User login
The Hospitalist only
CHAMP: A Real Winner at Teaching Geriatrics
The elderly constitute the fastest-growing segment of the U.S. population. According to one estimate, nearly one in five Americans will be 65 years old or older by 2050.1 Geriatric medicine has produced a plethora of information regarding older patients’ special needs, but when it comes to teaching medical students and residents, most curricular materials focus on the care and management of older outpatients, rather than inpatients. In an effort to fill this gap, faculty at the University of Chicago School of Medicine developed the Curriculum for the Hospitalized Aging Medical Patient (CHAMP). It is designed to help instructors teach the management of elderly inpatients. In this month’s issue of the Journal of Hospital Medicine, lead author Paula Podrazik, MD, associate professor in the section of geriatrics, department of medicine, University of Chicago, and her co-authors explain CHAMP as it was perceived by a targeted group of faculty learners.
—Paula Podrazik, MD, associate professor in the section of geriatrics, department of medicine, University of Chicago
CHAMP incorporates knowledge gleaned from first-hand experience and a review of the literature and existing models of care. “Our goal was to improve patient care and systems of hospital care through education by faculty development,” Dr. Podrazik tells The Hospitalist. The CHAMP program emphasizes issues of particular importance in geriatric hospital medicine, including frailty, avoiding hazards of hospitalization, palliation, and care transitions.
For example, “hospitalists need to know certain aspects of dementia care, such as how to recognize it and screen for it,” she explains. “They have to determine whether a particular patient is able to make decisions, and they have to understand what it is about this condition that puts these patients at higher risk in the hospital.” Another example includes medication review and “communicating medication changes when transitioning the patient to a skilled nursing facility, home, or a rehabilitation center.”
Dr. Podrazik and her colleagues hope CHAMP might entice more medical students and residents to consider entering geriatric medicine. “Half of the [hospital] beds in the U.S. are filled with patients who are at least 65 years old. Many students and residents base their career decisions on what they see during their hospital rotation, so this was a great opportunity for us, as geriatricians, to influence that decision.”
The program consists of learning modules presented in 12, four-hour sessions. The modules address four basic themes:
- Identification of the frail or vulnerable elderly patient;
- Recognition and avoidance of hospitalization hazards, such as falls and dementia;
- Palliative care and end-of-life issues; and
- Improving transitions of care.
Each module has specific learning objectives and an evaluation process based on the standard precepts of curriculum design. The first part of each session covers topics on geriatric inpatient medicine, such as high-risk medications, medication reconciliation, restraint use, care transitions, and other aspects of mandates from The Joint Commission, which have particular relevance to the care of elderly people. Faculty participants listen to 30- to 90-minute lectures on each topic, with an emphasis on applying the content during bedside teaching rounds.
Modules presented in the second half of the session cover teaching techniques, such as the Stanford Faculty Development Program for Medical Teachers, which uses case scenarios and practice sessions to polish participants’ teaching skills. Another component specifically developed for CHAMP is a mini-course called “Teaching on Today’s Wards.” It is designed to help non-geriatric faculty put more geriatrics content in their bedside rounds, and to improve bedside teaching techniques in the inpatient wards.
The CHAMP curriculum also addresses the core competencies designated by the Accreditation Council for Graduate Medical Education (ACGME), namely professionalism, communication, systems-based practice, and practice-based learning and improvement.
The basic principles of geriatric care already exist, Dr. Podrazik says. “It was our job to pull it all together,” she explains. “A program of this size and magnitude couldn’t have been done without the participation of people in a multitude of areas, including hospitalists, geriatricians, internists, and PhD educators. We had multiple champions who took different areas and just ran with them.”
With eight faculty scholars volunteering to serve as guinea pigs, Dr. Podrazik and her colleagues pilot-tested the program in the spring of 2004. By 2006, another 21 faculty members had participated in CHAMP, including nearly half of the university’s general medicine faculty and most of its hospitalists. The response was enthusiastic, she says, with learners praising the presentation of geriatric issues and concrete suggestions for incorporating the information in their own teaching sessions. Upon completion of the CHAMP series, participants reported feeling significantly more knowledgeable about geriatric content, had more positive attitudes toward older patients, and felt more confident in their ability to care for older patients and teach geriatric medicine.
A major challenge was “providing enough ongoing support to reinforce learning with an eye on the greater goal of changing teaching behaviors and clinical outcomes,” the authors wrote. To solve this problem, they added objective structural teaching evaluations (OSTEs), so participants could test their teaching skills and mastery of geriatric content. Practice-oriented games, exercises, and tutorials, and ongoing contact with CHAMP alumnae and faculty are provided, as well as access to support materials online. Efforts are under way to incorporate core CHAMP faculty members into hospitalist and general medicine lecture series. Also being considered is having a CHAMP core faculty member attend during inpatient ward rounds.
It appears as though CHAMP is starting to pay off, in terms of patient care, Dr. Podrazik says. Although she cautioned the findings are “really preliminary,” and data analysis is ongoing, clinical data “do show a beneficial effect on a number of patient care outcomes.” TH
Norra MacReady is a medical writer based in California.
Reference
1. Passel JS, Cohn D. U.S. population projections: 2005-2050. Pew Research Center. http://pewhispanic.org/reports/report.php?ReportID=85. Published February 11, 2008. Accessed Thursday, October 23, 2008.
The elderly constitute the fastest-growing segment of the U.S. population. According to one estimate, nearly one in five Americans will be 65 years old or older by 2050.1 Geriatric medicine has produced a plethora of information regarding older patients’ special needs, but when it comes to teaching medical students and residents, most curricular materials focus on the care and management of older outpatients, rather than inpatients. In an effort to fill this gap, faculty at the University of Chicago School of Medicine developed the Curriculum for the Hospitalized Aging Medical Patient (CHAMP). It is designed to help instructors teach the management of elderly inpatients. In this month’s issue of the Journal of Hospital Medicine, lead author Paula Podrazik, MD, associate professor in the section of geriatrics, department of medicine, University of Chicago, and her co-authors explain CHAMP as it was perceived by a targeted group of faculty learners.
—Paula Podrazik, MD, associate professor in the section of geriatrics, department of medicine, University of Chicago
CHAMP incorporates knowledge gleaned from first-hand experience and a review of the literature and existing models of care. “Our goal was to improve patient care and systems of hospital care through education by faculty development,” Dr. Podrazik tells The Hospitalist. The CHAMP program emphasizes issues of particular importance in geriatric hospital medicine, including frailty, avoiding hazards of hospitalization, palliation, and care transitions.
For example, “hospitalists need to know certain aspects of dementia care, such as how to recognize it and screen for it,” she explains. “They have to determine whether a particular patient is able to make decisions, and they have to understand what it is about this condition that puts these patients at higher risk in the hospital.” Another example includes medication review and “communicating medication changes when transitioning the patient to a skilled nursing facility, home, or a rehabilitation center.”
Dr. Podrazik and her colleagues hope CHAMP might entice more medical students and residents to consider entering geriatric medicine. “Half of the [hospital] beds in the U.S. are filled with patients who are at least 65 years old. Many students and residents base their career decisions on what they see during their hospital rotation, so this was a great opportunity for us, as geriatricians, to influence that decision.”
The program consists of learning modules presented in 12, four-hour sessions. The modules address four basic themes:
- Identification of the frail or vulnerable elderly patient;
- Recognition and avoidance of hospitalization hazards, such as falls and dementia;
- Palliative care and end-of-life issues; and
- Improving transitions of care.
Each module has specific learning objectives and an evaluation process based on the standard precepts of curriculum design. The first part of each session covers topics on geriatric inpatient medicine, such as high-risk medications, medication reconciliation, restraint use, care transitions, and other aspects of mandates from The Joint Commission, which have particular relevance to the care of elderly people. Faculty participants listen to 30- to 90-minute lectures on each topic, with an emphasis on applying the content during bedside teaching rounds.
Modules presented in the second half of the session cover teaching techniques, such as the Stanford Faculty Development Program for Medical Teachers, which uses case scenarios and practice sessions to polish participants’ teaching skills. Another component specifically developed for CHAMP is a mini-course called “Teaching on Today’s Wards.” It is designed to help non-geriatric faculty put more geriatrics content in their bedside rounds, and to improve bedside teaching techniques in the inpatient wards.
The CHAMP curriculum also addresses the core competencies designated by the Accreditation Council for Graduate Medical Education (ACGME), namely professionalism, communication, systems-based practice, and practice-based learning and improvement.
The basic principles of geriatric care already exist, Dr. Podrazik says. “It was our job to pull it all together,” she explains. “A program of this size and magnitude couldn’t have been done without the participation of people in a multitude of areas, including hospitalists, geriatricians, internists, and PhD educators. We had multiple champions who took different areas and just ran with them.”
With eight faculty scholars volunteering to serve as guinea pigs, Dr. Podrazik and her colleagues pilot-tested the program in the spring of 2004. By 2006, another 21 faculty members had participated in CHAMP, including nearly half of the university’s general medicine faculty and most of its hospitalists. The response was enthusiastic, she says, with learners praising the presentation of geriatric issues and concrete suggestions for incorporating the information in their own teaching sessions. Upon completion of the CHAMP series, participants reported feeling significantly more knowledgeable about geriatric content, had more positive attitudes toward older patients, and felt more confident in their ability to care for older patients and teach geriatric medicine.
A major challenge was “providing enough ongoing support to reinforce learning with an eye on the greater goal of changing teaching behaviors and clinical outcomes,” the authors wrote. To solve this problem, they added objective structural teaching evaluations (OSTEs), so participants could test their teaching skills and mastery of geriatric content. Practice-oriented games, exercises, and tutorials, and ongoing contact with CHAMP alumnae and faculty are provided, as well as access to support materials online. Efforts are under way to incorporate core CHAMP faculty members into hospitalist and general medicine lecture series. Also being considered is having a CHAMP core faculty member attend during inpatient ward rounds.
It appears as though CHAMP is starting to pay off, in terms of patient care, Dr. Podrazik says. Although she cautioned the findings are “really preliminary,” and data analysis is ongoing, clinical data “do show a beneficial effect on a number of patient care outcomes.” TH
Norra MacReady is a medical writer based in California.
Reference
1. Passel JS, Cohn D. U.S. population projections: 2005-2050. Pew Research Center. http://pewhispanic.org/reports/report.php?ReportID=85. Published February 11, 2008. Accessed Thursday, October 23, 2008.
The elderly constitute the fastest-growing segment of the U.S. population. According to one estimate, nearly one in five Americans will be 65 years old or older by 2050.1 Geriatric medicine has produced a plethora of information regarding older patients’ special needs, but when it comes to teaching medical students and residents, most curricular materials focus on the care and management of older outpatients, rather than inpatients. In an effort to fill this gap, faculty at the University of Chicago School of Medicine developed the Curriculum for the Hospitalized Aging Medical Patient (CHAMP). It is designed to help instructors teach the management of elderly inpatients. In this month’s issue of the Journal of Hospital Medicine, lead author Paula Podrazik, MD, associate professor in the section of geriatrics, department of medicine, University of Chicago, and her co-authors explain CHAMP as it was perceived by a targeted group of faculty learners.
—Paula Podrazik, MD, associate professor in the section of geriatrics, department of medicine, University of Chicago
CHAMP incorporates knowledge gleaned from first-hand experience and a review of the literature and existing models of care. “Our goal was to improve patient care and systems of hospital care through education by faculty development,” Dr. Podrazik tells The Hospitalist. The CHAMP program emphasizes issues of particular importance in geriatric hospital medicine, including frailty, avoiding hazards of hospitalization, palliation, and care transitions.
For example, “hospitalists need to know certain aspects of dementia care, such as how to recognize it and screen for it,” she explains. “They have to determine whether a particular patient is able to make decisions, and they have to understand what it is about this condition that puts these patients at higher risk in the hospital.” Another example includes medication review and “communicating medication changes when transitioning the patient to a skilled nursing facility, home, or a rehabilitation center.”
Dr. Podrazik and her colleagues hope CHAMP might entice more medical students and residents to consider entering geriatric medicine. “Half of the [hospital] beds in the U.S. are filled with patients who are at least 65 years old. Many students and residents base their career decisions on what they see during their hospital rotation, so this was a great opportunity for us, as geriatricians, to influence that decision.”
The program consists of learning modules presented in 12, four-hour sessions. The modules address four basic themes:
- Identification of the frail or vulnerable elderly patient;
- Recognition and avoidance of hospitalization hazards, such as falls and dementia;
- Palliative care and end-of-life issues; and
- Improving transitions of care.
Each module has specific learning objectives and an evaluation process based on the standard precepts of curriculum design. The first part of each session covers topics on geriatric inpatient medicine, such as high-risk medications, medication reconciliation, restraint use, care transitions, and other aspects of mandates from The Joint Commission, which have particular relevance to the care of elderly people. Faculty participants listen to 30- to 90-minute lectures on each topic, with an emphasis on applying the content during bedside teaching rounds.
Modules presented in the second half of the session cover teaching techniques, such as the Stanford Faculty Development Program for Medical Teachers, which uses case scenarios and practice sessions to polish participants’ teaching skills. Another component specifically developed for CHAMP is a mini-course called “Teaching on Today’s Wards.” It is designed to help non-geriatric faculty put more geriatrics content in their bedside rounds, and to improve bedside teaching techniques in the inpatient wards.
The CHAMP curriculum also addresses the core competencies designated by the Accreditation Council for Graduate Medical Education (ACGME), namely professionalism, communication, systems-based practice, and practice-based learning and improvement.
The basic principles of geriatric care already exist, Dr. Podrazik says. “It was our job to pull it all together,” she explains. “A program of this size and magnitude couldn’t have been done without the participation of people in a multitude of areas, including hospitalists, geriatricians, internists, and PhD educators. We had multiple champions who took different areas and just ran with them.”
With eight faculty scholars volunteering to serve as guinea pigs, Dr. Podrazik and her colleagues pilot-tested the program in the spring of 2004. By 2006, another 21 faculty members had participated in CHAMP, including nearly half of the university’s general medicine faculty and most of its hospitalists. The response was enthusiastic, she says, with learners praising the presentation of geriatric issues and concrete suggestions for incorporating the information in their own teaching sessions. Upon completion of the CHAMP series, participants reported feeling significantly more knowledgeable about geriatric content, had more positive attitudes toward older patients, and felt more confident in their ability to care for older patients and teach geriatric medicine.
A major challenge was “providing enough ongoing support to reinforce learning with an eye on the greater goal of changing teaching behaviors and clinical outcomes,” the authors wrote. To solve this problem, they added objective structural teaching evaluations (OSTEs), so participants could test their teaching skills and mastery of geriatric content. Practice-oriented games, exercises, and tutorials, and ongoing contact with CHAMP alumnae and faculty are provided, as well as access to support materials online. Efforts are under way to incorporate core CHAMP faculty members into hospitalist and general medicine lecture series. Also being considered is having a CHAMP core faculty member attend during inpatient ward rounds.
It appears as though CHAMP is starting to pay off, in terms of patient care, Dr. Podrazik says. Although she cautioned the findings are “really preliminary,” and data analysis is ongoing, clinical data “do show a beneficial effect on a number of patient care outcomes.” TH
Norra MacReady is a medical writer based in California.
Reference
1. Passel JS, Cohn D. U.S. population projections: 2005-2050. Pew Research Center. http://pewhispanic.org/reports/report.php?ReportID=85. Published February 11, 2008. Accessed Thursday, October 23, 2008.
End of '08 Drug Update
The FDA has approved the first nucleic acid HBV viral DNA test for measuring HBV viral load from a patient’s blood. Via HBV viral load assessment, healthcare professionals now have a highly sensitive method for gauging antiviral therapy progress in patients with chronic HBV infections.
The test is known as the COBAS TaqMan HBV Test (Roche Diagnostic Division). It is used to measure HBV levels before beginning treatment, and then follow-up levels during treatment to assess therapy response. It is estimated that approximately 1.25 million people in the U.S. are infected with HBV, with approximately 60,000 becoming infected each year. About 5,000 people die from HBV-related complications each year.8
New Warnings
In October 2007, the Federal Drug and Food Administration (FDA) issued information for healthcare professionals regarding the subcutaneous use of exenatide (Byetta, Amylin Pharmaceuti-cals).9 Since then, the FDA has received at least six additional case reports of necrotizing or pancreatitis in patients taking exenatide.
Of these six cases, all patients needed hospitalization, two patients died, and four were recovering at the time of the reporting. Exenatide was discontinued in all of these patients.
If pancreatitis is suspected, exenatide and other potentially suspect drugs should be discontinued. There are no signs or symptoms distinguishing acute hemorrhagic or necrotizing pancreatitis associated with exenatide from less severe forms of pancreatitis. If pancreatitis is confirmed, appropriate treatment should be initiated and patients should be carefully monitored until they fully recover. Exenatide should not be restarted. The FDA is working with Amylin Pharmaceuticals to add stronger and more prominent warnings to the product label regarding the noted risks.
Since the last warning of natalizumab injection (Tysabri, Biogen IDEC), the FDA has informed healthcare professionals of two new cases of progressive multifocal leukoencephalopathy (PML) in European patients receiving it for more than a year as monotherapy for multiple sclerosis (MS).10
The agent currently is FDA approved to treat multiple sclerosis and Crohn’s disease. Approximately 39,000 patients have received treatment worldwide, with approximately 12,000 patients receiving treatment for at least a year. No new cases have been reported in the U.S., where approximately 7,500 patients have received the drug for more than a year and approximately 3,300 have received the drug for more than 18 months.
The FDA still believes natalizumab monotherapy may confer a lower risk of PML than usage with other immunomodulatory medications. Prescribing information for natalizumab has been revised to reflect this new information. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a registered pharmacist based in New York City.
References:
1. Peck P. IV calcium channel blocker wins FDA okay. www.medpagetoday.com/ProductAlert/Prescriptions/10431. Published August 5, 2008. Accessed October 28, 2008.
2. Riley K. www.fda.gov. FDA approves first bone marrow stimulator to treat immune-related low platelet counts. www.fda.gov/bbs/topics/NEWS/2008/NEW01876.html Published August 22, 2008. Accessed October 28, 2008.
4. Waknine Y. www.fda.org. FDA approvals: stavzor, cardene IV, eovist. www.medscape.com/viewarticle/ 579068. Published August 14, 2008. Accessed October 28, 2008.
5. Eisai Pharmaceutical Company. www.eisai.com. FDA approves ALOXI® (palonosetron HCl) capsules for prevention of acute chemotherapy-induced nausea and vomiting. www.eisai.com/view_press_ release.asp? ID=147&press=195. Published August 23, 2008. Accessed October 28, 2008.
6. Monthly Prescribing Reference. www. prescribingreference.com. FDA approves viread for hepatitis B. www. prescribingreference.com/news/showNews/ which/FDAApprovesVireadForHepatitisB8123/. Published August 12, 2008. Accessed October 28, 2008.
7. Nainggolan L. theheart.org. First ARB/CCB combo approved for initial therapy. www.theheart.org/ article/886011.do. Published August 5, 2008. Accessed October 28. 2008.
8. Long P. U.S. Food & Drug Administration. www.fda.org. FDA approves DNA test to measure hepatitis B virus levels. www.fda.gov/bbs/topics/ NEWS/2008/NEW01880.html. Published September 4, 2008. Accessed October 28, 2008.
9. U.S. Food & Drug Administration. www.fda.org. 2007 safety alerts for human medical products—Byetta (exenatide). www.fda.gov/medwatch/safety/2007 /safety07.htm#Byetta. Published August 18, 2008. Accessed October 28, 2008.
10. U.S. Food & Drug Administration. www.fda.org. 2008 safety alerts for human medical products–Tysabri (natalizumab). www.fda.gov/medwatch/ safety/2008/safety08.htm#Tysabri2. Published August 25, 2008. Accessed October 28, 2008.
The FDA has approved the first nucleic acid HBV viral DNA test for measuring HBV viral load from a patient’s blood. Via HBV viral load assessment, healthcare professionals now have a highly sensitive method for gauging antiviral therapy progress in patients with chronic HBV infections.
The test is known as the COBAS TaqMan HBV Test (Roche Diagnostic Division). It is used to measure HBV levels before beginning treatment, and then follow-up levels during treatment to assess therapy response. It is estimated that approximately 1.25 million people in the U.S. are infected with HBV, with approximately 60,000 becoming infected each year. About 5,000 people die from HBV-related complications each year.8
New Warnings
In October 2007, the Federal Drug and Food Administration (FDA) issued information for healthcare professionals regarding the subcutaneous use of exenatide (Byetta, Amylin Pharmaceuti-cals).9 Since then, the FDA has received at least six additional case reports of necrotizing or pancreatitis in patients taking exenatide.
Of these six cases, all patients needed hospitalization, two patients died, and four were recovering at the time of the reporting. Exenatide was discontinued in all of these patients.
If pancreatitis is suspected, exenatide and other potentially suspect drugs should be discontinued. There are no signs or symptoms distinguishing acute hemorrhagic or necrotizing pancreatitis associated with exenatide from less severe forms of pancreatitis. If pancreatitis is confirmed, appropriate treatment should be initiated and patients should be carefully monitored until they fully recover. Exenatide should not be restarted. The FDA is working with Amylin Pharmaceuticals to add stronger and more prominent warnings to the product label regarding the noted risks.
Since the last warning of natalizumab injection (Tysabri, Biogen IDEC), the FDA has informed healthcare professionals of two new cases of progressive multifocal leukoencephalopathy (PML) in European patients receiving it for more than a year as monotherapy for multiple sclerosis (MS).10
The agent currently is FDA approved to treat multiple sclerosis and Crohn’s disease. Approximately 39,000 patients have received treatment worldwide, with approximately 12,000 patients receiving treatment for at least a year. No new cases have been reported in the U.S., where approximately 7,500 patients have received the drug for more than a year and approximately 3,300 have received the drug for more than 18 months.
The FDA still believes natalizumab monotherapy may confer a lower risk of PML than usage with other immunomodulatory medications. Prescribing information for natalizumab has been revised to reflect this new information. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a registered pharmacist based in New York City.
References:
1. Peck P. IV calcium channel blocker wins FDA okay. www.medpagetoday.com/ProductAlert/Prescriptions/10431. Published August 5, 2008. Accessed October 28, 2008.
2. Riley K. www.fda.gov. FDA approves first bone marrow stimulator to treat immune-related low platelet counts. www.fda.gov/bbs/topics/NEWS/2008/NEW01876.html Published August 22, 2008. Accessed October 28, 2008.
4. Waknine Y. www.fda.org. FDA approvals: stavzor, cardene IV, eovist. www.medscape.com/viewarticle/ 579068. Published August 14, 2008. Accessed October 28, 2008.
5. Eisai Pharmaceutical Company. www.eisai.com. FDA approves ALOXI® (palonosetron HCl) capsules for prevention of acute chemotherapy-induced nausea and vomiting. www.eisai.com/view_press_ release.asp? ID=147&press=195. Published August 23, 2008. Accessed October 28, 2008.
6. Monthly Prescribing Reference. www. prescribingreference.com. FDA approves viread for hepatitis B. www. prescribingreference.com/news/showNews/ which/FDAApprovesVireadForHepatitisB8123/. Published August 12, 2008. Accessed October 28, 2008.
7. Nainggolan L. theheart.org. First ARB/CCB combo approved for initial therapy. www.theheart.org/ article/886011.do. Published August 5, 2008. Accessed October 28. 2008.
