User login
Locally advanced non–small cell lung cancer: What is the optimal concurrent chemoradiation regimen?
The population of patients with stage III non–small cell lung cancer (NSCLC) presents a management challenge for clinicians. The standard of care for locally advanced NSCLC is chemotherapy plus radiation, but the optimal chemoradiation regimen is a work in progress, building upon decades of clinical trial research. Optimal therapy may require patient participation in a current phase 3 clinical trial.
Understanding the background behind the design of phase 3 clinical trials may permit better understanding of optimal chemoradiation. Most recent research has focused on optimization of chemotherapy with less attention paid to radiation dose and technique, the use of targeted agents, and imaging and planning.
A dilemma in the management of stage III NSCLC is how best to combine the correct treatments in the right sequence to achieve simultaneous local, regional, and distant control, as the disease occurs at multiple levels and cure is not possible without local disease control. Another dilemma concerns administration of radiation therapy when the lung, heart, esophagus, or spinal cord may impede delivery of treatment. Additionally, patients may not present with symptoms until an advanced stage of disease, and their performance status is frequently impaired and often influenced by comorbidities such as smoking.
FACTORS RELATED TO PROGNOSIS AND CHOICE OF TREATMENT
Most potentially curable patients with NSCLC present with locally advanced mediastinal disease. Despite improvements in staging procedures and therapy, however, the prognosis of locally advanced NSCLC remains poor with a survival rate of less than 20% at 5 years.
Prognostic indicators
Factors that affect treatment choice
Clinical and patient factors can influence the choice of concurrent chemoradiation therapy. Weight loss, performance status, comorbidity, and pulmonary reserve influence survival and patient outcome. Comorbidities are frequently observed in elderly patients and smokers. More than one-half of patients with stage III NSCLC are currently thought to be ineligible for concurrent regimens if inclusion is restricted to patients younger than 75 years and those with fewer than two serious comorbidities. The exact contribution of comorbidity, age, and other clinical parameters to the reported toxicity is unclear.
Tumor biology
The biology of different types of NSCLC can vary considerably (eg, bronchoalveolar vs squamous cell vs adenocarcinoma). Sometimes cancer grows indolently, even with nodal presentations. Molecular profiling to understand this phenomenon is still in its infancy.
CURRENT APPROACHES TO CHEMORADIATION
Treatment of unresectable stage III NSCLC requires control of local disease and distant metastases. Much work has been undertaken to determine the safety and efficacy of sequential chemoradiation (chemotherapy followed by radiation therapy) and concurrent chemoradiation (chemotherapy during radiation therapy).
Sequential chemoradiation
Dillman et al2,3 ushered in an era of combined modality therapy when in 1990 they demonstrated that a 5-week course of induction chemotherapy followed by radiotherapy in stage III NSCLC resulted in improved median survival compared with radiotherapy alone (13.8 months vs 9.7 months) in a randomized trial.
Sause et al4,5 later showed that in “good risk” patients (Karnofsky Performance Status > 70) with surgically unresectable NSCLC, induction chemotherapy followed by radiation therapy produced superior short-term survival compared with hyperfractionated radiation therapy or standard radiation therapy alone.
Concurrent chemoradiation
The next step in the search for optimal sequencing was the study of concurrent chemotherapy and radiation. In phase 3 studies that compared sequential chemoradiation with concurrent chemoradiation, a consistent advantage in overall survival was conferred by concurrent chemoradiation therapy. Even with concurrent chemoradiation, however, survival was still modest (16% and 21% to 5 years in the two largest comparisons), and median survival improved only from 14.5 months with sequential therapy to 17.1 months with concurrent therapy in the largest comparison.6
Further support for concurrent chemoradiation on the end point of overall survival comes from two meta-analyses. A Cochrane meta-analysis demonstrated a significant 14% reduction in the risk of death with concurrent chemoradiation compared with sequential treatment.7 The NSCLC Collaborative Group discovered a significant survival advantage with concurrent chemoradiation compared with sequential treatment (hazard ratio: 0.84) with an absolute benefit of 5.7% at 3 years (3-year survival of 18.1% with sequential chemoradiation vs 23.8% with concurrent chemoradiation).8
Applying the results of clinical trials to appropriate patients offers the best chance to improve outcomes. The heterogeneity of the NSCLC population makes application of therapeutic advances challenging. One must consider that the selection criteria used in clinical trials, including performance status, weight loss, disease stage, and volume of disease have a great bearing on the results achieved.
When toxicity between the two multimodality approaches was compared, the risk of grade 3 or 4 acute esophagitis was found to increase from 4% with sequential chemoradiation therapy to 18% with concurrent treatment, but no difference in acute pulmonary toxicity has been observed.8
Some investigators used lower doses of chemotherapy in the concurrent chemoradiation arms to minimize radiation toxicity. However, the dose intensity in sequential treatment should be maintained so that the advantage of controlling micrometastatic disease is not lost.
These clinical trials highlight that timing of chemoradiation precludes a significant proportion of patients from receiving uninterrupted radiation therapy, either because of toxicity from chemotherapy, leading to a reduction in performance status, or disease progression during sequential chemotherapy.
ATTEMPTS TO IMPROVE RADIOTHERAPY
Methods to improve radiotherapy have centered on evolving radiologic imaging and computer technology, with the objective of enhanced precision of radiation delivery. The routine use of PET in planning radiotherapy allows for dose escalation and control of toxicity.
