User login
Ramelteon
In clinical trials, ramelteon has helped patients fall asleep more quickly. Whereas other sleep-promoting medications sedate through effects on gamma-butyric acid (GABA) receptors, ramelteon interacts with melatonin receptors to regulate sleep patterns. It is FDA-approved for treating insomnia characterized by sleep-onset difficulty (Table 1)
Table 1
Ramelteon: Fast facts
| Brand name: |
| Rozerem |
| Class: |
| Nonbenzodiazepine hypnotic |
| FDA-approved indication: |
| Insomnia characterized by sleep-onset difficulty |
| Approval date: |
| August 18, 2005 |
| Manufacturer: |
| Takeda Pharmaceuticals North America |
| Dosing form: |
| 8-mg tablets |
| Recommended dosage: |
| 8 mg within 30 minutes of going to bed |
| Additional prescribing information: |
| www.rozerem.com |
How it works
Ramelteon, a melatonin receptor agonist, has high affinity for the MT1 and MT2 (melatonin) receptors. Although the precise mechanism by which ramelteon affects sleep remains unknown, its effect on sleep is hypothesized to be similar to that of the neurohormone melatonin.
Melatonin is important to maintaining the circadian rhythm that underlies the sleep-wake cycle. Sunlight influences neurohormones that mediate daytime-specific physiologic events. An increase in melatonin—a change that accompanies darkness—is believed to mediate changes in physiology that are characteristic of nighttime. Melatonin thus may be more of a circadian “clock” regulator than a sedative.
Ramelteon shows some features of melatonin that differentiate it from the GABA-related sedating agents. Both ramelteon and melatonin lack abuse potential and a dose-response relationship.
Pharmacokinetics
Ramelteon is absorbed rapidly from the GI tract and reaches median peak concentrations within 30 to 90 minutes of dosing. Taking ramelteon with a high-fat meal reduces its maximum concentration by 22% and slows hypnotic onset by approximately 45 minutes.
The drug is metabolized mostly through the 1A2 isoenzyme of the cytochrome P (CYP)-450 system, although CYP 2C and 3A4 isoenzymes are also involved. About 90% of the dose is excreted.
Ramelteon’s elimination half-life averages 1 to 2.6 hours, so blood levels upon awakening will likely be too low to cause residual effects. Interestingly, in one placebo-controlled study,1 subjects who received a single 64-mg dose reported significantly reduced alertness and diminished ability to concentrate upon awakening. Subjects who took a 16-mg dose did not report this effect. Whether this finding is clinically relevant or relates to a residual effect, sedation, or cognitive impairment is unclear.
Efficacy
In a randomized, double-blind, placebo-controlled trial, ramelteon shortened sleep latency (time between going to bed and falling asleep) among patients with transient insomnia.
Roth et al1 studied 375 healthy adults ages 35 to 60 who reported sleeping 6.5 to 8.5 hours nightly and usually taking ≥30 minutes to fall asleep. In sleep research centers, subjects received one dose of ramelteon, 16 or 64 mg, or placebo 30 minutes before bedtime.
Mean latency to persistent sleep, measured with polysomnography, was 10 minutes shorter among both ramelteon dosage groups than among the placebo group. Mean total sleep time was 11 to 14 minutes longer among both ramelteon groups based on polysomnography, although subjective sleep estimates the next morning were similar among all three groups.
Roth et al2 also assessed efficacy of ramelteon across 5 weeks among 829 older patients (mean age 72) with insomnia (as defined by DSM-IV-TR) for ≥3 months, total nightly sleep time ≤6.5 hours for 3 nights, and self-reported sleep latency ≥45 minutes nightly for ≥3 nights.
Mean sleep latency decreased 25 to 30 minutes among subjects taking ramelteon, 4 or 8 mg nightly, compared with a mean 15-minute decrease among the placebo group. Average total sleep time was 5 to 8 minutes longer among both ramelteon groups compared with placebo.
