User login
The protein-sparing modified fast for obese patients with type 2 diabetes: What to expect
Eighty percent of people with type 2 diabetes mellitus are obese or overweight.1 Excess adipose tissue can lead to endocrine dysregulation,2 contributing to the pathogenesis of type 2 diabetes, and obesity is one of the strongest predictors of this disease.3
For obese people with type 2 diabetes, diet and exercise can lead to weight loss and many other benefits, such as better glycemic control, less insulin resistance, lower risk of diabetes-related comorbidities and complications, fewer diabetic medications needed, and lower health care costs.4–7 Intensive lifestyle interventions have also been shown to induce partial remission of diabetes and to prevent the onset of type 2 diabetes in people at high risk of it.5–7
A very-low-calorie diet is one of many dietary options available to patients with type 2 diabetes who are overweight or obese. The protein-sparing modified fast (PSMF) is a type of very-low-calorie diet with a high protein content and simultaneous restriction of carbohydrate and fat.8,9 It was developed in the 1970s, and since then various permutations have been used in weight loss and health care clinics worldwide.
MOSTLY PROTEIN, VERY LITTLE CARBOHYDRATE AND FAT
The PSMF is a medically supervised diet that provides less than 800 kcal/day during an initial intensive phase of about 6 months, followed by the gradual reintroduction of calories during a refeeding phase of about 6 to 8 weeks.10
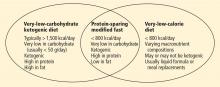
During the intensive phase, patients obtain most of their calories from protein, approximately 1.2 to 1.5 g/kg of ideal body weight per day. At the same time, carbohydrate intake is restricted to less than 20 to 50 g/day; additional fats outside of protein sources are not allowed.9 Thus, the PSMF shares features of both very-low-calorie diets and very-low-carbohydrate ketogenic diets (eg, the Atkins diet), though some differences exist among the three (Figure 1).
Patients rapidly lose weight during the intensive phase, typically between 1 and 3 kg per week, with even greater losses during the first 2 weeks.8,9 Weight loss typically plateaus within 6 months, at which point patients begin the refeeding period. During refeeding, complex carbohydrates and low-glycemic, high-fiber cereals, fruits, vegetables, and fats are gradually reintroduced. Meanwhile, protein intake is reduced to individually tailored amounts as part of a weight-maintenance diet.
LIPOLYSIS, KETOSIS, DIURESIS
The specific macronutrient composition of the PSMF during the intensive phase is designed so that patients enter ketosis and lose as much fat as they can while preserving lean body mass.9,11 Figure 2 illustrates the mechanisms of ketosis and the metabolic impact of the PSMF.
With dietary carbohydrate restriction, serum glucose and insulin levels decline and glycogen stores are depleted. The drop in serum insulin allows lipolysis to occur, resulting in loss of adipose tissue and production of ketone bodies in the liver. Ketone bodies become the primary source of energy for the brain and other tissues during fasting and have metabolic and neuroprotective benefits.12,13
Some studies suggest that ketosis also suppresses appetite, helping curb total caloric intake throughout the diet.14 Protein itself may increase satiety.15
Glycogen in the liver is bound to water, so the depletion of glycogen also results in loss of attached water. As a result, diuresis contributes significantly to the initial weight loss within the first 2 weeks on the PSMF.9
WHO IS A CANDIDATE FOR THE PSMF?
The PSMF is indicated only for adults with a body mass index (BMI) of at least 30 kg/m2 or a BMI of at least 27 kg/m2 and at least one comorbidity such as type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, osteoarthritis, or fatty liver.12 Patients must also be sufficiently committed and motivated to make the intensive dietary and behavioral changes the program calls for.
The PSMF should be considered when more conventional low-calorie approaches to weight loss fail or when patients become discouraged by the slower results seen with traditional diets.8 Patients undergoing a PSMF are usually encouraged by the initial period of rapid weight loss, and such diets have lower dropout rates.16
This diet may also be recommended for obese patients who have poorly controlled type 2 diabetes and growing resistance to medications, to bring down the blood glucose level. Another use is before bariatric surgery to reduce the risk of obesity-related complications.8 Patients who regain weight after bariatric surgery may also benefit.
MEAL REPLACEMENTS OR A DIET PLAN?
The PSMF program at Cleveland Clinic is based on modified preparation and selection of conventional foods. Details of the program are described in Table 1. Protein sources must be of high biologic value, containing the right mix of essential amino acids (eg, lean meat, fish, poultry, egg whites).9
Some commercially available very-low-calorie diets (eg, OPTIFAST, Medifast) that are advertised as PSMFs consist mainly of meal replacements. In the program at Cleveland Clinic, meal replacements in the form of commercial high-protein shakes or bars can be used occasionally for convenience and to maintain adherence to the diet.
However, preparation of PSMF meals from natural, conventional foods is thought to play an important role in long-term behavior modification and so is strongly encouraged. Patients learn low-fat cooking methods, portion control, and how to make appropriate choices in shopping, eating, and dining out. These lessons are valuable for those who struggle with long-term weight loss. Learning these behaviors through the program may help ease the transition to the weight-maintenance phase and beyond. For some patients, cooking is also a source of enjoyment, as is the sight, smell, and taste of nonliquid foods.10
In addition, patients appreciate being able to eat the same foods as others in their household, except for omitting high-carbohydrate foods. It has also been reported that patients on a food-based PSMF were significantly less hungry and preoccupied with eating than those on a liquid formula diet.17
CONTRAINDICATIONS AND SAFETY CONCERNS
Contraindications to the PSMF include a BMI less than 27 kg/m2, recent myocardial infarction, angina, significant arrhythmia, decompensated congestive heart failure, cerebrovascular insufficiency or recent stroke, end-stage renal disease, liver failure, malignancy, major psychiatric illness, pregnancy or lactation, and wasting disorders. It is also not recommended for patients under age 16 or over age 65.
In view of the risk of diabetic ketoacidosis and the difficulty of titrating required doses ofinsulin, patients with type 1 diabetes mellitus are usually not advised to undergo a low-carbohydrate or very-low-calorie diet.8,12 However, we and others have found that the PSMF can be used in some obese patients with type 1 diabetes if it is combined with appropriate education and careful monitoring.12
Major concerns about the safety of the PSMF stem from experiences with the first very-low-calorie diets in the 1970s, which were associated with fatal cardiac arrhythmias and sudden death.18 These early diets used liquid formulas with hydrolyzed collagen protein of poor biologic value and were deficient in many vitamins and minerals. Today’s very-low-calorie diets use protein sources of high biologic value (chiefly animal, soy, and egg for the PSMF) and are supplemented with necessary vitamins and minerals, reducing the risk of electrolyte and cardiac abnormalities.9,19,20 Furthermore, before starting the PSMF all patients must have an electrocardiogram to be sure they have no arrhythmias (eg, heart block, QT interval prolongation) or ischemia.
Relative contraindications
A known history of cholelithiasis is a relative contraindication to a very-low-calorie diet and may be of concern for some patients and providers. While obesity itself is already a risk factor for gallstones, gallstone formation has also been associated with bile stasis, which occurs from rapid weight loss with liquid formula diets of low fat intake (< 10 g/day).21 However, in the PSMF, fat intake from protein sources, though low (45–70 g/day), is considered high enough to allow adequate gallbladder contraction, thus decreasing the risk of gallstone formation.22
Gout is another relative contraindication, as hyperuricemia with risk of gout is also linked to high-protein diets.9 Palgi et al23 found that uric acid levels rose by a mean of 0.4 mg/dL during the diet. The risk of gout, however, seemed to be small, occurring in fewer than 1% of patients in the study. Furthermore, in a recent study by Li et al,24 uric acid levels were found to significantly decrease in patients on a high-protein, very-low-calorie diet. Nonetheless, uric acid levels should be monitored regularly in patients on the PSMF.
SIDE EFFECTS OF THE DIET
Common side effects of the PSMF include headache, fatigue, orthostatic hypotension, muscle cramps, cold intolerance, constipation, diarrhea, fatigue, halitosis, menstrual changes, and hair thinning. Most of these are transient and may be alleviated by adjusting fluid, salt, and supplement intake. Other side effects may disappear as the patient is weaned off the diet.8,9
REGULAR FOLLOW-UP WITH HEALTH CARE PROVIDERS
Current PSMF programs are considered safe when used in combination with regular follow-up with health care providers.8,12
At Cleveland Clinic, patients meet with a dietitian twice in the first month and monthly thereafter (or more frequently if needed) for weight monitoring and education on nutrition and behavior modification (Table 1). Since the PSMF does not provide complete nutrition, daily supplementation with vitamins and minerals is required.
