User login
Managing metabolic syndrome in patients with schizophrenia
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.
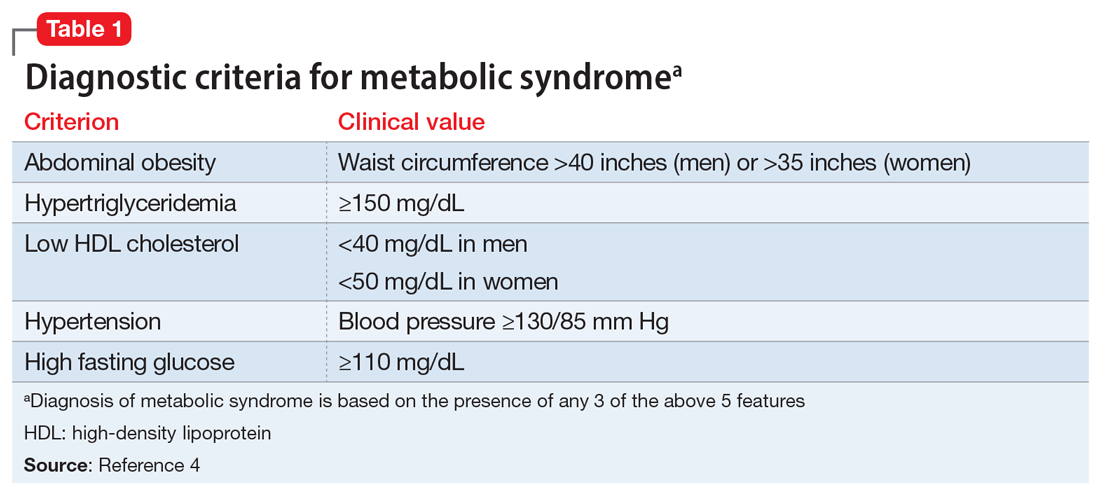
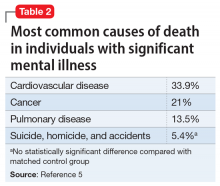
Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
Mr. N, age 55, has a long, documented history of schizophrenia. His overall baseline functioning has been poor because he is socially isolated, does not work, and lives in subsidized housing paid for by the county where he lives. His psychosocial circumstances have limited his ability to afford or otherwise obtain nutritious food or participate in any type of regular exercise program. He has been maintained on olanzapine, 20 mg nightly, for the past 5 years. During the past year, his functioning and overall quality of life have declined even further after he was diagnosed with hypertension. Mr. N’s in-office blood pressure was 160/95 mm Hg (normal range: systolic blood pressure, 90 to 120 mm Hg, and diastolic blood pressure, 60 to 80 mm Hg). He says his primary care physician informed him that he is pre-diabetic after his hemoglobin A1c came back at 6.0 mg/dL (normal range <5.7 mg/dL) and his body mass index was 32 kg/m2 (normal range 18.5 to 24.9 kg/m2). Currently, Mr. N’s psychiatric symptoms are stable, but his functional decline is now largely driven by metabolic parameters. Along with lifestyle changes and nonpharmacologic interventions, what else should you consider to help him?
In addition to positive, negative, and cognitive symptoms, schizophrenia is accompanied by disturbances in metabolism,1 inflammatory markers,2 and sleep/wake cycles.3 Current treatment strategies focus on addressing symptoms and functioning, but the metabolic and inflammatory targets that account for significant morbidity and mortality remain largely unaddressed.

Some patients with schizophrenia meet the criteria for metabolic syndrome, a cluster of conditions—including obesity, insulin resistance, dyslipidemia, and hypertension—that increase the risk of cardiovascular disease and type 2 diabetes mellitus (Table 14). Metabolic syndrome and its related consequences are a major barrier to the successful treatment of patients with schizophrenia, and lead to increased mortality. Druss et al5 found that individuals with significant mental illness died on average 8.2 years earlier than age-matched controls. The most common cause of death was cardiovascular disease (Table 25).
“Off-label” prescribing has been used in an attempt to delay or treat emerging metabolic syndrome in individuals with schizophrenia. Unfortunately, comprehensive strategies with a uniform application in clinical settings remain elusive. In this article, we review 3 off-label agents—metformin, topiramate, and melatonin—that may be used to address weight gain and metabolic syndrome in patients with schizophrenia.