Radiotherapy dose and outcomes
Three-dimensional (3D) conformal radiation techniques permit the use of higher doses of targeted radiation to spare normal tissue. A meta-analysis of six trials of concurrent chemoradiation therapy concluded that an increased dose of radiation improves both local control and survival.9 A better understanding of normal lung tolerability to radiation therapy is needed to optimize radiation dose.
A clinical trial to test the efficacy of high-dose conformal radiation therapy is in progress. Patients with unresectable stage IIIA or IIIB NSCLC are being randomized to concurrent chemoradiation therapy with carboplatin and weekly paclitaxel with either 74 Gy of radiation in 37 fractions over 7.5 weeks, or 60 Gy of radiation in 30 fractions over 6 weeks. Results will be stratified by radiation therapy technique (3D conformal radiation or intensity-modulated radiation therapy). Following an impressive survival rate (median overall survival: 22.7 months) obtained with the addition of cetuximab to the chemoradiation regimen in the phase 2 Radiation Therapy Oncology Group 0324 trial, an amendment to the design further randomized patients in each radiotherapy group to cetuximab or no cetuximab.10 Those randomized to cetuximab will continue on consolidation therapy with carboplatin, paclitaxel, and cetuximab, while the group randomized to no cetuximab will receive consolidation therapy with carboplatin and paclitaxel only.
Another approach in stage III NSCLC is the use of molecular biomarkers to predict response. Tumor typing for specific molecular sensitivities is generally thought to help predict response to systemic chemotherapy, but within the setting of radiotherapy, patients with a mutation of the epidermal growth factor receptor (EGFR) were found to have more radiosensitive tumors and decreased local recurrence rates than those without the EGFR mutation.11,12 Interactions between systemic therapy and radiation may also prove to be important in response to therapy and prognosis.
ATTEMPTS TO IMPROVE SYSTEMIC THERAPY
A three-arm study compared sequential chemotherapy/radiotherapy, induction chemotherapy followed by concurrent chemoradiation, and concurrent chemoradiation followed by consolidation chemotherapy.14 In the sequential and induction arms, paclitaxel and carboplatin were administered for two cycles prior to radiation therapy; in the consolidation arm, the drugs were given following radiation therapy. The median survival was 16.3 months in the consolidation arm, 12.7 months in the induction arm, and 13.0 months in the sequential arm. The induction and consolidation arms were associated with greater toxicity. The incidences of grade 3/4 esophagitis and pulmonary toxicity were highest in the consolidation arm (28% and 16%, respectively). Although the study was not powered for direct comparison of the three treatment arms, the prolonged median survival for concurrent treatment followed by consolidation chemotherapy adds support to the argument that providing the definitive treatment up front followed by systemically active doses of chemotherapy is the preferred therapeutic approach in stage III NSCLC.
The Southwest Oncology Group (SWOG) study 9504 conducted in patients with stage IIIB NSCLC adds to the evidence of a benefit with consolidation chemotherapy after definitive chemoradiation.15 In this trial, consolidation with docetaxel following concurrent cisplatin-etoposide and radiotherapy extended median overall survival to 26 months.
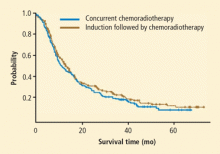
In the Hoosier Oncology Group (HOG) LUN 01-24 study, consolidation with docetaxel after cisplatin-etoposide did not have a survival advantage over cisplatin-etoposide and concurrent radiation alone, but it was associated with increased toxicity in patients with stage III inoperable NSCLC (Figure 2).16
The dose intensity and delivery of consolidation docetaxel were similar in the SWOG 9504 and the HOG LUN 01-24 studies. Although no difference in median survival was observed between the consolidation and observation arms in HOG LUN 01-24, the median survival for the observation arm in this trial was much higher than the 15 months demonstrated with the same concurrent regimen (cisplatin-etoposide and chest radiotherapy) in the SWOG 9019 trial.17 A difference in stage distribution across the two trials might explain the differences in survival in the observation arms.
LITTLE PROGRESS WITH BIOLOGIC THERAPIES
The improvements observed when combining chemotherapy with radiation therapy in sequence with systemically active doses of third-generation agents have come at a price of increased toxicity, and most patients will still suffer relapse and ultimately die of metastatic disease. A significant proportion of patients will not be fit enough for more aggressive regimens.
The addition of thalidomide as an immunomodulator agent to chemoradiation did not improve overall or progression-free survival; it was also associated with a higher rate of grade 3+ toxicities in patients with stage IIIA/B NSCLC.18
In CALBG 30407, a regimen of pemetrexed disodium and carboplatin together with radiation therapy with or without cetuximab was studied in patients with stage III unresectable NSCLC.19 Median survival was 22.3 months with pemetrexed-carboplatin; the addition of cetuximab conferred no significant benefit, with maintenance beyond 4 cycles being unfeasible in nearly 50% the patients enrolled.
Integrating the vascular endothelial growth factor inhibitor bevacizumab into combined modality therapy was tested in SWOG 0533. The study consisted of 3 treatment arms in which bevacizumab was introduced at different times in the concurrent chemoradiation setting in patients with stage III NSCLC. Accrual into the trial was terminated because of an unacceptable level of toxicity. Despite the risk stratification, restrictive eligibility criteria, and careful bevacizumab deployment, the approach still proved to be unfeasible.