8. Long P. U.S. Food & Drug Administration. www.fda.org. FDA approves DNA test to measure hepatitis B virus levels. www.fda.gov/bbs/topics/ NEWS/2008/NEW01880.html. Published September 4, 2008. Accessed October 28, 2008.
9. U.S. Food & Drug Administration. www.fda.org. 2007 safety alerts for human medical products—Byetta (exenatide). www.fda.gov/medwatch/safety/2007 /safety07.htm#Byetta. Published August 18, 2008. Accessed October 28, 2008.
10. U.S. Food & Drug Administration. www.fda.org. 2008 safety alerts for human medical products–Tysabri (natalizumab). www.fda.gov/medwatch/ safety/2008/safety08.htm#Tysabri2. Published August 25, 2008. Accessed October 28, 2008.
The FDA has approved the first nucleic acid HBV viral DNA test for measuring HBV viral load from a patient’s blood. Via HBV viral load assessment, healthcare professionals now have a highly sensitive method for gauging antiviral therapy progress in patients with chronic HBV infections.
The test is known as the COBAS TaqMan HBV Test (Roche Diagnostic Division). It is used to measure HBV levels before beginning treatment, and then follow-up levels during treatment to assess therapy response. It is estimated that approximately 1.25 million people in the U.S. are infected with HBV, with approximately 60,000 becoming infected each year. About 5,000 people die from HBV-related complications each year.8
New Warnings
In October 2007, the Federal Drug and Food Administration (FDA) issued information for healthcare professionals regarding the subcutaneous use of exenatide (Byetta, Amylin Pharmaceuti-cals).9 Since then, the FDA has received at least six additional case reports of necrotizing or pancreatitis in patients taking exenatide.
Of these six cases, all patients needed hospitalization, two patients died, and four were recovering at the time of the reporting. Exenatide was discontinued in all of these patients.
If pancreatitis is suspected, exenatide and other potentially suspect drugs should be discontinued. There are no signs or symptoms distinguishing acute hemorrhagic or necrotizing pancreatitis associated with exenatide from less severe forms of pancreatitis. If pancreatitis is confirmed, appropriate treatment should be initiated and patients should be carefully monitored until they fully recover. Exenatide should not be restarted. The FDA is working with Amylin Pharmaceuticals to add stronger and more prominent warnings to the product label regarding the noted risks.
Since the last warning of natalizumab injection (Tysabri, Biogen IDEC), the FDA has informed healthcare professionals of two new cases of progressive multifocal leukoencephalopathy (PML) in European patients receiving it for more than a year as monotherapy for multiple sclerosis (MS).10
The agent currently is FDA approved to treat multiple sclerosis and Crohn’s disease. Approximately 39,000 patients have received treatment worldwide, with approximately 12,000 patients receiving treatment for at least a year. No new cases have been reported in the U.S., where approximately 7,500 patients have received the drug for more than a year and approximately 3,300 have received the drug for more than 18 months.
The FDA still believes natalizumab monotherapy may confer a lower risk of PML than usage with other immunomodulatory medications. Prescribing information for natalizumab has been revised to reflect this new information. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a registered pharmacist based in New York City.
References:
1. Peck P. IV calcium channel blocker wins FDA okay. www.medpagetoday.com/ProductAlert/Prescriptions/10431. Published August 5, 2008. Accessed October 28, 2008.
2. Riley K. www.fda.gov. FDA approves first bone marrow stimulator to treat immune-related low platelet counts. www.fda.gov/bbs/topics/NEWS/2008/NEW01876.html Published August 22, 2008. Accessed October 28, 2008.
4. Waknine Y. www.fda.org. FDA approvals: stavzor, cardene IV, eovist. www.medscape.com/viewarticle/ 579068. Published August 14, 2008. Accessed October 28, 2008.
5. Eisai Pharmaceutical Company. www.eisai.com. FDA approves ALOXI® (palonosetron HCl) capsules for prevention of acute chemotherapy-induced nausea and vomiting. www.eisai.com/view_press_ release.asp? ID=147&press=195. Published August 23, 2008. Accessed October 28, 2008.
6. Monthly Prescribing Reference. www. prescribingreference.com. FDA approves viread for hepatitis B. www. prescribingreference.com/news/showNews/ which/FDAApprovesVireadForHepatitisB8123/. Published August 12, 2008. Accessed October 28, 2008.
7. Nainggolan L. theheart.org. First ARB/CCB combo approved for initial therapy. www.theheart.org/ article/886011.do. Published August 5, 2008. Accessed October 28. 2008.
8. Long P. U.S. Food & Drug Administration. www.fda.org. FDA approves DNA test to measure hepatitis B virus levels. www.fda.gov/bbs/topics/ NEWS/2008/NEW01880.html. Published September 4, 2008. Accessed October 28, 2008.
9. U.S. Food & Drug Administration. www.fda.org. 2007 safety alerts for human medical products—Byetta (exenatide). www.fda.gov/medwatch/safety/2007 /safety07.htm#Byetta. Published August 18, 2008. Accessed October 28, 2008.
10. U.S. Food & Drug Administration. www.fda.org. 2008 safety alerts for human medical products–Tysabri (natalizumab). www.fda.gov/medwatch/ safety/2008/safety08.htm#Tysabri2. Published August 25, 2008. Accessed October 28, 2008.
When should lipid-lowering therapy be started in the hospitalized patient?
Case
A 52-year-old man with no medical history other than a transient ischemic attack (TIA) three months ago presents to the emergency department (ED) following multiple episodes of substernal (ST) chest pressure. He takes no medication. His electrocardiogram (ECG) revealed lateral ST segment depressions, and his cardiac biomarkers were elevated. He underwent cardiac catheterization, and a single drug-eluting stent was successfully placed to a culprit left circumflex lesion. He is now stable less than 24 hours following his initial presentation, without any evidence of heart failure. His providers prescribe aspirin, clopidogrel, metoprolol, and lisinopril. His fasting LDL level is 92 mg/dL.
What, if any, is the role for lipid-lowering therapy at this time?
Overview
Long-term therapy with HMG CoA reductase inhibitors (statins) has been shown through several large, randomized, controlled trials to reduce the risk for death, myocardial infarction (MI), and stroke in patients with established coronary disease. The most significant effects were evident after approximately two years of treatment.1,2,3,4
Subsequent trials have shown earlier and more significant reductions in the rates of recurrent ischemic cardiovascular events following acute coronary syndromes (ACS) when statins are administered early—within days of the initial event. This is a window of time in which most patients still are hospitalized.4,5,6,7
In addition to this data regarding statin use following ACS, a large, randomized, controlled trial demonstrated similar reductions in the incidence of strokes and cardiovascular events when high-dose atorvastatin was administered within one to six months following TIA or stroke in patients without established coronary disease.8 There is growing data supporting the hypothesis that statins have pleiotropic (non cholesterol-lowering), neuroprotective, properties that may improve patient outcomes following cerebrovascular events.9,10,11 There are ongoing trials investing the role of statins in the acute management of stroke.12,13
Hospitalists frequently manage patients in the stages immediately following ACS and stroke. Based on the large and evolving volume of data regarding the use of statins following these events, when and how should a statin be started in the hospital?
Review of the Data
Following Acute Coronary Syndrome: Death and recurrent ischemic events following ACS are most likely to occur in the early phase of recovery. Based on this observation and evidence supporting the early (in some cases within hours of administration) ‘pleiotropic’ or non-cholesterol lowering effects of statins, including improvement in endothelial function and decreases in platelet aggregation, thrombus deposition, and vascular inflammation, the MIRACL study was designed to answer the question of whether the initiation of treatment with a statin within 24 to 96 hours following ACS would reduce the occurrence of death and recurrent ischemia.4,7,14 Investigators randomized 3,086 patients within 24-96 hours (mean 63 hours) following admission for non-ST segment myocardial infarction (NSTEMI) or unstable angina (UA) to receive either atorvastatin 80 mg/d or placebo.
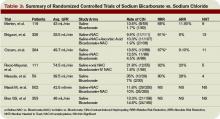
Investigators monitored patients for the primary end points of ischemic events (death, non-fatal MI, cardiac arrest with resuscitation, symptomatic myocardial ischemia with objective evidence) during a 16-week period. In the treatment arm, the risk of the primary combined end point was significantly reduced—relative risk (RR) 0.84; 95% confidence interval (CI), 0.70-1.00; p=0.048. (See Figure 1, pg. 39)
No significant differences were found between atorvastatin and placebo in the risk of death, non-fatal MI, or cardiac arrest with resuscitation. There was, however, a significantly lower risk of recurrent symptomatic myocardial ischemia with objective evidence requiring emergent re-hospitalization in the treatment arm (RR, 0.74; 95% CI, 0.57-0.95; p=0.02). The mean baseline LDL level in the treatment arm was 124 mg/dL, a value that may represent, in part, suppression of the LDL level in the setting of acute ACS. This is a phenomenon previously described in an analysis of the LUNAR trial.15
Suppression of LDL level after ACS appeared to be minimal, however, and is unlikely to be clinically significant. The benefits of atorvastatin in the MIRACL trial did not appear to depend on baseline LDL level—suggesting the decision to initiate statin therapy after ACS should not be influenced by LDL level at the time of the event.
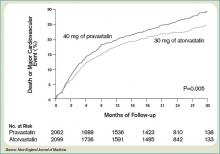
Only one dose of statin was used in the MIRACL trial, and the investigators commented they were unable to determine if a lower dose of atorvastatin or a gradual dose titration to a predetermined LDL target would have achieved similar benefits. The PROVE IT-TIMI 22 trial was designed to compare the reductions in death and major cardiovascular events following ACS between LDL lowering to approximately 100 mg/dL using 40 mg/d of pravastatin, and more intensive LDL lowering to approximately 70 mg/dL using 80 mg/d of atorvastatin.5 Investigators enrolled 4,162 patients for a median of seven days following ACS (STEMI, NSTEMI, or UA) to the two treatment arms. Investigators observed patients for a period of 18 to 36 months for the primary end points of death, MI, UA, revascularization, and stroke. The median LDL level at the time of enrollment was 106 mg/dL in both treatment arms. During follow up, the median LDL levels achieved were 95 mg/dL in the pravastatin group and 62 mg/dL in the atorvastatin group. After two years, a 16% reduction in the hazard ratio for any primary end point was seen favoring 80 mg/d of atorvastatin—p=0.005; 95% CI=5-26%. (See Figure 2, pg. 39) The benefit of high-dose atorvastatin was seen as early as 30 days after randomization and was sustained throughout the trial.
While the PROVE IT-TIMI 22 trial supported a specified dosing strategy for statin use following ACS, Phase Z of the A-to-Z trial was designed to evaluate the early initiation of intensive lipid lowering following ACS, as compared to a delayed and less-intensive strategy.5,6 Investigators randomized 4,497 patients (a mean of 3.7 days following either NSTEMI or STEMI) to receive either placebo for four months followed by simvastatin 20 mg/d or simvastatin 40 mg/d for one month followed by simvastatin 80 mg/d. They followed patients for 24 months for the primary end points of cardiovascular death, MI, readmission for ACS, or stroke. The primary end point occurred in 16.7% of the delayed, lower-intensity treatment group and in 14.4% of the early, higher-intensity treatment group (95% CI 0.76-1.04; p=0.14). Despite the lack of a significant difference in the composite primary end point between the two treatment arms, a significant reduction in the secondary end points of cardiovascular mortality (absolute risk reduction (ARR 1.3%; P=0.05) and congestive heart failure (ARR 1.3%; P=0.04) was evident favoring the early, intensive treatment strategy. These differences were not evident until at least four months after randomization. The A-to-Z trial investigators offered several possible explanations for the delay in evident clinical benefits in their trial when compared against the strong trend toward clinical benefit seen with 30 days following the early initiation of high-dose atorvastatin following ACS in the PROVE IT-TIMI 22 trial. In the PROVE IT trial, patients were enrolled an average of seven days after their index event, and as a result, 69% had undergone revascularization by this time. In the A-to-Z trial, patients were enrolled an average of three to four days earlier, and, therefore, were less likely to have undergone a revascularization procedure by the time of enrollment—and may have continued on with active thrombotic processes relatively less responsive to statin therapy.6 Another notable difference between PROVE IT and A-to-Z subjects was the C-reactive protein (CRP) concentrations in the A-to-Z subjects did not differ between treatment groups within 30 days despite significant differences in their LDL levels.16 This lack of a concurrent, pleiotropic, anti-inflammatory effect in the A-to-Z trial aggressive treatment arm may also have contributed to the delayed treatment effect.
In conclusion, the A-to-Z investigators suggest more intensive statin therapy (than the 40 mg Simvastatin in their intensive treatment arm) may be required to derive the most rapid and maximal clinical benefits during the highest risk period immediately following ACS.
Following stroke: Although there is more robust data supporting the benefits of early, intensive, statin therapy following ACS, there also is established and emerging data supporting similar treatment approaches following stroke.
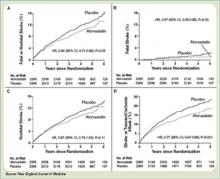
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was designed to determine whether or not atorvastatin 80 mg daily would reduce the risk of stroke in patients without known coronary heart disease who had suffered a TIA or stroke within the preceding six months.8 Patients who experienced a hemorrhagic or ischemic TIA or stroke between one to six months before study entry were randomized to receive either atorvastatin 80 mg/d or placebo.
Investigators followed patients for a mean period of 4.9 years for the primary end point of time to non-fatal or fatal stroke. Secondary composite end points included stroke or TIA, and any coronary or peripheral arterial event, including death from cardiac causes, non-fatal MI, ACS, revascularization (coronary, carotid, peripheral), and death from any cause. No difference in mean baseline LDL levels was witnessed between the treatment and placebo arms (132.7 and 133.7 mg/dL, respectively). Atorvastatin was associated with a 16% relative reduction in the risk of stroke—hazard ratio, 0.84; 95% CI 0.71–0.99; p=0.03. This was found despite an increase in hemorrhagic stroke in the atorvastatin group—a finding that supports an epidemiologic association between low cholesterol levels and brain hemorrhage. The risk of cardiovascular events also was significantly reduced, however, no significant difference in overall mortality was observed between the two groups.
In conclusion, the authors recommend the initiation of high-dose atorvastatin “soon” after stroke or TIA. One can only conclude, based on these data, statin therapy should be initiated within six months of TIA or stroke, in accordance with the study design. There is retrospective data suggesting benefit to statin therapy initiated within four weeks following ischemic stroke, and there are prospective trials in process evaluating the potential benefits of statins initiated within 24 hours following ischemic stroke, however, no large, randomized, controlled trial can demonstrate the effect of statins when used as acute stroke therapy.9,12,13,17
Back to the Case
The patient described in our case has a history of TIA and experienced an acute coronary syndrome (NSTEMI) within the preceding 24 hours. He underwent a revascularization procedure (PCI with stent), and is on appropriate therapy, including dual anti-platelet therapy with aspirin and clopidogrel, a beta-blocker, and an angiotensin-converting enzyme inhibitor. Based on the data and conclusions of the MIRACL, PROVE IT-TIMI 22, and SPARCL trials, high-dose statin therapy with atorvastatin 80 mg/d should be initiated immediately in the patient in order to significantly reduce his risk of recurrent ischemic cardiovascular events and stroke following his acute coronary syndrome and TIA.
Bottom Line
Following ACS, high-dose statin therapy with 80 mg of atorvastatin per day should be initiated when the patient is still in the hospital, irrespective of baseline LDL level. Statin therapy should also strongly be considered for secondary stroke prevention in most patients with a history of stroke or transient ischemic attack. TH
Caleb Hale, MD, is a hospitalist at Beth Israel Deaconess Medical Center in Boston. Joseph Ming Wah Li is director of the hospital medicine program and associate chief, division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston, and assistant professor of Medicine at Harvard Medical School.
References
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
2. Sacks RM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-1009.
3. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-1357.
4. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711-1718.
5. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
6. Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs. a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
7. Waters D, Schwartz GG, Olsson AG. The myocardial ischemia reduction with acute cholesterol lowering (MIRACL) trial: a new frontier for statins? Curr Control Trials Cardiovasc Med. 2001;2;111-114.
8. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
9. Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. 2005;36:1298-1300.
10. Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253-258.
11. Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969-1973.
12. Elkind MS, Sacco RL, MacArthur RB, et al. The neuroprotection with statin therapy for acute recovery trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3:210-218.
13. Montaner J, Chacon P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J of Neurol. 2008;15:82-90.
14. Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20-28.
15. Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. JACC. 2008;51:1440-1445.
16. Wiviott SD, de Lemos JA, Cannon CP, et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials of A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406-1414.
17. Elking MS. Statins as acute-stroke treatment. Int J Stroke. 2006;1:224-225.
Case
A 52-year-old man with no medical history other than a transient ischemic attack (TIA) three months ago presents to the emergency department (ED) following multiple episodes of substernal (ST) chest pressure. He takes no medication. His electrocardiogram (ECG) revealed lateral ST segment depressions, and his cardiac biomarkers were elevated. He underwent cardiac catheterization, and a single drug-eluting stent was successfully placed to a culprit left circumflex lesion. He is now stable less than 24 hours following his initial presentation, without any evidence of heart failure. His providers prescribe aspirin, clopidogrel, metoprolol, and lisinopril. His fasting LDL level is 92 mg/dL.
What, if any, is the role for lipid-lowering therapy at this time?
Overview
Long-term therapy with HMG CoA reductase inhibitors (statins) has been shown through several large, randomized, controlled trials to reduce the risk for death, myocardial infarction (MI), and stroke in patients with established coronary disease. The most significant effects were evident after approximately two years of treatment.1,2,3,4
Subsequent trials have shown earlier and more significant reductions in the rates of recurrent ischemic cardiovascular events following acute coronary syndromes (ACS) when statins are administered early—within days of the initial event. This is a window of time in which most patients still are hospitalized.4,5,6,7
In addition to this data regarding statin use following ACS, a large, randomized, controlled trial demonstrated similar reductions in the incidence of strokes and cardiovascular events when high-dose atorvastatin was administered within one to six months following TIA or stroke in patients without established coronary disease.8 There is growing data supporting the hypothesis that statins have pleiotropic (non cholesterol-lowering), neuroprotective, properties that may improve patient outcomes following cerebrovascular events.9,10,11 There are ongoing trials investing the role of statins in the acute management of stroke.12,13
Hospitalists frequently manage patients in the stages immediately following ACS and stroke. Based on the large and evolving volume of data regarding the use of statins following these events, when and how should a statin be started in the hospital?
Review of the Data
Following Acute Coronary Syndrome: Death and recurrent ischemic events following ACS are most likely to occur in the early phase of recovery. Based on this observation and evidence supporting the early (in some cases within hours of administration) ‘pleiotropic’ or non-cholesterol lowering effects of statins, including improvement in endothelial function and decreases in platelet aggregation, thrombus deposition, and vascular inflammation, the MIRACL study was designed to answer the question of whether the initiation of treatment with a statin within 24 to 96 hours following ACS would reduce the occurrence of death and recurrent ischemia.4,7,14 Investigators randomized 3,086 patients within 24-96 hours (mean 63 hours) following admission for non-ST segment myocardial infarction (NSTEMI) or unstable angina (UA) to receive either atorvastatin 80 mg/d or placebo.
Investigators monitored patients for the primary end points of ischemic events (death, non-fatal MI, cardiac arrest with resuscitation, symptomatic myocardial ischemia with objective evidence) during a 16-week period. In the treatment arm, the risk of the primary combined end point was significantly reduced—relative risk (RR) 0.84; 95% confidence interval (CI), 0.70-1.00; p=0.048. (See Figure 1, pg. 39)
No significant differences were found between atorvastatin and placebo in the risk of death, non-fatal MI, or cardiac arrest with resuscitation. There was, however, a significantly lower risk of recurrent symptomatic myocardial ischemia with objective evidence requiring emergent re-hospitalization in the treatment arm (RR, 0.74; 95% CI, 0.57-0.95; p=0.02). The mean baseline LDL level in the treatment arm was 124 mg/dL, a value that may represent, in part, suppression of the LDL level in the setting of acute ACS. This is a phenomenon previously described in an analysis of the LUNAR trial.15
Suppression of LDL level after ACS appeared to be minimal, however, and is unlikely to be clinically significant. The benefits of atorvastatin in the MIRACL trial did not appear to depend on baseline LDL level—suggesting the decision to initiate statin therapy after ACS should not be influenced by LDL level at the time of the event.
Only one dose of statin was used in the MIRACL trial, and the investigators commented they were unable to determine if a lower dose of atorvastatin or a gradual dose titration to a predetermined LDL target would have achieved similar benefits. The PROVE IT-TIMI 22 trial was designed to compare the reductions in death and major cardiovascular events following ACS between LDL lowering to approximately 100 mg/dL using 40 mg/d of pravastatin, and more intensive LDL lowering to approximately 70 mg/dL using 80 mg/d of atorvastatin.5 Investigators enrolled 4,162 patients for a median of seven days following ACS (STEMI, NSTEMI, or UA) to the two treatment arms. Investigators observed patients for a period of 18 to 36 months for the primary end points of death, MI, UA, revascularization, and stroke. The median LDL level at the time of enrollment was 106 mg/dL in both treatment arms. During follow up, the median LDL levels achieved were 95 mg/dL in the pravastatin group and 62 mg/dL in the atorvastatin group. After two years, a 16% reduction in the hazard ratio for any primary end point was seen favoring 80 mg/d of atorvastatin—p=0.005; 95% CI=5-26%. (See Figure 2, pg. 39) The benefit of high-dose atorvastatin was seen as early as 30 days after randomization and was sustained throughout the trial.
While the PROVE IT-TIMI 22 trial supported a specified dosing strategy for statin use following ACS, Phase Z of the A-to-Z trial was designed to evaluate the early initiation of intensive lipid lowering following ACS, as compared to a delayed and less-intensive strategy.5,6 Investigators randomized 4,497 patients (a mean of 3.7 days following either NSTEMI or STEMI) to receive either placebo for four months followed by simvastatin 20 mg/d or simvastatin 40 mg/d for one month followed by simvastatin 80 mg/d. They followed patients for 24 months for the primary end points of cardiovascular death, MI, readmission for ACS, or stroke. The primary end point occurred in 16.7% of the delayed, lower-intensity treatment group and in 14.4% of the early, higher-intensity treatment group (95% CI 0.76-1.04; p=0.14). Despite the lack of a significant difference in the composite primary end point between the two treatment arms, a significant reduction in the secondary end points of cardiovascular mortality (absolute risk reduction (ARR 1.3%; P=0.05) and congestive heart failure (ARR 1.3%; P=0.04) was evident favoring the early, intensive treatment strategy. These differences were not evident until at least four months after randomization. The A-to-Z trial investigators offered several possible explanations for the delay in evident clinical benefits in their trial when compared against the strong trend toward clinical benefit seen with 30 days following the early initiation of high-dose atorvastatin following ACS in the PROVE IT-TIMI 22 trial. In the PROVE IT trial, patients were enrolled an average of seven days after their index event, and as a result, 69% had undergone revascularization by this time. In the A-to-Z trial, patients were enrolled an average of three to four days earlier, and, therefore, were less likely to have undergone a revascularization procedure by the time of enrollment—and may have continued on with active thrombotic processes relatively less responsive to statin therapy.6 Another notable difference between PROVE IT and A-to-Z subjects was the C-reactive protein (CRP) concentrations in the A-to-Z subjects did not differ between treatment groups within 30 days despite significant differences in their LDL levels.16 This lack of a concurrent, pleiotropic, anti-inflammatory effect in the A-to-Z trial aggressive treatment arm may also have contributed to the delayed treatment effect.
