User login
More cognitive rigidity found in patients with depression plus fibromyalgia
Increasing cognitive complexity cited as a possible therapeutic target
More attention might need to be paid to the role of chronic pain in the treatment of patients with comorbid depression, researchers suggest.
“Maybe models of depression should differentiate between depressed patients with a chronic pain condition, such as [fibromyalgia], and those without pain, wrote Mari Aguilera of the department of cognition, development, and educational psychology at the University of Barcelona and associates.
The research involved 62 patients who had participated in a previous randomized controlled trial that had assessed the efficacy of a dilemma-focused intervention for depression. All patients in the trial had met the criteria for major depressive disorder and/or dysthymia and had a score of more than 19 on the Beck Depression Inventory-II (BDI-II) scale, the investigators reported in the International Journal of Clinical and Health Psychology.
For the current trial, the researchers studied 31 patients from the trial who had an average age of 50, a concurrent diagnosis of fibromyalgia for an average of 8.14 years, an average of 2.06 depressive episodes, and a mean pain intensity of 76.21 on the visual analog scale.
The matched group of 31 patients who were used as a comparator group did not have a diagnosis of fibromyalgia and did not report high levels of pain intensity. Results showed that, in line with the researchers’ expectations, depressed patients with fibromyalgia had significantly higher BDI-II scores than patients with depression alone.
The researchers noted that patients with comorbid fibromyalgia had higher scores in pessimism, irritability, concentration/difficulty, tiredness or fatigue, and loss of interest in sex, compared with the control group.
“The nature of the relationship between pain and depression needs further studies to develop a better understanding in the future,” they wrote. The study was published in the International Journal of Clinical and Health Psychology.
Patients with comorbid fibromyalgia had higher levels of depressive symptoms and greater cognitive rigidity than did controls, the researchers found. Those with comorbid depression and pain also displayed higher levels of polarization, compared with the matched patients, with a medium-sized effect.
The small study size was cited as a limitation for generalizability. However, if confirmed by other larger studies, the researchers said, the findings might have implications for the treatment of depressed patients with comorbid fibromyalgia. “For patients with chronic pain, increasing their cognitive complexity might lead to better therapeutic results,” they wrote. “Overall, our study points to the need for more attention to the role of chronic pain in the study and treatment of depressed patients.
The research was funded by Spain’s Ministry of Science and Innovation.
SOURCE: Aguilera M et al. Int J Clin Health Psychol. 2019 May;19(2):160-4.
Increasing cognitive complexity cited as a possible therapeutic target
Increasing cognitive complexity cited as a possible therapeutic target
More attention might need to be paid to the role of chronic pain in the treatment of patients with comorbid depression, researchers suggest.
“Maybe models of depression should differentiate between depressed patients with a chronic pain condition, such as [fibromyalgia], and those without pain, wrote Mari Aguilera of the department of cognition, development, and educational psychology at the University of Barcelona and associates.
The research involved 62 patients who had participated in a previous randomized controlled trial that had assessed the efficacy of a dilemma-focused intervention for depression. All patients in the trial had met the criteria for major depressive disorder and/or dysthymia and had a score of more than 19 on the Beck Depression Inventory-II (BDI-II) scale, the investigators reported in the International Journal of Clinical and Health Psychology.
For the current trial, the researchers studied 31 patients from the trial who had an average age of 50, a concurrent diagnosis of fibromyalgia for an average of 8.14 years, an average of 2.06 depressive episodes, and a mean pain intensity of 76.21 on the visual analog scale.
The matched group of 31 patients who were used as a comparator group did not have a diagnosis of fibromyalgia and did not report high levels of pain intensity. Results showed that, in line with the researchers’ expectations, depressed patients with fibromyalgia had significantly higher BDI-II scores than patients with depression alone.
The researchers noted that patients with comorbid fibromyalgia had higher scores in pessimism, irritability, concentration/difficulty, tiredness or fatigue, and loss of interest in sex, compared with the control group.
“The nature of the relationship between pain and depression needs further studies to develop a better understanding in the future,” they wrote. The study was published in the International Journal of Clinical and Health Psychology.
Patients with comorbid fibromyalgia had higher levels of depressive symptoms and greater cognitive rigidity than did controls, the researchers found. Those with comorbid depression and pain also displayed higher levels of polarization, compared with the matched patients, with a medium-sized effect.
The small study size was cited as a limitation for generalizability. However, if confirmed by other larger studies, the researchers said, the findings might have implications for the treatment of depressed patients with comorbid fibromyalgia. “For patients with chronic pain, increasing their cognitive complexity might lead to better therapeutic results,” they wrote. “Overall, our study points to the need for more attention to the role of chronic pain in the study and treatment of depressed patients.
The research was funded by Spain’s Ministry of Science and Innovation.
SOURCE: Aguilera M et al. Int J Clin Health Psychol. 2019 May;19(2):160-4.
More attention might need to be paid to the role of chronic pain in the treatment of patients with comorbid depression, researchers suggest.
“Maybe models of depression should differentiate between depressed patients with a chronic pain condition, such as [fibromyalgia], and those without pain, wrote Mari Aguilera of the department of cognition, development, and educational psychology at the University of Barcelona and associates.
The research involved 62 patients who had participated in a previous randomized controlled trial that had assessed the efficacy of a dilemma-focused intervention for depression. All patients in the trial had met the criteria for major depressive disorder and/or dysthymia and had a score of more than 19 on the Beck Depression Inventory-II (BDI-II) scale, the investigators reported in the International Journal of Clinical and Health Psychology.
For the current trial, the researchers studied 31 patients from the trial who had an average age of 50, a concurrent diagnosis of fibromyalgia for an average of 8.14 years, an average of 2.06 depressive episodes, and a mean pain intensity of 76.21 on the visual analog scale.
The matched group of 31 patients who were used as a comparator group did not have a diagnosis of fibromyalgia and did not report high levels of pain intensity. Results showed that, in line with the researchers’ expectations, depressed patients with fibromyalgia had significantly higher BDI-II scores than patients with depression alone.
The researchers noted that patients with comorbid fibromyalgia had higher scores in pessimism, irritability, concentration/difficulty, tiredness or fatigue, and loss of interest in sex, compared with the control group.
“The nature of the relationship between pain and depression needs further studies to develop a better understanding in the future,” they wrote. The study was published in the International Journal of Clinical and Health Psychology.
Patients with comorbid fibromyalgia had higher levels of depressive symptoms and greater cognitive rigidity than did controls, the researchers found. Those with comorbid depression and pain also displayed higher levels of polarization, compared with the matched patients, with a medium-sized effect.
The small study size was cited as a limitation for generalizability. However, if confirmed by other larger studies, the researchers said, the findings might have implications for the treatment of depressed patients with comorbid fibromyalgia. “For patients with chronic pain, increasing their cognitive complexity might lead to better therapeutic results,” they wrote. “Overall, our study points to the need for more attention to the role of chronic pain in the study and treatment of depressed patients.
The research was funded by Spain’s Ministry of Science and Innovation.
SOURCE: Aguilera M et al. Int J Clin Health Psychol. 2019 May;19(2):160-4.
FROM THE INTERNATIONAL JOURNAL OF CLINICAL AND HEALTH PSYCHOLOGY
Serotonin syndrome: How to keep your patients safe
Mr. S, age 55, comes to your clinic as a walk-in for management of major depressive disorder, insomnia, and migraines. He also has tobacco use disorder and hypertension. Several days ago, Mr. S had visited the clinic because he was continuing to experience depressive symptoms, so his sertraline was increased from 100 to 200 mg/d. His current medication regimen includes sertraline 200 mg/d, trazodone 100 mg/d, lisinopril 10 mg/d, and sumatriptan, 100 mg as needed for migraine. He says last week he used 4 or 5 doses of sumatriptan because he experienced several migraines. Mr. S also reports occasionally taking 2 tablets of trazodone instead of 1 on nights that he has trouble falling asleep.
Today, Mr. S presents with a low-grade fever, diarrhea, internal restlessness, and a racing heartbeat that started shortly after his last visit. During physical examination, he exhibits slow, continuous lateral eye movements. His vital signs are markedly elevated: blood pressure, 175/85 mm Hg; heart rate, 110 beats per minute; and temperature, 39°C (102.2°F). Based on his presentation, the treatment team decides to send Mr. S to urgent care for closer monitoring.
Serotonin syndrome is a drug-induced syndrome caused by overstimulation of serotonin receptors. The syndrome is characterized by a classic clinical triad consisting of mental status changes, autonomic hyperactivity, and neuromuscular abnormalities. The clinical presentation is highly variable, and the severity ranges from mild to life-threatening.1-3 The incidence and prevalence of serotonin syndrome has not been well defined.3 Serotonin syndrome may be underreported because mild cases are often overlooked due to nonspecific symptoms. In addition, lack of physician awareness of drug–drug interactions, signs and symptoms, and differential diagnoses may result in underdiagnosis or misdiagnosis.1-3
What causes it?
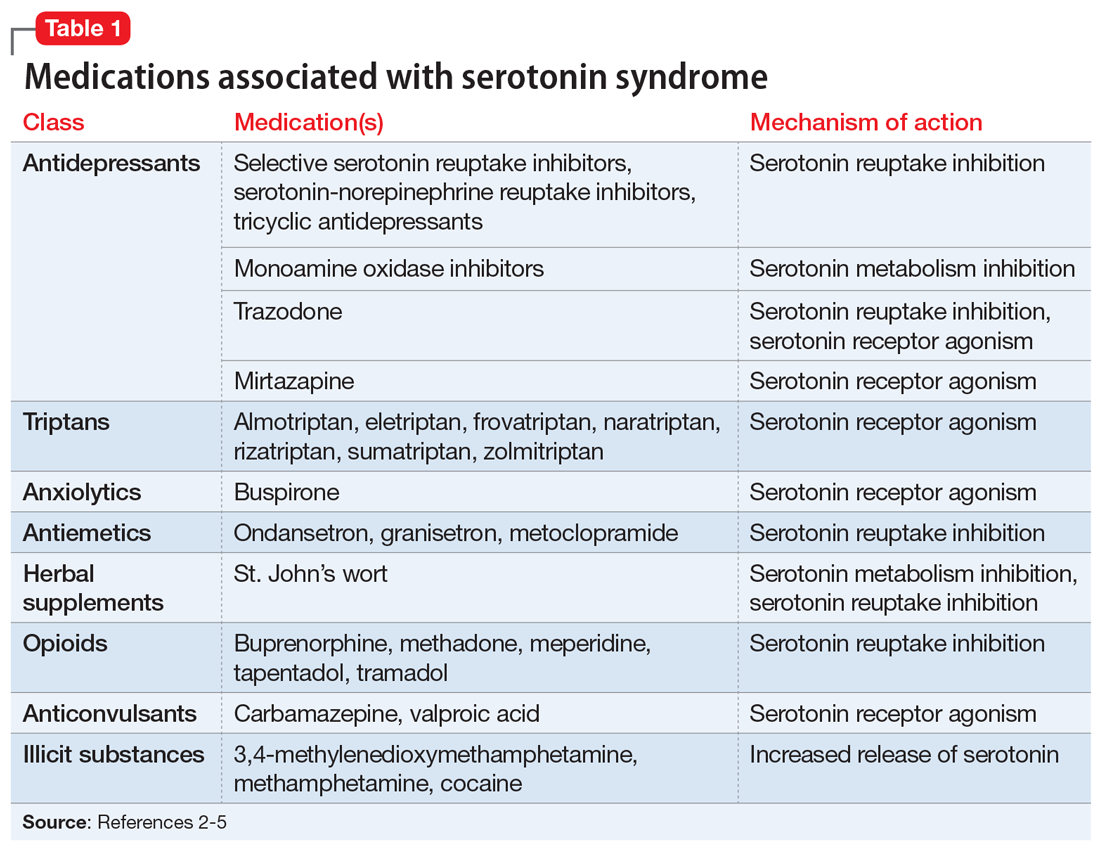
Serotonin syndrome is usually a consequence of a drug–drug interaction between 2 or more serotonergic agents.4 Serotonin syndrome may result following medication misuse, overdose, initiation of a serotonergic agent, or increase in the dose of a currently prescribed serotonergic agent.3,4 In addition to medication classes and specific agents, Table 12-5 lists the drug mechanisms associated with serotonin syndrome:
- inhibition of serotonin reuptake
- inhibition of serotonin metabolism
- increased serotonin synthesis
- agonism of the serotonin receptor.
