User login
Inpatient Smoking Cessation Program
In 1992, the Joint Commission on Accreditation of Healthcare Organizations (Joint Commission) introduced standards to make hospital buildings smoke‐free, resulting in the nation's first industry‐wide ban on smoking in the workplace. This hospital smoking ban has led to increased smoking cessation among employees.1 Since 2003, core measures from the Joint Commission and quality indicators from the Centers for Medicare and Medicaid Services have included inpatient smoking cessation counseling for acute myocardial infarction, pneumonia, and heart failure, as national guidelines strongly recommend smoking cessation counseling for patients with these diseases who smoke.25
The Department of Health and Human Services (DHHS) 2008 update on Clinical Practice Guidelines for Treating Tobacco Use and Dependence6 recommends that clinicians use hospitalization as an opportunity to promote smoking cessation and to prescribe medications to alleviate withdrawal symptoms. Hospitalization is an opportune time for smoking cessation because patients are restricted to a smoke‐free environment in the hospital and, increasingly, on hospital campuses.6 The illness leading to hospitalization may be attributable, at least in part, to tobacco use, thereby increasing the patient's receptivity to cessation counseling. Last, medications used in‐hospital to treat nicotine withdrawal symptoms may lead to continued or future use of these medications that, in turn, may ultimately lead to a successful quit attempt.
We report on the outcomes of our hospital's attempt to do this in the context of implementation of a smoke‐free medical campus.7 This study was designed to measure whether an inpatient smoking cessation intervention increases the likelihood of smoking cessation 6 months post‐hospital discharge. Because effectiveness studies are the next step to improving translation of research into health promotion practice,8 we set out to measure what the impact of this intervention would be in routine clinical practice as opposed to a carefully structured efficacy trial.
Methods
Intervention
The Smoking Cessation service for inpatients began on April 3, 2006. Upon admission, all patients were screened regarding their current smoking status. The nurse asked the patient if s/he currently smoked and then entered the responses into the hospital electronic medical record (EMR). A current smoker was defined as smoking every day or some days within the past 30 days. A roster of newly admitted current smokers was electronically transmitted to the Respiratory Care office daily. Only current smokers received counseling. The Smoking Cessation Specialist (SCS) subsequently saw inpatients within a 24‐hour time frame of admission, except for weekends and holidays. Each patient received 1 to 2 intensive follow‐up counseling sessions during hospitalization. An average of 10 patients per day were seen.
The goal of the inpatient smoking cessation service was to counsel patients on the health effects of smoking, address nicotine withdrawal symptoms, explain the different pharmacotherapies available, advise on how to quit, give self‐help materials, counsel family members, and refer to the New York State (NYS) Smokers' Fax‐to‐Quit program.9 Following the consult, the SCS documented the encounter in the patient's chart, including recommendations for nicotine replacement therapy (NRT) or bupropion (varenicline was not addressed as it was not on the formulary). The chart documentation informed the physician and nursing staff of the intervention and included the date/time, stage of change, and support action taken.
The SCS was part‐time, had nursing training, smoking cessation training,10 and was also trained by the Seton Health Cessation Center in the Butt Stops Here Program.11 She also implemented a performance improvement plan to increase the provision of smoking cessation counseling, increase NRT or bupropion prescriptions to smokers admitted to the hospital, and increase referrals to the NYS telephone quitline through the Fax‐to‐Quit program for outpatient resources and help following hospital discharge. The Fax‐to‐Quit program allows health care providers to refer patients to the NYS quitline via fax, with the patient's signature (patient permission) on the fax to quit form. After hospital discharge, the quitline then contacts the patient at a time that the patient requested.
The SCS visited patients with all admitting diagnoses on the medical, surgical, and special care units who were current smokers. Inpatients admitted to psychiatry, obstetrics, and the intensive care unit (ICU) were not seen by the SCS, except for ICU patients referred by a physician. Inpatients who had short stays or who were admitted and discharged in 1 day or during the weekend were not seen.
The intervention included either a brief 3‐minute to 5‐minute intervention or a more intensive intervention, that required 10 to 20 minutes (18 minutes average). The length of the intervention was determined by how receptive the patient was to the intervention. All interventions began with patient identification, an introduction to the SCS, and an explanation of the purpose of the visit. The SCS then inquired about the patient's comfort level vis‐a‐vis nicotine withdrawal and if s/he was receiving any NRT while in the hospital (NRT on the inpatient formulary included the nicotine patch or gum). If the patient was receptive to counseling, the SCS then began to work through the 5 A's, as described in the 2000 DHHS Clinical Practice Guidelines.12 The 2000 DHHS Clinical Practice Guidelines were used because the 2008 update had not been released at the time this study was initiated in 2006. These include: asking about smoking status, advising on how to quit, assessing readiness to quit, and assisting in arranging treatment options that include pharmacotherapy, counseling, as well as referral to the NYS Smokers' Quitline. A workbook was provided to reinforce counseling but was not necessarily used during counseling session. A compact disc (CD) with relaxation exercises5 was provided to those inpatients who were interested in stress reduction. If family members were present, and were also smokers, they were included in the counseling session, if willing. Each patient was offered a referral via the Fax‐to‐Quit program to continue treatment on an outpatient basis.
If the patient was not motivated to quit or declined the consult, the visits were short and focused on the patient's experience with nicotine withdrawal. These patients were also given self‐help materials and, if possible, the relevance of and roadblocks to quitting were reviewed. Patients were prompted to think about why quitting was relevant and often the reason for hospitalization was used to motivate the quitting process.
Upon hospital discharge, the patient's primary care provider was notified of the cessation intervention by a letter from the SCS. The letter described the intervention and stated whether or not the patient agreed to be referred to the Fax‐to‐Quit Program.
Study Participants
Patients were recruited from July 1, 2006 (after the smoking ban went into effect) through June 1, 2008. Inpatients who currently smoked were informed of this study and were asked to sign informed consent to participate after they were seen by the SCS. Current smokers of all admitting diagnoses were recruited into the study. Patients provided informed consent for a telephone interview 6 months posthospital discharge. A written Health Insurance Portability and Accountability Act (HIPAA) release was obtained to allow access to an individual's specific EMR.
A comparison group of inpatients who were also current smokers, but who did not receive the intervention were also contacted six months after hospitalization. Reasons for not receiving the intervention included the fact the SCS was part‐time and also took a leave of absence during the study and therefore could not see all inpatients who currently smoked. Other reasons for not receiving the intervention include too short a stay for the SCS to see the patient or the patient was out of the room for tests or procedures when the SCS was available. These patients provided informed consent to be interviewed 6 months after hospital discharge and HIPAA consent for access to their medical record. Not all inpatients in the comparison group provided written HIPAA release for use of their medical record; therefore, these patients were excluded because their baseline demographic and diagnostic data were missing.
Sample‐size considerations were driven around having adequate numbers of subjects to measure the prevalence of smoking cessation at 6 months post‐hospital discharge with an acceptable degree of precision. Prevalence estimates from previous studies for 6‐month cessation typically range from 20% to 30%, with cessation rates as high as 67% (this estimate applies to postmyocardial infarction patients.)13 For conservative estimation, we used 50% as the 6‐month prevalence of cessation in the current study, which placed binomial variance at its theoretical maximum. In this case, a sample of 300 subjects provides a margin of error of 0.058 for a 95% confidence interval around this point estimate.
Data Sources
The hospital EMR database was used to monitor several components of the program: nursing screening, smoking cessation counseling, and pharmacy dispensing of NRT and bupropion. The screening data were also used to monitor the proportion of current smokers admitted during the study period. Elements of the EMR were used to define the following covariates: patient age, gender, ethnicity, and the primary discharge diagnosis (via International Statistical Classification of Diseases and Related Health Problems, 9th edition [ICD9] codes) and readmission during the six month follow‐up period. Mean length of stay (LOS) was computed. The Elixhauser Comorbidity Index that utilizes ICD9 codes was used for comorbidity risk adjustment.14
Study participants were contacted by phone 6 months posthospital discharge. Data collection began July 1, 2006 and was completed January 1, 2009. The interview focused on self‐reported point prevalence of smoking and 6‐month quit status. The point prevalence for self‐reported abstinence was derived from the question Do you now smoke cigarettes every day, some days, or not at all?15 Self‐reported quit status was derived from question Have you quit smoking since you were discharged from the hospital? In addition, respondents were queried about their number of years smoked, post‐hospital discharge number of quit attempts, and cessation efforts (NRT, self‐help groups, quitline use, etc.). Last, they were surveyed about barriers to cessation (exposure to secondhand smoke, rules about smoking in the home or car), educational level, employment, and health ratings.
To determine the status of those lost to follow‐up, administrative and EMR databases for appointments and follow‐up visits were accessed to determine if the patient was alive during the 6 months between discharge and the follow‐up call. To confirm mortality, we searched the Internet, Ancestry.com, and/or local newspaper obituaries for dates of death for all patients to validate that they had not died during the 6‐month follow‐up. World wide web searches can identify 97% of deaths listed in the Centers for Disease Control and Prevention (CDC)/National Death Index, which is considered the gold standard in epidemiologic studies.16
Analysis
Univariate analysis of all covariates was completed to examine the normal distribution curves for these variables. Bivariate correlation analysis of all the independent variables by study group was performed to assess comparability of the study groups at baseline. The self‐reported cessation outcomes were calculated by dividing the number of patients who said they were not using tobacco or had quit, at 6 months posthospital discharge by the number of individuals in the study group at baseline minus those who had died. Both the intent to treat method, which assumes patients lost to follow‐up were still smoking, and the responder method, which does not include nonresponders in the analysis, were used to adjust the denominators for these outcomes.
Multivariate regression analysis was then used to model receipt of the intervention as predictor of self‐reported quit status adjusted for significant covariates. Statistical significance was defined by a P value of less than 0.05.
Survival analysis was employed to model differences in mortality between the study groups, controlling for any baseline imbalances (eg, comorbidity). Because baseline data were used in this model, the model includes only patients with signed a HIPAA release.
Internal review boards of our hospital and the NYS Department of Health reviewed and approved this study.
Results
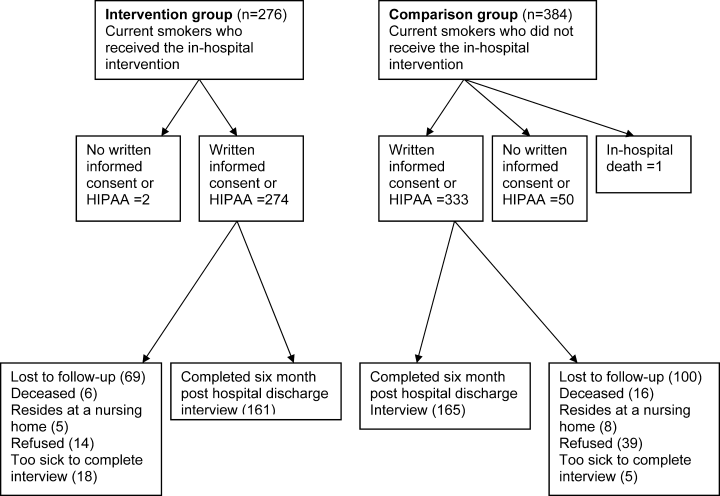
From January 1, 2007 to May 30, 2008, 660 inpatients who were current smokers were recruited into the study. Figure 1 summarizes patient flow through the study and explains the final sample size of 607. Exclusions include 52 inpatients from the study who completed the 6‐month interview but who did not return a written HIPAA release. Without a HIPAA release to access the EMR, baseline comparison of the study groups and adjustment for comorbidity could not be completed for these patients. At 6 months posthospital discharge, 53 subjects refused the interview when contacted by telephone.
As might be expected in a quasiexperimental design, the study groups were not equivalent at baseline (Table 1). The intervention and comparison groups differed with regard to age, length of stay (LOS), proportion of acute admissions, and the Elixhauser comorbidity index. These differences suggest that the intervention group was older, had a longer LOS, higher acuity at the time of admission, and more comorbidities. In addition, as a result of the intervention, the intervention group was more likely to receive NRT or bupropion in hospital and a Fax‐to‐Quit referral to the NYS Smokers' Quitline. In the intervention group, most patients received 1 visit from the SCS, only 2% received 2 visits. Family members were included in smoking cessation counseling for 58% of the intervention group.
| Intervention (n = 275) | Comparison (n = 335) (P value) | |
|---|---|---|
| ||
| Sex (% male) | 51 | 51 |
| Mean age (years) | 51.4 | 48.5 (0.03) |
| Ethnicity (% white) | 98 | 97 |
| Marital status (% married) | 48 | 47 |
| Elective admission (vs. acute) (%) | 25 | 31 |
| Inpatient (vs. outpatient observation or outpatient surgical admission) (%) | 86 | 68 (0.00) |
| LOS (days) | 3.80 | 2.68 (0.00) |
| Elixhauser comorbidity index (mean) | 1.66 | 1.17 (0.00) |
| Used NRT in hospital (%) | 37 | 19 (0.00) |
| Used bupropion in hospital (%) | 4 | 1 (0.00) |
| Referred to NYS Smokers' Quitline using Fax‐to‐Quit | 10 | 0 (0.00) |
| Mean cigarettes per day* | 17.7 (n = 213) | 15.9 (n = 192) |
As shown in Table 2, there was a significant amount of diagnostic heterogeneity in the discharge diagnoses codes of patients included in the study. However, there were significantly more patients in the intervention group with a first discharge diagnosis of cardiovascular disease (25%) compared to the comparison group (12%; P = 0.00).
| Intervention (n = 274)* (%) | Comparison (n = 333)* (%) | |
|---|---|---|
| ||
| Cardiovascular | 25 | 12, P = 0.00 |
| Pulmonary | 16 | 7 |
| Orthopedics | 12 | 12 |
| Injury | 10 | 15 |
| GI | 8 | 16 |
| Cancer | 6 | 8 |
| GU | 3 | 6 |
| Endocrine | 3 | 3 |
| Other | 17 | 21 |
Readmission outcomes based on EMR and administrative data were available for 607 inpatients with signed HIPAA releases. The readmission rate was higher for the intervention (41%) than the comparison group (20%). Despite a higher readmission rate, the crude mortality within the 6 months posthospital discharge was lower for the intervention group, ie, 0.02 (6/276), than the comparison group, which had a crude mortality of 0.04 (16/384) during this period.
A multivariate survival model, controlling for age, sex, Elixhauser comorbidity index, LOS, and cardiovascular diagnosis, showed a significantly reduced mortality in the intervention group (hazard ratio [HR] = 0.37; P = 0.04). Although cardiac status (P = 0.09) and LOS (P = 0.15) were not significant in this model, they were retained because both of these variables showed significantly higher levels (along with the Elixhauser Index) at baseline in the intervention group, implying that the intervention group was sicker than the comparison group. The Elixhauser comorbidity index (HR = 1.42; P < 0.00) and age (HR = 1.07; P < 0.00) were the only other significant predictors of mortality in this model.
Among those responding to the interview at 6 months post‐hospital discharge (n = 326), there were no significant differences between the study groups with regard to age first started smoking, gender, educational level, employment status, ethnicity, or physical health status (data not shown). Table 3 summarizes the outcomes by the intervention and comparison groups at 6 months post‐hospital discharge. The point prevalence for abstinence was 27% in the intervention group compared to 19% in the comparison group (P = 0.09). Using the intent to treat analysis, the point prevalence for abstinence was 16% in the intervention group compared to 9.8% in the comparison group (P = 0.02). Self‐reported quit status was 63% in the intervention group vs. 48% in the comparison group (P = 0.00). Using the intent to treat analysis, quit status was 44% in the intervention group vs. 30% in the comparison group (P = 0.00). Exclusion of the 52 patients without signed HIPAA releases (Figure 1) did not significantly alter these outcomes.
| Intervention (n = 161) | Comparison (n = 165) (P value) | |
|---|---|---|
| ||
| Self‐reported now smoking not at all (%) | 16 | 10 (0.02) |
| Self‐reported quit within 6 months (%) | 63 | 48 (0.00 |
| Tried to quit (%) | 68 | 62 |
| Used NRT post‐D/C (%) | 26 | 17 (0.04) |
| Used other intervention (%) | 21 | 14 |
| Heard of the NYS Smokers' Quitline (%) | 92 | 90 |
| Aware that NYS Quitline offers NRT (%) | 73 | 49 (0.00) |
| Received free NRT from NYS Smokers' Quitline (%) | 9 | 6 |
| Used NYS Smokers' Quitline (%) | 15 | 9 |
| Called by the NYS Smokers' Quitline (%) | 11 | 5 |
| Self‐rated health status as fair or poor (%) | 48 | 36 |
| Another smoker living at home (%) | 54 | 48 |
| Mean hours spent in same room where someone else was smoking (n) | 20 | 21 (0.04) |
| Households in which smoking is not allowed in the home (%) | 40 | 33 |
| Patients Still Smoking at the 6‐Month Interview | Intervention (n = 118) | Comparison (n = 134) |
| Mean cigarettes currently smoked (n) | 10.5 | 12.7 |
| Mean quit attempts post‐D/C (n) | 3.2 | 3.5 |
| Mean reduction in smoking* (cigarettes/day) | 5.83 | 4.09 |
Patients who received the inpatient smoking cessation counseling were more likely to be called by or use the NYS Smokers' Quitline; however, these differences were not statistically significant. There was no difference between the study groups in awareness of the Quitline but the intervention group was more aware that free NRT was offered by the NYS Quitline. In terms of quit methods used during the 6‐month period (Table 4), NRT or bupropion use was higher in the intervention group. There were no other significant differences between the study groups, except for the use of acupuncture.
| Intervention (n = 91) | Comparison (n = 93) (P value) | |
|---|---|---|
| ||
| Got help from friends or family (%) | 58 | 50 |
| Used any medication to quit (%) | 44 | 28 (0.02) |
| Used nicotine patch (%) | 43 | 25 (0.00) |
| Used bupropion (%) | 10 | 1 (0.00) |
| Used varenicline (%) | 32 | 25 |
| Cut back (%) | 43 | 46 |
| Quit with a friend (%) | 20 | 13 |
| Switched to lights (%) | 18 | 13 |
| Used print material (%) | 14 | 16 |
| Got help from the NYS Smokers' Quitline (%) | 11 | 9 |
| Called by the NYS Smokers' Quitline (%) | 11 | 5 (0.09) |
| Counseling (%) | 9 | 3 |
| Acupuncture (%) | 5 | 0 (0.02) |
| Switched to chew (%) | 2 | 4 |
| Attended classes (%) | 3 | 1 |
| Used NYS Smokers' Quitline website (%) | 2 | 4 |
Multivariate analysis predicting quit status at 6 months post‐hospital discharge included covariates controlling for age, sex, LOS, study group, and comorbidity. This analysis showed that patients with a cardiovascular discharge diagnosis were more likely to quit than patients who had other discharge diagnoses (odds ratio [OR], 3.02; 95% confidence interval [CI], 1.65.7; P = 0.00). Another statistically significant covariate in this model included sex (men were more likely to quit: OR, 0.61; 95% CI, 0.390.97; P = 0.04). Participating in the inpatient intervention group was marginally significant when controlling for these other variables (OR, 1.54; 95% CI, 0.982.45; P = 0.06). Hospital LOS, age, receipt of NRT in hospital, and the Elixhauser comorbidity index were not predictive of quit status at 6 months.
