User login
Increasingly violent storms may strain mental health
SAN FRANCISCO – Longer and more powerful storms caused by climate change will put increasing pressure on mental health care. The unusually powerful 2017 hurricane season, highlighted by damage done to Puerto Rico by Hurricane Maria and to Houston by Hurricane Harvey, may serve as a harbinger of more intense storm seasons to come, according to James M. Shultz, PhD, director of the Center for Disaster and Extreme Event Preparedness at the University of Miami.
Overall, 2017 was something of a “perfect storm” season. “We’ve had predictions about what climate change would do to extreme storms. It was quite exceptional in bringing together all of the elements we have seen predicted by climate scientists,” Dr. Shultz said during a press conference at the annual meeting of the American Psychiatric Association. Study coauthor Zelde Espinel, MD, MPH, also of the center at the university, presented the poster at the meeting.
Aside from greater intensity, climate change is causing a slowing of storms once they make landfall, which increases rainfall and the risks of floods. Nowhere was that more apparent than in Houston in the aftermath of Hurricane Harvey, where tens of thousands of spontaneous rescue efforts arose to rescue people trapped in their homes.
These storms put tremendous pressure on health care systems, as in Puerto Rico when Hurricane Maria knocked out electrical grids, some of which stayed down for 6 months or more. This kind of upheaval interrupts health care infrastructure, including psychiatric services, leaving vulnerable individuals at even greater risk.
Then there are the direct and indirect effects of storms on mental health. When air conditioning and fans are inoperative because of power outages, people get exposed to extreme and relentless heat. They may experience food and water shortages. In worst cases, they may be forced out of their homes on a temporary or even permanent basis. Dr. Shultz recounted research looking at victims of Hurricane Maria.
Researchers used standardized measures to assess both survivors who remained in Puerto Rico, and others who were forced to relocate, mostly to Florida. Sixty-six percent of those interviewed had clinically significant elevated symptoms of PTSD, major depression, or generalized anxiety. A study looking at people displaced from Puerto Rico and those who stayed also found high rates of posttraumatic stress disorder and depression in both samples, and rates were actually higher in those who were displaced to Florida, Dr. Shultz said (Disaster Med Public Health Prep. 2019 Feb;13[13]:24-7).
These effects will only worsen as climate change brings more and more powerful storms, and psychiatrists must be ready to help. The year 2017 “is just a snapshot. It may in fact be just a garden variety year when we look back later in this century.
Many countries most affected by climate change are poor in resources and may have few psychiatrists available in the first place. After a storm, infrastructure and the number of trained mental health professionals may further decline. That calls for outside assistance: “We’ve been talking about the possibility of bringing interpersonal psychotherapy (to affected areas) and to have lay personnel supervised by psychiatrists be able to deliver these sorts of interventions,” he said.
Dr. Shultz has no relevant financial disclosures.
SAN FRANCISCO – Longer and more powerful storms caused by climate change will put increasing pressure on mental health care. The unusually powerful 2017 hurricane season, highlighted by damage done to Puerto Rico by Hurricane Maria and to Houston by Hurricane Harvey, may serve as a harbinger of more intense storm seasons to come, according to James M. Shultz, PhD, director of the Center for Disaster and Extreme Event Preparedness at the University of Miami.
Overall, 2017 was something of a “perfect storm” season. “We’ve had predictions about what climate change would do to extreme storms. It was quite exceptional in bringing together all of the elements we have seen predicted by climate scientists,” Dr. Shultz said during a press conference at the annual meeting of the American Psychiatric Association. Study coauthor Zelde Espinel, MD, MPH, also of the center at the university, presented the poster at the meeting.
Aside from greater intensity, climate change is causing a slowing of storms once they make landfall, which increases rainfall and the risks of floods. Nowhere was that more apparent than in Houston in the aftermath of Hurricane Harvey, where tens of thousands of spontaneous rescue efforts arose to rescue people trapped in their homes.
These storms put tremendous pressure on health care systems, as in Puerto Rico when Hurricane Maria knocked out electrical grids, some of which stayed down for 6 months or more. This kind of upheaval interrupts health care infrastructure, including psychiatric services, leaving vulnerable individuals at even greater risk.
Then there are the direct and indirect effects of storms on mental health. When air conditioning and fans are inoperative because of power outages, people get exposed to extreme and relentless heat. They may experience food and water shortages. In worst cases, they may be forced out of their homes on a temporary or even permanent basis. Dr. Shultz recounted research looking at victims of Hurricane Maria.
Researchers used standardized measures to assess both survivors who remained in Puerto Rico, and others who were forced to relocate, mostly to Florida. Sixty-six percent of those interviewed had clinically significant elevated symptoms of PTSD, major depression, or generalized anxiety. A study looking at people displaced from Puerto Rico and those who stayed also found high rates of posttraumatic stress disorder and depression in both samples, and rates were actually higher in those who were displaced to Florida, Dr. Shultz said (Disaster Med Public Health Prep. 2019 Feb;13[13]:24-7).
These effects will only worsen as climate change brings more and more powerful storms, and psychiatrists must be ready to help. The year 2017 “is just a snapshot. It may in fact be just a garden variety year when we look back later in this century.
Many countries most affected by climate change are poor in resources and may have few psychiatrists available in the first place. After a storm, infrastructure and the number of trained mental health professionals may further decline. That calls for outside assistance: “We’ve been talking about the possibility of bringing interpersonal psychotherapy (to affected areas) and to have lay personnel supervised by psychiatrists be able to deliver these sorts of interventions,” he said.
Dr. Shultz has no relevant financial disclosures.
SAN FRANCISCO – Longer and more powerful storms caused by climate change will put increasing pressure on mental health care. The unusually powerful 2017 hurricane season, highlighted by damage done to Puerto Rico by Hurricane Maria and to Houston by Hurricane Harvey, may serve as a harbinger of more intense storm seasons to come, according to James M. Shultz, PhD, director of the Center for Disaster and Extreme Event Preparedness at the University of Miami.
Overall, 2017 was something of a “perfect storm” season. “We’ve had predictions about what climate change would do to extreme storms. It was quite exceptional in bringing together all of the elements we have seen predicted by climate scientists,” Dr. Shultz said during a press conference at the annual meeting of the American Psychiatric Association. Study coauthor Zelde Espinel, MD, MPH, also of the center at the university, presented the poster at the meeting.
Aside from greater intensity, climate change is causing a slowing of storms once they make landfall, which increases rainfall and the risks of floods. Nowhere was that more apparent than in Houston in the aftermath of Hurricane Harvey, where tens of thousands of spontaneous rescue efforts arose to rescue people trapped in their homes.
These storms put tremendous pressure on health care systems, as in Puerto Rico when Hurricane Maria knocked out electrical grids, some of which stayed down for 6 months or more. This kind of upheaval interrupts health care infrastructure, including psychiatric services, leaving vulnerable individuals at even greater risk.
Then there are the direct and indirect effects of storms on mental health. When air conditioning and fans are inoperative because of power outages, people get exposed to extreme and relentless heat. They may experience food and water shortages. In worst cases, they may be forced out of their homes on a temporary or even permanent basis. Dr. Shultz recounted research looking at victims of Hurricane Maria.
Researchers used standardized measures to assess both survivors who remained in Puerto Rico, and others who were forced to relocate, mostly to Florida. Sixty-six percent of those interviewed had clinically significant elevated symptoms of PTSD, major depression, or generalized anxiety. A study looking at people displaced from Puerto Rico and those who stayed also found high rates of posttraumatic stress disorder and depression in both samples, and rates were actually higher in those who were displaced to Florida, Dr. Shultz said (Disaster Med Public Health Prep. 2019 Feb;13[13]:24-7).
These effects will only worsen as climate change brings more and more powerful storms, and psychiatrists must be ready to help. The year 2017 “is just a snapshot. It may in fact be just a garden variety year when we look back later in this century.
Many countries most affected by climate change are poor in resources and may have few psychiatrists available in the first place. After a storm, infrastructure and the number of trained mental health professionals may further decline. That calls for outside assistance: “We’ve been talking about the possibility of bringing interpersonal psychotherapy (to affected areas) and to have lay personnel supervised by psychiatrists be able to deliver these sorts of interventions,” he said.
Dr. Shultz has no relevant financial disclosures.
REPORTING FROM APA 2019
Music shows promise for inpatient agitation
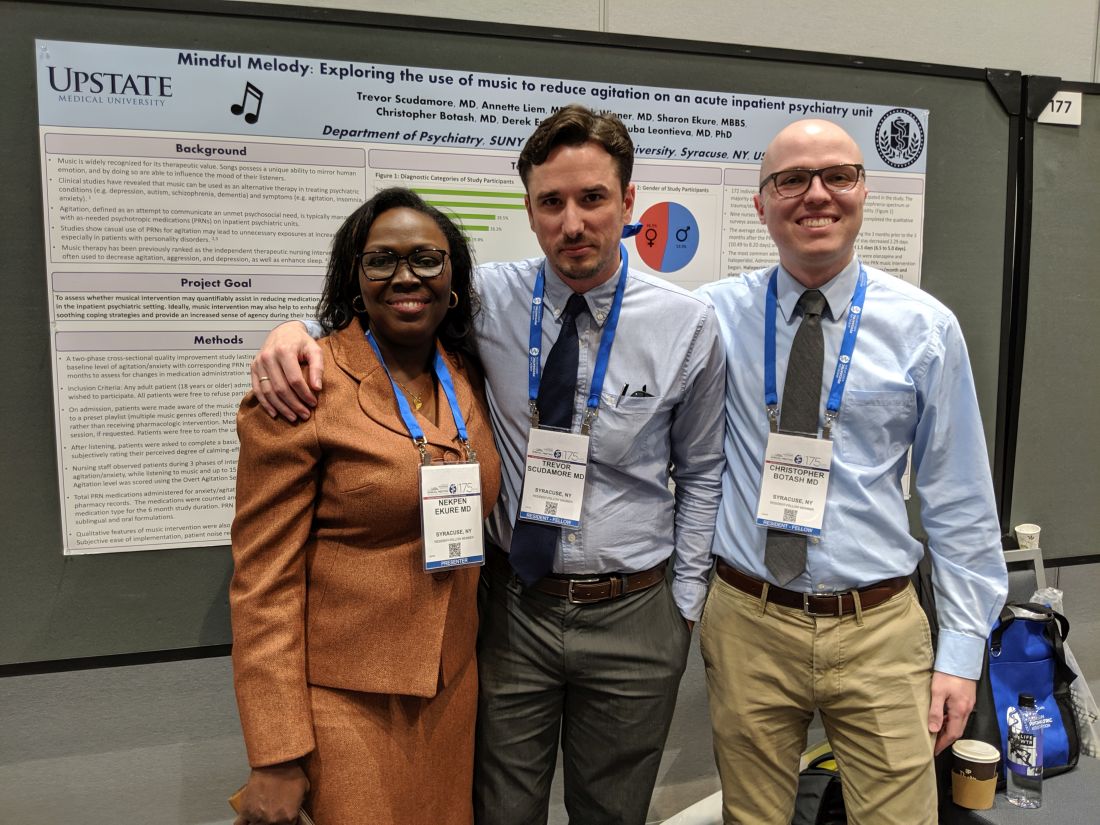
SAN FRANCISCO – In a proof-of-concept study, music provided an alternative to oral psychotropic medication in calming agitated patients at an inpatient psychiatric facility. Music has been studied as a treatment for agitation in dementia patients but not so much in psychiatric patients, according to Trevor Scudamore, MD, who is a resident fellow at State University of New York, Syracuse.
“The other thing is that music has been more looked at in group therapy settings than as adjunct therapy, or as an option as an as-needed medication for agitation,” Dr. Scudamore said in an interview. He presented the study at a poster session at the annual meeting of the American Psychiatric Association.
When agitation arose, the program allowed patients to choose between an oral medication or music, which entailed a 30-minute session listening to a preset playlist using a wireless headphone. Playlist options included a variety of musical genres, and participants could sit in one place or roam around while listening.
Traditionally, agitated patients had the choice of an oral medication. If the patient refused and then escalated, they had to accept an intravenous medication. “Now there’s a choice between an oral medication and music, almost like a third layer in defusing agitation in the patient. If they refuse music, then they could go for oral medication, and then [IV medication]. They’re given a little bit more options. Maybe they don’t feel so confined, which is an interesting way of helping possibly defuse anxiety from a situation. That needs to be explored further,” Dr. Scudamore said.
The study had a two-phase, cross-sectional design. The first 3 months, the study included 71 patients, who were used to establish a baseline of agitation and psychotropic medication use. They introduced the music intervention during the second 3-month period, with 101 participants. After they listened to music, the patients completed a self-report form using the Likert scale, and nursing staff observed the patients status during the initial anxiety/agitation, while they listened to music, and 15 minutes after the listening session.
That need for commitment from nurses presented a challenge to implementation. They had to hand out headphones, keep track of them, and make sure they got the headphones back. “It is a lot more work than just giving 50 mg of hydroxyzine,” said coauthor Christopher Botash, MD, who also is a resident at the university.
Study participants had a range of psychiatric ills, including substance abuse (61%), depression (51%), psychosis (28%), trauma (26%), personality disorder (20%), and anxiety (17%).
