User login
How is gene study adding to the overall knowledge of preterm birth?
The 2018 meeting of the American Gynecological and Obstetrical Society, held in Philadelphia, Pennsylvania, September 6 to 8, featured a talk by Louis J. Muglia, MD, PhD, on “Evolution, Genetics, and Preterm Birth.” Dr. Muglia, who is Co-Director of the Perinatal Institute, Director of the Center for Prevention of Preterm Birth, and Director of the Division of Human Genetics at the Cincinnati Children’s Hospital Medical Center, discussed his recent research on genetic associations of gestational duration and spontaneous preterm birth and some of his key findings. OBG
OBG Management : You discussed the “genetic architecture of human pregnancy.” Can you define what that is?
The genetic architecture tells us which pathways are activated that initiate birth to occur. By understanding that, we can begin to understand not only the genetic factors that the architecture describes, but also that the genetic architecture is going to be modified in response to environmental stimuli that will disrupt the outcomes. In the future, we will be able to develop biomarkers, predictive genetic algorithms, and other tools that will allow us to assess risk in a way that we can’t right now.
OBG Management : How is gene study adding to the overall knowledge of preterm birth?
Gene study is giving us new pathways to look at. It will give us biomarkers; it will give us targets for potential therapeutic interventions. I mentioned in my talk that one of the genes that we identified in our recent New England Journal of Medicine (NEJM) study pinpointed selenium as an important component and a whole process of determining risk for preterm birth that we never even thought of before. For instance, could there be preventive strategies for prematurity, like we have for neural tube defects and folic acid? The possibility of supplementation with selenium, or other micronutrients that some of the genetic studies will reveal to us in a nonbiased fashion, would not be discovered without the study of genes.
OBG Management : You mentioned your NEJM paper. Can you describe the large data sets that your team used in your gene research?
The discovery cohort, which refers to essentially the biggest compilation, was a wonderful collaboration that we had with the direct-to-consumer genotyping company 23andme. I had contacted them to determine if they had captured any pregnancy-related data, particularly birth-timing information related to individuals who had completed their research surveys. They indicated that they had asked the question, “What was the length of your first pregnancy?” With this information, we were able to get essentially 44,000 responses to that question. That really provided the foundation for the study in the NEJM.
Now, there are caveats with that information, since it was all recollection and self-reported data. We were unsure how accurate it would be. In addition, we did not always know why the delivery occurred—was it spontaneous or was it medically indicated? We were interested in the spontaneous, naturally-occurring preterm birth. Using that as a discovery cohort, with those reservations in mind, we identified 6 genes for birth length. We then asked in a carefully collected series of cohorts that we had amassed on our own, and with collaborators over the years from Finland, Norway, and Denmark, whether those same associations still existed. And every one of them did. Every one of them was validated in our carefully phenotyped cohort. In total, that was about 55,000 women that we had analyzed and studied between the discovery and the validation cohort. Since then, we have accessed another 3 or 4 cohorts, which has increased our sample size even more, so we have identified even more genes than we originally reported in our paper.
OBG Management : What do you identify as the next steps in your research after identifying several genes associated with the timing of birth?
The idea is not just to develop longer and longer lists of genes that are suggested or associated with birth timing phenotypes that we are interested in—either preterm birth or duration of gestation—but to actually understand what they are doing. That is a little bit trickier than saying we have identified genes. We have identified the precise region of mom’s genome that is involved in regulating birth timing, but in many cases I have indicated the closest gene that is involved in birth timing. For some of the regions, however, there are many genes involved, and so is it regulating one pathway, is it regulating many? Which tissue is involved in regulating? Is it in the uterus, in the cervix, or in the immune system? The next steps are to figure out how these things are acting so that we can design better strategies for prevention. The goal is to really bring down preterm birth rates by implementing strategies for prevention and treatment that we don’t currently have.
OBG Management : What is the significance of maternal selenium status and preterm birth risk?
Well, we really don’t know. We identified one of these gene regions, a variant near a factory involved in production of what are called selenoproteins—proteins that incorporate selenium into them. (There are about 25 of those in the human genome.) We identified a genetic risk factor in a region that is linked to the selenoprotein production chain. What we were brought to think about was this: In parts of the world where we know there is substantial selenium deficiency (parts of sub-Saharan Africa, parts of China, parts of Asia), could selenium deficiency itself be contributing to very high rates of preterm birth? Right now we are trying to figure out if there is an association by measuring maternal plasma selenium levels about halfway through pregnancy and then asking what was the outcome from the pregnancy. Are women with low levels of selenium at increased risk for preterm birth? There have been 2 studies published that do already suggest that women with lower selenium levels tend to give birth to premature babies often.
OBG Management : What is the HSPA1L pathway and why is it important for pregnancy outcomes?
In our study where we performed genome-wide association, we looked at what are called common variants in the human genome—common variants in general are carried by more people. They had to be carried by a couple percent of the population to be included in our study. But there is also the thought that individual, more severe variants (that do not necessarily get transmitted because of how severe their effects are), will also affect birth outcomes. So we did a study to sequence mom’s genome to look for these rarities, things that account for less than 1% of the whole population. We were able to identify this gene, HSPA1L, which again, as found in our genome-wide studies, seems to be involved in controlling the strength of the steroid hormone signal, which is very important for maintaining and ending pregnancy. Progesterone and estrogen are the yin and yang of maintaining and ending pregnancy, and we think HSPA1L, the variant we identified, decreases the steroid hormone signal function so that it is not able to regulate that progesterone/estrogen signal the same way anymore.
OBG Management : Why is this an exciting time to be studying genes in pregnancy?
To understand how gene study can optimize our knowledge of human pregnancy outcomes really requires a study of human pregnancy specifically, and one of the best opportunities we have is to gather these large data sets. And we can’t forget about collecting pregnancy outcomes on women as part of new National Institutes of Health initiatives that are developing personalized medicine strategies. We looked at 50,000 women in our research, but we have the capacity to look at 500,000 women. As we go from identifying 6 genes to 12 genes to 100 genes, we will be able to understand better how these things are talking to one another and better define the signatures of what tissues are being acted on. We will be able to get sequentially synergistic information that will allow us to solve this in a way we couldn’t before.
The 2018 meeting of the American Gynecological and Obstetrical Society, held in Philadelphia, Pennsylvania, September 6 to 8, featured a talk by Louis J. Muglia, MD, PhD, on “Evolution, Genetics, and Preterm Birth.” Dr. Muglia, who is Co-Director of the Perinatal Institute, Director of the Center for Prevention of Preterm Birth, and Director of the Division of Human Genetics at the Cincinnati Children’s Hospital Medical Center, discussed his recent research on genetic associations of gestational duration and spontaneous preterm birth and some of his key findings. OBG
OBG Management : You discussed the “genetic architecture of human pregnancy.” Can you define what that is?
The genetic architecture tells us which pathways are activated that initiate birth to occur. By understanding that, we can begin to understand not only the genetic factors that the architecture describes, but also that the genetic architecture is going to be modified in response to environmental stimuli that will disrupt the outcomes. In the future, we will be able to develop biomarkers, predictive genetic algorithms, and other tools that will allow us to assess risk in a way that we can’t right now.
OBG Management : How is gene study adding to the overall knowledge of preterm birth?
Gene study is giving us new pathways to look at. It will give us biomarkers; it will give us targets for potential therapeutic interventions. I mentioned in my talk that one of the genes that we identified in our recent New England Journal of Medicine (NEJM) study pinpointed selenium as an important component and a whole process of determining risk for preterm birth that we never even thought of before. For instance, could there be preventive strategies for prematurity, like we have for neural tube defects and folic acid? The possibility of supplementation with selenium, or other micronutrients that some of the genetic studies will reveal to us in a nonbiased fashion, would not be discovered without the study of genes.
OBG Management : You mentioned your NEJM paper. Can you describe the large data sets that your team used in your gene research?
The discovery cohort, which refers to essentially the biggest compilation, was a wonderful collaboration that we had with the direct-to-consumer genotyping company 23andme. I had contacted them to determine if they had captured any pregnancy-related data, particularly birth-timing information related to individuals who had completed their research surveys. They indicated that they had asked the question, “What was the length of your first pregnancy?” With this information, we were able to get essentially 44,000 responses to that question. That really provided the foundation for the study in the NEJM.
Now, there are caveats with that information, since it was all recollection and self-reported data. We were unsure how accurate it would be. In addition, we did not always know why the delivery occurred—was it spontaneous or was it medically indicated? We were interested in the spontaneous, naturally-occurring preterm birth. Using that as a discovery cohort, with those reservations in mind, we identified 6 genes for birth length. We then asked in a carefully collected series of cohorts that we had amassed on our own, and with collaborators over the years from Finland, Norway, and Denmark, whether those same associations still existed. And every one of them did. Every one of them was validated in our carefully phenotyped cohort. In total, that was about 55,000 women that we had analyzed and studied between the discovery and the validation cohort. Since then, we have accessed another 3 or 4 cohorts, which has increased our sample size even more, so we have identified even more genes than we originally reported in our paper.
OBG Management : What do you identify as the next steps in your research after identifying several genes associated with the timing of birth?
The idea is not just to develop longer and longer lists of genes that are suggested or associated with birth timing phenotypes that we are interested in—either preterm birth or duration of gestation—but to actually understand what they are doing. That is a little bit trickier than saying we have identified genes. We have identified the precise region of mom’s genome that is involved in regulating birth timing, but in many cases I have indicated the closest gene that is involved in birth timing. For some of the regions, however, there are many genes involved, and so is it regulating one pathway, is it regulating many? Which tissue is involved in regulating? Is it in the uterus, in the cervix, or in the immune system? The next steps are to figure out how these things are acting so that we can design better strategies for prevention. The goal is to really bring down preterm birth rates by implementing strategies for prevention and treatment that we don’t currently have.
OBG Management : What is the significance of maternal selenium status and preterm birth risk?
Well, we really don’t know. We identified one of these gene regions, a variant near a factory involved in production of what are called selenoproteins—proteins that incorporate selenium into them. (There are about 25 of those in the human genome.) We identified a genetic risk factor in a region that is linked to the selenoprotein production chain. What we were brought to think about was this: In parts of the world where we know there is substantial selenium deficiency (parts of sub-Saharan Africa, parts of China, parts of Asia), could selenium deficiency itself be contributing to very high rates of preterm birth? Right now we are trying to figure out if there is an association by measuring maternal plasma selenium levels about halfway through pregnancy and then asking what was the outcome from the pregnancy. Are women with low levels of selenium at increased risk for preterm birth? There have been 2 studies published that do already suggest that women with lower selenium levels tend to give birth to premature babies often.
OBG Management : What is the HSPA1L pathway and why is it important for pregnancy outcomes?
In our study where we performed genome-wide association, we looked at what are called common variants in the human genome—common variants in general are carried by more people. They had to be carried by a couple percent of the population to be included in our study. But there is also the thought that individual, more severe variants (that do not necessarily get transmitted because of how severe their effects are), will also affect birth outcomes. So we did a study to sequence mom’s genome to look for these rarities, things that account for less than 1% of the whole population. We were able to identify this gene, HSPA1L, which again, as found in our genome-wide studies, seems to be involved in controlling the strength of the steroid hormone signal, which is very important for maintaining and ending pregnancy. Progesterone and estrogen are the yin and yang of maintaining and ending pregnancy, and we think HSPA1L, the variant we identified, decreases the steroid hormone signal function so that it is not able to regulate that progesterone/estrogen signal the same way anymore.
OBG Management : Why is this an exciting time to be studying genes in pregnancy?
To understand how gene study can optimize our knowledge of human pregnancy outcomes really requires a study of human pregnancy specifically, and one of the best opportunities we have is to gather these large data sets. And we can’t forget about collecting pregnancy outcomes on women as part of new National Institutes of Health initiatives that are developing personalized medicine strategies. We looked at 50,000 women in our research, but we have the capacity to look at 500,000 women. As we go from identifying 6 genes to 12 genes to 100 genes, we will be able to understand better how these things are talking to one another and better define the signatures of what tissues are being acted on. We will be able to get sequentially synergistic information that will allow us to solve this in a way we couldn’t before.
The 2018 meeting of the American Gynecological and Obstetrical Society, held in Philadelphia, Pennsylvania, September 6 to 8, featured a talk by Louis J. Muglia, MD, PhD, on “Evolution, Genetics, and Preterm Birth.” Dr. Muglia, who is Co-Director of the Perinatal Institute, Director of the Center for Prevention of Preterm Birth, and Director of the Division of Human Genetics at the Cincinnati Children’s Hospital Medical Center, discussed his recent research on genetic associations of gestational duration and spontaneous preterm birth and some of his key findings. OBG
OBG Management : You discussed the “genetic architecture of human pregnancy.” Can you define what that is?
The genetic architecture tells us which pathways are activated that initiate birth to occur. By understanding that, we can begin to understand not only the genetic factors that the architecture describes, but also that the genetic architecture is going to be modified in response to environmental stimuli that will disrupt the outcomes. In the future, we will be able to develop biomarkers, predictive genetic algorithms, and other tools that will allow us to assess risk in a way that we can’t right now.
OBG Management : How is gene study adding to the overall knowledge of preterm birth?
Gene study is giving us new pathways to look at. It will give us biomarkers; it will give us targets for potential therapeutic interventions. I mentioned in my talk that one of the genes that we identified in our recent New England Journal of Medicine (NEJM) study pinpointed selenium as an important component and a whole process of determining risk for preterm birth that we never even thought of before. For instance, could there be preventive strategies for prematurity, like we have for neural tube defects and folic acid? The possibility of supplementation with selenium, or other micronutrients that some of the genetic studies will reveal to us in a nonbiased fashion, would not be discovered without the study of genes.
OBG Management : You mentioned your NEJM paper. Can you describe the large data sets that your team used in your gene research?
The discovery cohort, which refers to essentially the biggest compilation, was a wonderful collaboration that we had with the direct-to-consumer genotyping company 23andme. I had contacted them to determine if they had captured any pregnancy-related data, particularly birth-timing information related to individuals who had completed their research surveys. They indicated that they had asked the question, “What was the length of your first pregnancy?” With this information, we were able to get essentially 44,000 responses to that question. That really provided the foundation for the study in the NEJM.
Now, there are caveats with that information, since it was all recollection and self-reported data. We were unsure how accurate it would be. In addition, we did not always know why the delivery occurred—was it spontaneous or was it medically indicated? We were interested in the spontaneous, naturally-occurring preterm birth. Using that as a discovery cohort, with those reservations in mind, we identified 6 genes for birth length. We then asked in a carefully collected series of cohorts that we had amassed on our own, and with collaborators over the years from Finland, Norway, and Denmark, whether those same associations still existed. And every one of them did. Every one of them was validated in our carefully phenotyped cohort. In total, that was about 55,000 women that we had analyzed and studied between the discovery and the validation cohort. Since then, we have accessed another 3 or 4 cohorts, which has increased our sample size even more, so we have identified even more genes than we originally reported in our paper.
OBG Management : What do you identify as the next steps in your research after identifying several genes associated with the timing of birth?
The idea is not just to develop longer and longer lists of genes that are suggested or associated with birth timing phenotypes that we are interested in—either preterm birth or duration of gestation—but to actually understand what they are doing. That is a little bit trickier than saying we have identified genes. We have identified the precise region of mom’s genome that is involved in regulating birth timing, but in many cases I have indicated the closest gene that is involved in birth timing. For some of the regions, however, there are many genes involved, and so is it regulating one pathway, is it regulating many? Which tissue is involved in regulating? Is it in the uterus, in the cervix, or in the immune system? The next steps are to figure out how these things are acting so that we can design better strategies for prevention. The goal is to really bring down preterm birth rates by implementing strategies for prevention and treatment that we don’t currently have.
OBG Management : What is the significance of maternal selenium status and preterm birth risk?
Well, we really don’t know. We identified one of these gene regions, a variant near a factory involved in production of what are called selenoproteins—proteins that incorporate selenium into them. (There are about 25 of those in the human genome.) We identified a genetic risk factor in a region that is linked to the selenoprotein production chain. What we were brought to think about was this: In parts of the world where we know there is substantial selenium deficiency (parts of sub-Saharan Africa, parts of China, parts of Asia), could selenium deficiency itself be contributing to very high rates of preterm birth? Right now we are trying to figure out if there is an association by measuring maternal plasma selenium levels about halfway through pregnancy and then asking what was the outcome from the pregnancy. Are women with low levels of selenium at increased risk for preterm birth? There have been 2 studies published that do already suggest that women with lower selenium levels tend to give birth to premature babies often.
OBG Management : What is the HSPA1L pathway and why is it important for pregnancy outcomes?
In our study where we performed genome-wide association, we looked at what are called common variants in the human genome—common variants in general are carried by more people. They had to be carried by a couple percent of the population to be included in our study. But there is also the thought that individual, more severe variants (that do not necessarily get transmitted because of how severe their effects are), will also affect birth outcomes. So we did a study to sequence mom’s genome to look for these rarities, things that account for less than 1% of the whole population. We were able to identify this gene, HSPA1L, which again, as found in our genome-wide studies, seems to be involved in controlling the strength of the steroid hormone signal, which is very important for maintaining and ending pregnancy. Progesterone and estrogen are the yin and yang of maintaining and ending pregnancy, and we think HSPA1L, the variant we identified, decreases the steroid hormone signal function so that it is not able to regulate that progesterone/estrogen signal the same way anymore.
OBG Management : Why is this an exciting time to be studying genes in pregnancy?
To understand how gene study can optimize our knowledge of human pregnancy outcomes really requires a study of human pregnancy specifically, and one of the best opportunities we have is to gather these large data sets. And we can’t forget about collecting pregnancy outcomes on women as part of new National Institutes of Health initiatives that are developing personalized medicine strategies. We looked at 50,000 women in our research, but we have the capacity to look at 500,000 women. As we go from identifying 6 genes to 12 genes to 100 genes, we will be able to understand better how these things are talking to one another and better define the signatures of what tissues are being acted on. We will be able to get sequentially synergistic information that will allow us to solve this in a way we couldn’t before.
Importance of providing standardized management of hypertension in pregnancy
CASE Onset of nausea and headache, and elevated BP, at full term
A 24-year-old woman (G1P0) at 39 2/7 weeks of gestation without significant medical history and with uncomplicated prenatal care presents to labor and delivery reporting uterine contractions. She reports nausea and vomiting, and reports having a severe headache this morning. Blood pressure (BP) is 154/98 mm Hg. Urine dipstick analysis demonstrates absence of protein.
How should this patient be managed?
Although we have gained a greater understanding of hypertensive disorders in pregnancy—most notably, preeclampsia—during the past 15 years, management of these patients can, as evidenced in the case above, be complicated. Providers must respect this disease and be cognizant of the significant maternal, fetal, and neonatal complications that can be associated with hypertension during pregnancy—a leading cause of preterm birth and maternal mortality in the United States.1-3 Initiation of early and aggressive antihypertensive medical therapy, when indicated, plays a key role in preventing catastrophic complications of this disease.
Terminology and classification
Hypertension of pregnancy is classified as:
- chronic hypertension: BP≥140/90 mm Hg prior to pregnancy or prior to 20 weeks of gestation. Patients who have persistently elevated BP 12 weeks after delivery are also in this category.
- preeclampsia–eclampsia: hypertension along with multisystem involvement that occurs after 20 weeks of gestation.
- gestational hypertension: hypertension alone after 20 weeks of gestation; in approximately 15% to 25% of these patients, a diagnosis of preeclampsia will be made as pregnancy progresses.
- chronic hypertension with superimposed preeclampsia: hypertension complicated by development of multisystem involvement during the course of the pregnancy—often a challenging diagnosis, associated with greater perinatal morbidity than either chronic hypertension or preeclampsia alone.
Evaluation of the hypertensive gravida
Although most pregnant patients (approximately 90%) who have a diagnosis of chronic hypertension have primary or essential hypertension, a secondary cause—including thyroid disease, systemic lupus erythematosus (SLE), and underlying renal disease—might be present and should be sought out. It is important, therefore, to obtain a comprehensive history along with a directed physical examination and appropriate laboratory tests.
Ideally, a patient with chronic hypertension should be evaluated prior to pregnancy, but this rarely occurs. At the initial encounter, the patient should be informed of risks associated with chronic hypertension, as well as receive education on the signs and symptoms of preeclampsia. Obtain a thorough history—not only to evaluate for secondary causes of hypertension or end-organ involvement (eg, kidney disease), but to identify comorbidities (such as pregestational diabetes mellitus). The patient should be instructed to immediately discontinue any teratogenic medication (such as an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker).
Routine laboratory evaluation
Testing should comprise a chemistry panel to evaluate serum creatinine, electrolytes, and liver enzymes. A 24-hour urine collection for protein excretion and creatinine clearance or a urine protein–creatinine ratio should be obtained to record baseline kidney function.4 (Such testing is important, given that new-onset or worsening proteinuria is a manifestation of superimposed preeclampsia.) All pregnant patients with chronic hypertension also should have a complete blood count, including a platelet count, and an early screen for gestational diabetes.
Depending on what information is obtained from the history and physical examination, renal ultrasonography and any of several laboratory tests can be ordered, including thyroid function, an SLE panel, and vanillylmandelic acid/metanephrines. If the patient has a history of severe hypertension for greater than 5 years, is older than 40 years, or has cardiac symptoms, baseline electrocardio-graphy or echocardiography, or both, are recommended.
Clinical manifestations of chronic hypertension during pregnancy include5:
- in the mother: accelerated hypertension, with resulting target-organ damage involving heart, brain, and kidneys
- in the fetus: placental abruption, preterm birth, fetal growth restriction, and fetal death.
What should treatment seek to accomplish?
The goal of antihypertensive medication during pregnancy is to reduce maternal risk of stroke, congestive heart failure, renal failure, and severe hypertension. No convincing evidence exists that antihypertensive medications decrease the incidence of superimposed preeclampsia, preterm birth, placental abruption, or perinatal death.
According to the American College of Obstetricians and Gynecologists (ACOG), antihypertensive medication is not indicated in patients with uncomplicated chronic hypertension unless systolic BP is ≥ 160 mm Hg or diastolic BP is ≥ 105 mm Hg.3 The goal is to maintain systolic BP at 120–160 mm Hg and diastolic BP at 80–105 mm Hg. The National Institute for Health and Care Excellence recommends treatment of hypertension when systolic BP is ≥ 150 mm Hg or diastolic BP is ≥ 100 mm Hg.6 In patients with end-organ disease (chronic renal or cardiac disease) ACOG recommends treatment with an antihypertensive when systolic BP is >140 mm Hg or diastolic BP is >90 mm Hg.
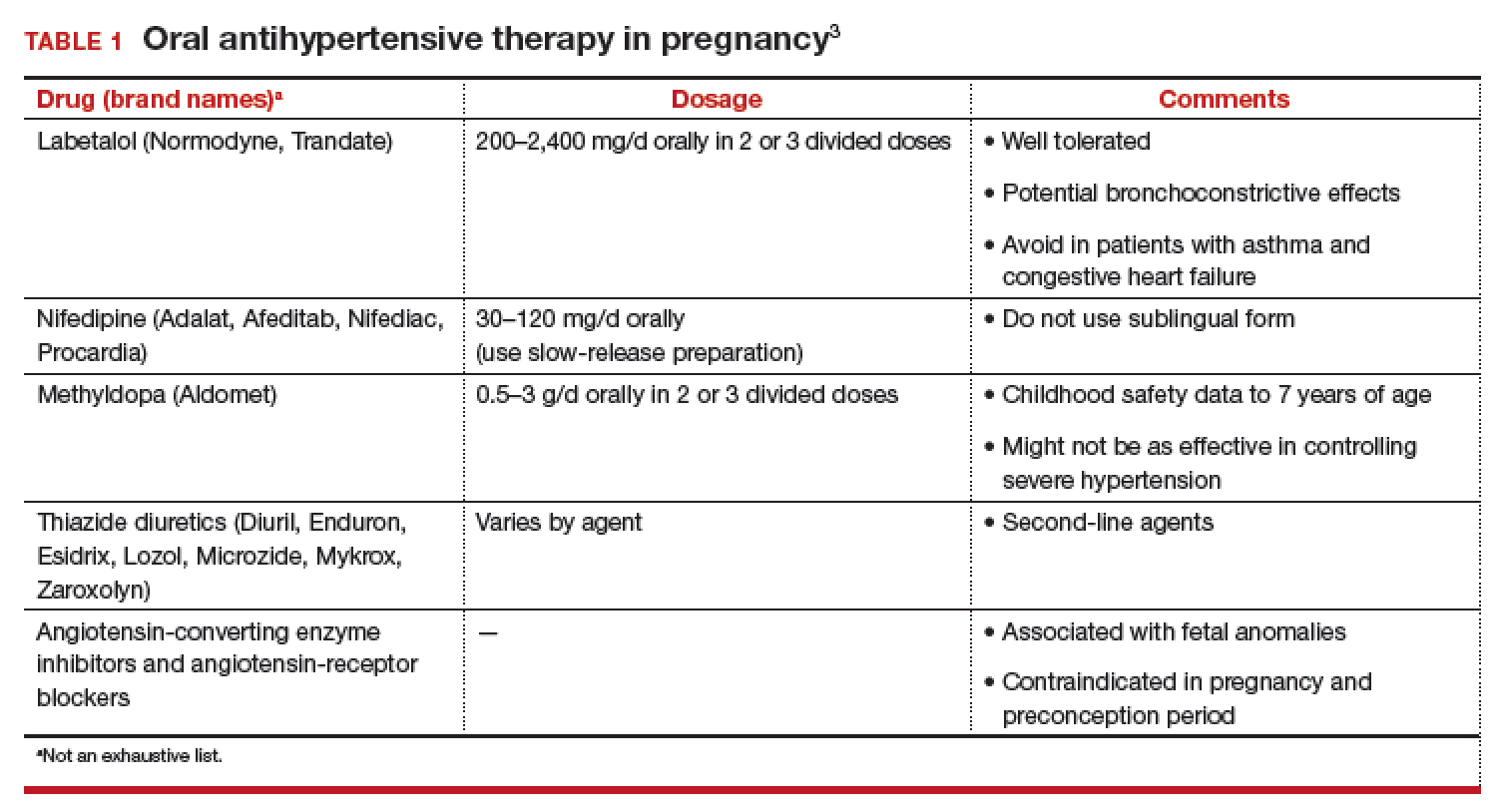
First-line antihypertensives consideredsafe during pregnancy are methyldopa, labetalol, and nifedipine. Thiazide diuretics, although considered second-line agents, may be used during pregnancy—especially if BP is adequately controlled prior to pregnancy. Again, angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are contraindicated during pregnancy (TABLE 1).3
Continuing care in chronic hypertension
Given the maternal and fetal consequences of chronic hypertension, it is recommended that a hypertensive patient be followed closely as an outpatient; in fact, it is advisablethat she check her BP at least twice daily. Beginning at 24 weeks of gestation, serial ultrasonography should be performed every 4 to 6 weeks to evaluate interval fetal growth. Twice-weekly antepartum testing should begin at 32 to 34 weeks of gestation.
During the course of the pregnancy, the chronically hypertensive patient should be observed closely for development of superimposed preeclampsia. If she does not develop preeclampsia or fetal growth restriction, and has no other pregnancy complications that necessitate early delivery, 3 recommendations regarding timing of delivery apply7:
- If the patient is not taking antihypertensive medication, delivery should occur at 38 to 39 6/7 weeks of gestation
- If hypertension is controlled with medication, delivery is recommended at 37 to 39 6/7 weeks of gestation.
- If the patient has severe hypertension that is difficult to control, delivery might be advisable as early as 36 weeks of gestation.
Be vigilant for maternal complications (including cardiac compromise, congestive heart failure, cerebrovascular accident, hypertensive encephalopathy, and worsening renal disease) and fetal complications (such as placental abruption, fetal growth restriction, and fetal death). If any of these occur, management must be tailored and individualized accordingly. Study results have demonstrated that superimposed preeclampsia occurs in 20% to 30% of patients who have underlying mild chronic hypertension. This increases to 50% in women with underlying severe hypertension.8
Antihypertensive medication is the mainstay of treatment for severely elevated blood pressure (BP). To avoid fetal heart rate decelerations and possible emergent cesarean delivery, however, do not decrease BP too quickly or lower to values that might compromise perfusion to the fetus. The BP goal should be 140-155 mm Hg (systolic) and 90-105 mm Hg (diastolic). A
Be prepared for eclampsia, which is unpredictable and can occur in patients without symptoms or severely elevated BP and even postpartum in patients in whom the diagnosis of preeclampsia was never made prior to delivery. The response to eclamptic seizure includes administering magnesium sulfate, which is the approved initial therapy for an eclamptic seizure. A
Make algorithms for acute treatment of severe hypertension and eclampsia readily available or posted in labor and delivery units and in the emergency department. C
Counsel high-risk patients about the potential benefit of low-dosage aspirin to prevent preeclampsia. A
Strength of recommendation:
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The complex challenge of managing preeclampsia
Chronic hypertension is not the only risk factor for preeclampsia; others include nulliparity, history of preeclampsia, multifetal gestation, underlying renal disease, SLE, antiphospolipid syndrome, thyroid disease, and pregestational diabetes. Furthermore, preeclampsia has a bimodal age distribution, occurring more often in adolescent pregnancies and women of advanced maternal age. Risk is also increased in the presence of abnormal levels of various serum analytes or biochemical markers, such as a low level of pregnancy-associated plasma protein A or estriol or an elevated level of maternal serum α-fetoprotein, human chorionic gonadotropin, or inhibin—findings that might reflect abnormal placentation.9
In fact, the findings of most studies that have looked at the pathophysiology of preeclampsia appear to show that several noteworthy pathophysiologic changes are evident in early pregnancy10,11:
- incomplete trophoblastic invasion of spiral arteries
- retention of thick-walled, muscular arteries
- decreased placental perfusion
- early placental hypoxia
- placental release of factors that lead to endothelial dysfunction and endothelial damage.
