User login
For heavy menstrual bleeding, are long-term outcomes similar for treatment with the LNG-IUS and radiofrequency endometrial ablation?
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
The role of hysteroscopy in diagnosing endometrial cancer
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
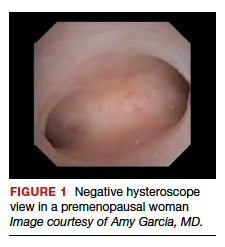
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.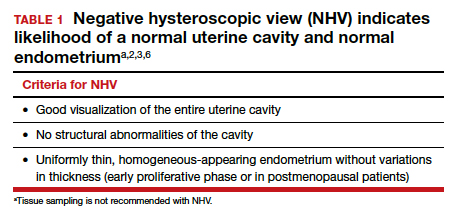
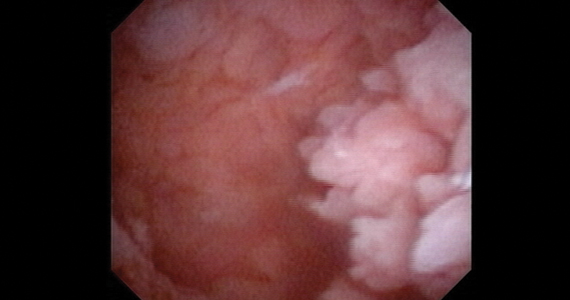
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).
Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1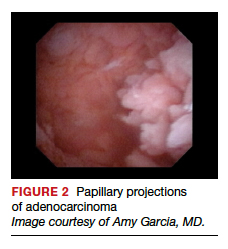
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
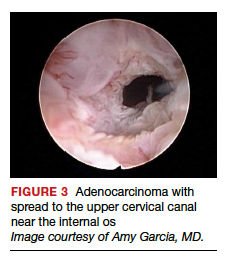
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

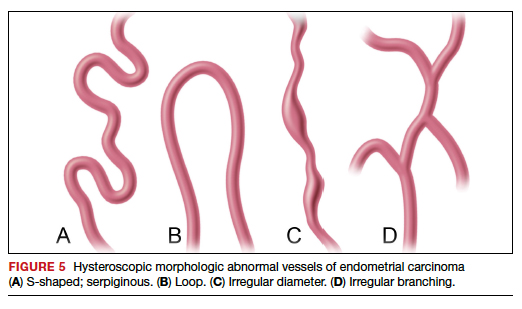
Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5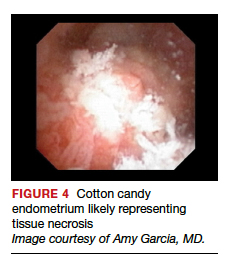
Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).
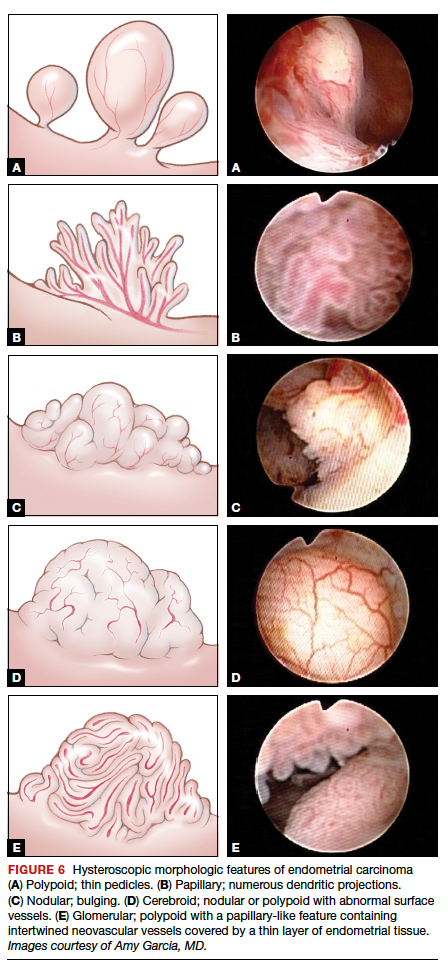
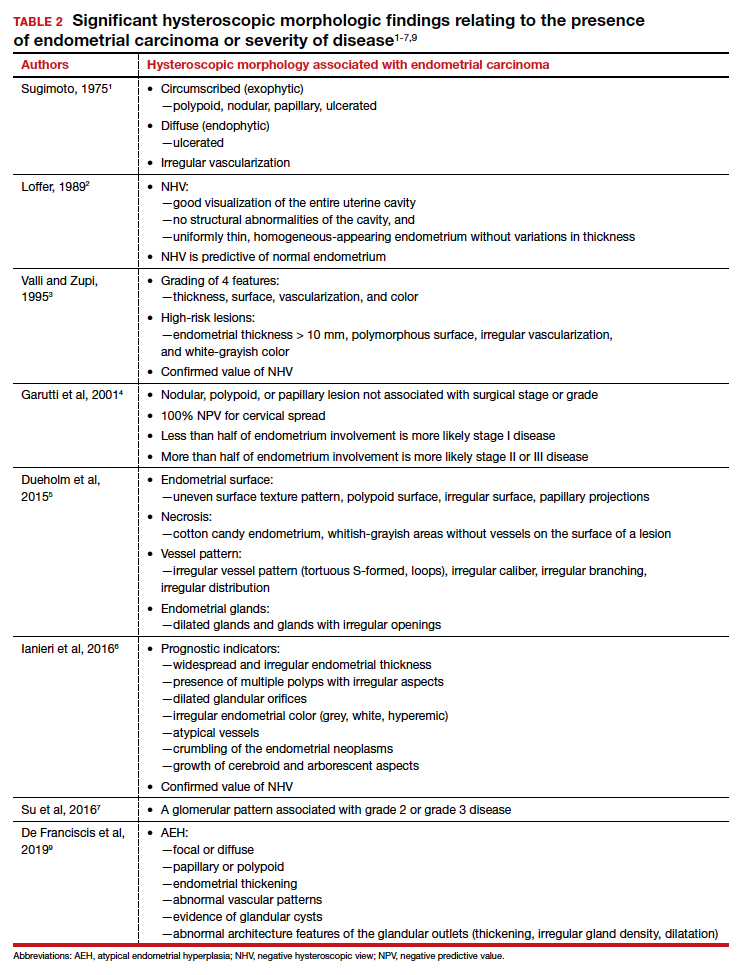
TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.
Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
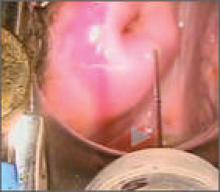
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).

Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4

Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5

Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).

TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.

Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).

Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4

Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5

Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).

TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.

Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
2015 Update on minimally invasive gynecologic surgery
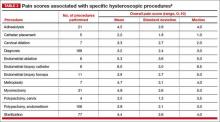
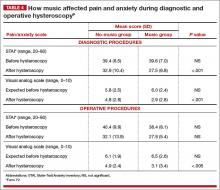
Office hysteroscopy offers many benefits and is becoming more acceptable among patients and gynecologists for both diagnostic and operative procedures (TABLE 1). Despite its clear advantages, however, many gynecologists remain hesitant to perform in-office procedures out of fear that the patient, who is generally awake, will experience significant discomfort.
Certainly, pain and low patient tolerance of discomfort have been the primary limitations to widespread use of office hysteroscopy without anesthesia.1 Data on the use of anesthesia for office hysteroscopy—especially diagnostic procedures—historically have been inconsistent in regard to the reduction of patient discomfort.2
Bettocchi and Selvaggi first reported a vaginoscopic approach to diagnostic hysteroscopy to reduce the discomfort of the procedure, compared with the conventional approach. They did not place a vaginal speculum or tenaculum and, therefore, avoided placing local anesthetic into the cervix.3
A randomized controlled trial by Sagiv and colleagues found reduced pain during diagnostic hysteroscopy with vaginoscopy (VIDEO).4

In 2004, Bettocchi and colleagues reported nearly 5,000 operative hysteroscopic procedures performed with this technique (the “no-touch” technique) in an outpatient setting, demonstrating very high patient tolerance and a low degree of procedural pain. More than 90% of patients experienced little to no pain, except for those undergoing polypectomy when the polyps were larger than the diameter of the cervical os, as well as those who had anatomic abnormalities, with moderate discomfort reported by 33.2% and 12.7% of these women, respectively.5 These few patients may have benefited from anesthetic intervention for the procedure.
In a 2010 review of the literature on hysteroscopy without anesthesia, Cicinelli found that diagnostic hysteroscopy was more successful, with less patient discomfort, when smaller hysteroscopes were used (3.5 mm or smaller, including flexible lenses) and when the approach was vaginoscopic.1 Reduced pain with operative procedures was associated with a number of variables, including:
- increased surgeon experience
- smaller instrument size
- shorter duration of the procedure
- premenopausal status.
Variables associated with increased pain during operative procedures included:
- chronic pelvic pain
- menopausal status
- previous cesarean delivery
- significant anxiety.
In the review, Cicinelli noted that not all patients are likely to have a successful hysteroscopic procedure without the use of anesthesia or analgesia, regardless of the approach used.1
Only 1 randomized controlled trial explored the use of anesthesia (versus placebo) during operative hysteroscopy, and the authors found a benefit for preprocedural paracervical block using local anesthetic to reduce cervical pain.6
The success of diagnostic and operative hysteroscopic procedures with minimal and acceptable levels of patient discomfort in the office depends, therefore, on multiple factors. Procedural factors affecting the outcome of hysteroscopy include the size of the instrument used, the type and length of the procedure, the use of preprocedure anesthesia or analgesia, and a vaginoscopic approach. The skill of the surgeon also affects the hysteroscopic experience and outcome. In addition, patient variables such as menopausal status, anatomic distortion (eg, cervical stenosis), and anxiety may adversely affect the patient’s experience.
In summary, it is possible for the gynecologist to appropriately accommodate any given patient and clinical scenario, keeping in mind that many patients will require a customized approach for ultimate success. In this article, I review 3 recent studies on office hysteroscopy, focusing on the reduction of procedural pain and anxiety. Because of the protective effect of a high degree of surgeon experience, it is important that we offer adequate education in hysteroscopy during residency and postgraduate courses.
Placement of local anesthetic at multiple anatomic sites facilitates patient comfort during hysteroscopy
Keyhan S, Munro MG. Office diagnostic and operative hysteroscopy using local anesthesia only: an analysis of patient reported pain and other procedural outcomes. J Minim Invasive Gynecol. 2014;21(5):791–798.
In a 2010 review of randomized controlled trials of the use of local anesthesia versus placebo during hysteroscopy, data from several studies indicated a significant decrease in procedural pain when local anesthesia was given, while other studies found no difference.2 Most of the studies in that review evaluated a single site of anesthesia placement and focused on diagnostic hysteroscopy. The findings of that review, as well as the differential innervation of the uterine cervix and fundus (FIGURE), prompted Keyhan and Munro to evaluate the efficacy of multimodal local anesthetic for office diagnostic and operative hysteroscopy without the use of any systemic agents except for preprocedural cyclooxygenase (COX) inhibitors (TABLE 2). Accordingly, they placed local anesthetic at multiple anatomic sites to alleviate patient pain and improve procedural success in a spectrum of office-based hysteroscopic procedures.
Details of the trial
Procedures generally were performed using a continuous-flow sheath with an outside diameter of 5.5 mm and a 5 French operative channel for placement of operative instruments such as scissors, graspers, and sterilization microinserts. Normal saline or sterile water was used as the uterine distention medium, with gravity inflow assisted by pressure cuff, when necessary. Occasionally, a sheath system with an outside diameter of 6.5 mm was used, or an outside diameter of 9 mm for resectoscopic procedures using a bipolar radiofrequency resectoscope. When needed, cervical dilation was performed to accommodate the specific instrument used.
The impact of the multimodal, multisite anesthetic protocol was evaluated using contemporaneous patient reporting of numeric pain scores (worst pain experienced) that included anesthesia-related pain, procedure-related pain, and overall pain.
A total of 478 women underwent 535 procedures. A patient verbal response scale (range, 0–10) was used to assess the worst pain experienced. The overall mean (SD) procedure pain score was 3.7 (2.5). The mean score for patients undergoing diagnostic hysteroscopy was 3.2 (2.5), and it was 4.1 (2.5) for operative hysteroscopy (P<.001).
TABLE 3 shows the procedures performed under the anesthetic protocol. Pain associated with placement of anesthesia was similar for diagnostic and operative procedures (mean score, 2.7), but the mean overall pain scores for diagnostic procedures were about 1 unit less than for operative procedures, regardless of age or delivery history.
Complications were limited to 3 transient vasovagal episodes. Five procedures could not be completed because of intolerable pain or inability to access the uterine cavity. There was no difference in pain scores between menopausal and premeno-pausal women.
Malcolm G. Munro, MD, offers a protocol for pain relief during hysteroscopy
In this video, Malcolm G. Munro, MD, makes use of both topical and injectable lidocaine at 5 anatomic sites
Dr. Munro is Professor of Obstetrics and Gynecology at the David Geffen School of Medicine at UCLA and Director of Gynecologic Services at Kaiser Permanente, Los Angeles Medical Center, in Los Angeles, California.
When placing anesthesia at multiple sites, allow time for onset of action
Keyhan and Munro demonstrated that successful completion of hysteroscopic procedures in the office environment can be achieved with acceptable levels of patient discomfort using a multimodal, multisite approach for preemptive placement of local anesthetic in the vagina, cervix, and endometrial cavity. They emphasize that the waiting time allotted for the onset of anesthesia is critical to the success of this approach. They also stress that no preprocedure oral sedative or narcotic is used with their approach. In addition, they note that the minimal discomfort experienced during placement of local anesthetic was overshadowed by general comfort during the wide spectrum of procedures performed.
What this EVIDENCE means for practice
The placement of preemptive local anesthesia at multiple anatomic sites facilitates diagnostic and operative hysteroscopy with an acceptable degree of patient comfort and successful completion of office procedures.
Music may reduce patient anxiety during hysteroscopy
Angioli R, De Cicco Nardone C, Plotti F, et al. Use of music to reduce anxiety during office hysteroscopy: prospective randomized trial. J Minim Invasive Gynecol. 2014;21(3):454–459.
