User login
Pharmacogenomic DNA chip
Genotyping for cytochrome (CYP) P-450 gene variations can identify patients who will not benefit from, or may react badly to, some psychotropics.1 Psychiatrists can then more accurately tailor initial dosages to improve response and prevent adverse reactions.
An FDA-approved pharmacogenomic diagnostic DNA chip is expected to be available to clinical laboratories this month (Table 1). The chip provides an accurate genotype for two drug-metabolizing enzymes—2D6 and 2C19.
Table 1
Pharmacogenomic DNA chip: Fast facts
| Brand name: |
| AmpliChip CYP 450 Test |
| FDA-approved indication: |
| Genotyping patients |
| Manufacturer: |
| Roche Diagnostics |
| Estimated availability: |
| July 2005 |
| Recommended use: |
| Determining cytochrome P-450 2D6 and 2C19 gene variations in patients before prescribing a psychotropic metabolized through these pathways. |
| Laboratories that process AmpliChip results: |
| Labcore, Mayo Medical Laboratories, Quest Diagnostics |
Genotyping’S Role in Psychiatry
CYP 2D6 and 2C19 enzymes help metabolize many commonly prescribed psychotropics, including:
- fluoxetine, paroxetine, and venlafaxine, which are among the psychotropics primarily metabolized by the cytochrome P-450 2D6 enzyme (Table 2).
- amitriptyline and citalopram, which are among the psychotropics metabolized in part by 2C19 (Table 3).
The chip can identify patients who are genetically predisposed to abnormal metabolism of 2D6 and 2C19 substrates. This information can help psychiatrists improve response for ultrarapid metabolizers and minimize adverse effects experienced by poor metabolizers of these substrates.
For example, if the patient is an ultrarapid metabolizer of 2D6 and/or 2C19 substrates, the psychiatrist can:
- exceed the recommended dosage to reach adequate serum levels
- or choose an antidepressant not primarily metabolized by either enzyme.
For a poor metabolizer of 2D6 and/or 2C19 substrates, the psychiatrist can:
- choose an antidepressant metabolized by a different enzyme
- or prescribe 2D6 and 2C19 substrates at very low dosages.
For example, some poor metabolizers of 2D6 substrates have been successfully treated with fluoxetine, 2 to 5 mg/d.2,3 This approach can help avoid side effects and potentially save the patient money. To prevent prescription errors, make sure the pharmacist understands your rationale for lower-than-recommended dosages.
Patients who are poor metabolizers of 2C19 and extensive metabolizers of 2D6 substrates can probably tolerate citalopram and amitriptyline dosages at the low end of the therapeutic range. Watch for high serum levels of either or both drugs if both enzyme systems are inactive.
Table 2
Evidence suggests these drugs are predominantly metabolized by the 2D6 enzyme*
| Antidepressants | Antipsychotics | Stimulants |
|---|---|---|
| Desipramine | Fluphenazine | Atomoxetine |
| Fluoxetine | Perphenazine | |
| Nortriptyline | Risperidone | |
| Paroxetine | Thioridazine | |
| Venlafaxine | ||
| *Use caution when prescribing these agents to patients who are poor 2D6 metabolizers. | ||
Table 3
Evidence suggests these drugs are predominantly metabolized by the 2C19 enzyme*
| Antidepressants | |
|---|---|
| Diazepam | Citalopram |
| Clomipramine | Escitalopram |
| Imipramine | Sertraline |
| Benzodiazepines | |
| Amitriptyline | |
| *Use caution when prescribing these agents to patients who are poor 2C19 metabolizers. | |
Pharmacogenomic Chip’s Accuracy
The 2D6 gene has more than 100 variations, many of which are very rare mutations. The pharmacogenomic DNA chip can detect 27 of these variants, allowing the chip to accurately genotype most patients. By contrast, early 2D6 genotyping techniques identified only four or five variants, resulting in too many false negatives for clinical use.4
The chip also can identify the normal form of the 2C19 gene and two of its variants. Both variants produce an inactive 2C19 enzyme form that is ineffective in metabolizing 2C19 substrates.
Clinical Use
When should a psychiatrist obtain 2D6 and 2C19 genotypes?
First, understand that the pharmacogenomic chip does not predict which medications will produce a therapeutic response. Gene chips that predict response are in development but probably will not be available before 2008.
The chip, however, can identify the relatively few ultrarapid metabolizers who will not benefit from 2D6 or 2C19 substrate medications at normal dosages, as well as “poor metabolizers” of these substrates.1 The approximately 1% of whites in the United States who have ≥3 copies of the 2D6 gene metabolize 2D6 substrates very rapidly and will not respond to recommended dosages. About 10% of whites in the United States metabolize 2D6 or 2C19 substrates poorly and face increased risk of adverse reactions from these medications.
There is some evidence that the prevalence of these genetic variations differ among ethnicities. Approximately 15% of Saudi Arabians and 20% of Ethiopians are ultrarapid metabolizers of 2D6 and 2C19 substrates.5,6
The most common 2D6 poor metabolizer allele (*4) has been found in 12% to 21% of whites, whereas 23% to 32% of Asians and 13% of whites have the most common 2C19 poor metabolizer allele (*2).6-10 Prevalence of poor 2D6 and/or 2C19 metabolism among African Americans, Hispanics, and Native Americans has not been established.
Clinical Practicality
Clinicians’ unfamiliarity with genotyping and cost concerns pose potential barriers to the test’s use.
Clinician knowledge. Pharmacogenomic 2D6 and 2C19 tests will soon be offered nationwide at reference laboratories such as Quest Diagnostics, Labcore, and Mayo Medical Laboratories. The psychiatrist can call the lab for instructions, then send a blood sample and receive results by mail within 2 to 3 days.
While I believe the test’s usefulness will soon be widely understood, courses are available to help clinicians learn about genetic testing. Mayo Clinic College of Medicine (http://www.mayo. edu/cme/genomics.html) offers an annual week-long CME course in August. The American Psychiatric Association, as part of its May 2006 annual meeting, will offer a similar half-day course led by Mayo Clinic psychiatrists.
Cost. The exact cost of using the pharmacogenomic chip varies, as each laboratory sets fees for genotyping. Even so, genotyping could offer enormous cost savings by preventing failed medication trials and reducing the need for more-intensive psychiatric care. Furthermore, many insurance companies cover genotype testing.
Related resources
- Pharmacogenomic diagnostic DNA chip product information. www.rochediagnostics.com/products_services/amplichip_cyp450.html.
- Kirchheiner J, Borsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;103(3):173-92.
Drug brand names
- Amitriptyline • Elavil
- Atomoxetine • Strattera
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Diazepam • Valium
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Risperidone • Risperdal
Disclosure
Dr. Mrazek is a consultant to Predix Pharmaceuticals.
1. Mrazek DA. New tool: genotyping makes prescribing safer, more effective. Current Psychiatry 2004;3(9):11-23.
2. Kirchheiner J, Borsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;103:173-92.
3. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry 2004;9:442-73.
4. Chou WH, Yan FX, Robbins-Weilert DK, et al. Comparison of two CYP2D6 genotyping methods and assessment of genotype-phenotype relationships. Clin Chem 2003;49:542-51.
5. Ingelman-Sundberg M, Oscarson M, McLellan RA. Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment. Trends Pharmacol Sci 1999;20:342-9.
6. Phillips KA, Veenstra DL, Oren E, et al. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA 2001;286:2270-9.
7. Ingelman-Sundberg M. Pharmacogenetics: an opportunity for a safer and more efficient pharmacotherapy. J Intern Med 2001;250:186-200.
8. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev 2003;35:99-106.
9. Griese EU, Ilett KF, Kitteringham NR, et al. Allele and genotype frequencies of polymorphic cytochromes P450 2D6, 2C19, and 2E1 in aborigines from western Australia. Pharmacogenetics 2001;11:69-76.
10. Sachse C, Brockmoller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 1997;60:284-95.
Genotyping for cytochrome (CYP) P-450 gene variations can identify patients who will not benefit from, or may react badly to, some psychotropics.1 Psychiatrists can then more accurately tailor initial dosages to improve response and prevent adverse reactions.
An FDA-approved pharmacogenomic diagnostic DNA chip is expected to be available to clinical laboratories this month (Table 1). The chip provides an accurate genotype for two drug-metabolizing enzymes—2D6 and 2C19.
