User login
How to assess a patient for a bisphosphonate drug holiday
Recorded at the 2017 meeting of the North American Menopause Society
Recorded at the 2017 meeting of the North American Menopause Society
Recorded at the 2017 meeting of the North American Menopause Society
What is the optimal opioid prescription length after women’s health surgical procedures?
WHAT DOES THIS MEAN FOR PRACTICE?
- 7-day opioid prescriptions should be sufficient after common gyn procedures
- Monitor patients closely
- Transfer patients as soon as possible to non-opioid pain medication
Are fewer nonpregnant women seeing ObGyns?
EXPERT COMMENTARY
Health care services for women are fragmented due to multiple types of providers who offer a variety of care. Simon and Uddin’s recent research analysis indicates that the percentage of nonpregnant women who visit a general ObGyn, whether alone or in combination with an internist, family physician, or general practitioner, has declined.
Details of the study
The authors used data from the National Health Interview Survey of a representative sample of US women age 18 or older. They sought to identify whether the women saw or talked to a physician who either specialized in women’s health (presumably an ObGyn) or treated a variety of illnesses during the previous 12 months.
While the percentage of women who saw a general physician remained essentially the same (70%–74%), it declined for seeing an ObGyn from 45% to 41% between 2003 and 2007, and from 42% to 38% between 2011 and 2015. Furthermore, the percentage of women who saw both an ObGyn and a general physician declined from a peak of 35% in 2003 to 30% in 2015.
Study strengths and weaknesses
The data used in this study were from a nationally representative, cross-sectional, multistage sample, population health survey conducted by the Centers for Disease Control and Prevention. The study period was sufficient to draw conclusions.
From my perspective, the study had 2 major limitations: 1) only physicians, not mid-level providers, were included in the analysis, and 2) no breakdown of the women’s age groups was provided.
Many ObGyn offices employ nurse practitioners and midwives, and these providers’ roles are increasingly important for improving frontline access to care and different levels of care. Women aged 19 to 39 seek almost all their health care from ObGyns or family physicians, and significant sharing of care exists across these provider groups.1 Women aged 45 to 64 are more likely to obtain care exclusively at the offices of family physicians or general internists than at those of ObGyns.2 Most ObGyns are engaged to some degree with women aged 65 years or older, especially for preventive care, disease screening and early detection, and urogenital conditions.3
The decline in the percentage of women seeking care from ObGyns is likely related to the patient's age, reason for seeking care, and access to care. The US population of adult women, especially those who are beyond the reproductive years, is rising in relation to the number of physicians in general ObGyn practice. Providing a team-based collaborative model of care should allow for improved access and value. Defining the roles of what constitutes evidence-based care also will impact when a person needs to see a women's health care specialist. Geographic distribution of ObGyns in relation to the patient population will invariably impact on the percentage of women who seek care at the office of an ObGyn alone, in combination with another general physician, or not at all. Given the overlap in care provided at more than one physician's office, continued surveillance is needed to minimize redundant costs and optimize resource utilization. I look forward to what unfolds over the next 15 years.
-- William F. Rayburn, MD, MBA
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Petterson SM, Bazemore AW, Phillips RL, Rayburn WF. Trends in office-based care for reproductive-aged women according to physician specialty: a ten-year study. J Womens Health (Larchmt). 2014;23(12):1021–1026.
- Raffoul MC, Petterson SM, Rayburn WF, Wingrove P, Bazemore AW. Office visits for women aged 45–64 years according to physician specialties. J Womens Health (Larchmt). 2016:25(12):1231–1236.
- Rayburn WF, Raglan GB, Herman CJ, Schulkin J. A survey of obstetrician-gynecologists regarding their care of women 65 years or older. J Geriatr Med Gerontol. 2015;1:2-5.
EXPERT COMMENTARY
Health care services for women are fragmented due to multiple types of providers who offer a variety of care. Simon and Uddin’s recent research analysis indicates that the percentage of nonpregnant women who visit a general ObGyn, whether alone or in combination with an internist, family physician, or general practitioner, has declined.
Details of the study
The authors used data from the National Health Interview Survey of a representative sample of US women age 18 or older. They sought to identify whether the women saw or talked to a physician who either specialized in women’s health (presumably an ObGyn) or treated a variety of illnesses during the previous 12 months.
While the percentage of women who saw a general physician remained essentially the same (70%–74%), it declined for seeing an ObGyn from 45% to 41% between 2003 and 2007, and from 42% to 38% between 2011 and 2015. Furthermore, the percentage of women who saw both an ObGyn and a general physician declined from a peak of 35% in 2003 to 30% in 2015.
Study strengths and weaknesses
The data used in this study were from a nationally representative, cross-sectional, multistage sample, population health survey conducted by the Centers for Disease Control and Prevention. The study period was sufficient to draw conclusions.
From my perspective, the study had 2 major limitations: 1) only physicians, not mid-level providers, were included in the analysis, and 2) no breakdown of the women’s age groups was provided.
Many ObGyn offices employ nurse practitioners and midwives, and these providers’ roles are increasingly important for improving frontline access to care and different levels of care. Women aged 19 to 39 seek almost all their health care from ObGyns or family physicians, and significant sharing of care exists across these provider groups.1 Women aged 45 to 64 are more likely to obtain care exclusively at the offices of family physicians or general internists than at those of ObGyns.2 Most ObGyns are engaged to some degree with women aged 65 years or older, especially for preventive care, disease screening and early detection, and urogenital conditions.3
The decline in the percentage of women seeking care from ObGyns is likely related to the patient's age, reason for seeking care, and access to care. The US population of adult women, especially those who are beyond the reproductive years, is rising in relation to the number of physicians in general ObGyn practice. Providing a team-based collaborative model of care should allow for improved access and value. Defining the roles of what constitutes evidence-based care also will impact when a person needs to see a women's health care specialist. Geographic distribution of ObGyns in relation to the patient population will invariably impact on the percentage of women who seek care at the office of an ObGyn alone, in combination with another general physician, or not at all. Given the overlap in care provided at more than one physician's office, continued surveillance is needed to minimize redundant costs and optimize resource utilization. I look forward to what unfolds over the next 15 years.
-- William F. Rayburn, MD, MBA
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Health care services for women are fragmented due to multiple types of providers who offer a variety of care. Simon and Uddin’s recent research analysis indicates that the percentage of nonpregnant women who visit a general ObGyn, whether alone or in combination with an internist, family physician, or general practitioner, has declined.
Details of the study
The authors used data from the National Health Interview Survey of a representative sample of US women age 18 or older. They sought to identify whether the women saw or talked to a physician who either specialized in women’s health (presumably an ObGyn) or treated a variety of illnesses during the previous 12 months.
While the percentage of women who saw a general physician remained essentially the same (70%–74%), it declined for seeing an ObGyn from 45% to 41% between 2003 and 2007, and from 42% to 38% between 2011 and 2015. Furthermore, the percentage of women who saw both an ObGyn and a general physician declined from a peak of 35% in 2003 to 30% in 2015.
Study strengths and weaknesses
The data used in this study were from a nationally representative, cross-sectional, multistage sample, population health survey conducted by the Centers for Disease Control and Prevention. The study period was sufficient to draw conclusions.
From my perspective, the study had 2 major limitations: 1) only physicians, not mid-level providers, were included in the analysis, and 2) no breakdown of the women’s age groups was provided.
Many ObGyn offices employ nurse practitioners and midwives, and these providers’ roles are increasingly important for improving frontline access to care and different levels of care. Women aged 19 to 39 seek almost all their health care from ObGyns or family physicians, and significant sharing of care exists across these provider groups.1 Women aged 45 to 64 are more likely to obtain care exclusively at the offices of family physicians or general internists than at those of ObGyns.2 Most ObGyns are engaged to some degree with women aged 65 years or older, especially for preventive care, disease screening and early detection, and urogenital conditions.3
The decline in the percentage of women seeking care from ObGyns is likely related to the patient's age, reason for seeking care, and access to care. The US population of adult women, especially those who are beyond the reproductive years, is rising in relation to the number of physicians in general ObGyn practice. Providing a team-based collaborative model of care should allow for improved access and value. Defining the roles of what constitutes evidence-based care also will impact when a person needs to see a women's health care specialist. Geographic distribution of ObGyns in relation to the patient population will invariably impact on the percentage of women who seek care at the office of an ObGyn alone, in combination with another general physician, or not at all. Given the overlap in care provided at more than one physician's office, continued surveillance is needed to minimize redundant costs and optimize resource utilization. I look forward to what unfolds over the next 15 years.
-- William F. Rayburn, MD, MBA
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Petterson SM, Bazemore AW, Phillips RL, Rayburn WF. Trends in office-based care for reproductive-aged women according to physician specialty: a ten-year study. J Womens Health (Larchmt). 2014;23(12):1021–1026.
- Raffoul MC, Petterson SM, Rayburn WF, Wingrove P, Bazemore AW. Office visits for women aged 45–64 years according to physician specialties. J Womens Health (Larchmt). 2016:25(12):1231–1236.
- Rayburn WF, Raglan GB, Herman CJ, Schulkin J. A survey of obstetrician-gynecologists regarding their care of women 65 years or older. J Geriatr Med Gerontol. 2015;1:2-5.
- Petterson SM, Bazemore AW, Phillips RL, Rayburn WF. Trends in office-based care for reproductive-aged women according to physician specialty: a ten-year study. J Womens Health (Larchmt). 2014;23(12):1021–1026.
- Raffoul MC, Petterson SM, Rayburn WF, Wingrove P, Bazemore AW. Office visits for women aged 45–64 years according to physician specialties. J Womens Health (Larchmt). 2016:25(12):1231–1236.
- Rayburn WF, Raglan GB, Herman CJ, Schulkin J. A survey of obstetrician-gynecologists regarding their care of women 65 years or older. J Geriatr Med Gerontol. 2015;1:2-5.
Career (of your dreams) advice for young MIGS surgeons
From the 46th AAGL Global Congress on MIGS
From the 46th AAGL Global Congress on MIGS
From the 46th AAGL Global Congress on MIGS
Hypoactive sexual desire disorder: The ideal patient for treatment with flibanserin
Your patient has a large symptomatic fibroid: Tools for decision making
Boston VA Medical Forum: HIV-Positive Veteran With Progressive Visual Changes
►Lakshmana Swamy, MD, chief medical resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. Dr. Serrao, when you hear about vision changes in a patient with HIV, what differential diagnosis is generated? What epidemiologic or historical factors can help distinguish these entities?
►Richard Serrao, MD, Infectious Disease Service, VABHS and assistant professor of medicine, Boston University School of Medicine. The differential diagnoses for vision changes in a patient with HIV is based on the overall immunosuppression of the patient: the lower the patient’s CD4 count, the higher the number of etiologies.1 The portions of the visual pathway as well as the pattern of vision loss are useful in narrowing the differential. For example, monocular visual disturbances with dermatomal vesicles within the ophthalmic division of the trigeminal nerve strongly implicates varicella zoster retinitis or keratitis; abducens nerve palsy could suggest granulomatous basilar meningitis from cryptococcosis. Likewise, ongoing fevers in an advanced AIDS patient with concomitant colitis, hepatitis, and pneumonitis is strongly suspicious for cytomegalovirus (CMV) retinitis with wide dissemination.
