User login
FDA approves first biosimilar product
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to be approved in the US.
A biosimilar product is approved based on data showing that it is highly similar to an already-approved biological product.
Sandoz Inc’s Zarxio is biosimilar to Amgen Inc’s Neupogen (filgrastim), which was originally licensed in 1991. Zarxio is now approved for the same indications as Neupogen.
Zarxio can be prescribed for:
- patients with cancer receiving myelosuppressive chemotherapy
- patients with acute myeloid leukemia receiving induction or consolidation chemotherapy
- patients with cancer undergoing bone marrow transplant
- patients undergoing autologous peripheral blood progenitor cell collection and therapy
- patients with severe chronic neutropenia.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
“Biosimilars will provide access to important therapies for patients who need them,” said FDA Commissioner Margaret A. Hamburg, MD.
“Patients and the healthcare community can be confident that biosimilar products approved by the FDA meet the agency’s rigorous safety, efficacy, and quality standards.”
Zarxio data
The FDA’s approval of Zarxio is based on a review of evidence that included structural and functional characterization, in vivo data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates Zarxio is biosimilar to Neupogen.
The PIONEER study was the final piece of data the FDA used to approve Zarxio as biosimilar to Neupogen. The data was sufficient to allow extrapolation of the use of Zarxio to all indications of Neupogen.
In the PIONEER study, Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in cancer patients undergoing myelosuppressive chemotherapy—1.17 and 1.20 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The most common side effects of Zarxio are aching in the bones or muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
About biosimilar approval
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) was passed as part of the Affordable Care Act that President Barack Obama signed into law in March 2010. The BPCI Act created an abbreviated licensure pathway for biological products shown to be “biosimilar” to or “interchangeable” with an FDA-licensed biological product, known as the reference product.
This abbreviated licensure pathway under section 351(k) of the Public Health Service Act permits reliance on certain existing scientific knowledge about the safety and effectiveness of the reference product, and it enables a biosimilar biological product to be licensed based on less than a full complement of product-specific preclinical and clinical data.
A biosimilar product can only be approved by the FDA if it has the same mechanism(s) of action, route(s) of administration, dosage form(s) and strength(s) as the reference product, and only for the indication(s) and condition(s) of use that have been approved for the reference product. The facilities where biosimilars are manufactured must also meet the FDA’s standards.
There must be no clinically meaningful differences between the biosimilar and the reference product in terms of safety and effectiveness. Only minor differences in clinically inactive components are allowable.
Zarxio has been approved as a biosimilar, not an interchangeable product. Under the BPCI Act, a biological product that has been approved as “interchangeable” may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product.
For Zarxio’s approval, the FDA has designated a placeholder nonproprietary name for this product as “filgrastim-sndz.” The provision of a placeholder nonproprietary name should not be viewed as reflective of the agency’s decision on a comprehensive naming policy for biosimilars and other biological products.
While the FDA has not yet issued draft guidance on how current and future biological products marketed in the US should be named, the agency intends to do so in the near future.
For more details on Zarxio, see the full prescribing information. ![]()
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to be approved in the US.
A biosimilar product is approved based on data showing that it is highly similar to an already-approved biological product.
Sandoz Inc’s Zarxio is biosimilar to Amgen Inc’s Neupogen (filgrastim), which was originally licensed in 1991. Zarxio is now approved for the same indications as Neupogen.
Zarxio can be prescribed for:
- patients with cancer receiving myelosuppressive chemotherapy
- patients with acute myeloid leukemia receiving induction or consolidation chemotherapy
- patients with cancer undergoing bone marrow transplant
- patients undergoing autologous peripheral blood progenitor cell collection and therapy
- patients with severe chronic neutropenia.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
“Biosimilars will provide access to important therapies for patients who need them,” said FDA Commissioner Margaret A. Hamburg, MD.
“Patients and the healthcare community can be confident that biosimilar products approved by the FDA meet the agency’s rigorous safety, efficacy, and quality standards.”
Zarxio data
The FDA’s approval of Zarxio is based on a review of evidence that included structural and functional characterization, in vivo data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates Zarxio is biosimilar to Neupogen.
The PIONEER study was the final piece of data the FDA used to approve Zarxio as biosimilar to Neupogen. The data was sufficient to allow extrapolation of the use of Zarxio to all indications of Neupogen.
In the PIONEER study, Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in cancer patients undergoing myelosuppressive chemotherapy—1.17 and 1.20 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The most common side effects of Zarxio are aching in the bones or muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
About biosimilar approval
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) was passed as part of the Affordable Care Act that President Barack Obama signed into law in March 2010. The BPCI Act created an abbreviated licensure pathway for biological products shown to be “biosimilar” to or “interchangeable” with an FDA-licensed biological product, known as the reference product.
This abbreviated licensure pathway under section 351(k) of the Public Health Service Act permits reliance on certain existing scientific knowledge about the safety and effectiveness of the reference product, and it enables a biosimilar biological product to be licensed based on less than a full complement of product-specific preclinical and clinical data.
A biosimilar product can only be approved by the FDA if it has the same mechanism(s) of action, route(s) of administration, dosage form(s) and strength(s) as the reference product, and only for the indication(s) and condition(s) of use that have been approved for the reference product. The facilities where biosimilars are manufactured must also meet the FDA’s standards.
There must be no clinically meaningful differences between the biosimilar and the reference product in terms of safety and effectiveness. Only minor differences in clinically inactive components are allowable.
Zarxio has been approved as a biosimilar, not an interchangeable product. Under the BPCI Act, a biological product that has been approved as “interchangeable” may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product.
For Zarxio’s approval, the FDA has designated a placeholder nonproprietary name for this product as “filgrastim-sndz.” The provision of a placeholder nonproprietary name should not be viewed as reflective of the agency’s decision on a comprehensive naming policy for biosimilars and other biological products.
While the FDA has not yet issued draft guidance on how current and future biological products marketed in the US should be named, the agency intends to do so in the near future.
For more details on Zarxio, see the full prescribing information. ![]()
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Zarxio (filgrastim-sndz), the first biosimilar product to be approved in the US.
A biosimilar product is approved based on data showing that it is highly similar to an already-approved biological product.
Sandoz Inc’s Zarxio is biosimilar to Amgen Inc’s Neupogen (filgrastim), which was originally licensed in 1991. Zarxio is now approved for the same indications as Neupogen.
Zarxio can be prescribed for:
- patients with cancer receiving myelosuppressive chemotherapy
- patients with acute myeloid leukemia receiving induction or consolidation chemotherapy
- patients with cancer undergoing bone marrow transplant
- patients undergoing autologous peripheral blood progenitor cell collection and therapy
- patients with severe chronic neutropenia.
Zarxio is marketed as Zarzio outside the US. The biosimilar is available in more than 60 countries worldwide.
“Biosimilars will provide access to important therapies for patients who need them,” said FDA Commissioner Margaret A. Hamburg, MD.
“Patients and the healthcare community can be confident that biosimilar products approved by the FDA meet the agency’s rigorous safety, efficacy, and quality standards.”
Zarxio data
The FDA’s approval of Zarxio is based on a review of evidence that included structural and functional characterization, in vivo data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrates Zarxio is biosimilar to Neupogen.
The PIONEER study was the final piece of data the FDA used to approve Zarxio as biosimilar to Neupogen. The data was sufficient to allow extrapolation of the use of Zarxio to all indications of Neupogen.
In the PIONEER study, Zarxio and Neupogen both produced the expected reduction in the duration of severe neutropenia in cancer patients undergoing myelosuppressive chemotherapy—1.17 and 1.20 days, respectively.
The mean time to absolute neutrophil count recovery in cycle 1 was also similar—1.8 ± 0.97 days in the Zarxio arm and 1.7 ± 0.81 days in the Neupogen arm. No immunogenicity or antibodies against rhG-CSF were detected throughout the study.
The most common side effects of Zarxio are aching in the bones or muscles and redness, swelling, or itching at the injection site. Serious side effects may include spleen rupture; serious allergic reactions that may cause rash, shortness of breath, wheezing and/or swelling around the mouth and eyes; fast pulse and sweating; and acute respiratory distress syndrome.
About biosimilar approval
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) was passed as part of the Affordable Care Act that President Barack Obama signed into law in March 2010. The BPCI Act created an abbreviated licensure pathway for biological products shown to be “biosimilar” to or “interchangeable” with an FDA-licensed biological product, known as the reference product.
This abbreviated licensure pathway under section 351(k) of the Public Health Service Act permits reliance on certain existing scientific knowledge about the safety and effectiveness of the reference product, and it enables a biosimilar biological product to be licensed based on less than a full complement of product-specific preclinical and clinical data.
A biosimilar product can only be approved by the FDA if it has the same mechanism(s) of action, route(s) of administration, dosage form(s) and strength(s) as the reference product, and only for the indication(s) and condition(s) of use that have been approved for the reference product. The facilities where biosimilars are manufactured must also meet the FDA’s standards.
There must be no clinically meaningful differences between the biosimilar and the reference product in terms of safety and effectiveness. Only minor differences in clinically inactive components are allowable.
Zarxio has been approved as a biosimilar, not an interchangeable product. Under the BPCI Act, a biological product that has been approved as “interchangeable” may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product.
For Zarxio’s approval, the FDA has designated a placeholder nonproprietary name for this product as “filgrastim-sndz.” The provision of a placeholder nonproprietary name should not be viewed as reflective of the agency’s decision on a comprehensive naming policy for biosimilars and other biological products.
While the FDA has not yet issued draft guidance on how current and future biological products marketed in the US should be named, the agency intends to do so in the near future.
For more details on Zarxio, see the full prescribing information. ![]()
NICE recommends apixaban for VTE

Image by Andre E.X. Brown
The UK’s National Institute for Health and Care Excellence (NICE) has issued a draft guidance recommending the anticoagulant apixaban (Eliquis) as an option for treating and preventing venous thromboembolism (VTE) in adults.
A NICE committee concluded that apixaban is clinically and cost-effective for this indication.
The draft guidance is now with consultees, who can appeal against it. Once NICE issues its final guidance on a technology, it replaces local recommendations.
“Apixaban, like the other newer oral anticoagulants already recommended by NICE for the treatment and secondary prevention of VTE, does not require frequent blood tests to monitor treatment and so represents a potential benefit for many people who have had a VTE,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“The committee also heard that apixaban is the only oral anticoagulant for which the licensed dose is lower for secondary prevention than for initial treatment of VTE. This could also be of potential benefit in terms of reducing the risk of bleeding where treatment is continued and therefore increase the chance that a person would take apixaban long-term.”
Clinical effectiveness
The NICE committee assessed the clinical effectiveness of apixaban based on results of the AMPLIFY and AMPLIFY-EXT studies.
Results of the AMPLIFY study indicated that apixaban is noninferior to standard treatment for recurrent VTE—initial parenteral enoxaparin overlapped with warfarin. Apixaban was comparable in efficacy to standard therapy and induced significantly less bleeding.
In AMPLIFY-EXT, researchers compared 12 months of treatment with apixaban at 2 doses—2.5 mg and 5 mg—to placebo in patients who had previously received anticoagulant therapy for 6 to 12 months to treat a prior VTE.
Both doses of apixaban effectively prevented VTE, VTE-related events, and death. And the incidence of bleeding events was low in all treatment arms.
The NICE committee noted that there were limited data in these trials pertaining to patients who needed less than 6 months of treatment and for patients still at high risk of recurrent VTE after 6 months of treatment.
However, the committee concluded that, despite these limitations, the AMPLIFY trials were the pivotal trials that informed the marketing authorization for apixaban. As such, they were sufficient to inform a recommendation for the whole population covered by the marketing authorization.
The committee did point out that there were no head-to-head trials evaluating the relative effectiveness of apixaban compared with rivaroxaban and dabigatran etexilate for treating and preventing VTE.
In addition, there were insufficient data to assess the effectiveness and safety of apixaban in patients with active cancer who had VTE, so it was not possible to make a specific recommendation for this group.
Cost-effectiveness
The recommended dose of apixaban as VTE treatment is 10 mg twice a day for the first 7 days, followed by 5 mg twice a day for at least 3 months. To prevent recurrent VTE, patients who have completed 6 months of VTE treatment should take apixaban at 2.5 mg twice a day.
The cost of apixaban is £1.10 per tablet for either the 2.5 mg or 5 mg dose (excluding tax). The daily cost of apixaban is £2.20. (Costs may vary in different settings because of negotiated procurement discounts.)
Analyses suggested that the incremental cost-effectiveness ratio of apixaban was less than £20,000 per quality-adjusted life-year gained for either 6 months or life-long treatment. Therefore, NICE concluded that apixaban is a cost-effective use of National Health Service resources. ![]()