Metformin
Metformin is an oral medication used to treat type 2 diabetes. It works by decreasing glucose absorption, suppressing gluconeogenesis in the liver, and increasing insulin sensitivity in peripheral tissues. It was FDA-approved for use in the United States in 1994. In addition to improving glucose homeostasis, metformin has also been associated with decreased body mass index (BMI), triglycerides, and low-density lipoprotein (LDL) cholesterol, and increased high-density lipoprotein (HDL) cholesterol in individuals at risk for diabetes.6
Recent consensus guidelines suggest that metformin has sufficient evidence to support its clinical use for preventing or treating antipsychotic-induced weight gain.7 A meta-analysis that included >40 randomized clinical trials (RCTs) found that metformin8-11:
- reduces antipsychotic-induced weight gain (approximately 3 kg, up to 5 kg in patients with first-episode psychosis)
- reduces fasting glucose levels, hemoglobin A1c, fasting insulin levels, and insulin resistance
- leads to a more favorable lipid profile (reduced triglycerides, LDL, and total cholesterol, and increased HDL).
Not surprisingly, metformin’s effects are augmented when used in conjunction with lifestyle interventions (diet and exercise), leading to further weight reductions of 1.5 kg and BMI reductions of 1.08 kg/m2 when compared with metformin alone.11 The mechanism underlying metformin’s attenuation of antipsychotic-induced weight gain is not fully understood, but preclinical studies suggest that it may prevent olanzapine-induced brown adipose tissue loss,12,13 alter Wnt signaling (an assortment of signal transduction pathways important for glucose homeostasis and metabolism),13 and influence the gut microbiome.14
Continue to: Metformin is generally...
Metformin is generally well tolerated. Common adverse effects include diarrhea, nausea, and abdominal pain, which are generally transient and can be ameliorated by using the extended-release formulation and lower starting doses.15 The frequency of medication discontinuation was minimal and similar in patients receiving metformin vs placebo.8,16 Despite these positive findings, most studies of metformin have had a follow-up of ≤24 weeks, and its long-term effects on antipsychotic-induced weight gain and metabolic parameters remain unknown.
When prescribing metformin for a patient with schizophrenia, consider a starting dose of 500 mg twice daily.
Topiramate
Topiramate is FDA-approved for treating generalized tonic-clonic and complex partial seizures17 and for migraine prophylaxis. More recently, it has been used off-label for weight loss in both psychiatric and non-psychiatric patients. Topiramate’s proposed mechanism for weight loss is by decreasing plasma leptin levels and increasing plasma adiponectin. A recent literature review of 8 RCTS that included 336 patients who received second-generation antipsychotics (SGAs) and adjunctive placebo or topiramate (100 to 300 mg/d) found that patients who received topiramate lost a statistically significant 2.83 kg vs placebo.18 Several case studies confirm similar findings, showing that patients with schizophrenia lost 2 to 5 kg when started on topiramate along with an SGA.19 Importantly, weight loss has been observed both in patients started on topiramate prophylactically along with an SGA, and those who had been receiving SGAs for an extended period of time before starting topiramate.
Tolerability has been a concern in patients receiving topiramate. Frequent complaints include cognitive dulling, sedation, and coldness or tingling of the extremities. In a meta-analysis of topiramate, metformin, and other medications used to induce weight loss in patients receiving SGAs, Zhuo et al20 found that topiramate was reported intolerable more frequently than other agents, although the difference was not statistically significant.
When prescribing topiramate for a patient with schizophrenia, consider a starting dose of 25 mg at bedtime.
Continue to: Melatonin
Melatonin
Melatonin is a naturally occurring hormone that is available over-the-counter and is frequently used to treat insomnia. Melatonin appears to have few adverse effects, is not habit-forming, and is inexpensive. It is a hormone produced primarily by the pineal gland, although it is also produced by many other cell types, including the skin, gut, bone marrow, thymus, and retina.21,22 Melatonin is a highly conserved essential hormone23 that acts via both G protein-coupled membrane bound receptors and nuclear receptors.23-25 Its ability to function both intra- and extracellularly implies it has an essential role in maintaining homeostatic mechanisms. Melatonin’s putative mechanism of action may derive from its effects on circadian rhythms, which in turn affect systolic blood pressure, glycemic control, and oxidative stress. In rodents, pinealectomy led to the rapid development of hypertension and metabolic syndrome. Daily administration of melatonin26 in these animals restored metabolism by decreasing abdominal fat and plasma leptin levels. These studies suggest that melatonin plays a central role in metabolism.
A recent study of patients with first-episode psychosis (n = 48) examined the effects of melatonin (3 mg/d) as an add-on treatment to olanzapine vs placebo.27 Compared with those in the placebo group, participants in the melatonin group experienced a statistically significant decrease in body weight, BMI, waist circumference, and triglyceride levels.27 In another study, the melatonin receptor agonist ramelteon was used in conjunction with SGAs.28 Augmentation with ramelteon led to significantly lower rises in total cholesterol levels compared with placebo.28
When recommending melatonin for a patient with schizophrenia, suggest that he/she begin by taking a starting dose of 3 mg nightly.
Weighing the options
Which medication to prescribe for a patient such as Mr. N would depend on the patient’s specific complaint/health target.
Weight gain or diabetes. If the patient’s primary concerns are avoiding weight gain or the development of diabetes, metformin is an excellent starting point.