Table 1
Pharmacogenomic DNA chip: Fast facts
| Brand name: |
| AmpliChip CYP 450 Test |
| FDA-approved indication: |
| Genotyping patients |
| Manufacturer: |
| Roche Diagnostics |
| Estimated availability: |
| July 2005 |
| Recommended use: |
| Determining cytochrome P-450 2D6 and 2C19 gene variations in patients before prescribing a psychotropic metabolized through these pathways. |
| Laboratories that process AmpliChip results: |
| Labcore, Mayo Medical Laboratories, Quest Diagnostics |
Genotyping’S Role in Psychiatry
CYP 2D6 and 2C19 enzymes help metabolize many commonly prescribed psychotropics, including:
- fluoxetine, paroxetine, and venlafaxine, which are among the psychotropics primarily metabolized by the cytochrome P-450 2D6 enzyme (Table 2).
- amitriptyline and citalopram, which are among the psychotropics metabolized in part by 2C19 (Table 3).
The chip can identify patients who are genetically predisposed to abnormal metabolism of 2D6 and 2C19 substrates. This information can help psychiatrists improve response for ultrarapid metabolizers and minimize adverse effects experienced by poor metabolizers of these substrates.
For example, if the patient is an ultrarapid metabolizer of 2D6 and/or 2C19 substrates, the psychiatrist can:
- exceed the recommended dosage to reach adequate serum levels
- or choose an antidepressant not primarily metabolized by either enzyme.
For a poor metabolizer of 2D6 and/or 2C19 substrates, the psychiatrist can:
- choose an antidepressant metabolized by a different enzyme
- or prescribe 2D6 and 2C19 substrates at very low dosages.
For example, some poor metabolizers of 2D6 substrates have been successfully treated with fluoxetine, 2 to 5 mg/d.2,3 This approach can help avoid side effects and potentially save the patient money. To prevent prescription errors, make sure the pharmacist understands your rationale for lower-than-recommended dosages.
Patients who are poor metabolizers of 2C19 and extensive metabolizers of 2D6 substrates can probably tolerate citalopram and amitriptyline dosages at the low end of the therapeutic range. Watch for high serum levels of either or both drugs if both enzyme systems are inactive.
Table 2
Evidence suggests these drugs are predominantly metabolized by the 2D6 enzyme*
| Antidepressants | Antipsychotics | Stimulants |
|---|---|---|
| Desipramine | Fluphenazine | Atomoxetine |
| Fluoxetine | Perphenazine | |
| Nortriptyline | Risperidone | |
| Paroxetine | Thioridazine | |
| Venlafaxine | ||
| *Use caution when prescribing these agents to patients who are poor 2D6 metabolizers. | ||
Table 3
Evidence suggests these drugs are predominantly metabolized by the 2C19 enzyme*
| Antidepressants | |
|---|---|
| Diazepam | Citalopram |
| Clomipramine | Escitalopram |
| Imipramine | Sertraline |
| Benzodiazepines | |
| Amitriptyline | |
| *Use caution when prescribing these agents to patients who are poor 2C19 metabolizers. | |
Pharmacogenomic Chip’s Accuracy
The 2D6 gene has more than 100 variations, many of which are very rare mutations. The pharmacogenomic DNA chip can detect 27 of these variants, allowing the chip to accurately genotype most patients. By contrast, early 2D6 genotyping techniques identified only four or five variants, resulting in too many false negatives for clinical use.4
The chip also can identify the normal form of the 2C19 gene and two of its variants. Both variants produce an inactive 2C19 enzyme form that is ineffective in metabolizing 2C19 substrates.
Clinical Use
When should a psychiatrist obtain 2D6 and 2C19 genotypes?
First, understand that the pharmacogenomic chip does not predict which medications will produce a therapeutic response. Gene chips that predict response are in development but probably will not be available before 2008.
The chip, however, can identify the relatively few ultrarapid metabolizers who will not benefit from 2D6 or 2C19 substrate medications at normal dosages, as well as “poor metabolizers” of these substrates.1 The approximately 1% of whites in the United States who have ≥3 copies of the 2D6 gene metabolize 2D6 substrates very rapidly and will not respond to recommended dosages. About 10% of whites in the United States metabolize 2D6 or 2C19 substrates poorly and face increased risk of adverse reactions from these medications.
There is some evidence that the prevalence of these genetic variations differ among ethnicities. Approximately 15% of Saudi Arabians and 20% of Ethiopians are ultrarapid metabolizers of 2D6 and 2C19 substrates.5,6
The most common 2D6 poor metabolizer allele (*4) has been found in 12% to 21% of whites, whereas 23% to 32% of Asians and 13% of whites have the most common 2C19 poor metabolizer allele (*2).6-10 Prevalence of poor 2D6 and/or 2C19 metabolism among African Americans, Hispanics, and Native Americans has not been established.
Clinical Practicality
Clinicians’ unfamiliarity with genotyping and cost concerns pose potential barriers to the test’s use.
Clinician knowledge. Pharmacogenomic 2D6 and 2C19 tests will soon be offered nationwide at reference laboratories such as Quest Diagnostics, Labcore, and Mayo Medical Laboratories. The psychiatrist can call the lab for instructions, then send a blood sample and receive results by mail within 2 to 3 days.
While I believe the test’s usefulness will soon be widely understood, courses are available to help clinicians learn about genetic testing. Mayo Clinic College of Medicine (http://www.mayo. edu/cme/genomics.html) offers an annual week-long CME course in August. The American Psychiatric Association, as part of its May 2006 annual meeting, will offer a similar half-day course led by Mayo Clinic psychiatrists.
Cost. The exact cost of using the pharmacogenomic chip varies, as each laboratory sets fees for genotyping. Even so, genotyping could offer enormous cost savings by preventing failed medication trials and reducing the need for more-intensive psychiatric care. Furthermore, many insurance companies cover genotype testing.
Related resources
- Pharmacogenomic diagnostic DNA chip product information. www.rochediagnostics.com/products_services/amplichip_cyp450.html.
- Kirchheiner J, Borsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;103(3):173-92.
Drug brand names
- Amitriptyline • Elavil
- Atomoxetine • Strattera
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Diazepam • Valium
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Risperidone • Risperdal
Disclosure
Dr. Mrazek is a consultant to Predix Pharmaceuticals.
Genotyping for cytochrome (CYP) P-450 gene variations can identify patients who will not benefit from, or may react badly to, some psychotropics.1 Psychiatrists can then more accurately tailor initial dosages to improve response and prevent adverse reactions.
An FDA-approved pharmacogenomic diagnostic DNA chip is expected to be available to clinical laboratories this month (Table 1). The chip provides an accurate genotype for two drug-metabolizing enzymes—2D6 and 2C19.
Table 1
Pharmacogenomic DNA chip: Fast facts
| Brand name: |
| AmpliChip CYP 450 Test |
| FDA-approved indication: |
| Genotyping patients |
| Manufacturer: |
| Roche Diagnostics |
| Estimated availability: |
| July 2005 |
| Recommended use: |
| Determining cytochrome P-450 2D6 and 2C19 gene variations in patients before prescribing a psychotropic metabolized through these pathways. |
| Laboratories that process AmpliChip results: |
| Labcore, Mayo Medical Laboratories, Quest Diagnostics |
Genotyping’S Role in Psychiatry
CYP 2D6 and 2C19 enzymes help metabolize many commonly prescribed psychotropics, including:
- fluoxetine, paroxetine, and venlafaxine, which are among the psychotropics primarily metabolized by the cytochrome P-450 2D6 enzyme (Table 2).
- amitriptyline and citalopram, which are among the psychotropics metabolized in part by 2C19 (Table 3).
The chip can identify patients who are genetically predisposed to abnormal metabolism of 2D6 and 2C19 substrates. This information can help psychiatrists improve response for ultrarapid metabolizers and minimize adverse effects experienced by poor metabolizers of these substrates.
For example, if the patient is an ultrarapid metabolizer of 2D6 and/or 2C19 substrates, the psychiatrist can:
- exceed the recommended dosage to reach adequate serum levels
- or choose an antidepressant not primarily metabolized by either enzyme.
For a poor metabolizer of 2D6 and/or 2C19 substrates, the psychiatrist can:
- choose an antidepressant metabolized by a different enzyme
- or prescribe 2D6 and 2C19 substrates at very low dosages.
For example, some poor metabolizers of 2D6 substrates have been successfully treated with fluoxetine, 2 to 5 mg/d.2,3 This approach can help avoid side effects and potentially save the patient money. To prevent prescription errors, make sure the pharmacist understands your rationale for lower-than-recommended dosages.
