User login
Antidepressants: The spectrum beyond depression
A molecule is a molecule is a molecule—until it becomes identified with a purpose. Consider, for example, (-)-trans-4R-(4’-fluorophenyl)-3S-[(3’,4’-methylenedioxyphenoxy) methyl] piperidine. You probably know this molecule as paroxetine—an antidepressant, of course, but it is more than that. If you examine paroxetine’s FDA-approved indications, it also has anti-panic, anti-social anxiety, anti-obsessive-compulsive disorder, anti-posttraumatic stress disorder, and anti-premenstrual dysphoric disorder effects.
“Antidepressants” have achieved fame as antidepressants; one could say these molecules’ search for meaning has been fulfilled. Yet even within psychiatry, their many other uses (Table) can create semantic misunderstandings. Beyond psychiatry, consider the nondepressed patient with neurocardiogenic syncope who wonders why he’s being treated with an antidepressant.
Rather than calling antidepressants “panaceas,” the better choice is to educate patients about the drugs’ wide spectrum of activity. Let’s look broadly across the so-called antidepressants and examine their varied uses in psychiatry and other medical specialties.
Table
FDA-approved psychiatric indications for serotonin uptake inhibitors*
| SSRIs | ||||||||
| Citalopram | X | |||||||
| Escitalopram | X | X | ||||||
| Fluoxetine | X | X | X | X | X | |||
| Fluvoxamine | X | |||||||
| Paroxetine | X | X | X | X | X | X | X | |
| Sertraline | X | X | X | X | X | X | ||
| SNRIs | ||||||||
| Duloxetine | X | X | ||||||
| Venlafaxine | X | X | X | X | ||||
| SSRIs: selective serotonin reuptake inhibitors; SNRIs: serotonin-norepinephrine reuptake inhibitors; MDD: major depressive disorder; PD: panic disorder; SAD: social anxiety disorder; PTSD: posttraumatic stress disorder; GAD: generalized anxiety disorder; OCD: obsessive-compulsive disorder; PMDD: premenstrual dysphoric disorder; BUL: bulimia | ||||||||
| * The absence of an X does not necessarily imply that a drug is ineffective for a given indication but, more likely, that definitive studies are lacking. | ||||||||
Pain syndromes
Peripheral neuropathy. The only antidepressant with an FDA-approved pain indication is duloxetine, a serotonin-norepinephrine reuptake inhibitor (SNRI). Its approval for diabetic peripheral neuropathic pain (DPNP) was based on two 12-week, randomized, double-blind, placebo-controlled studies using fixed doses of 60 mg once or twice daily.1,2 Another SNRI—venlafaxine XR, 150 to 225 mg/d, but not 75 mg/d—also was found to be more effective than placebo for this indication in a 6-week, double-blind study (Box 1).3
Using antidepressants to treat pain syndromes is neither new nor restricted to SNRIs, however. In combined double-blind, cross-over studies of patients with DPNP, Max et al4 found:
- moderate or greater pain relief in 74% and 61% of subjects, respectively, from the tricyclics amitriptyline, mean 105 mg/d, and desipramine, mean 111 mg/d—with pain reduced by equal amounts in depressed and nondepressed patients
- no statistically significant difference in pain relief between the selective serotonin reuptake inhibitor (SSRI) fluoxetine, 40 mg/d, and placebo.
- tricyclics: 1 in every 2 to 3 patients
- SNRIs: 1 in every 4 to 5 patients
- SSRIs: 1 in every 7 patients.
Chronic headache. A meta-analysis7 of randomized, placebo-controlled studies found antidepressants more effective than placebo for chronic migraine and tension headache prophylaxis. Although a subgroup meta-analysis found similar effects for tricyclics and SSRIs, the authors characterized the tricyclics’ results as well established and the SSRIs’ as “less certain.”
The results of this meta-analysis might not accurately reflect bona fide antidepressants, however. Some of the 38 studies (25 of migraine, 12 of tension headache, 1 of both) included treatment with serotonin antagonists—most commonly pizotifen, which is not available in the United States and does not appear to be an antidepressant.
Back pain. Patients with chronic low back pain (average 10 years) seem to benefit from antidepressants, according to a meta-analysis of 9 randomized, controlled trials by Salerno et al.8 The effect on pain in the total 504 patients was “small but significant,” and improvement in function was “small but nonsignificant.” Individual sample sizes also were small, however, and only 2 studies excluded depressed patients.
Fibromyalgia, with chronic generalized musculoskeletal pain and tenderness, has been a focus of antidepressant drug therapy. Goldenberg et al9 concluded from an ambitious literature review (505 articles) that evidence of efficacy was strong for amitripty-line and modest for SSRIs and SNRIs.
Overall, antidepressants are generally understood to have analgesic effects in the absence of depression. Benefits for patients with pain syndromes are well established for tricyclics (especially amitriptyline) and recently with SNRIs, whereas SSRIs are less effective.
Serotonin and norepinephrine are involved in pain modulation via descending inhibitory pathways in the brain and spinal cord. Serotonin-norepinephrine reuptake inhibitors (SNRIs) have been shown to reduce the severity of diabetic peripheral neuropathic pain (DPNP) in randomized controlled trials.
Duloxetine. In 2 double-blind studies,1,2 nondepressed patients with DPNP received duloxetine, 60 mg once daily; duloxetine, 60 mg bid; or placebo for 12 weeks. They rated the severity of neuropathic pain every 24 hours on an 11-point Likert scale, and weekly mean scores were the primary outcome measure. Average pain scores improved more in both duloxetine groups vs placebo. Duloxetine treatment did not interfere with diabetic control, and both dosages were well tolerated.
The FDA approved an added indication for duloxetine in the management of DPNP.
Venlafaxine. In a double-blind study,3 244 adult outpatients with moderately severe DPNP received venlafaxine ER, 75 or 150 to 225 mg/d, or placebo for 6 weeks. Daily scores on the Visual Analog Pain Intensity (VAS-PI) and Pain Relief (VAS-PR) scales were primary efficacy measures.
Patients receiving the higher venlafaxine dosage—but not 75 mg/d—showed statistically significant less-intensive pain vs placebo. VAS-PI scores were 27% lower than at enrollment with placebo, 32% lower with venlafaxine, 75 mg/d, and 50% lower with venlafaxine, 150 to 225 mg/d (P
Nausea and somnolence were the most common side effects; clinically important ECG changes occurred in 7 patients treated with venlafaxine, 150 to 225 mg/d.
Smoking cessation
Bupropion SR is FDA-approved to aid smoking cessation, and this effect is independent of the drug’s antidepressant activity. Bupropion may act as a nicotine receptor antagonist as well as a norepinephrine dopamine reuptake inhibitor.
Other antidepressants have been studied for smoking cessation, with nortriptyline showing benefit in 2 large placebo-controlled trials. Studies with doxepin, fluoxetine, and moclobemide found little or no benefit for this indication.
Cardiovascular uses
Angina. Monoamine oxidase inhibitor (MAOI) antidepressants were used to treat angina pectoris in the late 1950s and early 1960s. This practice stopped after evidence showed that whereas angina pain may have improved with MAOIs, stress-induced ischemia on ECG did not.
Antiarrhythmia. Tricyclics had a brief fling in cardiovascular therapeutics when their quinidine-like class I antiarrhythmic activity was recognized. Imipramine was one of several drugs included in the Cardiac Arrhythmia Pilot Study in the 1980s that involved 502 postmyocardial infarction patients with ventricular arrhythmias. Imipramine was the least effective of the 4 drugs studied and the least well tolerated.13
variety of medications. Options include the vasopressor midodrine, fludrocortisone, beta blockers, and SSRIs— none
FDA-approved for this indication. Paroxetine, 20 mg/d, was considerably more effective than placebo in preventing
recurrent syncope in 68 patients who had been unresponsive
to or intolerant of traditional medications. During a mean 25 months of treatment, 82% of patients remained syncope-free on paroxetine vs 47% on placebo.14
Selective serotonin reuptake inhibitors (SSRIs) often are used to treat irritable bowel syndrome (IBS), though evidence of their effectiveness is scarce. SSRIs can improve IBS patients’ quality of life, but effects on abdominal pain and bloating are less clear.
Paroxetine. In a randomized, double-blind trial,16 gastroenterologists tested a highfiber diet plus paroxetine in nondepressed patients with IBS. Ninety-eight patients ages 18 to 65 who experienced IBS symptoms on low- or average-fiber diets were first put on high-fiber diets and assessed for well-being and abdominal pain and bloating. Of these, 81 symptomatic patients continued highfiber diets with added paroxetine, 10 to 40 mg/d (n=38) or placebo (n=43).
With paroxetine, patients’ overall well-being improved more than with placebo, but abdominal pain and bloating and social functioning did not.
Fluoxetine. In a double-blind, randomized trial,17 44 patients with pain and constipation-predominant IBS received fluoxetine, 20 mg/d, or placebo for 12 weeks. These patients met Rome II criteria for IBS—abdominal discomfort/pain for ≥12 weeks in past year that met 2 of 3 criteria:
- relieved by defecation
- onset associated with change in stool frequency
- onset associated with change in stool appearance.
Patients receiving fluoxetine had less abdominal discomfort, less bloating, more frequent bowel movements, and decreased consistency of stool vs placebo 4 weeks after treatment stopped. Mean number of symptoms per patient decreased from 4.6 to 0.7 in the fluoxetine group vs 4.5 to 2.9 in controls (P
Citalopram. IBS symptom severity was the primary outcome in a crossover trial comparing citalopram (20 mg for 3 weeks and 40 mg for 3 weeks) with placebo in 23 nondepressed patients.18 Abdominal pain and bloating, impact of symptoms on daily life, and overall well-being improved significantly more with citalopram than with placebo after 3 and 6 weeks.
Symptom improvements were not related to changes in depression, anxiety, or colonic sensorimotor function.
Gastrointestinal
Peptic ulcer disease was shown in the 1980s to respond to tricyclic antidepressants. At the time, both anticholinergic and antihistaminic effects were thought to be responsible, but the later observation that trimipramine inhibited Campylobacter pylori in vitro suggested an additional explanation. Today, tricyclics are only of historic interest as treatments for peptic ulcer.
Irritable bowel syndrome (IBS) patients have responded favorably to antidepressants, although it is often difficult to know if the benefit is independent of improved coexisting anxiety or depression. A meta-analysis of 12 randomized, placebo-controlled trials—mostly with tricyclics—found an odds ratio for improvement of 4.2 and a number needed to treat of 3.2.15
More recently, a few placebo-controlled studies have shown SSRIs to be beneficial for IBS,16-18 although not all symptoms improved and some IBS subtypes might be more responsive than others (Box 2). In an editorial, Talley19 concluded that antidepressant therapy of IBS was “at best only a ‘band-aid’ approach to management.”
Genitourinary
Nocturnal enuresis. In the 1960s, imipramine was shown—in some but not all placebo-controlled studies—to be beneficial for nocturnal enuresis in children and adults. Although imipramine is not FDA-approved for this indication, it is thought to work by relaxing bladder muscle and contracting bladder neck smooth muscle. Imipramine appears to have a vasopressin-independent antidiuretic effect in enuretic patients with nocturnal polyuria.
Duloxetine is thought to improve stress urinary incontinence by increasing urethral sphincter tone and the force of sphincter contraction. This indication is not FDA- approved for duloxetine but is approved in the European Union.
Oncology
At one time antidepressants were suggested to promote tumors, based on observations that amitriptyline, fluoxetine, and several antihistamines promoted tumor growth in rodents.21 In 1995, a few case reports associated these 2 antidepressants with atypical cutaneous lymphoid infiltrates.22 A review by Sternback in 200323 concluded that a link between antidepressants and cancer was questionable but acknowledged the need for very long-term studies.
Recently, a nested case-control study found an association between high-dose SSRI use for ≤5 years and reduced risk of colorectal cancer, whereas no association was found with tricyclic use.24 A study of this design does not establish a causal relationship, how-ever, and one can only speculate whether SSRIs might have direct cytotoxic or anti-promoter effects.
At present, it seems reasonable to continue to treat depressed cancer patients with antidepressants without concern that cancer will worsen or hope that it will improve as a result.
Immunology
The pathogenesis of depression may be linked to pro-inflammatory cytokines—proteins such as tumor necrosis factor-alpha (TNF-α) and certain interleukins that mediate immune function. Bupropion markedly lowered pro-inflammatory cytokine levels in a mouse inflammation model, prompting the authors to suggest that this anti-inflammatory effect be explored in humans.25
Case reports have suggested benefit from bupropion in Crohn’s disease, recurrent aphthous ulcerations, psoriasis, atopic dermatitis, and Blau syndrome (a rare autosomal-dominant trait characterized by granulomatous arthritis, iritis, and skin rash). Whether this antidepressant has much anti-inflammatory potential remains to be determined, however.
Delayed ejaculation is among the sexual side effects commonly associated with antidepressant medication. In a 6-week trial,27 3 selective serotonin reuptake inhibitors (SSRIs)— paroxetine, fluoxetine, and sertraline— were shown to improve intravaginal ejaculatory latency time (IELT) in men with lifelong rapid ejaculation. Compared with baseline, the greatest delay in ejaculation was seen with paroxetine, 20 mg/d, followed by fluoxetine, 20 mg/d, and then sertraline, 50 mg/d, whereas delay with fluvoxamine, 100 mg/d, did not differ significantly from placebo.
Dapoxetine is a non-antidepressant SSRI under investigation for on-demand treatment of moderate-to-severe premature ejaculation.28 In two 12-week, randomized, double-blind, placebo-controlled trials, 870 men took placebo, 874 took 30-mg dapoxetine, and 870 took 60-mg dapoxetine 1 to 3 hours before sexual activity. Efficacy was determined by IELT measured at home by stopwatch.
Both dapoxetine doses improved IELT significantly more than placebo (P
Nausea, diarrhea, headache, and dizziness occurred in ≤20% of patients and were more common with the 60-mg than 30-mg dapoxetine dose.
Infectious disease
Pathogenic protozoa—such as Trypanosoma cruzi (Chagas disease), Leishmania donovani (Kala-azar), Leishmania major (Oriental sore), and Giardia lamblia (Giardiasis)—infect millions of humans worldwide. Clomipramine has been shown in vitro and in mice to inhibit or kill these protozoa, but these potential benefits have not been extended to humans.
Sertraline, on the other hand, might exert antifungal activity. Three patients with recurrent vulvovaginal candidiasis had no episodes while being treated with sertraline for premenstrual dysphoric disorder but relapsed when the drug was discontinued.26 Although sertraline demonstrated antifungal activity in vitro against several Candida species, this SSRI seems unlikely to gain prominence as an antifungal agent.
Sexual function
Premature ejaculation. SSRIs are well-known causes of delayed or absent orgasm, but a perceived liability can become an asset in treating premature ejaculation. By measuring intravaginal ejaculation latency time under double-blind, placebo-controlled conditions, Waldinger et al27 showed pronounced delay in ejaculation with sertraline, fluoxetine, and paroxetine in men with long-standing rapid ejaculation. Dapoxetine—a short-acting non-antidepressant SSRI—is being studied as a treatment for this condition (Box 3).28
Spermicidal effect. SSRIs—including fluoxetine— have demonstrated in vitro spermicidal and antitrichomonas activity29 but are unlikely to be developed as microbicidal contraceptives.
Related Resources
- Gorman JM, Kent JM. SSRIs and SMRIs: broad spectrum of efficacy beyond major depression. J Clin Psychiatry 1999;60(suppl 4):33-8.
- About.com: Mental Health. Antidepressants for more than depression. http://mentalhealth.about.com/cs/psychopharmacology/a/antimore.htm.
- Amitriptyline • Elavil, Endep
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin, Pertofrane
- Doxepin • Adapin, Sinequan
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fludrocortisone • Florinef
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Midodrine • ProAmitine
- Nortriptyline • Pamelor, Aventyl
- Paroxetine • Paxil
- Phenelzine • Nardil
- Sertraline • Zoloft
- Trimipramine • Surmontil
- Venlafaxine • Effexor
Dr. Jefferson receives research support from Bristol-Myers Squibb, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Eli Lilly and Company, Novartis, Pfizer, Roche, Solvay, UCB Pharma, and Wyeth. He is a consultant to GlaxoSmithKline, Schwarz Pharma, Shire, and Organon and a speaker for Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Forest Pharmaceuticals, GlaxoSmithKline, Eli Lilly and Company, Pfizer, Schwarz Pharma, Shire, and Wyeth. He holds stock in Bristol-Myers Squibb, GlaxoSmithKline, and SciClone.
1. Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006;67(8):1411-20.
2. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med 2005;6(5):346-56.
3. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain 2004;110:697-706.
4. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992;326:1250-6.
5. Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 2005;96:399-409.
6. Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology 2001;57:1583-8.
7. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001;111:54-63.
8. Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain. Arch Intern Med 2002;162:19-24.
9. Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA 2004;292:2388-95.
10. Littlejohn GO, Guymer EK. Fibromyalgia syndrome: which antidepressant drug should we choose. Curr Pharm Des 2006;12(1):3-9.
11. Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum 2004;50:2974-84.
12. Arnold LM, Rosen A, Pritchett YL, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 2005;119:5-15.
13. Effects of encainide, flecainide, imipramine and moricizine on ventricular arrhythmias during the year after acute myocardial infarction: The CAPS. The Cardiac Arrhythmia Pilot Study (CAPS) Investigators. Am J Cardiol 1988;61(8):501-9.
14. Di Girolamo E, Di Iorio C, Sabatini P, et al. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 1999;33:1227-30.
15. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000;108:65-72.
16. Tabas G, Beaves M, Wang J, et al. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol 2004;99(5):914-20.
17. Vahedi H, Merat S, Rashidioon A, et al. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Aliment Pharmacol Ther 2005;22:381-5.
18. Tack J, Broekaert D, Fischler B, et al. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55:1095-103.
19. Talley NJ. Antidepressants in IBS: are we deluding ourselves? [editorial]. Am J Gastroenterol 2004;99:921-3.
20. Mariappan P, Ballantyne Z, N’Dow JM, Alhasso AA. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database Syst Rev 2005;Jul 20;(3):CD004742.-
21. Brandes LJ, Arron RJ, Bogdanovic RP, et al. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res 1992;52:3796-800.
22. Crowson AN, Magro CM. Antidepressant therapy. Arch Dermatol 1995;131:925-9.
23. Sternbach H. Are antidepressants carcinogenic? A review of preclinical and clinical studies. J Clin Psychiatry 2003;64:1153-62.
24. Xu W, Tamim H, Shapiro S, et al. Use of antidepressants and risk of colorectal cancer: a nested case-control study. Lancet Oncol 2006;7:301-8.
25. Brustolim D, Ribeiro-dos-Santos R, Kast RE, et al. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol 2006;6:903-7.
26. Lass-Flörl C, Dierich MP, Fuchs D, et al. Antifungal activity against Candida species of the selective serotonin-reuptake inhibitor, sertraline. Clin Infect Dis 2001;33:e135-6.
27. Waldinger MD, Hengeveld MW, Zsinderman AH, Olivier B. Effect of SSRI antidepressants on ejaculation: a double-blind, randomized, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine, and sertraline. J Clin Psychopharmacol 1998;18(4):274-81.
28. Pryor JL, Althof SE, Steidle C, et al. Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials. Lancet 2006;368:929-37.
29. Kumar VS, Sharma VL, Tiwari P, et al. The spermicidal and antitrichomonas activities of SSRI antidepressants. Bioorg Med Chem Lett 2006;16:2509-12.
A molecule is a molecule is a molecule—until it becomes identified with a purpose. Consider, for example, (-)-trans-4R-(4’-fluorophenyl)-3S-[(3’,4’-methylenedioxyphenoxy) methyl] piperidine. You probably know this molecule as paroxetine—an antidepressant, of course, but it is more than that. If you examine paroxetine’s FDA-approved indications, it also has anti-panic, anti-social anxiety, anti-obsessive-compulsive disorder, anti-posttraumatic stress disorder, and anti-premenstrual dysphoric disorder effects.
“Antidepressants” have achieved fame as antidepressants; one could say these molecules’ search for meaning has been fulfilled. Yet even within psychiatry, their many other uses (Table) can create semantic misunderstandings. Beyond psychiatry, consider the nondepressed patient with neurocardiogenic syncope who wonders why he’s being treated with an antidepressant.
Rather than calling antidepressants “panaceas,” the better choice is to educate patients about the drugs’ wide spectrum of activity. Let’s look broadly across the so-called antidepressants and examine their varied uses in psychiatry and other medical specialties.
Table
FDA-approved psychiatric indications for serotonin uptake inhibitors*
| SSRIs | ||||||||
| Citalopram | X | |||||||
| Escitalopram | X | X | ||||||
| Fluoxetine | X | X | X | X | X | |||
| Fluvoxamine | X | |||||||
| Paroxetine | X | X | X | X | X | X | X | |
| Sertraline | X | X | X | X | X | X | ||
| SNRIs | ||||||||
| Duloxetine | X | X | ||||||
| Venlafaxine | X | X | X | X | ||||
| SSRIs: selective serotonin reuptake inhibitors; SNRIs: serotonin-norepinephrine reuptake inhibitors; MDD: major depressive disorder; PD: panic disorder; SAD: social anxiety disorder; PTSD: posttraumatic stress disorder; GAD: generalized anxiety disorder; OCD: obsessive-compulsive disorder; PMDD: premenstrual dysphoric disorder; BUL: bulimia | ||||||||
| * The absence of an X does not necessarily imply that a drug is ineffective for a given indication but, more likely, that definitive studies are lacking. | ||||||||
Pain syndromes
Peripheral neuropathy. The only antidepressant with an FDA-approved pain indication is duloxetine, a serotonin-norepinephrine reuptake inhibitor (SNRI). Its approval for diabetic peripheral neuropathic pain (DPNP) was based on two 12-week, randomized, double-blind, placebo-controlled studies using fixed doses of 60 mg once or twice daily.1,2 Another SNRI—venlafaxine XR, 150 to 225 mg/d, but not 75 mg/d—also was found to be more effective than placebo for this indication in a 6-week, double-blind study (Box 1).3
Using antidepressants to treat pain syndromes is neither new nor restricted to SNRIs, however. In combined double-blind, cross-over studies of patients with DPNP, Max et al4 found:
- moderate or greater pain relief in 74% and 61% of subjects, respectively, from the tricyclics amitriptyline, mean 105 mg/d, and desipramine, mean 111 mg/d—with pain reduced by equal amounts in depressed and nondepressed patients
- no statistically significant difference in pain relief between the selective serotonin reuptake inhibitor (SSRI) fluoxetine, 40 mg/d, and placebo.
- tricyclics: 1 in every 2 to 3 patients
- SNRIs: 1 in every 4 to 5 patients
- SSRIs: 1 in every 7 patients.
Chronic headache. A meta-analysis7 of randomized, placebo-controlled studies found antidepressants more effective than placebo for chronic migraine and tension headache prophylaxis. Although a subgroup meta-analysis found similar effects for tricyclics and SSRIs, the authors characterized the tricyclics’ results as well established and the SSRIs’ as “less certain.”
The results of this meta-analysis might not accurately reflect bona fide antidepressants, however. Some of the 38 studies (25 of migraine, 12 of tension headache, 1 of both) included treatment with serotonin antagonists—most commonly pizotifen, which is not available in the United States and does not appear to be an antidepressant.
Back pain. Patients with chronic low back pain (average 10 years) seem to benefit from antidepressants, according to a meta-analysis of 9 randomized, controlled trials by Salerno et al.8 The effect on pain in the total 504 patients was “small but significant,” and improvement in function was “small but nonsignificant.” Individual sample sizes also were small, however, and only 2 studies excluded depressed patients.
Fibromyalgia, with chronic generalized musculoskeletal pain and tenderness, has been a focus of antidepressant drug therapy. Goldenberg et al9 concluded from an ambitious literature review (505 articles) that evidence of efficacy was strong for amitripty-line and modest for SSRIs and SNRIs.
Overall, antidepressants are generally understood to have analgesic effects in the absence of depression. Benefits for patients with pain syndromes are well established for tricyclics (especially amitriptyline) and recently with SNRIs, whereas SSRIs are less effective.
