User login
Does provider self-reporting of etiquette behaviors improve patient experience? A randomized controlled trial
Physicians have historically had limited adoption of strategies to improve patient experience and often cite suboptimal data and lack of evidence-driven strategies. 1,2 However, public reporting of hospital-level physician domain Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) experience scores, and more recent linking of payments to performance on patient experience metrics, have been associated with significant increases in physician domain scores for most of the hospitals. 3 Hospitals and healthcare organizations have deployed a broad range of strategies to engage physicians. These include emphasizing the relationship between patient experience and patient compliance, complaints, and malpractice lawsuits; appealing to physicians’ sense of competitiveness by publishing individual provider experience scores; educating physicians on HCAHPS and providing them with regularly updated data; and development of specific techniques for improving patient-physician interaction. 4-8
Studies show that educational curricula on improving etiquette and communication skills for physicians lead to improvement in patient experience, and many such training programs are available to hospitals for a significant cost.9-15 Other studies that have focused on providing timely and individual feedback to physicians using tools other than HCAHPS have shown improvement in experience in some instances. 16,17 However, these strategies are resource intensive, require the presence of an independent observer in each patient room, and may not be practical in many settings. Further, long-term sustainability may be problematic.
Since the goal of any educational intervention targeting physicians is routinizing best practices, and since resource-intensive strategies of continuous assessment and feedback may not be practical, we sought to test the impact of periodic physician self-reporting of their etiquette-based behavior on their patient experience scores.
METHODS
Subjects
Hospitalists from 4 hospitals (2 community and 2 academic) that are part of the same healthcare system were the study subjects. Hospitalists who had at least 15 unique patients responding to the routinely administered Press Ganey experience survey during the baseline period were considered eligible. Eligible hospitalists were invited to enroll in the study if their site director confirmed that the provider was likely to stay with the group for the subsequent 12-month study period.
Randomization, Intervention and Control Group
Hospitalists were randomized to the study arm or control arm (1:1 randomization). Study arm participants received biweekly etiquette behavior (EB) surveys and were asked to report how frequently they performed 7 best-practice bedside etiquette behaviors during the previous 2-week period (Table 1). These behaviors were pre-defined by a consensus group of investigators as being amenable to self-report and commonly considered best practice as described in detail below. Control-arm participants received similarly worded survey on quality improvement behaviors (QIB) that would not be expected to impact patient experience (such as reviewing medications to ensure that antithrombotic prophylaxis was prescribed, Table 1).
Baseline and Study Periods
A 12-month period prior to the enrollment of each hospitalist was considered the baseline period for that individual. Hospitalist eligibility was assessed based on number of unique patients for each hospitalist who responded to the survey during this baseline period. Once enrolled, baseline provider-level patient experience scores were calculated based on the survey responses during this 12-month baseline period. Baseline etiquette behavior performance of the study was calculated from the first survey. After the initial survey, hospitalists received biweekly surveys (EB or QIB) for the 12-month study period for a total of 26 surveys (including the initial survey).
Survey Development, Nature of Survey, Survey Distribution Methods
The EB and QIB physician self-report surveys were developed through an iterative process by the study team. The EB survey included elements from an etiquette-based medicine checklist for hospitalized patients described by Kahn et al. 18 We conducted a review of literature to identify evidence-based practices.19-22 Research team members contributed items on best practices in etiquette-based medicine from their experience. Specifically, behaviors were selected if they met the following 4 criteria: 1) performing the behavior did not lead to significant increase in workload and was relatively easy to incorporate in the work flow; 2) occurrence of the behavior would be easy to note for any outside observer or the providers themselves; 3) the practice was considered to be either an evidence-based or consensus-based best-practice; 4) there was consensus among study team members on including the item. The survey was tested for understandability by hospitalists who were not eligible for the study.
The EB survey contained 7 items related to behaviors that were expected to impact patient experience. The QIB survey contained 4 items related to behaviors that were expected to improve quality (Table 1). The initial survey also included questions about demographic characteristics of the participants.
Survey questionnaires were sent via email every 2 weeks for a period of 12 months. The survey questionnaire became available every other week, between Friday morning and Tuesday midnight, during the study period. Hospitalists received daily email reminders on each of these days with a link to the survey website if they did not complete the survey. They had the opportunity to report that they were not on service in the prior week and opt out of the survey for the specific 2-week period. The survey questions were available online as well as on a mobile device format.
Provider Level Patient Experience Scores
Provider-level patient experience scores were calculated from the physician domain Press Ganey survey items, which included the time that the physician spent with patients, the physician addressed questions/worries, the physician kept patients informed, the friendliness/courtesy of physician, and the skill of physician. Press Ganey responses were scored from 1 to 5 based on the Likert scale responses on the survey such that a response “very good” was scored 5 and a response “very poor” was scored 1. Additionally, physician domain HCAHPS item (doctors treat with courtesy/respect, doctors listen carefully, doctors explain in way patients understand) responses were utilized to calculate another set of HCAHPS provider level experience scores. The responses were scored as 1 for “always” response and “0” for any other response, consistent with CMS dichotomization of these results for public reporting. Weighted scores were calculated for individual hospitalists based on the proportion of days each hospitalist billed for the hospitalization so that experience scores of patients who were cared for by multiple providers were assigned to each provider in proportion to the percent of care delivered.23 Separate composite physician scores were generated from the 5 Press Ganey and for the 3 HCAHPS physician items. Each item was weighted equally, with the maximum possible for Press Ganey composite score of 25 (sum of the maximum possible score of 5 on each of the 5 Press Ganey items) and the HCAHPS possible total was 3 (sum of the maximum possible score of 1 on each of the 3 HCAHPS items).
ANALYSIS AND STATISTICAL METHODS
We analyzed the data to assess for changes in frequency of self-reported behavior over the study period, changes in provider-level patient experience between baseline and study period, and the association between the these 2 outcomes. The self-reported etiquette-based behavior responses were scored as 1 for the lowest response (never) to 4 as the highest (always). With 7 questions, the maximum attainable score was 28. The maximum score was normalized to 100 for ease of interpretation (corresponding to percentage of time etiquette behaviors were employed, by self-report). Similarly, the maximum attainable self-reported QIB-related behavior score on the 4 questions was 16. This was also converted to 0-100 scale for ease of comparison.
Two additional sets of analyses were performed to evaluate changes in patient experience during the study period. First, the mean 12-month provider level patient experience composite score in the baseline period was compared with the 12-month composite score during the 12-month study period for the study group and the control group. These were assessed with and without adjusting for age, sex, race, and U.S. medical school graduate (USMG) status. In the second set of unadjusted and adjusted analyses, changes in biweekly composite scores during the study period were compared between the intervention and the control groups while accounting for correlation between observations from the same physician using mixed linear models. Linear mixed models were used to accommodate correlations among multiple observations made on the same physician by including random effects within each regression model. Furthermore, these models allowed us to account for unbalanced design in our data when not all physicians had an equal number of observations and data elements were collected asynchronously.24 Analyses were performed in R version 3.2.2 (The R Project for Statistical Computing, Vienna, Austria); linear mixed models were performed using the ‘nlme’ package.25
We hypothesized that self-reporting on biweekly surveys would result in increases in the frequency of the reported behavior in each arm. We also hypothesized that, because of biweekly reflection and self-reporting on etiquette-based bedside behavior, patient experience scores would increase in the study arm.
RESULTS
Of the 80 hospitalists approached to participate in the study, 64 elected to participate (80% participation rate). The mean response rate to the survey was 57.4% for the intervention arm and 85.7% for the control arm. Higher response rates were not associated with improved patient experience scores. Of the respondents, 43.1% were younger than 35 years of age, 51.5% practiced in academic settings, and 53.1% were female. There was no statistical difference between hospitalists’ baseline composite experience scores based on gender, age, academic hospitalist status, USMG status, and English as a second language status. Similarly, there were no differences in poststudy composite experience scores based on physician characteristics.
Physicians reported high rates of etiquette-based behavior at baseline (mean score, 83.9+/-3.3), and this showed moderate improvement over the study period (5.6 % [3.9%-7.3%, P < 0.0001]). Similarly, there was a moderate increase in frequency of self-reported behavior in the control arm (6.8% [3.5%-10.1%, P < 0.0001]). Hospitalists reported on 80.7% (77.6%-83.4%) of the biweekly surveys that they “almost always” wrapped up by asking, “Do you have any other questions or concerns” or something similar. In contrast, hospitalists reported on only 27.9% (24.7%-31.3%) of the biweekly survey that they “almost always” sat down in the patient room.
The composite physician domain Press Ganey experience scores were no different for the intervention arm and the control arm during the 12-month baseline period (21.8 vs. 21.7; P = 0.90) and the 12-month intervention period (21.6 vs. 21.5; P = 0.75). Baseline self-reported behaviors were not associated with baseline experience scores. Similarly, there were no differences between the arms on composite physician domain HCAHPS experience scores during baseline (2.1 vs. 2.3; P = 0.13) and intervention periods (2.2 vs. 2.1; P = 0.33).
The difference in difference analysis of the baseline and postintervention composite between the intervention arm and the control arm was not statistically significant for Press Ganey composite physician experience scores (-0.163 vs. -0.322; P = 0.71) or HCAHPS composite physician scores (-0.162 vs. -0.071; P = 0.06). The results did not change when controlled for survey response rate (percentage biweekly surveys completed by the hospitalist), age, gender, USMG status, English as a second language status, or percent clinical effort. The difference in difference analysis of the individual Press Ganey and HCAHPS physician domain items that were used to calculate the composite score was also not statistically significant (Table 2).
Changes in self-reported etiquette-based behavior were not associated with any changes in composite Press Ganey and HCAHPS experience score or individual items of the composite experience scores between baseline and intervention period. Similarly, biweekly self-reported etiquette behaviors were not associated with composite and individual item experience scores derived from responses of the patients discharged during the same 2-week reporting period. The intra-class correlation between observations from the same physician was only 0.02%, suggesting that most of the variation in scores was likely due to patient factors and did not result from differences between physicians.
DISCUSSION
This 12-month randomized multicenter study of hospitalists showed that repeated self-reporting of etiquette-based behavior results in modest reported increases in performance of these behaviors. However, there was no associated increase in provider level patient experience scores at the end of the study period when compared to baseline scores of the same physicians or when compared to the scores of the control group. The study demonstrated feasibility of self-reporting of behaviors by physicians with high participation when provided modest incentives.
Educational and feedback strategies used to improve patient experience are very resource intensive. Training sessions provided at some hospitals may take hours, and sustained effects are unproved. The presence of an independent observer in patient rooms to generate feedback for providers is not scalable and sustainable outside of a research study environment.9-11,15,17,26-29 We attempted to use physician repeated self-reporting to reinforce the important and easy to adopt components of etiquette-based behavior to develop a more easily sustainable strategy. This may have failed for several reasons.
When combining “always” and “usually” responses, the physicians in our study reported a high level of etiquette behavior at baseline. If physicians believe that they are performing well at baseline, they would not consider this to be an area in need of improvement. Bigger changes in behavior may have been possible had the physicians rated themselves less favorably at baseline. Inflated or high baseline self-assessment of performance might also have led to limited success of other types of educational interventions had they been employed.
Studies published since the rollout of our study have shown that physicians significantly overestimate how frequently they perform these etiquette behaviors.30,31 It is likely that was the case in our study subjects. This may, at best, indicate that a much higher change in the level of self-reported performance would be needed to result in meaningful actual changes, or worse, may render self-reported etiquette behavior entirely unreliable. Interventions designed to improve etiquette-based behavior might need to provide feedback about performance.
A program that provides education on the importance of etiquette-based behaviors, obtains objective measures of performance of these behaviors, and offers individualized feedback may be more likely to increase the desired behaviors. This is a limitation of our study. However, we aimed to test a method that required limited resources. Additionally, our method for attributing HCAHPS scores to an individual physician, based on weighted scores that were calculated according to the proportion of days each hospitalist billed for the hospitalization, may be inaccurate. It is possible that each interaction does not contribute equally to the overall score. A team-based intervention and experience measurements could overcome this limitation.
CONCLUSION
This randomized trial demonstrated the feasibility of self-assessment of bedside etiquette behaviors by hospitalists but failed to demonstrate a meaningful impact on patient experience through self-report. These findings suggest that more intensive interventions, perhaps involving direct observation, peer-to-peer mentoring, or other techniques may be required to impact significantly physician etiquette behaviors.
Disclosure
Johns Hopkins Hospitalist Scholars Program provided funding support. Dr. Qayyum is a consultant for Sunovion. The other authors have nothing to report.
1. Blumenthal D, Kilo CM. A report card on continuous quality improvement. Milbank Q. 1998;76(4):625-648. PubMed
2. Shortell SM, Bennett CL, Byck GR. Assessing the impact of continuous quality improvement on clinical practice: What it will take to accelerate progress. Milbank Q. 1998;76(4):593-624. PubMed
3. Mann RK, Siddiqui Z, Kurbanova N, Qayyum R. Effect of HCAHPS reporting on patient satisfaction with physician communication. J Hosp Med. 2015;11(2):105-110. PubMed
4. Rivers PA, Glover SH. Health care competition, strategic mission, and patient satisfaction: research model and propositions. J Health Organ Manag. 2008;22(6):627-641. PubMed
5. Kim SS, Kaplowitz S, Johnston MV. The effects of physician empathy on patient satisfaction and compliance. Eval Health Prof. 2004;27(3):237-251. PubMed
6. Stelfox HT, Gandhi TK, Orav EJ, Gustafson ML. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133. PubMed
7. Rodriguez HP, Rodday AM, Marshall RE, Nelson KL, Rogers WH, Safran DG. Relation of patients’ experiences with individual physicians to malpractice risk. Int J Qual Health Care. 2008;20(1):5-12. PubMed
8. Cydulka RK, Tamayo-Sarver J, Gage A, Bagnoli D. Association of patient satisfaction with complaints and risk management among emergency physicians. J Emerg Med. 2011;41(4):405-411. PubMed
9. Windover AK, Boissy A, Rice TW, Gilligan T, Velez VJ, Merlino J. The REDE model of healthcare communication: Optimizing relationship as a therapeutic agent. Journal of Patient Experience. 2014;1(1):8-13.
10. Chou CL, Hirschmann K, Fortin AH 6th, Lichstein PR. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89(7):1051-1056. PubMed
11. Kennedy M, Denise M, Fasolino M, John P, Gullen M, David J. Improving the patient experience through provider communication skills building. Patient Experience Journal. 2014;1(1):56-60.
12. Braverman AM, Kunkel EJ, Katz L, et al. Do I buy it? How AIDET™ training changes residents’ values about patient care. Journal of Patient Experience. 2015;2(1):13-20.
13. Riess H, Kelley JM, Bailey RW, Dunn EJ, Phillips M. Empathy training for resident physicians: a randomized controlled trial of a neuroscience-informed curriculum. J Gen Intern Med. 2012;27(10):1280-1286. PubMed
14. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Internl Med. 2012;27(2):185-189. PubMed
15. O’Leary KJ, Cyrus RM. Improving patient satisfaction: timely feedback to specific physicians is essential for success. J Hosp Med. 2015;10(8):555-556. PubMed
16. Indovina K, Keniston A, Reid M, et al. Real‐time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;10(8):497-502. PubMed
17. Banka G, Edgington S, Kyulo N, et al. Improving patient satisfaction through physician education, feedback, and incentives. J Hosp Med. 2015;10(8):497-502. PubMed
18. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
19. Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201. PubMed
20. Francis JJ, Pankratz VS, Huddleston JM. Patient satisfaction associated with correct identification of physicians’ photographs. Mayo Clin Proc. 2001;76(6):604-608. PubMed
21. Strasser F, Palmer JL, Willey J, et al. Impact of physician sitting versus standing during inpatient oncology consultations: patients’ preference and perception of compassion and duration. A randomized controlled trial. J Pain Symptom Manage. 2005;29(5):489-497. PubMed
22. Dudas RA, Lemerman H, Barone M, Serwint JR. PHACES (Photographs of Academic Clinicians and Their Educational Status): a tool to improve delivery of family-centered care. Acad Pediatr. 2010;10(2):138-145. PubMed
23. Herzke C, Michtalik H, Durkin N, et al. A method for attributing patient-level metrics to rotating providers in an inpatient setting. J Hosp Med. Under revision.
24. Holden JE, Kelley K, Agarwal R. Analyzing change: a primer on multilevel models with applications to nephrology. Am J Nephrol. 2008;28(5):792-801. PubMed
25. Pinheiro J, Bates D, DebRoy S, Sarkar D. Linear and nonlinear mixed effects models. R package version. 2007;3:57.
26. Braverman AM, Kunkel EJ, Katz L, et al. Do I buy it? How AIDET™ training changes residents’ values about patient care. Journal of Patient Experience. 2015;2(1):13-20.
27. Riess H, Kelley JM, Bailey RW, Dunn EJ, Phillips M. Empathy training for resident physicians: A randomized controlled trial of a neuroscience-informed curriculum. J Gen Intern Med. 2012;27(10):1280-1286. PubMed
28. Raper SE, Gupta M, Okusanya O, Morris JB. Improving communication skills: A course for academic medical center surgery residents and faculty. J Surg Educ. 2015;72(6):e202-e211. PubMed
29. Indovina K, Keniston A, Reid M, et al. Real‐time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;11(4):251-256. PubMed
30. Block L, Hutzler L, Habicht R, et al. Do internal medicine interns practice etiquette‐based communication? A critical look at the inpatient encounter. J Hosp Med. 2013;8(11):631-634. PubMed
31. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. PubMed
Physicians have historically had limited adoption of strategies to improve patient experience and often cite suboptimal data and lack of evidence-driven strategies. 1,2 However, public reporting of hospital-level physician domain Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) experience scores, and more recent linking of payments to performance on patient experience metrics, have been associated with significant increases in physician domain scores for most of the hospitals. 3 Hospitals and healthcare organizations have deployed a broad range of strategies to engage physicians. These include emphasizing the relationship between patient experience and patient compliance, complaints, and malpractice lawsuits; appealing to physicians’ sense of competitiveness by publishing individual provider experience scores; educating physicians on HCAHPS and providing them with regularly updated data; and development of specific techniques for improving patient-physician interaction. 4-8
Studies show that educational curricula on improving etiquette and communication skills for physicians lead to improvement in patient experience, and many such training programs are available to hospitals for a significant cost.9-15 Other studies that have focused on providing timely and individual feedback to physicians using tools other than HCAHPS have shown improvement in experience in some instances. 16,17 However, these strategies are resource intensive, require the presence of an independent observer in each patient room, and may not be practical in many settings. Further, long-term sustainability may be problematic.
Since the goal of any educational intervention targeting physicians is routinizing best practices, and since resource-intensive strategies of continuous assessment and feedback may not be practical, we sought to test the impact of periodic physician self-reporting of their etiquette-based behavior on their patient experience scores.
METHODS
Subjects
Hospitalists from 4 hospitals (2 community and 2 academic) that are part of the same healthcare system were the study subjects. Hospitalists who had at least 15 unique patients responding to the routinely administered Press Ganey experience survey during the baseline period were considered eligible. Eligible hospitalists were invited to enroll in the study if their site director confirmed that the provider was likely to stay with the group for the subsequent 12-month study period.
Randomization, Intervention and Control Group
Hospitalists were randomized to the study arm or control arm (1:1 randomization). Study arm participants received biweekly etiquette behavior (EB) surveys and were asked to report how frequently they performed 7 best-practice bedside etiquette behaviors during the previous 2-week period (Table 1). These behaviors were pre-defined by a consensus group of investigators as being amenable to self-report and commonly considered best practice as described in detail below. Control-arm participants received similarly worded survey on quality improvement behaviors (QIB) that would not be expected to impact patient experience (such as reviewing medications to ensure that antithrombotic prophylaxis was prescribed, Table 1).
Baseline and Study Periods
A 12-month period prior to the enrollment of each hospitalist was considered the baseline period for that individual. Hospitalist eligibility was assessed based on number of unique patients for each hospitalist who responded to the survey during this baseline period. Once enrolled, baseline provider-level patient experience scores were calculated based on the survey responses during this 12-month baseline period. Baseline etiquette behavior performance of the study was calculated from the first survey. After the initial survey, hospitalists received biweekly surveys (EB or QIB) for the 12-month study period for a total of 26 surveys (including the initial survey).
Survey Development, Nature of Survey, Survey Distribution Methods
The EB and QIB physician self-report surveys were developed through an iterative process by the study team. The EB survey included elements from an etiquette-based medicine checklist for hospitalized patients described by Kahn et al. 18 We conducted a review of literature to identify evidence-based practices.19-22 Research team members contributed items on best practices in etiquette-based medicine from their experience. Specifically, behaviors were selected if they met the following 4 criteria: 1) performing the behavior did not lead to significant increase in workload and was relatively easy to incorporate in the work flow; 2) occurrence of the behavior would be easy to note for any outside observer or the providers themselves; 3) the practice was considered to be either an evidence-based or consensus-based best-practice; 4) there was consensus among study team members on including the item. The survey was tested for understandability by hospitalists who were not eligible for the study.
The EB survey contained 7 items related to behaviors that were expected to impact patient experience. The QIB survey contained 4 items related to behaviors that were expected to improve quality (Table 1). The initial survey also included questions about demographic characteristics of the participants.
Survey questionnaires were sent via email every 2 weeks for a period of 12 months. The survey questionnaire became available every other week, between Friday morning and Tuesday midnight, during the study period. Hospitalists received daily email reminders on each of these days with a link to the survey website if they did not complete the survey. They had the opportunity to report that they were not on service in the prior week and opt out of the survey for the specific 2-week period. The survey questions were available online as well as on a mobile device format.
Provider Level Patient Experience Scores
Provider-level patient experience scores were calculated from the physician domain Press Ganey survey items, which included the time that the physician spent with patients, the physician addressed questions/worries, the physician kept patients informed, the friendliness/courtesy of physician, and the skill of physician. Press Ganey responses were scored from 1 to 5 based on the Likert scale responses on the survey such that a response “very good” was scored 5 and a response “very poor” was scored 1. Additionally, physician domain HCAHPS item (doctors treat with courtesy/respect, doctors listen carefully, doctors explain in way patients understand) responses were utilized to calculate another set of HCAHPS provider level experience scores. The responses were scored as 1 for “always” response and “0” for any other response, consistent with CMS dichotomization of these results for public reporting. Weighted scores were calculated for individual hospitalists based on the proportion of days each hospitalist billed for the hospitalization so that experience scores of patients who were cared for by multiple providers were assigned to each provider in proportion to the percent of care delivered.23 Separate composite physician scores were generated from the 5 Press Ganey and for the 3 HCAHPS physician items. Each item was weighted equally, with the maximum possible for Press Ganey composite score of 25 (sum of the maximum possible score of 5 on each of the 5 Press Ganey items) and the HCAHPS possible total was 3 (sum of the maximum possible score of 1 on each of the 3 HCAHPS items).
ANALYSIS AND STATISTICAL METHODS
We analyzed the data to assess for changes in frequency of self-reported behavior over the study period, changes in provider-level patient experience between baseline and study period, and the association between the these 2 outcomes. The self-reported etiquette-based behavior responses were scored as 1 for the lowest response (never) to 4 as the highest (always). With 7 questions, the maximum attainable score was 28. The maximum score was normalized to 100 for ease of interpretation (corresponding to percentage of time etiquette behaviors were employed, by self-report). Similarly, the maximum attainable self-reported QIB-related behavior score on the 4 questions was 16. This was also converted to 0-100 scale for ease of comparison.
Two additional sets of analyses were performed to evaluate changes in patient experience during the study period. First, the mean 12-month provider level patient experience composite score in the baseline period was compared with the 12-month composite score during the 12-month study period for the study group and the control group. These were assessed with and without adjusting for age, sex, race, and U.S. medical school graduate (USMG) status. In the second set of unadjusted and adjusted analyses, changes in biweekly composite scores during the study period were compared between the intervention and the control groups while accounting for correlation between observations from the same physician using mixed linear models. Linear mixed models were used to accommodate correlations among multiple observations made on the same physician by including random effects within each regression model. Furthermore, these models allowed us to account for unbalanced design in our data when not all physicians had an equal number of observations and data elements were collected asynchronously.24 Analyses were performed in R version 3.2.2 (The R Project for Statistical Computing, Vienna, Austria); linear mixed models were performed using the ‘nlme’ package.25
We hypothesized that self-reporting on biweekly surveys would result in increases in the frequency of the reported behavior in each arm. We also hypothesized that, because of biweekly reflection and self-reporting on etiquette-based bedside behavior, patient experience scores would increase in the study arm.
RESULTS
Of the 80 hospitalists approached to participate in the study, 64 elected to participate (80% participation rate). The mean response rate to the survey was 57.4% for the intervention arm and 85.7% for the control arm. Higher response rates were not associated with improved patient experience scores. Of the respondents, 43.1% were younger than 35 years of age, 51.5% practiced in academic settings, and 53.1% were female. There was no statistical difference between hospitalists’ baseline composite experience scores based on gender, age, academic hospitalist status, USMG status, and English as a second language status. Similarly, there were no differences in poststudy composite experience scores based on physician characteristics.
Physicians reported high rates of etiquette-based behavior at baseline (mean score, 83.9+/-3.3), and this showed moderate improvement over the study period (5.6 % [3.9%-7.3%, P < 0.0001]). Similarly, there was a moderate increase in frequency of self-reported behavior in the control arm (6.8% [3.5%-10.1%, P < 0.0001]). Hospitalists reported on 80.7% (77.6%-83.4%) of the biweekly surveys that they “almost always” wrapped up by asking, “Do you have any other questions or concerns” or something similar. In contrast, hospitalists reported on only 27.9% (24.7%-31.3%) of the biweekly survey that they “almost always” sat down in the patient room.
The composite physician domain Press Ganey experience scores were no different for the intervention arm and the control arm during the 12-month baseline period (21.8 vs. 21.7; P = 0.90) and the 12-month intervention period (21.6 vs. 21.5; P = 0.75). Baseline self-reported behaviors were not associated with baseline experience scores. Similarly, there were no differences between the arms on composite physician domain HCAHPS experience scores during baseline (2.1 vs. 2.3; P = 0.13) and intervention periods (2.2 vs. 2.1; P = 0.33).
The difference in difference analysis of the baseline and postintervention composite between the intervention arm and the control arm was not statistically significant for Press Ganey composite physician experience scores (-0.163 vs. -0.322; P = 0.71) or HCAHPS composite physician scores (-0.162 vs. -0.071; P = 0.06). The results did not change when controlled for survey response rate (percentage biweekly surveys completed by the hospitalist), age, gender, USMG status, English as a second language status, or percent clinical effort. The difference in difference analysis of the individual Press Ganey and HCAHPS physician domain items that were used to calculate the composite score was also not statistically significant (Table 2).
Changes in self-reported etiquette-based behavior were not associated with any changes in composite Press Ganey and HCAHPS experience score or individual items of the composite experience scores between baseline and intervention period. Similarly, biweekly self-reported etiquette behaviors were not associated with composite and individual item experience scores derived from responses of the patients discharged during the same 2-week reporting period. The intra-class correlation between observations from the same physician was only 0.02%, suggesting that most of the variation in scores was likely due to patient factors and did not result from differences between physicians.
DISCUSSION
This 12-month randomized multicenter study of hospitalists showed that repeated self-reporting of etiquette-based behavior results in modest reported increases in performance of these behaviors. However, there was no associated increase in provider level patient experience scores at the end of the study period when compared to baseline scores of the same physicians or when compared to the scores of the control group. The study demonstrated feasibility of self-reporting of behaviors by physicians with high participation when provided modest incentives.
Educational and feedback strategies used to improve patient experience are very resource intensive. Training sessions provided at some hospitals may take hours, and sustained effects are unproved. The presence of an independent observer in patient rooms to generate feedback for providers is not scalable and sustainable outside of a research study environment.9-11,15,17,26-29 We attempted to use physician repeated self-reporting to reinforce the important and easy to adopt components of etiquette-based behavior to develop a more easily sustainable strategy. This may have failed for several reasons.
When combining “always” and “usually” responses, the physicians in our study reported a high level of etiquette behavior at baseline. If physicians believe that they are performing well at baseline, they would not consider this to be an area in need of improvement. Bigger changes in behavior may have been possible had the physicians rated themselves less favorably at baseline. Inflated or high baseline self-assessment of performance might also have led to limited success of other types of educational interventions had they been employed.
Studies published since the rollout of our study have shown that physicians significantly overestimate how frequently they perform these etiquette behaviors.30,31 It is likely that was the case in our study subjects. This may, at best, indicate that a much higher change in the level of self-reported performance would be needed to result in meaningful actual changes, or worse, may render self-reported etiquette behavior entirely unreliable. Interventions designed to improve etiquette-based behavior might need to provide feedback about performance.
A program that provides education on the importance of etiquette-based behaviors, obtains objective measures of performance of these behaviors, and offers individualized feedback may be more likely to increase the desired behaviors. This is a limitation of our study. However, we aimed to test a method that required limited resources. Additionally, our method for attributing HCAHPS scores to an individual physician, based on weighted scores that were calculated according to the proportion of days each hospitalist billed for the hospitalization, may be inaccurate. It is possible that each interaction does not contribute equally to the overall score. A team-based intervention and experience measurements could overcome this limitation.
CONCLUSION
This randomized trial demonstrated the feasibility of self-assessment of bedside etiquette behaviors by hospitalists but failed to demonstrate a meaningful impact on patient experience through self-report. These findings suggest that more intensive interventions, perhaps involving direct observation, peer-to-peer mentoring, or other techniques may be required to impact significantly physician etiquette behaviors.
Disclosure
Johns Hopkins Hospitalist Scholars Program provided funding support. Dr. Qayyum is a consultant for Sunovion. The other authors have nothing to report.
Physicians have historically had limited adoption of strategies to improve patient experience and often cite suboptimal data and lack of evidence-driven strategies. 1,2 However, public reporting of hospital-level physician domain Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) experience scores, and more recent linking of payments to performance on patient experience metrics, have been associated with significant increases in physician domain scores for most of the hospitals. 3 Hospitals and healthcare organizations have deployed a broad range of strategies to engage physicians. These include emphasizing the relationship between patient experience and patient compliance, complaints, and malpractice lawsuits; appealing to physicians’ sense of competitiveness by publishing individual provider experience scores; educating physicians on HCAHPS and providing them with regularly updated data; and development of specific techniques for improving patient-physician interaction. 4-8
Studies show that educational curricula on improving etiquette and communication skills for physicians lead to improvement in patient experience, and many such training programs are available to hospitals for a significant cost.9-15 Other studies that have focused on providing timely and individual feedback to physicians using tools other than HCAHPS have shown improvement in experience in some instances. 16,17 However, these strategies are resource intensive, require the presence of an independent observer in each patient room, and may not be practical in many settings. Further, long-term sustainability may be problematic.
Since the goal of any educational intervention targeting physicians is routinizing best practices, and since resource-intensive strategies of continuous assessment and feedback may not be practical, we sought to test the impact of periodic physician self-reporting of their etiquette-based behavior on their patient experience scores.
METHODS
Subjects
Hospitalists from 4 hospitals (2 community and 2 academic) that are part of the same healthcare system were the study subjects. Hospitalists who had at least 15 unique patients responding to the routinely administered Press Ganey experience survey during the baseline period were considered eligible. Eligible hospitalists were invited to enroll in the study if their site director confirmed that the provider was likely to stay with the group for the subsequent 12-month study period.
Randomization, Intervention and Control Group
Hospitalists were randomized to the study arm or control arm (1:1 randomization). Study arm participants received biweekly etiquette behavior (EB) surveys and were asked to report how frequently they performed 7 best-practice bedside etiquette behaviors during the previous 2-week period (Table 1). These behaviors were pre-defined by a consensus group of investigators as being amenable to self-report and commonly considered best practice as described in detail below. Control-arm participants received similarly worded survey on quality improvement behaviors (QIB) that would not be expected to impact patient experience (such as reviewing medications to ensure that antithrombotic prophylaxis was prescribed, Table 1).
Baseline and Study Periods
A 12-month period prior to the enrollment of each hospitalist was considered the baseline period for that individual. Hospitalist eligibility was assessed based on number of unique patients for each hospitalist who responded to the survey during this baseline period. Once enrolled, baseline provider-level patient experience scores were calculated based on the survey responses during this 12-month baseline period. Baseline etiquette behavior performance of the study was calculated from the first survey. After the initial survey, hospitalists received biweekly surveys (EB or QIB) for the 12-month study period for a total of 26 surveys (including the initial survey).
Survey Development, Nature of Survey, Survey Distribution Methods
The EB and QIB physician self-report surveys were developed through an iterative process by the study team. The EB survey included elements from an etiquette-based medicine checklist for hospitalized patients described by Kahn et al. 18 We conducted a review of literature to identify evidence-based practices.19-22 Research team members contributed items on best practices in etiquette-based medicine from their experience. Specifically, behaviors were selected if they met the following 4 criteria: 1) performing the behavior did not lead to significant increase in workload and was relatively easy to incorporate in the work flow; 2) occurrence of the behavior would be easy to note for any outside observer or the providers themselves; 3) the practice was considered to be either an evidence-based or consensus-based best-practice; 4) there was consensus among study team members on including the item. The survey was tested for understandability by hospitalists who were not eligible for the study.
The EB survey contained 7 items related to behaviors that were expected to impact patient experience. The QIB survey contained 4 items related to behaviors that were expected to improve quality (Table 1). The initial survey also included questions about demographic characteristics of the participants.
Survey questionnaires were sent via email every 2 weeks for a period of 12 months. The survey questionnaire became available every other week, between Friday morning and Tuesday midnight, during the study period. Hospitalists received daily email reminders on each of these days with a link to the survey website if they did not complete the survey. They had the opportunity to report that they were not on service in the prior week and opt out of the survey for the specific 2-week period. The survey questions were available online as well as on a mobile device format.
Provider Level Patient Experience Scores
Provider-level patient experience scores were calculated from the physician domain Press Ganey survey items, which included the time that the physician spent with patients, the physician addressed questions/worries, the physician kept patients informed, the friendliness/courtesy of physician, and the skill of physician. Press Ganey responses were scored from 1 to 5 based on the Likert scale responses on the survey such that a response “very good” was scored 5 and a response “very poor” was scored 1. Additionally, physician domain HCAHPS item (doctors treat with courtesy/respect, doctors listen carefully, doctors explain in way patients understand) responses were utilized to calculate another set of HCAHPS provider level experience scores. The responses were scored as 1 for “always” response and “0” for any other response, consistent with CMS dichotomization of these results for public reporting. Weighted scores were calculated for individual hospitalists based on the proportion of days each hospitalist billed for the hospitalization so that experience scores of patients who were cared for by multiple providers were assigned to each provider in proportion to the percent of care delivered.23 Separate composite physician scores were generated from the 5 Press Ganey and for the 3 HCAHPS physician items. Each item was weighted equally, with the maximum possible for Press Ganey composite score of 25 (sum of the maximum possible score of 5 on each of the 5 Press Ganey items) and the HCAHPS possible total was 3 (sum of the maximum possible score of 1 on each of the 3 HCAHPS items).
ANALYSIS AND STATISTICAL METHODS
We analyzed the data to assess for changes in frequency of self-reported behavior over the study period, changes in provider-level patient experience between baseline and study period, and the association between the these 2 outcomes. The self-reported etiquette-based behavior responses were scored as 1 for the lowest response (never) to 4 as the highest (always). With 7 questions, the maximum attainable score was 28. The maximum score was normalized to 100 for ease of interpretation (corresponding to percentage of time etiquette behaviors were employed, by self-report). Similarly, the maximum attainable self-reported QIB-related behavior score on the 4 questions was 16. This was also converted to 0-100 scale for ease of comparison.
Two additional sets of analyses were performed to evaluate changes in patient experience during the study period. First, the mean 12-month provider level patient experience composite score in the baseline period was compared with the 12-month composite score during the 12-month study period for the study group and the control group. These were assessed with and without adjusting for age, sex, race, and U.S. medical school graduate (USMG) status. In the second set of unadjusted and adjusted analyses, changes in biweekly composite scores during the study period were compared between the intervention and the control groups while accounting for correlation between observations from the same physician using mixed linear models. Linear mixed models were used to accommodate correlations among multiple observations made on the same physician by including random effects within each regression model. Furthermore, these models allowed us to account for unbalanced design in our data when not all physicians had an equal number of observations and data elements were collected asynchronously.24 Analyses were performed in R version 3.2.2 (The R Project for Statistical Computing, Vienna, Austria); linear mixed models were performed using the ‘nlme’ package.25
We hypothesized that self-reporting on biweekly surveys would result in increases in the frequency of the reported behavior in each arm. We also hypothesized that, because of biweekly reflection and self-reporting on etiquette-based bedside behavior, patient experience scores would increase in the study arm.
RESULTS
Of the 80 hospitalists approached to participate in the study, 64 elected to participate (80% participation rate). The mean response rate to the survey was 57.4% for the intervention arm and 85.7% for the control arm. Higher response rates were not associated with improved patient experience scores. Of the respondents, 43.1% were younger than 35 years of age, 51.5% practiced in academic settings, and 53.1% were female. There was no statistical difference between hospitalists’ baseline composite experience scores based on gender, age, academic hospitalist status, USMG status, and English as a second language status. Similarly, there were no differences in poststudy composite experience scores based on physician characteristics.
Physicians reported high rates of etiquette-based behavior at baseline (mean score, 83.9+/-3.3), and this showed moderate improvement over the study period (5.6 % [3.9%-7.3%, P < 0.0001]). Similarly, there was a moderate increase in frequency of self-reported behavior in the control arm (6.8% [3.5%-10.1%, P < 0.0001]). Hospitalists reported on 80.7% (77.6%-83.4%) of the biweekly surveys that they “almost always” wrapped up by asking, “Do you have any other questions or concerns” or something similar. In contrast, hospitalists reported on only 27.9% (24.7%-31.3%) of the biweekly survey that they “almost always” sat down in the patient room.
The composite physician domain Press Ganey experience scores were no different for the intervention arm and the control arm during the 12-month baseline period (21.8 vs. 21.7; P = 0.90) and the 12-month intervention period (21.6 vs. 21.5; P = 0.75). Baseline self-reported behaviors were not associated with baseline experience scores. Similarly, there were no differences between the arms on composite physician domain HCAHPS experience scores during baseline (2.1 vs. 2.3; P = 0.13) and intervention periods (2.2 vs. 2.1; P = 0.33).
The difference in difference analysis of the baseline and postintervention composite between the intervention arm and the control arm was not statistically significant for Press Ganey composite physician experience scores (-0.163 vs. -0.322; P = 0.71) or HCAHPS composite physician scores (-0.162 vs. -0.071; P = 0.06). The results did not change when controlled for survey response rate (percentage biweekly surveys completed by the hospitalist), age, gender, USMG status, English as a second language status, or percent clinical effort. The difference in difference analysis of the individual Press Ganey and HCAHPS physician domain items that were used to calculate the composite score was also not statistically significant (Table 2).
Changes in self-reported etiquette-based behavior were not associated with any changes in composite Press Ganey and HCAHPS experience score or individual items of the composite experience scores between baseline and intervention period. Similarly, biweekly self-reported etiquette behaviors were not associated with composite and individual item experience scores derived from responses of the patients discharged during the same 2-week reporting period. The intra-class correlation between observations from the same physician was only 0.02%, suggesting that most of the variation in scores was likely due to patient factors and did not result from differences between physicians.
DISCUSSION
This 12-month randomized multicenter study of hospitalists showed that repeated self-reporting of etiquette-based behavior results in modest reported increases in performance of these behaviors. However, there was no associated increase in provider level patient experience scores at the end of the study period when compared to baseline scores of the same physicians or when compared to the scores of the control group. The study demonstrated feasibility of self-reporting of behaviors by physicians with high participation when provided modest incentives.
Educational and feedback strategies used to improve patient experience are very resource intensive. Training sessions provided at some hospitals may take hours, and sustained effects are unproved. The presence of an independent observer in patient rooms to generate feedback for providers is not scalable and sustainable outside of a research study environment.9-11,15,17,26-29 We attempted to use physician repeated self-reporting to reinforce the important and easy to adopt components of etiquette-based behavior to develop a more easily sustainable strategy. This may have failed for several reasons.
When combining “always” and “usually” responses, the physicians in our study reported a high level of etiquette behavior at baseline. If physicians believe that they are performing well at baseline, they would not consider this to be an area in need of improvement. Bigger changes in behavior may have been possible had the physicians rated themselves less favorably at baseline. Inflated or high baseline self-assessment of performance might also have led to limited success of other types of educational interventions had they been employed.
Studies published since the rollout of our study have shown that physicians significantly overestimate how frequently they perform these etiquette behaviors.30,31 It is likely that was the case in our study subjects. This may, at best, indicate that a much higher change in the level of self-reported performance would be needed to result in meaningful actual changes, or worse, may render self-reported etiquette behavior entirely unreliable. Interventions designed to improve etiquette-based behavior might need to provide feedback about performance.
A program that provides education on the importance of etiquette-based behaviors, obtains objective measures of performance of these behaviors, and offers individualized feedback may be more likely to increase the desired behaviors. This is a limitation of our study. However, we aimed to test a method that required limited resources. Additionally, our method for attributing HCAHPS scores to an individual physician, based on weighted scores that were calculated according to the proportion of days each hospitalist billed for the hospitalization, may be inaccurate. It is possible that each interaction does not contribute equally to the overall score. A team-based intervention and experience measurements could overcome this limitation.
CONCLUSION
This randomized trial demonstrated the feasibility of self-assessment of bedside etiquette behaviors by hospitalists but failed to demonstrate a meaningful impact on patient experience through self-report. These findings suggest that more intensive interventions, perhaps involving direct observation, peer-to-peer mentoring, or other techniques may be required to impact significantly physician etiquette behaviors.
Disclosure
Johns Hopkins Hospitalist Scholars Program provided funding support. Dr. Qayyum is a consultant for Sunovion. The other authors have nothing to report.
1. Blumenthal D, Kilo CM. A report card on continuous quality improvement. Milbank Q. 1998;76(4):625-648. PubMed
2. Shortell SM, Bennett CL, Byck GR. Assessing the impact of continuous quality improvement on clinical practice: What it will take to accelerate progress. Milbank Q. 1998;76(4):593-624. PubMed
3. Mann RK, Siddiqui Z, Kurbanova N, Qayyum R. Effect of HCAHPS reporting on patient satisfaction with physician communication. J Hosp Med. 2015;11(2):105-110. PubMed
4. Rivers PA, Glover SH. Health care competition, strategic mission, and patient satisfaction: research model and propositions. J Health Organ Manag. 2008;22(6):627-641. PubMed
5. Kim SS, Kaplowitz S, Johnston MV. The effects of physician empathy on patient satisfaction and compliance. Eval Health Prof. 2004;27(3):237-251. PubMed
6. Stelfox HT, Gandhi TK, Orav EJ, Gustafson ML. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133. PubMed
7. Rodriguez HP, Rodday AM, Marshall RE, Nelson KL, Rogers WH, Safran DG. Relation of patients’ experiences with individual physicians to malpractice risk. Int J Qual Health Care. 2008;20(1):5-12. PubMed
8. Cydulka RK, Tamayo-Sarver J, Gage A, Bagnoli D. Association of patient satisfaction with complaints and risk management among emergency physicians. J Emerg Med. 2011;41(4):405-411. PubMed
9. Windover AK, Boissy A, Rice TW, Gilligan T, Velez VJ, Merlino J. The REDE model of healthcare communication: Optimizing relationship as a therapeutic agent. Journal of Patient Experience. 2014;1(1):8-13.
10. Chou CL, Hirschmann K, Fortin AH 6th, Lichstein PR. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89(7):1051-1056. PubMed
11. Kennedy M, Denise M, Fasolino M, John P, Gullen M, David J. Improving the patient experience through provider communication skills building. Patient Experience Journal. 2014;1(1):56-60.
12. Braverman AM, Kunkel EJ, Katz L, et al. Do I buy it? How AIDET™ training changes residents’ values about patient care. Journal of Patient Experience. 2015;2(1):13-20.
13. Riess H, Kelley JM, Bailey RW, Dunn EJ, Phillips M. Empathy training for resident physicians: a randomized controlled trial of a neuroscience-informed curriculum. J Gen Intern Med. 2012;27(10):1280-1286. PubMed
14. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Internl Med. 2012;27(2):185-189. PubMed
15. O’Leary KJ, Cyrus RM. Improving patient satisfaction: timely feedback to specific physicians is essential for success. J Hosp Med. 2015;10(8):555-556. PubMed
16. Indovina K, Keniston A, Reid M, et al. Real‐time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;10(8):497-502. PubMed
17. Banka G, Edgington S, Kyulo N, et al. Improving patient satisfaction through physician education, feedback, and incentives. J Hosp Med. 2015;10(8):497-502. PubMed
18. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
19. Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201. PubMed
20. Francis JJ, Pankratz VS, Huddleston JM. Patient satisfaction associated with correct identification of physicians’ photographs. Mayo Clin Proc. 2001;76(6):604-608. PubMed
21. Strasser F, Palmer JL, Willey J, et al. Impact of physician sitting versus standing during inpatient oncology consultations: patients’ preference and perception of compassion and duration. A randomized controlled trial. J Pain Symptom Manage. 2005;29(5):489-497. PubMed
22. Dudas RA, Lemerman H, Barone M, Serwint JR. PHACES (Photographs of Academic Clinicians and Their Educational Status): a tool to improve delivery of family-centered care. Acad Pediatr. 2010;10(2):138-145. PubMed
23. Herzke C, Michtalik H, Durkin N, et al. A method for attributing patient-level metrics to rotating providers in an inpatient setting. J Hosp Med. Under revision.
24. Holden JE, Kelley K, Agarwal R. Analyzing change: a primer on multilevel models with applications to nephrology. Am J Nephrol. 2008;28(5):792-801. PubMed
25. Pinheiro J, Bates D, DebRoy S, Sarkar D. Linear and nonlinear mixed effects models. R package version. 2007;3:57.
26. Braverman AM, Kunkel EJ, Katz L, et al. Do I buy it? How AIDET™ training changes residents’ values about patient care. Journal of Patient Experience. 2015;2(1):13-20.
27. Riess H, Kelley JM, Bailey RW, Dunn EJ, Phillips M. Empathy training for resident physicians: A randomized controlled trial of a neuroscience-informed curriculum. J Gen Intern Med. 2012;27(10):1280-1286. PubMed
28. Raper SE, Gupta M, Okusanya O, Morris JB. Improving communication skills: A course for academic medical center surgery residents and faculty. J Surg Educ. 2015;72(6):e202-e211. PubMed
29. Indovina K, Keniston A, Reid M, et al. Real‐time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;11(4):251-256. PubMed
30. Block L, Hutzler L, Habicht R, et al. Do internal medicine interns practice etiquette‐based communication? A critical look at the inpatient encounter. J Hosp Med. 2013;8(11):631-634. PubMed
31. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. PubMed
1. Blumenthal D, Kilo CM. A report card on continuous quality improvement. Milbank Q. 1998;76(4):625-648. PubMed
2. Shortell SM, Bennett CL, Byck GR. Assessing the impact of continuous quality improvement on clinical practice: What it will take to accelerate progress. Milbank Q. 1998;76(4):593-624. PubMed
3. Mann RK, Siddiqui Z, Kurbanova N, Qayyum R. Effect of HCAHPS reporting on patient satisfaction with physician communication. J Hosp Med. 2015;11(2):105-110. PubMed
4. Rivers PA, Glover SH. Health care competition, strategic mission, and patient satisfaction: research model and propositions. J Health Organ Manag. 2008;22(6):627-641. PubMed
5. Kim SS, Kaplowitz S, Johnston MV. The effects of physician empathy on patient satisfaction and compliance. Eval Health Prof. 2004;27(3):237-251. PubMed
6. Stelfox HT, Gandhi TK, Orav EJ, Gustafson ML. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133. PubMed
7. Rodriguez HP, Rodday AM, Marshall RE, Nelson KL, Rogers WH, Safran DG. Relation of patients’ experiences with individual physicians to malpractice risk. Int J Qual Health Care. 2008;20(1):5-12. PubMed
8. Cydulka RK, Tamayo-Sarver J, Gage A, Bagnoli D. Association of patient satisfaction with complaints and risk management among emergency physicians. J Emerg Med. 2011;41(4):405-411. PubMed
9. Windover AK, Boissy A, Rice TW, Gilligan T, Velez VJ, Merlino J. The REDE model of healthcare communication: Optimizing relationship as a therapeutic agent. Journal of Patient Experience. 2014;1(1):8-13.
10. Chou CL, Hirschmann K, Fortin AH 6th, Lichstein PR. The impact of a faculty learning community on professional and personal development: the facilitator training program of the American Academy on Communication in Healthcare. Acad Med. 2014;89(7):1051-1056. PubMed
11. Kennedy M, Denise M, Fasolino M, John P, Gullen M, David J. Improving the patient experience through provider communication skills building. Patient Experience Journal. 2014;1(1):56-60.
12. Braverman AM, Kunkel EJ, Katz L, et al. Do I buy it? How AIDET™ training changes residents’ values about patient care. Journal of Patient Experience. 2015;2(1):13-20.
13. Riess H, Kelley JM, Bailey RW, Dunn EJ, Phillips M. Empathy training for resident physicians: a randomized controlled trial of a neuroscience-informed curriculum. J Gen Intern Med. 2012;27(10):1280-1286. PubMed
14. Rothberg MB, Steele JR, Wheeler J, Arora A, Priya A, Lindenauer PK. The relationship between time spent communicating and communication outcomes on a hospital medicine service. J Gen Internl Med. 2012;27(2):185-189. PubMed
15. O’Leary KJ, Cyrus RM. Improving patient satisfaction: timely feedback to specific physicians is essential for success. J Hosp Med. 2015;10(8):555-556. PubMed
16. Indovina K, Keniston A, Reid M, et al. Real‐time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;10(8):497-502. PubMed
17. Banka G, Edgington S, Kyulo N, et al. Improving patient satisfaction through physician education, feedback, and incentives. J Hosp Med. 2015;10(8):497-502. PubMed
18. Kahn MW. Etiquette-based medicine. N Engl J Med. 2008;358(19):1988-1989. PubMed
19. Arora V, Gangireddy S, Mehrotra A, Ginde R, Tormey M, Meltzer D. Ability of hospitalized patients to identify their in-hospital physicians. Arch Intern Med. 2009;169(2):199-201. PubMed
20. Francis JJ, Pankratz VS, Huddleston JM. Patient satisfaction associated with correct identification of physicians’ photographs. Mayo Clin Proc. 2001;76(6):604-608. PubMed
21. Strasser F, Palmer JL, Willey J, et al. Impact of physician sitting versus standing during inpatient oncology consultations: patients’ preference and perception of compassion and duration. A randomized controlled trial. J Pain Symptom Manage. 2005;29(5):489-497. PubMed
22. Dudas RA, Lemerman H, Barone M, Serwint JR. PHACES (Photographs of Academic Clinicians and Their Educational Status): a tool to improve delivery of family-centered care. Acad Pediatr. 2010;10(2):138-145. PubMed
23. Herzke C, Michtalik H, Durkin N, et al. A method for attributing patient-level metrics to rotating providers in an inpatient setting. J Hosp Med. Under revision.
24. Holden JE, Kelley K, Agarwal R. Analyzing change: a primer on multilevel models with applications to nephrology. Am J Nephrol. 2008;28(5):792-801. PubMed
25. Pinheiro J, Bates D, DebRoy S, Sarkar D. Linear and nonlinear mixed effects models. R package version. 2007;3:57.
26. Braverman AM, Kunkel EJ, Katz L, et al. Do I buy it? How AIDET™ training changes residents’ values about patient care. Journal of Patient Experience. 2015;2(1):13-20.
27. Riess H, Kelley JM, Bailey RW, Dunn EJ, Phillips M. Empathy training for resident physicians: A randomized controlled trial of a neuroscience-informed curriculum. J Gen Intern Med. 2012;27(10):1280-1286. PubMed
28. Raper SE, Gupta M, Okusanya O, Morris JB. Improving communication skills: A course for academic medical center surgery residents and faculty. J Surg Educ. 2015;72(6):e202-e211. PubMed
29. Indovina K, Keniston A, Reid M, et al. Real‐time patient experience surveys of hospitalized medical patients. J Hosp Med. 2016;11(4):251-256. PubMed
30. Block L, Hutzler L, Habicht R, et al. Do internal medicine interns practice etiquette‐based communication? A critical look at the inpatient encounter. J Hosp Med. 2013;8(11):631-634. PubMed
31. Tackett S, Tad-y D, Rios R, Kisuule F, Wright S. Appraising the practice of etiquette-based medicine in the inpatient setting. J Gen Intern Med. 2013;28(7):908-913. PubMed
© 2017 Society of Hospital Medicine
Prospective cohort study of hospitalized adults with advanced cancer: Associations between complications, comorbidity, and utilization
Of the major chronic conditions that affect adult patients in the United States, cancer accounts for the highest levels of per capita spending.1 Cost growth for cancer treatment has been substantial and persistent, from $72 billion in 2004 to $125 billion in 2010, and is projected to increase to $173 billion by 2020.2 Thirty-five percent of US direct medical cancer costs are attributable to inpatient hospital stays.3 Policy responses that can provide financially sustainable, high-quality models of care for patients with advanced cancer and other serious illness are urgently sought.4-7
Patterns and levels of resource utilization in providing healthcare to patients with serious illness reflect not only treatment choices but a complex set of relationships among demographic, clinical, and system factors.8-10 Patient-level factors previously identified as potentially significant drivers of resource utilization among cancer populations specifically include age,11 sex,12 primary diagnosis,13 and comorbidities.11 Among end-of-life populations, significant associations have been found between cost and ethnicity,14 socioeconomic status,15 advance directive status,16 insurance status,16 and functional status.17
Evidence on factors strongly associated with cost of hospital admission for patients with advanced cancer can therefore inform provision and planning of healthcare. For example, when a specific diagnosis or clinical condition is found to be associated with high cost, then improving coordination and provision of care for this patient group may reduce avoidable utilization. Determining associations between sociodemographics and hospital care cost can help in identifying possible disparities in care, such as those that might occur when care differs by race, class, or insurance status.
We conducted the Palliative Care for Cancer (PC4C) study, a prospective multisite cohort study of the palliative care consultation team intervention for hospitalized adults with advanced cancer.18,19 In our primary analysis, we controlled for receipt of palliative care and analyzed a rich patient-reported dataset to examine associations between hospital care cost, and sociodemographic factors, clinical variables, and prior healthcare utilization. The results provide evidence regarding the factors most associated with the cost of hospital-based cancer care.
METHODS
Design, Setting, Participants, Data Sources
The PC4C study has been described in detail by authors who estimated the impact of specialist palliative care consultation teams on hospitalization cost.19-21 We prospectively collected sociodemographic, clinical, prior utilization, and cost data for adult patients with a primary diagnosis of advanced cancer admitted to 4 large US hospitals between 2007 and 2011.
All 4 of these high-volume tertiary-care medical centers were selected for their high patient volume (to facilitate sample size) and research capacity (to facilitate proficient recruitment and data collection). Before the study was initiated, it was approved by the institutional review board of each facility. In addition, approval was sought from each attending physician at each hospital site; patients whose physician did not grant approval were not considered for enrollment. More than 95% of physicians gave their approval.
Patients were at least 18 years old and had a primary diagnosis of metastatic solid tumor; central nervous system malignancy; locally advanced head, neck, or pancreas cancer; metastatic melanoma; or transplant-ineligible lymphoma or multiple myeloma. Patients were excluded if they did not speak English, had a diagnosis of dementia, were unresponsive or nonverbal, had been admitted for routine chemotherapy, died or were discharged within 48 hours of admission, or had had a previous palliative care consultation.
Eligible patients were identified through daily review of admissions records and administrative databases. For each potential study patient identified, that patient’s bedside nurse inquired about willingness to participate in the study. Then, for each willing patient, a trained clinical interviewer approached to explain the study and obtain informed consent. With the patient’s consent, family members were also approached and enrolled with written informed consent.
Quantitative Variables
Independent variables. In the dataset, we identified 17 patient-level variables we hypothesized could be significantly associated with hospitalization cost. These variables covered 4 domains:
- Demographics: age, sex, race.
- Socioeconomics/systems: education level, insurance status, presence of advance directive (living will or healthcare proxy).
- Clinical care: primary cancer diagnosis, admitting diagnosis, comorbidities (Elixhauser index22), symptom burden and severity (Condensed Memorial Symptom Assessment Scale [CMSAS]23), and activities of daily living24 or presence of a hospital-acquired condition or complication.25
- Prior utilization: visiting homecare nurse and home health aide within 2 weeks before admission, and analgesic use in morphine sulfate equivalents within week before admission.
Data were collected through a combination of medical record review (age, sex, diagnoses, comorbidities, complications), patient interview (race, education, advance directive, CMSAS, activities of daily living, prior utilization), and hospital administrative databases (insurance). For use in regression, variables were divided into categories when appropriate. Table 1 lists these predictors and their prevalence in the analytic sample.
Dependent variable. The outcome of interest in this analysis was total direct cost of hospital stay. Direct costs are those attributable to the care of a specific patient, as distinct from indirect costs, the shared overhead costs of running a hospital.26 Cost data were extracted from hospital accounting databases and therefore reflect actual costs, the US dollar cost to the hospitals of care provided, also known as direct measurement.27 Costs were standardized for geographical region using the Medicare Wage Index28 and year using the Consumer Price Index29 and are presented here in US dollars for 2011, the final year of data collection.
Statistical Methods
Primary analyses. We regressed total direct hospital costs against all predictors listed in Table 1. To control for receipt of palliative care, we used additional independent variables—a fixed-effects variable for each of 3 hospitals (the fourth hospital was used as the reference case) and a binary treatment variable (whether or not the patient was seen by a palliative care consultation team within 2 days of hospital admission).19,20
Associations between cost and patient-level covariates were derived with use of a generalized linear model with a γ distribution and a log link,30 selected after comparative evaluation of performance for multiple linear and nonlinear modeling options.31
For each patient-level covariate, we estimated average marginal effects. For continuous variables, we estimated the marginal increase in cost associated with a 1-unit increase in the variable. For binary variables, we estimated the average incremental effect, the increase in cost associated with a move from the reference group, holding all other covariates to their original values. All analyses were performed with Stata Version 12.32
Secondary analyses. Primary analyses showed that number of patient comorbidities (Elixhauser index) was strongly associated with complications and comorbidity count. Prior analyses with these data have shown that palliative care had a larger cost-saving effect for patients with a larger number of comorbidities.20 Additional analyses were therefore performed to examine associations between complications, utilization, and palliative care. First, we cross-tabulated the sample by complications status (none; minor or major) and receipt of timely palliative care, and we present their summary utilization data. Second, we estimated the effect for each complications stratum (none; minor or major) of receiving timely palliative care on cost. These estimates are calculated consistent with prior work with these data: We used propensity scores to balance patients who received the treatment (palliative care) with patients who did not (usual care only),33,34 and we used a generalized linear model with a γ distribution and a log link to regress the direct hospital care cost on the binary treatment variable and all predictors listed in Table 1.19-21
RESULTS
Participants
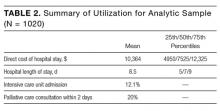
We have previously detailed that in our study there were 1023 patients eligible for cost analysis,19 of whom three were missing data in a field in Table 1 and excluded from this paper. The final analytic sample (N = 1020) is presented according to baseline covariates in Table 1 and according to summary utilization measures in Table 2.