The amount of serotonergic activity most likely to cause serotonin syndrome is unclear.4
Pathophysiology. Serotonin, also known as 5-hydroxytryptamine (5-HT), is a metabolite of the amino acid tryptophan. This neurotransmitter is located in both the CNS and the periphery. Regulation of the serotonergic system begins in the presynaptic neurons with decarboxylation and hydroxylation of tryptophan resulting in serotonin synthesis. Once serotonin is produced, it is released into the synaptic cleft, where it binds to serotonin receptors.1,4,5 After receptor binding, serotonin reuptake occurs in the presynaptic neurons, where it can be metabolized by the monoamine oxidase enzyme. Finally, the metabolites are excreted in the urine. Serotonin syndrome results when this regulatory system is disrupted due to hyperstimulation of the postsynaptic serotonin receptors, mainly via agonism of the 5-HT2A and 5-HT1A receptors.1,4,5
Continue to: A nonspecific presentation
A nonspecific presentation
Unfortunately, many of the symptoms of serotonin syndrome are nonspecific, and the severity varies among patients.2,3 The onset of symptoms usually occurs within 6 to 8 hours after ingestion of a serotonergic agent.5 It is important to immediately recognize the symptoms (Table 22-5) and formulate a differential diagnosis because sudden progression of symptoms is common and may lead to life-threatening circumstances.1,3
In mild cases of serotonin syndrome, patients may have a low-grade fever or be afebrile. Hyperthermia tends to be present in moderate and severe cases, with temperatures >41°C (105.8°F) during life-threatening cases. Diaphoresis and tachycardia may be present regardless of severity. Additional autonomic irregularities include hypertension, tachypnea, nausea, vomiting, diarrhea, and hyperactive bowel sounds. In terms of neuromuscular abnormalities, hyperreflexia is a primary concern, as well as myoclonus. As the severity progresses to life-threatening, the clonus may convert from inducible to spontaneous and slow, continuous lateral eye movements may be present. Additional neuromuscular symptoms include tremor, akathisia, and muscle rigidity.1,3-5
Common mental status changes during mild cases include restlessness and anxiety. Abnormal mentation during moderate cases may present as increased hypervigilance and agitation, and this may advance to delirium or coma in severe cases. As the severity intensifies, the risk of developing additional physiological complications also increases. Rhabdomyolysis may occur due to muscle damage and myoglobinuria secondary to hyperreflexia, myoclonus, hypertonicity, and muscle rigidity. Muscle breakdown may then progress to further complications, such as renal failure. In rare instances, serotonin syndrome can result in seizures or death.1,3-5
Medication history tips off the diagnosis
The first step in diagnosing serotonin syndrome is to conduct a thorough review of the patient’s medication history, specifically taking into account any recent exposure to serotonergic agents.3,5 It is important to ask about prescription medications as well as over-the-counter products, herbal supplements, and illicit substances.1,4 When reviewing the medication history, investigate whether there may have been a recent change in therapy with serotonergic agents. Also, determine when the patient’s symptoms began in relation to exposure to serotonergic agents.4
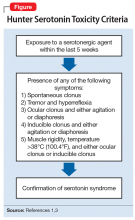
After the medication review, conduct a thorough physical and neurologic examination to identify current symptoms and severity.1,3 No specific laboratory test is available to definitively confirm the diagnosis of serotonin syndrome.1,4 Monitoring of serum serotonin is not recommended because the levels do not correlate with symptom severity.3 The recommended diagnostic tool is the Hunter Serotonin Toxicity Criteria (Figure1,3).3,4 Historically, the Sternbach’s Diagnostic Criteria for serotonin syndrome were used for diagnosis; however, the Hunter Serotonin Toxicity Criteria are more sensitive (96% vs 75%) and more specific (97% vs 84%) than the Sternbach’s Diagnostic Criteria for serotonin syndrome.1,3-5
Continue to: In addition to using the proper diagnostic tool...
In addition to using the proper diagnostic tool, conduct a differential diagnosis to rule out other drug-induced syndromes, such as anticholinergic toxidrome, neuroleptic malignant syndrome, or malignant hyperthermia.1,3,5 Autonomic instability, including hypertension, tachycardia, tachypnea, and hyperthermia, may be present in all of the aforementioned drug-induced syndromes.1 As a result, the clinician must monitor for other symptoms that may differentiate the disease states to establish a clear diagnosis.
Discontinue agents, offer supportive care
There are no official published guidelines for managing serotonin syndrome.5 Regardless of the severity of a patient’s presentation, all serotonergic agents should be discontinued immediately. In addition, supportive care should be initiated for symptom management. Intravenous fluid replacement is recommended for hydration and to treat hyperthermia. External cooling may also be warranted to reduce body temperatures. Vital signs should be stabilized with appropriate pharmacotherapy.1,3-5
Benzodiazepines are considered a mainstay for relief of agitation during serotonin syndrome of any severity. In life-threatening cases—which are characterized by hyperthermia >41°C (105.8°F)—sedation, paralysis, and intubation may be necessary to maintain the airway, breathing, and circulation.1,3-5 Because treatment of hyperthermia requires elimination of hyperreflexia, paralysis is recommended.1 Nondepolarizing neuromuscular blocking agents, such as vecuronium, are preferred over depolarizing agents due to their decreased potential for rhabdomyolysis.1,3
Cyproheptadine, a histamine-1 receptor antagonist and a 5-HT2A receptor antagonist, is recommended for off-label treatment of serotonin syndrome to help decrease the intensity of symptoms. This should be initiated as a single dose of 12 mg followed by 2 mg every 2 hours until symptoms improve.1,3,5 After stabilization, a maintenance dose of 8 mg every 6 hours is recommended. Doses should not exceed the maximum recommended dose of 0.5 mg/kg/d.1,3,6 The most common adverse reactions associated with cyproheptadine are sedation and anticholinergic adverse effects.1,4,6
Antipsychotics, such as olanzapine and chlorpromazine, have been considered treatment alternatives due to their associated 5-HT2A receptor antagonism. However, there is limited data supporting such use.1,4 Antipsychotics should be used with caution because neuroleptic malignant syndrome may be mistaken for serotonin syndrome. Use of antipyretics is not recommended for treating fever and hyperthermia because the increase in body temperature is secondary to excessive muscle activity rather than dysfunction of the hypothalamic temperature set point.1,3,5 Physical restraints are also not recommended because their use may provoke further hyperthermia and increase the risk of rhabdomyolysis.3,5
Continue to: Ultimately, the duration of treatment...
Ultimately, the duration of treatment will be influenced by the pharmacokinetics of the serotonergic agents that induced the serotonin syndrome. Following resolution, retrial of the offending serotonergic agents should be carefully assessed. A retrial should only be considered after an adequate washout period has been observed, and clinicians should consider utilizing lower doses.2,5
Take steps for prevention
Patients at highest risk of developing serotonin syndrome are those who have multiple comorbidities that result in treatment with multiple serotonergic agents.3 Clinicians and patients alike need to be educated about the signs and symptoms of serotonin syndrome to promote early recognition. Also consider modifying your prescribing practices to minimize the use of multiple serotonergic agents. When switching between serotonergic agents, institute safe washout periods. Encourage patients to adhere to their prescribed medication regimens. Using electronic ordering systems can help detect drug–drug interactions.1,3 Prophylaxis with cyproheptadine may be considered in high-risk patients; however, no clinical trials have been conducted to evaluate using cyproheptadine to prevent serotonin syndrome.7
CASE CONTINUED
Upon further assessment in urgent care, Mr. S is found to have muscle rigidity in addition to ocular clonus and a temperature >38°C (100.4°F). Because Mr. S’s symptoms coincide with a recent increase of sertraline and increased use of both trazodone and sumatriptan, he meets Hunter Serotonin Toxicity Criteria. Therefore, his symptoms are likely related to excessive increase in serotonergic activity. Mr. S is admitted to the hospital for closer monitoring, and his sertraline, trazodone, and sumatriptan are held. He receives IV fluids for several days as well as cyproheptadine, 8 mg every 6 hours after stabilization, until his symptoms resolve. On Day 4, Mr. S no longer experiences diarrhea and internal restlessness. His vital signs return to normal, and as a result of symptom resolution, he is discharged from the hospital. The treatment team discusses changing his medication regimen to avoid multiple serotonergic agents. Mr. S is switched from sertraline to bupropion XL, 150 mg/d. Sumatriptan, 100 mg/d as needed, is continued for acute migraine treatment. Trazodone is discontinued and replaced with melatonin, 3 mg/d. The team also counsels Mr. S on the importance of proper adherence to his medication regimen. He is advised to return to the clinic in 2 weeks for reassessment of safety and efficacy.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Almotriptan • Axert
Buprenorphine • Subutex
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cyproheptadine • Periactin
Eletriptan • Relpax
Frovatriptan • Frova
Granisetron • Kytril
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Methadone • Dolophine, Methadose
Metoclopramide • Reglan
Mirtazapine • Remeron
Naratriptan • Amerge
Olanzapine • Zyprexa
Ondansetron • Zofran
Rizatriptan • Maxalt
Sertraline • Zoloft
Sumatriptan • Imitrex tablets
Tapentadol • Nucynta
Tramadol • Conzip
Trazodone • Desyrel, Oleptro
Valproic acid • Depakene, Depakote
Vecuronium • Norcuron
Zolmitriptan • Zomig
1. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
2. Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18(4):395-400.
3. Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817.
4. Bartlett D. Drug-induced serotonin syndrome. Crit Care Nurse. 2017;37(1):49-54.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. Cyproheptadine hydrochloride tablets [package insert]. Hayward, CA: Impax Generics; 2017.
7. Deardorff OG, Khan T, Kulkarni G, et al. Serotonin syndrome: prophylactic treatment with cyproheptadine. Prim Care Companion CNS Disord. 2016;18(4). doi: 10.4088/PCC.16br01966.
Mr. S, age 55, comes to your clinic as a walk-in for management of major depressive disorder, insomnia, and migraines. He also has tobacco use disorder and hypertension. Several days ago, Mr. S had visited the clinic because he was continuing to experience depressive symptoms, so his sertraline was increased from 100 to 200 mg/d. His current medication regimen includes sertraline 200 mg/d, trazodone 100 mg/d, lisinopril 10 mg/d, and sumatriptan, 100 mg as needed for migraine. He says last week he used 4 or 5 doses of sumatriptan because he experienced several migraines. Mr. S also reports occasionally taking 2 tablets of trazodone instead of 1 on nights that he has trouble falling asleep.
Today, Mr. S presents with a low-grade fever, diarrhea, internal restlessness, and a racing heartbeat that started shortly after his last visit. During physical examination, he exhibits slow, continuous lateral eye movements. His vital signs are markedly elevated: blood pressure, 175/85 mm Hg; heart rate, 110 beats per minute; and temperature, 39°C (102.2°F). Based on his presentation, the treatment team decides to send Mr. S to urgent care for closer monitoring.
Serotonin syndrome is a drug-induced syndrome caused by overstimulation of serotonin receptors. The syndrome is characterized by a classic clinical triad consisting of mental status changes, autonomic hyperactivity, and neuromuscular abnormalities. The clinical presentation is highly variable, and the severity ranges from mild to life-threatening.1-3 The incidence and prevalence of serotonin syndrome has not been well defined.3 Serotonin syndrome may be underreported because mild cases are often overlooked due to nonspecific symptoms. In addition, lack of physician awareness of drug–drug interactions, signs and symptoms, and differential diagnoses may result in underdiagnosis or misdiagnosis.1-3
What causes it?
Serotonin syndrome is usually a consequence of a drug–drug interaction between 2 or more serotonergic agents.4 Serotonin syndrome may result following medication misuse, overdose, initiation of a serotonergic agent, or increase in the dose of a currently prescribed serotonergic agent.3,4 In addition to medication classes and specific agents, Table 12-5 lists the drug mechanisms associated with serotonin syndrome:
- inhibition of serotonin reuptake
- inhibition of serotonin metabolism
- increased serotonin synthesis
- agonism of the serotonin receptor.
The amount of serotonergic activity most likely to cause serotonin syndrome is unclear.4
Pathophysiology. Serotonin, also known as 5-hydroxytryptamine (5-HT), is a metabolite of the amino acid tryptophan. This neurotransmitter is located in both the CNS and the periphery. Regulation of the serotonergic system begins in the presynaptic neurons with decarboxylation and hydroxylation of tryptophan resulting in serotonin synthesis. Once serotonin is produced, it is released into the synaptic cleft, where it binds to serotonin receptors.1,4,5 After receptor binding, serotonin reuptake occurs in the presynaptic neurons, where it can be metabolized by the monoamine oxidase enzyme. Finally, the metabolites are excreted in the urine. Serotonin syndrome results when this regulatory system is disrupted due to hyperstimulation of the postsynaptic serotonin receptors, mainly via agonism of the 5-HT2A and 5-HT1A receptors.1,4,5
Continue to: A nonspecific presentation
A nonspecific presentation
Unfortunately, many of the symptoms of serotonin syndrome are nonspecific, and the severity varies among patients.2,3 The onset of symptoms usually occurs within 6 to 8 hours after ingestion of a serotonergic agent.5 It is important to immediately recognize the symptoms (Table 22-5) and formulate a differential diagnosis because sudden progression of symptoms is common and may lead to life-threatening circumstances.1,3
In mild cases of serotonin syndrome, patients may have a low-grade fever or be afebrile. Hyperthermia tends to be present in moderate and severe cases, with temperatures >41°C (105.8°F) during life-threatening cases. Diaphoresis and tachycardia may be present regardless of severity. Additional autonomic irregularities include hypertension, tachypnea, nausea, vomiting, diarrhea, and hyperactive bowel sounds. In terms of neuromuscular abnormalities, hyperreflexia is a primary concern, as well as myoclonus. As the severity progresses to life-threatening, the clonus may convert from inducible to spontaneous and slow, continuous lateral eye movements may be present. Additional neuromuscular symptoms include tremor, akathisia, and muscle rigidity.1,3-5
Common mental status changes during mild cases include restlessness and anxiety. Abnormal mentation during moderate cases may present as increased hypervigilance and agitation, and this may advance to delirium or coma in severe cases. As the severity intensifies, the risk of developing additional physiological complications also increases. Rhabdomyolysis may occur due to muscle damage and myoglobinuria secondary to hyperreflexia, myoclonus, hypertonicity, and muscle rigidity. Muscle breakdown may then progress to further complications, such as renal failure. In rare instances, serotonin syndrome can result in seizures or death.1,3-5
Medication history tips off the diagnosis
The first step in diagnosing serotonin syndrome is to conduct a thorough review of the patient’s medication history, specifically taking into account any recent exposure to serotonergic agents.3,5 It is important to ask about prescription medications as well as over-the-counter products, herbal supplements, and illicit substances.1,4 When reviewing the medication history, investigate whether there may have been a recent change in therapy with serotonergic agents. Also, determine when the patient’s symptoms began in relation to exposure to serotonergic agents.4
After the medication review, conduct a thorough physical and neurologic examination to identify current symptoms and severity.1,3 No specific laboratory test is available to definitively confirm the diagnosis of serotonin syndrome.1,4 Monitoring of serum serotonin is not recommended because the levels do not correlate with symptom severity.3 The recommended diagnostic tool is the Hunter Serotonin Toxicity Criteria (Figure1,3).3,4 Historically, the Sternbach’s Diagnostic Criteria for serotonin syndrome were used for diagnosis; however, the Hunter Serotonin Toxicity Criteria are more sensitive (96% vs 75%) and more specific (97% vs 84%) than the Sternbach’s Diagnostic Criteria for serotonin syndrome.1,3-5
Continue to: In addition to using the proper diagnostic tool...