Daily exercise is encouraged throughout the program to increase fitness and to help keep the weight off during the refeeding phase and after.
Patients also meet every 6 to 8 weeks with the referring nurse practitioner or physician for further monitoring and evaluation of vital signs, laboratory results, and side effects. The PSMF protocol at Cleveland Clinic enables both primary care physicians and specialists (including nurse practitioners) within our network to monitor the patient’s status. Use of a common electronic medical record system is particularly valuable for easy communication between providers. If a primary care physician feels unable to appropriately counsel and supervise a patient in the PSMF program, referral to an endocrinologist or weight loss specialist is recommended.
In addition to baseline electrocardiography and monitoring of uric acid levels, a comprehensive metabolic panel is drawn at baseline, twice in the first month, and monthly thereafter to check for electrolyte imbalances and metabolic and tissue dysfunction such as dehydration, excessive protein loss, and liver or kidney injury.
Patients should not attempt the PSMF without medical supervision. Many patients have friends or family members who want to try the PSMF along with them, but this can be dangerous, especially for those with hypertension or type 2 diabetes. The medications prescribed for these conditions can result in hypotension or hypoglycemia during the PSMF.
Although there are no standard guidelines for adjusting medication use before starting a patient on the PSMF, it is logical to taper off or discontinue antihypertensive agents in patients with tightly controlled hypertension to avoid possible dehydration and hypotension during the first few diuresis-inducing weeks of the diet. In particular, diuretic agents should be discontinued to prevent further electrolyte imbalance and fluid shifts.
Similarly, in patients with tightly controlled type 2 diabetes (hemoglobin A1c < 7.0%), oral hypoglycemic agents and insulin therapy should be reduced before starting the diet to avoid potential hypoglycemia. During the course of the diet, providers should then adjust medication dosages based on follow-up vital signs and laboratory results and daily glucose monitoring.8
EFFECTS OF THE PSMF IN PATIENTS WITH TYPE 2 DIABETES
Though few formal studies have been done, the PSMF may have major effects on hyperglycemia, cardiovascular risk factors, and diabetic nephropathy in obese patients with type 2 diabetes, at least in the short term (Table 2).
Weight loss
In one of the first PSMF studies,23 in 668 patients with or without type 2 diabetes (baseline weight 98 kg), the mean weight loss was 21 kg after the intensive phase and 19 kg by the end of the refeeding phase.
In another observational report,25 25% to 30% of patients lost even more weight, averaging 38.6 kg of weight loss. Typically, the higher the baseline weight, the greater the weight loss during the PSMF.23
Patients with type 2 diabetes lost a similar amount of weight (8.5 kg) compared with those without diabetes (9.4 kg, P = .64) in a study of meal-replacement PSMF (using OPTIFAST shakes and bars).26 In a large meal-replacement study of 2,093 patients, Li et al24 found that weight loss was similar between diabetic, prediabetic, and nondiabetic patients. Weight loss was also closely maintained in those patients who stayed on the diet for 12 months.
In a PSMF study in which all the participants had type 2 diabetes, the mean weight loss was 18.6 kg. Although the patients regained some of this weight, at 1 year they still weighed 8.6 kg less than at baseline. However, a conventional, balanced, low-calorie diet resulted in similar amounts of weight loss after 1 year.27 Furthermore, a second round of the PSMF did not result in significant additional weight loss but rather weight maintenance.28
Fat loss and smaller waist circumference
Most of the weight lost during a PSMF is from fat tissue.11,26 Abdominal (visceral) fat may be lost first, which is desirable for patients with type 2 diabetes, since a higher degree of abdominal fat is linked to insulin resistance.2,29
After a meal-replacement PSMF, waist circumference decreased significantly in patients both with and without type 2 diabetes.24,26 However, in one study, less fat was lost per unit of change of BMI in the group with type 2 diabetes than in the nondiabetic group.26 Since insulin inhibits lipolysis, it is possible that exogenous insulin use in diabetic patients may prevent greater reductions in fat mass, though this is likely not the only mechanism.26
Lower fasting serum glucose
Fasting serum glucose levels decreased significantly from baseline in patients with type 2 diabetes after a PSMF in all studies that measured this variable.23–28,30,31 Changes in fasting glucose are immediate and are associated with caloric restriction rather than weight loss itself.30,32 Furthermore, the observed decrease in serum glucose is even more impressive in view of the withdrawal or reduction of doses of insulin and oral hypoglycemic agents before starting the diet.
In a study that compared glycemic control in a PSMF diet vs a balanced low-calorie diet, the fasting serum glucose in the PSMF group declined 46%, from 255.9 mg/dL at baseline to 138.7 mg/dL at 20 weeks (P = .001). After 1 year, it had risen back to 187.4 mg/dL, which was still 27% lower than at baseline (P = .023). These results compared favorably with those in the low-calorie diet group (P < .05), which saw fasting serum glucose decline 27% after 20 weeks (from 230.6 mg/dL at baseline to 167.6 mg/dL) and then rise to 5% over baseline (243.2 mg/dL) after 1 year.27
In a later study, the decrease in fasting serum glucose was not maintained at 1 year, but a significantly higher percentage (55%) of participants in the PSMF group were still able to remain free of diabetic medications compared with those who followed a balanced low-calorie diet (31%, P = .01).28
Decrease in hemoglobin A1c
Declines in fasting serum glucose corresponded with short-term declines in hemoglobin A1c in several reports.27–31 Hemoglobin A1c declined significantly from an average of 10.4% to 7.3% (P = .001) after PSMF intervention in patients with type 2 diabetes. In contrast, hemoglobin A1c in the low-calorie diet control group declined from 10.4% to 8.6%.27 One year later, hemoglobin A1c remained lower than at baseline in the PSMF group (final 9.2%) and continued to compare favorably against the control group (final 11.8%, between-group P = .001). However, these 1-year post-intervention improvements were not seen in a second, more intensive study.28
Less insulin resistance
In several studies, fasting serum insulin levels declined along with serum glucose levels, implying decreased insulin resistance.25,27,28,30,31 In addition, insulin output was enhanced during glucose challenge after completion of the PSMF, suggesting possible improved (though still impaired) pancreatic beta-cell capacity.25,27,30
Improved lipid profile
The most common effect of the PSMF on the lipid profile is a significant decrease in triglycerides in patients both with and without type 2 diabetes.8,23,24,28 In addition, high-density lipoprotein cholesterol increased in two studies following PSMF intervention or after 1-year of follow-up.24,27,28 Total cholesterol and low-density lipoprotein cholesterol levels also improved after the PSMF, but these changes were not always maintained at follow-up visits.8,24,28
Lower blood pressure
Improvements in both systolic and diastolic blood pressure were noted in two studies, with mean decreases of 6 mm Hg to 13 mm Hg systolic and 8 mm Hg diastolic after PSMF intervention.23,28 In a third study, reductions in blood pressure were less dramatic, and only changes in diastolic but not systolic blood pressure remained significant at 12 months.24 While improvements were not observed in a fourth study, patients in this study also had impaired kidney function caused by diabetic nephropathy, and changes in medication were not taken into account.31
Kidney function tests
In a small study, Friedman et al showed that 12 weeks of the PSMF in six patients with advanced diabetic nephropathy (stage 3B or stage 4 chronic kidney disease) led to a loss of 12% of body weight (P = .03) as well as significant reductions in serum creatinine and cystatin C levels (P < .05).31 In addition, albuminuria decreased by 30% (P = .08). Side effects were minimal, and the diet was well tolerated despite its high protein content, which is a concern in patients with impaired kidney function.
Thus, weight loss via the PSMF may still be beneficial in type 2 diabetic patients with chronic kidney disease and may even improve the course of progression of diabetic nephropathy.
Long-term weight loss is elusive
Long-term weight loss has been an elusive goal for many diet programs. In a study using a very-low-calorie diet in obese patients with type 2 diabetes, substantial weight loss was maintained in half of the patients at 3 years after the intervention, but nearly all of the patients had regained most of their weight after 5 years.33
While commitment to behavior modification, maintenance of physical activity, and continued follow-up are all critical factors in sustaining weight loss, new and innovative approaches to battle weight regain are needed.34
Yet despite considerable weight regain in most patients, the Look AHEAD (Action for Health in Diabetes) study showed that participants in intensive lifestyle intervention programs still achieved greater weight loss after 4 years than those receiving standard care.35 Whether this holds true for those in intensive PSMF programs is unknown. In addition, conclusive PSMF studies regarding glycemic control, lipids, and blood pressure beyond 1 year of follow-up are lacking.
