User login
Women With Epilepsy Need More Contraceptive Counseling
Despite the fact that hormonal contraceptives interact with certain antiepileptic drugs, a recent study has shown that only 35% of 397 female patients with epilepsy were given any clinician counseling about contraceptive choices during their first clinic visit. And these patients were unlikely to receive contraceptive counseling during subsequent visits. The implications of these findings are troubling: Many antiepileptic agents decrease the efficacy of hormonal contraceptives by inducing hepatic enzymes, and estrogen-containing contraceptives are known to accelerate the metabolism of lamotrigine, an antiepileptic drug commonly prescribed in women of child-bearing age. Espinera et al also found that when women with epilepsy are given advice about the advantages of IUDs, they are far more likely to switch to IUDs, which are highly effective without AED drug interactions.
Espinera AR, Gavvala J, Bellinski I, et al. Counseling by epileptologists affects contraceptive choices of women with epilepsy. Epilepsy Behav. 2016;65:1-6.
Despite the fact that hormonal contraceptives interact with certain antiepileptic drugs, a recent study has shown that only 35% of 397 female patients with epilepsy were given any clinician counseling about contraceptive choices during their first clinic visit. And these patients were unlikely to receive contraceptive counseling during subsequent visits. The implications of these findings are troubling: Many antiepileptic agents decrease the efficacy of hormonal contraceptives by inducing hepatic enzymes, and estrogen-containing contraceptives are known to accelerate the metabolism of lamotrigine, an antiepileptic drug commonly prescribed in women of child-bearing age. Espinera et al also found that when women with epilepsy are given advice about the advantages of IUDs, they are far more likely to switch to IUDs, which are highly effective without AED drug interactions.
Espinera AR, Gavvala J, Bellinski I, et al. Counseling by epileptologists affects contraceptive choices of women with epilepsy. Epilepsy Behav. 2016;65:1-6.
Despite the fact that hormonal contraceptives interact with certain antiepileptic drugs, a recent study has shown that only 35% of 397 female patients with epilepsy were given any clinician counseling about contraceptive choices during their first clinic visit. And these patients were unlikely to receive contraceptive counseling during subsequent visits. The implications of these findings are troubling: Many antiepileptic agents decrease the efficacy of hormonal contraceptives by inducing hepatic enzymes, and estrogen-containing contraceptives are known to accelerate the metabolism of lamotrigine, an antiepileptic drug commonly prescribed in women of child-bearing age. Espinera et al also found that when women with epilepsy are given advice about the advantages of IUDs, they are far more likely to switch to IUDs, which are highly effective without AED drug interactions.
Espinera AR, Gavvala J, Bellinski I, et al. Counseling by epileptologists affects contraceptive choices of women with epilepsy. Epilepsy Behav. 2016;65:1-6.
Behavioral, Neurodevelopmental Disorders More Common in Young Adults With Epilepsy
Young adults with epilepsy are at greater risk for neurodevelopmental and behavioral disorders than some other patient populations, according to a recent case-control study that looked at hospital admissions, outpatients, and ED visits for adults with epilepsy, migraine, or leg fracture. The study cohort consisted of 5666 adult epilepsy patients between 19 and 25 years of age who were seen in hospitals and EDs, 17,507 patients with migraine, and 5966 patients with leg fractures. The researchers found that 51.8% of patients with epilepsy had behavioral health issues versus 37.6% of those with migraine and 21.6% of patients with fractures. Similarly, patients with epilepsy were 297% more likely to have a neurodevelopmental disorder compared with those with migraine.
Wagner JL, Wilson DA, Kellermann T, el al. Behavioral health in young adults with epilepsy: Implications for transition of care. Epilepsy Behav. 2016;65:7-12.
Young adults with epilepsy are at greater risk for neurodevelopmental and behavioral disorders than some other patient populations, according to a recent case-control study that looked at hospital admissions, outpatients, and ED visits for adults with epilepsy, migraine, or leg fracture. The study cohort consisted of 5666 adult epilepsy patients between 19 and 25 years of age who were seen in hospitals and EDs, 17,507 patients with migraine, and 5966 patients with leg fractures. The researchers found that 51.8% of patients with epilepsy had behavioral health issues versus 37.6% of those with migraine and 21.6% of patients with fractures. Similarly, patients with epilepsy were 297% more likely to have a neurodevelopmental disorder compared with those with migraine.
Wagner JL, Wilson DA, Kellermann T, el al. Behavioral health in young adults with epilepsy: Implications for transition of care. Epilepsy Behav. 2016;65:7-12.
Young adults with epilepsy are at greater risk for neurodevelopmental and behavioral disorders than some other patient populations, according to a recent case-control study that looked at hospital admissions, outpatients, and ED visits for adults with epilepsy, migraine, or leg fracture. The study cohort consisted of 5666 adult epilepsy patients between 19 and 25 years of age who were seen in hospitals and EDs, 17,507 patients with migraine, and 5966 patients with leg fractures. The researchers found that 51.8% of patients with epilepsy had behavioral health issues versus 37.6% of those with migraine and 21.6% of patients with fractures. Similarly, patients with epilepsy were 297% more likely to have a neurodevelopmental disorder compared with those with migraine.
Wagner JL, Wilson DA, Kellermann T, el al. Behavioral health in young adults with epilepsy: Implications for transition of care. Epilepsy Behav. 2016;65:7-12.
Can Mobile Apps Improve Medication Adherence in Pregnant Patients With Epilepsy?
To determine how well pregnant women with epilepsy adhere to their medication regimen, Ernst et al studied their intake of antiepileptic medication by providing them with an iPod Touch loaded with a mobile app specifically designed to track such data. Eighty-six women with epilepsy monitored their seizures and medication use. The study found that 75% of the women had tracked their medication usage for more than 80% of the days they were enrolled in the experiment. Among this subgroup, adherence to the anti-epilepsy drug regimen was 97.7%; 44% said they had missed taking their medication for at least one day. The investigators speculate that the high adherence rate may have been the result of using the mobile app itself.
Ernst L. Harden CL, Pennell PB, et al. Medication adherence in women with epilepsy who are planning pregnancy. Epilepsia. 2016; 57(12):2039-2044.
To determine how well pregnant women with epilepsy adhere to their medication regimen, Ernst et al studied their intake of antiepileptic medication by providing them with an iPod Touch loaded with a mobile app specifically designed to track such data. Eighty-six women with epilepsy monitored their seizures and medication use. The study found that 75% of the women had tracked their medication usage for more than 80% of the days they were enrolled in the experiment. Among this subgroup, adherence to the anti-epilepsy drug regimen was 97.7%; 44% said they had missed taking their medication for at least one day. The investigators speculate that the high adherence rate may have been the result of using the mobile app itself.
Ernst L. Harden CL, Pennell PB, et al. Medication adherence in women with epilepsy who are planning pregnancy. Epilepsia. 2016; 57(12):2039-2044.
To determine how well pregnant women with epilepsy adhere to their medication regimen, Ernst et al studied their intake of antiepileptic medication by providing them with an iPod Touch loaded with a mobile app specifically designed to track such data. Eighty-six women with epilepsy monitored their seizures and medication use. The study found that 75% of the women had tracked their medication usage for more than 80% of the days they were enrolled in the experiment. Among this subgroup, adherence to the anti-epilepsy drug regimen was 97.7%; 44% said they had missed taking their medication for at least one day. The investigators speculate that the high adherence rate may have been the result of using the mobile app itself.
Ernst L. Harden CL, Pennell PB, et al. Medication adherence in women with epilepsy who are planning pregnancy. Epilepsia. 2016; 57(12):2039-2044.
Understanding the Link between Traumatic Brain Injury and Posttraumatic Seizures
Among patients who have experienced traumatic brain injury, those who have had immediate or late seizures during an acute hospital stay are at increased of developing later posttraumatic seizures. Researchers found that new onset posttraumatic seizures were mostly likely to occur between the time patients were discharged from inpatient rehabilitation and 1 year (9.2%). By year 5, the cumulative incidence of such seizures was 20.5%. A patient’s race, intracranial pathology, and neurosurgical procedures also factored into their relative risk of posttraumatic seizures.
Ritter AC, Wagner AK, Fabio A, et al. Incidence and risk factors of posttraumatic seizures following traumatic brain injury: A Traumatic Brain Injury Model Systems Study. Epilepsia. 2016;57(12):1968-1977.