Main Results
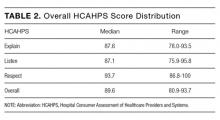
The results of the primary analysis, estimating the association between patient-level factors and cost of hospitalization, are presented in Table 3.
These results show the evidence of an association with cost is strongest for 3 clinical factors: a major complication (+$8267; 95% confidence interval [CI], $4509-$12,025), a minor but not a major complication (+$5289; CI, $3480-$7097), and number of comorbidities (+$852; CI, $550-$1153). In addition, there is evidence of associations between lower cost and admitting diagnosis of electrolyte disorders (–$4759; CI, –$7928 to –$1590) and older age (–$53; CI, –$99 to –$6). There is no significant association between primary diagnosis, symptom burden or other clinical factors, sociodemographic factors or healthcare utilization prior to admission and direct hospitalization costs.
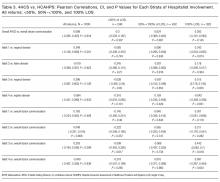
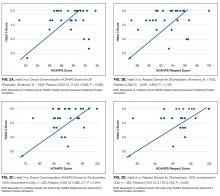
Results of the secondary analyses of associations between complications, utilization, and palliative care are listed in Table 4. Patients are stratified by complication (none; major | minor) and their direct cost of hospital care and hospital length of stay (LOS) presented by treatment group (palliative care; usual care only). The data show that within each strata patients who received palliative care had lower costs and LOS than those who received usual care only. Estimated effects of palliative care on utilization is found to be statistically significant in all four quadrants, with a larger cost-effect in the complications stratum than the non-complications stratum.
Sensitivity Analysis
Fifty-one patients died during admission. After removing these cases, because of concerns about possible unobserved heterogeneity,35 we checked our primary (Table 3) and secondary (Table 4) results. Patients discharged alive had results substantively similar to those of the entire sample.
DISCUSSION
Results from our primary analysis (Table 3) suggest that complications and number of comorbidities are the key drivers of hospitalization cost for adults with advanced cancer. Hospitalization for electrolyte disorders and age are both negatively associated with cost.
The association found between higher cost and hospital-acquired complications (HACs) is consistent with other studies’ finding that HACs often result in higher cost, longer LOS, and increased inhospital mortality.36 Since those studies were reported, policy attention has been increasingly focused on HACs.37 Our findings are notable in that, though prior evidence has also suggested high hospital cost is multifactorial, driven by a diversity of demographic, socioeconomic, and clinical factors, this rich patient-reported dataset suggests that, compared with other variables, HACs are emphatically the largest driver of cost. Moreover, cancer patients typically are a vulnerable population, more prone to complications and thus also to potentially avoidable treatments and higher cost. Our prior work suggested earlier palliative care consultation can reduce cost, in part by shortening LOS and reducing the opportunity for HACs to develop19,20; our secondary analysis (Table 4) suggested a palliative care team’s involvement in HAC treatment can significantly reduce cost of care as well. These associations possibly derive from changed treatment choices and shorter LOS. Further work is needed to better elucidate the role of palliative care in the prevention of HACs in seriously ill patients.
That the number of comorbidities was found to be a key driver of hospitalization cost is consistent with recent findings that high spending on seriously ill patients is associated with having multiple chronic conditions rather than any specific primary diagnosis.38,39 It is important to note that, unlike impending complications, serious chronic conditions generally are known at admission and can be addressed prospectively through provision and policy. A prior analysis with these data found that palliative care consultation was more cost-effective for patients with a larger number of comorbidities.20 Our 2 studies together suggest that, notwithstanding the preferable alternative of avoiding hospitalization entirely, palliative care and other skilled coordination of care services ought to be prioritized for inpatients with multiple serious illnesses and the highest medical complexity. This patient group has both the highest costs and the greatest amenability to skilled transdisciplinary intervention, possibly because multiple chronic conditions affect patients interactively, complicating identification of appropriate polypharmacy responses and prioritization of treatments.
Our findings also may help direct appropriate use of palliative care services. The recently published American Society of Clinical Oncology palliative care guidelines note that all patients with advanced cancer (eg, those enrolled in our study) should receive dedicated palliative care services, early in the disease course, concurrent with active treatment.40 Workforce estimates suggest that the current and future numbers of palliative care practitioners will be unable to meet the ASCO recommendations alone never mind patients with other serious illnesses (eg, advanced heart failure, COPD, CKD).41 As such, specialized palliative care services will need to be targeted to the patient populations that can benefit most from these services. Whereas cost should not be the principle driver specialized palliative care provision, it will likely be an important component due to both the necessity of allocating scarce resources in the most effective way and the evidence that in care of the seriously-ill lower costs are often a proxy for improved patient experience.
These findings also have implications for research: Different conditions and presumably different combinations of conditions have very different implications for hospital care costs for a cohort of adults with advanced cancer. Given the increasing number of co-occurring conditions among seriously ill patients, and the increasing costs of cancer care and of treating multimorbidity cases, it is essential to further our understanding of the relationship between comorbidities and costs in order to plan and finance care for advanced cancer patients.
Limitations and Generalizability
In this observational study, reported associations may be attributable to unobserved confounding that our analyses failed to control.
Our results reflect associations in a prospective multisite study of advanced cancer patients hospitalized in the United States. It is not clear how generalizable our findings are to patients without cancer, to patients in nonhospital settings, and to patients in other health systems and countries. Analyzing cost from the hospital perspective does not take into account that the most impactful way to reduce cost is to avoid hospitalization entirely.
Results of our secondary analysis will not necessarily be robust to patient groups, as specific weights likely will vary by sample. The idea that costs vary by condition, however, is important nevertheless. Elixhauser total was derived with use of the enhanced ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) algorithm from Quan et al.42 and does not include subsequent Elixhauser Comorbidity Software updates recommended by the Healthcare Cost and Utilization Project (HCUP; Agency for Healthcare Research and Quality).43 The Elixhauser index is recommended over Charlson and other comorbidity indices by both HCUP45 and a recent systematic review.44
One possible unobserved factor is prior chemotherapy, which is associated with increased hospitalization risk. Related factors that are somewhat controlled for in the study include cancer stage (advanced cancer was an eligibility criterion) and receipt of analgesics within the week before admission (patients admitted for routine chemotherapy were excluded from analyses at the outset).
CONCLUSION
Other studies have identified a wide range of sociodemographic, clinical, and health system factors associated with healthcare utilization. Our results suggest that, for cost of hospital admission among adults with advanced cancer, the most important drivers of utilization are complications and comorbidities. Hospital costs for patients with advanced cancer constitute a major part of US healthcare spending, and these results suggest the need to prioritize high-quality, cost-effective care for patients with multiple serious illnesses.
Acknowledgments
The authors thank Robert Arnold, Phil Santa Emma, Mary Beth Happ, Tim Smith, and David Weissman for contributing to the Palliative Care for Cancer (PC4C) project.
Disclosure
The study was funded by grant R01 CA116227 from the National Cancer Institute and the National Institute of Nursing Research. The study sponsors had no role in design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the US government. All authors are independent of the study sponsors. Dr. May was supported by a HRB/NCI Health Economics Fellowship during this work. Dr. Garrido is supported by a Veterans Affairs HSR&D career development award (CDA 11-201/CDP 12-255). Dr. Kelley’s time was funded by the National Institute on Aging (1K23AG040774-01A1) and the American Federation for Aging. Dr. Smith is funded by the NCI Core Grant P 30 006973, 1-R01 CA177562-01A1, 1-R01 NR014050 01, and the Harry J. Duffey Family Endowment for Palliative Care. Dr. Morrison was the recipient of a Midcareer Investigator Award in Patient-Oriented Research (5K24AG022345) during the course of this work. This work was supported by the NIA, Claude D. Pepper Older Americans Independence Center at the Icahn School of Medicine at Mount Sinai [5P30AG028741], and the National Palliative Care Research Center.
1. Soni A. Top 10 Most Costly Conditions Among Men and Women, 2008: Estimates for the U.S. Civilian Noninstitutionalized Adult Population, Age 18 and Older. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
2. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128. PubMed
3. American Cancer Society. Cancer Facts and Figures 2015. Atlanta, GA: American Cancer Society; 2015.
4. Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364(21):2060-2065. PubMed
5. Levit L, Balogh E, Nass S, Ganz PA, eds. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: Institute of Medicine/National Academies Press; 2013. PubMed
7. Anderson GF. Chronic Care: Making the Case for Ongoing Care. Princeton, NJ: Robert Wood Johnson Foundation; 2010.
8. Tibi-Levy Y, Le Vaillant M, de Pouvourville G. Determinants of resource utilization in four palliative care units. Palliat Med. 2006;20(2):95-106. PubMed
9. Simoens S, Kutten B, Keirse E, et al. The costs of treating terminal patients. J Pain Symptom Manage. 2010;40(3):436-448. PubMed
10. Groeneveld I, Murtagh F, Kaloki Y, Bausewein C, Higginson I. Determinants of healthcare costs in the last year of life. Annual Assembly of American Academy of Hospice and Palliative Medicine & Hospice and Palliative Nurses Association; March 14, 2013; New Orleans, LA.
11. Shugarman LR, Bird CE, Schuster CR, Lynn J. Age and gender differences in Medicare expenditures at the end of life for colorectal cancer decedents. J Womens Health. 2007;16(2):214-227. PubMed
12. Shugarman LR, Bird CE, Schuster CR, Lynn J. Age and gender differences in Medicare expenditures and service utilization at the end of life for lung cancer decedents. Womens Health Issues. 2008;18(3):199-209. PubMed
13. Walker H, Anderson M, Farahati F, et al. Resource use and costs of end-of-life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J Palliat Care. 2011;27(2):79-88. PubMed
14. Hanchate A, Kronman AC, Young-Xu Y, Ash AS, Emanuel E. Racial and ethnic differences in end-of-life costs: why do minorities cost more than whites? Arch Intern Med. 2009;169(5):493-501. PubMed
15. Hanratty B, Burstrom B, Walander A, Whitehead M. Inequality in the face of death? Public expenditure on health care for different socioeconomic groups in the last year of life. J Health Serv Res Policy. 2007;12(2):90-94. PubMed
16. Kelley AS, Ettner SL, Morrison RS, Du Q, Wenger NS, Sarkisian CA. Determinants of medical expenditures in the last 6 months of life. Ann Intern Med. 2011;154(4):235-242. PubMed
17. Guerriere DN, Zagorski B, Fassbender K, Masucci L, Librach L, Coyte PC. Cost variations in ambulatory and home-based palliative care. Palliat Med. 2010;24(5):523-532. PubMed
18. US Department of Health and Human Services, National Institutes of Health. Palliative Care for Hospitalized Cancer Patients [project information]. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health; 2006. Project 5R01CA116227-04. https://projectreporter.nih.gov/project_info_description.cfm?projectnumber=5R01CA116227-04. Published 2006. Accessed August 1, 2015.
19. May P, Garrido MM, Cassel JB, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015;33(25):2745-2752. PubMed
20. May P, Garrido MM, Cassel JB, et al. Palliative care teams’ cost-saving effect is larger for cancer patients with higher numbers of comorbidities. Health Aff. 2016;35(1):44-53. PubMed
21. May P, Garrido MM, Cassel JB, Morrison RS, Normand C. Using length of stay to control for unobserved heterogeneity when estimating treatment effect on hospital costs with observational data: issues of reliability, robustness and usefulness. Health Serv Res. 2016;51(5):2020-2043. PubMed
22. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
23. Chang VT, Hwang SS, Kasimis B, Thaler HT. Shorter symptom assessment instruments: the Condensed Memorial Symptom Assessment Scale (CMSAS). Cancer Invest. 2004;22(4):526-536. PubMed
24. Katz S, Ford A, Moskowitz R, Jackson B, Jaffe M. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185(12):914-919. PubMed
25. McLaughlin MA, Orosz GM, Magaziner J, et al. Preoperative status and risk of complications in patients with hip fracture. J Gen Intern Med. 2006;21(3):219-225. PubMed
26. Taheri PA, Butz D, Griffes LC, Morlock DR, Greenfield LJ. Physician impact on the total cost of care. Ann Surg. 2000;231(3):432-435. PubMed
27. US Department of Veterans Affairs, Health Economics Resource Center. Determining costs. Washington, DC: US Dept of Veterans Affairs, Health Economics Resource Center; 2016. http://www.herc.research.va.gov/include/page.asp?id=determining-costs. Published 2016. Accessed September 7, 2016.
28. US Department of Health and Human Services, Center for Medicare & Medicaid Services. FY 2011 Wage Index [Table 2]. Baltimore, MD: US Dept of Health and Human Services, Center for Medicare & Medicaid Services; 2011. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Wage-Index-Files-Items/CMS1234173.html. Published 2011. Accessed September 2, 2014.
29. US Department of Labor, Bureau of Labor Statistics. All Urban Consumers (Current Series) [Consumer Price Index database]. US Dept of Labor, Bureau of Labor Statistics; 2015. http://www.bls.gov/cpi/data.htm. Published 2015. Accessed August 15, 2016.
30. Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465-488. PubMed
31. Jones AM, Rice N, Bago d’Uva T, Balia S. Applied Health Economics. 2nd ed. Oxford, England: Routledge; 2013.
32. Stata [computer program]. Version 12. College Station, TX: StataCorp; 2011.
33. Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing
propensity scores. Health Serv Res. 2014;49(5):1701-1720. PubMed
34. R Core Team. R: A Language and Environment for Statistical Computing. Vienna,
Austria: R Foundation for Statistical Computing; 2016.
35. Cassel JB, Kerr K, Pantilat S, Smith TJ. Palliative care consultation and hospital
length of stay. J Palliat Med. 2010;13(6):761-767. PubMed
36. US Department of Health and Human Services, Agency for Healthcare Research
and Quality. Efforts to Improve Patient Safety Result in 1.3 Million Fewer Patient
Harms: Interim Update on 2013 Annual Hospital-Acquired Condition Rate and
Estimates of Cost Savings and Deaths Averted From 2010 to 2013. Rockville,
MD: US Dept of Health and Human Services, Agency for Healthcare Research
and Quality; 2015. http://www.ahrq.gov/professionals/quality-patient-safety/pfp/
interimhacrate2013.html. Published 2015. Updated November 2015. Accessed
November 18, 2016.
37. Cassidy A. Health Policy Brief: Medicare’s Hospital-Acquired Condition Reduction
Program. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_
id=142. Published August 6, 2015. Accessed April 24, 2017.
38. Davis MA, Nallamothu BK, Banerjee M, Bynum JP. Identification of four unique
spending patterns among older adults in the last year of life challenges standard
assumptions. Health Aff. 2016;35(7):1316-1323. PubMed
39. Aldridge MD, Kelley AS. The myth regarding the high cost of end-of-life care.
Am J Public Health. 2015;105(12):2411-2415. PubMed
40. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard
oncology care: American Society of Clinical Oncology clinical practice guideline
update. J Clin Oncol. 2017;35(1):96-112. PubMed
41. Spetz J, Dudley N, Trupin L, Rogers M, Meier DE, Dumanovsky T. Few hospital
palliative care programs meet national staffing recommendations. Health Aff.
2016;35(9):1690-1697. PubMed
42. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities
in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed
43. HCUP [Healthcare Cost and Utilization Project] Elixhauser Comorbidity Software
[computer program]. Version 3.7. Rockville, MD: Agency for Healthcare
Research and Quality; 2016. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/
comorbidity.jsp. Published 2016. Accessed November 9, 2016.
44. Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for
administrative data. Med Care. 2012;50(12):1109-1118. PubMed
Of the major chronic conditions that affect adult patients in the United States, cancer accounts for the highest levels of per capita spending.1 Cost growth for cancer treatment has been substantial and persistent, from $72 billion in 2004 to $125 billion in 2010, and is projected to increase to $173 billion by 2020.2 Thirty-five percent of US direct medical cancer costs are attributable to inpatient hospital stays.3 Policy responses that can provide financially sustainable, high-quality models of care for patients with advanced cancer and other serious illness are urgently sought.4-7
Patterns and levels of resource utilization in providing healthcare to patients with serious illness reflect not only treatment choices but a complex set of relationships among demographic, clinical, and system factors.8-10 Patient-level factors previously identified as potentially significant drivers of resource utilization among cancer populations specifically include age,11 sex,12 primary diagnosis,13 and comorbidities.11 Among end-of-life populations, significant associations have been found between cost and ethnicity,14 socioeconomic status,15 advance directive status,16 insurance status,16 and functional status.17
Evidence on factors strongly associated with cost of hospital admission for patients with advanced cancer can therefore inform provision and planning of healthcare. For example, when a specific diagnosis or clinical condition is found to be associated with high cost, then improving coordination and provision of care for this patient group may reduce avoidable utilization. Determining associations between sociodemographics and hospital care cost can help in identifying possible disparities in care, such as those that might occur when care differs by race, class, or insurance status.
We conducted the Palliative Care for Cancer (PC4C) study, a prospective multisite cohort study of the palliative care consultation team intervention for hospitalized adults with advanced cancer.18,19 In our primary analysis, we controlled for receipt of palliative care and analyzed a rich patient-reported dataset to examine associations between hospital care cost, and sociodemographic factors, clinical variables, and prior healthcare utilization. The results provide evidence regarding the factors most associated with the cost of hospital-based cancer care.
METHODS
Design, Setting, Participants, Data Sources
The PC4C study has been described in detail by authors who estimated the impact of specialist palliative care consultation teams on hospitalization cost.19-21 We prospectively collected sociodemographic, clinical, prior utilization, and cost data for adult patients with a primary diagnosis of advanced cancer admitted to 4 large US hospitals between 2007 and 2011.
All 4 of these high-volume tertiary-care medical centers were selected for their high patient volume (to facilitate sample size) and research capacity (to facilitate proficient recruitment and data collection). Before the study was initiated, it was approved by the institutional review board of each facility. In addition, approval was sought from each attending physician at each hospital site; patients whose physician did not grant approval were not considered for enrollment. More than 95% of physicians gave their approval.
Patients were at least 18 years old and had a primary diagnosis of metastatic solid tumor; central nervous system malignancy; locally advanced head, neck, or pancreas cancer; metastatic melanoma; or transplant-ineligible lymphoma or multiple myeloma. Patients were excluded if they did not speak English, had a diagnosis of dementia, were unresponsive or nonverbal, had been admitted for routine chemotherapy, died or were discharged within 48 hours of admission, or had had a previous palliative care consultation.
Eligible patients were identified through daily review of admissions records and administrative databases. For each potential study patient identified, that patient’s bedside nurse inquired about willingness to participate in the study. Then, for each willing patient, a trained clinical interviewer approached to explain the study and obtain informed consent. With the patient’s consent, family members were also approached and enrolled with written informed consent.
Quantitative Variables
Independent variables. In the dataset, we identified 17 patient-level variables we hypothesized could be significantly associated with hospitalization cost. These variables covered 4 domains:
- Demographics: age, sex, race.
- Socioeconomics/systems: education level, insurance status, presence of advance directive (living will or healthcare proxy).
- Clinical care: primary cancer diagnosis, admitting diagnosis, comorbidities (Elixhauser index22), symptom burden and severity (Condensed Memorial Symptom Assessment Scale [CMSAS]23), and activities of daily living24 or presence of a hospital-acquired condition or complication.25
- Prior utilization: visiting homecare nurse and home health aide within 2 weeks before admission, and analgesic use in morphine sulfate equivalents within week before admission.
Data were collected through a combination of medical record review (age, sex, diagnoses, comorbidities, complications), patient interview (race, education, advance directive, CMSAS, activities of daily living, prior utilization), and hospital administrative databases (insurance). For use in regression, variables were divided into categories when appropriate. Table 1 lists these predictors and their prevalence in the analytic sample.
Dependent variable. The outcome of interest in this analysis was total direct cost of hospital stay. Direct costs are those attributable to the care of a specific patient, as distinct from indirect costs, the shared overhead costs of running a hospital.26 Cost data were extracted from hospital accounting databases and therefore reflect actual costs, the US dollar cost to the hospitals of care provided, also known as direct measurement.27 Costs were standardized for geographical region using the Medicare Wage Index28 and year using the Consumer Price Index29 and are presented here in US dollars for 2011, the final year of data collection.
Statistical Methods
Primary analyses. We regressed total direct hospital costs against all predictors listed in Table 1. To control for receipt of palliative care, we used additional independent variables—a fixed-effects variable for each of 3 hospitals (the fourth hospital was used as the reference case) and a binary treatment variable (whether or not the patient was seen by a palliative care consultation team within 2 days of hospital admission).19,20
Associations between cost and patient-level covariates were derived with use of a generalized linear model with a γ distribution and a log link,30 selected after comparative evaluation of performance for multiple linear and nonlinear modeling options.31
For each patient-level covariate, we estimated average marginal effects. For continuous variables, we estimated the marginal increase in cost associated with a 1-unit increase in the variable. For binary variables, we estimated the average incremental effect, the increase in cost associated with a move from the reference group, holding all other covariates to their original values. All analyses were performed with Stata Version 12.32
Secondary analyses. Primary analyses showed that number of patient comorbidities (Elixhauser index) was strongly associated with complications and comorbidity count. Prior analyses with these data have shown that palliative care had a larger cost-saving effect for patients with a larger number of comorbidities.20 Additional analyses were therefore performed to examine associations between complications, utilization, and palliative care. First, we cross-tabulated the sample by complications status (none; minor or major) and receipt of timely palliative care, and we present their summary utilization data. Second, we estimated the effect for each complications stratum (none; minor or major) of receiving timely palliative care on cost. These estimates are calculated consistent with prior work with these data: We used propensity scores to balance patients who received the treatment (palliative care) with patients who did not (usual care only),33,34 and we used a generalized linear model with a γ distribution and a log link to regress the direct hospital care cost on the binary treatment variable and all predictors listed in Table 1.19-21
RESULTS
Participants
We have previously detailed that in our study there were 1023 patients eligible for cost analysis,19 of whom three were missing data in a field in Table 1 and excluded from this paper. The final analytic sample (N = 1020) is presented according to baseline covariates in Table 1 and according to summary utilization measures in Table 2.
Main Results
The results of the primary analysis, estimating the association between patient-level factors and cost of hospitalization, are presented in Table 3.
These results show the evidence of an association with cost is strongest for 3 clinical factors: a major complication (+$8267; 95% confidence interval [CI], $4509-$12,025), a minor but not a major complication (+$5289; CI, $3480-$7097), and number of comorbidities (+$852; CI, $550-$1153). In addition, there is evidence of associations between lower cost and admitting diagnosis of electrolyte disorders (–$4759; CI, –$7928 to –$1590) and older age (–$53; CI, –$99 to –$6). There is no significant association between primary diagnosis, symptom burden or other clinical factors, sociodemographic factors or healthcare utilization prior to admission and direct hospitalization costs.
Results of the secondary analyses of associations between complications, utilization, and palliative care are listed in Table 4. Patients are stratified by complication (none; major | minor) and their direct cost of hospital care and hospital length of stay (LOS) presented by treatment group (palliative care; usual care only). The data show that within each strata patients who received palliative care had lower costs and LOS than those who received usual care only. Estimated effects of palliative care on utilization is found to be statistically significant in all four quadrants, with a larger cost-effect in the complications stratum than the non-complications stratum.
Sensitivity Analysis
Fifty-one patients died during admission. After removing these cases, because of concerns about possible unobserved heterogeneity,35 we checked our primary (Table 3) and secondary (Table 4) results. Patients discharged alive had results substantively similar to those of the entire sample.
DISCUSSION
Results from our primary analysis (Table 3) suggest that complications and number of comorbidities are the key drivers of hospitalization cost for adults with advanced cancer. Hospitalization for electrolyte disorders and age are both negatively associated with cost.
The association found between higher cost and hospital-acquired complications (HACs) is consistent with other studies’ finding that HACs often result in higher cost, longer LOS, and increased inhospital mortality.36 Since those studies were reported, policy attention has been increasingly focused on HACs.37 Our findings are notable in that, though prior evidence has also suggested high hospital cost is multifactorial, driven by a diversity of demographic, socioeconomic, and clinical factors, this rich patient-reported dataset suggests that, compared with other variables, HACs are emphatically the largest driver of cost. Moreover, cancer patients typically are a vulnerable population, more prone to complications and thus also to potentially avoidable treatments and higher cost. Our prior work suggested earlier palliative care consultation can reduce cost, in part by shortening LOS and reducing the opportunity for HACs to develop19,20; our secondary analysis (Table 4) suggested a palliative care team’s involvement in HAC treatment can significantly reduce cost of care as well. These associations possibly derive from changed treatment choices and shorter LOS. Further work is needed to better elucidate the role of palliative care in the prevention of HACs in seriously ill patients.
That the number of comorbidities was found to be a key driver of hospitalization cost is consistent with recent findings that high spending on seriously ill patients is associated with having multiple chronic conditions rather than any specific primary diagnosis.38,39 It is important to note that, unlike impending complications, serious chronic conditions generally are known at admission and can be addressed prospectively through provision and policy. A prior analysis with these data found that palliative care consultation was more cost-effective for patients with a larger number of comorbidities.20 Our 2 studies together suggest that, notwithstanding the preferable alternative of avoiding hospitalization entirely, palliative care and other skilled coordination of care services ought to be prioritized for inpatients with multiple serious illnesses and the highest medical complexity. This patient group has both the highest costs and the greatest amenability to skilled transdisciplinary intervention, possibly because multiple chronic conditions affect patients interactively, complicating identification of appropriate polypharmacy responses and prioritization of treatments.
Our findings also may help direct appropriate use of palliative care services. The recently published American Society of Clinical Oncology palliative care guidelines note that all patients with advanced cancer (eg, those enrolled in our study) should receive dedicated palliative care services, early in the disease course, concurrent with active treatment.40 Workforce estimates suggest that the current and future numbers of palliative care practitioners will be unable to meet the ASCO recommendations alone never mind patients with other serious illnesses (eg, advanced heart failure, COPD, CKD).41 As such, specialized palliative care services will need to be targeted to the patient populations that can benefit most from these services. Whereas cost should not be the principle driver specialized palliative care provision, it will likely be an important component due to both the necessity of allocating scarce resources in the most effective way and the evidence that in care of the seriously-ill lower costs are often a proxy for improved patient experience.
These findings also have implications for research: Different conditions and presumably different combinations of conditions have very different implications for hospital care costs for a cohort of adults with advanced cancer. Given the increasing number of co-occurring conditions among seriously ill patients, and the increasing costs of cancer care and of treating multimorbidity cases, it is essential to further our understanding of the relationship between comorbidities and costs in order to plan and finance care for advanced cancer patients.
Limitations and Generalizability
In this observational study, reported associations may be attributable to unobserved confounding that our analyses failed to control.
Our results reflect associations in a prospective multisite study of advanced cancer patients hospitalized in the United States. It is not clear how generalizable our findings are to patients without cancer, to patients in nonhospital settings, and to patients in other health systems and countries. Analyzing cost from the hospital perspective does not take into account that the most impactful way to reduce cost is to avoid hospitalization entirely.
Results of our secondary analysis will not necessarily be robust to patient groups, as specific weights likely will vary by sample. The idea that costs vary by condition, however, is important nevertheless. Elixhauser total was derived with use of the enhanced ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) algorithm from Quan et al.42 and does not include subsequent Elixhauser Comorbidity Software updates recommended by the Healthcare Cost and Utilization Project (HCUP; Agency for Healthcare Research and Quality).43 The Elixhauser index is recommended over Charlson and other comorbidity indices by both HCUP45 and a recent systematic review.44
One possible unobserved factor is prior chemotherapy, which is associated with increased hospitalization risk. Related factors that are somewhat controlled for in the study include cancer stage (advanced cancer was an eligibility criterion) and receipt of analgesics within the week before admission (patients admitted for routine chemotherapy were excluded from analyses at the outset).
CONCLUSION
Other studies have identified a wide range of sociodemographic, clinical, and health system factors associated with healthcare utilization. Our results suggest that, for cost of hospital admission among adults with advanced cancer, the most important drivers of utilization are complications and comorbidities. Hospital costs for patients with advanced cancer constitute a major part of US healthcare spending, and these results suggest the need to prioritize high-quality, cost-effective care for patients with multiple serious illnesses.
Acknowledgments
The authors thank Robert Arnold, Phil Santa Emma, Mary Beth Happ, Tim Smith, and David Weissman for contributing to the Palliative Care for Cancer (PC4C) project.
Disclosure
The study was funded by grant R01 CA116227 from the National Cancer Institute and the National Institute of Nursing Research. The study sponsors had no role in design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the US government. All authors are independent of the study sponsors. Dr. May was supported by a HRB/NCI Health Economics Fellowship during this work. Dr. Garrido is supported by a Veterans Affairs HSR&D career development award (CDA 11-201/CDP 12-255). Dr. Kelley’s time was funded by the National Institute on Aging (1K23AG040774-01A1) and the American Federation for Aging. Dr. Smith is funded by the NCI Core Grant P 30 006973, 1-R01 CA177562-01A1, 1-R01 NR014050 01, and the Harry J. Duffey Family Endowment for Palliative Care. Dr. Morrison was the recipient of a Midcareer Investigator Award in Patient-Oriented Research (5K24AG022345) during the course of this work. This work was supported by the NIA, Claude D. Pepper Older Americans Independence Center at the Icahn School of Medicine at Mount Sinai [5P30AG028741], and the National Palliative Care Research Center.
Of the major chronic conditions that affect adult patients in the United States, cancer accounts for the highest levels of per capita spending.1 Cost growth for cancer treatment has been substantial and persistent, from $72 billion in 2004 to $125 billion in 2010, and is projected to increase to $173 billion by 2020.2 Thirty-five percent of US direct medical cancer costs are attributable to inpatient hospital stays.3 Policy responses that can provide financially sustainable, high-quality models of care for patients with advanced cancer and other serious illness are urgently sought.4-7
Patterns and levels of resource utilization in providing healthcare to patients with serious illness reflect not only treatment choices but a complex set of relationships among demographic, clinical, and system factors.8-10 Patient-level factors previously identified as potentially significant drivers of resource utilization among cancer populations specifically include age,11 sex,12 primary diagnosis,13 and comorbidities.11 Among end-of-life populations, significant associations have been found between cost and ethnicity,14 socioeconomic status,15 advance directive status,16 insurance status,16 and functional status.17
Evidence on factors strongly associated with cost of hospital admission for patients with advanced cancer can therefore inform provision and planning of healthcare. For example, when a specific diagnosis or clinical condition is found to be associated with high cost, then improving coordination and provision of care for this patient group may reduce avoidable utilization. Determining associations between sociodemographics and hospital care cost can help in identifying possible disparities in care, such as those that might occur when care differs by race, class, or insurance status.
We conducted the Palliative Care for Cancer (PC4C) study, a prospective multisite cohort study of the palliative care consultation team intervention for hospitalized adults with advanced cancer.18,19 In our primary analysis, we controlled for receipt of palliative care and analyzed a rich patient-reported dataset to examine associations between hospital care cost, and sociodemographic factors, clinical variables, and prior healthcare utilization. The results provide evidence regarding the factors most associated with the cost of hospital-based cancer care.
METHODS
Design, Setting, Participants, Data Sources
The PC4C study has been described in detail by authors who estimated the impact of specialist palliative care consultation teams on hospitalization cost.19-21 We prospectively collected sociodemographic, clinical, prior utilization, and cost data for adult patients with a primary diagnosis of advanced cancer admitted to 4 large US hospitals between 2007 and 2011.
All 4 of these high-volume tertiary-care medical centers were selected for their high patient volume (to facilitate sample size) and research capacity (to facilitate proficient recruitment and data collection). Before the study was initiated, it was approved by the institutional review board of each facility. In addition, approval was sought from each attending physician at each hospital site; patients whose physician did not grant approval were not considered for enrollment. More than 95% of physicians gave their approval.
Patients were at least 18 years old and had a primary diagnosis of metastatic solid tumor; central nervous system malignancy; locally advanced head, neck, or pancreas cancer; metastatic melanoma; or transplant-ineligible lymphoma or multiple myeloma. Patients were excluded if they did not speak English, had a diagnosis of dementia, were unresponsive or nonverbal, had been admitted for routine chemotherapy, died or were discharged within 48 hours of admission, or had had a previous palliative care consultation.
Eligible patients were identified through daily review of admissions records and administrative databases. For each potential study patient identified, that patient’s bedside nurse inquired about willingness to participate in the study. Then, for each willing patient, a trained clinical interviewer approached to explain the study and obtain informed consent. With the patient’s consent, family members were also approached and enrolled with written informed consent.
Quantitative Variables
Independent variables. In the dataset, we identified 17 patient-level variables we hypothesized could be significantly associated with hospitalization cost. These variables covered 4 domains:
- Demographics: age, sex, race.
- Socioeconomics/systems: education level, insurance status, presence of advance directive (living will or healthcare proxy).
- Clinical care: primary cancer diagnosis, admitting diagnosis, comorbidities (Elixhauser index22), symptom burden and severity (Condensed Memorial Symptom Assessment Scale [CMSAS]23), and activities of daily living24 or presence of a hospital-acquired condition or complication.25
- Prior utilization: visiting homecare nurse and home health aide within 2 weeks before admission, and analgesic use in morphine sulfate equivalents within week before admission.
Data were collected through a combination of medical record review (age, sex, diagnoses, comorbidities, complications), patient interview (race, education, advance directive, CMSAS, activities of daily living, prior utilization), and hospital administrative databases (insurance). For use in regression, variables were divided into categories when appropriate. Table 1 lists these predictors and their prevalence in the analytic sample.
Dependent variable. The outcome of interest in this analysis was total direct cost of hospital stay. Direct costs are those attributable to the care of a specific patient, as distinct from indirect costs, the shared overhead costs of running a hospital.26 Cost data were extracted from hospital accounting databases and therefore reflect actual costs, the US dollar cost to the hospitals of care provided, also known as direct measurement.27 Costs were standardized for geographical region using the Medicare Wage Index28 and year using the Consumer Price Index29 and are presented here in US dollars for 2011, the final year of data collection.
Statistical Methods
Primary analyses. We regressed total direct hospital costs against all predictors listed in Table 1. To control for receipt of palliative care, we used additional independent variables—a fixed-effects variable for each of 3 hospitals (the fourth hospital was used as the reference case) and a binary treatment variable (whether or not the patient was seen by a palliative care consultation team within 2 days of hospital admission).19,20
Associations between cost and patient-level covariates were derived with use of a generalized linear model with a γ distribution and a log link,30 selected after comparative evaluation of performance for multiple linear and nonlinear modeling options.31
For each patient-level covariate, we estimated average marginal effects. For continuous variables, we estimated the marginal increase in cost associated with a 1-unit increase in the variable. For binary variables, we estimated the average incremental effect, the increase in cost associated with a move from the reference group, holding all other covariates to their original values. All analyses were performed with Stata Version 12.32
Secondary analyses. Primary analyses showed that number of patient comorbidities (Elixhauser index) was strongly associated with complications and comorbidity count. Prior analyses with these data have shown that palliative care had a larger cost-saving effect for patients with a larger number of comorbidities.20 Additional analyses were therefore performed to examine associations between complications, utilization, and palliative care. First, we cross-tabulated the sample by complications status (none; minor or major) and receipt of timely palliative care, and we present their summary utilization data. Second, we estimated the effect for each complications stratum (none; minor or major) of receiving timely palliative care on cost. These estimates are calculated consistent with prior work with these data: We used propensity scores to balance patients who received the treatment (palliative care) with patients who did not (usual care only),33,34 and we used a generalized linear model with a γ distribution and a log link to regress the direct hospital care cost on the binary treatment variable and all predictors listed in Table 1.19-21
RESULTS
Participants
We have previously detailed that in our study there were 1023 patients eligible for cost analysis,19 of whom three were missing data in a field in Table 1 and excluded from this paper. The final analytic sample (N = 1020) is presented according to baseline covariates in Table 1 and according to summary utilization measures in Table 2.
Main Results
The results of the primary analysis, estimating the association between patient-level factors and cost of hospitalization, are presented in Table 3.
These results show the evidence of an association with cost is strongest for 3 clinical factors: a major complication (+$8267; 95% confidence interval [CI], $4509-$12,025), a minor but not a major complication (+$5289; CI, $3480-$7097), and number of comorbidities (+$852; CI, $550-$1153). In addition, there is evidence of associations between lower cost and admitting diagnosis of electrolyte disorders (–$4759; CI, –$7928 to –$1590) and older age (–$53; CI, –$99 to –$6). There is no significant association between primary diagnosis, symptom burden or other clinical factors, sociodemographic factors or healthcare utilization prior to admission and direct hospitalization costs.
Results of the secondary analyses of associations between complications, utilization, and palliative care are listed in Table 4. Patients are stratified by complication (none; major | minor) and their direct cost of hospital care and hospital length of stay (LOS) presented by treatment group (palliative care; usual care only). The data show that within each strata patients who received palliative care had lower costs and LOS than those who received usual care only. Estimated effects of palliative care on utilization is found to be statistically significant in all four quadrants, with a larger cost-effect in the complications stratum than the non-complications stratum.
Sensitivity Analysis
Fifty-one patients died during admission. After removing these cases, because of concerns about possible unobserved heterogeneity,35 we checked our primary (Table 3) and secondary (Table 4) results. Patients discharged alive had results substantively similar to those of the entire sample.
DISCUSSION
Results from our primary analysis (Table 3) suggest that complications and number of comorbidities are the key drivers of hospitalization cost for adults with advanced cancer. Hospitalization for electrolyte disorders and age are both negatively associated with cost.
The association found between higher cost and hospital-acquired complications (HACs) is consistent with other studies’ finding that HACs often result in higher cost, longer LOS, and increased inhospital mortality.36 Since those studies were reported, policy attention has been increasingly focused on HACs.37 Our findings are notable in that, though prior evidence has also suggested high hospital cost is multifactorial, driven by a diversity of demographic, socioeconomic, and clinical factors, this rich patient-reported dataset suggests that, compared with other variables, HACs are emphatically the largest driver of cost. Moreover, cancer patients typically are a vulnerable population, more prone to complications and thus also to potentially avoidable treatments and higher cost. Our prior work suggested earlier palliative care consultation can reduce cost, in part by shortening LOS and reducing the opportunity for HACs to develop19,20; our secondary analysis (Table 4) suggested a palliative care team’s involvement in HAC treatment can significantly reduce cost of care as well. These associations possibly derive from changed treatment choices and shorter LOS. Further work is needed to better elucidate the role of palliative care in the prevention of HACs in seriously ill patients.
That the number of comorbidities was found to be a key driver of hospitalization cost is consistent with recent findings that high spending on seriously ill patients is associated with having multiple chronic conditions rather than any specific primary diagnosis.38,39 It is important to note that, unlike impending complications, serious chronic conditions generally are known at admission and can be addressed prospectively through provision and policy. A prior analysis with these data found that palliative care consultation was more cost-effective for patients with a larger number of comorbidities.20 Our 2 studies together suggest that, notwithstanding the preferable alternative of avoiding hospitalization entirely, palliative care and other skilled coordination of care services ought to be prioritized for inpatients with multiple serious illnesses and the highest medical complexity. This patient group has both the highest costs and the greatest amenability to skilled transdisciplinary intervention, possibly because multiple chronic conditions affect patients interactively, complicating identification of appropriate polypharmacy responses and prioritization of treatments.
Our findings also may help direct appropriate use of palliative care services. The recently published American Society of Clinical Oncology palliative care guidelines note that all patients with advanced cancer (eg, those enrolled in our study) should receive dedicated palliative care services, early in the disease course, concurrent with active treatment.40 Workforce estimates suggest that the current and future numbers of palliative care practitioners will be unable to meet the ASCO recommendations alone never mind patients with other serious illnesses (eg, advanced heart failure, COPD, CKD).41 As such, specialized palliative care services will need to be targeted to the patient populations that can benefit most from these services. Whereas cost should not be the principle driver specialized palliative care provision, it will likely be an important component due to both the necessity of allocating scarce resources in the most effective way and the evidence that in care of the seriously-ill lower costs are often a proxy for improved patient experience.
These findings also have implications for research: Different conditions and presumably different combinations of conditions have very different implications for hospital care costs for a cohort of adults with advanced cancer. Given the increasing number of co-occurring conditions among seriously ill patients, and the increasing costs of cancer care and of treating multimorbidity cases, it is essential to further our understanding of the relationship between comorbidities and costs in order to plan and finance care for advanced cancer patients.
Limitations and Generalizability
In this observational study, reported associations may be attributable to unobserved confounding that our analyses failed to control.
Our results reflect associations in a prospective multisite study of advanced cancer patients hospitalized in the United States. It is not clear how generalizable our findings are to patients without cancer, to patients in nonhospital settings, and to patients in other health systems and countries. Analyzing cost from the hospital perspective does not take into account that the most impactful way to reduce cost is to avoid hospitalization entirely.
Results of our secondary analysis will not necessarily be robust to patient groups, as specific weights likely will vary by sample. The idea that costs vary by condition, however, is important nevertheless. Elixhauser total was derived with use of the enhanced ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) algorithm from Quan et al.42 and does not include subsequent Elixhauser Comorbidity Software updates recommended by the Healthcare Cost and Utilization Project (HCUP; Agency for Healthcare Research and Quality).43 The Elixhauser index is recommended over Charlson and other comorbidity indices by both HCUP45 and a recent systematic review.44
One possible unobserved factor is prior chemotherapy, which is associated with increased hospitalization risk. Related factors that are somewhat controlled for in the study include cancer stage (advanced cancer was an eligibility criterion) and receipt of analgesics within the week before admission (patients admitted for routine chemotherapy were excluded from analyses at the outset).
CONCLUSION
Other studies have identified a wide range of sociodemographic, clinical, and health system factors associated with healthcare utilization. Our results suggest that, for cost of hospital admission among adults with advanced cancer, the most important drivers of utilization are complications and comorbidities. Hospital costs for patients with advanced cancer constitute a major part of US healthcare spending, and these results suggest the need to prioritize high-quality, cost-effective care for patients with multiple serious illnesses.
Acknowledgments
The authors thank Robert Arnold, Phil Santa Emma, Mary Beth Happ, Tim Smith, and David Weissman for contributing to the Palliative Care for Cancer (PC4C) project.
Disclosure
The study was funded by grant R01 CA116227 from the National Cancer Institute and the National Institute of Nursing Research. The study sponsors had no role in design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the US government. All authors are independent of the study sponsors. Dr. May was supported by a HRB/NCI Health Economics Fellowship during this work. Dr. Garrido is supported by a Veterans Affairs HSR&D career development award (CDA 11-201/CDP 12-255). Dr. Kelley’s time was funded by the National Institute on Aging (1K23AG040774-01A1) and the American Federation for Aging. Dr. Smith is funded by the NCI Core Grant P 30 006973, 1-R01 CA177562-01A1, 1-R01 NR014050 01, and the Harry J. Duffey Family Endowment for Palliative Care. Dr. Morrison was the recipient of a Midcareer Investigator Award in Patient-Oriented Research (5K24AG022345) during the course of this work. This work was supported by the NIA, Claude D. Pepper Older Americans Independence Center at the Icahn School of Medicine at Mount Sinai [5P30AG028741], and the National Palliative Care Research Center.
1. Soni A. Top 10 Most Costly Conditions Among Men and Women, 2008: Estimates for the U.S. Civilian Noninstitutionalized Adult Population, Age 18 and Older. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
2. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128. PubMed
3. American Cancer Society. Cancer Facts and Figures 2015. Atlanta, GA: American Cancer Society; 2015.
4. Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364(21):2060-2065. PubMed
5. Levit L, Balogh E, Nass S, Ganz PA, eds. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: Institute of Medicine/National Academies Press; 2013. PubMed
7. Anderson GF. Chronic Care: Making the Case for Ongoing Care. Princeton, NJ: Robert Wood Johnson Foundation; 2010.
8. Tibi-Levy Y, Le Vaillant M, de Pouvourville G. Determinants of resource utilization in four palliative care units. Palliat Med. 2006;20(2):95-106. PubMed
9. Simoens S, Kutten B, Keirse E, et al. The costs of treating terminal patients. J Pain Symptom Manage. 2010;40(3):436-448. PubMed
10. Groeneveld I, Murtagh F, Kaloki Y, Bausewein C, Higginson I. Determinants of healthcare costs in the last year of life. Annual Assembly of American Academy of Hospice and Palliative Medicine & Hospice and Palliative Nurses Association; March 14, 2013; New Orleans, LA.
11. Shugarman LR, Bird CE, Schuster CR, Lynn J. Age and gender differences in Medicare expenditures at the end of life for colorectal cancer decedents. J Womens Health. 2007;16(2):214-227. PubMed
12. Shugarman LR, Bird CE, Schuster CR, Lynn J. Age and gender differences in Medicare expenditures and service utilization at the end of life for lung cancer decedents. Womens Health Issues. 2008;18(3):199-209. PubMed
13. Walker H, Anderson M, Farahati F, et al. Resource use and costs of end-of-life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J Palliat Care. 2011;27(2):79-88. PubMed
14. Hanchate A, Kronman AC, Young-Xu Y, Ash AS, Emanuel E. Racial and ethnic differences in end-of-life costs: why do minorities cost more than whites? Arch Intern Med. 2009;169(5):493-501. PubMed
15. Hanratty B, Burstrom B, Walander A, Whitehead M. Inequality in the face of death? Public expenditure on health care for different socioeconomic groups in the last year of life. J Health Serv Res Policy. 2007;12(2):90-94. PubMed
16. Kelley AS, Ettner SL, Morrison RS, Du Q, Wenger NS, Sarkisian CA. Determinants of medical expenditures in the last 6 months of life. Ann Intern Med. 2011;154(4):235-242. PubMed
17. Guerriere DN, Zagorski B, Fassbender K, Masucci L, Librach L, Coyte PC. Cost variations in ambulatory and home-based palliative care. Palliat Med. 2010;24(5):523-532. PubMed
18. US Department of Health and Human Services, National Institutes of Health. Palliative Care for Hospitalized Cancer Patients [project information]. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health; 2006. Project 5R01CA116227-04. https://projectreporter.nih.gov/project_info_description.cfm?projectnumber=5R01CA116227-04. Published 2006. Accessed August 1, 2015.
19. May P, Garrido MM, Cassel JB, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015;33(25):2745-2752. PubMed
20. May P, Garrido MM, Cassel JB, et al. Palliative care teams’ cost-saving effect is larger for cancer patients with higher numbers of comorbidities. Health Aff. 2016;35(1):44-53. PubMed
21. May P, Garrido MM, Cassel JB, Morrison RS, Normand C. Using length of stay to control for unobserved heterogeneity when estimating treatment effect on hospital costs with observational data: issues of reliability, robustness and usefulness. Health Serv Res. 2016;51(5):2020-2043. PubMed
22. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
23. Chang VT, Hwang SS, Kasimis B, Thaler HT. Shorter symptom assessment instruments: the Condensed Memorial Symptom Assessment Scale (CMSAS). Cancer Invest. 2004;22(4):526-536. PubMed
24. Katz S, Ford A, Moskowitz R, Jackson B, Jaffe M. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185(12):914-919. PubMed
25. McLaughlin MA, Orosz GM, Magaziner J, et al. Preoperative status and risk of complications in patients with hip fracture. J Gen Intern Med. 2006;21(3):219-225. PubMed
26. Taheri PA, Butz D, Griffes LC, Morlock DR, Greenfield LJ. Physician impact on the total cost of care. Ann Surg. 2000;231(3):432-435. PubMed
27. US Department of Veterans Affairs, Health Economics Resource Center. Determining costs. Washington, DC: US Dept of Veterans Affairs, Health Economics Resource Center; 2016. http://www.herc.research.va.gov/include/page.asp?id=determining-costs. Published 2016. Accessed September 7, 2016.
28. US Department of Health and Human Services, Center for Medicare & Medicaid Services. FY 2011 Wage Index [Table 2]. Baltimore, MD: US Dept of Health and Human Services, Center for Medicare & Medicaid Services; 2011. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Wage-Index-Files-Items/CMS1234173.html. Published 2011. Accessed September 2, 2014.
29. US Department of Labor, Bureau of Labor Statistics. All Urban Consumers (Current Series) [Consumer Price Index database]. US Dept of Labor, Bureau of Labor Statistics; 2015. http://www.bls.gov/cpi/data.htm. Published 2015. Accessed August 15, 2016.
30. Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465-488. PubMed
31. Jones AM, Rice N, Bago d’Uva T, Balia S. Applied Health Economics. 2nd ed. Oxford, England: Routledge; 2013.
32. Stata [computer program]. Version 12. College Station, TX: StataCorp; 2011.
33. Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing
propensity scores. Health Serv Res. 2014;49(5):1701-1720. PubMed
34. R Core Team. R: A Language and Environment for Statistical Computing. Vienna,
Austria: R Foundation for Statistical Computing; 2016.
35. Cassel JB, Kerr K, Pantilat S, Smith TJ. Palliative care consultation and hospital
length of stay. J Palliat Med. 2010;13(6):761-767. PubMed
36. US Department of Health and Human Services, Agency for Healthcare Research
and Quality. Efforts to Improve Patient Safety Result in 1.3 Million Fewer Patient
Harms: Interim Update on 2013 Annual Hospital-Acquired Condition Rate and
Estimates of Cost Savings and Deaths Averted From 2010 to 2013. Rockville,
MD: US Dept of Health and Human Services, Agency for Healthcare Research
and Quality; 2015. http://www.ahrq.gov/professionals/quality-patient-safety/pfp/
interimhacrate2013.html. Published 2015. Updated November 2015. Accessed
November 18, 2016.
37. Cassidy A. Health Policy Brief: Medicare’s Hospital-Acquired Condition Reduction
Program. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_
id=142. Published August 6, 2015. Accessed April 24, 2017.
38. Davis MA, Nallamothu BK, Banerjee M, Bynum JP. Identification of four unique
spending patterns among older adults in the last year of life challenges standard
assumptions. Health Aff. 2016;35(7):1316-1323. PubMed
39. Aldridge MD, Kelley AS. The myth regarding the high cost of end-of-life care.
Am J Public Health. 2015;105(12):2411-2415. PubMed
40. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard
oncology care: American Society of Clinical Oncology clinical practice guideline
update. J Clin Oncol. 2017;35(1):96-112. PubMed
41. Spetz J, Dudley N, Trupin L, Rogers M, Meier DE, Dumanovsky T. Few hospital
palliative care programs meet national staffing recommendations. Health Aff.
2016;35(9):1690-1697. PubMed
42. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities
in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed
43. HCUP [Healthcare Cost and Utilization Project] Elixhauser Comorbidity Software
[computer program]. Version 3.7. Rockville, MD: Agency for Healthcare
Research and Quality; 2016. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/
comorbidity.jsp. Published 2016. Accessed November 9, 2016.
44. Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for
administrative data. Med Care. 2012;50(12):1109-1118. PubMed
1. Soni A. Top 10 Most Costly Conditions Among Men and Women, 2008: Estimates for the U.S. Civilian Noninstitutionalized Adult Population, Age 18 and Older. Rockville, MD: Agency for Healthcare Research and Quality; 2011.
2. Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117-128. PubMed
3. American Cancer Society. Cancer Facts and Figures 2015. Atlanta, GA: American Cancer Society; 2015.
4. Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364(21):2060-2065. PubMed
5. Levit L, Balogh E, Nass S, Ganz PA, eds. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: Institute of Medicine/National Academies Press; 2013. PubMed
7. Anderson GF. Chronic Care: Making the Case for Ongoing Care. Princeton, NJ: Robert Wood Johnson Foundation; 2010.
8. Tibi-Levy Y, Le Vaillant M, de Pouvourville G. Determinants of resource utilization in four palliative care units. Palliat Med. 2006;20(2):95-106. PubMed
9. Simoens S, Kutten B, Keirse E, et al. The costs of treating terminal patients. J Pain Symptom Manage. 2010;40(3):436-448. PubMed
10. Groeneveld I, Murtagh F, Kaloki Y, Bausewein C, Higginson I. Determinants of healthcare costs in the last year of life. Annual Assembly of American Academy of Hospice and Palliative Medicine & Hospice and Palliative Nurses Association; March 14, 2013; New Orleans, LA.
11. Shugarman LR, Bird CE, Schuster CR, Lynn J. Age and gender differences in Medicare expenditures at the end of life for colorectal cancer decedents. J Womens Health. 2007;16(2):214-227. PubMed
12. Shugarman LR, Bird CE, Schuster CR, Lynn J. Age and gender differences in Medicare expenditures and service utilization at the end of life for lung cancer decedents. Womens Health Issues. 2008;18(3):199-209. PubMed
13. Walker H, Anderson M, Farahati F, et al. Resource use and costs of end-of-life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J Palliat Care. 2011;27(2):79-88. PubMed
14. Hanchate A, Kronman AC, Young-Xu Y, Ash AS, Emanuel E. Racial and ethnic differences in end-of-life costs: why do minorities cost more than whites? Arch Intern Med. 2009;169(5):493-501. PubMed
15. Hanratty B, Burstrom B, Walander A, Whitehead M. Inequality in the face of death? Public expenditure on health care for different socioeconomic groups in the last year of life. J Health Serv Res Policy. 2007;12(2):90-94. PubMed
16. Kelley AS, Ettner SL, Morrison RS, Du Q, Wenger NS, Sarkisian CA. Determinants of medical expenditures in the last 6 months of life. Ann Intern Med. 2011;154(4):235-242. PubMed
17. Guerriere DN, Zagorski B, Fassbender K, Masucci L, Librach L, Coyte PC. Cost variations in ambulatory and home-based palliative care. Palliat Med. 2010;24(5):523-532. PubMed
18. US Department of Health and Human Services, National Institutes of Health. Palliative Care for Hospitalized Cancer Patients [project information]. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health; 2006. Project 5R01CA116227-04. https://projectreporter.nih.gov/project_info_description.cfm?projectnumber=5R01CA116227-04. Published 2006. Accessed August 1, 2015.
19. May P, Garrido MM, Cassel JB, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015;33(25):2745-2752. PubMed
20. May P, Garrido MM, Cassel JB, et al. Palliative care teams’ cost-saving effect is larger for cancer patients with higher numbers of comorbidities. Health Aff. 2016;35(1):44-53. PubMed
21. May P, Garrido MM, Cassel JB, Morrison RS, Normand C. Using length of stay to control for unobserved heterogeneity when estimating treatment effect on hospital costs with observational data: issues of reliability, robustness and usefulness. Health Serv Res. 2016;51(5):2020-2043. PubMed
22. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
23. Chang VT, Hwang SS, Kasimis B, Thaler HT. Shorter symptom assessment instruments: the Condensed Memorial Symptom Assessment Scale (CMSAS). Cancer Invest. 2004;22(4):526-536. PubMed
24. Katz S, Ford A, Moskowitz R, Jackson B, Jaffe M. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185(12):914-919. PubMed
25. McLaughlin MA, Orosz GM, Magaziner J, et al. Preoperative status and risk of complications in patients with hip fracture. J Gen Intern Med. 2006;21(3):219-225. PubMed
26. Taheri PA, Butz D, Griffes LC, Morlock DR, Greenfield LJ. Physician impact on the total cost of care. Ann Surg. 2000;231(3):432-435. PubMed
27. US Department of Veterans Affairs, Health Economics Resource Center. Determining costs. Washington, DC: US Dept of Veterans Affairs, Health Economics Resource Center; 2016. http://www.herc.research.va.gov/include/page.asp?id=determining-costs. Published 2016. Accessed September 7, 2016.
28. US Department of Health and Human Services, Center for Medicare & Medicaid Services. FY 2011 Wage Index [Table 2]. Baltimore, MD: US Dept of Health and Human Services, Center for Medicare & Medicaid Services; 2011. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Wage-Index-Files-Items/CMS1234173.html. Published 2011. Accessed September 2, 2014.
29. US Department of Labor, Bureau of Labor Statistics. All Urban Consumers (Current Series) [Consumer Price Index database]. US Dept of Labor, Bureau of Labor Statistics; 2015. http://www.bls.gov/cpi/data.htm. Published 2015. Accessed August 15, 2016.
30. Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24(3):465-488. PubMed
31. Jones AM, Rice N, Bago d’Uva T, Balia S. Applied Health Economics. 2nd ed. Oxford, England: Routledge; 2013.
32. Stata [computer program]. Version 12. College Station, TX: StataCorp; 2011.
33. Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing
propensity scores. Health Serv Res. 2014;49(5):1701-1720. PubMed
34. R Core Team. R: A Language and Environment for Statistical Computing. Vienna,
Austria: R Foundation for Statistical Computing; 2016.
35. Cassel JB, Kerr K, Pantilat S, Smith TJ. Palliative care consultation and hospital
length of stay. J Palliat Med. 2010;13(6):761-767. PubMed
36. US Department of Health and Human Services, Agency for Healthcare Research
and Quality. Efforts to Improve Patient Safety Result in 1.3 Million Fewer Patient
Harms: Interim Update on 2013 Annual Hospital-Acquired Condition Rate and
Estimates of Cost Savings and Deaths Averted From 2010 to 2013. Rockville,
MD: US Dept of Health and Human Services, Agency for Healthcare Research
and Quality; 2015. http://www.ahrq.gov/professionals/quality-patient-safety/pfp/
interimhacrate2013.html. Published 2015. Updated November 2015. Accessed
November 18, 2016.
37. Cassidy A. Health Policy Brief: Medicare’s Hospital-Acquired Condition Reduction
Program. http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_
id=142. Published August 6, 2015. Accessed April 24, 2017.
38. Davis MA, Nallamothu BK, Banerjee M, Bynum JP. Identification of four unique
spending patterns among older adults in the last year of life challenges standard
assumptions. Health Aff. 2016;35(7):1316-1323. PubMed
39. Aldridge MD, Kelley AS. The myth regarding the high cost of end-of-life care.
Am J Public Health. 2015;105(12):2411-2415. PubMed
40. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard
oncology care: American Society of Clinical Oncology clinical practice guideline
update. J Clin Oncol. 2017;35(1):96-112. PubMed
41. Spetz J, Dudley N, Trupin L, Rogers M, Meier DE, Dumanovsky T. Few hospital
palliative care programs meet national staffing recommendations. Health Aff.