A VIABLE OPTION FOR MANY
Adherence to a very-low-calorie, ketogenic PSMF program results in major short-term health benefits for obese patients with type 2 diabetes. These benefits include significant weight loss, often more than 18 kg, within 6 months.23–28 In addition, significant improvements in fasting glucose23–28,30–32 and hemoglobin A1c levels27–31 are linked to the caloric and carbohydrate restriction of the PSMF. Insulin resistance was also attenuated, with possible partial restoration of pancreatic beta-cell capacity.25,27,28,30,31 In some studies, the PSMF resulted in lower systolic and diastolic blood pressure23,24,28 and triglyceride levels.8,23,24,28 One small study also suggested a possible improvement of diabetic nephropathy.31 Lastly, improvements in glycemia and hypertension were associated with a reduction in the need for antidiabetic and antihypertensive drugs.36
Still, weight loss and many of the associated improvements partially return to baseline levels 1 year after the intervention. Thus, more long-term studies are needed to explore factors for better weight maintenance after the PSMF.
Also, only a few studies have compared the effect of the PSMF between patients with or without type 2 diabetes. One study suggested that fat loss may be reduced in patients with type 2 diabetes.26
In conclusion, despite some risks and safety concerns, PSMF is a viable option for many obese, type 2 diabetic patients as a method of short-term weight loss, with evidence for improvement of glycemic control and cardiovascular risk factors for up to 1 year. To strengthen support for the PSMF, however, further research is warranted on the diet’s long-term effects in patients with type 2 diabetes and also in nondiabetic patients.
Acknowledgments: Many thanks to Cheryl Reitz, RD, LD, CDE, and Dawn Noe, RD, LD, CDE, for providing their expertise on the PSMF protocols carried out at Cleveland Clinic. Additional thanks to Tejas Kashyap for his initial assistance with this review.
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006; 12:75–80.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106:473–481.
- Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345:790–797.
- Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011; 378:129–139.
- Lindström J, Louheranta A, Mannelin M, et al; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003; 26:3230–3236.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012; 308:2489–2496.
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991; 14:802–823.
- Bistrian BR. Clinical use of a protein-sparing modified fast. JAMA 1978; 240:2299–2302.
- Walters JK, Hoogwerf BJ, Reddy SS. The protein-sparing modified fast for obesity-related medical problems. Cleve Clin J Med 1997; 64:242–244.
- Van Gaal LF, Snyders D, De Leeuw IH, Bekaert JL. Anthropometric and calorimetric evidence for the protein sparing effects of a new protein supplemented low calorie preparation. Am J Clin Nutr 1985; 41:540–544.
- Baker S, Jerums G, Proietto J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract 2009; 85:235–242.
- Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by ß-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339:211–214.
- Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008; 87:44–55.
- Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012; 108(suppl 2):S105–S112.
- Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr 2012; 96:953–961.
- Wadden TA, Stunkard AJ, Brownell KD, Day SC. A comparison of two very-low-calorie diets: protein-sparing-modified fast versus protein-formula-liquid diet. Am J Clin Nutr 1985; 41:533–539.
- Isner JM, Sours HE, Paris AL, Ferrans VJ, Roberts WC. Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation 1979; 60:1401–1412.
- Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 1986; 35:155–164.
- Seim HC, Mitchell JE, Pomeroy C, de Zwaan M. Electrocardiographic findings associated with very low calorie dieting. Int J Obes Relat Metab Disord 1995; 19:817–819.
- Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 2014; 38:279–284.
- Festi D, Colecchia A, Orsini M, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998; 22:592–600.
- Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: results in 668 outpatients. Am J Public Health 1985; 75:1190–1194.
- Li Z, Tseng CH, Li Q, Deng ML, Wang M, Heber D. Clinical efficacy of a medically supervised outpatient high-protein, low-calorie diet program is equivalent in prediabetic, diabetic and normoglycemic obese patients. Nutr Diabetes 2014 Feb 10; 4:e105.
- Genuth S. Supplemented fasting in the treatment of obesity and diabetes. Am J Clin Nutr 1979; 32:2579–2586.
- Baker ST, Jerums G, Prendergast LA, Panagiotopoulos S, Strauss BJ, Proietto J. Less fat reduction per unit weight loss in type 2 diabetic compared with nondiabetic obese individuals completing a very-low-calorie diet program. Metabolism 2012; 61:873–882.
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 1991; 151:1334–1340.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994; 97:354–362.
- Kawamura II, Chen CC, Yamazaki K, Miyazawa Y, Isono K. A clinical study of protein sparing modified fast (PSMF) administered preoperatively to morbidly obese patients: comparison of PSMF with natural food products to originally prepared PSMF. Obes Surg 1992; 2:33–40.
- Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 1984; 77:7–17.
- Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013; 8:1892–1898.
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17:30–36.
- Paisey RB, Frost J, Harvey P, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002; 15:121–127.
- Blackburn GL. Weight of the nation: moving forward, reversing the trend using medical care. Am J Clin Nutr 2012; 96:949–950.
- Wadden TA, Neiberg RH, Wing RR, et al; Look AHEAD Research Group. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011; 19:1987–1998.
- Redmon JB, Bertoni AG, Connelly S, et al; Look AHEAD Research Group. Effect of the Look AHEAD intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010; 33:1153–1158.
Eighty percent of people with type 2 diabetes mellitus are obese or overweight.1 Excess adipose tissue can lead to endocrine dysregulation,2 contributing to the pathogenesis of type 2 diabetes, and obesity is one of the strongest predictors of this disease.3
For obese people with type 2 diabetes, diet and exercise can lead to weight loss and many other benefits, such as better glycemic control, less insulin resistance, lower risk of diabetes-related comorbidities and complications, fewer diabetic medications needed, and lower health care costs.4–7 Intensive lifestyle interventions have also been shown to induce partial remission of diabetes and to prevent the onset of type 2 diabetes in people at high risk of it.5–7
A very-low-calorie diet is one of many dietary options available to patients with type 2 diabetes who are overweight or obese. The protein-sparing modified fast (PSMF) is a type of very-low-calorie diet with a high protein content and simultaneous restriction of carbohydrate and fat.8,9 It was developed in the 1970s, and since then various permutations have been used in weight loss and health care clinics worldwide.
MOSTLY PROTEIN, VERY LITTLE CARBOHYDRATE AND FAT
The PSMF is a medically supervised diet that provides less than 800 kcal/day during an initial intensive phase of about 6 months, followed by the gradual reintroduction of calories during a refeeding phase of about 6 to 8 weeks.10
During the intensive phase, patients obtain most of their calories from protein, approximately 1.2 to 1.5 g/kg of ideal body weight per day. At the same time, carbohydrate intake is restricted to less than 20 to 50 g/day; additional fats outside of protein sources are not allowed.9 Thus, the PSMF shares features of both very-low-calorie diets and very-low-carbohydrate ketogenic diets (eg, the Atkins diet), though some differences exist among the three (Figure 1).
Patients rapidly lose weight during the intensive phase, typically between 1 and 3 kg per week, with even greater losses during the first 2 weeks.8,9 Weight loss typically plateaus within 6 months, at which point patients begin the refeeding period. During refeeding, complex carbohydrates and low-glycemic, high-fiber cereals, fruits, vegetables, and fats are gradually reintroduced. Meanwhile, protein intake is reduced to individually tailored amounts as part of a weight-maintenance diet.
LIPOLYSIS, KETOSIS, DIURESIS
The specific macronutrient composition of the PSMF during the intensive phase is designed so that patients enter ketosis and lose as much fat as they can while preserving lean body mass.9,11 Figure 2 illustrates the mechanisms of ketosis and the metabolic impact of the PSMF.
With dietary carbohydrate restriction, serum glucose and insulin levels decline and glycogen stores are depleted. The drop in serum insulin allows lipolysis to occur, resulting in loss of adipose tissue and production of ketone bodies in the liver. Ketone bodies become the primary source of energy for the brain and other tissues during fasting and have metabolic and neuroprotective benefits.12,13
Some studies suggest that ketosis also suppresses appetite, helping curb total caloric intake throughout the diet.14 Protein itself may increase satiety.15
Glycogen in the liver is bound to water, so the depletion of glycogen also results in loss of attached water. As a result, diuresis contributes significantly to the initial weight loss within the first 2 weeks on the PSMF.9
WHO IS A CANDIDATE FOR THE PSMF?
The PSMF is indicated only for adults with a body mass index (BMI) of at least 30 kg/m2 or a BMI of at least 27 kg/m2 and at least one comorbidity such as type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, osteoarthritis, or fatty liver.12 Patients must also be sufficiently committed and motivated to make the intensive dietary and behavioral changes the program calls for.
