User login
Discrepant Advanced Directives and Code Status Orders: A Preventable Medical Error
The United States health system has been criticized for its overuse of aggressive and medically ineffective life-sustaining therapies (LST).1 Some professional societies have elevated dialog about end-of-life (EOL) care to a quality measure,2 expecting that more open discussion will achieve more “goal-concordant care”3 and appropriate use of LST. However, even when Advanced Directives (AD) or Physician Orders for Life-Sustaining Therapy (POLST) have been created, their directions are not always followed in the hospital. This perspective discusses how preventable errors allow for use of LST even when patients designated it as unwanted. Two cases, chosen from several similar ones, are highlighted, demonstrating both human and system errors.
During the time of these events, the hospital policy required admission orders to contain a “code status” designation in the electronic medical record (EMR). All active and historical code status orders were listed chronologically and all AD and POLST documents were scanned into a special section of the EMR. Hospital policy, consistent with professional society guidelines,4,5 stated that patients with AD/POLST limiting EOL support should have individualized discussion about resuscitation options in the event of a periprocedural critical event. Automatic suspension or reinstatement of limited code orders was not permitted.
CASE 1
A 62-year-old woman with refractory heart failure was admitted with recurrence. The admitting code order was “initiate CPR/intubation” even though a POLST order written 10 months earlier indicating “do not intubate” was visible in the EMR. A more recent POLST indicating “No CPR/No intubation” accompanied the patient in the ambulance and was placed at bedside, but not scanned. There was no documented discussion of code status that might have explained the POLST/code order disparity. Notably, during two prior admissions within the year, “full code” orders had also been placed. On the fifth hospital day, the patient was found in respiratory distress and unresponsive. A “code” was called. ICU staff, after confirming full code status, intubated the patient emergently and commenced other invasive ICU interventions. Family members brought the preexisting POLST to medical attention within hours of the code but could not agree on immediate extubation. Over the next week, multiple prognosis discussions were held with the patient (when responsive) and family. Ultimately, the patient failed to improve and indicated a desire to be extubated, dying a few hours later.
CASE 2
A 94-year-old woman was admitted from assisted living with a traumatic subcapital femur fracture. Admission code orders were “initiate CPR/intubation” despite the presence in the EMR of a POLST ordering “no CPR/no intubation.” The patient underwent hemiarthroplasty. There was no documented discussion of AD/POLST by the surgeon, anesthesiologist, or other operating room personnel even though the patient was alert and competent. On postoperative day one, she was found to be bradycardic and hypotensive. A code was called. After confirming full code status in the EMR, cardiac compressions were begun, followed by intubation. Immediately afterward, family members indicated that the patient had a POLST limiting EOL care. When the healthcare proxy was reached hours later, she directed the patient be extubated. The patient died 16 minutes later.
DISCUSSION
Data on the frequency of unwanted CPR/intubation due to medical error are scarce. In the US, several lawsuits arising from unwanted CPR and intubation have achieved notoriety, but registries of legal cases6 probably underestimate the frequency of this harm. In a study of incorrect code status orders at Canadian hospitals, 35% of 308 patients with limited care preferences had full code orders in the chart.7 It is unclear how many of these expressed preferences also had legal documents available. There was considerable variability among hospitals, suggesting that local practices and culture were important factors.
Spot audits of 121 of our own patient charts (median age 77 years) on oncology, geriatrics, and cardiac units at our institution found 36 (30%) with AD/POLST that clearly limited life-sustaining treatments. Of these, 14 (39%) had discrepant full code orders. A review of these discrepant orders showed no medical documentation to indicate that the discrepancy was purposeful.
A root cause analysis (RCA) of cases of unwanted resuscitation, including interviews with involved nurses, medical staff, and operating room, hospitalist, and medical informatics leadership, revealed several types of error, both human and system. These pitfalls are probably common to several hospitals, and the solutions developed may be helpful as well (Table).
ROOT CAUSE 1: HASTE
Haste leads to poor communication with the patient and family. Emergency departments and admitting services can be hectic. Clinicians facing time and acuity pressure may give short shrift to the essential activity of validating patient choices, regardless of whether an AD or POLST is available. Poor communication was the major factor allowing for discrepancy in the Canadian study.7 Avoiding prognostic frankness is a well-known coping strategy for both clinicians and patients8,9 but in all these cases, that obstacle had been overcome earlier in the clinical course of disease, leaving inattention or haste as the most likely culprit.
ROOT CAUSE 2: INADEQUATE COMMUNICATION
“It is not our hospital culture to surveille for code status discrepancies, discuss appropriateness on rounds or at sign out.”
In all reviewed cases of unwanted resuscitation, numerous admitting or attending physicians failed to discuss LST meaningfully despite clinical scenarios that were associated with poor prognosis and should have provoked discussion about medical ineffectiveness. The admitting hospitalist in case 2 stated later that she had listed code choices for the patient who chose full code despite having a POLST stating otherwise. However, that discussion was not in depth, not reviewed for match to her POLST, and not documented.
Moreover, all the cases of AD/POLST and code status discrepancy were on nursing units with daily multidisciplinary rounds and where there had been twice-daily nurse-to-nurse and medical staff–to–medical staff sign out. Queries about code status appropriateness and checks for discrepant AD/POLST and code orders were not standard work. Thus, the medical error was perpetuated.
Analysis of cases of unwanted intubation in postoperative cases indicated that contrary to guidelines,4,5 careful code status review was not part of the preoperative checklist or presurgical discussion.
ROOT CAUSE 3: DECEIVED BY THE EMR
The EMR is a well-recognized source of potential medical error.10,11 Clinicians may rely on the EMR for code status history or as a repository of relevant documents. These are important as a starting place for code status discussions, especially since patients and proxies often cannot accurately recall the existence of an AD/POLST or understand the options being presented.9,12 In case 1, clinicians partially relied upon the erroneous historical code status already in the chart from two prior admissions. This is a dangerous practice since code status choices have several options and depend upon the clinical situation. In the case of paper AD/POLST documents, the EMR is set up poorly to help the medical team find relevant documents. Furthermore, the EMR clinical decision support capabilities do not interact with paper documents, so no assistance in pointing out discrepancies is available. In addition, the scanning process itself can be problematic since scanning of paper documents was not performed until after the patient was discharged, thus hiding the most up-to-date documents from the personnel even if they had sought them. Moreover, our scanning process had been labeling documents with the date of scanning and not the date of completion, making it difficult to find the “active” order.
ROOT CAUSE 4: WE DID NOT KNOW
Interviews with different clinicians revealed widespread knowledge deficits, including appreciation of the POLST as durable across different medical institutions, effective differences between POLST and AD, location of POLST/AD within the EMR, recommendations of professional society guidelines on suspending DNR for procedures, hospital policy on same, the need to check for updates in bedside paper documents, and whether family members can overrule patients’ stated wishes. Education tends to be the most common form of recommendation after RCA and may be the least efficacious in risk mitigation,13 but in this case, education reinforced by new EMR capabilities was an essential part of the solutions bundle (Table).
AD/POLST and similar tools are complex, and the choices are not binary. They are subject to change depending upon the medical context and the patient status and may be poorly understood by patients and clinicians.14 Accordingly, writing a goal-concordant code status order demands time and attention and as much nuanced medical judgment as any other medical problem faced by hospital-based clinicians. Though time-consuming, discussion with the patient or the surrogate should be considered as “standard work.” To facilitate this, a mandatory affirmative statement about review of LST choices was added to admission templates, procedural areas, and clinician sign outs (Table).
Unwanted, and therefore unwarranted, resuscitation violates autonomy and creates distress, anger, and distrust among patients and families. The distress extends also to frontline clinicians who are committed to “do no harm” in every other aspect of their professional lives.
Respecting and translating patients’ AD/POLST or similar tools into goal-concordant code status order is an essential professional commitment. Respect for patient safety and autonomy demands that we do it well, teach it well, and hold each other accountable.
Disclosures
The authors have nothing disclose.
1. Institute of Medicine. Dying in America: improving quality and honoring individual preferences near end of life Washington, DC: National Academies Pr; 2015.