The small-molecule epidermal tyrosine kinase inhibitors gefitinib and erlotinib had demonstrated efficacy as single agents, but the randomized SWOG 0023 trial of maintenance gefitinib after concurrent chemoradiation and consolidation therapy with docetaxel was terminated early when an interim analysis suggested lack of efficacy of maintenance gefitinib.
CONCLUSIONS
Stage III NSCLC is a heterogeneous disease with considerable variations in prognosis and treatment options. The goals of treatment are local control through the use of radiation therapy and chemotherapy and eradication of distant micrometastases through chemotherapy. For patients with good performance status, concurrent chemoradiation is the standard of care.
Phase 3 trials of full-dose chemotherapy, as either induction or consolidation, have not optimized outcomes. Integration of targeted agents is now under investigation. Any future progress will likely rely on molecular selection, which will require accruing a large number of patients into many clinical trials.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010:297–330.
- Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990; 323:940–945.
- Dillman RO, Herndon J, Seagren SL, Eaton WL, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. J Natl Cancer Inst 1996; 88:1210–1215.
- Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88-08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst 1995; 87:198–205.
- Sause W, Kolesar P, Taylor S, et al. Final results of phase III trial in regionally advanced, unresectable non-small-cell lung cancer*: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000; 117:358–364.
- Bayman NA, Blackhall F, Jain P, et al. Management of unresectable stage III non-small-cell lung cancer with combined-modality therapy: a review of the current literature and recommendations for treatment. Clin Lung Cancer 2008; 9:92–101.
- Rowell NP, O’Rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004; 18:CD002140.
- Aupérin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotheapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010; 28:2181–2190.
- Machtay M, Swann S, Komaki R, et al. Higher BED is associated with improved local-regional control and survival for NSCLC treated with chemoradiotherapy [ASTRO abstract]. Int J Radiat Oncol Biol Phys 2005; 63( suppl 1):S40.
- Blumenschein GR, Paulus R, Curran WJ, et al. A phase II study of cetuximab (C225) in combination with chemoradiation (CRT) in patients (PTS) with stage IIIA/B non-small cell lung cancer (NSCLC): a report of the 2 year and median survival (MS) for the RTOG 0324 trial. J Clin Oncol 2008; 26( suppl). Abstract 7516.
- Riesterer O, Milas L, Ang KK. Use of molecular biomarkers for predicting the response to radiotherapy with or without chemotherapy. J Clin Oncol 2007; 26:4075–4083.
- Mak RH, Doran E, Muzikansky A, et al. Thoracic radiation therapy in locally advanced NSCLC patients (pts) with EGFR mutations. J Clin Oncol 2010; 28( suppl). Abstract 7016.
- Vokes EE, Herndon JE, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 2007; 25:1698–1704.
- Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol 2005; 23:5883–5891.
- Gandara DR, Chansky K, Gaspar LE, Albain KS, Lara PN, Crowley J. Long term survival in stage IIIb non-small cell lung cancer (NSCLC) treated with consolidation docetaxel following concurrent chemoradiotherapy (SWOG S9504). J Clin Oncol 2005; 23( suppl). Abstract 7059.
- Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008; 35:5755–5760.
- Albain KS, Crowley JJ, Turrisi AT, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol 2002; 20:3454–3460.
- Schiller JH, Dahlberg SE, Mehta M, et al. A phase III trial of carboplatin, paclitaxel, and thoracic radiation therapy with or without thalidomide in patients with stage III non-small cell carcinoma of the lung (NSCLC): E3598. J Clin Oncol 2009; 27 (suppl). Abstract 7503.
- Govindan R, Bogart J, Wang X, et al. Phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small cell lung cancer: CALGB 30407. J Clin Oncol 2009; 27 (suppl). Abstract 7505.
The population of patients with stage III non–small cell lung cancer (NSCLC) presents a management challenge for clinicians. The standard of care for locally advanced NSCLC is chemotherapy plus radiation, but the optimal chemoradiation regimen is a work in progress, building upon decades of clinical trial research. Optimal therapy may require patient participation in a current phase 3 clinical trial.
Understanding the background behind the design of phase 3 clinical trials may permit better understanding of optimal chemoradiation. Most recent research has focused on optimization of chemotherapy with less attention paid to radiation dose and technique, the use of targeted agents, and imaging and planning.
A dilemma in the management of stage III NSCLC is how best to combine the correct treatments in the right sequence to achieve simultaneous local, regional, and distant control, as the disease occurs at multiple levels and cure is not possible without local disease control. Another dilemma concerns administration of radiation therapy when the lung, heart, esophagus, or spinal cord may impede delivery of treatment. Additionally, patients may not present with symptoms until an advanced stage of disease, and their performance status is frequently impaired and often influenced by comorbidities such as smoking.
FACTORS RELATED TO PROGNOSIS AND CHOICE OF TREATMENT
Most potentially curable patients with NSCLC present with locally advanced mediastinal disease. Despite improvements in staging procedures and therapy, however, the prognosis of locally advanced NSCLC remains poor with a survival rate of less than 20% at 5 years.
Prognostic indicators
Factors that affect treatment choice
Clinical and patient factors can influence the choice of concurrent chemoradiation therapy. Weight loss, performance status, comorbidity, and pulmonary reserve influence survival and patient outcome. Comorbidities are frequently observed in elderly patients and smokers. More than one-half of patients with stage III NSCLC are currently thought to be ineligible for concurrent regimens if inclusion is restricted to patients younger than 75 years and those with fewer than two serious comorbidities. The exact contribution of comorbidity, age, and other clinical parameters to the reported toxicity is unclear.
