User login
Thyroid nodules: When is an aggressive evaluation warranted?
• Suspect malignancy if a patient with a thyroid nodule also exhibits hoarseness, persistent lymphadenopathy, or dysphagia. C
• Direct your evaluation toward hyperthyroidism instead of malignancy if the level of thyroid stimulating hormone is <0.5 μIU/mL. B
• Arrange for ultrasound imaging when there is a need to assess the size, consistency, and additional features of a nodule. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
You detect a solitary thyroid nodule during a patient’s annual physical examination. He’s 50 years old, in generally good health, and has no symptoms suggestive of thyroid disease. How far would you go in your investigation?
Thyroid nodules are fairly prevalent in the United States. Although the estimated prevalence of palpable thyroid nodules is 5%,1 autopsy studies show the prevalence of all nodules to be 49% to 57%.2 Ultrasound studies of asymptomatic individuals have reported incidental detection of thyroid nodules in 35% to 67% of patients,3-5 and similar findings occur with other imaging modalities.
The main concerns with thyroid nodules are malignancy and hyperactivity. The good news is that both palpable and nonpalpable nodules carry just a 5% risk of malignancy.6 Thus, it makes sense to limit screening of nodules to individuals at high risk—eg, males, those who are younger than 30 or older than 60 years, and patients who have had radiation treatment to the head and neck, have a family history of thyroid cancer or multiple endocrine neoplasia, or have had rapid growth of a nodule and associated lymphadenopathy.6
For the estimated 300,000 new nodules detected annually in the United States,7 we present a cost-effective approach to optimal diagnostic evaluation (ALGORITHM), reflecting guidelines issued by the American Thyroid Association and the American Association of Clinical Endocrinologists.6,8
ALGORITHM
Recommended evaluation of a thyroid nodule6,8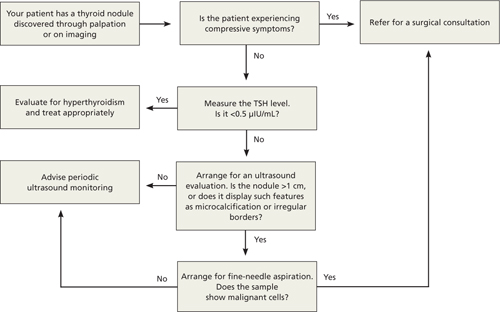
How signs and symptoms can direct your investigation
Most thyroid nodules are asymptomatic and are discovered during physical evaluation or during neck imaging for unrelated reasons. When symptoms occur, they are due either to hormonal dysfunction (hyper- or hypothyroidism) or mechanical compression.
Signs and symptoms of hyperthyroidism—eg, weight loss, tachycardia, irritability, sweating—suggest a “hot” nodule, which has very low malignancy potential.6
A sudden increase in nodule size accompanied by pain usually signifies acute hemorrhage into a cystic lesion. But because some cystic lesions also exhibit solid components, malignancy is a possibility.
Hoarseness, persistent lymphadenopathy, and dysphagia signal possible malignancy and should prompt aggressive evaluation.
Laboratory testing: Recommendations and cautions
Obtain a measurement of serum thyroid-stimulating hormone (TSH).6 A suppressed level (<0.5 μIU/mL) is found in about 10% of patients with a solitary nodule9; it significantly decreases the likelihood of thyroid malignancy10 and should redirect efforts toward uncovering a nonmalignant cause of hyperactivity. On the other hand, a normal or elevated TSH level does not preclude the presence of malignancy.
If the TSH level is normal, no additional information is gained by measuring thyroid hormone levels, serum thyroid autoantibodies titers (antithyroglobulin, antithyroid peroxidase), or serum thyroglobulin.
