User login
Do Combined Pharmacist and Prescriber Efforts on Medication Reconciliation Reduce Postdischarge Patient Emergency Department Visits and Hospital Readmissions?
Healthcare systems are targeting effective strategies to improve patient safety and reduce hospital readmissions. Hospital readmissions can be detrimental to patients’ health, a source of avoidable healthcare costs, and are frequently a reflection of the quality of patient care during transitions of care. Medication reconciliation (Med Rec) was identified as 1 of 12 interventions that may reduce 30-day readmissions; however, rigorously designed studies are scarce.1,2 Published systematic reviews and meta-analyses have produced mixed conclusions regarding the impact of Med Rec on unplanned 30-day readmissions.2-4
In several studies, researchers have established the positive impact of Med Rec on reducing patient medication discrepancies and potential adverse drug events.4-8 Pharmacy-led Med Rec interventions have been shown to easily identify more clinically relevant and higher impact medication discrepancies when compared to usual care.8 In a systematic review, Mueller et al.2 suggest that there are several interrelated elements that determine if a Med Rec intervention will influence hospital readmissions. These elements form a multicomponent “bundle” of interventions, including a systematic medication history process, admission reconciliation, patient education on discharge, discharge reconciliation, and communication to outpatient providers.9 Several prospective randomized controlled studies have demonstrated lower readmission rates and fewer visits to the emergency department (ED) after implementing a comprehensive, interprofessional, bundled intervention (including Med Rec) from admission to discharge.10-13 A 2016 systematic review and meta-analysis specifically evaluated pharmacy-led Med Rec programs (the majority of which included interventions involving multicomponent bundles) and demonstrated a significant reduction in posthospital healthcare utilization.14
Although comprehensive, interprofessional, bundled interventions have been shown to reduce readmission rates and ED visits in randomized controlled trials (RCTs), limited resources often prevent hospitals from consistently implementing all aspects of these multicomponent interventions. In practice, clinicians may provide varying components of the bundle, such as the combination of admission medication history by the pharmacist and discharge Med Rec completed by the physician alone. The unique impact of combined pharmacist and prescriber Med Rec interventions from admission to discharge on readmissions remains inconclusive. Further, it is unclear which high-risk patient groups will benefit the most from these interventions. We set out to evaluate the impact of an enhanced, interprofessional Med Rec process from admission to discharge (characterized within the context of a novel taxonomy continuum that specifies clinician involvement and intensity of services) on readmissions to hospital and ED visits within 30 days of discharge.
METHODS
We conducted a retrospective, observational, analytical cohort study using QuadraMed’s Computerized Patient Record and the EMITT (Electronic Medication Information Transfer Tool)15 to collect data from 2007 to 2011.
Setting
The study was conducted at a 417-bed tertiary care teaching hospital in Toronto, Ontario, Canada.
Med Rec Process and Description of Exposure (Intervention)
The targeted clinical areas had sustained interprofessional models of patient care in place from admission to discharge. They also were actively using an in-house EMITT to facilitate the documentation and tracking of Med Rec efforts throughout patient admission, transfer, and discharge.15 On admission, the pharmacist conducted a best possible medication history (BPMH). A BPMH provides the cornerstone for Med Rec. It differs from a routine medication history in that it involves (1) a systematic process for interviewing the patient (or family) and (2) a review of at least one other reliable source of information (eg, a provincial medication database, an inspection of medication vials, or contact with the community pharmacy) to obtain and verify patient medications (prescribed and nonprescribed). The pharmacist recorded the BPMH in the electronic patient record. The application supported admission and discharge Med Rec. On discharge, there were 2 options: (1) the prescriber alone would review and complete the discharge Med Rec and generate electronic prescriptions (Table 1, Silver level care) or (2) the pharmacist would collaborate with the prescriber to complete the discharge reconciliation and the prescriber would electronically generate prescriptions (Table 1, Gold level care). All clinical areas had a combined pharmacist and prescriber Med Rec model in place at admission, but the proportion of patients receiving discharge reconciliation completed by pharmacist and prescriber versus the prescriber-alone varied based on the individual clinician’s practices.
Patient Selection
All consecutive hospitalized patients admitted and discharged by the general internal medicine [GIM] service from March 2007 to December 2011 were included. The GIM service was chosen for the main analysis because they had been performing the intervention for the longest period of time and had the largest population of patients. Patients were identified via their hospital-specific medical record identification number and specific hospital-visit number. Patients were excluded if any of the following occurred: (1) the length of stay of their index admission was less than 24 hours; (2) they died during the visit; (3) they were transferred to a separate acute care inpatient facility; or (4) they left hospital against medical advice. Patient visits were excluded as index cases from the analysis if they were returning within 90 days of a previous discharge.
Outcomes
The primary study outcome was the occurrence of an inpatient readmission or ED visit within 30 days of discharge. In our secondary analyses, we examined the impact of the intervention on high-risk patient populations, such as those ≥65 years of age, with a length of stay, acuity of admission, Charlson comorbidity index, and emergency department visits in past 6 months (LACE) index score ≥10 (see supplementary Appendix 1 for LACE score description), on high-alert medications (1 or more of warfarin, insulin, digoxin, and opioids), and on ≥10 medications.
Data Collection
Identification of Exposure of Interest
We used the electronic database to capture all patients who received pharmacist and prescriber supported admission-to-discharge reconciliation. We explicitly defined increasing intensity of Med Rec care in categories of Bronze, Silver, and Gold care levels (Table 1). The exposed (intervention) group received an enhanced Med Rec bundle (patients receiving Gold level care). The control group was made of patients receiving a partial Med Rec Bundle (patients receiving Silver or Bronze level of care or below).
Determination of Hospital Visits
A search of administrative databases was used to determine if patients admitted to the targeted services had an ED visit or urgent inpatient admission to the study hospital within 30 days.
Statistical Analysis
A logistic regression for outcomes was performed. This yielded an adjusted odds ratio with a 95% confidence interval (CI) between the intervention and control groups. Statistical significance was determined with a 2-sided α level of 0.05. In the analysis, we used Statistical Analysis Software version 9.2.
In our multivariate logistic regression model, we adjusted for confounding factors that might influence the patients’ risk of readmission or the type of Med Rec they received upon discharge. By using administrative databases, patient level demographics, and the Charlson comorbidity index, the most responsible diagnosis and disease burden were collected. Medication-related factors collected included the number of medications on discharge and the presence of predefined high-alert medications. The number of medications on the medication discharge list was determined by using the electronic database. The final adjustment model included age, gender, the number of medications on discharge, and the LACE index score (supplementary Appendix 1). The LACE index score has been validated in Ontario, Canada, populations to quantify the risk of death or unplanned readmission within 30 days of discharge.24
Propensity Score Adjustment
Propensity scoring (probability of treatment assignment conditional on observed baseline characteristics) was planned a priori to account for possible factors that would impact whether a patient received the intervention or control care levels. The propensity score for receiving Med Rec was computed from a logistic model using Med Rec as the outcome. A structured iterative approach was used to refine this model to achieve covariate balance within the matched pairs. Covariate balance was measured by the standardized difference, in which an absolute standardized difference >10% represents meaningful imbalance.25 From the original cohort, we attempted to match patients who had the intervention to patients from the control by means of a matching algorithm using the logit of the propensity score for receiving the intervention.26
Subgroup Analysis
We also examined the impact of the intervention on high-risk patient populations such as those ≥65 years of age, with a LACE index score ≥10, on high-alert medications, and on ≥10 medications. A univariate analysis was conducted to identify patient-related risk predictors that may be independently correlated with a higher risk of hospital visits.
RESULTS
Baseline Characteristics
A total of 8678 patients representing 9931 unique visits met the inclusion criteria for analysis. There were 2541 unique visits (approximately 26% of visits) in the intervention group that received Gold level care and 7390 unique visits in the control group. The patients in the control group were largely patients who received the original standard of care at the institution, Silver level care (67% of the control group). Patients who received Bronze level care or less comprised 33% of the control group.
Patients in the intervention group were significantly older (average of 68 years old versus 64 years old) and on more medications. They also notably had a longer duration of stay in hospital, an increased percentage of visits with a LACE index score ≥10, and were more likely to be discharged home on a high-alert medication and with supports (Table 2).
Main Analysis
The main unadjusted analysis of GIM patients (n = 9931 visits) did not detect a difference in 30-day ED visits and readmissions between the intervention group (540 out of 2541; 21.2%) and control (1423 out of 7390; 19.3%; Table 3). By using a multivariate logistic regression model to account for age, sex, LACE index, and number of medications on discharge, the adjusted odds ratio was 1.06 (95% CI, 0.95-1.19; P = 0.33). After propensity score adjustment, the relative risk of readmission was 0.88 (16.7% vs 18.9%; 95% CI, 0.59-1.32; P = 0.54).
Secondary Analyses
In each predefined high-risk patient subgroup (age ≥65, LACE index score ≥10, number of discharge medications ≥10, and the presence of high-alert medications), analyses of our primary endpoint did not detect significant adjusted odds ratios (Table 4). In our univariate analysis, increasing number of medications, LACE index score, and male gender were independently correlated with a higher risk of hospital visits (supplementary Appendix 2).
DISCUSSION
Med Rec is widely recommended as a patient safety strategy to prevent clinically significant medication discrepancies at transitions in care.4-9 However, Med Rec varies widely in terms of what it entails and who delivers it, with the preponderance of evidence suggesting an impact on clinically significant medication discrepancies only when interprofessional care delivered includes a central role for pharmacists.27 Furthermore, Med Rec appears to impact short term readmissions only when embedded in a broader, multifaceted, bundled intervention in which pharmacists or other team members educate patients about their medications and deliver postdischarge follow-up phone calls.10-13
As very few hospitals have the resources to sustainably deliver intensive care bundles that are represented in RCTs (characterized by Platinum and Diamond levels of care in Table 1), in our observational study, we sought to explore whether a resource-attainable, enhanced Med Rec care bundle (Gold level) had an impact on hospital utilization compared to partial Med Rec care bundles (Bronze and Silver levels). In our findings, we did not detect a significant difference on ED visits and readmissions within 30 days between enhanced and partial care bundles. In a secondary analysis of the influence of the intervention on prespecified high-risk patient subgroups, we also did not detect a difference.
As far as we are aware, our long-term, observational study is the largest to date to explore a real-life, enhanced Med Rec intervention and examine its impact on meaningful patient outcomes. We extrapolated that our intervention group received several critical attributes of a successful bundle as discussed by Mueller in a systematic review.2 Our intervention included the following: (1) a systematic BPMH process on admission; (2) integrated admission-to-discharge reconciliation processes; (3) discharge delineation of medication changes since admission; (4) pharmacist involvement in reconciliation from admission to discharge; (5) an electronic platform; and (6) formal discharge reconciliation with interprofessional collaboration. Additional components in the bundle described by Mueller included the following: patient education at discharge, postdischarge communication with the patient, and communication with outpatient providers and medication management.
In our results, we did not find a difference in outcomes between the intervention and control groups. Therefore, it is possible that the enhanced bundle’s focus on interprofessional involvement in discharge reconciliation (Gold care level) has no impact on hospital utilization compared to partial care bundles (Silver and Bronze levels). Kwan et al.3 describe similar findings in their systematic review, in which they evaluated the effects of hospital-based Med Rec on unintentional discrepancies with nontrivial risks for harm to patients on 30-day postdischarge hospital visits. Kwan et al.3 concluded that larger well-designed studies are required to further evaluate this outcome, but authors of current published studies suggest that Med Rec alone probably does not reduce postdischarge hospital utilization within 30 days. Med Rec may have a more significant impact on utilization when bundled with other interventions that improve discharge coordination.3
There may be several reasons why we were unable to detect a significant difference between the intervention and control groups. One limitation is that our nonrandomized, retrospective design may have led to unmeasured confounders that impacted allocation into the intervention group versus the control group. It was notable that patients in the intervention group had an increased age, longer duration of hospital stay, more medications, and high-alert medications on discharge compared to the control group and that may have biased our results towards the null hypothesis. Although the propensity score analysis attempted to adjust for this, it also did not detect a significant difference between groups.
In addition, the existing standard of care during the study period allowed for patients in the control group to receive varying levels of Med Rec. Ideally, we would have compared the intervention to a placebo group that did not receive any Med Rec-related care elements. However, as this was a real-life observational study, the majority of patients received some Med Rec services as a part of the standard of care. As a result, 67% of patients in the control group received Silver level Med Rec with a BPMH, admission reconciliation, and prescriber-only discharge reconciliation. This may have made it more difficult to show an incremental benefit on readmissions between the intervention and control.
Also, our primary outcome of all-cause ED or hospital readmissions within 30 days may not have been sensitive enough to detect the effect of Med Rec interventions alone. Only a small proportion of readmissions within 30 days of discharge are preventable and many patient and community level factors responsible for readmissions cannot be controlled by the hospital’s actions.28 Comprehensive pharmacy interventions have demonstrated decreased hospitalizations and emergency visits at 12 months; however, the largest impact was seen on the more specific outcome of medication-related hospitalizations (80% reduction).29 Lastly, another limitation was that we were unable to capture hospital visits to other centres. However, in our region, almost 75% of readmissions are to the same site as the initial hospitalization.30
Overall, our findings in this study and novel characterization of Med Rec services are relevant to many hospital sites that are striving to implement integrated Med Rec with limited healthcare resources. Although interprofessional Med Rec likely reduces clinically significant medication discrepancies, enhanced interprofessional Med Rec on discharge (Gold Med Rec) alone may not be enough to impact hospital utilization compared to partial Med Rec services (Silver and Bronze Med Rec). Further research into practical, targeted Med Rec bundles on more specific outcomes (such as preventable postdischarge adverse events, “avoidable” hospital readmissions, and medication-related readmissions) may detect a significant benefit.
CONCLUSION
A long-term observational evaluation of interprofessional Med Rec did not detect a difference in 30-day postdischarge patient hospital visits between patients who received enhanced versus partial Med Rec patient care bundles. Researchers of future prospective studies could focus on evaluating high-risk populations or specific elements of Med Rec services on avoidable medication-related hospital admissions and postdischarge adverse drug events.
Acknowledgments
The authors thank Nita Dhir, MBA.
Presented as a poster and oral presentation at the 2012 American College of Clinical Pharmacy Annual Meeting, Hollywood, Florida, October 21-24, 2012, and as an encore poster presentation at the Canadian Society of Hospital Pharmacists Professional Practice Conference, Toronto, Canada, Feb 3, 2013.
Disclosure
The authors declare no conflicts of interest related to the manuscript submitted. All monies used for the research came from the University Health Network Department of Pharmacy Budget, including the pharmacy residency program.
1. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528. PubMed
2. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. PubMed
3. Kwan JL, Lo L, Sampson M, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:397-403. PubMed
4. Safer Health Care Now. Medication Reconciliation in Home Care Getting Started Kit. March 2015. www.ismp-canada.org/download/MedRec/Medrec_HC_English_GSK_v2.pdf. Accessed August 22, 2017.
5. Karapinar-Çarkit F, Borgsteede SD, Zoer J, Smit HJ, Egberts AC, van den Bemt PM. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010. PubMed
6. Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42(10):1373-1379. PubMed
7. Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169(8):771-780. PubMed
8. Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy-led medication reconciliation programmes at hospital transitions: a systematic review and meta-analysis. J Clin Pharm Ther. 2016;41(2):128-144. PubMed
9. Kaboli PJ, Fernandes O. Medication reconciliation: moving forward. Arch Intern Med. 2012;172(14):1069-1070. PubMed
10. Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4:211-218. PubMed
11. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178-187. PubMed
12. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older. Arch Intern Med. 2009:169(9):894-900. PubMed
13. Makowsky MJ, Koshman SL, Midodzi WK, Tsuyuki RT. Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study [NCT00351676]. Med Care. 2009;47(6):642-650. PubMed
14. Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e010003. PubMed
15. Cesta A, Bajcar JM, Ong SW, Fernandes OA. The EMITT study: development and evaluation of a medication information transfer tool. Ann Pharmacother. 2006:40(6):1074-1081 PubMed
16. Cornish P, et al. Unintended medication discrepancies at the time of hospital admission. Arch Internal Medicine, 2005, Feb: 165: 424-29. PubMed
17. Kwan Y, Fernandes OA, Nagge JJ, et al. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med. 2007;167(10):1034-1040 PubMed
18. Dedhia P, Kravet S, Bulger J, et al. A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc. 2009;57:1540–1546. PubMed
19. Murphy EM, Oxencis CJ, Klauck JA, et al. Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge. Am J Health Syst Pharm. 2009;66:2126–31 PubMed
20. , , , , , . A pharmacy discharge plan for hospitalized elderly patients - a randomized controlled trial. Age and Ageing. 2001;30(1):33-40. PubMed
21. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counselling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002 Dec;54(6):657–64. PubMed
22. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006 Mar 13;166(5):565–71. PubMed
23. Walker PC, Bernstein SJ, Jones JN, et al. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Arch Intern Med. 2009 Nov 23;169(21):2003–10. PubMed
24. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. PubMed
25. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following an acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387-398. PubMed
26. Rosenbaum PR., Donald BR. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38.
27. Fernandes O, Shojania KG. Medication reconciliation in the hospital: what, why, where, when, who and how? Healthc Q. 2012;15(Special Issue):42-49. PubMed
28. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
29. Zed PJ, Abu-Laban RB, Balen RM, et al. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. CMAJ. 2008;178(12):1563-1569. PubMed
30. Gruneir A, Dhalla IA, van Walraven C, et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104-e111. PubMed
Healthcare systems are targeting effective strategies to improve patient safety and reduce hospital readmissions. Hospital readmissions can be detrimental to patients’ health, a source of avoidable healthcare costs, and are frequently a reflection of the quality of patient care during transitions of care. Medication reconciliation (Med Rec) was identified as 1 of 12 interventions that may reduce 30-day readmissions; however, rigorously designed studies are scarce.1,2 Published systematic reviews and meta-analyses have produced mixed conclusions regarding the impact of Med Rec on unplanned 30-day readmissions.2-4
In several studies, researchers have established the positive impact of Med Rec on reducing patient medication discrepancies and potential adverse drug events.4-8 Pharmacy-led Med Rec interventions have been shown to easily identify more clinically relevant and higher impact medication discrepancies when compared to usual care.8 In a systematic review, Mueller et al.2 suggest that there are several interrelated elements that determine if a Med Rec intervention will influence hospital readmissions. These elements form a multicomponent “bundle” of interventions, including a systematic medication history process, admission reconciliation, patient education on discharge, discharge reconciliation, and communication to outpatient providers.9 Several prospective randomized controlled studies have demonstrated lower readmission rates and fewer visits to the emergency department (ED) after implementing a comprehensive, interprofessional, bundled intervention (including Med Rec) from admission to discharge.10-13 A 2016 systematic review and meta-analysis specifically evaluated pharmacy-led Med Rec programs (the majority of which included interventions involving multicomponent bundles) and demonstrated a significant reduction in posthospital healthcare utilization.14
Although comprehensive, interprofessional, bundled interventions have been shown to reduce readmission rates and ED visits in randomized controlled trials (RCTs), limited resources often prevent hospitals from consistently implementing all aspects of these multicomponent interventions. In practice, clinicians may provide varying components of the bundle, such as the combination of admission medication history by the pharmacist and discharge Med Rec completed by the physician alone. The unique impact of combined pharmacist and prescriber Med Rec interventions from admission to discharge on readmissions remains inconclusive. Further, it is unclear which high-risk patient groups will benefit the most from these interventions. We set out to evaluate the impact of an enhanced, interprofessional Med Rec process from admission to discharge (characterized within the context of a novel taxonomy continuum that specifies clinician involvement and intensity of services) on readmissions to hospital and ED visits within 30 days of discharge.
METHODS
We conducted a retrospective, observational, analytical cohort study using QuadraMed’s Computerized Patient Record and the EMITT (Electronic Medication Information Transfer Tool)15 to collect data from 2007 to 2011.
Setting
The study was conducted at a 417-bed tertiary care teaching hospital in Toronto, Ontario, Canada.
Med Rec Process and Description of Exposure (Intervention)
The targeted clinical areas had sustained interprofessional models of patient care in place from admission to discharge. They also were actively using an in-house EMITT to facilitate the documentation and tracking of Med Rec efforts throughout patient admission, transfer, and discharge.15 On admission, the pharmacist conducted a best possible medication history (BPMH). A BPMH provides the cornerstone for Med Rec. It differs from a routine medication history in that it involves (1) a systematic process for interviewing the patient (or family) and (2) a review of at least one other reliable source of information (eg, a provincial medication database, an inspection of medication vials, or contact with the community pharmacy) to obtain and verify patient medications (prescribed and nonprescribed). The pharmacist recorded the BPMH in the electronic patient record. The application supported admission and discharge Med Rec. On discharge, there were 2 options: (1) the prescriber alone would review and complete the discharge Med Rec and generate electronic prescriptions (Table 1, Silver level care) or (2) the pharmacist would collaborate with the prescriber to complete the discharge reconciliation and the prescriber would electronically generate prescriptions (Table 1, Gold level care). All clinical areas had a combined pharmacist and prescriber Med Rec model in place at admission, but the proportion of patients receiving discharge reconciliation completed by pharmacist and prescriber versus the prescriber-alone varied based on the individual clinician’s practices.
Patient Selection
All consecutive hospitalized patients admitted and discharged by the general internal medicine [GIM] service from March 2007 to December 2011 were included. The GIM service was chosen for the main analysis because they had been performing the intervention for the longest period of time and had the largest population of patients. Patients were identified via their hospital-specific medical record identification number and specific hospital-visit number. Patients were excluded if any of the following occurred: (1) the length of stay of their index admission was less than 24 hours; (2) they died during the visit; (3) they were transferred to a separate acute care inpatient facility; or (4) they left hospital against medical advice. Patient visits were excluded as index cases from the analysis if they were returning within 90 days of a previous discharge.
Outcomes
The primary study outcome was the occurrence of an inpatient readmission or ED visit within 30 days of discharge. In our secondary analyses, we examined the impact of the intervention on high-risk patient populations, such as those ≥65 years of age, with a length of stay, acuity of admission, Charlson comorbidity index, and emergency department visits in past 6 months (LACE) index score ≥10 (see supplementary Appendix 1 for LACE score description), on high-alert medications (1 or more of warfarin, insulin, digoxin, and opioids), and on ≥10 medications.
Data Collection
Identification of Exposure of Interest
We used the electronic database to capture all patients who received pharmacist and prescriber supported admission-to-discharge reconciliation. We explicitly defined increasing intensity of Med Rec care in categories of Bronze, Silver, and Gold care levels (Table 1). The exposed (intervention) group received an enhanced Med Rec bundle (patients receiving Gold level care). The control group was made of patients receiving a partial Med Rec Bundle (patients receiving Silver or Bronze level of care or below).
Determination of Hospital Visits
A search of administrative databases was used to determine if patients admitted to the targeted services had an ED visit or urgent inpatient admission to the study hospital within 30 days.
Statistical Analysis
A logistic regression for outcomes was performed. This yielded an adjusted odds ratio with a 95% confidence interval (CI) between the intervention and control groups. Statistical significance was determined with a 2-sided α level of 0.05. In the analysis, we used Statistical Analysis Software version 9.2.
In our multivariate logistic regression model, we adjusted for confounding factors that might influence the patients’ risk of readmission or the type of Med Rec they received upon discharge. By using administrative databases, patient level demographics, and the Charlson comorbidity index, the most responsible diagnosis and disease burden were collected. Medication-related factors collected included the number of medications on discharge and the presence of predefined high-alert medications. The number of medications on the medication discharge list was determined by using the electronic database. The final adjustment model included age, gender, the number of medications on discharge, and the LACE index score (supplementary Appendix 1). The LACE index score has been validated in Ontario, Canada, populations to quantify the risk of death or unplanned readmission within 30 days of discharge.24
Propensity Score Adjustment
Propensity scoring (probability of treatment assignment conditional on observed baseline characteristics) was planned a priori to account for possible factors that would impact whether a patient received the intervention or control care levels. The propensity score for receiving Med Rec was computed from a logistic model using Med Rec as the outcome. A structured iterative approach was used to refine this model to achieve covariate balance within the matched pairs. Covariate balance was measured by the standardized difference, in which an absolute standardized difference >10% represents meaningful imbalance.25 From the original cohort, we attempted to match patients who had the intervention to patients from the control by means of a matching algorithm using the logit of the propensity score for receiving the intervention.26
Subgroup Analysis
We also examined the impact of the intervention on high-risk patient populations such as those ≥65 years of age, with a LACE index score ≥10, on high-alert medications, and on ≥10 medications. A univariate analysis was conducted to identify patient-related risk predictors that may be independently correlated with a higher risk of hospital visits.
RESULTS
Baseline Characteristics
A total of 8678 patients representing 9931 unique visits met the inclusion criteria for analysis. There were 2541 unique visits (approximately 26% of visits) in the intervention group that received Gold level care and 7390 unique visits in the control group. The patients in the control group were largely patients who received the original standard of care at the institution, Silver level care (67% of the control group). Patients who received Bronze level care or less comprised 33% of the control group.
Patients in the intervention group were significantly older (average of 68 years old versus 64 years old) and on more medications. They also notably had a longer duration of stay in hospital, an increased percentage of visits with a LACE index score ≥10, and were more likely to be discharged home on a high-alert medication and with supports (Table 2).
Main Analysis
The main unadjusted analysis of GIM patients (n = 9931 visits) did not detect a difference in 30-day ED visits and readmissions between the intervention group (540 out of 2541; 21.2%) and control (1423 out of 7390; 19.3%; Table 3). By using a multivariate logistic regression model to account for age, sex, LACE index, and number of medications on discharge, the adjusted odds ratio was 1.06 (95% CI, 0.95-1.19; P = 0.33). After propensity score adjustment, the relative risk of readmission was 0.88 (16.7% vs 18.9%; 95% CI, 0.59-1.32; P = 0.54).
Secondary Analyses
In each predefined high-risk patient subgroup (age ≥65, LACE index score ≥10, number of discharge medications ≥10, and the presence of high-alert medications), analyses of our primary endpoint did not detect significant adjusted odds ratios (Table 4). In our univariate analysis, increasing number of medications, LACE index score, and male gender were independently correlated with a higher risk of hospital visits (supplementary Appendix 2).
DISCUSSION
Med Rec is widely recommended as a patient safety strategy to prevent clinically significant medication discrepancies at transitions in care.4-9 However, Med Rec varies widely in terms of what it entails and who delivers it, with the preponderance of evidence suggesting an impact on clinically significant medication discrepancies only when interprofessional care delivered includes a central role for pharmacists.27 Furthermore, Med Rec appears to impact short term readmissions only when embedded in a broader, multifaceted, bundled intervention in which pharmacists or other team members educate patients about their medications and deliver postdischarge follow-up phone calls.10-13
As very few hospitals have the resources to sustainably deliver intensive care bundles that are represented in RCTs (characterized by Platinum and Diamond levels of care in Table 1), in our observational study, we sought to explore whether a resource-attainable, enhanced Med Rec care bundle (Gold level) had an impact on hospital utilization compared to partial Med Rec care bundles (Bronze and Silver levels). In our findings, we did not detect a significant difference on ED visits and readmissions within 30 days between enhanced and partial care bundles. In a secondary analysis of the influence of the intervention on prespecified high-risk patient subgroups, we also did not detect a difference.
As far as we are aware, our long-term, observational study is the largest to date to explore a real-life, enhanced Med Rec intervention and examine its impact on meaningful patient outcomes. We extrapolated that our intervention group received several critical attributes of a successful bundle as discussed by Mueller in a systematic review.2 Our intervention included the following: (1) a systematic BPMH process on admission; (2) integrated admission-to-discharge reconciliation processes; (3) discharge delineation of medication changes since admission; (4) pharmacist involvement in reconciliation from admission to discharge; (5) an electronic platform; and (6) formal discharge reconciliation with interprofessional collaboration. Additional components in the bundle described by Mueller included the following: patient education at discharge, postdischarge communication with the patient, and communication with outpatient providers and medication management.
In our results, we did not find a difference in outcomes between the intervention and control groups. Therefore, it is possible that the enhanced bundle’s focus on interprofessional involvement in discharge reconciliation (Gold care level) has no impact on hospital utilization compared to partial care bundles (Silver and Bronze levels). Kwan et al.3 describe similar findings in their systematic review, in which they evaluated the effects of hospital-based Med Rec on unintentional discrepancies with nontrivial risks for harm to patients on 30-day postdischarge hospital visits. Kwan et al.3 concluded that larger well-designed studies are required to further evaluate this outcome, but authors of current published studies suggest that Med Rec alone probably does not reduce postdischarge hospital utilization within 30 days. Med Rec may have a more significant impact on utilization when bundled with other interventions that improve discharge coordination.3
There may be several reasons why we were unable to detect a significant difference between the intervention and control groups. One limitation is that our nonrandomized, retrospective design may have led to unmeasured confounders that impacted allocation into the intervention group versus the control group. It was notable that patients in the intervention group had an increased age, longer duration of hospital stay, more medications, and high-alert medications on discharge compared to the control group and that may have biased our results towards the null hypothesis. Although the propensity score analysis attempted to adjust for this, it also did not detect a significant difference between groups.
In addition, the existing standard of care during the study period allowed for patients in the control group to receive varying levels of Med Rec. Ideally, we would have compared the intervention to a placebo group that did not receive any Med Rec-related care elements. However, as this was a real-life observational study, the majority of patients received some Med Rec services as a part of the standard of care. As a result, 67% of patients in the control group received Silver level Med Rec with a BPMH, admission reconciliation, and prescriber-only discharge reconciliation. This may have made it more difficult to show an incremental benefit on readmissions between the intervention and control.
Also, our primary outcome of all-cause ED or hospital readmissions within 30 days may not have been sensitive enough to detect the effect of Med Rec interventions alone. Only a small proportion of readmissions within 30 days of discharge are preventable and many patient and community level factors responsible for readmissions cannot be controlled by the hospital’s actions.28 Comprehensive pharmacy interventions have demonstrated decreased hospitalizations and emergency visits at 12 months; however, the largest impact was seen on the more specific outcome of medication-related hospitalizations (80% reduction).29 Lastly, another limitation was that we were unable to capture hospital visits to other centres. However, in our region, almost 75% of readmissions are to the same site as the initial hospitalization.30
Overall, our findings in this study and novel characterization of Med Rec services are relevant to many hospital sites that are striving to implement integrated Med Rec with limited healthcare resources. Although interprofessional Med Rec likely reduces clinically significant medication discrepancies, enhanced interprofessional Med Rec on discharge (Gold Med Rec) alone may not be enough to impact hospital utilization compared to partial Med Rec services (Silver and Bronze Med Rec). Further research into practical, targeted Med Rec bundles on more specific outcomes (such as preventable postdischarge adverse events, “avoidable” hospital readmissions, and medication-related readmissions) may detect a significant benefit.
CONCLUSION
A long-term observational evaluation of interprofessional Med Rec did not detect a difference in 30-day postdischarge patient hospital visits between patients who received enhanced versus partial Med Rec patient care bundles. Researchers of future prospective studies could focus on evaluating high-risk populations or specific elements of Med Rec services on avoidable medication-related hospital admissions and postdischarge adverse drug events.
Acknowledgments
The authors thank Nita Dhir, MBA.
Presented as a poster and oral presentation at the 2012 American College of Clinical Pharmacy Annual Meeting, Hollywood, Florida, October 21-24, 2012, and as an encore poster presentation at the Canadian Society of Hospital Pharmacists Professional Practice Conference, Toronto, Canada, Feb 3, 2013.
Disclosure
The authors declare no conflicts of interest related to the manuscript submitted. All monies used for the research came from the University Health Network Department of Pharmacy Budget, including the pharmacy residency program.
Healthcare systems are targeting effective strategies to improve patient safety and reduce hospital readmissions. Hospital readmissions can be detrimental to patients’ health, a source of avoidable healthcare costs, and are frequently a reflection of the quality of patient care during transitions of care. Medication reconciliation (Med Rec) was identified as 1 of 12 interventions that may reduce 30-day readmissions; however, rigorously designed studies are scarce.1,2 Published systematic reviews and meta-analyses have produced mixed conclusions regarding the impact of Med Rec on unplanned 30-day readmissions.2-4
In several studies, researchers have established the positive impact of Med Rec on reducing patient medication discrepancies and potential adverse drug events.4-8 Pharmacy-led Med Rec interventions have been shown to easily identify more clinically relevant and higher impact medication discrepancies when compared to usual care.8 In a systematic review, Mueller et al.2 suggest that there are several interrelated elements that determine if a Med Rec intervention will influence hospital readmissions. These elements form a multicomponent “bundle” of interventions, including a systematic medication history process, admission reconciliation, patient education on discharge, discharge reconciliation, and communication to outpatient providers.9 Several prospective randomized controlled studies have demonstrated lower readmission rates and fewer visits to the emergency department (ED) after implementing a comprehensive, interprofessional, bundled intervention (including Med Rec) from admission to discharge.10-13 A 2016 systematic review and meta-analysis specifically evaluated pharmacy-led Med Rec programs (the majority of which included interventions involving multicomponent bundles) and demonstrated a significant reduction in posthospital healthcare utilization.14
Although comprehensive, interprofessional, bundled interventions have been shown to reduce readmission rates and ED visits in randomized controlled trials (RCTs), limited resources often prevent hospitals from consistently implementing all aspects of these multicomponent interventions. In practice, clinicians may provide varying components of the bundle, such as the combination of admission medication history by the pharmacist and discharge Med Rec completed by the physician alone. The unique impact of combined pharmacist and prescriber Med Rec interventions from admission to discharge on readmissions remains inconclusive. Further, it is unclear which high-risk patient groups will benefit the most from these interventions. We set out to evaluate the impact of an enhanced, interprofessional Med Rec process from admission to discharge (characterized within the context of a novel taxonomy continuum that specifies clinician involvement and intensity of services) on readmissions to hospital and ED visits within 30 days of discharge.
METHODS
We conducted a retrospective, observational, analytical cohort study using QuadraMed’s Computerized Patient Record and the EMITT (Electronic Medication Information Transfer Tool)15 to collect data from 2007 to 2011.
Setting
The study was conducted at a 417-bed tertiary care teaching hospital in Toronto, Ontario, Canada.
Med Rec Process and Description of Exposure (Intervention)
The targeted clinical areas had sustained interprofessional models of patient care in place from admission to discharge. They also were actively using an in-house EMITT to facilitate the documentation and tracking of Med Rec efforts throughout patient admission, transfer, and discharge.15 On admission, the pharmacist conducted a best possible medication history (BPMH). A BPMH provides the cornerstone for Med Rec. It differs from a routine medication history in that it involves (1) a systematic process for interviewing the patient (or family) and (2) a review of at least one other reliable source of information (eg, a provincial medication database, an inspection of medication vials, or contact with the community pharmacy) to obtain and verify patient medications (prescribed and nonprescribed). The pharmacist recorded the BPMH in the electronic patient record. The application supported admission and discharge Med Rec. On discharge, there were 2 options: (1) the prescriber alone would review and complete the discharge Med Rec and generate electronic prescriptions (Table 1, Silver level care) or (2) the pharmacist would collaborate with the prescriber to complete the discharge reconciliation and the prescriber would electronically generate prescriptions (Table 1, Gold level care). All clinical areas had a combined pharmacist and prescriber Med Rec model in place at admission, but the proportion of patients receiving discharge reconciliation completed by pharmacist and prescriber versus the prescriber-alone varied based on the individual clinician’s practices.
Patient Selection
All consecutive hospitalized patients admitted and discharged by the general internal medicine [GIM] service from March 2007 to December 2011 were included. The GIM service was chosen for the main analysis because they had been performing the intervention for the longest period of time and had the largest population of patients. Patients were identified via their hospital-specific medical record identification number and specific hospital-visit number. Patients were excluded if any of the following occurred: (1) the length of stay of their index admission was less than 24 hours; (2) they died during the visit; (3) they were transferred to a separate acute care inpatient facility; or (4) they left hospital against medical advice. Patient visits were excluded as index cases from the analysis if they were returning within 90 days of a previous discharge.
Outcomes
The primary study outcome was the occurrence of an inpatient readmission or ED visit within 30 days of discharge. In our secondary analyses, we examined the impact of the intervention on high-risk patient populations, such as those ≥65 years of age, with a length of stay, acuity of admission, Charlson comorbidity index, and emergency department visits in past 6 months (LACE) index score ≥10 (see supplementary Appendix 1 for LACE score description), on high-alert medications (1 or more of warfarin, insulin, digoxin, and opioids), and on ≥10 medications.
Data Collection
Identification of Exposure of Interest
We used the electronic database to capture all patients who received pharmacist and prescriber supported admission-to-discharge reconciliation. We explicitly defined increasing intensity of Med Rec care in categories of Bronze, Silver, and Gold care levels (Table 1). The exposed (intervention) group received an enhanced Med Rec bundle (patients receiving Gold level care). The control group was made of patients receiving a partial Med Rec Bundle (patients receiving Silver or Bronze level of care or below).
Determination of Hospital Visits
A search of administrative databases was used to determine if patients admitted to the targeted services had an ED visit or urgent inpatient admission to the study hospital within 30 days.
Statistical Analysis
A logistic regression for outcomes was performed. This yielded an adjusted odds ratio with a 95% confidence interval (CI) between the intervention and control groups. Statistical significance was determined with a 2-sided α level of 0.05. In the analysis, we used Statistical Analysis Software version 9.2.
In our multivariate logistic regression model, we adjusted for confounding factors that might influence the patients’ risk of readmission or the type of Med Rec they received upon discharge. By using administrative databases, patient level demographics, and the Charlson comorbidity index, the most responsible diagnosis and disease burden were collected. Medication-related factors collected included the number of medications on discharge and the presence of predefined high-alert medications. The number of medications on the medication discharge list was determined by using the electronic database. The final adjustment model included age, gender, the number of medications on discharge, and the LACE index score (supplementary Appendix 1). The LACE index score has been validated in Ontario, Canada, populations to quantify the risk of death or unplanned readmission within 30 days of discharge.24
Propensity Score Adjustment
Propensity scoring (probability of treatment assignment conditional on observed baseline characteristics) was planned a priori to account for possible factors that would impact whether a patient received the intervention or control care levels. The propensity score for receiving Med Rec was computed from a logistic model using Med Rec as the outcome. A structured iterative approach was used to refine this model to achieve covariate balance within the matched pairs. Covariate balance was measured by the standardized difference, in which an absolute standardized difference >10% represents meaningful imbalance.25 From the original cohort, we attempted to match patients who had the intervention to patients from the control by means of a matching algorithm using the logit of the propensity score for receiving the intervention.26
Subgroup Analysis
We also examined the impact of the intervention on high-risk patient populations such as those ≥65 years of age, with a LACE index score ≥10, on high-alert medications, and on ≥10 medications. A univariate analysis was conducted to identify patient-related risk predictors that may be independently correlated with a higher risk of hospital visits.
RESULTS
Baseline Characteristics
A total of 8678 patients representing 9931 unique visits met the inclusion criteria for analysis. There were 2541 unique visits (approximately 26% of visits) in the intervention group that received Gold level care and 7390 unique visits in the control group. The patients in the control group were largely patients who received the original standard of care at the institution, Silver level care (67% of the control group). Patients who received Bronze level care or less comprised 33% of the control group.
Patients in the intervention group were significantly older (average of 68 years old versus 64 years old) and on more medications. They also notably had a longer duration of stay in hospital, an increased percentage of visits with a LACE index score ≥10, and were more likely to be discharged home on a high-alert medication and with supports (Table 2).
Main Analysis
The main unadjusted analysis of GIM patients (n = 9931 visits) did not detect a difference in 30-day ED visits and readmissions between the intervention group (540 out of 2541; 21.2%) and control (1423 out of 7390; 19.3%; Table 3). By using a multivariate logistic regression model to account for age, sex, LACE index, and number of medications on discharge, the adjusted odds ratio was 1.06 (95% CI, 0.95-1.19; P = 0.33). After propensity score adjustment, the relative risk of readmission was 0.88 (16.7% vs 18.9%; 95% CI, 0.59-1.32; P = 0.54).
Secondary Analyses
In each predefined high-risk patient subgroup (age ≥65, LACE index score ≥10, number of discharge medications ≥10, and the presence of high-alert medications), analyses of our primary endpoint did not detect significant adjusted odds ratios (Table 4). In our univariate analysis, increasing number of medications, LACE index score, and male gender were independently correlated with a higher risk of hospital visits (supplementary Appendix 2).
DISCUSSION
Med Rec is widely recommended as a patient safety strategy to prevent clinically significant medication discrepancies at transitions in care.4-9 However, Med Rec varies widely in terms of what it entails and who delivers it, with the preponderance of evidence suggesting an impact on clinically significant medication discrepancies only when interprofessional care delivered includes a central role for pharmacists.27 Furthermore, Med Rec appears to impact short term readmissions only when embedded in a broader, multifaceted, bundled intervention in which pharmacists or other team members educate patients about their medications and deliver postdischarge follow-up phone calls.10-13
As very few hospitals have the resources to sustainably deliver intensive care bundles that are represented in RCTs (characterized by Platinum and Diamond levels of care in Table 1), in our observational study, we sought to explore whether a resource-attainable, enhanced Med Rec care bundle (Gold level) had an impact on hospital utilization compared to partial Med Rec care bundles (Bronze and Silver levels). In our findings, we did not detect a significant difference on ED visits and readmissions within 30 days between enhanced and partial care bundles. In a secondary analysis of the influence of the intervention on prespecified high-risk patient subgroups, we also did not detect a difference.
As far as we are aware, our long-term, observational study is the largest to date to explore a real-life, enhanced Med Rec intervention and examine its impact on meaningful patient outcomes. We extrapolated that our intervention group received several critical attributes of a successful bundle as discussed by Mueller in a systematic review.2 Our intervention included the following: (1) a systematic BPMH process on admission; (2) integrated admission-to-discharge reconciliation processes; (3) discharge delineation of medication changes since admission; (4) pharmacist involvement in reconciliation from admission to discharge; (5) an electronic platform; and (6) formal discharge reconciliation with interprofessional collaboration. Additional components in the bundle described by Mueller included the following: patient education at discharge, postdischarge communication with the patient, and communication with outpatient providers and medication management.
In our results, we did not find a difference in outcomes between the intervention and control groups. Therefore, it is possible that the enhanced bundle’s focus on interprofessional involvement in discharge reconciliation (Gold care level) has no impact on hospital utilization compared to partial care bundles (Silver and Bronze levels). Kwan et al.3 describe similar findings in their systematic review, in which they evaluated the effects of hospital-based Med Rec on unintentional discrepancies with nontrivial risks for harm to patients on 30-day postdischarge hospital visits. Kwan et al.3 concluded that larger well-designed studies are required to further evaluate this outcome, but authors of current published studies suggest that Med Rec alone probably does not reduce postdischarge hospital utilization within 30 days. Med Rec may have a more significant impact on utilization when bundled with other interventions that improve discharge coordination.3
There may be several reasons why we were unable to detect a significant difference between the intervention and control groups. One limitation is that our nonrandomized, retrospective design may have led to unmeasured confounders that impacted allocation into the intervention group versus the control group. It was notable that patients in the intervention group had an increased age, longer duration of hospital stay, more medications, and high-alert medications on discharge compared to the control group and that may have biased our results towards the null hypothesis. Although the propensity score analysis attempted to adjust for this, it also did not detect a significant difference between groups.
In addition, the existing standard of care during the study period allowed for patients in the control group to receive varying levels of Med Rec. Ideally, we would have compared the intervention to a placebo group that did not receive any Med Rec-related care elements. However, as this was a real-life observational study, the majority of patients received some Med Rec services as a part of the standard of care. As a result, 67% of patients in the control group received Silver level Med Rec with a BPMH, admission reconciliation, and prescriber-only discharge reconciliation. This may have made it more difficult to show an incremental benefit on readmissions between the intervention and control.
Also, our primary outcome of all-cause ED or hospital readmissions within 30 days may not have been sensitive enough to detect the effect of Med Rec interventions alone. Only a small proportion of readmissions within 30 days of discharge are preventable and many patient and community level factors responsible for readmissions cannot be controlled by the hospital’s actions.28 Comprehensive pharmacy interventions have demonstrated decreased hospitalizations and emergency visits at 12 months; however, the largest impact was seen on the more specific outcome of medication-related hospitalizations (80% reduction).29 Lastly, another limitation was that we were unable to capture hospital visits to other centres. However, in our region, almost 75% of readmissions are to the same site as the initial hospitalization.30
Overall, our findings in this study and novel characterization of Med Rec services are relevant to many hospital sites that are striving to implement integrated Med Rec with limited healthcare resources. Although interprofessional Med Rec likely reduces clinically significant medication discrepancies, enhanced interprofessional Med Rec on discharge (Gold Med Rec) alone may not be enough to impact hospital utilization compared to partial Med Rec services (Silver and Bronze Med Rec). Further research into practical, targeted Med Rec bundles on more specific outcomes (such as preventable postdischarge adverse events, “avoidable” hospital readmissions, and medication-related readmissions) may detect a significant benefit.
CONCLUSION
A long-term observational evaluation of interprofessional Med Rec did not detect a difference in 30-day postdischarge patient hospital visits between patients who received enhanced versus partial Med Rec patient care bundles. Researchers of future prospective studies could focus on evaluating high-risk populations or specific elements of Med Rec services on avoidable medication-related hospital admissions and postdischarge adverse drug events.
Acknowledgments
The authors thank Nita Dhir, MBA.
Presented as a poster and oral presentation at the 2012 American College of Clinical Pharmacy Annual Meeting, Hollywood, Florida, October 21-24, 2012, and as an encore poster presentation at the Canadian Society of Hospital Pharmacists Professional Practice Conference, Toronto, Canada, Feb 3, 2013.
Disclosure
The authors declare no conflicts of interest related to the manuscript submitted. All monies used for the research came from the University Health Network Department of Pharmacy Budget, including the pharmacy residency program.
1. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528. PubMed
2. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. PubMed
3. Kwan JL, Lo L, Sampson M, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:397-403. PubMed
4. Safer Health Care Now. Medication Reconciliation in Home Care Getting Started Kit. March 2015. www.ismp-canada.org/download/MedRec/Medrec_HC_English_GSK_v2.pdf. Accessed August 22, 2017.
5. Karapinar-Çarkit F, Borgsteede SD, Zoer J, Smit HJ, Egberts AC, van den Bemt PM. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010. PubMed
6. Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42(10):1373-1379. PubMed
7. Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169(8):771-780. PubMed
8. Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy-led medication reconciliation programmes at hospital transitions: a systematic review and meta-analysis. J Clin Pharm Ther. 2016;41(2):128-144. PubMed
9. Kaboli PJ, Fernandes O. Medication reconciliation: moving forward. Arch Intern Med. 2012;172(14):1069-1070. PubMed
10. Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4:211-218. PubMed
11. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178-187. PubMed
12. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older. Arch Intern Med. 2009:169(9):894-900. PubMed
13. Makowsky MJ, Koshman SL, Midodzi WK, Tsuyuki RT. Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study [NCT00351676]. Med Care. 2009;47(6):642-650. PubMed
14. Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e010003. PubMed
15. Cesta A, Bajcar JM, Ong SW, Fernandes OA. The EMITT study: development and evaluation of a medication information transfer tool. Ann Pharmacother. 2006:40(6):1074-1081 PubMed
16. Cornish P, et al. Unintended medication discrepancies at the time of hospital admission. Arch Internal Medicine, 2005, Feb: 165: 424-29. PubMed
17. Kwan Y, Fernandes OA, Nagge JJ, et al. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med. 2007;167(10):1034-1040 PubMed
18. Dedhia P, Kravet S, Bulger J, et al. A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc. 2009;57:1540–1546. PubMed
19. Murphy EM, Oxencis CJ, Klauck JA, et al. Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge. Am J Health Syst Pharm. 2009;66:2126–31 PubMed
20. , , , , , . A pharmacy discharge plan for hospitalized elderly patients - a randomized controlled trial. Age and Ageing. 2001;30(1):33-40. PubMed
21. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counselling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002 Dec;54(6):657–64. PubMed
22. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006 Mar 13;166(5):565–71. PubMed
23. Walker PC, Bernstein SJ, Jones JN, et al. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Arch Intern Med. 2009 Nov 23;169(21):2003–10. PubMed
24. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. PubMed
25. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following an acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387-398. PubMed
26. Rosenbaum PR., Donald BR. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38.
27. Fernandes O, Shojania KG. Medication reconciliation in the hospital: what, why, where, when, who and how? Healthc Q. 2012;15(Special Issue):42-49. PubMed
28. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
29. Zed PJ, Abu-Laban RB, Balen RM, et al. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. CMAJ. 2008;178(12):1563-1569. PubMed
30. Gruneir A, Dhalla IA, van Walraven C, et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104-e111. PubMed
1. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520-528. PubMed
2. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. PubMed
3. Kwan JL, Lo L, Sampson M, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:397-403. PubMed
4. Safer Health Care Now. Medication Reconciliation in Home Care Getting Started Kit. March 2015. www.ismp-canada.org/download/MedRec/Medrec_HC_English_GSK_v2.pdf. Accessed August 22, 2017.
