User login
High-sensitivity cardiac troponin levels linked to cardiovascular outcomes in COPD patients
, according to a post-hoc analysis of a clinical trial.
An increased risk of cardiovascular adverse events and cardiovascular death was seen in COPD patients in the highest quintile of plasma cardiac troponin concentrations at baseline, results of the analysis show.
The findings highlight the potential utility of high-sensitivity cardiac troponin in both clinical trials and clinical practice, according to researcher Nicholas L. Mills, MD, PhD, BHF/University Centre for Cardiovascular Science, The University of Edinburgh, Scotland, and co-investigators.
“Recognizing the risk associated with increased troponin concentrations might encourage clinicians to address cardiovascular risk due to lifestyle choices, and make patients more likely to engage with these recommendations,” Dr. Mills and co-authors wrote in the Journal of the American College of Cardiology.
Improved risk stratification may also help clinicians more appropriately target the use of preventive medications in COPD patients, they added in the report.
The analysis by Dr. Mills and colleagues was based on assessment of cardiac troponin I concentrations for patients in SUMMIT, a randomized trial assessing inhaled corticosteroids and long-acting beta agonists in COPD patients with ele-vated cardiovascular risk.
A total of 1,599 patients in the SUMMIT trial had a baseline cardiac troponin I assessment, and 1,258 had a follow-up assessment at 3 months following randomization.
Compared with those in the lowest quintile, patients in the highest quintile of baseline plasma cardiac troponin concentrations had an increased risk of a cardiovascular composite event, even after adjusting for confounding variables (hazard ratio, 3.67; 95% confidence interval, 1.33-10.13; P = .012)..
Increased risk of cardiovascular death was also seen in the highest quintile as compared with the lowest quintile (HR, 20.06; 95% CI, 2.44-165.15; P = .005), investigators said.
There was no difference in risk of COPD exacerbations between the highest and lowest quintiles, they added.
At 3 months, there were no differences in troponin concentrations related to COPD treatment, consistent with previous observations in the SUMMIT trial that treatment did not impact the cardiovascular composite endpoint, investigators said.
However, patients with a plasma troponin of 5 ng/L or greater recorded at either the baseline or 3-month assessment had an increased rate of the composite cardiovascular endpoint and a “markedly increased” risk of cardiovascular death, they wrote.
The research was supported by GlaxoSmithKline and a Butler British Heart Foundation Senior Clinical Research Fellowship received by Dr. Mills. Disclosures reported by Dr. Mills included consultancy, research grants, and speaker fees from Abbott Diagnostics, Roche, and Singulex. Study co-authors reported disclosures related to GlaxoSmithKline, Veramed Limited, AstraZeneca, Zambon, Bayer, Novartis, and others.
SOURCE: Adamson PD et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1126-37.
The current study data are “robust” and suggest a strong association between high-sensitivity cardiac troponin values and cardiovascular event risk in these COPD patients, according to authors of an editorial.
The study also showed that a change in high-sensitivity cardiac troponin at 3 months is associated with increased risk, noted editorial authors Allan S. Jaffe, MD, and H. Ari Jaffe, MD.
“Most of these events probably represent acceleration of atherosclerosis, given the effects of smoking on atherosclerotic disease and its progression,” the authors said in the Journal of the American College of Cardiology.
However, study authors could have more extensively addressed how to use that information to improve the care of COPD patients at elevated cardiovascular event risk, they added.
A “pilot algorithm” that could be used to apply this biomarker analysis in clinical practice was proposed in an editorial accompanying the research report.
They suggest repeating high-sensitivity cardiac troponin measurements to reduce variability, as well as repeating samples at 3 months to detect changes that could signal increased risk.
“In addition, one should avoid decisions based on small differences,” they wrote.
Allan S. Jaffe, MD, is with the department of cardiovascular medicine and the department of laboratory medicine and pathology at the Mayo Clinic in Rochester, Minn. He reported serving as a consultant for Beckman, Abbott, Siemens, ET Healthcare, Sphingotec, Becton Dickinson, Quindel, and Novartis. H. Ari Jaffe, MD, is with the department of medicine, pulmonary division at University of Illinois at Chicago, Jesse Brown VA Medicine Center, Chicago. He reported he has no relationships to disclose relevant to the contents of the editorial.
The current study data are “robust” and suggest a strong association between high-sensitivity cardiac troponin values and cardiovascular event risk in these COPD patients, according to authors of an editorial.
The study also showed that a change in high-sensitivity cardiac troponin at 3 months is associated with increased risk, noted editorial authors Allan S. Jaffe, MD, and H. Ari Jaffe, MD.
“Most of these events probably represent acceleration of atherosclerosis, given the effects of smoking on atherosclerotic disease and its progression,” the authors said in the Journal of the American College of Cardiology.
However, study authors could have more extensively addressed how to use that information to improve the care of COPD patients at elevated cardiovascular event risk, they added.
A “pilot algorithm” that could be used to apply this biomarker analysis in clinical practice was proposed in an editorial accompanying the research report.
They suggest repeating high-sensitivity cardiac troponin measurements to reduce variability, as well as repeating samples at 3 months to detect changes that could signal increased risk.
“In addition, one should avoid decisions based on small differences,” they wrote.
Allan S. Jaffe, MD, is with the department of cardiovascular medicine and the department of laboratory medicine and pathology at the Mayo Clinic in Rochester, Minn. He reported serving as a consultant for Beckman, Abbott, Siemens, ET Healthcare, Sphingotec, Becton Dickinson, Quindel, and Novartis. H. Ari Jaffe, MD, is with the department of medicine, pulmonary division at University of Illinois at Chicago, Jesse Brown VA Medicine Center, Chicago. He reported he has no relationships to disclose relevant to the contents of the editorial.
The current study data are “robust” and suggest a strong association between high-sensitivity cardiac troponin values and cardiovascular event risk in these COPD patients, according to authors of an editorial.
The study also showed that a change in high-sensitivity cardiac troponin at 3 months is associated with increased risk, noted editorial authors Allan S. Jaffe, MD, and H. Ari Jaffe, MD.
“Most of these events probably represent acceleration of atherosclerosis, given the effects of smoking on atherosclerotic disease and its progression,” the authors said in the Journal of the American College of Cardiology.
However, study authors could have more extensively addressed how to use that information to improve the care of COPD patients at elevated cardiovascular event risk, they added.
A “pilot algorithm” that could be used to apply this biomarker analysis in clinical practice was proposed in an editorial accompanying the research report.
They suggest repeating high-sensitivity cardiac troponin measurements to reduce variability, as well as repeating samples at 3 months to detect changes that could signal increased risk.
“In addition, one should avoid decisions based on small differences,” they wrote.
Allan S. Jaffe, MD, is with the department of cardiovascular medicine and the department of laboratory medicine and pathology at the Mayo Clinic in Rochester, Minn. He reported serving as a consultant for Beckman, Abbott, Siemens, ET Healthcare, Sphingotec, Becton Dickinson, Quindel, and Novartis. H. Ari Jaffe, MD, is with the department of medicine, pulmonary division at University of Illinois at Chicago, Jesse Brown VA Medicine Center, Chicago. He reported he has no relationships to disclose relevant to the contents of the editorial.
, according to a post-hoc analysis of a clinical trial.
An increased risk of cardiovascular adverse events and cardiovascular death was seen in COPD patients in the highest quintile of plasma cardiac troponin concentrations at baseline, results of the analysis show.
The findings highlight the potential utility of high-sensitivity cardiac troponin in both clinical trials and clinical practice, according to researcher Nicholas L. Mills, MD, PhD, BHF/University Centre for Cardiovascular Science, The University of Edinburgh, Scotland, and co-investigators.
“Recognizing the risk associated with increased troponin concentrations might encourage clinicians to address cardiovascular risk due to lifestyle choices, and make patients more likely to engage with these recommendations,” Dr. Mills and co-authors wrote in the Journal of the American College of Cardiology.
Improved risk stratification may also help clinicians more appropriately target the use of preventive medications in COPD patients, they added in the report.
The analysis by Dr. Mills and colleagues was based on assessment of cardiac troponin I concentrations for patients in SUMMIT, a randomized trial assessing inhaled corticosteroids and long-acting beta agonists in COPD patients with ele-vated cardiovascular risk.
A total of 1,599 patients in the SUMMIT trial had a baseline cardiac troponin I assessment, and 1,258 had a follow-up assessment at 3 months following randomization.
Compared with those in the lowest quintile, patients in the highest quintile of baseline plasma cardiac troponin concentrations had an increased risk of a cardiovascular composite event, even after adjusting for confounding variables (hazard ratio, 3.67; 95% confidence interval, 1.33-10.13; P = .012)..
Increased risk of cardiovascular death was also seen in the highest quintile as compared with the lowest quintile (HR, 20.06; 95% CI, 2.44-165.15; P = .005), investigators said.
There was no difference in risk of COPD exacerbations between the highest and lowest quintiles, they added.
At 3 months, there were no differences in troponin concentrations related to COPD treatment, consistent with previous observations in the SUMMIT trial that treatment did not impact the cardiovascular composite endpoint, investigators said.
However, patients with a plasma troponin of 5 ng/L or greater recorded at either the baseline or 3-month assessment had an increased rate of the composite cardiovascular endpoint and a “markedly increased” risk of cardiovascular death, they wrote.
The research was supported by GlaxoSmithKline and a Butler British Heart Foundation Senior Clinical Research Fellowship received by Dr. Mills. Disclosures reported by Dr. Mills included consultancy, research grants, and speaker fees from Abbott Diagnostics, Roche, and Singulex. Study co-authors reported disclosures related to GlaxoSmithKline, Veramed Limited, AstraZeneca, Zambon, Bayer, Novartis, and others.
SOURCE: Adamson PD et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1126-37.
, according to a post-hoc analysis of a clinical trial.
An increased risk of cardiovascular adverse events and cardiovascular death was seen in COPD patients in the highest quintile of plasma cardiac troponin concentrations at baseline, results of the analysis show.
The findings highlight the potential utility of high-sensitivity cardiac troponin in both clinical trials and clinical practice, according to researcher Nicholas L. Mills, MD, PhD, BHF/University Centre for Cardiovascular Science, The University of Edinburgh, Scotland, and co-investigators.
“Recognizing the risk associated with increased troponin concentrations might encourage clinicians to address cardiovascular risk due to lifestyle choices, and make patients more likely to engage with these recommendations,” Dr. Mills and co-authors wrote in the Journal of the American College of Cardiology.
Improved risk stratification may also help clinicians more appropriately target the use of preventive medications in COPD patients, they added in the report.
The analysis by Dr. Mills and colleagues was based on assessment of cardiac troponin I concentrations for patients in SUMMIT, a randomized trial assessing inhaled corticosteroids and long-acting beta agonists in COPD patients with ele-vated cardiovascular risk.
A total of 1,599 patients in the SUMMIT trial had a baseline cardiac troponin I assessment, and 1,258 had a follow-up assessment at 3 months following randomization.
Compared with those in the lowest quintile, patients in the highest quintile of baseline plasma cardiac troponin concentrations had an increased risk of a cardiovascular composite event, even after adjusting for confounding variables (hazard ratio, 3.67; 95% confidence interval, 1.33-10.13; P = .012)..
Increased risk of cardiovascular death was also seen in the highest quintile as compared with the lowest quintile (HR, 20.06; 95% CI, 2.44-165.15; P = .005), investigators said.
There was no difference in risk of COPD exacerbations between the highest and lowest quintiles, they added.
At 3 months, there were no differences in troponin concentrations related to COPD treatment, consistent with previous observations in the SUMMIT trial that treatment did not impact the cardiovascular composite endpoint, investigators said.
However, patients with a plasma troponin of 5 ng/L or greater recorded at either the baseline or 3-month assessment had an increased rate of the composite cardiovascular endpoint and a “markedly increased” risk of cardiovascular death, they wrote.
The research was supported by GlaxoSmithKline and a Butler British Heart Foundation Senior Clinical Research Fellowship received by Dr. Mills. Disclosures reported by Dr. Mills included consultancy, research grants, and speaker fees from Abbott Diagnostics, Roche, and Singulex. Study co-authors reported disclosures related to GlaxoSmithKline, Veramed Limited, AstraZeneca, Zambon, Bayer, Novartis, and others.
SOURCE: Adamson PD et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1126-37.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: In patients with COPD and heightened cardiovascular risk, high levels of high-sensitivity cardiac troponin were strongly associated with risk of cardiovascular outcomes.