Routine measurement of serum calcitonin has been proposed as a cost-effective screen for medullary thyroid carcinoma11 in individuals at high risk, such as those with a family history of multiple endocrine neoplasia.12 However, there is no clear recommendation for its use in screening all patients with thyroid nodules.6
Imaging: Generally limit to ultrasound
Ultrasound is the best imaging modality for evaluating the size and consistency (cystic vs solid) of the thyroid nodule.13 It can also detect microcalcifications, irregular margins, lymphadenopathy, and intranodular vascularity—features suggestive of malignancy, although not confirmatory. The ultrasound finding that probably correlates most strongly with benignity is a predominantly cystic lesion.14
I123 radioactive iodine uptake and scanning is not recommended for the routine evaluation of thyroid nodules. Its role is limited to cases with suppressed TSH, as only 5% of all thyroid nodules are “hot.”15 Computed tomography, magnetic resonance imaging, 18fluorodeoxyglucose positron emission tomography (18FDG-PET), and sestamibi scans are not cost effective in the work-up of thyroid nodules, although thyroid lesions commonly appear on these scans when they are obtained for other reasons. In particular, 18FDG-PET and sestamibi scans assess function rather than anatomy, and a finding of thyroid “incidentaloma” often leads to confusion regarding its clinical significance. Multiple reports have, however, suggested an increased incidence of cancer in 18FDG-PET-avid thyroid lesions (14%-30%)16-18; should a patient undergo this procedure and exhibit such a lesion, further investigation by fine-needle aspiration (FNA) is warranted.18,19 The significance of a positive sestamibi scan for a thyroid lesion appears to be more controversial, with conflicting reports about its ability to predict thyroid malignancy.20-22
Who is a candidate for fine-needle aspiration?
FNA is unequivocally the most cost-effective tool for establishing the benignity or malignancy of a thyroid nodule. Its estimated sensitivity is 83%, specificity is 92%, and positive predictive value is 75%.7 Selecting nodules and biopsy technique appropriately can decrease sampling error. Nodules that are purely cystic and those that appear “hot” on radioactive iodine scanning do not require sampling. Neither do nodules <1 cm in diameter that lack features associated with malignancy. However, any nodule >5 mm in diameter with high-risk or otherwise suspicious features (eg, microcalcifications, irregular margins) on ultrasound are candidates for FNA.6
With the exception of easily palpable nodules, ultrasound imaging is widely used to guide biopsy (UG-FNA) so as to minimize the rate of nondiagnostic samples. Historically, less than 10% of FNA results are malignant and 60% to 80% are confirmed benign.6
Management decisions
Confirmation of a benign nodule obviates the need for surgical resection, although follow-up is required with serial ultrasound evaluations to assess any increase in size. Slow growth is the natural history of thyroid nodules,23 and there are no clear data to indicate a rate or degree of growth suggestive of malignancy.24 Order a repeat ultrasound examination 6 to 18 months after the initial evaluation. Should a nodule show more than 50% change in volume (20% change in 2 dimensions), consider referring for UG-FNA6 or surgical resection.7 Referral for surgical resection is also warranted if compressive symptoms occur despite a nodule’s benign nature. A meta-analysis of the use of levothyroxine supplementation to prevent nodular growth did not show a statistically significant effect, although a trend toward shrinkage was noted.25
A clear diagnosis of malignancy on FNA necessitates a surgical referral for total thyroidectomy, as well as an endocrinology consultation for possible postoperative radioactive iodine remnant ablation and thyroid hormone replacement. Suspicious cytology results and follicular lesions in the presence of normal thyroid function usually require surgical resection due to high rates of malignancy confirmed postoperatively (60% and 20%, respectively).6
If the FNA result is nondiagnostic on the first attempt, a second attempt is warranted, preferably under ultrasound guidance (UG-FNA) to improve yield. A second nondiagnostic sample is an indication for surgical resection. Up to 12% of such scenarios lead to histologically confirmed malignancy postoperatively.6
Radioactive iodine therapy is commonly used to treat hyperfunctioning “hot” nodules, yielding a rate of return to normal function between 85% and 100%, and a median reduction in size of 45% at 2 years.6 Using radioactive iodine to treat nodular disease in euthyroid individuals with normal uptake on a scan had variable results, with 20% of patients having no change in nodule size and 80% having up to a 60% decrease in size at 5 years.6 Large-scale studies are needed to compare radioactive iodine therapy with surgical resection.
