User login
Study Suggests Sleep Habits May Help Prevent Health Risks
Insulin Spray May Help Combat Alzheimer Disease
$2.2 Billion in New Agent Orange Claims Paid Out
Report Shows Medical Errors Decreasing at VA Hospitals
CMS Bundled Payments Move Forward
Officials at the Centers for Medicare and Medicaid Services in August released a request for applications (RFA) inviting physicians, hospitals, and other health care providers to participate in the Bundled Payments for Care Improvement initiative. The program, which was mandated under the Affordable Care Act, offers options for bundling payments for a hospital stay, for post-discharge services, or for both the hospital stay and the post-discharge care.
Instead of paying hospitals, physicians, and other providers separately, this initiative would combine the payment over an episode of care for a specific condition. The aim of the program is to encourage clinicians to work together and provide better continuity of care, resulting in better quality and lower costs.
"Today, Medicare pays for care the wrong way," Health and Human Services Secretary Kathleen Sebelius said during a teleconference to announce the bundling program. "Payments are based on the quantity of care, the amount of services delivered, not the quality of that care. And that leaves us too often with a system that actually can punish the providers that are most successful at getting and keeping their patients healthy."
The new bundling program offers three ways for health care providers to receive payment retrospectively, and one way to receive a prospective payment. Under some of the retrospective payment models, CMS and the providers would agree on a target payment amount for the episode of care and providers would be paid under the original Medicare fee-for-service system, but at a negotiated discount of 2% to 3% or greater. At the end of the care episode, the total payment would be compared with the target price and providers would be able to share in the savings, according to CMS.
Under prospective payment model, CMS would make a single bundled payment to the hospital to cover all services provided during the inpatient stay by the hospital, physicians, and other providers. That payment would offer at least a 3% discount to Medicare. Under this option, physicians and other providers would submit "no pay" claims to Medicare and the hospital would pay them out of the single bundled payment.
In addition to the options of prospective or retrospective payment, providers could choose how long the episode of care will be and what conditions they want to bundle payment for, and what services would be included in the payment. CMS officials said they wanted to make the program flexible so that a range of hospitals, physicians, and other providers could participate.
The American College of Surgeons General Surgery Coding and Reimbursement Committee (GSCRC) has been actively studying how bundled payments could be applied in surgery. The ACS believes that critical to the success of any bundling initiative is ensuring that the bundle is clinically coherent. The ACS GSCRC will continue this work and their discussions with the administration, CMS, and other stakeholders to ensure that any possible bundled payments in surgery will improve patient care.
Organizations interested in applying must submit a letter of intent by Sept. 22 for Model 1 and by Nov. 4 for Models 2, 3, and 4. More information on the program and how to apply is available at www.innovations.cms.gov/areas-of-focus/patient-care-models/bundled-payments-for-care-improvement.html.
Dr. Richard Gilfillan, the acting director of the CMS Innovation Center, which is overseeing the bundling initiative, said he expects that hundreds of organizations will apply. The program is a unique opportunity for hospitals to redesign their systems to promote better care coordination, Dr. Gilfillan said, and have that effort supported through Medicare payments.
The idea is to eliminate the traditional barriers between physicians and other providers – both inpatient and outpatient – all of whom may be involved in the care of a single condition, said Dr. Nancy Nielson, senior advisor to the CMS Innovation Center and past president of the American Medical Association.
The AMA was still reviewing the bundled payment details at press time, but Dr. Cecil B. Wilson, AMA immediate past president, said the organization will urge federal officials to encourage applications for physician-led bundling projects. "For this to be successful, and for physicians to participate actively, then they need to be a part of that process rather than just some larger corporation or larger hospital system or health plan that’s organizing these," he said. "We think those are important as well, but we also think it’s important that physicians be a part of that leadership."
Health care consultant Robert Minkin urged physicians to seriously consider applying for the bundling program. The program is a sentinel event in the move from fee for service to more centralized, coordinated care model, he said.
This program should result in multiple benefits to everyone. By identifying and reducing frontline costs incurred by surgeons, physicians, and other providers, costs to the entire system are eliminated rather than simply shifted to another part of the system. We should applaud this kind of incisive "surgical strike" and help CMS identify other similar opportunities.
Dr. Magruder C. Donaldson is the chairman of surgery at Metrowest Medical Center in Framingham, Mass. He is also an associate medical editor of Vascular Specialist.
This following is the complete text of Dr. Donaldson's comments, which were abbreviated in print.
| Dr. Craig Donaldson |
The rationale for bundled payments is not only to reduce health care expenditures by removing the piece work, volume driven reimbursement system vulnerable to duplication, waste and fraud. More importantly, bundling should stimulate better organization, teamwork and coordination of services resulting in better care quality and outcomes.
Preventive measures and multidisciplinary disease-oriented programs should be strengthened. Evidence is strong that bundling will work, sufficient to convince the Centers for Medicare & Medicaid Services (CMS) to initiate a Request for Applications (RFA) on August 23 for providers to test and develop four models for bundling. The RFA can be viewed at www.innovations.cms.gov for more detail.
The four models focus on selected “episodes of care”, providing a negotiated bundled price for each episode. Unlike managed care which bases reimbursement on “covered lives”, payment for an episode of care such as a femoropopliteal bypass would bundle services for the episode, including inpatient, post-acute care rehabilitation, lab work and other services and related readmissions over a defined interval of time, depending on the model.
The four models include three retrospective plans and one prospective plan.
Under the retrospective plans (Models 1-3) CMS and the applicant provider set a target price for a defined episode of care by applying a discount to total costs for a similar episode of care based upon historical data. Payments would be made under the core Medicare system (not including Medicare Advantage plans) at the negotiated discount. At the end of the episode, total payments would be compared with the target price. Participating providers could then be able to share in any savings.
Under the prospective plan (Model 4) a single negotiated bundled payment for inpatient care would be made to the hospital for the episode, from which practitioners would be paid by the hospital.
The fundamental goal is alignment of incentives to create more efficient, better organized care. Disease prevention and improved care quality will result, with cost savings a byproduct. The fact that CMS will share any savings gained through the retrospective plans indicates which priority is their first.
All of the models will require strong leadership and cooperation among caregivers, most importantly hospitals and physicians. Integrated systems with employed physicians and surgeons would be more likely to apply for prospective bundling under Model 4. Less centralized systems would more likely choose Model 1 in which hospitals are paid a negotiated bundled and discounted price and physicians would be reimbursed per usual Medicare fee-for-service but could share in gains arising from better care coordination.
