User login
A systematic approach to chronic abnormal uterine bleeding
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.
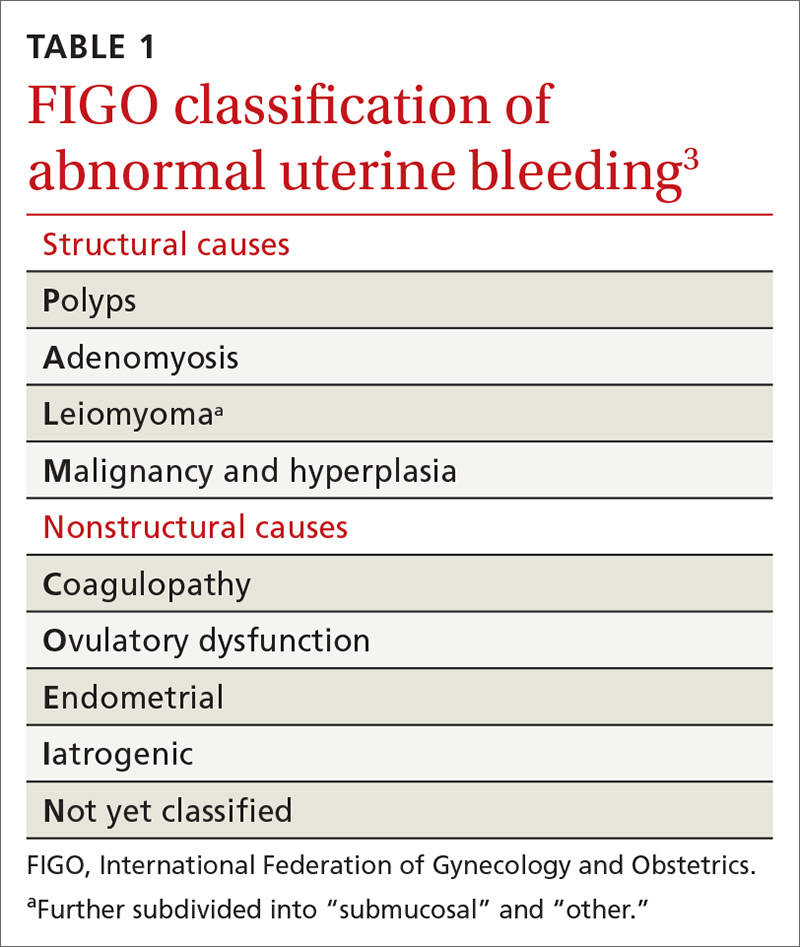
Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).
The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1
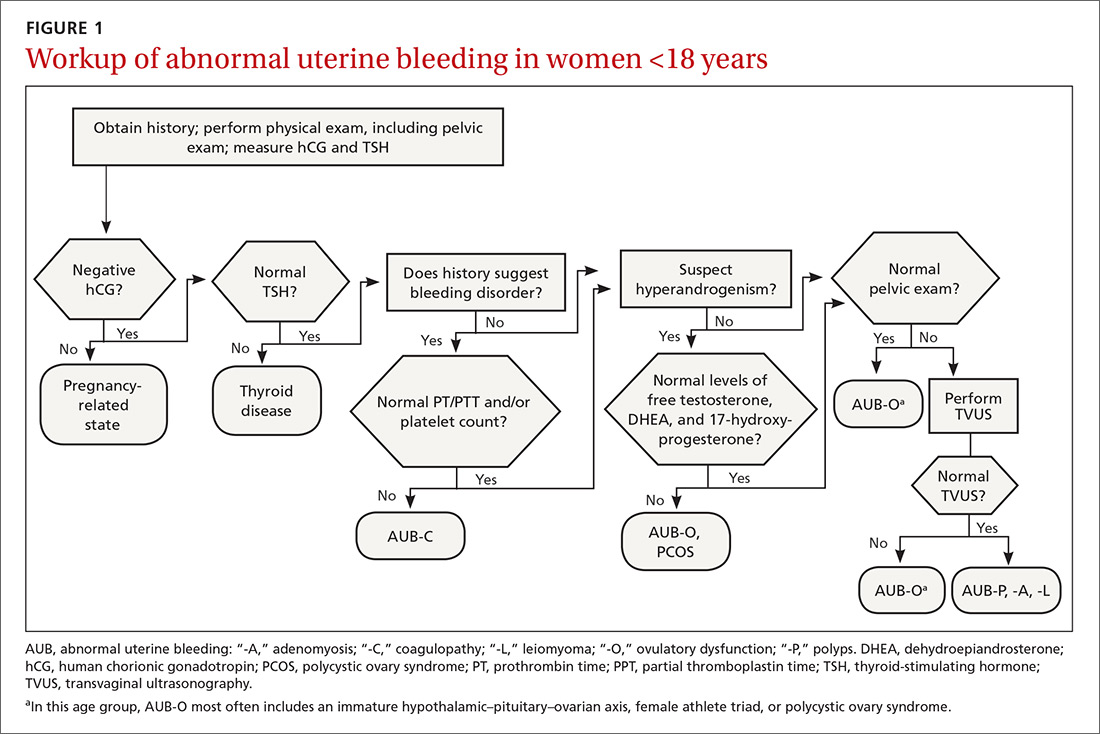
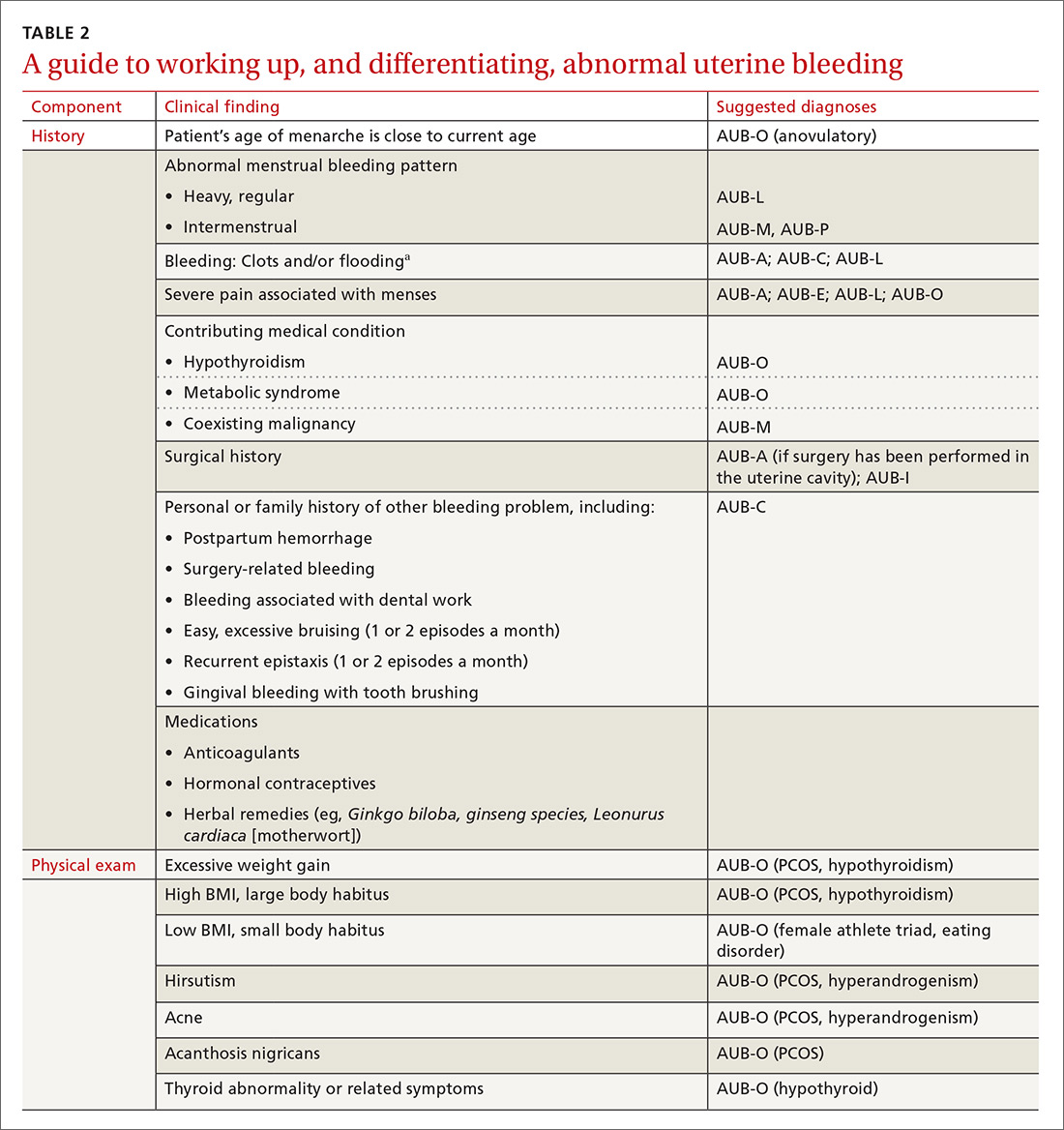
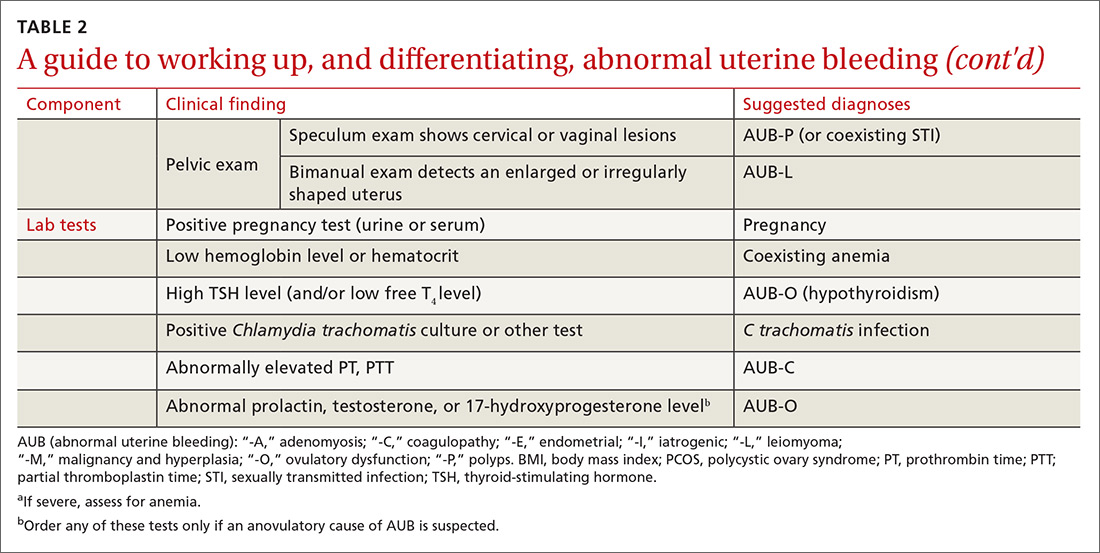
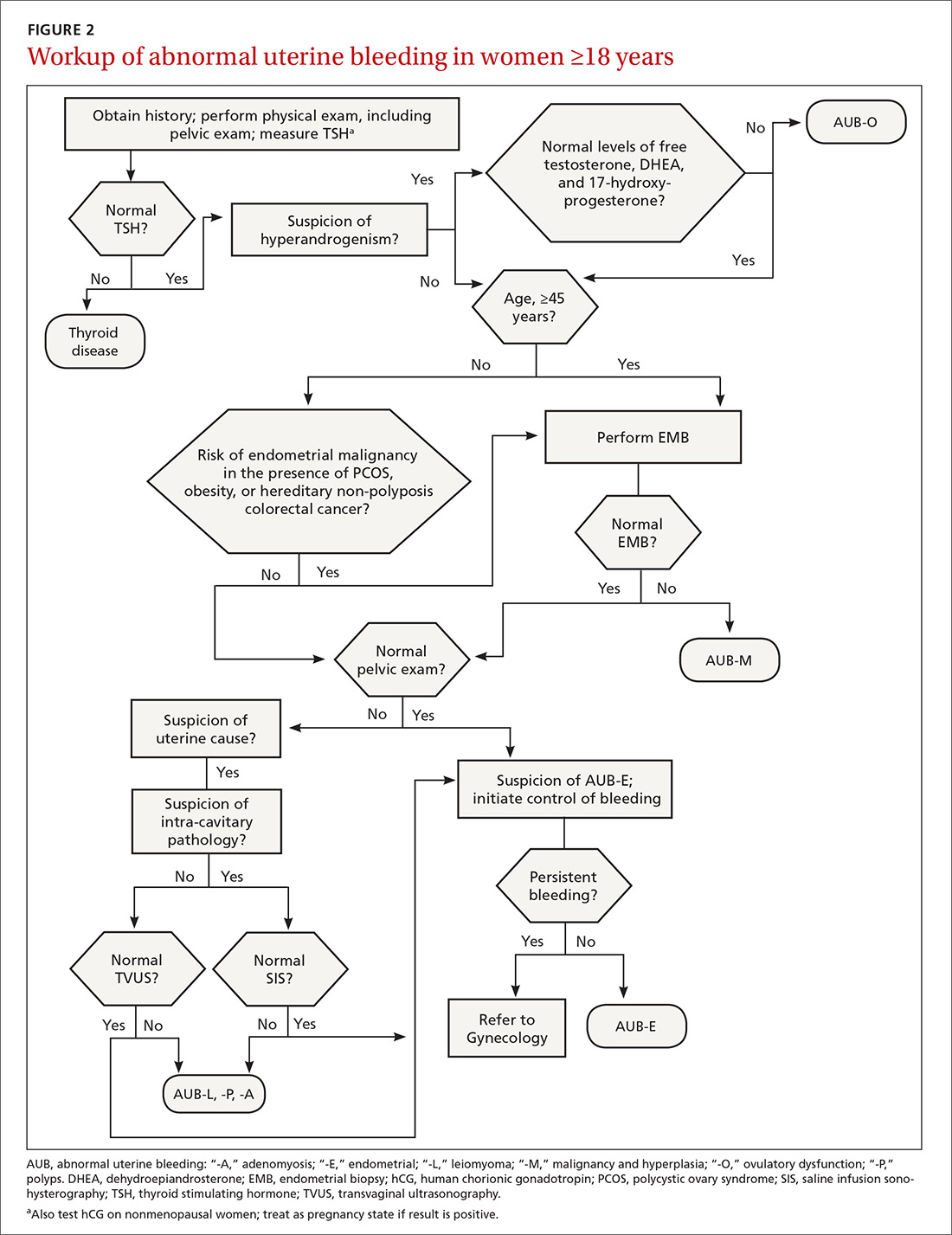
The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).
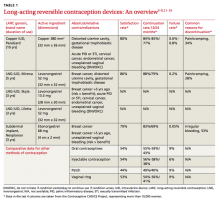
We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).

CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
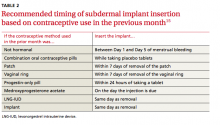
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; Mjordahl-iafrato@ecommunity.com.
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.

Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).


The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1

The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).

We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).

CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; Mjordahl-iafrato@ecommunity.com.
Menstrual bleeding is considered normal when it occurs regularly (every 21-35 days), lasts 4 to 8 days, and is not associated with heavy bleeding.1 During the first few years after menarche, it is normal for girls to experience irregular menstrual cycles but, by the third year, 60% to 80% of girls have an adult pattern of menstrual bleeding.2
Menstrual flow without normal volume, duration, regularity, or frequency is considered abnormal uterine bleeding (AUB). The condition is considered acute if there is need for immediate intervention. In the absence of the need for immediate intervention, recurrent AUB is classified as chronic.3 Chronic AUB is the focus of this article.

Invaluable tool: The FIGO classification
In 2011, the
- structural causes, recalled by “PALM” (Polyps, Adenomyosis, Leiomyoma, and Malignancy/hyperplasia)
- nonstructural causes, recalled by “COEIN” (Coagulopathy, Ovulatory dysfunction, Endometrial, Iatrogenic, and Not yet classified).


The PALM–COEIN system also uses descriptive terminology (heavy bleeding, intermenstrual bleeding) to characterize the bleeding pattern.3 The American College of Obstetricians and Gynecologists has adopted this classification system and recommends that such historically used terminology as “dysfunctional uterine bleeding,” “menorrhagia,” and “metrorrhagia” be abandoned.1

The initial workup of all causes of chronic uterine bleeding begins with a history; physical examination, including pelvic exam; and laboratory testing, including a urine pregnancy test, complete blood count, and a test of thyroid-stimulating hormone (TABLE 2). The need for additional laboratory testing, imaging, or endometrial biopsy depends on the suspected cause of AUB, detailed stepwise in FIGURE 1 (women <18 years) and FIGURE 2 (≥18 years).

We first briefly review the 9 categories of AUB in the PALM–COEIN system; discuss the most common causes in more detail; and review common treatment options (TABLE 3).