CORRESPONDENCE Armand Krikorian, MD, Division of Clinical and Molecular Endocrinology, Case Western Reserve University, 11100 Euclid Avenue, Cleveland, OH 44106; armand.krikorian@UHhospitals.org
1. Singer PA, Cooper DS, Daniels GH, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med. 1996;156:2165-2172.
2. Pinchera A. Thyroid incidentalomas. Horm Res. 2007;68(suppl 5):199-201.
3. Brander A, Viikinkoski P, Nickels J, et al. Thyroid gland: US screening in middle-aged women with no previous thyroid disease. Radiology. 1989;173:507-510.
4. Ezzat S, Sarti DA, Cain DR, et al. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838-1840.
5. Guth S, Theune U, Aberle J, et al. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699-706.
6. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2010;16(suppl 1):1-43.
7. Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007;36:707-735, vi.
8. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
9. Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am. 2010;43:229-238, vii.
10. Fiore E, Rago T, Provenzale MA, et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16:1251-1260.
11. Cheung K, Roman SA, Wang TS, et al. Calcitonin measurement in the evaluation of thyroid nodules in the United States: a cost-effectiveness and decision analysis. J Clin Endocr Metab. 2008;93:2173-2180.
12. Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658-5671.
13. Solbiati L, Osti V, Cova L, et al. Ultrasound of thyroid, parathyroid glands and neck lymph nodes. Eur Radiol. 2001;11:2411-2424.
14. Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411-3417.
15. Cases JA, Surks MI. The changing role of scintigraphy in the evaluation of thyroid nodules. Semin Nucl Med. 2000;30:81-87.
16. Chen YK, Ding HJ, Chen KT, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for cancer screening in healthy subjects. Anticancer Res. 2005;25:1421-1426.
17. Choi JY, Lee KS, Kim HJ, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47:609-615.
18. Shie P, Cardarelli R, Sprawls K, et al. Systematic review: prevalence of malignant incidental thyroid nodules identified on fluorine-18 fluorodeoxyglucose positron emission tomography. Nucl Med Commun. 2009;30:742-748.
19. Cibas ES. Fine-needle aspiration in the work-up of thyroid nodules. Otolaryngol Clin North Am. 2010;43:257-271, vii-viii.
20. Giovanella L, Suriano S, Maffioli M, et al. (99m)Tc-sestamibi scanning in thyroid nodules with nondiagnostic cytology. Head Neck. 2010;32:607-611.
21. Kresnik E, Gallowitsch HJ, Mikosch P, et al. Technetium-99m-MIBI scintigraphy of thyroid nodules in an endemic goiter area. J Nucl Med. 1997;38:62-65.
22. Sathekge MM, Mageza RB, Muthuphei MN, et al. Evaluation of thyroid nodules with technetium-99m MIBI and technetium-99m pertechnetate. Head Neck. 2001;23:305-310.
23. Alexander EK, Hurwitz S, Heering JP, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003;138:315-318.
24. Asanuma K, Kobayashi S, Shingu K, et al. The rate of tumour growth does not distinguish between malignant and benign thyroid nodules. Eur J Surg. 2001;167:102-105.
25. Castro MR, Caraballo PJ, Morris JC. Effectiveness of thyroid hormone suppressive therapy in benign solitary thyroid nodules: a meta-analysis. J Clin Endocrinol Metab. 2002;87:4154-4159.
• Suspect malignancy if a patient with a thyroid nodule also exhibits hoarseness, persistent lymphadenopathy, or dysphagia. C
• Direct your evaluation toward hyperthyroidism instead of malignancy if the level of thyroid stimulating hormone is <0.5 μIU/mL. B
• Arrange for ultrasound imaging when there is a need to assess the size, consistency, and additional features of a nodule. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
You detect a solitary thyroid nodule during a patient’s annual physical examination. He’s 50 years old, in generally good health, and has no symptoms suggestive of thyroid disease. How far would you go in your investigation?
Thyroid nodules are fairly prevalent in the United States. Although the estimated prevalence of palpable thyroid nodules is 5%,1 autopsy studies show the prevalence of all nodules to be 49% to 57%.2 Ultrasound studies of asymptomatic individuals have reported incidental detection of thyroid nodules in 35% to 67% of patients,3-5 and similar findings occur with other imaging modalities.