5. Karapinar-Çarkit F, Borgsteede SD, Zoer J, Smit HJ, Egberts AC, van den Bemt PM. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010. PubMed
6. Wong JD, Bajcar JM, Wong GG, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42(10):1373-1379. PubMed
7. Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169(8):771-780. PubMed
8. Mekonnen AB, McLachlan AJ, Brien JA. Pharmacy-led medication reconciliation programmes at hospital transitions: a systematic review and meta-analysis. J Clin Pharm Ther. 2016;41(2):128-144. PubMed
9. Kaboli PJ, Fernandes O. Medication reconciliation: moving forward. Arch Intern Med. 2012;172(14):1069-1070. PubMed
10. Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4:211-218. PubMed
11. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178-187. PubMed
12. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older. Arch Intern Med. 2009:169(9):894-900. PubMed
13. Makowsky MJ, Koshman SL, Midodzi WK, Tsuyuki RT. Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study [NCT00351676]. Med Care. 2009;47(6):642-650. PubMed
14. Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e010003. PubMed
15. Cesta A, Bajcar JM, Ong SW, Fernandes OA. The EMITT study: development and evaluation of a medication information transfer tool. Ann Pharmacother. 2006:40(6):1074-1081 PubMed
16. Cornish P, et al. Unintended medication discrepancies at the time of hospital admission. Arch Internal Medicine, 2005, Feb: 165: 424-29. PubMed
17. Kwan Y, Fernandes OA, Nagge JJ, et al. Pharmacist medication assessments in a surgical preadmission clinic. Arch Intern Med. 2007;167(10):1034-1040 PubMed
18. Dedhia P, Kravet S, Bulger J, et al. A quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc. 2009;57:1540–1546. PubMed
19. Murphy EM, Oxencis CJ, Klauck JA, et al. Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge. Am J Health Syst Pharm. 2009;66:2126–31 PubMed
20. , , , , , . A pharmacy discharge plan for hospitalized elderly patients - a randomized controlled trial. Age and Ageing. 2001;30(1):33-40. PubMed
21. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counselling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002 Dec;54(6):657–64. PubMed
22. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006 Mar 13;166(5):565–71. PubMed
23. Walker PC, Bernstein SJ, Jones JN, et al. Impact of a pharmacist-facilitated hospital discharge program: a quasi-experimental study. Arch Intern Med. 2009 Nov 23;169(21):2003–10. PubMed
24. van Walraven C, Dhalla IA, Bell C, et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551-557. PubMed
25. Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following an acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54(4):387-398. PubMed
26. Rosenbaum PR., Donald BR. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38.
27. Fernandes O, Shojania KG. Medication reconciliation in the hospital: what, why, where, when, who and how? Healthc Q. 2012;15(Special Issue):42-49. PubMed
28. Joynt KE, Jha AK. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366-1369. PubMed
29. Zed PJ, Abu-Laban RB, Balen RM, et al. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. CMAJ. 2008;178(12):1563-1569. PubMed
30. Gruneir A, Dhalla IA, van Walraven C, et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104-e111. PubMed
© 2018 Society of Hospital Medicine
Does the Week-End Justify the Means?
Let’s face it—rates of hospital admission are on the rise, but there are still just 7 days in a week. That means that patients are increasingly admitted on weekdays and on the weekend, requiring more nurses and doctors to look after them. Why then are there no lines for coffee on a Saturday? Does this reduced intensity of staffing translate into worse care for our patients?
Since one of its earliest descriptions in hospitalized patients, the “weekend effect” has been extensively studied in various patient populations and hospital settings.1-5 The results have been varied, depending on the place of care,6 reason for care, type of admission,5,7 or admitting diagnosis.1,8,9 Many researchers have posited the drivers behind the weekend effect, including understaffed wards, intensity of specialist care, delays in procedural treatments, or severity of illness, but the truth is that we still don’t know.
Pauls et al. performed a robust systematic review and meta-analysis examining the rates of in-hospital mortality in patients admitted on the weekend compared with those admitted on weekdays.10 They analyzed predetermined subgroups to identify system- and patient-level factors associated with a difference in weekend mortality.
A total of 97 studies—comprising an astounding 51 million patients—was included in the study. They found that individuals admitted on the weekend carried an almost 20% increase in the risk of death compared with those who landed in hospital on a weekday. The effect was present for both in-hospital deaths and when looking specifically at 30-day mortality. Translating these findings into practice, an additional 14 deaths per 1000 admissions occur when patients are admitted on the weekend. Brain surgery can be less risky.11
Despite this concerning finding, no individual factor was identified that could account for the effect. There was a 16% and 11% increase in mortality in weekend patients associated with decreased hospital staffing and delays to procedural therapies, respectively. No differences were found when examining reduced rates of procedures or illness severity on weekends compared with weekdays. But one must always interpret subgroup analyses, even prespecified ones, with caution because they often lack the statistical power to make concrete conclusions.
To this end, an important finding of the study by Pauls et al. highlights the variation in mortality risk as it relates to the weekend effect.10 Even for individuals with cancer, a disease with a relatively predictable rate of decline, there are weekend differences in mortality risk that depend upon the type of cancer.8,12 This heterogeneity persists when examining for the possible factors that contribute to the effect, introducing a significant amount of noise into the analysis, and may explain why research to date has been unable to find the proverbial black cat in the coal cellar.
One thing Pauls et al. makes clear is that the weekend effect appears to be a real phenomenon, despite significant heterogeneity in the literature.10 Only a high-quality, systematic review has the capability to draw such conclusions. Prior work demonstrates that this effect is substantial in some individuals,and this study confirms that it perseveres beyond an immediate time period following admission.1,9 The elements contributing to the weekend effect remain undefined and are likely as complex as our healthcare system itself.
Society and policy makers should resist the tantalizing urge to invoke interventions aimed at fixing this issue before fully understanding the drivers of a system problem. The government of the United Kingdom has decreed a manifesto to create a “7-day National Health Service,” in which weekend services and physician staffing will match that of the weekdays. Considering recent labor tensions between junior doctors in the United Kingdom over pay and working hours, the stakes are at an all-time high.
But such drastic measures violate a primary directive of quality improvement science to study and understand the problem before reflexively jumping to solutions. This will require new research endeavors aimed at determining the underlying factor(s) responsible for the weekend effect. Once we are confident in its cause, only then can careful evaluation of targeted interventions aimed at the highest-risk admissions be instituted. As global hospital and healthcare budgets bend under increasing strain, a critical component of any proposed intervention must be to examine the cost-effectiveness in doing so. Because the weekend effect is one of increased mortality, it will be hard to justify an acceptable price for an individual’s life. And it is not as straightforward as a randomized trial examining the efficacy of parachutes. Any formal evaluation must account for the unintended consequences and opportunity costs of implementing a potential fix aimed at minimizing the weekend effect.
The weekend effect has now been studied for over 15 years. Pauls et al. add to our knowledge of this phenomenon, confirming that the overall risk of mortality for patients admitted on the weekend is real, variable, and substantial.10 As more individuals are admitted to hospitals, resulting in increasing numbers of admissions on the weekend, a desperate search for the underlying cause must be carried out before we can fix it. Whatever the means to the end, our elation will continue to be tempered by a feeling of uneasiness every time our coworkers joyously exclaim, “TGIF!”
Disclosure
The authors have nothing to disclose.
1. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376. PubMed
2. Bell CM, Redelmeier DA. Waiting for urgent procedures on the weekend among emergently hospitalized patients. AJM. 2004;117(3):175-181. doi:10.1016/j.amjmed.2004.02.047. PubMed
3. Kalaitzakis E, Helgeson J, Strömdahl M, Tóth E. Weekend admission in upper GI bleeding: does it have an impact on outcome? Gastrointest Endosc. 2015;81(5):1295-1296. doi:10.1016/j.gie.2014.12.003. PubMed
4. Nanchal R, Kumar G, Taneja A, et al. Pulmonary embolism: the weekend effect. Chest. 2012;142(3):690-696. doi:10.1378/chest.11-2663. PubMed
5. Ricciardi R, Roberts PL, Read TE, Baxter NN, Marcello PW, Schoetz DJ. Mortality rate after nonelective hospital admission. Arch Surg. 2011;146(5):545-551. PubMed
6. Wunsch H, Mapstone J, Brady T, Hanks R, Rowan K. Hospital mortality associated with day and time of admission to intensive care units. Intensive Care Med. 2004;30(5):895-901. doi:10.1007/s00134-004-2170-3. PubMed
7. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009. PubMed
8. Lapointe-Shaw L, Bell CM. It’s not you, it’s me: time to narrow the gap in weekend care. BMJ Qual Saf. 2014;23(3):180-182. doi:10.1136/bmjqs-2013-002674. PubMed
9. Concha OP, Gallego B, Hillman K, Delaney GP, Coiera E. Do variations in hospital mortality patterns after weekend admission reflect reduced quality of care or different patient cohorts? A population-based study. BMJ Qual Saf. 2014;23(3):215-222. doi:10.1136/bmjqs-2013-002218. PubMed
10. Pauls LA, Johnson-Paben R, McGready J, Murphy JD, Pronovost PJ, Wu CL. The Weekend Effect in Hospitalized Patients: A Meta-analysis. J Hosp Med. 2017;12(9):760-766. PubMed
11. American College of Surgeons. NSQIP Risk Calculator. http://riskcalculator.facs.org/RiskCalculator/. Accessed on July 5, 2017.
12. Lapointe-Shaw L, Abushomar H, Chen XK, et al. Care and outcomes of patients with cancer admitted to the hospital on weekends and holidays: a retrospective cohort study. J Natl Compr Canc Netw. 2016;14(7):867-874. PubMed
Let’s face it—rates of hospital admission are on the rise, but there are still just 7 days in a week. That means that patients are increasingly admitted on weekdays and on the weekend, requiring more nurses and doctors to look after them. Why then are there no lines for coffee on a Saturday? Does this reduced intensity of staffing translate into worse care for our patients?
Since one of its earliest descriptions in hospitalized patients, the “weekend effect” has been extensively studied in various patient populations and hospital settings.1-5 The results have been varied, depending on the place of care,6 reason for care, type of admission,5,7 or admitting diagnosis.1,8,9 Many researchers have posited the drivers behind the weekend effect, including understaffed wards, intensity of specialist care, delays in procedural treatments, or severity of illness, but the truth is that we still don’t know.
Pauls et al. performed a robust systematic review and meta-analysis examining the rates of in-hospital mortality in patients admitted on the weekend compared with those admitted on weekdays.10 They analyzed predetermined subgroups to identify system- and patient-level factors associated with a difference in weekend mortality.
A total of 97 studies—comprising an astounding 51 million patients—was included in the study. They found that individuals admitted on the weekend carried an almost 20% increase in the risk of death compared with those who landed in hospital on a weekday. The effect was present for both in-hospital deaths and when looking specifically at 30-day mortality. Translating these findings into practice, an additional 14 deaths per 1000 admissions occur when patients are admitted on the weekend. Brain surgery can be less risky.11
Despite this concerning finding, no individual factor was identified that could account for the effect. There was a 16% and 11% increase in mortality in weekend patients associated with decreased hospital staffing and delays to procedural therapies, respectively. No differences were found when examining reduced rates of procedures or illness severity on weekends compared with weekdays. But one must always interpret subgroup analyses, even prespecified ones, with caution because they often lack the statistical power to make concrete conclusions.
To this end, an important finding of the study by Pauls et al. highlights the variation in mortality risk as it relates to the weekend effect.10 Even for individuals with cancer, a disease with a relatively predictable rate of decline, there are weekend differences in mortality risk that depend upon the type of cancer.8,12 This heterogeneity persists when examining for the possible factors that contribute to the effect, introducing a significant amount of noise into the analysis, and may explain why research to date has been unable to find the proverbial black cat in the coal cellar.
One thing Pauls et al. makes clear is that the weekend effect appears to be a real phenomenon, despite significant heterogeneity in the literature.10 Only a high-quality, systematic review has the capability to draw such conclusions. Prior work demonstrates that this effect is substantial in some individuals,and this study confirms that it perseveres beyond an immediate time period following admission.1,9 The elements contributing to the weekend effect remain undefined and are likely as complex as our healthcare system itself.
Society and policy makers should resist the tantalizing urge to invoke interventions aimed at fixing this issue before fully understanding the drivers of a system problem. The government of the United Kingdom has decreed a manifesto to create a “7-day National Health Service,” in which weekend services and physician staffing will match that of the weekdays. Considering recent labor tensions between junior doctors in the United Kingdom over pay and working hours, the stakes are at an all-time high.
But such drastic measures violate a primary directive of quality improvement science to study and understand the problem before reflexively jumping to solutions. This will require new research endeavors aimed at determining the underlying factor(s) responsible for the weekend effect. Once we are confident in its cause, only then can careful evaluation of targeted interventions aimed at the highest-risk admissions be instituted. As global hospital and healthcare budgets bend under increasing strain, a critical component of any proposed intervention must be to examine the cost-effectiveness in doing so. Because the weekend effect is one of increased mortality, it will be hard to justify an acceptable price for an individual’s life. And it is not as straightforward as a randomized trial examining the efficacy of parachutes. Any formal evaluation must account for the unintended consequences and opportunity costs of implementing a potential fix aimed at minimizing the weekend effect.
The weekend effect has now been studied for over 15 years. Pauls et al. add to our knowledge of this phenomenon, confirming that the overall risk of mortality for patients admitted on the weekend is real, variable, and substantial.10 As more individuals are admitted to hospitals, resulting in increasing numbers of admissions on the weekend, a desperate search for the underlying cause must be carried out before we can fix it. Whatever the means to the end, our elation will continue to be tempered by a feeling of uneasiness every time our coworkers joyously exclaim, “TGIF!”
Disclosure
The authors have nothing to disclose.
Let’s face it—rates of hospital admission are on the rise, but there are still just 7 days in a week. That means that patients are increasingly admitted on weekdays and on the weekend, requiring more nurses and doctors to look after them. Why then are there no lines for coffee on a Saturday? Does this reduced intensity of staffing translate into worse care for our patients?
Since one of its earliest descriptions in hospitalized patients, the “weekend effect” has been extensively studied in various patient populations and hospital settings.1-5 The results have been varied, depending on the place of care,6 reason for care, type of admission,5,7 or admitting diagnosis.1,8,9 Many researchers have posited the drivers behind the weekend effect, including understaffed wards, intensity of specialist care, delays in procedural treatments, or severity of illness, but the truth is that we still don’t know.
Pauls et al. performed a robust systematic review and meta-analysis examining the rates of in-hospital mortality in patients admitted on the weekend compared with those admitted on weekdays.10 They analyzed predetermined subgroups to identify system- and patient-level factors associated with a difference in weekend mortality.
A total of 97 studies—comprising an astounding 51 million patients—was included in the study. They found that individuals admitted on the weekend carried an almost 20% increase in the risk of death compared with those who landed in hospital on a weekday. The effect was present for both in-hospital deaths and when looking specifically at 30-day mortality. Translating these findings into practice, an additional 14 deaths per 1000 admissions occur when patients are admitted on the weekend. Brain surgery can be less risky.11
Despite this concerning finding, no individual factor was identified that could account for the effect. There was a 16% and 11% increase in mortality in weekend patients associated with decreased hospital staffing and delays to procedural therapies, respectively. No differences were found when examining reduced rates of procedures or illness severity on weekends compared with weekdays. But one must always interpret subgroup analyses, even prespecified ones, with caution because they often lack the statistical power to make concrete conclusions.
To this end, an important finding of the study by Pauls et al. highlights the variation in mortality risk as it relates to the weekend effect.10 Even for individuals with cancer, a disease with a relatively predictable rate of decline, there are weekend differences in mortality risk that depend upon the type of cancer.8,12 This heterogeneity persists when examining for the possible factors that contribute to the effect, introducing a significant amount of noise into the analysis, and may explain why research to date has been unable to find the proverbial black cat in the coal cellar.
One thing Pauls et al. makes clear is that the weekend effect appears to be a real phenomenon, despite significant heterogeneity in the literature.10 Only a high-quality, systematic review has the capability to draw such conclusions. Prior work demonstrates that this effect is substantial in some individuals,and this study confirms that it perseveres beyond an immediate time period following admission.1,9 The elements contributing to the weekend effect remain undefined and are likely as complex as our healthcare system itself.
Society and policy makers should resist the tantalizing urge to invoke interventions aimed at fixing this issue before fully understanding the drivers of a system problem. The government of the United Kingdom has decreed a manifesto to create a “7-day National Health Service,” in which weekend services and physician staffing will match that of the weekdays. Considering recent labor tensions between junior doctors in the United Kingdom over pay and working hours, the stakes are at an all-time high.
But such drastic measures violate a primary directive of quality improvement science to study and understand the problem before reflexively jumping to solutions. This will require new research endeavors aimed at determining the underlying factor(s) responsible for the weekend effect. Once we are confident in its cause, only then can careful evaluation of targeted interventions aimed at the highest-risk admissions be instituted. As global hospital and healthcare budgets bend under increasing strain, a critical component of any proposed intervention must be to examine the cost-effectiveness in doing so. Because the weekend effect is one of increased mortality, it will be hard to justify an acceptable price for an individual’s life. And it is not as straightforward as a randomized trial examining the efficacy of parachutes. Any formal evaluation must account for the unintended consequences and opportunity costs of implementing a potential fix aimed at minimizing the weekend effect.
The weekend effect has now been studied for over 15 years. Pauls et al. add to our knowledge of this phenomenon, confirming that the overall risk of mortality for patients admitted on the weekend is real, variable, and substantial.10 As more individuals are admitted to hospitals, resulting in increasing numbers of admissions on the weekend, a desperate search for the underlying cause must be carried out before we can fix it. Whatever the means to the end, our elation will continue to be tempered by a feeling of uneasiness every time our coworkers joyously exclaim, “TGIF!”
Disclosure
The authors have nothing to disclose.
1. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376. PubMed
2. Bell CM, Redelmeier DA. Waiting for urgent procedures on the weekend among emergently hospitalized patients. AJM. 2004;117(3):175-181. doi:10.1016/j.amjmed.2004.02.047. PubMed
3. Kalaitzakis E, Helgeson J, Strömdahl M, Tóth E. Weekend admission in upper GI bleeding: does it have an impact on outcome? Gastrointest Endosc. 2015;81(5):1295-1296. doi:10.1016/j.gie.2014.12.003. PubMed
4. Nanchal R, Kumar G, Taneja A, et al. Pulmonary embolism: the weekend effect. Chest. 2012;142(3):690-696. doi:10.1378/chest.11-2663. PubMed
5. Ricciardi R, Roberts PL, Read TE, Baxter NN, Marcello PW, Schoetz DJ. Mortality rate after nonelective hospital admission. Arch Surg. 2011;146(5):545-551. PubMed
6. Wunsch H, Mapstone J, Brady T, Hanks R, Rowan K. Hospital mortality associated with day and time of admission to intensive care units. Intensive Care Med. 2004;30(5):895-901. doi:10.1007/s00134-004-2170-3. PubMed
7. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009. PubMed
8. Lapointe-Shaw L, Bell CM. It’s not you, it’s me: time to narrow the gap in weekend care. BMJ Qual Saf. 2014;23(3):180-182. doi:10.1136/bmjqs-2013-002674. PubMed
9. Concha OP, Gallego B, Hillman K, Delaney GP, Coiera E. Do variations in hospital mortality patterns after weekend admission reflect reduced quality of care or different patient cohorts? A population-based study. BMJ Qual Saf. 2014;23(3):215-222. doi:10.1136/bmjqs-2013-002218. PubMed
10. Pauls LA, Johnson-Paben R, McGready J, Murphy JD, Pronovost PJ, Wu CL. The Weekend Effect in Hospitalized Patients: A Meta-analysis. J Hosp Med. 2017;12(9):760-766. PubMed
11. American College of Surgeons. NSQIP Risk Calculator. http://riskcalculator.facs.org/RiskCalculator/. Accessed on July 5, 2017.
12. Lapointe-Shaw L, Abushomar H, Chen XK, et al. Care and outcomes of patients with cancer admitted to the hospital on weekends and holidays: a retrospective cohort study. J Natl Compr Canc Netw. 2016;14(7):867-874. PubMed
1. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376. PubMed
2. Bell CM, Redelmeier DA. Waiting for urgent procedures on the weekend among emergently hospitalized patients. AJM. 2004;117(3):175-181. doi:10.1016/j.amjmed.2004.02.047. PubMed
3. Kalaitzakis E, Helgeson J, Strömdahl M, Tóth E. Weekend admission in upper GI bleeding: does it have an impact on outcome? Gastrointest Endosc. 2015;81(5):1295-1296. doi:10.1016/j.gie.2014.12.003. PubMed
4. Nanchal R, Kumar G, Taneja A, et al. Pulmonary embolism: the weekend effect. Chest. 2012;142(3):690-696. doi:10.1378/chest.11-2663. PubMed
5. Ricciardi R, Roberts PL, Read TE, Baxter NN, Marcello PW, Schoetz DJ. Mortality rate after nonelective hospital admission. Arch Surg. 2011;146(5):545-551. PubMed
6. Wunsch H, Mapstone J, Brady T, Hanks R, Rowan K. Hospital mortality associated with day and time of admission to intensive care units. Intensive Care Med. 2004;30(5):895-901. doi:10.1007/s00134-004-2170-3. PubMed
7. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009. PubMed
8. Lapointe-Shaw L, Bell CM. It’s not you, it’s me: time to narrow the gap in weekend care. BMJ Qual Saf. 2014;23(3):180-182. doi:10.1136/bmjqs-2013-002674. PubMed
9. Concha OP, Gallego B, Hillman K, Delaney GP, Coiera E. Do variations in hospital mortality patterns after weekend admission reflect reduced quality of care or different patient cohorts? A population-based study. BMJ Qual Saf. 2014;23(3):215-222. doi:10.1136/bmjqs-2013-002218. PubMed
10. Pauls LA, Johnson-Paben R, McGready J, Murphy JD, Pronovost PJ, Wu CL. The Weekend Effect in Hospitalized Patients: A Meta-analysis. J Hosp Med. 2017;12(9):760-766. PubMed
11. American College of Surgeons. NSQIP Risk Calculator. http://riskcalculator.facs.org/RiskCalculator/. Accessed on July 5, 2017.
12. Lapointe-Shaw L, Abushomar H, Chen XK, et al. Care and outcomes of patients with cancer admitted to the hospital on weekends and holidays: a retrospective cohort study. J Natl Compr Canc Netw. 2016;14(7):867-874. PubMed
© 2017 Society of Hospital Medicine
Impact of patient-centered discharge tools: A systematic review
Patient-centered care, defined by the Institute of Medicine as “health care that establishes a partnership among practitioners, patients, and their families to ensure that decisions respect patients’ wants, needs and preferences and that patients have the education and support they need to make decisions and participate in their own care,” has been recognized as an important factor in improving care transitions after discharge from the hospital.1 Previous efforts to improve the discharge process for hospitalized patients and reduce avoidable readmissions have focused on improving systems surrounding the patient, such as by increasing the availability of outpatient follow-up or standardizing communication between the inpatient and outpatient care teams.1,2 In fact, successful programs such as Project BOOST and the Care Transitions Interventions™ provide healthcare institutions with a “bundle” of evidence-based transitional care guidelines for discharge: they provide postdischarge transition coaches, assistance with medication self-management, timely follow-up tips, and improved patient records in order to improve postdischarge outcomes.3,4 Successful interventions, however, may not provide more services, but also engage the patient in their own care.5,6 The impact of engaging the patient in his or her own care by providing patient-friendly discharge instructions alone, however, is unknown.
A patient-centered discharge may use tools that were designed with patients, or may involve engaging patients in an interactive process of reviewing discharge instructions and empowering them to manage aspects of their own care after leaving the hospital. This endeavour may lead to more effective use of discharge instructions and reduce the need for additional or more intensive (and costly) interventions. For example, a patient-centered discharge tool could include an educational intervention that uses the “teach-back” method, in which patients are asked to restate in their own words what they thought they heard, or in which staff use additional media or a visual design tool meant to enhance comprehension of discharge instructions.6,7 Visual aids and the use of larger fonts are particularly useful design elements for improving comprehension among non-English speakers and patients with low health literacy, who tend to have poorer recall of instructions.8-10 What may constitute essential design elements to include in a discharge instruction tool, however, is not clear.
Moreover, whether the use of discharge tools with a specific focus on patient engagement may improve postdischarge outcomes is not known. Particularly, the ability of patient-centered discharge tools to improve outcomes beyond comprehension such as self-management, adherence to discharge instructions, a reduction in unplanned visits, and a reduction in mortality has not been studied systematically. The objective of this systematic review was to review the literature on discharge instruction tools with a focus on patient engagement and their impact among hospitalized patients.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was followed as a guideline for reporting throughout this review.11
Data Sources
A literature search was undertaken using the following databases from January 1994 or their inception date to May 2014: Medline, Embase, SIGLE, HTA, Bioethics, ASSIA, Psych Lit, CINAHL, Cochrane Library, EconLit, ERIC, and BioMed Central. We also searched relevant design-focused journals such as Design Issues, Journal of Design Research, Information Design Journal, Innovation, Design Studies, and International Journal of Design, as well as reference lists from studies obtained by electronic searching. The following key words and combination of key words were used with the assistance of a medical librarian: patient discharge, patient-centered discharge, patient-centered design, design thinking, user based design, patient education, discharge summary, education. Additional search terms were added when identified from relevant articles (Appendix).
Inclusion Criteria
We included all English-language studies with patients admitted to the hospital irrespective of age, sex, or medical condition, which included a control group or time period and which measured patient outcomes within 3 months of discharge. The 3-month period after discharge is often cited as a time when outcomes could reasonably be associated with an intervention at discharge.2
Exclusion Criteria
Studies that did not have clear implementation of a patient-centered tool, a control group, or those whose tool was used in the emergency department or as an outpatient were excluded. Studies that included postdischarge tools such as home visits or telephone calls were excluded unless independent effects of the predischarge interventions were measured. Studies with outcomes reported after 3 months were excluded unless outcomes before 3 months were also clearly noted.
All searches were entered into Endnote and duplicates were removed. A 2-stage inclusion process was used. Titles and abstracts of articles were first screened for meeting inclusion and exclusion criteria by 1 reviewer. A second reviewer independently checked a 10% random sample of all the abstracts that met the initial screening criteria. If the agreement to exclude studies was less than 95%, criteria were reviewed before checking the rest of the 90% sample. In the second stage, 2 independent reviewers examined paper copies of the full articles selected in the first stage. Disagreement between reviewers was resolved by discussion or a third reviewer if no agreement could be reached.
Data Analysis and Synthesis
The following information was extracted from the full reference: type of study, population studied, control group or time period, tool used, and outcomes measured. Based on the National Health Care Quality report’s priorities and goals on patient and/or family engagement during transitions of care, educational tools were further described based on method of teaching, involvement of the care team, involvement of the patient in the design or delivery of the tool, and/or the use of visual aids.12 All primary outcomes were classified according to 3 categories: improved knowledge/comprehension, patient experience (patient satisfaction, self-management/efficacy such as functional status, both physical and mental), and health outcomes (unscheduled visits or readmissions, adherence with medications, diet, exercise, or follow-up, and mortality).
No quantitative pooling of results or meta-analysis was done given the variability and heterogeneity of studies reviewed. However, following guidelines for Effect Practice and Organisation of Care (EPOC) Risk of Bias criteria,13 studies that had a higher risk of bias such as uncontrolled before-after studies or studies with only 1 intervention or control site (historical controls, eg) were excluded from the final review because of the difficulties in attributing causation. Only primary outcomes were reported in order to minimize type II errors.
RESULTS
Our search revealed a total of 3699 studies after duplicates had been removed (Figure). A total of 714 references were included after initial review by title and abstract and 30 studies after full-text review. Agreement on a 10% random sample of all abstracts and full text was 79% (k=0.58) and 86% (k=0.72), respectively. Discussion was needed for fewer than 100 references, and agreement was subsequently reached for 100%.
There were 22 randomized controlled trials and 8 nonrandomized studies (5 nonrandomized controlled trials and 3 controlled before-after studies). Most of these studies were conducted in the United States (13/30 studies), followed by other European countries (5 studies), and the United Kingdom (4 studies). A large number of studies were conducted among patients with cardiovascular disease or risk factors (10 studies), followed by postsurgical patients such as coronary artery bypass graft surgery or orthopaedic surgery (5 studies). Five of 30 studies were conducted among individuals older than 65 years. Most studies excluded patients who did not speak English or the country’s official language; only 3 studies included patients with limited literacy, patients who spoke other languages, or caregivers if the patients could not communicate.
Most studies tested the impact of educational discharge interventions (28 of 30 studies) (Table 1). Quite often, it was a member of the research team who carried out the patient education. Only 3 studies involved multiple members of the care team in designing or reviewing the discharge tool with the patient. Almost half (12 studies) targeted multiple aspects of postdischarge care, including medications and side effects, signs and symptoms to consider, plans for follow-up, dietary restrictions, and/or exercise modifications. Many (19 studies) provided education using one-on-one teaching in association with a discharge tool, accompanied by a written handout (13 studies), audiotape (2 studies), or video (3 studies). While 13 studies had patients involved in creating what content was discussed and 14 studies had patients involved in the delivery of the tool, only 6 studies had patients involved in both design and delivery of the tool. Nine studies also used visual aids such as pictures, larger font, or use of a tool enhanced for patients with language barriers or limited health literacy.
Among all 30 studies included, 16 studies tested the impact of their tool on comprehension postdischarge, with 10 studies demonstrating an improvement among patients who had received the tool (Table 2). Five studies evaluated healthcare utilization outcomes such as readmission, length of stay, or physician visits after discharge and 2 studies found improvements. Twelve studies also studied the impact on adherence with medications, diet, exercise, or follow-up instructions postdischarge. However, only 4 of these 12 studies showed a positive impact. Only 2 studies tested the impact on a patient’s ability to self-manage once at home, and both studies reported positive statistical outcomes. Few studies measured patient experience (such as patient satisfaction or improvement in self-efficacy) or mortality postdischarge.
DISCUSSION/CONCLUSION
Our systematic review found 30 studies that engaged patients during the design or the delivery of a discharge instruction tool and that tested the effect of the tool on postdischarge outcomes.6-10,14–38 Our review suggests that there is sufficient evidence that patient-centered discharge tools improve comprehension. However, evidence is currently insufficient to determine if patient-centered tools improve adherence with discharge instructions. Moreover, though limited studies show promising results, more studies are needed to determine if patient engagement improves self-efficacy and healthcare utilization after discharge.
A major limitation of current studies is the variability in the level of patient engagement in tool design or delivery. Patients were involved in the design mostly through targeted development of a discharge management plan and the delivery by encouraging them to ask questions. Few studies involved patients in the design of the tool such that patients were responsible for coming up with content that was of interest to them. The few that did, often with the additional use of video media, demonstrated significant outcomes. Only a minority of studies used an interactive process to assess understanding such as “teach-back” or maximize patient comprehension such as visual aids. Even fewer studies engaged patients in both developing the discharge tool and providing discharge instructions.
Several previous studies have demonstrated that most complications after discharge are the result of ineffective communication, which can be exacerbated by lack of fluency in English or by limited health literacy.2,39-43 As a result, poor understanding of discharge instructions by patients and their caregivers can create an important care gap.44 Therefore, the use of patient-centered tools to engage patients at discharge in their own care is needed. How to engage patients consistently and effectively is perhaps less evident, as demonstrated in this review of the literature in which different levels of patient engagement were found. Many of the tools tested placed attention on patient education, sometimes in the context of bundled care along with home visits or follow-up, all of which can require extensive resources and time. Providing patients with information that the patients themselves state is of value may be the easiest refinement to a discharge educational tool, although this was surprisingly uncommon.6,9,10,17,23,33,37 Only 2 studies were found that engaged patients in the initial stage of design of the discharge tool, by incorporating information of interest to them.23,32 For example, a study testing the impact of a computer-generated written education package on poststroke outcomes designed the information by asking patients to identify which topics they would like to receive information about (along with the amount of information and font size).23 Secondly, although most of the discharge tools reviewed included the use of one-on-one teaching and the use of media such as patient handouts, these tools were often used in such a way that patients were passive recipients. In fact, studies that used additional video media that incorporated personalized content were the most likely to demonstrate positive outcomes.17,34 The next level of patient engagement may therefore be to involve the patient as an interactive partner when delivering the tool in order to empower patients to self-care. For example, 1 study designed a structured education program by first assessing lifestyle risk factors related to hypertension that were modifiable along with preconceived notions through open-ended questions during a one-on-one interview.37 Patients were subsequently educated on any knowledge deficits regarding the management of their lifestyle. Another level of patient engagement may be to use visual aids during discussions, as a well-known complement to verbal instructions.45,46 For example, in a controlled study that randomized a ward of elderly patients with 4 or more prescriptions to predischarge counseling, the counseling session aimed to review reasons for their prescriptions along with corresponding side effects, doses, and dosage times with the help of a medicine reminder card. Other uses of visual aid tools identified in our review included the use of pictograms or illustrations or, at minimum, attention to font size.7,8,16,29,33,35 In the absence of a visual aid, asking the patient to repeat or demonstrate what was just communicated can be used to assess the amount of information retained.18,33
An important result discovered in our review of the literature was also the lack of studies that tested the impact of discharge tools on usability of discharge information once at home. Conducting an evaluation of the benefits to patients after discharge can help objectify vague outcomes like health gains or qualify benefits in patient’s views. This might also explain why many studies with documented patient engagement at the time of discharge were able to demonstrate improvements in comprehension but not adherence to instructions. Although patients and caregivers may understand the information, this comprehension does not necessarily mean they will find the information useful or adhere to it once at home. For example, in 1 study, patients discharged with at least 1 medication were randomized to a structured discharge interview during which the treatment plan was reviewed verbally and questions clarified along with a visually enhanced treatment card.26 Although knowledge of medications increased, no effect was found on adherence at 1 week postdischarge. However, use of the treatment card at home was not assessed. Similarly, another study tested the effect of an individualized video of exercises and failed to find a difference in patient adherence at 4 weeks.28 The authors suggested that the lack of benefit may have been because patients were not using the video once at home. This is in contrast to 2 studies that involved patients in their own care by requiring them to request their medication as part of a self-medication tool predischarge.16,30 Patients were engaged in the process such that increasing independence was given to patients based on their demonstration of understanding and adherence to their treatment while still in the hospital, a learning tool that can be applied once at home. Feeling knowledgeable and involved, as others have suggested, may be the intermediary outcomes that led to improved adherence.47 It is also possible that adherence to discharge instructions may vary based on complexity of the information provided, such that instructions focusing solely on medication use may require less patient engagement than discharge instructions that include information on medications, diet, exercise modifications, and follow-up.48
Our review has a few limitations. Previous systematic reviews have demonstrated that bundled discharge interventions that include patient-centered education have a positive effect on outcomes postdischarge.2,5 However, we sought to describe and study the individual and distinct impact of patient engagement in the creation and delivery of discharge tools on outcomes postdischarge. We hoped that this may provide others with key information regarding elements of patient engagement that were particularly useful when designing a new discharge tool. The variability of the studies we identified, however, made it difficult to ascertain what level of patient engagement is required to observe improvements in health outcomes. It is also possible that a higher level of patient engagement may have been used but not described in the studies we reviewed. As only primary outcomes were included, we may have underestimated the effect of patient-centered discharge tools on outcomes that were reported as secondary outcomes. As we were interested in reviewing as many studies of patient-centered discharge tools as possible, we did not assess the quality of the studies and cannot comment on the role of bias in these studies. However, we excluded studies with study designs known to have the highest risk of bias. Lastly, we also cannot comment on whether patient-centered tools may have an effect on outcomes more than 3 months after a hospital discharge. However, several studies included in this review suggest a sustained effect beyond this time period.8,25,32,37
Patient-centered discharge tools in which patients were engaged in the design or the delivery were found to improve comprehension of but not adherence with discharge instructions. The perceived lack of improved adherence may be due to a lack of studies that measured the usefulness and utilization of information for patients once at home. There was also substantial variability in the extent of patient involvement in designing the style and content of information provided to patients at discharge, as well as the extent of patient engagement when receiving discharge instructions. Future studies would benefit from detailing the level of patient engagement needed in designing and delivery of discharge tools. This information may lead to the discovery of barriers and facilitators to utilization of discharge information once at home and lead to a better understanding of the patient’s journey from hospital to home and onwards.
C.M.B. and this work were funded by a CIHR Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. Funding was provided to cover fees to obtain articles from the Donald J. Matthews Complex Care Fund of the University Health Network in Toronto, Canada. The Toronto Central Local Health Integration Network provided funding for the design and implementation of a patient-oriented discharge summary. None of the funding or supportive agencies were involved in the design or conduct of the present study, analysis, or interpretation of the data, or approval of the manuscript.
Disclosures
The authors report no conflicts of interest.
1. Hurtad
2. Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta-review. BMC Health Serv Res. 2007;7:47. PubMed
3. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
4. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
5. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
6. Osman LM, Calder C, Godden DJ, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax. 2002;57(10):869-874. PubMed
7. Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 suppl 1):S209-S216. PubMed
8. Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Resp Med. 2001;95(11):851-856. PubMed
9. Haerem JW, Ronning EJ, Leidal R. Home access to hospital discharge information on audiotape reduces sick leave and readmissions in patients with first-time myocardial infarction. Scand Cardiovasc J. 2000;34(2):219-222. PubMed
10. Legrain S, Tubach F, Bonnet-Zamponi D, et al. A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2011;59(11):2017-2028. PubMed
11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
12. Partnership NP. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, DC: National Quality Forum; 2008.
13. Effective Practice and Organisation of Care (EPOC). EPOC-specific resources for review authors. Oslo, Norway: Norwegian Knowledge Centre for the Health Services; 2013. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed December 21, 2016.
14. Manning DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care. 2007;16(1):71-76. PubMed
15. Perera KY, Ranasinghe P, Adikari AM, et al. Medium of language in discharge summaries: would the use of native language improve patients’ knowledge of their illness and medications? J Health Commun. 2012;17(2):141-148. PubMed
16. Lowe CJ, Raynor DK, Courtney EA, et al. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ. 1995;310(6989):1229-1231. PubMed
17. Mahler HI, Kulik JA, Tarazi RY. Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary bypass surgery. J Cardiopulm Rehabil. 1999;19(3):170-177. PubMed
18. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counseling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657-664. PubMed
19. Drenth-van Maanen AC, Wilting I, Jansen PA, et al. Effect of a discharge medication intervention on the incidence and nature of medication discrepancies in older adults. J Am Geriatr Soc. 2013;61(3):456-458. PubMed
20. Eshah NF. Predischarge education improves adherence to a healthy lifestyle among Jordanian patients with acute coronary syndrome. Nurs Health Sci. 2013;15(3):273-279. PubMed
21. Gwadry-Sridhar FH, Arnold JM, Zhang Y,et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. PubMed
22. Ho SM, Heh SS, Jevitt CM, et al. Effectiveness of a discharge education program in reducing the severity of postpartum depression: a randomized controlled evaluation study. Patient Educ Couns. 2009;77(1):68-71. PubMed
23. Hoffmann T, McKenna K, Worrall L, et al. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing. 2007;36(3):280-286. PubMed
24. Jenkins HM, Blank V, Miller K, et al. A randomized single-blind evaluation of a discharge teaching book for pediatric patients with burns. J Burn Care Rehabil. 1996;17(1):49-61. PubMed
25. Kommuri NV, Johnson ML, Koelling TM. Relationship between improvements in heart failure patient disease specific knowledge and clinical events as part of a randomized controlled trial. Patient Educ Couns. 2012;86(2):233-238. PubMed
26. Louis-Simonet M, Kossovsky MP, Sarasin FP, et al. Effects of a structured patient-centered discharge interview on patients’ knowledge about their medications. Am J Med. 2004;117(8):563-568. PubMed
27. Lucas KS. Outcomes evaluation of a pharmacist discharge medication teaching service. Am J Health Syst Pharm. 1998;55(24 suppl 4):S32-S35. PubMed
28. Lysack C, Dama M, Neufeld S, et al. A compliance and satisfaction with home exercise: a comparison of computer-assisted video instruction and routine rehabilitation practice. J Allied Health. 2005;34(2):76-82. PubMed
29. Moore SM. The effects of a discharge information intervention on recovery outcomes following coronary artery bypass surgery. Int J Nurs Stud. 1996;33(2):181-189. PubMed
30. Pereles L, Romonko L, Murzyn T, et al. Evaluation of a self-medication program. J Am Geriatr Soc. 1996;44(2):161-165. PubMed
31. Reynolds MA. Postoperative pain management discharge teaching in a rural population. Pain Manag Nurs. 2009;10(2):76-84. PubMed
32. Sabariego C, Barrera AE, Neubert S, et al. Evaluation of an ICF-based patient education programme for stroke patients: a randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br J Health Psychol. 2013;18(4):707-728. PubMed
33. Shieh SJ, Chen HL, Liu FC, et al. The effectiveness of structured discharge education on maternal confidence, caring knowledge and growth of premature newborns. J Clin Nurs. 2010;19(23-24):3307-3313. PubMed
34. Steinberg TG, Diercks MJ, Millspaugh J. An evaluation of the effectiveness of a videotape for discharge teaching of organ transplant recipients. J Transpl Coord. 1996;6(2):59-63. PubMed
35. Whitby M, McLaws ML, Doidge S, et al. Post-discharge surgical site surveillance: does patient education improve reliability of diagnosis? J Hosp Infect. 2007;66(3):237-242. PubMed
36. Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Mil Med. 1995;160(11):561–564. PubMed
37. Zernike W, Henderson A. Evaluating the effectiveness of two teaching strategies for patients diagnosed with hypertension. J Clin Nurs. 1998;7(1):37–44. PubMed
38. Press VG, Arora V, Constantine KL, et al. Forget me not: a randomized trial of the durability of hospital-based education on inhalers for patients with COPD or asthma [abstract]. J Gen Intern Med. 2014;29(1 suppl):S102.
39. Davis TC, Wolf MS, Bass PF, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. PubMed
40. McCarthy DM, Waite KR, Curtis LM, et al. What did the doctor say? Health literacy and recall of medical instructions. Med Care. 2012;50(4):277–282. PubMed
41. Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–1862. PubMed
42. Cawthon C, Walia S, Osborn CY, et al. Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324. PubMed
43. Karliner LS, Auerbach A, Nápoles A, et al. Language barriers and understanding of hospital discharge instructions. Med Care. 2012;50(4):283–289. PubMed
44. Enhancing the Continuum of Care. Report of the Avoidable Hospitalization Advisory Panel. http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Published November 2011. Accessed December 22, 2016.
45. Chugh A, Williams MV, Grigsby J, et al. Better transitions: improving comprehension of discharge instructions. Front Health Serv Manage. 2009;25(3):11–32. PubMed
46. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medication in an anticoagulation clinic: a comparison of verbal vs. visual assessment. J Health Commun. 2006;11(7):651–664. PubMed
47. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. PubMed
48. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498. PubMed
Patient-centered care, defined by the Institute of Medicine as “health care that establishes a partnership among practitioners, patients, and their families to ensure that decisions respect patients’ wants, needs and preferences and that patients have the education and support they need to make decisions and participate in their own care,” has been recognized as an important factor in improving care transitions after discharge from the hospital.1 Previous efforts to improve the discharge process for hospitalized patients and reduce avoidable readmissions have focused on improving systems surrounding the patient, such as by increasing the availability of outpatient follow-up or standardizing communication between the inpatient and outpatient care teams.1,2 In fact, successful programs such as Project BOOST and the Care Transitions Interventions™ provide healthcare institutions with a “bundle” of evidence-based transitional care guidelines for discharge: they provide postdischarge transition coaches, assistance with medication self-management, timely follow-up tips, and improved patient records in order to improve postdischarge outcomes.3,4 Successful interventions, however, may not provide more services, but also engage the patient in their own care.5,6 The impact of engaging the patient in his or her own care by providing patient-friendly discharge instructions alone, however, is unknown.
A patient-centered discharge may use tools that were designed with patients, or may involve engaging patients in an interactive process of reviewing discharge instructions and empowering them to manage aspects of their own care after leaving the hospital. This endeavour may lead to more effective use of discharge instructions and reduce the need for additional or more intensive (and costly) interventions. For example, a patient-centered discharge tool could include an educational intervention that uses the “teach-back” method, in which patients are asked to restate in their own words what they thought they heard, or in which staff use additional media or a visual design tool meant to enhance comprehension of discharge instructions.6,7 Visual aids and the use of larger fonts are particularly useful design elements for improving comprehension among non-English speakers and patients with low health literacy, who tend to have poorer recall of instructions.8-10 What may constitute essential design elements to include in a discharge instruction tool, however, is not clear.
Moreover, whether the use of discharge tools with a specific focus on patient engagement may improve postdischarge outcomes is not known. Particularly, the ability of patient-centered discharge tools to improve outcomes beyond comprehension such as self-management, adherence to discharge instructions, a reduction in unplanned visits, and a reduction in mortality has not been studied systematically. The objective of this systematic review was to review the literature on discharge instruction tools with a focus on patient engagement and their impact among hospitalized patients.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was followed as a guideline for reporting throughout this review.11
Data Sources
A literature search was undertaken using the following databases from January 1994 or their inception date to May 2014: Medline, Embase, SIGLE, HTA, Bioethics, ASSIA, Psych Lit, CINAHL, Cochrane Library, EconLit, ERIC, and BioMed Central. We also searched relevant design-focused journals such as Design Issues, Journal of Design Research, Information Design Journal, Innovation, Design Studies, and International Journal of Design, as well as reference lists from studies obtained by electronic searching. The following key words and combination of key words were used with the assistance of a medical librarian: patient discharge, patient-centered discharge, patient-centered design, design thinking, user based design, patient education, discharge summary, education. Additional search terms were added when identified from relevant articles (Appendix).
Inclusion Criteria
We included all English-language studies with patients admitted to the hospital irrespective of age, sex, or medical condition, which included a control group or time period and which measured patient outcomes within 3 months of discharge. The 3-month period after discharge is often cited as a time when outcomes could reasonably be associated with an intervention at discharge.2
Exclusion Criteria
Studies that did not have clear implementation of a patient-centered tool, a control group, or those whose tool was used in the emergency department or as an outpatient were excluded. Studies that included postdischarge tools such as home visits or telephone calls were excluded unless independent effects of the predischarge interventions were measured. Studies with outcomes reported after 3 months were excluded unless outcomes before 3 months were also clearly noted.
All searches were entered into Endnote and duplicates were removed. A 2-stage inclusion process was used. Titles and abstracts of articles were first screened for meeting inclusion and exclusion criteria by 1 reviewer. A second reviewer independently checked a 10% random sample of all the abstracts that met the initial screening criteria. If the agreement to exclude studies was less than 95%, criteria were reviewed before checking the rest of the 90% sample. In the second stage, 2 independent reviewers examined paper copies of the full articles selected in the first stage. Disagreement between reviewers was resolved by discussion or a third reviewer if no agreement could be reached.
