User login
Resource Utilization in Bacterial Meningitis
Bacterial meningitis can be a devastating disease in children. Overall mortality in children in the United States is 4%1 while long‐term morbidity is present in up to 25%2 of surviving children. The introduction of Haemophilus influenzae type B vaccine, heptavalent pneumococcal conjugate vaccine, and the quadrivalent meningococcal conjugate vaccine has altered the epidemiology of bacterial meningitis.24 Currently, little is known about the epidemiology of systemic complications and associated focal infections that occur during episodes of bacterial meningitis in children and how the presence of such complications affects in‐hospital healthcare resource utilization.
In a randomized controlled trial, the administration of adjuvant corticosteroids was associated with lower mortality rates in adults with bacterial meningitis due to all causes, with the greatest reduction in those with pneumococcal meningitis.5 In a post hoc analysis of data from this trial, reductions in systemic complications, such as septic shock, pneumonia, and acute respiratory distress syndrome, rather than neurologic complications were thought to be the underlying reason for the decrease in mortality associated with pneumococcal meningitis among corticosteroid recipients.6 However, children with bacterial meningitis have an overall 4‐fold lower mortality rate than adults with bacterial meningitis. An even greater difference in mortality rates exists between children and adults with pneumococcal meningitis.1, 5 Children do not benefit from adjuvant corticosteroids as adults do.1, 5, 7 Therefore, the pathogenesis of bacterial meningitis may differ in children from adults and account for the difference in response to adjuvant corticosteroids. Understanding the epidemiology of systemic complications and associated focal infections can aid in the understanding of the pathogenesis of the disease in varying age groups of children.
Previous studies in children have documented the frequency of certain bacterial meningitis‐associated conditions such as respiratory failure, pneumonia, endocarditis, and mastoiditis. Researchers have used the presence of such conditions to predict either mortality or neurologic sequelae in children.810 These studies were small and only included a few types of complications associated with bacterial meningitis. In‐hospital healthcare resource utilization, which may be an important indicator of in‐hospital morbidity, was also not considered as an outcome. In‐hospital morbidity may represent aspects of disease burden not captured by mortality rates or markers for long‐term morbidity alone. In future vaccine efficacy trails or novel therapeutics evaluations, consideration of these associated conditions is important.
The quantification of the use of in‐hospital healthcare utilization is also important for hospital planning and resource allocation in children with bacterial meningitis. A child presenting with bacterial meningitis and a systemic complication or an associated focal infection may require additional resource planning initially to expedite care to enhance recovery and decrease hospital length of stay (LOS).
Our goal was to document the frequency of bacterial meningitis‐associated conditions (systemic complications and associated focal infections) in a large cohort of children with bacterial meningitis treated at tertiary care children's hospitals in the United States, and determine how the presence of such conditions impacted in‐hospital healthcare resource utilization.
Patients and Methods
Data Source
Data for this retrospective cohort study was obtained from the Pediatric Health Information System (PHIS), a national administrative database containing data from 36 freestanding, tertiary care children's hospitals. These hospitals are affiliated with the Child Health Corporation of America (Shawnee Mission, KS), a business alliance of children's hospitals. Data quality and reliability are assured through a joint effort between the Child Health Corporation of America and participating hospitals. For the purposes of external benchmarking, participating hospitals provide discharge data including patient demographics, diagnoses, and procedures. Procedures to assure data validity were described previously.1 Total hospital charges are reported in the PHIS database and adjusted for hospital location using the Centers for Medicare and Medicaid price/wage index. A total of 27 participating hospitals also provide resource utilization data for each hospital discharge (ie, pharmaceutical dispensing, imaging, and laboratory studies); patients from these 27 hospitals were eligible for inclusion in this study. The protocol for the conduct of this study was reviewed and approved by The Children's Hospital of Philadelphia Committees for the Protection of Human Subjects.
Patients
Children less than 18 years of age with bacterial meningitis were eligible for this study if they were discharged from any of the 27 hospitals disclosing resource utilization data between January 1, 2001 and December 31, 2006. Study participants discharged with bacterial meningitis as their primary diagnosis were identified in the PHIS database using International Classification of Diseases, 9th revision, (ICD‐9) discharge diagnosis codes. The study population was limited to children without conditions predisposing to meningitis. Therefore, patients with ventricular shunts prior to the episode of bacterial meningitis were excluded using the following ICD‐9 procedure codes: ventricular shunt replacement (02.42); incision of peritoneum (54.95); removal of ventricular shunts (02.43); and the ICD‐9 discharge diagnosis code for mechanical complication of nervous system device, implant, and graft (996.2). Also, children with comorbid conditions that could predispose to meningitis or increase the likelihood of associated complications such as cancer (hematologic and nonhematologic), primary or secondary immunodeficiencies, prematurity, post‐operative infection, congenital cardiac disease, and sickle cell disease, were excluded from the analysis. Race and ethnicity were self‐reported by patients at time of admission.
Study Definitions
Study participants were identified from the PHIS database using ICD‐9 codes for the primary diagnosis of bacterial meningitis (codes 036.0‐036.1; 320.0‐320.3; 320.7; 320.81‐320.82; 320.89; 320.9). The sensitivity and specificity of ICD‐9 codes in identifying children with bacterial meningitis is unknown, however these codes have been used by previous investigators.1113 Bacterial meningitis associated‐conditions were classified as systemic complications (sepsis, systemic inflammatory response syndrome (SIRS), and respiratory failure) and associated focal infections (septic arthritis, mastoiditis, osteomyelitis, pneumonia and endocarditis). These associated conditions were identified by ICD‐9 discharge and procedural codes as listed in the Appendix (Supporting Information). Bone and joint infections were defined by the presence of either osteomyelitis or septic arthritis.
Primary Outcomes
The primary outcomes of interest were total in‐hospital charges and hospital LOS.
Measured Exposures
The primary exposures of interest were the occurrences of systemic complications, focal infections, or both conditions in children with bacterial meningitis.
Statistical Analysis
The data were initially described using frequencies and percentages for categorical variables and mean, median, interquartile ranges (IQRs) and range values for continuous variables. Analyses of bivariate associations between the outcomes (total in‐hospital charges and length of hospital stay) and potential covariates entailed either chi‐square tests or, for rare events with an expected frequency <5, Fishers Exact Test.
Following bivariate analysis, multivariable models were constructed to assess the adjusted impact of systemic complications and focal infection on total in‐hospital charges and hospital LOS. In evaluating total in‐hospital charges, the charge data were logarithmically transformed to account for the skewed distribution of charges. Multivariable linear regression was then performed to analyze the log transformed charges. The resulting beta‐coefficients were transformed to reflect the percent difference in total hospital charges between children with and without specific complications. In evaluating hospital LOS, negative binomial regression models were employed to estimate incidence rate ratios (IRRs) rather than log‐linear models, as to account for overdispersion in the outcome data. The negative binomial model produced a ratio of lengths of stay or IRR, where a ratio >1 indicates that the risk factor was associated with a longer LOS. The results were presented as percentage change to facilitate interpretation of the results.
The multivariable models were adjusted for the following confounders as determined a priori: age category, race, sex, vancomycin receipt, and adjuvant corticosteroid receipt within the first 24 hours of admission. Tests for interaction between systemic complications or focal infections and age were performed for each of these models. To address the possibility of referral bias which would lead us to overestimate the cost of caring for children with bacterial meningitis with an associated condition, the analyses were repeated restricting the sample to those children who had a lumber puncture performed at a PHIS‐participating hospital. The frequency of systemic complications and focal infections in those who were transferred was no different than in children who were not transferred; therefore the entire cohort was used in the final analyses. Sub‐group analyses were also performed for children identified with pneumococcal and meningococcal meningitis.
The standard errors for all estimates of covariate effects including metastatic effects under the above models were adjusted for the hospital to account for the increased variability due to clustering of individuals within hospitals. Two‐tailed P values <0.05 were considered statistically significant. Actual P values and 95% confidence intervals are reported. Data were analyzed using STATA, Version 10 (Stata Corporation, College Station, TX).
Results
Demographics
There were 2780 children admitted with bacterial meningitis during the study period; 461 (17%) children were excluded because of comorbid illness including malignancy (n = 37), congential heart disease (n = 231), prematurity (n = 104), human immunodeficiency virus infection (n = 4), sickle cell disease (n = 17), and post‐operative infection (n = 68). The remaining 2319 children with bacterial meningitis were included in the analyses. The mean age was 3.6 years (median, 1 year; IQR, 0‐6 years). Approximately half of the children were less than 1 year of age, 23% were 1 to 5 years, and 27% were >5 years. A total of 54% of children were white, 19% were black, 22% were Hispanic, and 5% were of other racial groups. Males accounted for 58% of the children. In this cohort of children, 9% received adjuvant corticosteroids within 24 hours of hospitalization.