Geographic epidemiologic factors can suggest pathogens more prevalent to certain regions of the world, such as histoplasma chorioretinitis in a resident of the central and eastern U.S. or tuberculosis in a returning traveler. Likewise, a cat owner or one who consumes steak tartare increases the likelihood for toxoplasma retinochoroiditis, or syphilis in men who have sex with men (MSM) in the U.S. given that the majority of new cases occur in this patient population. Other clues one should consider include the presence of splinter hemorrhages in the extremities in an intravenous drug user, raising the possibility of embolic endophthalmitis from bacterial or fungal endocarditis. A variety of other diagnoses can certainly occur as a result of drug treatment (uveitis from rifampin, for example), immune reconstitution from HAART, infections with other HIV-associated pathogens, such as Pneumocystis jiroveci, and many non-HIV-related ocular diseases.
►Dr. Swamy. Dr. Butler, what concerns do you have when you hear about an HIV-infected patient with vision loss from the ophthalmology perspective?
►Nicholas Butler, MD, Ophthalmology Service, Uveitis and Ocular Immunology, VABHS and assistant professor of ophthalmology, Harvard Medical School. Of course, patients with HIV suffer from common causes of vision loss—cataract, glaucoma, diabetes, macular degeneration, for instance—just like those without HIV infection. If there is no significant immunodeficiency, then the patient’s HIV status would be less relevant, and these more common causes of vision loss should be pursued. My first task would be to determine the patient’s most recent CD4 T-cell count.
Assuming an HIV-positive individual is experiencing visual symptoms related to his/her underlying HIV infection (especially in the setting of CD4 counts < 200 cells/mm3), ocular opportunistic infections (OOI) come to mind first. Despite a reduction in incidence of 75% to 80% in the HAART-era, CMV retinitis remains the most common OOI in patients with AIDS and carries the greatest risk of ocular morbidity.2 In fact, based on enrollment data for the Longitudinal Study of the Ocular Complications of AIDS (LSOCA), the prevalence of CMV retinitis among patients with AIDS is more than 20-fold higher than all other ocular complications of AIDS (OOIs and ocular neoplastic disease), including Kaposi sarcoma, lymphoma, herpes zoster ophthalmicus, ocular syphilis, ocular toxoplasma, necrotizing herpetic retinitis, cryptococcal choroiditis, and pneumocystis choroiditis.3 Beyond ocular opportunistic infections, the most common retinal finding in HIV-positive people is HIV retinopathy, nonspecific microvascular findings in the retina affecting nearly 70% of those with advanced HIV disease. Fortunately, HIV retinopathy is generally asymptomatic.4
►Dr. Swamy. Thank you for those explanations. Based on Dr. Serrao’s differential, it is worth noting that this patient is MSM. He was evaluated in urgent care with the initial examination showing a temperature of 98.0° F, pulse 83 beats per minute, and blood pressure 110/70 mm Hg. The eye exam showed no injection with normal extraocular movements. Initial laboratory data were notable for a CD4 count of 730 cells/mm3 with fewer than 20 HIV viral copies/mL. Cytomegalovirus immunoglobulin G (IgG) was positive, and immunoglobulin M (IgM) was negative. A Lyme antibody was positive with negative IgM and IgG by Western blot. Additional tests can be seen in Tables 1 and 2. The patient has good immunologic and virologic control. How does this change your thinking about the case?
►Dr. Serrao. His CD4 count is well above 350, increasing the likelihood of a relatively uncomplicated course and treatment. Cytomegalovirus antibodies reflect prior infection. As CMV generally does not manifest with disease of any variety (including CMV retinitis) at this high CD4 count, one can presume he does not have CMV retinitis as a cause for his visual changes. CMV retinitis occurs mainly when substantial CD4 depletion has occurred (typically less than 50 cells/mm3). A positive Lyme antibody screen, not specific to Lyme, can be falsely positive in other treponema diseases (eg, Treponema pallidum, the etiologic organism of syphilis) as evidenced by negative confirmatory Western blot IgG and IgM. Antineutrophil cystoplasmic antibodies, lysozyme, angiotensin-converting enzyme, rapid plasma reagin (RPR), herpes simplex virus, toxoplasma are generally included in the workup for the differential of uveitis, retinitis, choroiditis, etc.
►Dr. Swamy. Based on the visual changes, the patient was referred for urgent ophthalmologic evaluation. Dr. Butler, when should a generalist consider urgent ophthalmology referral?
►Dr. Butler. In general, all patients with acute (and significant) vision loss should be referred immediately to an ophthalmologist. The challenge for the general practitioner is determining the true extent of the reported vision loss. If possible, some assessment of visual acuity should be obtained, testing each eye independently and with the correct glasses correction (ie, the patient’s distance glasses if the test object is 12 feet or more from the patient or their reading glasses if the test object is held inside arm’s length). If the general practitioner does not have access to an eye chart or near card, any assessment of vision with an appropriate description will be useful (eg, the patient can quickly count fingers at 15 feet in the unaffected eye, but the eye with reported vision loss cannot reliably count fingers outside of 2 feet). Additional ocular symptoms associated with the vision loss, such as pain, redness, photophobia, new flashes or floaters, increase the urgency of the referral. The threshold for referral for any ocular complaint is lower compared with that of the general population for those with evidence of immunodeficiency, such as for this patient with HIV. Any CD4 count < 200 cells/mm3 should raise the practitioner’s concern for an ocular opportunistic infection, with the greatest concern with CD4 counts < 50 cells/mm3.
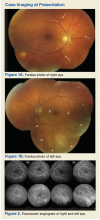
►Dr. Swamy. The patient underwent further testing in the ophthalmology clinic. Dr. Butler, can you please interpret the funduscopic exam?
►Dr. Butler. Both eyes demonstrate findings (microaneurysms and small dot-blot hemorrhages) consistent with moderate nonproliferative diabetic retinopathy (Figure 1A, white arrows). HIV-associated retinopathy could produce similar findings, but it is not generally seen with CD4 counts > 200 cells/mm3. Additionally, in the left eye, there is a diffuse patch of retinal whitening (retinitis) associated with the inferotemporal vascular arcades (Figure 1B, white arrows). The entire area involved is poorly circumscribed and the whitening is subtle in areas. Overlying some areas of deeper, ground-glass whitening there are scattered, punctate white spots (Figure 1B, green arrows). Wickremasinghe and colleagues described this pattern of retinitis and suggested that it had a high positive-predictive value in the diagnosis of ocular syphilis.5
►Dr. Swamy. The patient then underwent fluorescein angiography and optical coherence tomography (OCT). Dr. Butler, what did the fluorescein angiography show?
►Dr. Butler. The fluorescein angiogram in both eyes revealed leakage of dye consistent with diabetic retinopathy, with the right eye (OD) worse than the left (OS). Additionally, the areas of active retinitis in the left eye displayed gradual staining with leopard-spot changes, along with late leakage of fluorescein dye, indicating vasculopathy in the infected area (Figure 2, arrows). The patient also underwent OCT in the left eye (images not displayed) demonstrating vitreous cells (vitritis), patches of inner retinal thickening with hyperreflectivity, and hyperreflective nodules at the level of the retinal pigment epithelium with overlying photoreceptor disruption. These OCT findings are fairly stereotypic for syphilitic chorioretinitis.6
►Dr. Swamy. Based on the ophthalmic findings, a diagnosis of ocular syphilis was made. Dr. Serrao, what should internists consider as they evaluate and manage a patient with ocular syphilis?
►Dr. Serrao. Although isolated ocular involvement from syphilis is possible, the majority of patients (up to 85%) with HIV can present with concomitant central nervous system infection and about 30% present with symptomatic neurosyphilis (a typical late manifestation of this disease) that reflects the aggressiveness, accelerated course and propensity for wide dissemination of syphilis in this patient population.7
The presence of concomitant cutaneous rashes should prompt universal precautions, because transmission can occur via skin to skin contact. Clinicians should watch for the Jarisch-Herxheimer reaction during treatment, a syndrome of fever, myalgias, and headache, which results from circulating cytokines produced because of rapidly dying spirochetes that could mimic a penicillin drug reaction, yet is treated supportively.
As syphilis is sexually acquired, clinicians should test for coexistent sexually transmitted infections, vaccinate for those that are preventable (eg, hepatitis B), notify sexual partners via assistance from local departments of public health, and assess for coexistent drug use and offer counseling in order to optimize risk reduction. Special attention should be paid to virologic control of HIV since some studies have shown an increase in the propensity for breakthrough HIV viremia while on effective ART.9 This should warrant counseling for ongoing optimal ART adherence and close monitoring in the follow-up visits with a provider specialized in the treatment of syphilis and HIV.
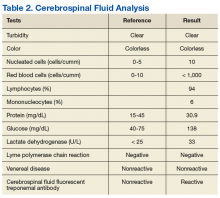
►Dr. Swamy. A lumbar puncture is performed with the results listed in Table 2. Dr. Serrao, is the CSF consistent with neurosyphilis? What would you do next?
►Dr. Serrao. The lumbar puncture is inflammatory with a lymphocytic predominance, consistent with active ocular/neurosyphilis. The CSF Venereal Disease Research Laboratory test is specific but not sensitive so a negative value does not rule out the presence of central nervous system infection.10 The CSF fluorescent treponemal antibody (CSF FTA-ABS) is sensitive but not specific. In this case, the ocular findings, positive serum RPR, CSF lymphocytic predominance, and CSF FTA ABS strongly supports the diagnosis of ocular/early neurosyphilis in a patient with HIV infection in whom early aggressive treatment is warranted to prevent rapid progression/potential loss of vision.11
►Dr. Swamy. Dr. Butler, how does syphilis behave in the eye as compared to other infectious or inflammatory diseases? Do visual symptoms respond well to treatment?
►Dr. Butler. As opposed to the dramatic reduction in rates and severity of CMV retinitis, HAART has had a negligible effect on ocular syphilis in the setting of HIV coinfection; in fact, rates of syphilis, including ocular syphilis, are currently surging world-wide, and HIV coinfection portends a worse prognosis.12 This is especially true among gay men. More so, there appears to be no correlation between CD4 count and incidence of developing ocular syphilis, as opposed to CMV retinitis, which occurs far more frequently in those with CD4 counts < 50 cells/mm3. In keeping with its epithet as one of the “Great Imitators,” syphilis can affect virtually every tissue of the eye—conjunctiva, sclera, cornea, iris, lens, vitreous, retina, choroid, optic nerve—unlike other OOI, such as CMV or toxoplasma, which generally hone to the retina. Nonetheless, various findings and patterns on clinical exam and ancillary testing, such as the more recently described punctate inner retinitis (as seen in our patient) and the more classic acute syphilitic posterior placoid chorioretinitis, carry high specificity for ocular syphilis.13
Patients with ocular syphilis should be treated according to neurosyphilis treatment protocols. In general, these patients respond very well to treatment with resolution of the ocular findings and recovery of complete, or nearly so, visual function, as long as an excessive delay between diagnosis and proper treatment does not occur.14
►Dr. Swamy. Following this testing, the patient completed 14 days of IV penicillin with resolution of symptoms. He had no further vision complaints. He was started on Triumeq (abacavir, dolutegravir, and lamivudine) with good adherence to therapy. Dr. Serrao, in 2016 the CDC released a clinical advisory about ocular syphilis. Can you tell us about why this is an important diagnosis to be aware of today?