Serotonin and norepinephrine are involved in pain modulation via descending inhibitory pathways in the brain and spinal cord. Serotonin-norepinephrine reuptake inhibitors (SNRIs) have been shown to reduce the severity of diabetic peripheral neuropathic pain (DPNP) in randomized controlled trials.
Duloxetine. In 2 double-blind studies,1,2 nondepressed patients with DPNP received duloxetine, 60 mg once daily; duloxetine, 60 mg bid; or placebo for 12 weeks. They rated the severity of neuropathic pain every 24 hours on an 11-point Likert scale, and weekly mean scores were the primary outcome measure. Average pain scores improved more in both duloxetine groups vs placebo. Duloxetine treatment did not interfere with diabetic control, and both dosages were well tolerated.
The FDA approved an added indication for duloxetine in the management of DPNP.
Venlafaxine. In a double-blind study,3 244 adult outpatients with moderately severe DPNP received venlafaxine ER, 75 or 150 to 225 mg/d, or placebo for 6 weeks. Daily scores on the Visual Analog Pain Intensity (VAS-PI) and Pain Relief (VAS-PR) scales were primary efficacy measures.
Patients receiving the higher venlafaxine dosage—but not 75 mg/d—showed statistically significant less-intensive pain vs placebo. VAS-PI scores were 27% lower than at enrollment with placebo, 32% lower with venlafaxine, 75 mg/d, and 50% lower with venlafaxine, 150 to 225 mg/d (P
Nausea and somnolence were the most common side effects; clinically important ECG changes occurred in 7 patients treated with venlafaxine, 150 to 225 mg/d.
Smoking cessation
Bupropion SR is FDA-approved to aid smoking cessation, and this effect is independent of the drug’s antidepressant activity. Bupropion may act as a nicotine receptor antagonist as well as a norepinephrine dopamine reuptake inhibitor.
Other antidepressants have been studied for smoking cessation, with nortriptyline showing benefit in 2 large placebo-controlled trials. Studies with doxepin, fluoxetine, and moclobemide found little or no benefit for this indication.
Cardiovascular uses
Angina. Monoamine oxidase inhibitor (MAOI) antidepressants were used to treat angina pectoris in the late 1950s and early 1960s. This practice stopped after evidence showed that whereas angina pain may have improved with MAOIs, stress-induced ischemia on ECG did not.
Antiarrhythmia. Tricyclics had a brief fling in cardiovascular therapeutics when their quinidine-like class I antiarrhythmic activity was recognized. Imipramine was one of several drugs included in the Cardiac Arrhythmia Pilot Study in the 1980s that involved 502 postmyocardial infarction patients with ventricular arrhythmias. Imipramine was the least effective of the 4 drugs studied and the least well tolerated.13
variety of medications. Options include the vasopressor midodrine, fludrocortisone, beta blockers, and SSRIs— none
FDA-approved for this indication. Paroxetine, 20 mg/d, was considerably more effective than placebo in preventing
recurrent syncope in 68 patients who had been unresponsive
to or intolerant of traditional medications. During a mean 25 months of treatment, 82% of patients remained syncope-free on paroxetine vs 47% on placebo.14
Selective serotonin reuptake inhibitors (SSRIs) often are used to treat irritable bowel syndrome (IBS), though evidence of their effectiveness is scarce. SSRIs can improve IBS patients’ quality of life, but effects on abdominal pain and bloating are less clear.
Paroxetine. In a randomized, double-blind trial,16 gastroenterologists tested a highfiber diet plus paroxetine in nondepressed patients with IBS. Ninety-eight patients ages 18 to 65 who experienced IBS symptoms on low- or average-fiber diets were first put on high-fiber diets and assessed for well-being and abdominal pain and bloating. Of these, 81 symptomatic patients continued highfiber diets with added paroxetine, 10 to 40 mg/d (n=38) or placebo (n=43).
With paroxetine, patients’ overall well-being improved more than with placebo, but abdominal pain and bloating and social functioning did not.
Fluoxetine. In a double-blind, randomized trial,17 44 patients with pain and constipation-predominant IBS received fluoxetine, 20 mg/d, or placebo for 12 weeks. These patients met Rome II criteria for IBS—abdominal discomfort/pain for ≥12 weeks in past year that met 2 of 3 criteria:
- relieved by defecation
- onset associated with change in stool frequency
- onset associated with change in stool appearance.
Patients receiving fluoxetine had less abdominal discomfort, less bloating, more frequent bowel movements, and decreased consistency of stool vs placebo 4 weeks after treatment stopped. Mean number of symptoms per patient decreased from 4.6 to 0.7 in the fluoxetine group vs 4.5 to 2.9 in controls (P
Citalopram. IBS symptom severity was the primary outcome in a crossover trial comparing citalopram (20 mg for 3 weeks and 40 mg for 3 weeks) with placebo in 23 nondepressed patients.18 Abdominal pain and bloating, impact of symptoms on daily life, and overall well-being improved significantly more with citalopram than with placebo after 3 and 6 weeks.
Symptom improvements were not related to changes in depression, anxiety, or colonic sensorimotor function.
Gastrointestinal
Peptic ulcer disease was shown in the 1980s to respond to tricyclic antidepressants. At the time, both anticholinergic and antihistaminic effects were thought to be responsible, but the later observation that trimipramine inhibited Campylobacter pylori in vitro suggested an additional explanation. Today, tricyclics are only of historic interest as treatments for peptic ulcer.
Irritable bowel syndrome (IBS) patients have responded favorably to antidepressants, although it is often difficult to know if the benefit is independent of improved coexisting anxiety or depression. A meta-analysis of 12 randomized, placebo-controlled trials—mostly with tricyclics—found an odds ratio for improvement of 4.2 and a number needed to treat of 3.2.15
More recently, a few placebo-controlled studies have shown SSRIs to be beneficial for IBS,16-18 although not all symptoms improved and some IBS subtypes might be more responsive than others (Box 2). In an editorial, Talley19 concluded that antidepressant therapy of IBS was “at best only a ‘band-aid’ approach to management.”
Genitourinary
Nocturnal enuresis. In the 1960s, imipramine was shown—in some but not all placebo-controlled studies—to be beneficial for nocturnal enuresis in children and adults. Although imipramine is not FDA-approved for this indication, it is thought to work by relaxing bladder muscle and contracting bladder neck smooth muscle. Imipramine appears to have a vasopressin-independent antidiuretic effect in enuretic patients with nocturnal polyuria.
Duloxetine is thought to improve stress urinary incontinence by increasing urethral sphincter tone and the force of sphincter contraction. This indication is not FDA- approved for duloxetine but is approved in the European Union.
Oncology
At one time antidepressants were suggested to promote tumors, based on observations that amitriptyline, fluoxetine, and several antihistamines promoted tumor growth in rodents.21 In 1995, a few case reports associated these 2 antidepressants with atypical cutaneous lymphoid infiltrates.22 A review by Sternback in 200323 concluded that a link between antidepressants and cancer was questionable but acknowledged the need for very long-term studies.
Recently, a nested case-control study found an association between high-dose SSRI use for ≤5 years and reduced risk of colorectal cancer, whereas no association was found with tricyclic use.24 A study of this design does not establish a causal relationship, how-ever, and one can only speculate whether SSRIs might have direct cytotoxic or anti-promoter effects.
At present, it seems reasonable to continue to treat depressed cancer patients with antidepressants without concern that cancer will worsen or hope that it will improve as a result.
Immunology
The pathogenesis of depression may be linked to pro-inflammatory cytokines—proteins such as tumor necrosis factor-alpha (TNF-α) and certain interleukins that mediate immune function. Bupropion markedly lowered pro-inflammatory cytokine levels in a mouse inflammation model, prompting the authors to suggest that this anti-inflammatory effect be explored in humans.25
Case reports have suggested benefit from bupropion in Crohn’s disease, recurrent aphthous ulcerations, psoriasis, atopic dermatitis, and Blau syndrome (a rare autosomal-dominant trait characterized by granulomatous arthritis, iritis, and skin rash). Whether this antidepressant has much anti-inflammatory potential remains to be determined, however.
Delayed ejaculation is among the sexual side effects commonly associated with antidepressant medication. In a 6-week trial,27 3 selective serotonin reuptake inhibitors (SSRIs)— paroxetine, fluoxetine, and sertraline— were shown to improve intravaginal ejaculatory latency time (IELT) in men with lifelong rapid ejaculation. Compared with baseline, the greatest delay in ejaculation was seen with paroxetine, 20 mg/d, followed by fluoxetine, 20 mg/d, and then sertraline, 50 mg/d, whereas delay with fluvoxamine, 100 mg/d, did not differ significantly from placebo.
Dapoxetine is a non-antidepressant SSRI under investigation for on-demand treatment of moderate-to-severe premature ejaculation.28 In two 12-week, randomized, double-blind, placebo-controlled trials, 870 men took placebo, 874 took 30-mg dapoxetine, and 870 took 60-mg dapoxetine 1 to 3 hours before sexual activity. Efficacy was determined by IELT measured at home by stopwatch.
Both dapoxetine doses improved IELT significantly more than placebo (P
Nausea, diarrhea, headache, and dizziness occurred in ≤20% of patients and were more common with the 60-mg than 30-mg dapoxetine dose.
Infectious disease
Pathogenic protozoa—such as Trypanosoma cruzi (Chagas disease), Leishmania donovani (Kala-azar), Leishmania major (Oriental sore), and Giardia lamblia (Giardiasis)—infect millions of humans worldwide. Clomipramine has been shown in vitro and in mice to inhibit or kill these protozoa, but these potential benefits have not been extended to humans.
Sertraline, on the other hand, might exert antifungal activity. Three patients with recurrent vulvovaginal candidiasis had no episodes while being treated with sertraline for premenstrual dysphoric disorder but relapsed when the drug was discontinued.26 Although sertraline demonstrated antifungal activity in vitro against several Candida species, this SSRI seems unlikely to gain prominence as an antifungal agent.
Sexual function
Premature ejaculation. SSRIs are well-known causes of delayed or absent orgasm, but a perceived liability can become an asset in treating premature ejaculation. By measuring intravaginal ejaculation latency time under double-blind, placebo-controlled conditions, Waldinger et al27 showed pronounced delay in ejaculation with sertraline, fluoxetine, and paroxetine in men with long-standing rapid ejaculation. Dapoxetine—a short-acting non-antidepressant SSRI—is being studied as a treatment for this condition (Box 3).28
Spermicidal effect. SSRIs—including fluoxetine— have demonstrated in vitro spermicidal and antitrichomonas activity29 but are unlikely to be developed as microbicidal contraceptives.
Related Resources
- Gorman JM, Kent JM. SSRIs and SMRIs: broad spectrum of efficacy beyond major depression. J Clin Psychiatry 1999;60(suppl 4):33-8.
- About.com: Mental Health. Antidepressants for more than depression. http://mentalhealth.about.com/cs/psychopharmacology/a/antimore.htm.
- Amitriptyline • Elavil, Endep
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin, Pertofrane
- Doxepin • Adapin, Sinequan
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fludrocortisone • Florinef
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Midodrine • ProAmitine
- Nortriptyline • Pamelor, Aventyl
- Paroxetine • Paxil
- Phenelzine • Nardil
- Sertraline • Zoloft
- Trimipramine • Surmontil
- Venlafaxine • Effexor
Dr. Jefferson receives research support from Bristol-Myers Squibb, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Eli Lilly and Company, Novartis, Pfizer, Roche, Solvay, UCB Pharma, and Wyeth. He is a consultant to GlaxoSmithKline, Schwarz Pharma, Shire, and Organon and a speaker for Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Forest Pharmaceuticals, GlaxoSmithKline, Eli Lilly and Company, Pfizer, Schwarz Pharma, Shire, and Wyeth. He holds stock in Bristol-Myers Squibb, GlaxoSmithKline, and SciClone.
A molecule is a molecule is a molecule—until it becomes identified with a purpose. Consider, for example, (-)-trans-4R-(4’-fluorophenyl)-3S-[(3’,4’-methylenedioxyphenoxy) methyl] piperidine. You probably know this molecule as paroxetine—an antidepressant, of course, but it is more than that. If you examine paroxetine’s FDA-approved indications, it also has anti-panic, anti-social anxiety, anti-obsessive-compulsive disorder, anti-posttraumatic stress disorder, and anti-premenstrual dysphoric disorder effects.
“Antidepressants” have achieved fame as antidepressants; one could say these molecules’ search for meaning has been fulfilled. Yet even within psychiatry, their many other uses (Table) can create semantic misunderstandings. Beyond psychiatry, consider the nondepressed patient with neurocardiogenic syncope who wonders why he’s being treated with an antidepressant.
Rather than calling antidepressants “panaceas,” the better choice is to educate patients about the drugs’ wide spectrum of activity. Let’s look broadly across the so-called antidepressants and examine their varied uses in psychiatry and other medical specialties.
Table
FDA-approved psychiatric indications for serotonin uptake inhibitors*
| SSRIs | ||||||||
| Citalopram | X | |||||||
| Escitalopram | X | X | ||||||
| Fluoxetine | X | X | X | X | X | |||
| Fluvoxamine | X | |||||||
| Paroxetine | X | X | X | X | X | X | X | |
| Sertraline | X | X | X | X | X | X | ||
| SNRIs | ||||||||
| Duloxetine | X | X | ||||||
| Venlafaxine | X | X | X | X | ||||
| SSRIs: selective serotonin reuptake inhibitors; SNRIs: serotonin-norepinephrine reuptake inhibitors; MDD: major depressive disorder; PD: panic disorder; SAD: social anxiety disorder; PTSD: posttraumatic stress disorder; GAD: generalized anxiety disorder; OCD: obsessive-compulsive disorder; PMDD: premenstrual dysphoric disorder; BUL: bulimia | ||||||||
| * The absence of an X does not necessarily imply that a drug is ineffective for a given indication but, more likely, that definitive studies are lacking. | ||||||||
Pain syndromes
Peripheral neuropathy. The only antidepressant with an FDA-approved pain indication is duloxetine, a serotonin-norepinephrine reuptake inhibitor (SNRI). Its approval for diabetic peripheral neuropathic pain (DPNP) was based on two 12-week, randomized, double-blind, placebo-controlled studies using fixed doses of 60 mg once or twice daily.1,2 Another SNRI—venlafaxine XR, 150 to 225 mg/d, but not 75 mg/d—also was found to be more effective than placebo for this indication in a 6-week, double-blind study (Box 1).3
Using antidepressants to treat pain syndromes is neither new nor restricted to SNRIs, however. In combined double-blind, cross-over studies of patients with DPNP, Max et al4 found:
- moderate or greater pain relief in 74% and 61% of subjects, respectively, from the tricyclics amitriptyline, mean 105 mg/d, and desipramine, mean 111 mg/d—with pain reduced by equal amounts in depressed and nondepressed patients
- no statistically significant difference in pain relief between the selective serotonin reuptake inhibitor (SSRI) fluoxetine, 40 mg/d, and placebo.
- tricyclics: 1 in every 2 to 3 patients
- SNRIs: 1 in every 4 to 5 patients
- SSRIs: 1 in every 7 patients.
Chronic headache. A meta-analysis7 of randomized, placebo-controlled studies found antidepressants more effective than placebo for chronic migraine and tension headache prophylaxis. Although a subgroup meta-analysis found similar effects for tricyclics and SSRIs, the authors characterized the tricyclics’ results as well established and the SSRIs’ as “less certain.”
The results of this meta-analysis might not accurately reflect bona fide antidepressants, however. Some of the 38 studies (25 of migraine, 12 of tension headache, 1 of both) included treatment with serotonin antagonists—most commonly pizotifen, which is not available in the United States and does not appear to be an antidepressant.
Back pain. Patients with chronic low back pain (average 10 years) seem to benefit from antidepressants, according to a meta-analysis of 9 randomized, controlled trials by Salerno et al.8 The effect on pain in the total 504 patients was “small but significant,” and improvement in function was “small but nonsignificant.” Individual sample sizes also were small, however, and only 2 studies excluded depressed patients.
Fibromyalgia, with chronic generalized musculoskeletal pain and tenderness, has been a focus of antidepressant drug therapy. Goldenberg et al9 concluded from an ambitious literature review (505 articles) that evidence of efficacy was strong for amitripty-line and modest for SSRIs and SNRIs.
Overall, antidepressants are generally understood to have analgesic effects in the absence of depression. Benefits for patients with pain syndromes are well established for tricyclics (especially amitriptyline) and recently with SNRIs, whereas SSRIs are less effective.
Serotonin and norepinephrine are involved in pain modulation via descending inhibitory pathways in the brain and spinal cord. Serotonin-norepinephrine reuptake inhibitors (SNRIs) have been shown to reduce the severity of diabetic peripheral neuropathic pain (DPNP) in randomized controlled trials.
Duloxetine. In 2 double-blind studies,1,2 nondepressed patients with DPNP received duloxetine, 60 mg once daily; duloxetine, 60 mg bid; or placebo for 12 weeks. They rated the severity of neuropathic pain every 24 hours on an 11-point Likert scale, and weekly mean scores were the primary outcome measure. Average pain scores improved more in both duloxetine groups vs placebo. Duloxetine treatment did not interfere with diabetic control, and both dosages were well tolerated.
The FDA approved an added indication for duloxetine in the management of DPNP.
Venlafaxine. In a double-blind study,3 244 adult outpatients with moderately severe DPNP received venlafaxine ER, 75 or 150 to 225 mg/d, or placebo for 6 weeks. Daily scores on the Visual Analog Pain Intensity (VAS-PI) and Pain Relief (VAS-PR) scales were primary efficacy measures.
Patients receiving the higher venlafaxine dosage—but not 75 mg/d—showed statistically significant less-intensive pain vs placebo. VAS-PI scores were 27% lower than at enrollment with placebo, 32% lower with venlafaxine, 75 mg/d, and 50% lower with venlafaxine, 150 to 225 mg/d (P
Nausea and somnolence were the most common side effects; clinically important ECG changes occurred in 7 patients treated with venlafaxine, 150 to 225 mg/d.
Smoking cessation
Bupropion SR is FDA-approved to aid smoking cessation, and this effect is independent of the drug’s antidepressant activity. Bupropion may act as a nicotine receptor antagonist as well as a norepinephrine dopamine reuptake inhibitor.
Other antidepressants have been studied for smoking cessation, with nortriptyline showing benefit in 2 large placebo-controlled trials. Studies with doxepin, fluoxetine, and moclobemide found little or no benefit for this indication.
Cardiovascular uses
Angina. Monoamine oxidase inhibitor (MAOI) antidepressants were used to treat angina pectoris in the late 1950s and early 1960s. This practice stopped after evidence showed that whereas angina pain may have improved with MAOIs, stress-induced ischemia on ECG did not.
Antiarrhythmia. Tricyclics had a brief fling in cardiovascular therapeutics when their quinidine-like class I antiarrhythmic activity was recognized. Imipramine was one of several drugs included in the Cardiac Arrhythmia Pilot Study in the 1980s that involved 502 postmyocardial infarction patients with ventricular arrhythmias. Imipramine was the least effective of the 4 drugs studied and the least well tolerated.13
variety of medications. Options include the vasopressor midodrine, fludrocortisone, beta blockers, and SSRIs— none
FDA-approved for this indication. Paroxetine, 20 mg/d, was considerably more effective than placebo in preventing
recurrent syncope in 68 patients who had been unresponsive
to or intolerant of traditional medications. During a mean 25 months of treatment, 82% of patients remained syncope-free on paroxetine vs 47% on placebo.14
Selective serotonin reuptake inhibitors (SSRIs) often are used to treat irritable bowel syndrome (IBS), though evidence of their effectiveness is scarce. SSRIs can improve IBS patients’ quality of life, but effects on abdominal pain and bloating are less clear.
Paroxetine. In a randomized, double-blind trial,16 gastroenterologists tested a highfiber diet plus paroxetine in nondepressed patients with IBS. Ninety-eight patients ages 18 to 65 who experienced IBS symptoms on low- or average-fiber diets were first put on high-fiber diets and assessed for well-being and abdominal pain and bloating. Of these, 81 symptomatic patients continued highfiber diets with added paroxetine, 10 to 40 mg/d (n=38) or placebo (n=43).
With paroxetine, patients’ overall well-being improved more than with placebo, but abdominal pain and bloating and social functioning did not.
Fluoxetine. In a double-blind, randomized trial,17 44 patients with pain and constipation-predominant IBS received fluoxetine, 20 mg/d, or placebo for 12 weeks. These patients met Rome II criteria for IBS—abdominal discomfort/pain for ≥12 weeks in past year that met 2 of 3 criteria:
- relieved by defecation
- onset associated with change in stool frequency
- onset associated with change in stool appearance.
Patients receiving fluoxetine had less abdominal discomfort, less bloating, more frequent bowel movements, and decreased consistency of stool vs placebo 4 weeks after treatment stopped. Mean number of symptoms per patient decreased from 4.6 to 0.7 in the fluoxetine group vs 4.5 to 2.9 in controls (P
Citalopram. IBS symptom severity was the primary outcome in a crossover trial comparing citalopram (20 mg for 3 weeks and 40 mg for 3 weeks) with placebo in 23 nondepressed patients.18 Abdominal pain and bloating, impact of symptoms on daily life, and overall well-being improved significantly more with citalopram than with placebo after 3 and 6 weeks.
Symptom improvements were not related to changes in depression, anxiety, or colonic sensorimotor function.
Gastrointestinal
Peptic ulcer disease was shown in the 1980s to respond to tricyclic antidepressants. At the time, both anticholinergic and antihistaminic effects were thought to be responsible, but the later observation that trimipramine inhibited Campylobacter pylori in vitro suggested an additional explanation. Today, tricyclics are only of historic interest as treatments for peptic ulcer.
Irritable bowel syndrome (IBS) patients have responded favorably to antidepressants, although it is often difficult to know if the benefit is independent of improved coexisting anxiety or depression. A meta-analysis of 12 randomized, placebo-controlled trials—mostly with tricyclics—found an odds ratio for improvement of 4.2 and a number needed to treat of 3.2.15
More recently, a few placebo-controlled studies have shown SSRIs to be beneficial for IBS,16-18 although not all symptoms improved and some IBS subtypes might be more responsive than others (Box 2). In an editorial, Talley19 concluded that antidepressant therapy of IBS was “at best only a ‘band-aid’ approach to management.”
Genitourinary
Nocturnal enuresis. In the 1960s, imipramine was shown—in some but not all placebo-controlled studies—to be beneficial for nocturnal enuresis in children and adults. Although imipramine is not FDA-approved for this indication, it is thought to work by relaxing bladder muscle and contracting bladder neck smooth muscle. Imipramine appears to have a vasopressin-independent antidiuretic effect in enuretic patients with nocturnal polyuria.
Duloxetine is thought to improve stress urinary incontinence by increasing urethral sphincter tone and the force of sphincter contraction. This indication is not FDA- approved for duloxetine but is approved in the European Union.
Oncology
At one time antidepressants were suggested to promote tumors, based on observations that amitriptyline, fluoxetine, and several antihistamines promoted tumor growth in rodents.21 In 1995, a few case reports associated these 2 antidepressants with atypical cutaneous lymphoid infiltrates.22 A review by Sternback in 200323 concluded that a link between antidepressants and cancer was questionable but acknowledged the need for very long-term studies.
Recently, a nested case-control study found an association between high-dose SSRI use for ≤5 years and reduced risk of colorectal cancer, whereas no association was found with tricyclic use.24 A study of this design does not establish a causal relationship, how-ever, and one can only speculate whether SSRIs might have direct cytotoxic or anti-promoter effects.
At present, it seems reasonable to continue to treat depressed cancer patients with antidepressants without concern that cancer will worsen or hope that it will improve as a result.