In addition to using the proper diagnostic tool, conduct a differential diagnosis to rule out other drug-induced syndromes, such as anticholinergic toxidrome, neuroleptic malignant syndrome, or malignant hyperthermia.1,3,5 Autonomic instability, including hypertension, tachycardia, tachypnea, and hyperthermia, may be present in all of the aforementioned drug-induced syndromes.1 As a result, the clinician must monitor for other symptoms that may differentiate the disease states to establish a clear diagnosis.
Discontinue agents, offer supportive care
There are no official published guidelines for managing serotonin syndrome.5 Regardless of the severity of a patient’s presentation, all serotonergic agents should be discontinued immediately. In addition, supportive care should be initiated for symptom management. Intravenous fluid replacement is recommended for hydration and to treat hyperthermia. External cooling may also be warranted to reduce body temperatures. Vital signs should be stabilized with appropriate pharmacotherapy.1,3-5
Benzodiazepines are considered a mainstay for relief of agitation during serotonin syndrome of any severity. In life-threatening cases—which are characterized by hyperthermia >41°C (105.8°F)—sedation, paralysis, and intubation may be necessary to maintain the airway, breathing, and circulation.1,3-5 Because treatment of hyperthermia requires elimination of hyperreflexia, paralysis is recommended.1 Nondepolarizing neuromuscular blocking agents, such as vecuronium, are preferred over depolarizing agents due to their decreased potential for rhabdomyolysis.1,3
Cyproheptadine, a histamine-1 receptor antagonist and a 5-HT2A receptor antagonist, is recommended for off-label treatment of serotonin syndrome to help decrease the intensity of symptoms. This should be initiated as a single dose of 12 mg followed by 2 mg every 2 hours until symptoms improve.1,3,5 After stabilization, a maintenance dose of 8 mg every 6 hours is recommended. Doses should not exceed the maximum recommended dose of 0.5 mg/kg/d.1,3,6 The most common adverse reactions associated with cyproheptadine are sedation and anticholinergic adverse effects.1,4,6
Antipsychotics, such as olanzapine and chlorpromazine, have been considered treatment alternatives due to their associated 5-HT2A receptor antagonism. However, there is limited data supporting such use.1,4 Antipsychotics should be used with caution because neuroleptic malignant syndrome may be mistaken for serotonin syndrome. Use of antipyretics is not recommended for treating fever and hyperthermia because the increase in body temperature is secondary to excessive muscle activity rather than dysfunction of the hypothalamic temperature set point.1,3,5 Physical restraints are also not recommended because their use may provoke further hyperthermia and increase the risk of rhabdomyolysis.3,5
Continue to: Ultimately, the duration of treatment...
Ultimately, the duration of treatment will be influenced by the pharmacokinetics of the serotonergic agents that induced the serotonin syndrome. Following resolution, retrial of the offending serotonergic agents should be carefully assessed. A retrial should only be considered after an adequate washout period has been observed, and clinicians should consider utilizing lower doses.2,5
Take steps for prevention
Patients at highest risk of developing serotonin syndrome are those who have multiple comorbidities that result in treatment with multiple serotonergic agents.3 Clinicians and patients alike need to be educated about the signs and symptoms of serotonin syndrome to promote early recognition. Also consider modifying your prescribing practices to minimize the use of multiple serotonergic agents. When switching between serotonergic agents, institute safe washout periods. Encourage patients to adhere to their prescribed medication regimens. Using electronic ordering systems can help detect drug–drug interactions.1,3 Prophylaxis with cyproheptadine may be considered in high-risk patients; however, no clinical trials have been conducted to evaluate using cyproheptadine to prevent serotonin syndrome.7
CASE CONTINUED
Upon further assessment in urgent care, Mr. S is found to have muscle rigidity in addition to ocular clonus and a temperature >38°C (100.4°F). Because Mr. S’s symptoms coincide with a recent increase of sertraline and increased use of both trazodone and sumatriptan, he meets Hunter Serotonin Toxicity Criteria. Therefore, his symptoms are likely related to excessive increase in serotonergic activity. Mr. S is admitted to the hospital for closer monitoring, and his sertraline, trazodone, and sumatriptan are held. He receives IV fluids for several days as well as cyproheptadine, 8 mg every 6 hours after stabilization, until his symptoms resolve. On Day 4, Mr. S no longer experiences diarrhea and internal restlessness. His vital signs return to normal, and as a result of symptom resolution, he is discharged from the hospital. The treatment team discusses changing his medication regimen to avoid multiple serotonergic agents. Mr. S is switched from sertraline to bupropion XL, 150 mg/d. Sumatriptan, 100 mg/d as needed, is continued for acute migraine treatment. Trazodone is discontinued and replaced with melatonin, 3 mg/d. The team also counsels Mr. S on the importance of proper adherence to his medication regimen. He is advised to return to the clinic in 2 weeks for reassessment of safety and efficacy.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Almotriptan • Axert
Buprenorphine • Subutex
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cyproheptadine • Periactin
Eletriptan • Relpax
Frovatriptan • Frova
Granisetron • Kytril
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Methadone • Dolophine, Methadose
Metoclopramide • Reglan
Mirtazapine • Remeron
Naratriptan • Amerge
Olanzapine • Zyprexa
Ondansetron • Zofran
Rizatriptan • Maxalt
Sertraline • Zoloft
Sumatriptan • Imitrex tablets
Tapentadol • Nucynta
Tramadol • Conzip
Trazodone • Desyrel, Oleptro
Valproic acid • Depakene, Depakote
Vecuronium • Norcuron
Zolmitriptan • Zomig
Mr. S, age 55, comes to your clinic as a walk-in for management of major depressive disorder, insomnia, and migraines. He also has tobacco use disorder and hypertension. Several days ago, Mr. S had visited the clinic because he was continuing to experience depressive symptoms, so his sertraline was increased from 100 to 200 mg/d. His current medication regimen includes sertraline 200 mg/d, trazodone 100 mg/d, lisinopril 10 mg/d, and sumatriptan, 100 mg as needed for migraine. He says last week he used 4 or 5 doses of sumatriptan because he experienced several migraines. Mr. S also reports occasionally taking 2 tablets of trazodone instead of 1 on nights that he has trouble falling asleep.
Today, Mr. S presents with a low-grade fever, diarrhea, internal restlessness, and a racing heartbeat that started shortly after his last visit. During physical examination, he exhibits slow, continuous lateral eye movements. His vital signs are markedly elevated: blood pressure, 175/85 mm Hg; heart rate, 110 beats per minute; and temperature, 39°C (102.2°F). Based on his presentation, the treatment team decides to send Mr. S to urgent care for closer monitoring.
Serotonin syndrome is a drug-induced syndrome caused by overstimulation of serotonin receptors. The syndrome is characterized by a classic clinical triad consisting of mental status changes, autonomic hyperactivity, and neuromuscular abnormalities. The clinical presentation is highly variable, and the severity ranges from mild to life-threatening.1-3 The incidence and prevalence of serotonin syndrome has not been well defined.3 Serotonin syndrome may be underreported because mild cases are often overlooked due to nonspecific symptoms. In addition, lack of physician awareness of drug–drug interactions, signs and symptoms, and differential diagnoses may result in underdiagnosis or misdiagnosis.1-3
What causes it?
Serotonin syndrome is usually a consequence of a drug–drug interaction between 2 or more serotonergic agents.4 Serotonin syndrome may result following medication misuse, overdose, initiation of a serotonergic agent, or increase in the dose of a currently prescribed serotonergic agent.3,4 In addition to medication classes and specific agents, Table 12-5 lists the drug mechanisms associated with serotonin syndrome:
- inhibition of serotonin reuptake
- inhibition of serotonin metabolism
- increased serotonin synthesis
- agonism of the serotonin receptor.
The amount of serotonergic activity most likely to cause serotonin syndrome is unclear.4
Pathophysiology. Serotonin, also known as 5-hydroxytryptamine (5-HT), is a metabolite of the amino acid tryptophan. This neurotransmitter is located in both the CNS and the periphery. Regulation of the serotonergic system begins in the presynaptic neurons with decarboxylation and hydroxylation of tryptophan resulting in serotonin synthesis. Once serotonin is produced, it is released into the synaptic cleft, where it binds to serotonin receptors.1,4,5 After receptor binding, serotonin reuptake occurs in the presynaptic neurons, where it can be metabolized by the monoamine oxidase enzyme. Finally, the metabolites are excreted in the urine. Serotonin syndrome results when this regulatory system is disrupted due to hyperstimulation of the postsynaptic serotonin receptors, mainly via agonism of the 5-HT2A and 5-HT1A receptors.1,4,5
Continue to: A nonspecific presentation
A nonspecific presentation
Unfortunately, many of the symptoms of serotonin syndrome are nonspecific, and the severity varies among patients.2,3 The onset of symptoms usually occurs within 6 to 8 hours after ingestion of a serotonergic agent.5 It is important to immediately recognize the symptoms (Table 22-5) and formulate a differential diagnosis because sudden progression of symptoms is common and may lead to life-threatening circumstances.1,3
In mild cases of serotonin syndrome, patients may have a low-grade fever or be afebrile. Hyperthermia tends to be present in moderate and severe cases, with temperatures >41°C (105.8°F) during life-threatening cases. Diaphoresis and tachycardia may be present regardless of severity. Additional autonomic irregularities include hypertension, tachypnea, nausea, vomiting, diarrhea, and hyperactive bowel sounds. In terms of neuromuscular abnormalities, hyperreflexia is a primary concern, as well as myoclonus. As the severity progresses to life-threatening, the clonus may convert from inducible to spontaneous and slow, continuous lateral eye movements may be present. Additional neuromuscular symptoms include tremor, akathisia, and muscle rigidity.1,3-5
Common mental status changes during mild cases include restlessness and anxiety. Abnormal mentation during moderate cases may present as increased hypervigilance and agitation, and this may advance to delirium or coma in severe cases. As the severity intensifies, the risk of developing additional physiological complications also increases. Rhabdomyolysis may occur due to muscle damage and myoglobinuria secondary to hyperreflexia, myoclonus, hypertonicity, and muscle rigidity. Muscle breakdown may then progress to further complications, such as renal failure. In rare instances, serotonin syndrome can result in seizures or death.1,3-5
Medication history tips off the diagnosis
The first step in diagnosing serotonin syndrome is to conduct a thorough review of the patient’s medication history, specifically taking into account any recent exposure to serotonergic agents.3,5 It is important to ask about prescription medications as well as over-the-counter products, herbal supplements, and illicit substances.1,4 When reviewing the medication history, investigate whether there may have been a recent change in therapy with serotonergic agents. Also, determine when the patient’s symptoms began in relation to exposure to serotonergic agents.4
After the medication review, conduct a thorough physical and neurologic examination to identify current symptoms and severity.1,3 No specific laboratory test is available to definitively confirm the diagnosis of serotonin syndrome.1,4 Monitoring of serum serotonin is not recommended because the levels do not correlate with symptom severity.3 The recommended diagnostic tool is the Hunter Serotonin Toxicity Criteria (Figure1,3).3,4 Historically, the Sternbach’s Diagnostic Criteria for serotonin syndrome were used for diagnosis; however, the Hunter Serotonin Toxicity Criteria are more sensitive (96% vs 75%) and more specific (97% vs 84%) than the Sternbach’s Diagnostic Criteria for serotonin syndrome.1,3-5
Continue to: In addition to using the proper diagnostic tool...
In addition to using the proper diagnostic tool, conduct a differential diagnosis to rule out other drug-induced syndromes, such as anticholinergic toxidrome, neuroleptic malignant syndrome, or malignant hyperthermia.1,3,5 Autonomic instability, including hypertension, tachycardia, tachypnea, and hyperthermia, may be present in all of the aforementioned drug-induced syndromes.1 As a result, the clinician must monitor for other symptoms that may differentiate the disease states to establish a clear diagnosis.