Patients who are poor metabolizers of 2C19 and extensive metabolizers of 2D6 substrates can probably tolerate citalopram and amitriptyline dosages at the low end of the therapeutic range. Watch for high serum levels of either or both drugs if both enzyme systems are inactive.
Table 2
Evidence suggests these drugs are predominantly metabolized by the 2D6 enzyme*
| Antidepressants | Antipsychotics | Stimulants |
|---|---|---|
| Desipramine | Fluphenazine | Atomoxetine |
| Fluoxetine | Perphenazine | |
| Nortriptyline | Risperidone | |
| Paroxetine | Thioridazine | |
| Venlafaxine | ||
| *Use caution when prescribing these agents to patients who are poor 2D6 metabolizers. | ||
Table 3
Evidence suggests these drugs are predominantly metabolized by the 2C19 enzyme*
| Antidepressants | |
|---|---|
| Diazepam | Citalopram |
| Clomipramine | Escitalopram |
| Imipramine | Sertraline |
| Benzodiazepines | |
| Amitriptyline | |
| *Use caution when prescribing these agents to patients who are poor 2C19 metabolizers. | |
Pharmacogenomic Chip’s Accuracy
The 2D6 gene has more than 100 variations, many of which are very rare mutations. The pharmacogenomic DNA chip can detect 27 of these variants, allowing the chip to accurately genotype most patients. By contrast, early 2D6 genotyping techniques identified only four or five variants, resulting in too many false negatives for clinical use.4
The chip also can identify the normal form of the 2C19 gene and two of its variants. Both variants produce an inactive 2C19 enzyme form that is ineffective in metabolizing 2C19 substrates.
Clinical Use
When should a psychiatrist obtain 2D6 and 2C19 genotypes?
First, understand that the pharmacogenomic chip does not predict which medications will produce a therapeutic response. Gene chips that predict response are in development but probably will not be available before 2008.
The chip, however, can identify the relatively few ultrarapid metabolizers who will not benefit from 2D6 or 2C19 substrate medications at normal dosages, as well as “poor metabolizers” of these substrates.1 The approximately 1% of whites in the United States who have ≥3 copies of the 2D6 gene metabolize 2D6 substrates very rapidly and will not respond to recommended dosages. About 10% of whites in the United States metabolize 2D6 or 2C19 substrates poorly and face increased risk of adverse reactions from these medications.
There is some evidence that the prevalence of these genetic variations differ among ethnicities. Approximately 15% of Saudi Arabians and 20% of Ethiopians are ultrarapid metabolizers of 2D6 and 2C19 substrates.5,6
The most common 2D6 poor metabolizer allele (*4) has been found in 12% to 21% of whites, whereas 23% to 32% of Asians and 13% of whites have the most common 2C19 poor metabolizer allele (*2).6-10 Prevalence of poor 2D6 and/or 2C19 metabolism among African Americans, Hispanics, and Native Americans has not been established.
Clinical Practicality
Clinicians’ unfamiliarity with genotyping and cost concerns pose potential barriers to the test’s use.
Clinician knowledge. Pharmacogenomic 2D6 and 2C19 tests will soon be offered nationwide at reference laboratories such as Quest Diagnostics, Labcore, and Mayo Medical Laboratories. The psychiatrist can call the lab for instructions, then send a blood sample and receive results by mail within 2 to 3 days.
While I believe the test’s usefulness will soon be widely understood, courses are available to help clinicians learn about genetic testing. Mayo Clinic College of Medicine (http://www.mayo. edu/cme/genomics.html) offers an annual week-long CME course in August. The American Psychiatric Association, as part of its May 2006 annual meeting, will offer a similar half-day course led by Mayo Clinic psychiatrists.
Cost. The exact cost of using the pharmacogenomic chip varies, as each laboratory sets fees for genotyping. Even so, genotyping could offer enormous cost savings by preventing failed medication trials and reducing the need for more-intensive psychiatric care. Furthermore, many insurance companies cover genotype testing.
Related resources
- Pharmacogenomic diagnostic DNA chip product information. www.rochediagnostics.com/products_services/amplichip_cyp450.html.
- Kirchheiner J, Borsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;103(3):173-92.
Drug brand names
- Amitriptyline • Elavil
- Atomoxetine • Strattera
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin
- Diazepam • Valium
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Nortriptyline • Pamelor
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Risperidone • Risperdal
Disclosure
Dr. Mrazek is a consultant to Predix Pharmaceuticals.
1. Mrazek DA. New tool: genotyping makes prescribing safer, more effective. Current Psychiatry 2004;3(9):11-23.
2. Kirchheiner J, Borsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;103:173-92.
3. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry 2004;9:442-73.
4. Chou WH, Yan FX, Robbins-Weilert DK, et al. Comparison of two CYP2D6 genotyping methods and assessment of genotype-phenotype relationships. Clin Chem 2003;49:542-51.
5. Ingelman-Sundberg M, Oscarson M, McLellan RA. Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment. Trends Pharmacol Sci 1999;20:342-9.
6. Phillips KA, Veenstra DL, Oren E, et al. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA 2001;286:2270-9.
7. Ingelman-Sundberg M. Pharmacogenetics: an opportunity for a safer and more efficient pharmacotherapy. J Intern Med 2001;250:186-200.
8. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev 2003;35:99-106.
9. Griese EU, Ilett KF, Kitteringham NR, et al. Allele and genotype frequencies of polymorphic cytochromes P450 2D6, 2C19, and 2E1 in aborigines from western Australia. Pharmacogenetics 2001;11:69-76.
10. Sachse C, Brockmoller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 1997;60:284-95.
1. Mrazek DA. New tool: genotyping makes prescribing safer, more effective. Current Psychiatry 2004;3(9):11-23.
2. Kirchheiner J, Borsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;103:173-92.
3. Kirchheiner J, Nickchen K, Bauer M, et al. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Mol Psychiatry 2004;9:442-73.
4. Chou WH, Yan FX, Robbins-Weilert DK, et al. Comparison of two CYP2D6 genotyping methods and assessment of genotype-phenotype relationships. Clin Chem 2003;49:542-51.
5. Ingelman-Sundberg M, Oscarson M, McLellan RA. Polymorphic human cytochrome P450 enzymes: an opportunity for individualized drug treatment. Trends Pharmacol Sci 1999;20:342-9.
6. Phillips KA, Veenstra DL, Oren E, et al. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA 2001;286:2270-9.
7. Ingelman-Sundberg M. Pharmacogenetics: an opportunity for a safer and more efficient pharmacotherapy. J Intern Med 2001;250:186-200.
8. Mizutani T. PM frequencies of major CYPs in Asians and Caucasians. Drug Metab Rev 2003;35:99-106.
9. Griese EU, Ilett KF, Kitteringham NR, et al. Allele and genotype frequencies of polymorphic cytochromes P450 2D6, 2C19, and 2E1 in aborigines from western Australia. Pharmacogenetics 2001;11:69-76.
10. Sachse C, Brockmoller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 1997;60:284-95.
New tool: Genotyping makes prescribing safer, more effective
Genotyping for cytochrome P-450 2D6 gene variations is emerging as a valuable clinical tool to help psychiatrists identify patients who:
Genetic variation has long been known to influence how individuals metabolize drugs, but only recently could we apply this information.5 Many academic medical centers and the two largest U.S. reference laboratories offer 2D6 testing at costs of $200 to $500.
Before long, psychiatrists may adopt routine genotyping before prescribing 2D6 substrate medications. This article and four vignettes illustrate the clinical benefits of psychiatric pharmacogenomics and suggest when prospective genotyping could help you select and dose medications.
Table 1
Drugs metabolized by the 2D6 enzyme*
| Antidepressants | Antipsychotics | Stimulants | Other medications |
|---|---|---|---|
| Desipramine | Fluphenazine | Atomoxetine | Codeine |
| Fluoxetine | Perphenazine | Dextromethorphan | |
| Nortriptyline | Risperidone | Oxycodone | |
| Paroxetine | Thioridazine | ||
| Venlafaxine | |||
| *Evidence suggests that these medications are predominantly metabolized by the 2D6 enzyme. | |||
| Be careful when prescribing these agents to patients who are poor 2D6 metabolizers. | |||
Why test for the 2D6 gene?
The 2D6 gene codes for the 2D6 enzyme, the primary enzyme required to metabolize many psychotropics Table 1.