Ultimately, vasoconstriction becomes evident, which leads to clinical manifestations of the disorder. In addition, there is an increase in the level of thromboxane (a vasoconstrictor and platelet aggregator), compared to the level of prostacyclin (a vasodilator).
ACOG revises nomenclature, provides recommendations
The considerable expansion of knowledge about preeclampsia over the past 10 to 15 years has not translated to better outcomes. In 2012, ACOG, in response to troubling observations about the condition (see “ACOG finds compelling motivation to boost understanding, management of preeclampsia,”), created a Task Force to investigate hypertension in pregnancy.
Findings and recommendations of the Task Force were published in November 2013,3 and have been endorsed and supported by professional organizations, including the American Academy of Neurology, American Society of Hypertension, Preeclampsia Foundation, and the Society for Maternal-Fetal Medicine. A major premise of the Task Force that has had a direct impact on recommendations for management of preeclampsia is that the condition is a progressive and dynamic process that involves multiple organ systems and is not specifically confined to the antepartum period.
The nomenclature of mild preeclampsia and severe preeclampsia was changed in the Task Force report to preeclampsia without severe features and preeclampsia with severe features. Preeclampsia without severe features is diagnosed when a patient has:
- systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg (measured twice at least 4 hours apart)
- proteinuria, defined as a 24-hour urine collection of ≥ 300 mg of protein or a urine protein–creatinine ratio of 0.3.
If a patient has elevated BP by those criteria, plus any of several laboratory indicators of multisystem involvement (platelet count, <100 × 103/μL; serum creatinine level, >1.1 mg/dL; doubling in the serum creatinine concentration; liver transaminase concentrations twice normal) or other findings (pulmonary edema, visual disturbance, headaches), she has preeclampsia with severe features. A diagnosis of preeclampsia without severe features is upgraded to preeclampsia with severe features if systolic BP increases to >160 mm Hgor diastolic BP increases to >110 mm Hg (determined by 2 measurements 4 hours apart) or if “severe”-range BP occurs with such rapidity that acute antihypertensive medication is required.
- Incidence of preeclampsia in the United States has increased by 25% over the past 2 decades
- Etiology remains unclear
- Leading cause of maternal and perinatal morbidity and mortality
- Risk factor for future cardiovascular disease and metabolic disease in women
- Hypertensive disorders of pregnancy are major contributors to prematurity
- New best-practice recommendations are urgently needed to guide clinicians in the care of women with all forms of preeclampsia and hypertension during pregnancy
- Improved patient education and counseling strategies are needed to convey, more effectively, the dangers of preeclampsia and hypertension during pregnancy
Reference
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
Pharmacotherapy for hypertensive emergency
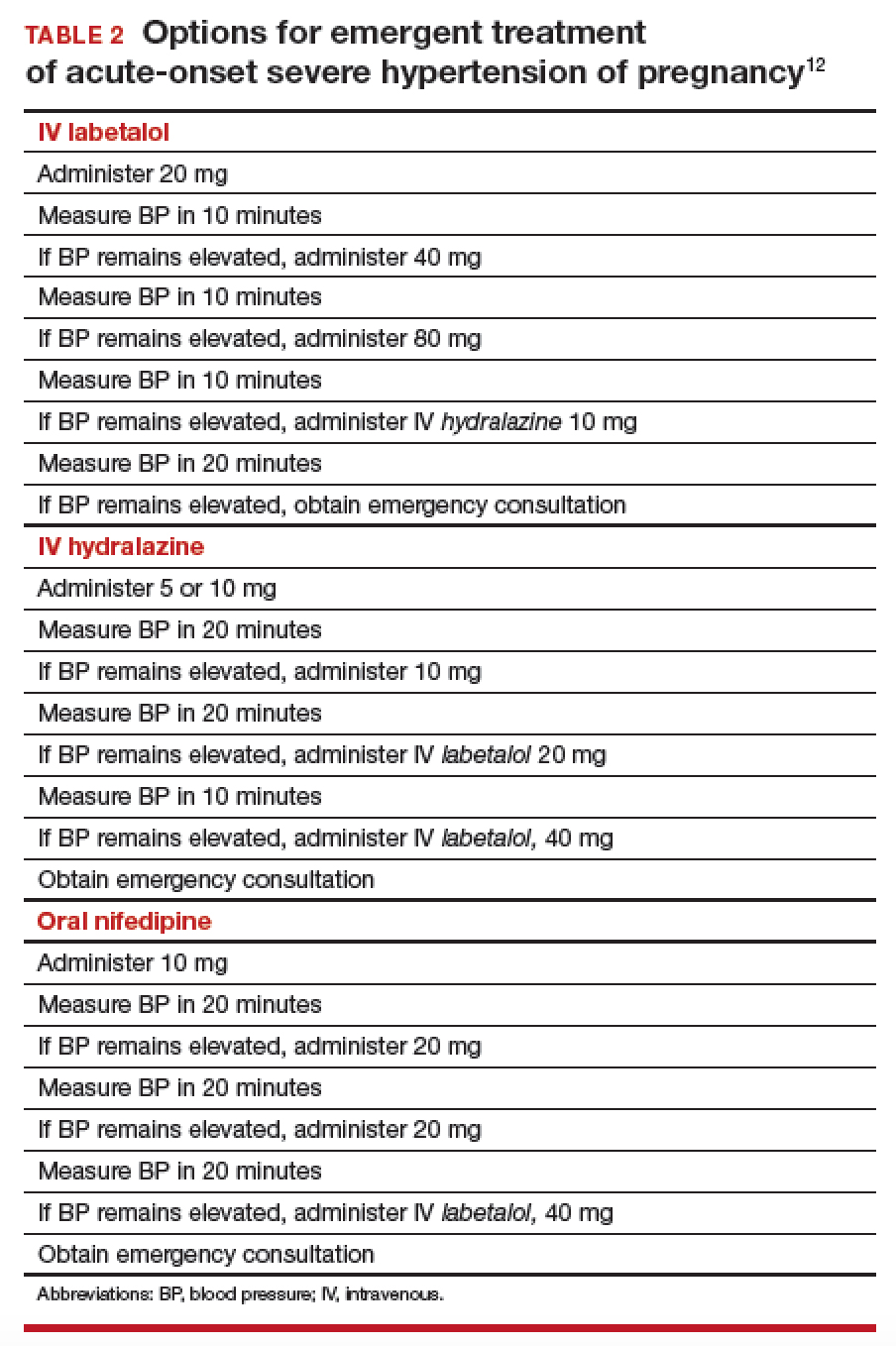
Acute BP control with intravenous (IV) labetalol or hydralazine or oral nifedipine is recommended when a patient has a hypertensive emergency, defined as acute-onset severe hypertension that persists for ≥ 15 minutes (TABLE 2).12 The goal of management is not to completely normalize BP but to lower BP to the range of 140 to 155 mm Hg (systolic) and 90 to 105 mm Hg (diastolic). Of all proposed interventions, these agents are likely the most effective in preventing a maternal cerebrovascular or cardiovascular event. (Note: Labetalol is contraindicated in patients with severe asthma and in the setting of acute cocaine or methamphetamine intoxication. Hydralazine can cause tachycardia.)13,14
Once a diagnosis of preeclampsia with severe features or superimposed preeclampsia with severe features is made, the patient should remain hospitalized until delivery. If either of these diagnoses is made at ≥ 34 weeks of gestation, there is no reason to prolong pregnancy. Rather, the patient should be given prophylactic magnesium sulfate to prevent seizures and delivery should be accomplished.15,16 Earlier than 36 6/7 weeks of gestation, consider a late preterm course of corticosteroids; however, do not delay delivery in this situation.17
Planning for delivery
Route of delivery depends on customary obstetric indications. Before 34 weeks of gestation, corticosteroids, magnesium sulfate, and prolonging the pregnancy until 34 weeks of gestation are recommended. If, at any time, maternal or fetal condition deteriorates, delivery should be accomplished regardless of gestational age. If the patient is unwilling to accept the risks of expectant management of preeclampsia with severe features remote from term, delivery is indicated.18,19 If delivery is not likely to occur, magnesium sulfate can be discontinued after the patient has received a second dose of corticosteroids, with the plan to resume magnesium sulfate if she develops signs of worsening preeclampsia or eclampsia, or once the plan for delivery is made.
In patients who have either gestational hypertension or preeclampsia without severe features, the recommendation is to accomplish delivery no later than 37 weeks of gestation. While the patient is being expectantly managed, close maternal and fetal surveillance are necessary, comprising serial assessment of maternal symptoms and fetal movement; serial BP measurement (twice weekly); and weekly measurement of the platelet count, serum creatinine, and liver enzymes. At 34 weeks of gestation, conventional antepartum testing should begin. Again, if there is deterioration of the maternal or fetal condition, the patient should be hospitalized and delivery should be accomplished according to the recommendations above.3
Seizure management
If a patient has a tonic–clonic seizure consistent with eclampsia, management should be as follows:
- Preserve the airway and immediately tilt the head forward to prevent aspiration.
- If the patient is not receiving magnesium sulfate, immediately administer a loading dose of 4-6 g IV or 10 mg intramuscularly if IV access has not been established.20
- If the patient is already receiving magnesium sulfate, administer a loading dose of 2 g IV over 5 minutes.
- If the patient continues to have seizure activity, administer anticonvulsant medication(lorazepam, diazepam, midazolam, or phenytoin).
Eclamptic seizures are usually self-limited, lasting no longer than 1 or 2 minutes. Regrettably, these seizures are unpredictable and contribute significantly to maternal morbidity and mortality.21,22 A maternal seizure causes a significant interruption in the oxygen pathway to the fetus, with resultant late decelerations, prolonged decelerations, or bradycardia.
Resist the temptation to perform emergent cesarean delivery when eclamptic seizure occurs; rather, allow time for fetal recovery and then proceed with delivery in a controlled fashion. In many circumstances, the patient can undergo vaginal delivery after an eclamptic seizure. Keep in mind that the differential diagnosis of new-onset seizure in pregnancy includes cerebral pathology, such as a bleeding arteriovenous malformation or ruptured aneurysm. Therefore, brain-imaging studies might be indicated, especially in patients who have focal neurologic deficits, or who have seizures either while receiving magnesium sulfate or 48 to 72 hours after delivery.
Preeclampsia postpartum
More recent studies have demonstrated that preeclampsia can be exacerbated after delivery or might even present initially postpartum.23,24 In all women in whom gestational hypertension, preeclampsia, or superimposed preeclampsia is diagnosed, therefore, recommendations are that BP be monitored in the hospital or on an outpatient basis for at least 72 hours postpartum and again 7 to 10 days after delivery. For all women postpartum, the recommendation is that discharge instructions 1) include information about signs and symptoms of preeclampsia and 2) emphasize the importance of promptly reporting such developments to providers.25 Remember: Sequelae of preeclampsia have been reported as late as 4 to 6 weeks postpartum.
Magnesium sulfate is recommended when a patient presents postpartum with new-onset hypertension associated with headache or blurred vision, or with preeclampsia with severe hypertension. Because nonsteroidal anti-inflammatory drugs can be associated with elevated BP, these medications should be replaced by other analgesics in women with hypertension that persists for more than 1 day postpartum.
Prevention of preeclampsia
Given the significant maternal, fetal, and neonatal complications associated with preeclampsia, a number of studies have sought to determine ways in which this condition can be prevented. Currently, although no interventions appear to prevent preeclampsia in all patients, significant strides have been made in prevention for high-risk patients. Specifically, beginning low-dosage aspirin (most commonly, 81 mg/d, beginning at less than 16 weeks of gestation) has been shown to mitigate—although not eliminate—risk in patients with a history of preeclampsia and those who have chronic hypertension, multifetal gestation, pregestational diabetes, renal disease, SLE, or antiphospholipid syndrome.26,27Aspirin appears to act by preferentially blocking production of thromboxane, thus reducing the vasoconstrictive properties of this hormone.
Summing up
Hypertensive disorders during pregnancy are associated with significant morbidity and mortality for mother, fetus, and newborn. Preeclampsia, specifically, is recognized as a dynamic and progressive disease that has the potential to involve multiple organ systems, might present for the first time after delivery, and might be associated with long-term risk of hypertension, heart disease, stroke, and venous thromboembolism.28,29
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991-2003. Am J Obstet Gynecol. 2008; 199:133.e1-e8.
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299-1306.
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
- Wheeler TL 2nd, Blackhurst DW, Dellinger EH, Ramsey PS. Usage of spot urine protein to creatinine ratios in the evaluation of preeclampsia. Am J Obstet Gynecol. 2007;196:465.e1-e4.
- Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LL. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. CG107, August 2010. https://www.nice.org.uk/guidance/cg107. Accessed August 27, 2018. Last updated January 2011.
- Spong CY, Mercer BM, D'Alton M, et al. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118:323-333.
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100(2):369-377.
- Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers or aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115:1052-1061.
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "great obstetrical syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193-201.
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970-975.
- The American College of Obstetricians and Gynecologists Committee on Obstetric Practice; El-Sayed YY, Borders AE. Committee Opinion Number 692. Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period; April 2017. https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co692.pdf?dmc=1. Accessed August 8, 2018.
- Hollander JE. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
- Ghuran A, Nolan J. Recreational drug misuse: issues for the cardiologist. Heart. 2000;83:627-633.
- Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
- Sibai BM. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks' gestation. Am J Obstet Gynecol. 2011;205:191-198.
- Norwitz E, Funai E. Expectant management of severe preeclampsia remote from term: hope for the best, but expect the worst. Am J Obstet Gynecol. 2008;199:209-212.
- Gordon R, Magee LA, Payne B, et al. Magnesium sulphate for the management of preeclampsia and eclampsia in low and middle income countries: a systematic review of tested dosing regimens. J Obstet Gynaecol Can. 2014;36(2):154-163.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402-410.
- Liu S, Joseph KS, Liston, RM, et al; Maternal Health Study Group of Canadian Perinatal Surveillance System (Public Health Agency of Canada). Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987-994.
- Yancey LM, Withers E, Bakes K, Abbot J. Postpartum preeclampsia: emergency department presentation and management. J Emerg Med. 2011;40:380-384.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206:470-475.
- You WB, Wolf MS, Bailey SC, Grobman WA. Improving patient understanding of preeclampsia: a randomized controlled trial. Am J Obstet Gynecol. 2012;206:431.e1-e5.
- Henderson JT, Whitlock EP, O'Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110-120.e6.
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974-986.
- McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918-930.
CASE Onset of nausea and headache, and elevated BP, at full term
A 24-year-old woman (G1P0) at 39 2/7 weeks of gestation without significant medical history and with uncomplicated prenatal care presents to labor and delivery reporting uterine contractions. She reports nausea and vomiting, and reports having a severe headache this morning. Blood pressure (BP) is 154/98 mm Hg. Urine dipstick analysis demonstrates absence of protein.
How should this patient be managed?
Although we have gained a greater understanding of hypertensive disorders in pregnancy—most notably, preeclampsia—during the past 15 years, management of these patients can, as evidenced in the case above, be complicated. Providers must respect this disease and be cognizant of the significant maternal, fetal, and neonatal complications that can be associated with hypertension during pregnancy—a leading cause of preterm birth and maternal mortality in the United States.1-3 Initiation of early and aggressive antihypertensive medical therapy, when indicated, plays a key role in preventing catastrophic complications of this disease.
Terminology and classification
Hypertension of pregnancy is classified as:
- chronic hypertension: BP≥140/90 mm Hg prior to pregnancy or prior to 20 weeks of gestation. Patients who have persistently elevated BP 12 weeks after delivery are also in this category.
- preeclampsia–eclampsia: hypertension along with multisystem involvement that occurs after 20 weeks of gestation.
- gestational hypertension: hypertension alone after 20 weeks of gestation; in approximately 15% to 25% of these patients, a diagnosis of preeclampsia will be made as pregnancy progresses.
- chronic hypertension with superimposed preeclampsia: hypertension complicated by development of multisystem involvement during the course of the pregnancy—often a challenging diagnosis, associated with greater perinatal morbidity than either chronic hypertension or preeclampsia alone.
Evaluation of the hypertensive gravida
Although most pregnant patients (approximately 90%) who have a diagnosis of chronic hypertension have primary or essential hypertension, a secondary cause—including thyroid disease, systemic lupus erythematosus (SLE), and underlying renal disease—might be present and should be sought out. It is important, therefore, to obtain a comprehensive history along with a directed physical examination and appropriate laboratory tests.
Ideally, a patient with chronic hypertension should be evaluated prior to pregnancy, but this rarely occurs. At the initial encounter, the patient should be informed of risks associated with chronic hypertension, as well as receive education on the signs and symptoms of preeclampsia. Obtain a thorough history—not only to evaluate for secondary causes of hypertension or end-organ involvement (eg, kidney disease), but to identify comorbidities (such as pregestational diabetes mellitus). The patient should be instructed to immediately discontinue any teratogenic medication (such as an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker).
Routine laboratory evaluation
Testing should comprise a chemistry panel to evaluate serum creatinine, electrolytes, and liver enzymes. A 24-hour urine collection for protein excretion and creatinine clearance or a urine protein–creatinine ratio should be obtained to record baseline kidney function.4 (Such testing is important, given that new-onset or worsening proteinuria is a manifestation of superimposed preeclampsia.) All pregnant patients with chronic hypertension also should have a complete blood count, including a platelet count, and an early screen for gestational diabetes.
Depending on what information is obtained from the history and physical examination, renal ultrasonography and any of several laboratory tests can be ordered, including thyroid function, an SLE panel, and vanillylmandelic acid/metanephrines. If the patient has a history of severe hypertension for greater than 5 years, is older than 40 years, or has cardiac symptoms, baseline electrocardio-graphy or echocardiography, or both, are recommended.
Clinical manifestations of chronic hypertension during pregnancy include5:
- in the mother: accelerated hypertension, with resulting target-organ damage involving heart, brain, and kidneys
- in the fetus: placental abruption, preterm birth, fetal growth restriction, and fetal death.
What should treatment seek to accomplish?
The goal of antihypertensive medication during pregnancy is to reduce maternal risk of stroke, congestive heart failure, renal failure, and severe hypertension. No convincing evidence exists that antihypertensive medications decrease the incidence of superimposed preeclampsia, preterm birth, placental abruption, or perinatal death.
According to the American College of Obstetricians and Gynecologists (ACOG), antihypertensive medication is not indicated in patients with uncomplicated chronic hypertension unless systolic BP is ≥ 160 mm Hg or diastolic BP is ≥ 105 mm Hg.3 The goal is to maintain systolic BP at 120–160 mm Hg and diastolic BP at 80–105 mm Hg. The National Institute for Health and Care Excellence recommends treatment of hypertension when systolic BP is ≥ 150 mm Hg or diastolic BP is ≥ 100 mm Hg.6 In patients with end-organ disease (chronic renal or cardiac disease) ACOG recommends treatment with an antihypertensive when systolic BP is >140 mm Hg or diastolic BP is >90 mm Hg.
First-line antihypertensives consideredsafe during pregnancy are methyldopa, labetalol, and nifedipine. Thiazide diuretics, although considered second-line agents, may be used during pregnancy—especially if BP is adequately controlled prior to pregnancy. Again, angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are contraindicated during pregnancy (TABLE 1).3
Continuing care in chronic hypertension
Given the maternal and fetal consequences of chronic hypertension, it is recommended that a hypertensive patient be followed closely as an outpatient; in fact, it is advisablethat she check her BP at least twice daily. Beginning at 24 weeks of gestation, serial ultrasonography should be performed every 4 to 6 weeks to evaluate interval fetal growth. Twice-weekly antepartum testing should begin at 32 to 34 weeks of gestation.
During the course of the pregnancy, the chronically hypertensive patient should be observed closely for development of superimposed preeclampsia. If she does not develop preeclampsia or fetal growth restriction, and has no other pregnancy complications that necessitate early delivery, 3 recommendations regarding timing of delivery apply7:
- If the patient is not taking antihypertensive medication, delivery should occur at 38 to 39 6/7 weeks of gestation
- If hypertension is controlled with medication, delivery is recommended at 37 to 39 6/7 weeks of gestation.
- If the patient has severe hypertension that is difficult to control, delivery might be advisable as early as 36 weeks of gestation.
Be vigilant for maternal complications (including cardiac compromise, congestive heart failure, cerebrovascular accident, hypertensive encephalopathy, and worsening renal disease) and fetal complications (such as placental abruption, fetal growth restriction, and fetal death). If any of these occur, management must be tailored and individualized accordingly. Study results have demonstrated that superimposed preeclampsia occurs in 20% to 30% of patients who have underlying mild chronic hypertension. This increases to 50% in women with underlying severe hypertension.8
Antihypertensive medication is the mainstay of treatment for severely elevated blood pressure (BP). To avoid fetal heart rate decelerations and possible emergent cesarean delivery, however, do not decrease BP too quickly or lower to values that might compromise perfusion to the fetus. The BP goal should be 140-155 mm Hg (systolic) and 90-105 mm Hg (diastolic). A
Be prepared for eclampsia, which is unpredictable and can occur in patients without symptoms or severely elevated BP and even postpartum in patients in whom the diagnosis of preeclampsia was never made prior to delivery. The response to eclamptic seizure includes administering magnesium sulfate, which is the approved initial therapy for an eclamptic seizure. A
Make algorithms for acute treatment of severe hypertension and eclampsia readily available or posted in labor and delivery units and in the emergency department. C
Counsel high-risk patients about the potential benefit of low-dosage aspirin to prevent preeclampsia. A
Strength of recommendation:
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The complex challenge of managing preeclampsia
Chronic hypertension is not the only risk factor for preeclampsia; others include nulliparity, history of preeclampsia, multifetal gestation, underlying renal disease, SLE, antiphospolipid syndrome, thyroid disease, and pregestational diabetes. Furthermore, preeclampsia has a bimodal age distribution, occurring more often in adolescent pregnancies and women of advanced maternal age. Risk is also increased in the presence of abnormal levels of various serum analytes or biochemical markers, such as a low level of pregnancy-associated plasma protein A or estriol or an elevated level of maternal serum α-fetoprotein, human chorionic gonadotropin, or inhibin—findings that might reflect abnormal placentation.9
In fact, the findings of most studies that have looked at the pathophysiology of preeclampsia appear to show that several noteworthy pathophysiologic changes are evident in early pregnancy10,11:
- incomplete trophoblastic invasion of spiral arteries
- retention of thick-walled, muscular arteries
- decreased placental perfusion
- early placental hypoxia
- placental release of factors that lead to endothelial dysfunction and endothelial damage.
Ultimately, vasoconstriction becomes evident, which leads to clinical manifestations of the disorder. In addition, there is an increase in the level of thromboxane (a vasoconstrictor and platelet aggregator), compared to the level of prostacyclin (a vasodilator).
ACOG revises nomenclature, provides recommendations
The considerable expansion of knowledge about preeclampsia over the past 10 to 15 years has not translated to better outcomes. In 2012, ACOG, in response to troubling observations about the condition (see “ACOG finds compelling motivation to boost understanding, management of preeclampsia,”), created a Task Force to investigate hypertension in pregnancy.
Findings and recommendations of the Task Force were published in November 2013,3 and have been endorsed and supported by professional organizations, including the American Academy of Neurology, American Society of Hypertension, Preeclampsia Foundation, and the Society for Maternal-Fetal Medicine. A major premise of the Task Force that has had a direct impact on recommendations for management of preeclampsia is that the condition is a progressive and dynamic process that involves multiple organ systems and is not specifically confined to the antepartum period.
The nomenclature of mild preeclampsia and severe preeclampsia was changed in the Task Force report to preeclampsia without severe features and preeclampsia with severe features. Preeclampsia without severe features is diagnosed when a patient has:
- systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg (measured twice at least 4 hours apart)
- proteinuria, defined as a 24-hour urine collection of ≥ 300 mg of protein or a urine protein–creatinine ratio of 0.3.
If a patient has elevated BP by those criteria, plus any of several laboratory indicators of multisystem involvement (platelet count, <100 × 103/μL; serum creatinine level, >1.1 mg/dL; doubling in the serum creatinine concentration; liver transaminase concentrations twice normal) or other findings (pulmonary edema, visual disturbance, headaches), she has preeclampsia with severe features. A diagnosis of preeclampsia without severe features is upgraded to preeclampsia with severe features if systolic BP increases to >160 mm Hgor diastolic BP increases to >110 mm Hg (determined by 2 measurements 4 hours apart) or if “severe”-range BP occurs with such rapidity that acute antihypertensive medication is required.
- Incidence of preeclampsia in the United States has increased by 25% over the past 2 decades
- Etiology remains unclear
- Leading cause of maternal and perinatal morbidity and mortality
- Risk factor for future cardiovascular disease and metabolic disease in women
- Hypertensive disorders of pregnancy are major contributors to prematurity
- New best-practice recommendations are urgently needed to guide clinicians in the care of women with all forms of preeclampsia and hypertension during pregnancy
- Improved patient education and counseling strategies are needed to convey, more effectively, the dangers of preeclampsia and hypertension during pregnancy
Reference
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
Pharmacotherapy for hypertensive emergency
Acute BP control with intravenous (IV) labetalol or hydralazine or oral nifedipine is recommended when a patient has a hypertensive emergency, defined as acute-onset severe hypertension that persists for ≥ 15 minutes (TABLE 2).12 The goal of management is not to completely normalize BP but to lower BP to the range of 140 to 155 mm Hg (systolic) and 90 to 105 mm Hg (diastolic). Of all proposed interventions, these agents are likely the most effective in preventing a maternal cerebrovascular or cardiovascular event. (Note: Labetalol is contraindicated in patients with severe asthma and in the setting of acute cocaine or methamphetamine intoxication. Hydralazine can cause tachycardia.)13,14
Once a diagnosis of preeclampsia with severe features or superimposed preeclampsia with severe features is made, the patient should remain hospitalized until delivery. If either of these diagnoses is made at ≥ 34 weeks of gestation, there is no reason to prolong pregnancy. Rather, the patient should be given prophylactic magnesium sulfate to prevent seizures and delivery should be accomplished.15,16 Earlier than 36 6/7 weeks of gestation, consider a late preterm course of corticosteroids; however, do not delay delivery in this situation.17
Planning for delivery
Route of delivery depends on customary obstetric indications. Before 34 weeks of gestation, corticosteroids, magnesium sulfate, and prolonging the pregnancy until 34 weeks of gestation are recommended. If, at any time, maternal or fetal condition deteriorates, delivery should be accomplished regardless of gestational age. If the patient is unwilling to accept the risks of expectant management of preeclampsia with severe features remote from term, delivery is indicated.18,19 If delivery is not likely to occur, magnesium sulfate can be discontinued after the patient has received a second dose of corticosteroids, with the plan to resume magnesium sulfate if she develops signs of worsening preeclampsia or eclampsia, or once the plan for delivery is made.
In patients who have either gestational hypertension or preeclampsia without severe features, the recommendation is to accomplish delivery no later than 37 weeks of gestation. While the patient is being expectantly managed, close maternal and fetal surveillance are necessary, comprising serial assessment of maternal symptoms and fetal movement; serial BP measurement (twice weekly); and weekly measurement of the platelet count, serum creatinine, and liver enzymes. At 34 weeks of gestation, conventional antepartum testing should begin. Again, if there is deterioration of the maternal or fetal condition, the patient should be hospitalized and delivery should be accomplished according to the recommendations above.3
Seizure management
If a patient has a tonic–clonic seizure consistent with eclampsia, management should be as follows:
- Preserve the airway and immediately tilt the head forward to prevent aspiration.
- If the patient is not receiving magnesium sulfate, immediately administer a loading dose of 4-6 g IV or 10 mg intramuscularly if IV access has not been established.20
- If the patient is already receiving magnesium sulfate, administer a loading dose of 2 g IV over 5 minutes.
- If the patient continues to have seizure activity, administer anticonvulsant medication(lorazepam, diazepam, midazolam, or phenytoin).
Eclamptic seizures are usually self-limited, lasting no longer than 1 or 2 minutes. Regrettably, these seizures are unpredictable and contribute significantly to maternal morbidity and mortality.21,22 A maternal seizure causes a significant interruption in the oxygen pathway to the fetus, with resultant late decelerations, prolonged decelerations, or bradycardia.
Resist the temptation to perform emergent cesarean delivery when eclamptic seizure occurs; rather, allow time for fetal recovery and then proceed with delivery in a controlled fashion. In many circumstances, the patient can undergo vaginal delivery after an eclamptic seizure. Keep in mind that the differential diagnosis of new-onset seizure in pregnancy includes cerebral pathology, such as a bleeding arteriovenous malformation or ruptured aneurysm. Therefore, brain-imaging studies might be indicated, especially in patients who have focal neurologic deficits, or who have seizures either while receiving magnesium sulfate or 48 to 72 hours after delivery.