Discontinue agents, offer supportive care
There are no official published guidelines for managing serotonin syndrome.5 Regardless of the severity of a patient’s presentation, all serotonergic agents should be discontinued immediately. In addition, supportive care should be initiated for symptom management. Intravenous fluid replacement is recommended for hydration and to treat hyperthermia. External cooling may also be warranted to reduce body temperatures. Vital signs should be stabilized with appropriate pharmacotherapy.1,3-5
Benzodiazepines are considered a mainstay for relief of agitation during serotonin syndrome of any severity. In life-threatening cases—which are characterized by hyperthermia >41°C (105.8°F)—sedation, paralysis, and intubation may be necessary to maintain the airway, breathing, and circulation.1,3-5 Because treatment of hyperthermia requires elimination of hyperreflexia, paralysis is recommended.1 Nondepolarizing neuromuscular blocking agents, such as vecuronium, are preferred over depolarizing agents due to their decreased potential for rhabdomyolysis.1,3
Cyproheptadine, a histamine-1 receptor antagonist and a 5-HT2A receptor antagonist, is recommended for off-label treatment of serotonin syndrome to help decrease the intensity of symptoms. This should be initiated as a single dose of 12 mg followed by 2 mg every 2 hours until symptoms improve.1,3,5 After stabilization, a maintenance dose of 8 mg every 6 hours is recommended. Doses should not exceed the maximum recommended dose of 0.5 mg/kg/d.1,3,6 The most common adverse reactions associated with cyproheptadine are sedation and anticholinergic adverse effects.1,4,6
Antipsychotics, such as olanzapine and chlorpromazine, have been considered treatment alternatives due to their associated 5-HT2A receptor antagonism. However, there is limited data supporting such use.1,4 Antipsychotics should be used with caution because neuroleptic malignant syndrome may be mistaken for serotonin syndrome. Use of antipyretics is not recommended for treating fever and hyperthermia because the increase in body temperature is secondary to excessive muscle activity rather than dysfunction of the hypothalamic temperature set point.1,3,5 Physical restraints are also not recommended because their use may provoke further hyperthermia and increase the risk of rhabdomyolysis.3,5
Continue to: Ultimately, the duration of treatment...
Ultimately, the duration of treatment will be influenced by the pharmacokinetics of the serotonergic agents that induced the serotonin syndrome. Following resolution, retrial of the offending serotonergic agents should be carefully assessed. A retrial should only be considered after an adequate washout period has been observed, and clinicians should consider utilizing lower doses.2,5
Take steps for prevention
Patients at highest risk of developing serotonin syndrome are those who have multiple comorbidities that result in treatment with multiple serotonergic agents.3 Clinicians and patients alike need to be educated about the signs and symptoms of serotonin syndrome to promote early recognition. Also consider modifying your prescribing practices to minimize the use of multiple serotonergic agents. When switching between serotonergic agents, institute safe washout periods. Encourage patients to adhere to their prescribed medication regimens. Using electronic ordering systems can help detect drug–drug interactions.1,3 Prophylaxis with cyproheptadine may be considered in high-risk patients; however, no clinical trials have been conducted to evaluate using cyproheptadine to prevent serotonin syndrome.7
CASE CONTINUED
Upon further assessment in urgent care, Mr. S is found to have muscle rigidity in addition to ocular clonus and a temperature >38°C (100.4°F). Because Mr. S’s symptoms coincide with a recent increase of sertraline and increased use of both trazodone and sumatriptan, he meets Hunter Serotonin Toxicity Criteria. Therefore, his symptoms are likely related to excessive increase in serotonergic activity. Mr. S is admitted to the hospital for closer monitoring, and his sertraline, trazodone, and sumatriptan are held. He receives IV fluids for several days as well as cyproheptadine, 8 mg every 6 hours after stabilization, until his symptoms resolve. On Day 4, Mr. S no longer experiences diarrhea and internal restlessness. His vital signs return to normal, and as a result of symptom resolution, he is discharged from the hospital. The treatment team discusses changing his medication regimen to avoid multiple serotonergic agents. Mr. S is switched from sertraline to bupropion XL, 150 mg/d. Sumatriptan, 100 mg/d as needed, is continued for acute migraine treatment. Trazodone is discontinued and replaced with melatonin, 3 mg/d. The team also counsels Mr. S on the importance of proper adherence to his medication regimen. He is advised to return to the clinic in 2 weeks for reassessment of safety and efficacy.
Related Resource
- Turner AH, Kim JJ, McCarron RM. Differentiating serotonin syndrome and neuroleptic malignant syndrome. Current Psychiatry. 2019;18(2):30-36.
Drug Brand Names
Almotriptan • Axert
Buprenorphine • Subutex
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Cyproheptadine • Periactin
Eletriptan • Relpax
Frovatriptan • Frova
Granisetron • Kytril
Lisinopril • Prinivil, Zestril
Meperidine • Demerol
Methadone • Dolophine, Methadose
Metoclopramide • Reglan
Mirtazapine • Remeron
Naratriptan • Amerge
Olanzapine • Zyprexa
Ondansetron • Zofran
Rizatriptan • Maxalt
Sertraline • Zoloft
Sumatriptan • Imitrex tablets
Tapentadol • Nucynta
Tramadol • Conzip
Trazodone • Desyrel, Oleptro
Valproic acid • Depakene, Depakote
Vecuronium • Norcuron
Zolmitriptan • Zomig
1. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
2. Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18(4):395-400.
3. Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817.
4. Bartlett D. Drug-induced serotonin syndrome. Crit Care Nurse. 2017;37(1):49-54.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. Cyproheptadine hydrochloride tablets [package insert]. Hayward, CA: Impax Generics; 2017.
7. Deardorff OG, Khan T, Kulkarni G, et al. Serotonin syndrome: prophylactic treatment with cyproheptadine. Prim Care Companion CNS Disord. 2016;18(4). doi: 10.4088/PCC.16br01966.
1. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
2. Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18(4):395-400.
3. Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: preventing, recognizing, and treating it. Cleve Clin J Med. 2016;83(11):810-817.
4. Bartlett D. Drug-induced serotonin syndrome. Crit Care Nurse. 2017;37(1):49-54.
5. Frank C. Recognition and treatment of serotonin syndrome. Can Fam Physician. 2008;54(7):988-992.
6. Cyproheptadine hydrochloride tablets [package insert]. Hayward, CA: Impax Generics; 2017.
7. Deardorff OG, Khan T, Kulkarni G, et al. Serotonin syndrome: prophylactic treatment with cyproheptadine. Prim Care Companion CNS Disord. 2016;18(4). doi: 10.4088/PCC.16br01966.
Nothing to sneeze at: Upper respiratory infections and mood disorders
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
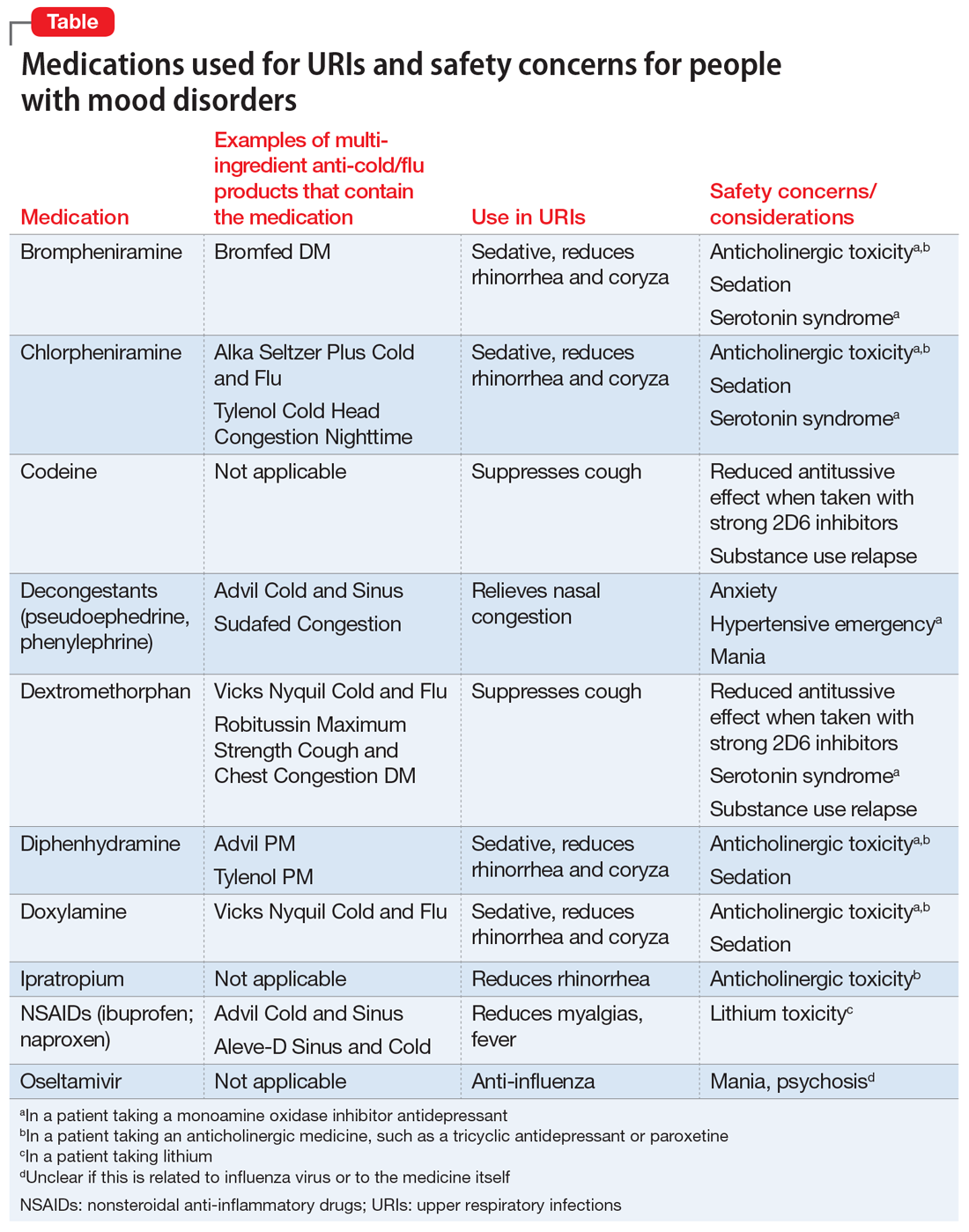
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Ketamine edges out ECT for refractory depression in small study
SAN FRANCISCO – Electroconvulsive therapy and ketamine both work well for refractory depression, but ketamine had the edge in a small, open label trial at the University of California, Los Angeles.
“Over the short term,” even a single ketamine infusion may “be as effective as ... ECT for reducing overall depression, apathy, anhedonia, and suicidal ideation,” but ECT may be more durable, said investigator Katherine Narr, PhD, an associate professor of neurology, psychiatry, and biobehavioral sciences at the school.
The study begins to address an issue that’s probably on the minds of many these days: ECT or ketamine for refractory depression? ELEKT-D (Clinicaltrials.gov NCT03113968), a large randomized, trial is underway to answer the question, but results aren’t expected for a couple of years.
In the meantime, although there was no randomization or blinding, Dr. Narr’s results are informative.
Twenty-six adults received one ketamine infusion, 0.5 mg/kg over 40 minutes, while 36 had four over about 2 weeks. Ketamine patients were allowed to stay on antidepressants. Forty-seven subjects, meanwhile, had 11 ECT treatments over 3 weeks, before which all psychiatric medications were stopped. The Hamilton Depression Rating Scale (HDRS) was used to assess outcomes.
Suicidal ideation probability dropped from 86% to 51% in the ECT group, but from 75% to 37% after one ketamine infusion, and to 11% after four (P less than .0001). A single “ketamine infusion showed similar probability of suicidal ideation reduction as a full course of ECT,” Dr. Narr said at the American Psychiatric Association annual meeting.
Improvements in overall HDRS scores were also greater after both single and serial ketamine (P less than .001).
However, HDRS scores – particularly for suicidal ideation – were beginning to creep up in the ketamine arm after just 5 weeks, but remained largely stable in the ECT group even at 3 months. In both groups, “therapeutic benefits for apathy and anhedonia last longer than for suicidal ideation,” Dr. Narr said.
At the moment, “you can’t predict who’s going to respond” better to one option or the other, “but I’m sure” biomarkers for that “are coming,” she said. Patients were 40 years old, on average, with depression first diagnosed in their early 20s. ECT subjects were equally split between the sexes, while there were more men than women in the ketamine arm, and current episodes were longer (average 6.6 years ketamine versus 3.7 years ECT). Baseline apathy scores were slightly higher in the ketamine group.
The work was funded by the National Institutes of Health. Dr. Narr didn’t have any disclosures.
SOURCE: Narr K et al., Presented at APA 2019
SAN FRANCISCO – Electroconvulsive therapy and ketamine both work well for refractory depression, but ketamine had the edge in a small, open label trial at the University of California, Los Angeles.
“Over the short term,” even a single ketamine infusion may “be as effective as ... ECT for reducing overall depression, apathy, anhedonia, and suicidal ideation,” but ECT may be more durable, said investigator Katherine Narr, PhD, an associate professor of neurology, psychiatry, and biobehavioral sciences at the school.
The study begins to address an issue that’s probably on the minds of many these days: ECT or ketamine for refractory depression? ELEKT-D (Clinicaltrials.gov NCT03113968), a large randomized, trial is underway to answer the question, but results aren’t expected for a couple of years.
In the meantime, although there was no randomization or blinding, Dr. Narr’s results are informative.
Twenty-six adults received one ketamine infusion, 0.5 mg/kg over 40 minutes, while 36 had four over about 2 weeks. Ketamine patients were allowed to stay on antidepressants. Forty-seven subjects, meanwhile, had 11 ECT treatments over 3 weeks, before which all psychiatric medications were stopped. The Hamilton Depression Rating Scale (HDRS) was used to assess outcomes.