Now more critical than ever, development and strengthening of physician hospital organizations (PHOs) and especially Accountable Care Organizations (ACOs) will be central to the CMS initiative. Since bundling involves sharing in multiple ways, physicians and especially surgeons will need to work energetically with other members of the care team on matters ranging from governance to database perfection to fair quantitation and monitoring of disbursements to team members for each episode of care.
For surgeons in general and many vascular surgeons, it will be important to continue mending fences with the forces of integration and organizational innovation in our communities. Changes such as the electronic medical record, e-prescribing and membership in the local PHO or ACO will keep us “in the loop”. Institutional participation in quality programs such as the American College’s National Surgical Quality Improvement Program (NSQIP) and the Society for Vascular Surgery’s Vascular Quality Initiative (VQI) will be increasingly important, along with ongoing support for hospital quality and safety efforts such as the Surgical Care improvement Program (SCIP) and enhanced use of the surgical checklist.
At heart, most physicians and surgeons know that we need significant change in our system. The CMS proposal looks like a step in the right direction. It is hoped that many of our institutions will respond to the RFA and participate in designing and testing plans under one of the four proposed models. This project cannot succeed without the wisdom and full involvement of physicians and surgeons.
This program should result in multiple benefits to everyone. By identifying and reducing frontline costs incurred by surgeons, physicians, and other providers, costs to the entire system are eliminated rather than simply shifted to another part of the system. We should applaud this kind of incisive "surgical strike" and help CMS identify other similar opportunities.
Dr. Magruder C. Donaldson is the chairman of surgery at Metrowest Medical Center in Framingham, Mass. He is also an associate medical editor of Vascular Specialist.
This following is the complete text of Dr. Donaldson's comments, which were abbreviated in print.
| Dr. Craig Donaldson |
The rationale for bundled payments is not only to reduce health care expenditures by removing the piece work, volume driven reimbursement system vulnerable to duplication, waste and fraud. More importantly, bundling should stimulate better organization, teamwork and coordination of services resulting in better care quality and outcomes.
Preventive measures and multidisciplinary disease-oriented programs should be strengthened. Evidence is strong that bundling will work, sufficient to convince the Centers for Medicare & Medicaid Services (CMS) to initiate a Request for Applications (RFA) on August 23 for providers to test and develop four models for bundling. The RFA can be viewed at www.innovations.cms.gov for more detail.
The four models focus on selected “episodes of care”, providing a negotiated bundled price for each episode. Unlike managed care which bases reimbursement on “covered lives”, payment for an episode of care such as a femoropopliteal bypass would bundle services for the episode, including inpatient, post-acute care rehabilitation, lab work and other services and related readmissions over a defined interval of time, depending on the model.
The four models include three retrospective plans and one prospective plan.
Under the retrospective plans (Models 1-3) CMS and the applicant provider set a target price for a defined episode of care by applying a discount to total costs for a similar episode of care based upon historical data. Payments would be made under the core Medicare system (not including Medicare Advantage plans) at the negotiated discount. At the end of the episode, total payments would be compared with the target price. Participating providers could then be able to share in any savings.
Under the prospective plan (Model 4) a single negotiated bundled payment for inpatient care would be made to the hospital for the episode, from which practitioners would be paid by the hospital.
The fundamental goal is alignment of incentives to create more efficient, better organized care. Disease prevention and improved care quality will result, with cost savings a byproduct. The fact that CMS will share any savings gained through the retrospective plans indicates which priority is their first.
All of the models will require strong leadership and cooperation among caregivers, most importantly hospitals and physicians. Integrated systems with employed physicians and surgeons would be more likely to apply for prospective bundling under Model 4. Less centralized systems would more likely choose Model 1 in which hospitals are paid a negotiated bundled and discounted price and physicians would be reimbursed per usual Medicare fee-for-service but could share in gains arising from better care coordination.
Now more critical than ever, development and strengthening of physician hospital organizations (PHOs) and especially Accountable Care Organizations (ACOs) will be central to the CMS initiative. Since bundling involves sharing in multiple ways, physicians and especially surgeons will need to work energetically with other members of the care team on matters ranging from governance to database perfection to fair quantitation and monitoring of disbursements to team members for each episode of care.
For surgeons in general and many vascular surgeons, it will be important to continue mending fences with the forces of integration and organizational innovation in our communities. Changes such as the electronic medical record, e-prescribing and membership in the local PHO or ACO will keep us “in the loop”. Institutional participation in quality programs such as the American College’s National Surgical Quality Improvement Program (NSQIP) and the Society for Vascular Surgery’s Vascular Quality Initiative (VQI) will be increasingly important, along with ongoing support for hospital quality and safety efforts such as the Surgical Care improvement Program (SCIP) and enhanced use of the surgical checklist.
At heart, most physicians and surgeons know that we need significant change in our system. The CMS proposal looks like a step in the right direction. It is hoped that many of our institutions will respond to the RFA and participate in designing and testing plans under one of the four proposed models. This project cannot succeed without the wisdom and full involvement of physicians and surgeons.
This program should result in multiple benefits to everyone. By identifying and reducing frontline costs incurred by surgeons, physicians, and other providers, costs to the entire system are eliminated rather than simply shifted to another part of the system. We should applaud this kind of incisive "surgical strike" and help CMS identify other similar opportunities.
Dr. Magruder C. Donaldson is the chairman of surgery at Metrowest Medical Center in Framingham, Mass. He is also an associate medical editor of Vascular Specialist.
This following is the complete text of Dr. Donaldson's comments, which were abbreviated in print.
| Dr. Craig Donaldson |
The rationale for bundled payments is not only to reduce health care expenditures by removing the piece work, volume driven reimbursement system vulnerable to duplication, waste and fraud. More importantly, bundling should stimulate better organization, teamwork and coordination of services resulting in better care quality and outcomes.
Preventive measures and multidisciplinary disease-oriented programs should be strengthened. Evidence is strong that bundling will work, sufficient to convince the Centers for Medicare & Medicaid Services (CMS) to initiate a Request for Applications (RFA) on August 23 for providers to test and develop four models for bundling. The RFA can be viewed at www.innovations.cms.gov for more detail.
The four models focus on selected “episodes of care”, providing a negotiated bundled price for each episode. Unlike managed care which bases reimbursement on “covered lives”, payment for an episode of care such as a femoropopliteal bypass would bundle services for the episode, including inpatient, post-acute care rehabilitation, lab work and other services and related readmissions over a defined interval of time, depending on the model.
The four models include three retrospective plans and one prospective plan.