Discussion
This study demonstrates how effective an inpatient smoking cessation program can be for increasing the success of quitting smoking after hospital discharge. At 6 months posthospital discharge, the intervention group had significantly higher intent to treat outcomes for point prevalence abstinence and quit status as well as lower crude and adjusted mortality than the comparison group.
Although at baseline the intervention group was older, had a longer LOS, more cardiovascular diagnoses, and higher comorbidity index, crude post‐hospital discharge mortality was significantly less in the intervention group (0.02) than in the comparison group (0.04). This finding is more significant in light of the higher comorbidity and acuity of the intervention group at baseline. Our multivariate survival model that controlled for these imbalances at baseline demonstrated that the intervention group had significantly less mortality than the comparison group (HR = 0.37; P = 0.04). Reduction in mortality, as soon as 30 days after inpatient smoking cessation counseling, has been demonstrated postmyocardial infarction.17, 18 Intensive smoking cessation quit services were also linked with lower all cause mortality among cardiovascular disease patients 2 years posthospitalization.19 Our study, despite its relatively small sample size, demonstrates that the intervention retains its impact on mortality in real‐world settings.
Following participation in an inpatient smoking cessation program, self‐reported quit status at 6 months post‐hospital discharge in the intervention group was significantly higher in the intervention group (63%) than the comparison group (48%; P = 0.00). Using the intent to treat method, the differences between the study groups was still significant (44% in the intervention group, 30% in the comparison group; P = 0.00). Given the limitations of self‐report and responder bias, the actual outcomes fall somewhere between these 2 estimates. In an effectiveness study of inpatient smoking cessation involving 6 hospitals in California, self‐reported quit rates of 26% at 6 months were reported; however, different methods were used so the results are not strictly comparable.20
Our multivariate analysis suggests that patients with cardiovascular discharge diagnosis were more likely to quit than patients who had other discharge diagnoses (OR, 3.02; 95% CI, 1.65.7; P = 0.0007). This study extends findings of other studies that show that the success of smoking cessation may vary by diagnosis, particularly for smokers admitted for cardiovascular disease.21, 22 Other studies have shown that smoking cessation rates among patients post‐myocardial infarction were higher in admitting facilities that had hospital‐based smoking cessation programs and for those patients referred to cardiac rehabilitation.23 Thus, the availability of a hospital‐based smoking cessation program may be considered a structural measure of health care quality as suggested by Dawood et al.23
The use of NRT greatly increased in our hospital, coincident with the start of the inpatient cessation program7 and, in this study, NRT use appears to continue after hospital discharge. Some studies show an additive effect of NRT combined with cessation counseling.24, 25 Although a Cochrane review did not find a statistically significant difference, there was a trend toward higher quit rates with the addition of NRT.21, 22
Because hospitalized smokers may be more motivated to stop smoking, the updated 2008 DHHS clinical practice guidelines for Treating Tobacco Use and Dependence now recommend that all inpatients who currently smoke be given medications, advised, counseled, and receive follow‐up after discharge.26 Although our inpatient cessation program was started before these clinical practice guidelines were updated, we have had the opportunity to evaluate the recommended practice of inpatient tobacco cessation counseling. Compared to effects shown in efficacy studies, clinical interventions often lose effect size in daily practice and real‐world settings.27, 28 It is reassuring that, in this effectiveness study, the impact of this intervention is still demonstrable.
Provision of inpatient smoking cessation has been shown to be an effective smoking cessation intervention if combined with outpatient follow‐up.29 Reviews by Rigotti et al.21, 22 recommend that inpatient high‐intensity behavioral interventions should be followed by at least 1 month of supportive contact after discharge to promote smoking cessation among hospitalized patients. In our study, specific cessation‐related outpatient follow‐up was not provided by our program. Although letters were sent to primary care providers describing the cessation service provided during the inpatient stay, our study could not ascertain what specific cessation service was offered by either primary‐care or specialty‐care providers during posthospitalization follow‐up visits. An efficient alternative to outpatient visits may be follow‐up delivered via a quitline. Follow‐up in our study included referrals to the NYS Smokers' Quitline; however, only about 10% of inpatient reported using this service. While feasible, the effectiveness of quitline follow‐up is as yet unknown.30
Limitations
This study targets a later phase in research progression from hypothesis development, pilot studies, efficacy (empirically supported) trials, effectiveness trials (real‐world settings), and dissemination studies.31 Because this study addresses the effectiveness rather than the efficacy of inpatient smoking cessation counseling, the use of a quasiexperimental rather than randomized controlled clinical trial design led to measured differences in the study groups at baseline. An important imbalance arose in the intervention group that had twice the percentage of patients with cardiovascular‐related discharge diagnoses as the comparison group. While we were able to adjust for these differences in our analysis, there may be unmeasured differences due to the fact that the inpatients were not randomized to the study groups.
The outcomes of this study cannot be attributed to any one component of the intervention (eg, NRT) vs. the combined effect of the inpatient smoking cessation program. The program components were implemented simultaneously in order to maximize synergistic effects; therefore, the effects of program components are difficult to disaggregate.
The results are limited by the validity of self‐report of smoking status. It is well known that research studies which validate smoking status biochemically have lower efficacy (OR, 1.44; 95% CI, 0.992.11) than those that do not validate smoking status (OR, 1.92; 95% CI, 1.262.93).32 Although it was impractical in this effectiveness study to biochemically validate smoking status 6 months posthospital discharge, we have documented a significant difference between the study groups that confirms the direction of the effect, if not the effect size.
Self‐reports tend to underestimate smoking status in population studies33; however, the discrepancy between self‐reported smoking and biochemical measurements among clinical trial participants is small.34 However, a small but significant bias toward a socially desirable response in intervention groups compared to control groups of 3% with carbon monoxide and 5% for cotinine has been documented.35 If social desirability bias is operative in this study, and if we apply the above correction factor of 4% to correct this classification error, then the difference between the intervention and comparison group would be 10 percentage points (40% in the intervention group vs. 30% in the comparison group using the intention to treat estimates). That difference is still clinically relevant.
The observed difference between the intervention and comparison group is underestimated because the comparison group was exposed to smoking cessation as well both at the time of admission and following discharge (Table 4). The comparison group in this study could thus be viewed as a usual care group rather than a control group. That exposure does cloud the measurement of quit rates as the comparison group is contaminated to some degree by exposure to various cessation methods. The impact of this exposure is to reduce the effect size observed in this study or underestimate the effect of the inpatient smoking cessation counseling because the comparison group was exposed other cessation methods, although to a lesser extent.
The social desirability bias inherent in self‐reported smoking status may increase the effect size while the use of comparison group that received usual care may decrease the effect size. Because neither of these biases could be measured in this study, it is impossible to say whether they negated each other.
As with any administrative database, use of EMR as a data source in this study led to missing data that precluded use of certain variables in the analysis. In addition, lack of written and signed HIPAA releases also precluded inclusion of several inpatients, mostly in the comparison group, in the analytic database. However, it is reassuring that the results of the 6‐month survey did not differ significantly when these individuals were included or excluded from a separate analysis of the survey data. Last, our study population is almost 100% Caucasian thus limiting how generalizable the results are to more heterogenous patient populations.
Conclusions
This quasiexperimental effectiveness study showed that inpatient smoking cessation intervention improved smoking cessation outcomes, use of NRT, and was associated with a decreased mortality 6 months post‐hospital discharge. The effectiveness of this inpatient intervention is maintained in real world settings but may be improved with posthospital discharge follow‐up.
Acknowledgements
The authors are grateful to the many Mary Imogene Bassett Hospital staff in administration, nursing, inpatient pharmacy, medical education, patient care service, and respiratory care who provided data needed to evaluate this program. They also acknowledge the NYS Smokers' Quitline website for data provided about monthly Fax‐to‐Quit program referrals from our county.
- , , , , , .“Implementing smoking bans in American hospitals: results of a national survey.Tob Control.1998;7(1):47–55.
- The Smoking Cessation Clinical Practice Guideline Panel and Staff: the Agency for Health Care Policy and Research Smoking Cessation Clinical Practice Guideline.JAMA.1996;275(16):1270–1280.
- , , , et al.ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures). Endorsed by the Heart Failure Society of America.J Am Coll Cardiol.2005;46(6):1144–1178.
- , , , et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction).Circulation.2004;110(9):e82–e292.
- , , , et al.ACC/AHA clinical performance measures for adults with ST‐elevation and non‐ST elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures on ST‐Elevation and Non‐ST‐Elevation Myocardial Infarction).J Am Coll Cardiol.2006;47(1):236–265.
- Treating Tobacco Use and Dependence‐Clinicians Packet. A How‐To Guide For Implementing the Public Health Service Clinical Practice Guideline, March2003. Rockville, MD: U.S. Public Health Service, Agency for Healthcare Research and Quality. Available at: http://www.ahrq.gov/clinic/tobacco. Accessed November 2009.
- , , , .Implementing a smoke‐free medical campus: impact on inpatient and employee outcomes.J Hosp Med.2010;5(1):51–54.
- , , , et al.The future of behavior change research: what is needed to improve translation of research into health promotion practice?Ann Behav Med.2004;27:3–12.
- The New York State Smokers' Quitline. Available at: http://www.nysmokefree.com. Accessed November2009.
- Tobacco Cessation Continuing Education for Healthcare Professionals and Counselors. Available at: http://www.tobaccocme.com. Accessed November2009.
- Seton Health Cessation Center. The Butt Stops Here. Relaxation Exercises for Smoking Cessation. 2001. The Butt Stops Here Program. Available at: http://www.setonhealth.org. Accessed November2009.
- U.S. Department of Health and Human Services.Treating Tobacco Use and Dependence. Clinical Practice Guideline.Rockville, MD:Public Health Service;2000.
- , , , , .A randomized controlled trial of smoking cessation counseling after myocardial infarction.Prev Med.2000;30(4):261–268.
- , , , .Comorbidity measures for use with administrative data.Med Care.1998:36(1):8–27.
- Tobacco Use Supplement to the Current Population Survey (TUS‐CPS). Available at: http://riskfactor.cancer.gov/studies/tus‐cps/info.html. Accessed November 2009.
- , , .Comparison of National Death Index and world wide web death searches.Am J of Epidemiol.2000;152(2):107–111.
- , , , et al.Post‐myocardial infarction smoking cessation counseling: associations with immediate and late mortality in older Medicare patients.Am J Med.2005;118(3):269–275.
- , , .Inpatient smoking‐cessation counseling and all‐cause mortality in patients with acute myocardial infarction.Am Heart J.2007;154(2):213–220.
- , , , et al.Intensive smoking cessation intervention reduces mortality in high‐risk smokers with cardiovascular disease.Chest.2007;131:446–452.
- , , , , .Dissemination of an effective inpatient tobacco use cessation program.Nicotine Tob Res.2005;7(1):129–137.
- , , .Interventions for smoking cessation in hospitalized patients.Cochrane Database Syst Rev.2007;3:CD001837.
- , , .Smoking cessation interventions for hospitalized smokers.Arch Intern Med.2008;168(18):1950–1960.
- , , , et al.Predictors of smoking cessation after a myocardial infarction.Arch Int Med.2008;168(18):1961–1967.
- , , , .The effectiveness of smoking cessation interventions prior to surgery: a systematic review.Nicotine Tob Res.2008;10(3):407–412.
- , , , et al.Clinical trial comparing nicotine replacement therapy (NRT) plus brief counseling, brief counseling alone, and minimal intervention on smoking cessation in hospital inpatients.Thorax.2003;58:484–488.
- Department of Health and Human Services (DHHS). Treating Tobacco Use and Dependence: 2008 Update. Chapter 7. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat2.section.28504. Accessed November2009.
- , .Implementation of evidence‐based tobacco use cessation guidelines in managed care organizations.Ann Behav Med.2004;27(1):13–21.
- .Decreasing effect sizes for effectiveness studies—implications for the transport of evidence‐based treatments: comment on Curtis, Ronan, and Borduin (2004).J Fam Psychol.2004;18(3):420–423.
- , , , , , .Efficacy of a smoking cessation program for hospital patients.Arch Intern Med.1997;157(22):2653–2660.
- , , , et al.Feasibility, acceptability, and cost of referring surgical patients for postdischarge cessation support from a quitline.Nicotine Tob Res.2008;10(6):1105–1108.
- .Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programs.Prev Med.1986;15:451–474.
- , , .Psychosocial interventions for smoking cessation in patients with coronary heart disease.Cochrane Database Syst Rev.2008;23(1):CD006886.
- , , , , .The accuracy of self‐reported smoking: a systematic review of the relationship between self‐reported and cotinine‐assessed smoking status.Nicotine Tob Res2009;11(1):12–24.
- , , , , .Relations of cotinine and carbon monoxide to self‐reported smoking in a cohort of smokers and ex‐smokers followed over 5 years.Nicotine Tob Res.2002;4(3):287–294.
- , , , .Error in smoking measures: effects of intervention on relations of cotinine and carbon monoxide to self‐reported smoking. The Lung Health Study Research Group.Am J Public Health.1993;83(9):1251–1257.
In 1992, the Joint Commission on Accreditation of Healthcare Organizations (Joint Commission) introduced standards to make hospital buildings smoke‐free, resulting in the nation's first industry‐wide ban on smoking in the workplace. This hospital smoking ban has led to increased smoking cessation among employees.1 Since 2003, core measures from the Joint Commission and quality indicators from the Centers for Medicare and Medicaid Services have included inpatient smoking cessation counseling for acute myocardial infarction, pneumonia, and heart failure, as national guidelines strongly recommend smoking cessation counseling for patients with these diseases who smoke.25
The Department of Health and Human Services (DHHS) 2008 update on Clinical Practice Guidelines for Treating Tobacco Use and Dependence6 recommends that clinicians use hospitalization as an opportunity to promote smoking cessation and to prescribe medications to alleviate withdrawal symptoms. Hospitalization is an opportune time for smoking cessation because patients are restricted to a smoke‐free environment in the hospital and, increasingly, on hospital campuses.6 The illness leading to hospitalization may be attributable, at least in part, to tobacco use, thereby increasing the patient's receptivity to cessation counseling. Last, medications used in‐hospital to treat nicotine withdrawal symptoms may lead to continued or future use of these medications that, in turn, may ultimately lead to a successful quit attempt.
We report on the outcomes of our hospital's attempt to do this in the context of implementation of a smoke‐free medical campus.7 This study was designed to measure whether an inpatient smoking cessation intervention increases the likelihood of smoking cessation 6 months post‐hospital discharge. Because effectiveness studies are the next step to improving translation of research into health promotion practice,8 we set out to measure what the impact of this intervention would be in routine clinical practice as opposed to a carefully structured efficacy trial.
Methods
Intervention
The Smoking Cessation service for inpatients began on April 3, 2006. Upon admission, all patients were screened regarding their current smoking status. The nurse asked the patient if s/he currently smoked and then entered the responses into the hospital electronic medical record (EMR). A current smoker was defined as smoking every day or some days within the past 30 days. A roster of newly admitted current smokers was electronically transmitted to the Respiratory Care office daily. Only current smokers received counseling. The Smoking Cessation Specialist (SCS) subsequently saw inpatients within a 24‐hour time frame of admission, except for weekends and holidays. Each patient received 1 to 2 intensive follow‐up counseling sessions during hospitalization. An average of 10 patients per day were seen.