Preeclampsia postpartum
More recent studies have demonstrated that preeclampsia can be exacerbated after delivery or might even present initially postpartum.23,24 In all women in whom gestational hypertension, preeclampsia, or superimposed preeclampsia is diagnosed, therefore, recommendations are that BP be monitored in the hospital or on an outpatient basis for at least 72 hours postpartum and again 7 to 10 days after delivery. For all women postpartum, the recommendation is that discharge instructions 1) include information about signs and symptoms of preeclampsia and 2) emphasize the importance of promptly reporting such developments to providers.25 Remember: Sequelae of preeclampsia have been reported as late as 4 to 6 weeks postpartum.
Magnesium sulfate is recommended when a patient presents postpartum with new-onset hypertension associated with headache or blurred vision, or with preeclampsia with severe hypertension. Because nonsteroidal anti-inflammatory drugs can be associated with elevated BP, these medications should be replaced by other analgesics in women with hypertension that persists for more than 1 day postpartum.
Prevention of preeclampsia
Given the significant maternal, fetal, and neonatal complications associated with preeclampsia, a number of studies have sought to determine ways in which this condition can be prevented. Currently, although no interventions appear to prevent preeclampsia in all patients, significant strides have been made in prevention for high-risk patients. Specifically, beginning low-dosage aspirin (most commonly, 81 mg/d, beginning at less than 16 weeks of gestation) has been shown to mitigate—although not eliminate—risk in patients with a history of preeclampsia and those who have chronic hypertension, multifetal gestation, pregestational diabetes, renal disease, SLE, or antiphospholipid syndrome.26,27Aspirin appears to act by preferentially blocking production of thromboxane, thus reducing the vasoconstrictive properties of this hormone.
Summing up
Hypertensive disorders during pregnancy are associated with significant morbidity and mortality for mother, fetus, and newborn. Preeclampsia, specifically, is recognized as a dynamic and progressive disease that has the potential to involve multiple organ systems, might present for the first time after delivery, and might be associated with long-term risk of hypertension, heart disease, stroke, and venous thromboembolism.28,29
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
CASE Onset of nausea and headache, and elevated BP, at full term
A 24-year-old woman (G1P0) at 39 2/7 weeks of gestation without significant medical history and with uncomplicated prenatal care presents to labor and delivery reporting uterine contractions. She reports nausea and vomiting, and reports having a severe headache this morning. Blood pressure (BP) is 154/98 mm Hg. Urine dipstick analysis demonstrates absence of protein.
How should this patient be managed?
Although we have gained a greater understanding of hypertensive disorders in pregnancy—most notably, preeclampsia—during the past 15 years, management of these patients can, as evidenced in the case above, be complicated. Providers must respect this disease and be cognizant of the significant maternal, fetal, and neonatal complications that can be associated with hypertension during pregnancy—a leading cause of preterm birth and maternal mortality in the United States.1-3 Initiation of early and aggressive antihypertensive medical therapy, when indicated, plays a key role in preventing catastrophic complications of this disease.
Terminology and classification
Hypertension of pregnancy is classified as:
- chronic hypertension: BP≥140/90 mm Hg prior to pregnancy or prior to 20 weeks of gestation. Patients who have persistently elevated BP 12 weeks after delivery are also in this category.
- preeclampsia–eclampsia: hypertension along with multisystem involvement that occurs after 20 weeks of gestation.
- gestational hypertension: hypertension alone after 20 weeks of gestation; in approximately 15% to 25% of these patients, a diagnosis of preeclampsia will be made as pregnancy progresses.
- chronic hypertension with superimposed preeclampsia: hypertension complicated by development of multisystem involvement during the course of the pregnancy—often a challenging diagnosis, associated with greater perinatal morbidity than either chronic hypertension or preeclampsia alone.
Evaluation of the hypertensive gravida
Although most pregnant patients (approximately 90%) who have a diagnosis of chronic hypertension have primary or essential hypertension, a secondary cause—including thyroid disease, systemic lupus erythematosus (SLE), and underlying renal disease—might be present and should be sought out. It is important, therefore, to obtain a comprehensive history along with a directed physical examination and appropriate laboratory tests.
Ideally, a patient with chronic hypertension should be evaluated prior to pregnancy, but this rarely occurs. At the initial encounter, the patient should be informed of risks associated with chronic hypertension, as well as receive education on the signs and symptoms of preeclampsia. Obtain a thorough history—not only to evaluate for secondary causes of hypertension or end-organ involvement (eg, kidney disease), but to identify comorbidities (such as pregestational diabetes mellitus). The patient should be instructed to immediately discontinue any teratogenic medication (such as an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker).
Routine laboratory evaluation
Testing should comprise a chemistry panel to evaluate serum creatinine, electrolytes, and liver enzymes. A 24-hour urine collection for protein excretion and creatinine clearance or a urine protein–creatinine ratio should be obtained to record baseline kidney function.4 (Such testing is important, given that new-onset or worsening proteinuria is a manifestation of superimposed preeclampsia.) All pregnant patients with chronic hypertension also should have a complete blood count, including a platelet count, and an early screen for gestational diabetes.
Depending on what information is obtained from the history and physical examination, renal ultrasonography and any of several laboratory tests can be ordered, including thyroid function, an SLE panel, and vanillylmandelic acid/metanephrines. If the patient has a history of severe hypertension for greater than 5 years, is older than 40 years, or has cardiac symptoms, baseline electrocardio-graphy or echocardiography, or both, are recommended.
Clinical manifestations of chronic hypertension during pregnancy include5:
- in the mother: accelerated hypertension, with resulting target-organ damage involving heart, brain, and kidneys
- in the fetus: placental abruption, preterm birth, fetal growth restriction, and fetal death.
What should treatment seek to accomplish?
The goal of antihypertensive medication during pregnancy is to reduce maternal risk of stroke, congestive heart failure, renal failure, and severe hypertension. No convincing evidence exists that antihypertensive medications decrease the incidence of superimposed preeclampsia, preterm birth, placental abruption, or perinatal death.
According to the American College of Obstetricians and Gynecologists (ACOG), antihypertensive medication is not indicated in patients with uncomplicated chronic hypertension unless systolic BP is ≥ 160 mm Hg or diastolic BP is ≥ 105 mm Hg.3 The goal is to maintain systolic BP at 120–160 mm Hg and diastolic BP at 80–105 mm Hg. The National Institute for Health and Care Excellence recommends treatment of hypertension when systolic BP is ≥ 150 mm Hg or diastolic BP is ≥ 100 mm Hg.6 In patients with end-organ disease (chronic renal or cardiac disease) ACOG recommends treatment with an antihypertensive when systolic BP is >140 mm Hg or diastolic BP is >90 mm Hg.
First-line antihypertensives consideredsafe during pregnancy are methyldopa, labetalol, and nifedipine. Thiazide diuretics, although considered second-line agents, may be used during pregnancy—especially if BP is adequately controlled prior to pregnancy. Again, angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers are contraindicated during pregnancy (TABLE 1).3
Continuing care in chronic hypertension
Given the maternal and fetal consequences of chronic hypertension, it is recommended that a hypertensive patient be followed closely as an outpatient; in fact, it is advisablethat she check her BP at least twice daily. Beginning at 24 weeks of gestation, serial ultrasonography should be performed every 4 to 6 weeks to evaluate interval fetal growth. Twice-weekly antepartum testing should begin at 32 to 34 weeks of gestation.
During the course of the pregnancy, the chronically hypertensive patient should be observed closely for development of superimposed preeclampsia. If she does not develop preeclampsia or fetal growth restriction, and has no other pregnancy complications that necessitate early delivery, 3 recommendations regarding timing of delivery apply7:
- If the patient is not taking antihypertensive medication, delivery should occur at 38 to 39 6/7 weeks of gestation
- If hypertension is controlled with medication, delivery is recommended at 37 to 39 6/7 weeks of gestation.
- If the patient has severe hypertension that is difficult to control, delivery might be advisable as early as 36 weeks of gestation.
Be vigilant for maternal complications (including cardiac compromise, congestive heart failure, cerebrovascular accident, hypertensive encephalopathy, and worsening renal disease) and fetal complications (such as placental abruption, fetal growth restriction, and fetal death). If any of these occur, management must be tailored and individualized accordingly. Study results have demonstrated that superimposed preeclampsia occurs in 20% to 30% of patients who have underlying mild chronic hypertension. This increases to 50% in women with underlying severe hypertension.8
Antihypertensive medication is the mainstay of treatment for severely elevated blood pressure (BP). To avoid fetal heart rate decelerations and possible emergent cesarean delivery, however, do not decrease BP too quickly or lower to values that might compromise perfusion to the fetus. The BP goal should be 140-155 mm Hg (systolic) and 90-105 mm Hg (diastolic). A
Be prepared for eclampsia, which is unpredictable and can occur in patients without symptoms or severely elevated BP and even postpartum in patients in whom the diagnosis of preeclampsia was never made prior to delivery. The response to eclamptic seizure includes administering magnesium sulfate, which is the approved initial therapy for an eclamptic seizure. A
Make algorithms for acute treatment of severe hypertension and eclampsia readily available or posted in labor and delivery units and in the emergency department. C
Counsel high-risk patients about the potential benefit of low-dosage aspirin to prevent preeclampsia. A
Strength of recommendation:
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The complex challenge of managing preeclampsia
Chronic hypertension is not the only risk factor for preeclampsia; others include nulliparity, history of preeclampsia, multifetal gestation, underlying renal disease, SLE, antiphospolipid syndrome, thyroid disease, and pregestational diabetes. Furthermore, preeclampsia has a bimodal age distribution, occurring more often in adolescent pregnancies and women of advanced maternal age. Risk is also increased in the presence of abnormal levels of various serum analytes or biochemical markers, such as a low level of pregnancy-associated plasma protein A or estriol or an elevated level of maternal serum α-fetoprotein, human chorionic gonadotropin, or inhibin—findings that might reflect abnormal placentation.9
In fact, the findings of most studies that have looked at the pathophysiology of preeclampsia appear to show that several noteworthy pathophysiologic changes are evident in early pregnancy10,11:
- incomplete trophoblastic invasion of spiral arteries
- retention of thick-walled, muscular arteries
- decreased placental perfusion
- early placental hypoxia
- placental release of factors that lead to endothelial dysfunction and endothelial damage.
Ultimately, vasoconstriction becomes evident, which leads to clinical manifestations of the disorder. In addition, there is an increase in the level of thromboxane (a vasoconstrictor and platelet aggregator), compared to the level of prostacyclin (a vasodilator).
ACOG revises nomenclature, provides recommendations
The considerable expansion of knowledge about preeclampsia over the past 10 to 15 years has not translated to better outcomes. In 2012, ACOG, in response to troubling observations about the condition (see “ACOG finds compelling motivation to boost understanding, management of preeclampsia,”), created a Task Force to investigate hypertension in pregnancy.
Findings and recommendations of the Task Force were published in November 2013,3 and have been endorsed and supported by professional organizations, including the American Academy of Neurology, American Society of Hypertension, Preeclampsia Foundation, and the Society for Maternal-Fetal Medicine. A major premise of the Task Force that has had a direct impact on recommendations for management of preeclampsia is that the condition is a progressive and dynamic process that involves multiple organ systems and is not specifically confined to the antepartum period.
The nomenclature of mild preeclampsia and severe preeclampsia was changed in the Task Force report to preeclampsia without severe features and preeclampsia with severe features. Preeclampsia without severe features is diagnosed when a patient has:
- systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg (measured twice at least 4 hours apart)
- proteinuria, defined as a 24-hour urine collection of ≥ 300 mg of protein or a urine protein–creatinine ratio of 0.3.
If a patient has elevated BP by those criteria, plus any of several laboratory indicators of multisystem involvement (platelet count, <100 × 103/μL; serum creatinine level, >1.1 mg/dL; doubling in the serum creatinine concentration; liver transaminase concentrations twice normal) or other findings (pulmonary edema, visual disturbance, headaches), she has preeclampsia with severe features. A diagnosis of preeclampsia without severe features is upgraded to preeclampsia with severe features if systolic BP increases to >160 mm Hgor diastolic BP increases to >110 mm Hg (determined by 2 measurements 4 hours apart) or if “severe”-range BP occurs with such rapidity that acute antihypertensive medication is required.
- Incidence of preeclampsia in the United States has increased by 25% over the past 2 decades
- Etiology remains unclear
- Leading cause of maternal and perinatal morbidity and mortality
- Risk factor for future cardiovascular disease and metabolic disease in women
- Hypertensive disorders of pregnancy are major contributors to prematurity
- New best-practice recommendations are urgently needed to guide clinicians in the care of women with all forms of preeclampsia and hypertension during pregnancy
- Improved patient education and counseling strategies are needed to convey, more effectively, the dangers of preeclampsia and hypertension during pregnancy
Reference
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
Pharmacotherapy for hypertensive emergency
Acute BP control with intravenous (IV) labetalol or hydralazine or oral nifedipine is recommended when a patient has a hypertensive emergency, defined as acute-onset severe hypertension that persists for ≥ 15 minutes (TABLE 2).12 The goal of management is not to completely normalize BP but to lower BP to the range of 140 to 155 mm Hg (systolic) and 90 to 105 mm Hg (diastolic). Of all proposed interventions, these agents are likely the most effective in preventing a maternal cerebrovascular or cardiovascular event. (Note: Labetalol is contraindicated in patients with severe asthma and in the setting of acute cocaine or methamphetamine intoxication. Hydralazine can cause tachycardia.)13,14
Once a diagnosis of preeclampsia with severe features or superimposed preeclampsia with severe features is made, the patient should remain hospitalized until delivery. If either of these diagnoses is made at ≥ 34 weeks of gestation, there is no reason to prolong pregnancy. Rather, the patient should be given prophylactic magnesium sulfate to prevent seizures and delivery should be accomplished.15,16 Earlier than 36 6/7 weeks of gestation, consider a late preterm course of corticosteroids; however, do not delay delivery in this situation.17
Planning for delivery
Route of delivery depends on customary obstetric indications. Before 34 weeks of gestation, corticosteroids, magnesium sulfate, and prolonging the pregnancy until 34 weeks of gestation are recommended. If, at any time, maternal or fetal condition deteriorates, delivery should be accomplished regardless of gestational age. If the patient is unwilling to accept the risks of expectant management of preeclampsia with severe features remote from term, delivery is indicated.18,19 If delivery is not likely to occur, magnesium sulfate can be discontinued after the patient has received a second dose of corticosteroids, with the plan to resume magnesium sulfate if she develops signs of worsening preeclampsia or eclampsia, or once the plan for delivery is made.
In patients who have either gestational hypertension or preeclampsia without severe features, the recommendation is to accomplish delivery no later than 37 weeks of gestation. While the patient is being expectantly managed, close maternal and fetal surveillance are necessary, comprising serial assessment of maternal symptoms and fetal movement; serial BP measurement (twice weekly); and weekly measurement of the platelet count, serum creatinine, and liver enzymes. At 34 weeks of gestation, conventional antepartum testing should begin. Again, if there is deterioration of the maternal or fetal condition, the patient should be hospitalized and delivery should be accomplished according to the recommendations above.3
Seizure management
If a patient has a tonic–clonic seizure consistent with eclampsia, management should be as follows:
- Preserve the airway and immediately tilt the head forward to prevent aspiration.
- If the patient is not receiving magnesium sulfate, immediately administer a loading dose of 4-6 g IV or 10 mg intramuscularly if IV access has not been established.20
- If the patient is already receiving magnesium sulfate, administer a loading dose of 2 g IV over 5 minutes.
- If the patient continues to have seizure activity, administer anticonvulsant medication(lorazepam, diazepam, midazolam, or phenytoin).
Eclamptic seizures are usually self-limited, lasting no longer than 1 or 2 minutes. Regrettably, these seizures are unpredictable and contribute significantly to maternal morbidity and mortality.21,22 A maternal seizure causes a significant interruption in the oxygen pathway to the fetus, with resultant late decelerations, prolonged decelerations, or bradycardia.
Resist the temptation to perform emergent cesarean delivery when eclamptic seizure occurs; rather, allow time for fetal recovery and then proceed with delivery in a controlled fashion. In many circumstances, the patient can undergo vaginal delivery after an eclamptic seizure. Keep in mind that the differential diagnosis of new-onset seizure in pregnancy includes cerebral pathology, such as a bleeding arteriovenous malformation or ruptured aneurysm. Therefore, brain-imaging studies might be indicated, especially in patients who have focal neurologic deficits, or who have seizures either while receiving magnesium sulfate or 48 to 72 hours after delivery.
Preeclampsia postpartum
More recent studies have demonstrated that preeclampsia can be exacerbated after delivery or might even present initially postpartum.23,24 In all women in whom gestational hypertension, preeclampsia, or superimposed preeclampsia is diagnosed, therefore, recommendations are that BP be monitored in the hospital or on an outpatient basis for at least 72 hours postpartum and again 7 to 10 days after delivery. For all women postpartum, the recommendation is that discharge instructions 1) include information about signs and symptoms of preeclampsia and 2) emphasize the importance of promptly reporting such developments to providers.25 Remember: Sequelae of preeclampsia have been reported as late as 4 to 6 weeks postpartum.
Magnesium sulfate is recommended when a patient presents postpartum with new-onset hypertension associated with headache or blurred vision, or with preeclampsia with severe hypertension. Because nonsteroidal anti-inflammatory drugs can be associated with elevated BP, these medications should be replaced by other analgesics in women with hypertension that persists for more than 1 day postpartum.
Prevention of preeclampsia
Given the significant maternal, fetal, and neonatal complications associated with preeclampsia, a number of studies have sought to determine ways in which this condition can be prevented. Currently, although no interventions appear to prevent preeclampsia in all patients, significant strides have been made in prevention for high-risk patients. Specifically, beginning low-dosage aspirin (most commonly, 81 mg/d, beginning at less than 16 weeks of gestation) has been shown to mitigate—although not eliminate—risk in patients with a history of preeclampsia and those who have chronic hypertension, multifetal gestation, pregestational diabetes, renal disease, SLE, or antiphospholipid syndrome.26,27Aspirin appears to act by preferentially blocking production of thromboxane, thus reducing the vasoconstrictive properties of this hormone.
Summing up
Hypertensive disorders during pregnancy are associated with significant morbidity and mortality for mother, fetus, and newborn. Preeclampsia, specifically, is recognized as a dynamic and progressive disease that has the potential to involve multiple organ systems, might present for the first time after delivery, and might be associated with long-term risk of hypertension, heart disease, stroke, and venous thromboembolism.28,29
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991-2003. Am J Obstet Gynecol. 2008; 199:133.e1-e8.
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299-1306.
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
- Wheeler TL 2nd, Blackhurst DW, Dellinger EH, Ramsey PS. Usage of spot urine protein to creatinine ratios in the evaluation of preeclampsia. Am J Obstet Gynecol. 2007;196:465.e1-e4.
- Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LL. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. CG107, August 2010. https://www.nice.org.uk/guidance/cg107. Accessed August 27, 2018. Last updated January 2011.
- Spong CY, Mercer BM, D'Alton M, et al. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118:323-333.
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100(2):369-377.
- Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers or aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115:1052-1061.
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "great obstetrical syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193-201.
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970-975.
- The American College of Obstetricians and Gynecologists Committee on Obstetric Practice; El-Sayed YY, Borders AE. Committee Opinion Number 692. Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period; April 2017. https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co692.pdf?dmc=1. Accessed August 8, 2018.
- Hollander JE. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
- Ghuran A, Nolan J. Recreational drug misuse: issues for the cardiologist. Heart. 2000;83:627-633.
- Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
- Sibai BM. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks' gestation. Am J Obstet Gynecol. 2011;205:191-198.
- Norwitz E, Funai E. Expectant management of severe preeclampsia remote from term: hope for the best, but expect the worst. Am J Obstet Gynecol. 2008;199:209-212.
- Gordon R, Magee LA, Payne B, et al. Magnesium sulphate for the management of preeclampsia and eclampsia in low and middle income countries: a systematic review of tested dosing regimens. J Obstet Gynaecol Can. 2014;36(2):154-163.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402-410.
- Liu S, Joseph KS, Liston, RM, et al; Maternal Health Study Group of Canadian Perinatal Surveillance System (Public Health Agency of Canada). Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987-994.
- Yancey LM, Withers E, Bakes K, Abbot J. Postpartum preeclampsia: emergency department presentation and management. J Emerg Med. 2011;40:380-384.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206:470-475.
- You WB, Wolf MS, Bailey SC, Grobman WA. Improving patient understanding of preeclampsia: a randomized controlled trial. Am J Obstet Gynecol. 2012;206:431.e1-e5.
- Henderson JT, Whitlock EP, O'Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110-120.e6.
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974-986.
- McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918-930.
- Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991-2003. Am J Obstet Gynecol. 2008; 199:133.e1-e8.
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299-1306.
- The American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. November 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. Accessed August 8, 2018.
- Wheeler TL 2nd, Blackhurst DW, Dellinger EH, Ramsey PS. Usage of spot urine protein to creatinine ratios in the evaluation of preeclampsia. Am J Obstet Gynecol. 2007;196:465.e1-e4.
- Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L, Chappell LL. Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ. 2014;348:g2301.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. CG107, August 2010. https://www.nice.org.uk/guidance/cg107. Accessed August 27, 2018. Last updated January 2011.
- Spong CY, Mercer BM, D'Alton M, et al. Timing of indicated late-preterm and early-term birth. Obstet Gynecol. 2011;118:323-333.
- Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100(2):369-377.
- Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers or aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115:1052-1061.
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The "great obstetrical syndromes" are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193-201.
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51:970-975.
- The American College of Obstetricians and Gynecologists Committee on Obstetric Practice; El-Sayed YY, Borders AE. Committee Opinion Number 692. Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period; April 2017. https://www.acog.org/-/media/Committee-Opinions/Committee-on-Obstetric-Practice/co692.pdf?dmc=1. Accessed August 8, 2018.
- Hollander JE. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
- Ghuran A, Nolan J. Recreational drug misuse: issues for the cardiologist. Heart. 2000;83:627-633.
- Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877-1890.
- Sibai BM. Magnesium sulfate prophylaxis in preeclampsia: lessons learned from recent trials. Am J Obstet Gynecol. 2004;190:1520-1526.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
- Publications Committee, Society for Maternal-Fetal Medicine, Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks' gestation. Am J Obstet Gynecol. 2011;205:191-198.
- Norwitz E, Funai E. Expectant management of severe preeclampsia remote from term: hope for the best, but expect the worst. Am J Obstet Gynecol. 2008;199:209-212.
- Gordon R, Magee LA, Payne B, et al. Magnesium sulphate for the management of preeclampsia and eclampsia in low and middle income countries: a systematic review of tested dosing regimens. J Obstet Gynaecol Can. 2014;36(2):154-163.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402-410.
- Liu S, Joseph KS, Liston, RM, et al; Maternal Health Study Group of Canadian Perinatal Surveillance System (Public Health Agency of Canada). Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987-994.
- Yancey LM, Withers E, Bakes K, Abbot J. Postpartum preeclampsia: emergency department presentation and management. J Emerg Med. 2011;40:380-384.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206:470-475.
- You WB, Wolf MS, Bailey SC, Grobman WA. Improving patient understanding of preeclampsia: a randomized controlled trial. Am J Obstet Gynecol. 2012;206:431.e1-e5.
- Henderson JT, Whitlock EP, O'Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(2):110-120.e6.
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974-986.
- McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918-930.
Making a difference: ACOG’s guidance on low-dose aspirin for preventing superimposed preeclampsia
Investigators at Thomas Jefferson University found that low-dose aspirin therapy in pregnant women with chronic hypertension—as recommended by the American College of Obstetricians and Gynecologists (ACOG) in 20161—was associated with a 57% decrease in superimposed preeclampsia. Chaitra Banala, BS, presented the study’s results in a poster presentation at the ACOG 2018 annual meeting (April 27–30, 2018, Austin, Texas).2

The study’s goal was to evaluate the incidence of superimposed preeclampsia in women with chronic hypertension in the periods before and after the ACOG recommendation was published.
Study participants. Pregnant women with chronic hypertension who delivered at Thomas Jefferson University Hospital from January 2008 to July 2017 were included in this retrospective cohort study. Women with multiple gestations were excluded.
The cohort included 715 pregnant patients with chronic hypertension divided into 2 groups: 635 pre-ACOG patients and 80 post-ACOG patients (that is, patients who delivered before and after the ACOG recommendation). The investigators offered daily low-dose (81 mg) aspirin.
The cohort was further stratified by additional risk factors for superimposed preeclampsia, including a history of preeclampsia and pregestational diabetes.
Outcomes. The primary outcome was the incidence of superimposed preeclampsia. Secondary outcomes included the incidence of superimposed preeclampsia with severe features (SIPSF), small for gestational age, and preterm birth.
Findings. The incidence of superimposed preeclampsia in women with chronic hypertension was 20 (25%) in the post-ACOG group versus 232 (37%) in the pre-ACOG group (odds ratio [OR], 0.43; 95% confidence interval [CI], 0.26–0.73).
In the subgroup of women with chronic hypertension who did not have other risk factors, superimposed preeclampsia and SIPSF were significantly decreased: 4/41 (10%) versus 106/355 (30%) (OR, 0.25 [95% CI, 0.08–0.73]) and 2/41 (5%) versus 65/355 (18%) (OR, 0.22 [95% CI, 0.54–0.97]), respectively. The maternal demographics and secondary outcomes did not differ significantly.
After the ACOG guidance was released, low-dose aspirin decreased superimposed preeclampsia by 57% in all women with chronic hypertension. Of those with chronic hypertension without other risk factors, there were decreases of 75% in superimposed preeclampsia and 78% in SIPSF.
Final thoughts. Ms. Banala said in an interview with OBG Management following her presentation, “When we stratified the cohort based on their risk factors, we found that aspirin had the highest benefit in patients with only chronic hypertension, so without other risk factors. And we found that there was a benefit in patients with chronic hypertension who were not on antihypertensive medication. So overall our study concluded that this guideline has made a significant impact in decreasing the frequency of superimposed preeclampsia.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Low-Dose-Aspirin-and-Prevention-of-Preeclampsia-Updated-Recommendations. Published July 11, 2016. Accessed May 3, 2018.
- Banala C, Cruz Y, Moreno C, Schoen C, Berghella V, Roman A. Impact of ACOG guideline regarding low dose aspirin for prevention of superimposed preeclampsia [abstract 27O]. Obstet Gynecol. 2018;131(suppl 1):170S.
Investigators at Thomas Jefferson University found that low-dose aspirin therapy in pregnant women with chronic hypertension—as recommended by the American College of Obstetricians and Gynecologists (ACOG) in 20161—was associated with a 57% decrease in superimposed preeclampsia. Chaitra Banala, BS, presented the study’s results in a poster presentation at the ACOG 2018 annual meeting (April 27–30, 2018, Austin, Texas).2

The study’s goal was to evaluate the incidence of superimposed preeclampsia in women with chronic hypertension in the periods before and after the ACOG recommendation was published.
Study participants. Pregnant women with chronic hypertension who delivered at Thomas Jefferson University Hospital from January 2008 to July 2017 were included in this retrospective cohort study. Women with multiple gestations were excluded.
The cohort included 715 pregnant patients with chronic hypertension divided into 2 groups: 635 pre-ACOG patients and 80 post-ACOG patients (that is, patients who delivered before and after the ACOG recommendation). The investigators offered daily low-dose (81 mg) aspirin.
The cohort was further stratified by additional risk factors for superimposed preeclampsia, including a history of preeclampsia and pregestational diabetes.
Outcomes. The primary outcome was the incidence of superimposed preeclampsia. Secondary outcomes included the incidence of superimposed preeclampsia with severe features (SIPSF), small for gestational age, and preterm birth.
Findings. The incidence of superimposed preeclampsia in women with chronic hypertension was 20 (25%) in the post-ACOG group versus 232 (37%) in the pre-ACOG group (odds ratio [OR], 0.43; 95% confidence interval [CI], 0.26–0.73).
In the subgroup of women with chronic hypertension who did not have other risk factors, superimposed preeclampsia and SIPSF were significantly decreased: 4/41 (10%) versus 106/355 (30%) (OR, 0.25 [95% CI, 0.08–0.73]) and 2/41 (5%) versus 65/355 (18%) (OR, 0.22 [95% CI, 0.54–0.97]), respectively. The maternal demographics and secondary outcomes did not differ significantly.
After the ACOG guidance was released, low-dose aspirin decreased superimposed preeclampsia by 57% in all women with chronic hypertension. Of those with chronic hypertension without other risk factors, there were decreases of 75% in superimposed preeclampsia and 78% in SIPSF.
Final thoughts. Ms. Banala said in an interview with OBG Management following her presentation, “When we stratified the cohort based on their risk factors, we found that aspirin had the highest benefit in patients with only chronic hypertension, so without other risk factors. And we found that there was a benefit in patients with chronic hypertension who were not on antihypertensive medication. So overall our study concluded that this guideline has made a significant impact in decreasing the frequency of superimposed preeclampsia.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
Investigators at Thomas Jefferson University found that low-dose aspirin therapy in pregnant women with chronic hypertension—as recommended by the American College of Obstetricians and Gynecologists (ACOG) in 20161—was associated with a 57% decrease in superimposed preeclampsia. Chaitra Banala, BS, presented the study’s results in a poster presentation at the ACOG 2018 annual meeting (April 27–30, 2018, Austin, Texas).2

The study’s goal was to evaluate the incidence of superimposed preeclampsia in women with chronic hypertension in the periods before and after the ACOG recommendation was published.