In conclusion, the A-to-Z investigators suggest more intensive statin therapy (than the 40 mg Simvastatin in their intensive treatment arm) may be required to derive the most rapid and maximal clinical benefits during the highest risk period immediately following ACS.
Following stroke: Although there is more robust data supporting the benefits of early, intensive, statin therapy following ACS, there also is established and emerging data supporting similar treatment approaches following stroke.
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was designed to determine whether or not atorvastatin 80 mg daily would reduce the risk of stroke in patients without known coronary heart disease who had suffered a TIA or stroke within the preceding six months.8 Patients who experienced a hemorrhagic or ischemic TIA or stroke between one to six months before study entry were randomized to receive either atorvastatin 80 mg/d or placebo.
Investigators followed patients for a mean period of 4.9 years for the primary end point of time to non-fatal or fatal stroke. Secondary composite end points included stroke or TIA, and any coronary or peripheral arterial event, including death from cardiac causes, non-fatal MI, ACS, revascularization (coronary, carotid, peripheral), and death from any cause. No difference in mean baseline LDL levels was witnessed between the treatment and placebo arms (132.7 and 133.7 mg/dL, respectively). Atorvastatin was associated with a 16% relative reduction in the risk of stroke—hazard ratio, 0.84; 95% CI 0.71–0.99; p=0.03. This was found despite an increase in hemorrhagic stroke in the atorvastatin group—a finding that supports an epidemiologic association between low cholesterol levels and brain hemorrhage. The risk of cardiovascular events also was significantly reduced, however, no significant difference in overall mortality was observed between the two groups.
In conclusion, the authors recommend the initiation of high-dose atorvastatin “soon” after stroke or TIA. One can only conclude, based on these data, statin therapy should be initiated within six months of TIA or stroke, in accordance with the study design. There is retrospective data suggesting benefit to statin therapy initiated within four weeks following ischemic stroke, and there are prospective trials in process evaluating the potential benefits of statins initiated within 24 hours following ischemic stroke, however, no large, randomized, controlled trial can demonstrate the effect of statins when used as acute stroke therapy.9,12,13,17
Back to the Case
The patient described in our case has a history of TIA and experienced an acute coronary syndrome (NSTEMI) within the preceding 24 hours. He underwent a revascularization procedure (PCI with stent), and is on appropriate therapy, including dual anti-platelet therapy with aspirin and clopidogrel, a beta-blocker, and an angiotensin-converting enzyme inhibitor. Based on the data and conclusions of the MIRACL, PROVE IT-TIMI 22, and SPARCL trials, high-dose statin therapy with atorvastatin 80 mg/d should be initiated immediately in the patient in order to significantly reduce his risk of recurrent ischemic cardiovascular events and stroke following his acute coronary syndrome and TIA.
Bottom Line
Following ACS, high-dose statin therapy with 80 mg of atorvastatin per day should be initiated when the patient is still in the hospital, irrespective of baseline LDL level. Statin therapy should also strongly be considered for secondary stroke prevention in most patients with a history of stroke or transient ischemic attack. TH
Caleb Hale, MD, is a hospitalist at Beth Israel Deaconess Medical Center in Boston. Joseph Ming Wah Li is director of the hospital medicine program and associate chief, division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston, and assistant professor of Medicine at Harvard Medical School.
References
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
2. Sacks RM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-1009.
3. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-1357.
4. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711-1718.
5. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
6. Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs. a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
7. Waters D, Schwartz GG, Olsson AG. The myocardial ischemia reduction with acute cholesterol lowering (MIRACL) trial: a new frontier for statins? Curr Control Trials Cardiovasc Med. 2001;2;111-114.
8. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
9. Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. 2005;36:1298-1300.
10. Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253-258.
11. Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969-1973.
12. Elkind MS, Sacco RL, MacArthur RB, et al. The neuroprotection with statin therapy for acute recovery trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3:210-218.
13. Montaner J, Chacon P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J of Neurol. 2008;15:82-90.
14. Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20-28.
15. Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. JACC. 2008;51:1440-1445.
16. Wiviott SD, de Lemos JA, Cannon CP, et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials of A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406-1414.
17. Elking MS. Statins as acute-stroke treatment. Int J Stroke. 2006;1:224-225.
Case
A 52-year-old man with no medical history other than a transient ischemic attack (TIA) three months ago presents to the emergency department (ED) following multiple episodes of substernal (ST) chest pressure. He takes no medication. His electrocardiogram (ECG) revealed lateral ST segment depressions, and his cardiac biomarkers were elevated. He underwent cardiac catheterization, and a single drug-eluting stent was successfully placed to a culprit left circumflex lesion. He is now stable less than 24 hours following his initial presentation, without any evidence of heart failure. His providers prescribe aspirin, clopidogrel, metoprolol, and lisinopril. His fasting LDL level is 92 mg/dL.
What, if any, is the role for lipid-lowering therapy at this time?
Overview
Long-term therapy with HMG CoA reductase inhibitors (statins) has been shown through several large, randomized, controlled trials to reduce the risk for death, myocardial infarction (MI), and stroke in patients with established coronary disease. The most significant effects were evident after approximately two years of treatment.1,2,3,4
Subsequent trials have shown earlier and more significant reductions in the rates of recurrent ischemic cardiovascular events following acute coronary syndromes (ACS) when statins are administered early—within days of the initial event. This is a window of time in which most patients still are hospitalized.4,5,6,7
In addition to this data regarding statin use following ACS, a large, randomized, controlled trial demonstrated similar reductions in the incidence of strokes and cardiovascular events when high-dose atorvastatin was administered within one to six months following TIA or stroke in patients without established coronary disease.8 There is growing data supporting the hypothesis that statins have pleiotropic (non cholesterol-lowering), neuroprotective, properties that may improve patient outcomes following cerebrovascular events.9,10,11 There are ongoing trials investing the role of statins in the acute management of stroke.12,13
Hospitalists frequently manage patients in the stages immediately following ACS and stroke. Based on the large and evolving volume of data regarding the use of statins following these events, when and how should a statin be started in the hospital?
Review of the Data
Following Acute Coronary Syndrome: Death and recurrent ischemic events following ACS are most likely to occur in the early phase of recovery. Based on this observation and evidence supporting the early (in some cases within hours of administration) ‘pleiotropic’ or non-cholesterol lowering effects of statins, including improvement in endothelial function and decreases in platelet aggregation, thrombus deposition, and vascular inflammation, the MIRACL study was designed to answer the question of whether the initiation of treatment with a statin within 24 to 96 hours following ACS would reduce the occurrence of death and recurrent ischemia.4,7,14 Investigators randomized 3,086 patients within 24-96 hours (mean 63 hours) following admission for non-ST segment myocardial infarction (NSTEMI) or unstable angina (UA) to receive either atorvastatin 80 mg/d or placebo.
Investigators monitored patients for the primary end points of ischemic events (death, non-fatal MI, cardiac arrest with resuscitation, symptomatic myocardial ischemia with objective evidence) during a 16-week period. In the treatment arm, the risk of the primary combined end point was significantly reduced—relative risk (RR) 0.84; 95% confidence interval (CI), 0.70-1.00; p=0.048. (See Figure 1, pg. 39)
No significant differences were found between atorvastatin and placebo in the risk of death, non-fatal MI, or cardiac arrest with resuscitation. There was, however, a significantly lower risk of recurrent symptomatic myocardial ischemia with objective evidence requiring emergent re-hospitalization in the treatment arm (RR, 0.74; 95% CI, 0.57-0.95; p=0.02). The mean baseline LDL level in the treatment arm was 124 mg/dL, a value that may represent, in part, suppression of the LDL level in the setting of acute ACS. This is a phenomenon previously described in an analysis of the LUNAR trial.15
Suppression of LDL level after ACS appeared to be minimal, however, and is unlikely to be clinically significant. The benefits of atorvastatin in the MIRACL trial did not appear to depend on baseline LDL level—suggesting the decision to initiate statin therapy after ACS should not be influenced by LDL level at the time of the event.
Only one dose of statin was used in the MIRACL trial, and the investigators commented they were unable to determine if a lower dose of atorvastatin or a gradual dose titration to a predetermined LDL target would have achieved similar benefits. The PROVE IT-TIMI 22 trial was designed to compare the reductions in death and major cardiovascular events following ACS between LDL lowering to approximately 100 mg/dL using 40 mg/d of pravastatin, and more intensive LDL lowering to approximately 70 mg/dL using 80 mg/d of atorvastatin.5 Investigators enrolled 4,162 patients for a median of seven days following ACS (STEMI, NSTEMI, or UA) to the two treatment arms. Investigators observed patients for a period of 18 to 36 months for the primary end points of death, MI, UA, revascularization, and stroke. The median LDL level at the time of enrollment was 106 mg/dL in both treatment arms. During follow up, the median LDL levels achieved were 95 mg/dL in the pravastatin group and 62 mg/dL in the atorvastatin group. After two years, a 16% reduction in the hazard ratio for any primary end point was seen favoring 80 mg/d of atorvastatin—p=0.005; 95% CI=5-26%. (See Figure 2, pg. 39) The benefit of high-dose atorvastatin was seen as early as 30 days after randomization and was sustained throughout the trial.
While the PROVE IT-TIMI 22 trial supported a specified dosing strategy for statin use following ACS, Phase Z of the A-to-Z trial was designed to evaluate the early initiation of intensive lipid lowering following ACS, as compared to a delayed and less-intensive strategy.5,6 Investigators randomized 4,497 patients (a mean of 3.7 days following either NSTEMI or STEMI) to receive either placebo for four months followed by simvastatin 20 mg/d or simvastatin 40 mg/d for one month followed by simvastatin 80 mg/d. They followed patients for 24 months for the primary end points of cardiovascular death, MI, readmission for ACS, or stroke. The primary end point occurred in 16.7% of the delayed, lower-intensity treatment group and in 14.4% of the early, higher-intensity treatment group (95% CI 0.76-1.04; p=0.14). Despite the lack of a significant difference in the composite primary end point between the two treatment arms, a significant reduction in the secondary end points of cardiovascular mortality (absolute risk reduction (ARR 1.3%; P=0.05) and congestive heart failure (ARR 1.3%; P=0.04) was evident favoring the early, intensive treatment strategy. These differences were not evident until at least four months after randomization. The A-to-Z trial investigators offered several possible explanations for the delay in evident clinical benefits in their trial when compared against the strong trend toward clinical benefit seen with 30 days following the early initiation of high-dose atorvastatin following ACS in the PROVE IT-TIMI 22 trial. In the PROVE IT trial, patients were enrolled an average of seven days after their index event, and as a result, 69% had undergone revascularization by this time. In the A-to-Z trial, patients were enrolled an average of three to four days earlier, and, therefore, were less likely to have undergone a revascularization procedure by the time of enrollment—and may have continued on with active thrombotic processes relatively less responsive to statin therapy.6 Another notable difference between PROVE IT and A-to-Z subjects was the C-reactive protein (CRP) concentrations in the A-to-Z subjects did not differ between treatment groups within 30 days despite significant differences in their LDL levels.16 This lack of a concurrent, pleiotropic, anti-inflammatory effect in the A-to-Z trial aggressive treatment arm may also have contributed to the delayed treatment effect.
In conclusion, the A-to-Z investigators suggest more intensive statin therapy (than the 40 mg Simvastatin in their intensive treatment arm) may be required to derive the most rapid and maximal clinical benefits during the highest risk period immediately following ACS.
Following stroke: Although there is more robust data supporting the benefits of early, intensive, statin therapy following ACS, there also is established and emerging data supporting similar treatment approaches following stroke.
The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial was designed to determine whether or not atorvastatin 80 mg daily would reduce the risk of stroke in patients without known coronary heart disease who had suffered a TIA or stroke within the preceding six months.8 Patients who experienced a hemorrhagic or ischemic TIA or stroke between one to six months before study entry were randomized to receive either atorvastatin 80 mg/d or placebo.
Investigators followed patients for a mean period of 4.9 years for the primary end point of time to non-fatal or fatal stroke. Secondary composite end points included stroke or TIA, and any coronary or peripheral arterial event, including death from cardiac causes, non-fatal MI, ACS, revascularization (coronary, carotid, peripheral), and death from any cause. No difference in mean baseline LDL levels was witnessed between the treatment and placebo arms (132.7 and 133.7 mg/dL, respectively). Atorvastatin was associated with a 16% relative reduction in the risk of stroke—hazard ratio, 0.84; 95% CI 0.71–0.99; p=0.03. This was found despite an increase in hemorrhagic stroke in the atorvastatin group—a finding that supports an epidemiologic association between low cholesterol levels and brain hemorrhage. The risk of cardiovascular events also was significantly reduced, however, no significant difference in overall mortality was observed between the two groups.
In conclusion, the authors recommend the initiation of high-dose atorvastatin “soon” after stroke or TIA. One can only conclude, based on these data, statin therapy should be initiated within six months of TIA or stroke, in accordance with the study design. There is retrospective data suggesting benefit to statin therapy initiated within four weeks following ischemic stroke, and there are prospective trials in process evaluating the potential benefits of statins initiated within 24 hours following ischemic stroke, however, no large, randomized, controlled trial can demonstrate the effect of statins when used as acute stroke therapy.9,12,13,17
Back to the Case
The patient described in our case has a history of TIA and experienced an acute coronary syndrome (NSTEMI) within the preceding 24 hours. He underwent a revascularization procedure (PCI with stent), and is on appropriate therapy, including dual anti-platelet therapy with aspirin and clopidogrel, a beta-blocker, and an angiotensin-converting enzyme inhibitor. Based on the data and conclusions of the MIRACL, PROVE IT-TIMI 22, and SPARCL trials, high-dose statin therapy with atorvastatin 80 mg/d should be initiated immediately in the patient in order to significantly reduce his risk of recurrent ischemic cardiovascular events and stroke following his acute coronary syndrome and TIA.
Bottom Line
Following ACS, high-dose statin therapy with 80 mg of atorvastatin per day should be initiated when the patient is still in the hospital, irrespective of baseline LDL level. Statin therapy should also strongly be considered for secondary stroke prevention in most patients with a history of stroke or transient ischemic attack. TH
Caleb Hale, MD, is a hospitalist at Beth Israel Deaconess Medical Center in Boston. Joseph Ming Wah Li is director of the hospital medicine program and associate chief, division of general medicine and primary care at Beth Israel Deaconess Medical Center in Boston, and assistant professor of Medicine at Harvard Medical School.
References
1. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
2. Sacks RM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001-1009.
3. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349-1357.
4. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes. The MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711-1718.
5. Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495-1504.
6. Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs. a delayed conservative simvastatin strategy in patients with acute coronary syndromes. Phase Z of the A to Z trial. JAMA. 2004;292:1307-1316.
7. Waters D, Schwartz GG, Olsson AG. The myocardial ischemia reduction with acute cholesterol lowering (MIRACL) trial: a new frontier for statins? Curr Control Trials Cardiovasc Med. 2001;2;111-114.
8. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549-559.
9. Moonis M, Kane K, Schwiderski U, Sandage BW, Fisher M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. 2005;36:1298-1300.
10. Elkind MS, Flint AC, Sciacca RR, Sacco RL. Lipid-lowering agent use at ischemic stroke onset is associated with decreased mortality. Neurology. 2005;65:253-258.
11. Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969-1973.
12. Elkind MS, Sacco RL, MacArthur RB, et al. The neuroprotection with statin therapy for acute recovery trial (NeuSTART): an adaptive design phase I dose-escalation study of high-dose lovastatin in acute ischemic stroke. Int J Stroke. 2008;3:210-218.
13. Montaner J, Chacon P, Krupinski J, et al. Simvastatin in the acute phase of ischemic stroke: a safety and efficacy pilot trial. Eur J of Neurol. 2008;15:82-90.
14. Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20-28.
15. Pitt B, Loscalzo J, Ycas J, Raichlen JS. Lipid levels after acute coronary syndromes. JACC. 2008;51:1440-1445.
16. Wiviott SD, de Lemos JA, Cannon CP, et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials of A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406-1414.
17. Elking MS. Statins as acute-stroke treatment. Int J Stroke. 2006;1:224-225.
Industry Innovator Eyes HM Challenges Ahead
Brian Bossard, MD, was practicing as a hospitalist before he even knew what a hospitalist was. In 1993, Dr. Bossard, then a private practice internist, initiated a contract with Lincoln General Hospital in Lincoln, Neb. He agreed to care for hospital patients who didn’t have physicians. The hospital signed the contract—three years before HM pioneer Bob Wachter, MD, professor and chief of the division of hospital medicine at the University of California San Francisco, former SHM president and author of the blog “Wachter’s World” (www.wachtersworld.com), coined the term “hospitalist.”
Dr. Bossard, director and CEO of Inpatient Physician Associates in Lincoln, recently spoke with The Hospitalist about being at the forefront of the hospital medicine movement.
Q: How did you come to form your own hospitalist group?
—Brian Bossard, MD
A: [Starting in 1993], I was providing hospital medicine service while at the same time working in a private practice model. I took care of my own patients and also took care of all the assigned patients through the hospital. During that period, I started getting referrals from other physicians who wanted to turn their patients’ care over to me. It became clear after just a few years of doing that I was getting very busy and that there was a need for a more formal hospital medicine program. So, beginning in 1998, I started going to national hospital medicine meetings. I took my hospital administrator with me to the first meeting, and during the next four years developed an infrastructure for a mature hospital medicine program.
Q: What trends have you identified in HM since that time?
A: In the case of academic medicine models, hospital medicine developed because they needed to have a system to provide a cap for the residents—both in terms of number of hours they worked and the number of patients they saw. That was a new development and one that wasn’t in place when I went through training.
Private practice or community-based hospitals had physicians who were no longer interested in providing community call for taking care of patients that didn’t have physicians, or maybe didn’t have insurance. Community hospitals were finding that many physicians were opting out of that community call so they needed hospital medicine support to take care of those patients.
Q: What is the most significant change you’ve witnessed?
A: It’s become clear hospital medicine programs not only provide staffing to take care of those patients who otherwise wouldn’t be taken care of, but also provide a structure to take care of patients better. Probably the most positive and meaningful change since the mid-’90s is that hospital medicine programs are seen as quality drivers, efficiency drivers, and as a source of leadership within hospital policy making and decision making.
Q: What are your responsibilities as CEO of your group?
A: I run the business from top to bottom. Since I started the group in 2002, we’ve grown from just six physicians to 18 physicians and three nurse coordinators. So, I’ve had an opportunity during the last seven years to develop leadership roles within our group and delegate some activities to other leaders in the group. Where I once oversaw every little detail, I am now able to turn over some things to other, very talented group members. What I really focus on now is recruitment, the clinical aspects, public relations, and those sorts of things. But I never lose sight of the importance of developing data to drive our decisions, so I’m very involved in that, as well. As we add more and more physicians, I have to dedicate more time to management of the group. My clinical time goes down as the group grows.
Q: You mentioned that you collect data?
A: I work with the folks in the IT and Division Analysis departments in the hospital to identify what data we can get, what is important for me to know … so we can make decisions for the better of the group and the hospital. Some of that involves knowing what time of the day we have the highest admissions consults and what days of the week we’re busiest, and then organizing our schedule accordingly. It’s important to look at numbers and data, as opposed to going by when you feel you’re busy and when you’re not, because sometimes the feel is different from what is actually happening.
Q: What are the challenges facing your HMG?
A: Recruitment is a huge challenge. The growth of hospital medicine is much greater than anticipated even five years ago. Many programs are understaffed right now. That’s not because they don’t have financing, but because they don’t have physicians available to staff the slots. When I started my group, I was able to recruit a strong, core group of five physicians in six months. I don’t think there is any way you could do that now. That’s a trend that’s changed for the worst. I don’t think internal medicine is going to be able to support the need for care providers within hospital medicine programs.
Q: How should hospital medicine groups look to fill their vacancies?
A: I think opportunities will exist for well-trained and motivated family medicine physicians. Many more rural or community-based hospitals are turning to family physicians to staff programs. Typically, family physicians represent only 3% of hospital medicine program slots. I see that percentage increasing fairly significantly in the next five years. TH
Brian Bossard, MD, was practicing as a hospitalist before he even knew what a hospitalist was. In 1993, Dr. Bossard, then a private practice internist, initiated a contract with Lincoln General Hospital in Lincoln, Neb. He agreed to care for hospital patients who didn’t have physicians. The hospital signed the contract—three years before HM pioneer Bob Wachter, MD, professor and chief of the division of hospital medicine at the University of California San Francisco, former SHM president and author of the blog “Wachter’s World” (www.wachtersworld.com), coined the term “hospitalist.”
Dr. Bossard, director and CEO of Inpatient Physician Associates in Lincoln, recently spoke with The Hospitalist about being at the forefront of the hospital medicine movement.
Q: How did you come to form your own hospitalist group?
—Brian Bossard, MD
A: [Starting in 1993], I was providing hospital medicine service while at the same time working in a private practice model. I took care of my own patients and also took care of all the assigned patients through the hospital. During that period, I started getting referrals from other physicians who wanted to turn their patients’ care over to me. It became clear after just a few years of doing that I was getting very busy and that there was a need for a more formal hospital medicine program. So, beginning in 1998, I started going to national hospital medicine meetings. I took my hospital administrator with me to the first meeting, and during the next four years developed an infrastructure for a mature hospital medicine program.
Q: What trends have you identified in HM since that time?
A: In the case of academic medicine models, hospital medicine developed because they needed to have a system to provide a cap for the residents—both in terms of number of hours they worked and the number of patients they saw. That was a new development and one that wasn’t in place when I went through training.
Private practice or community-based hospitals had physicians who were no longer interested in providing community call for taking care of patients that didn’t have physicians, or maybe didn’t have insurance. Community hospitals were finding that many physicians were opting out of that community call so they needed hospital medicine support to take care of those patients.
Q: What is the most significant change you’ve witnessed?
A: It’s become clear hospital medicine programs not only provide staffing to take care of those patients who otherwise wouldn’t be taken care of, but also provide a structure to take care of patients better. Probably the most positive and meaningful change since the mid-’90s is that hospital medicine programs are seen as quality drivers, efficiency drivers, and as a source of leadership within hospital policy making and decision making.
Q: What are your responsibilities as CEO of your group?
A: I run the business from top to bottom. Since I started the group in 2002, we’ve grown from just six physicians to 18 physicians and three nurse coordinators. So, I’ve had an opportunity during the last seven years to develop leadership roles within our group and delegate some activities to other leaders in the group. Where I once oversaw every little detail, I am now able to turn over some things to other, very talented group members. What I really focus on now is recruitment, the clinical aspects, public relations, and those sorts of things. But I never lose sight of the importance of developing data to drive our decisions, so I’m very involved in that, as well. As we add more and more physicians, I have to dedicate more time to management of the group. My clinical time goes down as the group grows.
Q: You mentioned that you collect data?
A: I work with the folks in the IT and Division Analysis departments in the hospital to identify what data we can get, what is important for me to know … so we can make decisions for the better of the group and the hospital. Some of that involves knowing what time of the day we have the highest admissions consults and what days of the week we’re busiest, and then organizing our schedule accordingly. It’s important to look at numbers and data, as opposed to going by when you feel you’re busy and when you’re not, because sometimes the feel is different from what is actually happening.
Q: What are the challenges facing your HMG?