►Dr. Serrao. As with any disease, the epidemiologic characteristics of an infection like syphilis allow the clinician to more carefully entertain such a diagnosis in any one individual by improving the index of suspicion for a particular disease. Awareness of an increase in ocular syphilis in HIV positive MSM allows for a more timely assessment and subsequent treatment with the goal of preventing loss of vision.15
1. Cunningham ET Jr, Margolis TP. Ocular manifestations of HIV infection. N Engl J Med. 1998;339(4):236-244.
2. Holtzer CD, Jacobson MA, Hadley WK, et al. Decline in the rate of specific opportunistic infections at San Francisco General Hospital, 1994-1997. AIDS. 1998;12(14):1931-1933.
3. Gangaputra S, Drye L, Vaidya V, Thorne JE, Jabs DA, Lyon AT. Non-cytomegalovirus ocular opportunistic infections in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 2013;155(2):206-212.e205.
4. Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114(4):780-786.
5. Wickremasinghe S, Ling C, Stawell R, Yeoh J, Hall A, Zamir E. Syphilitic punctate inner retinitis in immunocompetent gay men. Ophthalmology. 2009;116(6):1195-1200.
6. Burkholder BM, Leung TG, Ostheimer TA, Butler NJ, Thorne JE, Dunn JP. Spectral domain optical coherence tomography findings in acute syphilitic posterior placoid chorioretinitis. J Ophthalmic Inflamm Infect. 2014;4(1):2.
7. Musher DM, Hamill RJ, Baughn RE. Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med. 1990;113(11):872-881.
8. Lukehart SA, Hook EW 3rd, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med. 1988;109(11):855-862.
9. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
10. Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. 2012;39(6):453-457.
11. Harding AS, Ghanem KG. The performance of cerebrospinal fluid treponemal-specific antibody tests in neurosyphilis: a systematic review. Sex Transm Dis. 2012;39(4):291-297.
12. Butler NJ, Thorne JE. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol. 2012;23(6):517-522.
13. Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97(10):1288-1297.
14. Davis JL. Ocular syphilis. Curr Opin Ophthalmol. 2014;25(6):513-518.
15. Clinical Advisory: Ocular Syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Accessed September 11, 2017.
►Lakshmana Swamy, MD, chief medical resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. Dr. Serrao, when you hear about vision changes in a patient with HIV, what differential diagnosis is generated? What epidemiologic or historical factors can help distinguish these entities?
►Richard Serrao, MD, Infectious Disease Service, VABHS and assistant professor of medicine, Boston University School of Medicine. The differential diagnoses for vision changes in a patient with HIV is based on the overall immunosuppression of the patient: the lower the patient’s CD4 count, the higher the number of etiologies.1 The portions of the visual pathway as well as the pattern of vision loss are useful in narrowing the differential. For example, monocular visual disturbances with dermatomal vesicles within the ophthalmic division of the trigeminal nerve strongly implicates varicella zoster retinitis or keratitis; abducens nerve palsy could suggest granulomatous basilar meningitis from cryptococcosis. Likewise, ongoing fevers in an advanced AIDS patient with concomitant colitis, hepatitis, and pneumonitis is strongly suspicious for cytomegalovirus (CMV) retinitis with wide dissemination.
Geographic epidemiologic factors can suggest pathogens more prevalent to certain regions of the world, such as histoplasma chorioretinitis in a resident of the central and eastern U.S. or tuberculosis in a returning traveler. Likewise, a cat owner or one who consumes steak tartare increases the likelihood for toxoplasma retinochoroiditis, or syphilis in men who have sex with men (MSM) in the U.S. given that the majority of new cases occur in this patient population. Other clues one should consider include the presence of splinter hemorrhages in the extremities in an intravenous drug user, raising the possibility of embolic endophthalmitis from bacterial or fungal endocarditis. A variety of other diagnoses can certainly occur as a result of drug treatment (uveitis from rifampin, for example), immune reconstitution from HAART, infections with other HIV-associated pathogens, such as Pneumocystis jiroveci, and many non-HIV-related ocular diseases.
►Dr. Swamy. Dr. Butler, what concerns do you have when you hear about an HIV-infected patient with vision loss from the ophthalmology perspective?
►Nicholas Butler, MD, Ophthalmology Service, Uveitis and Ocular Immunology, VABHS and assistant professor of ophthalmology, Harvard Medical School. Of course, patients with HIV suffer from common causes of vision loss—cataract, glaucoma, diabetes, macular degeneration, for instance—just like those without HIV infection. If there is no significant immunodeficiency, then the patient’s HIV status would be less relevant, and these more common causes of vision loss should be pursued. My first task would be to determine the patient’s most recent CD4 T-cell count.
Assuming an HIV-positive individual is experiencing visual symptoms related to his/her underlying HIV infection (especially in the setting of CD4 counts < 200 cells/mm3), ocular opportunistic infections (OOI) come to mind first. Despite a reduction in incidence of 75% to 80% in the HAART-era, CMV retinitis remains the most common OOI in patients with AIDS and carries the greatest risk of ocular morbidity.2 In fact, based on enrollment data for the Longitudinal Study of the Ocular Complications of AIDS (LSOCA), the prevalence of CMV retinitis among patients with AIDS is more than 20-fold higher than all other ocular complications of AIDS (OOIs and ocular neoplastic disease), including Kaposi sarcoma, lymphoma, herpes zoster ophthalmicus, ocular syphilis, ocular toxoplasma, necrotizing herpetic retinitis, cryptococcal choroiditis, and pneumocystis choroiditis.3 Beyond ocular opportunistic infections, the most common retinal finding in HIV-positive people is HIV retinopathy, nonspecific microvascular findings in the retina affecting nearly 70% of those with advanced HIV disease. Fortunately, HIV retinopathy is generally asymptomatic.4
►Dr. Swamy. Thank you for those explanations. Based on Dr. Serrao’s differential, it is worth noting that this patient is MSM. He was evaluated in urgent care with the initial examination showing a temperature of 98.0° F, pulse 83 beats per minute, and blood pressure 110/70 mm Hg. The eye exam showed no injection with normal extraocular movements. Initial laboratory data were notable for a CD4 count of 730 cells/mm3 with fewer than 20 HIV viral copies/mL. Cytomegalovirus immunoglobulin G (IgG) was positive, and immunoglobulin M (IgM) was negative. A Lyme antibody was positive with negative IgM and IgG by Western blot. Additional tests can be seen in Tables 1 and 2. The patient has good immunologic and virologic control. How does this change your thinking about the case?
►Dr. Serrao. His CD4 count is well above 350, increasing the likelihood of a relatively uncomplicated course and treatment. Cytomegalovirus antibodies reflect prior infection. As CMV generally does not manifest with disease of any variety (including CMV retinitis) at this high CD4 count, one can presume he does not have CMV retinitis as a cause for his visual changes. CMV retinitis occurs mainly when substantial CD4 depletion has occurred (typically less than 50 cells/mm3). A positive Lyme antibody screen, not specific to Lyme, can be falsely positive in other treponema diseases (eg, Treponema pallidum, the etiologic organism of syphilis) as evidenced by negative confirmatory Western blot IgG and IgM. Antineutrophil cystoplasmic antibodies, lysozyme, angiotensin-converting enzyme, rapid plasma reagin (RPR), herpes simplex virus, toxoplasma are generally included in the workup for the differential of uveitis, retinitis, choroiditis, etc.
►Dr. Swamy. Based on the visual changes, the patient was referred for urgent ophthalmologic evaluation. Dr. Butler, when should a generalist consider urgent ophthalmology referral?
►Dr. Butler. In general, all patients with acute (and significant) vision loss should be referred immediately to an ophthalmologist. The challenge for the general practitioner is determining the true extent of the reported vision loss. If possible, some assessment of visual acuity should be obtained, testing each eye independently and with the correct glasses correction (ie, the patient’s distance glasses if the test object is 12 feet or more from the patient or their reading glasses if the test object is held inside arm’s length). If the general practitioner does not have access to an eye chart or near card, any assessment of vision with an appropriate description will be useful (eg, the patient can quickly count fingers at 15 feet in the unaffected eye, but the eye with reported vision loss cannot reliably count fingers outside of 2 feet). Additional ocular symptoms associated with the vision loss, such as pain, redness, photophobia, new flashes or floaters, increase the urgency of the referral. The threshold for referral for any ocular complaint is lower compared with that of the general population for those with evidence of immunodeficiency, such as for this patient with HIV. Any CD4 count < 200 cells/mm3 should raise the practitioner’s concern for an ocular opportunistic infection, with the greatest concern with CD4 counts < 50 cells/mm3.
►Dr. Swamy. The patient underwent further testing in the ophthalmology clinic. Dr. Butler, can you please interpret the funduscopic exam?
►Dr. Butler. Both eyes demonstrate findings (microaneurysms and small dot-blot hemorrhages) consistent with moderate nonproliferative diabetic retinopathy (Figure 1A, white arrows). HIV-associated retinopathy could produce similar findings, but it is not generally seen with CD4 counts > 200 cells/mm3. Additionally, in the left eye, there is a diffuse patch of retinal whitening (retinitis) associated with the inferotemporal vascular arcades (Figure 1B, white arrows). The entire area involved is poorly circumscribed and the whitening is subtle in areas. Overlying some areas of deeper, ground-glass whitening there are scattered, punctate white spots (Figure 1B, green arrows). Wickremasinghe and colleagues described this pattern of retinitis and suggested that it had a high positive-predictive value in the diagnosis of ocular syphilis.5
►Dr. Swamy. The patient then underwent fluorescein angiography and optical coherence tomography (OCT). Dr. Butler, what did the fluorescein angiography show?
►Dr. Butler. The fluorescein angiogram in both eyes revealed leakage of dye consistent with diabetic retinopathy, with the right eye (OD) worse than the left (OS). Additionally, the areas of active retinitis in the left eye displayed gradual staining with leopard-spot changes, along with late leakage of fluorescein dye, indicating vasculopathy in the infected area (Figure 2, arrows). The patient also underwent OCT in the left eye (images not displayed) demonstrating vitreous cells (vitritis), patches of inner retinal thickening with hyperreflectivity, and hyperreflective nodules at the level of the retinal pigment epithelium with overlying photoreceptor disruption. These OCT findings are fairly stereotypic for syphilitic chorioretinitis.6
►Dr. Swamy. Based on the ophthalmic findings, a diagnosis of ocular syphilis was made. Dr. Serrao, what should internists consider as they evaluate and manage a patient with ocular syphilis?