The main concerns with thyroid nodules are malignancy and hyperactivity. The good news is that both palpable and nonpalpable nodules carry just a 5% risk of malignancy.6 Thus, it makes sense to limit screening of nodules to individuals at high risk—eg, males, those who are younger than 30 or older than 60 years, and patients who have had radiation treatment to the head and neck, have a family history of thyroid cancer or multiple endocrine neoplasia, or have had rapid growth of a nodule and associated lymphadenopathy.6
For the estimated 300,000 new nodules detected annually in the United States,7 we present a cost-effective approach to optimal diagnostic evaluation (ALGORITHM), reflecting guidelines issued by the American Thyroid Association and the American Association of Clinical Endocrinologists.6,8
ALGORITHM
Recommended evaluation of a thyroid nodule6,8
How signs and symptoms can direct your investigation
Most thyroid nodules are asymptomatic and are discovered during physical evaluation or during neck imaging for unrelated reasons. When symptoms occur, they are due either to hormonal dysfunction (hyper- or hypothyroidism) or mechanical compression.
Signs and symptoms of hyperthyroidism—eg, weight loss, tachycardia, irritability, sweating—suggest a “hot” nodule, which has very low malignancy potential.6
A sudden increase in nodule size accompanied by pain usually signifies acute hemorrhage into a cystic lesion. But because some cystic lesions also exhibit solid components, malignancy is a possibility.
Hoarseness, persistent lymphadenopathy, and dysphagia signal possible malignancy and should prompt aggressive evaluation.
Laboratory testing: Recommendations and cautions
Obtain a measurement of serum thyroid-stimulating hormone (TSH).6 A suppressed level (<0.5 μIU/mL) is found in about 10% of patients with a solitary nodule9; it significantly decreases the likelihood of thyroid malignancy10 and should redirect efforts toward uncovering a nonmalignant cause of hyperactivity. On the other hand, a normal or elevated TSH level does not preclude the presence of malignancy.
If the TSH level is normal, no additional information is gained by measuring thyroid hormone levels, serum thyroid autoantibodies titers (antithyroglobulin, antithyroid peroxidase), or serum thyroglobulin.
Routine measurement of serum calcitonin has been proposed as a cost-effective screen for medullary thyroid carcinoma11 in individuals at high risk, such as those with a family history of multiple endocrine neoplasia.12 However, there is no clear recommendation for its use in screening all patients with thyroid nodules.6
Imaging: Generally limit to ultrasound
Ultrasound is the best imaging modality for evaluating the size and consistency (cystic vs solid) of the thyroid nodule.13 It can also detect microcalcifications, irregular margins, lymphadenopathy, and intranodular vascularity—features suggestive of malignancy, although not confirmatory. The ultrasound finding that probably correlates most strongly with benignity is a predominantly cystic lesion.14
I123 radioactive iodine uptake and scanning is not recommended for the routine evaluation of thyroid nodules. Its role is limited to cases with suppressed TSH, as only 5% of all thyroid nodules are “hot.”15 Computed tomography, magnetic resonance imaging, 18fluorodeoxyglucose positron emission tomography (18FDG-PET), and sestamibi scans are not cost effective in the work-up of thyroid nodules, although thyroid lesions commonly appear on these scans when they are obtained for other reasons. In particular, 18FDG-PET and sestamibi scans assess function rather than anatomy, and a finding of thyroid “incidentaloma” often leads to confusion regarding its clinical significance. Multiple reports have, however, suggested an increased incidence of cancer in 18FDG-PET-avid thyroid lesions (14%-30%)16-18; should a patient undergo this procedure and exhibit such a lesion, further investigation by fine-needle aspiration (FNA) is warranted.18,19 The significance of a positive sestamibi scan for a thyroid lesion appears to be more controversial, with conflicting reports about its ability to predict thyroid malignancy.20-22
Who is a candidate for fine-needle aspiration?