Tumor biology
The biology of different types of NSCLC can vary considerably (eg, bronchoalveolar vs squamous cell vs adenocarcinoma). Sometimes cancer grows indolently, even with nodal presentations. Molecular profiling to understand this phenomenon is still in its infancy.
CURRENT APPROACHES TO CHEMORADIATION
Treatment of unresectable stage III NSCLC requires control of local disease and distant metastases. Much work has been undertaken to determine the safety and efficacy of sequential chemoradiation (chemotherapy followed by radiation therapy) and concurrent chemoradiation (chemotherapy during radiation therapy).
Sequential chemoradiation
Dillman et al2,3 ushered in an era of combined modality therapy when in 1990 they demonstrated that a 5-week course of induction chemotherapy followed by radiotherapy in stage III NSCLC resulted in improved median survival compared with radiotherapy alone (13.8 months vs 9.7 months) in a randomized trial.
Sause et al4,5 later showed that in “good risk” patients (Karnofsky Performance Status > 70) with surgically unresectable NSCLC, induction chemotherapy followed by radiation therapy produced superior short-term survival compared with hyperfractionated radiation therapy or standard radiation therapy alone.
Concurrent chemoradiation
The next step in the search for optimal sequencing was the study of concurrent chemotherapy and radiation. In phase 3 studies that compared sequential chemoradiation with concurrent chemoradiation, a consistent advantage in overall survival was conferred by concurrent chemoradiation therapy. Even with concurrent chemoradiation, however, survival was still modest (16% and 21% to 5 years in the two largest comparisons), and median survival improved only from 14.5 months with sequential therapy to 17.1 months with concurrent therapy in the largest comparison.6
Further support for concurrent chemoradiation on the end point of overall survival comes from two meta-analyses. A Cochrane meta-analysis demonstrated a significant 14% reduction in the risk of death with concurrent chemoradiation compared with sequential treatment.7 The NSCLC Collaborative Group discovered a significant survival advantage with concurrent chemoradiation compared with sequential treatment (hazard ratio: 0.84) with an absolute benefit of 5.7% at 3 years (3-year survival of 18.1% with sequential chemoradiation vs 23.8% with concurrent chemoradiation).8
Applying the results of clinical trials to appropriate patients offers the best chance to improve outcomes. The heterogeneity of the NSCLC population makes application of therapeutic advances challenging. One must consider that the selection criteria used in clinical trials, including performance status, weight loss, disease stage, and volume of disease have a great bearing on the results achieved.
When toxicity between the two multimodality approaches was compared, the risk of grade 3 or 4 acute esophagitis was found to increase from 4% with sequential chemoradiation therapy to 18% with concurrent treatment, but no difference in acute pulmonary toxicity has been observed.8
Some investigators used lower doses of chemotherapy in the concurrent chemoradiation arms to minimize radiation toxicity. However, the dose intensity in sequential treatment should be maintained so that the advantage of controlling micrometastatic disease is not lost.
These clinical trials highlight that timing of chemoradiation precludes a significant proportion of patients from receiving uninterrupted radiation therapy, either because of toxicity from chemotherapy, leading to a reduction in performance status, or disease progression during sequential chemotherapy.
ATTEMPTS TO IMPROVE RADIOTHERAPY
Methods to improve radiotherapy have centered on evolving radiologic imaging and computer technology, with the objective of enhanced precision of radiation delivery. The routine use of PET in planning radiotherapy allows for dose escalation and control of toxicity.
Radiotherapy dose and outcomes
Three-dimensional (3D) conformal radiation techniques permit the use of higher doses of targeted radiation to spare normal tissue. A meta-analysis of six trials of concurrent chemoradiation therapy concluded that an increased dose of radiation improves both local control and survival.9 A better understanding of normal lung tolerability to radiation therapy is needed to optimize radiation dose.
A clinical trial to test the efficacy of high-dose conformal radiation therapy is in progress. Patients with unresectable stage IIIA or IIIB NSCLC are being randomized to concurrent chemoradiation therapy with carboplatin and weekly paclitaxel with either 74 Gy of radiation in 37 fractions over 7.5 weeks, or 60 Gy of radiation in 30 fractions over 6 weeks. Results will be stratified by radiation therapy technique (3D conformal radiation or intensity-modulated radiation therapy). Following an impressive survival rate (median overall survival: 22.7 months) obtained with the addition of cetuximab to the chemoradiation regimen in the phase 2 Radiation Therapy Oncology Group 0324 trial, an amendment to the design further randomized patients in each radiotherapy group to cetuximab or no cetuximab.10 Those randomized to cetuximab will continue on consolidation therapy with carboplatin, paclitaxel, and cetuximab, while the group randomized to no cetuximab will receive consolidation therapy with carboplatin and paclitaxel only.
Another approach in stage III NSCLC is the use of molecular biomarkers to predict response. Tumor typing for specific molecular sensitivities is generally thought to help predict response to systemic chemotherapy, but within the setting of radiotherapy, patients with a mutation of the epidermal growth factor receptor (EGFR) were found to have more radiosensitive tumors and decreased local recurrence rates than those without the EGFR mutation.11,12 Interactions between systemic therapy and radiation may also prove to be important in response to therapy and prognosis.