2016;35(9):1690-1697. PubMed
42. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities
in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed
43. HCUP [Healthcare Cost and Utilization Project] Elixhauser Comorbidity Software
[computer program]. Version 3.7. Rockville, MD: Agency for Healthcare
Research and Quality; 2016. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/
comorbidity.jsp. Published 2016. Accessed November 9, 2016.
44. Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for
administrative data. Med Care. 2012;50(12):1109-1118. PubMed
© 2017 Society of Hospital Medicine
Quality of care of hospitalized infective endocarditis patients: Report from a tertiary medical center
Infective endocarditis (IE) affected an estimated 46,800 Americans in 2011, and its incidence is increasing due to greater numbers of invasive procedures and prevalence of IE risk factors.1-3 Despite recent advances in the treatment of IE, morbidity and mortality remain high: in-hospital mortality in IE patients is 15% to 20%, and the 1-year mortality rate is approximately 40%.2,4,5
Poor IE outcomes may be the result of difficulty in diagnosing IE and identifying its optimal treatments. The American Heart Association (AHA), the American College of Cardiology (ACC), and the European Society of Cardiology (ESC) have published guidelines to address these challenges. Recent guidelines recommend a multidisciplinary approach that includes cardiology, cardiac surgery, and infectious disease (ID) specialty involvement in decision-making.5,6
In the absence of published quality measures for IE management, guidelines can be used to evaluate the quality of care of IE. Studies have showed poor concordance with guideline recommendations but did not examine agreement with more recently published guidelines.7,8 Furthermore, few studies have examined the management, outcomes, and quality of care received by IE patients. Therefore, we aimed to describe a modern cohort of patients with IE admitted to a tertiary medical center over a 4-year period. In particular, we aimed to assess quality of care received by this cohort, as measured by concordance with AHA and ACC guidelines to identify gaps in care and spur quality improvement (QI) efforts.
METHODS
Design and Study Population
We conducted a retrospective cohort study of adult IE patients admitted to Baystate Medical Center (BMC), a 716-bed tertiary academic center that covers a population of 800,000 people throughout western New England. We used the International Classification of Diseases (ICD)–Ninth Revision, to identify IE patients discharged with a principal or secondary diagnosis of IE between 2007 and 2011 (codes 421.0, 421.1, 421.9, 424.9, 424.90, and 424.91). Three co-authors confirmed the diagnosis by conducting a review of the electronic health records.
We included only patients who met modified Duke criteria for definite or possible IE.5 Definite IE defines patients with pathological criteria (microorganisms demonstrated by culture or histologic examination or a histologic examination showing active endocarditis); or patients with 2 major criteria (positive blood culture and evidence of endocardial involvement by echocardiogram), 1 major criterion and 3 minor criteria (minor criteria: predisposing heart conditions or intravenous drug (IVD) use, fever, vascular phenomena, immunologic phenomena, and microbiologic evidence that do not meet the major criteria) or 5 minor criteria. Possible IE defines patients with 1 major and 1 minor criterion or 3 minor criteria.5
Data Collection
We used billing and clinical databases to collect demographics, comorbidities, antibiotic treatment, 6-month readmission and 1-year mortality. Comorbid conditions were classified into Elixhauser comorbidities using software provided by the Healthcare Costs and Utilization Project of the Agency for Healthcare Research and Quality.9,10
We obtained all other data through electronic health record abstraction. These included microbiology, type of endocarditis (native valve endocarditis [NVE] or prosthetic valve endocarditis [PVE]), echocardiographic location of the vegetation, and complications involving the valve (eg, valve perforation, ruptured chorda, perivalvular abscess, or valvular insufficiency).
Using 2006 AHA/ACC guidelines,11 we identified quality metrics, including the presence of at least 2 sets of blood cultures prior to start of antibiotics and use of transthoracic echocardiogram (TTE) and transesophageal echocardiogram (TEE). Guidelines recommend using TTE as first-line to detect valvular vegetations and assess IE complications. TEE is recommended if TTE is nondiagnostic and also as first-line to diagnose PVE. We assessed the rate of consultation with ID, cardiology, and cardiac surgery specialties. While these consultations were not explicitly emphasized in the 2006 AHA/ACC guidelines, there is a class I recommendation in 2014 AHA/ACC guidelines5 to manage IE patients with consultation of all these specialties.
We reported the number of patients with intracardiac leads (pacemaker or defibrillator) who had documentation of intracardiac lead removal. Complete removal of intracardiac leads is indicated in IE patients with infection of leads or device (class I) and suggested for IE caused by Staphylococcus aureus or fungi (even without evidence of device or lead infection), and for patients undergoing valve surgery (class IIa).5 We entered abstracted data elements into a RedCap database, hosted by Tufts Clinical and Translational Science Institute.12
Outcomes
Outcomes included embolic events, strokes, need for cardiac surgery, LOS, inhospital mortality, 6-month readmission, and 1-year mortality. We identified embolic events using documentation of clinical or imaging evidence of an embolic event to the cerebral, coronary, peripheral arterial, renal, splenic, or pulmonary vasculature. We used record extraction to identify incidence of valve surgery. Nearly all patients who require surgery at BMC have it done onsite. We compared outcomes among patients who received less than 3 vs. 3 consultations provided by ID, cardiology, and cardiac surgery specialties. We also compared outcomes among patients who received 0, 1, 2, or 3 consultations to look for a trend.
Statistical Analysis
We divided the cohort into patients with NVE and PVE because there are differences in pathophysiology, treatment, and outcomes of these groups. We calculated descriptive statistics, including means/standard deviation (SD) and n (%). We conducted univariable analyses using Fisher exact (categorical), unpaired t tests (Gaussian), or Kruskal-Wallis equality-of-populations rank test (non-Gaussian). Common language effect sizes were also calculated to quantify group differences without respect to sample size.13,14 Analyses were performed using Stata 14.1. (StataCorp LLC, College Station, Texas). The BMC Institutional Review Board approved the protocol.
RESULTS
We identified a total of 317 hospitalizations at BMC meeting criteria for IE. Of these, 147 hospitalizations were readmissions or did not meet the clinical criteria of definite or possible IE. Thus, we included a total of 170 patients in the final analysis. Definite IE was present in 135 (79.4%) and possible IE in 35 (20.6%) patients.
Patient Characteristics
Of 170 patients, 127 (74.7%) had NVE and 43 (25.3%) had PVE. Mean ± SD age was 60.0 ± 17.9 years, 66.5% (n = 113) of patients were male, and 79.4% (n = 135) were white (Table 1). Hypertension and chronic kidney disease were the most common comorbidities. The median Gagne score15 was 4, corresponding to a 1-year mortality risk of 15%. Predisposing factors for IE included previous history of IE (n = 14 or 8.2%), IVD use (n = 23 or 13.5%), and presence of long-term venous catheters (n = 19 or 11.2%). Intracardiac leads were present in 17.1% (n = 29) of patients. Bicuspid aortic valve was reported in 6.5% (n = 11) of patients with NVE. Patients with PVE were older (+11.5 years, 95% confidence interval [CI] 5.5, 17.5) and more likely to have intracardiac leads (44.2% vs. 7.9%; P < 0.001; Table 1).
Microbiology and Antibiotics
Staphylococcus aureus was isolated in 40.0% of patients (methicillin-sensitive: 21.2%, n = 36; methicillin-resistant: 18.8%, n = 32) and vancomycin (88.2%, n = 150) was the most common initial antibiotic used. Nearly half (44.7%, n = 76) of patients received gentamicin as part of their initial antibiotic regimen. Appendix 1 provides information on final blood culture results, prosthetic versus native valve IE, and antimicrobial agents that each patient received. PVE patients were more likely to receive gentamicin as their initial antibiotic regimen than NVE (58.1% vs. 40.2%; P = 0.051; Table 1).
Echocardiography and Affected Valves
As per study inclusion criteria, all patients received echocardiography (either TTE, TEE, or both). Overall, the most common infected valve was mitral (41.3%), n = 59), followed by aortic valve (28.7%), n = 41). Patients in whom the location of infected valve could not be determined (15.9%, n = 27) had echocardiographic features of intracardiac device infection or intracardiac mass (Table 1).
Quality of Care
Nearly all (n = 165, 97.1%) of patients had at least 2 sets of blood cultures drawn, most on the first day of admission (71.2%). The vast majority of patients (n = 152, 89.4%) also received their first dose of antibiotics on the day of admission. Ten (5.9%) patients did not receive any consults, and 160 (94.1%) received at least 1 consultation. An ID consultation was obtained for most (147, 86.5%) patients; cardiac surgery consultation was obtained for about half of patients (92, 54.1%), and cardiology consultation was also obtained for nearly half of patients (80, 47.1%). One-third (53, 31.2%) did not receive a cardiology or cardiac surgery consult, two-thirds (117, 68.8%) received either a cardiology or a cardiac surgery consult, and one-third (55, 32.4%) received both.
Of the 29 patients who had an intracardiac lead, 6 patients had documentation of the device removal during the index hospitalization (5 or 50.0% of patients with NVE and 1 or 5.3% of patients with PVE; P = 0.02; Table 2).
Outcomes
Evidence of any embolic events was seen in 27.7% (n = 47) of patients, including stroke in 17.1% (n = 29). Median LOS for all patients was 13.5 days, and 6-month readmission among patients who survived their index admission was 51.0% (n = 74/145; 95% CI, 45.9%-62.7%). Inhospital mortality was 14.7% (n = 25; 95% CI: 10.1%-20.9%) and 12-month mortality was 22.4% (n = 38; 95% CI, 16.7%-29.3%). Inhospital mortality was more frequent among patients with PVE than NVE (20.9% vs. 12.6%; P = 0.21), although this difference was not statistically significant. Complications were more common in NVE than PVE (any embolic event: 32.3% vs. 14.0%, P = 0.03; stroke, 20.5% vs. 7.0%, P = 0.06; Table 3).
Although there was a trend toward reduction in 6-month readmission and 12-month mortality with incremental increase in the number of specialties consulted (ID, cardiology and cardiac surgery), the difference was not statistically significant (Figure). In addition, comparing outcomes of embolic events, stroke, 6-month readmission, and 12-month mortality between those who received 3 consults (28.8%, n = 49) to those with fewer than 3 (71.2%, n = 121) did not show statistically significant differences.
Of 92 patients who received a cardiac surgery consult, 73 had NVE and 19 had PVE. Of these, 47 underwent valve surgery, 39 (of 73) with NVE (53.42%) and 8 (of 19) with PVE (42.1%). Most of the NVE patients (73.2%) had more than 1 indication for surgery. The most common indications for surgery among NVE patients were significant valvular dysfunction resulting in heart failure (65.9%), followed by mobile vegetation (56.1%) and recurrent embolic events (26.8%). The most common indication for surgery in PVE was persistent bacteremia or recurrent embolic events (75.0%).
DISCUSSION
In this study, we described the management, quality of care, and outcomes of IE patients in a tertiary medical center. We found that the majority of hospitalized patients with IE were older white men with comorbidities and IE risk factors. The complication rate was high (27.7% with embolic events) and the inhospital mortality rate was in the lower range reported by prior studies [14.7% vs. 15%-20%].5 Nearly one-third of patients (n = 47, 27.7%) received valve surgery. Quality of care received was generally good, with most patients receiving early blood cultures, echocardiograms, early antibiotics, and timely ID consultation. We identified important gaps in care, including a failure to consult cardiac surgery in nearly half of patients and failure to consult cardiology in more than half of patients.
Our findings support work suggesting that IE is no longer primarily a chronic or subacute disease of younger patients with IVD use, positive human immunodeficiency virus status, or bicuspid aortic valves.1,4,16,17 The International Collaboration on Endocarditis-Prospective Cohort Study,4 a multinational prospective cohort study (2000-2005) of 2781 adults with IE, reported a higher prevalence of patients with diabetes or on hemodialysis, IVD users, and patients with long-term venous catheter and intracardiac leads than we found. Yet both studies suggest that the demographics of IE are changing. This may partially explain why IE mortality has not improved in recent years:2,3 patients with older age and higher comorbidity burden may not be considered good surgical candidates.
This study is among the first to contribute information on concordance with IE guidelines in a cohort of U.S. patients. Our findings suggest that most patients received timely blood culture, same-day administration of empiric antibiotics, and ID consultation, which is similar to European studies.7,18 Guideline concordance could be improved in some areas. Overall documentation of the management plan regarding the intracardiac leads could be improved. Only 6 of 29 patients with intracardiac leads had documentation of their removal during the index hospitalization.
The 2014 AHA/ACC guidelines5 and the ESC guidelines6 emphasized the importance of multidisciplinary management of IE. As part of the Heart Valve Team at BMC, cardiologists provide expertise in diagnosis, imaging and clinical management of IE, and cardiac surgeons provide consultation on whether to pursue surgery and optimal timing of surgery. Early discussion with surgical team is considered mandatory in all complicated cases of IE.6,18 Infectious disease consultation has been shown to improve the rate of IE diagnosis, reduce the 6-month relapse rate,19 and improve outcomes in patients with S aureus bacteremia.20 In our study 86.5% of patients had documentation of an ID consultation; cardiac surgery consultation was obtained in 54.1% and cardiology consultation in 47.1% of patients.
We observed a trend towards lower rates of 6-month readmission and 12-month mortality among patients who received all 3 consults (Figure 1), despite the fact that rates of embolic events and stroke were higher in patients with 3 consults compared to those with fewer than 3. Obviously, the lack of confounder adjustment and lack of power limits our ability to make inferences about this association, but it generates hypotheses for future work. Because subjects in our study were cared for prior to 2014, multidisciplinary management of IE with involvement of cardiology, cardiac surgery, and ID physicians was observed in only one-third of patients. However, 117 (68.8%) patients received either cardiology or cardiac surgery consults. It is possible that some physicians considered involving both cardiology and cardiac surgery consultants as unnecessary and, therefore, did not consult both specialties. We will focus future QI efforts in our institution on educating physicians about the benefits of multidisciplinary care and the importance of fully implementing the 2014 AHA/ACC guidelines.
Our findings around quality of care should be placed in the context of 2 studies by González de Molina et al8 and Delahaye et al7 These studies described considerable discordance between guideline recommendations and real-world IE care. However, these studies were performed more than a decade ago and were conducted before current recommendations to consult cardiology and cardiac surgery were published.
In the 2014 AHA/ACC guidelines, surgery prior to completion of antibiotics is indicated in patients with valve dysfunction resulting in heart failure; left-sided IE caused by highly resistant organisms (including fungus or S aureus); IE complicated by heart block, aortic abscess, or penetrating lesions; and presence of persistent infection (bacteremia or fever lasting longer than 5 to 7 days) after onset of appropriate antimicrobial therapy. In addition, there is a Class IIa indication of early surgery in patients with recurrent emboli and persistent vegetation despite appropriate antibiotic therapy and a Class IIb indication of early surgery in patients with NVE with mobile vegetation greater than 10 mm in length. Surgery is recommended for patients with PVE and relapsing infection.
It is recommended that IE patients be cared for in centers with immediate access to cardiac surgery because the urgent need for surgical intervention can arise rapidly.5 We found that nearly one-third of included patients underwent surgery. Although we did not collect data on indications for surgery in patients who did not receive surgery, we observed that 50% had a surgery consult, suggesting the presence of 1 or more surgical indications. Of these, half underwent valve surgery. Most of the NVE patients who underwent surgery had more than 1 indication for surgery. Our surgical rate is similar to a study from Italy3 and overall in the lower range of reported surgical rate (25%-50%) from other studies.21 The low rate of surgery at our center may be related to the fact that the use of surgery for IE has been hotly debated in the literature,21 and may also be due to the low rate of cardiac surgery consultation.
Our study has several limitations. We identified eligible patients using a discharge ICD-9 coding of IE and then confirmed the presence of Duke criteria using record review. Using discharge diagnosis codes for endocarditis has been validated, and our additional manual chart review to confirm Duke criteria likely improved the specificity significantly. However, by excluding patients who did not have documented evidence of Duke criteria, we may have missed some cases, lowering sensitivity. The performance on selected quality metrics may also have been affected by our inclusion criteria. Because we included only patients who met Duke criteria, we tended to include patients who had received blood cultures and echocardiograms, which are part of the criteria. Thus, we cannot comment on use of diagnostic testing or specialty consultation in patients with suspected IE. This was a single-center study and may not represent patients or current practices seen in other institutions. We did not collect data on some of the predisposing factors to NVE (for example, baseline rheumatic heart disease or preexisting valvular heart disease) because it is estimated that less than 5% of IE in the U.S. is superimposed on rheumatic heart disease.4 We likely underestimated 12-month mortality rate because we did not cross-reference our findings again the National Death Index; however, this should not affect the comparison of this outcome between groups.
CONCLUSION
Our study confirms reports that IE epidemiology has changed significantly in recent years. It also suggests that concordance with guideline recommendations is good for some aspects of care (eg, echocardiogram, blood cultures), but can be improved in other areas, particularly in use of specialty consultation during the hospitalization. Future QI efforts should emphasize the role of the heart valve team or endocarditis team that consists of an internist, ID physician, cardiologist, cardiac surgeon, and nursing. Finally, efforts should be made to develop strategies for community hospitals that do not have access to all of these specialists (eg, early transfer, telehealth).
Disclosure
Nothing to report.
1. Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65(19):2070-2076. PubMed
2. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PloS One. 2013;8(3):e60033. PubMed
3. Fedeli U, Schievano E, Buonfrate D, Pellizzer G, Spolaore P. Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC Infect Dis. 2011;11:48. PubMed
4. Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169(5):463-473. PubMed
5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):2438-2488. PubMed
6. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. PubMed
7. Delahaye F, Rial MO, de Gevigney G, Ecochard R, Delaye J. A critical appraisal of the quality of the management of infective endocarditis. J Am Coll Cardiol. 1999;33(3):788-793. PubMed
8. González De Molina M, Fernández-Guerrero JC, Azpitarte J. [Infectious endocarditis: degree of discordance between clinical guidelines recommendations and clinical practice]. Rev Esp Cardiol. 2002;55(8):793-800. PubMed
9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
10. Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived rom ICD-9-CCM administrative data. Med Care. 2002;40(8):675-685. PubMed
11. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, Bonow RO, Carabello BA, Kanu C, deLeon AC Jr, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114(5):e84-e231.
12. REDCap [Internet]. [cited 2016 May 14]. Available from: https://collaborate.tuftsctsi.org/redcap/.
13. McGraw KO, Wong SP. A common language effect-size statistic. Psychol Bull. 1992;111:361-365.
14. Cohen J. The statistical power of abnormal-social psychological research: a review. J Abnorm Soc Psychol. 1962;65:145-153. PubMed
15. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. PubMed
16. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. PubMed
17. Cecchi E, Chirillo F, Castiglione A, Faggiano P, Cecconi M, Moreo A, et al. Clinical epidemiology in Italian Registry of Infective Endocarditis (RIEI): Focus on age, intravascular devices and enterococci. Int J Cardiol. 2015;190:151-156. PubMed
18. Tornos P, Iung B, Permanyer-Miralda G, Baron G, Delahaye F, Gohlke-Bärwolf Ch, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart. 2005;91(5):571-575. PubMed
19. Yamamoto S, Hosokawa N, Sogi M, Inakaku M, Imoto K, Ohji G, et al. Impact of infectious diseases service consultation on diagnosis of infective endocarditis. Scand J Infect Dis. 2012;44(4):270-275. PubMed
20. Rieg S, Küpper MF. Infectious diseases consultations can make the difference: a brief review and a plea for more infectious diseases specialists in Germany. Infection. 2016;(2):159-166. PubMed
21. Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010;121(9):11411152. PubMed
Infective endocarditis (IE) affected an estimated 46,800 Americans in 2011, and its incidence is increasing due to greater numbers of invasive procedures and prevalence of IE risk factors.1-3 Despite recent advances in the treatment of IE, morbidity and mortality remain high: in-hospital mortality in IE patients is 15% to 20%, and the 1-year mortality rate is approximately 40%.2,4,5
Poor IE outcomes may be the result of difficulty in diagnosing IE and identifying its optimal treatments. The American Heart Association (AHA), the American College of Cardiology (ACC), and the European Society of Cardiology (ESC) have published guidelines to address these challenges. Recent guidelines recommend a multidisciplinary approach that includes cardiology, cardiac surgery, and infectious disease (ID) specialty involvement in decision-making.5,6
In the absence of published quality measures for IE management, guidelines can be used to evaluate the quality of care of IE. Studies have showed poor concordance with guideline recommendations but did not examine agreement with more recently published guidelines.7,8 Furthermore, few studies have examined the management, outcomes, and quality of care received by IE patients. Therefore, we aimed to describe a modern cohort of patients with IE admitted to a tertiary medical center over a 4-year period. In particular, we aimed to assess quality of care received by this cohort, as measured by concordance with AHA and ACC guidelines to identify gaps in care and spur quality improvement (QI) efforts.
METHODS
Design and Study Population
We conducted a retrospective cohort study of adult IE patients admitted to Baystate Medical Center (BMC), a 716-bed tertiary academic center that covers a population of 800,000 people throughout western New England. We used the International Classification of Diseases (ICD)–Ninth Revision, to identify IE patients discharged with a principal or secondary diagnosis of IE between 2007 and 2011 (codes 421.0, 421.1, 421.9, 424.9, 424.90, and 424.91). Three co-authors confirmed the diagnosis by conducting a review of the electronic health records.
We included only patients who met modified Duke criteria for definite or possible IE.5 Definite IE defines patients with pathological criteria (microorganisms demonstrated by culture or histologic examination or a histologic examination showing active endocarditis); or patients with 2 major criteria (positive blood culture and evidence of endocardial involvement by echocardiogram), 1 major criterion and 3 minor criteria (minor criteria: predisposing heart conditions or intravenous drug (IVD) use, fever, vascular phenomena, immunologic phenomena, and microbiologic evidence that do not meet the major criteria) or 5 minor criteria. Possible IE defines patients with 1 major and 1 minor criterion or 3 minor criteria.5
Data Collection
We used billing and clinical databases to collect demographics, comorbidities, antibiotic treatment, 6-month readmission and 1-year mortality. Comorbid conditions were classified into Elixhauser comorbidities using software provided by the Healthcare Costs and Utilization Project of the Agency for Healthcare Research and Quality.9,10
We obtained all other data through electronic health record abstraction. These included microbiology, type of endocarditis (native valve endocarditis [NVE] or prosthetic valve endocarditis [PVE]), echocardiographic location of the vegetation, and complications involving the valve (eg, valve perforation, ruptured chorda, perivalvular abscess, or valvular insufficiency).
Using 2006 AHA/ACC guidelines,11 we identified quality metrics, including the presence of at least 2 sets of blood cultures prior to start of antibiotics and use of transthoracic echocardiogram (TTE) and transesophageal echocardiogram (TEE). Guidelines recommend using TTE as first-line to detect valvular vegetations and assess IE complications. TEE is recommended if TTE is nondiagnostic and also as first-line to diagnose PVE. We assessed the rate of consultation with ID, cardiology, and cardiac surgery specialties. While these consultations were not explicitly emphasized in the 2006 AHA/ACC guidelines, there is a class I recommendation in 2014 AHA/ACC guidelines5 to manage IE patients with consultation of all these specialties.
We reported the number of patients with intracardiac leads (pacemaker or defibrillator) who had documentation of intracardiac lead removal. Complete removal of intracardiac leads is indicated in IE patients with infection of leads or device (class I) and suggested for IE caused by Staphylococcus aureus or fungi (even without evidence of device or lead infection), and for patients undergoing valve surgery (class IIa).5 We entered abstracted data elements into a RedCap database, hosted by Tufts Clinical and Translational Science Institute.12
Outcomes
Outcomes included embolic events, strokes, need for cardiac surgery, LOS, inhospital mortality, 6-month readmission, and 1-year mortality. We identified embolic events using documentation of clinical or imaging evidence of an embolic event to the cerebral, coronary, peripheral arterial, renal, splenic, or pulmonary vasculature. We used record extraction to identify incidence of valve surgery. Nearly all patients who require surgery at BMC have it done onsite. We compared outcomes among patients who received less than 3 vs. 3 consultations provided by ID, cardiology, and cardiac surgery specialties. We also compared outcomes among patients who received 0, 1, 2, or 3 consultations to look for a trend.
Statistical Analysis
We divided the cohort into patients with NVE and PVE because there are differences in pathophysiology, treatment, and outcomes of these groups. We calculated descriptive statistics, including means/standard deviation (SD) and n (%). We conducted univariable analyses using Fisher exact (categorical), unpaired t tests (Gaussian), or Kruskal-Wallis equality-of-populations rank test (non-Gaussian). Common language effect sizes were also calculated to quantify group differences without respect to sample size.13,14 Analyses were performed using Stata 14.1. (StataCorp LLC, College Station, Texas). The BMC Institutional Review Board approved the protocol.
RESULTS
We identified a total of 317 hospitalizations at BMC meeting criteria for IE. Of these, 147 hospitalizations were readmissions or did not meet the clinical criteria of definite or possible IE. Thus, we included a total of 170 patients in the final analysis. Definite IE was present in 135 (79.4%) and possible IE in 35 (20.6%) patients.
Patient Characteristics
Of 170 patients, 127 (74.7%) had NVE and 43 (25.3%) had PVE. Mean ± SD age was 60.0 ± 17.9 years, 66.5% (n = 113) of patients were male, and 79.4% (n = 135) were white (Table 1). Hypertension and chronic kidney disease were the most common comorbidities. The median Gagne score15 was 4, corresponding to a 1-year mortality risk of 15%. Predisposing factors for IE included previous history of IE (n = 14 or 8.2%), IVD use (n = 23 or 13.5%), and presence of long-term venous catheters (n = 19 or 11.2%). Intracardiac leads were present in 17.1% (n = 29) of patients. Bicuspid aortic valve was reported in 6.5% (n = 11) of patients with NVE. Patients with PVE were older (+11.5 years, 95% confidence interval [CI] 5.5, 17.5) and more likely to have intracardiac leads (44.2% vs. 7.9%; P < 0.001; Table 1).
Microbiology and Antibiotics
Staphylococcus aureus was isolated in 40.0% of patients (methicillin-sensitive: 21.2%, n = 36; methicillin-resistant: 18.8%, n = 32) and vancomycin (88.2%, n = 150) was the most common initial antibiotic used. Nearly half (44.7%, n = 76) of patients received gentamicin as part of their initial antibiotic regimen. Appendix 1 provides information on final blood culture results, prosthetic versus native valve IE, and antimicrobial agents that each patient received. PVE patients were more likely to receive gentamicin as their initial antibiotic regimen than NVE (58.1% vs. 40.2%; P = 0.051; Table 1).
Echocardiography and Affected Valves
As per study inclusion criteria, all patients received echocardiography (either TTE, TEE, or both). Overall, the most common infected valve was mitral (41.3%), n = 59), followed by aortic valve (28.7%), n = 41). Patients in whom the location of infected valve could not be determined (15.9%, n = 27) had echocardiographic features of intracardiac device infection or intracardiac mass (Table 1).
Quality of Care
Nearly all (n = 165, 97.1%) of patients had at least 2 sets of blood cultures drawn, most on the first day of admission (71.2%). The vast majority of patients (n = 152, 89.4%) also received their first dose of antibiotics on the day of admission. Ten (5.9%) patients did not receive any consults, and 160 (94.1%) received at least 1 consultation. An ID consultation was obtained for most (147, 86.5%) patients; cardiac surgery consultation was obtained for about half of patients (92, 54.1%), and cardiology consultation was also obtained for nearly half of patients (80, 47.1%). One-third (53, 31.2%) did not receive a cardiology or cardiac surgery consult, two-thirds (117, 68.8%) received either a cardiology or a cardiac surgery consult, and one-third (55, 32.4%) received both.
Of the 29 patients who had an intracardiac lead, 6 patients had documentation of the device removal during the index hospitalization (5 or 50.0% of patients with NVE and 1 or 5.3% of patients with PVE; P = 0.02; Table 2).
Outcomes
Evidence of any embolic events was seen in 27.7% (n = 47) of patients, including stroke in 17.1% (n = 29). Median LOS for all patients was 13.5 days, and 6-month readmission among patients who survived their index admission was 51.0% (n = 74/145; 95% CI, 45.9%-62.7%). Inhospital mortality was 14.7% (n = 25; 95% CI: 10.1%-20.9%) and 12-month mortality was 22.4% (n = 38; 95% CI, 16.7%-29.3%). Inhospital mortality was more frequent among patients with PVE than NVE (20.9% vs. 12.6%; P = 0.21), although this difference was not statistically significant. Complications were more common in NVE than PVE (any embolic event: 32.3% vs. 14.0%, P = 0.03; stroke, 20.5% vs. 7.0%, P = 0.06; Table 3).
Although there was a trend toward reduction in 6-month readmission and 12-month mortality with incremental increase in the number of specialties consulted (ID, cardiology and cardiac surgery), the difference was not statistically significant (Figure). In addition, comparing outcomes of embolic events, stroke, 6-month readmission, and 12-month mortality between those who received 3 consults (28.8%, n = 49) to those with fewer than 3 (71.2%, n = 121) did not show statistically significant differences.
Of 92 patients who received a cardiac surgery consult, 73 had NVE and 19 had PVE. Of these, 47 underwent valve surgery, 39 (of 73) with NVE (53.42%) and 8 (of 19) with PVE (42.1%). Most of the NVE patients (73.2%) had more than 1 indication for surgery. The most common indications for surgery among NVE patients were significant valvular dysfunction resulting in heart failure (65.9%), followed by mobile vegetation (56.1%) and recurrent embolic events (26.8%). The most common indication for surgery in PVE was persistent bacteremia or recurrent embolic events (75.0%).
DISCUSSION
In this study, we described the management, quality of care, and outcomes of IE patients in a tertiary medical center. We found that the majority of hospitalized patients with IE were older white men with comorbidities and IE risk factors. The complication rate was high (27.7% with embolic events) and the inhospital mortality rate was in the lower range reported by prior studies [14.7% vs. 15%-20%].5 Nearly one-third of patients (n = 47, 27.7%) received valve surgery. Quality of care received was generally good, with most patients receiving early blood cultures, echocardiograms, early antibiotics, and timely ID consultation. We identified important gaps in care, including a failure to consult cardiac surgery in nearly half of patients and failure to consult cardiology in more than half of patients.
Our findings support work suggesting that IE is no longer primarily a chronic or subacute disease of younger patients with IVD use, positive human immunodeficiency virus status, or bicuspid aortic valves.1,4,16,17 The International Collaboration on Endocarditis-Prospective Cohort Study,4 a multinational prospective cohort study (2000-2005) of 2781 adults with IE, reported a higher prevalence of patients with diabetes or on hemodialysis, IVD users, and patients with long-term venous catheter and intracardiac leads than we found. Yet both studies suggest that the demographics of IE are changing. This may partially explain why IE mortality has not improved in recent years:2,3 patients with older age and higher comorbidity burden may not be considered good surgical candidates.
This study is among the first to contribute information on concordance with IE guidelines in a cohort of U.S. patients. Our findings suggest that most patients received timely blood culture, same-day administration of empiric antibiotics, and ID consultation, which is similar to European studies.7,18 Guideline concordance could be improved in some areas. Overall documentation of the management plan regarding the intracardiac leads could be improved. Only 6 of 29 patients with intracardiac leads had documentation of their removal during the index hospitalization.
The 2014 AHA/ACC guidelines5 and the ESC guidelines6 emphasized the importance of multidisciplinary management of IE. As part of the Heart Valve Team at BMC, cardiologists provide expertise in diagnosis, imaging and clinical management of IE, and cardiac surgeons provide consultation on whether to pursue surgery and optimal timing of surgery. Early discussion with surgical team is considered mandatory in all complicated cases of IE.6,18 Infectious disease consultation has been shown to improve the rate of IE diagnosis, reduce the 6-month relapse rate,19 and improve outcomes in patients with S aureus bacteremia.20 In our study 86.5% of patients had documentation of an ID consultation; cardiac surgery consultation was obtained in 54.1% and cardiology consultation in 47.1% of patients.
We observed a trend towards lower rates of 6-month readmission and 12-month mortality among patients who received all 3 consults (Figure 1), despite the fact that rates of embolic events and stroke were higher in patients with 3 consults compared to those with fewer than 3. Obviously, the lack of confounder adjustment and lack of power limits our ability to make inferences about this association, but it generates hypotheses for future work. Because subjects in our study were cared for prior to 2014, multidisciplinary management of IE with involvement of cardiology, cardiac surgery, and ID physicians was observed in only one-third of patients. However, 117 (68.8%) patients received either cardiology or cardiac surgery consults. It is possible that some physicians considered involving both cardiology and cardiac surgery consultants as unnecessary and, therefore, did not consult both specialties. We will focus future QI efforts in our institution on educating physicians about the benefits of multidisciplinary care and the importance of fully implementing the 2014 AHA/ACC guidelines.
Our findings around quality of care should be placed in the context of 2 studies by González de Molina et al8 and Delahaye et al7 These studies described considerable discordance between guideline recommendations and real-world IE care. However, these studies were performed more than a decade ago and were conducted before current recommendations to consult cardiology and cardiac surgery were published.
In the 2014 AHA/ACC guidelines, surgery prior to completion of antibiotics is indicated in patients with valve dysfunction resulting in heart failure; left-sided IE caused by highly resistant organisms (including fungus or S aureus); IE complicated by heart block, aortic abscess, or penetrating lesions; and presence of persistent infection (bacteremia or fever lasting longer than 5 to 7 days) after onset of appropriate antimicrobial therapy. In addition, there is a Class IIa indication of early surgery in patients with recurrent emboli and persistent vegetation despite appropriate antibiotic therapy and a Class IIb indication of early surgery in patients with NVE with mobile vegetation greater than 10 mm in length. Surgery is recommended for patients with PVE and relapsing infection.
It is recommended that IE patients be cared for in centers with immediate access to cardiac surgery because the urgent need for surgical intervention can arise rapidly.5 We found that nearly one-third of included patients underwent surgery. Although we did not collect data on indications for surgery in patients who did not receive surgery, we observed that 50% had a surgery consult, suggesting the presence of 1 or more surgical indications. Of these, half underwent valve surgery. Most of the NVE patients who underwent surgery had more than 1 indication for surgery. Our surgical rate is similar to a study from Italy3 and overall in the lower range of reported surgical rate (25%-50%) from other studies.21 The low rate of surgery at our center may be related to the fact that the use of surgery for IE has been hotly debated in the literature,21 and may also be due to the low rate of cardiac surgery consultation.
Our study has several limitations. We identified eligible patients using a discharge ICD-9 coding of IE and then confirmed the presence of Duke criteria using record review. Using discharge diagnosis codes for endocarditis has been validated, and our additional manual chart review to confirm Duke criteria likely improved the specificity significantly. However, by excluding patients who did not have documented evidence of Duke criteria, we may have missed some cases, lowering sensitivity. The performance on selected quality metrics may also have been affected by our inclusion criteria. Because we included only patients who met Duke criteria, we tended to include patients who had received blood cultures and echocardiograms, which are part of the criteria. Thus, we cannot comment on use of diagnostic testing or specialty consultation in patients with suspected IE. This was a single-center study and may not represent patients or current practices seen in other institutions. We did not collect data on some of the predisposing factors to NVE (for example, baseline rheumatic heart disease or preexisting valvular heart disease) because it is estimated that less than 5% of IE in the U.S. is superimposed on rheumatic heart disease.4 We likely underestimated 12-month mortality rate because we did not cross-reference our findings again the National Death Index; however, this should not affect the comparison of this outcome between groups.
CONCLUSION
Our study confirms reports that IE epidemiology has changed significantly in recent years. It also suggests that concordance with guideline recommendations is good for some aspects of care (eg, echocardiogram, blood cultures), but can be improved in other areas, particularly in use of specialty consultation during the hospitalization. Future QI efforts should emphasize the role of the heart valve team or endocarditis team that consists of an internist, ID physician, cardiologist, cardiac surgeon, and nursing. Finally, efforts should be made to develop strategies for community hospitals that do not have access to all of these specialists (eg, early transfer, telehealth).
Disclosure
Nothing to report.
Infective endocarditis (IE) affected an estimated 46,800 Americans in 2011, and its incidence is increasing due to greater numbers of invasive procedures and prevalence of IE risk factors.1-3 Despite recent advances in the treatment of IE, morbidity and mortality remain high: in-hospital mortality in IE patients is 15% to 20%, and the 1-year mortality rate is approximately 40%.2,4,5
Poor IE outcomes may be the result of difficulty in diagnosing IE and identifying its optimal treatments. The American Heart Association (AHA), the American College of Cardiology (ACC), and the European Society of Cardiology (ESC) have published guidelines to address these challenges. Recent guidelines recommend a multidisciplinary approach that includes cardiology, cardiac surgery, and infectious disease (ID) specialty involvement in decision-making.5,6
In the absence of published quality measures for IE management, guidelines can be used to evaluate the quality of care of IE. Studies have showed poor concordance with guideline recommendations but did not examine agreement with more recently published guidelines.7,8 Furthermore, few studies have examined the management, outcomes, and quality of care received by IE patients. Therefore, we aimed to describe a modern cohort of patients with IE admitted to a tertiary medical center over a 4-year period. In particular, we aimed to assess quality of care received by this cohort, as measured by concordance with AHA and ACC guidelines to identify gaps in care and spur quality improvement (QI) efforts.
METHODS
Design and Study Population
We conducted a retrospective cohort study of adult IE patients admitted to Baystate Medical Center (BMC), a 716-bed tertiary academic center that covers a population of 800,000 people throughout western New England. We used the International Classification of Diseases (ICD)–Ninth Revision, to identify IE patients discharged with a principal or secondary diagnosis of IE between 2007 and 2011 (codes 421.0, 421.1, 421.9, 424.9, 424.90, and 424.91). Three co-authors confirmed the diagnosis by conducting a review of the electronic health records.
We included only patients who met modified Duke criteria for definite or possible IE.5 Definite IE defines patients with pathological criteria (microorganisms demonstrated by culture or histologic examination or a histologic examination showing active endocarditis); or patients with 2 major criteria (positive blood culture and evidence of endocardial involvement by echocardiogram), 1 major criterion and 3 minor criteria (minor criteria: predisposing heart conditions or intravenous drug (IVD) use, fever, vascular phenomena, immunologic phenomena, and microbiologic evidence that do not meet the major criteria) or 5 minor criteria. Possible IE defines patients with 1 major and 1 minor criterion or 3 minor criteria.5
Data Collection
We used billing and clinical databases to collect demographics, comorbidities, antibiotic treatment, 6-month readmission and 1-year mortality. Comorbid conditions were classified into Elixhauser comorbidities using software provided by the Healthcare Costs and Utilization Project of the Agency for Healthcare Research and Quality.9,10
We obtained all other data through electronic health record abstraction. These included microbiology, type of endocarditis (native valve endocarditis [NVE] or prosthetic valve endocarditis [PVE]), echocardiographic location of the vegetation, and complications involving the valve (eg, valve perforation, ruptured chorda, perivalvular abscess, or valvular insufficiency).
Using 2006 AHA/ACC guidelines,11 we identified quality metrics, including the presence of at least 2 sets of blood cultures prior to start of antibiotics and use of transthoracic echocardiogram (TTE) and transesophageal echocardiogram (TEE). Guidelines recommend using TTE as first-line to detect valvular vegetations and assess IE complications. TEE is recommended if TTE is nondiagnostic and also as first-line to diagnose PVE. We assessed the rate of consultation with ID, cardiology, and cardiac surgery specialties. While these consultations were not explicitly emphasized in the 2006 AHA/ACC guidelines, there is a class I recommendation in 2014 AHA/ACC guidelines5 to manage IE patients with consultation of all these specialties.
We reported the number of patients with intracardiac leads (pacemaker or defibrillator) who had documentation of intracardiac lead removal. Complete removal of intracardiac leads is indicated in IE patients with infection of leads or device (class I) and suggested for IE caused by Staphylococcus aureus or fungi (even without evidence of device or lead infection), and for patients undergoing valve surgery (class IIa).5 We entered abstracted data elements into a RedCap database, hosted by Tufts Clinical and Translational Science Institute.12
Outcomes
Outcomes included embolic events, strokes, need for cardiac surgery, LOS, inhospital mortality, 6-month readmission, and 1-year mortality. We identified embolic events using documentation of clinical or imaging evidence of an embolic event to the cerebral, coronary, peripheral arterial, renal, splenic, or pulmonary vasculature. We used record extraction to identify incidence of valve surgery. Nearly all patients who require surgery at BMC have it done onsite. We compared outcomes among patients who received less than 3 vs. 3 consultations provided by ID, cardiology, and cardiac surgery specialties. We also compared outcomes among patients who received 0, 1, 2, or 3 consultations to look for a trend.
Statistical Analysis
We divided the cohort into patients with NVE and PVE because there are differences in pathophysiology, treatment, and outcomes of these groups. We calculated descriptive statistics, including means/standard deviation (SD) and n (%). We conducted univariable analyses using Fisher exact (categorical), unpaired t tests (Gaussian), or Kruskal-Wallis equality-of-populations rank test (non-Gaussian). Common language effect sizes were also calculated to quantify group differences without respect to sample size.13,14 Analyses were performed using Stata 14.1. (StataCorp LLC, College Station, Texas). The BMC Institutional Review Board approved the protocol.
RESULTS
We identified a total of 317 hospitalizations at BMC meeting criteria for IE. Of these, 147 hospitalizations were readmissions or did not meet the clinical criteria of definite or possible IE. Thus, we included a total of 170 patients in the final analysis. Definite IE was present in 135 (79.4%) and possible IE in 35 (20.6%) patients.
Patient Characteristics
Of 170 patients, 127 (74.7%) had NVE and 43 (25.3%) had PVE. Mean ± SD age was 60.0 ± 17.9 years, 66.5% (n = 113) of patients were male, and 79.4% (n = 135) were white (Table 1). Hypertension and chronic kidney disease were the most common comorbidities. The median Gagne score15 was 4, corresponding to a 1-year mortality risk of 15%. Predisposing factors for IE included previous history of IE (n = 14 or 8.2%), IVD use (n = 23 or 13.5%), and presence of long-term venous catheters (n = 19 or 11.2%). Intracardiac leads were present in 17.1% (n = 29) of patients. Bicuspid aortic valve was reported in 6.5% (n = 11) of patients with NVE. Patients with PVE were older (+11.5 years, 95% confidence interval [CI] 5.5, 17.5) and more likely to have intracardiac leads (44.2% vs. 7.9%; P < 0.001; Table 1).
Microbiology and Antibiotics
Staphylococcus aureus was isolated in 40.0% of patients (methicillin-sensitive: 21.2%, n = 36; methicillin-resistant: 18.8%, n = 32) and vancomycin (88.2%, n = 150) was the most common initial antibiotic used. Nearly half (44.7%, n = 76) of patients received gentamicin as part of their initial antibiotic regimen. Appendix 1 provides information on final blood culture results, prosthetic versus native valve IE, and antimicrobial agents that each patient received. PVE patients were more likely to receive gentamicin as their initial antibiotic regimen than NVE (58.1% vs. 40.2%; P = 0.051; Table 1).
Echocardiography and Affected Valves
As per study inclusion criteria, all patients received echocardiography (either TTE, TEE, or both). Overall, the most common infected valve was mitral (41.3%), n = 59), followed by aortic valve (28.7%), n = 41). Patients in whom the location of infected valve could not be determined (15.9%, n = 27) had echocardiographic features of intracardiac device infection or intracardiac mass (Table 1).
Quality of Care
Nearly all (n = 165, 97.1%) of patients had at least 2 sets of blood cultures drawn, most on the first day of admission (71.2%). The vast majority of patients (n = 152, 89.4%) also received their first dose of antibiotics on the day of admission. Ten (5.9%) patients did not receive any consults, and 160 (94.1%) received at least 1 consultation. An ID consultation was obtained for most (147, 86.5%) patients; cardiac surgery consultation was obtained for about half of patients (92, 54.1%), and cardiology consultation was also obtained for nearly half of patients (80, 47.1%). One-third (53, 31.2%) did not receive a cardiology or cardiac surgery consult, two-thirds (117, 68.8%) received either a cardiology or a cardiac surgery consult, and one-third (55, 32.4%) received both.
Of the 29 patients who had an intracardiac lead, 6 patients had documentation of the device removal during the index hospitalization (5 or 50.0% of patients with NVE and 1 or 5.3% of patients with PVE; P = 0.02; Table 2).
Outcomes
Evidence of any embolic events was seen in 27.7% (n = 47) of patients, including stroke in 17.1% (n = 29). Median LOS for all patients was 13.5 days, and 6-month readmission among patients who survived their index admission was 51.0% (n = 74/145; 95% CI, 45.9%-62.7%). Inhospital mortality was 14.7% (n = 25; 95% CI: 10.1%-20.9%) and 12-month mortality was 22.4% (n = 38; 95% CI, 16.7%-29.3%). Inhospital mortality was more frequent among patients with PVE than NVE (20.9% vs. 12.6%; P = 0.21), although this difference was not statistically significant. Complications were more common in NVE than PVE (any embolic event: 32.3% vs. 14.0%, P = 0.03; stroke, 20.5% vs. 7.0%, P = 0.06; Table 3).
Although there was a trend toward reduction in 6-month readmission and 12-month mortality with incremental increase in the number of specialties consulted (ID, cardiology and cardiac surgery), the difference was not statistically significant (Figure). In addition, comparing outcomes of embolic events, stroke, 6-month readmission, and 12-month mortality between those who received 3 consults (28.8%, n = 49) to those with fewer than 3 (71.2%, n = 121) did not show statistically significant differences.
Of 92 patients who received a cardiac surgery consult, 73 had NVE and 19 had PVE. Of these, 47 underwent valve surgery, 39 (of 73) with NVE (53.42%) and 8 (of 19) with PVE (42.1%). Most of the NVE patients (73.2%) had more than 1 indication for surgery. The most common indications for surgery among NVE patients were significant valvular dysfunction resulting in heart failure (65.9%), followed by mobile vegetation (56.1%) and recurrent embolic events (26.8%). The most common indication for surgery in PVE was persistent bacteremia or recurrent embolic events (75.0%).
DISCUSSION
In this study, we described the management, quality of care, and outcomes of IE patients in a tertiary medical center. We found that the majority of hospitalized patients with IE were older white men with comorbidities and IE risk factors. The complication rate was high (27.7% with embolic events) and the inhospital mortality rate was in the lower range reported by prior studies [14.7% vs. 15%-20%].5 Nearly one-third of patients (n = 47, 27.7%) received valve surgery. Quality of care received was generally good, with most patients receiving early blood cultures, echocardiograms, early antibiotics, and timely ID consultation. We identified important gaps in care, including a failure to consult cardiac surgery in nearly half of patients and failure to consult cardiology in more than half of patients.
Our findings support work suggesting that IE is no longer primarily a chronic or subacute disease of younger patients with IVD use, positive human immunodeficiency virus status, or bicuspid aortic valves.1,4,16,17 The International Collaboration on Endocarditis-Prospective Cohort Study,4 a multinational prospective cohort study (2000-2005) of 2781 adults with IE, reported a higher prevalence of patients with diabetes or on hemodialysis, IVD users, and patients with long-term venous catheter and intracardiac leads than we found. Yet both studies suggest that the demographics of IE are changing. This may partially explain why IE mortality has not improved in recent years:2,3 patients with older age and higher comorbidity burden may not be considered good surgical candidates.
This study is among the first to contribute information on concordance with IE guidelines in a cohort of U.S. patients. Our findings suggest that most patients received timely blood culture, same-day administration of empiric antibiotics, and ID consultation, which is similar to European studies.7,18 Guideline concordance could be improved in some areas. Overall documentation of the management plan regarding the intracardiac leads could be improved. Only 6 of 29 patients with intracardiac leads had documentation of their removal during the index hospitalization.
The 2014 AHA/ACC guidelines5 and the ESC guidelines6 emphasized the importance of multidisciplinary management of IE. As part of the Heart Valve Team at BMC, cardiologists provide expertise in diagnosis, imaging and clinical management of IE, and cardiac surgeons provide consultation on whether to pursue surgery and optimal timing of surgery. Early discussion with surgical team is considered mandatory in all complicated cases of IE.6,18 Infectious disease consultation has been shown to improve the rate of IE diagnosis, reduce the 6-month relapse rate,19 and improve outcomes in patients with S aureus bacteremia.20 In our study 86.5% of patients had documentation of an ID consultation; cardiac surgery consultation was obtained in 54.1% and cardiology consultation in 47.1% of patients.
We observed a trend towards lower rates of 6-month readmission and 12-month mortality among patients who received all 3 consults (Figure 1), despite the fact that rates of embolic events and stroke were higher in patients with 3 consults compared to those with fewer than 3. Obviously, the lack of confounder adjustment and lack of power limits our ability to make inferences about this association, but it generates hypotheses for future work. Because subjects in our study were cared for prior to 2014, multidisciplinary management of IE with involvement of cardiology, cardiac surgery, and ID physicians was observed in only one-third of patients. However, 117 (68.8%) patients received either cardiology or cardiac surgery consults. It is possible that some physicians considered involving both cardiology and cardiac surgery consultants as unnecessary and, therefore, did not consult both specialties. We will focus future QI efforts in our institution on educating physicians about the benefits of multidisciplinary care and the importance of fully implementing the 2014 AHA/ACC guidelines.
Our findings around quality of care should be placed in the context of 2 studies by González de Molina et al8 and Delahaye et al7 These studies described considerable discordance between guideline recommendations and real-world IE care. However, these studies were performed more than a decade ago and were conducted before current recommendations to consult cardiology and cardiac surgery were published.
In the 2014 AHA/ACC guidelines, surgery prior to completion of antibiotics is indicated in patients with valve dysfunction resulting in heart failure; left-sided IE caused by highly resistant organisms (including fungus or S aureus); IE complicated by heart block, aortic abscess, or penetrating lesions; and presence of persistent infection (bacteremia or fever lasting longer than 5 to 7 days) after onset of appropriate antimicrobial therapy. In addition, there is a Class IIa indication of early surgery in patients with recurrent emboli and persistent vegetation despite appropriate antibiotic therapy and a Class IIb indication of early surgery in patients with NVE with mobile vegetation greater than 10 mm in length. Surgery is recommended for patients with PVE and relapsing infection.
It is recommended that IE patients be cared for in centers with immediate access to cardiac surgery because the urgent need for surgical intervention can arise rapidly.5 We found that nearly one-third of included patients underwent surgery. Although we did not collect data on indications for surgery in patients who did not receive surgery, we observed that 50% had a surgery consult, suggesting the presence of 1 or more surgical indications. Of these, half underwent valve surgery. Most of the NVE patients who underwent surgery had more than 1 indication for surgery. Our surgical rate is similar to a study from Italy3 and overall in the lower range of reported surgical rate (25%-50%) from other studies.21 The low rate of surgery at our center may be related to the fact that the use of surgery for IE has been hotly debated in the literature,21 and may also be due to the low rate of cardiac surgery consultation.
Our study has several limitations. We identified eligible patients using a discharge ICD-9 coding of IE and then confirmed the presence of Duke criteria using record review. Using discharge diagnosis codes for endocarditis has been validated, and our additional manual chart review to confirm Duke criteria likely improved the specificity significantly. However, by excluding patients who did not have documented evidence of Duke criteria, we may have missed some cases, lowering sensitivity. The performance on selected quality metrics may also have been affected by our inclusion criteria. Because we included only patients who met Duke criteria, we tended to include patients who had received blood cultures and echocardiograms, which are part of the criteria. Thus, we cannot comment on use of diagnostic testing or specialty consultation in patients with suspected IE. This was a single-center study and may not represent patients or current practices seen in other institutions. We did not collect data on some of the predisposing factors to NVE (for example, baseline rheumatic heart disease or preexisting valvular heart disease) because it is estimated that less than 5% of IE in the U.S. is superimposed on rheumatic heart disease.4 We likely underestimated 12-month mortality rate because we did not cross-reference our findings again the National Death Index; however, this should not affect the comparison of this outcome between groups.
CONCLUSION
Our study confirms reports that IE epidemiology has changed significantly in recent years. It also suggests that concordance with guideline recommendations is good for some aspects of care (eg, echocardiogram, blood cultures), but can be improved in other areas, particularly in use of specialty consultation during the hospitalization. Future QI efforts should emphasize the role of the heart valve team or endocarditis team that consists of an internist, ID physician, cardiologist, cardiac surgeon, and nursing. Finally, efforts should be made to develop strategies for community hospitals that do not have access to all of these specialists (eg, early transfer, telehealth).
Disclosure
Nothing to report.
1. Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65(19):2070-2076. PubMed
2. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PloS One. 2013;8(3):e60033. PubMed
3. Fedeli U, Schievano E, Buonfrate D, Pellizzer G, Spolaore P. Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC Infect Dis. 2011;11:48. PubMed
4. Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169(5):463-473. PubMed
5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):2438-2488. PubMed
6. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. PubMed
7. Delahaye F, Rial MO, de Gevigney G, Ecochard R, Delaye J. A critical appraisal of the quality of the management of infective endocarditis. J Am Coll Cardiol. 1999;33(3):788-793. PubMed
8. González De Molina M, Fernández-Guerrero JC, Azpitarte J. [Infectious endocarditis: degree of discordance between clinical guidelines recommendations and clinical practice]. Rev Esp Cardiol. 2002;55(8):793-800. PubMed
9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
10. Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived rom ICD-9-CCM administrative data. Med Care. 2002;40(8):675-685. PubMed
11. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, Bonow RO, Carabello BA, Kanu C, deLeon AC Jr, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114(5):e84-e231.
12. REDCap [Internet]. [cited 2016 May 14]. Available from: https://collaborate.tuftsctsi.org/redcap/.
13. McGraw KO, Wong SP. A common language effect-size statistic. Psychol Bull. 1992;111:361-365.
14. Cohen J. The statistical power of abnormal-social psychological research: a review. J Abnorm Soc Psychol. 1962;65:145-153. PubMed
15. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. PubMed
16. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. PubMed
17. Cecchi E, Chirillo F, Castiglione A, Faggiano P, Cecconi M, Moreo A, et al. Clinical epidemiology in Italian Registry of Infective Endocarditis (RIEI): Focus on age, intravascular devices and enterococci. Int J Cardiol. 2015;190:151-156. PubMed
18. Tornos P, Iung B, Permanyer-Miralda G, Baron G, Delahaye F, Gohlke-Bärwolf Ch, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart. 2005;91(5):571-575. PubMed
19. Yamamoto S, Hosokawa N, Sogi M, Inakaku M, Imoto K, Ohji G, et al. Impact of infectious diseases service consultation on diagnosis of infective endocarditis. Scand J Infect Dis. 2012;44(4):270-275. PubMed
20. Rieg S, Küpper MF. Infectious diseases consultations can make the difference: a brief review and a plea for more infectious diseases specialists in Germany. Infection. 2016;(2):159-166. PubMed
21. Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010;121(9):11411152. PubMed
1. Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, et al. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65(19):2070-2076. PubMed
2. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PloS One. 2013;8(3):e60033. PubMed
3. Fedeli U, Schievano E, Buonfrate D, Pellizzer G, Spolaore P. Increasing incidence and mortality of infective endocarditis: a population-based study through a record-linkage system. BMC Infect Dis. 2011;11:48. PubMed
4. Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169(5):463-473. PubMed
5. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):2438-2488. PubMed
6. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. PubMed
7. Delahaye F, Rial MO, de Gevigney G, Ecochard R, Delaye J. A critical appraisal of the quality of the management of infective endocarditis. J Am Coll Cardiol. 1999;33(3):788-793. PubMed
8. González De Molina M, Fernández-Guerrero JC, Azpitarte J. [Infectious endocarditis: degree of discordance between clinical guidelines recommendations and clinical practice]. Rev Esp Cardiol. 2002;55(8):793-800. PubMed
9. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
10. Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived rom ICD-9-CCM administrative data. Med Care. 2002;40(8):675-685. PubMed
11. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, Bonow RO, Carabello BA, Kanu C, deLeon AC Jr, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114(5):e84-e231.
12. REDCap [Internet]. [cited 2016 May 14]. Available from: https://collaborate.tuftsctsi.org/redcap/.
13. McGraw KO, Wong SP. A common language effect-size statistic. Psychol Bull. 1992;111:361-365.
14. Cohen J. The statistical power of abnormal-social psychological research: a review. J Abnorm Soc Psychol. 1962;65:145-153. PubMed
15. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749-759. PubMed
16. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. PubMed
17. Cecchi E, Chirillo F, Castiglione A, Faggiano P, Cecconi M, Moreo A, et al. Clinical epidemiology in Italian Registry of Infective Endocarditis (RIEI): Focus on age, intravascular devices and enterococci. Int J Cardiol. 2015;190:151-156. PubMed
18. Tornos P, Iung B, Permanyer-Miralda G, Baron G, Delahaye F, Gohlke-Bärwolf Ch, et al. Infective endocarditis in Europe: lessons from the Euro heart survey. Heart. 2005;91(5):571-575. PubMed
19. Yamamoto S, Hosokawa N, Sogi M, Inakaku M, Imoto K, Ohji G, et al. Impact of infectious diseases service consultation on diagnosis of infective endocarditis. Scand J Infect Dis. 2012;44(4):270-275. PubMed
20. Rieg S, Küpper MF. Infectious diseases consultations can make the difference: a brief review and a plea for more infectious diseases specialists in Germany. Infection. 2016;(2):159-166. PubMed
21. Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010;121(9):11411152. PubMed
© 2017 Society of Hospital Medicine
Do HCAHPS doctor communication scores reflect the communication skills of the attending on record? A cautionary tale from a tertiary-care medical service
Communication is the foundation of medical care.1 Effective communication can improve health outcomes, safety, adherence, satisfaction, trust, and enable genuine informed consent and decision-making.2-9 Furthermore, high-quality communication increases provider engagement and workplace satisfaction, while reducing stress and malpractice risk.10-15
Direct measurement of communication in the healthcare setting can be challenging. The “Four Habits Model,” which is derived from a synthesis of empiric studies8,16-20 and theoretical models21-24 of communication, offers 1 framework for assessing healthcare communication. The conceptual model underlying the 4 habits has been validated in studies of physician and patient satisfaction.1,4,25-27 The 4 habits are: investing in the beginning, eliciting the patient’s perspective, demonstrating empathy, and investing in the end. Each habit is divided into several identifiable tasks or skill sets, which can be reliably measured using validated tools and checklists.28 One such instrument, the Four Habits Coding Scheme (4HCS), has been evaluated against other tools and demonstrated overall satisfactory inter-rater reliability and validity.29,30
The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, developed under the direction of the Centers for Medicare and Medicaid Services (CMS) and the Agency for Healthcare Research and Quality, is an established national standard for measuring patient perceptions of care. HCAHPS retrospectively measures global perceptions of communication, support and empathy from physicians and staff, processes of care, and the overall patient experience. HCAHPS scores were first collected nationally in 2006 and have been publicly reported since 2008.31 With the introduction of value-based purchasing in 2012, health system revenues are now tied to HCAHPS survey performance.32 As a result, hospitals are financially motivated to improve HCAHPS scores but lack evidence-based methods for doing so. Some healthcare organizations have invested in communication training programs based on the available literature and best practices.2,33-35 However, it is not known how, if at all, HCAHPS scores relate to physicians’ real-time observed communication skills.
