User login
Impact of a Multicenter, Mentored Quality Collaborative on Hospital-Associated Venous Thromboembolism
Deep venous thrombosis and pulmonary embolism, collectively known as venous thromboembolism (VTE), affect up to 600,000 Americans a year.1 Most of these are hospital-associated venous thromboembolisms (HA-VTE).1,2 VTE poses a substantial risk of mortality and long-term morbidity, and its treatment poses a risk of major bleeding.1 As appropriate VTE prophylaxis (“prophylaxis”) can reduce the risk of VTE by 40% to 80% depending on the patient population,3 VTE risk assessment and prophylaxis is endorsed by multiple guidelines4-7 and supported by regulatory agencies.8-10
However, despite extensive study, consensus about the impact of prophylaxis4,11 and the optimal method of risk assessment4,5,7,12 is lacking. Meanwhile, implementation of prophylaxis in real-world settings is poor; only 40% to 60% of at-risk patients receive prophylaxis,13 and as few as <20% receive optimal prophylaxis.14 Both systematic reviews15,16 and experience with VTE prevention collaboratives17,18 found that multifaceted interventions and alerts may be most effective in improving prophylaxis rates, but without proof of improved VTE rates.15 There is limited experience with large-scale VTE prevention. Organizations like The Joint Commission (TJC)8 and the Surgical Care Improvement Project have promoted quality measures but without clear evidence of improvement.19 In addition, an analysis of over 20,000 medical patients at 35 hospitals found no difference in VTE rates between high- and low-performing hospitals,20 suggesting that aggressive prophylaxis efforts may not reduce VTE, at least among medical patients.21 However, a 5-hospital University of California collaborative was associated with improved VTE rates, chiefly among surgical patients.22
In 2011, Dignity Health targeted VTE for improvement after investigations of potentially preventable HA-VTE revealed variable patterns of prophylaxis. In addition, improvement seemed feasible because there is a proven framework for VTE quality improvement (QI) projects17,18 and a record of success with the following 3 specific strategies: quality mentorship,23 use of a simple VTE risk assessment method, and active surveillance (real-time monitoring targeting suboptimal prophylaxis with concurrent intervention). This active surveillance technique has been used successfully in prior improvement efforts, often termed measure-vention.17,18,22,24
METHODS
Setting and Participants
The QI collaborative was performed at 35 Dignity Health community hospitals in California, Arizona, and Nevada. Facilities ranged from 25 to 571 beds in size with a mixture of teaching and nonteaching hospitals. Prior to the initiative, prophylaxis improvement efforts were incomplete and inconsistent at study facilities. All adult acute care inpatients at all facilities were included except rehabilitation, behavioral health, skilled nursing, hospice, other nonacute care, and inpatient deliveries.
Design Overview
We performed a prospective, unblinded, open-intervention study of a QI collaborative in 35 community hospitals and studied the effect on prophylaxis and VTE rates with historical controls. The 35 hospitals were organized into 2 cohorts. In the “pilot” cohort, 9 hospitals (chosen to be representative of the various settings, size, and teaching status within the Dignity system) received funding from the Gordon and Betty Moore Foundation (GBMF) for intensive, individualized QI mentorship from experts as well as active surveillance (see “Interventions”). The pilot sites led the development of the VTE risk assessment and prophylaxis protocol (“VTE protocol”), measures, order sets, implementation tactics, and lessons learned, assisted by the mentor experts. Dissemination to the 26-hospital “spread” cohort was facilitated by the Dignity Health Hospital Engagement Network (HEN) infrastructure.
Timeline
Two of the pilot sites, acting as leads on the development of protocol and order set tools, formed improvement teams in March 2011, 6 to 12 months earlier than other Dignity sites. Planning and design work occurred from March 2011 to September 2012. Most implementation at the 35 hospitals occurred in a staggered fashion during calendar year (CY) 2012 and 2013 (see Figure 1). As few changes were made until mid-2012, we considered CY 2011 the baseline for comparison, CY 2012 to 2013 the implementation years, and CY 2014 the postimplementation period.
The project was reviewed by the Institutional Review Board (IRB) of Dignity Health and determined to be an IRB-exempt QI project.
Interventions
Collaborative Infrastructure
Multidisciplinary Teams
Improvement teams formed between March 2011 and September 2012. Members included a physician champion, frontline nurses and physicians, an administrative liaison, pharmacists, quality and data specialists, clinical informatics staff, and stakeholders from key clinical services. Teams met at least monthly at each site.
Physician Mentors
The 9 pilot sites received individualized mentorship provided by outside experts (IJ or GM) based on a model pioneered by the Society of Hospital Medicine’s (SHM) Mentored Implementation programs.23 Each pilot site completed a self-assessment survey17 (see supplementary Appendix A) about past efforts, team composition, current performance, aims, barriers, and opportunities. The mentors reviewed the completed questionnaire with each hospital and provided advice on the VTE protocol and order set design, measurement, and benchmarking during 3 webinar meetings scheduled at 0, 3, and 9 months, plus as-needed e-mail and phone correspondence. After each webinar, the mentors provided detailed improvement suggestions (see supplementary Appendix B). Several hospitals received mentor site visits, which focused on unit rounding, active surveillance, staff and provider education, and problem-solving sessions with senior leadership, physician leadership, and the improvement team.
VTE Protocol
After a literature review and consultation with the mentors, Dignity Health developed and implemented a VTE protocol, modified from a model used in previous improvement efforts.18,22-24 Its risk assessment method is often referred to as a “3 bucket” model because it assigns patients to high-, moderate-, or low-risk categories based on clinical factors (eg, major orthopedic surgery, prior VTE, and others), and the VTE protocol recommends interventions based on the risk category (see supplementary Appendix C). Dignity Health was transitioning to a single electronic health record (Cerner Corporation, North Kansas City, MO) during the study, and study hospitals were using multiple platforms, necessitating the development of both paper and electronic versions of the VTE protocol. The electronic version required completion of the VTE protocol for all inpatient admissions and transfers. The VTE protocol was completed in November 2011 and disseminated to other sites in a staggered fashion through November 2012. Completed protocols and improvement tips were shared by the project lead and by webinar sessions. Sites were also encouraged to implement a standardized practice that allowed nurses to apply sequential compression devices to at-risk patients without physician orders when indicated by protocol, when contraindications such as vascular disease or ulceration were absent.
Education
Staff were educated about the VTE protocol by local teams, starting between late 2011 and September 2012. The audience (physicians, nurses, pharmacists, etc.) and methods (conferences, fliers, etc.) were determined by local teams, following guidance by mentors and webinar content. Active surveillance provided opportunities for in-the-moment, patient-specific education and protocol reinforcement. Both mentors delivered educational presentations at pilot sites.
Active Surveillance
Sites were encouraged to perform daily review of prophylaxis adequacy for inpatients and correct lapses in real time (both under- and overprophylaxis). Inappropriate prophylaxis orders were addressed by contacting providers to change the order or document the rationale not to. Lapses in adherence to prophylaxis were addressed by nursing correction and education of involved staff. Active surveillance was funded for 10 hours a week at pilot sites. Spread sites received only minimal support from HEN monies. All sites used daily prophylaxis reports, enhanced to include contraindications like thrombocytopenia and coagulopathy, to facilitate efforts. Active surveillance began in May 2012 in the lead pilot hospitals and was implemented in other sites between October 2012 and February 2013.
Metrics
Prophylaxis Rates
Measurement of prophylaxis did not begin until 2012 to 2013; thus, the true baseline rate for prophylaxis was not captured. TJC metrics (VTE-1 and VTE-2)25 were consolidated into a composite TJC prophylaxis rate from January 2012 to December 2014 for both pilot and spread hospitals. These measures assess the percentage of adult inpatients who received VTE prophylaxis or have documentation of why no prophylaxis was given the day of or day after hospital admission (VTE-1) or the day of or day after ICU admission or transfer (VTE-2). These measures are met if any mechanical or pharmacologic prophylaxis was delivered.
In addition to the TJC metric, the 9 pilot hospitals monitored rates of protocol-compliant prophylaxis for 12 to 20 months. Each patient’s prophylaxis was considered protocol compliant if it was consistent with the prophylaxis protocol at the time of the audit or if contraindications were documented (eg, patients eligible for, but with contraindications to, pharmacologic prophylaxis had to have an order for mechanical prophylaxis or documented contraindication to both modalities). As this measure was initiated in a staggered fashion, the rate of protocol-compliant prophylaxis is summarized for consecutive months of measurement rather than consecutive calendar months.
HA-VTE Rates
VTE events were captured by review of electronic coding data for the International Classification of Diseases, 9th Revision (ICD-9) codes 415.11-415.19, 453.2, 453.40-453.42, and 453.8-453.89. HA-VTE was defined as either new VTE not present on admission (NPOA HA-VTE) or new VTE presenting in a readmitted patient within 30 days of discharge (Readmit HA-VTE). Cases were stratified based on whether the patient had undergone a major operation (surgery patients) or not (medical patients) as identified by Medicare Services diagnosis-related group codes.
Control Measures
Potential adverse events were captured by review of electronic coding data for ICD-9 codes 289.84 (heparin-induced thrombocytopenia [HIT]) and E934.2 (adverse effects because of anticoagulants).
Statistical Analysis
Statistical process control charts were used to depict changes in prophylaxis rates over the 3 years for which data was collected. For VTE and safety outcomes, Pearson χ2 value with relative risk (RR) calculations and 95% confidence intervals (CIs) were used to compare proportions between groups at baseline (CY 2011) versus postimplementation (CY 2014). Differences between the means of normally distributed data were calculated, and a 95% CI for the difference between the means was performed to assess statistical difference. Nonparametric characteristics were described by quartiles and interquartile range, and the 2-sided Mann-Whitney U test was performed to assess statistical difference between the CY 2011 and CY 2014 period.
Role of the Funding Source
The GBMF funded the collaborative and supported authorship of the manuscript but had no role in the design or conduct of the intervention, the collection or analysis of data, or the drafting of the manuscript.
RESULTS
Population Demographics
There were 1,155,069 adult inpatient admissions during the 4-year study period (264,280 in the 9 pilot sites, 890,789 in the 26 spread sites). There were no clinically relevant changes in gender distribution, mortality rate, median age, case mix index, or hospital length of stay in 2011 versus 2014. Men comprised 47.1% of the patient population in 2011 and 47.7% in 2014. The mortality rate was 2.7% in both years. Median age was 62 in 2011 and 63 in 2014. The mean case mix index (1.58 vs 1.65) and mean length of stay (4.29 vs 4.33 days) were similar in the 2 time periods.
Prophylaxis Rates
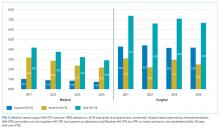
TJC Prophylaxis rates
Rates of Protocol-Compliant Prophylaxis
There were 34,071 active surveillance audits across the 20 months of reporting in the pilot cohort (mean, 1817 audits per month). The rate of protocol-compliant prophylaxis improved from 89% at month 1 of observation to 93% during month 2 and 97% by the last 3 months (Pearson χ2 P < .001 for both comparisons).
HA-VTE
HA-VTE characteristics
Improved HA-VTE over Time
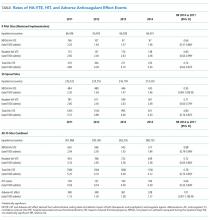
Four hundred twenty-eight fewer HA-VTEs occurred in 2014 than in 2011 (RR 0.78; 95% CI, 0.73-0.85) (Table and Figure 3). Readmission HA-VTEs were reduced by 315 (RR 0.72; 95% CI, 0.65-0.80), while the reduction in NPOA HA-VTEs was less robust (RR 0.88; 95% CI, 0.79-0.99). Pilot sites enjoyed a more robust reduction in HA-VTEs than spread sites (26% vs 20%), largely because the pilot cohort enjoyed a 34% reduction in NPOA HA-VTEs and a 20% reduction in Readmit HA-VTEs, while the spread cohort only achieved reductions in Readmit HA-VTEs.
Safety
Rates of HIT and adverse effects because of anticoagulants were low (Table). The rate of HIT declined from 178 events in 2011 to 109 in 2014 (RR 0.66; 95% CI, 0.52-0.84), and the RR of anticoagulant adverse events remained stable (RR 1.01; 95% CI, 0.87-1.15).
DISCUSSION
Our QI project, based on a proven collaborative approach and mentorship,18,22,24 order set redesign, and active surveillance, was associated with 26% less VTEs in the pilot cohort and 20% less VTEs in the spread cohort. These gains, down to a final rate of approximately 4 HA-VTEs per 1000 admissions, occurred despite a low baseline HA-VTE rate. Dignity Health achieved these improvements in 35 hospitals with varied sizes, settings, ordering systems, and teaching statuses, achieving what is to our knowledge the largest VTE QI initiative yet reported.
Implementation experiences were not systematically recorded, and techniques were not compared with a control group. However, we believe that Dignity Health’s organizational commitment to improvement and centralized support were crucial for success. In addition, the pilot sites received grant support from the GBMF for intensive quality mentoring, a strategy with demonstrated value.23 Mentors and team members noted that system-wide revision to the computerized physician order entry system was easiest to implement, while active surveillance represented the most labor-intensive intervention. Other experiences echoed lessons from previous VTE mentorship efforts.17,18
The selection of a VTE protocol conducive to implementation and provider use was a key strategy. The ideal approach to VTE risk assessment is not known,12,26 but guidelines either offer no specific guidance7 or would require implementation of 3 different systems per hospital.4,5 Several of these are point scoring systems, which may have lower clinician acceptance or require programming to improve real-world use18,26,27; the Padua score was derived from a patient population that differs significantly from those in the United States.12 Our study provides more practical experience with a “3-bucket” model, which has previously shown high interobserver reliability, good clinician acceptance, and meaningful reductions of VTE, including in American patient populations.18,22,24
The value of VTE prophylaxis is still disputed in many inpatient groups. The overall rate of HA-VTE is low, so the per-patient benefit of prophylaxis is low, and many patients may be overprophylaxed.4,11,12 Recently, Flanders et al.20 reported that HA-VTE rates among 20,800 medical inpatients in Michigan were low (about 1%) and similar at hospitals in the top (mean prophylaxis rate 86%) or bottom (mean prophylaxis rate 56%) tertiles of performance. Possible explanations for the differences between their multicenter experience and ours include our sample size (55 times larger) and the possibility that targeting prophylaxis to patients at highest need (captured in our protocol-compliant prophylaxis rates) matters more than prophylaxing a percent of the population.
Further research is needed to develop simple, easy-to-implement methods to identify inpatients who do not, or no longer, require prophylaxis.12 Hospital systems also need methods to determine if prophylaxis improvement efforts can lower their HA-VTE rates and in which subpopulations. For example, a collaborative effort at the University of California lowered HA-VTE rates toward a common improved rate of 0.65% to 0.73%,22 while Dignity Health achieved improvement despite starting with an even lower baseline. In the University of California collaborative, benefits were limited chiefly to surgical patients, while Dignity Health achieved most improvement in medical patients, particularly in Readmit HA-VTE. If future research uncovers the reasons for these differences, it could help hospitals decide where to target improvement efforts.
Our study has several limitations. First, we used a nonrandomized time series design, so we cannot exclude other potential explanations for the change in VTE rates. However, there were no major changes in patient populations or concurrent projects likely to have influenced event rates. While we did not collect detailed demographic information on subjects, the broad inclusion criteria and multicenter design suggests a high degree of generalizability. Second, we followed inpatient VTE events and VTE-related readmissions, but not VTE treated in the outpatient setting. This did not change over the study, but the availability of all-oral therapy for VTE could have caused underdetection if clinic or emergency room doctors sent home more patients on oral therapy instead of readmitting them to the hospital. Third, implementation was enhanced by GBMF funds (at 9 sites, with the remainder benefitting from their experience), a shared electronic med
Strengths of our study include reductions in HA-VTE, both with and without access to GBMF funds, by using broadly available QI strategies.17 This real-world success and ease of dissemination are particularly important because the clinical trials of prophylaxis have been criticized for using highly selected patient populations,11 and prophylaxis QI studies show an inconsistent impact on VTE outcomes.15 In previous studies, two of the authors monitored orders for prophylaxis22,24; during this project, delivery for both pharmacologic and mechanical VTE prophylaxis was monitored, confirming that patient care actually changed.
CONCLUSION
Our multicenter VTE prophylaxis initiative, featuring a “3-bucket” VTE protocol, QI mentorship, and active surveillance as key interventions, was associated with improved prophylaxis rates and a reduction in HA-VTE by 22% with no increase in adverse events. This project provides a model for hospital systems seeking to optimize their prophylaxis efforts, and it supports the use of collaborative QI initiatives and SHM’s quality mentorship program as methods to drive improvement across health systems.
Disclosure
None of the authors have any conflicts of interest related to any topics or products discussed in the article. Dignity Health provided a stipend for writing the manuscript to GM and IJ, as noted in the article, but had no role in data analysis, writing, or decision to submit.
1. U.S. Department of Health and Human Services; National Heart, Lung, and Blood Institute. Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville: Office of the Surgeon General; 2008.
2. Heit JA, Melton LJ, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients versus community residents. Mayo Clin Proc. 2001;76(11):1102-1110. PubMed
3. Guyatt GH, Eikelboom JW, Gould MK. Approach to Outcome Measurement in the Prevention of Thrombosis in Surgical and Medical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e185S-e194S. doi:10.1378/chest.11-2289. PubMed
4. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in Nonsurgical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S. doi:10.1378/chest.11-2296. PubMed
5. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in Nonorthopedic Surgical Patients. Chest. 2012;141(2 suppl):e227S-e277S. PubMed
6. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in Orthopedic Surgery Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S. doi:10.1378/chest.11-2404. PubMed
7. Qaseem A, Chou R, Humphrey LL. Venous Thromboembolism Prophylaxis in Hospitalized Patients: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. PubMed
8. The Joint Commission. Performance Measurement Initiatives. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement. Accessed June 14, 2012.
9. National Quality Forum. National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures. http://www.qualityforum.org/Publications/2006/12/National_Voluntary_Consensus_Standards_for_Prevention_and_Care_of_Venous_Thromboembolism__Policy,_Preferred_Practices,_and_Initial_Performance_Measures.aspx. Accessed June 14, 2012.
10. Medicare Quality Improvement Committee. SCIP Project Information. Agency for Healthcare Research and Quality. http://www.qualitymeasures.ahrq.gov/content.aspx?id=35538&search=scip. Accessed March 2013.
11. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients and Those with Stroke: A Background Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. PubMed
12. Rothberg MB. Venous thromboembolism prophylaxis for medical patients: who needs it? JAMA Intern Med. 2014;174(10):1585-1586. PubMed
13. Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross-sectional study. Lancet. 2008;371(9610):387-394. PubMed
14. Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician’s recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4(8):E15-E21. PubMed
15. Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201. doi:10.1002/14651858.CD008201.pub2. PubMed
16. Lau BD, Haut ER. Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23(3):187-195. PubMed
17. Maynard G. Preventing hospital-associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. Rockville: Agency for Healthcare Research and Quality; 2015. https://www.ahrq.gov/sites/default/files/publications/files/vteguide.pdf. Accessed October 29, 2017.
18. Maynard G, Stein J. Designing and Implementing Effective VTE Prevention Protocols: Lessons from Collaboratives. J Thromb Thrombolysis. 2010;29(2):159-166. PubMed
19. Altom LK, Deierhoi RJ, Grams J, et al. Association between Surgical Care Improvement Program venous thromboembolism measures and postoperative events. Am J Surg. 2012;204(5):591-597. PubMed
20. Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. PubMed
21. Finn KM, Greenwald JL. Update in Hospital Medicine: Evidence You Should Know. J Hosp Med. 2015;10(12):817-826. PubMed
22. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: Findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. PubMed
23. Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg Patient Safety and Quality Award. Mentored Implementation: Building Leaders and Achieving Results Through a Collaborative Improvement Model at the National Level. Jt Comm J Qual Patient Saf. 2012;38(7):301-310.
24. Maynard GA, Morris TA, Jenkins IH, et al. Optimizing prevention of hospital-acquired venous thromboembolism (VTE): Prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10-18. PubMed
25. The Joint Commission. Venous Thromboembolism Quality Measures. https://www.jointcommission.org/venous_thromboembolism/. Accessed October 13, 2017.
26. Maynard GA, Jenkins IH, Merli GJ. Venous thromboembolism prevention guidelines for medical inpatients: Mind the (implementation) Gap. J Hosp Med. 2013;8(10):582-588. PubMed
27. Elias P, Khanna R, Dudley A, et al. Automating Venous Thromboembolism Risk Calculation Using Electronic Health Record Data upon Hospital Admission: The Automated Padua Prediction Score. J Hosp Med. 2017;12(4):231-237. PubMed
Deep venous thrombosis and pulmonary embolism, collectively known as venous thromboembolism (VTE), affect up to 600,000 Americans a year.1 Most of these are hospital-associated venous thromboembolisms (HA-VTE).1,2 VTE poses a substantial risk of mortality and long-term morbidity, and its treatment poses a risk of major bleeding.1 As appropriate VTE prophylaxis (“prophylaxis”) can reduce the risk of VTE by 40% to 80% depending on the patient population,3 VTE risk assessment and prophylaxis is endorsed by multiple guidelines4-7 and supported by regulatory agencies.8-10
However, despite extensive study, consensus about the impact of prophylaxis4,11 and the optimal method of risk assessment4,5,7,12 is lacking. Meanwhile, implementation of prophylaxis in real-world settings is poor; only 40% to 60% of at-risk patients receive prophylaxis,13 and as few as <20% receive optimal prophylaxis.14 Both systematic reviews15,16 and experience with VTE prevention collaboratives17,18 found that multifaceted interventions and alerts may be most effective in improving prophylaxis rates, but without proof of improved VTE rates.15 There is limited experience with large-scale VTE prevention. Organizations like The Joint Commission (TJC)8 and the Surgical Care Improvement Project have promoted quality measures but without clear evidence of improvement.19 In addition, an analysis of over 20,000 medical patients at 35 hospitals found no difference in VTE rates between high- and low-performing hospitals,20 suggesting that aggressive prophylaxis efforts may not reduce VTE, at least among medical patients.21 However, a 5-hospital University of California collaborative was associated with improved VTE rates, chiefly among surgical patients.22
In 2011, Dignity Health targeted VTE for improvement after investigations of potentially preventable HA-VTE revealed variable patterns of prophylaxis. In addition, improvement seemed feasible because there is a proven framework for VTE quality improvement (QI) projects17,18 and a record of success with the following 3 specific strategies: quality mentorship,23 use of a simple VTE risk assessment method, and active surveillance (real-time monitoring targeting suboptimal prophylaxis with concurrent intervention). This active surveillance technique has been used successfully in prior improvement efforts, often termed measure-vention.17,18,22,24
METHODS
Setting and Participants
The QI collaborative was performed at 35 Dignity Health community hospitals in California, Arizona, and Nevada. Facilities ranged from 25 to 571 beds in size with a mixture of teaching and nonteaching hospitals. Prior to the initiative, prophylaxis improvement efforts were incomplete and inconsistent at study facilities. All adult acute care inpatients at all facilities were included except rehabilitation, behavioral health, skilled nursing, hospice, other nonacute care, and inpatient deliveries.
Design Overview
We performed a prospective, unblinded, open-intervention study of a QI collaborative in 35 community hospitals and studied the effect on prophylaxis and VTE rates with historical controls. The 35 hospitals were organized into 2 cohorts. In the “pilot” cohort, 9 hospitals (chosen to be representative of the various settings, size, and teaching status within the Dignity system) received funding from the Gordon and Betty Moore Foundation (GBMF) for intensive, individualized QI mentorship from experts as well as active surveillance (see “Interventions”). The pilot sites led the development of the VTE risk assessment and prophylaxis protocol (“VTE protocol”), measures, order sets, implementation tactics, and lessons learned, assisted by the mentor experts. Dissemination to the 26-hospital “spread” cohort was facilitated by the Dignity Health Hospital Engagement Network (HEN) infrastructure.
Timeline
Two of the pilot sites, acting as leads on the development of protocol and order set tools, formed improvement teams in March 2011, 6 to 12 months earlier than other Dignity sites. Planning and design work occurred from March 2011 to September 2012. Most implementation at the 35 hospitals occurred in a staggered fashion during calendar year (CY) 2012 and 2013 (see Figure 1). As few changes were made until mid-2012, we considered CY 2011 the baseline for comparison, CY 2012 to 2013 the implementation years, and CY 2014 the postimplementation period.
The project was reviewed by the Institutional Review Board (IRB) of Dignity Health and determined to be an IRB-exempt QI project.
Interventions
Collaborative Infrastructure
Multidisciplinary Teams
Improvement teams formed between March 2011 and September 2012. Members included a physician champion, frontline nurses and physicians, an administrative liaison, pharmacists, quality and data specialists, clinical informatics staff, and stakeholders from key clinical services. Teams met at least monthly at each site.
Physician Mentors
The 9 pilot sites received individualized mentorship provided by outside experts (IJ or GM) based on a model pioneered by the Society of Hospital Medicine’s (SHM) Mentored Implementation programs.23 Each pilot site completed a self-assessment survey17 (see supplementary Appendix A) about past efforts, team composition, current performance, aims, barriers, and opportunities. The mentors reviewed the completed questionnaire with each hospital and provided advice on the VTE protocol and order set design, measurement, and benchmarking during 3 webinar meetings scheduled at 0, 3, and 9 months, plus as-needed e-mail and phone correspondence. After each webinar, the mentors provided detailed improvement suggestions (see supplementary Appendix B). Several hospitals received mentor site visits, which focused on unit rounding, active surveillance, staff and provider education, and problem-solving sessions with senior leadership, physician leadership, and the improvement team.
VTE Protocol
After a literature review and consultation with the mentors, Dignity Health developed and implemented a VTE protocol, modified from a model used in previous improvement efforts.18,22-24 Its risk assessment method is often referred to as a “3 bucket” model because it assigns patients to high-, moderate-, or low-risk categories based on clinical factors (eg, major orthopedic surgery, prior VTE, and others), and the VTE protocol recommends interventions based on the risk category (see supplementary Appendix C). Dignity Health was transitioning to a single electronic health record (Cerner Corporation, North Kansas City, MO) during the study, and study hospitals were using multiple platforms, necessitating the development of both paper and electronic versions of the VTE protocol. The electronic version required completion of the VTE protocol for all inpatient admissions and transfers. The VTE protocol was completed in November 2011 and disseminated to other sites in a staggered fashion through November 2012. Completed protocols and improvement tips were shared by the project lead and by webinar sessions. Sites were also encouraged to implement a standardized practice that allowed nurses to apply sequential compression devices to at-risk patients without physician orders when indicated by protocol, when contraindications such as vascular disease or ulceration were absent.
Education
Staff were educated about the VTE protocol by local teams, starting between late 2011 and September 2012. The audience (physicians, nurses, pharmacists, etc.) and methods (conferences, fliers, etc.) were determined by local teams, following guidance by mentors and webinar content. Active surveillance provided opportunities for in-the-moment, patient-specific education and protocol reinforcement. Both mentors delivered educational presentations at pilot sites.
Active Surveillance
Sites were encouraged to perform daily review of prophylaxis adequacy for inpatients and correct lapses in real time (both under- and overprophylaxis). Inappropriate prophylaxis orders were addressed by contacting providers to change the order or document the rationale not to. Lapses in adherence to prophylaxis were addressed by nursing correction and education of involved staff. Active surveillance was funded for 10 hours a week at pilot sites. Spread sites received only minimal support from HEN monies. All sites used daily prophylaxis reports, enhanced to include contraindications like thrombocytopenia and coagulopathy, to facilitate efforts. Active surveillance began in May 2012 in the lead pilot hospitals and was implemented in other sites between October 2012 and February 2013.
Metrics
Prophylaxis Rates
Measurement of prophylaxis did not begin until 2012 to 2013; thus, the true baseline rate for prophylaxis was not captured. TJC metrics (VTE-1 and VTE-2)25 were consolidated into a composite TJC prophylaxis rate from January 2012 to December 2014 for both pilot and spread hospitals. These measures assess the percentage of adult inpatients who received VTE prophylaxis or have documentation of why no prophylaxis was given the day of or day after hospital admission (VTE-1) or the day of or day after ICU admission or transfer (VTE-2). These measures are met if any mechanical or pharmacologic prophylaxis was delivered.
In addition to the TJC metric, the 9 pilot hospitals monitored rates of protocol-compliant prophylaxis for 12 to 20 months. Each patient’s prophylaxis was considered protocol compliant if it was consistent with the prophylaxis protocol at the time of the audit or if contraindications were documented (eg, patients eligible for, but with contraindications to, pharmacologic prophylaxis had to have an order for mechanical prophylaxis or documented contraindication to both modalities). As this measure was initiated in a staggered fashion, the rate of protocol-compliant prophylaxis is summarized for consecutive months of measurement rather than consecutive calendar months.
HA-VTE Rates
VTE events were captured by review of electronic coding data for the International Classification of Diseases, 9th Revision (ICD-9) codes 415.11-415.19, 453.2, 453.40-453.42, and 453.8-453.89. HA-VTE was defined as either new VTE not present on admission (NPOA HA-VTE) or new VTE presenting in a readmitted patient within 30 days of discharge (Readmit HA-VTE). Cases were stratified based on whether the patient had undergone a major operation (surgery patients) or not (medical patients) as identified by Medicare Services diagnosis-related group codes.
Control Measures
Potential adverse events were captured by review of electronic coding data for ICD-9 codes 289.84 (heparin-induced thrombocytopenia [HIT]) and E934.2 (adverse effects because of anticoagulants).
Statistical Analysis
Statistical process control charts were used to depict changes in prophylaxis rates over the 3 years for which data was collected. For VTE and safety outcomes, Pearson χ2 value with relative risk (RR) calculations and 95% confidence intervals (CIs) were used to compare proportions between groups at baseline (CY 2011) versus postimplementation (CY 2014). Differences between the means of normally distributed data were calculated, and a 95% CI for the difference between the means was performed to assess statistical difference. Nonparametric characteristics were described by quartiles and interquartile range, and the 2-sided Mann-Whitney U test was performed to assess statistical difference between the CY 2011 and CY 2014 period.
Role of the Funding Source
The GBMF funded the collaborative and supported authorship of the manuscript but had no role in the design or conduct of the intervention, the collection or analysis of data, or the drafting of the manuscript.
RESULTS
Population Demographics
There were 1,155,069 adult inpatient admissions during the 4-year study period (264,280 in the 9 pilot sites, 890,789 in the 26 spread sites). There were no clinically relevant changes in gender distribution, mortality rate, median age, case mix index, or hospital length of stay in 2011 versus 2014. Men comprised 47.1% of the patient population in 2011 and 47.7% in 2014. The mortality rate was 2.7% in both years. Median age was 62 in 2011 and 63 in 2014. The mean case mix index (1.58 vs 1.65) and mean length of stay (4.29 vs 4.33 days) were similar in the 2 time periods.
Prophylaxis Rates
TJC Prophylaxis rates
Rates of Protocol-Compliant Prophylaxis
There were 34,071 active surveillance audits across the 20 months of reporting in the pilot cohort (mean, 1817 audits per month). The rate of protocol-compliant prophylaxis improved from 89% at month 1 of observation to 93% during month 2 and 97% by the last 3 months (Pearson χ2 P < .001 for both comparisons).
HA-VTE
HA-VTE characteristics
Improved HA-VTE over Time
Four hundred twenty-eight fewer HA-VTEs occurred in 2014 than in 2011 (RR 0.78; 95% CI, 0.73-0.85) (Table and Figure 3). Readmission HA-VTEs were reduced by 315 (RR 0.72; 95% CI, 0.65-0.80), while the reduction in NPOA HA-VTEs was less robust (RR 0.88; 95% CI, 0.79-0.99). Pilot sites enjoyed a more robust reduction in HA-VTEs than spread sites (26% vs 20%), largely because the pilot cohort enjoyed a 34% reduction in NPOA HA-VTEs and a 20% reduction in Readmit HA-VTEs, while the spread cohort only achieved reductions in Readmit HA-VTEs.
Safety
Rates of HIT and adverse effects because of anticoagulants were low (Table). The rate of HIT declined from 178 events in 2011 to 109 in 2014 (RR 0.66; 95% CI, 0.52-0.84), and the RR of anticoagulant adverse events remained stable (RR 1.01; 95% CI, 0.87-1.15).
DISCUSSION
Our QI project, based on a proven collaborative approach and mentorship,18,22,24 order set redesign, and active surveillance, was associated with 26% less VTEs in the pilot cohort and 20% less VTEs in the spread cohort. These gains, down to a final rate of approximately 4 HA-VTEs per 1000 admissions, occurred despite a low baseline HA-VTE rate. Dignity Health achieved these improvements in 35 hospitals with varied sizes, settings, ordering systems, and teaching statuses, achieving what is to our knowledge the largest VTE QI initiative yet reported.
Implementation experiences were not systematically recorded, and techniques were not compared with a control group. However, we believe that Dignity Health’s organizational commitment to improvement and centralized support were crucial for success. In addition, the pilot sites received grant support from the GBMF for intensive quality mentoring, a strategy with demonstrated value.23 Mentors and team members noted that system-wide revision to the computerized physician order entry system was easiest to implement, while active surveillance represented the most labor-intensive intervention. Other experiences echoed lessons from previous VTE mentorship efforts.17,18
The selection of a VTE protocol conducive to implementation and provider use was a key strategy. The ideal approach to VTE risk assessment is not known,12,26 but guidelines either offer no specific guidance7 or would require implementation of 3 different systems per hospital.4,5 Several of these are point scoring systems, which may have lower clinician acceptance or require programming to improve real-world use18,26,27; the Padua score was derived from a patient population that differs significantly from those in the United States.12 Our study provides more practical experience with a “3-bucket” model, which has previously shown high interobserver reliability, good clinician acceptance, and meaningful reductions of VTE, including in American patient populations.18,22,24
The value of VTE prophylaxis is still disputed in many inpatient groups. The overall rate of HA-VTE is low, so the per-patient benefit of prophylaxis is low, and many patients may be overprophylaxed.4,11,12 Recently, Flanders et al.20 reported that HA-VTE rates among 20,800 medical inpatients in Michigan were low (about 1%) and similar at hospitals in the top (mean prophylaxis rate 86%) or bottom (mean prophylaxis rate 56%) tertiles of performance. Possible explanations for the differences between their multicenter experience and ours include our sample size (55 times larger) and the possibility that targeting prophylaxis to patients at highest need (captured in our protocol-compliant prophylaxis rates) matters more than prophylaxing a percent of the population.
Further research is needed to develop simple, easy-to-implement methods to identify inpatients who do not, or no longer, require prophylaxis.12 Hospital systems also need methods to determine if prophylaxis improvement efforts can lower their HA-VTE rates and in which subpopulations. For example, a collaborative effort at the University of California lowered HA-VTE rates toward a common improved rate of 0.65% to 0.73%,22 while Dignity Health achieved improvement despite starting with an even lower baseline. In the University of California collaborative, benefits were limited chiefly to surgical patients, while Dignity Health achieved most improvement in medical patients, particularly in Readmit HA-VTE. If future research uncovers the reasons for these differences, it could help hospitals decide where to target improvement efforts.
Our study has several limitations. First, we used a nonrandomized time series design, so we cannot exclude other potential explanations for the change in VTE rates. However, there were no major changes in patient populations or concurrent projects likely to have influenced event rates. While we did not collect detailed demographic information on subjects, the broad inclusion criteria and multicenter design suggests a high degree of generalizability. Second, we followed inpatient VTE events and VTE-related readmissions, but not VTE treated in the outpatient setting. This did not change over the study, but the availability of all-oral therapy for VTE could have caused underdetection if clinic or emergency room doctors sent home more patients on oral therapy instead of readmitting them to the hospital. Third, implementation was enhanced by GBMF funds (at 9 sites, with the remainder benefitting from their experience), a shared electronic med
Strengths of our study include reductions in HA-VTE, both with and without access to GBMF funds, by using broadly available QI strategies.17 This real-world success and ease of dissemination are particularly important because the clinical trials of prophylaxis have been criticized for using highly selected patient populations,11 and prophylaxis QI studies show an inconsistent impact on VTE outcomes.15 In previous studies, two of the authors monitored orders for prophylaxis22,24; during this project, delivery for both pharmacologic and mechanical VTE prophylaxis was monitored, confirming that patient care actually changed.
CONCLUSION
Our multicenter VTE prophylaxis initiative, featuring a “3-bucket” VTE protocol, QI mentorship, and active surveillance as key interventions, was associated with improved prophylaxis rates and a reduction in HA-VTE by 22% with no increase in adverse events. This project provides a model for hospital systems seeking to optimize their prophylaxis efforts, and it supports the use of collaborative QI initiatives and SHM’s quality mentorship program as methods to drive improvement across health systems.