CASE 1
Marsha R, a 41-year-old-woman, complains of heavy menstrual bleeding for the past year that has become worse over the past 2 months. Her menstrual cycles have occurred every 28 days and last 10 days; she uses 10 to 12 pads a day.
Continue to: Recently, Ms. R reports...
Recently, Ms. R reports, she has been bleeding continuously for 14 days, with episodes of lighter bleeding followed by heavier bleeding. She also complains of fatigue.
Bimanual examination is notable for an enlarged uterus.
How would you proceed with the workup of this patient, to determine the cause of her bleeding and tailor management accordingly?
Structural AUB: The “PALM” mnemonic
A structural cause of AUB must be considered when you encounter an abnormality on physical exam (TABLE 1).3 In obese women or other patients in whom the physical exam is difficult, historical clues—including postcoital bleeding, intermenstrual bleeding, or pelvic pain or pressure—also suggest a structural abnormality.4
Transvaginal ultrasonography (TVUS) is the initial method of evaluation when a structural abnormality is suspected.1,4 However, although TVUS is excellent at visualizing the myometrium, lesions within the uterine cavity can be missed. If intracavitary pathology, such as submucosal fibroids or endometrial polyps, is suspected, additional imaging with saline infusion sonohysterography (SIS) should be performed. If a cavitary abnormality is confirmed, hysteroscopy is indicated.1 Magnetic resonance imaging (MRI) is reserved for cases in which a uterine cavity abnormality is found on TVUS but cannot be further characterized by SIS or hysteroscopy.1
Continue to: Endometrial biopsy...
Endometrial biopsy (EMB) is indicated as part of the initial evaluation of AUB in all women >45 years and in younger women who have risk factors for endometrial cancer, including polycystic ovary syndrome (PCOS), obesity, and hereditary nonpolyposis colorectal cancer. Such biopsy is necessary in these women whether or not another condition is the cause of the AUB and regardless of findings on TVUS.1-4 Endometrial biopsy should also be performed in women with AUB that persists despite medical management. If office EMB is nondiagnostic, hysteroscopy or SIS can be used to obtain tissue samples for further evaluation.5
Polyps. An endometrial polyp is a benign growth of endometrial tissue that is covered with epithelial cells. Polyps are often diagnosed by EMB or TVUS when these techniques are performed as part of the workup for AUB.6 Endometrial polyps are found more commonly in postmenopausal women, but should be considered as a cause of AUB in premenopausal women, too, especially those with intermenstrual bleeding or postcoital bleeding (or both) that is unresponsive to medical management.7 Risk factors for polyps include older age, obesity, and treatment with tamoxifen.7 The usual treatment for symptomatic endometrial polyps is removal by operative hysteroscopy.7
Adenomyosis. Ectopic endometrial tissue in the myometrium that leads to hypertrophy of the myometrium and uterine enlargement is known as adenomyosis. The disorder is most often diagnosed in women 40 to 50 years of age, who commonly complain of heavy uterine bleeding (40%-60% of cases) and dysmenorrhea (65%).8 Although definitive diagnosis is made histologically at hysterectomy, TVUS and MRI can be useful tools to help narrow the differential diagnosis in women with unexplained AUB.
According to a systematic review,9 the sensitivity and specificity of imaging in the diagnosis of adenomyosis is 72% and 81%, respectively, for TVUS and 77% and 89%, respectively, for MRI. Needle biopsy, performed hysteroscopically or laparoscopically, is less useful because the technique has low sensitivity (reported variously as 8%-56%) in diagnosing adenomyosis.8
Treatment options for adenomyosis are medical management with agents that reduce bleeding (eg, a combination oral contraceptive [OC], nonsteroidal anti-inflammatory drugs [NSAIDs], the antifibrinolytic tranexamic acid, and, when there is no distortion of the uterine cavity, a levonorgestrel intrauterine device [LNG-IUD]); uterine artery embolization; and hysterectomy.8
Continue to: Leiomyoma
Leiomyoma. Uterine fibroids, or leiomyomas, are benign, fibromuscular solid tumors, thought to be hormone-dependent because many regress after menopause. In women of reproductive age, uterine fibroids are the most common cause of structural AUB, with a cumulative incidence of 70% to 80% among women in this age group.3,10 Fibroids are more common in African-American women, women who experienced early menarche, and women who are obese, have PCOS, or had a late first pregnancy.3-10
Many fibroids are asymptomatic, and are found incidentally on sonographic examination performed for other reasons; in one-third of affected patients, the fibroids result in heavy menstrual bleeding.10 Intermenstrual bleeding and postcoital bleeding can occur, but are not common symptoms with fibroids. Consider other causes of AUB, such as endometrial polyps, when these symptoms are present.
Treatment of fibroids is medical or surgical. Medical management is a reasonable first-line option, especially in women who have not completed childbearing and who have small (<3 cm in diameter) fibroids. Options include a combination OC, NSAIDs, tranexamic acid, and, when the uterine cavity is not distorted, an LNG-IUD.4,10,11
For women with larger fibroids, those for whom the aforementioned medical treatments are unsuccessful, and those who are seeking more definitive treatment, uterine artery embolization, myomectomy, or hysterectomy can be considered.
› Uterine artery embolization is performed by an interventional radiologist under local anesthesia and, if necessary, moderate sedation.12 After the procedure, fibroids decrease in size due to avascular necrosis, but the remainder of the myometrium is relatively unaffected because collateral blood supply develops.13,14 Patients might experience abdominal cramping for 2 or 3 days following the procedure, which can be managed with an oral NSAID.12 Approximately 90% of women treated with embolization note improvement in AUB by 3 months after the procedure.15 Uterine artery embolization is not recommended in women who have not completed childbearing.12,16,17
Continue to: Myomectomy
› Myomectomy (removal of the leiomyoma) is the surgical treatment of choice for women who want to maintain fertility. Depending on the size and location of the fibroid(s), myomectomy can be performed as an open surgical procedure, laparoscopically, or hysteroscopically. At the discretion of the surgeon, leuprolide acetate, a gonadotropin-releasing hormone agonist, can be prescribed for 3 months before myomectomy to reduce intraoperative blood loss by decreasing the vascularity of the fibroids.4,18 Reduction in bleeding is reported in 70% to 90% of patients who undergo myomectomy.19
› Hysterectomy, the definitive treatment for uterine fibroids, should be reserved for women who have completed childbearing and who have failed (or have a contraindication to) other treatment options.
Malignancy/hyperplasia. EMB should be performed when endometrial malignancy/hyperplasia is suspected. As noted, endometrial cancer should be considered as a diagnostic possibility in women >45 years, in younger women with risk factors, and in women who have failed to respond to medical treatment for other suspected causes of AUB.5
When hyperplasia without atypia is diagnosed, the LNG-IUD or oral progesterone is an acceptable treatment option; note that fewer women who have an LNG-IUD eventually require hysterectomy, compared to women who take oral hormone therapy for AUB.20 When hyperplasia with atypia is diagnosed, hysterectomy is the treatment of choice. If a woman wishes to maintain fertility, however, oral progesterone therapy can be offered.21
When the diagnosis is cancer, the patient should be referred to a gynecologic oncologist for staging and treatment. Treatment varies depending on stage, but generally requires hysterectomy including bilateral salpingo-oophorectomy, with possible chemotherapy or radiation, or both.22
Continue to: CASE 1
CASE 1
Ms. R undergoes a sonogram that reveals a 4-cm fibroid in the uterine fundus that has not distorted the uterine cavity. Although she has completed childbearing, Ms. R is not interested in a surgical procedure at this time. You recommend insertion of an LNG-IUD; she accepts your advice.
CASE 2
Claire G, 27 years old, with a body mass index of 41,* complains of irregular menses for several months. Her menstrual cycle is irregular, as is the duration of menses and amount of bleeding. She has some mild fatigue without dizziness.
The physical exam is notable for mild hirsutism, without abnormalities on pelvic examination. Lab testing reveals iron-deficiency anemia; a pregnancy test is negative.
The questions that were raised by Ms. R’s case challenge you here, too: What is the appropriate workup of Ms. G’s bleeding? Once the cause is confirmed, how should you treat her?
Nonstructural AUB: The “COEIN” mnemonic
In the absence of abnormalities on a pelvic exam, and after excluding endometrial malignancy/hyperplasia in patients with the aforementioned risk factors, a nonstructural cause of AUB should be considered (TABLE 1).3 In women 20 to 40 years of age, the primary common cause of nonstructural uterine bleeding is ovulatory dysfunction, most often caused by PCOS or anovulatory bleeding.
Continue to: For nonstructual causes of AUB...
For nonstructural causes of AUB, the recommended laboratory workup varies with the suspected diagnosis. In addition, recently pregnant women should have a quantitative assay of β human chorionic gonadotropin to evaluate for trophoblastic disease.5,23
Imaging is not usually recommended when the cause of AUB is suspected to be nonstructural. However, when PCOS is suspected, TVUS can be used to confirm the presence of polycystic ovaries.23
As noted, EMB should be performed when AUB is present in women >45 years, in patients of any age group who fail to respond to medical therapy, and in those at increased risk for endometrial cancer.
Coagulopathy. When heavy bleeding has been present since the onset of menarche, inherited bleeding disorders must be considered, the most common of which is von Willebrand disease, a disorder of platelet adhesion.24 It is estimated that just under 50% of adolescents with abnormal uterine bleeding have a coagulopathy, most often a platelet function disorder.25 Additional clues to the presence of a coagulation disorder include a family history of bleeding disorder, a personal history of bleeding problems associated with surgery, and a history of iron-deficiency anemia.26 Abnormal uterine bleeding might resolve with treatment of the underlying coagulopathy; if it does not, consider consultation with a hematologist before prescribing an NSAID or an OC.
Heavy bleeding in patients taking an anticoagulant falls into the category of coagulopathy-related AUB. No further workup is generally needed for these women.3
Continue to: Ovulatory dysfunction
