTSD who also have comorbid conditions. In animal models, 3,4-methylenedioxymethamphetamine (MDMA), an empathogen/entactogen with stimulant properties, has been shown to enhance fear memory extinction and modulate fear memory reconsolidation. This study evaluated the efficacy and safety of MDMA-assisted therapy for treating patients with severe PTSD, including those with common comorbidities.11
Study design
- This randomized, double-blind, placebo-controlled, multi-site, phase 3 clinical trial evaluated individuals randomized to receive manualized therapy with MDMA or with placebo, combined with 3 preparatory and 9 integrative therapy sessions.
- Participants were 90 individuals (46 randomized to MDMA and 44 to placebo) with PTSD with a symptom duration ≥6 months and CAPS-5 total severity score ≥35 at baseline.
- Exclusion criteria included primary psychotic disorder, bipolar I disorder, eating disorders with active purging, major depressive disorder with psychotic features, dissociative identity disorder, personality disorders, current alcohol and substance use disorders, lactation or pregnancy, and any condition that could make receiving a sympathomimetic medication dangerous due to hypertension or tachycardia, including uncontrolled hypertension, history of arrhythmia, or marked baseline prolongation of QT and/or QTc interval.
- Three 8-hour experimental sessions of either therapy with MDMA assistance or therapy with a placebo control were given during the treatment period, and they were spaced approximately 4 weeks apart.
- In each session, participants received placebo or a single divided dose of MDMA 80 to 180 mg.
- At baseline and 2 months after the last experimental sessions, PTSD symptoms were measured with CAPS-5, and functional impairment was measured with Sheehan Disability Scale (SDS).
- The primary outcome measure was CAPS-5 total severity score at 18 weeks compared to baseline for MDMA-assisted therapy vs placebo-assisted therapy.
- The secondary outcome measure was clinician-rated functional impairment using the mean difference in SDS total scores from baseline to 18 weeks for MDMA-assisted therapy vs placebo-assisted therapy.
Outcomes
- MDMA was found to induce significant and robust attenuation in CAPS-5 score compared to placebo.
- The mean change in CAPS-5 score in completers was –24.4 in the MDMA group and –13.9 in the placebo group.
- MDMA significantly decreased the SDS total score.
- MDMA did not induce suicidality, misuse, or QT prolongation.
Continue to: Conclusions/limitations
Conclusions/limitations
- MDMA-assisted therapy is significantly more effective than manualized therapy with placebo in treating patients with severe PTSD, and it is also safe and well-tolerated, even in individuals with comorbidities.
- No major safety issues were associated with MDMA-assisted treatment.
- MDMA-assisted therapy should be promptly assessed for clinical usage because it has the potential to significantly transform the way PTSD is treated.
- Limitations of this study include a smaller sample size (due to the COVID-19 pandemic); lack of ethnic and racial diversity; short duration; safety data were collected by site therapist, which limited the blinding; and the blinding of participants was difficult due to the subjective effects of MDMA, which could have resulted in expectation effects.
6. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990
Sertraline and paroxetine are the only FDA-approved medications for treating PTSD. Some evidence suggests cannabis may provide a therapeutic benefit for PTSD.15 This study examined the effects of 3 different preparations of cannabis for treating PTSD symptoms.12
Study design
- This double-blind, randomized, placebo-controlled, crossover trial used 3 active treatment groups of cannabis: high delta-9-tetrahydrocannabinol (THC)/low cannabidiol (CBD), high CBD/low THC, and high THC/high CBD (THC+CBD). A low THC/low CBD preparation was used as a placebo. “High” content contained 9% to 15% concentration by weight of the respective cannabinoid, and “low” content contained <2% concentration by weight.
- Inclusion criteria included being a US military veteran, meeting DSM-5 PTSD criteria for ≥6 months, having moderate symptom severity (CAPS-5 score ≥25), abstaining from cannabis 2 weeks prior to study and agreeing not to use any non-study cannabis during the trial, and being stable on medications/therapy prior to the study.
- Exclusion criteria included women who were pregnant/nursing/child-bearing age and not taking an effective means of birth control; current/past serious mental illness, including psychotic and personality disorders; having a first-degree relative with a psychotic or bipolar disorder; having a high suicide risk based on Columbia-Suicide Severity Rating Scale; meeting DSM-5 criteria for moderate-severe cannabis use disorder; screening positive for illicit substances; or having significant medical disease.
- Participants in Stage 1 (n = 80) were randomized to 1 of the 3 active treatments or placebo for 3 weeks. After a 2-week washout, participants in Stage 2 (n = 74) were randomized to receive for 3 weeks 1 of the 3 active treatments they had not previously received.
- During each stage, participants had ad libitum use for a maximum of 1.8 g/d.
- The primary outcome was change in PTSD symptom severity by the end of Stage 1 as assessed with CAPS-5.
- Secondary outcomes included the PTSD Checklist for DSM-5 (PCL-5), the general depression subscale and anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms (IDAS), the Inventory of Psychosocial Functioning, and the Insomnia Severity Index.
Outcomes
- Six participants did not continue to Stage 2. Three participants did not finish Stage 2 due to adverse effects, and 7 did not complete outcome measurements. The overall attrition rate was 16.3%.
- There was no significant difference in total grams of smoked cannabis or placebo between the 4 treatment groups in Stage 1 at the end of 3 weeks. In Stage 2, there was a significant difference, with the THC+CBD group using more cannabis compared to the other 2 groups.
- Each of the 4 groups had significant reductions in total CAPS-5 scores at the end of Stage 1, and there was no significant difference in CAPS-5 severity scores between the 4 groups.
- In Stage 1, PCL-5 scores were not significantly different between treatment groups from baseline to the end of stage. There was a significant difference in Stage 2 between the high CBD and THC+CBD groups, with the combined group reporting greater improvement of symptoms.
- In Stage 2, the THC+CBD group reported greater reductions in pre/post IDAS social anxiety scores and IDAS general depression scores, and the high THC group reported greater reductions in pre/post IDAS social anxiety scores.
- In Stage 1, 37 of 60 participants in the active groups reported at least 1 adverse event, and 45 of the 74 Stage 2 participants reported at least 1 adverse event. The most common adverse events were cough, throat irritation, and anxiety. Participants in the Stage 1 high THC group had a significant increase in reported withdrawal symptoms after 1 week of stopping use.
Continue to: Conclusions/limitations
Conclusions/limitations
- This first randomized, placebo-control trial of cannabis in US veterans did not show a significant difference among treatment groups, including placebo, on the primary outcome of CAPS-5 score. All 4 groups had significant reductions in symptom severity on CAPS-5 and showed good tolerability.
- Prior beliefs about the effects of cannabis may have played a role in the reduction of PTSD symptoms in the placebo group.
- Many participants (n =34) were positive for THC during the screening process, so previous cannabis use/chronicity of cannabis use may have contributed.
- One limitation was that participants assigned to the Stage 1 high THC group had Cannabis Use Disorders Identification Test scores (which assesses cannabis use disorder risk) about 2 times greater than participants in other conditions.
- Another limitation was that total cannabis use was lower than expected, as participants in Stage 1 used 8.2 g to 14.6 g over 3 weeks, though they had access to up to 37.8 g.
- There was no placebo in Stage 2.
- Future studies should look at longer treatment periods with more participants.
7. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444
Bright light therapy is an inexpensive treatment approach that may affect serotonergic pathways.16 This study examined bright light therapy for reducing PTSD symptoms and examined if improvement of PTSD is related to a shift in circadian rhythm.13
Study design
- Veterans with combat-related PTSD had to have been stable on treatment for at least 8 weeks or to have not received any other PTSD treatments prior to the study.
- Participants were randomized to active treatment of 30 minutes daily 10,000 lux ultraviolet-filtered white light while sitting within 18 inches (n = 34) or a control condition of 30 minutes daily inactivated negative ion generator (n = 35) for 4 weeks.
- Inclusion criteria included a CAPS score ≥30.
- Exclusion criteria included high suicidality, high probability of alcohol/substance abuse in the past 3 months, bipolar disorder/mania/schizophrenia/psychosis, ophthalmologic deformities, shift work in past 2 months or travel across time zones in past 2 weeks, head trauma, high outdoor light exposure, history of winter depression, history of seizures, or myocardial infarction/stroke/cancer within 3 years.
- Primary outcomes were improvement on CAPS and Clinical Global Impressions-Improvement scale (CGI-IM) score at Week 4.
- Wrist actigraphy recordings measured sleep.
- Other measurements included the Hamilton Depression Rating Scale (HAM-D), Hamilton atypical symptoms (HAM-AS), PCL-Military (PCL-M), Pittsburg Sleep Quality Index (PSQI), BDI, Spielberger State-Trait Anxiety Inventory (STAI Form Y-2), Beck Suicide Scale, and Systematic Assessment for Treatment Emergent Effects questionnaire.
Outcomes
- There was a significant decrease in CAPS score in participants who received bright light therapy compared to controls. Treatment response (defined as ≥33% reduction in score) was significantly greater in the bright light (44%) vs control (8.6%) group. No participants achieved remission.
- There was a significant improvement in CGI-IM scores in the bright light group, but no significant difference in participants who were judged to improve “much” or “very much.”
- PCL-M scores did not change significantly between groups, although a significantly greater proportion of participants had treatment response in the bright light group (33%) vs control (6%).
- There were no significant changes in HAM-D, HAM-AS, STAI, BDI, actigraphic estimates of sleep, or PSQI scores.
- Bright light therapy resulted in phase advancement while control treatment had phase delay.