Among patients who have experienced traumatic brain injury, those who have had immediate or late seizures during an acute hospital stay are at increased of developing later posttraumatic seizures. Researchers found that new onset posttraumatic seizures were mostly likely to occur between the time patients were discharged from inpatient rehabilitation and 1 year (9.2%). By year 5, the cumulative incidence of such seizures was 20.5%. A patient’s race, intracranial pathology, and neurosurgical procedures also factored into their relative risk of posttraumatic seizures.
Ritter AC, Wagner AK, Fabio A, et al. Incidence and risk factors of posttraumatic seizures following traumatic brain injury: A Traumatic Brain Injury Model Systems Study. Epilepsia. 2016;57(12):1968-1977.
Among patients who have experienced traumatic brain injury, those who have had immediate or late seizures during an acute hospital stay are at increased of developing later posttraumatic seizures. Researchers found that new onset posttraumatic seizures were mostly likely to occur between the time patients were discharged from inpatient rehabilitation and 1 year (9.2%). By year 5, the cumulative incidence of such seizures was 20.5%. A patient’s race, intracranial pathology, and neurosurgical procedures also factored into their relative risk of posttraumatic seizures.
Ritter AC, Wagner AK, Fabio A, et al. Incidence and risk factors of posttraumatic seizures following traumatic brain injury: A Traumatic Brain Injury Model Systems Study. Epilepsia. 2016;57(12):1968-1977.
Hypothermia Offers Little Benefit to Patients with Convulsive Status Epilepticus
Induced hypothermia does not improve clinical outcomes among patients with convulsive status epilepticus (CSE), according to a new multicenter study published in the New England Journal of Medicine. To reach that conclusion, investigators assigned 270 critically ill patients with CSE on mechanical ventilation to either standard care or standard care plus hypothermia, which lowered their body temperature to 32 to 34⁰C for 24 hours. To measure the impact of hypothermia, researchers used the Glasgow Outcome Scale (GOS) score and defined success as a GOS score of 5, which represents no or minimal neurologic deficit, at 90 days. Forty-nine percent of patients on hypothermia achieved a score of 5, compared to 43% of those on standard care, which was statistically insignificant (P=.43).
Legriel S, Lemiale V, Schenck, M, et al. Hypothermia for Neuroprotection in Convulsive Status Epilepticus. N Engl J Med. 2016;375(25):2457-2467.
Induced hypothermia does not improve clinical outcomes among patients with convulsive status epilepticus (CSE), according to a new multicenter study published in the New England Journal of Medicine. To reach that conclusion, investigators assigned 270 critically ill patients with CSE on mechanical ventilation to either standard care or standard care plus hypothermia, which lowered their body temperature to 32 to 34⁰C for 24 hours. To measure the impact of hypothermia, researchers used the Glasgow Outcome Scale (GOS) score and defined success as a GOS score of 5, which represents no or minimal neurologic deficit, at 90 days. Forty-nine percent of patients on hypothermia achieved a score of 5, compared to 43% of those on standard care, which was statistically insignificant (P=.43).
Legriel S, Lemiale V, Schenck, M, et al. Hypothermia for Neuroprotection in Convulsive Status Epilepticus. N Engl J Med. 2016;375(25):2457-2467.
Induced hypothermia does not improve clinical outcomes among patients with convulsive status epilepticus (CSE), according to a new multicenter study published in the New England Journal of Medicine. To reach that conclusion, investigators assigned 270 critically ill patients with CSE on mechanical ventilation to either standard care or standard care plus hypothermia, which lowered their body temperature to 32 to 34⁰C for 24 hours. To measure the impact of hypothermia, researchers used the Glasgow Outcome Scale (GOS) score and defined success as a GOS score of 5, which represents no or minimal neurologic deficit, at 90 days. Forty-nine percent of patients on hypothermia achieved a score of 5, compared to 43% of those on standard care, which was statistically insignificant (P=.43).
Legriel S, Lemiale V, Schenck, M, et al. Hypothermia for Neuroprotection in Convulsive Status Epilepticus. N Engl J Med. 2016;375(25):2457-2467.
Statins May Reduce the Risk of Alzheimer’s Disease
Statins may lower the risk of developing Alzheimer’s disease, but the decrease in risk varies across statin molecules, and by gender, race, and ethnicity, according to research published online ahead of print December 12 in JAMA Neurology.
None of the statins assessed in the study affected the risk of developing Alzheimer’s disease among black men, said Julie M. Zissimopoulos, PhD, Vice Dean for Academic Affairs and Assistant Professor in the Sol Price School of Public Policy at the University of Southern California in Los Angeles, and her associates.
Several studies have suggested that statins exert a protective effect against Alzheimer’s disease, but they have had insufficient follow-up times, lacked minorities, and removed hyperlipidemic participants. For these reasons, Dr. Zissimopoulos and her colleagues analyzed medical and pharmacy data for a large, diverse sample of Medicare beneficiaries.
The researchers examined 399,979 adults aged 65 and older who initiated statin therapy during a two-year period. Beneficiaries were followed for approximately seven years. The mean interval between statin exposure and Alzheimer’s disease diagnosis was 5.4 years.
The study population included 310,240 non-Hispanic whites, 32,658 Hispanics, 32,278 non-Hispanic blacks, and 24,803 participants of Asian, Native American, other, or unknown race or ethnicity. The investigators confined their analysis to the four most commonly prescribed statins: simvastatin, atorvastatin, pravastatin, and rosuvastatin.
Overall, 1.72% of women and 1.32% of men were diagnosed as having Alzheimer’s disease during each year of follow-up. Study participants who were exposed to higher statin levels during the two-year exposure period were 10% less likely to receive a diagnosis of Alzheimer’s disease during follow-up, compared with those exposed to lower levels of statins. High exposure to statins reduced the risk of Alzheimer’s disease among women of all races (hazard ratio [HR], 0.85) and men of all races (HR, 0.88).
This association, however, varied across gender, race, and ethnicity. Statins decreased the risk of Alzheimer’s disease the most among Hispanic men (HR, 0.71), followed by black women (HR, 0.82), white women (HR, 0.86), and white men (HR, 0.89), but they did not decrease the risk of Alzheimer’s disease among black men, said Dr. Zissimopoulos. Simvastatin significantly decreased the risk of Alzheimer’s disease among white, Hispanic, and black women, compared with other subgroups, and atorvastatin significantly decreased the risk among white women, Hispanic women, and Hispanic men. Pravastatin and rosuvastatin decreased the risk of Alzheimer’s disease significantly among white women only.
These findings suggest that “certain patients facing multiple, otherwise equal statin alternatives for hyperlipidemia treatment, may reduce Alzheimer’s risk by using a particular statin. The right statin type for the right person at the right time may provide a relatively inexpensive means to lessen the burden of Alzheimer’s disease,” the investigators said.
This study was supported by the National Institute on Aging, the University of Southern California Zumberge Research Fund, and the Schaeffer-Amgen Fellowship Program.
—Mary Ann Moon
Suggested Reading
Zissimopoulos JM, Barthold D, Brinton RD, Joyce G. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. 2016 Dec 12 [Epub ahead of print].
Statins may lower the risk of developing Alzheimer’s disease, but the decrease in risk varies across statin molecules, and by gender, race, and ethnicity, according to research published online ahead of print December 12 in JAMA Neurology.
None of the statins assessed in the study affected the risk of developing Alzheimer’s disease among black men, said Julie M. Zissimopoulos, PhD, Vice Dean for Academic Affairs and Assistant Professor in the Sol Price School of Public Policy at the University of Southern California in Los Angeles, and her associates.
Several studies have suggested that statins exert a protective effect against Alzheimer’s disease, but they have had insufficient follow-up times, lacked minorities, and removed hyperlipidemic participants. For these reasons, Dr. Zissimopoulos and her colleagues analyzed medical and pharmacy data for a large, diverse sample of Medicare beneficiaries.
The researchers examined 399,979 adults aged 65 and older who initiated statin therapy during a two-year period. Beneficiaries were followed for approximately seven years. The mean interval between statin exposure and Alzheimer’s disease diagnosis was 5.4 years.
The study population included 310,240 non-Hispanic whites, 32,658 Hispanics, 32,278 non-Hispanic blacks, and 24,803 participants of Asian, Native American, other, or unknown race or ethnicity. The investigators confined their analysis to the four most commonly prescribed statins: simvastatin, atorvastatin, pravastatin, and rosuvastatin.
Overall, 1.72% of women and 1.32% of men were diagnosed as having Alzheimer’s disease during each year of follow-up. Study participants who were exposed to higher statin levels during the two-year exposure period were 10% less likely to receive a diagnosis of Alzheimer’s disease during follow-up, compared with those exposed to lower levels of statins. High exposure to statins reduced the risk of Alzheimer’s disease among women of all races (hazard ratio [HR], 0.85) and men of all races (HR, 0.88).