2. ASCO Institute for Quality: QCDR measures. http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI 2015 QCDR Measures - Narrative_0.pdf. Accessed March 3, 2019.
3. Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43(12):1847-1849. https://doi.org/10.1007/s00134-017-4873-2
4. American Society of Anesthesiology Ethics Committee. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment-last amended October 2013. Accessed March 12, 2019
5. American College of Surgeons Committee on Ethics. Statement on advanced directives by patients: “do not resuscitate” in the operating room. Bull Am Coll Surg. 2014;99(1):42-43
6. Pope TM. Legal briefing: new penalties for disregarding advance directives and do-not-resuscitate orders. J Clin Ethics. 2017;28(1):74-81.
7. Heyland DH, Ilan R, Jiang X, You JJ, Dodek P. The prevalence of medical error related to end-of-life communication in Canadian hospitals: results of a mutlicentre observational study. BMJ Qual Saf. 2016;25:671-679. https://doi.org/10.1136/bmjqs-2015-004567.
8. Robinson JD, Jagsi R. Physician-patient communication—an actionable target for reducing overly aggressive care near the end of life. JAMA Oncol. 2016;2(11):1407-1408. doi:10.1001/jamaoncol.2016.1948
9. Ugalde A, O’Callaghan C, Byard C, et al. Does implementation matter if comprehension is lacking? A qualitative investigation into perceptions of advanced care planning in people with cancer. Support Care Cancer. 2018;26:3765-3771. https://doi.org/10.1007/s00520-018-4241-y.
10. Silversetein S. The Syndrome of inappropriate overconfidence in computing. An invasion of medicine by the information technology industry? J Am Phys Surg. 2009;14:49-50
11. Ratwani RM, Reider, J and Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321(8):743-744. doi:10.1001/jama.2019.0161
12. Turnbull AE, Chessare CM, Coffin RK, Needham DM. More than one in three proxies do not know their loved one’s current code status: an observational study in a Maryland ICU. PLoS ONE. 2019;14(1):e0211531. https//doi.org/10.1371/journal.pone.0211531
13. Wu AW, Lipshutz AKM, Pronovost PJ. Effectiveness and efficiency of root cause analysis in medicine. JAMA. 2008;299(6):685-687. doi:10.1001/jama.299.6.685
14. Mirarchi F, Doshi AA, Zerkle SW, Cooney TE. TRIAD VI: how well do emergency physicians understand Physician Orders for Life-Sustaining Treatment (POLST) forms? J Patient Saf. 2015;11(1):1-8. https://doi.org/10.1097/PTS.0000000000000165.
The United States health system has been criticized for its overuse of aggressive and medically ineffective life-sustaining therapies (LST).1 Some professional societies have elevated dialog about end-of-life (EOL) care to a quality measure,2 expecting that more open discussion will achieve more “goal-concordant care”3 and appropriate use of LST. However, even when Advanced Directives (AD) or Physician Orders for Life-Sustaining Therapy (POLST) have been created, their directions are not always followed in the hospital. This perspective discusses how preventable errors allow for use of LST even when patients designated it as unwanted. Two cases, chosen from several similar ones, are highlighted, demonstrating both human and system errors.
During the time of these events, the hospital policy required admission orders to contain a “code status” designation in the electronic medical record (EMR). All active and historical code status orders were listed chronologically and all AD and POLST documents were scanned into a special section of the EMR. Hospital policy, consistent with professional society guidelines,4,5 stated that patients with AD/POLST limiting EOL support should have individualized discussion about resuscitation options in the event of a periprocedural critical event. Automatic suspension or reinstatement of limited code orders was not permitted.
CASE 1
A 62-year-old woman with refractory heart failure was admitted with recurrence. The admitting code order was “initiate CPR/intubation” even though a POLST order written 10 months earlier indicating “do not intubate” was visible in the EMR. A more recent POLST indicating “No CPR/No intubation” accompanied the patient in the ambulance and was placed at bedside, but not scanned. There was no documented discussion of code status that might have explained the POLST/code order disparity. Notably, during two prior admissions within the year, “full code” orders had also been placed. On the fifth hospital day, the patient was found in respiratory distress and unresponsive. A “code” was called. ICU staff, after confirming full code status, intubated the patient emergently and commenced other invasive ICU interventions. Family members brought the preexisting POLST to medical attention within hours of the code but could not agree on immediate extubation. Over the next week, multiple prognosis discussions were held with the patient (when responsive) and family. Ultimately, the patient failed to improve and indicated a desire to be extubated, dying a few hours later.
CASE 2
A 94-year-old woman was admitted from assisted living with a traumatic subcapital femur fracture. Admission code orders were “initiate CPR/intubation” despite the presence in the EMR of a POLST ordering “no CPR/no intubation.” The patient underwent hemiarthroplasty. There was no documented discussion of AD/POLST by the surgeon, anesthesiologist, or other operating room personnel even though the patient was alert and competent. On postoperative day one, she was found to be bradycardic and hypotensive. A code was called. After confirming full code status in the EMR, cardiac compressions were begun, followed by intubation. Immediately afterward, family members indicated that the patient had a POLST limiting EOL care. When the healthcare proxy was reached hours later, she directed the patient be extubated. The patient died 16 minutes later.
DISCUSSION
Data on the frequency of unwanted CPR/intubation due to medical error are scarce. In the US, several lawsuits arising from unwanted CPR and intubation have achieved notoriety, but registries of legal cases6 probably underestimate the frequency of this harm. In a study of incorrect code status orders at Canadian hospitals, 35% of 308 patients with limited care preferences had full code orders in the chart.7 It is unclear how many of these expressed preferences also had legal documents available. There was considerable variability among hospitals, suggesting that local practices and culture were important factors.
Spot audits of 121 of our own patient charts (median age 77 years) on oncology, geriatrics, and cardiac units at our institution found 36 (30%) with AD/POLST that clearly limited life-sustaining treatments. Of these, 14 (39%) had discrepant full code orders. A review of these discrepant orders showed no medical documentation to indicate that the discrepancy was purposeful.
A root cause analysis (RCA) of cases of unwanted resuscitation, including interviews with involved nurses, medical staff, and operating room, hospitalist, and medical informatics leadership, revealed several types of error, both human and system. These pitfalls are probably common to several hospitals, and the solutions developed may be helpful as well (Table).
ROOT CAUSE 1: HASTE
Haste leads to poor communication with the patient and family. Emergency departments and admitting services can be hectic. Clinicians facing time and acuity pressure may give short shrift to the essential activity of validating patient choices, regardless of whether an AD or POLST is available. Poor communication was the major factor allowing for discrepancy in the Canadian study.7 Avoiding prognostic frankness is a well-known coping strategy for both clinicians and patients8,9 but in all these cases, that obstacle had been overcome earlier in the clinical course of disease, leaving inattention or haste as the most likely culprit.
ROOT CAUSE 2: INADEQUATE COMMUNICATION
“It is not our hospital culture to surveille for code status discrepancies, discuss appropriateness on rounds or at sign out.”
In all reviewed cases of unwanted resuscitation, numerous admitting or attending physicians failed to discuss LST meaningfully despite clinical scenarios that were associated with poor prognosis and should have provoked discussion about medical ineffectiveness. The admitting hospitalist in case 2 stated later that she had listed code choices for the patient who chose full code despite having a POLST stating otherwise. However, that discussion was not in depth, not reviewed for match to her POLST, and not documented.
Moreover, all the cases of AD/POLST and code status discrepancy were on nursing units with daily multidisciplinary rounds and where there had been twice-daily nurse-to-nurse and medical staff–to–medical staff sign out. Queries about code status appropriateness and checks for discrepant AD/POLST and code orders were not standard work. Thus, the medical error was perpetuated.
Analysis of cases of unwanted intubation in postoperative cases indicated that contrary to guidelines,4,5 careful code status review was not part of the preoperative checklist or presurgical discussion.