Ovulatory dysfunction. Abnormal uterine bleeding caused by ovulatory dysfunction is generally due to PCOS or anovulatory bleeding. Other causes, beyond the scope of this discussion, include hypothyroidism, hyperandrogenism, female athlete triad, stress, and hyperprolactinemia.
› Polycystic ovary syndrome. A diagnosis of PCOS is made using any of several recognized criteria. The commonly used Rotterdam 2003 criteria27 require that at least 2 of the following be present to make a diagnosis of PCOS:
- oligo-ovulation or anovulation
- hyperandrogenism
- polycystic ovaries seen on ultrasonography.
In addition, women with PCOS are frequently obese, show signs of insulin resistance (diabetes, prediabetes, acanthosis nigricans), or hyperandrogenism (hirsutism, acne). Even if these latter findings are not present at diagnosis, women with PCOS are at risk for a metabolic disorder. Once a diagnosis of PCOS has been established, therefore, screening tests for diabetes and cardiac risk factors (eg, dyslipidemia) should be performed.28.29
To evaluate for hyperandrogenism, free testosterone should be measured using a high-sensitivity immunoassay in all women in whom PCOS is suspected. Because of a higher prevalence of nonclassical (ie, late-onset) congenital adrenal hyperplasia (CAH) in women of Ashkenazi Jewish (estimated prevalence, 3.7%), Hispanic (1.9%), Slavic (1.6%), and Italian (0.3%) descent, screening for CAH as a possible cause of hyperandrogenism is also recommended, by a test of a morning 17-hydroxyprogesterone level.23,29,30 (Note: The general Caucasian population has an estimated prevalence of nonclassical CAH of 0.1%.30)
Treatment of PCOS should be individualized, based on a patient’s symptoms and comorbidities. For overweight and obese women, weight loss, exercise, and metformin (1500-2000 mg/d) are the mainstays of therapy, and might reduce AUB.29,31 If these measures do not reduce AUB, other options include an OC, an LNG-IUD, and NSAIDs.
Continue to: Information on treating other PCOS-related symptoms...
Information on treating other PCOS-related symptoms (acne, hirsutism) is available from many sources29; these treatments do not typically help the patient’s AUB, however, and are therefore not addressed in this article.
› Anovulatory bleeding. In adolescence, the most common cause of AUB is anovulation resulting from immaturity of the hypothalamic–pituitary–ovarian axis. During anovulatory cycles, the imbalance of estrogen and progesterone creates a fragile endometrium, leading to unpredictable bleeding and irregular cycles. Other less common causes of AUB, such as ovarian or adrenal tumor, should be considered in adolescents who have hirsutism but do not meet the criteria for PCOS.5
When seeing an adolescent for evaluation of AUB, be aware that emotional barriers might be present that make it difficult for her to talk about menses and sexual activity. Be patient and normalize the patient’s symptoms when appropriate. Pelvic exam can be deferred, especially in adolescents who have not yet had vaginal intercourse. When AUB occurs in an adolescent and the cause is thought to be immaturity of the hypothalamic–pituitary–ovarian axis, there is no need for laboratory testing or imaging studies, other than excluding hypothyroidism and pregnancy as the cause.
Oral contraceptives, NSAIDs, tranexamic acid, and the LNG-IUD are all options for treating patients who have anovulatory bleeding4,5 (TABLE 3). An OC has a major advantage for adolescents because it alleviates other complaints related to adolescent hormonal changes, such as acne, and provides contraception when taken on a regular basis.
Alternatively, the LNG-IUD has the benefit of ease of use once inserted, while still providing the added benefit of contraception. In women who have not yet had vaginal intercourse, an intrauterine device might not be the first choice of treatment, however, and should be prescribed only after discussion with the patient. For both OCs and the LNG-IUD, myths surrounding the use of these medications must be addressed with the patient and, if she is a minor, her parents or guardian.32
Continue to: NSAIDS can be effective because...
NSAIDs can be effective because they reduce bleeding by causing vasoconstriction, but they provide the greatest benefit when started before menses, which can be difficult for a patient who has irregular cycles.
Endometrial causes of AUB should be suspected when a patient has heavy menstrual bleeding with regular menstrual cycles and no other causes can be identified. Endometrial dysfunction as the cause of AUB stems from aberrations in the biochemical pathways of endometrial hemostasis and repair, and therefore is difficult to confirm by laboratory analysis or histologic evaluation.3 Medical management focuses on alleviating heavy menstrual bleeding (TABLE 3).
Iatrogenic. The most common type of iatrogenic AUB is unscheduled bleeding, also known as breakthrough bleeding, that occurs during hormonal treatment with an OC or during the first few months after insertion of an LNG-IUD or contraceptive implant.3 In most cases, no specific treatment is required; bleeding resolves upon continued use of the contraceptive.
Not yet classified. This category is difficult to define; it was created for causes of AUB that have not yet been identified and remain unclear. For example, a condition known as chronic endometritis is under study as a possible cause of AUB, but has not been assigned to a PALM–COEIN category.3 As more data become available and understanding of pathophysiologic mechanisms lead to better definitions of disease, this and other poorly understood conditions will be moved to an appropriate category in the FIGO classification system.
CASE 2
Ms. G is given a diagnosis of PCOS, based on her history. You recommend weight loss and exercise; screen her for diabetes and dyslipidemia; and prescribe metformin.
ACKNOWLEDGMENT
Barry D. Weiss, MD, University of Arizona College of Medicine, Department of Family and Community Medicine, Tucson, assisted with the editing of this manuscript.
CORRESPONDENCE
Melody A. Jordahl-Iafrato, MD, Community Hospital East Family Medicine Residency, 10122 East 10th Street, Suite 100, Indianapolis, IN 46229; Mjordahl-iafrato@ecommunity.com.
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
1. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 128, July 2012: Diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
2. American College of Obstetricians and Gynecologists Committee Opinion No. 651: Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126:e143-e146.
3. Munro MG, Critchley HO, Broder MS, et al; FIGO Working Group on Menstrual Disorders. FIGO classifcation system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;133:3-13.
4. National Institute for Health and Care Excellence (NICE). Heavy menstrual bleeding: assessment and management [NG88]. www.nice.org.uk/guidance/ng88. Accessed February 28, 2019.
5. Committee on Practice Bulletins—Gynecology. American College of Obstetricians and Gynecologists Practice Bulletin Number 136, July 2013: Management of abnormal uterine bleeding associated with ovulatory dysfunction. Obstet Gynecol. 2013;122:176-185.
6. Hassa H, Tekin B, Senses T, et al. Are the site, diameter, and number of endometrial polyps related with symptomatology? Am J Obstet Gynecol. 2006;194:718-721.
7. Salim S, Won H, Nesbitt-Hawes E, et al. Diagnosis and management of endometrial polyps: a critical review of the literature. J Minim Invasive Gynecol. 2011;18:569-581.
8. Struble J, Reid S, Bedaiwy MA. Adenomyosis: A clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23:164-185.
9. Champaneria R, Abedin P, Daniels J, et al. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89:1374-1384.
10. Bartels CB, Cayton KC, Chuong FS, et al. An evidence-based approach to the medical management of fibroids: a systematic review. Clin Obstet Gynecol. 2016;59:30-52.
11. Lethaby A, Cooke I, Rees MC. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.
12. Spies JB. Current role of uterine artery embolization in the management of uterine fibroids. Clin Obstet Gynecol. 2016;59:93-102.
13. Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;(12):CD005073.
14. Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356:360-370.
15. Pron G, Bennett J, Common A, et al; Ontario Uterine Fibroid Embolization Collaboration Group. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
16. Torre A, Fauconnier A, Kahn V, et al. Fertility after uterine artery embolization for symptomatic multiple fibroids with no other infertility factors. Eur Radiol. 2017;27:2850-2859.
17. Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31:73-85.
18. Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;(2): CD000547.
19. Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332-338.
20. Abu Hashim H, Ghayaty E, El Rakhawy M. Levonorgestrel-releasing intrauterine system vs oral progestins for non-atypical endometrial hyperplasia: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:469-478.
21. Reed SD, Voigt LF, Newton KM, et al. Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113;655-662.
22. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387:1094-1108.
23. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 1. Endocr Pract. 2015;21:1291-1300.
24. Shankar M, Lee CA, Sabin CA, et al. von Willebrand disease in women with menorrhagia: a systematic review. BJOG. 2004;111:734-740.
25. Seravalli V, Linari S, Peruzzi E, et al. Prevalence of hemostatic disorders in adolescents with abnormal uterine bleeding. J Pediatr Adolesc Gynecol. 2013;26:285-289.
26. Philipp CS, Faiz A, Dowling NF, et al. Development of a screening tool for identifying women with menorrhagia for hemostatic evaluation. Am J Obstet Gynecol. 2008;198:163.e1-e8.
27. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41-47.
28. Goodman NF, Cobin RH, Futterweit W, et al; American Association of Clinical Endocrinologists (AACE); American College of Endocrinology (ACE); Androgen Excess and PCOS Society (AES). American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21:1415-26.
29. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 194: Polycystic ovary syndrome. Obstet Gynecol. 2018;131:e157-e171.
30. Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650-667.
31. Naderpoor N, Shorakae S, de Courten B, et al. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta-analysis. Hum Reprod Update. 2015;21:560-574.
32. Kolman KB, Hadley SK, Jordahl-Iafrato MA. Long-acting reversible contraception: who, what, when, and how. J Fam Pract. 2015;64:479-484.
PRACTICE RECOMMENDATIONS
› Perform endometrial biopsy on all women who have abnormal uterine bleeding and risk factors for endometrial cancer and on all women ≥45 years, regardless of risk. C
› Initiate a workup for a coagulation disorder in women who are close to the onset of menarche and have a history of heavy menstrual bleeding. C
› Promote lifestyle changes and weight loss as primary treatments for polycystic ovary syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Worsening dyspnea
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.
ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9
The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; karyn.kolman@bannerhealth.com.
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.

ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9

The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; karyn.kolman@bannerhealth.com.
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.

ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9

The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; karyn.kolman@bannerhealth.com.
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.
Long-acting reversible contraception: Who, what, when, and how
› Suggest long-acting reversible contraception (LARC), including intrauterine devices (IUDs), as a first-line method of contraception to most women, including adolescents and nulliparous women. A
› Offer immediate post-placental insertion of LARC when counseling women who have barriers to seeking contraception at a postpartum visit or are unlikely to return for a postpartum visit. B
› Treat sexually transmitted infections in most cases without removing an IUD that is already in place. Consider removing the IUD, however, if there is no clinical improvement after 2 to 3 days of antibiotics. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The number of women using long-acting reversible contraception (LARC) in the United States has been increasing, with current use accounting for approximately 18% of reversible contraception, according to the National Survey of Family Growth.1,2 LARC includes any method of contraception that lasts ≥3 years, is easily reversed, and does not rely on the user to maintain efficacy. Five LARC devices are available in the United States: 4 intrauterine devices (IUDs) and one subdermal implant.
The number of women using LARC is surprisingly low, given that it is considered a first-line contraceptive method for most women and adolescents,3 and when compared with other forms of reversible contraception, is more efficacious,4-6 has higher satisfaction rates,7-9 and higher rates of continuation.9 In fact, the Contraceptive CHOICE Project—a St. Louis community-based research program promoting and enabling access to reversible contraceptive methods—has shown that when appropriate counseling is provided and cost barriers are removed, up to 79% of women choose LARC as their preferred method of contraception.10
CASES › Jenny, who is 16 years old, comes to your office with her mother to discuss contraceptive options. She is nulliparous, has regular menses, and, aside from a body mass index (BMI) of 28, has no medical problems. Her mother is concerned about Jenny becoming pregnant while she is still in high school.
Maria D, a 32-year-old G2P1, comes in for a prenatal visit with her husband. She tells you that after delivery she is interested in a long-acting contraceptive, but is planning on breastfeeding and does not want anything to interfere with that.
What LARC options do these and other patients have?
The 4 IUDs and one implant approved for use are all viable options depending on a patient’s preference and comorbidities (TABLE 1).3-9,11-15 The copper IUD is the oldest method of LARC available and the only one that is nonhormonal. It is approved by the Food and Drug Administration (FDA) for use up to 10 years,11 but studies support its effectiveness for up to 12 years.16
The remaining IUDs (Skyla, Liletta, Mirena) contain varying amounts of the progestin levonorgestrel (LNG), released by each device at a slightly different rate that declines over time. Skyla releases a significantly lower dose of hormone than Liletta or Mirena.12-14 Skyla and Liletta are FDA-approved for up to 3 years of use,12,13 and Liletta is currently undergoing trials to gain approval for use up to 5 years. Mirena is FDA-approved for use up to 5 years,14 but studies have shown that it can be effective for 7 years.4,16
The only implant available in the US is Nexplanon, a plastic rod containing 68 mg of etonorgestrel. It is inserted subdermally and is FDA-approved for use up to 3 years.15
Through systemic hormonal effects, the primary mechanism of action of the implant is prevention of ovulation. Additionally, the implant has been shown to inhibit endometrial proliferation and cervical mucus thickening, both of which may contribute to the implant’s overall effectiveness.17 In contrast, both the copper IUD and the LNG-IUDs work primarily by preventing fertilization. The LNG-IUDs also exhibit local hormonal effects (endometrial atrophy and thickened cervical mucus) that contribute to their effectiveness.17
Who is eligible for LARC?
LARC is suitable for the vast majority of women of reproductive age. For most multiparous women ≥20 years, all LARC devices are classified as category 1 (use without restriction) in the Centers for Disease Control and Prevention’s (CDC) US Medical Eligibility Criteria (US MEC).3 For women <20 years, the implant is also considered category 1, but IUDs in this age group are classified as category 2 (recommended with the caution that advantages usually outweigh risks) because of concerns about an increased risk of IUD expulsion and the increased prevalence of sexually transmitted infections (STIs) in adolescents.3 Contraindications to use of LARC vary depending on the method chosen (TABLE 1).3
There has been concern about the efficacy of implants in overweight women because the original trials of subdermal implants excluded women >130% of ideal body weight. However, according to the Contraceptive CHOICE Project, overweight and obese women enrolled in its program did not experience reduced contraceptive efficacy when using the implant when compared with normal-weight women.18
When can LARC devices be inserted?
LARC device insertion is possible at any time during the menstrual cycle. An algorithm to guide initiation of LARC is available through the Reproductive Health Access Project’s Web site at http://www.reproductiveaccess.org/wp-content/uploads/2014/12/quickstart_algorithm.pdf.
Rule out pregnancy before placing any LARC device. The copper IUD can be inserted at any time during the menstrual cycle without the need for back-up contraception.11,19 In contrast, for LNG-IUDs, back-up contraception is recommended for 7 days unless the insertion is done during the first 7 days of the menstrual cycle.12-14,19
For the implant, recommendations about when to insert are based on a woman’s previous method of contraception (TABLE 2).15 If insertion is done at a time other than when recommended, advise patients to use barrier protection for 7 days after insertion.4,15,19
Other issues often arise and cause concern about whether and when a LARC device can be inserted, including the possibility of undiagnosed STI, time elapsed since delivery, and advisability of use when breastfeeding.
Sexually transmitted infections and IUDs
Whether or not a woman chooses to receive an IUD, follow routine CDC guidelines in determining if a patient is a candidate for STI screening.20 If a woman wants an IUD and routine screening is recommended, you can perform screening on the day of IUD insertion.4,19 For women with an IUD already in place who are diagnosed with an STI, treat the infection while leaving the IUD in place.19 For women with a known or suspected STI who do not have an IUD already, treat the STI before inserting the IUD. The American Congress of Obstetricians and Gynecologists (ACOG) advises postponing insertion of an IUD until a negative STI test result is obtained 3 to 4 weeks after treatment completion.4
Breastfeeding concerns and timing of insertion postpartum
The US MEC classifies insertion of the copper IUD as category 1 for all postpartum women, regardless of breastfeeding status, if placed >4 weeks postpartum or immediately postpartum (defined as within 10 minutes of the delivery of the placenta). IUD placement is category 2 (recommended with the caution that advantages usually outweigh risks) if placed ≥10 minutes after placental delivery (until 4 weeks postpartum) because of an increased risk of expulsion.3
The US MEC also considers use of the implant and LNG-IUDs in breastfeeding women as category 1 if the device is placed at ≥4 weeks postpartum. Insertion at <4 weeks postpartum is considered category 2 because of concerns for decreased breast milk supply.3 However, studies on whether progestin-containing LARC devices affect breastfeeding have yielded varying results. In one randomized controlled trial (RCT) of 69 breastfeeding women using the implant, breastfeeding duration and milk production were not dependent on the timing of insertion after delivery.21 Another RCT of 96 women using LNG-IUDs showed fewer women continued to breastfeed at 6 months when their LNG-IUD was inserted immediately postpartum, compared with waiting 6 weeks.22
In addition to a concern about breast milk supply, breastfeeding women have a higher risk for uterine perforation from IUDs, especially during the first 36 weeks after delivery.23
Several studies have shown that there is a lower repeat pregnancy rate among women who receive immediate postpartum LARC placement.24 However, even if IUD insertion is performed immediately postpartum, there is a higher expulsion rate than when the IUD is inserted ≥4 weeks postpartum. The expulsion rates for insertion <10 minutes after vaginal delivery range from 9.5% to 15% for the copper IUD to as high as 24% for the LNG-IUDs. Expulsion rates for all IUDs are slightly lower for cesarean delivery.4,25,26 ACOG supports immediate post-placental placement for women with barriers to postpartum care or limited access to contraception.4
How can I help my patients make an informed choice?
Provide counseling on efficacy, common adverse effects, risks, and complications.
Efficacy is high
The failure rate of LARC is equal to, or lower than, that of female sterilization and is significantly lower than that of oral contraceptives (TABLE 1).4-6 Not only are LARC devices extremely effective, they have a higher rate of satisfaction than any other reversible contraceptive (TABLE 1).7,8
Common adverse effects
The most common adverse effect seen with all LARC devices is an alteration in menstrual bleeding, and a frequent adverse effect with IUDs is pain. Vaginitis is less common and can be seen with any of the devices. The progestin-containing LARC devices are associated with hormonal effects: vaginitis, headache, weight gain, acne, breast pain, hair loss, and emotional lability.12-15
Copper IUD. Many women using the copper IUD experience either a transient increase in menstrual bleeding lasting for a few months or inter-menstrual bleeding that tends to continue for the duration of use.4,17 However, according to data from the Contraceptive CHOICE Project, the most common reason cited for early discontinuation of the copper IUD is pain and cramping.9
LNG-IUDs. Like the copper IUD, many users of LNG-IUDs experience an initial increase in menstrual bleeding. However, unlike the other LARC devices, 20% to 33% of Mirena users are likely to experience amenorrhea after one year of use and 70% at 2 years.4,14 According to package inserts, amenorrhea after 3 years is less common with both Skyla (12%) and Liletta (38%).12,13 As with the copper IUD, based on data from the Contraceptive CHOICE Project, the most common reason cited for early discontinuation of LNG-IUDs is pain and cramping.9
Subdermal implant. Changes in menses in women using the subdermal implant range from amenorrhea (22%) to prolonged bleeding (18%).15,17 Although it is difficult to predict which pattern a particular woman will experience, heavier women are more likely to have heavier bleeding patterns, and initial bleeding patterns are predictive of future ones.4 The most common reason women choose to discontinue use of the implant is abnormal bleeding.4,9,27,28
Newer IUDs do not increase risk of STIs
Many patients and clinicians erroneously believe that IUDs increase the risk of STIs and therefore assume that patients with a history of STI are not appropriate candidates for an IUD.29 There is a slightly increased risk of pelvic inflammatory disease (PID) in the first 21 days after insertion of an IUD. However, in contrast to older IUDs, currently available IUDs do not increase the general risk for STIs.17,30
Risk of infertility is nil
There is no risk of infertility from use of currently available LARCs. For those who want to become pregnant, fertility typically returns immediately after removal of the device, regardless of which method of LARC is used.11-15,30
Complications of IUD insertion
Uterine perforation. Uterine perforation occurs in 0.8 to 2.1 per 1000 women, usually at the time of IUD placement. If IUD strings are not visible during a speculum examination, locate the IUD with ultrasound.4,17,30 If the IUD is in the abdomen, refer to a gynecologist for laparoscopic removal and select another form of contraception for use in the interim.30
Expulsion. Rates of expulsion are low, occurring in less than 10% of women4,17 and are not affected by parity or BMI.31 Expulsion rates are higher when the IUD is inserted immediately postpartum.4,25,26 Adolescents also have a 2-fold higher risk of uterine expulsion than older women.31
Ectopic pregnancy. Although a woman’s overall risk of ectopic pregnancy is not increased by using an IUD,4 it is true that if a woman becomes pregnant with an IUD in place, the pregnancy is more likely to be ectopic. Thus, if pregnancy is confirmed in a woman with an IUD in place, rule out ectopic pregnancy.
The FDA and the World Health Organization recommend that if an intrauterine pregnancy is confirmed with an IUD in place and the strings are visible, the IUD should be removed.4 Although removing the IUD increases the risk of spontaneous abortion (SAB) as compared with pregnancies without an IUD in place, the risk of SAB is still lower than if the IUD is left in place.4 Additional risks of continuing a pregnancy with an IUD in place include increased risks of preterm labor, chorioamnionitis, and septic abortion.4,30
Complications of subdermal implant insertion
After insertion of the implant, women usually experience temporary bruising and soreness at the insertion site. Less than 1% of women develop an infection or hematoma.17 There is a low risk of nerve damage if the implant is inserted too deeply.15 Removal of the subdermal implant is recommended if pregnancy occurs.15
