User login
Stroke management: A 30-year retrospective
In 1993, managing patients with stroke had long remained an elusive and somewhat intimidating task for the neurological world. Previous efforts to treat the condition had produced more frustration than success, leaving clinicians and patients alike in despair for a solution. However, some successes in treating coronary thrombosis during that era rejuvenated researchers’ efforts to crack the code. An international team of researchers had studied a Streptococcus derivative (streptokinase) and others had begun to study a natural substance termed tissue plasminogen activator (tPA) as thrombolytic agents to lyse coronary clots and to treat pulmonary embolism. The adverse event of excessive bleeding found in Australian studies done on streptokinase intervention in patients with stroke prompted researchers to contemplate use of tPA in stroke management.
A group of German, Japanese, and American investigators began to research thrombolysis in acute stroke patients during the mid-1980s.
“What was unique is that patients had a CT scan followed by a catheter angiogram,” said Louis Caplan, MD, a senior member of the division of cerebrovascular disease at Beth Israel Deaconess Medical Center, Boston, professor of neurology at Harvard Medical School, Boston, and founder of the Harvard Stroke Registry at Beth Israel Deaconess Medical Center.
“If they had a blocked vessel, they got the drug, delivered either intravenously or intra-arterially.”
The process involved keeping the catheter open after drug administration to determine whether the vessel had opened or remained occluded. The researchers learned which blocked vessels opened when the drug was given intravenously and which required direct introduction of the drug into the clots.
A group of investigators in the United States funded by the National Institute of Neurological Disease and Stroke then performed a randomized therapeutic trial of intravenous tPA given within 90 minutes and 180 minutes after stroke symptom onset. The study was reported in the New England Journal of Medicine. Soon thereafter, in 1995, the Food and Drug Administration approved the use of tPA following the inclusion and exclusion rules used in the NINDS trial.
After the FDA approved tPA in 1995, stroke management was never the same.
tPA was just one factor in optimizing stroke management
Despite the major therapeutic breakthrough with tPA’s approval, it took the clinics, hospitals, and other acute care systems a while to catch up. “Neurologists and hospitals weren’t ready for acute stroke intervention and proper stroke management in the mid-90s,” Dr. Caplan recalled. “At the time, stroke wasn’t at the forefront of treatment, general neurologists weren’t trained, and there weren’t enough stroke neurologists.”
The preparation and training deficit was further exacerbated by low reimbursement for services. As a result, only about 5% of patients who were eligible for acute stroke management were treated with tPA.
According to Dr. Caplan, during the next 15-20 years, the accumulation of stroke data from MRI and CT vascular imaging clarified further which patients, with what extent of infarction, with which blocked vessels, would be good candidates for treatment.
More patients received interventional treatment using catheters directed into the area of clotting in attempt to remove the blockages. In addition, information regarding intervention at different periods (10-16 hours, up to 24 hours) and conditions (for example, patients with varying degrees of disability, infarct) were tested.
Eventually, hospitals became more attuned to emergency stroke treatment. More neurologists became trained, more stroke centers emerged, and clinicians enjoyed the benefit of technological advancements that allowed them to explore perfusion.
While decentralized care enhances outcomes in stroke management, more progress is needed
As of early 2023, stroke is one of the leading emergency diagnoses, and patients have access to primary and secondary stroke centers that are sprinkled throughout the United States. As impressive as the feat may seem, health care systems still have major strides to make to truly optimize therapy and outcomes in this patient population.
For example, location and access remain important issues. Secondary centers are typically located in large, metropolitan areas. While an urban location makes a primary center geographically more accessible to a larger patient population, traffic frequently hinders door-to-door access.
In the case of rural centers, distance can retard access, but they also face the challenges of how to route patients – especially patients who require more specialized care offered by secondary centers. Fortunately, primary centers have some ways to help better support their patients.
“One thing that happened is that primary centers made agreements with secondary centers via telemedicine to determine whether patients should be treated at the primary center or whether they should be routed to the higher-level center. These arrangements were termed ‘spoke and wheel,’ ” Dr. Caplan told this publication.
However, not all patients who are candidates for transport to a secondary center are able to be transported. In such cases, primary centers can use telemedicine to collaborate with secondary centers for support.
Logistics aside, perhaps today’s greatest challenge for clinicians is ensuring their patients and families receive education to increase their awareness of stroke centers as an important option for treatment and outcome optimization. Many patients and their loved ones do not realize that these centers exist or how to utilize them if and when the time comes.
Right now, some cities have stroke ambulances staffed with physicians to treat patients in the field. This decentralized model helps address access burdens such as door-to-needle delays and transportation while improving survival and recovery. Dr. Caplan said these services are available in Munich, and in a few select U.S. cities such as Cleveland and Houston, which helped pioneer the concept.
Better access in the future?
Looking ahead, Dr. Caplan seems optimistic about how stroke management will continue to evolve. Many cities will have stroke ambulances to provide on-site care, while stroke institutions will improve their cross-collaborative efforts to support their patient populations.
At the crux of cross-collaboration lies enhanced communication between peripheral and urban hospitals.
“Peripheral and urban hospitals and state organizations will engage in smoother integration to figure out when to take patient to the bigger hospitals,” Dr. Caplan said. “I also believe we will see greater emphasis on rehabilitation and recovery.”
As promising as the future looks, only time will tell.
In 1993, managing patients with stroke had long remained an elusive and somewhat intimidating task for the neurological world. Previous efforts to treat the condition had produced more frustration than success, leaving clinicians and patients alike in despair for a solution. However, some successes in treating coronary thrombosis during that era rejuvenated researchers’ efforts to crack the code. An international team of researchers had studied a Streptococcus derivative (streptokinase) and others had begun to study a natural substance termed tissue plasminogen activator (tPA) as thrombolytic agents to lyse coronary clots and to treat pulmonary embolism. The adverse event of excessive bleeding found in Australian studies done on streptokinase intervention in patients with stroke prompted researchers to contemplate use of tPA in stroke management.
A group of German, Japanese, and American investigators began to research thrombolysis in acute stroke patients during the mid-1980s.
“What was unique is that patients had a CT scan followed by a catheter angiogram,” said Louis Caplan, MD, a senior member of the division of cerebrovascular disease at Beth Israel Deaconess Medical Center, Boston, professor of neurology at Harvard Medical School, Boston, and founder of the Harvard Stroke Registry at Beth Israel Deaconess Medical Center.
“If they had a blocked vessel, they got the drug, delivered either intravenously or intra-arterially.”
The process involved keeping the catheter open after drug administration to determine whether the vessel had opened or remained occluded. The researchers learned which blocked vessels opened when the drug was given intravenously and which required direct introduction of the drug into the clots.
A group of investigators in the United States funded by the National Institute of Neurological Disease and Stroke then performed a randomized therapeutic trial of intravenous tPA given within 90 minutes and 180 minutes after stroke symptom onset. The study was reported in the New England Journal of Medicine. Soon thereafter, in 1995, the Food and Drug Administration approved the use of tPA following the inclusion and exclusion rules used in the NINDS trial.
After the FDA approved tPA in 1995, stroke management was never the same.
tPA was just one factor in optimizing stroke management
Despite the major therapeutic breakthrough with tPA’s approval, it took the clinics, hospitals, and other acute care systems a while to catch up. “Neurologists and hospitals weren’t ready for acute stroke intervention and proper stroke management in the mid-90s,” Dr. Caplan recalled. “At the time, stroke wasn’t at the forefront of treatment, general neurologists weren’t trained, and there weren’t enough stroke neurologists.”
The preparation and training deficit was further exacerbated by low reimbursement for services. As a result, only about 5% of patients who were eligible for acute stroke management were treated with tPA.
According to Dr. Caplan, during the next 15-20 years, the accumulation of stroke data from MRI and CT vascular imaging clarified further which patients, with what extent of infarction, with which blocked vessels, would be good candidates for treatment.
More patients received interventional treatment using catheters directed into the area of clotting in attempt to remove the blockages. In addition, information regarding intervention at different periods (10-16 hours, up to 24 hours) and conditions (for example, patients with varying degrees of disability, infarct) were tested.
Eventually, hospitals became more attuned to emergency stroke treatment. More neurologists became trained, more stroke centers emerged, and clinicians enjoyed the benefit of technological advancements that allowed them to explore perfusion.
While decentralized care enhances outcomes in stroke management, more progress is needed
As of early 2023, stroke is one of the leading emergency diagnoses, and patients have access to primary and secondary stroke centers that are sprinkled throughout the United States. As impressive as the feat may seem, health care systems still have major strides to make to truly optimize therapy and outcomes in this patient population.
For example, location and access remain important issues. Secondary centers are typically located in large, metropolitan areas. While an urban location makes a primary center geographically more accessible to a larger patient population, traffic frequently hinders door-to-door access.
In the case of rural centers, distance can retard access, but they also face the challenges of how to route patients – especially patients who require more specialized care offered by secondary centers. Fortunately, primary centers have some ways to help better support their patients.
“One thing that happened is that primary centers made agreements with secondary centers via telemedicine to determine whether patients should be treated at the primary center or whether they should be routed to the higher-level center. These arrangements were termed ‘spoke and wheel,’ ” Dr. Caplan told this publication.
However, not all patients who are candidates for transport to a secondary center are able to be transported. In such cases, primary centers can use telemedicine to collaborate with secondary centers for support.
Logistics aside, perhaps today’s greatest challenge for clinicians is ensuring their patients and families receive education to increase their awareness of stroke centers as an important option for treatment and outcome optimization. Many patients and their loved ones do not realize that these centers exist or how to utilize them if and when the time comes.
Right now, some cities have stroke ambulances staffed with physicians to treat patients in the field. This decentralized model helps address access burdens such as door-to-needle delays and transportation while improving survival and recovery. Dr. Caplan said these services are available in Munich, and in a few select U.S. cities such as Cleveland and Houston, which helped pioneer the concept.
Better access in the future?
Looking ahead, Dr. Caplan seems optimistic about how stroke management will continue to evolve. Many cities will have stroke ambulances to provide on-site care, while stroke institutions will improve their cross-collaborative efforts to support their patient populations.
At the crux of cross-collaboration lies enhanced communication between peripheral and urban hospitals.
“Peripheral and urban hospitals and state organizations will engage in smoother integration to figure out when to take patient to the bigger hospitals,” Dr. Caplan said. “I also believe we will see greater emphasis on rehabilitation and recovery.”
As promising as the future looks, only time will tell.
In 1993, managing patients with stroke had long remained an elusive and somewhat intimidating task for the neurological world. Previous efforts to treat the condition had produced more frustration than success, leaving clinicians and patients alike in despair for a solution. However, some successes in treating coronary thrombosis during that era rejuvenated researchers’ efforts to crack the code. An international team of researchers had studied a Streptococcus derivative (streptokinase) and others had begun to study a natural substance termed tissue plasminogen activator (tPA) as thrombolytic agents to lyse coronary clots and to treat pulmonary embolism. The adverse event of excessive bleeding found in Australian studies done on streptokinase intervention in patients with stroke prompted researchers to contemplate use of tPA in stroke management.
A group of German, Japanese, and American investigators began to research thrombolysis in acute stroke patients during the mid-1980s.