- There were no significant differences in adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Bright light therapy may be a treatment option or adjunct for combat-related PTSD as seen by improvement on CAPS and CGI scores, as well as a greater treatment response seen on CAPS and PCL-5 scores in the bright light group.
- There was no significant difference for other measures, including depression, anxiety, and sleep.
- Limitations include excluding patients with a wide variety of medical or psychiatric comorbidities, as well as limited long-term follow up data.
- Other limitations include not knowing the precise amount of time participants stayed in front of the light device and loss of some actigraphic data (data from only 49 of 69 participants).
8. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41 doi:10.1186/s12888-022-03699-4
Cognitive processing therapy (CPT), a type of trauma-focused psychotherapy, is an effective treatment for PTSD in the military population.17,18 However, patients may not be able to or want to participate in such therapy due to barriers such as difficulty arranging transportation, being homebound due to injury, concerns about COVID-19, stigma, familial obligations, and job constraints. This study looked at if CPT delivered face-to-face at the patient’s home or via telehealth in home would be effective and increase accessibility.14
Study design
- Participants (n = 120) were active-duty military and veterans who met DSM-5 criteria for PTSD. They were randomized to receive CPT in the office, in their home, or via telehealth. Participants could choose not to partake in 1 modality but were then randomized to 1 of the other 2.
- Exclusion criteria included suicide/homicide risk needing intervention, items/situations pertaining to danger (ie, aggressive pet or unsafe neighborhood), significant alcohol/substance use, active psychosis, and impaired cognitive functioning.
- The primary outcome measurement was change in PCL-5 and CAPS-5 score over 6 months. The BDI-II was used to assess depressive symptoms.
- Secondary outcomes included the Reliable Change Index (defined as “an improvement of 10 or more points that was sustained at all subsequent assessments”) on the PCL-5 and remission on the CAPS-5.
- CPT was delivered in 60-minute sessions twice a week for 6 weeks. Participants who did not have electronic resources were loaned a telehealth apparatus.
Outcomes
- Overall, 57% of participants opted out of 1 modality, which resulted in fewer participants being placed into the in-home arm (n = 32). Most participants chose not to do in-home treatments (54%), followed by in-office (29%), and telehealth (17%).
- There was a significant posttreatment improvement in PCL-5 scores in all treatment arms, with improvement greater with in-home (d = 2.1) and telehealth (d = 2.0) vs in-office (d=1.3). The in-home and telehealth scores were significantly improved compared to in-office, and the difference between in-home and telehealth PCL-5 scores was minimal.
- At 6 months posttreatment, the differences between the 3 treatment groups on PCL-5 score were negligible.
- CAPS-5 scores were significantly improved in all treatment arms, with improvement largest with in-home treatment; however, the differences between the groups were not significant.
- BDI-II scores improved in all modalities but were larger in the in-home (d = 1.2) and telehealth (d = 1.1) arms than the in-office arm (d = 0.52).
- Therapist time commitment was greater for the in-home and in-office arms (2 hours/session) than the telehealth arm (1 hour/session). This difference was due to commuting time for the patient or therapist.
- The dropout rate was not statistically significant between the groups.
- Adverse events did not significantly differ per group. The most commonly reported ones included nightmares, sleep difficulty, depression, anxiety, and irritability.
Conclusions/limitations
- Patients undergoing CPT had significant improvement in PTSD symptoms, with posttreatment PCL-5 improvement approximately twice as large in those who received the in-home and telehealth modalities vs in-office treatment.
- The group differences were not seen on CAPS-5 scores at posttreatment, or PCL-5 or CAPS-5 scores at 6 months posttreatment.
- In-home CPT was declined the most, which suggests that in-home distractions or the stigma of a mental health clinician being in their home played a role in patients’ decision-making. However, in-home CPT produced the greatest amount of improvement in PTSD symptoms. The authors concluded that in-home therapy should be reserved for those who are homebound or have travel limitations.
- This study shows evidence that telehealth may be a good modality for CPT, as seen by improvement in PTSD symptoms and good acceptability and retention.
- Limitations include more patients opting out of in-home CPT, and reimbursement for travel may not be available in the real-world setting.
Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder. The lifetime prevalence among American adults is 6.8%.1 Management of PTSD includes treating distressing symptoms, reducing avoidant behaviors, treating comorbid conditions (eg, depression, substance use disorders, or mood dysregulation), and improving adaptive functioning, which includes restoring a psychological sense of safety and trust. PTSD can be treated using evidence-based psychotherapies, pharmacotherapy, or a combination of both modalities. For adults, evidence-based treatment guidelines recommend the use of cognitive-behavioral therapy, cognitive processing therapy, cognitive therapy, and prolonged exposure therapy.2 These guidelines also recommend (with some reservations) the use of brief eclectic psychotherapy, eye movement desensitization and reprocessing, and narrative exposure therapy.2 Although the evidence base for the use of medications is not as strong as that for the psychotherapies listed above, the guidelines recommend the use of fluoxetine, paroxetine, sertraline, and venlafaxine.2
Currently available treatments for PTSD have significant limitations. For example, trauma-focused psychotherapies can have significant rates of nonresponse, partial response, or treatment dropout.3,4 Additionally, such therapies are not widely accessible. As for pharmacotherapy, very few available options are supported by evidence, and the efficacy of these options is limited, as shown by the reports that only 60% of patients with PTSD show a response to selective serotonin reuptake inhibitors (SSRIs), and only 20% to 30% achieve complete remission.5 Additionally, it may take months for patients to achieve an acceptable level of improvement with medications. As a result, a substantial proportion of patients who seek treatment continue to remain symptomatic, with impaired levels of functioning. This lack of progress in PTSD treatment has been labeled as a national crisis, calling for an urgent need to find effective pharmacologic treatments for PTSD.6
In this article, we review 8 randomized controlled trials (RCTs) of treatments for PTSD published within the last 5 years (Table7-14).

1. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202
Feder et al had previously found a significant and quick decrease in PTSD symptoms after a single dose of IV ketamine had. This is the first RCT to examine the effectiveness and safety of repeated IV ketamine infusions for the treatment of persistent PTSD.7
Study design
- This randomized, double-blind, parallel-arm controlled trial treated 30 individuals with chronic PTSD with 6 infusions of either ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg) over 2 consecutive weeks.
- Participants were individuals age 18 to 70 with a primary diagnosis of chronic PTSD according to the DSM-5 criteria and determined by The Structure Clinical Interview for DSM-5, with a score ≥30 on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5).
- Any severe or unstable medical condition, active suicidal or homicidal ideation, lifetime history of psychotic or bipolar disorder, current anorexia nervosa or bulimia, alcohol or substance use disorder within 3 months of screening, history of recreational ketamine or phencyclidine use on more than 1 occasion or any use in the previous 2 years, and ongoing treatment with a long-acting benzodiazepine or opioid medication were all considered exclusion criteria. Individuals who took short-acting benzodiazepines had their morning doses held on infusion days. Marijuana or cannabis derivatives were allowed.
- The primary outcome measure was a change in PTSD symptom severity as measured with CAPS-5. This was administered before the first infusion and weekly thereafter. The Impact of Event Scale-Revised, the Montgomery–Åsberg Depression Rating Scale, and adverse effect measurements were used as secondary outcome measures.
- Treatment response was defined as ≥30% symptom improvement 2 weeks after the first infusion as assessed with CAPS-5.
- Individuals who responded to treatment were followed naturalistically weekly for up to 4 weeks and then monthly until loss of responder status, or up to 6 months if there was no loss of response.
Outcomes
- At the second week, the mean CAPS-5 total score in the ketamine group was 11.88 points (SE = 3.96) lower than in the midazolam group (d = 1.13; 95% CI, 0.36 to 1.91).
- In the ketamine group, 67% of patients responded to therapy, compared to 20% in the midazolam group.
- Following the 2-week course of infusions, the median period until loss of response among ketamine responders was 27.5 days.
- Ketamine infusions showed good tolerability and safety. There were no clinically significant adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Repeated ketamine infusions are effective in reducing symptom severity in individuals with chronic PTSD.
- Limitations to this study include the exclusion of individuals with comorbid bipolar disorder, current alcohol or substance use disorder, or suicidal ideations, the small sample size, and a higher rate of transient dissociative symptoms in the ketamine group.
- Future studies could evaluate the efficacy of repeated ketamine infusions in individuals with treatment-resistant PTSD. Also, further studies are required to assess the efficacy of novel interventions to prevent relapse and evaluate the efficacy, safety, and tolerability of periodic IV ketamine use as maintenance.
- Additional research might determine whether pairing psychotherapy with ketamine administration can lessen the risk of recurrence for PTSD patients after stopping ketamine infusions.
2. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126
Clinical practice recommendations for PTSD have identified trauma-focused psychotherapies and SSRIs as very effective treatments. The few studies that have compared trauma-focused psychotherapy to SSRIs or to a combination of treatments are not generalizable, have significant limitations, or are primarily concerned with refractory disorders or augmentation techniques. This study evaluated the efficacy of prolonged exposure therapy (PE) plus placebo, PE plus sertraline, and sertraline plus enhanced medication management in the treatment of PTSD.8
Study design
- This randomized, 4-site, 24-week clinical trial divided participants into 3 subgroups: PE plus placebo, PE plus sertraline, and sertraline plus enhanced medication management.
- Participants were veterans or service members of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment as indicated by a CAPS score ≥50 for at least 3 months. The DSM-IV-TR version of CAPS was used because the DSM-5 version was not available at the time of the study.