Major finding: Compared to those in the lowest quintile, patients in the highest quintile of baseline plasma cardiac troponin concentrations had an increased risk of a cardiovascular composite event (hazard ratio, 3.67; 95% CI, 1.33-10.13; P = 0.012).
Study details: Post-hoc analysis of 1,599 patients in the SUMMIT trial who had a baseline cardiac troponin I assessment and 1,258 who had a 3-month follow-up assessment.
Disclosures: The study was supported by GlaxoSmithKline and a Butler British Heart Foundation Senior Clinical Research Fellowship. Authors reported disclosures related to GlaxoSmithKline, Veramed Limited, Abbott Diagnostics, Roche, Singulex, AstraZeneca, Zambon, Bayer, Novartis, and others.
Source: Adamson PD et al. J Am Coll Cardiol. 2018 Sep 4;72(10):1126-37.
Long-acting beta2 agonists don’t impact cardiovascular risk factors
Neither heart rate nor blood pressure worsened under long-term use of , according to a post hoc pooled analysis published in Pulmonary Pharmacology & Therapeutics.
The study was conducted by Stefan Andreas, MD, department of cardiology and pneumology, University Medical Centre Göttingen, and Lung Clinic Immenhausen, Germany. The analysis evaluated data from four studies and included a total of 3,104 patients with moderate to very severe COPD, which was defined as Global Initiative for Chronic Obstructive Lung Disease stage 2-4. Patients were randomized to either once-daily olodaterol (5 or 10 mcg), twice-daily formoterol (12 mcg), or placebo. Heart rate and blood pressure were measured before and after dosing at baseline and at four time points during the study: 6 weeks, 12 weeks, 24 weeks, and 48 weeks.
At all time points, the increases seen in the placebo group were greater than seen in the treatment groups; both systolic and diastolic blood pressure showed either slight decreases from or similarities with those seen at baseline, depending on time point. Furthermore, short-term effects were seen around dosing, from before administration to after, although these changes were quantitatively small.
One limitation of the study is that it couldn’t include patients with unstable COPD because of safety reasons; this prevents the findings from being more broadly generalizable.
“These findings, in a large COPD database, speak against the potential negative cardiovascular effects of olodaterol, as well as those of formoterol,” the researchers concluded.
They reported personal fees from various industry entities, such as Novartis, AstraZeneca, and GlaxoSmithKline. Some also reported receiving personal fees from or working for Boehringer Ingelheim, which funded the work.
SOURCE: Andreas S et al. Pulm Pharmacol Ther. 2018 Aug 2. doi: 10.1016/j.pupt.2018.08.002.
Neither heart rate nor blood pressure worsened under long-term use of , according to a post hoc pooled analysis published in Pulmonary Pharmacology & Therapeutics.
The study was conducted by Stefan Andreas, MD, department of cardiology and pneumology, University Medical Centre Göttingen, and Lung Clinic Immenhausen, Germany. The analysis evaluated data from four studies and included a total of 3,104 patients with moderate to very severe COPD, which was defined as Global Initiative for Chronic Obstructive Lung Disease stage 2-4. Patients were randomized to either once-daily olodaterol (5 or 10 mcg), twice-daily formoterol (12 mcg), or placebo. Heart rate and blood pressure were measured before and after dosing at baseline and at four time points during the study: 6 weeks, 12 weeks, 24 weeks, and 48 weeks.
At all time points, the increases seen in the placebo group were greater than seen in the treatment groups; both systolic and diastolic blood pressure showed either slight decreases from or similarities with those seen at baseline, depending on time point. Furthermore, short-term effects were seen around dosing, from before administration to after, although these changes were quantitatively small.
One limitation of the study is that it couldn’t include patients with unstable COPD because of safety reasons; this prevents the findings from being more broadly generalizable.
“These findings, in a large COPD database, speak against the potential negative cardiovascular effects of olodaterol, as well as those of formoterol,” the researchers concluded.
They reported personal fees from various industry entities, such as Novartis, AstraZeneca, and GlaxoSmithKline. Some also reported receiving personal fees from or working for Boehringer Ingelheim, which funded the work.
SOURCE: Andreas S et al. Pulm Pharmacol Ther. 2018 Aug 2. doi: 10.1016/j.pupt.2018.08.002.
Neither heart rate nor blood pressure worsened under long-term use of , according to a post hoc pooled analysis published in Pulmonary Pharmacology & Therapeutics.
The study was conducted by Stefan Andreas, MD, department of cardiology and pneumology, University Medical Centre Göttingen, and Lung Clinic Immenhausen, Germany. The analysis evaluated data from four studies and included a total of 3,104 patients with moderate to very severe COPD, which was defined as Global Initiative for Chronic Obstructive Lung Disease stage 2-4. Patients were randomized to either once-daily olodaterol (5 or 10 mcg), twice-daily formoterol (12 mcg), or placebo. Heart rate and blood pressure were measured before and after dosing at baseline and at four time points during the study: 6 weeks, 12 weeks, 24 weeks, and 48 weeks.
At all time points, the increases seen in the placebo group were greater than seen in the treatment groups; both systolic and diastolic blood pressure showed either slight decreases from or similarities with those seen at baseline, depending on time point. Furthermore, short-term effects were seen around dosing, from before administration to after, although these changes were quantitatively small.
One limitation of the study is that it couldn’t include patients with unstable COPD because of safety reasons; this prevents the findings from being more broadly generalizable.
“These findings, in a large COPD database, speak against the potential negative cardiovascular effects of olodaterol, as well as those of formoterol,” the researchers concluded.
They reported personal fees from various industry entities, such as Novartis, AstraZeneca, and GlaxoSmithKline. Some also reported receiving personal fees from or working for Boehringer Ingelheim, which funded the work.
SOURCE: Andreas S et al. Pulm Pharmacol Ther. 2018 Aug 2. doi: 10.1016/j.pupt.2018.08.002.
FROM PULMONARY PHARMACOLOGY & THERAPEUTICS
Key clinical point: Olodaterol and formoterol had a minimal impact on cardiovascular factors.
Major finding: Patients who were randomized to once-daily olodaterol (5 or 10 mcg), twice-daily formoterol (12 mcg), or placebo showed little change in heart rate and blood pressure at 6, 12, 24, or 48 weeks.
Study details: Post hoc pooled analysis from four studies comprising a total of 3,104 patients with moderate to very severe COPD.
Disclosures: Investigators reported personal fees from various industry entities, such as Novartis, AstraZeneca, and GlaxoSmithKline. Some also reported receiving personal fees from or working for Boehringer Ingelheim.
Source: Andreas S et al. Pulm Pharmacol Ther. 2018 Aug 2. doi: 10.1016/j.pupt.2018.08.002.
COPD opposites: Utah and West Virginia
New estimates of chronic obstructive pulmonary disease (COPD) may have Utah residents breathing a sigh of relief. West Virginians, not so much.
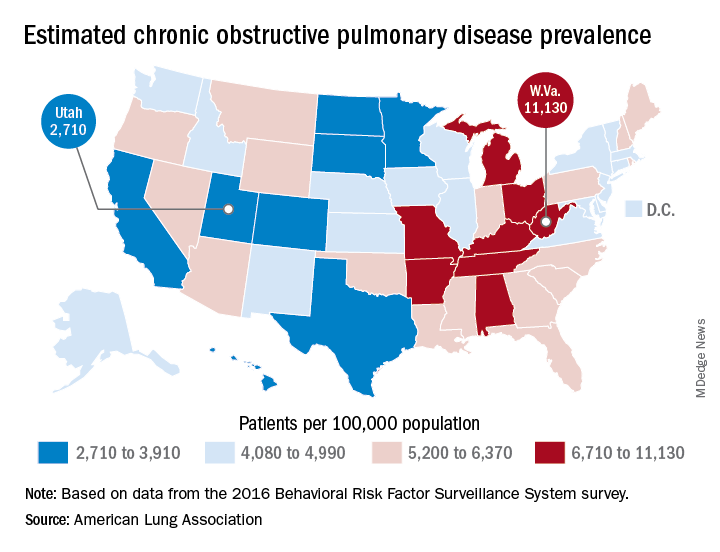
The Beehive State has the lowest prevalence of COPD in the country at 2,710 per 100,000 population, while the Mountain State tops the charts at 11,130 per 100,000, according to estimates from the American Lung Association. (Crude rates were calculated by MDedge News using the ALA’s estimates for total persons with COPD in each state and Census Bureau estimates for population.)
Other states with freer-breathing residents include Minnesota, which was just behind Utah with an estimated rate of 3,000 per 100,000 population, Hawaii (3,182), Colorado (3,334), and California (3,409). West Virginia’s rate, however, seems to be an outlier. The state with the next-highest rate, Kentucky, has a calculated prevalence of 8,890 per 100,000 population, followed by Tennessee at 7,880, Alabama at 7,400, and Arkansas at 7,330, using the ALA’s estimates, which were based on data from the 2016 Behavioral Risk Factor Surveillance System survey.
New estimates of chronic obstructive pulmonary disease (COPD) may have Utah residents breathing a sigh of relief. West Virginians, not so much.

The Beehive State has the lowest prevalence of COPD in the country at 2,710 per 100,000 population, while the Mountain State tops the charts at 11,130 per 100,000, according to estimates from the American Lung Association. (Crude rates were calculated by MDedge News using the ALA’s estimates for total persons with COPD in each state and Census Bureau estimates for population.)
Other states with freer-breathing residents include Minnesota, which was just behind Utah with an estimated rate of 3,000 per 100,000 population, Hawaii (3,182), Colorado (3,334), and California (3,409). West Virginia’s rate, however, seems to be an outlier. The state with the next-highest rate, Kentucky, has a calculated prevalence of 8,890 per 100,000 population, followed by Tennessee at 7,880, Alabama at 7,400, and Arkansas at 7,330, using the ALA’s estimates, which were based on data from the 2016 Behavioral Risk Factor Surveillance System survey.
New estimates of chronic obstructive pulmonary disease (COPD) may have Utah residents breathing a sigh of relief. West Virginians, not so much.

The Beehive State has the lowest prevalence of COPD in the country at 2,710 per 100,000 population, while the Mountain State tops the charts at 11,130 per 100,000, according to estimates from the American Lung Association. (Crude rates were calculated by MDedge News using the ALA’s estimates for total persons with COPD in each state and Census Bureau estimates for population.)
Other states with freer-breathing residents include Minnesota, which was just behind Utah with an estimated rate of 3,000 per 100,000 population, Hawaii (3,182), Colorado (3,334), and California (3,409). West Virginia’s rate, however, seems to be an outlier. The state with the next-highest rate, Kentucky, has a calculated prevalence of 8,890 per 100,000 population, followed by Tennessee at 7,880, Alabama at 7,400, and Arkansas at 7,330, using the ALA’s estimates, which were based on data from the 2016 Behavioral Risk Factor Surveillance System survey.
Telomere length linked to COPD exacerbations, mortality
according to a study published in Chest.
The evidence suggests that chronic obstructive pulmonary disease (COPD) may be a disease of accelerated aging, partly because of its relation to other senescence-related disorders such as osteoporosis and dementia, but also because it shows an exponential increase in prevalence in older age.
Telomere lengths are a measure of cellular senescence, and previous research has found that the telomeres are shortened in the peripheral leukocytes of patients with COPD, compared with healthy controls.
In this study, researchers examined the absolute telomere length of 576 people with moderate to severe COPD who were participating in the MACRO (Macrolide Azithromycin for Prevention of Exacerbations of COPD) study.
They found that individuals in the lowest quartile of telomere lengths had significantly worse health status and a higher exacerbation rate after accounting for treatment, compared with individuals in the higher quartile.
Patients with shorter telomere length had worse health status, as defined by higher St. George’s Respiratory Questionnaire scores. In the placebo arm of the study, the exacerbation rate (rate ratio, 1.50; 95% confidence interval, 1.16-1.95; P = .002) and mortality risk (hazard ratio, 9.45; 95% CI, 2.85-31.36; P = .015) were significantly higher in the shorter telomere group than in the longer telomere group; these differences were not observed in the azithromycin arm.
Patients with shorter telomeres also had a 800% higher risk of total mortality, compared with individuals with longer telomeres, although this was only evident in the placebo arm of the study, not the azithromycin arm. However, the authors noted that these data should be interpreted with caution because of the small number of deaths during the study.