Bacterial Meningitis‐Associated Conditions
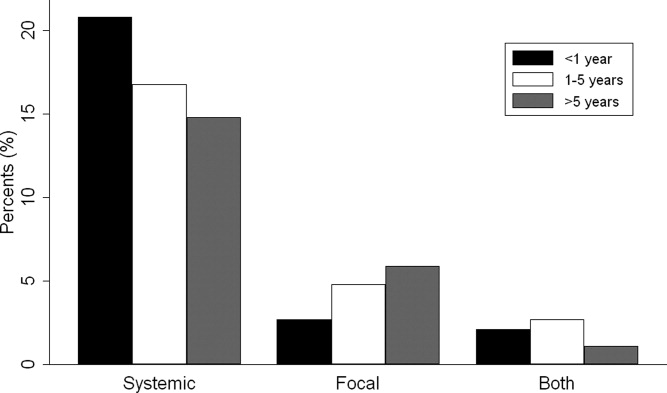
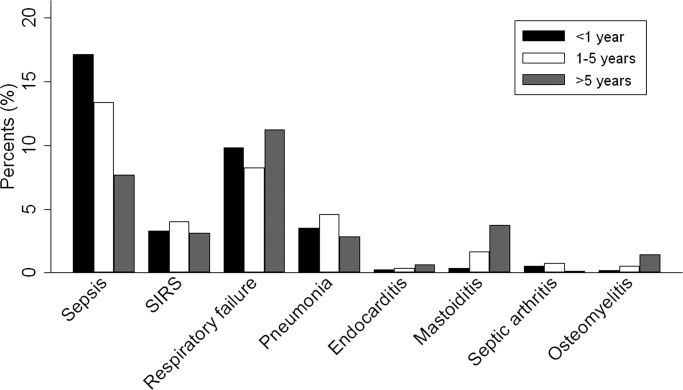
Overall, 574 (25%) of children with bacterial meningitis suffered a systemic complication or an associated focal infection. Figure 1 shows the types of associated condition stratified by age category. Older children had a higher frequency of associated focal infections while younger children had a higher frequency of systemic complications (P = 0.002, chi‐square test for trend). Figure 2 shows the distribution of specific conditions among children in each age category. The frequency of sepsis decreased with age (P < 0.001, chi‐square test) while the frequency of mastoiditis (P < 0.001, Fisher's exact test) and osteomyelitis (P = 0.005, Fisher's exact test) increased with age. There did not appear to be substantial variability in the proportion of patients with SIRS or sepsis across hospitals, suggesting that hospital‐level variability in coding for these conditions was likely minimal. The median proportion of patients with SIRS by hospital was 2.4% (IQR, 1.2‐4.8%) while the median proportion of patients with sepsis by hospital was 13.4% (IQR, 10.0‐16.9%).
Of the 151 children with an associated focal infection, only 3 (2%) of children had more than 1 infection (1 child had mastoiditis and endocarditis, 1 child had pneumonia and osteomyelitis, and 1 child had pneumonia and endocarditis). However, of the 479 children with systemic complications, 116 (24%) had more than 1 systemic disease (Table 1).
| Types of Systemic Complications | Systemic Complications in All Bacterial Meningitis, n (%) | Systemic Complications in Meningococcal Meningitis, n (%) | Systemic Complications in Pneumococcal Meningitis, n (%) |
|---|---|---|---|
| |||
| Sepsis only | 209 (44) | 16 (21) | 69 (54) |
| Respiratory failure only | 139 (29) | 38 (49) | 30 (24) |
| SIRS only | 15 (3) | 9 (12) | 1 (1) |
| Sepsis and respiratory failure | 52 (11) | 4 (5) | 18 (14) |
| SIRS and sepsis | 27 (6) | 2 (3) | 4 (3) |
| SIRS and respiratory failure | 9 (2) | 5 (6) | 0 (0) |
| SIRS and respiratory failure and sepsis | 28 (6) | 3 (4) | 5 (4) |
| Total systemic complications | 479 | 77 | 127 |
In sub‐group analyses, 269 children had meningococcal meningitis and 470 children had pneumococcal meningitis. Of the children with meningococcal meningitis, 31.2% had a meningitis‐associated condition: 26.4% had a systemic complication, 2.6% had a focal infection, and 2.2% had both conditions. The most common associated conditions in children with meningococcal meningitis were respiratory failure (18.6%; n = 50), sepsis (9.3%; n = 25), and SIRS (7.1%; n = 19). In children with pneumococcal meningitis, 32.3% had a meningitis‐associated complication: 24.7% had a systemic complication, 5.3% had a focal infection, and 2.3% had both conditions. The most common associated conditions in children with pneumococcal meningitis were sepsis (20.4%; n = 96), respiratory failure (11.3%; n = 53), and pneumonia (4.7%; n = 22); mastoiditis was present in 2.3% (n = 11) of children with pneumococcal meningitis. Respiratory failure was more common in meningococcal meningitis (18.6%) than in pneumococcal meningitis (11.3%; P = 0.006). In contrast, sepsis was less common in meningococcal meningitis (9.3%) than in pneumococcal meningitis (20.4%; P < 0.001).
Hospital Charges
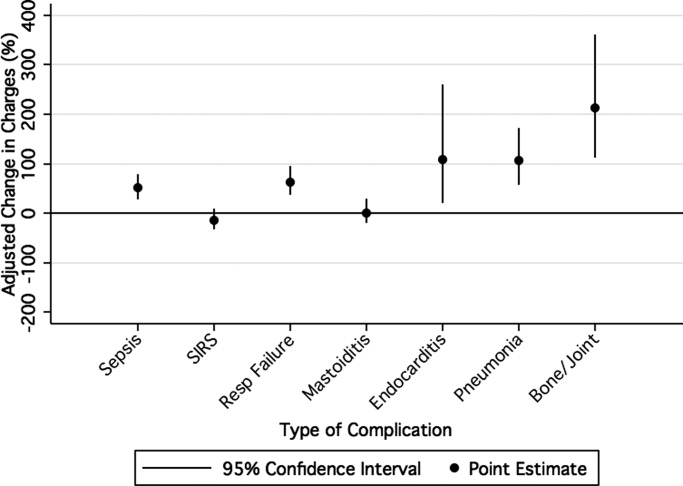
Overall, the median charges per hospital ranged from $20,158 to $53,823. In‐hospital charges for children with bacterial meningitis with and without any identified associated conditions are presented in Table 2. In multivariate analyses, the presence of systemic conditions, associated focal infections, or both conditions was independently associated with significantly higher total in‐hospital charges (Table 2). When conditions were considered individually, bone and joint infections (213% increase; 95% CI, 113‐260%), endocarditis (108% increase; 95% CI, 23‐258%), and pneumonia (107% increase; 95% CI, 58‐171%) were associated with the highest increases in total hospital charges (Figure 3). In contrast, SIRS and mastoiditis were not associated with higher hospital charges (Figure 3).
| Charges | LOS | |||
|---|---|---|---|---|
| Median, $ (IQR) | Adjusted Increase,* % (95% CI) | Median, days (IQR) | Adjusted Increase,* % (95% CI) | |
| ||||
| None (n = 1,745) | $27,110 (15,823‐48,307) | Reference** | 9 (6‐14) | Reference |
| Systemic (n = 423) | $66,690 (39,546136,756) | 136 (108269) | 14 (923) | 72 (5196) |
| Focal Infection (n = 95) | $58,016 (29,056125,813) | 118 (77168) | 13 (928) | 78 (40126) |
| Both (n = 56) | $130,744 (62,397299,288) | 351 (237503) | 21.5 (1245) | 211 (142303) |
LOS
The median LOS was 9 days (IQR, 6‐15 days); 5% of children had a LOS >42 days. Table 2 summarizes difference in LOS by the presence and absence of systemic conditions and focal infections. In multivariate analyses, the presence of systemic conditions, associated focal infections, or both conditions was independently associated with a significantly longer LOS (Table 2). When conditions were considered individually, endocarditis (152% increase; 95% CI, 60‐300%) and pneumonia (136% increase; 95% CI, 85‐201%) were associated with the greatest adjusted increases in LOS (Figure 4); only mastoiditis was not associated with an increased LOS compared with those without complications.

Discussion
To our knowledge, this is the first study to examine bacterial meningitis‐associated conditions in children and their impact on in‐hospital resource utilization. We found that 25% of the cohort of children with bacterial meningitis suffered from at least one focal infection or systemic complication. This represents a significant invasive disease burden among children with bacterial meningitis who do not have underlying comorbid conditions. Younger children were more likely to have systemic complications when compared with older children, specifically due to a higher frequency of sepsis in children <1 year. Older children were more likely to have an associated focal infection, specifically due to an increase in mastoiditis and osteomyelitis in children >1 year. Only 2% of children had more than 1 focal infection, while 24% of children had more than 1 systemic complication.
Importantly, the presence of a systemic complication in a child with bacterial meningitis increased their in‐hospital adjusted charges by 136%. The presence of a focal infection increased in‐hospital adjusted charges by 118%. A child with both a systemic complication and a focal infection and had a 351% increase in in‐hospital adjusted charges.