Image by Andre E.X. Brown
The UK’s National Institute for Health and Care Excellence (NICE) has issued a draft guidance recommending the anticoagulant apixaban (Eliquis) as an option for treating and preventing venous thromboembolism (VTE) in adults.
A NICE committee concluded that apixaban is clinically and cost-effective for this indication.
The draft guidance is now with consultees, who can appeal against it. Once NICE issues its final guidance on a technology, it replaces local recommendations.
“Apixaban, like the other newer oral anticoagulants already recommended by NICE for the treatment and secondary prevention of VTE, does not require frequent blood tests to monitor treatment and so represents a potential benefit for many people who have had a VTE,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“The committee also heard that apixaban is the only oral anticoagulant for which the licensed dose is lower for secondary prevention than for initial treatment of VTE. This could also be of potential benefit in terms of reducing the risk of bleeding where treatment is continued and therefore increase the chance that a person would take apixaban long-term.”
Clinical effectiveness
The NICE committee assessed the clinical effectiveness of apixaban based on results of the AMPLIFY and AMPLIFY-EXT studies.
Results of the AMPLIFY study indicated that apixaban is noninferior to standard treatment for recurrent VTE—initial parenteral enoxaparin overlapped with warfarin. Apixaban was comparable in efficacy to standard therapy and induced significantly less bleeding.
In AMPLIFY-EXT, researchers compared 12 months of treatment with apixaban at 2 doses—2.5 mg and 5 mg—to placebo in patients who had previously received anticoagulant therapy for 6 to 12 months to treat a prior VTE.
Both doses of apixaban effectively prevented VTE, VTE-related events, and death. And the incidence of bleeding events was low in all treatment arms.
The NICE committee noted that there were limited data in these trials pertaining to patients who needed less than 6 months of treatment and for patients still at high risk of recurrent VTE after 6 months of treatment.
However, the committee concluded that, despite these limitations, the AMPLIFY trials were the pivotal trials that informed the marketing authorization for apixaban. As such, they were sufficient to inform a recommendation for the whole population covered by the marketing authorization.
The committee did point out that there were no head-to-head trials evaluating the relative effectiveness of apixaban compared with rivaroxaban and dabigatran etexilate for treating and preventing VTE.
In addition, there were insufficient data to assess the effectiveness and safety of apixaban in patients with active cancer who had VTE, so it was not possible to make a specific recommendation for this group.
Cost-effectiveness
The recommended dose of apixaban as VTE treatment is 10 mg twice a day for the first 7 days, followed by 5 mg twice a day for at least 3 months. To prevent recurrent VTE, patients who have completed 6 months of VTE treatment should take apixaban at 2.5 mg twice a day.
The cost of apixaban is £1.10 per tablet for either the 2.5 mg or 5 mg dose (excluding tax). The daily cost of apixaban is £2.20. (Costs may vary in different settings because of negotiated procurement discounts.)
Analyses suggested that the incremental cost-effectiveness ratio of apixaban was less than £20,000 per quality-adjusted life-year gained for either 6 months or life-long treatment. Therefore, NICE concluded that apixaban is a cost-effective use of National Health Service resources. ![]()

Image by Andre E.X. Brown
The UK’s National Institute for Health and Care Excellence (NICE) has issued a draft guidance recommending the anticoagulant apixaban (Eliquis) as an option for treating and preventing venous thromboembolism (VTE) in adults.
A NICE committee concluded that apixaban is clinically and cost-effective for this indication.
The draft guidance is now with consultees, who can appeal against it. Once NICE issues its final guidance on a technology, it replaces local recommendations.
“Apixaban, like the other newer oral anticoagulants already recommended by NICE for the treatment and secondary prevention of VTE, does not require frequent blood tests to monitor treatment and so represents a potential benefit for many people who have had a VTE,” said Carole Longson, NICE Health Technology Evaluation Centre Director.
“The committee also heard that apixaban is the only oral anticoagulant for which the licensed dose is lower for secondary prevention than for initial treatment of VTE. This could also be of potential benefit in terms of reducing the risk of bleeding where treatment is continued and therefore increase the chance that a person would take apixaban long-term.”
Clinical effectiveness
The NICE committee assessed the clinical effectiveness of apixaban based on results of the AMPLIFY and AMPLIFY-EXT studies.
Results of the AMPLIFY study indicated that apixaban is noninferior to standard treatment for recurrent VTE—initial parenteral enoxaparin overlapped with warfarin. Apixaban was comparable in efficacy to standard therapy and induced significantly less bleeding.
In AMPLIFY-EXT, researchers compared 12 months of treatment with apixaban at 2 doses—2.5 mg and 5 mg—to placebo in patients who had previously received anticoagulant therapy for 6 to 12 months to treat a prior VTE.
Both doses of apixaban effectively prevented VTE, VTE-related events, and death. And the incidence of bleeding events was low in all treatment arms.
The NICE committee noted that there were limited data in these trials pertaining to patients who needed less than 6 months of treatment and for patients still at high risk of recurrent VTE after 6 months of treatment.
However, the committee concluded that, despite these limitations, the AMPLIFY trials were the pivotal trials that informed the marketing authorization for apixaban. As such, they were sufficient to inform a recommendation for the whole population covered by the marketing authorization.
The committee did point out that there were no head-to-head trials evaluating the relative effectiveness of apixaban compared with rivaroxaban and dabigatran etexilate for treating and preventing VTE.
In addition, there were insufficient data to assess the effectiveness and safety of apixaban in patients with active cancer who had VTE, so it was not possible to make a specific recommendation for this group.
Cost-effectiveness
The recommended dose of apixaban as VTE treatment is 10 mg twice a day for the first 7 days, followed by 5 mg twice a day for at least 3 months. To prevent recurrent VTE, patients who have completed 6 months of VTE treatment should take apixaban at 2.5 mg twice a day.
The cost of apixaban is £1.10 per tablet for either the 2.5 mg or 5 mg dose (excluding tax). The daily cost of apixaban is £2.20. (Costs may vary in different settings because of negotiated procurement discounts.)
Analyses suggested that the incremental cost-effectiveness ratio of apixaban was less than £20,000 per quality-adjusted life-year gained for either 6 months or life-long treatment. Therefore, NICE concluded that apixaban is a cost-effective use of National Health Service resources. ![]()
FDA approves new antifungal drug

The US Food and Drug Administration (FDA) has approved isavuconazonium sulfate (Cresemba) to treat adults with invasive aspergillosis and invasive mucormycosis, life-threatening fungal infections that predominantly occur in immunocompromised patients.
Isavuconazonium sulfate is an azole antifungal agent that works by targeting the cell wall of a fungus. The drug is available in oral and intravenous formulations.
“[The] approval provides a new treatment option for patients with serious fungal infections and underscores the importance of having available safe and effective antifungal drugs,” said Edward Cox, MD, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research.
Clinical trials
The FDA approved isavuconazonium sulfate to treat invasive aspergillosis based on results of the phase 3 SECURE trial. The study included 516 adults with invasive aspergillosis who were randomized to receive isavuconazonium sulfate or voriconazole.
Isavuconazonium sulfate demonstrated non-inferiority to voriconazole on the primary endpoint of all-cause mortality. All-cause mortality through day 42 was 18.6% in the isavuconazonium sulfate arm and 20.2% in the voriconazole arm.
In addition, isavuconazonium sulfate demonstrated similar rates of mortality and non-fatal adverse events as voriconazole
The FDA approved isavuconazonium sulfate to treat invasive mucormycosis based on results of the phase 3 VITAL trial. This single-arm study included 37 patients with invasive mucormycosis who received isavuconazonium sulfate.
All-cause mortality in these patients was 38%. The efficacy of isavuconazonium sulfate as a treatment for invasive mucormycosis has not been evaluated in concurrent, controlled clinical trials.
The most frequent adverse events for patients treated with isavuconazonium sulfate in clinical trials were nausea (26%), vomiting (25%), diarrhea (22%), headache (17%), elevated liver chemistry tests (17%), hypokalemia (14%), constipation (13%), dyspnea (12%), cough (12%), peripheral edema (11%), and back pain (10%).
QIDP status
Isavuconazonium sulfate is the sixth approved antifungal/antibacterial drug product designated as a qualified infectious disease product (QIDP). This designation is given to antibacterial or antifungal products that treat serious or life-threatening infections.
As part of its QIDP designation, isavuconazonium sulfate was given priority review. The QIDP designation also qualifies the drug for an additional 5 years of marketing exclusivity to be added to certain exclusivity periods already provided by the Food, Drug, and Cosmetic Act.
As invasive aspergillosis and mucormycosis are rare, the FDA also granted isavuconazonium sulfate orphan drug designations to treat these infections.
Isavuconazonium sulfate is marketed as Cresemba by Astellas Pharma US, Inc., which is based in Northbrook, Illinois. For more information on the drug, see the full prescribing information. ![]()

The US Food and Drug Administration (FDA) has approved isavuconazonium sulfate (Cresemba) to treat adults with invasive aspergillosis and invasive mucormycosis, life-threatening fungal infections that predominantly occur in immunocompromised patients.
Isavuconazonium sulfate is an azole antifungal agent that works by targeting the cell wall of a fungus. The drug is available in oral and intravenous formulations.
“[The] approval provides a new treatment option for patients with serious fungal infections and underscores the importance of having available safe and effective antifungal drugs,” said Edward Cox, MD, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research.
Clinical trials
The FDA approved isavuconazonium sulfate to treat invasive aspergillosis based on results of the phase 3 SECURE trial. The study included 516 adults with invasive aspergillosis who were randomized to receive isavuconazonium sulfate or voriconazole.
Isavuconazonium sulfate demonstrated non-inferiority to voriconazole on the primary endpoint of all-cause mortality. All-cause mortality through day 42 was 18.6% in the isavuconazonium sulfate arm and 20.2% in the voriconazole arm.
In addition, isavuconazonium sulfate demonstrated similar rates of mortality and non-fatal adverse events as voriconazole
The FDA approved isavuconazonium sulfate to treat invasive mucormycosis based on results of the phase 3 VITAL trial. This single-arm study included 37 patients with invasive mucormycosis who received isavuconazonium sulfate.
All-cause mortality in these patients was 38%. The efficacy of isavuconazonium sulfate as a treatment for invasive mucormycosis has not been evaluated in concurrent, controlled clinical trials.
The most frequent adverse events for patients treated with isavuconazonium sulfate in clinical trials were nausea (26%), vomiting (25%), diarrhea (22%), headache (17%), elevated liver chemistry tests (17%), hypokalemia (14%), constipation (13%), dyspnea (12%), cough (12%), peripheral edema (11%), and back pain (10%).
QIDP status
Isavuconazonium sulfate is the sixth approved antifungal/antibacterial drug product designated as a qualified infectious disease product (QIDP). This designation is given to antibacterial or antifungal products that treat serious or life-threatening infections.
As part of its QIDP designation, isavuconazonium sulfate was given priority review. The QIDP designation also qualifies the drug for an additional 5 years of marketing exclusivity to be added to certain exclusivity periods already provided by the Food, Drug, and Cosmetic Act.
As invasive aspergillosis and mucormycosis are rare, the FDA also granted isavuconazonium sulfate orphan drug designations to treat these infections.
Isavuconazonium sulfate is marketed as Cresemba by Astellas Pharma US, Inc., which is based in Northbrook, Illinois. For more information on the drug, see the full prescribing information. ![]()