Data Analysis and Synthesis
The following information was extracted from the full reference: type of study, population studied, control group or time period, tool used, and outcomes measured. Based on the National Health Care Quality report’s priorities and goals on patient and/or family engagement during transitions of care, educational tools were further described based on method of teaching, involvement of the care team, involvement of the patient in the design or delivery of the tool, and/or the use of visual aids.12 All primary outcomes were classified according to 3 categories: improved knowledge/comprehension, patient experience (patient satisfaction, self-management/efficacy such as functional status, both physical and mental), and health outcomes (unscheduled visits or readmissions, adherence with medications, diet, exercise, or follow-up, and mortality).
No quantitative pooling of results or meta-analysis was done given the variability and heterogeneity of studies reviewed. However, following guidelines for Effect Practice and Organisation of Care (EPOC) Risk of Bias criteria,13 studies that had a higher risk of bias such as uncontrolled before-after studies or studies with only 1 intervention or control site (historical controls, eg) were excluded from the final review because of the difficulties in attributing causation. Only primary outcomes were reported in order to minimize type II errors.
RESULTS
Our search revealed a total of 3699 studies after duplicates had been removed (Figure). A total of 714 references were included after initial review by title and abstract and 30 studies after full-text review. Agreement on a 10% random sample of all abstracts and full text was 79% (k=0.58) and 86% (k=0.72), respectively. Discussion was needed for fewer than 100 references, and agreement was subsequently reached for 100%.
There were 22 randomized controlled trials and 8 nonrandomized studies (5 nonrandomized controlled trials and 3 controlled before-after studies). Most of these studies were conducted in the United States (13/30 studies), followed by other European countries (5 studies), and the United Kingdom (4 studies). A large number of studies were conducted among patients with cardiovascular disease or risk factors (10 studies), followed by postsurgical patients such as coronary artery bypass graft surgery or orthopaedic surgery (5 studies). Five of 30 studies were conducted among individuals older than 65 years. Most studies excluded patients who did not speak English or the country’s official language; only 3 studies included patients with limited literacy, patients who spoke other languages, or caregivers if the patients could not communicate.
Most studies tested the impact of educational discharge interventions (28 of 30 studies) (Table 1). Quite often, it was a member of the research team who carried out the patient education. Only 3 studies involved multiple members of the care team in designing or reviewing the discharge tool with the patient. Almost half (12 studies) targeted multiple aspects of postdischarge care, including medications and side effects, signs and symptoms to consider, plans for follow-up, dietary restrictions, and/or exercise modifications. Many (19 studies) provided education using one-on-one teaching in association with a discharge tool, accompanied by a written handout (13 studies), audiotape (2 studies), or video (3 studies). While 13 studies had patients involved in creating what content was discussed and 14 studies had patients involved in the delivery of the tool, only 6 studies had patients involved in both design and delivery of the tool. Nine studies also used visual aids such as pictures, larger font, or use of a tool enhanced for patients with language barriers or limited health literacy.
Among all 30 studies included, 16 studies tested the impact of their tool on comprehension postdischarge, with 10 studies demonstrating an improvement among patients who had received the tool (Table 2). Five studies evaluated healthcare utilization outcomes such as readmission, length of stay, or physician visits after discharge and 2 studies found improvements. Twelve studies also studied the impact on adherence with medications, diet, exercise, or follow-up instructions postdischarge. However, only 4 of these 12 studies showed a positive impact. Only 2 studies tested the impact on a patient’s ability to self-manage once at home, and both studies reported positive statistical outcomes. Few studies measured patient experience (such as patient satisfaction or improvement in self-efficacy) or mortality postdischarge.
DISCUSSION/CONCLUSION
Our systematic review found 30 studies that engaged patients during the design or the delivery of a discharge instruction tool and that tested the effect of the tool on postdischarge outcomes.6-10,14–38 Our review suggests that there is sufficient evidence that patient-centered discharge tools improve comprehension. However, evidence is currently insufficient to determine if patient-centered tools improve adherence with discharge instructions. Moreover, though limited studies show promising results, more studies are needed to determine if patient engagement improves self-efficacy and healthcare utilization after discharge.
A major limitation of current studies is the variability in the level of patient engagement in tool design or delivery. Patients were involved in the design mostly through targeted development of a discharge management plan and the delivery by encouraging them to ask questions. Few studies involved patients in the design of the tool such that patients were responsible for coming up with content that was of interest to them. The few that did, often with the additional use of video media, demonstrated significant outcomes. Only a minority of studies used an interactive process to assess understanding such as “teach-back” or maximize patient comprehension such as visual aids. Even fewer studies engaged patients in both developing the discharge tool and providing discharge instructions.
Several previous studies have demonstrated that most complications after discharge are the result of ineffective communication, which can be exacerbated by lack of fluency in English or by limited health literacy.2,39-43 As a result, poor understanding of discharge instructions by patients and their caregivers can create an important care gap.44 Therefore, the use of patient-centered tools to engage patients at discharge in their own care is needed. How to engage patients consistently and effectively is perhaps less evident, as demonstrated in this review of the literature in which different levels of patient engagement were found. Many of the tools tested placed attention on patient education, sometimes in the context of bundled care along with home visits or follow-up, all of which can require extensive resources and time. Providing patients with information that the patients themselves state is of value may be the easiest refinement to a discharge educational tool, although this was surprisingly uncommon.6,9,10,17,23,33,37 Only 2 studies were found that engaged patients in the initial stage of design of the discharge tool, by incorporating information of interest to them.23,32 For example, a study testing the impact of a computer-generated written education package on poststroke outcomes designed the information by asking patients to identify which topics they would like to receive information about (along with the amount of information and font size).23 Secondly, although most of the discharge tools reviewed included the use of one-on-one teaching and the use of media such as patient handouts, these tools were often used in such a way that patients were passive recipients. In fact, studies that used additional video media that incorporated personalized content were the most likely to demonstrate positive outcomes.17,34 The next level of patient engagement may therefore be to involve the patient as an interactive partner when delivering the tool in order to empower patients to self-care. For example, 1 study designed a structured education program by first assessing lifestyle risk factors related to hypertension that were modifiable along with preconceived notions through open-ended questions during a one-on-one interview.37 Patients were subsequently educated on any knowledge deficits regarding the management of their lifestyle. Another level of patient engagement may be to use visual aids during discussions, as a well-known complement to verbal instructions.45,46 For example, in a controlled study that randomized a ward of elderly patients with 4 or more prescriptions to predischarge counseling, the counseling session aimed to review reasons for their prescriptions along with corresponding side effects, doses, and dosage times with the help of a medicine reminder card. Other uses of visual aid tools identified in our review included the use of pictograms or illustrations or, at minimum, attention to font size.7,8,16,29,33,35 In the absence of a visual aid, asking the patient to repeat or demonstrate what was just communicated can be used to assess the amount of information retained.18,33
An important result discovered in our review of the literature was also the lack of studies that tested the impact of discharge tools on usability of discharge information once at home. Conducting an evaluation of the benefits to patients after discharge can help objectify vague outcomes like health gains or qualify benefits in patient’s views. This might also explain why many studies with documented patient engagement at the time of discharge were able to demonstrate improvements in comprehension but not adherence to instructions. Although patients and caregivers may understand the information, this comprehension does not necessarily mean they will find the information useful or adhere to it once at home. For example, in 1 study, patients discharged with at least 1 medication were randomized to a structured discharge interview during which the treatment plan was reviewed verbally and questions clarified along with a visually enhanced treatment card.26 Although knowledge of medications increased, no effect was found on adherence at 1 week postdischarge. However, use of the treatment card at home was not assessed. Similarly, another study tested the effect of an individualized video of exercises and failed to find a difference in patient adherence at 4 weeks.28 The authors suggested that the lack of benefit may have been because patients were not using the video once at home. This is in contrast to 2 studies that involved patients in their own care by requiring them to request their medication as part of a self-medication tool predischarge.16,30 Patients were engaged in the process such that increasing independence was given to patients based on their demonstration of understanding and adherence to their treatment while still in the hospital, a learning tool that can be applied once at home. Feeling knowledgeable and involved, as others have suggested, may be the intermediary outcomes that led to improved adherence.47 It is also possible that adherence to discharge instructions may vary based on complexity of the information provided, such that instructions focusing solely on medication use may require less patient engagement than discharge instructions that include information on medications, diet, exercise modifications, and follow-up.48
Our review has a few limitations. Previous systematic reviews have demonstrated that bundled discharge interventions that include patient-centered education have a positive effect on outcomes postdischarge.2,5 However, we sought to describe and study the individual and distinct impact of patient engagement in the creation and delivery of discharge tools on outcomes postdischarge. We hoped that this may provide others with key information regarding elements of patient engagement that were particularly useful when designing a new discharge tool. The variability of the studies we identified, however, made it difficult to ascertain what level of patient engagement is required to observe improvements in health outcomes. It is also possible that a higher level of patient engagement may have been used but not described in the studies we reviewed. As only primary outcomes were included, we may have underestimated the effect of patient-centered discharge tools on outcomes that were reported as secondary outcomes. As we were interested in reviewing as many studies of patient-centered discharge tools as possible, we did not assess the quality of the studies and cannot comment on the role of bias in these studies. However, we excluded studies with study designs known to have the highest risk of bias. Lastly, we also cannot comment on whether patient-centered tools may have an effect on outcomes more than 3 months after a hospital discharge. However, several studies included in this review suggest a sustained effect beyond this time period.8,25,32,37
Patient-centered discharge tools in which patients were engaged in the design or the delivery were found to improve comprehension of but not adherence with discharge instructions. The perceived lack of improved adherence may be due to a lack of studies that measured the usefulness and utilization of information for patients once at home. There was also substantial variability in the extent of patient involvement in designing the style and content of information provided to patients at discharge, as well as the extent of patient engagement when receiving discharge instructions. Future studies would benefit from detailing the level of patient engagement needed in designing and delivery of discharge tools. This information may lead to the discovery of barriers and facilitators to utilization of discharge information once at home and lead to a better understanding of the patient’s journey from hospital to home and onwards.
C.M.B. and this work were funded by a CIHR Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. Funding was provided to cover fees to obtain articles from the Donald J. Matthews Complex Care Fund of the University Health Network in Toronto, Canada. The Toronto Central Local Health Integration Network provided funding for the design and implementation of a patient-oriented discharge summary. None of the funding or supportive agencies were involved in the design or conduct of the present study, analysis, or interpretation of the data, or approval of the manuscript.
Disclosures
The authors report no conflicts of interest.
Patient-centered care, defined by the Institute of Medicine as “health care that establishes a partnership among practitioners, patients, and their families to ensure that decisions respect patients’ wants, needs and preferences and that patients have the education and support they need to make decisions and participate in their own care,” has been recognized as an important factor in improving care transitions after discharge from the hospital.1 Previous efforts to improve the discharge process for hospitalized patients and reduce avoidable readmissions have focused on improving systems surrounding the patient, such as by increasing the availability of outpatient follow-up or standardizing communication between the inpatient and outpatient care teams.1,2 In fact, successful programs such as Project BOOST and the Care Transitions Interventions™ provide healthcare institutions with a “bundle” of evidence-based transitional care guidelines for discharge: they provide postdischarge transition coaches, assistance with medication self-management, timely follow-up tips, and improved patient records in order to improve postdischarge outcomes.3,4 Successful interventions, however, may not provide more services, but also engage the patient in their own care.5,6 The impact of engaging the patient in his or her own care by providing patient-friendly discharge instructions alone, however, is unknown.
A patient-centered discharge may use tools that were designed with patients, or may involve engaging patients in an interactive process of reviewing discharge instructions and empowering them to manage aspects of their own care after leaving the hospital. This endeavour may lead to more effective use of discharge instructions and reduce the need for additional or more intensive (and costly) interventions. For example, a patient-centered discharge tool could include an educational intervention that uses the “teach-back” method, in which patients are asked to restate in their own words what they thought they heard, or in which staff use additional media or a visual design tool meant to enhance comprehension of discharge instructions.6,7 Visual aids and the use of larger fonts are particularly useful design elements for improving comprehension among non-English speakers and patients with low health literacy, who tend to have poorer recall of instructions.8-10 What may constitute essential design elements to include in a discharge instruction tool, however, is not clear.
Moreover, whether the use of discharge tools with a specific focus on patient engagement may improve postdischarge outcomes is not known. Particularly, the ability of patient-centered discharge tools to improve outcomes beyond comprehension such as self-management, adherence to discharge instructions, a reduction in unplanned visits, and a reduction in mortality has not been studied systematically. The objective of this systematic review was to review the literature on discharge instruction tools with a focus on patient engagement and their impact among hospitalized patients.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement was followed as a guideline for reporting throughout this review.11
Data Sources
A literature search was undertaken using the following databases from January 1994 or their inception date to May 2014: Medline, Embase, SIGLE, HTA, Bioethics, ASSIA, Psych Lit, CINAHL, Cochrane Library, EconLit, ERIC, and BioMed Central. We also searched relevant design-focused journals such as Design Issues, Journal of Design Research, Information Design Journal, Innovation, Design Studies, and International Journal of Design, as well as reference lists from studies obtained by electronic searching. The following key words and combination of key words were used with the assistance of a medical librarian: patient discharge, patient-centered discharge, patient-centered design, design thinking, user based design, patient education, discharge summary, education. Additional search terms were added when identified from relevant articles (Appendix).
Inclusion Criteria
We included all English-language studies with patients admitted to the hospital irrespective of age, sex, or medical condition, which included a control group or time period and which measured patient outcomes within 3 months of discharge. The 3-month period after discharge is often cited as a time when outcomes could reasonably be associated with an intervention at discharge.2
Exclusion Criteria
Studies that did not have clear implementation of a patient-centered tool, a control group, or those whose tool was used in the emergency department or as an outpatient were excluded. Studies that included postdischarge tools such as home visits or telephone calls were excluded unless independent effects of the predischarge interventions were measured. Studies with outcomes reported after 3 months were excluded unless outcomes before 3 months were also clearly noted.
All searches were entered into Endnote and duplicates were removed. A 2-stage inclusion process was used. Titles and abstracts of articles were first screened for meeting inclusion and exclusion criteria by 1 reviewer. A second reviewer independently checked a 10% random sample of all the abstracts that met the initial screening criteria. If the agreement to exclude studies was less than 95%, criteria were reviewed before checking the rest of the 90% sample. In the second stage, 2 independent reviewers examined paper copies of the full articles selected in the first stage. Disagreement between reviewers was resolved by discussion or a third reviewer if no agreement could be reached.
Data Analysis and Synthesis
The following information was extracted from the full reference: type of study, population studied, control group or time period, tool used, and outcomes measured. Based on the National Health Care Quality report’s priorities and goals on patient and/or family engagement during transitions of care, educational tools were further described based on method of teaching, involvement of the care team, involvement of the patient in the design or delivery of the tool, and/or the use of visual aids.12 All primary outcomes were classified according to 3 categories: improved knowledge/comprehension, patient experience (patient satisfaction, self-management/efficacy such as functional status, both physical and mental), and health outcomes (unscheduled visits or readmissions, adherence with medications, diet, exercise, or follow-up, and mortality).
No quantitative pooling of results or meta-analysis was done given the variability and heterogeneity of studies reviewed. However, following guidelines for Effect Practice and Organisation of Care (EPOC) Risk of Bias criteria,13 studies that had a higher risk of bias such as uncontrolled before-after studies or studies with only 1 intervention or control site (historical controls, eg) were excluded from the final review because of the difficulties in attributing causation. Only primary outcomes were reported in order to minimize type II errors.
RESULTS
Our search revealed a total of 3699 studies after duplicates had been removed (Figure). A total of 714 references were included after initial review by title and abstract and 30 studies after full-text review. Agreement on a 10% random sample of all abstracts and full text was 79% (k=0.58) and 86% (k=0.72), respectively. Discussion was needed for fewer than 100 references, and agreement was subsequently reached for 100%.
There were 22 randomized controlled trials and 8 nonrandomized studies (5 nonrandomized controlled trials and 3 controlled before-after studies). Most of these studies were conducted in the United States (13/30 studies), followed by other European countries (5 studies), and the United Kingdom (4 studies). A large number of studies were conducted among patients with cardiovascular disease or risk factors (10 studies), followed by postsurgical patients such as coronary artery bypass graft surgery or orthopaedic surgery (5 studies). Five of 30 studies were conducted among individuals older than 65 years. Most studies excluded patients who did not speak English or the country’s official language; only 3 studies included patients with limited literacy, patients who spoke other languages, or caregivers if the patients could not communicate.
Most studies tested the impact of educational discharge interventions (28 of 30 studies) (Table 1). Quite often, it was a member of the research team who carried out the patient education. Only 3 studies involved multiple members of the care team in designing or reviewing the discharge tool with the patient. Almost half (12 studies) targeted multiple aspects of postdischarge care, including medications and side effects, signs and symptoms to consider, plans for follow-up, dietary restrictions, and/or exercise modifications. Many (19 studies) provided education using one-on-one teaching in association with a discharge tool, accompanied by a written handout (13 studies), audiotape (2 studies), or video (3 studies). While 13 studies had patients involved in creating what content was discussed and 14 studies had patients involved in the delivery of the tool, only 6 studies had patients involved in both design and delivery of the tool. Nine studies also used visual aids such as pictures, larger font, or use of a tool enhanced for patients with language barriers or limited health literacy.
Among all 30 studies included, 16 studies tested the impact of their tool on comprehension postdischarge, with 10 studies demonstrating an improvement among patients who had received the tool (Table 2). Five studies evaluated healthcare utilization outcomes such as readmission, length of stay, or physician visits after discharge and 2 studies found improvements. Twelve studies also studied the impact on adherence with medications, diet, exercise, or follow-up instructions postdischarge. However, only 4 of these 12 studies showed a positive impact. Only 2 studies tested the impact on a patient’s ability to self-manage once at home, and both studies reported positive statistical outcomes. Few studies measured patient experience (such as patient satisfaction or improvement in self-efficacy) or mortality postdischarge.
DISCUSSION/CONCLUSION
Our systematic review found 30 studies that engaged patients during the design or the delivery of a discharge instruction tool and that tested the effect of the tool on postdischarge outcomes.6-10,14–38 Our review suggests that there is sufficient evidence that patient-centered discharge tools improve comprehension. However, evidence is currently insufficient to determine if patient-centered tools improve adherence with discharge instructions. Moreover, though limited studies show promising results, more studies are needed to determine if patient engagement improves self-efficacy and healthcare utilization after discharge.
A major limitation of current studies is the variability in the level of patient engagement in tool design or delivery. Patients were involved in the design mostly through targeted development of a discharge management plan and the delivery by encouraging them to ask questions. Few studies involved patients in the design of the tool such that patients were responsible for coming up with content that was of interest to them. The few that did, often with the additional use of video media, demonstrated significant outcomes. Only a minority of studies used an interactive process to assess understanding such as “teach-back” or maximize patient comprehension such as visual aids. Even fewer studies engaged patients in both developing the discharge tool and providing discharge instructions.
Several previous studies have demonstrated that most complications after discharge are the result of ineffective communication, which can be exacerbated by lack of fluency in English or by limited health literacy.2,39-43 As a result, poor understanding of discharge instructions by patients and their caregivers can create an important care gap.44 Therefore, the use of patient-centered tools to engage patients at discharge in their own care is needed. How to engage patients consistently and effectively is perhaps less evident, as demonstrated in this review of the literature in which different levels of patient engagement were found. Many of the tools tested placed attention on patient education, sometimes in the context of bundled care along with home visits or follow-up, all of which can require extensive resources and time. Providing patients with information that the patients themselves state is of value may be the easiest refinement to a discharge educational tool, although this was surprisingly uncommon.6,9,10,17,23,33,37 Only 2 studies were found that engaged patients in the initial stage of design of the discharge tool, by incorporating information of interest to them.23,32 For example, a study testing the impact of a computer-generated written education package on poststroke outcomes designed the information by asking patients to identify which topics they would like to receive information about (along with the amount of information and font size).23 Secondly, although most of the discharge tools reviewed included the use of one-on-one teaching and the use of media such as patient handouts, these tools were often used in such a way that patients were passive recipients. In fact, studies that used additional video media that incorporated personalized content were the most likely to demonstrate positive outcomes.17,34 The next level of patient engagement may therefore be to involve the patient as an interactive partner when delivering the tool in order to empower patients to self-care. For example, 1 study designed a structured education program by first assessing lifestyle risk factors related to hypertension that were modifiable along with preconceived notions through open-ended questions during a one-on-one interview.37 Patients were subsequently educated on any knowledge deficits regarding the management of their lifestyle. Another level of patient engagement may be to use visual aids during discussions, as a well-known complement to verbal instructions.45,46 For example, in a controlled study that randomized a ward of elderly patients with 4 or more prescriptions to predischarge counseling, the counseling session aimed to review reasons for their prescriptions along with corresponding side effects, doses, and dosage times with the help of a medicine reminder card. Other uses of visual aid tools identified in our review included the use of pictograms or illustrations or, at minimum, attention to font size.7,8,16,29,33,35 In the absence of a visual aid, asking the patient to repeat or demonstrate what was just communicated can be used to assess the amount of information retained.18,33
An important result discovered in our review of the literature was also the lack of studies that tested the impact of discharge tools on usability of discharge information once at home. Conducting an evaluation of the benefits to patients after discharge can help objectify vague outcomes like health gains or qualify benefits in patient’s views. This might also explain why many studies with documented patient engagement at the time of discharge were able to demonstrate improvements in comprehension but not adherence to instructions. Although patients and caregivers may understand the information, this comprehension does not necessarily mean they will find the information useful or adhere to it once at home. For example, in 1 study, patients discharged with at least 1 medication were randomized to a structured discharge interview during which the treatment plan was reviewed verbally and questions clarified along with a visually enhanced treatment card.26 Although knowledge of medications increased, no effect was found on adherence at 1 week postdischarge. However, use of the treatment card at home was not assessed. Similarly, another study tested the effect of an individualized video of exercises and failed to find a difference in patient adherence at 4 weeks.28 The authors suggested that the lack of benefit may have been because patients were not using the video once at home. This is in contrast to 2 studies that involved patients in their own care by requiring them to request their medication as part of a self-medication tool predischarge.16,30 Patients were engaged in the process such that increasing independence was given to patients based on their demonstration of understanding and adherence to their treatment while still in the hospital, a learning tool that can be applied once at home. Feeling knowledgeable and involved, as others have suggested, may be the intermediary outcomes that led to improved adherence.47 It is also possible that adherence to discharge instructions may vary based on complexity of the information provided, such that instructions focusing solely on medication use may require less patient engagement than discharge instructions that include information on medications, diet, exercise modifications, and follow-up.48
Our review has a few limitations. Previous systematic reviews have demonstrated that bundled discharge interventions that include patient-centered education have a positive effect on outcomes postdischarge.2,5 However, we sought to describe and study the individual and distinct impact of patient engagement in the creation and delivery of discharge tools on outcomes postdischarge. We hoped that this may provide others with key information regarding elements of patient engagement that were particularly useful when designing a new discharge tool. The variability of the studies we identified, however, made it difficult to ascertain what level of patient engagement is required to observe improvements in health outcomes. It is also possible that a higher level of patient engagement may have been used but not described in the studies we reviewed. As only primary outcomes were included, we may have underestimated the effect of patient-centered discharge tools on outcomes that were reported as secondary outcomes. As we were interested in reviewing as many studies of patient-centered discharge tools as possible, we did not assess the quality of the studies and cannot comment on the role of bias in these studies. However, we excluded studies with study designs known to have the highest risk of bias. Lastly, we also cannot comment on whether patient-centered tools may have an effect on outcomes more than 3 months after a hospital discharge. However, several studies included in this review suggest a sustained effect beyond this time period.8,25,32,37
Patient-centered discharge tools in which patients were engaged in the design or the delivery were found to improve comprehension of but not adherence with discharge instructions. The perceived lack of improved adherence may be due to a lack of studies that measured the usefulness and utilization of information for patients once at home. There was also substantial variability in the extent of patient involvement in designing the style and content of information provided to patients at discharge, as well as the extent of patient engagement when receiving discharge instructions. Future studies would benefit from detailing the level of patient engagement needed in designing and delivery of discharge tools. This information may lead to the discovery of barriers and facilitators to utilization of discharge information once at home and lead to a better understanding of the patient’s journey from hospital to home and onwards.
C.M.B. and this work were funded by a CIHR Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. Funding was provided to cover fees to obtain articles from the Donald J. Matthews Complex Care Fund of the University Health Network in Toronto, Canada. The Toronto Central Local Health Integration Network provided funding for the design and implementation of a patient-oriented discharge summary. None of the funding or supportive agencies were involved in the design or conduct of the present study, analysis, or interpretation of the data, or approval of the manuscript.
Disclosures
The authors report no conflicts of interest.
1. Hurtad
2. Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta-review. BMC Health Serv Res. 2007;7:47. PubMed
3. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
4. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
5. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
6. Osman LM, Calder C, Godden DJ, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax. 2002;57(10):869-874. PubMed
7. Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 suppl 1):S209-S216. PubMed
8. Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Resp Med. 2001;95(11):851-856. PubMed
9. Haerem JW, Ronning EJ, Leidal R. Home access to hospital discharge information on audiotape reduces sick leave and readmissions in patients with first-time myocardial infarction. Scand Cardiovasc J. 2000;34(2):219-222. PubMed
10. Legrain S, Tubach F, Bonnet-Zamponi D, et al. A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2011;59(11):2017-2028. PubMed
11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
12. Partnership NP. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, DC: National Quality Forum; 2008.
13. Effective Practice and Organisation of Care (EPOC). EPOC-specific resources for review authors. Oslo, Norway: Norwegian Knowledge Centre for the Health Services; 2013. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed December 21, 2016.
14. Manning DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care. 2007;16(1):71-76. PubMed
15. Perera KY, Ranasinghe P, Adikari AM, et al. Medium of language in discharge summaries: would the use of native language improve patients’ knowledge of their illness and medications? J Health Commun. 2012;17(2):141-148. PubMed
16. Lowe CJ, Raynor DK, Courtney EA, et al. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ. 1995;310(6989):1229-1231. PubMed
17. Mahler HI, Kulik JA, Tarazi RY. Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary bypass surgery. J Cardiopulm Rehabil. 1999;19(3):170-177. PubMed
18. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counseling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657-664. PubMed
19. Drenth-van Maanen AC, Wilting I, Jansen PA, et al. Effect of a discharge medication intervention on the incidence and nature of medication discrepancies in older adults. J Am Geriatr Soc. 2013;61(3):456-458. PubMed
20. Eshah NF. Predischarge education improves adherence to a healthy lifestyle among Jordanian patients with acute coronary syndrome. Nurs Health Sci. 2013;15(3):273-279. PubMed
21. Gwadry-Sridhar FH, Arnold JM, Zhang Y,et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. PubMed
22. Ho SM, Heh SS, Jevitt CM, et al. Effectiveness of a discharge education program in reducing the severity of postpartum depression: a randomized controlled evaluation study. Patient Educ Couns. 2009;77(1):68-71. PubMed
23. Hoffmann T, McKenna K, Worrall L, et al. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing. 2007;36(3):280-286. PubMed
24. Jenkins HM, Blank V, Miller K, et al. A randomized single-blind evaluation of a discharge teaching book for pediatric patients with burns. J Burn Care Rehabil. 1996;17(1):49-61. PubMed
25. Kommuri NV, Johnson ML, Koelling TM. Relationship between improvements in heart failure patient disease specific knowledge and clinical events as part of a randomized controlled trial. Patient Educ Couns. 2012;86(2):233-238. PubMed
26. Louis-Simonet M, Kossovsky MP, Sarasin FP, et al. Effects of a structured patient-centered discharge interview on patients’ knowledge about their medications. Am J Med. 2004;117(8):563-568. PubMed
27. Lucas KS. Outcomes evaluation of a pharmacist discharge medication teaching service. Am J Health Syst Pharm. 1998;55(24 suppl 4):S32-S35. PubMed
28. Lysack C, Dama M, Neufeld S, et al. A compliance and satisfaction with home exercise: a comparison of computer-assisted video instruction and routine rehabilitation practice. J Allied Health. 2005;34(2):76-82. PubMed
29. Moore SM. The effects of a discharge information intervention on recovery outcomes following coronary artery bypass surgery. Int J Nurs Stud. 1996;33(2):181-189. PubMed
30. Pereles L, Romonko L, Murzyn T, et al. Evaluation of a self-medication program. J Am Geriatr Soc. 1996;44(2):161-165. PubMed
31. Reynolds MA. Postoperative pain management discharge teaching in a rural population. Pain Manag Nurs. 2009;10(2):76-84. PubMed
32. Sabariego C, Barrera AE, Neubert S, et al. Evaluation of an ICF-based patient education programme for stroke patients: a randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br J Health Psychol. 2013;18(4):707-728. PubMed
33. Shieh SJ, Chen HL, Liu FC, et al. The effectiveness of structured discharge education on maternal confidence, caring knowledge and growth of premature newborns. J Clin Nurs. 2010;19(23-24):3307-3313. PubMed
34. Steinberg TG, Diercks MJ, Millspaugh J. An evaluation of the effectiveness of a videotape for discharge teaching of organ transplant recipients. J Transpl Coord. 1996;6(2):59-63. PubMed
35. Whitby M, McLaws ML, Doidge S, et al. Post-discharge surgical site surveillance: does patient education improve reliability of diagnosis? J Hosp Infect. 2007;66(3):237-242. PubMed
36. Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Mil Med. 1995;160(11):561–564. PubMed
37. Zernike W, Henderson A. Evaluating the effectiveness of two teaching strategies for patients diagnosed with hypertension. J Clin Nurs. 1998;7(1):37–44. PubMed
38. Press VG, Arora V, Constantine KL, et al. Forget me not: a randomized trial of the durability of hospital-based education on inhalers for patients with COPD or asthma [abstract]. J Gen Intern Med. 2014;29(1 suppl):S102.
39. Davis TC, Wolf MS, Bass PF, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. PubMed
40. McCarthy DM, Waite KR, Curtis LM, et al. What did the doctor say? Health literacy and recall of medical instructions. Med Care. 2012;50(4):277–282. PubMed
41. Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–1862. PubMed
42. Cawthon C, Walia S, Osborn CY, et al. Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324. PubMed
43. Karliner LS, Auerbach A, Nápoles A, et al. Language barriers and understanding of hospital discharge instructions. Med Care. 2012;50(4):283–289. PubMed
44. Enhancing the Continuum of Care. Report of the Avoidable Hospitalization Advisory Panel. http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Published November 2011. Accessed December 22, 2016.
45. Chugh A, Williams MV, Grigsby J, et al. Better transitions: improving comprehension of discharge instructions. Front Health Serv Manage. 2009;25(3):11–32. PubMed
46. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medication in an anticoagulation clinic: a comparison of verbal vs. visual assessment. J Health Commun. 2006;11(7):651–664. PubMed
47. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. PubMed
48. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498. PubMed
1. Hurtad
2. Mistiaen P, Francke AL, Poot E. Interventions aimed at reducing problems in adult patients discharged from hospital to home: a systematic meta-review. BMC Health Serv Res. 2007;7:47. PubMed
3. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822-1828. PubMed
4. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
5. Hansen LO, Young RS, Hinami K, et al. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520-528. PubMed
6. Osman LM, Calder C, Godden DJ, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax. 2002;57(10):869-874. PubMed
7. Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 suppl 1):S209-S216. PubMed
8. Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Resp Med. 2001;95(11):851-856. PubMed
9. Haerem JW, Ronning EJ, Leidal R. Home access to hospital discharge information on audiotape reduces sick leave and readmissions in patients with first-time myocardial infarction. Scand Cardiovasc J. 2000;34(2):219-222. PubMed
10. Legrain S, Tubach F, Bonnet-Zamponi D, et al. A new multimodal geriatric discharge-planning intervention to prevent emergency visits and rehospitalizations of older adults: the optimization of medication in AGEd multicenter randomized controlled trial. J Am Geriatr Soc. 2011;59(11):2017-2028. PubMed
11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. PubMed
12. Partnership NP. National Priorities and Goals: Aligning Our Efforts to Transform America’s Healthcare. Washington, DC: National Quality Forum; 2008.
13. Effective Practice and Organisation of Care (EPOC). EPOC-specific resources for review authors. Oslo, Norway: Norwegian Knowledge Centre for the Health Services; 2013. http://epoc.cochrane.org/epoc-specific-resources-review-authors. Accessed December 21, 2016.
14. Manning DM, O’Meara JG, Williams AR, et al. 3D: a tool for medication discharge education. Qual Saf Health Care. 2007;16(1):71-76. PubMed
15. Perera KY, Ranasinghe P, Adikari AM, et al. Medium of language in discharge summaries: would the use of native language improve patients’ knowledge of their illness and medications? J Health Commun. 2012;17(2):141-148. PubMed
16. Lowe CJ, Raynor DK, Courtney EA, et al. Effects of self medication programme on knowledge of drugs and compliance with treatment in elderly patients. BMJ. 1995;310(6989):1229-1231. PubMed
17. Mahler HI, Kulik JA, Tarazi RY. Effects of a videotape information intervention at discharge on diet and exercise compliance after coronary bypass surgery. J Cardiopulm Rehabil. 1999;19(3):170-177. PubMed
18. Al-Rashed SA, Wright DJ, Roebuck N, et al. The value of inpatient pharmaceutical counseling to elderly patients prior to discharge. Br J Clin Pharmacol. 2002;54(6):657-664. PubMed
19. Drenth-van Maanen AC, Wilting I, Jansen PA, et al. Effect of a discharge medication intervention on the incidence and nature of medication discrepancies in older adults. J Am Geriatr Soc. 2013;61(3):456-458. PubMed
20. Eshah NF. Predischarge education improves adherence to a healthy lifestyle among Jordanian patients with acute coronary syndrome. Nurs Health Sci. 2013;15(3):273-279. PubMed
21. Gwadry-Sridhar FH, Arnold JM, Zhang Y,et al. Pilot study to determine the impact of a multidisciplinary educational intervention in patients hospitalized with heart failure. Am Heart J. 2005;150(5):982. PubMed
22. Ho SM, Heh SS, Jevitt CM, et al. Effectiveness of a discharge education program in reducing the severity of postpartum depression: a randomized controlled evaluation study. Patient Educ Couns. 2009;77(1):68-71. PubMed
23. Hoffmann T, McKenna K, Worrall L, et al. Randomised trial of a computer-generated tailored written education package for patients following stroke. Age Ageing. 2007;36(3):280-286. PubMed
24. Jenkins HM, Blank V, Miller K, et al. A randomized single-blind evaluation of a discharge teaching book for pediatric patients with burns. J Burn Care Rehabil. 1996;17(1):49-61. PubMed
25. Kommuri NV, Johnson ML, Koelling TM. Relationship between improvements in heart failure patient disease specific knowledge and clinical events as part of a randomized controlled trial. Patient Educ Couns. 2012;86(2):233-238. PubMed
26. Louis-Simonet M, Kossovsky MP, Sarasin FP, et al. Effects of a structured patient-centered discharge interview on patients’ knowledge about their medications. Am J Med. 2004;117(8):563-568. PubMed
27. Lucas KS. Outcomes evaluation of a pharmacist discharge medication teaching service. Am J Health Syst Pharm. 1998;55(24 suppl 4):S32-S35. PubMed
28. Lysack C, Dama M, Neufeld S, et al. A compliance and satisfaction with home exercise: a comparison of computer-assisted video instruction and routine rehabilitation practice. J Allied Health. 2005;34(2):76-82. PubMed
29. Moore SM. The effects of a discharge information intervention on recovery outcomes following coronary artery bypass surgery. Int J Nurs Stud. 1996;33(2):181-189. PubMed
30. Pereles L, Romonko L, Murzyn T, et al. Evaluation of a self-medication program. J Am Geriatr Soc. 1996;44(2):161-165. PubMed
31. Reynolds MA. Postoperative pain management discharge teaching in a rural population. Pain Manag Nurs. 2009;10(2):76-84. PubMed
32. Sabariego C, Barrera AE, Neubert S, et al. Evaluation of an ICF-based patient education programme for stroke patients: a randomized, single-blinded, controlled, multicentre trial of the effects on self-efficacy, life satisfaction and functioning. Br J Health Psychol. 2013;18(4):707-728. PubMed
33. Shieh SJ, Chen HL, Liu FC, et al. The effectiveness of structured discharge education on maternal confidence, caring knowledge and growth of premature newborns. J Clin Nurs. 2010;19(23-24):3307-3313. PubMed
34. Steinberg TG, Diercks MJ, Millspaugh J. An evaluation of the effectiveness of a videotape for discharge teaching of organ transplant recipients. J Transpl Coord. 1996;6(2):59-63. PubMed
35. Whitby M, McLaws ML, Doidge S, et al. Post-discharge surgical site surveillance: does patient education improve reliability of diagnosis? J Hosp Infect. 2007;66(3):237-242. PubMed
36. Williford SL, Johnson DF. Impact of pharmacist counseling on medication knowledge and compliance. Mil Med. 1995;160(11):561–564. PubMed
37. Zernike W, Henderson A. Evaluating the effectiveness of two teaching strategies for patients diagnosed with hypertension. J Clin Nurs. 1998;7(1):37–44. PubMed
38. Press VG, Arora V, Constantine KL, et al. Forget me not: a randomized trial of the durability of hospital-based education on inhalers for patients with COPD or asthma [abstract]. J Gen Intern Med. 2014;29(1 suppl):S102.
39. Davis TC, Wolf MS, Bass PF, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. PubMed
40. McCarthy DM, Waite KR, Curtis LM, et al. What did the doctor say? Health literacy and recall of medical instructions. Med Care. 2012;50(4):277–282. PubMed
41. Tarn DM, Heritage J, Paterniti DA, et al. Physician communication when prescribing new medications. Arch Intern Med. 2006;166(17):1855–1862. PubMed
42. Cawthon C, Walia S, Osborn CY, et al. Improving care transitions: the patient perspective. J Health Commun. 2012;17(suppl 3):312–324. PubMed
43. Karliner LS, Auerbach A, Nápoles A, et al. Language barriers and understanding of hospital discharge instructions. Med Care. 2012;50(4):283–289. PubMed
44. Enhancing the Continuum of Care. Report of the Avoidable Hospitalization Advisory Panel. http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Published November 2011. Accessed December 22, 2016.
45. Chugh A, Williams MV, Grigsby J, et al. Better transitions: improving comprehension of discharge instructions. Front Health Serv Manage. 2009;25(3):11–32. PubMed
46. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medication in an anticoagulation clinic: a comparison of verbal vs. visual assessment. J Health Commun. 2006;11(7):651–664. PubMed
47. Epstein RM, Street RL, Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100–103. PubMed
48. Albrecht JS, Gruber-Baldini AL, Hirshon JM, et al. Hospital discharge instructions: comprehension and compliance among older adults. J Gen Intern Med. 2014;29(11):1491–1498. PubMed
The plan-do-study-act cycle and data display
This month’s column is the second in a series of three articles written by a group from Toronto and Houston. The series imagined that a community of gastroenterologists set out to improve the adenoma detection rates of physicians in their practice. The first article described the design and launch of the project. This month, Dr. Bollegala and her colleagues explain the plan-do-study-act (PDSA) cycle of improvement within a small practice. The PDSA cycle is a fundamental component of successful quality improvement initiatives; it allows a group to systematically analyze what works and what doesn’t. The focus of this article is squarely on small community practices (still the majority of gastrointestinal practices nationally), so its relevance is high. PDSA cycles are small, narrowly focused projects that can be accomplished by all as we strive to improve our care of the patients we serve. Next month, we will learn how to embed a quality initiative within our practices so sustained improvement can be seen.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Article 1 of our series focused on the emergence of the adenoma detection rate (ADR) as a quality indicator for colonoscopy-based colorectal cancer screening programs.1 A target ADR of 25% has been established by several national gastroenterology societies and serves as a focus area for those seeking to develop quality improvement (QI) initiatives aimed at reducing the interval incidence of colorectal cancer.2 In this series, you are a community-based urban general gastroenterologist interested in improving your current group ADR of 19% to the established target of 25% for each individual endoscopist within the group over a 12-month period.
This article focuses on a clinician-friendly description of the plan-do-study-act (PDSA) cycle, a key construct within the Model for Improvement framework for QI initiatives. It also describes the importance and key elements of QI data reporting, including the run chart. All core concepts will be framed within the series example of the development of an institutional QI initiative for ADR improvement.
Plan-Do-Study-Act cycle
Conventional scientific research in health care generally is based on large-scale projects, performed over long periods of time and producing aggregate data analyzed through summary statistics. QI-related research, as it relates to PDSA, in contrast, is characterized by smaller-scale projects performed over shorter periods of time, with iterative protocols to accommodate local context and therefore optimize intervention success. As such, the framework for their development, implementation, and continual modification requires a conceptual and methodologic shift.
The PDSA cycle is characterized by four key steps. The first step is to plan. This step involves addressing the following questions: 1) what are we trying to accomplish? (aim); 2) how will we know that a change is an improvement? (measure); and 3) what changes can we make that will lead to improvement? (change). Additional considerations include ensuring that the stated goal is attainable, relevant, and that the timeline is feasible. An important aspect of the plan stage is gaining an understanding for the current local context, key participants and their roles, and areas in which performance is excelling or is challenged. This understanding is critical to conceptually linking the identified problem with its proposed solution. Formulating an impact prediction allows subsequent learning and adaptation.
The second step is to do. This step involves execution of the identified plan over a specified period of time. It also involves rigorous qualitative and quantitative data collection, allowing the research team to assess change and document unexpected events. The identification of an implementation leader or champion to ensure protocol adherence, effective communication among team members, and coordinate accurate data collection can be critical for overall success.
The third step is to study. This step requires evaluating whether a change in the outcome measure has occurred, which intervention was successful, and whether an identified change is sustained over time. It also requires interpretation of change within the local context, specifically with respect to unintended consequences, unanticipated events, and the sustainability of any gains. To interpret study findings appropriately, feedback with involved process members, endoscopists, and/or other stakeholder groups may be necessary. This can be important for explaining the results of each cycle, identifying protocol modifications for future cycles, and optimizing the opportunity for success. Studying the data generated by a QI initiative requires clear and accurate data display and rules for interpretation.
The fourth step is to act. This final step allows team members to reflect on the results generated and decide whether the same intervention should be continued, modified, or changed, thereby incorporating lessons learned from previous PDSA cycles (Figure 1).3
Despite these challenges, the PDSA framework allows for small-scale and fast-paced initiative testing that reduces patient and institutional risk while minimizing the commitment of resources.4,7 Successful cycles improve stakeholder confidence in the probability for success with larger-scale implementation.
In our series example, step 1 of the PDSA cycle, plan, can be described as follows: Aim: increase the ADR of all group endoscopists to 25% over a 12-month period. Measure: Outcome: the proportion of endoscopists at your institution with an ADR greater than 25%; process – withdrawal time; balancing – staff satisfaction, patient satisfaction, and procedure time. Change: Successive cycles will institute the following: audible timers to ensure adequate withdrawal time, publication of an endoscopist-specific composite score, and training to improve inspection technique.8
In step 2 of the PDSA cycle, do, a physician member of the gastroenterology division incorporates QI into their job description and leads a change team charged with PDSA cycle 1. An administrative assistant calculates the endoscopist-specific ADRs for that month. Documentation of related events for this cycle such as unexpected physician absence, delays in polyp histology reporting, and so forth, is performed.
In step 3 of the PDSA cycle, study, the data generated will be represented on a run chart plotting the proportion of endoscopists with an ADR greater than 25% on the y-axis, and time (in monthly intervals) on the x-axis. This will be described in further detail in a later section.
In the final step of the PDSA cycle, act, continuation and modification of the tested changes can be represented as follows.
Displaying data
The documentation, analysis, and interpretation of data generated by multiple PDSA cycles must be displayed accurately and succinctly. The run chart has been developed as a simple technique for identifying nonrandom patterns (that is, signals), which allows QI researchers to determine the impact of each cycle of change and the stability of that change over a given time period.9 This often is contrasted with conventional statistical approaches that aggregate data and perform summary statistical comparisons at static time points. Instead, the run chart allows for an appreciation of the dynamic nature of PDSA-driven process manipulation and resulting outcome changes.
Correct interpretation of the presented data requires an understanding of common cause variation (CCV) and special cause variation (SCV). CCV occurs randomly and is present in all health care processes. It can never be eliminated completely. SCV, in contrast, is the result of external factors that are imposed on normal processes. For example, the introduction of audible timers within endoscopy rooms to ensure adequate withdrawal time may result in an increase in the ADR. The relatively stable ADR measured in both the pre-intervention and postintervention periods are subject to CCV. However, the postintervention increase in ADR is the result of SCV.10
As shown in Figure 2, the horizontal axis shows the time scale and spans the entire duration of the intervention period. The y-axis shows the outcome measure of interest. A horizontal line representing the median is shown.9 A goal line also may be depicted. Annotations to indicate the implementation of change or other important events (such as unintended consequences or unexpected events) also may be added to facilitate data interpretation.
Shift: at least six consecutive data points above or below the median line are needed (points on the median line are skipped).9 To assess a shift appropriately, at least 10 data points are required.
Trend: at least five consecutive data points all increasing in value or all decreasing in value are needed (numerically equivalent points are skipped).9
Runs: a run refers to a series of data points on one side of the median.9 If a random pattern of data points exists on the run chart, there should be an appropriate number of runs on either side of the median. Values outside of this indicate a higher probability of a nonrandom pattern.9,11
Astronomic point: this refers to a data point that subjectively is found to be obviously different from the rest and prompts consideration of the events that led to this.9
Although straightforward to construct and interpret for clinicians without statistical training, the run chart has specific limitations. It is ideal for the display of early data but cannot be used to determine its durability.9 In addition, a run chart does not reflect discrete data with no clear median.
The example run chart in Figure 2 shows that there is a shift in data points from below the median to above the median, ultimately achieving 100% group adherence to the ADR target of greater than 25%. There are only two runs for a total of 12 data points within the 12-month study period, indicating that there is a 5% or less probability that this is a random pattern.11 It appears that our interventions have resulted in incremental improvements in the ADR to exceed the target level in a nonrandom fashion. Although the cumulative effect of these interventions has been successful, it is difficult to predict the durability of this change moving forward. In addition, it would be difficult to select only a single intervention, of the many trialed, that would result in a sustained ADR of 25% or greater.
Summary and next steps
This article selectively reviews the process of change framed by the PDSA cycle. We also discuss the role of data display and interpretation using a run chart. The final article in this series will cover how to sustain change and support a culture of continuous improvement.
References
1. Corley, D.A., Jensen, C.D., Marks, A.R., et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
2. Cohen, J., Schoenfeld, P., Park, W., et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
3. Module 5: Improvement Cycle. (2013). Available at: http://implementation.fpg.unc.edu/book/export/html/326. Accessed Feb. 1, 2016.
4. Taylor, M.J., McNicholas, C., Nicolay, C., et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290-8.
5. Davidoff, F., Batalden, P., Stevens, D. et al. Publication guidelines for quality improvement in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17:i3-9.
6. Ogrinc, G., Mooney, S., Estrada, C., et al. The SQUIRE (standards for Quality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17:i13-32.
7. Nelson, E.C., Batalden, B.P., Godfrey, M.M. Quality by design: a clinical microsystems approach. Jossey-Bass, San Francisco; 2007.
8. Coe, S.G.C.J., Diehl, N.N., Wallace, M.B. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol. 2013;108(2):219-26.
9. Perla, R.J., Provost, L.P., Murray, S.K. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51.
10. Neuhauser, D., Provost, L., Bergman, B. The meaning of variation to healthcare managers, clinical and health-services researchers, and individual patients. BMJ Qual Saf. 2011;20:i36-40.