Study participants. Pregnant women with chronic hypertension who delivered at Thomas Jefferson University Hospital from January 2008 to July 2017 were included in this retrospective cohort study. Women with multiple gestations were excluded.
The cohort included 715 pregnant patients with chronic hypertension divided into 2 groups: 635 pre-ACOG patients and 80 post-ACOG patients (that is, patients who delivered before and after the ACOG recommendation). The investigators offered daily low-dose (81 mg) aspirin.
The cohort was further stratified by additional risk factors for superimposed preeclampsia, including a history of preeclampsia and pregestational diabetes.
Outcomes. The primary outcome was the incidence of superimposed preeclampsia. Secondary outcomes included the incidence of superimposed preeclampsia with severe features (SIPSF), small for gestational age, and preterm birth.
Findings. The incidence of superimposed preeclampsia in women with chronic hypertension was 20 (25%) in the post-ACOG group versus 232 (37%) in the pre-ACOG group (odds ratio [OR], 0.43; 95% confidence interval [CI], 0.26–0.73).
In the subgroup of women with chronic hypertension who did not have other risk factors, superimposed preeclampsia and SIPSF were significantly decreased: 4/41 (10%) versus 106/355 (30%) (OR, 0.25 [95% CI, 0.08–0.73]) and 2/41 (5%) versus 65/355 (18%) (OR, 0.22 [95% CI, 0.54–0.97]), respectively. The maternal demographics and secondary outcomes did not differ significantly.
After the ACOG guidance was released, low-dose aspirin decreased superimposed preeclampsia by 57% in all women with chronic hypertension. Of those with chronic hypertension without other risk factors, there were decreases of 75% in superimposed preeclampsia and 78% in SIPSF.
Final thoughts. Ms. Banala said in an interview with OBG Management following her presentation, “When we stratified the cohort based on their risk factors, we found that aspirin had the highest benefit in patients with only chronic hypertension, so without other risk factors. And we found that there was a benefit in patients with chronic hypertension who were not on antihypertensive medication. So overall our study concluded that this guideline has made a significant impact in decreasing the frequency of superimposed preeclampsia.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Low-Dose-Aspirin-and-Prevention-of-Preeclampsia-Updated-Recommendations. Published July 11, 2016. Accessed May 3, 2018.
- Banala C, Cruz Y, Moreno C, Schoen C, Berghella V, Roman A. Impact of ACOG guideline regarding low dose aspirin for prevention of superimposed preeclampsia [abstract 27O]. Obstet Gynecol. 2018;131(suppl 1):170S.
- American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Low-Dose-Aspirin-and-Prevention-of-Preeclampsia-Updated-Recommendations. Published July 11, 2016. Accessed May 3, 2018.
- Banala C, Cruz Y, Moreno C, Schoen C, Berghella V, Roman A. Impact of ACOG guideline regarding low dose aspirin for prevention of superimposed preeclampsia [abstract 27O]. Obstet Gynecol. 2018;131(suppl 1):170S.
Take action to prevent maternal mortality
The facts
While other industrialized nations are seeing a decrease in their maternal mortality rates, the United States has noted a 26% increase over a 15-year period. This is especially true for women of color: black women are nearly 4 times as likely to die from pregnancy related causes as compared to non-Hispanic white women. Postpartum hemorrhage and preeclampsia are often the leading causes of maternal death; however, suicide and overdoses are becoming increasingly more common. This information is highlighted in the March 2018 OBG Management article “Factors critical to reducing US maternal mortality and morbidity,” by Lucia DiVenere, MA, Government and Political Affairs, at the American College of Obstetricians and Gynecologists (ACOG).1
Although there are efforts to improve these outcomes, programs vary by state. One initiative is the perinatal quality collaboratives (PQCs), state or multistate networks of teams working to improve the quality of care for mothers and babies (see “Has your state established a perinatal quality collaborative?”).
Currently, only 33 states have a maternal mortality review committee (MMRC) comprised of an interdisciplinary team of ObGyns, nurses, and other stakeholders. The MMRC reviews each maternal death in their state and provides recommendations and policy changes to help prevent further loss of life.
Many states currently have active collaboratives, and others are in development. The CDC’s Division of Reproductive Health (DRH) currently provides support for state-based PQCs in Colorado, Delaware, Florida, Georgia, Illinois, Louisiana, Massachusetts, Minnesota, Mississippi, New Jersey, New York, Oregon, and Wisconsin. The status of PQCs in Maine, Rhode Island, Pennsylvania, Missouri, South Dakota, and Wyoming is unknown.1
The CDC can help people establish a collaborative. Visit: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html.
Reference
- Centers for Disease Control and Prevention. Reproductive health: State Perinatal Quality Collaboratives. CDC website. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html Updated October 17, 2017. Accessed April 4, 2018.
The bill
Preventing Maternal Deaths Act/Maternal Health Accountability Act (H.R. 1318/S. 1112) is a bipartisan, bicameral effort to reduce maternal mortality and reduce health care disparities.
The bills authorize the Centers for Disease Control and Prevention (CDC) to help states create or expand state MMRCs through annual grant funding of $7 million through fiscal year 2022. Through the MMRCs, the CDC would have the ability to gather data on maternal mortality and health care disparities, allowing the agency to better understand leading causes of maternal death as well as a state’s successes and pitfalls in interventions.
Currently the House bill (H.R. 1318) has 102 cosponsors (https://cqrcengage.com/acog/app/bill/903056?0) and the Senate bill (S. 1112) has 17 cosponsors (https://cqrcengage.com/acog/app/bill/943204?1). Click these links to see if your representative is a cosponsor.
Not sure who your representative is? Click here to find out: http://cqrcengage.com/acog/app/lookup?1&m=29525.
Take action
Both the Senate and House bills have been referred to health committees. However, no advances have been made since March 2017. In order for the bills to move forward, your representatives need to hear from you.
If your representative is a cosponsor of the bill, thank them for their support, but also ask what we can do to ensure this bill becomes law.
If your representative is not a cosponsor, follow this link to email your representative: http://cqrcengage.com/acog/app/onestep-write-a-letter?0&engagementId=306574. You also can call your representative’s office and speak directly to a staff member.
When calling or emailing, highlight the following:
- I am an ObGyn and I am asking [your Representative/Senator] to support H.R. 1318 or S. 1112.
- While maternal mortality rates are decreasing in other parts of the world, they are increasing in the United States. We have the highest maternal mortality rate in the developing world.
- This bill gives all states the opportunity to have a maternal mortality review committee, allowing health care leaders to review each maternal death and analyze how further deaths can be prevented.
- Congress has invested in programs addressing infant mortality, birth defects, and preterm birth. It is time we put the same investment into saving our nation’s mothers.
- As an ObGyn, I urge you to support this bill.
More from ACOG
Want to know what other advocacy opportunities are available? Check out ACOG action at http://cqrcengage.com/acog/home?3.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- DiVenere L. Factors critical to reducing US maternal mortality and morbidity. OBG Manag. 2018;30(3):30−33.
The facts
While other industrialized nations are seeing a decrease in their maternal mortality rates, the United States has noted a 26% increase over a 15-year period. This is especially true for women of color: black women are nearly 4 times as likely to die from pregnancy related causes as compared to non-Hispanic white women. Postpartum hemorrhage and preeclampsia are often the leading causes of maternal death; however, suicide and overdoses are becoming increasingly more common. This information is highlighted in the March 2018 OBG Management article “Factors critical to reducing US maternal mortality and morbidity,” by Lucia DiVenere, MA, Government and Political Affairs, at the American College of Obstetricians and Gynecologists (ACOG).1
Although there are efforts to improve these outcomes, programs vary by state. One initiative is the perinatal quality collaboratives (PQCs), state or multistate networks of teams working to improve the quality of care for mothers and babies (see “Has your state established a perinatal quality collaborative?”).
Currently, only 33 states have a maternal mortality review committee (MMRC) comprised of an interdisciplinary team of ObGyns, nurses, and other stakeholders. The MMRC reviews each maternal death in their state and provides recommendations and policy changes to help prevent further loss of life.

Many states currently have active collaboratives, and others are in development. The CDC’s Division of Reproductive Health (DRH) currently provides support for state-based PQCs in Colorado, Delaware, Florida, Georgia, Illinois, Louisiana, Massachusetts, Minnesota, Mississippi, New Jersey, New York, Oregon, and Wisconsin. The status of PQCs in Maine, Rhode Island, Pennsylvania, Missouri, South Dakota, and Wyoming is unknown.1
The CDC can help people establish a collaborative. Visit: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html.
Reference
- Centers for Disease Control and Prevention. Reproductive health: State Perinatal Quality Collaboratives. CDC website. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html Updated October 17, 2017. Accessed April 4, 2018.
The bill
Preventing Maternal Deaths Act/Maternal Health Accountability Act (H.R. 1318/S. 1112) is a bipartisan, bicameral effort to reduce maternal mortality and reduce health care disparities.
The bills authorize the Centers for Disease Control and Prevention (CDC) to help states create or expand state MMRCs through annual grant funding of $7 million through fiscal year 2022. Through the MMRCs, the CDC would have the ability to gather data on maternal mortality and health care disparities, allowing the agency to better understand leading causes of maternal death as well as a state’s successes and pitfalls in interventions.
Currently the House bill (H.R. 1318) has 102 cosponsors (https://cqrcengage.com/acog/app/bill/903056?0) and the Senate bill (S. 1112) has 17 cosponsors (https://cqrcengage.com/acog/app/bill/943204?1). Click these links to see if your representative is a cosponsor.
Not sure who your representative is? Click here to find out: http://cqrcengage.com/acog/app/lookup?1&m=29525.
Take action
Both the Senate and House bills have been referred to health committees. However, no advances have been made since March 2017. In order for the bills to move forward, your representatives need to hear from you.
If your representative is a cosponsor of the bill, thank them for their support, but also ask what we can do to ensure this bill becomes law.
If your representative is not a cosponsor, follow this link to email your representative: http://cqrcengage.com/acog/app/onestep-write-a-letter?0&engagementId=306574. You also can call your representative’s office and speak directly to a staff member.
When calling or emailing, highlight the following:
- I am an ObGyn and I am asking [your Representative/Senator] to support H.R. 1318 or S. 1112.
- While maternal mortality rates are decreasing in other parts of the world, they are increasing in the United States. We have the highest maternal mortality rate in the developing world.
- This bill gives all states the opportunity to have a maternal mortality review committee, allowing health care leaders to review each maternal death and analyze how further deaths can be prevented.
- Congress has invested in programs addressing infant mortality, birth defects, and preterm birth. It is time we put the same investment into saving our nation’s mothers.
- As an ObGyn, I urge you to support this bill.
More from ACOG
Want to know what other advocacy opportunities are available? Check out ACOG action at http://cqrcengage.com/acog/home?3.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
The facts
While other industrialized nations are seeing a decrease in their maternal mortality rates, the United States has noted a 26% increase over a 15-year period. This is especially true for women of color: black women are nearly 4 times as likely to die from pregnancy related causes as compared to non-Hispanic white women. Postpartum hemorrhage and preeclampsia are often the leading causes of maternal death; however, suicide and overdoses are becoming increasingly more common. This information is highlighted in the March 2018 OBG Management article “Factors critical to reducing US maternal mortality and morbidity,” by Lucia DiVenere, MA, Government and Political Affairs, at the American College of Obstetricians and Gynecologists (ACOG).1
Although there are efforts to improve these outcomes, programs vary by state. One initiative is the perinatal quality collaboratives (PQCs), state or multistate networks of teams working to improve the quality of care for mothers and babies (see “Has your state established a perinatal quality collaborative?”).
Currently, only 33 states have a maternal mortality review committee (MMRC) comprised of an interdisciplinary team of ObGyns, nurses, and other stakeholders. The MMRC reviews each maternal death in their state and provides recommendations and policy changes to help prevent further loss of life.

Many states currently have active collaboratives, and others are in development. The CDC’s Division of Reproductive Health (DRH) currently provides support for state-based PQCs in Colorado, Delaware, Florida, Georgia, Illinois, Louisiana, Massachusetts, Minnesota, Mississippi, New Jersey, New York, Oregon, and Wisconsin. The status of PQCs in Maine, Rhode Island, Pennsylvania, Missouri, South Dakota, and Wyoming is unknown.1
The CDC can help people establish a collaborative. Visit: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html.
Reference
- Centers for Disease Control and Prevention. Reproductive health: State Perinatal Quality Collaboratives. CDC website. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html Updated October 17, 2017. Accessed April 4, 2018.
The bill
Preventing Maternal Deaths Act/Maternal Health Accountability Act (H.R. 1318/S. 1112) is a bipartisan, bicameral effort to reduce maternal mortality and reduce health care disparities.
The bills authorize the Centers for Disease Control and Prevention (CDC) to help states create or expand state MMRCs through annual grant funding of $7 million through fiscal year 2022. Through the MMRCs, the CDC would have the ability to gather data on maternal mortality and health care disparities, allowing the agency to better understand leading causes of maternal death as well as a state’s successes and pitfalls in interventions.
Currently the House bill (H.R. 1318) has 102 cosponsors (https://cqrcengage.com/acog/app/bill/903056?0) and the Senate bill (S. 1112) has 17 cosponsors (https://cqrcengage.com/acog/app/bill/943204?1). Click these links to see if your representative is a cosponsor.
Not sure who your representative is? Click here to find out: http://cqrcengage.com/acog/app/lookup?1&m=29525.
Take action
Both the Senate and House bills have been referred to health committees. However, no advances have been made since March 2017. In order for the bills to move forward, your representatives need to hear from you.
If your representative is a cosponsor of the bill, thank them for their support, but also ask what we can do to ensure this bill becomes law.
If your representative is not a cosponsor, follow this link to email your representative: http://cqrcengage.com/acog/app/onestep-write-a-letter?0&engagementId=306574. You also can call your representative’s office and speak directly to a staff member.
When calling or emailing, highlight the following:
- I am an ObGyn and I am asking [your Representative/Senator] to support H.R. 1318 or S. 1112.
- While maternal mortality rates are decreasing in other parts of the world, they are increasing in the United States. We have the highest maternal mortality rate in the developing world.
- This bill gives all states the opportunity to have a maternal mortality review committee, allowing health care leaders to review each maternal death and analyze how further deaths can be prevented.
- Congress has invested in programs addressing infant mortality, birth defects, and preterm birth. It is time we put the same investment into saving our nation’s mothers.
- As an ObGyn, I urge you to support this bill.
More from ACOG
Want to know what other advocacy opportunities are available? Check out ACOG action at http://cqrcengage.com/acog/home?3.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- DiVenere L. Factors critical to reducing US maternal mortality and morbidity. OBG Manag. 2018;30(3):30−33.
- DiVenere L. Factors critical to reducing US maternal mortality and morbidity. OBG Manag. 2018;30(3):30−33.
The role of patient-reported outcomes in women’s health
In its landmark publication, “Crossing the quality chasm: A new health system for the 21st century,” the Institute of Medicine (now the National Academy of Medicine) called for an emphasis on patient-centered care that it defined as “Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”1 Studies suggest that the patient’s view of health care delivery determines outcome and satisfaction.2 Therefore, we need to expend more effort to understand what patients need or want from their treatment or interaction with the health care system.
Measuring patient-reported outcomes (PROs) is an attempt to recognize and address patient concerns. Although currently PROs are focused primarily in the arena of clinical research, their use has the potential to transform daily clinical patient encounters and improve the cost and quality of health care.3
In this article, we provide a brief overview of PROs and describe how they can be used to improve individual patient care, clinical research, and health care quality. We also offer examples of how PROs can be used in specific women’s health conditions.

What exactly are PROs?
PROs are reports of the status of a patient’s health condition, health behavior, or experience with health care; they come directly from the patient, without anyone else (such as a clinician or caregiver) interpreting the patient’s response.4 PROs usually pertain to general health, quality of life, functional status, or preferences associated with health care or treatment.5 Usually PROs are elicited via a self-administered survey and provide the patient’s perspective on treatment benefits, side effects, change in symptoms, general perceptions of feelings or well-being, or satisfaction with care. Often they represent the outcomes that are most important to patients.6 The survey usually consists of several questions or items. It can be general or condition specific, and it may represent one or more health care dimensions.
The term patient-reported outcome measure (PROM) refers to the survey instrument used to collect PROs. Patient-reported experience measures (PREMs), such as satisfaction surveys, are considered a subset of PROMs.7
Standardized PROs developed out of clinical trials
The use of PROs evolved from clinical trials. The proliferation of PROs resulted in an inability to compare outcomes across trials or different conditions. This led to a need to standardize and possibly harmonize measures and to reach consensus about properties required for a “good” measure and requirements needed for “adequate” reporting. Many investigators and several national and international organizations have provided iterative guidance, including the US Food and Drug Administration (FDA), European Medicines Agency, National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), International Consortium for Health Outcomes Measurement (ICHOM), University of Oxford Patient Reported Outcomes Measurement Group, Cochrane Systematic Reviews, Consolidated Standards of Reporting Trials–Patient Reported Outcomes (CONSORT-PRO) extension (how to report PROs with the CONSORT checklist), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).4,5,8–18
In the United States, the RAND Medical Outcomes Study led to the development of the 12- and 36-item short form surveys, which are widely recognized and commonly used PROMs for health-related quality of life.19 The study generated multiple additional survey instruments that evaluate other domains and dimensions of health. These surveys have been translated into numerous languages, and the RAND website lists over 100 publications.19
In 2002, the NIH sponsored PROMIS, a cooperative program designed to develop, validate, and standardize item banks to measure PROs that were relevant across multiple, common medical conditions. Based on literature review, feedback from both healthy and sick patients, and clinical expert opinion, the PROMIS investigators developed a consensus-based framework for self-reported health that included the following domains: pain, fatigue, emotional distress, physical functioning, and social role participation; these domains were evaluated on paper or with computer-assisted technology.11–14 PROMIS is now a web-based resource with approximately 70 domains pertinent to children and adults in the general population and in those with chronic disease. Measures have been translated into more than 40 languages, and PROMIS-related work has resulted in more than 400 publications.14
In 2006, the FDA issued a draft document regarding the PRO standards that should be included in clinical trials for consideration of drug and device applications (TABLE 1). These recommendations, updated in 2009, were largely drawn from work published by PROMIS and University of Oxford investigators.4,14,16
Because PROs are infrequently measured in routine clinical practice and PROMs that are used vary between countries, global comparison is difficult. Hence, ICHOM convened in 2012 to develop consensus-based, globally agreed on sets of outcomes that are intended to reflect what matters most to patients.
ICHOM specified 2 goals: 1) the core sets should be used in routine clinical practice, and 2) the core sets should be used as end points in clinical studies.15
As of May 2015, 12 standard sets of outcomes have been developed, representing 35% of the global burden of disease. ICHOM currently is creating networks of hospitals around the world to begin measuring, benchmarking, and performing outcome comparisons that can ultimately be used to inform global health system learning and clinical care improvement.15
Read about the evolving use of PROs
Use of PROs is evolving
Historically, PROMs have been used primarily in clinical trials to document the relative benefits of an intervention. With today’s focus on patient-centered care, however, there is a growing mandate to integrate PROMs into clinical care, quality improvement, and ultimately reimbursement. Recently, Basch and colleagues eloquently described the benefit of routine collection of PROs for cancer patients and the opportunity for improved care across the health system.20
PROs can be applied on various levels. For example, if a patient reports a symptom (X), or a change in symptom X, the following options are possible:
- Clinician level: Symptom management with altered dose or change in medication. This is associated with improved self-efficacy for the patient, a shift toward goal-oriented care, improved communication with the provider, and improved patient satisfaction.
- Researcher level: PROs should be used as a primary end point, in addition to traditional outcomes (mortality, survival, physiologic markers), to allow for comparative effectiveness studies or patient-centered outcomes research studies that evaluate what matters most to patients relative to the specific health condition, intervention, and symptom management.
- Health system level: Quality assurance, quality improvement activities. How effective is the health system in the management of symptom X? Are all clinicians using the same medication or the same dose? Is there a best practice for managing symptom X?
- Population level: Provides evidence for other clinicians and patients to make decisions about what to expect with treatment for symptom X.
From a reimbursement level, clinicians and providers are paid based on performance—the more satisfied patients are about X, the higher the reimbursement. This has been pertinent particularly in high-volume orthopedic conditions in which anatomic correction of hip or knee joints has not consistently demonstrated improvement in quality of life as measured by the following PROs: perception of pain, mobility, physical functioning, social functioning, and emotional distress. Because of concerns about high volume, high cost, and inconsistent outcomes, the US Department of Health and Human Services has specified that 50% of Medicare and 90% of Medicaid reimbursements will be based on outcomes or value-based purchasing options.21
Studies have shown that it is possible to collect PRO data for cancer patients—despite age or severity of illness—and integrate it into clinical care delivery. These data can provide useful, actionable information, resulting in decreased emergency department visits, longer toleration of chemotherapy, and improved survival.22 Similar results have been demonstrated in other medical conditions, although challenges exist when transitioning from research settings to routine care. Challenges include privacy concerns, patient recruitment and tracking, encouraging patients to complete the PRO surveys (nonresponse leads to biased data), real and perceived administrative burden to staff, obtaining clinician buy in, and costs related to surveys and data analysis.23
Read about the benefits of PROs to patients and clients
Using PROs in women’s health care: Benefits for patients and clinicians
According to a study by Frosch, patients want to know if a prescribed therapy actually improves outcomes, not whether it changes an isolated biomarker that does not translate into subjective improvement.24 They want to know if the trade-off (adverse effects or higher cost) associated with a new drug or therapy is worth the improved mobility or time spent pain free.
Intuitively, all clinicians have similar opportunities for discussions with regard to the risks, benefits, and alternatives of medical treatment, surgical treatment, or expectant management. We routinely document this discussion daily. However, in this era of patient-centered care, when a patient asks, “What should I do, doctor?” we no longer can respond with a default recommendation. We must engage the patient and ask, “What do you want to do? What is most important to you?”
ObGyns are well suited to benefit from standardized efforts to collect PROs, as we frequently discuss with our patients trade-offs regarding treatment risks and benefits and their personal values and preferences. Examples include contraception options, hormone treatment for menopause, medication use during pregnancy, decisions at the limits of viability, preterm delivery for severe preeclampsia, induction/augmentation versus spontaneous labor, epidural versus physiologic labor, repeat cesarean versus vaginal birth after cesarean, and even elective primary cesarean versus vaginal birth.
Validated PROMs exist for benign gynecology, such as abnormal uterine bleeding, fibroids, polycystic ovary syndrome (PCOS), infertility, pelvic organ prolapse and/or urinary incontinence, and surgery for benign gynecology symptoms, as well as for cancer (breast, ovarian, cervical).25–39
From the PCOS literature we can glean a poignant example of the importance of PROs. Martin and colleagues compared patient and clinician interviews regarding important PROs from the patient perspective.29 Patients identified pain, cramping, heavy bleeding, and bloating as important, whereas clinicians did not consider these symptoms important to patients with PCOS. Clinicians thought “issues with menstruation,” characterized as irregular or no periods, were important, whereas patients were more concerned with heavy bleeding or bleeding of long duration. The authors concluded that concepts frequently expressed by patients and considered important from their perspective did not register with clinicians as being relevant and are not captured on current PRO instruments, emphasizing our knowledge gap and the need to pay attention to what patients want.29
Surprisingly, although pregnancy and childbirth is the number one cause for hospital admissions, a highly preference-driven condition, and a leading cause of morbidity, mortality, and costs, there are few published PROs in the field. In a systematic review of more than 1,700 articles describing PROs published in English through 2014, Martin found that fewer than 1% included PROs specific to pregnancy and childbirth.40
ICHOM has created a standard set of outcomes for pregnancy and childbirth based on consensus recommendations from physicians, measurement experts, and patients.41 The consortium describes 4 domains and 14 subdomains (TABLE 2) and provides suggestions for a validated PROM if known or where appropriate.
Similar domains and subdomains have been corroborated by our research team (the Maternal Quality Indicator [MQI] Work Group), the Childbirth Connection, and Gartner and colleagues.42–44 The MQI Work Group recently conducted a national survey of what women want and what they think is important for their childbirth experience. We identified 19 domains, consistent with those of other investigators.42 Gartner and colleagues advocate for a composite outcome measure that combines the core domains into one preference-based utility measure that is weighted.44 The rationale for this recommendation is that the levels of the domains might contribute differently to the overall birth experience. For example, communication might contribute more to an overall measure than pain management.44 The development of a childbirth-specific survey to evaluate patient-reported outcomes and patient-reported experiences with care is needed if we are to provide value-based care in this arena.45
Looking forward
PROs, PROMs, and PREMs are here to stay. They no longer are limited to clinical research, but increasingly will be incorporated into clinical care, providing us with opportunities to improve the quality of health care delivery, efficiency of patient/clinician interactions, and patients’ ratings of their health care experience.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001:6.
- Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- Rickert J. Patient-centered care: what it means and how to get there. Health Affairs website. http://healthaffairs.org/blog/2012/01/24/patient-centered-care-what-it-means-and-how-to-get-there/. Published January 24, 2012. Accessed October 15, 2017.
- US Food and Drug Administration. Guidance for industry: Patient reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Published December 2009. Accessed February 6, 2018.
- Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008. http://handbook.cochrane.org. Accessed October 15, 2017.
- Patrick DL, Guyatt PD, Acquadro C. Patient-reported outcomes. In: Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008:chap 17. http://handbook-5-1.cochrane.org/. Accessed October 15, 2017.
- Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68.
- McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163–169.
- European Medicines Agency, Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. https://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Published July 27, 2005. Accessed February 7, 2018.
- Venkatesan P. New European guidance on patient-reported outcomes. Lancet Oncol. 2016;17(6):e226.
- Cella D, Yount S, Rothrock N, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11.
- Cella D, Riley W, Stone A, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194.
- Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014;17(8):846–853.
- National Institutes of Health. Patient-Reported Outcomes Measurement Information System (PROMIS). https://commonfund.nih.gov/promis/index. Reviewed May 8, 2017. Accessed October 15, 2017.
- International Consortium for Health Outcomes Measurement (ICHOM). http://www.ichom.org/. Accessed October 15, 2017.
- University of Oxford, Patient Reported Outcomes Measurement Group http://phi.uhce.ox.ac.uk/. Accessed October 15, 2017.
- CONSORT. Patient-Reported Outcomes (CONSORT PRO). http://www.consort-statement.org/extensions/overview/consort-pro. Accessed October 15, 2017.
- International Society for Pharmacoeconomics and Outcomes Research. https://www.ispor.org/. Accessed October 15, 2017.
- RAND Health. RAND medical outcomes study: measures of quality of life core survey from RAND Health. https://www.rand.org/health/surveys_tools/mos.html. Accessed October 15, 2017.
- Basch EM, Deal AM, Dueck A, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment [abstract LBA2]. J Clin Oncol. 2017;35(18)(suppl).
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Accessed October 15, 2017.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Chenok K, Teleki S, SooHoo NF, Huddleston J, Bozic KJ. Collecting patient-reported outcomes: lessons from the California Joint Replacement Registry. EGEMS (Wash DC). 2015;3(1):1196.
- Frosch DL. Patient-reported outcomes as a measure of healthcare quality. J Gen Intern Med. 2015;30(10):1383–1384.
- Gibbons E, Mackintosh A, Fitzpatrick R; Patient-Reported Outcome Measurement Group, Oxford. A structured review of patient-reported outcome measures for people undergoing elective procedures for benign gynaecological conditions of the uterus, 2010. http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Gynaecological%20procedures_012011.pdf. Accessed October 23, 2017.
- Matteson KA, Boardman LA, Munro MG, Clark MA. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92(1):205–216.
- Matteson KA, Scott DM, Raker CA, Clark MA. The menstrual bleeding questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122(5):681–689.
- Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health. 2012;15(1):135–142.
- Martin ML, Halling K, Eek D, Krohe M, Paty J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Quality Life Outcomes. 2017;15(1):162.
- Malik-Aslam A, Reaney MD, Speight J. The suitability of polycystic ovary syndrome-specific questionnaires for measuring the impact of PCOS on quality of life in clinical trials. Value Health. 2010;13(4):440–446.
- Kitchen H, Aldhouse N, Trigg A, Palencia R, Mitchell S. A review of patient-reported outcome measures to assess female infertility-related quality of life. Health Qual Life Outcomes. 2017;15(1):86.
- Sung VW, Joo K, Marques F, Myers DL. Patient-reported outcomes after combined surgery for pelvic floor disorders in older compared to younger women. Am J Obstet Gynecol. 2009;201(5):534.e1–e5.
- Sung VW, Rogers RG, Barber MD, Clark MA. Conceptual framework for patient-important treatment outcomes for pelvic organ prolapse. Neurourol Urodynam. 2014;33(4):414–419.
- Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2016;215(5):659.e1–e7.
- Croke J. Cervical ca PROs in clinical practice. https://clinicaltrials.gov/ct2/show/NCT03048435. Accessed October 16, 2017.
- Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211–232.
- Jensen RE, Potosky AL, Moinpour CM, et al. United States population estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913–1920.
- Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials—lost opportunities and lessons learned. Ann Oncol. 2016;27(suppl 1):i66–i71.
- Joly F, Hilpert F, Okamoto A, Stuart G, Ochaia K, Friedlander M; 5th Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138.
- Martin A. Patient-reported outcomes in studies published in 2014: which disease areas have been the main focus of clinical research? Value Health. 2015;18(7):A742.