A: Recruitment is a huge challenge. The growth of hospital medicine is much greater than anticipated even five years ago. Many programs are understaffed right now. That’s not because they don’t have financing, but because they don’t have physicians available to staff the slots. When I started my group, I was able to recruit a strong, core group of five physicians in six months. I don’t think there is any way you could do that now. That’s a trend that’s changed for the worst. I don’t think internal medicine is going to be able to support the need for care providers within hospital medicine programs.
Q: How should hospital medicine groups look to fill their vacancies?
A: I think opportunities will exist for well-trained and motivated family medicine physicians. Many more rural or community-based hospitals are turning to family physicians to staff programs. Typically, family physicians represent only 3% of hospital medicine program slots. I see that percentage increasing fairly significantly in the next five years. TH
Brian Bossard, MD, was practicing as a hospitalist before he even knew what a hospitalist was. In 1993, Dr. Bossard, then a private practice internist, initiated a contract with Lincoln General Hospital in Lincoln, Neb. He agreed to care for hospital patients who didn’t have physicians. The hospital signed the contract—three years before HM pioneer Bob Wachter, MD, professor and chief of the division of hospital medicine at the University of California San Francisco, former SHM president and author of the blog “Wachter’s World” (www.wachtersworld.com), coined the term “hospitalist.”
Dr. Bossard, director and CEO of Inpatient Physician Associates in Lincoln, recently spoke with The Hospitalist about being at the forefront of the hospital medicine movement.
Q: How did you come to form your own hospitalist group?
—Brian Bossard, MD
A: [Starting in 1993], I was providing hospital medicine service while at the same time working in a private practice model. I took care of my own patients and also took care of all the assigned patients through the hospital. During that period, I started getting referrals from other physicians who wanted to turn their patients’ care over to me. It became clear after just a few years of doing that I was getting very busy and that there was a need for a more formal hospital medicine program. So, beginning in 1998, I started going to national hospital medicine meetings. I took my hospital administrator with me to the first meeting, and during the next four years developed an infrastructure for a mature hospital medicine program.
Q: What trends have you identified in HM since that time?
A: In the case of academic medicine models, hospital medicine developed because they needed to have a system to provide a cap for the residents—both in terms of number of hours they worked and the number of patients they saw. That was a new development and one that wasn’t in place when I went through training.
Private practice or community-based hospitals had physicians who were no longer interested in providing community call for taking care of patients that didn’t have physicians, or maybe didn’t have insurance. Community hospitals were finding that many physicians were opting out of that community call so they needed hospital medicine support to take care of those patients.
Q: What is the most significant change you’ve witnessed?
A: It’s become clear hospital medicine programs not only provide staffing to take care of those patients who otherwise wouldn’t be taken care of, but also provide a structure to take care of patients better. Probably the most positive and meaningful change since the mid-’90s is that hospital medicine programs are seen as quality drivers, efficiency drivers, and as a source of leadership within hospital policy making and decision making.
Q: What are your responsibilities as CEO of your group?
A: I run the business from top to bottom. Since I started the group in 2002, we’ve grown from just six physicians to 18 physicians and three nurse coordinators. So, I’ve had an opportunity during the last seven years to develop leadership roles within our group and delegate some activities to other leaders in the group. Where I once oversaw every little detail, I am now able to turn over some things to other, very talented group members. What I really focus on now is recruitment, the clinical aspects, public relations, and those sorts of things. But I never lose sight of the importance of developing data to drive our decisions, so I’m very involved in that, as well. As we add more and more physicians, I have to dedicate more time to management of the group. My clinical time goes down as the group grows.
Q: You mentioned that you collect data?
A: I work with the folks in the IT and Division Analysis departments in the hospital to identify what data we can get, what is important for me to know … so we can make decisions for the better of the group and the hospital. Some of that involves knowing what time of the day we have the highest admissions consults and what days of the week we’re busiest, and then organizing our schedule accordingly. It’s important to look at numbers and data, as opposed to going by when you feel you’re busy and when you’re not, because sometimes the feel is different from what is actually happening.
Q: What are the challenges facing your HMG?
A: Recruitment is a huge challenge. The growth of hospital medicine is much greater than anticipated even five years ago. Many programs are understaffed right now. That’s not because they don’t have financing, but because they don’t have physicians available to staff the slots. When I started my group, I was able to recruit a strong, core group of five physicians in six months. I don’t think there is any way you could do that now. That’s a trend that’s changed for the worst. I don’t think internal medicine is going to be able to support the need for care providers within hospital medicine programs.
Q: How should hospital medicine groups look to fill their vacancies?
A: I think opportunities will exist for well-trained and motivated family medicine physicians. Many more rural or community-based hospitals are turning to family physicians to staff programs. Typically, family physicians represent only 3% of hospital medicine program slots. I see that percentage increasing fairly significantly in the next five years. TH
The Bare Necessities
Medicare reimburses for procedures and services deemed “reasonable and necessary.” By statute, Medicare only may pay for items and services that are “reasonable and necessary for the diagnosis or treatment of illness or injury, or to improve the functioning of a malformed body member,” unless there is another statutory authorization for payment (e.g., colorectal cancer screening).1 Medical necessity is determined by evidence-based clinical standards of care, which guide the physician’s diagnostic and treatment process for certain patient populations, illnesses, or clinical circumstances.
National Coverage Determinations
The Centers for Medicare and Medicaid Services (CMS) develop national coverage determinations (NCDs) through an evidence-based process with opportunities for public participation. In some cases, CMS’ own research is supplemented by an outside technology assessment and/or consultation with the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC).
All Medicare contractors must adhere to NCDs and cannot create additional limitations or guidelines. As an example, the NCD for pronouncement of death states an individual only is considered to have died as of the time he orshe is pronounced dead by a person who is legally authorized to make such a pronouncement, usually a physician; and medical services rendered up to and including pronouncement are considered reasonable and necessary.2 Further guidance authorizes physicians to report discharge day management codes (99238-99239) for the face-to-face pronouncement encounter.3 See the Medicare National Coverage Determination Manual (www.cms.hhs.gov/Manuals/ IOM/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=1&sort Order=ascending&itemID=CMS014961&intNumPerPage=10) for other applicable NCDs.
Local Coverage Determinations
In the absence of a national coverage policy, an item or service may be covered at the discretion of the Medicare contractors based on a local coverage determination (LCD).4
An LCD, as established by Section 522 of the Benefits Improvement and Protection Act (BIPA), is a decision made by a fiscal intermediary or carrier to cover a particular service on an intermediary-wide or carrier-wide basis, in accordance with Section 1862(a)(1)(A) of the Social Security Act (i.e., a determination as to whether the service is reasonable and necessary).5 LCDs may vary by state, causing an inconsistent approach to medical coverage. Non-Medicare payers do not have to follow federal guidelines, unless the member participates in a Medicare managed care plan. A list of Medicare contractor LCDs can be found at www.cms.hhs.gov/DeterminationProcess/04_LCDs.asp.
Certain payers develop coverage requirements for frequent or problematic procedures or services. Coverage requirements identify specific conditions (i.e., ICD-9-CM codes) for which the services or procedures are considered medically necessary. For example, echocardiography (99307) may not be considered medically necessary for a patient who presents with chest pain unless documentation also supports suspected acute myocardial ischemia and baseline electrocardiogram (ECG) is nondiagnostic; or in cases when the physician suspects aortic dissection.6
Medical Review Program
It is insufficient to develop billing compliance policies and standards without enforcement of these guidelines. In an effort to verify the appropriateness of claims and payment, CMS contracts with Medicare Administrative Contractors (MACs), Fiscal Intermediaries (FIs), and Program Safeguard Contractors (PSCs) to perform medical reviews. The goals of the Medical Review Program are reducing Medicare claims payment errors; decreased denials and increased timely payments; and increased educational opportunities.7
In order to determine which providers should be subject to medical review, contractors must analyze provider compliance with coverage and coding rules and take corrective action when necessary. The corrective action aims to modify behavior in need of change, collect overpayments, and deny improper payments.8 Several types of review exist:
- Prepayment review: The Medicare contractor requests medical records prior to payment;
- Postpayment review: The contractor requests medical records after payment has been received by the physician; this may result in upholding or reversing the initial payment determination;
- Probe review: The contractor requests medical records associated with 20 to 40 claims based upon provider-specific issues; and
- Comprehensive error rate testing (CERT) review: CMS measures the error rate and estimates improper claim payments by randomly selecting and reviewing a sample of claims for compliance.9
Prepayment reviews seem to be expanding as a response to the error rate for certain services. For example, high-level consultation services (99245 and 99255) have prompted review over the last several years to ensure documentation and medical necessity are appropriately supported and maintained. Hospitalists may have noticed a recent increase in prepayment record requests for subsequent hospital care (99232 or 99233) and discharge day management (99239) services. Responses to these and other record requests must be timely in order to prevent claim denial or repayment requests. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References:
1. Exclusions from coverage and Medicare as a secondary payer. Social Security Online. www.ssa.gov/ OP_Home/ssact/title18/1862.htm. Updated October 28, 2008. Accessed October 15, 2008.
2. Centers for Medicare and Medicaid Services. Medicare national coverage determination manual: chapter 1, part 1, section 70.4. www.cms.hhs.gov/manuals/ downloads/ncd103c1_Part1.pdf. Accessed October 14, 2008.
3. Centers for Medicare and Medicaid Services. Transmittal 1460: Subsequent hospital visits and hospital discharge day management services (Codes 99231-99239). www.cms.hhs.gov/transmittals/downloads/R1460CP.pdf. Accessed October 14, 2008.
4. Centers for Medicare and Medicaid Services. Medicare coverage determination process: overview. www. cms.hhs.gov/DeterminationProcess/01_Overview.asp#TopOfPage. Updated August 5, 2008. Accessed October 15, 2008.
5. Centers for Medicare and Medicaid Services. Medicare coverage determination process: local coverage determinations. www.cms.hhs.gov/DeterminationProcess/ 04_LCDs.asp. Updated October 7, 2008. Accessed October 15, 2008.
6. Highmark Medicare Services. LCD L27536: transthoracic echocardiography. www.highmarkmedicareservices. com/policy/mac-ab/l27536-r3.html. Updated Septem-ber 23, 2008. Accessed October 16, 2008.
7. Centers for Medicare and Medicaid Services. The Medicare medical review program. www.cms. hhs.gov/MedicalReviewProcess/Downloads/mrfactsheet.pdf. Published September 2004. Accessed October 15, 2008.
8. Rudolph P, Shuren A. Dealing with Medicare. In: coding for chest medicine 2008. Northbrook, IL: Am Coll of Chest Physicians. 2008;23-35.
9. Centers for Medicare and Medicaid Services. Comprehensive error rate testing: overview. www. cms.hhs.gov/CERT/. Updated December 14, 2005. Accessed October 16, 2008.
10. Centers for Medicare and Medicaid Services. Medicare claims processing manual: chapter 12, section 30.6.10G. www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Updated July 9, 2008. Accessed October 16, 2008.
Medicare reimburses for procedures and services deemed “reasonable and necessary.” By statute, Medicare only may pay for items and services that are “reasonable and necessary for the diagnosis or treatment of illness or injury, or to improve the functioning of a malformed body member,” unless there is another statutory authorization for payment (e.g., colorectal cancer screening).1 Medical necessity is determined by evidence-based clinical standards of care, which guide the physician’s diagnostic and treatment process for certain patient populations, illnesses, or clinical circumstances.
National Coverage Determinations
The Centers for Medicare and Medicaid Services (CMS) develop national coverage determinations (NCDs) through an evidence-based process with opportunities for public participation. In some cases, CMS’ own research is supplemented by an outside technology assessment and/or consultation with the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC).
All Medicare contractors must adhere to NCDs and cannot create additional limitations or guidelines. As an example, the NCD for pronouncement of death states an individual only is considered to have died as of the time he orshe is pronounced dead by a person who is legally authorized to make such a pronouncement, usually a physician; and medical services rendered up to and including pronouncement are considered reasonable and necessary.2 Further guidance authorizes physicians to report discharge day management codes (99238-99239) for the face-to-face pronouncement encounter.3 See the Medicare National Coverage Determination Manual (www.cms.hhs.gov/Manuals/ IOM/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=1&sort Order=ascending&itemID=CMS014961&intNumPerPage=10) for other applicable NCDs.
Local Coverage Determinations
In the absence of a national coverage policy, an item or service may be covered at the discretion of the Medicare contractors based on a local coverage determination (LCD).4
An LCD, as established by Section 522 of the Benefits Improvement and Protection Act (BIPA), is a decision made by a fiscal intermediary or carrier to cover a particular service on an intermediary-wide or carrier-wide basis, in accordance with Section 1862(a)(1)(A) of the Social Security Act (i.e., a determination as to whether the service is reasonable and necessary).5 LCDs may vary by state, causing an inconsistent approach to medical coverage. Non-Medicare payers do not have to follow federal guidelines, unless the member participates in a Medicare managed care plan. A list of Medicare contractor LCDs can be found at www.cms.hhs.gov/DeterminationProcess/04_LCDs.asp.
Certain payers develop coverage requirements for frequent or problematic procedures or services. Coverage requirements identify specific conditions (i.e., ICD-9-CM codes) for which the services or procedures are considered medically necessary. For example, echocardiography (99307) may not be considered medically necessary for a patient who presents with chest pain unless documentation also supports suspected acute myocardial ischemia and baseline electrocardiogram (ECG) is nondiagnostic; or in cases when the physician suspects aortic dissection.6
Medical Review Program
It is insufficient to develop billing compliance policies and standards without enforcement of these guidelines. In an effort to verify the appropriateness of claims and payment, CMS contracts with Medicare Administrative Contractors (MACs), Fiscal Intermediaries (FIs), and Program Safeguard Contractors (PSCs) to perform medical reviews. The goals of the Medical Review Program are reducing Medicare claims payment errors; decreased denials and increased timely payments; and increased educational opportunities.7
In order to determine which providers should be subject to medical review, contractors must analyze provider compliance with coverage and coding rules and take corrective action when necessary. The corrective action aims to modify behavior in need of change, collect overpayments, and deny improper payments.8 Several types of review exist:
- Prepayment review: The Medicare contractor requests medical records prior to payment;
- Postpayment review: The contractor requests medical records after payment has been received by the physician; this may result in upholding or reversing the initial payment determination;
- Probe review: The contractor requests medical records associated with 20 to 40 claims based upon provider-specific issues; and
- Comprehensive error rate testing (CERT) review: CMS measures the error rate and estimates improper claim payments by randomly selecting and reviewing a sample of claims for compliance.9
Prepayment reviews seem to be expanding as a response to the error rate for certain services. For example, high-level consultation services (99245 and 99255) have prompted review over the last several years to ensure documentation and medical necessity are appropriately supported and maintained. Hospitalists may have noticed a recent increase in prepayment record requests for subsequent hospital care (99232 or 99233) and discharge day management (99239) services. Responses to these and other record requests must be timely in order to prevent claim denial or repayment requests. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References:
1. Exclusions from coverage and Medicare as a secondary payer. Social Security Online. www.ssa.gov/ OP_Home/ssact/title18/1862.htm. Updated October 28, 2008. Accessed October 15, 2008.
2. Centers for Medicare and Medicaid Services. Medicare national coverage determination manual: chapter 1, part 1, section 70.4. www.cms.hhs.gov/manuals/ downloads/ncd103c1_Part1.pdf. Accessed October 14, 2008.
3. Centers for Medicare and Medicaid Services. Transmittal 1460: Subsequent hospital visits and hospital discharge day management services (Codes 99231-99239). www.cms.hhs.gov/transmittals/downloads/R1460CP.pdf. Accessed October 14, 2008.
4. Centers for Medicare and Medicaid Services. Medicare coverage determination process: overview. www. cms.hhs.gov/DeterminationProcess/01_Overview.asp#TopOfPage. Updated August 5, 2008. Accessed October 15, 2008.
5. Centers for Medicare and Medicaid Services. Medicare coverage determination process: local coverage determinations. www.cms.hhs.gov/DeterminationProcess/ 04_LCDs.asp. Updated October 7, 2008. Accessed October 15, 2008.
6. Highmark Medicare Services. LCD L27536: transthoracic echocardiography. www.highmarkmedicareservices. com/policy/mac-ab/l27536-r3.html. Updated Septem-ber 23, 2008. Accessed October 16, 2008.
7. Centers for Medicare and Medicaid Services. The Medicare medical review program. www.cms. hhs.gov/MedicalReviewProcess/Downloads/mrfactsheet.pdf. Published September 2004. Accessed October 15, 2008.
8. Rudolph P, Shuren A. Dealing with Medicare. In: coding for chest medicine 2008. Northbrook, IL: Am Coll of Chest Physicians. 2008;23-35.
9. Centers for Medicare and Medicaid Services. Comprehensive error rate testing: overview. www. cms.hhs.gov/CERT/. Updated December 14, 2005. Accessed October 16, 2008.
10. Centers for Medicare and Medicaid Services. Medicare claims processing manual: chapter 12, section 30.6.10G. www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Updated July 9, 2008. Accessed October 16, 2008.
Medicare reimburses for procedures and services deemed “reasonable and necessary.” By statute, Medicare only may pay for items and services that are “reasonable and necessary for the diagnosis or treatment of illness or injury, or to improve the functioning of a malformed body member,” unless there is another statutory authorization for payment (e.g., colorectal cancer screening).1 Medical necessity is determined by evidence-based clinical standards of care, which guide the physician’s diagnostic and treatment process for certain patient populations, illnesses, or clinical circumstances.
National Coverage Determinations
The Centers for Medicare and Medicaid Services (CMS) develop national coverage determinations (NCDs) through an evidence-based process with opportunities for public participation. In some cases, CMS’ own research is supplemented by an outside technology assessment and/or consultation with the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC).
All Medicare contractors must adhere to NCDs and cannot create additional limitations or guidelines. As an example, the NCD for pronouncement of death states an individual only is considered to have died as of the time he orshe is pronounced dead by a person who is legally authorized to make such a pronouncement, usually a physician; and medical services rendered up to and including pronouncement are considered reasonable and necessary.2 Further guidance authorizes physicians to report discharge day management codes (99238-99239) for the face-to-face pronouncement encounter.3 See the Medicare National Coverage Determination Manual (www.cms.hhs.gov/Manuals/ IOM/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=1&sort Order=ascending&itemID=CMS014961&intNumPerPage=10) for other applicable NCDs.
Local Coverage Determinations
In the absence of a national coverage policy, an item or service may be covered at the discretion of the Medicare contractors based on a local coverage determination (LCD).4
An LCD, as established by Section 522 of the Benefits Improvement and Protection Act (BIPA), is a decision made by a fiscal intermediary or carrier to cover a particular service on an intermediary-wide or carrier-wide basis, in accordance with Section 1862(a)(1)(A) of the Social Security Act (i.e., a determination as to whether the service is reasonable and necessary).5 LCDs may vary by state, causing an inconsistent approach to medical coverage. Non-Medicare payers do not have to follow federal guidelines, unless the member participates in a Medicare managed care plan. A list of Medicare contractor LCDs can be found at www.cms.hhs.gov/DeterminationProcess/04_LCDs.asp.
Certain payers develop coverage requirements for frequent or problematic procedures or services. Coverage requirements identify specific conditions (i.e., ICD-9-CM codes) for which the services or procedures are considered medically necessary. For example, echocardiography (99307) may not be considered medically necessary for a patient who presents with chest pain unless documentation also supports suspected acute myocardial ischemia and baseline electrocardiogram (ECG) is nondiagnostic; or in cases when the physician suspects aortic dissection.6
Medical Review Program
It is insufficient to develop billing compliance policies and standards without enforcement of these guidelines. In an effort to verify the appropriateness of claims and payment, CMS contracts with Medicare Administrative Contractors (MACs), Fiscal Intermediaries (FIs), and Program Safeguard Contractors (PSCs) to perform medical reviews. The goals of the Medical Review Program are reducing Medicare claims payment errors; decreased denials and increased timely payments; and increased educational opportunities.7
In order to determine which providers should be subject to medical review, contractors must analyze provider compliance with coverage and coding rules and take corrective action when necessary. The corrective action aims to modify behavior in need of change, collect overpayments, and deny improper payments.8 Several types of review exist:
- Prepayment review: The Medicare contractor requests medical records prior to payment;
- Postpayment review: The contractor requests medical records after payment has been received by the physician; this may result in upholding or reversing the initial payment determination;
- Probe review: The contractor requests medical records associated with 20 to 40 claims based upon provider-specific issues; and
- Comprehensive error rate testing (CERT) review: CMS measures the error rate and estimates improper claim payments by randomly selecting and reviewing a sample of claims for compliance.9
Prepayment reviews seem to be expanding as a response to the error rate for certain services. For example, high-level consultation services (99245 and 99255) have prompted review over the last several years to ensure documentation and medical necessity are appropriately supported and maintained. Hospitalists may have noticed a recent increase in prepayment record requests for subsequent hospital care (99232 or 99233) and discharge day management (99239) services. Responses to these and other record requests must be timely in order to prevent claim denial or repayment requests. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References:
1. Exclusions from coverage and Medicare as a secondary payer. Social Security Online. www.ssa.gov/ OP_Home/ssact/title18/1862.htm. Updated October 28, 2008. Accessed October 15, 2008.
2. Centers for Medicare and Medicaid Services. Medicare national coverage determination manual: chapter 1, part 1, section 70.4. www.cms.hhs.gov/manuals/ downloads/ncd103c1_Part1.pdf. Accessed October 14, 2008.
3. Centers for Medicare and Medicaid Services. Transmittal 1460: Subsequent hospital visits and hospital discharge day management services (Codes 99231-99239). www.cms.hhs.gov/transmittals/downloads/R1460CP.pdf. Accessed October 14, 2008.
4. Centers for Medicare and Medicaid Services. Medicare coverage determination process: overview. www. cms.hhs.gov/DeterminationProcess/01_Overview.asp#TopOfPage. Updated August 5, 2008. Accessed October 15, 2008.
5. Centers for Medicare and Medicaid Services. Medicare coverage determination process: local coverage determinations. www.cms.hhs.gov/DeterminationProcess/ 04_LCDs.asp. Updated October 7, 2008. Accessed October 15, 2008.
6. Highmark Medicare Services. LCD L27536: transthoracic echocardiography. www.highmarkmedicareservices. com/policy/mac-ab/l27536-r3.html. Updated Septem-ber 23, 2008. Accessed October 16, 2008.
7. Centers for Medicare and Medicaid Services. The Medicare medical review program. www.cms. hhs.gov/MedicalReviewProcess/Downloads/mrfactsheet.pdf. Published September 2004. Accessed October 15, 2008.
8. Rudolph P, Shuren A. Dealing with Medicare. In: coding for chest medicine 2008. Northbrook, IL: Am Coll of Chest Physicians. 2008;23-35.
9. Centers for Medicare and Medicaid Services. Comprehensive error rate testing: overview. www. cms.hhs.gov/CERT/. Updated December 14, 2005. Accessed October 16, 2008.
10. Centers for Medicare and Medicaid Services. Medicare claims processing manual: chapter 12, section 30.6.10G. www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Updated July 9, 2008. Accessed October 16, 2008.
What interventions most effectively protect against contrast media-induced nephropathy?
Case
A 68-year-old diabetic woman hospitalized for non-ST-segment elevation myocardial infarction develops increasing chest pain despite maximal appropriate medical therapy and is referred for urgent coronary angiography. She is normotensive, weighs 60 kg, and is without signs of congestive heart failure on examination. The serum creatinine is 1.6 mg/dL (her baseline). What is her risk for contrast media-induced nephropathy (CIN)? What measures can be undertaken to reduce her risk?
