User login
Direct-to-patient telemedicine has benefits for providers, patients, and the practice
Innovations in care delivery, as previously introduced by Dr. Robert Gabbay, can enhance the patient and physician experience. Providing care via telemedicine can bring joy to work by introducing variety to practice. It also carries the satisfaction of easing access to care for the patient.
Broadly speaking, telemedicine can be seen as a tool for delivering care when a hands-on exam is not required. In direct-to-patient telemedicine, the patient can use a personal smartphone, tablet, or computer to connect with a provider in a real-time audio and/or video “visit” from home or work. The engagement can be scheduled or on demand. Although telemedicine is generally associated with the delivery of care to patients in remote or rural locations, it is increasingly being used in urban areas, especially with older patients and those for whom transport or time away from work might be difficult.
How the patient benefits
This built-in flexibility is appealing to patients – the easier access and convenience can translate into reduced time away from work or school and possibly a reduction in patient “no-shows.” Patients are more likely to enjoy the benefits of continuity of care with their own providers, rather than seeking independent, consumer-marketed services. In a nationwide survey of 4,345 respondents about attitudes toward telemedicine in primary care, 52% of respondents said they would like to see their own providers via telemedicine, 35% were willing to see a different provider from the same organization, and 15% said they would consider leaving their current provider to see one who offered telemedicine (BMC Health Services Research. 2017;17:784).
In addition, numerous studies have reported on the equivalent clinical outcomes and improved cost-of-care benefits in patients who receive diabetes care through telemedicine. Lui and colleagues looked at patients at the Denver VA Medical Center who were newly diagnosed with diabetes and they compared short-term glycemic control in patients who had telemedicine consultations with patients who had in-person visits. They found that the telemedicine consultations improved short-term glycemic control as effectively as the in-person visits, but with possible added financial benefits for both the patients and the health care system. (J Diabetes Sci Technol. 2016;10[5]:1079-86). Likewise, Fatehi and colleagues have reported that method of consultation – telemedicine or in-person consultation – did not affect concordance of advice between two endocrinologists (Diabetes Technol Ther. 2015;17[10]:717-25).
What telemedicine has to offer
When appropriate diagnostic labs have already been performed, newly diagnosed patients can be counseled on their diagnosis and started on therapy. For patients who have already been diagnosed, follow-up and monitoring of therapy adherence and glycemic control can be more convenient and done more routinely, compared with in-person visits, and thus yield better outcomes.
Use of cloud-based services to review data from glucometers, insulin pumps, and continuous glucose monitors allows the clinicians to access the same data they would in the office. Combining this data review with a video visit, rather than looking at the data in isolation, allows for increased patient engagement, shared decision making, and patient counseling.
Other diagnoses that readily fit at-home telemedicine care include gestational diabetes, as these patients need frequent follow-up, and doing some of their visits via telemedicine can reduce their burden of travel. Hypothyroidism follow-ups, with labs completed before the visit, can be very efficient via telemedicine. Internal surveys of direct-to-patient services at my institution demonstrated a high level of patient satisfaction, with 91% of patients indicating they were satisfied overall, and 81% saying that connection with the provider matched that of an in-person visit.
Gains for the provider, the care team, and the practice
Endocrinologists can derive benefit from telemedicine engagement with their patients, which could have positive implications for other members of the care team and for the practice as a whole. For the provider, being able to streamline clinical workflows and increase practice efficiency can help reduce personal and workplace-related stress and translate into greater personal satisfaction in one’s work and delivery of better-quality care.
At the practice level, the use of telemedicine presents opportunities for expanding the patient base and perhaps working more flexible hours to better accommodate the personal and professional time demands on providers and their staff. In addition, offering telemedicine as a medium of consultation could be a practice differentiator that could give you a competitive edge. That, along with smaller changes, such as enhancing or even reducing space utilization, could contribute to reduced overheads and a boost in revenue, which would have a positive impact on the practice’s bottom line.
Getting started
There is important groundwork to be done before a telemedicine program can get underway. First, bear in mind that there is considerable state-based variation in regulations and insurance coverage, so you need to be sure that you are in compliance with the requirements for your state. If direct-to-patient telemedicine is not widely reimbursed in your state, direct-payment models may be feasible. Providers who accept Medicare payments need to understand restrictions on self-payment for those patients. You may also be able to negotiate with payers to include reimbursement for telemedicine visits in your contracts. Negotiation with payers and direct-pay models may be possible.
Key guidelines. In addition to understanding your state’s regulations around telemedicine, there are specific aspects of practice about which you need to be clear, for example:
- You must be licensed in the state in which your patient is located at the time of their visit.
- Understand any restrictions on prescribing via telemedicine in your state.
- Be aware that Medicare has very specific guidelines and, at this time, does not recognize home as a place of service.
- You must be sure that you use HIPAA-compliant video software.
- If in any doubt, seek guidance from an attorney or your organization’s compliance office.
Infrastructure and outlay. Your infrastructure needs will depend on the specific services that you provide, but in general, you should include a communication platform and video conferencing equipment; sufficient bandwidth and a secure, reliable Internet connection; ready access to sound IT support; and comprehensive staff training at the outset, with subsequent refresher training sessions on a regular basis. Within the practice, you will need to think about adjustments to your existing workflow to accommodate the telemedicine services you plan to offer.
Resources. Two nonprofit groups that offer nonpartisan guidance in telemedicine are the Center for Connected Health Policy and the Regional Telehealth Resource Centers.
Dr. Griffith is assistant professor of medicine and medical director, Ambulatory Telehealth Services, Vanderbilt University Medical Center, in Nashville, Tenn. This article is part of a series based on presentations from the annual meeting of the Endocrine Society in March 2019. Dr. Griffith has no disclosures. Write to her at cenews@mdedge.com.
Innovations in care delivery, as previously introduced by Dr. Robert Gabbay, can enhance the patient and physician experience. Providing care via telemedicine can bring joy to work by introducing variety to practice. It also carries the satisfaction of easing access to care for the patient.
Broadly speaking, telemedicine can be seen as a tool for delivering care when a hands-on exam is not required. In direct-to-patient telemedicine, the patient can use a personal smartphone, tablet, or computer to connect with a provider in a real-time audio and/or video “visit” from home or work. The engagement can be scheduled or on demand. Although telemedicine is generally associated with the delivery of care to patients in remote or rural locations, it is increasingly being used in urban areas, especially with older patients and those for whom transport or time away from work might be difficult.
How the patient benefits
This built-in flexibility is appealing to patients – the easier access and convenience can translate into reduced time away from work or school and possibly a reduction in patient “no-shows.” Patients are more likely to enjoy the benefits of continuity of care with their own providers, rather than seeking independent, consumer-marketed services. In a nationwide survey of 4,345 respondents about attitudes toward telemedicine in primary care, 52% of respondents said they would like to see their own providers via telemedicine, 35% were willing to see a different provider from the same organization, and 15% said they would consider leaving their current provider to see one who offered telemedicine (BMC Health Services Research. 2017;17:784).
In addition, numerous studies have reported on the equivalent clinical outcomes and improved cost-of-care benefits in patients who receive diabetes care through telemedicine. Lui and colleagues looked at patients at the Denver VA Medical Center who were newly diagnosed with diabetes and they compared short-term glycemic control in patients who had telemedicine consultations with patients who had in-person visits. They found that the telemedicine consultations improved short-term glycemic control as effectively as the in-person visits, but with possible added financial benefits for both the patients and the health care system. (J Diabetes Sci Technol. 2016;10[5]:1079-86). Likewise, Fatehi and colleagues have reported that method of consultation – telemedicine or in-person consultation – did not affect concordance of advice between two endocrinologists (Diabetes Technol Ther. 2015;17[10]:717-25).
What telemedicine has to offer
When appropriate diagnostic labs have already been performed, newly diagnosed patients can be counseled on their diagnosis and started on therapy. For patients who have already been diagnosed, follow-up and monitoring of therapy adherence and glycemic control can be more convenient and done more routinely, compared with in-person visits, and thus yield better outcomes.
Use of cloud-based services to review data from glucometers, insulin pumps, and continuous glucose monitors allows the clinicians to access the same data they would in the office. Combining this data review with a video visit, rather than looking at the data in isolation, allows for increased patient engagement, shared decision making, and patient counseling.
Other diagnoses that readily fit at-home telemedicine care include gestational diabetes, as these patients need frequent follow-up, and doing some of their visits via telemedicine can reduce their burden of travel. Hypothyroidism follow-ups, with labs completed before the visit, can be very efficient via telemedicine. Internal surveys of direct-to-patient services at my institution demonstrated a high level of patient satisfaction, with 91% of patients indicating they were satisfied overall, and 81% saying that connection with the provider matched that of an in-person visit.
Gains for the provider, the care team, and the practice
Endocrinologists can derive benefit from telemedicine engagement with their patients, which could have positive implications for other members of the care team and for the practice as a whole. For the provider, being able to streamline clinical workflows and increase practice efficiency can help reduce personal and workplace-related stress and translate into greater personal satisfaction in one’s work and delivery of better-quality care.
At the practice level, the use of telemedicine presents opportunities for expanding the patient base and perhaps working more flexible hours to better accommodate the personal and professional time demands on providers and their staff. In addition, offering telemedicine as a medium of consultation could be a practice differentiator that could give you a competitive edge. That, along with smaller changes, such as enhancing or even reducing space utilization, could contribute to reduced overheads and a boost in revenue, which would have a positive impact on the practice’s bottom line.
Getting started
There is important groundwork to be done before a telemedicine program can get underway. First, bear in mind that there is considerable state-based variation in regulations and insurance coverage, so you need to be sure that you are in compliance with the requirements for your state. If direct-to-patient telemedicine is not widely reimbursed in your state, direct-payment models may be feasible. Providers who accept Medicare payments need to understand restrictions on self-payment for those patients. You may also be able to negotiate with payers to include reimbursement for telemedicine visits in your contracts. Negotiation with payers and direct-pay models may be possible.
Key guidelines. In addition to understanding your state’s regulations around telemedicine, there are specific aspects of practice about which you need to be clear, for example:
- You must be licensed in the state in which your patient is located at the time of their visit.
- Understand any restrictions on prescribing via telemedicine in your state.
- Be aware that Medicare has very specific guidelines and, at this time, does not recognize home as a place of service.
- You must be sure that you use HIPAA-compliant video software.
- If in any doubt, seek guidance from an attorney or your organization’s compliance office.
Infrastructure and outlay. Your infrastructure needs will depend on the specific services that you provide, but in general, you should include a communication platform and video conferencing equipment; sufficient bandwidth and a secure, reliable Internet connection; ready access to sound IT support; and comprehensive staff training at the outset, with subsequent refresher training sessions on a regular basis. Within the practice, you will need to think about adjustments to your existing workflow to accommodate the telemedicine services you plan to offer.
Resources. Two nonprofit groups that offer nonpartisan guidance in telemedicine are the Center for Connected Health Policy and the Regional Telehealth Resource Centers.
Dr. Griffith is assistant professor of medicine and medical director, Ambulatory Telehealth Services, Vanderbilt University Medical Center, in Nashville, Tenn. This article is part of a series based on presentations from the annual meeting of the Endocrine Society in March 2019. Dr. Griffith has no disclosures. Write to her at cenews@mdedge.com.
Innovations in care delivery, as previously introduced by Dr. Robert Gabbay, can enhance the patient and physician experience. Providing care via telemedicine can bring joy to work by introducing variety to practice. It also carries the satisfaction of easing access to care for the patient.
Broadly speaking, telemedicine can be seen as a tool for delivering care when a hands-on exam is not required. In direct-to-patient telemedicine, the patient can use a personal smartphone, tablet, or computer to connect with a provider in a real-time audio and/or video “visit” from home or work. The engagement can be scheduled or on demand. Although telemedicine is generally associated with the delivery of care to patients in remote or rural locations, it is increasingly being used in urban areas, especially with older patients and those for whom transport or time away from work might be difficult.
How the patient benefits
This built-in flexibility is appealing to patients – the easier access and convenience can translate into reduced time away from work or school and possibly a reduction in patient “no-shows.” Patients are more likely to enjoy the benefits of continuity of care with their own providers, rather than seeking independent, consumer-marketed services. In a nationwide survey of 4,345 respondents about attitudes toward telemedicine in primary care, 52% of respondents said they would like to see their own providers via telemedicine, 35% were willing to see a different provider from the same organization, and 15% said they would consider leaving their current provider to see one who offered telemedicine (BMC Health Services Research. 2017;17:784).
In addition, numerous studies have reported on the equivalent clinical outcomes and improved cost-of-care benefits in patients who receive diabetes care through telemedicine. Lui and colleagues looked at patients at the Denver VA Medical Center who were newly diagnosed with diabetes and they compared short-term glycemic control in patients who had telemedicine consultations with patients who had in-person visits. They found that the telemedicine consultations improved short-term glycemic control as effectively as the in-person visits, but with possible added financial benefits for both the patients and the health care system. (J Diabetes Sci Technol. 2016;10[5]:1079-86). Likewise, Fatehi and colleagues have reported that method of consultation – telemedicine or in-person consultation – did not affect concordance of advice between two endocrinologists (Diabetes Technol Ther. 2015;17[10]:717-25).
What telemedicine has to offer
When appropriate diagnostic labs have already been performed, newly diagnosed patients can be counseled on their diagnosis and started on therapy. For patients who have already been diagnosed, follow-up and monitoring of therapy adherence and glycemic control can be more convenient and done more routinely, compared with in-person visits, and thus yield better outcomes.
Use of cloud-based services to review data from glucometers, insulin pumps, and continuous glucose monitors allows the clinicians to access the same data they would in the office. Combining this data review with a video visit, rather than looking at the data in isolation, allows for increased patient engagement, shared decision making, and patient counseling.
Other diagnoses that readily fit at-home telemedicine care include gestational diabetes, as these patients need frequent follow-up, and doing some of their visits via telemedicine can reduce their burden of travel. Hypothyroidism follow-ups, with labs completed before the visit, can be very efficient via telemedicine. Internal surveys of direct-to-patient services at my institution demonstrated a high level of patient satisfaction, with 91% of patients indicating they were satisfied overall, and 81% saying that connection with the provider matched that of an in-person visit.
Gains for the provider, the care team, and the practice
Endocrinologists can derive benefit from telemedicine engagement with their patients, which could have positive implications for other members of the care team and for the practice as a whole. For the provider, being able to streamline clinical workflows and increase practice efficiency can help reduce personal and workplace-related stress and translate into greater personal satisfaction in one’s work and delivery of better-quality care.
At the practice level, the use of telemedicine presents opportunities for expanding the patient base and perhaps working more flexible hours to better accommodate the personal and professional time demands on providers and their staff. In addition, offering telemedicine as a medium of consultation could be a practice differentiator that could give you a competitive edge. That, along with smaller changes, such as enhancing or even reducing space utilization, could contribute to reduced overheads and a boost in revenue, which would have a positive impact on the practice’s bottom line.
Getting started
There is important groundwork to be done before a telemedicine program can get underway. First, bear in mind that there is considerable state-based variation in regulations and insurance coverage, so you need to be sure that you are in compliance with the requirements for your state. If direct-to-patient telemedicine is not widely reimbursed in your state, direct-payment models may be feasible. Providers who accept Medicare payments need to understand restrictions on self-payment for those patients. You may also be able to negotiate with payers to include reimbursement for telemedicine visits in your contracts. Negotiation with payers and direct-pay models may be possible.