Genetic variations. A common variation in a gene is frequently called an allele. More than 100 2D6 gene variations have been described. Consequently, the 2D6 gene’s enzyme activity also varies widely. Most mutations decrease the enzyme’s activity, but some polymorphisms change the gene’s promoter region, which can lead to upregulation and increased enzyme production.
Each 2D6 gene variation has been labeled with a standardized abbreviation (Table 2):
- *1 refers to the “normal” gene
- *2 stands for several variants with different activity levels.
- *3, *4, *6, *7, *8, *9, *10, *11, *12, *14, and *17 code for proteins with little or no activity.
- *5 indicates that the gene is deleted, and no enzyme can be produced.
Multiple copies. Another characteristic of the 2D6 gene is its unusually high propensity to accumulate in multiple copies on the 22nd chromosome. As many as 13 copies of the 2D6 gene have been shown on a single chromosome. Given that each gene can code for the 2D6 enzyme, patients with multiple copies can metabolize 2D6 substrate medications very rapidly.
Nonpsychiatric drugs. The 2D6 enzyme is also involved in metabolizing many nonpsychiatric drugs. To produce analgesia, for example, the 2D6 enzyme must metabolize the prodrug codeine to morphine. Thus, individuals with no 2D6 enzyme activity experience no analgesia with codeine. Approximately 7% of Caucasians metabolize codeine poorly. Conversely, individuals with multiple 2D6 gene copies metabolize codeine to morphine very rapidly, with potential for acute mental status changes, including psychosis.
4 metabolizer types. Based on variation in individual 2D6 genotype, a patient is usually categorized as being an ultrarapid, extensive, intermediate, or poor metabolizer (Table 3). The following case vignettes of patients in each category illustrate the clinical benefits of 2D6 genotyping.
Ultrarapid metabolizer: Extra 2D6 copies
Abdul, 49, is an Ethiopian businessman engaged in international commerce. While in the United States, he underwent a routine wisdom-tooth extraction and was treated with acetaminophen and codeine. Despite having no psychiatric history, he began to experience extreme discomfort and flashing visual hallucinations within 24 hours of taking two codeine doses. The oral surgeon instructed him to discontinue codeine, and his symptoms resolved within 24 hours.
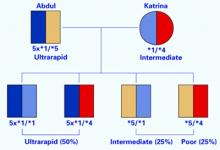
Because of this experience, Abdul underwent genotyping for the 2D6 gene. He was found to have five active copies on one 22nd chromosome and no copies on the other (Figure 1). This genotype is unusual in western European populations but common in North Africa. Abdul then received alternate analgesics; psychiatric symptoms did not recur.
A patient such as Abdul, with multiple copies of a functional 2D6 gene, is an ultrarapid metabolizer. The 22nd chromosome—where the 2D6 gene is located—is short and contains areas of high homology. As a result, uneven crossover events occur more frequently during meiosis than is typical of larger chromosomes. Uneven crossover results in one gamete with two copies of the 2D6 gene and the other gamete with none.
2D6 enzyme activity is not essential for survival, which raises fascinating questions about this gene’s evolutionary importance. In certain geographic regions, many individuals have multiple copies of the gene. In Ethiopia—the country with the highest documented number of ultrarapid metabolizers—more than 25% of the population has one chromosome with multiple copies of the 2D6 gene.6 Because these copies produce an increased amount of 2D6 active enzyme, normal doses of 2D6 substrate medications do not benefit these individuals.
Table 2
How common 2D6 gene variations (alleles) affect 2D6 enzyme activity
| Allele label | 2D6 enzyme activity | Allele frequency (%)† |
|---|---|---|
| *1 | Normal | 37 |
| *2 | Decreased | 3.3 |
| *2P | Modestly increased | 6 |
| *3 | None | 1 |
| *4 | None | 18 |
| *5 | None (gene deletion) | 4 |
| *6 | None | 1 |
| *7 | None | <1 |
| *9 | Decreased | 3 |
| *10 | Decreased | 2 |
| *11 | None | 0 |
| *12 | None | <1 |
| *14 | Decreased | <1 |
| *17 | Decreased | <1 |
| †In Caucasian populations | ||
Table 3
Four ways patients respond to 2D6 substrate drugs
| Category | Patient characteristics | % of Caucasian population |
|---|---|---|
| Ultrarapid | Metabolize 2D6 medications rapidly resulting in poor response | 1 to 2 |
| Extensive | Metabolize 2D6 medications at a normal rate | 73 to 82 |
| Intermediate | Metabolize 2D6 medications at a slower-than-normal rate | 10 to 15 |
| Poor | Metabolize 2D6 medications very slowly with increased risk of side effects | 7 to 10 |
When treating ultrarapid metabolizers one strategy is to increase the dosage to obtain a therapeutic effect Because some substrates have complex metabolic pathways, however, high concentrations of secondary or tertiary metabolites can accumulate. Thus, when a substance’s metabolic pathway is not well-documented, a more cautious approach is to choose a medication metabolized by another pathway.
Figure 1 Genotypes and metabolizer categories of 4 illustrative patients
Extensive metabolizer: The ‘norm’
George, a 31-year-old Ethiopian architect, is Abdul’s second cousin. He developed acute depression with intense suicidal ideation and sought psychiatric consultation. He had no history of atypical drug reactions, but—because of his ethnic background—his psychiatrist was concerned that George might be a rapid metabolizer.
2D6 genotyping showed that George’s genotype was *1/*1, which meant he had two functional 2D6 copies (Figure 1). This genotype suggests that he could tolerate many antidepressants. The psychiatrist concluded—with some confidence—that George would not experience adverse effects or low serum levels when prescribed fluoxetine at usual dosages.
Extensive metabolizers have two normal 2D6 gene copies and can produce adequate active 2D6 enzyme Patients with this genotype—common in Caucasians—are generally said to have “normal” 2D6 metabolism. This means they metabolize 2D6 substrate medications at a rate within the recommended dosage ranges determined from North American or European pharmacokinetic studies.
Intermediate metabolizer: Mixed message
Katrina, 27, represents the government of her native Sweden in trade agreements. When she developed depressive symptoms (insomnia, sense of hopelessness), Katrina saw her psychiatrist. She reported that her family has a history of adverse reactions to multiple medications, but she had tolerated most medications. In fact, she had twice been successfully treated with relatively high doses of codeine.
Her psychiatrist suspected she was an intermediate 2D6 metabolizer and ordered testing. Her genotype was *1/*4, with one normal copy and one that produced no functional 2D6 enzyme (Figure 1).
Based on her clinical history and this genotypic information, the psychiatrist prescribed sertraline—metabolized by both 2D6 and 3A4 enzymes— at 50 mg/d. Because Katrina metabolized sertraline at a slower-than-usual rate, she developed a therapeutic blood level and responded well to this low dosage.
Intermediate metabolizers have a chromosome with one functional 2D6 gene copy. The other chromosome has either a copy with a defective functional polymorphism or a deletion of the gene. These patients usually tolerate 2D6 substrate drugs in low dosages.
Poor metabolizer: ‘medication-sensitive’
Olga, Katrina’s mother, has always lived in northern Sweden. She has no psychiatric history except for one psychotic episode that required hospitalization.
Her psychotic illness began on the summer solstice, during an all-night celebration. In addition to using unspecified recreational drugs, she took three 20-mg capsules of fluoxetine that her friend told her would make her feel high. She instead developed acute mania and dramatic paranoid delusions.
Figure 2 Possible genotypes of Brad, son of Abdul and Katrina
Olga was hospitalized and treated with moderate doses of haloperidol that precipitated an acute dystonic reaction. She was subsequently given ben-ztropine, and her extrapyramidal symptoms resolved. After discharge, she was treated with haloperidol and benztropine for 2 years, after which she spontaneously discontinued these drugs against medical advice. Her psychotic illness has not recurred.
Knowing her own genotype, Katrina understood that her mother had a 50% probability of having one copy of the 2D6 *4 allele. Given her mother’s history of medication intolerance, Katrina believed that her mother’s psychiatric illness might have been related to a drug reaction. She persuaded her mother to send a blood sample to a laboratory in Stockholm.
Olga’s genotype was *4/*4, indicating that she would be unlikely to tolerate even moderate doses of 2D6 substrate medications (Figure 1). Given her complete recovery and continued good health without medication, the most probable retrospective diagnosis was drug-induced psychosis. Her 2-year neuroleptic treatment probably was unnecessary.