This association, however, varied across gender, race, and ethnicity. Statins decreased the risk of Alzheimer’s disease the most among Hispanic men (HR, 0.71), followed by black women (HR, 0.82), white women (HR, 0.86), and white men (HR, 0.89), but they did not decrease the risk of Alzheimer’s disease among black men, said Dr. Zissimopoulos. Simvastatin significantly decreased the risk of Alzheimer’s disease among white, Hispanic, and black women, compared with other subgroups, and atorvastatin significantly decreased the risk among white women, Hispanic women, and Hispanic men. Pravastatin and rosuvastatin decreased the risk of Alzheimer’s disease significantly among white women only.
These findings suggest that “certain patients facing multiple, otherwise equal statin alternatives for hyperlipidemia treatment, may reduce Alzheimer’s risk by using a particular statin. The right statin type for the right person at the right time may provide a relatively inexpensive means to lessen the burden of Alzheimer’s disease,” the investigators said.
This study was supported by the National Institute on Aging, the University of Southern California Zumberge Research Fund, and the Schaeffer-Amgen Fellowship Program.
—Mary Ann Moon
Suggested Reading
Zissimopoulos JM, Barthold D, Brinton RD, Joyce G. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. 2016 Dec 12 [Epub ahead of print].
Statins may lower the risk of developing Alzheimer’s disease, but the decrease in risk varies across statin molecules, and by gender, race, and ethnicity, according to research published online ahead of print December 12 in JAMA Neurology.
None of the statins assessed in the study affected the risk of developing Alzheimer’s disease among black men, said Julie M. Zissimopoulos, PhD, Vice Dean for Academic Affairs and Assistant Professor in the Sol Price School of Public Policy at the University of Southern California in Los Angeles, and her associates.
Several studies have suggested that statins exert a protective effect against Alzheimer’s disease, but they have had insufficient follow-up times, lacked minorities, and removed hyperlipidemic participants. For these reasons, Dr. Zissimopoulos and her colleagues analyzed medical and pharmacy data for a large, diverse sample of Medicare beneficiaries.
The researchers examined 399,979 adults aged 65 and older who initiated statin therapy during a two-year period. Beneficiaries were followed for approximately seven years. The mean interval between statin exposure and Alzheimer’s disease diagnosis was 5.4 years.
The study population included 310,240 non-Hispanic whites, 32,658 Hispanics, 32,278 non-Hispanic blacks, and 24,803 participants of Asian, Native American, other, or unknown race or ethnicity. The investigators confined their analysis to the four most commonly prescribed statins: simvastatin, atorvastatin, pravastatin, and rosuvastatin.
Overall, 1.72% of women and 1.32% of men were diagnosed as having Alzheimer’s disease during each year of follow-up. Study participants who were exposed to higher statin levels during the two-year exposure period were 10% less likely to receive a diagnosis of Alzheimer’s disease during follow-up, compared with those exposed to lower levels of statins. High exposure to statins reduced the risk of Alzheimer’s disease among women of all races (hazard ratio [HR], 0.85) and men of all races (HR, 0.88).
This association, however, varied across gender, race, and ethnicity. Statins decreased the risk of Alzheimer’s disease the most among Hispanic men (HR, 0.71), followed by black women (HR, 0.82), white women (HR, 0.86), and white men (HR, 0.89), but they did not decrease the risk of Alzheimer’s disease among black men, said Dr. Zissimopoulos. Simvastatin significantly decreased the risk of Alzheimer’s disease among white, Hispanic, and black women, compared with other subgroups, and atorvastatin significantly decreased the risk among white women, Hispanic women, and Hispanic men. Pravastatin and rosuvastatin decreased the risk of Alzheimer’s disease significantly among white women only.
These findings suggest that “certain patients facing multiple, otherwise equal statin alternatives for hyperlipidemia treatment, may reduce Alzheimer’s risk by using a particular statin. The right statin type for the right person at the right time may provide a relatively inexpensive means to lessen the burden of Alzheimer’s disease,” the investigators said.
This study was supported by the National Institute on Aging, the University of Southern California Zumberge Research Fund, and the Schaeffer-Amgen Fellowship Program.
—Mary Ann Moon
Suggested Reading
Zissimopoulos JM, Barthold D, Brinton RD, Joyce G. Sex and race differences in the association between statin use and the incidence of Alzheimer disease. JAMA Neurol. 2016 Dec 12 [Epub ahead of print].
What Are Safe and Efficacious Therapies for Restless Legs Syndrome in Adults?
The American Academy of Neurology (AAN) has published evidence-based recommendations for management of restless legs syndrome (RLS) in adults. The practice guideline was published online ahead of print November 16, 2016, in Neurology. The practice guideline addresses the question: What are safe and effective therapies, including both pharmacologic and nonpharmacologic approaches, for the symptoms and clinical consequences (eg, disturbed sleep, periodic limb movements in sleep, depression/anxiety, and decreased quality of life) of RLS in adults.
“When addressing RLS, clinicians and patients must first determine whether symptoms require treatment, the setting in which this practice guideline is relevant,” said John W. Winkelman, MD, PhD, and colleagues. Dr. Winkelman is an Associate Professor of Psychiatry at Harvard Medical School and Medical Director of the Sleep Health Center of Brigham and Women’s Hospital in Boston.
“Treatment should be considered if RLS symptoms interfere with sleep or daytime function to an important degree,” the guideline authors said. “Before determining the best treatment, it is important to first ensure there are no contributing factors to RLS symptoms (eg, iron deficiency or serotonergic antidepressants). The guidelines advise clinicians to consider prescribing a pharmacologic agent to reduce RLS symptoms in patients with moderate to severe primary RLS. There is strong (Level A) evidence for use of pramipexole, rotigotine, cabergoline, and gabapentin enacarbil; moderate evidence (Level B) supports ropinirole, pregabalin, and IV ferric carboxymaltose; and weak evidence (Level C) supports levodopa. When considering efficacy alone, clinicians may prefer cabergoline. It is rarely used in clinical practice for RLS, however, because it is associated with a risk of cardiac valvulopathy. Clinicians are also advised to consider the augmentation risks associated with dopaminergic agents.
For patients with periodic limb movement disorder, there is strong (Level A) evidence supporting ropinirole. Moderate evidence (Level B) supports pramipexole, rotigotine, cabergoline, and pregabalin; and weak evidence (Level C) supporting levodopa. The authors note insufficient evidence (Level U) for gabapentin enacarbil and ferric carboxymaltose. With regard to objective sleep measures (eg, total sleep time, sleep efficiency, sleep latency, wake after sleep onset) there is moderate evidence (Level B) supporting ropinirole, gabapentin, encarbil, and pregabalin. However, there is insufficient evidence (Level U) supporting pamipexole, rotigotine, cabergoline, or levodopa.
For subjective sleep measures, cabergoline and gabapentin enacarbil have Level A evidence; ropinirole, pramipexole, rotigotine, and pregabalin have Level B evidence; and levodopa and prolonged-release oxycodone/naloxone, and vibratory stimulation have Level C evidence. Insufficient evidence (Level U) exists for ferric carboxymaltose and iron sucrose.When patients with RLS fail to respond to other treatments, clinicians are advised to consider prescribing prolonged-release oxycodone/naloxone (Level C) or to consider nonpharmacologic options, including pneumatic compression (Level B), infrared spectroscopy or transcranial magnetic stimulation (Level C), and vibrating pads (Level C).
For iron-deficient patients with RLS (ferritin levels ≤ 75 µg/L), clinicians are advised to prescribe ferrous sulfate with vitamin C. In patients on hemodialysis with secondary RLS, clinicians are advised to prescribe vitamin C and E supplementation (Level B), ropinirole, levodopa, or exercise (Level C).
—Erica Tricarico
Suggested Reading
Winkelman JW, Armstrong MJ, Allen RP, et al. Practice guideline summary: treatment of restless legs syndrome in adults: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2016 Nov 16 [Epub ahead of print].
The American Academy of Neurology (AAN) has published evidence-based recommendations for management of restless legs syndrome (RLS) in adults. The practice guideline was published online ahead of print November 16, 2016, in Neurology. The practice guideline addresses the question: What are safe and effective therapies, including both pharmacologic and nonpharmacologic approaches, for the symptoms and clinical consequences (eg, disturbed sleep, periodic limb movements in sleep, depression/anxiety, and decreased quality of life) of RLS in adults.