11. Swed, F.S. Eisenhart, C. Tables for testing randomness of grouping in a sequence of alternatives. Ann Math Statist. 1943;14:66-87
Dr. Bollegala is in the division of gastroenterology, department of medicine, Women’s College Hospital; Dr. Mosko is in the division of gastroenterology, department of medicine, St. Michael’s Hospital, and the Institute of Health Policy, Management, and Evaluation; Dr. Bernstein is in the division of gastroenterology, department of medicine, Sunnybrook Health Sciences Centre; Dr. Brahmania is in the Toronto Center for Liver Diseases, division of gastroenterology, department of medicine, University Health Network; Dr. Liu is in the division of gastroenterology, department of medicine, University Health Network; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management, and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, department of medicine, Mount Sinai Hospital; Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine; Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation. All are at the University of Toronto. Dr. Patel is in the division of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. The authors disclose no conflicts.
This month’s column is the second in a series of three articles written by a group from Toronto and Houston. The series imagined that a community of gastroenterologists set out to improve the adenoma detection rates of physicians in their practice. The first article described the design and launch of the project. This month, Dr. Bollegala and her colleagues explain the plan-do-study-act (PDSA) cycle of improvement within a small practice. The PDSA cycle is a fundamental component of successful quality improvement initiatives; it allows a group to systematically analyze what works and what doesn’t. The focus of this article is squarely on small community practices (still the majority of gastrointestinal practices nationally), so its relevance is high. PDSA cycles are small, narrowly focused projects that can be accomplished by all as we strive to improve our care of the patients we serve. Next month, we will learn how to embed a quality initiative within our practices so sustained improvement can be seen.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Article 1 of our series focused on the emergence of the adenoma detection rate (ADR) as a quality indicator for colonoscopy-based colorectal cancer screening programs.1 A target ADR of 25% has been established by several national gastroenterology societies and serves as a focus area for those seeking to develop quality improvement (QI) initiatives aimed at reducing the interval incidence of colorectal cancer.2 In this series, you are a community-based urban general gastroenterologist interested in improving your current group ADR of 19% to the established target of 25% for each individual endoscopist within the group over a 12-month period.
This article focuses on a clinician-friendly description of the plan-do-study-act (PDSA) cycle, a key construct within the Model for Improvement framework for QI initiatives. It also describes the importance and key elements of QI data reporting, including the run chart. All core concepts will be framed within the series example of the development of an institutional QI initiative for ADR improvement.
Plan-Do-Study-Act cycle
Conventional scientific research in health care generally is based on large-scale projects, performed over long periods of time and producing aggregate data analyzed through summary statistics. QI-related research, as it relates to PDSA, in contrast, is characterized by smaller-scale projects performed over shorter periods of time, with iterative protocols to accommodate local context and therefore optimize intervention success. As such, the framework for their development, implementation, and continual modification requires a conceptual and methodologic shift.
The PDSA cycle is characterized by four key steps. The first step is to plan. This step involves addressing the following questions: 1) what are we trying to accomplish? (aim); 2) how will we know that a change is an improvement? (measure); and 3) what changes can we make that will lead to improvement? (change). Additional considerations include ensuring that the stated goal is attainable, relevant, and that the timeline is feasible. An important aspect of the plan stage is gaining an understanding for the current local context, key participants and their roles, and areas in which performance is excelling or is challenged. This understanding is critical to conceptually linking the identified problem with its proposed solution. Formulating an impact prediction allows subsequent learning and adaptation.
The second step is to do. This step involves execution of the identified plan over a specified period of time. It also involves rigorous qualitative and quantitative data collection, allowing the research team to assess change and document unexpected events. The identification of an implementation leader or champion to ensure protocol adherence, effective communication among team members, and coordinate accurate data collection can be critical for overall success.
The third step is to study. This step requires evaluating whether a change in the outcome measure has occurred, which intervention was successful, and whether an identified change is sustained over time. It also requires interpretation of change within the local context, specifically with respect to unintended consequences, unanticipated events, and the sustainability of any gains. To interpret study findings appropriately, feedback with involved process members, endoscopists, and/or other stakeholder groups may be necessary. This can be important for explaining the results of each cycle, identifying protocol modifications for future cycles, and optimizing the opportunity for success. Studying the data generated by a QI initiative requires clear and accurate data display and rules for interpretation.
The fourth step is to act. This final step allows team members to reflect on the results generated and decide whether the same intervention should be continued, modified, or changed, thereby incorporating lessons learned from previous PDSA cycles (Figure 1).3
Despite these challenges, the PDSA framework allows for small-scale and fast-paced initiative testing that reduces patient and institutional risk while minimizing the commitment of resources.4,7 Successful cycles improve stakeholder confidence in the probability for success with larger-scale implementation.
In our series example, step 1 of the PDSA cycle, plan, can be described as follows: Aim: increase the ADR of all group endoscopists to 25% over a 12-month period. Measure: Outcome: the proportion of endoscopists at your institution with an ADR greater than 25%; process – withdrawal time; balancing – staff satisfaction, patient satisfaction, and procedure time. Change: Successive cycles will institute the following: audible timers to ensure adequate withdrawal time, publication of an endoscopist-specific composite score, and training to improve inspection technique.8
In step 2 of the PDSA cycle, do, a physician member of the gastroenterology division incorporates QI into their job description and leads a change team charged with PDSA cycle 1. An administrative assistant calculates the endoscopist-specific ADRs for that month. Documentation of related events for this cycle such as unexpected physician absence, delays in polyp histology reporting, and so forth, is performed.
In step 3 of the PDSA cycle, study, the data generated will be represented on a run chart plotting the proportion of endoscopists with an ADR greater than 25% on the y-axis, and time (in monthly intervals) on the x-axis. This will be described in further detail in a later section.
In the final step of the PDSA cycle, act, continuation and modification of the tested changes can be represented as follows.
Displaying data
The documentation, analysis, and interpretation of data generated by multiple PDSA cycles must be displayed accurately and succinctly. The run chart has been developed as a simple technique for identifying nonrandom patterns (that is, signals), which allows QI researchers to determine the impact of each cycle of change and the stability of that change over a given time period.9 This often is contrasted with conventional statistical approaches that aggregate data and perform summary statistical comparisons at static time points. Instead, the run chart allows for an appreciation of the dynamic nature of PDSA-driven process manipulation and resulting outcome changes.
Correct interpretation of the presented data requires an understanding of common cause variation (CCV) and special cause variation (SCV). CCV occurs randomly and is present in all health care processes. It can never be eliminated completely. SCV, in contrast, is the result of external factors that are imposed on normal processes. For example, the introduction of audible timers within endoscopy rooms to ensure adequate withdrawal time may result in an increase in the ADR. The relatively stable ADR measured in both the pre-intervention and postintervention periods are subject to CCV. However, the postintervention increase in ADR is the result of SCV.10
As shown in Figure 2, the horizontal axis shows the time scale and spans the entire duration of the intervention period. The y-axis shows the outcome measure of interest. A horizontal line representing the median is shown.9 A goal line also may be depicted. Annotations to indicate the implementation of change or other important events (such as unintended consequences or unexpected events) also may be added to facilitate data interpretation.
Shift: at least six consecutive data points above or below the median line are needed (points on the median line are skipped).9 To assess a shift appropriately, at least 10 data points are required.
Trend: at least five consecutive data points all increasing in value or all decreasing in value are needed (numerically equivalent points are skipped).9
Runs: a run refers to a series of data points on one side of the median.9 If a random pattern of data points exists on the run chart, there should be an appropriate number of runs on either side of the median. Values outside of this indicate a higher probability of a nonrandom pattern.9,11
Astronomic point: this refers to a data point that subjectively is found to be obviously different from the rest and prompts consideration of the events that led to this.9
Although straightforward to construct and interpret for clinicians without statistical training, the run chart has specific limitations. It is ideal for the display of early data but cannot be used to determine its durability.9 In addition, a run chart does not reflect discrete data with no clear median.
The example run chart in Figure 2 shows that there is a shift in data points from below the median to above the median, ultimately achieving 100% group adherence to the ADR target of greater than 25%. There are only two runs for a total of 12 data points within the 12-month study period, indicating that there is a 5% or less probability that this is a random pattern.11 It appears that our interventions have resulted in incremental improvements in the ADR to exceed the target level in a nonrandom fashion. Although the cumulative effect of these interventions has been successful, it is difficult to predict the durability of this change moving forward. In addition, it would be difficult to select only a single intervention, of the many trialed, that would result in a sustained ADR of 25% or greater.
Summary and next steps
This article selectively reviews the process of change framed by the PDSA cycle. We also discuss the role of data display and interpretation using a run chart. The final article in this series will cover how to sustain change and support a culture of continuous improvement.
References
1. Corley, D.A., Jensen, C.D., Marks, A.R., et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
2. Cohen, J., Schoenfeld, P., Park, W., et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
3. Module 5: Improvement Cycle. (2013). Available at: http://implementation.fpg.unc.edu/book/export/html/326. Accessed Feb. 1, 2016.
4. Taylor, M.J., McNicholas, C., Nicolay, C., et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290-8.
5. Davidoff, F., Batalden, P., Stevens, D. et al. Publication guidelines for quality improvement in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17:i3-9.
6. Ogrinc, G., Mooney, S., Estrada, C., et al. The SQUIRE (standards for Quality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17:i13-32.
7. Nelson, E.C., Batalden, B.P., Godfrey, M.M. Quality by design: a clinical microsystems approach. Jossey-Bass, San Francisco; 2007.
8. Coe, S.G.C.J., Diehl, N.N., Wallace, M.B. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol. 2013;108(2):219-26.
9. Perla, R.J., Provost, L.P., Murray, S.K. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51.
10. Neuhauser, D., Provost, L., Bergman, B. The meaning of variation to healthcare managers, clinical and health-services researchers, and individual patients. BMJ Qual Saf. 2011;20:i36-40.
11. Swed, F.S. Eisenhart, C. Tables for testing randomness of grouping in a sequence of alternatives. Ann Math Statist. 1943;14:66-87
Dr. Bollegala is in the division of gastroenterology, department of medicine, Women’s College Hospital; Dr. Mosko is in the division of gastroenterology, department of medicine, St. Michael’s Hospital, and the Institute of Health Policy, Management, and Evaluation; Dr. Bernstein is in the division of gastroenterology, department of medicine, Sunnybrook Health Sciences Centre; Dr. Brahmania is in the Toronto Center for Liver Diseases, division of gastroenterology, department of medicine, University Health Network; Dr. Liu is in the division of gastroenterology, department of medicine, University Health Network; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management, and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, department of medicine, Mount Sinai Hospital; Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine; Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation. All are at the University of Toronto. Dr. Patel is in the division of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. The authors disclose no conflicts.
This month’s column is the second in a series of three articles written by a group from Toronto and Houston. The series imagined that a community of gastroenterologists set out to improve the adenoma detection rates of physicians in their practice. The first article described the design and launch of the project. This month, Dr. Bollegala and her colleagues explain the plan-do-study-act (PDSA) cycle of improvement within a small practice. The PDSA cycle is a fundamental component of successful quality improvement initiatives; it allows a group to systematically analyze what works and what doesn’t. The focus of this article is squarely on small community practices (still the majority of gastrointestinal practices nationally), so its relevance is high. PDSA cycles are small, narrowly focused projects that can be accomplished by all as we strive to improve our care of the patients we serve. Next month, we will learn how to embed a quality initiative within our practices so sustained improvement can be seen.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Article 1 of our series focused on the emergence of the adenoma detection rate (ADR) as a quality indicator for colonoscopy-based colorectal cancer screening programs.1 A target ADR of 25% has been established by several national gastroenterology societies and serves as a focus area for those seeking to develop quality improvement (QI) initiatives aimed at reducing the interval incidence of colorectal cancer.2 In this series, you are a community-based urban general gastroenterologist interested in improving your current group ADR of 19% to the established target of 25% for each individual endoscopist within the group over a 12-month period.
This article focuses on a clinician-friendly description of the plan-do-study-act (PDSA) cycle, a key construct within the Model for Improvement framework for QI initiatives. It also describes the importance and key elements of QI data reporting, including the run chart. All core concepts will be framed within the series example of the development of an institutional QI initiative for ADR improvement.
Plan-Do-Study-Act cycle
Conventional scientific research in health care generally is based on large-scale projects, performed over long periods of time and producing aggregate data analyzed through summary statistics. QI-related research, as it relates to PDSA, in contrast, is characterized by smaller-scale projects performed over shorter periods of time, with iterative protocols to accommodate local context and therefore optimize intervention success. As such, the framework for their development, implementation, and continual modification requires a conceptual and methodologic shift.
The PDSA cycle is characterized by four key steps. The first step is to plan. This step involves addressing the following questions: 1) what are we trying to accomplish? (aim); 2) how will we know that a change is an improvement? (measure); and 3) what changes can we make that will lead to improvement? (change). Additional considerations include ensuring that the stated goal is attainable, relevant, and that the timeline is feasible. An important aspect of the plan stage is gaining an understanding for the current local context, key participants and their roles, and areas in which performance is excelling or is challenged. This understanding is critical to conceptually linking the identified problem with its proposed solution. Formulating an impact prediction allows subsequent learning and adaptation.
The second step is to do. This step involves execution of the identified plan over a specified period of time. It also involves rigorous qualitative and quantitative data collection, allowing the research team to assess change and document unexpected events. The identification of an implementation leader or champion to ensure protocol adherence, effective communication among team members, and coordinate accurate data collection can be critical for overall success.
The third step is to study. This step requires evaluating whether a change in the outcome measure has occurred, which intervention was successful, and whether an identified change is sustained over time. It also requires interpretation of change within the local context, specifically with respect to unintended consequences, unanticipated events, and the sustainability of any gains. To interpret study findings appropriately, feedback with involved process members, endoscopists, and/or other stakeholder groups may be necessary. This can be important for explaining the results of each cycle, identifying protocol modifications for future cycles, and optimizing the opportunity for success. Studying the data generated by a QI initiative requires clear and accurate data display and rules for interpretation.
The fourth step is to act. This final step allows team members to reflect on the results generated and decide whether the same intervention should be continued, modified, or changed, thereby incorporating lessons learned from previous PDSA cycles (Figure 1).3
Despite these challenges, the PDSA framework allows for small-scale and fast-paced initiative testing that reduces patient and institutional risk while minimizing the commitment of resources.4,7 Successful cycles improve stakeholder confidence in the probability for success with larger-scale implementation.
In our series example, step 1 of the PDSA cycle, plan, can be described as follows: Aim: increase the ADR of all group endoscopists to 25% over a 12-month period. Measure: Outcome: the proportion of endoscopists at your institution with an ADR greater than 25%; process – withdrawal time; balancing – staff satisfaction, patient satisfaction, and procedure time. Change: Successive cycles will institute the following: audible timers to ensure adequate withdrawal time, publication of an endoscopist-specific composite score, and training to improve inspection technique.8
In step 2 of the PDSA cycle, do, a physician member of the gastroenterology division incorporates QI into their job description and leads a change team charged with PDSA cycle 1. An administrative assistant calculates the endoscopist-specific ADRs for that month. Documentation of related events for this cycle such as unexpected physician absence, delays in polyp histology reporting, and so forth, is performed.
In step 3 of the PDSA cycle, study, the data generated will be represented on a run chart plotting the proportion of endoscopists with an ADR greater than 25% on the y-axis, and time (in monthly intervals) on the x-axis. This will be described in further detail in a later section.
In the final step of the PDSA cycle, act, continuation and modification of the tested changes can be represented as follows.
Displaying data
The documentation, analysis, and interpretation of data generated by multiple PDSA cycles must be displayed accurately and succinctly. The run chart has been developed as a simple technique for identifying nonrandom patterns (that is, signals), which allows QI researchers to determine the impact of each cycle of change and the stability of that change over a given time period.9 This often is contrasted with conventional statistical approaches that aggregate data and perform summary statistical comparisons at static time points. Instead, the run chart allows for an appreciation of the dynamic nature of PDSA-driven process manipulation and resulting outcome changes.
Correct interpretation of the presented data requires an understanding of common cause variation (CCV) and special cause variation (SCV). CCV occurs randomly and is present in all health care processes. It can never be eliminated completely. SCV, in contrast, is the result of external factors that are imposed on normal processes. For example, the introduction of audible timers within endoscopy rooms to ensure adequate withdrawal time may result in an increase in the ADR. The relatively stable ADR measured in both the pre-intervention and postintervention periods are subject to CCV. However, the postintervention increase in ADR is the result of SCV.10
As shown in Figure 2, the horizontal axis shows the time scale and spans the entire duration of the intervention period. The y-axis shows the outcome measure of interest. A horizontal line representing the median is shown.9 A goal line also may be depicted. Annotations to indicate the implementation of change or other important events (such as unintended consequences or unexpected events) also may be added to facilitate data interpretation.
Shift: at least six consecutive data points above or below the median line are needed (points on the median line are skipped).9 To assess a shift appropriately, at least 10 data points are required.
Trend: at least five consecutive data points all increasing in value or all decreasing in value are needed (numerically equivalent points are skipped).9
Runs: a run refers to a series of data points on one side of the median.9 If a random pattern of data points exists on the run chart, there should be an appropriate number of runs on either side of the median. Values outside of this indicate a higher probability of a nonrandom pattern.9,11
Astronomic point: this refers to a data point that subjectively is found to be obviously different from the rest and prompts consideration of the events that led to this.9
Although straightforward to construct and interpret for clinicians without statistical training, the run chart has specific limitations. It is ideal for the display of early data but cannot be used to determine its durability.9 In addition, a run chart does not reflect discrete data with no clear median.
The example run chart in Figure 2 shows that there is a shift in data points from below the median to above the median, ultimately achieving 100% group adherence to the ADR target of greater than 25%. There are only two runs for a total of 12 data points within the 12-month study period, indicating that there is a 5% or less probability that this is a random pattern.11 It appears that our interventions have resulted in incremental improvements in the ADR to exceed the target level in a nonrandom fashion. Although the cumulative effect of these interventions has been successful, it is difficult to predict the durability of this change moving forward. In addition, it would be difficult to select only a single intervention, of the many trialed, that would result in a sustained ADR of 25% or greater.
Summary and next steps
This article selectively reviews the process of change framed by the PDSA cycle. We also discuss the role of data display and interpretation using a run chart. The final article in this series will cover how to sustain change and support a culture of continuous improvement.
References
1. Corley, D.A., Jensen, C.D., Marks, A.R., et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
2. Cohen, J., Schoenfeld, P., Park, W., et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
3. Module 5: Improvement Cycle. (2013). Available at: http://implementation.fpg.unc.edu/book/export/html/326. Accessed Feb. 1, 2016.
4. Taylor, M.J., McNicholas, C., Nicolay, C., et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290-8.
5. Davidoff, F., Batalden, P., Stevens, D. et al. Publication guidelines for quality improvement in health care: evolution of the SQUIRE project. Qual Saf Health Care. 2008;17:i3-9.
6. Ogrinc, G., Mooney, S., Estrada, C., et al. The SQUIRE (standards for Quality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17:i13-32.
7. Nelson, E.C., Batalden, B.P., Godfrey, M.M. Quality by design: a clinical microsystems approach. Jossey-Bass, San Francisco; 2007.
8. Coe, S.G.C.J., Diehl, N.N., Wallace, M.B. An endoscopic quality improvement program improves detection of colorectal adenomas. Am J Gastroenterol. 2013;108(2):219-26.
9. Perla, R.J., Provost, L.P., Murray, S.K. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20:46-51.
10. Neuhauser, D., Provost, L., Bergman, B. The meaning of variation to healthcare managers, clinical and health-services researchers, and individual patients. BMJ Qual Saf. 2011;20:i36-40.
11. Swed, F.S. Eisenhart, C. Tables for testing randomness of grouping in a sequence of alternatives. Ann Math Statist. 1943;14:66-87
Dr. Bollegala is in the division of gastroenterology, department of medicine, Women’s College Hospital; Dr. Mosko is in the division of gastroenterology, department of medicine, St. Michael’s Hospital, and the Institute of Health Policy, Management, and Evaluation; Dr. Bernstein is in the division of gastroenterology, department of medicine, Sunnybrook Health Sciences Centre; Dr. Brahmania is in the Toronto Center for Liver Diseases, division of gastroenterology, department of medicine, University Health Network; Dr. Liu is in the division of gastroenterology, department of medicine, University Health Network; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management, and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, department of medicine, Mount Sinai Hospital; Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine; Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation. All are at the University of Toronto. Dr. Patel is in the division of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. The authors disclose no conflicts.
Launching a quality improvement initiative
This article by Adam Weizman and colleagues is the first of a three-part series that will provide practical advice for practices that wish to develop a quality initiative. The first article, “Launching a quality improvement initiative” describes the infrastructure, personnel, and structure needed to approach an identified problem within a practice (variability in adenoma detection rates). This case-based approach helps us understand the step-by-step approach needed to reduce variability and improve quality. The authors present a plan (road map) in a straightforward and practical way that seems simple, but if followed carefully, leads to success. These articles are rich in resources and link to state-of-the-art advice.
John I. Allen, MD, MBA, AGAF, Special Section Editor
There has been increasing focus on measuring quality indicators in gastroenterology over the past few years. The adenoma detection rate (ADR) has emerged as one of the most important quality indicators because it is supported by robust clinical evidence.1-3 With every 1% increase in ADR, a 3% reduction in interval colorectal cancer has been noted.3 As such, an ADR of 25% has been designated as an important quality target for all endoscopists who perform colorectal cancer screening.1
You work at a community hospital in a large, metropolitan area. Your colleagues in a number of other departments across your hospital have been increasingly interested in quality improvement (QI) and have launched QI interventions, although none in your department. Moreover, there have been reforms in how hospital endoscopy units are funded in your jurisdiction, with a move toward volume-based funding with a quality overlay. In an effort to improve efficiency and better characterize performance, the hospital has been auditing the performance of all endoscopists at your institution over the past year. Among the eight endoscopists who work at your hospital, the overall ADR has been found to be 19%, decreasing to less than the generally accepted benchmark.1
In response to the results of the audit in your unit, you decide that you would like to develop an initiative to improve your group’s ADR.
Forming a quality improvement team
The first step in any QI project is to establish an improvement team. This working group consists of individuals with specific roles who perform interdependent tasks and share a common goal.4 Usually, frontline health care workers who are impacted most by the quality-of-care problem form the foundation of the team. A team lead is identified who will oversee the project. Content experts are also helpful members of the team who may have particular expertise in the clinical domain that will be the focus of the project. In addition, an improvement adviser, an individual with some expertise in QI, is needed on the team. This adviser may be from within your department or from outside. Although they may not possess expertise in the clinical problem you are trying to tackle, they should have skills in QI methodology and process to aid the team. An executive sponsor also needs to be identified. This should be an influential and well-respected individual who holds a senior administrative position at your institution who can help the team overcome barriers and secure resources. Physician engagement is a critical, often-overlooked step in any improvement effort. Regardless of the initiative, physicians continue to have tremendous influence over hospital-based outcomes.5 Identifying a physician champion, a prominent and respected physician at your organization to help spread the importance of your efforts and create a burning platform for change, is helpful. It also is valuable to have a patient on the improvement team to provide unique perspectives that only the end user of health care can convey and to ensure that the project is patient centered, as all improvement efforts should be.6
Improvement framework
Before starting any improvement effort, there are several important considerations that need to be addressed when choosing a quality improvement target.7 It is important to have a good understanding of the burden and severity of the problem. This often requires audit and measuring. For example, although we may think there is a problem with ADR in our endoscopy unit based on a general impression, it is critical to have data to support this suspicion. This is part of a current state analysis (discussed later). It also is important to select a quality-of-care problem that is under you or your group’s direct control. For example, it would be difficult to initiate a quality improvement project aimed at changing the practice of radiology reporting as a gastroenterologist. It is important to pick a problem that is focused and within a narrow scope that is feasible to address and then improve. Consideration of the unintended consequences of an improvement initiative often is overlooked, but needs to be considered because not all that comes out of quality improvement efforts is good. Finally, the likelihood of success of a quality initiative is increased significantly if it can generate momentum and lead to other interventions both within your department and beyond.
There are several specific improvement frameworks that can be used by a team to address a quality-of-care problem and perform a quality improvement project. The framework chosen depends on the type of problem that is being targeted and the training of the individuals on the improvement team. Three of the most commonly used improvement frameworks include the following: 1) Six Sigma; 2) Lean; and 3) Model for Improvement.
Six Sigma
Six Sigma is focused on improvement by reducing variability.8 It is a highly analytic framework relying on statistical analysis and mathematical modeling. It is best suited for projects in which the root cause and contributors to the target problem remain unclear and the aim of the intervention is to reduce variation.
Lean
Lean emphasizes improvement through elimination of waste and classifies all parts of any process as value added and nonvalue added.9 It is estimated that 95% of activities in any health care process are nonvalue added and the objective of Lean is to identify opportunities to simplify and create efficiencies. It is best suited for target problems that directly can be observed and mapped out, for example, process of care, flow, and efficiency of an endoscopy unit.
Model for Improvement
The Model for Improvement has been popularized by the Institute for Healthcare Improvement.10,11 It is well suited for health care teams, and its advantages are its adaptability to many improvement targets and lack of extensive training, consultant support, or statistical training as required by the previous frameworks mentioned earlier. As a result, it is the most commonly used improvement framework.
Using the Model for Improvement
The Model for Improvement is organized around three main questions: 1) What are we trying to accomplish? 2) How will we know that a change is an improvement? and 3) What changes can result in improvement?
Question 1: What are we trying to accomplish?
The first stage using the Model for Improvement is developing a clear project aim. A good aim statement should be specific in defining what measures one is hoping to improve and setting a concrete deadline by which to achieve it.10,11 It should answer the questions of what the team is trying to improve, by how much, and by what date. It is more effective for the target to be an ambitious, stretch goal to ensure the effort is worth the resources and time that will be invested by the team. Not only does a good aim statement serve as the foundation for the project, but it can redirect the team if the improvement effort is getting off track. In the earlier example of improving ADR, an aim statement could be “to increase the ADR of all endoscopists who perform colonoscopy at your hospital to 25% over a 12-month period.”
Question 2: How will we know that a change is an improvement?
This step involves defining measures that will allow you to understand if changes implemented are impacting the system within which your target problem resides and if this represents an improvement. This usually involves continuous, real-time measurement. Outcome measures are clinically relevant outcomes and are the ultimate goal of what the project team is trying to accomplish. In the example of ADR, this could be the proportion of endoscopists at your institution with an ADR greater than 25%. Process measures are relevant to the system within which you are working and your target problem resides. Typically, the intervention that you implement will have impact that is measurable much earlier by process outcomes than outcomes measures, which are usually a downstream effect. As such, an improvement project still may be a success if it shows improvements in process measures only. For example, the proportion of endoscopists measuring withdrawal time would be a process measure in an intervention aimed at improving ADR. In time, improvement in process measures may translate to improvements in the outcome measure. Balancing measures are indicators of unintended consequences of the project. Not all that comes from an improvement effort is necessarily positive. If improvements in certain process measures come at the cost of harms shown by the balancing measures, such as deterioration in staff satisfaction or increase in time per procedure, the improvement project may not be worth continuing.
Importance of understanding the target problem: Current-state analysis
In contrast to classic enumerative research in which the clinical environment can be well controlled, quality improvement work focuses on sampling and intervening upon a less controlled and dynamic process or system with the intent of improving it.10 Just as treatment strategies in clinical medicine are based on diagnostic testing, so too in quality improvement work, the strategy of diagnosing the current state allows for linking the root cause of quality problems with solutions that can induce positive change.
Several common diagnostic tools are used to identify root causes of quality and safety issues. These include the following: 1) process mapping, 2) cause-and-effect diagrams, and 3) Pareto charts.
Process mapping
Process maps are tools used to understand the system that is being studied. A process map is a graphic depiction of the flow through a process, which creates a collaborative awareness of the current state and identifies opportunities for improvement. It is important that multiple individuals who have knowledge of the process in question are involved in its creation. Process maps are created by first establishing the start and end of the process. Second, the high-level steps are included. Third, a more detailed set of steps can be included within each of the high-level steps.
Cause-and-effect diagrams
Cause-and-effect diagrams, also known as Ishikawa or fishbone diagrams, are helpful brainstorming tools used to graphically display and explore potential causes of a target problem. They illustrate that there often are many contributing factors to one underlying problem and the relationship between contributing factors. Classic examples of categories include equipment, environment, materials, methods and process, people, and measurement.10 Figure 1 provides an example of these tools in an effort to improve ADR.
To identify the most important contributors to the target problem and thus where to focus improvement efforts, a Pareto chart, a bar graph that places all defects/causes in the order of the frequency in which they occur, is constructed. The x-axis is a list of possible defects (Figure 1). The y-axis is the frequency with which any one defect is occurring, and the third (x-2) axis is the cumulative frequency. In theory, it is expected that there will be a vital few defects that account for 80% of all occurrences (referred to by some as the 80:20 rule).10, 11 Populating this graph requires measurement, which, as discussed earlier, is the key to understanding any problem. Measurement can be accomplished through direct observation/audit, chart review, and/or multivoting.
Question 3: What changes can result in improvement?
Once the improvement team has defined an aim and established its family of measures, it is time to develop and implement an intervention. Rather than investing time and resources into one intervention that may or may not be successful, it is preferable to perform small change cycles in which the intervention is conducted on a small scale, refined, and either repeated or changed. As a result, most quality improvement projects consist of an iterative process. The Model for Improvement defines four steps that allow the improvement team to perform this: Plan, do, study, act (PDSA).4,10,11 The first two questions listed earlier allowed the improvement team to plan the intervention. The next step, do, involves implementing your project on a small scale, thereby testing your change while collecting continuous measurements. Study involves interpreting your data using both conventional methods and several improvement-specific methods (discussed later) that help answer the question of how will we know that a change is improvement? Finally, act involves making a conclusion about your first PDSA cycle, helping to inform subsequent cycles. This results in a series of small, rapid cycle changes, one building on the next, that lead to implementation of change(s) that ultimately serve to address your improvement problem and your project aim.
A change concept is an approach known to be useful in developing specific changes that result in improvement. Change concepts are used as a starting point to generate change ideas. A number of change concepts spanning nine main categories have been defined by the Associates for Process Improvement,10 including eliminating waste, improving work flow, managing variation, and designing systems to prevent error. For the purpose of improving ADR, your team may choose a few change concepts and ideas based on the diagnostic work-up. For example, the change concept of designing the system to prevent errors through standardizing withdrawal time for all physicians may lead to an improvement in ADR. This then is linked to the change idea of audible timers placed in endoscopy suites to ensure longer withdrawal times.12 The impact of this change would be measured and the next cycle would build on these results.
Summary and next steps
In this first article of the series, the QI team moved forward with their aim to increase ADR. A root cause analysis was undertaken using multiple diagnostic tools including a fishbone diagram and a Pareto chart. Finally, change ideas were generated based on the earlier-described root causes and established change concepts. The next steps involve undertaking PDSA cycles to test change ideas and monitor for improvement.
References
1. Rex, D.K., Schoenfeld, P.S., Cohen, J. et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
2. Rex, D.K., Bond, J.H., Winawer, S. et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-308.
3. Corley, D., Jensen, C.D., Marks, A.R. et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
4. Kotter, J.P. Leading change. Harvard Business Review Press, Boston; 2012
5. Taitz, J.M., Lee, T.H., and Sequist, T.D. A framework for engaging physicians in quality and safety. BMJ Qual Saf. 2012;21:722-8.
6. Carman, K.L., Dardess, P., Maurer, M. et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff (Millwood). 2013;33:223-31.
7.Ranji, S.R. and Shojania, S.G. Implementing patient safety interventions in your hospital: what to try and what to avoid. Med Clin North Am. 2008;92:275-93.
8. Antony, J. Six Sigma vs Lean: some perspectives from leading academics and practitioners. Int J Product Perform Manage. 2011;60:185-90.
9. Bercaw, R. Taking improvement from the assembly line to healthcare: the application of lean within the healthcare industry. Taylor and Francis, Boca Raton, FL; 2012
10. Langley, G.J., Nolan, K.M., Nolan, T.W. et al. The improvement guide: a practical approach to enhancing organizational performance. Jossey-Bass, San Francisco; 2009
11. Berwick, D.M. A primer on leading the improvement of systems. BMJ. 1996;312:619-22.
12. Corley, D.A., Jensen, C.D., and Marks, A.R. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011;74:656-65.
Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, and Institute of Health Policy, Management and Evaluation, department of medicine; Dr. Mosko is in the division of gastroenterology, St. Michael’s Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Bollegala is in the division of gastroenterology, Women’s College Hospital, department of medicine; Dr. Bernstein is in the division of gastroenterology, Sunnybrook Health Sciences Centre, department of medicine; Dr. Brahmania is at the Toronto Center for Liver Diseases, division of gastroenterology, University Health Network, department of medicine; Dr. Liu is in the division of gastroenterology, University Health Network, department of medicine; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, Mount Sinai Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; and Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management and Evaluation; all are at the University of Toronto. Dr. Steinhart is an advisory board member for Abbvie, Janssen, Takeda, Shire, Allergan, Pfizer, Merck, Ferring, and Pharmascience; has received research grants from Abbvie, Amgen, Genentech, Cellgene, Arena Pharmaceuticals, Red Hill Biopharma, Millenium, Roche, and Centocor; and has received speaking honoraria from Abbvie, Janssen, Takeda, Shire, Pfizer, Merck, and Ferring.
The remaining authors declare no conflicts for this article.
This article by Adam Weizman and colleagues is the first of a three-part series that will provide practical advice for practices that wish to develop a quality initiative. The first article, “Launching a quality improvement initiative” describes the infrastructure, personnel, and structure needed to approach an identified problem within a practice (variability in adenoma detection rates). This case-based approach helps us understand the step-by-step approach needed to reduce variability and improve quality. The authors present a plan (road map) in a straightforward and practical way that seems simple, but if followed carefully, leads to success. These articles are rich in resources and link to state-of-the-art advice.
John I. Allen, MD, MBA, AGAF, Special Section Editor
There has been increasing focus on measuring quality indicators in gastroenterology over the past few years. The adenoma detection rate (ADR) has emerged as one of the most important quality indicators because it is supported by robust clinical evidence.1-3 With every 1% increase in ADR, a 3% reduction in interval colorectal cancer has been noted.3 As such, an ADR of 25% has been designated as an important quality target for all endoscopists who perform colorectal cancer screening.1
You work at a community hospital in a large, metropolitan area. Your colleagues in a number of other departments across your hospital have been increasingly interested in quality improvement (QI) and have launched QI interventions, although none in your department. Moreover, there have been reforms in how hospital endoscopy units are funded in your jurisdiction, with a move toward volume-based funding with a quality overlay. In an effort to improve efficiency and better characterize performance, the hospital has been auditing the performance of all endoscopists at your institution over the past year. Among the eight endoscopists who work at your hospital, the overall ADR has been found to be 19%, decreasing to less than the generally accepted benchmark.1
In response to the results of the audit in your unit, you decide that you would like to develop an initiative to improve your group’s ADR.
Forming a quality improvement team
The first step in any QI project is to establish an improvement team. This working group consists of individuals with specific roles who perform interdependent tasks and share a common goal.4 Usually, frontline health care workers who are impacted most by the quality-of-care problem form the foundation of the team. A team lead is identified who will oversee the project. Content experts are also helpful members of the team who may have particular expertise in the clinical domain that will be the focus of the project. In addition, an improvement adviser, an individual with some expertise in QI, is needed on the team. This adviser may be from within your department or from outside. Although they may not possess expertise in the clinical problem you are trying to tackle, they should have skills in QI methodology and process to aid the team. An executive sponsor also needs to be identified. This should be an influential and well-respected individual who holds a senior administrative position at your institution who can help the team overcome barriers and secure resources. Physician engagement is a critical, often-overlooked step in any improvement effort. Regardless of the initiative, physicians continue to have tremendous influence over hospital-based outcomes.5 Identifying a physician champion, a prominent and respected physician at your organization to help spread the importance of your efforts and create a burning platform for change, is helpful. It also is valuable to have a patient on the improvement team to provide unique perspectives that only the end user of health care can convey and to ensure that the project is patient centered, as all improvement efforts should be.6
Improvement framework
Before starting any improvement effort, there are several important considerations that need to be addressed when choosing a quality improvement target.7 It is important to have a good understanding of the burden and severity of the problem. This often requires audit and measuring. For example, although we may think there is a problem with ADR in our endoscopy unit based on a general impression, it is critical to have data to support this suspicion. This is part of a current state analysis (discussed later). It also is important to select a quality-of-care problem that is under you or your group’s direct control. For example, it would be difficult to initiate a quality improvement project aimed at changing the practice of radiology reporting as a gastroenterologist. It is important to pick a problem that is focused and within a narrow scope that is feasible to address and then improve. Consideration of the unintended consequences of an improvement initiative often is overlooked, but needs to be considered because not all that comes out of quality improvement efforts is good. Finally, the likelihood of success of a quality initiative is increased significantly if it can generate momentum and lead to other interventions both within your department and beyond.
There are several specific improvement frameworks that can be used by a team to address a quality-of-care problem and perform a quality improvement project. The framework chosen depends on the type of problem that is being targeted and the training of the individuals on the improvement team. Three of the most commonly used improvement frameworks include the following: 1) Six Sigma; 2) Lean; and 3) Model for Improvement.
Six Sigma
Six Sigma is focused on improvement by reducing variability.8 It is a highly analytic framework relying on statistical analysis and mathematical modeling. It is best suited for projects in which the root cause and contributors to the target problem remain unclear and the aim of the intervention is to reduce variation.
Lean
Lean emphasizes improvement through elimination of waste and classifies all parts of any process as value added and nonvalue added.9 It is estimated that 95% of activities in any health care process are nonvalue added and the objective of Lean is to identify opportunities to simplify and create efficiencies. It is best suited for target problems that directly can be observed and mapped out, for example, process of care, flow, and efficiency of an endoscopy unit.
Model for Improvement
The Model for Improvement has been popularized by the Institute for Healthcare Improvement.10,11 It is well suited for health care teams, and its advantages are its adaptability to many improvement targets and lack of extensive training, consultant support, or statistical training as required by the previous frameworks mentioned earlier. As a result, it is the most commonly used improvement framework.
Using the Model for Improvement
The Model for Improvement is organized around three main questions: 1) What are we trying to accomplish? 2) How will we know that a change is an improvement? and 3) What changes can result in improvement?
Question 1: What are we trying to accomplish?
The first stage using the Model for Improvement is developing a clear project aim. A good aim statement should be specific in defining what measures one is hoping to improve and setting a concrete deadline by which to achieve it.10,11 It should answer the questions of what the team is trying to improve, by how much, and by what date. It is more effective for the target to be an ambitious, stretch goal to ensure the effort is worth the resources and time that will be invested by the team. Not only does a good aim statement serve as the foundation for the project, but it can redirect the team if the improvement effort is getting off track. In the earlier example of improving ADR, an aim statement could be “to increase the ADR of all endoscopists who perform colonoscopy at your hospital to 25% over a 12-month period.”
Question 2: How will we know that a change is an improvement?
This step involves defining measures that will allow you to understand if changes implemented are impacting the system within which your target problem resides and if this represents an improvement. This usually involves continuous, real-time measurement. Outcome measures are clinically relevant outcomes and are the ultimate goal of what the project team is trying to accomplish. In the example of ADR, this could be the proportion of endoscopists at your institution with an ADR greater than 25%. Process measures are relevant to the system within which you are working and your target problem resides. Typically, the intervention that you implement will have impact that is measurable much earlier by process outcomes than outcomes measures, which are usually a downstream effect. As such, an improvement project still may be a success if it shows improvements in process measures only. For example, the proportion of endoscopists measuring withdrawal time would be a process measure in an intervention aimed at improving ADR. In time, improvement in process measures may translate to improvements in the outcome measure. Balancing measures are indicators of unintended consequences of the project. Not all that comes from an improvement effort is necessarily positive. If improvements in certain process measures come at the cost of harms shown by the balancing measures, such as deterioration in staff satisfaction or increase in time per procedure, the improvement project may not be worth continuing.
Importance of understanding the target problem: Current-state analysis
In contrast to classic enumerative research in which the clinical environment can be well controlled, quality improvement work focuses on sampling and intervening upon a less controlled and dynamic process or system with the intent of improving it.10 Just as treatment strategies in clinical medicine are based on diagnostic testing, so too in quality improvement work, the strategy of diagnosing the current state allows for linking the root cause of quality problems with solutions that can induce positive change.
Several common diagnostic tools are used to identify root causes of quality and safety issues. These include the following: 1) process mapping, 2) cause-and-effect diagrams, and 3) Pareto charts.
Process mapping
Process maps are tools used to understand the system that is being studied. A process map is a graphic depiction of the flow through a process, which creates a collaborative awareness of the current state and identifies opportunities for improvement. It is important that multiple individuals who have knowledge of the process in question are involved in its creation. Process maps are created by first establishing the start and end of the process. Second, the high-level steps are included. Third, a more detailed set of steps can be included within each of the high-level steps.
Cause-and-effect diagrams
Cause-and-effect diagrams, also known as Ishikawa or fishbone diagrams, are helpful brainstorming tools used to graphically display and explore potential causes of a target problem. They illustrate that there often are many contributing factors to one underlying problem and the relationship between contributing factors. Classic examples of categories include equipment, environment, materials, methods and process, people, and measurement.10 Figure 1 provides an example of these tools in an effort to improve ADR.
To identify the most important contributors to the target problem and thus where to focus improvement efforts, a Pareto chart, a bar graph that places all defects/causes in the order of the frequency in which they occur, is constructed. The x-axis is a list of possible defects (Figure 1). The y-axis is the frequency with which any one defect is occurring, and the third (x-2) axis is the cumulative frequency. In theory, it is expected that there will be a vital few defects that account for 80% of all occurrences (referred to by some as the 80:20 rule).10, 11 Populating this graph requires measurement, which, as discussed earlier, is the key to understanding any problem. Measurement can be accomplished through direct observation/audit, chart review, and/or multivoting.
Question 3: What changes can result in improvement?
Once the improvement team has defined an aim and established its family of measures, it is time to develop and implement an intervention. Rather than investing time and resources into one intervention that may or may not be successful, it is preferable to perform small change cycles in which the intervention is conducted on a small scale, refined, and either repeated or changed. As a result, most quality improvement projects consist of an iterative process. The Model for Improvement defines four steps that allow the improvement team to perform this: Plan, do, study, act (PDSA).4,10,11 The first two questions listed earlier allowed the improvement team to plan the intervention. The next step, do, involves implementing your project on a small scale, thereby testing your change while collecting continuous measurements. Study involves interpreting your data using both conventional methods and several improvement-specific methods (discussed later) that help answer the question of how will we know that a change is improvement? Finally, act involves making a conclusion about your first PDSA cycle, helping to inform subsequent cycles. This results in a series of small, rapid cycle changes, one building on the next, that lead to implementation of change(s) that ultimately serve to address your improvement problem and your project aim.
A change concept is an approach known to be useful in developing specific changes that result in improvement. Change concepts are used as a starting point to generate change ideas. A number of change concepts spanning nine main categories have been defined by the Associates for Process Improvement,10 including eliminating waste, improving work flow, managing variation, and designing systems to prevent error. For the purpose of improving ADR, your team may choose a few change concepts and ideas based on the diagnostic work-up. For example, the change concept of designing the system to prevent errors through standardizing withdrawal time for all physicians may lead to an improvement in ADR. This then is linked to the change idea of audible timers placed in endoscopy suites to ensure longer withdrawal times.12 The impact of this change would be measured and the next cycle would build on these results.
Summary and next steps
In this first article of the series, the QI team moved forward with their aim to increase ADR. A root cause analysis was undertaken using multiple diagnostic tools including a fishbone diagram and a Pareto chart. Finally, change ideas were generated based on the earlier-described root causes and established change concepts. The next steps involve undertaking PDSA cycles to test change ideas and monitor for improvement.
References
1. Rex, D.K., Schoenfeld, P.S., Cohen, J. et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
2. Rex, D.K., Bond, J.H., Winawer, S. et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-308.
3. Corley, D., Jensen, C.D., Marks, A.R. et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
4. Kotter, J.P. Leading change. Harvard Business Review Press, Boston; 2012
5. Taitz, J.M., Lee, T.H., and Sequist, T.D. A framework for engaging physicians in quality and safety. BMJ Qual Saf. 2012;21:722-8.
6. Carman, K.L., Dardess, P., Maurer, M. et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff (Millwood). 2013;33:223-31.
7.Ranji, S.R. and Shojania, S.G. Implementing patient safety interventions in your hospital: what to try and what to avoid. Med Clin North Am. 2008;92:275-93.
8. Antony, J. Six Sigma vs Lean: some perspectives from leading academics and practitioners. Int J Product Perform Manage. 2011;60:185-90.
9. Bercaw, R. Taking improvement from the assembly line to healthcare: the application of lean within the healthcare industry. Taylor and Francis, Boca Raton, FL; 2012
10. Langley, G.J., Nolan, K.M., Nolan, T.W. et al. The improvement guide: a practical approach to enhancing organizational performance. Jossey-Bass, San Francisco; 2009
11. Berwick, D.M. A primer on leading the improvement of systems. BMJ. 1996;312:619-22.
12. Corley, D.A., Jensen, C.D., and Marks, A.R. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011;74:656-65.
Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, and Institute of Health Policy, Management and Evaluation, department of medicine; Dr. Mosko is in the division of gastroenterology, St. Michael’s Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Bollegala is in the division of gastroenterology, Women’s College Hospital, department of medicine; Dr. Bernstein is in the division of gastroenterology, Sunnybrook Health Sciences Centre, department of medicine; Dr. Brahmania is at the Toronto Center for Liver Diseases, division of gastroenterology, University Health Network, department of medicine; Dr. Liu is in the division of gastroenterology, University Health Network, department of medicine; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, Mount Sinai Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; and Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management and Evaluation; all are at the University of Toronto. Dr. Steinhart is an advisory board member for Abbvie, Janssen, Takeda, Shire, Allergan, Pfizer, Merck, Ferring, and Pharmascience; has received research grants from Abbvie, Amgen, Genentech, Cellgene, Arena Pharmaceuticals, Red Hill Biopharma, Millenium, Roche, and Centocor; and has received speaking honoraria from Abbvie, Janssen, Takeda, Shire, Pfizer, Merck, and Ferring.
The remaining authors declare no conflicts for this article.
This article by Adam Weizman and colleagues is the first of a three-part series that will provide practical advice for practices that wish to develop a quality initiative. The first article, “Launching a quality improvement initiative” describes the infrastructure, personnel, and structure needed to approach an identified problem within a practice (variability in adenoma detection rates). This case-based approach helps us understand the step-by-step approach needed to reduce variability and improve quality. The authors present a plan (road map) in a straightforward and practical way that seems simple, but if followed carefully, leads to success. These articles are rich in resources and link to state-of-the-art advice.
John I. Allen, MD, MBA, AGAF, Special Section Editor
There has been increasing focus on measuring quality indicators in gastroenterology over the past few years. The adenoma detection rate (ADR) has emerged as one of the most important quality indicators because it is supported by robust clinical evidence.1-3 With every 1% increase in ADR, a 3% reduction in interval colorectal cancer has been noted.3 As such, an ADR of 25% has been designated as an important quality target for all endoscopists who perform colorectal cancer screening.1
You work at a community hospital in a large, metropolitan area. Your colleagues in a number of other departments across your hospital have been increasingly interested in quality improvement (QI) and have launched QI interventions, although none in your department. Moreover, there have been reforms in how hospital endoscopy units are funded in your jurisdiction, with a move toward volume-based funding with a quality overlay. In an effort to improve efficiency and better characterize performance, the hospital has been auditing the performance of all endoscopists at your institution over the past year. Among the eight endoscopists who work at your hospital, the overall ADR has been found to be 19%, decreasing to less than the generally accepted benchmark.1
In response to the results of the audit in your unit, you decide that you would like to develop an initiative to improve your group’s ADR.