Under the retrospective plans (Models 1-3) CMS and the applicant provider set a target price for a defined episode of care by applying a discount to total costs for a similar episode of care based upon historical data. Payments would be made under the core Medicare system (not including Medicare Advantage plans) at the negotiated discount. At the end of the episode, total payments would be compared with the target price. Participating providers could then be able to share in any savings.
Under the prospective plan (Model 4) a single negotiated bundled payment for inpatient care would be made to the hospital for the episode, from which practitioners would be paid by the hospital.
The fundamental goal is alignment of incentives to create more efficient, better organized care. Disease prevention and improved care quality will result, with cost savings a byproduct. The fact that CMS will share any savings gained through the retrospective plans indicates which priority is their first.
All of the models will require strong leadership and cooperation among caregivers, most importantly hospitals and physicians. Integrated systems with employed physicians and surgeons would be more likely to apply for prospective bundling under Model 4. Less centralized systems would more likely choose Model 1 in which hospitals are paid a negotiated bundled and discounted price and physicians would be reimbursed per usual Medicare fee-for-service but could share in gains arising from better care coordination.
Now more critical than ever, development and strengthening of physician hospital organizations (PHOs) and especially Accountable Care Organizations (ACOs) will be central to the CMS initiative. Since bundling involves sharing in multiple ways, physicians and especially surgeons will need to work energetically with other members of the care team on matters ranging from governance to database perfection to fair quantitation and monitoring of disbursements to team members for each episode of care.
For surgeons in general and many vascular surgeons, it will be important to continue mending fences with the forces of integration and organizational innovation in our communities. Changes such as the electronic medical record, e-prescribing and membership in the local PHO or ACO will keep us “in the loop”. Institutional participation in quality programs such as the American College’s National Surgical Quality Improvement Program (NSQIP) and the Society for Vascular Surgery’s Vascular Quality Initiative (VQI) will be increasingly important, along with ongoing support for hospital quality and safety efforts such as the Surgical Care improvement Program (SCIP) and enhanced use of the surgical checklist.
At heart, most physicians and surgeons know that we need significant change in our system. The CMS proposal looks like a step in the right direction. It is hoped that many of our institutions will respond to the RFA and participate in designing and testing plans under one of the four proposed models. This project cannot succeed without the wisdom and full involvement of physicians and surgeons.
Officials at the Centers for Medicare and Medicaid Services in August released a request for applications (RFA) inviting physicians, hospitals, and other health care providers to participate in the Bundled Payments for Care Improvement initiative. The program, which was mandated under the Affordable Care Act, offers options for bundling payments for a hospital stay, for post-discharge services, or for both the hospital stay and the post-discharge care.
Instead of paying hospitals, physicians, and other providers separately, this initiative would combine the payment over an episode of care for a specific condition. The aim of the program is to encourage clinicians to work together and provide better continuity of care, resulting in better quality and lower costs.
"Today, Medicare pays for care the wrong way," Health and Human Services Secretary Kathleen Sebelius said during a teleconference to announce the bundling program. "Payments are based on the quantity of care, the amount of services delivered, not the quality of that care. And that leaves us too often with a system that actually can punish the providers that are most successful at getting and keeping their patients healthy."
The new bundling program offers three ways for health care providers to receive payment retrospectively, and one way to receive a prospective payment. Under some of the retrospective payment models, CMS and the providers would agree on a target payment amount for the episode of care and providers would be paid under the original Medicare fee-for-service system, but at a negotiated discount of 2% to 3% or greater. At the end of the care episode, the total payment would be compared with the target price and providers would be able to share in the savings, according to CMS.
Under prospective payment model, CMS would make a single bundled payment to the hospital to cover all services provided during the inpatient stay by the hospital, physicians, and other providers. That payment would offer at least a 3% discount to Medicare. Under this option, physicians and other providers would submit "no pay" claims to Medicare and the hospital would pay them out of the single bundled payment.
In addition to the options of prospective or retrospective payment, providers could choose how long the episode of care will be and what conditions they want to bundle payment for, and what services would be included in the payment. CMS officials said they wanted to make the program flexible so that a range of hospitals, physicians, and other providers could participate.
The American College of Surgeons General Surgery Coding and Reimbursement Committee (GSCRC) has been actively studying how bundled payments could be applied in surgery. The ACS believes that critical to the success of any bundling initiative is ensuring that the bundle is clinically coherent. The ACS GSCRC will continue this work and their discussions with the administration, CMS, and other stakeholders to ensure that any possible bundled payments in surgery will improve patient care.
Organizations interested in applying must submit a letter of intent by Sept. 22 for Model 1 and by Nov. 4 for Models 2, 3, and 4. More information on the program and how to apply is available at www.innovations.cms.gov/areas-of-focus/patient-care-models/bundled-payments-for-care-improvement.html.
Dr. Richard Gilfillan, the acting director of the CMS Innovation Center, which is overseeing the bundling initiative, said he expects that hundreds of organizations will apply. The program is a unique opportunity for hospitals to redesign their systems to promote better care coordination, Dr. Gilfillan said, and have that effort supported through Medicare payments.
The idea is to eliminate the traditional barriers between physicians and other providers – both inpatient and outpatient – all of whom may be involved in the care of a single condition, said Dr. Nancy Nielson, senior advisor to the CMS Innovation Center and past president of the American Medical Association.
The AMA was still reviewing the bundled payment details at press time, but Dr. Cecil B. Wilson, AMA immediate past president, said the organization will urge federal officials to encourage applications for physician-led bundling projects. "For this to be successful, and for physicians to participate actively, then they need to be a part of that process rather than just some larger corporation or larger hospital system or health plan that’s organizing these," he said. "We think those are important as well, but we also think it’s important that physicians be a part of that leadership."
Health care consultant Robert Minkin urged physicians to seriously consider applying for the bundling program. The program is a sentinel event in the move from fee for service to more centralized, coordinated care model, he said.
Officials at the Centers for Medicare and Medicaid Services in August released a request for applications (RFA) inviting physicians, hospitals, and other health care providers to participate in the Bundled Payments for Care Improvement initiative. The program, which was mandated under the Affordable Care Act, offers options for bundling payments for a hospital stay, for post-discharge services, or for both the hospital stay and the post-discharge care.
Instead of paying hospitals, physicians, and other providers separately, this initiative would combine the payment over an episode of care for a specific condition. The aim of the program is to encourage clinicians to work together and provide better continuity of care, resulting in better quality and lower costs.