CASE DECISIONS › Jenny has been using oral contraceptive pills, but not regularly. You suggest that LARC may be a better option and counsel her that if she does choose an IUD or the implant, it is likely that her menses will change. You provide information and reassurance that LARC is safe to use in adolescents. Jenny says she would like to try an implant. Six months later, Jenny returns and says the implant is working well. She has some irregular bleeding, but it is not bothersome.
You review with Ms. D the types of LARC devices available and reassure her that all are safe to use once breastfeeding is established. Ms. D says she would like to use an IUD and elects to wait until her postpartum visit to have an IUD inserted. Ms. D returns 6 months after IUD insertion; breastfeeding is going well, and she has not had any menstrual bleeding since delivery.
CORRESPONDENCE
Karyn Kolman, MD, 2800 East Ajo Way, Room 3006, Tucson, AZ 85713; karyn.kolman@bannerhealth.com
1. Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States 2011-2013. NCHS data brief, no. 173. Hyattsville, MD: National Center for Health Statistics, 2014.
2. Branum AM, Jones J. Trends in long-acting reversible contraception use among US women aged 15-44. NCHS data brief, no. 188. Hyattsville, MD: National Center for Health Statistics, 2015.
3. Centers for Disease Control and Prevention (CDC). US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59:1-86.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118:184-196.
5. Pickle S, Wu J, Burbank-Schmitt E. Prevention of unintended pregnancy: a focus on long-acting reversible contraception. Prim Care. 2014;41:239-260.
6. Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998-2007.
7. Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
8. O’Neil-Callahan M, Peipert JF, Zhao Q, et al. Twenty-four-month continuation of reversible contraception. Obstet Gynecol. 2013;122:1083-1091.
9. Grunloh DS, Casner T, Secura GM, et al. Characteristics associated with discontinuation of long-acting reversible contraception within the first 6 months of use. Obstet Gynecol. 2011;117:705-719.
10. Birgisson NE, Zhao Q, Secura GM, et al. Preventing unintended pregnancy: the contraceptive CHOICE project in review. J Womens Health (Larchmt). 2015;24:349-353.
11. ParaGard T 380A. (intrauterine copper contraceptive) [package insert]. Sellersville, PA : Teva Pharmaceuticals USA, Inc., 2013.
12. Skyla (levonorgestrel-releasing intrauterine system) [package insert]. Wayne, NJ : Bayer HealthCare Pharmaceuticals, Inc., 2013.
13. Liletta (levonorgestrel-releasing intrauterine system) [package insert]. Parsippany, NJ : Actavis Pharma, Inc., 2015.
14. Mirena (levonorgestrel-releasing intrauterine system) [package insert]. Whippany, NJ : Bayer HealthCare Pharmaceuticals, Inc., 2014.
15. Nexplanon (etongestrel implant) [package insert]. Whitehouse Station, NJ: Merck & Co Inc.; 2014.
16. Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89:495-503.
17. Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71:969-980.
18. Xu H, Wade JA, Peipert JF, et al. Contraceptive failure rates of etonogestrel subdermal implants in overweight and obese women. Obstet Gynecol. 2012;120:21-26.
19. Centers for Disease Control and Prevention (CDC). US selected practice recommendations for contraceptive use. MMWR Recomm Rep. 2013;62:1-60.
20. Centers for Disease Control and Prevention (CDC). Sexually transmitted disease treatment guidelines. MMWR Recomm Rep. 2010;59:1-110.
21. Gurtcheff SE, Turok DK, Stoddard G, et al. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstet Gynecol. 2011;117:1114-1121.
22. Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504.
23. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
24. Tocce K, Sheeder J, Python J, et al. Long acting reversible contraception in postpartum adolescents: early initiation of etonogestrel implant is superior to IUDs in the outpatient setting. J Pediatr Adolesc Gynecol. 2012;25:59-63.
25. Mwalwanda CS, Black KI. Immediate post-partum initiation of intrauterine contraception and implants: a review of the safety and guidelines for use. Aust N Z J Obstet Gynaecol. 2013;53:331-337.
26. Sober, S, Schreiber CA. Postpartum contraception. Clin Obstet Gynecol. 2014;57:763-776.
27. Dickerson LM, Diaz VA, Jordon J, et al. Satisfaction, early removal, and side effects associated with long-acting reversible contraception. Fam Med. 2013;45:701-707.
28. Berenson AB, Tan A, Hirth JM. Complications and continuation rates associated with 2 types of long-acting contraception. Am J Obstet Gynecol. 2015;212:e1-e8.
29. Kavanaugh ML, Frowirth L, Jerman J, et al. Long-acting reversible contraception for adolescents and young adults: patient and provider perspectives. J Pediatr Adolesc Gynecol. 2013;86:86-95.
30. Espey E, Ogburn T. Long-acting reversible contraceptives: intrauterine devices and the contraceptive implant. Obstet Gynecol. 2011;117:705-719.
31. Madden T, McNicholas C, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
› Suggest long-acting reversible contraception (LARC), including intrauterine devices (IUDs), as a first-line method of contraception to most women, including adolescents and nulliparous women. A
› Offer immediate post-placental insertion of LARC when counseling women who have barriers to seeking contraception at a postpartum visit or are unlikely to return for a postpartum visit. B
› Treat sexually transmitted infections in most cases without removing an IUD that is already in place. Consider removing the IUD, however, if there is no clinical improvement after 2 to 3 days of antibiotics. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The number of women using long-acting reversible contraception (LARC) in the United States has been increasing, with current use accounting for approximately 18% of reversible contraception, according to the National Survey of Family Growth.1,2 LARC includes any method of contraception that lasts ≥3 years, is easily reversed, and does not rely on the user to maintain efficacy. Five LARC devices are available in the United States: 4 intrauterine devices (IUDs) and one subdermal implant.
The number of women using LARC is surprisingly low, given that it is considered a first-line contraceptive method for most women and adolescents,3 and when compared with other forms of reversible contraception, is more efficacious,4-6 has higher satisfaction rates,7-9 and higher rates of continuation.9 In fact, the Contraceptive CHOICE Project—a St. Louis community-based research program promoting and enabling access to reversible contraceptive methods—has shown that when appropriate counseling is provided and cost barriers are removed, up to 79% of women choose LARC as their preferred method of contraception.10
CASES › Jenny, who is 16 years old, comes to your office with her mother to discuss contraceptive options. She is nulliparous, has regular menses, and, aside from a body mass index (BMI) of 28, has no medical problems. Her mother is concerned about Jenny becoming pregnant while she is still in high school.
Maria D, a 32-year-old G2P1, comes in for a prenatal visit with her husband. She tells you that after delivery she is interested in a long-acting contraceptive, but is planning on breastfeeding and does not want anything to interfere with that.
What LARC options do these and other patients have?
The 4 IUDs and one implant approved for use are all viable options depending on a patient’s preference and comorbidities (TABLE 1).3-9,11-15 The copper IUD is the oldest method of LARC available and the only one that is nonhormonal. It is approved by the Food and Drug Administration (FDA) for use up to 10 years,11 but studies support its effectiveness for up to 12 years.16
The remaining IUDs (Skyla, Liletta, Mirena) contain varying amounts of the progestin levonorgestrel (LNG), released by each device at a slightly different rate that declines over time. Skyla releases a significantly lower dose of hormone than Liletta or Mirena.12-14 Skyla and Liletta are FDA-approved for up to 3 years of use,12,13 and Liletta is currently undergoing trials to gain approval for use up to 5 years. Mirena is FDA-approved for use up to 5 years,14 but studies have shown that it can be effective for 7 years.4,16
The only implant available in the US is Nexplanon, a plastic rod containing 68 mg of etonorgestrel. It is inserted subdermally and is FDA-approved for use up to 3 years.15
Through systemic hormonal effects, the primary mechanism of action of the implant is prevention of ovulation. Additionally, the implant has been shown to inhibit endometrial proliferation and cervical mucus thickening, both of which may contribute to the implant’s overall effectiveness.17 In contrast, both the copper IUD and the LNG-IUDs work primarily by preventing fertilization. The LNG-IUDs also exhibit local hormonal effects (endometrial atrophy and thickened cervical mucus) that contribute to their effectiveness.17
Who is eligible for LARC?
LARC is suitable for the vast majority of women of reproductive age. For most multiparous women ≥20 years, all LARC devices are classified as category 1 (use without restriction) in the Centers for Disease Control and Prevention’s (CDC) US Medical Eligibility Criteria (US MEC).3 For women <20 years, the implant is also considered category 1, but IUDs in this age group are classified as category 2 (recommended with the caution that advantages usually outweigh risks) because of concerns about an increased risk of IUD expulsion and the increased prevalence of sexually transmitted infections (STIs) in adolescents.3 Contraindications to use of LARC vary depending on the method chosen (TABLE 1).3
There has been concern about the efficacy of implants in overweight women because the original trials of subdermal implants excluded women >130% of ideal body weight. However, according to the Contraceptive CHOICE Project, overweight and obese women enrolled in its program did not experience reduced contraceptive efficacy when using the implant when compared with normal-weight women.18
When can LARC devices be inserted?
LARC device insertion is possible at any time during the menstrual cycle. An algorithm to guide initiation of LARC is available through the Reproductive Health Access Project’s Web site at http://www.reproductiveaccess.org/wp-content/uploads/2014/12/quickstart_algorithm.pdf.
Rule out pregnancy before placing any LARC device. The copper IUD can be inserted at any time during the menstrual cycle without the need for back-up contraception.11,19 In contrast, for LNG-IUDs, back-up contraception is recommended for 7 days unless the insertion is done during the first 7 days of the menstrual cycle.12-14,19
For the implant, recommendations about when to insert are based on a woman’s previous method of contraception (TABLE 2).15 If insertion is done at a time other than when recommended, advise patients to use barrier protection for 7 days after insertion.4,15,19
Other issues often arise and cause concern about whether and when a LARC device can be inserted, including the possibility of undiagnosed STI, time elapsed since delivery, and advisability of use when breastfeeding.
Sexually transmitted infections and IUDs
Whether or not a woman chooses to receive an IUD, follow routine CDC guidelines in determining if a patient is a candidate for STI screening.20 If a woman wants an IUD and routine screening is recommended, you can perform screening on the day of IUD insertion.4,19 For women with an IUD already in place who are diagnosed with an STI, treat the infection while leaving the IUD in place.19 For women with a known or suspected STI who do not have an IUD already, treat the STI before inserting the IUD. The American Congress of Obstetricians and Gynecologists (ACOG) advises postponing insertion of an IUD until a negative STI test result is obtained 3 to 4 weeks after treatment completion.4
Breastfeeding concerns and timing of insertion postpartum
The US MEC classifies insertion of the copper IUD as category 1 for all postpartum women, regardless of breastfeeding status, if placed >4 weeks postpartum or immediately postpartum (defined as within 10 minutes of the delivery of the placenta). IUD placement is category 2 (recommended with the caution that advantages usually outweigh risks) if placed ≥10 minutes after placental delivery (until 4 weeks postpartum) because of an increased risk of expulsion.3
The US MEC also considers use of the implant and LNG-IUDs in breastfeeding women as category 1 if the device is placed at ≥4 weeks postpartum. Insertion at <4 weeks postpartum is considered category 2 because of concerns for decreased breast milk supply.3 However, studies on whether progestin-containing LARC devices affect breastfeeding have yielded varying results. In one randomized controlled trial (RCT) of 69 breastfeeding women using the implant, breastfeeding duration and milk production were not dependent on the timing of insertion after delivery.21 Another RCT of 96 women using LNG-IUDs showed fewer women continued to breastfeed at 6 months when their LNG-IUD was inserted immediately postpartum, compared with waiting 6 weeks.22
In addition to a concern about breast milk supply, breastfeeding women have a higher risk for uterine perforation from IUDs, especially during the first 36 weeks after delivery.23
Several studies have shown that there is a lower repeat pregnancy rate among women who receive immediate postpartum LARC placement.24 However, even if IUD insertion is performed immediately postpartum, there is a higher expulsion rate than when the IUD is inserted ≥4 weeks postpartum. The expulsion rates for insertion <10 minutes after vaginal delivery range from 9.5% to 15% for the copper IUD to as high as 24% for the LNG-IUDs. Expulsion rates for all IUDs are slightly lower for cesarean delivery.4,25,26 ACOG supports immediate post-placental placement for women with barriers to postpartum care or limited access to contraception.4
How can I help my patients make an informed choice?
Provide counseling on efficacy, common adverse effects, risks, and complications.
Efficacy is high
The failure rate of LARC is equal to, or lower than, that of female sterilization and is significantly lower than that of oral contraceptives (TABLE 1).4-6 Not only are LARC devices extremely effective, they have a higher rate of satisfaction than any other reversible contraceptive (TABLE 1).7,8
Common adverse effects