Forming a quality improvement team
The first step in any QI project is to establish an improvement team. This working group consists of individuals with specific roles who perform interdependent tasks and share a common goal.4 Usually, frontline health care workers who are impacted most by the quality-of-care problem form the foundation of the team. A team lead is identified who will oversee the project. Content experts are also helpful members of the team who may have particular expertise in the clinical domain that will be the focus of the project. In addition, an improvement adviser, an individual with some expertise in QI, is needed on the team. This adviser may be from within your department or from outside. Although they may not possess expertise in the clinical problem you are trying to tackle, they should have skills in QI methodology and process to aid the team. An executive sponsor also needs to be identified. This should be an influential and well-respected individual who holds a senior administrative position at your institution who can help the team overcome barriers and secure resources. Physician engagement is a critical, often-overlooked step in any improvement effort. Regardless of the initiative, physicians continue to have tremendous influence over hospital-based outcomes.5 Identifying a physician champion, a prominent and respected physician at your organization to help spread the importance of your efforts and create a burning platform for change, is helpful. It also is valuable to have a patient on the improvement team to provide unique perspectives that only the end user of health care can convey and to ensure that the project is patient centered, as all improvement efforts should be.6
Improvement framework
Before starting any improvement effort, there are several important considerations that need to be addressed when choosing a quality improvement target.7 It is important to have a good understanding of the burden and severity of the problem. This often requires audit and measuring. For example, although we may think there is a problem with ADR in our endoscopy unit based on a general impression, it is critical to have data to support this suspicion. This is part of a current state analysis (discussed later). It also is important to select a quality-of-care problem that is under you or your group’s direct control. For example, it would be difficult to initiate a quality improvement project aimed at changing the practice of radiology reporting as a gastroenterologist. It is important to pick a problem that is focused and within a narrow scope that is feasible to address and then improve. Consideration of the unintended consequences of an improvement initiative often is overlooked, but needs to be considered because not all that comes out of quality improvement efforts is good. Finally, the likelihood of success of a quality initiative is increased significantly if it can generate momentum and lead to other interventions both within your department and beyond.
There are several specific improvement frameworks that can be used by a team to address a quality-of-care problem and perform a quality improvement project. The framework chosen depends on the type of problem that is being targeted and the training of the individuals on the improvement team. Three of the most commonly used improvement frameworks include the following: 1) Six Sigma; 2) Lean; and 3) Model for Improvement.
Six Sigma
Six Sigma is focused on improvement by reducing variability.8 It is a highly analytic framework relying on statistical analysis and mathematical modeling. It is best suited for projects in which the root cause and contributors to the target problem remain unclear and the aim of the intervention is to reduce variation.
Lean
Lean emphasizes improvement through elimination of waste and classifies all parts of any process as value added and nonvalue added.9 It is estimated that 95% of activities in any health care process are nonvalue added and the objective of Lean is to identify opportunities to simplify and create efficiencies. It is best suited for target problems that directly can be observed and mapped out, for example, process of care, flow, and efficiency of an endoscopy unit.
Model for Improvement
The Model for Improvement has been popularized by the Institute for Healthcare Improvement.10,11 It is well suited for health care teams, and its advantages are its adaptability to many improvement targets and lack of extensive training, consultant support, or statistical training as required by the previous frameworks mentioned earlier. As a result, it is the most commonly used improvement framework.
Using the Model for Improvement
The Model for Improvement is organized around three main questions: 1) What are we trying to accomplish? 2) How will we know that a change is an improvement? and 3) What changes can result in improvement?
Question 1: What are we trying to accomplish?
The first stage using the Model for Improvement is developing a clear project aim. A good aim statement should be specific in defining what measures one is hoping to improve and setting a concrete deadline by which to achieve it.10,11 It should answer the questions of what the team is trying to improve, by how much, and by what date. It is more effective for the target to be an ambitious, stretch goal to ensure the effort is worth the resources and time that will be invested by the team. Not only does a good aim statement serve as the foundation for the project, but it can redirect the team if the improvement effort is getting off track. In the earlier example of improving ADR, an aim statement could be “to increase the ADR of all endoscopists who perform colonoscopy at your hospital to 25% over a 12-month period.”
Question 2: How will we know that a change is an improvement?
This step involves defining measures that will allow you to understand if changes implemented are impacting the system within which your target problem resides and if this represents an improvement. This usually involves continuous, real-time measurement. Outcome measures are clinically relevant outcomes and are the ultimate goal of what the project team is trying to accomplish. In the example of ADR, this could be the proportion of endoscopists at your institution with an ADR greater than 25%. Process measures are relevant to the system within which you are working and your target problem resides. Typically, the intervention that you implement will have impact that is measurable much earlier by process outcomes than outcomes measures, which are usually a downstream effect. As such, an improvement project still may be a success if it shows improvements in process measures only. For example, the proportion of endoscopists measuring withdrawal time would be a process measure in an intervention aimed at improving ADR. In time, improvement in process measures may translate to improvements in the outcome measure. Balancing measures are indicators of unintended consequences of the project. Not all that comes from an improvement effort is necessarily positive. If improvements in certain process measures come at the cost of harms shown by the balancing measures, such as deterioration in staff satisfaction or increase in time per procedure, the improvement project may not be worth continuing.
Importance of understanding the target problem: Current-state analysis
In contrast to classic enumerative research in which the clinical environment can be well controlled, quality improvement work focuses on sampling and intervening upon a less controlled and dynamic process or system with the intent of improving it.10 Just as treatment strategies in clinical medicine are based on diagnostic testing, so too in quality improvement work, the strategy of diagnosing the current state allows for linking the root cause of quality problems with solutions that can induce positive change.
Several common diagnostic tools are used to identify root causes of quality and safety issues. These include the following: 1) process mapping, 2) cause-and-effect diagrams, and 3) Pareto charts.
Process mapping
Process maps are tools used to understand the system that is being studied. A process map is a graphic depiction of the flow through a process, which creates a collaborative awareness of the current state and identifies opportunities for improvement. It is important that multiple individuals who have knowledge of the process in question are involved in its creation. Process maps are created by first establishing the start and end of the process. Second, the high-level steps are included. Third, a more detailed set of steps can be included within each of the high-level steps.
Cause-and-effect diagrams
Cause-and-effect diagrams, also known as Ishikawa or fishbone diagrams, are helpful brainstorming tools used to graphically display and explore potential causes of a target problem. They illustrate that there often are many contributing factors to one underlying problem and the relationship between contributing factors. Classic examples of categories include equipment, environment, materials, methods and process, people, and measurement.10 Figure 1 provides an example of these tools in an effort to improve ADR.
To identify the most important contributors to the target problem and thus where to focus improvement efforts, a Pareto chart, a bar graph that places all defects/causes in the order of the frequency in which they occur, is constructed. The x-axis is a list of possible defects (Figure 1). The y-axis is the frequency with which any one defect is occurring, and the third (x-2) axis is the cumulative frequency. In theory, it is expected that there will be a vital few defects that account for 80% of all occurrences (referred to by some as the 80:20 rule).10, 11 Populating this graph requires measurement, which, as discussed earlier, is the key to understanding any problem. Measurement can be accomplished through direct observation/audit, chart review, and/or multivoting.
Question 3: What changes can result in improvement?
Once the improvement team has defined an aim and established its family of measures, it is time to develop and implement an intervention. Rather than investing time and resources into one intervention that may or may not be successful, it is preferable to perform small change cycles in which the intervention is conducted on a small scale, refined, and either repeated or changed. As a result, most quality improvement projects consist of an iterative process. The Model for Improvement defines four steps that allow the improvement team to perform this: Plan, do, study, act (PDSA).4,10,11 The first two questions listed earlier allowed the improvement team to plan the intervention. The next step, do, involves implementing your project on a small scale, thereby testing your change while collecting continuous measurements. Study involves interpreting your data using both conventional methods and several improvement-specific methods (discussed later) that help answer the question of how will we know that a change is improvement? Finally, act involves making a conclusion about your first PDSA cycle, helping to inform subsequent cycles. This results in a series of small, rapid cycle changes, one building on the next, that lead to implementation of change(s) that ultimately serve to address your improvement problem and your project aim.
A change concept is an approach known to be useful in developing specific changes that result in improvement. Change concepts are used as a starting point to generate change ideas. A number of change concepts spanning nine main categories have been defined by the Associates for Process Improvement,10 including eliminating waste, improving work flow, managing variation, and designing systems to prevent error. For the purpose of improving ADR, your team may choose a few change concepts and ideas based on the diagnostic work-up. For example, the change concept of designing the system to prevent errors through standardizing withdrawal time for all physicians may lead to an improvement in ADR. This then is linked to the change idea of audible timers placed in endoscopy suites to ensure longer withdrawal times.12 The impact of this change would be measured and the next cycle would build on these results.
Summary and next steps
In this first article of the series, the QI team moved forward with their aim to increase ADR. A root cause analysis was undertaken using multiple diagnostic tools including a fishbone diagram and a Pareto chart. Finally, change ideas were generated based on the earlier-described root causes and established change concepts. The next steps involve undertaking PDSA cycles to test change ideas and monitor for improvement.
References
1. Rex, D.K., Schoenfeld, P.S., Cohen, J. et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
2. Rex, D.K., Bond, J.H., Winawer, S. et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-308.
3. Corley, D., Jensen, C.D., Marks, A.R. et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
4. Kotter, J.P. Leading change. Harvard Business Review Press, Boston; 2012
5. Taitz, J.M., Lee, T.H., and Sequist, T.D. A framework for engaging physicians in quality and safety. BMJ Qual Saf. 2012;21:722-8.
6. Carman, K.L., Dardess, P., Maurer, M. et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff (Millwood). 2013;33:223-31.
7.Ranji, S.R. and Shojania, S.G. Implementing patient safety interventions in your hospital: what to try and what to avoid. Med Clin North Am. 2008;92:275-93.
8. Antony, J. Six Sigma vs Lean: some perspectives from leading academics and practitioners. Int J Product Perform Manage. 2011;60:185-90.
9. Bercaw, R. Taking improvement from the assembly line to healthcare: the application of lean within the healthcare industry. Taylor and Francis, Boca Raton, FL; 2012
10. Langley, G.J., Nolan, K.M., Nolan, T.W. et al. The improvement guide: a practical approach to enhancing organizational performance. Jossey-Bass, San Francisco; 2009
11. Berwick, D.M. A primer on leading the improvement of systems. BMJ. 1996;312:619-22.
12. Corley, D.A., Jensen, C.D., and Marks, A.R. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011;74:656-65.
Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, and Institute of Health Policy, Management and Evaluation, department of medicine; Dr. Mosko is in the division of gastroenterology, St. Michael’s Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Bollegala is in the division of gastroenterology, Women’s College Hospital, department of medicine; Dr. Bernstein is in the division of gastroenterology, Sunnybrook Health Sciences Centre, department of medicine; Dr. Brahmania is at the Toronto Center for Liver Diseases, division of gastroenterology, University Health Network, department of medicine; Dr. Liu is in the division of gastroenterology, University Health Network, department of medicine; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, Mount Sinai Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; and Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management and Evaluation; all are at the University of Toronto. Dr. Steinhart is an advisory board member for Abbvie, Janssen, Takeda, Shire, Allergan, Pfizer, Merck, Ferring, and Pharmascience; has received research grants from Abbvie, Amgen, Genentech, Cellgene, Arena Pharmaceuticals, Red Hill Biopharma, Millenium, Roche, and Centocor; and has received speaking honoraria from Abbvie, Janssen, Takeda, Shire, Pfizer, Merck, and Ferring.
The remaining authors declare no conflicts for this article.
PCP Visits to Hospitalized Patients
Transitions in care are vulnerable periods. As patients are transferred between settings of care (such as from hospital back to the community), communication between healthcare providers is vital for care continuity.[1] A significant number of preventable adverse events may be related to ineffective communication between care providers.[1, 2, 3] The advent of specialized care, such as the introduction of hospitalists in acute care settings, has created an environment in which a patient's most responsible physician can often change multiple times as they move through the healthcare system.[4] Although there are many benefits to this type of concentrated care, the increase in care transitions may result in breakdowns in communication that may then be linked to risks in patient safety and suboptimal patient outcomes.[5, 6, 7, 8]
Improved continuity of care has been demonstrated to enhance patient safety during care transitions.[7] Efforts to develop continuity of care interventions are largely focused on care‐provider continuity, improved facilitation of communication, care planning, and increasing involvement of primary care physicians during follow‐up to hospitalizations and specialist visits.[9, 10] Such continuity of care efforts may provide a moderate benefit, but there remains room for improvement.[10, 11]
One dimension of continuity of care that has received limited attention is the potential impact of primary care physicians hospital visits to their hospitalized patients in a supportive‐care role.[12] In these situations, the primary care physician is neither the most responsible physician nor are they involved directly in their patient's hospital care. However, visiting their patient implies that they are aware of the hospitalization, thereby facilitating the potential for communication between care providers. Primary care physicians can also provide valuable contextual and relevant information as well as be involved in the discharge process. To identify the extent to which primary care physicians visit hospitalized patients and to measure the potential impact of primary care physician supportive visits on future outcomes, we used population‐level data to determine the frequency of supportive‐care visits by primary care physicians to hospitalized patients and to identify the association between these visits, patient outcomes, and health services utilization.
METHODS
Overview
We applied a retrospective cohort design utilizing linked population‐based administrative databases in the province of Ontario, Canada to examine outcome differences between patients who received a supportive‐care in‐hospital visit by their primary care physician compared to those who did not.
Databases
We assembled the cohort from linked and encrypted population‐based healthcare administrative databases. Data were derived from information on patients and physicians from the Ontario Health Insurance Plan, the Canadian Census, the Canadian Institute of Health Information Hospital Discharge Abstract Database, Registered Persons Databases, National Ambulatory Care Reporting System, Corporate Provider Database, Client Agency Program Enrolment, and Home Care Database. These databases have been validated and widely used in numerous studies.[13, 14, 15] All adults aged18 years who were discharged from the hospital in Ontario, Canada between January 1, 2008 and December 31, 2009 were included. Patients transferred to nursing homes or other acute care facilities following discharge, including rehabilitation centers, were excluded because they may have different readmission patterns. Among remaining hospitalized patients, only those with an identifiable primary care physician in the community were included. The patientprimary care physician pairings were identified using validated algorithms based on historical physician billing information.[16] This approach, adapted from previous studies, maximized the comparability among the study groups.[17, 18] In addition to having an historical relationship with the patients, primary care physicians had to have a history of conducting in‐hospital supportive visits (i.e., visits to at least 2 hospital patients within the previous year) for the patientprimary care physician pair to be included. This criterion was included to increase the likelihood that we were capturing a usual physician practice behavior and not a single circumstantial visit by a primary care physician. The history of supportive visits was also identified with physician billing data using a specific fee code.
Exposure
The exposure of interest was an in‐hospital visit in a supportive‐care role by the primary care physician during a patient's hospitalization and was obtained from physician fee codes. The fee paid for a visit during the study period was less than $20 CND.
Outcome Measures
Two different composite outcome measures were examined. The primary outcome was a composite of an emergent hospital readmission, death, or emergency department visit (without hospital admission). A composite measure was utilized to account for all outcomes simultaneously and thus be representative of the overall patient experience.[19] This approach has been applied in several studies examining continuity of care.[19, 20, 21] The secondary outcome examined processes of care. It was a composite evaluating ambulatory health services use postdischarge, specifically the number of primary care physician office visits and formal (ie, paid for by the universal provincial health plan) home‐care services. Home‐care services included both visits for nursing care as well as formal social support such as personal care. All outcome measures were assessed at 30 and 90 days following hospital discharge to assess for short and medium range outcomes.[22]
Patient Characteristics
Patient demographics including age, sex, low income (defined as individual income below $16,018 [CND] or couples income below $24,175 [CND]), living in a rural region, and the number of previous visits with primary care physicians were described from the available data. Readmission risk from the initial hospitalization was calculated based on the LACE score.[23] The LACE score is a validated measure of 30‐day readmission risk based on healthcare administrative data that account for (L) length of stay, (A) acute admission, (C) comorbid disease burden, and number of (E) emergency department visits in previous 6 months.[23] The LACE score ranges from 0 to 19, which correspond to a probability of readmissions of 2% to 43.7%, respectively. We considered individuals to have a high risk of readmission with a LACE score 10, which corresponds to a probability of readmission of 12.2%.[23]
Statistical Analyses
Descriptive statistics were used to compare patient characteristics among those with a primary care physician supportive‐care visit to those without. Logistic regression modeling was conducted to examine the impact of primary care physician visits on outcomes. The results reported here reflect the selection of adjusting for the confounders of age, sex, a history of primary care physician visits, low income, rurality, and the LACE score.
Ethics
The project analysis was conducted at the Institute for Clinical Evaluative Sciences (ICES) in Toronto, Ontario and was approved by the Sunnybrook Health Sciences Centre Research Ethics Board.
RESULTS
Overview
There were 11,316 primary care physicians identified as practicing in Ontario during the study period, of which 3236 had a history of conducting regular in‐hospital visits to 2 or more patients. The final patient cohort consisted of 164,059 hospitalized patients; 19,614 patients received a visit from their primary care physician, whereas 144,445 did not (Figure 1).
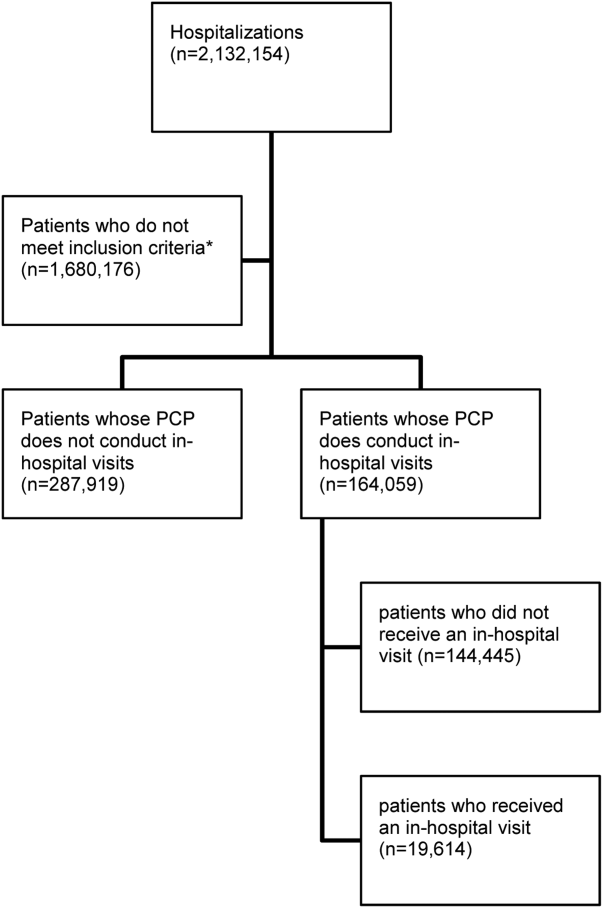
The hospitalized patients who received a visit from their primary care physician were significantly different than the patients who did not receive an in‐hospital visit (Table 1). Notably, patients who received a visit by their primary care physician had longer lengths of hospital stay (9.7 days vs 6.8 days, P<0.001). As well, a greater proportion had a high 30‐day readmission risk (LACE score10: 39.4% vs 29.9%, P<0.001) (Table 1).[21]
| Variablea | With PCP Visit (N=19,614) | Without PCP Visit (N=144,445) |
|---|---|---|
| ||
| Age, meanSD | 68.3716.85 | 65.7318.54 |
| Sex, no. of males | 9,393 (47.9%) | 67,030 (46.4%) |
| Low income | 3,937 (20.1%) | 30,157 (20.9%) |
| Individuals living in rural regions, no. | 1,951 (9.9%) | 25,731 (17.8%) |
| PCP visits in previous 6 months, meanSD | 4.764.47 | 4.174.28 |
| Length of stay, d, meanSD | 9.7217.40 | 6.7913.17 |
| Acute emergent visits, no. | 19,138 (97.6%) | 136,374 (94.4%) |
| Charlson score, meanSD | 1.061.60 | 0.921.49 |
| ED visits in previous 6 months, meanSD | 0.951.48 | 1.091.98 |
| LACE score, meanSDc | 9.022.88 | 8.103.02 |
| High risk for readmission (LACE score10), no. (%)c | 7,721 (39.4%) | 43,126 (29.9%) |
Patients who received an in‐hospital visit by their primary care physician were significantly different from those who did not (Table 2). They were older (68.4 years vs 65.7 years), and had a higher risk of readmission (LACE score of 9 vs 8). As well, proportionally fewer patients who received a visit were from rural regions than in the comparator group (9.9% of patients visited were from rural regions vs 17.8% of patients who did not receive a visit) (Table 2).
| Variable | Patients Who Received an In‐hospital Visit (N=19,614) | Patients Who Did Not Receive an In‐hospital Visit (N=144,445) | P Value |
|---|---|---|---|
| |||
| Primary outcome of emergency department visit, hospital readmission, or death | |||
| 30 days postdischarge, no. (%) | |||
| Readmission | 1,742 (8.9%) | 11,212 (7.8%) | <0.001 |
| ED visit | 2,039 (10.4%) | 16,823 (11.6%) | <0.001 |
| Death | 727 (3.7%) | 4,688 (3.2%) | <0.001 |
| Composite endpointa | 4,227 (21.6%) | 30,848 (21.4%) | 0.533 |
| 90 days postdischarge | |||
| Readmission | 2,791 (14.2%) | 18,257 (12.6%) | <0.001 |
| ED visit | 3,652 (18.6%) | 29,590 (20.5%) | <0.001 |
| Death | 1,507 (7.7%) | 9,821 (6.8%) | <0.001 |
| Composite endpointa | 7,125 (36.3%) | 52,245 (36.2%) | 0.668 |
| Secondary outcome of PCP office visits and home‐care services | |||
| 30 days postdischarge | |||
| Community PCP visits, meanSD | 3.85.1 | 3.14.6 | <0.001 |
| PCP visit, no. (%) | 15,732 (80.2%) | 108,266 (75%) | <0.001 |
| Home‐care services, no. (%) | 6,197 (31.6%) | 38,745 (26.8%) | <0.001 |
| Composite endpoint, no. (%)b | 16,851 (85.9%) | 117, 290 (81.2%) | <0.001 |
| 90 days postdischarge | |||
| Community PCP visits, meanSD | 8.210.1 | 6.99.3 | <0.001 |
| PCP visit, no. (%) | 18,112 (92.3%) | 128, 806 (89.2%) | <0.001 |
| Home‐care services, no. (%) | 7,256 (37.0%) | 45,675 (31.6%) | <0.001 |
| Composite endpoint, no. (%)b | 18, 504 (94.3%) | 132, 448 (91.7%) | <0.001 |
Individual Outcomes
Patients who received an in‐hospital visit by their primary care physician were also more likely to be readmitted within 30 days of discharge (8.9% vs 7.8%, P<0.001) and within 90 days of discharge (14.2% vs 12.6%, P<0.001). Additionally, patients who were visited by their primary care physician while hospitalized were more likely to die within 30 days postdischarge than those who did not receive an in‐hospital visit (3.7% vs 3.2%, P<0.001) and similarly by 90 days postdischarge (7.7% vs 6.8%, P<0.001) (Table 2).
Patients who received an in‐hospital visit were less likely to visit the emergency department at 30 days (10.4% vs 11.6%, P<0.001) and at 90 days (18.6% vs 20.5%, P<0.001) compared to patients who did not receive an in‐hospital visit (Table 2).
The patients who received in‐hospital visits by their primary care physician had a greater average number of primary care physician visits in the community at 30 days (3.8 vs 3.1, P<0.001) and 90 days (8.2 vs 6.9, P<0.001) (Table 2). Additionally, a higher proportion of patients who received an in‐hospital visit accessed home‐care services at 30 days postdischarge (31.6% vs 26.8%, P<0.001) and 90 days postdischarge (37.0% vs 31.6%, P<0.001) (Table 2).
Primary Outcome
There was no difference in proportion of patients who experienced the composite endpoint at 30 days (4227 [21.6%] vs 30,848 [21.4%], P>0.5) or 90 days (7125 [36.3%] vs 52,245 [36.2%], P>0.6) for patients who received an in‐hospital visit by their primary care physician compared to those who did not. The unadjusted model found no statistically significant difference between the 2 groups upon a primary care physician visit (odds ratio [OR]: 1.01; 95% confidence interval [CI]: 0.98‐1.04). However, once adjusting for differences in the groups for patient factors such as age, sex, location and health status, patients who received an in‐hospital visit by their primary care physician had lower adjusted risk for the composite outcome at 30 days postdischarge (adjusted OR [aOR]: 0.92; 95% CI: 0.89‐0.96) and 90 days postdischarge (aOR: 0.90; 95% CI: 0.87‐0.92) (Table 3). Estimates for each individual component of the composite outcome revealed significantly lower risk for ED visit and death but similar risk for readmission at both 30 days and 90 days after hospital discharge for patients who received and in‐hospital visit from their primary care physician and those who did not (Table 3).
| Variable | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)a |
|---|---|---|
| ||
| Primary outcome of emergency department visit, hospital readmission, or death | ||
| 30 days postdischarge | ||
| Readmission | 1.16 (1.10‐1.22) | 1.03 (0.97‐1.08) |
| ED visit | 0.88 (0.84‐0.92) | 0.88 (0.84‐0.92) |
| Death | 1.15 (1.06‐1.24) | 0.88 (0.81‐0.96) |
| Composite endpointb | 1.01 (0.98‐1.05) | 0.92 (0.89‐0.96) |
| 90 days postdischarge | ||
| Readmission | 1.15 (1.10‐1.20) | 1.00 (0.96‐1.04) |
| ED visit | 0.89 (0.86‐0.92) | 0.89 (0.86‐0.93) |
| Death | 1.14 (1.08‐1.21) | 0.87 (0.82‐0.93) |
| Composite endpointb | 1.01 (0.98‐1.04) | 0.90 (0.87‐0.92) |
| Secondary outcome of PCP office visits and home‐care services | ||
| 30 days postdischarge | ||
| Community PCP visits | 1.35 (1.31‐1.41) | 1.21 (1.16‐1.25) |
| Home‐care services | 1.26 (1.22‐1.30) | 1.05 (1.01‐1.09) |
| Composite endpointc | 1.41 (1.34‐1.47) | 1.16 (1.11‐1.21) |
| 90 days postdischarge | ||
| Community PCP visits | 1.46 (1.39‐1.55) | 1.25 (1.18‐1.33) |
| Home‐care services | 1.27 (1.23‐1.31) | 1.05 (1.01‐1.08) |
| Composite endpointc | 1.51 (1.42‐1.61) | 1.19 (1.12‐1.27) |
Secondary Outcome
Patients who received an in‐hospital visit by their primary care physician were more likely to experience the composite outcome of home‐care services and community primary care physician visits at 30 postdischarge (16,851 [85.9%] vs 117,290 [81.2%], P<0.001) and 90 days postdischarge (18,504 [94.3%] vs 132,448 [91.7%], P<0.001) compared to patients who did not receive an in‐hospital visit (Table 3). Once accounting for patient variables such as age, sex, location, and health status, patients who received an in‐hospital visit by their primary care physician had a higher adjusted risk for the composite outcome at 30 days postdischarge (aOR: 1.16; 95% CI: 1.11‐1.21) and 90 days postdischarge (aOR: 1.19; 95% CI: 1.12‐1.27) (Table 3).
DISCUSSION
Our population‐based study of primary care physicians is among the first to examine outcomes of patients whose primary care physicians have a history of providing supportive visits to hospitalized patients. After controlling for risk differences in patients at hospital discharge, we found that a primary care physician visit to a patient in the hospital was associated with a lower adjusted risk for the composite outcome of death, emergent hospital readmission, or emergency department visit at 30 and 90 days postdischarge compared to hospitalized patients who did not receive a visit by their primary care physician. We found this to be driven by patients having a lower risk of emergency department visits and death, whereas there was a similar risk of hospital readmission. We also found that visited patients were more likely to access home‐care services and have more primary care physician visits in the community following discharge.
The unadjusted model differs substantially from the adjusted model. On the surface this is an apparent paradox where the unadjusted results suggest an association with potential harm or no difference with a supportive visit. Conversely, the adjusted model suggests a reduction in harms. The differences between the unadjusted and adjusted model is driven by changes in the point estimates for readmission and death rates at both 30 and 90 day postdischarge. Prior to adjustment, it appears as if a primary care physician visit is associated with a significant increase of death; however, upon adjustment, it is associated with a significant reduction in death. Interestingly, this is a different effect than that observed with the secondary analysis, where the adjusted analyses demonstrate a more modest (but still positive) effect of supportive‐care visits. This observed change is likely due to differences in the patient groups. We can speculate that this may be an observed phenomenon of primary care physicians opting to visit their sicker patients, as perhaps it should be; however, further research is required to fully understand the real drivers of a supportive visit.
Our results are consistent with an earlier study that identified that a minority number of primary care physicians visit their hospitalized patients.[24] As well, findings from a randomized controlled trial of 364 patients over 60 years old identified a limited impact of primary care physician visits on patient outcomes but noted enhanced access to community health services.[12] Our work highlights the potential impact of primary care physician visits, which could, in theory, be leveraged and be an important role that primary care physicians can play in planning postdischarge care and improving the quality of care following hospitalization.
Our research study did not examine the impact of in‐hospital primary care physician visits on patient satisfaction directly. However, it has been demonstrated that patients have a strong desire for their primary care physician to be involved in their hospital care and their preference is for direct contact, with face‐to‐face visits compared to telephone or other communication.[25] This choice is important because dissatisfaction with services is associated with a loss of patient confidence in care quality and decreased adherence.[26] Also, primary care physicians acknowledge that information exchange is lacking when their patients are discharged, and that improving this aspect of a patient's care transition is important.[20] Research into discharge summaries as a tool to fill the communication gap has noted some success, yet there remains uncertainty regarding the type of information that should be included in a discharge summary, the time frame in which primary care physicians actually receive the summaries, and the accuracy of the information provided.[20, 27]
Our use of population‐based administrative data sources make the findings of our research generalizable to other similarly designed healthcare systems where a primary care physician may visit their hospitalized patients in a supportive‐care role. We were interested in a complex patientphysician interaction with a number of potential confounding factors, and our use of a composite measure represents the broad outcomes from this contact. Our cohort methodology was designed to isolate the exposure of interest while maximizing uniformity between the 2 study groups on other characteristics. Additionally a number of potential confounding factors were considered in an effort to isolate the effect of the primary care physician in‐hospital visit such as age, comorbid disease, and risk of hospital readmission.[12] The findings of our work support that of earlier research, but on a broader and more generalizable scale.[12]
There were notable differences between the intervention and control patient populations in the proportion of patients from rural regions who receive a supportive visit. This may be due to systemic differences between rural and nonrural regions with regard to access to care and ease of visit by primary care physicians. Alternatively, observed differences may be due to limitations of our study design in that some rural environments rely on primary care physicians to be involved in hospital care for the region. As such, they may actually be visiting their patients in a manner that was not captured as a supportive‐care visit. This is an important area that should be pursued in the future.
We acknowledge there are limits to our research findings. First, the nature of administrative data introduces challenges to causal inferences. As such, we are careful to describe associations and not draw causative links as there may be additional variables influencing outcomes including the patientphysician relationship, the location of the hospital relative to the physician practice and/or home, the time of the primary care physician visit, primary care physician hospital privileges for supportive‐care visits, and the number of other patients the primary care physician had in the same hospital at the same time. A second limitation is the use of the selected outcomes, which may not be direct measures of care quality.[28] However, the selected outcomes have been shown to be good quality measures in other work relevant to health policy.[8, 20, 21, 29] Third, the use of a composite outcome may over‐ or underestimate an exposure's impact.[19] Our composite outcome might have been dominated by some of its components. These observations may reflect the reality of primary care physicians visiting their sicker patients, or may be an attribute of the relatively short length of follow‐up of the study design. Fourth, we cannot determine whether there were additional interventions in place that assisted the continuity of care for primary care physician visits.[20, 27] However, this research included a broad range of hospitals throughout a large province where there were no system‐level quality interventions applied during this time. Fifth, our readmission rate may appear lower than other studies. However, our analysis is population based and not limited in focus to seniors.[30] As well, our posthospitalization death rates are similar to others, and the readmission rates are comparable to other Canadian studies.[31] Sixth, patients at higher risk for adverse outcomes may be identified as requiring more communication with their primary care physicians and we may not have fully captured this risk in our adjustment models, thereby underestimating the effect of exposure.[27] Further, primary care physicians may be involved in major medical decisions such as transitions to palliative care. A supportive‐care visit that facilitated these transitions and its ensuing outcomes may not have been included in our analysis. Seventh, our inherent assumption is that more care, such as posthospital primary care visits and home visits, denotes better care. This may not always be the case.[32] Eighth, physicians may find it difficult to visit their patient in the hospital, even when asked.[12] Finally, our findings are contingent on a system that supports primary care physicians being aware of their patients who become hospitalized. This is not only incumbent on any individual (eg, hospitalist) but a system where all providers work cohesively and seamlessly. On balance, however, these limitations do not overshadow our study's findings and conclusions.
Visits by primary care physicians to hospitalized patients are a longstanding tradition. The practice likely varies according to regional, patient, and individual physician characteristics.[16, 17, 18, 25] However, reimbursement codes for these services are present in a number of international healthcare systems' physician fee schedules with fairly modest remuneration amounts. The fairly nominal fee of less than $20 CND for a supportive‐care visit is similar to other systems and does not constitute a strong financial incentive to encourage this practice. The fee likely compensates the primary care physician for some of their time but comes with an opportunity cost to other aspects of their practice. Thus, results may differ in other environments or if the fee were higher, thereby incenting more primary care physicians to conduct visits. Indeed, the entire program for supportive hospital visits cost approximately $2.5 million CND per year for the 13 million people in the province of Ontario. Future work in this area could address the overall value and cost‐effectiveness of any potential fee changes. Still, it highlights the generalizability of our findings to other health systems and the ease in assessing the effect of the practice.
Overall, our findings underscore the importance and relevance for the practice of supportive‐care visits in its association with patient outcomes and health services utilization, which may prove to be an important key factor to improve quality healthcare. Our results suggest that an in‐hospital visit by a primary care physician may improve patient outcomes and increase subsequent support in the community. An in‐hospital supportive visit may be an additional method by which primary care physicians, and healthcare systems as a whole, strive to achieve the best care for patients.
Acknowledgements
Michael Manno, an analyst with the Institute of Clinical Evaluative Sciences (ICES) at the time of this study, assisted with the analyses.
Disclosures: This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by the ICES or the Ontario MOHLTC is intended or should be inferred. No researcher or persons involved in this study had any declared or otherwise known conflicts of interest. Stacey Brener received funding from a Canadian Institutes of Health Research (CIHR) Master's award in the area of primary care; the Ontario Graduate Student in Science and Technology award, an award from the CIHR Women's College Hospital Interdisciplinary Capacity Enhancement Team, and team grant OTG‐88591 from the CIHR. Susan Bronskill is supported by a CIHR New Investigator Award in the Area of Aging. Chaim Bell is supported by a CIHR/Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. These funding agencies had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , et al. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. 1991. Qual Saf Health Care. 2004;13(2):145–151; discussion 51–52.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- The College of Family Physicians of Canada. Family physicians caring for hospital inpatients. Available at: http://www.cfpc.ca/uploadedFiles/Resources/Resource_Items/FPs20Inpt20Hosp20Care_En.pdf. Published October 2003. Accessed August 15, 2015.
- , , . Is volume related to outcome in health care?. A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–520.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- , , , . Maintaining continuity of care: a look at the quality of communication between Ontario emergency departments and community physicians. CJEM. 2005;7(3):155–161.
- , , , et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med. 2010;5(7):398–405.
- , , , , , . Patient‐reported care coordination: associations with primary care continuity and specialty care use. Ann Fam Med. 2011;9(4):323–329.
- , . The Wellness Planner: empowerment, quality of life, and continuity of care in mental illness. Arch Psychiatr Nurs. 2011;25(4):284–293.
- , , , et al. Evaluation of the impact of interdisciplinarity in cancer care. BMC Health Serv Res. 2011;11:144.
- , , , , . Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial. Fam Pract. 1999;16(3):289–293.
- , , , et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840–847.
- , , , , , . Potentially unintended discontinuation of long‐term medication use after elective surgical procedures. Arch Intern Med. 2006;166(22):2525–2531.
- , , , , , , . Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Toronto: Institute for Clinical Evaluative Sciences; 2006. Available at: http://www.ices.on.ca/Publications/Atlases‐and‐Reports/2006/Canadian‐Institute‐for‐Health‐Information. Accessed August 15, 2015.
- , , , . Primary care physician workforce and Medicare beneficiaries' health outcomes. JAMA. 2011;305(20):2096–2104.
- , , , . Assigning ambulatory patients and their physicians to hospitals: a method for obtaining population‐based provider performance measurements. Health Serv Res. 2007;42(1 pt 1):45–62.
- , , , , , . Administrative data algorithms can describe ambulatory physician utilization. Health Serv Res. 2007;42(4):1783–1796.
- , , , et al. Validity of composite end points in clinical trials. BMJ. 2005;330(7491):594–596.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , , . Predicting readmission to the psychiatric hospital in a managed care environment: implications for quality indicators. Am J Psychiatry. 1997;154(3):337–340.
- , . The validity of readmission rate as a marker of the quality of hospital care, and a recommendation for its definition. N Z Med J. 2009;122(1289):63–70.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- , , , et al. Information exchange among physicians caring for the same patient in the community. CMAJ. 2008;179(10):1013–1018.
- . Supply of physicians' services in Ontario. Hosp Q. 1999;3(2):17.
- , , , , . Evaluation of outreach clinics held by specialists in general practice in England. J Epidemiol Community Health. 2000;54(2):149–156.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , . A conceptual framework for the study of early readmission as an indicator of quality of care. Soc Sci Med. 1996;43(11):1533–1541.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Effect of a postdischarge virtual ward on readmission or death for high‐risk patients: a randomized clinical trial. JAMA. 2014;312(13):1305–1312.
- , . Less is more: how less health care can result in better health. Arch Intern Med. 2010;170(9):749–750.
Transitions in care are vulnerable periods. As patients are transferred between settings of care (such as from hospital back to the community), communication between healthcare providers is vital for care continuity.[1] A significant number of preventable adverse events may be related to ineffective communication between care providers.[1, 2, 3] The advent of specialized care, such as the introduction of hospitalists in acute care settings, has created an environment in which a patient's most responsible physician can often change multiple times as they move through the healthcare system.[4] Although there are many benefits to this type of concentrated care, the increase in care transitions may result in breakdowns in communication that may then be linked to risks in patient safety and suboptimal patient outcomes.[5, 6, 7, 8]
Improved continuity of care has been demonstrated to enhance patient safety during care transitions.[7] Efforts to develop continuity of care interventions are largely focused on care‐provider continuity, improved facilitation of communication, care planning, and increasing involvement of primary care physicians during follow‐up to hospitalizations and specialist visits.[9, 10] Such continuity of care efforts may provide a moderate benefit, but there remains room for improvement.[10, 11]
One dimension of continuity of care that has received limited attention is the potential impact of primary care physicians hospital visits to their hospitalized patients in a supportive‐care role.[12] In these situations, the primary care physician is neither the most responsible physician nor are they involved directly in their patient's hospital care. However, visiting their patient implies that they are aware of the hospitalization, thereby facilitating the potential for communication between care providers. Primary care physicians can also provide valuable contextual and relevant information as well as be involved in the discharge process. To identify the extent to which primary care physicians visit hospitalized patients and to measure the potential impact of primary care physician supportive visits on future outcomes, we used population‐level data to determine the frequency of supportive‐care visits by primary care physicians to hospitalized patients and to identify the association between these visits, patient outcomes, and health services utilization.
METHODS
Overview
We applied a retrospective cohort design utilizing linked population‐based administrative databases in the province of Ontario, Canada to examine outcome differences between patients who received a supportive‐care in‐hospital visit by their primary care physician compared to those who did not.
Databases
We assembled the cohort from linked and encrypted population‐based healthcare administrative databases. Data were derived from information on patients and physicians from the Ontario Health Insurance Plan, the Canadian Census, the Canadian Institute of Health Information Hospital Discharge Abstract Database, Registered Persons Databases, National Ambulatory Care Reporting System, Corporate Provider Database, Client Agency Program Enrolment, and Home Care Database. These databases have been validated and widely used in numerous studies.[13, 14, 15] All adults aged18 years who were discharged from the hospital in Ontario, Canada between January 1, 2008 and December 31, 2009 were included. Patients transferred to nursing homes or other acute care facilities following discharge, including rehabilitation centers, were excluded because they may have different readmission patterns. Among remaining hospitalized patients, only those with an identifiable primary care physician in the community were included. The patientprimary care physician pairings were identified using validated algorithms based on historical physician billing information.[16] This approach, adapted from previous studies, maximized the comparability among the study groups.[17, 18] In addition to having an historical relationship with the patients, primary care physicians had to have a history of conducting in‐hospital supportive visits (i.e., visits to at least 2 hospital patients within the previous year) for the patientprimary care physician pair to be included. This criterion was included to increase the likelihood that we were capturing a usual physician practice behavior and not a single circumstantial visit by a primary care physician. The history of supportive visits was also identified with physician billing data using a specific fee code.
Exposure
The exposure of interest was an in‐hospital visit in a supportive‐care role by the primary care physician during a patient's hospitalization and was obtained from physician fee codes. The fee paid for a visit during the study period was less than $20 CND.
Outcome Measures
Two different composite outcome measures were examined. The primary outcome was a composite of an emergent hospital readmission, death, or emergency department visit (without hospital admission). A composite measure was utilized to account for all outcomes simultaneously and thus be representative of the overall patient experience.[19] This approach has been applied in several studies examining continuity of care.[19, 20, 21] The secondary outcome examined processes of care. It was a composite evaluating ambulatory health services use postdischarge, specifically the number of primary care physician office visits and formal (ie, paid for by the universal provincial health plan) home‐care services. Home‐care services included both visits for nursing care as well as formal social support such as personal care. All outcome measures were assessed at 30 and 90 days following hospital discharge to assess for short and medium range outcomes.[22]
Patient Characteristics
Patient demographics including age, sex, low income (defined as individual income below $16,018 [CND] or couples income below $24,175 [CND]), living in a rural region, and the number of previous visits with primary care physicians were described from the available data. Readmission risk from the initial hospitalization was calculated based on the LACE score.[23] The LACE score is a validated measure of 30‐day readmission risk based on healthcare administrative data that account for (L) length of stay, (A) acute admission, (C) comorbid disease burden, and number of (E) emergency department visits in previous 6 months.[23] The LACE score ranges from 0 to 19, which correspond to a probability of readmissions of 2% to 43.7%, respectively. We considered individuals to have a high risk of readmission with a LACE score 10, which corresponds to a probability of readmission of 12.2%.[23]
Statistical Analyses
Descriptive statistics were used to compare patient characteristics among those with a primary care physician supportive‐care visit to those without. Logistic regression modeling was conducted to examine the impact of primary care physician visits on outcomes. The results reported here reflect the selection of adjusting for the confounders of age, sex, a history of primary care physician visits, low income, rurality, and the LACE score.
Ethics
The project analysis was conducted at the Institute for Clinical Evaluative Sciences (ICES) in Toronto, Ontario and was approved by the Sunnybrook Health Sciences Centre Research Ethics Board.
RESULTS
Overview
There were 11,316 primary care physicians identified as practicing in Ontario during the study period, of which 3236 had a history of conducting regular in‐hospital visits to 2 or more patients. The final patient cohort consisted of 164,059 hospitalized patients; 19,614 patients received a visit from their primary care physician, whereas 144,445 did not (Figure 1).

The hospitalized patients who received a visit from their primary care physician were significantly different than the patients who did not receive an in‐hospital visit (Table 1). Notably, patients who received a visit by their primary care physician had longer lengths of hospital stay (9.7 days vs 6.8 days, P<0.001). As well, a greater proportion had a high 30‐day readmission risk (LACE score10: 39.4% vs 29.9%, P<0.001) (Table 1).[21]
| Variablea | With PCP Visit (N=19,614) | Without PCP Visit (N=144,445) |
|---|---|---|
| ||
| Age, meanSD | 68.3716.85 | 65.7318.54 |
| Sex, no. of males | 9,393 (47.9%) | 67,030 (46.4%) |
| Low income | 3,937 (20.1%) | 30,157 (20.9%) |
| Individuals living in rural regions, no. | 1,951 (9.9%) | 25,731 (17.8%) |
| PCP visits in previous 6 months, meanSD | 4.764.47 | 4.174.28 |
| Length of stay, d, meanSD | 9.7217.40 | 6.7913.17 |
| Acute emergent visits, no. | 19,138 (97.6%) | 136,374 (94.4%) |
| Charlson score, meanSD | 1.061.60 | 0.921.49 |
| ED visits in previous 6 months, meanSD | 0.951.48 | 1.091.98 |
| LACE score, meanSDc | 9.022.88 | 8.103.02 |
| High risk for readmission (LACE score10), no. (%)c | 7,721 (39.4%) | 43,126 (29.9%) |
Patients who received an in‐hospital visit by their primary care physician were significantly different from those who did not (Table 2). They were older (68.4 years vs 65.7 years), and had a higher risk of readmission (LACE score of 9 vs 8). As well, proportionally fewer patients who received a visit were from rural regions than in the comparator group (9.9% of patients visited were from rural regions vs 17.8% of patients who did not receive a visit) (Table 2).
| Variable | Patients Who Received an In‐hospital Visit (N=19,614) | Patients Who Did Not Receive an In‐hospital Visit (N=144,445) | P Value |
|---|---|---|---|
| |||
| Primary outcome of emergency department visit, hospital readmission, or death | |||
| 30 days postdischarge, no. (%) | |||
| Readmission | 1,742 (8.9%) | 11,212 (7.8%) | <0.001 |
| ED visit | 2,039 (10.4%) | 16,823 (11.6%) | <0.001 |
| Death | 727 (3.7%) | 4,688 (3.2%) | <0.001 |
| Composite endpointa | 4,227 (21.6%) | 30,848 (21.4%) | 0.533 |
| 90 days postdischarge | |||
| Readmission | 2,791 (14.2%) | 18,257 (12.6%) | <0.001 |
| ED visit | 3,652 (18.6%) | 29,590 (20.5%) | <0.001 |
| Death | 1,507 (7.7%) | 9,821 (6.8%) | <0.001 |
| Composite endpointa | 7,125 (36.3%) | 52,245 (36.2%) | 0.668 |
| Secondary outcome of PCP office visits and home‐care services | |||
| 30 days postdischarge | |||
| Community PCP visits, meanSD | 3.85.1 | 3.14.6 | <0.001 |
| PCP visit, no. (%) | 15,732 (80.2%) | 108,266 (75%) | <0.001 |
| Home‐care services, no. (%) | 6,197 (31.6%) | 38,745 (26.8%) | <0.001 |
| Composite endpoint, no. (%)b | 16,851 (85.9%) | 117, 290 (81.2%) | <0.001 |
| 90 days postdischarge | |||
| Community PCP visits, meanSD | 8.210.1 | 6.99.3 | <0.001 |
| PCP visit, no. (%) | 18,112 (92.3%) | 128, 806 (89.2%) | <0.001 |
| Home‐care services, no. (%) | 7,256 (37.0%) | 45,675 (31.6%) | <0.001 |
| Composite endpoint, no. (%)b | 18, 504 (94.3%) | 132, 448 (91.7%) | <0.001 |
Individual Outcomes
Patients who received an in‐hospital visit by their primary care physician were also more likely to be readmitted within 30 days of discharge (8.9% vs 7.8%, P<0.001) and within 90 days of discharge (14.2% vs 12.6%, P<0.001). Additionally, patients who were visited by their primary care physician while hospitalized were more likely to die within 30 days postdischarge than those who did not receive an in‐hospital visit (3.7% vs 3.2%, P<0.001) and similarly by 90 days postdischarge (7.7% vs 6.8%, P<0.001) (Table 2).