As part of a preliminary report, the researchers presented data from surveys completed by nine nurses and 31 patients. The two most commonly prescribed antipsychotics saw a decrease in administrations, from 3.37 to 2.93/month for haloperidol, and from 3.83 to 2.73 administrations/month for olanzapine.
A total of 56% of the nursing staff stated that the music therapy program helped calm down the patients. Nurses who disagreed cited the tendency for patients to intrude at the nursing station asking for the music, though this improved as patients learned the routine. Ninety-six percent of the patients reported satisfaction with the experience.
It was challenging for the researchers to implement the study, since offering music was a break in the routine. “The staff really does rely a lot on the meds, so oftentimes we would have to say, ‘Hey, have you offered the music yet?’ It’s a bit of a culture change,” Dr. Botash said.
The study, which Dr. Scudamore and Dr. Botash coauthored with Nekpen S. Ekure, MD, did not receive external funding. Dr. Scudamore and Dr. Botash had no relevant financial disclosures.
SAN FRANCISCO – In a proof-of-concept study, music provided an alternative to oral psychotropic medication in calming agitated patients at an inpatient psychiatric facility. Music has been studied as a treatment for agitation in dementia patients but not so much in psychiatric patients, according to Trevor Scudamore, MD, who is a resident fellow at State University of New York, Syracuse.
“The other thing is that music has been more looked at in group therapy settings than as adjunct therapy, or as an option as an as-needed medication for agitation,” Dr. Scudamore said in an interview. He presented the study at a poster session at the annual meeting of the American Psychiatric Association.
When agitation arose, the program allowed patients to choose between an oral medication or music, which entailed a 30-minute session listening to a preset playlist using a wireless headphone. Playlist options included a variety of musical genres, and participants could sit in one place or roam around while listening.
Traditionally, agitated patients had the choice of an oral medication. If the patient refused and then escalated, they had to accept an intravenous medication. “Now there’s a choice between an oral medication and music, almost like a third layer in defusing agitation in the patient. If they refuse music, then they could go for oral medication, and then [IV medication]. They’re given a little bit more options. Maybe they don’t feel so confined, which is an interesting way of helping possibly defuse anxiety from a situation. That needs to be explored further,” Dr. Scudamore said.
The study had a two-phase, cross-sectional design. The first 3 months, the study included 71 patients, who were used to establish a baseline of agitation and psychotropic medication use. They introduced the music intervention during the second 3-month period, with 101 participants. After they listened to music, the patients completed a self-report form using the Likert scale, and nursing staff observed the patients status during the initial anxiety/agitation, while they listened to music, and 15 minutes after the listening session.
That need for commitment from nurses presented a challenge to implementation. They had to hand out headphones, keep track of them, and make sure they got the headphones back. “It is a lot more work than just giving 50 mg of hydroxyzine,” said coauthor Christopher Botash, MD, who also is a resident at the university.
Study participants had a range of psychiatric ills, including substance abuse (61%), depression (51%), psychosis (28%), trauma (26%), personality disorder (20%), and anxiety (17%).
As part of a preliminary report, the researchers presented data from surveys completed by nine nurses and 31 patients. The two most commonly prescribed antipsychotics saw a decrease in administrations, from 3.37 to 2.93/month for haloperidol, and from 3.83 to 2.73 administrations/month for olanzapine.
A total of 56% of the nursing staff stated that the music therapy program helped calm down the patients. Nurses who disagreed cited the tendency for patients to intrude at the nursing station asking for the music, though this improved as patients learned the routine. Ninety-six percent of the patients reported satisfaction with the experience.
It was challenging for the researchers to implement the study, since offering music was a break in the routine. “The staff really does rely a lot on the meds, so oftentimes we would have to say, ‘Hey, have you offered the music yet?’ It’s a bit of a culture change,” Dr. Botash said.
The study, which Dr. Scudamore and Dr. Botash coauthored with Nekpen S. Ekure, MD, did not receive external funding. Dr. Scudamore and Dr. Botash had no relevant financial disclosures.
SAN FRANCISCO – In a proof-of-concept study, music provided an alternative to oral psychotropic medication in calming agitated patients at an inpatient psychiatric facility. Music has been studied as a treatment for agitation in dementia patients but not so much in psychiatric patients, according to Trevor Scudamore, MD, who is a resident fellow at State University of New York, Syracuse.
“The other thing is that music has been more looked at in group therapy settings than as adjunct therapy, or as an option as an as-needed medication for agitation,” Dr. Scudamore said in an interview. He presented the study at a poster session at the annual meeting of the American Psychiatric Association.
When agitation arose, the program allowed patients to choose between an oral medication or music, which entailed a 30-minute session listening to a preset playlist using a wireless headphone. Playlist options included a variety of musical genres, and participants could sit in one place or roam around while listening.
Traditionally, agitated patients had the choice of an oral medication. If the patient refused and then escalated, they had to accept an intravenous medication. “Now there’s a choice between an oral medication and music, almost like a third layer in defusing agitation in the patient. If they refuse music, then they could go for oral medication, and then [IV medication]. They’re given a little bit more options. Maybe they don’t feel so confined, which is an interesting way of helping possibly defuse anxiety from a situation. That needs to be explored further,” Dr. Scudamore said.
The study had a two-phase, cross-sectional design. The first 3 months, the study included 71 patients, who were used to establish a baseline of agitation and psychotropic medication use. They introduced the music intervention during the second 3-month period, with 101 participants. After they listened to music, the patients completed a self-report form using the Likert scale, and nursing staff observed the patients status during the initial anxiety/agitation, while they listened to music, and 15 minutes after the listening session.
That need for commitment from nurses presented a challenge to implementation. They had to hand out headphones, keep track of them, and make sure they got the headphones back. “It is a lot more work than just giving 50 mg of hydroxyzine,” said coauthor Christopher Botash, MD, who also is a resident at the university.
Study participants had a range of psychiatric ills, including substance abuse (61%), depression (51%), psychosis (28%), trauma (26%), personality disorder (20%), and anxiety (17%).
As part of a preliminary report, the researchers presented data from surveys completed by nine nurses and 31 patients. The two most commonly prescribed antipsychotics saw a decrease in administrations, from 3.37 to 2.93/month for haloperidol, and from 3.83 to 2.73 administrations/month for olanzapine.
A total of 56% of the nursing staff stated that the music therapy program helped calm down the patients. Nurses who disagreed cited the tendency for patients to intrude at the nursing station asking for the music, though this improved as patients learned the routine. Ninety-six percent of the patients reported satisfaction with the experience.
It was challenging for the researchers to implement the study, since offering music was a break in the routine. “The staff really does rely a lot on the meds, so oftentimes we would have to say, ‘Hey, have you offered the music yet?’ It’s a bit of a culture change,” Dr. Botash said.
The study, which Dr. Scudamore and Dr. Botash coauthored with Nekpen S. Ekure, MD, did not receive external funding. Dr. Scudamore and Dr. Botash had no relevant financial disclosures.
REPORTING FROM APA 2019
Synthetic drugs pose regulatory, diagnostic challenges
SAN FRANCISCO – Designer drugs, especially synthetic opioids and cannabinoids, are presenting increasing challenges to psychiatrists treating patients with overdoses or psychiatric adverse effects. In 2017, synthetic opioids caused more than 28,000 deaths in the United States, more than any other type. Some of these drugs are technically legal, because their modified chemical structures aren’t covered as legal definitions struggle to keep up with street drug identities.
... people are using drugs and they don’t even know what they’re using,” Vanessa Torres-Llenza, MD, assistant professor of psychiatry at George Washington University, Washington, said in an interview. Dr. Torres-Llenza moderated a session on synthetic opioids at the annual meeting of the American Psychiatric Association.
Of particular concern is the synthetic opioid fentanyl, which has a potency about 50 times that of heroin, and 100 times that of morphine. It is a legal pharmaceutical drug for use in severe pain, but it can be made illicitly, and it is frequently mixed with heroin or cocaine and put into counterfeit pills. The user often is not even aware of its presence. Another derivative, carfentanil, is even more dangerous. Used as a large-animal tranquilizer, and illegal for human use, carfentanil is about 100 times more potent than fentanyl.
These developments may require reconsideration of treatment using the opioid antagonist naloxone and similar drugs. The current guidance for naloxone is a 0.4- to 2-mg dose, followed by repeat dose at 2- to 3-minute intervals as needed. Considering the increasing presence of more potent drugs, “there may not be time to wait,” Dr. Torres-Llenza said.
Another concern is illicit manufacturing: By making even slight modifications to legal drugs, illegal operations can stay a step ahead of regulators because these derivatives are completely legal until legislation is passed to ban them. Estimates peg the number of such new derivatives at about 250 per year.
The recent history of the Food Drug Administration’s regulation of synthetic opioids, presented during the session by Gowri Ramachandran, MD, a resident at George Washington University, illustrates the challenges. The Controlled Substances Act of 1970 assigned every regulated drug into one of five classes based on medical use, and potential for abuse and dependence. Schedule I substances are flagged for a high potential of abuse, having no medical use in the United States, and a lack of accepted safety data for use under medical supervision. Schedule II substances have accepted medical uses.
In 2012, the Synthetic Drug Abuse Prevention Act amended the earlier legislation, declaring that any chemical or related derivative with cannabimimetic properties, as well as some other hallucinogenic molecules and their close relatives, were included as schedule I controlled substances.
The amended legislation also extended the potential length of temporary schedule I status, from 1 year with a 6-month extension, to 2 years with a 1-year extension, to give regulators more time to catch up with both legal and illegal synthetic changes to determine if a drug should be schedule I or II.
A recent example of this problem is bath salts, which are far more powerful, synthetic versions of a stimulant derived from the khat plant that is grown in East Africa and southern Arabia. Bath salts can produce hallucinogenic and euphoric effects similar to methamphetamine and ecstasy, but they are readily available online and in retail stores, labeled as “not for human use” and marketed as “bath salts,” “plant food,” “jewelry cleaner,” or “phone screen cleaner.”
Another concern is synthetic cannabinoids, which resemble the 100 or so cannabinoids found in marijuana, tetrahydrocannabinol (THC), and cannabidiol (CBD) being the most well-known examples. These began to appear in recreational use in 2005, representing legal forms of marijuana and sold with names like K2, Spice, and Kronic. They are sold in tobacco shops, again labeled “not for human consumption,” trumpeted instead as a “harmless incense blend” or “natural herbs.” Manufacture and content of these derivatives are completely unregulated, according to Dr. Ramachandran.
Like other drug classes, synthetic cannabinoids – many related to THC – have been structurally altered in recent years, posing challenges to regulation and even detection. This is especially concerning because a synthetic cannabinoid product could contain a potpourri of other drugs such as opioids or herbs, leading to unpredictable effects. It’s also nearly impossible to identify everything in a patient’s system, Dr. Torrez-Llenza said.
That makes diagnosis challenging given that synthetic cannabinoids can cause a wide range of symptoms, commonly violence, agitation, panic attacks, hallucinations, hyperglycemia, hyperkalemia, and tachycardia.
Synthetic cannabinoids usually do not contain CBD, which has some antipsychotic and anxiolytic effects. Instead they are generally derived from THC, which is associated with psychosis, and they are 40-660 times more potent than natural THC. This suggests that synthetic versions may pose a greater psychosis risk than natural cannabis. However, only case reports have examined the existence of an association between synthetic cannabinoids and psychosis, and it is difficult to distinguish a toxic syndrome from exacerbation of a previous prodromal syndrome, or new-onset illness.
Acute reactions can occur within minutes of use and last 2-5 hours or more. But this is all very unpredictable as it depends on the specific mixture used.
In the emergency department, agitation, aggression, and impulsive behaviors may signal exposure to synthetic cannabinoids. Most patients can be treated in the ED with antipsychotics or benzodiazepines to manage symptoms. There could be regional toxidromes that arise from local distribution of specific synthetic cannabinoid combinations.
While testing for synthetic cannabinoids remains challenging, Quest Diagnostics has a urine-based panel that includes them, and the company says it is working with information from the National Forensic Laboratory Information System, the Drug Enforcement Agency, industry sources, and the scientific literature to periodically update its standard panel.
Dr. Torres-Llenza had no relevant financial disclosures.
SAN FRANCISCO – Designer drugs, especially synthetic opioids and cannabinoids, are presenting increasing challenges to psychiatrists treating patients with overdoses or psychiatric adverse effects. In 2017, synthetic opioids caused more than 28,000 deaths in the United States, more than any other type. Some of these drugs are technically legal, because their modified chemical structures aren’t covered as legal definitions struggle to keep up with street drug identities.
... people are using drugs and they don’t even know what they’re using,” Vanessa Torres-Llenza, MD, assistant professor of psychiatry at George Washington University, Washington, said in an interview. Dr. Torres-Llenza moderated a session on synthetic opioids at the annual meeting of the American Psychiatric Association.
Of particular concern is the synthetic opioid fentanyl, which has a potency about 50 times that of heroin, and 100 times that of morphine. It is a legal pharmaceutical drug for use in severe pain, but it can be made illicitly, and it is frequently mixed with heroin or cocaine and put into counterfeit pills. The user often is not even aware of its presence. Another derivative, carfentanil, is even more dangerous. Used as a large-animal tranquilizer, and illegal for human use, carfentanil is about 100 times more potent than fentanyl.