"Today, Medicare pays for care the wrong way," Health and Human Services Secretary Kathleen Sebelius said during a teleconference to announce the bundling program. "Payments are based on the quantity of care, the amount of services delivered, not the quality of that care. And that leaves us too often with a system that actually can punish the providers that are most successful at getting and keeping their patients healthy."
The new bundling program offers three ways for health care providers to receive payment retrospectively, and one way to receive a prospective payment. Under some of the retrospective payment models, CMS and the providers would agree on a target payment amount for the episode of care and providers would be paid under the original Medicare fee-for-service system, but at a negotiated discount of 2% to 3% or greater. At the end of the care episode, the total payment would be compared with the target price and providers would be able to share in the savings, according to CMS.
Under prospective payment model, CMS would make a single bundled payment to the hospital to cover all services provided during the inpatient stay by the hospital, physicians, and other providers. That payment would offer at least a 3% discount to Medicare. Under this option, physicians and other providers would submit "no pay" claims to Medicare and the hospital would pay them out of the single bundled payment.
In addition to the options of prospective or retrospective payment, providers could choose how long the episode of care will be and what conditions they want to bundle payment for, and what services would be included in the payment. CMS officials said they wanted to make the program flexible so that a range of hospitals, physicians, and other providers could participate.
The American College of Surgeons General Surgery Coding and Reimbursement Committee (GSCRC) has been actively studying how bundled payments could be applied in surgery. The ACS believes that critical to the success of any bundling initiative is ensuring that the bundle is clinically coherent. The ACS GSCRC will continue this work and their discussions with the administration, CMS, and other stakeholders to ensure that any possible bundled payments in surgery will improve patient care.
Organizations interested in applying must submit a letter of intent by Sept. 22 for Model 1 and by Nov. 4 for Models 2, 3, and 4. More information on the program and how to apply is available at www.innovations.cms.gov/areas-of-focus/patient-care-models/bundled-payments-for-care-improvement.html.
Dr. Richard Gilfillan, the acting director of the CMS Innovation Center, which is overseeing the bundling initiative, said he expects that hundreds of organizations will apply. The program is a unique opportunity for hospitals to redesign their systems to promote better care coordination, Dr. Gilfillan said, and have that effort supported through Medicare payments.
The idea is to eliminate the traditional barriers between physicians and other providers – both inpatient and outpatient – all of whom may be involved in the care of a single condition, said Dr. Nancy Nielson, senior advisor to the CMS Innovation Center and past president of the American Medical Association.
The AMA was still reviewing the bundled payment details at press time, but Dr. Cecil B. Wilson, AMA immediate past president, said the organization will urge federal officials to encourage applications for physician-led bundling projects. "For this to be successful, and for physicians to participate actively, then they need to be a part of that process rather than just some larger corporation or larger hospital system or health plan that’s organizing these," he said. "We think those are important as well, but we also think it’s important that physicians be a part of that leadership."
Health care consultant Robert Minkin urged physicians to seriously consider applying for the bundling program. The program is a sentinel event in the move from fee for service to more centralized, coordinated care model, he said.
HHS Plans Revamp of Human Research Rules
The federal government plans to overhaul the rules for conducting research with human subjects with the aim of bringing the regulations in line with research in the 21st century.
The Department of Health and Human Services published the advance notice of proposed rule making on human subjects research in the Federal Register on July 26. The proposal seeks public comment on a series of possible changes, from relying on a single institutional review board for multicenter studies to simplifying informed consent forms. This is the first time the regulations on human subjects’ research, known as the Common Rule, have been updated since 1991.
While the Common Rule was a landmark development in the protection of research participants, those rules were developed during a "simpler time," Dr. Howard Koh, assistant secretary for health at HHS, said during a briefing with reporters on June 22. Twenty years later, human subjects’ research includes a variety of new areas such as genomics and behavioral and social science research, as well as studies utilizing the Internet and large-scale data networks.
"These changes in the research landscape have raised questions regarding the effectiveness of the current regulatory framework," he said.
With that in mind, HHS is proposing to offer greater protection to study participants in several ways, such as:
• Giving participants the right to say whether researchers can use their biospecimens in future research.
• Helping researchers craft easier to understand informed consent forms.
• Making security and information protections uniform across studies with potentially identifiable patient information.
• Developing a more systematic approach to collecting adverse event data from ongoing studies.
Officials also aim to ease regulatory burdens in the following ways:
• Designing review requirements to match the risk posed to research subjects.
• Ensuring that any guidance issued by the federal government is consistent across departments.
• Allowing multiple sites to be overseen by a single institutional review board.
HHS also seeks to expand the reach of the regulation by extending it to all studies conducted by institutions that receive federal funding for human subjects’ research from a Common Rule agency.
Mary Woolley, president and CEO of Research!America, a not-for-profit organization that advocates for public and private funding of medical research, said the proposal benefits both patients and researchers because it streamlines the process while adding patient protections.
"We’re going to speed the conduct of research and thus speed the day when we have more and more personalized medicine based on research," she said.
Dr. Holly A. Taylor, Ph.D., of the Berman Institute of Bioethics at the Johns Hopkins University, Baltimore, praised the regulation’s focus on improving the informed consent process.
Dr. Taylor, who has conducted research on informed consent, said she agrees with HHS that, in many cases, the forms have become too long and complex for patients to understand. She urged the agency to work with investigators, who aren’t trained to write for a consumer audience.
Comments are accepted on the proposed rule making at www.regulations.gov until 5 p.m. ET on Sept. 26.
The federal government plans to overhaul the rules for conducting research with human subjects with the aim of bringing the regulations in line with research in the 21st century.
The Department of Health and Human Services published the advance notice of proposed rule making on human subjects research in the Federal Register on July 26. The proposal seeks public comment on a series of possible changes, from relying on a single institutional review board for multicenter studies to simplifying informed consent forms. This is the first time the regulations on human subjects’ research, known as the Common Rule, have been updated since 1991.
While the Common Rule was a landmark development in the protection of research participants, those rules were developed during a "simpler time," Dr. Howard Koh, assistant secretary for health at HHS, said during a briefing with reporters on June 22. Twenty years later, human subjects’ research includes a variety of new areas such as genomics and behavioral and social science research, as well as studies utilizing the Internet and large-scale data networks.
"These changes in the research landscape have raised questions regarding the effectiveness of the current regulatory framework," he said.
With that in mind, HHS is proposing to offer greater protection to study participants in several ways, such as:
• Giving participants the right to say whether researchers can use their biospecimens in future research.
• Helping researchers craft easier to understand informed consent forms.