“Together, these data support the notion that COPD is a systemic disease of accelerated aging and that replicative senescence, denoted by peripheral blood telomeres, is associated with poor health outcomes in COPD,” wrote Minhee Jin, of the University of British Columbia, Vancouver, and coauthors.
“It is now well established that replicative senescence results in a change of cellular phenotype to a proinflammatory state, a process that has been referred to as senescence-associated secretory phenotype,” they added.
The study also found that the median value for telomere length across the study participants – who had a mean age of 66 years – was equivalent to the expected value for someone in their 80s, “suggesting that on average MACRO participants were biologically much older than their chronological age.”
Researchers also noted that patients in the lowest quartile of telomere length had significantly lower forced vital capacity values, which suggested shorter telomeres could be a biomarker of restrictive physiology.
MACRO was funded by the U.S. National Heart, Lung, and Blood Institute, and the biomarker component of the study was funded by the Canadian Respiratory Research Network, Genome Canada, and the St. Paul’s Hospital Foundation. One author was an employee of GenomeDx Biosciences, three declared funding from or consultancies with the pharmaceutical industry. No other conflicts of interest were reported.
SOURCE: Jin M et al. Chest. 2018 Jul 12. doi: 10.1016/j.chest.2018.05.022.
according to a study published in Chest.
The evidence suggests that chronic obstructive pulmonary disease (COPD) may be a disease of accelerated aging, partly because of its relation to other senescence-related disorders such as osteoporosis and dementia, but also because it shows an exponential increase in prevalence in older age.
Telomere lengths are a measure of cellular senescence, and previous research has found that the telomeres are shortened in the peripheral leukocytes of patients with COPD, compared with healthy controls.
In this study, researchers examined the absolute telomere length of 576 people with moderate to severe COPD who were participating in the MACRO (Macrolide Azithromycin for Prevention of Exacerbations of COPD) study.
They found that individuals in the lowest quartile of telomere lengths had significantly worse health status and a higher exacerbation rate after accounting for treatment, compared with individuals in the higher quartile.
Patients with shorter telomere length had worse health status, as defined by higher St. George’s Respiratory Questionnaire scores. In the placebo arm of the study, the exacerbation rate (rate ratio, 1.50; 95% confidence interval, 1.16-1.95; P = .002) and mortality risk (hazard ratio, 9.45; 95% CI, 2.85-31.36; P = .015) were significantly higher in the shorter telomere group than in the longer telomere group; these differences were not observed in the azithromycin arm.
Patients with shorter telomeres also had a 800% higher risk of total mortality, compared with individuals with longer telomeres, although this was only evident in the placebo arm of the study, not the azithromycin arm. However, the authors noted that these data should be interpreted with caution because of the small number of deaths during the study.
“Together, these data support the notion that COPD is a systemic disease of accelerated aging and that replicative senescence, denoted by peripheral blood telomeres, is associated with poor health outcomes in COPD,” wrote Minhee Jin, of the University of British Columbia, Vancouver, and coauthors.
“It is now well established that replicative senescence results in a change of cellular phenotype to a proinflammatory state, a process that has been referred to as senescence-associated secretory phenotype,” they added.
The study also found that the median value for telomere length across the study participants – who had a mean age of 66 years – was equivalent to the expected value for someone in their 80s, “suggesting that on average MACRO participants were biologically much older than their chronological age.”
Researchers also noted that patients in the lowest quartile of telomere length had significantly lower forced vital capacity values, which suggested shorter telomeres could be a biomarker of restrictive physiology.
MACRO was funded by the U.S. National Heart, Lung, and Blood Institute, and the biomarker component of the study was funded by the Canadian Respiratory Research Network, Genome Canada, and the St. Paul’s Hospital Foundation. One author was an employee of GenomeDx Biosciences, three declared funding from or consultancies with the pharmaceutical industry. No other conflicts of interest were reported.
SOURCE: Jin M et al. Chest. 2018 Jul 12. doi: 10.1016/j.chest.2018.05.022.
according to a study published in Chest.
The evidence suggests that chronic obstructive pulmonary disease (COPD) may be a disease of accelerated aging, partly because of its relation to other senescence-related disorders such as osteoporosis and dementia, but also because it shows an exponential increase in prevalence in older age.
Telomere lengths are a measure of cellular senescence, and previous research has found that the telomeres are shortened in the peripheral leukocytes of patients with COPD, compared with healthy controls.
In this study, researchers examined the absolute telomere length of 576 people with moderate to severe COPD who were participating in the MACRO (Macrolide Azithromycin for Prevention of Exacerbations of COPD) study.
They found that individuals in the lowest quartile of telomere lengths had significantly worse health status and a higher exacerbation rate after accounting for treatment, compared with individuals in the higher quartile.
Patients with shorter telomere length had worse health status, as defined by higher St. George’s Respiratory Questionnaire scores. In the placebo arm of the study, the exacerbation rate (rate ratio, 1.50; 95% confidence interval, 1.16-1.95; P = .002) and mortality risk (hazard ratio, 9.45; 95% CI, 2.85-31.36; P = .015) were significantly higher in the shorter telomere group than in the longer telomere group; these differences were not observed in the azithromycin arm.
Patients with shorter telomeres also had a 800% higher risk of total mortality, compared with individuals with longer telomeres, although this was only evident in the placebo arm of the study, not the azithromycin arm. However, the authors noted that these data should be interpreted with caution because of the small number of deaths during the study.
“Together, these data support the notion that COPD is a systemic disease of accelerated aging and that replicative senescence, denoted by peripheral blood telomeres, is associated with poor health outcomes in COPD,” wrote Minhee Jin, of the University of British Columbia, Vancouver, and coauthors.
“It is now well established that replicative senescence results in a change of cellular phenotype to a proinflammatory state, a process that has been referred to as senescence-associated secretory phenotype,” they added.
The study also found that the median value for telomere length across the study participants – who had a mean age of 66 years – was equivalent to the expected value for someone in their 80s, “suggesting that on average MACRO participants were biologically much older than their chronological age.”
Researchers also noted that patients in the lowest quartile of telomere length had significantly lower forced vital capacity values, which suggested shorter telomeres could be a biomarker of restrictive physiology.
MACRO was funded by the U.S. National Heart, Lung, and Blood Institute, and the biomarker component of the study was funded by the Canadian Respiratory Research Network, Genome Canada, and the St. Paul’s Hospital Foundation. One author was an employee of GenomeDx Biosciences, three declared funding from or consultancies with the pharmaceutical industry. No other conflicts of interest were reported.
SOURCE: Jin M et al. Chest. 2018 Jul 12. doi: 10.1016/j.chest.2018.05.022.
FROM CHEST
Key clinical point: Shorter telomeres are linked to an increased risk of chronic obstructive pulmonary disease exacerbations.
Major finding: Patients with shorter telomeres had a 800% higher risk of total mortality, compared with individuals with longer telomeres.
Study details: Data from 576 patients with chronic obstructive pulmonary disease who participated in the MACRO study.
Disclosures: MACRO was funded by the U.S. National Heart, Lung, and Blood Institute, and the biomarker component of the study was funded by the Canadian Respiratory Research Network and the Canadian Institutes of Health Research Genome Canada, and the St. Paul’s Hospital Foundation. One author was an employee of GenomeDx Biosciences, and three authors declared funding from or consultancies with the pharmaceutical industry. No other conflicts of interest were reported.
Source: Jin M et al. Chest. 2018 Jul 12. doi: 10.1016/j.chest.2018.05.022.
FDA rejects mepolizumab on efficacy, but supports safety for COPD
Asthma drug mepolizumab could be added safely to inhaled corticosteroids for maintenance therapy to help reduce exacerbations in chronic obstructive pulmonary disease (COPD) patients who meet criteria for eosinophil counts, but the current data do not support its efficacy strongly enough for approval, according to a majority of members of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
The committee voted 16-3 that there was insufficient evidence of efficacy to support guided by eosinophil levels; they also voted 16-3 that the risk-benefit profile was not adequate to support approval.
However, on a voting question of safety, the committee voted 17-2 that the safety data on mepolizumab were sufficient to support approval.
Mepolizumab, a humanized monoclonal antibody, is currently approved for the treatment of asthma with eosinophilic phenotype for patients aged 12 years and older and for adults with eosinophilic granulomatosis with polyangiitis. Manufacturer GlaxoSmithKline is seeking approval for its use as an add-on therapy in COPD patients at a subcutaneous dose of 100 mg every 4 weeks. Mepolizumab works by binding to interleukin-5 (IL-5) and reducing eosinophil maturation and survival, which prompted GlaxoSmithKline to pursue an indication for COPD patients in a high-eosinophil stratum.
The application was supported in part by two concurrent randomized trials of 52 weeks’ duration.
Banu A. Karimi-Shah, MD, clinical team leader of the FDA’s Division of Pulmonary, Allergy, and Rheumatology Products, presented data from the two studies, referred to as Study 106 and Study 113.
In Study 106, researchers found statistically significant reductions in exacerbations for patients in the highest eosinophil group. However, challenges of the studies included a lack of consensus over the definition and possible relevance of an eosinophilic COPD phenotype, Dr. Karimi-Shah said in a presentation at the meeting.
In Study 113, mepolizumab had no significant impact on reducing moderate to severe exacerbations at either a 100-mg or 300-mg dose, Dr. Karimi-Shah said. In addition, most secondary endpoints, with the exception of reducing time to the first exacerbation among patients in the highest eosinophil group, did not consistently support the primary endpoint of exacerbation reduction in either study, she said.
Robert Busch, MD, also of the FDA’s Division of Pulmonary, Allergy, and Rheumatology products, served as a clinical reviewer and presented data on safety, efficacy, and risk-benefit profile of mepolizumab.
Dr. Busch noted that the variability in blood eosinophils make it challenging to use as a potential marker to identify patients who would benefit from mepolizumab as an add-on therapy.
Overall, most of the committee agreed on the existence of an eosinophilic COPD phenotype, but expressed concern about the threshold being used.
“The studies were not particularly well controlled regarding the characterization of patients,” said William J. Calhoun, MD, of the University of Texas Medical Branch, Galveston, who cast one of the ‘no’ votes on the question of efficacy.
By contrast, Jeffrey S. Wagener, MD, of the University of Colorado at Denver, Aurora, referenced his background in cystic fibrosis, and voted “yes” on the question of efficacy. “For patients that have no other option, this is a step forward,” he said.
Committee members on both sides of the vote emphasized the need for more research with larger numbers, better patient characterization, and more female patients. The committee members reported no relevant conflicts of interest.
Asthma drug mepolizumab could be added safely to inhaled corticosteroids for maintenance therapy to help reduce exacerbations in chronic obstructive pulmonary disease (COPD) patients who meet criteria for eosinophil counts, but the current data do not support its efficacy strongly enough for approval, according to a majority of members of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
The committee voted 16-3 that there was insufficient evidence of efficacy to support guided by eosinophil levels; they also voted 16-3 that the risk-benefit profile was not adequate to support approval.
However, on a voting question of safety, the committee voted 17-2 that the safety data on mepolizumab were sufficient to support approval.
Mepolizumab, a humanized monoclonal antibody, is currently approved for the treatment of asthma with eosinophilic phenotype for patients aged 12 years and older and for adults with eosinophilic granulomatosis with polyangiitis. Manufacturer GlaxoSmithKline is seeking approval for its use as an add-on therapy in COPD patients at a subcutaneous dose of 100 mg every 4 weeks. Mepolizumab works by binding to interleukin-5 (IL-5) and reducing eosinophil maturation and survival, which prompted GlaxoSmithKline to pursue an indication for COPD patients in a high-eosinophil stratum.
The application was supported in part by two concurrent randomized trials of 52 weeks’ duration.
Banu A. Karimi-Shah, MD, clinical team leader of the FDA’s Division of Pulmonary, Allergy, and Rheumatology Products, presented data from the two studies, referred to as Study 106 and Study 113.
In Study 106, researchers found statistically significant reductions in exacerbations for patients in the highest eosinophil group. However, challenges of the studies included a lack of consensus over the definition and possible relevance of an eosinophilic COPD phenotype, Dr. Karimi-Shah said in a presentation at the meeting.
In Study 113, mepolizumab had no significant impact on reducing moderate to severe exacerbations at either a 100-mg or 300-mg dose, Dr. Karimi-Shah said. In addition, most secondary endpoints, with the exception of reducing time to the first exacerbation among patients in the highest eosinophil group, did not consistently support the primary endpoint of exacerbation reduction in either study, she said.