The most common adverse effect seen with all LARC devices is an alteration in menstrual bleeding, and a frequent adverse effect with IUDs is pain. Vaginitis is less common and can be seen with any of the devices. The progestin-containing LARC devices are associated with hormonal effects: vaginitis, headache, weight gain, acne, breast pain, hair loss, and emotional lability.12-15
Copper IUD. Many women using the copper IUD experience either a transient increase in menstrual bleeding lasting for a few months or inter-menstrual bleeding that tends to continue for the duration of use.4,17 However, according to data from the Contraceptive CHOICE Project, the most common reason cited for early discontinuation of the copper IUD is pain and cramping.9
LNG-IUDs. Like the copper IUD, many users of LNG-IUDs experience an initial increase in menstrual bleeding. However, unlike the other LARC devices, 20% to 33% of Mirena users are likely to experience amenorrhea after one year of use and 70% at 2 years.4,14 According to package inserts, amenorrhea after 3 years is less common with both Skyla (12%) and Liletta (38%).12,13 As with the copper IUD, based on data from the Contraceptive CHOICE Project, the most common reason cited for early discontinuation of LNG-IUDs is pain and cramping.9
Subdermal implant. Changes in menses in women using the subdermal implant range from amenorrhea (22%) to prolonged bleeding (18%).15,17 Although it is difficult to predict which pattern a particular woman will experience, heavier women are more likely to have heavier bleeding patterns, and initial bleeding patterns are predictive of future ones.4 The most common reason women choose to discontinue use of the implant is abnormal bleeding.4,9,27,28
Newer IUDs do not increase risk of STIs
Many patients and clinicians erroneously believe that IUDs increase the risk of STIs and therefore assume that patients with a history of STI are not appropriate candidates for an IUD.29 There is a slightly increased risk of pelvic inflammatory disease (PID) in the first 21 days after insertion of an IUD. However, in contrast to older IUDs, currently available IUDs do not increase the general risk for STIs.17,30
Risk of infertility is nil
There is no risk of infertility from use of currently available LARCs. For those who want to become pregnant, fertility typically returns immediately after removal of the device, regardless of which method of LARC is used.11-15,30
Complications of IUD insertion
Uterine perforation. Uterine perforation occurs in 0.8 to 2.1 per 1000 women, usually at the time of IUD placement. If IUD strings are not visible during a speculum examination, locate the IUD with ultrasound.4,17,30 If the IUD is in the abdomen, refer to a gynecologist for laparoscopic removal and select another form of contraception for use in the interim.30
Expulsion. Rates of expulsion are low, occurring in less than 10% of women4,17 and are not affected by parity or BMI.31 Expulsion rates are higher when the IUD is inserted immediately postpartum.4,25,26 Adolescents also have a 2-fold higher risk of uterine expulsion than older women.31
Ectopic pregnancy. Although a woman’s overall risk of ectopic pregnancy is not increased by using an IUD,4 it is true that if a woman becomes pregnant with an IUD in place, the pregnancy is more likely to be ectopic. Thus, if pregnancy is confirmed in a woman with an IUD in place, rule out ectopic pregnancy.
The FDA and the World Health Organization recommend that if an intrauterine pregnancy is confirmed with an IUD in place and the strings are visible, the IUD should be removed.4 Although removing the IUD increases the risk of spontaneous abortion (SAB) as compared with pregnancies without an IUD in place, the risk of SAB is still lower than if the IUD is left in place.4 Additional risks of continuing a pregnancy with an IUD in place include increased risks of preterm labor, chorioamnionitis, and septic abortion.4,30
Complications of subdermal implant insertion
After insertion of the implant, women usually experience temporary bruising and soreness at the insertion site. Less than 1% of women develop an infection or hematoma.17 There is a low risk of nerve damage if the implant is inserted too deeply.15 Removal of the subdermal implant is recommended if pregnancy occurs.15
CASE DECISIONS › Jenny has been using oral contraceptive pills, but not regularly. You suggest that LARC may be a better option and counsel her that if she does choose an IUD or the implant, it is likely that her menses will change. You provide information and reassurance that LARC is safe to use in adolescents. Jenny says she would like to try an implant. Six months later, Jenny returns and says the implant is working well. She has some irregular bleeding, but it is not bothersome.
You review with Ms. D the types of LARC devices available and reassure her that all are safe to use once breastfeeding is established. Ms. D says she would like to use an IUD and elects to wait until her postpartum visit to have an IUD inserted. Ms. D returns 6 months after IUD insertion; breastfeeding is going well, and she has not had any menstrual bleeding since delivery.
CORRESPONDENCE
Karyn Kolman, MD, 2800 East Ajo Way, Room 3006, Tucson, AZ 85713; karyn.kolman@bannerhealth.com
› Suggest long-acting reversible contraception (LARC), including intrauterine devices (IUDs), as a first-line method of contraception to most women, including adolescents and nulliparous women. A
› Offer immediate post-placental insertion of LARC when counseling women who have barriers to seeking contraception at a postpartum visit or are unlikely to return for a postpartum visit. B
› Treat sexually transmitted infections in most cases without removing an IUD that is already in place. Consider removing the IUD, however, if there is no clinical improvement after 2 to 3 days of antibiotics. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The number of women using long-acting reversible contraception (LARC) in the United States has been increasing, with current use accounting for approximately 18% of reversible contraception, according to the National Survey of Family Growth.1,2 LARC includes any method of contraception that lasts ≥3 years, is easily reversed, and does not rely on the user to maintain efficacy. Five LARC devices are available in the United States: 4 intrauterine devices (IUDs) and one subdermal implant.
The number of women using LARC is surprisingly low, given that it is considered a first-line contraceptive method for most women and adolescents,3 and when compared with other forms of reversible contraception, is more efficacious,4-6 has higher satisfaction rates,7-9 and higher rates of continuation.9 In fact, the Contraceptive CHOICE Project—a St. Louis community-based research program promoting and enabling access to reversible contraceptive methods—has shown that when appropriate counseling is provided and cost barriers are removed, up to 79% of women choose LARC as their preferred method of contraception.10
CASES › Jenny, who is 16 years old, comes to your office with her mother to discuss contraceptive options. She is nulliparous, has regular menses, and, aside from a body mass index (BMI) of 28, has no medical problems. Her mother is concerned about Jenny becoming pregnant while she is still in high school.
Maria D, a 32-year-old G2P1, comes in for a prenatal visit with her husband. She tells you that after delivery she is interested in a long-acting contraceptive, but is planning on breastfeeding and does not want anything to interfere with that.
What LARC options do these and other patients have?
The 4 IUDs and one implant approved for use are all viable options depending on a patient’s preference and comorbidities (TABLE 1).3-9,11-15 The copper IUD is the oldest method of LARC available and the only one that is nonhormonal. It is approved by the Food and Drug Administration (FDA) for use up to 10 years,11 but studies support its effectiveness for up to 12 years.16
The remaining IUDs (Skyla, Liletta, Mirena) contain varying amounts of the progestin levonorgestrel (LNG), released by each device at a slightly different rate that declines over time. Skyla releases a significantly lower dose of hormone than Liletta or Mirena.12-14 Skyla and Liletta are FDA-approved for up to 3 years of use,12,13 and Liletta is currently undergoing trials to gain approval for use up to 5 years. Mirena is FDA-approved for use up to 5 years,14 but studies have shown that it can be effective for 7 years.4,16
The only implant available in the US is Nexplanon, a plastic rod containing 68 mg of etonorgestrel. It is inserted subdermally and is FDA-approved for use up to 3 years.15
Through systemic hormonal effects, the primary mechanism of action of the implant is prevention of ovulation. Additionally, the implant has been shown to inhibit endometrial proliferation and cervical mucus thickening, both of which may contribute to the implant’s overall effectiveness.17 In contrast, both the copper IUD and the LNG-IUDs work primarily by preventing fertilization. The LNG-IUDs also exhibit local hormonal effects (endometrial atrophy and thickened cervical mucus) that contribute to their effectiveness.17
Who is eligible for LARC?
LARC is suitable for the vast majority of women of reproductive age. For most multiparous women ≥20 years, all LARC devices are classified as category 1 (use without restriction) in the Centers for Disease Control and Prevention’s (CDC) US Medical Eligibility Criteria (US MEC).3 For women <20 years, the implant is also considered category 1, but IUDs in this age group are classified as category 2 (recommended with the caution that advantages usually outweigh risks) because of concerns about an increased risk of IUD expulsion and the increased prevalence of sexually transmitted infections (STIs) in adolescents.3 Contraindications to use of LARC vary depending on the method chosen (TABLE 1).3
There has been concern about the efficacy of implants in overweight women because the original trials of subdermal implants excluded women >130% of ideal body weight. However, according to the Contraceptive CHOICE Project, overweight and obese women enrolled in its program did not experience reduced contraceptive efficacy when using the implant when compared with normal-weight women.18
When can LARC devices be inserted?
LARC device insertion is possible at any time during the menstrual cycle. An algorithm to guide initiation of LARC is available through the Reproductive Health Access Project’s Web site at http://www.reproductiveaccess.org/wp-content/uploads/2014/12/quickstart_algorithm.pdf.
Rule out pregnancy before placing any LARC device. The copper IUD can be inserted at any time during the menstrual cycle without the need for back-up contraception.11,19 In contrast, for LNG-IUDs, back-up contraception is recommended for 7 days unless the insertion is done during the first 7 days of the menstrual cycle.12-14,19
For the implant, recommendations about when to insert are based on a woman’s previous method of contraception (TABLE 2).15 If insertion is done at a time other than when recommended, advise patients to use barrier protection for 7 days after insertion.4,15,19
Other issues often arise and cause concern about whether and when a LARC device can be inserted, including the possibility of undiagnosed STI, time elapsed since delivery, and advisability of use when breastfeeding.
Sexually transmitted infections and IUDs
Whether or not a woman chooses to receive an IUD, follow routine CDC guidelines in determining if a patient is a candidate for STI screening.20 If a woman wants an IUD and routine screening is recommended, you can perform screening on the day of IUD insertion.4,19 For women with an IUD already in place who are diagnosed with an STI, treat the infection while leaving the IUD in place.19 For women with a known or suspected STI who do not have an IUD already, treat the STI before inserting the IUD. The American Congress of Obstetricians and Gynecologists (ACOG) advises postponing insertion of an IUD until a negative STI test result is obtained 3 to 4 weeks after treatment completion.4
Breastfeeding concerns and timing of insertion postpartum
The US MEC classifies insertion of the copper IUD as category 1 for all postpartum women, regardless of breastfeeding status, if placed >4 weeks postpartum or immediately postpartum (defined as within 10 minutes of the delivery of the placenta). IUD placement is category 2 (recommended with the caution that advantages usually outweigh risks) if placed ≥10 minutes after placental delivery (until 4 weeks postpartum) because of an increased risk of expulsion.3
The US MEC also considers use of the implant and LNG-IUDs in breastfeeding women as category 1 if the device is placed at ≥4 weeks postpartum. Insertion at <4 weeks postpartum is considered category 2 because of concerns for decreased breast milk supply.3 However, studies on whether progestin-containing LARC devices affect breastfeeding have yielded varying results. In one randomized controlled trial (RCT) of 69 breastfeeding women using the implant, breastfeeding duration and milk production were not dependent on the timing of insertion after delivery.21 Another RCT of 96 women using LNG-IUDs showed fewer women continued to breastfeed at 6 months when their LNG-IUD was inserted immediately postpartum, compared with waiting 6 weeks.22
In addition to a concern about breast milk supply, breastfeeding women have a higher risk for uterine perforation from IUDs, especially during the first 36 weeks after delivery.23
Several studies have shown that there is a lower repeat pregnancy rate among women who receive immediate postpartum LARC placement.24 However, even if IUD insertion is performed immediately postpartum, there is a higher expulsion rate than when the IUD is inserted ≥4 weeks postpartum. The expulsion rates for insertion <10 minutes after vaginal delivery range from 9.5% to 15% for the copper IUD to as high as 24% for the LNG-IUDs. Expulsion rates for all IUDs are slightly lower for cesarean delivery.4,25,26 ACOG supports immediate post-placental placement for women with barriers to postpartum care or limited access to contraception.4
How can I help my patients make an informed choice?
Provide counseling on efficacy, common adverse effects, risks, and complications.
Efficacy is high