Angioli and colleagues set out to address another factor that can impede patient comfort during office hysteroscopy—anxiety. Their randomized prospective trial is the only such trial evaluating the use of music to establish a calm and relaxing environment prior to office hysteroscopy for patients who are awake. Music supports an environment that “stimulates and maintains relaxation, well-being, and comfort and can be used as a self-management technique to reduce or control distress,” Angioli and colleagues write. The theory is that music distracts the patient by drawing her attention away from negative stimuli, thereby reducing pain, anxiety, and stress.
Details of the trial
A standardized visual analog scale (range, 0–10) was used to assess patient discomfort, and a State-Trait Anxiety Inventory (STAI; range, 20–80) also was given. Both tools were administered at baseline. The visual analog scale was measured again during the procedure, and the STAI was administered again after the procedure.
A hysteroscopic sheath with an outside diameter of 5 mm was used with a 5 French operative channel, and a vaginoscopic approach was used for each hysteroscopic procedure. A total of 372 women were enrolled and randomly allocated to either:
- music group (n = 185)
- no-music group (n = 187).
The surgical procedure was not completed in 15 patients (9 in the music group and 6 in the no-music group) because of stenosis of the cervix and/or excessive pain.
Women in the music group were allowed to select a playlist of classical, pop, jazz, or rock music that was played through a speaker system in the room. Of these patients, 50% preferred classical, 45% preferred pop, 5% chose jazz, and none selected rock music.
There were no statistically significant differences between the 2 groups in terms of preprocedure wait time, preprocedure scores on the visual analog scale or STAI, preprocedure vital signs, patient characteristics, type of procedure, or duration of the procedure. However, the music group had a lower visual analog score during the procedure and a lower postoperative STAI for diagnostic hysteroscopy than the no-music group did. The music group also had a statistically significant lower visual analog score for operative hysteroscopy than the no-music group did. In addition, the music group had a lower postoperative STAI score than the no-music group, but this difference was not statistically significant (TABLE 4).
Interestingly, in the music group, the STAI scores were lower after both diagnostic and operative hysteroscopy when classical music was selected rather than pop music.
Anxiety and pain are highly correlated
Angioli and colleagues found that patients who listened to music intraoperatively had a lower perception of pain and less anxiety. In addition, systolic blood pressure and heart rate were significantly lower in the music group than the no-music group, implying that patients who listened to music experienced less physical stress during the procedure.
Angioli and colleagues also noted that the level of anxiety and perception of pain were highly correlated. Pain is not an emotionally neutral experience but is almost always accompanied by distress. Investigators concluded that “anxiety can enhance painful sensations at all levels of the nervous system, from the peripheral receptors to the cortical level.”
What this EVIDENCE means for practice
Music is a useful complementary method to control patient anxiety and reduce the perception of pain during office hysteroscopy by creating a more relaxed and comfortable environment.
What are the risk factors for pain and discomfort during office hysteroscopy?
De Freitas FM, Sessa FV, Resende AD Jr, et al. Identifying predictors of unacceptable pain at office hysteroscopy. J Minim Invasive Gynecol. 2014;21(4):586–591.
De Freitas and colleagues conducted their prospective observational study to identify predictors of unacceptable pain during diagnostic office hysteroscopy (with or without directed or curette endometrial biopsy) and at the time of discharge. They hoped that any identifiable causes of unacceptable pain could be addressed individually in future patients undergoing office hysteroscopy to reduce their level of discomfort.
Details of the trial
A total of 558 procedures were evaluated. Hysteroscopists had varying levels of experience, with some having performed fewer than 50 procedures and others having performed more than 500.
A verbal response scale (range, 0–10) was used to assess pain at the end of each procedure and at the time of discharge. Investigators considered a score of 7 or more at the time of the procedure and a score of 4 or more at the time of discharge to be unacceptable.
A diagnostic, single-channel hysteroscope with an outside diameter of 3.5 mm was used with normal saline (at room temperature), along with a gravity system with pressure established to maintain intrauterine pressure at approximately 110 mm Hg. All hysteroscopic procedures were performed using a vaginoscopic approach, and biopsies were obtained as clinically indicated. Any patients who reported cramping at discharge (ie, a verbal response scale score of 4 or more) were offered an oral nonsteroidal anti-inflammatory drug.
Overall, the prevalence of unacceptable pain during office hysteroscopy was 32.3%. Experience of the hysteroscopist had a significant protective effect against pain. Longer procedures were significantly associated with unacceptable procedural pain.
The prevalence of unacceptable cramping at discharge was 28.6%. The risk of discomfort at discharge was significantly higher for women who reported dyspareunia or dysmenorrhea. Surgeon experience was significantly protective against unacceptable pain at discharge, and longer procedures were significantly associated with increased discomfort at discharge.
Dysmenorrhea and dyspareunia were significant predictors of pain at discharge
In this study, dysmenorrhea was a significant predictor of unacceptable pain at discharge, increasing the risk of unacceptable cramps by approximately threefold. Women who reported dyspareunia were nearly twice as likely to report unacceptable cramping at discharge.
Although a high level of expertise is not a prerequisite for office hysteroscopy, the skill and experience of the hysteroscopist, as well as shorter procedures, proved to be protective against procedural pain and discomfort at discharge but did not eliminate them altogether. Therefore, De Freitas and colleagues recommend that patients who can be identified as high-risk for procedural or discharge pain, such as women with dysmenorrhea or dyspareunia, should be offered preprocedure analgesia and/or anesthesia to reduce overall discomfort.
What this EVIDENCE means for practice
If a patient reports dysmenorrhea or dyspareunia preoperatively, she may benefit from preprocedure anesthesia or analgesia, or both, in an office setting.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17(6):703–708.
2. Munro MG, Brooks PG. Use of local anesthesia for office diagnostic and operative hysteroscopy. J Minim Invasive Gynecol. 2010;17(6):709–718.
3. Bettocchi S, Selvaggi L. A vaginoscopic approach to reduce the pain of office hysteroscopy. J Am Assoc Gynecol Laparosc. 1997;4(2):255–258.
4. Sagiv R, Sadan O, Boaz M, et al. A new approach to office hysteroscopy compared with traditional hysteroscopy. A randomized controlled trial. Am J Obstet Gynecol. 2006;108(2):387–392.
5.Bettocchi S, Ceci O, Nappi L, et al. Operative office hysteroscopy without anesthesia: analysis of 4,863 cases performed with mechanical instruments. J Am Assoc Gynecol Laparosc. 2004;11(1):59–61.
6. Chudnoff S, Einstein M, Levie M. Paracervical block efficacy in office hysteroscopic sterilization. A randomized controlled trial. Obstet Gynecol. 2010;115(1):26–34.
7. Garcia AL. Stop performing dilation and curettage for the evaluation of abnormal uterine bleeding. OBG Manag. 2013;25(6):44–48.
8. Keyhan S, Munro MG. Office diagnostic and operative hysteroscopy using local anesthesia only: an analysis of patient reported pain and other procedural outcomes. J Minim Invasive Gynecol. 2014;21(5):791–798.
9. Angioli R, de Cicco Nardone C, Plotti F, et al. Use of music to reduce anxiety during office hysteroscopy: prospective randomized trial. J Minim Invasive Gynecol. 2014;21(3):454–459.
Office hysteroscopy offers many benefits and is becoming more acceptable among patients and gynecologists for both diagnostic and operative procedures (TABLE 1). Despite its clear advantages, however, many gynecologists remain hesitant to perform in-office procedures out of fear that the patient, who is generally awake, will experience significant discomfort.
Certainly, pain and low patient tolerance of discomfort have been the primary limitations to widespread use of office hysteroscopy without anesthesia.1 Data on the use of anesthesia for office hysteroscopy—especially diagnostic procedures—historically have been inconsistent in regard to the reduction of patient discomfort.2
Bettocchi and Selvaggi first reported a vaginoscopic approach to diagnostic hysteroscopy to reduce the discomfort of the procedure, compared with the conventional approach. They did not place a vaginal speculum or tenaculum and, therefore, avoided placing local anesthetic into the cervix.3
A randomized controlled trial by Sagiv and colleagues found reduced pain during diagnostic hysteroscopy with vaginoscopy (VIDEO).4