“When addressing RLS, clinicians and patients must first determine whether symptoms require treatment, the setting in which this practice guideline is relevant,” said John W. Winkelman, MD, PhD, and colleagues. Dr. Winkelman is an Associate Professor of Psychiatry at Harvard Medical School and Medical Director of the Sleep Health Center of Brigham and Women’s Hospital in Boston.
“Treatment should be considered if RLS symptoms interfere with sleep or daytime function to an important degree,” the guideline authors said. “Before determining the best treatment, it is important to first ensure there are no contributing factors to RLS symptoms (eg, iron deficiency or serotonergic antidepressants). The guidelines advise clinicians to consider prescribing a pharmacologic agent to reduce RLS symptoms in patients with moderate to severe primary RLS. There is strong (Level A) evidence for use of pramipexole, rotigotine, cabergoline, and gabapentin enacarbil; moderate evidence (Level B) supports ropinirole, pregabalin, and IV ferric carboxymaltose; and weak evidence (Level C) supports levodopa. When considering efficacy alone, clinicians may prefer cabergoline. It is rarely used in clinical practice for RLS, however, because it is associated with a risk of cardiac valvulopathy. Clinicians are also advised to consider the augmentation risks associated with dopaminergic agents.
For patients with periodic limb movement disorder, there is strong (Level A) evidence supporting ropinirole. Moderate evidence (Level B) supports pramipexole, rotigotine, cabergoline, and pregabalin; and weak evidence (Level C) supporting levodopa. The authors note insufficient evidence (Level U) for gabapentin enacarbil and ferric carboxymaltose. With regard to objective sleep measures (eg, total sleep time, sleep efficiency, sleep latency, wake after sleep onset) there is moderate evidence (Level B) supporting ropinirole, gabapentin, encarbil, and pregabalin. However, there is insufficient evidence (Level U) supporting pamipexole, rotigotine, cabergoline, or levodopa.
For subjective sleep measures, cabergoline and gabapentin enacarbil have Level A evidence; ropinirole, pramipexole, rotigotine, and pregabalin have Level B evidence; and levodopa and prolonged-release oxycodone/naloxone, and vibratory stimulation have Level C evidence. Insufficient evidence (Level U) exists for ferric carboxymaltose and iron sucrose.When patients with RLS fail to respond to other treatments, clinicians are advised to consider prescribing prolonged-release oxycodone/naloxone (Level C) or to consider nonpharmacologic options, including pneumatic compression (Level B), infrared spectroscopy or transcranial magnetic stimulation (Level C), and vibrating pads (Level C).
For iron-deficient patients with RLS (ferritin levels ≤ 75 µg/L), clinicians are advised to prescribe ferrous sulfate with vitamin C. In patients on hemodialysis with secondary RLS, clinicians are advised to prescribe vitamin C and E supplementation (Level B), ropinirole, levodopa, or exercise (Level C).
—Erica Tricarico
Suggested Reading
Winkelman JW, Armstrong MJ, Allen RP, et al. Practice guideline summary: treatment of restless legs syndrome in adults: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2016 Nov 16 [Epub ahead of print].
The American Academy of Neurology (AAN) has published evidence-based recommendations for management of restless legs syndrome (RLS) in adults. The practice guideline was published online ahead of print November 16, 2016, in Neurology. The practice guideline addresses the question: What are safe and effective therapies, including both pharmacologic and nonpharmacologic approaches, for the symptoms and clinical consequences (eg, disturbed sleep, periodic limb movements in sleep, depression/anxiety, and decreased quality of life) of RLS in adults.
“When addressing RLS, clinicians and patients must first determine whether symptoms require treatment, the setting in which this practice guideline is relevant,” said John W. Winkelman, MD, PhD, and colleagues. Dr. Winkelman is an Associate Professor of Psychiatry at Harvard Medical School and Medical Director of the Sleep Health Center of Brigham and Women’s Hospital in Boston.
“Treatment should be considered if RLS symptoms interfere with sleep or daytime function to an important degree,” the guideline authors said. “Before determining the best treatment, it is important to first ensure there are no contributing factors to RLS symptoms (eg, iron deficiency or serotonergic antidepressants). The guidelines advise clinicians to consider prescribing a pharmacologic agent to reduce RLS symptoms in patients with moderate to severe primary RLS. There is strong (Level A) evidence for use of pramipexole, rotigotine, cabergoline, and gabapentin enacarbil; moderate evidence (Level B) supports ropinirole, pregabalin, and IV ferric carboxymaltose; and weak evidence (Level C) supports levodopa. When considering efficacy alone, clinicians may prefer cabergoline. It is rarely used in clinical practice for RLS, however, because it is associated with a risk of cardiac valvulopathy. Clinicians are also advised to consider the augmentation risks associated with dopaminergic agents.
For patients with periodic limb movement disorder, there is strong (Level A) evidence supporting ropinirole. Moderate evidence (Level B) supports pramipexole, rotigotine, cabergoline, and pregabalin; and weak evidence (Level C) supporting levodopa. The authors note insufficient evidence (Level U) for gabapentin enacarbil and ferric carboxymaltose. With regard to objective sleep measures (eg, total sleep time, sleep efficiency, sleep latency, wake after sleep onset) there is moderate evidence (Level B) supporting ropinirole, gabapentin, encarbil, and pregabalin. However, there is insufficient evidence (Level U) supporting pamipexole, rotigotine, cabergoline, or levodopa.
For subjective sleep measures, cabergoline and gabapentin enacarbil have Level A evidence; ropinirole, pramipexole, rotigotine, and pregabalin have Level B evidence; and levodopa and prolonged-release oxycodone/naloxone, and vibratory stimulation have Level C evidence. Insufficient evidence (Level U) exists for ferric carboxymaltose and iron sucrose.When patients with RLS fail to respond to other treatments, clinicians are advised to consider prescribing prolonged-release oxycodone/naloxone (Level C) or to consider nonpharmacologic options, including pneumatic compression (Level B), infrared spectroscopy or transcranial magnetic stimulation (Level C), and vibrating pads (Level C).
For iron-deficient patients with RLS (ferritin levels ≤ 75 µg/L), clinicians are advised to prescribe ferrous sulfate with vitamin C. In patients on hemodialysis with secondary RLS, clinicians are advised to prescribe vitamin C and E supplementation (Level B), ropinirole, levodopa, or exercise (Level C).
—Erica Tricarico
Suggested Reading
Winkelman JW, Armstrong MJ, Allen RP, et al. Practice guideline summary: treatment of restless legs syndrome in adults: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2016 Nov 16 [Epub ahead of print].
IV Ketamine May Be Effective as Subacute Treatment for Refractory Chronic Migraine
Ketamine may help to treat pain in patients with refractory chronic migraine, according to a case series published in the December 2016 Journal of Headache and Pain. IV ketamine treatment was associated with short-term improvement in pain severity in six of six patients with refractory chronic migraine.
“This study highlights the need for further research regarding new treatment options for patients who suffer daily consequences of refractory migraine and have failed many abortive and preventive medications,” said Clinton Lauritsen, MD, a Headache Fellow at Thomas Jefferson University Hospital in Philadelphia.
Ketamine is a dissociative anesthetic that acts on glutamate binding sites at the N-methyl-D-aspartate (NMDA) receptor, as well as at opioid, monoaminergic, cholinergic, nicotinic, and muscarinic receptors. IV ketamine was previously studied in several refractory pain conditions, including complex regional pain. While intranasal ketamine reduced the severity of migraine aura in a small randomized trial, the use of IV ketamine has only been reported in case series. Krusz et al showed improvement in pain scores in patients who used IV ketamine for refractory migraine; few side effects were reported.
Inpatient IV Ketamine
To further investigate the effect of IV ketamine in patients with intractable migraine, Dr. Lauritsen and colleagues conducted a retrospective chart review study. The researchers identified six patients with refractory chronic migraine admitted to Mount Sinai Beth Israel Hospital in New York from 2010 through 2014 for treatment with continuous IV ketamine.
Patients were given a starting dose of 0.1 mg/kg/h that was increased by 0.1 mg/kg/h every three to four hours as tolerated until the target pain score of 3 out of 10 was achieved and maintained for at least eight hours. Subsequently, the infusion was decreased by 0.2 mg/kg/h every three to four hours until the infusion rate reached 0 mg/kg/h.
The dose of ketamine was increased until maximum response was achieved or undesirable side effects, including psychomimetic and dysphoric effects, developed. Researchers used the Visual Analogue Score (VAS) at admission and during follow-up. VAS scores at different ketamine infusion rates were assessed from nursing and infusion records. Pain response was defined as a reduction in the initial VAS to a score of 3 or less. In addition, researchers attempted to contact patients for a telephone follow-up; however, they were only able to reach two of the six patients. During the telephone interview, researchers administered a questionnaire.