Disclosure
None of the authors have any conflicts of interest related to any topics or products discussed in the article. Dignity Health provided a stipend for writing the manuscript to GM and IJ, as noted in the article, but had no role in data analysis, writing, or decision to submit.
Deep venous thrombosis and pulmonary embolism, collectively known as venous thromboembolism (VTE), affect up to 600,000 Americans a year.1 Most of these are hospital-associated venous thromboembolisms (HA-VTE).1,2 VTE poses a substantial risk of mortality and long-term morbidity, and its treatment poses a risk of major bleeding.1 As appropriate VTE prophylaxis (“prophylaxis”) can reduce the risk of VTE by 40% to 80% depending on the patient population,3 VTE risk assessment and prophylaxis is endorsed by multiple guidelines4-7 and supported by regulatory agencies.8-10
However, despite extensive study, consensus about the impact of prophylaxis4,11 and the optimal method of risk assessment4,5,7,12 is lacking. Meanwhile, implementation of prophylaxis in real-world settings is poor; only 40% to 60% of at-risk patients receive prophylaxis,13 and as few as <20% receive optimal prophylaxis.14 Both systematic reviews15,16 and experience with VTE prevention collaboratives17,18 found that multifaceted interventions and alerts may be most effective in improving prophylaxis rates, but without proof of improved VTE rates.15 There is limited experience with large-scale VTE prevention. Organizations like The Joint Commission (TJC)8 and the Surgical Care Improvement Project have promoted quality measures but without clear evidence of improvement.19 In addition, an analysis of over 20,000 medical patients at 35 hospitals found no difference in VTE rates between high- and low-performing hospitals,20 suggesting that aggressive prophylaxis efforts may not reduce VTE, at least among medical patients.21 However, a 5-hospital University of California collaborative was associated with improved VTE rates, chiefly among surgical patients.22
In 2011, Dignity Health targeted VTE for improvement after investigations of potentially preventable HA-VTE revealed variable patterns of prophylaxis. In addition, improvement seemed feasible because there is a proven framework for VTE quality improvement (QI) projects17,18 and a record of success with the following 3 specific strategies: quality mentorship,23 use of a simple VTE risk assessment method, and active surveillance (real-time monitoring targeting suboptimal prophylaxis with concurrent intervention). This active surveillance technique has been used successfully in prior improvement efforts, often termed measure-vention.17,18,22,24
METHODS
Setting and Participants
The QI collaborative was performed at 35 Dignity Health community hospitals in California, Arizona, and Nevada. Facilities ranged from 25 to 571 beds in size with a mixture of teaching and nonteaching hospitals. Prior to the initiative, prophylaxis improvement efforts were incomplete and inconsistent at study facilities. All adult acute care inpatients at all facilities were included except rehabilitation, behavioral health, skilled nursing, hospice, other nonacute care, and inpatient deliveries.
Design Overview
We performed a prospective, unblinded, open-intervention study of a QI collaborative in 35 community hospitals and studied the effect on prophylaxis and VTE rates with historical controls. The 35 hospitals were organized into 2 cohorts. In the “pilot” cohort, 9 hospitals (chosen to be representative of the various settings, size, and teaching status within the Dignity system) received funding from the Gordon and Betty Moore Foundation (GBMF) for intensive, individualized QI mentorship from experts as well as active surveillance (see “Interventions”). The pilot sites led the development of the VTE risk assessment and prophylaxis protocol (“VTE protocol”), measures, order sets, implementation tactics, and lessons learned, assisted by the mentor experts. Dissemination to the 26-hospital “spread” cohort was facilitated by the Dignity Health Hospital Engagement Network (HEN) infrastructure.
Timeline
Two of the pilot sites, acting as leads on the development of protocol and order set tools, formed improvement teams in March 2011, 6 to 12 months earlier than other Dignity sites. Planning and design work occurred from March 2011 to September 2012. Most implementation at the 35 hospitals occurred in a staggered fashion during calendar year (CY) 2012 and 2013 (see Figure 1). As few changes were made until mid-2012, we considered CY 2011 the baseline for comparison, CY 2012 to 2013 the implementation years, and CY 2014 the postimplementation period.
The project was reviewed by the Institutional Review Board (IRB) of Dignity Health and determined to be an IRB-exempt QI project.
Interventions
Collaborative Infrastructure
Multidisciplinary Teams
Improvement teams formed between March 2011 and September 2012. Members included a physician champion, frontline nurses and physicians, an administrative liaison, pharmacists, quality and data specialists, clinical informatics staff, and stakeholders from key clinical services. Teams met at least monthly at each site.
Physician Mentors
The 9 pilot sites received individualized mentorship provided by outside experts (IJ or GM) based on a model pioneered by the Society of Hospital Medicine’s (SHM) Mentored Implementation programs.23 Each pilot site completed a self-assessment survey17 (see supplementary Appendix A) about past efforts, team composition, current performance, aims, barriers, and opportunities. The mentors reviewed the completed questionnaire with each hospital and provided advice on the VTE protocol and order set design, measurement, and benchmarking during 3 webinar meetings scheduled at 0, 3, and 9 months, plus as-needed e-mail and phone correspondence. After each webinar, the mentors provided detailed improvement suggestions (see supplementary Appendix B). Several hospitals received mentor site visits, which focused on unit rounding, active surveillance, staff and provider education, and problem-solving sessions with senior leadership, physician leadership, and the improvement team.
VTE Protocol
After a literature review and consultation with the mentors, Dignity Health developed and implemented a VTE protocol, modified from a model used in previous improvement efforts.18,22-24 Its risk assessment method is often referred to as a “3 bucket” model because it assigns patients to high-, moderate-, or low-risk categories based on clinical factors (eg, major orthopedic surgery, prior VTE, and others), and the VTE protocol recommends interventions based on the risk category (see supplementary Appendix C). Dignity Health was transitioning to a single electronic health record (Cerner Corporation, North Kansas City, MO) during the study, and study hospitals were using multiple platforms, necessitating the development of both paper and electronic versions of the VTE protocol. The electronic version required completion of the VTE protocol for all inpatient admissions and transfers. The VTE protocol was completed in November 2011 and disseminated to other sites in a staggered fashion through November 2012. Completed protocols and improvement tips were shared by the project lead and by webinar sessions. Sites were also encouraged to implement a standardized practice that allowed nurses to apply sequential compression devices to at-risk patients without physician orders when indicated by protocol, when contraindications such as vascular disease or ulceration were absent.
Education
Staff were educated about the VTE protocol by local teams, starting between late 2011 and September 2012. The audience (physicians, nurses, pharmacists, etc.) and methods (conferences, fliers, etc.) were determined by local teams, following guidance by mentors and webinar content. Active surveillance provided opportunities for in-the-moment, patient-specific education and protocol reinforcement. Both mentors delivered educational presentations at pilot sites.
Active Surveillance
Sites were encouraged to perform daily review of prophylaxis adequacy for inpatients and correct lapses in real time (both under- and overprophylaxis). Inappropriate prophylaxis orders were addressed by contacting providers to change the order or document the rationale not to. Lapses in adherence to prophylaxis were addressed by nursing correction and education of involved staff. Active surveillance was funded for 10 hours a week at pilot sites. Spread sites received only minimal support from HEN monies. All sites used daily prophylaxis reports, enhanced to include contraindications like thrombocytopenia and coagulopathy, to facilitate efforts. Active surveillance began in May 2012 in the lead pilot hospitals and was implemented in other sites between October 2012 and February 2013.
Metrics
Prophylaxis Rates
Measurement of prophylaxis did not begin until 2012 to 2013; thus, the true baseline rate for prophylaxis was not captured. TJC metrics (VTE-1 and VTE-2)25 were consolidated into a composite TJC prophylaxis rate from January 2012 to December 2014 for both pilot and spread hospitals. These measures assess the percentage of adult inpatients who received VTE prophylaxis or have documentation of why no prophylaxis was given the day of or day after hospital admission (VTE-1) or the day of or day after ICU admission or transfer (VTE-2). These measures are met if any mechanical or pharmacologic prophylaxis was delivered.
In addition to the TJC metric, the 9 pilot hospitals monitored rates of protocol-compliant prophylaxis for 12 to 20 months. Each patient’s prophylaxis was considered protocol compliant if it was consistent with the prophylaxis protocol at the time of the audit or if contraindications were documented (eg, patients eligible for, but with contraindications to, pharmacologic prophylaxis had to have an order for mechanical prophylaxis or documented contraindication to both modalities). As this measure was initiated in a staggered fashion, the rate of protocol-compliant prophylaxis is summarized for consecutive months of measurement rather than consecutive calendar months.
HA-VTE Rates
VTE events were captured by review of electronic coding data for the International Classification of Diseases, 9th Revision (ICD-9) codes 415.11-415.19, 453.2, 453.40-453.42, and 453.8-453.89. HA-VTE was defined as either new VTE not present on admission (NPOA HA-VTE) or new VTE presenting in a readmitted patient within 30 days of discharge (Readmit HA-VTE). Cases were stratified based on whether the patient had undergone a major operation (surgery patients) or not (medical patients) as identified by Medicare Services diagnosis-related group codes.
Control Measures
Potential adverse events were captured by review of electronic coding data for ICD-9 codes 289.84 (heparin-induced thrombocytopenia [HIT]) and E934.2 (adverse effects because of anticoagulants).
Statistical Analysis
Statistical process control charts were used to depict changes in prophylaxis rates over the 3 years for which data was collected. For VTE and safety outcomes, Pearson χ2 value with relative risk (RR) calculations and 95% confidence intervals (CIs) were used to compare proportions between groups at baseline (CY 2011) versus postimplementation (CY 2014). Differences between the means of normally distributed data were calculated, and a 95% CI for the difference between the means was performed to assess statistical difference. Nonparametric characteristics were described by quartiles and interquartile range, and the 2-sided Mann-Whitney U test was performed to assess statistical difference between the CY 2011 and CY 2014 period.
Role of the Funding Source
The GBMF funded the collaborative and supported authorship of the manuscript but had no role in the design or conduct of the intervention, the collection or analysis of data, or the drafting of the manuscript.
RESULTS
Population Demographics
There were 1,155,069 adult inpatient admissions during the 4-year study period (264,280 in the 9 pilot sites, 890,789 in the 26 spread sites). There were no clinically relevant changes in gender distribution, mortality rate, median age, case mix index, or hospital length of stay in 2011 versus 2014. Men comprised 47.1% of the patient population in 2011 and 47.7% in 2014. The mortality rate was 2.7% in both years. Median age was 62 in 2011 and 63 in 2014. The mean case mix index (1.58 vs 1.65) and mean length of stay (4.29 vs 4.33 days) were similar in the 2 time periods.
Prophylaxis Rates
TJC Prophylaxis rates
Rates of Protocol-Compliant Prophylaxis
There were 34,071 active surveillance audits across the 20 months of reporting in the pilot cohort (mean, 1817 audits per month). The rate of protocol-compliant prophylaxis improved from 89% at month 1 of observation to 93% during month 2 and 97% by the last 3 months (Pearson χ2 P < .001 for both comparisons).
HA-VTE
HA-VTE characteristics
Improved HA-VTE over Time
Four hundred twenty-eight fewer HA-VTEs occurred in 2014 than in 2011 (RR 0.78; 95% CI, 0.73-0.85) (Table and Figure 3). Readmission HA-VTEs were reduced by 315 (RR 0.72; 95% CI, 0.65-0.80), while the reduction in NPOA HA-VTEs was less robust (RR 0.88; 95% CI, 0.79-0.99). Pilot sites enjoyed a more robust reduction in HA-VTEs than spread sites (26% vs 20%), largely because the pilot cohort enjoyed a 34% reduction in NPOA HA-VTEs and a 20% reduction in Readmit HA-VTEs, while the spread cohort only achieved reductions in Readmit HA-VTEs.
Safety
Rates of HIT and adverse effects because of anticoagulants were low (Table). The rate of HIT declined from 178 events in 2011 to 109 in 2014 (RR 0.66; 95% CI, 0.52-0.84), and the RR of anticoagulant adverse events remained stable (RR 1.01; 95% CI, 0.87-1.15).
DISCUSSION
Our QI project, based on a proven collaborative approach and mentorship,18,22,24 order set redesign, and active surveillance, was associated with 26% less VTEs in the pilot cohort and 20% less VTEs in the spread cohort. These gains, down to a final rate of approximately 4 HA-VTEs per 1000 admissions, occurred despite a low baseline HA-VTE rate. Dignity Health achieved these improvements in 35 hospitals with varied sizes, settings, ordering systems, and teaching statuses, achieving what is to our knowledge the largest VTE QI initiative yet reported.
Implementation experiences were not systematically recorded, and techniques were not compared with a control group. However, we believe that Dignity Health’s organizational commitment to improvement and centralized support were crucial for success. In addition, the pilot sites received grant support from the GBMF for intensive quality mentoring, a strategy with demonstrated value.23 Mentors and team members noted that system-wide revision to the computerized physician order entry system was easiest to implement, while active surveillance represented the most labor-intensive intervention. Other experiences echoed lessons from previous VTE mentorship efforts.17,18
The selection of a VTE protocol conducive to implementation and provider use was a key strategy. The ideal approach to VTE risk assessment is not known,12,26 but guidelines either offer no specific guidance7 or would require implementation of 3 different systems per hospital.4,5 Several of these are point scoring systems, which may have lower clinician acceptance or require programming to improve real-world use18,26,27; the Padua score was derived from a patient population that differs significantly from those in the United States.12 Our study provides more practical experience with a “3-bucket” model, which has previously shown high interobserver reliability, good clinician acceptance, and meaningful reductions of VTE, including in American patient populations.18,22,24
The value of VTE prophylaxis is still disputed in many inpatient groups. The overall rate of HA-VTE is low, so the per-patient benefit of prophylaxis is low, and many patients may be overprophylaxed.4,11,12 Recently, Flanders et al.20 reported that HA-VTE rates among 20,800 medical inpatients in Michigan were low (about 1%) and similar at hospitals in the top (mean prophylaxis rate 86%) or bottom (mean prophylaxis rate 56%) tertiles of performance. Possible explanations for the differences between their multicenter experience and ours include our sample size (55 times larger) and the possibility that targeting prophylaxis to patients at highest need (captured in our protocol-compliant prophylaxis rates) matters more than prophylaxing a percent of the population.
Further research is needed to develop simple, easy-to-implement methods to identify inpatients who do not, or no longer, require prophylaxis.12 Hospital systems also need methods to determine if prophylaxis improvement efforts can lower their HA-VTE rates and in which subpopulations. For example, a collaborative effort at the University of California lowered HA-VTE rates toward a common improved rate of 0.65% to 0.73%,22 while Dignity Health achieved improvement despite starting with an even lower baseline. In the University of California collaborative, benefits were limited chiefly to surgical patients, while Dignity Health achieved most improvement in medical patients, particularly in Readmit HA-VTE. If future research uncovers the reasons for these differences, it could help hospitals decide where to target improvement efforts.
Our study has several limitations. First, we used a nonrandomized time series design, so we cannot exclude other potential explanations for the change in VTE rates. However, there were no major changes in patient populations or concurrent projects likely to have influenced event rates. While we did not collect detailed demographic information on subjects, the broad inclusion criteria and multicenter design suggests a high degree of generalizability. Second, we followed inpatient VTE events and VTE-related readmissions, but not VTE treated in the outpatient setting. This did not change over the study, but the availability of all-oral therapy for VTE could have caused underdetection if clinic or emergency room doctors sent home more patients on oral therapy instead of readmitting them to the hospital. Third, implementation was enhanced by GBMF funds (at 9 sites, with the remainder benefitting from their experience), a shared electronic med
Strengths of our study include reductions in HA-VTE, both with and without access to GBMF funds, by using broadly available QI strategies.17 This real-world success and ease of dissemination are particularly important because the clinical trials of prophylaxis have been criticized for using highly selected patient populations,11 and prophylaxis QI studies show an inconsistent impact on VTE outcomes.15 In previous studies, two of the authors monitored orders for prophylaxis22,24; during this project, delivery for both pharmacologic and mechanical VTE prophylaxis was monitored, confirming that patient care actually changed.
CONCLUSION
Our multicenter VTE prophylaxis initiative, featuring a “3-bucket” VTE protocol, QI mentorship, and active surveillance as key interventions, was associated with improved prophylaxis rates and a reduction in HA-VTE by 22% with no increase in adverse events. This project provides a model for hospital systems seeking to optimize their prophylaxis efforts, and it supports the use of collaborative QI initiatives and SHM’s quality mentorship program as methods to drive improvement across health systems.
Disclosure
None of the authors have any conflicts of interest related to any topics or products discussed in the article. Dignity Health provided a stipend for writing the manuscript to GM and IJ, as noted in the article, but had no role in data analysis, writing, or decision to submit.
1. U.S. Department of Health and Human Services; National Heart, Lung, and Blood Institute. Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville: Office of the Surgeon General; 2008.
2. Heit JA, Melton LJ, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients versus community residents. Mayo Clin Proc. 2001;76(11):1102-1110. PubMed
3. Guyatt GH, Eikelboom JW, Gould MK. Approach to Outcome Measurement in the Prevention of Thrombosis in Surgical and Medical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e185S-e194S. doi:10.1378/chest.11-2289. PubMed
4. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in Nonsurgical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S. doi:10.1378/chest.11-2296. PubMed
5. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in Nonorthopedic Surgical Patients. Chest. 2012;141(2 suppl):e227S-e277S. PubMed
6. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in Orthopedic Surgery Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S. doi:10.1378/chest.11-2404. PubMed
7. Qaseem A, Chou R, Humphrey LL. Venous Thromboembolism Prophylaxis in Hospitalized Patients: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. PubMed
8. The Joint Commission. Performance Measurement Initiatives. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement. Accessed June 14, 2012.
9. National Quality Forum. National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures. http://www.qualityforum.org/Publications/2006/12/National_Voluntary_Consensus_Standards_for_Prevention_and_Care_of_Venous_Thromboembolism__Policy,_Preferred_Practices,_and_Initial_Performance_Measures.aspx. Accessed June 14, 2012.
10. Medicare Quality Improvement Committee. SCIP Project Information. Agency for Healthcare Research and Quality. http://www.qualitymeasures.ahrq.gov/content.aspx?id=35538&search=scip. Accessed March 2013.
11. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients and Those with Stroke: A Background Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. PubMed
12. Rothberg MB. Venous thromboembolism prophylaxis for medical patients: who needs it? JAMA Intern Med. 2014;174(10):1585-1586. PubMed
13. Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross-sectional study. Lancet. 2008;371(9610):387-394. PubMed
14. Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician’s recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4(8):E15-E21. PubMed
15. Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201. doi:10.1002/14651858.CD008201.pub2. PubMed
16. Lau BD, Haut ER. Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23(3):187-195. PubMed
17. Maynard G. Preventing hospital-associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. Rockville: Agency for Healthcare Research and Quality; 2015. https://www.ahrq.gov/sites/default/files/publications/files/vteguide.pdf. Accessed October 29, 2017.
18. Maynard G, Stein J. Designing and Implementing Effective VTE Prevention Protocols: Lessons from Collaboratives. J Thromb Thrombolysis. 2010;29(2):159-166. PubMed
19. Altom LK, Deierhoi RJ, Grams J, et al. Association between Surgical Care Improvement Program venous thromboembolism measures and postoperative events. Am J Surg. 2012;204(5):591-597. PubMed
20. Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. PubMed
21. Finn KM, Greenwald JL. Update in Hospital Medicine: Evidence You Should Know. J Hosp Med. 2015;10(12):817-826. PubMed
22. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: Findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. PubMed
23. Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg Patient Safety and Quality Award. Mentored Implementation: Building Leaders and Achieving Results Through a Collaborative Improvement Model at the National Level. Jt Comm J Qual Patient Saf. 2012;38(7):301-310.
24. Maynard GA, Morris TA, Jenkins IH, et al. Optimizing prevention of hospital-acquired venous thromboembolism (VTE): Prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10-18. PubMed
25. The Joint Commission. Venous Thromboembolism Quality Measures. https://www.jointcommission.org/venous_thromboembolism/. Accessed October 13, 2017.
26. Maynard GA, Jenkins IH, Merli GJ. Venous thromboembolism prevention guidelines for medical inpatients: Mind the (implementation) Gap. J Hosp Med. 2013;8(10):582-588. PubMed
27. Elias P, Khanna R, Dudley A, et al. Automating Venous Thromboembolism Risk Calculation Using Electronic Health Record Data upon Hospital Admission: The Automated Padua Prediction Score. J Hosp Med. 2017;12(4):231-237. PubMed
1. U.S. Department of Health and Human Services; National Heart, Lung, and Blood Institute. Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville: Office of the Surgeon General; 2008.
2. Heit JA, Melton LJ, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients versus community residents. Mayo Clin Proc. 2001;76(11):1102-1110. PubMed
3. Guyatt GH, Eikelboom JW, Gould MK. Approach to Outcome Measurement in the Prevention of Thrombosis in Surgical and Medical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e185S-e194S. doi:10.1378/chest.11-2289. PubMed
4. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in Nonsurgical Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S-e226S. doi:10.1378/chest.11-2296. PubMed
5. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in Nonorthopedic Surgical Patients. Chest. 2012;141(2 suppl):e227S-e277S. PubMed
6. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in Orthopedic Surgery Patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e278S-e325S. doi:10.1378/chest.11-2404. PubMed
7. Qaseem A, Chou R, Humphrey LL. Venous Thromboembolism Prophylaxis in Hospitalized Patients: A Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. PubMed
8. The Joint Commission. Performance Measurement Initiatives. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement. Accessed June 14, 2012.
9. National Quality Forum. National Voluntary Consensus Standards for Prevention and Care of Venous Thromboembolism: Policy, Preferred Practices, and Initial Performance Measures. http://www.qualityforum.org/Publications/2006/12/National_Voluntary_Consensus_Standards_for_Prevention_and_Care_of_Venous_Thromboembolism__Policy,_Preferred_Practices,_and_Initial_Performance_Measures.aspx. Accessed June 14, 2012.
10. Medicare Quality Improvement Committee. SCIP Project Information. Agency for Healthcare Research and Quality. http://www.qualitymeasures.ahrq.gov/content.aspx?id=35538&search=scip. Accessed March 2013.
11. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients and Those with Stroke: A Background Review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. PubMed
12. Rothberg MB. Venous thromboembolism prophylaxis for medical patients: who needs it? JAMA Intern Med. 2014;174(10):1585-1586. PubMed
13. Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross-sectional study. Lancet. 2008;371(9610):387-394. PubMed
14. Amin AN, Stemkowski S, Lin J, Yang G. Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician’s recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4(8):E15-E21. PubMed
15. Kahn SR, Morrison DR, Cohen JM, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201. doi:10.1002/14651858.CD008201.pub2. PubMed
16. Lau BD, Haut ER. Practices to prevent venous thromboembolism: a brief review. BMJ Qual Saf. 2014;23(3):187-195. PubMed
17. Maynard G. Preventing hospital-associated venous thromboembolism: a guide for effective quality improvement, 2nd ed. Rockville: Agency for Healthcare Research and Quality; 2015. https://www.ahrq.gov/sites/default/files/publications/files/vteguide.pdf. Accessed October 29, 2017.
18. Maynard G, Stein J. Designing and Implementing Effective VTE Prevention Protocols: Lessons from Collaboratives. J Thromb Thrombolysis. 2010;29(2):159-166. PubMed
19. Altom LK, Deierhoi RJ, Grams J, et al. Association between Surgical Care Improvement Program venous thromboembolism measures and postoperative events. Am J Surg. 2012;204(5):591-597. PubMed
20. Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. PubMed
21. Finn KM, Greenwald JL. Update in Hospital Medicine: Evidence You Should Know. J Hosp Med. 2015;10(12):817-826. PubMed
22. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: Findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. PubMed
23. Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg Patient Safety and Quality Award. Mentored Implementation: Building Leaders and Achieving Results Through a Collaborative Improvement Model at the National Level. Jt Comm J Qual Patient Saf. 2012;38(7):301-310.
24. Maynard GA, Morris TA, Jenkins IH, et al. Optimizing prevention of hospital-acquired venous thromboembolism (VTE): Prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5(1):10-18. PubMed
25. The Joint Commission. Venous Thromboembolism Quality Measures. https://www.jointcommission.org/venous_thromboembolism/. Accessed October 13, 2017.
26. Maynard GA, Jenkins IH, Merli GJ. Venous thromboembolism prevention guidelines for medical inpatients: Mind the (implementation) Gap. J Hosp Med. 2013;8(10):582-588. PubMed
27. Elias P, Khanna R, Dudley A, et al. Automating Venous Thromboembolism Risk Calculation Using Electronic Health Record Data upon Hospital Admission: The Automated Padua Prediction Score. J Hosp Med. 2017;12(4):231-237. PubMed
© 2018 Society of Hospital Medicine
A Grumpy Old Man
Ms Chen acutely worse, altered, please assist, room 522Beth, chirped my pager. Ever increasing time pressures meant that hospitalists were supervising rounds almost daily. I had sent my resident, Beth, and the rest of the team to round separately that day, to foster their independence. It looked like we would be meeting ahead of schedule.
I'd received a similar page 2 years earlier when I was a junior resident myself. From the beginning of internship, our faculty never hesitated to challenge us. I will never forget when one of the hospitalists who had just come across an unresponsive patient tapped me on the shoulder and casually asked, Hey, you wanna run a code? and will never forget my inadequacy or the specific assistance I required in those tense few minutes. He, and the ICU team that arrived, gave me every chance to lead, and supported me each time I hesitated.
In similar fashion, I had sent my intern, David, to admit a patient with suspected CHF. I received his urgent update shortly after our patient arrived on the cardiology floor: Mr Johnson dropping sats, please help, room 207. I jogged to the patient's room, where I found David, 3 nurses, 2 medical students, and in the center, Mr Johnson: lethargic, gray, cachectic, and making no effort to rise from the 40 degree incline of his hospital bed. Weak respirations fogged his non‐rebreather mask about 28 times a minute.
David offered a quick report: 74‐year‐old male, CAD, hypertension, dementia CHF exacerbation hypertensive to 190. I think he needs IV nitroglycerin and another 80 of lasix.
I was pleased to hear him commit to a diagnosis and plan, but after sitting Mr Johnson up for a quick exam, I couldn't agree. Are you sure? He sounds more junky than crackly. Neck veins are flat.
His EF is 25% and he's been here 3 times with CHF.
Well, that won't protect him from anything else. Mr Johnson slumped forward, accessory muscles firing weakly, and only half‐opened his eyes to a loud command and vigorous shake. Well, let's get the diagnosis later, what does he need, now?
Well, the lasix and the nitro
Assuming this is CHF, looking at him now, will that work fast enough to prevent intubation? David shook his head no. He's full code, right? Let's just call a code before he gets any worse. Anyone disagree? A nurse made the call, then guarded the door to turn away everyone but anesthesia and the MICU as they arrived.
So what do you think it is? David asked.
This doesn't smell like failure. He's not anxious, he's more obtunded than dyspneic. He looks hypercarbic. He doesn't have COPD?
Nah, just vomiting, then weaker, more confused, restless.
Maybe he aspirated. We'll see. So what do you want to have ready for anesthesia?
Um, meds. An IV. Chest X‐ray ready.
Good they bring the meds he's got an IV how about we pull the bed from the wall and raise it up, get some suction ready, take the headboard off? Nurses sprang into action.
If he's hypercarbic, shouldn't we bag him? David asked.
Good point, I said. David took the mask from the bag of emergency gear from the wall and started to fit it on Mr Johnson. It's a 2‐person job, if you want to hold the mask2 hands, good. A nurse began ventilations, and I added some cricoid pressure. Keeps us from inflating his stomach.
Seconds later, anesthesia arrived, and David provided a concise, organized summary. Mr Johnson was intubated and whisked without incident to the MICU, where bronchoscopy extracted several mucus plugs. He was soon extubated, and later recovered from a delirium which began with promethazine for nausea. It was the last year before the 80‐hour workweek regulations, and not once in the entire processfrom admission, to emergency on the ward, to initial MICU managementdid I or my fellow residents think to call an attending, although I'm sure we would have learned something, as I hadn't suspected a mucous plug. We weren't hiding anything. We were just taking care of our patient.
Two years later, it didn't seem odd that my junior resident called me for assistance with Ms Cheninitially. In room 522, much as I found Mr Johnson, I found Ms Chen: elderly, lethargic, gray, frail, laboring to breathe, rhythmically fogging a non‐rebreather mask 30 times a minute, only half‐opening her eyes to a vigorous shake. It was day 4 of her fifth hospitalization for bronchiectasis‐related respiratory failure within 2 months.
She just got a treatment but she still sounds awful, offered Beth. Indeed, Ms Chen's chest was gurgly and wheezy throughout. We put her on a non‐rebreather, but that hasn't helped.
I glanced at her monitor. Sat's 99%. What was she before?
96%.
So hypoxia isn't the problemwho's this? I asked, as transportation staff arrived.
Stat head CT for Chen, he replied.
I'm sorry, she can't go off the floor right now. Thanks for coming, I apologized, and sent him away. Beth, can you lay her flat or send her off the unit right now?
She's altered and I need to rule out stroke.
Let's talk about that later. I did a quick neuro exam as I spoke: Besides, she resists weak but equal; pupils and face symmetricshe's not focal. What's a more likely cause?
Metabolic? We can repeat her morning labs
Will they be different? Why is she here? What's her exam telling you?
Beth took in the scene before her, as Ms Chen struggled weakly to ventilate her lungs, and after a brief pause she had it worked out. She's hypercarbic. She needs an ABG. You think she plugged? She shook her head, and grasped Ms Chen's hand in her own. But she really hates suctioning.
Well, she's DNI, and without it, she could die. Beth agreed; we also called for noninvasive ventilation. But the team missed much of the action. The medical student missed the entire eventaside from attempting to summarize it from second‐hand reports for rounds the following day. I realized only later that her intern had been pushed to the back of the room for the critical decisions (much like the students during Mr Johnson's emergency), and headed out midway to attend a mandatory teaching sessionthe chief residents had begun taking attendance. The resident soon left for noon conference and afternoon clinic, enlisting me to write transfer orders and call the family. Finished with her other work, and under pressure to bank time against work hour limitations, which she was at risk of violating, the intern signed her pager over to me and left in the early afternoon, after sheepishly asking me if I wouldn't mind keeping an eye on our patient.
Later, a translator and I met with the Chens to comfort them and plan care for the family matriarch, having found a quiet solarium we could use, with summery views of the city and ocean in the distance to belie the grim topic of discussion.
What is your understanding of her lung problem right now?
Nay yeega jee um'jee huigor fai ho jing yeung?
What were her hopes and fears about her health?
Nay jee um'jee huigor see seung hai mai ho tai hoi?
My mind drifted during the Cantonese as I thought about how I use the unique teaching opportunities offered by wholly translated meetings. Never check the time. This body language says I am listening. I am speaking to them, not the translator. I make notes because families don't remember much after the C‐word, I would whisper to trainees while families conversed with translators. Now, as I began to discuss hospice philosophy, I felt acutely alone.
My team had missed most of a great hospital medicine experience: applying knowledge to manage a physiologic crisis; using communication skills to ease the resulting human crisis. Recently, to manage the latest set of work hour restrictions, our residency program withdrew from medicine consultation at 2 of 3 sites, and from the medicine wards at the hospital that serves most of our insured, geriatric, and oncology patients. The cost of this experiment to the overall residency experience is unknown. But cases like Ms Chen's remind me how much I missed being the primary doctor. I do not mind the new tasks I perform for my trainees. But I worry about what they are missing: sufficient responsibility for making key clinical decisions while protected by supervision on demand. I am glad my internship challenged meit prepared me for residency, moonlighting, and attending positions. Without a doubt, residency remains challenging, but it seems that the greatestor firstchallenge imposed on residents is now to beat the clock, not to become a well‐rounded, capable, independent physician.
That night, I complained to my spouse, then a psychiatry intern: We weren't giving our trainees the best preparation for a career in medicine the lengthy shift I spent managing a hypotensive crisis would be forbidden now my pre‐work hours interns were much happier than their work hours successors a 4‐year residency must be around the corner. The response I got was more bemused smile than grave concern. You don't think that's important? I asked.
Of course I do. It's just that with all this talk about the days of the giants, he said gently, you're starting to sound like a grumpy old man. We chuckled. He was right. I expect a lot from myself, my trainees, and every clinician. I'd figured I'd be worthy of the title at some point.
But at 30?
Ms Chen acutely worse, altered, please assist, room 522Beth, chirped my pager. Ever increasing time pressures meant that hospitalists were supervising rounds almost daily. I had sent my resident, Beth, and the rest of the team to round separately that day, to foster their independence. It looked like we would be meeting ahead of schedule.
I'd received a similar page 2 years earlier when I was a junior resident myself. From the beginning of internship, our faculty never hesitated to challenge us. I will never forget when one of the hospitalists who had just come across an unresponsive patient tapped me on the shoulder and casually asked, Hey, you wanna run a code? and will never forget my inadequacy or the specific assistance I required in those tense few minutes. He, and the ICU team that arrived, gave me every chance to lead, and supported me each time I hesitated.
In similar fashion, I had sent my intern, David, to admit a patient with suspected CHF. I received his urgent update shortly after our patient arrived on the cardiology floor: Mr Johnson dropping sats, please help, room 207. I jogged to the patient's room, where I found David, 3 nurses, 2 medical students, and in the center, Mr Johnson: lethargic, gray, cachectic, and making no effort to rise from the 40 degree incline of his hospital bed. Weak respirations fogged his non‐rebreather mask about 28 times a minute.
David offered a quick report: 74‐year‐old male, CAD, hypertension, dementia CHF exacerbation hypertensive to 190. I think he needs IV nitroglycerin and another 80 of lasix.
I was pleased to hear him commit to a diagnosis and plan, but after sitting Mr Johnson up for a quick exam, I couldn't agree. Are you sure? He sounds more junky than crackly. Neck veins are flat.
His EF is 25% and he's been here 3 times with CHF.
Well, that won't protect him from anything else. Mr Johnson slumped forward, accessory muscles firing weakly, and only half‐opened his eyes to a loud command and vigorous shake. Well, let's get the diagnosis later, what does he need, now?
Well, the lasix and the nitro
Assuming this is CHF, looking at him now, will that work fast enough to prevent intubation? David shook his head no. He's full code, right? Let's just call a code before he gets any worse. Anyone disagree? A nurse made the call, then guarded the door to turn away everyone but anesthesia and the MICU as they arrived.
So what do you think it is? David asked.
This doesn't smell like failure. He's not anxious, he's more obtunded than dyspneic. He looks hypercarbic. He doesn't have COPD?
Nah, just vomiting, then weaker, more confused, restless.
Maybe he aspirated. We'll see. So what do you want to have ready for anesthesia?
Um, meds. An IV. Chest X‐ray ready.
Good they bring the meds he's got an IV how about we pull the bed from the wall and raise it up, get some suction ready, take the headboard off? Nurses sprang into action.
If he's hypercarbic, shouldn't we bag him? David asked.
Good point, I said. David took the mask from the bag of emergency gear from the wall and started to fit it on Mr Johnson. It's a 2‐person job, if you want to hold the mask2 hands, good. A nurse began ventilations, and I added some cricoid pressure. Keeps us from inflating his stomach.
Seconds later, anesthesia arrived, and David provided a concise, organized summary. Mr Johnson was intubated and whisked without incident to the MICU, where bronchoscopy extracted several mucus plugs. He was soon extubated, and later recovered from a delirium which began with promethazine for nausea. It was the last year before the 80‐hour workweek regulations, and not once in the entire processfrom admission, to emergency on the ward, to initial MICU managementdid I or my fellow residents think to call an attending, although I'm sure we would have learned something, as I hadn't suspected a mucous plug. We weren't hiding anything. We were just taking care of our patient.
Two years later, it didn't seem odd that my junior resident called me for assistance with Ms Cheninitially. In room 522, much as I found Mr Johnson, I found Ms Chen: elderly, lethargic, gray, frail, laboring to breathe, rhythmically fogging a non‐rebreather mask 30 times a minute, only half‐opening her eyes to a vigorous shake. It was day 4 of her fifth hospitalization for bronchiectasis‐related respiratory failure within 2 months.
She just got a treatment but she still sounds awful, offered Beth. Indeed, Ms Chen's chest was gurgly and wheezy throughout. We put her on a non‐rebreather, but that hasn't helped.
I glanced at her monitor. Sat's 99%. What was she before?
96%.
So hypoxia isn't the problemwho's this? I asked, as transportation staff arrived.