- Individuals who had a current, imminent risk of suicide; active psychosis; alcohol or substance dependence in the past 8 weeks; inability to attend weekly appointments for the treatment period; prior intolerance to or failure of an adequate trial of PE or sertraline; medical illness likely to result in hospitalization or contraindication to study treatment; serious cognitive impairment; mild traumatic brain injury; or concurrent use of antidepressants, antipsychotics, benzodiazepines, prazosin, or sleep agents were excluded.
- Participants completed up to thirteen 90-minute sessions of PE.
- The sertraline dosage was titrated during a 10-week period and continued until Week 24. Dosages were adjusted between 50 and 200 mg/d, with the last dose increase at Week 10.
- The primary outcome measure was symptom severity of PTSD in the past month as determined by CAPS score at Week 24.
- The secondary outcome was self-reported symptoms of PTSD (PTSD checklist [PCL] Specific Stressor Version), clinically meaningful change (reduction of ≥20 points or score ≤35 on CAPS), response (reduction of ≥50% in CAPS score), and remission (CAPS score ≤35).
Outcomes
- At Week 24, 149 participants completed the study; 207 were included in the intent-to-treat analysis.
- PTSD symptoms significantly decreased over 24 weeks, according to a modified intent-to-treat analysis utilizing a mixed model of repeated measurements; nevertheless, slopes were similar across therapy groups.
Continue to: Conclusions/limitations
Conclusions/limitations
- Although the severity of PTSD symptoms decreased in all 3 subgroups, there was no difference in PTSD symptom severity or change in symptoms at Week 24 among all 3 subgroups.
- The main limitation of this study was the inclusion of only combat veterans.
- Further research should focus on enhancing treatment retention and should include administering sustained exposure therapy at brief intervals.
3. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924
First-line therapy for PTSD includes cognitive-behavioral therapies such as PE. However, because many people still have major adverse effects after receiving medication, improving treatment efficacy is a concern. Glucocorticoids promote extinction learning, and alterations in glucocorticoid signaling pathways have been associated with PTSD. Lehrner et al previously showed that adding hydrocortisone (HCORT) to PE therapy increased patients’ glucocorticoid sensitivity at baseline, improved treatment retention, and resulted in greater treatment improvements. This study evaluated HCORT in conjunction with PE for combat veterans with PTSD following deployment to Iraq and Afghanistan.9
Study design
- This randomized, double-blind, placebo-controlled trial administered HCORT 30 mg oral or placebo to 96 combat veterans 30 minutes before PE sessions.
- Participants were veterans previously deployed to Afghanistan or Iraq with deployment-related PTSD >6 months with a minimum CAPS score of 60. They were unmedicated or on a stable psychotropic regimen for ≥4 weeks.
- Exclusion criteria included a lifetime history of a primary psychotic disorder (bipolar I disorder or obsessive-compulsive disorder), medical or mental health condition other than PTSD that required immediate clinical attention, moderate to severe traumatic brain injury (TBI), substance abuse or dependence within the past 3 months, medical illness that contraindicated ingestion of hydrocortisone, acute suicide risk, and pregnancy or intent to become pregnant.
- The primary outcome measures included PTSD severity as assessed with CAPS.
- Secondary outcome measures included self-reported PTSD symptoms as assessed with the Posttraumatic Diagnostic Scale (PDS) and depression as assessed with the Beck Depression Inventory-II (BDI). These scales were administered pretreatment, posttreatment, and at 3-months follow-up.
Outcomes
- Out of 96 veterans enrolled, 60 were randomized and 52 completed the treatment.
- Five participants were considered recovered early and completed <12 sessions.
- Of those who completed treatment, 50 completed the 1-week posttreatment evaluations and 49 completed the 3-month follow-up evaluation.
- There was no difference in the proportion of dropouts (13.33%) across the conditions.
- HCORT failed to significantly improve either secondary outcomes or PTSD symptoms, according to an intent-to-treat analysis.
- However, exploratory analyses revealed that veterans with recent post-concussive symptoms and moderate TBI exposure saw a larger decrease in hyperarousal symptoms after PE therapy with HCORT augmentation.
- The reduction in avoidance symptoms with HCORT augmentation was also larger in veterans with higher baseline glucocorticoid sensitivity.
Continue to: Conclusions/limitations
Conclusions/limitations
- HCORT does not improve PTSD symptoms as assessed with the CAPS and PDS, or depression as assessed with the BDI.
- The main limitation of this study is generalizability.
- Further studies are needed to determine whether PE with HCORT could benefit veterans with indicators of enhanced glucocorticoid sensitivity, mild TBI, or postconcussive syndrome.
4. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952
PE, one of the most well-researched therapies for PTSD, is based on fear extinction. Exploring pharmacotherapies that improve fear extinction learning and their potential as supplements to PE is gaining increased attention. Such pharmacotherapies aim to improve the clinical impact of PE on the extent and persistence of symptom reduction. This study evaluated the effects of HCORT and D-cycloserine (DCS), a partial agonist of the N-methyl-D-aspartate (NMDA) receptor, on the learning and consolidation of fear extinction in patients with PTSD.10
Study design
- This double-blind, placebo-controlled, 3-group experimental design evaluated 90 individuals with PTSD who underwent fear conditioning with stimuli that was paired (CS+) or unpaired (CS−) with shock.
- Participants were veterans and civilians age 18 to 65 recruited from VA outpatient and community clinics and internet advertisements who met the criteria for PTSD or subsyndromal PTSD (according to DSM-IV criteria) for at least 3 months.
- Exclusion criteria included schizophrenia, bipolar disorder, substance abuse or dependence, alcohol dependence, previous moderate or severe head injury, seizure or neurological disorder, current infectious illness, systemic illness affecting CNS function, or other conditions known to affect psychophysiological responses. Excluded medications were antipsychotics, mood stabilizers, alpha- and beta-adrenergics, benzodiazepines, anticonvulsants, antihypertensives, sympathomimetics, anticholinergics, and steroids.
- Extinction learning took place 72 hours after extinction, and extinction retention was evaluated 1 week later. Placebo, HCORT 25 mg, or DCS 50 mg was given 1 hour before extinction learning.
- Clinical measures included PTSD diagnosis and symptom levels as determined by interview using CAPS and skin conduction response.
Outcomes
- The mean shock level, mean pre-stimulus skin conductance level (SCL) during habituation, and mean SC orienting response during the habituation phase did not differ between groups and were not associated with differential fear conditioning. Therefore, variations in shock level preference, resting SCL, or SC orienting response magnitude are unlikely to account for differences between groups during extinction learning and retention.
- During extinction learning, the DCS and HCORT groups showed a reduced differential CS+/CS− skin conductance response (SCR) compared to placebo.
- One week later, during the retention testing, there was a nonsignificant trend toward a smaller differential CS+/CS− SCR in the DCS group compared to placebo. HCORT and DCS administered as a single dosage facilitated fear extinction learning in individuals with PTSD symptoms.
Continue to: Conclusions/limitations
Conclusions/limitations
- In traumatized people with PTSD symptoms, a single dosage of HCORT or DCS enhanced the learning of fear extinction compared to placebo. A nonsignificant trend toward better extinction retention in the DCS group but not the HCORT group was also visible.
- These results imply that glucocorticoids and NMDA agonists have the potential to promote extinction learning in PTSD.
- Limitations include a lack of measures of glucocorticoid receptor sensitivity or FKBP5.
- Further studies could evaluate these findings with the addition of blood biomarker measures such as glucocorticoid receptor sensitivity or FKBP5.
5. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
Poor PTSD treatment results are associated with numerous comorbid conditions, such as dissociation, depression, alcohol and substance use disorders, childhood trauma, and suicidal ideation, which frequently leads to treatment resistance. Therefore, it is crucial to find a treatment that works for individuals with PTSD who also have comorbid conditions. In animal models, 3,4-methylenedioxymethamphetamine (MDMA), an empathogen/entactogen with stimulant properties, has been shown to enhance fear memory extinction and modulate fear memory reconsolidation. This study evaluated the efficacy and safety of MDMA-assisted therapy for treating patients with severe PTSD, including those with common comorbidities.11
Study design
- This randomized, double-blind, placebo-controlled, multi-site, phase 3 clinical trial evaluated individuals randomized to receive manualized therapy with MDMA or with placebo, combined with 3 preparatory and 9 integrative therapy sessions.
- Participants were 90 individuals (46 randomized to MDMA and 44 to placebo) with PTSD with a symptom duration ≥6 months and CAPS-5 total severity score ≥35 at baseline.
- Exclusion criteria included primary psychotic disorder, bipolar I disorder, eating disorders with active purging, major depressive disorder with psychotic features, dissociative identity disorder, personality disorders, current alcohol and substance use disorders, lactation or pregnancy, and any condition that could make receiving a sympathomimetic medication dangerous due to hypertension or tachycardia, including uncontrolled hypertension, history of arrhythmia, or marked baseline prolongation of QT and/or QTc interval.
- Three 8-hour experimental sessions of either therapy with MDMA assistance or therapy with a placebo control were given during the treatment period, and they were spaced approximately 4 weeks apart.
- In each session, participants received placebo or a single divided dose of MDMA 80 to 180 mg.
- At baseline and 2 months after the last experimental sessions, PTSD symptoms were measured with CAPS-5, and functional impairment was measured with Sheehan Disability Scale (SDS).
- The primary outcome measure was CAPS-5 total severity score at 18 weeks compared to baseline for MDMA-assisted therapy vs placebo-assisted therapy.
- The secondary outcome measure was clinician-rated functional impairment using the mean difference in SDS total scores from baseline to 18 weeks for MDMA-assisted therapy vs placebo-assisted therapy.
Outcomes
- MDMA was found to induce significant and robust attenuation in CAPS-5 score compared to placebo.