Key guidelines. In addition to understanding your state’s regulations around telemedicine, there are specific aspects of practice about which you need to be clear, for example:
- You must be licensed in the state in which your patient is located at the time of their visit.
- Understand any restrictions on prescribing via telemedicine in your state.
- Be aware that Medicare has very specific guidelines and, at this time, does not recognize home as a place of service.
- You must be sure that you use HIPAA-compliant video software.
- If in any doubt, seek guidance from an attorney or your organization’s compliance office.
Infrastructure and outlay. Your infrastructure needs will depend on the specific services that you provide, but in general, you should include a communication platform and video conferencing equipment; sufficient bandwidth and a secure, reliable Internet connection; ready access to sound IT support; and comprehensive staff training at the outset, with subsequent refresher training sessions on a regular basis. Within the practice, you will need to think about adjustments to your existing workflow to accommodate the telemedicine services you plan to offer.
Resources. Two nonprofit groups that offer nonpartisan guidance in telemedicine are the Center for Connected Health Policy and the Regional Telehealth Resource Centers.
Dr. Griffith is assistant professor of medicine and medical director, Ambulatory Telehealth Services, Vanderbilt University Medical Center, in Nashville, Tenn. This article is part of a series based on presentations from the annual meeting of the Endocrine Society in March 2019. Dr. Griffith has no disclosures. Write to her at cenews@mdedge.com.
How innovation can bring more joy to your work
As the incidence of physician burnout increases, so does the overall cost to the health system, both in quality of care and financially, and findings from a recent study suggested that patients are becoming aware of burnout among providers and are concerned about its impact on the quality of the care that they are receiving.2
Burnout can be described as a form of extreme work-related stress manifesting as physical and/or emotional exhaustion that can generate a range of psychological ripple effects, such as depression, mood and anxiety disorders, a crippling sense of worthlessness, and a loss of sense of self. Anyone can be affected by burnout, but there seems to be a greater prevalence among women (50% vs 30% for men); younger doctors, especially residents; and providers of color.3,4
The fallout from burnout affects both our personal and professional lives. In our personal lives, it can translate into broken or strained relationships, alcohol and substance abuse, depression and mood or anxiety disorders, financial difficulties, and suicide (14% have reported thoughts of suicide; 1% have committed suicide). Juggling work and family can be overwhelming, and additional strains, such as caring for a parent or a sick family member, having a child, going through a divorce or a family bereavement, dealing with student debt, and pressure to achieve, can be cumulatively devastating for a hardworking provider.5
In the practice, we see burnout translate into an increase in the number of medical errors, diminished quality of care, lower patient satisfaction with care, decreased productivity and professional effort, dissatisfaction among staff, and an increase in physician turnover.6 Workplace-specific factors that can contribute to burnout include daily use of health information technology, especially the EHR; workplace inequities; pressures to keep abreast with changes in the specialty; and time management challenges and constraints that go along with the continual pressure to deliver better quality care, for less money, in less time.7
Our group wanted to devise innovative, practice-based strategies that would help our colleagues address the burnout crisis. We asked ourselves how – through new, innovative models of care – we could rediscover the joy in our work, and what the steppingstones of “meaningful work” would be. It seemed to us that working as a team and revisiting the care we deliver might be good starting points, and that, if we drilled down further, common themes to address burnout and find joy in work revolve around choice, camaraderie, equity, and cocreating solutions.
Evidence suggests that physicians who spend at least 20% of their professional effort focused on work they find most meaningful have a notably lower risk for burnout.8 Each 1% reduction below this threshold increases the risk of burnout, and there is a ceiling effect to the benefit at 20% – for example, spending 50% of your time in the most meaningful area is associated with similar rates of burnout as spending 20% on it.
So how do we to get to that meaningful threshold of 20%? You can begin with identifying your passion, making the business case, speaking to your boss, getting your colleagues’ buy-in, and even looking for grants and other funding if needed. We came up with five ways you might bring more joy to your work by adopting innovative models of diabetes care: the first – implementing a direct-to-patient telemedicine program – has been written by Michelle Griffith, MD, and is featured here. In coming articles, we will take a look at tackling the impediments of clinical inertia, coordinating care through use of a transfer summary, shifting to team-based care, how to use e-consultations to connect with primary care providers, and devising a business case for these and other innovations.
References
1. Kane L. National Physician Burnout, Depression & Suicide Report 2019. Medscape. Published online Jan 16, 2019.
2. American Society of Health-System Pharmacists. Press release. 2019 Jun 17.
3. Oakes K. Female family physicians come up short in burnout gender divide. Family Practice News. Published online November 27, 2017.
4. Dyrbye L. JAMA Network Open. 2019 Jul 26. doi: 10.1001/jamanetworkopen.2019.7457.
5. Yank V et al. JAMA Intern Med. 2019 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
6 . Panagioti M et al. JAMA Intern Med. 2018;178(10):1317-31.
7. Gardner R et al. J Am Med Inform Assoc. doi: 10.1093/jamia/ocy145.
8. Shanafelt T et al. Am J Med Qual. 2017;32(5):563-5.
Dr. Gabbay is chief medical officer at Joslin Diabetes Center and an associate professor of medicine at Harvard Medical School, both in Boston. This is the introduction to a series of articles based on presentations from the annual meeting of the Endocrine Society in March 2019. Dr. Gabbay reports being an adviser to Lark, Onduo, and HealthReveal. Write to him at cenews@mdedge.com.
As the incidence of physician burnout increases, so does the overall cost to the health system, both in quality of care and financially, and findings from a recent study suggested that patients are becoming aware of burnout among providers and are concerned about its impact on the quality of the care that they are receiving.2
Burnout can be described as a form of extreme work-related stress manifesting as physical and/or emotional exhaustion that can generate a range of psychological ripple effects, such as depression, mood and anxiety disorders, a crippling sense of worthlessness, and a loss of sense of self. Anyone can be affected by burnout, but there seems to be a greater prevalence among women (50% vs 30% for men); younger doctors, especially residents; and providers of color.3,4
The fallout from burnout affects both our personal and professional lives. In our personal lives, it can translate into broken or strained relationships, alcohol and substance abuse, depression and mood or anxiety disorders, financial difficulties, and suicide (14% have reported thoughts of suicide; 1% have committed suicide). Juggling work and family can be overwhelming, and additional strains, such as caring for a parent or a sick family member, having a child, going through a divorce or a family bereavement, dealing with student debt, and pressure to achieve, can be cumulatively devastating for a hardworking provider.5
In the practice, we see burnout translate into an increase in the number of medical errors, diminished quality of care, lower patient satisfaction with care, decreased productivity and professional effort, dissatisfaction among staff, and an increase in physician turnover.6 Workplace-specific factors that can contribute to burnout include daily use of health information technology, especially the EHR; workplace inequities; pressures to keep abreast with changes in the specialty; and time management challenges and constraints that go along with the continual pressure to deliver better quality care, for less money, in less time.7
Our group wanted to devise innovative, practice-based strategies that would help our colleagues address the burnout crisis. We asked ourselves how – through new, innovative models of care – we could rediscover the joy in our work, and what the steppingstones of “meaningful work” would be. It seemed to us that working as a team and revisiting the care we deliver might be good starting points, and that, if we drilled down further, common themes to address burnout and find joy in work revolve around choice, camaraderie, equity, and cocreating solutions.
Evidence suggests that physicians who spend at least 20% of their professional effort focused on work they find most meaningful have a notably lower risk for burnout.8 Each 1% reduction below this threshold increases the risk of burnout, and there is a ceiling effect to the benefit at 20% – for example, spending 50% of your time in the most meaningful area is associated with similar rates of burnout as spending 20% on it.
So how do we to get to that meaningful threshold of 20%? You can begin with identifying your passion, making the business case, speaking to your boss, getting your colleagues’ buy-in, and even looking for grants and other funding if needed. We came up with five ways you might bring more joy to your work by adopting innovative models of diabetes care: the first – implementing a direct-to-patient telemedicine program – has been written by Michelle Griffith, MD, and is featured here. In coming articles, we will take a look at tackling the impediments of clinical inertia, coordinating care through use of a transfer summary, shifting to team-based care, how to use e-consultations to connect with primary care providers, and devising a business case for these and other innovations.
References
1. Kane L. National Physician Burnout, Depression & Suicide Report 2019. Medscape. Published online Jan 16, 2019.
2. American Society of Health-System Pharmacists. Press release. 2019 Jun 17.
3. Oakes K. Female family physicians come up short in burnout gender divide. Family Practice News. Published online November 27, 2017.
4. Dyrbye L. JAMA Network Open. 2019 Jul 26. doi: 10.1001/jamanetworkopen.2019.7457.
5. Yank V et al. JAMA Intern Med. 2019 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
6 . Panagioti M et al. JAMA Intern Med. 2018;178(10):1317-31.
7. Gardner R et al. J Am Med Inform Assoc. doi: 10.1093/jamia/ocy145.
8. Shanafelt T et al. Am J Med Qual. 2017;32(5):563-5.
Dr. Gabbay is chief medical officer at Joslin Diabetes Center and an associate professor of medicine at Harvard Medical School, both in Boston. This is the introduction to a series of articles based on presentations from the annual meeting of the Endocrine Society in March 2019. Dr. Gabbay reports being an adviser to Lark, Onduo, and HealthReveal. Write to him at cenews@mdedge.com.
As the incidence of physician burnout increases, so does the overall cost to the health system, both in quality of care and financially, and findings from a recent study suggested that patients are becoming aware of burnout among providers and are concerned about its impact on the quality of the care that they are receiving.2
Burnout can be described as a form of extreme work-related stress manifesting as physical and/or emotional exhaustion that can generate a range of psychological ripple effects, such as depression, mood and anxiety disorders, a crippling sense of worthlessness, and a loss of sense of self. Anyone can be affected by burnout, but there seems to be a greater prevalence among women (50% vs 30% for men); younger doctors, especially residents; and providers of color.3,4
The fallout from burnout affects both our personal and professional lives. In our personal lives, it can translate into broken or strained relationships, alcohol and substance abuse, depression and mood or anxiety disorders, financial difficulties, and suicide (14% have reported thoughts of suicide; 1% have committed suicide). Juggling work and family can be overwhelming, and additional strains, such as caring for a parent or a sick family member, having a child, going through a divorce or a family bereavement, dealing with student debt, and pressure to achieve, can be cumulatively devastating for a hardworking provider.5
In the practice, we see burnout translate into an increase in the number of medical errors, diminished quality of care, lower patient satisfaction with care, decreased productivity and professional effort, dissatisfaction among staff, and an increase in physician turnover.6 Workplace-specific factors that can contribute to burnout include daily use of health information technology, especially the EHR; workplace inequities; pressures to keep abreast with changes in the specialty; and time management challenges and constraints that go along with the continual pressure to deliver better quality care, for less money, in less time.7
Our group wanted to devise innovative, practice-based strategies that would help our colleagues address the burnout crisis. We asked ourselves how – through new, innovative models of care – we could rediscover the joy in our work, and what the steppingstones of “meaningful work” would be. It seemed to us that working as a team and revisiting the care we deliver might be good starting points, and that, if we drilled down further, common themes to address burnout and find joy in work revolve around choice, camaraderie, equity, and cocreating solutions.
Evidence suggests that physicians who spend at least 20% of their professional effort focused on work they find most meaningful have a notably lower risk for burnout.8 Each 1% reduction below this threshold increases the risk of burnout, and there is a ceiling effect to the benefit at 20% – for example, spending 50% of your time in the most meaningful area is associated with similar rates of burnout as spending 20% on it.
So how do we to get to that meaningful threshold of 20%? You can begin with identifying your passion, making the business case, speaking to your boss, getting your colleagues’ buy-in, and even looking for grants and other funding if needed. We came up with five ways you might bring more joy to your work by adopting innovative models of diabetes care: the first – implementing a direct-to-patient telemedicine program – has been written by Michelle Griffith, MD, and is featured here. In coming articles, we will take a look at tackling the impediments of clinical inertia, coordinating care through use of a transfer summary, shifting to team-based care, how to use e-consultations to connect with primary care providers, and devising a business case for these and other innovations.
References
1. Kane L. National Physician Burnout, Depression & Suicide Report 2019. Medscape. Published online Jan 16, 2019.
2. American Society of Health-System Pharmacists. Press release. 2019 Jun 17.
3. Oakes K. Female family physicians come up short in burnout gender divide. Family Practice News. Published online November 27, 2017.
4. Dyrbye L. JAMA Network Open. 2019 Jul 26. doi: 10.1001/jamanetworkopen.2019.7457.
5. Yank V et al. JAMA Intern Med. 2019 Jan 28. doi: 10.1001/jamainternmed.2018.6411.
6 . Panagioti M et al. JAMA Intern Med. 2018;178(10):1317-31.
7. Gardner R et al. J Am Med Inform Assoc. doi: 10.1093/jamia/ocy145.
8. Shanafelt T et al. Am J Med Qual. 2017;32(5):563-5.
Dr. Gabbay is chief medical officer at Joslin Diabetes Center and an associate professor of medicine at Harvard Medical School, both in Boston. This is the introduction to a series of articles based on presentations from the annual meeting of the Endocrine Society in March 2019. Dr. Gabbay reports being an adviser to Lark, Onduo, and HealthReveal. Write to him at cenews@mdedge.com.
Ovarian cancer and perineal talc exposure: An epidemiologic dilemma
Many readers may be aware of large payments made by such companies as Johnson & Johnson to compensate women with a history of ovarian cancer who have claimed that perineal application of talc played a causative role in their cancer development. This column serves to review the purported role of perineal talc use in the development of ovarian cancer, and explore some of the pitfalls of observational science.
Talc, a hydrated magnesium silicate, is the softest mineral on earth, and has been sold as a personal hygiene product for many decades. Perineal application of talc to sanitary pads, perineal skin, undergarments, and diapers has been a common practice to decrease friction, moisture build-up, and as a deodorant. Talc is chemically similar, although not identical, to asbestos and is geologically located in close proximity to the known carcinogen. In the 1970s, there were concerns raised regarding the possible contamination of cosmetic-grade talc with asbestos, which led to the development of asbestos-free forms of the substance. Given that a strong causal relationship had been established between asbestos exposure and lung and pleural cancers, there was concern that exposure to perineal talc might increase cancer risk.
In the 1980s, an association between perineal talc exposure and ovarian cancer was observed in a case-control study.1 Since that time, multiple other observational studies, predominately case-control studies, have observed an increased ovarian cancer risk among users of perineal talc including the findings of a meta-analysis which estimated a 24%-39% increased risk for ovarian cancer among users.2 Does this establish a causal relationship? For the purposes of legal cases, these associations are adequate. However, science demands a different standard when determining cause and effect.
It is not unusual to rely on observational studies to establish a causal relationship between exposure and disease when it is unethical to randomize subjects in a clinical trial to exposure of the potential harmful agent. This was the necessary methodology behind establishing that smoking causes lung cancer. Several factors must be present when relying on observational studies to establish plausible causation including an observable biologic mechanism, dose-effect response, temporal relationship, consistent effect observed in multiple study populations, and statistical strength of response. These elements should be present in a consistent and powerful enough way to balance the pitfalls of observational studies, namely biases.
A particularly problematic bias is one of recall bias, which plagues case-control studies. Case-control studies are a popular tool to measure a relationship between an exposure and a rare disease, because they are more feasible than the prospective, observational cohort studies that require very large study populations observed over very long periods of time to capture enough events of interest (in this case, cases of ovarian cancer). In case-control studies, researchers identify a cohort of patients with the outcome of interest (ovarian cancer) and compare this population to a control group of similar demographic features. They then survey directly or indirectly (through medical records) for the exposure of interest (perineal talc use).
