User login
Aneurysmal Bone Cyst Involving the Metacarpal Bone in a Child
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
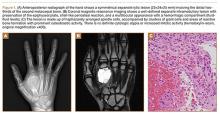
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
Subtrochanteric Femur Fracture After Removal of Screws for Femoral Neck Fracture in a Child
Subtrochanteric fractures and other complications related to hardware removal in children with slipped capital femoral epiphysis (SCFE) have been well documented.1-3 Subtrochanteric fractures after cannulated screw fixation of femoral neck fractures in adults have also been well recognized,4 and there are several reports on the topic.4,5 However, there are no reports on subtrochanteric fractures after removal of the screws for femoral neck fractures in children.
In this article, we report the case of a child who sustained a subtrochanteric fracture after the screw removal and healing that followed a femoral neck fracture. The patient’s parent provided written informed consent for print and electronic publication of this case report. In addition, our institutional review board approved this case report.
Case Report
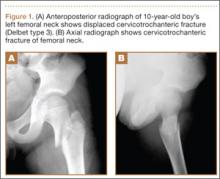
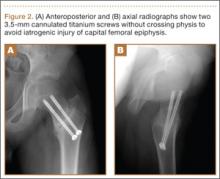
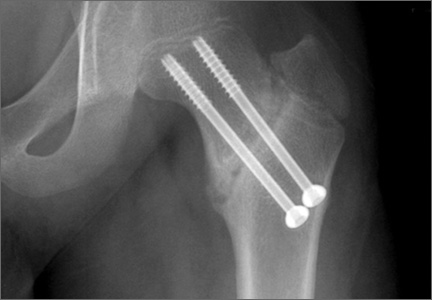
A 10-year-old boy was brought to our emergency department with the chief complaint of left hip pain after a car accident. Anteroposterior and axial lateral radiographs showed a displaced cervicotrochanteric femoral neck fracture (Figures 1A, 1B). The patient was admitted to the hospital and underwent closed reduction and internal fixation with two 3.5-mm cannulated titanium screws within 12 hours of arrival. The screws did not cross the physis to avoid iatrogenic injury of the capital femoral epiphysis (Figures 2A, 2B). The entry point was located at the lower level of the lesser trochanter. The lateral cortex was penetrated only once by the guide wire for the placement of each screw.
The patient was discharged to home care with a crutch and an ischial weight-bearing long leg brace for protection from unexpected external force. Two months after surgery, we allowed the patient to walk with the brace and without the crutch. Full-weight-bearing ambulation was allowed 3 months after surgery.
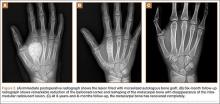
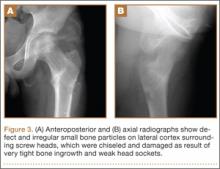
About 9 months after initial surgery, we removed 2 titanium screws, which were completely covered with growing new bone. The lateral cortex surrounding the screw heads was chiseled from the lower level of the lesser trochanter to remove the completely immersed screw heads (Figures 3A, 3B).
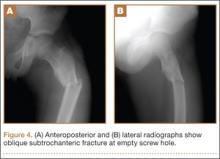
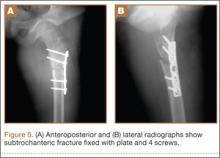
After screw removal, we recommended non-weight-bearing crutch-walking for 2 weeks followed by partial weight-bearing with crutch for another month. However, the patient started full weight-bearing 2 weeks after screw removal. One month after screw removal, he was brought to the emergency department with severe left hip pain after missing a step on a path. Anteroposterior and lateral radiographs showed an oblique subtrochanteric fracture at the empty screw holes (Figures 4A, 4B). A plate and 4 screws were placed to stabilize the subtrochanteric fracture, and a hip spica cast was applied and was to be worn for 3 weeks (Figures 5A, 5B).
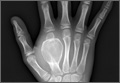
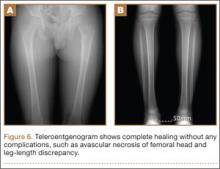
At final follow-up, 6 months after the second surgery, the fracture was healed, and there had been no complications, such as avascular necrosis of the femoral head and leg-length discrepancy (Figures 6A, 6B).
Discussion
Although in situ pinning of SCFE is a common procedure with good results, the rate of complications of hardware removal can be as high as 34%; these complications are well documented.5 Subtrochanteric fracture as a complication of proximal femoral neck pinning in adults is also well documented.4,5 However, there are no reports on subtrochanteric fractures after screw removal in the treatment of femoral neck fractures in children.