The US Food and Drug Administration (FDA) has approved isavuconazonium sulfate (Cresemba) to treat adults with invasive aspergillosis and invasive mucormycosis, life-threatening fungal infections that predominantly occur in immunocompromised patients.
Isavuconazonium sulfate is an azole antifungal agent that works by targeting the cell wall of a fungus. The drug is available in oral and intravenous formulations.
“[The] approval provides a new treatment option for patients with serious fungal infections and underscores the importance of having available safe and effective antifungal drugs,” said Edward Cox, MD, director of the Office of Antimicrobial Products in the FDA’s Center for Drug Evaluation and Research.
Clinical trials
The FDA approved isavuconazonium sulfate to treat invasive aspergillosis based on results of the phase 3 SECURE trial. The study included 516 adults with invasive aspergillosis who were randomized to receive isavuconazonium sulfate or voriconazole.
Isavuconazonium sulfate demonstrated non-inferiority to voriconazole on the primary endpoint of all-cause mortality. All-cause mortality through day 42 was 18.6% in the isavuconazonium sulfate arm and 20.2% in the voriconazole arm.
In addition, isavuconazonium sulfate demonstrated similar rates of mortality and non-fatal adverse events as voriconazole
The FDA approved isavuconazonium sulfate to treat invasive mucormycosis based on results of the phase 3 VITAL trial. This single-arm study included 37 patients with invasive mucormycosis who received isavuconazonium sulfate.
All-cause mortality in these patients was 38%. The efficacy of isavuconazonium sulfate as a treatment for invasive mucormycosis has not been evaluated in concurrent, controlled clinical trials.
The most frequent adverse events for patients treated with isavuconazonium sulfate in clinical trials were nausea (26%), vomiting (25%), diarrhea (22%), headache (17%), elevated liver chemistry tests (17%), hypokalemia (14%), constipation (13%), dyspnea (12%), cough (12%), peripheral edema (11%), and back pain (10%).
QIDP status
Isavuconazonium sulfate is the sixth approved antifungal/antibacterial drug product designated as a qualified infectious disease product (QIDP). This designation is given to antibacterial or antifungal products that treat serious or life-threatening infections.
As part of its QIDP designation, isavuconazonium sulfate was given priority review. The QIDP designation also qualifies the drug for an additional 5 years of marketing exclusivity to be added to certain exclusivity periods already provided by the Food, Drug, and Cosmetic Act.
As invasive aspergillosis and mucormycosis are rare, the FDA also granted isavuconazonium sulfate orphan drug designations to treat these infections.
Isavuconazonium sulfate is marketed as Cresemba by Astellas Pharma US, Inc., which is based in Northbrook, Illinois. For more information on the drug, see the full prescribing information. ![]()
FDA’s new app provides info on drug shortages

a Nokia smart phone
Photo by Halvard Lundgaard
The US Food and Drug Administration (FDA) has launched the agency’s first mobile application (app) designed to speed public access to information on drug shortages.
The app provides details regarding current drug shortages, resolved shortages, and discontinued drug products.
It works just like the FDA’s drug shortages website. App users can search for a drug by its generic name or active ingredient, or they can browse by therapeutic category.
The app can also be used to report a suspected drug shortage or supply issue to the FDA.
The app is available for free download via iTunes (for Apple devices) and the Google Play store (for Android devices). It can be found by searching “FDA Drug Shortages.”
The FDA developed the app to improve access to information about drug shortages, as part of the agency’s efforts outlined in the Strategic Plan for Preventing and Mitigating Drug Shortages.
“The FDA understands that healthcare professionals and pharmacists need real-time information about drug shortages to make treatment decisions,” said Valerie Jensen, associate director of the Drug Shortage Staff in the FDA’s Center for Drug Evaluation and Research.
“The new mobile app is an innovative tool that will offer easier and faster access to important drug shortage information.” ![]()

a Nokia smart phone
Photo by Halvard Lundgaard
The US Food and Drug Administration (FDA) has launched the agency’s first mobile application (app) designed to speed public access to information on drug shortages.
The app provides details regarding current drug shortages, resolved shortages, and discontinued drug products.
It works just like the FDA’s drug shortages website. App users can search for a drug by its generic name or active ingredient, or they can browse by therapeutic category.
The app can also be used to report a suspected drug shortage or supply issue to the FDA.
The app is available for free download via iTunes (for Apple devices) and the Google Play store (for Android devices). It can be found by searching “FDA Drug Shortages.”
The FDA developed the app to improve access to information about drug shortages, as part of the agency’s efforts outlined in the Strategic Plan for Preventing and Mitigating Drug Shortages.
“The FDA understands that healthcare professionals and pharmacists need real-time information about drug shortages to make treatment decisions,” said Valerie Jensen, associate director of the Drug Shortage Staff in the FDA’s Center for Drug Evaluation and Research.
“The new mobile app is an innovative tool that will offer easier and faster access to important drug shortage information.” ![]()

a Nokia smart phone
Photo by Halvard Lundgaard
The US Food and Drug Administration (FDA) has launched the agency’s first mobile application (app) designed to speed public access to information on drug shortages.
The app provides details regarding current drug shortages, resolved shortages, and discontinued drug products.
It works just like the FDA’s drug shortages website. App users can search for a drug by its generic name or active ingredient, or they can browse by therapeutic category.
The app can also be used to report a suspected drug shortage or supply issue to the FDA.
The app is available for free download via iTunes (for Apple devices) and the Google Play store (for Android devices). It can be found by searching “FDA Drug Shortages.”
The FDA developed the app to improve access to information about drug shortages, as part of the agency’s efforts outlined in the Strategic Plan for Preventing and Mitigating Drug Shortages.
“The FDA understands that healthcare professionals and pharmacists need real-time information about drug shortages to make treatment decisions,” said Valerie Jensen, associate director of the Drug Shortage Staff in the FDA’s Center for Drug Evaluation and Research.
“The new mobile app is an innovative tool that will offer easier and faster access to important drug shortage information.” ![]()
Too many blood tests can lead to anemia, transfusions

Photo by Juan D. Alfonso
A single-center study has shown that laboratory testing among patients undergoing cardiac surgery can lead to excessive bloodletting.
This can increase the risk of hospital-acquired anemia and, therefore, the need for blood transfusions.
Among cardiac surgery patients, transfusions have been associated with an increased risk of infection, more time spent on a ventilator, and a higher likelihood of death, said Colleen G. Koch, MD, of the Cleveland Clinic in Ohio.
She and her colleagues conducted this research and published their findings in The Annals of Thoracic Surgery.
The researchers recorded every laboratory test performed on 1894 patients who underwent cardiac surgery at the Cleveland Clinic from January to June 2012.
The team evaluated the number and type of blood tests performed from the time patients met their surgeons until hospital discharge, tallying up the total amount of blood taken from each patient.
‘Astonishing’ amount of blood drawn
There were 221,498 laboratory tests performed during the study period, or an average of 115 tests per patient. The most common tests were blood gas analyses (n=88,068), coagulation tests (n=39,535), complete blood counts (n=30,421), and metabolic panels (n=29,374).
The cumulative median phlebotomy volume for the entire hospital stay was 454 mL per patient. Patients tended to have more blood drawn if they were in the intensive care unit as compared to other hospital floors, with median phlebotomy volumes of 332 mL and 118 mL, respectively.
“We were astonished by the amount of blood taken from our patients for laboratory testing,” Dr Koch said. “Total phlebotomy volumes approached 1 to 2 units of red blood cells, which is roughly equivalent to 1 to 2 cans of soda.”
More complex procedures were associated with higher overall phlebotomy volume. Patients undergoing combined coronary artery bypass grafting surgery (CABG) and valve procedures had the highest median cumulative phlebotomy volume. The median volume was 653 mL for CABG-valve procedures, 448 mL for CABG alone, and 338 mL for valve procedures alone.
Transfusion need
The researchers also found that an increase in cumulative phlebotomy volume was linked to an increased need for blood products. Similarly, the longer a patient was hospitalized, the more blood was taken, which increased the subsequent need for a transfusion.
Overall, 49% of patients received red blood cells (RBCs), 25% fresh-frozen plasma (FFP), 33% platelets, and 15% cryoprecipitate.
Patients in the lowest phlebotomy volume quartile (0%-25th%) were much less likely to receive transfusions than patients in the highest quartile (75th% to 100th%).
In the lowest quartile, 2% of patients received cryoprecipitate, 3% FFP, 7% platelets, and 12% RBCs. In the highest quartile, 31% of patients received cryoprecipitate, 54% FFP, 61% platelets, and 87% RBCs.
So to reduce the use of transfusions, we must curb the use of blood tests, Dr Koch said, noting that patients can help.
“Patients should feel empowered to ask their doctors whether a specific test is necessary—’What is the indication for the test?,’ ‘Will it change my care?,’ and ‘If so, do you need to do it every day?,’” Dr Koch said.
“They should inquire whether smaller-volume test tubes could be used for the tests that are deemed necessary. Every attempt should be made to conserve the patient’s own blood. Every drop of blood counts.”
In an invited commentary, Milo Engoren, MD, of the University of Michigan in Ann Arbor, emphasized the importance of reducing blood loss to decrease possible complications during surgery.
“We make efforts to minimize intraoperative blood loss,” he noted. “Now, we need to make similar efforts postoperatively. While some may argue that transfusion itself is not harmful, but only a marker of a sicker patient, most would agree that avoiding anemia and transfusion is the best course for patients.” ![]()