These developments may require reconsideration of treatment using the opioid antagonist naloxone and similar drugs. The current guidance for naloxone is a 0.4- to 2-mg dose, followed by repeat dose at 2- to 3-minute intervals as needed. Considering the increasing presence of more potent drugs, “there may not be time to wait,” Dr. Torres-Llenza said.
Another concern is illicit manufacturing: By making even slight modifications to legal drugs, illegal operations can stay a step ahead of regulators because these derivatives are completely legal until legislation is passed to ban them. Estimates peg the number of such new derivatives at about 250 per year.
The recent history of the Food Drug Administration’s regulation of synthetic opioids, presented during the session by Gowri Ramachandran, MD, a resident at George Washington University, illustrates the challenges. The Controlled Substances Act of 1970 assigned every regulated drug into one of five classes based on medical use, and potential for abuse and dependence. Schedule I substances are flagged for a high potential of abuse, having no medical use in the United States, and a lack of accepted safety data for use under medical supervision. Schedule II substances have accepted medical uses.
In 2012, the Synthetic Drug Abuse Prevention Act amended the earlier legislation, declaring that any chemical or related derivative with cannabimimetic properties, as well as some other hallucinogenic molecules and their close relatives, were included as schedule I controlled substances.
The amended legislation also extended the potential length of temporary schedule I status, from 1 year with a 6-month extension, to 2 years with a 1-year extension, to give regulators more time to catch up with both legal and illegal synthetic changes to determine if a drug should be schedule I or II.
A recent example of this problem is bath salts, which are far more powerful, synthetic versions of a stimulant derived from the khat plant that is grown in East Africa and southern Arabia. Bath salts can produce hallucinogenic and euphoric effects similar to methamphetamine and ecstasy, but they are readily available online and in retail stores, labeled as “not for human use” and marketed as “bath salts,” “plant food,” “jewelry cleaner,” or “phone screen cleaner.”
Another concern is synthetic cannabinoids, which resemble the 100 or so cannabinoids found in marijuana, tetrahydrocannabinol (THC), and cannabidiol (CBD) being the most well-known examples. These began to appear in recreational use in 2005, representing legal forms of marijuana and sold with names like K2, Spice, and Kronic. They are sold in tobacco shops, again labeled “not for human consumption,” trumpeted instead as a “harmless incense blend” or “natural herbs.” Manufacture and content of these derivatives are completely unregulated, according to Dr. Ramachandran.
Like other drug classes, synthetic cannabinoids – many related to THC – have been structurally altered in recent years, posing challenges to regulation and even detection. This is especially concerning because a synthetic cannabinoid product could contain a potpourri of other drugs such as opioids or herbs, leading to unpredictable effects. It’s also nearly impossible to identify everything in a patient’s system, Dr. Torrez-Llenza said.
That makes diagnosis challenging given that synthetic cannabinoids can cause a wide range of symptoms, commonly violence, agitation, panic attacks, hallucinations, hyperglycemia, hyperkalemia, and tachycardia.
Synthetic cannabinoids usually do not contain CBD, which has some antipsychotic and anxiolytic effects. Instead they are generally derived from THC, which is associated with psychosis, and they are 40-660 times more potent than natural THC. This suggests that synthetic versions may pose a greater psychosis risk than natural cannabis. However, only case reports have examined the existence of an association between synthetic cannabinoids and psychosis, and it is difficult to distinguish a toxic syndrome from exacerbation of a previous prodromal syndrome, or new-onset illness.
Acute reactions can occur within minutes of use and last 2-5 hours or more. But this is all very unpredictable as it depends on the specific mixture used.
In the emergency department, agitation, aggression, and impulsive behaviors may signal exposure to synthetic cannabinoids. Most patients can be treated in the ED with antipsychotics or benzodiazepines to manage symptoms. There could be regional toxidromes that arise from local distribution of specific synthetic cannabinoid combinations.
While testing for synthetic cannabinoids remains challenging, Quest Diagnostics has a urine-based panel that includes them, and the company says it is working with information from the National Forensic Laboratory Information System, the Drug Enforcement Agency, industry sources, and the scientific literature to periodically update its standard panel.
Dr. Torres-Llenza had no relevant financial disclosures.
SAN FRANCISCO – Designer drugs, especially synthetic opioids and cannabinoids, are presenting increasing challenges to psychiatrists treating patients with overdoses or psychiatric adverse effects. In 2017, synthetic opioids caused more than 28,000 deaths in the United States, more than any other type. Some of these drugs are technically legal, because their modified chemical structures aren’t covered as legal definitions struggle to keep up with street drug identities.
... people are using drugs and they don’t even know what they’re using,” Vanessa Torres-Llenza, MD, assistant professor of psychiatry at George Washington University, Washington, said in an interview. Dr. Torres-Llenza moderated a session on synthetic opioids at the annual meeting of the American Psychiatric Association.
Of particular concern is the synthetic opioid fentanyl, which has a potency about 50 times that of heroin, and 100 times that of morphine. It is a legal pharmaceutical drug for use in severe pain, but it can be made illicitly, and it is frequently mixed with heroin or cocaine and put into counterfeit pills. The user often is not even aware of its presence. Another derivative, carfentanil, is even more dangerous. Used as a large-animal tranquilizer, and illegal for human use, carfentanil is about 100 times more potent than fentanyl.
These developments may require reconsideration of treatment using the opioid antagonist naloxone and similar drugs. The current guidance for naloxone is a 0.4- to 2-mg dose, followed by repeat dose at 2- to 3-minute intervals as needed. Considering the increasing presence of more potent drugs, “there may not be time to wait,” Dr. Torres-Llenza said.
Another concern is illicit manufacturing: By making even slight modifications to legal drugs, illegal operations can stay a step ahead of regulators because these derivatives are completely legal until legislation is passed to ban them. Estimates peg the number of such new derivatives at about 250 per year.
The recent history of the Food Drug Administration’s regulation of synthetic opioids, presented during the session by Gowri Ramachandran, MD, a resident at George Washington University, illustrates the challenges. The Controlled Substances Act of 1970 assigned every regulated drug into one of five classes based on medical use, and potential for abuse and dependence. Schedule I substances are flagged for a high potential of abuse, having no medical use in the United States, and a lack of accepted safety data for use under medical supervision. Schedule II substances have accepted medical uses.
In 2012, the Synthetic Drug Abuse Prevention Act amended the earlier legislation, declaring that any chemical or related derivative with cannabimimetic properties, as well as some other hallucinogenic molecules and their close relatives, were included as schedule I controlled substances.
The amended legislation also extended the potential length of temporary schedule I status, from 1 year with a 6-month extension, to 2 years with a 1-year extension, to give regulators more time to catch up with both legal and illegal synthetic changes to determine if a drug should be schedule I or II.
A recent example of this problem is bath salts, which are far more powerful, synthetic versions of a stimulant derived from the khat plant that is grown in East Africa and southern Arabia. Bath salts can produce hallucinogenic and euphoric effects similar to methamphetamine and ecstasy, but they are readily available online and in retail stores, labeled as “not for human use” and marketed as “bath salts,” “plant food,” “jewelry cleaner,” or “phone screen cleaner.”
Another concern is synthetic cannabinoids, which resemble the 100 or so cannabinoids found in marijuana, tetrahydrocannabinol (THC), and cannabidiol (CBD) being the most well-known examples. These began to appear in recreational use in 2005, representing legal forms of marijuana and sold with names like K2, Spice, and Kronic. They are sold in tobacco shops, again labeled “not for human consumption,” trumpeted instead as a “harmless incense blend” or “natural herbs.” Manufacture and content of these derivatives are completely unregulated, according to Dr. Ramachandran.
Like other drug classes, synthetic cannabinoids – many related to THC – have been structurally altered in recent years, posing challenges to regulation and even detection. This is especially concerning because a synthetic cannabinoid product could contain a potpourri of other drugs such as opioids or herbs, leading to unpredictable effects. It’s also nearly impossible to identify everything in a patient’s system, Dr. Torrez-Llenza said.
That makes diagnosis challenging given that synthetic cannabinoids can cause a wide range of symptoms, commonly violence, agitation, panic attacks, hallucinations, hyperglycemia, hyperkalemia, and tachycardia.
Synthetic cannabinoids usually do not contain CBD, which has some antipsychotic and anxiolytic effects. Instead they are generally derived from THC, which is associated with psychosis, and they are 40-660 times more potent than natural THC. This suggests that synthetic versions may pose a greater psychosis risk than natural cannabis. However, only case reports have examined the existence of an association between synthetic cannabinoids and psychosis, and it is difficult to distinguish a toxic syndrome from exacerbation of a previous prodromal syndrome, or new-onset illness.
Acute reactions can occur within minutes of use and last 2-5 hours or more. But this is all very unpredictable as it depends on the specific mixture used.
In the emergency department, agitation, aggression, and impulsive behaviors may signal exposure to synthetic cannabinoids. Most patients can be treated in the ED with antipsychotics or benzodiazepines to manage symptoms. There could be regional toxidromes that arise from local distribution of specific synthetic cannabinoid combinations.
While testing for synthetic cannabinoids remains challenging, Quest Diagnostics has a urine-based panel that includes them, and the company says it is working with information from the National Forensic Laboratory Information System, the Drug Enforcement Agency, industry sources, and the scientific literature to periodically update its standard panel.
Dr. Torres-Llenza had no relevant financial disclosures.
REPORTING FROM APA 2019
For borderline personality disorder, less may be more
SAN FRANCISCO – Borderline personality disorder is often treated with long-running psychotherapy, but this could be a disservice to patients. Instead, stepped-care models may offer more benefit in a shorter period of time, conserving resources and potentially expanding the reach of therapeutic interventions.
“There is this legacy of psychoanalysis, this idea that if you’ve had a condition for 20 years, it’s going to take 20 years of therapy to get rid of it – which is not true,” Joel Paris, MD, said in an interview. Dr. Paris moderated a session on stepped-care interventions for borderline personality disorder at the annual meeting of the American Psychiatric Association.
He also pointed out that psychoanalysis can prove self-sustaining: “There’s something to talk about, so it can go on and if there’s money to pay for the service, why stop?”
But psychoanalysis can have diminishing returns, and that in turn can strain resources. “Some people won’t get better or won’t go beyond a certain point. You help them to a certain extent, and then you have to be able to say, ‘That’s enough; stop there. If you get in to bad trouble, you can always come back,’ ” said Dr. Paris, professor of psychiatry at McGill University, Montreal.
That realization has led Dr. McGill to introduce a stepped-care model, which has provided a 12-week program for 15 years. Dr. Paris presented a retrospective look at the program, including 479 patients who received individual and group therapy. The dropout rate was high at 30%, but only 12% of patients returned asking for more therapy. A total of 145 patients deemed to be more chronic or who did not respond to the short-term program had the option of completing additional individual or group therapy over a period of 6-24 months.
“ There are also dropouts; maybe a quarter will not complete the program,” Dr. Paris said. “But that means 75% do, and most of them get better. There’s also good evidence that short therapy can help many people with substance abuse.”
Lois W. Choi-Kain, MD, assistant professor psychiatry at Harvard Medical School, Boston, presented evidence from several studies showing that shorter- and long-term courses of dialectical behavior therapy led to similar improvements, but that brief courses were associated with more rapid improvement. The short course consisted of weekly 1-hour individual sessions, while the longer course involved weekly 2-hour group sessions. The individual approach was associated with an 89% faster improvement in symptoms in the first 3 months (P less than .0001).
These kinds of findings underscore the need for shorter courses of therapy, at least for most patients, in order to conserve resources and broaden the availability of therapy, particularly in less affluent settings. “We saw in today’s symposium that most improvement takes place at the beginning of therapy, and you should quit while you’re ahead,” Dr. Paris said. “You should not try to do impossible things and make everybody into paradigms of mental health.”
Dr. Paris reported no relevant financial disclosures.
SAN FRANCISCO – Borderline personality disorder is often treated with long-running psychotherapy, but this could be a disservice to patients. Instead, stepped-care models may offer more benefit in a shorter period of time, conserving resources and potentially expanding the reach of therapeutic interventions.
“There is this legacy of psychoanalysis, this idea that if you’ve had a condition for 20 years, it’s going to take 20 years of therapy to get rid of it – which is not true,” Joel Paris, MD, said in an interview. Dr. Paris moderated a session on stepped-care interventions for borderline personality disorder at the annual meeting of the American Psychiatric Association.
He also pointed out that psychoanalysis can prove self-sustaining: “There’s something to talk about, so it can go on and if there’s money to pay for the service, why stop?”
But psychoanalysis can have diminishing returns, and that in turn can strain resources. “Some people won’t get better or won’t go beyond a certain point. You help them to a certain extent, and then you have to be able to say, ‘That’s enough; stop there. If you get in to bad trouble, you can always come back,’ ” said Dr. Paris, professor of psychiatry at McGill University, Montreal.
That realization has led Dr. McGill to introduce a stepped-care model, which has provided a 12-week program for 15 years. Dr. Paris presented a retrospective look at the program, including 479 patients who received individual and group therapy. The dropout rate was high at 30%, but only 12% of patients returned asking for more therapy. A total of 145 patients deemed to be more chronic or who did not respond to the short-term program had the option of completing additional individual or group therapy over a period of 6-24 months.
“ There are also dropouts; maybe a quarter will not complete the program,” Dr. Paris said. “But that means 75% do, and most of them get better. There’s also good evidence that short therapy can help many people with substance abuse.”