Suicidal ideation probability dropped from 86% to 51% in the ECT group, but from 75% to 37% after one ketamine infusion, and to 11% after four (P less than .0001). A single “ketamine infusion showed similar probability of suicidal ideation reduction as a full course of ECT,” Dr. Narr said at the American Psychiatric Association annual meeting.
Improvements in overall HDRS scores were also greater after both single and serial ketamine (P less than .001).
However, HDRS scores – particularly for suicidal ideation – were beginning to creep up in the ketamine arm after just 5 weeks, but remained largely stable in the ECT group even at 3 months. In both groups, “therapeutic benefits for apathy and anhedonia last longer than for suicidal ideation,” Dr. Narr said.
At the moment, “you can’t predict who’s going to respond” better to one option or the other, “but I’m sure” biomarkers for that “are coming,” she said. Patients were 40 years old, on average, with depression first diagnosed in their early 20s. ECT subjects were equally split between the sexes, while there were more men than women in the ketamine arm, and current episodes were longer (average 6.6 years ketamine versus 3.7 years ECT). Baseline apathy scores were slightly higher in the ketamine group.
The work was funded by the National Institutes of Health. Dr. Narr didn’t have any disclosures.
SOURCE: Narr K et al., Presented at APA 2019
SAN FRANCISCO – Electroconvulsive therapy and ketamine both work well for refractory depression, but ketamine had the edge in a small, open label trial at the University of California, Los Angeles.
“Over the short term,” even a single ketamine infusion may “be as effective as ... ECT for reducing overall depression, apathy, anhedonia, and suicidal ideation,” but ECT may be more durable, said investigator Katherine Narr, PhD, an associate professor of neurology, psychiatry, and biobehavioral sciences at the school.
The study begins to address an issue that’s probably on the minds of many these days: ECT or ketamine for refractory depression? ELEKT-D (Clinicaltrials.gov NCT03113968), a large randomized, trial is underway to answer the question, but results aren’t expected for a couple of years.
In the meantime, although there was no randomization or blinding, Dr. Narr’s results are informative.
Twenty-six adults received one ketamine infusion, 0.5 mg/kg over 40 minutes, while 36 had four over about 2 weeks. Ketamine patients were allowed to stay on antidepressants. Forty-seven subjects, meanwhile, had 11 ECT treatments over 3 weeks, before which all psychiatric medications were stopped. The Hamilton Depression Rating Scale (HDRS) was used to assess outcomes.
Suicidal ideation probability dropped from 86% to 51% in the ECT group, but from 75% to 37% after one ketamine infusion, and to 11% after four (P less than .0001). A single “ketamine infusion showed similar probability of suicidal ideation reduction as a full course of ECT,” Dr. Narr said at the American Psychiatric Association annual meeting.
Improvements in overall HDRS scores were also greater after both single and serial ketamine (P less than .001).
However, HDRS scores – particularly for suicidal ideation – were beginning to creep up in the ketamine arm after just 5 weeks, but remained largely stable in the ECT group even at 3 months. In both groups, “therapeutic benefits for apathy and anhedonia last longer than for suicidal ideation,” Dr. Narr said.
At the moment, “you can’t predict who’s going to respond” better to one option or the other, “but I’m sure” biomarkers for that “are coming,” she said. Patients were 40 years old, on average, with depression first diagnosed in their early 20s. ECT subjects were equally split between the sexes, while there were more men than women in the ketamine arm, and current episodes were longer (average 6.6 years ketamine versus 3.7 years ECT). Baseline apathy scores were slightly higher in the ketamine group.
The work was funded by the National Institutes of Health. Dr. Narr didn’t have any disclosures.
SOURCE: Narr K et al., Presented at APA 2019
REPORTING FROM APA 2019
Sexual assault in military linked to sexual pain
according to an observational study involving interviews with more than 1,000 military women.
Female veterans with histories of both childhood sexual abuse and sexual assault in the military were 4.33 times more likely to report sexual pain than female veterans with no history of sexual assault; women whose history of sexual assault occurred in the military only were 2.37 times more likely to report sexual pain. Those with histories of childhood sexual abuse but no military assaults were 1.75 times more likely to report sexual pain than those who had no history of sexual assault.
The findings suggest that sexual assault in the military is more detrimental to sexual function than childhood sexual abuse alone, which “is distinct from the pattern long observed in civilian women that childhood sexual abuse confers a greater risk for sexual pain than adulthood sexual assault,” Carey S. Pulverman, PhD, then of the Department of Veterans Affairs Center of Excellence for Research on Returning War Veterans in Waco, Tex., and coinvestigators wrote in Obstetrics & Gynecology.
The findings come from a secondary analysis of data collected for a larger project titled Sexual Violence and Women Veterans’ Gynecologic Health . The research team conducted telephone interviews with 1,004 female veterans younger than 52 years of age (mean, 38 years) who were enrolled at two large Midwestern VA medical centers and associated clinics. Sexual pain was assessed by one question: “Does it hurt you to have sexual intercourse or penetration?”
The study also identified high comorbidity between sexual pain and mental health concerns. As with sexual pain, rates of depression and PTSD were highest among female veterans with histories of both sexual abuse in childhood and sexual assault in the military, followed by women with histories of sexual assaults in the military alone, and then women with histories of childhood sexual abuse alone. Women with both histories were 6.35 times more likely to report PTSD, and 3.91 times more likely to report depression, compared with female veterans with no history of sexual assault.
Women who experienced sexual assault during their childhood and/or while serving in the military also may have been exposed to sexual assault during their pre- or postmilitary adulthood as well, but this was a small number and its effects were not evaluated, the authors noted.
Especially given the “growing numbers of women serving in the military and prevalence of sexual assault in this population,” there’s a need for more research on the sexual function of female veterans and development of “targeted treatments,” the investigators wrote.
For now, providers should be “more comprehensive in their assessment of sexual assault history” and should consider developing relationships with community providers who specialize in sexual health, they added.
The study was funded by the VA. The authors did not report any relevant financial disclosures.
SOURCE: Pulverman CS et al. Obstet Gynecol. 2019;134:63-71.
according to an observational study involving interviews with more than 1,000 military women.
Female veterans with histories of both childhood sexual abuse and sexual assault in the military were 4.33 times more likely to report sexual pain than female veterans with no history of sexual assault; women whose history of sexual assault occurred in the military only were 2.37 times more likely to report sexual pain. Those with histories of childhood sexual abuse but no military assaults were 1.75 times more likely to report sexual pain than those who had no history of sexual assault.
The findings suggest that sexual assault in the military is more detrimental to sexual function than childhood sexual abuse alone, which “is distinct from the pattern long observed in civilian women that childhood sexual abuse confers a greater risk for sexual pain than adulthood sexual assault,” Carey S. Pulverman, PhD, then of the Department of Veterans Affairs Center of Excellence for Research on Returning War Veterans in Waco, Tex., and coinvestigators wrote in Obstetrics & Gynecology.
The findings come from a secondary analysis of data collected for a larger project titled Sexual Violence and Women Veterans’ Gynecologic Health . The research team conducted telephone interviews with 1,004 female veterans younger than 52 years of age (mean, 38 years) who were enrolled at two large Midwestern VA medical centers and associated clinics. Sexual pain was assessed by one question: “Does it hurt you to have sexual intercourse or penetration?”
The study also identified high comorbidity between sexual pain and mental health concerns. As with sexual pain, rates of depression and PTSD were highest among female veterans with histories of both sexual abuse in childhood and sexual assault in the military, followed by women with histories of sexual assaults in the military alone, and then women with histories of childhood sexual abuse alone. Women with both histories were 6.35 times more likely to report PTSD, and 3.91 times more likely to report depression, compared with female veterans with no history of sexual assault.
Women who experienced sexual assault during their childhood and/or while serving in the military also may have been exposed to sexual assault during their pre- or postmilitary adulthood as well, but this was a small number and its effects were not evaluated, the authors noted.
Especially given the “growing numbers of women serving in the military and prevalence of sexual assault in this population,” there’s a need for more research on the sexual function of female veterans and development of “targeted treatments,” the investigators wrote.
For now, providers should be “more comprehensive in their assessment of sexual assault history” and should consider developing relationships with community providers who specialize in sexual health, they added.
The study was funded by the VA. The authors did not report any relevant financial disclosures.
SOURCE: Pulverman CS et al. Obstet Gynecol. 2019;134:63-71.
according to an observational study involving interviews with more than 1,000 military women.
Female veterans with histories of both childhood sexual abuse and sexual assault in the military were 4.33 times more likely to report sexual pain than female veterans with no history of sexual assault; women whose history of sexual assault occurred in the military only were 2.37 times more likely to report sexual pain. Those with histories of childhood sexual abuse but no military assaults were 1.75 times more likely to report sexual pain than those who had no history of sexual assault.
The findings suggest that sexual assault in the military is more detrimental to sexual function than childhood sexual abuse alone, which “is distinct from the pattern long observed in civilian women that childhood sexual abuse confers a greater risk for sexual pain than adulthood sexual assault,” Carey S. Pulverman, PhD, then of the Department of Veterans Affairs Center of Excellence for Research on Returning War Veterans in Waco, Tex., and coinvestigators wrote in Obstetrics & Gynecology.
The findings come from a secondary analysis of data collected for a larger project titled Sexual Violence and Women Veterans’ Gynecologic Health . The research team conducted telephone interviews with 1,004 female veterans younger than 52 years of age (mean, 38 years) who were enrolled at two large Midwestern VA medical centers and associated clinics. Sexual pain was assessed by one question: “Does it hurt you to have sexual intercourse or penetration?”
The study also identified high comorbidity between sexual pain and mental health concerns. As with sexual pain, rates of depression and PTSD were highest among female veterans with histories of both sexual abuse in childhood and sexual assault in the military, followed by women with histories of sexual assaults in the military alone, and then women with histories of childhood sexual abuse alone. Women with both histories were 6.35 times more likely to report PTSD, and 3.91 times more likely to report depression, compared with female veterans with no history of sexual assault.
Women who experienced sexual assault during their childhood and/or while serving in the military also may have been exposed to sexual assault during their pre- or postmilitary adulthood as well, but this was a small number and its effects were not evaluated, the authors noted.
Especially given the “growing numbers of women serving in the military and prevalence of sexual assault in this population,” there’s a need for more research on the sexual function of female veterans and development of “targeted treatments,” the investigators wrote.
For now, providers should be “more comprehensive in their assessment of sexual assault history” and should consider developing relationships with community providers who specialize in sexual health, they added.
The study was funded by the VA. The authors did not report any relevant financial disclosures.
SOURCE: Pulverman CS et al. Obstet Gynecol. 2019;134:63-71.
FROM OBSTETRICS & GYNECOLOGY
Anticholinergic drugs linked to dementia in older populations
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
Exposures to various types of anticholinergic medications were associated with a significantly increased risk of dementia in people aged 55 years or older in a large pharmacoepidemiologic study.
“This study was designed to assess the association between cumulative anticholinergic drug use and risk of dementia in a large, representative British population,” wrote Carol A. C. Coupland, PhD, of the division of primary care at the University of Nottingham (England), and colleagues. The findings were published in JAMA Internal Medicine.
The researchers conducted a large nested case-control study that included 58,769 patients with dementia and 225,574 matched controls from the QResearch database in England. Each study participant was matched to five controls based on various characteristics, including sex, age, and calendar time, among others.
Prescription data related to 56 different drugs with strong anticholinergic properties, including antipsychotics, bladder antimuscarinics, antiepileptics, antiparkinson agents, and antidepressants were used to measure drug exposure. The study data were analyzed from 2016 to 2018.
“The primary exposure was the total standardized daily doses (TSDDs) of anticholinergic drugs prescribed in the 1 to 11 years prior to the date of diagnosis of dementia or equivalent date in matched controls,” Dr. Coupland and colleagues wrote.
After analysis, the researchers found that exposure to antipsychotics (adjusted odds ratio, 1.70), bladder antimuscarinics (aOR, 1.65), antiepileptics (aOR, 1.39), antiparkinson agents (aOR, 1.52), and anticholinergic antidepressants (aOR, 1.29) was associated with an increased risk of dementia after adjustment for confounding factors.
“Associations were stronger in [dementia] cases diagnosed before the age of 80 years,” the researchers noted.
However, antihistamine, antivertigo/antiemetic, skeletal muscle relaxant, gastrointestinal antispasmodic, antiarrhythmic, and antimuscarinic bronchodilator anticholinergic agents were not associated with any increased risk of dementia.
One key limitation of the study was the absence of medication compliance assessment, which could result in exposure misclassification. Dr. Coupland and colleagues acknowledged this could underestimate some associations with medication exposure.
The stronger risk of dementia found among people who had dementia before age 80 “indicates that anticholinergic drugs should be prescribed with caution in middle-aged and older people,” they concluded.
One question that remains from the current study is whether anticholinergic drugs are a definite modifiable risk factor for Alzheimer’s disease and related dementias, Noll L. Campbell, PharmD, of Purdue University, West Lafayette, Ind., and colleagues wrote in an editorial accompanying the study by Dr. Coupland and associates (JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0676).
While a pharmacologic basis for this association has been proposed, causation has yet to be established by means of prospective randomized studies. The current supposition is that deprescribing anticholinergic medications has the potential to positively effect cholinergic neurotransmission in certain regions of the brain, which could lead to improved cognitive functioning, and lower the likelihood of developing Alzheimer’s disease and related dementias, they wrote in the editorial.