Subjects in both ramelteon groups then received placebo for 1 week, during which time their mean latency to persistent sleep improved further or stayed the same. This suggests that ramelteon did not cause rebound insomnia.
Safety and tolerability
Ramelteon was generally well tolerated in clinical and preclinical trials. Headaches (7% of subjects), somnolence (5%), dizziness (5%), fatigue (4%), nausea (3%), exacerbated insomnia (3%), and upper respiratory tract infection (3%) were most commonly reported.3 Less-common effects included diarrhea, myalgia, depression, dysgeusia, arthralgia, influenza, and blood cortisol decrease.
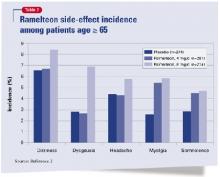
The most common side effects among subjects age ≥65 were dizziness, dysgeusia, headaches, myalgia, and somnolence (Table 2). These occurred less frequently over 5 weeks among patients taking 4 mg/d than among those who took 8 mg/d, the FDA-approved dosage.
Ramelteon also showed no abuse potential compared with triazolam and placebo in a trial of 14 patients with a history of anxiolytic or sedative/hypnotic abuse.4
Contraindications
Do not give ramelteon to patients taking fluvoxamine. The antidepressant has been shown to raise serum ramelteon approximately 70-fold, thus substantially increasing the risk of ramelteon-associated adverse events.3
Ramelteon has shown teratogenicity in animals, though at doses far exceeding human levels. Still, as with other sleep-promoting medications, avoid prescribing ramelteon to expectant mothers.
Concomitant use of a strong CYP enzyme inducer such as rifampin may increase ramelteon metabolism and reduce serum ramelteon, which might decrease its efficacy in some cases. Whether increasing the ramelteon dosage counters this interaction is unknown.
Strong CYP 2C9 inhibitors such as fluconazole or strong CYP 3A4 inhibitors such as ketoconazole can raise serum ramelteon and might increase the risk of adverse events in some persons.
Dosing
Start ramelteon at 8 mg nightly, and tell patients to take it within 30 minutes of going to bed.
Because high-fat food slows its absorption, advise patients not to take ramelteon within 1 hour of eating a high-fat meal.
Ramelteon’s efficacy and side effects do not appear to be dose-dependent when given at 8 to 64 mg/d. Whether dosages >64 mg/d increase side-effect risk or therapeutic effect is unknown.
As with other hypnotics, supplement ramelteon therapy with sleep hygiene education and relaxation techniques.
Clinical implications
Ramelteon appears to help patients who have trouble falling asleep.
Because no other prescription medication targets melatonin neurotransmitters, no precedent and little data exist to guide patient choice, dosing, and treatment duration. Effects of ramelteon use >5 weeks are unknown. Clinical use and future research should uncover more information about ramelteon’s properties.
Related resources
- Ramelteon Web site. www.rozerem.com.
- Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev 2005;9:25-39.
- Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev 2005;9:5-9.
Drug brand names
- Fluconazole • Diflucan
- Fluvoxamine • Luvox
- Ketoconazole • Nizoral
- Ramelteon • Rozerem
- Rifampin • Rifadin
- Triazolam • Halcion
Disclosures
Dr. Krystal receives research/grant support, is a consultant to, or is a speaker for Cephalon, Cyberonics, GlaxoSmithKline, Johnson & Johnson, King Pharmaceuticals, Mecta Corp., Merck and Co., Neurocrine Biosciences, Neurogen Corp., Neuronetics, Organon, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Somaxon Pharmaceuticals, Takeda Pharmaceuticals North America, and TransOral Pharmaceuticals.
1. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 2005;28:303-7.
2. Roth T, Seiden D, Sainati S, et al. Phase III outpatient trial of ramelteon for the treatment of chronic insomnia in elderly patients (poster presentation). Orlando, FL: American Geriatric Society annual meeting, 2005.