Pain Relief Achieved
Results from the data revealed a median age of 36.5 years; 83% of the patients were women. All of the patients were Caucasian, and the median age of migraine onset was 17. The median duration of the disease was 17 years. The mean number of failed acute migraine treatments was 18, and the mean number of failed preventive medications was 25. Pre-treatment pain scores ranged from 9 to 10.
In this small case series, all six patients with refractory migraine met
Overall, IV ketamine relieved pain in patients with chronic migraine without substantial adverse effects. “However, future study of this benefit on short-term headache relief needs to be conducted in a placebo-controlled fashion,” said Dr. Lauritsen.
“It is biologically plausible that ketamine could be an effective treatment for intractable headache,” the researchers said. “Ketamine is an antagonist at NMDA receptors, blocking the excitatory action of glutamate, a neurotransmitter long implicated in the pathophysiology of migraine. Glutamate has been … implicated in induction of cortical spreading depression [and] activation of trigeminal nociceptive neurons [and may] play a role in central sensitization.”
—Erica Tricarico
Suggested Reading
Lauritsen C, Mazuera S, Lipton RB, Ashina S. Intravenous ketamine for subacute treatment of refractory chronic migraine: a case series. J Headache Pain. 2016;17(1)106-110.
Ketamine may help to treat pain in patients with refractory chronic migraine, according to a case series published in the December 2016 Journal of Headache and Pain. IV ketamine treatment was associated with short-term improvement in pain severity in six of six patients with refractory chronic migraine.
“This study highlights the need for further research regarding new treatment options for patients who suffer daily consequences of refractory migraine and have failed many abortive and preventive medications,” said Clinton Lauritsen, MD, a Headache Fellow at Thomas Jefferson University Hospital in Philadelphia.
Ketamine is a dissociative anesthetic that acts on glutamate binding sites at the N-methyl-D-aspartate (NMDA) receptor, as well as at opioid, monoaminergic, cholinergic, nicotinic, and muscarinic receptors. IV ketamine was previously studied in several refractory pain conditions, including complex regional pain. While intranasal ketamine reduced the severity of migraine aura in a small randomized trial, the use of IV ketamine has only been reported in case series. Krusz et al showed improvement in pain scores in patients who used IV ketamine for refractory migraine; few side effects were reported.
Inpatient IV Ketamine
To further investigate the effect of IV ketamine in patients with intractable migraine, Dr. Lauritsen and colleagues conducted a retrospective chart review study. The researchers identified six patients with refractory chronic migraine admitted to Mount Sinai Beth Israel Hospital in New York from 2010 through 2014 for treatment with continuous IV ketamine.
Patients were given a starting dose of 0.1 mg/kg/h that was increased by 0.1 mg/kg/h every three to four hours as tolerated until the target pain score of 3 out of 10 was achieved and maintained for at least eight hours. Subsequently, the infusion was decreased by 0.2 mg/kg/h every three to four hours until the infusion rate reached 0 mg/kg/h.
The dose of ketamine was increased until maximum response was achieved or undesirable side effects, including psychomimetic and dysphoric effects, developed. Researchers used the Visual Analogue Score (VAS) at admission and during follow-up. VAS scores at different ketamine infusion rates were assessed from nursing and infusion records. Pain response was defined as a reduction in the initial VAS to a score of 3 or less. In addition, researchers attempted to contact patients for a telephone follow-up; however, they were only able to reach two of the six patients. During the telephone interview, researchers administered a questionnaire.
Pain Relief Achieved
Results from the data revealed a median age of 36.5 years; 83% of the patients were women. All of the patients were Caucasian, and the median age of migraine onset was 17. The median duration of the disease was 17 years. The mean number of failed acute migraine treatments was 18, and the mean number of failed preventive medications was 25. Pre-treatment pain scores ranged from 9 to 10.
In this small case series, all six patients with refractory migraine met
Overall, IV ketamine relieved pain in patients with chronic migraine without substantial adverse effects. “However, future study of this benefit on short-term headache relief needs to be conducted in a placebo-controlled fashion,” said Dr. Lauritsen.
“It is biologically plausible that ketamine could be an effective treatment for intractable headache,” the researchers said. “Ketamine is an antagonist at NMDA receptors, blocking the excitatory action of glutamate, a neurotransmitter long implicated in the pathophysiology of migraine. Glutamate has been … implicated in induction of cortical spreading depression [and] activation of trigeminal nociceptive neurons [and may] play a role in central sensitization.”
—Erica Tricarico
Suggested Reading
Lauritsen C, Mazuera S, Lipton RB, Ashina S. Intravenous ketamine for subacute treatment of refractory chronic migraine: a case series. J Headache Pain. 2016;17(1)106-110.
Ketamine may help to treat pain in patients with refractory chronic migraine, according to a case series published in the December 2016 Journal of Headache and Pain. IV ketamine treatment was associated with short-term improvement in pain severity in six of six patients with refractory chronic migraine.
“This study highlights the need for further research regarding new treatment options for patients who suffer daily consequences of refractory migraine and have failed many abortive and preventive medications,” said Clinton Lauritsen, MD, a Headache Fellow at Thomas Jefferson University Hospital in Philadelphia.
Ketamine is a dissociative anesthetic that acts on glutamate binding sites at the N-methyl-D-aspartate (NMDA) receptor, as well as at opioid, monoaminergic, cholinergic, nicotinic, and muscarinic receptors. IV ketamine was previously studied in several refractory pain conditions, including complex regional pain. While intranasal ketamine reduced the severity of migraine aura in a small randomized trial, the use of IV ketamine has only been reported in case series. Krusz et al showed improvement in pain scores in patients who used IV ketamine for refractory migraine; few side effects were reported.
Inpatient IV Ketamine
To further investigate the effect of IV ketamine in patients with intractable migraine, Dr. Lauritsen and colleagues conducted a retrospective chart review study. The researchers identified six patients with refractory chronic migraine admitted to Mount Sinai Beth Israel Hospital in New York from 2010 through 2014 for treatment with continuous IV ketamine.
Patients were given a starting dose of 0.1 mg/kg/h that was increased by 0.1 mg/kg/h every three to four hours as tolerated until the target pain score of 3 out of 10 was achieved and maintained for at least eight hours. Subsequently, the infusion was decreased by 0.2 mg/kg/h every three to four hours until the infusion rate reached 0 mg/kg/h.
The dose of ketamine was increased until maximum response was achieved or undesirable side effects, including psychomimetic and dysphoric effects, developed. Researchers used the Visual Analogue Score (VAS) at admission and during follow-up. VAS scores at different ketamine infusion rates were assessed from nursing and infusion records. Pain response was defined as a reduction in the initial VAS to a score of 3 or less. In addition, researchers attempted to contact patients for a telephone follow-up; however, they were only able to reach two of the six patients. During the telephone interview, researchers administered a questionnaire.
Pain Relief Achieved
Results from the data revealed a median age of 36.5 years; 83% of the patients were women. All of the patients were Caucasian, and the median age of migraine onset was 17. The median duration of the disease was 17 years. The mean number of failed acute migraine treatments was 18, and the mean number of failed preventive medications was 25. Pre-treatment pain scores ranged from 9 to 10.
In this small case series, all six patients with refractory migraine met
Overall, IV ketamine relieved pain in patients with chronic migraine without substantial adverse effects. “However, future study of this benefit on short-term headache relief needs to be conducted in a placebo-controlled fashion,” said Dr. Lauritsen.
“It is biologically plausible that ketamine could be an effective treatment for intractable headache,” the researchers said. “Ketamine is an antagonist at NMDA receptors, blocking the excitatory action of glutamate, a neurotransmitter long implicated in the pathophysiology of migraine. Glutamate has been … implicated in induction of cortical spreading depression [and] activation of trigeminal nociceptive neurons [and may] play a role in central sensitization.”
—Erica Tricarico
Suggested Reading
Lauritsen C, Mazuera S, Lipton RB, Ashina S. Intravenous ketamine for subacute treatment of refractory chronic migraine: a case series. J Headache Pain. 2016;17(1)106-110.
Pleth Variability Index shows promise for asthma assessments
Clinical question: Does pulse variability on plethysmography, or the Pleth Variability Index (PVI), correlate with disease severity in obstructive airway disease in children?