• Making security and information protections uniform across studies with potentially identifiable patient information.
• Developing a more systematic approach to collecting adverse event data from ongoing studies.
Officials also aim to ease regulatory burdens in the following ways:
• Designing review requirements to match the risk posed to research subjects.
• Ensuring that any guidance issued by the federal government is consistent across departments.
• Allowing multiple sites to be overseen by a single institutional review board.
HHS also seeks to expand the reach of the regulation by extending it to all studies conducted by institutions that receive federal funding for human subjects’ research from a Common Rule agency.
Mary Woolley, president and CEO of Research!America, a not-for-profit organization that advocates for public and private funding of medical research, said the proposal benefits both patients and researchers because it streamlines the process while adding patient protections.
"We’re going to speed the conduct of research and thus speed the day when we have more and more personalized medicine based on research," she said.
Dr. Holly A. Taylor, Ph.D., of the Berman Institute of Bioethics at the Johns Hopkins University, Baltimore, praised the regulation’s focus on improving the informed consent process.
Dr. Taylor, who has conducted research on informed consent, said she agrees with HHS that, in many cases, the forms have become too long and complex for patients to understand. She urged the agency to work with investigators, who aren’t trained to write for a consumer audience.
Comments are accepted on the proposed rule making at www.regulations.gov until 5 p.m. ET on Sept. 26.
The federal government plans to overhaul the rules for conducting research with human subjects with the aim of bringing the regulations in line with research in the 21st century.
The Department of Health and Human Services published the advance notice of proposed rule making on human subjects research in the Federal Register on July 26. The proposal seeks public comment on a series of possible changes, from relying on a single institutional review board for multicenter studies to simplifying informed consent forms. This is the first time the regulations on human subjects’ research, known as the Common Rule, have been updated since 1991.
While the Common Rule was a landmark development in the protection of research participants, those rules were developed during a "simpler time," Dr. Howard Koh, assistant secretary for health at HHS, said during a briefing with reporters on June 22. Twenty years later, human subjects’ research includes a variety of new areas such as genomics and behavioral and social science research, as well as studies utilizing the Internet and large-scale data networks.
"These changes in the research landscape have raised questions regarding the effectiveness of the current regulatory framework," he said.
With that in mind, HHS is proposing to offer greater protection to study participants in several ways, such as:
• Giving participants the right to say whether researchers can use their biospecimens in future research.
• Helping researchers craft easier to understand informed consent forms.
• Making security and information protections uniform across studies with potentially identifiable patient information.
• Developing a more systematic approach to collecting adverse event data from ongoing studies.
Officials also aim to ease regulatory burdens in the following ways:
• Designing review requirements to match the risk posed to research subjects.
• Ensuring that any guidance issued by the federal government is consistent across departments.
• Allowing multiple sites to be overseen by a single institutional review board.
HHS also seeks to expand the reach of the regulation by extending it to all studies conducted by institutions that receive federal funding for human subjects’ research from a Common Rule agency.
Mary Woolley, president and CEO of Research!America, a not-for-profit organization that advocates for public and private funding of medical research, said the proposal benefits both patients and researchers because it streamlines the process while adding patient protections.
"We’re going to speed the conduct of research and thus speed the day when we have more and more personalized medicine based on research," she said.
Dr. Holly A. Taylor, Ph.D., of the Berman Institute of Bioethics at the Johns Hopkins University, Baltimore, praised the regulation’s focus on improving the informed consent process.
Dr. Taylor, who has conducted research on informed consent, said she agrees with HHS that, in many cases, the forms have become too long and complex for patients to understand. She urged the agency to work with investigators, who aren’t trained to write for a consumer audience.
Comments are accepted on the proposed rule making at www.regulations.gov until 5 p.m. ET on Sept. 26.
Joint Commission Steps Up Efforts to Reduce Wrong-Site Surgery
Procedures performed on the wrong side of the body, the wrong site, and even the wrong patient continue to happen at a national rate as high as 40 times every week, according to Dr. Mark R. Chassin, president of the Joint Commission at a recent teleconference.
One example of improvements is Rhode Island Hospital in Providence. In November 2009, the hospital was facing a $150,000 fine from the state health department and an order to install video cameras in all operating rooms after reports of five wrong-site surgeries in 2 years.
Today, officials at the hospital say they have changed their ways and they have the safety record to prove it. There have been no wrong-site surgeries at the hospital in about 20 months, according to Dr. Mary Reich Cooper, chief quality officer for Lifespan Corp., which owns Rhode Island Hospital.
"We were able to show the front-line staff – as well as the surgeons and the patients coming into the hospital – that not only was safety our first priority, but we [also] were prepared to put a tremendous amount of resources into making safety our first priority," Dr. Cooper said.
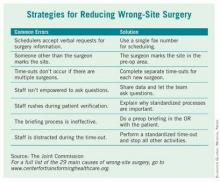
Lifespan’s Rhode Island Hospital is one of eight hospitals and ambulatory surgery centers that are working with the Joint Commission’s Center for Transforming Healthcare on a project to reduce wrong-site surgery. As a result of that project, which started at Rhode Island Hospital in 2009, the Joint Commission released a set of potential causes of wrong-site surgery and the targeted fixes that hospitals and surgery centers can use to eliminate them (see box). The plan is to begin adding those interventions to a Targeted Solutions Tool, an electronic application to allow all accredited or certified organizations to access the information and customize it.
The results of the project will give hospitals and surgery centers around the country a road map for pinpointing and measuring their risks of wrong-site surgery, said Dr. Chassin. He advised hospitals to start reviewing risks at the very beginning of the process, when an operation is scheduled. The Joint Commission’s project revealed that in 39% of cases, errors that increased the risk of wrong-site surgery were introduced during the scheduling process.
The scheduling process is ripe for errors, Dr. Chassin said, because the person supplying the patient and procedure information typically works in the surgeon’s office and often is not directly affiliated with the hospital or center where the surgery will take place. If that person is dealing with many different hospitals and surgery centers, all with different processes and requirements, it’s easy to get confused and relay incomplete or inaccurate information, he said.
And the scheduling process is just one area identified by the Joint Commission as having potential for errors that could lead to wrong-site surgery. The eight pilot organizations, some of which have never had a wrong-site surgery in their facility, found risks in all phases of their surgical processes ranging from inconsistent procedures for marking the surgical site to omissions in the "time-out" process just before surgery.