3. Rozerem prescribing information. Takeda Pharmaceuticals North America, 2005.
4. Griffiths R, Seuss P. Ramelteon and triazolam in humans: behavioral effects and abuse potential (poster). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
In clinical trials, ramelteon has helped patients fall asleep more quickly. Whereas other sleep-promoting medications sedate through effects on gamma-butyric acid (GABA) receptors, ramelteon interacts with melatonin receptors to regulate sleep patterns. It is FDA-approved for treating insomnia characterized by sleep-onset difficulty (Table 1)
Table 1
Ramelteon: Fast facts
| Brand name: |
| Rozerem |
| Class: |
| Nonbenzodiazepine hypnotic |
| FDA-approved indication: |
| Insomnia characterized by sleep-onset difficulty |
| Approval date: |
| August 18, 2005 |
| Manufacturer: |
| Takeda Pharmaceuticals North America |
| Dosing form: |
| 8-mg tablets |
| Recommended dosage: |
| 8 mg within 30 minutes of going to bed |
| Additional prescribing information: |
| www.rozerem.com |
How it works
Ramelteon, a melatonin receptor agonist, has high affinity for the MT1 and MT2 (melatonin) receptors. Although the precise mechanism by which ramelteon affects sleep remains unknown, its effect on sleep is hypothesized to be similar to that of the neurohormone melatonin.
Melatonin is important to maintaining the circadian rhythm that underlies the sleep-wake cycle. Sunlight influences neurohormones that mediate daytime-specific physiologic events. An increase in melatonin—a change that accompanies darkness—is believed to mediate changes in physiology that are characteristic of nighttime. Melatonin thus may be more of a circadian “clock” regulator than a sedative.
Ramelteon shows some features of melatonin that differentiate it from the GABA-related sedating agents. Both ramelteon and melatonin lack abuse potential and a dose-response relationship.
Pharmacokinetics
Ramelteon is absorbed rapidly from the GI tract and reaches median peak concentrations within 30 to 90 minutes of dosing. Taking ramelteon with a high-fat meal reduces its maximum concentration by 22% and slows hypnotic onset by approximately 45 minutes.
The drug is metabolized mostly through the 1A2 isoenzyme of the cytochrome P (CYP)-450 system, although CYP 2C and 3A4 isoenzymes are also involved. About 90% of the dose is excreted.
Ramelteon’s elimination half-life averages 1 to 2.6 hours, so blood levels upon awakening will likely be too low to cause residual effects. Interestingly, in one placebo-controlled study,1 subjects who received a single 64-mg dose reported significantly reduced alertness and diminished ability to concentrate upon awakening. Subjects who took a 16-mg dose did not report this effect. Whether this finding is clinically relevant or relates to a residual effect, sedation, or cognitive impairment is unclear.
Efficacy
In a randomized, double-blind, placebo-controlled trial, ramelteon shortened sleep latency (time between going to bed and falling asleep) among patients with transient insomnia.
Roth et al1 studied 375 healthy adults ages 35 to 60 who reported sleeping 6.5 to 8.5 hours nightly and usually taking ≥30 minutes to fall asleep. In sleep research centers, subjects received one dose of ramelteon, 16 or 64 mg, or placebo 30 minutes before bedtime.
Mean latency to persistent sleep, measured with polysomnography, was 10 minutes shorter among both ramelteon dosage groups than among the placebo group. Mean total sleep time was 11 to 14 minutes longer among both ramelteon groups based on polysomnography, although subjective sleep estimates the next morning were similar among all three groups.
Roth et al2 also assessed efficacy of ramelteon across 5 weeks among 829 older patients (mean age 72) with insomnia (as defined by DSM-IV-TR) for ≥3 months, total nightly sleep time ≤6.5 hours for 3 nights, and self-reported sleep latency ≥45 minutes nightly for ≥3 nights.
Mean sleep latency decreased 25 to 30 minutes among subjects taking ramelteon, 4 or 8 mg nightly, compared with a mean 15-minute decrease among the placebo group. Average total sleep time was 5 to 8 minutes longer among both ramelteon groups compared with placebo.