ROOT CAUSE 3: DECEIVED BY THE EMR
The EMR is a well-recognized source of potential medical error.10,11 Clinicians may rely on the EMR for code status history or as a repository of relevant documents. These are important as a starting place for code status discussions, especially since patients and proxies often cannot accurately recall the existence of an AD/POLST or understand the options being presented.9,12 In case 1, clinicians partially relied upon the erroneous historical code status already in the chart from two prior admissions. This is a dangerous practice since code status choices have several options and depend upon the clinical situation. In the case of paper AD/POLST documents, the EMR is set up poorly to help the medical team find relevant documents. Furthermore, the EMR clinical decision support capabilities do not interact with paper documents, so no assistance in pointing out discrepancies is available. In addition, the scanning process itself can be problematic since scanning of paper documents was not performed until after the patient was discharged, thus hiding the most up-to-date documents from the personnel even if they had sought them. Moreover, our scanning process had been labeling documents with the date of scanning and not the date of completion, making it difficult to find the “active” order.
ROOT CAUSE 4: WE DID NOT KNOW
Interviews with different clinicians revealed widespread knowledge deficits, including appreciation of the POLST as durable across different medical institutions, effective differences between POLST and AD, location of POLST/AD within the EMR, recommendations of professional society guidelines on suspending DNR for procedures, hospital policy on same, the need to check for updates in bedside paper documents, and whether family members can overrule patients’ stated wishes. Education tends to be the most common form of recommendation after RCA and may be the least efficacious in risk mitigation,13 but in this case, education reinforced by new EMR capabilities was an essential part of the solutions bundle (Table).
AD/POLST and similar tools are complex, and the choices are not binary. They are subject to change depending upon the medical context and the patient status and may be poorly understood by patients and clinicians.14 Accordingly, writing a goal-concordant code status order demands time and attention and as much nuanced medical judgment as any other medical problem faced by hospital-based clinicians. Though time-consuming, discussion with the patient or the surrogate should be considered as “standard work.” To facilitate this, a mandatory affirmative statement about review of LST choices was added to admission templates, procedural areas, and clinician sign outs (Table).
Unwanted, and therefore unwarranted, resuscitation violates autonomy and creates distress, anger, and distrust among patients and families. The distress extends also to frontline clinicians who are committed to “do no harm” in every other aspect of their professional lives.
Respecting and translating patients’ AD/POLST or similar tools into goal-concordant code status order is an essential professional commitment. Respect for patient safety and autonomy demands that we do it well, teach it well, and hold each other accountable.
Disclosures
The authors have nothing disclose.
The United States health system has been criticized for its overuse of aggressive and medically ineffective life-sustaining therapies (LST).1 Some professional societies have elevated dialog about end-of-life (EOL) care to a quality measure,2 expecting that more open discussion will achieve more “goal-concordant care”3 and appropriate use of LST. However, even when Advanced Directives (AD) or Physician Orders for Life-Sustaining Therapy (POLST) have been created, their directions are not always followed in the hospital. This perspective discusses how preventable errors allow for use of LST even when patients designated it as unwanted. Two cases, chosen from several similar ones, are highlighted, demonstrating both human and system errors.
During the time of these events, the hospital policy required admission orders to contain a “code status” designation in the electronic medical record (EMR). All active and historical code status orders were listed chronologically and all AD and POLST documents were scanned into a special section of the EMR. Hospital policy, consistent with professional society guidelines,4,5 stated that patients with AD/POLST limiting EOL support should have individualized discussion about resuscitation options in the event of a periprocedural critical event. Automatic suspension or reinstatement of limited code orders was not permitted.
CASE 1
A 62-year-old woman with refractory heart failure was admitted with recurrence. The admitting code order was “initiate CPR/intubation” even though a POLST order written 10 months earlier indicating “do not intubate” was visible in the EMR. A more recent POLST indicating “No CPR/No intubation” accompanied the patient in the ambulance and was placed at bedside, but not scanned. There was no documented discussion of code status that might have explained the POLST/code order disparity. Notably, during two prior admissions within the year, “full code” orders had also been placed. On the fifth hospital day, the patient was found in respiratory distress and unresponsive. A “code” was called. ICU staff, after confirming full code status, intubated the patient emergently and commenced other invasive ICU interventions. Family members brought the preexisting POLST to medical attention within hours of the code but could not agree on immediate extubation. Over the next week, multiple prognosis discussions were held with the patient (when responsive) and family. Ultimately, the patient failed to improve and indicated a desire to be extubated, dying a few hours later.
CASE 2
A 94-year-old woman was admitted from assisted living with a traumatic subcapital femur fracture. Admission code orders were “initiate CPR/intubation” despite the presence in the EMR of a POLST ordering “no CPR/no intubation.” The patient underwent hemiarthroplasty. There was no documented discussion of AD/POLST by the surgeon, anesthesiologist, or other operating room personnel even though the patient was alert and competent. On postoperative day one, she was found to be bradycardic and hypotensive. A code was called. After confirming full code status in the EMR, cardiac compressions were begun, followed by intubation. Immediately afterward, family members indicated that the patient had a POLST limiting EOL care. When the healthcare proxy was reached hours later, she directed the patient be extubated. The patient died 16 minutes later.
DISCUSSION
Data on the frequency of unwanted CPR/intubation due to medical error are scarce. In the US, several lawsuits arising from unwanted CPR and intubation have achieved notoriety, but registries of legal cases6 probably underestimate the frequency of this harm. In a study of incorrect code status orders at Canadian hospitals, 35% of 308 patients with limited care preferences had full code orders in the chart.7 It is unclear how many of these expressed preferences also had legal documents available. There was considerable variability among hospitals, suggesting that local practices and culture were important factors.
Spot audits of 121 of our own patient charts (median age 77 years) on oncology, geriatrics, and cardiac units at our institution found 36 (30%) with AD/POLST that clearly limited life-sustaining treatments. Of these, 14 (39%) had discrepant full code orders. A review of these discrepant orders showed no medical documentation to indicate that the discrepancy was purposeful.
A root cause analysis (RCA) of cases of unwanted resuscitation, including interviews with involved nurses, medical staff, and operating room, hospitalist, and medical informatics leadership, revealed several types of error, both human and system. These pitfalls are probably common to several hospitals, and the solutions developed may be helpful as well (Table).
ROOT CAUSE 1: HASTE
Haste leads to poor communication with the patient and family. Emergency departments and admitting services can be hectic. Clinicians facing time and acuity pressure may give short shrift to the essential activity of validating patient choices, regardless of whether an AD or POLST is available. Poor communication was the major factor allowing for discrepancy in the Canadian study.7 Avoiding prognostic frankness is a well-known coping strategy for both clinicians and patients8,9 but in all these cases, that obstacle had been overcome earlier in the clinical course of disease, leaving inattention or haste as the most likely culprit.
ROOT CAUSE 2: INADEQUATE COMMUNICATION
“It is not our hospital culture to surveille for code status discrepancies, discuss appropriateness on rounds or at sign out.”
In all reviewed cases of unwanted resuscitation, numerous admitting or attending physicians failed to discuss LST meaningfully despite clinical scenarios that were associated with poor prognosis and should have provoked discussion about medical ineffectiveness. The admitting hospitalist in case 2 stated later that she had listed code choices for the patient who chose full code despite having a POLST stating otherwise. However, that discussion was not in depth, not reviewed for match to her POLST, and not documented.
Moreover, all the cases of AD/POLST and code status discrepancy were on nursing units with daily multidisciplinary rounds and where there had been twice-daily nurse-to-nurse and medical staff–to–medical staff sign out. Queries about code status appropriateness and checks for discrepant AD/POLST and code orders were not standard work. Thus, the medical error was perpetuated.
Analysis of cases of unwanted intubation in postoperative cases indicated that contrary to guidelines,4,5 careful code status review was not part of the preoperative checklist or presurgical discussion.