- The mean change in CAPS-5 score in completers was –24.4 in the MDMA group and –13.9 in the placebo group.
- MDMA significantly decreased the SDS total score.
- MDMA did not induce suicidality, misuse, or QT prolongation.
Continue to: Conclusions/limitations
Conclusions/limitations
- MDMA-assisted therapy is significantly more effective than manualized therapy with placebo in treating patients with severe PTSD, and it is also safe and well-tolerated, even in individuals with comorbidities.
- No major safety issues were associated with MDMA-assisted treatment.
- MDMA-assisted therapy should be promptly assessed for clinical usage because it has the potential to significantly transform the way PTSD is treated.
- Limitations of this study include a smaller sample size (due to the COVID-19 pandemic); lack of ethnic and racial diversity; short duration; safety data were collected by site therapist, which limited the blinding; and the blinding of participants was difficult due to the subjective effects of MDMA, which could have resulted in expectation effects.
6. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990
Sertraline and paroxetine are the only FDA-approved medications for treating PTSD. Some evidence suggests cannabis may provide a therapeutic benefit for PTSD.15 This study examined the effects of 3 different preparations of cannabis for treating PTSD symptoms.12
Study design
- This double-blind, randomized, placebo-controlled, crossover trial used 3 active treatment groups of cannabis: high delta-9-tetrahydrocannabinol (THC)/low cannabidiol (CBD), high CBD/low THC, and high THC/high CBD (THC+CBD). A low THC/low CBD preparation was used as a placebo. “High” content contained 9% to 15% concentration by weight of the respective cannabinoid, and “low” content contained <2% concentration by weight.
- Inclusion criteria included being a US military veteran, meeting DSM-5 PTSD criteria for ≥6 months, having moderate symptom severity (CAPS-5 score ≥25), abstaining from cannabis 2 weeks prior to study and agreeing not to use any non-study cannabis during the trial, and being stable on medications/therapy prior to the study.
- Exclusion criteria included women who were pregnant/nursing/child-bearing age and not taking an effective means of birth control; current/past serious mental illness, including psychotic and personality disorders; having a first-degree relative with a psychotic or bipolar disorder; having a high suicide risk based on Columbia-Suicide Severity Rating Scale; meeting DSM-5 criteria for moderate-severe cannabis use disorder; screening positive for illicit substances; or having significant medical disease.
- Participants in Stage 1 (n = 80) were randomized to 1 of the 3 active treatments or placebo for 3 weeks. After a 2-week washout, participants in Stage 2 (n = 74) were randomized to receive for 3 weeks 1 of the 3 active treatments they had not previously received.
- During each stage, participants had ad libitum use for a maximum of 1.8 g/d.
- The primary outcome was change in PTSD symptom severity by the end of Stage 1 as assessed with CAPS-5.
- Secondary outcomes included the PTSD Checklist for DSM-5 (PCL-5), the general depression subscale and anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms (IDAS), the Inventory of Psychosocial Functioning, and the Insomnia Severity Index.
Outcomes
- Six participants did not continue to Stage 2. Three participants did not finish Stage 2 due to adverse effects, and 7 did not complete outcome measurements. The overall attrition rate was 16.3%.
- There was no significant difference in total grams of smoked cannabis or placebo between the 4 treatment groups in Stage 1 at the end of 3 weeks. In Stage 2, there was a significant difference, with the THC+CBD group using more cannabis compared to the other 2 groups.
- Each of the 4 groups had significant reductions in total CAPS-5 scores at the end of Stage 1, and there was no significant difference in CAPS-5 severity scores between the 4 groups.
- In Stage 1, PCL-5 scores were not significantly different between treatment groups from baseline to the end of stage. There was a significant difference in Stage 2 between the high CBD and THC+CBD groups, with the combined group reporting greater improvement of symptoms.
- In Stage 2, the THC+CBD group reported greater reductions in pre/post IDAS social anxiety scores and IDAS general depression scores, and the high THC group reported greater reductions in pre/post IDAS social anxiety scores.
- In Stage 1, 37 of 60 participants in the active groups reported at least 1 adverse event, and 45 of the 74 Stage 2 participants reported at least 1 adverse event. The most common adverse events were cough, throat irritation, and anxiety. Participants in the Stage 1 high THC group had a significant increase in reported withdrawal symptoms after 1 week of stopping use.
Continue to: Conclusions/limitations
Conclusions/limitations
- This first randomized, placebo-control trial of cannabis in US veterans did not show a significant difference among treatment groups, including placebo, on the primary outcome of CAPS-5 score. All 4 groups had significant reductions in symptom severity on CAPS-5 and showed good tolerability.
- Prior beliefs about the effects of cannabis may have played a role in the reduction of PTSD symptoms in the placebo group.
- Many participants (n =34) were positive for THC during the screening process, so previous cannabis use/chronicity of cannabis use may have contributed.
- One limitation was that participants assigned to the Stage 1 high THC group had Cannabis Use Disorders Identification Test scores (which assesses cannabis use disorder risk) about 2 times greater than participants in other conditions.
- Another limitation was that total cannabis use was lower than expected, as participants in Stage 1 used 8.2 g to 14.6 g over 3 weeks, though they had access to up to 37.8 g.
- There was no placebo in Stage 2.
- Future studies should look at longer treatment periods with more participants.
7. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444
Bright light therapy is an inexpensive treatment approach that may affect serotonergic pathways.16 This study examined bright light therapy for reducing PTSD symptoms and examined if improvement of PTSD is related to a shift in circadian rhythm.13
Study design
- Veterans with combat-related PTSD had to have been stable on treatment for at least 8 weeks or to have not received any other PTSD treatments prior to the study.
- Participants were randomized to active treatment of 30 minutes daily 10,000 lux ultraviolet-filtered white light while sitting within 18 inches (n = 34) or a control condition of 30 minutes daily inactivated negative ion generator (n = 35) for 4 weeks.
- Inclusion criteria included a CAPS score ≥30.
- Exclusion criteria included high suicidality, high probability of alcohol/substance abuse in the past 3 months, bipolar disorder/mania/schizophrenia/psychosis, ophthalmologic deformities, shift work in past 2 months or travel across time zones in past 2 weeks, head trauma, high outdoor light exposure, history of winter depression, history of seizures, or myocardial infarction/stroke/cancer within 3 years.
- Primary outcomes were improvement on CAPS and Clinical Global Impressions-Improvement scale (CGI-IM) score at Week 4.
- Wrist actigraphy recordings measured sleep.
- Other measurements included the Hamilton Depression Rating Scale (HAM-D), Hamilton atypical symptoms (HAM-AS), PCL-Military (PCL-M), Pittsburg Sleep Quality Index (PSQI), BDI, Spielberger State-Trait Anxiety Inventory (STAI Form Y-2), Beck Suicide Scale, and Systematic Assessment for Treatment Emergent Effects questionnaire.
Outcomes
- There was a significant decrease in CAPS score in participants who received bright light therapy compared to controls. Treatment response (defined as ≥33% reduction in score) was significantly greater in the bright light (44%) vs control (8.6%) group. No participants achieved remission.
- There was a significant improvement in CGI-IM scores in the bright light group, but no significant difference in participants who were judged to improve “much” or “very much.”
- PCL-M scores did not change significantly between groups, although a significantly greater proportion of participants had treatment response in the bright light group (33%) vs control (6%).
- There were no significant changes in HAM-D, HAM-AS, STAI, BDI, actigraphic estimates of sleep, or PSQI scores.
- Bright light therapy resulted in phase advancement while control treatment had phase delay.
- There were no significant differences in adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Bright light therapy may be a treatment option or adjunct for combat-related PTSD as seen by improvement on CAPS and CGI scores, as well as a greater treatment response seen on CAPS and PCL-5 scores in the bright light group.
- There was no significant difference for other measures, including depression, anxiety, and sleep.
- Limitations include excluding patients with a wide variety of medical or psychiatric comorbidities, as well as limited long-term follow up data.
- Other limitations include not knowing the precise amount of time participants stayed in front of the light device and loss of some actigraphic data (data from only 49 of 69 participants).
8. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41 doi:10.1186/s12888-022-03699-4
Cognitive processing therapy (CPT), a type of trauma-focused psychotherapy, is an effective treatment for PTSD in the military population.17,18 However, patients may not be able to or want to participate in such therapy due to barriers such as difficulty arranging transportation, being homebound due to injury, concerns about COVID-19, stigma, familial obligations, and job constraints. This study looked at if CPT delivered face-to-face at the patient’s home or via telehealth in home would be effective and increase accessibility.14
Study design
- Participants (n = 120) were active-duty military and veterans who met DSM-5 criteria for PTSD. They were randomized to receive CPT in the office, in their home, or via telehealth. Participants could choose not to partake in 1 modality but were then randomized to 1 of the other 2.
- Exclusion criteria included suicide/homicide risk needing intervention, items/situations pertaining to danger (ie, aggressive pet or unsafe neighborhood), significant alcohol/substance use, active psychosis, and impaired cognitive functioning.
- The primary outcome measurement was change in PCL-5 and CAPS-5 score over 6 months. The BDI-II was used to assess depressive symptoms.
- Secondary outcomes included the Reliable Change Index (defined as “an improvement of 10 or more points that was sustained at all subsequent assessments”) on the PCL-5 and remission on the CAPS-5.
- CPT was delivered in 60-minute sessions twice a week for 6 weeks. Participants who did not have electronic resources were loaned a telehealth apparatus.
Outcomes
- Overall, 57% of participants opted out of 1 modality, which resulted in fewer participants being placed into the in-home arm (n = 32). Most participants chose not to do in-home treatments (54%), followed by in-office (29%), and telehealth (17%).