The failure rate of LARC is equal to, or lower than, that of female sterilization and is significantly lower than that of oral contraceptives (TABLE 1).4-6 Not only are LARC devices extremely effective, they have a higher rate of satisfaction than any other reversible contraceptive (TABLE 1).7,8
Common adverse effects
The most common adverse effect seen with all LARC devices is an alteration in menstrual bleeding, and a frequent adverse effect with IUDs is pain. Vaginitis is less common and can be seen with any of the devices. The progestin-containing LARC devices are associated with hormonal effects: vaginitis, headache, weight gain, acne, breast pain, hair loss, and emotional lability.12-15
Copper IUD. Many women using the copper IUD experience either a transient increase in menstrual bleeding lasting for a few months or inter-menstrual bleeding that tends to continue for the duration of use.4,17 However, according to data from the Contraceptive CHOICE Project, the most common reason cited for early discontinuation of the copper IUD is pain and cramping.9
LNG-IUDs. Like the copper IUD, many users of LNG-IUDs experience an initial increase in menstrual bleeding. However, unlike the other LARC devices, 20% to 33% of Mirena users are likely to experience amenorrhea after one year of use and 70% at 2 years.4,14 According to package inserts, amenorrhea after 3 years is less common with both Skyla (12%) and Liletta (38%).12,13 As with the copper IUD, based on data from the Contraceptive CHOICE Project, the most common reason cited for early discontinuation of LNG-IUDs is pain and cramping.9
Subdermal implant. Changes in menses in women using the subdermal implant range from amenorrhea (22%) to prolonged bleeding (18%).15,17 Although it is difficult to predict which pattern a particular woman will experience, heavier women are more likely to have heavier bleeding patterns, and initial bleeding patterns are predictive of future ones.4 The most common reason women choose to discontinue use of the implant is abnormal bleeding.4,9,27,28
Newer IUDs do not increase risk of STIs
Many patients and clinicians erroneously believe that IUDs increase the risk of STIs and therefore assume that patients with a history of STI are not appropriate candidates for an IUD.29 There is a slightly increased risk of pelvic inflammatory disease (PID) in the first 21 days after insertion of an IUD. However, in contrast to older IUDs, currently available IUDs do not increase the general risk for STIs.17,30
Risk of infertility is nil
There is no risk of infertility from use of currently available LARCs. For those who want to become pregnant, fertility typically returns immediately after removal of the device, regardless of which method of LARC is used.11-15,30
Complications of IUD insertion
Uterine perforation. Uterine perforation occurs in 0.8 to 2.1 per 1000 women, usually at the time of IUD placement. If IUD strings are not visible during a speculum examination, locate the IUD with ultrasound.4,17,30 If the IUD is in the abdomen, refer to a gynecologist for laparoscopic removal and select another form of contraception for use in the interim.30
Expulsion. Rates of expulsion are low, occurring in less than 10% of women4,17 and are not affected by parity or BMI.31 Expulsion rates are higher when the IUD is inserted immediately postpartum.4,25,26 Adolescents also have a 2-fold higher risk of uterine expulsion than older women.31
Ectopic pregnancy. Although a woman’s overall risk of ectopic pregnancy is not increased by using an IUD,4 it is true that if a woman becomes pregnant with an IUD in place, the pregnancy is more likely to be ectopic. Thus, if pregnancy is confirmed in a woman with an IUD in place, rule out ectopic pregnancy.
The FDA and the World Health Organization recommend that if an intrauterine pregnancy is confirmed with an IUD in place and the strings are visible, the IUD should be removed.4 Although removing the IUD increases the risk of spontaneous abortion (SAB) as compared with pregnancies without an IUD in place, the risk of SAB is still lower than if the IUD is left in place.4 Additional risks of continuing a pregnancy with an IUD in place include increased risks of preterm labor, chorioamnionitis, and septic abortion.4,30
Complications of subdermal implant insertion
After insertion of the implant, women usually experience temporary bruising and soreness at the insertion site. Less than 1% of women develop an infection or hematoma.17 There is a low risk of nerve damage if the implant is inserted too deeply.15 Removal of the subdermal implant is recommended if pregnancy occurs.15
CASE DECISIONS › Jenny has been using oral contraceptive pills, but not regularly. You suggest that LARC may be a better option and counsel her that if she does choose an IUD or the implant, it is likely that her menses will change. You provide information and reassurance that LARC is safe to use in adolescents. Jenny says she would like to try an implant. Six months later, Jenny returns and says the implant is working well. She has some irregular bleeding, but it is not bothersome.
You review with Ms. D the types of LARC devices available and reassure her that all are safe to use once breastfeeding is established. Ms. D says she would like to use an IUD and elects to wait until her postpartum visit to have an IUD inserted. Ms. D returns 6 months after IUD insertion; breastfeeding is going well, and she has not had any menstrual bleeding since delivery.
CORRESPONDENCE
Karyn Kolman, MD, 2800 East Ajo Way, Room 3006, Tucson, AZ 85713; karyn.kolman@bannerhealth.com
1. Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States 2011-2013. NCHS data brief, no. 173. Hyattsville, MD: National Center for Health Statistics, 2014.
2. Branum AM, Jones J. Trends in long-acting reversible contraception use among US women aged 15-44. NCHS data brief, no. 188. Hyattsville, MD: National Center for Health Statistics, 2015.
3. Centers for Disease Control and Prevention (CDC). US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59:1-86.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118:184-196.
5. Pickle S, Wu J, Burbank-Schmitt E. Prevention of unintended pregnancy: a focus on long-acting reversible contraception. Prim Care. 2014;41:239-260.
6. Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998-2007.
7. Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
8. O’Neil-Callahan M, Peipert JF, Zhao Q, et al. Twenty-four-month continuation of reversible contraception. Obstet Gynecol. 2013;122:1083-1091.
9. Grunloh DS, Casner T, Secura GM, et al. Characteristics associated with discontinuation of long-acting reversible contraception within the first 6 months of use. Obstet Gynecol. 2011;117:705-719.
10. Birgisson NE, Zhao Q, Secura GM, et al. Preventing unintended pregnancy: the contraceptive CHOICE project in review. J Womens Health (Larchmt). 2015;24:349-353.
11. ParaGard T 380A. (intrauterine copper contraceptive) [package insert]. Sellersville, PA : Teva Pharmaceuticals USA, Inc., 2013.
12. Skyla (levonorgestrel-releasing intrauterine system) [package insert]. Wayne, NJ : Bayer HealthCare Pharmaceuticals, Inc., 2013.
13. Liletta (levonorgestrel-releasing intrauterine system) [package insert]. Parsippany, NJ : Actavis Pharma, Inc., 2015.
14. Mirena (levonorgestrel-releasing intrauterine system) [package insert]. Whippany, NJ : Bayer HealthCare Pharmaceuticals, Inc., 2014.
15. Nexplanon (etongestrel implant) [package insert]. Whitehouse Station, NJ: Merck & Co Inc.; 2014.
16. Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89:495-503.
17. Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71:969-980.
18. Xu H, Wade JA, Peipert JF, et al. Contraceptive failure rates of etonogestrel subdermal implants in overweight and obese women. Obstet Gynecol. 2012;120:21-26.
19. Centers for Disease Control and Prevention (CDC). US selected practice recommendations for contraceptive use. MMWR Recomm Rep. 2013;62:1-60.
20. Centers for Disease Control and Prevention (CDC). Sexually transmitted disease treatment guidelines. MMWR Recomm Rep. 2010;59:1-110.
21. Gurtcheff SE, Turok DK, Stoddard G, et al. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstet Gynecol. 2011;117:1114-1121.
22. Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504.
23. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
24. Tocce K, Sheeder J, Python J, et al. Long acting reversible contraception in postpartum adolescents: early initiation of etonogestrel implant is superior to IUDs in the outpatient setting. J Pediatr Adolesc Gynecol. 2012;25:59-63.
25. Mwalwanda CS, Black KI. Immediate post-partum initiation of intrauterine contraception and implants: a review of the safety and guidelines for use. Aust N Z J Obstet Gynaecol. 2013;53:331-337.
26. Sober, S, Schreiber CA. Postpartum contraception. Clin Obstet Gynecol. 2014;57:763-776.
27. Dickerson LM, Diaz VA, Jordon J, et al. Satisfaction, early removal, and side effects associated with long-acting reversible contraception. Fam Med. 2013;45:701-707.
28. Berenson AB, Tan A, Hirth JM. Complications and continuation rates associated with 2 types of long-acting contraception. Am J Obstet Gynecol. 2015;212:e1-e8.
29. Kavanaugh ML, Frowirth L, Jerman J, et al. Long-acting reversible contraception for adolescents and young adults: patient and provider perspectives. J Pediatr Adolesc Gynecol. 2013;86:86-95.
30. Espey E, Ogburn T. Long-acting reversible contraceptives: intrauterine devices and the contraceptive implant. Obstet Gynecol. 2011;117:705-719.
31. Madden T, McNicholas C, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.
1. Daniels K, Daugherty J, Jones J. Current contraceptive status among women aged 15-44: United States 2011-2013. NCHS data brief, no. 173. Hyattsville, MD: National Center for Health Statistics, 2014.
2. Branum AM, Jones J. Trends in long-acting reversible contraception use among US women aged 15-44. NCHS data brief, no. 188. Hyattsville, MD: National Center for Health Statistics, 2015.
3. Centers for Disease Control and Prevention (CDC). US medical eligibility criteria for contraceptive use, 2010. MMWR Recomm Rep. 2010;59:1-86.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 121: Long-acting reversible contraception: Implants and intrauterine devices. Obstet Gynecol. 2011;118:184-196.
5. Pickle S, Wu J, Burbank-Schmitt E. Prevention of unintended pregnancy: a focus on long-acting reversible contraception. Prim Care. 2014;41:239-260.
6. Winner B, Peipert JF, Zhao Q, et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998-2007.
7. Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
8. O’Neil-Callahan M, Peipert JF, Zhao Q, et al. Twenty-four-month continuation of reversible contraception. Obstet Gynecol. 2013;122:1083-1091.
9. Grunloh DS, Casner T, Secura GM, et al. Characteristics associated with discontinuation of long-acting reversible contraception within the first 6 months of use. Obstet Gynecol. 2011;117:705-719.
10. Birgisson NE, Zhao Q, Secura GM, et al. Preventing unintended pregnancy: the contraceptive CHOICE project in review. J Womens Health (Larchmt). 2015;24:349-353.
11. ParaGard T 380A. (intrauterine copper contraceptive) [package insert]. Sellersville, PA : Teva Pharmaceuticals USA, Inc., 2013.
12. Skyla (levonorgestrel-releasing intrauterine system) [package insert]. Wayne, NJ : Bayer HealthCare Pharmaceuticals, Inc., 2013.
13. Liletta (levonorgestrel-releasing intrauterine system) [package insert]. Parsippany, NJ : Actavis Pharma, Inc., 2015.
14. Mirena (levonorgestrel-releasing intrauterine system) [package insert]. Whippany, NJ : Bayer HealthCare Pharmaceuticals, Inc., 2014.
15. Nexplanon (etongestrel implant) [package insert]. Whitehouse Station, NJ: Merck & Co Inc.; 2014.
16. Wu JP, Pickle S. Extended use of the intrauterine device: a literature review and recommendations for clinical practice. Contraception. 2014;89:495-503.
17. Stoddard A, McNicholas C, Peipert JF. Efficacy and safety of long-acting reversible contraception. Drugs. 2011;71:969-980.
18. Xu H, Wade JA, Peipert JF, et al. Contraceptive failure rates of etonogestrel subdermal implants in overweight and obese women. Obstet Gynecol. 2012;120:21-26.
19. Centers for Disease Control and Prevention (CDC). US selected practice recommendations for contraceptive use. MMWR Recomm Rep. 2013;62:1-60.
20. Centers for Disease Control and Prevention (CDC). Sexually transmitted disease treatment guidelines. MMWR Recomm Rep. 2010;59:1-110.
21. Gurtcheff SE, Turok DK, Stoddard G, et al. Lactogenesis after early postpartum use of the contraceptive implant: a randomized controlled trial. Obstet Gynecol. 2011;117:1114-1121.
22. Chen BA, Reeves MF, Creinin MD, et al. Postplacental or delayed levonorgestrel intrauterine device insertion and breast-feeding duration. Contraception. 2011;84:499-504.
23. Heinemann K, Reed S, Moehner S, et al. Risk of uterine perforation with levonorgestrel-releasing and copper intrauterine devices in the European Active Surveillance Study on Intrauterine Devices. Contraception. 2015;91:274-279.
24. Tocce K, Sheeder J, Python J, et al. Long acting reversible contraception in postpartum adolescents: early initiation of etonogestrel implant is superior to IUDs in the outpatient setting. J Pediatr Adolesc Gynecol. 2012;25:59-63.
25. Mwalwanda CS, Black KI. Immediate post-partum initiation of intrauterine contraception and implants: a review of the safety and guidelines for use. Aust N Z J Obstet Gynaecol. 2013;53:331-337.
26. Sober, S, Schreiber CA. Postpartum contraception. Clin Obstet Gynecol. 2014;57:763-776.
27. Dickerson LM, Diaz VA, Jordon J, et al. Satisfaction, early removal, and side effects associated with long-acting reversible contraception. Fam Med. 2013;45:701-707.
28. Berenson AB, Tan A, Hirth JM. Complications and continuation rates associated with 2 types of long-acting contraception. Am J Obstet Gynecol. 2015;212:e1-e8.
29. Kavanaugh ML, Frowirth L, Jerman J, et al. Long-acting reversible contraception for adolescents and young adults: patient and provider perspectives. J Pediatr Adolesc Gynecol. 2013;86:86-95.
30. Espey E, Ogburn T. Long-acting reversible contraceptives: intrauterine devices and the contraceptive implant. Obstet Gynecol. 2011;117:705-719.
31. Madden T, McNicholas C, Zhao Q, et al. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124:718-726.