Recall bias occurs when subjects who have the disease are more likely to have memory of exposure than do control subjects because of the natural instincts individuals have toward attribution. This is emphasized when there is public commentary, justified or not, about the potential risks of that exposure. Given the significant publicity that these lawsuits have had with companies that produced cosmetic talc, it is plausible that ovarian cancer survivors are more likely to remember and negatively attribute their talc exposure to their cancer than are subjects without cancer. Additionally, their memory of volume and duration of exposure generally is enhanced by the same pressures. The potential for this bias is eliminated in prospective, cohort observational studies such as the Women’s Health Initiative Observational Study which, among 61,576 women, half of whom reported perineal talc exposure, did not measure a difference in the development of ovarian cancers during their 12 years of mean follow-up.3
Given these inherent biases, The biologic mechanism of talc carcinogenesis is largely theoretical. As mentioned earlier, prior to the 1970s, there was some observed contamination of talc with asbestos likely caused by the geologic proximity of these minerals. Asbestos is a known carcinogen, and therefore possibly could be harmful if a contaminant of talc. However, it is not known if this level of contamination was enough to be achieve ovarian carcinogenesis. Most theories of talc carcinogenesis are based on foreign body inflammatory reaction via talc particle ascent through the genital tract. This is proposed to induce an inflammatory release of prostaglandins and cytokines, which could cause a mutagenic effect promoting carcinogenesis. The foreign body inflammatory mechanism is further supported by the observation of a decreased incidence of ovarian cancer after hysterectomy or tubal ligation.4 However, inconsistently, a protective effect of NSAIDs has not been observed in ovarian cancer.5
A recent meta-analysis, which reviewed 27 of the largest, best-quality observational studies, identified a dose-effect response with an increased risk for ovarian cancer with greater than 3,600 lifetime applications, compared with less than 3,600 applications.2 The observed association between perineal talc exposure and increased risk of ovarian cancer appears to be consistent across a number of observational studies, including both case-control studies and prospective cohort studies (although somewhat mitigated in the latter). Additionally, there appears to be consistency in the finding that the risk is present for the epithelial subtypes of serous and endometrioid, but not mucinous or clear cell cancer. However, when considering the magnitude of effect, this remains somewhat small (odds ratio, 1.31; 95% confidence interval, 1.24-1.39) when compared with other better established carcinogenic relationships such as smoking and lung cancer where the hazard ratio is 12.12 (95% CI, 6.94-21.17).2,6
If talc does not cause ovarian cancer, why would this association be observed at all? One explanation could be that talc use is a confounder for the true causative mechanism. A theoretical example of this would be if the genital microbiome (a subject we have reviewed previously in this column) was the true culprit. If a particular microbiome profile promotes both oncogenic change in the ovary while also causing vaginal discharge and odor, it might increase the likelihood that perineal talc use is reported in the history of these cancer patients. This is purely speculative, but it always is important to consider the potential for confounding variables when utilizing observational studies to attribute cause and effect.
Therefore, there is a consistently observed association between perineal talc application and ovarian cancer, however, the relationship does not appear to be strong enough, associated with a proven carcinogenic mechanism, or free from interfering recall bias such to definitively state that perineal talc exposure causes ovarian cancer. Given these findings, it is reasonable to recommend patients avoid the use of perineal talc application until further definitive safety evidence is provided. In the meantime, it should be noted that even though talc-containing products are not commercially labeled as carcinogens, many pharmaceutical and cosmetic companies have replaced the mineral talc with corn starch in their powders.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Cancer. 1982 Jul 15;50(2):372-6.
2. Epidemiology. 2018 Jan;29(1):41-9.
3. J Natl Cancer Inst. 2014 Sep 10;106(9). pii: dju208.
4. Am J Epidemiol. 1991 Aug 15;134(4):362-9.
5. Int J Cancer. 2008 Jan 1;122(1):170-6.
6. J Natl Cancer Inst. 2018 Nov 1;110(11):1201-7.
Many readers may be aware of large payments made by such companies as Johnson & Johnson to compensate women with a history of ovarian cancer who have claimed that perineal application of talc played a causative role in their cancer development. This column serves to review the purported role of perineal talc use in the development of ovarian cancer, and explore some of the pitfalls of observational science.
Talc, a hydrated magnesium silicate, is the softest mineral on earth, and has been sold as a personal hygiene product for many decades. Perineal application of talc to sanitary pads, perineal skin, undergarments, and diapers has been a common practice to decrease friction, moisture build-up, and as a deodorant. Talc is chemically similar, although not identical, to asbestos and is geologically located in close proximity to the known carcinogen. In the 1970s, there were concerns raised regarding the possible contamination of cosmetic-grade talc with asbestos, which led to the development of asbestos-free forms of the substance. Given that a strong causal relationship had been established between asbestos exposure and lung and pleural cancers, there was concern that exposure to perineal talc might increase cancer risk.
In the 1980s, an association between perineal talc exposure and ovarian cancer was observed in a case-control study.1 Since that time, multiple other observational studies, predominately case-control studies, have observed an increased ovarian cancer risk among users of perineal talc including the findings of a meta-analysis which estimated a 24%-39% increased risk for ovarian cancer among users.2 Does this establish a causal relationship? For the purposes of legal cases, these associations are adequate. However, science demands a different standard when determining cause and effect.
It is not unusual to rely on observational studies to establish a causal relationship between exposure and disease when it is unethical to randomize subjects in a clinical trial to exposure of the potential harmful agent. This was the necessary methodology behind establishing that smoking causes lung cancer. Several factors must be present when relying on observational studies to establish plausible causation including an observable biologic mechanism, dose-effect response, temporal relationship, consistent effect observed in multiple study populations, and statistical strength of response. These elements should be present in a consistent and powerful enough way to balance the pitfalls of observational studies, namely biases.
A particularly problematic bias is one of recall bias, which plagues case-control studies. Case-control studies are a popular tool to measure a relationship between an exposure and a rare disease, because they are more feasible than the prospective, observational cohort studies that require very large study populations observed over very long periods of time to capture enough events of interest (in this case, cases of ovarian cancer). In case-control studies, researchers identify a cohort of patients with the outcome of interest (ovarian cancer) and compare this population to a control group of similar demographic features. They then survey directly or indirectly (through medical records) for the exposure of interest (perineal talc use).
Recall bias occurs when subjects who have the disease are more likely to have memory of exposure than do control subjects because of the natural instincts individuals have toward attribution. This is emphasized when there is public commentary, justified or not, about the potential risks of that exposure. Given the significant publicity that these lawsuits have had with companies that produced cosmetic talc, it is plausible that ovarian cancer survivors are more likely to remember and negatively attribute their talc exposure to their cancer than are subjects without cancer. Additionally, their memory of volume and duration of exposure generally is enhanced by the same pressures. The potential for this bias is eliminated in prospective, cohort observational studies such as the Women’s Health Initiative Observational Study which, among 61,576 women, half of whom reported perineal talc exposure, did not measure a difference in the development of ovarian cancers during their 12 years of mean follow-up.3
Given these inherent biases, The biologic mechanism of talc carcinogenesis is largely theoretical. As mentioned earlier, prior to the 1970s, there was some observed contamination of talc with asbestos likely caused by the geologic proximity of these minerals. Asbestos is a known carcinogen, and therefore possibly could be harmful if a contaminant of talc. However, it is not known if this level of contamination was enough to be achieve ovarian carcinogenesis. Most theories of talc carcinogenesis are based on foreign body inflammatory reaction via talc particle ascent through the genital tract. This is proposed to induce an inflammatory release of prostaglandins and cytokines, which could cause a mutagenic effect promoting carcinogenesis. The foreign body inflammatory mechanism is further supported by the observation of a decreased incidence of ovarian cancer after hysterectomy or tubal ligation.4 However, inconsistently, a protective effect of NSAIDs has not been observed in ovarian cancer.5
A recent meta-analysis, which reviewed 27 of the largest, best-quality observational studies, identified a dose-effect response with an increased risk for ovarian cancer with greater than 3,600 lifetime applications, compared with less than 3,600 applications.2 The observed association between perineal talc exposure and increased risk of ovarian cancer appears to be consistent across a number of observational studies, including both case-control studies and prospective cohort studies (although somewhat mitigated in the latter). Additionally, there appears to be consistency in the finding that the risk is present for the epithelial subtypes of serous and endometrioid, but not mucinous or clear cell cancer. However, when considering the magnitude of effect, this remains somewhat small (odds ratio, 1.31; 95% confidence interval, 1.24-1.39) when compared with other better established carcinogenic relationships such as smoking and lung cancer where the hazard ratio is 12.12 (95% CI, 6.94-21.17).2,6
If talc does not cause ovarian cancer, why would this association be observed at all? One explanation could be that talc use is a confounder for the true causative mechanism. A theoretical example of this would be if the genital microbiome (a subject we have reviewed previously in this column) was the true culprit. If a particular microbiome profile promotes both oncogenic change in the ovary while also causing vaginal discharge and odor, it might increase the likelihood that perineal talc use is reported in the history of these cancer patients. This is purely speculative, but it always is important to consider the potential for confounding variables when utilizing observational studies to attribute cause and effect.
Therefore, there is a consistently observed association between perineal talc application and ovarian cancer, however, the relationship does not appear to be strong enough, associated with a proven carcinogenic mechanism, or free from interfering recall bias such to definitively state that perineal talc exposure causes ovarian cancer. Given these findings, it is reasonable to recommend patients avoid the use of perineal talc application until further definitive safety evidence is provided. In the meantime, it should be noted that even though talc-containing products are not commercially labeled as carcinogens, many pharmaceutical and cosmetic companies have replaced the mineral talc with corn starch in their powders.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Cancer. 1982 Jul 15;50(2):372-6.
2. Epidemiology. 2018 Jan;29(1):41-9.
3. J Natl Cancer Inst. 2014 Sep 10;106(9). pii: dju208.
4. Am J Epidemiol. 1991 Aug 15;134(4):362-9.
5. Int J Cancer. 2008 Jan 1;122(1):170-6.
6. J Natl Cancer Inst. 2018 Nov 1;110(11):1201-7.
Many readers may be aware of large payments made by such companies as Johnson & Johnson to compensate women with a history of ovarian cancer who have claimed that perineal application of talc played a causative role in their cancer development. This column serves to review the purported role of perineal talc use in the development of ovarian cancer, and explore some of the pitfalls of observational science.
Talc, a hydrated magnesium silicate, is the softest mineral on earth, and has been sold as a personal hygiene product for many decades. Perineal application of talc to sanitary pads, perineal skin, undergarments, and diapers has been a common practice to decrease friction, moisture build-up, and as a deodorant. Talc is chemically similar, although not identical, to asbestos and is geologically located in close proximity to the known carcinogen. In the 1970s, there were concerns raised regarding the possible contamination of cosmetic-grade talc with asbestos, which led to the development of asbestos-free forms of the substance. Given that a strong causal relationship had been established between asbestos exposure and lung and pleural cancers, there was concern that exposure to perineal talc might increase cancer risk.
In the 1980s, an association between perineal talc exposure and ovarian cancer was observed in a case-control study.1 Since that time, multiple other observational studies, predominately case-control studies, have observed an increased ovarian cancer risk among users of perineal talc including the findings of a meta-analysis which estimated a 24%-39% increased risk for ovarian cancer among users.2 Does this establish a causal relationship? For the purposes of legal cases, these associations are adequate. However, science demands a different standard when determining cause and effect.
It is not unusual to rely on observational studies to establish a causal relationship between exposure and disease when it is unethical to randomize subjects in a clinical trial to exposure of the potential harmful agent. This was the necessary methodology behind establishing that smoking causes lung cancer. Several factors must be present when relying on observational studies to establish plausible causation including an observable biologic mechanism, dose-effect response, temporal relationship, consistent effect observed in multiple study populations, and statistical strength of response. These elements should be present in a consistent and powerful enough way to balance the pitfalls of observational studies, namely biases.
A particularly problematic bias is one of recall bias, which plagues case-control studies. Case-control studies are a popular tool to measure a relationship between an exposure and a rare disease, because they are more feasible than the prospective, observational cohort studies that require very large study populations observed over very long periods of time to capture enough events of interest (in this case, cases of ovarian cancer). In case-control studies, researchers identify a cohort of patients with the outcome of interest (ovarian cancer) and compare this population to a control group of similar demographic features. They then survey directly or indirectly (through medical records) for the exposure of interest (perineal talc use).
Recall bias occurs when subjects who have the disease are more likely to have memory of exposure than do control subjects because of the natural instincts individuals have toward attribution. This is emphasized when there is public commentary, justified or not, about the potential risks of that exposure. Given the significant publicity that these lawsuits have had with companies that produced cosmetic talc, it is plausible that ovarian cancer survivors are more likely to remember and negatively attribute their talc exposure to their cancer than are subjects without cancer. Additionally, their memory of volume and duration of exposure generally is enhanced by the same pressures. The potential for this bias is eliminated in prospective, cohort observational studies such as the Women’s Health Initiative Observational Study which, among 61,576 women, half of whom reported perineal talc exposure, did not measure a difference in the development of ovarian cancers during their 12 years of mean follow-up.3
Given these inherent biases, The biologic mechanism of talc carcinogenesis is largely theoretical. As mentioned earlier, prior to the 1970s, there was some observed contamination of talc with asbestos likely caused by the geologic proximity of these minerals. Asbestos is a known carcinogen, and therefore possibly could be harmful if a contaminant of talc. However, it is not known if this level of contamination was enough to be achieve ovarian carcinogenesis. Most theories of talc carcinogenesis are based on foreign body inflammatory reaction via talc particle ascent through the genital tract. This is proposed to induce an inflammatory release of prostaglandins and cytokines, which could cause a mutagenic effect promoting carcinogenesis. The foreign body inflammatory mechanism is further supported by the observation of a decreased incidence of ovarian cancer after hysterectomy or tubal ligation.4 However, inconsistently, a protective effect of NSAIDs has not been observed in ovarian cancer.5
A recent meta-analysis, which reviewed 27 of the largest, best-quality observational studies, identified a dose-effect response with an increased risk for ovarian cancer with greater than 3,600 lifetime applications, compared with less than 3,600 applications.2 The observed association between perineal talc exposure and increased risk of ovarian cancer appears to be consistent across a number of observational studies, including both case-control studies and prospective cohort studies (although somewhat mitigated in the latter). Additionally, there appears to be consistency in the finding that the risk is present for the epithelial subtypes of serous and endometrioid, but not mucinous or clear cell cancer. However, when considering the magnitude of effect, this remains somewhat small (odds ratio, 1.31; 95% confidence interval, 1.24-1.39) when compared with other better established carcinogenic relationships such as smoking and lung cancer where the hazard ratio is 12.12 (95% CI, 6.94-21.17).2,6
If talc does not cause ovarian cancer, why would this association be observed at all? One explanation could be that talc use is a confounder for the true causative mechanism. A theoretical example of this would be if the genital microbiome (a subject we have reviewed previously in this column) was the true culprit. If a particular microbiome profile promotes both oncogenic change in the ovary while also causing vaginal discharge and odor, it might increase the likelihood that perineal talc use is reported in the history of these cancer patients. This is purely speculative, but it always is important to consider the potential for confounding variables when utilizing observational studies to attribute cause and effect.