Subjects in both ramelteon groups then received placebo for 1 week, during which time their mean latency to persistent sleep improved further or stayed the same. This suggests that ramelteon did not cause rebound insomnia.
Safety and tolerability
Ramelteon was generally well tolerated in clinical and preclinical trials. Headaches (7% of subjects), somnolence (5%), dizziness (5%), fatigue (4%), nausea (3%), exacerbated insomnia (3%), and upper respiratory tract infection (3%) were most commonly reported.3 Less-common effects included diarrhea, myalgia, depression, dysgeusia, arthralgia, influenza, and blood cortisol decrease.
The most common side effects among subjects age ≥65 were dizziness, dysgeusia, headaches, myalgia, and somnolence (Table 2). These occurred less frequently over 5 weeks among patients taking 4 mg/d than among those who took 8 mg/d, the FDA-approved dosage.
Ramelteon also showed no abuse potential compared with triazolam and placebo in a trial of 14 patients with a history of anxiolytic or sedative/hypnotic abuse.4
Contraindications
Do not give ramelteon to patients taking fluvoxamine. The antidepressant has been shown to raise serum ramelteon approximately 70-fold, thus substantially increasing the risk of ramelteon-associated adverse events.3
Ramelteon has shown teratogenicity in animals, though at doses far exceeding human levels. Still, as with other sleep-promoting medications, avoid prescribing ramelteon to expectant mothers.
Concomitant use of a strong CYP enzyme inducer such as rifampin may increase ramelteon metabolism and reduce serum ramelteon, which might decrease its efficacy in some cases. Whether increasing the ramelteon dosage counters this interaction is unknown.
Strong CYP 2C9 inhibitors such as fluconazole or strong CYP 3A4 inhibitors such as ketoconazole can raise serum ramelteon and might increase the risk of adverse events in some persons.
Dosing
Start ramelteon at 8 mg nightly, and tell patients to take it within 30 minutes of going to bed.
Because high-fat food slows its absorption, advise patients not to take ramelteon within 1 hour of eating a high-fat meal.
Ramelteon’s efficacy and side effects do not appear to be dose-dependent when given at 8 to 64 mg/d. Whether dosages >64 mg/d increase side-effect risk or therapeutic effect is unknown.
As with other hypnotics, supplement ramelteon therapy with sleep hygiene education and relaxation techniques.
Clinical implications
Ramelteon appears to help patients who have trouble falling asleep.
Because no other prescription medication targets melatonin neurotransmitters, no precedent and little data exist to guide patient choice, dosing, and treatment duration. Effects of ramelteon use >5 weeks are unknown. Clinical use and future research should uncover more information about ramelteon’s properties.
Related resources
- Ramelteon Web site. www.rozerem.com.
- Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev 2005;9:25-39.
- Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev 2005;9:5-9.
Drug brand names
- Fluconazole • Diflucan
- Fluvoxamine • Luvox
- Ketoconazole • Nizoral
- Ramelteon • Rozerem
- Rifampin • Rifadin
- Triazolam • Halcion
Disclosures
Dr. Krystal receives research/grant support, is a consultant to, or is a speaker for Cephalon, Cyberonics, GlaxoSmithKline, Johnson & Johnson, King Pharmaceuticals, Mecta Corp., Merck and Co., Neurocrine Biosciences, Neurogen Corp., Neuronetics, Organon, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Somaxon Pharmaceuticals, Takeda Pharmaceuticals North America, and TransOral Pharmaceuticals.