FNA is unequivocally the most cost-effective tool for establishing the benignity or malignancy of a thyroid nodule. Its estimated sensitivity is 83%, specificity is 92%, and positive predictive value is 75%.7 Selecting nodules and biopsy technique appropriately can decrease sampling error. Nodules that are purely cystic and those that appear “hot” on radioactive iodine scanning do not require sampling. Neither do nodules <1 cm in diameter that lack features associated with malignancy. However, any nodule >5 mm in diameter with high-risk or otherwise suspicious features (eg, microcalcifications, irregular margins) on ultrasound are candidates for FNA.6
With the exception of easily palpable nodules, ultrasound imaging is widely used to guide biopsy (UG-FNA) so as to minimize the rate of nondiagnostic samples. Historically, less than 10% of FNA results are malignant and 60% to 80% are confirmed benign.6
Management decisions
Confirmation of a benign nodule obviates the need for surgical resection, although follow-up is required with serial ultrasound evaluations to assess any increase in size. Slow growth is the natural history of thyroid nodules,23 and there are no clear data to indicate a rate or degree of growth suggestive of malignancy.24 Order a repeat ultrasound examination 6 to 18 months after the initial evaluation. Should a nodule show more than 50% change in volume (20% change in 2 dimensions), consider referring for UG-FNA6 or surgical resection.7 Referral for surgical resection is also warranted if compressive symptoms occur despite a nodule’s benign nature. A meta-analysis of the use of levothyroxine supplementation to prevent nodular growth did not show a statistically significant effect, although a trend toward shrinkage was noted.25
A clear diagnosis of malignancy on FNA necessitates a surgical referral for total thyroidectomy, as well as an endocrinology consultation for possible postoperative radioactive iodine remnant ablation and thyroid hormone replacement. Suspicious cytology results and follicular lesions in the presence of normal thyroid function usually require surgical resection due to high rates of malignancy confirmed postoperatively (60% and 20%, respectively).6
If the FNA result is nondiagnostic on the first attempt, a second attempt is warranted, preferably under ultrasound guidance (UG-FNA) to improve yield. A second nondiagnostic sample is an indication for surgical resection. Up to 12% of such scenarios lead to histologically confirmed malignancy postoperatively.6
Radioactive iodine therapy is commonly used to treat hyperfunctioning “hot” nodules, yielding a rate of return to normal function between 85% and 100%, and a median reduction in size of 45% at 2 years.6 Using radioactive iodine to treat nodular disease in euthyroid individuals with normal uptake on a scan had variable results, with 20% of patients having no change in nodule size and 80% having up to a 60% decrease in size at 5 years.6 Large-scale studies are needed to compare radioactive iodine therapy with surgical resection.
CORRESPONDENCE Armand Krikorian, MD, Division of Clinical and Molecular Endocrinology, Case Western Reserve University, 11100 Euclid Avenue, Cleveland, OH 44106; armand.krikorian@UHhospitals.org
• Suspect malignancy if a patient with a thyroid nodule also exhibits hoarseness, persistent lymphadenopathy, or dysphagia. C
• Direct your evaluation toward hyperthyroidism instead of malignancy if the level of thyroid stimulating hormone is <0.5 μIU/mL. B
• Arrange for ultrasound imaging when there is a need to assess the size, consistency, and additional features of a nodule. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
You detect a solitary thyroid nodule during a patient’s annual physical examination. He’s 50 years old, in generally good health, and has no symptoms suggestive of thyroid disease. How far would you go in your investigation?
Thyroid nodules are fairly prevalent in the United States. Although the estimated prevalence of palpable thyroid nodules is 5%,1 autopsy studies show the prevalence of all nodules to be 49% to 57%.2 Ultrasound studies of asymptomatic individuals have reported incidental detection of thyroid nodules in 35% to 67% of patients,3-5 and similar findings occur with other imaging modalities.
The main concerns with thyroid nodules are malignancy and hyperactivity. The good news is that both palpable and nonpalpable nodules carry just a 5% risk of malignancy.6 Thus, it makes sense to limit screening of nodules to individuals at high risk—eg, males, those who are younger than 30 or older than 60 years, and patients who have had radiation treatment to the head and neck, have a family history of thyroid cancer or multiple endocrine neoplasia, or have had rapid growth of a nodule and associated lymphadenopathy.6
For the estimated 300,000 new nodules detected annually in the United States,7 we present a cost-effective approach to optimal diagnostic evaluation (ALGORITHM), reflecting guidelines issued by the American Thyroid Association and the American Association of Clinical Endocrinologists.6,8
ALGORITHM
Recommended evaluation of a thyroid nodule6,8
How signs and symptoms can direct your investigation
Most thyroid nodules are asymptomatic and are discovered during physical evaluation or during neck imaging for unrelated reasons. When symptoms occur, they are due either to hormonal dysfunction (hyper- or hypothyroidism) or mechanical compression.