Stat head CT for Chen, he replied.
I'm sorry, she can't go off the floor right now. Thanks for coming, I apologized, and sent him away. Beth, can you lay her flat or send her off the unit right now?
She's altered and I need to rule out stroke.
Let's talk about that later. I did a quick neuro exam as I spoke: Besides, she resists weak but equal; pupils and face symmetricshe's not focal. What's a more likely cause?
Metabolic? We can repeat her morning labs
Will they be different? Why is she here? What's her exam telling you?
Beth took in the scene before her, as Ms Chen struggled weakly to ventilate her lungs, and after a brief pause she had it worked out. She's hypercarbic. She needs an ABG. You think she plugged? She shook her head, and grasped Ms Chen's hand in her own. But she really hates suctioning.
Well, she's DNI, and without it, she could die. Beth agreed; we also called for noninvasive ventilation. But the team missed much of the action. The medical student missed the entire eventaside from attempting to summarize it from second‐hand reports for rounds the following day. I realized only later that her intern had been pushed to the back of the room for the critical decisions (much like the students during Mr Johnson's emergency), and headed out midway to attend a mandatory teaching sessionthe chief residents had begun taking attendance. The resident soon left for noon conference and afternoon clinic, enlisting me to write transfer orders and call the family. Finished with her other work, and under pressure to bank time against work hour limitations, which she was at risk of violating, the intern signed her pager over to me and left in the early afternoon, after sheepishly asking me if I wouldn't mind keeping an eye on our patient.
Later, a translator and I met with the Chens to comfort them and plan care for the family matriarch, having found a quiet solarium we could use, with summery views of the city and ocean in the distance to belie the grim topic of discussion.
What is your understanding of her lung problem right now?
Nay yeega jee um'jee huigor fai ho jing yeung?
What were her hopes and fears about her health?
Nay jee um'jee huigor see seung hai mai ho tai hoi?
My mind drifted during the Cantonese as I thought about how I use the unique teaching opportunities offered by wholly translated meetings. Never check the time. This body language says I am listening. I am speaking to them, not the translator. I make notes because families don't remember much after the C‐word, I would whisper to trainees while families conversed with translators. Now, as I began to discuss hospice philosophy, I felt acutely alone.
My team had missed most of a great hospital medicine experience: applying knowledge to manage a physiologic crisis; using communication skills to ease the resulting human crisis. Recently, to manage the latest set of work hour restrictions, our residency program withdrew from medicine consultation at 2 of 3 sites, and from the medicine wards at the hospital that serves most of our insured, geriatric, and oncology patients. The cost of this experiment to the overall residency experience is unknown. But cases like Ms Chen's remind me how much I missed being the primary doctor. I do not mind the new tasks I perform for my trainees. But I worry about what they are missing: sufficient responsibility for making key clinical decisions while protected by supervision on demand. I am glad my internship challenged meit prepared me for residency, moonlighting, and attending positions. Without a doubt, residency remains challenging, but it seems that the greatestor firstchallenge imposed on residents is now to beat the clock, not to become a well‐rounded, capable, independent physician.
That night, I complained to my spouse, then a psychiatry intern: We weren't giving our trainees the best preparation for a career in medicine the lengthy shift I spent managing a hypotensive crisis would be forbidden now my pre‐work hours interns were much happier than their work hours successors a 4‐year residency must be around the corner. The response I got was more bemused smile than grave concern. You don't think that's important? I asked.
Of course I do. It's just that with all this talk about the days of the giants, he said gently, you're starting to sound like a grumpy old man. We chuckled. He was right. I expect a lot from myself, my trainees, and every clinician. I'd figured I'd be worthy of the title at some point.
But at 30?
Ms Chen acutely worse, altered, please assist, room 522Beth, chirped my pager. Ever increasing time pressures meant that hospitalists were supervising rounds almost daily. I had sent my resident, Beth, and the rest of the team to round separately that day, to foster their independence. It looked like we would be meeting ahead of schedule.
I'd received a similar page 2 years earlier when I was a junior resident myself. From the beginning of internship, our faculty never hesitated to challenge us. I will never forget when one of the hospitalists who had just come across an unresponsive patient tapped me on the shoulder and casually asked, Hey, you wanna run a code? and will never forget my inadequacy or the specific assistance I required in those tense few minutes. He, and the ICU team that arrived, gave me every chance to lead, and supported me each time I hesitated.
In similar fashion, I had sent my intern, David, to admit a patient with suspected CHF. I received his urgent update shortly after our patient arrived on the cardiology floor: Mr Johnson dropping sats, please help, room 207. I jogged to the patient's room, where I found David, 3 nurses, 2 medical students, and in the center, Mr Johnson: lethargic, gray, cachectic, and making no effort to rise from the 40 degree incline of his hospital bed. Weak respirations fogged his non‐rebreather mask about 28 times a minute.
David offered a quick report: 74‐year‐old male, CAD, hypertension, dementia CHF exacerbation hypertensive to 190. I think he needs IV nitroglycerin and another 80 of lasix.
I was pleased to hear him commit to a diagnosis and plan, but after sitting Mr Johnson up for a quick exam, I couldn't agree. Are you sure? He sounds more junky than crackly. Neck veins are flat.
His EF is 25% and he's been here 3 times with CHF.
Well, that won't protect him from anything else. Mr Johnson slumped forward, accessory muscles firing weakly, and only half‐opened his eyes to a loud command and vigorous shake. Well, let's get the diagnosis later, what does he need, now?
Well, the lasix and the nitro
Assuming this is CHF, looking at him now, will that work fast enough to prevent intubation? David shook his head no. He's full code, right? Let's just call a code before he gets any worse. Anyone disagree? A nurse made the call, then guarded the door to turn away everyone but anesthesia and the MICU as they arrived.
So what do you think it is? David asked.
This doesn't smell like failure. He's not anxious, he's more obtunded than dyspneic. He looks hypercarbic. He doesn't have COPD?
Nah, just vomiting, then weaker, more confused, restless.
Maybe he aspirated. We'll see. So what do you want to have ready for anesthesia?
Um, meds. An IV. Chest X‐ray ready.
Good they bring the meds he's got an IV how about we pull the bed from the wall and raise it up, get some suction ready, take the headboard off? Nurses sprang into action.
If he's hypercarbic, shouldn't we bag him? David asked.
Good point, I said. David took the mask from the bag of emergency gear from the wall and started to fit it on Mr Johnson. It's a 2‐person job, if you want to hold the mask2 hands, good. A nurse began ventilations, and I added some cricoid pressure. Keeps us from inflating his stomach.
Seconds later, anesthesia arrived, and David provided a concise, organized summary. Mr Johnson was intubated and whisked without incident to the MICU, where bronchoscopy extracted several mucus plugs. He was soon extubated, and later recovered from a delirium which began with promethazine for nausea. It was the last year before the 80‐hour workweek regulations, and not once in the entire processfrom admission, to emergency on the ward, to initial MICU managementdid I or my fellow residents think to call an attending, although I'm sure we would have learned something, as I hadn't suspected a mucous plug. We weren't hiding anything. We were just taking care of our patient.
Two years later, it didn't seem odd that my junior resident called me for assistance with Ms Cheninitially. In room 522, much as I found Mr Johnson, I found Ms Chen: elderly, lethargic, gray, frail, laboring to breathe, rhythmically fogging a non‐rebreather mask 30 times a minute, only half‐opening her eyes to a vigorous shake. It was day 4 of her fifth hospitalization for bronchiectasis‐related respiratory failure within 2 months.
She just got a treatment but she still sounds awful, offered Beth. Indeed, Ms Chen's chest was gurgly and wheezy throughout. We put her on a non‐rebreather, but that hasn't helped.
I glanced at her monitor. Sat's 99%. What was she before?
96%.
So hypoxia isn't the problemwho's this? I asked, as transportation staff arrived.
Stat head CT for Chen, he replied.
I'm sorry, she can't go off the floor right now. Thanks for coming, I apologized, and sent him away. Beth, can you lay her flat or send her off the unit right now?
She's altered and I need to rule out stroke.
Let's talk about that later. I did a quick neuro exam as I spoke: Besides, she resists weak but equal; pupils and face symmetricshe's not focal. What's a more likely cause?
Metabolic? We can repeat her morning labs
Will they be different? Why is she here? What's her exam telling you?
Beth took in the scene before her, as Ms Chen struggled weakly to ventilate her lungs, and after a brief pause she had it worked out. She's hypercarbic. She needs an ABG. You think she plugged? She shook her head, and grasped Ms Chen's hand in her own. But she really hates suctioning.
Well, she's DNI, and without it, she could die. Beth agreed; we also called for noninvasive ventilation. But the team missed much of the action. The medical student missed the entire eventaside from attempting to summarize it from second‐hand reports for rounds the following day. I realized only later that her intern had been pushed to the back of the room for the critical decisions (much like the students during Mr Johnson's emergency), and headed out midway to attend a mandatory teaching sessionthe chief residents had begun taking attendance. The resident soon left for noon conference and afternoon clinic, enlisting me to write transfer orders and call the family. Finished with her other work, and under pressure to bank time against work hour limitations, which she was at risk of violating, the intern signed her pager over to me and left in the early afternoon, after sheepishly asking me if I wouldn't mind keeping an eye on our patient.
Later, a translator and I met with the Chens to comfort them and plan care for the family matriarch, having found a quiet solarium we could use, with summery views of the city and ocean in the distance to belie the grim topic of discussion.
What is your understanding of her lung problem right now?
Nay yeega jee um'jee huigor fai ho jing yeung?
What were her hopes and fears about her health?
Nay jee um'jee huigor see seung hai mai ho tai hoi?
My mind drifted during the Cantonese as I thought about how I use the unique teaching opportunities offered by wholly translated meetings. Never check the time. This body language says I am listening. I am speaking to them, not the translator. I make notes because families don't remember much after the C‐word, I would whisper to trainees while families conversed with translators. Now, as I began to discuss hospice philosophy, I felt acutely alone.
My team had missed most of a great hospital medicine experience: applying knowledge to manage a physiologic crisis; using communication skills to ease the resulting human crisis. Recently, to manage the latest set of work hour restrictions, our residency program withdrew from medicine consultation at 2 of 3 sites, and from the medicine wards at the hospital that serves most of our insured, geriatric, and oncology patients. The cost of this experiment to the overall residency experience is unknown. But cases like Ms Chen's remind me how much I missed being the primary doctor. I do not mind the new tasks I perform for my trainees. But I worry about what they are missing: sufficient responsibility for making key clinical decisions while protected by supervision on demand. I am glad my internship challenged meit prepared me for residency, moonlighting, and attending positions. Without a doubt, residency remains challenging, but it seems that the greatestor firstchallenge imposed on residents is now to beat the clock, not to become a well‐rounded, capable, independent physician.
That night, I complained to my spouse, then a psychiatry intern: We weren't giving our trainees the best preparation for a career in medicine the lengthy shift I spent managing a hypotensive crisis would be forbidden now my pre‐work hours interns were much happier than their work hours successors a 4‐year residency must be around the corner. The response I got was more bemused smile than grave concern. You don't think that's important? I asked.
Of course I do. It's just that with all this talk about the days of the giants, he said gently, you're starting to sound like a grumpy old man. We chuckled. He was right. I expect a lot from myself, my trainees, and every clinician. I'd figured I'd be worthy of the title at some point.
But at 30?
Diabetic ketoacidosis
To the Editor: I read with interest the article by Hu and Isaacson1 on methods to distinguish type 1 from type 2 diabetes.
While a laboratory workup may be helpful in some hyperglycemic patients, I am unsure what value C-peptide testing (it costs approximately $40 at ARUP Laboratories, Salt Lake City, UT) would offer to the patient in question. Even without considering his age (48), two diabetic parents, and weight of 278 lb, the fact that he had controlled his diabetes for 6 years with diet and metformin makes a history of type 1 diabetes impossible. Could he have new-onset autoimmune diabetes complicating type 2 diabetes? His age makes this highly unlikely, and as the authors note, this is the phase of type 1 diabetes when a C-peptide level may still be normal. My guess is that the level was actually sent just “to see,” or as a rough measure of whether his pancreatitis had so impaired his insulin secretion that he would have an insulin-deficiency diabetes in addition to his type 2 diabetes. One hopes, however, that the severity of pancreatitis would be the primary clue to this possibility.
One test won’t break the camel’s back, but I write to promote the “booger rule” coined by a former mentor: ordering a test is like picking your nose—you have to know what you’re going to do with the result before you go digging. This advice encourages clinical problem-solving and reduces phlebotomy-induced anemia, venous access issues, and costs. (In my academic hospitalist practice, I can frequently cancel hundreds of dollars of “morning labs” on a nightly basis.) In some cases it may be crucial: as resident, I was unable to stop a cardiac catheterization we knew could not influence care, and biopsy of a brain mass in an elderly patient (too ill for any cancer care) that caused a lethal hemorrhage. As an attending, I have prevented a diagnostic colonoscopy on a patient with less than a week to live.
- Hu M, Isaacson JH. A 48-year-old man with uncontrolled diabetes. Cleve Clin J Med 2009; 76:413–416.
To the Editor: I read with interest the article by Hu and Isaacson1 on methods to distinguish type 1 from type 2 diabetes.
While a laboratory workup may be helpful in some hyperglycemic patients, I am unsure what value C-peptide testing (it costs approximately $40 at ARUP Laboratories, Salt Lake City, UT) would offer to the patient in question. Even without considering his age (48), two diabetic parents, and weight of 278 lb, the fact that he had controlled his diabetes for 6 years with diet and metformin makes a history of type 1 diabetes impossible. Could he have new-onset autoimmune diabetes complicating type 2 diabetes? His age makes this highly unlikely, and as the authors note, this is the phase of type 1 diabetes when a C-peptide level may still be normal. My guess is that the level was actually sent just “to see,” or as a rough measure of whether his pancreatitis had so impaired his insulin secretion that he would have an insulin-deficiency diabetes in addition to his type 2 diabetes. One hopes, however, that the severity of pancreatitis would be the primary clue to this possibility.
One test won’t break the camel’s back, but I write to promote the “booger rule” coined by a former mentor: ordering a test is like picking your nose—you have to know what you’re going to do with the result before you go digging. This advice encourages clinical problem-solving and reduces phlebotomy-induced anemia, venous access issues, and costs. (In my academic hospitalist practice, I can frequently cancel hundreds of dollars of “morning labs” on a nightly basis.) In some cases it may be crucial: as resident, I was unable to stop a cardiac catheterization we knew could not influence care, and biopsy of a brain mass in an elderly patient (too ill for any cancer care) that caused a lethal hemorrhage. As an attending, I have prevented a diagnostic colonoscopy on a patient with less than a week to live.
To the Editor: I read with interest the article by Hu and Isaacson1 on methods to distinguish type 1 from type 2 diabetes.
While a laboratory workup may be helpful in some hyperglycemic patients, I am unsure what value C-peptide testing (it costs approximately $40 at ARUP Laboratories, Salt Lake City, UT) would offer to the patient in question. Even without considering his age (48), two diabetic parents, and weight of 278 lb, the fact that he had controlled his diabetes for 6 years with diet and metformin makes a history of type 1 diabetes impossible. Could he have new-onset autoimmune diabetes complicating type 2 diabetes? His age makes this highly unlikely, and as the authors note, this is the phase of type 1 diabetes when a C-peptide level may still be normal. My guess is that the level was actually sent just “to see,” or as a rough measure of whether his pancreatitis had so impaired his insulin secretion that he would have an insulin-deficiency diabetes in addition to his type 2 diabetes. One hopes, however, that the severity of pancreatitis would be the primary clue to this possibility.
One test won’t break the camel’s back, but I write to promote the “booger rule” coined by a former mentor: ordering a test is like picking your nose—you have to know what you’re going to do with the result before you go digging. This advice encourages clinical problem-solving and reduces phlebotomy-induced anemia, venous access issues, and costs. (In my academic hospitalist practice, I can frequently cancel hundreds of dollars of “morning labs” on a nightly basis.) In some cases it may be crucial: as resident, I was unable to stop a cardiac catheterization we knew could not influence care, and biopsy of a brain mass in an elderly patient (too ill for any cancer care) that caused a lethal hemorrhage. As an attending, I have prevented a diagnostic colonoscopy on a patient with less than a week to live.
- Hu M, Isaacson JH. A 48-year-old man with uncontrolled diabetes. Cleve Clin J Med 2009; 76:413–416.
- Hu M, Isaacson JH. A 48-year-old man with uncontrolled diabetes. Cleve Clin J Med 2009; 76:413–416.
The Devil is in the Details
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 47‐year‐old male presented to a community hospital with 5 weeks of daily fevers, accompanied by headache, myalgias, and malaise. He reported that his symptoms began abruptly 2 days after a weekend of camping in Connecticut.
This patient describes the onset of undifferentiated fever 2 days after a weekend of camping. Few infectious diseases have such short incubation periods, and either the accuracy of the history or the relationship of the camping trip to the present illness is thus questionable. However, more information about the onset and nature of the illness, and details about food, animal, water, mud, cave, wood chopping, and other environmental exposures during his trip is required. The exact dates of the camping trip may be helpful, as there is clear seasonality to vector‐borne diseases such as Lyme disease, babesiosis, ehrlichiosis, and rickettsial infections. Conditions unrelated to his camping trip, such as malignancies, rheumatologic conditions, and other infectious causes of prolonged fever, such as tuberculosis, endocarditis, or osteomyelitis, are more likely, given the duration of fever.
The fevers were accompanied by chills, without rigors, and subjectively worsened over the first 2 days. At that point, the patient began taking his temperature, and noted fevers of 38.5C to 40C occurring once or twice daily, generally in the afternoon or evening. The patient did not recall tick bites but did not carefully examine himself for ticks; he reported numerous mosquito bites during the trip. The patient camped in a tent and grilled meats and other food he had brought in a cooler. No family members or other travelers became ill. He denied spelunking, but had collected wood for camp fires, and acknowledged swimming in a freshwater pond during his trip, which occurred in August.
West Nile fever, St. Louis encephalitis, and eastern equine encephalitis are transmitted by mosquitoes in New England, but are unlikely causes of prolonged fever. Water exposure suggests the possibility of leptospirosis, and wood exposure suggests blastomycosis, but this usually presents with a pulmonary syndrome. Food‐borne illness seems unlikely. While no aspect of the history has pinpointed a specific diagnosis, exploring the progression of symptoms may offer a clue, and if he has undergone any previous evaluation, the results may significantly alter the differential diagnosis. For example, arthritis may develop weeks after fever in adult‐onset Still's disease, negative blood cultures would lower the probability of endocarditis, and common sites of pyrogenic malignancies (eg, liver, kidneys, and especially lymph nodes) may already have been imaged.
During the first 3 weeks of illness, the patient experienced daily fever and a gradual, 10‐pound weight loss. Over the next 10 days, he sought medical attention at 3 emergency departments. At one, a head computed tomography (CT) showed possible sinusitis, and he was prescribed a 7‐day course of clarithromycin, which he took without any improvement. At 2 others, he was told that his laboratory studies, and a CT of the abdomen, were normal, and that he had a viral syndrome. Several days later, and 5 weeks after the onset of symptoms, the development of dull right upper‐quadrant pain and mild nausea without vomiting prompted the current presentation to the community hospital. He reported several years of loose stools, but denied rash, arthritis, diarrhea, neck stiffness, cough, or other complaints.
A detailed past medical, social, and family history is required, with particular attention to ethnicity; immunocompromising conditions such as splenectomy or corticosteroid use; undiagnosed febrile diseases; severe, unusual, or recurrent infections; medication use; diet; sexual history; pet exposures; and any personal or family history of cancer. The development of right upper‐quadrant pain mandates attention to risk factors for viral hepatitis, known biliary pathology, or travel that might predispose the patient to pyogenic or amoebic liver abscess, and hematochezia, which could suggest a malignancy metastatic to the liver. Additionally, chronic diarrhea with new right upper‐quadrant pain may represent inflammatory bowel disease complicated by primary sclerosing cholangitis (PSC).
The patient was a Caucasian male of Mediterranean ancestry with thalassemia minor. He had undergone dilation of a benign esophageal stricture, but no surgical procedures, and he had never experienced unexplained fever or unusual infections. Medication exposure was limited to occasional use of acetaminophen for fever, and he had no known allergies. His diet was unremarkable and included no well water or unpasteurized dairy products. He denied risk factors for tuberculosis. He drank 2 to 10 beers a day, 5 times a week, had last smoked 10 years previously, and had never used illicit drugs. He denied any high‐risk sexual contacts and was monogamous with his wife, with whom he had 2 children. The family owned no pets and no relatives had suffered from malignant, rheumatologic, or febrile illness, with the exception of hand, foot, and mouth infection in an infant son, 1 year previously. The patient had never traveled outside of New England.
The history has uncovered several clues, but their relevance is doubtful. His ethnicity suggests possible familial Mediterranean fever, but recurrent abdominal pain and polyserositis, rather than a single prolonged episode, would be expected with this disease. A transfusion history should be obtained to explore the possibility of viral hepatitis. While iron overload can predispose patients to various infections including liver abscess, thalassemia minor should not require transfusion. Esophageal stricture could conceivably be due to histoplasmosis (complicated by mediastinal fibrosis) or tuberculosis, but is probably unrelated to his present illness. His excessive alcohol intake increases his risk for esophageal cancer and liver disease, but it is unlikely that metastatic disease to the liver would present with fever without preceding dysphagia, or that alcoholic hepatitis could have escaped detection after evaluations by several physicians.
We need to learn the details of the patient's physical examination. Given the development of right upper‐quadrant pain, I would particularly like to know if he had hepatosplenomegaly and if a Murphy's sign was present.
His temperature ranged from 36.9C to 39.8C, his pulse was 76 beats per minute with minimal elevations during fever spikes, and his respirations were 18 per minute. His blood pressure was 105/70 mm Hg. He was a well‐developed, overweight male with scleral icterus. He had good dentition and an oropharynx free of lesions. Cardiac examination demonstrated a regular rhythm with a normal S1 and S2, without murmurs or peripheral stigmata of infectious endocarditis. A smooth, minimally tender liver edge was palpable 2 cm below the costal margin; the spleen was nonpalpable. Murphy's sign was absent. There was no lymphadenopathy or rash. He had multiple, shallow, uninfected lacerations of both hands in various stages of healing. The remainder of his examination was normal.
The patient has obvious liver involvement. The pulse‐temperature dissociation suggests a variety of infections, including salmonellosis, psittacosis, typhoid fever, leptospirosis, tularemia, brucellosis, legionellosis, and mycoplasma pneumoniae infection. The patient should be asked how and when he injured his hands, as fresh water exposure can transmit leptospirosis across broken skin. However, while severe leptospirosis can cause fever and jaundice, the long duration of illness is not typical. The cryptogenic form of tularemiawhich can manifest as a typhoidal illnessshould be considered, given that tularemia is present in the area the patient visited; he should be asked about exposure to rabbits.
At this point, I would like to see a standard biochemical profile, a liver panel, a complete blood count and differential, urinalysis, chest X‐ray, and an electrocardiogram. I would examine thick and thin Wright‐Giemsa‐stained smears for evidence of babesiosis. Blood cultures should be held for at least 2 weeks to recover fastidious organisms like Francisella tularensis and Brucella sp. Bone marrow cultures should be obtained; they are more sensitive for mycobacteria and Brucella, and may also yield fungal pathogens. Serologies for a variety of infectious diseases, such as leptospirosis, typhoid fever, and tularemia, will be required if other diagnostic tests are unrevealing.
His white cell count was 8,100/L, with a normal differential, and his hemoglobin was 10 g/dL (normal range, 1417), with a mean corpuscular hemoglobin of 63 m3 (normal range, 8298). The platelet count was 303,000/L. Serum electrolytes were normal. His aspartate aminotransferase was 58 U/L and his alanine aminotransferase was 60 U/L (normal range for both, 1045). Bilirubin was 2.6 mg/dL (normal, 1.2); direct bilirubin was 0.9 mg/dL. Alkaline phosphatase was 150 U/L. Lactate dehydrogenase was 342 U/L (normal range, 2251). A lipase was 62 U/L. International normalized ratio (INR) was 1.4 with an activated partial thromboplastin time (aPTT) of 52 seconds (normal range, 2533). Erythrocyte sedimentation rate (ESR) was 50 mm/hour (normal range, 015). Iron studies showed a suppressed iron and iron‐binding capacity and elevated haptoglobin and ferritin (1878 ng/L; normal range, 22322). Several blood cultures obtained at admission showed no growth after 48 hours of incubation.
The anemia, low mean cell volume (MCV), and elevated ferritin and ESR are consistent with anemia of chronic disease, superimposed upon thalassemia minor. Transaminase elevations occur in a plethora of infectious processes. The elevated INR and aPTT are concerning, and may indicate a septic or malignant process with disseminated intravascular coagulation (DIC). While there is no mention of clinical DIC, it would be appropriate to obtain D‐dimers, fibrin degradation products, and a fibrinogen level. The platelet count is normal, which is reassuring.
Before initiating any empiric antimicrobials, I would obtain an abdominal ultrasound, and possibly an abdominal CT. Hepatitis (especially B and C), cytomegalovirus, and Epstein‐Barr virus serologies should be obtained. A variety of conditions including leptospirosis, tularemia, and babesiosis are possible; specific laboratory testing is required to guide therapy.
Ultrasound showed a thickened gallbladder; the liver was slightly enlarged with normal echotexture. Magnetic resonance cholangiopancreatography (MRCP) showed diffuse sequential beading and scarring of his extrahepatic biliary ducts.
There is no evidence of biliary stones, intrahepatic tumor, or abscess to explain the fever and hepatitis, although it would be helpful to know what other abdominal structures were imaged. The MRCP finding increases my suspicion of PSC, possibly complicated by infection, although the biliary abnormalities may be incidental, and an unrelated process may be responsible for the clinical presentation.
His physicians considered the possibilities of PSC and cholangiopathy due to as‐yet undiagnosed acquired immunodeficiency syndrome. Ampicillin‐sulbactam, ceftazidime, and gentamicin were administered for possible bacterial cholangitis, and endoscopic retrograde cholangiopancreatography was performed. This procedure showed only slight narrowing of his common bile duct, which was felt to be a normal variant. He felt no better after several days of antibiotic therapy, and was transferred to a tertiary care center for further evaluation. Repeat physical exam and laboratory studies were essentially unchanged. The patient explained that his hand lacerations were sustained during his work as a butcher who worked with lamb, beef, rabbit, and poultry. He rarely wore protective gloves because they induced contact dermatitis.
Tularemia becomes more likely given his history of rabbit butchering. Salmonellosis and leptospirosis also remain possible. Typhoid fever and brucellosis are unlikely unless the patient worked with imported exotic animals. At this point, given the systemic illness, empiric antibacterial therapy is reasonable. Of the chosen antimicrobials, only the gentamicin would reliably treat tularemia. I would stop ampicillin‐sulbactam and ceftazidime and replace gentamicin with ciprofloxacin, an effective and better‐tolerated agent for tularemia. Cultures of blood and bone marrow aspirate should be obtained. Stool should be cultured for Salmonella. Tularemia, leptospirosis, and typhoid serologies should be sent to a reference laboratory. At this point in the patient's illness, high‐titered antibodies should be present. However, it would be ideal to compare titers with those from previous serum sample, if possible.
The patient's antimicrobials were narrowed to doxycycline alone, for suspected zoonotic infection, but his fevers were unchanged after 1 week of treatment. Hepatitis serologies, human immunodeficiency virus (HIV) antibody, and smears for ehrlichiosis and babesiosis were negative. He had a positive immunoglobulin (Ig)G and a negative IgM for Epstein‐Barr virus and cytomegalovirus. Tularemia, ehrlichiosis, leptospirosis, brucellosis, and Query fever (Q fever) serologies were ordered. The elevated aPTT did not correct when his serum was mixed with normal serum. Thrombin was normal; factor VIII, von Willebrand (VW) factor, and VW cofactor were mildly elevated. Lupus anticoagulant was detected. A hepatologist declined to obtain a liver biopsy, citing the elevated aPTT and pending serologies. Given his clinical stability, the patient was discharged on doxycycline to await further results.
My highest suspicion is for tularemia, and I would switch antibiotic treatment to ciprofloxacin, awaiting serological results. Some in vitro studies have suggested that F. tularensis may often be resistant to doxycycline, and recent clinical experience has shown fluoroquinolones are superior to doxycycline in the treatment of tularemia.
His serologic results were as follows: tularemia, 1:32 (positive, 1:128); ehrlichia, 1:128 (granulocytic) and 1:64 (monocytic; normal for both, 1:64); leptospira, agglutinated nonspecifically; Brucella IgG and IgM 1 (negative, 9), Q fever (coxiella) IgG 1 + 2, IgM 1 + 2, all positive at 1:256 (1:16). A transesophageal echocardiogram showed no evidence of endocarditis. The patient was treated with 10 weeks of doxycycline for Q fever hepatitis. His fever, headache, and laboratory abnormalities resolved, and he remained well after the completion of therapy.
The serologies suggest the patient had Coxiella burnetii hepatitis, and illustrate the value of a precise exposure history. Most butchers work only with muscle tissue and have a negligible risk of Q fever. In retrospect, it became clear that he worked part‐time in a slaughterhouse, where highly infectious reproductive tract fluids can dry and aerosolize.
Commentary
Q fever was proposed as the name for a febrile illness affecting Australian slaughterhouse workers in 1937.1 The etiologic agent, C. burnetii, is a small, gram‐negative, obligate intracellular proteobacterium that exists in 2 distinct phases, specializing either in entering or persisting in macrophage lysosomes.2 Additionally, spores are formed and can persist in soil.
Q fever is an uncommonly recognized disease, in part because most infected persons have no symptoms or mild symptoms.3 In the United States, the estimated annual incidence has been 0.28 per million (about 50 cases per year) since 1999, when Q fever became a reportable disease due to bioterrorism concerns. In France, more frequent farming of goats and sheep may be responsible for the much higher annual incidence of 500 per million.4 Spread is usually occupational, via aerosol contact with the dried reproductive tract secretions of animals (mainly cattle, sheep, and goats), in a slaughterhouse or farm setting. However, wind‐borne dust can carry spores long distances, and spread can occur from household pets, unpasteurized dairy products, laboratory work, and possibly ticks.3 More than 30 cases have been reported in military personnel deployed to Iraq and Afghanistan, several without obvious exposures.5 One review noted a single reported case of intradermal inoculation,3 making this patient's lacerations a possible site of infection, but he was also at risk for inhalational exposurewhen he was later asked about the details of his work, he acknowledged working at a slaughterhouse as well as a supermarket.
Symptomatic patients are male in 77% of cases, can usually identify an occupational exposure, and have a mean age of 50 years.4 Fever, which lasts 5 to 57 days, as well as fatigue and headaches, begin after a 1‐week to 3‐week incubation period. Atypical pneumonia or rash may occur; meningoencephalitis and myocarditis portend a worse prognosis. As with this patient, 45% to 85% of patients suffer from hepatitis, although few have an abnormal bilirubin.3 Liver biopsy usually reveals granulomas, which may have a classic doughnut hole appearance,2, 3 although this patient ultimately received a diagnosis without the procedure. Acute Q fever rarely (5%) requires hospitalization, and fatalities are extremely rare.3
Chronic infection (ie, lasting >6 months) most often occurs as endocarditis, although chronic hepatitis, osteomyelitis, and infections of other sites occur. Interestingly, this patient's lupus anticoagulant may have been related to his underlying illness, as autoantibodies frequently occur in Q fever, especially in patients with hepatitis, many of whom develop smooth muscle antibodies, a positive Coombs test, antiprothrombinase, or other autoantibodies,3 and there is a high incidence of antiphospholipid antibodies, particularly anticardiolipin and lupus anticoagulant antibodies.6
Because C. burnetii is an obligate intracellular pathogen, culture requires either tissue or live animal inoculation, and the diagnosis is usually made serologically. Paired sera demonstrating seroconversion or a 4‐fold increase in titers are most conclusive, but a single sample may be used. Anti‐phase II antibodies are detectable in 90% of patients within 3 weeks of infection3 and peak at 2 months5; this patient's phase II sera (IgG > 1:200, IgM > 1:50) are said to be 100% predictive for acute Q fever.3 High‐titer anti‐phase I antibodies, in contrast, indicate chronic infection, and a titer 1:800 is one of the modified Duke criteria for endocarditis.5
Acute Q fever is generally treated with doxycycline for 14 days, although prolonged therapy may be advisable to prevent endocarditis if preexisting valvular lesions are present.2, 5 Fluoroquinolones are another option and may be especially useful for meningoencephalitis.5 Because acute Q fever is generally self‐limited, demonstrating a clear benefit to antibiotic therapy is difficult. The available evidence, which was largely obtained from Q fever pneumonia patients, suggests that tetracycline therapy shortens fever duration.3 Patients with Q fever hepatitis may have a protracted course. On the basis of anecdotal reports, some experts add prednisone (tapered from 40 mg daily over a week) for patients with Q fever hepatitis who fail to respond to doxycycline promptly.3 While this patient's fever was unchanged after a week of therapy, he was well into his treatment course when his diagnosis was ultimately confirmed. His physicians felt that prednisone would be of uncertain benefit and opted not to administer it.
Treatment of Q fever endocarditis is often delayed by the combination of negative blood cultures and a low (12%) rate of vegetation formation, increasing the risk of morbidity and mortality.3 Tetracycline monotherapy is associated with a greater than 50% risk of death,5 and even 4 years of treatment may fail to sterilize valve tissue.3 However, if hydroxychloroquine is given with doxycycline for at least 18 months to alkalize lysosomes and improve bacterial killing, the mortality rate can be lowered to about 5%.3, 5 Patients should be warned of the risk of photosensitivity, and monitored for retinal toxicity2 and serologic evidence of relapse.5
Before serologic results confirmed the diagnosis of Q fever, both the patient's clinicians and the discussant had to craft an antibiotic regimen for a suspected zoonosis. The patient received doxycycline, a good choice for leptospirosis,7 brucellosis,8 tularemia,9 and Q fever,3 all possible after livestock exposure, as well as ehrlichiosis.10 The discussant, who suspected tularemia, worried about the possibility of doxycycline resistance and selected ciprofloxacin instead. While fluoroquinolones are probably superior to doxycycline for mild to moderate tularemia,11, 12 aminoglycosides would be preferred for severe disease,9 and ciprofloxacin experience in leptospirosis7 and ehrlichiosis10 is limited. Neither selection would be optimal for brucellosis, for which either doxycycline or ciprofloxacin should be combined with another agent such as rifampin.8 The most reasonable empiric regimen is debatable, but in the absence of pathognomonic findings of tularemia, his treating physicians favored the broader activity of doxycycline.
Ultimately, the choice of antibiotics in this case hinged on the details of the patient's occupational exposures. His first 2 courses of antibiotics were based not on his exposure history, but on radiographic findings that were later proven spurious. The regimens selected by the discussant and by physicians at the referral hospital both targeted pathogens suggested by the patient's occupational history instead, but both were missing parts of the puzzle as well. The discussant thought the patient performed commercial butcher‐shop work, which is only rarely13 mentioned in the context of Q fever transmission. Several of the admitting physicians at the referral hospital were unaware of the importance of the butcher/slaughterhouse‐worker distinction. Physicians need a detailed understanding of both the exposure history and the biology of possible pathogens to craft an optimal differential diagnosis and empiric antibiotic regimen.
On the other hand, in most patients with fever of unknown origin (FUO; ie, >3 weeks with temperature >38.3 on multiple occasions, without a diagnosis after a weeklong evaluation),14 empiric antibiotic therapy is rarely a wise intervention. Clinicians should avoid blind administration of antibiotics as a diagnostic tool, given the inability to distinguish clinical responses from spontaneous resolution, or pinpoint a specific cause and thus a precise treatment plan and duration. However, empiric tetracyclines have been employed when intracellular pathogens were a suspected cause of FUO, as in one series of French patients in which Q fever was common.15 In this patient's case, no specific finding pointed to Q fever before the serologies became available, but the rare infections considered in this case can be considered doxycycline‐deficient states, meaning that empiric tetracycline therapy often leads to improvement. Recognizing doxycycline deficiency can guide therapy while definitive results are pending, and empiric doxycycline is particularly important if potentially aggressive zoonoses, such as Rocky Mountain spotted fever, are suspected.
Teaching Points:
-
A detailed and precise exposure history is crucial for the diagnosis of Q fever and other zoonoses and for the individualized evaluation of FUO in general.
-
Q fever is a rare disease that most commonly causes undifferentiated fever, pneumonia, hepatitis, and when chronic, often reflects endovascular infection, which is frequently difficult to eradicate.