Lois W. Choi-Kain, MD, assistant professor psychiatry at Harvard Medical School, Boston, presented evidence from several studies showing that shorter- and long-term courses of dialectical behavior therapy led to similar improvements, but that brief courses were associated with more rapid improvement. The short course consisted of weekly 1-hour individual sessions, while the longer course involved weekly 2-hour group sessions. The individual approach was associated with an 89% faster improvement in symptoms in the first 3 months (P less than .0001).
These kinds of findings underscore the need for shorter courses of therapy, at least for most patients, in order to conserve resources and broaden the availability of therapy, particularly in less affluent settings. “We saw in today’s symposium that most improvement takes place at the beginning of therapy, and you should quit while you’re ahead,” Dr. Paris said. “You should not try to do impossible things and make everybody into paradigms of mental health.”
Dr. Paris reported no relevant financial disclosures.
SAN FRANCISCO – Borderline personality disorder is often treated with long-running psychotherapy, but this could be a disservice to patients. Instead, stepped-care models may offer more benefit in a shorter period of time, conserving resources and potentially expanding the reach of therapeutic interventions.
“There is this legacy of psychoanalysis, this idea that if you’ve had a condition for 20 years, it’s going to take 20 years of therapy to get rid of it – which is not true,” Joel Paris, MD, said in an interview. Dr. Paris moderated a session on stepped-care interventions for borderline personality disorder at the annual meeting of the American Psychiatric Association.
He also pointed out that psychoanalysis can prove self-sustaining: “There’s something to talk about, so it can go on and if there’s money to pay for the service, why stop?”
But psychoanalysis can have diminishing returns, and that in turn can strain resources. “Some people won’t get better or won’t go beyond a certain point. You help them to a certain extent, and then you have to be able to say, ‘That’s enough; stop there. If you get in to bad trouble, you can always come back,’ ” said Dr. Paris, professor of psychiatry at McGill University, Montreal.
That realization has led Dr. McGill to introduce a stepped-care model, which has provided a 12-week program for 15 years. Dr. Paris presented a retrospective look at the program, including 479 patients who received individual and group therapy. The dropout rate was high at 30%, but only 12% of patients returned asking for more therapy. A total of 145 patients deemed to be more chronic or who did not respond to the short-term program had the option of completing additional individual or group therapy over a period of 6-24 months.
“ There are also dropouts; maybe a quarter will not complete the program,” Dr. Paris said. “But that means 75% do, and most of them get better. There’s also good evidence that short therapy can help many people with substance abuse.”
Lois W. Choi-Kain, MD, assistant professor psychiatry at Harvard Medical School, Boston, presented evidence from several studies showing that shorter- and long-term courses of dialectical behavior therapy led to similar improvements, but that brief courses were associated with more rapid improvement. The short course consisted of weekly 1-hour individual sessions, while the longer course involved weekly 2-hour group sessions. The individual approach was associated with an 89% faster improvement in symptoms in the first 3 months (P less than .0001).
These kinds of findings underscore the need for shorter courses of therapy, at least for most patients, in order to conserve resources and broaden the availability of therapy, particularly in less affluent settings. “We saw in today’s symposium that most improvement takes place at the beginning of therapy, and you should quit while you’re ahead,” Dr. Paris said. “You should not try to do impossible things and make everybody into paradigms of mental health.”
Dr. Paris reported no relevant financial disclosures.
REPORTING FROM APA 2019
Vet accuracy, privacy policies of mental health apps
SAN FRANCISCO – Smartphones are nearly ubiquitous, and the apps available on them run the gamut from entertainment to social media, organization tools, and references. It should be no surprise that mental health applications are also gaining traction, as people experiencing depression, anxiety, and other conditions turn to these programs for assistance.
Psychiatrists should be aware of any mental health apps that their patients are using, and they should do a little research to determine their safety and efficacy, according to Hephsibah M. Loeb, MD, a psychiatry resident at Jefferson Medical College, Philadelphia.
“It’s our responsibility to understand the medications we’re prescribing, so I don’t think this is any different,” said Dr. Loeb, who presented a poster outlining advice for clinicians at the annual meeting of the American Psychiatric Association.
One such app, called reSET, gained approval from Food and Drug Administration for the treatment of alcohol, cocaine, marijuana, and stimulant use disorders. Another app, called PRIME-D, designed for self-management of depression, showed some benefit in an open-label study. But there are many more apps out there, and how is a clinician to know of the potential benefits and hazards?
In her poster, Dr. Loeb outlined the APA’s App Evaluation tool, which is designed to help clinicians systematically investigate an app. The APA has no standing list of reviewed apps because they are changing all the time, and new apps are introduced and removed all the time.
“The evaluation model is a set of questions that the APA developed [representing] certain issues that if a patient reports using an app, a clinician should look into in order to determine if it’s an appropriate and safe tool. For example: Who developed the app? What is its privacy policy? Is the information correct?” Dr. Loeb asked.
Privacy is of particular concern, since the patient has no way of knowing how his or her data are being handled. “If you’re putting really personal stuff in there and you don’t know where it’s going, that’s a huge risk. We have HIPAA protection in the office, but needless to say, if someone downloads something from the app store, their information is not going to be protected by HIPAA,” Dr. Loeb said.
Despite these concerns, Dr. Loeb is not a naysayer. “I think that self-help is really interesting. Presumably, there are patients we’re not even seeing in the office,” she said. In some cases, people are not patients but have a complaint and need help. “If there weren’t an app or online therapy, they might not otherwise get help.”
But whether the help they’re getting is reliable is another question. “There’s the chance that the information is perfectly accurate; there’s the chance that there’s misinformation. There’s also the chance of mis–self-diagnosis,” Dr. Loeb said.
since better clinical outcomes with apps are associated with receiving support from a trained mental health coach.
Finally, there’s one sure way to determine the quality of an app: Dr. Loeb said that a coauthor on the poster, Ann Chandy, MD, a clinical professor of psychiatry at Jefferson Medical College, uses an app herself when a patient brings it to her attention.
Dr. Loeb reported no relevant financial disclosures.
SAN FRANCISCO – Smartphones are nearly ubiquitous, and the apps available on them run the gamut from entertainment to social media, organization tools, and references. It should be no surprise that mental health applications are also gaining traction, as people experiencing depression, anxiety, and other conditions turn to these programs for assistance.
Psychiatrists should be aware of any mental health apps that their patients are using, and they should do a little research to determine their safety and efficacy, according to Hephsibah M. Loeb, MD, a psychiatry resident at Jefferson Medical College, Philadelphia.
“It’s our responsibility to understand the medications we’re prescribing, so I don’t think this is any different,” said Dr. Loeb, who presented a poster outlining advice for clinicians at the annual meeting of the American Psychiatric Association.
One such app, called reSET, gained approval from Food and Drug Administration for the treatment of alcohol, cocaine, marijuana, and stimulant use disorders. Another app, called PRIME-D, designed for self-management of depression, showed some benefit in an open-label study. But there are many more apps out there, and how is a clinician to know of the potential benefits and hazards?
In her poster, Dr. Loeb outlined the APA’s App Evaluation tool, which is designed to help clinicians systematically investigate an app. The APA has no standing list of reviewed apps because they are changing all the time, and new apps are introduced and removed all the time.
“The evaluation model is a set of questions that the APA developed [representing] certain issues that if a patient reports using an app, a clinician should look into in order to determine if it’s an appropriate and safe tool. For example: Who developed the app? What is its privacy policy? Is the information correct?” Dr. Loeb asked.
Privacy is of particular concern, since the patient has no way of knowing how his or her data are being handled. “If you’re putting really personal stuff in there and you don’t know where it’s going, that’s a huge risk. We have HIPAA protection in the office, but needless to say, if someone downloads something from the app store, their information is not going to be protected by HIPAA,” Dr. Loeb said.
Despite these concerns, Dr. Loeb is not a naysayer. “I think that self-help is really interesting. Presumably, there are patients we’re not even seeing in the office,” she said. In some cases, people are not patients but have a complaint and need help. “If there weren’t an app or online therapy, they might not otherwise get help.”
But whether the help they’re getting is reliable is another question. “There’s the chance that the information is perfectly accurate; there’s the chance that there’s misinformation. There’s also the chance of mis–self-diagnosis,” Dr. Loeb said.
since better clinical outcomes with apps are associated with receiving support from a trained mental health coach.
Finally, there’s one sure way to determine the quality of an app: Dr. Loeb said that a coauthor on the poster, Ann Chandy, MD, a clinical professor of psychiatry at Jefferson Medical College, uses an app herself when a patient brings it to her attention.
Dr. Loeb reported no relevant financial disclosures.
SAN FRANCISCO – Smartphones are nearly ubiquitous, and the apps available on them run the gamut from entertainment to social media, organization tools, and references. It should be no surprise that mental health applications are also gaining traction, as people experiencing depression, anxiety, and other conditions turn to these programs for assistance.
Psychiatrists should be aware of any mental health apps that their patients are using, and they should do a little research to determine their safety and efficacy, according to Hephsibah M. Loeb, MD, a psychiatry resident at Jefferson Medical College, Philadelphia.
“It’s our responsibility to understand the medications we’re prescribing, so I don’t think this is any different,” said Dr. Loeb, who presented a poster outlining advice for clinicians at the annual meeting of the American Psychiatric Association.
One such app, called reSET, gained approval from Food and Drug Administration for the treatment of alcohol, cocaine, marijuana, and stimulant use disorders. Another app, called PRIME-D, designed for self-management of depression, showed some benefit in an open-label study. But there are many more apps out there, and how is a clinician to know of the potential benefits and hazards?
In her poster, Dr. Loeb outlined the APA’s App Evaluation tool, which is designed to help clinicians systematically investigate an app. The APA has no standing list of reviewed apps because they are changing all the time, and new apps are introduced and removed all the time.
“The evaluation model is a set of questions that the APA developed [representing] certain issues that if a patient reports using an app, a clinician should look into in order to determine if it’s an appropriate and safe tool. For example: Who developed the app? What is its privacy policy? Is the information correct?” Dr. Loeb asked.
Privacy is of particular concern, since the patient has no way of knowing how his or her data are being handled. “If you’re putting really personal stuff in there and you don’t know where it’s going, that’s a huge risk. We have HIPAA protection in the office, but needless to say, if someone downloads something from the app store, their information is not going to be protected by HIPAA,” Dr. Loeb said.
Despite these concerns, Dr. Loeb is not a naysayer. “I think that self-help is really interesting. Presumably, there are patients we’re not even seeing in the office,” she said. In some cases, people are not patients but have a complaint and need help. “If there weren’t an app or online therapy, they might not otherwise get help.”
But whether the help they’re getting is reliable is another question. “There’s the chance that the information is perfectly accurate; there’s the chance that there’s misinformation. There’s also the chance of mis–self-diagnosis,” Dr. Loeb said.
since better clinical outcomes with apps are associated with receiving support from a trained mental health coach.
Finally, there’s one sure way to determine the quality of an app: Dr. Loeb said that a coauthor on the poster, Ann Chandy, MD, a clinical professor of psychiatry at Jefferson Medical College, uses an app herself when a patient brings it to her attention.
Dr. Loeb reported no relevant financial disclosures.
EXPERT ANALYSIS FROM APA 2019
Ketamine may help OCD, but much work remains
SAN FRANCISCO – The recent Food and Drug Administration approval of intranasal esketamine for treatment-resistant depression has prompted interest in using this class of drugs in other conditions, including obsessive-compulsive disorder.
“OCD is severe, and one in seven people with OCD will attempt suicide in their lifetime,” said Carolyn Rodriguez, MD, PhD, associate professor of psychiatry and behavioral sciences at Stanford (Calif.) University.
Dr. Rodriguez presented some of her research on the mechanism of action of ketamine and its potential benefits for OCD during a session at the annual meeting of the American Psychiatric Association.
OCD patients experience a lengthy delay between treatment initiation and clinical benefit, sometimes 2 to 3 months, and most don’t achieve complete symptom remission, according to Dr. Rodriguez. “To help patients, I wanted to look at therapies that could be rapid acting, and given the converging lines of evidence that glutamate may play a role as an excitatory chemical messenger that helps neurons communicate, I looked at ketamine, which blocks the glutamate receptor,” she said.
A small study published in 2013 by her group was the first randomized, clinical trial of ketamine in OCD. It included 15 adults who experienced near-constant obsessions. A single dose given over 40 minutes led to a dramatic decrease in intrusive thoughts. One week after the infusion, four of eight patients who received ketamine met the criteria for a treatment response (35% or more reduction in Yale-Brown Obsessive Compulsive Scale scores), compared with none of the seven patients in the placebo group.
, with the intent of looking at the drug’s effects on the circuits involved in OCD. “We need a large study to see if this is something that can be replicated, and we don’t know how long the effects persist. We’re just at the tip of the iceberg with OCD. In depression, there are these large studies that have been replicated, and in OCD, at least for randomized studies, it’s just this one study (from 2013) and the one that we have coming,” Dr. Rodriguez said.
Given the severity of OCD, ketamine and esketamine have generated some excitement, especially as a bridge to other therapies, such as cognitive-behavioral therapy or selective serotonin reuptake inhibitors.