To examine the relationship between physician communication, as reported by global HCAHPS scores, and the quality of physician communication skills in specific encounters, we observed hospitalist physicians during inpatient bedside rounds and measured their communication skills using the 4HCS.
METHODS
Study Design
The study utilized a cross sectional design; physicians who consented were observed on rounds during 3 separate encounters, and we compared hospitalists’ 4HCS scores to their HCAHPS scores to assess the correlation. The study was approved by the Institutional Review Board of the Cleveland Clinic.
Population
The study was conducted at the main campus of the Cleveland Clinic. All physicians specializing in hospital medicine who had received 10 or more completed HCAHPS survey responses while rounding on a medicine service in the past year were invited to participate in the study. Participation was voluntary; night hospitalists were excluded. A research nurse was trained in the Four Habits Model28 and in the use of the 4HCS coding scheme by the principal investigator. The nurse observed each physician and ascertained the presence of communication behaviors using the 4HCS tool. Physicians were observed between August 2013 and August 2014. Multiple observations per physician could occur on the same day, but only 1 observation per patient was used for analysis. Observations consisted of a physician’s first encounter with a hospitalized patient, with the patient’s consent. Observations were conducted during encounters with English-speaking and cognitively intact patients only. Resident physicians were permitted to stay and conduct rounds per their normal routine. Patient information was not collected as part of the study.
Measures
HCAHPS. For each physician, we extracted all HCAHPS scores that were collected from our hospital’s Press Ganey database. The HCAHPS survey contains 22 core questions divided into 7 themes or domains, 1 of which is doctor communication. The survey uses frequency-based questions with possible answers fixed on a 4-point scale (4=always, 3=usually, 2=sometimes, 1=never). Our primary outcome was the doctor communication domain, which comprises 3 questions: 1) During this hospital stay, how often did the doctors treat you with respect? 2) During this hospital stay, how often did the doctors listen to you? and 3) During this hospital stay, how often did the doctors explain things in a language you can understand? Because CMS counts only the percentage of responses that are graded “always,” so-called “top box” scoring, we used the same measure.
The HCAHPS scores are always attributed to the physician at the time of discharge even if he may not have been responsible for the care of the patient during the entire hospital course. To mitigate contamination from patients seen by multiple providers, we cross-matched length of stay (LOS) data with billing data to determine the proportion of days a patient was seen by a single provider during the entire length of stay. We stratified patients seen by the attending providers to less than 50%, 50% to less than 100%, and at 100% of the LOS. However, we were unable to identify which patients were seen by other consultants or by residents due to limitations in data gathering and the nature of the database.
The Four Habits. The Four Habits are: invest in the beginning, elicit the patient’s perspective, demonstrate empathy, and invest in the end (Figure 1). Specific behaviors for Habits 1 to 4 are outlined in the Appendix, but we will briefly describe the themes as follows. Habit 1, invest in the beginning, describes the ability of the physician to set a welcoming environment for the patient, establish rapport, and collaborate on an agenda for the visit. Habit 2, elicit the patient’s perspective, describes the ability of the physician to explore the patients’ worries, ideas, expectations, and the impact of the illness on their lifestyle. Habit 3, demonstrate empathy, describes the physician’s openness to the patient’s emotions as well as the ability to explore, validate; express curiosity, and openly accept these feelings. Habit 4, invest in the end, is a measure of the physician’s ability to counsel patients in a language built around their original concerns or worries, as well as the ability to check the patients’ understanding of the plan.2,29-30
4HCS. The 4HCS tool (Appendix) measures discreet behaviors and phrases based on each of the Four Habits (Figure 1). With a scoring range from a low of 4 to a high of 20, the rater at bedside assigns a range of points on a scale of 1 to 5 for each habit. It is an instrument based on a teaching model used widely throughout Kaiser Permanente to improve clinicians’ communication skills. The 4HCS was first tested for interrater reliability and validity against the Roter Interaction Analysis System using 100 videotaped primary care physician encounters.29 It was further evaluated in a randomized control trial. Videotapes from 497 hospital encounters involving 71 doctors from a variety of clinical specialties were rated by 4 trained raters using the coding scheme. The total score Pearson’s R and intraclass correlation coefficient (ICC) exceeded 0.70 for all pairs of raters, and the interrater reliability was satisfactory for the 4HCS as applied to heterogeneous material.30
STATISTICAL ANALYSIS
Physician characteristics were summarized with standard descriptive statistics. Pearson correlation coefficients were computed between HCAHPS and 4HCS scores. All analyses were performed with RStudio (Boston, MA). The Pearson correlation between the averaged HCAHPS and 4HCS scores was also computed. A correlation with a P value less than 0.05 was considered statistically significant. With 28 physicians, the study had a power of 88% to detect a moderate correlation (greater than 0.50) with a 2-sided alpha of 0.05. We also computed the correlations based on the subgroups of data with patients seen by providers for less than 50%, 50% to less than 100%, and 100% of LOS. All analyses were conducted in SAS 9.2 (SAS Institute Inc., Cary, NC).36
RESULTS
There were 31 physicians who met our inclusion criteria. Of 29 volunteers, 28 were observed during 3 separate inpatient encounters and made up the final sample. A total of 1003 HCAHPS survey responses were available for these physicians. Participants were predominantly female (60.7%), with an average age of 39 years. They were in practice for an average of 4 years (12 were in practice more than 5 years), and 9 were observed on a teaching rotation.
The means of the overall 4HCS scores per observation were 17.39 ± 2.33 for the first, 17.00 ± 2.37 for the second, and 17.43 ± 2.36 for third bedside observation. The mean 4HCS scores per observation, broken down by habit, appear in Table 1. The ICC among the repeated scores within the same physician was 0.81. The median number of HCAHPS survey returns was 32 (range = [8, 85], with mean = 35.8, interquartile range = [16, 54]). The median overall HCAHPS doctor communication score was 89.6 (range = 80.9-93.7). Participants scored the highest in the respect subdomain and the lowest in the explain subdomain. Median HCAHPS scores and ranges appear in Table 2.
Because there were no significant associations between 4HCS scores or HCAHPS scores and physician age, sex, years in practice, or teaching site, correlations were not adjusted. Figure 2A and 2B show the association between mean 4HCS scores and HCAHPS scores by physician. There was no significant correlation between overall 4HCS and HCAHPS doctor communication scores (Pearson correlation coefficient 0.098; 95% confidence interval [CI], -0.285, 0.455). The individual habits also were not correlated with overall HCAHPS scores or with their corresponding HCAHPS domain (Table 3).
For 325 patients, 1 hospitalist was present for the entire LOS. In sensitivity analysis limiting observations to these patients (Figure 2C, Figure 2D, Table 3), we found a moderate correlation between habit 3 and the HCAHPS respect score (Pearson correlation coefficient 0.515; 95% CI, 0.176, 0.745; P = 0.005), and a weaker correlation between habit 3 and the HCAHPS overall doctor communication score (0.442; 95% CI, 0.082, 0.7; P = 0.019). There were no other significant correlations between specific habits and HCAHPS scores.
DISCUSSION
In this observational study of hospitalist physicians at a large tertiary care center, we found that communication skills, as measured by the 4HCS, varied substantially among physicians but were highly correlated within patients of the same physician. However, there was virtually no correlation between the attending physician of record’s 4HCS scores and their HCAHPS communication scores. When we limited our analysis to patients who saw only 1 hospitalist throughout their stay, there were moderate correlations between demonstration of empathy and both the HCAHPS respect score and overall doctor communication score. There were no trends across the strata of hospitalist involvement. It is important to note that the addition of even 1 different hospitalist to the LOS removes any association. Habits 1 and 2 are close to significance in the 100% subgroup, with a weak correlation. Interestingly, Habit 4, which focuses on creating a plan with the patient, showed no correlation at all with patients reporting that doctors explained things in language they could understand.
Development and testing of the HCAHPS survey began in 2002, commissioned by CMS and the Agency for Healthcare Research and Quality for the purpose of measuring patient experience in the hospital. The HCAHPS survey was endorsed by the National Quality Forum in 2005, with final approval of the national implementation granted by the Office of Management and Budget later that year. The CMS began implementation of the HCAHPS survey in 2006, with the first required public reporting of all hospitals taking place in March 2008.37-41 Based on CMS’ value-based purchasing initiative, hospitals with low HCAHPS scores have faced substantial penalties since 2012. Under these circumstances, it is important that the HCAHPS measures what it purports to measure. Because HCAHPS was designed to compare hospitals, testing was limited to assessment of internal reliability, hospital-level reliability, and construct validity. External validation with known measures of physician communication was not performed.41 Our study appears to be the first to compare HCAHPS scores to directly observed measures of physician communication skills. The lack of association between the 2 should sound a cautionary note to hospitals who seek to tie individual compensation to HCAHPS scores to improve them. In particular, the survey asks for a rating for all the patient’s doctors, not just the primary hospitalist. We found that, for hospital stays with just 1 hospitalist, the HCAHPS score reflected observed expression of empathy, although the correlation was only moderate, and HCAHPS were not correlated with other communication skills. Of all communication skills, empathy may be most important. Almost the entire body of research on physician communication cites empathy as a central skill. Empathy improves patient outcomes1-9,13-14,16-18,42 such as adherence to treatment, loyalty, and perception of care; and provider outcomes10-12,15 such as reduced burnout and a decreased likelihood of malpractice litigation.
It is less clear why other communication skills did not correlate with HCAHPS, but several differences in the measures themselves and how they were obtained might be responsible. It is possible that HCAHPS measures something broader than physician communication. In addition, the 4HCS was developed and normed on outpatient encounters as is true for virtually all doctor-patient coding schemes.43 Little is known about inpatient communication best practices. The timing of HCAHPS may also degrade the relationship between observed and reported communication. The HCAHPS questionnaires, collected after discharge, are retrospective reconstructions that are subject to recall bias and recency effects.44,45 In contrast, our observations took place in real time and were specific to the face-to-face interactions that take place when physicians engage patients at the bedside. Third, the response rate for HCAHPS surveys is only 30%, leading to potential sample bias.46 Respondents represent discharged patients who are willing and able to answer surveys, and may not be representative of all hospitalized patients. Finally, as with all global questions, the meaning any individual patient assigns to terms like “respect” may vary.
Our study has several limitations. The HCAHPS and 4HCS scores were not obtained from the same sample of patients. It is possible that the patients who were observed were not representative of the patients who completed the HCAHPS surveys. In addition, the only type of encounter observed was the initial visit between the hospitalist and the patient, and did not include communication during follow-up visits or on the day of discharge. However, there was a strong ICC among the 4HCS scores, implying that the 4HCS measures an inherent physician skill, which should be consistent across patients and encounters. Coding bias of the habits by a single observer could not be excluded. High intra-class correlation could be due in part to observer preferences for particular communication styles. Our sample included only 28 physicians. Although our study was powered to rule out a moderate correlation between 4HCS scores and HCAHPS scores (Pearson correlation coefficient greater than 0.5), we cannot exclude weaker correlations. Most correlations that we observed were so small that they would not be clinically meaningful, even in a much larger sample.
CONCLUSIONS
Our findings that HCAHPS scores did not correlate with the communication skills of the attending of record have some important implications. In an environment of value-based purchasing, most hospital systems are interested in identifying modifiable provider behaviors that optimize efficiency and payment structures. This study shows that directly measured communication skills do not correlate with HCAHPS scores as generally reported, indicating that HCAHPS may be measuring a broader domain than only physician communication skills. Better attribution based on the proportion of care provided by an individual physician could make the scores more useful for individual comparisons, but most institutions do not report their data in this way. Given this limitation, hospitals should refrain from comparing and incentivizing individual physicians based on their HCAHPS scores, because this measure was not designed for this purpose and does not appear to reflect an individual’s skills. This is important in the current environment in which hospitals face substantial penalties for underperformance but lack specific tools to improve their scores. Furthermore, there is concern that this type of measurement creates perverse incentives that may adversely alter clinical practice with the aim of improving scores.46
Training clinicians in communication and teaming skills is one potential means of increasing overall scores.15 Improving doctor-patient and team relationships is also the right thing to do. It is increasingly being demanded by patients and has always been a deep source of satisfaction for physicians.15,47 Moreover, there is an increasingly robust literature that relates face-to-face communication to biomedical and psychosocial outcomes of care.48 Identifying individual physicians who need help with communication skills is a worthwhile goal. Unfortunately, the HCAHPS survey does not appear to be the appropriate tool for this purpose.
Disclosure
The Cleveland Clinic Foundation, Division of Clinical Research, Research Programs Committees provided funding support. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors have no conflicts of interest for this study.
1. Glass RM. The patient-physician relationship. JAMA focuses on the center of medicine. JAMA. 1996;275(2):147-148. PubMed
2. Stein T, Frankel RM, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns. 2005;58(1):4-12. PubMed
3. Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796-804. PubMed
4. Safran DG, Taira DA, Rogers WH, Kosinski M, Ware JE, Tarlov AR. Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213-220. PubMed
5. Like R, Zyzanski SJ. Patient satisfaction with the clinical encounter: social psychological determinants. Soc Sci Med. 1987;24(4):351-357. PubMed
6. Williams S, Weinman J, Dale J. Doctor-patient communication and patient satisfaction: a review. Fam Pract. 1998;15(5):480-492. PubMed
7. Ciechanowski P, Katon WJ. The interpersonal experience of health care through the eyes of patients with diabetes. Soc Sci Med. 2006;63(12):3067-3079. PubMed
8. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
9. Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med. 2011;86(3):359-364. PubMed
10. Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277(7):553-559. PubMed
11. Ambady N, Laplante D, Nguyen T, Rosenthal R, Chaumeton N, Levinson W. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9. PubMed
12. Weng HC, Hung CM, Liu YT, et al. Associations between emotional intelligence and doctor burnout, job satisfaction and patient satisfaction. Med Educ. 2011;45(8):835-842. PubMed
13. Mauksch LB, Dugdale DC, Dodson S, Epstein R. Relationship, communication, and efficiency in the medical encounter: creating a clinical model from a literature review. Arch Intern Med. 2008;168(13):1387-1395. PubMed
14. Suchman AL, Roter D, Green M, Lipkin M Jr. Physician satisfaction with primary care office visits. Collaborative Study Group of the American Academy on Physician and Patient. Med Care. 1993;31(12):1083-1092. PubMed
15. Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31(7):755-761. PubMed
16. Brody DS, Miller SM, Lerman CE, Smith DG, Lazaro CG, Blum MJ. The relationship between patients’ satisfaction with their physicians and perceptions about interventions they desired and received. Med Care. 1989;27(11):1027-1035. PubMed
17. Wasserman RC, Inui TS, Barriatua RD, Carter WB, Lippincott P. Pediatric clinicians’ support for parents makes a difference: an outcome-based analysis of clinician-parent interaction. Pediatrics. 1984;74(6):1047-1053. PubMed
18. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528. PubMed
19. Inui TS, Carter WB. Problems and prospects for health services research on provider-patient communication. Med Care. 1985;23(5):521-538. PubMed
20. Beckman H, Frankel R, Kihm J, Kulesza G, Geheb M. Measurement and improvement of humanistic skills in first-year trainees. J Gen Intern Med. 1990;5(1):42-45. PubMed
21. Keller S, O’Malley AJ, Hays RD, et al. Methods used to streamline the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2057-2077. PubMed
22. Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137(5):535-544. PubMed
23. Lazare A, Eisenthal S, Wasserman L. The customer approach to patienthood. Attending to patient requests in a walk-in clinic. Arch Gen Psychiatry. 1975;32(5):553-558. PubMed
24. Eisenthal S, Lazare A. Evaluation of the initial interview in a walk-in clinic. The clinician’s perspective on a “negotiated approach”. J Nerv Ment Dis. 1977;164(1):30-35. PubMed
25. Kravitz RL, Callahan EJ, Paterniti D, Antonius D, Dunham M, Lewis CE. Prevalence and sources of patients’ unmet expectations for care. Ann Intern Med. 1996;125(9):730-737. PubMed
26. Froehlich GW, Welch HG. Meeting walk-in patients’ expectations for testing. Effects on satisfaction. J Gen Intern Med. 1996;11(8):470-474. PubMed
27. DiMatteo MR, Taranta A, Friedman HS, Prince LM. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387. PubMed
28. Frankel RM, Stein T. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16(4):184-191. PubMed
29. Krupat E, Frankel R, Stein T, Irish J. The Four Habits Coding Scheme: validation of an instrument to assess clinicians’ communication behavior. Patient Educ Couns. 2006;62(1):38-45. PubMed
30. Fossli Jensen B, Gulbrandsen P, Benth JS, Dahl FA, Krupat E, Finset A. Interrater reliability for the Four Habits Coding Scheme as part of a randomized controlled trial. Patient Educ Couns. 2010;80(3):405-409. PubMed
31. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. PubMed
32. Anonymous. CMS continues to shift emphasis to quality of care. Hosp Case Manag. 2012;20(10):150-151. PubMed
33. The R.E.D.E. Model 2015. Cleveland Clinic Center for Excellence in Healthcare
Communication. http://healthcarecommunication.info/. Accessed April 3 2016.
34. Empathetics, Inc. A Boston-based empathy training firm raises $1.5 million in
Series A Financing 2015. Empathetics Inc. http://www.prnewswire.com/news-releases/
empathetics-inc----a-boston-based-empathy-training-firm-raises-15-million-
in-series-a-financing-300072696.html). Accessed April 3, 2016.
35. Intensive Communication Skills 2016. Institute for Healthcare Communication.
http://healthcarecomm.org/. Accessed April 3, 2016.
36. Hu B, Palta M, Shao J. Variability explained by covariates in linear mixed-effect
models for longitudinal data. Canadian Journal of Statistics. 2010;38:352-368.
37. O’Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case-mix adjustment
of the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2162-2181. PubMed
38. O’Malley AJ, Zaslavsky AM, Hays RD, Hepner KA, Keller S, Cleary PD. Exploratory
factor analyses of the CAHPS Hospital Pilot Survey responses across
and within medical, surgical, and obstetric services. Health Serv Res. 2005;40(6 pt
2):2078-2095. PubMed
39. Goldstein E, Farquhar M, Crofton C, Darby C, Garfinkel S. Measuring hospital
care from the patients’ perspective: an overview of the CAHPS Hospital Survey
development process. Health Serv Res. 2005;40(6 pt 2):1977-1995. PubMed
40. Darby C, Hays RD, Kletke P. Development and evaluation of the CAHPS hospital
survey. Health Serv Res. 2005;40(6 pt 2):1973-1976. PubMed
41. Keller VF, Carroll JG. A new model for physician-patient communication. Patient
Educ Couns. 1994;23(2):131-140. PubMed
42. Quirk M, Mazor K, Haley HL, et al. How patients perceive a doctor’s caring attitude.
Patient Educ Couns. 2008;72(3):359-366. PubMed
43. Frankel RM, Levinson W. Back to the future: Can conversation analysis be used
to judge physicians’ malpractice history? Commun Med. 2014;11(1):27-39. PubMed
44. Furnham A. Response bias, social desirability and dissimulation. Personality and
individual differences 1986;7(3):385-400.
45. Shteingart H, Neiman T, Loewenstein Y. The role of first impression in operant
learning. J Exp Psychol Gen. 2013;142(2):476-488. PubMed
46. Tefera L, Lehrman WG, Conway P. Measurement of the patient experience: clarifying
facts, myths, and approaches. JAMA. 2016;315(2):2167-2168. PubMed
47. Horowitz CR, Suchman AL, Branch WT Jr, Frankel RM. What do doctors find
meaningful about their work? Ann Intern Med. 2003;138(9):772-775. PubMed
48. Rao JK, Anderson LA, Inui TS, Frankel RM. Communication interventions make
a difference in conversations between physicians and patients: a systematic review
of the evidence. Med Care. 2007;45(4):340-349. PubMed
Communication is the foundation of medical care.1 Effective communication can improve health outcomes, safety, adherence, satisfaction, trust, and enable genuine informed consent and decision-making.2-9 Furthermore, high-quality communication increases provider engagement and workplace satisfaction, while reducing stress and malpractice risk.10-15
Direct measurement of communication in the healthcare setting can be challenging. The “Four Habits Model,” which is derived from a synthesis of empiric studies8,16-20 and theoretical models21-24 of communication, offers 1 framework for assessing healthcare communication. The conceptual model underlying the 4 habits has been validated in studies of physician and patient satisfaction.1,4,25-27 The 4 habits are: investing in the beginning, eliciting the patient’s perspective, demonstrating empathy, and investing in the end. Each habit is divided into several identifiable tasks or skill sets, which can be reliably measured using validated tools and checklists.28 One such instrument, the Four Habits Coding Scheme (4HCS), has been evaluated against other tools and demonstrated overall satisfactory inter-rater reliability and validity.29,30
The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, developed under the direction of the Centers for Medicare and Medicaid Services (CMS) and the Agency for Healthcare Research and Quality, is an established national standard for measuring patient perceptions of care. HCAHPS retrospectively measures global perceptions of communication, support and empathy from physicians and staff, processes of care, and the overall patient experience. HCAHPS scores were first collected nationally in 2006 and have been publicly reported since 2008.31 With the introduction of value-based purchasing in 2012, health system revenues are now tied to HCAHPS survey performance.32 As a result, hospitals are financially motivated to improve HCAHPS scores but lack evidence-based methods for doing so. Some healthcare organizations have invested in communication training programs based on the available literature and best practices.2,33-35 However, it is not known how, if at all, HCAHPS scores relate to physicians’ real-time observed communication skills.
To examine the relationship between physician communication, as reported by global HCAHPS scores, and the quality of physician communication skills in specific encounters, we observed hospitalist physicians during inpatient bedside rounds and measured their communication skills using the 4HCS.
METHODS
Study Design
The study utilized a cross sectional design; physicians who consented were observed on rounds during 3 separate encounters, and we compared hospitalists’ 4HCS scores to their HCAHPS scores to assess the correlation. The study was approved by the Institutional Review Board of the Cleveland Clinic.
Population
The study was conducted at the main campus of the Cleveland Clinic. All physicians specializing in hospital medicine who had received 10 or more completed HCAHPS survey responses while rounding on a medicine service in the past year were invited to participate in the study. Participation was voluntary; night hospitalists were excluded. A research nurse was trained in the Four Habits Model28 and in the use of the 4HCS coding scheme by the principal investigator. The nurse observed each physician and ascertained the presence of communication behaviors using the 4HCS tool. Physicians were observed between August 2013 and August 2014. Multiple observations per physician could occur on the same day, but only 1 observation per patient was used for analysis. Observations consisted of a physician’s first encounter with a hospitalized patient, with the patient’s consent. Observations were conducted during encounters with English-speaking and cognitively intact patients only. Resident physicians were permitted to stay and conduct rounds per their normal routine. Patient information was not collected as part of the study.
Measures
HCAHPS. For each physician, we extracted all HCAHPS scores that were collected from our hospital’s Press Ganey database. The HCAHPS survey contains 22 core questions divided into 7 themes or domains, 1 of which is doctor communication. The survey uses frequency-based questions with possible answers fixed on a 4-point scale (4=always, 3=usually, 2=sometimes, 1=never). Our primary outcome was the doctor communication domain, which comprises 3 questions: 1) During this hospital stay, how often did the doctors treat you with respect? 2) During this hospital stay, how often did the doctors listen to you? and 3) During this hospital stay, how often did the doctors explain things in a language you can understand? Because CMS counts only the percentage of responses that are graded “always,” so-called “top box” scoring, we used the same measure.
The HCAHPS scores are always attributed to the physician at the time of discharge even if he may not have been responsible for the care of the patient during the entire hospital course. To mitigate contamination from patients seen by multiple providers, we cross-matched length of stay (LOS) data with billing data to determine the proportion of days a patient was seen by a single provider during the entire length of stay. We stratified patients seen by the attending providers to less than 50%, 50% to less than 100%, and at 100% of the LOS. However, we were unable to identify which patients were seen by other consultants or by residents due to limitations in data gathering and the nature of the database.
The Four Habits. The Four Habits are: invest in the beginning, elicit the patient’s perspective, demonstrate empathy, and invest in the end (Figure 1). Specific behaviors for Habits 1 to 4 are outlined in the Appendix, but we will briefly describe the themes as follows. Habit 1, invest in the beginning, describes the ability of the physician to set a welcoming environment for the patient, establish rapport, and collaborate on an agenda for the visit. Habit 2, elicit the patient’s perspective, describes the ability of the physician to explore the patients’ worries, ideas, expectations, and the impact of the illness on their lifestyle. Habit 3, demonstrate empathy, describes the physician’s openness to the patient’s emotions as well as the ability to explore, validate; express curiosity, and openly accept these feelings. Habit 4, invest in the end, is a measure of the physician’s ability to counsel patients in a language built around their original concerns or worries, as well as the ability to check the patients’ understanding of the plan.2,29-30
4HCS. The 4HCS tool (Appendix) measures discreet behaviors and phrases based on each of the Four Habits (Figure 1). With a scoring range from a low of 4 to a high of 20, the rater at bedside assigns a range of points on a scale of 1 to 5 for each habit. It is an instrument based on a teaching model used widely throughout Kaiser Permanente to improve clinicians’ communication skills. The 4HCS was first tested for interrater reliability and validity against the Roter Interaction Analysis System using 100 videotaped primary care physician encounters.29 It was further evaluated in a randomized control trial. Videotapes from 497 hospital encounters involving 71 doctors from a variety of clinical specialties were rated by 4 trained raters using the coding scheme. The total score Pearson’s R and intraclass correlation coefficient (ICC) exceeded 0.70 for all pairs of raters, and the interrater reliability was satisfactory for the 4HCS as applied to heterogeneous material.30
STATISTICAL ANALYSIS
Physician characteristics were summarized with standard descriptive statistics. Pearson correlation coefficients were computed between HCAHPS and 4HCS scores. All analyses were performed with RStudio (Boston, MA). The Pearson correlation between the averaged HCAHPS and 4HCS scores was also computed. A correlation with a P value less than 0.05 was considered statistically significant. With 28 physicians, the study had a power of 88% to detect a moderate correlation (greater than 0.50) with a 2-sided alpha of 0.05. We also computed the correlations based on the subgroups of data with patients seen by providers for less than 50%, 50% to less than 100%, and 100% of LOS. All analyses were conducted in SAS 9.2 (SAS Institute Inc., Cary, NC).36
RESULTS
There were 31 physicians who met our inclusion criteria. Of 29 volunteers, 28 were observed during 3 separate inpatient encounters and made up the final sample. A total of 1003 HCAHPS survey responses were available for these physicians. Participants were predominantly female (60.7%), with an average age of 39 years. They were in practice for an average of 4 years (12 were in practice more than 5 years), and 9 were observed on a teaching rotation.
The means of the overall 4HCS scores per observation were 17.39 ± 2.33 for the first, 17.00 ± 2.37 for the second, and 17.43 ± 2.36 for third bedside observation. The mean 4HCS scores per observation, broken down by habit, appear in Table 1. The ICC among the repeated scores within the same physician was 0.81. The median number of HCAHPS survey returns was 32 (range = [8, 85], with mean = 35.8, interquartile range = [16, 54]). The median overall HCAHPS doctor communication score was 89.6 (range = 80.9-93.7). Participants scored the highest in the respect subdomain and the lowest in the explain subdomain. Median HCAHPS scores and ranges appear in Table 2.
Because there were no significant associations between 4HCS scores or HCAHPS scores and physician age, sex, years in practice, or teaching site, correlations were not adjusted. Figure 2A and 2B show the association between mean 4HCS scores and HCAHPS scores by physician. There was no significant correlation between overall 4HCS and HCAHPS doctor communication scores (Pearson correlation coefficient 0.098; 95% confidence interval [CI], -0.285, 0.455). The individual habits also were not correlated with overall HCAHPS scores or with their corresponding HCAHPS domain (Table 3).
For 325 patients, 1 hospitalist was present for the entire LOS. In sensitivity analysis limiting observations to these patients (Figure 2C, Figure 2D, Table 3), we found a moderate correlation between habit 3 and the HCAHPS respect score (Pearson correlation coefficient 0.515; 95% CI, 0.176, 0.745; P = 0.005), and a weaker correlation between habit 3 and the HCAHPS overall doctor communication score (0.442; 95% CI, 0.082, 0.7; P = 0.019). There were no other significant correlations between specific habits and HCAHPS scores.
DISCUSSION
In this observational study of hospitalist physicians at a large tertiary care center, we found that communication skills, as measured by the 4HCS, varied substantially among physicians but were highly correlated within patients of the same physician. However, there was virtually no correlation between the attending physician of record’s 4HCS scores and their HCAHPS communication scores. When we limited our analysis to patients who saw only 1 hospitalist throughout their stay, there were moderate correlations between demonstration of empathy and both the HCAHPS respect score and overall doctor communication score. There were no trends across the strata of hospitalist involvement. It is important to note that the addition of even 1 different hospitalist to the LOS removes any association. Habits 1 and 2 are close to significance in the 100% subgroup, with a weak correlation. Interestingly, Habit 4, which focuses on creating a plan with the patient, showed no correlation at all with patients reporting that doctors explained things in language they could understand.
Development and testing of the HCAHPS survey began in 2002, commissioned by CMS and the Agency for Healthcare Research and Quality for the purpose of measuring patient experience in the hospital. The HCAHPS survey was endorsed by the National Quality Forum in 2005, with final approval of the national implementation granted by the Office of Management and Budget later that year. The CMS began implementation of the HCAHPS survey in 2006, with the first required public reporting of all hospitals taking place in March 2008.37-41 Based on CMS’ value-based purchasing initiative, hospitals with low HCAHPS scores have faced substantial penalties since 2012. Under these circumstances, it is important that the HCAHPS measures what it purports to measure. Because HCAHPS was designed to compare hospitals, testing was limited to assessment of internal reliability, hospital-level reliability, and construct validity. External validation with known measures of physician communication was not performed.41 Our study appears to be the first to compare HCAHPS scores to directly observed measures of physician communication skills. The lack of association between the 2 should sound a cautionary note to hospitals who seek to tie individual compensation to HCAHPS scores to improve them. In particular, the survey asks for a rating for all the patient’s doctors, not just the primary hospitalist. We found that, for hospital stays with just 1 hospitalist, the HCAHPS score reflected observed expression of empathy, although the correlation was only moderate, and HCAHPS were not correlated with other communication skills. Of all communication skills, empathy may be most important. Almost the entire body of research on physician communication cites empathy as a central skill. Empathy improves patient outcomes1-9,13-14,16-18,42 such as adherence to treatment, loyalty, and perception of care; and provider outcomes10-12,15 such as reduced burnout and a decreased likelihood of malpractice litigation.
It is less clear why other communication skills did not correlate with HCAHPS, but several differences in the measures themselves and how they were obtained might be responsible. It is possible that HCAHPS measures something broader than physician communication. In addition, the 4HCS was developed and normed on outpatient encounters as is true for virtually all doctor-patient coding schemes.43 Little is known about inpatient communication best practices. The timing of HCAHPS may also degrade the relationship between observed and reported communication. The HCAHPS questionnaires, collected after discharge, are retrospective reconstructions that are subject to recall bias and recency effects.44,45 In contrast, our observations took place in real time and were specific to the face-to-face interactions that take place when physicians engage patients at the bedside. Third, the response rate for HCAHPS surveys is only 30%, leading to potential sample bias.46 Respondents represent discharged patients who are willing and able to answer surveys, and may not be representative of all hospitalized patients. Finally, as with all global questions, the meaning any individual patient assigns to terms like “respect” may vary.
Our study has several limitations. The HCAHPS and 4HCS scores were not obtained from the same sample of patients. It is possible that the patients who were observed were not representative of the patients who completed the HCAHPS surveys. In addition, the only type of encounter observed was the initial visit between the hospitalist and the patient, and did not include communication during follow-up visits or on the day of discharge. However, there was a strong ICC among the 4HCS scores, implying that the 4HCS measures an inherent physician skill, which should be consistent across patients and encounters. Coding bias of the habits by a single observer could not be excluded. High intra-class correlation could be due in part to observer preferences for particular communication styles. Our sample included only 28 physicians. Although our study was powered to rule out a moderate correlation between 4HCS scores and HCAHPS scores (Pearson correlation coefficient greater than 0.5), we cannot exclude weaker correlations. Most correlations that we observed were so small that they would not be clinically meaningful, even in a much larger sample.
CONCLUSIONS
Our findings that HCAHPS scores did not correlate with the communication skills of the attending of record have some important implications. In an environment of value-based purchasing, most hospital systems are interested in identifying modifiable provider behaviors that optimize efficiency and payment structures. This study shows that directly measured communication skills do not correlate with HCAHPS scores as generally reported, indicating that HCAHPS may be measuring a broader domain than only physician communication skills. Better attribution based on the proportion of care provided by an individual physician could make the scores more useful for individual comparisons, but most institutions do not report their data in this way. Given this limitation, hospitals should refrain from comparing and incentivizing individual physicians based on their HCAHPS scores, because this measure was not designed for this purpose and does not appear to reflect an individual’s skills. This is important in the current environment in which hospitals face substantial penalties for underperformance but lack specific tools to improve their scores. Furthermore, there is concern that this type of measurement creates perverse incentives that may adversely alter clinical practice with the aim of improving scores.46
Training clinicians in communication and teaming skills is one potential means of increasing overall scores.15 Improving doctor-patient and team relationships is also the right thing to do. It is increasingly being demanded by patients and has always been a deep source of satisfaction for physicians.15,47 Moreover, there is an increasingly robust literature that relates face-to-face communication to biomedical and psychosocial outcomes of care.48 Identifying individual physicians who need help with communication skills is a worthwhile goal. Unfortunately, the HCAHPS survey does not appear to be the appropriate tool for this purpose.
Disclosure
The Cleveland Clinic Foundation, Division of Clinical Research, Research Programs Committees provided funding support. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors have no conflicts of interest for this study.
Communication is the foundation of medical care.1 Effective communication can improve health outcomes, safety, adherence, satisfaction, trust, and enable genuine informed consent and decision-making.2-9 Furthermore, high-quality communication increases provider engagement and workplace satisfaction, while reducing stress and malpractice risk.10-15
Direct measurement of communication in the healthcare setting can be challenging. The “Four Habits Model,” which is derived from a synthesis of empiric studies8,16-20 and theoretical models21-24 of communication, offers 1 framework for assessing healthcare communication. The conceptual model underlying the 4 habits has been validated in studies of physician and patient satisfaction.1,4,25-27 The 4 habits are: investing in the beginning, eliciting the patient’s perspective, demonstrating empathy, and investing in the end. Each habit is divided into several identifiable tasks or skill sets, which can be reliably measured using validated tools and checklists.28 One such instrument, the Four Habits Coding Scheme (4HCS), has been evaluated against other tools and demonstrated overall satisfactory inter-rater reliability and validity.29,30
The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, developed under the direction of the Centers for Medicare and Medicaid Services (CMS) and the Agency for Healthcare Research and Quality, is an established national standard for measuring patient perceptions of care. HCAHPS retrospectively measures global perceptions of communication, support and empathy from physicians and staff, processes of care, and the overall patient experience. HCAHPS scores were first collected nationally in 2006 and have been publicly reported since 2008.31 With the introduction of value-based purchasing in 2012, health system revenues are now tied to HCAHPS survey performance.32 As a result, hospitals are financially motivated to improve HCAHPS scores but lack evidence-based methods for doing so. Some healthcare organizations have invested in communication training programs based on the available literature and best practices.2,33-35 However, it is not known how, if at all, HCAHPS scores relate to physicians’ real-time observed communication skills.
To examine the relationship between physician communication, as reported by global HCAHPS scores, and the quality of physician communication skills in specific encounters, we observed hospitalist physicians during inpatient bedside rounds and measured their communication skills using the 4HCS.
METHODS
Study Design
The study utilized a cross sectional design; physicians who consented were observed on rounds during 3 separate encounters, and we compared hospitalists’ 4HCS scores to their HCAHPS scores to assess the correlation. The study was approved by the Institutional Review Board of the Cleveland Clinic.
Population
The study was conducted at the main campus of the Cleveland Clinic. All physicians specializing in hospital medicine who had received 10 or more completed HCAHPS survey responses while rounding on a medicine service in the past year were invited to participate in the study. Participation was voluntary; night hospitalists were excluded. A research nurse was trained in the Four Habits Model28 and in the use of the 4HCS coding scheme by the principal investigator. The nurse observed each physician and ascertained the presence of communication behaviors using the 4HCS tool. Physicians were observed between August 2013 and August 2014. Multiple observations per physician could occur on the same day, but only 1 observation per patient was used for analysis. Observations consisted of a physician’s first encounter with a hospitalized patient, with the patient’s consent. Observations were conducted during encounters with English-speaking and cognitively intact patients only. Resident physicians were permitted to stay and conduct rounds per their normal routine. Patient information was not collected as part of the study.
Measures
HCAHPS. For each physician, we extracted all HCAHPS scores that were collected from our hospital’s Press Ganey database. The HCAHPS survey contains 22 core questions divided into 7 themes or domains, 1 of which is doctor communication. The survey uses frequency-based questions with possible answers fixed on a 4-point scale (4=always, 3=usually, 2=sometimes, 1=never). Our primary outcome was the doctor communication domain, which comprises 3 questions: 1) During this hospital stay, how often did the doctors treat you with respect? 2) During this hospital stay, how often did the doctors listen to you? and 3) During this hospital stay, how often did the doctors explain things in a language you can understand? Because CMS counts only the percentage of responses that are graded “always,” so-called “top box” scoring, we used the same measure.
The HCAHPS scores are always attributed to the physician at the time of discharge even if he may not have been responsible for the care of the patient during the entire hospital course. To mitigate contamination from patients seen by multiple providers, we cross-matched length of stay (LOS) data with billing data to determine the proportion of days a patient was seen by a single provider during the entire length of stay. We stratified patients seen by the attending providers to less than 50%, 50% to less than 100%, and at 100% of the LOS. However, we were unable to identify which patients were seen by other consultants or by residents due to limitations in data gathering and the nature of the database.
The Four Habits. The Four Habits are: invest in the beginning, elicit the patient’s perspective, demonstrate empathy, and invest in the end (Figure 1). Specific behaviors for Habits 1 to 4 are outlined in the Appendix, but we will briefly describe the themes as follows. Habit 1, invest in the beginning, describes the ability of the physician to set a welcoming environment for the patient, establish rapport, and collaborate on an agenda for the visit. Habit 2, elicit the patient’s perspective, describes the ability of the physician to explore the patients’ worries, ideas, expectations, and the impact of the illness on their lifestyle. Habit 3, demonstrate empathy, describes the physician’s openness to the patient’s emotions as well as the ability to explore, validate; express curiosity, and openly accept these feelings. Habit 4, invest in the end, is a measure of the physician’s ability to counsel patients in a language built around their original concerns or worries, as well as the ability to check the patients’ understanding of the plan.2,29-30
4HCS. The 4HCS tool (Appendix) measures discreet behaviors and phrases based on each of the Four Habits (Figure 1). With a scoring range from a low of 4 to a high of 20, the rater at bedside assigns a range of points on a scale of 1 to 5 for each habit. It is an instrument based on a teaching model used widely throughout Kaiser Permanente to improve clinicians’ communication skills. The 4HCS was first tested for interrater reliability and validity against the Roter Interaction Analysis System using 100 videotaped primary care physician encounters.29 It was further evaluated in a randomized control trial. Videotapes from 497 hospital encounters involving 71 doctors from a variety of clinical specialties were rated by 4 trained raters using the coding scheme. The total score Pearson’s R and intraclass correlation coefficient (ICC) exceeded 0.70 for all pairs of raters, and the interrater reliability was satisfactory for the 4HCS as applied to heterogeneous material.30
STATISTICAL ANALYSIS
Physician characteristics were summarized with standard descriptive statistics. Pearson correlation coefficients were computed between HCAHPS and 4HCS scores. All analyses were performed with RStudio (Boston, MA). The Pearson correlation between the averaged HCAHPS and 4HCS scores was also computed. A correlation with a P value less than 0.05 was considered statistically significant. With 28 physicians, the study had a power of 88% to detect a moderate correlation (greater than 0.50) with a 2-sided alpha of 0.05. We also computed the correlations based on the subgroups of data with patients seen by providers for less than 50%, 50% to less than 100%, and 100% of LOS. All analyses were conducted in SAS 9.2 (SAS Institute Inc., Cary, NC).36
RESULTS
There were 31 physicians who met our inclusion criteria. Of 29 volunteers, 28 were observed during 3 separate inpatient encounters and made up the final sample. A total of 1003 HCAHPS survey responses were available for these physicians. Participants were predominantly female (60.7%), with an average age of 39 years. They were in practice for an average of 4 years (12 were in practice more than 5 years), and 9 were observed on a teaching rotation.
The means of the overall 4HCS scores per observation were 17.39 ± 2.33 for the first, 17.00 ± 2.37 for the second, and 17.43 ± 2.36 for third bedside observation. The mean 4HCS scores per observation, broken down by habit, appear in Table 1. The ICC among the repeated scores within the same physician was 0.81. The median number of HCAHPS survey returns was 32 (range = [8, 85], with mean = 35.8, interquartile range = [16, 54]). The median overall HCAHPS doctor communication score was 89.6 (range = 80.9-93.7). Participants scored the highest in the respect subdomain and the lowest in the explain subdomain. Median HCAHPS scores and ranges appear in Table 2.
Because there were no significant associations between 4HCS scores or HCAHPS scores and physician age, sex, years in practice, or teaching site, correlations were not adjusted. Figure 2A and 2B show the association between mean 4HCS scores and HCAHPS scores by physician. There was no significant correlation between overall 4HCS and HCAHPS doctor communication scores (Pearson correlation coefficient 0.098; 95% confidence interval [CI], -0.285, 0.455). The individual habits also were not correlated with overall HCAHPS scores or with their corresponding HCAHPS domain (Table 3).
For 325 patients, 1 hospitalist was present for the entire LOS. In sensitivity analysis limiting observations to these patients (Figure 2C, Figure 2D, Table 3), we found a moderate correlation between habit 3 and the HCAHPS respect score (Pearson correlation coefficient 0.515; 95% CI, 0.176, 0.745; P = 0.005), and a weaker correlation between habit 3 and the HCAHPS overall doctor communication score (0.442; 95% CI, 0.082, 0.7; P = 0.019). There were no other significant correlations between specific habits and HCAHPS scores.
DISCUSSION
In this observational study of hospitalist physicians at a large tertiary care center, we found that communication skills, as measured by the 4HCS, varied substantially among physicians but were highly correlated within patients of the same physician. However, there was virtually no correlation between the attending physician of record’s 4HCS scores and their HCAHPS communication scores. When we limited our analysis to patients who saw only 1 hospitalist throughout their stay, there were moderate correlations between demonstration of empathy and both the HCAHPS respect score and overall doctor communication score. There were no trends across the strata of hospitalist involvement. It is important to note that the addition of even 1 different hospitalist to the LOS removes any association. Habits 1 and 2 are close to significance in the 100% subgroup, with a weak correlation. Interestingly, Habit 4, which focuses on creating a plan with the patient, showed no correlation at all with patients reporting that doctors explained things in language they could understand.
Development and testing of the HCAHPS survey began in 2002, commissioned by CMS and the Agency for Healthcare Research and Quality for the purpose of measuring patient experience in the hospital. The HCAHPS survey was endorsed by the National Quality Forum in 2005, with final approval of the national implementation granted by the Office of Management and Budget later that year. The CMS began implementation of the HCAHPS survey in 2006, with the first required public reporting of all hospitals taking place in March 2008.37-41 Based on CMS’ value-based purchasing initiative, hospitals with low HCAHPS scores have faced substantial penalties since 2012. Under these circumstances, it is important that the HCAHPS measures what it purports to measure. Because HCAHPS was designed to compare hospitals, testing was limited to assessment of internal reliability, hospital-level reliability, and construct validity. External validation with known measures of physician communication was not performed.41 Our study appears to be the first to compare HCAHPS scores to directly observed measures of physician communication skills. The lack of association between the 2 should sound a cautionary note to hospitals who seek to tie individual compensation to HCAHPS scores to improve them. In particular, the survey asks for a rating for all the patient’s doctors, not just the primary hospitalist. We found that, for hospital stays with just 1 hospitalist, the HCAHPS score reflected observed expression of empathy, although the correlation was only moderate, and HCAHPS were not correlated with other communication skills. Of all communication skills, empathy may be most important. Almost the entire body of research on physician communication cites empathy as a central skill. Empathy improves patient outcomes1-9,13-14,16-18,42 such as adherence to treatment, loyalty, and perception of care; and provider outcomes10-12,15 such as reduced burnout and a decreased likelihood of malpractice litigation.
It is less clear why other communication skills did not correlate with HCAHPS, but several differences in the measures themselves and how they were obtained might be responsible. It is possible that HCAHPS measures something broader than physician communication. In addition, the 4HCS was developed and normed on outpatient encounters as is true for virtually all doctor-patient coding schemes.43 Little is known about inpatient communication best practices. The timing of HCAHPS may also degrade the relationship between observed and reported communication. The HCAHPS questionnaires, collected after discharge, are retrospective reconstructions that are subject to recall bias and recency effects.44,45 In contrast, our observations took place in real time and were specific to the face-to-face interactions that take place when physicians engage patients at the bedside. Third, the response rate for HCAHPS surveys is only 30%, leading to potential sample bias.46 Respondents represent discharged patients who are willing and able to answer surveys, and may not be representative of all hospitalized patients. Finally, as with all global questions, the meaning any individual patient assigns to terms like “respect” may vary.
Our study has several limitations. The HCAHPS and 4HCS scores were not obtained from the same sample of patients. It is possible that the patients who were observed were not representative of the patients who completed the HCAHPS surveys. In addition, the only type of encounter observed was the initial visit between the hospitalist and the patient, and did not include communication during follow-up visits or on the day of discharge. However, there was a strong ICC among the 4HCS scores, implying that the 4HCS measures an inherent physician skill, which should be consistent across patients and encounters. Coding bias of the habits by a single observer could not be excluded. High intra-class correlation could be due in part to observer preferences for particular communication styles. Our sample included only 28 physicians. Although our study was powered to rule out a moderate correlation between 4HCS scores and HCAHPS scores (Pearson correlation coefficient greater than 0.5), we cannot exclude weaker correlations. Most correlations that we observed were so small that they would not be clinically meaningful, even in a much larger sample.
CONCLUSIONS
Our findings that HCAHPS scores did not correlate with the communication skills of the attending of record have some important implications. In an environment of value-based purchasing, most hospital systems are interested in identifying modifiable provider behaviors that optimize efficiency and payment structures. This study shows that directly measured communication skills do not correlate with HCAHPS scores as generally reported, indicating that HCAHPS may be measuring a broader domain than only physician communication skills. Better attribution based on the proportion of care provided by an individual physician could make the scores more useful for individual comparisons, but most institutions do not report their data in this way. Given this limitation, hospitals should refrain from comparing and incentivizing individual physicians based on their HCAHPS scores, because this measure was not designed for this purpose and does not appear to reflect an individual’s skills. This is important in the current environment in which hospitals face substantial penalties for underperformance but lack specific tools to improve their scores. Furthermore, there is concern that this type of measurement creates perverse incentives that may adversely alter clinical practice with the aim of improving scores.46
Training clinicians in communication and teaming skills is one potential means of increasing overall scores.15 Improving doctor-patient and team relationships is also the right thing to do. It is increasingly being demanded by patients and has always been a deep source of satisfaction for physicians.15,47 Moreover, there is an increasingly robust literature that relates face-to-face communication to biomedical and psychosocial outcomes of care.48 Identifying individual physicians who need help with communication skills is a worthwhile goal. Unfortunately, the HCAHPS survey does not appear to be the appropriate tool for this purpose.
Disclosure
The Cleveland Clinic Foundation, Division of Clinical Research, Research Programs Committees provided funding support. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors have no conflicts of interest for this study.
1. Glass RM. The patient-physician relationship. JAMA focuses on the center of medicine. JAMA. 1996;275(2):147-148. PubMed
2. Stein T, Frankel RM, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns. 2005;58(1):4-12. PubMed
3. Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796-804. PubMed
4. Safran DG, Taira DA, Rogers WH, Kosinski M, Ware JE, Tarlov AR. Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213-220. PubMed
5. Like R, Zyzanski SJ. Patient satisfaction with the clinical encounter: social psychological determinants. Soc Sci Med. 1987;24(4):351-357. PubMed
6. Williams S, Weinman J, Dale J. Doctor-patient communication and patient satisfaction: a review. Fam Pract. 1998;15(5):480-492. PubMed
7. Ciechanowski P, Katon WJ. The interpersonal experience of health care through the eyes of patients with diabetes. Soc Sci Med. 2006;63(12):3067-3079. PubMed
8. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
9. Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med. 2011;86(3):359-364. PubMed
10. Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277(7):553-559. PubMed
11. Ambady N, Laplante D, Nguyen T, Rosenthal R, Chaumeton N, Levinson W. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9. PubMed
12. Weng HC, Hung CM, Liu YT, et al. Associations between emotional intelligence and doctor burnout, job satisfaction and patient satisfaction. Med Educ. 2011;45(8):835-842. PubMed
13. Mauksch LB, Dugdale DC, Dodson S, Epstein R. Relationship, communication, and efficiency in the medical encounter: creating a clinical model from a literature review. Arch Intern Med. 2008;168(13):1387-1395. PubMed
14. Suchman AL, Roter D, Green M, Lipkin M Jr. Physician satisfaction with primary care office visits. Collaborative Study Group of the American Academy on Physician and Patient. Med Care. 1993;31(12):1083-1092. PubMed
15. Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31(7):755-761. PubMed
16. Brody DS, Miller SM, Lerman CE, Smith DG, Lazaro CG, Blum MJ. The relationship between patients’ satisfaction with their physicians and perceptions about interventions they desired and received. Med Care. 1989;27(11):1027-1035. PubMed
17. Wasserman RC, Inui TS, Barriatua RD, Carter WB, Lippincott P. Pediatric clinicians’ support for parents makes a difference: an outcome-based analysis of clinician-parent interaction. Pediatrics. 1984;74(6):1047-1053. PubMed
18. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528. PubMed
19. Inui TS, Carter WB. Problems and prospects for health services research on provider-patient communication. Med Care. 1985;23(5):521-538. PubMed
20. Beckman H, Frankel R, Kihm J, Kulesza G, Geheb M. Measurement and improvement of humanistic skills in first-year trainees. J Gen Intern Med. 1990;5(1):42-45. PubMed
21. Keller S, O’Malley AJ, Hays RD, et al. Methods used to streamline the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2057-2077. PubMed
22. Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137(5):535-544. PubMed
23. Lazare A, Eisenthal S, Wasserman L. The customer approach to patienthood. Attending to patient requests in a walk-in clinic. Arch Gen Psychiatry. 1975;32(5):553-558. PubMed
24. Eisenthal S, Lazare A. Evaluation of the initial interview in a walk-in clinic. The clinician’s perspective on a “negotiated approach”. J Nerv Ment Dis. 1977;164(1):30-35. PubMed
25. Kravitz RL, Callahan EJ, Paterniti D, Antonius D, Dunham M, Lewis CE. Prevalence and sources of patients’ unmet expectations for care. Ann Intern Med. 1996;125(9):730-737. PubMed
26. Froehlich GW, Welch HG. Meeting walk-in patients’ expectations for testing. Effects on satisfaction. J Gen Intern Med. 1996;11(8):470-474. PubMed
27. DiMatteo MR, Taranta A, Friedman HS, Prince LM. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387. PubMed
28. Frankel RM, Stein T. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16(4):184-191. PubMed
29. Krupat E, Frankel R, Stein T, Irish J. The Four Habits Coding Scheme: validation of an instrument to assess clinicians’ communication behavior. Patient Educ Couns. 2006;62(1):38-45. PubMed
30. Fossli Jensen B, Gulbrandsen P, Benth JS, Dahl FA, Krupat E, Finset A. Interrater reliability for the Four Habits Coding Scheme as part of a randomized controlled trial. Patient Educ Couns. 2010;80(3):405-409. PubMed
31. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. PubMed
32. Anonymous. CMS continues to shift emphasis to quality of care. Hosp Case Manag. 2012;20(10):150-151. PubMed
33. The R.E.D.E. Model 2015. Cleveland Clinic Center for Excellence in Healthcare
Communication. http://healthcarecommunication.info/. Accessed April 3 2016.
34. Empathetics, Inc. A Boston-based empathy training firm raises $1.5 million in
Series A Financing 2015. Empathetics Inc. http://www.prnewswire.com/news-releases/
empathetics-inc----a-boston-based-empathy-training-firm-raises-15-million-
in-series-a-financing-300072696.html). Accessed April 3, 2016.
35. Intensive Communication Skills 2016. Institute for Healthcare Communication.
http://healthcarecomm.org/. Accessed April 3, 2016.
36. Hu B, Palta M, Shao J. Variability explained by covariates in linear mixed-effect
models for longitudinal data. Canadian Journal of Statistics. 2010;38:352-368.
37. O’Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case-mix adjustment
of the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2162-2181. PubMed
38. O’Malley AJ, Zaslavsky AM, Hays RD, Hepner KA, Keller S, Cleary PD. Exploratory
factor analyses of the CAHPS Hospital Pilot Survey responses across
and within medical, surgical, and obstetric services. Health Serv Res. 2005;40(6 pt
2):2078-2095. PubMed
39. Goldstein E, Farquhar M, Crofton C, Darby C, Garfinkel S. Measuring hospital
care from the patients’ perspective: an overview of the CAHPS Hospital Survey
development process. Health Serv Res. 2005;40(6 pt 2):1977-1995. PubMed
40. Darby C, Hays RD, Kletke P. Development and evaluation of the CAHPS hospital
survey. Health Serv Res. 2005;40(6 pt 2):1973-1976. PubMed
41. Keller VF, Carroll JG. A new model for physician-patient communication. Patient
Educ Couns. 1994;23(2):131-140. PubMed
42. Quirk M, Mazor K, Haley HL, et al. How patients perceive a doctor’s caring attitude.
Patient Educ Couns. 2008;72(3):359-366. PubMed
43. Frankel RM, Levinson W. Back to the future: Can conversation analysis be used
to judge physicians’ malpractice history? Commun Med. 2014;11(1):27-39. PubMed
44. Furnham A. Response bias, social desirability and dissimulation. Personality and
individual differences 1986;7(3):385-400.
45. Shteingart H, Neiman T, Loewenstein Y. The role of first impression in operant
learning. J Exp Psychol Gen. 2013;142(2):476-488. PubMed
46. Tefera L, Lehrman WG, Conway P. Measurement of the patient experience: clarifying
facts, myths, and approaches. JAMA. 2016;315(2):2167-2168. PubMed
47. Horowitz CR, Suchman AL, Branch WT Jr, Frankel RM. What do doctors find
meaningful about their work? Ann Intern Med. 2003;138(9):772-775. PubMed
48. Rao JK, Anderson LA, Inui TS, Frankel RM. Communication interventions make
a difference in conversations between physicians and patients: a systematic review
of the evidence. Med Care. 2007;45(4):340-349. PubMed
1. Glass RM. The patient-physician relationship. JAMA focuses on the center of medicine. JAMA. 1996;275(2):147-148. PubMed
2. Stein T, Frankel RM, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns. 2005;58(1):4-12. PubMed
3. Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796-804. PubMed
4. Safran DG, Taira DA, Rogers WH, Kosinski M, Ware JE, Tarlov AR. Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213-220. PubMed
5. Like R, Zyzanski SJ. Patient satisfaction with the clinical encounter: social psychological determinants. Soc Sci Med. 1987;24(4):351-357. PubMed
6. Williams S, Weinman J, Dale J. Doctor-patient communication and patient satisfaction: a review. Fam Pract. 1998;15(5):480-492. PubMed
7. Ciechanowski P, Katon WJ. The interpersonal experience of health care through the eyes of patients with diabetes. Soc Sci Med. 2006;63(12):3067-3079. PubMed
8. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
9. Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med. 2011;86(3):359-364. PubMed
10. Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277(7):553-559. PubMed
11. Ambady N, Laplante D, Nguyen T, Rosenthal R, Chaumeton N, Levinson W. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9. PubMed
12. Weng HC, Hung CM, Liu YT, et al. Associations between emotional intelligence and doctor burnout, job satisfaction and patient satisfaction. Med Educ. 2011;45(8):835-842. PubMed
13. Mauksch LB, Dugdale DC, Dodson S, Epstein R. Relationship, communication, and efficiency in the medical encounter: creating a clinical model from a literature review. Arch Intern Med. 2008;168(13):1387-1395. PubMed
14. Suchman AL, Roter D, Green M, Lipkin M Jr. Physician satisfaction with primary care office visits. Collaborative Study Group of the American Academy on Physician and Patient. Med Care. 1993;31(12):1083-1092. PubMed
15. Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31(7):755-761. PubMed
16. Brody DS, Miller SM, Lerman CE, Smith DG, Lazaro CG, Blum MJ. The relationship between patients’ satisfaction with their physicians and perceptions about interventions they desired and received. Med Care. 1989;27(11):1027-1035. PubMed
17. Wasserman RC, Inui TS, Barriatua RD, Carter WB, Lippincott P. Pediatric clinicians’ support for parents makes a difference: an outcome-based analysis of clinician-parent interaction. Pediatrics. 1984;74(6):1047-1053. PubMed
18. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528. PubMed
19. Inui TS, Carter WB. Problems and prospects for health services research on provider-patient communication. Med Care. 1985;23(5):521-538. PubMed
20. Beckman H, Frankel R, Kihm J, Kulesza G, Geheb M. Measurement and improvement of humanistic skills in first-year trainees. J Gen Intern Med. 1990;5(1):42-45. PubMed
21. Keller S, O’Malley AJ, Hays RD, et al. Methods used to streamline the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2057-2077. PubMed
22. Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137(5):535-544. PubMed
23. Lazare A, Eisenthal S, Wasserman L. The customer approach to patienthood. Attending to patient requests in a walk-in clinic. Arch Gen Psychiatry. 1975;32(5):553-558. PubMed
24. Eisenthal S, Lazare A. Evaluation of the initial interview in a walk-in clinic. The clinician’s perspective on a “negotiated approach”. J Nerv Ment Dis. 1977;164(1):30-35. PubMed
25. Kravitz RL, Callahan EJ, Paterniti D, Antonius D, Dunham M, Lewis CE. Prevalence and sources of patients’ unmet expectations for care. Ann Intern Med. 1996;125(9):730-737. PubMed
26. Froehlich GW, Welch HG. Meeting walk-in patients’ expectations for testing. Effects on satisfaction. J Gen Intern Med. 1996;11(8):470-474. PubMed
27. DiMatteo MR, Taranta A, Friedman HS, Prince LM. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387. PubMed
28. Frankel RM, Stein T. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16(4):184-191. PubMed
29. Krupat E, Frankel R, Stein T, Irish J. The Four Habits Coding Scheme: validation of an instrument to assess clinicians’ communication behavior. Patient Educ Couns. 2006;62(1):38-45. PubMed
30. Fossli Jensen B, Gulbrandsen P, Benth JS, Dahl FA, Krupat E, Finset A. Interrater reliability for the Four Habits Coding Scheme as part of a randomized controlled trial. Patient Educ Couns. 2010;80(3):405-409. PubMed
31. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. PubMed
32. Anonymous. CMS continues to shift emphasis to quality of care. Hosp Case Manag. 2012;20(10):150-151. PubMed
33. The R.E.D.E. Model 2015. Cleveland Clinic Center for Excellence in Healthcare
Communication. http://healthcarecommunication.info/. Accessed April 3 2016.