In clinical trials, ramelteon has helped patients fall asleep more quickly. Whereas other sleep-promoting medications sedate through effects on gamma-butyric acid (GABA) receptors, ramelteon interacts with melatonin receptors to regulate sleep patterns. It is FDA-approved for treating insomnia characterized by sleep-onset difficulty (Table 1)
Table 1
Ramelteon: Fast facts
| Brand name: |
| Rozerem |
| Class: |
| Nonbenzodiazepine hypnotic |
| FDA-approved indication: |
| Insomnia characterized by sleep-onset difficulty |
| Approval date: |
| August 18, 2005 |
| Manufacturer: |
| Takeda Pharmaceuticals North America |
| Dosing form: |
| 8-mg tablets |
| Recommended dosage: |
| 8 mg within 30 minutes of going to bed |
| Additional prescribing information: |
| www.rozerem.com |
How it works
Ramelteon, a melatonin receptor agonist, has high affinity for the MT1 and MT2 (melatonin) receptors. Although the precise mechanism by which ramelteon affects sleep remains unknown, its effect on sleep is hypothesized to be similar to that of the neurohormone melatonin.
Melatonin is important to maintaining the circadian rhythm that underlies the sleep-wake cycle. Sunlight influences neurohormones that mediate daytime-specific physiologic events. An increase in melatonin—a change that accompanies darkness—is believed to mediate changes in physiology that are characteristic of nighttime. Melatonin thus may be more of a circadian “clock” regulator than a sedative.
Ramelteon shows some features of melatonin that differentiate it from the GABA-related sedating agents. Both ramelteon and melatonin lack abuse potential and a dose-response relationship.
Pharmacokinetics
Ramelteon is absorbed rapidly from the GI tract and reaches median peak concentrations within 30 to 90 minutes of dosing. Taking ramelteon with a high-fat meal reduces its maximum concentration by 22% and slows hypnotic onset by approximately 45 minutes.
The drug is metabolized mostly through the 1A2 isoenzyme of the cytochrome P (CYP)-450 system, although CYP 2C and 3A4 isoenzymes are also involved. About 90% of the dose is excreted.
Ramelteon’s elimination half-life averages 1 to 2.6 hours, so blood levels upon awakening will likely be too low to cause residual effects. Interestingly, in one placebo-controlled study,1 subjects who received a single 64-mg dose reported significantly reduced alertness and diminished ability to concentrate upon awakening. Subjects who took a 16-mg dose did not report this effect. Whether this finding is clinically relevant or relates to a residual effect, sedation, or cognitive impairment is unclear.
Efficacy
In a randomized, double-blind, placebo-controlled trial, ramelteon shortened sleep latency (time between going to bed and falling asleep) among patients with transient insomnia.
Roth et al1 studied 375 healthy adults ages 35 to 60 who reported sleeping 6.5 to 8.5 hours nightly and usually taking ≥30 minutes to fall asleep. In sleep research centers, subjects received one dose of ramelteon, 16 or 64 mg, or placebo 30 minutes before bedtime.
Mean latency to persistent sleep, measured with polysomnography, was 10 minutes shorter among both ramelteon dosage groups than among the placebo group. Mean total sleep time was 11 to 14 minutes longer among both ramelteon groups based on polysomnography, although subjective sleep estimates the next morning were similar among all three groups.
Roth et al2 also assessed efficacy of ramelteon across 5 weeks among 829 older patients (mean age 72) with insomnia (as defined by DSM-IV-TR) for ≥3 months, total nightly sleep time ≤6.5 hours for 3 nights, and self-reported sleep latency ≥45 minutes nightly for ≥3 nights.
Mean sleep latency decreased 25 to 30 minutes among subjects taking ramelteon, 4 or 8 mg nightly, compared with a mean 15-minute decrease among the placebo group. Average total sleep time was 5 to 8 minutes longer among both ramelteon groups compared with placebo.
Subjects in both ramelteon groups then received placebo for 1 week, during which time their mean latency to persistent sleep improved further or stayed the same. This suggests that ramelteon did not cause rebound insomnia.
Safety and tolerability
Ramelteon was generally well tolerated in clinical and preclinical trials. Headaches (7% of subjects), somnolence (5%), dizziness (5%), fatigue (4%), nausea (3%), exacerbated insomnia (3%), and upper respiratory tract infection (3%) were most commonly reported.3 Less-common effects included diarrhea, myalgia, depression, dysgeusia, arthralgia, influenza, and blood cortisol decrease.