Signs and symptoms of hyperthyroidism—eg, weight loss, tachycardia, irritability, sweating—suggest a “hot” nodule, which has very low malignancy potential.6
A sudden increase in nodule size accompanied by pain usually signifies acute hemorrhage into a cystic lesion. But because some cystic lesions also exhibit solid components, malignancy is a possibility.
Hoarseness, persistent lymphadenopathy, and dysphagia signal possible malignancy and should prompt aggressive evaluation.
Laboratory testing: Recommendations and cautions
Obtain a measurement of serum thyroid-stimulating hormone (TSH).6 A suppressed level (<0.5 μIU/mL) is found in about 10% of patients with a solitary nodule9; it significantly decreases the likelihood of thyroid malignancy10 and should redirect efforts toward uncovering a nonmalignant cause of hyperactivity. On the other hand, a normal or elevated TSH level does not preclude the presence of malignancy.
If the TSH level is normal, no additional information is gained by measuring thyroid hormone levels, serum thyroid autoantibodies titers (antithyroglobulin, antithyroid peroxidase), or serum thyroglobulin.
Routine measurement of serum calcitonin has been proposed as a cost-effective screen for medullary thyroid carcinoma11 in individuals at high risk, such as those with a family history of multiple endocrine neoplasia.12 However, there is no clear recommendation for its use in screening all patients with thyroid nodules.6
Imaging: Generally limit to ultrasound
Ultrasound is the best imaging modality for evaluating the size and consistency (cystic vs solid) of the thyroid nodule.13 It can also detect microcalcifications, irregular margins, lymphadenopathy, and intranodular vascularity—features suggestive of malignancy, although not confirmatory. The ultrasound finding that probably correlates most strongly with benignity is a predominantly cystic lesion.14
I123 radioactive iodine uptake and scanning is not recommended for the routine evaluation of thyroid nodules. Its role is limited to cases with suppressed TSH, as only 5% of all thyroid nodules are “hot.”15 Computed tomography, magnetic resonance imaging, 18fluorodeoxyglucose positron emission tomography (18FDG-PET), and sestamibi scans are not cost effective in the work-up of thyroid nodules, although thyroid lesions commonly appear on these scans when they are obtained for other reasons. In particular, 18FDG-PET and sestamibi scans assess function rather than anatomy, and a finding of thyroid “incidentaloma” often leads to confusion regarding its clinical significance. Multiple reports have, however, suggested an increased incidence of cancer in 18FDG-PET-avid thyroid lesions (14%-30%)16-18; should a patient undergo this procedure and exhibit such a lesion, further investigation by fine-needle aspiration (FNA) is warranted.18,19 The significance of a positive sestamibi scan for a thyroid lesion appears to be more controversial, with conflicting reports about its ability to predict thyroid malignancy.20-22
Who is a candidate for fine-needle aspiration?