Figure 3 Genogram for Brad, son of Abdul and Katrina
Poor metabolizers without a functional 2D6 gene copy have low tolerance for many medications and often become labeled as “medication sensitive.” When genotyping reveals that an individual is a poor metabolizer, prescribing medications that do not require 2D6 metabolism is usually prudent.
In rare cases, poor metabolizers have died from normal doses of 2D6 substrate medications.7 Far more commonly, however, they spontaneously discontinue taking these drugs because of adverse side effects.
Benefits of prospective testing
When used in clinical practice, pharmacogenomic testing’s two goals are to identify:
- ultrarapid metabolizers, who will not benefit from a medication
- poor metabolizers, who likely will have adverse responses to a medication.
The following case demonstrates the benefit of prospective 2D6 genotyping:
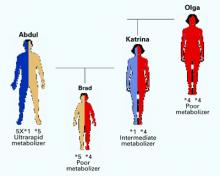
Brad, age 14, is the son of Abdul and Katrina, whose genotypes have been described. Brad developed a serious depression that was similar in severity and onset to an illness his mother experienced as a teen.
Brad’s parents want him to get the maximum benefit from psychopharmacologic treatment while avoiding distressing side effects. He had been healthy and had received no prescriptions other than antibiotics in the past.
How would you proceed? Without knowing Brad’s parents’ genotypes, you might reason that Brad would resemble one of them in drug response. However, when you review each parent’s genotype, you realize four scenarios are possible (Figure 2):
- Brad has a high likelihood of being an ultrarapid metabolizer because he has a 50% chance of inheriting a chromosome with five copies of the 2D6 gene from his father. He inherited the *1 or *4 form from his mother, but the effect of either will be clinically irrelevant.
- If Brad inherited the chromosome with the deletion from his father and the *1 form from his mother, he would be an intermediate metabolizer, as is his mother.
- If he inherited the chromosome with the deletion from his father and the *4 form from his mother, he would be a poor metabolizer like his grandmother, Olga. He would be at substantial risk for adverse reactions (such as intense headaches or vomiting) to 2D6 substrate medications.
On testing, Brad was found to be a poor metabolizer (Figure 3) The psychiatrist prescribed bupropion, which is metabolized by the 2B6 enzyme rather than the 2D6 enzyme.
Conclusion. To introduce the concept of genotypic testing, this review has focused on simple illustrations of variations in a single gene. However, many genes in the P-450 family play important roles in metabolizing psychotropics. In the future, genotyping of panels of these genes will likely provide more-specific guidance than can be achieved by simply testing one gene at a time.
Related resources
- Lerer B (ed). Pharmacogenetics of psychotropic drugs. Cambridge, UK: Cambridge University Press, 2002.
- Kirchheiner J, Borsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;103(3):173-92.
- Indiana University School of Medicine, Division of Clinical Pharmacology. Drug Interactions—Defining Genetic Influences on Pharmacologic Responses. http://medicine.iupui.edu/flockhart.
Drug brand names
- Acetaminophen w/codeine phosphate • Tylenol w/codeine
- Atomoxetine • Strattera
- Benztropine mesylate • Cogentin
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Nortriptyline • Aventyl, Pamelor
- Oxycodone • Oxycontin
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Risperidone • Risperdal
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Venlafaxine • Effexor
Disclosure
Dr. Mrazek reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mrazek DA. Clinical genomic testing. In: Wiener J, Dulcan M (eds). Textbook of child and adolescent psychiatry (3rd ed). Washington, DC: American Psychiatric Publishing, Inc., 2001;193-203.
2. Mrazek DA. Pharmacogenomic screening for depressed children and adolescents (scientific proceedings). Miami Beach, FL: American Academy of Child and Adolescent Psychiatry annual meeting, 2003;159.-
3. Phillips KA, Veenstra DL, Oren E, et al. Potential role of pharmacogenomics in reducing adverse drug reactions. JAMA 2001;286(18):2270-9.
4. Shi MM, Mehrens D, Dacus K. Pharmacogenomics: Changing the health care paradigm. Modern Drug Discovery 2001;4(7):27-32.
5. Kirchheiner J, Brosen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;104:173-92.
6. Masimirembwa CM, Hasler JA. Genetic polymorphism of drug metabolising enzymes in African populations: implications for the use of neuroleptics and antidepressants. Brain Res Bull 1997;44(5):561-71.
7. Sallee FR, DeVane CL, Ferrell RE. Fluoxetine-related death in a child with cytochrome P-450 2D6 genetic deficiency. J Child Adolesc Psychopharmacol 2000;10(1):27-34.
8. Gaedigk A, Gotschall RR, Forbes NS, et al. Optimization of cytochrome P4502D6 (CYP2D6) phenotype assignment using a genotyping algorithm based on allele frequency data. Pharmacogenetics 1999;9(6):669-82.
Genotyping for cytochrome P-450 2D6 gene variations is emerging as a valuable clinical tool to help psychiatrists identify patients who:
Genetic variation has long been known to influence how individuals metabolize drugs, but only recently could we apply this information.5 Many academic medical centers and the two largest U.S. reference laboratories offer 2D6 testing at costs of $200 to $500.
Before long, psychiatrists may adopt routine genotyping before prescribing 2D6 substrate medications. This article and four vignettes illustrate the clinical benefits of psychiatric pharmacogenomics and suggest when prospective genotyping could help you select and dose medications.
Table 1
Drugs metabolized by the 2D6 enzyme*
| Antidepressants | Antipsychotics | Stimulants | Other medications |
|---|---|---|---|
| Desipramine | Fluphenazine | Atomoxetine | Codeine |
| Fluoxetine | Perphenazine | Dextromethorphan | |
| Nortriptyline | Risperidone | Oxycodone | |
| Paroxetine | Thioridazine | ||
| Venlafaxine | |||
| *Evidence suggests that these medications are predominantly metabolized by the 2D6 enzyme. | |||
| Be careful when prescribing these agents to patients who are poor 2D6 metabolizers. | |||
Why test for the 2D6 gene?
The 2D6 gene codes for the 2D6 enzyme, the primary enzyme required to metabolize many psychotropics Table 1.
Genetic variations. A common variation in a gene is frequently called an allele. More than 100 2D6 gene variations have been described. Consequently, the 2D6 gene’s enzyme activity also varies widely. Most mutations decrease the enzyme’s activity, but some polymorphisms change the gene’s promoter region, which can lead to upregulation and increased enzyme production.
Each 2D6 gene variation has been labeled with a standardized abbreviation (Table 2):
- *1 refers to the “normal” gene
- *2 stands for several variants with different activity levels.
- *3, *4, *6, *7, *8, *9, *10, *11, *12, *14, and *17 code for proteins with little or no activity.
- *5 indicates that the gene is deleted, and no enzyme can be produced.
Multiple copies. Another characteristic of the 2D6 gene is its unusually high propensity to accumulate in multiple copies on the 22nd chromosome. As many as 13 copies of the 2D6 gene have been shown on a single chromosome. Given that each gene can code for the 2D6 enzyme, patients with multiple copies can metabolize 2D6 substrate medications very rapidly.
Nonpsychiatric drugs. The 2D6 enzyme is also involved in metabolizing many nonpsychiatric drugs. To produce analgesia, for example, the 2D6 enzyme must metabolize the prodrug codeine to morphine. Thus, individuals with no 2D6 enzyme activity experience no analgesia with codeine. Approximately 7% of Caucasians metabolize codeine poorly. Conversely, individuals with multiple 2D6 gene copies metabolize codeine to morphine very rapidly, with potential for acute mental status changes, including psychosis.
4 metabolizer types. Based on variation in individual 2D6 genotype, a patient is usually categorized as being an ultrarapid, extensive, intermediate, or poor metabolizer (Table 3). The following case vignettes of patients in each category illustrate the clinical benefits of 2D6 genotyping.
Ultrarapid metabolizer: Extra 2D6 copies
Abdul, 49, is an Ethiopian businessman engaged in international commerce. While in the United States, he underwent a routine wisdom-tooth extraction and was treated with acetaminophen and codeine. Despite having no psychiatric history, he began to experience extreme discomfort and flashing visual hallucinations within 24 hours of taking two codeine doses. The oral surgeon instructed him to discontinue codeine, and his symptoms resolved within 24 hours.