ATTEMPTS TO IMPROVE SYSTEMIC THERAPY
A three-arm study compared sequential chemotherapy/radiotherapy, induction chemotherapy followed by concurrent chemoradiation, and concurrent chemoradiation followed by consolidation chemotherapy.14 In the sequential and induction arms, paclitaxel and carboplatin were administered for two cycles prior to radiation therapy; in the consolidation arm, the drugs were given following radiation therapy. The median survival was 16.3 months in the consolidation arm, 12.7 months in the induction arm, and 13.0 months in the sequential arm. The induction and consolidation arms were associated with greater toxicity. The incidences of grade 3/4 esophagitis and pulmonary toxicity were highest in the consolidation arm (28% and 16%, respectively). Although the study was not powered for direct comparison of the three treatment arms, the prolonged median survival for concurrent treatment followed by consolidation chemotherapy adds support to the argument that providing the definitive treatment up front followed by systemically active doses of chemotherapy is the preferred therapeutic approach in stage III NSCLC.
The Southwest Oncology Group (SWOG) study 9504 conducted in patients with stage IIIB NSCLC adds to the evidence of a benefit with consolidation chemotherapy after definitive chemoradiation.15 In this trial, consolidation with docetaxel following concurrent cisplatin-etoposide and radiotherapy extended median overall survival to 26 months.
In the Hoosier Oncology Group (HOG) LUN 01-24 study, consolidation with docetaxel after cisplatin-etoposide did not have a survival advantage over cisplatin-etoposide and concurrent radiation alone, but it was associated with increased toxicity in patients with stage III inoperable NSCLC (Figure 2).16
The dose intensity and delivery of consolidation docetaxel were similar in the SWOG 9504 and the HOG LUN 01-24 studies. Although no difference in median survival was observed between the consolidation and observation arms in HOG LUN 01-24, the median survival for the observation arm in this trial was much higher than the 15 months demonstrated with the same concurrent regimen (cisplatin-etoposide and chest radiotherapy) in the SWOG 9019 trial.17 A difference in stage distribution across the two trials might explain the differences in survival in the observation arms.
LITTLE PROGRESS WITH BIOLOGIC THERAPIES
The improvements observed when combining chemotherapy with radiation therapy in sequence with systemically active doses of third-generation agents have come at a price of increased toxicity, and most patients will still suffer relapse and ultimately die of metastatic disease. A significant proportion of patients will not be fit enough for more aggressive regimens.
The addition of thalidomide as an immunomodulator agent to chemoradiation did not improve overall or progression-free survival; it was also associated with a higher rate of grade 3+ toxicities in patients with stage IIIA/B NSCLC.18
In CALBG 30407, a regimen of pemetrexed disodium and carboplatin together with radiation therapy with or without cetuximab was studied in patients with stage III unresectable NSCLC.19 Median survival was 22.3 months with pemetrexed-carboplatin; the addition of cetuximab conferred no significant benefit, with maintenance beyond 4 cycles being unfeasible in nearly 50% the patients enrolled.
Integrating the vascular endothelial growth factor inhibitor bevacizumab into combined modality therapy was tested in SWOG 0533. The study consisted of 3 treatment arms in which bevacizumab was introduced at different times in the concurrent chemoradiation setting in patients with stage III NSCLC. Accrual into the trial was terminated because of an unacceptable level of toxicity. Despite the risk stratification, restrictive eligibility criteria, and careful bevacizumab deployment, the approach still proved to be unfeasible.
The small-molecule epidermal tyrosine kinase inhibitors gefitinib and erlotinib had demonstrated efficacy as single agents, but the randomized SWOG 0023 trial of maintenance gefitinib after concurrent chemoradiation and consolidation therapy with docetaxel was terminated early when an interim analysis suggested lack of efficacy of maintenance gefitinib.
CONCLUSIONS
Stage III NSCLC is a heterogeneous disease with considerable variations in prognosis and treatment options. The goals of treatment are local control through the use of radiation therapy and chemotherapy and eradication of distant micrometastases through chemotherapy. For patients with good performance status, concurrent chemoradiation is the standard of care.
Phase 3 trials of full-dose chemotherapy, as either induction or consolidation, have not optimized outcomes. Integration of targeted agents is now under investigation. Any future progress will likely rely on molecular selection, which will require accruing a large number of patients into many clinical trials.
The population of patients with stage III non–small cell lung cancer (NSCLC) presents a management challenge for clinicians. The standard of care for locally advanced NSCLC is chemotherapy plus radiation, but the optimal chemoradiation regimen is a work in progress, building upon decades of clinical trial research. Optimal therapy may require patient participation in a current phase 3 clinical trial.
Understanding the background behind the design of phase 3 clinical trials may permit better understanding of optimal chemoradiation. Most recent research has focused on optimization of chemotherapy with less attention paid to radiation dose and technique, the use of targeted agents, and imaging and planning.
A dilemma in the management of stage III NSCLC is how best to combine the correct treatments in the right sequence to achieve simultaneous local, regional, and distant control, as the disease occurs at multiple levels and cure is not possible without local disease control. Another dilemma concerns administration of radiation therapy when the lung, heart, esophagus, or spinal cord may impede delivery of treatment. Additionally, patients may not present with symptoms until an advanced stage of disease, and their performance status is frequently impaired and often influenced by comorbidities such as smoking.