The PSMF should be considered when more conventional low-calorie approaches to weight loss fail or when patients become discouraged by the slower results seen with traditional diets.8 Patients undergoing a PSMF are usually encouraged by the initial period of rapid weight loss, and such diets have lower dropout rates.16
This diet may also be recommended for obese patients who have poorly controlled type 2 diabetes and growing resistance to medications, to bring down the blood glucose level. Another use is before bariatric surgery to reduce the risk of obesity-related complications.8 Patients who regain weight after bariatric surgery may also benefit.
MEAL REPLACEMENTS OR A DIET PLAN?
The PSMF program at Cleveland Clinic is based on modified preparation and selection of conventional foods. Details of the program are described in Table 1. Protein sources must be of high biologic value, containing the right mix of essential amino acids (eg, lean meat, fish, poultry, egg whites).9
Some commercially available very-low-calorie diets (eg, OPTIFAST, Medifast) that are advertised as PSMFs consist mainly of meal replacements. In the program at Cleveland Clinic, meal replacements in the form of commercial high-protein shakes or bars can be used occasionally for convenience and to maintain adherence to the diet.
However, preparation of PSMF meals from natural, conventional foods is thought to play an important role in long-term behavior modification and so is strongly encouraged. Patients learn low-fat cooking methods, portion control, and how to make appropriate choices in shopping, eating, and dining out. These lessons are valuable for those who struggle with long-term weight loss. Learning these behaviors through the program may help ease the transition to the weight-maintenance phase and beyond. For some patients, cooking is also a source of enjoyment, as is the sight, smell, and taste of nonliquid foods.10
In addition, patients appreciate being able to eat the same foods as others in their household, except for omitting high-carbohydrate foods. It has also been reported that patients on a food-based PSMF were significantly less hungry and preoccupied with eating than those on a liquid formula diet.17
CONTRAINDICATIONS AND SAFETY CONCERNS
Contraindications to the PSMF include a BMI less than 27 kg/m2, recent myocardial infarction, angina, significant arrhythmia, decompensated congestive heart failure, cerebrovascular insufficiency or recent stroke, end-stage renal disease, liver failure, malignancy, major psychiatric illness, pregnancy or lactation, and wasting disorders. It is also not recommended for patients under age 16 or over age 65.
In view of the risk of diabetic ketoacidosis and the difficulty of titrating required doses ofinsulin, patients with type 1 diabetes mellitus are usually not advised to undergo a low-carbohydrate or very-low-calorie diet.8,12 However, we and others have found that the PSMF can be used in some obese patients with type 1 diabetes if it is combined with appropriate education and careful monitoring.12
Major concerns about the safety of the PSMF stem from experiences with the first very-low-calorie diets in the 1970s, which were associated with fatal cardiac arrhythmias and sudden death.18 These early diets used liquid formulas with hydrolyzed collagen protein of poor biologic value and were deficient in many vitamins and minerals. Today’s very-low-calorie diets use protein sources of high biologic value (chiefly animal, soy, and egg for the PSMF) and are supplemented with necessary vitamins and minerals, reducing the risk of electrolyte and cardiac abnormalities.9,19,20 Furthermore, before starting the PSMF all patients must have an electrocardiogram to be sure they have no arrhythmias (eg, heart block, QT interval prolongation) or ischemia.
Relative contraindications
A known history of cholelithiasis is a relative contraindication to a very-low-calorie diet and may be of concern for some patients and providers. While obesity itself is already a risk factor for gallstones, gallstone formation has also been associated with bile stasis, which occurs from rapid weight loss with liquid formula diets of low fat intake (< 10 g/day).21 However, in the PSMF, fat intake from protein sources, though low (45–70 g/day), is considered high enough to allow adequate gallbladder contraction, thus decreasing the risk of gallstone formation.22
Gout is another relative contraindication, as hyperuricemia with risk of gout is also linked to high-protein diets.9 Palgi et al23 found that uric acid levels rose by a mean of 0.4 mg/dL during the diet. The risk of gout, however, seemed to be small, occurring in fewer than 1% of patients in the study. Furthermore, in a recent study by Li et al,24 uric acid levels were found to significantly decrease in patients on a high-protein, very-low-calorie diet. Nonetheless, uric acid levels should be monitored regularly in patients on the PSMF.
SIDE EFFECTS OF THE DIET
Common side effects of the PSMF include headache, fatigue, orthostatic hypotension, muscle cramps, cold intolerance, constipation, diarrhea, fatigue, halitosis, menstrual changes, and hair thinning. Most of these are transient and may be alleviated by adjusting fluid, salt, and supplement intake. Other side effects may disappear as the patient is weaned off the diet.8,9
REGULAR FOLLOW-UP WITH HEALTH CARE PROVIDERS
Current PSMF programs are considered safe when used in combination with regular follow-up with health care providers.8,12
At Cleveland Clinic, patients meet with a dietitian twice in the first month and monthly thereafter (or more frequently if needed) for weight monitoring and education on nutrition and behavior modification (Table 1). Since the PSMF does not provide complete nutrition, daily supplementation with vitamins and minerals is required.
Daily exercise is encouraged throughout the program to increase fitness and to help keep the weight off during the refeeding phase and after.
Patients also meet every 6 to 8 weeks with the referring nurse practitioner or physician for further monitoring and evaluation of vital signs, laboratory results, and side effects. The PSMF protocol at Cleveland Clinic enables both primary care physicians and specialists (including nurse practitioners) within our network to monitor the patient’s status. Use of a common electronic medical record system is particularly valuable for easy communication between providers. If a primary care physician feels unable to appropriately counsel and supervise a patient in the PSMF program, referral to an endocrinologist or weight loss specialist is recommended.
In addition to baseline electrocardiography and monitoring of uric acid levels, a comprehensive metabolic panel is drawn at baseline, twice in the first month, and monthly thereafter to check for electrolyte imbalances and metabolic and tissue dysfunction such as dehydration, excessive protein loss, and liver or kidney injury.
Patients should not attempt the PSMF without medical supervision. Many patients have friends or family members who want to try the PSMF along with them, but this can be dangerous, especially for those with hypertension or type 2 diabetes. The medications prescribed for these conditions can result in hypotension or hypoglycemia during the PSMF.
Although there are no standard guidelines for adjusting medication use before starting a patient on the PSMF, it is logical to taper off or discontinue antihypertensive agents in patients with tightly controlled hypertension to avoid possible dehydration and hypotension during the first few diuresis-inducing weeks of the diet. In particular, diuretic agents should be discontinued to prevent further electrolyte imbalance and fluid shifts.
Similarly, in patients with tightly controlled type 2 diabetes (hemoglobin A1c < 7.0%), oral hypoglycemic agents and insulin therapy should be reduced before starting the diet to avoid potential hypoglycemia. During the course of the diet, providers should then adjust medication dosages based on follow-up vital signs and laboratory results and daily glucose monitoring.8
EFFECTS OF THE PSMF IN PATIENTS WITH TYPE 2 DIABETES
Though few formal studies have been done, the PSMF may have major effects on hyperglycemia, cardiovascular risk factors, and diabetic nephropathy in obese patients with type 2 diabetes, at least in the short term (Table 2).
Weight loss
In one of the first PSMF studies,23 in 668 patients with or without type 2 diabetes (baseline weight 98 kg), the mean weight loss was 21 kg after the intensive phase and 19 kg by the end of the refeeding phase.
In another observational report,25 25% to 30% of patients lost even more weight, averaging 38.6 kg of weight loss. Typically, the higher the baseline weight, the greater the weight loss during the PSMF.23
Patients with type 2 diabetes lost a similar amount of weight (8.5 kg) compared with those without diabetes (9.4 kg, P = .64) in a study of meal-replacement PSMF (using OPTIFAST shakes and bars).26 In a large meal-replacement study of 2,093 patients, Li et al24 found that weight loss was similar between diabetic, prediabetic, and nondiabetic patients. Weight loss was also closely maintained in those patients who stayed on the diet for 12 months.
In a PSMF study in which all the participants had type 2 diabetes, the mean weight loss was 18.6 kg. Although the patients regained some of this weight, at 1 year they still weighed 8.6 kg less than at baseline. However, a conventional, balanced, low-calorie diet resulted in similar amounts of weight loss after 1 year.27 Furthermore, a second round of the PSMF did not result in significant additional weight loss but rather weight maintenance.28
Fat loss and smaller waist circumference
Most of the weight lost during a PSMF is from fat tissue.11,26 Abdominal (visceral) fat may be lost first, which is desirable for patients with type 2 diabetes, since a higher degree of abdominal fat is linked to insulin resistance.2,29
After a meal-replacement PSMF, waist circumference decreased significantly in patients both with and without type 2 diabetes.24,26 However, in one study, less fat was lost per unit of change of BMI in the group with type 2 diabetes than in the nondiabetic group.26 Since insulin inhibits lipolysis, it is possible that exogenous insulin use in diabetic patients may prevent greater reductions in fat mass, though this is likely not the only mechanism.26
Lower fasting serum glucose
Fasting serum glucose levels decreased significantly from baseline in patients with type 2 diabetes after a PSMF in all studies that measured this variable.23–28,30,31 Changes in fasting glucose are immediate and are associated with caloric restriction rather than weight loss itself.30,32 Furthermore, the observed decrease in serum glucose is even more impressive in view of the withdrawal or reduction of doses of insulin and oral hypoglycemic agents before starting the diet.