“What was unique is that patients had a CT scan followed by a catheter angiogram,” said Louis Caplan, MD, a senior member of the division of cerebrovascular disease at Beth Israel Deaconess Medical Center, Boston, professor of neurology at Harvard Medical School, Boston, and founder of the Harvard Stroke Registry at Beth Israel Deaconess Medical Center.
“If they had a blocked vessel, they got the drug, delivered either intravenously or intra-arterially.”
The process involved keeping the catheter open after drug administration to determine whether the vessel had opened or remained occluded. The researchers learned which blocked vessels opened when the drug was given intravenously and which required direct introduction of the drug into the clots.
A group of investigators in the United States funded by the National Institute of Neurological Disease and Stroke then performed a randomized therapeutic trial of intravenous tPA given within 90 minutes and 180 minutes after stroke symptom onset. The study was reported in the New England Journal of Medicine. Soon thereafter, in 1995, the Food and Drug Administration approved the use of tPA following the inclusion and exclusion rules used in the NINDS trial.
After the FDA approved tPA in 1995, stroke management was never the same.
tPA was just one factor in optimizing stroke management
Despite the major therapeutic breakthrough with tPA’s approval, it took the clinics, hospitals, and other acute care systems a while to catch up. “Neurologists and hospitals weren’t ready for acute stroke intervention and proper stroke management in the mid-90s,” Dr. Caplan recalled. “At the time, stroke wasn’t at the forefront of treatment, general neurologists weren’t trained, and there weren’t enough stroke neurologists.”
The preparation and training deficit was further exacerbated by low reimbursement for services. As a result, only about 5% of patients who were eligible for acute stroke management were treated with tPA.
According to Dr. Caplan, during the next 15-20 years, the accumulation of stroke data from MRI and CT vascular imaging clarified further which patients, with what extent of infarction, with which blocked vessels, would be good candidates for treatment.
More patients received interventional treatment using catheters directed into the area of clotting in attempt to remove the blockages. In addition, information regarding intervention at different periods (10-16 hours, up to 24 hours) and conditions (for example, patients with varying degrees of disability, infarct) were tested.
Eventually, hospitals became more attuned to emergency stroke treatment. More neurologists became trained, more stroke centers emerged, and clinicians enjoyed the benefit of technological advancements that allowed them to explore perfusion.
While decentralized care enhances outcomes in stroke management, more progress is needed
As of early 2023, stroke is one of the leading emergency diagnoses, and patients have access to primary and secondary stroke centers that are sprinkled throughout the United States. As impressive as the feat may seem, health care systems still have major strides to make to truly optimize therapy and outcomes in this patient population.
For example, location and access remain important issues. Secondary centers are typically located in large, metropolitan areas. While an urban location makes a primary center geographically more accessible to a larger patient population, traffic frequently hinders door-to-door access.
In the case of rural centers, distance can retard access, but they also face the challenges of how to route patients – especially patients who require more specialized care offered by secondary centers. Fortunately, primary centers have some ways to help better support their patients.
“One thing that happened is that primary centers made agreements with secondary centers via telemedicine to determine whether patients should be treated at the primary center or whether they should be routed to the higher-level center. These arrangements were termed ‘spoke and wheel,’ ” Dr. Caplan told this publication.
However, not all patients who are candidates for transport to a secondary center are able to be transported. In such cases, primary centers can use telemedicine to collaborate with secondary centers for support.
Logistics aside, perhaps today’s greatest challenge for clinicians is ensuring their patients and families receive education to increase their awareness of stroke centers as an important option for treatment and outcome optimization. Many patients and their loved ones do not realize that these centers exist or how to utilize them if and when the time comes.
Right now, some cities have stroke ambulances staffed with physicians to treat patients in the field. This decentralized model helps address access burdens such as door-to-needle delays and transportation while improving survival and recovery. Dr. Caplan said these services are available in Munich, and in a few select U.S. cities such as Cleveland and Houston, which helped pioneer the concept.
Better access in the future?
Looking ahead, Dr. Caplan seems optimistic about how stroke management will continue to evolve. Many cities will have stroke ambulances to provide on-site care, while stroke institutions will improve their cross-collaborative efforts to support their patient populations.
At the crux of cross-collaboration lies enhanced communication between peripheral and urban hospitals.
“Peripheral and urban hospitals and state organizations will engage in smoother integration to figure out when to take patient to the bigger hospitals,” Dr. Caplan said. “I also believe we will see greater emphasis on rehabilitation and recovery.”
As promising as the future looks, only time will tell.
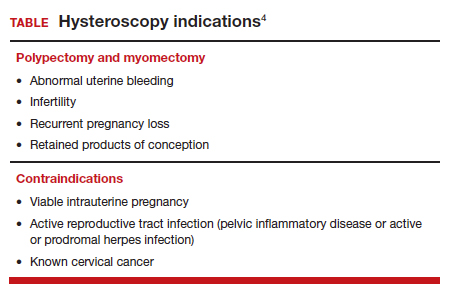
Should every scheduled cesarean birth use an Enhanced Recovery after Surgery (ERAS) pathway?

Cesarean birth is one of the most common major surgical procedures performed in developed countries1 with over 1,170,000 cesarean births in the United States in 2021.2 Many surgeons and anesthesiologists believe that Enhanced Recovery after Surgery (ERAS) pathways improve surgical outcomes.3,4 Important goals of ERAS include setting patient expectations for the surgical procedure, accelerating patient recovery to full function, and minimizing perioperative complications such as severe nausea, aspiration, surgical site infection, wound complications, and perioperative anemia. The ERAS Society in 20185-7 and the Society for Obstetric Anesthesia and Perinatology (SOAP) in 20218 proposed ERAS pathways for cesarean birth. Both societies recommended that obstetric units consider adopting an ERAS pathway compatible with local clinical resources. In addition, the American College of Obstetricians and Gynecologists (ACOG) has provided guidance for implementing ERAS pathways for gynecologic surgery.9 The consistent use of standardized protocols to improve surgical care in obstetrics should lead to a reduction in care variation and improve health equity outcomes.
The clinical interventions recommended for ERAS cesarean birth occur sequentially in the preoperative, intraoperative, and postoperative phases of care. The recommendations associated with each of these phases are reviewed below. It is important to note that each obstetric unit should use a multidisciplinary process to develop an ERAS pathway that best supports local practice given clinician preferences, patient characteristics, and resource availability.
Preoperative components of ERAS
Standardized patient education (SPE). SPE is an important component of ERAS, although evidence to support the recommendation is limited. At a minimum a written handout describing steps in the cesarean birth process, or a patient-education video should be part of patient education. The University of Michigan Medical Center has produced a 3-minute video for patients explaining ERAS cesarean birth.10 The University of Maryland Medical Center has produced a 2.5-minute video in English and Spanish, explaining ERAS cesarean birth for patients.11 Some surgeons place a telephone call to patients the evening before surgery to help orient the patient to ERAS cesarean birth.
Breastfeeding education. An important goal of obstetric care is to optimize the rate of exclusive breastfeeding at birth. Breastfeeding education, including a commitment to support the initiation of breastfeeding within 1 hour of birth, may enhance the rate of exclusive breastfeeding. There are numerous videos available for patients about breastfeeding after cesarean birth (as an example, see: https://www.youtube.com/watch?v=9iOGn85NdTg).
Limit fasting. In the past, surgical guidelines recommended fasting after midnight prior to surgery. The ERAS Society recommends that patients should be encouraged to drink clear fluids up to 2 hours before surgery and may have a light meal up to 6 hours before surgery (Part 1).
Carbohydrate loading. Surgery causes a metabolic stress that is increased by fasting. Carbohydrate loading prior to surgery reduces the magnitude of the catabolic state caused by the combination of surgery and fasting.12 SOAP and the ERAS Society recommend oral carbohydrate fluid supplementation 2 hours before surgery for nondiabetic patients. SOAP suggests 32 oz of Gatorade or 16 oz of clear apple juice as options for carbohydrate loading. For diabetic patients, the carbohydrate load can be omitted. In fasting pregnant patients at term, gastric emptying was near complete 2 hours after consumption of 400 mL of a carbohydrate drink.13 In one study, consumption of 400 mL of a carbohydrate drink 2 hours before cesarean resulted in a 7% increase in the newborn blood glucose level at 20 min after delivery.14
Minimize preoperative anemia. Approximately 50% of pregnant women are iron deficient and approximately 10% are anemic in the third trimester.15,16 Cesarean birth is associated with significant blood loss necessitating the need to optimize red blood cell mass before surgery. Measuring ferritin to identify patients with iron deficiency and aggressive iron replacement, including intravenous iron if necessary, will reduce the prevalence of anemia prior to cesarean birth.17 Another cause of anemia in pregnancy is vitamin B12 (cobalamin) deficiency. Low vitamin B12 is especially common in pregnant patients who have previously had bariatric surgery. One study reported that, of 113 pregnant patients who were, on average, 3 years from a bariatric surgery procedure, 12% had vitamin B12 circulating levels < 130 pg/mL.18 Among pregnant patients who are anemic, and do not have a hemoglobinopathy, measuring ferritin, folic acid, and vitamin B12 will help identify the cause of anemia and guide treatment.19
Optimize preoperative physical condition. Improving healthy behaviors and reducing unhealthy behaviors preoperatively may enhance patient recovery to full function. In the weeks before scheduled cesarean birth, cessation of the use of tobacco products, optimizing activity and improving diet quality, including increasing protein intake, may best prepare patients for the metabolic stress of surgery.
Continue to: Intraoperative components of ERAS...
Intraoperative components of ERAS
Reduce the risk of surgical site infection (SSI) and wound complications. Bundles that include antibiotics, chlorhexidine (or an alternative antibacterial soap) and clippers have been shown to reduce SSI.20 Routine administration of preoperative antibiotics is a consensus recommendation and there is high adherence with this recommendation in the United States. Chlorhexidine-alcohol is the preferred solution for skin preparation. Vaginal preparation with povidine-iodine or chlorhexidine may be considered.6
Surgical technique. Blunt extension of a transverse hysterotomy may reduce blood loss. Closure of the hysterotomy incision in 2 layers is recommended to reduce uterine scar dehiscence in a subsequent pregnancy. If the patient has ≥2 cm of subcutaneous tissue, this layer should be approximated with sutures. Skin closure should be with subcuticular suture.6
Optimize uterotonic administration. Routine use of uterotonics reduces the risk of blood loss, transfusion, and postoperative anemia. There is high adherence with the use of uterotonic administration after birth in the United States.6,8
Ensure normothermia. Many patients become hypothermic during a cesarean birth. Active warming of the patient with an in-line IV fluid warmer and forced air warming over the patient’s body can reduce the risk of hypothermia.8
Initiate multimodal anesthesia. Anesthesiologists often use intrathecal or epidural morphine to enhance analgesia. Ketorolac administration prior to completion of the cesarean procedure and perioperative administration of acetaminophen may reduce postoperative pain.8 The use of preoperative antiemetics will reduce intraoperative and postoperative nausea and vomiting.
Initiate VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.6
Postoperative components of ERAS
Patient education to prepare for discharge home when ready. Patient education focused on home when ready is important in preparing the patient for discharge home.7 Completion of required newborn testing, lactation education, and contraception planning plus coordination of newborn pediatric follow-up is necessary before discharge.