Background
Radiocontrast agents are well-recognized nephrotoxins that can cause a usually reversible, non-oliguric form of renal failure within 24 hours and up to five days following administration. Contrast nephropathy is associated with longer hospital stays and higher mortality. The incidence varies widely according to patient characteristics and the type and quantity of contrast agent used.
The pathogenesis of CIN is not completely understood, but likely represents a combination of contrast-mediated renal vasoconstriction, oxidative damage, and direct cytotoxic effects. Newer low-osmolar or iso-osmolar contrast agents are associated with lower rates of CIN than high-osmolar contrast agents. Multiple pharmacologic strategies for CIN prevention have been investigated, with several important trials published in the past two years. This review summarizes the risk assessment and prophylactic strategies required for optimal protection of patients from CIN.
Assesment of Patient Risk
Contrast-induced nephropathy is defined variably in clinical trials, most commonly as a 25% increase in serum creatinine above baseline at 48 hours after contrast administration. The most important risk factor for CIN is pre-existing kidney disease—more specifically, a diminished glomerular filtration rate (GFR) below 60 mL/minute/1.73 m2 body surface area.1 The serum creatinine concentration can be misleading. Advancing age, female gender, low lean body mass, or unstable rising creatinine all can lead to overestimation of the GFR. The Modification of Diet in Renal Disease (MDRD) estimate of GFR and the Cockcroft-Gault estimate of creatinine clearance are calculated in a basic formula. (see Table 1, left)
Several other factors have been linked to increased risk for CIN. Table 2 (left) summarizes these risk factors and assigns them various point scores. In general, patients with chronic kidney disease or any of these risk factors should have a serum creatinine drawn before the contrast study to clarify their CIN risk and facilitate decisions regarding prophylaxis. Patients with a score of six or more are at substantial risk for CIN.1
Strategy for Prophylaxis
Low-osmolar and iso-osmolar contrast agents have been associated with lower rates of CIN compared to high-osmolar contrast. However, the referring hospitalist rarely determines the type and volume of contrast used. Fortunately, high-osmolar contrast is used infrequently today. The primary strategy for CIN prophylaxis is to:
1) Determine CIN risk using a validated tool (see Table 2).
2) If “at risk,” consider alternate diagnostic modalities that do not involve the intravenous administration of iodinated contrast. Consider delaying testing with contrast agents until potentially reversible conditions affecting GFR are addressed, such as volume depletion, recent contrast use, or concomitant use of nonsteroidal anti-inflammatory drugs or angiotensin-converting enzyme inhibitors.
3) Provide pharmacologic and intravenous fluid prophylaxis as described below.
Pharmacologic Prophylaxis
Multiple agents have been investigated in the prevention of CIN: mannitol, furosemide, theophylline, fenoldopam, dopamine, N-acetylcysteine, and others. The most effective noteworthy of these is N-acetylcysteine (NAC). The first major trial of NAC for CIN prevention was published in 2000.2 Since then, more than two dozen studies, mostly randomized controlled trials (RCTs), and nearly a dozen meta-analyses have been published, with inconsistent results.
Of particular note, systematic reviews and meta-analyses have reached differing conclusions on the overall efficacy of NAC in the prevention of CIN. One recent study including NAC trials published before June 2006 concluded there has been “significant publication bias throughout the life cycle of this clinical question … further amplified by meta-analyses.”3 It has been estimated a single trial enrolling 1,800 patients (about 10 times larger than most completed trials) would be needed to definitively answer this question.4 The latest meta-analysis includes at least one large RCT of NAC not included in prior meta-analyses and concludes that NAC is effective in the prevention of CIN.5 The pooled relative risk for CIN was 0.62 (95% C.I. 0.44-0.88). These investigators concluded there was no significant publication bias.
Taken together, the primary literature and secondary meta-analyses suggest that NAC is probably effective in the prevention of CIN, although there may be some publication bias. Practically speaking, NAC is essentially without side effects, and the likelihood that it affords some degree of protection suggests it should be used routinely, unless or until larger studies demonstrate otherwise. A NAC dose of 1,200 mg twice daily beginning the day prior and continuing through the day of contrast administration was part of the successful protocol published by Brigouri, et al., in 2007.
Intravenous Crystalloids Trials
A landmark trial published in 1994 showed half-normal saline in 5% dextrose given 12 hours before and 12 hours after administering a radiocontrast agent was superior to half-normal saline plus mannitol or half-normal saline plus furosemide in preventing CIN.6 This regimen remained the standard of care until 2002, when a large RCT compared half- normal saline in 5% dextrose to isotonic normal saline in 1,620 patients undergoing coronary angioplasty.7 About 20% of the patients had underlying renal dysfunction and about 15% were diabetic. The rate of CIN decreased from 2% (14/698) to 0.7% (5/685), a modest-but-statistically-significant difference. After this study, practice generally shifted to using normal saline at 1 mL/kg/hr 12 hours before and 12 hours after contrast procedures. One notable review article published in 2006 concluded that isotonic saline was the best-proven strategy for the prevention of CIN.8
How does intravenous sodium chloride reduce the rate of CIN? The mechanism is unclear, but it may work simply by treating subclinical states of volume depletion. But as free radical oxidation has been implicated in the pathophysiology of CIN, investigators hypothesized that alkalinizing the urine (reducing free radical formation) with isotonic sodium bicarbonate might better protect patients from CIN than saline. In 2004, the first trial demonstrating the efficacy of bicarbonate was stopped early after the rate of CIN had decreased from 13.6% (8/59) in the saline arm to 1.7% (1/60) in the bicarbonate arm.9 The editorial accompanying this small trial cautioned “prospective confirmation should be required before accepting new therapies into routine clinical practice.”
In 2007, four prospective trials comparing various hydration regimens were published; each concluding that bicarbonate is superior to saline. The largest of these studies was the REMEDIAL trial.10 Patients were referred for coronary angiography and had a baseline serum creatinine of 2.0 mg/dl or higher or an estimated GFR below 40 mL/minute/1.73 m2 (or both). In double-blind fashion, patients were randomized to one of three preventative strategies: normal saline plus NAC (n=111), sodium bicarbonate plus NAC (n=108), or normal saline plus NAC plus ascorbic acid (n=107). The primary endpoint was defined as a 25% or higher increase in serum creatinine at 48 hours. This occurred in 9.9% (11/111) of the normal saline plus NAC group, 1.9% (2/108) of the sodium bicarbonate plus NAC group, and 10.3% (11/107) of the normal saline plus NAC plus ascorbic acid group (p=0.019 for sodium bicarbonate plus NAC versus normal saline plus NAC).
The sodium bicarbonate regimen was the same as that reported by Merten in 2004—namely, 154 mEq/L of sodium bicarbonate in 5% dextrose solution, given at 3 mL/kg/hr for one hour before contrast administration and 1 mL/kg/hr for six hours afterward. The saline regimen (154 mEq/L) was the same as that reported by Mueller in 2002—1 mL/kg/hr for 12 hours before contrast administration and 12 hours afterward. All patients received NAC at a dose of 1,200 mg twice daily the day before and the day of contrast administration. It is not possible to conclude from this trial whether sodium bicarbonate without NAC would have been as effective as the regimen studied. Ascorbic acid was included in this trial as another antioxidant to compare with NAC. The three other RCTs published in 2007 are summarized in Table 3 (see p. 21).11,12,13
Recently, two large RCTs of saline versus bicarbonate concluded there was no difference between the two.14,15 These trials were the largest to date, each of them single center and unblinded, and using slightly different methods than the REMEDIAL trial. CIN also was defined more broadly as a 0.5mg/dL or 25% change in creatinine within five days after contrast. Follow-up was only 88% in one trial. Nevertheless, these two new trials reach quite different conclusions than those before. Table 3 (see p. 21) summarizes seven RCTs of saline versus bicarbonate in the prevention of CIN. Differences in design and methods, definitions of CIN, completeness of follow-up, and severity of renal dysfunction among patients studied, make direct comparisons among these trials difficult. But as five of the seven RCTs of saline versus bicarbonate have concluded that bicarbonate is superior, and none have concluded saline is superior, this author recommends that at the present time intravenous sodium bicarbonate be used according to the Merten protocol when providing IVF for the prevention of CIN.
Back to the Case
The patient in the vignette has an estimated GFR of about 32 mL/min by the MDRD equation. With this level of renal dysfunction, the presence of diabetes mellitus, mellitus and assuming at least a 100 cc contrast bolus with the angiography, her risk for CIN is about 14% (eight points on the Mehran scale illustrated in Table 21). Alternatives to coronary angiography are limited in this example, and pharmacologic and IVF measures to prevent CIN are indicated. Borrowing from the regimen used in the REMEDIAL trial, she should ideally receive NAC 1200 mg orally BID for two days, starting one day prior to the procedure (in this case, would begin as soon as the risk for CIN is appreciated and continue for four doses). More importantly, she should receive sodium bicarbonate 154mEq/L at a rate of 3 mL/kg/hr one hour prior to contrast and 1 mL/kg/hr during and for six hours following the contrast procedure.
Bottom Line
Contrast nephropathy risk varies inversely with GFR and can be estimated according to a validated tool. Patients at risk for CIN should be identified early and offered NAC and sodium bicarbonate, if there are no alternatives to administering intravenous contrast. Intravenous saline also is effective, but may not be as effective as bicarbonate. TH
Dr. Anderson is an assistant professor of medicine at the University of Colorado Denver and the associate chief, Medical Service, at the Denver VA Medical Center.
References
1. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-1399.
2. Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-184.
3. Vaitkus PT and Brar C. N-Acetylcysteine in the prevention of contrast-induced nephropathy: publication bias perpetuated by meta-analyses. Am Heart J. 2007;153:275-280.
4. Bagshaw SM, McAlister FA, Manns BJ, Ghali WA. Acetylcysteine in the prevention of contrast-induced nephropathy: a case study of the pitfalls in the evolution of evidence. Arch Intern Med. 2006;166:161-166.
5. Kelly AM, Dwamena B, Cronin P, Bernstein SJ and Carlos RC. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148:284-294.
6. Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:14-16.
7. Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast-media associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329-336.
8. Barrett BJ and Parfrey PS. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379-386.
9. Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328-2334.
10. Brigouri C, Airoldi F, D.Andrea, et al. Renal insufficiency following contrast media administration trial (remedial): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211-1217.
11. Masuda M, Yamada T, Mine T, et al. Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. Am J Cardiol. 2007;100:781-786.
12. Recio-Mayoral A, Chaparro M, Prado B, et al. The reno-protective effect of hydration with sodium bicarbonate plus n-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the reno study. J Am Coll Cardiol. 2007;49:1283-1288.
13. Ozcan EE, Guneri S, Akdeniz B, et al. Sodium bicarbonate, n-acetylcysteine, and saline for the prevention of radiocontrast-induced nephropathy. a comparison of 3 regimens for protecting contrast-induced nephropathy (sic) in patients undergoing coronary procedures. a single center prospective controlled trial. Am Heart J. 2007;154:539-544.
14. Maioli M, Toso A, Leoncini M, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52:599-604.
15. Brar SS, Shen AYJ, Jorgensen MB, et al. Sodium bicarbonate vs. sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA. 2008;300:1038-1046.
Case
A 68-year-old diabetic woman hospitalized for non-ST-segment elevation myocardial infarction develops increasing chest pain despite maximal appropriate medical therapy and is referred for urgent coronary angiography. She is normotensive, weighs 60 kg, and is without signs of congestive heart failure on examination. The serum creatinine is 1.6 mg/dL (her baseline). What is her risk for contrast media-induced nephropathy (CIN)? What measures can be undertaken to reduce her risk?
Background
Radiocontrast agents are well-recognized nephrotoxins that can cause a usually reversible, non-oliguric form of renal failure within 24 hours and up to five days following administration. Contrast nephropathy is associated with longer hospital stays and higher mortality. The incidence varies widely according to patient characteristics and the type and quantity of contrast agent used.
The pathogenesis of CIN is not completely understood, but likely represents a combination of contrast-mediated renal vasoconstriction, oxidative damage, and direct cytotoxic effects. Newer low-osmolar or iso-osmolar contrast agents are associated with lower rates of CIN than high-osmolar contrast agents. Multiple pharmacologic strategies for CIN prevention have been investigated, with several important trials published in the past two years. This review summarizes the risk assessment and prophylactic strategies required for optimal protection of patients from CIN.
Assesment of Patient Risk
Contrast-induced nephropathy is defined variably in clinical trials, most commonly as a 25% increase in serum creatinine above baseline at 48 hours after contrast administration. The most important risk factor for CIN is pre-existing kidney disease—more specifically, a diminished glomerular filtration rate (GFR) below 60 mL/minute/1.73 m2 body surface area.1 The serum creatinine concentration can be misleading. Advancing age, female gender, low lean body mass, or unstable rising creatinine all can lead to overestimation of the GFR. The Modification of Diet in Renal Disease (MDRD) estimate of GFR and the Cockcroft-Gault estimate of creatinine clearance are calculated in a basic formula. (see Table 1, left)
Several other factors have been linked to increased risk for CIN. Table 2 (left) summarizes these risk factors and assigns them various point scores. In general, patients with chronic kidney disease or any of these risk factors should have a serum creatinine drawn before the contrast study to clarify their CIN risk and facilitate decisions regarding prophylaxis. Patients with a score of six or more are at substantial risk for CIN.1
Strategy for Prophylaxis
Low-osmolar and iso-osmolar contrast agents have been associated with lower rates of CIN compared to high-osmolar contrast. However, the referring hospitalist rarely determines the type and volume of contrast used. Fortunately, high-osmolar contrast is used infrequently today. The primary strategy for CIN prophylaxis is to:
1) Determine CIN risk using a validated tool (see Table 2).
2) If “at risk,” consider alternate diagnostic modalities that do not involve the intravenous administration of iodinated contrast. Consider delaying testing with contrast agents until potentially reversible conditions affecting GFR are addressed, such as volume depletion, recent contrast use, or concomitant use of nonsteroidal anti-inflammatory drugs or angiotensin-converting enzyme inhibitors.
3) Provide pharmacologic and intravenous fluid prophylaxis as described below.
Pharmacologic Prophylaxis
Multiple agents have been investigated in the prevention of CIN: mannitol, furosemide, theophylline, fenoldopam, dopamine, N-acetylcysteine, and others. The most effective noteworthy of these is N-acetylcysteine (NAC). The first major trial of NAC for CIN prevention was published in 2000.2 Since then, more than two dozen studies, mostly randomized controlled trials (RCTs), and nearly a dozen meta-analyses have been published, with inconsistent results.
Of particular note, systematic reviews and meta-analyses have reached differing conclusions on the overall efficacy of NAC in the prevention of CIN. One recent study including NAC trials published before June 2006 concluded there has been “significant publication bias throughout the life cycle of this clinical question … further amplified by meta-analyses.”3 It has been estimated a single trial enrolling 1,800 patients (about 10 times larger than most completed trials) would be needed to definitively answer this question.4 The latest meta-analysis includes at least one large RCT of NAC not included in prior meta-analyses and concludes that NAC is effective in the prevention of CIN.5 The pooled relative risk for CIN was 0.62 (95% C.I. 0.44-0.88). These investigators concluded there was no significant publication bias.
Taken together, the primary literature and secondary meta-analyses suggest that NAC is probably effective in the prevention of CIN, although there may be some publication bias. Practically speaking, NAC is essentially without side effects, and the likelihood that it affords some degree of protection suggests it should be used routinely, unless or until larger studies demonstrate otherwise. A NAC dose of 1,200 mg twice daily beginning the day prior and continuing through the day of contrast administration was part of the successful protocol published by Brigouri, et al., in 2007.
Intravenous Crystalloids Trials
A landmark trial published in 1994 showed half-normal saline in 5% dextrose given 12 hours before and 12 hours after administering a radiocontrast agent was superior to half-normal saline plus mannitol or half-normal saline plus furosemide in preventing CIN.6 This regimen remained the standard of care until 2002, when a large RCT compared half- normal saline in 5% dextrose to isotonic normal saline in 1,620 patients undergoing coronary angioplasty.7 About 20% of the patients had underlying renal dysfunction and about 15% were diabetic. The rate of CIN decreased from 2% (14/698) to 0.7% (5/685), a modest-but-statistically-significant difference. After this study, practice generally shifted to using normal saline at 1 mL/kg/hr 12 hours before and 12 hours after contrast procedures. One notable review article published in 2006 concluded that isotonic saline was the best-proven strategy for the prevention of CIN.8
How does intravenous sodium chloride reduce the rate of CIN? The mechanism is unclear, but it may work simply by treating subclinical states of volume depletion. But as free radical oxidation has been implicated in the pathophysiology of CIN, investigators hypothesized that alkalinizing the urine (reducing free radical formation) with isotonic sodium bicarbonate might better protect patients from CIN than saline. In 2004, the first trial demonstrating the efficacy of bicarbonate was stopped early after the rate of CIN had decreased from 13.6% (8/59) in the saline arm to 1.7% (1/60) in the bicarbonate arm.9 The editorial accompanying this small trial cautioned “prospective confirmation should be required before accepting new therapies into routine clinical practice.”
In 2007, four prospective trials comparing various hydration regimens were published; each concluding that bicarbonate is superior to saline. The largest of these studies was the REMEDIAL trial.10 Patients were referred for coronary angiography and had a baseline serum creatinine of 2.0 mg/dl or higher or an estimated GFR below 40 mL/minute/1.73 m2 (or both). In double-blind fashion, patients were randomized to one of three preventative strategies: normal saline plus NAC (n=111), sodium bicarbonate plus NAC (n=108), or normal saline plus NAC plus ascorbic acid (n=107). The primary endpoint was defined as a 25% or higher increase in serum creatinine at 48 hours. This occurred in 9.9% (11/111) of the normal saline plus NAC group, 1.9% (2/108) of the sodium bicarbonate plus NAC group, and 10.3% (11/107) of the normal saline plus NAC plus ascorbic acid group (p=0.019 for sodium bicarbonate plus NAC versus normal saline plus NAC).
The sodium bicarbonate regimen was the same as that reported by Merten in 2004—namely, 154 mEq/L of sodium bicarbonate in 5% dextrose solution, given at 3 mL/kg/hr for one hour before contrast administration and 1 mL/kg/hr for six hours afterward. The saline regimen (154 mEq/L) was the same as that reported by Mueller in 2002—1 mL/kg/hr for 12 hours before contrast administration and 12 hours afterward. All patients received NAC at a dose of 1,200 mg twice daily the day before and the day of contrast administration. It is not possible to conclude from this trial whether sodium bicarbonate without NAC would have been as effective as the regimen studied. Ascorbic acid was included in this trial as another antioxidant to compare with NAC. The three other RCTs published in 2007 are summarized in Table 3 (see p. 21).11,12,13
Recently, two large RCTs of saline versus bicarbonate concluded there was no difference between the two.14,15 These trials were the largest to date, each of them single center and unblinded, and using slightly different methods than the REMEDIAL trial. CIN also was defined more broadly as a 0.5mg/dL or 25% change in creatinine within five days after contrast. Follow-up was only 88% in one trial. Nevertheless, these two new trials reach quite different conclusions than those before. Table 3 (see p. 21) summarizes seven RCTs of saline versus bicarbonate in the prevention of CIN. Differences in design and methods, definitions of CIN, completeness of follow-up, and severity of renal dysfunction among patients studied, make direct comparisons among these trials difficult. But as five of the seven RCTs of saline versus bicarbonate have concluded that bicarbonate is superior, and none have concluded saline is superior, this author recommends that at the present time intravenous sodium bicarbonate be used according to the Merten protocol when providing IVF for the prevention of CIN.
Back to the Case
The patient in the vignette has an estimated GFR of about 32 mL/min by the MDRD equation. With this level of renal dysfunction, the presence of diabetes mellitus, mellitus and assuming at least a 100 cc contrast bolus with the angiography, her risk for CIN is about 14% (eight points on the Mehran scale illustrated in Table 21). Alternatives to coronary angiography are limited in this example, and pharmacologic and IVF measures to prevent CIN are indicated. Borrowing from the regimen used in the REMEDIAL trial, she should ideally receive NAC 1200 mg orally BID for two days, starting one day prior to the procedure (in this case, would begin as soon as the risk for CIN is appreciated and continue for four doses). More importantly, she should receive sodium bicarbonate 154mEq/L at a rate of 3 mL/kg/hr one hour prior to contrast and 1 mL/kg/hr during and for six hours following the contrast procedure.
Bottom Line
Contrast nephropathy risk varies inversely with GFR and can be estimated according to a validated tool. Patients at risk for CIN should be identified early and offered NAC and sodium bicarbonate, if there are no alternatives to administering intravenous contrast. Intravenous saline also is effective, but may not be as effective as bicarbonate. TH
Dr. Anderson is an assistant professor of medicine at the University of Colorado Denver and the associate chief, Medical Service, at the Denver VA Medical Center.
References
1. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-1399.
2. Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-184.
3. Vaitkus PT and Brar C. N-Acetylcysteine in the prevention of contrast-induced nephropathy: publication bias perpetuated by meta-analyses. Am Heart J. 2007;153:275-280.
4. Bagshaw SM, McAlister FA, Manns BJ, Ghali WA. Acetylcysteine in the prevention of contrast-induced nephropathy: a case study of the pitfalls in the evolution of evidence. Arch Intern Med. 2006;166:161-166.
5. Kelly AM, Dwamena B, Cronin P, Bernstein SJ and Carlos RC. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148:284-294.
6. Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:14-16.
7. Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast-media associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329-336.
8. Barrett BJ and Parfrey PS. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379-386.
9. Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328-2334.
10. Brigouri C, Airoldi F, D.Andrea, et al. Renal insufficiency following contrast media administration trial (remedial): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211-1217.
11. Masuda M, Yamada T, Mine T, et al. Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. Am J Cardiol. 2007;100:781-786.
12. Recio-Mayoral A, Chaparro M, Prado B, et al. The reno-protective effect of hydration with sodium bicarbonate plus n-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the reno study. J Am Coll Cardiol. 2007;49:1283-1288.
13. Ozcan EE, Guneri S, Akdeniz B, et al. Sodium bicarbonate, n-acetylcysteine, and saline for the prevention of radiocontrast-induced nephropathy. a comparison of 3 regimens for protecting contrast-induced nephropathy (sic) in patients undergoing coronary procedures. a single center prospective controlled trial. Am Heart J. 2007;154:539-544.
14. Maioli M, Toso A, Leoncini M, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52:599-604.
15. Brar SS, Shen AYJ, Jorgensen MB, et al. Sodium bicarbonate vs. sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA. 2008;300:1038-1046.
Case
A 68-year-old diabetic woman hospitalized for non-ST-segment elevation myocardial infarction develops increasing chest pain despite maximal appropriate medical therapy and is referred for urgent coronary angiography. She is normotensive, weighs 60 kg, and is without signs of congestive heart failure on examination. The serum creatinine is 1.6 mg/dL (her baseline). What is her risk for contrast media-induced nephropathy (CIN)? What measures can be undertaken to reduce her risk?
Background
Radiocontrast agents are well-recognized nephrotoxins that can cause a usually reversible, non-oliguric form of renal failure within 24 hours and up to five days following administration. Contrast nephropathy is associated with longer hospital stays and higher mortality. The incidence varies widely according to patient characteristics and the type and quantity of contrast agent used.