Robert Busch, MD, also of the FDA’s Division of Pulmonary, Allergy, and Rheumatology products, served as a clinical reviewer and presented data on safety, efficacy, and risk-benefit profile of mepolizumab.
Dr. Busch noted that the variability in blood eosinophils make it challenging to use as a potential marker to identify patients who would benefit from mepolizumab as an add-on therapy.
Overall, most of the committee agreed on the existence of an eosinophilic COPD phenotype, but expressed concern about the threshold being used.
“The studies were not particularly well controlled regarding the characterization of patients,” said William J. Calhoun, MD, of the University of Texas Medical Branch, Galveston, who cast one of the ‘no’ votes on the question of efficacy.
By contrast, Jeffrey S. Wagener, MD, of the University of Colorado at Denver, Aurora, referenced his background in cystic fibrosis, and voted “yes” on the question of efficacy. “For patients that have no other option, this is a step forward,” he said.
Committee members on both sides of the vote emphasized the need for more research with larger numbers, better patient characterization, and more female patients. The committee members reported no relevant conflicts of interest.
Asthma drug mepolizumab could be added safely to inhaled corticosteroids for maintenance therapy to help reduce exacerbations in chronic obstructive pulmonary disease (COPD) patients who meet criteria for eosinophil counts, but the current data do not support its efficacy strongly enough for approval, according to a majority of members of the Food and Drug Administration’s Pulmonary-Allergy Drugs Advisory Committee.
The committee voted 16-3 that there was insufficient evidence of efficacy to support guided by eosinophil levels; they also voted 16-3 that the risk-benefit profile was not adequate to support approval.
However, on a voting question of safety, the committee voted 17-2 that the safety data on mepolizumab were sufficient to support approval.
Mepolizumab, a humanized monoclonal antibody, is currently approved for the treatment of asthma with eosinophilic phenotype for patients aged 12 years and older and for adults with eosinophilic granulomatosis with polyangiitis. Manufacturer GlaxoSmithKline is seeking approval for its use as an add-on therapy in COPD patients at a subcutaneous dose of 100 mg every 4 weeks. Mepolizumab works by binding to interleukin-5 (IL-5) and reducing eosinophil maturation and survival, which prompted GlaxoSmithKline to pursue an indication for COPD patients in a high-eosinophil stratum.
The application was supported in part by two concurrent randomized trials of 52 weeks’ duration.
Banu A. Karimi-Shah, MD, clinical team leader of the FDA’s Division of Pulmonary, Allergy, and Rheumatology Products, presented data from the two studies, referred to as Study 106 and Study 113.
In Study 106, researchers found statistically significant reductions in exacerbations for patients in the highest eosinophil group. However, challenges of the studies included a lack of consensus over the definition and possible relevance of an eosinophilic COPD phenotype, Dr. Karimi-Shah said in a presentation at the meeting.
In Study 113, mepolizumab had no significant impact on reducing moderate to severe exacerbations at either a 100-mg or 300-mg dose, Dr. Karimi-Shah said. In addition, most secondary endpoints, with the exception of reducing time to the first exacerbation among patients in the highest eosinophil group, did not consistently support the primary endpoint of exacerbation reduction in either study, she said.
Robert Busch, MD, also of the FDA’s Division of Pulmonary, Allergy, and Rheumatology products, served as a clinical reviewer and presented data on safety, efficacy, and risk-benefit profile of mepolizumab.
Dr. Busch noted that the variability in blood eosinophils make it challenging to use as a potential marker to identify patients who would benefit from mepolizumab as an add-on therapy.
Overall, most of the committee agreed on the existence of an eosinophilic COPD phenotype, but expressed concern about the threshold being used.
“The studies were not particularly well controlled regarding the characterization of patients,” said William J. Calhoun, MD, of the University of Texas Medical Branch, Galveston, who cast one of the ‘no’ votes on the question of efficacy.
By contrast, Jeffrey S. Wagener, MD, of the University of Colorado at Denver, Aurora, referenced his background in cystic fibrosis, and voted “yes” on the question of efficacy. “For patients that have no other option, this is a step forward,” he said.
Committee members on both sides of the vote emphasized the need for more research with larger numbers, better patient characterization, and more female patients. The committee members reported no relevant conflicts of interest.
FROM AN FDA ADVISORY COMMITTEE MEETING
Ten tips for managing patients with both heart failure and COPD
Patients with both are prone to hospital readmissions that detract from quality of life and dramatically drive up care costs.
Because the two chronic diseases spring from the same root cause and share overlapping symptoms, strategies that improve clinical outcomes in one can also benefit the other, Ravi Kalhan, MD, and R. Kannan Mutharasan, MD, wrote in CHEST Journal (doi: 10.1016/j.chest.2018.06.001).
“Both conditions are characterized by periods of clinical stability punctuated by episodes of exacerbation and are typified by gradual functional decline,” wrote the colleagues, both of Northwestern University, Chicago. “From a patient perspective, both conditions lead to highly overlapping patterns of symptoms, involve complicated medication regimens, and have courses highly sensitive to adherence and lifestyle modification. Therefore, disease management strategies for both conditions can be synergistic.”
The team came up with a “Top 10 list” of practical tips for reducing readmissions in patients with this challenging combination.
Diagnose accurately
An acute hospitalization is often the first time these patients pop up on the radar. This is a great time to employ spirometry to accurately diagnose COPD. It’s also appropriate to conduct a chest CT and check right heart size, diameter of the pulmonary artery, and the presence of coronary calcification. The authors noted that relatively little is known about the course of patients with combined asthma and HF in contrast to COPD and HF.
Detect admissions for exacerbations early
Check soon to find out if this is a readmission, get an acute plan going, and don’t wait to implement multidisciplinary interventions. “First, specialist involvement can occur more rapidly, allowing for faster identification of any root causes driving the HF or COPD syndromes, and allowing for more rapid institution of treatment plans to control the acute exacerbation. Second, early identification during hospitalization allows time to deploy multidisciplinary interventions, such as disease management education, social work evaluation, follow-up appointment scheduling, and coordination of home services. These interventions are less effective, and are often not implemented, if initiated toward the end of hospitalization.”
Use specialist management in the hospital
Get experts on board fast. An integrated team means a coordinated treatment plan that’s easier to follow and more effective therapeutically. Specialist care may impact rates of readmission: weight loss with diuretics; discharge doses of guideline-directed medical therapy for heart failure; and higher rates of discharge on long-acting beta-agonists, long-acting muscarinic antagonists, inhaled corticosteroids, and home supplemental oxygen.
Modify the underlying disease substrate
Heart failure is more likely to arise from a correctable pathophysiology, so find it early and treat it thoroughly – especially in younger patients. Ischemic heart disease, valvular heart disease, systemic hypertension, and pulmonary hypertension all have potential to make the HF syndrome more tractable.
Apply and intensify evidence-based therapies
Start in the hospital if possible; if not, begin upon discharge. “The order of application of these therapies can be bewildering, as many strategies for initiation and up-titration of these medications are reasonable. Not only are there long-term outcome benefits for these therapies, evidence suggests early initiation of HF therapies can reduce 30-day readmissions.”
Activate the patient and develop critical health behaviors
Medical regimens for these diseases can be complex, and they must be supported by patient engagement. “Many strategies for engaging patients in care have been tested, including teaching to goal, motivational interviewing, and teach-back methods of activation and engagement. Often these methods are time intensive. Because physician time is increasingly constrained, a team approach is particularly useful.”
Set up feedback loops
“Course correction should the patient decompensate is critically important to maintaining outpatient success. Feedback loops can allow for clinical stabilization before rehospitalization is necessary.” Self-monitoring with individually set benchmarks is critical.
Arrange an early follow-up appointment prior to discharge
About half of Medicare patients with these conditions are readmitted before they’ve even had a postdischarge follow-up appointment. Ideally this should occur within 7 days. The purpose of early follow-up is to identify and address gaps in the discharge plan of care, revise the discharge plan of care to adapt to the outpatient environment, and reinforce critical health behaviors.
Consider and address other comorbidities
Comorbidities are the rule rather than the exception and contribute to many readmissions. Get primary care on the team and enlist their help in managing these issues before they lead to an exacerbation. “Meticulous control – even perfect control were it possible – of cardiopulmonary disease would still leave patients vulnerable to significant risk of readmission from other causes.”
Consider ancillary supportive services at home
Patients may be overwhelmed by the complexity of postdischarge care. Home health assistance can help in getting patients to physical therapy, continuing patient education, and providing a home clinical assessment.
Neither of the authors had any financial disclosures.
Patients with both are prone to hospital readmissions that detract from quality of life and dramatically drive up care costs.
Because the two chronic diseases spring from the same root cause and share overlapping symptoms, strategies that improve clinical outcomes in one can also benefit the other, Ravi Kalhan, MD, and R. Kannan Mutharasan, MD, wrote in CHEST Journal (doi: 10.1016/j.chest.2018.06.001).
“Both conditions are characterized by periods of clinical stability punctuated by episodes of exacerbation and are typified by gradual functional decline,” wrote the colleagues, both of Northwestern University, Chicago. “From a patient perspective, both conditions lead to highly overlapping patterns of symptoms, involve complicated medication regimens, and have courses highly sensitive to adherence and lifestyle modification. Therefore, disease management strategies for both conditions can be synergistic.”
The team came up with a “Top 10 list” of practical tips for reducing readmissions in patients with this challenging combination.
Diagnose accurately
An acute hospitalization is often the first time these patients pop up on the radar. This is a great time to employ spirometry to accurately diagnose COPD. It’s also appropriate to conduct a chest CT and check right heart size, diameter of the pulmonary artery, and the presence of coronary calcification. The authors noted that relatively little is known about the course of patients with combined asthma and HF in contrast to COPD and HF.
Detect admissions for exacerbations early
Check soon to find out if this is a readmission, get an acute plan going, and don’t wait to implement multidisciplinary interventions. “First, specialist involvement can occur more rapidly, allowing for faster identification of any root causes driving the HF or COPD syndromes, and allowing for more rapid institution of treatment plans to control the acute exacerbation. Second, early identification during hospitalization allows time to deploy multidisciplinary interventions, such as disease management education, social work evaluation, follow-up appointment scheduling, and coordination of home services. These interventions are less effective, and are often not implemented, if initiated toward the end of hospitalization.”
Use specialist management in the hospital
Get experts on board fast. An integrated team means a coordinated treatment plan that’s easier to follow and more effective therapeutically. Specialist care may impact rates of readmission: weight loss with diuretics; discharge doses of guideline-directed medical therapy for heart failure; and higher rates of discharge on long-acting beta-agonists, long-acting muscarinic antagonists, inhaled corticosteroids, and home supplemental oxygen.
Modify the underlying disease substrate
Heart failure is more likely to arise from a correctable pathophysiology, so find it early and treat it thoroughly – especially in younger patients. Ischemic heart disease, valvular heart disease, systemic hypertension, and pulmonary hypertension all have potential to make the HF syndrome more tractable.
Apply and intensify evidence-based therapies
Start in the hospital if possible; if not, begin upon discharge. “The order of application of these therapies can be bewildering, as many strategies for initiation and up-titration of these medications are reasonable. Not only are there long-term outcome benefits for these therapies, evidence suggests early initiation of HF therapies can reduce 30-day readmissions.”
Activate the patient and develop critical health behaviors
Medical regimens for these diseases can be complex, and they must be supported by patient engagement. “Many strategies for engaging patients in care have been tested, including teaching to goal, motivational interviewing, and teach-back methods of activation and engagement. Often these methods are time intensive. Because physician time is increasingly constrained, a team approach is particularly useful.”
Set up feedback loops
“Course correction should the patient decompensate is critically important to maintaining outpatient success. Feedback loops can allow for clinical stabilization before rehospitalization is necessary.” Self-monitoring with individually set benchmarks is critical.
Arrange an early follow-up appointment prior to discharge
About half of Medicare patients with these conditions are readmitted before they’ve even had a postdischarge follow-up appointment. Ideally this should occur within 7 days. The purpose of early follow-up is to identify and address gaps in the discharge plan of care, revise the discharge plan of care to adapt to the outpatient environment, and reinforce critical health behaviors.
Consider and address other comorbidities
Comorbidities are the rule rather than the exception and contribute to many readmissions. Get primary care on the team and enlist their help in managing these issues before they lead to an exacerbation. “Meticulous control – even perfect control were it possible – of cardiopulmonary disease would still leave patients vulnerable to significant risk of readmission from other causes.”