The presence of systemic complications or associated focal infections was significantly associated with higher in‐hospital charges and longer hospital LOS. Most individual meningitis‐associated conditions included in this study were associated with higher in‐hospital charges with the exception of SIRS and mastoiditis. All individual meningitis‐associated conditions were associated with a longer LOS except mastoiditis. This finding is not surprising as the LOS for children with mastoiditis is typically shorter than for children with bacterial meningitis. Glikich et al.14 reported a mean LOS of approximately 8 days for children with mastoiditis. As meningitis in the context of mastoiditis is likely caused by direct extension of infection, patients with meningitis and mastoiditis likely required extended hospitalization to treat meningitis rather than mastoiditis. In contrast, patients with meningitis occurring in the context of metastatic dissemination of infection (eg, endocarditis, pneumonia) often have hemodynamic instability requiring prolonged intensive care support.
A study of children with sepsis found that increasing severity of illness was associated with greater hospital resource utilization.15 Our study shows that this may also be true in children with bacterial meningitis. We found that in children with bacterial meningitis, having systemic complications or an associated focal infection was associated with greater in‐hospital resource utilization. This finding may therefore indicate greater in‐hospital morbidity among children with a bacterial meningitis‐associated condition. Since mortality rates for bacterial meningitis are low in children, in‐hospital morbidity may be a better indicator of disease burden.
Our data show that, in contrast to adults, bacterial meningitis in children is not typically associated with other focal infections. Some focal complications such as mastoiditis and osteomyelitis disproportionately affect older children. These complications are typically accompanied by overt clinical manifestations. Therefore, we believe that the evaluation for the presence of concomitant focal infections can be guided by clinical examination findings and that routine radiologic evaluation for focal complications may not be necessary. Additionally, focal infections tend to occur in the absence of concomitant systemic complications. Of the 151 children with at least 1 associated focal infection, only 37% had a systemic complication. Bacterial meningitis may lie on a continuum of invasive disease depending on the virulence factors of the invading pathogen as well as specific host factors. Understanding the epidemiology of these associated conditions can enhance our understanding of the pathogenesis of bacterial meningitis in children. Understanding why some children suffer from septicemia rather than bacteremia may help in developing novel therapeutics.
There are several limitations to our study. First, since we identified focal infections and systemic complications using billing charges and ICD‐9 discharge diagnosis codes, it was impossible to determine when these conditions represented true complications of bacterial meningitis and when they represented the primary source of infection. Therefore, some of our primary outcomes may represent the cause of meningitis rather than a direct complication. We attempted to minimize such misclassification by limiting the cohort to those with a primary discharge diagnosis of bacterial meningitis though such misclassification is still possible.
Second, the use of ICD‐9 codes to accurately identify systemic complications and associated focal infections is a potential limitation. For example, respiratory failure, defined as the requirement of endotracheal intubation in our study, may not capture children receiving non‐invasive mechanical ventilation (eg, bilevel positive airway pressure). If use of noninvasive ventilation strategies did not depend exclusively on illness severity, our study would underestimate the frequency of respiratory failure. Furthermore, there may be inconsistencies among pediatric physicians in coding conditions such as SIRS and sepsis. Even in the clinical setting, a uniform definition of SIRS and sepsis is problematic due to physiologic differences between adults and children of varying age groups.16 An international panel of pediatricians proposed age‐specific definitions for sepsis and SIRS, while acknowledging the paucity of evidence to support some of their recommendations.16 None of the proposed definitions could be applied using administrative data. Limitations in the use of ICD‐9 discharge diagnosis codes to identify children with bacterial meningitis were discussed previously.1
Third, only free‐standing children's hospitals were included in the analysis. It is likely that many children with uncomplicated bacterial meningitis are treated at community hospitals or smaller academic centers. Our study may overestimate the rate of bacterial meningitis‐associated focal infections and systemic complications since participating hospitals serve as regional referral centers. To address the potential for such referral bias, we repeated the analysis while restricting the cohort to those children who had a lumbar puncture performed at the treating facility. No difference in frequency of associated conditions or in‐hospital resource utilization was found between children transferred and children not transferred. Finally, the PHIS database reports billed charge data rather than cost data. Billed data may overestimate the actual economic impact of bacterial meningitis‐associated complications since payers often reimburse at lesser rates. Resource utilization may also vary widely between hospitals and geographic locations as previously shown.15
In conclusion, bacterial meningitis remains an important cause of morbidity in children. Systemic complications such as sepsis and respiratory failure are common. Respiratory failure occurred more commonly among patients with meningococcal meningitis while sepsis occurred more commonly among patients with pneumococcal meningitis. While focal complications are uncommon, children >5 years of age are more likely than younger children to have concomitant mastoiditis or osteomyelitis. The presence of both systemic and focal complications is associated with substantially greater resource utilization than either complication alone.
Dr. Shah had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. Study concept and design: Shah, Mongelluzzo; acquisition of data: Shah, Mohamad; analysis and interpretation of data: Mongelluzzo, Mohamad, Ten Have, Shah; drafting of the manuscript: Mongelluzzo; critical revision of the manuscript for important intellectual content: Mongelluzzo, Mohamad, Ten Have, Shah; statistical analysis: Shah, Mongelluzzo, Ten Have; obtained funding: Shah, Mongelluzzo; administrative, technical, or material support: Shah; study supervision: Shah.
Appendix
Diagnosis Codes:
Endocarditis: 421.0, 421.1, 421.9
Mastoiditis: 383.0, 383.1, 383.2, 383.8, 383.9
Osteomyelitis: 730.0, 730.1, 730.2, 730.3, 730.7, 730.8, 730.9
Septic arthritis: 711.0, 711.1, 711.2, 711.3, 711.4, 711.5, 711.6, 711.7, 711.8, 711.9
Sepsis: 038.0, 038.1, 038.2, 038.3, 038.4, 038.8, 038.9
Systemic Inflammatory Response Syndrome: 995.92
Pneumonia: 480.0, 480.1, 480.2, 480.3, 480.8, 480.9, 481, 482.0, 482.1, 482.2, 482.3, 482.4, 482.8, 482.9, 483.0, 483.1, 483.8, 484.1, 484.3, 484.5, 484.6, 484.7, 484.8, 485, 486
Procedure Codes:
Endotracheal Intubation: 96.04
- , , , .Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- , , , et al.Bacterial meningitis in the United States in 1995. Active surveillance team.N Engl J Med.1997;337:970–976.
- Progress toward elimination of Haemophilus influenzae type b disease among infants and children–United States, 1987–1995.MMWR Morb Mortal Wkly Rep.1996;45:901–906.
- , .Bacterial meningitis in children.Lancet.2003;361:2139–2148.
- , .Dexamethasone in adults with bacterial meningitis.N Engl J Med.2002;347:1549–1556.
- , .Dexamethasone and pneumococcal meningitis.Ann Intern Med.2004;141:327.
- , , , et al.Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis.N Engl J Med.2007;357:2431–2440.
- , , , , .Bacterial meningitis in an urban area: etiologic study and prognostic factors.Infection.2007;35:406–413.
- , , .Clinical features and prognostic factors in childhood pneumococcal meningitis.J Microbiol Immunol Infect.2008;41:48–53.
- , , .Clinical presentation and prognostic factors of Streptococcus pneumoniae meningitis according to the focus of infection.BMC Infect Dis.2005;5:93.
- , .Trends in invasive pneumococcal disease‐associated hospitalizations.Clin Infect Dis.2006;42:e1–e5.
- , , .Managing meningococcal disease in the United States: Hospital case characteristics and costs by age.Value Health.2006;9:236–243.
- , , , , , .Population‐based analysis of meningococcal disease mortality in the United States: 1990–2002.Pediatr Infect Dis J.2006;25:191–194.
- , , , .A contemporary analysis of acute mastoiditis.Arch Otolaryngol Head Neck Surg.1996;122:135–139.
- , , .Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis.Pediatrics.2007;119:487–494.
- , , .International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics.Pediatr Crit Care Med.2005;6:2–8.
Bacterial meningitis can be a devastating disease in children. Overall mortality in children in the United States is 4%1 while long‐term morbidity is present in up to 25%2 of surviving children. The introduction of Haemophilus influenzae type B vaccine, heptavalent pneumococcal conjugate vaccine, and the quadrivalent meningococcal conjugate vaccine has altered the epidemiology of bacterial meningitis.24 Currently, little is known about the epidemiology of systemic complications and associated focal infections that occur during episodes of bacterial meningitis in children and how the presence of such complications affects in‐hospital healthcare resource utilization.
In a randomized controlled trial, the administration of adjuvant corticosteroids was associated with lower mortality rates in adults with bacterial meningitis due to all causes, with the greatest reduction in those with pneumococcal meningitis.5 In a post hoc analysis of data from this trial, reductions in systemic complications, such as septic shock, pneumonia, and acute respiratory distress syndrome, rather than neurologic complications were thought to be the underlying reason for the decrease in mortality associated with pneumococcal meningitis among corticosteroid recipients.6 However, children with bacterial meningitis have an overall 4‐fold lower mortality rate than adults with bacterial meningitis. An even greater difference in mortality rates exists between children and adults with pneumococcal meningitis.1, 5 Children do not benefit from adjuvant corticosteroids as adults do.1, 5, 7 Therefore, the pathogenesis of bacterial meningitis may differ in children from adults and account for the difference in response to adjuvant corticosteroids. Understanding the epidemiology of systemic complications and associated focal infections can aid in the understanding of the pathogenesis of the disease in varying age groups of children.