"It turns out that this is a much more complicated problem than it might seem to be at first," Dr. Chassin said. But developing specific fixes for each of those risks has helped to sharply reduce the chances of wrong-site surgeries at the eight pilot sites, he added For example, addressing documentation and verification issues in the preoperative holding areas decreased the percentage of cases with risks from a baseline of 52% to 19%.
At Rhode Island Hospital, efforts were made to improve the "time-out" before surgery. All other activities were stopped to allow operating room personnel to focus, and they used a script to ensure that all last-minute safety checks – such as asking everyone in the operating room if they could see the surgical mark – were completed, Dr. Cooper said.
The Joint Commission has been working on wrong-site surgery for a number of years, issuing Sentinel Event alerts in 1998 and 2001 and developing the Universal Protocol, a standardized approach to eliminating risks for wrong-site surgery.
Procedures performed on the wrong side of the body, the wrong site, and even the wrong patient continue to happen at a national rate as high as 40 times every week, according to Dr. Mark R. Chassin, president of the Joint Commission at a recent teleconference.
One example of improvements is Rhode Island Hospital in Providence. In November 2009, the hospital was facing a $150,000 fine from the state health department and an order to install video cameras in all operating rooms after reports of five wrong-site surgeries in 2 years.
Today, officials at the hospital say they have changed their ways and they have the safety record to prove it. There have been no wrong-site surgeries at the hospital in about 20 months, according to Dr. Mary Reich Cooper, chief quality officer for Lifespan Corp., which owns Rhode Island Hospital.
"We were able to show the front-line staff – as well as the surgeons and the patients coming into the hospital – that not only was safety our first priority, but we [also] were prepared to put a tremendous amount of resources into making safety our first priority," Dr. Cooper said.
Lifespan’s Rhode Island Hospital is one of eight hospitals and ambulatory surgery centers that are working with the Joint Commission’s Center for Transforming Healthcare on a project to reduce wrong-site surgery. As a result of that project, which started at Rhode Island Hospital in 2009, the Joint Commission released a set of potential causes of wrong-site surgery and the targeted fixes that hospitals and surgery centers can use to eliminate them (see box). The plan is to begin adding those interventions to a Targeted Solutions Tool, an electronic application to allow all accredited or certified organizations to access the information and customize it.
The results of the project will give hospitals and surgery centers around the country a road map for pinpointing and measuring their risks of wrong-site surgery, said Dr. Chassin. He advised hospitals to start reviewing risks at the very beginning of the process, when an operation is scheduled. The Joint Commission’s project revealed that in 39% of cases, errors that increased the risk of wrong-site surgery were introduced during the scheduling process.
The scheduling process is ripe for errors, Dr. Chassin said, because the person supplying the patient and procedure information typically works in the surgeon’s office and often is not directly affiliated with the hospital or center where the surgery will take place. If that person is dealing with many different hospitals and surgery centers, all with different processes and requirements, it’s easy to get confused and relay incomplete or inaccurate information, he said.
And the scheduling process is just one area identified by the Joint Commission as having potential for errors that could lead to wrong-site surgery. The eight pilot organizations, some of which have never had a wrong-site surgery in their facility, found risks in all phases of their surgical processes ranging from inconsistent procedures for marking the surgical site to omissions in the "time-out" process just before surgery.
"It turns out that this is a much more complicated problem than it might seem to be at first," Dr. Chassin said. But developing specific fixes for each of those risks has helped to sharply reduce the chances of wrong-site surgeries at the eight pilot sites, he added For example, addressing documentation and verification issues in the preoperative holding areas decreased the percentage of cases with risks from a baseline of 52% to 19%.
At Rhode Island Hospital, efforts were made to improve the "time-out" before surgery. All other activities were stopped to allow operating room personnel to focus, and they used a script to ensure that all last-minute safety checks – such as asking everyone in the operating room if they could see the surgical mark – were completed, Dr. Cooper said.
The Joint Commission has been working on wrong-site surgery for a number of years, issuing Sentinel Event alerts in 1998 and 2001 and developing the Universal Protocol, a standardized approach to eliminating risks for wrong-site surgery.
Procedures performed on the wrong side of the body, the wrong site, and even the wrong patient continue to happen at a national rate as high as 40 times every week, according to Dr. Mark R. Chassin, president of the Joint Commission at a recent teleconference.
One example of improvements is Rhode Island Hospital in Providence. In November 2009, the hospital was facing a $150,000 fine from the state health department and an order to install video cameras in all operating rooms after reports of five wrong-site surgeries in 2 years.
Today, officials at the hospital say they have changed their ways and they have the safety record to prove it. There have been no wrong-site surgeries at the hospital in about 20 months, according to Dr. Mary Reich Cooper, chief quality officer for Lifespan Corp., which owns Rhode Island Hospital.
"We were able to show the front-line staff – as well as the surgeons and the patients coming into the hospital – that not only was safety our first priority, but we [also] were prepared to put a tremendous amount of resources into making safety our first priority," Dr. Cooper said.
Lifespan’s Rhode Island Hospital is one of eight hospitals and ambulatory surgery centers that are working with the Joint Commission’s Center for Transforming Healthcare on a project to reduce wrong-site surgery. As a result of that project, which started at Rhode Island Hospital in 2009, the Joint Commission released a set of potential causes of wrong-site surgery and the targeted fixes that hospitals and surgery centers can use to eliminate them (see box). The plan is to begin adding those interventions to a Targeted Solutions Tool, an electronic application to allow all accredited or certified organizations to access the information and customize it.
The results of the project will give hospitals and surgery centers around the country a road map for pinpointing and measuring their risks of wrong-site surgery, said Dr. Chassin. He advised hospitals to start reviewing risks at the very beginning of the process, when an operation is scheduled. The Joint Commission’s project revealed that in 39% of cases, errors that increased the risk of wrong-site surgery were introduced during the scheduling process.
The scheduling process is ripe for errors, Dr. Chassin said, because the person supplying the patient and procedure information typically works in the surgeon’s office and often is not directly affiliated with the hospital or center where the surgery will take place. If that person is dealing with many different hospitals and surgery centers, all with different processes and requirements, it’s easy to get confused and relay incomplete or inaccurate information, he said.
And the scheduling process is just one area identified by the Joint Commission as having potential for errors that could lead to wrong-site surgery. The eight pilot organizations, some of which have never had a wrong-site surgery in their facility, found risks in all phases of their surgical processes ranging from inconsistent procedures for marking the surgical site to omissions in the "time-out" process just before surgery.
"It turns out that this is a much more complicated problem than it might seem to be at first," Dr. Chassin said. But developing specific fixes for each of those risks has helped to sharply reduce the chances of wrong-site surgeries at the eight pilot sites, he added For example, addressing documentation and verification issues in the preoperative holding areas decreased the percentage of cases with risks from a baseline of 52% to 19%.