-
Doxycycline is effective for many, but not all zoonoses (babesia is a notable exception). Empiric therapy is reasonable if suspicion is high.
- .“Q” fever, new fever entity: clinical features, diagnosis and laboratory investigation.Med J Aust.1937;2:281–299.
- ,,.Q fever.Lancet.2006;367(9511):679–688.
- ,.Q fever.Clin Microbiol Rev.1999;12:518–553.
- ,,, et al.National surveillance and the epidemiology of human Q fever in the United States, 1978–2004.Am J Trop Med Hyg.2006;75:36–40.
- ,,,.Q fever: epidemiology, diagnosis, and treatment.Mayo Clin Proc.2008;83(5):574–579.
- ,,, et al.Prevalence, significance, and specificity of antibodies to phospholipids in Q fever.Clin Infect Dis.1994;18:213–218.
- ,,.Antimicrobial therapy of leptospirosis.Curr Opin Infect Dis.2006;19:533–537.
- ,,, et al.Treatment of human brucellosis with doxycycline plus rifampin or doxycycline plus streptomycin. A randomized, double‐blind study.Ann Intern Med.1992;117:25–30.
- ,,,.Tularemia: current epidemiology and disease management.Infect Dis Clin North Am.2006;20:289–311, ix.
- ,,,.Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment.Clin Infect Dis.2007;45(Suppl 1):S45–S51.
- ,.New approaches to diagnosis and therapy of tularemia.Ann NY Acad Sci.2007;1105:378–404.
- ,,, et al.Evaluation of clinical, laboratory, and therapeutic features of 145 tularemia cases: the role of quinolones in oropharyngeal tularemia.APMIS.2008;116:66–73.
- ,.A survey of Q fever antibodies in a high risk population in Panamá.Am J Trop Med Hyg.1980;29(5):1007–1011.
- ,.Fever of unknown origin.Lancet.1997;350:575–580.
- .Fever of unknown origin in adults: evaluation of 144 cases in a non‐university hospital.Scand J Infect Dis.2006;38:632–638.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 47‐year‐old male presented to a community hospital with 5 weeks of daily fevers, accompanied by headache, myalgias, and malaise. He reported that his symptoms began abruptly 2 days after a weekend of camping in Connecticut.
This patient describes the onset of undifferentiated fever 2 days after a weekend of camping. Few infectious diseases have such short incubation periods, and either the accuracy of the history or the relationship of the camping trip to the present illness is thus questionable. However, more information about the onset and nature of the illness, and details about food, animal, water, mud, cave, wood chopping, and other environmental exposures during his trip is required. The exact dates of the camping trip may be helpful, as there is clear seasonality to vector‐borne diseases such as Lyme disease, babesiosis, ehrlichiosis, and rickettsial infections. Conditions unrelated to his camping trip, such as malignancies, rheumatologic conditions, and other infectious causes of prolonged fever, such as tuberculosis, endocarditis, or osteomyelitis, are more likely, given the duration of fever.
The fevers were accompanied by chills, without rigors, and subjectively worsened over the first 2 days. At that point, the patient began taking his temperature, and noted fevers of 38.5C to 40C occurring once or twice daily, generally in the afternoon or evening. The patient did not recall tick bites but did not carefully examine himself for ticks; he reported numerous mosquito bites during the trip. The patient camped in a tent and grilled meats and other food he had brought in a cooler. No family members or other travelers became ill. He denied spelunking, but had collected wood for camp fires, and acknowledged swimming in a freshwater pond during his trip, which occurred in August.
West Nile fever, St. Louis encephalitis, and eastern equine encephalitis are transmitted by mosquitoes in New England, but are unlikely causes of prolonged fever. Water exposure suggests the possibility of leptospirosis, and wood exposure suggests blastomycosis, but this usually presents with a pulmonary syndrome. Food‐borne illness seems unlikely. While no aspect of the history has pinpointed a specific diagnosis, exploring the progression of symptoms may offer a clue, and if he has undergone any previous evaluation, the results may significantly alter the differential diagnosis. For example, arthritis may develop weeks after fever in adult‐onset Still's disease, negative blood cultures would lower the probability of endocarditis, and common sites of pyrogenic malignancies (eg, liver, kidneys, and especially lymph nodes) may already have been imaged.
During the first 3 weeks of illness, the patient experienced daily fever and a gradual, 10‐pound weight loss. Over the next 10 days, he sought medical attention at 3 emergency departments. At one, a head computed tomography (CT) showed possible sinusitis, and he was prescribed a 7‐day course of clarithromycin, which he took without any improvement. At 2 others, he was told that his laboratory studies, and a CT of the abdomen, were normal, and that he had a viral syndrome. Several days later, and 5 weeks after the onset of symptoms, the development of dull right upper‐quadrant pain and mild nausea without vomiting prompted the current presentation to the community hospital. He reported several years of loose stools, but denied rash, arthritis, diarrhea, neck stiffness, cough, or other complaints.
A detailed past medical, social, and family history is required, with particular attention to ethnicity; immunocompromising conditions such as splenectomy or corticosteroid use; undiagnosed febrile diseases; severe, unusual, or recurrent infections; medication use; diet; sexual history; pet exposures; and any personal or family history of cancer. The development of right upper‐quadrant pain mandates attention to risk factors for viral hepatitis, known biliary pathology, or travel that might predispose the patient to pyogenic or amoebic liver abscess, and hematochezia, which could suggest a malignancy metastatic to the liver. Additionally, chronic diarrhea with new right upper‐quadrant pain may represent inflammatory bowel disease complicated by primary sclerosing cholangitis (PSC).
The patient was a Caucasian male of Mediterranean ancestry with thalassemia minor. He had undergone dilation of a benign esophageal stricture, but no surgical procedures, and he had never experienced unexplained fever or unusual infections. Medication exposure was limited to occasional use of acetaminophen for fever, and he had no known allergies. His diet was unremarkable and included no well water or unpasteurized dairy products. He denied risk factors for tuberculosis. He drank 2 to 10 beers a day, 5 times a week, had last smoked 10 years previously, and had never used illicit drugs. He denied any high‐risk sexual contacts and was monogamous with his wife, with whom he had 2 children. The family owned no pets and no relatives had suffered from malignant, rheumatologic, or febrile illness, with the exception of hand, foot, and mouth infection in an infant son, 1 year previously. The patient had never traveled outside of New England.
The history has uncovered several clues, but their relevance is doubtful. His ethnicity suggests possible familial Mediterranean fever, but recurrent abdominal pain and polyserositis, rather than a single prolonged episode, would be expected with this disease. A transfusion history should be obtained to explore the possibility of viral hepatitis. While iron overload can predispose patients to various infections including liver abscess, thalassemia minor should not require transfusion. Esophageal stricture could conceivably be due to histoplasmosis (complicated by mediastinal fibrosis) or tuberculosis, but is probably unrelated to his present illness. His excessive alcohol intake increases his risk for esophageal cancer and liver disease, but it is unlikely that metastatic disease to the liver would present with fever without preceding dysphagia, or that alcoholic hepatitis could have escaped detection after evaluations by several physicians.
We need to learn the details of the patient's physical examination. Given the development of right upper‐quadrant pain, I would particularly like to know if he had hepatosplenomegaly and if a Murphy's sign was present.
His temperature ranged from 36.9C to 39.8C, his pulse was 76 beats per minute with minimal elevations during fever spikes, and his respirations were 18 per minute. His blood pressure was 105/70 mm Hg. He was a well‐developed, overweight male with scleral icterus. He had good dentition and an oropharynx free of lesions. Cardiac examination demonstrated a regular rhythm with a normal S1 and S2, without murmurs or peripheral stigmata of infectious endocarditis. A smooth, minimally tender liver edge was palpable 2 cm below the costal margin; the spleen was nonpalpable. Murphy's sign was absent. There was no lymphadenopathy or rash. He had multiple, shallow, uninfected lacerations of both hands in various stages of healing. The remainder of his examination was normal.
The patient has obvious liver involvement. The pulse‐temperature dissociation suggests a variety of infections, including salmonellosis, psittacosis, typhoid fever, leptospirosis, tularemia, brucellosis, legionellosis, and mycoplasma pneumoniae infection. The patient should be asked how and when he injured his hands, as fresh water exposure can transmit leptospirosis across broken skin. However, while severe leptospirosis can cause fever and jaundice, the long duration of illness is not typical. The cryptogenic form of tularemiawhich can manifest as a typhoidal illnessshould be considered, given that tularemia is present in the area the patient visited; he should be asked about exposure to rabbits.
At this point, I would like to see a standard biochemical profile, a liver panel, a complete blood count and differential, urinalysis, chest X‐ray, and an electrocardiogram. I would examine thick and thin Wright‐Giemsa‐stained smears for evidence of babesiosis. Blood cultures should be held for at least 2 weeks to recover fastidious organisms like Francisella tularensis and Brucella sp. Bone marrow cultures should be obtained; they are more sensitive for mycobacteria and Brucella, and may also yield fungal pathogens. Serologies for a variety of infectious diseases, such as leptospirosis, typhoid fever, and tularemia, will be required if other diagnostic tests are unrevealing.
His white cell count was 8,100/L, with a normal differential, and his hemoglobin was 10 g/dL (normal range, 1417), with a mean corpuscular hemoglobin of 63 m3 (normal range, 8298). The platelet count was 303,000/L. Serum electrolytes were normal. His aspartate aminotransferase was 58 U/L and his alanine aminotransferase was 60 U/L (normal range for both, 1045). Bilirubin was 2.6 mg/dL (normal, 1.2); direct bilirubin was 0.9 mg/dL. Alkaline phosphatase was 150 U/L. Lactate dehydrogenase was 342 U/L (normal range, 2251). A lipase was 62 U/L. International normalized ratio (INR) was 1.4 with an activated partial thromboplastin time (aPTT) of 52 seconds (normal range, 2533). Erythrocyte sedimentation rate (ESR) was 50 mm/hour (normal range, 015). Iron studies showed a suppressed iron and iron‐binding capacity and elevated haptoglobin and ferritin (1878 ng/L; normal range, 22322). Several blood cultures obtained at admission showed no growth after 48 hours of incubation.
The anemia, low mean cell volume (MCV), and elevated ferritin and ESR are consistent with anemia of chronic disease, superimposed upon thalassemia minor. Transaminase elevations occur in a plethora of infectious processes. The elevated INR and aPTT are concerning, and may indicate a septic or malignant process with disseminated intravascular coagulation (DIC). While there is no mention of clinical DIC, it would be appropriate to obtain D‐dimers, fibrin degradation products, and a fibrinogen level. The platelet count is normal, which is reassuring.
Before initiating any empiric antimicrobials, I would obtain an abdominal ultrasound, and possibly an abdominal CT. Hepatitis (especially B and C), cytomegalovirus, and Epstein‐Barr virus serologies should be obtained. A variety of conditions including leptospirosis, tularemia, and babesiosis are possible; specific laboratory testing is required to guide therapy.
Ultrasound showed a thickened gallbladder; the liver was slightly enlarged with normal echotexture. Magnetic resonance cholangiopancreatography (MRCP) showed diffuse sequential beading and scarring of his extrahepatic biliary ducts.
There is no evidence of biliary stones, intrahepatic tumor, or abscess to explain the fever and hepatitis, although it would be helpful to know what other abdominal structures were imaged. The MRCP finding increases my suspicion of PSC, possibly complicated by infection, although the biliary abnormalities may be incidental, and an unrelated process may be responsible for the clinical presentation.
His physicians considered the possibilities of PSC and cholangiopathy due to as‐yet undiagnosed acquired immunodeficiency syndrome. Ampicillin‐sulbactam, ceftazidime, and gentamicin were administered for possible bacterial cholangitis, and endoscopic retrograde cholangiopancreatography was performed. This procedure showed only slight narrowing of his common bile duct, which was felt to be a normal variant. He felt no better after several days of antibiotic therapy, and was transferred to a tertiary care center for further evaluation. Repeat physical exam and laboratory studies were essentially unchanged. The patient explained that his hand lacerations were sustained during his work as a butcher who worked with lamb, beef, rabbit, and poultry. He rarely wore protective gloves because they induced contact dermatitis.
Tularemia becomes more likely given his history of rabbit butchering. Salmonellosis and leptospirosis also remain possible. Typhoid fever and brucellosis are unlikely unless the patient worked with imported exotic animals. At this point, given the systemic illness, empiric antibacterial therapy is reasonable. Of the chosen antimicrobials, only the gentamicin would reliably treat tularemia. I would stop ampicillin‐sulbactam and ceftazidime and replace gentamicin with ciprofloxacin, an effective and better‐tolerated agent for tularemia. Cultures of blood and bone marrow aspirate should be obtained. Stool should be cultured for Salmonella. Tularemia, leptospirosis, and typhoid serologies should be sent to a reference laboratory. At this point in the patient's illness, high‐titered antibodies should be present. However, it would be ideal to compare titers with those from previous serum sample, if possible.
The patient's antimicrobials were narrowed to doxycycline alone, for suspected zoonotic infection, but his fevers were unchanged after 1 week of treatment. Hepatitis serologies, human immunodeficiency virus (HIV) antibody, and smears for ehrlichiosis and babesiosis were negative. He had a positive immunoglobulin (Ig)G and a negative IgM for Epstein‐Barr virus and cytomegalovirus. Tularemia, ehrlichiosis, leptospirosis, brucellosis, and Query fever (Q fever) serologies were ordered. The elevated aPTT did not correct when his serum was mixed with normal serum. Thrombin was normal; factor VIII, von Willebrand (VW) factor, and VW cofactor were mildly elevated. Lupus anticoagulant was detected. A hepatologist declined to obtain a liver biopsy, citing the elevated aPTT and pending serologies. Given his clinical stability, the patient was discharged on doxycycline to await further results.
My highest suspicion is for tularemia, and I would switch antibiotic treatment to ciprofloxacin, awaiting serological results. Some in vitro studies have suggested that F. tularensis may often be resistant to doxycycline, and recent clinical experience has shown fluoroquinolones are superior to doxycycline in the treatment of tularemia.
His serologic results were as follows: tularemia, 1:32 (positive, 1:128); ehrlichia, 1:128 (granulocytic) and 1:64 (monocytic; normal for both, 1:64); leptospira, agglutinated nonspecifically; Brucella IgG and IgM 1 (negative, 9), Q fever (coxiella) IgG 1 + 2, IgM 1 + 2, all positive at 1:256 (1:16). A transesophageal echocardiogram showed no evidence of endocarditis. The patient was treated with 10 weeks of doxycycline for Q fever hepatitis. His fever, headache, and laboratory abnormalities resolved, and he remained well after the completion of therapy.
The serologies suggest the patient had Coxiella burnetii hepatitis, and illustrate the value of a precise exposure history. Most butchers work only with muscle tissue and have a negligible risk of Q fever. In retrospect, it became clear that he worked part‐time in a slaughterhouse, where highly infectious reproductive tract fluids can dry and aerosolize.
Commentary
Q fever was proposed as the name for a febrile illness affecting Australian slaughterhouse workers in 1937.1 The etiologic agent, C. burnetii, is a small, gram‐negative, obligate intracellular proteobacterium that exists in 2 distinct phases, specializing either in entering or persisting in macrophage lysosomes.2 Additionally, spores are formed and can persist in soil.
Q fever is an uncommonly recognized disease, in part because most infected persons have no symptoms or mild symptoms.3 In the United States, the estimated annual incidence has been 0.28 per million (about 50 cases per year) since 1999, when Q fever became a reportable disease due to bioterrorism concerns. In France, more frequent farming of goats and sheep may be responsible for the much higher annual incidence of 500 per million.4 Spread is usually occupational, via aerosol contact with the dried reproductive tract secretions of animals (mainly cattle, sheep, and goats), in a slaughterhouse or farm setting. However, wind‐borne dust can carry spores long distances, and spread can occur from household pets, unpasteurized dairy products, laboratory work, and possibly ticks.3 More than 30 cases have been reported in military personnel deployed to Iraq and Afghanistan, several without obvious exposures.5 One review noted a single reported case of intradermal inoculation,3 making this patient's lacerations a possible site of infection, but he was also at risk for inhalational exposurewhen he was later asked about the details of his work, he acknowledged working at a slaughterhouse as well as a supermarket.
Symptomatic patients are male in 77% of cases, can usually identify an occupational exposure, and have a mean age of 50 years.4 Fever, which lasts 5 to 57 days, as well as fatigue and headaches, begin after a 1‐week to 3‐week incubation period. Atypical pneumonia or rash may occur; meningoencephalitis and myocarditis portend a worse prognosis. As with this patient, 45% to 85% of patients suffer from hepatitis, although few have an abnormal bilirubin.3 Liver biopsy usually reveals granulomas, which may have a classic doughnut hole appearance,2, 3 although this patient ultimately received a diagnosis without the procedure. Acute Q fever rarely (5%) requires hospitalization, and fatalities are extremely rare.3
Chronic infection (ie, lasting >6 months) most often occurs as endocarditis, although chronic hepatitis, osteomyelitis, and infections of other sites occur. Interestingly, this patient's lupus anticoagulant may have been related to his underlying illness, as autoantibodies frequently occur in Q fever, especially in patients with hepatitis, many of whom develop smooth muscle antibodies, a positive Coombs test, antiprothrombinase, or other autoantibodies,3 and there is a high incidence of antiphospholipid antibodies, particularly anticardiolipin and lupus anticoagulant antibodies.6
Because C. burnetii is an obligate intracellular pathogen, culture requires either tissue or live animal inoculation, and the diagnosis is usually made serologically. Paired sera demonstrating seroconversion or a 4‐fold increase in titers are most conclusive, but a single sample may be used. Anti‐phase II antibodies are detectable in 90% of patients within 3 weeks of infection3 and peak at 2 months5; this patient's phase II sera (IgG > 1:200, IgM > 1:50) are said to be 100% predictive for acute Q fever.3 High‐titer anti‐phase I antibodies, in contrast, indicate chronic infection, and a titer 1:800 is one of the modified Duke criteria for endocarditis.5
Acute Q fever is generally treated with doxycycline for 14 days, although prolonged therapy may be advisable to prevent endocarditis if preexisting valvular lesions are present.2, 5 Fluoroquinolones are another option and may be especially useful for meningoencephalitis.5 Because acute Q fever is generally self‐limited, demonstrating a clear benefit to antibiotic therapy is difficult. The available evidence, which was largely obtained from Q fever pneumonia patients, suggests that tetracycline therapy shortens fever duration.3 Patients with Q fever hepatitis may have a protracted course. On the basis of anecdotal reports, some experts add prednisone (tapered from 40 mg daily over a week) for patients with Q fever hepatitis who fail to respond to doxycycline promptly.3 While this patient's fever was unchanged after a week of therapy, he was well into his treatment course when his diagnosis was ultimately confirmed. His physicians felt that prednisone would be of uncertain benefit and opted not to administer it.
Treatment of Q fever endocarditis is often delayed by the combination of negative blood cultures and a low (12%) rate of vegetation formation, increasing the risk of morbidity and mortality.3 Tetracycline monotherapy is associated with a greater than 50% risk of death,5 and even 4 years of treatment may fail to sterilize valve tissue.3 However, if hydroxychloroquine is given with doxycycline for at least 18 months to alkalize lysosomes and improve bacterial killing, the mortality rate can be lowered to about 5%.3, 5 Patients should be warned of the risk of photosensitivity, and monitored for retinal toxicity2 and serologic evidence of relapse.5
Before serologic results confirmed the diagnosis of Q fever, both the patient's clinicians and the discussant had to craft an antibiotic regimen for a suspected zoonosis. The patient received doxycycline, a good choice for leptospirosis,7 brucellosis,8 tularemia,9 and Q fever,3 all possible after livestock exposure, as well as ehrlichiosis.10 The discussant, who suspected tularemia, worried about the possibility of doxycycline resistance and selected ciprofloxacin instead. While fluoroquinolones are probably superior to doxycycline for mild to moderate tularemia,11, 12 aminoglycosides would be preferred for severe disease,9 and ciprofloxacin experience in leptospirosis7 and ehrlichiosis10 is limited. Neither selection would be optimal for brucellosis, for which either doxycycline or ciprofloxacin should be combined with another agent such as rifampin.8 The most reasonable empiric regimen is debatable, but in the absence of pathognomonic findings of tularemia, his treating physicians favored the broader activity of doxycycline.
Ultimately, the choice of antibiotics in this case hinged on the details of the patient's occupational exposures. His first 2 courses of antibiotics were based not on his exposure history, but on radiographic findings that were later proven spurious. The regimens selected by the discussant and by physicians at the referral hospital both targeted pathogens suggested by the patient's occupational history instead, but both were missing parts of the puzzle as well. The discussant thought the patient performed commercial butcher‐shop work, which is only rarely13 mentioned in the context of Q fever transmission. Several of the admitting physicians at the referral hospital were unaware of the importance of the butcher/slaughterhouse‐worker distinction. Physicians need a detailed understanding of both the exposure history and the biology of possible pathogens to craft an optimal differential diagnosis and empiric antibiotic regimen.
On the other hand, in most patients with fever of unknown origin (FUO; ie, >3 weeks with temperature >38.3 on multiple occasions, without a diagnosis after a weeklong evaluation),14 empiric antibiotic therapy is rarely a wise intervention. Clinicians should avoid blind administration of antibiotics as a diagnostic tool, given the inability to distinguish clinical responses from spontaneous resolution, or pinpoint a specific cause and thus a precise treatment plan and duration. However, empiric tetracyclines have been employed when intracellular pathogens were a suspected cause of FUO, as in one series of French patients in which Q fever was common.15 In this patient's case, no specific finding pointed to Q fever before the serologies became available, but the rare infections considered in this case can be considered doxycycline‐deficient states, meaning that empiric tetracycline therapy often leads to improvement. Recognizing doxycycline deficiency can guide therapy while definitive results are pending, and empiric doxycycline is particularly important if potentially aggressive zoonoses, such as Rocky Mountain spotted fever, are suspected.
Teaching Points:
-
A detailed and precise exposure history is crucial for the diagnosis of Q fever and other zoonoses and for the individualized evaluation of FUO in general.
-
Q fever is a rare disease that most commonly causes undifferentiated fever, pneumonia, hepatitis, and when chronic, often reflects endovascular infection, which is frequently difficult to eradicate.
-
Doxycycline is effective for many, but not all zoonoses (babesia is a notable exception). Empiric therapy is reasonable if suspicion is high.
The approach to clinical conundrums by an expert clinician is revealed through presentation of an actual patient's case in an approach typical of morning report. Similar to patient care, sequential pieces of information are provided to the clinician who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A 47‐year‐old male presented to a community hospital with 5 weeks of daily fevers, accompanied by headache, myalgias, and malaise. He reported that his symptoms began abruptly 2 days after a weekend of camping in Connecticut.
This patient describes the onset of undifferentiated fever 2 days after a weekend of camping. Few infectious diseases have such short incubation periods, and either the accuracy of the history or the relationship of the camping trip to the present illness is thus questionable. However, more information about the onset and nature of the illness, and details about food, animal, water, mud, cave, wood chopping, and other environmental exposures during his trip is required. The exact dates of the camping trip may be helpful, as there is clear seasonality to vector‐borne diseases such as Lyme disease, babesiosis, ehrlichiosis, and rickettsial infections. Conditions unrelated to his camping trip, such as malignancies, rheumatologic conditions, and other infectious causes of prolonged fever, such as tuberculosis, endocarditis, or osteomyelitis, are more likely, given the duration of fever.
The fevers were accompanied by chills, without rigors, and subjectively worsened over the first 2 days. At that point, the patient began taking his temperature, and noted fevers of 38.5C to 40C occurring once or twice daily, generally in the afternoon or evening. The patient did not recall tick bites but did not carefully examine himself for ticks; he reported numerous mosquito bites during the trip. The patient camped in a tent and grilled meats and other food he had brought in a cooler. No family members or other travelers became ill. He denied spelunking, but had collected wood for camp fires, and acknowledged swimming in a freshwater pond during his trip, which occurred in August.
West Nile fever, St. Louis encephalitis, and eastern equine encephalitis are transmitted by mosquitoes in New England, but are unlikely causes of prolonged fever. Water exposure suggests the possibility of leptospirosis, and wood exposure suggests blastomycosis, but this usually presents with a pulmonary syndrome. Food‐borne illness seems unlikely. While no aspect of the history has pinpointed a specific diagnosis, exploring the progression of symptoms may offer a clue, and if he has undergone any previous evaluation, the results may significantly alter the differential diagnosis. For example, arthritis may develop weeks after fever in adult‐onset Still's disease, negative blood cultures would lower the probability of endocarditis, and common sites of pyrogenic malignancies (eg, liver, kidneys, and especially lymph nodes) may already have been imaged.
During the first 3 weeks of illness, the patient experienced daily fever and a gradual, 10‐pound weight loss. Over the next 10 days, he sought medical attention at 3 emergency departments. At one, a head computed tomography (CT) showed possible sinusitis, and he was prescribed a 7‐day course of clarithromycin, which he took without any improvement. At 2 others, he was told that his laboratory studies, and a CT of the abdomen, were normal, and that he had a viral syndrome. Several days later, and 5 weeks after the onset of symptoms, the development of dull right upper‐quadrant pain and mild nausea without vomiting prompted the current presentation to the community hospital. He reported several years of loose stools, but denied rash, arthritis, diarrhea, neck stiffness, cough, or other complaints.
A detailed past medical, social, and family history is required, with particular attention to ethnicity; immunocompromising conditions such as splenectomy or corticosteroid use; undiagnosed febrile diseases; severe, unusual, or recurrent infections; medication use; diet; sexual history; pet exposures; and any personal or family history of cancer. The development of right upper‐quadrant pain mandates attention to risk factors for viral hepatitis, known biliary pathology, or travel that might predispose the patient to pyogenic or amoebic liver abscess, and hematochezia, which could suggest a malignancy metastatic to the liver. Additionally, chronic diarrhea with new right upper‐quadrant pain may represent inflammatory bowel disease complicated by primary sclerosing cholangitis (PSC).
The patient was a Caucasian male of Mediterranean ancestry with thalassemia minor. He had undergone dilation of a benign esophageal stricture, but no surgical procedures, and he had never experienced unexplained fever or unusual infections. Medication exposure was limited to occasional use of acetaminophen for fever, and he had no known allergies. His diet was unremarkable and included no well water or unpasteurized dairy products. He denied risk factors for tuberculosis. He drank 2 to 10 beers a day, 5 times a week, had last smoked 10 years previously, and had never used illicit drugs. He denied any high‐risk sexual contacts and was monogamous with his wife, with whom he had 2 children. The family owned no pets and no relatives had suffered from malignant, rheumatologic, or febrile illness, with the exception of hand, foot, and mouth infection in an infant son, 1 year previously. The patient had never traveled outside of New England.
The history has uncovered several clues, but their relevance is doubtful. His ethnicity suggests possible familial Mediterranean fever, but recurrent abdominal pain and polyserositis, rather than a single prolonged episode, would be expected with this disease. A transfusion history should be obtained to explore the possibility of viral hepatitis. While iron overload can predispose patients to various infections including liver abscess, thalassemia minor should not require transfusion. Esophageal stricture could conceivably be due to histoplasmosis (complicated by mediastinal fibrosis) or tuberculosis, but is probably unrelated to his present illness. His excessive alcohol intake increases his risk for esophageal cancer and liver disease, but it is unlikely that metastatic disease to the liver would present with fever without preceding dysphagia, or that alcoholic hepatitis could have escaped detection after evaluations by several physicians.
We need to learn the details of the patient's physical examination. Given the development of right upper‐quadrant pain, I would particularly like to know if he had hepatosplenomegaly and if a Murphy's sign was present.
His temperature ranged from 36.9C to 39.8C, his pulse was 76 beats per minute with minimal elevations during fever spikes, and his respirations were 18 per minute. His blood pressure was 105/70 mm Hg. He was a well‐developed, overweight male with scleral icterus. He had good dentition and an oropharynx free of lesions. Cardiac examination demonstrated a regular rhythm with a normal S1 and S2, without murmurs or peripheral stigmata of infectious endocarditis. A smooth, minimally tender liver edge was palpable 2 cm below the costal margin; the spleen was nonpalpable. Murphy's sign was absent. There was no lymphadenopathy or rash. He had multiple, shallow, uninfected lacerations of both hands in various stages of healing. The remainder of his examination was normal.
The patient has obvious liver involvement. The pulse‐temperature dissociation suggests a variety of infections, including salmonellosis, psittacosis, typhoid fever, leptospirosis, tularemia, brucellosis, legionellosis, and mycoplasma pneumoniae infection. The patient should be asked how and when he injured his hands, as fresh water exposure can transmit leptospirosis across broken skin. However, while severe leptospirosis can cause fever and jaundice, the long duration of illness is not typical. The cryptogenic form of tularemiawhich can manifest as a typhoidal illnessshould be considered, given that tularemia is present in the area the patient visited; he should be asked about exposure to rabbits.
At this point, I would like to see a standard biochemical profile, a liver panel, a complete blood count and differential, urinalysis, chest X‐ray, and an electrocardiogram. I would examine thick and thin Wright‐Giemsa‐stained smears for evidence of babesiosis. Blood cultures should be held for at least 2 weeks to recover fastidious organisms like Francisella tularensis and Brucella sp. Bone marrow cultures should be obtained; they are more sensitive for mycobacteria and Brucella, and may also yield fungal pathogens. Serologies for a variety of infectious diseases, such as leptospirosis, typhoid fever, and tularemia, will be required if other diagnostic tests are unrevealing.
His white cell count was 8,100/L, with a normal differential, and his hemoglobin was 10 g/dL (normal range, 1417), with a mean corpuscular hemoglobin of 63 m3 (normal range, 8298). The platelet count was 303,000/L. Serum electrolytes were normal. His aspartate aminotransferase was 58 U/L and his alanine aminotransferase was 60 U/L (normal range for both, 1045). Bilirubin was 2.6 mg/dL (normal, 1.2); direct bilirubin was 0.9 mg/dL. Alkaline phosphatase was 150 U/L. Lactate dehydrogenase was 342 U/L (normal range, 2251). A lipase was 62 U/L. International normalized ratio (INR) was 1.4 with an activated partial thromboplastin time (aPTT) of 52 seconds (normal range, 2533). Erythrocyte sedimentation rate (ESR) was 50 mm/hour (normal range, 015). Iron studies showed a suppressed iron and iron‐binding capacity and elevated haptoglobin and ferritin (1878 ng/L; normal range, 22322). Several blood cultures obtained at admission showed no growth after 48 hours of incubation.
The anemia, low mean cell volume (MCV), and elevated ferritin and ESR are consistent with anemia of chronic disease, superimposed upon thalassemia minor. Transaminase elevations occur in a plethora of infectious processes. The elevated INR and aPTT are concerning, and may indicate a septic or malignant process with disseminated intravascular coagulation (DIC). While there is no mention of clinical DIC, it would be appropriate to obtain D‐dimers, fibrin degradation products, and a fibrinogen level. The platelet count is normal, which is reassuring.
Before initiating any empiric antimicrobials, I would obtain an abdominal ultrasound, and possibly an abdominal CT. Hepatitis (especially B and C), cytomegalovirus, and Epstein‐Barr virus serologies should be obtained. A variety of conditions including leptospirosis, tularemia, and babesiosis are possible; specific laboratory testing is required to guide therapy.
Ultrasound showed a thickened gallbladder; the liver was slightly enlarged with normal echotexture. Magnetic resonance cholangiopancreatography (MRCP) showed diffuse sequential beading and scarring of his extrahepatic biliary ducts.
There is no evidence of biliary stones, intrahepatic tumor, or abscess to explain the fever and hepatitis, although it would be helpful to know what other abdominal structures were imaged. The MRCP finding increases my suspicion of PSC, possibly complicated by infection, although the biliary abnormalities may be incidental, and an unrelated process may be responsible for the clinical presentation.
His physicians considered the possibilities of PSC and cholangiopathy due to as‐yet undiagnosed acquired immunodeficiency syndrome. Ampicillin‐sulbactam, ceftazidime, and gentamicin were administered for possible bacterial cholangitis, and endoscopic retrograde cholangiopancreatography was performed. This procedure showed only slight narrowing of his common bile duct, which was felt to be a normal variant. He felt no better after several days of antibiotic therapy, and was transferred to a tertiary care center for further evaluation. Repeat physical exam and laboratory studies were essentially unchanged. The patient explained that his hand lacerations were sustained during his work as a butcher who worked with lamb, beef, rabbit, and poultry. He rarely wore protective gloves because they induced contact dermatitis.
Tularemia becomes more likely given his history of rabbit butchering. Salmonellosis and leptospirosis also remain possible. Typhoid fever and brucellosis are unlikely unless the patient worked with imported exotic animals. At this point, given the systemic illness, empiric antibacterial therapy is reasonable. Of the chosen antimicrobials, only the gentamicin would reliably treat tularemia. I would stop ampicillin‐sulbactam and ceftazidime and replace gentamicin with ciprofloxacin, an effective and better‐tolerated agent for tularemia. Cultures of blood and bone marrow aspirate should be obtained. Stool should be cultured for Salmonella. Tularemia, leptospirosis, and typhoid serologies should be sent to a reference laboratory. At this point in the patient's illness, high‐titered antibodies should be present. However, it would be ideal to compare titers with those from previous serum sample, if possible.
The patient's antimicrobials were narrowed to doxycycline alone, for suspected zoonotic infection, but his fevers were unchanged after 1 week of treatment. Hepatitis serologies, human immunodeficiency virus (HIV) antibody, and smears for ehrlichiosis and babesiosis were negative. He had a positive immunoglobulin (Ig)G and a negative IgM for Epstein‐Barr virus and cytomegalovirus. Tularemia, ehrlichiosis, leptospirosis, brucellosis, and Query fever (Q fever) serologies were ordered. The elevated aPTT did not correct when his serum was mixed with normal serum. Thrombin was normal; factor VIII, von Willebrand (VW) factor, and VW cofactor were mildly elevated. Lupus anticoagulant was detected. A hepatologist declined to obtain a liver biopsy, citing the elevated aPTT and pending serologies. Given his clinical stability, the patient was discharged on doxycycline to await further results.
My highest suspicion is for tularemia, and I would switch antibiotic treatment to ciprofloxacin, awaiting serological results. Some in vitro studies have suggested that F. tularensis may often be resistant to doxycycline, and recent clinical experience has shown fluoroquinolones are superior to doxycycline in the treatment of tularemia.
His serologic results were as follows: tularemia, 1:32 (positive, 1:128); ehrlichia, 1:128 (granulocytic) and 1:64 (monocytic; normal for both, 1:64); leptospira, agglutinated nonspecifically; Brucella IgG and IgM 1 (negative, 9), Q fever (coxiella) IgG 1 + 2, IgM 1 + 2, all positive at 1:256 (1:16). A transesophageal echocardiogram showed no evidence of endocarditis. The patient was treated with 10 weeks of doxycycline for Q fever hepatitis. His fever, headache, and laboratory abnormalities resolved, and he remained well after the completion of therapy.
The serologies suggest the patient had Coxiella burnetii hepatitis, and illustrate the value of a precise exposure history. Most butchers work only with muscle tissue and have a negligible risk of Q fever. In retrospect, it became clear that he worked part‐time in a slaughterhouse, where highly infectious reproductive tract fluids can dry and aerosolize.
Commentary
Q fever was proposed as the name for a febrile illness affecting Australian slaughterhouse workers in 1937.1 The etiologic agent, C. burnetii, is a small, gram‐negative, obligate intracellular proteobacterium that exists in 2 distinct phases, specializing either in entering or persisting in macrophage lysosomes.2 Additionally, spores are formed and can persist in soil.
Q fever is an uncommonly recognized disease, in part because most infected persons have no symptoms or mild symptoms.3 In the United States, the estimated annual incidence has been 0.28 per million (about 50 cases per year) since 1999, when Q fever became a reportable disease due to bioterrorism concerns. In France, more frequent farming of goats and sheep may be responsible for the much higher annual incidence of 500 per million.4 Spread is usually occupational, via aerosol contact with the dried reproductive tract secretions of animals (mainly cattle, sheep, and goats), in a slaughterhouse or farm setting. However, wind‐borne dust can carry spores long distances, and spread can occur from household pets, unpasteurized dairy products, laboratory work, and possibly ticks.3 More than 30 cases have been reported in military personnel deployed to Iraq and Afghanistan, several without obvious exposures.5 One review noted a single reported case of intradermal inoculation,3 making this patient's lacerations a possible site of infection, but he was also at risk for inhalational exposurewhen he was later asked about the details of his work, he acknowledged working at a slaughterhouse as well as a supermarket.