The pathogenesis of CIN is not completely understood, but likely represents a combination of contrast-mediated renal vasoconstriction, oxidative damage, and direct cytotoxic effects. Newer low-osmolar or iso-osmolar contrast agents are associated with lower rates of CIN than high-osmolar contrast agents. Multiple pharmacologic strategies for CIN prevention have been investigated, with several important trials published in the past two years. This review summarizes the risk assessment and prophylactic strategies required for optimal protection of patients from CIN.
Assesment of Patient Risk
Contrast-induced nephropathy is defined variably in clinical trials, most commonly as a 25% increase in serum creatinine above baseline at 48 hours after contrast administration. The most important risk factor for CIN is pre-existing kidney disease—more specifically, a diminished glomerular filtration rate (GFR) below 60 mL/minute/1.73 m2 body surface area.1 The serum creatinine concentration can be misleading. Advancing age, female gender, low lean body mass, or unstable rising creatinine all can lead to overestimation of the GFR. The Modification of Diet in Renal Disease (MDRD) estimate of GFR and the Cockcroft-Gault estimate of creatinine clearance are calculated in a basic formula. (see Table 1, left)
Several other factors have been linked to increased risk for CIN. Table 2 (left) summarizes these risk factors and assigns them various point scores. In general, patients with chronic kidney disease or any of these risk factors should have a serum creatinine drawn before the contrast study to clarify their CIN risk and facilitate decisions regarding prophylaxis. Patients with a score of six or more are at substantial risk for CIN.1
Strategy for Prophylaxis
Low-osmolar and iso-osmolar contrast agents have been associated with lower rates of CIN compared to high-osmolar contrast. However, the referring hospitalist rarely determines the type and volume of contrast used. Fortunately, high-osmolar contrast is used infrequently today. The primary strategy for CIN prophylaxis is to:
1) Determine CIN risk using a validated tool (see Table 2).
2) If “at risk,” consider alternate diagnostic modalities that do not involve the intravenous administration of iodinated contrast. Consider delaying testing with contrast agents until potentially reversible conditions affecting GFR are addressed, such as volume depletion, recent contrast use, or concomitant use of nonsteroidal anti-inflammatory drugs or angiotensin-converting enzyme inhibitors.
3) Provide pharmacologic and intravenous fluid prophylaxis as described below.
Pharmacologic Prophylaxis
Multiple agents have been investigated in the prevention of CIN: mannitol, furosemide, theophylline, fenoldopam, dopamine, N-acetylcysteine, and others. The most effective noteworthy of these is N-acetylcysteine (NAC). The first major trial of NAC for CIN prevention was published in 2000.2 Since then, more than two dozen studies, mostly randomized controlled trials (RCTs), and nearly a dozen meta-analyses have been published, with inconsistent results.
Of particular note, systematic reviews and meta-analyses have reached differing conclusions on the overall efficacy of NAC in the prevention of CIN. One recent study including NAC trials published before June 2006 concluded there has been “significant publication bias throughout the life cycle of this clinical question … further amplified by meta-analyses.”3 It has been estimated a single trial enrolling 1,800 patients (about 10 times larger than most completed trials) would be needed to definitively answer this question.4 The latest meta-analysis includes at least one large RCT of NAC not included in prior meta-analyses and concludes that NAC is effective in the prevention of CIN.5 The pooled relative risk for CIN was 0.62 (95% C.I. 0.44-0.88). These investigators concluded there was no significant publication bias.
Taken together, the primary literature and secondary meta-analyses suggest that NAC is probably effective in the prevention of CIN, although there may be some publication bias. Practically speaking, NAC is essentially without side effects, and the likelihood that it affords some degree of protection suggests it should be used routinely, unless or until larger studies demonstrate otherwise. A NAC dose of 1,200 mg twice daily beginning the day prior and continuing through the day of contrast administration was part of the successful protocol published by Brigouri, et al., in 2007.
Intravenous Crystalloids Trials
A landmark trial published in 1994 showed half-normal saline in 5% dextrose given 12 hours before and 12 hours after administering a radiocontrast agent was superior to half-normal saline plus mannitol or half-normal saline plus furosemide in preventing CIN.6 This regimen remained the standard of care until 2002, when a large RCT compared half- normal saline in 5% dextrose to isotonic normal saline in 1,620 patients undergoing coronary angioplasty.7 About 20% of the patients had underlying renal dysfunction and about 15% were diabetic. The rate of CIN decreased from 2% (14/698) to 0.7% (5/685), a modest-but-statistically-significant difference. After this study, practice generally shifted to using normal saline at 1 mL/kg/hr 12 hours before and 12 hours after contrast procedures. One notable review article published in 2006 concluded that isotonic saline was the best-proven strategy for the prevention of CIN.8
How does intravenous sodium chloride reduce the rate of CIN? The mechanism is unclear, but it may work simply by treating subclinical states of volume depletion. But as free radical oxidation has been implicated in the pathophysiology of CIN, investigators hypothesized that alkalinizing the urine (reducing free radical formation) with isotonic sodium bicarbonate might better protect patients from CIN than saline. In 2004, the first trial demonstrating the efficacy of bicarbonate was stopped early after the rate of CIN had decreased from 13.6% (8/59) in the saline arm to 1.7% (1/60) in the bicarbonate arm.9 The editorial accompanying this small trial cautioned “prospective confirmation should be required before accepting new therapies into routine clinical practice.”
In 2007, four prospective trials comparing various hydration regimens were published; each concluding that bicarbonate is superior to saline. The largest of these studies was the REMEDIAL trial.10 Patients were referred for coronary angiography and had a baseline serum creatinine of 2.0 mg/dl or higher or an estimated GFR below 40 mL/minute/1.73 m2 (or both). In double-blind fashion, patients were randomized to one of three preventative strategies: normal saline plus NAC (n=111), sodium bicarbonate plus NAC (n=108), or normal saline plus NAC plus ascorbic acid (n=107). The primary endpoint was defined as a 25% or higher increase in serum creatinine at 48 hours. This occurred in 9.9% (11/111) of the normal saline plus NAC group, 1.9% (2/108) of the sodium bicarbonate plus NAC group, and 10.3% (11/107) of the normal saline plus NAC plus ascorbic acid group (p=0.019 for sodium bicarbonate plus NAC versus normal saline plus NAC).
The sodium bicarbonate regimen was the same as that reported by Merten in 2004—namely, 154 mEq/L of sodium bicarbonate in 5% dextrose solution, given at 3 mL/kg/hr for one hour before contrast administration and 1 mL/kg/hr for six hours afterward. The saline regimen (154 mEq/L) was the same as that reported by Mueller in 2002—1 mL/kg/hr for 12 hours before contrast administration and 12 hours afterward. All patients received NAC at a dose of 1,200 mg twice daily the day before and the day of contrast administration. It is not possible to conclude from this trial whether sodium bicarbonate without NAC would have been as effective as the regimen studied. Ascorbic acid was included in this trial as another antioxidant to compare with NAC. The three other RCTs published in 2007 are summarized in Table 3 (see p. 21).11,12,13
Recently, two large RCTs of saline versus bicarbonate concluded there was no difference between the two.14,15 These trials were the largest to date, each of them single center and unblinded, and using slightly different methods than the REMEDIAL trial. CIN also was defined more broadly as a 0.5mg/dL or 25% change in creatinine within five days after contrast. Follow-up was only 88% in one trial. Nevertheless, these two new trials reach quite different conclusions than those before. Table 3 (see p. 21) summarizes seven RCTs of saline versus bicarbonate in the prevention of CIN. Differences in design and methods, definitions of CIN, completeness of follow-up, and severity of renal dysfunction among patients studied, make direct comparisons among these trials difficult. But as five of the seven RCTs of saline versus bicarbonate have concluded that bicarbonate is superior, and none have concluded saline is superior, this author recommends that at the present time intravenous sodium bicarbonate be used according to the Merten protocol when providing IVF for the prevention of CIN.
Back to the Case
The patient in the vignette has an estimated GFR of about 32 mL/min by the MDRD equation. With this level of renal dysfunction, the presence of diabetes mellitus, mellitus and assuming at least a 100 cc contrast bolus with the angiography, her risk for CIN is about 14% (eight points on the Mehran scale illustrated in Table 21). Alternatives to coronary angiography are limited in this example, and pharmacologic and IVF measures to prevent CIN are indicated. Borrowing from the regimen used in the REMEDIAL trial, she should ideally receive NAC 1200 mg orally BID for two days, starting one day prior to the procedure (in this case, would begin as soon as the risk for CIN is appreciated and continue for four doses). More importantly, she should receive sodium bicarbonate 154mEq/L at a rate of 3 mL/kg/hr one hour prior to contrast and 1 mL/kg/hr during and for six hours following the contrast procedure.
Bottom Line
Contrast nephropathy risk varies inversely with GFR and can be estimated according to a validated tool. Patients at risk for CIN should be identified early and offered NAC and sodium bicarbonate, if there are no alternatives to administering intravenous contrast. Intravenous saline also is effective, but may not be as effective as bicarbonate. TH
Dr. Anderson is an assistant professor of medicine at the University of Colorado Denver and the associate chief, Medical Service, at the Denver VA Medical Center.
References
1. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393-1399.
2. Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-184.
3. Vaitkus PT and Brar C. N-Acetylcysteine in the prevention of contrast-induced nephropathy: publication bias perpetuated by meta-analyses. Am Heart J. 2007;153:275-280.
4. Bagshaw SM, McAlister FA, Manns BJ, Ghali WA. Acetylcysteine in the prevention of contrast-induced nephropathy: a case study of the pitfalls in the evolution of evidence. Arch Intern Med. 2006;166:161-166.
5. Kelly AM, Dwamena B, Cronin P, Bernstein SJ and Carlos RC. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148:284-294.
6. Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide on acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:14-16.
7. Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast-media associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329-336.
8. Barrett BJ and Parfrey PS. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354:379-386.
9. Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328-2334.
10. Brigouri C, Airoldi F, D.Andrea, et al. Renal insufficiency following contrast media administration trial (remedial): a randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211-1217.
11. Masuda M, Yamada T, Mine T, et al. Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. Am J Cardiol. 2007;100:781-786.
12. Recio-Mayoral A, Chaparro M, Prado B, et al. The reno-protective effect of hydration with sodium bicarbonate plus n-acetylcysteine in patients undergoing emergency percutaneous coronary intervention: the reno study. J Am Coll Cardiol. 2007;49:1283-1288.
13. Ozcan EE, Guneri S, Akdeniz B, et al. Sodium bicarbonate, n-acetylcysteine, and saline for the prevention of radiocontrast-induced nephropathy. a comparison of 3 regimens for protecting contrast-induced nephropathy (sic) in patients undergoing coronary procedures. a single center prospective controlled trial. Am Heart J. 2007;154:539-544.
14. Maioli M, Toso A, Leoncini M, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. J Am Coll Cardiol. 2008;52:599-604.
15. Brar SS, Shen AYJ, Jorgensen MB, et al. Sodium bicarbonate vs. sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA. 2008;300:1038-1046.
A Truly Different World
(Maj) Heather Cereste, MD, chair of the Bioethics Committee at Wilford Hall Medical Center at Lackland Air Force Base near San Antonio, Texas, and a member of Team Hospitalist, is the only geriatric-trained internist in the U.S. Air Force. From January through May 2007, she served as the attending primary care physician at Balad Trauma Hospital in Balad, Iraq. She recently spoke with The Hospitalist about her experience as a wartime physician.
Q: What motivated you to join the Air Force?
A: I talked to the Air Force near end of third year in residency. A number of things played into my decision. I was in Manhattan during 9/11 and got caught up in the surge of patriotism. I had thought about the military before, and was at a point when I was about to enter geriatrics and wasn’t sure if wanted to go into the traditional workforce or explore something else. I joined the reserves in 2004 and went active in 2006. To be honest with you, I never thought I would be deployed to a combat zone.
—Heather Cereste, MD
Q: What type of training did you receive before going to Iraq?
A: I was just undergoing the credentialing process when I was asked by my commander [to] deploy with her in a few months. I was a little shocked and taken aback, and didn’t feel at all prepared. So I inquired about further training and was referred to the shock trauma group in Baltimore, Md. It was the closest I could get to warfare type of injuries because it’s an urban warfare they fight in Baltimore. There, I was able to gain confidence in doing some procedures, including chest tubes, and refreshing myself about central lines and the acuity of care.
Q: What was it like working in Iraq?
A: Our team worked seven days a week in the intensive care unit. We were on call every fifth night, overnight. We took care of the critically ill patients who came in through ER or who were directed to us. For the most part, we interacted with the coalition people for only 24–48 hours before they were transported out. The American and British people often went to Germany for more definitive care.
Q: What medical conditions did you see?
A: Over five months we managed about 528 critically ill people. There were certainly a lot of postoperative cases. We took care of burns and head wounds, which were increasing in number, a lot of limb amputations, as well as blast injuries and gunshot wounds. Civilians would present at our gates and we could triage them, if we had enough room.
Q: Did you feel like you were in a war zone?
A: It was very surreal. I was one of the last rotations to go when it was a tent hospital, so when we had rain and weather, we’d have to deal with floods, etc. It was a very rustic environment; there was dust was everywhere. The helicopters would come in and land right outside our tents.
Our hospital was right next to the wire–that’s a barbed wire fence that separated our base from the outside of the base–so we heard machine guns constantly while we were doing our rounds. We also got mortared frequently. Disgruntled people on the other side would set up across the river. They had some Russian mortars that they would throw over to our side. Whenever we could identify that the mortars were coming over the wall, sirens would go off and we’d have to dive for cover.
You’re constantly reminded of war, if not by the sounds, than certainly with the injuries. And people were carrying their guns all the time. It was strange to be a physician carrying a gun.
Q: How did your background in geriatrics come into play?
A: Believe it or not, many of the Iraqi civilians we treated were not chronically aged, but were physiology aged. We saw a lot of geriatric syndromes, even in 45-year-olds. Diet and access to care were common issues.
Q: Did you have enough resources?
A: As far as combat hospitals go, in my limited experience, I think we had excellent resources. But sometimes, if patients required extended intensive care and if we didn’t have the dialysis or the level of burn care, we just couldn’t treat them. It was a challenge every day to deal with certain patients who we knew under normal circumstances we could take care of, but because of the circumstance we had to stop care. That made it really hard.
Q: Is there one case that stands out as an example of what can be done in a combat zone?
A: There was one young baby who was a medical case. He was 28 days old when he first presented. He came to the gate with his parents with an infected arm. He had been seen at an outside facility and was treated for some kind of infection.
We thought from an initial admitting diagnosis that he had pericardial infusion. He had a long, protracted course where he required intubation. He was quite the enigma, and required a lot of attention and care and resources. Everyone at the hospital, from the nursing staff, to the medical technicians, to chaplains, would stop by say hello to the baby. We all did our best to keep him alive. He ended up getting discharged; the last we heard he was doing all right. My hope is that he would grow very strong.
It was nice to have a child around. It was also great because the family had entrusted us to take care of him. They seemed grateful when they were finally able to take him home.
Q: Would you go back?
A: Definitely. It was probably the most amazing experience in my life, professional and personally. It’s a wonderful place to do medicine because you’re forced to practice outside your comfort zone. You also feel that your efforts are playing a positive role. You get out of that whole humdrum, “beaten-by-the-system” feeling that I think people may feel here. I got to meet interesting people and be a part of history. And I survived, so that was good. TH
(Maj) Heather Cereste, MD, chair of the Bioethics Committee at Wilford Hall Medical Center at Lackland Air Force Base near San Antonio, Texas, and a member of Team Hospitalist, is the only geriatric-trained internist in the U.S. Air Force. From January through May 2007, she served as the attending primary care physician at Balad Trauma Hospital in Balad, Iraq. She recently spoke with The Hospitalist about her experience as a wartime physician.
Q: What motivated you to join the Air Force?
A: I talked to the Air Force near end of third year in residency. A number of things played into my decision. I was in Manhattan during 9/11 and got caught up in the surge of patriotism. I had thought about the military before, and was at a point when I was about to enter geriatrics and wasn’t sure if wanted to go into the traditional workforce or explore something else. I joined the reserves in 2004 and went active in 2006. To be honest with you, I never thought I would be deployed to a combat zone.
—Heather Cereste, MD
Q: What type of training did you receive before going to Iraq?
A: I was just undergoing the credentialing process when I was asked by my commander [to] deploy with her in a few months. I was a little shocked and taken aback, and didn’t feel at all prepared. So I inquired about further training and was referred to the shock trauma group in Baltimore, Md. It was the closest I could get to warfare type of injuries because it’s an urban warfare they fight in Baltimore. There, I was able to gain confidence in doing some procedures, including chest tubes, and refreshing myself about central lines and the acuity of care.
Q: What was it like working in Iraq?
A: Our team worked seven days a week in the intensive care unit. We were on call every fifth night, overnight. We took care of the critically ill patients who came in through ER or who were directed to us. For the most part, we interacted with the coalition people for only 24–48 hours before they were transported out. The American and British people often went to Germany for more definitive care.
Q: What medical conditions did you see?
A: Over five months we managed about 528 critically ill people. There were certainly a lot of postoperative cases. We took care of burns and head wounds, which were increasing in number, a lot of limb amputations, as well as blast injuries and gunshot wounds. Civilians would present at our gates and we could triage them, if we had enough room.
Q: Did you feel like you were in a war zone?
A: It was very surreal. I was one of the last rotations to go when it was a tent hospital, so when we had rain and weather, we’d have to deal with floods, etc. It was a very rustic environment; there was dust was everywhere. The helicopters would come in and land right outside our tents.
Our hospital was right next to the wire–that’s a barbed wire fence that separated our base from the outside of the base–so we heard machine guns constantly while we were doing our rounds. We also got mortared frequently. Disgruntled people on the other side would set up across the river. They had some Russian mortars that they would throw over to our side. Whenever we could identify that the mortars were coming over the wall, sirens would go off and we’d have to dive for cover.
You’re constantly reminded of war, if not by the sounds, than certainly with the injuries. And people were carrying their guns all the time. It was strange to be a physician carrying a gun.
Q: How did your background in geriatrics come into play?
A: Believe it or not, many of the Iraqi civilians we treated were not chronically aged, but were physiology aged. We saw a lot of geriatric syndromes, even in 45-year-olds. Diet and access to care were common issues.
Q: Did you have enough resources?
A: As far as combat hospitals go, in my limited experience, I think we had excellent resources. But sometimes, if patients required extended intensive care and if we didn’t have the dialysis or the level of burn care, we just couldn’t treat them. It was a challenge every day to deal with certain patients who we knew under normal circumstances we could take care of, but because of the circumstance we had to stop care. That made it really hard.
Q: Is there one case that stands out as an example of what can be done in a combat zone?
A: There was one young baby who was a medical case. He was 28 days old when he first presented. He came to the gate with his parents with an infected arm. He had been seen at an outside facility and was treated for some kind of infection.
We thought from an initial admitting diagnosis that he had pericardial infusion. He had a long, protracted course where he required intubation. He was quite the enigma, and required a lot of attention and care and resources. Everyone at the hospital, from the nursing staff, to the medical technicians, to chaplains, would stop by say hello to the baby. We all did our best to keep him alive. He ended up getting discharged; the last we heard he was doing all right. My hope is that he would grow very strong.
It was nice to have a child around. It was also great because the family had entrusted us to take care of him. They seemed grateful when they were finally able to take him home.
Q: Would you go back?
A: Definitely. It was probably the most amazing experience in my life, professional and personally. It’s a wonderful place to do medicine because you’re forced to practice outside your comfort zone. You also feel that your efforts are playing a positive role. You get out of that whole humdrum, “beaten-by-the-system” feeling that I think people may feel here. I got to meet interesting people and be a part of history. And I survived, so that was good. TH
(Maj) Heather Cereste, MD, chair of the Bioethics Committee at Wilford Hall Medical Center at Lackland Air Force Base near San Antonio, Texas, and a member of Team Hospitalist, is the only geriatric-trained internist in the U.S. Air Force. From January through May 2007, she served as the attending primary care physician at Balad Trauma Hospital in Balad, Iraq. She recently spoke with The Hospitalist about her experience as a wartime physician.
Q: What motivated you to join the Air Force?
A: I talked to the Air Force near end of third year in residency. A number of things played into my decision. I was in Manhattan during 9/11 and got caught up in the surge of patriotism. I had thought about the military before, and was at a point when I was about to enter geriatrics and wasn’t sure if wanted to go into the traditional workforce or explore something else. I joined the reserves in 2004 and went active in 2006. To be honest with you, I never thought I would be deployed to a combat zone.
—Heather Cereste, MD
Q: What type of training did you receive before going to Iraq?
A: I was just undergoing the credentialing process when I was asked by my commander [to] deploy with her in a few months. I was a little shocked and taken aback, and didn’t feel at all prepared. So I inquired about further training and was referred to the shock trauma group in Baltimore, Md. It was the closest I could get to warfare type of injuries because it’s an urban warfare they fight in Baltimore. There, I was able to gain confidence in doing some procedures, including chest tubes, and refreshing myself about central lines and the acuity of care.
Q: What was it like working in Iraq?
A: Our team worked seven days a week in the intensive care unit. We were on call every fifth night, overnight. We took care of the critically ill patients who came in through ER or who were directed to us. For the most part, we interacted with the coalition people for only 24–48 hours before they were transported out. The American and British people often went to Germany for more definitive care.
Q: What medical conditions did you see?
A: Over five months we managed about 528 critically ill people. There were certainly a lot of postoperative cases. We took care of burns and head wounds, which were increasing in number, a lot of limb amputations, as well as blast injuries and gunshot wounds. Civilians would present at our gates and we could triage them, if we had enough room.
Q: Did you feel like you were in a war zone?
A: It was very surreal. I was one of the last rotations to go when it was a tent hospital, so when we had rain and weather, we’d have to deal with floods, etc. It was a very rustic environment; there was dust was everywhere. The helicopters would come in and land right outside our tents.
Our hospital was right next to the wire–that’s a barbed wire fence that separated our base from the outside of the base–so we heard machine guns constantly while we were doing our rounds. We also got mortared frequently. Disgruntled people on the other side would set up across the river. They had some Russian mortars that they would throw over to our side. Whenever we could identify that the mortars were coming over the wall, sirens would go off and we’d have to dive for cover.
You’re constantly reminded of war, if not by the sounds, than certainly with the injuries. And people were carrying their guns all the time. It was strange to be a physician carrying a gun.
Q: How did your background in geriatrics come into play?
A: Believe it or not, many of the Iraqi civilians we treated were not chronically aged, but were physiology aged. We saw a lot of geriatric syndromes, even in 45-year-olds. Diet and access to care were common issues.
Q: Did you have enough resources?
A: As far as combat hospitals go, in my limited experience, I think we had excellent resources. But sometimes, if patients required extended intensive care and if we didn’t have the dialysis or the level of burn care, we just couldn’t treat them. It was a challenge every day to deal with certain patients who we knew under normal circumstances we could take care of, but because of the circumstance we had to stop care. That made it really hard.
Q: Is there one case that stands out as an example of what can be done in a combat zone?
A: There was one young baby who was a medical case. He was 28 days old when he first presented. He came to the gate with his parents with an infected arm. He had been seen at an outside facility and was treated for some kind of infection.
We thought from an initial admitting diagnosis that he had pericardial infusion. He had a long, protracted course where he required intubation. He was quite the enigma, and required a lot of attention and care and resources. Everyone at the hospital, from the nursing staff, to the medical technicians, to chaplains, would stop by say hello to the baby. We all did our best to keep him alive. He ended up getting discharged; the last we heard he was doing all right. My hope is that he would grow very strong.