►Dr. Serrao. Although isolated ocular involvement from syphilis is possible, the majority of patients (up to 85%) with HIV can present with concomitant central nervous system infection and about 30% present with symptomatic neurosyphilis (a typical late manifestation of this disease) that reflects the aggressiveness, accelerated course and propensity for wide dissemination of syphilis in this patient population.7
The presence of concomitant cutaneous rashes should prompt universal precautions, because transmission can occur via skin to skin contact. Clinicians should watch for the Jarisch-Herxheimer reaction during treatment, a syndrome of fever, myalgias, and headache, which results from circulating cytokines produced because of rapidly dying spirochetes that could mimic a penicillin drug reaction, yet is treated supportively.
As syphilis is sexually acquired, clinicians should test for coexistent sexually transmitted infections, vaccinate for those that are preventable (eg, hepatitis B), notify sexual partners via assistance from local departments of public health, and assess for coexistent drug use and offer counseling in order to optimize risk reduction. Special attention should be paid to virologic control of HIV since some studies have shown an increase in the propensity for breakthrough HIV viremia while on effective ART.9 This should warrant counseling for ongoing optimal ART adherence and close monitoring in the follow-up visits with a provider specialized in the treatment of syphilis and HIV.
►Dr. Swamy. A lumbar puncture is performed with the results listed in Table 2. Dr. Serrao, is the CSF consistent with neurosyphilis? What would you do next?
►Dr. Serrao. The lumbar puncture is inflammatory with a lymphocytic predominance, consistent with active ocular/neurosyphilis. The CSF Venereal Disease Research Laboratory test is specific but not sensitive so a negative value does not rule out the presence of central nervous system infection.10 The CSF fluorescent treponemal antibody (CSF FTA-ABS) is sensitive but not specific. In this case, the ocular findings, positive serum RPR, CSF lymphocytic predominance, and CSF FTA ABS strongly supports the diagnosis of ocular/early neurosyphilis in a patient with HIV infection in whom early aggressive treatment is warranted to prevent rapid progression/potential loss of vision.11
►Dr. Swamy. Dr. Butler, how does syphilis behave in the eye as compared to other infectious or inflammatory diseases? Do visual symptoms respond well to treatment?
►Dr. Butler. As opposed to the dramatic reduction in rates and severity of CMV retinitis, HAART has had a negligible effect on ocular syphilis in the setting of HIV coinfection; in fact, rates of syphilis, including ocular syphilis, are currently surging world-wide, and HIV coinfection portends a worse prognosis.12 This is especially true among gay men. More so, there appears to be no correlation between CD4 count and incidence of developing ocular syphilis, as opposed to CMV retinitis, which occurs far more frequently in those with CD4 counts < 50 cells/mm3. In keeping with its epithet as one of the “Great Imitators,” syphilis can affect virtually every tissue of the eye—conjunctiva, sclera, cornea, iris, lens, vitreous, retina, choroid, optic nerve—unlike other OOI, such as CMV or toxoplasma, which generally hone to the retina. Nonetheless, various findings and patterns on clinical exam and ancillary testing, such as the more recently described punctate inner retinitis (as seen in our patient) and the more classic acute syphilitic posterior placoid chorioretinitis, carry high specificity for ocular syphilis.13
Patients with ocular syphilis should be treated according to neurosyphilis treatment protocols. In general, these patients respond very well to treatment with resolution of the ocular findings and recovery of complete, or nearly so, visual function, as long as an excessive delay between diagnosis and proper treatment does not occur.14
►Dr. Swamy. Following this testing, the patient completed 14 days of IV penicillin with resolution of symptoms. He had no further vision complaints. He was started on Triumeq (abacavir, dolutegravir, and lamivudine) with good adherence to therapy. Dr. Serrao, in 2016 the CDC released a clinical advisory about ocular syphilis. Can you tell us about why this is an important diagnosis to be aware of today?
►Dr. Serrao. As with any disease, the epidemiologic characteristics of an infection like syphilis allow the clinician to more carefully entertain such a diagnosis in any one individual by improving the index of suspicion for a particular disease. Awareness of an increase in ocular syphilis in HIV positive MSM allows for a more timely assessment and subsequent treatment with the goal of preventing loss of vision.15
►Lakshmana Swamy, MD, chief medical resident, VA Boston Healthcare System (VABHS) and Boston Medical Center. Dr. Serrao, when you hear about vision changes in a patient with HIV, what differential diagnosis is generated? What epidemiologic or historical factors can help distinguish these entities?
►Richard Serrao, MD, Infectious Disease Service, VABHS and assistant professor of medicine, Boston University School of Medicine. The differential diagnoses for vision changes in a patient with HIV is based on the overall immunosuppression of the patient: the lower the patient’s CD4 count, the higher the number of etiologies.1 The portions of the visual pathway as well as the pattern of vision loss are useful in narrowing the differential. For example, monocular visual disturbances with dermatomal vesicles within the ophthalmic division of the trigeminal nerve strongly implicates varicella zoster retinitis or keratitis; abducens nerve palsy could suggest granulomatous basilar meningitis from cryptococcosis. Likewise, ongoing fevers in an advanced AIDS patient with concomitant colitis, hepatitis, and pneumonitis is strongly suspicious for cytomegalovirus (CMV) retinitis with wide dissemination.
Geographic epidemiologic factors can suggest pathogens more prevalent to certain regions of the world, such as histoplasma chorioretinitis in a resident of the central and eastern U.S. or tuberculosis in a returning traveler. Likewise, a cat owner or one who consumes steak tartare increases the likelihood for toxoplasma retinochoroiditis, or syphilis in men who have sex with men (MSM) in the U.S. given that the majority of new cases occur in this patient population. Other clues one should consider include the presence of splinter hemorrhages in the extremities in an intravenous drug user, raising the possibility of embolic endophthalmitis from bacterial or fungal endocarditis. A variety of other diagnoses can certainly occur as a result of drug treatment (uveitis from rifampin, for example), immune reconstitution from HAART, infections with other HIV-associated pathogens, such as Pneumocystis jiroveci, and many non-HIV-related ocular diseases.
►Dr. Swamy. Dr. Butler, what concerns do you have when you hear about an HIV-infected patient with vision loss from the ophthalmology perspective?
►Nicholas Butler, MD, Ophthalmology Service, Uveitis and Ocular Immunology, VABHS and assistant professor of ophthalmology, Harvard Medical School. Of course, patients with HIV suffer from common causes of vision loss—cataract, glaucoma, diabetes, macular degeneration, for instance—just like those without HIV infection. If there is no significant immunodeficiency, then the patient’s HIV status would be less relevant, and these more common causes of vision loss should be pursued. My first task would be to determine the patient’s most recent CD4 T-cell count.
Assuming an HIV-positive individual is experiencing visual symptoms related to his/her underlying HIV infection (especially in the setting of CD4 counts < 200 cells/mm3), ocular opportunistic infections (OOI) come to mind first. Despite a reduction in incidence of 75% to 80% in the HAART-era, CMV retinitis remains the most common OOI in patients with AIDS and carries the greatest risk of ocular morbidity.2 In fact, based on enrollment data for the Longitudinal Study of the Ocular Complications of AIDS (LSOCA), the prevalence of CMV retinitis among patients with AIDS is more than 20-fold higher than all other ocular complications of AIDS (OOIs and ocular neoplastic disease), including Kaposi sarcoma, lymphoma, herpes zoster ophthalmicus, ocular syphilis, ocular toxoplasma, necrotizing herpetic retinitis, cryptococcal choroiditis, and pneumocystis choroiditis.3 Beyond ocular opportunistic infections, the most common retinal finding in HIV-positive people is HIV retinopathy, nonspecific microvascular findings in the retina affecting nearly 70% of those with advanced HIV disease. Fortunately, HIV retinopathy is generally asymptomatic.4
►Dr. Swamy. Thank you for those explanations. Based on Dr. Serrao’s differential, it is worth noting that this patient is MSM. He was evaluated in urgent care with the initial examination showing a temperature of 98.0° F, pulse 83 beats per minute, and blood pressure 110/70 mm Hg. The eye exam showed no injection with normal extraocular movements. Initial laboratory data were notable for a CD4 count of 730 cells/mm3 with fewer than 20 HIV viral copies/mL. Cytomegalovirus immunoglobulin G (IgG) was positive, and immunoglobulin M (IgM) was negative. A Lyme antibody was positive with negative IgM and IgG by Western blot. Additional tests can be seen in Tables 1 and 2. The patient has good immunologic and virologic control. How does this change your thinking about the case?
►Dr. Serrao. His CD4 count is well above 350, increasing the likelihood of a relatively uncomplicated course and treatment. Cytomegalovirus antibodies reflect prior infection. As CMV generally does not manifest with disease of any variety (including CMV retinitis) at this high CD4 count, one can presume he does not have CMV retinitis as a cause for his visual changes. CMV retinitis occurs mainly when substantial CD4 depletion has occurred (typically less than 50 cells/mm3). A positive Lyme antibody screen, not specific to Lyme, can be falsely positive in other treponema diseases (eg, Treponema pallidum, the etiologic organism of syphilis) as evidenced by negative confirmatory Western blot IgG and IgM. Antineutrophil cystoplasmic antibodies, lysozyme, angiotensin-converting enzyme, rapid plasma reagin (RPR), herpes simplex virus, toxoplasma are generally included in the workup for the differential of uveitis, retinitis, choroiditis, etc.
►Dr. Swamy. Based on the visual changes, the patient was referred for urgent ophthalmologic evaluation. Dr. Butler, when should a generalist consider urgent ophthalmology referral?
►Dr. Butler. In general, all patients with acute (and significant) vision loss should be referred immediately to an ophthalmologist. The challenge for the general practitioner is determining the true extent of the reported vision loss. If possible, some assessment of visual acuity should be obtained, testing each eye independently and with the correct glasses correction (ie, the patient’s distance glasses if the test object is 12 feet or more from the patient or their reading glasses if the test object is held inside arm’s length). If the general practitioner does not have access to an eye chart or near card, any assessment of vision with an appropriate description will be useful (eg, the patient can quickly count fingers at 15 feet in the unaffected eye, but the eye with reported vision loss cannot reliably count fingers outside of 2 feet). Additional ocular symptoms associated with the vision loss, such as pain, redness, photophobia, new flashes or floaters, increase the urgency of the referral. The threshold for referral for any ocular complaint is lower compared with that of the general population for those with evidence of immunodeficiency, such as for this patient with HIV. Any CD4 count < 200 cells/mm3 should raise the practitioner’s concern for an ocular opportunistic infection, with the greatest concern with CD4 counts < 50 cells/mm3.