Support early return of bowel function. Early return of bowel function is best supported by a multimodal approach including initiation of clear fluid intake immediately following surgery, encouraging consumption of a regular diet within 27 to 4 hours8 following surgery. Gum chewing for at least 5 minutes 3 times daily accelerates return of bowel function.8 In a meta-analysis of 10 randomized studies examining the effect of gum chewing after cesarean, the investigators reported that gum chewing shortened the time to passage of flatus and defecation.21
Early ambulation.
Sequentially advanced activity, starting with sitting on the edge of the bed, sitting in a chair, and ambulation within 8 hours of surgery, is recommended to facilitate faster recovery, reduce rates of complications, and enable transition to home.8
Early removal of the urinary catheter. It is recommended that the urinary catheter be removed within 12 hours after cesarean birth.8 Early removal of the urinary catheter increases patient mobility and reduces the length of hospitalization. Early removal of the urinary catheter may be associated with postoperative urinary retention and recatheterization in a small number of patients.
Prescribe routinely scheduled acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) and ketorolac. A key component of ERAS cesarean birth is the standardized administration of nonopioid pain medicines, alternating doses of acetaminophen and an NSAID. ERAS cesarean birth is likely to result in a reduction in inpatient and postdischarge opioid use.22-24
VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.8
Auditing and reporting adherence with components of ERAS
In clinical practice there may be a gap between a clinician’s subjective perception of their performance and an independent audit of their clinical performance. ERAS pathways should be implemented with a commitment to performing audits and providing quantitative feedback to clinicians. Consistent use of measurement, feedback, and coaching can improve performance and reduce variation among individual clinicians. As an example, in one study of the use of a surgical safety checklist, 99% of the surgeons reported that they routinely used a surgical safety checklist, but the audit showed that the checklist was used in only 60% of cases.25 Gaps between self-reported performance and audited performance are common in clinical practice. Audits with feedback are critical to improving adherence with the components of an ERAS pathway.
Three independent systematic reviews and meta-analyses report that ERAS pathways reduce hospital length of stay without increasing the readmission rate.26-28 One meta-analysis reported that ERAS may also reduce time to first mobilization and result in earlier removal of the urinary catheter.26 ERAS pathways also may reduce postoperative complications, lower pain scores, and decrease opioid use.27 The general consensus among quality and safety experts is that reducing variation through standardization of pathways is generally associated with improved quality and enhanced safety. ERAS pathways have been widely accepted in multiple surgical fields. ERAS pathways should become the standard for performing cesarean procedures.●
1. Molina G, Weiser RG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314:2263-2270.
2. Hamilton BE, Martin JA, Osterman MJK. Births: provisional data for 2021. Vital Statistics Release; No. 20. Hyattsville, MD: National Center for Health Statistics. May 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf.
3. Berian JR, Ban KA, Liu JB, et al. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019;486-493.
4. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-298.
5. Wilson RD, Caughey AB, Wood SL, et al. Guidelines for antenatal and preoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 1). Am J Obstet Gynecol. 2018;219:523.e1-523.e15.
6. Caughey AB, Wood SL, Macones GA, et al Guidelines for intraoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 2). Am J Obstet Gynecol. 2018;219:533-544.
7. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 3). Am J Obstet Gynecol. 2019;221:247.e1-247.e9.
8. Bollag L, Lim G, Sultan P, et al. Society for Obstetric Anesthesia and Perinatology: Consensus statement and recommendations for enhanced recovery after cesarean. Anesth Analg. 2021;132:1362-1377.
9. Perioperative pathways: enhanced recovery after surgery. ACOG Committee Opinion No 750. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e120-130.
10. University of Michigan. ERAS: A patient education video. https://www.youtube.com/watch?v=CoFtgdluBc0. Accessed October 24, 2022.
11. University of Maryland. ERAS. https://www.umms.org/ummc/health-services/womens-health/ostetrics-gynecology/pregnancy-childbirth/labor-delivery/enhanced-recovery-after-cesarean. Accessed October 24, 2022.
12. Bilku DK, Dennison AR, Hall TC, et al. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
13. Popivanov P, Irwin R, Walsh M, et al. Gastric emptying of carbohydrate drinks in term parturients before elective caesarean surgery: an observational study. Int J Obstet Anesth. 2020;41:29-34.
14. He Y, Liu C, Han Y, et al. The impact of carbohydrate-rich supplement taken two hours before caesarean delivery on maternal and neonatal perioperative outcomes- a randomized clinical trial. BMC Pregnancy Childbirth. 2021;21:682.
15. Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
16. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
17. Nour N, Barbieri RL. Optimize detection and treatment of iron deficiency in pregnancy. OBG Manag. 2022;34:9-11.
18. Mead NC, Sakkatos P, Sakellaropoulos GC, et al. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis. 2014;10:1166-1173.
19. Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin and folate. Blood. 2017;129:940-949.
20. Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:735-746.
21. Wen Z, Shen M, Wu C, et al. Chewing gum for intestinal function recovery after caesarean section: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:105.
22. McCoy JA, Gutman S, Hamm RF, et al. The association between implementation of an enhanced recovery after cesarean pathway with standardized discharge prescriptions and opioid use and pain experience after cesarean delivery. Am J Perinatol. 2021;38:1341-1347.
23. Mullman L, Hilden P, Goral J, et al. Improved outcomes with an enhanced recovery approach to cesarean delivery. Obstet Gynecol. 2020;136:685-691.
24. Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
25. Sendlhofer G, Lumenta DB, Leitgeb K, et al. The gap between individual perception and compliance: a quantitative follow-up study of the surgical safety checklist application. PLoS One. 2016;11:e0149212.
26. Sultan P, Sharawi N, Blake L, et al. Impact of enhanced recovery after cesarean delivery on maternal outcomes: a systematic review and meta-analysis. Anaesth Crit Care Pain Med. 2021;40:100935.
27. Meng X, Chen K, Yang C, et al. The clinical efficacy and safety of enhanced recovery after surgery for cesarean section: a systematic review and meta-analysis of randomized controlled trials and observational studies. Front Med. 2021;8:694385.
28. Corson E, Hind D, Beever D, et al. Enhanced recovery after elective caesarean: a rapid review of clinical protocols and an umbrella review of systematic reviews. BMC Pregnancy Childbirth. 2017;17:91.

Cesarean birth is one of the most common major surgical procedures performed in developed countries1 with over 1,170,000 cesarean births in the United States in 2021.2 Many surgeons and anesthesiologists believe that Enhanced Recovery after Surgery (ERAS) pathways improve surgical outcomes.3,4 Important goals of ERAS include setting patient expectations for the surgical procedure, accelerating patient recovery to full function, and minimizing perioperative complications such as severe nausea, aspiration, surgical site infection, wound complications, and perioperative anemia. The ERAS Society in 20185-7 and the Society for Obstetric Anesthesia and Perinatology (SOAP) in 20218 proposed ERAS pathways for cesarean birth. Both societies recommended that obstetric units consider adopting an ERAS pathway compatible with local clinical resources. In addition, the American College of Obstetricians and Gynecologists (ACOG) has provided guidance for implementing ERAS pathways for gynecologic surgery.9 The consistent use of standardized protocols to improve surgical care in obstetrics should lead to a reduction in care variation and improve health equity outcomes.
The clinical interventions recommended for ERAS cesarean birth occur sequentially in the preoperative, intraoperative, and postoperative phases of care. The recommendations associated with each of these phases are reviewed below. It is important to note that each obstetric unit should use a multidisciplinary process to develop an ERAS pathway that best supports local practice given clinician preferences, patient characteristics, and resource availability.
Preoperative components of ERAS
Standardized patient education (SPE). SPE is an important component of ERAS, although evidence to support the recommendation is limited. At a minimum a written handout describing steps in the cesarean birth process, or a patient-education video should be part of patient education. The University of Michigan Medical Center has produced a 3-minute video for patients explaining ERAS cesarean birth.10 The University of Maryland Medical Center has produced a 2.5-minute video in English and Spanish, explaining ERAS cesarean birth for patients.11 Some surgeons place a telephone call to patients the evening before surgery to help orient the patient to ERAS cesarean birth.
Breastfeeding education. An important goal of obstetric care is to optimize the rate of exclusive breastfeeding at birth. Breastfeeding education, including a commitment to support the initiation of breastfeeding within 1 hour of birth, may enhance the rate of exclusive breastfeeding. There are numerous videos available for patients about breastfeeding after cesarean birth (as an example, see: https://www.youtube.com/watch?v=9iOGn85NdTg).
Limit fasting. In the past, surgical guidelines recommended fasting after midnight prior to surgery. The ERAS Society recommends that patients should be encouraged to drink clear fluids up to 2 hours before surgery and may have a light meal up to 6 hours before surgery (Part 1).
Carbohydrate loading. Surgery causes a metabolic stress that is increased by fasting. Carbohydrate loading prior to surgery reduces the magnitude of the catabolic state caused by the combination of surgery and fasting.12 SOAP and the ERAS Society recommend oral carbohydrate fluid supplementation 2 hours before surgery for nondiabetic patients. SOAP suggests 32 oz of Gatorade or 16 oz of clear apple juice as options for carbohydrate loading. For diabetic patients, the carbohydrate load can be omitted. In fasting pregnant patients at term, gastric emptying was near complete 2 hours after consumption of 400 mL of a carbohydrate drink.13 In one study, consumption of 400 mL of a carbohydrate drink 2 hours before cesarean resulted in a 7% increase in the newborn blood glucose level at 20 min after delivery.14
Minimize preoperative anemia. Approximately 50% of pregnant women are iron deficient and approximately 10% are anemic in the third trimester.15,16 Cesarean birth is associated with significant blood loss necessitating the need to optimize red blood cell mass before surgery. Measuring ferritin to identify patients with iron deficiency and aggressive iron replacement, including intravenous iron if necessary, will reduce the prevalence of anemia prior to cesarean birth.17 Another cause of anemia in pregnancy is vitamin B12 (cobalamin) deficiency. Low vitamin B12 is especially common in pregnant patients who have previously had bariatric surgery. One study reported that, of 113 pregnant patients who were, on average, 3 years from a bariatric surgery procedure, 12% had vitamin B12 circulating levels < 130 pg/mL.18 Among pregnant patients who are anemic, and do not have a hemoglobinopathy, measuring ferritin, folic acid, and vitamin B12 will help identify the cause of anemia and guide treatment.19
Optimize preoperative physical condition. Improving healthy behaviors and reducing unhealthy behaviors preoperatively may enhance patient recovery to full function. In the weeks before scheduled cesarean birth, cessation of the use of tobacco products, optimizing activity and improving diet quality, including increasing protein intake, may best prepare patients for the metabolic stress of surgery.
Continue to: Intraoperative components of ERAS...