FACTORS RELATED TO PROGNOSIS AND CHOICE OF TREATMENT
Most potentially curable patients with NSCLC present with locally advanced mediastinal disease. Despite improvements in staging procedures and therapy, however, the prognosis of locally advanced NSCLC remains poor with a survival rate of less than 20% at 5 years.
Prognostic indicators
Factors that affect treatment choice
Clinical and patient factors can influence the choice of concurrent chemoradiation therapy. Weight loss, performance status, comorbidity, and pulmonary reserve influence survival and patient outcome. Comorbidities are frequently observed in elderly patients and smokers. More than one-half of patients with stage III NSCLC are currently thought to be ineligible for concurrent regimens if inclusion is restricted to patients younger than 75 years and those with fewer than two serious comorbidities. The exact contribution of comorbidity, age, and other clinical parameters to the reported toxicity is unclear.
Tumor biology
The biology of different types of NSCLC can vary considerably (eg, bronchoalveolar vs squamous cell vs adenocarcinoma). Sometimes cancer grows indolently, even with nodal presentations. Molecular profiling to understand this phenomenon is still in its infancy.
CURRENT APPROACHES TO CHEMORADIATION
Treatment of unresectable stage III NSCLC requires control of local disease and distant metastases. Much work has been undertaken to determine the safety and efficacy of sequential chemoradiation (chemotherapy followed by radiation therapy) and concurrent chemoradiation (chemotherapy during radiation therapy).
Sequential chemoradiation
Dillman et al2,3 ushered in an era of combined modality therapy when in 1990 they demonstrated that a 5-week course of induction chemotherapy followed by radiotherapy in stage III NSCLC resulted in improved median survival compared with radiotherapy alone (13.8 months vs 9.7 months) in a randomized trial.
Sause et al4,5 later showed that in “good risk” patients (Karnofsky Performance Status > 70) with surgically unresectable NSCLC, induction chemotherapy followed by radiation therapy produced superior short-term survival compared with hyperfractionated radiation therapy or standard radiation therapy alone.
Concurrent chemoradiation
The next step in the search for optimal sequencing was the study of concurrent chemotherapy and radiation. In phase 3 studies that compared sequential chemoradiation with concurrent chemoradiation, a consistent advantage in overall survival was conferred by concurrent chemoradiation therapy. Even with concurrent chemoradiation, however, survival was still modest (16% and 21% to 5 years in the two largest comparisons), and median survival improved only from 14.5 months with sequential therapy to 17.1 months with concurrent therapy in the largest comparison.6
Further support for concurrent chemoradiation on the end point of overall survival comes from two meta-analyses. A Cochrane meta-analysis demonstrated a significant 14% reduction in the risk of death with concurrent chemoradiation compared with sequential treatment.7 The NSCLC Collaborative Group discovered a significant survival advantage with concurrent chemoradiation compared with sequential treatment (hazard ratio: 0.84) with an absolute benefit of 5.7% at 3 years (3-year survival of 18.1% with sequential chemoradiation vs 23.8% with concurrent chemoradiation).8
Applying the results of clinical trials to appropriate patients offers the best chance to improve outcomes. The heterogeneity of the NSCLC population makes application of therapeutic advances challenging. One must consider that the selection criteria used in clinical trials, including performance status, weight loss, disease stage, and volume of disease have a great bearing on the results achieved.
When toxicity between the two multimodality approaches was compared, the risk of grade 3 or 4 acute esophagitis was found to increase from 4% with sequential chemoradiation therapy to 18% with concurrent treatment, but no difference in acute pulmonary toxicity has been observed.8
Some investigators used lower doses of chemotherapy in the concurrent chemoradiation arms to minimize radiation toxicity. However, the dose intensity in sequential treatment should be maintained so that the advantage of controlling micrometastatic disease is not lost.
These clinical trials highlight that timing of chemoradiation precludes a significant proportion of patients from receiving uninterrupted radiation therapy, either because of toxicity from chemotherapy, leading to a reduction in performance status, or disease progression during sequential chemotherapy.
ATTEMPTS TO IMPROVE RADIOTHERAPY
Methods to improve radiotherapy have centered on evolving radiologic imaging and computer technology, with the objective of enhanced precision of radiation delivery. The routine use of PET in planning radiotherapy allows for dose escalation and control of toxicity.
Radiotherapy dose and outcomes
Three-dimensional (3D) conformal radiation techniques permit the use of higher doses of targeted radiation to spare normal tissue. A meta-analysis of six trials of concurrent chemoradiation therapy concluded that an increased dose of radiation improves both local control and survival.9 A better understanding of normal lung tolerability to radiation therapy is needed to optimize radiation dose.
A clinical trial to test the efficacy of high-dose conformal radiation therapy is in progress. Patients with unresectable stage IIIA or IIIB NSCLC are being randomized to concurrent chemoradiation therapy with carboplatin and weekly paclitaxel with either 74 Gy of radiation in 37 fractions over 7.5 weeks, or 60 Gy of radiation in 30 fractions over 6 weeks. Results will be stratified by radiation therapy technique (3D conformal radiation or intensity-modulated radiation therapy). Following an impressive survival rate (median overall survival: 22.7 months) obtained with the addition of cetuximab to the chemoradiation regimen in the phase 2 Radiation Therapy Oncology Group 0324 trial, an amendment to the design further randomized patients in each radiotherapy group to cetuximab or no cetuximab.10 Those randomized to cetuximab will continue on consolidation therapy with carboplatin, paclitaxel, and cetuximab, while the group randomized to no cetuximab will receive consolidation therapy with carboplatin and paclitaxel only.