Patients who received an in‐hospital visit were less likely to visit the emergency department at 30 days (10.4% vs 11.6%, P<0.001) and at 90 days (18.6% vs 20.5%, P<0.001) compared to patients who did not receive an in‐hospital visit (Table 2).
The patients who received in‐hospital visits by their primary care physician had a greater average number of primary care physician visits in the community at 30 days (3.8 vs 3.1, P<0.001) and 90 days (8.2 vs 6.9, P<0.001) (Table 2). Additionally, a higher proportion of patients who received an in‐hospital visit accessed home‐care services at 30 days postdischarge (31.6% vs 26.8%, P<0.001) and 90 days postdischarge (37.0% vs 31.6%, P<0.001) (Table 2).
Primary Outcome
There was no difference in proportion of patients who experienced the composite endpoint at 30 days (4227 [21.6%] vs 30,848 [21.4%], P>0.5) or 90 days (7125 [36.3%] vs 52,245 [36.2%], P>0.6) for patients who received an in‐hospital visit by their primary care physician compared to those who did not. The unadjusted model found no statistically significant difference between the 2 groups upon a primary care physician visit (odds ratio [OR]: 1.01; 95% confidence interval [CI]: 0.98‐1.04). However, once adjusting for differences in the groups for patient factors such as age, sex, location and health status, patients who received an in‐hospital visit by their primary care physician had lower adjusted risk for the composite outcome at 30 days postdischarge (adjusted OR [aOR]: 0.92; 95% CI: 0.89‐0.96) and 90 days postdischarge (aOR: 0.90; 95% CI: 0.87‐0.92) (Table 3). Estimates for each individual component of the composite outcome revealed significantly lower risk for ED visit and death but similar risk for readmission at both 30 days and 90 days after hospital discharge for patients who received and in‐hospital visit from their primary care physician and those who did not (Table 3).
| Variable | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)a |
|---|---|---|
| ||
| Primary outcome of emergency department visit, hospital readmission, or death | ||
| 30 days postdischarge | ||
| Readmission | 1.16 (1.10‐1.22) | 1.03 (0.97‐1.08) |
| ED visit | 0.88 (0.84‐0.92) | 0.88 (0.84‐0.92) |
| Death | 1.15 (1.06‐1.24) | 0.88 (0.81‐0.96) |
| Composite endpointb | 1.01 (0.98‐1.05) | 0.92 (0.89‐0.96) |
| 90 days postdischarge | ||
| Readmission | 1.15 (1.10‐1.20) | 1.00 (0.96‐1.04) |
| ED visit | 0.89 (0.86‐0.92) | 0.89 (0.86‐0.93) |
| Death | 1.14 (1.08‐1.21) | 0.87 (0.82‐0.93) |
| Composite endpointb | 1.01 (0.98‐1.04) | 0.90 (0.87‐0.92) |
| Secondary outcome of PCP office visits and home‐care services | ||
| 30 days postdischarge | ||
| Community PCP visits | 1.35 (1.31‐1.41) | 1.21 (1.16‐1.25) |
| Home‐care services | 1.26 (1.22‐1.30) | 1.05 (1.01‐1.09) |
| Composite endpointc | 1.41 (1.34‐1.47) | 1.16 (1.11‐1.21) |
| 90 days postdischarge | ||
| Community PCP visits | 1.46 (1.39‐1.55) | 1.25 (1.18‐1.33) |
| Home‐care services | 1.27 (1.23‐1.31) | 1.05 (1.01‐1.08) |
| Composite endpointc | 1.51 (1.42‐1.61) | 1.19 (1.12‐1.27) |
Secondary Outcome
Patients who received an in‐hospital visit by their primary care physician were more likely to experience the composite outcome of home‐care services and community primary care physician visits at 30 postdischarge (16,851 [85.9%] vs 117,290 [81.2%], P<0.001) and 90 days postdischarge (18,504 [94.3%] vs 132,448 [91.7%], P<0.001) compared to patients who did not receive an in‐hospital visit (Table 3). Once accounting for patient variables such as age, sex, location, and health status, patients who received an in‐hospital visit by their primary care physician had a higher adjusted risk for the composite outcome at 30 days postdischarge (aOR: 1.16; 95% CI: 1.11‐1.21) and 90 days postdischarge (aOR: 1.19; 95% CI: 1.12‐1.27) (Table 3).
DISCUSSION
Our population‐based study of primary care physicians is among the first to examine outcomes of patients whose primary care physicians have a history of providing supportive visits to hospitalized patients. After controlling for risk differences in patients at hospital discharge, we found that a primary care physician visit to a patient in the hospital was associated with a lower adjusted risk for the composite outcome of death, emergent hospital readmission, or emergency department visit at 30 and 90 days postdischarge compared to hospitalized patients who did not receive a visit by their primary care physician. We found this to be driven by patients having a lower risk of emergency department visits and death, whereas there was a similar risk of hospital readmission. We also found that visited patients were more likely to access home‐care services and have more primary care physician visits in the community following discharge.
The unadjusted model differs substantially from the adjusted model. On the surface this is an apparent paradox where the unadjusted results suggest an association with potential harm or no difference with a supportive visit. Conversely, the adjusted model suggests a reduction in harms. The differences between the unadjusted and adjusted model is driven by changes in the point estimates for readmission and death rates at both 30 and 90 day postdischarge. Prior to adjustment, it appears as if a primary care physician visit is associated with a significant increase of death; however, upon adjustment, it is associated with a significant reduction in death. Interestingly, this is a different effect than that observed with the secondary analysis, where the adjusted analyses demonstrate a more modest (but still positive) effect of supportive‐care visits. This observed change is likely due to differences in the patient groups. We can speculate that this may be an observed phenomenon of primary care physicians opting to visit their sicker patients, as perhaps it should be; however, further research is required to fully understand the real drivers of a supportive visit.
Our results are consistent with an earlier study that identified that a minority number of primary care physicians visit their hospitalized patients.[24] As well, findings from a randomized controlled trial of 364 patients over 60 years old identified a limited impact of primary care physician visits on patient outcomes but noted enhanced access to community health services.[12] Our work highlights the potential impact of primary care physician visits, which could, in theory, be leveraged and be an important role that primary care physicians can play in planning postdischarge care and improving the quality of care following hospitalization.
Our research study did not examine the impact of in‐hospital primary care physician visits on patient satisfaction directly. However, it has been demonstrated that patients have a strong desire for their primary care physician to be involved in their hospital care and their preference is for direct contact, with face‐to‐face visits compared to telephone or other communication.[25] This choice is important because dissatisfaction with services is associated with a loss of patient confidence in care quality and decreased adherence.[26] Also, primary care physicians acknowledge that information exchange is lacking when their patients are discharged, and that improving this aspect of a patient's care transition is important.[20] Research into discharge summaries as a tool to fill the communication gap has noted some success, yet there remains uncertainty regarding the type of information that should be included in a discharge summary, the time frame in which primary care physicians actually receive the summaries, and the accuracy of the information provided.[20, 27]
Our use of population‐based administrative data sources make the findings of our research generalizable to other similarly designed healthcare systems where a primary care physician may visit their hospitalized patients in a supportive‐care role. We were interested in a complex patientphysician interaction with a number of potential confounding factors, and our use of a composite measure represents the broad outcomes from this contact. Our cohort methodology was designed to isolate the exposure of interest while maximizing uniformity between the 2 study groups on other characteristics. Additionally a number of potential confounding factors were considered in an effort to isolate the effect of the primary care physician in‐hospital visit such as age, comorbid disease, and risk of hospital readmission.[12] The findings of our work support that of earlier research, but on a broader and more generalizable scale.[12]
There were notable differences between the intervention and control patient populations in the proportion of patients from rural regions who receive a supportive visit. This may be due to systemic differences between rural and nonrural regions with regard to access to care and ease of visit by primary care physicians. Alternatively, observed differences may be due to limitations of our study design in that some rural environments rely on primary care physicians to be involved in hospital care for the region. As such, they may actually be visiting their patients in a manner that was not captured as a supportive‐care visit. This is an important area that should be pursued in the future.
We acknowledge there are limits to our research findings. First, the nature of administrative data introduces challenges to causal inferences. As such, we are careful to describe associations and not draw causative links as there may be additional variables influencing outcomes including the patientphysician relationship, the location of the hospital relative to the physician practice and/or home, the time of the primary care physician visit, primary care physician hospital privileges for supportive‐care visits, and the number of other patients the primary care physician had in the same hospital at the same time. A second limitation is the use of the selected outcomes, which may not be direct measures of care quality.[28] However, the selected outcomes have been shown to be good quality measures in other work relevant to health policy.[8, 20, 21, 29] Third, the use of a composite outcome may over‐ or underestimate an exposure's impact.[19] Our composite outcome might have been dominated by some of its components. These observations may reflect the reality of primary care physicians visiting their sicker patients, or may be an attribute of the relatively short length of follow‐up of the study design. Fourth, we cannot determine whether there were additional interventions in place that assisted the continuity of care for primary care physician visits.[20, 27] However, this research included a broad range of hospitals throughout a large province where there were no system‐level quality interventions applied during this time. Fifth, our readmission rate may appear lower than other studies. However, our analysis is population based and not limited in focus to seniors.[30] As well, our posthospitalization death rates are similar to others, and the readmission rates are comparable to other Canadian studies.[31] Sixth, patients at higher risk for adverse outcomes may be identified as requiring more communication with their primary care physicians and we may not have fully captured this risk in our adjustment models, thereby underestimating the effect of exposure.[27] Further, primary care physicians may be involved in major medical decisions such as transitions to palliative care. A supportive‐care visit that facilitated these transitions and its ensuing outcomes may not have been included in our analysis. Seventh, our inherent assumption is that more care, such as posthospital primary care visits and home visits, denotes better care. This may not always be the case.[32] Eighth, physicians may find it difficult to visit their patient in the hospital, even when asked.[12] Finally, our findings are contingent on a system that supports primary care physicians being aware of their patients who become hospitalized. This is not only incumbent on any individual (eg, hospitalist) but a system where all providers work cohesively and seamlessly. On balance, however, these limitations do not overshadow our study's findings and conclusions.
Visits by primary care physicians to hospitalized patients are a longstanding tradition. The practice likely varies according to regional, patient, and individual physician characteristics.[16, 17, 18, 25] However, reimbursement codes for these services are present in a number of international healthcare systems' physician fee schedules with fairly modest remuneration amounts. The fairly nominal fee of less than $20 CND for a supportive‐care visit is similar to other systems and does not constitute a strong financial incentive to encourage this practice. The fee likely compensates the primary care physician for some of their time but comes with an opportunity cost to other aspects of their practice. Thus, results may differ in other environments or if the fee were higher, thereby incenting more primary care physicians to conduct visits. Indeed, the entire program for supportive hospital visits cost approximately $2.5 million CND per year for the 13 million people in the province of Ontario. Future work in this area could address the overall value and cost‐effectiveness of any potential fee changes. Still, it highlights the generalizability of our findings to other health systems and the ease in assessing the effect of the practice.
Overall, our findings underscore the importance and relevance for the practice of supportive‐care visits in its association with patient outcomes and health services utilization, which may prove to be an important key factor to improve quality healthcare. Our results suggest that an in‐hospital visit by a primary care physician may improve patient outcomes and increase subsequent support in the community. An in‐hospital supportive visit may be an additional method by which primary care physicians, and healthcare systems as a whole, strive to achieve the best care for patients.
Acknowledgements
Michael Manno, an analyst with the Institute of Clinical Evaluative Sciences (ICES) at the time of this study, assisted with the analyses.
Disclosures: This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by the ICES or the Ontario MOHLTC is intended or should be inferred. No researcher or persons involved in this study had any declared or otherwise known conflicts of interest. Stacey Brener received funding from a Canadian Institutes of Health Research (CIHR) Master's award in the area of primary care; the Ontario Graduate Student in Science and Technology award, an award from the CIHR Women's College Hospital Interdisciplinary Capacity Enhancement Team, and team grant OTG‐88591 from the CIHR. Susan Bronskill is supported by a CIHR New Investigator Award in the Area of Aging. Chaim Bell is supported by a CIHR/Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. These funding agencies had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
Transitions in care are vulnerable periods. As patients are transferred between settings of care (such as from hospital back to the community), communication between healthcare providers is vital for care continuity.[1] A significant number of preventable adverse events may be related to ineffective communication between care providers.[1, 2, 3] The advent of specialized care, such as the introduction of hospitalists in acute care settings, has created an environment in which a patient's most responsible physician can often change multiple times as they move through the healthcare system.[4] Although there are many benefits to this type of concentrated care, the increase in care transitions may result in breakdowns in communication that may then be linked to risks in patient safety and suboptimal patient outcomes.[5, 6, 7, 8]
Improved continuity of care has been demonstrated to enhance patient safety during care transitions.[7] Efforts to develop continuity of care interventions are largely focused on care‐provider continuity, improved facilitation of communication, care planning, and increasing involvement of primary care physicians during follow‐up to hospitalizations and specialist visits.[9, 10] Such continuity of care efforts may provide a moderate benefit, but there remains room for improvement.[10, 11]
One dimension of continuity of care that has received limited attention is the potential impact of primary care physicians hospital visits to their hospitalized patients in a supportive‐care role.[12] In these situations, the primary care physician is neither the most responsible physician nor are they involved directly in their patient's hospital care. However, visiting their patient implies that they are aware of the hospitalization, thereby facilitating the potential for communication between care providers. Primary care physicians can also provide valuable contextual and relevant information as well as be involved in the discharge process. To identify the extent to which primary care physicians visit hospitalized patients and to measure the potential impact of primary care physician supportive visits on future outcomes, we used population‐level data to determine the frequency of supportive‐care visits by primary care physicians to hospitalized patients and to identify the association between these visits, patient outcomes, and health services utilization.
METHODS
Overview
We applied a retrospective cohort design utilizing linked population‐based administrative databases in the province of Ontario, Canada to examine outcome differences between patients who received a supportive‐care in‐hospital visit by their primary care physician compared to those who did not.
Databases
We assembled the cohort from linked and encrypted population‐based healthcare administrative databases. Data were derived from information on patients and physicians from the Ontario Health Insurance Plan, the Canadian Census, the Canadian Institute of Health Information Hospital Discharge Abstract Database, Registered Persons Databases, National Ambulatory Care Reporting System, Corporate Provider Database, Client Agency Program Enrolment, and Home Care Database. These databases have been validated and widely used in numerous studies.[13, 14, 15] All adults aged18 years who were discharged from the hospital in Ontario, Canada between January 1, 2008 and December 31, 2009 were included. Patients transferred to nursing homes or other acute care facilities following discharge, including rehabilitation centers, were excluded because they may have different readmission patterns. Among remaining hospitalized patients, only those with an identifiable primary care physician in the community were included. The patientprimary care physician pairings were identified using validated algorithms based on historical physician billing information.[16] This approach, adapted from previous studies, maximized the comparability among the study groups.[17, 18] In addition to having an historical relationship with the patients, primary care physicians had to have a history of conducting in‐hospital supportive visits (i.e., visits to at least 2 hospital patients within the previous year) for the patientprimary care physician pair to be included. This criterion was included to increase the likelihood that we were capturing a usual physician practice behavior and not a single circumstantial visit by a primary care physician. The history of supportive visits was also identified with physician billing data using a specific fee code.
Exposure
The exposure of interest was an in‐hospital visit in a supportive‐care role by the primary care physician during a patient's hospitalization and was obtained from physician fee codes. The fee paid for a visit during the study period was less than $20 CND.
Outcome Measures
Two different composite outcome measures were examined. The primary outcome was a composite of an emergent hospital readmission, death, or emergency department visit (without hospital admission). A composite measure was utilized to account for all outcomes simultaneously and thus be representative of the overall patient experience.[19] This approach has been applied in several studies examining continuity of care.[19, 20, 21] The secondary outcome examined processes of care. It was a composite evaluating ambulatory health services use postdischarge, specifically the number of primary care physician office visits and formal (ie, paid for by the universal provincial health plan) home‐care services. Home‐care services included both visits for nursing care as well as formal social support such as personal care. All outcome measures were assessed at 30 and 90 days following hospital discharge to assess for short and medium range outcomes.[22]
Patient Characteristics
Patient demographics including age, sex, low income (defined as individual income below $16,018 [CND] or couples income below $24,175 [CND]), living in a rural region, and the number of previous visits with primary care physicians were described from the available data. Readmission risk from the initial hospitalization was calculated based on the LACE score.[23] The LACE score is a validated measure of 30‐day readmission risk based on healthcare administrative data that account for (L) length of stay, (A) acute admission, (C) comorbid disease burden, and number of (E) emergency department visits in previous 6 months.[23] The LACE score ranges from 0 to 19, which correspond to a probability of readmissions of 2% to 43.7%, respectively. We considered individuals to have a high risk of readmission with a LACE score 10, which corresponds to a probability of readmission of 12.2%.[23]
Statistical Analyses
Descriptive statistics were used to compare patient characteristics among those with a primary care physician supportive‐care visit to those without. Logistic regression modeling was conducted to examine the impact of primary care physician visits on outcomes. The results reported here reflect the selection of adjusting for the confounders of age, sex, a history of primary care physician visits, low income, rurality, and the LACE score.
Ethics
The project analysis was conducted at the Institute for Clinical Evaluative Sciences (ICES) in Toronto, Ontario and was approved by the Sunnybrook Health Sciences Centre Research Ethics Board.
RESULTS
Overview
There were 11,316 primary care physicians identified as practicing in Ontario during the study period, of which 3236 had a history of conducting regular in‐hospital visits to 2 or more patients. The final patient cohort consisted of 164,059 hospitalized patients; 19,614 patients received a visit from their primary care physician, whereas 144,445 did not (Figure 1).

The hospitalized patients who received a visit from their primary care physician were significantly different than the patients who did not receive an in‐hospital visit (Table 1). Notably, patients who received a visit by their primary care physician had longer lengths of hospital stay (9.7 days vs 6.8 days, P<0.001). As well, a greater proportion had a high 30‐day readmission risk (LACE score10: 39.4% vs 29.9%, P<0.001) (Table 1).[21]
| Variablea | With PCP Visit (N=19,614) | Without PCP Visit (N=144,445) |
|---|---|---|
| ||
| Age, meanSD | 68.3716.85 | 65.7318.54 |
| Sex, no. of males | 9,393 (47.9%) | 67,030 (46.4%) |
| Low income | 3,937 (20.1%) | 30,157 (20.9%) |
| Individuals living in rural regions, no. | 1,951 (9.9%) | 25,731 (17.8%) |
| PCP visits in previous 6 months, meanSD | 4.764.47 | 4.174.28 |
| Length of stay, d, meanSD | 9.7217.40 | 6.7913.17 |
| Acute emergent visits, no. | 19,138 (97.6%) | 136,374 (94.4%) |
| Charlson score, meanSD | 1.061.60 | 0.921.49 |
| ED visits in previous 6 months, meanSD | 0.951.48 | 1.091.98 |
| LACE score, meanSDc | 9.022.88 | 8.103.02 |
| High risk for readmission (LACE score10), no. (%)c | 7,721 (39.4%) | 43,126 (29.9%) |
Patients who received an in‐hospital visit by their primary care physician were significantly different from those who did not (Table 2). They were older (68.4 years vs 65.7 years), and had a higher risk of readmission (LACE score of 9 vs 8). As well, proportionally fewer patients who received a visit were from rural regions than in the comparator group (9.9% of patients visited were from rural regions vs 17.8% of patients who did not receive a visit) (Table 2).
| Variable | Patients Who Received an In‐hospital Visit (N=19,614) | Patients Who Did Not Receive an In‐hospital Visit (N=144,445) | P Value |
|---|---|---|---|
| |||
| Primary outcome of emergency department visit, hospital readmission, or death | |||
| 30 days postdischarge, no. (%) | |||
| Readmission | 1,742 (8.9%) | 11,212 (7.8%) | <0.001 |
| ED visit | 2,039 (10.4%) | 16,823 (11.6%) | <0.001 |
| Death | 727 (3.7%) | 4,688 (3.2%) | <0.001 |
| Composite endpointa | 4,227 (21.6%) | 30,848 (21.4%) | 0.533 |
| 90 days postdischarge | |||
| Readmission | 2,791 (14.2%) | 18,257 (12.6%) | <0.001 |
| ED visit | 3,652 (18.6%) | 29,590 (20.5%) | <0.001 |
| Death | 1,507 (7.7%) | 9,821 (6.8%) | <0.001 |
| Composite endpointa | 7,125 (36.3%) | 52,245 (36.2%) | 0.668 |
| Secondary outcome of PCP office visits and home‐care services | |||
| 30 days postdischarge | |||
| Community PCP visits, meanSD | 3.85.1 | 3.14.6 | <0.001 |
| PCP visit, no. (%) | 15,732 (80.2%) | 108,266 (75%) | <0.001 |
| Home‐care services, no. (%) | 6,197 (31.6%) | 38,745 (26.8%) | <0.001 |
| Composite endpoint, no. (%)b | 16,851 (85.9%) | 117, 290 (81.2%) | <0.001 |
| 90 days postdischarge | |||
| Community PCP visits, meanSD | 8.210.1 | 6.99.3 | <0.001 |
| PCP visit, no. (%) | 18,112 (92.3%) | 128, 806 (89.2%) | <0.001 |
| Home‐care services, no. (%) | 7,256 (37.0%) | 45,675 (31.6%) | <0.001 |
| Composite endpoint, no. (%)b | 18, 504 (94.3%) | 132, 448 (91.7%) | <0.001 |
Individual Outcomes
Patients who received an in‐hospital visit by their primary care physician were also more likely to be readmitted within 30 days of discharge (8.9% vs 7.8%, P<0.001) and within 90 days of discharge (14.2% vs 12.6%, P<0.001). Additionally, patients who were visited by their primary care physician while hospitalized were more likely to die within 30 days postdischarge than those who did not receive an in‐hospital visit (3.7% vs 3.2%, P<0.001) and similarly by 90 days postdischarge (7.7% vs 6.8%, P<0.001) (Table 2).
Patients who received an in‐hospital visit were less likely to visit the emergency department at 30 days (10.4% vs 11.6%, P<0.001) and at 90 days (18.6% vs 20.5%, P<0.001) compared to patients who did not receive an in‐hospital visit (Table 2).
The patients who received in‐hospital visits by their primary care physician had a greater average number of primary care physician visits in the community at 30 days (3.8 vs 3.1, P<0.001) and 90 days (8.2 vs 6.9, P<0.001) (Table 2). Additionally, a higher proportion of patients who received an in‐hospital visit accessed home‐care services at 30 days postdischarge (31.6% vs 26.8%, P<0.001) and 90 days postdischarge (37.0% vs 31.6%, P<0.001) (Table 2).
Primary Outcome
There was no difference in proportion of patients who experienced the composite endpoint at 30 days (4227 [21.6%] vs 30,848 [21.4%], P>0.5) or 90 days (7125 [36.3%] vs 52,245 [36.2%], P>0.6) for patients who received an in‐hospital visit by their primary care physician compared to those who did not. The unadjusted model found no statistically significant difference between the 2 groups upon a primary care physician visit (odds ratio [OR]: 1.01; 95% confidence interval [CI]: 0.98‐1.04). However, once adjusting for differences in the groups for patient factors such as age, sex, location and health status, patients who received an in‐hospital visit by their primary care physician had lower adjusted risk for the composite outcome at 30 days postdischarge (adjusted OR [aOR]: 0.92; 95% CI: 0.89‐0.96) and 90 days postdischarge (aOR: 0.90; 95% CI: 0.87‐0.92) (Table 3). Estimates for each individual component of the composite outcome revealed significantly lower risk for ED visit and death but similar risk for readmission at both 30 days and 90 days after hospital discharge for patients who received and in‐hospital visit from their primary care physician and those who did not (Table 3).
| Variable | Unadjusted Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI)a |
|---|---|---|
| ||
| Primary outcome of emergency department visit, hospital readmission, or death | ||
| 30 days postdischarge | ||
| Readmission | 1.16 (1.10‐1.22) | 1.03 (0.97‐1.08) |
| ED visit | 0.88 (0.84‐0.92) | 0.88 (0.84‐0.92) |
| Death | 1.15 (1.06‐1.24) | 0.88 (0.81‐0.96) |
| Composite endpointb | 1.01 (0.98‐1.05) | 0.92 (0.89‐0.96) |
| 90 days postdischarge | ||
| Readmission | 1.15 (1.10‐1.20) | 1.00 (0.96‐1.04) |
| ED visit | 0.89 (0.86‐0.92) | 0.89 (0.86‐0.93) |
| Death | 1.14 (1.08‐1.21) | 0.87 (0.82‐0.93) |
| Composite endpointb | 1.01 (0.98‐1.04) | 0.90 (0.87‐0.92) |
| Secondary outcome of PCP office visits and home‐care services | ||
| 30 days postdischarge | ||
| Community PCP visits | 1.35 (1.31‐1.41) | 1.21 (1.16‐1.25) |
| Home‐care services | 1.26 (1.22‐1.30) | 1.05 (1.01‐1.09) |
| Composite endpointc | 1.41 (1.34‐1.47) | 1.16 (1.11‐1.21) |
| 90 days postdischarge | ||
| Community PCP visits | 1.46 (1.39‐1.55) | 1.25 (1.18‐1.33) |
| Home‐care services | 1.27 (1.23‐1.31) | 1.05 (1.01‐1.08) |
| Composite endpointc | 1.51 (1.42‐1.61) | 1.19 (1.12‐1.27) |
Secondary Outcome
Patients who received an in‐hospital visit by their primary care physician were more likely to experience the composite outcome of home‐care services and community primary care physician visits at 30 postdischarge (16,851 [85.9%] vs 117,290 [81.2%], P<0.001) and 90 days postdischarge (18,504 [94.3%] vs 132,448 [91.7%], P<0.001) compared to patients who did not receive an in‐hospital visit (Table 3). Once accounting for patient variables such as age, sex, location, and health status, patients who received an in‐hospital visit by their primary care physician had a higher adjusted risk for the composite outcome at 30 days postdischarge (aOR: 1.16; 95% CI: 1.11‐1.21) and 90 days postdischarge (aOR: 1.19; 95% CI: 1.12‐1.27) (Table 3).
DISCUSSION
Our population‐based study of primary care physicians is among the first to examine outcomes of patients whose primary care physicians have a history of providing supportive visits to hospitalized patients. After controlling for risk differences in patients at hospital discharge, we found that a primary care physician visit to a patient in the hospital was associated with a lower adjusted risk for the composite outcome of death, emergent hospital readmission, or emergency department visit at 30 and 90 days postdischarge compared to hospitalized patients who did not receive a visit by their primary care physician. We found this to be driven by patients having a lower risk of emergency department visits and death, whereas there was a similar risk of hospital readmission. We also found that visited patients were more likely to access home‐care services and have more primary care physician visits in the community following discharge.
The unadjusted model differs substantially from the adjusted model. On the surface this is an apparent paradox where the unadjusted results suggest an association with potential harm or no difference with a supportive visit. Conversely, the adjusted model suggests a reduction in harms. The differences between the unadjusted and adjusted model is driven by changes in the point estimates for readmission and death rates at both 30 and 90 day postdischarge. Prior to adjustment, it appears as if a primary care physician visit is associated with a significant increase of death; however, upon adjustment, it is associated with a significant reduction in death. Interestingly, this is a different effect than that observed with the secondary analysis, where the adjusted analyses demonstrate a more modest (but still positive) effect of supportive‐care visits. This observed change is likely due to differences in the patient groups. We can speculate that this may be an observed phenomenon of primary care physicians opting to visit their sicker patients, as perhaps it should be; however, further research is required to fully understand the real drivers of a supportive visit.
Our results are consistent with an earlier study that identified that a minority number of primary care physicians visit their hospitalized patients.[24] As well, findings from a randomized controlled trial of 364 patients over 60 years old identified a limited impact of primary care physician visits on patient outcomes but noted enhanced access to community health services.[12] Our work highlights the potential impact of primary care physician visits, which could, in theory, be leveraged and be an important role that primary care physicians can play in planning postdischarge care and improving the quality of care following hospitalization.
Our research study did not examine the impact of in‐hospital primary care physician visits on patient satisfaction directly. However, it has been demonstrated that patients have a strong desire for their primary care physician to be involved in their hospital care and their preference is for direct contact, with face‐to‐face visits compared to telephone or other communication.[25] This choice is important because dissatisfaction with services is associated with a loss of patient confidence in care quality and decreased adherence.[26] Also, primary care physicians acknowledge that information exchange is lacking when their patients are discharged, and that improving this aspect of a patient's care transition is important.[20] Research into discharge summaries as a tool to fill the communication gap has noted some success, yet there remains uncertainty regarding the type of information that should be included in a discharge summary, the time frame in which primary care physicians actually receive the summaries, and the accuracy of the information provided.[20, 27]
Our use of population‐based administrative data sources make the findings of our research generalizable to other similarly designed healthcare systems where a primary care physician may visit their hospitalized patients in a supportive‐care role. We were interested in a complex patientphysician interaction with a number of potential confounding factors, and our use of a composite measure represents the broad outcomes from this contact. Our cohort methodology was designed to isolate the exposure of interest while maximizing uniformity between the 2 study groups on other characteristics. Additionally a number of potential confounding factors were considered in an effort to isolate the effect of the primary care physician in‐hospital visit such as age, comorbid disease, and risk of hospital readmission.[12] The findings of our work support that of earlier research, but on a broader and more generalizable scale.[12]
There were notable differences between the intervention and control patient populations in the proportion of patients from rural regions who receive a supportive visit. This may be due to systemic differences between rural and nonrural regions with regard to access to care and ease of visit by primary care physicians. Alternatively, observed differences may be due to limitations of our study design in that some rural environments rely on primary care physicians to be involved in hospital care for the region. As such, they may actually be visiting their patients in a manner that was not captured as a supportive‐care visit. This is an important area that should be pursued in the future.
We acknowledge there are limits to our research findings. First, the nature of administrative data introduces challenges to causal inferences. As such, we are careful to describe associations and not draw causative links as there may be additional variables influencing outcomes including the patientphysician relationship, the location of the hospital relative to the physician practice and/or home, the time of the primary care physician visit, primary care physician hospital privileges for supportive‐care visits, and the number of other patients the primary care physician had in the same hospital at the same time. A second limitation is the use of the selected outcomes, which may not be direct measures of care quality.[28] However, the selected outcomes have been shown to be good quality measures in other work relevant to health policy.[8, 20, 21, 29] Third, the use of a composite outcome may over‐ or underestimate an exposure's impact.[19] Our composite outcome might have been dominated by some of its components. These observations may reflect the reality of primary care physicians visiting their sicker patients, or may be an attribute of the relatively short length of follow‐up of the study design. Fourth, we cannot determine whether there were additional interventions in place that assisted the continuity of care for primary care physician visits.[20, 27] However, this research included a broad range of hospitals throughout a large province where there were no system‐level quality interventions applied during this time. Fifth, our readmission rate may appear lower than other studies. However, our analysis is population based and not limited in focus to seniors.[30] As well, our posthospitalization death rates are similar to others, and the readmission rates are comparable to other Canadian studies.[31] Sixth, patients at higher risk for adverse outcomes may be identified as requiring more communication with their primary care physicians and we may not have fully captured this risk in our adjustment models, thereby underestimating the effect of exposure.[27] Further, primary care physicians may be involved in major medical decisions such as transitions to palliative care. A supportive‐care visit that facilitated these transitions and its ensuing outcomes may not have been included in our analysis. Seventh, our inherent assumption is that more care, such as posthospital primary care visits and home visits, denotes better care. This may not always be the case.[32] Eighth, physicians may find it difficult to visit their patient in the hospital, even when asked.[12] Finally, our findings are contingent on a system that supports primary care physicians being aware of their patients who become hospitalized. This is not only incumbent on any individual (eg, hospitalist) but a system where all providers work cohesively and seamlessly. On balance, however, these limitations do not overshadow our study's findings and conclusions.
Visits by primary care physicians to hospitalized patients are a longstanding tradition. The practice likely varies according to regional, patient, and individual physician characteristics.[16, 17, 18, 25] However, reimbursement codes for these services are present in a number of international healthcare systems' physician fee schedules with fairly modest remuneration amounts. The fairly nominal fee of less than $20 CND for a supportive‐care visit is similar to other systems and does not constitute a strong financial incentive to encourage this practice. The fee likely compensates the primary care physician for some of their time but comes with an opportunity cost to other aspects of their practice. Thus, results may differ in other environments or if the fee were higher, thereby incenting more primary care physicians to conduct visits. Indeed, the entire program for supportive hospital visits cost approximately $2.5 million CND per year for the 13 million people in the province of Ontario. Future work in this area could address the overall value and cost‐effectiveness of any potential fee changes. Still, it highlights the generalizability of our findings to other health systems and the ease in assessing the effect of the practice.
Overall, our findings underscore the importance and relevance for the practice of supportive‐care visits in its association with patient outcomes and health services utilization, which may prove to be an important key factor to improve quality healthcare. Our results suggest that an in‐hospital visit by a primary care physician may improve patient outcomes and increase subsequent support in the community. An in‐hospital supportive visit may be an additional method by which primary care physicians, and healthcare systems as a whole, strive to achieve the best care for patients.
Acknowledgements
Michael Manno, an analyst with the Institute of Clinical Evaluative Sciences (ICES) at the time of this study, assisted with the analyses.
Disclosures: This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by the ICES or the Ontario MOHLTC is intended or should be inferred. No researcher or persons involved in this study had any declared or otherwise known conflicts of interest. Stacey Brener received funding from a Canadian Institutes of Health Research (CIHR) Master's award in the area of primary care; the Ontario Graduate Student in Science and Technology award, an award from the CIHR Women's College Hospital Interdisciplinary Capacity Enhancement Team, and team grant OTG‐88591 from the CIHR. Susan Bronskill is supported by a CIHR New Investigator Award in the Area of Aging. Chaim Bell is supported by a CIHR/Canadian Patient Safety Institute Chair in Patient Safety and Continuity of Care. These funding agencies had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , et al. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. 1991. Qual Saf Health Care. 2004;13(2):145–151; discussion 51–52.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- The College of Family Physicians of Canada. Family physicians caring for hospital inpatients. Available at: http://www.cfpc.ca/uploadedFiles/Resources/Resource_Items/FPs20Inpt20Hosp20Care_En.pdf. Published October 2003. Accessed August 15, 2015.
- , , . Is volume related to outcome in health care?. A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–520.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- , , , . Maintaining continuity of care: a look at the quality of communication between Ontario emergency departments and community physicians. CJEM. 2005;7(3):155–161.
- , , , et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med. 2010;5(7):398–405.
- , , , , , . Patient‐reported care coordination: associations with primary care continuity and specialty care use. Ann Fam Med. 2011;9(4):323–329.
- , . The Wellness Planner: empowerment, quality of life, and continuity of care in mental illness. Arch Psychiatr Nurs. 2011;25(4):284–293.
- , , , et al. Evaluation of the impact of interdisciplinarity in cancer care. BMC Health Serv Res. 2011;11:144.
- , , , , . Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial. Fam Pract. 1999;16(3):289–293.
- , , , et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840–847.
- , , , , , . Potentially unintended discontinuation of long‐term medication use after elective surgical procedures. Arch Intern Med. 2006;166(22):2525–2531.
- , , , , , , . Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Toronto: Institute for Clinical Evaluative Sciences; 2006. Available at: http://www.ices.on.ca/Publications/Atlases‐and‐Reports/2006/Canadian‐Institute‐for‐Health‐Information. Accessed August 15, 2015.
- , , , . Primary care physician workforce and Medicare beneficiaries' health outcomes. JAMA. 2011;305(20):2096–2104.
- , , , . Assigning ambulatory patients and their physicians to hospitals: a method for obtaining population‐based provider performance measurements. Health Serv Res. 2007;42(1 pt 1):45–62.
- , , , , , . Administrative data algorithms can describe ambulatory physician utilization. Health Serv Res. 2007;42(4):1783–1796.
- , , , et al. Validity of composite end points in clinical trials. BMJ. 2005;330(7491):594–596.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , , . Predicting readmission to the psychiatric hospital in a managed care environment: implications for quality indicators. Am J Psychiatry. 1997;154(3):337–340.
- , . The validity of readmission rate as a marker of the quality of hospital care, and a recommendation for its definition. N Z Med J. 2009;122(1289):63–70.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- , , , et al. Information exchange among physicians caring for the same patient in the community. CMAJ. 2008;179(10):1013–1018.
- . Supply of physicians' services in Ontario. Hosp Q. 1999;3(2):17.
- , , , , . Evaluation of outreach clinics held by specialists in general practice in England. J Epidemiol Community Health. 2000;54(2):149–156.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , . A conceptual framework for the study of early readmission as an indicator of quality of care. Soc Sci Med. 1996;43(11):1533–1541.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Effect of a postdischarge virtual ward on readmission or death for high‐risk patients: a randomized clinical trial. JAMA. 2014;312(13):1305–1312.
- , . Less is more: how less health care can result in better health. Arch Intern Med. 2010;170(9):749–750.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , et al. Incidence of adverse events and negligence in hospitalized patients: results of the Harvard Medical Practice Study I. 1991. Qual Saf Health Care. 2004;13(2):145–151; discussion 51–52.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- The College of Family Physicians of Canada. Family physicians caring for hospital inpatients. Available at: http://www.cfpc.ca/uploadedFiles/Resources/Resource_Items/FPs20Inpt20Hosp20Care_En.pdf. Published October 2003. Accessed August 15, 2015.
- , , . Is volume related to outcome in health care?. A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137(6):511–520.
- , , , , , . Outcomes of care by hospitalists, general internists, and family physicians. N Engl J Med. 2007;357(25):2589–2600.
- , , , . Maintaining continuity of care: a look at the quality of communication between Ontario emergency departments and community physicians. CJEM. 2005;7(3):155–161.
- , , , et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med. 2010;5(7):398–405.
- , , , , , . Patient‐reported care coordination: associations with primary care continuity and specialty care use. Ann Fam Med. 2011;9(4):323–329.
- , . The Wellness Planner: empowerment, quality of life, and continuity of care in mental illness. Arch Psychiatr Nurs. 2011;25(4):284–293.
- , , , et al. Evaluation of the impact of interdisciplinarity in cancer care. BMC Health Serv Res. 2011;11:144.
- , , , , . Can GP input into discharge planning result in better outcomes for the frail aged: results from a randomized controlled trial. Fam Pract. 1999;16(3):289–293.
- , , , et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840–847.
- , , , , , . Potentially unintended discontinuation of long‐term medication use after elective surgical procedures. Arch Intern Med. 2006;166(22):2525–2531.
- , , , , , , . Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Toronto: Institute for Clinical Evaluative Sciences; 2006. Available at: http://www.ices.on.ca/Publications/Atlases‐and‐Reports/2006/Canadian‐Institute‐for‐Health‐Information. Accessed August 15, 2015.
- , , , . Primary care physician workforce and Medicare beneficiaries' health outcomes. JAMA. 2011;305(20):2096–2104.
- , , , . Assigning ambulatory patients and their physicians to hospitals: a method for obtaining population‐based provider performance measurements. Health Serv Res. 2007;42(1 pt 1):45–62.
- , , , , , . Administrative data algorithms can describe ambulatory physician utilization. Health Serv Res. 2007;42(4):1783–1796.
- , , , et al. Validity of composite end points in clinical trials. BMJ. 2005;330(7491):594–596.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , , . Predicting readmission to the psychiatric hospital in a managed care environment: implications for quality indicators. Am J Psychiatry. 1997;154(3):337–340.
- , . The validity of readmission rate as a marker of the quality of hospital care, and a recommendation for its definition. N Z Med J. 2009;122(1289):63–70.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- , , , et al. Information exchange among physicians caring for the same patient in the community. CMAJ. 2008;179(10):1013–1018.
- . Supply of physicians' services in Ontario. Hosp Q. 1999;3(2):17.
- , , , , . Evaluation of outreach clinics held by specialists in general practice in England. J Epidemiol Community Health. 2000;54(2):149–156.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- , , , et al. Relationship between hospital readmission and mortality rates for patients hospitalized with acute myocardial infarction, heart failure, or pneumonia. JAMA. 2013;309(6):587–593.
- , . A conceptual framework for the study of early readmission as an indicator of quality of care. Soc Sci Med. 1996;43(11):1533–1541.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Effect of a postdischarge virtual ward on readmission or death for high‐risk patients: a randomized clinical trial. JAMA. 2014;312(13):1305–1312.
- , . Less is more: how less health care can result in better health. Arch Intern Med. 2010;170(9):749–750.
© 2016 Society of Hospital Medicine
Impact of an Inpatient PN Program
Inpatient medicine is becoming increasingly complex. A growing number of patients with multiple chronic conditions coupled with mounting care fragmentation leave patients vulnerable to adverse events and readmission to the hospital.[1, 2, 3] Moreover, efforts to minimize hospital length of stay (LOS) have resulted in patients being discharged quicker and sicker than ever before.[4]
A cornerstone of safe and high‐quality healthcare is effective communication.[5] Ineffective communication between and among healthcare providers and patients is a leading cause of medical errors and patient harm. An analysis of sentinel events reported to The Joint Commission revealed that communication failure was the root cause in 59% of these events.[6]
The current climate of increasing healthcare complexity has prompted the need for adaptive innovation.[7] However, there are limited data describing interventions targeting improvements in both communication and transitional care planning. We created a new position, the patient navigator (PN), a dedicated patient‐care facilitator not responsible for clinical care. PNs were integrated into the inpatient multidisciplinary clinical team to facilitate patient and provider navigation through the complexity of a hospital admission by enhancing communication between and among patients and providers. The objective of this study was to determine whether this intervention would reduce hospital LOS and 30‐day unplanned readmissions.
METHODS
Setting
Mount Sinai Hospital is a 446‐bed acute care urban academic health center in Toronto, Ontario, Canada. The general internal medicine service operates as a 90‐bed clinical teaching unit physically distributed over 4 inpatient wards. The service is structurally divided into 4 nongeographically based multidisciplinary care teams (teams A, B, C, and D) comprised of the medical team (attending physician, senior resident physician, 23 junior resident physicians, and 23 medical students), pharmacist, social worker, physiotherapist, occupational therapist, speech and language pathologist, dietician, respiratory therapist, and nursing staff allocated by ward. Each team is on call approximately 1 night in 4 with no night float system. At our institution, attending physicians rotate on a 2‐ or 4‐week schedule, resident physicians rotate on a 1‐ or 2‐month schedule, and medical students rotate on a 2‐month schedule. Preintervention, communication occurred in person and by telephone between members of the medical team. Other members of the multidisciplinary care team communicated with the medical team in person at daily multidisciplinary rounds focused on discharge planning, by pager, or using a Web‐based communication tool.
Intervention
PNs were dedicated patient‐care facilitators not responsible for clinical care. They acted as liaisons between and among providers and patients. Each PN was a fully integrated member of their multidisciplinary care team. With ongoing medical team rotations, the PN was notably the only consistent member on the clinical team. Each patient saw the same PN throughout his or her hospital stay, as both the patient and the PN were team based. The average number of patients for whom each PN was responsible daily was dictated by the patient census for their team. On average, each team had a census between 20 and 30 patients daily. PNs worked during the daytime from Monday to Friday, and did not have any overnight or weekend responsibilities.
A PN's typical day began by reviewing and rounding on overnight admissions as a formal member of the clinical team. This was followed by participating in daily multidisciplinary rounds, then documenting and circulating the resultant action items. Thereafter, they expedited consultations and tests by liaising with departmental staff, and proactively established contact with the patient and their family. They answered simple factual questions related to test scheduling, consultations, diagnosis, medications, and treatments as discussed and outlined by the clinical team, and promptly relayed care questions beyond the scope of their knowledge to the clinical team. They were available to patients, family members, and providers via a dedicated mobile number using phone calls and text messages. If indicated, they assisted in discharge coordination by arranging follow‐up appointments and placing postdischarge phone calls. In addition, they served as primary contact for every patient admitted to their clinical team following discharge to ensure appropriate follow through on discharge plans. There were no set criteria for PNs to disengage from a patient's care. They could always be reached using their dedicated mobile number during business hours, with a voicemail system in place for after‐hours calls.
The role was filled by individuals skilled in communication and/or healthcare, such as registered nurses, a masters degreetrained educator, internationally trained physicians, and professionals from the hospitality and human resources industries. There were no prespecified training or degree requirements. Each PN underwent on‐the‐job training and participated in twice monthly PN meetings for ongoing feedback and education.
Program Implementation
We implemented the PN program on the inpatient general internal medicine service in June 2010 on 2 of 4 multidisciplinary clinical teams. Because a PN became an integrated member of 1 of 4 clinical teams, patient assignment to a PN was determined by the team to which the patient was admitted. On average, each of the 4 teams admitted equally on a daily basis. Initially, there were only sufficient resources to fund 2 PNs. Thus, from June 2010 to May 2011, only teams A and C were assigned PNs. To create fairness between the 4 teams, these 2 PNs moved to teams B and D from June 2011 to November 2011, and then back to teams A and C from December 2011 to April 2012. Following this initial pilot period, the program was allocated further resources, and so expanded to all 4 teams in May 2012. PN salaries were the only program costs. These costs were funded by matching donations from physicians within the Mount Sinai Hospital Department of Medicine and donations to the hospital from community members directed to support the implementation and evaluation of novel care delivery systems.
Study Design
We evaluated the PN program using a retrospective cohort study that included all general medical admissions between July 2010 and March 2014 matched by case mix group, age category, and resource intensity weight (a relative value measuring total patient resource use compared with average typical acute inpatients).[8]
Our primary outcomes were LOS and 30‐day readmission rate. These outcomes were stratified by exposure status to a PN. There were no exclusion criteria for the LOS analysis. Patients who died, were transferred to or from an acute care facility, or signed out against medical advice were excluded from the 30‐day readmission analysis. A secondary analysis restricted the timeframe from July 2010 to April 2011, when only 2 of 4 teams were exposed to PNs.
Average LOS has been observed to be higher in Canadian hospitals as compared to their US counterparts across different admission diagnoses, such as coronary artery bypass graft surgery and heart failure.[9, 10] We hypothesize that these differences are party due to systems‐level differences, including posthospital care. Specifically, the Canadian system does not utilize posthospital acute care, such as skilled nursing facilities, which may in part account for these differences. To help contextualize our data, we standardized LOS using an LOS index called the LOS/expected LOS (ELOS) ratio. It takes the LOS and divides it by the ELOS, a validated estimate of the expected LOS for a given patient generated using a national administrative database for acute hospital care in Canada that takes into account case mix group, age, comorbidity level, and intervention factors.[8]
Additionally, We performed an interrupted time‐series analysis, whereby a log‐linear model was fit on LOS and adjusted for weekly and monthly trends, age category, resource intensity weight, major clinical category (a surrogate for case mix group), admission location, and discharge location. The cohort was divided into 3 groups: before program implementation (July 2009June 2010), after program implementation with PN (July 2010March 2014), and after program implementation without PN (July 2010March 2014).
This study was approved by the research ethics board at Mount Sinai Hospital. No patient consent was deemed necessary. Data were obtained from institutional databases monitored by the hospital's performance measurement office.