Intraoperative components of ERAS
Reduce the risk of surgical site infection (SSI) and wound complications. Bundles that include antibiotics, chlorhexidine (or an alternative antibacterial soap) and clippers have been shown to reduce SSI.20 Routine administration of preoperative antibiotics is a consensus recommendation and there is high adherence with this recommendation in the United States. Chlorhexidine-alcohol is the preferred solution for skin preparation. Vaginal preparation with povidine-iodine or chlorhexidine may be considered.6
Surgical technique. Blunt extension of a transverse hysterotomy may reduce blood loss. Closure of the hysterotomy incision in 2 layers is recommended to reduce uterine scar dehiscence in a subsequent pregnancy. If the patient has ≥2 cm of subcutaneous tissue, this layer should be approximated with sutures. Skin closure should be with subcuticular suture.6
Optimize uterotonic administration. Routine use of uterotonics reduces the risk of blood loss, transfusion, and postoperative anemia. There is high adherence with the use of uterotonic administration after birth in the United States.6,8
Ensure normothermia. Many patients become hypothermic during a cesarean birth. Active warming of the patient with an in-line IV fluid warmer and forced air warming over the patient’s body can reduce the risk of hypothermia.8
Initiate multimodal anesthesia. Anesthesiologists often use intrathecal or epidural morphine to enhance analgesia. Ketorolac administration prior to completion of the cesarean procedure and perioperative administration of acetaminophen may reduce postoperative pain.8 The use of preoperative antiemetics will reduce intraoperative and postoperative nausea and vomiting.
Initiate VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.6
Postoperative components of ERAS
Patient education to prepare for discharge home when ready. Patient education focused on home when ready is important in preparing the patient for discharge home.7 Completion of required newborn testing, lactation education, and contraception planning plus coordination of newborn pediatric follow-up is necessary before discharge.
Support early return of bowel function. Early return of bowel function is best supported by a multimodal approach including initiation of clear fluid intake immediately following surgery, encouraging consumption of a regular diet within 27 to 4 hours8 following surgery. Gum chewing for at least 5 minutes 3 times daily accelerates return of bowel function.8 In a meta-analysis of 10 randomized studies examining the effect of gum chewing after cesarean, the investigators reported that gum chewing shortened the time to passage of flatus and defecation.21
Early ambulation.
Sequentially advanced activity, starting with sitting on the edge of the bed, sitting in a chair, and ambulation within 8 hours of surgery, is recommended to facilitate faster recovery, reduce rates of complications, and enable transition to home.8
Early removal of the urinary catheter. It is recommended that the urinary catheter be removed within 12 hours after cesarean birth.8 Early removal of the urinary catheter increases patient mobility and reduces the length of hospitalization. Early removal of the urinary catheter may be associated with postoperative urinary retention and recatheterization in a small number of patients.
Prescribe routinely scheduled acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) and ketorolac. A key component of ERAS cesarean birth is the standardized administration of nonopioid pain medicines, alternating doses of acetaminophen and an NSAID. ERAS cesarean birth is likely to result in a reduction in inpatient and postdischarge opioid use.22-24
VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.8
Auditing and reporting adherence with components of ERAS
In clinical practice there may be a gap between a clinician’s subjective perception of their performance and an independent audit of their clinical performance. ERAS pathways should be implemented with a commitment to performing audits and providing quantitative feedback to clinicians. Consistent use of measurement, feedback, and coaching can improve performance and reduce variation among individual clinicians. As an example, in one study of the use of a surgical safety checklist, 99% of the surgeons reported that they routinely used a surgical safety checklist, but the audit showed that the checklist was used in only 60% of cases.25 Gaps between self-reported performance and audited performance are common in clinical practice. Audits with feedback are critical to improving adherence with the components of an ERAS pathway.
Three independent systematic reviews and meta-analyses report that ERAS pathways reduce hospital length of stay without increasing the readmission rate.26-28 One meta-analysis reported that ERAS may also reduce time to first mobilization and result in earlier removal of the urinary catheter.26 ERAS pathways also may reduce postoperative complications, lower pain scores, and decrease opioid use.27 The general consensus among quality and safety experts is that reducing variation through standardization of pathways is generally associated with improved quality and enhanced safety. ERAS pathways have been widely accepted in multiple surgical fields. ERAS pathways should become the standard for performing cesarean procedures.●

Cesarean birth is one of the most common major surgical procedures performed in developed countries1 with over 1,170,000 cesarean births in the United States in 2021.2 Many surgeons and anesthesiologists believe that Enhanced Recovery after Surgery (ERAS) pathways improve surgical outcomes.3,4 Important goals of ERAS include setting patient expectations for the surgical procedure, accelerating patient recovery to full function, and minimizing perioperative complications such as severe nausea, aspiration, surgical site infection, wound complications, and perioperative anemia. The ERAS Society in 20185-7 and the Society for Obstetric Anesthesia and Perinatology (SOAP) in 20218 proposed ERAS pathways for cesarean birth. Both societies recommended that obstetric units consider adopting an ERAS pathway compatible with local clinical resources. In addition, the American College of Obstetricians and Gynecologists (ACOG) has provided guidance for implementing ERAS pathways for gynecologic surgery.9 The consistent use of standardized protocols to improve surgical care in obstetrics should lead to a reduction in care variation and improve health equity outcomes.
The clinical interventions recommended for ERAS cesarean birth occur sequentially in the preoperative, intraoperative, and postoperative phases of care. The recommendations associated with each of these phases are reviewed below. It is important to note that each obstetric unit should use a multidisciplinary process to develop an ERAS pathway that best supports local practice given clinician preferences, patient characteristics, and resource availability.
Preoperative components of ERAS
Standardized patient education (SPE). SPE is an important component of ERAS, although evidence to support the recommendation is limited. At a minimum a written handout describing steps in the cesarean birth process, or a patient-education video should be part of patient education. The University of Michigan Medical Center has produced a 3-minute video for patients explaining ERAS cesarean birth.10 The University of Maryland Medical Center has produced a 2.5-minute video in English and Spanish, explaining ERAS cesarean birth for patients.11 Some surgeons place a telephone call to patients the evening before surgery to help orient the patient to ERAS cesarean birth.
Breastfeeding education. An important goal of obstetric care is to optimize the rate of exclusive breastfeeding at birth. Breastfeeding education, including a commitment to support the initiation of breastfeeding within 1 hour of birth, may enhance the rate of exclusive breastfeeding. There are numerous videos available for patients about breastfeeding after cesarean birth (as an example, see: https://www.youtube.com/watch?v=9iOGn85NdTg).
Limit fasting. In the past, surgical guidelines recommended fasting after midnight prior to surgery. The ERAS Society recommends that patients should be encouraged to drink clear fluids up to 2 hours before surgery and may have a light meal up to 6 hours before surgery (Part 1).
Carbohydrate loading. Surgery causes a metabolic stress that is increased by fasting. Carbohydrate loading prior to surgery reduces the magnitude of the catabolic state caused by the combination of surgery and fasting.12 SOAP and the ERAS Society recommend oral carbohydrate fluid supplementation 2 hours before surgery for nondiabetic patients. SOAP suggests 32 oz of Gatorade or 16 oz of clear apple juice as options for carbohydrate loading. For diabetic patients, the carbohydrate load can be omitted. In fasting pregnant patients at term, gastric emptying was near complete 2 hours after consumption of 400 mL of a carbohydrate drink.13 In one study, consumption of 400 mL of a carbohydrate drink 2 hours before cesarean resulted in a 7% increase in the newborn blood glucose level at 20 min after delivery.14
Minimize preoperative anemia. Approximately 50% of pregnant women are iron deficient and approximately 10% are anemic in the third trimester.15,16 Cesarean birth is associated with significant blood loss necessitating the need to optimize red blood cell mass before surgery. Measuring ferritin to identify patients with iron deficiency and aggressive iron replacement, including intravenous iron if necessary, will reduce the prevalence of anemia prior to cesarean birth.17 Another cause of anemia in pregnancy is vitamin B12 (cobalamin) deficiency. Low vitamin B12 is especially common in pregnant patients who have previously had bariatric surgery. One study reported that, of 113 pregnant patients who were, on average, 3 years from a bariatric surgery procedure, 12% had vitamin B12 circulating levels < 130 pg/mL.18 Among pregnant patients who are anemic, and do not have a hemoglobinopathy, measuring ferritin, folic acid, and vitamin B12 will help identify the cause of anemia and guide treatment.19
Optimize preoperative physical condition. Improving healthy behaviors and reducing unhealthy behaviors preoperatively may enhance patient recovery to full function. In the weeks before scheduled cesarean birth, cessation of the use of tobacco products, optimizing activity and improving diet quality, including increasing protein intake, may best prepare patients for the metabolic stress of surgery.
Continue to: Intraoperative components of ERAS...
Intraoperative components of ERAS
Reduce the risk of surgical site infection (SSI) and wound complications. Bundles that include antibiotics, chlorhexidine (or an alternative antibacterial soap) and clippers have been shown to reduce SSI.20 Routine administration of preoperative antibiotics is a consensus recommendation and there is high adherence with this recommendation in the United States. Chlorhexidine-alcohol is the preferred solution for skin preparation. Vaginal preparation with povidine-iodine or chlorhexidine may be considered.6
Surgical technique. Blunt extension of a transverse hysterotomy may reduce blood loss. Closure of the hysterotomy incision in 2 layers is recommended to reduce uterine scar dehiscence in a subsequent pregnancy. If the patient has ≥2 cm of subcutaneous tissue, this layer should be approximated with sutures. Skin closure should be with subcuticular suture.6
Optimize uterotonic administration. Routine use of uterotonics reduces the risk of blood loss, transfusion, and postoperative anemia. There is high adherence with the use of uterotonic administration after birth in the United States.6,8
Ensure normothermia. Many patients become hypothermic during a cesarean birth. Active warming of the patient with an in-line IV fluid warmer and forced air warming over the patient’s body can reduce the risk of hypothermia.8
Initiate multimodal anesthesia. Anesthesiologists often use intrathecal or epidural morphine to enhance analgesia. Ketorolac administration prior to completion of the cesarean procedure and perioperative administration of acetaminophen may reduce postoperative pain.8 The use of preoperative antiemetics will reduce intraoperative and postoperative nausea and vomiting.
Initiate VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.6
Postoperative components of ERAS
Patient education to prepare for discharge home when ready. Patient education focused on home when ready is important in preparing the patient for discharge home.7 Completion of required newborn testing, lactation education, and contraception planning plus coordination of newborn pediatric follow-up is necessary before discharge.
Support early return of bowel function. Early return of bowel function is best supported by a multimodal approach including initiation of clear fluid intake immediately following surgery, encouraging consumption of a regular diet within 27 to 4 hours8 following surgery. Gum chewing for at least 5 minutes 3 times daily accelerates return of bowel function.8 In a meta-analysis of 10 randomized studies examining the effect of gum chewing after cesarean, the investigators reported that gum chewing shortened the time to passage of flatus and defecation.21
Early ambulation.
Sequentially advanced activity, starting with sitting on the edge of the bed, sitting in a chair, and ambulation within 8 hours of surgery, is recommended to facilitate faster recovery, reduce rates of complications, and enable transition to home.8
Early removal of the urinary catheter. It is recommended that the urinary catheter be removed within 12 hours after cesarean birth.8 Early removal of the urinary catheter increases patient mobility and reduces the length of hospitalization. Early removal of the urinary catheter may be associated with postoperative urinary retention and recatheterization in a small number of patients.