34. Empathetics, Inc. A Boston-based empathy training firm raises $1.5 million in
Series A Financing 2015. Empathetics Inc. http://www.prnewswire.com/news-releases/
empathetics-inc----a-boston-based-empathy-training-firm-raises-15-million-
in-series-a-financing-300072696.html). Accessed April 3, 2016.
35. Intensive Communication Skills 2016. Institute for Healthcare Communication.
http://healthcarecomm.org/. Accessed April 3, 2016.
36. Hu B, Palta M, Shao J. Variability explained by covariates in linear mixed-effect
models for longitudinal data. Canadian Journal of Statistics. 2010;38:352-368.
37. O’Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case-mix adjustment
of the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2162-2181. PubMed
38. O’Malley AJ, Zaslavsky AM, Hays RD, Hepner KA, Keller S, Cleary PD. Exploratory
factor analyses of the CAHPS Hospital Pilot Survey responses across
and within medical, surgical, and obstetric services. Health Serv Res. 2005;40(6 pt
2):2078-2095. PubMed
39. Goldstein E, Farquhar M, Crofton C, Darby C, Garfinkel S. Measuring hospital
care from the patients’ perspective: an overview of the CAHPS Hospital Survey
development process. Health Serv Res. 2005;40(6 pt 2):1977-1995. PubMed
40. Darby C, Hays RD, Kletke P. Development and evaluation of the CAHPS hospital
survey. Health Serv Res. 2005;40(6 pt 2):1973-1976. PubMed
41. Keller VF, Carroll JG. A new model for physician-patient communication. Patient
Educ Couns. 1994;23(2):131-140. PubMed
42. Quirk M, Mazor K, Haley HL, et al. How patients perceive a doctor’s caring attitude.
Patient Educ Couns. 2008;72(3):359-366. PubMed
43. Frankel RM, Levinson W. Back to the future: Can conversation analysis be used
to judge physicians’ malpractice history? Commun Med. 2014;11(1):27-39. PubMed
44. Furnham A. Response bias, social desirability and dissimulation. Personality and
individual differences 1986;7(3):385-400.
45. Shteingart H, Neiman T, Loewenstein Y. The role of first impression in operant
learning. J Exp Psychol Gen. 2013;142(2):476-488. PubMed
46. Tefera L, Lehrman WG, Conway P. Measurement of the patient experience: clarifying
facts, myths, and approaches. JAMA. 2016;315(2):2167-2168. PubMed
47. Horowitz CR, Suchman AL, Branch WT Jr, Frankel RM. What do doctors find
meaningful about their work? Ann Intern Med. 2003;138(9):772-775. PubMed
48. Rao JK, Anderson LA, Inui TS, Frankel RM. Communication interventions make
a difference in conversations between physicians and patients: a systematic review
of the evidence. Med Care. 2007;45(4):340-349. PubMed
© 2017 Society of Hospital Medicine
Association between opioid and benzodiazepine use and clinical deterioration in ward patients
Chronic opioid and benzodiazepine use is common and increasing.1-5 Outpatient use of these medications has been associated with hospital readmission and death,6-12 with concurrent use associated with particularly increased risk.13,14 Less is known about outcomes for hospitalized patients receiving these medications.
More than half of hospital inpatients in the United States receive opioids,15 many of which are new prescriptions rather than continuation of chronic therapy.16,17 Less is known about inpatient benzodiazepine administration, but the prevalence may exceed 10% among elderly populations.18 Hospitalized patients often have comorbidities or physiological disturbances that might increase their risk related to use of these medications. Opioids can cause central and obstructive sleep apneas,19-21 and benzodiazepines contribute to respiratory depression and airway relaxation.22 Benzodiazepines also impair psychomotor function and recall,23 which could mediate the recognized risk for delirium and falls in the hospital.24,25 These findings suggest pathways by which these medications might contribute to clinical deterioration.
Most studies in hospitalized patients have been limited to specific populations15,26-28 and have not explicitly controlled for severity of illness over time. It remains unclear whether associations identified within particular groups of patients hold true for the broader population of general ward inpatients. Therefore, we aimed to determine the independent association between opioid and benzodiazepine administration and clinical deterioration in ward patients.
MATERIALS AND METHODS
Setting and Study Population
We performed an observational cohort study at a 500-bed urban academic hospital. Data were obtained from all adults hospitalized on the wards between November 1, 2008, and January 21, 2016. The study protocol was approved by the University of Chicago Institutional Review Board (IRB#15-0195).
Data Collection
The study utilized de-identified data from the electronic health record (EHR; Epic Systems Corporation, Verona, Wisconsin) and administrative databases collected by the University of Chicago Clinical Research Data Warehouse. Patient age, sex, race, body mass index (BMI), and ward admission source (ie, emergency department (ED), transferred from the intensive care unit (ICU), or directly admitted to the wards) were collected. International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes were used to identify Elixhauser Comorbidity Index categories.29,30 Because patients with similar diagnoses (eg, active cancer) are cohorted within particular areas in our hospital, we obtained the ward unit for all patients. Patients who underwent surgery were identified using the hospital’s admission-transfer-discharge database.
To determine severity of illness, routinely collected vital signs and laboratory values were utilized to calculate the electronic cardiac arrest risk triage (eCART) score, an accurate risk score we previously developed and validated for predicting adverse events among ward patients.31 If any vital sign or laboratory value was missing, the next available measurement was carried forward. If any value remained missing after this change, the median value for that location (ie, wards, ICU, or ED) was imputed.32,33 Additionally, patient-reported pain scores at the time of opioid administration were extracted from nursing flowsheets. If no pain score was present at the time of opioid administration, the patient’s previous score was carried forward.
We excluded patients with sickle-cell disease or seizure history and admissions with diagnoses of alcohol withdrawal from the analysis, because these diagnoses were expected to be associated with different medication administration practices compared to other inpatients. We also excluded patients with a tracheostomy because we expected their respiratory monitoring to differ from the other patients in our cohort. Finally, because ward deaths resulting from a comfort care scenario often involve opioids and/or benzodiazepines, ward segments involving comfort care deaths (defined as death without attempted resuscitation) were excluded from the analysis (Supplemental Figure 1). Patients with sickle-cell disease were identified using ICD-9 codes, and encounters during which a seizure may have occurred were identified using a combination of ICD-9 codes and receipt of anti-epileptic medication (Supplemental Table 1). Patients at risk for alcohol withdrawal were identified by the presence of any Clinical Institute Withdrawal Assessment for Alcohol score within nursing flowsheets, and patients with tracheostomies were identified using documentation of ventilator support within their first 12 hours on the wards. In addition to these exclusion criteria, patients with obstructive sleep apnea (OSA) were identified by the following ICD-9 codes: 278.03, 327.23, 780.51, 780.53, and 780.57.
Medications
Ward administrations of opioids and benzodiazepines—dose, route, and administration time—were collected from the EHR. We excluded all administrations in nonward locations such as the ED, ICU, operating room, or procedure suite. Additionally, because patients emergently intubated may receive sedative and analgesic medications to facilitate intubation, and because patients experiencing cardiac arrest are frequently intubated periresuscitation, we a priori excluded all administrations within 15 minutes of a ward cardiac arrest or an intubation.
For consistent comparisons, opioid doses were converted to oral morphine equivalents34 and adjusted by a factor of 15 to reflect the smallest routinely available oral morphine tablet in our hospital (Supplemental Table 2). Benzodiazepine doses were converted to oral lorazepam equivalents (Supplemental Table 2).34 Thus, the independent variables were oral morphine or lorazepam equivalents administered within each 6-hour window. We a priori presumed opioid doses greater than the 99th percentile (1200 mg) or benzodiazepine doses greater than 10 mg oral lorazepam equivalents within a 6-hour window to be erroneous entries, and replaced these outlier values with the median value for each medication category.
Outcomes
The primary outcome was the composite of ICU transfer or cardiac arrest (loss of pulse with attempted resuscitation) on the wards, with individual outcomes investigated secondarily. An ICU transfer (patient movement from a ward directly to the ICU) was identified using the hospital’s admission-transfer-discharge database. Cardiac arrests were identified using a prospectively validated quality improvement database.35
Because deaths on the wards resulted either from cardiac arrest or from a comfort care scenario, mortality was not studied as an outcome.
Statistical Analysis
Patient characteristics were compared using Student t tests, Wilcoxon rank sum tests, and chi-squared statistics, as appropriate. Unadjusted and adjusted models were created using discrete-time survival analysis,36-39 which involved dividing time into discrete 6-hour intervals and employing the predictor variables chronologically closest to the beginning of each time window to forecast whether the outcome occurred within each interval. Predictor variables in the adjusted model included patient characteristics (age, sex, BMI, and Elixhauser Agency for Healthcare Research and Quality-Web comorbidities30 [a priori excluding comorbidities recorded for fewer than 1000 admissions from the model]), ward unit, surgical status, prior ICU admission during the hospitalization, cumulative opioid or benzodiazepine dose during the previous 24 hours, and severity of illness (measured by eCART score). The adjusted model for opioids also included the patient’s pain score. Age, eCART score, and pain score were entered linearly while race, BMI (underweight, less than 18.5 kg/m2; normal, 18.5-24.9 kg/m2; overweight, 25.0-29.9 kg/m2; obese, 30-39.9 kg/m2; and severely obese, 40 mg/m2 or greater), and ward unit were modeled as categorical variables.
Since repeat hospitalization could confound the results of our study, we performed a sensitivity analysis including only 1 randomly selected hospital admission per patient. We also performed a sensitivity analysis including receipt of both opioids and benzodiazepines, and an interaction term within each ward segment, as well as an analysis in which zolpidem—the most commonly administered nonbenzodiazepine hypnotic medication in our hospital—was included along with both opioids and benzodiazepines. Finally, we performed a sensitivity analysis replacing missing pain scores with imputed values ranging from 0 to the median ward pain score.
We also performed subgroup analyses of adjusted models across age quartiles and for each BMI category, as well as for surgical status, OSA status, gender, time of medication administration, and route of administration (intravenous vs. oral). We also performed an analysis across pain score severity40 to determine whether these medications produce differential effects at various levels of pain.
All tests of significance used a 2-sided P value less than 0.05. Statistical analyses were completed using Stata version 14.1 (StataCorp, LLC, College Station, Texas).
RESULTS
Patient Characteristics
A total of 144,895 admissions, from 75,369 patients, had ward vital signs or laboratory values documented during the study period. Ward segments from 634 admissions were excluded due to comfort care status, which resulted in exclusion of 479 complete patient admissions. Additionally, 139 patients with tracheostomies were excluded. Furthermore, 2934 patient admissions with a sickle-cell diagnosis were excluded, of which 95% (n = 2791) received an opioid and 11% (n = 310) received a benzodiazepine. Another 14,029 admissions associated with seizures, 6134 admissions involving alcohol withdrawal, and 1332 with both were excluded, of which 66% (n = 14,174) received an opioid and 35% (n = 7504) received a benzodiazepine. After exclusions, 120,518 admissions were included in the final analysis, with 67% (n = 80,463) associated with at least 1 administration of an opioid and 21% (n = 25,279) associated with at least 1 benzodiazepine administration.
In total, there were 672,851 intervals when an opioid was administered during the study, with a median dose of 12 mg oral morphine equivalents (interquartile range, 8-30). Of these, 21,634 doses were replaced due to outlier status outside the 99th percentile. Patients receiving opioids were younger (median age 56 vs 61 years), less likely to be African American (48% vs 59%), more likely to have undergone surgery (18% vs 6%), and less likely to have most noncancer medical comorbidities than those who never received an opioid (all P < 0.001) (Table 1).
Additionally, there were a total of 98,286 6-hour intervals in which a benzodiazepine was administered in the study, with a median dose of 1 mg oral lorazepam (interquartile range, 0.5-1). A total of 790 doses of benzodiazepines (less than 1%) were replaced due to outlier status. Patients who received benzodiazepines were more likely to be male (49% vs. 41%), less likely to be African-American, less likely to be obese or morbidly obese (33% vs. 39%), and more likely to have medical comorbidities compared to patients who never received a benzodiazepine (all P < 0.001) (Table 1).
The eCART scores were similar between all patient groups. The frequency of missing variables differed by data type, with vital signs rarely missing (all less than 1.1% except AVPU [10%]), followed by hematology labs (8%-9%), electrolytes and renal function results (12%-15%), and hepatic function tests (40%-45%). In addition to imputed data for missing vital signs and laboratory values, our model omitted human immunodeficiency virus/acquired immune deficiency syndrome and peptic ulcer disease from the adjusted models on the basis of fewer than 1000 admissions with these diagnoses listed.
Patient Outcomes
The incidence of the composite outcome was higher in admissions with at least 1 opioid medication than those without an opioid (7% vs. 4%, P < 0.001), and in admissions with at least 1 dose of benzodiazepines compared to those without a benzodiazepine (11% vs. 4%, P < 0.001) (Table 2).
Within 6-hour segments, increasing doses of opioids were associated with an initial decrease in the frequency of the composite outcome followed by a dose-related increase in the frequency of the composite outcome with morphine equivalents greater than 45 mg. By contrast, the frequency of the composite outcome increased with additional benzodiazepine equivalents (Figure).
In the adjusted model, opioid administration was associated with increased risk for the composite outcome (Table 3) in a dose-dependent fashion, with each 15 mg oral morphine equivalent associated with a 1.9% increase in the odds of ICU transfer or cardiac arrest within the subsequent 6-hour time interval (odds ratio [OR], 1.019; 95% confidence interval [CI], 1.013-1.026; P < 0.001).
Similarly, benzodiazepine administration was also associated with increased adjusted risk for the composite outcome within 6 hours in a dose-dependent manner. Each 1 mg oral lorazepam equivalent was associated with a 29% increase in the odds of ward cardiac arrest or ICU transfer (OR, 1.29; 95% CI, 1.16-1.44; P < 0.001) (Table 3).
Sensitivity Analyses
A sensitivity analysis including 1 randomly selected hospitalization per patient involved 67,097 admissions and found results similar to the primary analysis, with each 15 mg oral morphine equivalent associated with a 1.9% increase in the odds of the composite outcome (OR, 1.019; 95% CI, 1.011-1.028; P < 0.001) and each 1 mg oral lorazepam equivalent associated with a 41% increase in the odds of the composite outcome (OR, 1.41; 95% CI, 1.21-1.65; P < 0.001). Inclusion of both opioids and benzodiazepines in the adjusted model again yielded results similar to the main analysis for both opioids (OR, 1.020; 95% CI, 1.013-1.026; P < 0.001) and benzodiazepines (OR, 1.35; 95% CI, 1.18-1.54; P < 0.001), without a significant interaction detected (P = 0.09). These results were unchanged with the addition of zolpidem to the model as an additional potential confounder, and zolpidem did not increase the risk of the study outcomes (P = 0.2).
A final sensitivity analysis for the opioid model involved replacing missing pain scores with imputed values ranging from 0 to the median ward score, which was 5. The results of these analyses did not differ from the primary model and were consistent regardless of imputation value (OR, 1.018; 95% CI, 1.012-1.023; P < 0.001).
Subgroup Analyses
Analyses of opioid administration by subgroup (sex, age quartiles, BMI categories, OSA diagnosis, surgical status, daytime/nighttime medication administration, IV/PO administration, and pain severity) yielded similar results to the overall analysis (Supplemental Figure 2). Subgroup analysis of patients receiving benzodiazepines revealed similarly increased adjusted odds of the composite outcome across strata of gender, BMI, surgical status, and medication administration time (Supplemental Figure 3). Notably, patients older than 70 years who received a benzodiazepine were at 64% increased odds of the composite outcome (OR, 1.64; 95% CI, 1.30-2.08), compared to 2% to 38% increased risk for patients under 70 years. Finally, IV doses of benzodiazepines were associated with 48% increased odds for deterioration (OR, 1.48; 95% CI, 1.18-1.84; P = 0.001), compared to a nonsignificant 14% increase in the odds for PO doses (OR, 1.14; 95% CI, 0.99-1.31; P = 0.066).
DISCUSSION
In a large, single-center, observational study of ward inpatients, we found that opioid use was associated with a small but significant increased risk for clinical deterioration on the wards, with every 15 mg oral morphine equivalent increasing the odds of ICU transfer or cardiac arrest in the next 6 hours by 1.9%. Benzodiazepines were associated with a much higher risk: each equivalent of 1 mg of oral lorazepam increased the odds of ICU transfer or cardiac arrest by almost 30%. These results have important implications for care at the bedside of hospitalized ward patients and suggest the need for closer monitoring after receipt of these medications, particularly benzodiazepines.
Previous work has described negative effects of opioid medications among select inpatient populations. In surgical patients, opioids have been associated with hospital readmission, increased length of stay, and hospital mortality.26,28 More recently, Herzig et al.15 found more adverse events in nonsurgical ward patients within the hospitals prescribing opioids the most frequently. These studies may have been limited by the populations studied and the inability to control for confounders such as severity of illness and pain score. Our study expands these findings to a more generalizable population and shows that even after adjustment for potential confounders, such as severity of illness, pain score, and medication dose, opioids are associated with increased short-term risk of clinical deterioration.
By contrast, few studies have characterized the risks associated with benzodiazepine use among ward inpatients. Recently, Overdyk et al.27 found that inpatient use of opioids and sedatives was associated with increased risk for cardiac arrest and hospital death. However, this study included ICU patients, which may confound the results, as ICU patients often receive high doses of opioids or benzodiazepines to facilitate mechanical ventilation or other invasive procedures, while also having a particularly high risk of adverse outcomes like cardiac arrest and inhospital death.
Several mechanisms may explain the magnitude of effect seen with regard to benzodiazepines. First, benzodiazepines may directly produce clinical deterioration by decreased respiratory drive, diminished airway tone, or hemodynamic decompensation. It is possible that the broad spectrum of cardiorespiratory side effects of benzodiazepines—and potential unpredictability of these effects—increases the difficulty of observation and management for patients receiving them. This difficulty may be compounded with intravenous administration of benzodiazepines, which was associated with a higher risk for deterioration than oral doses in our cohort. Alternatively, benzodiazepines may contribute to clinical decompensation by masking signs of deterioration such as encephalopathy or vital sign instability like tachycardia or tachypnea that may be mistaken as anxiety. Notably, while our hospital has a nursing-driven protocol for monitoring patients receiving opioids (in which pain is serially assessed, leading to additional bedside observation), we do not have protocols for ward patients receiving benzodiazepines. Finally, although we found that orders for opioids and benzodiazepines were more common in white patients than African American patients, this finding may be due to differences in the types or number of medical comorbidities experienced by these patients.
Our study has several strengths, including the large number of admissions we included. Additionally, we included a broad range of medical and surgical ward admissions, which should increase the generalizability of our results. Further, our rates of ICU transfer are in line with data reported from other groups,41,42 which again may add to the generalizability of our findings. We also addressed many potential confounders by including patient characteristics, individual ward units, and (for opioids) pain score in our model, and by controlling for severity of illness with the eCART score, an accurate predictor of ICU transfer and ward cardiac arrest within our population.32,37 Finally, our robust methodology allowed us to include acute and cumulative medication doses, as well as time, in the model. By performing a discrete-time survival analysis, we were able to evaluate receipt of opioids and benzodiazepines—as well as risk for clinical deterioration—longitudinally, lending strength to our results.
Limitations of our study include its single-center cohort, which may reduce generalizability to other populations. Additionally, because we could not validate the accuracy of—or adherence to—outpatient medication lists, we were unable to identify chronic opioid or benzodiazepine users by these lists. However, patients chronically taking opioids or benzodiazepines would likely receive doses each hospital day; by including 24-hour cumulative doses in our model, we attempted to adjust for some portion of their chronic use. Also, because evaluation of delirium was not objectively recorded in our dataset, we were unable to evaluate the relationship between receipt of these medications and development of delirium, which is an important outcome for hospitalized patients. Finally, neither the diagnoses for which these medications were prescribed, nor the reason for ICU transfer, were present in our dataset, which leaves open the possibility of unmeasured confounding.
CONCLUSION
After adjustment for important confounders including severity of illness, medication dose, and time, opioids were associated with a slight increase in clinical deterioration on the wards, while benzodiazepines were associated with a much larger risk for deterioration. This finding raises concern about the safety of benzodiazepine use among ward patients and suggests that increased monitoring of patients receiving these medications may be warranted.
Acknowledgment
The authors thank Nicole Twu for administrative support.
Disclosure
Drs. Churpek and Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080). Dr. Churpek has received honoraria from Chest for invited speaking engagements. In addition, Dr. Edelson has received research support from Philips Healthcare (Andover, Massachusetts), research support from the American Heart Association (Dallas, Texas) and Laerdal Medical (Stavanger, Norway), and research support from Early Sense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, Illinois), which is developing products for risk stratification of hospitalized patients. Dr. Mokhlesi is supported by National Institutes of Health grant R01HL119161. Dr. Mokhlesi has served as a consultant to Philips/Respironics and has received research support from Philips/Respironics. Preliminary versions of these data were presented as a poster presentation at the 2016 meeting of the American Thoracic Society, May 17, 2016; San Francisco, California.
1. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
2. Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Public Health. 2016;106(4):686-688. PubMed
3. Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138(3):507-513. PubMed
4. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142. PubMed
5. Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002−2014. Am J Prev Med. 2016;51(2):151-160. PubMed
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. PubMed
7. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248. PubMed
8. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers---United States, 1999--2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492. PubMed
9. Lan TY, Zeng YF, Tang GJ, et al. The use of hypnotics and mortality - a population-based retrospective cohort study. PLoS One. 2015;10(12):e0145271. PubMed
10. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli P, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy: prior opioid use among veterans. J Hosp Med. 2014;9(2):82-87. PubMed
11. Palmaro A, Dupouy J, Lapeyre-Mestre M. Benzodiazepines and risk of death: results from two large cohort studies in France and UK. Eur Neuropsychopharmacol. 2015;25(10):1566-1577. PubMed
12. Parsaik AK, Mascarenhas SS, Khosh-Chashm D, et al. Mortality associated with anxiolytic and hypnotic drugs–a systematic review and meta-analysis. Aust N Z J Psychiatry. 2016;50(6):520-533. PubMed
13. Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. PubMed
14. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501. PubMed
15. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
16. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to Medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
17. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
18. Garrido MM, Prigerson HG, Penrod JD, Jones SC, Boockvar KS. Benzodiazepine and sedative-hypnotic use among older seriously ill veterans: choosing wisely? Clin Ther. 2014;36(11):1547-1554. PubMed
19. Doufas AG, Tian L, Padrez KA, et al. Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea. PloS One. 2013;8(1):e54807. PubMed
20. Gislason T, Almqvist M, Boman G, Lindholm CE, Terenius L. Increased CSF opioid activity in sleep apnea syndrome. Regression after successful treatment. Chest. 1989;96(2):250-254. PubMed
21. Van Ryswyk E, Antic N. Opioids and sleep disordered breathing. Chest. 2016;150(4):934-944. PubMed
22. Koga Y, Sato S, Sodeyama N, et al. Comparison of the relaxant effects of diazepam, flunitrazepam and midazolam on airway smooth muscle. Br J Anaesth. 1992;69(1):65-69. PubMed
23. Pomara N, Lee SH, Bruno D, et al. Adverse performance effects of acute lorazepam administration in elderly long-term users: pharmacokinetic and clinical predictors. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:129-135. PubMed
24. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21-26. PubMed
25. O’Neil CA, Krauss MJ, Bettale J, et al. Medications and patient characteristics associated with falling in the hospital. J Patient Saf. 2015 (epub ahead of print). PubMed
26. Kessler ER, Shah M, K Gruschkus S, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383-391. PubMed
27. Overdyk FJ, Dowling O, Marino J, et al. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11(2):e0150214. PubMed
28. Minkowitz HS, Gruschkus SK, Shah M, Raju A. Adverse drug events among patients receiving postsurgical opioids in a large health system: risk factors and outcomes. Am J Health Syst Pharm. 2014;71(18):1556-1565. PubMed
29. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
30. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed
31. Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190(6):649-655. PubMed
32. Knaus WA, Wagner DP, Draper EA, Z et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619-1636. PubMed
33. van den Boogaard M, Pickkers P, Slooter AJC, et al. Development and validation
of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction
model for intensive care patients: observational multicentre study. BMJ.
2012;344:e420. PubMed
34. Clinical calculators. ClinCalc.com. http://www.clincalc.com. Accessed February
21, 2016.
35. Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting
cardiac arrest on the wards: a nested case-control study. Chest. 2012;141(5):
1170-1176. PubMed
36. Churpek MM, Yuen TC, Park SY, Gibbons R, Edelson DP. Using electronic
health record data to develop and validate a prediction model for adverse outcomes
in the wards. Crit Care Med. 2014;42(4):841-848. PubMed
37. Efron B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J Am
Stat Assoc. 1988;83(402):414-425.
38. Gibbons RD, Duan N, Meltzer D, et al; Institute of Medicine Committee. Waiting
for organ transplantation: results of an analysis by an Institute of Medicine Committee.
Biostatistics. 2003;4(2):207-222. PubMed
39. Singer JD, Willett JB. It’s about time: using discrete-time survival analysis to study
duration and the timing of events. J Educ Behav Stat. 1993;18(2):155-195.
40. World Health Organization. Cancer pain relief and palliative care. Report of a
WHO Expert Committee. World Health Organ Tech Rep Ser. 1990;804:1-75. PubMed
41. Bailey TC, Chen Y, Mao Y, et al. A trial of a real-time alert for clinical deterioration
in patients hospitalized on general medical wards. J Hosp Med. 2013;8(5):236-242. PubMed
42. Liu V, Kipnis P, Rizk NW, Escobar GJ. Adverse outcomes associated with delayed
intensive care unit transfers in an integrated healthcare system. J Hosp Med.
2012;7(3):224-230. PubMed
Chronic opioid and benzodiazepine use is common and increasing.1-5 Outpatient use of these medications has been associated with hospital readmission and death,6-12 with concurrent use associated with particularly increased risk.13,14 Less is known about outcomes for hospitalized patients receiving these medications.
More than half of hospital inpatients in the United States receive opioids,15 many of which are new prescriptions rather than continuation of chronic therapy.16,17 Less is known about inpatient benzodiazepine administration, but the prevalence may exceed 10% among elderly populations.18 Hospitalized patients often have comorbidities or physiological disturbances that might increase their risk related to use of these medications. Opioids can cause central and obstructive sleep apneas,19-21 and benzodiazepines contribute to respiratory depression and airway relaxation.22 Benzodiazepines also impair psychomotor function and recall,23 which could mediate the recognized risk for delirium and falls in the hospital.24,25 These findings suggest pathways by which these medications might contribute to clinical deterioration.
Most studies in hospitalized patients have been limited to specific populations15,26-28 and have not explicitly controlled for severity of illness over time. It remains unclear whether associations identified within particular groups of patients hold true for the broader population of general ward inpatients. Therefore, we aimed to determine the independent association between opioid and benzodiazepine administration and clinical deterioration in ward patients.
MATERIALS AND METHODS
Setting and Study Population
We performed an observational cohort study at a 500-bed urban academic hospital. Data were obtained from all adults hospitalized on the wards between November 1, 2008, and January 21, 2016. The study protocol was approved by the University of Chicago Institutional Review Board (IRB#15-0195).
Data Collection
The study utilized de-identified data from the electronic health record (EHR; Epic Systems Corporation, Verona, Wisconsin) and administrative databases collected by the University of Chicago Clinical Research Data Warehouse. Patient age, sex, race, body mass index (BMI), and ward admission source (ie, emergency department (ED), transferred from the intensive care unit (ICU), or directly admitted to the wards) were collected. International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes were used to identify Elixhauser Comorbidity Index categories.29,30 Because patients with similar diagnoses (eg, active cancer) are cohorted within particular areas in our hospital, we obtained the ward unit for all patients. Patients who underwent surgery were identified using the hospital’s admission-transfer-discharge database.
To determine severity of illness, routinely collected vital signs and laboratory values were utilized to calculate the electronic cardiac arrest risk triage (eCART) score, an accurate risk score we previously developed and validated for predicting adverse events among ward patients.31 If any vital sign or laboratory value was missing, the next available measurement was carried forward. If any value remained missing after this change, the median value for that location (ie, wards, ICU, or ED) was imputed.32,33 Additionally, patient-reported pain scores at the time of opioid administration were extracted from nursing flowsheets. If no pain score was present at the time of opioid administration, the patient’s previous score was carried forward.
We excluded patients with sickle-cell disease or seizure history and admissions with diagnoses of alcohol withdrawal from the analysis, because these diagnoses were expected to be associated with different medication administration practices compared to other inpatients. We also excluded patients with a tracheostomy because we expected their respiratory monitoring to differ from the other patients in our cohort. Finally, because ward deaths resulting from a comfort care scenario often involve opioids and/or benzodiazepines, ward segments involving comfort care deaths (defined as death without attempted resuscitation) were excluded from the analysis (Supplemental Figure 1). Patients with sickle-cell disease were identified using ICD-9 codes, and encounters during which a seizure may have occurred were identified using a combination of ICD-9 codes and receipt of anti-epileptic medication (Supplemental Table 1). Patients at risk for alcohol withdrawal were identified by the presence of any Clinical Institute Withdrawal Assessment for Alcohol score within nursing flowsheets, and patients with tracheostomies were identified using documentation of ventilator support within their first 12 hours on the wards. In addition to these exclusion criteria, patients with obstructive sleep apnea (OSA) were identified by the following ICD-9 codes: 278.03, 327.23, 780.51, 780.53, and 780.57.
Medications
Ward administrations of opioids and benzodiazepines—dose, route, and administration time—were collected from the EHR. We excluded all administrations in nonward locations such as the ED, ICU, operating room, or procedure suite. Additionally, because patients emergently intubated may receive sedative and analgesic medications to facilitate intubation, and because patients experiencing cardiac arrest are frequently intubated periresuscitation, we a priori excluded all administrations within 15 minutes of a ward cardiac arrest or an intubation.
For consistent comparisons, opioid doses were converted to oral morphine equivalents34 and adjusted by a factor of 15 to reflect the smallest routinely available oral morphine tablet in our hospital (Supplemental Table 2). Benzodiazepine doses were converted to oral lorazepam equivalents (Supplemental Table 2).34 Thus, the independent variables were oral morphine or lorazepam equivalents administered within each 6-hour window. We a priori presumed opioid doses greater than the 99th percentile (1200 mg) or benzodiazepine doses greater than 10 mg oral lorazepam equivalents within a 6-hour window to be erroneous entries, and replaced these outlier values with the median value for each medication category.
Outcomes
The primary outcome was the composite of ICU transfer or cardiac arrest (loss of pulse with attempted resuscitation) on the wards, with individual outcomes investigated secondarily. An ICU transfer (patient movement from a ward directly to the ICU) was identified using the hospital’s admission-transfer-discharge database. Cardiac arrests were identified using a prospectively validated quality improvement database.35
Because deaths on the wards resulted either from cardiac arrest or from a comfort care scenario, mortality was not studied as an outcome.
Statistical Analysis
Patient characteristics were compared using Student t tests, Wilcoxon rank sum tests, and chi-squared statistics, as appropriate. Unadjusted and adjusted models were created using discrete-time survival analysis,36-39 which involved dividing time into discrete 6-hour intervals and employing the predictor variables chronologically closest to the beginning of each time window to forecast whether the outcome occurred within each interval. Predictor variables in the adjusted model included patient characteristics (age, sex, BMI, and Elixhauser Agency for Healthcare Research and Quality-Web comorbidities30 [a priori excluding comorbidities recorded for fewer than 1000 admissions from the model]), ward unit, surgical status, prior ICU admission during the hospitalization, cumulative opioid or benzodiazepine dose during the previous 24 hours, and severity of illness (measured by eCART score). The adjusted model for opioids also included the patient’s pain score. Age, eCART score, and pain score were entered linearly while race, BMI (underweight, less than 18.5 kg/m2; normal, 18.5-24.9 kg/m2; overweight, 25.0-29.9 kg/m2; obese, 30-39.9 kg/m2; and severely obese, 40 mg/m2 or greater), and ward unit were modeled as categorical variables.
Since repeat hospitalization could confound the results of our study, we performed a sensitivity analysis including only 1 randomly selected hospital admission per patient. We also performed a sensitivity analysis including receipt of both opioids and benzodiazepines, and an interaction term within each ward segment, as well as an analysis in which zolpidem—the most commonly administered nonbenzodiazepine hypnotic medication in our hospital—was included along with both opioids and benzodiazepines. Finally, we performed a sensitivity analysis replacing missing pain scores with imputed values ranging from 0 to the median ward pain score.
We also performed subgroup analyses of adjusted models across age quartiles and for each BMI category, as well as for surgical status, OSA status, gender, time of medication administration, and route of administration (intravenous vs. oral). We also performed an analysis across pain score severity40 to determine whether these medications produce differential effects at various levels of pain.
All tests of significance used a 2-sided P value less than 0.05. Statistical analyses were completed using Stata version 14.1 (StataCorp, LLC, College Station, Texas).
RESULTS
Patient Characteristics
A total of 144,895 admissions, from 75,369 patients, had ward vital signs or laboratory values documented during the study period. Ward segments from 634 admissions were excluded due to comfort care status, which resulted in exclusion of 479 complete patient admissions. Additionally, 139 patients with tracheostomies were excluded. Furthermore, 2934 patient admissions with a sickle-cell diagnosis were excluded, of which 95% (n = 2791) received an opioid and 11% (n = 310) received a benzodiazepine. Another 14,029 admissions associated with seizures, 6134 admissions involving alcohol withdrawal, and 1332 with both were excluded, of which 66% (n = 14,174) received an opioid and 35% (n = 7504) received a benzodiazepine. After exclusions, 120,518 admissions were included in the final analysis, with 67% (n = 80,463) associated with at least 1 administration of an opioid and 21% (n = 25,279) associated with at least 1 benzodiazepine administration.
In total, there were 672,851 intervals when an opioid was administered during the study, with a median dose of 12 mg oral morphine equivalents (interquartile range, 8-30). Of these, 21,634 doses were replaced due to outlier status outside the 99th percentile. Patients receiving opioids were younger (median age 56 vs 61 years), less likely to be African American (48% vs 59%), more likely to have undergone surgery (18% vs 6%), and less likely to have most noncancer medical comorbidities than those who never received an opioid (all P < 0.001) (Table 1).
Additionally, there were a total of 98,286 6-hour intervals in which a benzodiazepine was administered in the study, with a median dose of 1 mg oral lorazepam (interquartile range, 0.5-1). A total of 790 doses of benzodiazepines (less than 1%) were replaced due to outlier status. Patients who received benzodiazepines were more likely to be male (49% vs. 41%), less likely to be African-American, less likely to be obese or morbidly obese (33% vs. 39%), and more likely to have medical comorbidities compared to patients who never received a benzodiazepine (all P < 0.001) (Table 1).
The eCART scores were similar between all patient groups. The frequency of missing variables differed by data type, with vital signs rarely missing (all less than 1.1% except AVPU [10%]), followed by hematology labs (8%-9%), electrolytes and renal function results (12%-15%), and hepatic function tests (40%-45%). In addition to imputed data for missing vital signs and laboratory values, our model omitted human immunodeficiency virus/acquired immune deficiency syndrome and peptic ulcer disease from the adjusted models on the basis of fewer than 1000 admissions with these diagnoses listed.
Patient Outcomes
The incidence of the composite outcome was higher in admissions with at least 1 opioid medication than those without an opioid (7% vs. 4%, P < 0.001), and in admissions with at least 1 dose of benzodiazepines compared to those without a benzodiazepine (11% vs. 4%, P < 0.001) (Table 2).
Within 6-hour segments, increasing doses of opioids were associated with an initial decrease in the frequency of the composite outcome followed by a dose-related increase in the frequency of the composite outcome with morphine equivalents greater than 45 mg. By contrast, the frequency of the composite outcome increased with additional benzodiazepine equivalents (Figure).
In the adjusted model, opioid administration was associated with increased risk for the composite outcome (Table 3) in a dose-dependent fashion, with each 15 mg oral morphine equivalent associated with a 1.9% increase in the odds of ICU transfer or cardiac arrest within the subsequent 6-hour time interval (odds ratio [OR], 1.019; 95% confidence interval [CI], 1.013-1.026; P < 0.001).
Similarly, benzodiazepine administration was also associated with increased adjusted risk for the composite outcome within 6 hours in a dose-dependent manner. Each 1 mg oral lorazepam equivalent was associated with a 29% increase in the odds of ward cardiac arrest or ICU transfer (OR, 1.29; 95% CI, 1.16-1.44; P < 0.001) (Table 3).
Sensitivity Analyses
A sensitivity analysis including 1 randomly selected hospitalization per patient involved 67,097 admissions and found results similar to the primary analysis, with each 15 mg oral morphine equivalent associated with a 1.9% increase in the odds of the composite outcome (OR, 1.019; 95% CI, 1.011-1.028; P < 0.001) and each 1 mg oral lorazepam equivalent associated with a 41% increase in the odds of the composite outcome (OR, 1.41; 95% CI, 1.21-1.65; P < 0.001). Inclusion of both opioids and benzodiazepines in the adjusted model again yielded results similar to the main analysis for both opioids (OR, 1.020; 95% CI, 1.013-1.026; P < 0.001) and benzodiazepines (OR, 1.35; 95% CI, 1.18-1.54; P < 0.001), without a significant interaction detected (P = 0.09). These results were unchanged with the addition of zolpidem to the model as an additional potential confounder, and zolpidem did not increase the risk of the study outcomes (P = 0.2).
A final sensitivity analysis for the opioid model involved replacing missing pain scores with imputed values ranging from 0 to the median ward score, which was 5. The results of these analyses did not differ from the primary model and were consistent regardless of imputation value (OR, 1.018; 95% CI, 1.012-1.023; P < 0.001).
Subgroup Analyses
Analyses of opioid administration by subgroup (sex, age quartiles, BMI categories, OSA diagnosis, surgical status, daytime/nighttime medication administration, IV/PO administration, and pain severity) yielded similar results to the overall analysis (Supplemental Figure 2). Subgroup analysis of patients receiving benzodiazepines revealed similarly increased adjusted odds of the composite outcome across strata of gender, BMI, surgical status, and medication administration time (Supplemental Figure 3). Notably, patients older than 70 years who received a benzodiazepine were at 64% increased odds of the composite outcome (OR, 1.64; 95% CI, 1.30-2.08), compared to 2% to 38% increased risk for patients under 70 years. Finally, IV doses of benzodiazepines were associated with 48% increased odds for deterioration (OR, 1.48; 95% CI, 1.18-1.84; P = 0.001), compared to a nonsignificant 14% increase in the odds for PO doses (OR, 1.14; 95% CI, 0.99-1.31; P = 0.066).
DISCUSSION
In a large, single-center, observational study of ward inpatients, we found that opioid use was associated with a small but significant increased risk for clinical deterioration on the wards, with every 15 mg oral morphine equivalent increasing the odds of ICU transfer or cardiac arrest in the next 6 hours by 1.9%. Benzodiazepines were associated with a much higher risk: each equivalent of 1 mg of oral lorazepam increased the odds of ICU transfer or cardiac arrest by almost 30%. These results have important implications for care at the bedside of hospitalized ward patients and suggest the need for closer monitoring after receipt of these medications, particularly benzodiazepines.
Previous work has described negative effects of opioid medications among select inpatient populations. In surgical patients, opioids have been associated with hospital readmission, increased length of stay, and hospital mortality.26,28 More recently, Herzig et al.15 found more adverse events in nonsurgical ward patients within the hospitals prescribing opioids the most frequently. These studies may have been limited by the populations studied and the inability to control for confounders such as severity of illness and pain score. Our study expands these findings to a more generalizable population and shows that even after adjustment for potential confounders, such as severity of illness, pain score, and medication dose, opioids are associated with increased short-term risk of clinical deterioration.
By contrast, few studies have characterized the risks associated with benzodiazepine use among ward inpatients. Recently, Overdyk et al.27 found that inpatient use of opioids and sedatives was associated with increased risk for cardiac arrest and hospital death. However, this study included ICU patients, which may confound the results, as ICU patients often receive high doses of opioids or benzodiazepines to facilitate mechanical ventilation or other invasive procedures, while also having a particularly high risk of adverse outcomes like cardiac arrest and inhospital death.
Several mechanisms may explain the magnitude of effect seen with regard to benzodiazepines. First, benzodiazepines may directly produce clinical deterioration by decreased respiratory drive, diminished airway tone, or hemodynamic decompensation. It is possible that the broad spectrum of cardiorespiratory side effects of benzodiazepines—and potential unpredictability of these effects—increases the difficulty of observation and management for patients receiving them. This difficulty may be compounded with intravenous administration of benzodiazepines, which was associated with a higher risk for deterioration than oral doses in our cohort. Alternatively, benzodiazepines may contribute to clinical decompensation by masking signs of deterioration such as encephalopathy or vital sign instability like tachycardia or tachypnea that may be mistaken as anxiety. Notably, while our hospital has a nursing-driven protocol for monitoring patients receiving opioids (in which pain is serially assessed, leading to additional bedside observation), we do not have protocols for ward patients receiving benzodiazepines. Finally, although we found that orders for opioids and benzodiazepines were more common in white patients than African American patients, this finding may be due to differences in the types or number of medical comorbidities experienced by these patients.
Our study has several strengths, including the large number of admissions we included. Additionally, we included a broad range of medical and surgical ward admissions, which should increase the generalizability of our results. Further, our rates of ICU transfer are in line with data reported from other groups,41,42 which again may add to the generalizability of our findings. We also addressed many potential confounders by including patient characteristics, individual ward units, and (for opioids) pain score in our model, and by controlling for severity of illness with the eCART score, an accurate predictor of ICU transfer and ward cardiac arrest within our population.32,37 Finally, our robust methodology allowed us to include acute and cumulative medication doses, as well as time, in the model. By performing a discrete-time survival analysis, we were able to evaluate receipt of opioids and benzodiazepines—as well as risk for clinical deterioration—longitudinally, lending strength to our results.
Limitations of our study include its single-center cohort, which may reduce generalizability to other populations. Additionally, because we could not validate the accuracy of—or adherence to—outpatient medication lists, we were unable to identify chronic opioid or benzodiazepine users by these lists. However, patients chronically taking opioids or benzodiazepines would likely receive doses each hospital day; by including 24-hour cumulative doses in our model, we attempted to adjust for some portion of their chronic use. Also, because evaluation of delirium was not objectively recorded in our dataset, we were unable to evaluate the relationship between receipt of these medications and development of delirium, which is an important outcome for hospitalized patients. Finally, neither the diagnoses for which these medications were prescribed, nor the reason for ICU transfer, were present in our dataset, which leaves open the possibility of unmeasured confounding.
CONCLUSION
After adjustment for important confounders including severity of illness, medication dose, and time, opioids were associated with a slight increase in clinical deterioration on the wards, while benzodiazepines were associated with a much larger risk for deterioration. This finding raises concern about the safety of benzodiazepine use among ward patients and suggests that increased monitoring of patients receiving these medications may be warranted.
Acknowledgment
The authors thank Nicole Twu for administrative support.
Disclosure
Drs. Churpek and Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080). Dr. Churpek has received honoraria from Chest for invited speaking engagements. In addition, Dr. Edelson has received research support from Philips Healthcare (Andover, Massachusetts), research support from the American Heart Association (Dallas, Texas) and Laerdal Medical (Stavanger, Norway), and research support from Early Sense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, Illinois), which is developing products for risk stratification of hospitalized patients. Dr. Mokhlesi is supported by National Institutes of Health grant R01HL119161. Dr. Mokhlesi has served as a consultant to Philips/Respironics and has received research support from Philips/Respironics. Preliminary versions of these data were presented as a poster presentation at the 2016 meeting of the American Thoracic Society, May 17, 2016; San Francisco, California.
Chronic opioid and benzodiazepine use is common and increasing.1-5 Outpatient use of these medications has been associated with hospital readmission and death,6-12 with concurrent use associated with particularly increased risk.13,14 Less is known about outcomes for hospitalized patients receiving these medications.
More than half of hospital inpatients in the United States receive opioids,15 many of which are new prescriptions rather than continuation of chronic therapy.16,17 Less is known about inpatient benzodiazepine administration, but the prevalence may exceed 10% among elderly populations.18 Hospitalized patients often have comorbidities or physiological disturbances that might increase their risk related to use of these medications. Opioids can cause central and obstructive sleep apneas,19-21 and benzodiazepines contribute to respiratory depression and airway relaxation.22 Benzodiazepines also impair psychomotor function and recall,23 which could mediate the recognized risk for delirium and falls in the hospital.24,25 These findings suggest pathways by which these medications might contribute to clinical deterioration.
Most studies in hospitalized patients have been limited to specific populations15,26-28 and have not explicitly controlled for severity of illness over time. It remains unclear whether associations identified within particular groups of patients hold true for the broader population of general ward inpatients. Therefore, we aimed to determine the independent association between opioid and benzodiazepine administration and clinical deterioration in ward patients.
MATERIALS AND METHODS
Setting and Study Population
We performed an observational cohort study at a 500-bed urban academic hospital. Data were obtained from all adults hospitalized on the wards between November 1, 2008, and January 21, 2016. The study protocol was approved by the University of Chicago Institutional Review Board (IRB#15-0195).
Data Collection
The study utilized de-identified data from the electronic health record (EHR; Epic Systems Corporation, Verona, Wisconsin) and administrative databases collected by the University of Chicago Clinical Research Data Warehouse. Patient age, sex, race, body mass index (BMI), and ward admission source (ie, emergency department (ED), transferred from the intensive care unit (ICU), or directly admitted to the wards) were collected. International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes were used to identify Elixhauser Comorbidity Index categories.29,30 Because patients with similar diagnoses (eg, active cancer) are cohorted within particular areas in our hospital, we obtained the ward unit for all patients. Patients who underwent surgery were identified using the hospital’s admission-transfer-discharge database.
To determine severity of illness, routinely collected vital signs and laboratory values were utilized to calculate the electronic cardiac arrest risk triage (eCART) score, an accurate risk score we previously developed and validated for predicting adverse events among ward patients.31 If any vital sign or laboratory value was missing, the next available measurement was carried forward. If any value remained missing after this change, the median value for that location (ie, wards, ICU, or ED) was imputed.32,33 Additionally, patient-reported pain scores at the time of opioid administration were extracted from nursing flowsheets. If no pain score was present at the time of opioid administration, the patient’s previous score was carried forward.
We excluded patients with sickle-cell disease or seizure history and admissions with diagnoses of alcohol withdrawal from the analysis, because these diagnoses were expected to be associated with different medication administration practices compared to other inpatients. We also excluded patients with a tracheostomy because we expected their respiratory monitoring to differ from the other patients in our cohort. Finally, because ward deaths resulting from a comfort care scenario often involve opioids and/or benzodiazepines, ward segments involving comfort care deaths (defined as death without attempted resuscitation) were excluded from the analysis (Supplemental Figure 1). Patients with sickle-cell disease were identified using ICD-9 codes, and encounters during which a seizure may have occurred were identified using a combination of ICD-9 codes and receipt of anti-epileptic medication (Supplemental Table 1). Patients at risk for alcohol withdrawal were identified by the presence of any Clinical Institute Withdrawal Assessment for Alcohol score within nursing flowsheets, and patients with tracheostomies were identified using documentation of ventilator support within their first 12 hours on the wards. In addition to these exclusion criteria, patients with obstructive sleep apnea (OSA) were identified by the following ICD-9 codes: 278.03, 327.23, 780.51, 780.53, and 780.57.
Medications
Ward administrations of opioids and benzodiazepines—dose, route, and administration time—were collected from the EHR. We excluded all administrations in nonward locations such as the ED, ICU, operating room, or procedure suite. Additionally, because patients emergently intubated may receive sedative and analgesic medications to facilitate intubation, and because patients experiencing cardiac arrest are frequently intubated periresuscitation, we a priori excluded all administrations within 15 minutes of a ward cardiac arrest or an intubation.
For consistent comparisons, opioid doses were converted to oral morphine equivalents34 and adjusted by a factor of 15 to reflect the smallest routinely available oral morphine tablet in our hospital (Supplemental Table 2). Benzodiazepine doses were converted to oral lorazepam equivalents (Supplemental Table 2).34 Thus, the independent variables were oral morphine or lorazepam equivalents administered within each 6-hour window. We a priori presumed opioid doses greater than the 99th percentile (1200 mg) or benzodiazepine doses greater than 10 mg oral lorazepam equivalents within a 6-hour window to be erroneous entries, and replaced these outlier values with the median value for each medication category.
Outcomes
The primary outcome was the composite of ICU transfer or cardiac arrest (loss of pulse with attempted resuscitation) on the wards, with individual outcomes investigated secondarily. An ICU transfer (patient movement from a ward directly to the ICU) was identified using the hospital’s admission-transfer-discharge database. Cardiac arrests were identified using a prospectively validated quality improvement database.35
Because deaths on the wards resulted either from cardiac arrest or from a comfort care scenario, mortality was not studied as an outcome.
Statistical Analysis
Patient characteristics were compared using Student t tests, Wilcoxon rank sum tests, and chi-squared statistics, as appropriate. Unadjusted and adjusted models were created using discrete-time survival analysis,36-39 which involved dividing time into discrete 6-hour intervals and employing the predictor variables chronologically closest to the beginning of each time window to forecast whether the outcome occurred within each interval. Predictor variables in the adjusted model included patient characteristics (age, sex, BMI, and Elixhauser Agency for Healthcare Research and Quality-Web comorbidities30 [a priori excluding comorbidities recorded for fewer than 1000 admissions from the model]), ward unit, surgical status, prior ICU admission during the hospitalization, cumulative opioid or benzodiazepine dose during the previous 24 hours, and severity of illness (measured by eCART score). The adjusted model for opioids also included the patient’s pain score. Age, eCART score, and pain score were entered linearly while race, BMI (underweight, less than 18.5 kg/m2; normal, 18.5-24.9 kg/m2; overweight, 25.0-29.9 kg/m2; obese, 30-39.9 kg/m2; and severely obese, 40 mg/m2 or greater), and ward unit were modeled as categorical variables.
Since repeat hospitalization could confound the results of our study, we performed a sensitivity analysis including only 1 randomly selected hospital admission per patient. We also performed a sensitivity analysis including receipt of both opioids and benzodiazepines, and an interaction term within each ward segment, as well as an analysis in which zolpidem—the most commonly administered nonbenzodiazepine hypnotic medication in our hospital—was included along with both opioids and benzodiazepines. Finally, we performed a sensitivity analysis replacing missing pain scores with imputed values ranging from 0 to the median ward pain score.
We also performed subgroup analyses of adjusted models across age quartiles and for each BMI category, as well as for surgical status, OSA status, gender, time of medication administration, and route of administration (intravenous vs. oral). We also performed an analysis across pain score severity40 to determine whether these medications produce differential effects at various levels of pain.
All tests of significance used a 2-sided P value less than 0.05. Statistical analyses were completed using Stata version 14.1 (StataCorp, LLC, College Station, Texas).
RESULTS
Patient Characteristics
A total of 144,895 admissions, from 75,369 patients, had ward vital signs or laboratory values documented during the study period. Ward segments from 634 admissions were excluded due to comfort care status, which resulted in exclusion of 479 complete patient admissions. Additionally, 139 patients with tracheostomies were excluded. Furthermore, 2934 patient admissions with a sickle-cell diagnosis were excluded, of which 95% (n = 2791) received an opioid and 11% (n = 310) received a benzodiazepine. Another 14,029 admissions associated with seizures, 6134 admissions involving alcohol withdrawal, and 1332 with both were excluded, of which 66% (n = 14,174) received an opioid and 35% (n = 7504) received a benzodiazepine. After exclusions, 120,518 admissions were included in the final analysis, with 67% (n = 80,463) associated with at least 1 administration of an opioid and 21% (n = 25,279) associated with at least 1 benzodiazepine administration.
In total, there were 672,851 intervals when an opioid was administered during the study, with a median dose of 12 mg oral morphine equivalents (interquartile range, 8-30). Of these, 21,634 doses were replaced due to outlier status outside the 99th percentile. Patients receiving opioids were younger (median age 56 vs 61 years), less likely to be African American (48% vs 59%), more likely to have undergone surgery (18% vs 6%), and less likely to have most noncancer medical comorbidities than those who never received an opioid (all P < 0.001) (Table 1).
Additionally, there were a total of 98,286 6-hour intervals in which a benzodiazepine was administered in the study, with a median dose of 1 mg oral lorazepam (interquartile range, 0.5-1). A total of 790 doses of benzodiazepines (less than 1%) were replaced due to outlier status. Patients who received benzodiazepines were more likely to be male (49% vs. 41%), less likely to be African-American, less likely to be obese or morbidly obese (33% vs. 39%), and more likely to have medical comorbidities compared to patients who never received a benzodiazepine (all P < 0.001) (Table 1).
The eCART scores were similar between all patient groups. The frequency of missing variables differed by data type, with vital signs rarely missing (all less than 1.1% except AVPU [10%]), followed by hematology labs (8%-9%), electrolytes and renal function results (12%-15%), and hepatic function tests (40%-45%). In addition to imputed data for missing vital signs and laboratory values, our model omitted human immunodeficiency virus/acquired immune deficiency syndrome and peptic ulcer disease from the adjusted models on the basis of fewer than 1000 admissions with these diagnoses listed.
Patient Outcomes
The incidence of the composite outcome was higher in admissions with at least 1 opioid medication than those without an opioid (7% vs. 4%, P < 0.001), and in admissions with at least 1 dose of benzodiazepines compared to those without a benzodiazepine (11% vs. 4%, P < 0.001) (Table 2).
Within 6-hour segments, increasing doses of opioids were associated with an initial decrease in the frequency of the composite outcome followed by a dose-related increase in the frequency of the composite outcome with morphine equivalents greater than 45 mg. By contrast, the frequency of the composite outcome increased with additional benzodiazepine equivalents (Figure).
In the adjusted model, opioid administration was associated with increased risk for the composite outcome (Table 3) in a dose-dependent fashion, with each 15 mg oral morphine equivalent associated with a 1.9% increase in the odds of ICU transfer or cardiac arrest within the subsequent 6-hour time interval (odds ratio [OR], 1.019; 95% confidence interval [CI], 1.013-1.026; P < 0.001).
Similarly, benzodiazepine administration was also associated with increased adjusted risk for the composite outcome within 6 hours in a dose-dependent manner. Each 1 mg oral lorazepam equivalent was associated with a 29% increase in the odds of ward cardiac arrest or ICU transfer (OR, 1.29; 95% CI, 1.16-1.44; P < 0.001) (Table 3).
Sensitivity Analyses
A sensitivity analysis including 1 randomly selected hospitalization per patient involved 67,097 admissions and found results similar to the primary analysis, with each 15 mg oral morphine equivalent associated with a 1.9% increase in the odds of the composite outcome (OR, 1.019; 95% CI, 1.011-1.028; P < 0.001) and each 1 mg oral lorazepam equivalent associated with a 41% increase in the odds of the composite outcome (OR, 1.41; 95% CI, 1.21-1.65; P < 0.001). Inclusion of both opioids and benzodiazepines in the adjusted model again yielded results similar to the main analysis for both opioids (OR, 1.020; 95% CI, 1.013-1.026; P < 0.001) and benzodiazepines (OR, 1.35; 95% CI, 1.18-1.54; P < 0.001), without a significant interaction detected (P = 0.09). These results were unchanged with the addition of zolpidem to the model as an additional potential confounder, and zolpidem did not increase the risk of the study outcomes (P = 0.2).
A final sensitivity analysis for the opioid model involved replacing missing pain scores with imputed values ranging from 0 to the median ward score, which was 5. The results of these analyses did not differ from the primary model and were consistent regardless of imputation value (OR, 1.018; 95% CI, 1.012-1.023; P < 0.001).
Subgroup Analyses
Analyses of opioid administration by subgroup (sex, age quartiles, BMI categories, OSA diagnosis, surgical status, daytime/nighttime medication administration, IV/PO administration, and pain severity) yielded similar results to the overall analysis (Supplemental Figure 2). Subgroup analysis of patients receiving benzodiazepines revealed similarly increased adjusted odds of the composite outcome across strata of gender, BMI, surgical status, and medication administration time (Supplemental Figure 3). Notably, patients older than 70 years who received a benzodiazepine were at 64% increased odds of the composite outcome (OR, 1.64; 95% CI, 1.30-2.08), compared to 2% to 38% increased risk for patients under 70 years. Finally, IV doses of benzodiazepines were associated with 48% increased odds for deterioration (OR, 1.48; 95% CI, 1.18-1.84; P = 0.001), compared to a nonsignificant 14% increase in the odds for PO doses (OR, 1.14; 95% CI, 0.99-1.31; P = 0.066).
DISCUSSION
In a large, single-center, observational study of ward inpatients, we found that opioid use was associated with a small but significant increased risk for clinical deterioration on the wards, with every 15 mg oral morphine equivalent increasing the odds of ICU transfer or cardiac arrest in the next 6 hours by 1.9%. Benzodiazepines were associated with a much higher risk: each equivalent of 1 mg of oral lorazepam increased the odds of ICU transfer or cardiac arrest by almost 30%. These results have important implications for care at the bedside of hospitalized ward patients and suggest the need for closer monitoring after receipt of these medications, particularly benzodiazepines.
Previous work has described negative effects of opioid medications among select inpatient populations. In surgical patients, opioids have been associated with hospital readmission, increased length of stay, and hospital mortality.26,28 More recently, Herzig et al.15 found more adverse events in nonsurgical ward patients within the hospitals prescribing opioids the most frequently. These studies may have been limited by the populations studied and the inability to control for confounders such as severity of illness and pain score. Our study expands these findings to a more generalizable population and shows that even after adjustment for potential confounders, such as severity of illness, pain score, and medication dose, opioids are associated with increased short-term risk of clinical deterioration.
By contrast, few studies have characterized the risks associated with benzodiazepine use among ward inpatients. Recently, Overdyk et al.27 found that inpatient use of opioids and sedatives was associated with increased risk for cardiac arrest and hospital death. However, this study included ICU patients, which may confound the results, as ICU patients often receive high doses of opioids or benzodiazepines to facilitate mechanical ventilation or other invasive procedures, while also having a particularly high risk of adverse outcomes like cardiac arrest and inhospital death.