Symptomatic patients are male in 77% of cases, can usually identify an occupational exposure, and have a mean age of 50 years.4 Fever, which lasts 5 to 57 days, as well as fatigue and headaches, begin after a 1‐week to 3‐week incubation period. Atypical pneumonia or rash may occur; meningoencephalitis and myocarditis portend a worse prognosis. As with this patient, 45% to 85% of patients suffer from hepatitis, although few have an abnormal bilirubin.3 Liver biopsy usually reveals granulomas, which may have a classic doughnut hole appearance,2, 3 although this patient ultimately received a diagnosis without the procedure. Acute Q fever rarely (5%) requires hospitalization, and fatalities are extremely rare.3
Chronic infection (ie, lasting >6 months) most often occurs as endocarditis, although chronic hepatitis, osteomyelitis, and infections of other sites occur. Interestingly, this patient's lupus anticoagulant may have been related to his underlying illness, as autoantibodies frequently occur in Q fever, especially in patients with hepatitis, many of whom develop smooth muscle antibodies, a positive Coombs test, antiprothrombinase, or other autoantibodies,3 and there is a high incidence of antiphospholipid antibodies, particularly anticardiolipin and lupus anticoagulant antibodies.6
Because C. burnetii is an obligate intracellular pathogen, culture requires either tissue or live animal inoculation, and the diagnosis is usually made serologically. Paired sera demonstrating seroconversion or a 4‐fold increase in titers are most conclusive, but a single sample may be used. Anti‐phase II antibodies are detectable in 90% of patients within 3 weeks of infection3 and peak at 2 months5; this patient's phase II sera (IgG > 1:200, IgM > 1:50) are said to be 100% predictive for acute Q fever.3 High‐titer anti‐phase I antibodies, in contrast, indicate chronic infection, and a titer 1:800 is one of the modified Duke criteria for endocarditis.5
Acute Q fever is generally treated with doxycycline for 14 days, although prolonged therapy may be advisable to prevent endocarditis if preexisting valvular lesions are present.2, 5 Fluoroquinolones are another option and may be especially useful for meningoencephalitis.5 Because acute Q fever is generally self‐limited, demonstrating a clear benefit to antibiotic therapy is difficult. The available evidence, which was largely obtained from Q fever pneumonia patients, suggests that tetracycline therapy shortens fever duration.3 Patients with Q fever hepatitis may have a protracted course. On the basis of anecdotal reports, some experts add prednisone (tapered from 40 mg daily over a week) for patients with Q fever hepatitis who fail to respond to doxycycline promptly.3 While this patient's fever was unchanged after a week of therapy, he was well into his treatment course when his diagnosis was ultimately confirmed. His physicians felt that prednisone would be of uncertain benefit and opted not to administer it.
Treatment of Q fever endocarditis is often delayed by the combination of negative blood cultures and a low (12%) rate of vegetation formation, increasing the risk of morbidity and mortality.3 Tetracycline monotherapy is associated with a greater than 50% risk of death,5 and even 4 years of treatment may fail to sterilize valve tissue.3 However, if hydroxychloroquine is given with doxycycline for at least 18 months to alkalize lysosomes and improve bacterial killing, the mortality rate can be lowered to about 5%.3, 5 Patients should be warned of the risk of photosensitivity, and monitored for retinal toxicity2 and serologic evidence of relapse.5
Before serologic results confirmed the diagnosis of Q fever, both the patient's clinicians and the discussant had to craft an antibiotic regimen for a suspected zoonosis. The patient received doxycycline, a good choice for leptospirosis,7 brucellosis,8 tularemia,9 and Q fever,3 all possible after livestock exposure, as well as ehrlichiosis.10 The discussant, who suspected tularemia, worried about the possibility of doxycycline resistance and selected ciprofloxacin instead. While fluoroquinolones are probably superior to doxycycline for mild to moderate tularemia,11, 12 aminoglycosides would be preferred for severe disease,9 and ciprofloxacin experience in leptospirosis7 and ehrlichiosis10 is limited. Neither selection would be optimal for brucellosis, for which either doxycycline or ciprofloxacin should be combined with another agent such as rifampin.8 The most reasonable empiric regimen is debatable, but in the absence of pathognomonic findings of tularemia, his treating physicians favored the broader activity of doxycycline.
Ultimately, the choice of antibiotics in this case hinged on the details of the patient's occupational exposures. His first 2 courses of antibiotics were based not on his exposure history, but on radiographic findings that were later proven spurious. The regimens selected by the discussant and by physicians at the referral hospital both targeted pathogens suggested by the patient's occupational history instead, but both were missing parts of the puzzle as well. The discussant thought the patient performed commercial butcher‐shop work, which is only rarely13 mentioned in the context of Q fever transmission. Several of the admitting physicians at the referral hospital were unaware of the importance of the butcher/slaughterhouse‐worker distinction. Physicians need a detailed understanding of both the exposure history and the biology of possible pathogens to craft an optimal differential diagnosis and empiric antibiotic regimen.
On the other hand, in most patients with fever of unknown origin (FUO; ie, >3 weeks with temperature >38.3 on multiple occasions, without a diagnosis after a weeklong evaluation),14 empiric antibiotic therapy is rarely a wise intervention. Clinicians should avoid blind administration of antibiotics as a diagnostic tool, given the inability to distinguish clinical responses from spontaneous resolution, or pinpoint a specific cause and thus a precise treatment plan and duration. However, empiric tetracyclines have been employed when intracellular pathogens were a suspected cause of FUO, as in one series of French patients in which Q fever was common.15 In this patient's case, no specific finding pointed to Q fever before the serologies became available, but the rare infections considered in this case can be considered doxycycline‐deficient states, meaning that empiric tetracycline therapy often leads to improvement. Recognizing doxycycline deficiency can guide therapy while definitive results are pending, and empiric doxycycline is particularly important if potentially aggressive zoonoses, such as Rocky Mountain spotted fever, are suspected.
Teaching Points:
-
A detailed and precise exposure history is crucial for the diagnosis of Q fever and other zoonoses and for the individualized evaluation of FUO in general.
-
Q fever is a rare disease that most commonly causes undifferentiated fever, pneumonia, hepatitis, and when chronic, often reflects endovascular infection, which is frequently difficult to eradicate.
-
Doxycycline is effective for many, but not all zoonoses (babesia is a notable exception). Empiric therapy is reasonable if suspicion is high.
- .“Q” fever, new fever entity: clinical features, diagnosis and laboratory investigation.Med J Aust.1937;2:281–299.
- ,,.Q fever.Lancet.2006;367(9511):679–688.
- ,.Q fever.Clin Microbiol Rev.1999;12:518–553.
- ,,, et al.National surveillance and the epidemiology of human Q fever in the United States, 1978–2004.Am J Trop Med Hyg.2006;75:36–40.
- ,,,.Q fever: epidemiology, diagnosis, and treatment.Mayo Clin Proc.2008;83(5):574–579.
- ,,, et al.Prevalence, significance, and specificity of antibodies to phospholipids in Q fever.Clin Infect Dis.1994;18:213–218.
- ,,.Antimicrobial therapy of leptospirosis.Curr Opin Infect Dis.2006;19:533–537.
- ,,, et al.Treatment of human brucellosis with doxycycline plus rifampin or doxycycline plus streptomycin. A randomized, double‐blind study.Ann Intern Med.1992;117:25–30.
- ,,,.Tularemia: current epidemiology and disease management.Infect Dis Clin North Am.2006;20:289–311, ix.
- ,,,.Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment.Clin Infect Dis.2007;45(Suppl 1):S45–S51.
- ,.New approaches to diagnosis and therapy of tularemia.Ann NY Acad Sci.2007;1105:378–404.
- ,,, et al.Evaluation of clinical, laboratory, and therapeutic features of 145 tularemia cases: the role of quinolones in oropharyngeal tularemia.APMIS.2008;116:66–73.
- ,.A survey of Q fever antibodies in a high risk population in Panamá.Am J Trop Med Hyg.1980;29(5):1007–1011.
- ,.Fever of unknown origin.Lancet.1997;350:575–580.
- .Fever of unknown origin in adults: evaluation of 144 cases in a non‐university hospital.Scand J Infect Dis.2006;38:632–638.
- .“Q” fever, new fever entity: clinical features, diagnosis and laboratory investigation.Med J Aust.1937;2:281–299.
- ,,.Q fever.Lancet.2006;367(9511):679–688.
- ,.Q fever.Clin Microbiol Rev.1999;12:518–553.
- ,,, et al.National surveillance and the epidemiology of human Q fever in the United States, 1978–2004.Am J Trop Med Hyg.2006;75:36–40.
- ,,,.Q fever: epidemiology, diagnosis, and treatment.Mayo Clin Proc.2008;83(5):574–579.
- ,,, et al.Prevalence, significance, and specificity of antibodies to phospholipids in Q fever.Clin Infect Dis.1994;18:213–218.
- ,,.Antimicrobial therapy of leptospirosis.Curr Opin Infect Dis.2006;19:533–537.
- ,,, et al.Treatment of human brucellosis with doxycycline plus rifampin or doxycycline plus streptomycin. A randomized, double‐blind study.Ann Intern Med.1992;117:25–30.
- ,,,.Tularemia: current epidemiology and disease management.Infect Dis Clin North Am.2006;20:289–311, ix.
- ,,,.Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment.Clin Infect Dis.2007;45(Suppl 1):S45–S51.
- ,.New approaches to diagnosis and therapy of tularemia.Ann NY Acad Sci.2007;1105:378–404.
- ,,, et al.Evaluation of clinical, laboratory, and therapeutic features of 145 tularemia cases: the role of quinolones in oropharyngeal tularemia.APMIS.2008;116:66–73.
- ,.A survey of Q fever antibodies in a high risk population in Panamá.Am J Trop Med Hyg.1980;29(5):1007–1011.
- ,.Fever of unknown origin.Lancet.1997;350:575–580.
- .Fever of unknown origin in adults: evaluation of 144 cases in a non‐university hospital.Scand J Infect Dis.2006;38:632–638.
Linezolid‐ and vancomycin‐resistant Enterococcus faecium endocarditis: Successful treatment with tigecycline and daptomycin
Enterococci are a leading cause of endocarditis and nosocomial infections. Vancomycin‐resistant enterococci (VRE) emerged in the 1980s and now represent most nosocomial isolates in the United States. The first case of VRE endocarditis was reported in 1996.1 Although increasing enterococcal antibiotic resistance has prompted increasing reliance on newer antibiotics,2 a recent review of VRE endocarditis noted that survival rates were similar to those for vancomycin‐sensitive enterococcal endocarditis.1 Cure was achieved in several patients with bacteriostatic agents in the absence of valve replacement, but no patients were infected with truly linezolid‐resistant organisms. This case of linezolid‐resistant VRE endocarditis represents the first reported cure of infective endocarditis with a tigecycline‐containing regimen.
CASE REPORT
A 62‐year‐old man presented with hypoglycemia and delirium. His medical history included diabetes mellitus, coronary and peripheral arterial disease, and end‐stage renal disease. He had had endocarditis of an unknown type 12 years prior to admission. He had recently developed septic shock because of a Candida parapsilosis, Enterobacter cloacae, and Staphylococcus epidermidis infection of a peripherally inserted central catheter (PICC) and received 14 days of vancomycin, meropenem, and fluconazole administered through a new PICC. This catheter was not removed, and 39 days after completion of the antibiotic therapy, he developed hypoglycemia, which was attributed to weight loss without adjustment of his insulin regimen. He was afebrile; examination revealed a new 3/6 holosystolic murmur radiating to the axilla. There were no other stigmata of infective endocarditis, and his PICC and arteriovenous fistula sites appeared normal. Delirium resolved after administration of intravenous glucose.
E. faecium grew from all 6 initial blood cultures. A transesophageal echocardiogram revealed a new 3‐mm mitral valve vegetation with perforation and severe regurgitation. He had definite endocarditis on the basis of 2 major criteria.3 He was given vancomycin (1 g IV, then administered by levels), then switched to linezolid (600 mg orally every 12 hours), and finally tigecycline (100 mg IV followed by 50 mg IV every 12 hours) plus daptomycin (6 mg/kg IV every 48 hours) as further sensitivity data became available.
The organism was resistant to ampicillin, chloramphenicol, and linezolid (MIC > 20 g/mL), as well as vancomycin (MIC > 50 g/mL), quinupristin/dalfopristin (MIC 2.5 g/mL), and gentamicin (MIC > 200 g/mL), and demonstrated high‐level streptomycin resistance (>2000 g/mL). It was intermediate to doxycycline (MIC 5 g/mL). It was susceptible to daptomycin (MIC 4 g/mL) and tigecycline (MIC 0.06 g/mL).
Blood cultures done on hospital days 1, 4, 6, and 7 (day 1 of tigecycline) were positive, and multiple cultures were negative from day 10 on. Because of the lack of experience with tigecycline in infective endocarditis, unrevascularized left‐main coronary artery disease, and severe mitral regurgitation, the patient was advised to undergo valve replacement and coronary artery bypass surgery after antibiotic therapy. Because he feared surgical complications, he refused and received 70 days of tigecycline plus daptomycin therapy, which was complicated only by nausea. He remained clinically well and had negative blood cultures 16 weeks after completion of therapy.
DISCUSSION
Tigecycline, the first available glycylcycline, is a minocycline‐derived antibiotic that remains active in the presence of the ribosomal modifications and efflux pumps that mediate tetracycline resistance. Thus, it possesses broad‐spectrum bacteriostatic activity, including activity against VRE. A PubMed search revealed no published data about the use of tigecycline for endocarditis in humans. However, tetracyclines have been used to treat endocarditis due to such organisms as Bartonella, Coxiella burnetti, or methicillin‐resistant Staphylococcus aureus (MRSA), frequently for prolonged courses. Tetracyclines were combined with other antibiotics in 5 published cases of VRE endocarditis. All patients survived; 3 were cured with the tetracycline regimen and 2 with other antimicrobials.1 In animal models of endocarditis, tigecycline stabilized vegetation counts of E. faecalis and reduced vegetation counts of MRSA and 1 strain of E. faecium.4
Daptomycin, the first available cyclic lipopeptide, kills by nonlytic depolarization of the bacterial cell membrane. In a recent study, daptomycin was non‐inferior to vancomycin or antistaphylococcal penicillins for S. aureus bacteremia or endocarditis. Although a few patients had left‐sided endocarditis, only 1 of them experienced a successful outcome with daptomycin therapy, and daptomycin displayed a trend toward higher rates of persistent or relapsing infection.5 Less evidence supports the use of daptomycin for serious enterococcal infections.2 One report noted the deaths of 6 of 10 patients treated with daptomycin for VRE bacteremia, including both patients with endocarditis.6 Daptomycin was used successfully in a case of VRE endocarditis in combination with gentamicin and rifampin for 11 weeks1 and at least 6 other reported cases of VRE bacteremia.7, 8
In summary, despite tigecycline's lack of bactericidal activity or proven efficacy in endocarditis, daptomycin's prior performance in VRE bacteremia, and the isolate's borderline daptomycin susceptibility, prolonged combination therapy resulted in a cure of VRE endocarditis. This success extends the experience with using both agents in the treatment of resistant infections. As linezolid‐resistant VRE and other resistant pathogens become more common, the need for research on treatment options becomes more urgent, and familiarity with novel and lesser‐used antibiotics becomes more crucial for hospitalists.
- ,.Endocarditis due to vancomycin‐resistant enterococci: case report and review of the literature.Clin Infect Dis.2005;41:1134–1142.
- ,.Approaches to vancomycin resistant enterococci.Curr Opin Infect Dis.2004;17:541–547.
- ,,, et al.Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis.Clin Infect Dis.2000;4:633–638.
- ,,, et al.Activity and diffusion of tigecycline (GAR‐936) in experimental enterococcal endocarditis.Antimicrob Agents Chemother.2003;47:216–222.
- ,,, et al.Daptomycin versus standard therapy for bacteremia and endocarditis caused by staphylococcus aureus.New Engl J Med.2006;355:653–665.
- ,,.Daptomycin for the treatment of gram‐positive bacteremia and infective endocarditis: a retrospective case series of 31 patients.Pharmacotherapy.2006;26:347–352.
- ,,,,.Daptomycin in the treatment of vancomycin‐resistant Enterococcus faecium bacteremia in neutropenic patients.J Infect.2007;54:567–571.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2006;38:290–292.
Enterococci are a leading cause of endocarditis and nosocomial infections. Vancomycin‐resistant enterococci (VRE) emerged in the 1980s and now represent most nosocomial isolates in the United States. The first case of VRE endocarditis was reported in 1996.1 Although increasing enterococcal antibiotic resistance has prompted increasing reliance on newer antibiotics,2 a recent review of VRE endocarditis noted that survival rates were similar to those for vancomycin‐sensitive enterococcal endocarditis.1 Cure was achieved in several patients with bacteriostatic agents in the absence of valve replacement, but no patients were infected with truly linezolid‐resistant organisms. This case of linezolid‐resistant VRE endocarditis represents the first reported cure of infective endocarditis with a tigecycline‐containing regimen.
CASE REPORT
A 62‐year‐old man presented with hypoglycemia and delirium. His medical history included diabetes mellitus, coronary and peripheral arterial disease, and end‐stage renal disease. He had had endocarditis of an unknown type 12 years prior to admission. He had recently developed septic shock because of a Candida parapsilosis, Enterobacter cloacae, and Staphylococcus epidermidis infection of a peripherally inserted central catheter (PICC) and received 14 days of vancomycin, meropenem, and fluconazole administered through a new PICC. This catheter was not removed, and 39 days after completion of the antibiotic therapy, he developed hypoglycemia, which was attributed to weight loss without adjustment of his insulin regimen. He was afebrile; examination revealed a new 3/6 holosystolic murmur radiating to the axilla. There were no other stigmata of infective endocarditis, and his PICC and arteriovenous fistula sites appeared normal. Delirium resolved after administration of intravenous glucose.
E. faecium grew from all 6 initial blood cultures. A transesophageal echocardiogram revealed a new 3‐mm mitral valve vegetation with perforation and severe regurgitation. He had definite endocarditis on the basis of 2 major criteria.3 He was given vancomycin (1 g IV, then administered by levels), then switched to linezolid (600 mg orally every 12 hours), and finally tigecycline (100 mg IV followed by 50 mg IV every 12 hours) plus daptomycin (6 mg/kg IV every 48 hours) as further sensitivity data became available.
The organism was resistant to ampicillin, chloramphenicol, and linezolid (MIC > 20 g/mL), as well as vancomycin (MIC > 50 g/mL), quinupristin/dalfopristin (MIC 2.5 g/mL), and gentamicin (MIC > 200 g/mL), and demonstrated high‐level streptomycin resistance (>2000 g/mL). It was intermediate to doxycycline (MIC 5 g/mL). It was susceptible to daptomycin (MIC 4 g/mL) and tigecycline (MIC 0.06 g/mL).
Blood cultures done on hospital days 1, 4, 6, and 7 (day 1 of tigecycline) were positive, and multiple cultures were negative from day 10 on. Because of the lack of experience with tigecycline in infective endocarditis, unrevascularized left‐main coronary artery disease, and severe mitral regurgitation, the patient was advised to undergo valve replacement and coronary artery bypass surgery after antibiotic therapy. Because he feared surgical complications, he refused and received 70 days of tigecycline plus daptomycin therapy, which was complicated only by nausea. He remained clinically well and had negative blood cultures 16 weeks after completion of therapy.
DISCUSSION
Tigecycline, the first available glycylcycline, is a minocycline‐derived antibiotic that remains active in the presence of the ribosomal modifications and efflux pumps that mediate tetracycline resistance. Thus, it possesses broad‐spectrum bacteriostatic activity, including activity against VRE. A PubMed search revealed no published data about the use of tigecycline for endocarditis in humans. However, tetracyclines have been used to treat endocarditis due to such organisms as Bartonella, Coxiella burnetti, or methicillin‐resistant Staphylococcus aureus (MRSA), frequently for prolonged courses. Tetracyclines were combined with other antibiotics in 5 published cases of VRE endocarditis. All patients survived; 3 were cured with the tetracycline regimen and 2 with other antimicrobials.1 In animal models of endocarditis, tigecycline stabilized vegetation counts of E. faecalis and reduced vegetation counts of MRSA and 1 strain of E. faecium.4
Daptomycin, the first available cyclic lipopeptide, kills by nonlytic depolarization of the bacterial cell membrane. In a recent study, daptomycin was non‐inferior to vancomycin or antistaphylococcal penicillins for S. aureus bacteremia or endocarditis. Although a few patients had left‐sided endocarditis, only 1 of them experienced a successful outcome with daptomycin therapy, and daptomycin displayed a trend toward higher rates of persistent or relapsing infection.5 Less evidence supports the use of daptomycin for serious enterococcal infections.2 One report noted the deaths of 6 of 10 patients treated with daptomycin for VRE bacteremia, including both patients with endocarditis.6 Daptomycin was used successfully in a case of VRE endocarditis in combination with gentamicin and rifampin for 11 weeks1 and at least 6 other reported cases of VRE bacteremia.7, 8
In summary, despite tigecycline's lack of bactericidal activity or proven efficacy in endocarditis, daptomycin's prior performance in VRE bacteremia, and the isolate's borderline daptomycin susceptibility, prolonged combination therapy resulted in a cure of VRE endocarditis. This success extends the experience with using both agents in the treatment of resistant infections. As linezolid‐resistant VRE and other resistant pathogens become more common, the need for research on treatment options becomes more urgent, and familiarity with novel and lesser‐used antibiotics becomes more crucial for hospitalists.
Enterococci are a leading cause of endocarditis and nosocomial infections. Vancomycin‐resistant enterococci (VRE) emerged in the 1980s and now represent most nosocomial isolates in the United States. The first case of VRE endocarditis was reported in 1996.1 Although increasing enterococcal antibiotic resistance has prompted increasing reliance on newer antibiotics,2 a recent review of VRE endocarditis noted that survival rates were similar to those for vancomycin‐sensitive enterococcal endocarditis.1 Cure was achieved in several patients with bacteriostatic agents in the absence of valve replacement, but no patients were infected with truly linezolid‐resistant organisms. This case of linezolid‐resistant VRE endocarditis represents the first reported cure of infective endocarditis with a tigecycline‐containing regimen.
CASE REPORT
A 62‐year‐old man presented with hypoglycemia and delirium. His medical history included diabetes mellitus, coronary and peripheral arterial disease, and end‐stage renal disease. He had had endocarditis of an unknown type 12 years prior to admission. He had recently developed septic shock because of a Candida parapsilosis, Enterobacter cloacae, and Staphylococcus epidermidis infection of a peripherally inserted central catheter (PICC) and received 14 days of vancomycin, meropenem, and fluconazole administered through a new PICC. This catheter was not removed, and 39 days after completion of the antibiotic therapy, he developed hypoglycemia, which was attributed to weight loss without adjustment of his insulin regimen. He was afebrile; examination revealed a new 3/6 holosystolic murmur radiating to the axilla. There were no other stigmata of infective endocarditis, and his PICC and arteriovenous fistula sites appeared normal. Delirium resolved after administration of intravenous glucose.
E. faecium grew from all 6 initial blood cultures. A transesophageal echocardiogram revealed a new 3‐mm mitral valve vegetation with perforation and severe regurgitation. He had definite endocarditis on the basis of 2 major criteria.3 He was given vancomycin (1 g IV, then administered by levels), then switched to linezolid (600 mg orally every 12 hours), and finally tigecycline (100 mg IV followed by 50 mg IV every 12 hours) plus daptomycin (6 mg/kg IV every 48 hours) as further sensitivity data became available.
The organism was resistant to ampicillin, chloramphenicol, and linezolid (MIC > 20 g/mL), as well as vancomycin (MIC > 50 g/mL), quinupristin/dalfopristin (MIC 2.5 g/mL), and gentamicin (MIC > 200 g/mL), and demonstrated high‐level streptomycin resistance (>2000 g/mL). It was intermediate to doxycycline (MIC 5 g/mL). It was susceptible to daptomycin (MIC 4 g/mL) and tigecycline (MIC 0.06 g/mL).
Blood cultures done on hospital days 1, 4, 6, and 7 (day 1 of tigecycline) were positive, and multiple cultures were negative from day 10 on. Because of the lack of experience with tigecycline in infective endocarditis, unrevascularized left‐main coronary artery disease, and severe mitral regurgitation, the patient was advised to undergo valve replacement and coronary artery bypass surgery after antibiotic therapy. Because he feared surgical complications, he refused and received 70 days of tigecycline plus daptomycin therapy, which was complicated only by nausea. He remained clinically well and had negative blood cultures 16 weeks after completion of therapy.
DISCUSSION
Tigecycline, the first available glycylcycline, is a minocycline‐derived antibiotic that remains active in the presence of the ribosomal modifications and efflux pumps that mediate tetracycline resistance. Thus, it possesses broad‐spectrum bacteriostatic activity, including activity against VRE. A PubMed search revealed no published data about the use of tigecycline for endocarditis in humans. However, tetracyclines have been used to treat endocarditis due to such organisms as Bartonella, Coxiella burnetti, or methicillin‐resistant Staphylococcus aureus (MRSA), frequently for prolonged courses. Tetracyclines were combined with other antibiotics in 5 published cases of VRE endocarditis. All patients survived; 3 were cured with the tetracycline regimen and 2 with other antimicrobials.1 In animal models of endocarditis, tigecycline stabilized vegetation counts of E. faecalis and reduced vegetation counts of MRSA and 1 strain of E. faecium.4
Daptomycin, the first available cyclic lipopeptide, kills by nonlytic depolarization of the bacterial cell membrane. In a recent study, daptomycin was non‐inferior to vancomycin or antistaphylococcal penicillins for S. aureus bacteremia or endocarditis. Although a few patients had left‐sided endocarditis, only 1 of them experienced a successful outcome with daptomycin therapy, and daptomycin displayed a trend toward higher rates of persistent or relapsing infection.5 Less evidence supports the use of daptomycin for serious enterococcal infections.2 One report noted the deaths of 6 of 10 patients treated with daptomycin for VRE bacteremia, including both patients with endocarditis.6 Daptomycin was used successfully in a case of VRE endocarditis in combination with gentamicin and rifampin for 11 weeks1 and at least 6 other reported cases of VRE bacteremia.7, 8
In summary, despite tigecycline's lack of bactericidal activity or proven efficacy in endocarditis, daptomycin's prior performance in VRE bacteremia, and the isolate's borderline daptomycin susceptibility, prolonged combination therapy resulted in a cure of VRE endocarditis. This success extends the experience with using both agents in the treatment of resistant infections. As linezolid‐resistant VRE and other resistant pathogens become more common, the need for research on treatment options becomes more urgent, and familiarity with novel and lesser‐used antibiotics becomes more crucial for hospitalists.
- ,.Endocarditis due to vancomycin‐resistant enterococci: case report and review of the literature.Clin Infect Dis.2005;41:1134–1142.
- ,.Approaches to vancomycin resistant enterococci.Curr Opin Infect Dis.2004;17:541–547.
- ,,, et al.Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis.Clin Infect Dis.2000;4:633–638.
- ,,, et al.Activity and diffusion of tigecycline (GAR‐936) in experimental enterococcal endocarditis.Antimicrob Agents Chemother.2003;47:216–222.
- ,,, et al.Daptomycin versus standard therapy for bacteremia and endocarditis caused by staphylococcus aureus.New Engl J Med.2006;355:653–665.
- ,,.Daptomycin for the treatment of gram‐positive bacteremia and infective endocarditis: a retrospective case series of 31 patients.Pharmacotherapy.2006;26:347–352.
- ,,,,.Daptomycin in the treatment of vancomycin‐resistant Enterococcus faecium bacteremia in neutropenic patients.J Infect.2007;54:567–571.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2006;38:290–292.
- ,.Endocarditis due to vancomycin‐resistant enterococci: case report and review of the literature.Clin Infect Dis.2005;41:1134–1142.
- ,.Approaches to vancomycin resistant enterococci.Curr Opin Infect Dis.2004;17:541–547.
- ,,, et al.Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis.Clin Infect Dis.2000;4:633–638.
- ,,, et al.Activity and diffusion of tigecycline (GAR‐936) in experimental enterococcal endocarditis.Antimicrob Agents Chemother.2003;47:216–222.
- ,,, et al.Daptomycin versus standard therapy for bacteremia and endocarditis caused by staphylococcus aureus.New Engl J Med.2006;355:653–665.
- ,,.Daptomycin for the treatment of gram‐positive bacteremia and infective endocarditis: a retrospective case series of 31 patients.Pharmacotherapy.2006;26:347–352.
- ,,,,.Daptomycin in the treatment of vancomycin‐resistant Enterococcus faecium bacteremia in neutropenic patients.J Infect.2007;54:567–571.
- ,,,,.Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia.Scand J Infect Dis.2006;38:290–292.
Evidence‐Based Sepsis Therapy
Despite decades of intensive research and improvements in medical care, severe sepsis affects an estimated 751,000 patients in the United States every year, killing 215,000 of them at an annual cost of 16.7 billion dollars.1 Because the elderly experience a 100‐fold increase in incidence, as compared with children, and a nearly 4‐fold increase in mortality (38.4% of those more than 85 years old), this burden is expected to increase with the aging population.1 Patients with severe sepsis have prolonged ICU14 and hospital stays and incur substantially increased costs compared with other patients.36
New research continues to explore the complex pathophysiology of sepsis,7 and clinicians, who once relied primarily on clinical experience and expert opinion to guide therapy, now have an increasing array of evidenced‐based sepsis therapies to employ. Recent meta‐analyses have evaluated several major treatments for severe sepsis,810 and recommendations (the Surviving Sepsis Campaign guidelines) for the treatment of severe sepsis were recently endorsed by 11 international critical care and infectious disease organizations.11 This article summarizes the current definitions of sepsis syndromes, the trials supporting the specific therapies for sepsis that are currently recommended, ongoing controversies and research, and implications for hospitalists, with a focus on early, effective antibiotics, activated protein C, early goal‐directed therapy, stress dose steroids, and intensive insulin therapy. For space considerations, readers are directed elsewhere for data supporting prophylaxis for deep venous thrombosis (DVT)12 and stress ulcer bleeding13 and for therapies less often directed by hospitalists, such as lung protective ventilation.14
DEFINITIONS
Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined in 1992 to standardize the terminology of sepsis.15 These definitions have recently been reviewed and supported by a variety of American and European intensive care societies.16
SIRS is defined by the presence of at least 2 of the following:
-
Temperature > 38C or 36C;
-
Heart rate > 90 beats/min;
-
Respiratory rate > 20 breaths/min or PaCO2 32 mm Hg;
-
WBC >12,000 or 4000 cells/mm3, or >10% immature (band) forms.
Sepsis is SIRS due to documented or strongly suspected infection.
Severe sepsis is sepsis with organ dysfunction (such as lactic acidosis, oliguria, thrombocytopenia, or delirium), hypoperfusion, or hypotension ( 90 mm Hg systolic or more than 40 mm Hg below baseline).
Septic shock is severe sepsis complicated by hypotension or pressor dependence despite adequate (20‐30 mL/kg; 1.5‐3 liters in most patients) fluid resuscitation.
Sepsis terminology must be applied carefully. Many hospitalized patients meet criteria for SIRS, yet it is inaccurate to say a patient who has acute leukemia with leukocytosis, anemia‐induced tachycardia, and thrombocytopenia has severe sepsis if those abnormalities are not a result of inflammation or infection. Accurate documentation of sepsis syndromes can improve professional and institutional reimbursement and provide prognostic information: the in‐hospital mortality rates for severe sepsis and septic shock are approximately 30% and 50%, respectively.17 More importantly, thoughtful application of these definitions can help a hospitalist identify septic patients who qualify for one of the proven therapies for severe sepsis.
EARLY, EFFECTIVE ANTIBIOTICS
For obvious ethical reasons, randomized, controlled trials to study the impact of inappropriate or delayed antibiotic therapy for serious infections are not possible. However, the evidence supporting early, effective antibiotic therapy is still compelling, and because many hospitalists often initiate treatment with antibiotics before transferring a patient to intensive care, this may represent the most important intervention hospitalists can provide to patients with serious infections. Several studies have estimated the impact of early, effective antibiotics on outcomes.
Houck et al. retrospectively reviewed 13,771 cases of community‐acquired pneumonia among elderly Medicare patients. They found that 39.1% of the patients waited more than 4 hours for antibiotics and 7.6% waited more than 12 hours; three quarters of these delays resulted from delayed ordering of antibiotics.18 Further, 21.2% received an antibiotic selection incompatible with recent professional guidelines. Receiving antibiotics within 4 hours reduced in‐hospital and 30‐day mortality by 15% and length of stay by 0.4 days.18 Similar conclusions were reported by 3 of 4 previous analyses.1922 Extending these findings to critically ill patients, Iregui et al. found that delayed treatment with appropriate antibiotics (odds ratio, 7.68) was a greater predictor of mortality for 107 patients with ventilator‐acquired pneumonia than were APACHE II scores and malignancy; 31% failed to receive appropriate antibiotics within 24 hours, and again, three quarters of these delays resulted from delays in writing antibiotic orders.23
Not surprisingly, antibiotic therapy must be effective as well as timely. MacArthur et al. studied the impact of adequate (ie, active against cultured organisms, if isolated) antibiotics on the outcomes of 2634 septic patients enrolled in a randomized trial of an anti‐TNF antibody. Nearly 91% received appropriate antibiotics; their mortality rate was 33%, 10% lower than that of the patients whose initial antibiotics were inadequate (P .001).24 Leibovici et al. reported similar findings in a prospective study of patients with bacteremia. Only 63% of 3413 subjects received an antibiotic active against the infecting pathogen, and their mortality was 20%, 14% lower than that in the group that received ineffective antibiotics (P = .0001).25 Other authors have reported even worse outcomes with ineffective therapy: 62% mortality among inadequately treated bacteremic or fungemic ICU patients, compared with 28.4% among those who were adequately treated26 and an odds ratio of dying of 8.14 for the 46 of 270 septic ICU patients who received inadequate initial antibiotics,27 making inadequate antibiotic therapy the strongest risk factor for death. Finally, Kollef et al. reported that 26% of 655 infected ICU patients received inadequate antibiotics and suffered an infection‐related mortality rate of 40.2%, more than twice the 17.7% rate among adequately treated patients (P .001). Inadequate antimicrobial therapy was a greater risk factor for death than early respiratory failure or sepsis‐related organ failure assessment scores.28
Guidelines for anti‐infective care now recommend obtaining appropriate cultures and administering broad‐spectrum antibiotics (appropriate for suspected infections, local susceptibility patterns, and any relevant prior culture data from individual patients) within 1 hour of presentation.11 In addition, any removable focus of infection must be identified and managed (eg, an abscess, infected catheter, tampon, or infection requiring surgery).
ACTIVATED PROTEIN C
Recombinant human activated protein C (APC) is a protein with anticoagulant and anti‐inflammatory properties that is relatively deficient in approximately 87% of septic patients.29 Although numerous trials of other anticoagulants (antithrombin III and tissue factor pathway inhibitor) and immunosuppressives (tumor necrosis factor inhibitors, high‐dose steroids, interleukin‐1 receptor antagonists, and others) have failed to show any benefit,7 in 2001 APC became the first proven therapy specifically for sepsis. The PROWESS trial, which established its efficacy, randomized 1690 patients who met 3 SIRS criteria and dysfunction of at least 1 organ system to APC (24 g/kg IV per hour for 96 hours interrupted for bleeding or urgent procedures) or placebo. APC reduced 28‐day mortality from 30.8% to 24.7%, yielding an absolute risk reduction of 6.1% and a corresponding number needed to treat (NNT) of 16.4. This benefit was seen across all subgroups including those with normal baseline APC levels.29
Not surprisingly, APC increases the risk of serious bleeding. Although this effect was of borderline significance in PROWESS (3.5% vs. 2% in the placebo group, P = .06),29 it was confirmed in subsequent trials (3.9% vs. 2.2%, P = .01)30 and may be larger still in open‐label use, at 6.5%.17, 31 Intracerebral hemorrhage (ICH), a particularly devastating complication, occurred in 0.2% of the PROWESS patients and 0.5% of patients in 2 subsequent studies30, 32; in both major trials, there was a single extra event in the APC arm.29, 30 Like serious bleeding in general, ICH was more common in open‐label use, occurring in 1.5% of patients.31, 33 Therefore, it is vital to have strict adherence to exclusion criteria and familiarity with the risk factors for serious bleeding. In the PROWESS trial, after randomization, risk factors for serious bleeding included procedures and injury to vascular organs, an activated partial‐thromboplastin time of more than 120 seconds, an international normalized ratio greater than 3, gastrointestinal ulceration, and development of severe thrombocytopenia ( 30,000/mm3)29; in a 2002 study of 2786 APC recipients, ICH was largely confined to patients with meningitis or a platelet count less than 30,000/mm3.32
APC therapy has several other limitations and drawbacks. Multiple contraindications, including predisposition to bleeding, a recent history of bleeding, anticoagulant use, immunosuppression, liver disease, dialysis dependence, and hypercoagulable states, restrict its use. APC appears to work best when administered early, within 24 hours of the onset of organ dysfunction.31 In addition, APC is indicated only in adults with Acute Physiology and Chronic Health Evaluation (APACHE II) scores greater than 24 and multiorgan failure. Post hoc analysis of the PROWESS data showed that although the relative risk (RR) of death for those with APACHE II scores of 25 or more was .71 and statistically significant, the RR for those with scores below 25 was a nonsignificant .99.34 A subsequent study, ADDRESS, confirmed there was no benefit to septic patients with a low risk of death.30 In the ADDRESS study 2613 patients with severe sepsis and either an APACHE II score less than 25 or single organ failure were randomized to APC or placebo. No differences were found in 28‐day and in‐hospital mortality; among patients who had undergone surgery in the previous 30 days, those receiving APC had a significantly increased risk of death (20.7% vs. 14.1%, P = .03).