Prescribe routinely scheduled acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs) and ketorolac. A key component of ERAS cesarean birth is the standardized administration of nonopioid pain medicines, alternating doses of acetaminophen and an NSAID. ERAS cesarean birth is likely to result in a reduction in inpatient and postdischarge opioid use.22-24
VTE prophylaxis. Pneumatic compression stockings are recommended. Anticoagulation should not be routinely used for VTE prophylaxis.8
Auditing and reporting adherence with components of ERAS
In clinical practice there may be a gap between a clinician’s subjective perception of their performance and an independent audit of their clinical performance. ERAS pathways should be implemented with a commitment to performing audits and providing quantitative feedback to clinicians. Consistent use of measurement, feedback, and coaching can improve performance and reduce variation among individual clinicians. As an example, in one study of the use of a surgical safety checklist, 99% of the surgeons reported that they routinely used a surgical safety checklist, but the audit showed that the checklist was used in only 60% of cases.25 Gaps between self-reported performance and audited performance are common in clinical practice. Audits with feedback are critical to improving adherence with the components of an ERAS pathway.
Three independent systematic reviews and meta-analyses report that ERAS pathways reduce hospital length of stay without increasing the readmission rate.26-28 One meta-analysis reported that ERAS may also reduce time to first mobilization and result in earlier removal of the urinary catheter.26 ERAS pathways also may reduce postoperative complications, lower pain scores, and decrease opioid use.27 The general consensus among quality and safety experts is that reducing variation through standardization of pathways is generally associated with improved quality and enhanced safety. ERAS pathways have been widely accepted in multiple surgical fields. ERAS pathways should become the standard for performing cesarean procedures.●
1. Molina G, Weiser RG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314:2263-2270.
2. Hamilton BE, Martin JA, Osterman MJK. Births: provisional data for 2021. Vital Statistics Release; No. 20. Hyattsville, MD: National Center for Health Statistics. May 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf.
3. Berian JR, Ban KA, Liu JB, et al. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019;486-493.
4. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-298.
5. Wilson RD, Caughey AB, Wood SL, et al. Guidelines for antenatal and preoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 1). Am J Obstet Gynecol. 2018;219:523.e1-523.e15.
6. Caughey AB, Wood SL, Macones GA, et al Guidelines for intraoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 2). Am J Obstet Gynecol. 2018;219:533-544.
7. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 3). Am J Obstet Gynecol. 2019;221:247.e1-247.e9.
8. Bollag L, Lim G, Sultan P, et al. Society for Obstetric Anesthesia and Perinatology: Consensus statement and recommendations for enhanced recovery after cesarean. Anesth Analg. 2021;132:1362-1377.
9. Perioperative pathways: enhanced recovery after surgery. ACOG Committee Opinion No 750. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e120-130.
10. University of Michigan. ERAS: A patient education video. https://www.youtube.com/watch?v=CoFtgdluBc0. Accessed October 24, 2022.
11. University of Maryland. ERAS. https://www.umms.org/ummc/health-services/womens-health/ostetrics-gynecology/pregnancy-childbirth/labor-delivery/enhanced-recovery-after-cesarean. Accessed October 24, 2022.
12. Bilku DK, Dennison AR, Hall TC, et al. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
13. Popivanov P, Irwin R, Walsh M, et al. Gastric emptying of carbohydrate drinks in term parturients before elective caesarean surgery: an observational study. Int J Obstet Anesth. 2020;41:29-34.
14. He Y, Liu C, Han Y, et al. The impact of carbohydrate-rich supplement taken two hours before caesarean delivery on maternal and neonatal perioperative outcomes- a randomized clinical trial. BMC Pregnancy Childbirth. 2021;21:682.
15. Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
16. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
17. Nour N, Barbieri RL. Optimize detection and treatment of iron deficiency in pregnancy. OBG Manag. 2022;34:9-11.
18. Mead NC, Sakkatos P, Sakellaropoulos GC, et al. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis. 2014;10:1166-1173.
19. Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin and folate. Blood. 2017;129:940-949.
20. Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:735-746.
21. Wen Z, Shen M, Wu C, et al. Chewing gum for intestinal function recovery after caesarean section: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:105.
22. McCoy JA, Gutman S, Hamm RF, et al. The association between implementation of an enhanced recovery after cesarean pathway with standardized discharge prescriptions and opioid use and pain experience after cesarean delivery. Am J Perinatol. 2021;38:1341-1347.
23. Mullman L, Hilden P, Goral J, et al. Improved outcomes with an enhanced recovery approach to cesarean delivery. Obstet Gynecol. 2020;136:685-691.
24. Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
25. Sendlhofer G, Lumenta DB, Leitgeb K, et al. The gap between individual perception and compliance: a quantitative follow-up study of the surgical safety checklist application. PLoS One. 2016;11:e0149212.
26. Sultan P, Sharawi N, Blake L, et al. Impact of enhanced recovery after cesarean delivery on maternal outcomes: a systematic review and meta-analysis. Anaesth Crit Care Pain Med. 2021;40:100935.
27. Meng X, Chen K, Yang C, et al. The clinical efficacy and safety of enhanced recovery after surgery for cesarean section: a systematic review and meta-analysis of randomized controlled trials and observational studies. Front Med. 2021;8:694385.
28. Corson E, Hind D, Beever D, et al. Enhanced recovery after elective caesarean: a rapid review of clinical protocols and an umbrella review of systematic reviews. BMC Pregnancy Childbirth. 2017;17:91.
1. Molina G, Weiser RG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314:2263-2270.
2. Hamilton BE, Martin JA, Osterman MJK. Births: provisional data for 2021. Vital Statistics Release; No. 20. Hyattsville, MD: National Center for Health Statistics. May 2022. https://www.cdc.gov/nchs/data/vsrr/vsrr020.pdf.
3. Berian JR, Ban KA, Liu JB, et al. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019;486-493.
4. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292-298.
5. Wilson RD, Caughey AB, Wood SL, et al. Guidelines for antenatal and preoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 1). Am J Obstet Gynecol. 2018;219:523.e1-523.e15.
6. Caughey AB, Wood SL, Macones GA, et al Guidelines for intraoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 2). Am J Obstet Gynecol. 2018;219:533-544.
7. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery after Surgery Society recommendations (Part 3). Am J Obstet Gynecol. 2019;221:247.e1-247.e9.
8. Bollag L, Lim G, Sultan P, et al. Society for Obstetric Anesthesia and Perinatology: Consensus statement and recommendations for enhanced recovery after cesarean. Anesth Analg. 2021;132:1362-1377.
9. Perioperative pathways: enhanced recovery after surgery. ACOG Committee Opinion No 750. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e120-130.
10. University of Michigan. ERAS: A patient education video. https://www.youtube.com/watch?v=CoFtgdluBc0. Accessed October 24, 2022.
11. University of Maryland. ERAS. https://www.umms.org/ummc/health-services/womens-health/ostetrics-gynecology/pregnancy-childbirth/labor-delivery/enhanced-recovery-after-cesarean. Accessed October 24, 2022.
12. Bilku DK, Dennison AR, Hall TC, et al. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96:15-22.
13. Popivanov P, Irwin R, Walsh M, et al. Gastric emptying of carbohydrate drinks in term parturients before elective caesarean surgery: an observational study. Int J Obstet Anesth. 2020;41:29-34.
14. He Y, Liu C, Han Y, et al. The impact of carbohydrate-rich supplement taken two hours before caesarean delivery on maternal and neonatal perioperative outcomes- a randomized clinical trial. BMC Pregnancy Childbirth. 2021;21:682.
15. Auerbach M, Abernathy J, Juul S, et al. Prevalence of iron deficiency in first trimester, nonanemic pregnant women. J Matern Fetal Neonatal Med. 2021;34:1002-1005.
16. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1996-2006. Am J Clin Nutr. 2011;93:1312-1320.
17. Nour N, Barbieri RL. Optimize detection and treatment of iron deficiency in pregnancy. OBG Manag. 2022;34:9-11.
18. Mead NC, Sakkatos P, Sakellaropoulos GC, et al. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis. 2014;10:1166-1173.
19. Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin and folate. Blood. 2017;129:940-949.
20. Carter EB, Temming LA, Fowler S, et al. Evidence-based bundles and cesarean delivery surgical site infections: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:735-746.
21. Wen Z, Shen M, Wu C, et al. Chewing gum for intestinal function recovery after caesarean section: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2017;17:105.
22. McCoy JA, Gutman S, Hamm RF, et al. The association between implementation of an enhanced recovery after cesarean pathway with standardized discharge prescriptions and opioid use and pain experience after cesarean delivery. Am J Perinatol. 2021;38:1341-1347.
23. Mullman L, Hilden P, Goral J, et al. Improved outcomes with an enhanced recovery approach to cesarean delivery. Obstet Gynecol. 2020;136:685-691.
24. Hedderson M, Lee D, Hunt E, et al. Enhanced recovery after surgery to change process measures and reduce opioid use after cesarean delivery: a quality improvement initiative. Obstet Gynecol. 2019;134:511-519.
25. Sendlhofer G, Lumenta DB, Leitgeb K, et al. The gap between individual perception and compliance: a quantitative follow-up study of the surgical safety checklist application. PLoS One. 2016;11:e0149212.
26. Sultan P, Sharawi N, Blake L, et al. Impact of enhanced recovery after cesarean delivery on maternal outcomes: a systematic review and meta-analysis. Anaesth Crit Care Pain Med. 2021;40:100935.
27. Meng X, Chen K, Yang C, et al. The clinical efficacy and safety of enhanced recovery after surgery for cesarean section: a systematic review and meta-analysis of randomized controlled trials and observational studies. Front Med. 2021;8:694385.
28. Corson E, Hind D, Beever D, et al. Enhanced recovery after elective caesarean: a rapid review of clinical protocols and an umbrella review of systematic reviews. BMC Pregnancy Childbirth. 2017;17:91.
“Blind” endometrial sampling: A call to end the practice
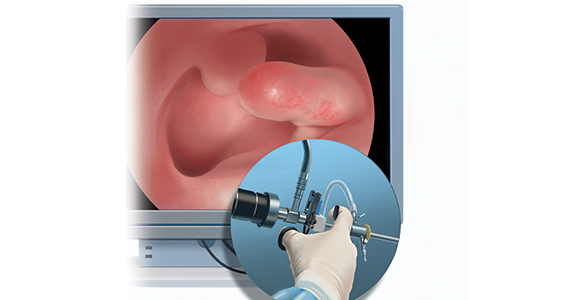
Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.
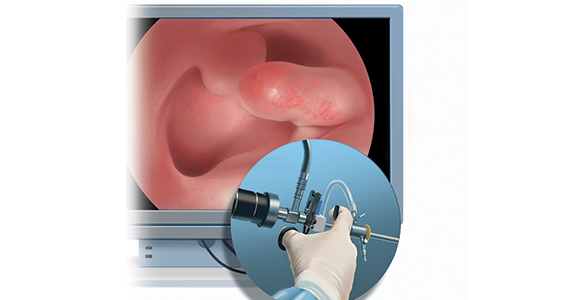
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.

Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.

OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.

Linda Bradley, MD: The standard in ObGyn for many years has been our reliance on the blind dilation and curettage (D&C)—it has been the mainstay for evaluation of the endometrial cavity. We know that it has risks, but most importantly, the procedure has low sensitivity for detecting focal pathology. This basic lack of confirmation of lesions makes a diagnosis impossible and patients are challenged in getting adequate treatment, and will not, since they may not know what options they have for the treatment of intrauterine pathology.