Therefore, there is a consistently observed association between perineal talc application and ovarian cancer, however, the relationship does not appear to be strong enough, associated with a proven carcinogenic mechanism, or free from interfering recall bias such to definitively state that perineal talc exposure causes ovarian cancer. Given these findings, it is reasonable to recommend patients avoid the use of perineal talc application until further definitive safety evidence is provided. In the meantime, it should be noted that even though talc-containing products are not commercially labeled as carcinogens, many pharmaceutical and cosmetic companies have replaced the mineral talc with corn starch in their powders.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Cancer. 1982 Jul 15;50(2):372-6.
2. Epidemiology. 2018 Jan;29(1):41-9.
3. J Natl Cancer Inst. 2014 Sep 10;106(9). pii: dju208.
4. Am J Epidemiol. 1991 Aug 15;134(4):362-9.
5. Int J Cancer. 2008 Jan 1;122(1):170-6.
6. J Natl Cancer Inst. 2018 Nov 1;110(11):1201-7.
Clinical Psychiatry News welcomes new board members
Clinical Psychiatry News is pleased to announce the addition of two new Editorial Advisory Board members.
Pooja Lakshmin, MD, is a board-certified psychiatrist specializing in women’s mental health and perinatal psychiatry, and a clinical assistant professor of psychiatry at the George Washington University, Washington. She maintains a private practice in Washington and is a clinical supervisor in the university’s Five Trimesters Perinatal Psychiatry Clinic. She is passionate about empowering women through psychoeducation and advocacy. She takes an integrative approach to psychiatry and frequently writes about topics related to women’s mental health, maternal mental health, and self-care.
Steven Starks, MD, a geriatric psychiatrist, is a congressional fellow at the Health and Aging Policy Fellows Program in Washington. Dr. Starks, a representative to American Psychiatric Association assembly for the APA Caucus of Black Psychiatrists, is keenly interested in understanding the cultural and social effects of geriatric mental health conditions on the lives of patients and families. Previously, Dr. Starks served as an assistant professor in the Baylor College of Medicine Menninger department of psychiatry and behavioral sciences, and in the department of neurology, Houston. He also was a faculty member at Baylor’s Alzheimer’s Disease and Memory Disorder Center.
Clinical Psychiatry News is pleased to announce the addition of two new Editorial Advisory Board members.
Pooja Lakshmin, MD, is a board-certified psychiatrist specializing in women’s mental health and perinatal psychiatry, and a clinical assistant professor of psychiatry at the George Washington University, Washington. She maintains a private practice in Washington and is a clinical supervisor in the university’s Five Trimesters Perinatal Psychiatry Clinic. She is passionate about empowering women through psychoeducation and advocacy. She takes an integrative approach to psychiatry and frequently writes about topics related to women’s mental health, maternal mental health, and self-care.
Steven Starks, MD, a geriatric psychiatrist, is a congressional fellow at the Health and Aging Policy Fellows Program in Washington. Dr. Starks, a representative to American Psychiatric Association assembly for the APA Caucus of Black Psychiatrists, is keenly interested in understanding the cultural and social effects of geriatric mental health conditions on the lives of patients and families. Previously, Dr. Starks served as an assistant professor in the Baylor College of Medicine Menninger department of psychiatry and behavioral sciences, and in the department of neurology, Houston. He also was a faculty member at Baylor’s Alzheimer’s Disease and Memory Disorder Center.
Clinical Psychiatry News is pleased to announce the addition of two new Editorial Advisory Board members.
Pooja Lakshmin, MD, is a board-certified psychiatrist specializing in women’s mental health and perinatal psychiatry, and a clinical assistant professor of psychiatry at the George Washington University, Washington. She maintains a private practice in Washington and is a clinical supervisor in the university’s Five Trimesters Perinatal Psychiatry Clinic. She is passionate about empowering women through psychoeducation and advocacy. She takes an integrative approach to psychiatry and frequently writes about topics related to women’s mental health, maternal mental health, and self-care.
Steven Starks, MD, a geriatric psychiatrist, is a congressional fellow at the Health and Aging Policy Fellows Program in Washington. Dr. Starks, a representative to American Psychiatric Association assembly for the APA Caucus of Black Psychiatrists, is keenly interested in understanding the cultural and social effects of geriatric mental health conditions on the lives of patients and families. Previously, Dr. Starks served as an assistant professor in the Baylor College of Medicine Menninger department of psychiatry and behavioral sciences, and in the department of neurology, Houston. He also was a faculty member at Baylor’s Alzheimer’s Disease and Memory Disorder Center.
Insurance networks: Is it time to abandon them?
Bob has been coming to therapy for a few months now. Initially, we met every week, but as his depression lifted, he asked to space the sessions out to twice a month, even as he continues to struggle with many challenges in his life. The cost, he says, is prohibitive, and while Bob believed he had good commercial insurance coverage, he’s learned a few things about insurance and mental health care.
Bob was referred to me by his internist. He knew I did not participate in his insurance network, but his policy covers out-of-network care. He’d had a number of imaging studies and then knee surgery earlier in the year, so he believed he’d met his deductible. He learned that, while he’d met his in-network deductible, he’d had no out-of-network expenses and there was a separate, much higher deductible – one he was not likely to meet with outpatient psychiatric care. In fact, the full cost of his treatment was not being subtracted from the deductible he needed to meet, but rather he was getting credit for lower usual and customary evaluation and management and psychotherapy fees for each session. It became clear that it would be many months – if ever – before Bob could expect any reimbursement for his out-of-network visits.
What Bob didn’t know was that, had he decided to switch to an in-network psychiatrist, he might well have trouble finding one, since half of psychiatrists don’t participate with any health insurance plans. And if he did see an in-network psychiatrist, he would likely need to find a separate in-network social worker or psychologist for psychotherapy, because most in-network psychiatrists see patients for short medication-management appointments. While the insurance companies would give Bob a list of providers, those lists are not kept up to date and include psychiatrists who have died, moved, aren’t taking new patients, or who have retired. The insurance company clearly states on its voicemail that verification of services does not guarantee payment, and Bob was told that the only way he could be certain of the reimbursement would be to submit the claims and wait. He went into treatment fully understanding that he might get no help with the cost from his health insurance.
When I first started in private practice in the 1990s, I joined only one panel. An older colleague told me I was foolish to hesitate, and that soon the panels would fill and it would be too late; psychiatrists wouldn’t be able to get on to the panels and would be unable to attract patients. A few years later, that same psychiatrist withdrew from all the insurance panels he was on; working on their terms was not rewarding. This division of in-network and out-of-network care is crucial to managed care: They must attract panels of doctors who will work for lower rates or with stipulations on how the doctor practices in order to save money.
But managed care came with a price: An entire administration was created to oversee the regulation of treatment. With time, some aspects of care management have vanished; it has been years since I have been asked to fill out a treatment plan to justify a need for outpatient psychotherapy. In Bob’s case, it’s clear how they save money; since he will not reach his high deductible, he will bear the full cost of his psychotherapy. Other patients who cannot afford to go out of network may give up searching and decide to go without treatment – this is not always an easy service to negotiate when one is distressed and compromised. Half of people with serious psychiatric disorders are not in treatment, and barriers to getting care are certainly one reason why.
Many psychiatrists have discovered that they can maintain a practice without being on insurance panels, as managed care only works if there are enough players willing to toss the ball. As psychiatrists have shied away from these panels, insurers have raised their reimbursement rates, and in Maryland, Medicaid also has had to raise their rates. The struggle has become one of how to get enough mental health professionals, and psychiatrists in particular, to join insurance panels in what is a shortage field.
Perhaps there are better ways to spend health care dollars than on the administration and management that come with limiting which doctors patients can see. The logistics of in-network versus out-of-network care are an expensive one, and create unconscionable scenarios in other fields. For example, such scenarios include ones in which a patient is brought in for emergency care to a facility where the doctors are not in network, or a patient has a procedure with an in-network surgeon but is unaware that the anesthesiologist or other members of the care team are not on the panel.
What if insurers controlled costs by setting a reasonable fee they would pay for services and paid any licensed physician for these services? Would market forces sort this out? Would fees then set to one which insurers would be willing to pay and physicians would be willing to accept? Or would those who are ill and impoverished be blocked from getting any care? What if we tried a whole new paradigm for psychiatric care?
Richard G. Frank, PhD, is a professor of health economics at Harvard. He specializes in mental health economics and is coauthor of the book “Better But Not Well: Mental Health Policy in the United States Since 1950” (Baltimore: Johns Hopkins University Press, 2006). Dr. Frank is a proponent of insurance panels.
“In the world we live in, we need to have panels; they are essential to controlling cost. They create a balance in terms of cost and utilization control in ways that protect patients, and they create a way for the insurance companies to bargain,” he said.
Dr. Frank noted that the concept that insurers should pay a set reasonable fee to any physician a patient wants to see has been tried. “It’s called ‘reference pricing,’ and when they did that with hip replacement surgery in California, it just hasn’t worked out. Patients ended up getting larger bills than they anticipated.
“The issues with psychiatry are different,” he continued. “There is a lot of bad behavior on the part of insurers and it’s an issue of parity. We shouldn’t let insurers differentially pay psychiatrists less. They have had an incentive to reduce the availability of mental health care and it’s bad for patients. It’s about lower payments to psychiatrists and the way those payments are currently structured drives patients out of care and defeats the purpose of insurance.”
Steven Sharfstein, MD, is the former CEO of Sheppard Pratt Health Systems, a past president of the American Psychiatric Association, and coauthor of several books on the economics of psychiatry. He refers to the current practice of credentialing network psychiatrists as a means of “rationing by supply.”
“The networks create a barrier to accessing care. I don’t think it’s an efficient way to take on the high cost of care, and it creates a tiered system.” Like Dr. Frank, Dr. Sharfstein believes parity is a large part of the problem. When asked about the idea of ending networks and establishing uniform deductibles and reimbursement rates, Dr. Sharfstein replied: “It really depends. We would need fees to hit a sweet spot that supports care while controlling costs.”
One thing is clear: With the current paradigm, it is often difficult to access treatment, and the well-insured patients may bear a significant and disproportionate cost (if not the entire cost) for getting care. Many who need care do not get it, regardless of their insurance status. Our status quo for psychiatric care falls short and I don’t predict that psychiatrists will rush to join networks so long as the demand for psychiatrists is greater than the supply.
Might another financial model work better? We know the system is lacking and one option, as Dr. Frank suggests, is to find solutions within the current model. But perhaps the question should not be one of how to get more psychiatrists to join networks, but of how to rework the system without the assumption that networks are the only way. While Bob is pleased that his symptoms are getting better and he’s managed a way to tackle his own bills, it’s certainly time to explore new ways of delivering and reimbursing psychiatric care.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle of Inpatient Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and has a private practice in Baltimore.
Bob has been coming to therapy for a few months now. Initially, we met every week, but as his depression lifted, he asked to space the sessions out to twice a month, even as he continues to struggle with many challenges in his life. The cost, he says, is prohibitive, and while Bob believed he had good commercial insurance coverage, he’s learned a few things about insurance and mental health care.
Bob was referred to me by his internist. He knew I did not participate in his insurance network, but his policy covers out-of-network care. He’d had a number of imaging studies and then knee surgery earlier in the year, so he believed he’d met his deductible. He learned that, while he’d met his in-network deductible, he’d had no out-of-network expenses and there was a separate, much higher deductible – one he was not likely to meet with outpatient psychiatric care. In fact, the full cost of his treatment was not being subtracted from the deductible he needed to meet, but rather he was getting credit for lower usual and customary evaluation and management and psychotherapy fees for each session. It became clear that it would be many months – if ever – before Bob could expect any reimbursement for his out-of-network visits.
What Bob didn’t know was that, had he decided to switch to an in-network psychiatrist, he might well have trouble finding one, since half of psychiatrists don’t participate with any health insurance plans. And if he did see an in-network psychiatrist, he would likely need to find a separate in-network social worker or psychologist for psychotherapy, because most in-network psychiatrists see patients for short medication-management appointments. While the insurance companies would give Bob a list of providers, those lists are not kept up to date and include psychiatrists who have died, moved, aren’t taking new patients, or who have retired. The insurance company clearly states on its voicemail that verification of services does not guarantee payment, and Bob was told that the only way he could be certain of the reimbursement would be to submit the claims and wait. He went into treatment fully understanding that he might get no help with the cost from his health insurance.
When I first started in private practice in the 1990s, I joined only one panel. An older colleague told me I was foolish to hesitate, and that soon the panels would fill and it would be too late; psychiatrists wouldn’t be able to get on to the panels and would be unable to attract patients. A few years later, that same psychiatrist withdrew from all the insurance panels he was on; working on their terms was not rewarding. This division of in-network and out-of-network care is crucial to managed care: They must attract panels of doctors who will work for lower rates or with stipulations on how the doctor practices in order to save money.
But managed care came with a price: An entire administration was created to oversee the regulation of treatment. With time, some aspects of care management have vanished; it has been years since I have been asked to fill out a treatment plan to justify a need for outpatient psychotherapy. In Bob’s case, it’s clear how they save money; since he will not reach his high deductible, he will bear the full cost of his psychotherapy. Other patients who cannot afford to go out of network may give up searching and decide to go without treatment – this is not always an easy service to negotiate when one is distressed and compromised. Half of people with serious psychiatric disorders are not in treatment, and barriers to getting care are certainly one reason why.
Many psychiatrists have discovered that they can maintain a practice without being on insurance panels, as managed care only works if there are enough players willing to toss the ball. As psychiatrists have shied away from these panels, insurers have raised their reimbursement rates, and in Maryland, Medicaid also has had to raise their rates. The struggle has become one of how to get enough mental health professionals, and psychiatrists in particular, to join insurance panels in what is a shortage field.
Perhaps there are better ways to spend health care dollars than on the administration and management that come with limiting which doctors patients can see. The logistics of in-network versus out-of-network care are an expensive one, and create unconscionable scenarios in other fields. For example, such scenarios include ones in which a patient is brought in for emergency care to a facility where the doctors are not in network, or a patient has a procedure with an in-network surgeon but is unaware that the anesthesiologist or other members of the care team are not on the panel.
What if insurers controlled costs by setting a reasonable fee they would pay for services and paid any licensed physician for these services? Would market forces sort this out? Would fees then set to one which insurers would be willing to pay and physicians would be willing to accept? Or would those who are ill and impoverished be blocked from getting any care? What if we tried a whole new paradigm for psychiatric care?
Richard G. Frank, PhD, is a professor of health economics at Harvard. He specializes in mental health economics and is coauthor of the book “Better But Not Well: Mental Health Policy in the United States Since 1950” (Baltimore: Johns Hopkins University Press, 2006). Dr. Frank is a proponent of insurance panels.
“In the world we live in, we need to have panels; they are essential to controlling cost. They create a balance in terms of cost and utilization control in ways that protect patients, and they create a way for the insurance companies to bargain,” he said.
Dr. Frank noted that the concept that insurers should pay a set reasonable fee to any physician a patient wants to see has been tried. “It’s called ‘reference pricing,’ and when they did that with hip replacement surgery in California, it just hasn’t worked out. Patients ended up getting larger bills than they anticipated.
“The issues with psychiatry are different,” he continued. “There is a lot of bad behavior on the part of insurers and it’s an issue of parity. We shouldn’t let insurers differentially pay psychiatrists less. They have had an incentive to reduce the availability of mental health care and it’s bad for patients. It’s about lower payments to psychiatrists and the way those payments are currently structured drives patients out of care and defeats the purpose of insurance.”