In 2004, Bettocchi and colleagues reported nearly 5,000 operative hysteroscopic procedures performed with this technique (the “no-touch” technique) in an outpatient setting, demonstrating very high patient tolerance and a low degree of procedural pain. More than 90% of patients experienced little to no pain, except for those undergoing polypectomy when the polyps were larger than the diameter of the cervical os, as well as those who had anatomic abnormalities, with moderate discomfort reported by 33.2% and 12.7% of these women, respectively.5 These few patients may have benefited from anesthetic intervention for the procedure.
In a 2010 review of the literature on hysteroscopy without anesthesia, Cicinelli found that diagnostic hysteroscopy was more successful, with less patient discomfort, when smaller hysteroscopes were used (3.5 mm or smaller, including flexible lenses) and when the approach was vaginoscopic.1 Reduced pain with operative procedures was associated with a number of variables, including:
- increased surgeon experience
- smaller instrument size
- shorter duration of the procedure
- premenopausal status.
Variables associated with increased pain during operative procedures included:
- chronic pelvic pain
- menopausal status
- previous cesarean delivery
- significant anxiety.
In the review, Cicinelli noted that not all patients are likely to have a successful hysteroscopic procedure without the use of anesthesia or analgesia, regardless of the approach used.1
Only 1 randomized controlled trial explored the use of anesthesia (versus placebo) during operative hysteroscopy, and the authors found a benefit for preprocedural paracervical block using local anesthetic to reduce cervical pain.6
The success of diagnostic and operative hysteroscopic procedures with minimal and acceptable levels of patient discomfort in the office depends, therefore, on multiple factors. Procedural factors affecting the outcome of hysteroscopy include the size of the instrument used, the type and length of the procedure, the use of preprocedure anesthesia or analgesia, and a vaginoscopic approach. The skill of the surgeon also affects the hysteroscopic experience and outcome. In addition, patient variables such as menopausal status, anatomic distortion (eg, cervical stenosis), and anxiety may adversely affect the patient’s experience.
In summary, it is possible for the gynecologist to appropriately accommodate any given patient and clinical scenario, keeping in mind that many patients will require a customized approach for ultimate success. In this article, I review 3 recent studies on office hysteroscopy, focusing on the reduction of procedural pain and anxiety. Because of the protective effect of a high degree of surgeon experience, it is important that we offer adequate education in hysteroscopy during residency and postgraduate courses.
Placement of local anesthetic at multiple anatomic sites facilitates patient comfort during hysteroscopy
Keyhan S, Munro MG. Office diagnostic and operative hysteroscopy using local anesthesia only: an analysis of patient reported pain and other procedural outcomes. J Minim Invasive Gynecol. 2014;21(5):791–798.
In a 2010 review of randomized controlled trials of the use of local anesthesia versus placebo during hysteroscopy, data from several studies indicated a significant decrease in procedural pain when local anesthesia was given, while other studies found no difference.2 Most of the studies in that review evaluated a single site of anesthesia placement and focused on diagnostic hysteroscopy. The findings of that review, as well as the differential innervation of the uterine cervix and fundus (FIGURE), prompted Keyhan and Munro to evaluate the efficacy of multimodal local anesthetic for office diagnostic and operative hysteroscopy without the use of any systemic agents except for preprocedural cyclooxygenase (COX) inhibitors (TABLE 2). Accordingly, they placed local anesthetic at multiple anatomic sites to alleviate patient pain and improve procedural success in a spectrum of office-based hysteroscopic procedures.
Details of the trial
Procedures generally were performed using a continuous-flow sheath with an outside diameter of 5.5 mm and a 5 French operative channel for placement of operative instruments such as scissors, graspers, and sterilization microinserts. Normal saline or sterile water was used as the uterine distention medium, with gravity inflow assisted by pressure cuff, when necessary. Occasionally, a sheath system with an outside diameter of 6.5 mm was used, or an outside diameter of 9 mm for resectoscopic procedures using a bipolar radiofrequency resectoscope. When needed, cervical dilation was performed to accommodate the specific instrument used.
The impact of the multimodal, multisite anesthetic protocol was evaluated using contemporaneous patient reporting of numeric pain scores (worst pain experienced) that included anesthesia-related pain, procedure-related pain, and overall pain.
A total of 478 women underwent 535 procedures. A patient verbal response scale (range, 0–10) was used to assess the worst pain experienced. The overall mean (SD) procedure pain score was 3.7 (2.5). The mean score for patients undergoing diagnostic hysteroscopy was 3.2 (2.5), and it was 4.1 (2.5) for operative hysteroscopy (P<.001).
TABLE 3 shows the procedures performed under the anesthetic protocol. Pain associated with placement of anesthesia was similar for diagnostic and operative procedures (mean score, 2.7), but the mean overall pain scores for diagnostic procedures were about 1 unit less than for operative procedures, regardless of age or delivery history.
Complications were limited to 3 transient vasovagal episodes. Five procedures could not be completed because of intolerable pain or inability to access the uterine cavity. There was no difference in pain scores between menopausal and premeno-pausal women.
Malcolm G. Munro, MD, offers a protocol for pain relief during hysteroscopy
In this video, Malcolm G. Munro, MD, makes use of both topical and injectable lidocaine at 5 anatomic sites
Dr. Munro is Professor of Obstetrics and Gynecology at the David Geffen School of Medicine at UCLA and Director of Gynecologic Services at Kaiser Permanente, Los Angeles Medical Center, in Los Angeles, California.
When placing anesthesia at multiple sites, allow time for onset of action
Keyhan and Munro demonstrated that successful completion of hysteroscopic procedures in the office environment can be achieved with acceptable levels of patient discomfort using a multimodal, multisite approach for preemptive placement of local anesthetic in the vagina, cervix, and endometrial cavity. They emphasize that the waiting time allotted for the onset of anesthesia is critical to the success of this approach. They also stress that no preprocedure oral sedative or narcotic is used with their approach. In addition, they note that the minimal discomfort experienced during placement of local anesthetic was overshadowed by general comfort during the wide spectrum of procedures performed.
What this EVIDENCE means for practice
The placement of preemptive local anesthesia at multiple anatomic sites facilitates diagnostic and operative hysteroscopy with an acceptable degree of patient comfort and successful completion of office procedures.
Music may reduce patient anxiety during hysteroscopy
Angioli R, De Cicco Nardone C, Plotti F, et al. Use of music to reduce anxiety during office hysteroscopy: prospective randomized trial. J Minim Invasive Gynecol. 2014;21(3):454–459.