►Dr. Swamy. The patient underwent further testing in the ophthalmology clinic. Dr. Butler, can you please interpret the funduscopic exam?
►Dr. Butler. Both eyes demonstrate findings (microaneurysms and small dot-blot hemorrhages) consistent with moderate nonproliferative diabetic retinopathy (Figure 1A, white arrows). HIV-associated retinopathy could produce similar findings, but it is not generally seen with CD4 counts > 200 cells/mm3. Additionally, in the left eye, there is a diffuse patch of retinal whitening (retinitis) associated with the inferotemporal vascular arcades (Figure 1B, white arrows). The entire area involved is poorly circumscribed and the whitening is subtle in areas. Overlying some areas of deeper, ground-glass whitening there are scattered, punctate white spots (Figure 1B, green arrows). Wickremasinghe and colleagues described this pattern of retinitis and suggested that it had a high positive-predictive value in the diagnosis of ocular syphilis.5
►Dr. Swamy. The patient then underwent fluorescein angiography and optical coherence tomography (OCT). Dr. Butler, what did the fluorescein angiography show?
►Dr. Butler. The fluorescein angiogram in both eyes revealed leakage of dye consistent with diabetic retinopathy, with the right eye (OD) worse than the left (OS). Additionally, the areas of active retinitis in the left eye displayed gradual staining with leopard-spot changes, along with late leakage of fluorescein dye, indicating vasculopathy in the infected area (Figure 2, arrows). The patient also underwent OCT in the left eye (images not displayed) demonstrating vitreous cells (vitritis), patches of inner retinal thickening with hyperreflectivity, and hyperreflective nodules at the level of the retinal pigment epithelium with overlying photoreceptor disruption. These OCT findings are fairly stereotypic for syphilitic chorioretinitis.6
►Dr. Swamy. Based on the ophthalmic findings, a diagnosis of ocular syphilis was made. Dr. Serrao, what should internists consider as they evaluate and manage a patient with ocular syphilis?
►Dr. Serrao. Although isolated ocular involvement from syphilis is possible, the majority of patients (up to 85%) with HIV can present with concomitant central nervous system infection and about 30% present with symptomatic neurosyphilis (a typical late manifestation of this disease) that reflects the aggressiveness, accelerated course and propensity for wide dissemination of syphilis in this patient population.7
The presence of concomitant cutaneous rashes should prompt universal precautions, because transmission can occur via skin to skin contact. Clinicians should watch for the Jarisch-Herxheimer reaction during treatment, a syndrome of fever, myalgias, and headache, which results from circulating cytokines produced because of rapidly dying spirochetes that could mimic a penicillin drug reaction, yet is treated supportively.
As syphilis is sexually acquired, clinicians should test for coexistent sexually transmitted infections, vaccinate for those that are preventable (eg, hepatitis B), notify sexual partners via assistance from local departments of public health, and assess for coexistent drug use and offer counseling in order to optimize risk reduction. Special attention should be paid to virologic control of HIV since some studies have shown an increase in the propensity for breakthrough HIV viremia while on effective ART.9 This should warrant counseling for ongoing optimal ART adherence and close monitoring in the follow-up visits with a provider specialized in the treatment of syphilis and HIV.
►Dr. Swamy. A lumbar puncture is performed with the results listed in Table 2. Dr. Serrao, is the CSF consistent with neurosyphilis? What would you do next?
►Dr. Serrao. The lumbar puncture is inflammatory with a lymphocytic predominance, consistent with active ocular/neurosyphilis. The CSF Venereal Disease Research Laboratory test is specific but not sensitive so a negative value does not rule out the presence of central nervous system infection.10 The CSF fluorescent treponemal antibody (CSF FTA-ABS) is sensitive but not specific. In this case, the ocular findings, positive serum RPR, CSF lymphocytic predominance, and CSF FTA ABS strongly supports the diagnosis of ocular/early neurosyphilis in a patient with HIV infection in whom early aggressive treatment is warranted to prevent rapid progression/potential loss of vision.11
►Dr. Swamy. Dr. Butler, how does syphilis behave in the eye as compared to other infectious or inflammatory diseases? Do visual symptoms respond well to treatment?
►Dr. Butler. As opposed to the dramatic reduction in rates and severity of CMV retinitis, HAART has had a negligible effect on ocular syphilis in the setting of HIV coinfection; in fact, rates of syphilis, including ocular syphilis, are currently surging world-wide, and HIV coinfection portends a worse prognosis.12 This is especially true among gay men. More so, there appears to be no correlation between CD4 count and incidence of developing ocular syphilis, as opposed to CMV retinitis, which occurs far more frequently in those with CD4 counts < 50 cells/mm3. In keeping with its epithet as one of the “Great Imitators,” syphilis can affect virtually every tissue of the eye—conjunctiva, sclera, cornea, iris, lens, vitreous, retina, choroid, optic nerve—unlike other OOI, such as CMV or toxoplasma, which generally hone to the retina. Nonetheless, various findings and patterns on clinical exam and ancillary testing, such as the more recently described punctate inner retinitis (as seen in our patient) and the more classic acute syphilitic posterior placoid chorioretinitis, carry high specificity for ocular syphilis.13
Patients with ocular syphilis should be treated according to neurosyphilis treatment protocols. In general, these patients respond very well to treatment with resolution of the ocular findings and recovery of complete, or nearly so, visual function, as long as an excessive delay between diagnosis and proper treatment does not occur.14
►Dr. Swamy. Following this testing, the patient completed 14 days of IV penicillin with resolution of symptoms. He had no further vision complaints. He was started on Triumeq (abacavir, dolutegravir, and lamivudine) with good adherence to therapy. Dr. Serrao, in 2016 the CDC released a clinical advisory about ocular syphilis. Can you tell us about why this is an important diagnosis to be aware of today?
►Dr. Serrao. As with any disease, the epidemiologic characteristics of an infection like syphilis allow the clinician to more carefully entertain such a diagnosis in any one individual by improving the index of suspicion for a particular disease. Awareness of an increase in ocular syphilis in HIV positive MSM allows for a more timely assessment and subsequent treatment with the goal of preventing loss of vision.15
1. Cunningham ET Jr, Margolis TP. Ocular manifestations of HIV infection. N Engl J Med. 1998;339(4):236-244.
2. Holtzer CD, Jacobson MA, Hadley WK, et al. Decline in the rate of specific opportunistic infections at San Francisco General Hospital, 1994-1997. AIDS. 1998;12(14):1931-1933.
3. Gangaputra S, Drye L, Vaidya V, Thorne JE, Jabs DA, Lyon AT. Non-cytomegalovirus ocular opportunistic infections in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 2013;155(2):206-212.e205.
4. Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114(4):780-786.
5. Wickremasinghe S, Ling C, Stawell R, Yeoh J, Hall A, Zamir E. Syphilitic punctate inner retinitis in immunocompetent gay men. Ophthalmology. 2009;116(6):1195-1200.
6. Burkholder BM, Leung TG, Ostheimer TA, Butler NJ, Thorne JE, Dunn JP. Spectral domain optical coherence tomography findings in acute syphilitic posterior placoid chorioretinitis. J Ophthalmic Inflamm Infect. 2014;4(1):2.
7. Musher DM, Hamill RJ, Baughn RE. Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med. 1990;113(11):872-881.
8. Lukehart SA, Hook EW 3rd, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med. 1988;109(11):855-862.
9. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
10. Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. 2012;39(6):453-457.
11. Harding AS, Ghanem KG. The performance of cerebrospinal fluid treponemal-specific antibody tests in neurosyphilis: a systematic review. Sex Transm Dis. 2012;39(4):291-297.
12. Butler NJ, Thorne JE. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol. 2012;23(6):517-522.
13. Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97(10):1288-1297.
14. Davis JL. Ocular syphilis. Curr Opin Ophthalmol. 2014;25(6):513-518.
15. Clinical Advisory: Ocular Syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Accessed September 11, 2017.
1. Cunningham ET Jr, Margolis TP. Ocular manifestations of HIV infection. N Engl J Med. 1998;339(4):236-244.
2. Holtzer CD, Jacobson MA, Hadley WK, et al. Decline in the rate of specific opportunistic infections at San Francisco General Hospital, 1994-1997. AIDS. 1998;12(14):1931-1933.
3. Gangaputra S, Drye L, Vaidya V, Thorne JE, Jabs DA, Lyon AT. Non-cytomegalovirus ocular opportunistic infections in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 2013;155(2):206-212.e205.
4. Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114(4):780-786.
5. Wickremasinghe S, Ling C, Stawell R, Yeoh J, Hall A, Zamir E. Syphilitic punctate inner retinitis in immunocompetent gay men. Ophthalmology. 2009;116(6):1195-1200.
6. Burkholder BM, Leung TG, Ostheimer TA, Butler NJ, Thorne JE, Dunn JP. Spectral domain optical coherence tomography findings in acute syphilitic posterior placoid chorioretinitis. J Ophthalmic Inflamm Infect. 2014;4(1):2.
7. Musher DM, Hamill RJ, Baughn RE. Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med. 1990;113(11):872-881.
8. Lukehart SA, Hook EW 3rd, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med. 1988;109(11):855-862.
9. Golden MR, Marra CM, Holmes KK. Update on syphilis: resurgence of an old problem. JAMA. 2003;290(11):1510-1514.
10. Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sex Transm Dis. 2012;39(6):453-457.
11. Harding AS, Ghanem KG. The performance of cerebrospinal fluid treponemal-specific antibody tests in neurosyphilis: a systematic review. Sex Transm Dis. 2012;39(4):291-297.
12. Butler NJ, Thorne JE. Current status of HIV infection and ocular disease. Curr Opin Ophthalmol. 2012;23(6):517-522.
13. Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97(10):1288-1297.
14. Davis JL. Ocular syphilis. Curr Opin Ophthalmol. 2014;25(6):513-518.
15. Clinical Advisory: Ocular Syphilis in the United States. https://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm. Accessed September 11, 2017.
Breast cancer screening: Is the controversy of benefits versus harms resolved?
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States, with an estimated 252,710 new cases and 40,610 deaths in 2017.1 Breast cancer mortality is prevented by the use of regular screening mammography, as demonstrated by randomized controlled trials (20% reduction), incidence-based mortality studies (38% to 40% reduction), and service screening studies (48% to 49% reduction).2
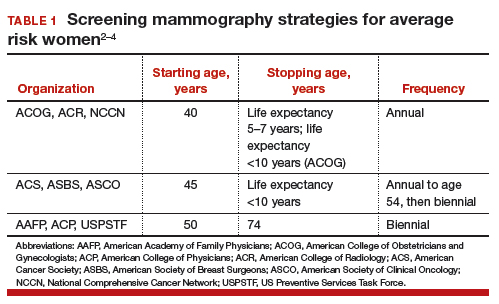
Controversy continues, however, on when to start mammography screening, when to stop screening, and the frequency with which screening should be performed for women at average risk for breast cancer. Indeed, 3 national recommendations—written by the American College of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the US Preventive Services Task Force (USPSTF)—offer different guidelines for mammography screening (TABLE 1).2–4
There are 2 principal reasons for the controversy over screening:
- mammography has both benefits and harms, and individuals place differential weight on the importance of these relative to each other
- randomized controlled trials on screening mammography did not include all of the starting age, stopping age, and screening intervals that are included in screening recommendations.