- International Consortium for Health Outcomes Management (ICHOM). Pregnancy and childbirth. http://www.ichom.org/medical-conditions/pregnancy-and-childbirth/. Accessed October 10, 2017.
- El Haj Ibrahim S, McCulloch J, Korst LM, Fridman M, Fink A, Gregory KD. Communication with staff during hospitalization for childbirth: the patient’s perspective [1R]. Obstet Gynecol. 2016;127.
- National Partnership for Women and Families. Childbirth Connection. Listening to mothers III: report of the third national US survey of women’s childbearing experience. http://transform.childbirthconnection.org/reports/listeningtomothers/. Accessed October 23, 2017.
- Gartner FR, Freeman LM, Rijnders ME, et al. A comprehensive representation of the birth-experience: identification and prioritization of birth-specific domains based on a mixed-method design. BMC Pregnancy Childbirth. 2014;14:147.
- National Partnership for Women and Families. The priority of developing and implementing CAHPS maternity care facility, clinician and health plan surveys. 2015. http://www.nationalpartnership.org/research-library/maternal-health/cahps-maternity-care-fact-sheet.pdf. Accessed October 23, 2017.
In its landmark publication, “Crossing the quality chasm: A new health system for the 21st century,” the Institute of Medicine (now the National Academy of Medicine) called for an emphasis on patient-centered care that it defined as “Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”1 Studies suggest that the patient’s view of health care delivery determines outcome and satisfaction.2 Therefore, we need to expend more effort to understand what patients need or want from their treatment or interaction with the health care system.
Measuring patient-reported outcomes (PROs) is an attempt to recognize and address patient concerns. Although currently PROs are focused primarily in the arena of clinical research, their use has the potential to transform daily clinical patient encounters and improve the cost and quality of health care.3
In this article, we provide a brief overview of PROs and describe how they can be used to improve individual patient care, clinical research, and health care quality. We also offer examples of how PROs can be used in specific women’s health conditions.

What exactly are PROs?
PROs are reports of the status of a patient’s health condition, health behavior, or experience with health care; they come directly from the patient, without anyone else (such as a clinician or caregiver) interpreting the patient’s response.4 PROs usually pertain to general health, quality of life, functional status, or preferences associated with health care or treatment.5 Usually PROs are elicited via a self-administered survey and provide the patient’s perspective on treatment benefits, side effects, change in symptoms, general perceptions of feelings or well-being, or satisfaction with care. Often they represent the outcomes that are most important to patients.6 The survey usually consists of several questions or items. It can be general or condition specific, and it may represent one or more health care dimensions.
The term patient-reported outcome measure (PROM) refers to the survey instrument used to collect PROs. Patient-reported experience measures (PREMs), such as satisfaction surveys, are considered a subset of PROMs.7
Standardized PROs developed out of clinical trials
The use of PROs evolved from clinical trials. The proliferation of PROs resulted in an inability to compare outcomes across trials or different conditions. This led to a need to standardize and possibly harmonize measures and to reach consensus about properties required for a “good” measure and requirements needed for “adequate” reporting. Many investigators and several national and international organizations have provided iterative guidance, including the US Food and Drug Administration (FDA), European Medicines Agency, National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), International Consortium for Health Outcomes Measurement (ICHOM), University of Oxford Patient Reported Outcomes Measurement Group, Cochrane Systematic Reviews, Consolidated Standards of Reporting Trials–Patient Reported Outcomes (CONSORT-PRO) extension (how to report PROs with the CONSORT checklist), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).4,5,8–18
In the United States, the RAND Medical Outcomes Study led to the development of the 12- and 36-item short form surveys, which are widely recognized and commonly used PROMs for health-related quality of life.19 The study generated multiple additional survey instruments that evaluate other domains and dimensions of health. These surveys have been translated into numerous languages, and the RAND website lists over 100 publications.19
In 2002, the NIH sponsored PROMIS, a cooperative program designed to develop, validate, and standardize item banks to measure PROs that were relevant across multiple, common medical conditions. Based on literature review, feedback from both healthy and sick patients, and clinical expert opinion, the PROMIS investigators developed a consensus-based framework for self-reported health that included the following domains: pain, fatigue, emotional distress, physical functioning, and social role participation; these domains were evaluated on paper or with computer-assisted technology.11–14 PROMIS is now a web-based resource with approximately 70 domains pertinent to children and adults in the general population and in those with chronic disease. Measures have been translated into more than 40 languages, and PROMIS-related work has resulted in more than 400 publications.14
In 2006, the FDA issued a draft document regarding the PRO standards that should be included in clinical trials for consideration of drug and device applications (TABLE 1). These recommendations, updated in 2009, were largely drawn from work published by PROMIS and University of Oxford investigators.4,14,16
Because PROs are infrequently measured in routine clinical practice and PROMs that are used vary between countries, global comparison is difficult. Hence, ICHOM convened in 2012 to develop consensus-based, globally agreed on sets of outcomes that are intended to reflect what matters most to patients.
ICHOM specified 2 goals: 1) the core sets should be used in routine clinical practice, and 2) the core sets should be used as end points in clinical studies.15
As of May 2015, 12 standard sets of outcomes have been developed, representing 35% of the global burden of disease. ICHOM currently is creating networks of hospitals around the world to begin measuring, benchmarking, and performing outcome comparisons that can ultimately be used to inform global health system learning and clinical care improvement.15
Read about the evolving use of PROs
Use of PROs is evolving
Historically, PROMs have been used primarily in clinical trials to document the relative benefits of an intervention. With today’s focus on patient-centered care, however, there is a growing mandate to integrate PROMs into clinical care, quality improvement, and ultimately reimbursement. Recently, Basch and colleagues eloquently described the benefit of routine collection of PROs for cancer patients and the opportunity for improved care across the health system.20
PROs can be applied on various levels. For example, if a patient reports a symptom (X), or a change in symptom X, the following options are possible:
- Clinician level: Symptom management with altered dose or change in medication. This is associated with improved self-efficacy for the patient, a shift toward goal-oriented care, improved communication with the provider, and improved patient satisfaction.
- Researcher level: PROs should be used as a primary end point, in addition to traditional outcomes (mortality, survival, physiologic markers), to allow for comparative effectiveness studies or patient-centered outcomes research studies that evaluate what matters most to patients relative to the specific health condition, intervention, and symptom management.
- Health system level: Quality assurance, quality improvement activities. How effective is the health system in the management of symptom X? Are all clinicians using the same medication or the same dose? Is there a best practice for managing symptom X?
- Population level: Provides evidence for other clinicians and patients to make decisions about what to expect with treatment for symptom X.
From a reimbursement level, clinicians and providers are paid based on performance—the more satisfied patients are about X, the higher the reimbursement. This has been pertinent particularly in high-volume orthopedic conditions in which anatomic correction of hip or knee joints has not consistently demonstrated improvement in quality of life as measured by the following PROs: perception of pain, mobility, physical functioning, social functioning, and emotional distress. Because of concerns about high volume, high cost, and inconsistent outcomes, the US Department of Health and Human Services has specified that 50% of Medicare and 90% of Medicaid reimbursements will be based on outcomes or value-based purchasing options.21
Studies have shown that it is possible to collect PRO data for cancer patients—despite age or severity of illness—and integrate it into clinical care delivery. These data can provide useful, actionable information, resulting in decreased emergency department visits, longer toleration of chemotherapy, and improved survival.22 Similar results have been demonstrated in other medical conditions, although challenges exist when transitioning from research settings to routine care. Challenges include privacy concerns, patient recruitment and tracking, encouraging patients to complete the PRO surveys (nonresponse leads to biased data), real and perceived administrative burden to staff, obtaining clinician buy in, and costs related to surveys and data analysis.23
Read about the benefits of PROs to patients and clients
Using PROs in women’s health care: Benefits for patients and clinicians
According to a study by Frosch, patients want to know if a prescribed therapy actually improves outcomes, not whether it changes an isolated biomarker that does not translate into subjective improvement.24 They want to know if the trade-off (adverse effects or higher cost) associated with a new drug or therapy is worth the improved mobility or time spent pain free.
Intuitively, all clinicians have similar opportunities for discussions with regard to the risks, benefits, and alternatives of medical treatment, surgical treatment, or expectant management. We routinely document this discussion daily. However, in this era of patient-centered care, when a patient asks, “What should I do, doctor?” we no longer can respond with a default recommendation. We must engage the patient and ask, “What do you want to do? What is most important to you?”
ObGyns are well suited to benefit from standardized efforts to collect PROs, as we frequently discuss with our patients trade-offs regarding treatment risks and benefits and their personal values and preferences. Examples include contraception options, hormone treatment for menopause, medication use during pregnancy, decisions at the limits of viability, preterm delivery for severe preeclampsia, induction/augmentation versus spontaneous labor, epidural versus physiologic labor, repeat cesarean versus vaginal birth after cesarean, and even elective primary cesarean versus vaginal birth.
Validated PROMs exist for benign gynecology, such as abnormal uterine bleeding, fibroids, polycystic ovary syndrome (PCOS), infertility, pelvic organ prolapse and/or urinary incontinence, and surgery for benign gynecology symptoms, as well as for cancer (breast, ovarian, cervical).25–39
From the PCOS literature we can glean a poignant example of the importance of PROs. Martin and colleagues compared patient and clinician interviews regarding important PROs from the patient perspective.29 Patients identified pain, cramping, heavy bleeding, and bloating as important, whereas clinicians did not consider these symptoms important to patients with PCOS. Clinicians thought “issues with menstruation,” characterized as irregular or no periods, were important, whereas patients were more concerned with heavy bleeding or bleeding of long duration. The authors concluded that concepts frequently expressed by patients and considered important from their perspective did not register with clinicians as being relevant and are not captured on current PRO instruments, emphasizing our knowledge gap and the need to pay attention to what patients want.29
Surprisingly, although pregnancy and childbirth is the number one cause for hospital admissions, a highly preference-driven condition, and a leading cause of morbidity, mortality, and costs, there are few published PROs in the field. In a systematic review of more than 1,700 articles describing PROs published in English through 2014, Martin found that fewer than 1% included PROs specific to pregnancy and childbirth.40
ICHOM has created a standard set of outcomes for pregnancy and childbirth based on consensus recommendations from physicians, measurement experts, and patients.41 The consortium describes 4 domains and 14 subdomains (TABLE 2) and provides suggestions for a validated PROM if known or where appropriate.
Similar domains and subdomains have been corroborated by our research team (the Maternal Quality Indicator [MQI] Work Group), the Childbirth Connection, and Gartner and colleagues.42–44 The MQI Work Group recently conducted a national survey of what women want and what they think is important for their childbirth experience. We identified 19 domains, consistent with those of other investigators.42 Gartner and colleagues advocate for a composite outcome measure that combines the core domains into one preference-based utility measure that is weighted.44 The rationale for this recommendation is that the levels of the domains might contribute differently to the overall birth experience. For example, communication might contribute more to an overall measure than pain management.44 The development of a childbirth-specific survey to evaluate patient-reported outcomes and patient-reported experiences with care is needed if we are to provide value-based care in this arena.45
Looking forward
PROs, PROMs, and PREMs are here to stay. They no longer are limited to clinical research, but increasingly will be incorporated into clinical care, providing us with opportunities to improve the quality of health care delivery, efficiency of patient/clinician interactions, and patients’ ratings of their health care experience.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
In its landmark publication, “Crossing the quality chasm: A new health system for the 21st century,” the Institute of Medicine (now the National Academy of Medicine) called for an emphasis on patient-centered care that it defined as “Providing care that is respectful of and responsive to individual patient preferences, needs, and values and ensuring that patient values guide all clinical decisions.”1 Studies suggest that the patient’s view of health care delivery determines outcome and satisfaction.2 Therefore, we need to expend more effort to understand what patients need or want from their treatment or interaction with the health care system.
Measuring patient-reported outcomes (PROs) is an attempt to recognize and address patient concerns. Although currently PROs are focused primarily in the arena of clinical research, their use has the potential to transform daily clinical patient encounters and improve the cost and quality of health care.3
In this article, we provide a brief overview of PROs and describe how they can be used to improve individual patient care, clinical research, and health care quality. We also offer examples of how PROs can be used in specific women’s health conditions.

What exactly are PROs?
PROs are reports of the status of a patient’s health condition, health behavior, or experience with health care; they come directly from the patient, without anyone else (such as a clinician or caregiver) interpreting the patient’s response.4 PROs usually pertain to general health, quality of life, functional status, or preferences associated with health care or treatment.5 Usually PROs are elicited via a self-administered survey and provide the patient’s perspective on treatment benefits, side effects, change in symptoms, general perceptions of feelings or well-being, or satisfaction with care. Often they represent the outcomes that are most important to patients.6 The survey usually consists of several questions or items. It can be general or condition specific, and it may represent one or more health care dimensions.
The term patient-reported outcome measure (PROM) refers to the survey instrument used to collect PROs. Patient-reported experience measures (PREMs), such as satisfaction surveys, are considered a subset of PROMs.7
Standardized PROs developed out of clinical trials
The use of PROs evolved from clinical trials. The proliferation of PROs resulted in an inability to compare outcomes across trials or different conditions. This led to a need to standardize and possibly harmonize measures and to reach consensus about properties required for a “good” measure and requirements needed for “adequate” reporting. Many investigators and several national and international organizations have provided iterative guidance, including the US Food and Drug Administration (FDA), European Medicines Agency, National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS), International Consortium for Health Outcomes Measurement (ICHOM), University of Oxford Patient Reported Outcomes Measurement Group, Cochrane Systematic Reviews, Consolidated Standards of Reporting Trials–Patient Reported Outcomes (CONSORT-PRO) extension (how to report PROs with the CONSORT checklist), and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR).4,5,8–18
In the United States, the RAND Medical Outcomes Study led to the development of the 12- and 36-item short form surveys, which are widely recognized and commonly used PROMs for health-related quality of life.19 The study generated multiple additional survey instruments that evaluate other domains and dimensions of health. These surveys have been translated into numerous languages, and the RAND website lists over 100 publications.19
In 2002, the NIH sponsored PROMIS, a cooperative program designed to develop, validate, and standardize item banks to measure PROs that were relevant across multiple, common medical conditions. Based on literature review, feedback from both healthy and sick patients, and clinical expert opinion, the PROMIS investigators developed a consensus-based framework for self-reported health that included the following domains: pain, fatigue, emotional distress, physical functioning, and social role participation; these domains were evaluated on paper or with computer-assisted technology.11–14 PROMIS is now a web-based resource with approximately 70 domains pertinent to children and adults in the general population and in those with chronic disease. Measures have been translated into more than 40 languages, and PROMIS-related work has resulted in more than 400 publications.14
In 2006, the FDA issued a draft document regarding the PRO standards that should be included in clinical trials for consideration of drug and device applications (TABLE 1). These recommendations, updated in 2009, were largely drawn from work published by PROMIS and University of Oxford investigators.4,14,16
Because PROs are infrequently measured in routine clinical practice and PROMs that are used vary between countries, global comparison is difficult. Hence, ICHOM convened in 2012 to develop consensus-based, globally agreed on sets of outcomes that are intended to reflect what matters most to patients.
ICHOM specified 2 goals: 1) the core sets should be used in routine clinical practice, and 2) the core sets should be used as end points in clinical studies.15
As of May 2015, 12 standard sets of outcomes have been developed, representing 35% of the global burden of disease. ICHOM currently is creating networks of hospitals around the world to begin measuring, benchmarking, and performing outcome comparisons that can ultimately be used to inform global health system learning and clinical care improvement.15
Read about the evolving use of PROs
Use of PROs is evolving
Historically, PROMs have been used primarily in clinical trials to document the relative benefits of an intervention. With today’s focus on patient-centered care, however, there is a growing mandate to integrate PROMs into clinical care, quality improvement, and ultimately reimbursement. Recently, Basch and colleagues eloquently described the benefit of routine collection of PROs for cancer patients and the opportunity for improved care across the health system.20
PROs can be applied on various levels. For example, if a patient reports a symptom (X), or a change in symptom X, the following options are possible:
- Clinician level: Symptom management with altered dose or change in medication. This is associated with improved self-efficacy for the patient, a shift toward goal-oriented care, improved communication with the provider, and improved patient satisfaction.
- Researcher level: PROs should be used as a primary end point, in addition to traditional outcomes (mortality, survival, physiologic markers), to allow for comparative effectiveness studies or patient-centered outcomes research studies that evaluate what matters most to patients relative to the specific health condition, intervention, and symptom management.
- Health system level: Quality assurance, quality improvement activities. How effective is the health system in the management of symptom X? Are all clinicians using the same medication or the same dose? Is there a best practice for managing symptom X?
- Population level: Provides evidence for other clinicians and patients to make decisions about what to expect with treatment for symptom X.
From a reimbursement level, clinicians and providers are paid based on performance—the more satisfied patients are about X, the higher the reimbursement. This has been pertinent particularly in high-volume orthopedic conditions in which anatomic correction of hip or knee joints has not consistently demonstrated improvement in quality of life as measured by the following PROs: perception of pain, mobility, physical functioning, social functioning, and emotional distress. Because of concerns about high volume, high cost, and inconsistent outcomes, the US Department of Health and Human Services has specified that 50% of Medicare and 90% of Medicaid reimbursements will be based on outcomes or value-based purchasing options.21
Studies have shown that it is possible to collect PRO data for cancer patients—despite age or severity of illness—and integrate it into clinical care delivery. These data can provide useful, actionable information, resulting in decreased emergency department visits, longer toleration of chemotherapy, and improved survival.22 Similar results have been demonstrated in other medical conditions, although challenges exist when transitioning from research settings to routine care. Challenges include privacy concerns, patient recruitment and tracking, encouraging patients to complete the PRO surveys (nonresponse leads to biased data), real and perceived administrative burden to staff, obtaining clinician buy in, and costs related to surveys and data analysis.23
Read about the benefits of PROs to patients and clients
Using PROs in women’s health care: Benefits for patients and clinicians
According to a study by Frosch, patients want to know if a prescribed therapy actually improves outcomes, not whether it changes an isolated biomarker that does not translate into subjective improvement.24 They want to know if the trade-off (adverse effects or higher cost) associated with a new drug or therapy is worth the improved mobility or time spent pain free.
Intuitively, all clinicians have similar opportunities for discussions with regard to the risks, benefits, and alternatives of medical treatment, surgical treatment, or expectant management. We routinely document this discussion daily. However, in this era of patient-centered care, when a patient asks, “What should I do, doctor?” we no longer can respond with a default recommendation. We must engage the patient and ask, “What do you want to do? What is most important to you?”
ObGyns are well suited to benefit from standardized efforts to collect PROs, as we frequently discuss with our patients trade-offs regarding treatment risks and benefits and their personal values and preferences. Examples include contraception options, hormone treatment for menopause, medication use during pregnancy, decisions at the limits of viability, preterm delivery for severe preeclampsia, induction/augmentation versus spontaneous labor, epidural versus physiologic labor, repeat cesarean versus vaginal birth after cesarean, and even elective primary cesarean versus vaginal birth.
Validated PROMs exist for benign gynecology, such as abnormal uterine bleeding, fibroids, polycystic ovary syndrome (PCOS), infertility, pelvic organ prolapse and/or urinary incontinence, and surgery for benign gynecology symptoms, as well as for cancer (breast, ovarian, cervical).25–39
From the PCOS literature we can glean a poignant example of the importance of PROs. Martin and colleagues compared patient and clinician interviews regarding important PROs from the patient perspective.29 Patients identified pain, cramping, heavy bleeding, and bloating as important, whereas clinicians did not consider these symptoms important to patients with PCOS. Clinicians thought “issues with menstruation,” characterized as irregular or no periods, were important, whereas patients were more concerned with heavy bleeding or bleeding of long duration. The authors concluded that concepts frequently expressed by patients and considered important from their perspective did not register with clinicians as being relevant and are not captured on current PRO instruments, emphasizing our knowledge gap and the need to pay attention to what patients want.29
Surprisingly, although pregnancy and childbirth is the number one cause for hospital admissions, a highly preference-driven condition, and a leading cause of morbidity, mortality, and costs, there are few published PROs in the field. In a systematic review of more than 1,700 articles describing PROs published in English through 2014, Martin found that fewer than 1% included PROs specific to pregnancy and childbirth.40
ICHOM has created a standard set of outcomes for pregnancy and childbirth based on consensus recommendations from physicians, measurement experts, and patients.41 The consortium describes 4 domains and 14 subdomains (TABLE 2) and provides suggestions for a validated PROM if known or where appropriate.
Similar domains and subdomains have been corroborated by our research team (the Maternal Quality Indicator [MQI] Work Group), the Childbirth Connection, and Gartner and colleagues.42–44 The MQI Work Group recently conducted a national survey of what women want and what they think is important for their childbirth experience. We identified 19 domains, consistent with those of other investigators.42 Gartner and colleagues advocate for a composite outcome measure that combines the core domains into one preference-based utility measure that is weighted.44 The rationale for this recommendation is that the levels of the domains might contribute differently to the overall birth experience. For example, communication might contribute more to an overall measure than pain management.44 The development of a childbirth-specific survey to evaluate patient-reported outcomes and patient-reported experiences with care is needed if we are to provide value-based care in this arena.45
Looking forward
PROs, PROMs, and PREMs are here to stay. They no longer are limited to clinical research, but increasingly will be incorporated into clinical care, providing us with opportunities to improve the quality of health care delivery, efficiency of patient/clinician interactions, and patients’ ratings of their health care experience.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001:6.
- Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- Rickert J. Patient-centered care: what it means and how to get there. Health Affairs website. http://healthaffairs.org/blog/2012/01/24/patient-centered-care-what-it-means-and-how-to-get-there/. Published January 24, 2012. Accessed October 15, 2017.
- US Food and Drug Administration. Guidance for industry: Patient reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Published December 2009. Accessed February 6, 2018.
- Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008. http://handbook.cochrane.org. Accessed October 15, 2017.
- Patrick DL, Guyatt PD, Acquadro C. Patient-reported outcomes. In: Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008:chap 17. http://handbook-5-1.cochrane.org/. Accessed October 15, 2017.
- Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68.
- McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163–169.
- European Medicines Agency, Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. https://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Published July 27, 2005. Accessed February 7, 2018.
- Venkatesan P. New European guidance on patient-reported outcomes. Lancet Oncol. 2016;17(6):e226.
- Cella D, Yount S, Rothrock N, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11.
- Cella D, Riley W, Stone A, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194.
- Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014;17(8):846–853.
- National Institutes of Health. Patient-Reported Outcomes Measurement Information System (PROMIS). https://commonfund.nih.gov/promis/index. Reviewed May 8, 2017. Accessed October 15, 2017.
- International Consortium for Health Outcomes Measurement (ICHOM). http://www.ichom.org/. Accessed October 15, 2017.
- University of Oxford, Patient Reported Outcomes Measurement Group http://phi.uhce.ox.ac.uk/. Accessed October 15, 2017.
- CONSORT. Patient-Reported Outcomes (CONSORT PRO). http://www.consort-statement.org/extensions/overview/consort-pro. Accessed October 15, 2017.
- International Society for Pharmacoeconomics and Outcomes Research. https://www.ispor.org/. Accessed October 15, 2017.
- RAND Health. RAND medical outcomes study: measures of quality of life core survey from RAND Health. https://www.rand.org/health/surveys_tools/mos.html. Accessed October 15, 2017.
- Basch EM, Deal AM, Dueck A, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment [abstract LBA2]. J Clin Oncol. 2017;35(18)(suppl).
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Accessed October 15, 2017.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Chenok K, Teleki S, SooHoo NF, Huddleston J, Bozic KJ. Collecting patient-reported outcomes: lessons from the California Joint Replacement Registry. EGEMS (Wash DC). 2015;3(1):1196.
- Frosch DL. Patient-reported outcomes as a measure of healthcare quality. J Gen Intern Med. 2015;30(10):1383–1384.
- Gibbons E, Mackintosh A, Fitzpatrick R; Patient-Reported Outcome Measurement Group, Oxford. A structured review of patient-reported outcome measures for people undergoing elective procedures for benign gynaecological conditions of the uterus, 2010. http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Gynaecological%20procedures_012011.pdf. Accessed October 23, 2017.
- Matteson KA, Boardman LA, Munro MG, Clark MA. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92(1):205–216.
- Matteson KA, Scott DM, Raker CA, Clark MA. The menstrual bleeding questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122(5):681–689.
- Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health. 2012;15(1):135–142.
- Martin ML, Halling K, Eek D, Krohe M, Paty J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Quality Life Outcomes. 2017;15(1):162.
- Malik-Aslam A, Reaney MD, Speight J. The suitability of polycystic ovary syndrome-specific questionnaires for measuring the impact of PCOS on quality of life in clinical trials. Value Health. 2010;13(4):440–446.
- Kitchen H, Aldhouse N, Trigg A, Palencia R, Mitchell S. A review of patient-reported outcome measures to assess female infertility-related quality of life. Health Qual Life Outcomes. 2017;15(1):86.
- Sung VW, Joo K, Marques F, Myers DL. Patient-reported outcomes after combined surgery for pelvic floor disorders in older compared to younger women. Am J Obstet Gynecol. 2009;201(5):534.e1–e5.
- Sung VW, Rogers RG, Barber MD, Clark MA. Conceptual framework for patient-important treatment outcomes for pelvic organ prolapse. Neurourol Urodynam. 2014;33(4):414–419.
- Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2016;215(5):659.e1–e7.
- Croke J. Cervical ca PROs in clinical practice. https://clinicaltrials.gov/ct2/show/NCT03048435. Accessed October 16, 2017.
- Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211–232.
- Jensen RE, Potosky AL, Moinpour CM, et al. United States population estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913–1920.
- Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials—lost opportunities and lessons learned. Ann Oncol. 2016;27(suppl 1):i66–i71.
- Joly F, Hilpert F, Okamoto A, Stuart G, Ochaia K, Friedlander M; 5th Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138.
- Martin A. Patient-reported outcomes in studies published in 2014: which disease areas have been the main focus of clinical research? Value Health. 2015;18(7):A742.
- International Consortium for Health Outcomes Management (ICHOM). Pregnancy and childbirth. http://www.ichom.org/medical-conditions/pregnancy-and-childbirth/. Accessed October 10, 2017.
- El Haj Ibrahim S, McCulloch J, Korst LM, Fridman M, Fink A, Gregory KD. Communication with staff during hospitalization for childbirth: the patient’s perspective [1R]. Obstet Gynecol. 2016;127.
- National Partnership for Women and Families. Childbirth Connection. Listening to mothers III: report of the third national US survey of women’s childbearing experience. http://transform.childbirthconnection.org/reports/listeningtomothers/. Accessed October 23, 2017.
- Gartner FR, Freeman LM, Rijnders ME, et al. A comprehensive representation of the birth-experience: identification and prioritization of birth-specific domains based on a mixed-method design. BMC Pregnancy Childbirth. 2014;14:147.
- National Partnership for Women and Families. The priority of developing and implementing CAHPS maternity care facility, clinician and health plan surveys. 2015. http://www.nationalpartnership.org/research-library/maternal-health/cahps-maternity-care-fact-sheet.pdf. Accessed October 23, 2017.
- Committee on Quality of Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001:6.
- Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796–804.
- Rickert J. Patient-centered care: what it means and how to get there. Health Affairs website. http://healthaffairs.org/blog/2012/01/24/patient-centered-care-what-it-means-and-how-to-get-there/. Published January 24, 2012. Accessed October 15, 2017.
- US Food and Drug Administration. Guidance for industry: Patient reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Published December 2009. Accessed February 6, 2018.
- Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008. http://handbook.cochrane.org. Accessed October 15, 2017.
- Patrick DL, Guyatt PD, Acquadro C. Patient-reported outcomes. In: Higgins JP, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011). Chichester, UK: John Wiley & Sons; 2008:chap 17. http://handbook-5-1.cochrane.org/. Accessed October 15, 2017.
- Weldring T, Smith SM. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights. 2013;6:61–68.
- McLeod LD, Coon CD, Martin SA, Fehnel SE, Hays RD. Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):163–169.
- European Medicines Agency, Committee for Medicinal Products for Human Use. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. https://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Published July 27, 2005. Accessed February 7, 2018.
- Venkatesan P. New European guidance on patient-reported outcomes. Lancet Oncol. 2016;17(6):e226.
- Cella D, Yount S, Rothrock N, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3–S11.
- Cella D, Riley W, Stone A, et al; PROMIS Cooperative Group. The Patient-Reported Outcomes Mesurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194.
- Craig BM, Reeve BB, Brown PM, et al. US valuation of health outcomes measured using the PROMIS-29. Value Health. 2014;17(8):846–853.
- National Institutes of Health. Patient-Reported Outcomes Measurement Information System (PROMIS). https://commonfund.nih.gov/promis/index. Reviewed May 8, 2017. Accessed October 15, 2017.
- International Consortium for Health Outcomes Measurement (ICHOM). http://www.ichom.org/. Accessed October 15, 2017.
- University of Oxford, Patient Reported Outcomes Measurement Group http://phi.uhce.ox.ac.uk/. Accessed October 15, 2017.
- CONSORT. Patient-Reported Outcomes (CONSORT PRO). http://www.consort-statement.org/extensions/overview/consort-pro. Accessed October 15, 2017.
- International Society for Pharmacoeconomics and Outcomes Research. https://www.ispor.org/. Accessed October 15, 2017.