The goal of the inpatient smoking cessation service was to counsel patients on the health effects of smoking, address nicotine withdrawal symptoms, explain the different pharmacotherapies available, advise on how to quit, give self‐help materials, counsel family members, and refer to the New York State (NYS) Smokers' Fax‐to‐Quit program.9 Following the consult, the SCS documented the encounter in the patient's chart, including recommendations for nicotine replacement therapy (NRT) or bupropion (varenicline was not addressed as it was not on the formulary). The chart documentation informed the physician and nursing staff of the intervention and included the date/time, stage of change, and support action taken.
The SCS was part‐time, had nursing training, smoking cessation training,10 and was also trained by the Seton Health Cessation Center in the Butt Stops Here Program.11 She also implemented a performance improvement plan to increase the provision of smoking cessation counseling, increase NRT or bupropion prescriptions to smokers admitted to the hospital, and increase referrals to the NYS telephone quitline through the Fax‐to‐Quit program for outpatient resources and help following hospital discharge. The Fax‐to‐Quit program allows health care providers to refer patients to the NYS quitline via fax, with the patient's signature (patient permission) on the fax to quit form. After hospital discharge, the quitline then contacts the patient at a time that the patient requested.
The SCS visited patients with all admitting diagnoses on the medical, surgical, and special care units who were current smokers. Inpatients admitted to psychiatry, obstetrics, and the intensive care unit (ICU) were not seen by the SCS, except for ICU patients referred by a physician. Inpatients who had short stays or who were admitted and discharged in 1 day or during the weekend were not seen.
The intervention included either a brief 3‐minute to 5‐minute intervention or a more intensive intervention, that required 10 to 20 minutes (18 minutes average). The length of the intervention was determined by how receptive the patient was to the intervention. All interventions began with patient identification, an introduction to the SCS, and an explanation of the purpose of the visit. The SCS then inquired about the patient's comfort level vis‐a‐vis nicotine withdrawal and if s/he was receiving any NRT while in the hospital (NRT on the inpatient formulary included the nicotine patch or gum). If the patient was receptive to counseling, the SCS then began to work through the 5 A's, as described in the 2000 DHHS Clinical Practice Guidelines.12 The 2000 DHHS Clinical Practice Guidelines were used because the 2008 update had not been released at the time this study was initiated in 2006. These include: asking about smoking status, advising on how to quit, assessing readiness to quit, and assisting in arranging treatment options that include pharmacotherapy, counseling, as well as referral to the NYS Smokers' Quitline. A workbook was provided to reinforce counseling but was not necessarily used during counseling session. A compact disc (CD) with relaxation exercises5 was provided to those inpatients who were interested in stress reduction. If family members were present, and were also smokers, they were included in the counseling session, if willing. Each patient was offered a referral via the Fax‐to‐Quit program to continue treatment on an outpatient basis.
If the patient was not motivated to quit or declined the consult, the visits were short and focused on the patient's experience with nicotine withdrawal. These patients were also given self‐help materials and, if possible, the relevance of and roadblocks to quitting were reviewed. Patients were prompted to think about why quitting was relevant and often the reason for hospitalization was used to motivate the quitting process.
Upon hospital discharge, the patient's primary care provider was notified of the cessation intervention by a letter from the SCS. The letter described the intervention and stated whether or not the patient agreed to be referred to the Fax‐to‐Quit Program.
Study Participants
Patients were recruited from July 1, 2006 (after the smoking ban went into effect) through June 1, 2008. Inpatients who currently smoked were informed of this study and were asked to sign informed consent to participate after they were seen by the SCS. Current smokers of all admitting diagnoses were recruited into the study. Patients provided informed consent for a telephone interview 6 months posthospital discharge. A written Health Insurance Portability and Accountability Act (HIPAA) release was obtained to allow access to an individual's specific EMR.
A comparison group of inpatients who were also current smokers, but who did not receive the intervention were also contacted six months after hospitalization. Reasons for not receiving the intervention included the fact the SCS was part‐time and also took a leave of absence during the study and therefore could not see all inpatients who currently smoked. Other reasons for not receiving the intervention include too short a stay for the SCS to see the patient or the patient was out of the room for tests or procedures when the SCS was available. These patients provided informed consent to be interviewed 6 months after hospital discharge and HIPAA consent for access to their medical record. Not all inpatients in the comparison group provided written HIPAA release for use of their medical record; therefore, these patients were excluded because their baseline demographic and diagnostic data were missing.
Sample‐size considerations were driven around having adequate numbers of subjects to measure the prevalence of smoking cessation at 6 months post‐hospital discharge with an acceptable degree of precision. Prevalence estimates from previous studies for 6‐month cessation typically range from 20% to 30%, with cessation rates as high as 67% (this estimate applies to postmyocardial infarction patients.)13 For conservative estimation, we used 50% as the 6‐month prevalence of cessation in the current study, which placed binomial variance at its theoretical maximum. In this case, a sample of 300 subjects provides a margin of error of 0.058 for a 95% confidence interval around this point estimate.
Data Sources
The hospital EMR database was used to monitor several components of the program: nursing screening, smoking cessation counseling, and pharmacy dispensing of NRT and bupropion. The screening data were also used to monitor the proportion of current smokers admitted during the study period. Elements of the EMR were used to define the following covariates: patient age, gender, ethnicity, and the primary discharge diagnosis (via International Statistical Classification of Diseases and Related Health Problems, 9th edition [ICD9] codes) and readmission during the six month follow‐up period. Mean length of stay (LOS) was computed. The Elixhauser Comorbidity Index that utilizes ICD9 codes was used for comorbidity risk adjustment.14
Study participants were contacted by phone 6 months posthospital discharge. Data collection began July 1, 2006 and was completed January 1, 2009. The interview focused on self‐reported point prevalence of smoking and 6‐month quit status. The point prevalence for self‐reported abstinence was derived from the question Do you now smoke cigarettes every day, some days, or not at all?15 Self‐reported quit status was derived from question Have you quit smoking since you were discharged from the hospital? In addition, respondents were queried about their number of years smoked, post‐hospital discharge number of quit attempts, and cessation efforts (NRT, self‐help groups, quitline use, etc.). Last, they were surveyed about barriers to cessation (exposure to secondhand smoke, rules about smoking in the home or car), educational level, employment, and health ratings.
To determine the status of those lost to follow‐up, administrative and EMR databases for appointments and follow‐up visits were accessed to determine if the patient was alive during the 6 months between discharge and the follow‐up call. To confirm mortality, we searched the Internet, Ancestry.com, and/or local newspaper obituaries for dates of death for all patients to validate that they had not died during the 6‐month follow‐up. World wide web searches can identify 97% of deaths listed in the Centers for Disease Control and Prevention (CDC)/National Death Index, which is considered the gold standard in epidemiologic studies.16
Analysis
Univariate analysis of all covariates was completed to examine the normal distribution curves for these variables. Bivariate correlation analysis of all the independent variables by study group was performed to assess comparability of the study groups at baseline. The self‐reported cessation outcomes were calculated by dividing the number of patients who said they were not using tobacco or had quit, at 6 months posthospital discharge by the number of individuals in the study group at baseline minus those who had died. Both the intent to treat method, which assumes patients lost to follow‐up were still smoking, and the responder method, which does not include nonresponders in the analysis, were used to adjust the denominators for these outcomes.
Multivariate regression analysis was then used to model receipt of the intervention as predictor of self‐reported quit status adjusted for significant covariates. Statistical significance was defined by a P value of less than 0.05.
Survival analysis was employed to model differences in mortality between the study groups, controlling for any baseline imbalances (eg, comorbidity). Because baseline data were used in this model, the model includes only patients with signed a HIPAA release.
Internal review boards of our hospital and the NYS Department of Health reviewed and approved this study.
Results
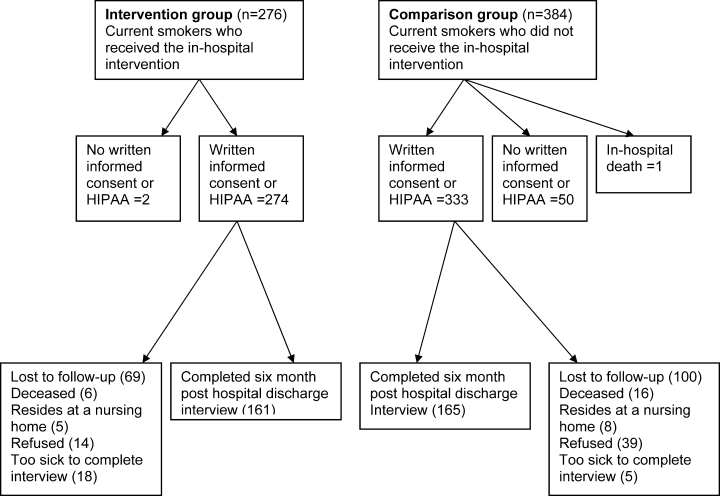
From January 1, 2007 to May 30, 2008, 660 inpatients who were current smokers were recruited into the study. Figure 1 summarizes patient flow through the study and explains the final sample size of 607. Exclusions include 52 inpatients from the study who completed the 6‐month interview but who did not return a written HIPAA release. Without a HIPAA release to access the EMR, baseline comparison of the study groups and adjustment for comorbidity could not be completed for these patients. At 6 months posthospital discharge, 53 subjects refused the interview when contacted by telephone.

As might be expected in a quasiexperimental design, the study groups were not equivalent at baseline (Table 1). The intervention and comparison groups differed with regard to age, length of stay (LOS), proportion of acute admissions, and the Elixhauser comorbidity index. These differences suggest that the intervention group was older, had a longer LOS, higher acuity at the time of admission, and more comorbidities. In addition, as a result of the intervention, the intervention group was more likely to receive NRT or bupropion in hospital and a Fax‐to‐Quit referral to the NYS Smokers' Quitline. In the intervention group, most patients received 1 visit from the SCS, only 2% received 2 visits. Family members were included in smoking cessation counseling for 58% of the intervention group.
| Intervention (n = 275) | Comparison (n = 335) (P value) | |
|---|---|---|
| ||
| Sex (% male) | 51 | 51 |
| Mean age (years) | 51.4 | 48.5 (0.03) |
| Ethnicity (% white) | 98 | 97 |
| Marital status (% married) | 48 | 47 |
| Elective admission (vs. acute) (%) | 25 | 31 |
| Inpatient (vs. outpatient observation or outpatient surgical admission) (%) | 86 | 68 (0.00) |
| LOS (days) | 3.80 | 2.68 (0.00) |
| Elixhauser comorbidity index (mean) | 1.66 | 1.17 (0.00) |
| Used NRT in hospital (%) | 37 | 19 (0.00) |
| Used bupropion in hospital (%) | 4 | 1 (0.00) |
| Referred to NYS Smokers' Quitline using Fax‐to‐Quit | 10 | 0 (0.00) |
| Mean cigarettes per day* | 17.7 (n = 213) | 15.9 (n = 192) |
As shown in Table 2, there was a significant amount of diagnostic heterogeneity in the discharge diagnoses codes of patients included in the study. However, there were significantly more patients in the intervention group with a first discharge diagnosis of cardiovascular disease (25%) compared to the comparison group (12%; P = 0.00).
| Intervention (n = 274)* (%) | Comparison (n = 333)* (%) | |
|---|---|---|
| ||
| Cardiovascular | 25 | 12, P = 0.00 |
| Pulmonary | 16 | 7 |
| Orthopedics | 12 | 12 |
| Injury | 10 | 15 |
| GI | 8 | 16 |
| Cancer | 6 | 8 |
| GU | 3 | 6 |
| Endocrine | 3 | 3 |
| Other | 17 | 21 |
Readmission outcomes based on EMR and administrative data were available for 607 inpatients with signed HIPAA releases. The readmission rate was higher for the intervention (41%) than the comparison group (20%). Despite a higher readmission rate, the crude mortality within the 6 months posthospital discharge was lower for the intervention group, ie, 0.02 (6/276), than the comparison group, which had a crude mortality of 0.04 (16/384) during this period.
A multivariate survival model, controlling for age, sex, Elixhauser comorbidity index, LOS, and cardiovascular diagnosis, showed a significantly reduced mortality in the intervention group (hazard ratio [HR] = 0.37; P = 0.04). Although cardiac status (P = 0.09) and LOS (P = 0.15) were not significant in this model, they were retained because both of these variables showed significantly higher levels (along with the Elixhauser Index) at baseline in the intervention group, implying that the intervention group was sicker than the comparison group. The Elixhauser comorbidity index (HR = 1.42; P < 0.00) and age (HR = 1.07; P < 0.00) were the only other significant predictors of mortality in this model.
Among those responding to the interview at 6 months post‐hospital discharge (n = 326), there were no significant differences between the study groups with regard to age first started smoking, gender, educational level, employment status, ethnicity, or physical health status (data not shown). Table 3 summarizes the outcomes by the intervention and comparison groups at 6 months post‐hospital discharge. The point prevalence for abstinence was 27% in the intervention group compared to 19% in the comparison group (P = 0.09). Using the intent to treat analysis, the point prevalence for abstinence was 16% in the intervention group compared to 9.8% in the comparison group (P = 0.02). Self‐reported quit status was 63% in the intervention group vs. 48% in the comparison group (P = 0.00). Using the intent to treat analysis, quit status was 44% in the intervention group vs. 30% in the comparison group (P = 0.00). Exclusion of the 52 patients without signed HIPAA releases (Figure 1) did not significantly alter these outcomes.
| Intervention (n = 161) | Comparison (n = 165) (P value) | |
|---|---|---|
| ||
| Self‐reported now smoking not at all (%) | 16 | 10 (0.02) |
| Self‐reported quit within 6 months (%) | 63 | 48 (0.00 |
| Tried to quit (%) | 68 | 62 |
| Used NRT post‐D/C (%) | 26 | 17 (0.04) |
| Used other intervention (%) | 21 | 14 |
| Heard of the NYS Smokers' Quitline (%) | 92 | 90 |
| Aware that NYS Quitline offers NRT (%) | 73 | 49 (0.00) |
| Received free NRT from NYS Smokers' Quitline (%) | 9 | 6 |
| Used NYS Smokers' Quitline (%) | 15 | 9 |
| Called by the NYS Smokers' Quitline (%) | 11 | 5 |
| Self‐rated health status as fair or poor (%) | 48 | 36 |
| Another smoker living at home (%) | 54 | 48 |
| Mean hours spent in same room where someone else was smoking (n) | 20 | 21 (0.04) |
| Households in which smoking is not allowed in the home (%) | 40 | 33 |
| Patients Still Smoking at the 6‐Month Interview | Intervention (n = 118) | Comparison (n = 134) |
| Mean cigarettes currently smoked (n) | 10.5 | 12.7 |
| Mean quit attempts post‐D/C (n) | 3.2 | 3.5 |
| Mean reduction in smoking* (cigarettes/day) | 5.83 | 4.09 |
Patients who received the inpatient smoking cessation counseling were more likely to be called by or use the NYS Smokers' Quitline; however, these differences were not statistically significant. There was no difference between the study groups in awareness of the Quitline but the intervention group was more aware that free NRT was offered by the NYS Quitline. In terms of quit methods used during the 6‐month period (Table 4), NRT or bupropion use was higher in the intervention group. There were no other significant differences between the study groups, except for the use of acupuncture.
| Intervention (n = 91) | Comparison (n = 93) (P value) | |
|---|---|---|
| ||
| Got help from friends or family (%) | 58 | 50 |
| Used any medication to quit (%) | 44 | 28 (0.02) |
| Used nicotine patch (%) | 43 | 25 (0.00) |
| Used bupropion (%) | 10 | 1 (0.00) |
| Used varenicline (%) | 32 | 25 |
| Cut back (%) | 43 | 46 |
| Quit with a friend (%) | 20 | 13 |
| Switched to lights (%) | 18 | 13 |
| Used print material (%) | 14 | 16 |
| Got help from the NYS Smokers' Quitline (%) | 11 | 9 |
| Called by the NYS Smokers' Quitline (%) | 11 | 5 (0.09) |
| Counseling (%) | 9 | 3 |
| Acupuncture (%) | 5 | 0 (0.02) |
| Switched to chew (%) | 2 | 4 |
| Attended classes (%) | 3 | 1 |
| Used NYS Smokers' Quitline website (%) | 2 | 4 |
Multivariate analysis predicting quit status at 6 months post‐hospital discharge included covariates controlling for age, sex, LOS, study group, and comorbidity. This analysis showed that patients with a cardiovascular discharge diagnosis were more likely to quit than patients who had other discharge diagnoses (odds ratio [OR], 3.02; 95% confidence interval [CI], 1.65.7; P = 0.00). Another statistically significant covariate in this model included sex (men were more likely to quit: OR, 0.61; 95% CI, 0.390.97; P = 0.04). Participating in the inpatient intervention group was marginally significant when controlling for these other variables (OR, 1.54; 95% CI, 0.982.45; P = 0.06). Hospital LOS, age, receipt of NRT in hospital, and the Elixhauser comorbidity index were not predictive of quit status at 6 months.