In a study that compared glycemic control in a PSMF diet vs a balanced low-calorie diet, the fasting serum glucose in the PSMF group declined 46%, from 255.9 mg/dL at baseline to 138.7 mg/dL at 20 weeks (P = .001). After 1 year, it had risen back to 187.4 mg/dL, which was still 27% lower than at baseline (P = .023). These results compared favorably with those in the low-calorie diet group (P < .05), which saw fasting serum glucose decline 27% after 20 weeks (from 230.6 mg/dL at baseline to 167.6 mg/dL) and then rise to 5% over baseline (243.2 mg/dL) after 1 year.27
In a later study, the decrease in fasting serum glucose was not maintained at 1 year, but a significantly higher percentage (55%) of participants in the PSMF group were still able to remain free of diabetic medications compared with those who followed a balanced low-calorie diet (31%, P = .01).28
Decrease in hemoglobin A1c
Declines in fasting serum glucose corresponded with short-term declines in hemoglobin A1c in several reports.27–31 Hemoglobin A1c declined significantly from an average of 10.4% to 7.3% (P = .001) after PSMF intervention in patients with type 2 diabetes. In contrast, hemoglobin A1c in the low-calorie diet control group declined from 10.4% to 8.6%.27 One year later, hemoglobin A1c remained lower than at baseline in the PSMF group (final 9.2%) and continued to compare favorably against the control group (final 11.8%, between-group P = .001). However, these 1-year post-intervention improvements were not seen in a second, more intensive study.28
Less insulin resistance
In several studies, fasting serum insulin levels declined along with serum glucose levels, implying decreased insulin resistance.25,27,28,30,31 In addition, insulin output was enhanced during glucose challenge after completion of the PSMF, suggesting possible improved (though still impaired) pancreatic beta-cell capacity.25,27,30
Improved lipid profile
The most common effect of the PSMF on the lipid profile is a significant decrease in triglycerides in patients both with and without type 2 diabetes.8,23,24,28 In addition, high-density lipoprotein cholesterol increased in two studies following PSMF intervention or after 1-year of follow-up.24,27,28 Total cholesterol and low-density lipoprotein cholesterol levels also improved after the PSMF, but these changes were not always maintained at follow-up visits.8,24,28
Lower blood pressure
Improvements in both systolic and diastolic blood pressure were noted in two studies, with mean decreases of 6 mm Hg to 13 mm Hg systolic and 8 mm Hg diastolic after PSMF intervention.23,28 In a third study, reductions in blood pressure were less dramatic, and only changes in diastolic but not systolic blood pressure remained significant at 12 months.24 While improvements were not observed in a fourth study, patients in this study also had impaired kidney function caused by diabetic nephropathy, and changes in medication were not taken into account.31
Kidney function tests
In a small study, Friedman et al showed that 12 weeks of the PSMF in six patients with advanced diabetic nephropathy (stage 3B or stage 4 chronic kidney disease) led to a loss of 12% of body weight (P = .03) as well as significant reductions in serum creatinine and cystatin C levels (P < .05).31 In addition, albuminuria decreased by 30% (P = .08). Side effects were minimal, and the diet was well tolerated despite its high protein content, which is a concern in patients with impaired kidney function.
Thus, weight loss via the PSMF may still be beneficial in type 2 diabetic patients with chronic kidney disease and may even improve the course of progression of diabetic nephropathy.
Long-term weight loss is elusive
Long-term weight loss has been an elusive goal for many diet programs. In a study using a very-low-calorie diet in obese patients with type 2 diabetes, substantial weight loss was maintained in half of the patients at 3 years after the intervention, but nearly all of the patients had regained most of their weight after 5 years.33
While commitment to behavior modification, maintenance of physical activity, and continued follow-up are all critical factors in sustaining weight loss, new and innovative approaches to battle weight regain are needed.34
Yet despite considerable weight regain in most patients, the Look AHEAD (Action for Health in Diabetes) study showed that participants in intensive lifestyle intervention programs still achieved greater weight loss after 4 years than those receiving standard care.35 Whether this holds true for those in intensive PSMF programs is unknown. In addition, conclusive PSMF studies regarding glycemic control, lipids, and blood pressure beyond 1 year of follow-up are lacking.
A VIABLE OPTION FOR MANY
Adherence to a very-low-calorie, ketogenic PSMF program results in major short-term health benefits for obese patients with type 2 diabetes. These benefits include significant weight loss, often more than 18 kg, within 6 months.23–28 In addition, significant improvements in fasting glucose23–28,30–32 and hemoglobin A1c levels27–31 are linked to the caloric and carbohydrate restriction of the PSMF. Insulin resistance was also attenuated, with possible partial restoration of pancreatic beta-cell capacity.25,27,28,30,31 In some studies, the PSMF resulted in lower systolic and diastolic blood pressure23,24,28 and triglyceride levels.8,23,24,28 One small study also suggested a possible improvement of diabetic nephropathy.31 Lastly, improvements in glycemia and hypertension were associated with a reduction in the need for antidiabetic and antihypertensive drugs.36
Still, weight loss and many of the associated improvements partially return to baseline levels 1 year after the intervention. Thus, more long-term studies are needed to explore factors for better weight maintenance after the PSMF.
Also, only a few studies have compared the effect of the PSMF between patients with or without type 2 diabetes. One study suggested that fat loss may be reduced in patients with type 2 diabetes.26
In conclusion, despite some risks and safety concerns, PSMF is a viable option for many obese, type 2 diabetic patients as a method of short-term weight loss, with evidence for improvement of glycemic control and cardiovascular risk factors for up to 1 year. To strengthen support for the PSMF, however, further research is warranted on the diet’s long-term effects in patients with type 2 diabetes and also in nondiabetic patients.
Acknowledgments: Many thanks to Cheryl Reitz, RD, LD, CDE, and Dawn Noe, RD, LD, CDE, for providing their expertise on the PSMF protocols carried out at Cleveland Clinic. Additional thanks to Tejas Kashyap for his initial assistance with this review.
Eighty percent of people with type 2 diabetes mellitus are obese or overweight.1 Excess adipose tissue can lead to endocrine dysregulation,2 contributing to the pathogenesis of type 2 diabetes, and obesity is one of the strongest predictors of this disease.3
For obese people with type 2 diabetes, diet and exercise can lead to weight loss and many other benefits, such as better glycemic control, less insulin resistance, lower risk of diabetes-related comorbidities and complications, fewer diabetic medications needed, and lower health care costs.4–7 Intensive lifestyle interventions have also been shown to induce partial remission of diabetes and to prevent the onset of type 2 diabetes in people at high risk of it.5–7
A very-low-calorie diet is one of many dietary options available to patients with type 2 diabetes who are overweight or obese. The protein-sparing modified fast (PSMF) is a type of very-low-calorie diet with a high protein content and simultaneous restriction of carbohydrate and fat.8,9 It was developed in the 1970s, and since then various permutations have been used in weight loss and health care clinics worldwide.
MOSTLY PROTEIN, VERY LITTLE CARBOHYDRATE AND FAT
The PSMF is a medically supervised diet that provides less than 800 kcal/day during an initial intensive phase of about 6 months, followed by the gradual reintroduction of calories during a refeeding phase of about 6 to 8 weeks.10
During the intensive phase, patients obtain most of their calories from protein, approximately 1.2 to 1.5 g/kg of ideal body weight per day. At the same time, carbohydrate intake is restricted to less than 20 to 50 g/day; additional fats outside of protein sources are not allowed.9 Thus, the PSMF shares features of both very-low-calorie diets and very-low-carbohydrate ketogenic diets (eg, the Atkins diet), though some differences exist among the three (Figure 1).
Patients rapidly lose weight during the intensive phase, typically between 1 and 3 kg per week, with even greater losses during the first 2 weeks.8,9 Weight loss typically plateaus within 6 months, at which point patients begin the refeeding period. During refeeding, complex carbohydrates and low-glycemic, high-fiber cereals, fruits, vegetables, and fats are gradually reintroduced. Meanwhile, protein intake is reduced to individually tailored amounts as part of a weight-maintenance diet.