Photo by Juan D. Alfonso
A single-center study has shown that laboratory testing among patients undergoing cardiac surgery can lead to excessive bloodletting.
This can increase the risk of hospital-acquired anemia and, therefore, the need for blood transfusions.
Among cardiac surgery patients, transfusions have been associated with an increased risk of infection, more time spent on a ventilator, and a higher likelihood of death, said Colleen G. Koch, MD, of the Cleveland Clinic in Ohio.
She and her colleagues conducted this research and published their findings in The Annals of Thoracic Surgery.
The researchers recorded every laboratory test performed on 1894 patients who underwent cardiac surgery at the Cleveland Clinic from January to June 2012.
The team evaluated the number and type of blood tests performed from the time patients met their surgeons until hospital discharge, tallying up the total amount of blood taken from each patient.
‘Astonishing’ amount of blood drawn
There were 221,498 laboratory tests performed during the study period, or an average of 115 tests per patient. The most common tests were blood gas analyses (n=88,068), coagulation tests (n=39,535), complete blood counts (n=30,421), and metabolic panels (n=29,374).
The cumulative median phlebotomy volume for the entire hospital stay was 454 mL per patient. Patients tended to have more blood drawn if they were in the intensive care unit as compared to other hospital floors, with median phlebotomy volumes of 332 mL and 118 mL, respectively.
“We were astonished by the amount of blood taken from our patients for laboratory testing,” Dr Koch said. “Total phlebotomy volumes approached 1 to 2 units of red blood cells, which is roughly equivalent to 1 to 2 cans of soda.”
More complex procedures were associated with higher overall phlebotomy volume. Patients undergoing combined coronary artery bypass grafting surgery (CABG) and valve procedures had the highest median cumulative phlebotomy volume. The median volume was 653 mL for CABG-valve procedures, 448 mL for CABG alone, and 338 mL for valve procedures alone.
Transfusion need
The researchers also found that an increase in cumulative phlebotomy volume was linked to an increased need for blood products. Similarly, the longer a patient was hospitalized, the more blood was taken, which increased the subsequent need for a transfusion.
Overall, 49% of patients received red blood cells (RBCs), 25% fresh-frozen plasma (FFP), 33% platelets, and 15% cryoprecipitate.
Patients in the lowest phlebotomy volume quartile (0%-25th%) were much less likely to receive transfusions than patients in the highest quartile (75th% to 100th%).
In the lowest quartile, 2% of patients received cryoprecipitate, 3% FFP, 7% platelets, and 12% RBCs. In the highest quartile, 31% of patients received cryoprecipitate, 54% FFP, 61% platelets, and 87% RBCs.
So to reduce the use of transfusions, we must curb the use of blood tests, Dr Koch said, noting that patients can help.
“Patients should feel empowered to ask their doctors whether a specific test is necessary—’What is the indication for the test?,’ ‘Will it change my care?,’ and ‘If so, do you need to do it every day?,’” Dr Koch said.
“They should inquire whether smaller-volume test tubes could be used for the tests that are deemed necessary. Every attempt should be made to conserve the patient’s own blood. Every drop of blood counts.”
In an invited commentary, Milo Engoren, MD, of the University of Michigan in Ann Arbor, emphasized the importance of reducing blood loss to decrease possible complications during surgery.
“We make efforts to minimize intraoperative blood loss,” he noted. “Now, we need to make similar efforts postoperatively. While some may argue that transfusion itself is not harmful, but only a marker of a sicker patient, most would agree that avoiding anemia and transfusion is the best course for patients.” ![]()

Photo by Juan D. Alfonso
A single-center study has shown that laboratory testing among patients undergoing cardiac surgery can lead to excessive bloodletting.
This can increase the risk of hospital-acquired anemia and, therefore, the need for blood transfusions.
Among cardiac surgery patients, transfusions have been associated with an increased risk of infection, more time spent on a ventilator, and a higher likelihood of death, said Colleen G. Koch, MD, of the Cleveland Clinic in Ohio.
She and her colleagues conducted this research and published their findings in The Annals of Thoracic Surgery.
The researchers recorded every laboratory test performed on 1894 patients who underwent cardiac surgery at the Cleveland Clinic from January to June 2012.
The team evaluated the number and type of blood tests performed from the time patients met their surgeons until hospital discharge, tallying up the total amount of blood taken from each patient.
‘Astonishing’ amount of blood drawn
There were 221,498 laboratory tests performed during the study period, or an average of 115 tests per patient. The most common tests were blood gas analyses (n=88,068), coagulation tests (n=39,535), complete blood counts (n=30,421), and metabolic panels (n=29,374).
The cumulative median phlebotomy volume for the entire hospital stay was 454 mL per patient. Patients tended to have more blood drawn if they were in the intensive care unit as compared to other hospital floors, with median phlebotomy volumes of 332 mL and 118 mL, respectively.
“We were astonished by the amount of blood taken from our patients for laboratory testing,” Dr Koch said. “Total phlebotomy volumes approached 1 to 2 units of red blood cells, which is roughly equivalent to 1 to 2 cans of soda.”
More complex procedures were associated with higher overall phlebotomy volume. Patients undergoing combined coronary artery bypass grafting surgery (CABG) and valve procedures had the highest median cumulative phlebotomy volume. The median volume was 653 mL for CABG-valve procedures, 448 mL for CABG alone, and 338 mL for valve procedures alone.
Transfusion need
The researchers also found that an increase in cumulative phlebotomy volume was linked to an increased need for blood products. Similarly, the longer a patient was hospitalized, the more blood was taken, which increased the subsequent need for a transfusion.
Overall, 49% of patients received red blood cells (RBCs), 25% fresh-frozen plasma (FFP), 33% platelets, and 15% cryoprecipitate.
Patients in the lowest phlebotomy volume quartile (0%-25th%) were much less likely to receive transfusions than patients in the highest quartile (75th% to 100th%).
In the lowest quartile, 2% of patients received cryoprecipitate, 3% FFP, 7% platelets, and 12% RBCs. In the highest quartile, 31% of patients received cryoprecipitate, 54% FFP, 61% platelets, and 87% RBCs.
So to reduce the use of transfusions, we must curb the use of blood tests, Dr Koch said, noting that patients can help.
“Patients should feel empowered to ask their doctors whether a specific test is necessary—’What is the indication for the test?,’ ‘Will it change my care?,’ and ‘If so, do you need to do it every day?,’” Dr Koch said.
“They should inquire whether smaller-volume test tubes could be used for the tests that are deemed necessary. Every attempt should be made to conserve the patient’s own blood. Every drop of blood counts.”
In an invited commentary, Milo Engoren, MD, of the University of Michigan in Ann Arbor, emphasized the importance of reducing blood loss to decrease possible complications during surgery.
“We make efforts to minimize intraoperative blood loss,” he noted. “Now, we need to make similar efforts postoperatively. While some may argue that transfusion itself is not harmful, but only a marker of a sicker patient, most would agree that avoiding anemia and transfusion is the best course for patients.” ![]()
FDA approves first HDAC inhibitor for MM

The US Food and Drug Administration (FDA) has granted accelerated approval for panobinostat (Farydak) to treat patients with multiple myeloma (MM).
Panobinostat is the first histone deacetylase (HDAC) inhibitor approved to treat MM.
The drug can now be used in combination with bortezomib and dexamethasone to treat patients who have received at least 2 prior standard therapies, including bortezomib and an immunomodulatory agent (IMiD).
Panobinostat was approved with a boxed warning alerting patients and healthcare professionals that severe diarrhea and severe and fatal cardiac events, arrhythmias, and electrocardiogram changes have occurred in patients receiving the drug.
Panobinostat was approved with a Risk Evaluation and Mitigation Strategy as well, which consists of a communication plan to inform healthcare professionals of these risks and how to minimize them.
Data supporting approval
In November 2014, the FDA’s Oncologic Drugs Advisory Committee advised the agency that, based on the data reviewed, the benefits of panobinostat did not outweigh its risks for patients with relapsed MM.
After the meeting, Novartis, the company developing the HDAC inhibitor, submitted additional information supporting the use of panobinostat for a different indication: MM patients who have received at least 2 prior standard therapies, including bortezomib and an IMiD.
The FDA’s accelerated approval of panobinostat is based on that data—efficacy and safety results in a subgroup analysis of 193 patients enrolled in the phase 3 PANORAMA-1 trial. These patients had received prior treatment with both bortezomib and an IMiD.
In these patients, treatment with panobinostat, bortezomib, and dexamethasone resulted in superior progression-free survival, when compared to treatment with bortezomib, dexamethasone, and placebo—10.6 months and 5.8 months, respectively (hazard ratio=0.52).
The most common adverse events (incidence ≥ 20%) in clinical studies of panobinostat have been diarrhea, fatigue, nausea, peripheral edema, decreased appetite, pyrexia, and vomiting.
The most common non-hematologic laboratory abnormalities (incidence ≥ 40%) were hypophosphatemia, hypokalemia, hyponatremia, and increased creatinine. The most common hematologic laboratory abnormalities (incidence ≥ 60%) were thrombocytopenia, lymphopenia, leukopenia, neutropenia, and anemia.
Panobinostat can cause fatal and serious toxicities, including severe diarrhea and cardiac toxicities.
The most frequent (≥ 5%) treatment-emergent serious adverse events for patients treated with the HDAC inhibitor were pneumonia (18%), diarrhea (11%), thrombocytopenia (7%), fatigue (6%), and sepsis (6%). Additional serious adverse events included hemorrhage, myelosuppression, infections, hepatotoxicity, and embryo-fetal toxicity.
Panobinostat development
The FDA previously granted panobinostat priority review and orphan product designation. Priority review provides an expedited review of drugs that are intended to treat a serious disease or condition and may provide a significant improvement over available therapy. Orphan product designation is given to drugs intended to treat rare diseases.
Now, the FDA has granted panobinostat accelerated approval, which allows for conditional approval of a drug based on clinical data showing the drug has an effect on a surrogate endpoint reasonably likely to predict clinical benefit to patients.
Continued approval of panobinostat may be contingent upon verification of a clinical benefit in confirmatory trials conducted by Novartis. An improvement in overall survival or disease-related symptoms has not yet been established for the HDAC inhibitor.
For more details on panobinostat, see the full prescribing information. ![]()

The US Food and Drug Administration (FDA) has granted accelerated approval for panobinostat (Farydak) to treat patients with multiple myeloma (MM).
Panobinostat is the first histone deacetylase (HDAC) inhibitor approved to treat MM.
The drug can now be used in combination with bortezomib and dexamethasone to treat patients who have received at least 2 prior standard therapies, including bortezomib and an immunomodulatory agent (IMiD).
Panobinostat was approved with a boxed warning alerting patients and healthcare professionals that severe diarrhea and severe and fatal cardiac events, arrhythmias, and electrocardiogram changes have occurred in patients receiving the drug.
Panobinostat was approved with a Risk Evaluation and Mitigation Strategy as well, which consists of a communication plan to inform healthcare professionals of these risks and how to minimize them.
Data supporting approval
In November 2014, the FDA’s Oncologic Drugs Advisory Committee advised the agency that, based on the data reviewed, the benefits of panobinostat did not outweigh its risks for patients with relapsed MM.
After the meeting, Novartis, the company developing the HDAC inhibitor, submitted additional information supporting the use of panobinostat for a different indication: MM patients who have received at least 2 prior standard therapies, including bortezomib and an IMiD.
The FDA’s accelerated approval of panobinostat is based on that data—efficacy and safety results in a subgroup analysis of 193 patients enrolled in the phase 3 PANORAMA-1 trial. These patients had received prior treatment with both bortezomib and an IMiD.
In these patients, treatment with panobinostat, bortezomib, and dexamethasone resulted in superior progression-free survival, when compared to treatment with bortezomib, dexamethasone, and placebo—10.6 months and 5.8 months, respectively (hazard ratio=0.52).
The most common adverse events (incidence ≥ 20%) in clinical studies of panobinostat have been diarrhea, fatigue, nausea, peripheral edema, decreased appetite, pyrexia, and vomiting.
The most common non-hematologic laboratory abnormalities (incidence ≥ 40%) were hypophosphatemia, hypokalemia, hyponatremia, and increased creatinine. The most common hematologic laboratory abnormalities (incidence ≥ 60%) were thrombocytopenia, lymphopenia, leukopenia, neutropenia, and anemia.
Panobinostat can cause fatal and serious toxicities, including severe diarrhea and cardiac toxicities.
The most frequent (≥ 5%) treatment-emergent serious adverse events for patients treated with the HDAC inhibitor were pneumonia (18%), diarrhea (11%), thrombocytopenia (7%), fatigue (6%), and sepsis (6%). Additional serious adverse events included hemorrhage, myelosuppression, infections, hepatotoxicity, and embryo-fetal toxicity.
Panobinostat development
The FDA previously granted panobinostat priority review and orphan product designation. Priority review provides an expedited review of drugs that are intended to treat a serious disease or condition and may provide a significant improvement over available therapy. Orphan product designation is given to drugs intended to treat rare diseases.
Now, the FDA has granted panobinostat accelerated approval, which allows for conditional approval of a drug based on clinical data showing the drug has an effect on a surrogate endpoint reasonably likely to predict clinical benefit to patients.
Continued approval of panobinostat may be contingent upon verification of a clinical benefit in confirmatory trials conducted by Novartis. An improvement in overall survival or disease-related symptoms has not yet been established for the HDAC inhibitor.
For more details on panobinostat, see the full prescribing information. ![]()