Background: Asthma is the most common reason for hospitalization in the United S. for children 3-12 years old. Asthma accounts for a quarter of ED visits for children aged 1-9 years old.1 Although systems have been developed to assess asthma exacerbation severity and the need for hospitalization, many of these depend on reassessments over time or have been proven to be invalid in larger studies.2,3,4 Pulsus paradoxus (PP), which is defined as a drop in systolic blood pressure greater than 10 mm Hg, correlates with the severity of obstruction in asthma exacerbations, but it is not practical in the children being evaluated in the ED or hospital.5,6 PP measurement using plethysmography has been found to correlate with measurement by sphygmomanometry.7 Furthermore, PVI, which is derived from amplitude variability in the pulse oximeter waveform, has been found to correlate with fluid responsiveness in mechanically ventilated patients. To this date, no study has assessed the correlation between PVI and exacerbation severity in asthma.
Setting: A 137-bed, tertiary-care children’s hospital.
Synopsis: Over a 6-month period on weekdays, researchers enrolled patients aged 1-18 years evaluated in the ED for asthma exacerbations or reactive airway disease. ED staff diagnosed patients clinically, and other patients with conditions known to affect PP – such as dehydration, croup, and cardiac disease – were excluded. PVI was calculated by measuring the minimum perfusion index (PImin) and the maximum perfusion index (PImax) using the following formula: 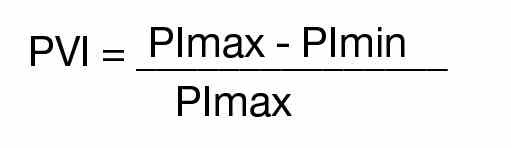
A printout of the first ED pulse oximetry reading was used to obtain the PImax and PImin as below:
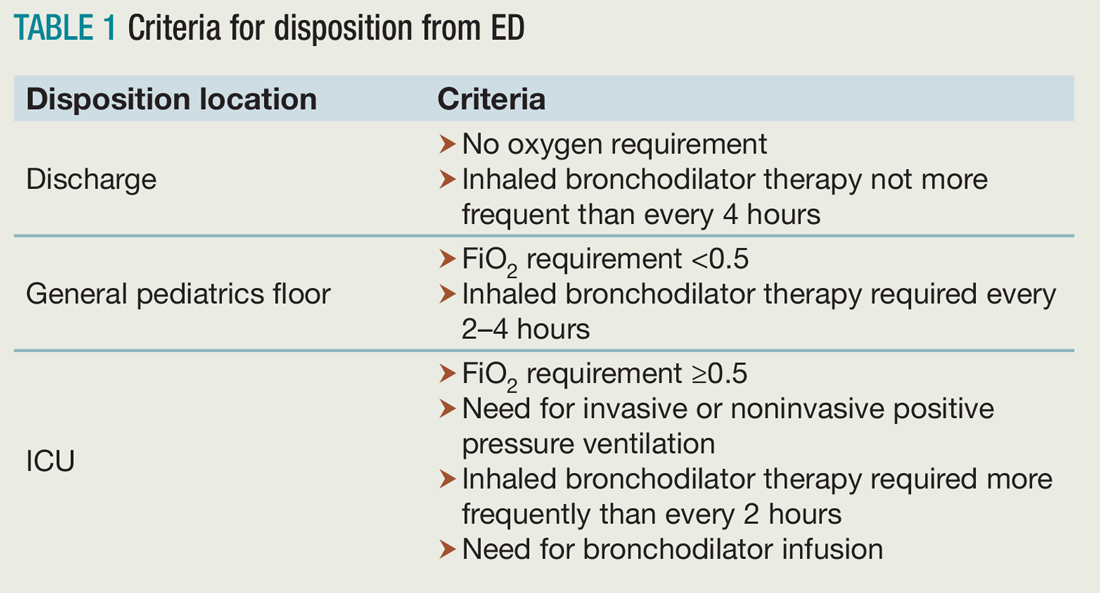
Researchers followed patients after the initial evaluation to determine disposition from the ED, which included either discharge to home, admission to a general pediatrics floor, or admission to the PICU. The hospital utilized specific criteria for disposition from the ED (see Table 1).
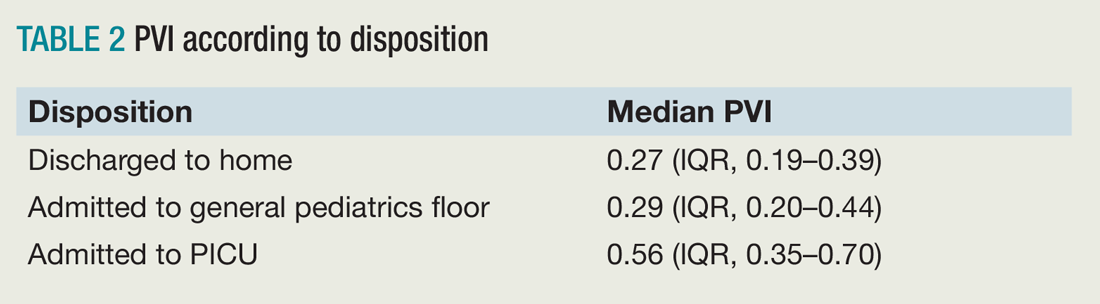
Of the 117 patients who were analyzed after application of exclusion criteria, 48 were discharged to home, 61 were admitted to a general pediatrics floor, and eight were admitted to the PICU. The three groups were found to be demographically similar. Researchers found a significant difference between the PVI of the three groups, but pairwise analysis showed no significant difference between the PVI of patients admitted to the general pediatrics floor versus discharged to home (see Table 2).
Bottom line: PVI shows promise as a tool to rapidly assess disease severity in pediatric patients being evaluated and treated for asthma, but further studies are needed to validate this in the ED and hospital setting.
Citation: Brandwein A, Patel K, Kline M, Silver P, Gangadharan S. Using pleth variability as a triage tool for children with obstructive airway disease in a pediatric emergency department [published online ahead of print Oct. 6, 2016]. Pediatr Emerg Care. doi: 10.1097/PEC.0000000000000887.
References
1. Care of children and adolescents in U.S. hospitals. Agency for Healthcare Research and Quality website. Available at: https://archive.ahrq.gov/data/hcup/factbk4/factbk4.htm. Accessed Nov. 18, 2016.
2. Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respir Med. 2004;98(8):777-781.
3. Keogh KA, Macarthur C, Parkin PC, et al. Predictors of hospitalization in children with acute asthma. J Pediatr. 2001;139(2):273-277.
4. Keahey L, Bulloch B, Becker AB, et al. Initial oxygen saturation as a predictor of admission in children presenting to the emergency department with acute asthma. Ann Emerg Med. 2002;40(3):300-307.
5. Guntheroth WG, Morgan BC, Mullins GL. Effect of respiration on venous return and stroke volume in cardiac tamponade. Mechanism of pulsus paradoxus. Circ Res. 1967;20(4):381-390.
6. Frey B, Freezer N. Diagnostic value and pathophysiologic basis of pulsus paradoxus in infants and children with respiratory disease. Pediatr Pulmonol. 2001;31(2):138-143.
7. Clark JA, Lieh-Lai M, Thomas R, Raghavan K, Sarnaik AP. Comparison of traditional and plethysmographic methods for measuring pulsus paradoxus. Arch Pediatr Adolesc Med. 2004;158(1):48-51.
Clinical question: Does pulse variability on plethysmography, or the Pleth Variability Index (PVI), correlate with disease severity in obstructive airway disease in children?
Background: Asthma is the most common reason for hospitalization in the United S. for children 3-12 years old. Asthma accounts for a quarter of ED visits for children aged 1-9 years old.1 Although systems have been developed to assess asthma exacerbation severity and the need for hospitalization, many of these depend on reassessments over time or have been proven to be invalid in larger studies.2,3,4 Pulsus paradoxus (PP), which is defined as a drop in systolic blood pressure greater than 10 mm Hg, correlates with the severity of obstruction in asthma exacerbations, but it is not practical in the children being evaluated in the ED or hospital.5,6 PP measurement using plethysmography has been found to correlate with measurement by sphygmomanometry.7 Furthermore, PVI, which is derived from amplitude variability in the pulse oximeter waveform, has been found to correlate with fluid responsiveness in mechanically ventilated patients. To this date, no study has assessed the correlation between PVI and exacerbation severity in asthma.
Setting: A 137-bed, tertiary-care children’s hospital.