Discussion
This study demonstrates how effective an inpatient smoking cessation program can be for increasing the success of quitting smoking after hospital discharge. At 6 months posthospital discharge, the intervention group had significantly higher intent to treat outcomes for point prevalence abstinence and quit status as well as lower crude and adjusted mortality than the comparison group.
Although at baseline the intervention group was older, had a longer LOS, more cardiovascular diagnoses, and higher comorbidity index, crude post‐hospital discharge mortality was significantly less in the intervention group (0.02) than in the comparison group (0.04). This finding is more significant in light of the higher comorbidity and acuity of the intervention group at baseline. Our multivariate survival model that controlled for these imbalances at baseline demonstrated that the intervention group had significantly less mortality than the comparison group (HR = 0.37; P = 0.04). Reduction in mortality, as soon as 30 days after inpatient smoking cessation counseling, has been demonstrated postmyocardial infarction.17, 18 Intensive smoking cessation quit services were also linked with lower all cause mortality among cardiovascular disease patients 2 years posthospitalization.19 Our study, despite its relatively small sample size, demonstrates that the intervention retains its impact on mortality in real‐world settings.
Following participation in an inpatient smoking cessation program, self‐reported quit status at 6 months post‐hospital discharge in the intervention group was significantly higher in the intervention group (63%) than the comparison group (48%; P = 0.00). Using the intent to treat method, the differences between the study groups was still significant (44% in the intervention group, 30% in the comparison group; P = 0.00). Given the limitations of self‐report and responder bias, the actual outcomes fall somewhere between these 2 estimates. In an effectiveness study of inpatient smoking cessation involving 6 hospitals in California, self‐reported quit rates of 26% at 6 months were reported; however, different methods were used so the results are not strictly comparable.20
Our multivariate analysis suggests that patients with cardiovascular discharge diagnosis were more likely to quit than patients who had other discharge diagnoses (OR, 3.02; 95% CI, 1.65.7; P = 0.0007). This study extends findings of other studies that show that the success of smoking cessation may vary by diagnosis, particularly for smokers admitted for cardiovascular disease.21, 22 Other studies have shown that smoking cessation rates among patients post‐myocardial infarction were higher in admitting facilities that had hospital‐based smoking cessation programs and for those patients referred to cardiac rehabilitation.23 Thus, the availability of a hospital‐based smoking cessation program may be considered a structural measure of health care quality as suggested by Dawood et al.23
The use of NRT greatly increased in our hospital, coincident with the start of the inpatient cessation program7 and, in this study, NRT use appears to continue after hospital discharge. Some studies show an additive effect of NRT combined with cessation counseling.24, 25 Although a Cochrane review did not find a statistically significant difference, there was a trend toward higher quit rates with the addition of NRT.21, 22
Because hospitalized smokers may be more motivated to stop smoking, the updated 2008 DHHS clinical practice guidelines for Treating Tobacco Use and Dependence now recommend that all inpatients who currently smoke be given medications, advised, counseled, and receive follow‐up after discharge.26 Although our inpatient cessation program was started before these clinical practice guidelines were updated, we have had the opportunity to evaluate the recommended practice of inpatient tobacco cessation counseling. Compared to effects shown in efficacy studies, clinical interventions often lose effect size in daily practice and real‐world settings.27, 28 It is reassuring that, in this effectiveness study, the impact of this intervention is still demonstrable.
Provision of inpatient smoking cessation has been shown to be an effective smoking cessation intervention if combined with outpatient follow‐up.29 Reviews by Rigotti et al.21, 22 recommend that inpatient high‐intensity behavioral interventions should be followed by at least 1 month of supportive contact after discharge to promote smoking cessation among hospitalized patients. In our study, specific cessation‐related outpatient follow‐up was not provided by our program. Although letters were sent to primary care providers describing the cessation service provided during the inpatient stay, our study could not ascertain what specific cessation service was offered by either primary‐care or specialty‐care providers during posthospitalization follow‐up visits. An efficient alternative to outpatient visits may be follow‐up delivered via a quitline. Follow‐up in our study included referrals to the NYS Smokers' Quitline; however, only about 10% of inpatient reported using this service. While feasible, the effectiveness of quitline follow‐up is as yet unknown.30
Limitations
This study targets a later phase in research progression from hypothesis development, pilot studies, efficacy (empirically supported) trials, effectiveness trials (real‐world settings), and dissemination studies.31 Because this study addresses the effectiveness rather than the efficacy of inpatient smoking cessation counseling, the use of a quasiexperimental rather than randomized controlled clinical trial design led to measured differences in the study groups at baseline. An important imbalance arose in the intervention group that had twice the percentage of patients with cardiovascular‐related discharge diagnoses as the comparison group. While we were able to adjust for these differences in our analysis, there may be unmeasured differences due to the fact that the inpatients were not randomized to the study groups.
The outcomes of this study cannot be attributed to any one component of the intervention (eg, NRT) vs. the combined effect of the inpatient smoking cessation program. The program components were implemented simultaneously in order to maximize synergistic effects; therefore, the effects of program components are difficult to disaggregate.
The results are limited by the validity of self‐report of smoking status. It is well known that research studies which validate smoking status biochemically have lower efficacy (OR, 1.44; 95% CI, 0.992.11) than those that do not validate smoking status (OR, 1.92; 95% CI, 1.262.93).32 Although it was impractical in this effectiveness study to biochemically validate smoking status 6 months posthospital discharge, we have documented a significant difference between the study groups that confirms the direction of the effect, if not the effect size.
Self‐reports tend to underestimate smoking status in population studies33; however, the discrepancy between self‐reported smoking and biochemical measurements among clinical trial participants is small.34 However, a small but significant bias toward a socially desirable response in intervention groups compared to control groups of 3% with carbon monoxide and 5% for cotinine has been documented.35 If social desirability bias is operative in this study, and if we apply the above correction factor of 4% to correct this classification error, then the difference between the intervention and comparison group would be 10 percentage points (40% in the intervention group vs. 30% in the comparison group using the intention to treat estimates). That difference is still clinically relevant.
The observed difference between the intervention and comparison group is underestimated because the comparison group was exposed to smoking cessation as well both at the time of admission and following discharge (Table 4). The comparison group in this study could thus be viewed as a usual care group rather than a control group. That exposure does cloud the measurement of quit rates as the comparison group is contaminated to some degree by exposure to various cessation methods. The impact of this exposure is to reduce the effect size observed in this study or underestimate the effect of the inpatient smoking cessation counseling because the comparison group was exposed other cessation methods, although to a lesser extent.
The social desirability bias inherent in self‐reported smoking status may increase the effect size while the use of comparison group that received usual care may decrease the effect size. Because neither of these biases could be measured in this study, it is impossible to say whether they negated each other.
As with any administrative database, use of EMR as a data source in this study led to missing data that precluded use of certain variables in the analysis. In addition, lack of written and signed HIPAA releases also precluded inclusion of several inpatients, mostly in the comparison group, in the analytic database. However, it is reassuring that the results of the 6‐month survey did not differ significantly when these individuals were included or excluded from a separate analysis of the survey data. Last, our study population is almost 100% Caucasian thus limiting how generalizable the results are to more heterogenous patient populations.
Conclusions
This quasiexperimental effectiveness study showed that inpatient smoking cessation intervention improved smoking cessation outcomes, use of NRT, and was associated with a decreased mortality 6 months post‐hospital discharge. The effectiveness of this inpatient intervention is maintained in real world settings but may be improved with posthospital discharge follow‐up.
Acknowledgements
The authors are grateful to the many Mary Imogene Bassett Hospital staff in administration, nursing, inpatient pharmacy, medical education, patient care service, and respiratory care who provided data needed to evaluate this program. They also acknowledge the NYS Smokers' Quitline website for data provided about monthly Fax‐to‐Quit program referrals from our county.
In 1992, the Joint Commission on Accreditation of Healthcare Organizations (Joint Commission) introduced standards to make hospital buildings smoke‐free, resulting in the nation's first industry‐wide ban on smoking in the workplace. This hospital smoking ban has led to increased smoking cessation among employees.1 Since 2003, core measures from the Joint Commission and quality indicators from the Centers for Medicare and Medicaid Services have included inpatient smoking cessation counseling for acute myocardial infarction, pneumonia, and heart failure, as national guidelines strongly recommend smoking cessation counseling for patients with these diseases who smoke.25
The Department of Health and Human Services (DHHS) 2008 update on Clinical Practice Guidelines for Treating Tobacco Use and Dependence6 recommends that clinicians use hospitalization as an opportunity to promote smoking cessation and to prescribe medications to alleviate withdrawal symptoms. Hospitalization is an opportune time for smoking cessation because patients are restricted to a smoke‐free environment in the hospital and, increasingly, on hospital campuses.6 The illness leading to hospitalization may be attributable, at least in part, to tobacco use, thereby increasing the patient's receptivity to cessation counseling. Last, medications used in‐hospital to treat nicotine withdrawal symptoms may lead to continued or future use of these medications that, in turn, may ultimately lead to a successful quit attempt.
We report on the outcomes of our hospital's attempt to do this in the context of implementation of a smoke‐free medical campus.7 This study was designed to measure whether an inpatient smoking cessation intervention increases the likelihood of smoking cessation 6 months post‐hospital discharge. Because effectiveness studies are the next step to improving translation of research into health promotion practice,8 we set out to measure what the impact of this intervention would be in routine clinical practice as opposed to a carefully structured efficacy trial.
Methods
Intervention
The Smoking Cessation service for inpatients began on April 3, 2006. Upon admission, all patients were screened regarding their current smoking status. The nurse asked the patient if s/he currently smoked and then entered the responses into the hospital electronic medical record (EMR). A current smoker was defined as smoking every day or some days within the past 30 days. A roster of newly admitted current smokers was electronically transmitted to the Respiratory Care office daily. Only current smokers received counseling. The Smoking Cessation Specialist (SCS) subsequently saw inpatients within a 24‐hour time frame of admission, except for weekends and holidays. Each patient received 1 to 2 intensive follow‐up counseling sessions during hospitalization. An average of 10 patients per day were seen.
The goal of the inpatient smoking cessation service was to counsel patients on the health effects of smoking, address nicotine withdrawal symptoms, explain the different pharmacotherapies available, advise on how to quit, give self‐help materials, counsel family members, and refer to the New York State (NYS) Smokers' Fax‐to‐Quit program.9 Following the consult, the SCS documented the encounter in the patient's chart, including recommendations for nicotine replacement therapy (NRT) or bupropion (varenicline was not addressed as it was not on the formulary). The chart documentation informed the physician and nursing staff of the intervention and included the date/time, stage of change, and support action taken.
The SCS was part‐time, had nursing training, smoking cessation training,10 and was also trained by the Seton Health Cessation Center in the Butt Stops Here Program.11 She also implemented a performance improvement plan to increase the provision of smoking cessation counseling, increase NRT or bupropion prescriptions to smokers admitted to the hospital, and increase referrals to the NYS telephone quitline through the Fax‐to‐Quit program for outpatient resources and help following hospital discharge. The Fax‐to‐Quit program allows health care providers to refer patients to the NYS quitline via fax, with the patient's signature (patient permission) on the fax to quit form. After hospital discharge, the quitline then contacts the patient at a time that the patient requested.
The SCS visited patients with all admitting diagnoses on the medical, surgical, and special care units who were current smokers. Inpatients admitted to psychiatry, obstetrics, and the intensive care unit (ICU) were not seen by the SCS, except for ICU patients referred by a physician. Inpatients who had short stays or who were admitted and discharged in 1 day or during the weekend were not seen.
The intervention included either a brief 3‐minute to 5‐minute intervention or a more intensive intervention, that required 10 to 20 minutes (18 minutes average). The length of the intervention was determined by how receptive the patient was to the intervention. All interventions began with patient identification, an introduction to the SCS, and an explanation of the purpose of the visit. The SCS then inquired about the patient's comfort level vis‐a‐vis nicotine withdrawal and if s/he was receiving any NRT while in the hospital (NRT on the inpatient formulary included the nicotine patch or gum). If the patient was receptive to counseling, the SCS then began to work through the 5 A's, as described in the 2000 DHHS Clinical Practice Guidelines.12 The 2000 DHHS Clinical Practice Guidelines were used because the 2008 update had not been released at the time this study was initiated in 2006. These include: asking about smoking status, advising on how to quit, assessing readiness to quit, and assisting in arranging treatment options that include pharmacotherapy, counseling, as well as referral to the NYS Smokers' Quitline. A workbook was provided to reinforce counseling but was not necessarily used during counseling session. A compact disc (CD) with relaxation exercises5 was provided to those inpatients who were interested in stress reduction. If family members were present, and were also smokers, they were included in the counseling session, if willing. Each patient was offered a referral via the Fax‐to‐Quit program to continue treatment on an outpatient basis.
If the patient was not motivated to quit or declined the consult, the visits were short and focused on the patient's experience with nicotine withdrawal. These patients were also given self‐help materials and, if possible, the relevance of and roadblocks to quitting were reviewed. Patients were prompted to think about why quitting was relevant and often the reason for hospitalization was used to motivate the quitting process.
Upon hospital discharge, the patient's primary care provider was notified of the cessation intervention by a letter from the SCS. The letter described the intervention and stated whether or not the patient agreed to be referred to the Fax‐to‐Quit Program.
Study Participants
Patients were recruited from July 1, 2006 (after the smoking ban went into effect) through June 1, 2008. Inpatients who currently smoked were informed of this study and were asked to sign informed consent to participate after they were seen by the SCS. Current smokers of all admitting diagnoses were recruited into the study. Patients provided informed consent for a telephone interview 6 months posthospital discharge. A written Health Insurance Portability and Accountability Act (HIPAA) release was obtained to allow access to an individual's specific EMR.
A comparison group of inpatients who were also current smokers, but who did not receive the intervention were also contacted six months after hospitalization. Reasons for not receiving the intervention included the fact the SCS was part‐time and also took a leave of absence during the study and therefore could not see all inpatients who currently smoked. Other reasons for not receiving the intervention include too short a stay for the SCS to see the patient or the patient was out of the room for tests or procedures when the SCS was available. These patients provided informed consent to be interviewed 6 months after hospital discharge and HIPAA consent for access to their medical record. Not all inpatients in the comparison group provided written HIPAA release for use of their medical record; therefore, these patients were excluded because their baseline demographic and diagnostic data were missing.
Sample‐size considerations were driven around having adequate numbers of subjects to measure the prevalence of smoking cessation at 6 months post‐hospital discharge with an acceptable degree of precision. Prevalence estimates from previous studies for 6‐month cessation typically range from 20% to 30%, with cessation rates as high as 67% (this estimate applies to postmyocardial infarction patients.)13 For conservative estimation, we used 50% as the 6‐month prevalence of cessation in the current study, which placed binomial variance at its theoretical maximum. In this case, a sample of 300 subjects provides a margin of error of 0.058 for a 95% confidence interval around this point estimate.
Data Sources
The hospital EMR database was used to monitor several components of the program: nursing screening, smoking cessation counseling, and pharmacy dispensing of NRT and bupropion. The screening data were also used to monitor the proportion of current smokers admitted during the study period. Elements of the EMR were used to define the following covariates: patient age, gender, ethnicity, and the primary discharge diagnosis (via International Statistical Classification of Diseases and Related Health Problems, 9th edition [ICD9] codes) and readmission during the six month follow‐up period. Mean length of stay (LOS) was computed. The Elixhauser Comorbidity Index that utilizes ICD9 codes was used for comorbidity risk adjustment.14
Study participants were contacted by phone 6 months posthospital discharge. Data collection began July 1, 2006 and was completed January 1, 2009. The interview focused on self‐reported point prevalence of smoking and 6‐month quit status. The point prevalence for self‐reported abstinence was derived from the question Do you now smoke cigarettes every day, some days, or not at all?15 Self‐reported quit status was derived from question Have you quit smoking since you were discharged from the hospital? In addition, respondents were queried about their number of years smoked, post‐hospital discharge number of quit attempts, and cessation efforts (NRT, self‐help groups, quitline use, etc.). Last, they were surveyed about barriers to cessation (exposure to secondhand smoke, rules about smoking in the home or car), educational level, employment, and health ratings.
To determine the status of those lost to follow‐up, administrative and EMR databases for appointments and follow‐up visits were accessed to determine if the patient was alive during the 6 months between discharge and the follow‐up call. To confirm mortality, we searched the Internet, Ancestry.com, and/or local newspaper obituaries for dates of death for all patients to validate that they had not died during the 6‐month follow‐up. World wide web searches can identify 97% of deaths listed in the Centers for Disease Control and Prevention (CDC)/National Death Index, which is considered the gold standard in epidemiologic studies.16
Analysis
Univariate analysis of all covariates was completed to examine the normal distribution curves for these variables. Bivariate correlation analysis of all the independent variables by study group was performed to assess comparability of the study groups at baseline. The self‐reported cessation outcomes were calculated by dividing the number of patients who said they were not using tobacco or had quit, at 6 months posthospital discharge by the number of individuals in the study group at baseline minus those who had died. Both the intent to treat method, which assumes patients lost to follow‐up were still smoking, and the responder method, which does not include nonresponders in the analysis, were used to adjust the denominators for these outcomes.