Statistical Analysis
In Tables 1, 2, mean values were compared using a 2‐tailed t test, and the relationship between categorical groups was determined using a 2 test. For the interrupted time‐series analysis, 2‐tailed t tests were used to test null hypotheses of no association between the parameter value and the outcome, and 2 tests were used to test for the equivalence of 2 given parameters. P0.05 indicated statistical significance for all comparisons and analyses. All data were analyzed using Stata version 13 (StataCorp, College Station, TX) or R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
| With PN, n = 5,628 | Without PN, n = 2,213 | |
|---|---|---|
| ||
| Age, y, mean (SD)* | 69 (20) | 68 (20) |
| Female sex, n (%) | 3,018 (53.6) | 1,196 (54.0) |
| Most responsible diagnosis, n (%) | ||
| Pneumonia | 374 (6.6) | 135 (6.1) |
| Chronic obstructive pulmonary disease | 271 (4.8) | 88 (4.0) |
| Congestive heart failure | 217 (3.9) | 87 (3.9) |
| Admission location, n (%) | ||
| Home | 4,665 (82.9) | 1,943 (87.8) |
| Long‐term care* | 524 (9.3) | 158 (7.1) |
| Other* | 439 (7.8) | 112 (5.1) |
| Discharge location, n (%) | ||
| Home | 3,824 (67.9) | 1,578 (71.3) |
| Long‐term care | 779 (13.8) | 267 (12.1) |
| Other | 1,025 (18.3) | 368 (16.6) |
RESULTS
Our matched cohort included 7841 admissions (6141 patients), with 5628 admissions (4592 patients) exposed and 2213 admissions (1920 patients) not exposed to PNs. The discrepancy between the total number of patients and the sum of exposed and nonexposed patients is resultant from patients admitted more than once over the study period, as patients admitted to at least 1 team staffed with a PN and another team not staffed with a PN over the study period were counted in both groups. The 2 groups were similar with respect to several characteristics (Table 1). However, the 2 groups were significantly different for age (P = 0.046) and admissions from long‐term care (P < 0.01) and other facilities (P < 0.01).
Admissions with PNs were 1.3 days (21%) shorter than admission without PNs (6.2 vs 7.5 days, P < 0.001). Moreover, admissions with PNs had a smaller mean LOS/ELOS ratio compared to admissions without PNs (0.93 vs 1.05, P < 0.001). The restricted analysis found a 1.2‐day (18%) lower LOS (6.4 vs 7.6 days, P < 0.05) and a smaller mean LOS/ELOS ratio (0.91 vs 1.06, P < 0.001). Thirty‐day readmission rate was not different between the 2 groups (13.1 vs 13.8%, P = 0.48) or in the restricted analysis (12.0 vs 13.5%, P = 0.40) (Table 2).
| With PN | Without PN | P Value | |
|---|---|---|---|
| |||
| July 2010‐March 2014 | |||
| LOS, d (95% confidence interval) [n] | 6.2 (6.06.4) [5,628] | 7.5 (7.17.9) [2,213] | <0.001 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.93 (0.910.95) [5,628] | 1.05 (1.001.09) [2,213] | <0.001 |
| 30‐day readmission rate, % [n] | 13.1 [5,055] | 13.8 [2,012] | 0.48 |
| July 2010 to April 2011 | |||
| LOS, d (95% confidence interval) [n] | 6.4 (5.87.0) [713] | 7.6 (6.88.3) [753) | <0.05 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.91 (0.850.96) [713] | 1.06 (1.001.11) [753] | <0.001 |
| 30‐day readmission rate, % [n] | 12.0 [627] | 13.5 [681] | 0.40 |
In the interrupted time‐series analysis, prior to the implementation of the PN program, there was a positive relationship between LOS and time. After the implementation of the program, this relationship became inverse, meaning the curve plotting LOS against time had a negative slope. Furthermore, there was a statistically significant drop in LOS at the time of program implementation (P < 0.05). However, there was no difference in slope between the groups with and without PN after program implementation.
DISCUSSION
We describe an innovative inpatient intervention featuring an integrated patient‐care facilitator not responsible for clinical care charged with enhancing communication between and among patients and providers. Data from the almost 4‐year period demonstrated that implementation was associated with a 21% reduction in hospital LOS, with no difference in 30‐day readmission rates.
The patient navigator was first conceptualized in 1990 to help African American women in Harlem with breast cancer negotiate the complex world of oncology.[11] It was later implemented by the National Cancer Institute as an outpatient intervention spanning the continuum of cancer care. This concept has since expanded to other domains of complex single disease outpatient care, including asthma and fertility.[12, 13] To our knowledge, there has been limited evidence in the literature describing implementation of such programs in the inpatient general medical setting.
This study contributes to the growing literature on interventions targeting improvements in transitional care, such as transition coaches and discharge advocates.[14, 15] Balaban et al. recently described a PN intervention in the safety‐net population.[16] A common theme to these interventions was the prioritization of safe care transitions. However, this goal was achieved using related, yet different approaches: transition coaches focused on encouraging the patient and caregiver to assert a more active role,[14] discharge advocates focused on providing a comprehensive discharge plan for patients,[15] PNs from Balaban's study focused on coaching and assistance in navigating patients through the transition from hospital to home, and our study's PNs focused on enhancing communication between and among patients and providers. Additionally, unlike transition coaches and discharge advocates, who were nurses by training, and PNs from Balaban's study, who were community health workers, our PNs did not have any prespecified training or degree requirements.
Patients are at risk of being inadequately informed about important issues related to their care, such as hospital medications, diagnoses, and treatment plans during their hospital stay.[17, 18] Furthermore, we know that ineffective communication is a common cause of poor patient outcomes in hospital‐based care.[6] This phenomenon can be amplified from external pressures to maximize productivity. For example, Elliott and colleagues found that increasing hospitalist workload is associated with higher hospital LOS and cost.[19] PNs may offload care demands by enhancing communication for providers and patients.
Our study has several strengths. By matching admissions by case mix group, age category, and resource intensity weight, we aimed to reduce potential bias contributed by these covariates. Moreover, a staged rollout of the intervention, whereby over a 10‐month period, 2 of the multidisciplinary care teams were assigned PNs, while the remaining 2 were not, enabled contemporaneous comparison. Our study had few exclusion criteria, thus making it potentially generalizable to other inpatient general medicine settings of a similar nature. The relative simplicity of this intervention makes it amenable to scalability. Of note, the intervention has been deemed to show great promise at our institution, and has currently expanded to the cardiology, gastroenterology, and surgical oncology units.
Our study's limitations include a single‐center design. Moreover, although we demonstrate similarity in the majority of measurable covariates between the groups, we cannot exclude the existence of unmeasured confounders. Of the covariates that were found to be different between the groups, we suspect the difference in admissions from long‐term care and other facilities did not largely influence our study's main findings. Furthermore, though age was found to be statistically different between the groups, we postulate that the 1‐year difference between the groups is not particularly relevant clinically. Additionally, 30‐day readmission rates were only captured for our institution. However, the vast majority of readmissions in our region are to the index facility, and are unlikely to differ between the 2 groups.[20]
There may have been secular trends at play. In the interrupted time‐series analysis, there was a statistically significant drop in LOS at the time of program implementation. There was however, no difference in slope between the groups with and without PNs after program implementation. There are some plausible explanations for this lack of difference in slope. The study may not have been powered to detect such a difference, as this analysis was not prespecified. Furthermore, there may have been a spillover effect of the program, such that PNs may have improved efficiency for the teams to which they were assigned, thereby improving the efficiency of the other members of the multidisciplinary team, many of whom cared for patients assigned and not assigned a PN. Additionally, we measured the LOS in a preintervention control group between July 2009 and June 2010 using the same inclusion criteria as the matched cohort. It was found to be 8.5 days, which suggests a secular trend toward improvement in LOS over time at our institution. We are, however, reassured that our restricted analysis enabling contemporaneous comparison between patients exposed and not exposed to PNs was still found to be significant.
The implementation of this intervention could have implications for policymakers‐at‐large. Establishment of criteria for qualifications and a clear educational curriculum to train future PNs is needed, especially in the context of ongoing program expansion. These initiatives are currently underway at our institution. Furthermore, evaluation of the program's operating cost and calculation of its return on investment should include balanced metrics incorporating patient‐, provider‐, organizational‐, and system‐level measures. The current cost to the hospital per PN is approximately $73,800 CAD ($58,700 USD), which covers 1 PN's annual salary and benefits. Thus, the implementation of 4 PNs for each of the 4 multidisciplinary teams costs the hospital approximately $295,000 CAD ($234,700 USD) per year. Although the details of our preliminary calculations are outside the scope of this report, it suggests that the savings incurred from shorter LOS outweigh program costs.
We found that implementation of this innovative inpatient intervention targeting improvements in communication was associated with a reduction in LOS without an increase in 30‐day readmission. Our experience shows promise and may inform others considering similar interventions. Patient and provider experience and generalizability should be evaluated in future work.
Acknowledgements
The authors thank Dr. Allan Detsky and David Wells for their review of the manuscript. They are also grateful to Chin‐Chin Chua, Ningmei Wang, and Joann Bon in the Office of Quality and Performance Measurement for their help with data collection, and John Matelski for his help with data analysis.
Disclosure: This program was funded by matched donations from physicians in the Mount Sinai Hospital Department of Medicine and donations to Mount Sinai Hospital from community members directed to support the implementation and evaluation of novel care delivery systems. The authors report no conflicts of interest. Preliminary abstracts of this study were presented in the online forum, Leading Health Care Innovation, November 12, 2013 (
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(S3):391–395.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Prospective payment system and impairment at discharge. The “quicker‐and‐sicker” story revisited. JAMA. 1990;264(15):1980–1983.
- . The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13(suppl 1):i85–i90.
- The Joint Commission. Sentinel event data root: causes by event type (2004–June 2014). Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004‐2014.pdf. Accessed March 12, 2014.
- , . Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628.
- Canadian Institute for Health Information. Case mix. Available at: http://www.cihi.ca/CIHI‐ext‐portal/internet/EN/TabbedContent/standards+and+data+submission/standards/case+mix/cihi010690. Accessed April 12, 2015.
- , , , , , . Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med. 2005;165(13):1506–1513.
- , , , et al. Differences in treatment, outcomes, and quality of life among patients with heart failure in Canada and the United States. JACC Heart Fail. 2013;1(6):523–530.
- , , . Expanding access to cancer screening and clinical follow‐up among the medically underserved. Cancer Pract. 1995;3(1):19–30.
- , , , et al. Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma. 2010;47(8):913–919.
- . The role of a patient navigator in fertility preservation. In: Cancer Treatment and Research. Vol 156. Boston, MA: Springer US; 2010:469–470.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , , , . A patient navigator intervention to reduce hospital readmissions among high‐risk safety‐net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–915.
- , , . Lack of patient knowledge regarding hospital medications. J Hosp Med. 2010;5(2):83–86.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , , . Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
Inpatient medicine is becoming increasingly complex. A growing number of patients with multiple chronic conditions coupled with mounting care fragmentation leave patients vulnerable to adverse events and readmission to the hospital.[1, 2, 3] Moreover, efforts to minimize hospital length of stay (LOS) have resulted in patients being discharged quicker and sicker than ever before.[4]
A cornerstone of safe and high‐quality healthcare is effective communication.[5] Ineffective communication between and among healthcare providers and patients is a leading cause of medical errors and patient harm. An analysis of sentinel events reported to The Joint Commission revealed that communication failure was the root cause in 59% of these events.[6]
The current climate of increasing healthcare complexity has prompted the need for adaptive innovation.[7] However, there are limited data describing interventions targeting improvements in both communication and transitional care planning. We created a new position, the patient navigator (PN), a dedicated patient‐care facilitator not responsible for clinical care. PNs were integrated into the inpatient multidisciplinary clinical team to facilitate patient and provider navigation through the complexity of a hospital admission by enhancing communication between and among patients and providers. The objective of this study was to determine whether this intervention would reduce hospital LOS and 30‐day unplanned readmissions.
METHODS
Setting
Mount Sinai Hospital is a 446‐bed acute care urban academic health center in Toronto, Ontario, Canada. The general internal medicine service operates as a 90‐bed clinical teaching unit physically distributed over 4 inpatient wards. The service is structurally divided into 4 nongeographically based multidisciplinary care teams (teams A, B, C, and D) comprised of the medical team (attending physician, senior resident physician, 23 junior resident physicians, and 23 medical students), pharmacist, social worker, physiotherapist, occupational therapist, speech and language pathologist, dietician, respiratory therapist, and nursing staff allocated by ward. Each team is on call approximately 1 night in 4 with no night float system. At our institution, attending physicians rotate on a 2‐ or 4‐week schedule, resident physicians rotate on a 1‐ or 2‐month schedule, and medical students rotate on a 2‐month schedule. Preintervention, communication occurred in person and by telephone between members of the medical team. Other members of the multidisciplinary care team communicated with the medical team in person at daily multidisciplinary rounds focused on discharge planning, by pager, or using a Web‐based communication tool.
Intervention
PNs were dedicated patient‐care facilitators not responsible for clinical care. They acted as liaisons between and among providers and patients. Each PN was a fully integrated member of their multidisciplinary care team. With ongoing medical team rotations, the PN was notably the only consistent member on the clinical team. Each patient saw the same PN throughout his or her hospital stay, as both the patient and the PN were team based. The average number of patients for whom each PN was responsible daily was dictated by the patient census for their team. On average, each team had a census between 20 and 30 patients daily. PNs worked during the daytime from Monday to Friday, and did not have any overnight or weekend responsibilities.
A PN's typical day began by reviewing and rounding on overnight admissions as a formal member of the clinical team. This was followed by participating in daily multidisciplinary rounds, then documenting and circulating the resultant action items. Thereafter, they expedited consultations and tests by liaising with departmental staff, and proactively established contact with the patient and their family. They answered simple factual questions related to test scheduling, consultations, diagnosis, medications, and treatments as discussed and outlined by the clinical team, and promptly relayed care questions beyond the scope of their knowledge to the clinical team. They were available to patients, family members, and providers via a dedicated mobile number using phone calls and text messages. If indicated, they assisted in discharge coordination by arranging follow‐up appointments and placing postdischarge phone calls. In addition, they served as primary contact for every patient admitted to their clinical team following discharge to ensure appropriate follow through on discharge plans. There were no set criteria for PNs to disengage from a patient's care. They could always be reached using their dedicated mobile number during business hours, with a voicemail system in place for after‐hours calls.
The role was filled by individuals skilled in communication and/or healthcare, such as registered nurses, a masters degreetrained educator, internationally trained physicians, and professionals from the hospitality and human resources industries. There were no prespecified training or degree requirements. Each PN underwent on‐the‐job training and participated in twice monthly PN meetings for ongoing feedback and education.
Program Implementation
We implemented the PN program on the inpatient general internal medicine service in June 2010 on 2 of 4 multidisciplinary clinical teams. Because a PN became an integrated member of 1 of 4 clinical teams, patient assignment to a PN was determined by the team to which the patient was admitted. On average, each of the 4 teams admitted equally on a daily basis. Initially, there were only sufficient resources to fund 2 PNs. Thus, from June 2010 to May 2011, only teams A and C were assigned PNs. To create fairness between the 4 teams, these 2 PNs moved to teams B and D from June 2011 to November 2011, and then back to teams A and C from December 2011 to April 2012. Following this initial pilot period, the program was allocated further resources, and so expanded to all 4 teams in May 2012. PN salaries were the only program costs. These costs were funded by matching donations from physicians within the Mount Sinai Hospital Department of Medicine and donations to the hospital from community members directed to support the implementation and evaluation of novel care delivery systems.
Study Design
We evaluated the PN program using a retrospective cohort study that included all general medical admissions between July 2010 and March 2014 matched by case mix group, age category, and resource intensity weight (a relative value measuring total patient resource use compared with average typical acute inpatients).[8]
Our primary outcomes were LOS and 30‐day readmission rate. These outcomes were stratified by exposure status to a PN. There were no exclusion criteria for the LOS analysis. Patients who died, were transferred to or from an acute care facility, or signed out against medical advice were excluded from the 30‐day readmission analysis. A secondary analysis restricted the timeframe from July 2010 to April 2011, when only 2 of 4 teams were exposed to PNs.
Average LOS has been observed to be higher in Canadian hospitals as compared to their US counterparts across different admission diagnoses, such as coronary artery bypass graft surgery and heart failure.[9, 10] We hypothesize that these differences are party due to systems‐level differences, including posthospital care. Specifically, the Canadian system does not utilize posthospital acute care, such as skilled nursing facilities, which may in part account for these differences. To help contextualize our data, we standardized LOS using an LOS index called the LOS/expected LOS (ELOS) ratio. It takes the LOS and divides it by the ELOS, a validated estimate of the expected LOS for a given patient generated using a national administrative database for acute hospital care in Canada that takes into account case mix group, age, comorbidity level, and intervention factors.[8]
Additionally, We performed an interrupted time‐series analysis, whereby a log‐linear model was fit on LOS and adjusted for weekly and monthly trends, age category, resource intensity weight, major clinical category (a surrogate for case mix group), admission location, and discharge location. The cohort was divided into 3 groups: before program implementation (July 2009June 2010), after program implementation with PN (July 2010March 2014), and after program implementation without PN (July 2010March 2014).
This study was approved by the research ethics board at Mount Sinai Hospital. No patient consent was deemed necessary. Data were obtained from institutional databases monitored by the hospital's performance measurement office.
Statistical Analysis
In Tables 1, 2, mean values were compared using a 2‐tailed t test, and the relationship between categorical groups was determined using a 2 test. For the interrupted time‐series analysis, 2‐tailed t tests were used to test null hypotheses of no association between the parameter value and the outcome, and 2 tests were used to test for the equivalence of 2 given parameters. P0.05 indicated statistical significance for all comparisons and analyses. All data were analyzed using Stata version 13 (StataCorp, College Station, TX) or R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
| With PN, n = 5,628 | Without PN, n = 2,213 | |
|---|---|---|
| ||
| Age, y, mean (SD)* | 69 (20) | 68 (20) |
| Female sex, n (%) | 3,018 (53.6) | 1,196 (54.0) |
| Most responsible diagnosis, n (%) | ||
| Pneumonia | 374 (6.6) | 135 (6.1) |
| Chronic obstructive pulmonary disease | 271 (4.8) | 88 (4.0) |
| Congestive heart failure | 217 (3.9) | 87 (3.9) |
| Admission location, n (%) | ||
| Home | 4,665 (82.9) | 1,943 (87.8) |
| Long‐term care* | 524 (9.3) | 158 (7.1) |
| Other* | 439 (7.8) | 112 (5.1) |
| Discharge location, n (%) | ||
| Home | 3,824 (67.9) | 1,578 (71.3) |
| Long‐term care | 779 (13.8) | 267 (12.1) |
| Other | 1,025 (18.3) | 368 (16.6) |
RESULTS
Our matched cohort included 7841 admissions (6141 patients), with 5628 admissions (4592 patients) exposed and 2213 admissions (1920 patients) not exposed to PNs. The discrepancy between the total number of patients and the sum of exposed and nonexposed patients is resultant from patients admitted more than once over the study period, as patients admitted to at least 1 team staffed with a PN and another team not staffed with a PN over the study period were counted in both groups. The 2 groups were similar with respect to several characteristics (Table 1). However, the 2 groups were significantly different for age (P = 0.046) and admissions from long‐term care (P < 0.01) and other facilities (P < 0.01).
Admissions with PNs were 1.3 days (21%) shorter than admission without PNs (6.2 vs 7.5 days, P < 0.001). Moreover, admissions with PNs had a smaller mean LOS/ELOS ratio compared to admissions without PNs (0.93 vs 1.05, P < 0.001). The restricted analysis found a 1.2‐day (18%) lower LOS (6.4 vs 7.6 days, P < 0.05) and a smaller mean LOS/ELOS ratio (0.91 vs 1.06, P < 0.001). Thirty‐day readmission rate was not different between the 2 groups (13.1 vs 13.8%, P = 0.48) or in the restricted analysis (12.0 vs 13.5%, P = 0.40) (Table 2).
| With PN | Without PN | P Value | |
|---|---|---|---|
| |||
| July 2010‐March 2014 | |||
| LOS, d (95% confidence interval) [n] | 6.2 (6.06.4) [5,628] | 7.5 (7.17.9) [2,213] | <0.001 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.93 (0.910.95) [5,628] | 1.05 (1.001.09) [2,213] | <0.001 |
| 30‐day readmission rate, % [n] | 13.1 [5,055] | 13.8 [2,012] | 0.48 |
| July 2010 to April 2011 | |||
| LOS, d (95% confidence interval) [n] | 6.4 (5.87.0) [713] | 7.6 (6.88.3) [753) | <0.05 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.91 (0.850.96) [713] | 1.06 (1.001.11) [753] | <0.001 |
| 30‐day readmission rate, % [n] | 12.0 [627] | 13.5 [681] | 0.40 |
In the interrupted time‐series analysis, prior to the implementation of the PN program, there was a positive relationship between LOS and time. After the implementation of the program, this relationship became inverse, meaning the curve plotting LOS against time had a negative slope. Furthermore, there was a statistically significant drop in LOS at the time of program implementation (P < 0.05). However, there was no difference in slope between the groups with and without PN after program implementation.
DISCUSSION
We describe an innovative inpatient intervention featuring an integrated patient‐care facilitator not responsible for clinical care charged with enhancing communication between and among patients and providers. Data from the almost 4‐year period demonstrated that implementation was associated with a 21% reduction in hospital LOS, with no difference in 30‐day readmission rates.
The patient navigator was first conceptualized in 1990 to help African American women in Harlem with breast cancer negotiate the complex world of oncology.[11] It was later implemented by the National Cancer Institute as an outpatient intervention spanning the continuum of cancer care. This concept has since expanded to other domains of complex single disease outpatient care, including asthma and fertility.[12, 13] To our knowledge, there has been limited evidence in the literature describing implementation of such programs in the inpatient general medical setting.
This study contributes to the growing literature on interventions targeting improvements in transitional care, such as transition coaches and discharge advocates.[14, 15] Balaban et al. recently described a PN intervention in the safety‐net population.[16] A common theme to these interventions was the prioritization of safe care transitions. However, this goal was achieved using related, yet different approaches: transition coaches focused on encouraging the patient and caregiver to assert a more active role,[14] discharge advocates focused on providing a comprehensive discharge plan for patients,[15] PNs from Balaban's study focused on coaching and assistance in navigating patients through the transition from hospital to home, and our study's PNs focused on enhancing communication between and among patients and providers. Additionally, unlike transition coaches and discharge advocates, who were nurses by training, and PNs from Balaban's study, who were community health workers, our PNs did not have any prespecified training or degree requirements.
Patients are at risk of being inadequately informed about important issues related to their care, such as hospital medications, diagnoses, and treatment plans during their hospital stay.[17, 18] Furthermore, we know that ineffective communication is a common cause of poor patient outcomes in hospital‐based care.[6] This phenomenon can be amplified from external pressures to maximize productivity. For example, Elliott and colleagues found that increasing hospitalist workload is associated with higher hospital LOS and cost.[19] PNs may offload care demands by enhancing communication for providers and patients.
Our study has several strengths. By matching admissions by case mix group, age category, and resource intensity weight, we aimed to reduce potential bias contributed by these covariates. Moreover, a staged rollout of the intervention, whereby over a 10‐month period, 2 of the multidisciplinary care teams were assigned PNs, while the remaining 2 were not, enabled contemporaneous comparison. Our study had few exclusion criteria, thus making it potentially generalizable to other inpatient general medicine settings of a similar nature. The relative simplicity of this intervention makes it amenable to scalability. Of note, the intervention has been deemed to show great promise at our institution, and has currently expanded to the cardiology, gastroenterology, and surgical oncology units.
Our study's limitations include a single‐center design. Moreover, although we demonstrate similarity in the majority of measurable covariates between the groups, we cannot exclude the existence of unmeasured confounders. Of the covariates that were found to be different between the groups, we suspect the difference in admissions from long‐term care and other facilities did not largely influence our study's main findings. Furthermore, though age was found to be statistically different between the groups, we postulate that the 1‐year difference between the groups is not particularly relevant clinically. Additionally, 30‐day readmission rates were only captured for our institution. However, the vast majority of readmissions in our region are to the index facility, and are unlikely to differ between the 2 groups.[20]
There may have been secular trends at play. In the interrupted time‐series analysis, there was a statistically significant drop in LOS at the time of program implementation. There was however, no difference in slope between the groups with and without PNs after program implementation. There are some plausible explanations for this lack of difference in slope. The study may not have been powered to detect such a difference, as this analysis was not prespecified. Furthermore, there may have been a spillover effect of the program, such that PNs may have improved efficiency for the teams to which they were assigned, thereby improving the efficiency of the other members of the multidisciplinary team, many of whom cared for patients assigned and not assigned a PN. Additionally, we measured the LOS in a preintervention control group between July 2009 and June 2010 using the same inclusion criteria as the matched cohort. It was found to be 8.5 days, which suggests a secular trend toward improvement in LOS over time at our institution. We are, however, reassured that our restricted analysis enabling contemporaneous comparison between patients exposed and not exposed to PNs was still found to be significant.
The implementation of this intervention could have implications for policymakers‐at‐large. Establishment of criteria for qualifications and a clear educational curriculum to train future PNs is needed, especially in the context of ongoing program expansion. These initiatives are currently underway at our institution. Furthermore, evaluation of the program's operating cost and calculation of its return on investment should include balanced metrics incorporating patient‐, provider‐, organizational‐, and system‐level measures. The current cost to the hospital per PN is approximately $73,800 CAD ($58,700 USD), which covers 1 PN's annual salary and benefits. Thus, the implementation of 4 PNs for each of the 4 multidisciplinary teams costs the hospital approximately $295,000 CAD ($234,700 USD) per year. Although the details of our preliminary calculations are outside the scope of this report, it suggests that the savings incurred from shorter LOS outweigh program costs.
We found that implementation of this innovative inpatient intervention targeting improvements in communication was associated with a reduction in LOS without an increase in 30‐day readmission. Our experience shows promise and may inform others considering similar interventions. Patient and provider experience and generalizability should be evaluated in future work.
Acknowledgements
The authors thank Dr. Allan Detsky and David Wells for their review of the manuscript. They are also grateful to Chin‐Chin Chua, Ningmei Wang, and Joann Bon in the Office of Quality and Performance Measurement for their help with data collection, and John Matelski for his help with data analysis.
Disclosure: This program was funded by matched donations from physicians in the Mount Sinai Hospital Department of Medicine and donations to Mount Sinai Hospital from community members directed to support the implementation and evaluation of novel care delivery systems. The authors report no conflicts of interest. Preliminary abstracts of this study were presented in the online forum, Leading Health Care Innovation, November 12, 2013 (
Inpatient medicine is becoming increasingly complex. A growing number of patients with multiple chronic conditions coupled with mounting care fragmentation leave patients vulnerable to adverse events and readmission to the hospital.[1, 2, 3] Moreover, efforts to minimize hospital length of stay (LOS) have resulted in patients being discharged quicker and sicker than ever before.[4]
A cornerstone of safe and high‐quality healthcare is effective communication.[5] Ineffective communication between and among healthcare providers and patients is a leading cause of medical errors and patient harm. An analysis of sentinel events reported to The Joint Commission revealed that communication failure was the root cause in 59% of these events.[6]
The current climate of increasing healthcare complexity has prompted the need for adaptive innovation.[7] However, there are limited data describing interventions targeting improvements in both communication and transitional care planning. We created a new position, the patient navigator (PN), a dedicated patient‐care facilitator not responsible for clinical care. PNs were integrated into the inpatient multidisciplinary clinical team to facilitate patient and provider navigation through the complexity of a hospital admission by enhancing communication between and among patients and providers. The objective of this study was to determine whether this intervention would reduce hospital LOS and 30‐day unplanned readmissions.
METHODS
Setting
Mount Sinai Hospital is a 446‐bed acute care urban academic health center in Toronto, Ontario, Canada. The general internal medicine service operates as a 90‐bed clinical teaching unit physically distributed over 4 inpatient wards. The service is structurally divided into 4 nongeographically based multidisciplinary care teams (teams A, B, C, and D) comprised of the medical team (attending physician, senior resident physician, 23 junior resident physicians, and 23 medical students), pharmacist, social worker, physiotherapist, occupational therapist, speech and language pathologist, dietician, respiratory therapist, and nursing staff allocated by ward. Each team is on call approximately 1 night in 4 with no night float system. At our institution, attending physicians rotate on a 2‐ or 4‐week schedule, resident physicians rotate on a 1‐ or 2‐month schedule, and medical students rotate on a 2‐month schedule. Preintervention, communication occurred in person and by telephone between members of the medical team. Other members of the multidisciplinary care team communicated with the medical team in person at daily multidisciplinary rounds focused on discharge planning, by pager, or using a Web‐based communication tool.
Intervention
PNs were dedicated patient‐care facilitators not responsible for clinical care. They acted as liaisons between and among providers and patients. Each PN was a fully integrated member of their multidisciplinary care team. With ongoing medical team rotations, the PN was notably the only consistent member on the clinical team. Each patient saw the same PN throughout his or her hospital stay, as both the patient and the PN were team based. The average number of patients for whom each PN was responsible daily was dictated by the patient census for their team. On average, each team had a census between 20 and 30 patients daily. PNs worked during the daytime from Monday to Friday, and did not have any overnight or weekend responsibilities.
A PN's typical day began by reviewing and rounding on overnight admissions as a formal member of the clinical team. This was followed by participating in daily multidisciplinary rounds, then documenting and circulating the resultant action items. Thereafter, they expedited consultations and tests by liaising with departmental staff, and proactively established contact with the patient and their family. They answered simple factual questions related to test scheduling, consultations, diagnosis, medications, and treatments as discussed and outlined by the clinical team, and promptly relayed care questions beyond the scope of their knowledge to the clinical team. They were available to patients, family members, and providers via a dedicated mobile number using phone calls and text messages. If indicated, they assisted in discharge coordination by arranging follow‐up appointments and placing postdischarge phone calls. In addition, they served as primary contact for every patient admitted to their clinical team following discharge to ensure appropriate follow through on discharge plans. There were no set criteria for PNs to disengage from a patient's care. They could always be reached using their dedicated mobile number during business hours, with a voicemail system in place for after‐hours calls.
The role was filled by individuals skilled in communication and/or healthcare, such as registered nurses, a masters degreetrained educator, internationally trained physicians, and professionals from the hospitality and human resources industries. There were no prespecified training or degree requirements. Each PN underwent on‐the‐job training and participated in twice monthly PN meetings for ongoing feedback and education.
Program Implementation
We implemented the PN program on the inpatient general internal medicine service in June 2010 on 2 of 4 multidisciplinary clinical teams. Because a PN became an integrated member of 1 of 4 clinical teams, patient assignment to a PN was determined by the team to which the patient was admitted. On average, each of the 4 teams admitted equally on a daily basis. Initially, there were only sufficient resources to fund 2 PNs. Thus, from June 2010 to May 2011, only teams A and C were assigned PNs. To create fairness between the 4 teams, these 2 PNs moved to teams B and D from June 2011 to November 2011, and then back to teams A and C from December 2011 to April 2012. Following this initial pilot period, the program was allocated further resources, and so expanded to all 4 teams in May 2012. PN salaries were the only program costs. These costs were funded by matching donations from physicians within the Mount Sinai Hospital Department of Medicine and donations to the hospital from community members directed to support the implementation and evaluation of novel care delivery systems.
Study Design
We evaluated the PN program using a retrospective cohort study that included all general medical admissions between July 2010 and March 2014 matched by case mix group, age category, and resource intensity weight (a relative value measuring total patient resource use compared with average typical acute inpatients).[8]
Our primary outcomes were LOS and 30‐day readmission rate. These outcomes were stratified by exposure status to a PN. There were no exclusion criteria for the LOS analysis. Patients who died, were transferred to or from an acute care facility, or signed out against medical advice were excluded from the 30‐day readmission analysis. A secondary analysis restricted the timeframe from July 2010 to April 2011, when only 2 of 4 teams were exposed to PNs.
Average LOS has been observed to be higher in Canadian hospitals as compared to their US counterparts across different admission diagnoses, such as coronary artery bypass graft surgery and heart failure.[9, 10] We hypothesize that these differences are party due to systems‐level differences, including posthospital care. Specifically, the Canadian system does not utilize posthospital acute care, such as skilled nursing facilities, which may in part account for these differences. To help contextualize our data, we standardized LOS using an LOS index called the LOS/expected LOS (ELOS) ratio. It takes the LOS and divides it by the ELOS, a validated estimate of the expected LOS for a given patient generated using a national administrative database for acute hospital care in Canada that takes into account case mix group, age, comorbidity level, and intervention factors.[8]
Additionally, We performed an interrupted time‐series analysis, whereby a log‐linear model was fit on LOS and adjusted for weekly and monthly trends, age category, resource intensity weight, major clinical category (a surrogate for case mix group), admission location, and discharge location. The cohort was divided into 3 groups: before program implementation (July 2009June 2010), after program implementation with PN (July 2010March 2014), and after program implementation without PN (July 2010March 2014).
This study was approved by the research ethics board at Mount Sinai Hospital. No patient consent was deemed necessary. Data were obtained from institutional databases monitored by the hospital's performance measurement office.
Statistical Analysis
In Tables 1, 2, mean values were compared using a 2‐tailed t test, and the relationship between categorical groups was determined using a 2 test. For the interrupted time‐series analysis, 2‐tailed t tests were used to test null hypotheses of no association between the parameter value and the outcome, and 2 tests were used to test for the equivalence of 2 given parameters. P0.05 indicated statistical significance for all comparisons and analyses. All data were analyzed using Stata version 13 (StataCorp, College Station, TX) or R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
| With PN, n = 5,628 | Without PN, n = 2,213 | |
|---|---|---|
| ||
| Age, y, mean (SD)* | 69 (20) | 68 (20) |
| Female sex, n (%) | 3,018 (53.6) | 1,196 (54.0) |
| Most responsible diagnosis, n (%) | ||
| Pneumonia | 374 (6.6) | 135 (6.1) |
| Chronic obstructive pulmonary disease | 271 (4.8) | 88 (4.0) |
| Congestive heart failure | 217 (3.9) | 87 (3.9) |
| Admission location, n (%) | ||
| Home | 4,665 (82.9) | 1,943 (87.8) |
| Long‐term care* | 524 (9.3) | 158 (7.1) |
| Other* | 439 (7.8) | 112 (5.1) |
| Discharge location, n (%) | ||
| Home | 3,824 (67.9) | 1,578 (71.3) |
| Long‐term care | 779 (13.8) | 267 (12.1) |
| Other | 1,025 (18.3) | 368 (16.6) |
RESULTS
Our matched cohort included 7841 admissions (6141 patients), with 5628 admissions (4592 patients) exposed and 2213 admissions (1920 patients) not exposed to PNs. The discrepancy between the total number of patients and the sum of exposed and nonexposed patients is resultant from patients admitted more than once over the study period, as patients admitted to at least 1 team staffed with a PN and another team not staffed with a PN over the study period were counted in both groups. The 2 groups were similar with respect to several characteristics (Table 1). However, the 2 groups were significantly different for age (P = 0.046) and admissions from long‐term care (P < 0.01) and other facilities (P < 0.01).
Admissions with PNs were 1.3 days (21%) shorter than admission without PNs (6.2 vs 7.5 days, P < 0.001). Moreover, admissions with PNs had a smaller mean LOS/ELOS ratio compared to admissions without PNs (0.93 vs 1.05, P < 0.001). The restricted analysis found a 1.2‐day (18%) lower LOS (6.4 vs 7.6 days, P < 0.05) and a smaller mean LOS/ELOS ratio (0.91 vs 1.06, P < 0.001). Thirty‐day readmission rate was not different between the 2 groups (13.1 vs 13.8%, P = 0.48) or in the restricted analysis (12.0 vs 13.5%, P = 0.40) (Table 2).
| With PN | Without PN | P Value | |
|---|---|---|---|
| |||
| July 2010‐March 2014 | |||
| LOS, d (95% confidence interval) [n] | 6.2 (6.06.4) [5,628] | 7.5 (7.17.9) [2,213] | <0.001 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.93 (0.910.95) [5,628] | 1.05 (1.001.09) [2,213] | <0.001 |
| 30‐day readmission rate, % [n] | 13.1 [5,055] | 13.8 [2,012] | 0.48 |
| July 2010 to April 2011 | |||
| LOS, d (95% confidence interval) [n] | 6.4 (5.87.0) [713] | 7.6 (6.88.3) [753) | <0.05 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.91 (0.850.96) [713] | 1.06 (1.001.11) [753] | <0.001 |
| 30‐day readmission rate, % [n] | 12.0 [627] | 13.5 [681] | 0.40 |
In the interrupted time‐series analysis, prior to the implementation of the PN program, there was a positive relationship between LOS and time. After the implementation of the program, this relationship became inverse, meaning the curve plotting LOS against time had a negative slope. Furthermore, there was a statistically significant drop in LOS at the time of program implementation (P < 0.05). However, there was no difference in slope between the groups with and without PN after program implementation.
DISCUSSION
We describe an innovative inpatient intervention featuring an integrated patient‐care facilitator not responsible for clinical care charged with enhancing communication between and among patients and providers. Data from the almost 4‐year period demonstrated that implementation was associated with a 21% reduction in hospital LOS, with no difference in 30‐day readmission rates.
The patient navigator was first conceptualized in 1990 to help African American women in Harlem with breast cancer negotiate the complex world of oncology.[11] It was later implemented by the National Cancer Institute as an outpatient intervention spanning the continuum of cancer care. This concept has since expanded to other domains of complex single disease outpatient care, including asthma and fertility.[12, 13] To our knowledge, there has been limited evidence in the literature describing implementation of such programs in the inpatient general medical setting.
This study contributes to the growing literature on interventions targeting improvements in transitional care, such as transition coaches and discharge advocates.[14, 15] Balaban et al. recently described a PN intervention in the safety‐net population.[16] A common theme to these interventions was the prioritization of safe care transitions. However, this goal was achieved using related, yet different approaches: transition coaches focused on encouraging the patient and caregiver to assert a more active role,[14] discharge advocates focused on providing a comprehensive discharge plan for patients,[15] PNs from Balaban's study focused on coaching and assistance in navigating patients through the transition from hospital to home, and our study's PNs focused on enhancing communication between and among patients and providers. Additionally, unlike transition coaches and discharge advocates, who were nurses by training, and PNs from Balaban's study, who were community health workers, our PNs did not have any prespecified training or degree requirements.
Patients are at risk of being inadequately informed about important issues related to their care, such as hospital medications, diagnoses, and treatment plans during their hospital stay.[17, 18] Furthermore, we know that ineffective communication is a common cause of poor patient outcomes in hospital‐based care.[6] This phenomenon can be amplified from external pressures to maximize productivity. For example, Elliott and colleagues found that increasing hospitalist workload is associated with higher hospital LOS and cost.[19] PNs may offload care demands by enhancing communication for providers and patients.
Our study has several strengths. By matching admissions by case mix group, age category, and resource intensity weight, we aimed to reduce potential bias contributed by these covariates. Moreover, a staged rollout of the intervention, whereby over a 10‐month period, 2 of the multidisciplinary care teams were assigned PNs, while the remaining 2 were not, enabled contemporaneous comparison. Our study had few exclusion criteria, thus making it potentially generalizable to other inpatient general medicine settings of a similar nature. The relative simplicity of this intervention makes it amenable to scalability. Of note, the intervention has been deemed to show great promise at our institution, and has currently expanded to the cardiology, gastroenterology, and surgical oncology units.
Our study's limitations include a single‐center design. Moreover, although we demonstrate similarity in the majority of measurable covariates between the groups, we cannot exclude the existence of unmeasured confounders. Of the covariates that were found to be different between the groups, we suspect the difference in admissions from long‐term care and other facilities did not largely influence our study's main findings. Furthermore, though age was found to be statistically different between the groups, we postulate that the 1‐year difference between the groups is not particularly relevant clinically. Additionally, 30‐day readmission rates were only captured for our institution. However, the vast majority of readmissions in our region are to the index facility, and are unlikely to differ between the 2 groups.[20]
There may have been secular trends at play. In the interrupted time‐series analysis, there was a statistically significant drop in LOS at the time of program implementation. There was however, no difference in slope between the groups with and without PNs after program implementation. There are some plausible explanations for this lack of difference in slope. The study may not have been powered to detect such a difference, as this analysis was not prespecified. Furthermore, there may have been a spillover effect of the program, such that PNs may have improved efficiency for the teams to which they were assigned, thereby improving the efficiency of the other members of the multidisciplinary team, many of whom cared for patients assigned and not assigned a PN. Additionally, we measured the LOS in a preintervention control group between July 2009 and June 2010 using the same inclusion criteria as the matched cohort. It was found to be 8.5 days, which suggests a secular trend toward improvement in LOS over time at our institution. We are, however, reassured that our restricted analysis enabling contemporaneous comparison between patients exposed and not exposed to PNs was still found to be significant.
The implementation of this intervention could have implications for policymakers‐at‐large. Establishment of criteria for qualifications and a clear educational curriculum to train future PNs is needed, especially in the context of ongoing program expansion. These initiatives are currently underway at our institution. Furthermore, evaluation of the program's operating cost and calculation of its return on investment should include balanced metrics incorporating patient‐, provider‐, organizational‐, and system‐level measures. The current cost to the hospital per PN is approximately $73,800 CAD ($58,700 USD), which covers 1 PN's annual salary and benefits. Thus, the implementation of 4 PNs for each of the 4 multidisciplinary teams costs the hospital approximately $295,000 CAD ($234,700 USD) per year. Although the details of our preliminary calculations are outside the scope of this report, it suggests that the savings incurred from shorter LOS outweigh program costs.
We found that implementation of this innovative inpatient intervention targeting improvements in communication was associated with a reduction in LOS without an increase in 30‐day readmission. Our experience shows promise and may inform others considering similar interventions. Patient and provider experience and generalizability should be evaluated in future work.
Acknowledgements
The authors thank Dr. Allan Detsky and David Wells for their review of the manuscript. They are also grateful to Chin‐Chin Chua, Ningmei Wang, and Joann Bon in the Office of Quality and Performance Measurement for their help with data collection, and John Matelski for his help with data analysis.
Disclosure: This program was funded by matched donations from physicians in the Mount Sinai Hospital Department of Medicine and donations to Mount Sinai Hospital from community members directed to support the implementation and evaluation of novel care delivery systems. The authors report no conflicts of interest. Preliminary abstracts of this study were presented in the online forum, Leading Health Care Innovation, November 12, 2013 (
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(S3):391–395.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Prospective payment system and impairment at discharge. The “quicker‐and‐sicker” story revisited. JAMA. 1990;264(15):1980–1983.
- . The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13(suppl 1):i85–i90.
- The Joint Commission. Sentinel event data root: causes by event type (2004–June 2014). Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004‐2014.pdf. Accessed March 12, 2014.
- , . Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628.
- Canadian Institute for Health Information. Case mix. Available at: http://www.cihi.ca/CIHI‐ext‐portal/internet/EN/TabbedContent/standards+and+data+submission/standards/case+mix/cihi010690. Accessed April 12, 2015.
- , , , , , . Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med. 2005;165(13):1506–1513.
- , , , et al. Differences in treatment, outcomes, and quality of life among patients with heart failure in Canada and the United States. JACC Heart Fail. 2013;1(6):523–530.
- , , . Expanding access to cancer screening and clinical follow‐up among the medically underserved. Cancer Pract. 1995;3(1):19–30.
- , , , et al. Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma. 2010;47(8):913–919.
- . The role of a patient navigator in fertility preservation. In: Cancer Treatment and Research. Vol 156. Boston, MA: Springer US; 2010:469–470.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , , , . A patient navigator intervention to reduce hospital readmissions among high‐risk safety‐net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–915.
- , , . Lack of patient knowledge regarding hospital medications. J Hosp Med. 2010;5(2):83–86.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , , . Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(S3):391–395.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Prospective payment system and impairment at discharge. The “quicker‐and‐sicker” story revisited. JAMA. 1990;264(15):1980–1983.
- . The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13(suppl 1):i85–i90.
- The Joint Commission. Sentinel event data root: causes by event type (2004–June 2014). Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004‐2014.pdf. Accessed March 12, 2014.
- , . Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628.
- Canadian Institute for Health Information. Case mix. Available at: http://www.cihi.ca/CIHI‐ext‐portal/internet/EN/TabbedContent/standards+and+data+submission/standards/case+mix/cihi010690. Accessed April 12, 2015.
- , , , , , . Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med. 2005;165(13):1506–1513.
- , , , et al. Differences in treatment, outcomes, and quality of life among patients with heart failure in Canada and the United States. JACC Heart Fail. 2013;1(6):523–530.
- , , . Expanding access to cancer screening and clinical follow‐up among the medically underserved. Cancer Pract. 1995;3(1):19–30.
- , , , et al. Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma. 2010;47(8):913–919.
- . The role of a patient navigator in fertility preservation. In: Cancer Treatment and Research. Vol 156. Boston, MA: Springer US; 2010:469–470.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , , , . A patient navigator intervention to reduce hospital readmissions among high‐risk safety‐net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–915.
- , , . Lack of patient knowledge regarding hospital medications. J Hosp Med. 2010;5(2):83–86.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , , . Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
© 2015 Society of Hospital Medicine
Checklist of Safe Discharge Practices
The transition from hospital to home can expose patients to adverse events during the postdischarge period.[1, 2] Deficits in communication at hospital discharge are common,[3] and accurate information on important hospital events is often inadequately transmitted to outpatient providers, which may adversely affect patient outcomes.[4, 5, 6] Discharge bundles (multifaceted interventions including patient education, structured discharge planning, medication reconciliation, and follow‐up visits or phone calls) are strategies that provide support to patients returning home and facilitate transfer of information to primary‐care providers (PCPs).[7, 8, 9] These interventions collectively may improve patient satisfaction and possibly reduce rehospitalization.[10]
Beginning in 2012, the Centers for Medicare and Medicaid Services will be reducing payments to facilities with high rates of readmissions.[11] Thus, improving care transitions and thereby reducing avoidable readmissions are now priorities in many jurisdictions in the United States. There is a similar focus on readmission rates in the province of Ontario.[12] The Ontario Ministry of Health and Long‐Term Care convened an expert advisory panel with a mandate to provide guidance on evidence‐based practices that ensure efficient, effective, safe, and patient‐centered care transitions.[13] The objective of this study is to describe a structured panel approach to safe discharge practices, including a checklist of a recommended sequence of steps that can be followed throughout the hospital stay. This tool can aid efforts to optimize patient discharge from the hospital and improve outcomes.
METHODS
Literature Review
The research team reviewed the literature to determine the nature and format of the core information to be contained in a discharge checklist for hospitalized patients. We searched Medline (through January 2011) for relevant articles. We used combined Medical Subject Headings and keywords using patient discharge, transition, and medication reconciliation. Bibliographies of all relevant articles were reviewed to identify additional studies. In addition, we conducted a focused study of select resources, such as the systematic review examining interventions to reduce rehospitalization by Hansen and colleagues,[10] the Transitional Care Initiative for heart failure patients,[14] the Care Transitions Intervention,[15] Project RED (Re‐Engineered Hospital Discharge),[7] Project BOOST (Better Outcomes by Optimizing Safe Transitions),[16] and The King's Fund report on avoiding hospital admissions.[17] Available toolkit resources including those developed by the Commonwealth Fund in partnership with the Institute for Healthcare Improvement,[18] the World Health Organization,[19] and the Safer Healthcare Now![20] were examined in detail.
Consultation With Experts
The panel was composed of expert members from multiple disciplines and across several healthcare sectors, including PCPs, hospitalists, rehabilitation clinicians, nurses, researchers, pharmacists, academics, and hospital administrators. The aim was to create a discharge checklist to aid in transition planning based on best practices.
Checklist‐Development Process
An improvement consultant (N.Z.) facilitated the process (Figure 1). The results of the literature review were circulated prior to the first meeting. The panel met 3 times in person over a period of 3 months, from January 2011 to March 2011. At the first meeting, the panel reviewed existing toolkits and evidence‐based recommendations around best discharge practices. During the meeting, panel members were assigned to 1 of 6 groups (based on specialty area) and instructed to review toolkits and literature using a context‐specific lens (primary care, home care, follow‐up plans, communication to providers and caregivers, medication, and education). The goal of this exercise was to ensure that elements necessary for a successful discharge were viewed through the perspectives of interprofessional groups involved in the care of a patient. For example, PCPs in group 1 were asked to consider an ideal discharge from the perspective of primary care. Following the meeting, each group communicated via e‐mail to generate a list of evidence‐based items necessary for a safe discharge within the context of the group's assigned lens. Every group reached consensus on items specific to its context. A preliminary draft checklist was produced based on input from all groups. The checklist was created using recommended human‐factors engineering concepts.[21] The second meeting provided the opportunity for individual comments and feedback on the draft checklist. Three cycles of checklist revision followed by comments and feedback were conducted after the meeting, through e‐mail exchange. A final meeting provided consensus of the panel on every element of the Safe Discharge Practices Checklist.