Angioli and colleagues set out to address another factor that can impede patient comfort during office hysteroscopy—anxiety. Their randomized prospective trial is the only such trial evaluating the use of music to establish a calm and relaxing environment prior to office hysteroscopy for patients who are awake. Music supports an environment that “stimulates and maintains relaxation, well-being, and comfort and can be used as a self-management technique to reduce or control distress,” Angioli and colleagues write. The theory is that music distracts the patient by drawing her attention away from negative stimuli, thereby reducing pain, anxiety, and stress.
Details of the trial
A standardized visual analog scale (range, 0–10) was used to assess patient discomfort, and a State-Trait Anxiety Inventory (STAI; range, 20–80) also was given. Both tools were administered at baseline. The visual analog scale was measured again during the procedure, and the STAI was administered again after the procedure.
A hysteroscopic sheath with an outside diameter of 5 mm was used with a 5 French operative channel, and a vaginoscopic approach was used for each hysteroscopic procedure. A total of 372 women were enrolled and randomly allocated to either:
- music group (n = 185)
- no-music group (n = 187).
The surgical procedure was not completed in 15 patients (9 in the music group and 6 in the no-music group) because of stenosis of the cervix and/or excessive pain.
Women in the music group were allowed to select a playlist of classical, pop, jazz, or rock music that was played through a speaker system in the room. Of these patients, 50% preferred classical, 45% preferred pop, 5% chose jazz, and none selected rock music.
There were no statistically significant differences between the 2 groups in terms of preprocedure wait time, preprocedure scores on the visual analog scale or STAI, preprocedure vital signs, patient characteristics, type of procedure, or duration of the procedure. However, the music group had a lower visual analog score during the procedure and a lower postoperative STAI for diagnostic hysteroscopy than the no-music group did. The music group also had a statistically significant lower visual analog score for operative hysteroscopy than the no-music group did. In addition, the music group had a lower postoperative STAI score than the no-music group, but this difference was not statistically significant (TABLE 4).
Interestingly, in the music group, the STAI scores were lower after both diagnostic and operative hysteroscopy when classical music was selected rather than pop music.
Anxiety and pain are highly correlated
Angioli and colleagues found that patients who listened to music intraoperatively had a lower perception of pain and less anxiety. In addition, systolic blood pressure and heart rate were significantly lower in the music group than the no-music group, implying that patients who listened to music experienced less physical stress during the procedure.
Angioli and colleagues also noted that the level of anxiety and perception of pain were highly correlated. Pain is not an emotionally neutral experience but is almost always accompanied by distress. Investigators concluded that “anxiety can enhance painful sensations at all levels of the nervous system, from the peripheral receptors to the cortical level.”
What this EVIDENCE means for practice
Music is a useful complementary method to control patient anxiety and reduce the perception of pain during office hysteroscopy by creating a more relaxed and comfortable environment.
What are the risk factors for pain and discomfort during office hysteroscopy?
De Freitas FM, Sessa FV, Resende AD Jr, et al. Identifying predictors of unacceptable pain at office hysteroscopy. J Minim Invasive Gynecol. 2014;21(4):586–591.
De Freitas and colleagues conducted their prospective observational study to identify predictors of unacceptable pain during diagnostic office hysteroscopy (with or without directed or curette endometrial biopsy) and at the time of discharge. They hoped that any identifiable causes of unacceptable pain could be addressed individually in future patients undergoing office hysteroscopy to reduce their level of discomfort.
Details of the trial
A total of 558 procedures were evaluated. Hysteroscopists had varying levels of experience, with some having performed fewer than 50 procedures and others having performed more than 500.
A verbal response scale (range, 0–10) was used to assess pain at the end of each procedure and at the time of discharge. Investigators considered a score of 7 or more at the time of the procedure and a score of 4 or more at the time of discharge to be unacceptable.
A diagnostic, single-channel hysteroscope with an outside diameter of 3.5 mm was used with normal saline (at room temperature), along with a gravity system with pressure established to maintain intrauterine pressure at approximately 110 mm Hg. All hysteroscopic procedures were performed using a vaginoscopic approach, and biopsies were obtained as clinically indicated. Any patients who reported cramping at discharge (ie, a verbal response scale score of 4 or more) were offered an oral nonsteroidal anti-inflammatory drug.
Overall, the prevalence of unacceptable pain during office hysteroscopy was 32.3%. Experience of the hysteroscopist had a significant protective effect against pain. Longer procedures were significantly associated with unacceptable procedural pain.
The prevalence of unacceptable cramping at discharge was 28.6%. The risk of discomfort at discharge was significantly higher for women who reported dyspareunia or dysmenorrhea. Surgeon experience was significantly protective against unacceptable pain at discharge, and longer procedures were significantly associated with increased discomfort at discharge.
Dysmenorrhea and dyspareunia were significant predictors of pain at discharge
In this study, dysmenorrhea was a significant predictor of unacceptable pain at discharge, increasing the risk of unacceptable cramps by approximately threefold. Women who reported dyspareunia were nearly twice as likely to report unacceptable cramping at discharge.
Although a high level of expertise is not a prerequisite for office hysteroscopy, the skill and experience of the hysteroscopist, as well as shorter procedures, proved to be protective against procedural pain and discomfort at discharge but did not eliminate them altogether. Therefore, De Freitas and colleagues recommend that patients who can be identified as high-risk for procedural or discharge pain, such as women with dysmenorrhea or dyspareunia, should be offered preprocedure analgesia and/or anesthesia to reduce overall discomfort.
What this EVIDENCE means for practice
If a patient reports dysmenorrhea or dyspareunia preoperatively, she may benefit from preprocedure anesthesia or analgesia, or both, in an office setting.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Office hysteroscopy offers many benefits and is becoming more acceptable among patients and gynecologists for both diagnostic and operative procedures (TABLE 1). Despite its clear advantages, however, many gynecologists remain hesitant to perform in-office procedures out of fear that the patient, who is generally awake, will experience significant discomfort.
Certainly, pain and low patient tolerance of discomfort have been the primary limitations to widespread use of office hysteroscopy without anesthesia.1 Data on the use of anesthesia for office hysteroscopy—especially diagnostic procedures—historically have been inconsistent in regard to the reduction of patient discomfort.2
Bettocchi and Selvaggi first reported a vaginoscopic approach to diagnostic hysteroscopy to reduce the discomfort of the procedure, compared with the conventional approach. They did not place a vaginal speculum or tenaculum and, therefore, avoided placing local anesthetic into the cervix.3
A randomized controlled trial by Sagiv and colleagues found reduced pain during diagnostic hysteroscopy with vaginoscopy (VIDEO).4