Synopsis: Over a 6-month period on weekdays, researchers enrolled patients aged 1-18 years evaluated in the ED for asthma exacerbations or reactive airway disease. ED staff diagnosed patients clinically, and other patients with conditions known to affect PP – such as dehydration, croup, and cardiac disease – were excluded. PVI was calculated by measuring the minimum perfusion index (PImin) and the maximum perfusion index (PImax) using the following formula: 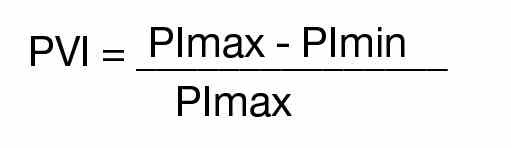
A printout of the first ED pulse oximetry reading was used to obtain the PImax and PImin as below:
Researchers followed patients after the initial evaluation to determine disposition from the ED, which included either discharge to home, admission to a general pediatrics floor, or admission to the PICU. The hospital utilized specific criteria for disposition from the ED (see Table 1).
Of the 117 patients who were analyzed after application of exclusion criteria, 48 were discharged to home, 61 were admitted to a general pediatrics floor, and eight were admitted to the PICU. The three groups were found to be demographically similar. Researchers found a significant difference between the PVI of the three groups, but pairwise analysis showed no significant difference between the PVI of patients admitted to the general pediatrics floor versus discharged to home (see Table 2).
Bottom line: PVI shows promise as a tool to rapidly assess disease severity in pediatric patients being evaluated and treated for asthma, but further studies are needed to validate this in the ED and hospital setting.
Citation: Brandwein A, Patel K, Kline M, Silver P, Gangadharan S. Using pleth variability as a triage tool for children with obstructive airway disease in a pediatric emergency department [published online ahead of print Oct. 6, 2016]. Pediatr Emerg Care. doi: 10.1097/PEC.0000000000000887.
References
1. Care of children and adolescents in U.S. hospitals. Agency for Healthcare Research and Quality website. Available at: https://archive.ahrq.gov/data/hcup/factbk4/factbk4.htm. Accessed Nov. 18, 2016.
2. Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respir Med. 2004;98(8):777-781.
3. Keogh KA, Macarthur C, Parkin PC, et al. Predictors of hospitalization in children with acute asthma. J Pediatr. 2001;139(2):273-277.
4. Keahey L, Bulloch B, Becker AB, et al. Initial oxygen saturation as a predictor of admission in children presenting to the emergency department with acute asthma. Ann Emerg Med. 2002;40(3):300-307.
5. Guntheroth WG, Morgan BC, Mullins GL. Effect of respiration on venous return and stroke volume in cardiac tamponade. Mechanism of pulsus paradoxus. Circ Res. 1967;20(4):381-390.
6. Frey B, Freezer N. Diagnostic value and pathophysiologic basis of pulsus paradoxus in infants and children with respiratory disease. Pediatr Pulmonol. 2001;31(2):138-143.
7. Clark JA, Lieh-Lai M, Thomas R, Raghavan K, Sarnaik AP. Comparison of traditional and plethysmographic methods for measuring pulsus paradoxus. Arch Pediatr Adolesc Med. 2004;158(1):48-51.
Clinical question: Does pulse variability on plethysmography, or the Pleth Variability Index (PVI), correlate with disease severity in obstructive airway disease in children?
Background: Asthma is the most common reason for hospitalization in the United S. for children 3-12 years old. Asthma accounts for a quarter of ED visits for children aged 1-9 years old.1 Although systems have been developed to assess asthma exacerbation severity and the need for hospitalization, many of these depend on reassessments over time or have been proven to be invalid in larger studies.2,3,4 Pulsus paradoxus (PP), which is defined as a drop in systolic blood pressure greater than 10 mm Hg, correlates with the severity of obstruction in asthma exacerbations, but it is not practical in the children being evaluated in the ED or hospital.5,6 PP measurement using plethysmography has been found to correlate with measurement by sphygmomanometry.7 Furthermore, PVI, which is derived from amplitude variability in the pulse oximeter waveform, has been found to correlate with fluid responsiveness in mechanically ventilated patients. To this date, no study has assessed the correlation between PVI and exacerbation severity in asthma.
Setting: A 137-bed, tertiary-care children’s hospital.
Synopsis: Over a 6-month period on weekdays, researchers enrolled patients aged 1-18 years evaluated in the ED for asthma exacerbations or reactive airway disease. ED staff diagnosed patients clinically, and other patients with conditions known to affect PP – such as dehydration, croup, and cardiac disease – were excluded. PVI was calculated by measuring the minimum perfusion index (PImin) and the maximum perfusion index (PImax) using the following formula: 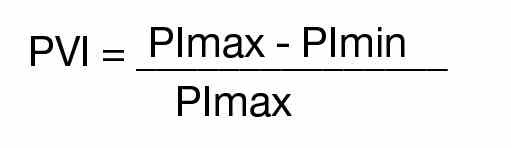
A printout of the first ED pulse oximetry reading was used to obtain the PImax and PImin as below:
Researchers followed patients after the initial evaluation to determine disposition from the ED, which included either discharge to home, admission to a general pediatrics floor, or admission to the PICU. The hospital utilized specific criteria for disposition from the ED (see Table 1).
Of the 117 patients who were analyzed after application of exclusion criteria, 48 were discharged to home, 61 were admitted to a general pediatrics floor, and eight were admitted to the PICU. The three groups were found to be demographically similar. Researchers found a significant difference between the PVI of the three groups, but pairwise analysis showed no significant difference between the PVI of patients admitted to the general pediatrics floor versus discharged to home (see Table 2).
Bottom line: PVI shows promise as a tool to rapidly assess disease severity in pediatric patients being evaluated and treated for asthma, but further studies are needed to validate this in the ED and hospital setting.
Citation: Brandwein A, Patel K, Kline M, Silver P, Gangadharan S. Using pleth variability as a triage tool for children with obstructive airway disease in a pediatric emergency department [published online ahead of print Oct. 6, 2016]. Pediatr Emerg Care. doi: 10.1097/PEC.0000000000000887.
References
1. Care of children and adolescents in U.S. hospitals. Agency for Healthcare Research and Quality website. Available at: https://archive.ahrq.gov/data/hcup/factbk4/factbk4.htm. Accessed Nov. 18, 2016.
2. Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respir Med. 2004;98(8):777-781.
3. Keogh KA, Macarthur C, Parkin PC, et al. Predictors of hospitalization in children with acute asthma. J Pediatr. 2001;139(2):273-277.
4. Keahey L, Bulloch B, Becker AB, et al. Initial oxygen saturation as a predictor of admission in children presenting to the emergency department with acute asthma. Ann Emerg Med. 2002;40(3):300-307.
5. Guntheroth WG, Morgan BC, Mullins GL. Effect of respiration on venous return and stroke volume in cardiac tamponade. Mechanism of pulsus paradoxus. Circ Res. 1967;20(4):381-390.
6. Frey B, Freezer N. Diagnostic value and pathophysiologic basis of pulsus paradoxus in infants and children with respiratory disease. Pediatr Pulmonol. 2001;31(2):138-143.
7. Clark JA, Lieh-Lai M, Thomas R, Raghavan K, Sarnaik AP. Comparison of traditional and plethysmographic methods for measuring pulsus paradoxus. Arch Pediatr Adolesc Med. 2004;158(1):48-51.
Gene Mutation Linked to Early Onset of Parkinson’s Disease
A defect in a gene that produces dopamine in nigrostriatal cells appears to accelerate the onset of Parkinson’s disease, according to a study published in the February issue of Neurobiology of Aging. The effect is particularly pronounced for people younger than 50.
Auriel Willette, PhD, an Assistant Professor in the Departments of Food Science and Human Nutrition and Psychology at Iowa State University in Ames, and Joseph Webb, a graduate research assistant also at Iowa State, found on average that Caucasians with one mutated version of the gene—guanosine triphosphate cyclohydrolase-1 (GCH1)—had a 23% increased risk for Parkinson’s disease and developed symptoms five years earlier than those without the gene mutation.
However, young-to-middle-aged adults with the mutation had a 45% increased risk of developing Parkinson’s disease. Researchers said that the presence of the mutated gene in older adults had minimal effect.
The Potential for Personalized Medicine
It is widely known that rigidity and loss of muscle function associated with Parkinson’s disease are linked to a depletion of dopamine in the substantia nigra. Taking a holistic approach to their study, Dr. Willette and Mr. Webb sought to better understand how the GCH1 gene affects the course of Parkinson’s disease and certain outcomes such as motor skills and anxiety.