Consider ancillary supportive services at home
Patients may be overwhelmed by the complexity of postdischarge care. Home health assistance can help in getting patients to physical therapy, continuing patient education, and providing a home clinical assessment.
Neither of the authors had any financial disclosures.
Patients with both are prone to hospital readmissions that detract from quality of life and dramatically drive up care costs.
Because the two chronic diseases spring from the same root cause and share overlapping symptoms, strategies that improve clinical outcomes in one can also benefit the other, Ravi Kalhan, MD, and R. Kannan Mutharasan, MD, wrote in CHEST Journal (doi: 10.1016/j.chest.2018.06.001).
“Both conditions are characterized by periods of clinical stability punctuated by episodes of exacerbation and are typified by gradual functional decline,” wrote the colleagues, both of Northwestern University, Chicago. “From a patient perspective, both conditions lead to highly overlapping patterns of symptoms, involve complicated medication regimens, and have courses highly sensitive to adherence and lifestyle modification. Therefore, disease management strategies for both conditions can be synergistic.”
The team came up with a “Top 10 list” of practical tips for reducing readmissions in patients with this challenging combination.
Diagnose accurately
An acute hospitalization is often the first time these patients pop up on the radar. This is a great time to employ spirometry to accurately diagnose COPD. It’s also appropriate to conduct a chest CT and check right heart size, diameter of the pulmonary artery, and the presence of coronary calcification. The authors noted that relatively little is known about the course of patients with combined asthma and HF in contrast to COPD and HF.
Detect admissions for exacerbations early
Check soon to find out if this is a readmission, get an acute plan going, and don’t wait to implement multidisciplinary interventions. “First, specialist involvement can occur more rapidly, allowing for faster identification of any root causes driving the HF or COPD syndromes, and allowing for more rapid institution of treatment plans to control the acute exacerbation. Second, early identification during hospitalization allows time to deploy multidisciplinary interventions, such as disease management education, social work evaluation, follow-up appointment scheduling, and coordination of home services. These interventions are less effective, and are often not implemented, if initiated toward the end of hospitalization.”
Use specialist management in the hospital
Get experts on board fast. An integrated team means a coordinated treatment plan that’s easier to follow and more effective therapeutically. Specialist care may impact rates of readmission: weight loss with diuretics; discharge doses of guideline-directed medical therapy for heart failure; and higher rates of discharge on long-acting beta-agonists, long-acting muscarinic antagonists, inhaled corticosteroids, and home supplemental oxygen.
Modify the underlying disease substrate
Heart failure is more likely to arise from a correctable pathophysiology, so find it early and treat it thoroughly – especially in younger patients. Ischemic heart disease, valvular heart disease, systemic hypertension, and pulmonary hypertension all have potential to make the HF syndrome more tractable.
Apply and intensify evidence-based therapies
Start in the hospital if possible; if not, begin upon discharge. “The order of application of these therapies can be bewildering, as many strategies for initiation and up-titration of these medications are reasonable. Not only are there long-term outcome benefits for these therapies, evidence suggests early initiation of HF therapies can reduce 30-day readmissions.”
Activate the patient and develop critical health behaviors
Medical regimens for these diseases can be complex, and they must be supported by patient engagement. “Many strategies for engaging patients in care have been tested, including teaching to goal, motivational interviewing, and teach-back methods of activation and engagement. Often these methods are time intensive. Because physician time is increasingly constrained, a team approach is particularly useful.”
Set up feedback loops
“Course correction should the patient decompensate is critically important to maintaining outpatient success. Feedback loops can allow for clinical stabilization before rehospitalization is necessary.” Self-monitoring with individually set benchmarks is critical.
Arrange an early follow-up appointment prior to discharge
About half of Medicare patients with these conditions are readmitted before they’ve even had a postdischarge follow-up appointment. Ideally this should occur within 7 days. The purpose of early follow-up is to identify and address gaps in the discharge plan of care, revise the discharge plan of care to adapt to the outpatient environment, and reinforce critical health behaviors.
Consider and address other comorbidities
Comorbidities are the rule rather than the exception and contribute to many readmissions. Get primary care on the team and enlist their help in managing these issues before they lead to an exacerbation. “Meticulous control – even perfect control were it possible – of cardiopulmonary disease would still leave patients vulnerable to significant risk of readmission from other causes.”
Consider ancillary supportive services at home
Patients may be overwhelmed by the complexity of postdischarge care. Home health assistance can help in getting patients to physical therapy, continuing patient education, and providing a home clinical assessment.
Neither of the authors had any financial disclosures.
EXPERT ANALYSIS FROM CHEST JOURNAL
Medicare’s bundled pay plan didn’t deliver big cost savings
Participation in Medicare’s bundled payments initiative didn’t significantly change payments per episode or care outcomes for the top five medical conditions selected under the program, a new analysis shows.
Payments for the common conditions remained around $24,000 per episode before and during participation in the Bundled Payments for Care Improvement (BPCI) initiative for the 125 participating hospitals evaluated in this study, conducted by Karen E. Joynt Maddox, MD, of Washington University, St. Louis, and her coauthors.
The finding contrasts with a previous study showing that hospitals in BPCI successfully lowered overall Medicare payments for patients who underwent joint replacement.
“Bundling of services to encourage more efficient care has great face validity and enjoys bipartisan support,” Dr. Joynt Maddox and her colleagues wrote. “For such bundling to work for medical conditions, however, more time, new care strategies and partnerships, or additional incentives may be required.”
The Center for Medicare & Medicaid Innovation initiated the voluntary BPCI demonstration project in 2013. The program targets 48 conditions that account for about 70% of Medicare spending. Hospitals that achieve cost targets for a specific condition get to keep a portion of the savings, and they reimburse Medicare for part of the difference when costs are exceeded.
The present study focused on 2013-2015 Medicare claims for the five medical conditions that account for two-thirds of patients enrolled in medical bundles: congestive heart failure, pneumonia, chronic obstructive pulmonary disease, sepsis, and acute myocardial infarction.
Mean baseline payments per episode for those conditions were $24,280 before participation in the BPCI. After hospitals joined, their average payments per episode were $23,993 (P = .41). For a set of matched control hospitals, payments were a mean of $23,901 at baseline and $23,503 in the corresponding follow-up period (P = .08).
That amounted to a $286 payment reduction for BPCI hospitals and a $398 reduction for controls, a difference of $112 (P = .79), the study investigators reported.
Changes in length of stay, readmissions, emergency department use, and clinical complexity of cases from baseline to follow-up periods was not significantly different between BPCI and control hospitals. For example, 90-day mortality increases were seen in both groups, and the degree of increase was not statistically different between the groups.
Those data help fill a gap in research on the BPCI program and BPCI Advanced, a related version of the demonstration project that will have its first cohort of participants starting Oct. 1, 2018.
“Despite the importance of episode-based payment, there has been little research examining its efficacy or determining whether it has unintended consequences, such as hospitals’ selecting patients with relatively less complex conditions to reduce costs and improve outcomes,” Dr. Joynt Maddox and her colleagues cautioned.
It’s unclear why the previous joint replacement study showed a successful reduction in costs under BPCI, while the new study did not. However, patients in the new analysis of the most common bundled conditions were older and had higher rates of poverty and disability.
“As a result of these complexities, patients admitted for medical conditions may have had post-acute care needs that were less amenable to intervention,” Dr. Joynt Maddox said.
The investigators added that hospitals’ lack of effective influence on post–acute-care services may blunt their ability to achieve greater savings under BPCI. Better relationships with skilled nursing facilities, long-term care hospitals, home health agencies, and inpatient rehabilitation facilities could make a difference.
The Commonwealth Fund supported the study. One study author reported personal fees from HHS outside the submitted work, and another reported that he is an associate editor for the New England Journal of Medicine. No other disclosures were reported.
SOURCE: Joynt Maddox KE et al. N Engl J Med. 2018 Jul 19;379(3):260-9.
Participation in Medicare’s bundled payments initiative didn’t significantly change payments per episode or care outcomes for the top five medical conditions selected under the program, a new analysis shows.
Payments for the common conditions remained around $24,000 per episode before and during participation in the Bundled Payments for Care Improvement (BPCI) initiative for the 125 participating hospitals evaluated in this study, conducted by Karen E. Joynt Maddox, MD, of Washington University, St. Louis, and her coauthors.
The finding contrasts with a previous study showing that hospitals in BPCI successfully lowered overall Medicare payments for patients who underwent joint replacement.
“Bundling of services to encourage more efficient care has great face validity and enjoys bipartisan support,” Dr. Joynt Maddox and her colleagues wrote. “For such bundling to work for medical conditions, however, more time, new care strategies and partnerships, or additional incentives may be required.”
The Center for Medicare & Medicaid Innovation initiated the voluntary BPCI demonstration project in 2013. The program targets 48 conditions that account for about 70% of Medicare spending. Hospitals that achieve cost targets for a specific condition get to keep a portion of the savings, and they reimburse Medicare for part of the difference when costs are exceeded.
The present study focused on 2013-2015 Medicare claims for the five medical conditions that account for two-thirds of patients enrolled in medical bundles: congestive heart failure, pneumonia, chronic obstructive pulmonary disease, sepsis, and acute myocardial infarction.
Mean baseline payments per episode for those conditions were $24,280 before participation in the BPCI. After hospitals joined, their average payments per episode were $23,993 (P = .41). For a set of matched control hospitals, payments were a mean of $23,901 at baseline and $23,503 in the corresponding follow-up period (P = .08).
That amounted to a $286 payment reduction for BPCI hospitals and a $398 reduction for controls, a difference of $112 (P = .79), the study investigators reported.
Changes in length of stay, readmissions, emergency department use, and clinical complexity of cases from baseline to follow-up periods was not significantly different between BPCI and control hospitals. For example, 90-day mortality increases were seen in both groups, and the degree of increase was not statistically different between the groups.
Those data help fill a gap in research on the BPCI program and BPCI Advanced, a related version of the demonstration project that will have its first cohort of participants starting Oct. 1, 2018.
“Despite the importance of episode-based payment, there has been little research examining its efficacy or determining whether it has unintended consequences, such as hospitals’ selecting patients with relatively less complex conditions to reduce costs and improve outcomes,” Dr. Joynt Maddox and her colleagues cautioned.
It’s unclear why the previous joint replacement study showed a successful reduction in costs under BPCI, while the new study did not. However, patients in the new analysis of the most common bundled conditions were older and had higher rates of poverty and disability.
“As a result of these complexities, patients admitted for medical conditions may have had post-acute care needs that were less amenable to intervention,” Dr. Joynt Maddox said.
The investigators added that hospitals’ lack of effective influence on post–acute-care services may blunt their ability to achieve greater savings under BPCI. Better relationships with skilled nursing facilities, long-term care hospitals, home health agencies, and inpatient rehabilitation facilities could make a difference.
The Commonwealth Fund supported the study. One study author reported personal fees from HHS outside the submitted work, and another reported that he is an associate editor for the New England Journal of Medicine. No other disclosures were reported.
SOURCE: Joynt Maddox KE et al. N Engl J Med. 2018 Jul 19;379(3):260-9.
Participation in Medicare’s bundled payments initiative didn’t significantly change payments per episode or care outcomes for the top five medical conditions selected under the program, a new analysis shows.
Payments for the common conditions remained around $24,000 per episode before and during participation in the Bundled Payments for Care Improvement (BPCI) initiative for the 125 participating hospitals evaluated in this study, conducted by Karen E. Joynt Maddox, MD, of Washington University, St. Louis, and her coauthors.
The finding contrasts with a previous study showing that hospitals in BPCI successfully lowered overall Medicare payments for patients who underwent joint replacement.
“Bundling of services to encourage more efficient care has great face validity and enjoys bipartisan support,” Dr. Joynt Maddox and her colleagues wrote. “For such bundling to work for medical conditions, however, more time, new care strategies and partnerships, or additional incentives may be required.”
The Center for Medicare & Medicaid Innovation initiated the voluntary BPCI demonstration project in 2013. The program targets 48 conditions that account for about 70% of Medicare spending. Hospitals that achieve cost targets for a specific condition get to keep a portion of the savings, and they reimburse Medicare for part of the difference when costs are exceeded.
The present study focused on 2013-2015 Medicare claims for the five medical conditions that account for two-thirds of patients enrolled in medical bundles: congestive heart failure, pneumonia, chronic obstructive pulmonary disease, sepsis, and acute myocardial infarction.