Brooks and colleagues6 emphasized the point that multiple passes weakened the lateral cortex, decreased the energy-absorbing capacity by 55.2%, and increased local stress. Even if a screw is placed in a relatively safe zone above the lesser trochanter, pie-crusting of the cortex can weaken it enough to predispose it to failure under a relatively normal load.7 We inserted 2 cannulated titanium screws without repositioning or multiple drilling, and the femoral neck fracture was united.
The common denominator for subtrochanteric fractures after screw or pin fixation of femoral neck fractures in adults seemed to be the entry point of the lateral cortex below the level of the most inferior edge of the lesser trochanter.4 The pin should have its entry site proximal to the level of the lesser trochnater. Paloski and colleagues7 and Canale and colleagues8 hypothesized that this screw acted as a stress riser to the normal bone, which underwent abnormal loads caused by the patient’s habitus and later mechanism of injury. In our patient’s case, the appropriate starting point for perpendicular penetration of the femoral neck fracture line was on the lateral femoral cortex at the level of the lesser trochanter. We thought this entry on the lateral cortex might predispose the patient to a subtrochanteric fracture. The starting point of the screw is considered the most important factor in preventing fracture after screw removal.
As titanium pins cause very tight bone ingrowth,9,10 the surface of titanium screws used for femoral neck fractures in children are smoothed to reduce turning force.1 The hexagonal sockets wore off rapidly and proved to be too weak to overcome the necessary torque for loosening the pin from the bone.
Lee and colleagues10 found that significantly more operative time was needed to remove titanium pins (vs steel pins) after 12 months or longer. When Asnis III pins (Howmedica, Rutherford, New Jersey) were used in the treatment of femoral neck fractures in aged patients, similar problems did not occur. One possible explanation is that bone density is higher in adolescents than in adults. In addition, more bone ingrowth and higher bone compression might occur in adolescent bones.1 Given the considerable disadvantages noted in their series, Ilchmann and Parsch1 concluded that use of cannulated titanium screws should be suspended and that stainless steel pins are safe to use in SCFE.
In our patient’s case, we also struggled to remove titanium screws. Subtrochanteric fractures can be complications after removal of screws for femoral neck fractures in children. If there are no specific screw-related symptoms, one should consider leaving the screw in place and avoiding screw removal.
1. Ilchmann T, Parsch K. Complications at screw removal in slipped capital femoral epiphysis treated by cannulated titanium screws. Arch Orthop Trauma Surg. 2006;126(6):359-363.
2. Raney EM, Freccero LA, Dolan DE, Lighter R, Fillman L, Chambers HG. Evidence-based analysis of removal of orthopaedic implants in the pediatric population. J Pediatr Orthop. 2008;28(7):701-704.
3. Karagkevrekis CB, Rahman H. Subtrochanteric femoral fracture following removal of screw for slipped capital femoral epiphysis. Injury. 2003;38(4):320-321.
4. Kloen P, Rubel IF, Lyden JP, Helfet DL. Subtrochanteric fracture after cannulated screw fixation of femoral neck fractures: a report of four cases. J Orthop Trauma. 2003;17(3):225-229.
5. Karr RK, Schwab JP. Subtrochanteric fracture as complication of proximal femoral pinning. Clin Orthop. 1985;(194):214-217.
6. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
7. Paloski M, Taylor BC, Willits M. Subtrochanteric femur fracture after slipped capital femoral epiphysis pinning: a novel treatment. Adv Orthop. 2011;2011:809136.
8. Canale ST, Casillas M, Banta JV. Displaced femoral neck fractures at the bone–screw interface after in situ fixation of slipped capital femoral epiphysis. J Pediatr Orthop. 1997;17(2):212-215.
9. Vresilovic EJ, Spindler KP, Robertson WW Jr, Davidson RS, Drummond DS. Failure of pin removal after in situ pinning of slipped capital femoral epiphysis: a comparison of different pin types. J Pediatr Orthop. 1990;10(6):764-768.
10. Lee TK, Haynes RJ, Longo JA, Chu JR. Pin removal in slipped capital femoral epiphysis: the unsuitability of titanium devices. J Pediatr Orthop. 1996;16(1):49-52.
Subtrochanteric fractures and other complications related to hardware removal in children with slipped capital femoral epiphysis (SCFE) have been well documented.1-3 Subtrochanteric fractures after cannulated screw fixation of femoral neck fractures in adults have also been well recognized,4 and there are several reports on the topic.4,5 However, there are no reports on subtrochanteric fractures after removal of the screws for femoral neck fractures in children.