However, the discontinuation of some anticholinergic agents may pose other risks, such as worsening pain or depressive symptoms, in addition to increasing the utilization of acute care facilities. As a result, high-quality, well-designed, randomized trials are needed to better understand the long-term effects of deprescribing anticholinergic medications. These trials would help inform clinicians, patients, and policymakers about the risks and benefits of deprescribing interventions, Dr. Campbell and coauthors said.
The study was supported by the National Institute for Health Research and the University of Nottingham. The authors reported financial affiliations with ClinRisk Ltd. The authors of the editorial reported receiving support from the National Institute on Aging and the Agency for Healthcare Research and Quality. Dr. Campbell reported receiving personal fees from Astellas Pharma US.
SOURCE: Coupland C et al. JAMA Intern Med. 2019 Jun 24. doi: 10.1001/jamainternmed.2019.0677
FROM JAMA INTERNAL MEDICINE
Case shows power of collaborative care for depression
Remission rate for Boeing employees climbed from 10% to 35%
SAN FRANCISCO – Under an accountable care contract with airplane maker Boeing, the University of Washington, Seattle, increased the rate of depression remission from about 10% to 35%, and the number of people in remission improved, based on Patient Health Questionnaire (PHQ-9) scores, from 20% to 70% – both in less than a year.
Boeing was particularly concerned about depression among its roughly 27,000 Puget Sound–area employees when it entered a contract with the University of Washington (UW) a few years ago for health services. Workers with depression are less likely to show up to work, and when they do, they are more likely to make mistakes and cause safety problems. To ensure that the university addressed the problem, Boeing tied payments to improved depression scores.
It didn’t take UW long to meet the PHQ-9 targets for improvement and remission, meaning a score below 5 points. Boeing also wanted its employees to be screened annually for depression and repeated testing of patients with depression to track how well they were doing. The university increased the number of patients rescreened within 8 weeks of their first PHQ-9 from about 45% to 75% – also in less than a year.
It simply scaled up the approach to meet Boeing’s targets.
“This has been an interesting journey,” said Jürgen Unützer, MD, MPH, who has been key to the efforts. “It’s required quite a bit of work, but it can be done. We’ve made a lot of progress,” he said at the American Psychiatric Association annual meeting.
Key components, besides the primary care provider, include evidence-based treatment, a mental health case manager, a system to track outcomes, and a psychiatrist to consult when patients do not improve. It’s a team approach.
Dr. Unützer and his colleagues have proved that it can work among older adults with depression and, in the end, save money (Am J Manag Care. 2008 Feb;14[2]:95-100). They’ve even published a how-to book, “Integrated Care: Creating Effective Mental and Primary Health Care Teams” (John Wiley & Sons, 2016).
A key challenge with Boeing was making sure that depressed patients returned for follow-up care and repeat PHQ-9s, and that they did not languish on ineffective treatments.
“We explain [to them that] this is not just a one-time thing,” said Dr. Unützer, chair of psychiatry and behavioral sciences at UW. “We [will] keep with them until they are well.”
Patients are enrolled in the patient portal on UW’s Epic records system to facilitate communication. The system sends out follow-up reminders, and sometimes it is used to send PHQ-9s directly to patients.
“We have automated this as much as possible.” When there’s no response, patients often are sent text messages or called by phone to make sure that they are doing OK and taking their medicine, he said.
Chart reviews are used to identify patients who are not improving. “We reach out to primary care and say, ‘We think you could use some help.’ It’s not always ”a comfortable conversation. “A lot of us like to assume our patients are getting better,” Dr. Unützer said.
Overall, “this notion of population-based care – the idea that ... you have a whole bucket of patients out there you might have seen at some point” but are still responsible for – “is a total change for most of us who are practicing clinicians,” he said.
Dr. Unützer did not report any disclosures.
Remission rate for Boeing employees climbed from 10% to 35%
Remission rate for Boeing employees climbed from 10% to 35%
SAN FRANCISCO – Under an accountable care contract with airplane maker Boeing, the University of Washington, Seattle, increased the rate of depression remission from about 10% to 35%, and the number of people in remission improved, based on Patient Health Questionnaire (PHQ-9) scores, from 20% to 70% – both in less than a year.
Boeing was particularly concerned about depression among its roughly 27,000 Puget Sound–area employees when it entered a contract with the University of Washington (UW) a few years ago for health services. Workers with depression are less likely to show up to work, and when they do, they are more likely to make mistakes and cause safety problems. To ensure that the university addressed the problem, Boeing tied payments to improved depression scores.
It didn’t take UW long to meet the PHQ-9 targets for improvement and remission, meaning a score below 5 points. Boeing also wanted its employees to be screened annually for depression and repeated testing of patients with depression to track how well they were doing. The university increased the number of patients rescreened within 8 weeks of their first PHQ-9 from about 45% to 75% – also in less than a year.
It simply scaled up the approach to meet Boeing’s targets.
“This has been an interesting journey,” said Jürgen Unützer, MD, MPH, who has been key to the efforts. “It’s required quite a bit of work, but it can be done. We’ve made a lot of progress,” he said at the American Psychiatric Association annual meeting.
Key components, besides the primary care provider, include evidence-based treatment, a mental health case manager, a system to track outcomes, and a psychiatrist to consult when patients do not improve. It’s a team approach.
Dr. Unützer and his colleagues have proved that it can work among older adults with depression and, in the end, save money (Am J Manag Care. 2008 Feb;14[2]:95-100). They’ve even published a how-to book, “Integrated Care: Creating Effective Mental and Primary Health Care Teams” (John Wiley & Sons, 2016).
A key challenge with Boeing was making sure that depressed patients returned for follow-up care and repeat PHQ-9s, and that they did not languish on ineffective treatments.
“We explain [to them that] this is not just a one-time thing,” said Dr. Unützer, chair of psychiatry and behavioral sciences at UW. “We [will] keep with them until they are well.”
Patients are enrolled in the patient portal on UW’s Epic records system to facilitate communication. The system sends out follow-up reminders, and sometimes it is used to send PHQ-9s directly to patients.
“We have automated this as much as possible.” When there’s no response, patients often are sent text messages or called by phone to make sure that they are doing OK and taking their medicine, he said.
Chart reviews are used to identify patients who are not improving. “We reach out to primary care and say, ‘We think you could use some help.’ It’s not always ”a comfortable conversation. “A lot of us like to assume our patients are getting better,” Dr. Unützer said.
Overall, “this notion of population-based care – the idea that ... you have a whole bucket of patients out there you might have seen at some point” but are still responsible for – “is a total change for most of us who are practicing clinicians,” he said.
Dr. Unützer did not report any disclosures.
SAN FRANCISCO – Under an accountable care contract with airplane maker Boeing, the University of Washington, Seattle, increased the rate of depression remission from about 10% to 35%, and the number of people in remission improved, based on Patient Health Questionnaire (PHQ-9) scores, from 20% to 70% – both in less than a year.
Boeing was particularly concerned about depression among its roughly 27,000 Puget Sound–area employees when it entered a contract with the University of Washington (UW) a few years ago for health services. Workers with depression are less likely to show up to work, and when they do, they are more likely to make mistakes and cause safety problems. To ensure that the university addressed the problem, Boeing tied payments to improved depression scores.
It didn’t take UW long to meet the PHQ-9 targets for improvement and remission, meaning a score below 5 points. Boeing also wanted its employees to be screened annually for depression and repeated testing of patients with depression to track how well they were doing. The university increased the number of patients rescreened within 8 weeks of their first PHQ-9 from about 45% to 75% – also in less than a year.
It simply scaled up the approach to meet Boeing’s targets.
“This has been an interesting journey,” said Jürgen Unützer, MD, MPH, who has been key to the efforts. “It’s required quite a bit of work, but it can be done. We’ve made a lot of progress,” he said at the American Psychiatric Association annual meeting.
Key components, besides the primary care provider, include evidence-based treatment, a mental health case manager, a system to track outcomes, and a psychiatrist to consult when patients do not improve. It’s a team approach.
Dr. Unützer and his colleagues have proved that it can work among older adults with depression and, in the end, save money (Am J Manag Care. 2008 Feb;14[2]:95-100). They’ve even published a how-to book, “Integrated Care: Creating Effective Mental and Primary Health Care Teams” (John Wiley & Sons, 2016).
A key challenge with Boeing was making sure that depressed patients returned for follow-up care and repeat PHQ-9s, and that they did not languish on ineffective treatments.
“We explain [to them that] this is not just a one-time thing,” said Dr. Unützer, chair of psychiatry and behavioral sciences at UW. “We [will] keep with them until they are well.”
Patients are enrolled in the patient portal on UW’s Epic records system to facilitate communication. The system sends out follow-up reminders, and sometimes it is used to send PHQ-9s directly to patients.
“We have automated this as much as possible.” When there’s no response, patients often are sent text messages or called by phone to make sure that they are doing OK and taking their medicine, he said.
Chart reviews are used to identify patients who are not improving. “We reach out to primary care and say, ‘We think you could use some help.’ It’s not always ”a comfortable conversation. “A lot of us like to assume our patients are getting better,” Dr. Unützer said.
Overall, “this notion of population-based care – the idea that ... you have a whole bucket of patients out there you might have seen at some point” but are still responsible for – “is a total change for most of us who are practicing clinicians,” he said.
Dr. Unützer did not report any disclosures.
REPORTING FROM APA 2019
Why we need another article on suicide contracts
Every guideline and lecture on suicide risk assessment includes the message: “Do not use suicide contracts.” Yet, as forensic psychiatrists, we continue to see medical records that rely solely on the patient verbalizing, agreeing, or signing that they will be safe, in order to justify medical decision-making. A recent case we reviewed involving a grossly psychotic male spotlighted the meaninglessness of suicide contracts. In an attempt to understand the impulse by clinicians to use suicide contracts, we decided to review the topic.
Suicide risk assessment is a confusing and poorly explained skill in our field. Suicide risk assessment tools are well-intended. They are meant to identify and stratify risk, and help guide medical decision-making. Popular tools are startlingly different. How can two scales represent adequate psychiatric knowledge yet be completely different? SADPERSONS1 is widely used and still considered standard of care yet has nothing in common with the Columbia–Suicide Severity Rating Scale (CSSRS).2
For those of us working in forensic settings, we are aghast that neither assessment is modified for use in correctional settings or accounts for essential risk factors of suicide in jails and prisons (placement in solitary, significant charges, homeless, etc.) Yet, they are widely used in jails and prisons across the country. This can be extrapolated to all of us who work with specific populations yet are asked to follow generic scales by administrators.
In reviewing the literature, we are surprised to see the lack of acknowledgment that many tools used in suicide risk assessment have little to no evidence. Despite their numerous appearances in medical records that we review, we are not aware of existing evidence for asking patients whether patients are suicidal on an hourly basis, for psychotropic treatment other than lithium and clozapine (Clozaril), and for safety plans that involve telling the patient to call 911. Of even greater concern, suicide risk assessments themselves may have limited value because of a lack of evidence as suggested by large study findings. It may surprise some to learn that the National Institute for Health and Care Excellence (NICE) in the United Kingdom includes the following statement in its guidelines: “Do not use risk assessment tools and scales to predict future suicide or repetition of self-harm.”3
In 2017, Carter et al.4 reviewed 70 studies using suicide risk scales to stratify patients in higher-risk groups for self-harm or suicide, during a follow-up period. The study reviewed biological tests such as the dexamethasone suppression test and 5-hydroxyindoleacetic acid; as well as psychological scales, including Buglass & Horton, SADPERSONS, the Beck Hopelessness Scale, the Beck’s Depression Inventory, Manchester Self Harm Rule, and the Edinburgh Risk Rating Scale. Their conclusion was clear: “No individual predictive instrument or pooled subgroups of instruments were able to classify patients as being at high risk of suicidal behavior with a level of accuracy suitable to be used to allocate treatment.”
Despite the bad reputation, one must admit that suicide contracts intuitively feel right. Just as we ask patients whether they believe they will stay sober in the future, or ask patients if they will be compliant with their psychotropics, asking them if they feel that they can maintain safety seems relevant. Reading through the literature, one can even find articles promoting this approach. In 2011, researchers simply asked 147 patients in psychiatric hospitals considered to be high risk for suicide whether they would engage in self-harm in the following weeks. They followed those patients for 15 weeks after their discharge for acts of self-harm. They concluded that “self-perceptions of risk seem to perform as well as the best [standardized assessment tools] the field has to offer” for the prediction of self-harm.5 We are unconvinced that juries would find suicide contracts irrelevant despite the lack of evidence. American society values individual autonomy and self-decision making. Patients telling their clinicians, “I will be OK” is relevant to suicide risk assessment. One can argue that the problem is not with the suicide contract itself, but with its blind use as a marker of safety.
The standard of care dictates that we try to assess suicide risk using evidence-based techniques. To the providers who see merit in asking patients whether they will be able to maintain their safety, we empathize with this impulse despite the lack of evidence. This will contribute in our shared effort to minimize suicide.
We acknowledge that the evidence of any assessment is limited and might miss a greater point in this entire discussion: Why are new iterations of suicide risk assessments not an improvement on the prior ones but a competing theory? New assessments emphasizing different facets of suicidal thinking do not include key demographic factors, while older tools do not include more recent understanding, such as the importance of hopelessness. From a provider’s perspective, the debate appears to be a battle of trends, theories, and acronyms rather than comprehensive analysis of the latest evidence. We, therefore, are concerned by “suicide experts” who advocate for any one assessment as the only gold standard and give false hopes about its efficacy.