New comparison of recommendations
An ongoing project funded by the National Cancer Institute, known as the Cancer Intervention and Surveillance Modeling Network (CISNET), models different starting and stopping ages and screening intervals for mammography to assess their impact on both benefits (mortality improvement, life-years gained) and harms (callbacks, benign breast biopsies). Recently, Arleo and colleagues used CISNET model data to compare the breast cancer screening recommendations from ACOG, the ACS, and the USPSTF, focusing on the differential effect on benefits and harms.5
Benefits vs harms of screening in perspective
Without question, the principal goal of cancer screening strategies is to effectively and efficiently reduce cancer mortality. Because mammography screening has both benefits and harms, a clear understanding of the relative frequency of these events among the different screening recommendations should be an important element in patient counseling.
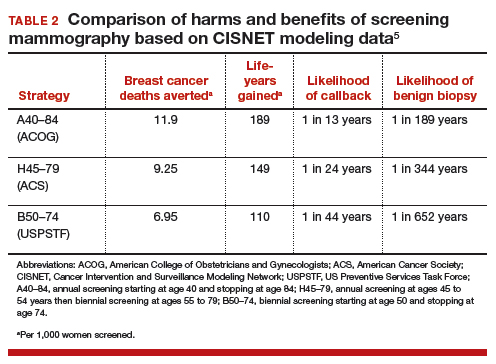
Based on CISNET-modeled estimates, TABLE 2, illustrates the differences in both benefits and harms of the 3 screening strategies. With all strategies, there is a clear benefit in both fewer breast cancer–related deaths and life-years gained per 1,000 women screened.
The greatest benefit is seen in the A40–84 group, that is, women who undergo the most intensive screening strategy with annual screening starting at age 40 and ending at age 84 (ACOG) compared with the USPSTF’s least intensive screening strategy, B50–74, which includes biennial screening starting at age 50 and stopping at age 74; benefits of the ACS’s H45–79 strategy (annual screening at ages 45 to 54 years then biennial screening at ages 55 to 79) were in-between. Not surprisingly, the A40–84 screening strategy was also associated with the most harms, with more recalls and benign breast biopsies; the least harms occurred with the USPSTF strategy, with the ACS strategy again in-between in terms of harms.
Related articles:
Breast density and optimal screening for breast cancer
To further demonstrate differences between the 3 strategies, CISNET also modeled results by looking at all women born in a single birth year cohort (1960) who were still alive at age 40 (2.468 million women). The modeling estimates the number of women who would die from breast cancer without screening mammography and compares that with the number of women who would die from breast cancer using any of the 3 screening strategies. Using this 1960 birth year cohort analysis, there would be approximately 12,000 fewer breast cancer deaths using the ACOG-recommended screening strategy compared with the USPSTF-recommended approach.4
These data show that while there are more harms associated with the most intense screening recommendation, the less frequent screening recommendations will result in higher mortality and more life-years lost. It is reasonable to assume that most patients would value mortality reduction and life-years gained over a likelihood of more benign biopsies or callbacks. As a result, each of the guidelines recommends that by age 40, women at average risk for breast cancer should be counseled and offered mammography screening based on their personal values.
Read about how Dr. Pearlman counsels his patients on screening.
My counseling approach on screening
Notably, the Women’s Preventive Services Initiative recommends that average risk women initiate mammography screening no earlier than age 40 and no later than age 50.6 This creates more flexibility around starting time for screening. In the population of women that I personally counsel, we discuss that fewer women (1 in 68) will experience breast cancer in their 40s compared with in their 50s (1 in 43); therefore as a population, more women will benefit from screening mammography in their 50s. However, there is clear evidence of mortality benefit for a woman in either decade should she develop breast cancer.
We also discuss that the frequency of harms is fairly comparable in either decade, but women who choose to start screening at age 50 will obviously not experience any callbacks or screening-associated benign breast biopsies in their 40s. With this understanding of benefits and harms, most (but not all) average risk women in my practice choose to start screening at age 40.
Related articles:
Breast cancer screening: My practices and response to the USPSTF guidelines
Be mindful of study limitations
The study by Arleo and colleagues has several weaknesses.5
Simulation studies/computer models have limitations. They are only as accurate as the assumptions that are used in the model. However, CISNET modeling has the benefit of having 6 different models with different assumptions on mortality, efficacy of mammography, and efficacy of treatment, and Arleo and colleagues’ analysis takes the mean of these 6 different models.5 It is reassuring to know that the modeling results are consistent with virtually all studies that show that annual screening mammography has a mortality benefit for women in their 40s.
Cost differences are not included. The actual cost of differences between the strategies is difficult to calculate and was not analyzed in this study. While it is easy to calculate the “front end” costs in a study like this (for example, how many more mammograms or biopsies in the different strategies), it is very difficult to calculate the “back end” costs (such as avoided chemotherapy or end-of-life care).
Overtreatment and overdiagnosis have been discussed extensively with regard to the different screening strategies. For example, approximately 80% of women with ductal carcinoma in situ (DCIS) have these tumors detected on screening mammography, and DCIS is not an obligate precursor to invasive breast cancer. Because the natural history of DCIS cannot be predicted, treatment is recommended for all women with DCIS, even though many of these tumors will remain indolent and never cause harm. As a result, concerns have been raised that more intensive screening strategies may result in more overdiagnosis and overtreatment compared with less intensive strategies.
Increasingly, this argument has been questioned, since the prevailing thought is that DCIS does not regress or disappear on mammography. In other words, if DCIS is present at age 40, it will be detected whenever screening starts (age 40, 45, or 50), and age of starting screening or the screening interval will not impact overdiagnosis or overtreatment.7
Related articles:
More than one-third of tumors found on breast cancer screening represent overdiagnosis
Counsel patients, offer screening at age 40
While 3 different breast cancer mammography screening strategies are recommended in the United States, the study by Arleo and colleagues suggests that based on CISNET data, the A40–84 strategy appears to be the most effective at reducing breast cancer mortality and resulting in the most life-years gained. This strategy also requires the most lifetime mammograms and results in the most callbacks and benign biopsies. Women should be offered annual screening mammography starting at age 40 and should start no later than age 50 after receiving counseling about benefits and harms.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed October 4, 2017.
- Oeffinger KC, Frontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 179: Breast cancer risk assessment and screening in average risk women. Obstet Gynecol. 2017;130(1):e1–e16.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- Women’s Preventive Services Initiative. Breast cancer screening for average-risk women. https://www.womenspreventivehealth.org/recommendations/breast-cancer-screening-for-average-risk-women/. Published 2016. Accessed October 4, 2017.
- Arleo EK, Monticciolo DL, Monsees B, McGinty G, Sickles EA. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14(7):863–867.
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States, with an estimated 252,710 new cases and 40,610 deaths in 2017.1 Breast cancer mortality is prevented by the use of regular screening mammography, as demonstrated by randomized controlled trials (20% reduction), incidence-based mortality studies (38% to 40% reduction), and service screening studies (48% to 49% reduction).2
Controversy continues, however, on when to start mammography screening, when to stop screening, and the frequency with which screening should be performed for women at average risk for breast cancer. Indeed, 3 national recommendations—written by the American College of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the US Preventive Services Task Force (USPSTF)—offer different guidelines for mammography screening (TABLE 1).2–4
There are 2 principal reasons for the controversy over screening:
- mammography has both benefits and harms, and individuals place differential weight on the importance of these relative to each other
- randomized controlled trials on screening mammography did not include all of the starting age, stopping age, and screening intervals that are included in screening recommendations.
New comparison of recommendations
An ongoing project funded by the National Cancer Institute, known as the Cancer Intervention and Surveillance Modeling Network (CISNET), models different starting and stopping ages and screening intervals for mammography to assess their impact on both benefits (mortality improvement, life-years gained) and harms (callbacks, benign breast biopsies). Recently, Arleo and colleagues used CISNET model data to compare the breast cancer screening recommendations from ACOG, the ACS, and the USPSTF, focusing on the differential effect on benefits and harms.5
Benefits vs harms of screening in perspective
Without question, the principal goal of cancer screening strategies is to effectively and efficiently reduce cancer mortality. Because mammography screening has both benefits and harms, a clear understanding of the relative frequency of these events among the different screening recommendations should be an important element in patient counseling.
Based on CISNET-modeled estimates, TABLE 2, illustrates the differences in both benefits and harms of the 3 screening strategies. With all strategies, there is a clear benefit in both fewer breast cancer–related deaths and life-years gained per 1,000 women screened.
The greatest benefit is seen in the A40–84 group, that is, women who undergo the most intensive screening strategy with annual screening starting at age 40 and ending at age 84 (ACOG) compared with the USPSTF’s least intensive screening strategy, B50–74, which includes biennial screening starting at age 50 and stopping at age 74; benefits of the ACS’s H45–79 strategy (annual screening at ages 45 to 54 years then biennial screening at ages 55 to 79) were in-between. Not surprisingly, the A40–84 screening strategy was also associated with the most harms, with more recalls and benign breast biopsies; the least harms occurred with the USPSTF strategy, with the ACS strategy again in-between in terms of harms.
Related articles:
Breast density and optimal screening for breast cancer
To further demonstrate differences between the 3 strategies, CISNET also modeled results by looking at all women born in a single birth year cohort (1960) who were still alive at age 40 (2.468 million women). The modeling estimates the number of women who would die from breast cancer without screening mammography and compares that with the number of women who would die from breast cancer using any of the 3 screening strategies. Using this 1960 birth year cohort analysis, there would be approximately 12,000 fewer breast cancer deaths using the ACOG-recommended screening strategy compared with the USPSTF-recommended approach.4
These data show that while there are more harms associated with the most intense screening recommendation, the less frequent screening recommendations will result in higher mortality and more life-years lost. It is reasonable to assume that most patients would value mortality reduction and life-years gained over a likelihood of more benign biopsies or callbacks. As a result, each of the guidelines recommends that by age 40, women at average risk for breast cancer should be counseled and offered mammography screening based on their personal values.
Read about how Dr. Pearlman counsels his patients on screening.
My counseling approach on screening
Notably, the Women’s Preventive Services Initiative recommends that average risk women initiate mammography screening no earlier than age 40 and no later than age 50.6 This creates more flexibility around starting time for screening. In the population of women that I personally counsel, we discuss that fewer women (1 in 68) will experience breast cancer in their 40s compared with in their 50s (1 in 43); therefore as a population, more women will benefit from screening mammography in their 50s. However, there is clear evidence of mortality benefit for a woman in either decade should she develop breast cancer.