In this article, we report the case of a child who sustained a subtrochanteric fracture after the screw removal and healing that followed a femoral neck fracture. The patient’s parent provided written informed consent for print and electronic publication of this case report. In addition, our institutional review board approved this case report.
Case Report
A 10-year-old boy was brought to our emergency department with the chief complaint of left hip pain after a car accident. Anteroposterior and axial lateral radiographs showed a displaced cervicotrochanteric femoral neck fracture (Figures 1A, 1B). The patient was admitted to the hospital and underwent closed reduction and internal fixation with two 3.5-mm cannulated titanium screws within 12 hours of arrival. The screws did not cross the physis to avoid iatrogenic injury of the capital femoral epiphysis (Figures 2A, 2B). The entry point was located at the lower level of the lesser trochanter. The lateral cortex was penetrated only once by the guide wire for the placement of each screw.
The patient was discharged to home care with a crutch and an ischial weight-bearing long leg brace for protection from unexpected external force. Two months after surgery, we allowed the patient to walk with the brace and without the crutch. Full-weight-bearing ambulation was allowed 3 months after surgery.
About 9 months after initial surgery, we removed 2 titanium screws, which were completely covered with growing new bone. The lateral cortex surrounding the screw heads was chiseled from the lower level of the lesser trochanter to remove the completely immersed screw heads (Figures 3A, 3B).
After screw removal, we recommended non-weight-bearing crutch-walking for 2 weeks followed by partial weight-bearing with crutch for another month. However, the patient started full weight-bearing 2 weeks after screw removal. One month after screw removal, he was brought to the emergency department with severe left hip pain after missing a step on a path. Anteroposterior and lateral radiographs showed an oblique subtrochanteric fracture at the empty screw holes (Figures 4A, 4B). A plate and 4 screws were placed to stabilize the subtrochanteric fracture, and a hip spica cast was applied and was to be worn for 3 weeks (Figures 5A, 5B).
At final follow-up, 6 months after the second surgery, the fracture was healed, and there had been no complications, such as avascular necrosis of the femoral head and leg-length discrepancy (Figures 6A, 6B).
Discussion
Although in situ pinning of SCFE is a common procedure with good results, the rate of complications of hardware removal can be as high as 34%; these complications are well documented.5 Subtrochanteric fracture as a complication of proximal femoral neck pinning in adults is also well documented.4,5 However, there are no reports on subtrochanteric fractures after screw removal in the treatment of femoral neck fractures in children.
Brooks and colleagues6 emphasized the point that multiple passes weakened the lateral cortex, decreased the energy-absorbing capacity by 55.2%, and increased local stress. Even if a screw is placed in a relatively safe zone above the lesser trochanter, pie-crusting of the cortex can weaken it enough to predispose it to failure under a relatively normal load.7 We inserted 2 cannulated titanium screws without repositioning or multiple drilling, and the femoral neck fracture was united.
The common denominator for subtrochanteric fractures after screw or pin fixation of femoral neck fractures in adults seemed to be the entry point of the lateral cortex below the level of the most inferior edge of the lesser trochanter.4 The pin should have its entry site proximal to the level of the lesser trochnater. Paloski and colleagues7 and Canale and colleagues8 hypothesized that this screw acted as a stress riser to the normal bone, which underwent abnormal loads caused by the patient’s habitus and later mechanism of injury. In our patient’s case, the appropriate starting point for perpendicular penetration of the femoral neck fracture line was on the lateral femoral cortex at the level of the lesser trochanter. We thought this entry on the lateral cortex might predispose the patient to a subtrochanteric fracture. The starting point of the screw is considered the most important factor in preventing fracture after screw removal.
As titanium pins cause very tight bone ingrowth,9,10 the surface of titanium screws used for femoral neck fractures in children are smoothed to reduce turning force.1 The hexagonal sockets wore off rapidly and proved to be too weak to overcome the necessary torque for loosening the pin from the bone.
Lee and colleagues10 found that significantly more operative time was needed to remove titanium pins (vs steel pins) after 12 months or longer. When Asnis III pins (Howmedica, Rutherford, New Jersey) were used in the treatment of femoral neck fractures in aged patients, similar problems did not occur. One possible explanation is that bone density is higher in adolescents than in adults. In addition, more bone ingrowth and higher bone compression might occur in adolescent bones.1 Given the considerable disadvantages noted in their series, Ilchmann and Parsch1 concluded that use of cannulated titanium screws should be suspended and that stainless steel pins are safe to use in SCFE.