- RAND Health. RAND medical outcomes study: measures of quality of life core survey from RAND Health. https://www.rand.org/health/surveys_tools/mos.html. Accessed October 15, 2017.
- Basch EM, Deal AM, Dueck A, et al. Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment [abstract LBA2]. J Clin Oncol. 2017;35(18)(suppl).
- Centers for Medicare and Medicaid Services. Better care. Smarter spending. Healthier people: paying providers for value, not volume. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-01-26-3.html. Accessed October 15, 2017.
- Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565.
- Chenok K, Teleki S, SooHoo NF, Huddleston J, Bozic KJ. Collecting patient-reported outcomes: lessons from the California Joint Replacement Registry. EGEMS (Wash DC). 2015;3(1):1196.
- Frosch DL. Patient-reported outcomes as a measure of healthcare quality. J Gen Intern Med. 2015;30(10):1383–1384.
- Gibbons E, Mackintosh A, Fitzpatrick R; Patient-Reported Outcome Measurement Group, Oxford. A structured review of patient-reported outcome measures for people undergoing elective procedures for benign gynaecological conditions of the uterus, 2010. http://phi.uhce.ox.ac.uk/pdf/ElectiveProcedures/PROMs_Oxford_Gynaecological%20procedures_012011.pdf. Accessed October 23, 2017.
- Matteson KA, Boardman LA, Munro MG, Clark MA. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92(1):205–216.
- Matteson KA, Scott DM, Raker CA, Clark MA. The menstrual bleeding questionnaire: development and validation of a comprehensive patient-reported outcome instrument for heavy menstrual bleeding. BJOG. 2015;122(5):681–689.
- Coyne KS, Margolis MK, Bradley LD, Guido R, Maxwell GL, Spies JB. Further validation of the uterine fibroid symptom and quality-of-life questionnaire. Value Health. 2012;15(1):135–142.
- Martin ML, Halling K, Eek D, Krohe M, Paty J. Understanding polycystic ovary syndrome from the patient perspective: a concept elicitation patient interview study. Health Quality Life Outcomes. 2017;15(1):162.
- Malik-Aslam A, Reaney MD, Speight J. The suitability of polycystic ovary syndrome-specific questionnaires for measuring the impact of PCOS on quality of life in clinical trials. Value Health. 2010;13(4):440–446.
- Kitchen H, Aldhouse N, Trigg A, Palencia R, Mitchell S. A review of patient-reported outcome measures to assess female infertility-related quality of life. Health Qual Life Outcomes. 2017;15(1):86.
- Sung VW, Joo K, Marques F, Myers DL. Patient-reported outcomes after combined surgery for pelvic floor disorders in older compared to younger women. Am J Obstet Gynecol. 2009;201(5):534.e1–e5.
- Sung VW, Rogers RG, Barber MD, Clark MA. Conceptual framework for patient-important treatment outcomes for pelvic organ prolapse. Neurourol Urodynam. 2014;33(4):414–419.
- Sung VW, Wohlrab KJ, Madsen A, Raker C. Patient-reported goal attainment and comprehensive functioning outcomes after surgery compared with pessary for pelvic organ prolapse. Am J Obstet Gynecol. 2016;215(5):659.e1–e7.
- Croke J. Cervical ca PROs in clinical practice. https://clinicaltrials.gov/ct2/show/NCT03048435. Accessed October 16, 2017.
- Catt S, Starkings R, Shilling V, Fallowfield L. Patient-reported outcome measures of the impact of cancer on patients’ everyday lives: a systematic review. J Cancer Surviv. 2017;11(2):211–232.
- Jensen RE, Potosky AL, Moinpour CM, et al. United States population estimates of patient-reported outcomes measurement information system symptom and functional status reference values for individuals with cancer. J Clin Oncol. 2017;35(17):1913–1920.
- Friedlander M, Mercieca-Bebber RL, King MT. Patient-reported outcomes (PRO) in ovarian cancer clinical trials—lost opportunities and lessons learned. Ann Oncol. 2016;27(suppl 1):i66–i71.
- Joly F, Hilpert F, Okamoto A, Stuart G, Ochaia K, Friedlander M; 5th Ovarian Cancer Consensus Conference. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: recommendations on incorporating patient-reported outcomes in clinical trials in epithelial ovarian cancer. Eur J Cancer. 2017;78:133–138.
- Martin A. Patient-reported outcomes in studies published in 2014: which disease areas have been the main focus of clinical research? Value Health. 2015;18(7):A742.
- International Consortium for Health Outcomes Management (ICHOM). Pregnancy and childbirth. http://www.ichom.org/medical-conditions/pregnancy-and-childbirth/. Accessed October 10, 2017.
- El Haj Ibrahim S, McCulloch J, Korst LM, Fridman M, Fink A, Gregory KD. Communication with staff during hospitalization for childbirth: the patient’s perspective [1R]. Obstet Gynecol. 2016;127.
- National Partnership for Women and Families. Childbirth Connection. Listening to mothers III: report of the third national US survey of women’s childbearing experience. http://transform.childbirthconnection.org/reports/listeningtomothers/. Accessed October 23, 2017.
- Gartner FR, Freeman LM, Rijnders ME, et al. A comprehensive representation of the birth-experience: identification and prioritization of birth-specific domains based on a mixed-method design. BMC Pregnancy Childbirth. 2014;14:147.
- National Partnership for Women and Families. The priority of developing and implementing CAHPS maternity care facility, clinician and health plan surveys. 2015. http://www.nationalpartnership.org/research-library/maternal-health/cahps-maternity-care-fact-sheet.pdf. Accessed October 23, 2017.
Read all parts of this series
PART 1 Value-based payment: What does it mean and how can ObGyns get out ahead
PART 2 What makes a “quality” quality measure?
PART 3 The role of patient-reported outcomes in women’s health
PART 4 It costs what?! How we can educate residents and students on how much things cost
Which antibiotics should be used with caution in pregnant women with UTI?
EXPERT COMMENTARY
Lower urinary tract infection (UTI) is one of the most common medical complications of pregnancy. Approximately 5% to 10% of all pregnant women have asymptomatic bacteriuria, which usually antedates the pregnancy and is detected at the time of the first prenatal appointment. Another 2% to 3% develop acute cystitis during pregnancy. The dominant organisms that cause lower UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, Proteus species, group B streptococci, enterococci, and Staphylococcus saprophyticus.
One goal of treating asymptomatic bacteriuria and acute cystitis is to prevent ascending infection (pyelonephritis), which can be associated with preterm delivery, sepsis, and adult respiratory distress syndrome. Another key goal is to use an antibiotic that eradicates the uropathogen without causing harm to either the mother or fetus.
In 2009, Crider and colleagues reported that 2 of the most commonly used antibiotics for UTIs, sulfonamides and nitrofurantoin, were associated with a disturbing spectrum of birth defects.1 Following that report, in 2011 the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion that recommended against the use of these 2 agents in the first trimester of pregnancy unless other antibiotics were unlikely to be effective.2
Details of the study
Centers for Disease Control and Prevention investigators recently conducted a study to assess the effect of these ACOG recommendations on clinical practice. Ailes and co-workers used the Truven Health MarketScan Commercial Database to examine antibiotic prescriptions filled by pregnant women with UTIs.
The database included 482,917 pregnancies in 2014 eligible for analysis. A total of 7.2% (n = 34,864) of pregnant women were treated as outpatients for a UTI within the 90-day interval before the last menstrual period or during the pregnancy. Among these women, the most commonly prescribed antibiotics during the first trimester were nitrofurantoin (34.7%), ciprofloxacin (10.5%), cephalexin (10.3%), and trimethoprim-sulfamethoxazole (7.6%).
The authors concluded that 43% of women used an antibiotic (nitrofurantoin or trimethoprim-sulfamethoxazole) in the first trimester that had potential teratogenicity, despite the precautionary statement articulated in the ACOG committee opinion.2
Antibiotic-associated effects
Of all the antibiotics that could be used to treat a lower UTI in pregnancy, nitrofurantoin probably has the greatest appeal. The drug is highly concentrated in the urine and is very active against all the common uropathogens except Proteus species. It is not absorbed significantly outside the lower urinary tract, and thus it does not alter the natural flora of the bowel or vagina (such alteration would predispose the patient to antibiotic-associated diarrhea or vulvovaginal candidiasis). Nitrofurantoin is inexpensive and usually is very well tolerated.
In the National Birth Defects Prevention Study by Crider and colleagues, nitrofurantoin was associated with anophthalmia or microphthalmos (adjusted odds ratio [AOR], 3.7; 95% confidence interval [CI], 1.1–12.2), hypoplastic left heart syndrome (AOR, 4.2; 95% CI, 1.9–9.1), atrial septal defects (AOR, 1.9; 95% CI, 1.1–3.4), and cleft lip with cleft palate (AOR, 2.1; 95% CI, 1.2–3.9).1 Other investigations, including one published as recently as 2013, have not documented these same associations.3
Similarly, the combination of trimethoprim-sulfamethoxazole also has considerable appeal for treating lower UTIs in pregnancy because it is highly active against most uropathogens, is inexpensive, and usually is very well tolerated. The report by Crider and colleagues, however, was even more worrisome with respect to the possible teratogenicity of this antibiotic.1 The authors found that use of this antibiotic in the first trimester was associated with anencephaly (AOR, 3.4; 95% CI, 1.3–8.8), coarctation of the aorta (AOR, 2.7; 95% CI, 1.3–5.6), hypoplastic left heart (AOR, 3.2; 95% CI, 1.3–7.6), choanal atresia (AOR, 8.0; 95% CI, 2.7–23.5), transverse limb deficiency (AOR, 2.5; 95% CI, 1.0–5.9), and diaphragmatic hernia (AOR, 2.4; 95% CI, 1.1–5.4). Again, other authors, using different epidemiologic methods, have not found the same associations.3
Study strengths and weaknesses
The National Birth Defects Prevention Study by Crider and colleagues was a large, well-funded, and well-designed epidemiologic study. It included more than 13,000 patients from 10 different states.
Nevertheless, the study had certain limitations.4 The findings are subject to recall bias because the investigators questioned patients about antibiotic use after, rather than during, pregnancy. Understandably, the investigators were not able to verify the prescriptions for antibiotics by reviewing each individual medical record. In fact, one-third of study participants were unable to recall the exact name of the antibiotic they received. The authors did not precisely distinguish between single-agent sulfonamides and the combination drug, trimethoprim-sulfamethoxazole, although it seems reasonable to assume that the majority of the prescriptions were for the latter. Finally, given the observational nature of the study, the authors could not be certain that the observed associations were due to the antibiotic, the infection for which the drug was prescribed, or another confounding factor.
Pending the publication of additional investigations, I believe that the guidance outlined below is prudent.
Trimethoprim-sulfamethoxazole should not be used for treating UTIs in the first trimester of pregnancy unless no other antibiotic is likely to be effective. This drug also should be avoided just prior to expected delivery because it can displace bilirubin from protein-binding sites in the newborn and increase the risk of neonatal jaundice.
There may be instances in which trimethoprim-sulfamethoxazole should be used even early in pregnancy, such as to provide prophylaxis against Pneumocystis jiroveci infection in women with human immunodeficiency virus.
To exercise an abundance of caution, I recommend that nitrofurantoin not be used in the first trimester of pregnancy unless no other antibiotic is likely to be effective.
Alternative antibiotics that might be used in the first trimester for treatment of UTIs include ampicillin, amoxicillin, cephalexin, and amoxicillin-clavulanic acid. Substantial evidence supports the safety of these antibiotics in early pregnancy. Unless no other drug is likely to be effective, I would not recommend use of a quinolone antibiotic, such as ciprofloxacin, because of concern about the possible injurious effect of these agents on cartilaginous tissue in the developing fetus.
Neither trimethoprim-sulfamethoxazole nor nitrofurantoin should be used at any time in pregnancy in a patient who has glucose-6-phosphate dehydrogenase deficiency or who may be at increased risk for this disorder.2
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–985.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 494: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2011;117(6):1484–1485.
- Nordeng H, Lupattelli A, Romoren M, Koren G. Neonatal outcomes after gestational exposure to nitrofurantoin. Obstet Gynecol. 2013;121(2 pt 1):306–313.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 717: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130(3):e150–e152. doi:10.1097/AOG.0000000000002300.
EXPERT COMMENTARY
Lower urinary tract infection (UTI) is one of the most common medical complications of pregnancy. Approximately 5% to 10% of all pregnant women have asymptomatic bacteriuria, which usually antedates the pregnancy and is detected at the time of the first prenatal appointment. Another 2% to 3% develop acute cystitis during pregnancy. The dominant organisms that cause lower UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, Proteus species, group B streptococci, enterococci, and Staphylococcus saprophyticus.
One goal of treating asymptomatic bacteriuria and acute cystitis is to prevent ascending infection (pyelonephritis), which can be associated with preterm delivery, sepsis, and adult respiratory distress syndrome. Another key goal is to use an antibiotic that eradicates the uropathogen without causing harm to either the mother or fetus.
In 2009, Crider and colleagues reported that 2 of the most commonly used antibiotics for UTIs, sulfonamides and nitrofurantoin, were associated with a disturbing spectrum of birth defects.1 Following that report, in 2011 the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion that recommended against the use of these 2 agents in the first trimester of pregnancy unless other antibiotics were unlikely to be effective.2
Details of the study
Centers for Disease Control and Prevention investigators recently conducted a study to assess the effect of these ACOG recommendations on clinical practice. Ailes and co-workers used the Truven Health MarketScan Commercial Database to examine antibiotic prescriptions filled by pregnant women with UTIs.
The database included 482,917 pregnancies in 2014 eligible for analysis. A total of 7.2% (n = 34,864) of pregnant women were treated as outpatients for a UTI within the 90-day interval before the last menstrual period or during the pregnancy. Among these women, the most commonly prescribed antibiotics during the first trimester were nitrofurantoin (34.7%), ciprofloxacin (10.5%), cephalexin (10.3%), and trimethoprim-sulfamethoxazole (7.6%).
The authors concluded that 43% of women used an antibiotic (nitrofurantoin or trimethoprim-sulfamethoxazole) in the first trimester that had potential teratogenicity, despite the precautionary statement articulated in the ACOG committee opinion.2
Antibiotic-associated effects
Of all the antibiotics that could be used to treat a lower UTI in pregnancy, nitrofurantoin probably has the greatest appeal. The drug is highly concentrated in the urine and is very active against all the common uropathogens except Proteus species. It is not absorbed significantly outside the lower urinary tract, and thus it does not alter the natural flora of the bowel or vagina (such alteration would predispose the patient to antibiotic-associated diarrhea or vulvovaginal candidiasis). Nitrofurantoin is inexpensive and usually is very well tolerated.
In the National Birth Defects Prevention Study by Crider and colleagues, nitrofurantoin was associated with anophthalmia or microphthalmos (adjusted odds ratio [AOR], 3.7; 95% confidence interval [CI], 1.1–12.2), hypoplastic left heart syndrome (AOR, 4.2; 95% CI, 1.9–9.1), atrial septal defects (AOR, 1.9; 95% CI, 1.1–3.4), and cleft lip with cleft palate (AOR, 2.1; 95% CI, 1.2–3.9).1 Other investigations, including one published as recently as 2013, have not documented these same associations.3
Similarly, the combination of trimethoprim-sulfamethoxazole also has considerable appeal for treating lower UTIs in pregnancy because it is highly active against most uropathogens, is inexpensive, and usually is very well tolerated. The report by Crider and colleagues, however, was even more worrisome with respect to the possible teratogenicity of this antibiotic.1 The authors found that use of this antibiotic in the first trimester was associated with anencephaly (AOR, 3.4; 95% CI, 1.3–8.8), coarctation of the aorta (AOR, 2.7; 95% CI, 1.3–5.6), hypoplastic left heart (AOR, 3.2; 95% CI, 1.3–7.6), choanal atresia (AOR, 8.0; 95% CI, 2.7–23.5), transverse limb deficiency (AOR, 2.5; 95% CI, 1.0–5.9), and diaphragmatic hernia (AOR, 2.4; 95% CI, 1.1–5.4). Again, other authors, using different epidemiologic methods, have not found the same associations.3
Study strengths and weaknesses
The National Birth Defects Prevention Study by Crider and colleagues was a large, well-funded, and well-designed epidemiologic study. It included more than 13,000 patients from 10 different states.
Nevertheless, the study had certain limitations.4 The findings are subject to recall bias because the investigators questioned patients about antibiotic use after, rather than during, pregnancy. Understandably, the investigators were not able to verify the prescriptions for antibiotics by reviewing each individual medical record. In fact, one-third of study participants were unable to recall the exact name of the antibiotic they received. The authors did not precisely distinguish between single-agent sulfonamides and the combination drug, trimethoprim-sulfamethoxazole, although it seems reasonable to assume that the majority of the prescriptions were for the latter. Finally, given the observational nature of the study, the authors could not be certain that the observed associations were due to the antibiotic, the infection for which the drug was prescribed, or another confounding factor.
Pending the publication of additional investigations, I believe that the guidance outlined below is prudent.
Trimethoprim-sulfamethoxazole should not be used for treating UTIs in the first trimester of pregnancy unless no other antibiotic is likely to be effective. This drug also should be avoided just prior to expected delivery because it can displace bilirubin from protein-binding sites in the newborn and increase the risk of neonatal jaundice.
There may be instances in which trimethoprim-sulfamethoxazole should be used even early in pregnancy, such as to provide prophylaxis against Pneumocystis jiroveci infection in women with human immunodeficiency virus.
To exercise an abundance of caution, I recommend that nitrofurantoin not be used in the first trimester of pregnancy unless no other antibiotic is likely to be effective.
Alternative antibiotics that might be used in the first trimester for treatment of UTIs include ampicillin, amoxicillin, cephalexin, and amoxicillin-clavulanic acid. Substantial evidence supports the safety of these antibiotics in early pregnancy. Unless no other drug is likely to be effective, I would not recommend use of a quinolone antibiotic, such as ciprofloxacin, because of concern about the possible injurious effect of these agents on cartilaginous tissue in the developing fetus.
Neither trimethoprim-sulfamethoxazole nor nitrofurantoin should be used at any time in pregnancy in a patient who has glucose-6-phosphate dehydrogenase deficiency or who may be at increased risk for this disorder.2
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Lower urinary tract infection (UTI) is one of the most common medical complications of pregnancy. Approximately 5% to 10% of all pregnant women have asymptomatic bacteriuria, which usually antedates the pregnancy and is detected at the time of the first prenatal appointment. Another 2% to 3% develop acute cystitis during pregnancy. The dominant organisms that cause lower UTIs in pregnant women are Escherichia coli, Klebsiella pneumoniae, Proteus species, group B streptococci, enterococci, and Staphylococcus saprophyticus.
One goal of treating asymptomatic bacteriuria and acute cystitis is to prevent ascending infection (pyelonephritis), which can be associated with preterm delivery, sepsis, and adult respiratory distress syndrome. Another key goal is to use an antibiotic that eradicates the uropathogen without causing harm to either the mother or fetus.
In 2009, Crider and colleagues reported that 2 of the most commonly used antibiotics for UTIs, sulfonamides and nitrofurantoin, were associated with a disturbing spectrum of birth defects.1 Following that report, in 2011 the American College of Obstetricians and Gynecologists (ACOG) published a committee opinion that recommended against the use of these 2 agents in the first trimester of pregnancy unless other antibiotics were unlikely to be effective.2
Details of the study
Centers for Disease Control and Prevention investigators recently conducted a study to assess the effect of these ACOG recommendations on clinical practice. Ailes and co-workers used the Truven Health MarketScan Commercial Database to examine antibiotic prescriptions filled by pregnant women with UTIs.
The database included 482,917 pregnancies in 2014 eligible for analysis. A total of 7.2% (n = 34,864) of pregnant women were treated as outpatients for a UTI within the 90-day interval before the last menstrual period or during the pregnancy. Among these women, the most commonly prescribed antibiotics during the first trimester were nitrofurantoin (34.7%), ciprofloxacin (10.5%), cephalexin (10.3%), and trimethoprim-sulfamethoxazole (7.6%).
The authors concluded that 43% of women used an antibiotic (nitrofurantoin or trimethoprim-sulfamethoxazole) in the first trimester that had potential teratogenicity, despite the precautionary statement articulated in the ACOG committee opinion.2
Antibiotic-associated effects
Of all the antibiotics that could be used to treat a lower UTI in pregnancy, nitrofurantoin probably has the greatest appeal. The drug is highly concentrated in the urine and is very active against all the common uropathogens except Proteus species. It is not absorbed significantly outside the lower urinary tract, and thus it does not alter the natural flora of the bowel or vagina (such alteration would predispose the patient to antibiotic-associated diarrhea or vulvovaginal candidiasis). Nitrofurantoin is inexpensive and usually is very well tolerated.
In the National Birth Defects Prevention Study by Crider and colleagues, nitrofurantoin was associated with anophthalmia or microphthalmos (adjusted odds ratio [AOR], 3.7; 95% confidence interval [CI], 1.1–12.2), hypoplastic left heart syndrome (AOR, 4.2; 95% CI, 1.9–9.1), atrial septal defects (AOR, 1.9; 95% CI, 1.1–3.4), and cleft lip with cleft palate (AOR, 2.1; 95% CI, 1.2–3.9).1 Other investigations, including one published as recently as 2013, have not documented these same associations.3
Similarly, the combination of trimethoprim-sulfamethoxazole also has considerable appeal for treating lower UTIs in pregnancy because it is highly active against most uropathogens, is inexpensive, and usually is very well tolerated. The report by Crider and colleagues, however, was even more worrisome with respect to the possible teratogenicity of this antibiotic.1 The authors found that use of this antibiotic in the first trimester was associated with anencephaly (AOR, 3.4; 95% CI, 1.3–8.8), coarctation of the aorta (AOR, 2.7; 95% CI, 1.3–5.6), hypoplastic left heart (AOR, 3.2; 95% CI, 1.3–7.6), choanal atresia (AOR, 8.0; 95% CI, 2.7–23.5), transverse limb deficiency (AOR, 2.5; 95% CI, 1.0–5.9), and diaphragmatic hernia (AOR, 2.4; 95% CI, 1.1–5.4). Again, other authors, using different epidemiologic methods, have not found the same associations.3
Study strengths and weaknesses
The National Birth Defects Prevention Study by Crider and colleagues was a large, well-funded, and well-designed epidemiologic study. It included more than 13,000 patients from 10 different states.
Nevertheless, the study had certain limitations.4 The findings are subject to recall bias because the investigators questioned patients about antibiotic use after, rather than during, pregnancy. Understandably, the investigators were not able to verify the prescriptions for antibiotics by reviewing each individual medical record. In fact, one-third of study participants were unable to recall the exact name of the antibiotic they received. The authors did not precisely distinguish between single-agent sulfonamides and the combination drug, trimethoprim-sulfamethoxazole, although it seems reasonable to assume that the majority of the prescriptions were for the latter. Finally, given the observational nature of the study, the authors could not be certain that the observed associations were due to the antibiotic, the infection for which the drug was prescribed, or another confounding factor.
Pending the publication of additional investigations, I believe that the guidance outlined below is prudent.
Trimethoprim-sulfamethoxazole should not be used for treating UTIs in the first trimester of pregnancy unless no other antibiotic is likely to be effective. This drug also should be avoided just prior to expected delivery because it can displace bilirubin from protein-binding sites in the newborn and increase the risk of neonatal jaundice.
There may be instances in which trimethoprim-sulfamethoxazole should be used even early in pregnancy, such as to provide prophylaxis against Pneumocystis jiroveci infection in women with human immunodeficiency virus.
To exercise an abundance of caution, I recommend that nitrofurantoin not be used in the first trimester of pregnancy unless no other antibiotic is likely to be effective.
Alternative antibiotics that might be used in the first trimester for treatment of UTIs include ampicillin, amoxicillin, cephalexin, and amoxicillin-clavulanic acid. Substantial evidence supports the safety of these antibiotics in early pregnancy. Unless no other drug is likely to be effective, I would not recommend use of a quinolone antibiotic, such as ciprofloxacin, because of concern about the possible injurious effect of these agents on cartilaginous tissue in the developing fetus.
Neither trimethoprim-sulfamethoxazole nor nitrofurantoin should be used at any time in pregnancy in a patient who has glucose-6-phosphate dehydrogenase deficiency or who may be at increased risk for this disorder.2
-- Patrick Duff, MD
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–985.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 494: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2011;117(6):1484–1485.
- Nordeng H, Lupattelli A, Romoren M, Koren G. Neonatal outcomes after gestational exposure to nitrofurantoin. Obstet Gynecol. 2013;121(2 pt 1):306–313.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 717: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130(3):e150–e152. doi:10.1097/AOG.0000000000002300.
- Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med. 2009;163(11):978–985.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 494: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2011;117(6):1484–1485.
- Nordeng H, Lupattelli A, Romoren M, Koren G. Neonatal outcomes after gestational exposure to nitrofurantoin. Obstet Gynecol. 2013;121(2 pt 1):306–313.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 717: Sulfonamides, nitrofurantoin, and risk of birth defects. Obstet Gynecol. 2017;130(3):e150–e152. doi:10.1097/AOG.0000000000002300.
The beginning of the end of the Pap?
EXPERT COMMENTARY
Realistic prospective performance data are needed to quantify the additional benefit of the cytology component of cotesting on top of what is already known to be highly sensitive molecular HPV testing. While the addition of cytology to HPV testing can add performance, it also can add further costs and the potential for unnecessary colposcopies for what are merely cytomorphologic manifestations of an active HPV infection. Frequent invasive procedures such as colposcopy, which can be costly and lead to anxiety and distress in generally young women and the potential for overtreatment of likely regressive lesions, has been defined as a harm of screening by the US Preventive Services Task Force (USPSTF).
Details of the study
In a cohort from Kaiser Permanente Northern California, 1,208,710 women aged 30 years or older were screened with cotesting from 2003 to 2015. Those who cotested HPV negative and cytology negative were offered triennial screening. Positive cotest results were managed according to Kaiser protocol. Women with cytologic abnormalities were referred for colposcopy. Those with HPV positive/cytology negative results or HPV negative/cytology equivocal results underwent accelerated testing at 1 year. A total of 623 cervical cancers were identified and included in the analyses.
Using multiple analyses, Schiffman and colleagues demonstrated the sensitivity advantage of HPV testing. They clearly showed that the cytology component to cotesting performance over many years is very limited for detecting precancers and early curable cancers. For example, prediagnostic HPV testing (76.7%) was more likely to be positive than cytology (59.1%; P<.001 for paired comparison); 82.6% of all prediagnostic cotests were positive by HPV and/or cytology; and only 5.9% of the cotests were positive by cytology alone (HPV negative.)
Primary HPV testing is recommended as a potential screening strategy by an interim guidance group led by the Society of Gynecologic Oncology and the American Society for Colposcopy and Cervical Pathology, and it is the primary cervical cancer screening recommendation of USPSTF draft guidelines.1 There have been reports that reliance on primary HPV testing would encourage cervical cancer mortality; Schiffman and colleagues point out, however, that according to their study data, such reports are overstated.
Despite these data, practically speaking, shifting away from standard cotesting poses numerous challenges for clinicians and laboratories alike; however, these data clearly show the limited value of cytology and, due to the overtreatment of likely regressive cervical intraepithelial neoplasia grade 2, the possible increased risk of preterm birth and its subsequent harm as well.
Study strengths and weaknesses
The authors examined the long-term relative history of HPV testing and cytology prior to cancer diagnosis in a large, prospectively followed US cohort where hundreds of women in this cohort developed cancer. There will not be a validation study of this size and scale in the near future. Further, the authors showed that the relative value of cytology to cotesting is minimal. Multiple subsequent rounds of cotesting after negative results also can be questioned.
One weakness of the study is that the data were collected from only one health care system and therefore may not be representative of all populations. Additionally, cotesting was performed on 2 separately collected specimens, which may have reduced HPV testing performance.
Excessive cervical cancer screening, including frequent cotesting, could have minimal cancer prevention benefits while increasing the harms of screening. These data confirm guidance showing HPV testing alone is an effective cervical cancer screening strategy.
-- Mark H. Einstein, MD, MS
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330-337.
EXPERT COMMENTARY
Realistic prospective performance data are needed to quantify the additional benefit of the cytology component of cotesting on top of what is already known to be highly sensitive molecular HPV testing. While the addition of cytology to HPV testing can add performance, it also can add further costs and the potential for unnecessary colposcopies for what are merely cytomorphologic manifestations of an active HPV infection. Frequent invasive procedures such as colposcopy, which can be costly and lead to anxiety and distress in generally young women and the potential for overtreatment of likely regressive lesions, has been defined as a harm of screening by the US Preventive Services Task Force (USPSTF).
Details of the study
In a cohort from Kaiser Permanente Northern California, 1,208,710 women aged 30 years or older were screened with cotesting from 2003 to 2015. Those who cotested HPV negative and cytology negative were offered triennial screening. Positive cotest results were managed according to Kaiser protocol. Women with cytologic abnormalities were referred for colposcopy. Those with HPV positive/cytology negative results or HPV negative/cytology equivocal results underwent accelerated testing at 1 year. A total of 623 cervical cancers were identified and included in the analyses.
Using multiple analyses, Schiffman and colleagues demonstrated the sensitivity advantage of HPV testing. They clearly showed that the cytology component to cotesting performance over many years is very limited for detecting precancers and early curable cancers. For example, prediagnostic HPV testing (76.7%) was more likely to be positive than cytology (59.1%; P<.001 for paired comparison); 82.6% of all prediagnostic cotests were positive by HPV and/or cytology; and only 5.9% of the cotests were positive by cytology alone (HPV negative.)