Several mechanisms may explain the magnitude of effect seen with regard to benzodiazepines. First, benzodiazepines may directly produce clinical deterioration by decreased respiratory drive, diminished airway tone, or hemodynamic decompensation. It is possible that the broad spectrum of cardiorespiratory side effects of benzodiazepines—and potential unpredictability of these effects—increases the difficulty of observation and management for patients receiving them. This difficulty may be compounded with intravenous administration of benzodiazepines, which was associated with a higher risk for deterioration than oral doses in our cohort. Alternatively, benzodiazepines may contribute to clinical decompensation by masking signs of deterioration such as encephalopathy or vital sign instability like tachycardia or tachypnea that may be mistaken as anxiety. Notably, while our hospital has a nursing-driven protocol for monitoring patients receiving opioids (in which pain is serially assessed, leading to additional bedside observation), we do not have protocols for ward patients receiving benzodiazepines. Finally, although we found that orders for opioids and benzodiazepines were more common in white patients than African American patients, this finding may be due to differences in the types or number of medical comorbidities experienced by these patients.
Our study has several strengths, including the large number of admissions we included. Additionally, we included a broad range of medical and surgical ward admissions, which should increase the generalizability of our results. Further, our rates of ICU transfer are in line with data reported from other groups,41,42 which again may add to the generalizability of our findings. We also addressed many potential confounders by including patient characteristics, individual ward units, and (for opioids) pain score in our model, and by controlling for severity of illness with the eCART score, an accurate predictor of ICU transfer and ward cardiac arrest within our population.32,37 Finally, our robust methodology allowed us to include acute and cumulative medication doses, as well as time, in the model. By performing a discrete-time survival analysis, we were able to evaluate receipt of opioids and benzodiazepines—as well as risk for clinical deterioration—longitudinally, lending strength to our results.
Limitations of our study include its single-center cohort, which may reduce generalizability to other populations. Additionally, because we could not validate the accuracy of—or adherence to—outpatient medication lists, we were unable to identify chronic opioid or benzodiazepine users by these lists. However, patients chronically taking opioids or benzodiazepines would likely receive doses each hospital day; by including 24-hour cumulative doses in our model, we attempted to adjust for some portion of their chronic use. Also, because evaluation of delirium was not objectively recorded in our dataset, we were unable to evaluate the relationship between receipt of these medications and development of delirium, which is an important outcome for hospitalized patients. Finally, neither the diagnoses for which these medications were prescribed, nor the reason for ICU transfer, were present in our dataset, which leaves open the possibility of unmeasured confounding.
CONCLUSION
After adjustment for important confounders including severity of illness, medication dose, and time, opioids were associated with a slight increase in clinical deterioration on the wards, while benzodiazepines were associated with a much larger risk for deterioration. This finding raises concern about the safety of benzodiazepine use among ward patients and suggests that increased monitoring of patients receiving these medications may be warranted.
Acknowledgment
The authors thank Nicole Twu for administrative support.
Disclosure
Drs. Churpek and Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080). Dr. Churpek has received honoraria from Chest for invited speaking engagements. In addition, Dr. Edelson has received research support from Philips Healthcare (Andover, Massachusetts), research support from the American Heart Association (Dallas, Texas) and Laerdal Medical (Stavanger, Norway), and research support from Early Sense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, Illinois), which is developing products for risk stratification of hospitalized patients. Dr. Mokhlesi is supported by National Institutes of Health grant R01HL119161. Dr. Mokhlesi has served as a consultant to Philips/Respironics and has received research support from Philips/Respironics. Preliminary versions of these data were presented as a poster presentation at the 2016 meeting of the American Thoracic Society, May 17, 2016; San Francisco, California.
1. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
2. Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Public Health. 2016;106(4):686-688. PubMed
3. Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138(3):507-513. PubMed
4. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142. PubMed
5. Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002−2014. Am J Prev Med. 2016;51(2):151-160. PubMed
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. PubMed
7. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248. PubMed
8. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers---United States, 1999--2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492. PubMed
9. Lan TY, Zeng YF, Tang GJ, et al. The use of hypnotics and mortality - a population-based retrospective cohort study. PLoS One. 2015;10(12):e0145271. PubMed
10. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli P, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy: prior opioid use among veterans. J Hosp Med. 2014;9(2):82-87. PubMed
11. Palmaro A, Dupouy J, Lapeyre-Mestre M. Benzodiazepines and risk of death: results from two large cohort studies in France and UK. Eur Neuropsychopharmacol. 2015;25(10):1566-1577. PubMed
12. Parsaik AK, Mascarenhas SS, Khosh-Chashm D, et al. Mortality associated with anxiolytic and hypnotic drugs–a systematic review and meta-analysis. Aust N Z J Psychiatry. 2016;50(6):520-533. PubMed
13. Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. PubMed
14. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501. PubMed
15. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
16. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to Medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
17. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
18. Garrido MM, Prigerson HG, Penrod JD, Jones SC, Boockvar KS. Benzodiazepine and sedative-hypnotic use among older seriously ill veterans: choosing wisely? Clin Ther. 2014;36(11):1547-1554. PubMed
19. Doufas AG, Tian L, Padrez KA, et al. Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea. PloS One. 2013;8(1):e54807. PubMed
20. Gislason T, Almqvist M, Boman G, Lindholm CE, Terenius L. Increased CSF opioid activity in sleep apnea syndrome. Regression after successful treatment. Chest. 1989;96(2):250-254. PubMed
21. Van Ryswyk E, Antic N. Opioids and sleep disordered breathing. Chest. 2016;150(4):934-944. PubMed
22. Koga Y, Sato S, Sodeyama N, et al. Comparison of the relaxant effects of diazepam, flunitrazepam and midazolam on airway smooth muscle. Br J Anaesth. 1992;69(1):65-69. PubMed
23. Pomara N, Lee SH, Bruno D, et al. Adverse performance effects of acute lorazepam administration in elderly long-term users: pharmacokinetic and clinical predictors. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:129-135. PubMed
24. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21-26. PubMed
25. O’Neil CA, Krauss MJ, Bettale J, et al. Medications and patient characteristics associated with falling in the hospital. J Patient Saf. 2015 (epub ahead of print). PubMed
26. Kessler ER, Shah M, K Gruschkus S, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383-391. PubMed
27. Overdyk FJ, Dowling O, Marino J, et al. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11(2):e0150214. PubMed
28. Minkowitz HS, Gruschkus SK, Shah M, Raju A. Adverse drug events among patients receiving postsurgical opioids in a large health system: risk factors and outcomes. Am J Health Syst Pharm. 2014;71(18):1556-1565. PubMed
29. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
30. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed
31. Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190(6):649-655. PubMed
32. Knaus WA, Wagner DP, Draper EA, Z et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619-1636. PubMed
33. van den Boogaard M, Pickkers P, Slooter AJC, et al. Development and validation
of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction
model for intensive care patients: observational multicentre study. BMJ.
2012;344:e420. PubMed
34. Clinical calculators. ClinCalc.com. http://www.clincalc.com. Accessed February
21, 2016.
35. Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting
cardiac arrest on the wards: a nested case-control study. Chest. 2012;141(5):
1170-1176. PubMed
36. Churpek MM, Yuen TC, Park SY, Gibbons R, Edelson DP. Using electronic
health record data to develop and validate a prediction model for adverse outcomes
in the wards. Crit Care Med. 2014;42(4):841-848. PubMed
37. Efron B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J Am
Stat Assoc. 1988;83(402):414-425.
38. Gibbons RD, Duan N, Meltzer D, et al; Institute of Medicine Committee. Waiting
for organ transplantation: results of an analysis by an Institute of Medicine Committee.
Biostatistics. 2003;4(2):207-222. PubMed
39. Singer JD, Willett JB. It’s about time: using discrete-time survival analysis to study
duration and the timing of events. J Educ Behav Stat. 1993;18(2):155-195.
40. World Health Organization. Cancer pain relief and palliative care. Report of a
WHO Expert Committee. World Health Organ Tech Rep Ser. 1990;804:1-75. PubMed
41. Bailey TC, Chen Y, Mao Y, et al. A trial of a real-time alert for clinical deterioration
in patients hospitalized on general medical wards. J Hosp Med. 2013;8(5):236-242. PubMed
42. Liu V, Kipnis P, Rizk NW, Escobar GJ. Adverse outcomes associated with delayed
intensive care unit transfers in an integrated healthcare system. J Hosp Med.
2012;7(3):224-230. PubMed
1. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
2. Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996–2013. Am J Public Health. 2016;106(4):686-688. PubMed
3. Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138(3):507-513. PubMed
4. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142. PubMed
5. Hwang CS, Kang EM, Kornegay CJ, Staffa JA, Jones CM, McAninch JK. Trends in the concomitant prescribing of opioids and benzodiazepines, 2002−2014. Am J Prev Med. 2016;51(2):151-160. PubMed
6. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321. PubMed
7. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248. PubMed
8. Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers---United States, 1999--2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487-1492. PubMed
9. Lan TY, Zeng YF, Tang GJ, et al. The use of hypnotics and mortality - a population-based retrospective cohort study. PLoS One. 2015;10(12):e0145271. PubMed
10. Mosher HJ, Jiang L, Vaughan Sarrazin MS, Cram P, Kaboli P, Vander Weg MW. Prevalence and characteristics of hospitalized adults on chronic opioid therapy: prior opioid use among veterans. J Hosp Med. 2014;9(2):82-87. PubMed
11. Palmaro A, Dupouy J, Lapeyre-Mestre M. Benzodiazepines and risk of death: results from two large cohort studies in France and UK. Eur Neuropsychopharmacol. 2015;25(10):1566-1577. PubMed
12. Parsaik AK, Mascarenhas SS, Khosh-Chashm D, et al. Mortality associated with anxiolytic and hypnotic drugs–a systematic review and meta-analysis. Aust N Z J Psychiatry. 2016;50(6):520-533. PubMed
13. Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. PubMed
14. Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493-501. PubMed
15. Herzig SJ, Rothberg MB, Cheung M, Ngo LH, Marcantonio ER. Opioid utilization and opioid-related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2014;9(2):73-81. PubMed
16. Jena AB, Goldman D, Karaca-Mandic P. Hospital prescribing of opioids to Medicare beneficiaries. JAMA Intern Med. 2016;176(7):990-997. PubMed
17. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
18. Garrido MM, Prigerson HG, Penrod JD, Jones SC, Boockvar KS. Benzodiazepine and sedative-hypnotic use among older seriously ill veterans: choosing wisely? Clin Ther. 2014;36(11):1547-1554. PubMed
19. Doufas AG, Tian L, Padrez KA, et al. Experimental pain and opioid analgesia in volunteers at high risk for obstructive sleep apnea. PloS One. 2013;8(1):e54807. PubMed
20. Gislason T, Almqvist M, Boman G, Lindholm CE, Terenius L. Increased CSF opioid activity in sleep apnea syndrome. Regression after successful treatment. Chest. 1989;96(2):250-254. PubMed
21. Van Ryswyk E, Antic N. Opioids and sleep disordered breathing. Chest. 2016;150(4):934-944. PubMed
22. Koga Y, Sato S, Sodeyama N, et al. Comparison of the relaxant effects of diazepam, flunitrazepam and midazolam on airway smooth muscle. Br J Anaesth. 1992;69(1):65-69. PubMed
23. Pomara N, Lee SH, Bruno D, et al. Adverse performance effects of acute lorazepam administration in elderly long-term users: pharmacokinetic and clinical predictors. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:129-135. PubMed
24. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21-26. PubMed
25. O’Neil CA, Krauss MJ, Bettale J, et al. Medications and patient characteristics associated with falling in the hospital. J Patient Saf. 2015 (epub ahead of print). PubMed
26. Kessler ER, Shah M, K Gruschkus S, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383-391. PubMed
27. Overdyk FJ, Dowling O, Marino J, et al. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11(2):e0150214. PubMed
28. Minkowitz HS, Gruschkus SK, Shah M, Raju A. Adverse drug events among patients receiving postsurgical opioids in a large health system: risk factors and outcomes. Am J Health Syst Pharm. 2014;71(18):1556-1565. PubMed
29. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
30. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. PubMed
31. Churpek MM, Yuen TC, Winslow C, et al. Multicenter development and validation of a risk stratification tool for ward patients. Am J Respir Crit Care Med. 2014;190(6):649-655. PubMed
32. Knaus WA, Wagner DP, Draper EA, Z et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619-1636. PubMed
33. van den Boogaard M, Pickkers P, Slooter AJC, et al. Development and validation
of PRE-DELIRIC (PREdiction of DELIRium in ICu patients) delirium prediction
model for intensive care patients: observational multicentre study. BMJ.
2012;344:e420. PubMed
34. Clinical calculators. ClinCalc.com. http://www.clincalc.com. Accessed February
21, 2016.
35. Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting
cardiac arrest on the wards: a nested case-control study. Chest. 2012;141(5):
1170-1176. PubMed
36. Churpek MM, Yuen TC, Park SY, Gibbons R, Edelson DP. Using electronic
health record data to develop and validate a prediction model for adverse outcomes
in the wards. Crit Care Med. 2014;42(4):841-848. PubMed
37. Efron B. Logistic regression, survival analysis, and the Kaplan-Meier curve. J Am
Stat Assoc. 1988;83(402):414-425.
38. Gibbons RD, Duan N, Meltzer D, et al; Institute of Medicine Committee. Waiting
for organ transplantation: results of an analysis by an Institute of Medicine Committee.
Biostatistics. 2003;4(2):207-222. PubMed
39. Singer JD, Willett JB. It’s about time: using discrete-time survival analysis to study
duration and the timing of events. J Educ Behav Stat. 1993;18(2):155-195.
40. World Health Organization. Cancer pain relief and palliative care. Report of a
WHO Expert Committee. World Health Organ Tech Rep Ser. 1990;804:1-75. PubMed
41. Bailey TC, Chen Y, Mao Y, et al. A trial of a real-time alert for clinical deterioration
in patients hospitalized on general medical wards. J Hosp Med. 2013;8(5):236-242. PubMed
42. Liu V, Kipnis P, Rizk NW, Escobar GJ. Adverse outcomes associated with delayed
intensive care unit transfers in an integrated healthcare system. J Hosp Med.
2012;7(3):224-230. PubMed
© 2017 Society of Hospital Medicine
The shifting landscape in utilization of inpatient, observation, and emergency department services across payers
For over a decade, private and public payers have implemented policies aimed at reducing rates of inpatient hospitalization. One approach for doing so is to improve ambulatory care, which can reduce the need for hospital-based acute care. Another approach is to stabilize acutely ill patients and discharge them from the emergency department (ED) or following a period of observation.1 Private payers are entering into value-based contracting arrangements with hospitals and health systems to improve the quality of ambulatory care and lower healthcare expenditures.2 Enrollment in managed care programs has grown among Medicaid recipients for similar reasons.3 Policies of the Centers for Medicare & Medicaid Services (CMS) encourage improvements in ambulatory care as well as observation of Medicare beneficiaries instead of inpatient admission in certain situations.4
Recent studies have documented declines in inpatient admissions and increases in treat-and-release observation stays and ED visits among Medicare beneficiaries.4-7 However, almost half of all hospitalizations unrelated to childbirth occur among patients with private insurance, Medicaid, or no insurance.8 Less is known about shifts in the nature of hospital-based acute care among these populations. Such shifts would have implications for quality of care, patient outcomes, and costs. Therefore, further investigation is warranted.
Our objective was to investigate recent trends in payer-specific population-based rates of adults using inpatient, observation, and ED services. We focused on 10 medical conditions that are common reasons for hospital-based acute care: heart failure, bacterial pneumonia, chronic obstructive pulmonary disease, asthma, dehydration, urinary tract infection, uncontrolled diabetes, diabetes with long-term complications, diabetes with short-term complications, and hypertension. These conditions constitute more than 20% of inpatient stays in the general medical service line, can be affected by improvements in ambulatory care, and provided a consistent set of diagnoses to track trends over time.9 We used 2009 and 2013 data from four states to examine trends among individuals with private insurance, Medicare, Medicaid, and no insurance.
METHODS
We obtained encounter-level data for Georgia, Nebraska, South Carolina, and Tennessee from the Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP).10 Using encrypted patient identifiers, we linked inpatient admissions from the 2009 and 2013 State Inpatient Databases, observation stays from the State Ambulatory Surgery and Services Databases, and ED visits from State Emergency Department Databases.
We defined the 10 medical conditions using numerator specifications from the ICD-9-CM v 5.0 AHRQ Prevention Quality Indicators (see Appendix). At most, 1 inpatient admission, 1 observation stay, and 1 ED visit for a study condition was counted for each adult in each year. Limiting the number of visits minimized the skew caused by multiple uses of the same service.
Using the American Community Survey, we calculated utilization rates for each type of service per 100,000 population in four payer and age groups: privately insured adults, Medicaid recipients, and uninsured adults 18 to 64 years, as well as Medicare beneficiaries 65 years and older. For each group, we also examined the origin of inpatient admissions—those who were directly admitted without evaluation in the ED, those admitted from the ED, and ED visits leading to observation stays and then inpatient admission.
RESULTS
Comparing 2009 and 2013, population-based rates of adults with 1 or more inpatient admissions for 10 common medical conditions declined, whereas rates of adults with treat-and-release observation stays rose. Changes in rates of treat-and-release ED visits varied across payers but were small relative to the substantial declines in inpatient admissions (Figure 1). In addition, a growing percentage of inpatient admissions began as observation stays and fewer adults were admitted directly, except among uninsured individuals (Figure 2).
Private Payers, 18 to 64 Years
The rate of adults with treat-and-release observation stays rose (+12.0%, 30 to 33 per 100,000 private payer population, P < 0.001). The rate of adults with treat-and-release ED visits declined (–9.0%, 713 to 648 per 100,000 population, P < 0.001), but by less than for inpatient admissions (–28.2%, 231 to 166 per 100,000 population, P < 0.001; Figure 1A). The percentage of inpatient admissions that began as observation stays rose (from 4.1% to 5.4%, P = 0.041), as did the percentage of admissions originating in the ED (from 66.4% to 71.5%, P ≤ 0.001; Figure 2).
Medicare, 65 Years and Older
The rate of adults with inpatient admissions declined (–17.0%, 2669 to 2216 per 100,000 Medicare population, P < 0.001). Rates rose for adults with treat-and-release ED visits (+3.9%, 1887 to 1961 per 100,000 population, P < 0.001) and treat-and-release observation stays (+32.9%, 234 to 311 per 100,000 population, P < 0.001; Figure 1B). The percentage of inpatient admissions that began as observation stays also rose (5.4% to 9.1%, P < 0.001; Figure 2).
Medicaid, 18 to 64 Years
The rate of adults with inpatient admissions declined (–15.3%, 1100 to 931 per 100,000 Medicaid population, P < 0.001), whereas treat-and-release ED visits remained flat (–1.5%, 4867 to 4792 per 100,000 population, P = 0.413) and treat-and-release observation stays rose (+18.1%, 196 to 232 per 100,000 population, P < 0.001; Figure 1C). The percentage of inpatient admissions that began as observation stays rose (5.9% to 8.1%, P = 0.022; Figure 2).
Uninsured, 18 to 64 Years
The rate of adults with inpatient admissions declined (–5.2%, 296 to 281 per 100,000 uninsured population, P = 0.003), whereas rates rose for treat-and-release ED visits (+8.9%, 1888 to 2057 per 100,000 population, P < 0.001) and treat-and-release observation stays (34.7%, 54 to 73 per 100,000 population, P < 0.001; Figure 1D). The source of inpatient admissions remained stable (Figure 2).
DISCUSSION
Data on hospital encounters from four states show that both ED visits and observation stays are playing an increasing role in hospital-based acute care for 10 common conditions among populations insured by private payers, Medicare, and Medicaid, as well as those without insurance. Compared with 2009, in 2013 substantially fewer individuals had inpatient admissions, and patients were more likely to be discharged from the ED or discharged following observation without receiving inpatient care. Additionally, an increasing percentage of inpatient admissions followed observation stays, whereas direct admissions declined.
Previous authors also have reported declines in inpatient stays for these same conditions.11 Others have reported increases in the use of observation stays for diverse conditions among patients with private insurance, Medicare beneficiaries, and veterans.4,12,13 The unique attributes of HCUP databases from these four states (eg, all-payer data including patient linkage numbers across inpatient, observation, and ED care) enabled us to assess concurrent shifts in hospital-based acute care from inpatient to outpatient care among multiple payer populations. A recent analysis reported declines in readmissions and increases in observation visits occurring within 30 days after hospitalization among Medicare beneficiaries with heart failure, acute myocardial infarction, or pneumonia.14 Future research should examine trends in readmissions and observation visits following hospitalization among multiple payer populations.
These shifts raise two important questions. The first pertains to quality of care, including outcomes. Although dedicated observation units with condition-specific care pathways can be associated with shorter stays and fewer admissions, many patients placed under observation are neither in dedicated units nor subject to care pathways.15,16 Systems for monitoring quality of care are less developed for observation care. The CMS publicly reports hospital-level data on quality of ED and inpatient care, including for several of the conditions we studied, but no measures apply to observation stays.17 Little is known about whether shifts from inpatient care to observation status or discharge from the ED are associated with different health outcomes.
The second issue is patients’ out-of-pocket costs. Although shifts from inpatient admissions to observation stays can reduce costs to payers,15 effects on patient out-of-pocket costs are uncertain and may vary. For privately insured patients, out-of-pocket costs may be up to four times higher for observation than for inpatient care.18 For Medicare beneficiaries, out-of-pocket costs can be higher for observation than for inpatient stays, particularly when patients receive costly medications or are discharged to skilled nursing facilities;19,20 however, having secondary insurance dramatically reduces out-of-pocket costs.21 We are not aware of data on Medicaid recipients or uninsured individuals.
This study has limitations. Only four states had data needed for these analyses, so generalization to other states is limited. Our analysis was descriptive and did not control for case mix, evaluate specific policies by any payer, or assess the full volume of visits among high utilizers. Movement of healthier or sicker individuals across payers could have contributed to temporal trends, but findings were similar across payers.
In conclusion, among 10 common medical conditions and three major payer populations and uninsured individuals in four states, inpatient admissions declined, and care shifted toward treat-and-release ED visits and observation stays. The number of inpatient admissions that began as observation stays also increased. Given these trends and the possibility that such shifts may be widespread and continue beyond 2013, quality of care, outcomes, and costs to patients warrant further evaluation.
Acknowledgments
The authors gratefully acknowledge Minya Sheng, MS (Truven Health Analytics) for assistance in programming and data management, and Paige Jackson, MS and Linda Lee, PhD, (Truven Health Analytics) for providing editorial review of the manuscript. They also wish to acknowledge the four HCUP Partner organizations that contributed to the 2009 and 2013 HCUP state databases used in this study: Georgia Hospital Association, Nebraska Hospital Association, South Carolina Revenue and Fiscal Affairs Office, and Tennessee Hospital Association.
Disclosure
Funding for this study was provided by the Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP) (Contract No. HHSA-290-2013-00002-C). The views expressed in this article are those of the authors and do not necessarily reflect those of the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services. The authors have no conflicts of interest to declare or financial disclosures.
1. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156 PubMed
2. Song Z. Accountable care organizations in the U.S. health care system. J Clin Outcomes Manag. 2014;21(8):364-371. PubMed
3. Kaiser Family Foundation. Total Medicaid MCOs. State Health Facts. 2016. http://kff.org/other/state-indicator/total-medicaid-mcos/. Accessed July 19, 2016.
4. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. PubMed
5. Skinner HG, Blanchard J, Elixhauser A. Trends in emergency department visits, 2006–2011. HCUP Statistical Brief #179. September 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb179-Emergency-Department-Trends.pdf. Accessed July 21, 2016.
6. Medicare Payment Advisory Commission. Report to the Congress: Medicare and the Health Care Delivery System. June 2015. http://www.medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed October 6, 2016.
7. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. March 2016. http://www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed October 6, 2016.
8. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. HCUPnet. Agency for Healthcare Research and Quality, Rockville, MD. http://hcupnet.ahrq.gov/. Accessed October 6, 2016.
9. Fingar KR, Barrett ML, Elixhauser A, Stocks C, Steiner CA. Trends in potentially preventable inpatient hospital admissions and emergency department visits. HCUP Statistical Brief #195. November 2015. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb195-Potentially-Preventable-Hospitalizations.pdf. Accessed August 9, 2016.
10. Agency for Healthcare Research and Quality. HCUP Databases. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/databases.jsp. Accessed August 8, 2016.
11. Torio CM, Andrews RM. Geographic variation in potentially preventable hospitalizations for acute and chronic conditions, 2005–2011. HCUP Statistical Brief, #178. September 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb178-Preventable-Hospitalizations-by-Region.pdf. Accessed November 8, 2015.
12. Wright B, O’Shea AM, Ayyagari P, Ugwi PG, Kaboli P, Vaughan Sarrazin M. Observation rates at veterans’ hospitals more than doubled during 2005-13, similar to Medicare trends. Health Aff (Millwood). 2015;34(10):1730-1737. PubMed
13. Noel-Miller C, Lind K. Is observation status substituting for hospital readmission? Health Affairs Blog. October 28, 2015. Project Hope: The People-to-People Health Foundation, Inc., Millwood, VA. http://healthaffairs.org/blog/2015/10/28/is-observation-status-substituting-for-hospital-readmission/. Accessed November 8, 2015.
14. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. PubMed
15. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. PubMed
16. Sheehy AM. Dedicated observation unit for patients with “observation status” -- reply. JAMA Intern Med. 2014;174(2):301-302. PubMed
17. Medicare.gov. Measures and current data collection periods. Centers for Medicare and Medicaid Services, Baltimore, MD. https://www.medicare.gov/hospitalcompare/Data/Data-Updated.html#. Accessed July 19, 2016.
18. Jaffe S. You’re being observed in the hospital? Patients with private insurance better off than seniors. September 11, 2014. Kaiser Health News, Kaiser Family Foundation, Menlo Park, CA. http://khn.org/news/youre-being-observed-in-the-hospital-patients-with-private-insurance-are-better-off-than-seniors/. Accessed November 8, 2015.
19. Kangovi S, Cafardi SG, Smith RA, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10(11):718-723. PubMed
20. U.S. Department of Health and Human Services, Office of Inspector General. Hospitals’ use of observation stays and short inpatient stays for Medicare beneficiaries. Memorandum Report OEI-02-12-00040. July 29, 2013. U.S. Department of Health and Human Services, Washington, DC. https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed October 6, 2016.
21. Doyle BJ, Ettner SL, Nuckols TK. Supplemental insurance reduces out-of-pocket costs in Medicare observation services. J Hosp Med. 2016;11(7):502-504. PubMed
For over a decade, private and public payers have implemented policies aimed at reducing rates of inpatient hospitalization. One approach for doing so is to improve ambulatory care, which can reduce the need for hospital-based acute care. Another approach is to stabilize acutely ill patients and discharge them from the emergency department (ED) or following a period of observation.1 Private payers are entering into value-based contracting arrangements with hospitals and health systems to improve the quality of ambulatory care and lower healthcare expenditures.2 Enrollment in managed care programs has grown among Medicaid recipients for similar reasons.3 Policies of the Centers for Medicare & Medicaid Services (CMS) encourage improvements in ambulatory care as well as observation of Medicare beneficiaries instead of inpatient admission in certain situations.4
Recent studies have documented declines in inpatient admissions and increases in treat-and-release observation stays and ED visits among Medicare beneficiaries.4-7 However, almost half of all hospitalizations unrelated to childbirth occur among patients with private insurance, Medicaid, or no insurance.8 Less is known about shifts in the nature of hospital-based acute care among these populations. Such shifts would have implications for quality of care, patient outcomes, and costs. Therefore, further investigation is warranted.
Our objective was to investigate recent trends in payer-specific population-based rates of adults using inpatient, observation, and ED services. We focused on 10 medical conditions that are common reasons for hospital-based acute care: heart failure, bacterial pneumonia, chronic obstructive pulmonary disease, asthma, dehydration, urinary tract infection, uncontrolled diabetes, diabetes with long-term complications, diabetes with short-term complications, and hypertension. These conditions constitute more than 20% of inpatient stays in the general medical service line, can be affected by improvements in ambulatory care, and provided a consistent set of diagnoses to track trends over time.9 We used 2009 and 2013 data from four states to examine trends among individuals with private insurance, Medicare, Medicaid, and no insurance.
METHODS
We obtained encounter-level data for Georgia, Nebraska, South Carolina, and Tennessee from the Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP).10 Using encrypted patient identifiers, we linked inpatient admissions from the 2009 and 2013 State Inpatient Databases, observation stays from the State Ambulatory Surgery and Services Databases, and ED visits from State Emergency Department Databases.
We defined the 10 medical conditions using numerator specifications from the ICD-9-CM v 5.0 AHRQ Prevention Quality Indicators (see Appendix). At most, 1 inpatient admission, 1 observation stay, and 1 ED visit for a study condition was counted for each adult in each year. Limiting the number of visits minimized the skew caused by multiple uses of the same service.
Using the American Community Survey, we calculated utilization rates for each type of service per 100,000 population in four payer and age groups: privately insured adults, Medicaid recipients, and uninsured adults 18 to 64 years, as well as Medicare beneficiaries 65 years and older. For each group, we also examined the origin of inpatient admissions—those who were directly admitted without evaluation in the ED, those admitted from the ED, and ED visits leading to observation stays and then inpatient admission.
RESULTS
Comparing 2009 and 2013, population-based rates of adults with 1 or more inpatient admissions for 10 common medical conditions declined, whereas rates of adults with treat-and-release observation stays rose. Changes in rates of treat-and-release ED visits varied across payers but were small relative to the substantial declines in inpatient admissions (Figure 1). In addition, a growing percentage of inpatient admissions began as observation stays and fewer adults were admitted directly, except among uninsured individuals (Figure 2).
Private Payers, 18 to 64 Years
The rate of adults with treat-and-release observation stays rose (+12.0%, 30 to 33 per 100,000 private payer population, P < 0.001). The rate of adults with treat-and-release ED visits declined (–9.0%, 713 to 648 per 100,000 population, P < 0.001), but by less than for inpatient admissions (–28.2%, 231 to 166 per 100,000 population, P < 0.001; Figure 1A). The percentage of inpatient admissions that began as observation stays rose (from 4.1% to 5.4%, P = 0.041), as did the percentage of admissions originating in the ED (from 66.4% to 71.5%, P ≤ 0.001; Figure 2).
Medicare, 65 Years and Older
The rate of adults with inpatient admissions declined (–17.0%, 2669 to 2216 per 100,000 Medicare population, P < 0.001). Rates rose for adults with treat-and-release ED visits (+3.9%, 1887 to 1961 per 100,000 population, P < 0.001) and treat-and-release observation stays (+32.9%, 234 to 311 per 100,000 population, P < 0.001; Figure 1B). The percentage of inpatient admissions that began as observation stays also rose (5.4% to 9.1%, P < 0.001; Figure 2).
Medicaid, 18 to 64 Years
The rate of adults with inpatient admissions declined (–15.3%, 1100 to 931 per 100,000 Medicaid population, P < 0.001), whereas treat-and-release ED visits remained flat (–1.5%, 4867 to 4792 per 100,000 population, P = 0.413) and treat-and-release observation stays rose (+18.1%, 196 to 232 per 100,000 population, P < 0.001; Figure 1C). The percentage of inpatient admissions that began as observation stays rose (5.9% to 8.1%, P = 0.022; Figure 2).
Uninsured, 18 to 64 Years
The rate of adults with inpatient admissions declined (–5.2%, 296 to 281 per 100,000 uninsured population, P = 0.003), whereas rates rose for treat-and-release ED visits (+8.9%, 1888 to 2057 per 100,000 population, P < 0.001) and treat-and-release observation stays (34.7%, 54 to 73 per 100,000 population, P < 0.001; Figure 1D). The source of inpatient admissions remained stable (Figure 2).
DISCUSSION
Data on hospital encounters from four states show that both ED visits and observation stays are playing an increasing role in hospital-based acute care for 10 common conditions among populations insured by private payers, Medicare, and Medicaid, as well as those without insurance. Compared with 2009, in 2013 substantially fewer individuals had inpatient admissions, and patients were more likely to be discharged from the ED or discharged following observation without receiving inpatient care. Additionally, an increasing percentage of inpatient admissions followed observation stays, whereas direct admissions declined.
Previous authors also have reported declines in inpatient stays for these same conditions.11 Others have reported increases in the use of observation stays for diverse conditions among patients with private insurance, Medicare beneficiaries, and veterans.4,12,13 The unique attributes of HCUP databases from these four states (eg, all-payer data including patient linkage numbers across inpatient, observation, and ED care) enabled us to assess concurrent shifts in hospital-based acute care from inpatient to outpatient care among multiple payer populations. A recent analysis reported declines in readmissions and increases in observation visits occurring within 30 days after hospitalization among Medicare beneficiaries with heart failure, acute myocardial infarction, or pneumonia.14 Future research should examine trends in readmissions and observation visits following hospitalization among multiple payer populations.
These shifts raise two important questions. The first pertains to quality of care, including outcomes. Although dedicated observation units with condition-specific care pathways can be associated with shorter stays and fewer admissions, many patients placed under observation are neither in dedicated units nor subject to care pathways.15,16 Systems for monitoring quality of care are less developed for observation care. The CMS publicly reports hospital-level data on quality of ED and inpatient care, including for several of the conditions we studied, but no measures apply to observation stays.17 Little is known about whether shifts from inpatient care to observation status or discharge from the ED are associated with different health outcomes.
The second issue is patients’ out-of-pocket costs. Although shifts from inpatient admissions to observation stays can reduce costs to payers,15 effects on patient out-of-pocket costs are uncertain and may vary. For privately insured patients, out-of-pocket costs may be up to four times higher for observation than for inpatient care.18 For Medicare beneficiaries, out-of-pocket costs can be higher for observation than for inpatient stays, particularly when patients receive costly medications or are discharged to skilled nursing facilities;19,20 however, having secondary insurance dramatically reduces out-of-pocket costs.21 We are not aware of data on Medicaid recipients or uninsured individuals.
This study has limitations. Only four states had data needed for these analyses, so generalization to other states is limited. Our analysis was descriptive and did not control for case mix, evaluate specific policies by any payer, or assess the full volume of visits among high utilizers. Movement of healthier or sicker individuals across payers could have contributed to temporal trends, but findings were similar across payers.
In conclusion, among 10 common medical conditions and three major payer populations and uninsured individuals in four states, inpatient admissions declined, and care shifted toward treat-and-release ED visits and observation stays. The number of inpatient admissions that began as observation stays also increased. Given these trends and the possibility that such shifts may be widespread and continue beyond 2013, quality of care, outcomes, and costs to patients warrant further evaluation.
Acknowledgments
The authors gratefully acknowledge Minya Sheng, MS (Truven Health Analytics) for assistance in programming and data management, and Paige Jackson, MS and Linda Lee, PhD, (Truven Health Analytics) for providing editorial review of the manuscript. They also wish to acknowledge the four HCUP Partner organizations that contributed to the 2009 and 2013 HCUP state databases used in this study: Georgia Hospital Association, Nebraska Hospital Association, South Carolina Revenue and Fiscal Affairs Office, and Tennessee Hospital Association.
Disclosure
Funding for this study was provided by the Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP) (Contract No. HHSA-290-2013-00002-C). The views expressed in this article are those of the authors and do not necessarily reflect those of the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services. The authors have no conflicts of interest to declare or financial disclosures.
For over a decade, private and public payers have implemented policies aimed at reducing rates of inpatient hospitalization. One approach for doing so is to improve ambulatory care, which can reduce the need for hospital-based acute care. Another approach is to stabilize acutely ill patients and discharge them from the emergency department (ED) or following a period of observation.1 Private payers are entering into value-based contracting arrangements with hospitals and health systems to improve the quality of ambulatory care and lower healthcare expenditures.2 Enrollment in managed care programs has grown among Medicaid recipients for similar reasons.3 Policies of the Centers for Medicare & Medicaid Services (CMS) encourage improvements in ambulatory care as well as observation of Medicare beneficiaries instead of inpatient admission in certain situations.4
Recent studies have documented declines in inpatient admissions and increases in treat-and-release observation stays and ED visits among Medicare beneficiaries.4-7 However, almost half of all hospitalizations unrelated to childbirth occur among patients with private insurance, Medicaid, or no insurance.8 Less is known about shifts in the nature of hospital-based acute care among these populations. Such shifts would have implications for quality of care, patient outcomes, and costs. Therefore, further investigation is warranted.
Our objective was to investigate recent trends in payer-specific population-based rates of adults using inpatient, observation, and ED services. We focused on 10 medical conditions that are common reasons for hospital-based acute care: heart failure, bacterial pneumonia, chronic obstructive pulmonary disease, asthma, dehydration, urinary tract infection, uncontrolled diabetes, diabetes with long-term complications, diabetes with short-term complications, and hypertension. These conditions constitute more than 20% of inpatient stays in the general medical service line, can be affected by improvements in ambulatory care, and provided a consistent set of diagnoses to track trends over time.9 We used 2009 and 2013 data from four states to examine trends among individuals with private insurance, Medicare, Medicaid, and no insurance.
METHODS
We obtained encounter-level data for Georgia, Nebraska, South Carolina, and Tennessee from the Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP).10 Using encrypted patient identifiers, we linked inpatient admissions from the 2009 and 2013 State Inpatient Databases, observation stays from the State Ambulatory Surgery and Services Databases, and ED visits from State Emergency Department Databases.
We defined the 10 medical conditions using numerator specifications from the ICD-9-CM v 5.0 AHRQ Prevention Quality Indicators (see Appendix). At most, 1 inpatient admission, 1 observation stay, and 1 ED visit for a study condition was counted for each adult in each year. Limiting the number of visits minimized the skew caused by multiple uses of the same service.
Using the American Community Survey, we calculated utilization rates for each type of service per 100,000 population in four payer and age groups: privately insured adults, Medicaid recipients, and uninsured adults 18 to 64 years, as well as Medicare beneficiaries 65 years and older. For each group, we also examined the origin of inpatient admissions—those who were directly admitted without evaluation in the ED, those admitted from the ED, and ED visits leading to observation stays and then inpatient admission.
RESULTS
Comparing 2009 and 2013, population-based rates of adults with 1 or more inpatient admissions for 10 common medical conditions declined, whereas rates of adults with treat-and-release observation stays rose. Changes in rates of treat-and-release ED visits varied across payers but were small relative to the substantial declines in inpatient admissions (Figure 1). In addition, a growing percentage of inpatient admissions began as observation stays and fewer adults were admitted directly, except among uninsured individuals (Figure 2).
Private Payers, 18 to 64 Years
The rate of adults with treat-and-release observation stays rose (+12.0%, 30 to 33 per 100,000 private payer population, P < 0.001). The rate of adults with treat-and-release ED visits declined (–9.0%, 713 to 648 per 100,000 population, P < 0.001), but by less than for inpatient admissions (–28.2%, 231 to 166 per 100,000 population, P < 0.001; Figure 1A). The percentage of inpatient admissions that began as observation stays rose (from 4.1% to 5.4%, P = 0.041), as did the percentage of admissions originating in the ED (from 66.4% to 71.5%, P ≤ 0.001; Figure 2).
Medicare, 65 Years and Older
The rate of adults with inpatient admissions declined (–17.0%, 2669 to 2216 per 100,000 Medicare population, P < 0.001). Rates rose for adults with treat-and-release ED visits (+3.9%, 1887 to 1961 per 100,000 population, P < 0.001) and treat-and-release observation stays (+32.9%, 234 to 311 per 100,000 population, P < 0.001; Figure 1B). The percentage of inpatient admissions that began as observation stays also rose (5.4% to 9.1%, P < 0.001; Figure 2).
Medicaid, 18 to 64 Years
The rate of adults with inpatient admissions declined (–15.3%, 1100 to 931 per 100,000 Medicaid population, P < 0.001), whereas treat-and-release ED visits remained flat (–1.5%, 4867 to 4792 per 100,000 population, P = 0.413) and treat-and-release observation stays rose (+18.1%, 196 to 232 per 100,000 population, P < 0.001; Figure 1C). The percentage of inpatient admissions that began as observation stays rose (5.9% to 8.1%, P = 0.022; Figure 2).
Uninsured, 18 to 64 Years
The rate of adults with inpatient admissions declined (–5.2%, 296 to 281 per 100,000 uninsured population, P = 0.003), whereas rates rose for treat-and-release ED visits (+8.9%, 1888 to 2057 per 100,000 population, P < 0.001) and treat-and-release observation stays (34.7%, 54 to 73 per 100,000 population, P < 0.001; Figure 1D). The source of inpatient admissions remained stable (Figure 2).
DISCUSSION
Data on hospital encounters from four states show that both ED visits and observation stays are playing an increasing role in hospital-based acute care for 10 common conditions among populations insured by private payers, Medicare, and Medicaid, as well as those without insurance. Compared with 2009, in 2013 substantially fewer individuals had inpatient admissions, and patients were more likely to be discharged from the ED or discharged following observation without receiving inpatient care. Additionally, an increasing percentage of inpatient admissions followed observation stays, whereas direct admissions declined.
Previous authors also have reported declines in inpatient stays for these same conditions.11 Others have reported increases in the use of observation stays for diverse conditions among patients with private insurance, Medicare beneficiaries, and veterans.4,12,13 The unique attributes of HCUP databases from these four states (eg, all-payer data including patient linkage numbers across inpatient, observation, and ED care) enabled us to assess concurrent shifts in hospital-based acute care from inpatient to outpatient care among multiple payer populations. A recent analysis reported declines in readmissions and increases in observation visits occurring within 30 days after hospitalization among Medicare beneficiaries with heart failure, acute myocardial infarction, or pneumonia.14 Future research should examine trends in readmissions and observation visits following hospitalization among multiple payer populations.
These shifts raise two important questions. The first pertains to quality of care, including outcomes. Although dedicated observation units with condition-specific care pathways can be associated with shorter stays and fewer admissions, many patients placed under observation are neither in dedicated units nor subject to care pathways.15,16 Systems for monitoring quality of care are less developed for observation care. The CMS publicly reports hospital-level data on quality of ED and inpatient care, including for several of the conditions we studied, but no measures apply to observation stays.17 Little is known about whether shifts from inpatient care to observation status or discharge from the ED are associated with different health outcomes.
The second issue is patients’ out-of-pocket costs. Although shifts from inpatient admissions to observation stays can reduce costs to payers,15 effects on patient out-of-pocket costs are uncertain and may vary. For privately insured patients, out-of-pocket costs may be up to four times higher for observation than for inpatient care.18 For Medicare beneficiaries, out-of-pocket costs can be higher for observation than for inpatient stays, particularly when patients receive costly medications or are discharged to skilled nursing facilities;19,20 however, having secondary insurance dramatically reduces out-of-pocket costs.21 We are not aware of data on Medicaid recipients or uninsured individuals.
This study has limitations. Only four states had data needed for these analyses, so generalization to other states is limited. Our analysis was descriptive and did not control for case mix, evaluate specific policies by any payer, or assess the full volume of visits among high utilizers. Movement of healthier or sicker individuals across payers could have contributed to temporal trends, but findings were similar across payers.
In conclusion, among 10 common medical conditions and three major payer populations and uninsured individuals in four states, inpatient admissions declined, and care shifted toward treat-and-release ED visits and observation stays. The number of inpatient admissions that began as observation stays also increased. Given these trends and the possibility that such shifts may be widespread and continue beyond 2013, quality of care, outcomes, and costs to patients warrant further evaluation.
Acknowledgments
The authors gratefully acknowledge Minya Sheng, MS (Truven Health Analytics) for assistance in programming and data management, and Paige Jackson, MS and Linda Lee, PhD, (Truven Health Analytics) for providing editorial review of the manuscript. They also wish to acknowledge the four HCUP Partner organizations that contributed to the 2009 and 2013 HCUP state databases used in this study: Georgia Hospital Association, Nebraska Hospital Association, South Carolina Revenue and Fiscal Affairs Office, and Tennessee Hospital Association.
Disclosure
Funding for this study was provided by the Agency for Healthcare Research and Quality (AHRQ), Healthcare Cost and Utilization Project (HCUP) (Contract No. HHSA-290-2013-00002-C). The views expressed in this article are those of the authors and do not necessarily reflect those of the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services. The authors have no conflicts of interest to declare or financial disclosures.
1. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156 PubMed
2. Song Z. Accountable care organizations in the U.S. health care system. J Clin Outcomes Manag. 2014;21(8):364-371. PubMed
3. Kaiser Family Foundation. Total Medicaid MCOs. State Health Facts. 2016. http://kff.org/other/state-indicator/total-medicaid-mcos/. Accessed July 19, 2016.
4. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. PubMed
5. Skinner HG, Blanchard J, Elixhauser A. Trends in emergency department visits, 2006–2011. HCUP Statistical Brief #179. September 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb179-Emergency-Department-Trends.pdf. Accessed July 21, 2016.
6. Medicare Payment Advisory Commission. Report to the Congress: Medicare and the Health Care Delivery System. June 2015. http://www.medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed October 6, 2016.
7. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. March 2016. http://www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed October 6, 2016.
8. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. HCUPnet. Agency for Healthcare Research and Quality, Rockville, MD. http://hcupnet.ahrq.gov/. Accessed October 6, 2016.
9. Fingar KR, Barrett ML, Elixhauser A, Stocks C, Steiner CA. Trends in potentially preventable inpatient hospital admissions and emergency department visits. HCUP Statistical Brief #195. November 2015. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb195-Potentially-Preventable-Hospitalizations.pdf. Accessed August 9, 2016.
10. Agency for Healthcare Research and Quality. HCUP Databases. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/databases.jsp. Accessed August 8, 2016.
11. Torio CM, Andrews RM. Geographic variation in potentially preventable hospitalizations for acute and chronic conditions, 2005–2011. HCUP Statistical Brief, #178. September 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb178-Preventable-Hospitalizations-by-Region.pdf. Accessed November 8, 2015.
12. Wright B, O’Shea AM, Ayyagari P, Ugwi PG, Kaboli P, Vaughan Sarrazin M. Observation rates at veterans’ hospitals more than doubled during 2005-13, similar to Medicare trends. Health Aff (Millwood). 2015;34(10):1730-1737. PubMed
13. Noel-Miller C, Lind K. Is observation status substituting for hospital readmission? Health Affairs Blog. October 28, 2015. Project Hope: The People-to-People Health Foundation, Inc., Millwood, VA. http://healthaffairs.org/blog/2015/10/28/is-observation-status-substituting-for-hospital-readmission/. Accessed November 8, 2015.
14. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. PubMed
15. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. PubMed
16. Sheehy AM. Dedicated observation unit for patients with “observation status” -- reply. JAMA Intern Med. 2014;174(2):301-302. PubMed
17. Medicare.gov. Measures and current data collection periods. Centers for Medicare and Medicaid Services, Baltimore, MD. https://www.medicare.gov/hospitalcompare/Data/Data-Updated.html#. Accessed July 19, 2016.
18. Jaffe S. You’re being observed in the hospital? Patients with private insurance better off than seniors. September 11, 2014. Kaiser Health News, Kaiser Family Foundation, Menlo Park, CA. http://khn.org/news/youre-being-observed-in-the-hospital-patients-with-private-insurance-are-better-off-than-seniors/. Accessed November 8, 2015.
19. Kangovi S, Cafardi SG, Smith RA, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10(11):718-723. PubMed
20. U.S. Department of Health and Human Services, Office of Inspector General. Hospitals’ use of observation stays and short inpatient stays for Medicare beneficiaries. Memorandum Report OEI-02-12-00040. July 29, 2013. U.S. Department of Health and Human Services, Washington, DC. https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed October 6, 2016.
21. Doyle BJ, Ettner SL, Nuckols TK. Supplemental insurance reduces out-of-pocket costs in Medicare observation services. J Hosp Med. 2016;11(7):502-504. PubMed
1. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156 PubMed
2. Song Z. Accountable care organizations in the U.S. health care system. J Clin Outcomes Manag. 2014;21(8):364-371. PubMed
3. Kaiser Family Foundation. Total Medicaid MCOs. State Health Facts. 2016. http://kff.org/other/state-indicator/total-medicaid-mcos/. Accessed July 19, 2016.
4. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. PubMed
5. Skinner HG, Blanchard J, Elixhauser A. Trends in emergency department visits, 2006–2011. HCUP Statistical Brief #179. September 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb179-Emergency-Department-Trends.pdf. Accessed July 21, 2016.
6. Medicare Payment Advisory Commission. Report to the Congress: Medicare and the Health Care Delivery System. June 2015. http://www.medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed October 6, 2016.
7. Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy. March 2016. http://www.medpac.gov/docs/default-source/reports/march-2016-report-to-the-congress-medicare-payment-policy.pdf?sfvrsn=0. Accessed October 6, 2016.
8. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. HCUPnet. Agency for Healthcare Research and Quality, Rockville, MD. http://hcupnet.ahrq.gov/. Accessed October 6, 2016.
9. Fingar KR, Barrett ML, Elixhauser A, Stocks C, Steiner CA. Trends in potentially preventable inpatient hospital admissions and emergency department visits. HCUP Statistical Brief #195. November 2015. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb195-Potentially-Preventable-Hospitalizations.pdf. Accessed August 9, 2016.
10. Agency for Healthcare Research and Quality. HCUP Databases. Agency for Healthcare Research and Quality, Rockville, MD. www.hcup-us.ahrq.gov/databases.jsp. Accessed August 8, 2016.
11. Torio CM, Andrews RM. Geographic variation in potentially preventable hospitalizations for acute and chronic conditions, 2005–2011. HCUP Statistical Brief, #178. September 2014. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb178-Preventable-Hospitalizations-by-Region.pdf. Accessed November 8, 2015.
12. Wright B, O’Shea AM, Ayyagari P, Ugwi PG, Kaboli P, Vaughan Sarrazin M. Observation rates at veterans’ hospitals more than doubled during 2005-13, similar to Medicare trends. Health Aff (Millwood). 2015;34(10):1730-1737. PubMed
13. Noel-Miller C, Lind K. Is observation status substituting for hospital readmission? Health Affairs Blog. October 28, 2015. Project Hope: The People-to-People Health Foundation, Inc., Millwood, VA. http://healthaffairs.org/blog/2015/10/28/is-observation-status-substituting-for-hospital-readmission/. Accessed November 8, 2015.
14. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374(16):1543-1551. PubMed
15. Ross MA, Hockenberry JM, Mutter R, Barrett M, Wheatley M, Pitts SR. Protocol-driven emergency department observation units offer savings, shorter stays, and reduced admissions. Health Aff (Millwood). 2013;32(12):2149-2156. PubMed
16. Sheehy AM. Dedicated observation unit for patients with “observation status” -- reply. JAMA Intern Med. 2014;174(2):301-302. PubMed
17. Medicare.gov. Measures and current data collection periods. Centers for Medicare and Medicaid Services, Baltimore, MD. https://www.medicare.gov/hospitalcompare/Data/Data-Updated.html#. Accessed July 19, 2016.
18. Jaffe S. You’re being observed in the hospital? Patients with private insurance better off than seniors. September 11, 2014. Kaiser Health News, Kaiser Family Foundation, Menlo Park, CA. http://khn.org/news/youre-being-observed-in-the-hospital-patients-with-private-insurance-are-better-off-than-seniors/. Accessed November 8, 2015.
19. Kangovi S, Cafardi SG, Smith RA, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10(11):718-723. PubMed
20. U.S. Department of Health and Human Services, Office of Inspector General. Hospitals’ use of observation stays and short inpatient stays for Medicare beneficiaries. Memorandum Report OEI-02-12-00040. July 29, 2013. U.S. Department of Health and Human Services, Washington, DC. https://oig.hhs.gov/oei/reports/oei-02-12-00040.pdf. Accessed October 6, 2016.
21. Doyle BJ, Ettner SL, Nuckols TK. Supplemental insurance reduces out-of-pocket costs in Medicare observation services. J Hosp Med. 2016;11(7):502-504. PubMed
© 2017 Society of Hospital Medicine
Telemetry monitor watchers reduce bedside nurses’ exposure to alarms by intercepting a high number of nonactionable alarms
Cardiac telemetry, designed to monitor hospitalized patients with active cardiac conditions, is highly utilized outside the intensive care unit (ICU) and generates a large number of automated alarms. Telemetry is also costly and requires substantial time and attention commitments from nursing and technician staff, who place and maintain the recording devices and address monitoring results. 1,2 The staff address and dismiss invalid alarms caused by telemetry artifacts, 2 such as the misreporting of patient movement as ventricular tachycardia/fibrillation (VT/VF) or the mimicking of asystole by a lead disconnection.
One strategy for addressing telemetry alarms is to have dedicated staff observe telemetry monitors and notify nurses with any events or findings. Studies conducted in the 1990s showed that dedicated monitor watchers, compared with automatically generated alarms alone, did not affect most outcomes 3 but can improve accuracy of arrhythmia detection. 4 Since then, given the advances in telemetry detection software, the effect of monitor watchers has not been evaluated. Mindful of the perceived burden of nonactionable telemetry alerts, we wanted to quantify the frequency of automated telemetry alerts in the wards and analyze the proportion of alerts deemed nonactionable by monitor watchers.
METHODS
We conducted this retrospective study at a 545-bed urban academic hospital in the United States. We reviewed the cases of all non-ICU patients with telemetry monitoring ordered. The telemetry order requires providers specify the indication for monitoring and adjust alert parameters for variables such as heart rate (preset to 60 and 100 beats per minute) and baseline rhythm (preset to normal sinus). Once a telemetry order is received, 5 leads are attached to the patient, and electrocardiographic data begin transmitting to a portable wireless telemetry monitor, or telemeter (Philips Intellispace Telemetry System), which in turn transmits to a central monitoring station in the progressive care unit (PCU; cardiac/pulmonary unit). The majority of patients on telemetry are in the PCU. Telemeters are also located in the general medicine, surgical, and neurologic non-ICU units. Data from a maximum of 96 telemeters in the hospital are simultaneously displayed in the central monitoring station.
At all times, two dedicated monitor watchers oversee the central monitoring station. Watchers are certified medical assistants with extra telemetry-specific training. Each receives a salary of $17 per hour (no benefits), or about $800 per 24-hour day for two watchers. Their role is to respond to audiovisual alerts triggered by the monitoring system—they either contact the bedside nurse or intercept the alert if deemed nonactionable. Consistent with the literature, 5 nonactionable alerts and alarms were defined as either “invalid” or “nuisance.” Invalid alerts and alarms misrepresent patient status (eg, patient motion is electronically interpreted as VT/VF), and nuisance alerts and alarms do not require clinical intervention (eg, persistent sinus tachycardia has already been communicated to the nurse or provider). Monitor watchers must intercept the alert within a limited amount of time: 15 seconds for suspected lethal alerts (asystole, VT/VF), 30 seconds for extreme tachycardia/bradycardia, and 60 seconds for lead displacement or low battery.
If a watcher does not intercept an alert—either intentionally or because time ran out—the alert generates an alarm, which automatically sends a text message to the patient’s nurse’s wireless phone. The nurse acknowledges the alarm and decides on further action. If the bedside nurse does not acknowledge the alarm within the same time frames as mentioned, the alarm is escalated, first to the unit charge nurse and then to the monitoring station charge nurse (Figure). All alerts are available for provider review at the central monitoring station for the duration of the telemetry order, and select telemetry strips are printed and filed in the patient’s paper chart.
For this study, we analyzed telemetry system data for all monitored non-ICU ward patients from August 1 through September 30, 2014. We focused on the rate and relevance of alerts (system-generated) and alarms (text message to nurse). As cardiac arrhythmias leading to cardiopulmonary arrest can potentially be detected by telemetry, we also reviewed all code team activations, which are recorded in a separate database that details time of code team activation, to evaluate for correlation with telemetry alerts.
RESULTS
Within the 2-month study period, there were 1917 admissions to, and 1370 transfers to, non-ICU floors, for a total of 3287 unique patient-admissions and 9704 total patient-days. There were 1199 patient admissions with telemetry orders (36.5% of all admissions), 4044 total patient-days of telemetry, and an average of 66.3 patients monitored per day. In addition, the system generated 20,775 alerts, an average of 341 per day, 5.1 per patient-day, 1 every 4 minutes. Overall, 18,051 alerts (87%) were intercepted by monitor watchers, preventing nurse text-alarms. Of all alerts, 91% were from patients on medicine services, including pulmonary and cardiology; 6% were from patients on the neurology floor; and 3% were from patients on the surgery floor.
Forty percent of all alerts were for heart rates deviating outside the ranges set by the provider; of these, the overwhelming majority were intercepted as nuisance alerts (Table). In addition, 26% of all alerts were for maintenance reasons, including issues with batteries or leads. Finally, 34% (6954) were suspected lethal alerts (asystole, VT/VF); of these, 74% (5170) were intercepted by monitor watchers, suggesting they were deemed invalid. None of the suspected lethal alerts triggered a code team activation, indicating there were no telemetry-documented asystole or VT/VF episodes prompting resuscitative efforts. During the study period, there were 7 code team activations. Of the 7 patients, 2 were on telemetry, and their code team activation was for hypoxia detected by pulse oximetry; the other 5 patients, not on telemetry, were found unresponsive or apneic, and 4 of them had confirmed pulseless electrical activity.
DISCUSSION
In small studies, other investigators have directly observed nurses for hours at a time and assessed their response to telemetry-related alarms. 1,2 In the present study, we found a very large number of telemetry-detected alerts over a continuous 2-month period. The large majority (87%) of alerts were manually intercepted by monitor watchers before being communicated to a nurse or provider, indicating these alerts did not affect clinical management and likely were either false positives or nonactionable. It is possible that repeat nonactionable alerts, like continued sinus tachycardia or bradycardia, affect decision making, but this may be outside the role of continuous cardiac telemetry. In addition, it is likely that all the lethal alarms (asystole, VT/VF) forwarded to the nurses were invalid, as none resulted in code team activations.
Addressing these alerts is a major issue, as frequent telemetry alarms can lead to alarm fatigue, a widely acknowledged safety concern. 6 Furthermore, nonactionable alarms are a time sink, diverting nursing attention from other patient care needs. Finally, nonactionable alarms, especially invalid alarms, can lead to adverse patient outcomes. Although we did not specifically evaluate for harm, an earlier case series found a potential for unnecessary interventions and device implantation as a result of reporting artifactual arrhythmias. 7
Our results also highlight the role of monitor watchers in intercepting nonactionable alarms and reducing the alarm burden on nurses. Other investigators have reported on computerized paging systems that directly alert only nurses, 8 or on escalated alarm paging systems that let noncrisis alarms self-resolve. 9 In contrast, our study used a hybrid 2-step telemetry-monitoring system—an escalated paging system designed to be sensitive and less likely than human monitoring to overlook events, followed by dedicated monitor watchers who are first-responders for a large number of alarms and who increase the specificity of alarms by screening for nonactionable alarms, thereby reducing the number of alarms transmitted to nurses. We think that, for most hospitals, monitor watchers are cost-effective, as their hourly wage is lower than that of registered nurses. Furthermore, monitor watchers can screen alerts faster because they are always at the monitoring station. Their presence reduces the amount of time that nurses need to divert from other clinical tasks in order to walk to the monitoring station to evaluate alerts.