In 2004, Bettocchi and colleagues reported nearly 5,000 operative hysteroscopic procedures performed with this technique (the “no-touch” technique) in an outpatient setting, demonstrating very high patient tolerance and a low degree of procedural pain. More than 90% of patients experienced little to no pain, except for those undergoing polypectomy when the polyps were larger than the diameter of the cervical os, as well as those who had anatomic abnormalities, with moderate discomfort reported by 33.2% and 12.7% of these women, respectively.5 These few patients may have benefited from anesthetic intervention for the procedure.
In a 2010 review of the literature on hysteroscopy without anesthesia, Cicinelli found that diagnostic hysteroscopy was more successful, with less patient discomfort, when smaller hysteroscopes were used (3.5 mm or smaller, including flexible lenses) and when the approach was vaginoscopic.1 Reduced pain with operative procedures was associated with a number of variables, including:
- increased surgeon experience
- smaller instrument size
- shorter duration of the procedure
- premenopausal status.
Variables associated with increased pain during operative procedures included:
- chronic pelvic pain
- menopausal status
- previous cesarean delivery
- significant anxiety.
In the review, Cicinelli noted that not all patients are likely to have a successful hysteroscopic procedure without the use of anesthesia or analgesia, regardless of the approach used.1
Only 1 randomized controlled trial explored the use of anesthesia (versus placebo) during operative hysteroscopy, and the authors found a benefit for preprocedural paracervical block using local anesthetic to reduce cervical pain.6
The success of diagnostic and operative hysteroscopic procedures with minimal and acceptable levels of patient discomfort in the office depends, therefore, on multiple factors. Procedural factors affecting the outcome of hysteroscopy include the size of the instrument used, the type and length of the procedure, the use of preprocedure anesthesia or analgesia, and a vaginoscopic approach. The skill of the surgeon also affects the hysteroscopic experience and outcome. In addition, patient variables such as menopausal status, anatomic distortion (eg, cervical stenosis), and anxiety may adversely affect the patient’s experience.
In summary, it is possible for the gynecologist to appropriately accommodate any given patient and clinical scenario, keeping in mind that many patients will require a customized approach for ultimate success. In this article, I review 3 recent studies on office hysteroscopy, focusing on the reduction of procedural pain and anxiety. Because of the protective effect of a high degree of surgeon experience, it is important that we offer adequate education in hysteroscopy during residency and postgraduate courses.
Placement of local anesthetic at multiple anatomic sites facilitates patient comfort during hysteroscopy
Keyhan S, Munro MG. Office diagnostic and operative hysteroscopy using local anesthesia only: an analysis of patient reported pain and other procedural outcomes. J Minim Invasive Gynecol. 2014;21(5):791–798.
In a 2010 review of randomized controlled trials of the use of local anesthesia versus placebo during hysteroscopy, data from several studies indicated a significant decrease in procedural pain when local anesthesia was given, while other studies found no difference.2 Most of the studies in that review evaluated a single site of anesthesia placement and focused on diagnostic hysteroscopy. The findings of that review, as well as the differential innervation of the uterine cervix and fundus (FIGURE), prompted Keyhan and Munro to evaluate the efficacy of multimodal local anesthetic for office diagnostic and operative hysteroscopy without the use of any systemic agents except for preprocedural cyclooxygenase (COX) inhibitors (TABLE 2). Accordingly, they placed local anesthetic at multiple anatomic sites to alleviate patient pain and improve procedural success in a spectrum of office-based hysteroscopic procedures.
Details of the trial
Procedures generally were performed using a continuous-flow sheath with an outside diameter of 5.5 mm and a 5 French operative channel for placement of operative instruments such as scissors, graspers, and sterilization microinserts. Normal saline or sterile water was used as the uterine distention medium, with gravity inflow assisted by pressure cuff, when necessary. Occasionally, a sheath system with an outside diameter of 6.5 mm was used, or an outside diameter of 9 mm for resectoscopic procedures using a bipolar radiofrequency resectoscope. When needed, cervical dilation was performed to accommodate the specific instrument used.
The impact of the multimodal, multisite anesthetic protocol was evaluated using contemporaneous patient reporting of numeric pain scores (worst pain experienced) that included anesthesia-related pain, procedure-related pain, and overall pain.
A total of 478 women underwent 535 procedures. A patient verbal response scale (range, 0–10) was used to assess the worst pain experienced. The overall mean (SD) procedure pain score was 3.7 (2.5). The mean score for patients undergoing diagnostic hysteroscopy was 3.2 (2.5), and it was 4.1 (2.5) for operative hysteroscopy (P<.001).
TABLE 3 shows the procedures performed under the anesthetic protocol. Pain associated with placement of anesthesia was similar for diagnostic and operative procedures (mean score, 2.7), but the mean overall pain scores for diagnostic procedures were about 1 unit less than for operative procedures, regardless of age or delivery history.
Complications were limited to 3 transient vasovagal episodes. Five procedures could not be completed because of intolerable pain or inability to access the uterine cavity. There was no difference in pain scores between menopausal and premeno-pausal women.
Malcolm G. Munro, MD, offers a protocol for pain relief during hysteroscopy
In this video, Malcolm G. Munro, MD, makes use of both topical and injectable lidocaine at 5 anatomic sites
Dr. Munro is Professor of Obstetrics and Gynecology at the David Geffen School of Medicine at UCLA and Director of Gynecologic Services at Kaiser Permanente, Los Angeles Medical Center, in Los Angeles, California.
When placing anesthesia at multiple sites, allow time for onset of action