An additional drawback of APC therapy is its cost, approximately $6800 per infusion, although the cost per year of life gained, $24,484, or $52,360 per life saved (NNT $6800), is reasonable for those with APACHE II scores greater than 24.34 Concerns have also been raised about the PROWESS trial itself: the production of the study drug and some exclusion criteria were changed midtrial, after which the effectiveness of APC improved. APACHE II scores had not been validated for selection of patients for therapies and may have varied with time or by observer. The original PROWESS study population may have been skewed away from chronically ill patients.35 Experts differ on the significance of these concerns and even whether APC therapy should be considered the standard of care pending further research.32, 35 The ADDRESS trial also failed to demonstrate a benefit in a subgroup of patients with APACHE II scores above 24, although it was underpowered to do so, and according to enrollment criteria, none of those patients had multiorgan failure.30 However, in the subgroup of PROWESS patients with APACHE II scores greater than 24, the absolute reduction in mortality was a full 13%,17 with a corresponding NNT of 7.7, and although the PROWESS findings have not been duplicated in a second randomized trial, a single‐arm, open‐label study of APC (ENHANCE) showed a nearly identical mortality rate.31 Pending confirmatory trials, APC remains a recommended therapy for selected patients sick enough to benefit and without excessive bleeding risk.11
EARLY GOAL‐DIRECTED THERAPY
Because physician‐directed resuscitation for sepsis may normalize vital signs, central venous pressures (CVP), and urine output without correcting hypoperfusion, Rivers et al. tested a resuscitation protocol that incorporated a central line that continuously monitored mixed‐venous oxygen saturation as a surrogate for cardiac output.36 They randomized 263 patients with septic shock (defined as hypotension 90 mm Hg after a 20‐30 mL/kg bolus, or lactate > 4 mmol/L, which is associated with at least a 3‐fold increase in the mortality of emergency department patients with suspected infection37) to either standard care or early goal‐directed therapy (EGDT) for the initial 6 hours of hospital care. Patients with acute coronary ischemia, pulmonary edema, stroke, asthma, overdose, trauma, dysrhythmia, immunosuppression, uncontrolled cancer, or a need for urgent procedures were excluded. Standard care was directed by physiologic parameters such as vital signs, urine output, and CVP. EGDT used sequential therapies designed to support organ perfusion: 500 mL of normal saline was given every half hour until the CVP was at least 8‐12 mm Hg. Pressors were given until the mean arterial pressure was 65‐90 mm Hg (norepinephrine36 or dopamine were preferred agents, and vasopressin [0.01‐0.04 units/min] was an option for shock refractory to first‐line pressors)11, 38 Transfusion (to a hematocrit goal of 30) and dobutamine were given until mixed‐venous oxygenation saturation was 70% or better (Fig. 1). Lastly, patients who did not achieve this goal were sedated and mechanically ventilated.
Results were dramatic: mortality was reduced from 46.5% to 30.5%, with an ARR of 16% and an NNT of 6.25. Study patients received similar amounts of crystalloid, but received it earlier than the standard care patients and received more transfusions and inotropes. Substantially more patients in the EGDT group than the standard care group achieved a mixed venous oxygen saturation of 70%; 13.7% of the EGDT patients had occult hypoperfusion (low mixed‐venous oxygenation that responded to inotropes despite satisfactory vital signs). EGDT improved length of stay (4 days shorter among survivors) and duration of intubation, as well as APACHE scores and several physiologic parameters.36
Critics of this trial note the impossibility of adequate blinding and the high mortality in the placebo group. Further, because the trial tested the EGDT protocol as a whole, there was no way to know if each step was optimal. For example, a different CVP goal could have been used or adjustments made for mechanical ventilation, which can falsely elevate a low CVP into the desired range (the Surviving Sepsis Campaign guidelines recommend a CVP goal of 12‐15 mm Hg in mechanically ventilated patients11). Also, the selection of pressor, the use of inotropes, and the transfusion threshold were chosen on the basis of physiologic rationales, but all of these are arguable.39 This was also a single trial, and earlier goal‐directed protocols for ICU patients actually showed harm,40, 41 although those trials targeted supranormal physiologic goals in more established critical illness.42 Finally, on a practical level, hospitals and particularly emergency departments must commit resources to train physicians and staff, purchase the appropriate central venous catheters, and convince eligible patients to undergo an invasive procedure. In a survey of 30 attending physicians in academic referral hospitals, only 7% reported standard use of EGDT. Barriers included the requirement for specialty monitoring equipment and other resources, and central venous cannulation.43
Despite these concerns, the striking reduction in mortality associated with EGDT led to its endorsement by the Surviving Sepsis Campaign guidelines and underscores the principle of aggressive early resuscitation for patients who do not meet eligibility criteria but appear at risk for worsening sepsis. As yet, however, no strong evidence mandates a specific approach to the septic patient without shock.
STRESS DOSE STEROIDS
Because of the importance of the inflammatory cascade in severe sepsis, a potential role for steroids in the management of sepsis has been repeatedly studied. More than 50 studies have been performed since the 1950s, generally with pharmacologic doses of steroid; a meta‐analysis showed that such a practice was ineffective.44, 45 However, data accumulated that relative adrenal insufficiency during severe sepsis was common and associated with an increased risk of death and that physiologic doses of steroids could reverse refractory hypotension.46 To define the role of a physiologic course of steroids in septic shock, Annane et al. randomized 299 critically ill adults to either 7 days of stress dose hydrocortisone (50 mg IV every 6 hours) and fludrocortisone (50 g NG every 24 hours) or matched placebos. Enrolled patients were severely ill; the placebo group had a 63% mortality, and patients had to have septic shock, oliguria or hypoxia, hypotension despite low‐dose dopamine, and lactate greater than 2 mmol/L and require mechanical ventilation. Pregnant women, those with myocardial infarction or pulmonary embolus, advanced malignancies, or immunodeficiency, and those with clear indications or contraindications to steroids were excluded.47 Enrollment criteria were modified midstudy; changes included the exclusion of patients who had recently received etomidate, which inhibits 11‐‐hydroxylase and has been identified as a risk factor for adrenal insufficiency in intensive care patients.48 All patients received a 250‐g cosyntropin stimulation test. The authors considered patients nonresponders to consyntropin if serum cortisol failed to increase to 9 g/dL or more.
Steroids reduced the duration that a vasopressor was required and reduced mortality from 63% to 53% among nonresponders, giving an NNT of only 10 to prevent 1 death at 28 days. Although the authors described no evidence of adverse effects, among the subset of 70 patients who responded appropriately to cosyntropin, there was a nonsignificant trend toward increased mortality, and rates of hyperglycemia were not provided.47 The authors concluded that physicians should test appropriate patients for adrenal reserve, give the studied steroid regimen while results are pending, and discontinue treatment if a patient retains adrenal reserve.
The literature on steroids and critical illness is complex, with more than 1300 articles on steroids and sepsis published since 1988, and several concerns were raised about the Annane study. For example, did much of the benefit for those patients enrolled before the protocol amendment come from reducing an adverse effect of etomidate?49 Does the high‐dose, 250‐g cosyntropin stimulation test overcome (and conceal) partial ACTH resistance that might benefit from treatment?50 Might not a robust baseline cortisol suggest sufficient adrenal function regardless of the incremental response to cosyntropin?51 Partial answers were provided by 2 subsequent meta‐analyses. Both found that more recent studies gave lower doses of steroids in longer, 5‐ to 7‐day courses to sicker patients and demonstrated improvement in mortality and shock reversal, with relative risk reductions of 14%‐22%; the NNT ranged from 8 to 11. One analysis found no difference in outcomes between adrenally sufficient and adrenally insufficient patients, and those authors advised considering treatment for all patients regardless of their adrenal function test results.8 The other analysis concluded that the data on steroids for those with adrenal reserve was too limited to recommend treating adrenally sufficient patients.9
Disputes about certain details, such as whether patients should be treated without regard to adrenal reserve, continue in the literature.45, 52 An ongoing randomized, controlled trial, CORTICUS, is expected to provide additional guidance on the use of low‐dose steroids in sepsis; in the meantime, the literature clearly supports a longer course of low‐dose steroid therapy for patients with pressor‐dependent septic shock with inadequate adrenal reserve by cosyntropin testing, and guidelines allow discretion about whether patients with adequate adrenal reserve should also be treated.11 Hospitalists may also want to treat septic shock with equivalent doses of dexamethasone (approximately 2 mg IV every 6 hours) if adrenal evaluation may be delayed, as this agent will not confound cosyntropin stimulation test results, and they may want to avoid etomidate in septic patients53, 54 for whom they perform or supervise intubations.
INTENSIVE INSULIN THERAPY
Mounting evidence supports the short‐term role of hyperglycemia in morbidity and mortality, especially in critical illness. Hyperglycemia impairs neutrophil and endothelial cell function as well as protective responses to cardiac and neuronal ischemia,55 whereas insulin has anti‐inflammatory and antiapoptotic effects,7, 56 suggesting that intensive insulin might improve the outcomes of critically ill patients. To test this theory, van den Berghe and colleagues randomized 1548 mostly surgical ICU patients to insulin infusions titrated for glucose goals of either 80‐110 or 180‐200 mg/dL, followed by subcutaneous insulin after ICU discharge. Although blinding was impossible, in both cases glucose management was performed by a separate research team. Multiple benefits were noted: ICU and total in‐hospital deaths were reduced, mostly among patients with an ICU stay of more than 5 days, whose risk of death fell from 20.2% to 10.6%. Intensive insulin also reduced septicemia, renal impairment, critical illness polyneuropathy, and duration of intensive care.57
Subsequently, a meta‐analysis of 35 trials suggested that insulin reduced the mortality of critically ill patients by 15%.10 Van den Berghe et al.'s results were also duplicated in a broad, medical‐surgical ICU population, although the reductions in morbidity and mortality were measured against historical controls.58 However, whether the results of the influential surgical ICU study could be applied to medical patients was not known until 2006, when the van den Berghe group reported the effects of similar insulin protocols on 1200 patients in the medical ICU who were expected to need intensive care for at least 3 days.59 In this study, intensive insulin failed to reduce overall mortality (40% and 37.3%, P = .33). However, intensive insulin did reduce mortality among the 64% of patients who stayed in the ICU 3 or more days from 52.5% to 43% (NNT 10.5, P = .009). This benefit was offset by an increased number of deaths in the intensive insulin group among patients with ICU stays of less than 3 days (P = .05‐.35 depending on the method used).59 Intensive insulin did reduce newly acquired kidney injury, duration of mechanical ventilation, and lengths of ICU and hospital stays, and the reduction in morbidity increased with the duration of intensive insulin therapy. Hypoglycemia (mean 32 mg/dL) occurred in 25% of patients with prolonged stays6.4 times as often as in the usual care group.60 Liver and renal failure were associated with hypoglycemia.59
Critics of the surgical ICU trial noted the high mortality among the usual care patients (5.1%), a robust 34% mortality reduction for a relatively small 50 mg/dL reduction in morning glucose levels, and the aggressive use of parenteral nutrition, raising the question of whether intensive insulin merely attenuated the side effects of intravenous glucose.61, 62 Also, the ideal blood glucose target is not known with certainty. Retrospective studies suggested the upper limit for target blood glucoses could be 145 mg/dL63 and found differing thresholds at which hyperglycemia increased mortality in nondiabetics (144 mg/dL) and diabetics (200 mg/dL).64 However, in the surgical ICU trial, there was no threshold below which there was no further reduction in risk; patients whose mean blood glucose was below 110 mg/dL had lower mortality than those whose levels were between 110 and 150 mg/dL (P = .026).65 Finally, the effects of hyperglycemia and intensive insulin may vary by population: retrospective studies found that ICU hyperglycemia was more strongly associated with mortality among nondiabetics,64, 66 and van den Berghe et al. noted no benefit from intensive insulin in a small subgroup of diabetics.59
In summary, large, well‐designed trials have demonstrated that intensive insulin reduced mortality in critically ill patients after a delay of 3‐5 days, but this benefit did not extend to all patients in the medical ICU.57, 59 Some authors have suggested deferring intensive insulin for 3 days,67 but because early therapy probably contributes to the delayed mortality benefit, this approach may deprive patients of the observed benefits. Ongoing clinical trials (NICE‐SUGAR) are likely to provide useful information about how hyperglycemia should be managed in different populations, including septic ICU patients.61 In the meantime, institutions can select the intensity of their insulin therapy by weighing morbidity and long‐term mortality benefits against possible short‐term harms and ensuring that hospital staff members are sufficiently trained to control hyperglycemia safely. For example, in critical illness, intravenous insulin is preferable to subcutaneous insulin, and the frequent measurement of whole‐blood glucose instead of finger‐stick glucose helps to avoid errors.55, 68 And although researchers were unable to prospectively identify patients with long ICU stays,59 severely septic patients have long ICU stays (generally 7.5‐16.6 days),14 and individual ICUs might observe enough stays of more than 2 days in their patient population to justify intensive insulin for this subgroup. And finally, although no conclusive evidence mandates a specific approach to hyperglycemia outside the ICU, the ICU data provide a physiologic rationale for cautious but tight control of glucose in more moderately ill patients. Guidelines for the management of inpatient hyperglycemia were published previously.55
SEPSIS AND THE HOSPITALIST
Hospitalists who provide critical care may make frequent decisions about the inclusion and exclusion criteria for the major trials of sepsis, weigh their relative benefits against risks and costs, contemplate gray areas such as adrenal testing in shock, and employ evidence‐based therapies for severe sepsis. However, hospitalists may also see patients who qualify for these therapies when they are called to see septic patients in the emergency department, when severe sepsis develops in patients on the medicine ward, or when they provide consultation services in an ICU. Sepsis care must be implemented urgently; patients in the pivotal trial of steroids had to be randomized within 3 hours of shock onset,47 data suggest that the window for optimal antibiotic therapy may be no greater than 4 hours from diagnosis,18 whereas guidelines suggest therapy within 1 hour,11 and early goal‐directed therapy was studied only for the first 6 or more hours of hospitalization.36 Thus, hospitalists who do not provide ICU care should be able to identify patients with severe sepsis and either deliver initial care or recognize the need for immediate consultation. Specifically, hospitalists can:
-
Recognize that both absolute ( 90 mm Hg) and relative hypotension (> 40 mm Hg below baseline) indicate septic shock;
-
Identify normotensive candidates for EGDT (severe sepsis with serum lactate > 4 mmol/L) by requesting a serum lactate in addition to prompt appropriate cultures for severe acute infection69;
-
Recognize atypical presentations of sepsis (tachypnea, tachycardia, confusion, etc.) and maintain a high suspicion for sepsis in patients who may be predisposed to infection and to atypical presentation because of age, immunosuppression, neutropenia, diabetes, or other conditions;
-
Initiate effective antibiotics and EGDT promptly for individual patients or by coordinating efforts to improve sepsis care at an institutional level, for example, as a component of medical emergency team services70, 71;
-
Rapidly identify and manage removable foci of infection such as abscesses, empyemas, necrotizing fasciitis, or infected vascular catheters; and
-
Competently educate hospital staff, residents, and medical students about sepsis care.
Hospitalists are busy physicians, and the task of reviewing sepsis literature and implementing recommendations is daunting. However, hospitalists can turn to resources such as the Surviving Sepsis Campaign Guidelines, a series of recommendations for managing severe sepsis that were endorsed by 11 international critical care and infectious disease societies and published in Critical Care Medicine in 2004.11 The Institute for Healthcare Improvement has also published a series of online severe sepsis bundles, or groups of proven interventions, complete with implementation tips and supporting literature, available at
CONCLUSION: DEADLY YET TREATABLE
The death toll from severe sepsis in the United States exceeds that of lung, breast, and colon cancer combined and equals that of myocardial infarction (MI),1 a condition that appropriately triggers a series of emergency interventions. Physicians now have an arsenal of therapies for severe sepsis analogous to those employed for MI, and a comparison between the 2 conditions underscores the high mortality rate of severe sepsis and the enormous impact on patient outcomes provided by evidence‐based sepsis therapy. Figure 2 compares the 9.5%‐16% ARR for death associated with APC in patients with APACHE 2 scores greater than 24 and multiorgan failure,29 EGDT,36 stress dose steroids in shock complicated by adrenal insufficiency,47 and intensive insulin in patients with medical ICU stays longer than 3 days,59 with the benefits of thrombolysis for ST‐elevation MI (2%‐3%)73 or antiplatelet therapy for acute MI (2.3%).74 Figure 3 compares the corresponding NNT values to save 1 life; according to the available data, a hospitalist is 5‐8 times more likely to save a life with EGDT than with fibrinolysis.
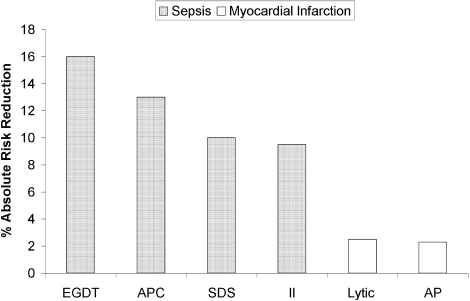
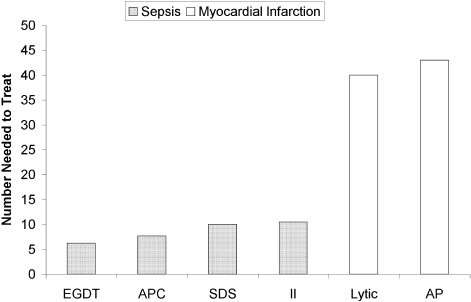
Because the literature supporting several major sepsis therapies have been limited to retrospective studies1828 and single randomized, controlled trials29, 36 and because key trials are still underway (CORTICUS, NICE‐SUGAR), the benefits of sepsis therapies are less certain than are those for the treatment of MI. This was underscored by the finding that the benefit in reduced mortality of intensive insulin in the surgical ICU57 did not extend to all patients in the medical ICU.59 However, the potentially marked survival benefit of early effective antibiotics, APC, EGDT, stress dose steroids, and intensive insulin and the urgency with which they must be applied demand that all hospitalists become or remain familiar with the evolving sepsis literature.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29:1303–1310.
- ,,,.Prevalence and incidence of severe sepsis in Dutch intensive care units.Crit Care.2004;8:R153–R162.
- ,, et al.Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use.Intensive Care Med.2002;28:1440–1446.
- ,,,,.Effects of severity of illness on resource use by survivors and nonsurvivors of severe sepsis at intensive care unit admission.Crit Care Med.2002;30:2413–2419.
- ,,, et al.Resource utilization among patients with sepsis syndrome.Infect Control Hosp Epidemiol.2003;24:62–70.
- ,,,,.The costs of septic syndromes in the intensive care unit and influence of hospital‐acquired sepsis.Intensive Care Med,2003;29:1464–1471.
- ,.The pathophysiology and treatment of sepsis.N Engl J Med.2003;348:138–150.
- ,,,,.Meta‐analysis: the effect of steroids on survival and shock during sepsis depends on the dose.Ann Intern Med.2004;141:47–56.
- ,,,,,.Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis.Brit Med J.2004;329:480–488.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32:858–873.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26:115–117.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome.N Engl J Med.2000;342:1301–1308.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Crit Care Med.1992;20:864–874.
- ;;, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- .Severe sepsis and therapy with activated protein C.New Engl J Med.2005;353:1398–1399.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Measuring quality of care with explicit process criteria before and after implementation of the DRG‐based prospective payment system.JAMA.1990;264:1969–1973.
- ,.Pneumonia mortality reduction and quality improvement in a community hospital.Qual Rev Bull.1993;19:124–130.
- ,,, et al.Quality of care, process and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS Trial.Clin Infect Dis.2004;38:284–288.
- ,,,,,.The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection.J Intern Med.1998;244:379–386.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- ,,,,,.Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
- ,,, et al.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Efficacy and safety of recombinant human activated protein C for severe sepsis.N Engl J Med.2001;344:699–709.
- ,,, et al.Drotecogin alfa (activated) for adults with severe sepsis and a low risk of death.New Eng J Med.2005;353:1332–1341.
- ,,, et al.Drotecogin alfa (activated) treatment in severe sepsis from the global open label trial ENHANCE.Crit Care Med.2005;10:2266–2277.
- ,,.Activated protein C for severe sepsis.N Engl J Med.2002;347;1035–1036.
- .Assessing the use of activated protein C in the treatment of severe sepsis.N Engl J Med.2002;347:1030–1034.
- ,,,,.An economic evaluation of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:993–1000.
- ,,,.Risks and benefits of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:1027–1030.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Serum lactate as a predictor of mortality in emergency department patients with infection.Ann Emerg Med.2005;45:524–528.
- ,,,.Vasopressor and inotropic support in septic shock: an evidence‐based review.Crit Care Med.2004;32(11 Suppl):S455–S465.
- ,,, et al.Goal‐directed therapy for severe sepsis [letter].N Engl J Med.2002;346:1025–1026.
- ,,,,,.Elevation of systemic oxygen delivery in the treatment of critically ill patients.New Engl J Med.1994;330:1717–1722.
- ,, et al.A trial of goal‐oriented hemodynamic therapy in critically ill patients.N Engl J Med.1995;333:1025–1032.
- .Hemodynamic and metabolic therapy in critically ill patients.New Engl J Med.2001;345:1417–1418.
- ,.Use of goal directed therapy for severe sepsis and septic shock in academic emergency departments.Crit Care Med.2005;33:1888–1889.
- ,,, et al.Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature.Crit Care Med.1995;23:1430–1439.
- .Physicians should administer low‐dose corticosteroids selectively to septic patients until an ongoing trial is completed.Ann Intern Med.2004;141:70–72.
- ,.Corticosteroids and septic shock.JAMA.2002;288:886–887.
- ,,, et al.Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock.JAMA.2002;288:862–871.
- ,,, et al.Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation.Intensive Care Med.2005;31:388–392.
- ,.Editorial III: Corticosteroids for septic shock—a standard of care?Br J Anaesth.2004;93:178–180.
- ,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for septic shock [letter].Ann Intern Med.2004;141:742–743.
- .Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal.Chest.2005;127:1031–1038.
- .ICU physicians should abandon the use of etomidate!Intensive Care Med.2005;31:325–326.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,,.Intensive insulin therapy exerts anti‐inflammatory effects in critically ill patients and counteracts the adverse effects of low mannose binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000. Published erratum appears in Mayo Clin Proc.year="2005"2005;80:1101
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- Supplement to:,,, et al.Intensive insulin therapy in the medical ICU.N Eng J Med.2006;354:449–461. Available at: http://content.nejm.org/cgi/data/354/5/449/DC1/1.
- .Glycemic control in the intensive care unit: why we should wait for NICE‐SUGAR.Mayo Clin Proc.2005;80:1546–1548.
- ,,.Intensive insulin therapy in critically ill patients [letter].New Engl J Med.2002;346:1586–1588.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,,,.Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus.Mayo Clin Proc.2005;80:1558–1567.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:634–635.
- ,,,,.Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations.Crit Care Med.2005;33:2772–2777.
- .Intensive insulin in intensive care.New Engl J Med.2006;354:516–518.
- ,,,,.Fingerstick glucose determination in shock.Ann Intern Med.1991;114:1020–1024.
- ,,.A blueprint for a sepsis protocol.Acad Emerg Med.2005;12:352–359.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome.Crit Care Med.2004;32:S595–S597.
- Fibrinolytic Therapy Trialists' Collaborative Group.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients.Lancet.1994;343:311–322.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.Brit Med J.2002;324:71–86.
Despite decades of intensive research and improvements in medical care, severe sepsis affects an estimated 751,000 patients in the United States every year, killing 215,000 of them at an annual cost of 16.7 billion dollars.1 Because the elderly experience a 100‐fold increase in incidence, as compared with children, and a nearly 4‐fold increase in mortality (38.4% of those more than 85 years old), this burden is expected to increase with the aging population.1 Patients with severe sepsis have prolonged ICU14 and hospital stays and incur substantially increased costs compared with other patients.36
New research continues to explore the complex pathophysiology of sepsis,7 and clinicians, who once relied primarily on clinical experience and expert opinion to guide therapy, now have an increasing array of evidenced‐based sepsis therapies to employ. Recent meta‐analyses have evaluated several major treatments for severe sepsis,810 and recommendations (the Surviving Sepsis Campaign guidelines) for the treatment of severe sepsis were recently endorsed by 11 international critical care and infectious disease organizations.11 This article summarizes the current definitions of sepsis syndromes, the trials supporting the specific therapies for sepsis that are currently recommended, ongoing controversies and research, and implications for hospitalists, with a focus on early, effective antibiotics, activated protein C, early goal‐directed therapy, stress dose steroids, and intensive insulin therapy. For space considerations, readers are directed elsewhere for data supporting prophylaxis for deep venous thrombosis (DVT)12 and stress ulcer bleeding13 and for therapies less often directed by hospitalists, such as lung protective ventilation.14
DEFINITIONS
Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined in 1992 to standardize the terminology of sepsis.15 These definitions have recently been reviewed and supported by a variety of American and European intensive care societies.16
SIRS is defined by the presence of at least 2 of the following:
-
Temperature > 38C or 36C;
-
Heart rate > 90 beats/min;
-
Respiratory rate > 20 breaths/min or PaCO2 32 mm Hg;
-
WBC >12,000 or 4000 cells/mm3, or >10% immature (band) forms.
Sepsis is SIRS due to documented or strongly suspected infection.
Severe sepsis is sepsis with organ dysfunction (such as lactic acidosis, oliguria, thrombocytopenia, or delirium), hypoperfusion, or hypotension ( 90 mm Hg systolic or more than 40 mm Hg below baseline).
Septic shock is severe sepsis complicated by hypotension or pressor dependence despite adequate (20‐30 mL/kg; 1.5‐3 liters in most patients) fluid resuscitation.
Sepsis terminology must be applied carefully. Many hospitalized patients meet criteria for SIRS, yet it is inaccurate to say a patient who has acute leukemia with leukocytosis, anemia‐induced tachycardia, and thrombocytopenia has severe sepsis if those abnormalities are not a result of inflammation or infection. Accurate documentation of sepsis syndromes can improve professional and institutional reimbursement and provide prognostic information: the in‐hospital mortality rates for severe sepsis and septic shock are approximately 30% and 50%, respectively.17 More importantly, thoughtful application of these definitions can help a hospitalist identify septic patients who qualify for one of the proven therapies for severe sepsis.
EARLY, EFFECTIVE ANTIBIOTICS
For obvious ethical reasons, randomized, controlled trials to study the impact of inappropriate or delayed antibiotic therapy for serious infections are not possible. However, the evidence supporting early, effective antibiotic therapy is still compelling, and because many hospitalists often initiate treatment with antibiotics before transferring a patient to intensive care, this may represent the most important intervention hospitalists can provide to patients with serious infections. Several studies have estimated the impact of early, effective antibiotics on outcomes.
Houck et al. retrospectively reviewed 13,771 cases of community‐acquired pneumonia among elderly Medicare patients. They found that 39.1% of the patients waited more than 4 hours for antibiotics and 7.6% waited more than 12 hours; three quarters of these delays resulted from delayed ordering of antibiotics.18 Further, 21.2% received an antibiotic selection incompatible with recent professional guidelines. Receiving antibiotics within 4 hours reduced in‐hospital and 30‐day mortality by 15% and length of stay by 0.4 days.18 Similar conclusions were reported by 3 of 4 previous analyses.1922 Extending these findings to critically ill patients, Iregui et al. found that delayed treatment with appropriate antibiotics (odds ratio, 7.68) was a greater predictor of mortality for 107 patients with ventilator‐acquired pneumonia than were APACHE II scores and malignancy; 31% failed to receive appropriate antibiotics within 24 hours, and again, three quarters of these delays resulted from delays in writing antibiotic orders.23
Not surprisingly, antibiotic therapy must be effective as well as timely. MacArthur et al. studied the impact of adequate (ie, active against cultured organisms, if isolated) antibiotics on the outcomes of 2634 septic patients enrolled in a randomized trial of an anti‐TNF antibody. Nearly 91% received appropriate antibiotics; their mortality rate was 33%, 10% lower than that of the patients whose initial antibiotics were inadequate (P .001).24 Leibovici et al. reported similar findings in a prospective study of patients with bacteremia. Only 63% of 3413 subjects received an antibiotic active against the infecting pathogen, and their mortality was 20%, 14% lower than that in the group that received ineffective antibiotics (P = .0001).25 Other authors have reported even worse outcomes with ineffective therapy: 62% mortality among inadequately treated bacteremic or fungemic ICU patients, compared with 28.4% among those who were adequately treated26 and an odds ratio of dying of 8.14 for the 46 of 270 septic ICU patients who received inadequate initial antibiotics,27 making inadequate antibiotic therapy the strongest risk factor for death. Finally, Kollef et al. reported that 26% of 655 infected ICU patients received inadequate antibiotics and suffered an infection‐related mortality rate of 40.2%, more than twice the 17.7% rate among adequately treated patients (P .001). Inadequate antimicrobial therapy was a greater risk factor for death than early respiratory failure or sepsis‐related organ failure assessment scores.28
Guidelines for anti‐infective care now recommend obtaining appropriate cultures and administering broad‐spectrum antibiotics (appropriate for suspected infections, local susceptibility patterns, and any relevant prior culture data from individual patients) within 1 hour of presentation.11 In addition, any removable focus of infection must be identified and managed (eg, an abscess, infected catheter, tampon, or infection requiring surgery).
ACTIVATED PROTEIN C
Recombinant human activated protein C (APC) is a protein with anticoagulant and anti‐inflammatory properties that is relatively deficient in approximately 87% of septic patients.29 Although numerous trials of other anticoagulants (antithrombin III and tissue factor pathway inhibitor) and immunosuppressives (tumor necrosis factor inhibitors, high‐dose steroids, interleukin‐1 receptor antagonists, and others) have failed to show any benefit,7 in 2001 APC became the first proven therapy specifically for sepsis. The PROWESS trial, which established its efficacy, randomized 1690 patients who met 3 SIRS criteria and dysfunction of at least 1 organ system to APC (24 g/kg IV per hour for 96 hours interrupted for bleeding or urgent procedures) or placebo. APC reduced 28‐day mortality from 30.8% to 24.7%, yielding an absolute risk reduction of 6.1% and a corresponding number needed to treat (NNT) of 16.4. This benefit was seen across all subgroups including those with normal baseline APC levels.29
Not surprisingly, APC increases the risk of serious bleeding. Although this effect was of borderline significance in PROWESS (3.5% vs. 2% in the placebo group, P = .06),29 it was confirmed in subsequent trials (3.9% vs. 2.2%, P = .01)30 and may be larger still in open‐label use, at 6.5%.17, 31 Intracerebral hemorrhage (ICH), a particularly devastating complication, occurred in 0.2% of the PROWESS patients and 0.5% of patients in 2 subsequent studies30, 32; in both major trials, there was a single extra event in the APC arm.29, 30 Like serious bleeding in general, ICH was more common in open‐label use, occurring in 1.5% of patients.31, 33 Therefore, it is vital to have strict adherence to exclusion criteria and familiarity with the risk factors for serious bleeding. In the PROWESS trial, after randomization, risk factors for serious bleeding included procedures and injury to vascular organs, an activated partial‐thromboplastin time of more than 120 seconds, an international normalized ratio greater than 3, gastrointestinal ulceration, and development of severe thrombocytopenia ( 30,000/mm3)29; in a 2002 study of 2786 APC recipients, ICH was largely confined to patients with meningitis or a platelet count less than 30,000/mm3.32
APC therapy has several other limitations and drawbacks. Multiple contraindications, including predisposition to bleeding, a recent history of bleeding, anticoagulant use, immunosuppression, liver disease, dialysis dependence, and hypercoagulable states, restrict its use. APC appears to work best when administered early, within 24 hours of the onset of organ dysfunction.31 In addition, APC is indicated only in adults with Acute Physiology and Chronic Health Evaluation (APACHE II) scores greater than 24 and multiorgan failure. Post hoc analysis of the PROWESS data showed that although the relative risk (RR) of death for those with APACHE II scores of 25 or more was .71 and statistically significant, the RR for those with scores below 25 was a nonsignificant .99.34 A subsequent study, ADDRESS, confirmed there was no benefit to septic patients with a low risk of death.30 In the ADDRESS study 2613 patients with severe sepsis and either an APACHE II score less than 25 or single organ failure were randomized to APC or placebo. No differences were found in 28‐day and in‐hospital mortality; among patients who had undergone surgery in the previous 30 days, those receiving APC had a significantly increased risk of death (20.7% vs. 14.1%, P = .03).
An additional drawback of APC therapy is its cost, approximately $6800 per infusion, although the cost per year of life gained, $24,484, or $52,360 per life saved (NNT $6800), is reasonable for those with APACHE II scores greater than 24.34 Concerns have also been raised about the PROWESS trial itself: the production of the study drug and some exclusion criteria were changed midtrial, after which the effectiveness of APC improved. APACHE II scores had not been validated for selection of patients for therapies and may have varied with time or by observer. The original PROWESS study population may have been skewed away from chronically ill patients.35 Experts differ on the significance of these concerns and even whether APC therapy should be considered the standard of care pending further research.32, 35 The ADDRESS trial also failed to demonstrate a benefit in a subgroup of patients with APACHE II scores above 24, although it was underpowered to do so, and according to enrollment criteria, none of those patients had multiorgan failure.30 However, in the subgroup of PROWESS patients with APACHE II scores greater than 24, the absolute reduction in mortality was a full 13%,17 with a corresponding NNT of 7.7, and although the PROWESS findings have not been duplicated in a second randomized trial, a single‐arm, open‐label study of APC (ENHANCE) showed a nearly identical mortality rate.31 Pending confirmatory trials, APC remains a recommended therapy for selected patients sick enough to benefit and without excessive bleeding risk.11
EARLY GOAL‐DIRECTED THERAPY
Because physician‐directed resuscitation for sepsis may normalize vital signs, central venous pressures (CVP), and urine output without correcting hypoperfusion, Rivers et al. tested a resuscitation protocol that incorporated a central line that continuously monitored mixed‐venous oxygen saturation as a surrogate for cardiac output.36 They randomized 263 patients with septic shock (defined as hypotension 90 mm Hg after a 20‐30 mL/kg bolus, or lactate > 4 mmol/L, which is associated with at least a 3‐fold increase in the mortality of emergency department patients with suspected infection37) to either standard care or early goal‐directed therapy (EGDT) for the initial 6 hours of hospital care. Patients with acute coronary ischemia, pulmonary edema, stroke, asthma, overdose, trauma, dysrhythmia, immunosuppression, uncontrolled cancer, or a need for urgent procedures were excluded. Standard care was directed by physiologic parameters such as vital signs, urine output, and CVP. EGDT used sequential therapies designed to support organ perfusion: 500 mL of normal saline was given every half hour until the CVP was at least 8‐12 mm Hg. Pressors were given until the mean arterial pressure was 65‐90 mm Hg (norepinephrine36 or dopamine were preferred agents, and vasopressin [0.01‐0.04 units/min] was an option for shock refractory to first‐line pressors)11, 38 Transfusion (to a hematocrit goal of 30) and dobutamine were given until mixed‐venous oxygenation saturation was 70% or better (Fig. 1). Lastly, patients who did not achieve this goal were sedated and mechanically ventilated.