We also discuss that the frequency of harms is fairly comparable in either decade, but women who choose to start screening at age 50 will obviously not experience any callbacks or screening-associated benign breast biopsies in their 40s. With this understanding of benefits and harms, most (but not all) average risk women in my practice choose to start screening at age 40.
Related articles:
Breast cancer screening: My practices and response to the USPSTF guidelines
Be mindful of study limitations
The study by Arleo and colleagues has several weaknesses.5
Simulation studies/computer models have limitations. They are only as accurate as the assumptions that are used in the model. However, CISNET modeling has the benefit of having 6 different models with different assumptions on mortality, efficacy of mammography, and efficacy of treatment, and Arleo and colleagues’ analysis takes the mean of these 6 different models.5 It is reassuring to know that the modeling results are consistent with virtually all studies that show that annual screening mammography has a mortality benefit for women in their 40s.
Cost differences are not included. The actual cost of differences between the strategies is difficult to calculate and was not analyzed in this study. While it is easy to calculate the “front end” costs in a study like this (for example, how many more mammograms or biopsies in the different strategies), it is very difficult to calculate the “back end” costs (such as avoided chemotherapy or end-of-life care).
Overtreatment and overdiagnosis have been discussed extensively with regard to the different screening strategies. For example, approximately 80% of women with ductal carcinoma in situ (DCIS) have these tumors detected on screening mammography, and DCIS is not an obligate precursor to invasive breast cancer. Because the natural history of DCIS cannot be predicted, treatment is recommended for all women with DCIS, even though many of these tumors will remain indolent and never cause harm. As a result, concerns have been raised that more intensive screening strategies may result in more overdiagnosis and overtreatment compared with less intensive strategies.
Increasingly, this argument has been questioned, since the prevailing thought is that DCIS does not regress or disappear on mammography. In other words, if DCIS is present at age 40, it will be detected whenever screening starts (age 40, 45, or 50), and age of starting screening or the screening interval will not impact overdiagnosis or overtreatment.7
Related articles:
More than one-third of tumors found on breast cancer screening represent overdiagnosis
Counsel patients, offer screening at age 40
While 3 different breast cancer mammography screening strategies are recommended in the United States, the study by Arleo and colleagues suggests that based on CISNET data, the A40–84 strategy appears to be the most effective at reducing breast cancer mortality and resulting in the most life-years gained. This strategy also requires the most lifetime mammograms and results in the most callbacks and benign biopsies. Women should be offered annual screening mammography starting at age 40 and should start no later than age 50 after receiving counseling about benefits and harms.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States, with an estimated 252,710 new cases and 40,610 deaths in 2017.1 Breast cancer mortality is prevented by the use of regular screening mammography, as demonstrated by randomized controlled trials (20% reduction), incidence-based mortality studies (38% to 40% reduction), and service screening studies (48% to 49% reduction).2
Controversy continues, however, on when to start mammography screening, when to stop screening, and the frequency with which screening should be performed for women at average risk for breast cancer. Indeed, 3 national recommendations—written by the American College of Obstetricians and Gynecologists (ACOG), the American Cancer Society (ACS), and the US Preventive Services Task Force (USPSTF)—offer different guidelines for mammography screening (TABLE 1).2–4
There are 2 principal reasons for the controversy over screening:
- mammography has both benefits and harms, and individuals place differential weight on the importance of these relative to each other
- randomized controlled trials on screening mammography did not include all of the starting age, stopping age, and screening intervals that are included in screening recommendations.
New comparison of recommendations
An ongoing project funded by the National Cancer Institute, known as the Cancer Intervention and Surveillance Modeling Network (CISNET), models different starting and stopping ages and screening intervals for mammography to assess their impact on both benefits (mortality improvement, life-years gained) and harms (callbacks, benign breast biopsies). Recently, Arleo and colleagues used CISNET model data to compare the breast cancer screening recommendations from ACOG, the ACS, and the USPSTF, focusing on the differential effect on benefits and harms.5
Benefits vs harms of screening in perspective
Without question, the principal goal of cancer screening strategies is to effectively and efficiently reduce cancer mortality. Because mammography screening has both benefits and harms, a clear understanding of the relative frequency of these events among the different screening recommendations should be an important element in patient counseling.
Based on CISNET-modeled estimates, TABLE 2, illustrates the differences in both benefits and harms of the 3 screening strategies. With all strategies, there is a clear benefit in both fewer breast cancer–related deaths and life-years gained per 1,000 women screened.
The greatest benefit is seen in the A40–84 group, that is, women who undergo the most intensive screening strategy with annual screening starting at age 40 and ending at age 84 (ACOG) compared with the USPSTF’s least intensive screening strategy, B50–74, which includes biennial screening starting at age 50 and stopping at age 74; benefits of the ACS’s H45–79 strategy (annual screening at ages 45 to 54 years then biennial screening at ages 55 to 79) were in-between. Not surprisingly, the A40–84 screening strategy was also associated with the most harms, with more recalls and benign breast biopsies; the least harms occurred with the USPSTF strategy, with the ACS strategy again in-between in terms of harms.
Related articles:
Breast density and optimal screening for breast cancer
To further demonstrate differences between the 3 strategies, CISNET also modeled results by looking at all women born in a single birth year cohort (1960) who were still alive at age 40 (2.468 million women). The modeling estimates the number of women who would die from breast cancer without screening mammography and compares that with the number of women who would die from breast cancer using any of the 3 screening strategies. Using this 1960 birth year cohort analysis, there would be approximately 12,000 fewer breast cancer deaths using the ACOG-recommended screening strategy compared with the USPSTF-recommended approach.4
These data show that while there are more harms associated with the most intense screening recommendation, the less frequent screening recommendations will result in higher mortality and more life-years lost. It is reasonable to assume that most patients would value mortality reduction and life-years gained over a likelihood of more benign biopsies or callbacks. As a result, each of the guidelines recommends that by age 40, women at average risk for breast cancer should be counseled and offered mammography screening based on their personal values.
Read about how Dr. Pearlman counsels his patients on screening.
My counseling approach on screening
Notably, the Women’s Preventive Services Initiative recommends that average risk women initiate mammography screening no earlier than age 40 and no later than age 50.6 This creates more flexibility around starting time for screening. In the population of women that I personally counsel, we discuss that fewer women (1 in 68) will experience breast cancer in their 40s compared with in their 50s (1 in 43); therefore as a population, more women will benefit from screening mammography in their 50s. However, there is clear evidence of mortality benefit for a woman in either decade should she develop breast cancer.
We also discuss that the frequency of harms is fairly comparable in either decade, but women who choose to start screening at age 50 will obviously not experience any callbacks or screening-associated benign breast biopsies in their 40s. With this understanding of benefits and harms, most (but not all) average risk women in my practice choose to start screening at age 40.
Related articles:
Breast cancer screening: My practices and response to the USPSTF guidelines
Be mindful of study limitations
The study by Arleo and colleagues has several weaknesses.5
Simulation studies/computer models have limitations. They are only as accurate as the assumptions that are used in the model. However, CISNET modeling has the benefit of having 6 different models with different assumptions on mortality, efficacy of mammography, and efficacy of treatment, and Arleo and colleagues’ analysis takes the mean of these 6 different models.5 It is reassuring to know that the modeling results are consistent with virtually all studies that show that annual screening mammography has a mortality benefit for women in their 40s.
Cost differences are not included. The actual cost of differences between the strategies is difficult to calculate and was not analyzed in this study. While it is easy to calculate the “front end” costs in a study like this (for example, how many more mammograms or biopsies in the different strategies), it is very difficult to calculate the “back end” costs (such as avoided chemotherapy or end-of-life care).
Overtreatment and overdiagnosis have been discussed extensively with regard to the different screening strategies. For example, approximately 80% of women with ductal carcinoma in situ (DCIS) have these tumors detected on screening mammography, and DCIS is not an obligate precursor to invasive breast cancer. Because the natural history of DCIS cannot be predicted, treatment is recommended for all women with DCIS, even though many of these tumors will remain indolent and never cause harm. As a result, concerns have been raised that more intensive screening strategies may result in more overdiagnosis and overtreatment compared with less intensive strategies.
Increasingly, this argument has been questioned, since the prevailing thought is that DCIS does not regress or disappear on mammography. In other words, if DCIS is present at age 40, it will be detected whenever screening starts (age 40, 45, or 50), and age of starting screening or the screening interval will not impact overdiagnosis or overtreatment.7
Related articles:
More than one-third of tumors found on breast cancer screening represent overdiagnosis
Counsel patients, offer screening at age 40
While 3 different breast cancer mammography screening strategies are recommended in the United States, the study by Arleo and colleagues suggests that based on CISNET data, the A40–84 strategy appears to be the most effective at reducing breast cancer mortality and resulting in the most life-years gained. This strategy also requires the most lifetime mammograms and results in the most callbacks and benign biopsies. Women should be offered annual screening mammography starting at age 40 and should start no later than age 50 after receiving counseling about benefits and harms.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed October 4, 2017.
- Oeffinger KC, Frontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 179: Breast cancer risk assessment and screening in average risk women. Obstet Gynecol. 2017;130(1):e1–e16.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- Women’s Preventive Services Initiative. Breast cancer screening for average-risk women. https://www.womenspreventivehealth.org/recommendations/breast-cancer-screening-for-average-risk-women/. Published 2016. Accessed October 4, 2017.
- Arleo EK, Monticciolo DL, Monsees B, McGinty G, Sickles EA. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14(7):863–867.
- Cancer Facts & Figures 2017. American Cancer Society website. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf. Accessed October 4, 2017.
- Oeffinger KC, Frontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599–1614.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 179: Breast cancer risk assessment and screening in average risk women. Obstet Gynecol. 2017;130(1):e1–e16.
- Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279–296.
- Arleo EK, Hendrick RE, Helvie MA, Sickles EA. Comparison of recommendations for screening mammography using CISNET models. Cancer. 2017;123(19):3673–3680.
- Women’s Preventive Services Initiative. Breast cancer screening for average-risk women. https://www.womenspreventivehealth.org/recommendations/breast-cancer-screening-for-average-risk-women/. Published 2016. Accessed October 4, 2017.
- Arleo EK, Monticciolo DL, Monsees B, McGinty G, Sickles EA. Persistent untreated screening-detected breast cancer: an argument against delaying screening or increasing the interval between screenings. J Am Coll Radiol. 2017;14(7):863–867.
Did long-term follow-up of WHI participants reveal any mortality increase among women who received HT?
EXPERT COMMENTARY
A 2013 report from the Women’s Health Initiative (WHI), the large National Institutes of Health–funded placebo-controlledrandomized trial of postmenopausal hormone therapy (HT) with oral estrogen (for women with hysterectomy) or estrogen-progestin (for women with an intact uterus), with 13 years of cumulative follow-up, documented the safety of systemic HT when initiated by women younger than 60 years of age or within 10 years of menopause onset.1 Now, with 18 years of cumulative follow-up data available (intervention and extended postintervention phases), the WHI investigators present all-cause and cause-specific mortality outcomes from the 2 HT trials.