Because it is a “blind procedure,” done without looking, we don’t know the endpoints, such as when is the procedure completed, how do we know we removed all of the lesions? Let’s look at our colleagues, like GI and colorectal physicians. If a patient presents with rectal bleeding, we would perform an exam, followed by either a colonoscopy or sigmoidoscopy. If a patient were vomiting up blood, a gastroenterologist would perform an upper endoscopy, look with a tube to see if there is an ulcer or something else as a source of the bleeding. If a patient were bleeding from the bladder, a urologist would use a cystoscope for direct inspection.
Unfortunately for gynecologists, only about 15% to 25% of us will use hysteroscopy as a diagnostic method2—a method that has excellent sensitivity in detecting endocervical disease, intrauterine disease, and proximal tubal pathology. Compared with blind curettage, we can visualize the cavity; we can sample the cavity directly; we can determine what the patient has and determine the proper surgical procedure, medical therapy, or reassurance that a patient may be offered. We often are looking at focal lesions, lesions in the uterine cavity that could be cancer, so we can make a diagnosis. Or we may be looking at small things, like endometrial hyperplasia, endocervical or endometrial polyps, retained products of conception, or fibroids. We can look at uterine pathology as well as anatomic issues and malformations—such as bicornuate or septate uterus.
I actually say, “My hysteroscope is my stethoscope” because it allows us to evaluate for many things. The beauty of the new office hysteroscopes is that they are miniaturized. Doctors now have the ability to use reusable devices that are as small as 3 millimeters. There are disposable ones that are up to 3.5 to 4 millimeters in size. Gynecologists have the options to choose from reusuable rigid or flexible hysteroscopes or completely disposable devices. So, truly, we now should not have an excuse for evaluating a woman’s anatomy, especially for bleeding. We should no longer rely, as we have for the last century or more, just on blind sampling, because we miss focal lesions.
OBG Management: When was the hysteroscope first introduced into the field?
Dr. Bradley: The technology employed in hysteroscopy has been around really since the last 150+ years, introduced by Dr. Pantaleoni. We just have not embraced its usefulness in our clinical practice for many years. Today, about 15% to 25% of gynecologists practicing in the United States are performing hysteroscopy in the office.1
OBG Management: How does using hysteroscopy contribute to better patient outcomes?
Dr. Bradley: We can get a more accurate diagnosis—fewer false-negatives and a high degree of sensitivity in detecting focal lesions. With D&C, much focal pathology can be left behind. In a 2001 study, 105 symptomatic postmenopausal women with bleeding and thickened lining of the uterus greater than 5 mm on ultrasound underwent blind D&C. They found that 80% of the women had intracavitary lesions and 90% had focal lesions. In fact, 87% of the patients with focal lesions still had residual pathology after the blind D&C.3 The D&C procedure missed 58% of polyps, 50% of endometrial hyperplasia, 60% of cases of complex atypical hyperplasia, and even 11% of endometrial cancers. So these numbers are just not very good. Direct inspection of the uterus, with uninterrupted visualization through hysteroscopy, with removal of lesions under direct visualization, should be our goal.
Blind sampling also poses greater risk for things like perforation. In addition, you not only can miss lesions by just scraping the endometrium, D&C also can leave lesions just floating around in the uterine cavity, with those lesions never retrieved. With office hysteroscopy, the physician can be more successful in treating a condition because once you see what is going on in the uterine cavity, you can say, “Okay, I can fix this with a surgical procedure. What instruments do I need? How much time is it going to take? Is this a straightforward case? Is it more complicated? Do I let an intern do the case? Is this for a more senior resident or fellow?” So I think it helps to direct the next steps for surgical management and even medical management, which also could be what we call “one-stop shopping.” For instance, for directed biopsies for removal of small polyps, for patients that can tolerate the procedure a little longer, the diagnostic hysteroscopy then becomes a management, an operative procedure, that really, for myself, can be done in the office. Removal of larger fibroids, because of fluid management and other concerns, would not be done in the office. Most patients tolerate office procedures, but it also depends on a patient’s weight, and her ability to relax during the procedure.
The ultimate goal for hysteroscopy is a minimum of diagnosis, meaning in less than 2, 3 minutes, you can look inside the uterus. Our devices are 3 millimeters in size; I tell my patients, it’s the size of “a piece of spaghetti or pasta,” and we will just take a look. If we see a polyp, okay, if your office is not equipped, because then you need a different type of equipment for removal, then take her to the operating room. The patient would be under brief anesthesia and go home an hour or 2 later. So really, for physicians, we just need to embrace the technology to make a diagnosis, just look, and then from there decide what is next.

OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?...
OBG Management: What techniques do you use to minimize or eliminate patient discomfort during hysteroscopy?
Dr. Bradley: I think first is always be patient-centric. Let patients be prepared for the procedure. We have reading materials; our nurses explain the procedure. In the office, I try to prepare the patient for success. I let her know what is going on. A friend, family member can be with her. We have a nurse that understands the procedure; she explains it well. We have a type of bed that allows the patients’ legs to rest more comfortably in the stirrups—a leg rest kind of stirrup. We use a heating pad. Some patients like to hear music. Some patients like to have aromatherapy. We are quick and efficient, and typically just talk to the patient throughout the procedure. Although some patients don’t like this explanatory, “talkative” approach—they say, “Dr. Bradley, just do the procedure. I don’t want to know you are touching the cervix. I don’t want to know that you’re prepping. Just do it.”
But I like what we called it when I was growing up: vocal-local (talk to your patient and explain as you proceed). It’s like local anesthesia. For these procedures in the office you usually do not have to use numbing medicine or a paracervical block. Look at the patient’s age, number of years in menopause, whether or not she has delivered vaginally, and what her cervix looks like. Does she have a sexually transmitted infection or pelvic inflammatory disease? Sometimes we will use misoprostol, my personal preference is oral, but there are data to suggest that vaginal can be of help.4 We suggest Motrin, Tylenol an hour or 2 before, and we always want patients to not come in on an empty stomach. There is also the option of primrose oil, a supplement, that patients buy at the drug store in the vitamin section. It’s used for cervical softening. It is taken orally.5-7
If they want, patients can watch a video—similar to watching childbirth videos when I used to deliver babies. At some point we started putting mirrors where women could see their efforts of pushing a baby out, as it might give them more willpower to push harder. Some people don’t want to look. But the majority of women will do well in this setting. I do have a small number of women that just say, “I can’t do this in the office,” and so in those cases, they can go to the operating room. But the main idea is, even in an operating room, you are not just doing a D&C. You are still going to look inside with a hysteroscope and have a great panoramic view of what is going on, and remove a lesion with an instrument while you watch. Not a process of looking with the hysteroscope, scraping with a curettage, and thinking that you are complete. Targeted removal of focal lesions under continuous visualization is the goal.
OBG Management: Can you describe the goals of the consensus document on ending blind sampling co-created by the European Society of Gynecologic Endoscopy, AAGL, and the Global Community on Hysteroscopy?
Dr. Bradley: Our goal for this year is to get a systematic review and guidelines paper written that speaks to what we have just talked about. We want to have as many articles about why blind sampling is not beneficial, with too many misses, and now we have new technology available. We want to speak to physicians to solve the conundrum of bleeding, with equivocal ultrasounds, equivocal saline infusion, sonograms, equivocal MRIs—be able to take a look. Let’s come up to speed like our other colleagues in other specialties that “look.” A systematic review guideline document will provide the evidence that blind D&C is fraught with problems and how often we miss disease and its inherent risk.
We need to, by itself, for most of our patients, abandon D&C because we have too many missed diagnoses. As doctors we have to be lifelong learners. There was no robot back in the day. We were not able to do laparoscopic hysterectomies, there were no MRIs. I remember in our city, there was one CT scan. We just did not have a lot of technology. The half-life of medical knowledge used to be decades—you graduated in the ‘60s, you could be a great gynecologist for the next 30 years because there was not that much going on. When I finished in the mid to late ‘80s, there was no hysteroscopy training. But I have come to see its value, the science behind it.
So what I say to doctors is, “We learn so many new things, we shouldn’t get stuck in just saying, ‘I didn’t do this when I was in training.’” And if your thought is, “Oh, in my practice, I don’t have that many cases,” you still need to be able to know who in your community can be a resource to your patients. As Maya Angelou says, “When you know better, you should do better.” And that’s where I am now—to be a lifelong learner, and just do it.
Lastly, patient influence is very important. If patients ask, “How are you going to do the procedure?” it’s a driver for change. By utilizing hysteroscopy in the evaluation of the intrauterine cavity, we have the opportunity to change the face of evaluation and treatment for abnormal uterine bleeding.●
To maximize visualization and procedure ease, schedule office hysteroscopy shortly after menstruation for reproductive-age women with regular menstrual cycles, which corresponds to timing of the thinnest endometrial lining.1 By contrast, the luteal phase of the menstrual cycle may be associated with the presence of secretory endometrium, which may mimic endometrial polyps or obscure intrauterine pathology, including FIGO type 1 and 2 submucous leiomyomas.
The following patients can have their procedures scheduled at any time, as they do not regularly cycle:
- those receiving continuous hormonal contraception
- women taking menopausal hormonal therapy
- women on progestin therapy (including those using intrauterine devices).
For patients with irregular cycles, timing is crucial as the topography of the endometrium can be variable. To increase successful visualization and diagnostic accuracy, a short course of combined hormonal contraceptives2 or progestin therapy3,4 can be considered for 10-14 days, followed by a withdrawal menses, and immediate procedure scheduling after bleeding subsides, as this will produce a thin endometrium. This approach may be especially beneficial for operative procedures such as polypectomy in order to promote complete specimen extraction.
Pharmacologic endometrial preparation also is an option and has been associated with decreased procedure time and improved patient and clinician satisfaction during operative hysteroscopy.2,3 We discourage the use of hormonal pre-treatment for diagnostic hysteroscopy alone, as this may alter endometrial histology and provide misleading results. Overall, data related to pharmacologic endometrial preparation are limited to small studies with varying treatment protocols, and an optimal regimen has yet to be determined.
References
1. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/AOG.0000000000003712.
2. Cicinelli E, Pinto V, Quattromini P, et al. Endometrial preparation with estradiol plus dienogest (Qlaira) for office hysteroscopic polypectomy: randomized pilot study. J Minim Invasive Gynecol. 2012;19:356-359. doi:10.1016/j.jmig.2011.12.020.
3. Laganà AS, Vitale SG, Muscia V, et al. Endometrial preparation with dienogest before hysteroscopic surgery: a systematic review. Arch Gynecol Obstet. 2017;295:661-667. doi:10.1007/s00404-016-4244-1.
4. Ciebiera M, Zgliczyńska M, Zgliczyński S, et al. Oral desogestrel as endometrial preparation before operative hysteroscopy: a systematic review. Gynecol Obstet Invest. 2021;86:209-217. doi:10.1159/000514584.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.
- Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstet Gynecol. August 3, 2022. doi: 10.1097/ AOG.0000000000004898.
- Salazar CA, Isaacson KB. Office operative hysteroscopy: an update. J Minim Invasive Gynecol. 2018;25:199-208.
- Epstein E, Ramirez A, Skoog L, et al. Dilatation and curettage fails to detect most focal lesions in the uterine cavity in women with postmenopausal bleeding. Acta Obstet Gynecol Scand. 2001;80:1131-1136. doi:10.1034/j.1600-0412.2001.801210.x.