Results were dramatic: mortality was reduced from 46.5% to 30.5%, with an ARR of 16% and an NNT of 6.25. Study patients received similar amounts of crystalloid, but received it earlier than the standard care patients and received more transfusions and inotropes. Substantially more patients in the EGDT group than the standard care group achieved a mixed venous oxygen saturation of 70%; 13.7% of the EGDT patients had occult hypoperfusion (low mixed‐venous oxygenation that responded to inotropes despite satisfactory vital signs). EGDT improved length of stay (4 days shorter among survivors) and duration of intubation, as well as APACHE scores and several physiologic parameters.36
Critics of this trial note the impossibility of adequate blinding and the high mortality in the placebo group. Further, because the trial tested the EGDT protocol as a whole, there was no way to know if each step was optimal. For example, a different CVP goal could have been used or adjustments made for mechanical ventilation, which can falsely elevate a low CVP into the desired range (the Surviving Sepsis Campaign guidelines recommend a CVP goal of 12‐15 mm Hg in mechanically ventilated patients11). Also, the selection of pressor, the use of inotropes, and the transfusion threshold were chosen on the basis of physiologic rationales, but all of these are arguable.39 This was also a single trial, and earlier goal‐directed protocols for ICU patients actually showed harm,40, 41 although those trials targeted supranormal physiologic goals in more established critical illness.42 Finally, on a practical level, hospitals and particularly emergency departments must commit resources to train physicians and staff, purchase the appropriate central venous catheters, and convince eligible patients to undergo an invasive procedure. In a survey of 30 attending physicians in academic referral hospitals, only 7% reported standard use of EGDT. Barriers included the requirement for specialty monitoring equipment and other resources, and central venous cannulation.43
Despite these concerns, the striking reduction in mortality associated with EGDT led to its endorsement by the Surviving Sepsis Campaign guidelines and underscores the principle of aggressive early resuscitation for patients who do not meet eligibility criteria but appear at risk for worsening sepsis. As yet, however, no strong evidence mandates a specific approach to the septic patient without shock.
STRESS DOSE STEROIDS
Because of the importance of the inflammatory cascade in severe sepsis, a potential role for steroids in the management of sepsis has been repeatedly studied. More than 50 studies have been performed since the 1950s, generally with pharmacologic doses of steroid; a meta‐analysis showed that such a practice was ineffective.44, 45 However, data accumulated that relative adrenal insufficiency during severe sepsis was common and associated with an increased risk of death and that physiologic doses of steroids could reverse refractory hypotension.46 To define the role of a physiologic course of steroids in septic shock, Annane et al. randomized 299 critically ill adults to either 7 days of stress dose hydrocortisone (50 mg IV every 6 hours) and fludrocortisone (50 g NG every 24 hours) or matched placebos. Enrolled patients were severely ill; the placebo group had a 63% mortality, and patients had to have septic shock, oliguria or hypoxia, hypotension despite low‐dose dopamine, and lactate greater than 2 mmol/L and require mechanical ventilation. Pregnant women, those with myocardial infarction or pulmonary embolus, advanced malignancies, or immunodeficiency, and those with clear indications or contraindications to steroids were excluded.47 Enrollment criteria were modified midstudy; changes included the exclusion of patients who had recently received etomidate, which inhibits 11‐‐hydroxylase and has been identified as a risk factor for adrenal insufficiency in intensive care patients.48 All patients received a 250‐g cosyntropin stimulation test. The authors considered patients nonresponders to consyntropin if serum cortisol failed to increase to 9 g/dL or more.
Steroids reduced the duration that a vasopressor was required and reduced mortality from 63% to 53% among nonresponders, giving an NNT of only 10 to prevent 1 death at 28 days. Although the authors described no evidence of adverse effects, among the subset of 70 patients who responded appropriately to cosyntropin, there was a nonsignificant trend toward increased mortality, and rates of hyperglycemia were not provided.47 The authors concluded that physicians should test appropriate patients for adrenal reserve, give the studied steroid regimen while results are pending, and discontinue treatment if a patient retains adrenal reserve.
The literature on steroids and critical illness is complex, with more than 1300 articles on steroids and sepsis published since 1988, and several concerns were raised about the Annane study. For example, did much of the benefit for those patients enrolled before the protocol amendment come from reducing an adverse effect of etomidate?49 Does the high‐dose, 250‐g cosyntropin stimulation test overcome (and conceal) partial ACTH resistance that might benefit from treatment?50 Might not a robust baseline cortisol suggest sufficient adrenal function regardless of the incremental response to cosyntropin?51 Partial answers were provided by 2 subsequent meta‐analyses. Both found that more recent studies gave lower doses of steroids in longer, 5‐ to 7‐day courses to sicker patients and demonstrated improvement in mortality and shock reversal, with relative risk reductions of 14%‐22%; the NNT ranged from 8 to 11. One analysis found no difference in outcomes between adrenally sufficient and adrenally insufficient patients, and those authors advised considering treatment for all patients regardless of their adrenal function test results.8 The other analysis concluded that the data on steroids for those with adrenal reserve was too limited to recommend treating adrenally sufficient patients.9
Disputes about certain details, such as whether patients should be treated without regard to adrenal reserve, continue in the literature.45, 52 An ongoing randomized, controlled trial, CORTICUS, is expected to provide additional guidance on the use of low‐dose steroids in sepsis; in the meantime, the literature clearly supports a longer course of low‐dose steroid therapy for patients with pressor‐dependent septic shock with inadequate adrenal reserve by cosyntropin testing, and guidelines allow discretion about whether patients with adequate adrenal reserve should also be treated.11 Hospitalists may also want to treat septic shock with equivalent doses of dexamethasone (approximately 2 mg IV every 6 hours) if adrenal evaluation may be delayed, as this agent will not confound cosyntropin stimulation test results, and they may want to avoid etomidate in septic patients53, 54 for whom they perform or supervise intubations.
INTENSIVE INSULIN THERAPY
Mounting evidence supports the short‐term role of hyperglycemia in morbidity and mortality, especially in critical illness. Hyperglycemia impairs neutrophil and endothelial cell function as well as protective responses to cardiac and neuronal ischemia,55 whereas insulin has anti‐inflammatory and antiapoptotic effects,7, 56 suggesting that intensive insulin might improve the outcomes of critically ill patients. To test this theory, van den Berghe and colleagues randomized 1548 mostly surgical ICU patients to insulin infusions titrated for glucose goals of either 80‐110 or 180‐200 mg/dL, followed by subcutaneous insulin after ICU discharge. Although blinding was impossible, in both cases glucose management was performed by a separate research team. Multiple benefits were noted: ICU and total in‐hospital deaths were reduced, mostly among patients with an ICU stay of more than 5 days, whose risk of death fell from 20.2% to 10.6%. Intensive insulin also reduced septicemia, renal impairment, critical illness polyneuropathy, and duration of intensive care.57
Subsequently, a meta‐analysis of 35 trials suggested that insulin reduced the mortality of critically ill patients by 15%.10 Van den Berghe et al.'s results were also duplicated in a broad, medical‐surgical ICU population, although the reductions in morbidity and mortality were measured against historical controls.58 However, whether the results of the influential surgical ICU study could be applied to medical patients was not known until 2006, when the van den Berghe group reported the effects of similar insulin protocols on 1200 patients in the medical ICU who were expected to need intensive care for at least 3 days.59 In this study, intensive insulin failed to reduce overall mortality (40% and 37.3%, P = .33). However, intensive insulin did reduce mortality among the 64% of patients who stayed in the ICU 3 or more days from 52.5% to 43% (NNT 10.5, P = .009). This benefit was offset by an increased number of deaths in the intensive insulin group among patients with ICU stays of less than 3 days (P = .05‐.35 depending on the method used).59 Intensive insulin did reduce newly acquired kidney injury, duration of mechanical ventilation, and lengths of ICU and hospital stays, and the reduction in morbidity increased with the duration of intensive insulin therapy. Hypoglycemia (mean 32 mg/dL) occurred in 25% of patients with prolonged stays6.4 times as often as in the usual care group.60 Liver and renal failure were associated with hypoglycemia.59
Critics of the surgical ICU trial noted the high mortality among the usual care patients (5.1%), a robust 34% mortality reduction for a relatively small 50 mg/dL reduction in morning glucose levels, and the aggressive use of parenteral nutrition, raising the question of whether intensive insulin merely attenuated the side effects of intravenous glucose.61, 62 Also, the ideal blood glucose target is not known with certainty. Retrospective studies suggested the upper limit for target blood glucoses could be 145 mg/dL63 and found differing thresholds at which hyperglycemia increased mortality in nondiabetics (144 mg/dL) and diabetics (200 mg/dL).64 However, in the surgical ICU trial, there was no threshold below which there was no further reduction in risk; patients whose mean blood glucose was below 110 mg/dL had lower mortality than those whose levels were between 110 and 150 mg/dL (P = .026).65 Finally, the effects of hyperglycemia and intensive insulin may vary by population: retrospective studies found that ICU hyperglycemia was more strongly associated with mortality among nondiabetics,64, 66 and van den Berghe et al. noted no benefit from intensive insulin in a small subgroup of diabetics.59
In summary, large, well‐designed trials have demonstrated that intensive insulin reduced mortality in critically ill patients after a delay of 3‐5 days, but this benefit did not extend to all patients in the medical ICU.57, 59 Some authors have suggested deferring intensive insulin for 3 days,67 but because early therapy probably contributes to the delayed mortality benefit, this approach may deprive patients of the observed benefits. Ongoing clinical trials (NICE‐SUGAR) are likely to provide useful information about how hyperglycemia should be managed in different populations, including septic ICU patients.61 In the meantime, institutions can select the intensity of their insulin therapy by weighing morbidity and long‐term mortality benefits against possible short‐term harms and ensuring that hospital staff members are sufficiently trained to control hyperglycemia safely. For example, in critical illness, intravenous insulin is preferable to subcutaneous insulin, and the frequent measurement of whole‐blood glucose instead of finger‐stick glucose helps to avoid errors.55, 68 And although researchers were unable to prospectively identify patients with long ICU stays,59 severely septic patients have long ICU stays (generally 7.5‐16.6 days),14 and individual ICUs might observe enough stays of more than 2 days in their patient population to justify intensive insulin for this subgroup. And finally, although no conclusive evidence mandates a specific approach to hyperglycemia outside the ICU, the ICU data provide a physiologic rationale for cautious but tight control of glucose in more moderately ill patients. Guidelines for the management of inpatient hyperglycemia were published previously.55
SEPSIS AND THE HOSPITALIST
Hospitalists who provide critical care may make frequent decisions about the inclusion and exclusion criteria for the major trials of sepsis, weigh their relative benefits against risks and costs, contemplate gray areas such as adrenal testing in shock, and employ evidence‐based therapies for severe sepsis. However, hospitalists may also see patients who qualify for these therapies when they are called to see septic patients in the emergency department, when severe sepsis develops in patients on the medicine ward, or when they provide consultation services in an ICU. Sepsis care must be implemented urgently; patients in the pivotal trial of steroids had to be randomized within 3 hours of shock onset,47 data suggest that the window for optimal antibiotic therapy may be no greater than 4 hours from diagnosis,18 whereas guidelines suggest therapy within 1 hour,11 and early goal‐directed therapy was studied only for the first 6 or more hours of hospitalization.36 Thus, hospitalists who do not provide ICU care should be able to identify patients with severe sepsis and either deliver initial care or recognize the need for immediate consultation. Specifically, hospitalists can:
-
Recognize that both absolute ( 90 mm Hg) and relative hypotension (> 40 mm Hg below baseline) indicate septic shock;
-
Identify normotensive candidates for EGDT (severe sepsis with serum lactate > 4 mmol/L) by requesting a serum lactate in addition to prompt appropriate cultures for severe acute infection69;
-
Recognize atypical presentations of sepsis (tachypnea, tachycardia, confusion, etc.) and maintain a high suspicion for sepsis in patients who may be predisposed to infection and to atypical presentation because of age, immunosuppression, neutropenia, diabetes, or other conditions;
-
Initiate effective antibiotics and EGDT promptly for individual patients or by coordinating efforts to improve sepsis care at an institutional level, for example, as a component of medical emergency team services70, 71;
-
Rapidly identify and manage removable foci of infection such as abscesses, empyemas, necrotizing fasciitis, or infected vascular catheters; and
-
Competently educate hospital staff, residents, and medical students about sepsis care.
Hospitalists are busy physicians, and the task of reviewing sepsis literature and implementing recommendations is daunting. However, hospitalists can turn to resources such as the Surviving Sepsis Campaign Guidelines, a series of recommendations for managing severe sepsis that were endorsed by 11 international critical care and infectious disease societies and published in Critical Care Medicine in 2004.11 The Institute for Healthcare Improvement has also published a series of online severe sepsis bundles, or groups of proven interventions, complete with implementation tips and supporting literature, available at
CONCLUSION: DEADLY YET TREATABLE
The death toll from severe sepsis in the United States exceeds that of lung, breast, and colon cancer combined and equals that of myocardial infarction (MI),1 a condition that appropriately triggers a series of emergency interventions. Physicians now have an arsenal of therapies for severe sepsis analogous to those employed for MI, and a comparison between the 2 conditions underscores the high mortality rate of severe sepsis and the enormous impact on patient outcomes provided by evidence‐based sepsis therapy. Figure 2 compares the 9.5%‐16% ARR for death associated with APC in patients with APACHE 2 scores greater than 24 and multiorgan failure,29 EGDT,36 stress dose steroids in shock complicated by adrenal insufficiency,47 and intensive insulin in patients with medical ICU stays longer than 3 days,59 with the benefits of thrombolysis for ST‐elevation MI (2%‐3%)73 or antiplatelet therapy for acute MI (2.3%).74 Figure 3 compares the corresponding NNT values to save 1 life; according to the available data, a hospitalist is 5‐8 times more likely to save a life with EGDT than with fibrinolysis.


Because the literature supporting several major sepsis therapies have been limited to retrospective studies1828 and single randomized, controlled trials29, 36 and because key trials are still underway (CORTICUS, NICE‐SUGAR), the benefits of sepsis therapies are less certain than are those for the treatment of MI. This was underscored by the finding that the benefit in reduced mortality of intensive insulin in the surgical ICU57 did not extend to all patients in the medical ICU.59 However, the potentially marked survival benefit of early effective antibiotics, APC, EGDT, stress dose steroids, and intensive insulin and the urgency with which they must be applied demand that all hospitalists become or remain familiar with the evolving sepsis literature.
Despite decades of intensive research and improvements in medical care, severe sepsis affects an estimated 751,000 patients in the United States every year, killing 215,000 of them at an annual cost of 16.7 billion dollars.1 Because the elderly experience a 100‐fold increase in incidence, as compared with children, and a nearly 4‐fold increase in mortality (38.4% of those more than 85 years old), this burden is expected to increase with the aging population.1 Patients with severe sepsis have prolonged ICU14 and hospital stays and incur substantially increased costs compared with other patients.36
New research continues to explore the complex pathophysiology of sepsis,7 and clinicians, who once relied primarily on clinical experience and expert opinion to guide therapy, now have an increasing array of evidenced‐based sepsis therapies to employ. Recent meta‐analyses have evaluated several major treatments for severe sepsis,810 and recommendations (the Surviving Sepsis Campaign guidelines) for the treatment of severe sepsis were recently endorsed by 11 international critical care and infectious disease organizations.11 This article summarizes the current definitions of sepsis syndromes, the trials supporting the specific therapies for sepsis that are currently recommended, ongoing controversies and research, and implications for hospitalists, with a focus on early, effective antibiotics, activated protein C, early goal‐directed therapy, stress dose steroids, and intensive insulin therapy. For space considerations, readers are directed elsewhere for data supporting prophylaxis for deep venous thrombosis (DVT)12 and stress ulcer bleeding13 and for therapies less often directed by hospitalists, such as lung protective ventilation.14
DEFINITIONS
Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined in 1992 to standardize the terminology of sepsis.15 These definitions have recently been reviewed and supported by a variety of American and European intensive care societies.16
SIRS is defined by the presence of at least 2 of the following:
-
Temperature > 38C or 36C;
-
Heart rate > 90 beats/min;
-
Respiratory rate > 20 breaths/min or PaCO2 32 mm Hg;
-
WBC >12,000 or 4000 cells/mm3, or >10% immature (band) forms.
Sepsis is SIRS due to documented or strongly suspected infection.
Severe sepsis is sepsis with organ dysfunction (such as lactic acidosis, oliguria, thrombocytopenia, or delirium), hypoperfusion, or hypotension ( 90 mm Hg systolic or more than 40 mm Hg below baseline).
Septic shock is severe sepsis complicated by hypotension or pressor dependence despite adequate (20‐30 mL/kg; 1.5‐3 liters in most patients) fluid resuscitation.
Sepsis terminology must be applied carefully. Many hospitalized patients meet criteria for SIRS, yet it is inaccurate to say a patient who has acute leukemia with leukocytosis, anemia‐induced tachycardia, and thrombocytopenia has severe sepsis if those abnormalities are not a result of inflammation or infection. Accurate documentation of sepsis syndromes can improve professional and institutional reimbursement and provide prognostic information: the in‐hospital mortality rates for severe sepsis and septic shock are approximately 30% and 50%, respectively.17 More importantly, thoughtful application of these definitions can help a hospitalist identify septic patients who qualify for one of the proven therapies for severe sepsis.
EARLY, EFFECTIVE ANTIBIOTICS
For obvious ethical reasons, randomized, controlled trials to study the impact of inappropriate or delayed antibiotic therapy for serious infections are not possible. However, the evidence supporting early, effective antibiotic therapy is still compelling, and because many hospitalists often initiate treatment with antibiotics before transferring a patient to intensive care, this may represent the most important intervention hospitalists can provide to patients with serious infections. Several studies have estimated the impact of early, effective antibiotics on outcomes.
Houck et al. retrospectively reviewed 13,771 cases of community‐acquired pneumonia among elderly Medicare patients. They found that 39.1% of the patients waited more than 4 hours for antibiotics and 7.6% waited more than 12 hours; three quarters of these delays resulted from delayed ordering of antibiotics.18 Further, 21.2% received an antibiotic selection incompatible with recent professional guidelines. Receiving antibiotics within 4 hours reduced in‐hospital and 30‐day mortality by 15% and length of stay by 0.4 days.18 Similar conclusions were reported by 3 of 4 previous analyses.1922 Extending these findings to critically ill patients, Iregui et al. found that delayed treatment with appropriate antibiotics (odds ratio, 7.68) was a greater predictor of mortality for 107 patients with ventilator‐acquired pneumonia than were APACHE II scores and malignancy; 31% failed to receive appropriate antibiotics within 24 hours, and again, three quarters of these delays resulted from delays in writing antibiotic orders.23
Not surprisingly, antibiotic therapy must be effective as well as timely. MacArthur et al. studied the impact of adequate (ie, active against cultured organisms, if isolated) antibiotics on the outcomes of 2634 septic patients enrolled in a randomized trial of an anti‐TNF antibody. Nearly 91% received appropriate antibiotics; their mortality rate was 33%, 10% lower than that of the patients whose initial antibiotics were inadequate (P .001).24 Leibovici et al. reported similar findings in a prospective study of patients with bacteremia. Only 63% of 3413 subjects received an antibiotic active against the infecting pathogen, and their mortality was 20%, 14% lower than that in the group that received ineffective antibiotics (P = .0001).25 Other authors have reported even worse outcomes with ineffective therapy: 62% mortality among inadequately treated bacteremic or fungemic ICU patients, compared with 28.4% among those who were adequately treated26 and an odds ratio of dying of 8.14 for the 46 of 270 septic ICU patients who received inadequate initial antibiotics,27 making inadequate antibiotic therapy the strongest risk factor for death. Finally, Kollef et al. reported that 26% of 655 infected ICU patients received inadequate antibiotics and suffered an infection‐related mortality rate of 40.2%, more than twice the 17.7% rate among adequately treated patients (P .001). Inadequate antimicrobial therapy was a greater risk factor for death than early respiratory failure or sepsis‐related organ failure assessment scores.28
Guidelines for anti‐infective care now recommend obtaining appropriate cultures and administering broad‐spectrum antibiotics (appropriate for suspected infections, local susceptibility patterns, and any relevant prior culture data from individual patients) within 1 hour of presentation.11 In addition, any removable focus of infection must be identified and managed (eg, an abscess, infected catheter, tampon, or infection requiring surgery).
ACTIVATED PROTEIN C
Recombinant human activated protein C (APC) is a protein with anticoagulant and anti‐inflammatory properties that is relatively deficient in approximately 87% of septic patients.29 Although numerous trials of other anticoagulants (antithrombin III and tissue factor pathway inhibitor) and immunosuppressives (tumor necrosis factor inhibitors, high‐dose steroids, interleukin‐1 receptor antagonists, and others) have failed to show any benefit,7 in 2001 APC became the first proven therapy specifically for sepsis. The PROWESS trial, which established its efficacy, randomized 1690 patients who met 3 SIRS criteria and dysfunction of at least 1 organ system to APC (24 g/kg IV per hour for 96 hours interrupted for bleeding or urgent procedures) or placebo. APC reduced 28‐day mortality from 30.8% to 24.7%, yielding an absolute risk reduction of 6.1% and a corresponding number needed to treat (NNT) of 16.4. This benefit was seen across all subgroups including those with normal baseline APC levels.29
Not surprisingly, APC increases the risk of serious bleeding. Although this effect was of borderline significance in PROWESS (3.5% vs. 2% in the placebo group, P = .06),29 it was confirmed in subsequent trials (3.9% vs. 2.2%, P = .01)30 and may be larger still in open‐label use, at 6.5%.17, 31 Intracerebral hemorrhage (ICH), a particularly devastating complication, occurred in 0.2% of the PROWESS patients and 0.5% of patients in 2 subsequent studies30, 32; in both major trials, there was a single extra event in the APC arm.29, 30 Like serious bleeding in general, ICH was more common in open‐label use, occurring in 1.5% of patients.31, 33 Therefore, it is vital to have strict adherence to exclusion criteria and familiarity with the risk factors for serious bleeding. In the PROWESS trial, after randomization, risk factors for serious bleeding included procedures and injury to vascular organs, an activated partial‐thromboplastin time of more than 120 seconds, an international normalized ratio greater than 3, gastrointestinal ulceration, and development of severe thrombocytopenia ( 30,000/mm3)29; in a 2002 study of 2786 APC recipients, ICH was largely confined to patients with meningitis or a platelet count less than 30,000/mm3.32
APC therapy has several other limitations and drawbacks. Multiple contraindications, including predisposition to bleeding, a recent history of bleeding, anticoagulant use, immunosuppression, liver disease, dialysis dependence, and hypercoagulable states, restrict its use. APC appears to work best when administered early, within 24 hours of the onset of organ dysfunction.31 In addition, APC is indicated only in adults with Acute Physiology and Chronic Health Evaluation (APACHE II) scores greater than 24 and multiorgan failure. Post hoc analysis of the PROWESS data showed that although the relative risk (RR) of death for those with APACHE II scores of 25 or more was .71 and statistically significant, the RR for those with scores below 25 was a nonsignificant .99.34 A subsequent study, ADDRESS, confirmed there was no benefit to septic patients with a low risk of death.30 In the ADDRESS study 2613 patients with severe sepsis and either an APACHE II score less than 25 or single organ failure were randomized to APC or placebo. No differences were found in 28‐day and in‐hospital mortality; among patients who had undergone surgery in the previous 30 days, those receiving APC had a significantly increased risk of death (20.7% vs. 14.1%, P = .03).
An additional drawback of APC therapy is its cost, approximately $6800 per infusion, although the cost per year of life gained, $24,484, or $52,360 per life saved (NNT $6800), is reasonable for those with APACHE II scores greater than 24.34 Concerns have also been raised about the PROWESS trial itself: the production of the study drug and some exclusion criteria were changed midtrial, after which the effectiveness of APC improved. APACHE II scores had not been validated for selection of patients for therapies and may have varied with time or by observer. The original PROWESS study population may have been skewed away from chronically ill patients.35 Experts differ on the significance of these concerns and even whether APC therapy should be considered the standard of care pending further research.32, 35 The ADDRESS trial also failed to demonstrate a benefit in a subgroup of patients with APACHE II scores above 24, although it was underpowered to do so, and according to enrollment criteria, none of those patients had multiorgan failure.30 However, in the subgroup of PROWESS patients with APACHE II scores greater than 24, the absolute reduction in mortality was a full 13%,17 with a corresponding NNT of 7.7, and although the PROWESS findings have not been duplicated in a second randomized trial, a single‐arm, open‐label study of APC (ENHANCE) showed a nearly identical mortality rate.31 Pending confirmatory trials, APC remains a recommended therapy for selected patients sick enough to benefit and without excessive bleeding risk.11
EARLY GOAL‐DIRECTED THERAPY
Because physician‐directed resuscitation for sepsis may normalize vital signs, central venous pressures (CVP), and urine output without correcting hypoperfusion, Rivers et al. tested a resuscitation protocol that incorporated a central line that continuously monitored mixed‐venous oxygen saturation as a surrogate for cardiac output.36 They randomized 263 patients with septic shock (defined as hypotension 90 mm Hg after a 20‐30 mL/kg bolus, or lactate > 4 mmol/L, which is associated with at least a 3‐fold increase in the mortality of emergency department patients with suspected infection37) to either standard care or early goal‐directed therapy (EGDT) for the initial 6 hours of hospital care. Patients with acute coronary ischemia, pulmonary edema, stroke, asthma, overdose, trauma, dysrhythmia, immunosuppression, uncontrolled cancer, or a need for urgent procedures were excluded. Standard care was directed by physiologic parameters such as vital signs, urine output, and CVP. EGDT used sequential therapies designed to support organ perfusion: 500 mL of normal saline was given every half hour until the CVP was at least 8‐12 mm Hg. Pressors were given until the mean arterial pressure was 65‐90 mm Hg (norepinephrine36 or dopamine were preferred agents, and vasopressin [0.01‐0.04 units/min] was an option for shock refractory to first‐line pressors)11, 38 Transfusion (to a hematocrit goal of 30) and dobutamine were given until mixed‐venous oxygenation saturation was 70% or better (Fig. 1). Lastly, patients who did not achieve this goal were sedated and mechanically ventilated.

Results were dramatic: mortality was reduced from 46.5% to 30.5%, with an ARR of 16% and an NNT of 6.25. Study patients received similar amounts of crystalloid, but received it earlier than the standard care patients and received more transfusions and inotropes. Substantially more patients in the EGDT group than the standard care group achieved a mixed venous oxygen saturation of 70%; 13.7% of the EGDT patients had occult hypoperfusion (low mixed‐venous oxygenation that responded to inotropes despite satisfactory vital signs). EGDT improved length of stay (4 days shorter among survivors) and duration of intubation, as well as APACHE scores and several physiologic parameters.36
Critics of this trial note the impossibility of adequate blinding and the high mortality in the placebo group. Further, because the trial tested the EGDT protocol as a whole, there was no way to know if each step was optimal. For example, a different CVP goal could have been used or adjustments made for mechanical ventilation, which can falsely elevate a low CVP into the desired range (the Surviving Sepsis Campaign guidelines recommend a CVP goal of 12‐15 mm Hg in mechanically ventilated patients11). Also, the selection of pressor, the use of inotropes, and the transfusion threshold were chosen on the basis of physiologic rationales, but all of these are arguable.39 This was also a single trial, and earlier goal‐directed protocols for ICU patients actually showed harm,40, 41 although those trials targeted supranormal physiologic goals in more established critical illness.42 Finally, on a practical level, hospitals and particularly emergency departments must commit resources to train physicians and staff, purchase the appropriate central venous catheters, and convince eligible patients to undergo an invasive procedure. In a survey of 30 attending physicians in academic referral hospitals, only 7% reported standard use of EGDT. Barriers included the requirement for specialty monitoring equipment and other resources, and central venous cannulation.43
Despite these concerns, the striking reduction in mortality associated with EGDT led to its endorsement by the Surviving Sepsis Campaign guidelines and underscores the principle of aggressive early resuscitation for patients who do not meet eligibility criteria but appear at risk for worsening sepsis. As yet, however, no strong evidence mandates a specific approach to the septic patient without shock.
STRESS DOSE STEROIDS
Because of the importance of the inflammatory cascade in severe sepsis, a potential role for steroids in the management of sepsis has been repeatedly studied. More than 50 studies have been performed since the 1950s, generally with pharmacologic doses of steroid; a meta‐analysis showed that such a practice was ineffective.44, 45 However, data accumulated that relative adrenal insufficiency during severe sepsis was common and associated with an increased risk of death and that physiologic doses of steroids could reverse refractory hypotension.46 To define the role of a physiologic course of steroids in septic shock, Annane et al. randomized 299 critically ill adults to either 7 days of stress dose hydrocortisone (50 mg IV every 6 hours) and fludrocortisone (50 g NG every 24 hours) or matched placebos. Enrolled patients were severely ill; the placebo group had a 63% mortality, and patients had to have septic shock, oliguria or hypoxia, hypotension despite low‐dose dopamine, and lactate greater than 2 mmol/L and require mechanical ventilation. Pregnant women, those with myocardial infarction or pulmonary embolus, advanced malignancies, or immunodeficiency, and those with clear indications or contraindications to steroids were excluded.47 Enrollment criteria were modified midstudy; changes included the exclusion of patients who had recently received etomidate, which inhibits 11‐‐hydroxylase and has been identified as a risk factor for adrenal insufficiency in intensive care patients.48 All patients received a 250‐g cosyntropin stimulation test. The authors considered patients nonresponders to consyntropin if serum cortisol failed to increase to 9 g/dL or more.
Steroids reduced the duration that a vasopressor was required and reduced mortality from 63% to 53% among nonresponders, giving an NNT of only 10 to prevent 1 death at 28 days. Although the authors described no evidence of adverse effects, among the subset of 70 patients who responded appropriately to cosyntropin, there was a nonsignificant trend toward increased mortality, and rates of hyperglycemia were not provided.47 The authors concluded that physicians should test appropriate patients for adrenal reserve, give the studied steroid regimen while results are pending, and discontinue treatment if a patient retains adrenal reserve.
The literature on steroids and critical illness is complex, with more than 1300 articles on steroids and sepsis published since 1988, and several concerns were raised about the Annane study. For example, did much of the benefit for those patients enrolled before the protocol amendment come from reducing an adverse effect of etomidate?49 Does the high‐dose, 250‐g cosyntropin stimulation test overcome (and conceal) partial ACTH resistance that might benefit from treatment?50 Might not a robust baseline cortisol suggest sufficient adrenal function regardless of the incremental response to cosyntropin?51 Partial answers were provided by 2 subsequent meta‐analyses. Both found that more recent studies gave lower doses of steroids in longer, 5‐ to 7‐day courses to sicker patients and demonstrated improvement in mortality and shock reversal, with relative risk reductions of 14%‐22%; the NNT ranged from 8 to 11. One analysis found no difference in outcomes between adrenally sufficient and adrenally insufficient patients, and those authors advised considering treatment for all patients regardless of their adrenal function test results.8 The other analysis concluded that the data on steroids for those with adrenal reserve was too limited to recommend treating adrenally sufficient patients.9
Disputes about certain details, such as whether patients should be treated without regard to adrenal reserve, continue in the literature.45, 52 An ongoing randomized, controlled trial, CORTICUS, is expected to provide additional guidance on the use of low‐dose steroids in sepsis; in the meantime, the literature clearly supports a longer course of low‐dose steroid therapy for patients with pressor‐dependent septic shock with inadequate adrenal reserve by cosyntropin testing, and guidelines allow discretion about whether patients with adequate adrenal reserve should also be treated.11 Hospitalists may also want to treat septic shock with equivalent doses of dexamethasone (approximately 2 mg IV every 6 hours) if adrenal evaluation may be delayed, as this agent will not confound cosyntropin stimulation test results, and they may want to avoid etomidate in septic patients53, 54 for whom they perform or supervise intubations.
INTENSIVE INSULIN THERAPY
Mounting evidence supports the short‐term role of hyperglycemia in morbidity and mortality, especially in critical illness. Hyperglycemia impairs neutrophil and endothelial cell function as well as protective responses to cardiac and neuronal ischemia,55 whereas insulin has anti‐inflammatory and antiapoptotic effects,7, 56 suggesting that intensive insulin might improve the outcomes of critically ill patients. To test this theory, van den Berghe and colleagues randomized 1548 mostly surgical ICU patients to insulin infusions titrated for glucose goals of either 80‐110 or 180‐200 mg/dL, followed by subcutaneous insulin after ICU discharge. Although blinding was impossible, in both cases glucose management was performed by a separate research team. Multiple benefits were noted: ICU and total in‐hospital deaths were reduced, mostly among patients with an ICU stay of more than 5 days, whose risk of death fell from 20.2% to 10.6%. Intensive insulin also reduced septicemia, renal impairment, critical illness polyneuropathy, and duration of intensive care.57
Subsequently, a meta‐analysis of 35 trials suggested that insulin reduced the mortality of critically ill patients by 15%.10 Van den Berghe et al.'s results were also duplicated in a broad, medical‐surgical ICU population, although the reductions in morbidity and mortality were measured against historical controls.58 However, whether the results of the influential surgical ICU study could be applied to medical patients was not known until 2006, when the van den Berghe group reported the effects of similar insulin protocols on 1200 patients in the medical ICU who were expected to need intensive care for at least 3 days.59 In this study, intensive insulin failed to reduce overall mortality (40% and 37.3%, P = .33). However, intensive insulin did reduce mortality among the 64% of patients who stayed in the ICU 3 or more days from 52.5% to 43% (NNT 10.5, P = .009). This benefit was offset by an increased number of deaths in the intensive insulin group among patients with ICU stays of less than 3 days (P = .05‐.35 depending on the method used).59 Intensive insulin did reduce newly acquired kidney injury, duration of mechanical ventilation, and lengths of ICU and hospital stays, and the reduction in morbidity increased with the duration of intensive insulin therapy. Hypoglycemia (mean 32 mg/dL) occurred in 25% of patients with prolonged stays6.4 times as often as in the usual care group.60 Liver and renal failure were associated with hypoglycemia.59
Critics of the surgical ICU trial noted the high mortality among the usual care patients (5.1%), a robust 34% mortality reduction for a relatively small 50 mg/dL reduction in morning glucose levels, and the aggressive use of parenteral nutrition, raising the question of whether intensive insulin merely attenuated the side effects of intravenous glucose.61, 62 Also, the ideal blood glucose target is not known with certainty. Retrospective studies suggested the upper limit for target blood glucoses could be 145 mg/dL63 and found differing thresholds at which hyperglycemia increased mortality in nondiabetics (144 mg/dL) and diabetics (200 mg/dL).64 However, in the surgical ICU trial, there was no threshold below which there was no further reduction in risk; patients whose mean blood glucose was below 110 mg/dL had lower mortality than those whose levels were between 110 and 150 mg/dL (P = .026).65 Finally, the effects of hyperglycemia and intensive insulin may vary by population: retrospective studies found that ICU hyperglycemia was more strongly associated with mortality among nondiabetics,64, 66 and van den Berghe et al. noted no benefit from intensive insulin in a small subgroup of diabetics.59
In summary, large, well‐designed trials have demonstrated that intensive insulin reduced mortality in critically ill patients after a delay of 3‐5 days, but this benefit did not extend to all patients in the medical ICU.57, 59 Some authors have suggested deferring intensive insulin for 3 days,67 but because early therapy probably contributes to the delayed mortality benefit, this approach may deprive patients of the observed benefits. Ongoing clinical trials (NICE‐SUGAR) are likely to provide useful information about how hyperglycemia should be managed in different populations, including septic ICU patients.61 In the meantime, institutions can select the intensity of their insulin therapy by weighing morbidity and long‐term mortality benefits against possible short‐term harms and ensuring that hospital staff members are sufficiently trained to control hyperglycemia safely. For example, in critical illness, intravenous insulin is preferable to subcutaneous insulin, and the frequent measurement of whole‐blood glucose instead of finger‐stick glucose helps to avoid errors.55, 68 And although researchers were unable to prospectively identify patients with long ICU stays,59 severely septic patients have long ICU stays (generally 7.5‐16.6 days),14 and individual ICUs might observe enough stays of more than 2 days in their patient population to justify intensive insulin for this subgroup. And finally, although no conclusive evidence mandates a specific approach to hyperglycemia outside the ICU, the ICU data provide a physiologic rationale for cautious but tight control of glucose in more moderately ill patients. Guidelines for the management of inpatient hyperglycemia were published previously.55
SEPSIS AND THE HOSPITALIST
Hospitalists who provide critical care may make frequent decisions about the inclusion and exclusion criteria for the major trials of sepsis, weigh their relative benefits against risks and costs, contemplate gray areas such as adrenal testing in shock, and employ evidence‐based therapies for severe sepsis. However, hospitalists may also see patients who qualify for these therapies when they are called to see septic patients in the emergency department, when severe sepsis develops in patients on the medicine ward, or when they provide consultation services in an ICU. Sepsis care must be implemented urgently; patients in the pivotal trial of steroids had to be randomized within 3 hours of shock onset,47 data suggest that the window for optimal antibiotic therapy may be no greater than 4 hours from diagnosis,18 whereas guidelines suggest therapy within 1 hour,11 and early goal‐directed therapy was studied only for the first 6 or more hours of hospitalization.36 Thus, hospitalists who do not provide ICU care should be able to identify patients with severe sepsis and either deliver initial care or recognize the need for immediate consultation. Specifically, hospitalists can:
-
Recognize that both absolute ( 90 mm Hg) and relative hypotension (> 40 mm Hg below baseline) indicate septic shock;
-
Identify normotensive candidates for EGDT (severe sepsis with serum lactate > 4 mmol/L) by requesting a serum lactate in addition to prompt appropriate cultures for severe acute infection69;
-
Recognize atypical presentations of sepsis (tachypnea, tachycardia, confusion, etc.) and maintain a high suspicion for sepsis in patients who may be predisposed to infection and to atypical presentation because of age, immunosuppression, neutropenia, diabetes, or other conditions;
-
Initiate effective antibiotics and EGDT promptly for individual patients or by coordinating efforts to improve sepsis care at an institutional level, for example, as a component of medical emergency team services70, 71;
-
Rapidly identify and manage removable foci of infection such as abscesses, empyemas, necrotizing fasciitis, or infected vascular catheters; and
-
Competently educate hospital staff, residents, and medical students about sepsis care.