Another approach in stage III NSCLC is the use of molecular biomarkers to predict response. Tumor typing for specific molecular sensitivities is generally thought to help predict response to systemic chemotherapy, but within the setting of radiotherapy, patients with a mutation of the epidermal growth factor receptor (EGFR) were found to have more radiosensitive tumors and decreased local recurrence rates than those without the EGFR mutation.11,12 Interactions between systemic therapy and radiation may also prove to be important in response to therapy and prognosis.
ATTEMPTS TO IMPROVE SYSTEMIC THERAPY
A three-arm study compared sequential chemotherapy/radiotherapy, induction chemotherapy followed by concurrent chemoradiation, and concurrent chemoradiation followed by consolidation chemotherapy.14 In the sequential and induction arms, paclitaxel and carboplatin were administered for two cycles prior to radiation therapy; in the consolidation arm, the drugs were given following radiation therapy. The median survival was 16.3 months in the consolidation arm, 12.7 months in the induction arm, and 13.0 months in the sequential arm. The induction and consolidation arms were associated with greater toxicity. The incidences of grade 3/4 esophagitis and pulmonary toxicity were highest in the consolidation arm (28% and 16%, respectively). Although the study was not powered for direct comparison of the three treatment arms, the prolonged median survival for concurrent treatment followed by consolidation chemotherapy adds support to the argument that providing the definitive treatment up front followed by systemically active doses of chemotherapy is the preferred therapeutic approach in stage III NSCLC.
The Southwest Oncology Group (SWOG) study 9504 conducted in patients with stage IIIB NSCLC adds to the evidence of a benefit with consolidation chemotherapy after definitive chemoradiation.15 In this trial, consolidation with docetaxel following concurrent cisplatin-etoposide and radiotherapy extended median overall survival to 26 months.
In the Hoosier Oncology Group (HOG) LUN 01-24 study, consolidation with docetaxel after cisplatin-etoposide did not have a survival advantage over cisplatin-etoposide and concurrent radiation alone, but it was associated with increased toxicity in patients with stage III inoperable NSCLC (Figure 2).16
The dose intensity and delivery of consolidation docetaxel were similar in the SWOG 9504 and the HOG LUN 01-24 studies. Although no difference in median survival was observed between the consolidation and observation arms in HOG LUN 01-24, the median survival for the observation arm in this trial was much higher than the 15 months demonstrated with the same concurrent regimen (cisplatin-etoposide and chest radiotherapy) in the SWOG 9019 trial.17 A difference in stage distribution across the two trials might explain the differences in survival in the observation arms.
LITTLE PROGRESS WITH BIOLOGIC THERAPIES
The improvements observed when combining chemotherapy with radiation therapy in sequence with systemically active doses of third-generation agents have come at a price of increased toxicity, and most patients will still suffer relapse and ultimately die of metastatic disease. A significant proportion of patients will not be fit enough for more aggressive regimens.
The addition of thalidomide as an immunomodulator agent to chemoradiation did not improve overall or progression-free survival; it was also associated with a higher rate of grade 3+ toxicities in patients with stage IIIA/B NSCLC.18
In CALBG 30407, a regimen of pemetrexed disodium and carboplatin together with radiation therapy with or without cetuximab was studied in patients with stage III unresectable NSCLC.19 Median survival was 22.3 months with pemetrexed-carboplatin; the addition of cetuximab conferred no significant benefit, with maintenance beyond 4 cycles being unfeasible in nearly 50% the patients enrolled.
Integrating the vascular endothelial growth factor inhibitor bevacizumab into combined modality therapy was tested in SWOG 0533. The study consisted of 3 treatment arms in which bevacizumab was introduced at different times in the concurrent chemoradiation setting in patients with stage III NSCLC. Accrual into the trial was terminated because of an unacceptable level of toxicity. Despite the risk stratification, restrictive eligibility criteria, and careful bevacizumab deployment, the approach still proved to be unfeasible.
The small-molecule epidermal tyrosine kinase inhibitors gefitinib and erlotinib had demonstrated efficacy as single agents, but the randomized SWOG 0023 trial of maintenance gefitinib after concurrent chemoradiation and consolidation therapy with docetaxel was terminated early when an interim analysis suggested lack of efficacy of maintenance gefitinib.
CONCLUSIONS
Stage III NSCLC is a heterogeneous disease with considerable variations in prognosis and treatment options. The goals of treatment are local control through the use of radiation therapy and chemotherapy and eradication of distant micrometastases through chemotherapy. For patients with good performance status, concurrent chemoradiation is the standard of care.
Phase 3 trials of full-dose chemotherapy, as either induction or consolidation, have not optimized outcomes. Integration of targeted agents is now under investigation. Any future progress will likely rely on molecular selection, which will require accruing a large number of patients into many clinical trials.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010:297–330.
- Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990; 323:940–945.
- Dillman RO, Herndon J, Seagren SL, Eaton WL, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. J Natl Cancer Inst 1996; 88:1210–1215.
- Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88-08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst 1995; 87:198–205.
- Sause W, Kolesar P, Taylor S, et al. Final results of phase III trial in regionally advanced, unresectable non-small-cell lung cancer*: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000; 117:358–364.