LIPOLYSIS, KETOSIS, DIURESIS
The specific macronutrient composition of the PSMF during the intensive phase is designed so that patients enter ketosis and lose as much fat as they can while preserving lean body mass.9,11 Figure 2 illustrates the mechanisms of ketosis and the metabolic impact of the PSMF.
With dietary carbohydrate restriction, serum glucose and insulin levels decline and glycogen stores are depleted. The drop in serum insulin allows lipolysis to occur, resulting in loss of adipose tissue and production of ketone bodies in the liver. Ketone bodies become the primary source of energy for the brain and other tissues during fasting and have metabolic and neuroprotective benefits.12,13
Some studies suggest that ketosis also suppresses appetite, helping curb total caloric intake throughout the diet.14 Protein itself may increase satiety.15
Glycogen in the liver is bound to water, so the depletion of glycogen also results in loss of attached water. As a result, diuresis contributes significantly to the initial weight loss within the first 2 weeks on the PSMF.9
WHO IS A CANDIDATE FOR THE PSMF?
The PSMF is indicated only for adults with a body mass index (BMI) of at least 30 kg/m2 or a BMI of at least 27 kg/m2 and at least one comorbidity such as type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, osteoarthritis, or fatty liver.12 Patients must also be sufficiently committed and motivated to make the intensive dietary and behavioral changes the program calls for.
The PSMF should be considered when more conventional low-calorie approaches to weight loss fail or when patients become discouraged by the slower results seen with traditional diets.8 Patients undergoing a PSMF are usually encouraged by the initial period of rapid weight loss, and such diets have lower dropout rates.16
This diet may also be recommended for obese patients who have poorly controlled type 2 diabetes and growing resistance to medications, to bring down the blood glucose level. Another use is before bariatric surgery to reduce the risk of obesity-related complications.8 Patients who regain weight after bariatric surgery may also benefit.
MEAL REPLACEMENTS OR A DIET PLAN?
The PSMF program at Cleveland Clinic is based on modified preparation and selection of conventional foods. Details of the program are described in Table 1. Protein sources must be of high biologic value, containing the right mix of essential amino acids (eg, lean meat, fish, poultry, egg whites).9
Some commercially available very-low-calorie diets (eg, OPTIFAST, Medifast) that are advertised as PSMFs consist mainly of meal replacements. In the program at Cleveland Clinic, meal replacements in the form of commercial high-protein shakes or bars can be used occasionally for convenience and to maintain adherence to the diet.
However, preparation of PSMF meals from natural, conventional foods is thought to play an important role in long-term behavior modification and so is strongly encouraged. Patients learn low-fat cooking methods, portion control, and how to make appropriate choices in shopping, eating, and dining out. These lessons are valuable for those who struggle with long-term weight loss. Learning these behaviors through the program may help ease the transition to the weight-maintenance phase and beyond. For some patients, cooking is also a source of enjoyment, as is the sight, smell, and taste of nonliquid foods.10
In addition, patients appreciate being able to eat the same foods as others in their household, except for omitting high-carbohydrate foods. It has also been reported that patients on a food-based PSMF were significantly less hungry and preoccupied with eating than those on a liquid formula diet.17
CONTRAINDICATIONS AND SAFETY CONCERNS
Contraindications to the PSMF include a BMI less than 27 kg/m2, recent myocardial infarction, angina, significant arrhythmia, decompensated congestive heart failure, cerebrovascular insufficiency or recent stroke, end-stage renal disease, liver failure, malignancy, major psychiatric illness, pregnancy or lactation, and wasting disorders. It is also not recommended for patients under age 16 or over age 65.
In view of the risk of diabetic ketoacidosis and the difficulty of titrating required doses ofinsulin, patients with type 1 diabetes mellitus are usually not advised to undergo a low-carbohydrate or very-low-calorie diet.8,12 However, we and others have found that the PSMF can be used in some obese patients with type 1 diabetes if it is combined with appropriate education and careful monitoring.12
Major concerns about the safety of the PSMF stem from experiences with the first very-low-calorie diets in the 1970s, which were associated with fatal cardiac arrhythmias and sudden death.18 These early diets used liquid formulas with hydrolyzed collagen protein of poor biologic value and were deficient in many vitamins and minerals. Today’s very-low-calorie diets use protein sources of high biologic value (chiefly animal, soy, and egg for the PSMF) and are supplemented with necessary vitamins and minerals, reducing the risk of electrolyte and cardiac abnormalities.9,19,20 Furthermore, before starting the PSMF all patients must have an electrocardiogram to be sure they have no arrhythmias (eg, heart block, QT interval prolongation) or ischemia.
Relative contraindications
A known history of cholelithiasis is a relative contraindication to a very-low-calorie diet and may be of concern for some patients and providers. While obesity itself is already a risk factor for gallstones, gallstone formation has also been associated with bile stasis, which occurs from rapid weight loss with liquid formula diets of low fat intake (< 10 g/day).21 However, in the PSMF, fat intake from protein sources, though low (45–70 g/day), is considered high enough to allow adequate gallbladder contraction, thus decreasing the risk of gallstone formation.22
Gout is another relative contraindication, as hyperuricemia with risk of gout is also linked to high-protein diets.9 Palgi et al23 found that uric acid levels rose by a mean of 0.4 mg/dL during the diet. The risk of gout, however, seemed to be small, occurring in fewer than 1% of patients in the study. Furthermore, in a recent study by Li et al,24 uric acid levels were found to significantly decrease in patients on a high-protein, very-low-calorie diet. Nonetheless, uric acid levels should be monitored regularly in patients on the PSMF.
SIDE EFFECTS OF THE DIET
Common side effects of the PSMF include headache, fatigue, orthostatic hypotension, muscle cramps, cold intolerance, constipation, diarrhea, fatigue, halitosis, menstrual changes, and hair thinning. Most of these are transient and may be alleviated by adjusting fluid, salt, and supplement intake. Other side effects may disappear as the patient is weaned off the diet.8,9
REGULAR FOLLOW-UP WITH HEALTH CARE PROVIDERS
Current PSMF programs are considered safe when used in combination with regular follow-up with health care providers.8,12
At Cleveland Clinic, patients meet with a dietitian twice in the first month and monthly thereafter (or more frequently if needed) for weight monitoring and education on nutrition and behavior modification (Table 1). Since the PSMF does not provide complete nutrition, daily supplementation with vitamins and minerals is required.
Daily exercise is encouraged throughout the program to increase fitness and to help keep the weight off during the refeeding phase and after.
Patients also meet every 6 to 8 weeks with the referring nurse practitioner or physician for further monitoring and evaluation of vital signs, laboratory results, and side effects. The PSMF protocol at Cleveland Clinic enables both primary care physicians and specialists (including nurse practitioners) within our network to monitor the patient’s status. Use of a common electronic medical record system is particularly valuable for easy communication between providers. If a primary care physician feels unable to appropriately counsel and supervise a patient in the PSMF program, referral to an endocrinologist or weight loss specialist is recommended.
In addition to baseline electrocardiography and monitoring of uric acid levels, a comprehensive metabolic panel is drawn at baseline, twice in the first month, and monthly thereafter to check for electrolyte imbalances and metabolic and tissue dysfunction such as dehydration, excessive protein loss, and liver or kidney injury.
Patients should not attempt the PSMF without medical supervision. Many patients have friends or family members who want to try the PSMF along with them, but this can be dangerous, especially for those with hypertension or type 2 diabetes. The medications prescribed for these conditions can result in hypotension or hypoglycemia during the PSMF.
Although there are no standard guidelines for adjusting medication use before starting a patient on the PSMF, it is logical to taper off or discontinue antihypertensive agents in patients with tightly controlled hypertension to avoid possible dehydration and hypotension during the first few diuresis-inducing weeks of the diet. In particular, diuretic agents should be discontinued to prevent further electrolyte imbalance and fluid shifts.
Similarly, in patients with tightly controlled type 2 diabetes (hemoglobin A1c < 7.0%), oral hypoglycemic agents and insulin therapy should be reduced before starting the diet to avoid potential hypoglycemia. During the course of the diet, providers should then adjust medication dosages based on follow-up vital signs and laboratory results and daily glucose monitoring.8
EFFECTS OF THE PSMF IN PATIENTS WITH TYPE 2 DIABETES
Though few formal studies have been done, the PSMF may have major effects on hyperglycemia, cardiovascular risk factors, and diabetic nephropathy in obese patients with type 2 diabetes, at least in the short term (Table 2).
Weight loss
In one of the first PSMF studies,23 in 668 patients with or without type 2 diabetes (baseline weight 98 kg), the mean weight loss was 21 kg after the intensive phase and 19 kg by the end of the refeeding phase.