- There was a significant posttreatment improvement in PCL-5 scores in all treatment arms, with improvement greater with in-home (d = 2.1) and telehealth (d = 2.0) vs in-office (d=1.3). The in-home and telehealth scores were significantly improved compared to in-office, and the difference between in-home and telehealth PCL-5 scores was minimal.
- At 6 months posttreatment, the differences between the 3 treatment groups on PCL-5 score were negligible.
- CAPS-5 scores were significantly improved in all treatment arms, with improvement largest with in-home treatment; however, the differences between the groups were not significant.
- BDI-II scores improved in all modalities but were larger in the in-home (d = 1.2) and telehealth (d = 1.1) arms than the in-office arm (d = 0.52).
- Therapist time commitment was greater for the in-home and in-office arms (2 hours/session) than the telehealth arm (1 hour/session). This difference was due to commuting time for the patient or therapist.
- The dropout rate was not statistically significant between the groups.
- Adverse events did not significantly differ per group. The most commonly reported ones included nightmares, sleep difficulty, depression, anxiety, and irritability.
Conclusions/limitations
- Patients undergoing CPT had significant improvement in PTSD symptoms, with posttreatment PCL-5 improvement approximately twice as large in those who received the in-home and telehealth modalities vs in-office treatment.
- The group differences were not seen on CAPS-5 scores at posttreatment, or PCL-5 or CAPS-5 scores at 6 months posttreatment.
- In-home CPT was declined the most, which suggests that in-home distractions or the stigma of a mental health clinician being in their home played a role in patients’ decision-making. However, in-home CPT produced the greatest amount of improvement in PTSD symptoms. The authors concluded that in-home therapy should be reserved for those who are homebound or have travel limitations.
- This study shows evidence that telehealth may be a good modality for CPT, as seen by improvement in PTSD symptoms and good acceptability and retention.
- Limitations include more patients opting out of in-home CPT, and reimbursement for travel may not be available in the real-world setting.
1. Kessler RC, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019;74(5):596-607. doi: 10.1037/amp0000473
3. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
4. Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656-657.
5. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):169-180.
6. Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59.
7. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202. doi:10.1176/appi.ajp.2020.20050596
8. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126. doi:10.1001/jamapsychiatry.2018.3412
9. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924. doi:10.1016/j.brat.2021.103924
10. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952. doi:10.1038/s41386-021-01222-z
11. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
12. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. doi:10.1371/journal.pone.0246990
13. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444. doi:10.1093/milmed/usab014
14. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41. doi:10.1186/s12888-022-03699-4
15. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14:78-83. doi:10.1016/j.copsyc.2016.12.001
16. Neumeister A, Praschak-Rieder N, Besselmann B, et al. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54(2):133-138. doi:10.1001/archpsyc.1997.01830140043008
17. Kaysen D, Schumm J, Pedersen ER, et al. Cognitive processing therapy for veterans with comorbid PTSD and alcohol use disorders. Addict Behav. 2014;39(2):420-427. doi:10.1016/j.addbeh.2013.08.016
18. Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83(6):1058-1068. doi:10.1037/ccp0000016
1. Kessler RC, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019;74(5):596-607. doi: 10.1037/amp0000473
3. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
4. Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656-657.
5. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):169-180.
6. Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59.
7. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202. doi:10.1176/appi.ajp.2020.20050596
8. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126. doi:10.1001/jamapsychiatry.2018.3412
9. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924. doi:10.1016/j.brat.2021.103924
10. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952. doi:10.1038/s41386-021-01222-z
11. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
12. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. doi:10.1371/journal.pone.0246990
13. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444. doi:10.1093/milmed/usab014
14. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41. doi:10.1186/s12888-022-03699-4
15. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14:78-83. doi:10.1016/j.copsyc.2016.12.001
16. Neumeister A, Praschak-Rieder N, Besselmann B, et al. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54(2):133-138. doi:10.1001/archpsyc.1997.01830140043008
17. Kaysen D, Schumm J, Pedersen ER, et al. Cognitive processing therapy for veterans with comorbid PTSD and alcohol use disorders. Addict Behav. 2014;39(2):420-427. doi:10.1016/j.addbeh.2013.08.016
18. Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83(6):1058-1068. doi:10.1037/ccp0000016
Positive psychotherapy: Core principles
In a time of great national and global upheaval, increasing social problems, migration, climate crisis, globalization, and increasingly multicultural societies, our patients and their needs are unique, diverse, and changing. We need a new understanding of mental health to be able to adequately meet the demands of an ever-changing world. Treatment exclusively with psychotropic medications or years of psychoanalysis will not meet these needs.
Psychiatrists and psychotherapists feel (and actually have) a social responsibility, particularly in a multifaceted global society. Psychotherapeutic interventions may contribute to a more peaceful society1 by reducing individuals’ inner stress, solving (unconscious) conflicts, and conveying a humanistic worldview. As an integrative and transcultural method, positive psychotherapy has been applied for more than 45 years in more than 60 countries and is an active force within a “positive mental health movement.”2
The term “positive psychotherapy” describes 2 different approaches3: positive psychotherapy (1977) by Nossrat Peseschkian,4 which is a humanistic psychodynamic approach, and positive psychotherapy (2006) by Martin E.P. Seligman, Tayyab Rashid, and Acacia C. Parks,5 which is a more cognitive-behavioral therapy (CBT)–based approach. This article focuses on the first approach.
Why ‘positive’ psychotherapy?
The term “positive” implies that positive psychotherapy focuses on the patient’s possibilities and capacities. Symptoms and disorders are seen as capacities to react to a conflict. The Latin term “positum” or “positivus” is applied in its original meaning—the factual, the given, the actual. Factual and given are not only the disorder, the symptoms, and the problems but also the capacity to become healthy and/or cope with this situation. This positive meaning confronts the patient (and the therapist) with a lesser-known aspect of the illness, but one that is just as important for the understanding and clinical treatment of the affliction: its function, its meaning, and, consequently, its positive aspects.6
Positive psychotherapy is a humanistic psychodynamic psychotherapy approach developed by Nossrat Peseschkian (1933-2010).4,7 Positive psychotherapy has been developed since the 1970s in the clinical setting with neurotic and psychosomatic patients. It integrates approaches of the 4 main modalities of psychotherapy:
- a humanistic view of human beings
- a systemic approach toward culture, work, and environment
- a psychodynamic understanding of disorders
- a practical, goal-oriented approach with some cognitive-behavioral techniques.
The concept of balance
Based on a humanistic view of human beings and the resources every patient possesses, a key concept of positive psychotherapy is the importance of balance in one’s life. The balance model (Figure) is the core of positive psychotherapy and is applied in clinical and nonclinical settings. This model is based on the concept that there are 4 main areas of life in which a human being lives and functions. These areas influence one’s satisfaction in life, one’s feelings of self-worth, and the way one deals with conflicts and challenges. Although all 4 capacities are latent in every human being, depending on one`s education, environment, and zeitgeist, some will be more developed than others. Our life energies, activities, and reactions belong to these 4 areas of life:
- physical: eating, tenderness, sexuality, sleep, relaxation, sports, appearance, clothing
- achievement: work, job, career, money
- relationships: partner, family, friends, acquaintances and strangers, community life
- meaning and future: existential questions, spirituality, religious practices, future plans, fantasy.
A goal of treatment is to help the patient recognize their own resources and mobilize them with the goal of bringing them into a dynamic equilibrium. This goal places value on a balanced distribution of energy (25% to each area), not of time. According to positive psychotherapy, a person does not become ill because one sphere of life is overemphasized but because of the areas that have been neglected. In the case vignette described in the Box, the problem is not the patient’s work but that his physical health, family and friends, and existential questions are being neglected. That the therapist is not critical from the start of treatment is a constructive experience for the patient and is important and fruitful for building the relationship between the therapist and the patient. Instead of emphasizing the deficits or the disorders, the patient and his family hear that he has neglected other areas of life and not developed them yet.
Box
Mr. M, a 52-year-old manager, is “sent” by his wife to see a psychotherapist. “My wife says I am married to my job, and I should spend more time with her and the children. I understand this, but I love my job. It is no stress for me, but a few minutes at home, and I feel totally stressed out,” he says. During the first interview, the therapist asks Mr. M to draw his energy distribution in the balance model (Figure), and it becomes clear he spends more than 80% of his time and energy on his job.
That is not such a surprise for him. But after some explanation, the therapist tells him that he should continue to do so and that it is an ability to be able to spend so much time every day for his job. Mr. M says, “You are the first person to tell me that it is good that I am working so much. I expected you, like all the others, to tell me I must reduce my working hours immediately, go on vacation, etc.”
Continue to: The balance model...
The balance model also embodies the 4 potential sources of self-esteem. Usually, only 1 or 2 areas provide self-esteem, but in the therapeutic process a patient can learn to uncover the neglected areas so that their self-esteem will have additional pillars of support. By emphasizing how therapy can help to develop one’s self-esteem, many patients can be motivated for the therapeutic process. The balance model, with its concept of devoting 25% of one’s energy to each sphere of life, gives the patient a clear vision about their life and how they can be healthy over the long run by avoiding one-sidedness.8
The transcultural approach
In positive psychotherapy, the term “transcultural” (or cross-cultural) means not only consideration of cultural factors when the therapist and patient come from diverse cultural backgrounds (intercultural psychotherapy or “migrant psychotherapy”) but specifically the consideration of cultural factors in every therapeutic relationship, as a therapeutic attitude and consequently as a sociopolitical dimension of our thinking and behavior. This consideration of the uniqueness of each person, of the relativity of human behavior, and of “unity in diversity” is an essential reason positive psychotherapy is not a “Western” method in the sense of “psychological colonization.”9 Rather, this approach is a culture-sensitive method that can be modified to adapt to particular cultures and life situations.