Nonetheless, there remains a large number of nonactionable alerts forwarded as alarms to nurses, likely because of monitor watchers’ inability to address the multitude of alerts, and perhaps because of alarm fatigue. Although this study showed the utility of monitor watchers in decreasing telemetry alarms to nurses, other steps can be taken to reduce telemetry alarm fatigue. A systematic review of alarm frequency interventions 5 noted that detection algorithms can be improved to decrease telemetry alert false positives. Another solution, likely easier to implement, is to encourage appropriate alterations in telemetry alarm parameters, which can decrease the alarm proportion. 10 An essential step is to decrease inappropriate telemetry use regarding the indication for and duration of monitoring, as emphasized by the Choosing Wisely campaign championing American Heart Association (AHA) guidelines for appropriate telemetry use. 11 At our institution, 20.2% of telemetry orders were for indications outside AHA guidelines, and that percentage likely is an underestimate, as this was required self-reporting on ordering. 12 Telemetry may not frequently result in changes in management in the non-ICU setting, 13 and may lead to other harms such as worsening delirium, 14 so it needs to be evaluated for harm versus benefit per patient before order.
Cardiac telemetry in the non-ICU setting produces a large number of alerts and alarms. The vast majority are not seen or addressed by nurses or physicians, leading to a negligible impact on patient care decisions. Monitor watchers reduce the nursing burden in dealing with telemetry alerts, but we emphasize the need to take additional measures to reduce telemetry-related alerts and thereby reduce alarm-related harms and alarm fatigue.
Acknowledgments
The authors thank Torberg Tonnessen, who was instrumental in providing the telemetry and clinical data used in this study, as well as the numerous Johns Hopkins Bayview Medical Center nurses, patient care technicians, and monitor watchers who answered questions about telemetry processes and allowed their work to be observed.
Disclosure
Nothing to report.
1. Gazarian PK. Nurses’ response to frequency and types of electrocardiography alarms in a non-critical care setting: a descriptive study. Int J Nurs Stud . 2014;51(2):190-197. PubMed
2. Varpio L, Kuziemsky C, MacDonald C, King WJ. The helpful or hindering effects of in-hospital patient monitor alarms on nurses. Comput Inform Nurs . 2012;30(4):210-217. PubMed
3. Funk M, Parkosewich J, Johnson C, Stukshis I. Effect of dedicated monitor watchers on patients’ outcomes. Am J Crit Care . 1997;6(4):318-323. PubMed
4. Stukshis I, Funk M, Johnson C, Parkosewich J. Accuracy of detection of clinically important dysrhythmias with and without a dedicated monitor watcher. Am J Crit Care . 1997;6(4):312-317. PubMed
5. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med . 2016;11(2):136-144. PubMed
6. Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 national patient safety goal. Jt Comm Perspect . 2013;33(7):1, 3-4. PubMed
7. Knight BP, Pelosi F, Michaud GF, Strickberger SA, Morady F. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med . 1999;341(17):1270-1274. PubMed
8. Zwieg FH, Karfonta TL, Jeske LJ, et al. Arrhythmia detection and response in a monitoring technician and pocket paging system. Prog Cardiovasc Nurs . 1998;13(1):16-22, 33. PubMed
9. Cvach MM, Frank RJ, Doyle P, Stevens ZK. Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual . 2013;29(1):9-18. PubMed
10. Gross B, Dahl D, Nielsen L. Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol . 2011;Spring(suppl):29-36. PubMed
11. Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses [published correction appears in Circulation . 2005;111(3):378]. Circulation . 2004;110(17):2721-2746. PubMed
12. Chen S, Palchaudhuri S, Johnson A, Trost J, Ponor I, Zakaria S. Does this patient need telemetry? An analysis of telemetry ordering practices at an academic medical center. J Eval Clin Pract . 2017 Jan 27 [Epub ahead of print] . PubMed
13. Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol . 1995;76(12):960-965. PubMed
14. Chen S, Zakaria S. Behind the monitor—the trouble with telemetry: a teachable moment. JAMA Intern Med . 2015;175(6):894. PubMed
Cardiac telemetry, designed to monitor hospitalized patients with active cardiac conditions, is highly utilized outside the intensive care unit (ICU) and generates a large number of automated alarms. Telemetry is also costly and requires substantial time and attention commitments from nursing and technician staff, who place and maintain the recording devices and address monitoring results. 1,2 The staff address and dismiss invalid alarms caused by telemetry artifacts, 2 such as the misreporting of patient movement as ventricular tachycardia/fibrillation (VT/VF) or the mimicking of asystole by a lead disconnection.
One strategy for addressing telemetry alarms is to have dedicated staff observe telemetry monitors and notify nurses with any events or findings. Studies conducted in the 1990s showed that dedicated monitor watchers, compared with automatically generated alarms alone, did not affect most outcomes 3 but can improve accuracy of arrhythmia detection. 4 Since then, given the advances in telemetry detection software, the effect of monitor watchers has not been evaluated. Mindful of the perceived burden of nonactionable telemetry alerts, we wanted to quantify the frequency of automated telemetry alerts in the wards and analyze the proportion of alerts deemed nonactionable by monitor watchers.
METHODS
We conducted this retrospective study at a 545-bed urban academic hospital in the United States. We reviewed the cases of all non-ICU patients with telemetry monitoring ordered. The telemetry order requires providers specify the indication for monitoring and adjust alert parameters for variables such as heart rate (preset to 60 and 100 beats per minute) and baseline rhythm (preset to normal sinus). Once a telemetry order is received, 5 leads are attached to the patient, and electrocardiographic data begin transmitting to a portable wireless telemetry monitor, or telemeter (Philips Intellispace Telemetry System), which in turn transmits to a central monitoring station in the progressive care unit (PCU; cardiac/pulmonary unit). The majority of patients on telemetry are in the PCU. Telemeters are also located in the general medicine, surgical, and neurologic non-ICU units. Data from a maximum of 96 telemeters in the hospital are simultaneously displayed in the central monitoring station.
At all times, two dedicated monitor watchers oversee the central monitoring station. Watchers are certified medical assistants with extra telemetry-specific training. Each receives a salary of $17 per hour (no benefits), or about $800 per 24-hour day for two watchers. Their role is to respond to audiovisual alerts triggered by the monitoring system—they either contact the bedside nurse or intercept the alert if deemed nonactionable. Consistent with the literature, 5 nonactionable alerts and alarms were defined as either “invalid” or “nuisance.” Invalid alerts and alarms misrepresent patient status (eg, patient motion is electronically interpreted as VT/VF), and nuisance alerts and alarms do not require clinical intervention (eg, persistent sinus tachycardia has already been communicated to the nurse or provider). Monitor watchers must intercept the alert within a limited amount of time: 15 seconds for suspected lethal alerts (asystole, VT/VF), 30 seconds for extreme tachycardia/bradycardia, and 60 seconds for lead displacement or low battery.
If a watcher does not intercept an alert—either intentionally or because time ran out—the alert generates an alarm, which automatically sends a text message to the patient’s nurse’s wireless phone. The nurse acknowledges the alarm and decides on further action. If the bedside nurse does not acknowledge the alarm within the same time frames as mentioned, the alarm is escalated, first to the unit charge nurse and then to the monitoring station charge nurse (Figure). All alerts are available for provider review at the central monitoring station for the duration of the telemetry order, and select telemetry strips are printed and filed in the patient’s paper chart.
For this study, we analyzed telemetry system data for all monitored non-ICU ward patients from August 1 through September 30, 2014. We focused on the rate and relevance of alerts (system-generated) and alarms (text message to nurse). As cardiac arrhythmias leading to cardiopulmonary arrest can potentially be detected by telemetry, we also reviewed all code team activations, which are recorded in a separate database that details time of code team activation, to evaluate for correlation with telemetry alerts.
RESULTS
Within the 2-month study period, there were 1917 admissions to, and 1370 transfers to, non-ICU floors, for a total of 3287 unique patient-admissions and 9704 total patient-days. There were 1199 patient admissions with telemetry orders (36.5% of all admissions), 4044 total patient-days of telemetry, and an average of 66.3 patients monitored per day. In addition, the system generated 20,775 alerts, an average of 341 per day, 5.1 per patient-day, 1 every 4 minutes. Overall, 18,051 alerts (87%) were intercepted by monitor watchers, preventing nurse text-alarms. Of all alerts, 91% were from patients on medicine services, including pulmonary and cardiology; 6% were from patients on the neurology floor; and 3% were from patients on the surgery floor.
Forty percent of all alerts were for heart rates deviating outside the ranges set by the provider; of these, the overwhelming majority were intercepted as nuisance alerts (Table). In addition, 26% of all alerts were for maintenance reasons, including issues with batteries or leads. Finally, 34% (6954) were suspected lethal alerts (asystole, VT/VF); of these, 74% (5170) were intercepted by monitor watchers, suggesting they were deemed invalid. None of the suspected lethal alerts triggered a code team activation, indicating there were no telemetry-documented asystole or VT/VF episodes prompting resuscitative efforts. During the study period, there were 7 code team activations. Of the 7 patients, 2 were on telemetry, and their code team activation was for hypoxia detected by pulse oximetry; the other 5 patients, not on telemetry, were found unresponsive or apneic, and 4 of them had confirmed pulseless electrical activity.
DISCUSSION
In small studies, other investigators have directly observed nurses for hours at a time and assessed their response to telemetry-related alarms. 1,2 In the present study, we found a very large number of telemetry-detected alerts over a continuous 2-month period. The large majority (87%) of alerts were manually intercepted by monitor watchers before being communicated to a nurse or provider, indicating these alerts did not affect clinical management and likely were either false positives or nonactionable. It is possible that repeat nonactionable alerts, like continued sinus tachycardia or bradycardia, affect decision making, but this may be outside the role of continuous cardiac telemetry. In addition, it is likely that all the lethal alarms (asystole, VT/VF) forwarded to the nurses were invalid, as none resulted in code team activations.
Addressing these alerts is a major issue, as frequent telemetry alarms can lead to alarm fatigue, a widely acknowledged safety concern. 6 Furthermore, nonactionable alarms are a time sink, diverting nursing attention from other patient care needs. Finally, nonactionable alarms, especially invalid alarms, can lead to adverse patient outcomes. Although we did not specifically evaluate for harm, an earlier case series found a potential for unnecessary interventions and device implantation as a result of reporting artifactual arrhythmias. 7
Our results also highlight the role of monitor watchers in intercepting nonactionable alarms and reducing the alarm burden on nurses. Other investigators have reported on computerized paging systems that directly alert only nurses, 8 or on escalated alarm paging systems that let noncrisis alarms self-resolve. 9 In contrast, our study used a hybrid 2-step telemetry-monitoring system—an escalated paging system designed to be sensitive and less likely than human monitoring to overlook events, followed by dedicated monitor watchers who are first-responders for a large number of alarms and who increase the specificity of alarms by screening for nonactionable alarms, thereby reducing the number of alarms transmitted to nurses. We think that, for most hospitals, monitor watchers are cost-effective, as their hourly wage is lower than that of registered nurses. Furthermore, monitor watchers can screen alerts faster because they are always at the monitoring station. Their presence reduces the amount of time that nurses need to divert from other clinical tasks in order to walk to the monitoring station to evaluate alerts.
Nonetheless, there remains a large number of nonactionable alerts forwarded as alarms to nurses, likely because of monitor watchers’ inability to address the multitude of alerts, and perhaps because of alarm fatigue. Although this study showed the utility of monitor watchers in decreasing telemetry alarms to nurses, other steps can be taken to reduce telemetry alarm fatigue. A systematic review of alarm frequency interventions 5 noted that detection algorithms can be improved to decrease telemetry alert false positives. Another solution, likely easier to implement, is to encourage appropriate alterations in telemetry alarm parameters, which can decrease the alarm proportion. 10 An essential step is to decrease inappropriate telemetry use regarding the indication for and duration of monitoring, as emphasized by the Choosing Wisely campaign championing American Heart Association (AHA) guidelines for appropriate telemetry use. 11 At our institution, 20.2% of telemetry orders were for indications outside AHA guidelines, and that percentage likely is an underestimate, as this was required self-reporting on ordering. 12 Telemetry may not frequently result in changes in management in the non-ICU setting, 13 and may lead to other harms such as worsening delirium, 14 so it needs to be evaluated for harm versus benefit per patient before order.
Cardiac telemetry in the non-ICU setting produces a large number of alerts and alarms. The vast majority are not seen or addressed by nurses or physicians, leading to a negligible impact on patient care decisions. Monitor watchers reduce the nursing burden in dealing with telemetry alerts, but we emphasize the need to take additional measures to reduce telemetry-related alerts and thereby reduce alarm-related harms and alarm fatigue.
Acknowledgments
The authors thank Torberg Tonnessen, who was instrumental in providing the telemetry and clinical data used in this study, as well as the numerous Johns Hopkins Bayview Medical Center nurses, patient care technicians, and monitor watchers who answered questions about telemetry processes and allowed their work to be observed.
Disclosure
Nothing to report.
Cardiac telemetry, designed to monitor hospitalized patients with active cardiac conditions, is highly utilized outside the intensive care unit (ICU) and generates a large number of automated alarms. Telemetry is also costly and requires substantial time and attention commitments from nursing and technician staff, who place and maintain the recording devices and address monitoring results. 1,2 The staff address and dismiss invalid alarms caused by telemetry artifacts, 2 such as the misreporting of patient movement as ventricular tachycardia/fibrillation (VT/VF) or the mimicking of asystole by a lead disconnection.
One strategy for addressing telemetry alarms is to have dedicated staff observe telemetry monitors and notify nurses with any events or findings. Studies conducted in the 1990s showed that dedicated monitor watchers, compared with automatically generated alarms alone, did not affect most outcomes 3 but can improve accuracy of arrhythmia detection. 4 Since then, given the advances in telemetry detection software, the effect of monitor watchers has not been evaluated. Mindful of the perceived burden of nonactionable telemetry alerts, we wanted to quantify the frequency of automated telemetry alerts in the wards and analyze the proportion of alerts deemed nonactionable by monitor watchers.
METHODS
We conducted this retrospective study at a 545-bed urban academic hospital in the United States. We reviewed the cases of all non-ICU patients with telemetry monitoring ordered. The telemetry order requires providers specify the indication for monitoring and adjust alert parameters for variables such as heart rate (preset to 60 and 100 beats per minute) and baseline rhythm (preset to normal sinus). Once a telemetry order is received, 5 leads are attached to the patient, and electrocardiographic data begin transmitting to a portable wireless telemetry monitor, or telemeter (Philips Intellispace Telemetry System), which in turn transmits to a central monitoring station in the progressive care unit (PCU; cardiac/pulmonary unit). The majority of patients on telemetry are in the PCU. Telemeters are also located in the general medicine, surgical, and neurologic non-ICU units. Data from a maximum of 96 telemeters in the hospital are simultaneously displayed in the central monitoring station.
At all times, two dedicated monitor watchers oversee the central monitoring station. Watchers are certified medical assistants with extra telemetry-specific training. Each receives a salary of $17 per hour (no benefits), or about $800 per 24-hour day for two watchers. Their role is to respond to audiovisual alerts triggered by the monitoring system—they either contact the bedside nurse or intercept the alert if deemed nonactionable. Consistent with the literature, 5 nonactionable alerts and alarms were defined as either “invalid” or “nuisance.” Invalid alerts and alarms misrepresent patient status (eg, patient motion is electronically interpreted as VT/VF), and nuisance alerts and alarms do not require clinical intervention (eg, persistent sinus tachycardia has already been communicated to the nurse or provider). Monitor watchers must intercept the alert within a limited amount of time: 15 seconds for suspected lethal alerts (asystole, VT/VF), 30 seconds for extreme tachycardia/bradycardia, and 60 seconds for lead displacement or low battery.
If a watcher does not intercept an alert—either intentionally or because time ran out—the alert generates an alarm, which automatically sends a text message to the patient’s nurse’s wireless phone. The nurse acknowledges the alarm and decides on further action. If the bedside nurse does not acknowledge the alarm within the same time frames as mentioned, the alarm is escalated, first to the unit charge nurse and then to the monitoring station charge nurse (Figure). All alerts are available for provider review at the central monitoring station for the duration of the telemetry order, and select telemetry strips are printed and filed in the patient’s paper chart.
For this study, we analyzed telemetry system data for all monitored non-ICU ward patients from August 1 through September 30, 2014. We focused on the rate and relevance of alerts (system-generated) and alarms (text message to nurse). As cardiac arrhythmias leading to cardiopulmonary arrest can potentially be detected by telemetry, we also reviewed all code team activations, which are recorded in a separate database that details time of code team activation, to evaluate for correlation with telemetry alerts.
RESULTS
Within the 2-month study period, there were 1917 admissions to, and 1370 transfers to, non-ICU floors, for a total of 3287 unique patient-admissions and 9704 total patient-days. There were 1199 patient admissions with telemetry orders (36.5% of all admissions), 4044 total patient-days of telemetry, and an average of 66.3 patients monitored per day. In addition, the system generated 20,775 alerts, an average of 341 per day, 5.1 per patient-day, 1 every 4 minutes. Overall, 18,051 alerts (87%) were intercepted by monitor watchers, preventing nurse text-alarms. Of all alerts, 91% were from patients on medicine services, including pulmonary and cardiology; 6% were from patients on the neurology floor; and 3% were from patients on the surgery floor.
Forty percent of all alerts were for heart rates deviating outside the ranges set by the provider; of these, the overwhelming majority were intercepted as nuisance alerts (Table). In addition, 26% of all alerts were for maintenance reasons, including issues with batteries or leads. Finally, 34% (6954) were suspected lethal alerts (asystole, VT/VF); of these, 74% (5170) were intercepted by monitor watchers, suggesting they were deemed invalid. None of the suspected lethal alerts triggered a code team activation, indicating there were no telemetry-documented asystole or VT/VF episodes prompting resuscitative efforts. During the study period, there were 7 code team activations. Of the 7 patients, 2 were on telemetry, and their code team activation was for hypoxia detected by pulse oximetry; the other 5 patients, not on telemetry, were found unresponsive or apneic, and 4 of them had confirmed pulseless electrical activity.
DISCUSSION
In small studies, other investigators have directly observed nurses for hours at a time and assessed their response to telemetry-related alarms. 1,2 In the present study, we found a very large number of telemetry-detected alerts over a continuous 2-month period. The large majority (87%) of alerts were manually intercepted by monitor watchers before being communicated to a nurse or provider, indicating these alerts did not affect clinical management and likely were either false positives or nonactionable. It is possible that repeat nonactionable alerts, like continued sinus tachycardia or bradycardia, affect decision making, but this may be outside the role of continuous cardiac telemetry. In addition, it is likely that all the lethal alarms (asystole, VT/VF) forwarded to the nurses were invalid, as none resulted in code team activations.
Addressing these alerts is a major issue, as frequent telemetry alarms can lead to alarm fatigue, a widely acknowledged safety concern. 6 Furthermore, nonactionable alarms are a time sink, diverting nursing attention from other patient care needs. Finally, nonactionable alarms, especially invalid alarms, can lead to adverse patient outcomes. Although we did not specifically evaluate for harm, an earlier case series found a potential for unnecessary interventions and device implantation as a result of reporting artifactual arrhythmias. 7
Our results also highlight the role of monitor watchers in intercepting nonactionable alarms and reducing the alarm burden on nurses. Other investigators have reported on computerized paging systems that directly alert only nurses, 8 or on escalated alarm paging systems that let noncrisis alarms self-resolve. 9 In contrast, our study used a hybrid 2-step telemetry-monitoring system—an escalated paging system designed to be sensitive and less likely than human monitoring to overlook events, followed by dedicated monitor watchers who are first-responders for a large number of alarms and who increase the specificity of alarms by screening for nonactionable alarms, thereby reducing the number of alarms transmitted to nurses. We think that, for most hospitals, monitor watchers are cost-effective, as their hourly wage is lower than that of registered nurses. Furthermore, monitor watchers can screen alerts faster because they are always at the monitoring station. Their presence reduces the amount of time that nurses need to divert from other clinical tasks in order to walk to the monitoring station to evaluate alerts.
Nonetheless, there remains a large number of nonactionable alerts forwarded as alarms to nurses, likely because of monitor watchers’ inability to address the multitude of alerts, and perhaps because of alarm fatigue. Although this study showed the utility of monitor watchers in decreasing telemetry alarms to nurses, other steps can be taken to reduce telemetry alarm fatigue. A systematic review of alarm frequency interventions 5 noted that detection algorithms can be improved to decrease telemetry alert false positives. Another solution, likely easier to implement, is to encourage appropriate alterations in telemetry alarm parameters, which can decrease the alarm proportion. 10 An essential step is to decrease inappropriate telemetry use regarding the indication for and duration of monitoring, as emphasized by the Choosing Wisely campaign championing American Heart Association (AHA) guidelines for appropriate telemetry use. 11 At our institution, 20.2% of telemetry orders were for indications outside AHA guidelines, and that percentage likely is an underestimate, as this was required self-reporting on ordering. 12 Telemetry may not frequently result in changes in management in the non-ICU setting, 13 and may lead to other harms such as worsening delirium, 14 so it needs to be evaluated for harm versus benefit per patient before order.
Cardiac telemetry in the non-ICU setting produces a large number of alerts and alarms. The vast majority are not seen or addressed by nurses or physicians, leading to a negligible impact on patient care decisions. Monitor watchers reduce the nursing burden in dealing with telemetry alerts, but we emphasize the need to take additional measures to reduce telemetry-related alerts and thereby reduce alarm-related harms and alarm fatigue.
Acknowledgments
The authors thank Torberg Tonnessen, who was instrumental in providing the telemetry and clinical data used in this study, as well as the numerous Johns Hopkins Bayview Medical Center nurses, patient care technicians, and monitor watchers who answered questions about telemetry processes and allowed their work to be observed.
Disclosure
Nothing to report.
1. Gazarian PK. Nurses’ response to frequency and types of electrocardiography alarms in a non-critical care setting: a descriptive study. Int J Nurs Stud . 2014;51(2):190-197. PubMed
2. Varpio L, Kuziemsky C, MacDonald C, King WJ. The helpful or hindering effects of in-hospital patient monitor alarms on nurses. Comput Inform Nurs . 2012;30(4):210-217. PubMed
3. Funk M, Parkosewich J, Johnson C, Stukshis I. Effect of dedicated monitor watchers on patients’ outcomes. Am J Crit Care . 1997;6(4):318-323. PubMed
4. Stukshis I, Funk M, Johnson C, Parkosewich J. Accuracy of detection of clinically important dysrhythmias with and without a dedicated monitor watcher. Am J Crit Care . 1997;6(4):312-317. PubMed
5. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med . 2016;11(2):136-144. PubMed
6. Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 national patient safety goal. Jt Comm Perspect . 2013;33(7):1, 3-4. PubMed
7. Knight BP, Pelosi F, Michaud GF, Strickberger SA, Morady F. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med . 1999;341(17):1270-1274. PubMed
8. Zwieg FH, Karfonta TL, Jeske LJ, et al. Arrhythmia detection and response in a monitoring technician and pocket paging system. Prog Cardiovasc Nurs . 1998;13(1):16-22, 33. PubMed
9. Cvach MM, Frank RJ, Doyle P, Stevens ZK. Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual . 2013;29(1):9-18. PubMed
10. Gross B, Dahl D, Nielsen L. Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol . 2011;Spring(suppl):29-36. PubMed
11. Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses [published correction appears in Circulation . 2005;111(3):378]. Circulation . 2004;110(17):2721-2746. PubMed
12. Chen S, Palchaudhuri S, Johnson A, Trost J, Ponor I, Zakaria S. Does this patient need telemetry? An analysis of telemetry ordering practices at an academic medical center. J Eval Clin Pract . 2017 Jan 27 [Epub ahead of print] . PubMed
13. Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol . 1995;76(12):960-965. PubMed
14. Chen S, Zakaria S. Behind the monitor—the trouble with telemetry: a teachable moment. JAMA Intern Med . 2015;175(6):894. PubMed
1. Gazarian PK. Nurses’ response to frequency and types of electrocardiography alarms in a non-critical care setting: a descriptive study. Int J Nurs Stud . 2014;51(2):190-197. PubMed
2. Varpio L, Kuziemsky C, MacDonald C, King WJ. The helpful or hindering effects of in-hospital patient monitor alarms on nurses. Comput Inform Nurs . 2012;30(4):210-217. PubMed
3. Funk M, Parkosewich J, Johnson C, Stukshis I. Effect of dedicated monitor watchers on patients’ outcomes. Am J Crit Care . 1997;6(4):318-323. PubMed
4. Stukshis I, Funk M, Johnson C, Parkosewich J. Accuracy of detection of clinically important dysrhythmias with and without a dedicated monitor watcher. Am J Crit Care . 1997;6(4):312-317. PubMed
5. Paine CW, Goel VV, Ely E, et al. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med . 2016;11(2):136-144. PubMed
6. Joint Commission on Accreditation of Healthcare Organizations. The Joint Commission announces 2014 national patient safety goal. Jt Comm Perspect . 2013;33(7):1, 3-4. PubMed
7. Knight BP, Pelosi F, Michaud GF, Strickberger SA, Morady F. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med . 1999;341(17):1270-1274. PubMed
8. Zwieg FH, Karfonta TL, Jeske LJ, et al. Arrhythmia detection and response in a monitoring technician and pocket paging system. Prog Cardiovasc Nurs . 1998;13(1):16-22, 33. PubMed
9. Cvach MM, Frank RJ, Doyle P, Stevens ZK. Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual . 2013;29(1):9-18. PubMed
10. Gross B, Dahl D, Nielsen L. Physiologic monitoring alarm load on medical/surgical floors of a community hospital. Biomed Instrum Technol . 2011;Spring(suppl):29-36. PubMed
11. Drew BJ, Califf RM, Funk M, et al; American Heart Association; Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses [published correction appears in Circulation . 2005;111(3):378]. Circulation . 2004;110(17):2721-2746. PubMed
12. Chen S, Palchaudhuri S, Johnson A, Trost J, Ponor I, Zakaria S. Does this patient need telemetry? An analysis of telemetry ordering practices at an academic medical center. J Eval Clin Pract . 2017 Jan 27 [Epub ahead of print] . PubMed
13. Estrada CA, Rosman HS, Prasad NK, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol . 1995;76(12):960-965. PubMed
14. Chen S, Zakaria S. Behind the monitor—the trouble with telemetry: a teachable moment. JAMA Intern Med . 2015;175(6):894. PubMed
© 2017 Society of Hospital Medicine
Perceptions of hospital-dependent patients on their needs for hospitalization
In the United States, patients 65 years old or older accounted for more than one third of inpatient stays and 42% of inpatient care spending in 2012.1 Despite the identification of risk factors, the implementation of an array of interventions, and the institution of penalties on hospitals, a subset of older adults continues to spend significant time in the hospital.2,3
Hospital dependency is a concept that was only recently described. It identifies patients who improve while in the hospital but quickly deteriorate after leaving the hospital, resulting in recurring hospitalizations.4 Although little is known about hospital-dependent patients, studies have explored patients’ perspectives on readmissions.5,6 Nevertheless, it remains unclear whether there are individuals for whom frequent and prolonged hospitalizations are appropriate, and whether there are undisclosed factors that, if addressed, could decrease their hospital dependency. We conducted an exploratory study to ascertain hospital-dependent patients’ perspectives on their needs for hospitalizations.
METHODS
Study Design
This study was approved by the Yale University Institutional Review Board. From March 2015 to September 2015, Dr. Liu conducted semistructured explorative interviews with patients on the medical units of an academic medical center. Dr. Liu was not directly involved in the care of these patients. An interview guide that includes open-ended questions was created to elicit patients’ perspectives on their need for hospitalizations, health status, and outside-hospital support. This guide was pilot-tested with 6 patients, whose transcripts were not included in the final analysis, to assess for ease of understanding. After the pilot interviews, the questions were revised, and the final guide consists of 12 questions (Supplemental Table).
Recruitment
We used predetermined criteria and a purposeful sampling strategy to select potential study participants. We identified participants by periodically (~once a week) reviewing the electronic medical records of all patients admitted to the medicine service during the study period. Eligible patients were 65 years old or older and had at least 3 hospitalizations over the preceding 6 months. Patients were excluded if they met our chronic critical illness criteria: mechanical ventilation for more than 21 days, history of tracheotomy for failed weaning from mechanical ventilation,7 presence of a conservator, or admission only for comfort measures. Participants were recruited until no new themes emerged.
Data Collection
Twenty-nine patients were eligible. We obtained permission from their inpatient providers to approach them about the study. Of the 29 patients, 26 agreed to be interviewed, and 3 declined. Of the 26 participants, 6 underwent pilot interviews, and 20 underwent formal interviews with use of the finalized interview guide. The interviews, conducted in the hospital while the participants were hospitalized, lasted 17 minutes on average. The interviews were transcribed and iteratively analyzed. The themes that emerged from the initial interviews were further explored and validated in subsequent interviews. Interviews were conducted until theoretical saturation was reached and no new themes were derived from them. Demographic information, including age, sex, ethnicity, and marital status, was also collected.
Analysis
Interviews were digitally recorded and transcribed. Independently, two investigators used Atlas Ti software to analyze and code the interview transcriptions. An inductive approach was used to identify new codes from the data.8 The coders then met to discuss repeating ideas based on the codes. When a code was identified by one coder but not the other, or when there was disagreement about interpretation of a code, the coders returned to the relevant text to reach consensus and to determine whether to include or discard the code.9 We then organized and reorganized repeating ideas based on their conceptual similarities to determine the themes and subthemes.9
RESULTS
Twenty patients participated in the formal interviews. Participants’ baseline characteristics are listed in Table 1, and four dominant themes, and their subthemes and exemplary quotations, are listed in Table 2.
Perspectives on Hospital Care
Participants perceived their hospitalizations as inevitable and necessary for survival: “I think if I haven’t come to the hospital, I probably would have died.” Furthermore, participants thought only the hospital had the resources to help them (“The medications they were giving me … you can get that in the hospital but not outside the hospital”) and sustain them (“You are like an old car, and it breaks down little by little, so you have to go in periodically and get the problem fixed, so you will drive it around for a while”).
Feeling Safe in Hospital. Asked how being in the hospital makes them feel, participants attributed their feelings of safety to the constant observation, the availability of providers and nurses, and the idea that hospital care is helping. As one participant stated, “Makes me feel safer in case you go into something like cardiac arrest. You are right here where they can help you.”
Outside-Hospital Support. Despite multiple hospitalizations, most participants reported having social support (“I have the aide. I got the nurses come in. I have my daughter …”), physical support, and medical support (“I have all the doctors”) outside the hospital. A minority of participants questioned the usefulness of the services. One participant described declining the help of visiting nurses because she wanted to be independent and thought that, despite recurrent hospitalizations for physical symptoms, she still had the ability to manage her own medications.
Goals-of-Care Discussion. Some participants reported inadequate discussions about goals of care, health priorities, and health trajectories. In their reports, this inadequacy included not thinking about their goals, despite continued health decline. One participant stated, “Oh, God, I don’t know if I had any conversation like that. … I think until it is really brought to the front, you don’t make a decision really if you don’t have to.” Citing the value of a more established relationship and deeper trust, participants preferred having these serious and personal discussions with their ambulatory care clinicians: “Because I know my doctor much closer. I have been with him for a number of years. The doctors in the hospital seem to be nice and competent, but I don’t know them.”
DISCUSSION
Participants considered their hospitalizations a necessity and reported feeling safe in the hospital. Given that most already had support outside the hospital, increasing community services may be inadequate to alter participants’ perceived hospital care needs. On the other hand, a few participants reported declining services that might have prevented hospitalizations. Although there has been a study of treatment refusal among older adults with advanced illnesses,10 not much is known about refusal of services among this population. Investigators should examine the reasons for refusing services and the effect that refusal has on hospitalizations. Furthermore, although it would have been informative to ascertain clinician perspectives as well, we focused on patient perspectives because less is known on this topic.
Some participants noted their lack of discussion with their clinicians about healthcare goals and probable health trajectories. Barriers to goals-of-care discussion among this highly vulnerable population have been researched from the perspectives of clinicians and other health professionals but not patients themselves.11,12 Of particular concern in our study is the participant-noted lack of discussion about health trajectories and health priorities, given the decline that occurs in this population and even in those with good care. This inadequacy in discussion suggests continued hospital care may not always be consistent with a patient’s goals. Patients’ desire to have this discussion with their clinicians, with whom they have a relationship, supports the need to involve ambulatory care clinicians, or ensure these patients are cared for by the same clinicians, across healthcare settings.13,14 Whoever provides the care, the clinician must align treatment with the patient’s goal, whether it is to continue hospital-level care or to transition to palliative care. Such an approach also reflects the core elements of person-centered care.15
Study Limitations
Participants were recruited from the medicine service at a single large academic center, limiting the study’s generalizability to patients admitted to surgical services or community hospitals. The patients in this small sample were English-speaking and predominantly Caucasian, so our findings may not represent the perspectives of non-English-speaking or minority patients. We did not perform statistical analysis to quantify intercoder reliability. Last, as this was a qualitative study, we cannot comment on the relative importance or prevalence of the reasons cited for frequent hospitalizations, and we cannot estimate the proportion of patients who had recurrent hospitalizations and were hospital-dependent.
Implication
Although quantitative research is needed to confirm our findings, the hospital-dependent patients in this study thought their survival required hospital-level care and resources. From their perspective, increasing posthospital and community support may be insufficient to prevent some hospitalizations. The lack of goals-of-care discussion supports attempts to increase efforts to facilitate discussion about health trajectories and health priorities between patients and their preferred clinicians.
Acknowledgments
The authors thank Dr. Grace Jenq for providing feedback on the study design.
Disclosure
Nothing to report.
1. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012: Statistical Brief 180. Rockville, MD: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project; 2014. http://www.ncbi.nlm.nih.gov/books/NBK259100/. Published October 2014. Accessed February 17, 2016.
2. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
3. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. PubMed
4. Reuben DB, Tinetti ME. The hospital-dependent patient. N Engl J Med. 2014;370(8):694-697. PubMed
5. Enguidanos S, Coulourides Kogan AM, Schreibeis-Baum H, Lendon J, Lorenz K. “Because I was sick”: seriously ill veterans’ perspectives on reason for 30-day readmissions. J Am Geriatr Soc. 2015;63(3):537-542. PubMed
6. Kangovi S, Grande D, Meehan P, Mitra N, Shannon R, Long JA. Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709-712. PubMed
7. Lamas D. Chronic critical illness. N Engl J Med. 2014;370(2):175-177. PubMed
8. Saldana J. Fundamentals of Qualitative Research. Cary, NC: Oxford University Press; 2011.
9. Auerbach CF, Silverstein LB. Qualitative Data: An Introduction to Coding and Analysis. New York, NY: New York University Press; 2003.
10. Rothman MD, Van Ness PH, O’Leary JR, Fried TR. Refusal of medical and surgical interventions by older persons with advanced chronic disease. J Gen Intern Med. 2007;22(7):982-987. PubMed
11. You JJ, Downar J, Fowler RA, et al; Canadian Researchers at the End of Life Network. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549-556. PubMed
12. Schoenborn NL, Bowman TL 2nd, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678. PubMed
13. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. PubMed
14. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. PubMed
15. American Geriatrics Society Expert Panel on Person-Centered Care. Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64(1):15-18. PubMed
In the United States, patients 65 years old or older accounted for more than one third of inpatient stays and 42% of inpatient care spending in 2012.1 Despite the identification of risk factors, the implementation of an array of interventions, and the institution of penalties on hospitals, a subset of older adults continues to spend significant time in the hospital.2,3
Hospital dependency is a concept that was only recently described. It identifies patients who improve while in the hospital but quickly deteriorate after leaving the hospital, resulting in recurring hospitalizations.4 Although little is known about hospital-dependent patients, studies have explored patients’ perspectives on readmissions.5,6 Nevertheless, it remains unclear whether there are individuals for whom frequent and prolonged hospitalizations are appropriate, and whether there are undisclosed factors that, if addressed, could decrease their hospital dependency. We conducted an exploratory study to ascertain hospital-dependent patients’ perspectives on their needs for hospitalizations.
METHODS
Study Design
This study was approved by the Yale University Institutional Review Board. From March 2015 to September 2015, Dr. Liu conducted semistructured explorative interviews with patients on the medical units of an academic medical center. Dr. Liu was not directly involved in the care of these patients. An interview guide that includes open-ended questions was created to elicit patients’ perspectives on their need for hospitalizations, health status, and outside-hospital support. This guide was pilot-tested with 6 patients, whose transcripts were not included in the final analysis, to assess for ease of understanding. After the pilot interviews, the questions were revised, and the final guide consists of 12 questions (Supplemental Table).
Recruitment
We used predetermined criteria and a purposeful sampling strategy to select potential study participants. We identified participants by periodically (~once a week) reviewing the electronic medical records of all patients admitted to the medicine service during the study period. Eligible patients were 65 years old or older and had at least 3 hospitalizations over the preceding 6 months. Patients were excluded if they met our chronic critical illness criteria: mechanical ventilation for more than 21 days, history of tracheotomy for failed weaning from mechanical ventilation,7 presence of a conservator, or admission only for comfort measures. Participants were recruited until no new themes emerged.
Data Collection
Twenty-nine patients were eligible. We obtained permission from their inpatient providers to approach them about the study. Of the 29 patients, 26 agreed to be interviewed, and 3 declined. Of the 26 participants, 6 underwent pilot interviews, and 20 underwent formal interviews with use of the finalized interview guide. The interviews, conducted in the hospital while the participants were hospitalized, lasted 17 minutes on average. The interviews were transcribed and iteratively analyzed. The themes that emerged from the initial interviews were further explored and validated in subsequent interviews. Interviews were conducted until theoretical saturation was reached and no new themes were derived from them. Demographic information, including age, sex, ethnicity, and marital status, was also collected.
Analysis
Interviews were digitally recorded and transcribed. Independently, two investigators used Atlas Ti software to analyze and code the interview transcriptions. An inductive approach was used to identify new codes from the data.8 The coders then met to discuss repeating ideas based on the codes. When a code was identified by one coder but not the other, or when there was disagreement about interpretation of a code, the coders returned to the relevant text to reach consensus and to determine whether to include or discard the code.9 We then organized and reorganized repeating ideas based on their conceptual similarities to determine the themes and subthemes.9
RESULTS
Twenty patients participated in the formal interviews. Participants’ baseline characteristics are listed in Table 1, and four dominant themes, and their subthemes and exemplary quotations, are listed in Table 2.
Perspectives on Hospital Care
Participants perceived their hospitalizations as inevitable and necessary for survival: “I think if I haven’t come to the hospital, I probably would have died.” Furthermore, participants thought only the hospital had the resources to help them (“The medications they were giving me … you can get that in the hospital but not outside the hospital”) and sustain them (“You are like an old car, and it breaks down little by little, so you have to go in periodically and get the problem fixed, so you will drive it around for a while”).
Feeling Safe in Hospital. Asked how being in the hospital makes them feel, participants attributed their feelings of safety to the constant observation, the availability of providers and nurses, and the idea that hospital care is helping. As one participant stated, “Makes me feel safer in case you go into something like cardiac arrest. You are right here where they can help you.”
Outside-Hospital Support. Despite multiple hospitalizations, most participants reported having social support (“I have the aide. I got the nurses come in. I have my daughter …”), physical support, and medical support (“I have all the doctors”) outside the hospital. A minority of participants questioned the usefulness of the services. One participant described declining the help of visiting nurses because she wanted to be independent and thought that, despite recurrent hospitalizations for physical symptoms, she still had the ability to manage her own medications.
Goals-of-Care Discussion. Some participants reported inadequate discussions about goals of care, health priorities, and health trajectories. In their reports, this inadequacy included not thinking about their goals, despite continued health decline. One participant stated, “Oh, God, I don’t know if I had any conversation like that. … I think until it is really brought to the front, you don’t make a decision really if you don’t have to.” Citing the value of a more established relationship and deeper trust, participants preferred having these serious and personal discussions with their ambulatory care clinicians: “Because I know my doctor much closer. I have been with him for a number of years. The doctors in the hospital seem to be nice and competent, but I don’t know them.”
DISCUSSION
Participants considered their hospitalizations a necessity and reported feeling safe in the hospital. Given that most already had support outside the hospital, increasing community services may be inadequate to alter participants’ perceived hospital care needs. On the other hand, a few participants reported declining services that might have prevented hospitalizations. Although there has been a study of treatment refusal among older adults with advanced illnesses,10 not much is known about refusal of services among this population. Investigators should examine the reasons for refusing services and the effect that refusal has on hospitalizations. Furthermore, although it would have been informative to ascertain clinician perspectives as well, we focused on patient perspectives because less is known on this topic.
Some participants noted their lack of discussion with their clinicians about healthcare goals and probable health trajectories. Barriers to goals-of-care discussion among this highly vulnerable population have been researched from the perspectives of clinicians and other health professionals but not patients themselves.11,12 Of particular concern in our study is the participant-noted lack of discussion about health trajectories and health priorities, given the decline that occurs in this population and even in those with good care. This inadequacy in discussion suggests continued hospital care may not always be consistent with a patient’s goals. Patients’ desire to have this discussion with their clinicians, with whom they have a relationship, supports the need to involve ambulatory care clinicians, or ensure these patients are cared for by the same clinicians, across healthcare settings.13,14 Whoever provides the care, the clinician must align treatment with the patient’s goal, whether it is to continue hospital-level care or to transition to palliative care. Such an approach also reflects the core elements of person-centered care.15
Study Limitations
Participants were recruited from the medicine service at a single large academic center, limiting the study’s generalizability to patients admitted to surgical services or community hospitals. The patients in this small sample were English-speaking and predominantly Caucasian, so our findings may not represent the perspectives of non-English-speaking or minority patients. We did not perform statistical analysis to quantify intercoder reliability. Last, as this was a qualitative study, we cannot comment on the relative importance or prevalence of the reasons cited for frequent hospitalizations, and we cannot estimate the proportion of patients who had recurrent hospitalizations and were hospital-dependent.
Implication
Although quantitative research is needed to confirm our findings, the hospital-dependent patients in this study thought their survival required hospital-level care and resources. From their perspective, increasing posthospital and community support may be insufficient to prevent some hospitalizations. The lack of goals-of-care discussion supports attempts to increase efforts to facilitate discussion about health trajectories and health priorities between patients and their preferred clinicians.
Acknowledgments
The authors thank Dr. Grace Jenq for providing feedback on the study design.
Disclosure
Nothing to report.
In the United States, patients 65 years old or older accounted for more than one third of inpatient stays and 42% of inpatient care spending in 2012.1 Despite the identification of risk factors, the implementation of an array of interventions, and the institution of penalties on hospitals, a subset of older adults continues to spend significant time in the hospital.2,3
Hospital dependency is a concept that was only recently described. It identifies patients who improve while in the hospital but quickly deteriorate after leaving the hospital, resulting in recurring hospitalizations.4 Although little is known about hospital-dependent patients, studies have explored patients’ perspectives on readmissions.5,6 Nevertheless, it remains unclear whether there are individuals for whom frequent and prolonged hospitalizations are appropriate, and whether there are undisclosed factors that, if addressed, could decrease their hospital dependency. We conducted an exploratory study to ascertain hospital-dependent patients’ perspectives on their needs for hospitalizations.
METHODS
Study Design
This study was approved by the Yale University Institutional Review Board. From March 2015 to September 2015, Dr. Liu conducted semistructured explorative interviews with patients on the medical units of an academic medical center. Dr. Liu was not directly involved in the care of these patients. An interview guide that includes open-ended questions was created to elicit patients’ perspectives on their need for hospitalizations, health status, and outside-hospital support. This guide was pilot-tested with 6 patients, whose transcripts were not included in the final analysis, to assess for ease of understanding. After the pilot interviews, the questions were revised, and the final guide consists of 12 questions (Supplemental Table).
Recruitment
We used predetermined criteria and a purposeful sampling strategy to select potential study participants. We identified participants by periodically (~once a week) reviewing the electronic medical records of all patients admitted to the medicine service during the study period. Eligible patients were 65 years old or older and had at least 3 hospitalizations over the preceding 6 months. Patients were excluded if they met our chronic critical illness criteria: mechanical ventilation for more than 21 days, history of tracheotomy for failed weaning from mechanical ventilation,7 presence of a conservator, or admission only for comfort measures. Participants were recruited until no new themes emerged.
Data Collection
Twenty-nine patients were eligible. We obtained permission from their inpatient providers to approach them about the study. Of the 29 patients, 26 agreed to be interviewed, and 3 declined. Of the 26 participants, 6 underwent pilot interviews, and 20 underwent formal interviews with use of the finalized interview guide. The interviews, conducted in the hospital while the participants were hospitalized, lasted 17 minutes on average. The interviews were transcribed and iteratively analyzed. The themes that emerged from the initial interviews were further explored and validated in subsequent interviews. Interviews were conducted until theoretical saturation was reached and no new themes were derived from them. Demographic information, including age, sex, ethnicity, and marital status, was also collected.
Analysis
Interviews were digitally recorded and transcribed. Independently, two investigators used Atlas Ti software to analyze and code the interview transcriptions. An inductive approach was used to identify new codes from the data.8 The coders then met to discuss repeating ideas based on the codes. When a code was identified by one coder but not the other, or when there was disagreement about interpretation of a code, the coders returned to the relevant text to reach consensus and to determine whether to include or discard the code.9 We then organized and reorganized repeating ideas based on their conceptual similarities to determine the themes and subthemes.9
RESULTS
Twenty patients participated in the formal interviews. Participants’ baseline characteristics are listed in Table 1, and four dominant themes, and their subthemes and exemplary quotations, are listed in Table 2.
Perspectives on Hospital Care
Participants perceived their hospitalizations as inevitable and necessary for survival: “I think if I haven’t come to the hospital, I probably would have died.” Furthermore, participants thought only the hospital had the resources to help them (“The medications they were giving me … you can get that in the hospital but not outside the hospital”) and sustain them (“You are like an old car, and it breaks down little by little, so you have to go in periodically and get the problem fixed, so you will drive it around for a while”).
Feeling Safe in Hospital. Asked how being in the hospital makes them feel, participants attributed their feelings of safety to the constant observation, the availability of providers and nurses, and the idea that hospital care is helping. As one participant stated, “Makes me feel safer in case you go into something like cardiac arrest. You are right here where they can help you.”
Outside-Hospital Support. Despite multiple hospitalizations, most participants reported having social support (“I have the aide. I got the nurses come in. I have my daughter …”), physical support, and medical support (“I have all the doctors”) outside the hospital. A minority of participants questioned the usefulness of the services. One participant described declining the help of visiting nurses because she wanted to be independent and thought that, despite recurrent hospitalizations for physical symptoms, she still had the ability to manage her own medications.
Goals-of-Care Discussion. Some participants reported inadequate discussions about goals of care, health priorities, and health trajectories. In their reports, this inadequacy included not thinking about their goals, despite continued health decline. One participant stated, “Oh, God, I don’t know if I had any conversation like that. … I think until it is really brought to the front, you don’t make a decision really if you don’t have to.” Citing the value of a more established relationship and deeper trust, participants preferred having these serious and personal discussions with their ambulatory care clinicians: “Because I know my doctor much closer. I have been with him for a number of years. The doctors in the hospital seem to be nice and competent, but I don’t know them.”
DISCUSSION
Participants considered their hospitalizations a necessity and reported feeling safe in the hospital. Given that most already had support outside the hospital, increasing community services may be inadequate to alter participants’ perceived hospital care needs. On the other hand, a few participants reported declining services that might have prevented hospitalizations. Although there has been a study of treatment refusal among older adults with advanced illnesses,10 not much is known about refusal of services among this population. Investigators should examine the reasons for refusing services and the effect that refusal has on hospitalizations. Furthermore, although it would have been informative to ascertain clinician perspectives as well, we focused on patient perspectives because less is known on this topic.
Some participants noted their lack of discussion with their clinicians about healthcare goals and probable health trajectories. Barriers to goals-of-care discussion among this highly vulnerable population have been researched from the perspectives of clinicians and other health professionals but not patients themselves.11,12 Of particular concern in our study is the participant-noted lack of discussion about health trajectories and health priorities, given the decline that occurs in this population and even in those with good care. This inadequacy in discussion suggests continued hospital care may not always be consistent with a patient’s goals. Patients’ desire to have this discussion with their clinicians, with whom they have a relationship, supports the need to involve ambulatory care clinicians, or ensure these patients are cared for by the same clinicians, across healthcare settings.13,14 Whoever provides the care, the clinician must align treatment with the patient’s goal, whether it is to continue hospital-level care or to transition to palliative care. Such an approach also reflects the core elements of person-centered care.15
Study Limitations
Participants were recruited from the medicine service at a single large academic center, limiting the study’s generalizability to patients admitted to surgical services or community hospitals. The patients in this small sample were English-speaking and predominantly Caucasian, so our findings may not represent the perspectives of non-English-speaking or minority patients. We did not perform statistical analysis to quantify intercoder reliability. Last, as this was a qualitative study, we cannot comment on the relative importance or prevalence of the reasons cited for frequent hospitalizations, and we cannot estimate the proportion of patients who had recurrent hospitalizations and were hospital-dependent.
Implication
Although quantitative research is needed to confirm our findings, the hospital-dependent patients in this study thought their survival required hospital-level care and resources. From their perspective, increasing posthospital and community support may be insufficient to prevent some hospitalizations. The lack of goals-of-care discussion supports attempts to increase efforts to facilitate discussion about health trajectories and health priorities between patients and their preferred clinicians.
Acknowledgments
The authors thank Dr. Grace Jenq for providing feedback on the study design.
Disclosure
Nothing to report.
1. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012: Statistical Brief 180. Rockville, MD: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project; 2014. http://www.ncbi.nlm.nih.gov/books/NBK259100/. Published October 2014. Accessed February 17, 2016.
2. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
3. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. PubMed
4. Reuben DB, Tinetti ME. The hospital-dependent patient. N Engl J Med. 2014;370(8):694-697. PubMed
5. Enguidanos S, Coulourides Kogan AM, Schreibeis-Baum H, Lendon J, Lorenz K. “Because I was sick”: seriously ill veterans’ perspectives on reason for 30-day readmissions. J Am Geriatr Soc. 2015;63(3):537-542. PubMed
6. Kangovi S, Grande D, Meehan P, Mitra N, Shannon R, Long JA. Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709-712. PubMed
7. Lamas D. Chronic critical illness. N Engl J Med. 2014;370(2):175-177. PubMed
8. Saldana J. Fundamentals of Qualitative Research. Cary, NC: Oxford University Press; 2011.
9. Auerbach CF, Silverstein LB. Qualitative Data: An Introduction to Coding and Analysis. New York, NY: New York University Press; 2003.
10. Rothman MD, Van Ness PH, O’Leary JR, Fried TR. Refusal of medical and surgical interventions by older persons with advanced chronic disease. J Gen Intern Med. 2007;22(7):982-987. PubMed
11. You JJ, Downar J, Fowler RA, et al; Canadian Researchers at the End of Life Network. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549-556. PubMed
12. Schoenborn NL, Bowman TL 2nd, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678. PubMed
13. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. PubMed
14. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. PubMed
15. American Geriatrics Society Expert Panel on Person-Centered Care. Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64(1):15-18. PubMed
1. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012: Statistical Brief 180. Rockville, MD: Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project; 2014. http://www.ncbi.nlm.nih.gov/books/NBK259100/. Published October 2014. Accessed February 17, 2016.
2. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and causes of readmissions in a national cohort of general medicine patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
3. Donzé JD, Williams MV, Robinson EJ, et al. International validity of the HOSPITAL score to predict 30-day potentially avoidable hospital readmissions. JAMA Intern Med. 2016;176(4):496-502. PubMed
4. Reuben DB, Tinetti ME. The hospital-dependent patient. N Engl J Med. 2014;370(8):694-697. PubMed
5. Enguidanos S, Coulourides Kogan AM, Schreibeis-Baum H, Lendon J, Lorenz K. “Because I was sick”: seriously ill veterans’ perspectives on reason for 30-day readmissions. J Am Geriatr Soc. 2015;63(3):537-542. PubMed
6. Kangovi S, Grande D, Meehan P, Mitra N, Shannon R, Long JA. Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709-712. PubMed
7. Lamas D. Chronic critical illness. N Engl J Med. 2014;370(2):175-177. PubMed
8. Saldana J. Fundamentals of Qualitative Research. Cary, NC: Oxford University Press; 2011.
9. Auerbach CF, Silverstein LB. Qualitative Data: An Introduction to Coding and Analysis. New York, NY: New York University Press; 2003.
10. Rothman MD, Van Ness PH, O’Leary JR, Fried TR. Refusal of medical and surgical interventions by older persons with advanced chronic disease. J Gen Intern Med. 2007;22(7):982-987. PubMed
11. You JJ, Downar J, Fowler RA, et al; Canadian Researchers at the End of Life Network. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549-556. PubMed
12. Schoenborn NL, Bowman TL 2nd, Cayea D, Pollack CE, Feeser S, Boyd C. Primary care practitioners’ views on incorporating long-term prognosis in the care of older adults. JAMA Intern Med. 2016;176(5):671-678. PubMed
13. Arora VM, Prochaska ML, Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: a mixed methods study. J Hosp Med. 2010;5(7):385-391. PubMed
14. Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417-424. PubMed
15. American Geriatrics Society Expert Panel on Person-Centered Care. Person-centered care: a definition and essential elements. J Am Geriatr Soc. 2016;64(1):15-18. PubMed
© 2017 Society of Hospital Medicine
Incidental pulmonary nodules reported on CT abdominal imaging: Frequency and factors affecting inclusion in the hospital discharge summary
Incidental findings create both medical and logistical challenges regarding communication.1,2 Pulmonary nodules are among the most frequent and medically relevant incidental findings, being noted in up to 8.4% of abdominal computed tomography (CT) scans.3 There are guidelines regarding proper follow-up and management of such incidental pulmonary nodules, but appropriate evidence-based surveillance imaging is often not performed, and many patients remain uninformed. Collins et al.4 reported that, before initiation of a standardized protocol, only 17.7% of incidental findings were communicated to patients admitted to the trauma service; after protocol initiation, the rate increased to 32.4%. The hospital discharge summary provides an opportunity to communicate incidental findings to patients and their medical care providers, but Kripalani et al.5 raised questions regarding the current completeness and accuracy of discharge summaries, reporting that 65% of discharge summaries omitted relevant diagnostic testing, and 30% omitted a follow-up plan.
We conducted a study to determine how often incidental pulmonary nodules found on abdominal CT are documented in the discharge summary, and to identify factors associated with pulmonary nodule inclusion.
METHODS
This was a retrospective cohort study of hospitalized patients ≥35 years of age who underwent in-patient abdominal CT between January 1, 2012 and December 31, 2014. Patients were identified by cross-referencing hospital admissions with Current Procedural Terminology (CPT) codes indicating abdominal CT (74176, 74177, 74178, 74160, 74150, 74170). Patients with chest CT (CPT codes 71260, 71250, 71270) during that hospitalization or within 30 days before admission were excluded to ensure that pulmonary nodules were incidental and asymptomatic. The index hospitalization was defined as the first hospitalization during which the patient was diagnosed with an incidental pulmonary nodule on abdominal CT, or the first hospitalization during the study period for patients without pulmonary nodules. All patient charts were manually reviewed, and baseline age, sex, and smoking status data collected.
Radiology reports were electronically screened for the words nodule and nodules and then confirmed through manual review of the full text reports. Nodules described as tiny (without other size description) were assumed to be <4 mm in size, per manual review of a small sample. Nodules were deemed as falling outside the Fleischner Society criteria guidelines (designed for indeterminate pulmonary nodules), and were therefore excluded, if any of seven criteria were met: The nodule was (1) cavitary, (2) associated with a known metastatic disease, (3) associated with a known granulomatous disease, (4) associated with a known inflammatory process, (5) reported likely to represent atelectasis, (6) reported likely to be a lymph node, or (7) previously biopsied.4
For each patient with pulmonary nodules, a personal history of cancer was obtained. Nodule size, characteristics, and stability compared with available prior imaging were recorded. Radiology reports were reviewed to determine if pulmonary nodules were mentioned in the summary headings of the reports or in the body of the reports and whether specific follow-up recommendations were provided. Hospital discharge summaries were reviewed for documentation of pulmonary nodule(s) and follow-up recommendations. Discharging service (medical/medical subspecialty, surgical/surgical subspecialty) was noted, along with the patients’ condition at discharge (alive, alive on hospice, deceased).
The frequency of incidental pulmonary nodules on abdominal CT during hospitalization and the frequency of nodules requiring follow-up were reported using a point estimate and corresponding 95% confidence interval (CI). The χ2 test was used to compare the frequency of pulmonary nodules across patient groups. In addition, for patients found to have incidental nodules requiring follow-up, the χ2 test was used to compare across groups the percentage of patients with discharge documentation of the incidental nodule. In all cases, 2-tailed Ps are reported, with P ≤ 0.05 considered statistically significant.
RESULTS
Between January 1, 2012 and December 31, 2014, 7173 patients ≥35 years old underwent in-patient abdominal CT without concurrent chest CT. Of these patients, 62.2% were ≥60 years old, 50.6% were men, and 45.5% were current or former smokers. Incidental pulmonary nodules were noted in 402 patients (5.6%; 95% CI, 5.1%-6.2%), of whom 68.7% were ≥60 years old, 56.5% were men, and 46.3% were current or former smokers. Increasing age (P = 0.004) and male sex (P = 0.015) were associated with increased frequency of incidental pulmonary nodules, but smoking status (P = 0.586) was not. Of patients with incidental nodules, 71.6% had solitary nodules, and 58.5% had a maximum nodule size of ≤4 mm (Table 1). Based on smoking status, nodule size, and reported size stability, 208 patients (2.9%; 95% CI, 2.5%-3.3%) required follow-up surveillance as per 2005 Fleischner Society guidelines. Among solitary pulmonary nodules requiring further surveillance (n = 147), the mean risk of malignancy based on the Mayo Clinic solitary pulmonary nodule risk calculator was 7.9% (interquartile range, 3.0%-10.5%), with 28% having a malignancy risk of ≥10%.6
Of the 208 patients with nodules requiring further surveillance, only 48 (23%) received discharge summaries documenting the nodule; 34 of these summaries included a recommendation for nodule follow-up, with 19 of the recommendations including a time frame for repeat CT. Three factors were positively associated with documentation of the pulmonary nodule in the discharge summary: mention of the pulmonary nodule in the summary headings of the radiology report (P < 0.001), radiologist recommendation for further surveillance (P < 0.001), and medical discharging service (P = 0.016) (Table 2). The highest rate of pulmonary nodule inclusion in the discharge summary (42%) was noted among patients for whom the radiology report included specific recommendations.