- The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, number 800. Obstet Gynecol. 2020;135:e138-e148. doi:10.1097/ AOG.0000000000003712.
- Vahdat M, Tahermanesh K, Mehdizadeh Kashi A, et al. Evening Primrose Oil effect on the ease of cervical ripening and dilatation before operative hysteroscopy. Thrita. 2015;4:7-10. doi:10.5812/thrita.29876
- Nouri B, Baghestani A, Pooransari P. Evening primrose versus misoprostol for cervical dilatation before gynecologic surgeries: a double-blind randomized clinical trial. J Obstet Gynecol Cancer Res. 2021;6:87-94. doi:10.30699/jogcr.6.2.87
- Verano RMA, Veloso-borromeo MG. The efficacy of evening primrose oil as a cervical ripening agent for gynecologic procedures: a single-blinded, randomized controlled trial. PJOG. 2015;39:24-28.
What is the best management strategy for complicated appendicitis in pregnancy?
Ashbrook M, et al. Management of complicated appendicitis during pregnancy in the US. JAMA Network Open. 2022;5:e227555. doi:10.1001/jamanetworkopen.2022.7555.
Expert Commentary
Over the last decade, the management of acute appendicitis in the nonpregnant adult has evolved such that some authorities favor first-line nonoperative therapy in the appropriate candidate, including some individuals with complicated appendicitis. While the conventional teaching regarding appendicitis in pregnancy has always been immediate surgery, favorable outcomes from nonoperative management in the nonpregnant population have led to an increasing application of conservative therapy in pregnancy, particularly among patients with uncomplicated appendicitis. However, optimal management of complicated appendicitis in pregnancy is unclear, as the risks of both operative and nonoperative management can be significant.
Details about the study
This retrospective cohort study using data from the National Inpatient Sample (NIS) focuses on outcomes of various management options among pregnant women with complicated appendicitis from January 2003 to September 2015. Complicated appendicitis refers to individuals with appendiceal perforation with peritonitis (a free perforation) or phlegmon/abscess (a walled-off perforation). Women included in the study were identified using ICD-9 codes for both pregnancy and complicated appendicitis; they were categorized into 3 groups: immediate operative management, successful nonoperative management, and failed nonoperative management (defined as surgical intervention >1 day after admission). The clinical and other outcomes of interest included maternal death, preterm labor/delivery or pregnancy loss, amniotic infection, sepsis, pneumonia, antenatal hemorrhage, and premature rupture of membranes. Outcomes included are those that occurred during the hospitalization for appendicitis; outcomes that may have occurred between discharge from the appendicitis hospitalization to the delivery hospitalization are not included in this study.
A total of 8,087 pregnant women with complicated appendicitis were included in this study, of whom 954 (11.8%) had successful nonoperative management, 2,646 (32.7%) had failed nonoperative management, and 4,487 (55.5%) had immediate operative management. First, when comparing successful nonoperative management to immediate operative management, there were no differences in preterm labor/delivery or pregnancy loss, or antenatal hemorrhage; however, successful nonoperative management was also associated with higher risks of maternal infectious complications, including risks of amniotic infection, pneumonia, and sepsis. When comparing failed nonoperative management (women who required surgical intervention during the index hospitalization) to immediate operative management, failed conservative management was associated with higher risks of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, amniotic infection, pneumonia, and sepsis. For every 1 day that surgery was delayed in the group of women who failed nonoperative management, the odds of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, sepsis, amniotic infection, and pneumonia increased.
Study strengths and weaknesses
Database studies have inherent limitations that are overcome with strength in numbers. In this study, our understanding of outcomes associated with management of complicated appendicitis assumes that women were correctly identified as both being pregnant and having complicated appendicitis (as opposed to uncomplicated appendicitis but miscoded). Clinical data that may have led to selection of one management strategy over another, or specific clinical management decisions, are not possible to extract from the NIS. For instance, did nonoperative management systematically include percutaneous guided drainage if an abscess was noted, and appropriately targeted antibiotic therapy? If delayed operative intervention with IV antibiotics to allow for “cooling off” of the abdomen prior to surgery was planned, this strategy would have been included in the failed nonoperative management group, when in fact nonoperative management was never the plan. Whether gestational age (which is not known in this study), or any other clinical data contributed to the initially chosen management strategy is not known.
The treating clinicians, obstetricians and surgeons alike, would like to know the pregnancy outcome when considering the various management strategies for complicated appendicitis. However, this study only provides insight into the outcomes for the hospitalization for appendicitis. Whether or not women categorized as successful nonoperative management go on to require surgery or have preterm labor in the future, or whether women with successful immediate surgical management might be readmitted with complications, is not known. This is a significant limitation of the database, which does not allow for linking of individual hospitalizations, and rather can provide only a snapshot in time.
This study includes a fairly long timespan–2003 to 2015–during which the management of complicated appendicitis was actively evolving. Early in this time frame, nonoperative management outside of pregnancy was uncommon, and nonoperative management may have been even rarer and perhaps reserved for the most ill of pregnant women on presentation (for whom surgery may have been considered too risky without a short time with IV antibiotics to “cool off” the abdomen). As time progressed over the study span, nonoperative management was likely offered with greater frequency and among women with lesser degrees of illness. However, the year of presentation was not controlled for in this study.
Finally, given the differences noted in management strategy by race/ethnicity and type of hospital, it is not clear how this bias influences the findings from this study. ●
Immediate operative intervention for complicated appendicitis in pregnancy remains a mainstay of management. Perinatal risks associated with surgical intervention are low and are comparable in many respects to successful nonoperative intervention. However, characteristics that predict successful nonoperative intervention are not known, and nonoperative therapy still carries higher risks of maternal infectious complications. When nonoperative intervention is the chosen approach in pregnant women with complicated appendicitis, clinicians must maintain a low threshold for conversion to operative management to avoid maternal morbidity. In addition, clinicians must closely monitor women discharged after successful appendicitis treatment for subsequent complications, as the long-term risks of conservative management or delayed operative intervention are not clear.
Ashbrook M, et al. Management of complicated appendicitis during pregnancy in the US. JAMA Network Open. 2022;5:e227555. doi:10.1001/jamanetworkopen.2022.7555.
Expert Commentary
Over the last decade, the management of acute appendicitis in the nonpregnant adult has evolved such that some authorities favor first-line nonoperative therapy in the appropriate candidate, including some individuals with complicated appendicitis. While the conventional teaching regarding appendicitis in pregnancy has always been immediate surgery, favorable outcomes from nonoperative management in the nonpregnant population have led to an increasing application of conservative therapy in pregnancy, particularly among patients with uncomplicated appendicitis. However, optimal management of complicated appendicitis in pregnancy is unclear, as the risks of both operative and nonoperative management can be significant.
Details about the study
This retrospective cohort study using data from the National Inpatient Sample (NIS) focuses on outcomes of various management options among pregnant women with complicated appendicitis from January 2003 to September 2015. Complicated appendicitis refers to individuals with appendiceal perforation with peritonitis (a free perforation) or phlegmon/abscess (a walled-off perforation). Women included in the study were identified using ICD-9 codes for both pregnancy and complicated appendicitis; they were categorized into 3 groups: immediate operative management, successful nonoperative management, and failed nonoperative management (defined as surgical intervention >1 day after admission). The clinical and other outcomes of interest included maternal death, preterm labor/delivery or pregnancy loss, amniotic infection, sepsis, pneumonia, antenatal hemorrhage, and premature rupture of membranes. Outcomes included are those that occurred during the hospitalization for appendicitis; outcomes that may have occurred between discharge from the appendicitis hospitalization to the delivery hospitalization are not included in this study.
A total of 8,087 pregnant women with complicated appendicitis were included in this study, of whom 954 (11.8%) had successful nonoperative management, 2,646 (32.7%) had failed nonoperative management, and 4,487 (55.5%) had immediate operative management. First, when comparing successful nonoperative management to immediate operative management, there were no differences in preterm labor/delivery or pregnancy loss, or antenatal hemorrhage; however, successful nonoperative management was also associated with higher risks of maternal infectious complications, including risks of amniotic infection, pneumonia, and sepsis. When comparing failed nonoperative management (women who required surgical intervention during the index hospitalization) to immediate operative management, failed conservative management was associated with higher risks of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, amniotic infection, pneumonia, and sepsis. For every 1 day that surgery was delayed in the group of women who failed nonoperative management, the odds of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, sepsis, amniotic infection, and pneumonia increased.
Study strengths and weaknesses
Database studies have inherent limitations that are overcome with strength in numbers. In this study, our understanding of outcomes associated with management of complicated appendicitis assumes that women were correctly identified as both being pregnant and having complicated appendicitis (as opposed to uncomplicated appendicitis but miscoded). Clinical data that may have led to selection of one management strategy over another, or specific clinical management decisions, are not possible to extract from the NIS. For instance, did nonoperative management systematically include percutaneous guided drainage if an abscess was noted, and appropriately targeted antibiotic therapy? If delayed operative intervention with IV antibiotics to allow for “cooling off” of the abdomen prior to surgery was planned, this strategy would have been included in the failed nonoperative management group, when in fact nonoperative management was never the plan. Whether gestational age (which is not known in this study), or any other clinical data contributed to the initially chosen management strategy is not known.
The treating clinicians, obstetricians and surgeons alike, would like to know the pregnancy outcome when considering the various management strategies for complicated appendicitis. However, this study only provides insight into the outcomes for the hospitalization for appendicitis. Whether or not women categorized as successful nonoperative management go on to require surgery or have preterm labor in the future, or whether women with successful immediate surgical management might be readmitted with complications, is not known. This is a significant limitation of the database, which does not allow for linking of individual hospitalizations, and rather can provide only a snapshot in time.
This study includes a fairly long timespan–2003 to 2015–during which the management of complicated appendicitis was actively evolving. Early in this time frame, nonoperative management outside of pregnancy was uncommon, and nonoperative management may have been even rarer and perhaps reserved for the most ill of pregnant women on presentation (for whom surgery may have been considered too risky without a short time with IV antibiotics to “cool off” the abdomen). As time progressed over the study span, nonoperative management was likely offered with greater frequency and among women with lesser degrees of illness. However, the year of presentation was not controlled for in this study.
Finally, given the differences noted in management strategy by race/ethnicity and type of hospital, it is not clear how this bias influences the findings from this study. ●
Immediate operative intervention for complicated appendicitis in pregnancy remains a mainstay of management. Perinatal risks associated with surgical intervention are low and are comparable in many respects to successful nonoperative intervention. However, characteristics that predict successful nonoperative intervention are not known, and nonoperative therapy still carries higher risks of maternal infectious complications. When nonoperative intervention is the chosen approach in pregnant women with complicated appendicitis, clinicians must maintain a low threshold for conversion to operative management to avoid maternal morbidity. In addition, clinicians must closely monitor women discharged after successful appendicitis treatment for subsequent complications, as the long-term risks of conservative management or delayed operative intervention are not clear.
Ashbrook M, et al. Management of complicated appendicitis during pregnancy in the US. JAMA Network Open. 2022;5:e227555. doi:10.1001/jamanetworkopen.2022.7555.