Multivariate regression analysis was then used to model receipt of the intervention as predictor of self‐reported quit status adjusted for significant covariates. Statistical significance was defined by a P value of less than 0.05.
Survival analysis was employed to model differences in mortality between the study groups, controlling for any baseline imbalances (eg, comorbidity). Because baseline data were used in this model, the model includes only patients with signed a HIPAA release.
Internal review boards of our hospital and the NYS Department of Health reviewed and approved this study.
Results
From January 1, 2007 to May 30, 2008, 660 inpatients who were current smokers were recruited into the study. Figure 1 summarizes patient flow through the study and explains the final sample size of 607. Exclusions include 52 inpatients from the study who completed the 6‐month interview but who did not return a written HIPAA release. Without a HIPAA release to access the EMR, baseline comparison of the study groups and adjustment for comorbidity could not be completed for these patients. At 6 months posthospital discharge, 53 subjects refused the interview when contacted by telephone.

As might be expected in a quasiexperimental design, the study groups were not equivalent at baseline (Table 1). The intervention and comparison groups differed with regard to age, length of stay (LOS), proportion of acute admissions, and the Elixhauser comorbidity index. These differences suggest that the intervention group was older, had a longer LOS, higher acuity at the time of admission, and more comorbidities. In addition, as a result of the intervention, the intervention group was more likely to receive NRT or bupropion in hospital and a Fax‐to‐Quit referral to the NYS Smokers' Quitline. In the intervention group, most patients received 1 visit from the SCS, only 2% received 2 visits. Family members were included in smoking cessation counseling for 58% of the intervention group.
| Intervention (n = 275) | Comparison (n = 335) (P value) | |
|---|---|---|
| ||
| Sex (% male) | 51 | 51 |
| Mean age (years) | 51.4 | 48.5 (0.03) |
| Ethnicity (% white) | 98 | 97 |
| Marital status (% married) | 48 | 47 |
| Elective admission (vs. acute) (%) | 25 | 31 |
| Inpatient (vs. outpatient observation or outpatient surgical admission) (%) | 86 | 68 (0.00) |
| LOS (days) | 3.80 | 2.68 (0.00) |
| Elixhauser comorbidity index (mean) | 1.66 | 1.17 (0.00) |
| Used NRT in hospital (%) | 37 | 19 (0.00) |
| Used bupropion in hospital (%) | 4 | 1 (0.00) |
| Referred to NYS Smokers' Quitline using Fax‐to‐Quit | 10 | 0 (0.00) |
| Mean cigarettes per day* | 17.7 (n = 213) | 15.9 (n = 192) |
As shown in Table 2, there was a significant amount of diagnostic heterogeneity in the discharge diagnoses codes of patients included in the study. However, there were significantly more patients in the intervention group with a first discharge diagnosis of cardiovascular disease (25%) compared to the comparison group (12%; P = 0.00).
| Intervention (n = 274)* (%) | Comparison (n = 333)* (%) | |
|---|---|---|
| ||
| Cardiovascular | 25 | 12, P = 0.00 |
| Pulmonary | 16 | 7 |
| Orthopedics | 12 | 12 |
| Injury | 10 | 15 |
| GI | 8 | 16 |
| Cancer | 6 | 8 |
| GU | 3 | 6 |
| Endocrine | 3 | 3 |
| Other | 17 | 21 |
Readmission outcomes based on EMR and administrative data were available for 607 inpatients with signed HIPAA releases. The readmission rate was higher for the intervention (41%) than the comparison group (20%). Despite a higher readmission rate, the crude mortality within the 6 months posthospital discharge was lower for the intervention group, ie, 0.02 (6/276), than the comparison group, which had a crude mortality of 0.04 (16/384) during this period.
A multivariate survival model, controlling for age, sex, Elixhauser comorbidity index, LOS, and cardiovascular diagnosis, showed a significantly reduced mortality in the intervention group (hazard ratio [HR] = 0.37; P = 0.04). Although cardiac status (P = 0.09) and LOS (P = 0.15) were not significant in this model, they were retained because both of these variables showed significantly higher levels (along with the Elixhauser Index) at baseline in the intervention group, implying that the intervention group was sicker than the comparison group. The Elixhauser comorbidity index (HR = 1.42; P < 0.00) and age (HR = 1.07; P < 0.00) were the only other significant predictors of mortality in this model.
Among those responding to the interview at 6 months post‐hospital discharge (n = 326), there were no significant differences between the study groups with regard to age first started smoking, gender, educational level, employment status, ethnicity, or physical health status (data not shown). Table 3 summarizes the outcomes by the intervention and comparison groups at 6 months post‐hospital discharge. The point prevalence for abstinence was 27% in the intervention group compared to 19% in the comparison group (P = 0.09). Using the intent to treat analysis, the point prevalence for abstinence was 16% in the intervention group compared to 9.8% in the comparison group (P = 0.02). Self‐reported quit status was 63% in the intervention group vs. 48% in the comparison group (P = 0.00). Using the intent to treat analysis, quit status was 44% in the intervention group vs. 30% in the comparison group (P = 0.00). Exclusion of the 52 patients without signed HIPAA releases (Figure 1) did not significantly alter these outcomes.
| Intervention (n = 161) | Comparison (n = 165) (P value) | |
|---|---|---|
| ||
| Self‐reported now smoking not at all (%) | 16 | 10 (0.02) |
| Self‐reported quit within 6 months (%) | 63 | 48 (0.00 |
| Tried to quit (%) | 68 | 62 |
| Used NRT post‐D/C (%) | 26 | 17 (0.04) |
| Used other intervention (%) | 21 | 14 |
| Heard of the NYS Smokers' Quitline (%) | 92 | 90 |
| Aware that NYS Quitline offers NRT (%) | 73 | 49 (0.00) |
| Received free NRT from NYS Smokers' Quitline (%) | 9 | 6 |
| Used NYS Smokers' Quitline (%) | 15 | 9 |
| Called by the NYS Smokers' Quitline (%) | 11 | 5 |
| Self‐rated health status as fair or poor (%) | 48 | 36 |
| Another smoker living at home (%) | 54 | 48 |
| Mean hours spent in same room where someone else was smoking (n) | 20 | 21 (0.04) |
| Households in which smoking is not allowed in the home (%) | 40 | 33 |
| Patients Still Smoking at the 6‐Month Interview | Intervention (n = 118) | Comparison (n = 134) |
| Mean cigarettes currently smoked (n) | 10.5 | 12.7 |
| Mean quit attempts post‐D/C (n) | 3.2 | 3.5 |
| Mean reduction in smoking* (cigarettes/day) | 5.83 | 4.09 |
Patients who received the inpatient smoking cessation counseling were more likely to be called by or use the NYS Smokers' Quitline; however, these differences were not statistically significant. There was no difference between the study groups in awareness of the Quitline but the intervention group was more aware that free NRT was offered by the NYS Quitline. In terms of quit methods used during the 6‐month period (Table 4), NRT or bupropion use was higher in the intervention group. There were no other significant differences between the study groups, except for the use of acupuncture.
| Intervention (n = 91) | Comparison (n = 93) (P value) | |
|---|---|---|
| ||
| Got help from friends or family (%) | 58 | 50 |
| Used any medication to quit (%) | 44 | 28 (0.02) |
| Used nicotine patch (%) | 43 | 25 (0.00) |
| Used bupropion (%) | 10 | 1 (0.00) |
| Used varenicline (%) | 32 | 25 |
| Cut back (%) | 43 | 46 |
| Quit with a friend (%) | 20 | 13 |
| Switched to lights (%) | 18 | 13 |
| Used print material (%) | 14 | 16 |
| Got help from the NYS Smokers' Quitline (%) | 11 | 9 |
| Called by the NYS Smokers' Quitline (%) | 11 | 5 (0.09) |
| Counseling (%) | 9 | 3 |
| Acupuncture (%) | 5 | 0 (0.02) |
| Switched to chew (%) | 2 | 4 |
| Attended classes (%) | 3 | 1 |
| Used NYS Smokers' Quitline website (%) | 2 | 4 |
Multivariate analysis predicting quit status at 6 months post‐hospital discharge included covariates controlling for age, sex, LOS, study group, and comorbidity. This analysis showed that patients with a cardiovascular discharge diagnosis were more likely to quit than patients who had other discharge diagnoses (odds ratio [OR], 3.02; 95% confidence interval [CI], 1.65.7; P = 0.00). Another statistically significant covariate in this model included sex (men were more likely to quit: OR, 0.61; 95% CI, 0.390.97; P = 0.04). Participating in the inpatient intervention group was marginally significant when controlling for these other variables (OR, 1.54; 95% CI, 0.982.45; P = 0.06). Hospital LOS, age, receipt of NRT in hospital, and the Elixhauser comorbidity index were not predictive of quit status at 6 months.
Discussion
This study demonstrates how effective an inpatient smoking cessation program can be for increasing the success of quitting smoking after hospital discharge. At 6 months posthospital discharge, the intervention group had significantly higher intent to treat outcomes for point prevalence abstinence and quit status as well as lower crude and adjusted mortality than the comparison group.
Although at baseline the intervention group was older, had a longer LOS, more cardiovascular diagnoses, and higher comorbidity index, crude post‐hospital discharge mortality was significantly less in the intervention group (0.02) than in the comparison group (0.04). This finding is more significant in light of the higher comorbidity and acuity of the intervention group at baseline. Our multivariate survival model that controlled for these imbalances at baseline demonstrated that the intervention group had significantly less mortality than the comparison group (HR = 0.37; P = 0.04). Reduction in mortality, as soon as 30 days after inpatient smoking cessation counseling, has been demonstrated postmyocardial infarction.17, 18 Intensive smoking cessation quit services were also linked with lower all cause mortality among cardiovascular disease patients 2 years posthospitalization.19 Our study, despite its relatively small sample size, demonstrates that the intervention retains its impact on mortality in real‐world settings.
Following participation in an inpatient smoking cessation program, self‐reported quit status at 6 months post‐hospital discharge in the intervention group was significantly higher in the intervention group (63%) than the comparison group (48%; P = 0.00). Using the intent to treat method, the differences between the study groups was still significant (44% in the intervention group, 30% in the comparison group; P = 0.00). Given the limitations of self‐report and responder bias, the actual outcomes fall somewhere between these 2 estimates. In an effectiveness study of inpatient smoking cessation involving 6 hospitals in California, self‐reported quit rates of 26% at 6 months were reported; however, different methods were used so the results are not strictly comparable.20
Our multivariate analysis suggests that patients with cardiovascular discharge diagnosis were more likely to quit than patients who had other discharge diagnoses (OR, 3.02; 95% CI, 1.65.7; P = 0.0007). This study extends findings of other studies that show that the success of smoking cessation may vary by diagnosis, particularly for smokers admitted for cardiovascular disease.21, 22 Other studies have shown that smoking cessation rates among patients post‐myocardial infarction were higher in admitting facilities that had hospital‐based smoking cessation programs and for those patients referred to cardiac rehabilitation.23 Thus, the availability of a hospital‐based smoking cessation program may be considered a structural measure of health care quality as suggested by Dawood et al.23
The use of NRT greatly increased in our hospital, coincident with the start of the inpatient cessation program7 and, in this study, NRT use appears to continue after hospital discharge. Some studies show an additive effect of NRT combined with cessation counseling.24, 25 Although a Cochrane review did not find a statistically significant difference, there was a trend toward higher quit rates with the addition of NRT.21, 22
Because hospitalized smokers may be more motivated to stop smoking, the updated 2008 DHHS clinical practice guidelines for Treating Tobacco Use and Dependence now recommend that all inpatients who currently smoke be given medications, advised, counseled, and receive follow‐up after discharge.26 Although our inpatient cessation program was started before these clinical practice guidelines were updated, we have had the opportunity to evaluate the recommended practice of inpatient tobacco cessation counseling. Compared to effects shown in efficacy studies, clinical interventions often lose effect size in daily practice and real‐world settings.27, 28 It is reassuring that, in this effectiveness study, the impact of this intervention is still demonstrable.
Provision of inpatient smoking cessation has been shown to be an effective smoking cessation intervention if combined with outpatient follow‐up.29 Reviews by Rigotti et al.21, 22 recommend that inpatient high‐intensity behavioral interventions should be followed by at least 1 month of supportive contact after discharge to promote smoking cessation among hospitalized patients. In our study, specific cessation‐related outpatient follow‐up was not provided by our program. Although letters were sent to primary care providers describing the cessation service provided during the inpatient stay, our study could not ascertain what specific cessation service was offered by either primary‐care or specialty‐care providers during posthospitalization follow‐up visits. An efficient alternative to outpatient visits may be follow‐up delivered via a quitline. Follow‐up in our study included referrals to the NYS Smokers' Quitline; however, only about 10% of inpatient reported using this service. While feasible, the effectiveness of quitline follow‐up is as yet unknown.30
Limitations
This study targets a later phase in research progression from hypothesis development, pilot studies, efficacy (empirically supported) trials, effectiveness trials (real‐world settings), and dissemination studies.31 Because this study addresses the effectiveness rather than the efficacy of inpatient smoking cessation counseling, the use of a quasiexperimental rather than randomized controlled clinical trial design led to measured differences in the study groups at baseline. An important imbalance arose in the intervention group that had twice the percentage of patients with cardiovascular‐related discharge diagnoses as the comparison group. While we were able to adjust for these differences in our analysis, there may be unmeasured differences due to the fact that the inpatients were not randomized to the study groups.
The outcomes of this study cannot be attributed to any one component of the intervention (eg, NRT) vs. the combined effect of the inpatient smoking cessation program. The program components were implemented simultaneously in order to maximize synergistic effects; therefore, the effects of program components are difficult to disaggregate.
The results are limited by the validity of self‐report of smoking status. It is well known that research studies which validate smoking status biochemically have lower efficacy (OR, 1.44; 95% CI, 0.992.11) than those that do not validate smoking status (OR, 1.92; 95% CI, 1.262.93).32 Although it was impractical in this effectiveness study to biochemically validate smoking status 6 months posthospital discharge, we have documented a significant difference between the study groups that confirms the direction of the effect, if not the effect size.
Self‐reports tend to underestimate smoking status in population studies33; however, the discrepancy between self‐reported smoking and biochemical measurements among clinical trial participants is small.34 However, a small but significant bias toward a socially desirable response in intervention groups compared to control groups of 3% with carbon monoxide and 5% for cotinine has been documented.35 If social desirability bias is operative in this study, and if we apply the above correction factor of 4% to correct this classification error, then the difference between the intervention and comparison group would be 10 percentage points (40% in the intervention group vs. 30% in the comparison group using the intention to treat estimates). That difference is still clinically relevant.
The observed difference between the intervention and comparison group is underestimated because the comparison group was exposed to smoking cessation as well both at the time of admission and following discharge (Table 4). The comparison group in this study could thus be viewed as a usual care group rather than a control group. That exposure does cloud the measurement of quit rates as the comparison group is contaminated to some degree by exposure to various cessation methods. The impact of this exposure is to reduce the effect size observed in this study or underestimate the effect of the inpatient smoking cessation counseling because the comparison group was exposed other cessation methods, although to a lesser extent.
The social desirability bias inherent in self‐reported smoking status may increase the effect size while the use of comparison group that received usual care may decrease the effect size. Because neither of these biases could be measured in this study, it is impossible to say whether they negated each other.
As with any administrative database, use of EMR as a data source in this study led to missing data that precluded use of certain variables in the analysis. In addition, lack of written and signed HIPAA releases also precluded inclusion of several inpatients, mostly in the comparison group, in the analytic database. However, it is reassuring that the results of the 6‐month survey did not differ significantly when these individuals were included or excluded from a separate analysis of the survey data. Last, our study population is almost 100% Caucasian thus limiting how generalizable the results are to more heterogenous patient populations.
Conclusions
This quasiexperimental effectiveness study showed that inpatient smoking cessation intervention improved smoking cessation outcomes, use of NRT, and was associated with a decreased mortality 6 months post‐hospital discharge. The effectiveness of this inpatient intervention is maintained in real world settings but may be improved with posthospital discharge follow‐up.
Acknowledgements
The authors are grateful to the many Mary Imogene Bassett Hospital staff in administration, nursing, inpatient pharmacy, medical education, patient care service, and respiratory care who provided data needed to evaluate this program. They also acknowledge the NYS Smokers' Quitline website for data provided about monthly Fax‐to‐Quit program referrals from our county.
- , , , , , .“Implementing smoking bans in American hospitals: results of a national survey.Tob Control.1998;7(1):47–55.
- The Smoking Cessation Clinical Practice Guideline Panel and Staff: the Agency for Health Care Policy and Research Smoking Cessation Clinical Practice Guideline.JAMA.1996;275(16):1270–1280.
- , , , et al.ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures). Endorsed by the Heart Failure Society of America.J Am Coll Cardiol.2005;46(6):1144–1178.
- , , , et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction).Circulation.2004;110(9):e82–e292.
- , , , et al.ACC/AHA clinical performance measures for adults with ST‐elevation and non‐ST elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures on ST‐Elevation and Non‐ST‐Elevation Myocardial Infarction).J Am Coll Cardiol.2006;47(1):236–265.