In another observational report,25 25% to 30% of patients lost even more weight, averaging 38.6 kg of weight loss. Typically, the higher the baseline weight, the greater the weight loss during the PSMF.23
Patients with type 2 diabetes lost a similar amount of weight (8.5 kg) compared with those without diabetes (9.4 kg, P = .64) in a study of meal-replacement PSMF (using OPTIFAST shakes and bars).26 In a large meal-replacement study of 2,093 patients, Li et al24 found that weight loss was similar between diabetic, prediabetic, and nondiabetic patients. Weight loss was also closely maintained in those patients who stayed on the diet for 12 months.
In a PSMF study in which all the participants had type 2 diabetes, the mean weight loss was 18.6 kg. Although the patients regained some of this weight, at 1 year they still weighed 8.6 kg less than at baseline. However, a conventional, balanced, low-calorie diet resulted in similar amounts of weight loss after 1 year.27 Furthermore, a second round of the PSMF did not result in significant additional weight loss but rather weight maintenance.28
Fat loss and smaller waist circumference
Most of the weight lost during a PSMF is from fat tissue.11,26 Abdominal (visceral) fat may be lost first, which is desirable for patients with type 2 diabetes, since a higher degree of abdominal fat is linked to insulin resistance.2,29
After a meal-replacement PSMF, waist circumference decreased significantly in patients both with and without type 2 diabetes.24,26 However, in one study, less fat was lost per unit of change of BMI in the group with type 2 diabetes than in the nondiabetic group.26 Since insulin inhibits lipolysis, it is possible that exogenous insulin use in diabetic patients may prevent greater reductions in fat mass, though this is likely not the only mechanism.26
Lower fasting serum glucose
Fasting serum glucose levels decreased significantly from baseline in patients with type 2 diabetes after a PSMF in all studies that measured this variable.23–28,30,31 Changes in fasting glucose are immediate and are associated with caloric restriction rather than weight loss itself.30,32 Furthermore, the observed decrease in serum glucose is even more impressive in view of the withdrawal or reduction of doses of insulin and oral hypoglycemic agents before starting the diet.
In a study that compared glycemic control in a PSMF diet vs a balanced low-calorie diet, the fasting serum glucose in the PSMF group declined 46%, from 255.9 mg/dL at baseline to 138.7 mg/dL at 20 weeks (P = .001). After 1 year, it had risen back to 187.4 mg/dL, which was still 27% lower than at baseline (P = .023). These results compared favorably with those in the low-calorie diet group (P < .05), which saw fasting serum glucose decline 27% after 20 weeks (from 230.6 mg/dL at baseline to 167.6 mg/dL) and then rise to 5% over baseline (243.2 mg/dL) after 1 year.27
In a later study, the decrease in fasting serum glucose was not maintained at 1 year, but a significantly higher percentage (55%) of participants in the PSMF group were still able to remain free of diabetic medications compared with those who followed a balanced low-calorie diet (31%, P = .01).28
Decrease in hemoglobin A1c
Declines in fasting serum glucose corresponded with short-term declines in hemoglobin A1c in several reports.27–31 Hemoglobin A1c declined significantly from an average of 10.4% to 7.3% (P = .001) after PSMF intervention in patients with type 2 diabetes. In contrast, hemoglobin A1c in the low-calorie diet control group declined from 10.4% to 8.6%.27 One year later, hemoglobin A1c remained lower than at baseline in the PSMF group (final 9.2%) and continued to compare favorably against the control group (final 11.8%, between-group P = .001). However, these 1-year post-intervention improvements were not seen in a second, more intensive study.28
Less insulin resistance
In several studies, fasting serum insulin levels declined along with serum glucose levels, implying decreased insulin resistance.25,27,28,30,31 In addition, insulin output was enhanced during glucose challenge after completion of the PSMF, suggesting possible improved (though still impaired) pancreatic beta-cell capacity.25,27,30
Improved lipid profile
The most common effect of the PSMF on the lipid profile is a significant decrease in triglycerides in patients both with and without type 2 diabetes.8,23,24,28 In addition, high-density lipoprotein cholesterol increased in two studies following PSMF intervention or after 1-year of follow-up.24,27,28 Total cholesterol and low-density lipoprotein cholesterol levels also improved after the PSMF, but these changes were not always maintained at follow-up visits.8,24,28
Lower blood pressure
Improvements in both systolic and diastolic blood pressure were noted in two studies, with mean decreases of 6 mm Hg to 13 mm Hg systolic and 8 mm Hg diastolic after PSMF intervention.23,28 In a third study, reductions in blood pressure were less dramatic, and only changes in diastolic but not systolic blood pressure remained significant at 12 months.24 While improvements were not observed in a fourth study, patients in this study also had impaired kidney function caused by diabetic nephropathy, and changes in medication were not taken into account.31
Kidney function tests
In a small study, Friedman et al showed that 12 weeks of the PSMF in six patients with advanced diabetic nephropathy (stage 3B or stage 4 chronic kidney disease) led to a loss of 12% of body weight (P = .03) as well as significant reductions in serum creatinine and cystatin C levels (P < .05).31 In addition, albuminuria decreased by 30% (P = .08). Side effects were minimal, and the diet was well tolerated despite its high protein content, which is a concern in patients with impaired kidney function.
Thus, weight loss via the PSMF may still be beneficial in type 2 diabetic patients with chronic kidney disease and may even improve the course of progression of diabetic nephropathy.
Long-term weight loss is elusive
Long-term weight loss has been an elusive goal for many diet programs. In a study using a very-low-calorie diet in obese patients with type 2 diabetes, substantial weight loss was maintained in half of the patients at 3 years after the intervention, but nearly all of the patients had regained most of their weight after 5 years.33
While commitment to behavior modification, maintenance of physical activity, and continued follow-up are all critical factors in sustaining weight loss, new and innovative approaches to battle weight regain are needed.34
Yet despite considerable weight regain in most patients, the Look AHEAD (Action for Health in Diabetes) study showed that participants in intensive lifestyle intervention programs still achieved greater weight loss after 4 years than those receiving standard care.35 Whether this holds true for those in intensive PSMF programs is unknown. In addition, conclusive PSMF studies regarding glycemic control, lipids, and blood pressure beyond 1 year of follow-up are lacking.
A VIABLE OPTION FOR MANY
Adherence to a very-low-calorie, ketogenic PSMF program results in major short-term health benefits for obese patients with type 2 diabetes. These benefits include significant weight loss, often more than 18 kg, within 6 months.23–28 In addition, significant improvements in fasting glucose23–28,30–32 and hemoglobin A1c levels27–31 are linked to the caloric and carbohydrate restriction of the PSMF. Insulin resistance was also attenuated, with possible partial restoration of pancreatic beta-cell capacity.25,27,28,30,31 In some studies, the PSMF resulted in lower systolic and diastolic blood pressure23,24,28 and triglyceride levels.8,23,24,28 One small study also suggested a possible improvement of diabetic nephropathy.31 Lastly, improvements in glycemia and hypertension were associated with a reduction in the need for antidiabetic and antihypertensive drugs.36
Still, weight loss and many of the associated improvements partially return to baseline levels 1 year after the intervention. Thus, more long-term studies are needed to explore factors for better weight maintenance after the PSMF.
Also, only a few studies have compared the effect of the PSMF between patients with or without type 2 diabetes. One study suggested that fat loss may be reduced in patients with type 2 diabetes.26
In conclusion, despite some risks and safety concerns, PSMF is a viable option for many obese, type 2 diabetic patients as a method of short-term weight loss, with evidence for improvement of glycemic control and cardiovascular risk factors for up to 1 year. To strengthen support for the PSMF, however, further research is warranted on the diet’s long-term effects in patients with type 2 diabetes and also in nondiabetic patients.
Acknowledgments: Many thanks to Cheryl Reitz, RD, LD, CDE, and Dawn Noe, RD, LD, CDE, for providing their expertise on the PSMF protocols carried out at Cleveland Clinic. Additional thanks to Tejas Kashyap for his initial assistance with this review.
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006; 12:75–80.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106:473–481.
- Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345:790–797.
- Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011; 378:129–139.
- Lindström J, Louheranta A, Mannelin M, et al; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003; 26:3230–3236.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012; 308:2489–2496.
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991; 14:802–823.
- Bistrian BR. Clinical use of a protein-sparing modified fast. JAMA 1978; 240:2299–2302.
- Walters JK, Hoogwerf BJ, Reddy SS. The protein-sparing modified fast for obesity-related medical problems. Cleve Clin J Med 1997; 64:242–244.
- Van Gaal LF, Snyders D, De Leeuw IH, Bekaert JL. Anthropometric and calorimetric evidence for the protein sparing effects of a new protein supplemented low calorie preparation. Am J Clin Nutr 1985; 41:540–544.