RESULTS
Evidence‐based interventions pre‐, post‐, and bridging discharge were categorized into 7 domains: (1) indication for hospitalization, (2) primary care, (3) medication safety, (4) follow‐up plans, (5) home‐care referral, (6) communication with outpatient providers, and (7) patient education (Table 1). The panel reached 100% agreement on the recommended timeline to implement elements of the discharge checklist. Given the diverse interprofessional membership of the panel, it was felt that a daily reminder of tasks to be performed would provide the best format and have the highest likelihood of engaging team members in patient care coordination. It was also felt that daily interdisciplinary (ie, bullet) rounds would serve as the most appropriate venue to utilize the checklist tool.
| Day of Admission | Subsequent Hospital Days | Discharge Day | Discharge Day +3 | |
|---|---|---|---|---|
| ||||
| 1. Hospital | ||||
| a. Assess patient to see if hospitalization is still required. | ||||
| 2. Primary care | ||||
| a. Identify and/or confirm patient has an active PCP; alert care team if no PCP and/or begin PCP search. | ||||
| b. Contact PCP and notify of patient's admission, diagnosis, and predicted discharge date. | ||||
| c. Book postdischarge PCP follow‐up appointment within 714 days of discharge (according to patient/caregiver availability and transportation needs). | ||||
| 3. Medication safety | ||||
| a. Develop BPMH and reconcile this to admission's medication orders. | ||||
| b. Teach patient how to properly use discharge medications and how these relate to the medications patient was taking prior to admission. | ||||
| c. Reconcile discharge medication order/prescription with BPMH and medications prescribed while in hospital. | ||||
| 4. Follow‐up | ||||
| a.Perform postdischarge follow‐up phone call to patient (for patients with high LACE scoresa). During call, ask: | ||||
| Has patient received new meds (if any)? | ||||
| Has patient received home care? | ||||
| Remind patient of upcoming appointments. | ||||
| If necessary, schedule patient and caregiver to come back to facility for education and training. | ||||
| b. If necessary, arrange outpatient investigations (laboratory, radiology, etc.). | ||||
| c. If necessary, book specialty‐clinic follow‐up appointment. | ||||
| 5. Home care | ||||
| a. Home‐care agency shares information, where available, about patient's existing community services. | ||||
| b. Engage home‐care agencies (eg, interdisciplinary rounds). | ||||
| c. If necessary, schedule postdischarge care. | ||||
| 6. Communication | ||||
| a. Provide patient, community pharmacy, PCP, and formal caregiver (family, LTC, home‐care agency) with copy of Discharge Summary Plan/Note and the Medication Reconciliation Form, and contact information of attending hospital physician and inpatient unit. | ||||
| 7. Patient education | ||||
| a. Clinical team performs teach‐back to patient.b | ||||
| b. Explain to patient how new medications relate to diagnosis. | ||||
| c. Thoroughly explain discharge summary to patient (use teach‐back if needed). | ||||
| d. Explain potential symptoms, what to expect while at home, and under what circumstances patient should visit ED. | ||||
The panel chose daily reminders to perform patient education around medications and clinical care for several reasons. Daily teaching provides an opportunity to assess information carried over and accurate understanding of treatment plans, as well as to review changes in care plans that may be evolving during a hospitalization. Although education starting on day 1 of admission may seem premature, we felt there was merit in addressing issues early. For example, patients admitted with heart failure can benefit from daily inpatient education around self‐monitoring, diet, and lifestyle counseling.[22]
The literature review identified communication with PCPs as an important focus to prevent adverse events when patients transition from hospital to home.[3] The expert panel agreed on admission notification, follow‐up appointment scheduling, and transfer of a high‐quality discharge summary to the patient's PCP, such as one described by Maslove and colleagues.[23] For example, summaries containing structured sections such as relevant inpatient provider contacts, diagnoses, course in hospital, results of investigations (including pending results), discharge instructions and follow‐up, and medication reconciliation have been recommended to improve communication to outpatient providers.[3] Use of validated scores such as the LACE index (a score calculated based on 4 factors: [L] length of hospital stay, [A] acuity on admission, [C] comorbidity, and [E] emergency department visits) to identify patients at high risk of readmission and targeting these individuals when arranging postdischarge follow‐up is encouraged.[24, 25] Patients with high LACE scores (10) would benefit from postdischarge follow‐up phone calls within the first 3 days of returning home. In addition, high‐risk patients may require an earlier follow‐up appointment with the PCP, and the panel supports attempts to arrange follow‐up within 7 days for at‐risk individuals. For those without a PCP, it was recommended that a search should be initiated to assist the patient in obtaining a PCP.
Medication safety is a significant source of adverse events for patients returning home from the hospital.[2, 26, 27, 28] The discharge checklist provides prompts to reconcile medications on admission and discharge, in addition to daily patient education on proper use of medications. Formal medication reconciliation programs should be tailored to the individual hospital's own resources and requirements.[29, 30]
Postdischarge care plays an important role in supporting the patient upon discharge and when part of a multifaceted discharge plan can result in decreased readmission rates and hospital utilization.[7, 9, 15, 31] The panel incorporated these elements by recommending performing postdischarge phone calls, arranging outpatient follow‐up if necessary, and coordinating home‐care services through local agencies.
To facilitate transfer of information, patients, caregivers, outpatient providers, and community pharmacies are to be provided copies of a comprehensive discharge summary, medication reconciliation, and contact information of the inpatient team under the category of Communication. Finally, as the teach‐back method is an effective tool to ensure patient understanding of their health issues, the panel recommended its use when educating patients on medication use, plan of care, and discharge instructions.[32, 33] Examples of scenarios where teach‐back would be of benefit include changes in medications with a high risk of adverse events, such as warfarin or furosemide, to ensure patients understand the dosing, frequency, and monitoring required; and self‐management skills (eg, daily weights and dietary changes) in patients with heart failure.
Finally, the panel noted that it was important to link the checklist items with relevant measures, audit, and feedback to determine associations between process and outcomes. The group avoided specific detailed recommendations to allow each institution to locally tailor appropriate process and outcome measures to assess fidelity of each component of the checklist.
DISCUSSION
A standardized, evidence‐based discharge process is critical to safe transitions for the hospitalized patient. We have used a consensus process of stakeholders to develop a Checklist of Safe Discharge Practices for Hospital Patients that details the steps of events that need to be completed for every day of a typical hospitalization. The day of discharge is often a confusing and chaotic time, with patients receiving an overwhelming volume of information on their last day in the hospital. We believe that discharge planning starts from the day of admission with daily patient education and a coordinated interdisciplinary team approach. The components of the discharge checklist should be completed throughout a patient's hospitalization to ensure a successful discharge and transmission of knowledge.
Discharge checklists have been described previously. Halasyamani and colleagues developed a checklist for elderly inpatients created through a process of literature and peer review followed by a panel discussion at the Society of Hospital Medicine Annual Meeting.[34] The resultant tool described important data elements necessary for a successful discharge and which processes were most appropriate to facilitate the transfer of information. This differs significantly from our discharge checklist, which provides specific recommendations on methods and processes to effect a safe discharge in addition to an expected timeline of when to complete each step. Kripalani et al reviewed the literature for suggested methods of promoting effective transitions of care at discharge, and their results are consistent with those summarized in our discharge checklist.[29] In contrast to both efforts, our group was multidisciplinary and had broad representation from the acute care, chronic care, home care, rehabilitation medicine, and long‐term care sectors, thereby incorporating all possible aspects of the transition process. Coordinating discharge care requires significant teamwork; our tool extends beyond a checklist of tasks to be performed, and rather serves as a platform to facilitate interprofessional collaboration. In addition, this checklist was designed to integrate discharge planning into interprofessional care rounds occurring throughout a hospital admission. As well, our paper follows an explicit and defined consensus process. Finally, our proposed tool better follows a recommended checklist format.[21]
The discharge process occurring during a patient's hospitalization is a complex, multifaceted care‐coordination plan that must begin on the first day of admission. Often, transfer of important information and medication review take place only hours before a patient leaves the hospital, a suboptimal time for patient education.[28, 35] Just as standardized treatment protocols and care plans can improve outcomes,[36] a similar approach for discharge processes may facilitate safe transition from hospital to home. Our discharge checklist prompts hospital providers to initiate steps necessary for a successful discharge while allowing for local adaptation in how each element is performed. We suggest using the checklist during daily interprofessional team rounds to ensure each task is completed, if appropriate. Institutions may consider measuring process measures such as adherence and completion of checklist, audits of discharge summaries for completion and transmission rates to PCPs (by fax or through health record departments), and documentation of patient education or medication reconciliation. Example outcome measures, if feasible, include Care Transitions Measure (CTM) scores, patient satisfaction surveys, and readmission rates.
Several limitations of this study should be considered. First, current literature on safe discharge practices is limited by low study‐design quality, with a paucity of randomized controlled trials. However, a recent systematic review found that bundled discharge interventions are likely to be most effective.[10] Individual items of the checklist may not have had an extensive evidence base; however, some of these suggested elements (eg, contact home care) have clinical face validity. Second, the heterogeneity of interventions studied pose challenges in determining generalizable best practices without considering local factors. To mitigate this, we suggest adapting the checklist to local contexts and resource availability. Third, the checklist has not been tested. The next step of this project is to pilot checklist use through small‐scale Plan‐Do‐Study‐Act (PDSA) cycles followed by large‐scale implementation. We plan to collect baseline, process, and outcome measures before and after implementation of the checklist at multiple institutions to determine utility.
Standardization of discharge practices is critical to safe transitions and preventing avoidable admissions to hospital. Our discharge checklist is an expanded tool that provides explicit guidance for each day of hospitalization and can be adapted for any hospital admission to aid interdisciplinary efforts toward a successful discharge. Future studies to evaluate the checklist in improving care‐transition processes are required to determine association with outcomes.
Disclosures
Nothing to report.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–128.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , , . “I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223–2228.
- , . Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533–536.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. A Quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc. 57(9):1540–1546.
- , , , et al. Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211–218.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- Centers for Medicare and Medicaid Services. Readmissions reduction program. Available at: http://cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/AcuteInpatientPPS/Readmissions‐Reduction‐Program.html.Accessed September 5, 2012.
- Ontario Ministry of Health and Long‐Term Care. The Excellent Care for All Act, 2010. Available at: http://health.gov.on.ca/en/public/programs/ecfa/default.aspx/. Accessed February 28, 2013.
- Ontario Ministry of Health and Long‐Term Care; Baker GR, ed. Enhancing the Continuum of Care: Report of the Avoidable Hospitalization Advisory Panel, November 2011. Available at: http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Accessed August 8, 2012.
- , , , , , . Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial [published correction appears in J Am Geriatr Soc. 2004;52(7):1228]. J Am Geriatr Soc. 2004;52(5):675–684.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- Society of Hospital Medicine. Project BOOST: Better Outcomes by Optimizing Safe Transitions. Available at: http://www.hospitalmedicine.org/BOOST/. Accessed October 31, 2012.
- The King's Fund; , , . Avoiding Hospital Admissions: Lessons From Evidence and Experience. Available at: http://www.kingsfund.org.uk/publications/articles/avoiding‐hospital‐admissions‐lessons‐evidence. Published October 28, 2010. Accessed September 4, 2012.
- Nielsen GA, Rutherford P, Taylor J, eds. How‐To Guide: Creating an Ideal Transition Home. Cambridge, MA: Institute for Healthcare Improvement; 2009. Available at: http://www.ihi.org or http://ah.cms‐plus.com/files/IHI_How_to_Guide_Creating_an_Ideal_Transition_Home.pdf. Accessed August 8, 2012.
- World Health Organization. Action on Patient Safety—High 5s. Available at: http://www.who.int/patientsafety/implementation/solutions/high5s/en/index.html. Accessed October 29, 2012.
- Safer Healthcare Now! Medication Reconciliation in Acute Care: Getting Started Kit. Available at: http://www.ismp‐canada.org/download/MedRec/Medrec_AC_English_GSK_V3.pdf. Accessed October 29, 2012.
- US Agency for Healthcare Research and Quality. PSNet: Patient‐safety primers, checklists. Available at: http://www.psnet.ahrq.gov/primer.aspx?primerID=14. Accessed November 1, 2012.
- , , , . Benefits of comprehensive inpatient education and discharge planning combined with outpatient support in elderly patients with congestive heart failure. Congest Heart Fail. 2005;11(6):315–321.
- , , , et al. Electronic versus dictated hospital discharge summaries: a randomized controlled trial. J Gen Intern Med. 2009;24(9):995–1001.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- . Will, ideas, and execution: their role in reducing adverse medication events. J Pediatr. 2005;147(6):727–728.
- , , , . Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014–2018.
- , , , et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , . Medication reconciliation in the hospital. Healthc Q. 2012;15:42–49.
- , , , , , . Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial. Ann Intern Med. 1994;120(12):999–1006.
- , , , et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163(1):83–90.
- , , . The effects of patient communication skills training on compliance. Arch Fam Med. 2000;9(1):57–64.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354–360.
- , , , , . Influence of a “discharge interview” on patient knowledge, compliance, and functional status after hospitalization. Med Care. 1983;21(8):755–767.
- , , , , . Critical pathways intervention to reduce length of hospital stay. Am J Med. 2001;110(3):175–180.
The transition from hospital to home can expose patients to adverse events during the postdischarge period.[1, 2] Deficits in communication at hospital discharge are common,[3] and accurate information on important hospital events is often inadequately transmitted to outpatient providers, which may adversely affect patient outcomes.[4, 5, 6] Discharge bundles (multifaceted interventions including patient education, structured discharge planning, medication reconciliation, and follow‐up visits or phone calls) are strategies that provide support to patients returning home and facilitate transfer of information to primary‐care providers (PCPs).[7, 8, 9] These interventions collectively may improve patient satisfaction and possibly reduce rehospitalization.[10]
Beginning in 2012, the Centers for Medicare and Medicaid Services will be reducing payments to facilities with high rates of readmissions.[11] Thus, improving care transitions and thereby reducing avoidable readmissions are now priorities in many jurisdictions in the United States. There is a similar focus on readmission rates in the province of Ontario.[12] The Ontario Ministry of Health and Long‐Term Care convened an expert advisory panel with a mandate to provide guidance on evidence‐based practices that ensure efficient, effective, safe, and patient‐centered care transitions.[13] The objective of this study is to describe a structured panel approach to safe discharge practices, including a checklist of a recommended sequence of steps that can be followed throughout the hospital stay. This tool can aid efforts to optimize patient discharge from the hospital and improve outcomes.
METHODS
Literature Review
The research team reviewed the literature to determine the nature and format of the core information to be contained in a discharge checklist for hospitalized patients. We searched Medline (through January 2011) for relevant articles. We used combined Medical Subject Headings and keywords using patient discharge, transition, and medication reconciliation. Bibliographies of all relevant articles were reviewed to identify additional studies. In addition, we conducted a focused study of select resources, such as the systematic review examining interventions to reduce rehospitalization by Hansen and colleagues,[10] the Transitional Care Initiative for heart failure patients,[14] the Care Transitions Intervention,[15] Project RED (Re‐Engineered Hospital Discharge),[7] Project BOOST (Better Outcomes by Optimizing Safe Transitions),[16] and The King's Fund report on avoiding hospital admissions.[17] Available toolkit resources including those developed by the Commonwealth Fund in partnership with the Institute for Healthcare Improvement,[18] the World Health Organization,[19] and the Safer Healthcare Now![20] were examined in detail.
Consultation With Experts
The panel was composed of expert members from multiple disciplines and across several healthcare sectors, including PCPs, hospitalists, rehabilitation clinicians, nurses, researchers, pharmacists, academics, and hospital administrators. The aim was to create a discharge checklist to aid in transition planning based on best practices.
Checklist‐Development Process
An improvement consultant (N.Z.) facilitated the process (Figure 1). The results of the literature review were circulated prior to the first meeting. The panel met 3 times in person over a period of 3 months, from January 2011 to March 2011. At the first meeting, the panel reviewed existing toolkits and evidence‐based recommendations around best discharge practices. During the meeting, panel members were assigned to 1 of 6 groups (based on specialty area) and instructed to review toolkits and literature using a context‐specific lens (primary care, home care, follow‐up plans, communication to providers and caregivers, medication, and education). The goal of this exercise was to ensure that elements necessary for a successful discharge were viewed through the perspectives of interprofessional groups involved in the care of a patient. For example, PCPs in group 1 were asked to consider an ideal discharge from the perspective of primary care. Following the meeting, each group communicated via e‐mail to generate a list of evidence‐based items necessary for a safe discharge within the context of the group's assigned lens. Every group reached consensus on items specific to its context. A preliminary draft checklist was produced based on input from all groups. The checklist was created using recommended human‐factors engineering concepts.[21] The second meeting provided the opportunity for individual comments and feedback on the draft checklist. Three cycles of checklist revision followed by comments and feedback were conducted after the meeting, through e‐mail exchange. A final meeting provided consensus of the panel on every element of the Safe Discharge Practices Checklist.

RESULTS
Evidence‐based interventions pre‐, post‐, and bridging discharge were categorized into 7 domains: (1) indication for hospitalization, (2) primary care, (3) medication safety, (4) follow‐up plans, (5) home‐care referral, (6) communication with outpatient providers, and (7) patient education (Table 1). The panel reached 100% agreement on the recommended timeline to implement elements of the discharge checklist. Given the diverse interprofessional membership of the panel, it was felt that a daily reminder of tasks to be performed would provide the best format and have the highest likelihood of engaging team members in patient care coordination. It was also felt that daily interdisciplinary (ie, bullet) rounds would serve as the most appropriate venue to utilize the checklist tool.
| Day of Admission | Subsequent Hospital Days | Discharge Day | Discharge Day +3 | |
|---|---|---|---|---|
| ||||
| 1. Hospital | ||||
| a. Assess patient to see if hospitalization is still required. | ||||
| 2. Primary care | ||||
| a. Identify and/or confirm patient has an active PCP; alert care team if no PCP and/or begin PCP search. | ||||
| b. Contact PCP and notify of patient's admission, diagnosis, and predicted discharge date. | ||||
| c. Book postdischarge PCP follow‐up appointment within 714 days of discharge (according to patient/caregiver availability and transportation needs). | ||||
| 3. Medication safety | ||||
| a. Develop BPMH and reconcile this to admission's medication orders. | ||||
| b. Teach patient how to properly use discharge medications and how these relate to the medications patient was taking prior to admission. | ||||
| c. Reconcile discharge medication order/prescription with BPMH and medications prescribed while in hospital. | ||||
| 4. Follow‐up | ||||
| a.Perform postdischarge follow‐up phone call to patient (for patients with high LACE scoresa). During call, ask: | ||||
| Has patient received new meds (if any)? | ||||
| Has patient received home care? | ||||
| Remind patient of upcoming appointments. | ||||
| If necessary, schedule patient and caregiver to come back to facility for education and training. | ||||
| b. If necessary, arrange outpatient investigations (laboratory, radiology, etc.). | ||||
| c. If necessary, book specialty‐clinic follow‐up appointment. | ||||
| 5. Home care | ||||
| a. Home‐care agency shares information, where available, about patient's existing community services. | ||||
| b. Engage home‐care agencies (eg, interdisciplinary rounds). | ||||
| c. If necessary, schedule postdischarge care. | ||||
| 6. Communication | ||||
| a. Provide patient, community pharmacy, PCP, and formal caregiver (family, LTC, home‐care agency) with copy of Discharge Summary Plan/Note and the Medication Reconciliation Form, and contact information of attending hospital physician and inpatient unit. | ||||
| 7. Patient education | ||||
| a. Clinical team performs teach‐back to patient.b | ||||
| b. Explain to patient how new medications relate to diagnosis. | ||||
| c. Thoroughly explain discharge summary to patient (use teach‐back if needed). | ||||
| d. Explain potential symptoms, what to expect while at home, and under what circumstances patient should visit ED. | ||||
The panel chose daily reminders to perform patient education around medications and clinical care for several reasons. Daily teaching provides an opportunity to assess information carried over and accurate understanding of treatment plans, as well as to review changes in care plans that may be evolving during a hospitalization. Although education starting on day 1 of admission may seem premature, we felt there was merit in addressing issues early. For example, patients admitted with heart failure can benefit from daily inpatient education around self‐monitoring, diet, and lifestyle counseling.[22]
The literature review identified communication with PCPs as an important focus to prevent adverse events when patients transition from hospital to home.[3] The expert panel agreed on admission notification, follow‐up appointment scheduling, and transfer of a high‐quality discharge summary to the patient's PCP, such as one described by Maslove and colleagues.[23] For example, summaries containing structured sections such as relevant inpatient provider contacts, diagnoses, course in hospital, results of investigations (including pending results), discharge instructions and follow‐up, and medication reconciliation have been recommended to improve communication to outpatient providers.[3] Use of validated scores such as the LACE index (a score calculated based on 4 factors: [L] length of hospital stay, [A] acuity on admission, [C] comorbidity, and [E] emergency department visits) to identify patients at high risk of readmission and targeting these individuals when arranging postdischarge follow‐up is encouraged.[24, 25] Patients with high LACE scores (10) would benefit from postdischarge follow‐up phone calls within the first 3 days of returning home. In addition, high‐risk patients may require an earlier follow‐up appointment with the PCP, and the panel supports attempts to arrange follow‐up within 7 days for at‐risk individuals. For those without a PCP, it was recommended that a search should be initiated to assist the patient in obtaining a PCP.
Medication safety is a significant source of adverse events for patients returning home from the hospital.[2, 26, 27, 28] The discharge checklist provides prompts to reconcile medications on admission and discharge, in addition to daily patient education on proper use of medications. Formal medication reconciliation programs should be tailored to the individual hospital's own resources and requirements.[29, 30]
Postdischarge care plays an important role in supporting the patient upon discharge and when part of a multifaceted discharge plan can result in decreased readmission rates and hospital utilization.[7, 9, 15, 31] The panel incorporated these elements by recommending performing postdischarge phone calls, arranging outpatient follow‐up if necessary, and coordinating home‐care services through local agencies.
To facilitate transfer of information, patients, caregivers, outpatient providers, and community pharmacies are to be provided copies of a comprehensive discharge summary, medication reconciliation, and contact information of the inpatient team under the category of Communication. Finally, as the teach‐back method is an effective tool to ensure patient understanding of their health issues, the panel recommended its use when educating patients on medication use, plan of care, and discharge instructions.[32, 33] Examples of scenarios where teach‐back would be of benefit include changes in medications with a high risk of adverse events, such as warfarin or furosemide, to ensure patients understand the dosing, frequency, and monitoring required; and self‐management skills (eg, daily weights and dietary changes) in patients with heart failure.
Finally, the panel noted that it was important to link the checklist items with relevant measures, audit, and feedback to determine associations between process and outcomes. The group avoided specific detailed recommendations to allow each institution to locally tailor appropriate process and outcome measures to assess fidelity of each component of the checklist.
DISCUSSION
A standardized, evidence‐based discharge process is critical to safe transitions for the hospitalized patient. We have used a consensus process of stakeholders to develop a Checklist of Safe Discharge Practices for Hospital Patients that details the steps of events that need to be completed for every day of a typical hospitalization. The day of discharge is often a confusing and chaotic time, with patients receiving an overwhelming volume of information on their last day in the hospital. We believe that discharge planning starts from the day of admission with daily patient education and a coordinated interdisciplinary team approach. The components of the discharge checklist should be completed throughout a patient's hospitalization to ensure a successful discharge and transmission of knowledge.
Discharge checklists have been described previously. Halasyamani and colleagues developed a checklist for elderly inpatients created through a process of literature and peer review followed by a panel discussion at the Society of Hospital Medicine Annual Meeting.[34] The resultant tool described important data elements necessary for a successful discharge and which processes were most appropriate to facilitate the transfer of information. This differs significantly from our discharge checklist, which provides specific recommendations on methods and processes to effect a safe discharge in addition to an expected timeline of when to complete each step. Kripalani et al reviewed the literature for suggested methods of promoting effective transitions of care at discharge, and their results are consistent with those summarized in our discharge checklist.[29] In contrast to both efforts, our group was multidisciplinary and had broad representation from the acute care, chronic care, home care, rehabilitation medicine, and long‐term care sectors, thereby incorporating all possible aspects of the transition process. Coordinating discharge care requires significant teamwork; our tool extends beyond a checklist of tasks to be performed, and rather serves as a platform to facilitate interprofessional collaboration. In addition, this checklist was designed to integrate discharge planning into interprofessional care rounds occurring throughout a hospital admission. As well, our paper follows an explicit and defined consensus process. Finally, our proposed tool better follows a recommended checklist format.[21]
The discharge process occurring during a patient's hospitalization is a complex, multifaceted care‐coordination plan that must begin on the first day of admission. Often, transfer of important information and medication review take place only hours before a patient leaves the hospital, a suboptimal time for patient education.[28, 35] Just as standardized treatment protocols and care plans can improve outcomes,[36] a similar approach for discharge processes may facilitate safe transition from hospital to home. Our discharge checklist prompts hospital providers to initiate steps necessary for a successful discharge while allowing for local adaptation in how each element is performed. We suggest using the checklist during daily interprofessional team rounds to ensure each task is completed, if appropriate. Institutions may consider measuring process measures such as adherence and completion of checklist, audits of discharge summaries for completion and transmission rates to PCPs (by fax or through health record departments), and documentation of patient education or medication reconciliation. Example outcome measures, if feasible, include Care Transitions Measure (CTM) scores, patient satisfaction surveys, and readmission rates.
Several limitations of this study should be considered. First, current literature on safe discharge practices is limited by low study‐design quality, with a paucity of randomized controlled trials. However, a recent systematic review found that bundled discharge interventions are likely to be most effective.[10] Individual items of the checklist may not have had an extensive evidence base; however, some of these suggested elements (eg, contact home care) have clinical face validity. Second, the heterogeneity of interventions studied pose challenges in determining generalizable best practices without considering local factors. To mitigate this, we suggest adapting the checklist to local contexts and resource availability. Third, the checklist has not been tested. The next step of this project is to pilot checklist use through small‐scale Plan‐Do‐Study‐Act (PDSA) cycles followed by large‐scale implementation. We plan to collect baseline, process, and outcome measures before and after implementation of the checklist at multiple institutions to determine utility.
Standardization of discharge practices is critical to safe transitions and preventing avoidable admissions to hospital. Our discharge checklist is an expanded tool that provides explicit guidance for each day of hospitalization and can be adapted for any hospital admission to aid interdisciplinary efforts toward a successful discharge. Future studies to evaluate the checklist in improving care‐transition processes are required to determine association with outcomes.
Disclosures
Nothing to report.
The transition from hospital to home can expose patients to adverse events during the postdischarge period.[1, 2] Deficits in communication at hospital discharge are common,[3] and accurate information on important hospital events is often inadequately transmitted to outpatient providers, which may adversely affect patient outcomes.[4, 5, 6] Discharge bundles (multifaceted interventions including patient education, structured discharge planning, medication reconciliation, and follow‐up visits or phone calls) are strategies that provide support to patients returning home and facilitate transfer of information to primary‐care providers (PCPs).[7, 8, 9] These interventions collectively may improve patient satisfaction and possibly reduce rehospitalization.[10]
Beginning in 2012, the Centers for Medicare and Medicaid Services will be reducing payments to facilities with high rates of readmissions.[11] Thus, improving care transitions and thereby reducing avoidable readmissions are now priorities in many jurisdictions in the United States. There is a similar focus on readmission rates in the province of Ontario.[12] The Ontario Ministry of Health and Long‐Term Care convened an expert advisory panel with a mandate to provide guidance on evidence‐based practices that ensure efficient, effective, safe, and patient‐centered care transitions.[13] The objective of this study is to describe a structured panel approach to safe discharge practices, including a checklist of a recommended sequence of steps that can be followed throughout the hospital stay. This tool can aid efforts to optimize patient discharge from the hospital and improve outcomes.
METHODS
Literature Review
The research team reviewed the literature to determine the nature and format of the core information to be contained in a discharge checklist for hospitalized patients. We searched Medline (through January 2011) for relevant articles. We used combined Medical Subject Headings and keywords using patient discharge, transition, and medication reconciliation. Bibliographies of all relevant articles were reviewed to identify additional studies. In addition, we conducted a focused study of select resources, such as the systematic review examining interventions to reduce rehospitalization by Hansen and colleagues,[10] the Transitional Care Initiative for heart failure patients,[14] the Care Transitions Intervention,[15] Project RED (Re‐Engineered Hospital Discharge),[7] Project BOOST (Better Outcomes by Optimizing Safe Transitions),[16] and The King's Fund report on avoiding hospital admissions.[17] Available toolkit resources including those developed by the Commonwealth Fund in partnership with the Institute for Healthcare Improvement,[18] the World Health Organization,[19] and the Safer Healthcare Now![20] were examined in detail.
Consultation With Experts
The panel was composed of expert members from multiple disciplines and across several healthcare sectors, including PCPs, hospitalists, rehabilitation clinicians, nurses, researchers, pharmacists, academics, and hospital administrators. The aim was to create a discharge checklist to aid in transition planning based on best practices.
Checklist‐Development Process
An improvement consultant (N.Z.) facilitated the process (Figure 1). The results of the literature review were circulated prior to the first meeting. The panel met 3 times in person over a period of 3 months, from January 2011 to March 2011. At the first meeting, the panel reviewed existing toolkits and evidence‐based recommendations around best discharge practices. During the meeting, panel members were assigned to 1 of 6 groups (based on specialty area) and instructed to review toolkits and literature using a context‐specific lens (primary care, home care, follow‐up plans, communication to providers and caregivers, medication, and education). The goal of this exercise was to ensure that elements necessary for a successful discharge were viewed through the perspectives of interprofessional groups involved in the care of a patient. For example, PCPs in group 1 were asked to consider an ideal discharge from the perspective of primary care. Following the meeting, each group communicated via e‐mail to generate a list of evidence‐based items necessary for a safe discharge within the context of the group's assigned lens. Every group reached consensus on items specific to its context. A preliminary draft checklist was produced based on input from all groups. The checklist was created using recommended human‐factors engineering concepts.[21] The second meeting provided the opportunity for individual comments and feedback on the draft checklist. Three cycles of checklist revision followed by comments and feedback were conducted after the meeting, through e‐mail exchange. A final meeting provided consensus of the panel on every element of the Safe Discharge Practices Checklist.

RESULTS
Evidence‐based interventions pre‐, post‐, and bridging discharge were categorized into 7 domains: (1) indication for hospitalization, (2) primary care, (3) medication safety, (4) follow‐up plans, (5) home‐care referral, (6) communication with outpatient providers, and (7) patient education (Table 1). The panel reached 100% agreement on the recommended timeline to implement elements of the discharge checklist. Given the diverse interprofessional membership of the panel, it was felt that a daily reminder of tasks to be performed would provide the best format and have the highest likelihood of engaging team members in patient care coordination. It was also felt that daily interdisciplinary (ie, bullet) rounds would serve as the most appropriate venue to utilize the checklist tool.
| Day of Admission | Subsequent Hospital Days | Discharge Day | Discharge Day +3 | |
|---|---|---|---|---|
| ||||
| 1. Hospital | ||||
| a. Assess patient to see if hospitalization is still required. | ||||
| 2. Primary care | ||||
| a. Identify and/or confirm patient has an active PCP; alert care team if no PCP and/or begin PCP search. | ||||
| b. Contact PCP and notify of patient's admission, diagnosis, and predicted discharge date. | ||||
| c. Book postdischarge PCP follow‐up appointment within 714 days of discharge (according to patient/caregiver availability and transportation needs). | ||||
| 3. Medication safety | ||||
| a. Develop BPMH and reconcile this to admission's medication orders. | ||||
| b. Teach patient how to properly use discharge medications and how these relate to the medications patient was taking prior to admission. | ||||
| c. Reconcile discharge medication order/prescription with BPMH and medications prescribed while in hospital. | ||||
| 4. Follow‐up | ||||
| a.Perform postdischarge follow‐up phone call to patient (for patients with high LACE scoresa). During call, ask: | ||||
| Has patient received new meds (if any)? | ||||
| Has patient received home care? | ||||
| Remind patient of upcoming appointments. | ||||
| If necessary, schedule patient and caregiver to come back to facility for education and training. | ||||
| b. If necessary, arrange outpatient investigations (laboratory, radiology, etc.). | ||||
| c. If necessary, book specialty‐clinic follow‐up appointment. | ||||
| 5. Home care | ||||
| a. Home‐care agency shares information, where available, about patient's existing community services. | ||||
| b. Engage home‐care agencies (eg, interdisciplinary rounds). | ||||
| c. If necessary, schedule postdischarge care. | ||||
| 6. Communication | ||||
| a. Provide patient, community pharmacy, PCP, and formal caregiver (family, LTC, home‐care agency) with copy of Discharge Summary Plan/Note and the Medication Reconciliation Form, and contact information of attending hospital physician and inpatient unit. | ||||
| 7. Patient education | ||||
| a. Clinical team performs teach‐back to patient.b | ||||
| b. Explain to patient how new medications relate to diagnosis. | ||||
| c. Thoroughly explain discharge summary to patient (use teach‐back if needed). | ||||
| d. Explain potential symptoms, what to expect while at home, and under what circumstances patient should visit ED. | ||||
The panel chose daily reminders to perform patient education around medications and clinical care for several reasons. Daily teaching provides an opportunity to assess information carried over and accurate understanding of treatment plans, as well as to review changes in care plans that may be evolving during a hospitalization. Although education starting on day 1 of admission may seem premature, we felt there was merit in addressing issues early. For example, patients admitted with heart failure can benefit from daily inpatient education around self‐monitoring, diet, and lifestyle counseling.[22]
The literature review identified communication with PCPs as an important focus to prevent adverse events when patients transition from hospital to home.[3] The expert panel agreed on admission notification, follow‐up appointment scheduling, and transfer of a high‐quality discharge summary to the patient's PCP, such as one described by Maslove and colleagues.[23] For example, summaries containing structured sections such as relevant inpatient provider contacts, diagnoses, course in hospital, results of investigations (including pending results), discharge instructions and follow‐up, and medication reconciliation have been recommended to improve communication to outpatient providers.[3] Use of validated scores such as the LACE index (a score calculated based on 4 factors: [L] length of hospital stay, [A] acuity on admission, [C] comorbidity, and [E] emergency department visits) to identify patients at high risk of readmission and targeting these individuals when arranging postdischarge follow‐up is encouraged.[24, 25] Patients with high LACE scores (10) would benefit from postdischarge follow‐up phone calls within the first 3 days of returning home. In addition, high‐risk patients may require an earlier follow‐up appointment with the PCP, and the panel supports attempts to arrange follow‐up within 7 days for at‐risk individuals. For those without a PCP, it was recommended that a search should be initiated to assist the patient in obtaining a PCP.
Medication safety is a significant source of adverse events for patients returning home from the hospital.[2, 26, 27, 28] The discharge checklist provides prompts to reconcile medications on admission and discharge, in addition to daily patient education on proper use of medications. Formal medication reconciliation programs should be tailored to the individual hospital's own resources and requirements.[29, 30]
Postdischarge care plays an important role in supporting the patient upon discharge and when part of a multifaceted discharge plan can result in decreased readmission rates and hospital utilization.[7, 9, 15, 31] The panel incorporated these elements by recommending performing postdischarge phone calls, arranging outpatient follow‐up if necessary, and coordinating home‐care services through local agencies.
To facilitate transfer of information, patients, caregivers, outpatient providers, and community pharmacies are to be provided copies of a comprehensive discharge summary, medication reconciliation, and contact information of the inpatient team under the category of Communication. Finally, as the teach‐back method is an effective tool to ensure patient understanding of their health issues, the panel recommended its use when educating patients on medication use, plan of care, and discharge instructions.[32, 33] Examples of scenarios where teach‐back would be of benefit include changes in medications with a high risk of adverse events, such as warfarin or furosemide, to ensure patients understand the dosing, frequency, and monitoring required; and self‐management skills (eg, daily weights and dietary changes) in patients with heart failure.
Finally, the panel noted that it was important to link the checklist items with relevant measures, audit, and feedback to determine associations between process and outcomes. The group avoided specific detailed recommendations to allow each institution to locally tailor appropriate process and outcome measures to assess fidelity of each component of the checklist.
DISCUSSION
A standardized, evidence‐based discharge process is critical to safe transitions for the hospitalized patient. We have used a consensus process of stakeholders to develop a Checklist of Safe Discharge Practices for Hospital Patients that details the steps of events that need to be completed for every day of a typical hospitalization. The day of discharge is often a confusing and chaotic time, with patients receiving an overwhelming volume of information on their last day in the hospital. We believe that discharge planning starts from the day of admission with daily patient education and a coordinated interdisciplinary team approach. The components of the discharge checklist should be completed throughout a patient's hospitalization to ensure a successful discharge and transmission of knowledge.
Discharge checklists have been described previously. Halasyamani and colleagues developed a checklist for elderly inpatients created through a process of literature and peer review followed by a panel discussion at the Society of Hospital Medicine Annual Meeting.[34] The resultant tool described important data elements necessary for a successful discharge and which processes were most appropriate to facilitate the transfer of information. This differs significantly from our discharge checklist, which provides specific recommendations on methods and processes to effect a safe discharge in addition to an expected timeline of when to complete each step. Kripalani et al reviewed the literature for suggested methods of promoting effective transitions of care at discharge, and their results are consistent with those summarized in our discharge checklist.[29] In contrast to both efforts, our group was multidisciplinary and had broad representation from the acute care, chronic care, home care, rehabilitation medicine, and long‐term care sectors, thereby incorporating all possible aspects of the transition process. Coordinating discharge care requires significant teamwork; our tool extends beyond a checklist of tasks to be performed, and rather serves as a platform to facilitate interprofessional collaboration. In addition, this checklist was designed to integrate discharge planning into interprofessional care rounds occurring throughout a hospital admission. As well, our paper follows an explicit and defined consensus process. Finally, our proposed tool better follows a recommended checklist format.[21]
The discharge process occurring during a patient's hospitalization is a complex, multifaceted care‐coordination plan that must begin on the first day of admission. Often, transfer of important information and medication review take place only hours before a patient leaves the hospital, a suboptimal time for patient education.[28, 35] Just as standardized treatment protocols and care plans can improve outcomes,[36] a similar approach for discharge processes may facilitate safe transition from hospital to home. Our discharge checklist prompts hospital providers to initiate steps necessary for a successful discharge while allowing for local adaptation in how each element is performed. We suggest using the checklist during daily interprofessional team rounds to ensure each task is completed, if appropriate. Institutions may consider measuring process measures such as adherence and completion of checklist, audits of discharge summaries for completion and transmission rates to PCPs (by fax or through health record departments), and documentation of patient education or medication reconciliation. Example outcome measures, if feasible, include Care Transitions Measure (CTM) scores, patient satisfaction surveys, and readmission rates.
Several limitations of this study should be considered. First, current literature on safe discharge practices is limited by low study‐design quality, with a paucity of randomized controlled trials. However, a recent systematic review found that bundled discharge interventions are likely to be most effective.[10] Individual items of the checklist may not have had an extensive evidence base; however, some of these suggested elements (eg, contact home care) have clinical face validity. Second, the heterogeneity of interventions studied pose challenges in determining generalizable best practices without considering local factors. To mitigate this, we suggest adapting the checklist to local contexts and resource availability. Third, the checklist has not been tested. The next step of this project is to pilot checklist use through small‐scale Plan‐Do‐Study‐Act (PDSA) cycles followed by large‐scale implementation. We plan to collect baseline, process, and outcome measures before and after implementation of the checklist at multiple institutions to determine utility.
Standardization of discharge practices is critical to safe transitions and preventing avoidable admissions to hospital. Our discharge checklist is an expanded tool that provides explicit guidance for each day of hospitalization and can be adapted for any hospital admission to aid interdisciplinary efforts toward a successful discharge. Future studies to evaluate the checklist in improving care‐transition processes are required to determine association with outcomes.
Disclosures
Nothing to report.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–128.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , , . “I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223–2228.
- , . Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533–536.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. A Quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc. 57(9):1540–1546.
- , , , et al. Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211–218.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- Centers for Medicare and Medicaid Services. Readmissions reduction program. Available at: http://cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/AcuteInpatientPPS/Readmissions‐Reduction‐Program.html.Accessed September 5, 2012.
- Ontario Ministry of Health and Long‐Term Care. The Excellent Care for All Act, 2010. Available at: http://health.gov.on.ca/en/public/programs/ecfa/default.aspx/. Accessed February 28, 2013.
- Ontario Ministry of Health and Long‐Term Care; Baker GR, ed. Enhancing the Continuum of Care: Report of the Avoidable Hospitalization Advisory Panel, November 2011. Available at: http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Accessed August 8, 2012.
- , , , , , . Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial [published correction appears in J Am Geriatr Soc. 2004;52(7):1228]. J Am Geriatr Soc. 2004;52(5):675–684.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- Society of Hospital Medicine. Project BOOST: Better Outcomes by Optimizing Safe Transitions. Available at: http://www.hospitalmedicine.org/BOOST/. Accessed October 31, 2012.
- The King's Fund; , , . Avoiding Hospital Admissions: Lessons From Evidence and Experience. Available at: http://www.kingsfund.org.uk/publications/articles/avoiding‐hospital‐admissions‐lessons‐evidence. Published October 28, 2010. Accessed September 4, 2012.
- Nielsen GA, Rutherford P, Taylor J, eds. How‐To Guide: Creating an Ideal Transition Home. Cambridge, MA: Institute for Healthcare Improvement; 2009. Available at: http://www.ihi.org or http://ah.cms‐plus.com/files/IHI_How_to_Guide_Creating_an_Ideal_Transition_Home.pdf. Accessed August 8, 2012.
- World Health Organization. Action on Patient Safety—High 5s. Available at: http://www.who.int/patientsafety/implementation/solutions/high5s/en/index.html. Accessed October 29, 2012.
- Safer Healthcare Now! Medication Reconciliation in Acute Care: Getting Started Kit. Available at: http://www.ismp‐canada.org/download/MedRec/Medrec_AC_English_GSK_V3.pdf. Accessed October 29, 2012.
- US Agency for Healthcare Research and Quality. PSNet: Patient‐safety primers, checklists. Available at: http://www.psnet.ahrq.gov/primer.aspx?primerID=14. Accessed November 1, 2012.
- , , , . Benefits of comprehensive inpatient education and discharge planning combined with outpatient support in elderly patients with congestive heart failure. Congest Heart Fail. 2005;11(6):315–321.
- , , , et al. Electronic versus dictated hospital discharge summaries: a randomized controlled trial. J Gen Intern Med. 2009;24(9):995–1001.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- . Will, ideas, and execution: their role in reducing adverse medication events. J Pediatr. 2005;147(6):727–728.
- , , , . Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014–2018.
- , , , et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , . Medication reconciliation in the hospital. Healthc Q. 2012;15:42–49.
- , , , , , . Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial. Ann Intern Med. 1994;120(12):999–1006.
- , , , et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163(1):83–90.
- , , . The effects of patient communication skills training on compliance. Arch Fam Med. 2000;9(1):57–64.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354–360.
- , , , , . Influence of a “discharge interview” on patient knowledge, compliance, and functional status after hospitalization. Med Care. 1983;21(8):755–767.
- , , , , . Critical pathways intervention to reduce length of hospital stay. Am J Med. 2001;110(3):175–180.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med. 2005;143(2):121–128.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , , , . “I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223–2228.
- , . Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141(7):533–536.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , et al. A Quality improvement intervention to facilitate the transition of older adults from three hospitals back to their homes. J Am Geriatr Soc. 57(9):1540–1546.
- , , , et al. Reduction of 30‐day postdischarge hospital readmission or emergency department (ED) visit rates in high‐risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009;4(4):211–218.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- Centers for Medicare and Medicaid Services. Readmissions reduction program. Available at: http://cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/AcuteInpatientPPS/Readmissions‐Reduction‐Program.html.Accessed September 5, 2012.
- Ontario Ministry of Health and Long‐Term Care. The Excellent Care for All Act, 2010. Available at: http://health.gov.on.ca/en/public/programs/ecfa/default.aspx/. Accessed February 28, 2013.
- Ontario Ministry of Health and Long‐Term Care; Baker GR, ed. Enhancing the Continuum of Care: Report of the Avoidable Hospitalization Advisory Panel, November 2011. Available at: http://www.health.gov.on.ca/en/common/ministry/publications/reports/baker_2011/baker_2011.pdf. Accessed August 8, 2012.
- , , , , , . Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial [published correction appears in J Am Geriatr Soc. 2004;52(7):1228]. J Am Geriatr Soc. 2004;52(5):675–684.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- Society of Hospital Medicine. Project BOOST: Better Outcomes by Optimizing Safe Transitions. Available at: http://www.hospitalmedicine.org/BOOST/. Accessed October 31, 2012.
- The King's Fund; , , . Avoiding Hospital Admissions: Lessons From Evidence and Experience. Available at: http://www.kingsfund.org.uk/publications/articles/avoiding‐hospital‐admissions‐lessons‐evidence. Published October 28, 2010. Accessed September 4, 2012.
- Nielsen GA, Rutherford P, Taylor J, eds. How‐To Guide: Creating an Ideal Transition Home. Cambridge, MA: Institute for Healthcare Improvement; 2009. Available at: http://www.ihi.org or http://ah.cms‐plus.com/files/IHI_How_to_Guide_Creating_an_Ideal_Transition_Home.pdf. Accessed August 8, 2012.
- World Health Organization. Action on Patient Safety—High 5s. Available at: http://www.who.int/patientsafety/implementation/solutions/high5s/en/index.html. Accessed October 29, 2012.
- Safer Healthcare Now! Medication Reconciliation in Acute Care: Getting Started Kit. Available at: http://www.ismp‐canada.org/download/MedRec/Medrec_AC_English_GSK_V3.pdf. Accessed October 29, 2012.
- US Agency for Healthcare Research and Quality. PSNet: Patient‐safety primers, checklists. Available at: http://www.psnet.ahrq.gov/primer.aspx?primerID=14. Accessed November 1, 2012.
- , , , . Benefits of comprehensive inpatient education and discharge planning combined with outpatient support in elderly patients with congestive heart failure. Congest Heart Fail. 2005;11(6):315–321.
- , , , et al. Electronic versus dictated hospital discharge summaries: a randomized controlled trial. J Gen Intern Med. 2009;24(9):995–1001.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- , , , et al. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557.
- . Will, ideas, and execution: their role in reducing adverse medication events. J Pediatr. 2005;147(6):727–728.
- , , , . Pharmacists on rounding teams reduce preventable adverse drug events in hospital general medicine units. Arch Intern Med. 2003;163(17):2014–2018.
- , , , et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166(5):565–571.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314–323.
- , . Medication reconciliation in the hospital. Healthc Q. 2012;15:42–49.
- , , , , , . Comprehensive discharge planning for the hospitalized elderly: a randomized clinical trial. Ann Intern Med. 1994;120(12):999–1006.
- , , , et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163(1):83–90.
- , , . The effects of patient communication skills training on compliance. Arch Fam Med. 2000;9(1):57–64.
- , , , et al. Transition of care for hospitalized elderly patients—development of a discharge checklist for hospitalists. J Hosp Med. 2006;1(6):354–360.
- , , , , . Influence of a “discharge interview” on patient knowledge, compliance, and functional status after hospitalization. Med Care. 1983;21(8):755–767.
- , , , , . Critical pathways intervention to reduce length of hospital stay. Am J Med. 2001;110(3):175–180.
Copyright © 2013 Society of Hospital Medicine