Mean baseline payments per episode for those conditions were $24,280 before participation in the BPCI. After hospitals joined, their average payments per episode were $23,993 (P = .41). For a set of matched control hospitals, payments were a mean of $23,901 at baseline and $23,503 in the corresponding follow-up period (P = .08).
That amounted to a $286 payment reduction for BPCI hospitals and a $398 reduction for controls, a difference of $112 (P = .79), the study investigators reported.
Changes in length of stay, readmissions, emergency department use, and clinical complexity of cases from baseline to follow-up periods was not significantly different between BPCI and control hospitals. For example, 90-day mortality increases were seen in both groups, and the degree of increase was not statistically different between the groups.
Those data help fill a gap in research on the BPCI program and BPCI Advanced, a related version of the demonstration project that will have its first cohort of participants starting Oct. 1, 2018.
“Despite the importance of episode-based payment, there has been little research examining its efficacy or determining whether it has unintended consequences, such as hospitals’ selecting patients with relatively less complex conditions to reduce costs and improve outcomes,” Dr. Joynt Maddox and her colleagues cautioned.
It’s unclear why the previous joint replacement study showed a successful reduction in costs under BPCI, while the new study did not. However, patients in the new analysis of the most common bundled conditions were older and had higher rates of poverty and disability.
“As a result of these complexities, patients admitted for medical conditions may have had post-acute care needs that were less amenable to intervention,” Dr. Joynt Maddox said.
The investigators added that hospitals’ lack of effective influence on post–acute-care services may blunt their ability to achieve greater savings under BPCI. Better relationships with skilled nursing facilities, long-term care hospitals, home health agencies, and inpatient rehabilitation facilities could make a difference.
The Commonwealth Fund supported the study. One study author reported personal fees from HHS outside the submitted work, and another reported that he is an associate editor for the New England Journal of Medicine. No other disclosures were reported.
SOURCE: Joynt Maddox KE et al. N Engl J Med. 2018 Jul 19;379(3):260-9.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Participation in Medicare’s Bundled Payments for Care Improvement (BPCI) initiative didn’t significantly change payments per episode for the top five medical conditions selected under the program.
Major finding: Baseline payments per episode for those conditions were a mean of $24,280 before participation in the BPCI, and $23,993 after adoption (P = .41).
Study details: A retrospective analysis of Medicare data for 125 hospitals participating in the program and matched control hospitals.
Disclosures: The Commonwealth Fund supported the study. One study author reported personal fees from HHS outside the submitted work, and another reported that he is an associate editor for the New England Journal of Medicine.
Source: Joynt Maddox KE et al. N Engl J Med. 2018 Jul 19;379(3):260-9.
Noninvasive ventilation during exercise benefited a subgroup of COPD patients
SAN DIEGO – Use of noninvasive ventilation during an exercise session in hypercapnic patients with very severe chronic obstructive pulmonary disease (COPD) led to a clinically relevant increase in endurance time, a randomized trial showed.
At an international conference of the American Thoracic Society, lead study author Tessa Schneeberger noted that nocturnal noninvasive ventilation (NIV) in hypercapnic COPD patients has been shown to improve quality of life and survival (Lancet Resp Med. 2014;2[9]:698-705). Another study found that NIV with unchanged nocturnal settings during a 6-minute walk test in hypercapnic COPD patients can increase oxygenation, decrease dyspnea, and increase walking distance (Eur Respir J. 2007;29:930-6).
For the current study, Ms. Schneeberger, a physiotherapist at the Institute for Pulmonary Rehabilitation Research, Schoenau am Koenigssee, Germany, and her associates set out to investigate short-term effects of using NIV during exercise in hypercapnic patients with very severe COPD, as part of a 3-week inpatient physical rehabilitation program. The researchers limited their analysis to 20 Global Initiative for Chronic Obstructive Lung Disease stage IV patients aged 40-80 years with a carbon dioxide partial pressure (PCO2) of greater than 50 mm Hg at rest and/or during exercise and who were non-naive to NIV, and excluded patients with concomitant conditions that make cycling impossible, those with acute exacerbations, and those with exercise-limiting cardiovascular diseases.
The day after an initial incremental cycle ergometer test, patients performed two constant work rate tests (CWRT) at 60% of the peak work rate, with and without NIV, in randomized order and with a resting time of 1 hour between tests. The inspiratory positive airway pressure (IPAP) was individually adjusted from each patient’s nocturnal settings to provide sufficient pressure to relieve the work on breathing muscles and to decrease transcutaneous PCO2 (TcPCO2) levels during NIV. The primary outcome was cycle endurance time. Other outcomes of interest were TcPCO2, oxygen saturation (SpO2) and perceived dyspnea/leg fatigue via the 10-point Borg scale during CWRTs.
The mean age of the study participants was 60 years, their mean body mass index was 23 kg/m2, their mean forced expiratory volume in1 second was 19% predicted, their mean PaCO2 was 51 mm Hg, their mean PaO2 was 54.5 mm Hg, their mean distance on the 6-minute walk test was 243 meters, and their mean peak work rate was 42 watts.
NIV via full face mask and assisted pressure control ventilation mode was performed with mean IPAP/expiratory PAP levels of 27/6 cm H2O.
During CWRTs patients cycled with NIV for 663 seconds and without NIV for 476 seconds, a significant difference (P = .013) and one that was clinically relevant. At isotime (the time of CWRT with shortest duration), TcPCO2 was significantly lower with NIV (a mean of –6.1 mm Hg), while SpO2 was significantly higher with NIV (a mean of 3.6%). In addition, after CWRT, NIV patients perceived less dyspnea (P = .008) with comparable leg fatigue (P = .79).
“We found that NIV during cycling exercise in hypercapnic patients with very severe COPD can lead to an acutely significant increase in exercise duration, with lower TcPCO2 and a reduced sensation of dyspnea,” Ms. Schneeberger concluded. “It can be performed with high-pressure assisted-controlled ventilation comparable as that used nocturnally to effectively reduce TcPCO2 in people with COPD.”
She emphasized that this approach requires appropriate equipment and special staff expertise for setup titration. “We will continue this research to look into the underlying physiological mechanisms to define nonresponders and responders, and also to look how at this might improve outcomes of an exercise training program.”
Ms. Schneeberger reported having no financial disclosures.
SOURCE: Schneeberger T et al. ATS 2018, Abstract A2453.
SAN DIEGO – Use of noninvasive ventilation during an exercise session in hypercapnic patients with very severe chronic obstructive pulmonary disease (COPD) led to a clinically relevant increase in endurance time, a randomized trial showed.
At an international conference of the American Thoracic Society, lead study author Tessa Schneeberger noted that nocturnal noninvasive ventilation (NIV) in hypercapnic COPD patients has been shown to improve quality of life and survival (Lancet Resp Med. 2014;2[9]:698-705). Another study found that NIV with unchanged nocturnal settings during a 6-minute walk test in hypercapnic COPD patients can increase oxygenation, decrease dyspnea, and increase walking distance (Eur Respir J. 2007;29:930-6).
For the current study, Ms. Schneeberger, a physiotherapist at the Institute for Pulmonary Rehabilitation Research, Schoenau am Koenigssee, Germany, and her associates set out to investigate short-term effects of using NIV during exercise in hypercapnic patients with very severe COPD, as part of a 3-week inpatient physical rehabilitation program. The researchers limited their analysis to 20 Global Initiative for Chronic Obstructive Lung Disease stage IV patients aged 40-80 years with a carbon dioxide partial pressure (PCO2) of greater than 50 mm Hg at rest and/or during exercise and who were non-naive to NIV, and excluded patients with concomitant conditions that make cycling impossible, those with acute exacerbations, and those with exercise-limiting cardiovascular diseases.
The day after an initial incremental cycle ergometer test, patients performed two constant work rate tests (CWRT) at 60% of the peak work rate, with and without NIV, in randomized order and with a resting time of 1 hour between tests. The inspiratory positive airway pressure (IPAP) was individually adjusted from each patient’s nocturnal settings to provide sufficient pressure to relieve the work on breathing muscles and to decrease transcutaneous PCO2 (TcPCO2) levels during NIV. The primary outcome was cycle endurance time. Other outcomes of interest were TcPCO2, oxygen saturation (SpO2) and perceived dyspnea/leg fatigue via the 10-point Borg scale during CWRTs.
The mean age of the study participants was 60 years, their mean body mass index was 23 kg/m2, their mean forced expiratory volume in1 second was 19% predicted, their mean PaCO2 was 51 mm Hg, their mean PaO2 was 54.5 mm Hg, their mean distance on the 6-minute walk test was 243 meters, and their mean peak work rate was 42 watts.
NIV via full face mask and assisted pressure control ventilation mode was performed with mean IPAP/expiratory PAP levels of 27/6 cm H2O.
During CWRTs patients cycled with NIV for 663 seconds and without NIV for 476 seconds, a significant difference (P = .013) and one that was clinically relevant. At isotime (the time of CWRT with shortest duration), TcPCO2 was significantly lower with NIV (a mean of –6.1 mm Hg), while SpO2 was significantly higher with NIV (a mean of 3.6%). In addition, after CWRT, NIV patients perceived less dyspnea (P = .008) with comparable leg fatigue (P = .79).
“We found that NIV during cycling exercise in hypercapnic patients with very severe COPD can lead to an acutely significant increase in exercise duration, with lower TcPCO2 and a reduced sensation of dyspnea,” Ms. Schneeberger concluded. “It can be performed with high-pressure assisted-controlled ventilation comparable as that used nocturnally to effectively reduce TcPCO2 in people with COPD.”
She emphasized that this approach requires appropriate equipment and special staff expertise for setup titration. “We will continue this research to look into the underlying physiological mechanisms to define nonresponders and responders, and also to look how at this might improve outcomes of an exercise training program.”
Ms. Schneeberger reported having no financial disclosures.
SOURCE: Schneeberger T et al. ATS 2018, Abstract A2453.
SAN DIEGO – Use of noninvasive ventilation during an exercise session in hypercapnic patients with very severe chronic obstructive pulmonary disease (COPD) led to a clinically relevant increase in endurance time, a randomized trial showed.
At an international conference of the American Thoracic Society, lead study author Tessa Schneeberger noted that nocturnal noninvasive ventilation (NIV) in hypercapnic COPD patients has been shown to improve quality of life and survival (Lancet Resp Med. 2014;2[9]:698-705). Another study found that NIV with unchanged nocturnal settings during a 6-minute walk test in hypercapnic COPD patients can increase oxygenation, decrease dyspnea, and increase walking distance (Eur Respir J. 2007;29:930-6).
For the current study, Ms. Schneeberger, a physiotherapist at the Institute for Pulmonary Rehabilitation Research, Schoenau am Koenigssee, Germany, and her associates set out to investigate short-term effects of using NIV during exercise in hypercapnic patients with very severe COPD, as part of a 3-week inpatient physical rehabilitation program. The researchers limited their analysis to 20 Global Initiative for Chronic Obstructive Lung Disease stage IV patients aged 40-80 years with a carbon dioxide partial pressure (PCO2) of greater than 50 mm Hg at rest and/or during exercise and who were non-naive to NIV, and excluded patients with concomitant conditions that make cycling impossible, those with acute exacerbations, and those with exercise-limiting cardiovascular diseases.
The day after an initial incremental cycle ergometer test, patients performed two constant work rate tests (CWRT) at 60% of the peak work rate, with and without NIV, in randomized order and with a resting time of 1 hour between tests. The inspiratory positive airway pressure (IPAP) was individually adjusted from each patient’s nocturnal settings to provide sufficient pressure to relieve the work on breathing muscles and to decrease transcutaneous PCO2 (TcPCO2) levels during NIV. The primary outcome was cycle endurance time. Other outcomes of interest were TcPCO2, oxygen saturation (SpO2) and perceived dyspnea/leg fatigue via the 10-point Borg scale during CWRTs.
The mean age of the study participants was 60 years, their mean body mass index was 23 kg/m2, their mean forced expiratory volume in1 second was 19% predicted, their mean PaCO2 was 51 mm Hg, their mean PaO2 was 54.5 mm Hg, their mean distance on the 6-minute walk test was 243 meters, and their mean peak work rate was 42 watts.
NIV via full face mask and assisted pressure control ventilation mode was performed with mean IPAP/expiratory PAP levels of 27/6 cm H2O.