Transcultural positive psychotherapy begins with answering 2 questions: “How are people different?” and “What do all people have in common?”4 During the therapeutic process, the therapist gives examples from other cultures to the patient to help them relativize their own perspective and broaden their repertoire of behavior.
The use of stories, tales, proverbs, and anecdotes
A special technique of positive psychotherapy is the therapeutic use of stories, tales, proverbs, and anecdotes.10 Often stories from other cultures are used because they offer another perspective when the patient sees none. This has been shown to be highly effective in psychiatric settings, especially in group settings. Psychiatric patients can often easily relate to the images created by stories. In psychiatry and psychotherapy, stories can be a means of changing a patient’s point of view. Such narratives can free up the listener’s feelings and thoughts and often lead to “Aha!” moments. The mirror function of storytelling leads to identification. In the narratives, the reader or listener recognizes themself as well as their needs and situation. They can reflect on the stories without personally becoming the focus of these reflections and remember their own experiences. Stories present solutions that can be models against which one’s own approach can be compared but that also leave room for broader interpretation. Storytelling is particularly useful in bringing about change in patients who are holding fast to old and outworn ideas.
The positive interpretation of disorders
Positive psychotherapy is based on a humanistic view that every human being is good by nature and endowed with unique capacities.11 This positive perspective leads not only to a new quality of relationship between the therapist and patient but also to a new perspective on disorders (Table). Thus, disorders can be “interpreted” in a positive way6: What does the patient unconsciously want to express with their symptoms? What is the function of their disorder? The positive process brings with it a change in perspective to all those concerned: the patient, their family, and the therapist/physician. In this way, one moves from the symptom (which is the disorder and often already has been very thoroughly examined) to the conflict (and the function of the disorder). The positive interpretations are only offered to the patient (“What do you say to this explanation?” “Can you apply this to your own situation?”).

Continue to: This process also helps us...
This process also helps us focus on the “true” patient, who often is not our patient. The patient who comes to us functions as a symptom carrier and can be seen as the “weakest link” in the family chain. The “real patient” is often sitting at home. The positive interpretation of illnesses confronts the patient with the possible function and psychodynamic meaning of their illness for themself and their social milieu, encouraging the patient (and their family) to see their abilities and not merely the pathological aspects.12
Fields of application of positive psychotherapy
As a method positioned between manualized CBT and process-oriented analytical psychotherapy, positive psychotherapy pursues a semi-structured approach in diagnostics (first interview), treatment, posttherapeutic self-help, and training. Positive psychotherapy is applied for the treatment of mood (affective), neurotic, stress-related, and somatoform disorders; behavioral syndromes; and, to some extent, personality disorders. Positive psychotherapy has been employed successfully side-by-side with classical individual therapy as well as in the settings of couple, family, and group therapy.13
What makes positive psychotherapy attractive for mental health professionals?
- As a method that integrates the 4 main modalities of psychotherapy, it does not engage in the conflicts between different schools but combines effective elements into a single approach.
- As an integrative approach, it adjusts to the patient and not vice versa. It gives the therapist the possibility of focusing more on either the actual problems (supportive approach) or the basic conflict (psychodynamic approach).
- It uses vocabulary and terms that can be understood by patients from all strata of society.
- As a culturally sensitive method, it can be applied to patients from different cultures and does not require cultural adaptation.
- As a psychodynamic method, it does not stop after early life conflicts have become more conscious but helps the patient to apply the gained insights using practical techniques.
- It starts with positive affirmations and encouragement but does not later “forget” the unconscious conflicts that have led to disorders. It is not perceived as superficial.
- As a method originally coming from psychiatry and medical practice, it builds a bridge between a scientific basis and psychotherapeutic insights. It favors the biopsychosocial approach.
Bottom Line
Positive psychotherapy combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. It is based on a resource-oriented view of human beings in which disorders are interpreted as capacities to react in a specific and unique way to life events and circumstances. Positive psychotherapy can be applied in psychiatry and psychotherapy. This short-term method is easily understood by patients from diverse cultures and social backgrounds.
Related Resources
- Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32. https://doi.org/10.1007/978-3-030-33264-8_2
- Tritt K, Loew T, Meyer M, et al. Positive psychotherapy: effectiveness of an interdisciplinary approach. Eur J Psychiatry. 1999;13(4):231-241.
- World Association for Positive and Transcultural Psychotherapy. http://www.positum.org
1. Mackenthun G. Passt Psychotherapie an ‚die Gesellschaft’ an? Dynamische Psychiatrie. 1991;24(5-6):326-333.
2. Jeste DV. Foreword: positive mental health. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:vii-xiii.
3. Dobiała E, Winkler P. ‘Positive psychotherapy’ according to Seligman and ‘positive psychotherapy’ according to Peseschkian: a comparison. Int J Psychother. 2016;20(3):5-17.
4. Peseschkian N. Positive Psychotherapy: Theory and Practice of a New Method. Springer; 1987.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Peseschkian N. Positive Psychosomatics: Clinical Manual of Positive Psychotherapy. AuthorHouse; 2016.
7. Peseschkian N. Positive psychotherapy. In: Pritz A, ed. Globalized Psychotherapy. Facultas Universitätsverlag; 2002.
8. Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32.
9. Moghaddam FM, Harre R. But is it science? Traditional and alternative approaches to the study of social behavior. World Psychol. 1995;1(4):47-78.
10. Peseschkian N. Oriental Stories as Techniques in Positive Psychotherapy. AuthorHouse; 2016.
11. Cope TA. Positive psychotherapy’s theory of the capacity to know as explication of unconscious contents. J Relig Health. 2009;48(1):79-89.
12. Huebner G. Health-illness from the perspective of positive psychotherapy. Global Psychother. 2021;1(1):57-61.
13. Sinici E. A ‘balance model’ for patients with post-traumatic stress disorder. Int J Psychother. 2015;19(3):13-19.
In a time of great national and global upheaval, increasing social problems, migration, climate crisis, globalization, and increasingly multicultural societies, our patients and their needs are unique, diverse, and changing. We need a new understanding of mental health to be able to adequately meet the demands of an ever-changing world. Treatment exclusively with psychotropic medications or years of psychoanalysis will not meet these needs.
Psychiatrists and psychotherapists feel (and actually have) a social responsibility, particularly in a multifaceted global society. Psychotherapeutic interventions may contribute to a more peaceful society1 by reducing individuals’ inner stress, solving (unconscious) conflicts, and conveying a humanistic worldview. As an integrative and transcultural method, positive psychotherapy has been applied for more than 45 years in more than 60 countries and is an active force within a “positive mental health movement.”2
The term “positive psychotherapy” describes 2 different approaches3: positive psychotherapy (1977) by Nossrat Peseschkian,4 which is a humanistic psychodynamic approach, and positive psychotherapy (2006) by Martin E.P. Seligman, Tayyab Rashid, and Acacia C. Parks,5 which is a more cognitive-behavioral therapy (CBT)–based approach. This article focuses on the first approach.
Why ‘positive’ psychotherapy?
The term “positive” implies that positive psychotherapy focuses on the patient’s possibilities and capacities. Symptoms and disorders are seen as capacities to react to a conflict. The Latin term “positum” or “positivus” is applied in its original meaning—the factual, the given, the actual. Factual and given are not only the disorder, the symptoms, and the problems but also the capacity to become healthy and/or cope with this situation. This positive meaning confronts the patient (and the therapist) with a lesser-known aspect of the illness, but one that is just as important for the understanding and clinical treatment of the affliction: its function, its meaning, and, consequently, its positive aspects.6
Positive psychotherapy is a humanistic psychodynamic psychotherapy approach developed by Nossrat Peseschkian (1933-2010).4,7 Positive psychotherapy has been developed since the 1970s in the clinical setting with neurotic and psychosomatic patients. It integrates approaches of the 4 main modalities of psychotherapy:
- a humanistic view of human beings
- a systemic approach toward culture, work, and environment
- a psychodynamic understanding of disorders
- a practical, goal-oriented approach with some cognitive-behavioral techniques.
The concept of balance
Based on a humanistic view of human beings and the resources every patient possesses, a key concept of positive psychotherapy is the importance of balance in one’s life. The balance model (Figure) is the core of positive psychotherapy and is applied in clinical and nonclinical settings. This model is based on the concept that there are 4 main areas of life in which a human being lives and functions. These areas influence one’s satisfaction in life, one’s feelings of self-worth, and the way one deals with conflicts and challenges. Although all 4 capacities are latent in every human being, depending on one`s education, environment, and zeitgeist, some will be more developed than others. Our life energies, activities, and reactions belong to these 4 areas of life:
- physical: eating, tenderness, sexuality, sleep, relaxation, sports, appearance, clothing
- achievement: work, job, career, money
- relationships: partner, family, friends, acquaintances and strangers, community life
- meaning and future: existential questions, spirituality, religious practices, future plans, fantasy.

A goal of treatment is to help the patient recognize their own resources and mobilize them with the goal of bringing them into a dynamic equilibrium. This goal places value on a balanced distribution of energy (25% to each area), not of time. According to positive psychotherapy, a person does not become ill because one sphere of life is overemphasized but because of the areas that have been neglected. In the case vignette described in the Box, the problem is not the patient’s work but that his physical health, family and friends, and existential questions are being neglected. That the therapist is not critical from the start of treatment is a constructive experience for the patient and is important and fruitful for building the relationship between the therapist and the patient. Instead of emphasizing the deficits or the disorders, the patient and his family hear that he has neglected other areas of life and not developed them yet.