Primary HPV testing is recommended as a potential screening strategy by an interim guidance group led by the Society of Gynecologic Oncology and the American Society for Colposcopy and Cervical Pathology, and it is the primary cervical cancer screening recommendation of USPSTF draft guidelines.1 There have been reports that reliance on primary HPV testing would encourage cervical cancer mortality; Schiffman and colleagues point out, however, that according to their study data, such reports are overstated.
Despite these data, practically speaking, shifting away from standard cotesting poses numerous challenges for clinicians and laboratories alike; however, these data clearly show the limited value of cytology and, due to the overtreatment of likely regressive cervical intraepithelial neoplasia grade 2, the possible increased risk of preterm birth and its subsequent harm as well.
Study strengths and weaknesses
The authors examined the long-term relative history of HPV testing and cytology prior to cancer diagnosis in a large, prospectively followed US cohort where hundreds of women in this cohort developed cancer. There will not be a validation study of this size and scale in the near future. Further, the authors showed that the relative value of cytology to cotesting is minimal. Multiple subsequent rounds of cotesting after negative results also can be questioned.
One weakness of the study is that the data were collected from only one health care system and therefore may not be representative of all populations. Additionally, cotesting was performed on 2 separately collected specimens, which may have reduced HPV testing performance.
Excessive cervical cancer screening, including frequent cotesting, could have minimal cancer prevention benefits while increasing the harms of screening. These data confirm guidance showing HPV testing alone is an effective cervical cancer screening strategy.
-- Mark H. Einstein, MD, MS
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Realistic prospective performance data are needed to quantify the additional benefit of the cytology component of cotesting on top of what is already known to be highly sensitive molecular HPV testing. While the addition of cytology to HPV testing can add performance, it also can add further costs and the potential for unnecessary colposcopies for what are merely cytomorphologic manifestations of an active HPV infection. Frequent invasive procedures such as colposcopy, which can be costly and lead to anxiety and distress in generally young women and the potential for overtreatment of likely regressive lesions, has been defined as a harm of screening by the US Preventive Services Task Force (USPSTF).
Details of the study
In a cohort from Kaiser Permanente Northern California, 1,208,710 women aged 30 years or older were screened with cotesting from 2003 to 2015. Those who cotested HPV negative and cytology negative were offered triennial screening. Positive cotest results were managed according to Kaiser protocol. Women with cytologic abnormalities were referred for colposcopy. Those with HPV positive/cytology negative results or HPV negative/cytology equivocal results underwent accelerated testing at 1 year. A total of 623 cervical cancers were identified and included in the analyses.
Using multiple analyses, Schiffman and colleagues demonstrated the sensitivity advantage of HPV testing. They clearly showed that the cytology component to cotesting performance over many years is very limited for detecting precancers and early curable cancers. For example, prediagnostic HPV testing (76.7%) was more likely to be positive than cytology (59.1%; P<.001 for paired comparison); 82.6% of all prediagnostic cotests were positive by HPV and/or cytology; and only 5.9% of the cotests were positive by cytology alone (HPV negative.)
Primary HPV testing is recommended as a potential screening strategy by an interim guidance group led by the Society of Gynecologic Oncology and the American Society for Colposcopy and Cervical Pathology, and it is the primary cervical cancer screening recommendation of USPSTF draft guidelines.1 There have been reports that reliance on primary HPV testing would encourage cervical cancer mortality; Schiffman and colleagues point out, however, that according to their study data, such reports are overstated.
Despite these data, practically speaking, shifting away from standard cotesting poses numerous challenges for clinicians and laboratories alike; however, these data clearly show the limited value of cytology and, due to the overtreatment of likely regressive cervical intraepithelial neoplasia grade 2, the possible increased risk of preterm birth and its subsequent harm as well.
Study strengths and weaknesses
The authors examined the long-term relative history of HPV testing and cytology prior to cancer diagnosis in a large, prospectively followed US cohort where hundreds of women in this cohort developed cancer. There will not be a validation study of this size and scale in the near future. Further, the authors showed that the relative value of cytology to cotesting is minimal. Multiple subsequent rounds of cotesting after negative results also can be questioned.
One weakness of the study is that the data were collected from only one health care system and therefore may not be representative of all populations. Additionally, cotesting was performed on 2 separately collected specimens, which may have reduced HPV testing performance.
Excessive cervical cancer screening, including frequent cotesting, could have minimal cancer prevention benefits while increasing the harms of screening. These data confirm guidance showing HPV testing alone is an effective cervical cancer screening strategy.
-- Mark H. Einstein, MD, MS
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330-337.
- Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Obstet Gynecol. 2015;125(2):330-337.
When could use of antenatal corticosteroids in the late preterm birth period be beneficial?
The use of antenatal corticosteroids for preterm deliveries between 24 and 34 weeks has been standard of care in obstetric practice. But approximately 70% of preterm deliveries in the United States occur after 34 weeks, in the so-called late preterm period (34 weeks 0 days to 36 weeks 6 days). Recently, Gyamfi-Bannerman and colleagues at the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network completed a trial that examined the use of antenatal betamethasone in women at risk for delivery in the late preterm period.
Details of the study
The Antenatal Late Preterm Steroids (ALPS) trial was a randomized, double-blind, placebo-controlled study that included women with a singleton gestation between 34 weeks 0 days and 36 weeks 5 days who had a high probability risk of delivery in the late preterm period. The authors defined “high probability of delivery” as spontaneous labor with cervical change (at least 3-cm dilation or 75% effacement), preterm premature rupture of the membranes, or a planned delivery scheduled in the late preterm period for specific obstetric indications, such as oligohydramnios, preeclampsia, gestational hypertension, and intrauterine growth restriction.
Women were excluded from the study if they had previously received a course of steroids or had multiple gestations, pregestational diabetes, chorioamnionitis, or were expected to deliver in less than 12 hours due to advanced labor, vaginal bleeding, or nonreassuring fetal status.
Study participants were randomly assigned to receive 2 doses (12 mg intramuscularly) of betamethasone 24 hours apart (1,429 participants) or identical-appearing placebo (1,402 participants). Tocolysis was not allowed in the protocol.
Positive outcomes for neonates
The use of corticosteroids was associated with a significant reduction in the primary outcome of need for respiratory support in the first 72 hours of life (14.4% in the placebo group vs 11.6% in the betamethasone group; relative risk [RR], 0.80; 95% confidence interval [CI], 0.66–0.97; P = .02). Steroid use also decreased the incidence of severe respiratory complications, the need for resuscitation at birth, the need for surfactant therapy, the incidence of transient tachypnea of the newborn, and the incidence of bronchopulmonary dysplasia. Neonatal hypoglycemia was more frequent among infants exposed to betamethasone (24% vs 15%; RR, 1.6; 95% CI, 1.37–1.87; P<.001).
New guidelines issued
The ALPS study is the largest randomized trial to evaluate the benefit of antenatal steroids during the late preterm period. The study’s findings certainly will change clinical practice. Based on the study’s large sample size, rigorous design and protocol, and a cohort generalizable to the US population, SMFM has issued new recommendations for practitioners on using antenatal steroids in the late preterm period in women at risk for preterm delivery.
What this EVIDENCE means for practice
In light of the new SMFM recommendations, in my practice, I will adhere to the inclusion criteria used in the ALPS study, and be careful not to apply the same approach used before 34 weeks, when delivery is often delayed intentionally in order to achieve steroid benefit. If considering adoption of this same practice, clinicians should not use tocolytics when administering corticosteroids in the late preterm period. When indicated, such as in women with severe preeclampsia or ruptured membranes, delivery should not be delayed. A patient with high probability of delivery in the late preterm period is eligible for treatment as long as the clinician thinks that she is not going to deliver within 12 hours. On the other hand, clinicians should not overtreat women, and should maintain a high suspicion for delivery in patients with preterm labor (a cervix that is at least 3 cm dilated or 75% effaced).
The ALPS trial did not allow the administration of more than one course of steroids. The eligibility criteria for corticosteroid use in the late preterm period should not be extended to include subpopulations that were not studied in the trial (including patients with multiple gestations, pregestational diabetes, or those who already had received a complete course of steroids).
— Luis Pacheco, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The use of antenatal corticosteroids for preterm deliveries between 24 and 34 weeks has been standard of care in obstetric practice. But approximately 70% of preterm deliveries in the United States occur after 34 weeks, in the so-called late preterm period (34 weeks 0 days to 36 weeks 6 days). Recently, Gyamfi-Bannerman and colleagues at the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network completed a trial that examined the use of antenatal betamethasone in women at risk for delivery in the late preterm period.
Details of the study
The Antenatal Late Preterm Steroids (ALPS) trial was a randomized, double-blind, placebo-controlled study that included women with a singleton gestation between 34 weeks 0 days and 36 weeks 5 days who had a high probability risk of delivery in the late preterm period. The authors defined “high probability of delivery” as spontaneous labor with cervical change (at least 3-cm dilation or 75% effacement), preterm premature rupture of the membranes, or a planned delivery scheduled in the late preterm period for specific obstetric indications, such as oligohydramnios, preeclampsia, gestational hypertension, and intrauterine growth restriction.
Women were excluded from the study if they had previously received a course of steroids or had multiple gestations, pregestational diabetes, chorioamnionitis, or were expected to deliver in less than 12 hours due to advanced labor, vaginal bleeding, or nonreassuring fetal status.
Study participants were randomly assigned to receive 2 doses (12 mg intramuscularly) of betamethasone 24 hours apart (1,429 participants) or identical-appearing placebo (1,402 participants). Tocolysis was not allowed in the protocol.
Positive outcomes for neonates
The use of corticosteroids was associated with a significant reduction in the primary outcome of need for respiratory support in the first 72 hours of life (14.4% in the placebo group vs 11.6% in the betamethasone group; relative risk [RR], 0.80; 95% confidence interval [CI], 0.66–0.97; P = .02). Steroid use also decreased the incidence of severe respiratory complications, the need for resuscitation at birth, the need for surfactant therapy, the incidence of transient tachypnea of the newborn, and the incidence of bronchopulmonary dysplasia. Neonatal hypoglycemia was more frequent among infants exposed to betamethasone (24% vs 15%; RR, 1.6; 95% CI, 1.37–1.87; P<.001).
New guidelines issued
The ALPS study is the largest randomized trial to evaluate the benefit of antenatal steroids during the late preterm period. The study’s findings certainly will change clinical practice. Based on the study’s large sample size, rigorous design and protocol, and a cohort generalizable to the US population, SMFM has issued new recommendations for practitioners on using antenatal steroids in the late preterm period in women at risk for preterm delivery.
What this EVIDENCE means for practice
In light of the new SMFM recommendations, in my practice, I will adhere to the inclusion criteria used in the ALPS study, and be careful not to apply the same approach used before 34 weeks, when delivery is often delayed intentionally in order to achieve steroid benefit. If considering adoption of this same practice, clinicians should not use tocolytics when administering corticosteroids in the late preterm period. When indicated, such as in women with severe preeclampsia or ruptured membranes, delivery should not be delayed. A patient with high probability of delivery in the late preterm period is eligible for treatment as long as the clinician thinks that she is not going to deliver within 12 hours. On the other hand, clinicians should not overtreat women, and should maintain a high suspicion for delivery in patients with preterm labor (a cervix that is at least 3 cm dilated or 75% effaced).
The ALPS trial did not allow the administration of more than one course of steroids. The eligibility criteria for corticosteroid use in the late preterm period should not be extended to include subpopulations that were not studied in the trial (including patients with multiple gestations, pregestational diabetes, or those who already had received a complete course of steroids).
— Luis Pacheco, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The use of antenatal corticosteroids for preterm deliveries between 24 and 34 weeks has been standard of care in obstetric practice. But approximately 70% of preterm deliveries in the United States occur after 34 weeks, in the so-called late preterm period (34 weeks 0 days to 36 weeks 6 days). Recently, Gyamfi-Bannerman and colleagues at the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network completed a trial that examined the use of antenatal betamethasone in women at risk for delivery in the late preterm period.
Details of the study
The Antenatal Late Preterm Steroids (ALPS) trial was a randomized, double-blind, placebo-controlled study that included women with a singleton gestation between 34 weeks 0 days and 36 weeks 5 days who had a high probability risk of delivery in the late preterm period. The authors defined “high probability of delivery” as spontaneous labor with cervical change (at least 3-cm dilation or 75% effacement), preterm premature rupture of the membranes, or a planned delivery scheduled in the late preterm period for specific obstetric indications, such as oligohydramnios, preeclampsia, gestational hypertension, and intrauterine growth restriction.
Women were excluded from the study if they had previously received a course of steroids or had multiple gestations, pregestational diabetes, chorioamnionitis, or were expected to deliver in less than 12 hours due to advanced labor, vaginal bleeding, or nonreassuring fetal status.
Study participants were randomly assigned to receive 2 doses (12 mg intramuscularly) of betamethasone 24 hours apart (1,429 participants) or identical-appearing placebo (1,402 participants). Tocolysis was not allowed in the protocol.
Positive outcomes for neonates
The use of corticosteroids was associated with a significant reduction in the primary outcome of need for respiratory support in the first 72 hours of life (14.4% in the placebo group vs 11.6% in the betamethasone group; relative risk [RR], 0.80; 95% confidence interval [CI], 0.66–0.97; P = .02). Steroid use also decreased the incidence of severe respiratory complications, the need for resuscitation at birth, the need for surfactant therapy, the incidence of transient tachypnea of the newborn, and the incidence of bronchopulmonary dysplasia. Neonatal hypoglycemia was more frequent among infants exposed to betamethasone (24% vs 15%; RR, 1.6; 95% CI, 1.37–1.87; P<.001).
New guidelines issued
The ALPS study is the largest randomized trial to evaluate the benefit of antenatal steroids during the late preterm period. The study’s findings certainly will change clinical practice. Based on the study’s large sample size, rigorous design and protocol, and a cohort generalizable to the US population, SMFM has issued new recommendations for practitioners on using antenatal steroids in the late preterm period in women at risk for preterm delivery.
What this EVIDENCE means for practice
In light of the new SMFM recommendations, in my practice, I will adhere to the inclusion criteria used in the ALPS study, and be careful not to apply the same approach used before 34 weeks, when delivery is often delayed intentionally in order to achieve steroid benefit. If considering adoption of this same practice, clinicians should not use tocolytics when administering corticosteroids in the late preterm period. When indicated, such as in women with severe preeclampsia or ruptured membranes, delivery should not be delayed. A patient with high probability of delivery in the late preterm period is eligible for treatment as long as the clinician thinks that she is not going to deliver within 12 hours. On the other hand, clinicians should not overtreat women, and should maintain a high suspicion for delivery in patients with preterm labor (a cervix that is at least 3 cm dilated or 75% effaced).
The ALPS trial did not allow the administration of more than one course of steroids. The eligibility criteria for corticosteroid use in the late preterm period should not be extended to include subpopulations that were not studied in the trial (including patients with multiple gestations, pregestational diabetes, or those who already had received a complete course of steroids).
— Luis Pacheco, MD
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Start offering aspirin to pregnant women at high risk for preeclampsia
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7
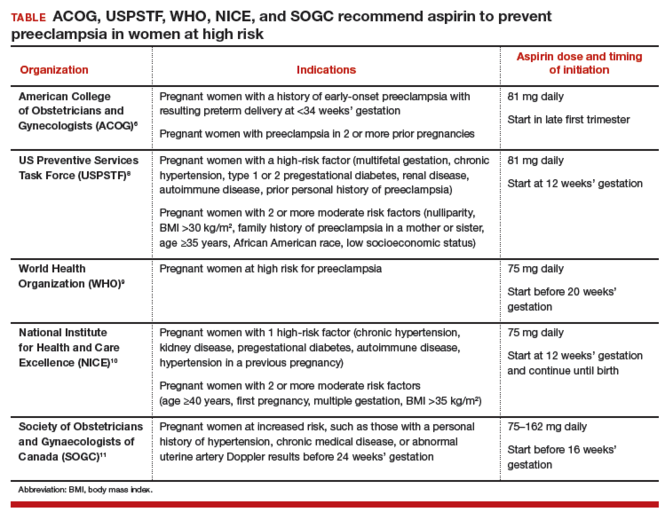
An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to rbarbieri@frontlinemedcom.com. Please include the city and state in which you practice.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7

An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to rbarbieri@frontlinemedcom.com. Please include the city and state in which you practice.
Obstetricians work diligently to anticipate, diagnose, and treat preeclampsia because the maternal and perinatal health burden of the disease is enormous. Many meta-analyses have reported that aspirin treatment of women at high risk for preeclampsia reduces the risk of developing the disease by about 10% to 23%.1–5 In addition, for women at high risk for preeclampsia, aspirin treatment reduces the risk of preterm birth and intrauterine growth restriction (IUGR). In your practice you should start offering aspirin to pregnant women at high risk for preeclampsia.
Aspirin reduces the risk of preeclampsia, preterm birth, and IUGRBased on the results of multiple meta-analyses of clinical trials involving more than 35,000 women, investigators consistently have concluded that aspirin treatment reduces the risk of preeclampsia in women at high risk for the disease.1–5 The magnitude of the effect is difficult to define with precision, but the risk reduction is likely in the range of 10% to 23%.1
In addition to reducing the risk of preeclampsia, aspirin also reduces the risk of 2 associated problems: preterm birth and IUGR. For preterm birth, the risk reduction is estimated to be in the range of 11% to 31%. For IUGR, the estimation for risk reduction is in the range of 7% to 24%.1 Although these benefits are modest, the burden of maternal and perinatal morbidity associated with preeclampsia is great, making even a modest benefit clinically significant.
Potential harms of aspirin treatmentIn the most recent meta-analysis from the US Preventive Services Task Force (USPSTF),1 low-dose aspirin treatment was associated with no significant perinatal or maternal harms, but rare harms could not be ruled out. A small increase in the risk of placental abruption was noted, but this increase did not reach significance (relative risk [RR], 1.17; 95% confidence interval [CI], 0.93–1.48).1 There was no increased risk of maternal postpartum hemorrhage or blood loss at delivery.1 In one meta-analysis, aspirin treatment did not increase the risk of newborn intracranial hemorrhage.1
Other potential adverse effects of aspirin treatment include maternal gastrointestinal bleeding and exacerbation of respiratory disorders such as asthma, but these effects have not been reported as significant associations in clinical trials of preeclampsia prevention.
Dueling recommendations: Restrictive or liberal use of aspirin?The American College of Obstetricians and Gynecologists (ACOG) recommends use of aspirin to prevent preeclampsia in women who have a personal history of early-onset preeclampsia with delivery before 34 weeks of gestation and in women with preeclampsia in 2 or more prior pregnancies.6 The restrictive ACOG guideline recommends aspirin treatment for a very small group of women. In one analysis, using the ACOG guideline, only 0.35% of all pregnant women would be eligible for treatment with aspirin to prevent preeclampsia.7
The USPSTF recommends that all pregnant women with one major risk factor for preeclampsia—including multifetal gestation, chronic hypertension, type 1 or 2 pregestational diabetes, renal disease, autoimmune disease, or prior personal history of preeclampsia—receive treatment with aspirin to prevent preeclampsia.8 The Task Force also recommends that women with multiple moderate risk factors for preeclampsia, such as nulliparity, body mass index greater than 30 kg/m2, family history of preeclampsia in a mother or sister, age 35 years or older, and certain sociodemographic risk factors (African American race, low socioeconomic status) also be offered aspirin treatment.
The USPSTF guideline advises aspirin treatment for many women. According to one analysis, the USPSTFguideline would result in approximately 24% of all pregnant women being offered aspirin treatment.7
The USPSTF guideline would result in 67 times more pregnant women being treated with aspirin than the ACOG guideline. The narrowly focused ACOG recommendation is problematic because it recommends against aspirin treatment in women who are at very high risk for developing preeclampsia, for example, a 41-year-old woman in her first pregnancy with twins and pregestational diabetes. In addition, the ACOG recommendation is not consistent with the recommendations of most other major health organizations.
The World Health Organization,9 the United Kingdom’s National Institute for Health and Care Excellence (NICE),10 and the Society of Obstetricians and Gynaecologists of Canada11 all recommend aspirin treatment to prevent preeclampsia in pregnant women at high risk for the disease and utilize an expanded definition of “high risk” (TABLE). Some experts have observed that, in actual clinical practice, it is often difficult to consistently implement a prevention plan based on a complex assessment of clinical risk factors.7

An alternative to guidelines that use clinical risk factors to identify women at high risk is universal treatment. With universal treatment all pregnant women are prescribed aspirin, thereby maximizing the clinical benefit but unnecessarily treating many women with aspirin.7 Universal treatment of pregnant women with aspirin appears to be cost-effective and would be associated with annual health care savings of $365 million.7
Timing of aspirin initiationIn one meta-analysis, initiating aspirin before 16 weeks’ gestation resulted in a greater reduction in preeclampsia than starting aspirin after 16 weeks.12 The USPSTF cautions that meta-analysis of the available data is not well suited for identifying the optimal time to initiate aspirin therapy.13 ACOG, USPSTF, and NICE recommend initiating aspirin therapy at approximately 12 weeks’ gestation—the end of the first trimester.
Ideal aspirin doseThe optimal dose of aspirin to prevent preeclampsia is not precisely defined. Aspirin doses ranging from 50 mg to 162 mg have been proposed for the prevention of preeclampsia. Most authorities recommend a daily dose between 80 mg and less than 300 mg to prevent preeclampsia.14 ACOG and USPSTF recommend aspirin at a dose of 81 mg daily,6,8 because this dose is widely available in the United States.
Let’s close the gap between current and optimal practiceAccording to the USPSTF guidelines, approximately 24% of the pregnant women in our practices have risk factors that would justify the initiation of aspirin treatment for the prevention of preeclampsia.8 This approach would modestly reduce the rate of preeclampsia and the associated problems of preterm birth and IUGR with little cost and few adverse effects. Yet relatively few pregnant women in the United States are currently receiving aspirin therapy. We could close this clinical gap between current and optimal practice by reflecting on the USPSTF recommendations and implementing them in our practices, as appropriate.
Tell us…What are your thoughts about the use of aspirin in pregnant women who are at high risk for preeclampsia?
Send your letter to the editor to rbarbieri@frontlinemedcom.com. Please include the city and state in which you practice.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.
- Henderson JT, Whitlock EP, O'Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(10):695-703.
- Roberge S, Nicolaides KH, Demers S, Villa P, Bujold E. Prevention of perinatal death and adverse perinatal outcome using low-dose aspirin: a meta-analysis. Ultrasound Obstet Gynecol. 2013;41(5):491-499.
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402-414.
- Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791-1798.
- American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122-1131.
- Werner EF, Hauspurg AK, Rouse DJ. A cost-benefit analysis of low-dose aspirin prophylaxis for the prevention of preeclampsia in the United States. Obstet Gynecol. 2015;126(6):1242-1250.
- LeFevre ML; US Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(11):819-826.
- World Health Organization. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia. Geneva, Switzerland: WHO; 2011:13-15. https://www.preeclampsia.org/images/pdf/2011c-who_pe_final.pdf. Accessed January 4, 2016.
- National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. Clinical guideline 107. Manchester, United Kingdom: NICE; 2010:7. https://www.nice.org.uk/guidance/cg107/resources/hypertension-in-pregnancy-diagnosis-and-management-35109334009285. Accessed April 4, 2016.
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36(5):416-441.
- Roberge S, Demers S, Bujold E. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613.
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia [letter to the editor]. Ann Intern Med. 2014;161(8):613-614.
- Bujold E, Roberge S, Nicolaides KH. Low-dose aspirin for prevention of adverse outcomes related to abnormal placentation. Prenat Diagn. 2014;34(7):642-648.
Need for caution before extending the use of antenatal corticosteroids beyond 34 weeks’ gestation
The results of the highly anticipated Antenatal Late Preterm Study recently have become available.1 Data from this randomized controlled trial, conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network, demonstrated that administration of betamethasone to women at risk for preterm delivery between 34 weeks 0 days and 36 weeks 6 days of gestation significantly reduces the rate of neonatal respiratory complications. It may represent the largest study of antenatal corticosteroids (ACS) to date, with 2,827 infants studied, and its results inevitably lead to the logical practical question: Should ACS use be extended beyond the 34 weeks’ gestation limit previously recommended by professional guidelines in the United States2?
There are some issues that bear discussion before such a significant change in standard of care should be promoted.2
Antenatal Late Preterm Study outcomesThe primary outcome in the study was a composite end point describing the need for respiratory support within 72 hours after birth. Based on a pilot study, the investigators had anticipated a 33% decrease in the rate of the primary outcome; however, the reduction was only 20% (relative risk [RR], 0.80; 95% confidence interval [CI], 0.66−0.97). Although the effect size was statistically significant, one could question the clinical relevance of such a small difference.
A 33% reduction effect, more consistent with the preliminary expectations, was noted in the prespecified secondary composite outcome of severe respiratory complications (RR, 0.67; 95% CI, 0.53−0.84). Occurrences included in the secondary composite outcome that also showed significant rate reductions were:
- the use of continuous positive airway pressure (CPAP) or high-flow oxygen via nasal cannula for at least 12 hours (RR, 0.62; 95% CI, 0.48−0.80)
- need for resuscitation at birth (RR, 0.78; 95% CI, 0.66−0.92)
- surfactant use (RR, 0.59; 95% CI, 0.37−0.96)
- transient tachypnea of the newborn (RR, 0.68; 95% CI, 0.53−0.87).
The reported reduction in bronchopulmonary dysplasia (RR, 0.22; 95% CI, 0.02−0.92) cannot plausibly be attributed to ACS. Randomized data aggregated by the Cochrane Database of Systematic Reviews3 do not show improvement in chronic lung disease with ACS use. Moreover, the authors recognize that the assessment for bronchopulmonary dysplasia at only 28 days of life is only partially informative and that longer childhood follow-up is required to confirm the finding.
- Although corticosteroids have been shown to reduce the risk of the baby needing breathing support by 20%, they are associated with a 60% increase in risk for low blood sugar in the newborn (hypoglycemia). Hypoglycemia can place the baby at risk for seizures and even brain damage.
- There is an unknown safety profile for corticosteroid administration at this gestational age. The fetal brain is still developing during this period, and there is some information to suggest that corticosteroids could have an unfavorable effect on brain development.
- Corticosteroids are potent hormones and potentially can have undesired hormonal effects at this gestational age.
- If corticosteroids are given and the mother carries the baby to term (37 weeks or later) there are some studies that suggest the baby is at an increased risk for neurologic, cognitive, metabolic, and/or behavioral abnormalities in later life.
We recommend caution before changing current practiceWe propose prudence with ACS use after 34 weeks’ gestation for the following reasons: the increased risk for neonatal hypoglycemia associated with ACS, the increased risk for ACS-related harm in term-born babies, and safety concerns with ACS in the late preterm period.
Evidence shows an increased risk for neonatal hypoglycemiaThe most profound effect modification observed in the study was an adverse effect—namely, a 60% increase in neonatal hypoglycemia with ACS administration (RR, 1.6; 95% CI, 1.37−1.87). The rate of neonatal hypoglycemia was 24% in the ACS group, compared with 15% in the placebo group.
Results of prior studies have demonstrated either no increased risk of hypoglycemia with ACS use4−7 or a much smaller increase (from 4.2% to 5.7%).8 The higher rate of neonatal hypoglycemia seen in this study suggests the possibility that the late preterm population may be more vulnerable to the negative impact of ACS on neonatal glucose/insulin homeostasis. Presumed mechanisms of action are either maternal hyperglycemia or fetal adrenal suppression or both, with potential for fetal adrenal suppression resulting from betamethasone exposure to affect long-term metabolic outcomes.9
Of note, women with pregestational diabetes were excluded from the study and, in routine practice, inclusion of such patients may further increase the risk of neonatal hypoglycemia.
There are few data on the prognostic significance of neonatal hypoglycemia in preterm infants, with the exception of a single study, the results of which show that it is associated with adverse neurodevelopment at 18 months of age.10
Data reveal increased risk for harm in term-born babiesIn spite of strict protocol specifications to increase the probability of delivery before 37 weeks’ gestation, 16% of women in the trial delivered at term. Investigators of prior randomized studies of ACS, aimed at reducing the risks of prematurity, have reported a rate of term delivery of about one-third,4,11 and in routine practice, administration of ACS after 34 weeks may be associated with even higher rates of term delivery.