At Rhode Island Hospital, efforts were made to improve the "time-out" before surgery. All other activities were stopped to allow operating room personnel to focus, and they used a script to ensure that all last-minute safety checks – such as asking everyone in the operating room if they could see the surgical mark – were completed, Dr. Cooper said.
The Joint Commission has been working on wrong-site surgery for a number of years, issuing Sentinel Event alerts in 1998 and 2001 and developing the Universal Protocol, a standardized approach to eliminating risks for wrong-site surgery.
CMS Proposes 30% Pay Cut for 2012
As expected, the Centers for Medicare and Medicaid Services proposed that physician fees for 2012 would be reduced by 29.5%. The proposed rule was released in the Federal Register July 1. The 29.5% pay cut is scheduled to take effect Jan. 1, 2012, unless Congress once again intervenes.
The reduction is required by the Sustainable Growth Rate (SGR) formula that was part of the Balanced Budget Act of 1997. But Dr. Donald M. Berwick, CMS administrator, said in a statement that the agency is hoping to find a way to avoid the statutory decrease.
"We need a permanent SGR fix to solve this problem once and for all. That’s why the president’s budget and his fiscal framework call for averting these cuts and why we are determined to pass and implement a permanent and sustainable fix," Dr. Berwick said.
"We are pleased that there is support from the administration and bipartisan members of Congress for permanent reform of this broken system, but agreement is not enough – action is needed," said Dr. Peter W. Carmel, president of the American Medical Association, in a statement.
The AMA has been seeking a review and revision of the Medicare Economic Index (MEI), a measure of cost increases that affect physician practices. That review has not yet begun, but revisions in the MEI could significantly reduce the legislative cost of permanent reform of the Medicare physician payment formula, said Dr. Carmel, noting that cost is an estimated $300 billion over the next 10 years, and is on its way to hit half a trillion dollars in a few years.
The reductions could be deeper for some specialties – especially for radiation oncology and diagnostic imaging – based on the impact of the Physician Practice Information Survey. The changes would reflect the third year of a 4-year transition to new practice expense relative value units. Additional changes will also be made because of the implementation of some recommendations of the American Medical Association/Specialty Society Resource Based Relative Value Scale Update Committee (RUC).
The CMS said in a statement that it is proposing to continue efforts to identify what it calls "potentially misvalued codes." As part of those efforts, the agency will look at all evaluation and management (E/M) codes to determine if they are undervalued. The agency also proposes to examine the highest non–E/M expenditure codes for each specialty to see if they are overvalued.
CMS estimates that the additional changes included in the proposed fee schedule will result in a 0% total payment change for general surgery services. Whereas general surgery still will be subject to the 29.5% Medicare payment reduction if Congress fails to act, this update also means that, unlike some other specialties, general surgery will not be subject to any further payment reductions in 2012.
CMS wants to extend the multiple procedure payment reduction (MPPR) policy to the professional component of advanced imaging services, which includes computed tomography (CT) scans, MRI, and ultrasound. The agency said the reduction would affect about 100 types of services. Reducing that component by 50% for subsequent procedures furnished to the same patient, on the same day, in the same session would result in an estimated $200 million in savings, according to the CMS.
For the first time, the agency is proposing quality and cost measures to be used in setting incentive payments for physicians who provide higher quality and more efficient care. That lays the groundwork for 2015, when the Affordable Care Act requires the CMS to begin making payment adjustments for certain physicians and physician groups. The requirement goes into effect for all physicians in 2017. The agency is proposing to use 2013 as the initial performance year.
Also included in the rule are proposals that would update a number of physician incentive programs, including the Physician Quality Reporting System, the e-Prescribing Incentive Program, and the Electronic Health Records Incentive Program. Additionally, it calls for expanding the multiple procedure payment reduction policy and for using quality and cost measures to establish a new physician value-based payment modifier.
The American College of Surgeons (ACS) continues to work with CMS on all of the programs addressed in the current proposed rule. Ten surgical organizations, including the ACS, recently sent a letter to U.S. Department of Health and Human Services (HHS) Secretary Kathleen Sebelius and CMS Administrator Berwick expressing concern regarding CMS’s action in the last Medicare Physician Fee Schedule. The organizations identified CMS’s departure from past practice in which it has traditionally accepted more than 90% of the RUC’s recommendations. The letter noted that CMS accepted only 71% of the 2010 RUC recommendations.
Citing the need to accurately reflect the work associated with a variety of physician services, the surgical organizations asked CMS to adopt the work values that were developed by the RUC. The organizations also stated that CMS’s decision regarding the valuation of work for physician services "must be made using a transparent, consistent process, and must be based on credible data."
A final rule is expected by Nov. 1.
As expected, the Centers for Medicare and Medicaid Services proposed that physician fees for 2012 would be reduced by 29.5%. The proposed rule was released in the Federal Register July 1. The 29.5% pay cut is scheduled to take effect Jan. 1, 2012, unless Congress once again intervenes.
The reduction is required by the Sustainable Growth Rate (SGR) formula that was part of the Balanced Budget Act of 1997. But Dr. Donald M. Berwick, CMS administrator, said in a statement that the agency is hoping to find a way to avoid the statutory decrease.
"We need a permanent SGR fix to solve this problem once and for all. That’s why the president’s budget and his fiscal framework call for averting these cuts and why we are determined to pass and implement a permanent and sustainable fix," Dr. Berwick said.
"We are pleased that there is support from the administration and bipartisan members of Congress for permanent reform of this broken system, but agreement is not enough – action is needed," said Dr. Peter W. Carmel, president of the American Medical Association, in a statement.
The AMA has been seeking a review and revision of the Medicare Economic Index (MEI), a measure of cost increases that affect physician practices. That review has not yet begun, but revisions in the MEI could significantly reduce the legislative cost of permanent reform of the Medicare physician payment formula, said Dr. Carmel, noting that cost is an estimated $300 billion over the next 10 years, and is on its way to hit half a trillion dollars in a few years.
The reductions could be deeper for some specialties – especially for radiation oncology and diagnostic imaging – based on the impact of the Physician Practice Information Survey. The changes would reflect the third year of a 4-year transition to new practice expense relative value units. Additional changes will also be made because of the implementation of some recommendations of the American Medical Association/Specialty Society Resource Based Relative Value Scale Update Committee (RUC).