Immunology
The pathogenesis of depression may be linked to pro-inflammatory cytokines—proteins such as tumor necrosis factor-alpha (TNF-α) and certain interleukins that mediate immune function. Bupropion markedly lowered pro-inflammatory cytokine levels in a mouse inflammation model, prompting the authors to suggest that this anti-inflammatory effect be explored in humans.25
Case reports have suggested benefit from bupropion in Crohn’s disease, recurrent aphthous ulcerations, psoriasis, atopic dermatitis, and Blau syndrome (a rare autosomal-dominant trait characterized by granulomatous arthritis, iritis, and skin rash). Whether this antidepressant has much anti-inflammatory potential remains to be determined, however.
Delayed ejaculation is among the sexual side effects commonly associated with antidepressant medication. In a 6-week trial,27 3 selective serotonin reuptake inhibitors (SSRIs)— paroxetine, fluoxetine, and sertraline— were shown to improve intravaginal ejaculatory latency time (IELT) in men with lifelong rapid ejaculation. Compared with baseline, the greatest delay in ejaculation was seen with paroxetine, 20 mg/d, followed by fluoxetine, 20 mg/d, and then sertraline, 50 mg/d, whereas delay with fluvoxamine, 100 mg/d, did not differ significantly from placebo.
Dapoxetine is a non-antidepressant SSRI under investigation for on-demand treatment of moderate-to-severe premature ejaculation.28 In two 12-week, randomized, double-blind, placebo-controlled trials, 870 men took placebo, 874 took 30-mg dapoxetine, and 870 took 60-mg dapoxetine 1 to 3 hours before sexual activity. Efficacy was determined by IELT measured at home by stopwatch.
Both dapoxetine doses improved IELT significantly more than placebo (P
Nausea, diarrhea, headache, and dizziness occurred in ≤20% of patients and were more common with the 60-mg than 30-mg dapoxetine dose.
Infectious disease
Pathogenic protozoa—such as Trypanosoma cruzi (Chagas disease), Leishmania donovani (Kala-azar), Leishmania major (Oriental sore), and Giardia lamblia (Giardiasis)—infect millions of humans worldwide. Clomipramine has been shown in vitro and in mice to inhibit or kill these protozoa, but these potential benefits have not been extended to humans.
Sertraline, on the other hand, might exert antifungal activity. Three patients with recurrent vulvovaginal candidiasis had no episodes while being treated with sertraline for premenstrual dysphoric disorder but relapsed when the drug was discontinued.26 Although sertraline demonstrated antifungal activity in vitro against several Candida species, this SSRI seems unlikely to gain prominence as an antifungal agent.
Sexual function
Premature ejaculation. SSRIs are well-known causes of delayed or absent orgasm, but a perceived liability can become an asset in treating premature ejaculation. By measuring intravaginal ejaculation latency time under double-blind, placebo-controlled conditions, Waldinger et al27 showed pronounced delay in ejaculation with sertraline, fluoxetine, and paroxetine in men with long-standing rapid ejaculation. Dapoxetine—a short-acting non-antidepressant SSRI—is being studied as a treatment for this condition (Box 3).28
Spermicidal effect. SSRIs—including fluoxetine— have demonstrated in vitro spermicidal and antitrichomonas activity29 but are unlikely to be developed as microbicidal contraceptives.
Related Resources
- Gorman JM, Kent JM. SSRIs and SMRIs: broad spectrum of efficacy beyond major depression. J Clin Psychiatry 1999;60(suppl 4):33-8.
- About.com: Mental Health. Antidepressants for more than depression. http://mentalhealth.about.com/cs/psychopharmacology/a/antimore.htm.
- Amitriptyline • Elavil, Endep
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clomipramine • Anafranil
- Desipramine • Norpramin, Pertofrane
- Doxepin • Adapin, Sinequan
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fludrocortisone • Florinef
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Midodrine • ProAmitine
- Nortriptyline • Pamelor, Aventyl
- Paroxetine • Paxil
- Phenelzine • Nardil
- Sertraline • Zoloft
- Trimipramine • Surmontil
- Venlafaxine • Effexor
Dr. Jefferson receives research support from Bristol-Myers Squibb, Forest Pharmaceuticals, GlaxoSmithKline, Janssen Pharmaceutica, Eli Lilly and Company, Novartis, Pfizer, Roche, Solvay, UCB Pharma, and Wyeth. He is a consultant to GlaxoSmithKline, Schwarz Pharma, Shire, and Organon and a speaker for Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Forest Pharmaceuticals, GlaxoSmithKline, Eli Lilly and Company, Pfizer, Schwarz Pharma, Shire, and Wyeth. He holds stock in Bristol-Myers Squibb, GlaxoSmithKline, and SciClone.
1. Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006;67(8):1411-20.
2. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med 2005;6(5):346-56.
3. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain 2004;110:697-706.
4. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992;326:1250-6.
5. Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 2005;96:399-409.
6. Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology 2001;57:1583-8.
7. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001;111:54-63.
8. Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain. Arch Intern Med 2002;162:19-24.
9. Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA 2004;292:2388-95.
10. Littlejohn GO, Guymer EK. Fibromyalgia syndrome: which antidepressant drug should we choose. Curr Pharm Des 2006;12(1):3-9.
11. Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum 2004;50:2974-84.
12. Arnold LM, Rosen A, Pritchett YL, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 2005;119:5-15.
13. Effects of encainide, flecainide, imipramine and moricizine on ventricular arrhythmias during the year after acute myocardial infarction: The CAPS. The Cardiac Arrhythmia Pilot Study (CAPS) Investigators. Am J Cardiol 1988;61(8):501-9.
14. Di Girolamo E, Di Iorio C, Sabatini P, et al. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 1999;33:1227-30.
15. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000;108:65-72.
16. Tabas G, Beaves M, Wang J, et al. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol 2004;99(5):914-20.
17. Vahedi H, Merat S, Rashidioon A, et al. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Aliment Pharmacol Ther 2005;22:381-5.
18. Tack J, Broekaert D, Fischler B, et al. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55:1095-103.
19. Talley NJ. Antidepressants in IBS: are we deluding ourselves? [editorial]. Am J Gastroenterol 2004;99:921-3.
20. Mariappan P, Ballantyne Z, N’Dow JM, Alhasso AA. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database Syst Rev 2005;Jul 20;(3):CD004742.-
21. Brandes LJ, Arron RJ, Bogdanovic RP, et al. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res 1992;52:3796-800.
22. Crowson AN, Magro CM. Antidepressant therapy. Arch Dermatol 1995;131:925-9.
23. Sternbach H. Are antidepressants carcinogenic? A review of preclinical and clinical studies. J Clin Psychiatry 2003;64:1153-62.
24. Xu W, Tamim H, Shapiro S, et al. Use of antidepressants and risk of colorectal cancer: a nested case-control study. Lancet Oncol 2006;7:301-8.
25. Brustolim D, Ribeiro-dos-Santos R, Kast RE, et al. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol 2006;6:903-7.
26. Lass-Flörl C, Dierich MP, Fuchs D, et al. Antifungal activity against Candida species of the selective serotonin-reuptake inhibitor, sertraline. Clin Infect Dis 2001;33:e135-6.
27. Waldinger MD, Hengeveld MW, Zsinderman AH, Olivier B. Effect of SSRI antidepressants on ejaculation: a double-blind, randomized, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine, and sertraline. J Clin Psychopharmacol 1998;18(4):274-81.
28. Pryor JL, Althof SE, Steidle C, et al. Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials. Lancet 2006;368:929-37.
29. Kumar VS, Sharma VL, Tiwari P, et al. The spermicidal and antitrichomonas activities of SSRI antidepressants. Bioorg Med Chem Lett 2006;16:2509-12.
1. Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology 2006;67(8):1411-20.
2. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med 2005;6(5):346-56.
3. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain 2004;110:697-706.
4. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992;326:1250-6.
5. Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 2005;96:399-409.
6. Semenchuk MR, Sherman S, Davis B. Double-blind, randomized trial of bupropion SR for the treatment of neuropathic pain. Neurology 2001;57:1583-8.
7. Tomkins GE, Jackson JL, O’Malley PG, et al. Treatment of chronic headache with antidepressants: a meta-analysis. Am J Med 2001;111:54-63.
8. Salerno SM, Browning R, Jackson JL. The effect of antidepressant treatment on chronic back pain. Arch Intern Med 2002;162:19-24.
9. Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA 2004;292:2388-95.
10. Littlejohn GO, Guymer EK. Fibromyalgia syndrome: which antidepressant drug should we choose. Curr Pharm Des 2006;12(1):3-9.
11. Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum 2004;50:2974-84.
12. Arnold LM, Rosen A, Pritchett YL, et al. A randomized, double-blind, placebo-controlled trial of duloxetine in the treatment of women with fibromyalgia with or without major depressive disorder. Pain 2005;119:5-15.
13. Effects of encainide, flecainide, imipramine and moricizine on ventricular arrhythmias during the year after acute myocardial infarction: The CAPS. The Cardiac Arrhythmia Pilot Study (CAPS) Investigators. Am J Cardiol 1988;61(8):501-9.
14. Di Girolamo E, Di Iorio C, Sabatini P, et al. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 1999;33:1227-30.
15. Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000;108:65-72.
16. Tabas G, Beaves M, Wang J, et al. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol 2004;99(5):914-20.
17. Vahedi H, Merat S, Rashidioon A, et al. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Aliment Pharmacol Ther 2005;22:381-5.
18. Tack J, Broekaert D, Fischler B, et al. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55:1095-103.
19. Talley NJ. Antidepressants in IBS: are we deluding ourselves? [editorial]. Am J Gastroenterol 2004;99:921-3.
20. Mariappan P, Ballantyne Z, N’Dow JM, Alhasso AA. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database Syst Rev 2005;Jul 20;(3):CD004742.-
21. Brandes LJ, Arron RJ, Bogdanovic RP, et al. Stimulation of malignant growth in rodents by antidepressant drugs at clinically relevant doses. Cancer Res 1992;52:3796-800.
22. Crowson AN, Magro CM. Antidepressant therapy. Arch Dermatol 1995;131:925-9.
23. Sternbach H. Are antidepressants carcinogenic? A review of preclinical and clinical studies. J Clin Psychiatry 2003;64:1153-62.
24. Xu W, Tamim H, Shapiro S, et al. Use of antidepressants and risk of colorectal cancer: a nested case-control study. Lancet Oncol 2006;7:301-8.
25. Brustolim D, Ribeiro-dos-Santos R, Kast RE, et al. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol 2006;6:903-7.
26. Lass-Flörl C, Dierich MP, Fuchs D, et al. Antifungal activity against Candida species of the selective serotonin-reuptake inhibitor, sertraline. Clin Infect Dis 2001;33:e135-6.
27. Waldinger MD, Hengeveld MW, Zsinderman AH, Olivier B. Effect of SSRI antidepressants on ejaculation: a double-blind, randomized, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine, and sertraline. J Clin Psychopharmacol 1998;18(4):274-81.
28. Pryor JL, Althof SE, Steidle C, et al. Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials. Lancet 2006;368:929-37.
29. Kumar VS, Sharma VL, Tiwari P, et al. The spermicidal and antitrichomonas activities of SSRI antidepressants. Bioorg Med Chem Lett 2006;16:2509-12.
Finger-stick lithium test
A new, FDA-approved in-office lithium test (Table) can eliminate the inconvenience and fallibility of testing venous blood samples that often discourage lithium use. The test, which measures lithium in capillary blood drawn from a finger stick, has shown reliability when compared in clinical trials with established testing methods.
Why finger-stick testing?
Periodically monitoring serum or plasma lithium minimizes side effects and toxicity, maintains therapeutic dosing, and ensures treatment adherence. Laboratories generally use flame photometry, atomic absorption (AA) spectrophotometry, or ion-selective electrode analysis to measure lithium in blood drawn via venipuncture. A colorimetric assay is also available.1
Table
Lithium fingerstick test: Fast facts
| Brand name: |
| InstaRead Lithium System |
| Indication: |
| Testing plasma lithium levels in-office |
| Manufacturer: |
| ReliaLAB |
| Recommended use: |
| Testing plasma lithium levels 12 hours after dosing; repeat test after 5 minutes to confirm abnormal reading |
| Reimbursement information: |
| 1-866-467-8273 or www.relialab.com/Reimbursement.html |
For years, researchers have investigated alternatives to venipuncture lithium testing. Aside from being inconvenient, venipuncture draws can increase risk of excessive bleeding, hematoma, infection, vasovagal syncope, and multiple punctures to locate a vein. In some cases:
- psychiatrists wait 2 or more days for a laboratory to return results
- patients forget to have blood drawn before the office visit
- samples are incorrectly timed in relation to the last dose
- results are filed away unnoticed
- or the psychiatrist needs to call the patient 3 days or so after the visit to discuss an abnormal reading.
With the new in-office test, clinicians can ensure they will obtain a valid blood sample in minutes, 12 hours after dosing. Psychiatrists then can immediately discuss the result with patients, perform a repeat test 5 minutes later to check an abnormal reading, and counsel patients on raising low lithium levels. This instant feedback can powerfully reinforce a physician’s advice and promote treatment adherence.2
How it works
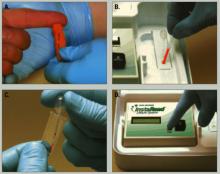
A 50-μl blood sample is drawn via finger stick and converted to plasma in a lectin-coated membrane separator. The clinician then adds 0.2 μl of the plasma to a micro-cuvette containing a colorimetric reagent that is photometrically analyzed for lithium. The test takes 5 minutes or less (Figure).
The assay has been shown to be sensitive to 0.1 mEq/L of lithium and linear between 0.1 and 2.5 mEq/L.3
Figure How finger-stick lithium test works
Clinician obtains blood sample (a) and empties it into a separator (b), which processes blood to plasma. Clinician then adds plasma to a reagent vial (c), which is inserted into a reader (d) to obtain a lithium level.
Reliability
In clinical trials during which patients were tested and retested, the colorimetric assay showed reliability when compared with:
- routine lithium spectrophotometry. Researchers compared venipuncture blood samples split for colorimetric and spectrophotometric testing
- atomic absorption spectrophotometry of venipuncture blood from psychiatric patients
- standard spectrophotometry of venipuncture samples to which a known amount of lithium was added.4
Colorimetric finger-stick testing also was compared with AA spectrophotometry testing of 88 matched venipuncture samples from 56 bipolar patients.5 Results were not identical, but most fingerstick results varied no more than±0.2 mEq/L from the AA results. Differences were positive and negative, indicating random variation between the two methods rather than systematic bias.
Clinical applicability
In-office finger-stick blood testing for lithium levels could improve quality of care for patients taking lithium.
The manufacturer, ReliaLAB, says the test costs $399, plus $264 for a refill kit containing 24 patient test packs. A certain volume of patients taking lithium would seem to be necessary to justify purchasing the instrument.
The test may be reimbursable under certain circumstances. ReliaLAB offers information on coding and reimbursement for in-office lithium monitoring (Table).
Also, because instant in-office creatinine and thyroid-stimulating hormone tests are not available, lithium therapy monitoring will still require laboratory visits when these tests are needed. Nonetheless, point-of-care plasma lithium level determination should improve convenience, compliance, and overall comprehensiveness of care.
Related resources
- Online information on in-office lithium test. www.relialab.com/Lith.html.
- Johnson FN. The origins of lithium therapy. Rev Contemp Pharmacother 1999;10:193-265.
Drug brand names
- Lithium • Eskalith, others
Disclosure
Dr. Jefferson reports no financial relationship with or proprietary interest in ReliaLAB.
1. Jefferson JW, Greist JH. Lithium. In: Sadock BJ, Sadock VA (eds). Comprehensive textbook of psychiatry, vol. 2 (8th ed). Philadelphia: Lippincott Williams & Wilkins; 2005;2839-51.
2. Srinivasan DP, Birch NJ. Instant lithium monitoring: A clinical revolution in the making. Br J Clin Pract 1996;50:386-88.
3. Glazer WM, Sonnenberg JG, Reinstein MJ, Akers RF. A novel, point-of-care test for lithium levels: Description and reliability. J Clin Psychiatry 2004;652-5.
4. Vrouwe EX, Luttge R, van den Berg A. Direct measurement of lithium in whole blood using microchip capillary electrophoresis with integrated conductivity detection. Electrophoresis 2004;25:1660-7.
5. Glazer WM, Sonnenberg J, Reinstein MJ. A novel, “point of care” test for lithium levels (poster presentation). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
A new, FDA-approved in-office lithium test (Table) can eliminate the inconvenience and fallibility of testing venous blood samples that often discourage lithium use. The test, which measures lithium in capillary blood drawn from a finger stick, has shown reliability when compared in clinical trials with established testing methods.
Why finger-stick testing?
Periodically monitoring serum or plasma lithium minimizes side effects and toxicity, maintains therapeutic dosing, and ensures treatment adherence. Laboratories generally use flame photometry, atomic absorption (AA) spectrophotometry, or ion-selective electrode analysis to measure lithium in blood drawn via venipuncture. A colorimetric assay is also available.1
Table
Lithium fingerstick test: Fast facts
| Brand name: |
| InstaRead Lithium System |
| Indication: |
| Testing plasma lithium levels in-office |
| Manufacturer: |
| ReliaLAB |
| Recommended use: |
| Testing plasma lithium levels 12 hours after dosing; repeat test after 5 minutes to confirm abnormal reading |
| Reimbursement information: |
| 1-866-467-8273 or www.relialab.com/Reimbursement.html |
For years, researchers have investigated alternatives to venipuncture lithium testing. Aside from being inconvenient, venipuncture draws can increase risk of excessive bleeding, hematoma, infection, vasovagal syncope, and multiple punctures to locate a vein. In some cases:
- psychiatrists wait 2 or more days for a laboratory to return results
- patients forget to have blood drawn before the office visit
- samples are incorrectly timed in relation to the last dose
- results are filed away unnoticed
- or the psychiatrist needs to call the patient 3 days or so after the visit to discuss an abnormal reading.
With the new in-office test, clinicians can ensure they will obtain a valid blood sample in minutes, 12 hours after dosing. Psychiatrists then can immediately discuss the result with patients, perform a repeat test 5 minutes later to check an abnormal reading, and counsel patients on raising low lithium levels. This instant feedback can powerfully reinforce a physician’s advice and promote treatment adherence.2
How it works
A 50-μl blood sample is drawn via finger stick and converted to plasma in a lectin-coated membrane separator. The clinician then adds 0.2 μl of the plasma to a micro-cuvette containing a colorimetric reagent that is photometrically analyzed for lithium. The test takes 5 minutes or less (Figure).
The assay has been shown to be sensitive to 0.1 mEq/L of lithium and linear between 0.1 and 2.5 mEq/L.3
Figure How finger-stick lithium test works
Clinician obtains blood sample (a) and empties it into a separator (b), which processes blood to plasma. Clinician then adds plasma to a reagent vial (c), which is inserted into a reader (d) to obtain a lithium level.
Reliability
In clinical trials during which patients were tested and retested, the colorimetric assay showed reliability when compared with:
- routine lithium spectrophotometry. Researchers compared venipuncture blood samples split for colorimetric and spectrophotometric testing
- atomic absorption spectrophotometry of venipuncture blood from psychiatric patients
- standard spectrophotometry of venipuncture samples to which a known amount of lithium was added.4
Colorimetric finger-stick testing also was compared with AA spectrophotometry testing of 88 matched venipuncture samples from 56 bipolar patients.5 Results were not identical, but most fingerstick results varied no more than±0.2 mEq/L from the AA results. Differences were positive and negative, indicating random variation between the two methods rather than systematic bias.
Clinical applicability
In-office finger-stick blood testing for lithium levels could improve quality of care for patients taking lithium.
The manufacturer, ReliaLAB, says the test costs $399, plus $264 for a refill kit containing 24 patient test packs. A certain volume of patients taking lithium would seem to be necessary to justify purchasing the instrument.
The test may be reimbursable under certain circumstances. ReliaLAB offers information on coding and reimbursement for in-office lithium monitoring (Table).
Also, because instant in-office creatinine and thyroid-stimulating hormone tests are not available, lithium therapy monitoring will still require laboratory visits when these tests are needed. Nonetheless, point-of-care plasma lithium level determination should improve convenience, compliance, and overall comprehensiveness of care.
Related resources
- Online information on in-office lithium test. www.relialab.com/Lith.html.
- Johnson FN. The origins of lithium therapy. Rev Contemp Pharmacother 1999;10:193-265.
Drug brand names
- Lithium • Eskalith, others
Disclosure
Dr. Jefferson reports no financial relationship with or proprietary interest in ReliaLAB.
A new, FDA-approved in-office lithium test (Table) can eliminate the inconvenience and fallibility of testing venous blood samples that often discourage lithium use. The test, which measures lithium in capillary blood drawn from a finger stick, has shown reliability when compared in clinical trials with established testing methods.
Why finger-stick testing?
Periodically monitoring serum or plasma lithium minimizes side effects and toxicity, maintains therapeutic dosing, and ensures treatment adherence. Laboratories generally use flame photometry, atomic absorption (AA) spectrophotometry, or ion-selective electrode analysis to measure lithium in blood drawn via venipuncture. A colorimetric assay is also available.1
Table
Lithium fingerstick test: Fast facts
| Brand name: |
| InstaRead Lithium System |
| Indication: |
| Testing plasma lithium levels in-office |
| Manufacturer: |
| ReliaLAB |
| Recommended use: |
| Testing plasma lithium levels 12 hours after dosing; repeat test after 5 minutes to confirm abnormal reading |
| Reimbursement information: |
| 1-866-467-8273 or www.relialab.com/Reimbursement.html |
For years, researchers have investigated alternatives to venipuncture lithium testing. Aside from being inconvenient, venipuncture draws can increase risk of excessive bleeding, hematoma, infection, vasovagal syncope, and multiple punctures to locate a vein. In some cases:
- psychiatrists wait 2 or more days for a laboratory to return results
- patients forget to have blood drawn before the office visit
- samples are incorrectly timed in relation to the last dose
- results are filed away unnoticed
- or the psychiatrist needs to call the patient 3 days or so after the visit to discuss an abnormal reading.
With the new in-office test, clinicians can ensure they will obtain a valid blood sample in minutes, 12 hours after dosing. Psychiatrists then can immediately discuss the result with patients, perform a repeat test 5 minutes later to check an abnormal reading, and counsel patients on raising low lithium levels. This instant feedback can powerfully reinforce a physician’s advice and promote treatment adherence.2
How it works
A 50-μl blood sample is drawn via finger stick and converted to plasma in a lectin-coated membrane separator. The clinician then adds 0.2 μl of the plasma to a micro-cuvette containing a colorimetric reagent that is photometrically analyzed for lithium. The test takes 5 minutes or less (Figure).
The assay has been shown to be sensitive to 0.1 mEq/L of lithium and linear between 0.1 and 2.5 mEq/L.3
Figure How finger-stick lithium test works
Clinician obtains blood sample (a) and empties it into a separator (b), which processes blood to plasma. Clinician then adds plasma to a reagent vial (c), which is inserted into a reader (d) to obtain a lithium level.
Reliability
In clinical trials during which patients were tested and retested, the colorimetric assay showed reliability when compared with:
- routine lithium spectrophotometry. Researchers compared venipuncture blood samples split for colorimetric and spectrophotometric testing
- atomic absorption spectrophotometry of venipuncture blood from psychiatric patients
- standard spectrophotometry of venipuncture samples to which a known amount of lithium was added.4
Colorimetric finger-stick testing also was compared with AA spectrophotometry testing of 88 matched venipuncture samples from 56 bipolar patients.5 Results were not identical, but most fingerstick results varied no more than±0.2 mEq/L from the AA results. Differences were positive and negative, indicating random variation between the two methods rather than systematic bias.
Clinical applicability
In-office finger-stick blood testing for lithium levels could improve quality of care for patients taking lithium.
The manufacturer, ReliaLAB, says the test costs $399, plus $264 for a refill kit containing 24 patient test packs. A certain volume of patients taking lithium would seem to be necessary to justify purchasing the instrument.
The test may be reimbursable under certain circumstances. ReliaLAB offers information on coding and reimbursement for in-office lithium monitoring (Table).
Also, because instant in-office creatinine and thyroid-stimulating hormone tests are not available, lithium therapy monitoring will still require laboratory visits when these tests are needed. Nonetheless, point-of-care plasma lithium level determination should improve convenience, compliance, and overall comprehensiveness of care.