The US Food and Drug Administration (FDA) has granted accelerated approval for panobinostat (Farydak) to treat patients with multiple myeloma (MM).
Panobinostat is the first histone deacetylase (HDAC) inhibitor approved to treat MM.
The drug can now be used in combination with bortezomib and dexamethasone to treat patients who have received at least 2 prior standard therapies, including bortezomib and an immunomodulatory agent (IMiD).
Panobinostat was approved with a boxed warning alerting patients and healthcare professionals that severe diarrhea and severe and fatal cardiac events, arrhythmias, and electrocardiogram changes have occurred in patients receiving the drug.
Panobinostat was approved with a Risk Evaluation and Mitigation Strategy as well, which consists of a communication plan to inform healthcare professionals of these risks and how to minimize them.
Data supporting approval
In November 2014, the FDA’s Oncologic Drugs Advisory Committee advised the agency that, based on the data reviewed, the benefits of panobinostat did not outweigh its risks for patients with relapsed MM.
After the meeting, Novartis, the company developing the HDAC inhibitor, submitted additional information supporting the use of panobinostat for a different indication: MM patients who have received at least 2 prior standard therapies, including bortezomib and an IMiD.
The FDA’s accelerated approval of panobinostat is based on that data—efficacy and safety results in a subgroup analysis of 193 patients enrolled in the phase 3 PANORAMA-1 trial. These patients had received prior treatment with both bortezomib and an IMiD.
In these patients, treatment with panobinostat, bortezomib, and dexamethasone resulted in superior progression-free survival, when compared to treatment with bortezomib, dexamethasone, and placebo—10.6 months and 5.8 months, respectively (hazard ratio=0.52).
The most common adverse events (incidence ≥ 20%) in clinical studies of panobinostat have been diarrhea, fatigue, nausea, peripheral edema, decreased appetite, pyrexia, and vomiting.
The most common non-hematologic laboratory abnormalities (incidence ≥ 40%) were hypophosphatemia, hypokalemia, hyponatremia, and increased creatinine. The most common hematologic laboratory abnormalities (incidence ≥ 60%) were thrombocytopenia, lymphopenia, leukopenia, neutropenia, and anemia.
Panobinostat can cause fatal and serious toxicities, including severe diarrhea and cardiac toxicities.
The most frequent (≥ 5%) treatment-emergent serious adverse events for patients treated with the HDAC inhibitor were pneumonia (18%), diarrhea (11%), thrombocytopenia (7%), fatigue (6%), and sepsis (6%). Additional serious adverse events included hemorrhage, myelosuppression, infections, hepatotoxicity, and embryo-fetal toxicity.
Panobinostat development
The FDA previously granted panobinostat priority review and orphan product designation. Priority review provides an expedited review of drugs that are intended to treat a serious disease or condition and may provide a significant improvement over available therapy. Orphan product designation is given to drugs intended to treat rare diseases.
Now, the FDA has granted panobinostat accelerated approval, which allows for conditional approval of a drug based on clinical data showing the drug has an effect on a surrogate endpoint reasonably likely to predict clinical benefit to patients.
Continued approval of panobinostat may be contingent upon verification of a clinical benefit in confirmatory trials conducted by Novartis. An improvement in overall survival or disease-related symptoms has not yet been established for the HDAC inhibitor.
For more details on panobinostat, see the full prescribing information. ![]()
EC expands indication for lenalidomide in MM

Photo courtesy of Celgene
The European Commission (EC) has expanded the marketing authorization for lenalidomide (Revlimid), just 2 days after the US Food and Drug Administration did the same.
Lenalidomide is now approved in the European Union (EU) to treat adults with previously untreated multiple myeloma (MM) who are not eligible for hematopoietic stem cell transplant. These patients can receive the drug continuously until
disease progression.
Lenalidomide was already approved in the EU for use in combination with dexamethasone to treat adults with MM who have received at least 1 prior therapy.
Lenalidomide is also approved in the EU to treat patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with 5q deletion when other therapeutic options are insufficient or inadequate.
“Having a new treatment option now available for patients newly diagnosed with multiple myeloma is a real step forward,” said Thierry Facon, MD, of CHRU Lille in France.
“Treating patients continuously until disease progression is supported by several clinical studies and will have an important impact on how we manage the disease over the long-term.”
The EC’s decision to extend the approved use of lenalidomide was based on the results of 2 studies: MM-015 and MM-020, also known as FIRST.
The FIRST trial
In the phase 3 FIRST trial, researchers enrolled 1623 patients who were newly diagnosed with MM and not eligible for transplant.
Patients were randomized to receive lenalidomide and dexamethasone (Rd) in 28-day cycles until disease progression (n=535), 18 cycles of lenalidomide and dexamethasone (Rd18) for 72 weeks (n=541), or melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
Response rates were significantly better with continuous Rd (75%) and Rd18 (73%) than with MPT (62%, P<0.001 for both comparisons). Complete response rates were 15%, 14%, and 9%, respectively.
The median progression-free survival was 25.5 months with continuous Rd, 20.7 months with Rd18, and 21.2 months with MPT.
This resulted in a 28% reduction in the risk of progression or death for patients treated with continuous Rd compared with those treated with MPT (hazard ratio[HR]=0.72, P<0.001) and a 30% reduction compared with Rd18 (HR=0.70, P<0.001).
The pre-planned interim analysis of overall survival showed a 22% reduction in the risk of death for continuous Rd vs MPT (HR=0.78, P=0.02), but the difference did not cross the pre-specified superiority boundary (P<0.0096).
Adverse events reported in 20% or more of patients in the continuous Rd, Rd18, or MPT arms included diarrhea (45.5%, 38.5%, 16.5%), anemia (43.8%, 35.7%, 42.3%), neutropenia (35.0%, 33.0%, 60.6%), fatigue (32.5%, 32.8%, 28.5%), back pain (32.0%, 26.9%, 21.4%), insomnia (27.6%, 23.5%, 9.8%), asthenia (28.2%, 22.8%, 22.9%), rash (26.1%, 28.0%, 19.4%), decreased appetite (23.1%, 21.3%, 13.3%), cough (22.7%, 17.4%, 12.6%), pyrexia (21.4%, 18.9%, 14.0%), muscle spasms (20.5%, 18.9%, 11.3%), and abdominal pain (20.5%, 14.4%, 11.1%).
The incidence of invasive second primary malignancies was 3% in patients taking continuous Rd, 6% in patients taking Rd18, and 5% in those taking MPT. The overall incidence of solid tumors was identical in the continuous Rd and MPT arms (3%) and 5% in the Rd18 arm.
The MM-015 trial
In the phase 3 MM-015 study, researchers enrolled 459 patients who were 65 or older and newly diagnosed with MM.
The team compared melphalan-prednisone-lenalidomide induction followed by lenalidomide maintenance (MPR-R) with melphalan-prednisone-lenalidomide (MPR) or melphalan-prednisone (MP) followed by placebo maintenance.
Patients who received MPR-R or MPR had significantly better response rates than patients who received MP, at 77%, 68%, and 50%, respectively (P<0.001 and P=0.002, respectively, for the comparison with MP).
And the median progression-free survival was significantly longer with MPR-R (31 months) than with MPR (14 months, HR=0.49, P<0.001) or MP (13 months, HR=0.40, P<0.001).
During induction, the most frequent adverse events were hematologic. Grade 4 neutropenia occurred in 35% of patients in the MPR-R arm, 32% in the MPR arm, and 8% in the MP arm. The 3-year rate of second primary malignancies was 7%, 7%, and 3%, respectively. ![]()

Photo courtesy of Celgene
The European Commission (EC) has expanded the marketing authorization for lenalidomide (Revlimid), just 2 days after the US Food and Drug Administration did the same.
Lenalidomide is now approved in the European Union (EU) to treat adults with previously untreated multiple myeloma (MM) who are not eligible for hematopoietic stem cell transplant. These patients can receive the drug continuously until
disease progression.
Lenalidomide was already approved in the EU for use in combination with dexamethasone to treat adults with MM who have received at least 1 prior therapy.
Lenalidomide is also approved in the EU to treat patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with 5q deletion when other therapeutic options are insufficient or inadequate.
“Having a new treatment option now available for patients newly diagnosed with multiple myeloma is a real step forward,” said Thierry Facon, MD, of CHRU Lille in France.
“Treating patients continuously until disease progression is supported by several clinical studies and will have an important impact on how we manage the disease over the long-term.”
The EC’s decision to extend the approved use of lenalidomide was based on the results of 2 studies: MM-015 and MM-020, also known as FIRST.
The FIRST trial
In the phase 3 FIRST trial, researchers enrolled 1623 patients who were newly diagnosed with MM and not eligible for transplant.
Patients were randomized to receive lenalidomide and dexamethasone (Rd) in 28-day cycles until disease progression (n=535), 18 cycles of lenalidomide and dexamethasone (Rd18) for 72 weeks (n=541), or melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
Response rates were significantly better with continuous Rd (75%) and Rd18 (73%) than with MPT (62%, P<0.001 for both comparisons). Complete response rates were 15%, 14%, and 9%, respectively.
The median progression-free survival was 25.5 months with continuous Rd, 20.7 months with Rd18, and 21.2 months with MPT.
This resulted in a 28% reduction in the risk of progression or death for patients treated with continuous Rd compared with those treated with MPT (hazard ratio[HR]=0.72, P<0.001) and a 30% reduction compared with Rd18 (HR=0.70, P<0.001).
The pre-planned interim analysis of overall survival showed a 22% reduction in the risk of death for continuous Rd vs MPT (HR=0.78, P=0.02), but the difference did not cross the pre-specified superiority boundary (P<0.0096).
Adverse events reported in 20% or more of patients in the continuous Rd, Rd18, or MPT arms included diarrhea (45.5%, 38.5%, 16.5%), anemia (43.8%, 35.7%, 42.3%), neutropenia (35.0%, 33.0%, 60.6%), fatigue (32.5%, 32.8%, 28.5%), back pain (32.0%, 26.9%, 21.4%), insomnia (27.6%, 23.5%, 9.8%), asthenia (28.2%, 22.8%, 22.9%), rash (26.1%, 28.0%, 19.4%), decreased appetite (23.1%, 21.3%, 13.3%), cough (22.7%, 17.4%, 12.6%), pyrexia (21.4%, 18.9%, 14.0%), muscle spasms (20.5%, 18.9%, 11.3%), and abdominal pain (20.5%, 14.4%, 11.1%).
The incidence of invasive second primary malignancies was 3% in patients taking continuous Rd, 6% in patients taking Rd18, and 5% in those taking MPT. The overall incidence of solid tumors was identical in the continuous Rd and MPT arms (3%) and 5% in the Rd18 arm.
The MM-015 trial
In the phase 3 MM-015 study, researchers enrolled 459 patients who were 65 or older and newly diagnosed with MM.
The team compared melphalan-prednisone-lenalidomide induction followed by lenalidomide maintenance (MPR-R) with melphalan-prednisone-lenalidomide (MPR) or melphalan-prednisone (MP) followed by placebo maintenance.
Patients who received MPR-R or MPR had significantly better response rates than patients who received MP, at 77%, 68%, and 50%, respectively (P<0.001 and P=0.002, respectively, for the comparison with MP).
And the median progression-free survival was significantly longer with MPR-R (31 months) than with MPR (14 months, HR=0.49, P<0.001) or MP (13 months, HR=0.40, P<0.001).
During induction, the most frequent adverse events were hematologic. Grade 4 neutropenia occurred in 35% of patients in the MPR-R arm, 32% in the MPR arm, and 8% in the MP arm. The 3-year rate of second primary malignancies was 7%, 7%, and 3%, respectively. ![]()