This is important because recent evidence shows an unfavorable impact of ACS exposure in term-born children.12 The 5-year follow-up of the largest randomized trial in which multiple ACS courses were used noted that babies born at term had a 4-fold increased odds ratio for neurosensory disability.11 There was no dose−response interaction, with the same adverse odds ratio after 1 or 4 additional ACS courses. This observation was consistent with a previously reported Swedish national cohort, pointing to an unfavorable impact of even a single course of ACS in term-born children, with a greater likelihood of harm than benefit.13
In a UK follow-up of children aged 8 to 15 years who were enrolled in an RCT of ACS before cesarean delivery at term, low academic achievement was significantly more common in the group whose mothers had received ACS.14 In another study of 304 children born at term after exposure to a single course of ACS, investigators noted significantly increased cortisol reactivity to acute psychological stress at ages 6 to 11 years in the ACS-exposed patients, compared with 212 babies of women with threatened preterm labor who did not receive ACS and 372 babies from uncomplicated term pregnancies.15
The relevance of such study findings extends beyond childhood given the fact that elevated hypothalamic-pituitary-adrenal (HPA) axis reactivity has been linked to the pathogenesis of metabolic syndrome and depression in adult life.16 As recently as 2015, investigators of a randomized trial of ACS in 6 low- and middle-income countries highlighted their concern regarding “potentially harmful use of antenatal corticosteroids for infants not delivered preterm.”17
There are safety concerns with ACS in the late preterm periodThe effects of ACS are more pleiotropic than those reflected in a lower incidence of respiratory difficulties. Knowledge of the overall consequences of ACS exposure in infants born late-preterm or at term is still limited. The close-to-term fetus exposed to exogenous corticosteroids is also exposed to the physiologic endogenous surge of cortisol known to occur in the maternal circulation in late pregnancy, which reaches levels 3 times higher than those seen in nonpregnant women.18 Although placental 11 beta-hydroxysteroid dehydrogenase type 2 plays a protective role by allowing no more than 10% to 20% of maternal corticosteroids to cross the placenta, fetal overexposure from concomitant exogenous maternal corticosteroid administration remains a theoretical concern close to term. This is especially worrisome if there is a gestational age−related increase in the sensitivity to corticosteroid-induced in utero fetal programming. It has been reported that fetal overexposure to corticosteroids in late pregnancy can permanently increase the activity of the HPA-axis, with likely consequences in adult life.19
Another concern relates to oligodendrocytes development. Although the neuronal division process in humans usually is completed by 24 weeks’ gestation, the most rapid growth for oligodendrocytes occurs between 34 and 36 weeks’ gestation; these are the cells responsible for the synthesis of myelin. Overexposure to corticosteroids at this vulnerable time in the late preterm fetus potentially may have unanticipated negative neurologic consequences.20
This is the only scenario in which we feel antenatal corticosteroids could be used in a fetus aged 34 weeks to 36 weeks 5 days. In the setting of a scheduled cesarean delivery between 34 weeks and 35 weeks, the concerns relative to term delivery after corticosteroid exposure may not apply, but the concerns in relation to the administration of corticosteroid in the late preterm period—which is a time of possibly increased neurohormonal and neurologic vulnerability—still apply. With regard to the risk of neonatal hypoglycemia, one might argue that close neonatal monitoring of babies so exposed may ensure that any associated neonatal hypoglycemia does not go unnoticed or untreated. However, the prognostic significance of even short periods of neonatal hypoglycemia has not been established.
Where should future studies focus?There is clear neonatal benefit from a single course of ACS given to women who will deliver before 34 weeks’ gestation. It is widely accepted, based on the evidence provided by the 30-year follow-up of the cohort of 534 participants from the Auckland trial (the longest follow-up for any pregnancy trial), that administration of ACS at less than 34 weeks’ gestation is not associated with any obvious major developmental risk.21−23
However, the reassurances provided by the Auckland cohort should be neither directly extrapolated to the administration of ACS in the late preterm period nor applied to term-born babies exposed to ACS, for the simple reason that these subgroups never have been analyzed separately. The risk:benefit ratio of ACS use in the late-preterm period is as yet unknown, and in term-born babies the ratio may be unfavorable.
Follow-up studies are neededWe consider that there is a vital need for long-term follow-up studies. The focus of research on the effects of ACS no longer is on the immediate neonatal outcomes and now is on safety and the long-term outcomes of this exposure.
Bottom lineWe regard the large, high-quality study conducted by the NICHD MFMU Network1 as an opportunity to answer current concerns. It is hoped that the resources necessaryfor in-depth follow-up of the children involved in this study will be provided to the investigators and to the NICHD. It is only with such follow-up that mid- and long-term adverse effects can be assessed. We believe that, at a minimum, mid-term follow-up data should be available before it is wise to make any definitive recommendations for a sweeping change in clinical practice.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery [published online ahead of print February 4, 2016]. N Engl J Med.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117(2 pt 1):422–424.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454.
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525.
- Sann L, Burnod J, Lasne Y, Bethenod M. Antenatal administration of betamethasone: effects upon neonatal blood glucose in premature infants [in French]. Nouv Presse Med. 1979;8(39):3147–3148.
- Rokicki W, Krasnodebski J. Antenatal glucocorticoid administration and neonatal glycemia. Dev Pharmacol Ther. 1987;10(4):307–311.
- Gazquez Serrano IM, Arroyos Plana A, Diaz Morales O, Herraiz Perea C, Holgueras Bragado A. Antenatal corticosteroid therapy and late preterm infant morbidity and mortality [in Spanish]. An Pediatr (Barc). 2014;81(6):374–382.
- Pettit KE, Tran SH, Lee E, Caughey AB. The association of antenatal corticosteroids with neonatal hypoglycemia and hyperbilirubinemia. J Matern Fetal Neonatal Med. 2014;27(7):683–686.
- Aydin M, Derveci U, Hakan N. Neonatal hypoglycemia associated with the antenatal corticosteroids may be secondary to fetal adrenal suppression. J Matern Fetal Neonatal Med. 2015;28(8):892.
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308.
- Asztalos EV, Murphy KE, Willan AR, et al; MACS-5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102–1110.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications [published online ahead of print January 18, 2016]. BJOG. doi:10.1111/1471-0528.13853.
- Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Health consequences of prophylactic exposure to antenatal corticosteroids among children born late preterm or term. Acta Obstet Gynecol Scand. 2012;91(12):1415–1421.
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F195–F200.
- Alexander N, Rosenlocher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381.
- Althabe F, Belizan JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639.
- Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11ß-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdale GR mRNA expression and anxiety-like behavior in the offspring. Eur J Neurosci. 2000;12(3):1047–1054.
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F154–F157.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862.
- Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11Dalziel SR, Walker NK, Parag V, et al. Dalziel SR, Lim VK, Lambert A, et al. Dalziel SR, Rea HH, Walker NK, et al.
The results of the highly anticipated Antenatal Late Preterm Study recently have become available.1 Data from this randomized controlled trial, conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network, demonstrated that administration of betamethasone to women at risk for preterm delivery between 34 weeks 0 days and 36 weeks 6 days of gestation significantly reduces the rate of neonatal respiratory complications. It may represent the largest study of antenatal corticosteroids (ACS) to date, with 2,827 infants studied, and its results inevitably lead to the logical practical question: Should ACS use be extended beyond the 34 weeks’ gestation limit previously recommended by professional guidelines in the United States2?
There are some issues that bear discussion before such a significant change in standard of care should be promoted.2
Antenatal Late Preterm Study outcomesThe primary outcome in the study was a composite end point describing the need for respiratory support within 72 hours after birth. Based on a pilot study, the investigators had anticipated a 33% decrease in the rate of the primary outcome; however, the reduction was only 20% (relative risk [RR], 0.80; 95% confidence interval [CI], 0.66−0.97). Although the effect size was statistically significant, one could question the clinical relevance of such a small difference.
A 33% reduction effect, more consistent with the preliminary expectations, was noted in the prespecified secondary composite outcome of severe respiratory complications (RR, 0.67; 95% CI, 0.53−0.84). Occurrences included in the secondary composite outcome that also showed significant rate reductions were:
- the use of continuous positive airway pressure (CPAP) or high-flow oxygen via nasal cannula for at least 12 hours (RR, 0.62; 95% CI, 0.48−0.80)
- need for resuscitation at birth (RR, 0.78; 95% CI, 0.66−0.92)
- surfactant use (RR, 0.59; 95% CI, 0.37−0.96)
- transient tachypnea of the newborn (RR, 0.68; 95% CI, 0.53−0.87).
The reported reduction in bronchopulmonary dysplasia (RR, 0.22; 95% CI, 0.02−0.92) cannot plausibly be attributed to ACS. Randomized data aggregated by the Cochrane Database of Systematic Reviews3 do not show improvement in chronic lung disease with ACS use. Moreover, the authors recognize that the assessment for bronchopulmonary dysplasia at only 28 days of life is only partially informative and that longer childhood follow-up is required to confirm the finding.
- Although corticosteroids have been shown to reduce the risk of the baby needing breathing support by 20%, they are associated with a 60% increase in risk for low blood sugar in the newborn (hypoglycemia). Hypoglycemia can place the baby at risk for seizures and even brain damage.
- There is an unknown safety profile for corticosteroid administration at this gestational age. The fetal brain is still developing during this period, and there is some information to suggest that corticosteroids could have an unfavorable effect on brain development.
- Corticosteroids are potent hormones and potentially can have undesired hormonal effects at this gestational age.
- If corticosteroids are given and the mother carries the baby to term (37 weeks or later) there are some studies that suggest the baby is at an increased risk for neurologic, cognitive, metabolic, and/or behavioral abnormalities in later life.
We recommend caution before changing current practiceWe propose prudence with ACS use after 34 weeks’ gestation for the following reasons: the increased risk for neonatal hypoglycemia associated with ACS, the increased risk for ACS-related harm in term-born babies, and safety concerns with ACS in the late preterm period.
Evidence shows an increased risk for neonatal hypoglycemiaThe most profound effect modification observed in the study was an adverse effect—namely, a 60% increase in neonatal hypoglycemia with ACS administration (RR, 1.6; 95% CI, 1.37−1.87). The rate of neonatal hypoglycemia was 24% in the ACS group, compared with 15% in the placebo group.
Results of prior studies have demonstrated either no increased risk of hypoglycemia with ACS use4−7 or a much smaller increase (from 4.2% to 5.7%).8 The higher rate of neonatal hypoglycemia seen in this study suggests the possibility that the late preterm population may be more vulnerable to the negative impact of ACS on neonatal glucose/insulin homeostasis. Presumed mechanisms of action are either maternal hyperglycemia or fetal adrenal suppression or both, with potential for fetal adrenal suppression resulting from betamethasone exposure to affect long-term metabolic outcomes.9
Of note, women with pregestational diabetes were excluded from the study and, in routine practice, inclusion of such patients may further increase the risk of neonatal hypoglycemia.
There are few data on the prognostic significance of neonatal hypoglycemia in preterm infants, with the exception of a single study, the results of which show that it is associated with adverse neurodevelopment at 18 months of age.10
Data reveal increased risk for harm in term-born babiesIn spite of strict protocol specifications to increase the probability of delivery before 37 weeks’ gestation, 16% of women in the trial delivered at term. Investigators of prior randomized studies of ACS, aimed at reducing the risks of prematurity, have reported a rate of term delivery of about one-third,4,11 and in routine practice, administration of ACS after 34 weeks may be associated with even higher rates of term delivery.
This is important because recent evidence shows an unfavorable impact of ACS exposure in term-born children.12 The 5-year follow-up of the largest randomized trial in which multiple ACS courses were used noted that babies born at term had a 4-fold increased odds ratio for neurosensory disability.11 There was no dose−response interaction, with the same adverse odds ratio after 1 or 4 additional ACS courses. This observation was consistent with a previously reported Swedish national cohort, pointing to an unfavorable impact of even a single course of ACS in term-born children, with a greater likelihood of harm than benefit.13
In a UK follow-up of children aged 8 to 15 years who were enrolled in an RCT of ACS before cesarean delivery at term, low academic achievement was significantly more common in the group whose mothers had received ACS.14 In another study of 304 children born at term after exposure to a single course of ACS, investigators noted significantly increased cortisol reactivity to acute psychological stress at ages 6 to 11 years in the ACS-exposed patients, compared with 212 babies of women with threatened preterm labor who did not receive ACS and 372 babies from uncomplicated term pregnancies.15
The relevance of such study findings extends beyond childhood given the fact that elevated hypothalamic-pituitary-adrenal (HPA) axis reactivity has been linked to the pathogenesis of metabolic syndrome and depression in adult life.16 As recently as 2015, investigators of a randomized trial of ACS in 6 low- and middle-income countries highlighted their concern regarding “potentially harmful use of antenatal corticosteroids for infants not delivered preterm.”17
There are safety concerns with ACS in the late preterm periodThe effects of ACS are more pleiotropic than those reflected in a lower incidence of respiratory difficulties. Knowledge of the overall consequences of ACS exposure in infants born late-preterm or at term is still limited. The close-to-term fetus exposed to exogenous corticosteroids is also exposed to the physiologic endogenous surge of cortisol known to occur in the maternal circulation in late pregnancy, which reaches levels 3 times higher than those seen in nonpregnant women.18 Although placental 11 beta-hydroxysteroid dehydrogenase type 2 plays a protective role by allowing no more than 10% to 20% of maternal corticosteroids to cross the placenta, fetal overexposure from concomitant exogenous maternal corticosteroid administration remains a theoretical concern close to term. This is especially worrisome if there is a gestational age−related increase in the sensitivity to corticosteroid-induced in utero fetal programming. It has been reported that fetal overexposure to corticosteroids in late pregnancy can permanently increase the activity of the HPA-axis, with likely consequences in adult life.19
Another concern relates to oligodendrocytes development. Although the neuronal division process in humans usually is completed by 24 weeks’ gestation, the most rapid growth for oligodendrocytes occurs between 34 and 36 weeks’ gestation; these are the cells responsible for the synthesis of myelin. Overexposure to corticosteroids at this vulnerable time in the late preterm fetus potentially may have unanticipated negative neurologic consequences.20
This is the only scenario in which we feel antenatal corticosteroids could be used in a fetus aged 34 weeks to 36 weeks 5 days. In the setting of a scheduled cesarean delivery between 34 weeks and 35 weeks, the concerns relative to term delivery after corticosteroid exposure may not apply, but the concerns in relation to the administration of corticosteroid in the late preterm period—which is a time of possibly increased neurohormonal and neurologic vulnerability—still apply. With regard to the risk of neonatal hypoglycemia, one might argue that close neonatal monitoring of babies so exposed may ensure that any associated neonatal hypoglycemia does not go unnoticed or untreated. However, the prognostic significance of even short periods of neonatal hypoglycemia has not been established.
Where should future studies focus?There is clear neonatal benefit from a single course of ACS given to women who will deliver before 34 weeks’ gestation. It is widely accepted, based on the evidence provided by the 30-year follow-up of the cohort of 534 participants from the Auckland trial (the longest follow-up for any pregnancy trial), that administration of ACS at less than 34 weeks’ gestation is not associated with any obvious major developmental risk.21−23
However, the reassurances provided by the Auckland cohort should be neither directly extrapolated to the administration of ACS in the late preterm period nor applied to term-born babies exposed to ACS, for the simple reason that these subgroups never have been analyzed separately. The risk:benefit ratio of ACS use in the late-preterm period is as yet unknown, and in term-born babies the ratio may be unfavorable.
Follow-up studies are neededWe consider that there is a vital need for long-term follow-up studies. The focus of research on the effects of ACS no longer is on the immediate neonatal outcomes and now is on safety and the long-term outcomes of this exposure.
Bottom lineWe regard the large, high-quality study conducted by the NICHD MFMU Network1 as an opportunity to answer current concerns. It is hoped that the resources necessaryfor in-depth follow-up of the children involved in this study will be provided to the investigators and to the NICHD. It is only with such follow-up that mid- and long-term adverse effects can be assessed. We believe that, at a minimum, mid-term follow-up data should be available before it is wise to make any definitive recommendations for a sweeping change in clinical practice.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
The results of the highly anticipated Antenatal Late Preterm Study recently have become available.1 Data from this randomized controlled trial, conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network, demonstrated that administration of betamethasone to women at risk for preterm delivery between 34 weeks 0 days and 36 weeks 6 days of gestation significantly reduces the rate of neonatal respiratory complications. It may represent the largest study of antenatal corticosteroids (ACS) to date, with 2,827 infants studied, and its results inevitably lead to the logical practical question: Should ACS use be extended beyond the 34 weeks’ gestation limit previously recommended by professional guidelines in the United States2?
There are some issues that bear discussion before such a significant change in standard of care should be promoted.2
Antenatal Late Preterm Study outcomesThe primary outcome in the study was a composite end point describing the need for respiratory support within 72 hours after birth. Based on a pilot study, the investigators had anticipated a 33% decrease in the rate of the primary outcome; however, the reduction was only 20% (relative risk [RR], 0.80; 95% confidence interval [CI], 0.66−0.97). Although the effect size was statistically significant, one could question the clinical relevance of such a small difference.
A 33% reduction effect, more consistent with the preliminary expectations, was noted in the prespecified secondary composite outcome of severe respiratory complications (RR, 0.67; 95% CI, 0.53−0.84). Occurrences included in the secondary composite outcome that also showed significant rate reductions were:
- the use of continuous positive airway pressure (CPAP) or high-flow oxygen via nasal cannula for at least 12 hours (RR, 0.62; 95% CI, 0.48−0.80)
- need for resuscitation at birth (RR, 0.78; 95% CI, 0.66−0.92)
- surfactant use (RR, 0.59; 95% CI, 0.37−0.96)
- transient tachypnea of the newborn (RR, 0.68; 95% CI, 0.53−0.87).
The reported reduction in bronchopulmonary dysplasia (RR, 0.22; 95% CI, 0.02−0.92) cannot plausibly be attributed to ACS. Randomized data aggregated by the Cochrane Database of Systematic Reviews3 do not show improvement in chronic lung disease with ACS use. Moreover, the authors recognize that the assessment for bronchopulmonary dysplasia at only 28 days of life is only partially informative and that longer childhood follow-up is required to confirm the finding.
- Although corticosteroids have been shown to reduce the risk of the baby needing breathing support by 20%, they are associated with a 60% increase in risk for low blood sugar in the newborn (hypoglycemia). Hypoglycemia can place the baby at risk for seizures and even brain damage.
- There is an unknown safety profile for corticosteroid administration at this gestational age. The fetal brain is still developing during this period, and there is some information to suggest that corticosteroids could have an unfavorable effect on brain development.
- Corticosteroids are potent hormones and potentially can have undesired hormonal effects at this gestational age.
- If corticosteroids are given and the mother carries the baby to term (37 weeks or later) there are some studies that suggest the baby is at an increased risk for neurologic, cognitive, metabolic, and/or behavioral abnormalities in later life.
We recommend caution before changing current practiceWe propose prudence with ACS use after 34 weeks’ gestation for the following reasons: the increased risk for neonatal hypoglycemia associated with ACS, the increased risk for ACS-related harm in term-born babies, and safety concerns with ACS in the late preterm period.
Evidence shows an increased risk for neonatal hypoglycemiaThe most profound effect modification observed in the study was an adverse effect—namely, a 60% increase in neonatal hypoglycemia with ACS administration (RR, 1.6; 95% CI, 1.37−1.87). The rate of neonatal hypoglycemia was 24% in the ACS group, compared with 15% in the placebo group.
Results of prior studies have demonstrated either no increased risk of hypoglycemia with ACS use4−7 or a much smaller increase (from 4.2% to 5.7%).8 The higher rate of neonatal hypoglycemia seen in this study suggests the possibility that the late preterm population may be more vulnerable to the negative impact of ACS on neonatal glucose/insulin homeostasis. Presumed mechanisms of action are either maternal hyperglycemia or fetal adrenal suppression or both, with potential for fetal adrenal suppression resulting from betamethasone exposure to affect long-term metabolic outcomes.9
Of note, women with pregestational diabetes were excluded from the study and, in routine practice, inclusion of such patients may further increase the risk of neonatal hypoglycemia.
There are few data on the prognostic significance of neonatal hypoglycemia in preterm infants, with the exception of a single study, the results of which show that it is associated with adverse neurodevelopment at 18 months of age.10
Data reveal increased risk for harm in term-born babiesIn spite of strict protocol specifications to increase the probability of delivery before 37 weeks’ gestation, 16% of women in the trial delivered at term. Investigators of prior randomized studies of ACS, aimed at reducing the risks of prematurity, have reported a rate of term delivery of about one-third,4,11 and in routine practice, administration of ACS after 34 weeks may be associated with even higher rates of term delivery.
This is important because recent evidence shows an unfavorable impact of ACS exposure in term-born children.12 The 5-year follow-up of the largest randomized trial in which multiple ACS courses were used noted that babies born at term had a 4-fold increased odds ratio for neurosensory disability.11 There was no dose−response interaction, with the same adverse odds ratio after 1 or 4 additional ACS courses. This observation was consistent with a previously reported Swedish national cohort, pointing to an unfavorable impact of even a single course of ACS in term-born children, with a greater likelihood of harm than benefit.13
In a UK follow-up of children aged 8 to 15 years who were enrolled in an RCT of ACS before cesarean delivery at term, low academic achievement was significantly more common in the group whose mothers had received ACS.14 In another study of 304 children born at term after exposure to a single course of ACS, investigators noted significantly increased cortisol reactivity to acute psychological stress at ages 6 to 11 years in the ACS-exposed patients, compared with 212 babies of women with threatened preterm labor who did not receive ACS and 372 babies from uncomplicated term pregnancies.15
The relevance of such study findings extends beyond childhood given the fact that elevated hypothalamic-pituitary-adrenal (HPA) axis reactivity has been linked to the pathogenesis of metabolic syndrome and depression in adult life.16 As recently as 2015, investigators of a randomized trial of ACS in 6 low- and middle-income countries highlighted their concern regarding “potentially harmful use of antenatal corticosteroids for infants not delivered preterm.”17
There are safety concerns with ACS in the late preterm periodThe effects of ACS are more pleiotropic than those reflected in a lower incidence of respiratory difficulties. Knowledge of the overall consequences of ACS exposure in infants born late-preterm or at term is still limited. The close-to-term fetus exposed to exogenous corticosteroids is also exposed to the physiologic endogenous surge of cortisol known to occur in the maternal circulation in late pregnancy, which reaches levels 3 times higher than those seen in nonpregnant women.18 Although placental 11 beta-hydroxysteroid dehydrogenase type 2 plays a protective role by allowing no more than 10% to 20% of maternal corticosteroids to cross the placenta, fetal overexposure from concomitant exogenous maternal corticosteroid administration remains a theoretical concern close to term. This is especially worrisome if there is a gestational age−related increase in the sensitivity to corticosteroid-induced in utero fetal programming. It has been reported that fetal overexposure to corticosteroids in late pregnancy can permanently increase the activity of the HPA-axis, with likely consequences in adult life.19
Another concern relates to oligodendrocytes development. Although the neuronal division process in humans usually is completed by 24 weeks’ gestation, the most rapid growth for oligodendrocytes occurs between 34 and 36 weeks’ gestation; these are the cells responsible for the synthesis of myelin. Overexposure to corticosteroids at this vulnerable time in the late preterm fetus potentially may have unanticipated negative neurologic consequences.20
This is the only scenario in which we feel antenatal corticosteroids could be used in a fetus aged 34 weeks to 36 weeks 5 days. In the setting of a scheduled cesarean delivery between 34 weeks and 35 weeks, the concerns relative to term delivery after corticosteroid exposure may not apply, but the concerns in relation to the administration of corticosteroid in the late preterm period—which is a time of possibly increased neurohormonal and neurologic vulnerability—still apply. With regard to the risk of neonatal hypoglycemia, one might argue that close neonatal monitoring of babies so exposed may ensure that any associated neonatal hypoglycemia does not go unnoticed or untreated. However, the prognostic significance of even short periods of neonatal hypoglycemia has not been established.
Where should future studies focus?There is clear neonatal benefit from a single course of ACS given to women who will deliver before 34 weeks’ gestation. It is widely accepted, based on the evidence provided by the 30-year follow-up of the cohort of 534 participants from the Auckland trial (the longest follow-up for any pregnancy trial), that administration of ACS at less than 34 weeks’ gestation is not associated with any obvious major developmental risk.21−23
However, the reassurances provided by the Auckland cohort should be neither directly extrapolated to the administration of ACS in the late preterm period nor applied to term-born babies exposed to ACS, for the simple reason that these subgroups never have been analyzed separately. The risk:benefit ratio of ACS use in the late-preterm period is as yet unknown, and in term-born babies the ratio may be unfavorable.
Follow-up studies are neededWe consider that there is a vital need for long-term follow-up studies. The focus of research on the effects of ACS no longer is on the immediate neonatal outcomes and now is on safety and the long-term outcomes of this exposure.
Bottom lineWe regard the large, high-quality study conducted by the NICHD MFMU Network1 as an opportunity to answer current concerns. It is hoped that the resources necessaryfor in-depth follow-up of the children involved in this study will be provided to the investigators and to the NICHD. It is only with such follow-up that mid- and long-term adverse effects can be assessed. We believe that, at a minimum, mid-term follow-up data should be available before it is wise to make any definitive recommendations for a sweeping change in clinical practice.
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery [published online ahead of print February 4, 2016]. N Engl J Med.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117(2 pt 1):422–424.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454.
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525.
- Sann L, Burnod J, Lasne Y, Bethenod M. Antenatal administration of betamethasone: effects upon neonatal blood glucose in premature infants [in French]. Nouv Presse Med. 1979;8(39):3147–3148.
- Rokicki W, Krasnodebski J. Antenatal glucocorticoid administration and neonatal glycemia. Dev Pharmacol Ther. 1987;10(4):307–311.
- Gazquez Serrano IM, Arroyos Plana A, Diaz Morales O, Herraiz Perea C, Holgueras Bragado A. Antenatal corticosteroid therapy and late preterm infant morbidity and mortality [in Spanish]. An Pediatr (Barc). 2014;81(6):374–382.
- Pettit KE, Tran SH, Lee E, Caughey AB. The association of antenatal corticosteroids with neonatal hypoglycemia and hyperbilirubinemia. J Matern Fetal Neonatal Med. 2014;27(7):683–686.
- Aydin M, Derveci U, Hakan N. Neonatal hypoglycemia associated with the antenatal corticosteroids may be secondary to fetal adrenal suppression. J Matern Fetal Neonatal Med. 2015;28(8):892.
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308.
- Asztalos EV, Murphy KE, Willan AR, et al; MACS-5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102–1110.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications [published online ahead of print January 18, 2016]. BJOG. doi:10.1111/1471-0528.13853.
- Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Health consequences of prophylactic exposure to antenatal corticosteroids among children born late preterm or term. Acta Obstet Gynecol Scand. 2012;91(12):1415–1421.
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F195–F200.
- Alexander N, Rosenlocher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381.
- Althabe F, Belizan JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639.
- Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11ß-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdale GR mRNA expression and anxiety-like behavior in the offspring. Eur J Neurosci. 2000;12(3):1047–1054.
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F154–F157.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862.
- Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11Dalziel SR, Walker NK, Parag V, et al. Dalziel SR, Lim VK, Lambert A, et al. Dalziel SR, Rea HH, Walker NK, et al.
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal-Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery [published online ahead of print February 4, 2016]. N Engl J Med.
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 475: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117(2 pt 1):422–424.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;(3):CD004454.
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50(4):515–525.
- Sann L, Burnod J, Lasne Y, Bethenod M. Antenatal administration of betamethasone: effects upon neonatal blood glucose in premature infants [in French]. Nouv Presse Med. 1979;8(39):3147–3148.
- Rokicki W, Krasnodebski J. Antenatal glucocorticoid administration and neonatal glycemia. Dev Pharmacol Ther. 1987;10(4):307–311.
- Gazquez Serrano IM, Arroyos Plana A, Diaz Morales O, Herraiz Perea C, Holgueras Bragado A. Antenatal corticosteroid therapy and late preterm infant morbidity and mortality [in Spanish]. An Pediatr (Barc). 2014;81(6):374–382.
- Pettit KE, Tran SH, Lee E, Caughey AB. The association of antenatal corticosteroids with neonatal hypoglycemia and hyperbilirubinemia. J Matern Fetal Neonatal Med. 2014;27(7):683–686.
- Aydin M, Derveci U, Hakan N. Neonatal hypoglycemia associated with the antenatal corticosteroids may be secondary to fetal adrenal suppression. J Matern Fetal Neonatal Med. 2015;28(8):892.
- Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–1308.
- Asztalos EV, Murphy KE, Willan AR, et al; MACS-5 Collaborative Group. Multiple courses of antenatal corticosteroids for preterm birth study: outcomes in children at 5 years of age (MACS-5). JAMA Pediatr. 2013;167(12):1102–1110.
- Vidaeff AC, Belfort MA, Steer PJ. Antenatal corticosteroids: a time for more careful scrutiny of the indications [published online ahead of print January 18, 2016]. BJOG. doi:10.1111/1471-0528.13853.
- Eriksson L, Haglund B, Ewald U, Odlind V, Kieler H. Health consequences of prophylactic exposure to antenatal corticosteroids among children born late preterm or term. Acta Obstet Gynecol Scand. 2012;91(12):1415–1421.
- Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial). Arch Dis Child Fetal Neonatal Ed. 2013;98(3):F195–F200.
- Alexander N, Rosenlocher F, Stalder T, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97(10):3538–3544.
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5(7):374–381.
- Althabe F, Belizan JM, McClure EM, et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster-randomised trial. Lancet. 2015;385(9968):629–639.
- Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11ß-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdale GR mRNA expression and anxiety-like behavior in the offspring. Eur J Neurosci. 2000;12(3):1047–1054.
- Whitelaw A, Thoresen M. Antenatal steroids and the developing brain. Arch Dis Child Fetal Neonatal Ed. 2000;83(2):F154–F157.
- Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365(9474):1856–1862.
- Dalziel SR, Lim VK, Lambert A, et al. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331(7518):665.
- Welberg LA, Seckl JR, Holmes MC. Inhibition of 11Dalziel SR, Walker NK, Parag V, et al. Dalziel SR, Lim VK, Lambert A, et al. Dalziel SR, Rea HH, Walker NK, et al.