Box
Mr. M, a 52-year-old manager, is “sent” by his wife to see a psychotherapist. “My wife says I am married to my job, and I should spend more time with her and the children. I understand this, but I love my job. It is no stress for me, but a few minutes at home, and I feel totally stressed out,” he says. During the first interview, the therapist asks Mr. M to draw his energy distribution in the balance model (Figure), and it becomes clear he spends more than 80% of his time and energy on his job.
That is not such a surprise for him. But after some explanation, the therapist tells him that he should continue to do so and that it is an ability to be able to spend so much time every day for his job. Mr. M says, “You are the first person to tell me that it is good that I am working so much. I expected you, like all the others, to tell me I must reduce my working hours immediately, go on vacation, etc.”
Continue to: The balance model...
The balance model also embodies the 4 potential sources of self-esteem. Usually, only 1 or 2 areas provide self-esteem, but in the therapeutic process a patient can learn to uncover the neglected areas so that their self-esteem will have additional pillars of support. By emphasizing how therapy can help to develop one’s self-esteem, many patients can be motivated for the therapeutic process. The balance model, with its concept of devoting 25% of one’s energy to each sphere of life, gives the patient a clear vision about their life and how they can be healthy over the long run by avoiding one-sidedness.8
The transcultural approach
In positive psychotherapy, the term “transcultural” (or cross-cultural) means not only consideration of cultural factors when the therapist and patient come from diverse cultural backgrounds (intercultural psychotherapy or “migrant psychotherapy”) but specifically the consideration of cultural factors in every therapeutic relationship, as a therapeutic attitude and consequently as a sociopolitical dimension of our thinking and behavior. This consideration of the uniqueness of each person, of the relativity of human behavior, and of “unity in diversity” is an essential reason positive psychotherapy is not a “Western” method in the sense of “psychological colonization.”9 Rather, this approach is a culture-sensitive method that can be modified to adapt to particular cultures and life situations.
Transcultural positive psychotherapy begins with answering 2 questions: “How are people different?” and “What do all people have in common?”4 During the therapeutic process, the therapist gives examples from other cultures to the patient to help them relativize their own perspective and broaden their repertoire of behavior.
The use of stories, tales, proverbs, and anecdotes
A special technique of positive psychotherapy is the therapeutic use of stories, tales, proverbs, and anecdotes.10 Often stories from other cultures are used because they offer another perspective when the patient sees none. This has been shown to be highly effective in psychiatric settings, especially in group settings. Psychiatric patients can often easily relate to the images created by stories. In psychiatry and psychotherapy, stories can be a means of changing a patient’s point of view. Such narratives can free up the listener’s feelings and thoughts and often lead to “Aha!” moments. The mirror function of storytelling leads to identification. In the narratives, the reader or listener recognizes themself as well as their needs and situation. They can reflect on the stories without personally becoming the focus of these reflections and remember their own experiences. Stories present solutions that can be models against which one’s own approach can be compared but that also leave room for broader interpretation. Storytelling is particularly useful in bringing about change in patients who are holding fast to old and outworn ideas.
The positive interpretation of disorders
Positive psychotherapy is based on a humanistic view that every human being is good by nature and endowed with unique capacities.11 This positive perspective leads not only to a new quality of relationship between the therapist and patient but also to a new perspective on disorders (Table). Thus, disorders can be “interpreted” in a positive way6: What does the patient unconsciously want to express with their symptoms? What is the function of their disorder? The positive process brings with it a change in perspective to all those concerned: the patient, their family, and the therapist/physician. In this way, one moves from the symptom (which is the disorder and often already has been very thoroughly examined) to the conflict (and the function of the disorder). The positive interpretations are only offered to the patient (“What do you say to this explanation?” “Can you apply this to your own situation?”).

Continue to: This process also helps us...
This process also helps us focus on the “true” patient, who often is not our patient. The patient who comes to us functions as a symptom carrier and can be seen as the “weakest link” in the family chain. The “real patient” is often sitting at home. The positive interpretation of illnesses confronts the patient with the possible function and psychodynamic meaning of their illness for themself and their social milieu, encouraging the patient (and their family) to see their abilities and not merely the pathological aspects.12
Fields of application of positive psychotherapy
As a method positioned between manualized CBT and process-oriented analytical psychotherapy, positive psychotherapy pursues a semi-structured approach in diagnostics (first interview), treatment, posttherapeutic self-help, and training. Positive psychotherapy is applied for the treatment of mood (affective), neurotic, stress-related, and somatoform disorders; behavioral syndromes; and, to some extent, personality disorders. Positive psychotherapy has been employed successfully side-by-side with classical individual therapy as well as in the settings of couple, family, and group therapy.13
What makes positive psychotherapy attractive for mental health professionals?
- As a method that integrates the 4 main modalities of psychotherapy, it does not engage in the conflicts between different schools but combines effective elements into a single approach.
- As an integrative approach, it adjusts to the patient and not vice versa. It gives the therapist the possibility of focusing more on either the actual problems (supportive approach) or the basic conflict (psychodynamic approach).
- It uses vocabulary and terms that can be understood by patients from all strata of society.
- As a culturally sensitive method, it can be applied to patients from different cultures and does not require cultural adaptation.
- As a psychodynamic method, it does not stop after early life conflicts have become more conscious but helps the patient to apply the gained insights using practical techniques.
- It starts with positive affirmations and encouragement but does not later “forget” the unconscious conflicts that have led to disorders. It is not perceived as superficial.
- As a method originally coming from psychiatry and medical practice, it builds a bridge between a scientific basis and psychotherapeutic insights. It favors the biopsychosocial approach.
Bottom Line
Positive psychotherapy combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. It is based on a resource-oriented view of human beings in which disorders are interpreted as capacities to react in a specific and unique way to life events and circumstances. Positive psychotherapy can be applied in psychiatry and psychotherapy. This short-term method is easily understood by patients from diverse cultures and social backgrounds.
Related Resources
- Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32. https://doi.org/10.1007/978-3-030-33264-8_2
- Tritt K, Loew T, Meyer M, et al. Positive psychotherapy: effectiveness of an interdisciplinary approach. Eur J Psychiatry. 1999;13(4):231-241.
- World Association for Positive and Transcultural Psychotherapy. http://www.positum.org
In a time of great national and global upheaval, increasing social problems, migration, climate crisis, globalization, and increasingly multicultural societies, our patients and their needs are unique, diverse, and changing. We need a new understanding of mental health to be able to adequately meet the demands of an ever-changing world. Treatment exclusively with psychotropic medications or years of psychoanalysis will not meet these needs.
Psychiatrists and psychotherapists feel (and actually have) a social responsibility, particularly in a multifaceted global society. Psychotherapeutic interventions may contribute to a more peaceful society1 by reducing individuals’ inner stress, solving (unconscious) conflicts, and conveying a humanistic worldview. As an integrative and transcultural method, positive psychotherapy has been applied for more than 45 years in more than 60 countries and is an active force within a “positive mental health movement.”2
The term “positive psychotherapy” describes 2 different approaches3: positive psychotherapy (1977) by Nossrat Peseschkian,4 which is a humanistic psychodynamic approach, and positive psychotherapy (2006) by Martin E.P. Seligman, Tayyab Rashid, and Acacia C. Parks,5 which is a more cognitive-behavioral therapy (CBT)–based approach. This article focuses on the first approach.
Why ‘positive’ psychotherapy?
The term “positive” implies that positive psychotherapy focuses on the patient’s possibilities and capacities. Symptoms and disorders are seen as capacities to react to a conflict. The Latin term “positum” or “positivus” is applied in its original meaning—the factual, the given, the actual. Factual and given are not only the disorder, the symptoms, and the problems but also the capacity to become healthy and/or cope with this situation. This positive meaning confronts the patient (and the therapist) with a lesser-known aspect of the illness, but one that is just as important for the understanding and clinical treatment of the affliction: its function, its meaning, and, consequently, its positive aspects.6
Positive psychotherapy is a humanistic psychodynamic psychotherapy approach developed by Nossrat Peseschkian (1933-2010).4,7 Positive psychotherapy has been developed since the 1970s in the clinical setting with neurotic and psychosomatic patients. It integrates approaches of the 4 main modalities of psychotherapy:
- a humanistic view of human beings
- a systemic approach toward culture, work, and environment
- a psychodynamic understanding of disorders
- a practical, goal-oriented approach with some cognitive-behavioral techniques.
The concept of balance
Based on a humanistic view of human beings and the resources every patient possesses, a key concept of positive psychotherapy is the importance of balance in one’s life. The balance model (Figure) is the core of positive psychotherapy and is applied in clinical and nonclinical settings. This model is based on the concept that there are 4 main areas of life in which a human being lives and functions. These areas influence one’s satisfaction in life, one’s feelings of self-worth, and the way one deals with conflicts and challenges. Although all 4 capacities are latent in every human being, depending on one`s education, environment, and zeitgeist, some will be more developed than others. Our life energies, activities, and reactions belong to these 4 areas of life:
- physical: eating, tenderness, sexuality, sleep, relaxation, sports, appearance, clothing
- achievement: work, job, career, money
- relationships: partner, family, friends, acquaintances and strangers, community life
- meaning and future: existential questions, spirituality, religious practices, future plans, fantasy.