The FDA’s approval of esketamine in March further boosted interest, but Dr. Rodriguez cautions that more research needs to be done. There is also at least one potential twist to use of an inhaled version of the drug. Contamination OCD patients may be unwilling to use a spray. In fact, Dr. Rodriguez’s team had to cancel a study looking at an inhaled form of racemic ketamine in OCD because they couldn’t recruit enough subjects. “There are variants [in OCD], and that’s why it’s important to study all populations and not assume that depression studies will cover the whole spectrum of our patients,” she said.
Dr. Rodriguez has consulted for Epiodyne, Allergan, BlackThorn, and Rugen.
SAN FRANCISCO – The recent Food and Drug Administration approval of intranasal esketamine for treatment-resistant depression has prompted interest in using this class of drugs in other conditions, including obsessive-compulsive disorder.
“OCD is severe, and one in seven people with OCD will attempt suicide in their lifetime,” said Carolyn Rodriguez, MD, PhD, associate professor of psychiatry and behavioral sciences at Stanford (Calif.) University.
Dr. Rodriguez presented some of her research on the mechanism of action of ketamine and its potential benefits for OCD during a session at the annual meeting of the American Psychiatric Association.
OCD patients experience a lengthy delay between treatment initiation and clinical benefit, sometimes 2 to 3 months, and most don’t achieve complete symptom remission, according to Dr. Rodriguez. “To help patients, I wanted to look at therapies that could be rapid acting, and given the converging lines of evidence that glutamate may play a role as an excitatory chemical messenger that helps neurons communicate, I looked at ketamine, which blocks the glutamate receptor,” she said.
A small study published in 2013 by her group was the first randomized, clinical trial of ketamine in OCD. It included 15 adults who experienced near-constant obsessions. A single dose given over 40 minutes led to a dramatic decrease in intrusive thoughts. One week after the infusion, four of eight patients who received ketamine met the criteria for a treatment response (35% or more reduction in Yale-Brown Obsessive Compulsive Scale scores), compared with none of the seven patients in the placebo group.
, with the intent of looking at the drug’s effects on the circuits involved in OCD. “We need a large study to see if this is something that can be replicated, and we don’t know how long the effects persist. We’re just at the tip of the iceberg with OCD. In depression, there are these large studies that have been replicated, and in OCD, at least for randomized studies, it’s just this one study (from 2013) and the one that we have coming,” Dr. Rodriguez said.
Given the severity of OCD, ketamine and esketamine have generated some excitement, especially as a bridge to other therapies, such as cognitive-behavioral therapy or selective serotonin reuptake inhibitors.
The FDA’s approval of esketamine in March further boosted interest, but Dr. Rodriguez cautions that more research needs to be done. There is also at least one potential twist to use of an inhaled version of the drug. Contamination OCD patients may be unwilling to use a spray. In fact, Dr. Rodriguez’s team had to cancel a study looking at an inhaled form of racemic ketamine in OCD because they couldn’t recruit enough subjects. “There are variants [in OCD], and that’s why it’s important to study all populations and not assume that depression studies will cover the whole spectrum of our patients,” she said.
Dr. Rodriguez has consulted for Epiodyne, Allergan, BlackThorn, and Rugen.
SAN FRANCISCO – The recent Food and Drug Administration approval of intranasal esketamine for treatment-resistant depression has prompted interest in using this class of drugs in other conditions, including obsessive-compulsive disorder.
“OCD is severe, and one in seven people with OCD will attempt suicide in their lifetime,” said Carolyn Rodriguez, MD, PhD, associate professor of psychiatry and behavioral sciences at Stanford (Calif.) University.
Dr. Rodriguez presented some of her research on the mechanism of action of ketamine and its potential benefits for OCD during a session at the annual meeting of the American Psychiatric Association.
OCD patients experience a lengthy delay between treatment initiation and clinical benefit, sometimes 2 to 3 months, and most don’t achieve complete symptom remission, according to Dr. Rodriguez. “To help patients, I wanted to look at therapies that could be rapid acting, and given the converging lines of evidence that glutamate may play a role as an excitatory chemical messenger that helps neurons communicate, I looked at ketamine, which blocks the glutamate receptor,” she said.
A small study published in 2013 by her group was the first randomized, clinical trial of ketamine in OCD. It included 15 adults who experienced near-constant obsessions. A single dose given over 40 minutes led to a dramatic decrease in intrusive thoughts. One week after the infusion, four of eight patients who received ketamine met the criteria for a treatment response (35% or more reduction in Yale-Brown Obsessive Compulsive Scale scores), compared with none of the seven patients in the placebo group.
, with the intent of looking at the drug’s effects on the circuits involved in OCD. “We need a large study to see if this is something that can be replicated, and we don’t know how long the effects persist. We’re just at the tip of the iceberg with OCD. In depression, there are these large studies that have been replicated, and in OCD, at least for randomized studies, it’s just this one study (from 2013) and the one that we have coming,” Dr. Rodriguez said.
Given the severity of OCD, ketamine and esketamine have generated some excitement, especially as a bridge to other therapies, such as cognitive-behavioral therapy or selective serotonin reuptake inhibitors.
The FDA’s approval of esketamine in March further boosted interest, but Dr. Rodriguez cautions that more research needs to be done. There is also at least one potential twist to use of an inhaled version of the drug. Contamination OCD patients may be unwilling to use a spray. In fact, Dr. Rodriguez’s team had to cancel a study looking at an inhaled form of racemic ketamine in OCD because they couldn’t recruit enough subjects. “There are variants [in OCD], and that’s why it’s important to study all populations and not assume that depression studies will cover the whole spectrum of our patients,” she said.
Dr. Rodriguez has consulted for Epiodyne, Allergan, BlackThorn, and Rugen.
REPORTING FROM APA 2019
Ketamine may rely on opioid receptors for antidepressive effect
SAN FRANCISCO – Ketamine and the more recently Food and Drug Administration–approved esketamine have generated a great deal of excitement in psychiatry over their potential to treat depression and other psychiatric disorders. However, the mechanism of action is not completely understood, and efforts are underway to better understand the drugs.
Much has been made of ketamine’s interaction with the N-methyl-D-aspartate (NMDA) receptors. One belief is that chronic stress leads to the accumulation of extracellular glutamate, which leads to a range of negative consequences. Ketamine blocks excess glutamate in the synapse and may thus reverse these downstream effects.
But it may not be so clear cut, according to Nolan Williams, MD. During a session on ketamine’s mechanism of action at the annual meeting of the American Psychiatric Association, Dr. Williams, assistant professor of psychiatry and behavioral sciences at Stanford (Calif.) University, noted that ketamine is “one of the most dirty drugs we know. It affects most neurotransmitter systems and has all sorts of downstream effects.”
Pain research has already shown that ketamine’s mechanisms can be complex. It affects the opioid system, both directly and indirectly, and an opioid receptor antagonist blocks ketamine’s pain-relieving effect.
Since opioids are also known to have antidepressant effects, it’s natural to wonder if ketamine’s mechanism also involves the opioid system. The opioid antagonist naltrexone provides a tool to study the problem. Dr. Williams reasoned that if ketamine relies in whole or in part on the opioid system for its antidepressive effect, then administering naltrexone ahead of ketamine should blunt or even eliminate its efficacy.
To examine the question, the team conducted a study of 11 patients with treatment-resistant depression. Right away, it was clear that the participants had some bias toward the treatment. “They all said the same thing to me: ‘My psychiatrist said that your study doesn’t make any sense because this is an NMDA antagonist. I’m going to get two free ketamine infusions.’ So they were preloaded with this idea that they were going to get very potent antidepressant effects (even with naltrexone),” said Dr. Williams.
The study had a crossover design and included two ketamine infusions. Participants were randomized to get either naltrexone or placebo 1 hour before the first ketamine infusion, and then were allowed to relapse back to within 20% of their baseline depression score before receiving the second ketamine infusion, when they received naltrexone or placebo, whichever hadn’t been given in the first treatment.
Of 12 who completed the study, seven responded to placebo plus ketamine treatment, and six remitted. But when they crossed over to the naltrexone arm, the result was very different: In three of four measures, there was no significant difference between the pretreatment and posttreatment results. “The same people who were almost exclusively in remission during the ketamine plus placebo condition got nothing out of ketamine plus naltrexone,” said Dr. Williams. Naltrexone had a similar negating effect on the positive response to ketamine on the suicide item of the 17-item Hamilton Rating Scale for Depression.
The researchers also looked at whether ketamine’s dissociative effect could be responsible for its activity through altering the participant’s mental state, but determined that it may be necessary for the antidepressant effect – but it is not sufficient.
We’re not saying it’s sufficient, but it appears to be quite necessary,” Dr. Williams said. He added that the results don’t mean that ketamine’s effect on NMDA is unimportant. In fact, “it’s probable that the ketamine is driving the mood in a direction, and these glutamine-related changes are probably what’s maintaining the mood in that direction,” he added.
As to the clinical impacts of his findings, Dr. Williams emphasized the need to understand the mechanisms behind the ketamine class. He pointed out that in pivotal trials of esketamine, there were six deaths, three by suicide, all of them in the esketamine group. Surprisingly, two of those who took their own lives had scored a 0 on the Columbia-Suicide Severity Scale, both at baseline and at the visit immediately preceding their deaths. The score wasn’t available for the third. “These weren’t people who were particularly dangerously suicidal. So understanding the mechanism is really important to understanding the safety of this drug, who we should give it to, and what sorts of things we should watch out for,” Dr. Williams said.
The study was funded by the National Institutes of Health, Spectrum, the Brain and Behavior Research Foundation, and Stanford Bio-X. Dr. Williams had no disclosures.
SAN FRANCISCO – Ketamine and the more recently Food and Drug Administration–approved esketamine have generated a great deal of excitement in psychiatry over their potential to treat depression and other psychiatric disorders. However, the mechanism of action is not completely understood, and efforts are underway to better understand the drugs.
Much has been made of ketamine’s interaction with the N-methyl-D-aspartate (NMDA) receptors. One belief is that chronic stress leads to the accumulation of extracellular glutamate, which leads to a range of negative consequences. Ketamine blocks excess glutamate in the synapse and may thus reverse these downstream effects.
But it may not be so clear cut, according to Nolan Williams, MD. During a session on ketamine’s mechanism of action at the annual meeting of the American Psychiatric Association, Dr. Williams, assistant professor of psychiatry and behavioral sciences at Stanford (Calif.) University, noted that ketamine is “one of the most dirty drugs we know. It affects most neurotransmitter systems and has all sorts of downstream effects.”
Pain research has already shown that ketamine’s mechanisms can be complex. It affects the opioid system, both directly and indirectly, and an opioid receptor antagonist blocks ketamine’s pain-relieving effect.
Since opioids are also known to have antidepressant effects, it’s natural to wonder if ketamine’s mechanism also involves the opioid system. The opioid antagonist naltrexone provides a tool to study the problem. Dr. Williams reasoned that if ketamine relies in whole or in part on the opioid system for its antidepressive effect, then administering naltrexone ahead of ketamine should blunt or even eliminate its efficacy.
To examine the question, the team conducted a study of 11 patients with treatment-resistant depression. Right away, it was clear that the participants had some bias toward the treatment. “They all said the same thing to me: ‘My psychiatrist said that your study doesn’t make any sense because this is an NMDA antagonist. I’m going to get two free ketamine infusions.’ So they were preloaded with this idea that they were going to get very potent antidepressant effects (even with naltrexone),” said Dr. Williams.
The study had a crossover design and included two ketamine infusions. Participants were randomized to get either naltrexone or placebo 1 hour before the first ketamine infusion, and then were allowed to relapse back to within 20% of their baseline depression score before receiving the second ketamine infusion, when they received naltrexone or placebo, whichever hadn’t been given in the first treatment.
Of 12 who completed the study, seven responded to placebo plus ketamine treatment, and six remitted. But when they crossed over to the naltrexone arm, the result was very different: In three of four measures, there was no significant difference between the pretreatment and posttreatment results. “The same people who were almost exclusively in remission during the ketamine plus placebo condition got nothing out of ketamine plus naltrexone,” said Dr. Williams. Naltrexone had a similar negating effect on the positive response to ketamine on the suicide item of the 17-item Hamilton Rating Scale for Depression.
The researchers also looked at whether ketamine’s dissociative effect could be responsible for its activity through altering the participant’s mental state, but determined that it may be necessary for the antidepressant effect – but it is not sufficient.
We’re not saying it’s sufficient, but it appears to be quite necessary,” Dr. Williams said. He added that the results don’t mean that ketamine’s effect on NMDA is unimportant. In fact, “it’s probable that the ketamine is driving the mood in a direction, and these glutamine-related changes are probably what’s maintaining the mood in that direction,” he added.
As to the clinical impacts of his findings, Dr. Williams emphasized the need to understand the mechanisms behind the ketamine class. He pointed out that in pivotal trials of esketamine, there were six deaths, three by suicide, all of them in the esketamine group. Surprisingly, two of those who took their own lives had scored a 0 on the Columbia-Suicide Severity Scale, both at baseline and at the visit immediately preceding their deaths. The score wasn’t available for the third. “These weren’t people who were particularly dangerously suicidal. So understanding the mechanism is really important to understanding the safety of this drug, who we should give it to, and what sorts of things we should watch out for,” Dr. Williams said.
The study was funded by the National Institutes of Health, Spectrum, the Brain and Behavior Research Foundation, and Stanford Bio-X. Dr. Williams had no disclosures.