It was nice to have a child around. It was also great because the family had entrusted us to take care of him. They seemed grateful when they were finally able to take him home.
Q: Would you go back?
A: Definitely. It was probably the most amazing experience in my life, professional and personally. It’s a wonderful place to do medicine because you’re forced to practice outside your comfort zone. You also feel that your efforts are playing a positive role. You get out of that whole humdrum, “beaten-by-the-system” feeling that I think people may feel here. I got to meet interesting people and be a part of history. And I survived, so that was good. TH
Reimbursement Rights
Recent changes in healthcare have forced academic medical centers to seek additional resources in the delivery of quality care. In response to internal and external pressures to minimize length of stay, adhere to limitations on the maximum number of admitted patients, focus on evidence-based care, and improve outcomes of care, hospitalists have incorporated non-physician providers (NPPs), such as acute care nurse practitioners (ACNPs), into their group practices.1
Whereas traditional nurse practitioners focus on the promotion of health and management of chronic illness, ACNPs focus on the care of acutely ill patients. Hospitalists utilize NPPs to expand medical service capacity and improve the efficiency and quality of patient care.2
Research indicates physician/nurse practitioner collaboration in the multidisciplinary management of hospitalized medical patients reduces length of stay and improves hospital profit without altering readmissions or mortality.3 Billing and documentation standards for NPP services must comply with current state and federal regulations. Hospitalist groups should become familiar with these guidelines prior to billing for NPP services involved in this patient care model.
The following highlights inpatient services provided by nurse practitioners (NPs) and physician assistants (PAs).
Covered Services
Medicare pays for services considered reasonable and necessary and not otherwise excluded from coverage. NPPs may provide any service permitted by the state scope of practice and performed in conjunction with the appropriate level of supervision or collaboration, as outlined in licensure or billing requirements. Being only limited by state and/or facility regulations, NPP services comprise visits or procedures typically rendered by ancillary staff or considered a physician service (a doctor of medicine, MD, or osteopathy, DO). Additionally, NPPs must meet the insurer-specified qualifications.
Independent Billing
Since 1998, designated NPPs are allowed to submit Medicare Part B claims for services, including procedures, provided in any inpatient or outpatient setting. For billing purposes, these “independent” services do not require physician involvement (e.g. physician initiation of care plan, physician-patient encounter, or physician presence on patient floor/unit) unless otherwise specified by state legislation or facility standards of practice. NPPs do not need to be employed by the physician group. The entity employing the physician group also may employ the NPP.
Claim requirements mandate the use of a national provider identifier (NPI) on all claims, therefore, all NPPs receive an NPI for claim submission. However, not all NPPs may directly bill Medicare or receive direct payment (e.g., physician assistant).1 In this situation, the NPP employer (i.e., physician or group), reports the service with the physician or group provider number and the NPP’s NPI included for identification of who actually provided the service.
Medicare Part B processes NPP claims reported under the independent billing option. Duplicate payments from any other Medicare Part A or Part B source is strictly prohibited and may result in refunds, fines and penalties. Generally, Medicare payment for NPP services is limited to 85% of the allowable physician rate. Financial impact of the 15% rate reduction is typically offset by the increase in physician time. Physicians may use this time to provide more comprehensive or complex services (admissions or consultations), potentially generating more revenue. Consistent with all provider documentation, NPP documentation must support the reported service.
Shared/Split Billing
The shared/split billing option first appeared in 2002 to address facility-based services provided to a single patient by an NPP and physician from the same group practice on the same calendar day. This option only applies to evaluation and management services provided in an emergency department, outpatient or inpatient hospital. It excludes consultations and critical care services. Unlike the independent billing option, the shared/split billing option only involves service provided by nurse practitioners, physician assistants, clinical nurse specialists, and certified nurse-midwives.
In order to qualify as a shared/split service, the NPP and the physician each must have a face-to-face encounter with the patient, although the extent of each provider’s involvement is left to provider discretion and/or local Medicare contractor requirements. The timing of each provider’s visit is irrelevant, as long as the two services are performed on the same date. For example, the NPP may see a hospital inpatient in the morning with a follow-up visit by the physician later in the day.4 When documenting, both the NPP and the physician should identify the name of the individual with whom the service is shared/split. This will allow for appropriate service capture, and ensure that the correct notes are sent to the payer in the event of claim denial and subsequent appeal. Each provider must document their portion of the rendered service and select the visit level supported by the cumulative encounter. The physician need not duplicate the elements performed and documented by the NPP, but merely perform and record the physician-determined critical or key portions. Do not confuse this billing option with teaching physician regulations. Physician and the specified NPPs cannot share or split a service with any other provider type (e.g., residents, medical or nursing students).
Only one claim may be submitted for a shared/split service. The physician may choose to report the service under his own name or under the NPP name. Reimbursement is dependent upon this selection. The physician name secures 100% of the Medicare allowable rate; the NPP name earns 85% of the allowable physician rate.
While the physician has the opportunity to report the service under his own name for the full service rate, the shared/split billing option requires the efforts of two individuals and may be an impractical approach for some physician groups.
“Incident-to”
Hospitalists, or their staff, may have encountered the term “incident-to” and wondered how this billing option applies to hospitalist services. “Incident-to” guidelines only apply to procedures and services performed in a private physician office. In this setting, the patient establishes care with the physician and the physician develops a patient-specific plan of care. Subsequent services may be provided to the established patient by the NPP, yet reported under the physician’s name for 100% of the allowable physician rate. “Incident-to” services cannot be reported by a hospitalist, since hospitalist services only take place in facility-based locations.
Summary
NPPs currently are involved in an extensive number of services within the hospital, and Medicare has two billing options for NPP services provided on behalf of or in conjunction with hospitalists. Each option involves specific rules and regulations with which NPPs and physician groups must comply.
Successful reporting requires understanding of and adherence to federal, state, and facility guidelines. It is important to identify NPP employment relationships, the NPP’s role in the provision of services, the state supervisory or collaborative rules, and local payer interpretations to prevent misrepresentation, misunderstanding, or erroneous reporting. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
References
1. Centers for Medicare and Medicaid Services. Medicare benefit policy manual. www.cms.hhs.gov/manuals/Downloads/bp102c15.pdf. Accessed September 12, 2008.
2. Howie J, Erickson M. Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service. Am J of Critical Care. 2002;11:448-458.
3. Cowan M, Shapiro M, et al.. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nursing Admin. 2006;36:79-85.
4. CMS. Medicare claims processing manual: Chapter 12, Section 30.6.1B. www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Accessed September 14, 2008.
5. Pohlig C. Nonphysician providers in your practice. In: coding for chest medicine 2008. Northbrook, IL: Am Coll Chest Phy. 2008;249-254.
Recent changes in healthcare have forced academic medical centers to seek additional resources in the delivery of quality care. In response to internal and external pressures to minimize length of stay, adhere to limitations on the maximum number of admitted patients, focus on evidence-based care, and improve outcomes of care, hospitalists have incorporated non-physician providers (NPPs), such as acute care nurse practitioners (ACNPs), into their group practices.1
Whereas traditional nurse practitioners focus on the promotion of health and management of chronic illness, ACNPs focus on the care of acutely ill patients. Hospitalists utilize NPPs to expand medical service capacity and improve the efficiency and quality of patient care.2
Research indicates physician/nurse practitioner collaboration in the multidisciplinary management of hospitalized medical patients reduces length of stay and improves hospital profit without altering readmissions or mortality.3 Billing and documentation standards for NPP services must comply with current state and federal regulations. Hospitalist groups should become familiar with these guidelines prior to billing for NPP services involved in this patient care model.
The following highlights inpatient services provided by nurse practitioners (NPs) and physician assistants (PAs).
Covered Services
Medicare pays for services considered reasonable and necessary and not otherwise excluded from coverage. NPPs may provide any service permitted by the state scope of practice and performed in conjunction with the appropriate level of supervision or collaboration, as outlined in licensure or billing requirements. Being only limited by state and/or facility regulations, NPP services comprise visits or procedures typically rendered by ancillary staff or considered a physician service (a doctor of medicine, MD, or osteopathy, DO). Additionally, NPPs must meet the insurer-specified qualifications.
Independent Billing
Since 1998, designated NPPs are allowed to submit Medicare Part B claims for services, including procedures, provided in any inpatient or outpatient setting. For billing purposes, these “independent” services do not require physician involvement (e.g. physician initiation of care plan, physician-patient encounter, or physician presence on patient floor/unit) unless otherwise specified by state legislation or facility standards of practice. NPPs do not need to be employed by the physician group. The entity employing the physician group also may employ the NPP.
Claim requirements mandate the use of a national provider identifier (NPI) on all claims, therefore, all NPPs receive an NPI for claim submission. However, not all NPPs may directly bill Medicare or receive direct payment (e.g., physician assistant).1 In this situation, the NPP employer (i.e., physician or group), reports the service with the physician or group provider number and the NPP’s NPI included for identification of who actually provided the service.
Medicare Part B processes NPP claims reported under the independent billing option. Duplicate payments from any other Medicare Part A or Part B source is strictly prohibited and may result in refunds, fines and penalties. Generally, Medicare payment for NPP services is limited to 85% of the allowable physician rate. Financial impact of the 15% rate reduction is typically offset by the increase in physician time. Physicians may use this time to provide more comprehensive or complex services (admissions or consultations), potentially generating more revenue. Consistent with all provider documentation, NPP documentation must support the reported service.
Shared/Split Billing
The shared/split billing option first appeared in 2002 to address facility-based services provided to a single patient by an NPP and physician from the same group practice on the same calendar day. This option only applies to evaluation and management services provided in an emergency department, outpatient or inpatient hospital. It excludes consultations and critical care services. Unlike the independent billing option, the shared/split billing option only involves service provided by nurse practitioners, physician assistants, clinical nurse specialists, and certified nurse-midwives.
In order to qualify as a shared/split service, the NPP and the physician each must have a face-to-face encounter with the patient, although the extent of each provider’s involvement is left to provider discretion and/or local Medicare contractor requirements. The timing of each provider’s visit is irrelevant, as long as the two services are performed on the same date. For example, the NPP may see a hospital inpatient in the morning with a follow-up visit by the physician later in the day.4 When documenting, both the NPP and the physician should identify the name of the individual with whom the service is shared/split. This will allow for appropriate service capture, and ensure that the correct notes are sent to the payer in the event of claim denial and subsequent appeal. Each provider must document their portion of the rendered service and select the visit level supported by the cumulative encounter. The physician need not duplicate the elements performed and documented by the NPP, but merely perform and record the physician-determined critical or key portions. Do not confuse this billing option with teaching physician regulations. Physician and the specified NPPs cannot share or split a service with any other provider type (e.g., residents, medical or nursing students).
Only one claim may be submitted for a shared/split service. The physician may choose to report the service under his own name or under the NPP name. Reimbursement is dependent upon this selection. The physician name secures 100% of the Medicare allowable rate; the NPP name earns 85% of the allowable physician rate.
While the physician has the opportunity to report the service under his own name for the full service rate, the shared/split billing option requires the efforts of two individuals and may be an impractical approach for some physician groups.
“Incident-to”
Hospitalists, or their staff, may have encountered the term “incident-to” and wondered how this billing option applies to hospitalist services. “Incident-to” guidelines only apply to procedures and services performed in a private physician office. In this setting, the patient establishes care with the physician and the physician develops a patient-specific plan of care. Subsequent services may be provided to the established patient by the NPP, yet reported under the physician’s name for 100% of the allowable physician rate. “Incident-to” services cannot be reported by a hospitalist, since hospitalist services only take place in facility-based locations.
Summary
NPPs currently are involved in an extensive number of services within the hospital, and Medicare has two billing options for NPP services provided on behalf of or in conjunction with hospitalists. Each option involves specific rules and regulations with which NPPs and physician groups must comply.
Successful reporting requires understanding of and adherence to federal, state, and facility guidelines. It is important to identify NPP employment relationships, the NPP’s role in the provision of services, the state supervisory or collaborative rules, and local payer interpretations to prevent misrepresentation, misunderstanding, or erroneous reporting. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
References
1. Centers for Medicare and Medicaid Services. Medicare benefit policy manual. www.cms.hhs.gov/manuals/Downloads/bp102c15.pdf. Accessed September 12, 2008.
2. Howie J, Erickson M. Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service. Am J of Critical Care. 2002;11:448-458.
3. Cowan M, Shapiro M, et al.. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nursing Admin. 2006;36:79-85.
4. CMS. Medicare claims processing manual: Chapter 12, Section 30.6.1B. www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Accessed September 14, 2008.
5. Pohlig C. Nonphysician providers in your practice. In: coding for chest medicine 2008. Northbrook, IL: Am Coll Chest Phy. 2008;249-254.
Recent changes in healthcare have forced academic medical centers to seek additional resources in the delivery of quality care. In response to internal and external pressures to minimize length of stay, adhere to limitations on the maximum number of admitted patients, focus on evidence-based care, and improve outcomes of care, hospitalists have incorporated non-physician providers (NPPs), such as acute care nurse practitioners (ACNPs), into their group practices.1
Whereas traditional nurse practitioners focus on the promotion of health and management of chronic illness, ACNPs focus on the care of acutely ill patients. Hospitalists utilize NPPs to expand medical service capacity and improve the efficiency and quality of patient care.2
Research indicates physician/nurse practitioner collaboration in the multidisciplinary management of hospitalized medical patients reduces length of stay and improves hospital profit without altering readmissions or mortality.3 Billing and documentation standards for NPP services must comply with current state and federal regulations. Hospitalist groups should become familiar with these guidelines prior to billing for NPP services involved in this patient care model.
The following highlights inpatient services provided by nurse practitioners (NPs) and physician assistants (PAs).
Covered Services
Medicare pays for services considered reasonable and necessary and not otherwise excluded from coverage. NPPs may provide any service permitted by the state scope of practice and performed in conjunction with the appropriate level of supervision or collaboration, as outlined in licensure or billing requirements. Being only limited by state and/or facility regulations, NPP services comprise visits or procedures typically rendered by ancillary staff or considered a physician service (a doctor of medicine, MD, or osteopathy, DO). Additionally, NPPs must meet the insurer-specified qualifications.
Independent Billing
Since 1998, designated NPPs are allowed to submit Medicare Part B claims for services, including procedures, provided in any inpatient or outpatient setting. For billing purposes, these “independent” services do not require physician involvement (e.g. physician initiation of care plan, physician-patient encounter, or physician presence on patient floor/unit) unless otherwise specified by state legislation or facility standards of practice. NPPs do not need to be employed by the physician group. The entity employing the physician group also may employ the NPP.
Claim requirements mandate the use of a national provider identifier (NPI) on all claims, therefore, all NPPs receive an NPI for claim submission. However, not all NPPs may directly bill Medicare or receive direct payment (e.g., physician assistant).1 In this situation, the NPP employer (i.e., physician or group), reports the service with the physician or group provider number and the NPP’s NPI included for identification of who actually provided the service.
Medicare Part B processes NPP claims reported under the independent billing option. Duplicate payments from any other Medicare Part A or Part B source is strictly prohibited and may result in refunds, fines and penalties. Generally, Medicare payment for NPP services is limited to 85% of the allowable physician rate. Financial impact of the 15% rate reduction is typically offset by the increase in physician time. Physicians may use this time to provide more comprehensive or complex services (admissions or consultations), potentially generating more revenue. Consistent with all provider documentation, NPP documentation must support the reported service.
Shared/Split Billing
The shared/split billing option first appeared in 2002 to address facility-based services provided to a single patient by an NPP and physician from the same group practice on the same calendar day. This option only applies to evaluation and management services provided in an emergency department, outpatient or inpatient hospital. It excludes consultations and critical care services. Unlike the independent billing option, the shared/split billing option only involves service provided by nurse practitioners, physician assistants, clinical nurse specialists, and certified nurse-midwives.
In order to qualify as a shared/split service, the NPP and the physician each must have a face-to-face encounter with the patient, although the extent of each provider’s involvement is left to provider discretion and/or local Medicare contractor requirements. The timing of each provider’s visit is irrelevant, as long as the two services are performed on the same date. For example, the NPP may see a hospital inpatient in the morning with a follow-up visit by the physician later in the day.4 When documenting, both the NPP and the physician should identify the name of the individual with whom the service is shared/split. This will allow for appropriate service capture, and ensure that the correct notes are sent to the payer in the event of claim denial and subsequent appeal. Each provider must document their portion of the rendered service and select the visit level supported by the cumulative encounter. The physician need not duplicate the elements performed and documented by the NPP, but merely perform and record the physician-determined critical or key portions. Do not confuse this billing option with teaching physician regulations. Physician and the specified NPPs cannot share or split a service with any other provider type (e.g., residents, medical or nursing students).
Only one claim may be submitted for a shared/split service. The physician may choose to report the service under his own name or under the NPP name. Reimbursement is dependent upon this selection. The physician name secures 100% of the Medicare allowable rate; the NPP name earns 85% of the allowable physician rate.
While the physician has the opportunity to report the service under his own name for the full service rate, the shared/split billing option requires the efforts of two individuals and may be an impractical approach for some physician groups.
“Incident-to”
Hospitalists, or their staff, may have encountered the term “incident-to” and wondered how this billing option applies to hospitalist services. “Incident-to” guidelines only apply to procedures and services performed in a private physician office. In this setting, the patient establishes care with the physician and the physician develops a patient-specific plan of care. Subsequent services may be provided to the established patient by the NPP, yet reported under the physician’s name for 100% of the allowable physician rate. “Incident-to” services cannot be reported by a hospitalist, since hospitalist services only take place in facility-based locations.
Summary
NPPs currently are involved in an extensive number of services within the hospital, and Medicare has two billing options for NPP services provided on behalf of or in conjunction with hospitalists. Each option involves specific rules and regulations with which NPPs and physician groups must comply.
Successful reporting requires understanding of and adherence to federal, state, and facility guidelines. It is important to identify NPP employment relationships, the NPP’s role in the provision of services, the state supervisory or collaborative rules, and local payer interpretations to prevent misrepresentation, misunderstanding, or erroneous reporting. TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
References
1. Centers for Medicare and Medicaid Services. Medicare benefit policy manual. www.cms.hhs.gov/manuals/Downloads/bp102c15.pdf. Accessed September 12, 2008.
2. Howie J, Erickson M. Acute care nurse practitioners: creating and implementing a model of care for an inpatient general medical service. Am J of Critical Care. 2002;11:448-458.
3. Cowan M, Shapiro M, et al.. The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs. J Nursing Admin. 2006;36:79-85.
4. CMS. Medicare claims processing manual: Chapter 12, Section 30.6.1B. www.cms.hhs.gov/manuals/downloads/clm104c12.pdf. Accessed September 14, 2008.
5. Pohlig C. Nonphysician providers in your practice. In: coding for chest medicine 2008. Northbrook, IL: Am Coll Chest Phy. 2008;249-254.
Enough Is Enough, I’m Calling a Lawyer
Physicians are no strangers to specialized careers. In narrowing the scope of their practice, specialists develop the expertise and experience to benefit patients and colleagues alike.
Specialization is common in the legal profession, as well, and some legal issues present the need to obtain specialized legal assistance. Just as a patient needing an appendectomy shouldn’t visit a psychiatrist, a medical malpractice client shouldn’t visit a tax attorney.
Before working with an attorney, pose the following questions:
- How many times have you represented clients with my particular legal problem?
- How many of those cases have gone to trial?
- Have you received any specialized training in the area of my legal problem?
- Have you written any articles or taught any courses in the area of my legal problem?
- And, most importantly, what is your philosophy towards handling legal matters?
Some legal issues will require an aggressive attorney; others may need a softer touch, an attorney who will work toward resolving a matter amicably. You should feel comfortable your attorney has the experience to handle the claim and the right philosophy toward litigation. If you want confirmation, feel free to ask for the name of a prior client.
Here are some brief descriptions of the different types of specialized legal services available. Choosing the right attorney will save you time, money and should maximize the possibility that you will have a successful outcome.
Medical Malpractice Defense Counsel
In the unfortunate event you are sued for medical malpractice, you want to make sure your insurance company assigns you an attorney who has substantial experience in defending medical malpractice. These lawsuits are very complex and require defense attorneys to understand not only the legal requirements of the claim, but also the medical conditions and interventions undertaken on the patients’ behalf.
Professional Licensure Defense Counsel
Some attorneys focus on defending health care professionals before licensing agencies, such as the Board of Medical Examiners or the Drug Enforcement Agency. These proceedings often involve issues that are non-medical in nature, such as fraud, sexual misconduct and substance abuse. Attorneys specialized in representing clients before licensing agencies will have a better understanding of how the agency views the issues and will be able to recommend prospective courses of action, such as peer assistance or continuing education programs, making formal disciplinary proceedings less likely.
Labor and Employment Litigator
There are numerous laws governing the workplace, so when an employment issue surfaces, it’s important to work with an experienced labor and employment attorney. Most attorneys further specialize and represent plaintiffs or defendants, so make sure that you consult with an attorney on the right side of your issue.
Personal Injury Litigator
Some personal injury attorneys work on a volume basis and defer much of the process to paralegals and staff members. Other counselors take on a smaller volume of cases and give each case more individual attention. If you are injured in the workplace and need to find a personal injury attorney, you might want to ask a medical malpractice defense lawyer or your insurance company for a referral.
Matrimonial
One of the most common reasons a physician needs to hire counsel is the dissolution of a marriage. These cases raise intense, personal issues dealing with the division of assets, sale of property, and the allocation of parental responsibilities. Many of these issues are the subject of state laws, which attempt to compel an equitable determination. Working with an experienced matrimonial attorney will keep the focus on the legal merits of the case.
Tax Counsel
Tax law is one of the areas in which law schools offer an advanced degree, known as an LLM. It is the equivalent of a post-doctoral training program. These professionals have tremendous experience in representing individuals and businesses in the formation of business entities and in dealing with federal and state taxing authorities.
Medical Entity Formation
Depending on the state you live in, you may have a choice of business entities for your practice, such as corporations, partnerships, limited liability partnerships (LLP), and professional corporations. In choosing and structuring a business entity, you should consult with an attorney who has experience in representing health care professionals. State and federal regulations may affect your choice of an entity. A good attorney also can help clients anticipate and avoid potential dissolution issues, such as disputes over non-compete provisions, distribution of accounts receivable, and transfer of patient files.
Real Property
When attorneys refer to “real property,” they are describing the purchase and development of land, which can raise complex legal issues related to zoning, easements, assessments, restrictive covenants, and leasing.
Intellectual Property
When lawyers refer to “intellectual property,” they are describing the protections provided to a person’s creative efforts, such as copyright, trademarks and patents. Attorneys can earn a formal advanced degree in this area through an LLM program. If you develop an invention or write a book, intellectual property attorneys are best suited to make sure you receive the benefits of your creative efforts.
Trust and Estate
When people die, they leave an estate, which can be the subject of extensive probate proceedings to determine the heirs’ rights. Even if there are no disputes between heirs, there can be probate proceedings to determine the value of the estate and the taxes that might be assessed against it. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
Physicians are no strangers to specialized careers. In narrowing the scope of their practice, specialists develop the expertise and experience to benefit patients and colleagues alike.
Specialization is common in the legal profession, as well, and some legal issues present the need to obtain specialized legal assistance. Just as a patient needing an appendectomy shouldn’t visit a psychiatrist, a medical malpractice client shouldn’t visit a tax attorney.