Steven Sharfstein, MD, is the former CEO of Sheppard Pratt Health Systems, a past president of the American Psychiatric Association, and coauthor of several books on the economics of psychiatry. He refers to the current practice of credentialing network psychiatrists as a means of “rationing by supply.”
“The networks create a barrier to accessing care. I don’t think it’s an efficient way to take on the high cost of care, and it creates a tiered system.” Like Dr. Frank, Dr. Sharfstein believes parity is a large part of the problem. When asked about the idea of ending networks and establishing uniform deductibles and reimbursement rates, Dr. Sharfstein replied: “It really depends. We would need fees to hit a sweet spot that supports care while controlling costs.”
One thing is clear: With the current paradigm, it is often difficult to access treatment, and the well-insured patients may bear a significant and disproportionate cost (if not the entire cost) for getting care. Many who need care do not get it, regardless of their insurance status. Our status quo for psychiatric care falls short and I don’t predict that psychiatrists will rush to join networks so long as the demand for psychiatrists is greater than the supply.
Might another financial model work better? We know the system is lacking and one option, as Dr. Frank suggests, is to find solutions within the current model. But perhaps the question should not be one of how to get more psychiatrists to join networks, but of how to rework the system without the assumption that networks are the only way. While Bob is pleased that his symptoms are getting better and he’s managed a way to tackle his own bills, it’s certainly time to explore new ways of delivering and reimbursing psychiatric care.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle of Inpatient Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and has a private practice in Baltimore.
Bob has been coming to therapy for a few months now. Initially, we met every week, but as his depression lifted, he asked to space the sessions out to twice a month, even as he continues to struggle with many challenges in his life. The cost, he says, is prohibitive, and while Bob believed he had good commercial insurance coverage, he’s learned a few things about insurance and mental health care.
Bob was referred to me by his internist. He knew I did not participate in his insurance network, but his policy covers out-of-network care. He’d had a number of imaging studies and then knee surgery earlier in the year, so he believed he’d met his deductible. He learned that, while he’d met his in-network deductible, he’d had no out-of-network expenses and there was a separate, much higher deductible – one he was not likely to meet with outpatient psychiatric care. In fact, the full cost of his treatment was not being subtracted from the deductible he needed to meet, but rather he was getting credit for lower usual and customary evaluation and management and psychotherapy fees for each session. It became clear that it would be many months – if ever – before Bob could expect any reimbursement for his out-of-network visits.
What Bob didn’t know was that, had he decided to switch to an in-network psychiatrist, he might well have trouble finding one, since half of psychiatrists don’t participate with any health insurance plans. And if he did see an in-network psychiatrist, he would likely need to find a separate in-network social worker or psychologist for psychotherapy, because most in-network psychiatrists see patients for short medication-management appointments. While the insurance companies would give Bob a list of providers, those lists are not kept up to date and include psychiatrists who have died, moved, aren’t taking new patients, or who have retired. The insurance company clearly states on its voicemail that verification of services does not guarantee payment, and Bob was told that the only way he could be certain of the reimbursement would be to submit the claims and wait. He went into treatment fully understanding that he might get no help with the cost from his health insurance.
When I first started in private practice in the 1990s, I joined only one panel. An older colleague told me I was foolish to hesitate, and that soon the panels would fill and it would be too late; psychiatrists wouldn’t be able to get on to the panels and would be unable to attract patients. A few years later, that same psychiatrist withdrew from all the insurance panels he was on; working on their terms was not rewarding. This division of in-network and out-of-network care is crucial to managed care: They must attract panels of doctors who will work for lower rates or with stipulations on how the doctor practices in order to save money.
But managed care came with a price: An entire administration was created to oversee the regulation of treatment. With time, some aspects of care management have vanished; it has been years since I have been asked to fill out a treatment plan to justify a need for outpatient psychotherapy. In Bob’s case, it’s clear how they save money; since he will not reach his high deductible, he will bear the full cost of his psychotherapy. Other patients who cannot afford to go out of network may give up searching and decide to go without treatment – this is not always an easy service to negotiate when one is distressed and compromised. Half of people with serious psychiatric disorders are not in treatment, and barriers to getting care are certainly one reason why.
Many psychiatrists have discovered that they can maintain a practice without being on insurance panels, as managed care only works if there are enough players willing to toss the ball. As psychiatrists have shied away from these panels, insurers have raised their reimbursement rates, and in Maryland, Medicaid also has had to raise their rates. The struggle has become one of how to get enough mental health professionals, and psychiatrists in particular, to join insurance panels in what is a shortage field.
Perhaps there are better ways to spend health care dollars than on the administration and management that come with limiting which doctors patients can see. The logistics of in-network versus out-of-network care are an expensive one, and create unconscionable scenarios in other fields. For example, such scenarios include ones in which a patient is brought in for emergency care to a facility where the doctors are not in network, or a patient has a procedure with an in-network surgeon but is unaware that the anesthesiologist or other members of the care team are not on the panel.
What if insurers controlled costs by setting a reasonable fee they would pay for services and paid any licensed physician for these services? Would market forces sort this out? Would fees then set to one which insurers would be willing to pay and physicians would be willing to accept? Or would those who are ill and impoverished be blocked from getting any care? What if we tried a whole new paradigm for psychiatric care?
Richard G. Frank, PhD, is a professor of health economics at Harvard. He specializes in mental health economics and is coauthor of the book “Better But Not Well: Mental Health Policy in the United States Since 1950” (Baltimore: Johns Hopkins University Press, 2006). Dr. Frank is a proponent of insurance panels.
“In the world we live in, we need to have panels; they are essential to controlling cost. They create a balance in terms of cost and utilization control in ways that protect patients, and they create a way for the insurance companies to bargain,” he said.
Dr. Frank noted that the concept that insurers should pay a set reasonable fee to any physician a patient wants to see has been tried. “It’s called ‘reference pricing,’ and when they did that with hip replacement surgery in California, it just hasn’t worked out. Patients ended up getting larger bills than they anticipated.
“The issues with psychiatry are different,” he continued. “There is a lot of bad behavior on the part of insurers and it’s an issue of parity. We shouldn’t let insurers differentially pay psychiatrists less. They have had an incentive to reduce the availability of mental health care and it’s bad for patients. It’s about lower payments to psychiatrists and the way those payments are currently structured drives patients out of care and defeats the purpose of insurance.”
Steven Sharfstein, MD, is the former CEO of Sheppard Pratt Health Systems, a past president of the American Psychiatric Association, and coauthor of several books on the economics of psychiatry. He refers to the current practice of credentialing network psychiatrists as a means of “rationing by supply.”
“The networks create a barrier to accessing care. I don’t think it’s an efficient way to take on the high cost of care, and it creates a tiered system.” Like Dr. Frank, Dr. Sharfstein believes parity is a large part of the problem. When asked about the idea of ending networks and establishing uniform deductibles and reimbursement rates, Dr. Sharfstein replied: “It really depends. We would need fees to hit a sweet spot that supports care while controlling costs.”
One thing is clear: With the current paradigm, it is often difficult to access treatment, and the well-insured patients may bear a significant and disproportionate cost (if not the entire cost) for getting care. Many who need care do not get it, regardless of their insurance status. Our status quo for psychiatric care falls short and I don’t predict that psychiatrists will rush to join networks so long as the demand for psychiatrists is greater than the supply.
Might another financial model work better? We know the system is lacking and one option, as Dr. Frank suggests, is to find solutions within the current model. But perhaps the question should not be one of how to get more psychiatrists to join networks, but of how to rework the system without the assumption that networks are the only way. While Bob is pleased that his symptoms are getting better and he’s managed a way to tackle his own bills, it’s certainly time to explore new ways of delivering and reimbursing psychiatric care.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle of Inpatient Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016), and has a private practice in Baltimore.
Researchers win grants to study real-world cancer data
Three researchers have won CancerLinQ Discovery Research Support Grants from the American Society of Clinical Oncology, and two researchers have received a National Institutes of Health R21 grant.
Igor Astsaturov, MD, PhD, and Edna Cukierman, PhD, both of Fox Chase Cancer Center in Philadelphia, won the R21 grant. The pair will receive $432,410 over 2 years for their research on pancreatic cancer.
With their work, Dr. Astsaturov and Dr. Cukierman are “hoping to describe the structural and functional nature of cell-cell contact, or oncogenic synapses, associated with cancer-associated fibroblasts and pancreatic cells,” according to Fox Chase.
Three other researchers have won ASCO’s CancerLinQ Discovery Research Support Grants. The recipients will conduct projects using CancerLinQ, which collects and analyzes real-world data from cancer patients at practices across the United States.
Each 1-year grant covers the cost of a CancerLinQ Discovery data set and a meeting at ASCO headquarters. The grants also contribute to personnel and/or research expenses. The grants are funded by the ASCO Foundation’s Mission Endowment of Conquer Cancer.
With his CancerLinQ Discovery grant, Sadiq Rehmani, MD, of Icahn School of Medicine at Mount Sinai in New York, will study immunotherapy in older lung cancer patients with comorbidities.
Grant recipient Yasmin Karimi, MD, of Stanford (Calif.) University, will study how osteoclast inhibitors affect skeletal-related events and mortality in “real-world” patients with metastatic breast cancer and bone metastasis.
Grant recipient Vinayak Muralidhar, MD, of Brigham and Women’s Hospital and the radiation oncology program at Harvard Medical School, Boston, will study the use of androgen-deprivation therapy and hypofractionation in prostate cancer.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
Three researchers have won CancerLinQ Discovery Research Support Grants from the American Society of Clinical Oncology, and two researchers have received a National Institutes of Health R21 grant.
Igor Astsaturov, MD, PhD, and Edna Cukierman, PhD, both of Fox Chase Cancer Center in Philadelphia, won the R21 grant. The pair will receive $432,410 over 2 years for their research on pancreatic cancer.
With their work, Dr. Astsaturov and Dr. Cukierman are “hoping to describe the structural and functional nature of cell-cell contact, or oncogenic synapses, associated with cancer-associated fibroblasts and pancreatic cells,” according to Fox Chase.
Three other researchers have won ASCO’s CancerLinQ Discovery Research Support Grants. The recipients will conduct projects using CancerLinQ, which collects and analyzes real-world data from cancer patients at practices across the United States.
Each 1-year grant covers the cost of a CancerLinQ Discovery data set and a meeting at ASCO headquarters. The grants also contribute to personnel and/or research expenses. The grants are funded by the ASCO Foundation’s Mission Endowment of Conquer Cancer.
With his CancerLinQ Discovery grant, Sadiq Rehmani, MD, of Icahn School of Medicine at Mount Sinai in New York, will study immunotherapy in older lung cancer patients with comorbidities.
Grant recipient Yasmin Karimi, MD, of Stanford (Calif.) University, will study how osteoclast inhibitors affect skeletal-related events and mortality in “real-world” patients with metastatic breast cancer and bone metastasis.
Grant recipient Vinayak Muralidhar, MD, of Brigham and Women’s Hospital and the radiation oncology program at Harvard Medical School, Boston, will study the use of androgen-deprivation therapy and hypofractionation in prostate cancer.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
Three researchers have won CancerLinQ Discovery Research Support Grants from the American Society of Clinical Oncology, and two researchers have received a National Institutes of Health R21 grant.
Igor Astsaturov, MD, PhD, and Edna Cukierman, PhD, both of Fox Chase Cancer Center in Philadelphia, won the R21 grant. The pair will receive $432,410 over 2 years for their research on pancreatic cancer.
With their work, Dr. Astsaturov and Dr. Cukierman are “hoping to describe the structural and functional nature of cell-cell contact, or oncogenic synapses, associated with cancer-associated fibroblasts and pancreatic cells,” according to Fox Chase.
Three other researchers have won ASCO’s CancerLinQ Discovery Research Support Grants. The recipients will conduct projects using CancerLinQ, which collects and analyzes real-world data from cancer patients at practices across the United States.
Each 1-year grant covers the cost of a CancerLinQ Discovery data set and a meeting at ASCO headquarters. The grants also contribute to personnel and/or research expenses. The grants are funded by the ASCO Foundation’s Mission Endowment of Conquer Cancer.
With his CancerLinQ Discovery grant, Sadiq Rehmani, MD, of Icahn School of Medicine at Mount Sinai in New York, will study immunotherapy in older lung cancer patients with comorbidities.
Grant recipient Yasmin Karimi, MD, of Stanford (Calif.) University, will study how osteoclast inhibitors affect skeletal-related events and mortality in “real-world” patients with metastatic breast cancer and bone metastasis.
Grant recipient Vinayak Muralidhar, MD, of Brigham and Women’s Hospital and the radiation oncology program at Harvard Medical School, Boston, will study the use of androgen-deprivation therapy and hypofractionation in prostate cancer.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at hematologynews@mdedge.com, and you could be featured in Movers in Medicine.
A Call to Address Sexual Harassment and Gender Discrimination in Medicine
PART I
Reports of sexual harassment and gender discrimination have dominated news headlines, and the #MeToo movement has brought the scope and severity of discriminatory behavior to the forefront of public consciousness. The #MeToo movement has raised national and global awareness of gender discrimination and sexual harassment in all industries and has given rise to Time’s Up initiative within health care.
Academic medicine has not been immune to workplace gender discrimination and sexual harassment as has been vastly reported in the literature and clearly documented in the 2018 National Academies of Sciences, Engineering, and Medicine report, which points out that … “the cumulative effect of sexual harassment is a significant and costly loss of talent in academic science, engineering, and medicine, which has consequences for advancing the nation’s economic and social well-being and its overall public health.”1
With the increasing recognition that healthcare is an environment especially prone to inequality, gender discrimination and sexual discrimination, the Time’s Up national organization, supported by the Time’s Up Legal Defense Fund, launched the Time’s Up initiative for health care workers on March 1, 2019.2,3 The overarching goal of this initiative is to expose workplace inequalities; drive policy and legislative changes focused on equal pay, equal opportunity, and equal work environments; and support safe, fair, and dignified work for women in health care. 2,3
This article, presented over the next three issues of Vascular Specialist, will present data on the ongoing problem of sexual harassment in medicine, discuss why the problem is prevalent in academic medicine, and provide recommendations for mitigating the problem in our workplace.
Defining & Measuring Sexual Harassment
Although commonly referred to as “sex discrimination,” sexual harassment differs from sexual discrimination. Sex discrimination refers to an employees’ denial of civil rights, raises, job opportunities, employment or a demotion or other mistreatments based on sex. On the other hand, sexual harassment relates to behavior that is inappropriate or offensive. A 2018 report from the National Academies Press defined sexual harassment (a form of discrimination) as comprising three categories of behavior: gender harassment – verbal and nonverbal behaviors that convey hostility, objectification, exclusion, or second-class status about members of one sex; unwanted sexual attention – verbal or physical unwelcome sexual advances, which can include assault; and sexual coercion – when favorable professional or educational treatment is conditional based on sexual activity.1
During 1995-2016, more than 7,000 health care service employees filed claims of sexual harassment with the Equal Employment Opportunity Commission. While this number may seem large, the number of official reports severely undervalues the prevalence of sexual discrimination in U.S. health care.1 Prevalence is best determined using representative validated surveys that rely on firsthand experience or observation of the behavior(s) without requiring the respondent to label those behaviors.