- Treating Tobacco Use and Dependence‐Clinicians Packet. A How‐To Guide For Implementing the Public Health Service Clinical Practice Guideline, March2003. Rockville, MD: U.S. Public Health Service, Agency for Healthcare Research and Quality. Available at: http://www.ahrq.gov/clinic/tobacco. Accessed November 2009.
- , , , .Implementing a smoke‐free medical campus: impact on inpatient and employee outcomes.J Hosp Med.2010;5(1):51–54.
- , , , et al.The future of behavior change research: what is needed to improve translation of research into health promotion practice?Ann Behav Med.2004;27:3–12.
- The New York State Smokers' Quitline. Available at: http://www.nysmokefree.com. Accessed November2009.
- Tobacco Cessation Continuing Education for Healthcare Professionals and Counselors. Available at: http://www.tobaccocme.com. Accessed November2009.
- Seton Health Cessation Center. The Butt Stops Here. Relaxation Exercises for Smoking Cessation. 2001. The Butt Stops Here Program. Available at: http://www.setonhealth.org. Accessed November2009.
- U.S. Department of Health and Human Services.Treating Tobacco Use and Dependence. Clinical Practice Guideline.Rockville, MD:Public Health Service;2000.
- , , , , .A randomized controlled trial of smoking cessation counseling after myocardial infarction.Prev Med.2000;30(4):261–268.
- , , , .Comorbidity measures for use with administrative data.Med Care.1998:36(1):8–27.
- Tobacco Use Supplement to the Current Population Survey (TUS‐CPS). Available at: http://riskfactor.cancer.gov/studies/tus‐cps/info.html. Accessed November 2009.
- , , .Comparison of National Death Index and world wide web death searches.Am J of Epidemiol.2000;152(2):107–111.
- , , , et al.Post‐myocardial infarction smoking cessation counseling: associations with immediate and late mortality in older Medicare patients.Am J Med.2005;118(3):269–275.
- , , .Inpatient smoking‐cessation counseling and all‐cause mortality in patients with acute myocardial infarction.Am Heart J.2007;154(2):213–220.
- , , , et al.Intensive smoking cessation intervention reduces mortality in high‐risk smokers with cardiovascular disease.Chest.2007;131:446–452.
- , , , , .Dissemination of an effective inpatient tobacco use cessation program.Nicotine Tob Res.2005;7(1):129–137.
- , , .Interventions for smoking cessation in hospitalized patients.Cochrane Database Syst Rev.2007;3:CD001837.
- , , .Smoking cessation interventions for hospitalized smokers.Arch Intern Med.2008;168(18):1950–1960.
- , , , et al.Predictors of smoking cessation after a myocardial infarction.Arch Int Med.2008;168(18):1961–1967.
- , , , .The effectiveness of smoking cessation interventions prior to surgery: a systematic review.Nicotine Tob Res.2008;10(3):407–412.
- , , , et al.Clinical trial comparing nicotine replacement therapy (NRT) plus brief counseling, brief counseling alone, and minimal intervention on smoking cessation in hospital inpatients.Thorax.2003;58:484–488.
- Department of Health and Human Services (DHHS). Treating Tobacco Use and Dependence: 2008 Update. Chapter 7. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat2.section.28504. Accessed November2009.
- , .Implementation of evidence‐based tobacco use cessation guidelines in managed care organizations.Ann Behav Med.2004;27(1):13–21.
- .Decreasing effect sizes for effectiveness studies—implications for the transport of evidence‐based treatments: comment on Curtis, Ronan, and Borduin (2004).J Fam Psychol.2004;18(3):420–423.
- , , , , , .Efficacy of a smoking cessation program for hospital patients.Arch Intern Med.1997;157(22):2653–2660.
- , , , et al.Feasibility, acceptability, and cost of referring surgical patients for postdischarge cessation support from a quitline.Nicotine Tob Res.2008;10(6):1105–1108.
- .Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programs.Prev Med.1986;15:451–474.
- , , .Psychosocial interventions for smoking cessation in patients with coronary heart disease.Cochrane Database Syst Rev.2008;23(1):CD006886.
- , , , , .The accuracy of self‐reported smoking: a systematic review of the relationship between self‐reported and cotinine‐assessed smoking status.Nicotine Tob Res2009;11(1):12–24.
- , , , , .Relations of cotinine and carbon monoxide to self‐reported smoking in a cohort of smokers and ex‐smokers followed over 5 years.Nicotine Tob Res.2002;4(3):287–294.
- , , , .Error in smoking measures: effects of intervention on relations of cotinine and carbon monoxide to self‐reported smoking. The Lung Health Study Research Group.Am J Public Health.1993;83(9):1251–1257.
- , , , , , .“Implementing smoking bans in American hospitals: results of a national survey.Tob Control.1998;7(1):47–55.
- The Smoking Cessation Clinical Practice Guideline Panel and Staff: the Agency for Health Care Policy and Research Smoking Cessation Clinical Practice Guideline.JAMA.1996;275(16):1270–1280.
- , , , et al.ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures). Endorsed by the Heart Failure Society of America.J Am Coll Cardiol.2005;46(6):1144–1178.
- , , , et al.ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction).Circulation.2004;110(9):e82–e292.
- , , , et al.ACC/AHA clinical performance measures for adults with ST‐elevation and non‐ST elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures on ST‐Elevation and Non‐ST‐Elevation Myocardial Infarction).J Am Coll Cardiol.2006;47(1):236–265.
- Treating Tobacco Use and Dependence‐Clinicians Packet. A How‐To Guide For Implementing the Public Health Service Clinical Practice Guideline, March2003. Rockville, MD: U.S. Public Health Service, Agency for Healthcare Research and Quality. Available at: http://www.ahrq.gov/clinic/tobacco. Accessed November 2009.
- , , , .Implementing a smoke‐free medical campus: impact on inpatient and employee outcomes.J Hosp Med.2010;5(1):51–54.
- , , , et al.The future of behavior change research: what is needed to improve translation of research into health promotion practice?Ann Behav Med.2004;27:3–12.
- The New York State Smokers' Quitline. Available at: http://www.nysmokefree.com. Accessed November2009.
- Tobacco Cessation Continuing Education for Healthcare Professionals and Counselors. Available at: http://www.tobaccocme.com. Accessed November2009.
- Seton Health Cessation Center. The Butt Stops Here. Relaxation Exercises for Smoking Cessation. 2001. The Butt Stops Here Program. Available at: http://www.setonhealth.org. Accessed November2009.
- U.S. Department of Health and Human Services.Treating Tobacco Use and Dependence. Clinical Practice Guideline.Rockville, MD:Public Health Service;2000.
- , , , , .A randomized controlled trial of smoking cessation counseling after myocardial infarction.Prev Med.2000;30(4):261–268.
- , , , .Comorbidity measures for use with administrative data.Med Care.1998:36(1):8–27.
- Tobacco Use Supplement to the Current Population Survey (TUS‐CPS). Available at: http://riskfactor.cancer.gov/studies/tus‐cps/info.html. Accessed November 2009.
- , , .Comparison of National Death Index and world wide web death searches.Am J of Epidemiol.2000;152(2):107–111.
- , , , et al.Post‐myocardial infarction smoking cessation counseling: associations with immediate and late mortality in older Medicare patients.Am J Med.2005;118(3):269–275.
- , , .Inpatient smoking‐cessation counseling and all‐cause mortality in patients with acute myocardial infarction.Am Heart J.2007;154(2):213–220.
- , , , et al.Intensive smoking cessation intervention reduces mortality in high‐risk smokers with cardiovascular disease.Chest.2007;131:446–452.
- , , , , .Dissemination of an effective inpatient tobacco use cessation program.Nicotine Tob Res.2005;7(1):129–137.
- , , .Interventions for smoking cessation in hospitalized patients.Cochrane Database Syst Rev.2007;3:CD001837.
- , , .Smoking cessation interventions for hospitalized smokers.Arch Intern Med.2008;168(18):1950–1960.
- , , , et al.Predictors of smoking cessation after a myocardial infarction.Arch Int Med.2008;168(18):1961–1967.
- , , , .The effectiveness of smoking cessation interventions prior to surgery: a systematic review.Nicotine Tob Res.2008;10(3):407–412.
- , , , et al.Clinical trial comparing nicotine replacement therapy (NRT) plus brief counseling, brief counseling alone, and minimal intervention on smoking cessation in hospital inpatients.Thorax.2003;58:484–488.
- Department of Health and Human Services (DHHS). Treating Tobacco Use and Dependence: 2008 Update. Chapter 7. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat2.section.28504. Accessed November2009.
- , .Implementation of evidence‐based tobacco use cessation guidelines in managed care organizations.Ann Behav Med.2004;27(1):13–21.
- .Decreasing effect sizes for effectiveness studies—implications for the transport of evidence‐based treatments: comment on Curtis, Ronan, and Borduin (2004).J Fam Psychol.2004;18(3):420–423.
- , , , , , .Efficacy of a smoking cessation program for hospital patients.Arch Intern Med.1997;157(22):2653–2660.
- , , , et al.Feasibility, acceptability, and cost of referring surgical patients for postdischarge cessation support from a quitline.Nicotine Tob Res.2008;10(6):1105–1108.
- .Efficacy and effectiveness trials (and other phases of research) in the development of health promotion programs.Prev Med.1986;15:451–474.
- , , .Psychosocial interventions for smoking cessation in patients with coronary heart disease.Cochrane Database Syst Rev.2008;23(1):CD006886.
- , , , , .The accuracy of self‐reported smoking: a systematic review of the relationship between self‐reported and cotinine‐assessed smoking status.Nicotine Tob Res2009;11(1):12–24.
- , , , , .Relations of cotinine and carbon monoxide to self‐reported smoking in a cohort of smokers and ex‐smokers followed over 5 years.Nicotine Tob Res.2002;4(3):287–294.
- , , , .Error in smoking measures: effects of intervention on relations of cotinine and carbon monoxide to self‐reported smoking. The Lung Health Study Research Group.Am J Public Health.1993;83(9):1251–1257.
Copyright © 2010 Society of Hospital Medicine
Implementing a Smoke‐Free Medical Campus
Even though imposition of smoke‐free policies and workplaces comprise one of the most effective antismoking strategies,1 hospital administrators hesitate to implement a smoke‐free medical campus policy.2 They fear losing patients who smoke because these patients will opt for other facilities that permit smoking.
Apart from studies evaluating Joint Commission on Accreditation of Healthcare Organizations (JCAHO)‐required indoor smoking bans in hospitals in 1992,3, 4 there are few published studies or formal evaluations of the impact of medical campuses going smoke‐free. One study of the implementation of a smoke‐free medical campus policy at a university hospital in Little Rock, AR, showed that the policy had no impact on employee retention, bed occupancy, or mean daily census; however, inpatient smoking status was not ascertained.5 Most (83%) employees were supportive of the policy. More importantly, employees at 2 university medical centers reported reduced cigarette consumption and increased attempts to quit after implementation of a smoke‐free medical campus policy.6, 7
Our hospital is 180‐bed, acute care inpatient teaching facility in upstate New York. Prior to the implementation of the smoke‐free medical campus policy, it was common to see employees, visitors, and patients lined up outdoors around the main hospital entrances and smoking just beyond the no smoking signage. Inpatients could look out their windows at the main entrance or into the courtyard and see hospital staff, other patients, and visitors smoking.
This study prospectively evaluates the impact of implementing the smoke‐free medical campus policy and starting an inpatient smoking cessation service. It addresses the following questions that have also been raised by the Task Force for Community Preventive Services.8 Does the institution of hospital smoking bans reduce the percentage of inpatients who smoke or increase the percentage who sign out against medical advice? What are the extended effects (beyond 1 year after implementation) of medical campus smoking bans on employee smoking rates?
Materials and Methods
Policy Implementation
As prior studies have shown that institution of a smoke‐free medical campus policy involves much more than just posting signage,9, 10 a detailed multidisciplinary work plan was implemented starting 1.5 years prior to the date our policy went into effect on July 1, 2006. The Implementing a Smoke‐Free Environment plan, produced by the University of Michigan,11 which includes a 15‐step checklist, was used to guide this policy change.12 As part of that plan, employees were offered on‐site smoking cessation services, including nicotine replacement therapy (NRT), and 150 employees participated in this program prior to July 1, 2006. Staff, community, and patient education was also completed. A new campus map delineating the smoke‐free border was disseminated. Signage was posted in areas used in the past for smoking. In addition to implementing this plan, an inpatient smoking cessation service was started 3 months prior to July 1, 2006. In addition to supporting the enforcement of the smoke‐free medical campus, our inpatient smoking cessation program was designed to help inpatients with nicotine withdrawal as well as smoking cessation, if they were ready to quit.
Data Collection and Analysis
The inpatient electronic medical record (EMR) was used to monitor the smoking status of patients admitted to hospital on a monthly basis. On admission to the hospital, the admitting nurse screened patients for current smoking status. This information was entered into the EMR starting in April 2006; therefore, pre‐ban screening data were limited to 2 months prior to the ban. Inpatients too sick to complete this screening process, women admitted for labor and delivery, and inpatients boarded in the emergency department were not screened. No identifiers were used in compiling these monthly data.
Nursing reports of inpatients signing out against medical advice (AMA) were compiled in order to compare incidence of AMA pre‐ban to post‐ban. AMA documentation in our hospital takes the form of a structured incident report that is reliably documented by nursing staff and signed by the attending physician of service.
Computerized inpatient doctors' orders to pharmacy for NRT, dispensed as gum or patch, were monitored 2 years preinitiation and postinitiation of the inpatient smoking cessation service on April 1, 2006. As varenicline was nonformulary and bupropion was used for other indications than smoking cessation, these medications were not included in this review. The Chow test was used to measure and test for significant breaks in a time series analysis of the NRT orders.
New York State law requires an annual occupational health review to be completed by every hospital employee. At our hospital, this review included a question on tobacco use Do you smoke or chew tobacco? Although there has been a smoker/nonsmoker differential in the rates offered for supplemental life insurance since 1992, there were no wellness credits or other incentives for medical insurance offered in employee benefits that may predispose employees to underreport tobacco use. Using this question, employees were categorized as self‐reported current smokers or chew users. Employee smoking rates were estimated using different denominators to validate the direction of the trend. First, self‐reported smoking rates were compared pre‐ban and post‐ban among a stable cohort of hospital employees (n = 489), defined as hospital‐based employees with anniversary dates from March to June who reported in both 2005 and 2007. The McNemar test was used to test the statistical significance of the 2 smoking rates of paired replicates in this stable cohort of employees reporting pre‐ban and post‐ban. Second, all employees in the database reporting smoking status pre‐ban, March to June 2005, and then post‐ban, March to June 2006 and 2007, were compared in order to monitor trends in employee smoking overall. A t‐test was used to compare the statistical significance of the difference in the overall rates of smoking among all employees pre‐ban and post‐ban.
Internal review boards of our hospital and the New York State Department of Health reviewed and approved this study.
Results
Inpatient Outcomes
An average of 959 patients were admitted per month in the 18‐month period pre‐ban (January 2005 to June 2006) vs. 988 per month in the 23‐month period post‐ban (July 2006 to September 2008). A monthly average of 89% of inpatients were screened for tobacco use when admitted. The monthly average for the percentage of inpatients who currently smoke has been approximately 21.6% following the implementation of the smoke‐free hospital plan. There has been little variation (Figure 1) in the percentage of inpatients who smoke pre‐ban and post‐ban except for the startup period in 2006 and the onset of the 2007 respiratory illness season.
Among all inpatients who currently smoke, 69.8% received a brief nursing intervention at the time of admission and 25% received an inpatient visit from our part‐time smoking cessation specialist.
The percentage of inpatients who signed out against medical advice (AMA) with the reason of having to smoke was 13.8% (4/29) 6 months pre‐ban, and 13.6% (3/22) 6 months post‐ban. In 2007, there were no inpatients who signed out AMA stating that they needed to smoke. Because the reason for signing out AMA may be underreported, we also examined the rate of smoking among all inpatients who sign out AMA. Six months pre‐ban, this percentage was 48.3% (14/29), but increased 6 months post‐ban to 59% (13/22). In 2007, the percentage of smokers among inpatients who sign out AMA leveled off at 50.8% (29/57).
Review of computerized inpatient prescription orders shows that orders for NRT nearly tripled after the inpatient smoking cessation service started April 1, 2006 (3 months prior to the ban) (Figure 2). Inpatient orders for these medications increased from 832 in a 2‐year period before the ban (April 1, 2004 to March 31, 2006) to 2475 in the 2 years following the initiation of the inpatient smoking service (April 1, 2006 to March 31, 2008). The Chow test is highly significant for a break point in June 2006 (P = 0.008), 1 month prior to the ban.

Employee Smoking Rates
Among a cohort of 489 hospital‐based employees reporting in both 2005 and 2007, 12% reported smoking in 2005 and 7.5% in 2007 (McNemar was significant at P < 0.001). Two employees reported using chewing tobacco in 2005 and only 1 in 2007.
Including all hospital employees reporting any 1 year during their anniversary dates, the self‐reported smoking rates were 14.3% (n = 624) in March to June 2005, 14.8% (n = 661) in March to June 2006, and 9.4% (n = 1,112) in March to June 2007 (P < 0.0002). Because promotions change the anniversary date, and the database was expanded in 2007 to include new hires and managerial staff, these estimates represent the point prevalence among employees whose anniversary dates fall between March and June.