Because of this experience, Abdul underwent genotyping for the 2D6 gene. He was found to have five active copies on one 22nd chromosome and no copies on the other (Figure 1). This genotype is unusual in western European populations but common in North Africa. Abdul then received alternate analgesics; psychiatric symptoms did not recur.
A patient such as Abdul, with multiple copies of a functional 2D6 gene, is an ultrarapid metabolizer. The 22nd chromosome—where the 2D6 gene is located—is short and contains areas of high homology. As a result, uneven crossover events occur more frequently during meiosis than is typical of larger chromosomes. Uneven crossover results in one gamete with two copies of the 2D6 gene and the other gamete with none.
2D6 enzyme activity is not essential for survival, which raises fascinating questions about this gene’s evolutionary importance. In certain geographic regions, many individuals have multiple copies of the gene. In Ethiopia—the country with the highest documented number of ultrarapid metabolizers—more than 25% of the population has one chromosome with multiple copies of the 2D6 gene.6 Because these copies produce an increased amount of 2D6 active enzyme, normal doses of 2D6 substrate medications do not benefit these individuals.
Table 2
How common 2D6 gene variations (alleles) affect 2D6 enzyme activity
| Allele label | 2D6 enzyme activity | Allele frequency (%)† |
|---|---|---|
| *1 | Normal | 37 |
| *2 | Decreased | 3.3 |
| *2P | Modestly increased | 6 |
| *3 | None | 1 |
| *4 | None | 18 |
| *5 | None (gene deletion) | 4 |
| *6 | None | 1 |
| *7 | None | <1 |
| *9 | Decreased | 3 |
| *10 | Decreased | 2 |
| *11 | None | 0 |
| *12 | None | <1 |
| *14 | Decreased | <1 |
| *17 | Decreased | <1 |
| †In Caucasian populations | ||
Table 3
Four ways patients respond to 2D6 substrate drugs
| Category | Patient characteristics | % of Caucasian population |
|---|---|---|
| Ultrarapid | Metabolize 2D6 medications rapidly resulting in poor response | 1 to 2 |
| Extensive | Metabolize 2D6 medications at a normal rate | 73 to 82 |
| Intermediate | Metabolize 2D6 medications at a slower-than-normal rate | 10 to 15 |
| Poor | Metabolize 2D6 medications very slowly with increased risk of side effects | 7 to 10 |
When treating ultrarapid metabolizers one strategy is to increase the dosage to obtain a therapeutic effect Because some substrates have complex metabolic pathways, however, high concentrations of secondary or tertiary metabolites can accumulate. Thus, when a substance’s metabolic pathway is not well-documented, a more cautious approach is to choose a medication metabolized by another pathway.
Figure 1 Genotypes and metabolizer categories of 4 illustrative patients
Extensive metabolizer: The ‘norm’
George, a 31-year-old Ethiopian architect, is Abdul’s second cousin. He developed acute depression with intense suicidal ideation and sought psychiatric consultation. He had no history of atypical drug reactions, but—because of his ethnic background—his psychiatrist was concerned that George might be a rapid metabolizer.
2D6 genotyping showed that George’s genotype was *1/*1, which meant he had two functional 2D6 copies (Figure 1). This genotype suggests that he could tolerate many antidepressants. The psychiatrist concluded—with some confidence—that George would not experience adverse effects or low serum levels when prescribed fluoxetine at usual dosages.
Extensive metabolizers have two normal 2D6 gene copies and can produce adequate active 2D6 enzyme Patients with this genotype—common in Caucasians—are generally said to have “normal” 2D6 metabolism. This means they metabolize 2D6 substrate medications at a rate within the recommended dosage ranges determined from North American or European pharmacokinetic studies.
Intermediate metabolizer: Mixed message
Katrina, 27, represents the government of her native Sweden in trade agreements. When she developed depressive symptoms (insomnia, sense of hopelessness), Katrina saw her psychiatrist. She reported that her family has a history of adverse reactions to multiple medications, but she had tolerated most medications. In fact, she had twice been successfully treated with relatively high doses of codeine.
Her psychiatrist suspected she was an intermediate 2D6 metabolizer and ordered testing. Her genotype was *1/*4, with one normal copy and one that produced no functional 2D6 enzyme (Figure 1).
Based on her clinical history and this genotypic information, the psychiatrist prescribed sertraline—metabolized by both 2D6 and 3A4 enzymes— at 50 mg/d. Because Katrina metabolized sertraline at a slower-than-usual rate, she developed a therapeutic blood level and responded well to this low dosage.
Intermediate metabolizers have a chromosome with one functional 2D6 gene copy. The other chromosome has either a copy with a defective functional polymorphism or a deletion of the gene. These patients usually tolerate 2D6 substrate drugs in low dosages.
Poor metabolizer: ‘medication-sensitive’
Olga, Katrina’s mother, has always lived in northern Sweden. She has no psychiatric history except for one psychotic episode that required hospitalization.
Her psychotic illness began on the summer solstice, during an all-night celebration. In addition to using unspecified recreational drugs, she took three 20-mg capsules of fluoxetine that her friend told her would make her feel high. She instead developed acute mania and dramatic paranoid delusions.
Figure 2 Possible genotypes of Brad, son of Abdul and Katrina
Olga was hospitalized and treated with moderate doses of haloperidol that precipitated an acute dystonic reaction. She was subsequently given ben-ztropine, and her extrapyramidal symptoms resolved. After discharge, she was treated with haloperidol and benztropine for 2 years, after which she spontaneously discontinued these drugs against medical advice. Her psychotic illness has not recurred.
Knowing her own genotype, Katrina understood that her mother had a 50% probability of having one copy of the 2D6 *4 allele. Given her mother’s history of medication intolerance, Katrina believed that her mother’s psychiatric illness might have been related to a drug reaction. She persuaded her mother to send a blood sample to a laboratory in Stockholm.
Olga’s genotype was *4/*4, indicating that she would be unlikely to tolerate even moderate doses of 2D6 substrate medications (Figure 1). Given her complete recovery and continued good health without medication, the most probable retrospective diagnosis was drug-induced psychosis. Her 2-year neuroleptic treatment probably was unnecessary.
Figure 3 Genogram for Brad, son of Abdul and Katrina
Poor metabolizers without a functional 2D6 gene copy have low tolerance for many medications and often become labeled as “medication sensitive.” When genotyping reveals that an individual is a poor metabolizer, prescribing medications that do not require 2D6 metabolism is usually prudent.
In rare cases, poor metabolizers have died from normal doses of 2D6 substrate medications.7 Far more commonly, however, they spontaneously discontinue taking these drugs because of adverse side effects.
Benefits of prospective testing
When used in clinical practice, pharmacogenomic testing’s two goals are to identify:
- ultrarapid metabolizers, who will not benefit from a medication
- poor metabolizers, who likely will have adverse responses to a medication.
The following case demonstrates the benefit of prospective 2D6 genotyping:
Brad, age 14, is the son of Abdul and Katrina, whose genotypes have been described. Brad developed a serious depression that was similar in severity and onset to an illness his mother experienced as a teen.
Brad’s parents want him to get the maximum benefit from psychopharmacologic treatment while avoiding distressing side effects. He had been healthy and had received no prescriptions other than antibiotics in the past.
How would you proceed? Without knowing Brad’s parents’ genotypes, you might reason that Brad would resemble one of them in drug response. However, when you review each parent’s genotype, you realize four scenarios are possible (Figure 2):
- Brad has a high likelihood of being an ultrarapid metabolizer because he has a 50% chance of inheriting a chromosome with five copies of the 2D6 gene from his father. He inherited the *1 or *4 form from his mother, but the effect of either will be clinically irrelevant.
- If Brad inherited the chromosome with the deletion from his father and the *1 form from his mother, he would be an intermediate metabolizer, as is his mother.
- If he inherited the chromosome with the deletion from his father and the *4 form from his mother, he would be a poor metabolizer like his grandmother, Olga. He would be at substantial risk for adverse reactions (such as intense headaches or vomiting) to 2D6 substrate medications.
On testing, Brad was found to be a poor metabolizer (Figure 3) The psychiatrist prescribed bupropion, which is metabolized by the 2B6 enzyme rather than the 2D6 enzyme.
Conclusion. To introduce the concept of genotypic testing, this review has focused on simple illustrations of variations in a single gene. However, many genes in the P-450 family play important roles in metabolizing psychotropics. In the future, genotyping of panels of these genes will likely provide more-specific guidance than can be achieved by simply testing one gene at a time.