Hospitalists are busy physicians, and the task of reviewing sepsis literature and implementing recommendations is daunting. However, hospitalists can turn to resources such as the Surviving Sepsis Campaign Guidelines, a series of recommendations for managing severe sepsis that were endorsed by 11 international critical care and infectious disease societies and published in Critical Care Medicine in 2004.11 The Institute for Healthcare Improvement has also published a series of online severe sepsis bundles, or groups of proven interventions, complete with implementation tips and supporting literature, available at
CONCLUSION: DEADLY YET TREATABLE
The death toll from severe sepsis in the United States exceeds that of lung, breast, and colon cancer combined and equals that of myocardial infarction (MI),1 a condition that appropriately triggers a series of emergency interventions. Physicians now have an arsenal of therapies for severe sepsis analogous to those employed for MI, and a comparison between the 2 conditions underscores the high mortality rate of severe sepsis and the enormous impact on patient outcomes provided by evidence‐based sepsis therapy. Figure 2 compares the 9.5%‐16% ARR for death associated with APC in patients with APACHE 2 scores greater than 24 and multiorgan failure,29 EGDT,36 stress dose steroids in shock complicated by adrenal insufficiency,47 and intensive insulin in patients with medical ICU stays longer than 3 days,59 with the benefits of thrombolysis for ST‐elevation MI (2%‐3%)73 or antiplatelet therapy for acute MI (2.3%).74 Figure 3 compares the corresponding NNT values to save 1 life; according to the available data, a hospitalist is 5‐8 times more likely to save a life with EGDT than with fibrinolysis.


Because the literature supporting several major sepsis therapies have been limited to retrospective studies1828 and single randomized, controlled trials29, 36 and because key trials are still underway (CORTICUS, NICE‐SUGAR), the benefits of sepsis therapies are less certain than are those for the treatment of MI. This was underscored by the finding that the benefit in reduced mortality of intensive insulin in the surgical ICU57 did not extend to all patients in the medical ICU.59 However, the potentially marked survival benefit of early effective antibiotics, APC, EGDT, stress dose steroids, and intensive insulin and the urgency with which they must be applied demand that all hospitalists become or remain familiar with the evolving sepsis literature.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29:1303–1310.
- ,,,.Prevalence and incidence of severe sepsis in Dutch intensive care units.Crit Care.2004;8:R153–R162.
- ,, et al.Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use.Intensive Care Med.2002;28:1440–1446.
- ,,,,.Effects of severity of illness on resource use by survivors and nonsurvivors of severe sepsis at intensive care unit admission.Crit Care Med.2002;30:2413–2419.
- ,,, et al.Resource utilization among patients with sepsis syndrome.Infect Control Hosp Epidemiol.2003;24:62–70.
- ,,,,.The costs of septic syndromes in the intensive care unit and influence of hospital‐acquired sepsis.Intensive Care Med,2003;29:1464–1471.
- ,.The pathophysiology and treatment of sepsis.N Engl J Med.2003;348:138–150.
- ,,,,.Meta‐analysis: the effect of steroids on survival and shock during sepsis depends on the dose.Ann Intern Med.2004;141:47–56.
- ,,,,,.Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis.Brit Med J.2004;329:480–488.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32:858–873.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26:115–117.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome.N Engl J Med.2000;342:1301–1308.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Crit Care Med.1992;20:864–874.
- ;;, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- .Severe sepsis and therapy with activated protein C.New Engl J Med.2005;353:1398–1399.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Measuring quality of care with explicit process criteria before and after implementation of the DRG‐based prospective payment system.JAMA.1990;264:1969–1973.
- ,.Pneumonia mortality reduction and quality improvement in a community hospital.Qual Rev Bull.1993;19:124–130.
- ,,, et al.Quality of care, process and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS Trial.Clin Infect Dis.2004;38:284–288.
- ,,,,,.The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection.J Intern Med.1998;244:379–386.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- ,,,,,.Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
- ,,, et al.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Efficacy and safety of recombinant human activated protein C for severe sepsis.N Engl J Med.2001;344:699–709.
- ,,, et al.Drotecogin alfa (activated) for adults with severe sepsis and a low risk of death.New Eng J Med.2005;353:1332–1341.
- ,,, et al.Drotecogin alfa (activated) treatment in severe sepsis from the global open label trial ENHANCE.Crit Care Med.2005;10:2266–2277.
- ,,.Activated protein C for severe sepsis.N Engl J Med.2002;347;1035–1036.
- .Assessing the use of activated protein C in the treatment of severe sepsis.N Engl J Med.2002;347:1030–1034.
- ,,,,.An economic evaluation of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:993–1000.
- ,,,.Risks and benefits of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:1027–1030.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Serum lactate as a predictor of mortality in emergency department patients with infection.Ann Emerg Med.2005;45:524–528.
- ,,,.Vasopressor and inotropic support in septic shock: an evidence‐based review.Crit Care Med.2004;32(11 Suppl):S455–S465.
- ,,, et al.Goal‐directed therapy for severe sepsis [letter].N Engl J Med.2002;346:1025–1026.
- ,,,,,.Elevation of systemic oxygen delivery in the treatment of critically ill patients.New Engl J Med.1994;330:1717–1722.
- ,, et al.A trial of goal‐oriented hemodynamic therapy in critically ill patients.N Engl J Med.1995;333:1025–1032.
- .Hemodynamic and metabolic therapy in critically ill patients.New Engl J Med.2001;345:1417–1418.
- ,.Use of goal directed therapy for severe sepsis and septic shock in academic emergency departments.Crit Care Med.2005;33:1888–1889.
- ,,, et al.Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature.Crit Care Med.1995;23:1430–1439.
- .Physicians should administer low‐dose corticosteroids selectively to septic patients until an ongoing trial is completed.Ann Intern Med.2004;141:70–72.
- ,.Corticosteroids and septic shock.JAMA.2002;288:886–887.
- ,,, et al.Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock.JAMA.2002;288:862–871.
- ,,, et al.Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation.Intensive Care Med.2005;31:388–392.
- ,.Editorial III: Corticosteroids for septic shock—a standard of care?Br J Anaesth.2004;93:178–180.
- ,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for septic shock [letter].Ann Intern Med.2004;141:742–743.
- .Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal.Chest.2005;127:1031–1038.
- .ICU physicians should abandon the use of etomidate!Intensive Care Med.2005;31:325–326.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,,.Intensive insulin therapy exerts anti‐inflammatory effects in critically ill patients and counteracts the adverse effects of low mannose binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000. Published erratum appears in Mayo Clin Proc.year="2005"2005;80:1101
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- Supplement to:,,, et al.Intensive insulin therapy in the medical ICU.N Eng J Med.2006;354:449–461. Available at: http://content.nejm.org/cgi/data/354/5/449/DC1/1.
- .Glycemic control in the intensive care unit: why we should wait for NICE‐SUGAR.Mayo Clin Proc.2005;80:1546–1548.
- ,,.Intensive insulin therapy in critically ill patients [letter].New Engl J Med.2002;346:1586–1588.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,,,.Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus.Mayo Clin Proc.2005;80:1558–1567.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:634–635.
- ,,,,.Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations.Crit Care Med.2005;33:2772–2777.
- .Intensive insulin in intensive care.New Engl J Med.2006;354:516–518.
- ,,,,.Fingerstick glucose determination in shock.Ann Intern Med.1991;114:1020–1024.
- ,,.A blueprint for a sepsis protocol.Acad Emerg Med.2005;12:352–359.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome.Crit Care Med.2004;32:S595–S597.
- Fibrinolytic Therapy Trialists' Collaborative Group.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients.Lancet.1994;343:311–322.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.Brit Med J.2002;324:71–86.
- ,,,,,.Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care.Crit Care Med.2001;29:1303–1310.
- ,,,.Prevalence and incidence of severe sepsis in Dutch intensive care units.Crit Care.2004;8:R153–R162.
- ,, et al.Direct costs of severe sepsis in three German intensive care units based on retrospective electronic patient record analysis of resource use.Intensive Care Med.2002;28:1440–1446.
- ,,,,.Effects of severity of illness on resource use by survivors and nonsurvivors of severe sepsis at intensive care unit admission.Crit Care Med.2002;30:2413–2419.
- ,,, et al.Resource utilization among patients with sepsis syndrome.Infect Control Hosp Epidemiol.2003;24:62–70.
- ,,,,.The costs of septic syndromes in the intensive care unit and influence of hospital‐acquired sepsis.Intensive Care Med,2003;29:1464–1471.
- ,.The pathophysiology and treatment of sepsis.N Engl J Med.2003;348:138–150.
- ,,,,.Meta‐analysis: the effect of steroids on survival and shock during sepsis depends on the dose.Ann Intern Med.2004;141:47–56.
- ,,,,,.Corticosteroids for severe sepsis and septic shock: a systematic review and meta‐analysis.Brit Med J.2004;329:480–488.
- ,,.Insulin therapy for critically ill hospitalized patients: a meta‐analysis of randomized controlled trials.Arch Intern Med.2004;164:2005–2011.
- ,,, et al.Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock.Crit Care Med.2004;32:858–873.
- ,,,,.Prevention of deep vein thrombosis in medical patients by low‐dose heparin.Scott Med J.1981;26:115–117.
- ,,, et al.A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group.N Engl J Med.1998;338:791–797.
- The Acute Respiratory Distress Syndrome Network.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome.N Engl J Med.2000;342:1301–1308.
- American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Crit Care Med.1992;20:864–874.
- ;;, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- .Severe sepsis and therapy with activated protein C.New Engl J Med.2005;353:1398–1399.
- ,,,,.Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia.Arch Intern Med.2004;164:637–644.
- ,,, et al.Measuring quality of care with explicit process criteria before and after implementation of the DRG‐based prospective payment system.JAMA.1990;264:1969–1973.
- ,.Pneumonia mortality reduction and quality improvement in a community hospital.Qual Rev Bull.1993;19:124–130.
- ,,, et al.Quality of care, process and outcomes in elderly patients with pneumonia.JAMA.1997;278:2080–2084.
- ,,,,.Processes of care, illness severity, and outcomes in the management of community‐acquired pneumonia at academic hospitals.Arch Intern Med.2001;161:2099–2104.
- ,,,,.Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator‐associated pneumonia.Chest.2002;122:262–268.
- ,,, et al.Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS Trial.Clin Infect Dis.2004;38:284–288.
- ,,,,,.The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection.J Intern Med.1998;244:379–386.
- ,,,,.The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting.Chest.2000;118:146–155.
- ,,,,,.Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis.Crit Care Med.2003;31:2742–2751.
- ,,, et al.Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients.Chest.1999;115:462–474.
- ,,, et al.Efficacy and safety of recombinant human activated protein C for severe sepsis.N Engl J Med.2001;344:699–709.
- ,,, et al.Drotecogin alfa (activated) for adults with severe sepsis and a low risk of death.New Eng J Med.2005;353:1332–1341.
- ,,, et al.Drotecogin alfa (activated) treatment in severe sepsis from the global open label trial ENHANCE.Crit Care Med.2005;10:2266–2277.
- ,,.Activated protein C for severe sepsis.N Engl J Med.2002;347;1035–1036.
- .Assessing the use of activated protein C in the treatment of severe sepsis.N Engl J Med.2002;347:1030–1034.
- ,,,,.An economic evaluation of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:993–1000.
- ,,,.Risks and benefits of activated protein C treatment for severe sepsis.N Engl J Med.2002;347:1027–1030.
- ,,, et al.Early goal‐directed therapy in the treatment of severe sepsis and septic shock.N Engl J Med.2001;345:1368–1377.
- ,,, et al.Serum lactate as a predictor of mortality in emergency department patients with infection.Ann Emerg Med.2005;45:524–528.
- ,,,.Vasopressor and inotropic support in septic shock: an evidence‐based review.Crit Care Med.2004;32(11 Suppl):S455–S465.
- ,,, et al.Goal‐directed therapy for severe sepsis [letter].N Engl J Med.2002;346:1025–1026.
- ,,,,,.Elevation of systemic oxygen delivery in the treatment of critically ill patients.New Engl J Med.1994;330:1717–1722.
- ,, et al.A trial of goal‐oriented hemodynamic therapy in critically ill patients.N Engl J Med.1995;333:1025–1032.
- .Hemodynamic and metabolic therapy in critically ill patients.New Engl J Med.2001;345:1417–1418.
- ,.Use of goal directed therapy for severe sepsis and septic shock in academic emergency departments.Crit Care Med.2005;33:1888–1889.
- ,,, et al.Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature.Crit Care Med.1995;23:1430–1439.
- .Physicians should administer low‐dose corticosteroids selectively to septic patients until an ongoing trial is completed.Ann Intern Med.2004;141:70–72.
- ,.Corticosteroids and septic shock.JAMA.2002;288:886–887.
- ,,, et al.Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock.JAMA.2002;288:862–871.
- ,,, et al.Risk factors of relative adrenocortical deficiency in intensive care patients needing mechanical ventilation.Intensive Care Med.2005;31:388–392.
- ,.Editorial III: Corticosteroids for septic shock—a standard of care?Br J Anaesth.2004;93:178–180.
- ,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for patients with septic shock [letter].JAMA.2003;289:42.
- ,,,,.Corticosteroids for septic shock [letter].Ann Intern Med.2004;141:742–743.
- .Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal.Chest.2005;127:1031–1038.
- .ICU physicians should abandon the use of etomidate!Intensive Care Med.2005;31:325–326.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,,,.Intensive insulin therapy exerts anti‐inflammatory effects in critically ill patients and counteracts the adverse effects of low mannose binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000. Published erratum appears in Mayo Clin Proc.year="2005"2005;80:1101
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- Supplement to:,,, et al.Intensive insulin therapy in the medical ICU.N Eng J Med.2006;354:449–461. Available at: http://content.nejm.org/cgi/data/354/5/449/DC1/1.
- .Glycemic control in the intensive care unit: why we should wait for NICE‐SUGAR.Mayo Clin Proc.2005;80:1546–1548.
- ,,.Intensive insulin therapy in critically ill patients [letter].New Engl J Med.2002;346:1586–1588.
- ,,,.Glucose control and mortality in critically ill patients.JAMA.2003;290:2041–2047.
- ,,,,.Influence of individual characteristics on outcome of glycemic control in intensive care unit patients with or without diabetes mellitus.Mayo Clin Proc.2005;80:1558–1567.
- ,,, et al.Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control.Crit Care Med.2003;31:634–635.
- ,,,,.Impact of admission hyperglycemia on hospital mortality in various intensive care unit populations.Crit Care Med.2005;33:2772–2777.
- .Intensive insulin in intensive care.New Engl J Med.2006;354:516–518.
- ,,,,.Fingerstick glucose determination in shock.Ann Intern Med.1991;114:1020–1024.
- ,,.A blueprint for a sepsis protocol.Acad Emerg Med.2005;12:352–359.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Sepsis change bundles: converting guidelines into meaningful change in behavior and clinical outcome.Crit Care Med.2004;32:S595–S597.
- Fibrinolytic Therapy Trialists' Collaborative Group.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients.Lancet.1994;343:311–322.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.Brit Med J.2002;324:71–86.
Handoffs
On my first day as a nervous, third‐year medical student, a nurse offered to orient me to the pediatric ICU. I expected a litany of facts to memorize. Instead, she pointed at each room in turn and described the tragedies they had hosted.
Room 1: a little girl just died of meningitis there. Room 2: that boy's liver transplant failed, and he had a massive stroke. The father sat holding the jaundiced hand of his unresponsive son, whose stapled abdomen held back tense ascites. His wife died of cancer 2 months ago. Now he has no one. Room 3: teen with cystic fibrosis; she'll be OK. Room 4 I will never forget. A teenager died of leukemia there and refused all painkillers. He wanted to be lucid for his family, and they huddled on his bed and sang Amazing Grace until he died. Most beautiful thing I have seen.
I had thought, Beautiful? How can you even come to work?
Five years later, I remembered that conversation as if it had just happened. I was the senior resident in the medical ICU, it was 3 AM, and I was gathering my thoughts amid the whooshes, beeps, and flickering monitors of the sleeping unit. I was preparing to go tell Betsy that Joe, her 31‐year‐old husband, needed prone ventilation. Joe lay dying from, of all things, chickenpox. He was receiving 12 infusions, including 4 pressors, sedatives, antibiotics, acyclovir, full‐strength bicarbonate, his 26th amp of calcium, and liter number‐who‐knows‐what of saline. He sprouted 2 IVs, 2 central lines, a Foley catheter, endotracheal and orogastric tubes, an arterial line, and an array of monitor leads. His blood pressure would plummetfrom a systolic of 80whenever we interrupted his bicarb drip to spike a new bag, so we knew moving him might kill him. Every nurse raced to finish tasks on other patients, preparing to help.
Joe's admission began, like several of his earlier ones, with a chief complaint of Crohn's flare. This time, however, he had a new rash, and although John's ward team suspected medications were to blame, they soon started him on acyclovir. In days, hepatitis, acute renal failure, and pneumonia prompted his ICU transfer. He required intubation hours later. His course since had been like watching a pedestrian struck by a truck in slow motion: a sudden, jolting, irreversible crueltydrawn out over hours. Anasarca had folded his blistering ears in half and forced us to revise his endotracheal tube taping 3 times so it would not incise his cheeks. He had unremitting hypotension. His transaminases climbed above 6000 and his creatinine to 6; his arterial pH dropped to 7.03, and his platelets fell to 16,000. His partial pressure of oxygen sank below 60 mm Hg despite paralysis, every conceivable ventilator adjustment, and 100% oxygen. Crossing that terrible threshold felt like drifting below hull‐crush depth in a submarine. I waited for the walls and windows of the ICU to groan with the strain as disaster neared.
My intern followed me to the waiting room where Betsy slept. She hadn't left the hospital in days. I knelt beside her cot and woke her, and she supported her pregnant abdomen with her hand as she rolled to face me. We smiled. Then she remembered where she was.
Is something wrong? she asked.
No, he's about the same. But the other things we tried didn't help. We need to do what I mentioned beforeturn him over so he can use his lungs better. She nodded. We're very careful, but he has so many IV lines right now. If he loses one, he could get much worse. So I wanted to make sure you spent some time with him now, just in case.
Her eyes teared. He could die?
Just a small chance. But possible.
And if it works, he might get better?
I paused. He's very sick.
There are other things you can do?
We have to really hope this works.
This isn't supposed to happen. I don't know if I can raise 2 children without Joe. I can't be a widow at 29. I sensed I could have talked hersleep deprived and stunnedback into sleep, into a conviction her nightmare would pass by morning. Instead I squeezed her hand and listened.
We need to do this, OK? You'll have 10 minutes to talk. Remember how his blood pressure rose when they cleaned him? He's still in there. I believe he can hear you. So you tell him to keep fighting.
Betsy wiped her eyes and searched for her shoes. As we walked briskly back to the unit, I composed myself and told my intern, I'll be 29 in 3 weeks.
Me too. What day?
May 28th.
Same as mine, he said.
It took 25 minutes to prone Joe with every nurse assisting, but the maneuver went well. His oxygenation improved, but his relentless decline resumed within hours. The following afternoon, Betsy held Joe's hand and told him it was OK for him to go, and that she would look after their children. Joe's blood pressure eventually dwindled to nothing, leaving only sinus tachycardia on the monitor and the rhythmic puffs of the ventilator. Then, within 2 weeks, the resident team managed a series of unexpected tragedies: we lost young mothers to acetaminophen overdose and lung cancer, and cared for 2 young adults with septic shock and a perimenopausal woman for whom the cost of pneumonia was her first and probably only pregnancy.
Five years before, when I first stepped into an ICU, I imagined the residents held a dozen lives in their hands and faced critical illness at all hoursalone. By the time Joe died of disseminated varicella, I realized the truth was far from that vision. Joe's nurse had worked in the ICU as long as I'd been alive, and expert respiratory therapists guided his mechanical ventilation. I had coresidents and consultantseven a rabbi when I guided a family meeting on declaring CPR not indicated. Our institution's overnight attending assisted me throughout the night, and the primary attending drove in at 2 AM to supervise nitric oxide therapy. At no point did I ever care for Joe alone.
Instead, the challenge lay in facing the winning smiles of our patient Joe and his 10 month‐old son Jacob waving from a recent photo taped by the head of his bed and a young wife refusing to leave her increasingly unrecognizable husband as his body failed, despite her conspicuous 7‐month pregnancy. And it lay in the surprising futility of all our interventions. Perhaps most of all, the challenge was in the persistence of the sights and sounds and smells of that night and many others. I've seen the expression a pathologist makes on learning his daughter has anaplastic thyroid cancer. I've heard the sound a daughter makes when her mother has a ventricular free‐wall rupture while welcoming us into her room. I've smelled a teenager who had burned to the bone while conscious yet pinned in his car. I've felt the crackle of subcutaneous emphysema after chest tubes for malignant pleural effusions that was so severe the patient could not open his eyes or close his hands. And the papery skin and tremulous handshake of a man after my news of his wife's prognosis promised their 64th year of marriage would be the last.
Far from alone, I spend much of my time in the company of these ghosts, as must many health care workers. How we make our peace with them is up to us. With tears? Humor? Alcohol? Sometimes it is by numb indifference; you might wonder from most of the businesslike discussions physicians hold if these ghosts even existed. Or, we can make our peace with words. I am grateful for a chance to speak with Betsy some days after Joe died to assure her that although we did ask Joe to fight, in the end no effort could have saved him. I am grateful she later wrote us to celebrate the healthy birth of their second son, Joshua. She assured me Joe would live on for her in their sons and live on for them through her memories. Her strength helped me welcome Joe's ghost, and many others, into my life.
After 5 years of clinical medicine, I finally understood the lesson I received from the pediatric ICU nurse. Our ghost stories help us grieve, and they celebrate healing, or if there was no healing, then release. At the very least, great tragedy reminds us of the great meaning of our calling.
On my first day as a nervous, third‐year medical student, a nurse offered to orient me to the pediatric ICU. I expected a litany of facts to memorize. Instead, she pointed at each room in turn and described the tragedies they had hosted.
Room 1: a little girl just died of meningitis there. Room 2: that boy's liver transplant failed, and he had a massive stroke. The father sat holding the jaundiced hand of his unresponsive son, whose stapled abdomen held back tense ascites. His wife died of cancer 2 months ago. Now he has no one. Room 3: teen with cystic fibrosis; she'll be OK. Room 4 I will never forget. A teenager died of leukemia there and refused all painkillers. He wanted to be lucid for his family, and they huddled on his bed and sang Amazing Grace until he died. Most beautiful thing I have seen.
I had thought, Beautiful? How can you even come to work?
Five years later, I remembered that conversation as if it had just happened. I was the senior resident in the medical ICU, it was 3 AM, and I was gathering my thoughts amid the whooshes, beeps, and flickering monitors of the sleeping unit. I was preparing to go tell Betsy that Joe, her 31‐year‐old husband, needed prone ventilation. Joe lay dying from, of all things, chickenpox. He was receiving 12 infusions, including 4 pressors, sedatives, antibiotics, acyclovir, full‐strength bicarbonate, his 26th amp of calcium, and liter number‐who‐knows‐what of saline. He sprouted 2 IVs, 2 central lines, a Foley catheter, endotracheal and orogastric tubes, an arterial line, and an array of monitor leads. His blood pressure would plummetfrom a systolic of 80whenever we interrupted his bicarb drip to spike a new bag, so we knew moving him might kill him. Every nurse raced to finish tasks on other patients, preparing to help.
Joe's admission began, like several of his earlier ones, with a chief complaint of Crohn's flare. This time, however, he had a new rash, and although John's ward team suspected medications were to blame, they soon started him on acyclovir. In days, hepatitis, acute renal failure, and pneumonia prompted his ICU transfer. He required intubation hours later. His course since had been like watching a pedestrian struck by a truck in slow motion: a sudden, jolting, irreversible crueltydrawn out over hours. Anasarca had folded his blistering ears in half and forced us to revise his endotracheal tube taping 3 times so it would not incise his cheeks. He had unremitting hypotension. His transaminases climbed above 6000 and his creatinine to 6; his arterial pH dropped to 7.03, and his platelets fell to 16,000. His partial pressure of oxygen sank below 60 mm Hg despite paralysis, every conceivable ventilator adjustment, and 100% oxygen. Crossing that terrible threshold felt like drifting below hull‐crush depth in a submarine. I waited for the walls and windows of the ICU to groan with the strain as disaster neared.
My intern followed me to the waiting room where Betsy slept. She hadn't left the hospital in days. I knelt beside her cot and woke her, and she supported her pregnant abdomen with her hand as she rolled to face me. We smiled. Then she remembered where she was.
Is something wrong? she asked.
No, he's about the same. But the other things we tried didn't help. We need to do what I mentioned beforeturn him over so he can use his lungs better. She nodded. We're very careful, but he has so many IV lines right now. If he loses one, he could get much worse. So I wanted to make sure you spent some time with him now, just in case.
Her eyes teared. He could die?
Just a small chance. But possible.
And if it works, he might get better?
I paused. He's very sick.
There are other things you can do?
We have to really hope this works.
This isn't supposed to happen. I don't know if I can raise 2 children without Joe. I can't be a widow at 29. I sensed I could have talked hersleep deprived and stunnedback into sleep, into a conviction her nightmare would pass by morning. Instead I squeezed her hand and listened.
We need to do this, OK? You'll have 10 minutes to talk. Remember how his blood pressure rose when they cleaned him? He's still in there. I believe he can hear you. So you tell him to keep fighting.
Betsy wiped her eyes and searched for her shoes. As we walked briskly back to the unit, I composed myself and told my intern, I'll be 29 in 3 weeks.
Me too. What day?
May 28th.
Same as mine, he said.
It took 25 minutes to prone Joe with every nurse assisting, but the maneuver went well. His oxygenation improved, but his relentless decline resumed within hours. The following afternoon, Betsy held Joe's hand and told him it was OK for him to go, and that she would look after their children. Joe's blood pressure eventually dwindled to nothing, leaving only sinus tachycardia on the monitor and the rhythmic puffs of the ventilator. Then, within 2 weeks, the resident team managed a series of unexpected tragedies: we lost young mothers to acetaminophen overdose and lung cancer, and cared for 2 young adults with septic shock and a perimenopausal woman for whom the cost of pneumonia was her first and probably only pregnancy.
Five years before, when I first stepped into an ICU, I imagined the residents held a dozen lives in their hands and faced critical illness at all hoursalone. By the time Joe died of disseminated varicella, I realized the truth was far from that vision. Joe's nurse had worked in the ICU as long as I'd been alive, and expert respiratory therapists guided his mechanical ventilation. I had coresidents and consultantseven a rabbi when I guided a family meeting on declaring CPR not indicated. Our institution's overnight attending assisted me throughout the night, and the primary attending drove in at 2 AM to supervise nitric oxide therapy. At no point did I ever care for Joe alone.
Instead, the challenge lay in facing the winning smiles of our patient Joe and his 10 month‐old son Jacob waving from a recent photo taped by the head of his bed and a young wife refusing to leave her increasingly unrecognizable husband as his body failed, despite her conspicuous 7‐month pregnancy. And it lay in the surprising futility of all our interventions. Perhaps most of all, the challenge was in the persistence of the sights and sounds and smells of that night and many others. I've seen the expression a pathologist makes on learning his daughter has anaplastic thyroid cancer. I've heard the sound a daughter makes when her mother has a ventricular free‐wall rupture while welcoming us into her room. I've smelled a teenager who had burned to the bone while conscious yet pinned in his car. I've felt the crackle of subcutaneous emphysema after chest tubes for malignant pleural effusions that was so severe the patient could not open his eyes or close his hands. And the papery skin and tremulous handshake of a man after my news of his wife's prognosis promised their 64th year of marriage would be the last.
Far from alone, I spend much of my time in the company of these ghosts, as must many health care workers. How we make our peace with them is up to us. With tears? Humor? Alcohol? Sometimes it is by numb indifference; you might wonder from most of the businesslike discussions physicians hold if these ghosts even existed. Or, we can make our peace with words. I am grateful for a chance to speak with Betsy some days after Joe died to assure her that although we did ask Joe to fight, in the end no effort could have saved him. I am grateful she later wrote us to celebrate the healthy birth of their second son, Joshua. She assured me Joe would live on for her in their sons and live on for them through her memories. Her strength helped me welcome Joe's ghost, and many others, into my life.
After 5 years of clinical medicine, I finally understood the lesson I received from the pediatric ICU nurse. Our ghost stories help us grieve, and they celebrate healing, or if there was no healing, then release. At the very least, great tragedy reminds us of the great meaning of our calling.
On my first day as a nervous, third‐year medical student, a nurse offered to orient me to the pediatric ICU. I expected a litany of facts to memorize. Instead, she pointed at each room in turn and described the tragedies they had hosted.
Room 1: a little girl just died of meningitis there. Room 2: that boy's liver transplant failed, and he had a massive stroke. The father sat holding the jaundiced hand of his unresponsive son, whose stapled abdomen held back tense ascites. His wife died of cancer 2 months ago. Now he has no one. Room 3: teen with cystic fibrosis; she'll be OK. Room 4 I will never forget. A teenager died of leukemia there and refused all painkillers. He wanted to be lucid for his family, and they huddled on his bed and sang Amazing Grace until he died. Most beautiful thing I have seen.
I had thought, Beautiful? How can you even come to work?
Five years later, I remembered that conversation as if it had just happened. I was the senior resident in the medical ICU, it was 3 AM, and I was gathering my thoughts amid the whooshes, beeps, and flickering monitors of the sleeping unit. I was preparing to go tell Betsy that Joe, her 31‐year‐old husband, needed prone ventilation. Joe lay dying from, of all things, chickenpox. He was receiving 12 infusions, including 4 pressors, sedatives, antibiotics, acyclovir, full‐strength bicarbonate, his 26th amp of calcium, and liter number‐who‐knows‐what of saline. He sprouted 2 IVs, 2 central lines, a Foley catheter, endotracheal and orogastric tubes, an arterial line, and an array of monitor leads. His blood pressure would plummetfrom a systolic of 80whenever we interrupted his bicarb drip to spike a new bag, so we knew moving him might kill him. Every nurse raced to finish tasks on other patients, preparing to help.
Joe's admission began, like several of his earlier ones, with a chief complaint of Crohn's flare. This time, however, he had a new rash, and although John's ward team suspected medications were to blame, they soon started him on acyclovir. In days, hepatitis, acute renal failure, and pneumonia prompted his ICU transfer. He required intubation hours later. His course since had been like watching a pedestrian struck by a truck in slow motion: a sudden, jolting, irreversible crueltydrawn out over hours. Anasarca had folded his blistering ears in half and forced us to revise his endotracheal tube taping 3 times so it would not incise his cheeks. He had unremitting hypotension. His transaminases climbed above 6000 and his creatinine to 6; his arterial pH dropped to 7.03, and his platelets fell to 16,000. His partial pressure of oxygen sank below 60 mm Hg despite paralysis, every conceivable ventilator adjustment, and 100% oxygen. Crossing that terrible threshold felt like drifting below hull‐crush depth in a submarine. I waited for the walls and windows of the ICU to groan with the strain as disaster neared.
My intern followed me to the waiting room where Betsy slept. She hadn't left the hospital in days. I knelt beside her cot and woke her, and she supported her pregnant abdomen with her hand as she rolled to face me. We smiled. Then she remembered where she was.
Is something wrong? she asked.
No, he's about the same. But the other things we tried didn't help. We need to do what I mentioned beforeturn him over so he can use his lungs better. She nodded. We're very careful, but he has so many IV lines right now. If he loses one, he could get much worse. So I wanted to make sure you spent some time with him now, just in case.
Her eyes teared. He could die?
Just a small chance. But possible.
And if it works, he might get better?
I paused. He's very sick.
There are other things you can do?
We have to really hope this works.
This isn't supposed to happen. I don't know if I can raise 2 children without Joe. I can't be a widow at 29. I sensed I could have talked hersleep deprived and stunnedback into sleep, into a conviction her nightmare would pass by morning. Instead I squeezed her hand and listened.
We need to do this, OK? You'll have 10 minutes to talk. Remember how his blood pressure rose when they cleaned him? He's still in there. I believe he can hear you. So you tell him to keep fighting.
Betsy wiped her eyes and searched for her shoes. As we walked briskly back to the unit, I composed myself and told my intern, I'll be 29 in 3 weeks.
Me too. What day?
May 28th.
Same as mine, he said.
It took 25 minutes to prone Joe with every nurse assisting, but the maneuver went well. His oxygenation improved, but his relentless decline resumed within hours. The following afternoon, Betsy held Joe's hand and told him it was OK for him to go, and that she would look after their children. Joe's blood pressure eventually dwindled to nothing, leaving only sinus tachycardia on the monitor and the rhythmic puffs of the ventilator. Then, within 2 weeks, the resident team managed a series of unexpected tragedies: we lost young mothers to acetaminophen overdose and lung cancer, and cared for 2 young adults with septic shock and a perimenopausal woman for whom the cost of pneumonia was her first and probably only pregnancy.
Five years before, when I first stepped into an ICU, I imagined the residents held a dozen lives in their hands and faced critical illness at all hoursalone. By the time Joe died of disseminated varicella, I realized the truth was far from that vision. Joe's nurse had worked in the ICU as long as I'd been alive, and expert respiratory therapists guided his mechanical ventilation. I had coresidents and consultantseven a rabbi when I guided a family meeting on declaring CPR not indicated. Our institution's overnight attending assisted me throughout the night, and the primary attending drove in at 2 AM to supervise nitric oxide therapy. At no point did I ever care for Joe alone.
Instead, the challenge lay in facing the winning smiles of our patient Joe and his 10 month‐old son Jacob waving from a recent photo taped by the head of his bed and a young wife refusing to leave her increasingly unrecognizable husband as his body failed, despite her conspicuous 7‐month pregnancy. And it lay in the surprising futility of all our interventions. Perhaps most of all, the challenge was in the persistence of the sights and sounds and smells of that night and many others. I've seen the expression a pathologist makes on learning his daughter has anaplastic thyroid cancer. I've heard the sound a daughter makes when her mother has a ventricular free‐wall rupture while welcoming us into her room. I've smelled a teenager who had burned to the bone while conscious yet pinned in his car. I've felt the crackle of subcutaneous emphysema after chest tubes for malignant pleural effusions that was so severe the patient could not open his eyes or close his hands. And the papery skin and tremulous handshake of a man after my news of his wife's prognosis promised their 64th year of marriage would be the last.
Far from alone, I spend much of my time in the company of these ghosts, as must many health care workers. How we make our peace with them is up to us. With tears? Humor? Alcohol? Sometimes it is by numb indifference; you might wonder from most of the businesslike discussions physicians hold if these ghosts even existed. Or, we can make our peace with words. I am grateful for a chance to speak with Betsy some days after Joe died to assure her that although we did ask Joe to fight, in the end no effort could have saved him. I am grateful she later wrote us to celebrate the healthy birth of their second son, Joshua. She assured me Joe would live on for her in their sons and live on for them through her memories. Her strength helped me welcome Joe's ghost, and many others, into my life.
After 5 years of clinical medicine, I finally understood the lesson I received from the pediatric ICU nurse. Our ghost stories help us grieve, and they celebrate healing, or if there was no healing, then release. At the very least, great tragedy reminds us of the great meaning of our calling.