The most common side effects among subjects age ≥65 were dizziness, dysgeusia, headaches, myalgia, and somnolence (Table 2). These occurred less frequently over 5 weeks among patients taking 4 mg/d than among those who took 8 mg/d, the FDA-approved dosage.
Ramelteon also showed no abuse potential compared with triazolam and placebo in a trial of 14 patients with a history of anxiolytic or sedative/hypnotic abuse.4
Contraindications
Do not give ramelteon to patients taking fluvoxamine. The antidepressant has been shown to raise serum ramelteon approximately 70-fold, thus substantially increasing the risk of ramelteon-associated adverse events.3
Ramelteon has shown teratogenicity in animals, though at doses far exceeding human levels. Still, as with other sleep-promoting medications, avoid prescribing ramelteon to expectant mothers.
Concomitant use of a strong CYP enzyme inducer such as rifampin may increase ramelteon metabolism and reduce serum ramelteon, which might decrease its efficacy in some cases. Whether increasing the ramelteon dosage counters this interaction is unknown.
Strong CYP 2C9 inhibitors such as fluconazole or strong CYP 3A4 inhibitors such as ketoconazole can raise serum ramelteon and might increase the risk of adverse events in some persons.
Dosing
Start ramelteon at 8 mg nightly, and tell patients to take it within 30 minutes of going to bed.
Because high-fat food slows its absorption, advise patients not to take ramelteon within 1 hour of eating a high-fat meal.
Ramelteon’s efficacy and side effects do not appear to be dose-dependent when given at 8 to 64 mg/d. Whether dosages >64 mg/d increase side-effect risk or therapeutic effect is unknown.
As with other hypnotics, supplement ramelteon therapy with sleep hygiene education and relaxation techniques.
Clinical implications
Ramelteon appears to help patients who have trouble falling asleep.
Because no other prescription medication targets melatonin neurotransmitters, no precedent and little data exist to guide patient choice, dosing, and treatment duration. Effects of ramelteon use >5 weeks are unknown. Clinical use and future research should uncover more information about ramelteon’s properties.
Related resources
- Ramelteon Web site. www.rozerem.com.
- Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev 2005;9:25-39.
- Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev 2005;9:5-9.
Drug brand names
- Fluconazole • Diflucan
- Fluvoxamine • Luvox
- Ketoconazole • Nizoral
- Ramelteon • Rozerem
- Rifampin • Rifadin
- Triazolam • Halcion
Disclosures
Dr. Krystal receives research/grant support, is a consultant to, or is a speaker for Cephalon, Cyberonics, GlaxoSmithKline, Johnson & Johnson, King Pharmaceuticals, Mecta Corp., Merck and Co., Neurocrine Biosciences, Neurogen Corp., Neuronetics, Organon, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Somaxon Pharmaceuticals, Takeda Pharmaceuticals North America, and TransOral Pharmaceuticals.
1. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 2005;28:303-7.
2. Roth T, Seiden D, Sainati S, et al. Phase III outpatient trial of ramelteon for the treatment of chronic insomnia in elderly patients (poster presentation). Orlando, FL: American Geriatric Society annual meeting, 2005.
3. Rozerem prescribing information. Takeda Pharmaceuticals North America, 2005.
4. Griffiths R, Seuss P. Ramelteon and triazolam in humans: behavioral effects and abuse potential (poster). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
1. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 2005;28:303-7.
2. Roth T, Seiden D, Sainati S, et al. Phase III outpatient trial of ramelteon for the treatment of chronic insomnia in elderly patients (poster presentation). Orlando, FL: American Geriatric Society annual meeting, 2005.
3. Rozerem prescribing information. Takeda Pharmaceuticals North America, 2005.
4. Griffiths R, Seuss P. Ramelteon and triazolam in humans: behavioral effects and abuse potential (poster). Atlanta, GA: American Psychiatric Association annual meeting, 2005.