ROOT CAUSE 3: DECEIVED BY THE EMR
The EMR is a well-recognized source of potential medical error.10,11 Clinicians may rely on the EMR for code status history or as a repository of relevant documents. These are important as a starting place for code status discussions, especially since patients and proxies often cannot accurately recall the existence of an AD/POLST or understand the options being presented.9,12 In case 1, clinicians partially relied upon the erroneous historical code status already in the chart from two prior admissions. This is a dangerous practice since code status choices have several options and depend upon the clinical situation. In the case of paper AD/POLST documents, the EMR is set up poorly to help the medical team find relevant documents. Furthermore, the EMR clinical decision support capabilities do not interact with paper documents, so no assistance in pointing out discrepancies is available. In addition, the scanning process itself can be problematic since scanning of paper documents was not performed until after the patient was discharged, thus hiding the most up-to-date documents from the personnel even if they had sought them. Moreover, our scanning process had been labeling documents with the date of scanning and not the date of completion, making it difficult to find the “active” order.
ROOT CAUSE 4: WE DID NOT KNOW
Interviews with different clinicians revealed widespread knowledge deficits, including appreciation of the POLST as durable across different medical institutions, effective differences between POLST and AD, location of POLST/AD within the EMR, recommendations of professional society guidelines on suspending DNR for procedures, hospital policy on same, the need to check for updates in bedside paper documents, and whether family members can overrule patients’ stated wishes. Education tends to be the most common form of recommendation after RCA and may be the least efficacious in risk mitigation,13 but in this case, education reinforced by new EMR capabilities was an essential part of the solutions bundle (Table).
AD/POLST and similar tools are complex, and the choices are not binary. They are subject to change depending upon the medical context and the patient status and may be poorly understood by patients and clinicians.14 Accordingly, writing a goal-concordant code status order demands time and attention and as much nuanced medical judgment as any other medical problem faced by hospital-based clinicians. Though time-consuming, discussion with the patient or the surrogate should be considered as “standard work.” To facilitate this, a mandatory affirmative statement about review of LST choices was added to admission templates, procedural areas, and clinician sign outs (Table).
Unwanted, and therefore unwarranted, resuscitation violates autonomy and creates distress, anger, and distrust among patients and families. The distress extends also to frontline clinicians who are committed to “do no harm” in every other aspect of their professional lives.
Respecting and translating patients’ AD/POLST or similar tools into goal-concordant code status order is an essential professional commitment. Respect for patient safety and autonomy demands that we do it well, teach it well, and hold each other accountable.
Disclosures
The authors have nothing disclose.
1. Institute of Medicine. Dying in America: improving quality and honoring individual preferences near end of life Washington, DC: National Academies Pr; 2015.
2. ASCO Institute for Quality: QCDR measures. http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI 2015 QCDR Measures - Narrative_0.pdf. Accessed March 3, 2019.
3. Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43(12):1847-1849. https://doi.org/10.1007/s00134-017-4873-2
4. American Society of Anesthesiology Ethics Committee. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment-last amended October 2013. Accessed March 12, 2019
5. American College of Surgeons Committee on Ethics. Statement on advanced directives by patients: “do not resuscitate” in the operating room. Bull Am Coll Surg. 2014;99(1):42-43
6. Pope TM. Legal briefing: new penalties for disregarding advance directives and do-not-resuscitate orders. J Clin Ethics. 2017;28(1):74-81.
7. Heyland DH, Ilan R, Jiang X, You JJ, Dodek P. The prevalence of medical error related to end-of-life communication in Canadian hospitals: results of a mutlicentre observational study. BMJ Qual Saf. 2016;25:671-679. https://doi.org/10.1136/bmjqs-2015-004567.
8. Robinson JD, Jagsi R. Physician-patient communication—an actionable target for reducing overly aggressive care near the end of life. JAMA Oncol. 2016;2(11):1407-1408. doi:10.1001/jamaoncol.2016.1948
9. Ugalde A, O’Callaghan C, Byard C, et al. Does implementation matter if comprehension is lacking? A qualitative investigation into perceptions of advanced care planning in people with cancer. Support Care Cancer. 2018;26:3765-3771. https://doi.org/10.1007/s00520-018-4241-y.
10. Silversetein S. The Syndrome of inappropriate overconfidence in computing. An invasion of medicine by the information technology industry? J Am Phys Surg. 2009;14:49-50
11. Ratwani RM, Reider, J and Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321(8):743-744. doi:10.1001/jama.2019.0161
12. Turnbull AE, Chessare CM, Coffin RK, Needham DM. More than one in three proxies do not know their loved one’s current code status: an observational study in a Maryland ICU. PLoS ONE. 2019;14(1):e0211531. https//doi.org/10.1371/journal.pone.0211531
13. Wu AW, Lipshutz AKM, Pronovost PJ. Effectiveness and efficiency of root cause analysis in medicine. JAMA. 2008;299(6):685-687. doi:10.1001/jama.299.6.685
14. Mirarchi F, Doshi AA, Zerkle SW, Cooney TE. TRIAD VI: how well do emergency physicians understand Physician Orders for Life-Sustaining Treatment (POLST) forms? J Patient Saf. 2015;11(1):1-8. https://doi.org/10.1097/PTS.0000000000000165.
1. Institute of Medicine. Dying in America: improving quality and honoring individual preferences near end of life Washington, DC: National Academies Pr; 2015.
2. ASCO Institute for Quality: QCDR measures. http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI 2015 QCDR Measures - Narrative_0.pdf. Accessed March 3, 2019.
3. Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 2017;43(12):1847-1849. https://doi.org/10.1007/s00134-017-4873-2
4. American Society of Anesthesiology Ethics Committee. Ethical guidelines for the anesthesia care of patients with do-not-resuscitate orders or other directives that limit treatment-last amended October 2013. Accessed March 12, 2019
5. American College of Surgeons Committee on Ethics. Statement on advanced directives by patients: “do not resuscitate” in the operating room. Bull Am Coll Surg. 2014;99(1):42-43
6. Pope TM. Legal briefing: new penalties for disregarding advance directives and do-not-resuscitate orders. J Clin Ethics. 2017;28(1):74-81.
7. Heyland DH, Ilan R, Jiang X, You JJ, Dodek P. The prevalence of medical error related to end-of-life communication in Canadian hospitals: results of a mutlicentre observational study. BMJ Qual Saf. 2016;25:671-679. https://doi.org/10.1136/bmjqs-2015-004567.
8. Robinson JD, Jagsi R. Physician-patient communication—an actionable target for reducing overly aggressive care near the end of life. JAMA Oncol. 2016;2(11):1407-1408. doi:10.1001/jamaoncol.2016.1948
9. Ugalde A, O’Callaghan C, Byard C, et al. Does implementation matter if comprehension is lacking? A qualitative investigation into perceptions of advanced care planning in people with cancer. Support Care Cancer. 2018;26:3765-3771. https://doi.org/10.1007/s00520-018-4241-y.
10. Silversetein S. The Syndrome of inappropriate overconfidence in computing. An invasion of medicine by the information technology industry? J Am Phys Surg. 2009;14:49-50
11. Ratwani RM, Reider, J and Singh H. A decade of health information technology usability challenges and the path forward. JAMA. 2019;321(8):743-744. doi:10.1001/jama.2019.0161
12. Turnbull AE, Chessare CM, Coffin RK, Needham DM. More than one in three proxies do not know their loved one’s current code status: an observational study in a Maryland ICU. PLoS ONE. 2019;14(1):e0211531. https//doi.org/10.1371/journal.pone.0211531
13. Wu AW, Lipshutz AKM, Pronovost PJ. Effectiveness and efficiency of root cause analysis in medicine. JAMA. 2008;299(6):685-687. doi:10.1001/jama.299.6.685
14. Mirarchi F, Doshi AA, Zerkle SW, Cooney TE. TRIAD VI: how well do emergency physicians understand Physician Orders for Life-Sustaining Treatment (POLST) forms? J Patient Saf. 2015;11(1):1-8. https://doi.org/10.1097/PTS.0000000000000165.
© 2019 Society of Hospital Medicine
The Value of Routine Transthoracic Echocardiography in Defining the Source of Stroke in a Community Hospital
From Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Background: Acute stroke or cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating consequences, particularly in the setting of recurrence. Cardiac sources are potentially remediable; thus, a transthoracic echocardiogram (TTE) is frequently ordered to evaluate for a cardiac source of embolism.
- Objective: To evaluate the utility of performing TTE on patients experiencing a CVA or transient ischemic attack (TIA) to evaluate for a cardiac source of embolism.