Photo courtesy of Celgene
The European Commission (EC) has expanded the marketing authorization for lenalidomide (Revlimid), just 2 days after the US Food and Drug Administration did the same.
Lenalidomide is now approved in the European Union (EU) to treat adults with previously untreated multiple myeloma (MM) who are not eligible for hematopoietic stem cell transplant. These patients can receive the drug continuously until
disease progression.
Lenalidomide was already approved in the EU for use in combination with dexamethasone to treat adults with MM who have received at least 1 prior therapy.
Lenalidomide is also approved in the EU to treat patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with 5q deletion when other therapeutic options are insufficient or inadequate.
“Having a new treatment option now available for patients newly diagnosed with multiple myeloma is a real step forward,” said Thierry Facon, MD, of CHRU Lille in France.
“Treating patients continuously until disease progression is supported by several clinical studies and will have an important impact on how we manage the disease over the long-term.”
The EC’s decision to extend the approved use of lenalidomide was based on the results of 2 studies: MM-015 and MM-020, also known as FIRST.
The FIRST trial
In the phase 3 FIRST trial, researchers enrolled 1623 patients who were newly diagnosed with MM and not eligible for transplant.
Patients were randomized to receive lenalidomide and dexamethasone (Rd) in 28-day cycles until disease progression (n=535), 18 cycles of lenalidomide and dexamethasone (Rd18) for 72 weeks (n=541), or melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
Response rates were significantly better with continuous Rd (75%) and Rd18 (73%) than with MPT (62%, P<0.001 for both comparisons). Complete response rates were 15%, 14%, and 9%, respectively.
The median progression-free survival was 25.5 months with continuous Rd, 20.7 months with Rd18, and 21.2 months with MPT.
This resulted in a 28% reduction in the risk of progression or death for patients treated with continuous Rd compared with those treated with MPT (hazard ratio[HR]=0.72, P<0.001) and a 30% reduction compared with Rd18 (HR=0.70, P<0.001).
The pre-planned interim analysis of overall survival showed a 22% reduction in the risk of death for continuous Rd vs MPT (HR=0.78, P=0.02), but the difference did not cross the pre-specified superiority boundary (P<0.0096).
Adverse events reported in 20% or more of patients in the continuous Rd, Rd18, or MPT arms included diarrhea (45.5%, 38.5%, 16.5%), anemia (43.8%, 35.7%, 42.3%), neutropenia (35.0%, 33.0%, 60.6%), fatigue (32.5%, 32.8%, 28.5%), back pain (32.0%, 26.9%, 21.4%), insomnia (27.6%, 23.5%, 9.8%), asthenia (28.2%, 22.8%, 22.9%), rash (26.1%, 28.0%, 19.4%), decreased appetite (23.1%, 21.3%, 13.3%), cough (22.7%, 17.4%, 12.6%), pyrexia (21.4%, 18.9%, 14.0%), muscle spasms (20.5%, 18.9%, 11.3%), and abdominal pain (20.5%, 14.4%, 11.1%).
The incidence of invasive second primary malignancies was 3% in patients taking continuous Rd, 6% in patients taking Rd18, and 5% in those taking MPT. The overall incidence of solid tumors was identical in the continuous Rd and MPT arms (3%) and 5% in the Rd18 arm.
The MM-015 trial
In the phase 3 MM-015 study, researchers enrolled 459 patients who were 65 or older and newly diagnosed with MM.
The team compared melphalan-prednisone-lenalidomide induction followed by lenalidomide maintenance (MPR-R) with melphalan-prednisone-lenalidomide (MPR) or melphalan-prednisone (MP) followed by placebo maintenance.
Patients who received MPR-R or MPR had significantly better response rates than patients who received MP, at 77%, 68%, and 50%, respectively (P<0.001 and P=0.002, respectively, for the comparison with MP).
And the median progression-free survival was significantly longer with MPR-R (31 months) than with MPR (14 months, HR=0.49, P<0.001) or MP (13 months, HR=0.40, P<0.001).
During induction, the most frequent adverse events were hematologic. Grade 4 neutropenia occurred in 35% of patients in the MPR-R arm, 32% in the MPR arm, and 8% in the MP arm. The 3-year rate of second primary malignancies was 7%, 7%, and 3%, respectively.
Generic enoxaparin launched in US

Image by Kevin MacKenzie
Teva Pharmaceutical Industries Ltd. has launched the generic equivalent of the low-molecular-weight heparin Lovenox (enoxaparin sodium injection) in 7 dosage strengths in the US.
Enoxaparin can be used to prevent deep vein thrombosis (DVT) in patients undergoing abdominal surgery, those receiving a hip or knee replacement, and patients at risk of thromboembolic complications due to severely restricted mobility during acute illness.
When administered with warfarin, enoxaparin can be used for inpatient treatment of acute DVT, with or without pulmonary embolism (PE). Enoxaparin given in conjunction with warfarin may also be used for outpatient treatment of acute DVT without PE.
When given concurrently with aspirin, enoxaparin can be used to prevent ischemic complications of unstable angina and non-Q-wave myocardial infarction. Enoxaparin may also be used to treat acute ST-segment elevation myocardial infarction that is managed medically or with subsequent percutaneous coronary intervention.
Teva’s Enoxaparin Sodium Injection USP is available in the following doses:
- 30 mg/0.3 mL syringe, 10 x 0.3 mL
- 40 mg/0.4 mL syringe, 10 x 0.4 mL
- 60 mg/0.6 mL syringe, 10 x 0.6 mL
- 80 mg/0.8 mL syringe, 10 x 0.8 mL
- 100 mg/mL syringe, 10 x 1 mL
- 120 mg/0.8 mL syringe, 10 x 0.8 mL
- 150 mg/mL syringe, 10 x 1 mL.
Safety information
Enoxaparin’s label contains a boxed warning detailing the risk of epidural or spinal hematomas that can occur in patients who are anticoagulated with low-molecular-weight heparins or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. The hematomas may result in long-term or permanent paralysis.
Enoxaparin is contraindicated in patients with active major bleeding, thrombocytopenia with a positive in vitro test for antiplatelet antibody in the presence of enoxaparin, or known hypersensitivity to enoxaparin, heparin, or pork products.
Serious adverse reactions reported with enoxaparin include increased risk of hemorrhage and thrombocytopenia.
Enoxaparin should be used with extreme caution in patients who have conditions with an increased risk of hemorrhage or in patients treated concomitantly with platelet inhibitors. Major hemorrhages, including retroperitoneal and intracranial bleeding, have been reported with enoxaparin. Some of these cases have been fatal.
Bleeding can occur at any site during enoxaparin treatment. The drug should be used with care in patients with a bleeding diathesis, uncontrolled arterial hypertension, or a history of recent gastrointestinal ulceration, diabetic retinopathy, renal dysfunction, and hemorrhage.
In clinical trials, the most common adverse reactions associated with enoxaparin (occurring in more than 1% of patients) were bleeding, anemia, thrombocytopenia, elevation of serum aminotransferase, diarrhea, and nausea. Mild local irritation, pain, hematoma, ecchymosis, and erythema may follow subcutaneous injection.
For additional information on enoxaparin, see the full prescribing information.

Image by Kevin MacKenzie
Teva Pharmaceutical Industries Ltd. has launched the generic equivalent of the low-molecular-weight heparin Lovenox (enoxaparin sodium injection) in 7 dosage strengths in the US.
Enoxaparin can be used to prevent deep vein thrombosis (DVT) in patients undergoing abdominal surgery, those receiving a hip or knee replacement, and patients at risk of thromboembolic complications due to severely restricted mobility during acute illness.
When administered with warfarin, enoxaparin can be used for inpatient treatment of acute DVT, with or without pulmonary embolism (PE). Enoxaparin given in conjunction with warfarin may also be used for outpatient treatment of acute DVT without PE.
When given concurrently with aspirin, enoxaparin can be used to prevent ischemic complications of unstable angina and non-Q-wave myocardial infarction. Enoxaparin may also be used to treat acute ST-segment elevation myocardial infarction that is managed medically or with subsequent percutaneous coronary intervention.
Teva’s Enoxaparin Sodium Injection USP is available in the following doses:
- 30 mg/0.3 mL syringe, 10 x 0.3 mL
- 40 mg/0.4 mL syringe, 10 x 0.4 mL
- 60 mg/0.6 mL syringe, 10 x 0.6 mL
- 80 mg/0.8 mL syringe, 10 x 0.8 mL
- 100 mg/mL syringe, 10 x 1 mL
- 120 mg/0.8 mL syringe, 10 x 0.8 mL
- 150 mg/mL syringe, 10 x 1 mL.
Safety information
Enoxaparin’s label contains a boxed warning detailing the risk of epidural or spinal hematomas that can occur in patients who are anticoagulated with low-molecular-weight heparins or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. The hematomas may result in long-term or permanent paralysis.
Enoxaparin is contraindicated in patients with active major bleeding, thrombocytopenia with a positive in vitro test for antiplatelet antibody in the presence of enoxaparin, or known hypersensitivity to enoxaparin, heparin, or pork products.
Serious adverse reactions reported with enoxaparin include increased risk of hemorrhage and thrombocytopenia.
Enoxaparin should be used with extreme caution in patients who have conditions with an increased risk of hemorrhage or in patients treated concomitantly with platelet inhibitors. Major hemorrhages, including retroperitoneal and intracranial bleeding, have been reported with enoxaparin. Some of these cases have been fatal.
Bleeding can occur at any site during enoxaparin treatment. The drug should be used with care in patients with a bleeding diathesis, uncontrolled arterial hypertension, or a history of recent gastrointestinal ulceration, diabetic retinopathy, renal dysfunction, and hemorrhage.
In clinical trials, the most common adverse reactions associated with enoxaparin (occurring in more than 1% of patients) were bleeding, anemia, thrombocytopenia, elevation of serum aminotransferase, diarrhea, and nausea. Mild local irritation, pain, hematoma, ecchymosis, and erythema may follow subcutaneous injection.
For additional information on enoxaparin, see the full prescribing information.