During CWRTs patients cycled with NIV for 663 seconds and without NIV for 476 seconds, a significant difference (P = .013) and one that was clinically relevant. At isotime (the time of CWRT with shortest duration), TcPCO2 was significantly lower with NIV (a mean of –6.1 mm Hg), while SpO2 was significantly higher with NIV (a mean of 3.6%). In addition, after CWRT, NIV patients perceived less dyspnea (P = .008) with comparable leg fatigue (P = .79).
“We found that NIV during cycling exercise in hypercapnic patients with very severe COPD can lead to an acutely significant increase in exercise duration, with lower TcPCO2 and a reduced sensation of dyspnea,” Ms. Schneeberger concluded. “It can be performed with high-pressure assisted-controlled ventilation comparable as that used nocturnally to effectively reduce TcPCO2 in people with COPD.”
She emphasized that this approach requires appropriate equipment and special staff expertise for setup titration. “We will continue this research to look into the underlying physiological mechanisms to define nonresponders and responders, and also to look how at this might improve outcomes of an exercise training program.”
Ms. Schneeberger reported having no financial disclosures.
SOURCE: Schneeberger T et al. ATS 2018, Abstract A2453.
REPORTING FROM ATS 2018
Key clinical point: Noninvasive ventilation (NIV) during exercise seems feasible and could provide an opportunity to improve endurance training outcomes in selected chronic obstructive pulmonary disease patients.
Major finding: During constant work rate tests patients cycled with NIV for 663 seconds and without NIV for 476 seconds, a significant difference (P = .013).
Study details: A randomized trial of short-term effects of NIV during exercise in 20 hypercapnic patients with very severe COPD.
Disclosures: Ms. Schneeberger reported having no financial disclosures.
Source: Schneeberger T et al. ATS 2018, Abstract A2453.
Lung volume reduction procedures on the rise since 2011
SAN DIEGO – The number of has been increasing since 2011, according to a large national analysis.
Lung volume–reduction surgery (LVRS) has been available for decades, but results from the National Emphysema Treatment Trial (NETT) in 2003 (N Engl J Med 2003;348:2059-73) demonstrated that certain COPD patients benefited from the surgery, Amy Attaway, MD, said at an international conference of the American Thoracic Society.
In that trial, overall mortality at 30 days was 3.6% for the surgical group. “If you excluded high-risk patients, the 30-day mortality was only 2.2%,” said Dr. Attaway, a staff physician at the Cleveland Clinic Respiratory Institute. “If you look at the upper lobe–predominant, low-exercise group, their mortality at 30 days was 1.4%.”
Subsequent studies that evaluated NETT patients over time showed continued improvements in their mortality. For example, one study found that in the upper lobe–predominant patients who received surgery in the NETT trial, their survival at 3 years was 81% vs. 74% in the medical group (P = .05), while survival at 5 years was 70% in the surgery group vs. 60% in the medical group (P = .02; J Thorac Cardiovasc Surg. 2010;140[3]:564-72). Despite this improved mortality, other studies have shown that LVRS remains underutilized. Once such analysis of the Nationwide Inpatient Sample showed a logarithmic drop from 2000 to 2010 (Chest 2014;146[6]:e228-9). The authors also noted that the overall mortality was 6% and that the need for a tracheostomy was 7.9%. Age greater than 65 years was associated with increased mortality (odds ratio 2.8).
Dr. Attaway and her associates hypothesized that availability of the long-term survival data from the NETT, support from GOLD guidelines, and the lack of a Food and Drug Administration–approved alternative may have increased utilization of this surgery from 2007 through 2013. With data from the Nationwide Inpatient Sample for 2007-2013, the researchers performed a retrospective cohort analysis of 2,805 patients who underwent LVRS. The composite primary outcome was mortality or need for tracheostomy. Logistic regression was performed to analyze factors associated with the composite primary outcome.
The average patient age was 59 years, and 64% were male. Medicare was the payer in nearly half of cases, in-hospital mortality and need for tracheostomy were both 5.5%, and the risk for tracheostomy or mortality was 10.5%. Linear regression analysis showed a significant increase in LVRS over time, with the 320 surgeries in 2007 and 605 in 2013 (P = .0016).
On univariate analysis, the following factors were found to be significantly associated with the composite primary outcome: in-hospital mortality or the need for tracheostomy (P less than .001), respiratory failure (P less than .001), septicemia (P = .01), shock (P less than .001), acute kidney injury (P less than .001), secondary pulmonary hypertension (P less than .001), and a higher mean number of diagnoses or number of chronic conditions on admission (P less than .001 and P = .017, respectively).
On multivariate logistic regression, only two factors were found to be significantly associated with the composite primary outcome: a higher number of diagnoses (adjusted OR of 1.17 per additional diagnosis), and the presence of secondary pulmonary hypertension (adjusted OR 4.4).
“Availability of long-term survival data from NETT, support from the GOLD guidelines, and lack of current FDA-approved alternatives are potential reasons for increased utilization [of LVRS],” Dr. Attaway said. “However, our study showed that in-hospital mortality and morbidity is high, compared to the NETT results. We also found that secondary pulmonary hypertension and comorbidities are associated with poor outcomes. This is important to keep in mind for patient selection.”
Dr. Attaway and her associates reported having no financial disclosures.
SOURCE: Attaway A et al. ATS 2018, Abstract 4436.
SAN DIEGO – The number of has been increasing since 2011, according to a large national analysis.
Lung volume–reduction surgery (LVRS) has been available for decades, but results from the National Emphysema Treatment Trial (NETT) in 2003 (N Engl J Med 2003;348:2059-73) demonstrated that certain COPD patients benefited from the surgery, Amy Attaway, MD, said at an international conference of the American Thoracic Society.
In that trial, overall mortality at 30 days was 3.6% for the surgical group. “If you excluded high-risk patients, the 30-day mortality was only 2.2%,” said Dr. Attaway, a staff physician at the Cleveland Clinic Respiratory Institute. “If you look at the upper lobe–predominant, low-exercise group, their mortality at 30 days was 1.4%.”
Subsequent studies that evaluated NETT patients over time showed continued improvements in their mortality. For example, one study found that in the upper lobe–predominant patients who received surgery in the NETT trial, their survival at 3 years was 81% vs. 74% in the medical group (P = .05), while survival at 5 years was 70% in the surgery group vs. 60% in the medical group (P = .02; J Thorac Cardiovasc Surg. 2010;140[3]:564-72). Despite this improved mortality, other studies have shown that LVRS remains underutilized. Once such analysis of the Nationwide Inpatient Sample showed a logarithmic drop from 2000 to 2010 (Chest 2014;146[6]:e228-9). The authors also noted that the overall mortality was 6% and that the need for a tracheostomy was 7.9%. Age greater than 65 years was associated with increased mortality (odds ratio 2.8).
Dr. Attaway and her associates hypothesized that availability of the long-term survival data from the NETT, support from GOLD guidelines, and the lack of a Food and Drug Administration–approved alternative may have increased utilization of this surgery from 2007 through 2013. With data from the Nationwide Inpatient Sample for 2007-2013, the researchers performed a retrospective cohort analysis of 2,805 patients who underwent LVRS. The composite primary outcome was mortality or need for tracheostomy. Logistic regression was performed to analyze factors associated with the composite primary outcome.
The average patient age was 59 years, and 64% were male. Medicare was the payer in nearly half of cases, in-hospital mortality and need for tracheostomy were both 5.5%, and the risk for tracheostomy or mortality was 10.5%. Linear regression analysis showed a significant increase in LVRS over time, with the 320 surgeries in 2007 and 605 in 2013 (P = .0016).
On univariate analysis, the following factors were found to be significantly associated with the composite primary outcome: in-hospital mortality or the need for tracheostomy (P less than .001), respiratory failure (P less than .001), septicemia (P = .01), shock (P less than .001), acute kidney injury (P less than .001), secondary pulmonary hypertension (P less than .001), and a higher mean number of diagnoses or number of chronic conditions on admission (P less than .001 and P = .017, respectively).
On multivariate logistic regression, only two factors were found to be significantly associated with the composite primary outcome: a higher number of diagnoses (adjusted OR of 1.17 per additional diagnosis), and the presence of secondary pulmonary hypertension (adjusted OR 4.4).
“Availability of long-term survival data from NETT, support from the GOLD guidelines, and lack of current FDA-approved alternatives are potential reasons for increased utilization [of LVRS],” Dr. Attaway said. “However, our study showed that in-hospital mortality and morbidity is high, compared to the NETT results. We also found that secondary pulmonary hypertension and comorbidities are associated with poor outcomes. This is important to keep in mind for patient selection.”
Dr. Attaway and her associates reported having no financial disclosures.
SOURCE: Attaway A et al. ATS 2018, Abstract 4436.
SAN DIEGO – The number of has been increasing since 2011, according to a large national analysis.
Lung volume–reduction surgery (LVRS) has been available for decades, but results from the National Emphysema Treatment Trial (NETT) in 2003 (N Engl J Med 2003;348:2059-73) demonstrated that certain COPD patients benefited from the surgery, Amy Attaway, MD, said at an international conference of the American Thoracic Society.
In that trial, overall mortality at 30 days was 3.6% for the surgical group. “If you excluded high-risk patients, the 30-day mortality was only 2.2%,” said Dr. Attaway, a staff physician at the Cleveland Clinic Respiratory Institute. “If you look at the upper lobe–predominant, low-exercise group, their mortality at 30 days was 1.4%.”
Subsequent studies that evaluated NETT patients over time showed continued improvements in their mortality. For example, one study found that in the upper lobe–predominant patients who received surgery in the NETT trial, their survival at 3 years was 81% vs. 74% in the medical group (P = .05), while survival at 5 years was 70% in the surgery group vs. 60% in the medical group (P = .02; J Thorac Cardiovasc Surg. 2010;140[3]:564-72). Despite this improved mortality, other studies have shown that LVRS remains underutilized. Once such analysis of the Nationwide Inpatient Sample showed a logarithmic drop from 2000 to 2010 (Chest 2014;146[6]:e228-9). The authors also noted that the overall mortality was 6% and that the need for a tracheostomy was 7.9%. Age greater than 65 years was associated with increased mortality (odds ratio 2.8).
Dr. Attaway and her associates hypothesized that availability of the long-term survival data from the NETT, support from GOLD guidelines, and the lack of a Food and Drug Administration–approved alternative may have increased utilization of this surgery from 2007 through 2013. With data from the Nationwide Inpatient Sample for 2007-2013, the researchers performed a retrospective cohort analysis of 2,805 patients who underwent LVRS. The composite primary outcome was mortality or need for tracheostomy. Logistic regression was performed to analyze factors associated with the composite primary outcome.
The average patient age was 59 years, and 64% were male. Medicare was the payer in nearly half of cases, in-hospital mortality and need for tracheostomy were both 5.5%, and the risk for tracheostomy or mortality was 10.5%. Linear regression analysis showed a significant increase in LVRS over time, with the 320 surgeries in 2007 and 605 in 2013 (P = .0016).
On univariate analysis, the following factors were found to be significantly associated with the composite primary outcome: in-hospital mortality or the need for tracheostomy (P less than .001), respiratory failure (P less than .001), septicemia (P = .01), shock (P less than .001), acute kidney injury (P less than .001), secondary pulmonary hypertension (P less than .001), and a higher mean number of diagnoses or number of chronic conditions on admission (P less than .001 and P = .017, respectively).
On multivariate logistic regression, only two factors were found to be significantly associated with the composite primary outcome: a higher number of diagnoses (adjusted OR of 1.17 per additional diagnosis), and the presence of secondary pulmonary hypertension (adjusted OR 4.4).
“Availability of long-term survival data from NETT, support from the GOLD guidelines, and lack of current FDA-approved alternatives are potential reasons for increased utilization [of LVRS],” Dr. Attaway said. “However, our study showed that in-hospital mortality and morbidity is high, compared to the NETT results. We also found that secondary pulmonary hypertension and comorbidities are associated with poor outcomes. This is important to keep in mind for patient selection.”
Dr. Attaway and her associates reported having no financial disclosures.
SOURCE: Attaway A et al. ATS 2018, Abstract 4436.
AT ATS 2018
Key clinical point: Lung volume–reduction surgery remains an evidence-based therapy for a cohort of severe COPD patients.
Major finding: Linear regression analysis showed a significant increase in LVRS over time, with the 320 surgeries in 2007 and 605 in 2013 (P = .0016).
Study details: A retrospective cohort analysis of 2,805 patients who underwent LVRS.
Disclosures: The researchers reported having no financial disclosures.
Source: Attaway A et al. Abstract 4436, ATS 2018.