SAN FRANCISCO – Ketamine and the more recently Food and Drug Administration–approved esketamine have generated a great deal of excitement in psychiatry over their potential to treat depression and other psychiatric disorders. However, the mechanism of action is not completely understood, and efforts are underway to better understand the drugs.
Much has been made of ketamine’s interaction with the N-methyl-D-aspartate (NMDA) receptors. One belief is that chronic stress leads to the accumulation of extracellular glutamate, which leads to a range of negative consequences. Ketamine blocks excess glutamate in the synapse and may thus reverse these downstream effects.
But it may not be so clear cut, according to Nolan Williams, MD. During a session on ketamine’s mechanism of action at the annual meeting of the American Psychiatric Association, Dr. Williams, assistant professor of psychiatry and behavioral sciences at Stanford (Calif.) University, noted that ketamine is “one of the most dirty drugs we know. It affects most neurotransmitter systems and has all sorts of downstream effects.”
Pain research has already shown that ketamine’s mechanisms can be complex. It affects the opioid system, both directly and indirectly, and an opioid receptor antagonist blocks ketamine’s pain-relieving effect.
Since opioids are also known to have antidepressant effects, it’s natural to wonder if ketamine’s mechanism also involves the opioid system. The opioid antagonist naltrexone provides a tool to study the problem. Dr. Williams reasoned that if ketamine relies in whole or in part on the opioid system for its antidepressive effect, then administering naltrexone ahead of ketamine should blunt or even eliminate its efficacy.
To examine the question, the team conducted a study of 11 patients with treatment-resistant depression. Right away, it was clear that the participants had some bias toward the treatment. “They all said the same thing to me: ‘My psychiatrist said that your study doesn’t make any sense because this is an NMDA antagonist. I’m going to get two free ketamine infusions.’ So they were preloaded with this idea that they were going to get very potent antidepressant effects (even with naltrexone),” said Dr. Williams.
The study had a crossover design and included two ketamine infusions. Participants were randomized to get either naltrexone or placebo 1 hour before the first ketamine infusion, and then were allowed to relapse back to within 20% of their baseline depression score before receiving the second ketamine infusion, when they received naltrexone or placebo, whichever hadn’t been given in the first treatment.
Of 12 who completed the study, seven responded to placebo plus ketamine treatment, and six remitted. But when they crossed over to the naltrexone arm, the result was very different: In three of four measures, there was no significant difference between the pretreatment and posttreatment results. “The same people who were almost exclusively in remission during the ketamine plus placebo condition got nothing out of ketamine plus naltrexone,” said Dr. Williams. Naltrexone had a similar negating effect on the positive response to ketamine on the suicide item of the 17-item Hamilton Rating Scale for Depression.
The researchers also looked at whether ketamine’s dissociative effect could be responsible for its activity through altering the participant’s mental state, but determined that it may be necessary for the antidepressant effect – but it is not sufficient.
We’re not saying it’s sufficient, but it appears to be quite necessary,” Dr. Williams said. He added that the results don’t mean that ketamine’s effect on NMDA is unimportant. In fact, “it’s probable that the ketamine is driving the mood in a direction, and these glutamine-related changes are probably what’s maintaining the mood in that direction,” he added.
As to the clinical impacts of his findings, Dr. Williams emphasized the need to understand the mechanisms behind the ketamine class. He pointed out that in pivotal trials of esketamine, there were six deaths, three by suicide, all of them in the esketamine group. Surprisingly, two of those who took their own lives had scored a 0 on the Columbia-Suicide Severity Scale, both at baseline and at the visit immediately preceding their deaths. The score wasn’t available for the third. “These weren’t people who were particularly dangerously suicidal. So understanding the mechanism is really important to understanding the safety of this drug, who we should give it to, and what sorts of things we should watch out for,” Dr. Williams said.
The study was funded by the National Institutes of Health, Spectrum, the Brain and Behavior Research Foundation, and Stanford Bio-X. Dr. Williams had no disclosures.
REPORTING FROM APA 2019
Intranasal esketamine combo tied to symptom improvement
Concern raised about drug’s abuse potential
SAN FRANCISCO – In treatment-resistant depression, a switch to a new antidepressant combined with inhaled esketamine (Spravato) led to better symptom improvement than did a switch to a new antidepressant plus placebo, results from a new trial show. The treatment effect of esketamine was modest but had a number needed to treat for remission of just five.
About 30% of patients with major depressive disorder are treatment resistant. They are at risk of suicidal behavior and self-harm during the interval between starting a new medication and onset of efficacy. Esketamine, with its rapid onset of efficacy associated with its stimulation of synaptogenesis through inhibition of the N-methyl-D-aspartate receptor, gained Food and Drug Administration approval in March for the treatment of treatment-resistant depression in adults.
The findings were part of a phase 3 study presented at a press conference at the annual meeting of the American Psychiatric Association and simultaneously published in the American Journal of Psychiatry (2019. doi: 10.1176/appi.ajp.2019.19020172).
Adding esketamine to the start of a new antidepressant was associated with a small improvement in symptoms (effect size, 0.30), and this benefit did not continue to accrue further advantage past the original separation, which led to some skepticism from the author of an accompanying editorial (Am J Psychiatry. 2019 May. doi: 10.1176/appi.ajp.2019.19040423). “The question is, do you need to keep a patient on the drug past 48 hours? There is no statistically significant difference (in patient response) past the first 48 hours. That to me is a concern,” said Alan F. Schatzberg, MD, at the press conference.
Dr. Schatzberg, who is a professor of psychiatry at the Stanford (Calif.) University, also expressed concern about the potential for abuse with esketamine, as well as withdrawal after discontinuation. “I think the drug ought to be used, but it needs to be recommended with considerable caution. We have not answered the many questions that are needed in a drug of potential abuse,” Dr. Schatzberg added.
Study coauthor Michael E. Thase, MD, who also presented at the press conference, discussed the possibility of alterations to the treatment regimen. “I have a hunch you can see this (positive response) within first week or 2, and you can at least concentrate the resource on patients that gain the largest benefit. We can be smarter about it,” said Dr. Thase, who is a professor of psychiatry at the University of Pennsylvania, Philadelphia. He predicted that protocols eventually will be put in place to streamline intranasal ketamine treatment.
Regardless of the effect size, the study produced high remission and response rates in this difficult to treat population, which is welcome news to Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., and director of the Yale Depression Research Program. he said in an interview.
In the phase 3 study, researchers randomized 223 patients from five countries to receive esketamine or placebo, along with either an SSRI (escitalopram or sertraline) or a selective norepinephrine reuptake inhibitor (duloxetine or venlafaxine extended release), based on investigator choice.
To be eligible, participants had to have failed two previous trials of antidepressants. After a 4-week observation period, patients began a 4-week regimen of a new antidepressant combined with twice-weekly nasal esketamine or a placebo.
Subjects in the treatment arm received esketamine on day 1 of the treatment phase with a 56-mg dose. On day 4, 45.8% were increased to a dose of 84 mg, and 66.7% were at the 84-mg dose at the end of the treatment phase.
From baseline to day 28, the esketamine group had a greater decrease in Montgomery-Åsberg Depression Rating Scale (MADRS) score (difference of least square means, –4.0; P = .020; effect size, 0.30). A scatter plot of individual MADRS data during that period revealed rapid onset and increasing rapid response during repeated dosing 24 hours after dosing (least square mean between-group difference, –3.3), day 8 (–2.9), day 15 (–2.0), and day 22 (–4.0; P = .020).
Researchers also examined subjects who achieved at least a 50% reduction MADRS score at day 2 and maintained the improvement at day 28, and the difference was not significant between the two groups. At day 2, 16.5% of esketamine patients achieved a 50% or greater reduction in MADRS, compared with 10.8% in the placebo arm, though the significance of this difference could not be determined.
A post hoc analysis showed that 69.3% of participants in the esketamine group had responded by day 28, compared with 52.0% (odds ratio, 2.4; 95% confidence interval, 1.30-4.54; number needed to treat, 6). More subjects in the treatment group were in remission at day 28 (52.5% vs. 31.0%; NNT, 5).
The most common treatment-emergent adverse events in the esketamine group were dizziness (20.9%), dissociation (26.1%), dysgeusia (24.3%), vertigo (26.1%), and nausea (26.1%).
The study was funded by Janssen Research and Development. Dr. Sanacora was an investigator on previous Janssen-funded studies and has consulted for Janssen. Dr. Schatzberg has received research support from and consulted for Janssen, and has consulted for numerous other companies. Dr. Thase has received research support from Janssen and consulted for a wide range of pharmaceutical companies.
SOURCE: Popova V et al. APA 2019, Am J Psychiatry 2019 May. doi: 10.1176/appi.ajp.2019.19020172.
Concern raised about drug’s abuse potential
Concern raised about drug’s abuse potential
SAN FRANCISCO – In treatment-resistant depression, a switch to a new antidepressant combined with inhaled esketamine (Spravato) led to better symptom improvement than did a switch to a new antidepressant plus placebo, results from a new trial show. The treatment effect of esketamine was modest but had a number needed to treat for remission of just five.
About 30% of patients with major depressive disorder are treatment resistant. They are at risk of suicidal behavior and self-harm during the interval between starting a new medication and onset of efficacy. Esketamine, with its rapid onset of efficacy associated with its stimulation of synaptogenesis through inhibition of the N-methyl-D-aspartate receptor, gained Food and Drug Administration approval in March for the treatment of treatment-resistant depression in adults.
The findings were part of a phase 3 study presented at a press conference at the annual meeting of the American Psychiatric Association and simultaneously published in the American Journal of Psychiatry (2019. doi: 10.1176/appi.ajp.2019.19020172).
Adding esketamine to the start of a new antidepressant was associated with a small improvement in symptoms (effect size, 0.30), and this benefit did not continue to accrue further advantage past the original separation, which led to some skepticism from the author of an accompanying editorial (Am J Psychiatry. 2019 May. doi: 10.1176/appi.ajp.2019.19040423). “The question is, do you need to keep a patient on the drug past 48 hours? There is no statistically significant difference (in patient response) past the first 48 hours. That to me is a concern,” said Alan F. Schatzberg, MD, at the press conference.
Dr. Schatzberg, who is a professor of psychiatry at the Stanford (Calif.) University, also expressed concern about the potential for abuse with esketamine, as well as withdrawal after discontinuation. “I think the drug ought to be used, but it needs to be recommended with considerable caution. We have not answered the many questions that are needed in a drug of potential abuse,” Dr. Schatzberg added.
Study coauthor Michael E. Thase, MD, who also presented at the press conference, discussed the possibility of alterations to the treatment regimen. “I have a hunch you can see this (positive response) within first week or 2, and you can at least concentrate the resource on patients that gain the largest benefit. We can be smarter about it,” said Dr. Thase, who is a professor of psychiatry at the University of Pennsylvania, Philadelphia. He predicted that protocols eventually will be put in place to streamline intranasal ketamine treatment.
Regardless of the effect size, the study produced high remission and response rates in this difficult to treat population, which is welcome news to Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., and director of the Yale Depression Research Program. he said in an interview.
In the phase 3 study, researchers randomized 223 patients from five countries to receive esketamine or placebo, along with either an SSRI (escitalopram or sertraline) or a selective norepinephrine reuptake inhibitor (duloxetine or venlafaxine extended release), based on investigator choice.
To be eligible, participants had to have failed two previous trials of antidepressants. After a 4-week observation period, patients began a 4-week regimen of a new antidepressant combined with twice-weekly nasal esketamine or a placebo.
Subjects in the treatment arm received esketamine on day 1 of the treatment phase with a 56-mg dose. On day 4, 45.8% were increased to a dose of 84 mg, and 66.7% were at the 84-mg dose at the end of the treatment phase.
From baseline to day 28, the esketamine group had a greater decrease in Montgomery-Åsberg Depression Rating Scale (MADRS) score (difference of least square means, –4.0; P = .020; effect size, 0.30). A scatter plot of individual MADRS data during that period revealed rapid onset and increasing rapid response during repeated dosing 24 hours after dosing (least square mean between-group difference, –3.3), day 8 (–2.9), day 15 (–2.0), and day 22 (–4.0; P = .020).
Researchers also examined subjects who achieved at least a 50% reduction MADRS score at day 2 and maintained the improvement at day 28, and the difference was not significant between the two groups. At day 2, 16.5% of esketamine patients achieved a 50% or greater reduction in MADRS, compared with 10.8% in the placebo arm, though the significance of this difference could not be determined.
A post hoc analysis showed that 69.3% of participants in the esketamine group had responded by day 28, compared with 52.0% (odds ratio, 2.4; 95% confidence interval, 1.30-4.54; number needed to treat, 6). More subjects in the treatment group were in remission at day 28 (52.5% vs. 31.0%; NNT, 5).
The most common treatment-emergent adverse events in the esketamine group were dizziness (20.9%), dissociation (26.1%), dysgeusia (24.3%), vertigo (26.1%), and nausea (26.1%).
The study was funded by Janssen Research and Development. Dr. Sanacora was an investigator on previous Janssen-funded studies and has consulted for Janssen. Dr. Schatzberg has received research support from and consulted for Janssen, and has consulted for numerous other companies. Dr. Thase has received research support from Janssen and consulted for a wide range of pharmaceutical companies.
SOURCE: Popova V et al. APA 2019, Am J Psychiatry 2019 May. doi: 10.1176/appi.ajp.2019.19020172.