- Baker S, Jerums G, Proietto J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract 2009; 85:235–242.
- Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by ß-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339:211–214.
- Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008; 87:44–55.
- Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012; 108(suppl 2):S105–S112.
- Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr 2012; 96:953–961.
- Wadden TA, Stunkard AJ, Brownell KD, Day SC. A comparison of two very-low-calorie diets: protein-sparing-modified fast versus protein-formula-liquid diet. Am J Clin Nutr 1985; 41:533–539.
- Isner JM, Sours HE, Paris AL, Ferrans VJ, Roberts WC. Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation 1979; 60:1401–1412.
- Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 1986; 35:155–164.
- Seim HC, Mitchell JE, Pomeroy C, de Zwaan M. Electrocardiographic findings associated with very low calorie dieting. Int J Obes Relat Metab Disord 1995; 19:817–819.
- Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 2014; 38:279–284.
- Festi D, Colecchia A, Orsini M, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998; 22:592–600.
- Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: results in 668 outpatients. Am J Public Health 1985; 75:1190–1194.
- Li Z, Tseng CH, Li Q, Deng ML, Wang M, Heber D. Clinical efficacy of a medically supervised outpatient high-protein, low-calorie diet program is equivalent in prediabetic, diabetic and normoglycemic obese patients. Nutr Diabetes 2014 Feb 10; 4:e105.
- Genuth S. Supplemented fasting in the treatment of obesity and diabetes. Am J Clin Nutr 1979; 32:2579–2586.
- Baker ST, Jerums G, Prendergast LA, Panagiotopoulos S, Strauss BJ, Proietto J. Less fat reduction per unit weight loss in type 2 diabetic compared with nondiabetic obese individuals completing a very-low-calorie diet program. Metabolism 2012; 61:873–882.
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 1991; 151:1334–1340.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994; 97:354–362.
- Kawamura II, Chen CC, Yamazaki K, Miyazawa Y, Isono K. A clinical study of protein sparing modified fast (PSMF) administered preoperatively to morbidly obese patients: comparison of PSMF with natural food products to originally prepared PSMF. Obes Surg 1992; 2:33–40.
- Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 1984; 77:7–17.
- Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013; 8:1892–1898.
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17:30–36.
- Paisey RB, Frost J, Harvey P, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002; 15:121–127.
- Blackburn GL. Weight of the nation: moving forward, reversing the trend using medical care. Am J Clin Nutr 2012; 96:949–950.
- Wadden TA, Neiberg RH, Wing RR, et al; Look AHEAD Research Group. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011; 19:1987–1998.
- Redmon JB, Bertoni AG, Connelly S, et al; Look AHEAD Research Group. Effect of the Look AHEAD intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010; 33:1153–1158.
- Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat Med 2006; 12:75–80.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000; 106:473–481.
- Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001; 345:790–797.
- Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011; 378:129–139.
- Lindström J, Louheranta A, Mannelin M, et al; Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003; 26:3230–3236.
- Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403.
- Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA 2012; 308:2489–2496.
- Henry RR, Gumbiner B. Benefits and limitations of very-low-calorie diet therapy in obese NIDDM. Diabetes Care 1991; 14:802–823.
- Bistrian BR. Clinical use of a protein-sparing modified fast. JAMA 1978; 240:2299–2302.
- Walters JK, Hoogwerf BJ, Reddy SS. The protein-sparing modified fast for obesity-related medical problems. Cleve Clin J Med 1997; 64:242–244.
- Van Gaal LF, Snyders D, De Leeuw IH, Bekaert JL. Anthropometric and calorimetric evidence for the protein sparing effects of a new protein supplemented low calorie preparation. Am J Clin Nutr 1985; 41:540–544.
- Baker S, Jerums G, Proietto J. Effects and clinical potential of very-low-calorie diets (VLCDs) in type 2 diabetes. Diabetes Res Clin Pract 2009; 85:235–242.
- Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by ß-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013; 339:211–214.
- Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am J Clin Nutr 2008; 87:44–55.
- Westerterp-Plantenga MS, Lemmens SG, Westerterp KR. Dietary protein—its role in satiety, energetics, weight loss and health. Br J Nutr 2012; 108(suppl 2):S105–S112.
- Hemmingsson E, Johansson K, Eriksson J, Sundström J, Neovius M, Marcus C. Weight loss and dropout during a commercial weight-loss program including a very-low-calorie diet, a low-calorie diet, or restricted normal food: observational cohort study. Am J Clin Nutr 2012; 96:953–961.
- Wadden TA, Stunkard AJ, Brownell KD, Day SC. A comparison of two very-low-calorie diets: protein-sparing-modified fast versus protein-formula-liquid diet. Am J Clin Nutr 1985; 41:533–539.
- Isner JM, Sours HE, Paris AL, Ferrans VJ, Roberts WC. Sudden, unexpected death in avid dieters using the liquid-protein-modified-fast diet. Observations in 17 patients and the role of the prolonged QT interval. Circulation 1979; 60:1401–1412.
- Henry RR, Wiest-Kent TA, Scheaffer L, Kolterman OG, Olefsky JM. Metabolic consequences of very-low-calorie diet therapy in obese non-insulin-dependent diabetic and nondiabetic subjects. Diabetes 1986; 35:155–164.
- Seim HC, Mitchell JE, Pomeroy C, de Zwaan M. Electrocardiographic findings associated with very low calorie dieting. Int J Obes Relat Metab Disord 1995; 19:817–819.
- Johansson K, Sundström J, Marcus C, Hemmingsson E, Neovius M. Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 2014; 38:279–284.
- Festi D, Colecchia A, Orsini M, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998; 22:592–600.
- Palgi A, Read JL, Greenberg I, Hoefer MA, Bistrian BR, Blackburn GL. Multidisciplinary treatment of obesity with a protein-sparing modified fast: results in 668 outpatients. Am J Public Health 1985; 75:1190–1194.
- Li Z, Tseng CH, Li Q, Deng ML, Wang M, Heber D. Clinical efficacy of a medically supervised outpatient high-protein, low-calorie diet program is equivalent in prediabetic, diabetic and normoglycemic obese patients. Nutr Diabetes 2014 Feb 10; 4:e105.
- Genuth S. Supplemented fasting in the treatment of obesity and diabetes. Am J Clin Nutr 1979; 32:2579–2586.
- Baker ST, Jerums G, Prendergast LA, Panagiotopoulos S, Strauss BJ, Proietto J. Less fat reduction per unit weight loss in type 2 diabetic compared with nondiabetic obese individuals completing a very-low-calorie diet program. Metabolism 2012; 61:873–882.
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very-low-calorie diet on long-term glycemic control in obese type 2 diabetic subjects. Arch Intern Med 1991; 151:1334–1340.
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year-long weight loss treatment for obese patients with type II diabetes: does including an intermittent very-low-calorie diet improve outcome? Am J Med 1994; 97:354–362.
- Kawamura II, Chen CC, Yamazaki K, Miyazawa Y, Isono K. A clinical study of protein sparing modified fast (PSMF) administered preoperatively to morbidly obese patients: comparison of PSMF with natural food products to originally prepared PSMF. Obes Surg 1992; 2:33–40.
- Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 1984; 77:7–17.
- Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013; 8:1892–1898.
- Wing RR, Blair EH, Bononi P, Marcus MD, Watanabe R, Bergman RN. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care 1994; 17:30–36.
- Paisey RB, Frost J, Harvey P, et al. Five year results of a prospective very low calorie diet or conventional weight loss programme in type 2 diabetes. J Hum Nutr Diet 2002; 15:121–127.
- Blackburn GL. Weight of the nation: moving forward, reversing the trend using medical care. Am J Clin Nutr 2012; 96:949–950.
- Wadden TA, Neiberg RH, Wing RR, et al; Look AHEAD Research Group. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011; 19:1987–1998.
- Redmon JB, Bertoni AG, Connelly S, et al; Look AHEAD Research Group. Effect of the Look AHEAD intervention on medication use and related cost to treat cardiovascular disease risk factors in individuals with type 2 diabetes. Diabetes Care 2010; 33:1153–1158.
KEY POINTS
- The PSMF is indicated in patients who have a body mass index (BMI) of 30 kg/m2 or more, or a BMI of 27 kg/m2 or more with one or more comorbidities such as type 2 diabetes.
- The PSMF provides less than 800 kcal/day during an initial intensive phase of about 6 months, with gradual reintroduction of calories during a refeeding phase lasting 6 to 8 weeks.
- Patients on the PSMF under medical supervision rapidly lose fat while maintaining lean body mass.
- Unfortunately, many patients tend to regain weight after completing a PSMF program. Additional strategies are needed to maintain weight loss.