The study is the first to look at these biological markers, as well as the first to examine how the gene’s impact on dopamine production specifically affects Caucasian populations. Previous studies have focused primarily on Chinese and Taiwanese populations, said Dr. Willette. The findings have the potential to help personalize medical care for people with a family history of Parkinson’s disease, he said, in a way similar to testing for the BRCA gene in women at risk for breast cancer.
“We want to have a more comprehensive understanding of what these genes related to Parkinson’s disease are doing at different points in someone’s lifetime,” Dr. Willette said. “Then, with genetic testing we can determine the risk for illness based on someone’s age, gender, weight, and other intervening factors.”
The Impact of Age
Data for the study were collected through the Parkinson’s Progression Markers Initiative, a public–private partnership sponsored by the Michael J. Fox Foundation for Parkinson’s Research. The initiative evaluates people with the disease to develop new and better treatments. The Iowa State study included 289 treatment-naïve people recently diagnosed with Parkinson’s disease and 233 healthy controls.
The researchers analyzed anxiety and motor function using the Unified Parkinson’s Disease Rating Scale (UPDRS). They found that those with polymorphisms at rs11158026 coding for the GCH1 enzyme, regardless of age, were more anxious and struggled more with daily activities. The defective gene was not as strong of a predictor of developing Parkinson’s disease in people older than 50, however.
“As we age, we make progressively less dopamine, and this effect strongly outweighs the genetic influences from the ‘bad version’ of this gene,” said Mr. Webb. “Simply by aging, our dopamine production decreases to the point that the effects from a mutation in this gene are not noticeable in older adults, but make a big difference in younger populations.”
The researchers noted that it is also important to pay attention to blood cholesterol levels. Cholesterol is directly related to the ability to produce dopamine. High low-density lipoprotein (LDL) is an established risk factor for Parkinson’s disease, Dr. Willette said. Their study shows that carriers of the mutated GCH1 gene had higher cholesterol than noncarriers, regardless of age.
Suggested Reading
Webb J, Willette AA. Aging modifies the effect of GCH1 RS11158026 on DAT uptake and Parkinson’s disease clinical severity. Neurobiol Aging. 2017;50:39-46.
A defect in a gene that produces dopamine in nigrostriatal cells appears to accelerate the onset of Parkinson’s disease, according to a study published in the February issue of Neurobiology of Aging. The effect is particularly pronounced for people younger than 50.
Auriel Willette, PhD, an Assistant Professor in the Departments of Food Science and Human Nutrition and Psychology at Iowa State University in Ames, and Joseph Webb, a graduate research assistant also at Iowa State, found on average that Caucasians with one mutated version of the gene—guanosine triphosphate cyclohydrolase-1 (GCH1)—had a 23% increased risk for Parkinson’s disease and developed symptoms five years earlier than those without the gene mutation.
However, young-to-middle-aged adults with the mutation had a 45% increased risk of developing Parkinson’s disease. Researchers said that the presence of the mutated gene in older adults had minimal effect.
The Potential for Personalized Medicine
It is widely known that rigidity and loss of muscle function associated with Parkinson’s disease are linked to a depletion of dopamine in the substantia nigra. Taking a holistic approach to their study, Dr. Willette and Mr. Webb sought to better understand how the GCH1 gene affects the course of Parkinson’s disease and certain outcomes such as motor skills and anxiety.
The study is the first to look at these biological markers, as well as the first to examine how the gene’s impact on dopamine production specifically affects Caucasian populations. Previous studies have focused primarily on Chinese and Taiwanese populations, said Dr. Willette. The findings have the potential to help personalize medical care for people with a family history of Parkinson’s disease, he said, in a way similar to testing for the BRCA gene in women at risk for breast cancer.
“We want to have a more comprehensive understanding of what these genes related to Parkinson’s disease are doing at different points in someone’s lifetime,” Dr. Willette said. “Then, with genetic testing we can determine the risk for illness based on someone’s age, gender, weight, and other intervening factors.”
The Impact of Age
Data for the study were collected through the Parkinson’s Progression Markers Initiative, a public–private partnership sponsored by the Michael J. Fox Foundation for Parkinson’s Research. The initiative evaluates people with the disease to develop new and better treatments. The Iowa State study included 289 treatment-naïve people recently diagnosed with Parkinson’s disease and 233 healthy controls.
The researchers analyzed anxiety and motor function using the Unified Parkinson’s Disease Rating Scale (UPDRS). They found that those with polymorphisms at rs11158026 coding for the GCH1 enzyme, regardless of age, were more anxious and struggled more with daily activities. The defective gene was not as strong of a predictor of developing Parkinson’s disease in people older than 50, however.
“As we age, we make progressively less dopamine, and this effect strongly outweighs the genetic influences from the ‘bad version’ of this gene,” said Mr. Webb. “Simply by aging, our dopamine production decreases to the point that the effects from a mutation in this gene are not noticeable in older adults, but make a big difference in younger populations.”
The researchers noted that it is also important to pay attention to blood cholesterol levels. Cholesterol is directly related to the ability to produce dopamine. High low-density lipoprotein (LDL) is an established risk factor for Parkinson’s disease, Dr. Willette said. Their study shows that carriers of the mutated GCH1 gene had higher cholesterol than noncarriers, regardless of age.
Suggested Reading
Webb J, Willette AA. Aging modifies the effect of GCH1 RS11158026 on DAT uptake and Parkinson’s disease clinical severity. Neurobiol Aging. 2017;50:39-46.
A defect in a gene that produces dopamine in nigrostriatal cells appears to accelerate the onset of Parkinson’s disease, according to a study published in the February issue of Neurobiology of Aging. The effect is particularly pronounced for people younger than 50.
Auriel Willette, PhD, an Assistant Professor in the Departments of Food Science and Human Nutrition and Psychology at Iowa State University in Ames, and Joseph Webb, a graduate research assistant also at Iowa State, found on average that Caucasians with one mutated version of the gene—guanosine triphosphate cyclohydrolase-1 (GCH1)—had a 23% increased risk for Parkinson’s disease and developed symptoms five years earlier than those without the gene mutation.
However, young-to-middle-aged adults with the mutation had a 45% increased risk of developing Parkinson’s disease. Researchers said that the presence of the mutated gene in older adults had minimal effect.
The Potential for Personalized Medicine
It is widely known that rigidity and loss of muscle function associated with Parkinson’s disease are linked to a depletion of dopamine in the substantia nigra. Taking a holistic approach to their study, Dr. Willette and Mr. Webb sought to better understand how the GCH1 gene affects the course of Parkinson’s disease and certain outcomes such as motor skills and anxiety.
The study is the first to look at these biological markers, as well as the first to examine how the gene’s impact on dopamine production specifically affects Caucasian populations. Previous studies have focused primarily on Chinese and Taiwanese populations, said Dr. Willette. The findings have the potential to help personalize medical care for people with a family history of Parkinson’s disease, he said, in a way similar to testing for the BRCA gene in women at risk for breast cancer.
“We want to have a more comprehensive understanding of what these genes related to Parkinson’s disease are doing at different points in someone’s lifetime,” Dr. Willette said. “Then, with genetic testing we can determine the risk for illness based on someone’s age, gender, weight, and other intervening factors.”
The Impact of Age
Data for the study were collected through the Parkinson’s Progression Markers Initiative, a public–private partnership sponsored by the Michael J. Fox Foundation for Parkinson’s Research. The initiative evaluates people with the disease to develop new and better treatments. The Iowa State study included 289 treatment-naïve people recently diagnosed with Parkinson’s disease and 233 healthy controls.
The researchers analyzed anxiety and motor function using the Unified Parkinson’s Disease Rating Scale (UPDRS). They found that those with polymorphisms at rs11158026 coding for the GCH1 enzyme, regardless of age, were more anxious and struggled more with daily activities. The defective gene was not as strong of a predictor of developing Parkinson’s disease in people older than 50, however.
“As we age, we make progressively less dopamine, and this effect strongly outweighs the genetic influences from the ‘bad version’ of this gene,” said Mr. Webb. “Simply by aging, our dopamine production decreases to the point that the effects from a mutation in this gene are not noticeable in older adults, but make a big difference in younger populations.”
The researchers noted that it is also important to pay attention to blood cholesterol levels. Cholesterol is directly related to the ability to produce dopamine. High low-density lipoprotein (LDL) is an established risk factor for Parkinson’s disease, Dr. Willette said. Their study shows that carriers of the mutated GCH1 gene had higher cholesterol than noncarriers, regardless of age.
Suggested Reading
Webb J, Willette AA. Aging modifies the effect of GCH1 RS11158026 on DAT uptake and Parkinson’s disease clinical severity. Neurobiol Aging. 2017;50:39-46.