- Methods: Retrospective review of TTE reports and patient electronic medical records at Anne Arundel Medical Center, a 385-bed community hospital. Medical charts for all CVA patients receiving a TTE between February 2012 to April 2013 were reviewed for TTEs showing unequivocal cardiac sources of embolism as evaluated by the reviewing cardiologist. Patient information and clinical morbidities were also noted to construct a composite demographic of CVA patients.
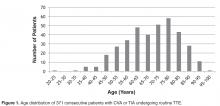
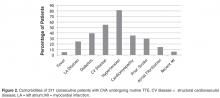
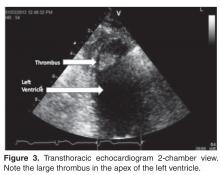
- Results: One TTE of 371 (0.270%) identified a clear cardiac embolus. Risk factors for stroke included hyper-tension (n = 302), cardiovascular disease (n = 204), cardiomyopathy (n = 131), and diabetes (n = 146).
- Conclusion: In the setting of stroke, TTE is of limited value when determining the etiology of stroke and should be used provisionally rather than routinely in evaluating patients experiencing CVA or TIA.
Acute cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating clinical consequences, particularly in the setting of recurrence. Defining the etiology of CVA and transient ischemic attacks (TIA) when they occur is important so that appropriate therapy can be initiated. Transthoracic echocardiograms (TTEs) are frequently ordered to evaluate for a cardiac source of embolism. No consensus exists about the use of imaging strategies to identify potential cardiovascular sources of emboli in patients who have had strokes.
A few published studies have investigated the yield of TTE in identifying cardiac sources of CVA. The yield has been reported to be between < 1% and as high as 37% [1–3]. However, some of the reported sources of CVA included mitral valve prolapse and patent foramen ovale [4,5], conditions for which the association with stroke has been questioned [6–8]. In addition, many of these studies were performed using clinical data from tertiary referral centers, which may have increased the yield of cardiac sources [9].
The purpose of this study was to evaluate the yield of TTE in evaluating for a clear cardiac source of embolism in a consecutive series of patients diagnosed with a CVA or TIA in a community hospital.
Methods
Setting
All data was collected from the echocardiography lab at Anne Arundel Medical Center, a 384-bed community hospital in Annapolis, MD. The medical center sees about 250 patients a day in its emergency department and admits about 30,000 patients annually. All echocardiograms are performed by a centralized laboratory accredited by the Intersocietal Commission for the Accreditation of Echocardiography Laboratories, which performs approximately 6000 echocardiograms annually.
All TTEs done for the diagnosis of CVA or TIA between 1 February 2013 and 1 May 2013 were evaluated in consecutive fashion by report review. Reports were searched for any cardiac source of embolism to include thrombus, tumor, vegetation, shunt, aortic atheroma, or any other finding that was felt to be a clear source of embolism by the interpreting cardiologist. We did not include entities such as mitral valve prolapse, patent foramen ovale, and isolated atrial septal aneurysms since their association with CVA/TIA has been questioned. Also not included was cardiomyopathy without aneurysm, apical wall motion abnormality, or intra-cavitary thrombus solely because the ejection fraction was less than 35%, as the literature does not support these conditions as clear causes of TIA or CVA.
In addition to reviewing echocardiogram reports, all patient records were evaluated for clinical variables including age, gender, presence of atrial fibrillation, hypertension, diabetes, past CVA, left atrial dilation by calculation of indexed direct left atrial volume, recent myocardial infarction, and known cardiovascular disease.
All echocardiograms were performed on Hewlet-Packard Vivid 7 or Vivid 9 (GE Healthcare, Wauwatosa, WI) by technicians who held registered diagnostic cardiac sonographer status. All TTEs contained the “standard” views in accordance with published guidelines [10].Saline contrast to look for shunts was not standard on these studies. Echocardiogram images were stored digitally and read from an EchoPac (GE Healthcare, Wauwatosa, WI) reading station. All echocardiograms were interpreted by one of 16 American Board of Internal Medicine–certified cardiologists, 5 of whom were testamurs (physicians who have passed one of the examinations of special competence in echocardiography). These 5 interpreted 20% of the studies.
Results
Discussion
Our data are in keeping with those of others, though our yield was even lower than that reported in previous studies [1–3]. The low yield may be explained by a number of factors. First, we did not include patent foramen ovale or atrial septal aneurysms (which account for a high percentage of embolic sources in other publications) since there is not a clear consensus that any of those entities are associated with an increased risk of embolic events. The exclusion of cardiomyopathy as a cause of CVA or TIA is arguable, but its link to CVA or TIA is also unproven. One study did associate cardiomyopathy with CVA [12]; however, the mechanism is not clear, as the incidence of CVA in cardiomyopathy has been described as similar regardless of the severity of left ventricular dysfunction [13].Many past reports have come from tertiary care centers, where there may be referral bias whereas our data come from consecutive patients at a single community hospital.
TTE is relatively quick to perform and interpret and carries no physical risk to a patient. However, our data suggest that ordering TTE routinely in the setting of CVA offers little value. With health care organizations turning their attention to reducing low-value care, which potentially wastes limited resources, considerations of value and effectiveness continue to be a priority. Our findings suggest TTE use in this setting conflicts with the current trajectory of value-based medical practice. As well, a prior Markov model decision analysis found that TTE is not cost-effective when used routinely to identify source of emboli in stroke [14].
Despite the low yield of TTE in evaluating for a cardiac source of CVA, TTEs continue to be frequently ordered. In our own institution, 48% of patients with a CVA or TIA underwent a TTE based on preferences and habits of individual admitting physicians and without any structured criteria. Order sets for CVA admissions do not include this test; physicians are adding it but not for any particular patient characteristic or exam finding.
There are a number of reasons that echocardiograms may be ordered more frequently by some. A documented decline in ordering echocardiograms was seen following education at one center [15], suggesting that lack of knowledge about the limitations of TTE may be a factor. A second potential factor is fear of medicolegal consequences. Indeed, the current American Heart Association/American Stroke Association guidelines for the early management of adults with ischemic stroke [16] offers no formal recommendations or clear indications.
Computerized decision support (CDS) that links the medical record to appropriateness criteria could potentially reduce the inappropriate use of TTE. CDS has been shown to be effective in reducing unnecessary ordering of tests in other settings [17–19].
Among the limitations in our analysis is the heterogeneity in echocardiogram readers. However, this heterogeneity may makes the study more relevant as it reflects the reality in most community hospitals. Another potential limitation is that saline contrast studies were not used routinely; however, this too is typical at community hospitals. Also, while all echocardiograms were interpreted by “board-certified” cardiologists, only 5 had passed the “examination of special competence” to be certified as a testamur of the National Board of Echocardiography, raising the question as to whether subtle findings could have been missed. However, there were no relevant findings in the 20% of studies interpreted by the testamurs, suggesting that the other echocardiographers were not missing diagnoses. Finally, we had only 10 patients younger than age 45 and so the study conclusions are less definitive for that age-group.
Conclusion
TTE was of limited utility in uncovering a cardiac source of embolism in a typical population with CVA or TIA.Based upon the data, we believe that TTE should not be used routinely in the setting of CVA; however, we do recognize that TTE may be of value in patients who have other comorbidities that would place them at increased risk of embolic CVA such as a recent anterior MI, those at risk for endocarditis, or those with brain imaging findings suggestive of embolic CVA [20]. Ordering a low-value test such as a TTE in the setting of TIA or CVA adds cost and does not often yield a clinically meaningful results. In addition, a “negative” TTE can be misinterpreted as a normal heart and forestall additional workup such as transesophageal echocardiography and long-term rhythm analysis, which may be of higher value. We suggest that in a community hospital setting the determination of need for TTE be made based on the clinical nuances of the case rather than by habit or as part of standardized order sets.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, meisenberg@aahs.org.
Financial disclosures: None.
Author contributions: conception and design, BM, WCM; analysis and interpretation of data, BM, WCM; drafting of article, RHB, BM, WCM; critical revision of the article, BM, WCM; administrative or technical support, JC; collection and assembly of data, RHB, JC.
1. Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke 1996;27:691.
2. Khan MA, Khealanj B, Kamal, A. Diagnostic yield of transthoracic echocardiography for stroke patients in a developing country. J Pak Med Assoc 2008;58:375–7.
3. de Abreu T, Mateus S, José Correia J. Therapy implications of transthoracic echocardiography in acute ischemic stroke patients. Stroke 2005;36:1565–6.
4. de Bruijn SFTM, Agema WRP, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006;37:2531–4.
5. Putaala J, Metso AJ, MD, Metso T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke. Stroke 2009;40:1195–203.
6. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent fora-men ovale in patients with stroke. N Engl J Med 1988;318:1148–52.
7. Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
8. Orencia AJ, Petty GW, Khandheria BK, et al. Risk of stroke with mitral valve prolapse in population-based cohort study; Stroke 1995;26:7–13.
9. Holmes M, Rathbone J, Littlewood C. Routine echocardiography in the management of stroke and transient ischaemic attack: a systematic review and economic evaluation. Health Technol Assess 2014;18:1–176.
10. Ryan T, Armstrong W. Feigenbaum’s echocardiography. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2009.
11. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010;137:263–72.
12. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–76.
13. Hays AG, Sacco RL, Rundek T. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke 2006;37:1715–9.
14. McNamara RL, Lima JA, Whelton PK, Powe NR. Echocardiographic identification of cardiovascular sources of emboli to guide clinical management of stroke: a cost-effectiveness analysis. Ann Intern Med 1997;127:775–87.
15. Alberts MJ, Bennett CA, Rutledge VR. Hospital charges for stroke patients. Stroke 1996;27:1825–8.
16. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947.
17. Levick DL, Stern G, Meyerhoefer CD, et al. Reducing unnecessary testing in a CPOE system through implementation of a targeted CDS intervention. BMC Med Inform Decis 2013;13:43.
18. Chen P, Tanasijevic MJ, Schoenenberger RA, et al. A computer-based intervention for improving the appropriateness of antiepileptic drug level monitoring. Am J Clin Pathol 2003;119:432–8.
19. Solberg LI, Wei F, Butler JC, et al. Effects of electronic decision support on high-tech diagnostic imaging orders and patients. Am J Manag Care 2010;16:102–6.
20. Menon BK, Coulter JI, Simerpret B, et al. Acute ischaemic stroke or transient ischaemic attack and the need for inpatient echocardiography. Postgrad Med J 2014;90:434–8.
From Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Background: Acute stroke or cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating consequences, particularly in the setting of recurrence. Cardiac sources are potentially remediable; thus, a transthoracic echocardiogram (TTE) is frequently ordered to evaluate for a cardiac source of embolism.
- Objective: To evaluate the utility of performing TTE on patients experiencing a CVA or transient ischemic attack (TIA) to evaluate for a cardiac source of embolism.
- Methods: Retrospective review of TTE reports and patient electronic medical records at Anne Arundel Medical Center, a 385-bed community hospital. Medical charts for all CVA patients receiving a TTE between February 2012 to April 2013 were reviewed for TTEs showing unequivocal cardiac sources of embolism as evaluated by the reviewing cardiologist. Patient information and clinical morbidities were also noted to construct a composite demographic of CVA patients.
- Results: One TTE of 371 (0.270%) identified a clear cardiac embolus. Risk factors for stroke included hyper-tension (n = 302), cardiovascular disease (n = 204), cardiomyopathy (n = 131), and diabetes (n = 146).
- Conclusion: In the setting of stroke, TTE is of limited value when determining the etiology of stroke and should be used provisionally rather than routinely in evaluating patients experiencing CVA or TIA.
Acute cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating clinical consequences, particularly in the setting of recurrence. Defining the etiology of CVA and transient ischemic attacks (TIA) when they occur is important so that appropriate therapy can be initiated. Transthoracic echocardiograms (TTEs) are frequently ordered to evaluate for a cardiac source of embolism. No consensus exists about the use of imaging strategies to identify potential cardiovascular sources of emboli in patients who have had strokes.
A few published studies have investigated the yield of TTE in identifying cardiac sources of CVA. The yield has been reported to be between < 1% and as high as 37% [1–3]. However, some of the reported sources of CVA included mitral valve prolapse and patent foramen ovale [4,5], conditions for which the association with stroke has been questioned [6–8]. In addition, many of these studies were performed using clinical data from tertiary referral centers, which may have increased the yield of cardiac sources [9].
The purpose of this study was to evaluate the yield of TTE in evaluating for a clear cardiac source of embolism in a consecutive series of patients diagnosed with a CVA or TIA in a community hospital.
Methods
Setting
All data was collected from the echocardiography lab at Anne Arundel Medical Center, a 384-bed community hospital in Annapolis, MD. The medical center sees about 250 patients a day in its emergency department and admits about 30,000 patients annually. All echocardiograms are performed by a centralized laboratory accredited by the Intersocietal Commission for the Accreditation of Echocardiography Laboratories, which performs approximately 6000 echocardiograms annually.
All TTEs done for the diagnosis of CVA or TIA between 1 February 2013 and 1 May 2013 were evaluated in consecutive fashion by report review. Reports were searched for any cardiac source of embolism to include thrombus, tumor, vegetation, shunt, aortic atheroma, or any other finding that was felt to be a clear source of embolism by the interpreting cardiologist. We did not include entities such as mitral valve prolapse, patent foramen ovale, and isolated atrial septal aneurysms since their association with CVA/TIA has been questioned. Also not included was cardiomyopathy without aneurysm, apical wall motion abnormality, or intra-cavitary thrombus solely because the ejection fraction was less than 35%, as the literature does not support these conditions as clear causes of TIA or CVA.
In addition to reviewing echocardiogram reports, all patient records were evaluated for clinical variables including age, gender, presence of atrial fibrillation, hypertension, diabetes, past CVA, left atrial dilation by calculation of indexed direct left atrial volume, recent myocardial infarction, and known cardiovascular disease.
All echocardiograms were performed on Hewlet-Packard Vivid 7 or Vivid 9 (GE Healthcare, Wauwatosa, WI) by technicians who held registered diagnostic cardiac sonographer status. All TTEs contained the “standard” views in accordance with published guidelines [10].Saline contrast to look for shunts was not standard on these studies. Echocardiogram images were stored digitally and read from an EchoPac (GE Healthcare, Wauwatosa, WI) reading station. All echocardiograms were interpreted by one of 16 American Board of Internal Medicine–certified cardiologists, 5 of whom were testamurs (physicians who have passed one of the examinations of special competence in echocardiography). These 5 interpreted 20% of the studies.
Results
Discussion
Our data are in keeping with those of others, though our yield was even lower than that reported in previous studies [1–3]. The low yield may be explained by a number of factors. First, we did not include patent foramen ovale or atrial septal aneurysms (which account for a high percentage of embolic sources in other publications) since there is not a clear consensus that any of those entities are associated with an increased risk of embolic events. The exclusion of cardiomyopathy as a cause of CVA or TIA is arguable, but its link to CVA or TIA is also unproven. One study did associate cardiomyopathy with CVA [12]; however, the mechanism is not clear, as the incidence of CVA in cardiomyopathy has been described as similar regardless of the severity of left ventricular dysfunction [13].Many past reports have come from tertiary care centers, where there may be referral bias whereas our data come from consecutive patients at a single community hospital.
TTE is relatively quick to perform and interpret and carries no physical risk to a patient. However, our data suggest that ordering TTE routinely in the setting of CVA offers little value. With health care organizations turning their attention to reducing low-value care, which potentially wastes limited resources, considerations of value and effectiveness continue to be a priority. Our findings suggest TTE use in this setting conflicts with the current trajectory of value-based medical practice. As well, a prior Markov model decision analysis found that TTE is not cost-effective when used routinely to identify source of emboli in stroke [14].
Despite the low yield of TTE in evaluating for a cardiac source of CVA, TTEs continue to be frequently ordered. In our own institution, 48% of patients with a CVA or TIA underwent a TTE based on preferences and habits of individual admitting physicians and without any structured criteria. Order sets for CVA admissions do not include this test; physicians are adding it but not for any particular patient characteristic or exam finding.