In our patient’s case, we also struggled to remove titanium screws. Subtrochanteric fractures can be complications after removal of screws for femoral neck fractures in children. If there are no specific screw-related symptoms, one should consider leaving the screw in place and avoiding screw removal.
Subtrochanteric fractures and other complications related to hardware removal in children with slipped capital femoral epiphysis (SCFE) have been well documented.1-3 Subtrochanteric fractures after cannulated screw fixation of femoral neck fractures in adults have also been well recognized,4 and there are several reports on the topic.4,5 However, there are no reports on subtrochanteric fractures after removal of the screws for femoral neck fractures in children.
In this article, we report the case of a child who sustained a subtrochanteric fracture after the screw removal and healing that followed a femoral neck fracture. The patient’s parent provided written informed consent for print and electronic publication of this case report. In addition, our institutional review board approved this case report.
Case Report
A 10-year-old boy was brought to our emergency department with the chief complaint of left hip pain after a car accident. Anteroposterior and axial lateral radiographs showed a displaced cervicotrochanteric femoral neck fracture (Figures 1A, 1B). The patient was admitted to the hospital and underwent closed reduction and internal fixation with two 3.5-mm cannulated titanium screws within 12 hours of arrival. The screws did not cross the physis to avoid iatrogenic injury of the capital femoral epiphysis (Figures 2A, 2B). The entry point was located at the lower level of the lesser trochanter. The lateral cortex was penetrated only once by the guide wire for the placement of each screw.
The patient was discharged to home care with a crutch and an ischial weight-bearing long leg brace for protection from unexpected external force. Two months after surgery, we allowed the patient to walk with the brace and without the crutch. Full-weight-bearing ambulation was allowed 3 months after surgery.
About 9 months after initial surgery, we removed 2 titanium screws, which were completely covered with growing new bone. The lateral cortex surrounding the screw heads was chiseled from the lower level of the lesser trochanter to remove the completely immersed screw heads (Figures 3A, 3B).
After screw removal, we recommended non-weight-bearing crutch-walking for 2 weeks followed by partial weight-bearing with crutch for another month. However, the patient started full weight-bearing 2 weeks after screw removal. One month after screw removal, he was brought to the emergency department with severe left hip pain after missing a step on a path. Anteroposterior and lateral radiographs showed an oblique subtrochanteric fracture at the empty screw holes (Figures 4A, 4B). A plate and 4 screws were placed to stabilize the subtrochanteric fracture, and a hip spica cast was applied and was to be worn for 3 weeks (Figures 5A, 5B).
At final follow-up, 6 months after the second surgery, the fracture was healed, and there had been no complications, such as avascular necrosis of the femoral head and leg-length discrepancy (Figures 6A, 6B).
Discussion
Although in situ pinning of SCFE is a common procedure with good results, the rate of complications of hardware removal can be as high as 34%; these complications are well documented.5 Subtrochanteric fracture as a complication of proximal femoral neck pinning in adults is also well documented.4,5 However, there are no reports on subtrochanteric fractures after screw removal in the treatment of femoral neck fractures in children.
Brooks and colleagues6 emphasized the point that multiple passes weakened the lateral cortex, decreased the energy-absorbing capacity by 55.2%, and increased local stress. Even if a screw is placed in a relatively safe zone above the lesser trochanter, pie-crusting of the cortex can weaken it enough to predispose it to failure under a relatively normal load.7 We inserted 2 cannulated titanium screws without repositioning or multiple drilling, and the femoral neck fracture was united.
The common denominator for subtrochanteric fractures after screw or pin fixation of femoral neck fractures in adults seemed to be the entry point of the lateral cortex below the level of the most inferior edge of the lesser trochanter.4 The pin should have its entry site proximal to the level of the lesser trochnater. Paloski and colleagues7 and Canale and colleagues8 hypothesized that this screw acted as a stress riser to the normal bone, which underwent abnormal loads caused by the patient’s habitus and later mechanism of injury. In our patient’s case, the appropriate starting point for perpendicular penetration of the femoral neck fracture line was on the lateral femoral cortex at the level of the lesser trochanter. We thought this entry on the lateral cortex might predispose the patient to a subtrochanteric fracture. The starting point of the screw is considered the most important factor in preventing fracture after screw removal.
As titanium pins cause very tight bone ingrowth,9,10 the surface of titanium screws used for femoral neck fractures in children are smoothed to reduce turning force.1 The hexagonal sockets wore off rapidly and proved to be too weak to overcome the necessary torque for loosening the pin from the bone.