Before working with an attorney, pose the following questions:
- How many times have you represented clients with my particular legal problem?
- How many of those cases have gone to trial?
- Have you received any specialized training in the area of my legal problem?
- Have you written any articles or taught any courses in the area of my legal problem?
- And, most importantly, what is your philosophy towards handling legal matters?
Some legal issues will require an aggressive attorney; others may need a softer touch, an attorney who will work toward resolving a matter amicably. You should feel comfortable your attorney has the experience to handle the claim and the right philosophy toward litigation. If you want confirmation, feel free to ask for the name of a prior client.
Here are some brief descriptions of the different types of specialized legal services available. Choosing the right attorney will save you time, money and should maximize the possibility that you will have a successful outcome.
Medical Malpractice Defense Counsel
In the unfortunate event you are sued for medical malpractice, you want to make sure your insurance company assigns you an attorney who has substantial experience in defending medical malpractice. These lawsuits are very complex and require defense attorneys to understand not only the legal requirements of the claim, but also the medical conditions and interventions undertaken on the patients’ behalf.
Professional Licensure Defense Counsel
Some attorneys focus on defending health care professionals before licensing agencies, such as the Board of Medical Examiners or the Drug Enforcement Agency. These proceedings often involve issues that are non-medical in nature, such as fraud, sexual misconduct and substance abuse. Attorneys specialized in representing clients before licensing agencies will have a better understanding of how the agency views the issues and will be able to recommend prospective courses of action, such as peer assistance or continuing education programs, making formal disciplinary proceedings less likely.
Labor and Employment Litigator
There are numerous laws governing the workplace, so when an employment issue surfaces, it’s important to work with an experienced labor and employment attorney. Most attorneys further specialize and represent plaintiffs or defendants, so make sure that you consult with an attorney on the right side of your issue.
Personal Injury Litigator
Some personal injury attorneys work on a volume basis and defer much of the process to paralegals and staff members. Other counselors take on a smaller volume of cases and give each case more individual attention. If you are injured in the workplace and need to find a personal injury attorney, you might want to ask a medical malpractice defense lawyer or your insurance company for a referral.
Matrimonial
One of the most common reasons a physician needs to hire counsel is the dissolution of a marriage. These cases raise intense, personal issues dealing with the division of assets, sale of property, and the allocation of parental responsibilities. Many of these issues are the subject of state laws, which attempt to compel an equitable determination. Working with an experienced matrimonial attorney will keep the focus on the legal merits of the case.
Tax Counsel
Tax law is one of the areas in which law schools offer an advanced degree, known as an LLM. It is the equivalent of a post-doctoral training program. These professionals have tremendous experience in representing individuals and businesses in the formation of business entities and in dealing with federal and state taxing authorities.
Medical Entity Formation
Depending on the state you live in, you may have a choice of business entities for your practice, such as corporations, partnerships, limited liability partnerships (LLP), and professional corporations. In choosing and structuring a business entity, you should consult with an attorney who has experience in representing health care professionals. State and federal regulations may affect your choice of an entity. A good attorney also can help clients anticipate and avoid potential dissolution issues, such as disputes over non-compete provisions, distribution of accounts receivable, and transfer of patient files.
Real Property
When attorneys refer to “real property,” they are describing the purchase and development of land, which can raise complex legal issues related to zoning, easements, assessments, restrictive covenants, and leasing.
Intellectual Property
When lawyers refer to “intellectual property,” they are describing the protections provided to a person’s creative efforts, such as copyright, trademarks and patents. Attorneys can earn a formal advanced degree in this area through an LLM program. If you develop an invention or write a book, intellectual property attorneys are best suited to make sure you receive the benefits of your creative efforts.
Trust and Estate
When people die, they leave an estate, which can be the subject of extensive probate proceedings to determine the heirs’ rights. Even if there are no disputes between heirs, there can be probate proceedings to determine the value of the estate and the taxes that might be assessed against it. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
Physicians are no strangers to specialized careers. In narrowing the scope of their practice, specialists develop the expertise and experience to benefit patients and colleagues alike.
Specialization is common in the legal profession, as well, and some legal issues present the need to obtain specialized legal assistance. Just as a patient needing an appendectomy shouldn’t visit a psychiatrist, a medical malpractice client shouldn’t visit a tax attorney.
Before working with an attorney, pose the following questions:
- How many times have you represented clients with my particular legal problem?
- How many of those cases have gone to trial?
- Have you received any specialized training in the area of my legal problem?
- Have you written any articles or taught any courses in the area of my legal problem?
- And, most importantly, what is your philosophy towards handling legal matters?
Some legal issues will require an aggressive attorney; others may need a softer touch, an attorney who will work toward resolving a matter amicably. You should feel comfortable your attorney has the experience to handle the claim and the right philosophy toward litigation. If you want confirmation, feel free to ask for the name of a prior client.
Here are some brief descriptions of the different types of specialized legal services available. Choosing the right attorney will save you time, money and should maximize the possibility that you will have a successful outcome.
Medical Malpractice Defense Counsel
In the unfortunate event you are sued for medical malpractice, you want to make sure your insurance company assigns you an attorney who has substantial experience in defending medical malpractice. These lawsuits are very complex and require defense attorneys to understand not only the legal requirements of the claim, but also the medical conditions and interventions undertaken on the patients’ behalf.
Professional Licensure Defense Counsel
Some attorneys focus on defending health care professionals before licensing agencies, such as the Board of Medical Examiners or the Drug Enforcement Agency. These proceedings often involve issues that are non-medical in nature, such as fraud, sexual misconduct and substance abuse. Attorneys specialized in representing clients before licensing agencies will have a better understanding of how the agency views the issues and will be able to recommend prospective courses of action, such as peer assistance or continuing education programs, making formal disciplinary proceedings less likely.
Labor and Employment Litigator
There are numerous laws governing the workplace, so when an employment issue surfaces, it’s important to work with an experienced labor and employment attorney. Most attorneys further specialize and represent plaintiffs or defendants, so make sure that you consult with an attorney on the right side of your issue.
Personal Injury Litigator
Some personal injury attorneys work on a volume basis and defer much of the process to paralegals and staff members. Other counselors take on a smaller volume of cases and give each case more individual attention. If you are injured in the workplace and need to find a personal injury attorney, you might want to ask a medical malpractice defense lawyer or your insurance company for a referral.
Matrimonial
One of the most common reasons a physician needs to hire counsel is the dissolution of a marriage. These cases raise intense, personal issues dealing with the division of assets, sale of property, and the allocation of parental responsibilities. Many of these issues are the subject of state laws, which attempt to compel an equitable determination. Working with an experienced matrimonial attorney will keep the focus on the legal merits of the case.
Tax Counsel
Tax law is one of the areas in which law schools offer an advanced degree, known as an LLM. It is the equivalent of a post-doctoral training program. These professionals have tremendous experience in representing individuals and businesses in the formation of business entities and in dealing with federal and state taxing authorities.
Medical Entity Formation
Depending on the state you live in, you may have a choice of business entities for your practice, such as corporations, partnerships, limited liability partnerships (LLP), and professional corporations. In choosing and structuring a business entity, you should consult with an attorney who has experience in representing health care professionals. State and federal regulations may affect your choice of an entity. A good attorney also can help clients anticipate and avoid potential dissolution issues, such as disputes over non-compete provisions, distribution of accounts receivable, and transfer of patient files.
Real Property
When attorneys refer to “real property,” they are describing the purchase and development of land, which can raise complex legal issues related to zoning, easements, assessments, restrictive covenants, and leasing.
Intellectual Property
When lawyers refer to “intellectual property,” they are describing the protections provided to a person’s creative efforts, such as copyright, trademarks and patents. Attorneys can earn a formal advanced degree in this area through an LLM program. If you develop an invention or write a book, intellectual property attorneys are best suited to make sure you receive the benefits of your creative efforts.
Trust and Estate
When people die, they leave an estate, which can be the subject of extensive probate proceedings to determine the heirs’ rights. Even if there are no disputes between heirs, there can be probate proceedings to determine the value of the estate and the taxes that might be assessed against it. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
Mind Your Manners
Beginning January 1, 2009, your on-the-job behavior—and that of other healthcare providers—will be held to a new standard. New Joint Commission standards include a requirement for healthcare organizations to create a code of conduct outlining acceptable and unacceptable behaviors for healthcare professionals, and to implement a process for managing problematic behavior. The reason for this unusual step is the belief that disruptive or intimidating behavior by physicians, nurses, and other healthcare workers has a negative impact on the quality of care.
“I think the standard shows that the Joint Commission is interested in behaviors within hospitals and other healthcare organizations, and how that affects quality of care, safety and the patient experience,” says Russell L. Holman, MD, immediate past president of SHM and chief operating officer for Cogent Healthcare, Nashville, Tenn. “By highlighting this as an area to be included in reviews and standards, it causes organizations to look for their own policies on disruptive behaviors.”
Here is a closer look at the new standard and how it might impact hospital medicine.
Not Physicians Only
The Joint Commission standard addresses “the problem of behaviors that threaten the performance of the healthcare team,” mentioning unprofessional behavior, specifically “intimidating and disruptive behaviors.” To many, this seems to target physicians. “In a hospital, there is an unwritten hierarchy, with physicians at the top,” Dr. Holman points out. “As such, some feel that different standards are applied to physician behaviors. For example, if a nurse or a pharmacist uses obscene language, they may be terminated. If a physician does this, they may receive feedback that the language was inappropriate.”
However, the Sentinel Event Alert released by the Joint Commission in July states, “While most formal research centers on intimidating and disruptive behaviors among physicians and nurses, there is evidence that these behaviors occur among other healthcare professionals, such as pharmacists, therapists, and support staff, as well as among administrators.” The alert does not single out physicians or any other healthcare profession regarding bad behaviors.
“I think the Joint Commission has been very clear in its intent that the standard applies equally to physicians and non-physicians,” Dr. Holman says.
When Hospitalists Cross the Line
How will this code of conduct standard affect hospitalists? Because of the nature of their work, they will be held to the standards of any hospital they work in. In the case of hospitalists who are directly employed by a hospital, the response should be straightforward. However, independent hospital medicine groups will have to work with their hospitals on behavior issues. First, these groups will need to decide whether they should have their own policies and procedures for code of conduct. “Hospital medicine groups need appropriate systems of identifying disruptive behavior, monitoring it, and taking any necessary actions to make sure the behavior is not continued,” Dr. Holman stresses.
Second, independent groups must communicate closely with the hospital when a behavior issue arises. “If you have a hospitalist who is not directly employed by the hospital, there is a dual responsibility for managing their disruptive behavior,” Dr. Holman says. “The hospital has medical staff standards, which are reflected in the medical staff bylaws and rules and regulations. These documents need to include policy and procedures around the incidence of disruptive physician behavior.”
But just because procedures are in place doesn’t mean the hospital will address a problem hospitalist. “This is where in practice, things can get a little fuzzy,” Dr. Holman admits. “The hospital may defer the responsibility for managing the physician to the employer. This is the scenario that has come up in hospital medicine.” He adds, “In my personal opinion, there is a dual responsibility. The hospital needs to apply its standard to all medical staff, regardless of specialty, tenure or employment status.” At the same time, the hospital medicine group/employer should have—and should implement—an approach to managing disruptive behavior.
“Different employers will have different capabilities,” Dr. Holman says. “For example, large, multi-specialty medical groups may have an infrastructure, including human resources professionals, risk managers and depth of medical and operational management, in place for dealing with disruptive behavior. … Small practices won’t have this. They may rely more heavily on the hospital’s infrastructure.”
—Russell L. Holman, MD, COO, Cogent Healthcare, Nashville, Tenn.
Regardless of the hospital medicine group’s size and capabilities, it should promote two-way communication with the hospital regarding problems with individual hospitalists. “If an incident occurs in the hospital, the employer needs to know the details so they can follow up,” Dr. Holman says. “They have to be careful about sharing appropriate information, and protect all privacies. And they have to balance this communication with the fact that it doesn’t absolve one or the other from acting. There must be follow through from both parties, including disciplinary or corrective action as necessary.”
Defining “Disruptive”
One concern healthcare leaders—and the people they lead—may have is deciding the standard used in crafting a policy that specifies what types of behavior are unprofessional. “The challenge is defining disruptive behavior,” Dr. Holman says. “Of course, it can be very clear sometimes. But a surgeon throwing instruments in the operating room is different than someone who is a little bit outspoken.” Consider a hospitalist or other physician who’s in the habit of questioning authority; could this requirement lead to efforts to shut them down?
“Naturally, there is a degree of concern amongst physicians that this is a physician-directed standard, and that there may be a tough time distinguishing between the good faith criticisms of outspoken physicians and those who demonstrate physically threatening behavior,” Dr. Holman says.
The best way for hospitals, hospital medicine groups and other healthcare organizations to avoid this is to find established policies on this subject that are fair, carefully phrased and comprehensive, then customize one or more to their own specifications and distribute to all affected employees.
“I think these policies are nice to include in new physician orientations or training programs, so that physicians are aware of them,” Dr. Holman suggests.
For more information on the code of conduct standard, visit www.jointcommis-sion.org/SentinelEvents/SentinelEventAlert/sea_40.htm. TH
Jane Jerrard is a medical writer based in Chicago.
Beginning January 1, 2009, your on-the-job behavior—and that of other healthcare providers—will be held to a new standard. New Joint Commission standards include a requirement for healthcare organizations to create a code of conduct outlining acceptable and unacceptable behaviors for healthcare professionals, and to implement a process for managing problematic behavior. The reason for this unusual step is the belief that disruptive or intimidating behavior by physicians, nurses, and other healthcare workers has a negative impact on the quality of care.
“I think the standard shows that the Joint Commission is interested in behaviors within hospitals and other healthcare organizations, and how that affects quality of care, safety and the patient experience,” says Russell L. Holman, MD, immediate past president of SHM and chief operating officer for Cogent Healthcare, Nashville, Tenn. “By highlighting this as an area to be included in reviews and standards, it causes organizations to look for their own policies on disruptive behaviors.”
Here is a closer look at the new standard and how it might impact hospital medicine.
Not Physicians Only
The Joint Commission standard addresses “the problem of behaviors that threaten the performance of the healthcare team,” mentioning unprofessional behavior, specifically “intimidating and disruptive behaviors.” To many, this seems to target physicians. “In a hospital, there is an unwritten hierarchy, with physicians at the top,” Dr. Holman points out. “As such, some feel that different standards are applied to physician behaviors. For example, if a nurse or a pharmacist uses obscene language, they may be terminated. If a physician does this, they may receive feedback that the language was inappropriate.”
However, the Sentinel Event Alert released by the Joint Commission in July states, “While most formal research centers on intimidating and disruptive behaviors among physicians and nurses, there is evidence that these behaviors occur among other healthcare professionals, such as pharmacists, therapists, and support staff, as well as among administrators.” The alert does not single out physicians or any other healthcare profession regarding bad behaviors.
“I think the Joint Commission has been very clear in its intent that the standard applies equally to physicians and non-physicians,” Dr. Holman says.
When Hospitalists Cross the Line
How will this code of conduct standard affect hospitalists? Because of the nature of their work, they will be held to the standards of any hospital they work in. In the case of hospitalists who are directly employed by a hospital, the response should be straightforward. However, independent hospital medicine groups will have to work with their hospitals on behavior issues. First, these groups will need to decide whether they should have their own policies and procedures for code of conduct. “Hospital medicine groups need appropriate systems of identifying disruptive behavior, monitoring it, and taking any necessary actions to make sure the behavior is not continued,” Dr. Holman stresses.
Second, independent groups must communicate closely with the hospital when a behavior issue arises. “If you have a hospitalist who is not directly employed by the hospital, there is a dual responsibility for managing their disruptive behavior,” Dr. Holman says. “The hospital has medical staff standards, which are reflected in the medical staff bylaws and rules and regulations. These documents need to include policy and procedures around the incidence of disruptive physician behavior.”
But just because procedures are in place doesn’t mean the hospital will address a problem hospitalist. “This is where in practice, things can get a little fuzzy,” Dr. Holman admits. “The hospital may defer the responsibility for managing the physician to the employer. This is the scenario that has come up in hospital medicine.” He adds, “In my personal opinion, there is a dual responsibility. The hospital needs to apply its standard to all medical staff, regardless of specialty, tenure or employment status.” At the same time, the hospital medicine group/employer should have—and should implement—an approach to managing disruptive behavior.
“Different employers will have different capabilities,” Dr. Holman says. “For example, large, multi-specialty medical groups may have an infrastructure, including human resources professionals, risk managers and depth of medical and operational management, in place for dealing with disruptive behavior. … Small practices won’t have this. They may rely more heavily on the hospital’s infrastructure.”
—Russell L. Holman, MD, COO, Cogent Healthcare, Nashville, Tenn.
Regardless of the hospital medicine group’s size and capabilities, it should promote two-way communication with the hospital regarding problems with individual hospitalists. “If an incident occurs in the hospital, the employer needs to know the details so they can follow up,” Dr. Holman says. “They have to be careful about sharing appropriate information, and protect all privacies. And they have to balance this communication with the fact that it doesn’t absolve one or the other from acting. There must be follow through from both parties, including disciplinary or corrective action as necessary.”
Defining “Disruptive”
One concern healthcare leaders—and the people they lead—may have is deciding the standard used in crafting a policy that specifies what types of behavior are unprofessional. “The challenge is defining disruptive behavior,” Dr. Holman says. “Of course, it can be very clear sometimes. But a surgeon throwing instruments in the operating room is different than someone who is a little bit outspoken.” Consider a hospitalist or other physician who’s in the habit of questioning authority; could this requirement lead to efforts to shut them down?
“Naturally, there is a degree of concern amongst physicians that this is a physician-directed standard, and that there may be a tough time distinguishing between the good faith criticisms of outspoken physicians and those who demonstrate physically threatening behavior,” Dr. Holman says.
The best way for hospitals, hospital medicine groups and other healthcare organizations to avoid this is to find established policies on this subject that are fair, carefully phrased and comprehensive, then customize one or more to their own specifications and distribute to all affected employees.
“I think these policies are nice to include in new physician orientations or training programs, so that physicians are aware of them,” Dr. Holman suggests.
For more information on the code of conduct standard, visit www.jointcommis-sion.org/SentinelEvents/SentinelEventAlert/sea_40.htm. TH
Jane Jerrard is a medical writer based in Chicago.
Beginning January 1, 2009, your on-the-job behavior—and that of other healthcare providers—will be held to a new standard. New Joint Commission standards include a requirement for healthcare organizations to create a code of conduct outlining acceptable and unacceptable behaviors for healthcare professionals, and to implement a process for managing problematic behavior. The reason for this unusual step is the belief that disruptive or intimidating behavior by physicians, nurses, and other healthcare workers has a negative impact on the quality of care.
“I think the standard shows that the Joint Commission is interested in behaviors within hospitals and other healthcare organizations, and how that affects quality of care, safety and the patient experience,” says Russell L. Holman, MD, immediate past president of SHM and chief operating officer for Cogent Healthcare, Nashville, Tenn. “By highlighting this as an area to be included in reviews and standards, it causes organizations to look for their own policies on disruptive behaviors.”
Here is a closer look at the new standard and how it might impact hospital medicine.
Not Physicians Only
The Joint Commission standard addresses “the problem of behaviors that threaten the performance of the healthcare team,” mentioning unprofessional behavior, specifically “intimidating and disruptive behaviors.” To many, this seems to target physicians. “In a hospital, there is an unwritten hierarchy, with physicians at the top,” Dr. Holman points out. “As such, some feel that different standards are applied to physician behaviors. For example, if a nurse or a pharmacist uses obscene language, they may be terminated. If a physician does this, they may receive feedback that the language was inappropriate.”
However, the Sentinel Event Alert released by the Joint Commission in July states, “While most formal research centers on intimidating and disruptive behaviors among physicians and nurses, there is evidence that these behaviors occur among other healthcare professionals, such as pharmacists, therapists, and support staff, as well as among administrators.” The alert does not single out physicians or any other healthcare profession regarding bad behaviors.
“I think the Joint Commission has been very clear in its intent that the standard applies equally to physicians and non-physicians,” Dr. Holman says.
When Hospitalists Cross the Line
How will this code of conduct standard affect hospitalists? Because of the nature of their work, they will be held to the standards of any hospital they work in. In the case of hospitalists who are directly employed by a hospital, the response should be straightforward. However, independent hospital medicine groups will have to work with their hospitals on behavior issues. First, these groups will need to decide whether they should have their own policies and procedures for code of conduct. “Hospital medicine groups need appropriate systems of identifying disruptive behavior, monitoring it, and taking any necessary actions to make sure the behavior is not continued,” Dr. Holman stresses.
Second, independent groups must communicate closely with the hospital when a behavior issue arises. “If you have a hospitalist who is not directly employed by the hospital, there is a dual responsibility for managing their disruptive behavior,” Dr. Holman says. “The hospital has medical staff standards, which are reflected in the medical staff bylaws and rules and regulations. These documents need to include policy and procedures around the incidence of disruptive physician behavior.”
But just because procedures are in place doesn’t mean the hospital will address a problem hospitalist. “This is where in practice, things can get a little fuzzy,” Dr. Holman admits. “The hospital may defer the responsibility for managing the physician to the employer. This is the scenario that has come up in hospital medicine.” He adds, “In my personal opinion, there is a dual responsibility. The hospital needs to apply its standard to all medical staff, regardless of specialty, tenure or employment status.” At the same time, the hospital medicine group/employer should have—and should implement—an approach to managing disruptive behavior.
“Different employers will have different capabilities,” Dr. Holman says. “For example, large, multi-specialty medical groups may have an infrastructure, including human resources professionals, risk managers and depth of medical and operational management, in place for dealing with disruptive behavior. … Small practices won’t have this. They may rely more heavily on the hospital’s infrastructure.”
—Russell L. Holman, MD, COO, Cogent Healthcare, Nashville, Tenn.
Regardless of the hospital medicine group’s size and capabilities, it should promote two-way communication with the hospital regarding problems with individual hospitalists. “If an incident occurs in the hospital, the employer needs to know the details so they can follow up,” Dr. Holman says. “They have to be careful about sharing appropriate information, and protect all privacies. And they have to balance this communication with the fact that it doesn’t absolve one or the other from acting. There must be follow through from both parties, including disciplinary or corrective action as necessary.”
Defining “Disruptive”
One concern healthcare leaders—and the people they lead—may have is deciding the standard used in crafting a policy that specifies what types of behavior are unprofessional. “The challenge is defining disruptive behavior,” Dr. Holman says. “Of course, it can be very clear sometimes. But a surgeon throwing instruments in the operating room is different than someone who is a little bit outspoken.” Consider a hospitalist or other physician who’s in the habit of questioning authority; could this requirement lead to efforts to shut them down?
“Naturally, there is a degree of concern amongst physicians that this is a physician-directed standard, and that there may be a tough time distinguishing between the good faith criticisms of outspoken physicians and those who demonstrate physically threatening behavior,” Dr. Holman says.
The best way for hospitals, hospital medicine groups and other healthcare organizations to avoid this is to find established policies on this subject that are fair, carefully phrased and comprehensive, then customize one or more to their own specifications and distribute to all affected employees.
“I think these policies are nice to include in new physician orientations or training programs, so that physicians are aware of them,” Dr. Holman suggests.
For more information on the code of conduct standard, visit www.jointcommis-sion.org/SentinelEvents/SentinelEventAlert/sea_40.htm. TH
Jane Jerrard is a medical writer based in Chicago.