Previous studies in children have documented the frequency of certain bacterial meningitis‐associated conditions such as respiratory failure, pneumonia, endocarditis, and mastoiditis. Researchers have used the presence of such conditions to predict either mortality or neurologic sequelae in children.810 These studies were small and only included a few types of complications associated with bacterial meningitis. In‐hospital healthcare resource utilization, which may be an important indicator of in‐hospital morbidity, was also not considered as an outcome. In‐hospital morbidity may represent aspects of disease burden not captured by mortality rates or markers for long‐term morbidity alone. In future vaccine efficacy trails or novel therapeutics evaluations, consideration of these associated conditions is important.
The quantification of the use of in‐hospital healthcare utilization is also important for hospital planning and resource allocation in children with bacterial meningitis. A child presenting with bacterial meningitis and a systemic complication or an associated focal infection may require additional resource planning initially to expedite care to enhance recovery and decrease hospital length of stay (LOS).
Our goal was to document the frequency of bacterial meningitis‐associated conditions (systemic complications and associated focal infections) in a large cohort of children with bacterial meningitis treated at tertiary care children's hospitals in the United States, and determine how the presence of such conditions impacted in‐hospital healthcare resource utilization.
Patients and Methods
Data Source
Data for this retrospective cohort study was obtained from the Pediatric Health Information System (PHIS), a national administrative database containing data from 36 freestanding, tertiary care children's hospitals. These hospitals are affiliated with the Child Health Corporation of America (Shawnee Mission, KS), a business alliance of children's hospitals. Data quality and reliability are assured through a joint effort between the Child Health Corporation of America and participating hospitals. For the purposes of external benchmarking, participating hospitals provide discharge data including patient demographics, diagnoses, and procedures. Procedures to assure data validity were described previously.1 Total hospital charges are reported in the PHIS database and adjusted for hospital location using the Centers for Medicare and Medicaid price/wage index. A total of 27 participating hospitals also provide resource utilization data for each hospital discharge (ie, pharmaceutical dispensing, imaging, and laboratory studies); patients from these 27 hospitals were eligible for inclusion in this study. The protocol for the conduct of this study was reviewed and approved by The Children's Hospital of Philadelphia Committees for the Protection of Human Subjects.
Patients
Children less than 18 years of age with bacterial meningitis were eligible for this study if they were discharged from any of the 27 hospitals disclosing resource utilization data between January 1, 2001 and December 31, 2006. Study participants discharged with bacterial meningitis as their primary diagnosis were identified in the PHIS database using International Classification of Diseases, 9th revision, (ICD‐9) discharge diagnosis codes. The study population was limited to children without conditions predisposing to meningitis. Therefore, patients with ventricular shunts prior to the episode of bacterial meningitis were excluded using the following ICD‐9 procedure codes: ventricular shunt replacement (02.42); incision of peritoneum (54.95); removal of ventricular shunts (02.43); and the ICD‐9 discharge diagnosis code for mechanical complication of nervous system device, implant, and graft (996.2). Also, children with comorbid conditions that could predispose to meningitis or increase the likelihood of associated complications such as cancer (hematologic and nonhematologic), primary or secondary immunodeficiencies, prematurity, post‐operative infection, congenital cardiac disease, and sickle cell disease, were excluded from the analysis. Race and ethnicity were self‐reported by patients at time of admission.
Study Definitions
Study participants were identified from the PHIS database using ICD‐9 codes for the primary diagnosis of bacterial meningitis (codes 036.0‐036.1; 320.0‐320.3; 320.7; 320.81‐320.82; 320.89; 320.9). The sensitivity and specificity of ICD‐9 codes in identifying children with bacterial meningitis is unknown, however these codes have been used by previous investigators.1113 Bacterial meningitis associated‐conditions were classified as systemic complications (sepsis, systemic inflammatory response syndrome (SIRS), and respiratory failure) and associated focal infections (septic arthritis, mastoiditis, osteomyelitis, pneumonia and endocarditis). These associated conditions were identified by ICD‐9 discharge and procedural codes as listed in the Appendix (Supporting Information). Bone and joint infections were defined by the presence of either osteomyelitis or septic arthritis.
Primary Outcomes
The primary outcomes of interest were total in‐hospital charges and hospital LOS.
Measured Exposures
The primary exposures of interest were the occurrences of systemic complications, focal infections, or both conditions in children with bacterial meningitis.
Statistical Analysis
The data were initially described using frequencies and percentages for categorical variables and mean, median, interquartile ranges (IQRs) and range values for continuous variables. Analyses of bivariate associations between the outcomes (total in‐hospital charges and length of hospital stay) and potential covariates entailed either chi‐square tests or, for rare events with an expected frequency <5, Fishers Exact Test.
Following bivariate analysis, multivariable models were constructed to assess the adjusted impact of systemic complications and focal infection on total in‐hospital charges and hospital LOS. In evaluating total in‐hospital charges, the charge data were logarithmically transformed to account for the skewed distribution of charges. Multivariable linear regression was then performed to analyze the log transformed charges. The resulting beta‐coefficients were transformed to reflect the percent difference in total hospital charges between children with and without specific complications. In evaluating hospital LOS, negative binomial regression models were employed to estimate incidence rate ratios (IRRs) rather than log‐linear models, as to account for overdispersion in the outcome data. The negative binomial model produced a ratio of lengths of stay or IRR, where a ratio >1 indicates that the risk factor was associated with a longer LOS. The results were presented as percentage change to facilitate interpretation of the results.
The multivariable models were adjusted for the following confounders as determined a priori: age category, race, sex, vancomycin receipt, and adjuvant corticosteroid receipt within the first 24 hours of admission. Tests for interaction between systemic complications or focal infections and age were performed for each of these models. To address the possibility of referral bias which would lead us to overestimate the cost of caring for children with bacterial meningitis with an associated condition, the analyses were repeated restricting the sample to those children who had a lumber puncture performed at a PHIS‐participating hospital. The frequency of systemic complications and focal infections in those who were transferred was no different than in children who were not transferred; therefore the entire cohort was used in the final analyses. Sub‐group analyses were also performed for children identified with pneumococcal and meningococcal meningitis.
The standard errors for all estimates of covariate effects including metastatic effects under the above models were adjusted for the hospital to account for the increased variability due to clustering of individuals within hospitals. Two‐tailed P values <0.05 were considered statistically significant. Actual P values and 95% confidence intervals are reported. Data were analyzed using STATA, Version 10 (Stata Corporation, College Station, TX).
Results
Demographics
There were 2780 children admitted with bacterial meningitis during the study period; 461 (17%) children were excluded because of comorbid illness including malignancy (n = 37), congential heart disease (n = 231), prematurity (n = 104), human immunodeficiency virus infection (n = 4), sickle cell disease (n = 17), and post‐operative infection (n = 68). The remaining 2319 children with bacterial meningitis were included in the analyses. The mean age was 3.6 years (median, 1 year; IQR, 0‐6 years). Approximately half of the children were less than 1 year of age, 23% were 1 to 5 years, and 27% were >5 years. A total of 54% of children were white, 19% were black, 22% were Hispanic, and 5% were of other racial groups. Males accounted for 58% of the children. In this cohort of children, 9% received adjuvant corticosteroids within 24 hours of hospitalization.
Bacterial Meningitis‐Associated Conditions
Overall, 574 (25%) of children with bacterial meningitis suffered a systemic complication or an associated focal infection. Figure 1 shows the types of associated condition stratified by age category. Older children had a higher frequency of associated focal infections while younger children had a higher frequency of systemic complications (P = 0.002, chi‐square test for trend). Figure 2 shows the distribution of specific conditions among children in each age category. The frequency of sepsis decreased with age (P < 0.001, chi‐square test) while the frequency of mastoiditis (P < 0.001, Fisher's exact test) and osteomyelitis (P = 0.005, Fisher's exact test) increased with age. There did not appear to be substantial variability in the proportion of patients with SIRS or sepsis across hospitals, suggesting that hospital‐level variability in coding for these conditions was likely minimal. The median proportion of patients with SIRS by hospital was 2.4% (IQR, 1.2‐4.8%) while the median proportion of patients with sepsis by hospital was 13.4% (IQR, 10.0‐16.9%).