There are a number of reasons that echocardiograms may be ordered more frequently by some. A documented decline in ordering echocardiograms was seen following education at one center [15], suggesting that lack of knowledge about the limitations of TTE may be a factor. A second potential factor is fear of medicolegal consequences. Indeed, the current American Heart Association/American Stroke Association guidelines for the early management of adults with ischemic stroke [16] offers no formal recommendations or clear indications.
Computerized decision support (CDS) that links the medical record to appropriateness criteria could potentially reduce the inappropriate use of TTE. CDS has been shown to be effective in reducing unnecessary ordering of tests in other settings [17–19].
Among the limitations in our analysis is the heterogeneity in echocardiogram readers. However, this heterogeneity may makes the study more relevant as it reflects the reality in most community hospitals. Another potential limitation is that saline contrast studies were not used routinely; however, this too is typical at community hospitals. Also, while all echocardiograms were interpreted by “board-certified” cardiologists, only 5 had passed the “examination of special competence” to be certified as a testamur of the National Board of Echocardiography, raising the question as to whether subtle findings could have been missed. However, there were no relevant findings in the 20% of studies interpreted by the testamurs, suggesting that the other echocardiographers were not missing diagnoses. Finally, we had only 10 patients younger than age 45 and so the study conclusions are less definitive for that age-group.
Conclusion
TTE was of limited utility in uncovering a cardiac source of embolism in a typical population with CVA or TIA.Based upon the data, we believe that TTE should not be used routinely in the setting of CVA; however, we do recognize that TTE may be of value in patients who have other comorbidities that would place them at increased risk of embolic CVA such as a recent anterior MI, those at risk for endocarditis, or those with brain imaging findings suggestive of embolic CVA [20]. Ordering a low-value test such as a TTE in the setting of TIA or CVA adds cost and does not often yield a clinically meaningful results. In addition, a “negative” TTE can be misinterpreted as a normal heart and forestall additional workup such as transesophageal echocardiography and long-term rhythm analysis, which may be of higher value. We suggest that in a community hospital setting the determination of need for TTE be made based on the clinical nuances of the case rather than by habit or as part of standardized order sets.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, meisenberg@aahs.org.
Financial disclosures: None.
Author contributions: conception and design, BM, WCM; analysis and interpretation of data, BM, WCM; drafting of article, RHB, BM, WCM; critical revision of the article, BM, WCM; administrative or technical support, JC; collection and assembly of data, RHB, JC.
From Anne Arundel Medical Center, Annapolis, MD.
Abstract
- Background: Acute stroke or cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating consequences, particularly in the setting of recurrence. Cardiac sources are potentially remediable; thus, a transthoracic echocardiogram (TTE) is frequently ordered to evaluate for a cardiac source of embolism.
- Objective: To evaluate the utility of performing TTE on patients experiencing a CVA or transient ischemic attack (TIA) to evaluate for a cardiac source of embolism.
- Methods: Retrospective review of TTE reports and patient electronic medical records at Anne Arundel Medical Center, a 385-bed community hospital. Medical charts for all CVA patients receiving a TTE between February 2012 to April 2013 were reviewed for TTEs showing unequivocal cardiac sources of embolism as evaluated by the reviewing cardiologist. Patient information and clinical morbidities were also noted to construct a composite demographic of CVA patients.
- Results: One TTE of 371 (0.270%) identified a clear cardiac embolus. Risk factors for stroke included hyper-tension (n = 302), cardiovascular disease (n = 204), cardiomyopathy (n = 131), and diabetes (n = 146).
- Conclusion: In the setting of stroke, TTE is of limited value when determining the etiology of stroke and should be used provisionally rather than routinely in evaluating patients experiencing CVA or TIA.
Acute cerebrovascular accident (CVA) is a common indication for hospitalization and can have devastating clinical consequences, particularly in the setting of recurrence. Defining the etiology of CVA and transient ischemic attacks (TIA) when they occur is important so that appropriate therapy can be initiated. Transthoracic echocardiograms (TTEs) are frequently ordered to evaluate for a cardiac source of embolism. No consensus exists about the use of imaging strategies to identify potential cardiovascular sources of emboli in patients who have had strokes.
A few published studies have investigated the yield of TTE in identifying cardiac sources of CVA. The yield has been reported to be between < 1% and as high as 37% [1–3]. However, some of the reported sources of CVA included mitral valve prolapse and patent foramen ovale [4,5], conditions for which the association with stroke has been questioned [6–8]. In addition, many of these studies were performed using clinical data from tertiary referral centers, which may have increased the yield of cardiac sources [9].
The purpose of this study was to evaluate the yield of TTE in evaluating for a clear cardiac source of embolism in a consecutive series of patients diagnosed with a CVA or TIA in a community hospital.
Methods
Setting
All data was collected from the echocardiography lab at Anne Arundel Medical Center, a 384-bed community hospital in Annapolis, MD. The medical center sees about 250 patients a day in its emergency department and admits about 30,000 patients annually. All echocardiograms are performed by a centralized laboratory accredited by the Intersocietal Commission for the Accreditation of Echocardiography Laboratories, which performs approximately 6000 echocardiograms annually.
All TTEs done for the diagnosis of CVA or TIA between 1 February 2013 and 1 May 2013 were evaluated in consecutive fashion by report review. Reports were searched for any cardiac source of embolism to include thrombus, tumor, vegetation, shunt, aortic atheroma, or any other finding that was felt to be a clear source of embolism by the interpreting cardiologist. We did not include entities such as mitral valve prolapse, patent foramen ovale, and isolated atrial septal aneurysms since their association with CVA/TIA has been questioned. Also not included was cardiomyopathy without aneurysm, apical wall motion abnormality, or intra-cavitary thrombus solely because the ejection fraction was less than 35%, as the literature does not support these conditions as clear causes of TIA or CVA.
In addition to reviewing echocardiogram reports, all patient records were evaluated for clinical variables including age, gender, presence of atrial fibrillation, hypertension, diabetes, past CVA, left atrial dilation by calculation of indexed direct left atrial volume, recent myocardial infarction, and known cardiovascular disease.
All echocardiograms were performed on Hewlet-Packard Vivid 7 or Vivid 9 (GE Healthcare, Wauwatosa, WI) by technicians who held registered diagnostic cardiac sonographer status. All TTEs contained the “standard” views in accordance with published guidelines [10].Saline contrast to look for shunts was not standard on these studies. Echocardiogram images were stored digitally and read from an EchoPac (GE Healthcare, Wauwatosa, WI) reading station. All echocardiograms were interpreted by one of 16 American Board of Internal Medicine–certified cardiologists, 5 of whom were testamurs (physicians who have passed one of the examinations of special competence in echocardiography). These 5 interpreted 20% of the studies.
Results
Discussion
Our data are in keeping with those of others, though our yield was even lower than that reported in previous studies [1–3]. The low yield may be explained by a number of factors. First, we did not include patent foramen ovale or atrial septal aneurysms (which account for a high percentage of embolic sources in other publications) since there is not a clear consensus that any of those entities are associated with an increased risk of embolic events. The exclusion of cardiomyopathy as a cause of CVA or TIA is arguable, but its link to CVA or TIA is also unproven. One study did associate cardiomyopathy with CVA [12]; however, the mechanism is not clear, as the incidence of CVA in cardiomyopathy has been described as similar regardless of the severity of left ventricular dysfunction [13].Many past reports have come from tertiary care centers, where there may be referral bias whereas our data come from consecutive patients at a single community hospital.
TTE is relatively quick to perform and interpret and carries no physical risk to a patient. However, our data suggest that ordering TTE routinely in the setting of CVA offers little value. With health care organizations turning their attention to reducing low-value care, which potentially wastes limited resources, considerations of value and effectiveness continue to be a priority. Our findings suggest TTE use in this setting conflicts with the current trajectory of value-based medical practice. As well, a prior Markov model decision analysis found that TTE is not cost-effective when used routinely to identify source of emboli in stroke [14].