Continue to: Migraines or desire to lose weight
Migraines or desire to lose weight. If the patient reports frequent migraines or a history of migraines, or if he/she is interested in weight loss, a trial of topiramate may be appropriate.
Sleep difficulties. If sleep is the patient’s primary concern, then adding melatonin might be a good first choice.
At this point, the available data points to metformin as the most efficacious medication in ameliorating some of the metabolic adverse effects associated with the long-term use of SGAs.8-11 Comprehensive treatment of patients with schizophrenia should include addressing underlying metabolic issues not only to improve health outcomes and reduce morbidity and mortality, but also to improve psychosocial functioning and quality of life.
Bottom Line
Preventing or treating metabolic syndrome is an important consideration in all patients with schizophrenia. Metformin, topiramate, and melatonin show some promise in helping ameliorate metabolic syndrome and its associated morbidity and mortality, and also may help improve patients’ functioning and quality of life.
Related Resources
- Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318.
- Majeed MH, Khalil HA. Cardiovascular adverse effects of psychotropics: what to look for. Current Psychiatry. 2018; 17(7):54-55
- Wake, LA, Balon R. Should psychiatrists prescribe nonpsychotropic medications? Current Psychiatry. 2019; 18(11):52-56.
Drug Brand Names
Metformin • Glucophage
Olanzapine • Zyprexa
Ramelteon • Rozerem
Topiramate • Topamax
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.
1. Bushe C, Holt R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl. 2004;184(suppl 47):S67-S71.
2. Harvey PD. Inflammation in schizophrenia: what it means and how to treat it. Am J Geriatr Psychiatry. 2017;25(1):62-63.
3. Chouinard S, Poulin J, Stip E. Sleep in untreated patients with schizophrenia: a meta-analysis. Schizophr Bull. 2004;30(4):957-967.
4. Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237.
5. Druss BG, Zhao L, Von Esenwein S, et al. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49(6):599-604.
6. Salpeter SR, Buckley NS, Kahn JA, et al. Meta-analysis: metformin treatment in persons at risk for diabetes mellitus. Am J Med. 2008;121(2):149-157.
7. Faulkner G, Duncan M. Metformin to reduce weight gain and metabolic disturbance in schizophrenia. Evid Based Ment Health. 2015;18(3):89.
8. Jarskog LF, Hamer RM, Catellier DJ, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
9. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403.
10. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. doi: 10.1371/journal.pone.0156208.
11. Wu T, Horowitz M, Rayner CK. New insights into the anti-diabetic actions of metformin: from the liver to the gut. Expert Rev Gastroenterol Hepatol. 2017;11(2):157-166.
12. Hu Y, Young AJ, Ehli EA, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats. PLoS One. 2014;9(3):e93310. doi: 10.1371/journal.pone.0093310.
13. Li R, Ou J, Li L, et al. The Wnt signaling pathway effector TCF7L2 mediates olanzapine-induced weight gain and insulin resistance. Front Pharmacol. 2018;9:379.
14. Luo C, Wang X, Huang H, et al. Effect of metformin on antipsychotic-induced metabolic dysfunction: the potential role of gut-brain axis. Front Pharmacol. 2019;10:371.
15. Flory JH, Keating SJ, Siscovick D, et al. Identifying prevalence and risk factors for metformin non-persistence: a retrospective cohort study using an electronic health record. BMJ Open. 2018;8(7):e021505. doi: 10.1136/bmjopen-2018-021505.
16. Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138(1):54-57.
17. Maryanoff BE. Phenotypic assessment and the discovery of topiramate. ACS Med Chem Lett. 2016;7(7):662-665.
18. Mahmood S, Booker I, Huang J, et al. Effect of topiramate on weight gain in patients receiving atypical antipsychotic agents. J Clin Psychopharmacol. 2013;33(1):90-94.
19. Lin YH, Liu CY, Hsiao MC. Management of atypical antipsychotic-induced weight gain in schizophrenic patients with topiramate. Psychiatry Clin Neurosci. 2005;59(5):613-615.
20. Zhuo C, Xu Y, Liu S, et al. Topiramate and metformin are effective add-on treatments in controlling antipsychotic-induced weight gain: a systematic review and network meta-analysis. Front Pharmacol. 2018;9:1393.
21. Nduhirabandi F, du Toit EF, Lochner A. Melatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities? Acta Physiol (Oxf). 2012;205(2):209-223.
22. Srinivasan V, Ohta Y, Espino J, et al. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11-25.
23. Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006;38(3):313-316.
24. Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93(3):350-384.
25. Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor Neurol Neurosci. 1998;12(2-3):143-150.
26. Nava M, Quiroz Y, Vaziri N, et al. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2003;284(3):F447-F454.
27. Modabbernia A, Heidari P, Soleimani R, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140.
28. Borba CP, Fan X, Copeland PM, et al. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(5):653-658.