Of the 151 children with an associated focal infection, only 3 (2%) of children had more than 1 infection (1 child had mastoiditis and endocarditis, 1 child had pneumonia and osteomyelitis, and 1 child had pneumonia and endocarditis). However, of the 479 children with systemic complications, 116 (24%) had more than 1 systemic disease (Table 1).
| Types of Systemic Complications | Systemic Complications in All Bacterial Meningitis, n (%) | Systemic Complications in Meningococcal Meningitis, n (%) | Systemic Complications in Pneumococcal Meningitis, n (%) |
|---|---|---|---|
| |||
| Sepsis only | 209 (44) | 16 (21) | 69 (54) |
| Respiratory failure only | 139 (29) | 38 (49) | 30 (24) |
| SIRS only | 15 (3) | 9 (12) | 1 (1) |
| Sepsis and respiratory failure | 52 (11) | 4 (5) | 18 (14) |
| SIRS and sepsis | 27 (6) | 2 (3) | 4 (3) |
| SIRS and respiratory failure | 9 (2) | 5 (6) | 0 (0) |
| SIRS and respiratory failure and sepsis | 28 (6) | 3 (4) | 5 (4) |
| Total systemic complications | 479 | 77 | 127 |
In sub‐group analyses, 269 children had meningococcal meningitis and 470 children had pneumococcal meningitis. Of the children with meningococcal meningitis, 31.2% had a meningitis‐associated condition: 26.4% had a systemic complication, 2.6% had a focal infection, and 2.2% had both conditions. The most common associated conditions in children with meningococcal meningitis were respiratory failure (18.6%; n = 50), sepsis (9.3%; n = 25), and SIRS (7.1%; n = 19). In children with pneumococcal meningitis, 32.3% had a meningitis‐associated complication: 24.7% had a systemic complication, 5.3% had a focal infection, and 2.3% had both conditions. The most common associated conditions in children with pneumococcal meningitis were sepsis (20.4%; n = 96), respiratory failure (11.3%; n = 53), and pneumonia (4.7%; n = 22); mastoiditis was present in 2.3% (n = 11) of children with pneumococcal meningitis. Respiratory failure was more common in meningococcal meningitis (18.6%) than in pneumococcal meningitis (11.3%; P = 0.006). In contrast, sepsis was less common in meningococcal meningitis (9.3%) than in pneumococcal meningitis (20.4%; P < 0.001).
Hospital Charges
Overall, the median charges per hospital ranged from $20,158 to $53,823. In‐hospital charges for children with bacterial meningitis with and without any identified associated conditions are presented in Table 2. In multivariate analyses, the presence of systemic conditions, associated focal infections, or both conditions was independently associated with significantly higher total in‐hospital charges (Table 2). When conditions were considered individually, bone and joint infections (213% increase; 95% CI, 113‐260%), endocarditis (108% increase; 95% CI, 23‐258%), and pneumonia (107% increase; 95% CI, 58‐171%) were associated with the highest increases in total hospital charges (Figure 3). In contrast, SIRS and mastoiditis were not associated with higher hospital charges (Figure 3).
| Charges | LOS | |||
|---|---|---|---|---|
| Median, $ (IQR) | Adjusted Increase,* % (95% CI) | Median, days (IQR) | Adjusted Increase,* % (95% CI) | |
| ||||
| None (n = 1,745) | $27,110 (15,823‐48,307) | Reference** | 9 (6‐14) | Reference |
| Systemic (n = 423) | $66,690 (39,546136,756) | 136 (108269) | 14 (923) | 72 (5196) |
| Focal Infection (n = 95) | $58,016 (29,056125,813) | 118 (77168) | 13 (928) | 78 (40126) |
| Both (n = 56) | $130,744 (62,397299,288) | 351 (237503) | 21.5 (1245) | 211 (142303) |

LOS
The median LOS was 9 days (IQR, 6‐15 days); 5% of children had a LOS >42 days. Table 2 summarizes difference in LOS by the presence and absence of systemic conditions and focal infections. In multivariate analyses, the presence of systemic conditions, associated focal infections, or both conditions was independently associated with a significantly longer LOS (Table 2). When conditions were considered individually, endocarditis (152% increase; 95% CI, 60‐300%) and pneumonia (136% increase; 95% CI, 85‐201%) were associated with the greatest adjusted increases in LOS (Figure 4); only mastoiditis was not associated with an increased LOS compared with those without complications.

Discussion
To our knowledge, this is the first study to examine bacterial meningitis‐associated conditions in children and their impact on in‐hospital resource utilization. We found that 25% of the cohort of children with bacterial meningitis suffered from at least one focal infection or systemic complication. This represents a significant invasive disease burden among children with bacterial meningitis who do not have underlying comorbid conditions. Younger children were more likely to have systemic complications when compared with older children, specifically due to a higher frequency of sepsis in children <1 year. Older children were more likely to have an associated focal infection, specifically due to an increase in mastoiditis and osteomyelitis in children >1 year. Only 2% of children had more than 1 focal infection, while 24% of children had more than 1 systemic complication.
Importantly, the presence of a systemic complication in a child with bacterial meningitis increased their in‐hospital adjusted charges by 136%. The presence of a focal infection increased in‐hospital adjusted charges by 118%. A child with both a systemic complication and a focal infection and had a 351% increase in in‐hospital adjusted charges.
The presence of systemic complications or associated focal infections was significantly associated with higher in‐hospital charges and longer hospital LOS. Most individual meningitis‐associated conditions included in this study were associated with higher in‐hospital charges with the exception of SIRS and mastoiditis. All individual meningitis‐associated conditions were associated with a longer LOS except mastoiditis. This finding is not surprising as the LOS for children with mastoiditis is typically shorter than for children with bacterial meningitis. Glikich et al.14 reported a mean LOS of approximately 8 days for children with mastoiditis. As meningitis in the context of mastoiditis is likely caused by direct extension of infection, patients with meningitis and mastoiditis likely required extended hospitalization to treat meningitis rather than mastoiditis. In contrast, patients with meningitis occurring in the context of metastatic dissemination of infection (eg, endocarditis, pneumonia) often have hemodynamic instability requiring prolonged intensive care support.
A study of children with sepsis found that increasing severity of illness was associated with greater hospital resource utilization.15 Our study shows that this may also be true in children with bacterial meningitis. We found that in children with bacterial meningitis, having systemic complications or an associated focal infection was associated with greater in‐hospital resource utilization. This finding may therefore indicate greater in‐hospital morbidity among children with a bacterial meningitis‐associated condition. Since mortality rates for bacterial meningitis are low in children, in‐hospital morbidity may be a better indicator of disease burden.
Our data show that, in contrast to adults, bacterial meningitis in children is not typically associated with other focal infections. Some focal complications such as mastoiditis and osteomyelitis disproportionately affect older children. These complications are typically accompanied by overt clinical manifestations. Therefore, we believe that the evaluation for the presence of concomitant focal infections can be guided by clinical examination findings and that routine radiologic evaluation for focal complications may not be necessary. Additionally, focal infections tend to occur in the absence of concomitant systemic complications. Of the 151 children with at least 1 associated focal infection, only 37% had a systemic complication. Bacterial meningitis may lie on a continuum of invasive disease depending on the virulence factors of the invading pathogen as well as specific host factors. Understanding the epidemiology of these associated conditions can enhance our understanding of the pathogenesis of bacterial meningitis in children. Understanding why some children suffer from septicemia rather than bacteremia may help in developing novel therapeutics.
There are several limitations to our study. First, since we identified focal infections and systemic complications using billing charges and ICD‐9 discharge diagnosis codes, it was impossible to determine when these conditions represented true complications of bacterial meningitis and when they represented the primary source of infection. Therefore, some of our primary outcomes may represent the cause of meningitis rather than a direct complication. We attempted to minimize such misclassification by limiting the cohort to those with a primary discharge diagnosis of bacterial meningitis though such misclassification is still possible.
Second, the use of ICD‐9 codes to accurately identify systemic complications and associated focal infections is a potential limitation. For example, respiratory failure, defined as the requirement of endotracheal intubation in our study, may not capture children receiving non‐invasive mechanical ventilation (eg, bilevel positive airway pressure). If use of noninvasive ventilation strategies did not depend exclusively on illness severity, our study would underestimate the frequency of respiratory failure. Furthermore, there may be inconsistencies among pediatric physicians in coding conditions such as SIRS and sepsis. Even in the clinical setting, a uniform definition of SIRS and sepsis is problematic due to physiologic differences between adults and children of varying age groups.16 An international panel of pediatricians proposed age‐specific definitions for sepsis and SIRS, while acknowledging the paucity of evidence to support some of their recommendations.16 None of the proposed definitions could be applied using administrative data. Limitations in the use of ICD‐9 discharge diagnosis codes to identify children with bacterial meningitis were discussed previously.1
Third, only free‐standing children's hospitals were included in the analysis. It is likely that many children with uncomplicated bacterial meningitis are treated at community hospitals or smaller academic centers. Our study may overestimate the rate of bacterial meningitis‐associated focal infections and systemic complications since participating hospitals serve as regional referral centers. To address the potential for such referral bias, we repeated the analysis while restricting the cohort to those children who had a lumbar puncture performed at the treating facility. No difference in frequency of associated conditions or in‐hospital resource utilization was found between children transferred and children not transferred. Finally, the PHIS database reports billed charge data rather than cost data. Billed data may overestimate the actual economic impact of bacterial meningitis‐associated complications since payers often reimburse at lesser rates. Resource utilization may also vary widely between hospitals and geographic locations as previously shown.15
In conclusion, bacterial meningitis remains an important cause of morbidity in children. Systemic complications such as sepsis and respiratory failure are common. Respiratory failure occurred more commonly among patients with meningococcal meningitis while sepsis occurred more commonly among patients with pneumococcal meningitis. While focal complications are uncommon, children >5 years of age are more likely than younger children to have concomitant mastoiditis or osteomyelitis. The presence of both systemic and focal complications is associated with substantially greater resource utilization than either complication alone.