SAN FRANCISCO – In treatment-resistant depression, a switch to a new antidepressant combined with inhaled esketamine (Spravato) led to better symptom improvement than did a switch to a new antidepressant plus placebo, results from a new trial show. The treatment effect of esketamine was modest but had a number needed to treat for remission of just five.
About 30% of patients with major depressive disorder are treatment resistant. They are at risk of suicidal behavior and self-harm during the interval between starting a new medication and onset of efficacy. Esketamine, with its rapid onset of efficacy associated with its stimulation of synaptogenesis through inhibition of the N-methyl-D-aspartate receptor, gained Food and Drug Administration approval in March for the treatment of treatment-resistant depression in adults.
The findings were part of a phase 3 study presented at a press conference at the annual meeting of the American Psychiatric Association and simultaneously published in the American Journal of Psychiatry (2019. doi: 10.1176/appi.ajp.2019.19020172).
Adding esketamine to the start of a new antidepressant was associated with a small improvement in symptoms (effect size, 0.30), and this benefit did not continue to accrue further advantage past the original separation, which led to some skepticism from the author of an accompanying editorial (Am J Psychiatry. 2019 May. doi: 10.1176/appi.ajp.2019.19040423). “The question is, do you need to keep a patient on the drug past 48 hours? There is no statistically significant difference (in patient response) past the first 48 hours. That to me is a concern,” said Alan F. Schatzberg, MD, at the press conference.
Dr. Schatzberg, who is a professor of psychiatry at the Stanford (Calif.) University, also expressed concern about the potential for abuse with esketamine, as well as withdrawal after discontinuation. “I think the drug ought to be used, but it needs to be recommended with considerable caution. We have not answered the many questions that are needed in a drug of potential abuse,” Dr. Schatzberg added.
Study coauthor Michael E. Thase, MD, who also presented at the press conference, discussed the possibility of alterations to the treatment regimen. “I have a hunch you can see this (positive response) within first week or 2, and you can at least concentrate the resource on patients that gain the largest benefit. We can be smarter about it,” said Dr. Thase, who is a professor of psychiatry at the University of Pennsylvania, Philadelphia. He predicted that protocols eventually will be put in place to streamline intranasal ketamine treatment.
Regardless of the effect size, the study produced high remission and response rates in this difficult to treat population, which is welcome news to Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., and director of the Yale Depression Research Program. he said in an interview.
In the phase 3 study, researchers randomized 223 patients from five countries to receive esketamine or placebo, along with either an SSRI (escitalopram or sertraline) or a selective norepinephrine reuptake inhibitor (duloxetine or venlafaxine extended release), based on investigator choice.
To be eligible, participants had to have failed two previous trials of antidepressants. After a 4-week observation period, patients began a 4-week regimen of a new antidepressant combined with twice-weekly nasal esketamine or a placebo.
Subjects in the treatment arm received esketamine on day 1 of the treatment phase with a 56-mg dose. On day 4, 45.8% were increased to a dose of 84 mg, and 66.7% were at the 84-mg dose at the end of the treatment phase.
From baseline to day 28, the esketamine group had a greater decrease in Montgomery-Åsberg Depression Rating Scale (MADRS) score (difference of least square means, –4.0; P = .020; effect size, 0.30). A scatter plot of individual MADRS data during that period revealed rapid onset and increasing rapid response during repeated dosing 24 hours after dosing (least square mean between-group difference, –3.3), day 8 (–2.9), day 15 (–2.0), and day 22 (–4.0; P = .020).
Researchers also examined subjects who achieved at least a 50% reduction MADRS score at day 2 and maintained the improvement at day 28, and the difference was not significant between the two groups. At day 2, 16.5% of esketamine patients achieved a 50% or greater reduction in MADRS, compared with 10.8% in the placebo arm, though the significance of this difference could not be determined.
A post hoc analysis showed that 69.3% of participants in the esketamine group had responded by day 28, compared with 52.0% (odds ratio, 2.4; 95% confidence interval, 1.30-4.54; number needed to treat, 6). More subjects in the treatment group were in remission at day 28 (52.5% vs. 31.0%; NNT, 5).
The most common treatment-emergent adverse events in the esketamine group were dizziness (20.9%), dissociation (26.1%), dysgeusia (24.3%), vertigo (26.1%), and nausea (26.1%).
The study was funded by Janssen Research and Development. Dr. Sanacora was an investigator on previous Janssen-funded studies and has consulted for Janssen. Dr. Schatzberg has received research support from and consulted for Janssen, and has consulted for numerous other companies. Dr. Thase has received research support from Janssen and consulted for a wide range of pharmaceutical companies.
SOURCE: Popova V et al. APA 2019, Am J Psychiatry 2019 May. doi: 10.1176/appi.ajp.2019.19020172.
REPORTING FROM APA 2019
Key clinical point: Pivotal study reports a modest benefit to symptoms when inhaled esketamine is combined with a new antidepressant in treatment-resistant depression.
Major finding: Combining esketamine with a new antidepressant led to a small improvement in symptoms, compared with the antidepressant alone (effect size, 0.30).
Study details: Results of a randomized, controlled trial (n = 223).
Disclosures: The study was funded by Janssen Research and Development. Dr. Sanacora was an investigator on previous Janssen-funded studies and has consulted for Janssen. Dr. Schatzberg has received research support from and consulted for Janssen, and has consulted for numerous other companies. Dr. Thase has received research support from Janssen and consulted for a wide range of pharmaceutical companies.
Source: Popova V et al. APA 2019, Am J Psychiatry. 2019 May. doi: 10.1176/appi.ajp.2019.19020172.
For some MST survivors, VA hospitals can trigger PTSD
Alternative treatment settings could be ‘easier access point’
SAN FRANCISCO – Veterans who are survivors of military sexual trauma during their service face unique challenges in their treatment and recovery. They are often reluctant to report their experiences – and understandably so.
“Military sexual assault represents a huge violation of that trust and safety. That’s what makes it so toxic and hard for participants to [come] forward, because they’re accused of breaking cohesion of their unit and breaking morale, and yet they have been mistreated,” Niranjan Karnik, MD, PhD, associate dean for community behavioral health at Rush Medical College, Chicago, said in an interview.
Dr. Karnik moderated a session on the prevalence and treatment of military sexual assault at the annual meeting of the American Psychiatric Association. Although the Department of Veterans Affairs treats many survivors of sexual assault, not all of them feel comfortable in that environment. “A VA hospital has a quasi-military feel to it, and that’s a reflection of what it is and the people who are there. That can be an inhibition – and can even be a trigger for [PTSD] symptoms,” Dr. Karnik said.
Survivors may also worry about being labeled, or about adverse entries going into their official record and how that could affect them in the future. The issue is a stark contrast to veterans who are suffering from combat-related trauma.
“When a combat trauma survivor goes to the VA, they feel protected because their colleagues are there. With military sexual trauma, because of that violation of trust from their peers, it can really exacerbate things,” Dr. Karnik said.
Fortunately, there are alternatives, such as the Road Home* Program at Rush Hospital, which has a few military accoutrements but more closely resembles a civilian center. “It can be an easier access point. The VA is taking care of a large majority of patients. We are a boutique program for the vets who can’t or feel unable to go through the VA program,” Dr. Karnik said.
Overall, 52.5% of women and 8.9% of men in the military report sexual harassment, and 23.6% of women and 1.9% of men report being sexually assaulted. That amounts to 14,900 service members, 8,600 women, and 6,300 men who were assaulted in 2016, according to Neeral K. Sheth, DO, assistant professor of psychiatry at Rush Medical College, who also presented at the session. The frequency of assault is higher among LGBTQ individuals, and African American men and women are more likely to experience sexual harassment.
There are options for treatment of military sexual trauma (MST). The 3-week Road Home intensive outpatient treatment program at Rush Hospital combines group and individual cognitive-processing therapy, which is a cognitive-behavioral therapy that has been shown to improve PTSD resulting from MST. The program places combat trauma and MST trauma patients into separate cohorts, each containing individual and group components. Individual sessions closely follow a manualized protocol, while group sessions offer an opportunity to practice cognitive-processing therapy skills.
The team adapted the program to MST treatment by incorporating dialectical-behavioral therapy skills modules in the first week of the program, and implemented one-on-one skills consultation by request throughout the program.
An analysis of 191 subjects participating in 19 cohorts (12 combat, 9 MST cohorts) showed a 92% completion rate, which was similar, regardless of gender or cohort type. Both cohorts had significant reductions in PTSD severity as measured by the PTSD Checklist for DSM-5, and depression symptoms as measured by the Patient Health Questionnaire–9.
Another program, Families OverComing Under Stress, can also be adapted to MST. It is designed to build resiliency and wellness within families dealing with trauma or loss. It incorporates family assessment, psychoeducation tailored to the needs of the entire family, family-level resilience skills, and a narrative component.
An important element is the identification and management of stress reminders – triggers that remind the individual of a trauma and may cause a sudden shift in mood or behavior. A family member’s knowledge that the survivor is experiencing a stress reminder can reduce misunderstandings or unhelpful interpretations of behavior.
In fact, family considerations are often what bring veterans in for help in the first place, according to Dr. Karnik. He or she may be concerned about behavioral problems in a child, which the VA cannot address because its federal funding dictates a sole focus on the veteran. “We will take care of the whole family,” Dr. Karnik said. “Often that’s the entry point, and that allows us to do some engagement with the veteran, and things start to get uncovered.”
Dr. Karnik has no relevant financial disclosures.
*CORRECTION, 5/21/2019
Alternative treatment settings could be ‘easier access point’
Alternative treatment settings could be ‘easier access point’
SAN FRANCISCO – Veterans who are survivors of military sexual trauma during their service face unique challenges in their treatment and recovery. They are often reluctant to report their experiences – and understandably so.
“Military sexual assault represents a huge violation of that trust and safety. That’s what makes it so toxic and hard for participants to [come] forward, because they’re accused of breaking cohesion of their unit and breaking morale, and yet they have been mistreated,” Niranjan Karnik, MD, PhD, associate dean for community behavioral health at Rush Medical College, Chicago, said in an interview.
Dr. Karnik moderated a session on the prevalence and treatment of military sexual assault at the annual meeting of the American Psychiatric Association. Although the Department of Veterans Affairs treats many survivors of sexual assault, not all of them feel comfortable in that environment. “A VA hospital has a quasi-military feel to it, and that’s a reflection of what it is and the people who are there. That can be an inhibition – and can even be a trigger for [PTSD] symptoms,” Dr. Karnik said.
Survivors may also worry about being labeled, or about adverse entries going into their official record and how that could affect them in the future. The issue is a stark contrast to veterans who are suffering from combat-related trauma.
“When a combat trauma survivor goes to the VA, they feel protected because their colleagues are there. With military sexual trauma, because of that violation of trust from their peers, it can really exacerbate things,” Dr. Karnik said.
Fortunately, there are alternatives, such as the Road Home* Program at Rush Hospital, which has a few military accoutrements but more closely resembles a civilian center. “It can be an easier access point. The VA is taking care of a large majority of patients. We are a boutique program for the vets who can’t or feel unable to go through the VA program,” Dr. Karnik said.
Overall, 52.5% of women and 8.9% of men in the military report sexual harassment, and 23.6% of women and 1.9% of men report being sexually assaulted. That amounts to 14,900 service members, 8,600 women, and 6,300 men who were assaulted in 2016, according to Neeral K. Sheth, DO, assistant professor of psychiatry at Rush Medical College, who also presented at the session. The frequency of assault is higher among LGBTQ individuals, and African American men and women are more likely to experience sexual harassment.
There are options for treatment of military sexual trauma (MST). The 3-week Road Home intensive outpatient treatment program at Rush Hospital combines group and individual cognitive-processing therapy, which is a cognitive-behavioral therapy that has been shown to improve PTSD resulting from MST. The program places combat trauma and MST trauma patients into separate cohorts, each containing individual and group components. Individual sessions closely follow a manualized protocol, while group sessions offer an opportunity to practice cognitive-processing therapy skills.
The team adapted the program to MST treatment by incorporating dialectical-behavioral therapy skills modules in the first week of the program, and implemented one-on-one skills consultation by request throughout the program.
An analysis of 191 subjects participating in 19 cohorts (12 combat, 9 MST cohorts) showed a 92% completion rate, which was similar, regardless of gender or cohort type. Both cohorts had significant reductions in PTSD severity as measured by the PTSD Checklist for DSM-5, and depression symptoms as measured by the Patient Health Questionnaire–9.
Another program, Families OverComing Under Stress, can also be adapted to MST. It is designed to build resiliency and wellness within families dealing with trauma or loss. It incorporates family assessment, psychoeducation tailored to the needs of the entire family, family-level resilience skills, and a narrative component.
An important element is the identification and management of stress reminders – triggers that remind the individual of a trauma and may cause a sudden shift in mood or behavior. A family member’s knowledge that the survivor is experiencing a stress reminder can reduce misunderstandings or unhelpful interpretations of behavior.
In fact, family considerations are often what bring veterans in for help in the first place, according to Dr. Karnik. He or she may be concerned about behavioral problems in a child, which the VA cannot address because its federal funding dictates a sole focus on the veteran. “We will take care of the whole family,” Dr. Karnik said. “Often that’s the entry point, and that allows us to do some engagement with the veteran, and things start to get uncovered.”