Lee and colleagues10 found that significantly more operative time was needed to remove titanium pins (vs steel pins) after 12 months or longer. When Asnis III pins (Howmedica, Rutherford, New Jersey) were used in the treatment of femoral neck fractures in aged patients, similar problems did not occur. One possible explanation is that bone density is higher in adolescents than in adults. In addition, more bone ingrowth and higher bone compression might occur in adolescent bones.1 Given the considerable disadvantages noted in their series, Ilchmann and Parsch1 concluded that use of cannulated titanium screws should be suspended and that stainless steel pins are safe to use in SCFE.
In our patient’s case, we also struggled to remove titanium screws. Subtrochanteric fractures can be complications after removal of screws for femoral neck fractures in children. If there are no specific screw-related symptoms, one should consider leaving the screw in place and avoiding screw removal.
1. Ilchmann T, Parsch K. Complications at screw removal in slipped capital femoral epiphysis treated by cannulated titanium screws. Arch Orthop Trauma Surg. 2006;126(6):359-363.
2. Raney EM, Freccero LA, Dolan DE, Lighter R, Fillman L, Chambers HG. Evidence-based analysis of removal of orthopaedic implants in the pediatric population. J Pediatr Orthop. 2008;28(7):701-704.
3. Karagkevrekis CB, Rahman H. Subtrochanteric femoral fracture following removal of screw for slipped capital femoral epiphysis. Injury. 2003;38(4):320-321.
4. Kloen P, Rubel IF, Lyden JP, Helfet DL. Subtrochanteric fracture after cannulated screw fixation of femoral neck fractures: a report of four cases. J Orthop Trauma. 2003;17(3):225-229.
5. Karr RK, Schwab JP. Subtrochanteric fracture as complication of proximal femoral pinning. Clin Orthop. 1985;(194):214-217.
6. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
7. Paloski M, Taylor BC, Willits M. Subtrochanteric femur fracture after slipped capital femoral epiphysis pinning: a novel treatment. Adv Orthop. 2011;2011:809136.
8. Canale ST, Casillas M, Banta JV. Displaced femoral neck fractures at the bone–screw interface after in situ fixation of slipped capital femoral epiphysis. J Pediatr Orthop. 1997;17(2):212-215.
9. Vresilovic EJ, Spindler KP, Robertson WW Jr, Davidson RS, Drummond DS. Failure of pin removal after in situ pinning of slipped capital femoral epiphysis: a comparison of different pin types. J Pediatr Orthop. 1990;10(6):764-768.
10. Lee TK, Haynes RJ, Longo JA, Chu JR. Pin removal in slipped capital femoral epiphysis: the unsuitability of titanium devices. J Pediatr Orthop. 1996;16(1):49-52.
1. Ilchmann T, Parsch K. Complications at screw removal in slipped capital femoral epiphysis treated by cannulated titanium screws. Arch Orthop Trauma Surg. 2006;126(6):359-363.
2. Raney EM, Freccero LA, Dolan DE, Lighter R, Fillman L, Chambers HG. Evidence-based analysis of removal of orthopaedic implants in the pediatric population. J Pediatr Orthop. 2008;28(7):701-704.
3. Karagkevrekis CB, Rahman H. Subtrochanteric femoral fracture following removal of screw for slipped capital femoral epiphysis. Injury. 2003;38(4):320-321.
4. Kloen P, Rubel IF, Lyden JP, Helfet DL. Subtrochanteric fracture after cannulated screw fixation of femoral neck fractures: a report of four cases. J Orthop Trauma. 2003;17(3):225-229.
5. Karr RK, Schwab JP. Subtrochanteric fracture as complication of proximal femoral pinning. Clin Orthop. 1985;(194):214-217.
6. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
7. Paloski M, Taylor BC, Willits M. Subtrochanteric femur fracture after slipped capital femoral epiphysis pinning: a novel treatment. Adv Orthop. 2011;2011:809136.
8. Canale ST, Casillas M, Banta JV. Displaced femoral neck fractures at the bone–screw interface after in situ fixation of slipped capital femoral epiphysis. J Pediatr Orthop. 1997;17(2):212-215.
9. Vresilovic EJ, Spindler KP, Robertson WW Jr, Davidson RS, Drummond DS. Failure of pin removal after in situ pinning of slipped capital femoral epiphysis: a comparison of different pin types. J Pediatr Orthop. 1990;10(6):764-768.
10. Lee TK, Haynes RJ, Longo JA, Chu JR. Pin removal in slipped capital femoral epiphysis: the unsuitability of titanium devices. J Pediatr Orthop. 1996;16(1):49-52.