Related resources
- Online information on in-office lithium test. www.relialab.com/Lith.html.
- Johnson FN. The origins of lithium therapy. Rev Contemp Pharmacother 1999;10:193-265.
Drug brand names
- Lithium • Eskalith, others
Disclosure
Dr. Jefferson reports no financial relationship with or proprietary interest in ReliaLAB.
1. Jefferson JW, Greist JH. Lithium. In: Sadock BJ, Sadock VA (eds). Comprehensive textbook of psychiatry, vol. 2 (8th ed). Philadelphia: Lippincott Williams & Wilkins; 2005;2839-51.
2. Srinivasan DP, Birch NJ. Instant lithium monitoring: A clinical revolution in the making. Br J Clin Pract 1996;50:386-88.
3. Glazer WM, Sonnenberg JG, Reinstein MJ, Akers RF. A novel, point-of-care test for lithium levels: Description and reliability. J Clin Psychiatry 2004;652-5.
4. Vrouwe EX, Luttge R, van den Berg A. Direct measurement of lithium in whole blood using microchip capillary electrophoresis with integrated conductivity detection. Electrophoresis 2004;25:1660-7.
5. Glazer WM, Sonnenberg J, Reinstein MJ. A novel, “point of care” test for lithium levels (poster presentation). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
1. Jefferson JW, Greist JH. Lithium. In: Sadock BJ, Sadock VA (eds). Comprehensive textbook of psychiatry, vol. 2 (8th ed). Philadelphia: Lippincott Williams & Wilkins; 2005;2839-51.
2. Srinivasan DP, Birch NJ. Instant lithium monitoring: A clinical revolution in the making. Br J Clin Pract 1996;50:386-88.
3. Glazer WM, Sonnenberg JG, Reinstein MJ, Akers RF. A novel, point-of-care test for lithium levels: Description and reliability. J Clin Psychiatry 2004;652-5.
4. Vrouwe EX, Luttge R, van den Berg A. Direct measurement of lithium in whole blood using microchip capillary electrophoresis with integrated conductivity detection. Electrophoresis 2004;25:1660-7.
5. Glazer WM, Sonnenberg J, Reinstein MJ. A novel, “point of care” test for lithium levels (poster presentation). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
Rediscovering the art of lithium therapy
As a mood stabilizer for patients with bipolar disorder, lithium was the darling of U.S. psychiatry from the 1970s to well into the 1990s. It then began an ill-deserved, gradual fall from grace and today could be considered a pharmaceutical endangered species. But why?
Did lithium lose effectiveness? Is it too toxic? Is its side effect burden too heavy? Does it interact adversely with too many medicines? Is it too cumbersome to use? Was it just a fad whose time came and went—a psychiatric pet rock? Did it fall prey to the marketing might behind patent-protected drugs? Was it replaced by more effective and safer drugs?
You are partially correct if you checked “all of the above,” because all contain a kernel of truth. At the same time, each is an exaggeration that does grave injustice to a remarkable medication. In addition, psychiatry appears to pay only lip service to convincing evidence that lithium is the only mood stabilizer that reduces the risk of suicide during long-term treatment.1
Some psychiatrists rationalize that “lithium is too difficult to use, so I never prescribe it.”2,3 My response is simply, “try it, and I think you’ll like it.” Measuring serum lithium concentrations is simple, accurate, and inexpensive. And we know quite a bit about how lithium dosage and blood level relate to response and tolerability.
Where does lithium stand?
Lithium is the first solid element in the periodic table (atomic number 3, atomic weight 6.94) (Box 1). As a treatment for bipolar disorder, lithium’s rise to prominence in the United States was far from rapid. Its antimanic properties were described by John Cade in Australia in 1949 in an open-label case series, but it was not FDA-approved for 20 years—for acute manic episodes in 1970 and for maintenance therapy “in those manic depressive patients with a history of mania” in 1974. Today, lithium shares FDA-approved manic episode billing with chlorpromazine (1973), divalproex (1995), and olanzapine (2000), but it remains the only FDA-approved drug for maintenance (although the FDA is considering a bipolar depression maintenance indication for lamotrigine).
Lithium has no meaningful protein binding and no metabolites, being excreted almost entirely by the kidneys. Its elimination half-life of 18 to 24 hours may be longer in the elderly and shorter in youth because of age-dependent variations in glomerular filtration rate. For unclear reasons, renal lithium clearance appears to be more rapid in obese persons.
Lithium preparations available in the United States include standard-release (150, 300, 600 mg), slow-release (Lithobid and generic 300 mg), and controlled-release (Eskalith CR 450 mg) forms of lithium carbonate and a lithium citrate liquid. Lithium carbonate, 300 mg, and lithium citrate, 5 cc, each contain about 8 mmols of lithium. Lithium and lithium carbonate are not the same—there are 56.36 mg of lithium in 300 mg of lithium carbonate. The correct formula for lithium carbonate is Li2CO3, not LiCO3 as is commonly and erroneously written.
With the standard-release preparation, peak serum levels are reached in about 1 1/2 hours and with the slow- and controlled-release forms in about 4 to 4 1/2 hours. At times, the slower-release forms may be better tolerated, but they are also a bit more costly (although all forms of lithium are inexpensive, compared with other mood stabilizers).
If you examine lithium’s status relative to other bipolar medications, you’ll find some inconsistencies. For example:
- Clinical practice guidelines from the Department of Veterans Affairs (January 1999) recommended lithium as the first-line agent for acute and prophylactic treatment of manic and mixed states, bipolar depression, and rapid cycling.4
- The Expert Consensus Guidelines (April 2000) gave at least equal billing—if not preferred status—to divalproex for those indications.5
- The American Psychiatric Association’s (APA) revised guidelines (April 2002) gave the nod to lithium for classic elated mania and bipolar depression but to divalproex for mixed mania and rapid cycling.6 Divalproex was rated comparable to lithium for maintenance therapy, despite the lack of convincing data.
- The European perspective (January 2002) is most similar to that of the Department of Veterans Affairs, favoring lithium for acute mania, bipolar depression, and long-term treatment.7
There is no clear winner (or loser) in the battle for bipolar marketplace supremacy. The belief that one drug does everything is a fantasy for all but a small minority of patients with bipolar disorder. Polypharmacy is the rule, and rational polypharmacy the goal. To exclude lithium from the arsenal of bipolar drugs would be folly, yet lithium prescribing seems to have become a vanishing art. One of my psychopharmacologist colleagues recently expressed bewilderment at the number of “treatment-resistant” bipolar patients referred to him who had never been treated with lithium.
Diagnosis matters
Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning. Its potential benefits, however, clearly extend to all other aspects of bipolar disorder, to augmentation for treatment-resistant major depressive disorder, to schizoaffective disorder, and—at times—to aggressive states. As the bipolar spectrum expands, it is hardly surprising that the effectiveness of lithium (or any other drug) lessens as we approach the periphery of the spectrum.
Blood levels and dosing
Recommended lithium serum concentrations are given as ranges, realizing that individual variability makes exact numbers impractical. Package inserts for lithium products list serum concentrations between 1.0 and 1.5 mEq/L for acute mania and 0.6 and 1.2 mEq/L for maintenance therapy. The APA’s revised guidelines are a bit more conservative, recommending 0.5 to 1.2 mEq/L for acute mania and waffling somewhat on maintenance.4 Many patients on maintenance therapy do well at levels between 0.6 and 0.8 mEq/L, and some prosper at even lower levels.
To avoid obtaining a misleading blood level:
- Samples should be drawn in the morning as close as possible to 12 hours after the last dose.
- Steady state conditions should exist, usually meaning 4 or 5 days on the same dosage without any missed or extra doses (Box 2).
Start treatment using divided dosages, but—following stabilization—once-daily dosing is possible for many patients. If lithium is taken as a single daily dose, 12-hour blood levels will be somewhat higher than with multiple daily dosing. Single and multiple daily dosing are similarly effective, but once-daily dosing may have a compliance and tolerability edge in some patients.
Considering individual patient variability, a lithium carbonate dosage of 1,200 to 1,800 mg/d is likely to be therapeutic for mania and 900 to 1,200 mg/d for maintenance in otherwise healthy, nongeriatric adults.
Starting and maintaining lithium
Medical history. Assuming that lithium theapy is indicated, obtain a detailed medical history. Focus on findings that increase the risk of lithium toxicity, such as renal impairment, drug interactions, and unstable fluid-electrolyte balance.
Although lithium is not contraindicated in patients with renal disease, using an alternate drug is probably preferable. On the other hand, because lithium does not adversely affect the liver or pancreas, it may be preferred to some other mood stabilizers if these organs are diseased.
A thorough diet and drug history is also important. Because low-sodium diets reduce renal lithium clearance, lower doses may be required to reach a given serum concentration. Some drugs alter lithium excretion and can increase or decrease blood levels (see “Drug combinations,”).
Advise women of childbearing age about lithium’s teratogenic potential (which is considerably less than that of carbamazepine or valproate). The risk of cardiovascular malformation of the fetus has been estimated at 1/1,000 to 1/2,000 births among women who took lithium during the first trimester of pregnancy.8
- Draw samples in the morning, as close as possible to 12 hours after the last dose.
- Measure serum levels at steady state, at least 4 or 5 days on the same dosage without any missed or extra doses.
Baseline lab tests. Assessing renal function is essential. A serum creatinine level will usually suffice, unless a history of renal disease suggests the need for a more extensive evaluation, such as creatinine clearance, renal ultrasound, or nephrology consultation.
A urinalysis is often part of the package. Because thyroid dysfunction can alter mood and lithium can disrupt thyroid function, baseline TSH and T4 tests are recommended. CBC is optional (lithium can cause leukocytosis). The medical history should determine whether additional blood work is necessary. An ECG is sometimes advised in older patients, especially if the history suggests cardiovascular disease. Finally, don’t forget a pregnancy test in women of childbearing potential (Box 3).
Monitoring. Early in the course of therapy, lithium blood levels are usually obtained at 5- to 7-day intervals until the patient is stabilized. After that, assuming all is well, routine monitoring can occur every 3, 4, or even 6 months, depending on the individual’s reliability and stability. Because ongoing assessment of renal and thyroid function is also important, it makes sense to obtain:
- a serum creatinine measurement linked to each lithium level
- and a serum TSH yearly or at the slightest indication of thyroid dysfunction, such as fatigue, weight gain, cognitive impairment, cold intolerance, or depression.
Stopping lithium. Lithium can be discontinued abruptly without side effects if it is ineffective or not tolerated. Stopping lithium after successful long-term use is another story. There is a high likelihood of illness recurrence and a small but real possibility that lithium will be ineffective when restarted. Also, abrupt or rapid discontinuation (within 1 to 14 days) is believed to increase the likelihood of earlier recurrence, compared with more gradual discontinuation.9
Side effects and toxicity
One reason for lithium’s slide in popularity is its perceived side-effect profile. Toxic amounts can be lethal, and therapeutic amounts can be bothersome. Yet concerns are often exaggerated because of lack of familiarity with the drug.10,11
Intoxication. Lithium does have a narrow therapeutic index, with toxicity related to serum concentration and duration of exposure. Acute overdoses, while not benign, are often better tolerated than gradual, more tissue-saturating exposures. Idiosyncratic factors are also involved, as evidenced by documented toxicity at “therapeutic” levels and tolerability despite very high levels.
Early warnings of impending toxicity include:
- neurologic findings such as dysarthria, new or worsening tremor, and ataxia
- gastrointestinal symptoms such as anorexia, nausea, vomiting, and diarrhea.
Severe toxicity can be fatal or cause permanent neurologic (often cerebellar) damage. Causes of intoxication range from deliberate overdose to renal impairment, low-sodium diets, drug interactions, and dehydration. At particular risk are patients with lithium-induced polyuria whose access to fluid replacement is compromised.
Treatment involves reducing absorption, increasing excretion, and restoring fluid-electrolyte balance. Severe intoxication, especially if renal function is impaired, is best treated with hemodialysis.
| Test | Indication |
|---|---|
| Serum creatinine, urinalysis | To screen for renal function |
| TSH and T4 | To establish baseline thyroid function |
| CBC (optional) | If indicated by patient’s overall medical condition or because some doctors prefer to do more general screening |
| ECG (optional) | For patients with risk factors for heart disease |
| Pregnancy test | For at-risk women because of lithium’s teratogenic potential |
Neurologic. Mild neurologic complaints such as memory impairment, slow reaction time, lack of spontaneity, and lost creativity have been ascribed to lithium and may lead to noncompliance. Under such circumstances, other diagnostic considerations include breakthrough depression, hypothyroidism, other illness, hypercalcemia, other medications, and absence of hypomania.
Like valproate, lithium can cause a benign postural tremor that is usually tolerable and often transient. Should the tremor be problematic, treatment considerations include dosage reduction, switching to a slow-release preparation, reducing caffeine intake, avoiding other tremor-causing drugs such as theophylline or stimulants, and treating associated anxiety. If an anti-tremor drug is needed, a beta-blocker such as propranolol is used most commonly; other options to consider are primidone and gabapentin. Don’t forget that a worsening tremor may indicate impending toxicity.
Very rarely, lithium has been associated with pseudotumor cerebri (benign intracranial hypertension), peripheral neuropathy, and a myasthenia gravis-like syndrome.
Cardiovascular. Like many drugs, lithium can cause benign ST-T wave changes on ECG.
More serious, but fortunately quite uncommon, is lithium-induced sinus node dysfunction manifesting as a variety of bradyarrhythmias and, at times, syncopal episodes. Since normal aging is associated with a gradual loss of sinus node pacemaker cells, the elderly may be especially sensitive to this problem. Unless a pacemaker is implanted, sinus node dysfunction usually requires lithium discontinuation.
Endocrine. The association between lithium and goiter and hypothyroidism is well-recognized, with elevated risk in women and in patients with pre-existing thyroid disease. Both clinical and symptomatic subclinical hypothyroidism will improve with supplemental thyroid hormone. Less well appreciated are reports of hyperthyroidism occurring during lithium therapy or shortly after its discontinuation. Because subclinical hyperthyroidism may not be benign, careful attention must be paid to maintaining thyroid function well within the normal range.
Reports continue to accrue of lithium-related hypercalcemia and increased parathyroid hormone levels, with an occasional patient developing parathyroid hyperplasia or adenoma requiring surgical intervention.12 No specific guidelines have been established for monitoring serum calcium, but some authors have recommended periodic testing.
Weight. At least one-third of patients on lithium gain weight for a variety of reasons, such as altered lipid and carbohydrate metabolism, use of high-calorie fluids to combat polydipsia and polyuria, hypothyroidism, and the use of other drugs associated with weight gain. If weight gain occurs, recognize it early (weigh your patients) and institute appropriate dietary and exercise measures.
Hematologic. A mild, benign leukocytosis is seen sometimes during treatment with lithium. This effect has been harnessed to treat some neutropenic conditions. Lithium does not increase the risk of blood dyscrasias.
Dermatologic. Acne, psoriasis, and follicular keratosis may first appear or worsen during lithium therapy. Occasionally, otherwise successful lithium therapy has been rendered impossible by a dramatic dermatologic flare-up. Hair loss has also been associated with lithium use for unclear reasons, although hypothyroidism is occasionally a factor.
Renal. Impaired urinary concentrating ability and polyuria are common adverse effects. Both may be reversed with timely treatment discontinuation, but they may persist even after discontinuation in patients on long-term lithium treatment.12
Polyuria is largely nephrogenic in origin and, at times, can be voluminous, cause great inconvenience, and pose a risk of dehydration and lithium intoxication. Patients sometimes believe that thirst drives the polyuria and attempt to deal with it by restricting fluid intake, which can be quite dangerous. More appropriate interventions include dosage reduction (if possible) and the use of a thiazide and/or potassium-sparing diuretic. If diuretics are used, serum lithium concentrations may rise. Debate remains as to whether slow-or controlled-release preparations or single daily dosing are “kinder to the kidney.”
- Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning.
- Lithium is the only mood stabilizer that has been shown to reduce the risk of suicide during long-term treatment.
- Renal impairment, drug interactions, and unstable fluid-electrolyte balance increase the risk of lithium toxicity.
- Lithium does not adversely affect the liver or pancreas and may be the preferred mood stabilizer if these organs are diseased.
- Lithium has teratogenic potential but less than that of carbamazepine or valproate.
- Because lithium can disrupt thyroid function, baseline and ongoing thyroid function tests are recommended.
In recent years, there has been a disturbing increase in reports of elevated serum creatinine and reduced creatinine clearance associated with long-term lithium use.13 Because renal impairment has many causes, evaluation by a nephrologist is strongly advised. Then if the finger of causation points strongly at lithium, a careful risk/benefit analysis is in order. Even if lithium is discontinued—and especially if it is continued—regular renal function assessment is essential.
Rarely, lithium can cause a nephrotic syndrome (proteinuria, edema, decreased serum albumin, and increased serum lipids) that tends to be reversible with drug discontinuation.
Drug combinations
First the good news. Lithium tends to combine well with all the anticonvulsant mood stabilizers, making it the favored drug for combination therapies. Lithium/antidepressant combinations can be useful for treatment-resistant depression, although serotonin syndrome occasionally has been reported when lithium is combined with selective serotonin reuptake inhibitors.10,11 Using lithium with atypical antipsychotics is common, often effective, and usually well-tolerated.
Drug-drug interactions. Some nonpsychiatric drugs are associated with reduced renal lithium clearance and potential lithium toxicity. Because nonpsychiatrists usually prescribe these drugs, encourage patients taking lithium to ask their doctors about the possibility of interactions whenever a new drug is prescribed. Pharmacists can be particularly helpful in avoiding drug-drug interactions.
In patients taking diuretics, serum lithium concentrations are definitely increased by thiazides, possibly by potassium-sparing types, and occasionally by loop types. Osmotic and xanthine diuretics do just the opposite. Because diuretics are often used in medically unstable patients, assume that all can disrupt lithium balance.
Most nonsteroidal anti-inflammatory drugs can increase serum lithium levels, although dose and treatment duration are important variables. Aspirin and acetaminophen should not cause problems. The effect of COX-2 inhibitors on lithium levels has not been studied adequately, so these drugs should remain under suspicion.14
Lithium toxicity has been reported with angiotensin-converting enzyme (ACE) inhibitors, and their package inserts caution about this possibility. More recently, a few cases of lithium toxicity have been reported in patients taking angiotensin II receptor type-1 (AT-1) antagonists (e.g., candesartan, losartan, valsartan).15
Other, less well-substantiated pharmacokinetic and pharmacodynamic interactions that have been reported with lithium and other drugs can be researched, by using a computer-based drug interaction program or consulting with a drug information center.
Patient and clinician education
Both patients and clinicians have an obligation to ensure that lithium (or any other drug) is used safely and effectively (Box 4). Excellent sources of continuing education are listed below in “Related resources.” Rather than fall prey to the illusion that lithium therapy is a “vanishing art,” it would be better for clinicians to heed these words from the APA’s 2002 practice guidelines for bipolar disorder:
“No other treatment has performed as well as lithium in as many aspects of long-term care of bipolar disorder patients, and despite some risks and limitations lithium remains the standard against which all proposed alternatives are compared.”6
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Lithium Information Center. www.miminc.org
Drug brand names
- Chlorpromazine • Thorazine
- Divalproex • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Primidone • Mysoline
- Propranolol • Inderal
Disclosure
Dr. Jefferson receives grant/research support from Abbott Laboratories, Bristol-Myers Squibb Co., Forest Laboratories Inc., GlaxoSmithKline, Eli Lilly and Co., Novartis Pharmaceuticals Corp., Organon, Janssen Pharmaceutica, Pfizer Inc., Solvay, and Wyeth Pharmaceuticals. He also serves as a consultant to GlaxoSmithKline, Novartis Pharmaceuticals Corp., Solvay, and UCB Pharma.
1. Tondo L, Hennen J, Baldessarini RJ, et al. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand 2001;104:163-72.
2. Baldessarini RJ, Tondo L, Hennen J, et al. Is lithium still worth using? An update of selected recent research. Harvard Rev Psychiatry 2002;10(2):59-75.
3. Sadock BJ. Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry. Philadelphia: Lippincott Williams & Wilkins, 2000;2377-90.
4. Bauer MS, Callahan AM, Jampala C, et al. Clinical practice guidelines for bipolar disorder from the Department of Veterans Affairs. J Clin Psychiatry 1999;60(1):9-21.
5. Sachs GS, Printz DJ, Kahn DA, et al. Medication treatment of bipolar disorder. Postgrad Med Special Report 2000;Apr:1-104.
6. American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159:1.-
7. Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002;356:241-7.
8. Cohen LS, Rosenbaum JP. Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry 1998;59(suppl 2):18-28.
9. Baldessarini RJ, Tondo L. Recurrence risk in bipolar manic-depressive disorders after discontinuing lithium maintenance treatment: an overview. Clin Drug Invest 1998;15(4):337-51.
10. Jefferson JW. Lithium. In: Dukes MNG, Aronson JK (eds). Meyler’s side effects of drugs (14th ed). Amsterdam: Elsevier Science BV, 2001;86-94.
11. Jefferson JW. Lithium. In: Aronson JK (ed). Side effects of drugs, annual 24. Amsterdam: Elsevier Science BV, 2001;22-31.
12. Abdull H, Bliss R, Guinea AE, et al. Pathology and outcome of surgical treatment for lithium-associated hyperparathyroidism. Br J Surg 1999;86:91-3.
13. Gitlin M. Lithium and the kidney. An updated review. Drug Safety 1999;20(3):231-43.
14. Lundmark J, Gunnarsson T, Bengtsson F. A possible interaction between lithium and rofecoxib (letter to the editor). Br J Clin Pharmacol 2002;53(4):403-4.
15. Zwanzger P, Marcuse A, Boerner RJ, et al. Lithium intoxication after administration of AT1 blockers. J Clin Psychiatry 2001;62(3):208-9.
As a mood stabilizer for patients with bipolar disorder, lithium was the darling of U.S. psychiatry from the 1970s to well into the 1990s. It then began an ill-deserved, gradual fall from grace and today could be considered a pharmaceutical endangered species. But why?
Did lithium lose effectiveness? Is it too toxic? Is its side effect burden too heavy? Does it interact adversely with too many medicines? Is it too cumbersome to use? Was it just a fad whose time came and went—a psychiatric pet rock? Did it fall prey to the marketing might behind patent-protected drugs? Was it replaced by more effective and safer drugs?
You are partially correct if you checked “all of the above,” because all contain a kernel of truth. At the same time, each is an exaggeration that does grave injustice to a remarkable medication. In addition, psychiatry appears to pay only lip service to convincing evidence that lithium is the only mood stabilizer that reduces the risk of suicide during long-term treatment.1
Some psychiatrists rationalize that “lithium is too difficult to use, so I never prescribe it.”2,3 My response is simply, “try it, and I think you’ll like it.” Measuring serum lithium concentrations is simple, accurate, and inexpensive. And we know quite a bit about how lithium dosage and blood level relate to response and tolerability.
Where does lithium stand?
Lithium is the first solid element in the periodic table (atomic number 3, atomic weight 6.94) (Box 1). As a treatment for bipolar disorder, lithium’s rise to prominence in the United States was far from rapid. Its antimanic properties were described by John Cade in Australia in 1949 in an open-label case series, but it was not FDA-approved for 20 years—for acute manic episodes in 1970 and for maintenance therapy “in those manic depressive patients with a history of mania” in 1974. Today, lithium shares FDA-approved manic episode billing with chlorpromazine (1973), divalproex (1995), and olanzapine (2000), but it remains the only FDA-approved drug for maintenance (although the FDA is considering a bipolar depression maintenance indication for lamotrigine).
Lithium has no meaningful protein binding and no metabolites, being excreted almost entirely by the kidneys. Its elimination half-life of 18 to 24 hours may be longer in the elderly and shorter in youth because of age-dependent variations in glomerular filtration rate. For unclear reasons, renal lithium clearance appears to be more rapid in obese persons.