Image by Kevin MacKenzie
Teva Pharmaceutical Industries Ltd. has launched the generic equivalent of the low-molecular-weight heparin Lovenox (enoxaparin sodium injection) in 7 dosage strengths in the US.
Enoxaparin can be used to prevent deep vein thrombosis (DVT) in patients undergoing abdominal surgery, those receiving a hip or knee replacement, and patients at risk of thromboembolic complications due to severely restricted mobility during acute illness.
When administered with warfarin, enoxaparin can be used for inpatient treatment of acute DVT, with or without pulmonary embolism (PE). Enoxaparin given in conjunction with warfarin may also be used for outpatient treatment of acute DVT without PE.
When given concurrently with aspirin, enoxaparin can be used to prevent ischemic complications of unstable angina and non-Q-wave myocardial infarction. Enoxaparin may also be used to treat acute ST-segment elevation myocardial infarction that is managed medically or with subsequent percutaneous coronary intervention.
Teva’s Enoxaparin Sodium Injection USP is available in the following doses:
- 30 mg/0.3 mL syringe, 10 x 0.3 mL
- 40 mg/0.4 mL syringe, 10 x 0.4 mL
- 60 mg/0.6 mL syringe, 10 x 0.6 mL
- 80 mg/0.8 mL syringe, 10 x 0.8 mL
- 100 mg/mL syringe, 10 x 1 mL
- 120 mg/0.8 mL syringe, 10 x 0.8 mL
- 150 mg/mL syringe, 10 x 1 mL.
Safety information
Enoxaparin’s label contains a boxed warning detailing the risk of epidural or spinal hematomas that can occur in patients who are anticoagulated with low-molecular-weight heparins or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. The hematomas may result in long-term or permanent paralysis.
Enoxaparin is contraindicated in patients with active major bleeding, thrombocytopenia with a positive in vitro test for antiplatelet antibody in the presence of enoxaparin, or known hypersensitivity to enoxaparin, heparin, or pork products.
Serious adverse reactions reported with enoxaparin include increased risk of hemorrhage and thrombocytopenia.
Enoxaparin should be used with extreme caution in patients who have conditions with an increased risk of hemorrhage or in patients treated concomitantly with platelet inhibitors. Major hemorrhages, including retroperitoneal and intracranial bleeding, have been reported with enoxaparin. Some of these cases have been fatal.
Bleeding can occur at any site during enoxaparin treatment. The drug should be used with care in patients with a bleeding diathesis, uncontrolled arterial hypertension, or a history of recent gastrointestinal ulceration, diabetic retinopathy, renal dysfunction, and hemorrhage.
In clinical trials, the most common adverse reactions associated with enoxaparin (occurring in more than 1% of patients) were bleeding, anemia, thrombocytopenia, elevation of serum aminotransferase, diarrhea, and nausea. Mild local irritation, pain, hematoma, ecchymosis, and erythema may follow subcutaneous injection.
For additional information on enoxaparin, see the full prescribing information.
FDA approves drug for newly diagnosed MM
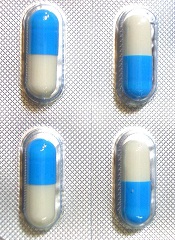
The US Food and Drug Administration (FDA) has expanded the existing indication for lenalidomide (Revlimid)—in combination with dexamethasone—to include patients with newly diagnosed multiple myeloma (MM).
The FDA previously approved lenalidomide in combination with dexamethasone to treat MM patients who had received at least 1 prior therapy.
Lenalidomide is also FDA-approved to treat mantle cell lymphoma patients who have failed 2 prior therapies, including bortezomib.
And the drug is approved to treat patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with 5q deletion, with or without additional cytogenetic abnormalities.
“The approval of Revlimid as an option for use in all patients with multiple myeloma represents a new paradigm in the management of this disease,” said Kenneth Anderson, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston, Massachusetts.
“We now have clinical evidence demonstrating that starting and keeping newly diagnosed multiple myeloma patients on Revlimid significantly improves progression-free survival.”
The FDA’s latest approval of lenalidomide was based on safety and efficacy results from phase 3 studies, particularly the FIRST trial.
The FIRST trial
In this phase 3 trial, researchers enrolled 1623 patients who were newly diagnosed with MM and not eligible for stem cell transplant.
Patients were randomized to receive lenalidomide and dexamethasone (Rd) in 28-day cycles until disease progression (n=535), 18 cycles of lenalidomide and dexamethasone (Rd18) for 72 weeks (n=541), or melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
Response rates were significantly better with continuous Rd (75%) and Rd18 (73%) than with MPT (62%, P<0.001 for both comparisons). Complete response rates were 15%, 14%, and 9%, respectively.
The median progression-free survival was 25.5 months with continuous Rd, 20.7 months with Rd18, and 21.2 months with MPT.
This resulted in a 28% reduction in the risk of progression or death for patients treated with continuous Rd compared with those treated with MPT (hazard ratio[HR]=0.72, P<0.001) and a 30% reduction compared with Rd18 (HR=0.70, P<0.001).
The pre-planned interim analysis of overall survival showed a 22% reduction in the risk of death for continuous Rd vs MPT (HR=0.78, P=0.02), but the difference did not cross the pre-specified superiority boundary (P<0.0096).
Adverse events reported in 20% or more of patients in the continuous Rd, Rd18, or MPT arms included diarrhea (45.5%, 38.5%, 16.5%), anemia (43.8%, 35.7%, 42.3%), neutropenia (35.0%, 33.0%, 60.6%), fatigue (32.5%, 32.8%, 28.5%), back pain (32.0%, 26.9%, 21.4%), insomnia (27.6%, 23.5%, 9.8%), asthenia (28.2%, 22.8%, 22.9%), rash (26.1%, 28.0%, 19.4%), decreased appetite (23.1%, 21.3%, 13.3%), cough (22.7%, 17.4%, 12.6%), pyrexia (21.4%, 18.9%, 14.0%), muscle spasms (20.5%, 18.9%, 11.3%) and abdominal pain (20.5%, 14.4%, 11.1%).
The most frequently reported grade 3/4 events in the continuous Rd arm (until disease progression) were neutropenia (27.8%), anemia (18.2%), thrombocytopenia (8.3%), pneumonia (11.3%), asthenia (7.7%), fatigue (7.3%), back pain (7%), hypokalemia (6.6%), rash (7.3%), cataract (5.8%), dyspnea (5.6%), deep vein thrombosis (5.6%), and hyperglycemia (5.3%).
The incidence of invasive second primary malignancies was 3% in the continuous Rd arm, 6% in the Rd18 arm, and 5% in the MPT arm. The overall incidence of solid tumors was identical in the continuous Rd and MPT arms (3%) and 5% in the Rd18 arm.

The US Food and Drug Administration (FDA) has expanded the existing indication for lenalidomide (Revlimid)—in combination with dexamethasone—to include patients with newly diagnosed multiple myeloma (MM).
The FDA previously approved lenalidomide in combination with dexamethasone to treat MM patients who had received at least 1 prior therapy.
Lenalidomide is also FDA-approved to treat mantle cell lymphoma patients who have failed 2 prior therapies, including bortezomib.
And the drug is approved to treat patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with 5q deletion, with or without additional cytogenetic abnormalities.
“The approval of Revlimid as an option for use in all patients with multiple myeloma represents a new paradigm in the management of this disease,” said Kenneth Anderson, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston, Massachusetts.
“We now have clinical evidence demonstrating that starting and keeping newly diagnosed multiple myeloma patients on Revlimid significantly improves progression-free survival.”
The FDA’s latest approval of lenalidomide was based on safety and efficacy results from phase 3 studies, particularly the FIRST trial.
The FIRST trial
In this phase 3 trial, researchers enrolled 1623 patients who were newly diagnosed with MM and not eligible for stem cell transplant.
Patients were randomized to receive lenalidomide and dexamethasone (Rd) in 28-day cycles until disease progression (n=535), 18 cycles of lenalidomide and dexamethasone (Rd18) for 72 weeks (n=541), or melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
Response rates were significantly better with continuous Rd (75%) and Rd18 (73%) than with MPT (62%, P<0.001 for both comparisons). Complete response rates were 15%, 14%, and 9%, respectively.
The median progression-free survival was 25.5 months with continuous Rd, 20.7 months with Rd18, and 21.2 months with MPT.
This resulted in a 28% reduction in the risk of progression or death for patients treated with continuous Rd compared with those treated with MPT (hazard ratio[HR]=0.72, P<0.001) and a 30% reduction compared with Rd18 (HR=0.70, P<0.001).
The pre-planned interim analysis of overall survival showed a 22% reduction in the risk of death for continuous Rd vs MPT (HR=0.78, P=0.02), but the difference did not cross the pre-specified superiority boundary (P<0.0096).
Adverse events reported in 20% or more of patients in the continuous Rd, Rd18, or MPT arms included diarrhea (45.5%, 38.5%, 16.5%), anemia (43.8%, 35.7%, 42.3%), neutropenia (35.0%, 33.0%, 60.6%), fatigue (32.5%, 32.8%, 28.5%), back pain (32.0%, 26.9%, 21.4%), insomnia (27.6%, 23.5%, 9.8%), asthenia (28.2%, 22.8%, 22.9%), rash (26.1%, 28.0%, 19.4%), decreased appetite (23.1%, 21.3%, 13.3%), cough (22.7%, 17.4%, 12.6%), pyrexia (21.4%, 18.9%, 14.0%), muscle spasms (20.5%, 18.9%, 11.3%) and abdominal pain (20.5%, 14.4%, 11.1%).
The most frequently reported grade 3/4 events in the continuous Rd arm (until disease progression) were neutropenia (27.8%), anemia (18.2%), thrombocytopenia (8.3%), pneumonia (11.3%), asthenia (7.7%), fatigue (7.3%), back pain (7%), hypokalemia (6.6%), rash (7.3%), cataract (5.8%), dyspnea (5.6%), deep vein thrombosis (5.6%), and hyperglycemia (5.3%).
The incidence of invasive second primary malignancies was 3% in the continuous Rd arm, 6% in the Rd18 arm, and 5% in the MPT arm. The overall incidence of solid tumors was identical in the continuous Rd and MPT arms (3%) and 5% in the Rd18 arm.

The US Food and Drug Administration (FDA) has expanded the existing indication for lenalidomide (Revlimid)—in combination with dexamethasone—to include patients with newly diagnosed multiple myeloma (MM).
The FDA previously approved lenalidomide in combination with dexamethasone to treat MM patients who had received at least 1 prior therapy.
Lenalidomide is also FDA-approved to treat mantle cell lymphoma patients who have failed 2 prior therapies, including bortezomib.
And the drug is approved to treat patients with transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with 5q deletion, with or without additional cytogenetic abnormalities.
“The approval of Revlimid as an option for use in all patients with multiple myeloma represents a new paradigm in the management of this disease,” said Kenneth Anderson, MD, of Dana-Farber/Brigham and Women’s Cancer Center in Boston, Massachusetts.
“We now have clinical evidence demonstrating that starting and keeping newly diagnosed multiple myeloma patients on Revlimid significantly improves progression-free survival.”
The FDA’s latest approval of lenalidomide was based on safety and efficacy results from phase 3 studies, particularly the FIRST trial.
The FIRST trial
In this phase 3 trial, researchers enrolled 1623 patients who were newly diagnosed with MM and not eligible for stem cell transplant.
Patients were randomized to receive lenalidomide and dexamethasone (Rd) in 28-day cycles until disease progression (n=535), 18 cycles of lenalidomide and dexamethasone (Rd18) for 72 weeks (n=541), or melphalan, prednisone, and thalidomide (MPT) for 72 weeks (n=547).
Response rates were significantly better with continuous Rd (75%) and Rd18 (73%) than with MPT (62%, P<0.001 for both comparisons). Complete response rates were 15%, 14%, and 9%, respectively.
The median progression-free survival was 25.5 months with continuous Rd, 20.7 months with Rd18, and 21.2 months with MPT.
This resulted in a 28% reduction in the risk of progression or death for patients treated with continuous Rd compared with those treated with MPT (hazard ratio[HR]=0.72, P<0.001) and a 30% reduction compared with Rd18 (HR=0.70, P<0.001).
The pre-planned interim analysis of overall survival showed a 22% reduction in the risk of death for continuous Rd vs MPT (HR=0.78, P=0.02), but the difference did not cross the pre-specified superiority boundary (P<0.0096).
Adverse events reported in 20% or more of patients in the continuous Rd, Rd18, or MPT arms included diarrhea (45.5%, 38.5%, 16.5%), anemia (43.8%, 35.7%, 42.3%), neutropenia (35.0%, 33.0%, 60.6%), fatigue (32.5%, 32.8%, 28.5%), back pain (32.0%, 26.9%, 21.4%), insomnia (27.6%, 23.5%, 9.8%), asthenia (28.2%, 22.8%, 22.9%), rash (26.1%, 28.0%, 19.4%), decreased appetite (23.1%, 21.3%, 13.3%), cough (22.7%, 17.4%, 12.6%), pyrexia (21.4%, 18.9%, 14.0%), muscle spasms (20.5%, 18.9%, 11.3%) and abdominal pain (20.5%, 14.4%, 11.1%).
The most frequently reported grade 3/4 events in the continuous Rd arm (until disease progression) were neutropenia (27.8%), anemia (18.2%), thrombocytopenia (8.3%), pneumonia (11.3%), asthenia (7.7%), fatigue (7.3%), back pain (7%), hypokalemia (6.6%), rash (7.3%), cataract (5.8%), dyspnea (5.6%), deep vein thrombosis (5.6%), and hyperglycemia (5.3%).
The incidence of invasive second primary malignancies was 3% in the continuous Rd arm, 6% in the Rd18 arm, and 5% in the MPT arm. The overall incidence of solid tumors was identical in the continuous Rd and MPT arms (3%) and 5% in the Rd18 arm.
FDA issues documents on drug compounding