Expert Commentary
Over the last decade, the management of acute appendicitis in the nonpregnant adult has evolved such that some authorities favor first-line nonoperative therapy in the appropriate candidate, including some individuals with complicated appendicitis. While the conventional teaching regarding appendicitis in pregnancy has always been immediate surgery, favorable outcomes from nonoperative management in the nonpregnant population have led to an increasing application of conservative therapy in pregnancy, particularly among patients with uncomplicated appendicitis. However, optimal management of complicated appendicitis in pregnancy is unclear, as the risks of both operative and nonoperative management can be significant.
Details about the study
This retrospective cohort study using data from the National Inpatient Sample (NIS) focuses on outcomes of various management options among pregnant women with complicated appendicitis from January 2003 to September 2015. Complicated appendicitis refers to individuals with appendiceal perforation with peritonitis (a free perforation) or phlegmon/abscess (a walled-off perforation). Women included in the study were identified using ICD-9 codes for both pregnancy and complicated appendicitis; they were categorized into 3 groups: immediate operative management, successful nonoperative management, and failed nonoperative management (defined as surgical intervention >1 day after admission). The clinical and other outcomes of interest included maternal death, preterm labor/delivery or pregnancy loss, amniotic infection, sepsis, pneumonia, antenatal hemorrhage, and premature rupture of membranes. Outcomes included are those that occurred during the hospitalization for appendicitis; outcomes that may have occurred between discharge from the appendicitis hospitalization to the delivery hospitalization are not included in this study.
A total of 8,087 pregnant women with complicated appendicitis were included in this study, of whom 954 (11.8%) had successful nonoperative management, 2,646 (32.7%) had failed nonoperative management, and 4,487 (55.5%) had immediate operative management. First, when comparing successful nonoperative management to immediate operative management, there were no differences in preterm labor/delivery or pregnancy loss, or antenatal hemorrhage; however, successful nonoperative management was also associated with higher risks of maternal infectious complications, including risks of amniotic infection, pneumonia, and sepsis. When comparing failed nonoperative management (women who required surgical intervention during the index hospitalization) to immediate operative management, failed conservative management was associated with higher risks of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, amniotic infection, pneumonia, and sepsis. For every 1 day that surgery was delayed in the group of women who failed nonoperative management, the odds of preterm labor/delivery or pregnancy loss, antenatal hemorrhage, sepsis, amniotic infection, and pneumonia increased.
Study strengths and weaknesses
Database studies have inherent limitations that are overcome with strength in numbers. In this study, our understanding of outcomes associated with management of complicated appendicitis assumes that women were correctly identified as both being pregnant and having complicated appendicitis (as opposed to uncomplicated appendicitis but miscoded). Clinical data that may have led to selection of one management strategy over another, or specific clinical management decisions, are not possible to extract from the NIS. For instance, did nonoperative management systematically include percutaneous guided drainage if an abscess was noted, and appropriately targeted antibiotic therapy? If delayed operative intervention with IV antibiotics to allow for “cooling off” of the abdomen prior to surgery was planned, this strategy would have been included in the failed nonoperative management group, when in fact nonoperative management was never the plan. Whether gestational age (which is not known in this study), or any other clinical data contributed to the initially chosen management strategy is not known.
The treating clinicians, obstetricians and surgeons alike, would like to know the pregnancy outcome when considering the various management strategies for complicated appendicitis. However, this study only provides insight into the outcomes for the hospitalization for appendicitis. Whether or not women categorized as successful nonoperative management go on to require surgery or have preterm labor in the future, or whether women with successful immediate surgical management might be readmitted with complications, is not known. This is a significant limitation of the database, which does not allow for linking of individual hospitalizations, and rather can provide only a snapshot in time.
This study includes a fairly long timespan–2003 to 2015–during which the management of complicated appendicitis was actively evolving. Early in this time frame, nonoperative management outside of pregnancy was uncommon, and nonoperative management may have been even rarer and perhaps reserved for the most ill of pregnant women on presentation (for whom surgery may have been considered too risky without a short time with IV antibiotics to “cool off” the abdomen). As time progressed over the study span, nonoperative management was likely offered with greater frequency and among women with lesser degrees of illness. However, the year of presentation was not controlled for in this study.
Finally, given the differences noted in management strategy by race/ethnicity and type of hospital, it is not clear how this bias influences the findings from this study. ●
Immediate operative intervention for complicated appendicitis in pregnancy remains a mainstay of management. Perinatal risks associated with surgical intervention are low and are comparable in many respects to successful nonoperative intervention. However, characteristics that predict successful nonoperative intervention are not known, and nonoperative therapy still carries higher risks of maternal infectious complications. When nonoperative intervention is the chosen approach in pregnant women with complicated appendicitis, clinicians must maintain a low threshold for conversion to operative management to avoid maternal morbidity. In addition, clinicians must closely monitor women discharged after successful appendicitis treatment for subsequent complications, as the long-term risks of conservative management or delayed operative intervention are not clear.
Should we rethink maternal monitoring of fetal movement through “kick counts”?

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.
Are single-incision mini-slings the new gold standard for stress urinary incontinence?
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
Abdel-Fattah M, Cooper D, Davidson T, et al. Single-incision mini-slings for stress urinary incontinence in women. N Engl J Med. 2022;386:1230-1243.
EXPERT COMMENTARY
A joint society position statement by the American Urogynecologic Society and the Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction published in December 2021 declared synthetic midurethral slings, first cleared for use in the United States in the early 1990s, the most extensively studied anti-incontinence operation and the standard of care for the treatment of female stress urinary incontinence.1 Full-length retropubic and transobturator (out-in and in-out) slings have been extensively evaluated for safety and efficacy in well-conducted randomized trials.2 Single-incision mini-slings (SIMS) were first cleared for use in 2006, but they lack the long-term safety and comparative effectiveness data of full-length standard midurethral slings (SMUS).3 Furthermore, several iterations of the mini-slings have come to market but have been withdrawn or modified to allow for adjustability.
The SIMS trial by Abdel-Fattah and colleagues, published recently in the New England Journal of Medicine, is one of the few randomized trials with long-term (3 year) subjective and objective outcome data based on comparison of adjustable single-incision mini-slings versus standard full-length midurethral slings.
Details of the study
The SIMS trial is a noninferiority multicenter randomized controlled trial funded by the National Institute for Health Research at 21 hospitals in the United Kingdom that compared adjustable mini-sling procedures performed under local anesthesia with full-length retrotropubic and transobturator sling procedures performed under general anesthesia. Patients and surgeons were not masked to study group assignment because of the differences in anesthesia, and patients with greater than stage 2 prolapse were excluded from the trial.
The primary outcome was Patient Global Impression of Improvement (PGI-I) based on a 7-point Likert scale, with success defined as very much improved or much improved at 15 months and failure defined as all other responses (improved, same, worse, much worse, and very much worse). A noninferiority margin was set at 10 percentage points at 15 months.
Secondary outcomes and adverse events at 36 months included postoperative pain, return to normal activities, objective success based on a 24-hour pad test weight of less than 8 g, and tape exposure, organ injury, new or worsening urinary urgency, dyspareunia, and need for prolonged catheterization.
A total of 596 women were enrolled in the study, 298 in the mini-sling arm and 298 in the standard midurethral sling arm. Baseline characteristics were similar in both groups with most sling procedures being performed by general consultant gynecologists (>60%) versus subspecialist urogynecologists.
Results. Success at 15 months, based on the PGI-I responses of very much better or much better, was noted in 79.1% of patients in the mini-sling group (212/268) versus 75.6% in the full-length sling group (189/250). The authors deemed mini-slings noninferior to standard full-length slings (adjusted risk difference, 4.6 percentage points; 95% confidence interval [CI], -2.7 to 11.8; P<.001 for noninferiority). Success rates declined but remained similar in both groups at 36 months: 72% in the mini-sling group (177/246) and 66.8% (157/235) in the full-length sling group.
More than 70% of mini-incision slings were Altis (Coloplast) and 22% were Ajust (CR Bard; since withdrawn from the market). The majority of standard midurethral full-length slings were transobturator slings (52.9%) versus retropubic slings (35.6%).
While blood loss, organ injury, and 36-month objective 24-hour pad test did not differ between groups, there were significant differences in other secondary outcomes. Dyspareunia and coital incontinence were more common with mini-slings at 15 and 36 months, reported in 11.7% of the mini-sling group and 4.8% of the full-length group (P<.01). Groin or thigh pain did not differ significantly between groups at 36 months (14.1% in mini-sling and 14.9% in full-length sling group, P = .61). Mesh exposure was noted in 3.3% of those with mini-slings and 1.9% of those with standard midurethral slings. The need for surgical intervention to treat recurrent stress incontinence or mesh removal for voiding dysfunction, pain, or mesh exposure also did not differ between groups (8.7% of the mini-sling group and 4.6% of the midurethral sling group; P = .12).
Study strengths and limitations
The strengths of this pragmatic randomized trial are in the use of clinically important and validated patient-reported subjective and objective outcomes in an adequately powered multisite trial of long duration (36 months). This study is important in demonstrating noninferiority of the mini-sling procedure compared with full-length slings, especially given this trial’s timing when there was a pause or suspension of sling mesh use in the United Kingdom beginning in 2018.
Study limitations include the loss to follow-up with diminished response rate of 87.1% at 15 months and 81.4% at 36 months and the inability to adequately assess for the uncommon outcomes, such as mesh-related complications and groin pain.
Further analysis needed
The high rate of dyspareunia (11.7%) with mini-slings deserves further analysis and consideration of whether or not to implant them in patients who are sexually active. Groin or thigh pain did not differ at 36 months but reported pain coincided with the higher percentage of transobturator slings placed over retropubic slings. Prior randomized trials of transobturator versus retropubic midurethral slings have demonstrated this same phenomenon of increased groin pain with the transobturator approach.2 Furthermore, this study by Abdel-Fattah and colleagues excluded patients with advanced anterior or apical prolapse, but one trial is currently underway in the United States.4
In conclusion, this trial suggests some advantages of single-incision mini-slings—ability to perform the procedure under local anesthesia, less synthetic mesh implantation with theoretically decreased risk of bladder perforation or bowel injury, and potential for easier removal compared with full-length slings. Disadvantages include dyspareunia and mesh exposure, which could be significant trade-offs for patients. ●
In the IDEAL framework for evaluating new surgical innovations, the recommended process begins with an idea, followed by development by a few surgeons in a few patients, then exploration in a feasibility randomized controlled trial, an assessment in larger trials by many surgeons, and long-term follow-up.5 The SIMS trial falls under the assessment tab of the IDEAL framework and represents a much-needed study prior to widespread adoption of single-incision mini-slings. The higher dyspareunia rate in women undergoing single-incision mini-slings deserves further evaluation.
CHERYL B. IGLESIA, MD
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.
- Joint position statement on midurethral slings for stress urinary incontinence. Female Pelvic Med Reconstr Surg. 2021;27:707-710. doi: 10.1097/SPV.0000000000001096.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Nambiar A, Cody JD, Jeffery ST. Single-incision sling operations for urinary incontinence in women. Cochrane Database Syst Rev. 2014;6:CD008709.
- National Institutes of Health. Retropubic vs single-incision mid-urethral sling for stress urinary incontinence. ClinicalTrials.gov identifier NCT03520114. Accessed July16, 2022. https://www.clinicaltrials.gov/ct2/show/NCT0352011 4?cond=altis+sling&draw=2&rank=6
- McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet. 2009;374:1105-1111.