Lithium preparations available in the United States include standard-release (150, 300, 600 mg), slow-release (Lithobid and generic 300 mg), and controlled-release (Eskalith CR 450 mg) forms of lithium carbonate and a lithium citrate liquid. Lithium carbonate, 300 mg, and lithium citrate, 5 cc, each contain about 8 mmols of lithium. Lithium and lithium carbonate are not the same—there are 56.36 mg of lithium in 300 mg of lithium carbonate. The correct formula for lithium carbonate is Li2CO3, not LiCO3 as is commonly and erroneously written.
With the standard-release preparation, peak serum levels are reached in about 1 1/2 hours and with the slow- and controlled-release forms in about 4 to 4 1/2 hours. At times, the slower-release forms may be better tolerated, but they are also a bit more costly (although all forms of lithium are inexpensive, compared with other mood stabilizers).
If you examine lithium’s status relative to other bipolar medications, you’ll find some inconsistencies. For example:
- Clinical practice guidelines from the Department of Veterans Affairs (January 1999) recommended lithium as the first-line agent for acute and prophylactic treatment of manic and mixed states, bipolar depression, and rapid cycling.4
- The Expert Consensus Guidelines (April 2000) gave at least equal billing—if not preferred status—to divalproex for those indications.5
- The American Psychiatric Association’s (APA) revised guidelines (April 2002) gave the nod to lithium for classic elated mania and bipolar depression but to divalproex for mixed mania and rapid cycling.6 Divalproex was rated comparable to lithium for maintenance therapy, despite the lack of convincing data.
- The European perspective (January 2002) is most similar to that of the Department of Veterans Affairs, favoring lithium for acute mania, bipolar depression, and long-term treatment.7
There is no clear winner (or loser) in the battle for bipolar marketplace supremacy. The belief that one drug does everything is a fantasy for all but a small minority of patients with bipolar disorder. Polypharmacy is the rule, and rational polypharmacy the goal. To exclude lithium from the arsenal of bipolar drugs would be folly, yet lithium prescribing seems to have become a vanishing art. One of my psychopharmacologist colleagues recently expressed bewilderment at the number of “treatment-resistant” bipolar patients referred to him who had never been treated with lithium.
Diagnosis matters
Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning. Its potential benefits, however, clearly extend to all other aspects of bipolar disorder, to augmentation for treatment-resistant major depressive disorder, to schizoaffective disorder, and—at times—to aggressive states. As the bipolar spectrum expands, it is hardly surprising that the effectiveness of lithium (or any other drug) lessens as we approach the periphery of the spectrum.
Blood levels and dosing
Recommended lithium serum concentrations are given as ranges, realizing that individual variability makes exact numbers impractical. Package inserts for lithium products list serum concentrations between 1.0 and 1.5 mEq/L for acute mania and 0.6 and 1.2 mEq/L for maintenance therapy. The APA’s revised guidelines are a bit more conservative, recommending 0.5 to 1.2 mEq/L for acute mania and waffling somewhat on maintenance.4 Many patients on maintenance therapy do well at levels between 0.6 and 0.8 mEq/L, and some prosper at even lower levels.
To avoid obtaining a misleading blood level:
- Samples should be drawn in the morning as close as possible to 12 hours after the last dose.
- Steady state conditions should exist, usually meaning 4 or 5 days on the same dosage without any missed or extra doses (Box 2).
Start treatment using divided dosages, but—following stabilization—once-daily dosing is possible for many patients. If lithium is taken as a single daily dose, 12-hour blood levels will be somewhat higher than with multiple daily dosing. Single and multiple daily dosing are similarly effective, but once-daily dosing may have a compliance and tolerability edge in some patients.
Considering individual patient variability, a lithium carbonate dosage of 1,200 to 1,800 mg/d is likely to be therapeutic for mania and 900 to 1,200 mg/d for maintenance in otherwise healthy, nongeriatric adults.
Starting and maintaining lithium
Medical history. Assuming that lithium theapy is indicated, obtain a detailed medical history. Focus on findings that increase the risk of lithium toxicity, such as renal impairment, drug interactions, and unstable fluid-electrolyte balance.
Although lithium is not contraindicated in patients with renal disease, using an alternate drug is probably preferable. On the other hand, because lithium does not adversely affect the liver or pancreas, it may be preferred to some other mood stabilizers if these organs are diseased.
A thorough diet and drug history is also important. Because low-sodium diets reduce renal lithium clearance, lower doses may be required to reach a given serum concentration. Some drugs alter lithium excretion and can increase or decrease blood levels (see “Drug combinations,”).
Advise women of childbearing age about lithium’s teratogenic potential (which is considerably less than that of carbamazepine or valproate). The risk of cardiovascular malformation of the fetus has been estimated at 1/1,000 to 1/2,000 births among women who took lithium during the first trimester of pregnancy.8
- Draw samples in the morning, as close as possible to 12 hours after the last dose.
- Measure serum levels at steady state, at least 4 or 5 days on the same dosage without any missed or extra doses.
Baseline lab tests. Assessing renal function is essential. A serum creatinine level will usually suffice, unless a history of renal disease suggests the need for a more extensive evaluation, such as creatinine clearance, renal ultrasound, or nephrology consultation.
A urinalysis is often part of the package. Because thyroid dysfunction can alter mood and lithium can disrupt thyroid function, baseline TSH and T4 tests are recommended. CBC is optional (lithium can cause leukocytosis). The medical history should determine whether additional blood work is necessary. An ECG is sometimes advised in older patients, especially if the history suggests cardiovascular disease. Finally, don’t forget a pregnancy test in women of childbearing potential (Box 3).
Monitoring. Early in the course of therapy, lithium blood levels are usually obtained at 5- to 7-day intervals until the patient is stabilized. After that, assuming all is well, routine monitoring can occur every 3, 4, or even 6 months, depending on the individual’s reliability and stability. Because ongoing assessment of renal and thyroid function is also important, it makes sense to obtain:
- a serum creatinine measurement linked to each lithium level
- and a serum TSH yearly or at the slightest indication of thyroid dysfunction, such as fatigue, weight gain, cognitive impairment, cold intolerance, or depression.
Stopping lithium. Lithium can be discontinued abruptly without side effects if it is ineffective or not tolerated. Stopping lithium after successful long-term use is another story. There is a high likelihood of illness recurrence and a small but real possibility that lithium will be ineffective when restarted. Also, abrupt or rapid discontinuation (within 1 to 14 days) is believed to increase the likelihood of earlier recurrence, compared with more gradual discontinuation.9
Side effects and toxicity
One reason for lithium’s slide in popularity is its perceived side-effect profile. Toxic amounts can be lethal, and therapeutic amounts can be bothersome. Yet concerns are often exaggerated because of lack of familiarity with the drug.10,11
Intoxication. Lithium does have a narrow therapeutic index, with toxicity related to serum concentration and duration of exposure. Acute overdoses, while not benign, are often better tolerated than gradual, more tissue-saturating exposures. Idiosyncratic factors are also involved, as evidenced by documented toxicity at “therapeutic” levels and tolerability despite very high levels.
Early warnings of impending toxicity include:
- neurologic findings such as dysarthria, new or worsening tremor, and ataxia
- gastrointestinal symptoms such as anorexia, nausea, vomiting, and diarrhea.
Severe toxicity can be fatal or cause permanent neurologic (often cerebellar) damage. Causes of intoxication range from deliberate overdose to renal impairment, low-sodium diets, drug interactions, and dehydration. At particular risk are patients with lithium-induced polyuria whose access to fluid replacement is compromised.
Treatment involves reducing absorption, increasing excretion, and restoring fluid-electrolyte balance. Severe intoxication, especially if renal function is impaired, is best treated with hemodialysis.
| Test | Indication |
|---|---|
| Serum creatinine, urinalysis | To screen for renal function |
| TSH and T4 | To establish baseline thyroid function |
| CBC (optional) | If indicated by patient’s overall medical condition or because some doctors prefer to do more general screening |
| ECG (optional) | For patients with risk factors for heart disease |
| Pregnancy test | For at-risk women because of lithium’s teratogenic potential |
Neurologic. Mild neurologic complaints such as memory impairment, slow reaction time, lack of spontaneity, and lost creativity have been ascribed to lithium and may lead to noncompliance. Under such circumstances, other diagnostic considerations include breakthrough depression, hypothyroidism, other illness, hypercalcemia, other medications, and absence of hypomania.
Like valproate, lithium can cause a benign postural tremor that is usually tolerable and often transient. Should the tremor be problematic, treatment considerations include dosage reduction, switching to a slow-release preparation, reducing caffeine intake, avoiding other tremor-causing drugs such as theophylline or stimulants, and treating associated anxiety. If an anti-tremor drug is needed, a beta-blocker such as propranolol is used most commonly; other options to consider are primidone and gabapentin. Don’t forget that a worsening tremor may indicate impending toxicity.
Very rarely, lithium has been associated with pseudotumor cerebri (benign intracranial hypertension), peripheral neuropathy, and a myasthenia gravis-like syndrome.
Cardiovascular. Like many drugs, lithium can cause benign ST-T wave changes on ECG.
More serious, but fortunately quite uncommon, is lithium-induced sinus node dysfunction manifesting as a variety of bradyarrhythmias and, at times, syncopal episodes. Since normal aging is associated with a gradual loss of sinus node pacemaker cells, the elderly may be especially sensitive to this problem. Unless a pacemaker is implanted, sinus node dysfunction usually requires lithium discontinuation.
Endocrine. The association between lithium and goiter and hypothyroidism is well-recognized, with elevated risk in women and in patients with pre-existing thyroid disease. Both clinical and symptomatic subclinical hypothyroidism will improve with supplemental thyroid hormone. Less well appreciated are reports of hyperthyroidism occurring during lithium therapy or shortly after its discontinuation. Because subclinical hyperthyroidism may not be benign, careful attention must be paid to maintaining thyroid function well within the normal range.
Reports continue to accrue of lithium-related hypercalcemia and increased parathyroid hormone levels, with an occasional patient developing parathyroid hyperplasia or adenoma requiring surgical intervention.12 No specific guidelines have been established for monitoring serum calcium, but some authors have recommended periodic testing.
Weight. At least one-third of patients on lithium gain weight for a variety of reasons, such as altered lipid and carbohydrate metabolism, use of high-calorie fluids to combat polydipsia and polyuria, hypothyroidism, and the use of other drugs associated with weight gain. If weight gain occurs, recognize it early (weigh your patients) and institute appropriate dietary and exercise measures.
Hematologic. A mild, benign leukocytosis is seen sometimes during treatment with lithium. This effect has been harnessed to treat some neutropenic conditions. Lithium does not increase the risk of blood dyscrasias.
Dermatologic. Acne, psoriasis, and follicular keratosis may first appear or worsen during lithium therapy. Occasionally, otherwise successful lithium therapy has been rendered impossible by a dramatic dermatologic flare-up. Hair loss has also been associated with lithium use for unclear reasons, although hypothyroidism is occasionally a factor.
Renal. Impaired urinary concentrating ability and polyuria are common adverse effects. Both may be reversed with timely treatment discontinuation, but they may persist even after discontinuation in patients on long-term lithium treatment.12
Polyuria is largely nephrogenic in origin and, at times, can be voluminous, cause great inconvenience, and pose a risk of dehydration and lithium intoxication. Patients sometimes believe that thirst drives the polyuria and attempt to deal with it by restricting fluid intake, which can be quite dangerous. More appropriate interventions include dosage reduction (if possible) and the use of a thiazide and/or potassium-sparing diuretic. If diuretics are used, serum lithium concentrations may rise. Debate remains as to whether slow-or controlled-release preparations or single daily dosing are “kinder to the kidney.”
- Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning.
- Lithium is the only mood stabilizer that has been shown to reduce the risk of suicide during long-term treatment.
- Renal impairment, drug interactions, and unstable fluid-electrolyte balance increase the risk of lithium toxicity.
- Lithium does not adversely affect the liver or pancreas and may be the preferred mood stabilizer if these organs are diseased.
- Lithium has teratogenic potential but less than that of carbamazepine or valproate.
- Because lithium can disrupt thyroid function, baseline and ongoing thyroid function tests are recommended.
In recent years, there has been a disturbing increase in reports of elevated serum creatinine and reduced creatinine clearance associated with long-term lithium use.13 Because renal impairment has many causes, evaluation by a nephrologist is strongly advised. Then if the finger of causation points strongly at lithium, a careful risk/benefit analysis is in order. Even if lithium is discontinued—and especially if it is continued—regular renal function assessment is essential.
Rarely, lithium can cause a nephrotic syndrome (proteinuria, edema, decreased serum albumin, and increased serum lipids) that tends to be reversible with drug discontinuation.
Drug combinations
First the good news. Lithium tends to combine well with all the anticonvulsant mood stabilizers, making it the favored drug for combination therapies. Lithium/antidepressant combinations can be useful for treatment-resistant depression, although serotonin syndrome occasionally has been reported when lithium is combined with selective serotonin reuptake inhibitors.10,11 Using lithium with atypical antipsychotics is common, often effective, and usually well-tolerated.
Drug-drug interactions. Some nonpsychiatric drugs are associated with reduced renal lithium clearance and potential lithium toxicity. Because nonpsychiatrists usually prescribe these drugs, encourage patients taking lithium to ask their doctors about the possibility of interactions whenever a new drug is prescribed. Pharmacists can be particularly helpful in avoiding drug-drug interactions.
In patients taking diuretics, serum lithium concentrations are definitely increased by thiazides, possibly by potassium-sparing types, and occasionally by loop types. Osmotic and xanthine diuretics do just the opposite. Because diuretics are often used in medically unstable patients, assume that all can disrupt lithium balance.
Most nonsteroidal anti-inflammatory drugs can increase serum lithium levels, although dose and treatment duration are important variables. Aspirin and acetaminophen should not cause problems. The effect of COX-2 inhibitors on lithium levels has not been studied adequately, so these drugs should remain under suspicion.14
Lithium toxicity has been reported with angiotensin-converting enzyme (ACE) inhibitors, and their package inserts caution about this possibility. More recently, a few cases of lithium toxicity have been reported in patients taking angiotensin II receptor type-1 (AT-1) antagonists (e.g., candesartan, losartan, valsartan).15
Other, less well-substantiated pharmacokinetic and pharmacodynamic interactions that have been reported with lithium and other drugs can be researched, by using a computer-based drug interaction program or consulting with a drug information center.
Patient and clinician education
Both patients and clinicians have an obligation to ensure that lithium (or any other drug) is used safely and effectively (Box 4). Excellent sources of continuing education are listed below in “Related resources.” Rather than fall prey to the illusion that lithium therapy is a “vanishing art,” it would be better for clinicians to heed these words from the APA’s 2002 practice guidelines for bipolar disorder:
“No other treatment has performed as well as lithium in as many aspects of long-term care of bipolar disorder patients, and despite some risks and limitations lithium remains the standard against which all proposed alternatives are compared.”6
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Lithium Information Center. www.miminc.org
Drug brand names
- Chlorpromazine • Thorazine
- Divalproex • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Primidone • Mysoline
- Propranolol • Inderal
Disclosure
Dr. Jefferson receives grant/research support from Abbott Laboratories, Bristol-Myers Squibb Co., Forest Laboratories Inc., GlaxoSmithKline, Eli Lilly and Co., Novartis Pharmaceuticals Corp., Organon, Janssen Pharmaceutica, Pfizer Inc., Solvay, and Wyeth Pharmaceuticals. He also serves as a consultant to GlaxoSmithKline, Novartis Pharmaceuticals Corp., Solvay, and UCB Pharma.
As a mood stabilizer for patients with bipolar disorder, lithium was the darling of U.S. psychiatry from the 1970s to well into the 1990s. It then began an ill-deserved, gradual fall from grace and today could be considered a pharmaceutical endangered species. But why?
Did lithium lose effectiveness? Is it too toxic? Is its side effect burden too heavy? Does it interact adversely with too many medicines? Is it too cumbersome to use? Was it just a fad whose time came and went—a psychiatric pet rock? Did it fall prey to the marketing might behind patent-protected drugs? Was it replaced by more effective and safer drugs?
You are partially correct if you checked “all of the above,” because all contain a kernel of truth. At the same time, each is an exaggeration that does grave injustice to a remarkable medication. In addition, psychiatry appears to pay only lip service to convincing evidence that lithium is the only mood stabilizer that reduces the risk of suicide during long-term treatment.1
Some psychiatrists rationalize that “lithium is too difficult to use, so I never prescribe it.”2,3 My response is simply, “try it, and I think you’ll like it.” Measuring serum lithium concentrations is simple, accurate, and inexpensive. And we know quite a bit about how lithium dosage and blood level relate to response and tolerability.
Where does lithium stand?
Lithium is the first solid element in the periodic table (atomic number 3, atomic weight 6.94) (Box 1). As a treatment for bipolar disorder, lithium’s rise to prominence in the United States was far from rapid. Its antimanic properties were described by John Cade in Australia in 1949 in an open-label case series, but it was not FDA-approved for 20 years—for acute manic episodes in 1970 and for maintenance therapy “in those manic depressive patients with a history of mania” in 1974. Today, lithium shares FDA-approved manic episode billing with chlorpromazine (1973), divalproex (1995), and olanzapine (2000), but it remains the only FDA-approved drug for maintenance (although the FDA is considering a bipolar depression maintenance indication for lamotrigine).
Lithium has no meaningful protein binding and no metabolites, being excreted almost entirely by the kidneys. Its elimination half-life of 18 to 24 hours may be longer in the elderly and shorter in youth because of age-dependent variations in glomerular filtration rate. For unclear reasons, renal lithium clearance appears to be more rapid in obese persons.
Lithium preparations available in the United States include standard-release (150, 300, 600 mg), slow-release (Lithobid and generic 300 mg), and controlled-release (Eskalith CR 450 mg) forms of lithium carbonate and a lithium citrate liquid. Lithium carbonate, 300 mg, and lithium citrate, 5 cc, each contain about 8 mmols of lithium. Lithium and lithium carbonate are not the same—there are 56.36 mg of lithium in 300 mg of lithium carbonate. The correct formula for lithium carbonate is Li2CO3, not LiCO3 as is commonly and erroneously written.
With the standard-release preparation, peak serum levels are reached in about 1 1/2 hours and with the slow- and controlled-release forms in about 4 to 4 1/2 hours. At times, the slower-release forms may be better tolerated, but they are also a bit more costly (although all forms of lithium are inexpensive, compared with other mood stabilizers).
If you examine lithium’s status relative to other bipolar medications, you’ll find some inconsistencies. For example:
- Clinical practice guidelines from the Department of Veterans Affairs (January 1999) recommended lithium as the first-line agent for acute and prophylactic treatment of manic and mixed states, bipolar depression, and rapid cycling.4
- The Expert Consensus Guidelines (April 2000) gave at least equal billing—if not preferred status—to divalproex for those indications.5
- The American Psychiatric Association’s (APA) revised guidelines (April 2002) gave the nod to lithium for classic elated mania and bipolar depression but to divalproex for mixed mania and rapid cycling.6 Divalproex was rated comparable to lithium for maintenance therapy, despite the lack of convincing data.
- The European perspective (January 2002) is most similar to that of the Department of Veterans Affairs, favoring lithium for acute mania, bipolar depression, and long-term treatment.7
There is no clear winner (or loser) in the battle for bipolar marketplace supremacy. The belief that one drug does everything is a fantasy for all but a small minority of patients with bipolar disorder. Polypharmacy is the rule, and rational polypharmacy the goal. To exclude lithium from the arsenal of bipolar drugs would be folly, yet lithium prescribing seems to have become a vanishing art. One of my psychopharmacologist colleagues recently expressed bewilderment at the number of “treatment-resistant” bipolar patients referred to him who had never been treated with lithium.
Diagnosis matters
Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning. Its potential benefits, however, clearly extend to all other aspects of bipolar disorder, to augmentation for treatment-resistant major depressive disorder, to schizoaffective disorder, and—at times—to aggressive states. As the bipolar spectrum expands, it is hardly surprising that the effectiveness of lithium (or any other drug) lessens as we approach the periphery of the spectrum.
Blood levels and dosing
Recommended lithium serum concentrations are given as ranges, realizing that individual variability makes exact numbers impractical. Package inserts for lithium products list serum concentrations between 1.0 and 1.5 mEq/L for acute mania and 0.6 and 1.2 mEq/L for maintenance therapy. The APA’s revised guidelines are a bit more conservative, recommending 0.5 to 1.2 mEq/L for acute mania and waffling somewhat on maintenance.4 Many patients on maintenance therapy do well at levels between 0.6 and 0.8 mEq/L, and some prosper at even lower levels.
To avoid obtaining a misleading blood level:
- Samples should be drawn in the morning as close as possible to 12 hours after the last dose.
- Steady state conditions should exist, usually meaning 4 or 5 days on the same dosage without any missed or extra doses (Box 2).
Start treatment using divided dosages, but—following stabilization—once-daily dosing is possible for many patients. If lithium is taken as a single daily dose, 12-hour blood levels will be somewhat higher than with multiple daily dosing. Single and multiple daily dosing are similarly effective, but once-daily dosing may have a compliance and tolerability edge in some patients.
Considering individual patient variability, a lithium carbonate dosage of 1,200 to 1,800 mg/d is likely to be therapeutic for mania and 900 to 1,200 mg/d for maintenance in otherwise healthy, nongeriatric adults.
Starting and maintaining lithium
Medical history. Assuming that lithium theapy is indicated, obtain a detailed medical history. Focus on findings that increase the risk of lithium toxicity, such as renal impairment, drug interactions, and unstable fluid-electrolyte balance.
Although lithium is not contraindicated in patients with renal disease, using an alternate drug is probably preferable. On the other hand, because lithium does not adversely affect the liver or pancreas, it may be preferred to some other mood stabilizers if these organs are diseased.
A thorough diet and drug history is also important. Because low-sodium diets reduce renal lithium clearance, lower doses may be required to reach a given serum concentration. Some drugs alter lithium excretion and can increase or decrease blood levels (see “Drug combinations,”).
Advise women of childbearing age about lithium’s teratogenic potential (which is considerably less than that of carbamazepine or valproate). The risk of cardiovascular malformation of the fetus has been estimated at 1/1,000 to 1/2,000 births among women who took lithium during the first trimester of pregnancy.8
- Draw samples in the morning, as close as possible to 12 hours after the last dose.
- Measure serum levels at steady state, at least 4 or 5 days on the same dosage without any missed or extra doses.
Baseline lab tests. Assessing renal function is essential. A serum creatinine level will usually suffice, unless a history of renal disease suggests the need for a more extensive evaluation, such as creatinine clearance, renal ultrasound, or nephrology consultation.
A urinalysis is often part of the package. Because thyroid dysfunction can alter mood and lithium can disrupt thyroid function, baseline TSH and T4 tests are recommended. CBC is optional (lithium can cause leukocytosis). The medical history should determine whether additional blood work is necessary. An ECG is sometimes advised in older patients, especially if the history suggests cardiovascular disease. Finally, don’t forget a pregnancy test in women of childbearing potential (Box 3).
Monitoring. Early in the course of therapy, lithium blood levels are usually obtained at 5- to 7-day intervals until the patient is stabilized. After that, assuming all is well, routine monitoring can occur every 3, 4, or even 6 months, depending on the individual’s reliability and stability. Because ongoing assessment of renal and thyroid function is also important, it makes sense to obtain:
- a serum creatinine measurement linked to each lithium level
- and a serum TSH yearly or at the slightest indication of thyroid dysfunction, such as fatigue, weight gain, cognitive impairment, cold intolerance, or depression.
Stopping lithium. Lithium can be discontinued abruptly without side effects if it is ineffective or not tolerated. Stopping lithium after successful long-term use is another story. There is a high likelihood of illness recurrence and a small but real possibility that lithium will be ineffective when restarted. Also, abrupt or rapid discontinuation (within 1 to 14 days) is believed to increase the likelihood of earlier recurrence, compared with more gradual discontinuation.9
Side effects and toxicity
One reason for lithium’s slide in popularity is its perceived side-effect profile. Toxic amounts can be lethal, and therapeutic amounts can be bothersome. Yet concerns are often exaggerated because of lack of familiarity with the drug.10,11
Intoxication. Lithium does have a narrow therapeutic index, with toxicity related to serum concentration and duration of exposure. Acute overdoses, while not benign, are often better tolerated than gradual, more tissue-saturating exposures. Idiosyncratic factors are also involved, as evidenced by documented toxicity at “therapeutic” levels and tolerability despite very high levels.