Dr. Shah had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. Study concept and design: Shah, Mongelluzzo; acquisition of data: Shah, Mohamad; analysis and interpretation of data: Mongelluzzo, Mohamad, Ten Have, Shah; drafting of the manuscript: Mongelluzzo; critical revision of the manuscript for important intellectual content: Mongelluzzo, Mohamad, Ten Have, Shah; statistical analysis: Shah, Mongelluzzo, Ten Have; obtained funding: Shah, Mongelluzzo; administrative, technical, or material support: Shah; study supervision: Shah.
Appendix
Diagnosis Codes:
Endocarditis: 421.0, 421.1, 421.9
Mastoiditis: 383.0, 383.1, 383.2, 383.8, 383.9
Osteomyelitis: 730.0, 730.1, 730.2, 730.3, 730.7, 730.8, 730.9
Septic arthritis: 711.0, 711.1, 711.2, 711.3, 711.4, 711.5, 711.6, 711.7, 711.8, 711.9
Sepsis: 038.0, 038.1, 038.2, 038.3, 038.4, 038.8, 038.9
Systemic Inflammatory Response Syndrome: 995.92
Pneumonia: 480.0, 480.1, 480.2, 480.3, 480.8, 480.9, 481, 482.0, 482.1, 482.2, 482.3, 482.4, 482.8, 482.9, 483.0, 483.1, 483.8, 484.1, 484.3, 484.5, 484.6, 484.7, 484.8, 485, 486
Procedure Codes:
Endotracheal Intubation: 96.04
Bacterial meningitis can be a devastating disease in children. Overall mortality in children in the United States is 4%1 while long‐term morbidity is present in up to 25%2 of surviving children. The introduction of Haemophilus influenzae type B vaccine, heptavalent pneumococcal conjugate vaccine, and the quadrivalent meningococcal conjugate vaccine has altered the epidemiology of bacterial meningitis.24 Currently, little is known about the epidemiology of systemic complications and associated focal infections that occur during episodes of bacterial meningitis in children and how the presence of such complications affects in‐hospital healthcare resource utilization.
In a randomized controlled trial, the administration of adjuvant corticosteroids was associated with lower mortality rates in adults with bacterial meningitis due to all causes, with the greatest reduction in those with pneumococcal meningitis.5 In a post hoc analysis of data from this trial, reductions in systemic complications, such as septic shock, pneumonia, and acute respiratory distress syndrome, rather than neurologic complications were thought to be the underlying reason for the decrease in mortality associated with pneumococcal meningitis among corticosteroid recipients.6 However, children with bacterial meningitis have an overall 4‐fold lower mortality rate than adults with bacterial meningitis. An even greater difference in mortality rates exists between children and adults with pneumococcal meningitis.1, 5 Children do not benefit from adjuvant corticosteroids as adults do.1, 5, 7 Therefore, the pathogenesis of bacterial meningitis may differ in children from adults and account for the difference in response to adjuvant corticosteroids. Understanding the epidemiology of systemic complications and associated focal infections can aid in the understanding of the pathogenesis of the disease in varying age groups of children.
Previous studies in children have documented the frequency of certain bacterial meningitis‐associated conditions such as respiratory failure, pneumonia, endocarditis, and mastoiditis. Researchers have used the presence of such conditions to predict either mortality or neurologic sequelae in children.810 These studies were small and only included a few types of complications associated with bacterial meningitis. In‐hospital healthcare resource utilization, which may be an important indicator of in‐hospital morbidity, was also not considered as an outcome. In‐hospital morbidity may represent aspects of disease burden not captured by mortality rates or markers for long‐term morbidity alone. In future vaccine efficacy trails or novel therapeutics evaluations, consideration of these associated conditions is important.
The quantification of the use of in‐hospital healthcare utilization is also important for hospital planning and resource allocation in children with bacterial meningitis. A child presenting with bacterial meningitis and a systemic complication or an associated focal infection may require additional resource planning initially to expedite care to enhance recovery and decrease hospital length of stay (LOS).
Our goal was to document the frequency of bacterial meningitis‐associated conditions (systemic complications and associated focal infections) in a large cohort of children with bacterial meningitis treated at tertiary care children's hospitals in the United States, and determine how the presence of such conditions impacted in‐hospital healthcare resource utilization.
Patients and Methods
Data Source
Data for this retrospective cohort study was obtained from the Pediatric Health Information System (PHIS), a national administrative database containing data from 36 freestanding, tertiary care children's hospitals. These hospitals are affiliated with the Child Health Corporation of America (Shawnee Mission, KS), a business alliance of children's hospitals. Data quality and reliability are assured through a joint effort between the Child Health Corporation of America and participating hospitals. For the purposes of external benchmarking, participating hospitals provide discharge data including patient demographics, diagnoses, and procedures. Procedures to assure data validity were described previously.1 Total hospital charges are reported in the PHIS database and adjusted for hospital location using the Centers for Medicare and Medicaid price/wage index. A total of 27 participating hospitals also provide resource utilization data for each hospital discharge (ie, pharmaceutical dispensing, imaging, and laboratory studies); patients from these 27 hospitals were eligible for inclusion in this study. The protocol for the conduct of this study was reviewed and approved by The Children's Hospital of Philadelphia Committees for the Protection of Human Subjects.
Patients
Children less than 18 years of age with bacterial meningitis were eligible for this study if they were discharged from any of the 27 hospitals disclosing resource utilization data between January 1, 2001 and December 31, 2006. Study participants discharged with bacterial meningitis as their primary diagnosis were identified in the PHIS database using International Classification of Diseases, 9th revision, (ICD‐9) discharge diagnosis codes. The study population was limited to children without conditions predisposing to meningitis. Therefore, patients with ventricular shunts prior to the episode of bacterial meningitis were excluded using the following ICD‐9 procedure codes: ventricular shunt replacement (02.42); incision of peritoneum (54.95); removal of ventricular shunts (02.43); and the ICD‐9 discharge diagnosis code for mechanical complication of nervous system device, implant, and graft (996.2). Also, children with comorbid conditions that could predispose to meningitis or increase the likelihood of associated complications such as cancer (hematologic and nonhematologic), primary or secondary immunodeficiencies, prematurity, post‐operative infection, congenital cardiac disease, and sickle cell disease, were excluded from the analysis. Race and ethnicity were self‐reported by patients at time of admission.
Study Definitions
Study participants were identified from the PHIS database using ICD‐9 codes for the primary diagnosis of bacterial meningitis (codes 036.0‐036.1; 320.0‐320.3; 320.7; 320.81‐320.82; 320.89; 320.9). The sensitivity and specificity of ICD‐9 codes in identifying children with bacterial meningitis is unknown, however these codes have been used by previous investigators.1113 Bacterial meningitis associated‐conditions were classified as systemic complications (sepsis, systemic inflammatory response syndrome (SIRS), and respiratory failure) and associated focal infections (septic arthritis, mastoiditis, osteomyelitis, pneumonia and endocarditis). These associated conditions were identified by ICD‐9 discharge and procedural codes as listed in the Appendix (Supporting Information). Bone and joint infections were defined by the presence of either osteomyelitis or septic arthritis.
Primary Outcomes
The primary outcomes of interest were total in‐hospital charges and hospital LOS.
Measured Exposures
The primary exposures of interest were the occurrences of systemic complications, focal infections, or both conditions in children with bacterial meningitis.
Statistical Analysis
The data were initially described using frequencies and percentages for categorical variables and mean, median, interquartile ranges (IQRs) and range values for continuous variables. Analyses of bivariate associations between the outcomes (total in‐hospital charges and length of hospital stay) and potential covariates entailed either chi‐square tests or, for rare events with an expected frequency <5, Fishers Exact Test.
Following bivariate analysis, multivariable models were constructed to assess the adjusted impact of systemic complications and focal infection on total in‐hospital charges and hospital LOS. In evaluating total in‐hospital charges, the charge data were logarithmically transformed to account for the skewed distribution of charges. Multivariable linear regression was then performed to analyze the log transformed charges. The resulting beta‐coefficients were transformed to reflect the percent difference in total hospital charges between children with and without specific complications. In evaluating hospital LOS, negative binomial regression models were employed to estimate incidence rate ratios (IRRs) rather than log‐linear models, as to account for overdispersion in the outcome data. The negative binomial model produced a ratio of lengths of stay or IRR, where a ratio >1 indicates that the risk factor was associated with a longer LOS. The results were presented as percentage change to facilitate interpretation of the results.