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has issued 5 draft documents related to drug compounding and repackaging that aim to help entities comply with public health provisions.
The agency said these draft documents are applicable to pharmacies, federal facilities, outsourcing facilities, and physicians.
The new category of outsourcing facilities was created under the Drug Quality and Security Act (DQSA), which was enacted by Congress in November 2013.
It was enacted in response to a deadly fungal meningitis outbreak that was linked to contaminated sterile compounded drug products.
Drugs compounded in an outsourcing facility that meet certain conditions may be entitled to exemptions from certain provisions of the Federal Food, Drug, and Cosmetic Act (FD&C Act), including the new drug approval requirements and the requirement to label drug products with adequate directions for use.
Outsourcing facilities are subject to current good manufacturing practice requirements and inspections by the FDA according to a risk-based schedule.
Drugs produced by compounders that are not registered as outsourcing facilities must meet certain other conditions described in the FD&C Act, or they will be subject to all of the requirements applicable to drugs produced by conventional drug manufacturers.
“The draft guidance documents provide information to pharmacies, outsourcing facilities, healthcare entities, and others about these FDA-proposed policies, which are critical to protecting the public health,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research.
Descriptions of these documents follow.
This draft guidance provides an entity considering whether to register with the FDA as an outsourcing facility with information about the regulatory impact of registering.
For example, it explains that a facility engaged in only certain activities, including repackaging human drugs and compounding non-sterile drugs, should not register as an outsourcing facility because its drug products will not qualify for the exemptions provided in section 503B, including the exemption from the new drug approval requirements.
This draft guidance describes the conditions under which the FDA does not intend to take action for certain violations of the law when state-licensed pharmacies, federal facilities, or outsourcing facilities repackage certain drug products.
Repackaged drug products are generally not exempt from any of the provisions of the FD&C Act related to the production of drugs, and the compounding provisions of the FD&C Act do not address repackaging. Therefore, the FDA is issuing guidance to describe how it intends to address repackaging when done in a state-licensed pharmacy, federal facility, or outsourcing facility.
This draft guidance describes the conditions under which the FDA does not intend to take action for violations of certain sections of the Public Health Service Act (PHS Act) and the FD&C Act when state-licensed pharmacies, federal facilities, or outsourcing facilities mix, dilute, or repackage specific biological products without an approved BLA, or when such facilities or physicians prepare prescription sets of allergenic extracts without an approved BLA.
The draft guidance notes that a biological product that is mixed, diluted, or repackaged outside the scope of an approved BLA is an unlicensed biological product under section 351 of the PHS Act and may not be legally marketed without an approved BLA.
Additionally, the compounding provisions of the FD&C Act do not address biological products subject to licensure under section 351 of the PHS Act. Therefore, the FDA is issuing the guidance to describe how it intends to address these practices.
Entities registered as outsourcing facilities are required to report adverse events to the FDA. This draft guidance explains adverse event reporting for such facilities.
The draft memorandum of understanding (MOU) under section 503A of the FD&C Act describes the responsibilities of a state that chooses to sign the MOU in investigating and responding to complaints related to compounded human drug products distributed outside the state, and in addressing the interstate distribution of “inordinate amounts” of compounded human drug products.
These documents are the latest in a series of policy documents related to FDA oversight of drugs produced by state-licensed pharmacies, federal facilities, and outsourcing facilities.
The draft guidance documents are available for public comment for 90 days. The public has 120 days to comment on the draft MOU between the states and the FDA.

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has issued 5 draft documents related to drug compounding and repackaging that aim to help entities comply with public health provisions.
The agency said these draft documents are applicable to pharmacies, federal facilities, outsourcing facilities, and physicians.
The new category of outsourcing facilities was created under the Drug Quality and Security Act (DQSA), which was enacted by Congress in November 2013.
It was enacted in response to a deadly fungal meningitis outbreak that was linked to contaminated sterile compounded drug products.
Drugs compounded in an outsourcing facility that meet certain conditions may be entitled to exemptions from certain provisions of the Federal Food, Drug, and Cosmetic Act (FD&C Act), including the new drug approval requirements and the requirement to label drug products with adequate directions for use.
Outsourcing facilities are subject to current good manufacturing practice requirements and inspections by the FDA according to a risk-based schedule.
Drugs produced by compounders that are not registered as outsourcing facilities must meet certain other conditions described in the FD&C Act, or they will be subject to all of the requirements applicable to drugs produced by conventional drug manufacturers.
“The draft guidance documents provide information to pharmacies, outsourcing facilities, healthcare entities, and others about these FDA-proposed policies, which are critical to protecting the public health,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research.
Descriptions of these documents follow.
This draft guidance provides an entity considering whether to register with the FDA as an outsourcing facility with information about the regulatory impact of registering.
For example, it explains that a facility engaged in only certain activities, including repackaging human drugs and compounding non-sterile drugs, should not register as an outsourcing facility because its drug products will not qualify for the exemptions provided in section 503B, including the exemption from the new drug approval requirements.
This draft guidance describes the conditions under which the FDA does not intend to take action for certain violations of the law when state-licensed pharmacies, federal facilities, or outsourcing facilities repackage certain drug products.
Repackaged drug products are generally not exempt from any of the provisions of the FD&C Act related to the production of drugs, and the compounding provisions of the FD&C Act do not address repackaging. Therefore, the FDA is issuing guidance to describe how it intends to address repackaging when done in a state-licensed pharmacy, federal facility, or outsourcing facility.
This draft guidance describes the conditions under which the FDA does not intend to take action for violations of certain sections of the Public Health Service Act (PHS Act) and the FD&C Act when state-licensed pharmacies, federal facilities, or outsourcing facilities mix, dilute, or repackage specific biological products without an approved BLA, or when such facilities or physicians prepare prescription sets of allergenic extracts without an approved BLA.
The draft guidance notes that a biological product that is mixed, diluted, or repackaged outside the scope of an approved BLA is an unlicensed biological product under section 351 of the PHS Act and may not be legally marketed without an approved BLA.
Additionally, the compounding provisions of the FD&C Act do not address biological products subject to licensure under section 351 of the PHS Act. Therefore, the FDA is issuing the guidance to describe how it intends to address these practices.
Entities registered as outsourcing facilities are required to report adverse events to the FDA. This draft guidance explains adverse event reporting for such facilities.
The draft memorandum of understanding (MOU) under section 503A of the FD&C Act describes the responsibilities of a state that chooses to sign the MOU in investigating and responding to complaints related to compounded human drug products distributed outside the state, and in addressing the interstate distribution of “inordinate amounts” of compounded human drug products.
These documents are the latest in a series of policy documents related to FDA oversight of drugs produced by state-licensed pharmacies, federal facilities, and outsourcing facilities.
The draft guidance documents are available for public comment for 90 days. The public has 120 days to comment on the draft MOU between the states and the FDA.

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has issued 5 draft documents related to drug compounding and repackaging that aim to help entities comply with public health provisions.
The agency said these draft documents are applicable to pharmacies, federal facilities, outsourcing facilities, and physicians.
The new category of outsourcing facilities was created under the Drug Quality and Security Act (DQSA), which was enacted by Congress in November 2013.
It was enacted in response to a deadly fungal meningitis outbreak that was linked to contaminated sterile compounded drug products.
Drugs compounded in an outsourcing facility that meet certain conditions may be entitled to exemptions from certain provisions of the Federal Food, Drug, and Cosmetic Act (FD&C Act), including the new drug approval requirements and the requirement to label drug products with adequate directions for use.
Outsourcing facilities are subject to current good manufacturing practice requirements and inspections by the FDA according to a risk-based schedule.
Drugs produced by compounders that are not registered as outsourcing facilities must meet certain other conditions described in the FD&C Act, or they will be subject to all of the requirements applicable to drugs produced by conventional drug manufacturers.
“The draft guidance documents provide information to pharmacies, outsourcing facilities, healthcare entities, and others about these FDA-proposed policies, which are critical to protecting the public health,” said Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research.
Descriptions of these documents follow.
This draft guidance provides an entity considering whether to register with the FDA as an outsourcing facility with information about the regulatory impact of registering.
For example, it explains that a facility engaged in only certain activities, including repackaging human drugs and compounding non-sterile drugs, should not register as an outsourcing facility because its drug products will not qualify for the exemptions provided in section 503B, including the exemption from the new drug approval requirements.
This draft guidance describes the conditions under which the FDA does not intend to take action for certain violations of the law when state-licensed pharmacies, federal facilities, or outsourcing facilities repackage certain drug products.
Repackaged drug products are generally not exempt from any of the provisions of the FD&C Act related to the production of drugs, and the compounding provisions of the FD&C Act do not address repackaging. Therefore, the FDA is issuing guidance to describe how it intends to address repackaging when done in a state-licensed pharmacy, federal facility, or outsourcing facility.
This draft guidance describes the conditions under which the FDA does not intend to take action for violations of certain sections of the Public Health Service Act (PHS Act) and the FD&C Act when state-licensed pharmacies, federal facilities, or outsourcing facilities mix, dilute, or repackage specific biological products without an approved BLA, or when such facilities or physicians prepare prescription sets of allergenic extracts without an approved BLA.
The draft guidance notes that a biological product that is mixed, diluted, or repackaged outside the scope of an approved BLA is an unlicensed biological product under section 351 of the PHS Act and may not be legally marketed without an approved BLA.
Additionally, the compounding provisions of the FD&C Act do not address biological products subject to licensure under section 351 of the PHS Act. Therefore, the FDA is issuing the guidance to describe how it intends to address these practices.
Entities registered as outsourcing facilities are required to report adverse events to the FDA. This draft guidance explains adverse event reporting for such facilities.
The draft memorandum of understanding (MOU) under section 503A of the FD&C Act describes the responsibilities of a state that chooses to sign the MOU in investigating and responding to complaints related to compounded human drug products distributed outside the state, and in addressing the interstate distribution of “inordinate amounts” of compounded human drug products.
These documents are the latest in a series of policy documents related to FDA oversight of drugs produced by state-licensed pharmacies, federal facilities, and outsourcing facilities.
The draft guidance documents are available for public comment for 90 days. The public has 120 days to comment on the draft MOU between the states and the FDA.