Early warnings of impending toxicity include:
- neurologic findings such as dysarthria, new or worsening tremor, and ataxia
- gastrointestinal symptoms such as anorexia, nausea, vomiting, and diarrhea.
Severe toxicity can be fatal or cause permanent neurologic (often cerebellar) damage. Causes of intoxication range from deliberate overdose to renal impairment, low-sodium diets, drug interactions, and dehydration. At particular risk are patients with lithium-induced polyuria whose access to fluid replacement is compromised.
Treatment involves reducing absorption, increasing excretion, and restoring fluid-electrolyte balance. Severe intoxication, especially if renal function is impaired, is best treated with hemodialysis.
| Test | Indication |
|---|---|
| Serum creatinine, urinalysis | To screen for renal function |
| TSH and T4 | To establish baseline thyroid function |
| CBC (optional) | If indicated by patient’s overall medical condition or because some doctors prefer to do more general screening |
| ECG (optional) | For patients with risk factors for heart disease |
| Pregnancy test | For at-risk women because of lithium’s teratogenic potential |
Neurologic. Mild neurologic complaints such as memory impairment, slow reaction time, lack of spontaneity, and lost creativity have been ascribed to lithium and may lead to noncompliance. Under such circumstances, other diagnostic considerations include breakthrough depression, hypothyroidism, other illness, hypercalcemia, other medications, and absence of hypomania.
Like valproate, lithium can cause a benign postural tremor that is usually tolerable and often transient. Should the tremor be problematic, treatment considerations include dosage reduction, switching to a slow-release preparation, reducing caffeine intake, avoiding other tremor-causing drugs such as theophylline or stimulants, and treating associated anxiety. If an anti-tremor drug is needed, a beta-blocker such as propranolol is used most commonly; other options to consider are primidone and gabapentin. Don’t forget that a worsening tremor may indicate impending toxicity.
Very rarely, lithium has been associated with pseudotumor cerebri (benign intracranial hypertension), peripheral neuropathy, and a myasthenia gravis-like syndrome.
Cardiovascular. Like many drugs, lithium can cause benign ST-T wave changes on ECG.
More serious, but fortunately quite uncommon, is lithium-induced sinus node dysfunction manifesting as a variety of bradyarrhythmias and, at times, syncopal episodes. Since normal aging is associated with a gradual loss of sinus node pacemaker cells, the elderly may be especially sensitive to this problem. Unless a pacemaker is implanted, sinus node dysfunction usually requires lithium discontinuation.
Endocrine. The association between lithium and goiter and hypothyroidism is well-recognized, with elevated risk in women and in patients with pre-existing thyroid disease. Both clinical and symptomatic subclinical hypothyroidism will improve with supplemental thyroid hormone. Less well appreciated are reports of hyperthyroidism occurring during lithium therapy or shortly after its discontinuation. Because subclinical hyperthyroidism may not be benign, careful attention must be paid to maintaining thyroid function well within the normal range.
Reports continue to accrue of lithium-related hypercalcemia and increased parathyroid hormone levels, with an occasional patient developing parathyroid hyperplasia or adenoma requiring surgical intervention.12 No specific guidelines have been established for monitoring serum calcium, but some authors have recommended periodic testing.
Weight. At least one-third of patients on lithium gain weight for a variety of reasons, such as altered lipid and carbohydrate metabolism, use of high-calorie fluids to combat polydipsia and polyuria, hypothyroidism, and the use of other drugs associated with weight gain. If weight gain occurs, recognize it early (weigh your patients) and institute appropriate dietary and exercise measures.
Hematologic. A mild, benign leukocytosis is seen sometimes during treatment with lithium. This effect has been harnessed to treat some neutropenic conditions. Lithium does not increase the risk of blood dyscrasias.
Dermatologic. Acne, psoriasis, and follicular keratosis may first appear or worsen during lithium therapy. Occasionally, otherwise successful lithium therapy has been rendered impossible by a dramatic dermatologic flare-up. Hair loss has also been associated with lithium use for unclear reasons, although hypothyroidism is occasionally a factor.
Renal. Impaired urinary concentrating ability and polyuria are common adverse effects. Both may be reversed with timely treatment discontinuation, but they may persist even after discontinuation in patients on long-term lithium treatment.12
Polyuria is largely nephrogenic in origin and, at times, can be voluminous, cause great inconvenience, and pose a risk of dehydration and lithium intoxication. Patients sometimes believe that thirst drives the polyuria and attempt to deal with it by restricting fluid intake, which can be quite dangerous. More appropriate interventions include dosage reduction (if possible) and the use of a thiazide and/or potassium-sparing diuretic. If diuretics are used, serum lithium concentrations may rise. Debate remains as to whether slow-or controlled-release preparations or single daily dosing are “kinder to the kidney.”
- Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning.
- Lithium is the only mood stabilizer that has been shown to reduce the risk of suicide during long-term treatment.
- Renal impairment, drug interactions, and unstable fluid-electrolyte balance increase the risk of lithium toxicity.
- Lithium does not adversely affect the liver or pancreas and may be the preferred mood stabilizer if these organs are diseased.
- Lithium has teratogenic potential but less than that of carbamazepine or valproate.
- Because lithium can disrupt thyroid function, baseline and ongoing thyroid function tests are recommended.
In recent years, there has been a disturbing increase in reports of elevated serum creatinine and reduced creatinine clearance associated with long-term lithium use.13 Because renal impairment has many causes, evaluation by a nephrologist is strongly advised. Then if the finger of causation points strongly at lithium, a careful risk/benefit analysis is in order. Even if lithium is discontinued—and especially if it is continued—regular renal function assessment is essential.
Rarely, lithium can cause a nephrotic syndrome (proteinuria, edema, decreased serum albumin, and increased serum lipids) that tends to be reversible with drug discontinuation.
Drug combinations
First the good news. Lithium tends to combine well with all the anticonvulsant mood stabilizers, making it the favored drug for combination therapies. Lithium/antidepressant combinations can be useful for treatment-resistant depression, although serotonin syndrome occasionally has been reported when lithium is combined with selective serotonin reuptake inhibitors.10,11 Using lithium with atypical antipsychotics is common, often effective, and usually well-tolerated.
Drug-drug interactions. Some nonpsychiatric drugs are associated with reduced renal lithium clearance and potential lithium toxicity. Because nonpsychiatrists usually prescribe these drugs, encourage patients taking lithium to ask their doctors about the possibility of interactions whenever a new drug is prescribed. Pharmacists can be particularly helpful in avoiding drug-drug interactions.
In patients taking diuretics, serum lithium concentrations are definitely increased by thiazides, possibly by potassium-sparing types, and occasionally by loop types. Osmotic and xanthine diuretics do just the opposite. Because diuretics are often used in medically unstable patients, assume that all can disrupt lithium balance.
Most nonsteroidal anti-inflammatory drugs can increase serum lithium levels, although dose and treatment duration are important variables. Aspirin and acetaminophen should not cause problems. The effect of COX-2 inhibitors on lithium levels has not been studied adequately, so these drugs should remain under suspicion.14
Lithium toxicity has been reported with angiotensin-converting enzyme (ACE) inhibitors, and their package inserts caution about this possibility. More recently, a few cases of lithium toxicity have been reported in patients taking angiotensin II receptor type-1 (AT-1) antagonists (e.g., candesartan, losartan, valsartan).15
Other, less well-substantiated pharmacokinetic and pharmacodynamic interactions that have been reported with lithium and other drugs can be researched, by using a computer-based drug interaction program or consulting with a drug information center.
Patient and clinician education
Both patients and clinicians have an obligation to ensure that lithium (or any other drug) is used safely and effectively (Box 4). Excellent sources of continuing education are listed below in “Related resources.” Rather than fall prey to the illusion that lithium therapy is a “vanishing art,” it would be better for clinicians to heed these words from the APA’s 2002 practice guidelines for bipolar disorder:
“No other treatment has performed as well as lithium in as many aspects of long-term care of bipolar disorder patients, and despite some risks and limitations lithium remains the standard against which all proposed alternatives are compared.”6
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Lithium Information Center. www.miminc.org
Drug brand names
- Chlorpromazine • Thorazine
- Divalproex • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Primidone • Mysoline
- Propranolol • Inderal
Disclosure
Dr. Jefferson receives grant/research support from Abbott Laboratories, Bristol-Myers Squibb Co., Forest Laboratories Inc., GlaxoSmithKline, Eli Lilly and Co., Novartis Pharmaceuticals Corp., Organon, Janssen Pharmaceutica, Pfizer Inc., Solvay, and Wyeth Pharmaceuticals. He also serves as a consultant to GlaxoSmithKline, Novartis Pharmaceuticals Corp., Solvay, and UCB Pharma.
1. Tondo L, Hennen J, Baldessarini RJ, et al. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand 2001;104:163-72.
2. Baldessarini RJ, Tondo L, Hennen J, et al. Is lithium still worth using? An update of selected recent research. Harvard Rev Psychiatry 2002;10(2):59-75.
3. Sadock BJ. Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry. Philadelphia: Lippincott Williams & Wilkins, 2000;2377-90.
4. Bauer MS, Callahan AM, Jampala C, et al. Clinical practice guidelines for bipolar disorder from the Department of Veterans Affairs. J Clin Psychiatry 1999;60(1):9-21.
5. Sachs GS, Printz DJ, Kahn DA, et al. Medication treatment of bipolar disorder. Postgrad Med Special Report 2000;Apr:1-104.
6. American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159:1.-
7. Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002;356:241-7.
8. Cohen LS, Rosenbaum JP. Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry 1998;59(suppl 2):18-28.
9. Baldessarini RJ, Tondo L. Recurrence risk in bipolar manic-depressive disorders after discontinuing lithium maintenance treatment: an overview. Clin Drug Invest 1998;15(4):337-51.
10. Jefferson JW. Lithium. In: Dukes MNG, Aronson JK (eds). Meyler’s side effects of drugs (14th ed). Amsterdam: Elsevier Science BV, 2001;86-94.
11. Jefferson JW. Lithium. In: Aronson JK (ed). Side effects of drugs, annual 24. Amsterdam: Elsevier Science BV, 2001;22-31.
12. Abdull H, Bliss R, Guinea AE, et al. Pathology and outcome of surgical treatment for lithium-associated hyperparathyroidism. Br J Surg 1999;86:91-3.
13. Gitlin M. Lithium and the kidney. An updated review. Drug Safety 1999;20(3):231-43.
14. Lundmark J, Gunnarsson T, Bengtsson F. A possible interaction between lithium and rofecoxib (letter to the editor). Br J Clin Pharmacol 2002;53(4):403-4.
15. Zwanzger P, Marcuse A, Boerner RJ, et al. Lithium intoxication after administration of AT1 blockers. J Clin Psychiatry 2001;62(3):208-9.
1. Tondo L, Hennen J, Baldessarini RJ, et al. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand 2001;104:163-72.
2. Baldessarini RJ, Tondo L, Hennen J, et al. Is lithium still worth using? An update of selected recent research. Harvard Rev Psychiatry 2002;10(2):59-75.
3. Sadock BJ. Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry. Philadelphia: Lippincott Williams & Wilkins, 2000;2377-90.
4. Bauer MS, Callahan AM, Jampala C, et al. Clinical practice guidelines for bipolar disorder from the Department of Veterans Affairs. J Clin Psychiatry 1999;60(1):9-21.
5. Sachs GS, Printz DJ, Kahn DA, et al. Medication treatment of bipolar disorder. Postgrad Med Special Report 2000;Apr:1-104.
6. American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159:1.-
7. Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002;356:241-7.
8. Cohen LS, Rosenbaum JP. Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry 1998;59(suppl 2):18-28.
9. Baldessarini RJ, Tondo L. Recurrence risk in bipolar manic-depressive disorders after discontinuing lithium maintenance treatment: an overview. Clin Drug Invest 1998;15(4):337-51.
10. Jefferson JW. Lithium. In: Dukes MNG, Aronson JK (eds). Meyler’s side effects of drugs (14th ed). Amsterdam: Elsevier Science BV, 2001;86-94.
11. Jefferson JW. Lithium. In: Aronson JK (ed). Side effects of drugs, annual 24. Amsterdam: Elsevier Science BV, 2001;22-31.
12. Abdull H, Bliss R, Guinea AE, et al. Pathology and outcome of surgical treatment for lithium-associated hyperparathyroidism. Br J Surg 1999;86:91-3.
13. Gitlin M. Lithium and the kidney. An updated review. Drug Safety 1999;20(3):231-43.
14. Lundmark J, Gunnarsson T, Bengtsson F. A possible interaction between lithium and rofecoxib (letter to the editor). Br J Clin Pharmacol 2002;53(4):403-4.
15. Zwanzger P, Marcuse A, Boerner RJ, et al. Lithium intoxication after administration of AT1 blockers. J Clin Psychiatry 2001;62(3):208-9.
Rediscovering the art of lithium therapy
As a mood stabilizer for patients with bipolar disorder, lithium was the darling of U.S. psychiatry from the 1970s to well into the 1990s. It then began an ill-deserved, gradual fall from grace and today could be considered a pharmaceutical endangered species. But why?
Did lithium lose effectiveness? Is it too toxic? Is its side effect burden too heavy? Does it interact adversely with too many medicines? Is it too cumbersome to use? Was it just a fad whose time came and went—a psychiatric pet rock? Did it fall prey to the marketing might behind patent-protected drugs? Was it replaced by more effective and safer drugs?
You are partially correct if you checked “all of the above,” because all contain a kernel of truth. At the same time, each is an exaggeration that does grave injustice to a remarkable medication. In addition, psychiatry appears to pay only lip service to convincing evidence that lithium is the only mood stabilizer that reduces the risk of suicide during long-term treatment.1
Some psychiatrists rationalize that “lithium is too difficult to use, so I never prescribe it.”2,3 My response is simply, “try it, and I think you’ll like it.” Measuring serum lithium concentrations is simple, accurate, and inexpensive. And we know quite a bit about how lithium dosage and blood level relate to response and tolerability.
Where does lithium stand?
Lithium is the first solid element in the periodic table (atomic number 3, atomic weight 6.94) (Box 1). As a treatment for bipolar disorder, lithium’s rise to prominence in the United States was far from rapid. Its antimanic properties were described by John Cade in Australia in 1949 in an open-label case series, but it was not FDA-approved for 20 years—for acute manic episodes in 1970 and for maintenance therapy “in those manic depressive patients with a history of mania” in 1974. Today, lithium shares FDA-approved manic episode billing with chlorpromazine (1973), divalproex (1995), and olanzapine (2000), but it remains the only FDA-approved drug for maintenance (although the FDA is considering a bipolar depression maintenance indication for lamotrigine).
Lithium has no meaningful protein binding and no metabolites, being excreted almost entirely by the kidneys. Its elimination half-life of 18 to 24 hours may be longer in the elderly and shorter in youth because of age-dependent variations in glomerular filtration rate. For unclear reasons, renal lithium clearance appears to be more rapid in obese persons.
Lithium preparations available in the United States include standard-release (150, 300, 600 mg), slow-release (Lithobid and generic 300 mg), and controlled-release (Eskalith CR 450 mg) forms of lithium carbonate and a lithium citrate liquid. Lithium carbonate, 300 mg, and lithium citrate, 5 cc, each contain about 8 mmols of lithium. Lithium and lithium carbonate are not the same—there are 56.36 mg of lithium in 300 mg of lithium carbonate. The correct formula for lithium carbonate is Li2CO3, not LiCO3 as is commonly and erroneously written.
With the standard-release preparation, peak serum levels are reached in about 1 1/2 hours and with the slow- and controlled-release forms in about 4 to 4 1/2 hours. At times, the slower-release forms may be better tolerated, but they are also a bit more costly (although all forms of lithium are inexpensive, compared with other mood stabilizers).
If you examine lithium’s status relative to other bipolar medications, you’ll find some inconsistencies. For example:
- Clinical practice guidelines from the Department of Veterans Affairs (January 1999) recommended lithium as the first-line agent for acute and prophylactic treatment of manic and mixed states, bipolar depression, and rapid cycling.4
- The Expert Consensus Guidelines (April 2000) gave at least equal billing—if not preferred status—to divalproex for those indications.5
- The American Psychiatric Association’s (APA) revised guidelines (April 2002) gave the nod to lithium for classic elated mania and bipolar depression but to divalproex for mixed mania and rapid cycling.6 Divalproex was rated comparable to lithium for maintenance therapy, despite the lack of convincing data.
- The European perspective (January 2002) is most similar to that of the Department of Veterans Affairs, favoring lithium for acute mania, bipolar depression, and long-term treatment.7
There is no clear winner (or loser) in the battle for bipolar marketplace supremacy. The belief that one drug does everything is a fantasy for all but a small minority of patients with bipolar disorder. Polypharmacy is the rule, and rational polypharmacy the goal. To exclude lithium from the arsenal of bipolar drugs would be folly, yet lithium prescribing seems to have become a vanishing art. One of my psychopharmacologist colleagues recently expressed bewilderment at the number of “treatment-resistant” bipolar patients referred to him who had never been treated with lithium.
Diagnosis matters
Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning. Its potential benefits, however, clearly extend to all other aspects of bipolar disorder, to augmentation for treatment-resistant major depressive disorder, to schizoaffective disorder, and—at times—to aggressive states. As the bipolar spectrum expands, it is hardly surprising that the effectiveness of lithium (or any other drug) lessens as we approach the periphery of the spectrum.
Blood levels and dosing
Recommended lithium serum concentrations are given as ranges, realizing that individual variability makes exact numbers impractical. Package inserts for lithium products list serum concentrations between 1.0 and 1.5 mEq/L for acute mania and 0.6 and 1.2 mEq/L for maintenance therapy. The APA’s revised guidelines are a bit more conservative, recommending 0.5 to 1.2 mEq/L for acute mania and waffling somewhat on maintenance.4 Many patients on maintenance therapy do well at levels between 0.6 and 0.8 mEq/L, and some prosper at even lower levels.
To avoid obtaining a misleading blood level:
- Samples should be drawn in the morning as close as possible to 12 hours after the last dose.
- Steady state conditions should exist, usually meaning 4 or 5 days on the same dosage without any missed or extra doses (Box 2).
Start treatment using divided dosages, but—following stabilization—once-daily dosing is possible for many patients. If lithium is taken as a single daily dose, 12-hour blood levels will be somewhat higher than with multiple daily dosing. Single and multiple daily dosing are similarly effective, but once-daily dosing may have a compliance and tolerability edge in some patients.
Considering individual patient variability, a lithium carbonate dosage of 1,200 to 1,800 mg/d is likely to be therapeutic for mania and 900 to 1,200 mg/d for maintenance in otherwise healthy, nongeriatric adults.
Starting and maintaining lithium
Medical history. Assuming that lithium theapy is indicated, obtain a detailed medical history. Focus on findings that increase the risk of lithium toxicity, such as renal impairment, drug interactions, and unstable fluid-electrolyte balance.
Although lithium is not contraindicated in patients with renal disease, using an alternate drug is probably preferable. On the other hand, because lithium does not adversely affect the liver or pancreas, it may be preferred to some other mood stabilizers if these organs are diseased.
A thorough diet and drug history is also important. Because low-sodium diets reduce renal lithium clearance, lower doses may be required to reach a given serum concentration. Some drugs alter lithium excretion and can increase or decrease blood levels (see “Drug combinations,”).
Advise women of childbearing age about lithium’s teratogenic potential (which is considerably less than that of carbamazepine or valproate). The risk of cardiovascular malformation of the fetus has been estimated at 1/1,000 to 1/2,000 births among women who took lithium during the first trimester of pregnancy.8
- Draw samples in the morning, as close as possible to 12 hours after the last dose.
- Measure serum levels at steady state, at least 4 or 5 days on the same dosage without any missed or extra doses.
Baseline lab tests. Assessing renal function is essential. A serum creatinine level will usually suffice, unless a history of renal disease suggests the need for a more extensive evaluation, such as creatinine clearance, renal ultrasound, or nephrology consultation.
A urinalysis is often part of the package. Because thyroid dysfunction can alter mood and lithium can disrupt thyroid function, baseline TSH and T4 tests are recommended. CBC is optional (lithium can cause leukocytosis). The medical history should determine whether additional blood work is necessary. An ECG is sometimes advised in older patients, especially if the history suggests cardiovascular disease. Finally, don’t forget a pregnancy test in women of childbearing potential (Box 3).
Monitoring. Early in the course of therapy, lithium blood levels are usually obtained at 5- to 7-day intervals until the patient is stabilized. After that, assuming all is well, routine monitoring can occur every 3, 4, or even 6 months, depending on the individual’s reliability and stability. Because ongoing assessment of renal and thyroid function is also important, it makes sense to obtain:
- a serum creatinine measurement linked to each lithium level
- and a serum TSH yearly or at the slightest indication of thyroid dysfunction, such as fatigue, weight gain, cognitive impairment, cold intolerance, or depression.
Stopping lithium. Lithium can be discontinued abruptly without side effects if it is ineffective or not tolerated. Stopping lithium after successful long-term use is another story. There is a high likelihood of illness recurrence and a small but real possibility that lithium will be ineffective when restarted. Also, abrupt or rapid discontinuation (within 1 to 14 days) is believed to increase the likelihood of earlier recurrence, compared with more gradual discontinuation.9
Side effects and toxicity
One reason for lithium’s slide in popularity is its perceived side-effect profile. Toxic amounts can be lethal, and therapeutic amounts can be bothersome. Yet concerns are often exaggerated because of lack of familiarity with the drug.10,11
Intoxication. Lithium does have a narrow therapeutic index, with toxicity related to serum concentration and duration of exposure. Acute overdoses, while not benign, are often better tolerated than gradual, more tissue-saturating exposures. Idiosyncratic factors are also involved, as evidenced by documented toxicity at “therapeutic” levels and tolerability despite very high levels.
Early warnings of impending toxicity include:
- neurologic findings such as dysarthria, new or worsening tremor, and ataxia
- gastrointestinal symptoms such as anorexia, nausea, vomiting, and diarrhea.
Severe toxicity can be fatal or cause permanent neurologic (often cerebellar) damage. Causes of intoxication range from deliberate overdose to renal impairment, low-sodium diets, drug interactions, and dehydration. At particular risk are patients with lithium-induced polyuria whose access to fluid replacement is compromised.
Treatment involves reducing absorption, increasing excretion, and restoring fluid-electrolyte balance. Severe intoxication, especially if renal function is impaired, is best treated with hemodialysis.
| Test | Indication |
|---|---|
| Serum creatinine, urinalysis | To screen for renal function |
| TSH and T4 | To establish baseline thyroid function |
| CBC (optional) | If indicated by patient’s overall medical condition or because some doctors prefer to do more general screening |
| ECG (optional) | For patients with risk factors for heart disease |
| Pregnancy test | For at-risk women because of lithium’s teratogenic potential |
Neurologic. Mild neurologic complaints such as memory impairment, slow reaction time, lack of spontaneity, and lost creativity have been ascribed to lithium and may lead to noncompliance. Under such circumstances, other diagnostic considerations include breakthrough depression, hypothyroidism, other illness, hypercalcemia, other medications, and absence of hypomania.
Like valproate, lithium can cause a benign postural tremor that is usually tolerable and often transient. Should the tremor be problematic, treatment considerations include dosage reduction, switching to a slow-release preparation, reducing caffeine intake, avoiding other tremor-causing drugs such as theophylline or stimulants, and treating associated anxiety. If an anti-tremor drug is needed, a beta-blocker such as propranolol is used most commonly; other options to consider are primidone and gabapentin. Don’t forget that a worsening tremor may indicate impending toxicity.
Very rarely, lithium has been associated with pseudotumor cerebri (benign intracranial hypertension), peripheral neuropathy, and a myasthenia gravis-like syndrome.
Cardiovascular. Like many drugs, lithium can cause benign ST-T wave changes on ECG.