Discussion
Following implementation of a smoke‐free medical campus, no adverse effects were observed on inpatient volume at our hospital. The percentage of inpatients who smoke and the percentage of inpatients signing out AMA have remained stable after the smoke‐free policy went into effect. In addition, self‐reported employee smoking rates decreased significantly. Fears about losing inpatients (who smoke) following the implementation of a smoke‐free hospital plan were unfounded.
This study employs the electronic medical record to not only monitor trends in the proportion of inpatients who smoke pre‐ban and post‐ban, but also to notify our inpatient smoking cessation specialist, on the day of admission, to consult on patients who currently smoke. Unfortunately, our cessation specialist, who is part‐time, was unable to see all inpatients who smoke on account of the inpatient's acuity, pain, hospice status, weekend or night admission, or not being available due to testing, surgery, or other procedures. Nevertheless, use of NRT increased sharply following the initiation of this program. As shown in Figure 2, a linear rise in NRT orders was already underway starting April 2005, probably in anticipation of the ban and coinciding with the start of the inpatient smoking cessation program. However, the Chow test is highly significant for a breakpoint in June 2006 (P = 0.008), 1 month prior to the ban, meaning that the slope was climbing even more steeply after that point.
As hospitalized smokers may be more motivated to stop smoking, the updated 2008 clinical practice guidelines for Treating Tobacco Use and Dependence now recommend that all patients in the hospital be given medications, advised, counseled, and receive follow‐up after discharge.13 Although our inpatient cessation program was started before these clinical practice guidelines were available, we are currently evaluating the efficacy of our inpatient program by assessing self‐reported quit rates 6‐months posthospitalization (data collection in process). Provision of inpatient smoking cessation has been shown to be an effective smoking cessation intervention if combined with outpatient follow‐up.14 Our current program will be expanded to include outpatient follow‐up, if the inpatient's primary care provider is unable to provide it or if the inpatient refuses faxed referral to the New York State quit line program.
This study evaluates the impact of simultaneously introduced interventions such as medical campus smoking ban, inpatient smoking cessation program, hospital staff education, and other elements of the University of Michigan Smoke‐Free Hospital Implementation Plan. The role of individual components of the plan cannot be evaluated in this study as they were intentionally implemented simultaneously in order to achieve a synergistic effect.
Another limitation of this study is that smoking status is self‐reported and not validated biochemically. Although validated smoking status measures such as salivary cotinine testing would be more scientifically valid, it was not feasible to validate the smoking status of inpatients, nor that of employees. Thus smoking status, as ascertained in this study, is subject to underreporting. Social desirability bias has been recognized as potential limitation of self‐reported smoking status in other evaluations of smoke‐free policies.3, 4, 15
In the 1990s, the employee benefits of instituting indoor smoking bans in hospitals were theorized to include reduced employee sick time, break time, and tobacco use, as well as increased motivation for smoking cessation and reduced legitimacy of tobacco use.16, 17 Peer pressure, workplace socialization, and being forced to stay away from cigarettes for the length of entire workdays have been credited with helping hospital workers to quit.4, 7 In our study, extending the ban to the outdoor areas of our medical campus as well as provision of employee smoking cessation services may augment these mechanisms. This study extends findings of older studies that showed hospital smoking bans (primarily indoor) decreased hospital employee smoking rates. Currently, our reduced employee smoking rate approaches the Healthy People 2010 goal of 12%.18
In conclusion, implementing a smoke‐free medical campus does not adversely affect inpatient volume (even among smokers), does not increase inpatient signing out AMA and can significantly increase inpatient NRT use, which in turn can increase the success of a quit attempt.19 In addition, implementing an outdoor smoking ban further reduces hospital employee smoking rates.
Acknowledgements
The authors are grateful to the many Mary Imogene Bassett Hospital staff in administration, employee health, facilities management, human resources, inpatient pharmacy, medical education, patient care service, respiratory care, and security who provided policy support and/or data needed to evaluate policy implementation.
- Institute of Medicine.Ending the Tobacco Problem: A Blueprint for the Nation.Washington, DC:National Academies Press;2007.
- ,.Smoke‐Free Hospital Campus Policies.Washington, DC,Advisory Board Original Inquiry Brief. 2/1/2005. Available at: http://www.roswellpark.org/files/1_2_1/prevention/3%20‐%20‐Advisory% 20Board%20smoke%20free%20policies.pdf. Accessed March 2009.
- ,,,,.Effects of the implementation of a smoke‐free policy in a medical center.Chest.1992;102:1531–1536.
- ,,, et al.Hospital smoking bans and employee smoking behavior: results of a national survey.JAMA.1996;275(16):1252–1257.
- ,,, et al.Impact of a smoke‐free hospital campus policy on employee and consumer behavior.Public Health Rep.2007;122(6):744–752.
- ,,,.Employee attitudes and smoking behavior at the City of Hope National Medical Center smoke–free campus.J Natl Compr Canc Netw.2006;4(6):535–542.
- ,.Effect of a total work‐site ban on employee smoking and attitudes.J Occup Med.1991;33(8):884–890.
- ,,, et al.Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke.Am J Prev Med.2001;20(2S):16–66.
- ,,.Smoking on hospital grounds and the impact of outdoor smoke‐free zones.Tob Control.1996;5:199–204.
- ,,,,.The making of a smoke free hospital may not be as easy as you think.Am J Prev Med.1991;7(4):214–218.
- University of Michigan Health System. Tobacco Consultation Service. Available at: http://www.med.umich.edu/mfit/tobacco/freeenvironment. htm. Accessed March2009.
- Michigan Health and Hospital Association. It's a matter of life and health: MHA campaign for smoke‐free hospitals. Available at: http://www. mhasmokefreecampus.org. Accessed March2009.
- Department of Health and Human Services (DHHS). Treating Tobacco Use and Dependence: 2008 Update. Chapter 7: Specific Populations and Other Topics. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi? rid=hstat2.section.28504. Accessed March2009.
- ,,.Interventions for smoking cessation in hospitalized patients.Cochrane Database Syst Rev.2007;(3):CD001837.
- ,,, et al.Ending smoking at the Johns Hopkins Medical Institutions: an evaluation of smoking prevalence and indoor air pollution.JAMA.1990;264:1565–1569.
- .Toward smoke‐free medical facilities.Chest.1990;97:1027–1028.
- .The benefits of smoke‐free health care campuses.Am Fam Physician.1994;49(1):28–33.
- U.S. Department of Health and Human Services.Healthy People 2010. Vol 12nd ed.Washington, DC:U.S. Department of Health and Human Services;2000.
- ,,,.Effectiveness of smoking cessation therapies: a systematic review and meta‐analysis.BMC Public Health.2006;6:300.
Even though imposition of smoke‐free policies and workplaces comprise one of the most effective antismoking strategies,1 hospital administrators hesitate to implement a smoke‐free medical campus policy.2 They fear losing patients who smoke because these patients will opt for other facilities that permit smoking.
Apart from studies evaluating Joint Commission on Accreditation of Healthcare Organizations (JCAHO)‐required indoor smoking bans in hospitals in 1992,3, 4 there are few published studies or formal evaluations of the impact of medical campuses going smoke‐free. One study of the implementation of a smoke‐free medical campus policy at a university hospital in Little Rock, AR, showed that the policy had no impact on employee retention, bed occupancy, or mean daily census; however, inpatient smoking status was not ascertained.5 Most (83%) employees were supportive of the policy. More importantly, employees at 2 university medical centers reported reduced cigarette consumption and increased attempts to quit after implementation of a smoke‐free medical campus policy.6, 7
Our hospital is 180‐bed, acute care inpatient teaching facility in upstate New York. Prior to the implementation of the smoke‐free medical campus policy, it was common to see employees, visitors, and patients lined up outdoors around the main hospital entrances and smoking just beyond the no smoking signage. Inpatients could look out their windows at the main entrance or into the courtyard and see hospital staff, other patients, and visitors smoking.
This study prospectively evaluates the impact of implementing the smoke‐free medical campus policy and starting an inpatient smoking cessation service. It addresses the following questions that have also been raised by the Task Force for Community Preventive Services.8 Does the institution of hospital smoking bans reduce the percentage of inpatients who smoke or increase the percentage who sign out against medical advice? What are the extended effects (beyond 1 year after implementation) of medical campus smoking bans on employee smoking rates?
Materials and Methods
Policy Implementation
As prior studies have shown that institution of a smoke‐free medical campus policy involves much more than just posting signage,9, 10 a detailed multidisciplinary work plan was implemented starting 1.5 years prior to the date our policy went into effect on July 1, 2006. The Implementing a Smoke‐Free Environment plan, produced by the University of Michigan,11 which includes a 15‐step checklist, was used to guide this policy change.12 As part of that plan, employees were offered on‐site smoking cessation services, including nicotine replacement therapy (NRT), and 150 employees participated in this program prior to July 1, 2006. Staff, community, and patient education was also completed. A new campus map delineating the smoke‐free border was disseminated. Signage was posted in areas used in the past for smoking. In addition to implementing this plan, an inpatient smoking cessation service was started 3 months prior to July 1, 2006. In addition to supporting the enforcement of the smoke‐free medical campus, our inpatient smoking cessation program was designed to help inpatients with nicotine withdrawal as well as smoking cessation, if they were ready to quit.
Data Collection and Analysis
The inpatient electronic medical record (EMR) was used to monitor the smoking status of patients admitted to hospital on a monthly basis. On admission to the hospital, the admitting nurse screened patients for current smoking status. This information was entered into the EMR starting in April 2006; therefore, pre‐ban screening data were limited to 2 months prior to the ban. Inpatients too sick to complete this screening process, women admitted for labor and delivery, and inpatients boarded in the emergency department were not screened. No identifiers were used in compiling these monthly data.
Nursing reports of inpatients signing out against medical advice (AMA) were compiled in order to compare incidence of AMA pre‐ban to post‐ban. AMA documentation in our hospital takes the form of a structured incident report that is reliably documented by nursing staff and signed by the attending physician of service.
Computerized inpatient doctors' orders to pharmacy for NRT, dispensed as gum or patch, were monitored 2 years preinitiation and postinitiation of the inpatient smoking cessation service on April 1, 2006. As varenicline was nonformulary and bupropion was used for other indications than smoking cessation, these medications were not included in this review. The Chow test was used to measure and test for significant breaks in a time series analysis of the NRT orders.
New York State law requires an annual occupational health review to be completed by every hospital employee. At our hospital, this review included a question on tobacco use Do you smoke or chew tobacco? Although there has been a smoker/nonsmoker differential in the rates offered for supplemental life insurance since 1992, there were no wellness credits or other incentives for medical insurance offered in employee benefits that may predispose employees to underreport tobacco use. Using this question, employees were categorized as self‐reported current smokers or chew users. Employee smoking rates were estimated using different denominators to validate the direction of the trend. First, self‐reported smoking rates were compared pre‐ban and post‐ban among a stable cohort of hospital employees (n = 489), defined as hospital‐based employees with anniversary dates from March to June who reported in both 2005 and 2007. The McNemar test was used to test the statistical significance of the 2 smoking rates of paired replicates in this stable cohort of employees reporting pre‐ban and post‐ban. Second, all employees in the database reporting smoking status pre‐ban, March to June 2005, and then post‐ban, March to June 2006 and 2007, were compared in order to monitor trends in employee smoking overall. A t‐test was used to compare the statistical significance of the difference in the overall rates of smoking among all employees pre‐ban and post‐ban.
Internal review boards of our hospital and the New York State Department of Health reviewed and approved this study.
Results
Inpatient Outcomes
An average of 959 patients were admitted per month in the 18‐month period pre‐ban (January 2005 to June 2006) vs. 988 per month in the 23‐month period post‐ban (July 2006 to September 2008). A monthly average of 89% of inpatients were screened for tobacco use when admitted. The monthly average for the percentage of inpatients who currently smoke has been approximately 21.6% following the implementation of the smoke‐free hospital plan. There has been little variation (Figure 1) in the percentage of inpatients who smoke pre‐ban and post‐ban except for the startup period in 2006 and the onset of the 2007 respiratory illness season.

Among all inpatients who currently smoke, 69.8% received a brief nursing intervention at the time of admission and 25% received an inpatient visit from our part‐time smoking cessation specialist.
The percentage of inpatients who signed out against medical advice (AMA) with the reason of having to smoke was 13.8% (4/29) 6 months pre‐ban, and 13.6% (3/22) 6 months post‐ban. In 2007, there were no inpatients who signed out AMA stating that they needed to smoke. Because the reason for signing out AMA may be underreported, we also examined the rate of smoking among all inpatients who sign out AMA. Six months pre‐ban, this percentage was 48.3% (14/29), but increased 6 months post‐ban to 59% (13/22). In 2007, the percentage of smokers among inpatients who sign out AMA leveled off at 50.8% (29/57).
Review of computerized inpatient prescription orders shows that orders for NRT nearly tripled after the inpatient smoking cessation service started April 1, 2006 (3 months prior to the ban) (Figure 2). Inpatient orders for these medications increased from 832 in a 2‐year period before the ban (April 1, 2004 to March 31, 2006) to 2475 in the 2 years following the initiation of the inpatient smoking service (April 1, 2006 to March 31, 2008). The Chow test is highly significant for a break point in June 2006 (P = 0.008), 1 month prior to the ban.

Employee Smoking Rates
Among a cohort of 489 hospital‐based employees reporting in both 2005 and 2007, 12% reported smoking in 2005 and 7.5% in 2007 (McNemar was significant at P < 0.001). Two employees reported using chewing tobacco in 2005 and only 1 in 2007.
Including all hospital employees reporting any 1 year during their anniversary dates, the self‐reported smoking rates were 14.3% (n = 624) in March to June 2005, 14.8% (n = 661) in March to June 2006, and 9.4% (n = 1,112) in March to June 2007 (P < 0.0002). Because promotions change the anniversary date, and the database was expanded in 2007 to include new hires and managerial staff, these estimates represent the point prevalence among employees whose anniversary dates fall between March and June.
Discussion
Following implementation of a smoke‐free medical campus, no adverse effects were observed on inpatient volume at our hospital. The percentage of inpatients who smoke and the percentage of inpatients signing out AMA have remained stable after the smoke‐free policy went into effect. In addition, self‐reported employee smoking rates decreased significantly. Fears about losing inpatients (who smoke) following the implementation of a smoke‐free hospital plan were unfounded.
This study employs the electronic medical record to not only monitor trends in the proportion of inpatients who smoke pre‐ban and post‐ban, but also to notify our inpatient smoking cessation specialist, on the day of admission, to consult on patients who currently smoke. Unfortunately, our cessation specialist, who is part‐time, was unable to see all inpatients who smoke on account of the inpatient's acuity, pain, hospice status, weekend or night admission, or not being available due to testing, surgery, or other procedures. Nevertheless, use of NRT increased sharply following the initiation of this program. As shown in Figure 2, a linear rise in NRT orders was already underway starting April 2005, probably in anticipation of the ban and coinciding with the start of the inpatient smoking cessation program. However, the Chow test is highly significant for a breakpoint in June 2006 (P = 0.008), 1 month prior to the ban, meaning that the slope was climbing even more steeply after that point.
As hospitalized smokers may be more motivated to stop smoking, the updated 2008 clinical practice guidelines for Treating Tobacco Use and Dependence now recommend that all patients in the hospital be given medications, advised, counseled, and receive follow‐up after discharge.13 Although our inpatient cessation program was started before these clinical practice guidelines were available, we are currently evaluating the efficacy of our inpatient program by assessing self‐reported quit rates 6‐months posthospitalization (data collection in process). Provision of inpatient smoking cessation has been shown to be an effective smoking cessation intervention if combined with outpatient follow‐up.14 Our current program will be expanded to include outpatient follow‐up, if the inpatient's primary care provider is unable to provide it or if the inpatient refuses faxed referral to the New York State quit line program.
This study evaluates the impact of simultaneously introduced interventions such as medical campus smoking ban, inpatient smoking cessation program, hospital staff education, and other elements of the University of Michigan Smoke‐Free Hospital Implementation Plan. The role of individual components of the plan cannot be evaluated in this study as they were intentionally implemented simultaneously in order to achieve a synergistic effect.
Another limitation of this study is that smoking status is self‐reported and not validated biochemically. Although validated smoking status measures such as salivary cotinine testing would be more scientifically valid, it was not feasible to validate the smoking status of inpatients, nor that of employees. Thus smoking status, as ascertained in this study, is subject to underreporting. Social desirability bias has been recognized as potential limitation of self‐reported smoking status in other evaluations of smoke‐free policies.3, 4, 15
In the 1990s, the employee benefits of instituting indoor smoking bans in hospitals were theorized to include reduced employee sick time, break time, and tobacco use, as well as increased motivation for smoking cessation and reduced legitimacy of tobacco use.16, 17 Peer pressure, workplace socialization, and being forced to stay away from cigarettes for the length of entire workdays have been credited with helping hospital workers to quit.4, 7 In our study, extending the ban to the outdoor areas of our medical campus as well as provision of employee smoking cessation services may augment these mechanisms. This study extends findings of older studies that showed hospital smoking bans (primarily indoor) decreased hospital employee smoking rates. Currently, our reduced employee smoking rate approaches the Healthy People 2010 goal of 12%.18
In conclusion, implementing a smoke‐free medical campus does not adversely affect inpatient volume (even among smokers), does not increase inpatient signing out AMA and can significantly increase inpatient NRT use, which in turn can increase the success of a quit attempt.19 In addition, implementing an outdoor smoking ban further reduces hospital employee smoking rates.