Related resources
- Lerer B (ed). Pharmacogenetics of psychotropic drugs. Cambridge, UK: Cambridge University Press, 2002.
- Kirchheiner J, Borsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;103(3):173-92.
- Indiana University School of Medicine, Division of Clinical Pharmacology. Drug Interactions—Defining Genetic Influences on Pharmacologic Responses. http://medicine.iupui.edu/flockhart.
Drug brand names
- Acetaminophen w/codeine phosphate • Tylenol w/codeine
- Atomoxetine • Strattera
- Benztropine mesylate • Cogentin
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Nortriptyline • Aventyl, Pamelor
- Oxycodone • Oxycontin
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Risperidone • Risperdal
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Venlafaxine • Effexor
Disclosure
Dr. Mrazek reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Genotyping for cytochrome P-450 2D6 gene variations is emerging as a valuable clinical tool to help psychiatrists identify patients who:
Genetic variation has long been known to influence how individuals metabolize drugs, but only recently could we apply this information.5 Many academic medical centers and the two largest U.S. reference laboratories offer 2D6 testing at costs of $200 to $500.
Before long, psychiatrists may adopt routine genotyping before prescribing 2D6 substrate medications. This article and four vignettes illustrate the clinical benefits of psychiatric pharmacogenomics and suggest when prospective genotyping could help you select and dose medications.
Table 1
Drugs metabolized by the 2D6 enzyme*
| Antidepressants | Antipsychotics | Stimulants | Other medications |
|---|---|---|---|
| Desipramine | Fluphenazine | Atomoxetine | Codeine |
| Fluoxetine | Perphenazine | Dextromethorphan | |
| Nortriptyline | Risperidone | Oxycodone | |
| Paroxetine | Thioridazine | ||
| Venlafaxine | |||
| *Evidence suggests that these medications are predominantly metabolized by the 2D6 enzyme. | |||
| Be careful when prescribing these agents to patients who are poor 2D6 metabolizers. | |||
Why test for the 2D6 gene?
The 2D6 gene codes for the 2D6 enzyme, the primary enzyme required to metabolize many psychotropics Table 1.
Genetic variations. A common variation in a gene is frequently called an allele. More than 100 2D6 gene variations have been described. Consequently, the 2D6 gene’s enzyme activity also varies widely. Most mutations decrease the enzyme’s activity, but some polymorphisms change the gene’s promoter region, which can lead to upregulation and increased enzyme production.
Each 2D6 gene variation has been labeled with a standardized abbreviation (Table 2):
- *1 refers to the “normal” gene
- *2 stands for several variants with different activity levels.
- *3, *4, *6, *7, *8, *9, *10, *11, *12, *14, and *17 code for proteins with little or no activity.
- *5 indicates that the gene is deleted, and no enzyme can be produced.
Multiple copies. Another characteristic of the 2D6 gene is its unusually high propensity to accumulate in multiple copies on the 22nd chromosome. As many as 13 copies of the 2D6 gene have been shown on a single chromosome. Given that each gene can code for the 2D6 enzyme, patients with multiple copies can metabolize 2D6 substrate medications very rapidly.
Nonpsychiatric drugs. The 2D6 enzyme is also involved in metabolizing many nonpsychiatric drugs. To produce analgesia, for example, the 2D6 enzyme must metabolize the prodrug codeine to morphine. Thus, individuals with no 2D6 enzyme activity experience no analgesia with codeine. Approximately 7% of Caucasians metabolize codeine poorly. Conversely, individuals with multiple 2D6 gene copies metabolize codeine to morphine very rapidly, with potential for acute mental status changes, including psychosis.
4 metabolizer types. Based on variation in individual 2D6 genotype, a patient is usually categorized as being an ultrarapid, extensive, intermediate, or poor metabolizer (Table 3). The following case vignettes of patients in each category illustrate the clinical benefits of 2D6 genotyping.
Ultrarapid metabolizer: Extra 2D6 copies
Abdul, 49, is an Ethiopian businessman engaged in international commerce. While in the United States, he underwent a routine wisdom-tooth extraction and was treated with acetaminophen and codeine. Despite having no psychiatric history, he began to experience extreme discomfort and flashing visual hallucinations within 24 hours of taking two codeine doses. The oral surgeon instructed him to discontinue codeine, and his symptoms resolved within 24 hours.
Because of this experience, Abdul underwent genotyping for the 2D6 gene. He was found to have five active copies on one 22nd chromosome and no copies on the other (Figure 1). This genotype is unusual in western European populations but common in North Africa. Abdul then received alternate analgesics; psychiatric symptoms did not recur.
A patient such as Abdul, with multiple copies of a functional 2D6 gene, is an ultrarapid metabolizer. The 22nd chromosome—where the 2D6 gene is located—is short and contains areas of high homology. As a result, uneven crossover events occur more frequently during meiosis than is typical of larger chromosomes. Uneven crossover results in one gamete with two copies of the 2D6 gene and the other gamete with none.
2D6 enzyme activity is not essential for survival, which raises fascinating questions about this gene’s evolutionary importance. In certain geographic regions, many individuals have multiple copies of the gene. In Ethiopia—the country with the highest documented number of ultrarapid metabolizers—more than 25% of the population has one chromosome with multiple copies of the 2D6 gene.6 Because these copies produce an increased amount of 2D6 active enzyme, normal doses of 2D6 substrate medications do not benefit these individuals.
Table 2
How common 2D6 gene variations (alleles) affect 2D6 enzyme activity
| Allele label | 2D6 enzyme activity | Allele frequency (%)† |
|---|---|---|
| *1 | Normal | 37 |
| *2 | Decreased | 3.3 |
| *2P | Modestly increased | 6 |
| *3 | None | 1 |
| *4 | None | 18 |
| *5 | None (gene deletion) | 4 |
| *6 | None | 1 |
| *7 | None | <1 |
| *9 | Decreased | 3 |
| *10 | Decreased | 2 |
| *11 | None | 0 |
| *12 | None | <1 |
| *14 | Decreased | <1 |
| *17 | Decreased | <1 |
| †In Caucasian populations | ||
Table 3
Four ways patients respond to 2D6 substrate drugs
| Category | Patient characteristics | % of Caucasian population |
|---|---|---|
| Ultrarapid | Metabolize 2D6 medications rapidly resulting in poor response | 1 to 2 |
| Extensive | Metabolize 2D6 medications at a normal rate | 73 to 82 |
| Intermediate | Metabolize 2D6 medications at a slower-than-normal rate | 10 to 15 |
| Poor | Metabolize 2D6 medications very slowly with increased risk of side effects | 7 to 10 |
When treating ultrarapid metabolizers one strategy is to increase the dosage to obtain a therapeutic effect Because some substrates have complex metabolic pathways, however, high concentrations of secondary or tertiary metabolites can accumulate. Thus, when a substance’s metabolic pathway is not well-documented, a more cautious approach is to choose a medication metabolized by another pathway.
Figure 1 Genotypes and metabolizer categories of 4 illustrative patients
Extensive metabolizer: The ‘norm’
George, a 31-year-old Ethiopian architect, is Abdul’s second cousin. He developed acute depression with intense suicidal ideation and sought psychiatric consultation. He had no history of atypical drug reactions, but—because of his ethnic background—his psychiatrist was concerned that George might be a rapid metabolizer.
2D6 genotyping showed that George’s genotype was *1/*1, which meant he had two functional 2D6 copies (Figure 1). This genotype suggests that he could tolerate many antidepressants. The psychiatrist concluded—with some confidence—that George would not experience adverse effects or low serum levels when prescribed fluoxetine at usual dosages.
Extensive metabolizers have two normal 2D6 gene copies and can produce adequate active 2D6 enzyme Patients with this genotype—common in Caucasians—are generally said to have “normal” 2D6 metabolism. This means they metabolize 2D6 substrate medications at a rate within the recommended dosage ranges determined from North American or European pharmacokinetic studies.
Intermediate metabolizer: Mixed message
Katrina, 27, represents the government of her native Sweden in trade agreements. When she developed depressive symptoms (insomnia, sense of hopelessness), Katrina saw her psychiatrist. She reported that her family has a history of adverse reactions to multiple medications, but she had tolerated most medications. In fact, she had twice been successfully treated with relatively high doses of codeine.
Her psychiatrist suspected she was an intermediate 2D6 metabolizer and ordered testing. Her genotype was *1/*4, with one normal copy and one that produced no functional 2D6 enzyme (Figure 1).