More serious, but fortunately quite uncommon, is lithium-induced sinus node dysfunction manifesting as a variety of bradyarrhythmias and, at times, syncopal episodes. Since normal aging is associated with a gradual loss of sinus node pacemaker cells, the elderly may be especially sensitive to this problem. Unless a pacemaker is implanted, sinus node dysfunction usually requires lithium discontinuation.
Endocrine. The association between lithium and goiter and hypothyroidism is well-recognized, with elevated risk in women and in patients with pre-existing thyroid disease. Both clinical and symptomatic subclinical hypothyroidism will improve with supplemental thyroid hormone. Less well appreciated are reports of hyperthyroidism occurring during lithium therapy or shortly after its discontinuation. Because subclinical hyperthyroidism may not be benign, careful attention must be paid to maintaining thyroid function well within the normal range.
Reports continue to accrue of lithium-related hypercalcemia and increased parathyroid hormone levels, with an occasional patient developing parathyroid hyperplasia or adenoma requiring surgical intervention.12 No specific guidelines have been established for monitoring serum calcium, but some authors have recommended periodic testing.
Weight. At least one-third of patients on lithium gain weight for a variety of reasons, such as altered lipid and carbohydrate metabolism, use of high-calorie fluids to combat polydipsia and polyuria, hypothyroidism, and the use of other drugs associated with weight gain. If weight gain occurs, recognize it early (weigh your patients) and institute appropriate dietary and exercise measures.
Hematologic. A mild, benign leukocytosis is seen sometimes during treatment with lithium. This effect has been harnessed to treat some neutropenic conditions. Lithium does not increase the risk of blood dyscrasias.
Dermatologic. Acne, psoriasis, and follicular keratosis may first appear or worsen during lithium therapy. Occasionally, otherwise successful lithium therapy has been rendered impossible by a dramatic dermatologic flare-up. Hair loss has also been associated with lithium use for unclear reasons, although hypothyroidism is occasionally a factor.
Renal. Impaired urinary concentrating ability and polyuria are common adverse effects. Both may be reversed with timely treatment discontinuation, but they may persist even after discontinuation in patients on long-term lithium treatment.12
Polyuria is largely nephrogenic in origin and, at times, can be voluminous, cause great inconvenience, and pose a risk of dehydration and lithium intoxication. Patients sometimes believe that thirst drives the polyuria and attempt to deal with it by restricting fluid intake, which can be quite dangerous. More appropriate interventions include dosage reduction (if possible) and the use of a thiazide and/or potassium-sparing diuretic. If diuretics are used, serum lithium concentrations may rise. Debate remains as to whether slow-or controlled-release preparations or single daily dosing are “kinder to the kidney.”
- Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning.
- Lithium is the only mood stabilizer that has been shown to reduce the risk of suicide during long-term treatment.
- Renal impairment, drug interactions, and unstable fluid-electrolyte balance increase the risk of lithium toxicity.
- Lithium does not adversely affect the liver or pancreas and may be the preferred mood stabilizer if these organs are diseased.
- Lithium has teratogenic potential but less than that of carbamazepine or valproate.
- Because lithium can disrupt thyroid function, baseline and ongoing thyroid function tests are recommended.
In recent years, there has been a disturbing increase in reports of elevated serum creatinine and reduced creatinine clearance associated with long-term lithium use.13 Because renal impairment has many causes, evaluation by a nephrologist is strongly advised. Then if the finger of causation points strongly at lithium, a careful risk/benefit analysis is in order. Even if lithium is discontinued—and especially if it is continued—regular renal function assessment is essential.
Rarely, lithium can cause a nephrotic syndrome (proteinuria, edema, decreased serum albumin, and increased serum lipids) that tends to be reversible with drug discontinuation.
Drug combinations
First the good news. Lithium tends to combine well with all the anticonvulsant mood stabilizers, making it the favored drug for combination therapies. Lithium/antidepressant combinations can be useful for treatment-resistant depression, although serotonin syndrome occasionally has been reported when lithium is combined with selective serotonin reuptake inhibitors.10,11 Using lithium with atypical antipsychotics is common, often effective, and usually well-tolerated.
Drug-drug interactions. Some nonpsychiatric drugs are associated with reduced renal lithium clearance and potential lithium toxicity. Because nonpsychiatrists usually prescribe these drugs, encourage patients taking lithium to ask their doctors about the possibility of interactions whenever a new drug is prescribed. Pharmacists can be particularly helpful in avoiding drug-drug interactions.
In patients taking diuretics, serum lithium concentrations are definitely increased by thiazides, possibly by potassium-sparing types, and occasionally by loop types. Osmotic and xanthine diuretics do just the opposite. Because diuretics are often used in medically unstable patients, assume that all can disrupt lithium balance.
Most nonsteroidal anti-inflammatory drugs can increase serum lithium levels, although dose and treatment duration are important variables. Aspirin and acetaminophen should not cause problems. The effect of COX-2 inhibitors on lithium levels has not been studied adequately, so these drugs should remain under suspicion.14
Lithium toxicity has been reported with angiotensin-converting enzyme (ACE) inhibitors, and their package inserts caution about this possibility. More recently, a few cases of lithium toxicity have been reported in patients taking angiotensin II receptor type-1 (AT-1) antagonists (e.g., candesartan, losartan, valsartan).15
Other, less well-substantiated pharmacokinetic and pharmacodynamic interactions that have been reported with lithium and other drugs can be researched, by using a computer-based drug interaction program or consulting with a drug information center.
Patient and clinician education
Both patients and clinicians have an obligation to ensure that lithium (or any other drug) is used safely and effectively (Box 4). Excellent sources of continuing education are listed below in “Related resources.” Rather than fall prey to the illusion that lithium therapy is a “vanishing art,” it would be better for clinicians to heed these words from the APA’s 2002 practice guidelines for bipolar disorder:
“No other treatment has performed as well as lithium in as many aspects of long-term care of bipolar disorder patients, and despite some risks and limitations lithium remains the standard against which all proposed alternatives are compared.”6
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Lithium Information Center. www.miminc.org
Drug brand names
- Chlorpromazine • Thorazine
- Divalproex • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Primidone • Mysoline
- Propranolol • Inderal
Disclosure
Dr. Jefferson receives grant/research support from Abbott Laboratories, Bristol-Myers Squibb Co., Forest Laboratories Inc., GlaxoSmithKline, Eli Lilly and Co., Novartis Pharmaceuticals Corp., Organon, Janssen Pharmaceutica, Pfizer Inc., Solvay, and Wyeth Pharmaceuticals. He also serves as a consultant to GlaxoSmithKline, Novartis Pharmaceuticals Corp., Solvay, and UCB Pharma.
1. Tondo L, Hennen J, Baldessarini RJ, et al. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand 2001;104:163-72.
2. Baldessarini RJ, Tondo L, Hennen J, et al. Is lithium still worth using? An update of selected recent research. Harvard Rev Psychiatry 2002;10(2):59-75.
3. Sadock BJ. Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry. Philadelphia: Lippincott Williams & Wilkins, 2000;2377-90.
4. Bauer MS, Callahan AM, Jampala C, et al. Clinical practice guidelines for bipolar disorder from the Department of Veterans Affairs. J Clin Psychiatry 1999;60(1):9-21.
5. Sachs GS, Printz DJ, Kahn DA, et al. Medication treatment of bipolar disorder. Postgrad Med Special Report 2000;Apr:1-104.
6. American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159:1.-
7. Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002;356:241-7.
8. Cohen LS, Rosenbaum JP. Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry 1998;59(suppl 2):18-28.
9. Baldessarini RJ, Tondo L. Recurrence risk in bipolar manic-depressive disorders after discontinuing lithium maintenance treatment: an overview. Clin Drug Invest 1998;15(4):337-51.
10. Jefferson JW. Lithium. In: Dukes MNG, Aronson JK (eds). Meyler’s side effects of drugs (14th ed). Amsterdam: Elsevier Science BV, 2001;86-94.
11. Jefferson JW. Lithium. In: Aronson JK (ed). Side effects of drugs, annual 24. Amsterdam: Elsevier Science BV, 2001;22-31.
12. Abdull H, Bliss R, Guinea AE, et al. Pathology and outcome of surgical treatment for lithium-associated hyperparathyroidism. Br J Surg 1999;86:91-3.
13. Gitlin M. Lithium and the kidney. An updated review. Drug Safety 1999;20(3):231-43.
14. Lundmark J, Gunnarsson T, Bengtsson F. A possible interaction between lithium and rofecoxib (letter to the editor). Br J Clin Pharmacol 2002;53(4):403-4.
15. Zwanzger P, Marcuse A, Boerner RJ, et al. Lithium intoxication after administration of AT1 blockers. J Clin Psychiatry 2001;62(3):208-9.
As a mood stabilizer for patients with bipolar disorder, lithium was the darling of U.S. psychiatry from the 1970s to well into the 1990s. It then began an ill-deserved, gradual fall from grace and today could be considered a pharmaceutical endangered species. But why?
Did lithium lose effectiveness? Is it too toxic? Is its side effect burden too heavy? Does it interact adversely with too many medicines? Is it too cumbersome to use? Was it just a fad whose time came and went—a psychiatric pet rock? Did it fall prey to the marketing might behind patent-protected drugs? Was it replaced by more effective and safer drugs?
You are partially correct if you checked “all of the above,” because all contain a kernel of truth. At the same time, each is an exaggeration that does grave injustice to a remarkable medication. In addition, psychiatry appears to pay only lip service to convincing evidence that lithium is the only mood stabilizer that reduces the risk of suicide during long-term treatment.1
Some psychiatrists rationalize that “lithium is too difficult to use, so I never prescribe it.”2,3 My response is simply, “try it, and I think you’ll like it.” Measuring serum lithium concentrations is simple, accurate, and inexpensive. And we know quite a bit about how lithium dosage and blood level relate to response and tolerability.
Where does lithium stand?
Lithium is the first solid element in the periodic table (atomic number 3, atomic weight 6.94) (Box 1). As a treatment for bipolar disorder, lithium’s rise to prominence in the United States was far from rapid. Its antimanic properties were described by John Cade in Australia in 1949 in an open-label case series, but it was not FDA-approved for 20 years—for acute manic episodes in 1970 and for maintenance therapy “in those manic depressive patients with a history of mania” in 1974. Today, lithium shares FDA-approved manic episode billing with chlorpromazine (1973), divalproex (1995), and olanzapine (2000), but it remains the only FDA-approved drug for maintenance (although the FDA is considering a bipolar depression maintenance indication for lamotrigine).
Lithium has no meaningful protein binding and no metabolites, being excreted almost entirely by the kidneys. Its elimination half-life of 18 to 24 hours may be longer in the elderly and shorter in youth because of age-dependent variations in glomerular filtration rate. For unclear reasons, renal lithium clearance appears to be more rapid in obese persons.
Lithium preparations available in the United States include standard-release (150, 300, 600 mg), slow-release (Lithobid and generic 300 mg), and controlled-release (Eskalith CR 450 mg) forms of lithium carbonate and a lithium citrate liquid. Lithium carbonate, 300 mg, and lithium citrate, 5 cc, each contain about 8 mmols of lithium. Lithium and lithium carbonate are not the same—there are 56.36 mg of lithium in 300 mg of lithium carbonate. The correct formula for lithium carbonate is Li2CO3, not LiCO3 as is commonly and erroneously written.
With the standard-release preparation, peak serum levels are reached in about 1 1/2 hours and with the slow- and controlled-release forms in about 4 to 4 1/2 hours. At times, the slower-release forms may be better tolerated, but they are also a bit more costly (although all forms of lithium are inexpensive, compared with other mood stabilizers).
If you examine lithium’s status relative to other bipolar medications, you’ll find some inconsistencies. For example:
- Clinical practice guidelines from the Department of Veterans Affairs (January 1999) recommended lithium as the first-line agent for acute and prophylactic treatment of manic and mixed states, bipolar depression, and rapid cycling.4
- The Expert Consensus Guidelines (April 2000) gave at least equal billing—if not preferred status—to divalproex for those indications.5
- The American Psychiatric Association’s (APA) revised guidelines (April 2002) gave the nod to lithium for classic elated mania and bipolar depression but to divalproex for mixed mania and rapid cycling.6 Divalproex was rated comparable to lithium for maintenance therapy, despite the lack of convincing data.
- The European perspective (January 2002) is most similar to that of the Department of Veterans Affairs, favoring lithium for acute mania, bipolar depression, and long-term treatment.7
There is no clear winner (or loser) in the battle for bipolar marketplace supremacy. The belief that one drug does everything is a fantasy for all but a small minority of patients with bipolar disorder. Polypharmacy is the rule, and rational polypharmacy the goal. To exclude lithium from the arsenal of bipolar drugs would be folly, yet lithium prescribing seems to have become a vanishing art. One of my psychopharmacologist colleagues recently expressed bewilderment at the number of “treatment-resistant” bipolar patients referred to him who had never been treated with lithium.
Diagnosis matters
Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning. Its potential benefits, however, clearly extend to all other aspects of bipolar disorder, to augmentation for treatment-resistant major depressive disorder, to schizoaffective disorder, and—at times—to aggressive states. As the bipolar spectrum expands, it is hardly surprising that the effectiveness of lithium (or any other drug) lessens as we approach the periphery of the spectrum.
Blood levels and dosing
Recommended lithium serum concentrations are given as ranges, realizing that individual variability makes exact numbers impractical. Package inserts for lithium products list serum concentrations between 1.0 and 1.5 mEq/L for acute mania and 0.6 and 1.2 mEq/L for maintenance therapy. The APA’s revised guidelines are a bit more conservative, recommending 0.5 to 1.2 mEq/L for acute mania and waffling somewhat on maintenance.4 Many patients on maintenance therapy do well at levels between 0.6 and 0.8 mEq/L, and some prosper at even lower levels.
To avoid obtaining a misleading blood level:
- Samples should be drawn in the morning as close as possible to 12 hours after the last dose.
- Steady state conditions should exist, usually meaning 4 or 5 days on the same dosage without any missed or extra doses (Box 2).
Start treatment using divided dosages, but—following stabilization—once-daily dosing is possible for many patients. If lithium is taken as a single daily dose, 12-hour blood levels will be somewhat higher than with multiple daily dosing. Single and multiple daily dosing are similarly effective, but once-daily dosing may have a compliance and tolerability edge in some patients.
Considering individual patient variability, a lithium carbonate dosage of 1,200 to 1,800 mg/d is likely to be therapeutic for mania and 900 to 1,200 mg/d for maintenance in otherwise healthy, nongeriatric adults.
Starting and maintaining lithium
Medical history. Assuming that lithium theapy is indicated, obtain a detailed medical history. Focus on findings that increase the risk of lithium toxicity, such as renal impairment, drug interactions, and unstable fluid-electrolyte balance.
Although lithium is not contraindicated in patients with renal disease, using an alternate drug is probably preferable. On the other hand, because lithium does not adversely affect the liver or pancreas, it may be preferred to some other mood stabilizers if these organs are diseased.
A thorough diet and drug history is also important. Because low-sodium diets reduce renal lithium clearance, lower doses may be required to reach a given serum concentration. Some drugs alter lithium excretion and can increase or decrease blood levels (see “Drug combinations,”).
Advise women of childbearing age about lithium’s teratogenic potential (which is considerably less than that of carbamazepine or valproate). The risk of cardiovascular malformation of the fetus has been estimated at 1/1,000 to 1/2,000 births among women who took lithium during the first trimester of pregnancy.8
- Draw samples in the morning, as close as possible to 12 hours after the last dose.
- Measure serum levels at steady state, at least 4 or 5 days on the same dosage without any missed or extra doses.
Baseline lab tests. Assessing renal function is essential. A serum creatinine level will usually suffice, unless a history of renal disease suggests the need for a more extensive evaluation, such as creatinine clearance, renal ultrasound, or nephrology consultation.
A urinalysis is often part of the package. Because thyroid dysfunction can alter mood and lithium can disrupt thyroid function, baseline TSH and T4 tests are recommended. CBC is optional (lithium can cause leukocytosis). The medical history should determine whether additional blood work is necessary. An ECG is sometimes advised in older patients, especially if the history suggests cardiovascular disease. Finally, don’t forget a pregnancy test in women of childbearing potential (Box 3).
Monitoring. Early in the course of therapy, lithium blood levels are usually obtained at 5- to 7-day intervals until the patient is stabilized. After that, assuming all is well, routine monitoring can occur every 3, 4, or even 6 months, depending on the individual’s reliability and stability. Because ongoing assessment of renal and thyroid function is also important, it makes sense to obtain:
- a serum creatinine measurement linked to each lithium level
- and a serum TSH yearly or at the slightest indication of thyroid dysfunction, such as fatigue, weight gain, cognitive impairment, cold intolerance, or depression.
Stopping lithium. Lithium can be discontinued abruptly without side effects if it is ineffective or not tolerated. Stopping lithium after successful long-term use is another story. There is a high likelihood of illness recurrence and a small but real possibility that lithium will be ineffective when restarted. Also, abrupt or rapid discontinuation (within 1 to 14 days) is believed to increase the likelihood of earlier recurrence, compared with more gradual discontinuation.9
Side effects and toxicity
One reason for lithium’s slide in popularity is its perceived side-effect profile. Toxic amounts can be lethal, and therapeutic amounts can be bothersome. Yet concerns are often exaggerated because of lack of familiarity with the drug.10,11
Intoxication. Lithium does have a narrow therapeutic index, with toxicity related to serum concentration and duration of exposure. Acute overdoses, while not benign, are often better tolerated than gradual, more tissue-saturating exposures. Idiosyncratic factors are also involved, as evidenced by documented toxicity at “therapeutic” levels and tolerability despite very high levels.
Early warnings of impending toxicity include:
- neurologic findings such as dysarthria, new or worsening tremor, and ataxia
- gastrointestinal symptoms such as anorexia, nausea, vomiting, and diarrhea.
Severe toxicity can be fatal or cause permanent neurologic (often cerebellar) damage. Causes of intoxication range from deliberate overdose to renal impairment, low-sodium diets, drug interactions, and dehydration. At particular risk are patients with lithium-induced polyuria whose access to fluid replacement is compromised.
Treatment involves reducing absorption, increasing excretion, and restoring fluid-electrolyte balance. Severe intoxication, especially if renal function is impaired, is best treated with hemodialysis.
| Test | Indication |
|---|---|
| Serum creatinine, urinalysis | To screen for renal function |
| TSH and T4 | To establish baseline thyroid function |
| CBC (optional) | If indicated by patient’s overall medical condition or because some doctors prefer to do more general screening |
| ECG (optional) | For patients with risk factors for heart disease |
| Pregnancy test | For at-risk women because of lithium’s teratogenic potential |
Neurologic. Mild neurologic complaints such as memory impairment, slow reaction time, lack of spontaneity, and lost creativity have been ascribed to lithium and may lead to noncompliance. Under such circumstances, other diagnostic considerations include breakthrough depression, hypothyroidism, other illness, hypercalcemia, other medications, and absence of hypomania.
Like valproate, lithium can cause a benign postural tremor that is usually tolerable and often transient. Should the tremor be problematic, treatment considerations include dosage reduction, switching to a slow-release preparation, reducing caffeine intake, avoiding other tremor-causing drugs such as theophylline or stimulants, and treating associated anxiety. If an anti-tremor drug is needed, a beta-blocker such as propranolol is used most commonly; other options to consider are primidone and gabapentin. Don’t forget that a worsening tremor may indicate impending toxicity.
Very rarely, lithium has been associated with pseudotumor cerebri (benign intracranial hypertension), peripheral neuropathy, and a myasthenia gravis-like syndrome.
Cardiovascular. Like many drugs, lithium can cause benign ST-T wave changes on ECG.
More serious, but fortunately quite uncommon, is lithium-induced sinus node dysfunction manifesting as a variety of bradyarrhythmias and, at times, syncopal episodes. Since normal aging is associated with a gradual loss of sinus node pacemaker cells, the elderly may be especially sensitive to this problem. Unless a pacemaker is implanted, sinus node dysfunction usually requires lithium discontinuation.
Endocrine. The association between lithium and goiter and hypothyroidism is well-recognized, with elevated risk in women and in patients with pre-existing thyroid disease. Both clinical and symptomatic subclinical hypothyroidism will improve with supplemental thyroid hormone. Less well appreciated are reports of hyperthyroidism occurring during lithium therapy or shortly after its discontinuation. Because subclinical hyperthyroidism may not be benign, careful attention must be paid to maintaining thyroid function well within the normal range.
Reports continue to accrue of lithium-related hypercalcemia and increased parathyroid hormone levels, with an occasional patient developing parathyroid hyperplasia or adenoma requiring surgical intervention.12 No specific guidelines have been established for monitoring serum calcium, but some authors have recommended periodic testing.
Weight. At least one-third of patients on lithium gain weight for a variety of reasons, such as altered lipid and carbohydrate metabolism, use of high-calorie fluids to combat polydipsia and polyuria, hypothyroidism, and the use of other drugs associated with weight gain. If weight gain occurs, recognize it early (weigh your patients) and institute appropriate dietary and exercise measures.
Hematologic. A mild, benign leukocytosis is seen sometimes during treatment with lithium. This effect has been harnessed to treat some neutropenic conditions. Lithium does not increase the risk of blood dyscrasias.
Dermatologic. Acne, psoriasis, and follicular keratosis may first appear or worsen during lithium therapy. Occasionally, otherwise successful lithium therapy has been rendered impossible by a dramatic dermatologic flare-up. Hair loss has also been associated with lithium use for unclear reasons, although hypothyroidism is occasionally a factor.
Renal. Impaired urinary concentrating ability and polyuria are common adverse effects. Both may be reversed with timely treatment discontinuation, but they may persist even after discontinuation in patients on long-term lithium treatment.12
Polyuria is largely nephrogenic in origin and, at times, can be voluminous, cause great inconvenience, and pose a risk of dehydration and lithium intoxication. Patients sometimes believe that thirst drives the polyuria and attempt to deal with it by restricting fluid intake, which can be quite dangerous. More appropriate interventions include dosage reduction (if possible) and the use of a thiazide and/or potassium-sparing diuretic. If diuretics are used, serum lithium concentrations may rise. Debate remains as to whether slow-or controlled-release preparations or single daily dosing are “kinder to the kidney.”
- Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning.
- Lithium is the only mood stabilizer that has been shown to reduce the risk of suicide during long-term treatment.
- Renal impairment, drug interactions, and unstable fluid-electrolyte balance increase the risk of lithium toxicity.
- Lithium does not adversely affect the liver or pancreas and may be the preferred mood stabilizer if these organs are diseased.
- Lithium has teratogenic potential but less than that of carbamazepine or valproate.
- Because lithium can disrupt thyroid function, baseline and ongoing thyroid function tests are recommended.
In recent years, there has been a disturbing increase in reports of elevated serum creatinine and reduced creatinine clearance associated with long-term lithium use.13 Because renal impairment has many causes, evaluation by a nephrologist is strongly advised. Then if the finger of causation points strongly at lithium, a careful risk/benefit analysis is in order. Even if lithium is discontinued—and especially if it is continued—regular renal function assessment is essential.
Rarely, lithium can cause a nephrotic syndrome (proteinuria, edema, decreased serum albumin, and increased serum lipids) that tends to be reversible with drug discontinuation.
Drug combinations
First the good news. Lithium tends to combine well with all the anticonvulsant mood stabilizers, making it the favored drug for combination therapies. Lithium/antidepressant combinations can be useful for treatment-resistant depression, although serotonin syndrome occasionally has been reported when lithium is combined with selective serotonin reuptake inhibitors.10,11 Using lithium with atypical antipsychotics is common, often effective, and usually well-tolerated.
Drug-drug interactions. Some nonpsychiatric drugs are associated with reduced renal lithium clearance and potential lithium toxicity. Because nonpsychiatrists usually prescribe these drugs, encourage patients taking lithium to ask their doctors about the possibility of interactions whenever a new drug is prescribed. Pharmacists can be particularly helpful in avoiding drug-drug interactions.