The CMS said in a statement that it is proposing to continue efforts to identify what it calls "potentially misvalued codes." As part of those efforts, the agency will look at all evaluation and management (E/M) codes to determine if they are undervalued. The agency also proposes to examine the highest non–E/M expenditure codes for each specialty to see if they are overvalued.
CMS estimates that the additional changes included in the proposed fee schedule will result in a 0% total payment change for general surgery services. Whereas general surgery still will be subject to the 29.5% Medicare payment reduction if Congress fails to act, this update also means that, unlike some other specialties, general surgery will not be subject to any further payment reductions in 2012.
CMS wants to extend the multiple procedure payment reduction (MPPR) policy to the professional component of advanced imaging services, which includes computed tomography (CT) scans, MRI, and ultrasound. The agency said the reduction would affect about 100 types of services. Reducing that component by 50% for subsequent procedures furnished to the same patient, on the same day, in the same session would result in an estimated $200 million in savings, according to the CMS.
For the first time, the agency is proposing quality and cost measures to be used in setting incentive payments for physicians who provide higher quality and more efficient care. That lays the groundwork for 2015, when the Affordable Care Act requires the CMS to begin making payment adjustments for certain physicians and physician groups. The requirement goes into effect for all physicians in 2017. The agency is proposing to use 2013 as the initial performance year.
Also included in the rule are proposals that would update a number of physician incentive programs, including the Physician Quality Reporting System, the e-Prescribing Incentive Program, and the Electronic Health Records Incentive Program. Additionally, it calls for expanding the multiple procedure payment reduction policy and for using quality and cost measures to establish a new physician value-based payment modifier.
The American College of Surgeons (ACS) continues to work with CMS on all of the programs addressed in the current proposed rule. Ten surgical organizations, including the ACS, recently sent a letter to U.S. Department of Health and Human Services (HHS) Secretary Kathleen Sebelius and CMS Administrator Berwick expressing concern regarding CMS’s action in the last Medicare Physician Fee Schedule. The organizations identified CMS’s departure from past practice in which it has traditionally accepted more than 90% of the RUC’s recommendations. The letter noted that CMS accepted only 71% of the 2010 RUC recommendations.
Citing the need to accurately reflect the work associated with a variety of physician services, the surgical organizations asked CMS to adopt the work values that were developed by the RUC. The organizations also stated that CMS’s decision regarding the valuation of work for physician services "must be made using a transparent, consistent process, and must be based on credible data."
A final rule is expected by Nov. 1.
As expected, the Centers for Medicare and Medicaid Services proposed that physician fees for 2012 would be reduced by 29.5%. The proposed rule was released in the Federal Register July 1. The 29.5% pay cut is scheduled to take effect Jan. 1, 2012, unless Congress once again intervenes.
The reduction is required by the Sustainable Growth Rate (SGR) formula that was part of the Balanced Budget Act of 1997. But Dr. Donald M. Berwick, CMS administrator, said in a statement that the agency is hoping to find a way to avoid the statutory decrease.
"We need a permanent SGR fix to solve this problem once and for all. That’s why the president’s budget and his fiscal framework call for averting these cuts and why we are determined to pass and implement a permanent and sustainable fix," Dr. Berwick said.
"We are pleased that there is support from the administration and bipartisan members of Congress for permanent reform of this broken system, but agreement is not enough – action is needed," said Dr. Peter W. Carmel, president of the American Medical Association, in a statement.
The AMA has been seeking a review and revision of the Medicare Economic Index (MEI), a measure of cost increases that affect physician practices. That review has not yet begun, but revisions in the MEI could significantly reduce the legislative cost of permanent reform of the Medicare physician payment formula, said Dr. Carmel, noting that cost is an estimated $300 billion over the next 10 years, and is on its way to hit half a trillion dollars in a few years.
The reductions could be deeper for some specialties – especially for radiation oncology and diagnostic imaging – based on the impact of the Physician Practice Information Survey. The changes would reflect the third year of a 4-year transition to new practice expense relative value units. Additional changes will also be made because of the implementation of some recommendations of the American Medical Association/Specialty Society Resource Based Relative Value Scale Update Committee (RUC).
The CMS said in a statement that it is proposing to continue efforts to identify what it calls "potentially misvalued codes." As part of those efforts, the agency will look at all evaluation and management (E/M) codes to determine if they are undervalued. The agency also proposes to examine the highest non–E/M expenditure codes for each specialty to see if they are overvalued.
CMS estimates that the additional changes included in the proposed fee schedule will result in a 0% total payment change for general surgery services. Whereas general surgery still will be subject to the 29.5% Medicare payment reduction if Congress fails to act, this update also means that, unlike some other specialties, general surgery will not be subject to any further payment reductions in 2012.
CMS wants to extend the multiple procedure payment reduction (MPPR) policy to the professional component of advanced imaging services, which includes computed tomography (CT) scans, MRI, and ultrasound. The agency said the reduction would affect about 100 types of services. Reducing that component by 50% for subsequent procedures furnished to the same patient, on the same day, in the same session would result in an estimated $200 million in savings, according to the CMS.
For the first time, the agency is proposing quality and cost measures to be used in setting incentive payments for physicians who provide higher quality and more efficient care. That lays the groundwork for 2015, when the Affordable Care Act requires the CMS to begin making payment adjustments for certain physicians and physician groups. The requirement goes into effect for all physicians in 2017. The agency is proposing to use 2013 as the initial performance year.
Also included in the rule are proposals that would update a number of physician incentive programs, including the Physician Quality Reporting System, the e-Prescribing Incentive Program, and the Electronic Health Records Incentive Program. Additionally, it calls for expanding the multiple procedure payment reduction policy and for using quality and cost measures to establish a new physician value-based payment modifier.
The American College of Surgeons (ACS) continues to work with CMS on all of the programs addressed in the current proposed rule. Ten surgical organizations, including the ACS, recently sent a letter to U.S. Department of Health and Human Services (HHS) Secretary Kathleen Sebelius and CMS Administrator Berwick expressing concern regarding CMS’s action in the last Medicare Physician Fee Schedule. The organizations identified CMS’s departure from past practice in which it has traditionally accepted more than 90% of the RUC’s recommendations. The letter noted that CMS accepted only 71% of the 2010 RUC recommendations.
Citing the need to accurately reflect the work associated with a variety of physician services, the surgical organizations asked CMS to adopt the work values that were developed by the RUC. The organizations also stated that CMS’s decision regarding the valuation of work for physician services "must be made using a transparent, consistent process, and must be based on credible data."
A final rule is expected by Nov. 1.