Keyhan and Munro demonstrated that successful completion of hysteroscopic procedures in the office environment can be achieved with acceptable levels of patient discomfort using a multimodal, multisite approach for preemptive placement of local anesthetic in the vagina, cervix, and endometrial cavity. They emphasize that the waiting time allotted for the onset of anesthesia is critical to the success of this approach. They also stress that no preprocedure oral sedative or narcotic is used with their approach. In addition, they note that the minimal discomfort experienced during placement of local anesthetic was overshadowed by general comfort during the wide spectrum of procedures performed.
What this EVIDENCE means for practice
The placement of preemptive local anesthesia at multiple anatomic sites facilitates diagnostic and operative hysteroscopy with an acceptable degree of patient comfort and successful completion of office procedures.
Music may reduce patient anxiety during hysteroscopy
Angioli R, De Cicco Nardone C, Plotti F, et al. Use of music to reduce anxiety during office hysteroscopy: prospective randomized trial. J Minim Invasive Gynecol. 2014;21(3):454–459.
Angioli and colleagues set out to address another factor that can impede patient comfort during office hysteroscopy—anxiety. Their randomized prospective trial is the only such trial evaluating the use of music to establish a calm and relaxing environment prior to office hysteroscopy for patients who are awake. Music supports an environment that “stimulates and maintains relaxation, well-being, and comfort and can be used as a self-management technique to reduce or control distress,” Angioli and colleagues write. The theory is that music distracts the patient by drawing her attention away from negative stimuli, thereby reducing pain, anxiety, and stress.
Details of the trial
A standardized visual analog scale (range, 0–10) was used to assess patient discomfort, and a State-Trait Anxiety Inventory (STAI; range, 20–80) also was given. Both tools were administered at baseline. The visual analog scale was measured again during the procedure, and the STAI was administered again after the procedure.
A hysteroscopic sheath with an outside diameter of 5 mm was used with a 5 French operative channel, and a vaginoscopic approach was used for each hysteroscopic procedure. A total of 372 women were enrolled and randomly allocated to either:
- music group (n = 185)
- no-music group (n = 187).
The surgical procedure was not completed in 15 patients (9 in the music group and 6 in the no-music group) because of stenosis of the cervix and/or excessive pain.
Women in the music group were allowed to select a playlist of classical, pop, jazz, or rock music that was played through a speaker system in the room. Of these patients, 50% preferred classical, 45% preferred pop, 5% chose jazz, and none selected rock music.
There were no statistically significant differences between the 2 groups in terms of preprocedure wait time, preprocedure scores on the visual analog scale or STAI, preprocedure vital signs, patient characteristics, type of procedure, or duration of the procedure. However, the music group had a lower visual analog score during the procedure and a lower postoperative STAI for diagnostic hysteroscopy than the no-music group did. The music group also had a statistically significant lower visual analog score for operative hysteroscopy than the no-music group did. In addition, the music group had a lower postoperative STAI score than the no-music group, but this difference was not statistically significant (TABLE 4).
Interestingly, in the music group, the STAI scores were lower after both diagnostic and operative hysteroscopy when classical music was selected rather than pop music.
Anxiety and pain are highly correlated
Angioli and colleagues found that patients who listened to music intraoperatively had a lower perception of pain and less anxiety. In addition, systolic blood pressure and heart rate were significantly lower in the music group than the no-music group, implying that patients who listened to music experienced less physical stress during the procedure.
Angioli and colleagues also noted that the level of anxiety and perception of pain were highly correlated. Pain is not an emotionally neutral experience but is almost always accompanied by distress. Investigators concluded that “anxiety can enhance painful sensations at all levels of the nervous system, from the peripheral receptors to the cortical level.”
What this EVIDENCE means for practice
Music is a useful complementary method to control patient anxiety and reduce the perception of pain during office hysteroscopy by creating a more relaxed and comfortable environment.
What are the risk factors for pain and discomfort during office hysteroscopy?
De Freitas FM, Sessa FV, Resende AD Jr, et al. Identifying predictors of unacceptable pain at office hysteroscopy. J Minim Invasive Gynecol. 2014;21(4):586–591.
De Freitas and colleagues conducted their prospective observational study to identify predictors of unacceptable pain during diagnostic office hysteroscopy (with or without directed or curette endometrial biopsy) and at the time of discharge. They hoped that any identifiable causes of unacceptable pain could be addressed individually in future patients undergoing office hysteroscopy to reduce their level of discomfort.
Details of the trial
A total of 558 procedures were evaluated. Hysteroscopists had varying levels of experience, with some having performed fewer than 50 procedures and others having performed more than 500.
A verbal response scale (range, 0–10) was used to assess pain at the end of each procedure and at the time of discharge. Investigators considered a score of 7 or more at the time of the procedure and a score of 4 or more at the time of discharge to be unacceptable.
A diagnostic, single-channel hysteroscope with an outside diameter of 3.5 mm was used with normal saline (at room temperature), along with a gravity system with pressure established to maintain intrauterine pressure at approximately 110 mm Hg. All hysteroscopic procedures were performed using a vaginoscopic approach, and biopsies were obtained as clinically indicated. Any patients who reported cramping at discharge (ie, a verbal response scale score of 4 or more) were offered an oral nonsteroidal anti-inflammatory drug.