Environments at Risk for Sexual Harassment
Research reveals that academic settings in the fields of science exhibit characteristics that create high levels of risk for sexual harassment to occur. These environments historically are male dominated, tolerate sexually harassing behavior, and create a hierarchy in which men hold most of the positions of power and authority. Moreover, dependent relationships often exist between these gatekeepers and those subordinate to them, with gatekeepers directly influencing the career advancement of those subordinates.1
The greatest predictor of sexual harassment in the workplace is the organizational climate, which refers to the tolerance for sexual harassment and is measured on three elements: a lack of sanctions against offenders; a perceived risk to those who report sexually harassing behavior; and the perception that one’s report of sexually harassing behavior will not be taken seriously.1 Women are less likely to be directly harassed in environments that do not tolerate harassing behaviors or have a strong, clear, transparent consequence for these behaviors.
Sexual Harassment in Academic Medicine
Academic medicine has the highest rate of gender and sexual harassment in the health care industry, with about 50% of female academic physicians reporting incidents of sexual harassment.1 A recent survey suggests that more than half (58%) of women surgeons experienced sexual harassment within just the previous year alone.4 The conditions that increase the risk of sexual harassment against women – male-dominated hierarchical environments and organizational tolerance of sexual harassment – still prevail in academic medicine.
Higher-education environments are perceived as permissive environments in part because when targets report sexual harassment, they are retaliated against or there are few consequences for the perpetrator. Academic institutions are replete with cases in which the conduct of offenders is regarded as an open secret, but there are no sanctions for that bad behavior. These offenders often are perceived as superstars in their particular substantive area. Because they hold valued grants or national status within their specialty area, they often receive preferential treatment and are not held accountable for gender-biased and sexually harassing behavior. Interview data regarding sexual harassment in academic medicine reveals that interview respondents and other colleagues often know which individuals have a history of sexually harassing behavior. Both men and women warn colleagues of these perpetrators – knowing that calling out or reporting these behaviors is fruitless – and that the best manner for dealing with their behavior is to avoid or ignore it. This normalization of sexual harassment and gender bias was noted, unfortunately, to fuel similar behavior in new cohorts of medicine faculty.1
Sexual harassment of women in academic medicine starts in medical school. Female medical students are significantly more likely to experience sexual harassment by faculty and staff than are graduate or undergraduate students. Sexual harassment continues into residency training with residency described as “breeding grounds for abusive behavior by superiors.”1 Interview studies report that both men and women trainees widely accept harassing behavior at this stage of their training. The expectation of abusive and grueling conditions during residency caused several respondents to view sexual harassment as part of a continuum that they were expected to endure. Female residents in surgery and emergency medicine are more likely to be harassed than those in other specialties because of the high value placed on a hierarchical and authoritative workplace. Once out of residency, the sexual harassment of women in the workplace continues. A recent meta-analysis reveals that 58% of women faculty experience sexual harassment at work. Academic medicine has the second-highest rate of sexual harassment, behind the military (69%), as compared with all other workplaces. Women physicians of color experience more harassment (as a combination of sexual and racial harassment) than do white women physicians.1
Why Women Are Not Likely to Report Sexual Harassment
Only 25% of targets file formal reports with their employer, with even fewer taking claims to court. These numbers are even lower for women in the military and academic medicine, where formal reporting is the last resort for the victims. The reluctance to use formal reporting mechanisms is rooted in the “fear of blame, disbelief, inaction, retaliation, humiliation, ostracism, and the damage to one’s career and reputation.”1 Targets may perceive that there seem to be few benefits and high costs for reporting. Women and nonwhites often resist calling bad behavior “discrimination” because that increases their loss of control and victimhood.1 Women frequently perceive that grievance procedures favor the institution over the individual, and research has proven that women face retaliation, both professional and social, for speaking out. Furthermore, stark power differentials between the target and the perpetrator exacerbate the reluctance to report and the fear of retaliation. The overall effects can be long lasting.
References:
1. National Academies of Sciences, Engineering, and Medicine. Sexual Harassment of Women: Climate, Culture, and Consequences in Academic Sciences, Engineering, and Medicine. The National Academies Press, Washington, DC; 2018. doi. 10.17226/24994.
2. Choo EK et al. From #MeToo to #TimesUp in Health Care: Can a Culture of Accountability End Inequity and Harassment? Lancet. 2019 Feb 9;393(10171):499-502.
3. Choo EK et al. Time’s Up for Medicine? Only Time Will Tell. N Engl J Med. 2018 Oct 25;379(17):1592-3.
4. Medicine Has Its Own #MeToo Problems. Can Time’s Up Healthcare Fix It?
Dr. Mitchell is a vascular surgeon at Salem (Ore.) Hospital; Dr. Drudi is as vascular surgery resident at McGill University, Montreal; Dr. Brown is a professor of surgery at the Medical College of Wisconsin. Milwaukee; Dr. Sachdev-Ost is an associate professor of surgery at the University of Pittsburgh Medical Center.
PART I
Reports of sexual harassment and gender discrimination have dominated news headlines, and the #MeToo movement has brought the scope and severity of discriminatory behavior to the forefront of public consciousness. The #MeToo movement has raised national and global awareness of gender discrimination and sexual harassment in all industries and has given rise to Time’s Up initiative within health care.
Academic medicine has not been immune to workplace gender discrimination and sexual harassment as has been vastly reported in the literature and clearly documented in the 2018 National Academies of Sciences, Engineering, and Medicine report, which points out that … “the cumulative effect of sexual harassment is a significant and costly loss of talent in academic science, engineering, and medicine, which has consequences for advancing the nation’s economic and social well-being and its overall public health.”1
With the increasing recognition that healthcare is an environment especially prone to inequality, gender discrimination and sexual discrimination, the Time’s Up national organization, supported by the Time’s Up Legal Defense Fund, launched the Time’s Up initiative for health care workers on March 1, 2019.2,3 The overarching goal of this initiative is to expose workplace inequalities; drive policy and legislative changes focused on equal pay, equal opportunity, and equal work environments; and support safe, fair, and dignified work for women in health care. 2,3
This article, presented over the next three issues of Vascular Specialist, will present data on the ongoing problem of sexual harassment in medicine, discuss why the problem is prevalent in academic medicine, and provide recommendations for mitigating the problem in our workplace.
Defining & Measuring Sexual Harassment
Although commonly referred to as “sex discrimination,” sexual harassment differs from sexual discrimination. Sex discrimination refers to an employees’ denial of civil rights, raises, job opportunities, employment or a demotion or other mistreatments based on sex. On the other hand, sexual harassment relates to behavior that is inappropriate or offensive. A 2018 report from the National Academies Press defined sexual harassment (a form of discrimination) as comprising three categories of behavior: gender harassment – verbal and nonverbal behaviors that convey hostility, objectification, exclusion, or second-class status about members of one sex; unwanted sexual attention – verbal or physical unwelcome sexual advances, which can include assault; and sexual coercion – when favorable professional or educational treatment is conditional based on sexual activity.1
During 1995-2016, more than 7,000 health care service employees filed claims of sexual harassment with the Equal Employment Opportunity Commission. While this number may seem large, the number of official reports severely undervalues the prevalence of sexual discrimination in U.S. health care.1 Prevalence is best determined using representative validated surveys that rely on firsthand experience or observation of the behavior(s) without requiring the respondent to label those behaviors.
Environments at Risk for Sexual Harassment
Research reveals that academic settings in the fields of science exhibit characteristics that create high levels of risk for sexual harassment to occur. These environments historically are male dominated, tolerate sexually harassing behavior, and create a hierarchy in which men hold most of the positions of power and authority. Moreover, dependent relationships often exist between these gatekeepers and those subordinate to them, with gatekeepers directly influencing the career advancement of those subordinates.1
The greatest predictor of sexual harassment in the workplace is the organizational climate, which refers to the tolerance for sexual harassment and is measured on three elements: a lack of sanctions against offenders; a perceived risk to those who report sexually harassing behavior; and the perception that one’s report of sexually harassing behavior will not be taken seriously.1 Women are less likely to be directly harassed in environments that do not tolerate harassing behaviors or have a strong, clear, transparent consequence for these behaviors.
Sexual Harassment in Academic Medicine
Academic medicine has the highest rate of gender and sexual harassment in the health care industry, with about 50% of female academic physicians reporting incidents of sexual harassment.1 A recent survey suggests that more than half (58%) of women surgeons experienced sexual harassment within just the previous year alone.4 The conditions that increase the risk of sexual harassment against women – male-dominated hierarchical environments and organizational tolerance of sexual harassment – still prevail in academic medicine.
Higher-education environments are perceived as permissive environments in part because when targets report sexual harassment, they are retaliated against or there are few consequences for the perpetrator. Academic institutions are replete with cases in which the conduct of offenders is regarded as an open secret, but there are no sanctions for that bad behavior. These offenders often are perceived as superstars in their particular substantive area. Because they hold valued grants or national status within their specialty area, they often receive preferential treatment and are not held accountable for gender-biased and sexually harassing behavior. Interview data regarding sexual harassment in academic medicine reveals that interview respondents and other colleagues often know which individuals have a history of sexually harassing behavior. Both men and women warn colleagues of these perpetrators – knowing that calling out or reporting these behaviors is fruitless – and that the best manner for dealing with their behavior is to avoid or ignore it. This normalization of sexual harassment and gender bias was noted, unfortunately, to fuel similar behavior in new cohorts of medicine faculty.1
Sexual harassment of women in academic medicine starts in medical school. Female medical students are significantly more likely to experience sexual harassment by faculty and staff than are graduate or undergraduate students. Sexual harassment continues into residency training with residency described as “breeding grounds for abusive behavior by superiors.”1 Interview studies report that both men and women trainees widely accept harassing behavior at this stage of their training. The expectation of abusive and grueling conditions during residency caused several respondents to view sexual harassment as part of a continuum that they were expected to endure. Female residents in surgery and emergency medicine are more likely to be harassed than those in other specialties because of the high value placed on a hierarchical and authoritative workplace. Once out of residency, the sexual harassment of women in the workplace continues. A recent meta-analysis reveals that 58% of women faculty experience sexual harassment at work. Academic medicine has the second-highest rate of sexual harassment, behind the military (69%), as compared with all other workplaces. Women physicians of color experience more harassment (as a combination of sexual and racial harassment) than do white women physicians.1
Why Women Are Not Likely to Report Sexual Harassment
Only 25% of targets file formal reports with their employer, with even fewer taking claims to court. These numbers are even lower for women in the military and academic medicine, where formal reporting is the last resort for the victims. The reluctance to use formal reporting mechanisms is rooted in the “fear of blame, disbelief, inaction, retaliation, humiliation, ostracism, and the damage to one’s career and reputation.”1 Targets may perceive that there seem to be few benefits and high costs for reporting. Women and nonwhites often resist calling bad behavior “discrimination” because that increases their loss of control and victimhood.1 Women frequently perceive that grievance procedures favor the institution over the individual, and research has proven that women face retaliation, both professional and social, for speaking out. Furthermore, stark power differentials between the target and the perpetrator exacerbate the reluctance to report and the fear of retaliation. The overall effects can be long lasting.
References:
1. National Academies of Sciences, Engineering, and Medicine. Sexual Harassment of Women: Climate, Culture, and Consequences in Academic Sciences, Engineering, and Medicine. The National Academies Press, Washington, DC; 2018. doi. 10.17226/24994.
2. Choo EK et al. From #MeToo to #TimesUp in Health Care: Can a Culture of Accountability End Inequity and Harassment? Lancet. 2019 Feb 9;393(10171):499-502.
3. Choo EK et al. Time’s Up for Medicine? Only Time Will Tell. N Engl J Med. 2018 Oct 25;379(17):1592-3.
4. Medicine Has Its Own #MeToo Problems. Can Time’s Up Healthcare Fix It?
Dr. Mitchell is a vascular surgeon at Salem (Ore.) Hospital; Dr. Drudi is as vascular surgery resident at McGill University, Montreal; Dr. Brown is a professor of surgery at the Medical College of Wisconsin. Milwaukee; Dr. Sachdev-Ost is an associate professor of surgery at the University of Pittsburgh Medical Center.
PART I
Reports of sexual harassment and gender discrimination have dominated news headlines, and the #MeToo movement has brought the scope and severity of discriminatory behavior to the forefront of public consciousness. The #MeToo movement has raised national and global awareness of gender discrimination and sexual harassment in all industries and has given rise to Time’s Up initiative within health care.
Academic medicine has not been immune to workplace gender discrimination and sexual harassment as has been vastly reported in the literature and clearly documented in the 2018 National Academies of Sciences, Engineering, and Medicine report, which points out that … “the cumulative effect of sexual harassment is a significant and costly loss of talent in academic science, engineering, and medicine, which has consequences for advancing the nation’s economic and social well-being and its overall public health.”1
With the increasing recognition that healthcare is an environment especially prone to inequality, gender discrimination and sexual discrimination, the Time’s Up national organization, supported by the Time’s Up Legal Defense Fund, launched the Time’s Up initiative for health care workers on March 1, 2019.2,3 The overarching goal of this initiative is to expose workplace inequalities; drive policy and legislative changes focused on equal pay, equal opportunity, and equal work environments; and support safe, fair, and dignified work for women in health care. 2,3
This article, presented over the next three issues of Vascular Specialist, will present data on the ongoing problem of sexual harassment in medicine, discuss why the problem is prevalent in academic medicine, and provide recommendations for mitigating the problem in our workplace.
Defining & Measuring Sexual Harassment
Although commonly referred to as “sex discrimination,” sexual harassment differs from sexual discrimination. Sex discrimination refers to an employees’ denial of civil rights, raises, job opportunities, employment or a demotion or other mistreatments based on sex. On the other hand, sexual harassment relates to behavior that is inappropriate or offensive. A 2018 report from the National Academies Press defined sexual harassment (a form of discrimination) as comprising three categories of behavior: gender harassment – verbal and nonverbal behaviors that convey hostility, objectification, exclusion, or second-class status about members of one sex; unwanted sexual attention – verbal or physical unwelcome sexual advances, which can include assault; and sexual coercion – when favorable professional or educational treatment is conditional based on sexual activity.1
During 1995-2016, more than 7,000 health care service employees filed claims of sexual harassment with the Equal Employment Opportunity Commission. While this number may seem large, the number of official reports severely undervalues the prevalence of sexual discrimination in U.S. health care.1 Prevalence is best determined using representative validated surveys that rely on firsthand experience or observation of the behavior(s) without requiring the respondent to label those behaviors.
Environments at Risk for Sexual Harassment
Research reveals that academic settings in the fields of science exhibit characteristics that create high levels of risk for sexual harassment to occur. These environments historically are male dominated, tolerate sexually harassing behavior, and create a hierarchy in which men hold most of the positions of power and authority. Moreover, dependent relationships often exist between these gatekeepers and those subordinate to them, with gatekeepers directly influencing the career advancement of those subordinates.1
The greatest predictor of sexual harassment in the workplace is the organizational climate, which refers to the tolerance for sexual harassment and is measured on three elements: a lack of sanctions against offenders; a perceived risk to those who report sexually harassing behavior; and the perception that one’s report of sexually harassing behavior will not be taken seriously.1 Women are less likely to be directly harassed in environments that do not tolerate harassing behaviors or have a strong, clear, transparent consequence for these behaviors.
Sexual Harassment in Academic Medicine
Academic medicine has the highest rate of gender and sexual harassment in the health care industry, with about 50% of female academic physicians reporting incidents of sexual harassment.1 A recent survey suggests that more than half (58%) of women surgeons experienced sexual harassment within just the previous year alone.4 The conditions that increase the risk of sexual harassment against women – male-dominated hierarchical environments and organizational tolerance of sexual harassment – still prevail in academic medicine.