Acknowledgements
The authors are grateful to the many Mary Imogene Bassett Hospital staff in administration, employee health, facilities management, human resources, inpatient pharmacy, medical education, patient care service, respiratory care, and security who provided policy support and/or data needed to evaluate policy implementation.
Even though imposition of smoke‐free policies and workplaces comprise one of the most effective antismoking strategies,1 hospital administrators hesitate to implement a smoke‐free medical campus policy.2 They fear losing patients who smoke because these patients will opt for other facilities that permit smoking.
Apart from studies evaluating Joint Commission on Accreditation of Healthcare Organizations (JCAHO)‐required indoor smoking bans in hospitals in 1992,3, 4 there are few published studies or formal evaluations of the impact of medical campuses going smoke‐free. One study of the implementation of a smoke‐free medical campus policy at a university hospital in Little Rock, AR, showed that the policy had no impact on employee retention, bed occupancy, or mean daily census; however, inpatient smoking status was not ascertained.5 Most (83%) employees were supportive of the policy. More importantly, employees at 2 university medical centers reported reduced cigarette consumption and increased attempts to quit after implementation of a smoke‐free medical campus policy.6, 7
Our hospital is 180‐bed, acute care inpatient teaching facility in upstate New York. Prior to the implementation of the smoke‐free medical campus policy, it was common to see employees, visitors, and patients lined up outdoors around the main hospital entrances and smoking just beyond the no smoking signage. Inpatients could look out their windows at the main entrance or into the courtyard and see hospital staff, other patients, and visitors smoking.
This study prospectively evaluates the impact of implementing the smoke‐free medical campus policy and starting an inpatient smoking cessation service. It addresses the following questions that have also been raised by the Task Force for Community Preventive Services.8 Does the institution of hospital smoking bans reduce the percentage of inpatients who smoke or increase the percentage who sign out against medical advice? What are the extended effects (beyond 1 year after implementation) of medical campus smoking bans on employee smoking rates?
Materials and Methods
Policy Implementation
As prior studies have shown that institution of a smoke‐free medical campus policy involves much more than just posting signage,9, 10 a detailed multidisciplinary work plan was implemented starting 1.5 years prior to the date our policy went into effect on July 1, 2006. The Implementing a Smoke‐Free Environment plan, produced by the University of Michigan,11 which includes a 15‐step checklist, was used to guide this policy change.12 As part of that plan, employees were offered on‐site smoking cessation services, including nicotine replacement therapy (NRT), and 150 employees participated in this program prior to July 1, 2006. Staff, community, and patient education was also completed. A new campus map delineating the smoke‐free border was disseminated. Signage was posted in areas used in the past for smoking. In addition to implementing this plan, an inpatient smoking cessation service was started 3 months prior to July 1, 2006. In addition to supporting the enforcement of the smoke‐free medical campus, our inpatient smoking cessation program was designed to help inpatients with nicotine withdrawal as well as smoking cessation, if they were ready to quit.
Data Collection and Analysis
The inpatient electronic medical record (EMR) was used to monitor the smoking status of patients admitted to hospital on a monthly basis. On admission to the hospital, the admitting nurse screened patients for current smoking status. This information was entered into the EMR starting in April 2006; therefore, pre‐ban screening data were limited to 2 months prior to the ban. Inpatients too sick to complete this screening process, women admitted for labor and delivery, and inpatients boarded in the emergency department were not screened. No identifiers were used in compiling these monthly data.
Nursing reports of inpatients signing out against medical advice (AMA) were compiled in order to compare incidence of AMA pre‐ban to post‐ban. AMA documentation in our hospital takes the form of a structured incident report that is reliably documented by nursing staff and signed by the attending physician of service.
Computerized inpatient doctors' orders to pharmacy for NRT, dispensed as gum or patch, were monitored 2 years preinitiation and postinitiation of the inpatient smoking cessation service on April 1, 2006. As varenicline was nonformulary and bupropion was used for other indications than smoking cessation, these medications were not included in this review. The Chow test was used to measure and test for significant breaks in a time series analysis of the NRT orders.
New York State law requires an annual occupational health review to be completed by every hospital employee. At our hospital, this review included a question on tobacco use Do you smoke or chew tobacco? Although there has been a smoker/nonsmoker differential in the rates offered for supplemental life insurance since 1992, there were no wellness credits or other incentives for medical insurance offered in employee benefits that may predispose employees to underreport tobacco use. Using this question, employees were categorized as self‐reported current smokers or chew users. Employee smoking rates were estimated using different denominators to validate the direction of the trend. First, self‐reported smoking rates were compared pre‐ban and post‐ban among a stable cohort of hospital employees (n = 489), defined as hospital‐based employees with anniversary dates from March to June who reported in both 2005 and 2007. The McNemar test was used to test the statistical significance of the 2 smoking rates of paired replicates in this stable cohort of employees reporting pre‐ban and post‐ban. Second, all employees in the database reporting smoking status pre‐ban, March to June 2005, and then post‐ban, March to June 2006 and 2007, were compared in order to monitor trends in employee smoking overall. A t‐test was used to compare the statistical significance of the difference in the overall rates of smoking among all employees pre‐ban and post‐ban.
Internal review boards of our hospital and the New York State Department of Health reviewed and approved this study.
Results
Inpatient Outcomes
An average of 959 patients were admitted per month in the 18‐month period pre‐ban (January 2005 to June 2006) vs. 988 per month in the 23‐month period post‐ban (July 2006 to September 2008). A monthly average of 89% of inpatients were screened for tobacco use when admitted. The monthly average for the percentage of inpatients who currently smoke has been approximately 21.6% following the implementation of the smoke‐free hospital plan. There has been little variation (Figure 1) in the percentage of inpatients who smoke pre‐ban and post‐ban except for the startup period in 2006 and the onset of the 2007 respiratory illness season.

Among all inpatients who currently smoke, 69.8% received a brief nursing intervention at the time of admission and 25% received an inpatient visit from our part‐time smoking cessation specialist.
The percentage of inpatients who signed out against medical advice (AMA) with the reason of having to smoke was 13.8% (4/29) 6 months pre‐ban, and 13.6% (3/22) 6 months post‐ban. In 2007, there were no inpatients who signed out AMA stating that they needed to smoke. Because the reason for signing out AMA may be underreported, we also examined the rate of smoking among all inpatients who sign out AMA. Six months pre‐ban, this percentage was 48.3% (14/29), but increased 6 months post‐ban to 59% (13/22). In 2007, the percentage of smokers among inpatients who sign out AMA leveled off at 50.8% (29/57).
Review of computerized inpatient prescription orders shows that orders for NRT nearly tripled after the inpatient smoking cessation service started April 1, 2006 (3 months prior to the ban) (Figure 2). Inpatient orders for these medications increased from 832 in a 2‐year period before the ban (April 1, 2004 to March 31, 2006) to 2475 in the 2 years following the initiation of the inpatient smoking service (April 1, 2006 to March 31, 2008). The Chow test is highly significant for a break point in June 2006 (P = 0.008), 1 month prior to the ban.

Employee Smoking Rates
Among a cohort of 489 hospital‐based employees reporting in both 2005 and 2007, 12% reported smoking in 2005 and 7.5% in 2007 (McNemar was significant at P < 0.001). Two employees reported using chewing tobacco in 2005 and only 1 in 2007.
Including all hospital employees reporting any 1 year during their anniversary dates, the self‐reported smoking rates were 14.3% (n = 624) in March to June 2005, 14.8% (n = 661) in March to June 2006, and 9.4% (n = 1,112) in March to June 2007 (P < 0.0002). Because promotions change the anniversary date, and the database was expanded in 2007 to include new hires and managerial staff, these estimates represent the point prevalence among employees whose anniversary dates fall between March and June.
Discussion
Following implementation of a smoke‐free medical campus, no adverse effects were observed on inpatient volume at our hospital. The percentage of inpatients who smoke and the percentage of inpatients signing out AMA have remained stable after the smoke‐free policy went into effect. In addition, self‐reported employee smoking rates decreased significantly. Fears about losing inpatients (who smoke) following the implementation of a smoke‐free hospital plan were unfounded.
This study employs the electronic medical record to not only monitor trends in the proportion of inpatients who smoke pre‐ban and post‐ban, but also to notify our inpatient smoking cessation specialist, on the day of admission, to consult on patients who currently smoke. Unfortunately, our cessation specialist, who is part‐time, was unable to see all inpatients who smoke on account of the inpatient's acuity, pain, hospice status, weekend or night admission, or not being available due to testing, surgery, or other procedures. Nevertheless, use of NRT increased sharply following the initiation of this program. As shown in Figure 2, a linear rise in NRT orders was already underway starting April 2005, probably in anticipation of the ban and coinciding with the start of the inpatient smoking cessation program. However, the Chow test is highly significant for a breakpoint in June 2006 (P = 0.008), 1 month prior to the ban, meaning that the slope was climbing even more steeply after that point.
As hospitalized smokers may be more motivated to stop smoking, the updated 2008 clinical practice guidelines for Treating Tobacco Use and Dependence now recommend that all patients in the hospital be given medications, advised, counseled, and receive follow‐up after discharge.13 Although our inpatient cessation program was started before these clinical practice guidelines were available, we are currently evaluating the efficacy of our inpatient program by assessing self‐reported quit rates 6‐months posthospitalization (data collection in process). Provision of inpatient smoking cessation has been shown to be an effective smoking cessation intervention if combined with outpatient follow‐up.14 Our current program will be expanded to include outpatient follow‐up, if the inpatient's primary care provider is unable to provide it or if the inpatient refuses faxed referral to the New York State quit line program.
This study evaluates the impact of simultaneously introduced interventions such as medical campus smoking ban, inpatient smoking cessation program, hospital staff education, and other elements of the University of Michigan Smoke‐Free Hospital Implementation Plan. The role of individual components of the plan cannot be evaluated in this study as they were intentionally implemented simultaneously in order to achieve a synergistic effect.
Another limitation of this study is that smoking status is self‐reported and not validated biochemically. Although validated smoking status measures such as salivary cotinine testing would be more scientifically valid, it was not feasible to validate the smoking status of inpatients, nor that of employees. Thus smoking status, as ascertained in this study, is subject to underreporting. Social desirability bias has been recognized as potential limitation of self‐reported smoking status in other evaluations of smoke‐free policies.3, 4, 15
In the 1990s, the employee benefits of instituting indoor smoking bans in hospitals were theorized to include reduced employee sick time, break time, and tobacco use, as well as increased motivation for smoking cessation and reduced legitimacy of tobacco use.16, 17 Peer pressure, workplace socialization, and being forced to stay away from cigarettes for the length of entire workdays have been credited with helping hospital workers to quit.4, 7 In our study, extending the ban to the outdoor areas of our medical campus as well as provision of employee smoking cessation services may augment these mechanisms. This study extends findings of older studies that showed hospital smoking bans (primarily indoor) decreased hospital employee smoking rates. Currently, our reduced employee smoking rate approaches the Healthy People 2010 goal of 12%.18
In conclusion, implementing a smoke‐free medical campus does not adversely affect inpatient volume (even among smokers), does not increase inpatient signing out AMA and can significantly increase inpatient NRT use, which in turn can increase the success of a quit attempt.19 In addition, implementing an outdoor smoking ban further reduces hospital employee smoking rates.
Acknowledgements
The authors are grateful to the many Mary Imogene Bassett Hospital staff in administration, employee health, facilities management, human resources, inpatient pharmacy, medical education, patient care service, respiratory care, and security who provided policy support and/or data needed to evaluate policy implementation.
- Institute of Medicine.Ending the Tobacco Problem: A Blueprint for the Nation.Washington, DC:National Academies Press;2007.
- ,.Smoke‐Free Hospital Campus Policies.Washington, DC,Advisory Board Original Inquiry Brief. 2/1/2005. Available at: http://www.roswellpark.org/files/1_2_1/prevention/3%20‐%20‐Advisory% 20Board%20smoke%20free%20policies.pdf. Accessed March 2009.
- ,,,,.Effects of the implementation of a smoke‐free policy in a medical center.Chest.1992;102:1531–1536.
- ,,, et al.Hospital smoking bans and employee smoking behavior: results of a national survey.JAMA.1996;275(16):1252–1257.
- ,,, et al.Impact of a smoke‐free hospital campus policy on employee and consumer behavior.Public Health Rep.2007;122(6):744–752.
- ,,,.Employee attitudes and smoking behavior at the City of Hope National Medical Center smoke–free campus.J Natl Compr Canc Netw.2006;4(6):535–542.
- ,.Effect of a total work‐site ban on employee smoking and attitudes.J Occup Med.1991;33(8):884–890.
- ,,, et al.Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke.Am J Prev Med.2001;20(2S):16–66.
- ,,.Smoking on hospital grounds and the impact of outdoor smoke‐free zones.Tob Control.1996;5:199–204.
- ,,,,.The making of a smoke free hospital may not be as easy as you think.Am J Prev Med.1991;7(4):214–218.
- University of Michigan Health System. Tobacco Consultation Service. Available at: http://www.med.umich.edu/mfit/tobacco/freeenvironment. htm. Accessed March2009.
- Michigan Health and Hospital Association. It's a matter of life and health: MHA campaign for smoke‐free hospitals. Available at: http://www. mhasmokefreecampus.org. Accessed March2009.
- Department of Health and Human Services (DHHS). Treating Tobacco Use and Dependence: 2008 Update. Chapter 7: Specific Populations and Other Topics. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi? rid=hstat2.section.28504. Accessed March2009.
- ,,.Interventions for smoking cessation in hospitalized patients.Cochrane Database Syst Rev.2007;(3):CD001837.
- ,,, et al.Ending smoking at the Johns Hopkins Medical Institutions: an evaluation of smoking prevalence and indoor air pollution.JAMA.1990;264:1565–1569.
- .Toward smoke‐free medical facilities.Chest.1990;97:1027–1028.
- .The benefits of smoke‐free health care campuses.Am Fam Physician.1994;49(1):28–33.
- U.S. Department of Health and Human Services.Healthy People 2010. Vol 12nd ed.Washington, DC:U.S. Department of Health and Human Services;2000.
- ,,,.Effectiveness of smoking cessation therapies: a systematic review and meta‐analysis.BMC Public Health.2006;6:300.
- Institute of Medicine.Ending the Tobacco Problem: A Blueprint for the Nation.Washington, DC:National Academies Press;2007.
- ,.Smoke‐Free Hospital Campus Policies.Washington, DC,Advisory Board Original Inquiry Brief. 2/1/2005. Available at: http://www.roswellpark.org/files/1_2_1/prevention/3%20‐%20‐Advisory% 20Board%20smoke%20free%20policies.pdf. Accessed March 2009.
- ,,,,.Effects of the implementation of a smoke‐free policy in a medical center.Chest.1992;102:1531–1536.
- ,,, et al.Hospital smoking bans and employee smoking behavior: results of a national survey.JAMA.1996;275(16):1252–1257.
- ,,, et al.Impact of a smoke‐free hospital campus policy on employee and consumer behavior.Public Health Rep.2007;122(6):744–752.
- ,,,.Employee attitudes and smoking behavior at the City of Hope National Medical Center smoke–free campus.J Natl Compr Canc Netw.2006;4(6):535–542.
- ,.Effect of a total work‐site ban on employee smoking and attitudes.J Occup Med.1991;33(8):884–890.
- ,,, et al.Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke.Am J Prev Med.2001;20(2S):16–66.
- ,,.Smoking on hospital grounds and the impact of outdoor smoke‐free zones.Tob Control.1996;5:199–204.
- ,,,,.The making of a smoke free hospital may not be as easy as you think.Am J Prev Med.1991;7(4):214–218.
- University of Michigan Health System. Tobacco Consultation Service. Available at: http://www.med.umich.edu/mfit/tobacco/freeenvironment. htm. Accessed March2009.
- Michigan Health and Hospital Association. It's a matter of life and health: MHA campaign for smoke‐free hospitals. Available at: http://www. mhasmokefreecampus.org. Accessed March2009.
- Department of Health and Human Services (DHHS). Treating Tobacco Use and Dependence: 2008 Update. Chapter 7: Specific Populations and Other Topics. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi? rid=hstat2.section.28504. Accessed March2009.
- ,,.Interventions for smoking cessation in hospitalized patients.Cochrane Database Syst Rev.2007;(3):CD001837.
- ,,, et al.Ending smoking at the Johns Hopkins Medical Institutions: an evaluation of smoking prevalence and indoor air pollution.JAMA.1990;264:1565–1569.
- .Toward smoke‐free medical facilities.Chest.1990;97:1027–1028.
- .The benefits of smoke‐free health care campuses.Am Fam Physician.1994;49(1):28–33.
- U.S. Department of Health and Human Services.Healthy People 2010. Vol 12nd ed.Washington, DC:U.S. Department of Health and Human Services;2000.
- ,,,.Effectiveness of smoking cessation therapies: a systematic review and meta‐analysis.BMC Public Health.2006;6:300.