As suicide rates continue to climb across the country, one wonders what we, as psychiatrists, are trying to achieve. Promises of zero suicides by hospitals,6 academic institutions,7 and even governments8 are well-meaning but possibly misleading to families and patients. Psychiatry should advocate within the standard of care for reasonable attempts at suicide risk assessment, including demographic factors (see SADPERSONS), as well as examination of the actual suicidality (see the CSSRS). Our professional organizations should clarify expectations for clinicians while also clarifying the limitations of our current knowledge base.
References
1. Patterson WM et al. Evaluation of suicidal patients: the SADPERSONS scale. Psychosomatics. 1983 Apr;24[4]:343-5, 348-9.
2. Posner K et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011 Dec;168(12):1266-77.
3. Kendall T et al. Longer term management of self harm: summary of NICE guidance. BMJ. 2011;343. doi: 10.1136/bmj.d7073.
4. Carter G et al. Predicting suicidal behaviors using clinical instruments: systematic review and meta-analysis of positive predictive values for risk scales. Br J Psychiatry. 2017 Jun;210(6):387-95.
5. Peterson J et al. If you want to know, consider asking: How likely is it that patients will hurt themselves in the future? Psychol Assess. 2011 Sep;23(3):626-34.
5. Byrne JM et al. Implementation and impact of the central district of California’s suicide prevention program for crime defendants. Federal Probation. 2012 Jun;76(1):3-13.
6. “R.I.’s Butler Hospital sets ‘zero suicide’ goal for patients”/audio. Providence Journal. May 15, 2018.
7. “NIMH funds 3 ‘zero suicide’ grants.” National Institute of Mental Health. Sep 16, 2016.
8. Rothschild N. “Is it possible to eliminate suicide?” Atlantic. Jun 5, 2015.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. Dr. Rao is a San Diego–based board-certified psychiatrist with expertise in forensic psychiatry, correctional psychiatry, telepsychiatry, and inpatient psychiatry.
Every guideline and lecture on suicide risk assessment includes the message: “Do not use suicide contracts.” Yet, as forensic psychiatrists, we continue to see medical records that rely solely on the patient verbalizing, agreeing, or signing that they will be safe, in order to justify medical decision-making. A recent case we reviewed involving a grossly psychotic male spotlighted the meaninglessness of suicide contracts. In an attempt to understand the impulse by clinicians to use suicide contracts, we decided to review the topic.
Suicide risk assessment is a confusing and poorly explained skill in our field. Suicide risk assessment tools are well-intended. They are meant to identify and stratify risk, and help guide medical decision-making. Popular tools are startlingly different. How can two scales represent adequate psychiatric knowledge yet be completely different? SADPERSONS1 is widely used and still considered standard of care yet has nothing in common with the Columbia–Suicide Severity Rating Scale (CSSRS).2
For those of us working in forensic settings, we are aghast that neither assessment is modified for use in correctional settings or accounts for essential risk factors of suicide in jails and prisons (placement in solitary, significant charges, homeless, etc.) Yet, they are widely used in jails and prisons across the country. This can be extrapolated to all of us who work with specific populations yet are asked to follow generic scales by administrators.
In reviewing the literature, we are surprised to see the lack of acknowledgment that many tools used in suicide risk assessment have little to no evidence. Despite their numerous appearances in medical records that we review, we are not aware of existing evidence for asking patients whether patients are suicidal on an hourly basis, for psychotropic treatment other than lithium and clozapine (Clozaril), and for safety plans that involve telling the patient to call 911. Of even greater concern, suicide risk assessments themselves may have limited value because of a lack of evidence as suggested by large study findings. It may surprise some to learn that the National Institute for Health and Care Excellence (NICE) in the United Kingdom includes the following statement in its guidelines: “Do not use risk assessment tools and scales to predict future suicide or repetition of self-harm.”3
In 2017, Carter et al.4 reviewed 70 studies using suicide risk scales to stratify patients in higher-risk groups for self-harm or suicide, during a follow-up period. The study reviewed biological tests such as the dexamethasone suppression test and 5-hydroxyindoleacetic acid; as well as psychological scales, including Buglass & Horton, SADPERSONS, the Beck Hopelessness Scale, the Beck’s Depression Inventory, Manchester Self Harm Rule, and the Edinburgh Risk Rating Scale. Their conclusion was clear: “No individual predictive instrument or pooled subgroups of instruments were able to classify patients as being at high risk of suicidal behavior with a level of accuracy suitable to be used to allocate treatment.”
Despite the bad reputation, one must admit that suicide contracts intuitively feel right. Just as we ask patients whether they believe they will stay sober in the future, or ask patients if they will be compliant with their psychotropics, asking them if they feel that they can maintain safety seems relevant. Reading through the literature, one can even find articles promoting this approach. In 2011, researchers simply asked 147 patients in psychiatric hospitals considered to be high risk for suicide whether they would engage in self-harm in the following weeks. They followed those patients for 15 weeks after their discharge for acts of self-harm. They concluded that “self-perceptions of risk seem to perform as well as the best [standardized assessment tools] the field has to offer” for the prediction of self-harm.5 We are unconvinced that juries would find suicide contracts irrelevant despite the lack of evidence. American society values individual autonomy and self-decision making. Patients telling their clinicians, “I will be OK” is relevant to suicide risk assessment. One can argue that the problem is not with the suicide contract itself, but with its blind use as a marker of safety.
The standard of care dictates that we try to assess suicide risk using evidence-based techniques. To the providers who see merit in asking patients whether they will be able to maintain their safety, we empathize with this impulse despite the lack of evidence. This will contribute in our shared effort to minimize suicide.
We acknowledge that the evidence of any assessment is limited and might miss a greater point in this entire discussion: Why are new iterations of suicide risk assessments not an improvement on the prior ones but a competing theory? New assessments emphasizing different facets of suicidal thinking do not include key demographic factors, while older tools do not include more recent understanding, such as the importance of hopelessness. From a provider’s perspective, the debate appears to be a battle of trends, theories, and acronyms rather than comprehensive analysis of the latest evidence. We, therefore, are concerned by “suicide experts” who advocate for any one assessment as the only gold standard and give false hopes about its efficacy.
As suicide rates continue to climb across the country, one wonders what we, as psychiatrists, are trying to achieve. Promises of zero suicides by hospitals,6 academic institutions,7 and even governments8 are well-meaning but possibly misleading to families and patients. Psychiatry should advocate within the standard of care for reasonable attempts at suicide risk assessment, including demographic factors (see SADPERSONS), as well as examination of the actual suicidality (see the CSSRS). Our professional organizations should clarify expectations for clinicians while also clarifying the limitations of our current knowledge base.
References
1. Patterson WM et al. Evaluation of suicidal patients: the SADPERSONS scale. Psychosomatics. 1983 Apr;24[4]:343-5, 348-9.
2. Posner K et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011 Dec;168(12):1266-77.
3. Kendall T et al. Longer term management of self harm: summary of NICE guidance. BMJ. 2011;343. doi: 10.1136/bmj.d7073.
4. Carter G et al. Predicting suicidal behaviors using clinical instruments: systematic review and meta-analysis of positive predictive values for risk scales. Br J Psychiatry. 2017 Jun;210(6):387-95.
5. Peterson J et al. If you want to know, consider asking: How likely is it that patients will hurt themselves in the future? Psychol Assess. 2011 Sep;23(3):626-34.
5. Byrne JM et al. Implementation and impact of the central district of California’s suicide prevention program for crime defendants. Federal Probation. 2012 Jun;76(1):3-13.
6. “R.I.’s Butler Hospital sets ‘zero suicide’ goal for patients”/audio. Providence Journal. May 15, 2018.
7. “NIMH funds 3 ‘zero suicide’ grants.” National Institute of Mental Health. Sep 16, 2016.
8. Rothschild N. “Is it possible to eliminate suicide?” Atlantic. Jun 5, 2015.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. Dr. Rao is a San Diego–based board-certified psychiatrist with expertise in forensic psychiatry, correctional psychiatry, telepsychiatry, and inpatient psychiatry.
Every guideline and lecture on suicide risk assessment includes the message: “Do not use suicide contracts.” Yet, as forensic psychiatrists, we continue to see medical records that rely solely on the patient verbalizing, agreeing, or signing that they will be safe, in order to justify medical decision-making. A recent case we reviewed involving a grossly psychotic male spotlighted the meaninglessness of suicide contracts. In an attempt to understand the impulse by clinicians to use suicide contracts, we decided to review the topic.
Suicide risk assessment is a confusing and poorly explained skill in our field. Suicide risk assessment tools are well-intended. They are meant to identify and stratify risk, and help guide medical decision-making. Popular tools are startlingly different. How can two scales represent adequate psychiatric knowledge yet be completely different? SADPERSONS1 is widely used and still considered standard of care yet has nothing in common with the Columbia–Suicide Severity Rating Scale (CSSRS).2
For those of us working in forensic settings, we are aghast that neither assessment is modified for use in correctional settings or accounts for essential risk factors of suicide in jails and prisons (placement in solitary, significant charges, homeless, etc.) Yet, they are widely used in jails and prisons across the country. This can be extrapolated to all of us who work with specific populations yet are asked to follow generic scales by administrators.
In reviewing the literature, we are surprised to see the lack of acknowledgment that many tools used in suicide risk assessment have little to no evidence. Despite their numerous appearances in medical records that we review, we are not aware of existing evidence for asking patients whether patients are suicidal on an hourly basis, for psychotropic treatment other than lithium and clozapine (Clozaril), and for safety plans that involve telling the patient to call 911. Of even greater concern, suicide risk assessments themselves may have limited value because of a lack of evidence as suggested by large study findings. It may surprise some to learn that the National Institute for Health and Care Excellence (NICE) in the United Kingdom includes the following statement in its guidelines: “Do not use risk assessment tools and scales to predict future suicide or repetition of self-harm.”3
In 2017, Carter et al.4 reviewed 70 studies using suicide risk scales to stratify patients in higher-risk groups for self-harm or suicide, during a follow-up period. The study reviewed biological tests such as the dexamethasone suppression test and 5-hydroxyindoleacetic acid; as well as psychological scales, including Buglass & Horton, SADPERSONS, the Beck Hopelessness Scale, the Beck’s Depression Inventory, Manchester Self Harm Rule, and the Edinburgh Risk Rating Scale. Their conclusion was clear: “No individual predictive instrument or pooled subgroups of instruments were able to classify patients as being at high risk of suicidal behavior with a level of accuracy suitable to be used to allocate treatment.”
Despite the bad reputation, one must admit that suicide contracts intuitively feel right. Just as we ask patients whether they believe they will stay sober in the future, or ask patients if they will be compliant with their psychotropics, asking them if they feel that they can maintain safety seems relevant. Reading through the literature, one can even find articles promoting this approach. In 2011, researchers simply asked 147 patients in psychiatric hospitals considered to be high risk for suicide whether they would engage in self-harm in the following weeks. They followed those patients for 15 weeks after their discharge for acts of self-harm. They concluded that “self-perceptions of risk seem to perform as well as the best [standardized assessment tools] the field has to offer” for the prediction of self-harm.5 We are unconvinced that juries would find suicide contracts irrelevant despite the lack of evidence. American society values individual autonomy and self-decision making. Patients telling their clinicians, “I will be OK” is relevant to suicide risk assessment. One can argue that the problem is not with the suicide contract itself, but with its blind use as a marker of safety.
The standard of care dictates that we try to assess suicide risk using evidence-based techniques. To the providers who see merit in asking patients whether they will be able to maintain their safety, we empathize with this impulse despite the lack of evidence. This will contribute in our shared effort to minimize suicide.
We acknowledge that the evidence of any assessment is limited and might miss a greater point in this entire discussion: Why are new iterations of suicide risk assessments not an improvement on the prior ones but a competing theory? New assessments emphasizing different facets of suicidal thinking do not include key demographic factors, while older tools do not include more recent understanding, such as the importance of hopelessness. From a provider’s perspective, the debate appears to be a battle of trends, theories, and acronyms rather than comprehensive analysis of the latest evidence. We, therefore, are concerned by “suicide experts” who advocate for any one assessment as the only gold standard and give false hopes about its efficacy.
As suicide rates continue to climb across the country, one wonders what we, as psychiatrists, are trying to achieve. Promises of zero suicides by hospitals,6 academic institutions,7 and even governments8 are well-meaning but possibly misleading to families and patients. Psychiatry should advocate within the standard of care for reasonable attempts at suicide risk assessment, including demographic factors (see SADPERSONS), as well as examination of the actual suicidality (see the CSSRS). Our professional organizations should clarify expectations for clinicians while also clarifying the limitations of our current knowledge base.
References
1. Patterson WM et al. Evaluation of suicidal patients: the SADPERSONS scale. Psychosomatics. 1983 Apr;24[4]:343-5, 348-9.
2. Posner K et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011 Dec;168(12):1266-77.
3. Kendall T et al. Longer term management of self harm: summary of NICE guidance. BMJ. 2011;343. doi: 10.1136/bmj.d7073.
4. Carter G et al. Predicting suicidal behaviors using clinical instruments: systematic review and meta-analysis of positive predictive values for risk scales. Br J Psychiatry. 2017 Jun;210(6):387-95.
5. Peterson J et al. If you want to know, consider asking: How likely is it that patients will hurt themselves in the future? Psychol Assess. 2011 Sep;23(3):626-34.
5. Byrne JM et al. Implementation and impact of the central district of California’s suicide prevention program for crime defendants. Federal Probation. 2012 Jun;76(1):3-13.