Higher-education environments are perceived as permissive environments in part because when targets report sexual harassment, they are retaliated against or there are few consequences for the perpetrator. Academic institutions are replete with cases in which the conduct of offenders is regarded as an open secret, but there are no sanctions for that bad behavior. These offenders often are perceived as superstars in their particular substantive area. Because they hold valued grants or national status within their specialty area, they often receive preferential treatment and are not held accountable for gender-biased and sexually harassing behavior. Interview data regarding sexual harassment in academic medicine reveals that interview respondents and other colleagues often know which individuals have a history of sexually harassing behavior. Both men and women warn colleagues of these perpetrators – knowing that calling out or reporting these behaviors is fruitless – and that the best manner for dealing with their behavior is to avoid or ignore it. This normalization of sexual harassment and gender bias was noted, unfortunately, to fuel similar behavior in new cohorts of medicine faculty.1
Sexual harassment of women in academic medicine starts in medical school. Female medical students are significantly more likely to experience sexual harassment by faculty and staff than are graduate or undergraduate students. Sexual harassment continues into residency training with residency described as “breeding grounds for abusive behavior by superiors.”1 Interview studies report that both men and women trainees widely accept harassing behavior at this stage of their training. The expectation of abusive and grueling conditions during residency caused several respondents to view sexual harassment as part of a continuum that they were expected to endure. Female residents in surgery and emergency medicine are more likely to be harassed than those in other specialties because of the high value placed on a hierarchical and authoritative workplace. Once out of residency, the sexual harassment of women in the workplace continues. A recent meta-analysis reveals that 58% of women faculty experience sexual harassment at work. Academic medicine has the second-highest rate of sexual harassment, behind the military (69%), as compared with all other workplaces. Women physicians of color experience more harassment (as a combination of sexual and racial harassment) than do white women physicians.1
Why Women Are Not Likely to Report Sexual Harassment
Only 25% of targets file formal reports with their employer, with even fewer taking claims to court. These numbers are even lower for women in the military and academic medicine, where formal reporting is the last resort for the victims. The reluctance to use formal reporting mechanisms is rooted in the “fear of blame, disbelief, inaction, retaliation, humiliation, ostracism, and the damage to one’s career and reputation.”1 Targets may perceive that there seem to be few benefits and high costs for reporting. Women and nonwhites often resist calling bad behavior “discrimination” because that increases their loss of control and victimhood.1 Women frequently perceive that grievance procedures favor the institution over the individual, and research has proven that women face retaliation, both professional and social, for speaking out. Furthermore, stark power differentials between the target and the perpetrator exacerbate the reluctance to report and the fear of retaliation. The overall effects can be long lasting.
References:
1. National Academies of Sciences, Engineering, and Medicine. Sexual Harassment of Women: Climate, Culture, and Consequences in Academic Sciences, Engineering, and Medicine. The National Academies Press, Washington, DC; 2018. doi. 10.17226/24994.
2. Choo EK et al. From #MeToo to #TimesUp in Health Care: Can a Culture of Accountability End Inequity and Harassment? Lancet. 2019 Feb 9;393(10171):499-502.
3. Choo EK et al. Time’s Up for Medicine? Only Time Will Tell. N Engl J Med. 2018 Oct 25;379(17):1592-3.
4. Medicine Has Its Own #MeToo Problems. Can Time’s Up Healthcare Fix It?
Dr. Mitchell is a vascular surgeon at Salem (Ore.) Hospital; Dr. Drudi is as vascular surgery resident at McGill University, Montreal; Dr. Brown is a professor of surgery at the Medical College of Wisconsin. Milwaukee; Dr. Sachdev-Ost is an associate professor of surgery at the University of Pittsburgh Medical Center.
Dermatologists lack training about skin of color
a small study in JAMA Dermatology suggests.
Lead author Kristina Gorbatenko-Roth, PhD, of the department of psychology at University of Wisconsin-Stout, Menomonie, Wis., and colleagues analyzed the perceptions of 19 black, adult patients who had received treatment in a skin of color clinic (SOCC). Patients were asked about their perspectives and experiences inside and outside of the clinic as it pertained to dermatologists’ interaction style, cultural awareness, and overall treatment. Two focus groups consisted of patients seen by a race-concordant dermatologist, and two focus groups consisted of patients seen by a race-discordant dermatologist. The patients also responded to a survey.
Of 19 adult black patients who participated in the study, 18 respondents were women, and the mean age was 50 years. Compared with non-SOCC dermatology treatment experiences, patients experienced higher levels of overall satisfaction with SOCC dermatologists, reporting that SOCC dermatologists were better trained to care for black patients, showed greater respect and dignity, and were more trustworthy, according to the study published Aug 21.
Care satisfaction appeared most related to doctors’ interpersonal style and specialized knowledge of black skin and hair, according to the study. Investigators gleaned nine major themes during the analysis, five of which included dermatologist behaviors: interaction style, knowledge, partnering with patients in focusing on outcomes, economic sensitivity, and shared life experiences. Four themes were specific to patients: comfort, confidence, education, and concordance preference. Across all participants, a dermatologist’s interaction style was identified as the most important factor, elements of which included oral communication, body language, and physical examination performance.
Regarding experiences outside the SOCC, participants reported that some providers performed only a cursory skin examination and appeared to avoid physical contact, which some patients interpreted as a sign of disrespect and a lack of racial sensitivity. Participants also expressed frustration with dermatologists outside the clinic who seemed to lack knowledge about black skin and hair disorders. Of all respondents, 71% reported they would prefer a black (or race concordant) dermatologist, including 91% of the race-concordant group and 33% of the race-discordant group.
The investigators wrote the findings underscore a number of needed changes to enhance the care of black dermatology patients, including enhanced dermatology residency and workforce education about treatment of skin of color and more training on culturally aware communication skills. Perceptions of racial and cost-of-care insensitivities identified by the study population also suggest the need for training in cultural competency and implicit bias, delivery of cost conscious care, and the social determinants of health.
As far as they know, the authors noted that, before this study, “little was known regarding black patients’ perceptions of their dermatology care, either within or external to an SOCC,” and that the study “appears to be the first to investigate and provide preliminary findings for addressing this knowledge gap.”
SOURCE: Gorbatenko-Roth K et al. JAMA Dermatol. 2019 Aug 21. doi: 10.1001/jamadermatol.2019.2063.
The study by Gorbatenko-Roth et al. will hopefully serve as a spring board for the field of dermatology to improve physicians’ cultural competence and eliminate persistent knowledge gaps that exist in the treatment of black skin and hair, according to Susan C. Taylor, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia.
In an accompanying editorial in JAMA Dermatology (2019 Aug 21. doi: 10.1001/jamadermatol.2019.1963), Dr. Taylor wrote that the analysis yields insight into the importance of recognizing and understanding that differences exist in the skin and hair of black patients, compared with those of white patients.
“Implicit in this statement is that black skin color, biology, disease, reactions, presentation, diagnosis, and treatment as well as hair types, texture, tensile strength, shape, diameter, growth pattern, follicular configuration, diseases, and treatments are different than those of whites and require the dermatologist to have an expanded knowledge base and cultural sensitivity when evaluating and treating black patients,” Dr. Taylor wrote.
Another important finding is the overall preference by black patients for a race-concordant dermatologist, Dr. Taylor wrote, noting that the ability to fulfill these preferences is limited. In 2016, black dermatologists constituted only 3% of all dermatologists in the United States, while the overall black population in the United States at the time was 12.8%.
“Although this was a small study, the authors have demonstrated the great need for improvement and opportunities for the field of dermatology, including enhanced residency training, lifelong education in skin of color, culturally sensitive and competent care, and greater diversity in the dermatology workforce,” Dr. Taylor wrote. “Let us use this article as a call to action to serve all patients, regardless of race or ethnicity, with equal excellence.”
Dr. Taylor is an associate professor of dermatology at the University of Pennsylvania, Philadelphia, and creator and inaugural director of the Skin of Color Center, St. Luke’s Roosevelt Hospital Center, New York (currently Mount Sinai St Luke’s Medical Center). She reported no disclosures other than her association with the Skin of Color Center.
The study by Gorbatenko-Roth et al. will hopefully serve as a spring board for the field of dermatology to improve physicians’ cultural competence and eliminate persistent knowledge gaps that exist in the treatment of black skin and hair, according to Susan C. Taylor, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia.
In an accompanying editorial in JAMA Dermatology (2019 Aug 21. doi: 10.1001/jamadermatol.2019.1963), Dr. Taylor wrote that the analysis yields insight into the importance of recognizing and understanding that differences exist in the skin and hair of black patients, compared with those of white patients.
“Implicit in this statement is that black skin color, biology, disease, reactions, presentation, diagnosis, and treatment as well as hair types, texture, tensile strength, shape, diameter, growth pattern, follicular configuration, diseases, and treatments are different than those of whites and require the dermatologist to have an expanded knowledge base and cultural sensitivity when evaluating and treating black patients,” Dr. Taylor wrote.
Another important finding is the overall preference by black patients for a race-concordant dermatologist, Dr. Taylor wrote, noting that the ability to fulfill these preferences is limited. In 2016, black dermatologists constituted only 3% of all dermatologists in the United States, while the overall black population in the United States at the time was 12.8%.
“Although this was a small study, the authors have demonstrated the great need for improvement and opportunities for the field of dermatology, including enhanced residency training, lifelong education in skin of color, culturally sensitive and competent care, and greater diversity in the dermatology workforce,” Dr. Taylor wrote. “Let us use this article as a call to action to serve all patients, regardless of race or ethnicity, with equal excellence.”
Dr. Taylor is an associate professor of dermatology at the University of Pennsylvania, Philadelphia, and creator and inaugural director of the Skin of Color Center, St. Luke’s Roosevelt Hospital Center, New York (currently Mount Sinai St Luke’s Medical Center). She reported no disclosures other than her association with the Skin of Color Center.
The study by Gorbatenko-Roth et al. will hopefully serve as a spring board for the field of dermatology to improve physicians’ cultural competence and eliminate persistent knowledge gaps that exist in the treatment of black skin and hair, according to Susan C. Taylor, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia.
In an accompanying editorial in JAMA Dermatology (2019 Aug 21. doi: 10.1001/jamadermatol.2019.1963), Dr. Taylor wrote that the analysis yields insight into the importance of recognizing and understanding that differences exist in the skin and hair of black patients, compared with those of white patients.
“Implicit in this statement is that black skin color, biology, disease, reactions, presentation, diagnosis, and treatment as well as hair types, texture, tensile strength, shape, diameter, growth pattern, follicular configuration, diseases, and treatments are different than those of whites and require the dermatologist to have an expanded knowledge base and cultural sensitivity when evaluating and treating black patients,” Dr. Taylor wrote.
Another important finding is the overall preference by black patients for a race-concordant dermatologist, Dr. Taylor wrote, noting that the ability to fulfill these preferences is limited. In 2016, black dermatologists constituted only 3% of all dermatologists in the United States, while the overall black population in the United States at the time was 12.8%.
“Although this was a small study, the authors have demonstrated the great need for improvement and opportunities for the field of dermatology, including enhanced residency training, lifelong education in skin of color, culturally sensitive and competent care, and greater diversity in the dermatology workforce,” Dr. Taylor wrote. “Let us use this article as a call to action to serve all patients, regardless of race or ethnicity, with equal excellence.”
Dr. Taylor is an associate professor of dermatology at the University of Pennsylvania, Philadelphia, and creator and inaugural director of the Skin of Color Center, St. Luke’s Roosevelt Hospital Center, New York (currently Mount Sinai St Luke’s Medical Center). She reported no disclosures other than her association with the Skin of Color Center.
a small study in JAMA Dermatology suggests.
Lead author Kristina Gorbatenko-Roth, PhD, of the department of psychology at University of Wisconsin-Stout, Menomonie, Wis., and colleagues analyzed the perceptions of 19 black, adult patients who had received treatment in a skin of color clinic (SOCC). Patients were asked about their perspectives and experiences inside and outside of the clinic as it pertained to dermatologists’ interaction style, cultural awareness, and overall treatment. Two focus groups consisted of patients seen by a race-concordant dermatologist, and two focus groups consisted of patients seen by a race-discordant dermatologist. The patients also responded to a survey.
Of 19 adult black patients who participated in the study, 18 respondents were women, and the mean age was 50 years. Compared with non-SOCC dermatology treatment experiences, patients experienced higher levels of overall satisfaction with SOCC dermatologists, reporting that SOCC dermatologists were better trained to care for black patients, showed greater respect and dignity, and were more trustworthy, according to the study published Aug 21.
Care satisfaction appeared most related to doctors’ interpersonal style and specialized knowledge of black skin and hair, according to the study. Investigators gleaned nine major themes during the analysis, five of which included dermatologist behaviors: interaction style, knowledge, partnering with patients in focusing on outcomes, economic sensitivity, and shared life experiences. Four themes were specific to patients: comfort, confidence, education, and concordance preference. Across all participants, a dermatologist’s interaction style was identified as the most important factor, elements of which included oral communication, body language, and physical examination performance.
Regarding experiences outside the SOCC, participants reported that some providers performed only a cursory skin examination and appeared to avoid physical contact, which some patients interpreted as a sign of disrespect and a lack of racial sensitivity. Participants also expressed frustration with dermatologists outside the clinic who seemed to lack knowledge about black skin and hair disorders. Of all respondents, 71% reported they would prefer a black (or race concordant) dermatologist, including 91% of the race-concordant group and 33% of the race-discordant group.
The investigators wrote the findings underscore a number of needed changes to enhance the care of black dermatology patients, including enhanced dermatology residency and workforce education about treatment of skin of color and more training on culturally aware communication skills. Perceptions of racial and cost-of-care insensitivities identified by the study population also suggest the need for training in cultural competency and implicit bias, delivery of cost conscious care, and the social determinants of health.
As far as they know, the authors noted that, before this study, “little was known regarding black patients’ perceptions of their dermatology care, either within or external to an SOCC,” and that the study “appears to be the first to investigate and provide preliminary findings for addressing this knowledge gap.”
SOURCE: Gorbatenko-Roth K et al. JAMA Dermatol. 2019 Aug 21. doi: 10.1001/jamadermatol.2019.2063.
a small study in JAMA Dermatology suggests.
Lead author Kristina Gorbatenko-Roth, PhD, of the department of psychology at University of Wisconsin-Stout, Menomonie, Wis., and colleagues analyzed the perceptions of 19 black, adult patients who had received treatment in a skin of color clinic (SOCC). Patients were asked about their perspectives and experiences inside and outside of the clinic as it pertained to dermatologists’ interaction style, cultural awareness, and overall treatment. Two focus groups consisted of patients seen by a race-concordant dermatologist, and two focus groups consisted of patients seen by a race-discordant dermatologist. The patients also responded to a survey.
Of 19 adult black patients who participated in the study, 18 respondents were women, and the mean age was 50 years. Compared with non-SOCC dermatology treatment experiences, patients experienced higher levels of overall satisfaction with SOCC dermatologists, reporting that SOCC dermatologists were better trained to care for black patients, showed greater respect and dignity, and were more trustworthy, according to the study published Aug 21.
Care satisfaction appeared most related to doctors’ interpersonal style and specialized knowledge of black skin and hair, according to the study. Investigators gleaned nine major themes during the analysis, five of which included dermatologist behaviors: interaction style, knowledge, partnering with patients in focusing on outcomes, economic sensitivity, and shared life experiences. Four themes were specific to patients: comfort, confidence, education, and concordance preference. Across all participants, a dermatologist’s interaction style was identified as the most important factor, elements of which included oral communication, body language, and physical examination performance.