Related article:
2017 Update on menopause
Details of the study
A total of 27,347 WHI participants (baseline mean age, 63.4 years; 80.6% white) used oral estrogen-progestin therapy (EPT) or placebo for a median of 5.6 years (n = 16,608) or estrogen-only therapy (ET) or placebo for a median of 7.2 years (n = 10,739). Each hazard ratio (HR) reported below refers to 18-year cumulative follow-up.
All-cause mortality. In the overall pooled cohort (EPT and ET groups), all-cause mortality was similar, with a rate of 27.1% in the HT group and 27.6% in the placebo group (HR, 0.99; 95% confidence interval [CI], 0.94–1.03). The mortality end points included deaths from all causes; cardiovascular disease (coronary heart disease, stroke, and other cardiovascular diseases); cancer (breast, colorectal, and other cancers); and other (Alzheimer disease, other dementia, chronic obstructive pulmonary disease, injuries and accidents, and other).
Stratifying by baseline participant age (comparing women aged 50–59 years with those aged 70–79 years), the HR for all-cause mortality in the pooled cohort during the intervention phase was 0.61 (95% CI, 0.43–0.87), and during the cumulative 18-year follow-up, the HR was 0.87 (95% CI, 0.76–1.00).
Cause-specific mortality. Neither cardiovascular disease mortality nor total cancer mortality was significantly impacted by HT use. In the pooled cohort, cardiovascular disease mortality was 8.9% in the HT group and 9.0% in the placebo group (HR, 1.00; 95% CI, 0.92–1.08), with no differences between the EPT and the ET trials. Cancer mortality rates in the pooled cohort also were similar, with 8.2% in the HT group and 8.0% in the placebo group (HR, 1.03; 95% CI, 0.95–1.12).
With respect to breast cancer mortality, the impact of HT diverged for EPT and ET. For the EPT group, the HR for breast cancer mortality was 1.44 (95% CI, 0.97–2.15; P = .07), while for the ET group the HR was 0.55 (95% CI, 0.33–0.92; P = .02).
Related articles:
Does the discontinuation of menopausal hormone therapy affect a woman’s cardiovascular risk?
Study strengths and weaknesses
The WHI represents the largest randomized placebo-controlled trials of HT. The current WHI trials report provides new, cumulative 18-year follow-up data on all-cause and cause-specific mortality in women treated with HT or placebo.
The authors noted, however, that the use of only one HT dose, formulation, and route of administration in each trial may limit the generalizability of the study results to other HT preparations. For example, the WHI did not examine the transdermal route of estrogen administration. Likewise, the WHI did not examine use of progestational agents other than medroxyprogesterone acetate. In addition, while almost all cohort deaths were captured through the National Death Index for the data analyses, specificity of cause of death may vary across outcomes. Further, since multiple outcomes and subgroups were examined, clinicians should interpret cause-specific mortality rates with caution.
Given the complex impact of HT, all-cause mortality represents an important summary outcome in assessing the safety of 5 to 7 years of HT use. This report's reassuring findings regarding the safety of HT support the guidance from The North American Menopause Society and the Endocrine Society, which endorse the use of HT for symptomatic recently menopausal women without contraindications.2,3
--ANDREW M. KAUNITZ, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728-753.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011.
EXPERT COMMENTARY
A 2013 report from the Women’s Health Initiative (WHI), the large National Institutes of Health–funded placebo-controlledrandomized trial of postmenopausal hormone therapy (HT) with oral estrogen (for women with hysterectomy) or estrogen-progestin (for women with an intact uterus), with 13 years of cumulative follow-up, documented the safety of systemic HT when initiated by women younger than 60 years of age or within 10 years of menopause onset.1 Now, with 18 years of cumulative follow-up data available (intervention and extended postintervention phases), the WHI investigators present all-cause and cause-specific mortality outcomes from the 2 HT trials.
Related article:
2017 Update on menopause
Details of the study
A total of 27,347 WHI participants (baseline mean age, 63.4 years; 80.6% white) used oral estrogen-progestin therapy (EPT) or placebo for a median of 5.6 years (n = 16,608) or estrogen-only therapy (ET) or placebo for a median of 7.2 years (n = 10,739). Each hazard ratio (HR) reported below refers to 18-year cumulative follow-up.
All-cause mortality. In the overall pooled cohort (EPT and ET groups), all-cause mortality was similar, with a rate of 27.1% in the HT group and 27.6% in the placebo group (HR, 0.99; 95% confidence interval [CI], 0.94–1.03). The mortality end points included deaths from all causes; cardiovascular disease (coronary heart disease, stroke, and other cardiovascular diseases); cancer (breast, colorectal, and other cancers); and other (Alzheimer disease, other dementia, chronic obstructive pulmonary disease, injuries and accidents, and other).
Stratifying by baseline participant age (comparing women aged 50–59 years with those aged 70–79 years), the HR for all-cause mortality in the pooled cohort during the intervention phase was 0.61 (95% CI, 0.43–0.87), and during the cumulative 18-year follow-up, the HR was 0.87 (95% CI, 0.76–1.00).
Cause-specific mortality. Neither cardiovascular disease mortality nor total cancer mortality was significantly impacted by HT use. In the pooled cohort, cardiovascular disease mortality was 8.9% in the HT group and 9.0% in the placebo group (HR, 1.00; 95% CI, 0.92–1.08), with no differences between the EPT and the ET trials. Cancer mortality rates in the pooled cohort also were similar, with 8.2% in the HT group and 8.0% in the placebo group (HR, 1.03; 95% CI, 0.95–1.12).
With respect to breast cancer mortality, the impact of HT diverged for EPT and ET. For the EPT group, the HR for breast cancer mortality was 1.44 (95% CI, 0.97–2.15; P = .07), while for the ET group the HR was 0.55 (95% CI, 0.33–0.92; P = .02).
Related articles:
Does the discontinuation of menopausal hormone therapy affect a woman’s cardiovascular risk?
Study strengths and weaknesses
The WHI represents the largest randomized placebo-controlled trials of HT. The current WHI trials report provides new, cumulative 18-year follow-up data on all-cause and cause-specific mortality in women treated with HT or placebo.
The authors noted, however, that the use of only one HT dose, formulation, and route of administration in each trial may limit the generalizability of the study results to other HT preparations. For example, the WHI did not examine the transdermal route of estrogen administration. Likewise, the WHI did not examine use of progestational agents other than medroxyprogesterone acetate. In addition, while almost all cohort deaths were captured through the National Death Index for the data analyses, specificity of cause of death may vary across outcomes. Further, since multiple outcomes and subgroups were examined, clinicians should interpret cause-specific mortality rates with caution.
Given the complex impact of HT, all-cause mortality represents an important summary outcome in assessing the safety of 5 to 7 years of HT use. This report's reassuring findings regarding the safety of HT support the guidance from The North American Menopause Society and the Endocrine Society, which endorse the use of HT for symptomatic recently menopausal women without contraindications.2,3
--ANDREW M. KAUNITZ, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
A 2013 report from the Women’s Health Initiative (WHI), the large National Institutes of Health–funded placebo-controlledrandomized trial of postmenopausal hormone therapy (HT) with oral estrogen (for women with hysterectomy) or estrogen-progestin (for women with an intact uterus), with 13 years of cumulative follow-up, documented the safety of systemic HT when initiated by women younger than 60 years of age or within 10 years of menopause onset.1 Now, with 18 years of cumulative follow-up data available (intervention and extended postintervention phases), the WHI investigators present all-cause and cause-specific mortality outcomes from the 2 HT trials.
Related article:
2017 Update on menopause
Details of the study
A total of 27,347 WHI participants (baseline mean age, 63.4 years; 80.6% white) used oral estrogen-progestin therapy (EPT) or placebo for a median of 5.6 years (n = 16,608) or estrogen-only therapy (ET) or placebo for a median of 7.2 years (n = 10,739). Each hazard ratio (HR) reported below refers to 18-year cumulative follow-up.
All-cause mortality. In the overall pooled cohort (EPT and ET groups), all-cause mortality was similar, with a rate of 27.1% in the HT group and 27.6% in the placebo group (HR, 0.99; 95% confidence interval [CI], 0.94–1.03). The mortality end points included deaths from all causes; cardiovascular disease (coronary heart disease, stroke, and other cardiovascular diseases); cancer (breast, colorectal, and other cancers); and other (Alzheimer disease, other dementia, chronic obstructive pulmonary disease, injuries and accidents, and other).
Stratifying by baseline participant age (comparing women aged 50–59 years with those aged 70–79 years), the HR for all-cause mortality in the pooled cohort during the intervention phase was 0.61 (95% CI, 0.43–0.87), and during the cumulative 18-year follow-up, the HR was 0.87 (95% CI, 0.76–1.00).
Cause-specific mortality. Neither cardiovascular disease mortality nor total cancer mortality was significantly impacted by HT use. In the pooled cohort, cardiovascular disease mortality was 8.9% in the HT group and 9.0% in the placebo group (HR, 1.00; 95% CI, 0.92–1.08), with no differences between the EPT and the ET trials. Cancer mortality rates in the pooled cohort also were similar, with 8.2% in the HT group and 8.0% in the placebo group (HR, 1.03; 95% CI, 0.95–1.12).
With respect to breast cancer mortality, the impact of HT diverged for EPT and ET. For the EPT group, the HR for breast cancer mortality was 1.44 (95% CI, 0.97–2.15; P = .07), while for the ET group the HR was 0.55 (95% CI, 0.33–0.92; P = .02).
Related articles:
Does the discontinuation of menopausal hormone therapy affect a woman’s cardiovascular risk?
Study strengths and weaknesses
The WHI represents the largest randomized placebo-controlled trials of HT. The current WHI trials report provides new, cumulative 18-year follow-up data on all-cause and cause-specific mortality in women treated with HT or placebo.
The authors noted, however, that the use of only one HT dose, formulation, and route of administration in each trial may limit the generalizability of the study results to other HT preparations. For example, the WHI did not examine the transdermal route of estrogen administration. Likewise, the WHI did not examine use of progestational agents other than medroxyprogesterone acetate. In addition, while almost all cohort deaths were captured through the National Death Index for the data analyses, specificity of cause of death may vary across outcomes. Further, since multiple outcomes and subgroups were examined, clinicians should interpret cause-specific mortality rates with caution.
Given the complex impact of HT, all-cause mortality represents an important summary outcome in assessing the safety of 5 to 7 years of HT use. This report's reassuring findings regarding the safety of HT support the guidance from The North American Menopause Society and the Endocrine Society, which endorse the use of HT for symptomatic recently menopausal women without contraindications.2,3
--ANDREW M. KAUNITZ, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728-753.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310(13):1353-1368.
- The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728-753.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(11):3975-4011.