DISCUSSION
The frequency of incidental pulmonary nodules reported on abdominal CT in our study (5.6%) is consistent with frequencies reported in similar studies. Wu et al.7 (reviewing 141,406 abdominal CT scans) and Alpert et al.8 (reviewing 12,287 abdominal CT scans) reported frequencies of 2.5% and 3%, respectively, while Rinaldi et al.3 (reviewing 243 abdominal CT scans) reported a higher frequency, 8.4%. Variation likely results from patient factors and the individual radiologist’s attention to incidental pulmonary findings. Rinaldi et al. suggested that up to 39% of abdominal CT scans include pulmonary nodules on independent review, raising the possibility of significant underreporting. In our study, we focused on pulmonary nodules included in the radiology report to tailor the relevance of our study to the hospital medicine community. We also included only those incidental nodules falling within the purview of the Fleischner Society criteria in order to analyze only findings with established follow-up guidelines.
The rate of pulmonary nodule documentation in our study was low overall (23%) but consistent with the literature. Collins et al.,4 for example, reported that only 17.7% of patients with trauma were notified of incidental CT findings by either the discharge summary or an appropriate specialist consultation. Various contributing factors can be hypothesized. First, incidental pulmonary nodules are discovered largely in the context of evaluation for other symptomatic conditions, which can overshadow their importance. Second, the lack of clear patient-friendly education materials regarding incidental pulmonary nodules can complicate discussions with patients. Third, many electronic health record (EHR) systems cannot automatically pull incidental findings into the discharge summary and instead rely on provider vigilance.
As our study does, the literature highlights the importance of the radiology report in communicating incidental findings. In a review of >1000 pulmonary angiographic CT studies, Blagev et al.9 reported an overall follow-up rate of 29% (28/96) among patients with incidental pulmonary nodules, but none of the 12 patients with pulmonary nodules mentioned in the body of the report (rather than in the summary headings) received adequate follow-up. Similarly, in Shuaib et al.,10 radiology reports that included follow-up recommendations were more likely to change patient treatment than reports without follow-up recommendations (70% vs 2%). However, our data also show that radiologist recommendations alone are insufficient to ensure adequate communication of incidental findings.
The literature regarding the most cost-effective means of addressing this quality gap is limited. Some institutions have integrated their EHR systems to allow radiologists to flag incidental findings for auto-population in a dedicated section of the discharge summary. Although these efforts can be helpful, documentation alone does not save lives without appropriate follow-up and intervention. Some institutions have hired dedicated nursing staff as incidental finding coordinators. For high-risk incidental findings, Sperry et al.11 reported that hiring an incidental findings coordinator helped their level I trauma center achieve nearly complete documentation, patient notification, and confirmation of posthospital follow-up appointments. Such solutions, however, are labor-intensive and still rely on appropriate primary care follow-up.
Strengths of our study include its relatively large size and particular focus on the issues and decisions facing hospital medicine providers. By focusing on incidental pulmonary nodules reported on abdominal CT, and excluding patients with concurrent chest CT, we avoided including patients with symptomatic or previously identified pulmonary findings. Study limitations include the cross-sectional, retrospective design, which did not include follow-up data regarding such outcomes as rates of appropriate follow-up surveillance and subsequent lung cancer diagnoses. Our single-center study findings may not apply to all hospital practice settings, though they are consistent with the literature with comparison data.
Our study results highlight the need for a multidisciplinary systems-based approach to incidental pulmonary nodule documentation, communication, and follow-up surveillance.
Disclosure
Nothing to report.
1. Armao D, Smith JK. Overuse of computed tomography and the onslaught of incidental findings. N C Med J. 2014;75(2):127. PubMed
2. Gould MK, Tang T, Liu IL, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. PubMed
3. Rinaldi MF, Bartalena T, Giannelli G, et al. Incidental lung nodules on CT examinations of the abdomen: prevalence and reporting rates in the PACS era. Eur J Radiol. 2010;74(3):e84-e88. PubMed
4. Collins CE, Cherng N, McDade T, et al. Improving patient notification of solid abdominal viscera incidental findings with a standardized protocol. J Trauma Manag Outcomes. 2015;9(1):1. PubMed
5. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
6. Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849-855. PubMed
7. Wu CC, Cronin CG, Chu JT, et al. Incidental pulmonary nodules detected on abdominal computed tomography. J Comput Assist Tomogr. 2012;36(6):641-645. PubMed
8. Alpert JB, Fantauzzi JP, Melamud K, Greenwood H, Naidich DP, Ko JP. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol. 2012;198(4):793-799. PubMed
9. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. PubMed
10. Shuaib W, Johnson JO, Salastekar N, Maddu KK, Khosa F. Incidental findings detected on abdomino-pelvic multidetector computed tomography performed in the acute setting [published correction appears in Am J Emerg Med. 2014;32(7):811. Waqas, Shuaib (corrected to Shuaib, Waqas)]. Am J Emerg Med. 2014;32(1):36-39. PubMed
11. Sperry JL, Massaro MS, Collage RD, et al. Incidental radiographic findings after injury: dedicated attention results in improved capture, documentation, and management. Surgery. 2010;148(4):618-624. PubMed
Incidental findings create both medical and logistical challenges regarding communication.1,2 Pulmonary nodules are among the most frequent and medically relevant incidental findings, being noted in up to 8.4% of abdominal computed tomography (CT) scans.3 There are guidelines regarding proper follow-up and management of such incidental pulmonary nodules, but appropriate evidence-based surveillance imaging is often not performed, and many patients remain uninformed. Collins et al.4 reported that, before initiation of a standardized protocol, only 17.7% of incidental findings were communicated to patients admitted to the trauma service; after protocol initiation, the rate increased to 32.4%. The hospital discharge summary provides an opportunity to communicate incidental findings to patients and their medical care providers, but Kripalani et al.5 raised questions regarding the current completeness and accuracy of discharge summaries, reporting that 65% of discharge summaries omitted relevant diagnostic testing, and 30% omitted a follow-up plan.
We conducted a study to determine how often incidental pulmonary nodules found on abdominal CT are documented in the discharge summary, and to identify factors associated with pulmonary nodule inclusion.
METHODS
This was a retrospective cohort study of hospitalized patients ≥35 years of age who underwent in-patient abdominal CT between January 1, 2012 and December 31, 2014. Patients were identified by cross-referencing hospital admissions with Current Procedural Terminology (CPT) codes indicating abdominal CT (74176, 74177, 74178, 74160, 74150, 74170). Patients with chest CT (CPT codes 71260, 71250, 71270) during that hospitalization or within 30 days before admission were excluded to ensure that pulmonary nodules were incidental and asymptomatic. The index hospitalization was defined as the first hospitalization during which the patient was diagnosed with an incidental pulmonary nodule on abdominal CT, or the first hospitalization during the study period for patients without pulmonary nodules. All patient charts were manually reviewed, and baseline age, sex, and smoking status data collected.
Radiology reports were electronically screened for the words nodule and nodules and then confirmed through manual review of the full text reports. Nodules described as tiny (without other size description) were assumed to be <4 mm in size, per manual review of a small sample. Nodules were deemed as falling outside the Fleischner Society criteria guidelines (designed for indeterminate pulmonary nodules), and were therefore excluded, if any of seven criteria were met: The nodule was (1) cavitary, (2) associated with a known metastatic disease, (3) associated with a known granulomatous disease, (4) associated with a known inflammatory process, (5) reported likely to represent atelectasis, (6) reported likely to be a lymph node, or (7) previously biopsied.4
For each patient with pulmonary nodules, a personal history of cancer was obtained. Nodule size, characteristics, and stability compared with available prior imaging were recorded. Radiology reports were reviewed to determine if pulmonary nodules were mentioned in the summary headings of the reports or in the body of the reports and whether specific follow-up recommendations were provided. Hospital discharge summaries were reviewed for documentation of pulmonary nodule(s) and follow-up recommendations. Discharging service (medical/medical subspecialty, surgical/surgical subspecialty) was noted, along with the patients’ condition at discharge (alive, alive on hospice, deceased).
The frequency of incidental pulmonary nodules on abdominal CT during hospitalization and the frequency of nodules requiring follow-up were reported using a point estimate and corresponding 95% confidence interval (CI). The χ2 test was used to compare the frequency of pulmonary nodules across patient groups. In addition, for patients found to have incidental nodules requiring follow-up, the χ2 test was used to compare across groups the percentage of patients with discharge documentation of the incidental nodule. In all cases, 2-tailed Ps are reported, with P ≤ 0.05 considered statistically significant.
RESULTS
Between January 1, 2012 and December 31, 2014, 7173 patients ≥35 years old underwent in-patient abdominal CT without concurrent chest CT. Of these patients, 62.2% were ≥60 years old, 50.6% were men, and 45.5% were current or former smokers. Incidental pulmonary nodules were noted in 402 patients (5.6%; 95% CI, 5.1%-6.2%), of whom 68.7% were ≥60 years old, 56.5% were men, and 46.3% were current or former smokers. Increasing age (P = 0.004) and male sex (P = 0.015) were associated with increased frequency of incidental pulmonary nodules, but smoking status (P = 0.586) was not. Of patients with incidental nodules, 71.6% had solitary nodules, and 58.5% had a maximum nodule size of ≤4 mm (Table 1). Based on smoking status, nodule size, and reported size stability, 208 patients (2.9%; 95% CI, 2.5%-3.3%) required follow-up surveillance as per 2005 Fleischner Society guidelines. Among solitary pulmonary nodules requiring further surveillance (n = 147), the mean risk of malignancy based on the Mayo Clinic solitary pulmonary nodule risk calculator was 7.9% (interquartile range, 3.0%-10.5%), with 28% having a malignancy risk of ≥10%.6
Of the 208 patients with nodules requiring further surveillance, only 48 (23%) received discharge summaries documenting the nodule; 34 of these summaries included a recommendation for nodule follow-up, with 19 of the recommendations including a time frame for repeat CT. Three factors were positively associated with documentation of the pulmonary nodule in the discharge summary: mention of the pulmonary nodule in the summary headings of the radiology report (P < 0.001), radiologist recommendation for further surveillance (P < 0.001), and medical discharging service (P = 0.016) (Table 2). The highest rate of pulmonary nodule inclusion in the discharge summary (42%) was noted among patients for whom the radiology report included specific recommendations.
DISCUSSION
The frequency of incidental pulmonary nodules reported on abdominal CT in our study (5.6%) is consistent with frequencies reported in similar studies. Wu et al.7 (reviewing 141,406 abdominal CT scans) and Alpert et al.8 (reviewing 12,287 abdominal CT scans) reported frequencies of 2.5% and 3%, respectively, while Rinaldi et al.3 (reviewing 243 abdominal CT scans) reported a higher frequency, 8.4%. Variation likely results from patient factors and the individual radiologist’s attention to incidental pulmonary findings. Rinaldi et al. suggested that up to 39% of abdominal CT scans include pulmonary nodules on independent review, raising the possibility of significant underreporting. In our study, we focused on pulmonary nodules included in the radiology report to tailor the relevance of our study to the hospital medicine community. We also included only those incidental nodules falling within the purview of the Fleischner Society criteria in order to analyze only findings with established follow-up guidelines.
The rate of pulmonary nodule documentation in our study was low overall (23%) but consistent with the literature. Collins et al.,4 for example, reported that only 17.7% of patients with trauma were notified of incidental CT findings by either the discharge summary or an appropriate specialist consultation. Various contributing factors can be hypothesized. First, incidental pulmonary nodules are discovered largely in the context of evaluation for other symptomatic conditions, which can overshadow their importance. Second, the lack of clear patient-friendly education materials regarding incidental pulmonary nodules can complicate discussions with patients. Third, many electronic health record (EHR) systems cannot automatically pull incidental findings into the discharge summary and instead rely on provider vigilance.
As our study does, the literature highlights the importance of the radiology report in communicating incidental findings. In a review of >1000 pulmonary angiographic CT studies, Blagev et al.9 reported an overall follow-up rate of 29% (28/96) among patients with incidental pulmonary nodules, but none of the 12 patients with pulmonary nodules mentioned in the body of the report (rather than in the summary headings) received adequate follow-up. Similarly, in Shuaib et al.,10 radiology reports that included follow-up recommendations were more likely to change patient treatment than reports without follow-up recommendations (70% vs 2%). However, our data also show that radiologist recommendations alone are insufficient to ensure adequate communication of incidental findings.
The literature regarding the most cost-effective means of addressing this quality gap is limited. Some institutions have integrated their EHR systems to allow radiologists to flag incidental findings for auto-population in a dedicated section of the discharge summary. Although these efforts can be helpful, documentation alone does not save lives without appropriate follow-up and intervention. Some institutions have hired dedicated nursing staff as incidental finding coordinators. For high-risk incidental findings, Sperry et al.11 reported that hiring an incidental findings coordinator helped their level I trauma center achieve nearly complete documentation, patient notification, and confirmation of posthospital follow-up appointments. Such solutions, however, are labor-intensive and still rely on appropriate primary care follow-up.
Strengths of our study include its relatively large size and particular focus on the issues and decisions facing hospital medicine providers. By focusing on incidental pulmonary nodules reported on abdominal CT, and excluding patients with concurrent chest CT, we avoided including patients with symptomatic or previously identified pulmonary findings. Study limitations include the cross-sectional, retrospective design, which did not include follow-up data regarding such outcomes as rates of appropriate follow-up surveillance and subsequent lung cancer diagnoses. Our single-center study findings may not apply to all hospital practice settings, though they are consistent with the literature with comparison data.
Our study results highlight the need for a multidisciplinary systems-based approach to incidental pulmonary nodule documentation, communication, and follow-up surveillance.
Disclosure
Nothing to report.
Incidental findings create both medical and logistical challenges regarding communication.1,2 Pulmonary nodules are among the most frequent and medically relevant incidental findings, being noted in up to 8.4% of abdominal computed tomography (CT) scans.3 There are guidelines regarding proper follow-up and management of such incidental pulmonary nodules, but appropriate evidence-based surveillance imaging is often not performed, and many patients remain uninformed. Collins et al.4 reported that, before initiation of a standardized protocol, only 17.7% of incidental findings were communicated to patients admitted to the trauma service; after protocol initiation, the rate increased to 32.4%. The hospital discharge summary provides an opportunity to communicate incidental findings to patients and their medical care providers, but Kripalani et al.5 raised questions regarding the current completeness and accuracy of discharge summaries, reporting that 65% of discharge summaries omitted relevant diagnostic testing, and 30% omitted a follow-up plan.
We conducted a study to determine how often incidental pulmonary nodules found on abdominal CT are documented in the discharge summary, and to identify factors associated with pulmonary nodule inclusion.
METHODS
This was a retrospective cohort study of hospitalized patients ≥35 years of age who underwent in-patient abdominal CT between January 1, 2012 and December 31, 2014. Patients were identified by cross-referencing hospital admissions with Current Procedural Terminology (CPT) codes indicating abdominal CT (74176, 74177, 74178, 74160, 74150, 74170). Patients with chest CT (CPT codes 71260, 71250, 71270) during that hospitalization or within 30 days before admission were excluded to ensure that pulmonary nodules were incidental and asymptomatic. The index hospitalization was defined as the first hospitalization during which the patient was diagnosed with an incidental pulmonary nodule on abdominal CT, or the first hospitalization during the study period for patients without pulmonary nodules. All patient charts were manually reviewed, and baseline age, sex, and smoking status data collected.
Radiology reports were electronically screened for the words nodule and nodules and then confirmed through manual review of the full text reports. Nodules described as tiny (without other size description) were assumed to be <4 mm in size, per manual review of a small sample. Nodules were deemed as falling outside the Fleischner Society criteria guidelines (designed for indeterminate pulmonary nodules), and were therefore excluded, if any of seven criteria were met: The nodule was (1) cavitary, (2) associated with a known metastatic disease, (3) associated with a known granulomatous disease, (4) associated with a known inflammatory process, (5) reported likely to represent atelectasis, (6) reported likely to be a lymph node, or (7) previously biopsied.4
For each patient with pulmonary nodules, a personal history of cancer was obtained. Nodule size, characteristics, and stability compared with available prior imaging were recorded. Radiology reports were reviewed to determine if pulmonary nodules were mentioned in the summary headings of the reports or in the body of the reports and whether specific follow-up recommendations were provided. Hospital discharge summaries were reviewed for documentation of pulmonary nodule(s) and follow-up recommendations. Discharging service (medical/medical subspecialty, surgical/surgical subspecialty) was noted, along with the patients’ condition at discharge (alive, alive on hospice, deceased).
The frequency of incidental pulmonary nodules on abdominal CT during hospitalization and the frequency of nodules requiring follow-up were reported using a point estimate and corresponding 95% confidence interval (CI). The χ2 test was used to compare the frequency of pulmonary nodules across patient groups. In addition, for patients found to have incidental nodules requiring follow-up, the χ2 test was used to compare across groups the percentage of patients with discharge documentation of the incidental nodule. In all cases, 2-tailed Ps are reported, with P ≤ 0.05 considered statistically significant.
RESULTS
Between January 1, 2012 and December 31, 2014, 7173 patients ≥35 years old underwent in-patient abdominal CT without concurrent chest CT. Of these patients, 62.2% were ≥60 years old, 50.6% were men, and 45.5% were current or former smokers. Incidental pulmonary nodules were noted in 402 patients (5.6%; 95% CI, 5.1%-6.2%), of whom 68.7% were ≥60 years old, 56.5% were men, and 46.3% were current or former smokers. Increasing age (P = 0.004) and male sex (P = 0.015) were associated with increased frequency of incidental pulmonary nodules, but smoking status (P = 0.586) was not. Of patients with incidental nodules, 71.6% had solitary nodules, and 58.5% had a maximum nodule size of ≤4 mm (Table 1). Based on smoking status, nodule size, and reported size stability, 208 patients (2.9%; 95% CI, 2.5%-3.3%) required follow-up surveillance as per 2005 Fleischner Society guidelines. Among solitary pulmonary nodules requiring further surveillance (n = 147), the mean risk of malignancy based on the Mayo Clinic solitary pulmonary nodule risk calculator was 7.9% (interquartile range, 3.0%-10.5%), with 28% having a malignancy risk of ≥10%.6
Of the 208 patients with nodules requiring further surveillance, only 48 (23%) received discharge summaries documenting the nodule; 34 of these summaries included a recommendation for nodule follow-up, with 19 of the recommendations including a time frame for repeat CT. Three factors were positively associated with documentation of the pulmonary nodule in the discharge summary: mention of the pulmonary nodule in the summary headings of the radiology report (P < 0.001), radiologist recommendation for further surveillance (P < 0.001), and medical discharging service (P = 0.016) (Table 2). The highest rate of pulmonary nodule inclusion in the discharge summary (42%) was noted among patients for whom the radiology report included specific recommendations.
DISCUSSION
The frequency of incidental pulmonary nodules reported on abdominal CT in our study (5.6%) is consistent with frequencies reported in similar studies. Wu et al.7 (reviewing 141,406 abdominal CT scans) and Alpert et al.8 (reviewing 12,287 abdominal CT scans) reported frequencies of 2.5% and 3%, respectively, while Rinaldi et al.3 (reviewing 243 abdominal CT scans) reported a higher frequency, 8.4%. Variation likely results from patient factors and the individual radiologist’s attention to incidental pulmonary findings. Rinaldi et al. suggested that up to 39% of abdominal CT scans include pulmonary nodules on independent review, raising the possibility of significant underreporting. In our study, we focused on pulmonary nodules included in the radiology report to tailor the relevance of our study to the hospital medicine community. We also included only those incidental nodules falling within the purview of the Fleischner Society criteria in order to analyze only findings with established follow-up guidelines.
The rate of pulmonary nodule documentation in our study was low overall (23%) but consistent with the literature. Collins et al.,4 for example, reported that only 17.7% of patients with trauma were notified of incidental CT findings by either the discharge summary or an appropriate specialist consultation. Various contributing factors can be hypothesized. First, incidental pulmonary nodules are discovered largely in the context of evaluation for other symptomatic conditions, which can overshadow their importance. Second, the lack of clear patient-friendly education materials regarding incidental pulmonary nodules can complicate discussions with patients. Third, many electronic health record (EHR) systems cannot automatically pull incidental findings into the discharge summary and instead rely on provider vigilance.
As our study does, the literature highlights the importance of the radiology report in communicating incidental findings. In a review of >1000 pulmonary angiographic CT studies, Blagev et al.9 reported an overall follow-up rate of 29% (28/96) among patients with incidental pulmonary nodules, but none of the 12 patients with pulmonary nodules mentioned in the body of the report (rather than in the summary headings) received adequate follow-up. Similarly, in Shuaib et al.,10 radiology reports that included follow-up recommendations were more likely to change patient treatment than reports without follow-up recommendations (70% vs 2%). However, our data also show that radiologist recommendations alone are insufficient to ensure adequate communication of incidental findings.
The literature regarding the most cost-effective means of addressing this quality gap is limited. Some institutions have integrated their EHR systems to allow radiologists to flag incidental findings for auto-population in a dedicated section of the discharge summary. Although these efforts can be helpful, documentation alone does not save lives without appropriate follow-up and intervention. Some institutions have hired dedicated nursing staff as incidental finding coordinators. For high-risk incidental findings, Sperry et al.11 reported that hiring an incidental findings coordinator helped their level I trauma center achieve nearly complete documentation, patient notification, and confirmation of posthospital follow-up appointments. Such solutions, however, are labor-intensive and still rely on appropriate primary care follow-up.
Strengths of our study include its relatively large size and particular focus on the issues and decisions facing hospital medicine providers. By focusing on incidental pulmonary nodules reported on abdominal CT, and excluding patients with concurrent chest CT, we avoided including patients with symptomatic or previously identified pulmonary findings. Study limitations include the cross-sectional, retrospective design, which did not include follow-up data regarding such outcomes as rates of appropriate follow-up surveillance and subsequent lung cancer diagnoses. Our single-center study findings may not apply to all hospital practice settings, though they are consistent with the literature with comparison data.
Our study results highlight the need for a multidisciplinary systems-based approach to incidental pulmonary nodule documentation, communication, and follow-up surveillance.
Disclosure
Nothing to report.
1. Armao D, Smith JK. Overuse of computed tomography and the onslaught of incidental findings. N C Med J. 2014;75(2):127. PubMed
2. Gould MK, Tang T, Liu IL, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. PubMed
3. Rinaldi MF, Bartalena T, Giannelli G, et al. Incidental lung nodules on CT examinations of the abdomen: prevalence and reporting rates in the PACS era. Eur J Radiol. 2010;74(3):e84-e88. PubMed
4. Collins CE, Cherng N, McDade T, et al. Improving patient notification of solid abdominal viscera incidental findings with a standardized protocol. J Trauma Manag Outcomes. 2015;9(1):1. PubMed
5. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
6. Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849-855. PubMed
7. Wu CC, Cronin CG, Chu JT, et al. Incidental pulmonary nodules detected on abdominal computed tomography. J Comput Assist Tomogr. 2012;36(6):641-645. PubMed
8. Alpert JB, Fantauzzi JP, Melamud K, Greenwood H, Naidich DP, Ko JP. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol. 2012;198(4):793-799. PubMed
9. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. PubMed
10. Shuaib W, Johnson JO, Salastekar N, Maddu KK, Khosa F. Incidental findings detected on abdomino-pelvic multidetector computed tomography performed in the acute setting [published correction appears in Am J Emerg Med. 2014;32(7):811. Waqas, Shuaib (corrected to Shuaib, Waqas)]. Am J Emerg Med. 2014;32(1):36-39. PubMed
11. Sperry JL, Massaro MS, Collage RD, et al. Incidental radiographic findings after injury: dedicated attention results in improved capture, documentation, and management. Surgery. 2010;148(4):618-624. PubMed
1. Armao D, Smith JK. Overuse of computed tomography and the onslaught of incidental findings. N C Med J. 2014;75(2):127. PubMed
2. Gould MK, Tang T, Liu IL, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214. PubMed
3. Rinaldi MF, Bartalena T, Giannelli G, et al. Incidental lung nodules on CT examinations of the abdomen: prevalence and reporting rates in the PACS era. Eur J Radiol. 2010;74(3):e84-e88. PubMed
4. Collins CE, Cherng N, McDade T, et al. Improving patient notification of solid abdominal viscera incidental findings with a standardized protocol. J Trauma Manag Outcomes. 2015;9(1):1. PubMed
5. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
6. Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849-855. PubMed
7. Wu CC, Cronin CG, Chu JT, et al. Incidental pulmonary nodules detected on abdominal computed tomography. J Comput Assist Tomogr. 2012;36(6):641-645. PubMed
8. Alpert JB, Fantauzzi JP, Melamud K, Greenwood H, Naidich DP, Ko JP. Clinical significance of lung nodules reported on abdominal CT. AJR Am J Roentgenol. 2012;198(4):793-799. PubMed
9. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. PubMed
10. Shuaib W, Johnson JO, Salastekar N, Maddu KK, Khosa F. Incidental findings detected on abdomino-pelvic multidetector computed tomography performed in the acute setting [published correction appears in Am J Emerg Med. 2014;32(7):811. Waqas, Shuaib (corrected to Shuaib, Waqas)]. Am J Emerg Med. 2014;32(1):36-39. PubMed
11. Sperry JL, Massaro MS, Collage RD, et al. Incidental radiographic findings after injury: dedicated attention results in improved capture, documentation, and management. Surgery. 2010;148(4):618-624. PubMed
© 2017 Society of Hospital Medicine
Empiric <i>Listeria monocytogenes</i> antibiotic coverage for febrile infants (age, 0-90 days)
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Evaluation and treatment of the febrile infant 0 to 90 days of age are common clinical issues in pediatrics, family medicine, emergency medicine, and pediatric hospital medicine. Traditional teaching has been that Listeria monocytogenes is 1 of the 3 most common pathogens causing neonatal sepsis. Many practitioners routinely use antibiotic regimens, including ampicillin, to specifically target Listeria. However, a large body of evidence, including a meta-analysis and several multicenter studies, has shown that listeriosis is extremely rare in the United States. The practice of empiric ampicillin thus exposes the patient to harms and costs with little if any potential benefit, while increasing pressure on the bacterial flora in the community to generate antibiotic resistance. Empiric ampicillin for all infants admitted for sepsis evaluation is a tradition-based practice no longer founded on the best available evidence.
CASE REPORT
A 32-day-old, full-term, previously healthy girl presented with fever of 1 day’s duration. Her parents reported she had appeared well until the evening before admission, when she became a bit less active and spent less time breastfeeding. The morning of admission, she was fussier than usual. Rectal temperature, taken by her parents, was 101°F. There were no other symptoms and no sick contacts.
On examination, the patient’s rectal temperature was 101.5°F. Her other vitals and the physical examination findings were unremarkable. Laboratory test results included a normal urinalysis and a peripheral white blood cell (WBC) count of 21,300 cells/µL. Cerebrospinal fluid (CSF) analysis revealed normal protein and glucose levels with 3 WBCs/µL and a negative gram stain. Due to stratifying at higher risk for serious bacterial infection (SBI), the child was admitted and started on ampicillin and cefotaxime while awaiting culture results.
BACKGROUND
Evaluation and treatment of febrile infants are common clinical issues in pediatrics, emergency medicine, and general practice. Practice guidelines for evaluation of febrile infants recommend hospitalization and parenteral antibiotics for children younger than 28 days and children 29 to 90 days old if stratified at high risk for SBI.1,2 Recommendations for empiric antibiotic regimens include ampicillin in addition to either gentamicin or cefotaxime.1,2
WHY YOU MIGHT THINK AMPICILLIN IS HELPFUL
Generations of pediatrics students have been taught that the 3 pathogens most likely to cause bacterial sepsis in infants are group B Streptococcus (GBS), Escherichia coli, and Listeria monocytogenes. This teaching is still espoused in the latest editions of pediatrics textbooks.3 Ampicillin is specifically recommended for covering Listeria, and studies have found that 62% to 78% of practitioners choose empiric ampicillin-containing antibiotic regimens for the treatment of febrile infants.4-6
WHY EMPIRIC AMPICILLIN IS UNNECESSARY
In the past, Listeria was a potential though still uncommon infant pathogen. Over the past few decades, however, the epidemiology of infant sepsis has changed significantly. Estimates of the rate of infection with Listeria now range from extremely rare to nonexistent across multiple studies4,7-15 (Table). In a 4-year retrospective case series at a single urban academic center in Washington, DC, Sadow et al.4 reported no instances of Listeria among 121 positive bacterial cultures in infants younger than 60 days seen in the emergency department (ED). Byington et al.7 examined all positive cultures for infants 0 to 90 days old at a large academic referral center in Utah over a 3-year period and reported no cases of Listeria (1298 patients, 105 SBI cases). A study at a North Carolina academic center found 1 case of Listeria meningitis among 72 SBIs (668 febrile infants) without a localizing source.8 At a large group-practice in northern California, Greenhow et al.9 examined all blood cultures (N = 4255) performed over 4 years for otherwise healthy infants 1 week to 3 months old and found no cases of Listeria. In a follow-up study, the same authors examined all blood (n = 5396), urine (n = 4599), and CSF (n = 1796) cultures in the same population and found no Listeria cases.10 Hassoun et al.11 studied SBI rates among infants younger than 28 days with any blood, urine, or CSF culture performed over 4 years at two Michigan EDs. One (0.08%) of the 1192 infants evaluated had bacteremia caused by Listeria.
Multicenter studies have reported similar results. In a study of 6 hospital systems in geographically diverse areas of the United States, Biondi et al.12 examined all positive blood cultures (N = 181) for febrile infants younger than 90 days admitted to a general pediatric ward, and found no listeriosis. Mischler et al.13 examined all positive blood cultures (N = 392) for otherwise healthy febrile infants 0 to 90 days old admitted to a hospital in 1 of 17 geographically diverse healthcare systems and found no cases of Listeria. A recent meta-analysis of studies that reported SBI rates for febrile infants 0 to 90 days old found the weighted prevalence of Listeria bacteremia to be 0.03% (2/20,703) and that of meningitis to be 0.02% (3/13,375).14 Veesenmeyer and Edmonson15 used a national inpatient database to identify all Listeria cases among infants over a 6-year period and estimated listeriosis rates for the US population. Over the 6 years, there were 212 total cases, which were extrapolated to 344 in the United States during that period, yielding a pooled annual incidence rate of 1.41 in 100,000 births.
Ampicillin offers no significant improvement in coverage for GBS or E coli beyond other β-lactam antibiotics, such as cefotaxime. Therefore, though the cost and potential harms of 24 to 48 hours of intravenous ampicillin are low for the individual patient, there is almost no potential benefit. Using the weighted prevalence of 0.03% for Listeria bacteremia reported in the recent meta-analysis,14 the number needed to treat to cover 1 case of Listeria bacteremia would be 3333. In addition, the increasing incidence of ampicillin resistance, particularly among gram-negative organisms,4,7,9 argues strongly for better antibiotic stewardship on a national level. A number of expert authors have advocated dropping empiric Listeria coverage as part of the treatment of febrile infants, particularly infants 29 to 90 days old.16,17 Some authors continue to advocate empiric Listeria coverage.6 It is interesting to note, however, that the incidence of Staph aureus bacteremia in recent case series is much higher than that reported for Listeria, accounting for 6-9% of bacteremia cases.9,11,13 Yet few if any authors advocate for empiric S. aureus coverage.
WHEN EMPIRIC AMPICILLIN COVERAGE MAY BE REASONABLE
The rate of listeriosis remains low across age groups in recent studies, but the rate is slightly higher in very young infants. In the recent national database study of listeriosis cases over a 6-year period, almost half involved infants younger than 7 days, and most of these infants showed no evidence of meningitis.15 Therefore, it may be reasonable to include empiric Listeria coverage in febrile infants younger than 7 days, though the study authors estimated 22.5 annual cases of Listeria in this age range in the United States. Eighty percent of the Listeria cases were in infants younger than 28 days, but more than 85% of infants 7 to 28 days old had meningitis. Therefore, broad antimicrobial coverage for infants with CSF pleocytosis and/or a high bacterial meningitis score is reasonable, especially for infants younger than 28 days.
Other potential indications for ampicillin are enterococcal infections. Though enteroccocal SBI rates in febrile infants are also quite low,7-9,11,12 if Enterococcus were highly suspected, such as in an infant with pyuria and gram positive organisms on gram stain, ampicillin offers good additional coverage. In the case of a local outbreak of listeriosis, or a specific exposure to Listeria-contaminated products on a patient history, antibiotics with efficacy against Listeria should be used. Last, in cases in which gentamicin is used as empiric coverage for gram-negative organisms, ampicillin offers important additional coverage for GBS.
Some practitioners advocate ampicillin and gentamicin over cefotaxime regimens on the basis of an often cited study that found a survival benefit for febrile neonates in the intensive care setting.18 There are a number of reasons that this study should not influence care for typical infants admitted with possible sepsis. First, the study was retrospective and limited by its use of administrative data. The authors acknowledged that a potential explanation for their results is unmeasured confounding. Second, the patients included in the study were dramatically different from the group of well infants admitted with possible sepsis; the study included neonatal critical care unit patients treated with antibiotics within the first 3 days of life. Third, the study’s results have not been replicated in otherwise healthy febrile infants.
WHAT YOU SHOULD USE INSTEAD OF AMPICILLIN FOR EMPIRIC LISTERIA COVERAGE
For febrile children 0 to 90 days old, empiric antibiotic coverage should be aimed at covering the current predominant pathogens, which include E coli and GBS. Therefore, for most children and US regions, a third-generation cephalosporin (eg, cefotaxime) is sufficient.
RECOMMENDATIONS
- Empiric antibiotics for treatment of febrile children 0-90 days should target E. coli and GBS; a third generation cephalosporin, (e.g. cefotaxime) alone is a reasonable choice for most patients.
- Prescribing ampicillin to specifically cover Listeria is unnecessary for the vast majority of febrile infants
- Prescribing ampicillin is reasonable in certain subgroups of febrile infants: those less than seven days of age, those with evidence of bacterial meningitis (especially if also <28 days of age), those in whom enterococcal infection is strongly suspected, and those with specific Listeria exposures related to local outbreaks.
CONCLUSION
The 32-day-old infant described in the clinical scenario was at extremely low risk for listeriosis. Antibiotic coverage with a third-generation cephalosporin is sufficient for the most likely pathogens. The common practice of empirically covering Listeria in otherwise healthy febrile infants considered to be at higher risk for SBI is no longer based on best available evidence and represents overtreatment with at least theoretical harms. Avoidance of the risks associated with the side effects of antibiotics, costs saved by forgoing multiple antibiotics, a decrease in medication dosing frequency, and improved antibiotic stewardship for the general population all argue forcefully for making empiric Listeria coverage a thing of the past.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. PubMed
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545. PubMed
3. Nield L, Kamat D. Fever without a focus. In: Kliegman R, Stanton B, eds. Nelson’s Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016.
4. Sadow KB, Derr R, Teach SJ. Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment. Arch Pediatr Adolesc Med. 1999;153(6):611-614. PubMed
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. PubMed
6. Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568-571. PubMed
7. Byington CL, Rittichier KK, Bassett KE, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111(5 pt 1):964-968. PubMed
8. Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5(8):e12448. PubMed
9. Greenhow TL, Hung YY, Herz AM. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics. 2012;129(3):e590-e596. PubMed
10. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. PubMed
11. Hassoun A, Stankovic C, Rogers A, et al. Listeria and enterococcal infections in neonates 28 days of age and younger: is empiric parenteral ampicillin still indicated? Pediatr Emerg Care. 2014;30(4):240-243. PubMed
12. Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990-996. PubMed
13. Mischler M, Ryan MS, Leyenaar JK, et al. Epidemiology of bacteremia in previously healthy febrile infants: a follow-up study. Hosp Pediatr. 2015;5(6):293-300. PubMed
14. Leazer R, Perkins AM, Shomaker K, Fine B. A meta-analysis of the rates of Listeria monocytogenes and Enterococcus in febrile infants. Hosp Pediatr. 2016;6(4):187-195. PubMed
15. Veesenmeyer AF, Edmonson MB. Trends in US hospital stays for listeriosis in infants. Hosp Pediatr. 2016;6(4):196-203. PubMed
16. Schroeder AR, Roberts KB. Is tradition trumping evidence in the treatment of young, febrile infants? Hosp Pediatr. 2016;6(4):252-253. PubMed
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. PubMed
18. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67-74. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Evaluation and treatment of the febrile infant 0 to 90 days of age are common clinical issues in pediatrics, family medicine, emergency medicine, and pediatric hospital medicine. Traditional teaching has been that Listeria monocytogenes is 1 of the 3 most common pathogens causing neonatal sepsis. Many practitioners routinely use antibiotic regimens, including ampicillin, to specifically target Listeria. However, a large body of evidence, including a meta-analysis and several multicenter studies, has shown that listeriosis is extremely rare in the United States. The practice of empiric ampicillin thus exposes the patient to harms and costs with little if any potential benefit, while increasing pressure on the bacterial flora in the community to generate antibiotic resistance. Empiric ampicillin for all infants admitted for sepsis evaluation is a tradition-based practice no longer founded on the best available evidence.
CASE REPORT
A 32-day-old, full-term, previously healthy girl presented with fever of 1 day’s duration. Her parents reported she had appeared well until the evening before admission, when she became a bit less active and spent less time breastfeeding. The morning of admission, she was fussier than usual. Rectal temperature, taken by her parents, was 101°F. There were no other symptoms and no sick contacts.
On examination, the patient’s rectal temperature was 101.5°F. Her other vitals and the physical examination findings were unremarkable. Laboratory test results included a normal urinalysis and a peripheral white blood cell (WBC) count of 21,300 cells/µL. Cerebrospinal fluid (CSF) analysis revealed normal protein and glucose levels with 3 WBCs/µL and a negative gram stain. Due to stratifying at higher risk for serious bacterial infection (SBI), the child was admitted and started on ampicillin and cefotaxime while awaiting culture results.
BACKGROUND
Evaluation and treatment of febrile infants are common clinical issues in pediatrics, emergency medicine, and general practice. Practice guidelines for evaluation of febrile infants recommend hospitalization and parenteral antibiotics for children younger than 28 days and children 29 to 90 days old if stratified at high risk for SBI.1,2 Recommendations for empiric antibiotic regimens include ampicillin in addition to either gentamicin or cefotaxime.1,2
WHY YOU MIGHT THINK AMPICILLIN IS HELPFUL
Generations of pediatrics students have been taught that the 3 pathogens most likely to cause bacterial sepsis in infants are group B Streptococcus (GBS), Escherichia coli, and Listeria monocytogenes. This teaching is still espoused in the latest editions of pediatrics textbooks.3 Ampicillin is specifically recommended for covering Listeria, and studies have found that 62% to 78% of practitioners choose empiric ampicillin-containing antibiotic regimens for the treatment of febrile infants.4-6
WHY EMPIRIC AMPICILLIN IS UNNECESSARY
In the past, Listeria was a potential though still uncommon infant pathogen. Over the past few decades, however, the epidemiology of infant sepsis has changed significantly. Estimates of the rate of infection with Listeria now range from extremely rare to nonexistent across multiple studies4,7-15 (Table). In a 4-year retrospective case series at a single urban academic center in Washington, DC, Sadow et al.4 reported no instances of Listeria among 121 positive bacterial cultures in infants younger than 60 days seen in the emergency department (ED). Byington et al.7 examined all positive cultures for infants 0 to 90 days old at a large academic referral center in Utah over a 3-year period and reported no cases of Listeria (1298 patients, 105 SBI cases). A study at a North Carolina academic center found 1 case of Listeria meningitis among 72 SBIs (668 febrile infants) without a localizing source.8 At a large group-practice in northern California, Greenhow et al.9 examined all blood cultures (N = 4255) performed over 4 years for otherwise healthy infants 1 week to 3 months old and found no cases of Listeria. In a follow-up study, the same authors examined all blood (n = 5396), urine (n = 4599), and CSF (n = 1796) cultures in the same population and found no Listeria cases.10 Hassoun et al.11 studied SBI rates among infants younger than 28 days with any blood, urine, or CSF culture performed over 4 years at two Michigan EDs. One (0.08%) of the 1192 infants evaluated had bacteremia caused by Listeria.
Multicenter studies have reported similar results. In a study of 6 hospital systems in geographically diverse areas of the United States, Biondi et al.12 examined all positive blood cultures (N = 181) for febrile infants younger than 90 days admitted to a general pediatric ward, and found no listeriosis. Mischler et al.13 examined all positive blood cultures (N = 392) for otherwise healthy febrile infants 0 to 90 days old admitted to a hospital in 1 of 17 geographically diverse healthcare systems and found no cases of Listeria. A recent meta-analysis of studies that reported SBI rates for febrile infants 0 to 90 days old found the weighted prevalence of Listeria bacteremia to be 0.03% (2/20,703) and that of meningitis to be 0.02% (3/13,375).14 Veesenmeyer and Edmonson15 used a national inpatient database to identify all Listeria cases among infants over a 6-year period and estimated listeriosis rates for the US population. Over the 6 years, there were 212 total cases, which were extrapolated to 344 in the United States during that period, yielding a pooled annual incidence rate of 1.41 in 100,000 births.
Ampicillin offers no significant improvement in coverage for GBS or E coli beyond other β-lactam antibiotics, such as cefotaxime. Therefore, though the cost and potential harms of 24 to 48 hours of intravenous ampicillin are low for the individual patient, there is almost no potential benefit. Using the weighted prevalence of 0.03% for Listeria bacteremia reported in the recent meta-analysis,14 the number needed to treat to cover 1 case of Listeria bacteremia would be 3333. In addition, the increasing incidence of ampicillin resistance, particularly among gram-negative organisms,4,7,9 argues strongly for better antibiotic stewardship on a national level. A number of expert authors have advocated dropping empiric Listeria coverage as part of the treatment of febrile infants, particularly infants 29 to 90 days old.16,17 Some authors continue to advocate empiric Listeria coverage.6 It is interesting to note, however, that the incidence of Staph aureus bacteremia in recent case series is much higher than that reported for Listeria, accounting for 6-9% of bacteremia cases.9,11,13 Yet few if any authors advocate for empiric S. aureus coverage.
WHEN EMPIRIC AMPICILLIN COVERAGE MAY BE REASONABLE
The rate of listeriosis remains low across age groups in recent studies, but the rate is slightly higher in very young infants. In the recent national database study of listeriosis cases over a 6-year period, almost half involved infants younger than 7 days, and most of these infants showed no evidence of meningitis.15 Therefore, it may be reasonable to include empiric Listeria coverage in febrile infants younger than 7 days, though the study authors estimated 22.5 annual cases of Listeria in this age range in the United States. Eighty percent of the Listeria cases were in infants younger than 28 days, but more than 85% of infants 7 to 28 days old had meningitis. Therefore, broad antimicrobial coverage for infants with CSF pleocytosis and/or a high bacterial meningitis score is reasonable, especially for infants younger than 28 days.
Other potential indications for ampicillin are enterococcal infections. Though enteroccocal SBI rates in febrile infants are also quite low,7-9,11,12 if Enterococcus were highly suspected, such as in an infant with pyuria and gram positive organisms on gram stain, ampicillin offers good additional coverage. In the case of a local outbreak of listeriosis, or a specific exposure to Listeria-contaminated products on a patient history, antibiotics with efficacy against Listeria should be used. Last, in cases in which gentamicin is used as empiric coverage for gram-negative organisms, ampicillin offers important additional coverage for GBS.
Some practitioners advocate ampicillin and gentamicin over cefotaxime regimens on the basis of an often cited study that found a survival benefit for febrile neonates in the intensive care setting.18 There are a number of reasons that this study should not influence care for typical infants admitted with possible sepsis. First, the study was retrospective and limited by its use of administrative data. The authors acknowledged that a potential explanation for their results is unmeasured confounding. Second, the patients included in the study were dramatically different from the group of well infants admitted with possible sepsis; the study included neonatal critical care unit patients treated with antibiotics within the first 3 days of life. Third, the study’s results have not been replicated in otherwise healthy febrile infants.
WHAT YOU SHOULD USE INSTEAD OF AMPICILLIN FOR EMPIRIC LISTERIA COVERAGE
For febrile children 0 to 90 days old, empiric antibiotic coverage should be aimed at covering the current predominant pathogens, which include E coli and GBS. Therefore, for most children and US regions, a third-generation cephalosporin (eg, cefotaxime) is sufficient.
RECOMMENDATIONS
- Empiric antibiotics for treatment of febrile children 0-90 days should target E. coli and GBS; a third generation cephalosporin, (e.g. cefotaxime) alone is a reasonable choice for most patients.
- Prescribing ampicillin to specifically cover Listeria is unnecessary for the vast majority of febrile infants
- Prescribing ampicillin is reasonable in certain subgroups of febrile infants: those less than seven days of age, those with evidence of bacterial meningitis (especially if also <28 days of age), those in whom enterococcal infection is strongly suspected, and those with specific Listeria exposures related to local outbreaks.
CONCLUSION
The 32-day-old infant described in the clinical scenario was at extremely low risk for listeriosis. Antibiotic coverage with a third-generation cephalosporin is sufficient for the most likely pathogens. The common practice of empirically covering Listeria in otherwise healthy febrile infants considered to be at higher risk for SBI is no longer based on best available evidence and represents overtreatment with at least theoretical harms. Avoidance of the risks associated with the side effects of antibiotics, costs saved by forgoing multiple antibiotics, a decrease in medication dosing frequency, and improved antibiotic stewardship for the general population all argue forcefully for making empiric Listeria coverage a thing of the past.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Evaluation and treatment of the febrile infant 0 to 90 days of age are common clinical issues in pediatrics, family medicine, emergency medicine, and pediatric hospital medicine. Traditional teaching has been that Listeria monocytogenes is 1 of the 3 most common pathogens causing neonatal sepsis. Many practitioners routinely use antibiotic regimens, including ampicillin, to specifically target Listeria. However, a large body of evidence, including a meta-analysis and several multicenter studies, has shown that listeriosis is extremely rare in the United States. The practice of empiric ampicillin thus exposes the patient to harms and costs with little if any potential benefit, while increasing pressure on the bacterial flora in the community to generate antibiotic resistance. Empiric ampicillin for all infants admitted for sepsis evaluation is a tradition-based practice no longer founded on the best available evidence.
CASE REPORT
A 32-day-old, full-term, previously healthy girl presented with fever of 1 day’s duration. Her parents reported she had appeared well until the evening before admission, when she became a bit less active and spent less time breastfeeding. The morning of admission, she was fussier than usual. Rectal temperature, taken by her parents, was 101°F. There were no other symptoms and no sick contacts.
On examination, the patient’s rectal temperature was 101.5°F. Her other vitals and the physical examination findings were unremarkable. Laboratory test results included a normal urinalysis and a peripheral white blood cell (WBC) count of 21,300 cells/µL. Cerebrospinal fluid (CSF) analysis revealed normal protein and glucose levels with 3 WBCs/µL and a negative gram stain. Due to stratifying at higher risk for serious bacterial infection (SBI), the child was admitted and started on ampicillin and cefotaxime while awaiting culture results.
BACKGROUND
Evaluation and treatment of febrile infants are common clinical issues in pediatrics, emergency medicine, and general practice. Practice guidelines for evaluation of febrile infants recommend hospitalization and parenteral antibiotics for children younger than 28 days and children 29 to 90 days old if stratified at high risk for SBI.1,2 Recommendations for empiric antibiotic regimens include ampicillin in addition to either gentamicin or cefotaxime.1,2
WHY YOU MIGHT THINK AMPICILLIN IS HELPFUL
Generations of pediatrics students have been taught that the 3 pathogens most likely to cause bacterial sepsis in infants are group B Streptococcus (GBS), Escherichia coli, and Listeria monocytogenes. This teaching is still espoused in the latest editions of pediatrics textbooks.3 Ampicillin is specifically recommended for covering Listeria, and studies have found that 62% to 78% of practitioners choose empiric ampicillin-containing antibiotic regimens for the treatment of febrile infants.4-6
WHY EMPIRIC AMPICILLIN IS UNNECESSARY
In the past, Listeria was a potential though still uncommon infant pathogen. Over the past few decades, however, the epidemiology of infant sepsis has changed significantly. Estimates of the rate of infection with Listeria now range from extremely rare to nonexistent across multiple studies4,7-15 (Table). In a 4-year retrospective case series at a single urban academic center in Washington, DC, Sadow et al.4 reported no instances of Listeria among 121 positive bacterial cultures in infants younger than 60 days seen in the emergency department (ED). Byington et al.7 examined all positive cultures for infants 0 to 90 days old at a large academic referral center in Utah over a 3-year period and reported no cases of Listeria (1298 patients, 105 SBI cases). A study at a North Carolina academic center found 1 case of Listeria meningitis among 72 SBIs (668 febrile infants) without a localizing source.8 At a large group-practice in northern California, Greenhow et al.9 examined all blood cultures (N = 4255) performed over 4 years for otherwise healthy infants 1 week to 3 months old and found no cases of Listeria. In a follow-up study, the same authors examined all blood (n = 5396), urine (n = 4599), and CSF (n = 1796) cultures in the same population and found no Listeria cases.10 Hassoun et al.11 studied SBI rates among infants younger than 28 days with any blood, urine, or CSF culture performed over 4 years at two Michigan EDs. One (0.08%) of the 1192 infants evaluated had bacteremia caused by Listeria.
Multicenter studies have reported similar results. In a study of 6 hospital systems in geographically diverse areas of the United States, Biondi et al.12 examined all positive blood cultures (N = 181) for febrile infants younger than 90 days admitted to a general pediatric ward, and found no listeriosis. Mischler et al.13 examined all positive blood cultures (N = 392) for otherwise healthy febrile infants 0 to 90 days old admitted to a hospital in 1 of 17 geographically diverse healthcare systems and found no cases of Listeria. A recent meta-analysis of studies that reported SBI rates for febrile infants 0 to 90 days old found the weighted prevalence of Listeria bacteremia to be 0.03% (2/20,703) and that of meningitis to be 0.02% (3/13,375).14 Veesenmeyer and Edmonson15 used a national inpatient database to identify all Listeria cases among infants over a 6-year period and estimated listeriosis rates for the US population. Over the 6 years, there were 212 total cases, which were extrapolated to 344 in the United States during that period, yielding a pooled annual incidence rate of 1.41 in 100,000 births.
Ampicillin offers no significant improvement in coverage for GBS or E coli beyond other β-lactam antibiotics, such as cefotaxime. Therefore, though the cost and potential harms of 24 to 48 hours of intravenous ampicillin are low for the individual patient, there is almost no potential benefit. Using the weighted prevalence of 0.03% for Listeria bacteremia reported in the recent meta-analysis,14 the number needed to treat to cover 1 case of Listeria bacteremia would be 3333. In addition, the increasing incidence of ampicillin resistance, particularly among gram-negative organisms,4,7,9 argues strongly for better antibiotic stewardship on a national level. A number of expert authors have advocated dropping empiric Listeria coverage as part of the treatment of febrile infants, particularly infants 29 to 90 days old.16,17 Some authors continue to advocate empiric Listeria coverage.6 It is interesting to note, however, that the incidence of Staph aureus bacteremia in recent case series is much higher than that reported for Listeria, accounting for 6-9% of bacteremia cases.9,11,13 Yet few if any authors advocate for empiric S. aureus coverage.
WHEN EMPIRIC AMPICILLIN COVERAGE MAY BE REASONABLE
The rate of listeriosis remains low across age groups in recent studies, but the rate is slightly higher in very young infants. In the recent national database study of listeriosis cases over a 6-year period, almost half involved infants younger than 7 days, and most of these infants showed no evidence of meningitis.15 Therefore, it may be reasonable to include empiric Listeria coverage in febrile infants younger than 7 days, though the study authors estimated 22.5 annual cases of Listeria in this age range in the United States. Eighty percent of the Listeria cases were in infants younger than 28 days, but more than 85% of infants 7 to 28 days old had meningitis. Therefore, broad antimicrobial coverage for infants with CSF pleocytosis and/or a high bacterial meningitis score is reasonable, especially for infants younger than 28 days.
Other potential indications for ampicillin are enterococcal infections. Though enteroccocal SBI rates in febrile infants are also quite low,7-9,11,12 if Enterococcus were highly suspected, such as in an infant with pyuria and gram positive organisms on gram stain, ampicillin offers good additional coverage. In the case of a local outbreak of listeriosis, or a specific exposure to Listeria-contaminated products on a patient history, antibiotics with efficacy against Listeria should be used. Last, in cases in which gentamicin is used as empiric coverage for gram-negative organisms, ampicillin offers important additional coverage for GBS.
Some practitioners advocate ampicillin and gentamicin over cefotaxime regimens on the basis of an often cited study that found a survival benefit for febrile neonates in the intensive care setting.18 There are a number of reasons that this study should not influence care for typical infants admitted with possible sepsis. First, the study was retrospective and limited by its use of administrative data. The authors acknowledged that a potential explanation for their results is unmeasured confounding. Second, the patients included in the study were dramatically different from the group of well infants admitted with possible sepsis; the study included neonatal critical care unit patients treated with antibiotics within the first 3 days of life. Third, the study’s results have not been replicated in otherwise healthy febrile infants.
WHAT YOU SHOULD USE INSTEAD OF AMPICILLIN FOR EMPIRIC LISTERIA COVERAGE
For febrile children 0 to 90 days old, empiric antibiotic coverage should be aimed at covering the current predominant pathogens, which include E coli and GBS. Therefore, for most children and US regions, a third-generation cephalosporin (eg, cefotaxime) is sufficient.
RECOMMENDATIONS
- Empiric antibiotics for treatment of febrile children 0-90 days should target E. coli and GBS; a third generation cephalosporin, (e.g. cefotaxime) alone is a reasonable choice for most patients.
- Prescribing ampicillin to specifically cover Listeria is unnecessary for the vast majority of febrile infants
- Prescribing ampicillin is reasonable in certain subgroups of febrile infants: those less than seven days of age, those with evidence of bacterial meningitis (especially if also <28 days of age), those in whom enterococcal infection is strongly suspected, and those with specific Listeria exposures related to local outbreaks.
CONCLUSION
The 32-day-old infant described in the clinical scenario was at extremely low risk for listeriosis. Antibiotic coverage with a third-generation cephalosporin is sufficient for the most likely pathogens. The common practice of empirically covering Listeria in otherwise healthy febrile infants considered to be at higher risk for SBI is no longer based on best available evidence and represents overtreatment with at least theoretical harms. Avoidance of the risks associated with the side effects of antibiotics, costs saved by forgoing multiple antibiotics, a decrease in medication dosing frequency, and improved antibiotic stewardship for the general population all argue forcefully for making empiric Listeria coverage a thing of the past.
Disclosure
Nothing to report.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and Liking It on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. PubMed
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545. PubMed
3. Nield L, Kamat D. Fever without a focus. In: Kliegman R, Stanton B, eds. Nelson’s Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016.
4. Sadow KB, Derr R, Teach SJ. Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment. Arch Pediatr Adolesc Med. 1999;153(6):611-614. PubMed
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. PubMed
6. Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568-571. PubMed
7. Byington CL, Rittichier KK, Bassett KE, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111(5 pt 1):964-968. PubMed
8. Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5(8):e12448. PubMed
9. Greenhow TL, Hung YY, Herz AM. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics. 2012;129(3):e590-e596. PubMed
10. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. PubMed
11. Hassoun A, Stankovic C, Rogers A, et al. Listeria and enterococcal infections in neonates 28 days of age and younger: is empiric parenteral ampicillin still indicated? Pediatr Emerg Care. 2014;30(4):240-243. PubMed
12. Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990-996. PubMed
13. Mischler M, Ryan MS, Leyenaar JK, et al. Epidemiology of bacteremia in previously healthy febrile infants: a follow-up study. Hosp Pediatr. 2015;5(6):293-300. PubMed
14. Leazer R, Perkins AM, Shomaker K, Fine B. A meta-analysis of the rates of Listeria monocytogenes and Enterococcus in febrile infants. Hosp Pediatr. 2016;6(4):187-195. PubMed
15. Veesenmeyer AF, Edmonson MB. Trends in US hospital stays for listeriosis in infants. Hosp Pediatr. 2016;6(4):196-203. PubMed
16. Schroeder AR, Roberts KB. Is tradition trumping evidence in the treatment of young, febrile infants? Hosp Pediatr. 2016;6(4):252-253. PubMed
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. PubMed
18. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67-74. PubMed
1. Baraff LJ, Bass JW, Fleisher GR, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Agency for Health Care Policy and Research. Ann Emerg Med. 1993;22(7):1198-1210. PubMed
2. American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42(4):530-545. PubMed
3. Nield L, Kamat D. Fever without a focus. In: Kliegman R, Stanton B, eds. Nelson’s Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier; 2016.
4. Sadow KB, Derr R, Teach SJ. Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment. Arch Pediatr Adolesc Med. 1999;153(6):611-614. PubMed
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134(4):667-677. PubMed
6. Cantey JB, Lopez-Medina E, Nguyen S, Doern C, Garcia C. Empiric antibiotics for serious bacterial infection in young infants: opportunities for stewardship. Pediatr Emerg Care. 2015;31(8):568-571. PubMed
7. Byington CL, Rittichier KK, Bassett KE, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111(5 pt 1):964-968. PubMed
8. Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PLoS One. 2010;5(8):e12448. PubMed
9. Greenhow TL, Hung YY, Herz AM. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics. 2012;129(3):e590-e596. PubMed
10. Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33(6):595-599. PubMed
11. Hassoun A, Stankovic C, Rogers A, et al. Listeria and enterococcal infections in neonates 28 days of age and younger: is empiric parenteral ampicillin still indicated? Pediatr Emerg Care. 2014;30(4):240-243. PubMed
12. Biondi E, Evans R, Mischler M, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132(6):990-996. PubMed
13. Mischler M, Ryan MS, Leyenaar JK, et al. Epidemiology of bacteremia in previously healthy febrile infants: a follow-up study. Hosp Pediatr. 2015;5(6):293-300. PubMed
14. Leazer R, Perkins AM, Shomaker K, Fine B. A meta-analysis of the rates of Listeria monocytogenes and Enterococcus in febrile infants. Hosp Pediatr. 2016;6(4):187-195. PubMed
15. Veesenmeyer AF, Edmonson MB. Trends in US hospital stays for listeriosis in infants. Hosp Pediatr. 2016;6(4):196-203. PubMed
16. Schroeder AR, Roberts KB. Is tradition trumping evidence in the treatment of young, febrile infants? Hosp Pediatr. 2016;6(4):252-253. PubMed
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. PubMed
18. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67-74. PubMed
© 2017 Society of Hospital Medicine