The multivariable models were adjusted for the following confounders as determined a priori: age category, race, sex, vancomycin receipt, and adjuvant corticosteroid receipt within the first 24 hours of admission. Tests for interaction between systemic complications or focal infections and age were performed for each of these models. To address the possibility of referral bias which would lead us to overestimate the cost of caring for children with bacterial meningitis with an associated condition, the analyses were repeated restricting the sample to those children who had a lumber puncture performed at a PHIS‐participating hospital. The frequency of systemic complications and focal infections in those who were transferred was no different than in children who were not transferred; therefore the entire cohort was used in the final analyses. Sub‐group analyses were also performed for children identified with pneumococcal and meningococcal meningitis.
The standard errors for all estimates of covariate effects including metastatic effects under the above models were adjusted for the hospital to account for the increased variability due to clustering of individuals within hospitals. Two‐tailed P values <0.05 were considered statistically significant. Actual P values and 95% confidence intervals are reported. Data were analyzed using STATA, Version 10 (Stata Corporation, College Station, TX).
Results
Demographics
There were 2780 children admitted with bacterial meningitis during the study period; 461 (17%) children were excluded because of comorbid illness including malignancy (n = 37), congential heart disease (n = 231), prematurity (n = 104), human immunodeficiency virus infection (n = 4), sickle cell disease (n = 17), and post‐operative infection (n = 68). The remaining 2319 children with bacterial meningitis were included in the analyses. The mean age was 3.6 years (median, 1 year; IQR, 0‐6 years). Approximately half of the children were less than 1 year of age, 23% were 1 to 5 years, and 27% were >5 years. A total of 54% of children were white, 19% were black, 22% were Hispanic, and 5% were of other racial groups. Males accounted for 58% of the children. In this cohort of children, 9% received adjuvant corticosteroids within 24 hours of hospitalization.
Bacterial Meningitis‐Associated Conditions
Overall, 574 (25%) of children with bacterial meningitis suffered a systemic complication or an associated focal infection. Figure 1 shows the types of associated condition stratified by age category. Older children had a higher frequency of associated focal infections while younger children had a higher frequency of systemic complications (P = 0.002, chi‐square test for trend). Figure 2 shows the distribution of specific conditions among children in each age category. The frequency of sepsis decreased with age (P < 0.001, chi‐square test) while the frequency of mastoiditis (P < 0.001, Fisher's exact test) and osteomyelitis (P = 0.005, Fisher's exact test) increased with age. There did not appear to be substantial variability in the proportion of patients with SIRS or sepsis across hospitals, suggesting that hospital‐level variability in coding for these conditions was likely minimal. The median proportion of patients with SIRS by hospital was 2.4% (IQR, 1.2‐4.8%) while the median proportion of patients with sepsis by hospital was 13.4% (IQR, 10.0‐16.9%).


Of the 151 children with an associated focal infection, only 3 (2%) of children had more than 1 infection (1 child had mastoiditis and endocarditis, 1 child had pneumonia and osteomyelitis, and 1 child had pneumonia and endocarditis). However, of the 479 children with systemic complications, 116 (24%) had more than 1 systemic disease (Table 1).
| Types of Systemic Complications | Systemic Complications in All Bacterial Meningitis, n (%) | Systemic Complications in Meningococcal Meningitis, n (%) | Systemic Complications in Pneumococcal Meningitis, n (%) |
|---|---|---|---|
| |||
| Sepsis only | 209 (44) | 16 (21) | 69 (54) |
| Respiratory failure only | 139 (29) | 38 (49) | 30 (24) |
| SIRS only | 15 (3) | 9 (12) | 1 (1) |
| Sepsis and respiratory failure | 52 (11) | 4 (5) | 18 (14) |
| SIRS and sepsis | 27 (6) | 2 (3) | 4 (3) |
| SIRS and respiratory failure | 9 (2) | 5 (6) | 0 (0) |
| SIRS and respiratory failure and sepsis | 28 (6) | 3 (4) | 5 (4) |
| Total systemic complications | 479 | 77 | 127 |
In sub‐group analyses, 269 children had meningococcal meningitis and 470 children had pneumococcal meningitis. Of the children with meningococcal meningitis, 31.2% had a meningitis‐associated condition: 26.4% had a systemic complication, 2.6% had a focal infection, and 2.2% had both conditions. The most common associated conditions in children with meningococcal meningitis were respiratory failure (18.6%; n = 50), sepsis (9.3%; n = 25), and SIRS (7.1%; n = 19). In children with pneumococcal meningitis, 32.3% had a meningitis‐associated complication: 24.7% had a systemic complication, 5.3% had a focal infection, and 2.3% had both conditions. The most common associated conditions in children with pneumococcal meningitis were sepsis (20.4%; n = 96), respiratory failure (11.3%; n = 53), and pneumonia (4.7%; n = 22); mastoiditis was present in 2.3% (n = 11) of children with pneumococcal meningitis. Respiratory failure was more common in meningococcal meningitis (18.6%) than in pneumococcal meningitis (11.3%; P = 0.006). In contrast, sepsis was less common in meningococcal meningitis (9.3%) than in pneumococcal meningitis (20.4%; P < 0.001).
Hospital Charges
Overall, the median charges per hospital ranged from $20,158 to $53,823. In‐hospital charges for children with bacterial meningitis with and without any identified associated conditions are presented in Table 2. In multivariate analyses, the presence of systemic conditions, associated focal infections, or both conditions was independently associated with significantly higher total in‐hospital charges (Table 2). When conditions were considered individually, bone and joint infections (213% increase; 95% CI, 113‐260%), endocarditis (108% increase; 95% CI, 23‐258%), and pneumonia (107% increase; 95% CI, 58‐171%) were associated with the highest increases in total hospital charges (Figure 3). In contrast, SIRS and mastoiditis were not associated with higher hospital charges (Figure 3).
| Charges | LOS | |||
|---|---|---|---|---|
| Median, $ (IQR) | Adjusted Increase,* % (95% CI) | Median, days (IQR) | Adjusted Increase,* % (95% CI) | |
| ||||
| None (n = 1,745) | $27,110 (15,823‐48,307) | Reference** | 9 (6‐14) | Reference |
| Systemic (n = 423) | $66,690 (39,546136,756) | 136 (108269) | 14 (923) | 72 (5196) |
| Focal Infection (n = 95) | $58,016 (29,056125,813) | 118 (77168) | 13 (928) | 78 (40126) |
| Both (n = 56) | $130,744 (62,397299,288) | 351 (237503) | 21.5 (1245) | 211 (142303) |

LOS
The median LOS was 9 days (IQR, 6‐15 days); 5% of children had a LOS >42 days. Table 2 summarizes difference in LOS by the presence and absence of systemic conditions and focal infections. In multivariate analyses, the presence of systemic conditions, associated focal infections, or both conditions was independently associated with a significantly longer LOS (Table 2). When conditions were considered individually, endocarditis (152% increase; 95% CI, 60‐300%) and pneumonia (136% increase; 95% CI, 85‐201%) were associated with the greatest adjusted increases in LOS (Figure 4); only mastoiditis was not associated with an increased LOS compared with those without complications.

Discussion
To our knowledge, this is the first study to examine bacterial meningitis‐associated conditions in children and their impact on in‐hospital resource utilization. We found that 25% of the cohort of children with bacterial meningitis suffered from at least one focal infection or systemic complication. This represents a significant invasive disease burden among children with bacterial meningitis who do not have underlying comorbid conditions. Younger children were more likely to have systemic complications when compared with older children, specifically due to a higher frequency of sepsis in children <1 year. Older children were more likely to have an associated focal infection, specifically due to an increase in mastoiditis and osteomyelitis in children >1 year. Only 2% of children had more than 1 focal infection, while 24% of children had more than 1 systemic complication.
Importantly, the presence of a systemic complication in a child with bacterial meningitis increased their in‐hospital adjusted charges by 136%. The presence of a focal infection increased in‐hospital adjusted charges by 118%. A child with both a systemic complication and a focal infection and had a 351% increase in in‐hospital adjusted charges.
The presence of systemic complications or associated focal infections was significantly associated with higher in‐hospital charges and longer hospital LOS. Most individual meningitis‐associated conditions included in this study were associated with higher in‐hospital charges with the exception of SIRS and mastoiditis. All individual meningitis‐associated conditions were associated with a longer LOS except mastoiditis. This finding is not surprising as the LOS for children with mastoiditis is typically shorter than for children with bacterial meningitis. Glikich et al.14 reported a mean LOS of approximately 8 days for children with mastoiditis. As meningitis in the context of mastoiditis is likely caused by direct extension of infection, patients with meningitis and mastoiditis likely required extended hospitalization to treat meningitis rather than mastoiditis. In contrast, patients with meningitis occurring in the context of metastatic dissemination of infection (eg, endocarditis, pneumonia) often have hemodynamic instability requiring prolonged intensive care support.