6. “R.I.’s Butler Hospital sets ‘zero suicide’ goal for patients”/audio. Providence Journal. May 15, 2018.
7. “NIMH funds 3 ‘zero suicide’ grants.” National Institute of Mental Health. Sep 16, 2016.
8. Rothschild N. “Is it possible to eliminate suicide?” Atlantic. Jun 5, 2015.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. Dr. Rao is a San Diego–based board-certified psychiatrist with expertise in forensic psychiatry, correctional psychiatry, telepsychiatry, and inpatient psychiatry.
Increased awareness needed of bipolar disorder in primary care
A significant number of patients with in primary care also have unrecognized bipolar disorder, according to researchers at the University of Manchester (England).
In a systematic review and meta-analysis published in General Hospital Psychiatry, the researchers searched Medline, Embase, Cochrane, and PsycINFO for that measured the rate of unrecognized bipolar disorder in primary care.
The overall prevalence of unrecognized bipolar disorder within the patient group was 17%; however, that rate varied significantly. Depending on the study, the range was 5%-28%. A subgroup analysis showed that studies that relied on clinical interviews for patients with confirmed bipolar disorder had lower rates than studies that relied on self-reporting. However, that difference did not reach statistical significance (14% vs. 22%; P = .121).
“There is ... an imperative need to improve the recognition of bipolar disorder in patients in primary care. A lack of effective training of primary care physicians, competing clinical demands, and reduced financial incentives ... are key reasons for the unrecognition of mental health conditions in primary care,” the investigators noted.
No conflicts of interest were reported.
SOURCE: Daveney J et al. Gen Hosp Psychiatry. 2019 Mar 27. doi: 10.1016/j.genhosppsych.2019.03.006.
A significant number of patients with in primary care also have unrecognized bipolar disorder, according to researchers at the University of Manchester (England).
In a systematic review and meta-analysis published in General Hospital Psychiatry, the researchers searched Medline, Embase, Cochrane, and PsycINFO for that measured the rate of unrecognized bipolar disorder in primary care.
The overall prevalence of unrecognized bipolar disorder within the patient group was 17%; however, that rate varied significantly. Depending on the study, the range was 5%-28%. A subgroup analysis showed that studies that relied on clinical interviews for patients with confirmed bipolar disorder had lower rates than studies that relied on self-reporting. However, that difference did not reach statistical significance (14% vs. 22%; P = .121).
“There is ... an imperative need to improve the recognition of bipolar disorder in patients in primary care. A lack of effective training of primary care physicians, competing clinical demands, and reduced financial incentives ... are key reasons for the unrecognition of mental health conditions in primary care,” the investigators noted.
No conflicts of interest were reported.
SOURCE: Daveney J et al. Gen Hosp Psychiatry. 2019 Mar 27. doi: 10.1016/j.genhosppsych.2019.03.006.
A significant number of patients with in primary care also have unrecognized bipolar disorder, according to researchers at the University of Manchester (England).
In a systematic review and meta-analysis published in General Hospital Psychiatry, the researchers searched Medline, Embase, Cochrane, and PsycINFO for that measured the rate of unrecognized bipolar disorder in primary care.
The overall prevalence of unrecognized bipolar disorder within the patient group was 17%; however, that rate varied significantly. Depending on the study, the range was 5%-28%. A subgroup analysis showed that studies that relied on clinical interviews for patients with confirmed bipolar disorder had lower rates than studies that relied on self-reporting. However, that difference did not reach statistical significance (14% vs. 22%; P = .121).
“There is ... an imperative need to improve the recognition of bipolar disorder in patients in primary care. A lack of effective training of primary care physicians, competing clinical demands, and reduced financial incentives ... are key reasons for the unrecognition of mental health conditions in primary care,” the investigators noted.
No conflicts of interest were reported.
SOURCE: Daveney J et al. Gen Hosp Psychiatry. 2019 Mar 27. doi: 10.1016/j.genhosppsych.2019.03.006.
FROM GENERAL HOSPITAL PSYCHIATRY
Consider iatrogenesis in patients with new psychiatric symptoms
CRYSTAL CITY, VA. – Be aware of the potential iatrogenic properties of medications prescribed when patients present with new psychiatric symptoms, Henry A. Nasrallah, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Drugs that can cause iatrogenic psychiatric symptoms include stimulants, anabolic steroids, ACE inhibitors, anticholinergics, tricyclic antidepressants, antiepileptics, benzodiazepines, beta-adrenergic blockers, dopamine receptor agonists, among many others. A diverse class of medications can cause depression, anxiety, mania, and psychotic symptoms, and some medications cause multiple iatrogenic effects.
“Iatrogenic psychopathology can occur with a wide array of medications that are used in general medical practice,” said Dr. Nasrallah, editor in chief of Current Psychiatry and professor and chairman of the department of neurology and psychiatry at Saint Louis University. For example, the drug reserpine can cause depression in about 10% of cases, and corticosteroids can cause mood disorders such as depression or mania in about 6% of cases.
In other situations, use of alcohol, cannabis, hallucinogens, opioids, and other recreational drugs can cause psychiatric symptoms, and withdrawal from alcohol and sedatives can induce psychosis.
The DSM-5 defines a psychiatric disorder as a disorder that is not caused by a general medical condition and is not attributable to recreational or prescription drugs. However, a direct causal connection is sometimes difficult to establish, said Dr. Nasrallah, because psychiatric symptoms that manifest during treatment with prescription medications also could be tied to an underlying medical illness, psychosocial factors, withdrawal from a different prescription medication, or an unrecognized psychopathology. To confirm the drug is causing the disorder, clinicians should also rechallenge the patient.
he said at the meeting presented by Global Academy for Medical Education. “First-episode psychiatric disorder is always suspect. Iatrogenesis can occur for the first time in a patient who never had that symptom before, so you suspect it might be iatrogenic.”
Some drugs might induce psychiatric symptoms at higher but not lower doses, he added.
Other risk factors for iatrogenesis include simultaneous use of prescription medications, administration method, narrow therapeutic index, and rapid titration. Patients with slow metabolisms or hepatic insufficiency are at risk for iatrogenesis, as are those who are very young or very old, in stressful settings, or in a postpartum period.
Evaluate when psychiatric symptoms occurred, whether symptoms worsened and when, the dates of medication use, rechallenge and dechallenge dates, and any previous history of psychiatric disorders, said Dr. Nasrallah, who holds the Sydney W. Souers Endowed Chair at the university. If a patient is using more than one medication at a time, record the dates of each drug and their discontinuations.
Determine when the iatrogenesis occurred with psychiatric drugs, Dr. Nasrallah noted. “Iatrogenesis can complicate the course and outcome of the main medical or psychiatric illness being treated. Sometimes psychiatric medication can cause iatrogenic medical conditions; it’s not just a one-way street.”
Dr. Nasrallah reported receiving research grants from Forest, Forum, and Otsuka. In addition, he is a consultant for Acadia, Alkermes, Boehringer Ingelheim, Forum, Janssen, Merck, Novartis, Otsuka, Sunovion, and Teva, and he serves on the speaker’s bureau for Acadia, Alkermes, Janssen, Otsuka, and Sunovion.
Global Academy and this news organization are owned by the same parent company.
CRYSTAL CITY, VA. – Be aware of the potential iatrogenic properties of medications prescribed when patients present with new psychiatric symptoms, Henry A. Nasrallah, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Drugs that can cause iatrogenic psychiatric symptoms include stimulants, anabolic steroids, ACE inhibitors, anticholinergics, tricyclic antidepressants, antiepileptics, benzodiazepines, beta-adrenergic blockers, dopamine receptor agonists, among many others. A diverse class of medications can cause depression, anxiety, mania, and psychotic symptoms, and some medications cause multiple iatrogenic effects.
“Iatrogenic psychopathology can occur with a wide array of medications that are used in general medical practice,” said Dr. Nasrallah, editor in chief of Current Psychiatry and professor and chairman of the department of neurology and psychiatry at Saint Louis University. For example, the drug reserpine can cause depression in about 10% of cases, and corticosteroids can cause mood disorders such as depression or mania in about 6% of cases.
In other situations, use of alcohol, cannabis, hallucinogens, opioids, and other recreational drugs can cause psychiatric symptoms, and withdrawal from alcohol and sedatives can induce psychosis.
The DSM-5 defines a psychiatric disorder as a disorder that is not caused by a general medical condition and is not attributable to recreational or prescription drugs. However, a direct causal connection is sometimes difficult to establish, said Dr. Nasrallah, because psychiatric symptoms that manifest during treatment with prescription medications also could be tied to an underlying medical illness, psychosocial factors, withdrawal from a different prescription medication, or an unrecognized psychopathology. To confirm the drug is causing the disorder, clinicians should also rechallenge the patient.
he said at the meeting presented by Global Academy for Medical Education. “First-episode psychiatric disorder is always suspect. Iatrogenesis can occur for the first time in a patient who never had that symptom before, so you suspect it might be iatrogenic.”
Some drugs might induce psychiatric symptoms at higher but not lower doses, he added.
Other risk factors for iatrogenesis include simultaneous use of prescription medications, administration method, narrow therapeutic index, and rapid titration. Patients with slow metabolisms or hepatic insufficiency are at risk for iatrogenesis, as are those who are very young or very old, in stressful settings, or in a postpartum period.
Evaluate when psychiatric symptoms occurred, whether symptoms worsened and when, the dates of medication use, rechallenge and dechallenge dates, and any previous history of psychiatric disorders, said Dr. Nasrallah, who holds the Sydney W. Souers Endowed Chair at the university. If a patient is using more than one medication at a time, record the dates of each drug and their discontinuations.
Determine when the iatrogenesis occurred with psychiatric drugs, Dr. Nasrallah noted. “Iatrogenesis can complicate the course and outcome of the main medical or psychiatric illness being treated. Sometimes psychiatric medication can cause iatrogenic medical conditions; it’s not just a one-way street.”
Dr. Nasrallah reported receiving research grants from Forest, Forum, and Otsuka. In addition, he is a consultant for Acadia, Alkermes, Boehringer Ingelheim, Forum, Janssen, Merck, Novartis, Otsuka, Sunovion, and Teva, and he serves on the speaker’s bureau for Acadia, Alkermes, Janssen, Otsuka, and Sunovion.
Global Academy and this news organization are owned by the same parent company.
CRYSTAL CITY, VA. – Be aware of the potential iatrogenic properties of medications prescribed when patients present with new psychiatric symptoms, Henry A. Nasrallah, MD, said at Focus on Neuropsychiatry presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
Drugs that can cause iatrogenic psychiatric symptoms include stimulants, anabolic steroids, ACE inhibitors, anticholinergics, tricyclic antidepressants, antiepileptics, benzodiazepines, beta-adrenergic blockers, dopamine receptor agonists, among many others. A diverse class of medications can cause depression, anxiety, mania, and psychotic symptoms, and some medications cause multiple iatrogenic effects.
“Iatrogenic psychopathology can occur with a wide array of medications that are used in general medical practice,” said Dr. Nasrallah, editor in chief of Current Psychiatry and professor and chairman of the department of neurology and psychiatry at Saint Louis University. For example, the drug reserpine can cause depression in about 10% of cases, and corticosteroids can cause mood disorders such as depression or mania in about 6% of cases.
In other situations, use of alcohol, cannabis, hallucinogens, opioids, and other recreational drugs can cause psychiatric symptoms, and withdrawal from alcohol and sedatives can induce psychosis.
The DSM-5 defines a psychiatric disorder as a disorder that is not caused by a general medical condition and is not attributable to recreational or prescription drugs. However, a direct causal connection is sometimes difficult to establish, said Dr. Nasrallah, because psychiatric symptoms that manifest during treatment with prescription medications also could be tied to an underlying medical illness, psychosocial factors, withdrawal from a different prescription medication, or an unrecognized psychopathology. To confirm the drug is causing the disorder, clinicians should also rechallenge the patient.
he said at the meeting presented by Global Academy for Medical Education. “First-episode psychiatric disorder is always suspect. Iatrogenesis can occur for the first time in a patient who never had that symptom before, so you suspect it might be iatrogenic.”
Some drugs might induce psychiatric symptoms at higher but not lower doses, he added.
Other risk factors for iatrogenesis include simultaneous use of prescription medications, administration method, narrow therapeutic index, and rapid titration. Patients with slow metabolisms or hepatic insufficiency are at risk for iatrogenesis, as are those who are very young or very old, in stressful settings, or in a postpartum period.
Evaluate when psychiatric symptoms occurred, whether symptoms worsened and when, the dates of medication use, rechallenge and dechallenge dates, and any previous history of psychiatric disorders, said Dr. Nasrallah, who holds the Sydney W. Souers Endowed Chair at the university. If a patient is using more than one medication at a time, record the dates of each drug and their discontinuations.
Determine when the iatrogenesis occurred with psychiatric drugs, Dr. Nasrallah noted. “Iatrogenesis can complicate the course and outcome of the main medical or psychiatric illness being treated. Sometimes psychiatric medication can cause iatrogenic medical conditions; it’s not just a one-way street.”
Dr. Nasrallah reported receiving research grants from Forest, Forum, and Otsuka. In addition, he is a consultant for Acadia, Alkermes, Boehringer Ingelheim, Forum, Janssen, Merck, Novartis, Otsuka, Sunovion, and Teva, and he serves on the speaker’s bureau for Acadia, Alkermes, Janssen, Otsuka, and Sunovion.
Global Academy and this news organization are owned by the same parent company.
REPORTING FROM FOCUS ON NEUROPSYCHIATRY 2019