FNA is unequivocally the most cost-effective tool for establishing the benignity or malignancy of a thyroid nodule. Its estimated sensitivity is 83%, specificity is 92%, and positive predictive value is 75%.7 Selecting nodules and biopsy technique appropriately can decrease sampling error. Nodules that are purely cystic and those that appear “hot” on radioactive iodine scanning do not require sampling. Neither do nodules <1 cm in diameter that lack features associated with malignancy. However, any nodule >5 mm in diameter with high-risk or otherwise suspicious features (eg, microcalcifications, irregular margins) on ultrasound are candidates for FNA.6
With the exception of easily palpable nodules, ultrasound imaging is widely used to guide biopsy (UG-FNA) so as to minimize the rate of nondiagnostic samples. Historically, less than 10% of FNA results are malignant and 60% to 80% are confirmed benign.6
Management decisions
Confirmation of a benign nodule obviates the need for surgical resection, although follow-up is required with serial ultrasound evaluations to assess any increase in size. Slow growth is the natural history of thyroid nodules,23 and there are no clear data to indicate a rate or degree of growth suggestive of malignancy.24 Order a repeat ultrasound examination 6 to 18 months after the initial evaluation. Should a nodule show more than 50% change in volume (20% change in 2 dimensions), consider referring for UG-FNA6 or surgical resection.7 Referral for surgical resection is also warranted if compressive symptoms occur despite a nodule’s benign nature. A meta-analysis of the use of levothyroxine supplementation to prevent nodular growth did not show a statistically significant effect, although a trend toward shrinkage was noted.25
A clear diagnosis of malignancy on FNA necessitates a surgical referral for total thyroidectomy, as well as an endocrinology consultation for possible postoperative radioactive iodine remnant ablation and thyroid hormone replacement. Suspicious cytology results and follicular lesions in the presence of normal thyroid function usually require surgical resection due to high rates of malignancy confirmed postoperatively (60% and 20%, respectively).6
If the FNA result is nondiagnostic on the first attempt, a second attempt is warranted, preferably under ultrasound guidance (UG-FNA) to improve yield. A second nondiagnostic sample is an indication for surgical resection. Up to 12% of such scenarios lead to histologically confirmed malignancy postoperatively.6
Radioactive iodine therapy is commonly used to treat hyperfunctioning “hot” nodules, yielding a rate of return to normal function between 85% and 100%, and a median reduction in size of 45% at 2 years.6 Using radioactive iodine to treat nodular disease in euthyroid individuals with normal uptake on a scan had variable results, with 20% of patients having no change in nodule size and 80% having up to a 60% decrease in size at 5 years.6 Large-scale studies are needed to compare radioactive iodine therapy with surgical resection.
CORRESPONDENCE Armand Krikorian, MD, Division of Clinical and Molecular Endocrinology, Case Western Reserve University, 11100 Euclid Avenue, Cleveland, OH 44106; armand.krikorian@UHhospitals.org
1. Singer PA, Cooper DS, Daniels GH, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med. 1996;156:2165-2172.
2. Pinchera A. Thyroid incidentalomas. Horm Res. 2007;68(suppl 5):199-201.
3. Brander A, Viikinkoski P, Nickels J, et al. Thyroid gland: US screening in middle-aged women with no previous thyroid disease. Radiology. 1989;173:507-510.
4. Ezzat S, Sarti DA, Cain DR, et al. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838-1840.
5. Guth S, Theune U, Aberle J, et al. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699-706.
6. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2010;16(suppl 1):1-43.
7. Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007;36:707-735, vi.
8. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
9. Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am. 2010;43:229-238, vii.
10. Fiore E, Rago T, Provenzale MA, et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16:1251-1260.
11. Cheung K, Roman SA, Wang TS, et al. Calcitonin measurement in the evaluation of thyroid nodules in the United States: a cost-effectiveness and decision analysis. J Clin Endocr Metab. 2008;93:2173-2180.
12. Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658-5671.
13. Solbiati L, Osti V, Cova L, et al. Ultrasound of thyroid, parathyroid glands and neck lymph nodes. Eur Radiol. 2001;11:2411-2424.
14. Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411-3417.
15. Cases JA, Surks MI. The changing role of scintigraphy in the evaluation of thyroid nodules. Semin Nucl Med. 2000;30:81-87.
16. Chen YK, Ding HJ, Chen KT, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for cancer screening in healthy subjects. Anticancer Res. 2005;25:1421-1426.
17. Choi JY, Lee KS, Kim HJ, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47:609-615.
18. Shie P, Cardarelli R, Sprawls K, et al. Systematic review: prevalence of malignant incidental thyroid nodules identified on fluorine-18 fluorodeoxyglucose positron emission tomography. Nucl Med Commun. 2009;30:742-748.
19. Cibas ES. Fine-needle aspiration in the work-up of thyroid nodules. Otolaryngol Clin North Am. 2010;43:257-271, vii-viii.
20. Giovanella L, Suriano S, Maffioli M, et al. (99m)Tc-sestamibi scanning in thyroid nodules with nondiagnostic cytology. Head Neck. 2010;32:607-611.