Overall, the prevalence of unacceptable pain during office hysteroscopy was 32.3%. Experience of the hysteroscopist had a significant protective effect against pain. Longer procedures were significantly associated with unacceptable procedural pain.
The prevalence of unacceptable cramping at discharge was 28.6%. The risk of discomfort at discharge was significantly higher for women who reported dyspareunia or dysmenorrhea. Surgeon experience was significantly protective against unacceptable pain at discharge, and longer procedures were significantly associated with increased discomfort at discharge.
Dysmenorrhea and dyspareunia were significant predictors of pain at discharge
In this study, dysmenorrhea was a significant predictor of unacceptable pain at discharge, increasing the risk of unacceptable cramps by approximately threefold. Women who reported dyspareunia were nearly twice as likely to report unacceptable cramping at discharge.
Although a high level of expertise is not a prerequisite for office hysteroscopy, the skill and experience of the hysteroscopist, as well as shorter procedures, proved to be protective against procedural pain and discomfort at discharge but did not eliminate them altogether. Therefore, De Freitas and colleagues recommend that patients who can be identified as high-risk for procedural or discharge pain, such as women with dysmenorrhea or dyspareunia, should be offered preprocedure analgesia and/or anesthesia to reduce overall discomfort.
What this EVIDENCE means for practice
If a patient reports dysmenorrhea or dyspareunia preoperatively, she may benefit from preprocedure anesthesia or analgesia, or both, in an office setting.
Share your thoughts on this article! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
1. Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17(6):703–708.
2. Munro MG, Brooks PG. Use of local anesthesia for office diagnostic and operative hysteroscopy. J Minim Invasive Gynecol. 2010;17(6):709–718.
3. Bettocchi S, Selvaggi L. A vaginoscopic approach to reduce the pain of office hysteroscopy. J Am Assoc Gynecol Laparosc. 1997;4(2):255–258.
4. Sagiv R, Sadan O, Boaz M, et al. A new approach to office hysteroscopy compared with traditional hysteroscopy. A randomized controlled trial. Am J Obstet Gynecol. 2006;108(2):387–392.
5.Bettocchi S, Ceci O, Nappi L, et al. Operative office hysteroscopy without anesthesia: analysis of 4,863 cases performed with mechanical instruments. J Am Assoc Gynecol Laparosc. 2004;11(1):59–61.
6. Chudnoff S, Einstein M, Levie M. Paracervical block efficacy in office hysteroscopic sterilization. A randomized controlled trial. Obstet Gynecol. 2010;115(1):26–34.
7. Garcia AL. Stop performing dilation and curettage for the evaluation of abnormal uterine bleeding. OBG Manag. 2013;25(6):44–48.
8. Keyhan S, Munro MG. Office diagnostic and operative hysteroscopy using local anesthesia only: an analysis of patient reported pain and other procedural outcomes. J Minim Invasive Gynecol. 2014;21(5):791–798.
9. Angioli R, de Cicco Nardone C, Plotti F, et al. Use of music to reduce anxiety during office hysteroscopy: prospective randomized trial. J Minim Invasive Gynecol. 2014;21(3):454–459.
1. Cicinelli E. Hysteroscopy without anesthesia: review of recent literature. J Minim Invasive Gynecol. 2010;17(6):703–708.
2. Munro MG, Brooks PG. Use of local anesthesia for office diagnostic and operative hysteroscopy. J Minim Invasive Gynecol. 2010;17(6):709–718.
3. Bettocchi S, Selvaggi L. A vaginoscopic approach to reduce the pain of office hysteroscopy. J Am Assoc Gynecol Laparosc. 1997;4(2):255–258.
4. Sagiv R, Sadan O, Boaz M, et al. A new approach to office hysteroscopy compared with traditional hysteroscopy. A randomized controlled trial. Am J Obstet Gynecol. 2006;108(2):387–392.
5.Bettocchi S, Ceci O, Nappi L, et al. Operative office hysteroscopy without anesthesia: analysis of 4,863 cases performed with mechanical instruments. J Am Assoc Gynecol Laparosc. 2004;11(1):59–61.
6. Chudnoff S, Einstein M, Levie M. Paracervical block efficacy in office hysteroscopic sterilization. A randomized controlled trial. Obstet Gynecol. 2010;115(1):26–34.
7. Garcia AL. Stop performing dilation and curettage for the evaluation of abnormal uterine bleeding. OBG Manag. 2013;25(6):44–48.
8. Keyhan S, Munro MG. Office diagnostic and operative hysteroscopy using local anesthesia only: an analysis of patient reported pain and other procedural outcomes. J Minim Invasive Gynecol. 2014;21(5):791–798.
9. Angioli R, de Cicco Nardone C, Plotti F, et al. Use of music to reduce anxiety during office hysteroscopy: prospective randomized trial. J Minim Invasive Gynecol. 2014;21(3):454–459.
In this Article
— Innervation of the uterus
— Pain scores associated with specific hysteroscopic procedures
A vaginoscopic approach to diagnostic hysteroscopy
In this video, diffuse complex endometrial hyperplasia is identified using a vaginoscopic approach with a 1.9-mm diagnostic rigid hysteroscope. The posterior fornix of the vagina is filled with saline until the cervix is elevated with the fluid and the cervical os is identified. The cervical canal is entered and gentle rotational movement and hydrodistension allows the canal to be traversed into the uterine cavity.
Video provided by Amy L. Garcia, MD

Read Dr. Garcia’s “Update on minimally invasive gynecology” (April 2015)
In this video, diffuse complex endometrial hyperplasia is identified using a vaginoscopic approach with a 1.9-mm diagnostic rigid hysteroscope. The posterior fornix of the vagina is filled with saline until the cervix is elevated with the fluid and the cervical os is identified. The cervical canal is entered and gentle rotational movement and hydrodistension allows the canal to be traversed into the uterine cavity.
Video provided by Amy L. Garcia, MD

Read Dr. Garcia’s “Update on minimally invasive gynecology” (April 2015)
In this video, diffuse complex endometrial hyperplasia is identified using a vaginoscopic approach with a 1.9-mm diagnostic rigid hysteroscope. The posterior fornix of the vagina is filled with saline until the cervix is elevated with the fluid and the cervical os is identified. The cervical canal is entered and gentle rotational movement and hydrodistension allows the canal to be traversed into the uterine cavity.
Video provided by Amy L. Garcia, MD

Read Dr. Garcia’s “Update on minimally invasive gynecology” (April 2015)