A goal of treatment is to help the patient recognize their own resources and mobilize them with the goal of bringing them into a dynamic equilibrium. This goal places value on a balanced distribution of energy (25% to each area), not of time. According to positive psychotherapy, a person does not become ill because one sphere of life is overemphasized but because of the areas that have been neglected. In the case vignette described in the Box, the problem is not the patient’s work but that his physical health, family and friends, and existential questions are being neglected. That the therapist is not critical from the start of treatment is a constructive experience for the patient and is important and fruitful for building the relationship between the therapist and the patient. Instead of emphasizing the deficits or the disorders, the patient and his family hear that he has neglected other areas of life and not developed them yet.
Box
Mr. M, a 52-year-old manager, is “sent” by his wife to see a psychotherapist. “My wife says I am married to my job, and I should spend more time with her and the children. I understand this, but I love my job. It is no stress for me, but a few minutes at home, and I feel totally stressed out,” he says. During the first interview, the therapist asks Mr. M to draw his energy distribution in the balance model (Figure), and it becomes clear he spends more than 80% of his time and energy on his job.
That is not such a surprise for him. But after some explanation, the therapist tells him that he should continue to do so and that it is an ability to be able to spend so much time every day for his job. Mr. M says, “You are the first person to tell me that it is good that I am working so much. I expected you, like all the others, to tell me I must reduce my working hours immediately, go on vacation, etc.”
Continue to: The balance model...
The balance model also embodies the 4 potential sources of self-esteem. Usually, only 1 or 2 areas provide self-esteem, but in the therapeutic process a patient can learn to uncover the neglected areas so that their self-esteem will have additional pillars of support. By emphasizing how therapy can help to develop one’s self-esteem, many patients can be motivated for the therapeutic process. The balance model, with its concept of devoting 25% of one’s energy to each sphere of life, gives the patient a clear vision about their life and how they can be healthy over the long run by avoiding one-sidedness.8
The transcultural approach
In positive psychotherapy, the term “transcultural” (or cross-cultural) means not only consideration of cultural factors when the therapist and patient come from diverse cultural backgrounds (intercultural psychotherapy or “migrant psychotherapy”) but specifically the consideration of cultural factors in every therapeutic relationship, as a therapeutic attitude and consequently as a sociopolitical dimension of our thinking and behavior. This consideration of the uniqueness of each person, of the relativity of human behavior, and of “unity in diversity” is an essential reason positive psychotherapy is not a “Western” method in the sense of “psychological colonization.”9 Rather, this approach is a culture-sensitive method that can be modified to adapt to particular cultures and life situations.
Transcultural positive psychotherapy begins with answering 2 questions: “How are people different?” and “What do all people have in common?”4 During the therapeutic process, the therapist gives examples from other cultures to the patient to help them relativize their own perspective and broaden their repertoire of behavior.
The use of stories, tales, proverbs, and anecdotes
A special technique of positive psychotherapy is the therapeutic use of stories, tales, proverbs, and anecdotes.10 Often stories from other cultures are used because they offer another perspective when the patient sees none. This has been shown to be highly effective in psychiatric settings, especially in group settings. Psychiatric patients can often easily relate to the images created by stories. In psychiatry and psychotherapy, stories can be a means of changing a patient’s point of view. Such narratives can free up the listener’s feelings and thoughts and often lead to “Aha!” moments. The mirror function of storytelling leads to identification. In the narratives, the reader or listener recognizes themself as well as their needs and situation. They can reflect on the stories without personally becoming the focus of these reflections and remember their own experiences. Stories present solutions that can be models against which one’s own approach can be compared but that also leave room for broader interpretation. Storytelling is particularly useful in bringing about change in patients who are holding fast to old and outworn ideas.
The positive interpretation of disorders
Positive psychotherapy is based on a humanistic view that every human being is good by nature and endowed with unique capacities.11 This positive perspective leads not only to a new quality of relationship between the therapist and patient but also to a new perspective on disorders (Table). Thus, disorders can be “interpreted” in a positive way6: What does the patient unconsciously want to express with their symptoms? What is the function of their disorder? The positive process brings with it a change in perspective to all those concerned: the patient, their family, and the therapist/physician. In this way, one moves from the symptom (which is the disorder and often already has been very thoroughly examined) to the conflict (and the function of the disorder). The positive interpretations are only offered to the patient (“What do you say to this explanation?” “Can you apply this to your own situation?”).

Continue to: This process also helps us...
This process also helps us focus on the “true” patient, who often is not our patient. The patient who comes to us functions as a symptom carrier and can be seen as the “weakest link” in the family chain. The “real patient” is often sitting at home. The positive interpretation of illnesses confronts the patient with the possible function and psychodynamic meaning of their illness for themself and their social milieu, encouraging the patient (and their family) to see their abilities and not merely the pathological aspects.12
Fields of application of positive psychotherapy
As a method positioned between manualized CBT and process-oriented analytical psychotherapy, positive psychotherapy pursues a semi-structured approach in diagnostics (first interview), treatment, posttherapeutic self-help, and training. Positive psychotherapy is applied for the treatment of mood (affective), neurotic, stress-related, and somatoform disorders; behavioral syndromes; and, to some extent, personality disorders. Positive psychotherapy has been employed successfully side-by-side with classical individual therapy as well as in the settings of couple, family, and group therapy.13
What makes positive psychotherapy attractive for mental health professionals?
- As a method that integrates the 4 main modalities of psychotherapy, it does not engage in the conflicts between different schools but combines effective elements into a single approach.
- As an integrative approach, it adjusts to the patient and not vice versa. It gives the therapist the possibility of focusing more on either the actual problems (supportive approach) or the basic conflict (psychodynamic approach).
- It uses vocabulary and terms that can be understood by patients from all strata of society.
- As a culturally sensitive method, it can be applied to patients from different cultures and does not require cultural adaptation.
- As a psychodynamic method, it does not stop after early life conflicts have become more conscious but helps the patient to apply the gained insights using practical techniques.
- It starts with positive affirmations and encouragement but does not later “forget” the unconscious conflicts that have led to disorders. It is not perceived as superficial.
- As a method originally coming from psychiatry and medical practice, it builds a bridge between a scientific basis and psychotherapeutic insights. It favors the biopsychosocial approach.
Bottom Line
Positive psychotherapy combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. It is based on a resource-oriented view of human beings in which disorders are interpreted as capacities to react in a specific and unique way to life events and circumstances. Positive psychotherapy can be applied in psychiatry and psychotherapy. This short-term method is easily understood by patients from diverse cultures and social backgrounds.
Related Resources
- Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32. https://doi.org/10.1007/978-3-030-33264-8_2
- Tritt K, Loew T, Meyer M, et al. Positive psychotherapy: effectiveness of an interdisciplinary approach. Eur J Psychiatry. 1999;13(4):231-241.
- World Association for Positive and Transcultural Psychotherapy. http://www.positum.org
1. Mackenthun G. Passt Psychotherapie an ‚die Gesellschaft’ an? Dynamische Psychiatrie. 1991;24(5-6):326-333.
2. Jeste DV. Foreword: positive mental health. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:vii-xiii.
3. Dobiała E, Winkler P. ‘Positive psychotherapy’ according to Seligman and ‘positive psychotherapy’ according to Peseschkian: a comparison. Int J Psychother. 2016;20(3):5-17.
4. Peseschkian N. Positive Psychotherapy: Theory and Practice of a New Method. Springer; 1987.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Peseschkian N. Positive Psychosomatics: Clinical Manual of Positive Psychotherapy. AuthorHouse; 2016.
7. Peseschkian N. Positive psychotherapy. In: Pritz A, ed. Globalized Psychotherapy. Facultas Universitätsverlag; 2002.
8. Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32.
9. Moghaddam FM, Harre R. But is it science? Traditional and alternative approaches to the study of social behavior. World Psychol. 1995;1(4):47-78.
10. Peseschkian N. Oriental Stories as Techniques in Positive Psychotherapy. AuthorHouse; 2016.
11. Cope TA. Positive psychotherapy’s theory of the capacity to know as explication of unconscious contents. J Relig Health. 2009;48(1):79-89.
12. Huebner G. Health-illness from the perspective of positive psychotherapy. Global Psychother. 2021;1(1):57-61.
13. Sinici E. A ‘balance model’ for patients with post-traumatic stress disorder. Int J Psychother. 2015;19(3):13-19.
1. Mackenthun G. Passt Psychotherapie an ‚die Gesellschaft’ an? Dynamische Psychiatrie. 1991;24(5-6):326-333.
2. Jeste DV. Foreword: positive mental health. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:vii-xiii.
3. Dobiała E, Winkler P. ‘Positive psychotherapy’ according to Seligman and ‘positive psychotherapy’ according to Peseschkian: a comparison. Int J Psychother. 2016;20(3):5-17.
4. Peseschkian N. Positive Psychotherapy: Theory and Practice of a New Method. Springer; 1987.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Peseschkian N. Positive Psychosomatics: Clinical Manual of Positive Psychotherapy. AuthorHouse; 2016.
7. Peseschkian N. Positive psychotherapy. In: Pritz A, ed. Globalized Psychotherapy. Facultas Universitätsverlag; 2002.
8. Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32.
9. Moghaddam FM, Harre R. But is it science? Traditional and alternative approaches to the study of social behavior. World Psychol. 1995;1(4):47-78.
10. Peseschkian N. Oriental Stories as Techniques in Positive Psychotherapy. AuthorHouse; 2016.
11. Cope TA. Positive psychotherapy’s theory of the capacity to know as explication of unconscious contents. J Relig Health. 2009;48(1):79-89.
12. Huebner G. Health-illness from the perspective of positive psychotherapy. Global Psychother. 2021;1(1):57-61.
13. Sinici E. A ‘balance model’ for patients with post-traumatic stress disorder. Int J Psychother. 2015;19(3):13-19.