A study of children with sepsis found that increasing severity of illness was associated with greater hospital resource utilization.15 Our study shows that this may also be true in children with bacterial meningitis. We found that in children with bacterial meningitis, having systemic complications or an associated focal infection was associated with greater in‐hospital resource utilization. This finding may therefore indicate greater in‐hospital morbidity among children with a bacterial meningitis‐associated condition. Since mortality rates for bacterial meningitis are low in children, in‐hospital morbidity may be a better indicator of disease burden.
Our data show that, in contrast to adults, bacterial meningitis in children is not typically associated with other focal infections. Some focal complications such as mastoiditis and osteomyelitis disproportionately affect older children. These complications are typically accompanied by overt clinical manifestations. Therefore, we believe that the evaluation for the presence of concomitant focal infections can be guided by clinical examination findings and that routine radiologic evaluation for focal complications may not be necessary. Additionally, focal infections tend to occur in the absence of concomitant systemic complications. Of the 151 children with at least 1 associated focal infection, only 37% had a systemic complication. Bacterial meningitis may lie on a continuum of invasive disease depending on the virulence factors of the invading pathogen as well as specific host factors. Understanding the epidemiology of these associated conditions can enhance our understanding of the pathogenesis of bacterial meningitis in children. Understanding why some children suffer from septicemia rather than bacteremia may help in developing novel therapeutics.
There are several limitations to our study. First, since we identified focal infections and systemic complications using billing charges and ICD‐9 discharge diagnosis codes, it was impossible to determine when these conditions represented true complications of bacterial meningitis and when they represented the primary source of infection. Therefore, some of our primary outcomes may represent the cause of meningitis rather than a direct complication. We attempted to minimize such misclassification by limiting the cohort to those with a primary discharge diagnosis of bacterial meningitis though such misclassification is still possible.
Second, the use of ICD‐9 codes to accurately identify systemic complications and associated focal infections is a potential limitation. For example, respiratory failure, defined as the requirement of endotracheal intubation in our study, may not capture children receiving non‐invasive mechanical ventilation (eg, bilevel positive airway pressure). If use of noninvasive ventilation strategies did not depend exclusively on illness severity, our study would underestimate the frequency of respiratory failure. Furthermore, there may be inconsistencies among pediatric physicians in coding conditions such as SIRS and sepsis. Even in the clinical setting, a uniform definition of SIRS and sepsis is problematic due to physiologic differences between adults and children of varying age groups.16 An international panel of pediatricians proposed age‐specific definitions for sepsis and SIRS, while acknowledging the paucity of evidence to support some of their recommendations.16 None of the proposed definitions could be applied using administrative data. Limitations in the use of ICD‐9 discharge diagnosis codes to identify children with bacterial meningitis were discussed previously.1
Third, only free‐standing children's hospitals were included in the analysis. It is likely that many children with uncomplicated bacterial meningitis are treated at community hospitals or smaller academic centers. Our study may overestimate the rate of bacterial meningitis‐associated focal infections and systemic complications since participating hospitals serve as regional referral centers. To address the potential for such referral bias, we repeated the analysis while restricting the cohort to those children who had a lumbar puncture performed at the treating facility. No difference in frequency of associated conditions or in‐hospital resource utilization was found between children transferred and children not transferred. Finally, the PHIS database reports billed charge data rather than cost data. Billed data may overestimate the actual economic impact of bacterial meningitis‐associated complications since payers often reimburse at lesser rates. Resource utilization may also vary widely between hospitals and geographic locations as previously shown.15
In conclusion, bacterial meningitis remains an important cause of morbidity in children. Systemic complications such as sepsis and respiratory failure are common. Respiratory failure occurred more commonly among patients with meningococcal meningitis while sepsis occurred more commonly among patients with pneumococcal meningitis. While focal complications are uncommon, children >5 years of age are more likely than younger children to have concomitant mastoiditis or osteomyelitis. The presence of both systemic and focal complications is associated with substantially greater resource utilization than either complication alone.
Dr. Shah had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. Study concept and design: Shah, Mongelluzzo; acquisition of data: Shah, Mohamad; analysis and interpretation of data: Mongelluzzo, Mohamad, Ten Have, Shah; drafting of the manuscript: Mongelluzzo; critical revision of the manuscript for important intellectual content: Mongelluzzo, Mohamad, Ten Have, Shah; statistical analysis: Shah, Mongelluzzo, Ten Have; obtained funding: Shah, Mongelluzzo; administrative, technical, or material support: Shah; study supervision: Shah.
Appendix
Diagnosis Codes:
Endocarditis: 421.0, 421.1, 421.9
Mastoiditis: 383.0, 383.1, 383.2, 383.8, 383.9
Osteomyelitis: 730.0, 730.1, 730.2, 730.3, 730.7, 730.8, 730.9
Septic arthritis: 711.0, 711.1, 711.2, 711.3, 711.4, 711.5, 711.6, 711.7, 711.8, 711.9
Sepsis: 038.0, 038.1, 038.2, 038.3, 038.4, 038.8, 038.9
Systemic Inflammatory Response Syndrome: 995.92
Pneumonia: 480.0, 480.1, 480.2, 480.3, 480.8, 480.9, 481, 482.0, 482.1, 482.2, 482.3, 482.4, 482.8, 482.9, 483.0, 483.1, 483.8, 484.1, 484.3, 484.5, 484.6, 484.7, 484.8, 485, 486
Procedure Codes:
Endotracheal Intubation: 96.04
- , , , .Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- , , , et al.Bacterial meningitis in the United States in 1995. Active surveillance team.N Engl J Med.1997;337:970–976.
- Progress toward elimination of Haemophilus influenzae type b disease among infants and children–United States, 1987–1995.MMWR Morb Mortal Wkly Rep.1996;45:901–906.
- , .Bacterial meningitis in children.Lancet.2003;361:2139–2148.
- , .Dexamethasone in adults with bacterial meningitis.N Engl J Med.2002;347:1549–1556.
- , .Dexamethasone and pneumococcal meningitis.Ann Intern Med.2004;141:327.
- , , , et al.Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis.N Engl J Med.2007;357:2431–2440.
- , , , , .Bacterial meningitis in an urban area: etiologic study and prognostic factors.Infection.2007;35:406–413.
- , , .Clinical features and prognostic factors in childhood pneumococcal meningitis.J Microbiol Immunol Infect.2008;41:48–53.
- , , .Clinical presentation and prognostic factors of Streptococcus pneumoniae meningitis according to the focus of infection.BMC Infect Dis.2005;5:93.
- , .Trends in invasive pneumococcal disease‐associated hospitalizations.Clin Infect Dis.2006;42:e1–e5.
- , , .Managing meningococcal disease in the United States: Hospital case characteristics and costs by age.Value Health.2006;9:236–243.
- , , , , , .Population‐based analysis of meningococcal disease mortality in the United States: 1990–2002.Pediatr Infect Dis J.2006;25:191–194.
- , , , .A contemporary analysis of acute mastoiditis.Arch Otolaryngol Head Neck Surg.1996;122:135–139.
- , , .Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis.Pediatrics.2007;119:487–494.
- , , .International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics.Pediatr Crit Care Med.2005;6:2–8.
- , , , .Corticosteroids and mortality in children with bacterial meningitis.JAMA.2008;299:2048–2055.
- , , , et al.Bacterial meningitis in the United States in 1995. Active surveillance team.N Engl J Med.1997;337:970–976.
- Progress toward elimination of Haemophilus influenzae type b disease among infants and children–United States, 1987–1995.MMWR Morb Mortal Wkly Rep.1996;45:901–906.
- , .Bacterial meningitis in children.Lancet.2003;361:2139–2148.
- , .Dexamethasone in adults with bacterial meningitis.N Engl J Med.2002;347:1549–1556.
- , .Dexamethasone and pneumococcal meningitis.Ann Intern Med.2004;141:327.
- , , , et al.Dexamethasone in Vietnamese adolescents and adults with bacterial meningitis.N Engl J Med.2007;357:2431–2440.
- , , , , .Bacterial meningitis in an urban area: etiologic study and prognostic factors.Infection.2007;35:406–413.
- , , .Clinical features and prognostic factors in childhood pneumococcal meningitis.J Microbiol Immunol Infect.2008;41:48–53.
- , , .Clinical presentation and prognostic factors of Streptococcus pneumoniae meningitis according to the focus of infection.BMC Infect Dis.2005;5:93.
- , .Trends in invasive pneumococcal disease‐associated hospitalizations.Clin Infect Dis.2006;42:e1–e5.
- , , .Managing meningococcal disease in the United States: Hospital case characteristics and costs by age.Value Health.2006;9:236–243.
- , , , , , .Population‐based analysis of meningococcal disease mortality in the United States: 1990–2002.Pediatr Infect Dis J.2006;25:191–194.
- , , , .A contemporary analysis of acute mastoiditis.Arch Otolaryngol Head Neck Surg.1996;122:135–139.
- , , .Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis.Pediatrics.2007;119:487–494.
- , , .International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics.Pediatr Crit Care Med.2005;6:2–8.
Copyright © 2010 Society of Hospital Medicine