Dr. Karnik has no relevant financial disclosures.
*CORRECTION, 5/21/2019
SAN FRANCISCO – Veterans who are survivors of military sexual trauma during their service face unique challenges in their treatment and recovery. They are often reluctant to report their experiences – and understandably so.
“Military sexual assault represents a huge violation of that trust and safety. That’s what makes it so toxic and hard for participants to [come] forward, because they’re accused of breaking cohesion of their unit and breaking morale, and yet they have been mistreated,” Niranjan Karnik, MD, PhD, associate dean for community behavioral health at Rush Medical College, Chicago, said in an interview.
Dr. Karnik moderated a session on the prevalence and treatment of military sexual assault at the annual meeting of the American Psychiatric Association. Although the Department of Veterans Affairs treats many survivors of sexual assault, not all of them feel comfortable in that environment. “A VA hospital has a quasi-military feel to it, and that’s a reflection of what it is and the people who are there. That can be an inhibition – and can even be a trigger for [PTSD] symptoms,” Dr. Karnik said.
Survivors may also worry about being labeled, or about adverse entries going into their official record and how that could affect them in the future. The issue is a stark contrast to veterans who are suffering from combat-related trauma.
“When a combat trauma survivor goes to the VA, they feel protected because their colleagues are there. With military sexual trauma, because of that violation of trust from their peers, it can really exacerbate things,” Dr. Karnik said.
Fortunately, there are alternatives, such as the Road Home* Program at Rush Hospital, which has a few military accoutrements but more closely resembles a civilian center. “It can be an easier access point. The VA is taking care of a large majority of patients. We are a boutique program for the vets who can’t or feel unable to go through the VA program,” Dr. Karnik said.
Overall, 52.5% of women and 8.9% of men in the military report sexual harassment, and 23.6% of women and 1.9% of men report being sexually assaulted. That amounts to 14,900 service members, 8,600 women, and 6,300 men who were assaulted in 2016, according to Neeral K. Sheth, DO, assistant professor of psychiatry at Rush Medical College, who also presented at the session. The frequency of assault is higher among LGBTQ individuals, and African American men and women are more likely to experience sexual harassment.
There are options for treatment of military sexual trauma (MST). The 3-week Road Home intensive outpatient treatment program at Rush Hospital combines group and individual cognitive-processing therapy, which is a cognitive-behavioral therapy that has been shown to improve PTSD resulting from MST. The program places combat trauma and MST trauma patients into separate cohorts, each containing individual and group components. Individual sessions closely follow a manualized protocol, while group sessions offer an opportunity to practice cognitive-processing therapy skills.
The team adapted the program to MST treatment by incorporating dialectical-behavioral therapy skills modules in the first week of the program, and implemented one-on-one skills consultation by request throughout the program.
An analysis of 191 subjects participating in 19 cohorts (12 combat, 9 MST cohorts) showed a 92% completion rate, which was similar, regardless of gender or cohort type. Both cohorts had significant reductions in PTSD severity as measured by the PTSD Checklist for DSM-5, and depression symptoms as measured by the Patient Health Questionnaire–9.
Another program, Families OverComing Under Stress, can also be adapted to MST. It is designed to build resiliency and wellness within families dealing with trauma or loss. It incorporates family assessment, psychoeducation tailored to the needs of the entire family, family-level resilience skills, and a narrative component.
An important element is the identification and management of stress reminders – triggers that remind the individual of a trauma and may cause a sudden shift in mood or behavior. A family member’s knowledge that the survivor is experiencing a stress reminder can reduce misunderstandings or unhelpful interpretations of behavior.
In fact, family considerations are often what bring veterans in for help in the first place, according to Dr. Karnik. He or she may be concerned about behavioral problems in a child, which the VA cannot address because its federal funding dictates a sole focus on the veteran. “We will take care of the whole family,” Dr. Karnik said. “Often that’s the entry point, and that allows us to do some engagement with the veteran, and things start to get uncovered.”
Dr. Karnik has no relevant financial disclosures.
*CORRECTION, 5/21/2019
REPORTING FROM APA 2019
About one-third of anxiety patients relapse after stopping antidepressants
SAN FRANCISCO – Relapse is more likely in the absence of medication and, if they resume their antidepressant after relapse, some patients experience adverse events or drug resistance.
“It’s important that we realize that anxiety disorders can be treated effectively in the short term, but it’s very difficult to treat them for the long term. We know that within a year, it’s better to continue the medication. There’s a lack of data to give evidence-based advice after 1 year,” Neeltje Batelaan, MD, PhD, a psychiatrist and senior researcher at VU University Medical Center, Amsterdam, said in an interview.
Dr. Batelaan moderated a session at the annual meeting of the American Psychiatric Association on discontinuation of antidepressant medications in these patients.
Anxiety disorders can often be successfully treated with antidepressants, but their adverse effects can become less tolerable over time, especially after patients go into remission. When patients begin treatment, they are willing to endure side effects in the service of resolving their symptoms. But when they go into remission, “they want to get on with their lives, so their sexual side effects or their weight gain rates a lot worse,” Dr. Batelaan said.
A meta-analysis of 28 studies, with follow-up periods ranging from 8 to 52 weeks, found that the anxiety relapse risk after discontinuation of antidepressants was 36.4%, compared with 16.4% in those who stayed on medication. Even continuing antidepressants isn’t completely protective, she noted. The study found a number needed to treat of five to prevent one relapse.
Researchers at the VU University Medical Center developed a cognitive-behavioral therapy (CBT) regimen aimed at reducing anxiety relapses. In their study, 87 patients with a remitted anxiety disorder who wanted to stop their antidepressants were randomized to do so either with or without CBT intervention.
Unfortunately, the study had to be stopped when an interim analysis showed a lack of efficacy. In fact, patients who received CBT actually had higher relapse rates. Surprisingly, just 37% of patients succeeded in completely discontinuing medication, which hints at the inherent challenges of the transition.
“Unfortunately, building a CBT relapse prevention did not come true, but we learned some valuable lessons that will guide further studies,” Willemijn Scholten, PhD, a postdoc researcher at VU University Medical Center, said during one of the presentations.
In his presentation, Anton (Ton) Van Balkom, MD, PhD, professor of psychiatry at VU University Medical Center, recounted the case of a woman who had been functioning well with an antidepressant but grew tired of the sexual side effects. She carefully discontinued her medication under his guidance, but in 2 months she experienced her first panic attack in 30 years. Reintroducing the medication failed to resolve the issue, and it took years of effort before cognitive behavioral therapy resulted in a remission.
Further, a meta-analysis of nine studies showed that 17% of patients with remitted anxiety who went off and then restarted their antidepressants experienced tachycardia.
To help reduce tachycardia, Dr. Van Balkom suggested alternative options to antidepressants in less-complicated patients with anxiety, and to anticipate long-term use of antidepressants once those medications are employed.
Dr. Batelaan agreed with that assessment, drawing an analogy with type 2 diabetes. “You first start with a diet, and advise the patient to lose weight, and then if that’s not successful you go to [medication] and you realize it’s lifelong. Maybe we have to differentiate antidepressant therapies and [not start them until necessary]. But if you have to start them, realize that there’s a difficult decision waiting ahead.”
SAN FRANCISCO – Relapse is more likely in the absence of medication and, if they resume their antidepressant after relapse, some patients experience adverse events or drug resistance.
“It’s important that we realize that anxiety disorders can be treated effectively in the short term, but it’s very difficult to treat them for the long term. We know that within a year, it’s better to continue the medication. There’s a lack of data to give evidence-based advice after 1 year,” Neeltje Batelaan, MD, PhD, a psychiatrist and senior researcher at VU University Medical Center, Amsterdam, said in an interview.
Dr. Batelaan moderated a session at the annual meeting of the American Psychiatric Association on discontinuation of antidepressant medications in these patients.
Anxiety disorders can often be successfully treated with antidepressants, but their adverse effects can become less tolerable over time, especially after patients go into remission. When patients begin treatment, they are willing to endure side effects in the service of resolving their symptoms. But when they go into remission, “they want to get on with their lives, so their sexual side effects or their weight gain rates a lot worse,” Dr. Batelaan said.
A meta-analysis of 28 studies, with follow-up periods ranging from 8 to 52 weeks, found that the anxiety relapse risk after discontinuation of antidepressants was 36.4%, compared with 16.4% in those who stayed on medication. Even continuing antidepressants isn’t completely protective, she noted. The study found a number needed to treat of five to prevent one relapse.
Researchers at the VU University Medical Center developed a cognitive-behavioral therapy (CBT) regimen aimed at reducing anxiety relapses. In their study, 87 patients with a remitted anxiety disorder who wanted to stop their antidepressants were randomized to do so either with or without CBT intervention.
Unfortunately, the study had to be stopped when an interim analysis showed a lack of efficacy. In fact, patients who received CBT actually had higher relapse rates. Surprisingly, just 37% of patients succeeded in completely discontinuing medication, which hints at the inherent challenges of the transition.
“Unfortunately, building a CBT relapse prevention did not come true, but we learned some valuable lessons that will guide further studies,” Willemijn Scholten, PhD, a postdoc researcher at VU University Medical Center, said during one of the presentations.
In his presentation, Anton (Ton) Van Balkom, MD, PhD, professor of psychiatry at VU University Medical Center, recounted the case of a woman who had been functioning well with an antidepressant but grew tired of the sexual side effects. She carefully discontinued her medication under his guidance, but in 2 months she experienced her first panic attack in 30 years. Reintroducing the medication failed to resolve the issue, and it took years of effort before cognitive behavioral therapy resulted in a remission.
Further, a meta-analysis of nine studies showed that 17% of patients with remitted anxiety who went off and then restarted their antidepressants experienced tachycardia.
To help reduce tachycardia, Dr. Van Balkom suggested alternative options to antidepressants in less-complicated patients with anxiety, and to anticipate long-term use of antidepressants once those medications are employed.
Dr. Batelaan agreed with that assessment, drawing an analogy with type 2 diabetes. “You first start with a diet, and advise the patient to lose weight, and then if that’s not successful you go to [medication] and you realize it’s lifelong. Maybe we have to differentiate antidepressant therapies and [not start them until necessary]. But if you have to start them, realize that there’s a difficult decision waiting ahead.”
SAN FRANCISCO – Relapse is more likely in the absence of medication and, if they resume their antidepressant after relapse, some patients experience adverse events or drug resistance.
“It’s important that we realize that anxiety disorders can be treated effectively in the short term, but it’s very difficult to treat them for the long term. We know that within a year, it’s better to continue the medication. There’s a lack of data to give evidence-based advice after 1 year,” Neeltje Batelaan, MD, PhD, a psychiatrist and senior researcher at VU University Medical Center, Amsterdam, said in an interview.
Dr. Batelaan moderated a session at the annual meeting of the American Psychiatric Association on discontinuation of antidepressant medications in these patients.
Anxiety disorders can often be successfully treated with antidepressants, but their adverse effects can become less tolerable over time, especially after patients go into remission. When patients begin treatment, they are willing to endure side effects in the service of resolving their symptoms. But when they go into remission, “they want to get on with their lives, so their sexual side effects or their weight gain rates a lot worse,” Dr. Batelaan said.
A meta-analysis of 28 studies, with follow-up periods ranging from 8 to 52 weeks, found that the anxiety relapse risk after discontinuation of antidepressants was 36.4%, compared with 16.4% in those who stayed on medication. Even continuing antidepressants isn’t completely protective, she noted. The study found a number needed to treat of five to prevent one relapse.
Researchers at the VU University Medical Center developed a cognitive-behavioral therapy (CBT) regimen aimed at reducing anxiety relapses. In their study, 87 patients with a remitted anxiety disorder who wanted to stop their antidepressants were randomized to do so either with or without CBT intervention.
Unfortunately, the study had to be stopped when an interim analysis showed a lack of efficacy. In fact, patients who received CBT actually had higher relapse rates. Surprisingly, just 37% of patients succeeded in completely discontinuing medication, which hints at the inherent challenges of the transition.
“Unfortunately, building a CBT relapse prevention did not come true, but we learned some valuable lessons that will guide further studies,” Willemijn Scholten, PhD, a postdoc researcher at VU University Medical Center, said during one of the presentations.
In his presentation, Anton (Ton) Van Balkom, MD, PhD, professor of psychiatry at VU University Medical Center, recounted the case of a woman who had been functioning well with an antidepressant but grew tired of the sexual side effects. She carefully discontinued her medication under his guidance, but in 2 months she experienced her first panic attack in 30 years. Reintroducing the medication failed to resolve the issue, and it took years of effort before cognitive behavioral therapy resulted in a remission.
Further, a meta-analysis of nine studies showed that 17% of patients with remitted anxiety who went off and then restarted their antidepressants experienced tachycardia.
To help reduce tachycardia, Dr. Van Balkom suggested alternative options to antidepressants in less-complicated patients with anxiety, and to anticipate long-term use of antidepressants once those medications are employed.
Dr. Batelaan agreed with that assessment, drawing an analogy with type 2 diabetes. “You first start with a diet, and advise the patient to lose weight, and then if that’s not successful you go to [medication] and you realize it’s lifelong. Maybe we have to differentiate antidepressant therapies and [not start them until necessary]. But if you have to start them, realize that there’s a difficult decision waiting ahead.”
REPORTING FROM APA 2019