In patients taking diuretics, serum lithium concentrations are definitely increased by thiazides, possibly by potassium-sparing types, and occasionally by loop types. Osmotic and xanthine diuretics do just the opposite. Because diuretics are often used in medically unstable patients, assume that all can disrupt lithium balance.
Most nonsteroidal anti-inflammatory drugs can increase serum lithium levels, although dose and treatment duration are important variables. Aspirin and acetaminophen should not cause problems. The effect of COX-2 inhibitors on lithium levels has not been studied adequately, so these drugs should remain under suspicion.14
Lithium toxicity has been reported with angiotensin-converting enzyme (ACE) inhibitors, and their package inserts caution about this possibility. More recently, a few cases of lithium toxicity have been reported in patients taking angiotensin II receptor type-1 (AT-1) antagonists (e.g., candesartan, losartan, valsartan).15
Other, less well-substantiated pharmacokinetic and pharmacodynamic interactions that have been reported with lithium and other drugs can be researched, by using a computer-based drug interaction program or consulting with a drug information center.
Patient and clinician education
Both patients and clinicians have an obligation to ensure that lithium (or any other drug) is used safely and effectively (Box 4). Excellent sources of continuing education are listed below in “Related resources.” Rather than fall prey to the illusion that lithium therapy is a “vanishing art,” it would be better for clinicians to heed these words from the APA’s 2002 practice guidelines for bipolar disorder:
“No other treatment has performed as well as lithium in as many aspects of long-term care of bipolar disorder patients, and despite some risks and limitations lithium remains the standard against which all proposed alternatives are compared.”6
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Lithium Information Center. www.miminc.org
Drug brand names
- Chlorpromazine • Thorazine
- Divalproex • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Primidone • Mysoline
- Propranolol • Inderal
Disclosure
Dr. Jefferson receives grant/research support from Abbott Laboratories, Bristol-Myers Squibb Co., Forest Laboratories Inc., GlaxoSmithKline, Eli Lilly and Co., Novartis Pharmaceuticals Corp., Organon, Janssen Pharmaceutica, Pfizer Inc., Solvay, and Wyeth Pharmaceuticals. He also serves as a consultant to GlaxoSmithKline, Novartis Pharmaceuticals Corp., Solvay, and UCB Pharma.
As a mood stabilizer for patients with bipolar disorder, lithium was the darling of U.S. psychiatry from the 1970s to well into the 1990s. It then began an ill-deserved, gradual fall from grace and today could be considered a pharmaceutical endangered species. But why?
Did lithium lose effectiveness? Is it too toxic? Is its side effect burden too heavy? Does it interact adversely with too many medicines? Is it too cumbersome to use? Was it just a fad whose time came and went—a psychiatric pet rock? Did it fall prey to the marketing might behind patent-protected drugs? Was it replaced by more effective and safer drugs?
You are partially correct if you checked “all of the above,” because all contain a kernel of truth. At the same time, each is an exaggeration that does grave injustice to a remarkable medication. In addition, psychiatry appears to pay only lip service to convincing evidence that lithium is the only mood stabilizer that reduces the risk of suicide during long-term treatment.1
Some psychiatrists rationalize that “lithium is too difficult to use, so I never prescribe it.”2,3 My response is simply, “try it, and I think you’ll like it.” Measuring serum lithium concentrations is simple, accurate, and inexpensive. And we know quite a bit about how lithium dosage and blood level relate to response and tolerability.
Where does lithium stand?
Lithium is the first solid element in the periodic table (atomic number 3, atomic weight 6.94) (Box 1). As a treatment for bipolar disorder, lithium’s rise to prominence in the United States was far from rapid. Its antimanic properties were described by John Cade in Australia in 1949 in an open-label case series, but it was not FDA-approved for 20 years—for acute manic episodes in 1970 and for maintenance therapy “in those manic depressive patients with a history of mania” in 1974. Today, lithium shares FDA-approved manic episode billing with chlorpromazine (1973), divalproex (1995), and olanzapine (2000), but it remains the only FDA-approved drug for maintenance (although the FDA is considering a bipolar depression maintenance indication for lamotrigine).
Lithium has no meaningful protein binding and no metabolites, being excreted almost entirely by the kidneys. Its elimination half-life of 18 to 24 hours may be longer in the elderly and shorter in youth because of age-dependent variations in glomerular filtration rate. For unclear reasons, renal lithium clearance appears to be more rapid in obese persons.
Lithium preparations available in the United States include standard-release (150, 300, 600 mg), slow-release (Lithobid and generic 300 mg), and controlled-release (Eskalith CR 450 mg) forms of lithium carbonate and a lithium citrate liquid. Lithium carbonate, 300 mg, and lithium citrate, 5 cc, each contain about 8 mmols of lithium. Lithium and lithium carbonate are not the same—there are 56.36 mg of lithium in 300 mg of lithium carbonate. The correct formula for lithium carbonate is Li2CO3, not LiCO3 as is commonly and erroneously written.
With the standard-release preparation, peak serum levels are reached in about 1 1/2 hours and with the slow- and controlled-release forms in about 4 to 4 1/2 hours. At times, the slower-release forms may be better tolerated, but they are also a bit more costly (although all forms of lithium are inexpensive, compared with other mood stabilizers).
If you examine lithium’s status relative to other bipolar medications, you’ll find some inconsistencies. For example:
- Clinical practice guidelines from the Department of Veterans Affairs (January 1999) recommended lithium as the first-line agent for acute and prophylactic treatment of manic and mixed states, bipolar depression, and rapid cycling.4
- The Expert Consensus Guidelines (April 2000) gave at least equal billing—if not preferred status—to divalproex for those indications.5
- The American Psychiatric Association’s (APA) revised guidelines (April 2002) gave the nod to lithium for classic elated mania and bipolar depression but to divalproex for mixed mania and rapid cycling.6 Divalproex was rated comparable to lithium for maintenance therapy, despite the lack of convincing data.
- The European perspective (January 2002) is most similar to that of the Department of Veterans Affairs, favoring lithium for acute mania, bipolar depression, and long-term treatment.7
There is no clear winner (or loser) in the battle for bipolar marketplace supremacy. The belief that one drug does everything is a fantasy for all but a small minority of patients with bipolar disorder. Polypharmacy is the rule, and rational polypharmacy the goal. To exclude lithium from the arsenal of bipolar drugs would be folly, yet lithium prescribing seems to have become a vanishing art. One of my psychopharmacologist colleagues recently expressed bewilderment at the number of “treatment-resistant” bipolar patients referred to him who had never been treated with lithium.
Diagnosis matters
Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning. Its potential benefits, however, clearly extend to all other aspects of bipolar disorder, to augmentation for treatment-resistant major depressive disorder, to schizoaffective disorder, and—at times—to aggressive states. As the bipolar spectrum expands, it is hardly surprising that the effectiveness of lithium (or any other drug) lessens as we approach the periphery of the spectrum.
Blood levels and dosing
Recommended lithium serum concentrations are given as ranges, realizing that individual variability makes exact numbers impractical. Package inserts for lithium products list serum concentrations between 1.0 and 1.5 mEq/L for acute mania and 0.6 and 1.2 mEq/L for maintenance therapy. The APA’s revised guidelines are a bit more conservative, recommending 0.5 to 1.2 mEq/L for acute mania and waffling somewhat on maintenance.4 Many patients on maintenance therapy do well at levels between 0.6 and 0.8 mEq/L, and some prosper at even lower levels.
To avoid obtaining a misleading blood level:
- Samples should be drawn in the morning as close as possible to 12 hours after the last dose.
- Steady state conditions should exist, usually meaning 4 or 5 days on the same dosage without any missed or extra doses (Box 2).
Start treatment using divided dosages, but—following stabilization—once-daily dosing is possible for many patients. If lithium is taken as a single daily dose, 12-hour blood levels will be somewhat higher than with multiple daily dosing. Single and multiple daily dosing are similarly effective, but once-daily dosing may have a compliance and tolerability edge in some patients.
Considering individual patient variability, a lithium carbonate dosage of 1,200 to 1,800 mg/d is likely to be therapeutic for mania and 900 to 1,200 mg/d for maintenance in otherwise healthy, nongeriatric adults.
Starting and maintaining lithium
Medical history. Assuming that lithium theapy is indicated, obtain a detailed medical history. Focus on findings that increase the risk of lithium toxicity, such as renal impairment, drug interactions, and unstable fluid-electrolyte balance.
Although lithium is not contraindicated in patients with renal disease, using an alternate drug is probably preferable. On the other hand, because lithium does not adversely affect the liver or pancreas, it may be preferred to some other mood stabilizers if these organs are diseased.
A thorough diet and drug history is also important. Because low-sodium diets reduce renal lithium clearance, lower doses may be required to reach a given serum concentration. Some drugs alter lithium excretion and can increase or decrease blood levels (see “Drug combinations,”).
Advise women of childbearing age about lithium’s teratogenic potential (which is considerably less than that of carbamazepine or valproate). The risk of cardiovascular malformation of the fetus has been estimated at 1/1,000 to 1/2,000 births among women who took lithium during the first trimester of pregnancy.8
- Draw samples in the morning, as close as possible to 12 hours after the last dose.
- Measure serum levels at steady state, at least 4 or 5 days on the same dosage without any missed or extra doses.
Baseline lab tests. Assessing renal function is essential. A serum creatinine level will usually suffice, unless a history of renal disease suggests the need for a more extensive evaluation, such as creatinine clearance, renal ultrasound, or nephrology consultation.
A urinalysis is often part of the package. Because thyroid dysfunction can alter mood and lithium can disrupt thyroid function, baseline TSH and T4 tests are recommended. CBC is optional (lithium can cause leukocytosis). The medical history should determine whether additional blood work is necessary. An ECG is sometimes advised in older patients, especially if the history suggests cardiovascular disease. Finally, don’t forget a pregnancy test in women of childbearing potential (Box 3).
Monitoring. Early in the course of therapy, lithium blood levels are usually obtained at 5- to 7-day intervals until the patient is stabilized. After that, assuming all is well, routine monitoring can occur every 3, 4, or even 6 months, depending on the individual’s reliability and stability. Because ongoing assessment of renal and thyroid function is also important, it makes sense to obtain:
- a serum creatinine measurement linked to each lithium level
- and a serum TSH yearly or at the slightest indication of thyroid dysfunction, such as fatigue, weight gain, cognitive impairment, cold intolerance, or depression.
Stopping lithium. Lithium can be discontinued abruptly without side effects if it is ineffective or not tolerated. Stopping lithium after successful long-term use is another story. There is a high likelihood of illness recurrence and a small but real possibility that lithium will be ineffective when restarted. Also, abrupt or rapid discontinuation (within 1 to 14 days) is believed to increase the likelihood of earlier recurrence, compared with more gradual discontinuation.9
Side effects and toxicity
One reason for lithium’s slide in popularity is its perceived side-effect profile. Toxic amounts can be lethal, and therapeutic amounts can be bothersome. Yet concerns are often exaggerated because of lack of familiarity with the drug.10,11
Intoxication. Lithium does have a narrow therapeutic index, with toxicity related to serum concentration and duration of exposure. Acute overdoses, while not benign, are often better tolerated than gradual, more tissue-saturating exposures. Idiosyncratic factors are also involved, as evidenced by documented toxicity at “therapeutic” levels and tolerability despite very high levels.
Early warnings of impending toxicity include:
- neurologic findings such as dysarthria, new or worsening tremor, and ataxia
- gastrointestinal symptoms such as anorexia, nausea, vomiting, and diarrhea.
Severe toxicity can be fatal or cause permanent neurologic (often cerebellar) damage. Causes of intoxication range from deliberate overdose to renal impairment, low-sodium diets, drug interactions, and dehydration. At particular risk are patients with lithium-induced polyuria whose access to fluid replacement is compromised.
Treatment involves reducing absorption, increasing excretion, and restoring fluid-electrolyte balance. Severe intoxication, especially if renal function is impaired, is best treated with hemodialysis.
| Test | Indication |
|---|---|
| Serum creatinine, urinalysis | To screen for renal function |
| TSH and T4 | To establish baseline thyroid function |
| CBC (optional) | If indicated by patient’s overall medical condition or because some doctors prefer to do more general screening |
| ECG (optional) | For patients with risk factors for heart disease |
| Pregnancy test | For at-risk women because of lithium’s teratogenic potential |
Neurologic. Mild neurologic complaints such as memory impairment, slow reaction time, lack of spontaneity, and lost creativity have been ascribed to lithium and may lead to noncompliance. Under such circumstances, other diagnostic considerations include breakthrough depression, hypothyroidism, other illness, hypercalcemia, other medications, and absence of hypomania.
Like valproate, lithium can cause a benign postural tremor that is usually tolerable and often transient. Should the tremor be problematic, treatment considerations include dosage reduction, switching to a slow-release preparation, reducing caffeine intake, avoiding other tremor-causing drugs such as theophylline or stimulants, and treating associated anxiety. If an anti-tremor drug is needed, a beta-blocker such as propranolol is used most commonly; other options to consider are primidone and gabapentin. Don’t forget that a worsening tremor may indicate impending toxicity.
Very rarely, lithium has been associated with pseudotumor cerebri (benign intracranial hypertension), peripheral neuropathy, and a myasthenia gravis-like syndrome.
Cardiovascular. Like many drugs, lithium can cause benign ST-T wave changes on ECG.
More serious, but fortunately quite uncommon, is lithium-induced sinus node dysfunction manifesting as a variety of bradyarrhythmias and, at times, syncopal episodes. Since normal aging is associated with a gradual loss of sinus node pacemaker cells, the elderly may be especially sensitive to this problem. Unless a pacemaker is implanted, sinus node dysfunction usually requires lithium discontinuation.
Endocrine. The association between lithium and goiter and hypothyroidism is well-recognized, with elevated risk in women and in patients with pre-existing thyroid disease. Both clinical and symptomatic subclinical hypothyroidism will improve with supplemental thyroid hormone. Less well appreciated are reports of hyperthyroidism occurring during lithium therapy or shortly after its discontinuation. Because subclinical hyperthyroidism may not be benign, careful attention must be paid to maintaining thyroid function well within the normal range.
Reports continue to accrue of lithium-related hypercalcemia and increased parathyroid hormone levels, with an occasional patient developing parathyroid hyperplasia or adenoma requiring surgical intervention.12 No specific guidelines have been established for monitoring serum calcium, but some authors have recommended periodic testing.
Weight. At least one-third of patients on lithium gain weight for a variety of reasons, such as altered lipid and carbohydrate metabolism, use of high-calorie fluids to combat polydipsia and polyuria, hypothyroidism, and the use of other drugs associated with weight gain. If weight gain occurs, recognize it early (weigh your patients) and institute appropriate dietary and exercise measures.
Hematologic. A mild, benign leukocytosis is seen sometimes during treatment with lithium. This effect has been harnessed to treat some neutropenic conditions. Lithium does not increase the risk of blood dyscrasias.
Dermatologic. Acne, psoriasis, and follicular keratosis may first appear or worsen during lithium therapy. Occasionally, otherwise successful lithium therapy has been rendered impossible by a dramatic dermatologic flare-up. Hair loss has also been associated with lithium use for unclear reasons, although hypothyroidism is occasionally a factor.
Renal. Impaired urinary concentrating ability and polyuria are common adverse effects. Both may be reversed with timely treatment discontinuation, but they may persist even after discontinuation in patients on long-term lithium treatment.12
Polyuria is largely nephrogenic in origin and, at times, can be voluminous, cause great inconvenience, and pose a risk of dehydration and lithium intoxication. Patients sometimes believe that thirst drives the polyuria and attempt to deal with it by restricting fluid intake, which can be quite dangerous. More appropriate interventions include dosage reduction (if possible) and the use of a thiazide and/or potassium-sparing diuretic. If diuretics are used, serum lithium concentrations may rise. Debate remains as to whether slow-or controlled-release preparations or single daily dosing are “kinder to the kidney.”
- Lithium is most effective in patients with euphoric mania, full remission between episodes, and normal interepisode functioning.
- Lithium is the only mood stabilizer that has been shown to reduce the risk of suicide during long-term treatment.
- Renal impairment, drug interactions, and unstable fluid-electrolyte balance increase the risk of lithium toxicity.
- Lithium does not adversely affect the liver or pancreas and may be the preferred mood stabilizer if these organs are diseased.
- Lithium has teratogenic potential but less than that of carbamazepine or valproate.
- Because lithium can disrupt thyroid function, baseline and ongoing thyroid function tests are recommended.
In recent years, there has been a disturbing increase in reports of elevated serum creatinine and reduced creatinine clearance associated with long-term lithium use.13 Because renal impairment has many causes, evaluation by a nephrologist is strongly advised. Then if the finger of causation points strongly at lithium, a careful risk/benefit analysis is in order. Even if lithium is discontinued—and especially if it is continued—regular renal function assessment is essential.
Rarely, lithium can cause a nephrotic syndrome (proteinuria, edema, decreased serum albumin, and increased serum lipids) that tends to be reversible with drug discontinuation.
Drug combinations
First the good news. Lithium tends to combine well with all the anticonvulsant mood stabilizers, making it the favored drug for combination therapies. Lithium/antidepressant combinations can be useful for treatment-resistant depression, although serotonin syndrome occasionally has been reported when lithium is combined with selective serotonin reuptake inhibitors.10,11 Using lithium with atypical antipsychotics is common, often effective, and usually well-tolerated.
Drug-drug interactions. Some nonpsychiatric drugs are associated with reduced renal lithium clearance and potential lithium toxicity. Because nonpsychiatrists usually prescribe these drugs, encourage patients taking lithium to ask their doctors about the possibility of interactions whenever a new drug is prescribed. Pharmacists can be particularly helpful in avoiding drug-drug interactions.
In patients taking diuretics, serum lithium concentrations are definitely increased by thiazides, possibly by potassium-sparing types, and occasionally by loop types. Osmotic and xanthine diuretics do just the opposite. Because diuretics are often used in medically unstable patients, assume that all can disrupt lithium balance.
Most nonsteroidal anti-inflammatory drugs can increase serum lithium levels, although dose and treatment duration are important variables. Aspirin and acetaminophen should not cause problems. The effect of COX-2 inhibitors on lithium levels has not been studied adequately, so these drugs should remain under suspicion.14
Lithium toxicity has been reported with angiotensin-converting enzyme (ACE) inhibitors, and their package inserts caution about this possibility. More recently, a few cases of lithium toxicity have been reported in patients taking angiotensin II receptor type-1 (AT-1) antagonists (e.g., candesartan, losartan, valsartan).15
Other, less well-substantiated pharmacokinetic and pharmacodynamic interactions that have been reported with lithium and other drugs can be researched, by using a computer-based drug interaction program or consulting with a drug information center.
Patient and clinician education
Both patients and clinicians have an obligation to ensure that lithium (or any other drug) is used safely and effectively (Box 4). Excellent sources of continuing education are listed below in “Related resources.” Rather than fall prey to the illusion that lithium therapy is a “vanishing art,” it would be better for clinicians to heed these words from the APA’s 2002 practice guidelines for bipolar disorder:
“No other treatment has performed as well as lithium in as many aspects of long-term care of bipolar disorder patients, and despite some risks and limitations lithium remains the standard against which all proposed alternatives are compared.”6
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Lithium Information Center. www.miminc.org
Drug brand names
- Chlorpromazine • Thorazine
- Divalproex • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Olanzapine • Zyprexa
- Primidone • Mysoline
- Propranolol • Inderal
Disclosure
Dr. Jefferson receives grant/research support from Abbott Laboratories, Bristol-Myers Squibb Co., Forest Laboratories Inc., GlaxoSmithKline, Eli Lilly and Co., Novartis Pharmaceuticals Corp., Organon, Janssen Pharmaceutica, Pfizer Inc., Solvay, and Wyeth Pharmaceuticals. He also serves as a consultant to GlaxoSmithKline, Novartis Pharmaceuticals Corp., Solvay, and UCB Pharma.
1. Tondo L, Hennen J, Baldessarini RJ, et al. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand 2001;104:163-72.
2. Baldessarini RJ, Tondo L, Hennen J, et al. Is lithium still worth using? An update of selected recent research. Harvard Rev Psychiatry 2002;10(2):59-75.
3. Sadock BJ. Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry. Philadelphia: Lippincott Williams & Wilkins, 2000;2377-90.
4. Bauer MS, Callahan AM, Jampala C, et al. Clinical practice guidelines for bipolar disorder from the Department of Veterans Affairs. J Clin Psychiatry 1999;60(1):9-21.
5. Sachs GS, Printz DJ, Kahn DA, et al. Medication treatment of bipolar disorder. Postgrad Med Special Report 2000;Apr:1-104.
6. American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159:1.-
7. Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002;356:241-7.
8. Cohen LS, Rosenbaum JP. Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry 1998;59(suppl 2):18-28.
9. Baldessarini RJ, Tondo L. Recurrence risk in bipolar manic-depressive disorders after discontinuing lithium maintenance treatment: an overview. Clin Drug Invest 1998;15(4):337-51.
10. Jefferson JW. Lithium. In: Dukes MNG, Aronson JK (eds). Meyler’s side effects of drugs (14th ed). Amsterdam: Elsevier Science BV, 2001;86-94.
11. Jefferson JW. Lithium. In: Aronson JK (ed). Side effects of drugs, annual 24. Amsterdam: Elsevier Science BV, 2001;22-31.
12. Abdull H, Bliss R, Guinea AE, et al. Pathology and outcome of surgical treatment for lithium-associated hyperparathyroidism. Br J Surg 1999;86:91-3.
13. Gitlin M. Lithium and the kidney. An updated review. Drug Safety 1999;20(3):231-43.
14. Lundmark J, Gunnarsson T, Bengtsson F. A possible interaction between lithium and rofecoxib (letter to the editor). Br J Clin Pharmacol 2002;53(4):403-4.
15. Zwanzger P, Marcuse A, Boerner RJ, et al. Lithium intoxication after administration of AT1 blockers. J Clin Psychiatry 2001;62(3):208-9.
1. Tondo L, Hennen J, Baldessarini RJ, et al. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand 2001;104:163-72.
2. Baldessarini RJ, Tondo L, Hennen J, et al. Is lithium still worth using? An update of selected recent research. Harvard Rev Psychiatry 2002;10(2):59-75.
3. Sadock BJ. Sadock VA (eds). Kaplan & Sadock’s comprehensive textbook of psychiatry. Philadelphia: Lippincott Williams & Wilkins, 2000;2377-90.
4. Bauer MS, Callahan AM, Jampala C, et al. Clinical practice guidelines for bipolar disorder from the Department of Veterans Affairs. J Clin Psychiatry 1999;60(1):9-21.
5. Sachs GS, Printz DJ, Kahn DA, et al. Medication treatment of bipolar disorder. Postgrad Med Special Report 2000;Apr:1-104.
6. American Psychiatric Association. Practice guidelines for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159:1.-
7. Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002;356:241-7.
8. Cohen LS, Rosenbaum JP. Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry 1998;59(suppl 2):18-28.
9. Baldessarini RJ, Tondo L. Recurrence risk in bipolar manic-depressive disorders after discontinuing lithium maintenance treatment: an overview. Clin Drug Invest 1998;15(4):337-51.
10. Jefferson JW. Lithium. In: Dukes MNG, Aronson JK (eds). Meyler’s side effects of drugs (14th ed). Amsterdam: Elsevier Science BV, 2001;86-94.
11. Jefferson JW. Lithium. In: Aronson JK (ed). Side effects of drugs, annual 24. Amsterdam: Elsevier Science BV, 2001;22-31.
12. Abdull H, Bliss R, Guinea AE, et al. Pathology and outcome of surgical treatment for lithium-associated hyperparathyroidism. Br J Surg 1999;86:91-3.
13. Gitlin M. Lithium and the kidney. An updated review. Drug Safety 1999;20(3):231-43.
14. Lundmark J, Gunnarsson T, Bengtsson F. A possible interaction between lithium and rofecoxib (letter to the editor). Br J Clin Pharmacol 2002;53(4):403-4.
15. Zwanzger P, Marcuse A, Boerner RJ, et al. Lithium intoxication after administration of AT1 blockers. J Clin Psychiatry 2001;62(3):208-9.