21. Kresnik E, Gallowitsch HJ, Mikosch P, et al. Technetium-99m-MIBI scintigraphy of thyroid nodules in an endemic goiter area. J Nucl Med. 1997;38:62-65.
22. Sathekge MM, Mageza RB, Muthuphei MN, et al. Evaluation of thyroid nodules with technetium-99m MIBI and technetium-99m pertechnetate. Head Neck. 2001;23:305-310.
23. Alexander EK, Hurwitz S, Heering JP, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003;138:315-318.
24. Asanuma K, Kobayashi S, Shingu K, et al. The rate of tumour growth does not distinguish between malignant and benign thyroid nodules. Eur J Surg. 2001;167:102-105.
25. Castro MR, Caraballo PJ, Morris JC. Effectiveness of thyroid hormone suppressive therapy in benign solitary thyroid nodules: a meta-analysis. J Clin Endocrinol Metab. 2002;87:4154-4159.
1. Singer PA, Cooper DS, Daniels GH, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med. 1996;156:2165-2172.
2. Pinchera A. Thyroid incidentalomas. Horm Res. 2007;68(suppl 5):199-201.
3. Brander A, Viikinkoski P, Nickels J, et al. Thyroid gland: US screening in middle-aged women with no previous thyroid disease. Radiology. 1989;173:507-510.
4. Ezzat S, Sarti DA, Cain DR, et al. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838-1840.
5. Guth S, Theune U, Aberle J, et al. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699-706.
6. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2010;16(suppl 1):1-43.
7. Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007;36:707-735, vi.
8. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214.
9. Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am. 2010;43:229-238, vii.
10. Fiore E, Rago T, Provenzale MA, et al. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16:1251-1260.
11. Cheung K, Roman SA, Wang TS, et al. Calcitonin measurement in the evaluation of thyroid nodules in the United States: a cost-effectiveness and decision analysis. J Clin Endocr Metab. 2008;93:2173-2180.
12. Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86:5658-5671.
13. Solbiati L, Osti V, Cova L, et al. Ultrasound of thyroid, parathyroid glands and neck lymph nodes. Eur Radiol. 2001;11:2411-2424.
14. Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411-3417.
15. Cases JA, Surks MI. The changing role of scintigraphy in the evaluation of thyroid nodules. Semin Nucl Med. 2000;30:81-87.
16. Chen YK, Ding HJ, Chen KT, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for cancer screening in healthy subjects. Anticancer Res. 2005;25:1421-1426.
17. Choi JY, Lee KS, Kim HJ, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47:609-615.
18. Shie P, Cardarelli R, Sprawls K, et al. Systematic review: prevalence of malignant incidental thyroid nodules identified on fluorine-18 fluorodeoxyglucose positron emission tomography. Nucl Med Commun. 2009;30:742-748.
19. Cibas ES. Fine-needle aspiration in the work-up of thyroid nodules. Otolaryngol Clin North Am. 2010;43:257-271, vii-viii.
20. Giovanella L, Suriano S, Maffioli M, et al. (99m)Tc-sestamibi scanning in thyroid nodules with nondiagnostic cytology. Head Neck. 2010;32:607-611.
21. Kresnik E, Gallowitsch HJ, Mikosch P, et al. Technetium-99m-MIBI scintigraphy of thyroid nodules in an endemic goiter area. J Nucl Med. 1997;38:62-65.
22. Sathekge MM, Mageza RB, Muthuphei MN, et al. Evaluation of thyroid nodules with technetium-99m MIBI and technetium-99m pertechnetate. Head Neck. 2001;23:305-310.
23. Alexander EK, Hurwitz S, Heering JP, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003;138:315-318.
24. Asanuma K, Kobayashi S, Shingu K, et al. The rate of tumour growth does not distinguish between malignant and benign thyroid nodules. Eur J Surg. 2001;167:102-105.
25. Castro MR, Caraballo PJ, Morris JC. Effectiveness of thyroid hormone suppressive therapy in benign solitary thyroid nodules: a meta-analysis. J Clin Endocrinol Metab. 2002;87:4154-4159.