Despite the low yield of TTE in evaluating for a cardiac source of CVA, TTEs continue to be frequently ordered. In our own institution, 48% of patients with a CVA or TIA underwent a TTE based on preferences and habits of individual admitting physicians and without any structured criteria. Order sets for CVA admissions do not include this test; physicians are adding it but not for any particular patient characteristic or exam finding.
There are a number of reasons that echocardiograms may be ordered more frequently by some. A documented decline in ordering echocardiograms was seen following education at one center [15], suggesting that lack of knowledge about the limitations of TTE may be a factor. A second potential factor is fear of medicolegal consequences. Indeed, the current American Heart Association/American Stroke Association guidelines for the early management of adults with ischemic stroke [16] offers no formal recommendations or clear indications.
Computerized decision support (CDS) that links the medical record to appropriateness criteria could potentially reduce the inappropriate use of TTE. CDS has been shown to be effective in reducing unnecessary ordering of tests in other settings [17–19].
Among the limitations in our analysis is the heterogeneity in echocardiogram readers. However, this heterogeneity may makes the study more relevant as it reflects the reality in most community hospitals. Another potential limitation is that saline contrast studies were not used routinely; however, this too is typical at community hospitals. Also, while all echocardiograms were interpreted by “board-certified” cardiologists, only 5 had passed the “examination of special competence” to be certified as a testamur of the National Board of Echocardiography, raising the question as to whether subtle findings could have been missed. However, there were no relevant findings in the 20% of studies interpreted by the testamurs, suggesting that the other echocardiographers were not missing diagnoses. Finally, we had only 10 patients younger than age 45 and so the study conclusions are less definitive for that age-group.
Conclusion
TTE was of limited utility in uncovering a cardiac source of embolism in a typical population with CVA or TIA.Based upon the data, we believe that TTE should not be used routinely in the setting of CVA; however, we do recognize that TTE may be of value in patients who have other comorbidities that would place them at increased risk of embolic CVA such as a recent anterior MI, those at risk for endocarditis, or those with brain imaging findings suggestive of embolic CVA [20]. Ordering a low-value test such as a TTE in the setting of TIA or CVA adds cost and does not often yield a clinically meaningful results. In addition, a “negative” TTE can be misinterpreted as a normal heart and forestall additional workup such as transesophageal echocardiography and long-term rhythm analysis, which may be of higher value. We suggest that in a community hospital setting the determination of need for TTE be made based on the clinical nuances of the case rather than by habit or as part of standardized order sets.
Corresponding author: Barry Meisenberg, MD, DeCesaris Cancer Institute, 2001 Medical Parkway, Annapolis, MD 21146, meisenberg@aahs.org.
Financial disclosures: None.
Author contributions: conception and design, BM, WCM; analysis and interpretation of data, BM, WCM; drafting of article, RHB, BM, WCM; critical revision of the article, BM, WCM; administrative or technical support, JC; collection and assembly of data, RHB, JC.
1. Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke 1996;27:691.
2. Khan MA, Khealanj B, Kamal, A. Diagnostic yield of transthoracic echocardiography for stroke patients in a developing country. J Pak Med Assoc 2008;58:375–7.
3. de Abreu T, Mateus S, José Correia J. Therapy implications of transthoracic echocardiography in acute ischemic stroke patients. Stroke 2005;36:1565–6.
4. de Bruijn SFTM, Agema WRP, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006;37:2531–4.
5. Putaala J, Metso AJ, MD, Metso T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke. Stroke 2009;40:1195–203.
6. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent fora-men ovale in patients with stroke. N Engl J Med 1988;318:1148–52.
7. Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
8. Orencia AJ, Petty GW, Khandheria BK, et al. Risk of stroke with mitral valve prolapse in population-based cohort study; Stroke 1995;26:7–13.
9. Holmes M, Rathbone J, Littlewood C. Routine echocardiography in the management of stroke and transient ischaemic attack: a systematic review and economic evaluation. Health Technol Assess 2014;18:1–176.
10. Ryan T, Armstrong W. Feigenbaum’s echocardiography. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2009.
11. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010;137:263–72.
12. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–76.
13. Hays AG, Sacco RL, Rundek T. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke 2006;37:1715–9.
14. McNamara RL, Lima JA, Whelton PK, Powe NR. Echocardiographic identification of cardiovascular sources of emboli to guide clinical management of stroke: a cost-effectiveness analysis. Ann Intern Med 1997;127:775–87.
15. Alberts MJ, Bennett CA, Rutledge VR. Hospital charges for stroke patients. Stroke 1996;27:1825–8.
16. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947.
17. Levick DL, Stern G, Meyerhoefer CD, et al. Reducing unnecessary testing in a CPOE system through implementation of a targeted CDS intervention. BMC Med Inform Decis 2013;13:43.
18. Chen P, Tanasijevic MJ, Schoenenberger RA, et al. A computer-based intervention for improving the appropriateness of antiepileptic drug level monitoring. Am J Clin Pathol 2003;119:432–8.
19. Solberg LI, Wei F, Butler JC, et al. Effects of electronic decision support on high-tech diagnostic imaging orders and patients. Am J Manag Care 2010;16:102–6.
20. Menon BK, Coulter JI, Simerpret B, et al. Acute ischaemic stroke or transient ischaemic attack and the need for inpatient echocardiography. Postgrad Med J 2014;90:434–8.
1. Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke 1996;27:691.
2. Khan MA, Khealanj B, Kamal, A. Diagnostic yield of transthoracic echocardiography for stroke patients in a developing country. J Pak Med Assoc 2008;58:375–7.
3. de Abreu T, Mateus S, José Correia J. Therapy implications of transthoracic echocardiography in acute ischemic stroke patients. Stroke 2005;36:1565–6.
4. de Bruijn SFTM, Agema WRP, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006;37:2531–4.
5. Putaala J, Metso AJ, MD, Metso T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke. Stroke 2009;40:1195–203.
6. Lechat P, Mas JL, Lascault G, et al. Prevalence of patent fora-men ovale in patients with stroke. N Engl J Med 1988;318:1148–52.
7. Di Tullio MR, Jin Z, Russo C, et al. Patent foramen ovale, subclinical cerebrovascular disease, and ischemic stroke in a population-based cohort. J Am Coll Cardiol 2013; 62:35–41.
8. Orencia AJ, Petty GW, Khandheria BK, et al. Risk of stroke with mitral valve prolapse in population-based cohort study; Stroke 1995;26:7–13.
9. Holmes M, Rathbone J, Littlewood C. Routine echocardiography in the management of stroke and transient ischaemic attack: a systematic review and economic evaluation. Health Technol Assess 2014;18:1–176.
10. Ryan T, Armstrong W. Feigenbaum’s echocardiography. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2009.
11. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010;137:263–72.
12. Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–76.
13. Hays AG, Sacco RL, Rundek T. Left ventricular systolic dysfunction and the risk of ischemic stroke in a multiethnic population. Stroke 2006;37:1715–9.
14. McNamara RL, Lima JA, Whelton PK, Powe NR. Echocardiographic identification of cardiovascular sources of emboli to guide clinical management of stroke: a cost-effectiveness analysis. Ann Intern Med 1997;127:775–87.
15. Alberts MJ, Bennett CA, Rutledge VR. Hospital charges for stroke patients. Stroke 1996;27:1825–8.
16. Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947.
17. Levick DL, Stern G, Meyerhoefer CD, et al. Reducing unnecessary testing in a CPOE system through implementation of a targeted CDS intervention. BMC Med Inform Decis 2013;13:43.
18. Chen P, Tanasijevic MJ, Schoenenberger RA, et al. A computer-based intervention for improving the appropriateness of antiepileptic drug level monitoring. Am J Clin Pathol 2003;119:432–8.
19. Solberg LI, Wei F, Butler JC, et al. Effects of electronic decision support on high-tech diagnostic imaging orders and patients. Am J Manag Care 2010;16:102–6.
20. Menon BK, Coulter JI, Simerpret B, et al. Acute ischaemic stroke or transient ischaemic attack and the need for inpatient echocardiography. Postgrad Med J 2014;90:434–8.