Based on her clinical history and this genotypic information, the psychiatrist prescribed sertraline—metabolized by both 2D6 and 3A4 enzymes— at 50 mg/d. Because Katrina metabolized sertraline at a slower-than-usual rate, she developed a therapeutic blood level and responded well to this low dosage.
Intermediate metabolizers have a chromosome with one functional 2D6 gene copy. The other chromosome has either a copy with a defective functional polymorphism or a deletion of the gene. These patients usually tolerate 2D6 substrate drugs in low dosages.
Poor metabolizer: ‘medication-sensitive’
Olga, Katrina’s mother, has always lived in northern Sweden. She has no psychiatric history except for one psychotic episode that required hospitalization.
Her psychotic illness began on the summer solstice, during an all-night celebration. In addition to using unspecified recreational drugs, she took three 20-mg capsules of fluoxetine that her friend told her would make her feel high. She instead developed acute mania and dramatic paranoid delusions.
Figure 2 Possible genotypes of Brad, son of Abdul and Katrina
Olga was hospitalized and treated with moderate doses of haloperidol that precipitated an acute dystonic reaction. She was subsequently given ben-ztropine, and her extrapyramidal symptoms resolved. After discharge, she was treated with haloperidol and benztropine for 2 years, after which she spontaneously discontinued these drugs against medical advice. Her psychotic illness has not recurred.
Knowing her own genotype, Katrina understood that her mother had a 50% probability of having one copy of the 2D6 *4 allele. Given her mother’s history of medication intolerance, Katrina believed that her mother’s psychiatric illness might have been related to a drug reaction. She persuaded her mother to send a blood sample to a laboratory in Stockholm.
Olga’s genotype was *4/*4, indicating that she would be unlikely to tolerate even moderate doses of 2D6 substrate medications (Figure 1). Given her complete recovery and continued good health without medication, the most probable retrospective diagnosis was drug-induced psychosis. Her 2-year neuroleptic treatment probably was unnecessary.
Figure 3 Genogram for Brad, son of Abdul and Katrina
Poor metabolizers without a functional 2D6 gene copy have low tolerance for many medications and often become labeled as “medication sensitive.” When genotyping reveals that an individual is a poor metabolizer, prescribing medications that do not require 2D6 metabolism is usually prudent.
In rare cases, poor metabolizers have died from normal doses of 2D6 substrate medications.7 Far more commonly, however, they spontaneously discontinue taking these drugs because of adverse side effects.
Benefits of prospective testing
When used in clinical practice, pharmacogenomic testing’s two goals are to identify:
- ultrarapid metabolizers, who will not benefit from a medication
- poor metabolizers, who likely will have adverse responses to a medication.
The following case demonstrates the benefit of prospective 2D6 genotyping:
Brad, age 14, is the son of Abdul and Katrina, whose genotypes have been described. Brad developed a serious depression that was similar in severity and onset to an illness his mother experienced as a teen.
Brad’s parents want him to get the maximum benefit from psychopharmacologic treatment while avoiding distressing side effects. He had been healthy and had received no prescriptions other than antibiotics in the past.
How would you proceed? Without knowing Brad’s parents’ genotypes, you might reason that Brad would resemble one of them in drug response. However, when you review each parent’s genotype, you realize four scenarios are possible (Figure 2):
- Brad has a high likelihood of being an ultrarapid metabolizer because he has a 50% chance of inheriting a chromosome with five copies of the 2D6 gene from his father. He inherited the *1 or *4 form from his mother, but the effect of either will be clinically irrelevant.
- If Brad inherited the chromosome with the deletion from his father and the *1 form from his mother, he would be an intermediate metabolizer, as is his mother.
- If he inherited the chromosome with the deletion from his father and the *4 form from his mother, he would be a poor metabolizer like his grandmother, Olga. He would be at substantial risk for adverse reactions (such as intense headaches or vomiting) to 2D6 substrate medications.
On testing, Brad was found to be a poor metabolizer (Figure 3) The psychiatrist prescribed bupropion, which is metabolized by the 2B6 enzyme rather than the 2D6 enzyme.
Conclusion. To introduce the concept of genotypic testing, this review has focused on simple illustrations of variations in a single gene. However, many genes in the P-450 family play important roles in metabolizing psychotropics. In the future, genotyping of panels of these genes will likely provide more-specific guidance than can be achieved by simply testing one gene at a time.
Related resources
- Lerer B (ed). Pharmacogenetics of psychotropic drugs. Cambridge, UK: Cambridge University Press, 2002.
- Kirchheiner J, Borsen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;103(3):173-92.
- Indiana University School of Medicine, Division of Clinical Pharmacology. Drug Interactions—Defining Genetic Influences on Pharmacologic Responses. http://medicine.iupui.edu/flockhart.
Drug brand names
- Acetaminophen w/codeine phosphate • Tylenol w/codeine
- Atomoxetine • Strattera
- Benztropine mesylate • Cogentin
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Fluoxetine • Prozac
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Nortriptyline • Aventyl, Pamelor
- Oxycodone • Oxycontin
- Paroxetine • Paxil
- Perphenazine • Trilafon
- Risperidone • Risperdal
- Sertraline • Zoloft
- Thioridazine • Mellaril
- Venlafaxine • Effexor
Disclosure
Dr. Mrazek reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mrazek DA. Clinical genomic testing. In: Wiener J, Dulcan M (eds). Textbook of child and adolescent psychiatry (3rd ed). Washington, DC: American Psychiatric Publishing, Inc., 2001;193-203.
2. Mrazek DA. Pharmacogenomic screening for depressed children and adolescents (scientific proceedings). Miami Beach, FL: American Academy of Child and Adolescent Psychiatry annual meeting, 2003;159.-
3. Phillips KA, Veenstra DL, Oren E, et al. Potential role of pharmacogenomics in reducing adverse drug reactions. JAMA 2001;286(18):2270-9.
4. Shi MM, Mehrens D, Dacus K. Pharmacogenomics: Changing the health care paradigm. Modern Drug Discovery 2001;4(7):27-32.
5. Kirchheiner J, Brosen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;104:173-92.
6. Masimirembwa CM, Hasler JA. Genetic polymorphism of drug metabolising enzymes in African populations: implications for the use of neuroleptics and antidepressants. Brain Res Bull 1997;44(5):561-71.
7. Sallee FR, DeVane CL, Ferrell RE. Fluoxetine-related death in a child with cytochrome P-450 2D6 genetic deficiency. J Child Adolesc Psychopharmacol 2000;10(1):27-34.
8. Gaedigk A, Gotschall RR, Forbes NS, et al. Optimization of cytochrome P4502D6 (CYP2D6) phenotype assignment using a genotyping algorithm based on allele frequency data. Pharmacogenetics 1999;9(6):669-82.
1. Mrazek DA. Clinical genomic testing. In: Wiener J, Dulcan M (eds). Textbook of child and adolescent psychiatry (3rd ed). Washington, DC: American Psychiatric Publishing, Inc., 2001;193-203.
2. Mrazek DA. Pharmacogenomic screening for depressed children and adolescents (scientific proceedings). Miami Beach, FL: American Academy of Child and Adolescent Psychiatry annual meeting, 2003;159.-
3. Phillips KA, Veenstra DL, Oren E, et al. Potential role of pharmacogenomics in reducing adverse drug reactions. JAMA 2001;286(18):2270-9.
4. Shi MM, Mehrens D, Dacus K. Pharmacogenomics: Changing the health care paradigm. Modern Drug Discovery 2001;4(7):27-32.
5. Kirchheiner J, Brosen K, Dahl ML, et al. CYP2D6 and CYP2C19 genotype-based dose recommendations for antidepressants: a first step towards subpopulation-specific dosages. Acta Psychiatr Scand 2001;104:173-92.
6. Masimirembwa CM, Hasler JA. Genetic polymorphism of drug metabolising enzymes in African populations: implications for the use of neuroleptics and antidepressants. Brain Res Bull 1997;44(5):561-71.
7. Sallee FR, DeVane CL, Ferrell RE. Fluoxetine-related death in a child with cytochrome P-450 2D6 genetic deficiency. J Child Adolesc Psychopharmacol 2000;10(1):27-34.
8. Gaedigk A, Gotschall RR, Forbes NS, et al. Optimization of cytochrome P4502D6 (CYP2D6) phenotype assignment using a genotyping algorithm based on allele frequency data. Pharmacogenetics 1999;9(6):669-82.