Regarding experiences outside the SOCC, participants reported that some providers performed only a cursory skin examination and appeared to avoid physical contact, which some patients interpreted as a sign of disrespect and a lack of racial sensitivity. Participants also expressed frustration with dermatologists outside the clinic who seemed to lack knowledge about black skin and hair disorders. Of all respondents, 71% reported they would prefer a black (or race concordant) dermatologist, including 91% of the race-concordant group and 33% of the race-discordant group.
The investigators wrote the findings underscore a number of needed changes to enhance the care of black dermatology patients, including enhanced dermatology residency and workforce education about treatment of skin of color and more training on culturally aware communication skills. Perceptions of racial and cost-of-care insensitivities identified by the study population also suggest the need for training in cultural competency and implicit bias, delivery of cost conscious care, and the social determinants of health.
As far as they know, the authors noted that, before this study, “little was known regarding black patients’ perceptions of their dermatology care, either within or external to an SOCC,” and that the study “appears to be the first to investigate and provide preliminary findings for addressing this knowledge gap.”
SOURCE: Gorbatenko-Roth K et al. JAMA Dermatol. 2019 Aug 21. doi: 10.1001/jamadermatol.2019.2063.
FROM JAMA DERMATOLOGY
Key clinical point: Dermatologists need more training about the treatment of skin of color.
Major finding: Compared with a non–skin of color clinic (non-SOCC), patients experienced higher levels of overall satisfaction with SOCC dermatologists, reporting that SOCC dermatologists were better trained to care for black patients.
Study details: A study of 19 black, adult patients through focus groups and a survey.
Disclosures: No disclosures were reported.
Source: Gorbatenko-Roth K et al. JAMA Dermatol. 2019 Aug 21. doi: 10.1001/jamadermatol.2019.2063.
Forging a path for gender equality in medicine
I read with cautious optimism the Diversity Pledge and No–All Male Panel Policy recently announced by the Lancet Group.1 This initiative comes at a crucial time, on the heels of a renewed interest and attention to gender inequities in medicine.
Despite 50-year-old federal legislation mandating that men and women receive equal compensation for equal work, pay inequities persist in all aspects of the workforce and medicine is no exception. In 2018, the American College of Physicians published a position paper acknowledging that, despite an increase in women in the active physician workforce and training positions, our numbers remain disproportionately low in leadership positions, with a mediocre track record for career advancement.2 Inadequate mentorship, discrimination, gender bias, imposter syndrome and a lack of work-life integration have all been cited as contributing to the problem.
Consensus and commitment statements are important first steps in legitimizing a problem, but they do not always engender change. They run the risk of creating an “illusion of fairness” that lulls us into a sense of complacency that change is coming and yet it never does. This is why the Lancet Group’s pledge is so remarkable in its scope. Less than a year after the timely ACP position paper, the Lancet Group published a theme issue addressing the systematic concerns surrounding gender bias in medicine.
Recognizing that publications are prime currency in medicine, the Lancet Group has declared its commitment to increase the representation of women, minorities, and underrepresented geographic areas in editorial boards, peer review, and authorship. Since their #LancetWomen issue, 8 of their 18 journals have refreshed their editorial boards to include at least 50% female membership, with a goal of all 18 journals achieving this landmark by year’s end. That this change is being catapulted by a major publishing group suggests that a necessary cultural transformation is underway.
Today, women are entering the medical field in equal numbers to men. While some may argue that we have achieved gender diversity in medicine, we must not confuse this with gender equity, which remains a distant goal. This is a ripe time in medicine. We have momentum and an opportunity for change.
In an editorial comment to the ACP position paper, Molly Carnes, MD, drew an analogy to the Surgeon General’s report in 1964, which announced that smoking was detrimental to health. The report legitimized the already overwhelming evidence that smoking negatively impacted clinical outcomes and it served as a trigger to propel change.3 Similarly, we hope the ACP position has legitimized the research highlighting gender disparities in medicine and that the Lancet Group’s initiative is among the first of many changes to come.
This change must be embraced and executed by leadership across medical schools, universities, and professional societies. We must collectively pursue interventions and solutions to the multifaceted problem of gender inequity. After all, women have become an integral part of the medical workforce and we all need to work together to ensure both genders have sustainable careers.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
References
1. Lancet. 2019 Aug 10; 394(10197): 452-3.
2. Ann Intern Med. 2018 May 15;168(10):721-3.
3. Ann Intern Med. 2018 May 15;168(10):741-3.
I read with cautious optimism the Diversity Pledge and No–All Male Panel Policy recently announced by the Lancet Group.1 This initiative comes at a crucial time, on the heels of a renewed interest and attention to gender inequities in medicine.
Despite 50-year-old federal legislation mandating that men and women receive equal compensation for equal work, pay inequities persist in all aspects of the workforce and medicine is no exception. In 2018, the American College of Physicians published a position paper acknowledging that, despite an increase in women in the active physician workforce and training positions, our numbers remain disproportionately low in leadership positions, with a mediocre track record for career advancement.2 Inadequate mentorship, discrimination, gender bias, imposter syndrome and a lack of work-life integration have all been cited as contributing to the problem.
Consensus and commitment statements are important first steps in legitimizing a problem, but they do not always engender change. They run the risk of creating an “illusion of fairness” that lulls us into a sense of complacency that change is coming and yet it never does. This is why the Lancet Group’s pledge is so remarkable in its scope. Less than a year after the timely ACP position paper, the Lancet Group published a theme issue addressing the systematic concerns surrounding gender bias in medicine.
Recognizing that publications are prime currency in medicine, the Lancet Group has declared its commitment to increase the representation of women, minorities, and underrepresented geographic areas in editorial boards, peer review, and authorship. Since their #LancetWomen issue, 8 of their 18 journals have refreshed their editorial boards to include at least 50% female membership, with a goal of all 18 journals achieving this landmark by year’s end. That this change is being catapulted by a major publishing group suggests that a necessary cultural transformation is underway.
Today, women are entering the medical field in equal numbers to men. While some may argue that we have achieved gender diversity in medicine, we must not confuse this with gender equity, which remains a distant goal. This is a ripe time in medicine. We have momentum and an opportunity for change.
In an editorial comment to the ACP position paper, Molly Carnes, MD, drew an analogy to the Surgeon General’s report in 1964, which announced that smoking was detrimental to health. The report legitimized the already overwhelming evidence that smoking negatively impacted clinical outcomes and it served as a trigger to propel change.3 Similarly, we hope the ACP position has legitimized the research highlighting gender disparities in medicine and that the Lancet Group’s initiative is among the first of many changes to come.
This change must be embraced and executed by leadership across medical schools, universities, and professional societies. We must collectively pursue interventions and solutions to the multifaceted problem of gender inequity. After all, women have become an integral part of the medical workforce and we all need to work together to ensure both genders have sustainable careers.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
References
1. Lancet. 2019 Aug 10; 394(10197): 452-3.
2. Ann Intern Med. 2018 May 15;168(10):721-3.
3. Ann Intern Med. 2018 May 15;168(10):741-3.
I read with cautious optimism the Diversity Pledge and No–All Male Panel Policy recently announced by the Lancet Group.1 This initiative comes at a crucial time, on the heels of a renewed interest and attention to gender inequities in medicine.
Despite 50-year-old federal legislation mandating that men and women receive equal compensation for equal work, pay inequities persist in all aspects of the workforce and medicine is no exception. In 2018, the American College of Physicians published a position paper acknowledging that, despite an increase in women in the active physician workforce and training positions, our numbers remain disproportionately low in leadership positions, with a mediocre track record for career advancement.2 Inadequate mentorship, discrimination, gender bias, imposter syndrome and a lack of work-life integration have all been cited as contributing to the problem.
Consensus and commitment statements are important first steps in legitimizing a problem, but they do not always engender change. They run the risk of creating an “illusion of fairness” that lulls us into a sense of complacency that change is coming and yet it never does. This is why the Lancet Group’s pledge is so remarkable in its scope. Less than a year after the timely ACP position paper, the Lancet Group published a theme issue addressing the systematic concerns surrounding gender bias in medicine.
Recognizing that publications are prime currency in medicine, the Lancet Group has declared its commitment to increase the representation of women, minorities, and underrepresented geographic areas in editorial boards, peer review, and authorship. Since their #LancetWomen issue, 8 of their 18 journals have refreshed their editorial boards to include at least 50% female membership, with a goal of all 18 journals achieving this landmark by year’s end. That this change is being catapulted by a major publishing group suggests that a necessary cultural transformation is underway.
Today, women are entering the medical field in equal numbers to men. While some may argue that we have achieved gender diversity in medicine, we must not confuse this with gender equity, which remains a distant goal. This is a ripe time in medicine. We have momentum and an opportunity for change.
In an editorial comment to the ACP position paper, Molly Carnes, MD, drew an analogy to the Surgeon General’s report in 1964, which announced that smoking was detrimental to health. The report legitimized the already overwhelming evidence that smoking negatively impacted clinical outcomes and it served as a trigger to propel change.3 Similarly, we hope the ACP position has legitimized the research highlighting gender disparities in medicine and that the Lancet Group’s initiative is among the first of many changes to come.
This change must be embraced and executed by leadership across medical schools, universities, and professional societies. We must collectively pursue interventions and solutions to the multifaceted problem of gender inequity. After all, women have become an integral part of the medical workforce and we all need to work together to ensure both genders have sustainable careers.
Dr. Sosa is a benign hematologist at Fox Chase Cancer Center in Philadelphia. Her research interests are in thromboembolic disease, with a focus in racial and gender disparities.
References
1. Lancet. 2019 Aug 10; 394(10197): 452-3.
2. Ann Intern Med. 2018 May 15;168(10):721-3.
3. Ann Intern Med. 2018 May 15;168(10):741-3.
Unsubsidized enrollees leaving insurance exchanges
Both overall and unsubsidized enrollment in the various state and federal health insurance exchanges dropped in 2018, but new data on payments for those policies show that more people paid their premiums in 2019, according to the Centers for Medicare & Medicaid Services.
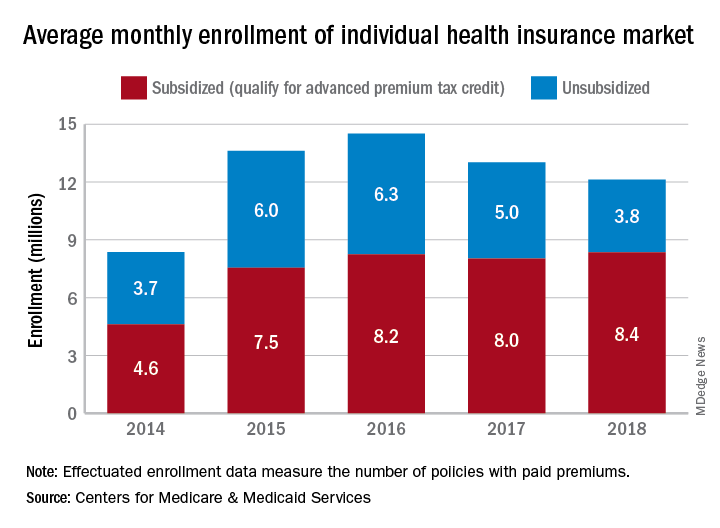
The number of policies selected with the individual insurance exchanges in late 2018 for which premiums were paid in February 2019 (termed the effectuated enrollment) was almost 10.6 million, or more than 92% of the 11.4 million plans selected during open enrollment, CMS reported. For February 2018, effectuated enrollment was just over 10.5 million, which represented 89.5% of the nearly 11.8 million policies selected during the previous open enrollment.
Over the longer term, the trend has been a rise and a fall as average monthly effectuated enrollment peaked in 2016 and dropped 16.5% by 2018, CMS data show.
A look at the advance premium tax credit (APTC) provides some insight into that decline. The population subsidized by the APTC has been fairly stable since 2016 – effectuated enrollment rose by just over 1% – but the number of unsubsidized enrollees has dropped 40% as 2.5 million people who did not qualify for the APTC left the market, the CMS said.
From 2017 to 2018, there were 47 states with declines in unsubsidized enrollment, with 9 states losing more than 40% of such enrollees. The largest drop in the unsubsidized population (85%) came in Iowa, while Alaska’s 7% gain was the largest increase, the CMS reported.
“As President Trump predicted, people are fleeing the individual market. Obamacare is failing the American people, and the ongoing exodus of the unsubsidized population from the market proves that Obamacare’s sky-high premiums are unaffordable,” CMS Administrator Seema Verma said in a written statement.
Both overall and unsubsidized enrollment in the various state and federal health insurance exchanges dropped in 2018, but new data on payments for those policies show that more people paid their premiums in 2019, according to the Centers for Medicare & Medicaid Services.

The number of policies selected with the individual insurance exchanges in late 2018 for which premiums were paid in February 2019 (termed the effectuated enrollment) was almost 10.6 million, or more than 92% of the 11.4 million plans selected during open enrollment, CMS reported. For February 2018, effectuated enrollment was just over 10.5 million, which represented 89.5% of the nearly 11.8 million policies selected during the previous open enrollment.
Over the longer term, the trend has been a rise and a fall as average monthly effectuated enrollment peaked in 2016 and dropped 16.5% by 2018, CMS data show.
A look at the advance premium tax credit (APTC) provides some insight into that decline. The population subsidized by the APTC has been fairly stable since 2016 – effectuated enrollment rose by just over 1% – but the number of unsubsidized enrollees has dropped 40% as 2.5 million people who did not qualify for the APTC left the market, the CMS said.
From 2017 to 2018, there were 47 states with declines in unsubsidized enrollment, with 9 states losing more than 40% of such enrollees. The largest drop in the unsubsidized population (85%) came in Iowa, while Alaska’s 7% gain was the largest increase, the CMS reported.
“As President Trump predicted, people are fleeing the individual market. Obamacare is failing the American people, and the ongoing exodus of the unsubsidized population from the market proves that Obamacare’s sky-high premiums are unaffordable,” CMS Administrator Seema Verma said in a written statement.
Both overall and unsubsidized enrollment in the various state and federal health insurance exchanges dropped in 2018, but new data on payments for those policies show that more people paid their premiums in 2019, according to the Centers for Medicare & Medicaid Services.

The number of policies selected with the individual insurance exchanges in late 2018 for which premiums were paid in February 2019 (termed the effectuated enrollment) was almost 10.6 million, or more than 92% of the 11.4 million plans selected during open enrollment, CMS reported. For February 2018, effectuated enrollment was just over 10.5 million, which represented 89.5% of the nearly 11.8 million policies selected during the previous open enrollment.
Over the longer term, the trend has been a rise and a fall as average monthly effectuated enrollment peaked in 2016 and dropped 16.5% by 2018, CMS data show.
A look at the advance premium tax credit (APTC) provides some insight into that decline. The population subsidized by the APTC has been fairly stable since 2016 – effectuated enrollment rose by just over 1% – but the number of unsubsidized enrollees has dropped 40% as 2.5 million people who did not qualify for the APTC left the market, the CMS said.
From 2017 to 2018, there were 47 states with declines in unsubsidized enrollment, with 9 states losing more than 40% of such enrollees. The largest drop in the unsubsidized population (85%) came in Iowa, while Alaska’s 7% gain was the largest increase, the CMS reported.
“As President Trump predicted, people are fleeing the individual market. Obamacare is failing the American people, and the ongoing exodus of the unsubsidized population from the market proves that Obamacare’s sky-high premiums are unaffordable,” CMS Administrator Seema Verma said in a written statement.





