Aclidinium bromide for COPD: No impact on MACE
SAN DIEGO – The use of aclidinium bromide 400 mcg b.i.d. did not increase the risk of major adverse cardiac events or mortality in patients with moderate to very severe , compared with placebo.
Those are two key findings from the ASCENT COPD trial presented by Robert A. Wise, MD, at an international conference of the American Thoracic Society. “Cardiovascular risk factors and comorbidities are prevalent in patients with COPD, and about 30% of COPD patients die of cardiovascular disease,” said Dr. Wise, who serves as director of research for the division of pulmonary and critical care medicine at the Johns Hopkins University School of Medicine, Baltimore. “However, patients who have cardiovascular disease are often excluded from, or not enrolled in, COPD clinical trials. Moreover, there has been controversy as to whether or not treatment with a long-acting muscarinic antagonist is associated with an increased risk of cardiovascular events. That’s been seen in randomized trials, meta-analyses, as well as in observational studies.”
Aclidinium bromide 400 mcg b.i.d., administered by the Pressair inhaler, is approved as a maintenance treatment for patients with COPD. However, during the registration studies, there were not an adequate number of cardiovascular events in order to ascertain clearly whether or not the drug was associated with increased risk, Dr. Wise said. Therefore, he and his associates in the ASCENT COPD study set out to assess the long-term cardiovascular safety profile of aclidinium 400 mcg b.i.d. in patients with moderate to very severe COPD at risk of major adverse cardiovascular events (MACE) for up to 3 years (Chronic Obstr Pulm Dis. 2018;5[1]:5-15). For the randomized, placebo-controlled, parallel-group study, patients received treatment with aclidinium bromide or a placebo inhaler of similar appearance. The study was designed to be terminated when at least 122 patients experienced an adjudicated MACE. The primary safety endpoint was time to first MACE during follow-up of up to 3 years, while the primary efficacy endpoint was the rate of moderate to severe exacerbations per patient per year during the first year of treatment.
To be included in the study, patients had to be at least 40 years of age with moderate to very severe stable COPD, have a smoking history of at least 10 pack-years, and have at least one of the following significant risk factors: cerebrovascular disease; coronary artery disease; peripheral vascular disease, or history of claudication; or at least two atherothrombotic risk factors (male at least 65 years of age, female at least 70 years of age; waist circumference of at least 40 inches among males or at least 38 inches among females; an estimated glomerular filtration rate of less than 60 mL/min and microalbuminuria; dyslipidemia; or hypertension).
The researchers randomized 1,791 patients to the aclidinium group and 1,798 to the placebo group. Their mean age was 67 years, and about 60% of patients had an exacerbation in the preceding year. Nearly two-thirds of patients (63%) were receiving concomitant long-acting beta 2-agonists (LABA) or LABA/inhaled corticosteroid therapy. In addition, 44% of patients entered the study with a history of a prior cardiovascular event plus at least two atherothrombotic risk factors, 52% reported at least two atherothrombotic risk factors without any prior cardiovascular events, and 4% had a history of a prior cardiovascular event only.
Dr. Wise reported that aclidinium did not increase the risk of MACE in patients with moderate to very severe COPD with significant cardiovascular risk factors, compared with placebo (hazard ratio 0.89; P = .469); non-inferiority was concluded as the upper bound of the 95% confidence interval was less than 1.8). In terms of all-cause mortality, aclidinium did not increase the risk of death, compared with placebo (HR 0.99; P = .929).
During the first year of treatment, Dr. Wise and his associates also observed a 22% reduction in COPD exacerbation rate for aclidinium vs. placebo groups (HR 0.44 vs. 0.57, respectively; P less than .001), and a 35% reduction in the rate of COPD exacerbations leading to hospitalizations (HR 0.07 vs. 0.10; P = .006). “The reduction in exacerbation risk was similar, whether or not patients had an exacerbation in the past year,” Dr. Wise said. He reported being a consultant to, and receiving research support from, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and ContraFect.
dbrunk@mdedge.com
SOURCE: Wise, R., et al., Abstract 7711, ATS 2018.
SAN DIEGO – The use of aclidinium bromide 400 mcg b.i.d. did not increase the risk of major adverse cardiac events or mortality in patients with moderate to very severe , compared with placebo.
Those are two key findings from the ASCENT COPD trial presented by Robert A. Wise, MD, at an international conference of the American Thoracic Society. “Cardiovascular risk factors and comorbidities are prevalent in patients with COPD, and about 30% of COPD patients die of cardiovascular disease,” said Dr. Wise, who serves as director of research for the division of pulmonary and critical care medicine at the Johns Hopkins University School of Medicine, Baltimore. “However, patients who have cardiovascular disease are often excluded from, or not enrolled in, COPD clinical trials. Moreover, there has been controversy as to whether or not treatment with a long-acting muscarinic antagonist is associated with an increased risk of cardiovascular events. That’s been seen in randomized trials, meta-analyses, as well as in observational studies.”
Aclidinium bromide 400 mcg b.i.d., administered by the Pressair inhaler, is approved as a maintenance treatment for patients with COPD. However, during the registration studies, there were not an adequate number of cardiovascular events in order to ascertain clearly whether or not the drug was associated with increased risk, Dr. Wise said. Therefore, he and his associates in the ASCENT COPD study set out to assess the long-term cardiovascular safety profile of aclidinium 400 mcg b.i.d. in patients with moderate to very severe COPD at risk of major adverse cardiovascular events (MACE) for up to 3 years (Chronic Obstr Pulm Dis. 2018;5[1]:5-15). For the randomized, placebo-controlled, parallel-group study, patients received treatment with aclidinium bromide or a placebo inhaler of similar appearance. The study was designed to be terminated when at least 122 patients experienced an adjudicated MACE. The primary safety endpoint was time to first MACE during follow-up of up to 3 years, while the primary efficacy endpoint was the rate of moderate to severe exacerbations per patient per year during the first year of treatment.
To be included in the study, patients had to be at least 40 years of age with moderate to very severe stable COPD, have a smoking history of at least 10 pack-years, and have at least one of the following significant risk factors: cerebrovascular disease; coronary artery disease; peripheral vascular disease, or history of claudication; or at least two atherothrombotic risk factors (male at least 65 years of age, female at least 70 years of age; waist circumference of at least 40 inches among males or at least 38 inches among females; an estimated glomerular filtration rate of less than 60 mL/min and microalbuminuria; dyslipidemia; or hypertension).
The researchers randomized 1,791 patients to the aclidinium group and 1,798 to the placebo group. Their mean age was 67 years, and about 60% of patients had an exacerbation in the preceding year. Nearly two-thirds of patients (63%) were receiving concomitant long-acting beta 2-agonists (LABA) or LABA/inhaled corticosteroid therapy. In addition, 44% of patients entered the study with a history of a prior cardiovascular event plus at least two atherothrombotic risk factors, 52% reported at least two atherothrombotic risk factors without any prior cardiovascular events, and 4% had a history of a prior cardiovascular event only.
Dr. Wise reported that aclidinium did not increase the risk of MACE in patients with moderate to very severe COPD with significant cardiovascular risk factors, compared with placebo (hazard ratio 0.89; P = .469); non-inferiority was concluded as the upper bound of the 95% confidence interval was less than 1.8). In terms of all-cause mortality, aclidinium did not increase the risk of death, compared with placebo (HR 0.99; P = .929).
During the first year of treatment, Dr. Wise and his associates also observed a 22% reduction in COPD exacerbation rate for aclidinium vs. placebo groups (HR 0.44 vs. 0.57, respectively; P less than .001), and a 35% reduction in the rate of COPD exacerbations leading to hospitalizations (HR 0.07 vs. 0.10; P = .006). “The reduction in exacerbation risk was similar, whether or not patients had an exacerbation in the past year,” Dr. Wise said. He reported being a consultant to, and receiving research support from, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and ContraFect.
dbrunk@mdedge.com
SOURCE: Wise, R., et al., Abstract 7711, ATS 2018.
SAN DIEGO – The use of aclidinium bromide 400 mcg b.i.d. did not increase the risk of major adverse cardiac events or mortality in patients with moderate to very severe , compared with placebo.
Those are two key findings from the ASCENT COPD trial presented by Robert A. Wise, MD, at an international conference of the American Thoracic Society. “Cardiovascular risk factors and comorbidities are prevalent in patients with COPD, and about 30% of COPD patients die of cardiovascular disease,” said Dr. Wise, who serves as director of research for the division of pulmonary and critical care medicine at the Johns Hopkins University School of Medicine, Baltimore. “However, patients who have cardiovascular disease are often excluded from, or not enrolled in, COPD clinical trials. Moreover, there has been controversy as to whether or not treatment with a long-acting muscarinic antagonist is associated with an increased risk of cardiovascular events. That’s been seen in randomized trials, meta-analyses, as well as in observational studies.”
Aclidinium bromide 400 mcg b.i.d., administered by the Pressair inhaler, is approved as a maintenance treatment for patients with COPD. However, during the registration studies, there were not an adequate number of cardiovascular events in order to ascertain clearly whether or not the drug was associated with increased risk, Dr. Wise said. Therefore, he and his associates in the ASCENT COPD study set out to assess the long-term cardiovascular safety profile of aclidinium 400 mcg b.i.d. in patients with moderate to very severe COPD at risk of major adverse cardiovascular events (MACE) for up to 3 years (Chronic Obstr Pulm Dis. 2018;5[1]:5-15). For the randomized, placebo-controlled, parallel-group study, patients received treatment with aclidinium bromide or a placebo inhaler of similar appearance. The study was designed to be terminated when at least 122 patients experienced an adjudicated MACE. The primary safety endpoint was time to first MACE during follow-up of up to 3 years, while the primary efficacy endpoint was the rate of moderate to severe exacerbations per patient per year during the first year of treatment.
To be included in the study, patients had to be at least 40 years of age with moderate to very severe stable COPD, have a smoking history of at least 10 pack-years, and have at least one of the following significant risk factors: cerebrovascular disease; coronary artery disease; peripheral vascular disease, or history of claudication; or at least two atherothrombotic risk factors (male at least 65 years of age, female at least 70 years of age; waist circumference of at least 40 inches among males or at least 38 inches among females; an estimated glomerular filtration rate of less than 60 mL/min and microalbuminuria; dyslipidemia; or hypertension).
The researchers randomized 1,791 patients to the aclidinium group and 1,798 to the placebo group. Their mean age was 67 years, and about 60% of patients had an exacerbation in the preceding year. Nearly two-thirds of patients (63%) were receiving concomitant long-acting beta 2-agonists (LABA) or LABA/inhaled corticosteroid therapy. In addition, 44% of patients entered the study with a history of a prior cardiovascular event plus at least two atherothrombotic risk factors, 52% reported at least two atherothrombotic risk factors without any prior cardiovascular events, and 4% had a history of a prior cardiovascular event only.
Dr. Wise reported that aclidinium did not increase the risk of MACE in patients with moderate to very severe COPD with significant cardiovascular risk factors, compared with placebo (hazard ratio 0.89; P = .469); non-inferiority was concluded as the upper bound of the 95% confidence interval was less than 1.8). In terms of all-cause mortality, aclidinium did not increase the risk of death, compared with placebo (HR 0.99; P = .929).
During the first year of treatment, Dr. Wise and his associates also observed a 22% reduction in COPD exacerbation rate for aclidinium vs. placebo groups (HR 0.44 vs. 0.57, respectively; P less than .001), and a 35% reduction in the rate of COPD exacerbations leading to hospitalizations (HR 0.07 vs. 0.10; P = .006). “The reduction in exacerbation risk was similar, whether or not patients had an exacerbation in the past year,” Dr. Wise said. He reported being a consultant to, and receiving research support from, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and ContraFect.
dbrunk@mdedge.com
SOURCE: Wise, R., et al., Abstract 7711, ATS 2018.
AT ATS 2018
Key clinical point: Researchers found no increased risk of MACE in at-risk patients with COPD receiving aclidinium.
Major finding: MACE risk and mortality in COPD patients with significant cardiovascular risk given aclidinium bromide had a hazard ratio 0.89 (P = .469), compared to placebo.
Study details: A randomized, placebo-controlled, parallel-group study of 3,589 patients with moderate to very severe COPD at risk of major adverse cardiovascular events.
Disclosures: Dr. Wise reported being a consultant to, and receiving research support from, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and ContraFect.
Source: Wise, R. et al, ATS 2018, Abstract 7711.