- Bayman NA, Blackhall F, Jain P, et al. Management of unresectable stage III non-small-cell lung cancer with combined-modality therapy: a review of the current literature and recommendations for treatment. Clin Lung Cancer 2008; 9:92–101.
- Rowell NP, O’Rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004; 18:CD002140.
- Aupérin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotheapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010; 28:2181–2190.
- Machtay M, Swann S, Komaki R, et al. Higher BED is associated with improved local-regional control and survival for NSCLC treated with chemoradiotherapy [ASTRO abstract]. Int J Radiat Oncol Biol Phys 2005; 63( suppl 1):S40.
- Blumenschein GR, Paulus R, Curran WJ, et al. A phase II study of cetuximab (C225) in combination with chemoradiation (CRT) in patients (PTS) with stage IIIA/B non-small cell lung cancer (NSCLC): a report of the 2 year and median survival (MS) for the RTOG 0324 trial. J Clin Oncol 2008; 26( suppl). Abstract 7516.
- Riesterer O, Milas L, Ang KK. Use of molecular biomarkers for predicting the response to radiotherapy with or without chemotherapy. J Clin Oncol 2007; 26:4075–4083.
- Mak RH, Doran E, Muzikansky A, et al. Thoracic radiation therapy in locally advanced NSCLC patients (pts) with EGFR mutations. J Clin Oncol 2010; 28( suppl). Abstract 7016.
- Vokes EE, Herndon JE, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 2007; 25:1698–1704.
- Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol 2005; 23:5883–5891.
- Gandara DR, Chansky K, Gaspar LE, Albain KS, Lara PN, Crowley J. Long term survival in stage IIIb non-small cell lung cancer (NSCLC) treated with consolidation docetaxel following concurrent chemoradiotherapy (SWOG S9504). J Clin Oncol 2005; 23( suppl). Abstract 7059.
- Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008; 35:5755–5760.
- Albain KS, Crowley JJ, Turrisi AT, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol 2002; 20:3454–3460.
- Schiller JH, Dahlberg SE, Mehta M, et al. A phase III trial of carboplatin, paclitaxel, and thoracic radiation therapy with or without thalidomide in patients with stage III non-small cell carcinoma of the lung (NSCLC): E3598. J Clin Oncol 2009; 27 (suppl). Abstract 7503.
- Govindan R, Bogart J, Wang X, et al. Phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small cell lung cancer: CALGB 30407. J Clin Oncol 2009; 27 (suppl). Abstract 7505.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010:297–330.
- Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990; 323:940–945.
- Dillman RO, Herndon J, Seagren SL, Eaton WL, Green MR. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of Cancer and Leukemia Group B (CALGB) 8433 trial. J Natl Cancer Inst 1996; 88:1210–1215.
- Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88-08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst 1995; 87:198–205.
- Sause W, Kolesar P, Taylor S, et al. Final results of phase III trial in regionally advanced, unresectable non-small-cell lung cancer*: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000; 117:358–364.
- Bayman NA, Blackhall F, Jain P, et al. Management of unresectable stage III non-small-cell lung cancer with combined-modality therapy: a review of the current literature and recommendations for treatment. Clin Lung Cancer 2008; 9:92–101.
- Rowell NP, O’Rourke NP. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev 2004; 18:CD002140.
- Aupérin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotheapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010; 28:2181–2190.
- Machtay M, Swann S, Komaki R, et al. Higher BED is associated with improved local-regional control and survival for NSCLC treated with chemoradiotherapy [ASTRO abstract]. Int J Radiat Oncol Biol Phys 2005; 63( suppl 1):S40.
- Blumenschein GR, Paulus R, Curran WJ, et al. A phase II study of cetuximab (C225) in combination with chemoradiation (CRT) in patients (PTS) with stage IIIA/B non-small cell lung cancer (NSCLC): a report of the 2 year and median survival (MS) for the RTOG 0324 trial. J Clin Oncol 2008; 26( suppl). Abstract 7516.
- Riesterer O, Milas L, Ang KK. Use of molecular biomarkers for predicting the response to radiotherapy with or without chemotherapy. J Clin Oncol 2007; 26:4075–4083.
- Mak RH, Doran E, Muzikansky A, et al. Thoracic radiation therapy in locally advanced NSCLC patients (pts) with EGFR mutations. J Clin Oncol 2010; 28( suppl). Abstract 7016.
- Vokes EE, Herndon JE, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 2007; 25:1698–1704.
- Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol 2005; 23:5883–5891.
- Gandara DR, Chansky K, Gaspar LE, Albain KS, Lara PN, Crowley J. Long term survival in stage IIIb non-small cell lung cancer (NSCLC) treated with consolidation docetaxel following concurrent chemoradiotherapy (SWOG S9504). J Clin Oncol 2005; 23( suppl). Abstract 7059.
- Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008; 35:5755–5760.
- Albain KS, Crowley JJ, Turrisi AT, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: a Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol 2002; 20:3454–3460.
- Schiller JH, Dahlberg SE, Mehta M, et al. A phase III trial of carboplatin, paclitaxel, and thoracic radiation therapy with or without thalidomide in patients with stage III non-small cell carcinoma of the lung (NSCLC): E3598. J Clin Oncol 2009; 27 (suppl). Abstract 7503.
- Govindan R, Bogart J, Wang X, et al. Phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small cell lung cancer: CALGB 30407. J Clin Oncol 2009; 27 (suppl). Abstract 7505.